|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

|
DELAWARE
|
95-4405754
|
|
(State or other jurisdiction of
|
(I.R.S. Employer
|
|
incorporation organization)
|
Identification No.)
|
|
|
|
|
500 NEWPORT CENTER DRIVE,
|
|
|
NEWPORT BEACH, CA
|
92660
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|
Common Stock, $0.001 par value
|
The NASDAQ Stock Market, LLC
|
|
Large accelerated filer
R
|
|
Accelerated filer
£
|
|
Non-accelerated filer
£
(Do not check if a smaller reporting company)
|
|
Smaller reporting company
£
|
|
|
Page
|
|
|
PART I
|
||
|
|
|
|
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
|
|
|
|
|
|
|
|
PART II
|
||
|
|
|
|
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
|
|
|
|
|
|
|
|
PART III
|
||
|
|
|
|
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
|
|
|
|
|
|
|
|
PART IV
|
||
|
|
|
|
|
Item 15.
|
||
|
•
|
our corporate code of conduct, our code of conduct for our board of directors and our fraud policy; and
|
|
•
|
charters for our audit committee, nominating and corporate governance committee, disclosure committee and compensation committee.
|

|
•
|
Identify Emerging Growth Areas where Patented Technologies will Play a Vital Role
|
|
•
|
Contact and Form Alliances with Owners of Core, Patented Technologies
|
|
•
|
Effectively and Efficiently Evaluate Patented Technologies for Acquisition, Licensing and Enforcement
|
|
•
|
Utilizing our staff of in-house intellectual property business development executives, patent attorneys, intellectual property licensing executives, and technology engineers to conduct our tailored patent acquisition and evaluation processes and procedures. We may also leverage the expertise of external specialists and technology consultants.
|
|
•
|
Identifying emerging growth areas where patented technologies will play a vital role in connection with the manufacture or sale of products and services.
|
|
•
|
Identifying core, patented technologies that have been or are anticipated to be widely adopted by third parties in connection with the manufacture or sale of products and services.
|
|
•
|
Considering the impact of subtleties in the language of a patent, recorded interactions with the patent office, evaluating prior art and literature and considering the impact on the potential licensing and enforcement revenue that can be derived from a patent or patent portfolio.
|
|
•
|
Evaluating the strength of a patent portfolio, including consideration of the types of claims and the number of claims potentially infringed by third parties, before the decision is made to allocate resources to an acquisition or an effective licensing and enforcement effort.
|
|
•
|
Identifying and considering potential problem areas, if any, and determining whether potential problem areas can be overcome prior to acquiring a patent portfolio or launching an effective licensing program.
|
|
•
|
Identifying potential infringers, industries within which the potential infringers exist, longevity of the patented technology, and a variety of other factors that directly impact the magnitude and potential success of a licensing and enforcement program.
|
|
•
|
Purchase or Acquire the Rights to Patented Technologies
|
|
•
|
Successfully License and Enforce Patents with Significant Royalty Potential
|
|
·
|
Aligned Wafer Bonding
|
·
|
Enhanced Internet Navigation
|
·
|
Online Ad Tracking
|
|
·
|
Audio Communications Fraud Detection
|
·
|
Enterprise Content Management
|
·
|
Online Auction Guarantees
|
|
·
|
Audio Storage and Retrieval System
|
·
|
Facilities Operation Management System
|
·
|
Online Promotion
|
|
·
|
Audio/Video Enhancement & Synchronization
|
·
|
File Locking in Shared Storage Networks
|
·
|
Optical Recording
|
|
·
|
Authorized Spending Accounts
|
·
|
File Systems and Development Environments
|
·
|
Optical Switching
|
|
·
|
Automated Communications
|
·
|
Flash Memory
|
·
|
Parallel Processing with Shared Memory
|
|
·
|
Automated Notification of Tax Return Status
|
·
|
Fluid Flow Control and Monitoring
|
·
|
Peer to Peer Communications
|
|
·
|
Automated Tax Reporting
|
·
|
Gemstone Grading
|
·
|
Physical Access Control
|
|
·
|
Automatic Image Labeling
|
·
|
GPS
|
·
|
Picture Archiving & Communication Systems
|
|
·
|
Biosensor
|
·
|
Greeting Card
|
·
|
Pointing Device
|
|
·
|
Broadcast Data Retrieval
|
·
|
Hearing Aid ECS
|
·
|
Portable Credit Card Processing
|
|
·
|
Business Process Modeling (BPM)
|
·
|
Heated Surgical Blades
|
·
|
Portable Storage Devices with Links
|
|
·
|
Camera Support
|
·
|
High Performance Computer Architecture
|
·
|
Power Management Within Integrated Circuits
|
|
·
|
Catheter Insertion
|
·
|
High Quality Image Processing
|
·
|
Product Activation
|
|
·
|
Child-Friendly Secure Mobile Phones
|
·
|
High Resolution Optics
|
·
|
Projector
|
|
·
|
Chip-Stacking
|
·
|
Image Resolution Enhancement
|
·
|
Purifying Nucleic Acids
|
|
·
|
Color Correction for Video Graphics Systems
|
·
|
Impact Instrument
|
·
|
Radio Communication with Graphics
|
|
·
|
Compact Disk
|
·
|
Improved Anti-Trap Safety Technology for Vehicles
|
·
|
Records Management
|
|
·
|
Compiler
|
·
|
Improved Commercial Print
|
·
|
Relational Database Access
|
|
·
|
Computer Architecture and Power Management
|
·
|
Improved Lighting
|
·
|
Remote Management of Imaging Devices
|
|
·
|
Computer Graphics
|
·
|
Improved Memory Manufacturing
|
·
|
Resource Scheduling
|
|
·
|
Computer Memory Cache Coherency
|
·
|
Improved Printing
|
·
|
Rule Based Monitoring
|
|
·
|
Computer Simulations
|
·
|
Information Portal Software
|
·
|
Shape Memory Alloys
|
|
·
|
Computer Storage Restoration
|
·
|
Information Storage, Searching and Retrieval
|
·
|
Software Activation
|
|
·
|
Consumer Rewards
|
·
|
Integrated Access
|
·
|
Software Installation
|
|
·
|
Continuous TV Viewer Measuring
|
·
|
Interactive Content in a Cable Distribution System
|
·
|
Software License Management
|
|
·
|
Copy Protection
|
·
|
Internet Radio Advertising
|
·
|
Spreadsheet Automation
|
|
·
|
Credit Card Fraud Protection
|
·
|
Interstitial Internet Advertising & Pop-Up Internet Advertising
|
·
|
Storage Technology
|
|
·
|
Database Access
|
·
|
Intraluminal Device Technology
|
·
|
Surgical Catheter
|
|
·
|
Database Management
|
·
|
|
·
|
Targeted Content Delivery
|
|
·
|
Database Retrieval Technology
|
