|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
British Columbia, Canada
|
98-0597776
|
|
|
(State or Other Jurisdiction of
|
(I.R.S. Employer
|
|
|
Incorporation or Organization)
|
Identification No.)
|
|
|
Title of each class
|
Trading Symbol(s)
|
Name of each exchange on which registered
|
|
Common Shares, without par value
|
ABUS
|
The Nasdaq Stock Market LLC
|
|
Large accelerated filer [ ]
|
Accelerated filer [X]
|
Non-accelerated filer [ ]
|
Smaller reporting company [X]
|
Emerging growth company [ ]
|
|
Page
|
||
|
|
|
|
|
|
||
|
|
September 30, 2019
|
December 31, 2018
|
|||||
|
Assets
|
|
|
|||||
|
Current assets:
|
|
||||||
|
Cash and cash equivalents (note 3)
|
$
|
90,082
|
|
$
|
36,942
|
|
|
|
Short-term investments (note 3)
|
—
|
|
87,675
|
|
|||
|
Accounts receivable
|
2,488
|
|
1,431
|
|
|||
|
Prepaid expenses and other current assets
|
1,771
|
|
3,181
|
|
|||
|
Total current assets
|
94,341
|
|
129,229
|
|
|||
|
Investment in Genevant (note 4)
|
10,969
|
|
22,224
|
|
|||
|
Property and equipment, net accumulated depreciation $8,612 (December 31, 2018: $7,090)
|
9,150
|
|
10,145
|
|
|||
|
Right of use asset (note 5)
|
2,817
|
|
—
|
|
|||
|
Intangible assets (note 6)
|
—
|
|
43,836
|
|
|||
|
Goodwill (note 6)
|
—
|
|
22,471
|
|
|||
|
Total assets
|
$
|
117,277
|
|
$
|
227,905
|
|
|
|
Liabilities and stockholders' equity
|
|
|
|||||
|
Current liabilities:
|
|
|
|||||
|
Accounts payable and accrued liabilities (note 7)
|
$
|
8,199
|
|
$
|
9,429
|
|
|
|
Site consolidation accrual (note 8)
|
203
|
|
1,331
|
|
|||
|
Liability-classified options (note 3)
|
86
|
|
479
|
|
|||
|
Lease liability, current (note 5)
|
329
|
|
—
|
|
|||
|
Total current liabilities
|
8,817
|
|
11,239
|
|
|||
|
Liability related to sale of future royalties (note 9)
|
18,675
|
|
—
|
|
|||
|
Deferred rent and inducements, non-current
|
—
|
|
645
|
|
|||
|
Contingent consideration (notes 3 and 10)
|
3,005
|
|
3,126
|
|
|||
|
Lease liability, non-current (note 5)
|
3,143
|
|
—
|
|
|||
|
Deferred tax liability (note 6)
|
—
|
|
12,661
|
|
|||
|
Total liabilities
|
33,640
|
|
27,671
|
|
|||
|
Stockholders’ equity:
|
|
|
|||||
|
Preferred shares (note 12)
|
|||||||
|
Authorized: 1,164,000 without par value
|
|||||||
|
Issued and outstanding: 1,164,000 (December 31, 2018: 1,164,000)
|
134,405
|
|
126,136
|
|
|||
|
Common shares
|
|||||||
|
Authorized: unlimited number without par value
|
|||||||
|
Issued and outstanding: 56,850,172 (December 31, 2018: 55,518,800)
|
884,623
|
|
879,405
|
|
|||
|
Additional paid-in capital
|
55,385
|
|
48,084
|
|
|||
|
Deficit
|
(942,559
|
)
|
(805,221
|
)
|
|||
|
Accumulated other comprehensive loss
|
(48,217
|
)
|
(48,170
|
)
|
|||
|
Total stockholders' equity
|
83,637
|
|
200,234
|
|
|||
|
Total liabilities and stockholders' equity
|
$
|
117,277
|
|
$
|
227,905
|
|
|
|
Three Months Ended September 30,
|
Nine Months Ended September 30,
|
||||||||||||||
|
2019
|
2018
|
2019
|
2018
|
||||||||||||
|
Revenue
(note 11)
|
$
|
3,061
|
|
$
|
1,587
|
|
$
|
4,393
|
|
$
|
4,267
|
|
|||
|
Operating expenses
|
|
|
|||||||||||||
|
Research and development
|
17,731
|
|
16,566
|
|
45,183
|
|
46,871
|
|
|||||||
|
General and administrative
|
3,249
|
|
2,631
|
|
15,850
|
|
10,075
|
|
|||||||
|
Depreciation
|
507
|
|
497
|
|
1,521
|
|
1,677
|
|
|||||||
|
Site consolidation (note 8)
|
182
|
|
(492
|
)
|
33
|
|
3,710
|
|
|||||||
|
Impairment of intangible assets (note 6)
|
43,836
|
|
14,811
|
|
43,836
|
|
14,811
|
|
|||||||
|
Impairment of goodwill (note 6)
|
22,471
|
|
—
|
|
22,471
|
|
—
|
|
|||||||
|
Arbitration (note 10)
|
6,486
|
|
—
|
|
6,486
|
|
—
|
|
|||||||
|
Total operating expenses
|
94,462
|
|
34,013
|
|
135,380
|
|
77,144
|
|
|||||||
|
Loss from operations
|
(91,401
|
)
|
(32,426
|
)
|
(130,987
|
)
|
(72,877
|
)
|
|||||||
|
Other income (loss)
|
|
|
|||||||||||||
|
Interest income
|
503
|
|
756
|
|
1,709
|
|
2,319
|
|
|||||||
|
Interest expense (note 9)
|
(1,100
|
)
|
—
|
|
(1,114
|
)
|
(104
|
)
|
|||||||
|
Foreign exchange gain (loss)
|
(25
|
)
|
145
|
|
43
|
|
(740
|
)
|
|||||||
|
Gain on investment (note 4)
|
—
|
|
—
|
|
—
|
|
24,884
|
|
|||||||
