|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
British Columbia, Canada
|
980597776
|
|
|
(State or Other Jurisdiction of
Incorporation or Organization)
|
(I.R.S. Employer
Identification No.)
|
|
|
100-8900 Glenlyon Parkway, Burnaby, BC V5J 5J8
(Address of Principal Executive Offices)
|
||
|
604-419-3200
(Registrant’s Telephone Number, Including Area Code):
|
||
|
Securities registered pursuant to Section 12(b) of the Act:
|
||
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|
|
Common shares, without par value
|
The NASDAQ Stock Market LLC
|
|
|
Securities registered pursuant to Section 12(g) of the Act:
|
||
|
Large accelerated filer
o
|
Accelerated filer
ý
|
Non-accelerated filer
o
|
Smaller reporting company
o
|
|
(Do not check if a smaller reporting company)
|
|||
|
Page
|
||
|
Item
1.
|
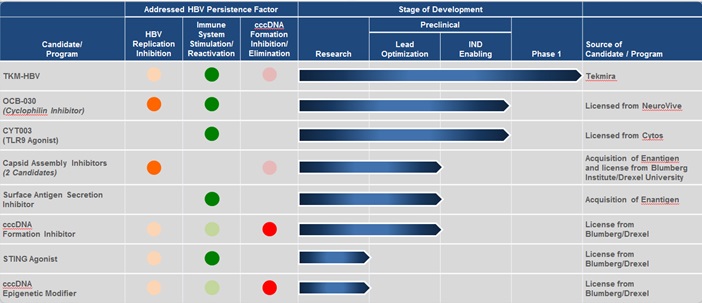
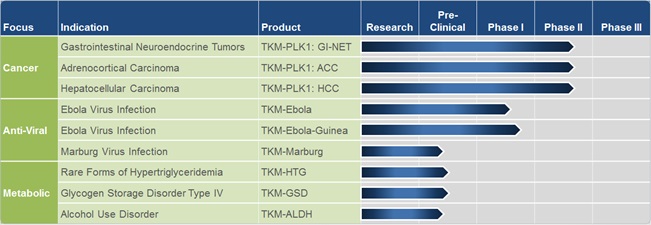
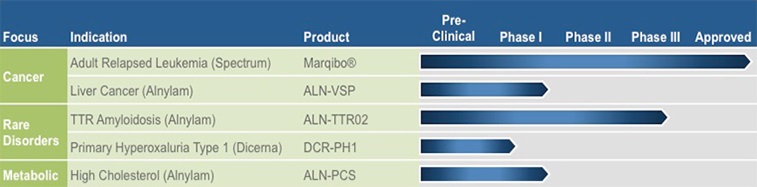
Business
|

|
Invention
Category
|
Title
|
Priority
Filing
Date*
|
Status**
|
Expiration
Date***
|
|
LNP
|
Lipid Encapsulated Interfering RNA
|
07/16/2003
|
U.S. Pat. No.7,982,027; applications pending in the U.S. and Europe
|
07/16/2024
|
|
LNP
|
Lipid Encapsulated Interfering RNA
|
06/07/2004
|
U.S. Pat. No. 7,799,565; European Pat. No.1766035; application pending in the U.S.
|
06/07/2025
|
|
LNP
|
Novel Lipid Formulations for Nucleic Acid Delivery
|
04/15/2008
|
U.S. Pat. Nos. 8,058,069; 8,492,359 and 8,822,668; applications pending in U.S. and Europe.
|
04/15/2029
|
|
LNP
|
Novel Lipid Formulations for Delivery of Therapeutic Agents to Solid Tumors
|
07/01/2009
|
U.S. Pat. No.8,283,333 Applications pending in the U.S. and Europe
|
06/30/2030
|
|
LNP
Manufacturing
|
Liposomal Apparatus and Manufacturing Methods
|
06/28/2002
|
U.S. Pat. Nos. 7,901,708 and 8,329,070; European Pat. No. 1519714; application pending in the U.S.; application allowed in Europe
|
06/30/2023
|
|
LNP
Manufacturing
|
Systems and Methods for Manufacturing Liposomes
|
07/27/2005
|
Application pending in the U.S. and Europe
|
07/27/2026
|
|
Novel Lipids
|
Cationic Lipids and Methods of Use
|
06/07/2004
|
U.S. Pat. No. 7,745,651; European Pat. No. 1781593; application pending in the U.S.
|
06/07/2025
|
|
Novel Lipids
|
Polyethyleneglycol-Modified Lipid Compounds and Uses Thereof
|
09/15/2003
|
U.S. Pat. No. 7,803,397; European Pat. No. 1664316; application pending in the U.S.
|
09/15/2024
|
|
Chemical
Modifications
|
Modified siRNA Molecules and Uses Thereof
|
11/02/2005
|
U.S. Pat. Nos. 8,101,741,8,188,263 and 8,513,403; applications pending in Europe and the U.S.
|
11/02/2026
|
|
Chemical
Modifications
|
Modified siRNA Molecules and Uses Thereof
|
06/09/2006
|
U.S. Pat. No. 7,915,399
|
06/08/2027
|
|
Therapeutic
Target
|
siRNA Silencing of Apolipoprotein B
|
11/17/2004
|
Application pending in Europe
|
11/17/2025
|
|
Therapeutic
Target
|
Compositions and Methods for Silencing Apolipoprotein B
|
07/01/2009
|
U.S. Pat. No. 8,236,943 application pending in Europe
|
06/30/2030
|
|
Therapeutic
Target
|
siRNA Silencing of Filovirus Gene Expression
|
10/20/2005
|
U.S. Pat. No. 7,838,658
|
10/20/2026
|
|
Therapeutic
Target
|
Compositions and Methods for Silencing Ebola Virus Gene Expression
|
07/20/2009
|
Application allowed in the U.S.
|
07/20/2030
|
|
Therapeutic
Target
|
Silencing of Polo-Like Kinase Expression using Interfering RNA
|
12/27/2007
|
Applications pending in the U.S. and Europe
|
12/23/2028
|
|
(1)
|
Patent information current as of January 8, 2015.
|
|
*
|
Priority filing dates are based on the filing dates of provisional patent applications. Provisional applications expire unless they are converted to non-provisional applications within one year.
|
|
**
|
An “allowed” patent application is an active case that has been found by the patent office to contain patentable subject matter, subject to the payment of issue/grant fees by the applicant.
|
| *** | Once issued, the term of a US patent first filed after mid-1995 generally extends until the 20th anniversary of the filing date of the first non-provisional application to which such patent claims priority. It is important to note, however, that the United States Patent & Trademark Office, or USPTO, sometimes requires the filing of a Terminal Disclaimer during prosecution, which may shorten the term of the patent. On the other hand, certain patent term adjustments may be available based on USPTO delays during prosecution. Similarly, in the pharmaceutical area, certain patent term extensions may be available based on the history of the drug in clinical trials. We cannot predict whether or not any such adjustments or extensions will be available or the length of any such adjustments or extensions. |
|
Item 1A.
|
Risk Factors
|
|
·
|
execute research and development activities using RNAi technology; and technologies involved in the development of HBV therapeutics;
|
|
|
·
|
build, maintain and protect a strong intellectual property portfolio;
|
|
|
·
|
gain acceptance for the development and commercialization of any product we develop;
|
|
|
·
|
develop and maintain successful strategic relationships; and
|
|
|
·
|
manage our spending and cash requirements as our expenses are expected to increase due to research and preclinical work, clinical trials, regulatory approvals, and commercialization and maintaining our intellectual property portfolio
|
|
·
|
we may not be able to attract and build a significant marketing or sales force;
|
|
|
·
|
the cost of establishing a marketing or sales force may not be justifiable in light of the revenues generated by any particular product; and
|
|
|
·
|
our direct sales and marketing efforts may not be successful.
|
|
·
|
revenues earned from our partners, including Alnylam, Spectrum, Monsanto, and Dicerna;
|
|
|
·
|
revenues earned from our DoD contract to develop TKM-Ebola;
|
|
|
·
|
the extent to which we continue the development of our product candidates or form collaborative relationships to advance our products;
|
|
|
·
|
our decisions to in-license or acquire additional products or technology for development,
|
|
|
·
|
our ability to attract and retain corporate partners, and their effectiveness in carrying out the development and ultimate commercialization of our product candidates;
|
|
|
·
|
whether batches of drugs that we manufacture fail to meet specifications resulting in delays and investigational and remanufacturing costs;
|
|
|
·
|
the decisions, and the timing of decisions, made by health regulatory agencies regarding our technology and products;
|
|
|
·
|
competing technological and market developments; and
|
|
|
·
|
prosecuting and enforcing our patent claims and other intellectual property rights.
|
|
·
|
controlled research and human clinical testing;
|
|
|
·
|
establishment of the safety and efficacy of the product for each use sought;
|
|
|
·
|
government review and approval of a submission containing manufacturing, pre-clinical and clinical data;
|
|
|
·
|
adherence to Good Manufacturing Practice Regulations during production and storage; and
|
|
|
·
|
control of marketing activities, including advertising and labelling
|
|
·
|
decreased demand for our product candidates;
|
|
|
·
|
impairment of our business reputation;
|
|
|
·
|
withdrawal of clinical trial participants;
|
|
|
·
|
costs of related litigation;
|
|
|
·
|
substantial monetary awards to patients or other claimants;
|
|
|
·
|
loss of revenues; and
|
|
|
·
|
inability to commercialize our product candidates.
|
|
·
|
some or all patent applications may not result in the issuance of a patent;
|
|
|
·
|
patents issued may not provide the holder with any competitive advantages;
|
|
|
·
|
patents could be challenged by third parties;
|
|
|
·
|
the patents of others, including Alnylam, could impede our ability to do business;
|
|
|
·
|
competitors may find ways to design around our patents; and
|
|
|
·
|
competitors could independently develop products which duplicate our products.
|
|
·
|
much greater financial, technical and human resources than we have at every stage of the discovery, development, manufacture and commercialization process;
|
|
|
·
|
more extensive experience in pre-clinical testing, conducting clinical trials, obtaining regulatory approvals, and in manufacturing, marketing and selling pharmaceutical products;
|
|
|
·
|
product candidates that are based on previously tested or accepted technologies;
|
|
|
·
|
products that have been approved or are in late stages of development; and
|
|
|
·
|
collaborative arrangements in our target markets with leading companies and research institutions.