·
|
Laparoscopic Surgery
|
·
|
Telematics
|
|
·
|
Digital Newspaper Delivery
|
·
|
Laptop Connectivity
|
·
|
Television Data Display
|
|
·
|
Digital Signal Processing Architecture
|
·
|
Lighting Ballast
|
·
|
Television Signal Scrambling
|
|
·
|
Digital Video Enhancement
|
·
|
Lighting Control
|
·
|
Text Auto-Completion
|
|
·
|
Digital Video Production
|
·
|
Line Screen Printing
|
·
|
User Programmable Engine Control
|
|
·
|
Disk Array Systems
|
·
|
Location Based Services
|
·
|
Vehicle Anti-Theft Parking Systems
|
|
·
|
Distributed Data Management and Synchronization
|
·
|
Manufacturing Data Transfer
|
·
|
Vehicle Maintenance
|
|
·
|
DMT®
|
·
|
Medical Image Manipulation
|
·
|
Vehicle Occupant Sensing
|
|
·
|
Document Generation
|
·
|
Medical Image Stabilization
|
·
|
Video Encoding
|
|
·
|
Document Retrieval Using Global Word Co-Occurrence Patterns
|
·
|
Medical Monitoring
|
·
|
Videoconferencing
|
|
·
|
Dynamic Manufacturing Modeling
|
·
|
MEMS
|
·
|
Virtual Computer Workspaces
|
|
·
|
Dynamic Random Access Memory
|
·
|
Messaging
|
·
|
Virtual Server
|
|
·
|
Electronic Address List Management
|
·
|
Micromirror Digital Display
|
·
|
Website Crawling
|
|
·
|
Electronic Message Advertising
|
·
|
Microprocessor Enhancement
|
·
|
Wireless Data
|
|
·
|
Electronic Securities Trading
|
·
|
Microprocessor Memory Management
|
·
|
Wireless Digital Messaging
|
|
·
|
Embedded Broadcast Data
|
·
|
Mobile Computer Synchronization
|
·
|
Wireless LAN
|
|
·
|
Encrypted Media & Playback Devices
|
·
|
Multi-Dimensional Database Compression
|
·
|
Wireless Monitoring
|
|
·
|
Energy Trading
|
·
|
Network Monitoring
|
·
|
Wireless Multimedia
|
|
·
|
Enhanced DRAM Architecture
|
·
|
Network Remote Access Technology
|
·
|
Workspace with Moving Viewpoint
|
|
•
|
our inability to enter into a definitive agreement with respect to any potential acquisition, or if we are able to enter into such agreement, our inability to consummate the potential acquisition;
|
|
•
|
difficulty integrating the operations, technology and personnel of the acquired entity;
|
|
•
|
our inability to achieve the anticipated financial and other benefits of the specific acquisition;
|
|
•
|
our inability to retain key personnel from the acquired company, if necessary;
|
|
•
|
difficulty in maintaining controls, procedures and policies during the transition and integration process;
|
|
•
|
diversion of our management's attention from other business concerns; and
|
|
•
|
failure of our due diligence process to identify significant issues, including issues with respect to patented technologies and patent portfolios, and other legal and financial contingencies.
|
|
•
|
Section 203 of the Delaware General Corporation Law, which prohibits a merger with a 15%-or-greater stockholder, such as a party that has completed a successful tender offer, until three years after that party became a 15%-or-greater stockholder;
|
|
•
|
amendment of our bylaws by the stockholders requires a two-thirds approval of the outstanding shares;
|
|
•
|
the authorization in our certificate of incorporation of undesignated preferred stock, which could be issued without stockholder approval in a manner designed to prevent or discourage a takeover;
|
|
•
|
provisions in our bylaws eliminating stockholders' rights to call a special meeting of stockholders, which could make it more difficult for stockholders to wage a proxy contest for control of our board of directors or to vote to repeal any of the anti-takeover provisions contained in our certificate of incorporation and bylaws; and
|
|
•
|
the division of our board of directors into three classes with staggered terms for each class, which could make it more difficult for an outsider to gain control of our board of directors.
|
|
•
|
merge or consolidate with another corporation;
|
|
•
|
liquidate or partially liquidate;
|
|
•
|
sell or transfer all or substantially all of its assets;
|
|
•
|
redeem or repurchase its stock (except in certain limited circumstances); or
|
|
•
|
take any other action which could reasonably be expected to cause Section 355(e) to apply to the distribution.
|
|
•
|
the dollar amount of agreements executed in each period, which is primarily driven by the nature and characteristics of the technology being licensed and the magnitude of infringement associated with a specific licensee;
|
|
•
|
the specific terms and conditions of agreements executed in each period and the periods of infringement contemplated by the respective payments;
|
|
•
|
fluctuations in the total number of agreements executed;
|
|
•
|
fluctuations in the sales results or other royalty-per-unit activities of our licensees that impact the calculation of license fees due;
|
|
•
|
the timing of the receipt of periodic license fee payments and/or reports from licensees;
|
|
•
|
fluctuations in the net number of active licensees period to period;
|
|
•
|
costs related to acquisitions, alliances, licenses and other efforts to expand our operations;
|
|
•
|
the timing of payments under the terms of any customer or license agreements into which our operating subsidiaries may enter; and
|
|
•
|
expenses related to, and the timing and results of, patent filings and other enforcement proceedings relating to intellectual property rights, as more fully described in this section.
|
|
•
|
announcements of developments in our patent enforcement actions;
|
|
•
|
developments or disputes concerning our patents;
|
|
•
|
our or our competitors' technological innovations;
|
|
•
|
developments in relationships with licensees;
|
|
•
|
variations in our quarterly operating results;
|
|
•
|
our failure to meet or exceed securities analysts' expectations of our financial results;
|
|
•
|
a change in financial estimates or securities analysts' recommendations;
|
|
•
|
changes in management's or securities analysts' estimates of our financial performance;
|
|
•
|
changes in market valuations of similar companies;
|
|
•
|
the current sovereign debt crises affecting several countries in the European Union and concerns about sovereign debt of the United States;
|
|
•
|
announcements by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures, capital commitments, new technologies, or patents; and
|
|
•
|
failure to complete significant transactions.