|
Equity investment loss (note 4)
|
(3,512
|
)
|
(2,838
|
)
|
(11,497
|
)
|
(2,838
|
)
|
|||||||
|
Change in fair value of contingent consideration (notes 3 and 10)
|
376
|
|
5,608
|
|
121
|
|
6,263
|
|
|||||||
|
Total other income (loss)
|
(3,758
|
)
|
3,671
|
|
(10,738
|
)
|
29,784
|
|
|||||||
|
Loss before income taxes
|
$
|
(95,159
|
)
|
$
|
(28,755
|
)
|
$
|
(141,725
|
)
|
$
|
(43,093
|
)
|
|||
|
Income tax benefit (note 6)
|
12,656
|
|
4,282
|
|
12,656
|
|
4,282
|
|
|||||||
|
Net loss
|
$
|
(82,503
|
)
|
$
|
(24,473
|
)
|
$
|
(129,069
|
)
|
$
|
(38,811
|
)
|
|||
|
Items applicable to preferred shares:
|
|||||||||||||||
|
Accrual of coupon on convertible preferred shares
|
(2,792
|
)
|
(2,567
|
)
|
(8,269
|
)
|
$
|
(7,444
|
)
|
||||||
|
Net loss attributable to common shareholders
(note 2)
|
$
|
(85,295
|
)
|
$
|
(27,040
|
)
|
$
|
(137,338
|
)
|
$
|
(46,255
|
)
|
|||
|
Net loss attributable to common shareholders, per share
|
|
|
|||||||||||||
|
Basic and diluted
|
$
|
(1.50
|
)
|
$
|
(0.49
|
)
|
$
|
(2.43
|
)
|
$
|
(0.84
|
)
|
|||
|
Weighted average number of common shares
|
|
|
|
||||||||||||
|
Basic and diluted
|
56,850,172
|
|
55,421,504
|
|
56,469,358
|
|
55,241,284
|
|
|||||||
|
|
Three Months Ended September 30,
|
Nine Months Ended September 30,
|
|||||||||||||
|
|
2019
|
2018
|
2019
|
2018
|
|||||||||||
|
Net loss
|
$
|
(82,503
|
)
|
$
|
(24,473
|
)
|
$
|
(129,069
|
)
|
$
|
(38,811
|
)
|
|||
|
Other comprehensive income (loss):
|
|||||||||||||||
|
Share of other comprehensive income (loss) of equity method investment (note 4)
|
27
|
|
(12
|
)
|
(47
|
)
|
(12
|
)
|
|||||||
|
Comprehensive loss
|
$
|
(82,476
|
)
|
$
|
(24,485
|
)
|
$
|
(129,116
|
)
|
$
|
(38,823
|
)
|
|||
|
Convertible Preferred Shares
|
Common Shares
|
|||||||||||||||||||||
|
|
Number of Shares
|
Share Capital
|
Number of Shares
|
Share Capital
|
Additional Paid-In Capital
|
Deficit
|
Accumulated Other Comprehensive Loss
|
Total Stockholders' Equity
|
||||||||||||||
|
Balance, December 31, 2018
|
1,164,000
|
|
$
|
126,136
|
|
55,518,800
|
|
$
|
879,405
|
|
$
|
48,084
|
|
$
|
(805,221
|
)
|
$
|
(48,170
|
)
|
$
|
200,234
|
|
|
Accretion of accumulated dividends on Preferred Shares
|
—
|
|
2,715
|
|
—
|
|
—
|
|
—
|
|
(2,715
|
)
|
—
|
|
—
|
|
||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
1,665
|
|
—
|
|
—
|
|
1,665
|
|
||||||
|
Certain fair value adjustments to liability-classified
|
—
|
|
—
|
|
—
|
|
—
|
|
47
|
|
—
|
|
—
|
|
47
|
|
||||||
|
Issuance of common shares pursuant to our ATM
|
—
|
|
—
|
|
614,401
|
|
2,248
|
|
—
|
|
—
|
|
—
|
|
2,248
|
|
||||||
|
Issuance of common shares pursuant to exercise of options
|
—
|
|
—
|
|
122,603
|
|
490
|
|
(202
|
)
|
—
|
|
—
|
|
288
|
|
||||||
|
Currency translation adjustment
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(22
|
)
|
(22
|
)
|
||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(23,251
|
)
|
—
|
|
(23,251
|
)
|
||||||
|
Balance, March 31, 2019
|
1,164,000
|
|
$
|
128,851
|
|
56,255,804
|
|
$
|
882,143
|
|
$
|
49,594
|
|
$
|
(831,187
|
)
|
$
|
(48,192
|
)
|
$
|
181,209
|
|
|
Accretion of accumulated dividends on Preferred Shares
|
—
|
|
2,762
|
|
—
|
|
—
|
|
—
|
|
(2,762
|
)
|
—
|
|
—
|
|
||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
3,915
|
|
—
|
|
—
|
|
3,915
|
|
||||||
|
Certain fair value adjustments to liability-classified
|
—
|
|
—
|
|
—
|
|
—
|
|
230
|
|
—
|
|
—
|
|
230
|
|
||||||
|
Issuance of common shares pursuant to our ATM
|
—
|
|
—
|
|
593,689
|
|
2,477
|
|
—
|
|
—
|
|
—
|
|
2,477
|
|
||||||
|
Issuance of common shares pursuant to exercise of options
|
—
|
|
—
|
|
679
|
|
3
|
|
(1
|
)
|
—
|
|
—
|
|
2
|
|
||||||
|
Currency translation adjustment
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(52
|
)
|
(52
|
)
|
||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(23,315
|
)
|
—
|
|
(23,315
|
)
|
||||||
|
Balance, June 30, 2019
|
1,164,000
|
|
$
|
131,613
|
|
56,850,172
|
|
$
|
884,623
|
|
$
|
53,738
|
|
$
|
(857,264
|
)
|
$
|
(48,244
|
)
|
$
|
164,466
|
|
|
Accretion of accumulated dividends on Preferred Shares
|
—
|
|
2,792
|
|
—
|
|
—
|
|
—
|
|
(2,792
|
)
|
—
|
|
—