|
|
·
|
safety and effectiveness of our products
|
|
|
·
|
ease with which our products can be administered and the extent to which patients and physicians accept new routes of administration;
|
|
|
·
|
timing and scope of regulatory approvals for these products;
|
|
|
·
|
availability and cost of manufacturing, marketing and sales capabilities;
|
|
|
·
|
price;
|
|
|
·
|
reimbursement coverage; and
|
|
|
·
|
patent position.
|
|
•
|
discover, develop and commercialize drugs that are superior to other products in the market;
|
|
•
|
demonstrate through our clinical trials that our drug candidates are differentiated from existing and future therapies;
|
|
•
|
attract qualified scientific, product development and commercial personnel;
|
|
•
|
obtain patent or other proprietary protection for our drugs and technologies;
|
|
•
|
obtain required regulatory approvals;
|
|
•
|
successfully collaborate with pharmaceutical companies in the discovery, development and commercialization of new drugs; and
|
|
•
|
negotiate competitive pricing and reimbursement with third party payors.
|
|
·
|
general economic and political conditions in Canada, the United States and globally;
|
|
|
·
|
governmental regulation of the health care and pharmaceutical industries;
|
|
|
·
|
failure to achieve desired drug discovery outcomes by us or our collaborators;
|
|
|
·
|
failure to obtain industry partner and other third party consents and approvals, when required;
|
|
|
·
|
stock market volatility and market valuations;
|
|
|
·
|
competition for, among other things, capital, drug targets and skilled personnel;
|
|
|
·
|
the need to obtain required approvals from regulatory authorities;
|
|
|
·
|
revenue and operating results failing to meet expectations in any particular period;
|
|
|
·
|
investor perception of the health care and pharmaceutical industries;
|
|
|
·
|
limited trading volume of our Common Shares;
|
|
|
·
|
announcements relating to our business or the businesses of our competitors; and
|
|
|
·
|
our ability or inability to raise additional funds.
|
|
Item 1B.
|
Unresolved Staff Comments
|
|
Item 2.
|
Properties
|
|
Item 3.
|
Legal Proceedings
|
|
Item 4.
|
Mine Safety Disclosures
|
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
|
|
NASDAQ
High
(US$)
|
|
NASDAQ
Low
(US$)
|
|
TSX
High
(C$)
|
|
TSX
Low
(C$)
|
|||||||||
|
Year Ended:
|
|
|
|
|
||||||||||||
|
December 31, 2014
|
|
$
|
31.48
|
|
|
$
|
7.65
|
|
|
$
|
34.66
|
|
|
$
|
8.14
|
|
|
December 31, 2013
|
|
$
|
11.42
|
|
|
$
|
4.18
|
|
|
$
|
11.62
|
|
|
$
|
4.31
|
|
|
Quarter Ended:
|
|
|
|
|
||||||||||||
|
December 31, 2014
|
|
$
|
29.93
|
|
|
$
|
12.54
|
|
|
$
|
33.69
|
|
|
$
|
14.37
|
|
|
September 30, 2014
|
|
$
|
26.05
|
|
|
$
|
8.86
|
|
|
$
|
28.56
|
|
|
$
|
9.55
|
|
|
June 30, 2014
|
|
$
|
24.47
|
|
|
$
|
10.20
|
|
|
$
|
26.99
|
|
|
$
|
11.08
|
|
|
March 31, 2014
|
|
$
|
31.48
|
|
|
$
|
7.65
|
|
|
$
|
34.66
|
|
|
$
|
8.14
|
|
|
December 31, 2013
|
|
$
|
11.42
|
|
|
$
|
6.93
|
|
|
$
|
11.62
|
|
|
$
|
7.16
|
|
|
September 30, 2013
|
|
$
|
7.72
|
|
|
$
|
4.70
|
|
|
$
|
7.90
|
|
|
$
|
4.96
|
|
|
June 30, 2013
|
|
$
|
5.25
|
|
|
$
|
4.25
|
|
|
$
|
5.34
|
|
|
$
|
4.35
|
|
|
March 31, 2013
|
|
$
|
5.53
|
|
|
$
|
4.18
|
|
|
$
|
5.45
|
|
|
$
|
4.31
|
|
|
Month Ended:
|
|
|
|
|
||||||||||||
|
February 28, 2015
|
|
$
|
25.49
|
|
|
$
|
17.50
|
|
|
$
|
33.76
|
|
|
$
|
17.05
|
|
|
January 31, 2015
|
|
$
|
26.73
|
|
|
$
|
14.50
|
|
|
$
|
32.19
|
|
|
$
|
21.90
|
|
|
Location
|
|
Number of Shares
|
|
Percentage of
Total Shares
|
Number of Registered
Shareholders of
Record
|
|||||||
|
Canada
|
|
15,776,736
|
|
|
33.9%
|
|
100
|
|
||||
|
United States
|
|
14,776,536
|
|
|
31.7%
|
22
|
|
|||||
|
Other
|
|
16,014,224
|
|
|
34.4%
|
4
|
|
|||||
|
|
|
|||||||||||
|
Total
|
|
46,567,496
|
|
|
100%
|
|
126
|
|
||||
|
Item 6.
|
Selected Consolidated Financial Data
|
|
Year Ended December 31,
|
||||||||||||||||||||
|
2014
|
2013
|
2012
|
2011
|
2010
|
||||||||||||||||
|
$
|
$
|
$
|
$
|
$
|
||||||||||||||||
|
Operating Data
|
||||||||||||||||||||
|
Revenue
|
14,953
|
15,465
|
14,105
|
16,812
|
20,745
|
|||||||||||||||
|
Expenses
|
47,925
|
27,617
|
27,050
|
27,505
|
32,900
|
|||||||||||||||
|
Loss from operations
|
(32,972
|
)
|
(12,152
|
)
|
(12,945
|
)
|
(10,694
|
)
|
(12,155
|
)
|
||||||||||
|
Net income (loss)
|
(38,837
|
)
|
(14,063
|
)
|
29,611
|
(10,083
|
)
|
(12,058
|
)
|
|||||||||||
|
Weighted average number of common shares—basic
(1)
|
21,603
|
15,303
|
13,728
|
11,319
|
10,333
|
|||||||||||||||
|
Weighted average number of common shares—diluted
(1)
|
21,603
|
15,303
|
14,321
|
11,319
|
10,333
|
|||||||||||||||
|
Income (loss) per common share—basic
|
(1.80
|
)
|
(0.92
|
)
|
2.16
|
(0.89
|
)
|
(1.17
|
)
|
|||||||||||
|
Income (loss) per common share—diluted
|
(1.80
|
)
|
(0.92
|
)
|
2.07
|
(0.89
|
)
|
(1.17
|
)
|
|||||||||||
|
Balance Sheet Data
|
||||||||||||||||||||
|
Total current assets
|
116,418
|
70,343
|
51,243
|
11,594
|
18,006
|
|||||||||||||||
|
Total assets
|
118,178
|
71,716
|
52,595
|
13,758
|
21,136
|
|||||||||||||||
|
Total liabilities
|
30,143
|
12,522
|
11,676
|
8,531
|
10,345
|
|||||||||||||||
|
Share capital
|
316,212
|
242,045
|
206,572
|
200,965
|
196,393
|
|||||||||||||||
|
Total stockholders’ equity
|
88,035
|
59,194
|
40,919
|
5,227
|
10,791
|
|||||||||||||||
|
Number of shares outstanding
(1)
|
22,438
|
19,049
|
14,305
|
12,149
|
10,339
|
|||||||||||||||
|
(1)
|
On November 4, 2010, Tekmira completed a consolidation of its common shares whereby five old common shares of Tekmira were exchanged for one new common share of Tekmira. Except as otherwise indicated, all references to common shares, common shares outstanding, average number of common shares outstanding, per share amounts and options in this document have been restated to reflect the common shares consolidation on a retroactive basis.