|
|
|
2011
|
2010
|
||||||||||||||
|
|
Fourth
Quarter
|
Third
Quarter
|
Second
Quarter
|
First
Quarter
|
Fourth
Quarter
|
Third
Quarter
|
Second
Quarter
|
First
Quarter
|
||||||||
|
High
|
$43.83
|
$47.24
|
$41.89
|
$36.44
|
$30.20
|
$17.75
|
$16.32
|
$11.34
|
||||||||
|
Low
|
$28.32
|
$32.39
|
$31.35
|
$22.12
|
$17.80
|
$12.87
|
$10.30
|
$7.79
|
||||||||
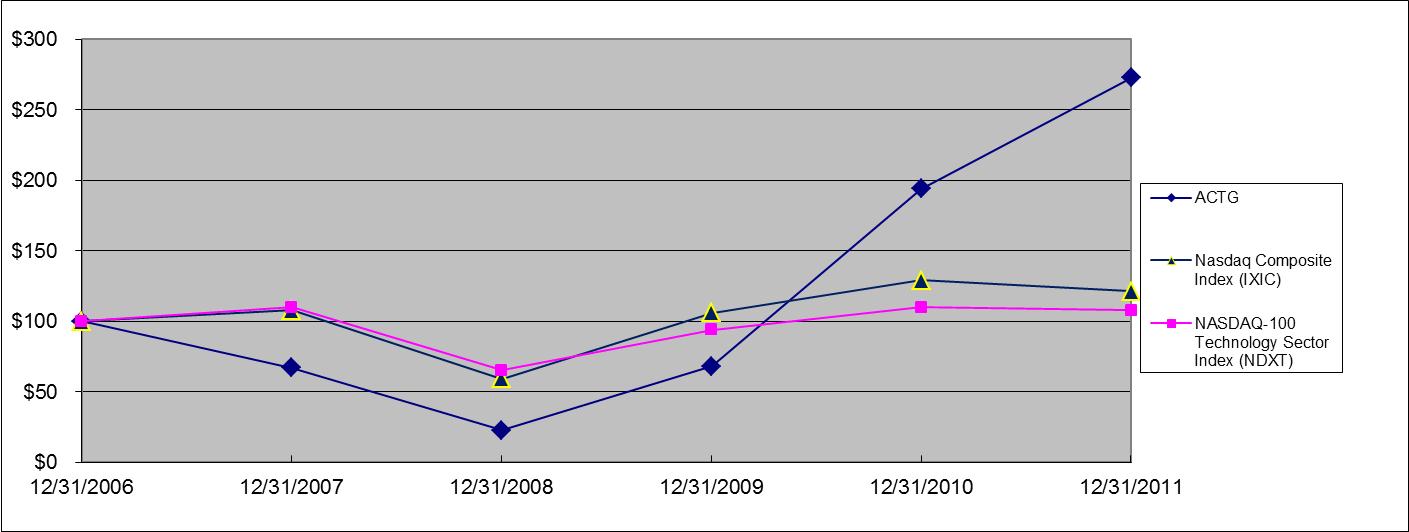
|
|
2007
|
2008
|
2009
|
2010
|
2011
|
|||||
|
Acacia Research Corporation common stock
|
$67
|
$23
|
$68
|
$194
|
$273
|
|||||
|
Nasdaq Composite Index (IXIC)
|
$108
|
$59
|
$106
|
$129
|
$121
|
|||||
|
NASDAQ-100 Technology Sector Index (NDXT)
|
$110
|
$65
|
$94
|
$110
|
$108
|
|||||
|
|
For the Years Ended December 31,
|
|||||||||||||||||||
|
|
2011
|
2010
|
2009
|
2008
|
2007
|
|||||||||||||||
|
Revenues and other operating income
(2)
|
$
|
184,707
|
|
$
|
131,829
|
|
$
|
67,340
|
|
$
|
48,227
|
|
$
|
52,597
|
|
|||||
|
Inventor royalties and contingent legal fees expense - patents
(2)
|
91,669
|
|
45,198
|
|
31,618
|
|
27,424
|
|
29,224
|
|
||||||||||
|
Litigation and licensing expenses - patents
|
13,005
|
|
13,891
|
|
14,055
|
|
6,900
|
|
7,799
|
|
||||||||||
|
Amortization of patents
|
9,745
|
|
6,931
|
|
4,634
|
|
6,043
|
|
5,583
|
|
||||||||||
|
Marketing, general and administrative expenses (including non-cash stock compensation expense)
|
35,693
|
|
25,067
|
|
21,070
|
|
21,130
|
|
18,381
|
|
||||||||||
|
Research, consulting and other expenses - business development
|
4,338
|
|
2,121
|
|
1,689
|
|
933
|
|
886
|
|
||||||||||
|
Operating income (loss)
|
30,257
|
|
38,621
|
|
(5,726
|
)
|
(14,203
|
)
|
(9,511
|
)
|
||||||||||
|
Other income, net
|
96
|
|
135
|
|
302
|
|
570
|
|
2,359
|
|
||||||||||
|
Income (loss) from continuing operations before provision for income taxes
|
30,353
|
|
38,756
|
|
(5,424
|
)
|
(13,633
|
)
|
(7,152
|
)
|
||||||||||
|
Provision for income taxes
|
(8,708
|
)
|
(1,740
|
)
|
(209
|
)
|
(124
|
)
|
(207
|
)
|
||||||||||
|
Net income (loss) from continuing operations including noncontrolling interests in operating subsidiaries
|
21,645
|
|
37,016
|
|
(5,633
|
)
|
(13,757
|
)
|
(7,359
|
)
|
||||||||||
|
Net income attributable to noncontrolling interests in operating subsidiaries
|
(539
|
)
|
(2,965
|
)
|
(5,657
|
)
|
—
|
|
—
|
|
||||||||||
|
Net income (loss) from continuing operations attributable to Acacia Research Corporation
|
21,106
|
|
34,051
|
|
(11,290
|
)
|
(13,757
|
)
|
(7,359
|
)
|
||||||||||
|
Discontinued operations
(1)
|
—
|
|
—
|
|
—
|
|
—
|
|
(8,086
|
)
|
||||||||||
|
Net income (loss) attributable to Acacia Research Corporation
|
21,106
|
|
34,051
|
|
(11,290
|
)
|
(13,757
|
)
|
(15,445
|
)
|
||||||||||
|
Net income (loss) per common share:
|
|
|
|
|
|
|
||||||||||||||
|
Net income (loss) from continuing operations attributable to Acacia Research Corporation
|
|
|
|
|
||||||||||||||||
|
Acacia Research Corporation common stock - basic
|
$
|
0.53
|
|
$
|
1.05
|
|
$
|
(0.38
|
)
|
$
|
(0.47
|
)
|
$
|
(0.26
|
)
|
|||||
|
Acacia Research Corporation common stock - diluted
|
$
|
0.51
|
|
$
|
0.97
|
|
$
|
(0.38
|
)
|
$
|
(0.47
|
)
|
$
|
(0.26
|
)
|
|||||
|
Discontinued operations
(1)
|
|
|
|
|
|
|
||||||||||||||
|
Acacia Research - CombiMatrix stock
(1)
|
—
|
|
—
|
|
—
|
|
$
|
—
|
|
$
|
(0.14
|
)
|
||||||||
|
Weighted-average number of common and potential common shares used in computation of income (loss) per common share
:
|
|
|
|
|
|
|
||||||||||||||
|
Acacia Research Corporation common stock:
|
|
|
|
|
|
|
||||||||||||||
|
Basic
|
39,743,433
|
|
32,306,322
|
|
29,914,801
|
|
29,423,998
|
|
28,503,314
|
|
||||||||||
|
Diluted
|
41,258,297
|
|
35,081,611
|
|
29,914,801
|
|
29,423,998
|
|
28,503,314
|
|
||||||||||
|
Acacia Research - CombiMatrix stock
(1)
:
|
|
|
|
|
|
|
||||||||||||||
|
Basic and diluted
|
—
|
|
—
|
|
—
|
|
—
|
|
55,862,707
|
|
||||||||||
|
|
At December 31,
|
|||||||||||||||||||
|
|
2011
|
2010
|
2009
|
2008
|
2007
|
|||||||||||||||
|
Total assets
|
$
|
352,877
|
|
$
|
134,784
|
|
$
|
78,256
|
|
$
|
73,074
|
|
$
|
71,051
|
|
|||||
|
Total liabilities
|
$
|
30,765
|
|
$
|
20,931
|
|
$
|
22,287
|
|
$
|
14,527
|
|
$
|
6,247
|
|
|||||
|
Noncontrolling interests in operating subsidiaries
|
$
|
2,163
|
|
$
|
2,982
|
|
$
|
2,507
|
|
$
|
—
|
|
$
|
—
|
|
|||||
|
Stockholders' equity
|
$
|
319,949
|
|
$
|
110,871
|
|
$
|
53,462
|
|
$
|
58,547
|
|
$
|
64,804
|
|
|||||
|
•
|
The increase in revenues and other operating income in fiscal year 2011, as compared to fiscal year 2010, was due primarily to an increase in the average revenue per executed agreement, which was partially offset by a decrease in the number of agreements executed in fiscal year 2011. The increase in revenues in fiscal year 2010, as compared to fiscal year 2009, was
due primarily to an increase in the average revenue per executed agreement and
an increase in the number of new licensing agreements executed during the period.