|
|
||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
1,592
|
|
—
|
|
—
|
|
1,592
|
|
||||||
|
Certain fair value adjustments to liability-classified
|
—
|
|
—
|
|
—
|
|
—
|
|
55
|
|
—
|
|
—
|
|
55
|
|
||||||
|
Currency translation adjustment
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
27
|
|
27
|
|
||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(82,503
|
)
|
—
|
|
(82,503
|
)
|
||||||
|
Balance, September 30, 2019
|
1,164,000
|
|
$
|
134,405
|
|
56,850,172
|
|
$
|
884,623
|
|
$
|
55,385
|
|
$
|
(942,559
|
)
|
$
|
(48,217
|
)
|
$
|
83,637
|
|
|
Convertible Preferred Shares
|
Common Shares
|
|||||||||||||||||||||
|
|
Number of Shares
|
Share Capital
|
Number of Shares
|
Share Capital
|
Additional Paid-In Capital
|
Deficit
|
Accumulated Other Comprehensive Loss
|
Total Stockholders' Equity
|
||||||||||||||
|
Balance, December 31, 2017
|
500,000
|
|
$
|
49,780
|
|
55,060,650
|
|
$
|
876,108
|
|
$
|
42,840
|
|
$
|
(738,070
|
)
|
$
|
(48,185
|
)
|
$
|
182,473
|
|
|
Issuance of Preferred Shares, net of issuance costs of $135
|
664,000
|
|
66,265
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
66,265
|
|
||||||
|
Accretion of coupon on Preferred Shares
|
—
|
|
2,336
|
|
—
|
|
—
|
|
—
|
|
(2,336
|
)
|
—
|
|
—
|
|
||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
1,510
|
|
—
|
|
—
|
|
1,510
|
|
||||||
|
Certain fair value adjustments to liability stock option awards
|
—
|
|
—
|
|
—
|
|
—
|
|
(504
|
)
|
—
|
|
—
|
|
(504
|
)
|
||||||
|
Issuance of common shares pursuant to exercise of options
|
—
|
|
—
|
|
26,541
|
|
180
|
|
(77
|
)
|
—
|
|
—
|
|
103
|
|
||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(17,429
|
)
|
—
|
|
(17,429
|
)
|
||||||
|
Balance, March 31, 2018
|
1,164,000
|
|
$
|
118,381
|
|
55,087,191
|
|
$
|
876,288
|
|
$
|
43,769
|
|
$
|
(757,835
|
)
|
$
|
(48,185
|
)
|
$
|
232,418
|
|
|
Accretion of coupon on Preferred Shares
|
—
|
|
2,541
|
|
—
|
|
—
|
|
—
|
|
(2,541
|
)
|
—
|
|
—
|
|
||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
1,862
|
|
—
|
|
—
|
|
1,862
|
|
||||||
|
Certain fair value adjustments to liability stock option awards
|
—
|
|
—
|
|
—
|
|
—
|
|
(34
|
)
|
—
|
|
—
|
|
(34
|
)
|
||||||
|
Issuance of common shares pursuant to exercise of options
|
—
|
|
—
|
|
238,059
|
|
1,903
|
|
(1,168
|
)
|
—
|
|
—
|
|
735
|
|
||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
3,091
|
|
—
|
|
3,091
|
|
||||||
|
Balance, June 30, 2018
|
1,164,000
|
|
$
|
120,922
|
|
55,325,250
|
|
$
|
878,191
|
|
$
|
44,429
|
|
$
|
(757,285
|
)
|
$
|
(48,185
|
)
|
$
|
238,072
|
|
|
Accretion of coupon on Preferred Shares
|
—
|
|
2,567
|
|
—
|
|
—
|
|
—
|
|
(2,567
|
)
|
—
|
|
—
|
|
||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
1,658
|
|
—
|
|
—
|
|
1,658
|
|
||||||
|
Certain fair value adjustments to liability stock option awards
|
—
|
|
—
|
|
—
|
|
—
|
|
(407
|
)
|
—
|
|
—
|
|
(407
|
)
|
||||||
|
Issuance of common shares pursuant to exercise of options
|
—
|
|
—
|
|
147,069
|
|
614
|
|
(180
|
)
|
—
|
|
—
|
|
434
|
|
||||||
|
Currency translation adjustment
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(12
|
)
|
(12
|
)
|
||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(24,473
|
)
|
—
|
|
(24,473
|
)
|
||||||
|
Balance, September 30, 2018
|
1,164,000
|
|
$
|
123,489
|
|
55,472,319
|
|
$
|
878,805
|
|
$
|
45,500
|
|
$
|
(784,325
|
)
|
$
|
(48,197
|
)
|
$
|
215,272
|
|
|
|
Three Months Ended September 30,
|
Nine Months Ended September 30,
|
|||||||||||||
|
|
2019
|
2018
|
2019
|
2018
|
|||||||||||
|
OPERATING ACTIVITIES
|
|
|
|
|
|||||||||||
|
Net loss
|
$
|
(82,503
|
)
|
$
|
(24,473
|
)
|
$
|
(129,069
|
)
|
$
|
(38,811
|
)
|
|||
|
Items not involving cash:
|
|||||||||||||||
|
Deferred income tax benefit
|
(12,661
|
)
|
(4,282
|
)
|
(12,661
|
)
|
(4,282
|
)
|
|||||||
|
Depreciation
|
507
|
|
497
|
|
1,521
|
|
1,677
|
|
|||||||
|
Loss (gain) on sale of property and equipment
|
—
|
|
(26
|
)
|
(11
|
)
|
(26
|
)
|
|||||||
|
Stock-based compensation expense
|
1,516
|
|
1,828
|
|
6,822
|
|
5,445
|
|
|||||||
|
Unrealized foreign exchange losses (gains)
|
24
|
|
(131
|
)
|
(71
|
)
|
795
|
|
|||||||