|
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
|
Q4
|
Q3
|
Q2
|
Q1
|
Q4
|
Q3
|
Q2
|
Q1
|
|||||||||||||||||||||||||
|
2014
|
2014
|
2014
|
2014
|
2013
|
2013
|
2013
|
2013
|
|||||||||||||||||||||||||
|
Revenue
|
||||||||||||||||||||||||||||||||
|
Collaborations and contracts:
|
||||||||||||||||||||||||||||||||
|
DoD
|
$
|
2.8
|
$
|
1.5
|
$
|
0.9
|
$
|
3.2
|
$
|
2.6
|
$
|
2.8
|
$
|
2.4
|
$
|
1.9
|
||||||||||||||||
|
Monsanto
|
0.3
|
0.3
|
0.2
|
0.3
|
—
|
—
|
—
|
—
|
||||||||||||||||||||||||
|
Dicerna
|
0.3
|
0.2
|
—
|
—
|
—
|
—
|
—
|
—
|
||||||||||||||||||||||||
|
Other
|
—
|
1.6
|
—
|
0.2
|
(0.1)
|
0.1
|
0.4
|
0.2
|
||||||||||||||||||||||||
|
3.4
|
3.6
|
1.1
|
3.7
|
2.6
|
2.9
|
2.8
|
2.1
|
|||||||||||||||||||||||||
|
Alnylam milestone payments
|
—
|
—
|
—
|
0.2
|
5.0
|
—
|
—
|
—
|
||||||||||||||||||||||||
|
Monsanto licensing fees and milestone payments
|
0.9
|
0.7
|
0.6
|
0.5
|
—
|
—
|
—
|
—
|
||||||||||||||||||||||||
|
Spectrum milestone and royalty payments
|
0.1
|
0.1
|
0.0
|
0.0
|
—
|
—
|
—
|
—
|
||||||||||||||||||||||||
|
Total revenue
|
4.4
|
4.4
|
1.8
|
4.4
|
7.6
|
2.9
|
2.8
|
2.1
|
||||||||||||||||||||||||
|
Expenses
|
(15.1)
|
(11.2)
|
(11.2)
|
(10.4)
|
(9.9)
|
(6.6)
|
(5.9)
|
(5.1)
|
||||||||||||||||||||||||
|
Other income (losses)
|
4.5
|
(1.8)
|
3.3
|
(12.0)
|
(0.2)
|
(2.2)
|
0.1
|
0.5
|
||||||||||||||||||||||||
|
Net loss
|
(6.2)
|
(8.6)
|
(6.1)
|
(18.0)
|
(2.6)
|
(5.9)
|
(3.0)
|
(2.5)
|
||||||||||||||||||||||||
|
Basic net loss per share
|
$
|
(0.27)
|
$
|
(0.39)
|
$
|
(0.28)
|
$
|
(0.91)
|
$
|
(0.15)
|
$
|
(0.41)
|
$
|
(0.21)
|
$
|
(0.17)
|
||||||||||||||||
|
Diluted net loss per share
|
$
|
(0.27)
|
$
|
(0.39)
|
$
|
(0.28)
|
$
|
(0.91)
|
$
|
(0.15)
|
$
|
(0.41)
|
$
|
(0.21)
|
$
|
(0.17)
|
||||||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Total revenue
|
15.0
|
15.5
|
14.1
|
|||||||||
|
Operating expenses
|
47.9
|
27.6
|
27.0
|
|||||||||
|
Loss from operations
|
(33.0
|
)
|
(12.2
|
)
|
(12.9
|
)
|
||||||
|
Net income (loss)
|
(38.8
|
)
|
(14.1
|
)
|
29.6
|
|||||||
|
Basic income (loss) per share
|
(1.80
|
)
|
(0.92
|
)
|
2.16
|
|||||||
|
Diluted income (loss) per share
|
(1.80
|
)
|
(0.92
|
)
|
2.07
|
|||||||
|
Total assets
|
118.2
|
71.7
|
52.6
|
|||||||||
|
Total liabilities
|
30.1
|
12.5
|
11.7
|
|||||||||
|
Total non-current liabilities
|
9.9
|
0.0
|
0.7
|
|||||||||
|
Deficit
|
(205.9
|
)
|
(167.0
|
)
|
(153.0
|
)
|
||||||
|
Accumulated other comprehensive loss
|
(22.3
|
)
|
(15.8
|
)
|
(12.7
|
)
|
||||||
|
Total stockholders’ equity
|
88.0
|
59.2
|
40.9
|
|||||||||
|
2014
|
% of Total
|
2013
|
% of Total
|
|||||||||||||
|
Collaborations and contracts
|
||||||||||||||||
|
DoD
|
8.4
|
56
|
%
|
9.8
|
63
|
%
|
||||||||||
|
Monsanto
|
1.1
|
7
|
%
|
-
|
-
|
|||||||||||
|
BMS
|
1.7
|
12
|
%
|
0.5
|
3
|
%
|
||||||||||
|
Other RNAi collaborators
|
0.5
|
3
|
%
|
0.1
|
1
|
%
|
||||||||||
|
Total collaborations and contracts
|
11.7
|
78
|
%
|
10.4
|
68
|
%
|
||||||||||
|
Monsanto licensing fees and milestone payments
|
2.7
|
19
|
%
|
-
|
-
|
|||||||||||
|
Alnylam milestone payments
|
0.2
|
1
|
%
|
5.0
|
32
|
%
|
||||||||||
|
Dicerna licensing fee
|
0.2
|
1
|
%
|
-
|
-
|
|||||||||||
|
Spectrum milestone and royalty payments
|
0.2
|
1
|
%
|
0.0
|
0
|
%
|
||||||||||
|
Total revenue
|
15.0
|
15.5
|
||||||||||||||
|
2014
|
% of Total
|
2013
|
% of Total
|
|||||||||||||
|
Research, development, collaborations and contracts
|
$
|
38.7
|
81
|
%
|
$
|
21.5
|
78
|
%
|
||||||||
|
General and administrative
|
8.7
|
17
|
%
|
5.5
|
20
|
%
|
||||||||||
|
Depreciation
|
0.5
|
1
|
%
|
0.6
|
2
|
%
|
||||||||||
|
Total operating expenses
|
$
|
47.9
|
$
|
27.6
|
||||||||||||
|
2014
|
2013
|
|||||||
|
Interest income
|
$
|
0.9
|
$
|
0.5
|
||||
|
Foreign exchange gains
|
4.1
|
1.1
|
||||||
|
Increase in fair value of warrant liability
|
(10.4
|
)
|
(3.5
|
)
|
||||
|
Acquisition costs
|
(0.5
|
)
|
-
|
|||||
|
Total other income (losses)
|
$
|
(5.9
|
)
|
$
|
(1.9
|
)
|
||
|
2013
|
% of Total
|
2012
|
% of Total
|
|||||||||||||
|
Collaborations and contracts
|
||||||||||||||||
|
DoD
|
$
|
9.8
|
63
|
%
|
$
|
11.5
|
82
|
%
|
||||||||
|
BMS
|
0.5
|
3
|
%
|
0.4
|
3
|
%
|
||||||||||
|
Other RNAi collaborators
|
0.1
|
1
|
%
|
0.1
|
1
|
%
|
||||||||||
|
Total collaborations and contracts
|
10.4
|
68
|
%
|
12.1
|
86
|
%
|
||||||||||
|
Alnylam milestone payments
|
5.0
|
32
|
%
|
1.0
|
7
|
%
|
||||||||||
|
Spectrum milestone and royalty payments
|
0.0
|
0
|
%
|
1.0
|
7
|
%
|
||||||||||
|
Total revenue
|
$
|
15.5
|
$
|
14.1
|
||||||||||||
|
2013
|
% of Total
|
2012
|
% of Total
|
|||||||||||||
|
Research, development, collaborations and contracts
|
$
|
21.5
|
78
|
%
|
$
|
18.0
|
67
|
%
|
||||||||
|
General and administrative
|
5.5
|
20
|
%
|
8.1
|
30
|
%
|
||||||||||
|
Depreciation
|
0.6
|
2
|
%
|
0.9
|
3
|
%
|
||||||||||
|
Total operating expenses
|
27.6
|
27.0
|
||||||||||||||
|
2013
|
2012
|
|||||||
|
Interest income
|
$
|
0.5
|
$
|
0.1
|
||||
|
Licensing settlement payment
|
-
|
65.0
|
||||||
|
Licensing settlement legal fees
|
-
|
(18.7
|
)
|
|||||
|
Foreign exchange gains
|
1.1
|
-
|
||||||
|
Increase in fair value of warrant liability
|
(3.5
|
)
|
(3.8
|
)
|
||||
|
Total other income (losses)
|
$
|
(1.9
|
)
|
$
|
42.6
|
|||
|
Year ended December 31
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Net (loss) income for the year
|
(38.8
|
)
|
(14.1
|
)
|
29.6
|
|||||||
|
Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activities
|
9.9
|
5.0
|
5.7
|
|||||||||
|
Changes in operating assets and liabilities
|
16.6
|
2.3
|
(2.4
|
)
|
||||||||
|
Net cash (used in) provided by operating activities
|
(12.3
|
)
|
(6.7
|
)
|
32.9
|
|||||||
|
Net cash used in investing activities
|
(43.0
|
)
|
(0.7
|
)
|
(0.0
|
)
|
||||||
|
Net cash provided by financing activities
|
60.7
|
32.7
|
4.5
|
|||||||||
|
Effect of foreign exchange rate changes on cash & cash equivalents
|
(1.8
|
)
|
(3.6
|
)
|
0.5
|
|||||||
|
Net increase in cash and cash equivalents
|
3.5
|
21.7
|
38.0
|
|||||||||
|
Cash and cash equivalents, beginning of year
|
68.7
|
47.0
|
9.0
|
|||||||||
|
Cash and cash equivalents, end of year
|
72.2
|
68.7
|
47.0
|
|||||||||
|
|
·
|
the need for additional capital to fund future business development programs, including the merger with OnCore;
|
|
|
·
|
revenues earned form our current collaborative partnership and licensing agreements with Monsanto and Dicerna;
|
|
|
·
|
revenues earned from our DoD contract to develop TKM-Ebola and TKM-Ebola-Guinea;
|
|
|
·
|
revenues earned from our legacy collaborative partnerships and licensing agreements, including milestone payments from Alnylam and royalties from sales of Marqibo from Spectrum;
|
|
|
·
|
the extent to which we continue the development of our product candidates, add new product candidates to our pipeline, or form collaborative relationships to advance our products;
|
|
|
·
|
our decisions to in-license or acquire additional products or technology for development, in particular for our RNAi therapeutics programs;
|
|
|
·
|
our ability to attract and retain corporate partners, and their effectiveness in carrying out the development and ultimate commercialization of our product candidates;
|
|
|
·
|
whether batches of drugs that we manufacture fail to meet specifications resulting in delays and investigational and remanufacturing costs;
|
|
|
·
|
the decisions, and the timing of decisions, made by health regulatory agencies regarding our technology and products;
|
|
|
·
|
competing technological and market developments; and
|
|
|
·
|
costs associated with prosecuting and enforcing our patent claims and other intellectual property rights, including litigation and arbitration arising in the course of our business activities.
|
|
(in millions $)
|
Payments Due by Period
|
|||||||||||||||||||
|
Total
|
Less
than 1 year
|
1 – 3
years
|
3 – 5
years
|
More than
5 years
|
||||||||||||||||
|
Contractual Obligations
|
||||||||||||||||||||
|
Facility lease
|
5.1
|
1.1
|
2.2
|
1.8
|
—
|
|||||||||||||||
|
Technology license obligations
(1)
|
0.3
|
0.3
|
—
|
—
|
—
|
|||||||||||||||
|
Total contractual obligations
|
5.4
|
1.4
|
2.2
|
1.8
|
—
|
|||||||||||||||
|
Item 7A.
|
Quantitative and Qualitative Disclosures about Market Risk
|
|
Item 8.