In fiscal 2011 and 2010, we entered into a number of separate significant revenue agreements with unrelated third parties resolving pending patent matters.
|
|
•
|
Net income attributable to noncontrolling interests in operating subsidiaries, or net income attributable to noncontrolling interests, represents the portion of net income or loss from the licensing and enforcement activities of our majority-owned operating subsidiaries that are distributable to the operating subsidiary's noncontrolling interest holders pursuant to the underlying operating agreements.
|
|
•
|
Inventor royalties, net income attributable to noncontrolling interests, and contingent legal fees, on a combined basis, increased
89%
in fiscal year 2011, as compared to fiscal year 2010, primarily reflecting the increase in related revenues for the same periods. The increase was greater than the percentage increase in related revenues for the same periods, due to, in the aggregate, lower or no inventor royalty or contingent legal fee arrangement obligations associated with a higher percentage of the portfolios generating revenues in fiscal year 2010, as compared to fiscal year 2011.
|
|
•
|
Inventor royalties, net income attributable to noncontrolling interests, and contingent legal fees, on a combined basis, increased 30% in fiscal year 2010, as compared to fiscal year 2009, primarily reflecting the increase in related revenues for 2010. The increase was less than the percentage increase in related revenues due to, in the aggregate, lower or no inventor royalty or contingent legal fee arrangement obligations associated with certain of the portfolios generating revenues in fiscal year 2010, as compared to fiscal year 2009.
|
|
•
|
The increase in provision for income taxes in fiscal year 2011 primarily reflects the impact of foreign withholding taxes, totaling $7.6 million, withheld by the applicable foreign tax authority pursuant to the requirements of the applicable income tax convention on payments in connection with certain licensing arrangements executed during fiscal year 2011.
|
|
•
|
In fiscal year 2011 and 2010, amortization of patents included the acceleration of patent amortization related to recoupable up-front patent portfolio acquisition costs that were recovered, pursuant to the provisions of the underlying inventor agreements, totaling
$3.1 million
and
$1.2 million
, respectively.
|
|
•
|
Marketing, general and administrative expenses included non-cash stock compensation expense totaling
$13.6 million
, $7.1 million, $7.1 million, $7.4 million and $5.9 million in 2011, 2010, 2009, 2008 and 2007, respectively.
|
|
•
|
In January 2006, our board of directors approved a plan for our former wholly owned subsidiary, CombiMatrix Corporation, or CombiMatrix, the primary component of our former life science business, known as the CombiMatrix group, to become an independent publicly-held company. On August 15, 2007, CombiMatrix was split-off from us through the redemption of all outstanding shares of Acacia Research-CombiMatrix common stock in exchange for the distribution of new shares of CombiMatrix common stock. We refer to this transaction as the Split-Off Transaction. Accordingly, the assets, liabilities, results of operations and cash flows for the CombiMatrix group are presented as “Discontinued Operations,” for the applicable historical periods above.
|
|
|
2011
|
|
2010
|
|
2009
|
||||||
|
|
|
|
|||||||||
|
Revenues and other operating income (in thousands)
|
$
|
184,707
|
|
|
$
|
131,829
|
|
|
$
|
67,340
|
|
|
New agreements executed
|
125
|
|
|
221
|
|
|
117
|
|
|||
|
Licensing and enforcement programs generating revenues - during the respective period
|
56
|
|
|
58
|
|
|
30
|
|
|||
|
Licensing and enforcement programs with initial revenues
|
21
|
|
|
31
|
|
|
12
|
|
|||
|
New patent portfolios
|
40
|
|
|
36
|
|
|
30
|
|
|||
|
Cumulative number of licensing and enforcement programs generating revenues - inception to date
|
112
|
|
91
|
|
60
|
|
|||||
|
As of Date:
|
Trailing Twelve -Month Revenues
|
% Change
|
|||||
|
December 31, 2011
|
$
|
184,707
|
|
4
|
%
|
||
|
September 30, 2011
|
177,014
|
|
(1
|
)%
|
|||
|
June 30, 2011
|
177,927
|
|
16
|
%
|
|||
|
March 31, 2011
|
153,187
|
|
16
|
%
|
|||
|
December 31, 2010
|
131,829
|
|
96
|
%
|
|||
|
December 31, 2009
|
67,340
|
|
—
|
|
|||
|
•
|
Audio Communications Fraud Detection technology
|
•
|
Magnetic Storage technology
(1)
|
|
|
•
|
Biosensor technology
(1)
|
•
|
Manufacturing Data Transfer technology
|
|
|
•
|
Camera Support technology
|
•
|
MEMS technology
(1)
|
|
|
•
|
Catheter Insertion technology
(1)
|
•
|
Messaging technology
(1)
|
|
|
•
|
Computer Architecture and Power Management technology
(1)
|
•
|
Microprocessor Enhancement technology
|
|
|
•
|
Computer Graphics technology
|
•
|
Mobile Computer Synchronization technology
|
|
|
•
|
Data Compression technology
(1)
|
•
|
Network Monitoring technology
|
|
|
•
|
Database Retrieval technology
(1)
|
•
|
Network Remote Access technology
|
|
|
•
|
DDR SDRAM technology
(1)
|
•
|
NOR Flash technology
(1)
|
|
|
•
|
Digital Signal Processing Architecture technology
|
•
|
Online Auction Guarantee technology
|
|
|
•
|
Digital Video Enhancement technology
|
•
|
Optical Recording technology
(1)
|
|
|
•
|
Disk Array Systems & Storage Area Network technology
|
•
|
Optical Switching technology
|
|
|
•
|
DMT® technology
|
•
|
Pop-up Internet Advertising technology
|
|
|
•
|
Document Generation technology
|
•
|
Power Management Within Integrated Circuits technology
(1)
|
|
|
•
|
DDR SDRAM
(1)
|
Power-over-Ethernet technology
(1)
|
||
|
•
|
DRAM Memory architecture technology
|
•
|
Rule Based Monitoring technology
|
|
|
•
|
Electronic Message Advertising technology
|
•
|
Semiconductor Manufacture technology
(1)
|
|
|
•
|
Facilities Operation Management System technology
|
•
|
Shape Memory Alloys technology
(1)
|
|
|
•
|
High Performance Computer Architecture technology
|
•
|
Short Messaging in Cellular Telephony technology
|
|
|
•
|
Image Resolution Enhancement technology
|
•
|
Software Installation technology