|
Change in fair value of contingent consideration
|
(376
|
)
|
(5,608
|
)
|
(121
|
)
|
(6,263
|
)
|
|||||||
|
Impairment of intangible assets
|
43,836
|
|
14,811
|
|
43,836
|
|
14,811
|
|
|||||||
|
Impairment of goodwill
|
22,471
|
|
—
|
|
22,471
|
|
—
|
|
|||||||
|
Site consolidation non-cash portion
|
—
|
|
—
|
|
—
|
|
396
|
|
|||||||
|
Gain on investment
|
—
|
|
—
|
|
—
|
|
(24,884
|
)
|
|||||||
|
Equity investment loss
|
3,512
|
|
2,838
|
|
11,497
|
|
2,838
|
|
|||||||
|
Non-cash royalty revenue
|
(979
|
)
|
—
|
|
(979
|
)
|
—
|
|
|||||||
|
Non-cash interest expense
|
1,106
|
|
—
|
|
1,106
|
|
—
|
|
|||||||
|
Net change in non-cash operating items:
|
|||||||||||||||
|
Accounts receivable
|
(957
|
)
|
784
|
|
(1,057
|
)
|
(136
|
)
|
|||||||
|
Prepaid expenses and other assets
|
1,080
|
|
109
|
|
1,839
|
|
1,017
|
|
|||||||
|
Accrued revenue
|
—
|
|
—
|
|
—
|
|
128
|
|
|||||||
|
Accounts payable and accrued liabilities
|
538
|
|
1,094
|
|
(1,320
|
)
|
(2,171
|
)
|
|||||||
|
Deferred revenue
|
—
|
|
(325
|
)
|
—
|
|
(2,093
|
)
|
|||||||
|
Restructuring accrual
|
(138
|
)
|
(320
|
)
|
(917
|
)
|
770
|
|
|||||||
|
Other liabilities
|
(446
|
)
|
—
|
|
(541
|
)
|
(2
|
)
|
|||||||
|
Net cash used in operating activities
|
(23,470
|
)
|
(13,204
|
)
|
(57,655
|
)
|
(50,791
|
)
|
|||||||
|
INVESTING ACTIVITIES
|
|
|
|||||||||||||
|
Acquisition of short and long-term investments
|
—
|
|
—
|
|
—
|
|
(48,025
|
)
|
|||||||
|
Disposition of short and long-term investments
|
16,410
|
|
24,590
|
|
87,675
|
|
—
|
|
|||||||
|
Proceeds from sale of property and equipment
|
—
|
|
25
|
|
11
|
|
25
|
|
|||||||
|
Acquisition of property and equipment
|
(255
|
)
|
(237
|
)
|
(526
|
)
|
(911
|
)
|
|||||||
|
Net cash provided by (used) in investing activities
|
16,155
|
|
24,378
|
|
87,160
|
|
(48,911
|
)
|
|||||||
|
FINANCING ACTIVITIES
|
|
|
|||||||||||||
|
Proceeds from sale of future royalties, net
|
18,549
|
|
—
|
|
18,549
|
|
—
|
|
|||||||
|
Promissory note repayment
|
—
|
|
—
|
|
—
|
|
(12,001
|
)
|
|||||||
|
Proceeds from sale of Series A Preferred Shares, net of issuance costs
|
—
|
|
—
|
|
—
|
|
66,265
|
|
|||||||
|
Issuance of common shares pursuant to the ATM
|
—
|
|
—
|
|
4,725
|
|
—
|
|
|||||||
|
Issuance of common shares pursuant to exercise of options
|
—
|
|
435
|
|
290
|
|
1,273
|
|
|||||||
|
Net cash provided by financing activities
|
18,549
|
|
435
|
|
23,564
|
|
55,537
|
|
|||||||
|
Effect of foreign exchange rate changes on cash and cash equivalents
|
(24
|
)
|
131
|
|
71
|
|
(795
|
)
|
|||||||
|
Increase in cash and cash equivalents
|
11,210
|
|
11,740
|
|
53,140
|
|
(44,960
|
)
|
|||||||
|
Cash and cash equivalents, beginning of period
|
78,872
|
|
10,193
|
|
36,942
|
|
66,893
|
|
|||||||
|
Cash and cash equivalents, end of period
|
$
|
90,082
|
|
$
|
21,933
|
|
$
|
90,082
|
|
$
|
21,933
|
|
|||
|
Supplemental cash flow information
|
|
|
|||||||||||||
|
Non-cash transactions:
|
|
|
|||||||||||||
|
Preferred shares dividends accrued
|
$
|
(2,792
|
)
|
$
|
(2,567
|
)
|
$
|
(8,269
|
)
|
$
|
(7,444
|
)
|
|||
|
Investment in Genevant
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
24,665
|
|
|||
|
Three Months Ended September 30,
|
Nine Months Ended September 30,
|
||||||||||||||
|
|
2019
|
2018
|
2019
|
2018
|
|||||||||||
|
(in thousands, except share and per share amounts)
|
|||||||||||||||
|
Numerator:
|
|||||||||||||||
|
Allocation of distributable earnings
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Allocation of undistributable loss
|
(85,295
|
)
|
(27,040
|
)
|
(137,338
|
)
|
(46,255
|
)
|
|||||||
|
Allocation of loss attributed to common shareholders
|
$
|
(85,295
|
)
|
$
|
(27,040
|
)
|
$
|
(137,338
|
)
|
$
|
(46,255
|
)
|
|||
|
Denominator:
|
|||||||||||||||
|
Weighted average number of common shares - basic and diluted
|
56,850,172
|
|
55,421,504
|
|
56,469,358
|
|
55,241,284
|
|
|||||||
|
Basic and diluted net loss attributable to common shareholders per share
|
$
|
(1.50
|
)
|
$
|
(0.49
|
)
|
$
|
(2.43
|
)
|
$
|
(0.84
|
)
|
|||
|
•
|
Level 1 inputs are quoted market prices for identical instruments available in active markets.