|
Financial Statements and Supplementary Data
|
|
Page
|
||
|
December 31
2014
|
December 31
2013
|
|||||||
|
Assets
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$ | 72,187 | $ | 68,717 | ||||
|
Short-term investments (note 2)
|
39,974 | - | ||||||
|
Accounts receivable
|
1,903 | 117 | ||||||
|
Accrued revenue
|
538 | 212 | ||||||
|
Deferred expenses
|
- | 173 | ||||||
|
Investment tax credits receivable
|
86 | 40 | ||||||
|
Prepaid expenses and other assets (note 6(a))
|
1,730 | 1,084 | ||||||
|
Total current assets
|
116,418 | 70,343 | ||||||
|
Property and equipment (note 4)
|
12,959 | 13,039 | ||||||
|
Less accumulated depreciation (note 4)
|
(11,199 | ) | (11,666 | ) | ||||
|
Property and equipment, net of accumulated
depreciation (note 4)
|
1,760 | 1,373 | ||||||
|
Total assets
|
$ | 118,178 | $ | 71,716 | ||||
|
Liabilities and stockholders' equity
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable and accrued liabilities (note 10)
|
$ | 9,328 | $ | 3,680 | ||||
|
Deferred revenue (note 3)
|
5,779 | 3,463 | ||||||
|
Warrants (note 2 and 5)
|
5,099 | 5,379 | ||||||
|
Total current liabilities
|
20,206 | 12,522 | ||||||
|
Deferred revenue, net of current portion (note 3)
|
9,937 | - | ||||||
|
Total liabilities
|
30,143 | 12,522 | ||||||
|
Stockholders’ equity:
|
||||||||
|
Common shares (note 5)
|
||||||||
|
Authorized - unlimited number with no par value
|
||||||||
|
Issued and outstanding:
22,438,169 (December 31, 2013 - 19,048,900)
|
290,004 | 216,702 | ||||||
|
Additional paid-in capital
|
26,208 | 25,343 | ||||||
|
Deficit
|
(205,864 | ) | (167,027 | ) | ||||
|
Accumulated other comprehensive loss
|
(22,313 | ) | (15,824 | ) | ||||
|
Total stockholders' equity
|
88,035 | 59,194 | ||||||
|
Total liabilities and stockholders' equity
|
$ | 118,178 | $ | 71,716 | ||||
|
Year ended
December 31
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Revenue (note 3)
|
||||||||||||
|
Collaborations and contracts
|
$ | 11,738 | $ | 10,425 | $ | 12,105 | ||||||
|
Licensing fees, milestone and
royalty payments
|
3,215 | 5,040 | 2,000 | |||||||||
|
Total revenue
|
14,953 | 15,465 | 14,105 | |||||||||
|
Expenses
|
||||||||||||
|
Research, development, collaborations
and contracts
|
38,713 | 21,458 | 18,043 | |||||||||
|
General and administrative
|
8,683 | 5,546 | 8,141 | |||||||||
|
Depreciation of property and equipment
|
529 | 613 | 866 | |||||||||
|
Total expenses
|
47,925 | 27,617 | 27,050 | |||||||||
|
Loss from operations
|
(32,972 | ) | (12,152 | ) | (12,945 | ) | ||||||
|
Other income (losses)
|
||||||||||||
|
Interest income
|
853 | 540 | 138 | |||||||||
|
Licensing settlement payment (note 3(c))
|
- | - | 65,000 | |||||||||
|
Licensing settlement legal fees (note 3(c))
|
- | - | (18,738 | ) | ||||||||
|
Foreign exchange gains
|
4,127 | 1,079 | 25 | |||||||||
|
Warrant issuance costs (note 5)
|
- | - | (47 | ) | ||||||||
|
Increase in fair value of warrant liability (note 2)
|
(10,383 | ) | (3,530 | ) | (3,822 | ) | ||||||
|
Acquisition costs
|
(462 | ) | - | - | ||||||||
|
Net income (loss)
|
$ | (38,837 | ) | $ | (14,063 | ) | $ | 29,611 | ||||
|
Income (loss) per common share
|
||||||||||||
|
Basic
|
$ | (1.80 | ) | $ | (0.92 | ) | $ | 2.16 | ||||
|
Diluted
|
$ | (1.80 | ) | $ | (0.92 | ) | $ | 2.07 | ||||
|
Weighted average number of common shares
|
||||||||||||
|
Basic
|
21,603,136 | 15,302,680 | 13,727,925 | |||||||||
|
Diluted
|
21,603,136 | 15,302,680 | 14,320,814 | |||||||||
|
Comprehensive income (loss)
|
||||||||||||
|
Cumulative translation adjustment
|
(6,489 | ) | (3,135 | ) | 474 | |||||||
|
Comprehensive loss
|
$ | (45,326 | ) | $ | (17,198 | ) | $ | 30,085 | ||||
|
Number
of shares
|
Share
capital
|
Additional paid-in
capital
|
Deficit
|
Accumulated
other comprehensive
|
Total
stockholders'
|
|||||||||||||||||||
|
Balance, December 31, 2011
|
12,148,635 | $ | 177,039 | $ | 23,927 | $ | (182,575 | ) | $ | (13,163 | ) | $ | 5,228 | |||||||||||
|
Stock-based compensation
|
- | - | 982 | - | - | 982 | ||||||||||||||||||
|
Issuance of common shares
pursuant to exercise of options
|
38,635 | 194 | (123 | ) | - | - | 71 | |||||||||||||||||
|
Issuance of common shares
pursuant to exercise of warrants
|
269,485 | 1,513 | - | - | - | 1,513 | ||||||||||||||||||
|
Issuance of common shares in conjunction with the private offering, net of issuance costs of $179,000 and net of initial fair value of warrants of $851,000
|
1,848,601 | 3,040 | - | - | - | 3,040 | ||||||||||||||||||
|
Currency translation adjustment
|
- | - | - | - | 474 | 474 | ||||||||||||||||||
|
Net income
|
- | - | - | 29,611 | - | 29,611 | ||||||||||||||||||
|
Balance, December 31, 2012
|
14,305,356 | $ | 181,786 | $ | 24,786 | $ | (152,964 | ) | $ | (12,689 | ) | $ | 40,919 | |||||||||||
|
Stock-based compensation
|
- | - | 903 | - | - | 903 | ||||||||||||||||||
|
Issuance of common shares
pursuant to exercise of options
|
125,596 | 735 | (346 | ) | - | - | 389 | |||||||||||||||||
|
|
||||||||||||||||||||||||
|
Issuance of common shares
pursuant to exercise of warrants
|
305,448 | 2,143 | - | - | - | 2,143 | ||||||||||||||||||
|
Issuance of common shares in conjunction with the
private offering, net of issuance costs of $
2,462,000
|
4,312,500 | 32,038 | - | - | - | 32,038 | ||||||||||||||||||
|
Currency translation adjustment
|
- | - | - | - | (3,135 | ) | (3,135 | ) | ||||||||||||||||
|
Net loss
|
- | - | - | (14,063 | ) | - | (14,063 | ) | ||||||||||||||||
|
Balance, December 31, 2013
|
19,048,900 | $ | 216,702 | $ | 25,343 | $ | (167,027 | ) | $ | (15,824 | ) | $ | 59,194 | |||||||||||
|
Stock-based compensation
|
- | - | 3,283 | - | - | 3,283 | ||||||||||||||||||
|
Issuance of common shares
pursuant to exercise of options
|
648,506 | 5,034 | (2,418 | ) | - | - | 2,616 | |||||||||||||||||
|
|
||||||||||||||||||||||||
|
Issuance of common shares
pursuant to exercise of warrants
|
615,763 | 11,791 | - | - | - | 11,791 | ||||||||||||||||||
|
Issuance of common shares in conjunction with the
private offering, net of issuance costs of $4,085,000
|
2,125,000 | 56,477 | - | - | - | 56,477 | ||||||||||||||||||
|
Currency translation adjustment
|
- | - | - | - | (6,489 | ) | (6,489 | ) | ||||||||||||||||
|
Net loss
|
- | - | - | (38,837 | ) | - | (38,837 | ) | ||||||||||||||||
|
Balance, December 31, 2014
|
22,438,169 | $ | 290,004 | $ | 26,208 | $ | (205,864 | ) | $ | (22,313 | ) | $ | 88,035 | |||||||||||
|
Year ended
December 31
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
OPERATING ACTIVITIES
|
||||||||||||
|
Net income (loss) for the period
|
$ | (38,837 | ) | $ | (14,063 | ) | $ | 29,611 | ||||
|
Items not involving cash:
|
||||||||||||
|
Depreciation of property and equipment
|
529 | 613 | 866 | |||||||||
|
Gain on sale of property and equipment
|
(80 | ) | - | - | ||||||||
|
Stock-based compensation - research, development, collaborations
and contract expenses
|
2,343 | 622 | 772 | |||||||||
|
Stock-based compensation - general and administrative expenses
|
940 | 281 | 210 | |||||||||
|
Unrealized foreign exchange (gains) losses
|
(4,218 | ) | (18 | ) | 29 | |||||||
|
Warrant issuance costs
|
- | 47 | ||||||||||
|
Change in fair value of warrant liability
|
10,383 | 3,530 | 3,822 | |||||||||
|
Net change in non-cash operating items:
|
||||||||||||
|
Accounts receivable
|
(1,887 | ) | 889 | (190 | ) | |||||||
|
Accrued revenue
|
(360 | ) | 2,008 | (2,188 | ) | |||||||
|
Deferred expenses
|
167 | 231 | 361 | |||||||||
|
Investment tax credits receivable
|
(52 | ) | (31 | ) | 323 | |||||||
|
Prepaid expenses and other assets
|
(773 | ) | (776 | ) | 97 | |||||||
|
Accounts payable and accrued liabilities
|
6,253 | 130 | (197 | ) | ||||||||
|
Deferred revenue
|
13,171 | (153 | ) | (655 | ) | |||||||
|
Net cash provided by (used in) operating activities
|
(12,421 | ) | (6,737 | ) | 32,908 | |||||||
|
INVESTING ACTIVITIES
|
||||||||||||
|
Acquisition of investments
|
(41,982 | ) | - | - | ||||||||
|
Proceeds from sale of property and equipment
|
80 | - | 3 | |||||||||
|
Acquisition of property and equipment
|
(1,056 | ) | (725 | ) | (15 | ) | ||||||
|
Net cash used in investing activities
|
(42,958 | ) | (725 | ) | (12 | ) | ||||||
|
FINANCING ACTIVITIES
|
||||||||||||
|
Proceeds from issuance of common shares, net of issuance costs
|
56,477 | 32,038 | 3,844 | |||||||||
|
Issuance of common shares pursuant to exercise of options
|
2,616 | 389 | 71 | |||||||||
|
Issuance of common shares pursuant to exercise of warrants
|
1,583 | 289 | 632 | |||||||||
|
Net cash provided by financing activities
|
60,676 | 32,716 | 4,547 | |||||||||
|
Effect of foreign exchange rate changes on cash and cash equivalents
|
(1,827 | ) | (3,561 | ) | 550 | |||||||
|
Increase (decrease) in cash and cash equivalents
|
3,470 | 21,693 | 37,993 | |||||||||
|
Cash and cash equivalents, beginning of period
|
68,717 | 47,024 | 9,031 | |||||||||
|
Cash and cash equivalents, end of period
|
$ | 72,187 | $ | 68,717 | $ | 47,024 | ||||||
|
Supplemental cash flow information
|
||||||||||||
|
Fair value of warrants exercised on a cashless basis
|
$ | 116 | $ | 1,404 | $ | 211 | ||||||
|
Investment tax credits received
|
$ | - | $ | 10 | $ | 323 | ||||||
|
Fair value of warrants issued in conjunction with public offering
|
$ | - | $ | - | $ | 851 | ||||||
|
1.