|
|
|
•
|
Impact Instrument technology
|
•
|
Storage technology
|
|
|
•
|
Improved Commercial Print technology
|
•
|
Targeted Content Delivery technology
(1)
|
|
|
•
|
Improved Lighting technology
|
•
|
Telematics technology
|
|
|
•
|
Interactive Content in a Cable Distribution System technology
(1)
|
•
|
User Programmable Engine Control technology
(1)
|
|
|
•
|
Interactive Mapping technology
|
•
|
Video Encoding technology
(1)
|
|
|
•
|
Item Identification technology
|
•
|
Virtual Server technology
|
|
|
•
|
Lighting Ballast technology
|
•
|
Visual Data Evaluation technology
|
|
|
•
|
Lighting Control technology
(1)
|
•
|
Website Crawling technology
|
|
|
•
|
Location Based Services technology
|
|||
|
•
|
Audio Communications Fraud Detection technology
|
•
|
Location Based Services technology
|
|
|
•
|
Authorized Spending Accounts technology
|
•
|
Manufacturing Data Transfer technology
(1)
|
|
|
•
|
Automatic Image Labeling technology
(1)
|
•
|
Medical Image Stabilization technology
|
|
|
•
|
Business Process Modeling (BPM) technology
(1)
|
•
|
Medical Monitoring technology
(1)
|
|
|
•
|
Camera Support technology
(1)
|
•
|
Microprocessor Enhancement technology
(1)
|
|
|
•
|
Child-friendly Secure Mobile Phones technology
|
•
|
Mobile Computer Synchronization technology
(1)
|
|
|
•
|
Compiler technology
(1)
|
•
|
Mutli-Dimensional Database Compression technology
|
|
|
•
|
Computer Graphics technology
(1)
|
•
|
Network Monitoring technology
(1)
|
|
|
•
|
Credit Card Fraud Protection technology
|
•
|
Network Remote Access technology
(1)
|
|
|
•
|
Database Access technology
|
•
|
Online Ad Tracking technology
(1)
|
|
|
•
|
Database Management technology
|
•
|
Online Auction Guarantee technology
|
|
|
•
|
Digital Signal Processing Architecture technology
(1)
|
•
|
Online Newsletters with Links technology
(1)
|
|
|
•
|
Digital Video Enhancement technology
(1)
|
•
|
Online Promotion technology
|
|
|
•
|
Disk Array Systems & Storage Area Network technology
(1)
|
•
|
Optical Switching technology
(1)
|
|
|
•
|
DMT® technology
|
•
|
Picture Archiving & Communications System technology
|
|
|
•
|
Document Generation technology
|
•
|
Pop-up Internet Advertising technology
|
|
|
•
|
DRAM Memory Architecture technology
(1)
|
•
|
Projector technology
|
|
|
•
|
Encrypted Media & Playback Devices technology
|
•
|
Records Management technology
(1)
|
|
|
•
|
Facilities Operation Management System technology
(1)
|
•
|
Rule Based Monitoring technology
|
|
|
•
|
File Locking In Shared Storage Networks technology
|
•
|
Short Messaging in Cellular Telephony technology
(1)
|
|
|
•
|
High Performance Computer Architecture technology
|
•
|
Software Installation technology
(1)
|
|
|
•
|
Image Resolution Enhancement technology
|
•
|
Storage technology
|
|
|
•
|
Improved Commercial Print technology
(1)
|
•
|
Telematics technology
|
|
|
•
|
Improved Lighting technology
(1)
|
•
|
Vehicle Occupant Sensing technology
(1)
|
|
|
•
|
Information Portal Software technology
(1)
|
•
|
Virtual Computer Workspace technology
|
|
|
•
|
Interactive Mapping technology
(1)
|
•
|
Virtual Server technology
|
|
|
•
|
Internet Radio Advertising technology
|
•
|
Visual Data Evaluation technology
(1)
|
|
|
•
|
|
•
|
Website Crawling technology
(1)
|
|
|
•
|
Lighting Ballast technology
|
•
|
Wireless Multimedia technology
(1)
|
|
|
•
|
Audio Communications Fraud Detection technology
|
•
|
Location Based Services technology
|
|
|
•
|
Audio Video Enhancement & Synchronization technology
|
•
|
Medical Image Stabilization technology
|
|
|
•
|
Authorized Spending Accounts technology
|
•
|
Multi-Dimensional Database Compression technology
(1)
|
|
|
•
|
Child-friendly Secure Mobile Phones technology
(1)
|
•
|
Online Auction Guarantee technology
|
|
|
•
|
Credit Card Fraud Protection technology
|
•
|
Online Promotion technology
(1)
|
|
|
•
|
Database Access technology
(1)
|
•
|
Picture Archiving & Communications System technology
|
|
|
•
|
DMT® technology
|
•
|
Pop-up Internet Advertising technology
|
|
|
•
|
Document Generation technology
(1)
|
•
|
Projector technology
|
|
|
•
|
eCommerce Pricing technology
|
•
|
Remote Management of Imaging Devices technology
|
|
|
•
|
Encrypted Media & Playback Devices technology
(1)
|
•
|
Rule Based Monitoring technology
|
|
|
•
|
Heated Surgical Blades technology
(1)
|
•
|
Storage technology
|
|
|
•
|
High Performance Computer Architecture technology
(1)
|
•
|
Surgical Catheter technology
(1)
|
|
|
•
|
High Quality Image Processing technology
|
•
|
Telematics technology
|
|
|
•
|
Internet Radio Advertising technology
(1)
|
•
|
Vehicle maintenance technology
|
|
|
•
|
Lighting Ballast technology
(1)
|
•
|
Virtual Server technology
(1)
|
|
|
Fiscal Year
|
% Change
|
||||||||||||||||
|
|
2011
|
|
2010
|
2009
|
|
2011 vs. 2010
|
|
2010 vs. 2009
|
|||||||||
|
|
|
|
|
|
|||||||||||||
|
Revenues
|
$
|
172,256
|
|
|
$
|
131,829
|
|
|
$
|
67,340
|
|
|
31
|
%
|
|
96
|
%
|
|
Verdict insurance proceeds
|
12,451
|
|
—
|
|
—
|
|
100
|
%
|
100
|
%
|
|||||||
|
Total revenues and other operating income
|
184,707
|
|
131,829
|
|
67,340
|
|
40
|
%
|
|
96
|
%
|
||||||
|
Operating costs and expenses**
|
154,450
|
|
|
93,208
|
|
|
73,066
|
|
|
66
|
%
|
|
28
|
%
|
|||
|
Operating income (loss)
|
30,257
|
|
38,621
|
|
(5,726
|
)
|
(22
|
)%
|
|
*
|
|
||||||
|
Provision for income taxes
|
(8,708
|
)
|
(1,740
|
)
|
(209
|
)
|
*
|
|
*
|
|
|||||||
|
Net income attributable to noncontrolling interests***
|
(539
|
)
|
|
(2,965
|
)
|
|
(5,657
|
)
|
|
(82
|
)%
|
|
(48
|
)%
|
|||
|
Net income (loss) attributable to Acacia Research Corporation
|
21,106
|
|
|
34,051
|
|
|
(11,290
|
)
|
|
(38
|
)%
|
|
*
|
|
|||
|
•
|
Revenues and other operating income increased
$52.9 million
, or
40%
,
due primarily to an increase in the average revenue per executed agreement, which was partially offset by a decrease in the total number of agreements executed in fiscal year 2011.
|
|
•
|
Other operating income includes verdict insurance proceeds totaling $12.5 million received during fiscal year 2011, as described below under "Consolidated Results of Operations."