|
|
•
|
Level 2 inputs are inputs other than quoted prices included within Level 1 that are observable for the asset or liability either directly or indirectly. If the asset or liability has a contractual term, the input must be observable for substantially the full term. An example includes quoted market prices for similar assets or liabilities in active markets.
|
|
•
|
Level 3 inputs are unobservable inputs for the asset or liability and will reflect management’s assumptions about market assumptions that would be used to price the asset or liability.
|
|
Level 1
|
Level 2
|
Level 3
|
Total
|
||||||||||||
|
As of September 30, 2019
|
(in thousands)
|
||||||||||||||
|
Assets
|
|||||||||||||||
|
Cash and cash equivalents
|
$
|
90,082
|
|
$
|
—
|
|
$
|
—
|
|
$
|
90,082
|
|
|||
|
Liabilities
|
|||||||||||||||
|
Liability-classified options
|
—
|
|
—
|
|
86
|
|
86
|
|
|||||||
|
Contingent consideration
|
—
|
|
—
|
|
3,005
|
|
3,005
|
|
|||||||
|
Total
|
$
|
—
|
|
$
|
—
|
|
$
|
3,091
|
|
$
|
3,091
|
|
|||
|
Level 1
|
Level 2
|
Level 3
|
Total
|
||||||||||||
|
As of December 31, 2018
|
(in thousands)
|
||||||||||||||
|
Assets
|
|||||||||||||||
|
Cash and cash equivalents
|
$
|
36,942
|
|
$
|
—
|
|
$
|
—
|
|
$
|
36,942
|
|
|||
|
Short-term investments
|
87,675
|
|
—
|
|
—
|
|
87,675
|
|
|||||||
|
Total
|
124,617
|
|
—
|
|
—
|
|
124,617
|
|
|||||||
|
Liabilities
|
|||||||||||||||
|
Liability-classified stock option awards
|
—
|
|
—
|
|
479
|
|
479
|
|
|||||||
|
Contingent consideration
|
—
|
|
—
|
|
3,126
|
|
3,126
|
|
|||||||
|
Total
|
$
|
—
|
|
$
|
—
|
|
$
|
3,605
|
|
$
|
3,605
|
|
|||
|
|
Liability at beginning of the period
|
Fair value of liability-classified options exercised in the period
|
Increase (decrease) in fair value of liability
|
Liability at end of the period
|
|||||||||||
|
(in thousands)
|
|||||||||||||||
|
Nine months ended September 30, 2018
|
$
|
1,239
|
|
$
|
—
|
|
$
|
1,499
|
|
$
|
2,738
|
|
|||
|
Nine months ended September 30, 2019
|
$
|
479
|
|
$
|
—
|
|
$
|
(393
|
)
|
$
|
86
|
|
|||
|
|
Liability at beginning of the period
|
Increase (decrease) in fair value of liability
|
Liability at end of the period
|
||||||||
|
(in thousands)
|
|||||||||||
|
Nine months ended September 30, 2018
|
$
|
10,424
|
|
$
|
(6,263
|
)
|
$
|
4,161
|
|
||
|
Nine months ended September 30, 2019
|
$
|
3,126
|
|
$
|
(121
|
)
|
$
|
3,005
|
|
||
|
5.
|
Leases
|
|
As of September 30, 2019
|
|
|
Weighted-average remaining lease term (years)
|
7.3
|
|
Weighted average discount rate
|
8.9%
|
|
Nine Months Ended September 30,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Cash paid for amounts included in the measurement of lease liabilities
|
$
|
921
|
|
$
|
—
|
|
|
|
As of September 30, 2019
|
|||
|
(in thousands)
|
|||
|
October through December 2019
|
$
|
188
|
|
|
2020
|
657
|
|
|
|
2021
|
677
|
|
|
|
2022
|
581
|
|
|
|
2023
|
598
|
|
|
|
Thereafter
|
2,039
|
|
|
|
Total Lease Payments
|
$
|
4,740
|
|
|
Less: interest
|
(1,268
|
)
|
|
|
Present value of lease payments
|
$
|
3,472
|
|
|
|
September 30, 2019
|
December 31, 2018
|
|||||
|
(in thousands)
|
|||||||
|
Trade accounts payable
|
$
|
1,400
|
|
$
|
3,192
|
|
|
|
Research and development accruals
|
4,112
|
|
2,716
|
|
|||
|
Professional fee accruals
|
1,177
|
|
871
|
|
|||
|
Payroll accruals
|
1,509
|
|
2,341
|
|
|||
|
Other accrued liabilities
|
1
|
|
309
|
|
|||
|
|
$
|
8,199
|
|
$
|
9,429
|
|
|
|
8.