|
Nature
of business and future operations
|
|
2.
|
Significant accounting policies
|
|
•
|
Level 1 inputs are quoted market prices for identical instruments available in active markets.
|
|
•
|
Level 2 inputs are inputs other than quoted prices included within Level 1 that are observable for the asset or liability either directly or indirectly. If the asset or liability has a contractual term, the input must be observable for substantially the full term. An example includes quoted market prices for similar assets or liabilities in active markets.
|
|
•
|
Level 3 inputs are unobservable inputs for the asset or liability and will reflect management’s assumptions about market assumptions that would be used to price the asset or liability.
|
|
Level 1
|
Level 2
|
Level 3
|
December 31, 2014
|
|||||||||||||
|
Assets
|
||||||||||||||||
|
Cash and cash equivalents
|
$ | 72,187 | - | - | $ | 72,187 | ||||||||||
|
Guaranteed investment certificates
|
39,974 | - | - | 39,974 | ||||||||||||
|
Total
|
$ | 112,161 | - | - | $ | 112,161 | ||||||||||
|
Liabilities
|
||||||||||||||||
|
Warrants
|
- | - | $ | 5,099 | $ | 5,099 | ||||||||||
|
Financial instrument
|
- | - | - | - | ||||||||||||
|
Total
|
- | - | $ | 5,099 | $ | 5,099 | ||||||||||
|
Level 1
|
Level 2
|
Level 3
|
December 31, 2013
|
|||||||||||||
|
Assets
|
||||||||||||||||
|
Cash and cash equivalents
|
$ | 68,717 | - | - | $ | 68,717 | ||||||||||
|
Liabilities
|
||||||||||||||||
|
Warrants
|
- | - | $ | 5,379 | $ | 5,379 | ||||||||||
|
Liability at beginning
of the period
|
Opening liability of
warrants issued in
the period
|
Fair value of
warrants exercised
in the period
|
Increase in fair
value of warrants
|
Foreign exchange
(gain) loss
|
Liability at end
of the period
|
|||||||||||||||||||
|
Year ended December 31, 2012
|
$ | 202 | $ | 851 | $ | (881 | ) | $ | 3,822 | $ | 21 | $ | 4,015 | |||||||||||
|
Year ended December 31, 2013
|
$ | 4,015 | - | $ | (1,854 | ) | $ | 3,530 | $ | (312 | ) | $ | 5,379 | |||||||||||
|
Year ended December 31, 2014
|
$ | 5,379 | - | $ | (10,208 | ) | $ | 10,383 | $ | (455 | ) | $ | 5,099 | |||||||||||
|
Rate
|
|||
|
Laboratory equipment (years)
|
5
|
||
|
Computer and office equipment (years)
|
2
|
-
|
5
|
|
Furniture and fixtures (years)
|
5
|
||
|
For the year ended December 31
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Numerator:
|
||||||||||||
|
Net income (loss)
|
$ | (38,837 | ) | $ | (14,063 | ) | $ | 29,611 | ||||
|
Denominator:
|
||||||||||||
|
Weighted average number of common shares
|
21,603,136 | 15,302,680 | 13,727,925 | |||||||||
|
Effect of dilutive securities:
|
||||||||||||
|
Warrants
|
- | - | 177,374 | |||||||||
|
Options
|
- | - | 415,515 | |||||||||
|
Diluted weighted average number of common shares
|
21,603,136 | 15,302,680 | 14,320,814 | |||||||||
|
Basic income (loss) per common share
|
$ | (1.80 | ) | $ | (0.92 | ) | $ | 2.16 | ||||
|
Diluted income (loss) per common share
|
$ | (1.80 | ) | $ | (0.92 | ) | $ | 2.07 | ||||
|
3.
|
Collaborations, contracts and licensing agreements
|
|
Year ended December 31
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Collaborations and contracts
|
||||||||||||
|
DoD (a)
|
$ | 8,407 | $ | 9,806 | $ | 11,536 | ||||||
|
Monsanto (b)
|
1,080 | - | - | |||||||||
|
Alnylam (c)
|
- | - | 10 | |||||||||
|
BMS (d)
|
1,741 | 526 | 440 | |||||||||
|
Dicerna (e)
|
510 | - | - | |||||||||
|
Other RNAi collaborators (g)
|
- | 93 | 119 | |||||||||
|
Total research and development collaborations and contracts
|
11,738 | 10,425 | 12,105 | |||||||||
|
Licensing fees, milestone and royalty payments
|
||||||||||||
|
Monsanto licensing fees and milestone payments (b)
|
2,744 | - | - | |||||||||
|
Alnylam milestone payments (c)
|
150 | 5,000 | 1,000 | |||||||||
|
Dicerna licensing fee (e )
|
131 | - | - | |||||||||
|
Spectrum royalty payments (f)
|
190 | 40 | 1,000 | |||||||||
|
Total licensing fees, milestone and royalty payments
|
3,215 | 5,040 | 2,000 | |||||||||
|
Total revenue
|
$ | 14,953 | $ | 15,465 | $ | 14,105 | ||||||
|
December 31, 2014
|
December 31, 2013
|
|||||||
|
DoD (a)
|
$ | 313 | $ | 1,655 | ||||
|
Monsanto current portion (b)
|
4,245 | - | ||||||
|
BMS current portion (d)
|
- | 1,808 | ||||||
|
Dicerna current portion (e)
|
1,221 | |||||||
|
Deferred revenue, current portion
|
5,779 | 3,463 | ||||||
|
Monsanto long-term portion (b)
|
8,666 | - | ||||||
|
Dicerna long-term portion (e)
|
1,271 | - | ||||||
|
Total deferred revenue
|
$ | 15,716 | $ | 3,463 | ||||
|
4.
|
Property and equipment
|
|
December 31, 2014
|
Cost
|
Accumulated
depreciation
|
Net
book value
|
|||||||||
|
Lab equipment
|
$ | 5,021 | (4,451 | ) | $ | 570 | ||||||
|
Leashold improvements
|
5,281 | (4,796 | ) | 485 | ||||||||
|
Computer hardware and software
|
2,293 | (1,588 | ) | 705 | ||||||||
|
Furniture and fixtures
|
364 | (364 | ) | - | ||||||||
| $ | 12,959 | (11,199 | ) | $ | 1,760 | |||||||
|
December 31, 2013
|
Cost
|
Accumulated
depreciation
|
Net
book value
|
|||||||||
|
Lab equipment
|
$ | 4,886 | (4,679 | ) | $ | 207 | ||||||
|
Leashold improvements
|
5,592 | (5,001 | ) | 591 | ||||||||
|
Computer hardware and software
|
1,992 | (1,590 | ) | 402 | ||||||||
|
Furniture and fixtures
|
396 | (396 | ) | - | ||||||||
|
Assets under construction
|
173 | - | 173 | |||||||||
| $ | 13,039 | (11,666 | ) | $ | 1,373 | |||||||
|
5.