|
|
•
|
Cost of Revenues and Other Operating Expenses:
|
|
◦
|
Inventor royalties, net income attributable to noncontrolling interests or noncontrolling interests, contingent legal fees, and applicable verdict insurance proceeds related costs, on a combined basis, increased
$43.3 million
, or
89%
, primarily reflecting the increase in related revenues and other operating income for fiscal year 2011. The increase was greater than the percentage increase in related revenues and other operating income due to, in the aggregate, lower or no inventor royalty or contingent legal fee arrangement obligations associated with a higher percentage of the portfolios generating revenues in fiscal year 2010, as compared to the portfolios generating revenues and other operating income in fiscal year 2011.
|
|
◦
|
Verdict insurance proceeds related costs for fiscal year 2011 totaled $7.7 million, as described below under "Consolidated Results of Operations".
|
|
◦
|
Litigation and licensing expenses-patents decreased $886,000, or 6%, to
$13.0 million
,
due to a lower net level of litigation support, third party technical consulting and professional expert expenses incurred in fiscal year 2011.
|
|
◦
|
Marketing, general and administrative expenses increased $10.6 million, or 42% to
$35.7 million
,
due primarily to an increase in non-cash stock compensation charges resulting from an increase in the average grant date fair value of restricted shares expensed during fiscal year 2011, an increase in annual one-time variable performance based compensation charges, an increase in other variable performance based compensation charges, a net increase in business development, engineering and other personnel since the end of the prior year period, and a net increase in corporate, general and administrative costs.
|
|
◦
|
Patent amortization increased
$2.8 million
, or
41%
to $9.7 million, due primarily to the acceleration of patent amortization related to recoupable up-front patent portfolio acquisition costs that were recovered in fiscal year 2011 and an increase in amortization related to new patent portfolios acquired in fiscal year 2011.
|
|
◦
|
The increase in provision for income taxes primarily reflects the impact of foreign withholding taxes, totaling $7.6 million, withheld by the applicable foreign tax authority pursuant to the requirements of the applicable income tax convention on payments in connection with certain licensing arrangements executed during fiscal year 2011.
|
|
•
|
Revenues increased $64.5 million, or 96%, due primarily to an increase in the average revenue per executed agreement and
an increase in the total number of new licensing agreements executed during the period.
|
|
•
|
Cost of Revenues and Other Operating Expenses:
|
|
◦
|
Inventor royalties, net income attributable to noncontrolling interests, and contingent legal fees, on a combined basis, increased 30%, primarily reflecting the increase in related revenues for 2010. The increase was less than the percentage increase in related revenues due to, in the aggregate, lower or no inventor royalty or contingent legal fee arrangement obligations associated with certain of the portfolios generating revenues in fiscal year 2010, as compared to fiscal year 2009.
|
|
◦
|
Litigation and licensing expenses-patents decreased $164,000, or 1% to $13.9 million,
due to a lower net level of litigation support, third party technical consulting and professional expert expenses incurred in fiscal year 2010. The decrease was partially offset by an increase in litigation and licensing expenses incurred in connection with our continued investment in ongoing licensing and enforcement programs and new licensing and enforcement programs commenced since the end of the prior year period.
|
|
◦
|
Marketing, general and administrative expenses increased $4.0 million, or 19% to $25.1 million,
due primarily to an increase in variable performance-based compensation costs, an increase in other variable personnel costs, and a minor net increase in engineering and licensing personnel, non-cash stock compensation charges and state related gross receipts taxes incurred on certain licensing revenues recognized in fiscal year 2010.
|
|
◦
|
Patent amortization increased $2.3 million, or 50%, to $6.9 million, due primarily to the acceleration of patent amortization related to recoupable up-front patent portfolio acquisition costs that were recovered in fiscal year 2010 and an increase in amortization related to new patent portfolios acquired in fiscal year 2010.
|
|
◦
|
The increase in the our tax expense in fiscal year 2010, as compared to fiscal year 2009, reflects the impact of the suspension of the use of NOLs in California, as described below, and the calculation of tax expense for financial reporting purposes without the excess tax benefit related to the exercise and vesting of equity-based incentive awards in fiscal year 2010.
|
|
•
|
Increases in patent-related legal expenses, including, but not limited to, increases in costs billed by outside legal counsel for discovery, depositions, economic analyses, damages assessments, expert witnesses and other consultants, case-related audio/video presentations and other litigation support and administrative costs could increase our operating costs and decrease our revenue generating opportunities;
|
|
•
|
Our patented technologies and enforcement actions are complex, and as a result, we may be required to appeal adverse decisions by trial courts in order to successfully enforce our patents;
|
|
•
|
New legislation, regulations or rules related to enforcement actions could significantly increase our operating costs and decrease our revenue generating opportunities; and
|
|
•
|
Courts may rule that our subsidiaries have violated certain statutory, regulatory, federal, local or governing rules or standards by pursuing such enforcement actions, which may expose us and our operating subsidiaries to material liabilities, which could harm our operating results and our financial position.
|
|
•
|
Flash Memory
. This patented technology consists of 16 flash memory patents relating to architecture, manufacturing and operation of flash memory, including NOR flash. The patented technology covers techniques for enhancing the performance and reliability of the flash memory cell. NOR flash memory is extensively used in cell phones.
|
|
•
|
Cellular Air Interface.
This patented technology has 200 patents covering 3G and 4G cellular air interface and infrastructure technologies. These technologies may be found in mobile handsets, base stations, routers and other related equipment.
|
|
•
|
Mobile Computer Synchronization.
This patented technology relates to mobile applications for use in smartphones and other wireless computing devices.
|
|
•
|
Cellular.
Acquired 6 patent portfolios relating to cellular technology, mobile handsets, wireless local area networks (WLAN), video processing, IPTV technology, and location based services technology.
|
|
•
|
Additional Patent Portfolios Acquired.
We also acquired, or acquired the rights to, additional patent portfolios related to Inhaler Drug Delivery technology, Enhanced Screensaver technology, Hearing Aid technology, Semiconductor Memory and Process technology, Online Gaming technology, Infusion Pump technology, Optical Networking technology, Circuit and Packaging technology, Radiation Therapy technology, Prescription Lens technology, Application Authentication technology, DDR SDRAM technology, Power-over-Ethernet technology, Targeted Marketing technology, Targeted Internet Advertising technology, Microprocessor and DSP technology, Data Compression technology, Heart-Lung Machine technology, Voice-Over-IP technology, HDTV technology, Mobile Communications technology, DRAM technology, Advanced Memory and Processor technology, 3G & 4G Wireless technology, Domain Name Redirection technology, Printer Document Assembly technology, Semiconductor Processing technology, Semiconductor Packaging technology, Computer-Aided Design technology and Heart Valve technology.
|
|
•
|
revenue recognition;
|
|
•
|
stock-based compensation expense;
|
|
•
|
valuation of long-lived and intangible assets; and
|
|
•
|
impairment of marketable securities;
|
|
•
|
significant underperformance relative to expected historical or projected future operating results;
|
|
•
|
significant changes in the manner of our use of the acquired assets or the strategy for our overall business;
|
|
•
|
significant negative industry or economic trends;
|
|
•
|
significant adverse changes in legal factors or in the business climate, including adverse regulatory actions or assessments; and
|
|
•
|
significant decline in our stock price for a sustained period.
|
|
•
|
Level 1 - Observable Inputs: Quoted prices in active markets for identical investments;
|
|
•
|
Level 2 - Pricing Models with Significant Observable Inputs: Other significant observable inputs, including quoted prices for similar investments, interest rates, credit risk, etc.; and
|
|
•
|
Level 3 - Unobservable Inputs: Significant unobservable inputs, including the entity’s own assumptions in determining the fair value of investments.