|
Site consolidation
|
|
Three Months Ended September 30,
|
Nine Months Ended September 30,
|
||||||||||||||
|
2019
|
2018
|
2019
|
2018
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Employee severance and relocation
|
$
|
231
|
|
$
|
198
|
|
$
|
429
|
|
$
|
3,399
|
|
|||
|
Facility and other expenses
|
(49
|
)
|
(690
|
)
|
(396
|
)
|
311
|
|
|||||||
|
Total site consolidation expense
|
$
|
182
|
|
$
|
(492
|
)
|
$
|
33
|
|
$
|
3,710
|
|
|||
|
Employee severance and relocation
|
Facility and other expenses
|
Total
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Site consolidation accrual as of December 31, 2018
|
$
|
697
|
|
$
|
634
|
|
$
|
1,331
|
|
||
|
Additional accruals and other adjustments
|
429
|
|
(396
|
)
|
33
|
|
|||||
|
Payments
|
(923
|
)
|
(238
|
)
|
(1,161
|
)
|
|||||
|
Site consolidation accrual as of September 30, 2019
|
$
|
203
|
|
$
|
—
|
|
$
|
203
|
|
||
|
9.
|
Sale of future royalties
|
|
Nine Months Ended September 30, 2019
|
|||
|
(in thousands)
|
|||
|
Net liability related to sale of future royalties - beginning balance
|
$
|
—
|
|
|
Initial recognition of liability
|
30,000
|
|
|
|
Debt discount and issuance costs
|
(11,451
|
)
|
|
|
Non-cash royalty revenue
|
(979
|
)
|
|
|
Non-cash interest expense
|
1,106
|
|
|
|
Net liability related to sale of future royalties - ending balance
|
$
|
18,675
|
|
|
•
|
our strategy, future operations, pre-clinical research, pre-clinical studies, clinical trials, prospects and the plans of management;
|
|
•
|
the discovery, development and commercialization of a cure for chronic hepatitis B infection, a disease of the liver caused by the hepatitis B virus ("HBV");
|
|
•
|
our beliefs and development path and strategy to achieve a cure for HBV;
|
|
•
|
obtaining necessary regulatory approvals;
|
|
•
|
obtaining adequate financing through a combination of financing activities and operations;
|
|
•
|
using the results from our HBV studies to adaptively design additional clinical trials to test the efficacy of the combination therapy and the duration of the result in patients;
|
|
•
|
the expected timing of and amount for payments related to Enantigen Therapeutics, Inc.'s (“Enantigen”) transaction and its programs;
|
|
•
|
the potential of our drug candidates to improve upon the standard of care and contribute to a curative combination treatment regimen;
|
|
•
|
the potential benefits of the reversion of the OMERS royalty monetization transaction for our ONPATTRO™ (Patisiran) royalty interest;
|
|
•
|
developing a suite of products that intervene at different points in the viral life cycle, with the potential to reactivate the host immune system;
|
|
•
|
using pre-clinical results to adaptively design clinical trials for additional cohorts of patients, testing the combination and the duration of therapy;
|
|
•
|
selecting combination therapy regimens and treatment durations to conduct Phase 3 clinical trials intended to ultimately support regulatory filings for marketing approval;
|
|
•
|
expanding our HBV drug candidate pipeline through internal development, acquisitions and in-licenses;
|
|
•
|
the potential of our assets, including our ownership stake in Genevant Sciences Ltd. (Genevant”) and the royalty entitlement on ONPATTRO, to provide significant non-dilutive capital;
|
|
•
|
our expectation to present results from the AB-506 Phase 1a/1b clinical trial along with further details regarding the two cases of acute hepatitis at the AASLD meeting in November 2019;
|
|
•
|
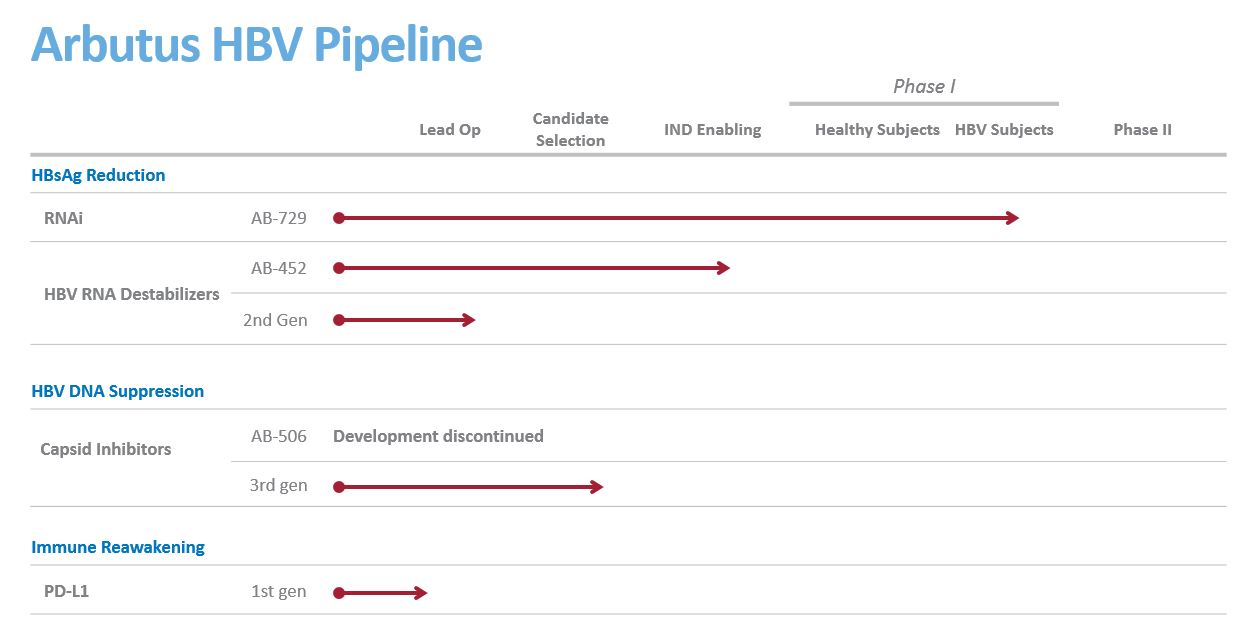
our expectation to select one of several oral next-generation capsid inhibitor lead compounds for IND-enabling studies in December of this year;
|
|
•
|
our expectation to make a decision regarding AB-452 clinical development in early 2020;
|
|
•
|
our expectation for AB-729 for preliminary safety and efficacy data from both healthy subjects and several single dose cohorts of subjects with CHB to be available in the first quarter of 2020.