|
Share capital
|
|
Common shares
purchasable upon
exercise of
warrants
|
Weighted average
exercise price (C$)
|
Weighted
average exercise
price (US$)
|
Range of
exercise prices
(C$)
|
Range of
exercise prices
(US$)
|
Weighted average remaining contractual life (years)
|
Aggregate
intrinsic value
(C$)
|
Aggregate
intrinsic value
(US$)
|
|||||||||||||||||||||||||||
|
Balance, December 31, 2012
|
1,588,411 | $ | 3.00 | $ | 3.02 | $2.50 | - | $3.35 | $2.51 | - | $3.37 | 3.8 | $ | 3,141 | $ | 3,157 | ||||||||||||||||||
|
Exercised
|
(573,683 | ) | $ | 3.19 | $ | 3.00 | $2.60 | - | $3.35 | $2.44 | - | $3.15 | ||||||||||||||||||||||
|
Balance, December 31, 2013
|
1,014,728 | $ | 2.90 | $ | 2.72 | $2.60 | - | $3.35 | $2.44 | - | $3.15 | 2.7 | $ | 5,635 | $ | 5,298 | ||||||||||||||||||
|
Exercised
|
(616,478 | ) | $ | 3.09 | $ | 2.80 | $2.60 | - | $3.35 | $2.35 | - | $3.03 | ||||||||||||||||||||||
|
Balance, December 31, 2014
|
398,250 | $ | 2.95 | $ | 2.67 | $2.60 | - | $3.35 | $2.35 | - | $3.03 | 1.8 | $ | 5,902 | $ | 5,343 | ||||||||||||||||||
|
Year ended December 31
|
||||||||
|
2014
|
2013
|
|||||||
|
Dividend yield
|
0.00 | % | 0.00 | % | ||||
|
Expected volatility
|
85.22 | % | 47.03 | % | ||||
|
Risk-free interest rate
|
1.00 | % | 1.13 | % | ||||
|
Expected average term (years)
|
0.5
|
1.6
|
||||||
|
Fair value of warrants outstanding
|
$ | 12.80 | $ | 5.30 | ||||
|
Aggregate fair value of warrants outstanding
|
$ | 5,099 | $ | 5,379 | ||||
|
Number of warrants outstanding
|
398,250 | 1,014,728 | ||||||
|
Number of
optioned
|
Weighted
average exercise
|
Weighted
average exercise
|
Aggregate
intrinsic
|
Aggregate
intrinsic
|
||||||||||||||||
|
Balance, December 31, 2011
|
1,413,318 | $ | 5.32 | $ | 5.38 | $ | 2 | $ | 2 | |||||||||||
|
Options granted
|
326,300 | $ | 4.16 | $ | 4.16 | |||||||||||||||
|
Options exercised
|
(28,417 | ) | $ | 2.34 | $ | 2.34 | $ | 82 | $ | 82 | ||||||||||
|
Options forfeited, cancelled or expired
|
(62,355 | ) | $ | 21.27 | $ | 21.29 | ||||||||||||||
|
Balance, December 31, 2012
|
1,648,846 | $ | 4.54 | $ | 4.54 | $ | 2,300 | $ | 2,301 | |||||||||||
|
Options granted
|
270,250 | $ | 7.52 | $ | 7.30 | |||||||||||||||
|
Options exercised
|
(124,246 | ) | $ | 3.22 | $ | 3.13 | $ | 551 | $ | 535 | ||||||||||
|
Options forfeited, cancelled or expired
|
(64,085 | ) | $ | 21.87 | $ | 21.23 | ||||||||||||||
|
Balance, December 31, 2013
|
1,730,765 | $ | 4.45 | $ | 4.32 | $ | 7,030 | $ | 6,826 | |||||||||||
|
Options granted
|
431,125 | $ | 13.63 | $ | 12.34 | |||||||||||||||
|
Options exercised
|
(622,752 | ) | $ | 4.62 | $ | 4.18 | $ | 7,650 | $ | 6,926 | ||||||||||
|
Options forfeited, cancelled or expired
|
(9,000 | ) | $ | 8.20 | $ | 7.42 | ||||||||||||||
|
Balance, December 31, 2014
|
1,530,138 | $ | 6.95 | $ | 6.29 | $ | 16,573 | $ | 15,004 | |||||||||||
|
Options outstanding December 31, 2014
|
Options exercisable December 31, 2014
|
|||||||||||||||||||||||||||||
|
Range of
Exercise prices
|
Number
of options
|
Weighted
average
|
Weighted
average
|
Weighted
average
|
Number
of options
|
Weighted
average
|
Weighted
average
|
|||||||||||||||||||||||
| $1.50 | to | $1.90 | 184,325 | 5.9 | $ | 1.71 | $ | 1.55 | 184,325 | $ | 1.71 | $ | 1.55 | |||||||||||||||||
| $2.10 | to | $2.60 | 189,299 | 6.7 | $ | 2.32 | $ | 2.10 | 169,404 | $ | 2.35 | $ | 2.13 | |||||||||||||||||
| $3.00 | to | $3.85 | 160,650 | 3.8 | $ | 3.57 | $ | 3.23 | 160,450 | $ | 3.57 | $ | 3.23 | |||||||||||||||||
| $4.49 | to | $6.50 | 411,356 | 6.4 | $ | 5.21 | $ | 4.72 | 323,052 | $ | 5.20 | $ | 4.71 | |||||||||||||||||
| $7.05 | to | $10.40 | 252,923 | 8.6 | $ | 8.78 | $ | 7.95 | 117,548 | $ | 8.81 | $ | 7.98 | |||||||||||||||||
| $11.60 | to | $13.26 | 156,085 | 9.1 | $ | 12.89 | $ | 11.67 | 76,879 | $ | 12.68 | $ | 11.48 | |||||||||||||||||
| $14.80 | to | $18.54 | 175,500 | 9.2 | $ | 16.67 | $ | 15.09 | 57,250 | $ | 16.45 | $ | 14.89 | |||||||||||||||||
| $1.50 | to | $18.54 | 1,530,138 | 7.1 | $ | 6.95 | $ | 6.29 | 1,088,908 | $ | 5.43 | $ | 4.92 | |||||||||||||||||
|
Number of
optioned
|
Weighted
average
|
Weighted
average
|
||||||||||
|
Non-vested at December 31, 2013
|
353,675 | $ | 5.44 | $ | 5.28 | |||||||
|
Options granted
|
431,125 | $ | 13.63 | 12.34 | ||||||||
|
Options vested
|
(334,994 | ) | $ | 8.35 | 7.56 | |||||||
|
Non-vested options forfeited
|
(8,576 | ) | $ | 6.89 | 6.24 | |||||||
|
Non-vested at December 31, 2014
|
441,230 | $ | 9.30 | $ | 8.42 | |||||||
|
Year ended December 31
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Dividend yield
|
0.00 | % | 0.00 | % | 0.00 | % | ||||||
|
Expected volatility
|
101.08 | % | 111.61 | % | 120.40 | % | ||||||
|
Risk-free interest rate
|
2.25 | % | 2.39 | % | 1.56 | % | ||||||
|
Expected average option term (years)
|
8.8
|
9.6
|
8.2
|
|||||||||
|
Fair value of options granted (C$)
|
$ | 11.68 | $ | 6.96 | $ | 3.83 | ||||||
|
Year ended December 31
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Research, development, collaborations
and contracts expenses
|
$ | 2,343 | $ | 622 | $ | 772 | ||||||
|
General and administrative expenses
|
940 | 281 | 210 | |||||||||
|
Total
|
$ | 3,283 | $ | 903 | $ | 982 | ||||||
|
Number of
Protiva
Options
|
Equivalent number
of Company
common shares
|
Weighted
average exercise
price (C$)
|
Weighted
average exercise
price (US$)
|
|||||||||||||
|
Balance, December 31, 2011
|
491,020 | 331,517 | $ | 0.30 | 0.30 | |||||||||||
|
Options exercised
|
(15,135 | ) | (10,218 | ) | 0.30 | 0.30 | ||||||||||
|
Options forfeited, cancelled or expired
|
- | - | - | - | ||||||||||||
|
Balance, December 31, 2012
|
475,885 | 321,299 | $ | 0.30 | $ | 0.30 | ||||||||||
|
Options exercised
|
(2,000 | ) | (1,350 | ) | 0.30 | 0.29 | ||||||||||
|
Options forfeited, cancelled or expired
|
(1,000 | ) | (675 | ) | 0.30 | $ | 0.29 | |||||||||
|
Balance, December 31, 2013
|
472,885 | 319,274 | $ | 0.30 | $ | 0.29 | ||||||||||
|
Options exercised
|
(38,145 | ) | (25,754 | ) | 0.30 | 0.27 | ||||||||||
|
Options forfeited, cancelled or expired
|
(1,000 | ) | (675 | ) | 0.30 | 0.27 | ||||||||||
|
Balance, December 31, 2014
|
433,740 | 292,845 | $ | 0.30 | 0.27 | |||||||||||
|
6.
|
Government grants and refundable investment tax credits
|
|
7.
|
Income taxes
|
|
Year ended December 31
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Computed taxes (recoveries) at Canadian federal and provincial tax rates
|
$
|
(6,893
|
) |
$
|
(2,380
|
)
|
$
|
7,486
|
||||
|
Differences due to change in enacted tax rates
|
-
|
(6
|
)
|
781
|
||||||||
|
Difference due to change in tax rate on opening deferred taxes
|
-
|
-
|
2,720
|
|||||||||
|
Permanent and other differences
|
1,342
|
1,150
|
(1,195
|
) | ||||||||
|
Change in valuation allowance - other
|
5,551
|
1,236
|
798
|
|
||||||||
|
Change in valuation allowance - utilization of investment tax credits
|
- | - | (10,590 | ) | ||||||||
|
Income tax (recovery) expense
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||
|
Year ended December 31
|
||||||||
|
2014
|
2013
|
|||||||
|
Deferred tax assets:
|
||||||||
|
Non-capital loss carryforwards
|
$
|
4,491
|
$
|
4,354
|
||||
|
Research and development deductions
|
9,562
|
8,859
|
||||||
|
Book amortization in excess of tax
|
1,874
|
2,171
|
||||||
|
Share issue costs
|
815
|
(136
|
)
|
|||||
|
Revenue recognized for tax purposes in excess of revenue recognized for accounting purposes
|
2,790
|
668
|
||||||
|
Tax value in excess of accounting value in lease inducements
|
45
|
(3
|
)
|
|||||
|
Federal investment tax credits
|
6,470
|
5,539
|
||||||
|
Provincial investment tax credits
|
3,347
|
2,391
|
||||||
|
Total deferred tax assets
|
29,394
|
23,843
|
||||||
|
Valuation allowance
|
(29,394
|
)
|
(23,843
|
)
|
||||
|
Net deferred tax assets
|
$
|
-
|
$
|
-
|
||||
|
8.
|
Contingencies and commitments
|
|
Year ended December 31, 2015
|
$ | 1,119,000 | ||
|
Year ended December 31, 2016
|
1,119,000 | |||
|
Year ended December 31, 2017
|
1,119,000 | |||
|
Year ended December 31, 2018
|
1,119,000 | |||
|
Year ended December 31, 2019
|
653,000 | |||
| $ | 5,129,000 |
|
9.
|
Concentrations of business risk
|
|
December 31, 2014
|
December 31, 2013
|
|||||||
|
Cash, cash equivalents and short-term investments
|
$ | 112,161 | $ | 68,717 | ||||
|
Less: Accounts payable and accrued liabilties
|
(9,328 | ) | (3,680 | ) | ||||
| $ | 102,833 | $ | 65,037 | |||||
|
(in C$)
|
December 31, 2014
|
December 31, 2013
|
||||||
|
Cash and cash equivalents and short-term investments
|
$ | 75,224 | $ | 38,901 | ||||
|
Accounts receivable
|
1,942 | 11 | ||||||
|
Accrued revenue
|
624 | 226 | ||||||
|
Accounts payable and accrued liabilities
|
(4,494 | ) | (1,889 | ) | ||||
| $ | 73,296 | $ | 37,248 | |||||
|
10.
|
Supplementary information
|
|
December 31, 2014
|
December 31, 2013
|
|||||||
|
Trade accounts payable
|
$ | 2,044 | $ | 1,217 | ||||
|
Research and development accruals
|
2,391 | 669 | ||||||
| License fee accruals | 250 | - | ||||||
|
Professional fee accruals
|
1,294 | 247 | ||||||
|
Deferred lease inducements
|
250 | 16 | ||||||
| Payroll accruals | 2,873 | 1,224 | ||||||
|
Other accrued liabilities
|
226 | 307 | ||||||
| $ | 9,328 | $ | 3,680 | |||||
|
11.