|
|
|
2011
|
|
2010
|
|
2009
|
|||||||
|
Revenues
|
$
|
172,256
|
|
|
$
|
131,829
|
|
|
$
|
67,340
|
|
|
|
Verdict insurance proceeds
|
12,451
|
|
—
|
|
—
|
|
||||||
|
$
|
184,707
|
|
$
|
131,829
|
|
$
|
67,340
|
|
||||
|
|
2011
|
|
2010
|
|
2009
|
||||
|
New agreements executed
|
125
|
|
|
221
|
|
|
117
|
|
|
|
Licensing and enforcement programs with initial revenues
|
21
|
|
|
31
|
|
|
12
|
|
|
|
•
|
the dollar amount of agreements executed each period, which can be driven by the nature and characteristics of the technology or technologies being licensed and the magnitude of infringement associated with a specific licensee;
|
|
•
|
the specific terms and conditions of agreements executed each period, including the nature and characteristics of rights granted, and the periods of infringement or term of use contemplated by the respective payments;
|
|
•
|
fluctuations in the total number of agreements executed;
|
|
•
|
fluctuations in the sales results or other royalty per unit activities of our licensees that impact the calculation of fees due;
|
|
•
|
the timing of the receipt of periodic payments and/or reports from licensees; and
|
|
•
|
fluctuations in the net number of active licensees from period to period.
|
|
|
2011
|
|
2010
|
|
2009
|
||||||
|
Cost of revenues and other operating income:
|
|
|
|||||||||
|
Inventor royalties**
|
$
|
46,614
|
|
|
$
|
25,292
|
|
|
$
|
15,673
|
|
|
Contingent legal fees**
|
44,247
|
|
|
19,906
|
|
|
15,945
|
|
|||
|
Other verdict insurance related costs
|
808
|
|
—
|
|
—
|
|
|||||
|
Net income attributable to noncontrolling interests*
|
—
|
|
|
(3,191
|
)
|
|
(5,657
|
)
|
|||
|
2011 vs. 2010
|
2010 vs. 2009
|
||||||
|
Increase in revenues and other operating income
|
40
|
%
|
|
96
|
%
|
|
|
|
Increase in inventor royalties and noncontrolling interests
(c)
|
64
|
%
|
Note (a)
|
34
|
%
|
Note (a)
|
|
|
Increase in contingent legal fees expense
(c)
|
122
|
%
|
Note (b)
|
25
|
%
|
Note (b)
|
|
|
Increase in inventor royalties expense, noncontrolling interests and contingent legal fees expense
(c)
|
88
|
%
|
Note (a),(b)
|
30
|
%
|
Note (a),(b)
|
|
|
As a Percentage of Revenue and Other Operating Income:
|
2011
|
|
2010
|
|
2009
|
|
|||||
|
|
|
|
|
||||||||
|
Inventor royalties and noncontrolling interests
(c)
|
25
|
%
|
|
22
|
%
|
|
32
|
%
|
|
Note (a)
|
|
|
Contingent legal fees expense
(c)
|
24
|
%
|
|
15
|
%
|
|
24
|
%
|
|
Note (b)
|
|
|
Inventor royalties, noncontrolling interests and contingent legal fees
(c)
|
49
|
%
|
|
37
|
%
|
|
55
|
%
|
|
Note (a),(b)
|
|
|
(a)
|
The percentage increase in inventor royalties and noncontrolling interests in fiscal year 2011, as compared to fiscal year 2010, was greater than the increase in revenues for the same periods, primarily due to a higher percentage of revenues recognized in fiscal year 2010 having no corresponding inventor royalty arrangement obligations, and in the aggregate, lower inventor royalty rates associated with the portfolios generating revenues in fiscal year 2010, as compared to fiscal year 2011.
|
|
(b)
|
The percentage increase in contingent legal fees expense in fiscal year 2011, as compared fiscal year 2010, was greater than the increase in revenues for the same periods, primarily due to a higher percentage of revenues recognized in fiscal year 2010 having no corresponding contingent legal fee arrangement obligations, and in the aggregate, lower contingent legal fee rates associated with the portfolios generating revenues in fiscal year 2010, as compared to fiscal year 2011.
|
|
(c)
|
Includes inventor royalties and contingent legal fees associated with the verdict insurance proceeds received, as described above.
|
|
|
2011
|
|
2010
|
|
2009
|
||||||
|
Litigation and licensing expenses - patents
|
$
|
13,005
|
|
|
$
|
13,891
|
|
|
$
|
14,055
|
|
|
Amortization of patents
|
9,745
|
|
|
6,931
|
|
|
4,634
|
|
|||
|
|
2011
|
|
2010
|
|
2009
|
|||||||
|
Marketing, general and administrative expenses (including non-cash stock compensation expense of $13,579 for 2011, $7,121 for 2010 and $7,065 for 2009)
|
$
|
35,693
|
|
$
|
25,067
|
|
$
|
21,070
|
|
|||
|
Research, consulting and other expenses - business development
|
4,338
|
|
2,121
|
|
1,689
|
|
||||||
|
2011 vs. 2010
|
2010 vs. 2009
|
||||||
|
Addition of licensing, business development and engineering personnel and other personnel costs, net
|
$
|
2,172
|
|
|
$
|
1,021
|
|
|
Increase in variable performance-based compensation and other variable personnel costs
|
1,303
|
|
2,153
|
|
|||
|
Corporate, general and administrative costs
|
796
|
|
|
622
|
|
||
|
State and foreign gross receipts taxes
|
(103
|
)
|
145
|
|
|||
|
Non-cash stock compensation expense
|
6,458
|
|
|
56
|
|
||
|
|
2011
|
|
2010
|
|
2009
|
||||||
|
|
|
||||||||||
|
Provision for income taxes
|
$
|
8,708
|
|
|
$
|
1,740
|
|
|
$
|
209
|
|
|
Effective tax rate
|
29
|
%
|
4
|
%
|
(4
|
)%
|
|||||
|
|
2011
|
2010
|
2009
|
|||||||||
|
Net cash provided by (used in):
|
|
|
|
|||||||||
|
Operating activities
|
$
|
60,590
|
|
$
|
44,922
|
|
$
|
16,118
|
|
|||
|
Investing activities
|
(23,237
|
)
|
(8,098
|
)
|
(8,652
|
)
|
||||||
|
Financing activities
|
174,865
|
|
13,956
|
|
(4,010
|
)
|
||||||
|
|
Payments Due by Period (In thousands)
|
|||||||||||||||
|
Total
|
Less than
1 year
|
1-3 years
|
More than 3 years
|
|||||||||||||
|
Operating leases
|
$
|
3,210
|
|
$
|
675
|
|
$
|
1,451
|
|
$
|
1,084
|
|
||||
|
Scheduled patent acquisition related payments
|
1,400
|
|
900
|
|
250
|
|
250
|
|
||||||||
|
Payments to consultants
|
200
|
|
200
|
|
—
|
|
—
|
|
||||||||
|
Total contractual obligations
|
$
|
4,810
|
|
$
|
1,775
|
|
$
|
1,701
|
|
$
|
1,334
|
|
||||
|
Plan Category
|
(a) Number of securities to be issued upon exercise of outstanding options
|
(b) Weighted-average exercise price of outstanding options
|
(c) Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a))
|
||||||
|
Equity compensation plans approved by security holders
|
|
|
|
||||||
|
2002 Acacia Technologies Stock Incentive Plan
(1)
|
434,000
|
|
$5.55
|
598,000
|
|
||||
|
2007 Acacia Technologies Stock Incentive Plan
(2)
|
—
|
|
—
|
|
—
|
|
|||
|
Subtotal
|
434,000
|
|
$5.55
|
598,000
|
|
||||
|
Equity compensation plans not approved by security holders
(3)
|
|
|
|
|
|
||||
|
|
N/A
|
|
N/A
|
N/A
|
|
||||
|
Total
|
434,000
|
|
$5.55
|
598,000
|
|
||||
|
(1)
|
The share reserve under the 2002 Acacia Technologies Stock Incentive Plan automatically increases on the first trading day in January each calendar year by an amount equal to three percent (3%) of the total number of shares of our common stock outstanding on the last trading day of December in the prior calendar year, but in no event will this annual increase exceed 500,000 shares and in no event will the total number of shares of common stock in the share reserve (as adjusted for all such annual increases) exceed twenty million shares. Column (a) excludes 1,333,000 in nonvested restricted stock awards and restricted stock units outstanding at December 31, 2011. Refer to Note 11 to our notes to consolidated financial statements included elsewhere herein.