|
|
•
|
payments from the Gritstone Oncology, Inc. ("Gritstone") licensing agreement;
|
|
•
|
the belief that current legal proceedings will not have a material adverse effect on our consolidated results of operations, cash flows, or financial condition;
|
|
•
|
the expected return from strategic alliances, licensing agreements, and research collaborations;
|
|
•
|
statements with respect to revenue and expense fluctuation and guidance;
|
|
•
|
the sufficiency of our cash and cash equivalents to extend into early 2021;
|
|
•
|
obtaining funding to maintain and advance our business from a variety of sources including public or private equity or debt financing, collaborative arrangements with pharmaceutical companies and government grants and contracts;
|
|
•
|
on-going arbitration; and
|
|
•
|
the amount and timing of potential funding,
|
|
•
|
developing a pipeline of proprietary therapeutic agents that target multiple elements of the HBV viral lifecycle, the most important of which we believe are HBV replication and hepatitis B surface antigen ("HBsAg") expression, and the host immune system; and
|
|
•
|
identifying an effective combination of complementary proprietary therapeutic agents administered for a finite treatment duration.
|
|
•
|
progress our clinical and pre-clinical product candidates through Phase 1 and Phase 2 clinical trials;
|
|
•
|
identify a safe and effective combination regimen to support a robust Phase 3 clinical registration program;
|
|
•
|
obtain regulatory approval for such combination regimen; and
|
|
•
|
commercialize such combination regimen.
|
|
|
Three Months Ended
|
Nine Months Ended
|
|||||||||||||
|
|
September 30,
|
September 30,
|
|||||||||||||
|
|
2019
|
2018
|
2019
|
2018
|
|||||||||||
|
(in thousands except per share amounts)
|
|||||||||||||||
|
Total revenue
|
$
|
3,061
|
|
$
|
1,587
|
|
$
|
4,393
|
|
$
|
4,267
|
|
|||
|
Operating expenses
|
94,462
|
|
34,013
|
|
135,380
|
|
77,144
|
|
|||||||
|
Loss from operations
|
(91,401
|
)
|
(32,426
|
)
|
(130,987
|
)
|
(72,877
|
)
|
|||||||
|
Net loss
|
(82,503
|
)
|
(24,473
|
)
|
(129,069
|
)
|
(38,811
|
)
|
|||||||
|
Net loss attributable to common shares
|
$
|
(85,295
|
)
|
$
|
(27,040
|
)
|
$
|
(137,338
|
)
|
$
|
(46,255
|
)
|
|||
|
Basic and diluted loss per common share
|
(1.50
|
)
|
(0.49
|
)
|
(2.43
|
)
|
(0.84
|
)
|
|||||||
|
Three Months Ended September 30,
|
|||||||||||||
|
2019
|
% of Total
|
2018
|
% of Total
|
||||||||||
|
(in thousands)
|
|||||||||||||
|
Research and development
|
$
|
17,731
|
|
19
|
%
|
$
|
16,566
|
|
49
|
%
|
|||
|
General and administrative
|
3,249
|
|
3
|
%
|
2,631
|
|
8
|
%
|
|||||
|
Depreciation
|
507
|
|
1
|
%
|
497
|
|
1
|
%
|
|||||
|
Site consolidation (note 8)
|
182
|
|
—
|
%
|
(492
|
)
|
(1
|
)%
|
|||||
|
Impairment of intangible assets (note 6)
|
43,836
|
|
46
|
%
|
14,811
|
|
44
|
%
|
|||||
|
Impairment of goodwill (note 6)
|
22,471
|
|
24
|
%
|
—
|
|
—
|
%
|
|||||
|
Arbitration (note 10)
|
6,486
|
|
7
|
%
|
—
|
|
—
|
%
|
|||||
|
Total operating expenses
|
$
|
94,462
|
|
100
|
%
|
$
|
34,013
|
|
100
|
%
|
|||
|
Nine Months Ended September 30,
|
|||||||||||||
|
2019
|
% of Total
|
2018
|
% of Total
|
||||||||||
|
(in thousands)
|
|||||||||||||
|
Research and development
|
$
|
45,183
|
|
33
|
%
|
$
|
46,871
|
|
61
|
%
|
|||
|
General and administrative
|
15,850
|
|
12
|
%
|
10,075
|
|
13
|
%
|
|||||
|
Depreciation
|
1,521
|
|
1
|
%
|
1,677
|
|
2
|
%
|
|||||
|
Site consolidation (note 8)
|
33
|
|
—
|
%
|
3,710
|
|
5
|
%
|
|||||
|
Impairment of intangible assets (note 6)
|
43,836
|
|
32
|
%
|
14,811
|
|
19
|
%
|
|||||
|
Impairment of goodwill (note 6)
|
22,471
|
|
17
|
%
|
—
|
|
—
|
%
|
|||||
|
Arbitration (note 10)
|
6,486
|
|
5
|
%
|
—
|
|
—
|
%
|
|||||
|
Total operating expenses
|
$
|
135,380
|
|
100
|
%
|
$
|
77,144
|
|
100
|
%
|
|||
|
|
Three Months Ended
|
Nine Months Ended
|
|||||||||||||
|
|
September 30,
|
September 30,
|
|||||||||||||
|
|
2019
|
2018
|
2019
|
2018
|
|||||||||||
|
(in thousands)
|
|||||||||||||||
|
Interest income
|
$
|
503
|
|