|
Interim financial data (unaudited)
|
|
2014
|
||||||||||||||||||||
|
Q1
|
Q2
|
Q3
|
Q4
|
Total
|
||||||||||||||||
|
Revenue
|
4,430
|
1,811
|
4,362
|
4,350
|
14,953
|
|||||||||||||||
|
Loss from operations
|
(5,958
|
)
|
(9,423
|
)
|
(6,844
|
)
|
(10,747
|
)
|
(32,972
|
)
|
||||||||||
|
Net loss
|
(17,984
|
)
|
(6,081
|
)
|
(8,604
|
)
|
(6,168
|
)
|
(38,837
|
)
|
||||||||||
|
Basic and diluted net loss per share
|
$
|
(0.91
|
)
|
$
|
(0.28
|
)
|
$
|
(0.39
|
)
|
$
|
(0.27
|
)
|
$
|
(1.80
|
)
|
|||||
|
2013
|
||||||||||||||||||||
|
Q1
|
Q2
|
Q3
|
Q4
|
Total
|
||||||||||||||||
|
Revenue
|
2,132
|
2,844
|
2,963
|
7,52
|
15,465
|
|||||||||||||||
|
Loss from operations
|
(2,994
|
)
|
(3,071
|
)
|
(3,652
|
)
|
(2,435
|
)
|
(12,152
|
)
|
||||||||||
|
Net loss
|
(2,546
|
)
|
(3,015
|
)
|
(5,906
|
)
|
(2,596
|
)
|
(14,063
|
)
|
||||||||||
|
Basic and diluted net loss per share
|
$
|
(0.18
|
)
|
$
|
(0.21
|
)
|
$
|
(0.41
|
)
|
$
|
(0.15
|
)
|
$
|
(0.92
|
)
|
|||||
|
12.
|
Subsequent events
|
|
Consideration paid:
|
|
|||
|
Common shares issued without subjects
|
|
$
|
371,553
|
|
|
Common shares issued subject to repurchase provision
|
|
9,262
|
|
|
|
Common shares issuable for OnCore stock options
|
|
1,127
|
|
|
|
|
$
|
381,942
|
|
|
|
|
||||
|
Identifiable assets acquired and liabilities assumed:
|
|
|||
|
Cash
|
|
$
|
325
|
|
|
Prepaid expenses and other assets
|
|
125
|
|
|
|
Accounts receivable
|
|
7
|
|
|
|
Property and equipment
|
|
149
|
|
|
|
Acquired intangible assets from combined OnCore
|
|
393,192
|
|
|
|
Accounts payable and accrued liabilities
|
|
(3,182
|
)
|
|
|
Other noncurrent liabilities
|
|
(8,674
|
)
|
|
|
Total purchase price allocation
|
|
$
|
381,942
|
|
|
Item 9.
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
|
|
Item 9A.
|
Controls and Procedures
|
|
Item 9B.
|
Other Information
|
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
|
Item 11.
|
Executive Compensation
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
|
Item 14.
|
Principal Accountant Fees and Services
|
|
Item 15.
|
Exhibits and Financial Statement Schedules
|
|
TEKMIRA PHARMACEUTICALS CORPORATION
|
||
|
By:
|
/s/ Mark Murray
|
|
|
Mark Murray
|
||
|
President and Chief Executive Officer
|
||
|
Signatures
|
Capacity in Which Signed
|
|
|
/s/ Vivek Ramaswamy
|
Director (Chairman)
|
|
|
Vivek Ramaswamy
|
||
|
/s/ Mark Murray
|
President and
Chief Executive Officer and Director
|
|
|
Mark Murray
|
(Principal Executive Officer)
|
|
|
/s/ Bruce Cousins
|
Executive Vice President, Finance and Chief Financial Officer
|
|
|
Bruce Cousins
|
(Principal Financial Officer and Accounting Officer)
|
|
|
/s/ Herbert J. Conrad
|
Director
|
|
|
Herbert J. Conrad
|
||
|
/s/ Richard C. Henriques
|
Director
|
|
|
Richard C. Henriques
|
||
|
/s/ Frank Karbe
|
Director
|
|
|
Frank Karbe
|
||
|
/s/ Keith Manchester
|
Director
|
|
|
Keith Manchester
|
|
|
| /s/ William T. Symonds |
Chief Development Officer
|
|
|
William T. Symonds
|
|
Exhibit
Number
|
Description
|
|
|
2.1*
|
Subscription Agreement, between the Company and Alnylam Pharmaceuticals, Inc., dated March 28, 2008 (incorporated herein by reference to Exhibit 2.1 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2010 filed with the SEC on June 3, 2011).
|
|
|
2.2*
|
Subscription Agreement, between the Company and Roche Finance Ltd., dated March 31, 2008 (incorporated herein by reference to Exhibit 2.2 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2010 filed with the SEC on June 3, 2011).
|
|
|
2.3*
|
Agreement and Plan of Merger and Reorganization, dated January 11, 2015, by and among Tekmira Pharmaceuticals Corporation, TKM Acquisition Corporation and OnCore Biopharma, Inc. (incorporated herein by reference to Exhibit 2.1 to the Registrant’s Current Report on Form 8-K/A filed with the SEC on January 26, 2015).
|
|
|
3.1*
|
Notice of Articles and Articles of the Company (incorporated herein by reference to Exhibit 1.1 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2010 filed with the SEC on June 3, 2011).
|
|
|
3.2*
|
Amendment to the Articles of the Company dated May 14, 2013 (incorporated herein by reference to Exhibit 3.2 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2013 filed with the SEC on March 28, 2014).
|
|
|
3.3*
|
Governance Amendment to the Articles of the Company dated March 4, 2015, (incorporated herein by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K filed with the SEC on March 4, 2015).
|
|
|
3.4*
|
Approval of Quorum Policy of the Company, adopted January 31, 2015 (incorporated herein by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K filed with the SEC on February 5, 2015).
|
|
|
4.1*
|
Governance Agreement between the Company and Roivant Sciences Ltd., a Bermuda exempted company, dated January 11, 2015 (incorporated herein by reference to Exhibit 2.1 to the Registrant’s Current Report on Form 8-K/A filed with the SEC on January 26, 2015).
|
|
|
10.1†*
|
Amendment No. 1 to the Amended and Restated Agreement, between the Company (formerly Inex Pharmaceuticals Corporation) and Hana Biosciences, Inc., effective as of May 27, 2009 (incorporated herein by reference to Exhibit 4.1 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2010 filed with the SEC on June 3, 2011).
|
|
|
10.2†*
|
Amended and Restated License Agreement, between Inex Pharmaceuticals Corporation and Hana Biosciences, Inc., dated April 30, 2007 (incorporated herein by reference to Exhibit 4.2 to the Registrant’s Amendment No. 1 to Form 20-F for the year ended December 31, 2010 filed with the SEC on January 31, 2012).
|
|
|
10.3†*
|
Sublicense Agreement, between Inex Pharmaceuticals Corporation and Alnylam Pharmaceuticals, Inc., dated January 8, 2007 (incorporated herein by reference to Exhibit 4.3 to the Registrant’s Amendment No. 1 to Form 20-F for the year ended December 31, 2010 filed with the SEC on January 31, 2012).
|
|
|
10.4†*
|
Amended and Restated License and Collaboration Agreement, between the Company and Alnylam Pharmaceuticals, Inc., effective as of May 30, 2008 (incorporated herein by reference to Exhibit 4.4 to the Registrant’s Amendment No. 1 to Form 20-F for the year ended December 31, 2010 filed with the SEC on January 31, 2012).
|
|
|
10.5†*
|
Amended and Restated Cross-License Agreement, between Alnylam Pharmaceuticals, Inc. and Protiva Biotherapeutics Inc., dated May 30, 2008 (incorporated herein by reference to Exhibit 4.5 to the Registrant’s Amendment No. 1 to Form 20-F for the year ended December 31, 2010 filed with the SEC on January 31, 2012).
|
|
|
10.6†*
|
License Agreement, between Inex Pharmaceuticals and Aradigm Corporation, dated December 8, 2004 (incorporated herein by reference to Exhibit 4.6 to the Registrant’s Amendment No. 1 to Form 20-F for the year ended December 31, 2010 filed with the SEC on January 31, 2012).
|
|
|
10.7†*
|
Settlement Agreement, between Sirna Therapeutics, Inc. and Merck & Co., Inc. and Protiva Biotherapeutics Inc. and Protiva Biotherapeutics (USA), Inc., effective as of October 9, 2007 (incorporated herein by reference to Exhibit 4.7 to the Registrant’s Amendment No. 1 to Form 20-F for the year ended December 31, 2010 filed with the SEC on January 31, 2012).
|
|
|
10.8†*
|
Development, Manufacturing and Supply Agreement, between the Company and Alnylam Pharmaceuticals, Inc., dated January 2, 2009 (incorporated herein by reference to Exhibit 4.8 to the Registrant’s Amendment No. 1 to Form 20-F for the year ended December 31, 2010 filed with the SEC on January 31, 2012).
|
|
|
10.9†*#
|
Executive Employment Agreement with Ian Mortimer, dated March 26, 2008 (incorporated herein by reference to Exhibit 4.9 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2010 filed with the SEC on June 3, 2011).
|
|
|
10.10*#
|
Executive Employment Agreement with Ian MacLachlan, dated May 30, 2008 (incorporated herein by reference to Exhibit 4.10 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2010 filed with the SEC on June 3, 2011).
|
|
|
10.11*#
|
Executive Employment Agreement with Mark Murray, dated May 30, 2008 (incorporated herein by reference to Exhibit 4.11 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2010 filed with the SEC on June 3, 2011).
|
|
|
10.12*#
|
Executive Employment Agreement with Peter Lutwyche, dated January 1, 2009 (incorporated herein by reference to Exhibit 4.12 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2010 filed with the SEC on June 3, 2011).
|
|
|
10.13*#
|
Share Option Plan amended through May 12, 2009 (including form stock option agreements) (incorporated herein by reference to Exhibit 4.13 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2010 filed with the SEC on June 3, 2011).
|
|
|
10.14*
|
Lease Agreement with Canada Lands Company CLC Limited dated December 15, 1997, as amended (incorporated herein by reference to Exhibit 4.14 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2010 filed with the SEC on June 3, 2011).
|
|
|
10.15*#
|
Form of Indemnity Agreement (incorporated herein by reference to Exhibit 4.15 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2010 filed with the SEC on June 3, 2011).
|
|
|
10.16*
|
Award Contract with USASMDC/ARSTRAT effective date July 14, 2010 (incorporated herein by reference to Exhibit 4.16 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2010 filed with the SEC on June 3, 2011).
|
|
|
10.17†*
|
License Agreement between the University of British Columbia and Inex Pharmaceuticals Corporation executed on July 30, 2001 (incorporated herein by reference to Exhibit 4.17 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2010 filed with the SEC on June 3, 2011).
|
|
|
10.18†*
|
Amendment Agreement between the University of British Columbia and Inex Pharmaceuticals Corporation dated July 11, 2006 (incorporated herein by reference to Exhibit 4.18 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2010 filed with the SEC on June 3, 2011).
|
|
|
10.19†*
|
Second Amendment Agreement between the University of British Columbia and Inex Pharmaceuticals Corporation dated January 8, 2007 (incorporated herein by reference to Exhibit 4.19 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2010 filed with the SEC on June 3, 2011).
|
|
|
10.20†*
|
Consent Agreement of the University of British Columbia to Inex/Alnylam Sublicense Agreement dated January 8, 2007 (incorporated herein by reference to Exhibit 4.20 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2010 filed with the SEC on June 3, 2011).
|
|
|
10.21†*
|
Amendment No. 2 to the Amended and Restated Agreement, between the Company (formerly Inex Pharmaceuticals Corporation) and Hana Biosciences, Inc., effective as of September 20, 2010 (incorporated herein by reference to Exhibit 4.21 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2010 filed with the SEC on June 3, 2011).
|
|
|
10.22†*
|
License and Collaboration Agreement between the Company and Halo-Bio RNAi Therapeutics, Inc. as of August 24, 2011 (incorporated herein by reference to Exhibit 4.22 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2011 filed with the SEC on March 27, 2012).
|
|
|
10.23*
|
Loan Agreement with Silicon Valley Bank dated as of December 21, 2011 (incorporated herein by reference to Exhibit 4.23 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2011 filed with the SEC on March 27, 2012).
|
|
|
10.24*#
|
Employment Agreement with Paul Brennan dated August 24, 2010 (incorporated herein by reference to Exhibit 4.24 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2011 filed with the SEC on March 27, 2012).
|
|
|
10.25*#
|
Tekmira 2011 Omnibus Share Compensation Plan approved by shareholders on June 22, 2011 (incorporated herein by reference to Exhibit 4.25 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2011 filed with the SEC on March 27, 2012).
|
|
|
10.26†*
|
Settlement Agreement and General Release, by and among Tekmira Pharmaceuticals Corporation, Protiva Biotherapeutics Inc., Alnylam Pharmaceuticals, Inc., and AlCana Technologies, Inc., dated November 12, 2012 (incorporated herein by reference to Exhibit 4.26 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2012 filed with the SEC on March 27, 2013).
|
|
10.27†*
|
Cross-License Agreement by and among Alnylam Pharmaceuticals, Inc., Tekmira Pharmaceuticals Corporation and Protiva Biotherapeutics Inc., dated November 12, 2012(incorporated herein by reference to Exhibit 4.27 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2012 filed with the SEC on March 27, 2013).
|
|
|
10.28†*
|
License Agreement by and among Protiva Biotherapeutics Inc. and Marina Biotech, Inc. dated November 28, 2012 (incorporated herein by reference to Exhibit 4.28 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2012 filed with the SEC on March 27, 2013).
|
|
|
10.29*#
|
Employment Agreement with Diane Gardiner dated March 1, 2013 (incorporated herein by reference to Exhibit 4.29 to the Registrant’s Annual Report on Form 20-F for the year ended December 31, 2012 filed with the SEC on March 27, 2013).
|
|
|
10.30*#
|
Employment Agreement with Mark Kowalski dated August 12, 2013 (incorporated herein by reference to Exhibit 10.30 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2013 filed with the SEC on March 28, 2014).
|
|
|
10.31*#
|
Employment Agreement with Bruce Cousins dated October 7, 2013 (incorporated herein by reference to Exhibit 10.31 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2013 filed with the SEC on March 28, 2014).
|
|
|
10.32†*
|
Services Agreement by and among Protiva Biotherapeutics Inc., Protiva Agricultural Development Company Inc. and Monsanto Company dated January 12, 2014 (incorporated herein by reference to Exhibit 10.32 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2013 filed with the SEC on March 28, 2014).
|
|
|
10.33†*
|
Option Agreement by and among Tekmira Pharmaceuticals Corporation, Protiva Biotherapeutics Inc., Protiva Agricultural Development Company Inc. and Monsanto Canada Inc. dated January 12, 2014 (incorporated herein by reference to Exhibit 10.33 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2013 filed with the SEC on March 28, 2014).
|
|
|
10.34†*
|
License and Services Agreement by and among Protiva Biotherapeutics Inc., Protiva Agricultural Development Company Inc. and Tekmira Pharmaceuticals Corporation dated January 12, 2014 (incorporated herein by reference to Exhibit 10.34 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2013 filed with the SEC on March 28, 2014).
|
|
|
10.35*
|
Forms of Lock-Up Agreement (incorporated herein by reference to Exhibit 2.1 to the Registrant’s Current Report on Form 8-K/A filed with the SEC on January 26, 2015).
|
|
|
10.36*
|
Form of Registration Rights Agreement (incorporated herein by reference to Exhibit 2.1 to the Registrant’s Current Report on Form 8-K/A filed with the SEC on January 26, 2015).
|
|
|
10.37*
|
Form of Standstill Agreement (incorporated herein by reference to Exhibit 2.1 to the Registrant’s Current Report on Form 8-K/A filed with the SEC on January 26, 2015).
|
|
|
10.38*
|
Form of Representation Letter (incorporated herein by reference to Exhibit 2.1 to the Registrant’s Current Report on Form 8-K/A filed with the SEC on January 26, 2015).
|
|
|
10.39**#
|
Executive Employment Agreement with Michael Abrams, dated November 14, 2013
|
|
|
10.40**#
|
Executive Employment Agreement with Kirk Rosemark, dated December 8, 2014
|
|
|
10.41**††
|
License Agreement, between Tekmira Pharmaceuticals and Protiva Biotherapeutics and Dicerna Pharmaceuticals dated November 16, 2014
|
|
|
10.42**††
|
Manufacturing and Clinical Trial Agreement between Tekmira Pharmaceuticals and Protiva Biotherapeutics and the Chancellor Masters and Scholars of the University of Oxford, dated December 18, 2014
|
|
|
10.43**
|
Modification Contract P0001, dated July 19, 2010, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.44**
|
Modification Contract P0002, dated April 15, 2011, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.45**
|
Modification Contract P0003, dated June 13, 2011, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.46**††
|
Modification Contract P0004, dated October 3, 2011, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.47**
|
Modification Contract P0005, dated December 2, 2011, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.48**
|
Modification Contract P0006, dated January 25, 2012, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.49**††
|
Modification Contract P0007, dated March 5, 2012, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.50**
|
Modification Contract P0008, dated April 23, 2012, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.51**
|
Modification Contract P0009, dated June 29, 2012, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.52**
|
Modification Contract P00010, dated July 16, 2012, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.53**
|
Modification Contract P00011, dated July 25, 2012, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.54**††
|
Modification Contract P00012, dated August 2, 2012, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.55**
|
Modification Contract P00013, dated August 27, 2012, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.56 **
|
Modification Contract P00014, dated August 31, 2012, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.57**
|
Modification Contract P00015, dated October 1, 2012, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.58**
|
Modification Contract P00016, dated October 2, 2012, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.59**
|
Modification Contract P00017, dated October 19, 2012, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.60**
|
Modification Contract P00018, dated December 31, 2012, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.61**
|
Modification Contract P00019, dated January 23, 2013, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.62 **
|
Modification Contract P00020, dated February 19, 2013, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.63 **
|
Modification Contract P00021, dated March 29, 2013, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
| 10.64**†† | Modification Contract P00022, dated April 30, 2013, to Award Contract, dated July 14, 2010 (Exhibit 10.16) | |
|
10.65**††
|
Modification Contract P00023, dated May 21, 2013, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.66 **
|
Modification Contract P00024, dated June 19, 2013, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.67**††
|
Modification Contract P00025, dated April 22, 2014, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.68**††
|
Modification Contract P00026, dated July 25, 2014, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.69**
|
Modification Contract P00027, dated July 25, 2014, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.70 **††
|
Modification Contract P00028, dated September 5, 2014, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.71 **
|
Modification Contract P00029, dated September 30, 2014, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.72**††
|
Modification Contract P00030, dated October 31, 2014, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.73**
|
Modification Contract P00031, dated November 17, 2014, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.74**††
|
Modification Contract P00032, dated March 4, 2015, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.75**††
|
Modification Contract P00033, dated March 4, 2015, to Award Contract, dated July 14, 2010 (Exhibit 10.16)
|
|
|
10.76**
|
Underwriting Agreement for 3,750,000 Common Shares with Stifel, Nicolaus & Company, dated October 17, 2013
|
|
|
10.77**
|
Underwriting Agreement for 2,125,000 Common Shares with Leerink Partners LLC, dated March 14, 2014
|
|
|
21.1**
|
List of Subsidiaries
|
|
|
23.1**
|
Consent of KPMG LLP, an Independent Registered Public Accounting Firm
|
|
|
31.1**
|
Certification of Chief Executive Officer pursuant to Rule 13a-14 or 15d-14 of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
|
31.2**
|
Certification of Chief Financial Officer pursuant to Rule 13a-14 or 15d-14 of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
|
32.1**
|
Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
32.2**
|
Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
101.INS**
|
XBRL Instance Document
|
|
|
101.SCH**
|
XBRL Taxonomy Extension Schema Document
|
|
|
101.CAL**
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
|
101.DEF**
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
|
101.LAB**
|
XBRL Taxonomy Extension Label Linkbase Document
|
|
|
101.PRE**
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
*
**
|
Previously filed
Filed herewith
|
|
†
|
Confidential treatment granted as to portions of this exhibit.
|
|
††
#
|
Confidential treatment has been requested as to portions of this exhibit.
Management Contract
|