$
|
756
|
|
$
|
1,709
|
|
$
|
2,319
|
|
|||
|
Interest expense (note 9)
|
(1,100
|
)
|
—
|
|
(1,114
|
)
|
(104
|
)
|
|||||||
|
Foreign exchange gain (loss)
|
(25
|
)
|
145
|
|
43
|
|
(740
|
)
|
|||||||
|
Gain on investment (note 4)
|
—
|
|
—
|
|
—
|
|
24,884
|
|
|||||||
|
Equity investment loss (note 4)
|
(3,512
|
)
|
(2,838
|
)
|
(11,497
|
)
|
(2,838
|
)
|
|||||||
|
Change in fair value of contingent consideration (notes 3 and 10)
|
376
|
|
5,608
|
|
121
|
|
6,263
|
|
|||||||
|
Total other income (loss)
|
$
|
(3,758
|
)
|
$
|
3,671
|
|
$
|
(10,738
|
)
|
$
|
29,784
|
|
|||
|
|
Three Months Ended September 30,
|
Nine Months Ended September 30,
|
|||||||||||||
|
|
2019
|
2018
|
2019
|
2018
|
|||||||||||
|
(in thousands)
|
|||||||||||||||
|
Net loss
|
$
|
(82,503
|
)
|
$
|
(24,473
|
)
|
$
|
(129,069
|
)
|
$
|
(38,811
|
)
|
|||
|
Items not involving cash:
|
58,956
|
|
9,927
|
|
73,410
|
|
(9,493
|
)
|
|||||||
|
Net change in non-cash operating items:
|
77
|
|
1,342
|
|
(1,996
|
)
|
(2,487
|
)
|
|||||||
|
Net cash used in operating activities
|
(23,470
|
)
|
(13,204
|
)
|
(57,655
|
)
|
(50,791
|
)
|
|||||||
|
Net cash provided by (used) in investing activities
|
16,155
|
|
24,378
|
|
87,160
|
|
(48,911
|
)
|
|||||||
|
Net cash provided by financing activities
|
18,549
|
|
435
|
|
23,564
|
|
55,537
|
|
|||||||
|
Effect of foreign exchange rate changes on cash and cash equivalents
|
(24
|
)
|
131
|
|
71
|
|
(795
|
)
|
|||||||
|
Increase in cash and cash equivalents
|
11,210
|
|
11,740
|
|
53,140
|
|
(44,960
|
)
|
|||||||
|
Cash and cash equivalents, beginning of period
|
78,872
|
|
10,193
|
|
36,942
|
|
66,893
|
|
|||||||
|
Cash and cash equivalents, end of period
|
$
|
90,082
|
|
$
|
21,933
|
|
$
|
90,082
|
|
21,933
|
|
||||
|
•
|
revenue earned from our legacy collaborative partnerships and licensing agreements, including potential royalty payments from Alnylam's ONPATTRO;
|
|
•
|
revenue earned from ongoing collaborative partnerships, including milestone and royalty payments;
|
|
•
|
the extent to which we continue the development of our product candidates, add new product candidates to our pipeline, or form collaborative relationships to advance our products;
|
|
•
|
delays in the development of our product candidates due to pre-clinical and clinical findings;
|
|
•
|
our decisions to in-license or acquire additional products, product candidates or technology for development, in particular for our HBV therapeutics programs;
|
|
•
|
our ability to attract and retain corporate partners, and their effectiveness in carrying out the development and ultimate commercialization of our product candidates;
|
|
•
|
whether batches of drugs that we manufacture fail to meet specifications resulting in delays and investigational and remanufacturing costs;
|
|
•
|
the decisions, and the timing of decisions, made by health regulatory agencies regarding our technology and products;
|
|
•
|
competing technological and market developments; and
|
|
•
|
costs associated with prosecuting and enforcing our patent claims and other intellectual property rights, including litigation and arbitration arising in the course of our business activities.
|
|
Number
|
Description
|
|
|
3.1
|
||
|
3.2
|
||
|
4.1
|
||
|
10.1*
|
||
|
10.2†
|
||
|
10.3*
|
||
|
31.1*
|
||
|
|
|
|
|
31.2*
|
||
|
|
||
|
32.1**
|
||
|
|
|
|
|
32.2**
|
||
|
|
|
|
|
101
|
The following materials from Arbutus Biopharma Corporation's Quarterly Report on Form 10-Q for the quarter ended September 30, 2019, formatted in XBRL (eXtensible Business Reporting Language): (i) Condensed Consolidated Balance Sheets; (ii) Condensed Consolidated Statements of Operations; (iii) Condensed Consolidated Statements of Comprehensive Loss; (iv) Condensed Consolidated Statements of Stockholders' Equity; (v) Condensed Consolidated Statements of Cash Flows; and (vi) Notes to Condensed Consolidated Financial Statements
|
|
|
ARBUTUS BIOPHARMA CORPORATION
|
||
|
|
By:
|
/s/ William H Collier
|
|
|
|
William H Collier
|
|
|
|
President and Chief Executive Officer
|