|
|
(2)
|
The initial share reserve under the 2007 Acacia Technologies Stock Incentive Plan, or the 2007 Plan, was 560,000 shares of our common stock. The share reserve under the 2007 Plan automatically increased on January 1, 2008 and 2009, by an amount equal to two percent (2%) of the total number of shares of our common stock outstanding on the last trading day of December in the prior calendar year. After January 1, 2009, no new additional shares will be added to the 2007 Plan without security holder approval (except for shares subject to outstanding awards that are forfeited or otherwise returned to the 2007 Plan). Column (a) excludes 270,000 in nonvested restricted stock awards outstanding at December 31, 2011. Refer to Note 11 to our notes to consolidated financial statements included elsewhere herein.
|
|
(3)
|
We have not authorized the issuance of equity securities under any plan not approved by security holders.
|
|
(a)
|
The following documents are filed as part of this report.
|
|
(1) Financial Statements
|
Page
|
|
|
|
|
|
|
Acacia Research Corporation Consolidated Financial Statements
|
|
|
|
|
|
|
|
(2) Financial Statement Schedules
|
|
|
|
|
|
|
|
Financial statement schedules are omitted because they are not applicable or the required information is shown in the Financial Statements or the Notes thereto.
|
||
|
|
|
|
|
(3) Exhibits
|
|
|
|
|
|
|
|
Refer to Item 15(b) below.
|
|
|
|
(b)
|
Exhibits. The following exhibits are either filed herewith or incorporated herein by reference:
|
|
Exhibit
Number
|
Description
|
|
|
|
|
2.1
|
Agreement and Plan of Merger, dated November 22, 2011, by and among Acacia Research Group LLC, Apollo Patent Corp., Adaptix, Inc., and Baker Communications Fund II (QP), L.P., solely in its capacity as representative for the shareholders of Adaptix, Inc.(16)
|
|
3.1
|
Amended and Restated Certificate of Incorporation (1)
|
|
3.2
|
Amended and Restated Bylaws (10)
|
|
3.2.1
|
Amendment to Amended and Restated Bylaws (11)
|
|
10.1*
|
Acacia Research Corporation 1996 Stock Option Plan, as amended (2)
|
|
10.2*
|
Form of Option Agreement constituting the Acacia Research Corporation 1996 Executive Stock Bonus Plan (3)
|
|
10.3*
|
2002 Acacia Technologies Stock Incentive Plan (4)
|
|
10.4*
|
2007 Acacia Technologies Stock Incentive Plan (5)
|
|
10.5*
|
Form of Acacia Technologies Stock Option Agreement for the 2007 Acacia Technologies Stock Incentive Plan (6)
|
|
10.6*
|
Form of Acacia Technologies Stock Issuance Agreement for the 2002 Acacia Technologies Stock Incentive Plan (6)
|
|
10.7*
|
Form of Acacia Technologies Stock Issuance Agreement for the 2007 Acacia Technologies Stock Incentive Plan (6)
|
|
10.8
|
Office Space Lease dated January 28, 2002, between Acacia Research Corporation and The Irvine Company (7)
|
|
10.10
|
Form of Indemnification Agreement (8)
|
|
10.11
|
Form of Subscription Agreement between Acacia Research Corporation and certain investors (9)
|
|
10.12
|
Third Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (9)
|
|
10.19*
|
Employment Agreement, dated January 28, 2005, by and between Acacia Technologies Services Corporation, and Dooyong Lee, as amended (10)
|
|
10.19.1*
|
Amendment to Employment Agreement, dated December 17, 2008, by and between Acacia Technologies, LLC and Dooyong Lee (14)
|
|
10.20*
|
Employment Agreement, dated April 12, 2004, by and between Acacia Media Technologies Corporation and Edward Treska (10)
|
|
10.20.1*
|
Addendum, dated March 31, 2008, to Employment Agreement by and between Acacia Media Technologies Corporation and Edward Treska (12)
|
|
10.21
|
Fourth Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (10)
|
|
10.22
|
Fifth Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (10)
|
|
10.23*
|
Employment Agreement, dated March 31, 2008, by and between Acacia Technologies, LLC and Paul Ryan (13)
|
|
10.23.1*
|
Amendment to Employment Agreement, dated December 17, 2008, by and between Acacia Technologies, LLC and Paul Ryan (13)
|
|
10.24*
|
Employment Agreement, dated March 31, 2008, by and between Acacia Technologies, LLC and Robert L. Harris (12)
|
|
10.24.1*
|
Amendment to Employment Agreement, dated December 17, 2008, by and between Acacia Technologies, LLC and Robert L. Harris (13)
|
|
10.25*
|
Amended Employment Agreement, dated March 31, 2008, by and between Acacia Technologies, LLC and Clayton J. Haynes (12)
|
|
10.25.1*
|
Amendment to Amended Employment Agreement, dated December 17, 2008, by and between Acacia Technologies, LLC and Clayton J. Haynes (13)
|
|
10.26*
|
Acacia Research Corporation Amended and Restated Executive Severance Policy (13)
|
|
10.27
|
Sixth Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (15)
|
|
10.28
|
Form of Purchase Agreement (17)
|
|
18.1
|
Preferability Letter dated February 25, 2010 from Grant Thornton LLP, independent registered public accounting firm, regarding change in accounting principle (14)
|
|
21.1
|
List of Subsidiaries
|
|
23.1
|
Consent of Independent Registered Public Accounting Firm
|
|
24.1
|
Power of Attorney (included in the signature page hereto).
|
|
31.1
|
Certification of Chief Executive Officer Pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934
|
|
31.2
|
Certification of Chief Financial Officer Pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934
|
|
32.1
|
Certification of Chief Executive Officer Pursuant to Rule 13a-14(b)/15d-14(b) of the Securities Exchange Act of 1934 and 18 U.S.C. Section 1350
|
|
32.2
|
Certification of Chief Financial Officer Pursuant to Rule 13a-14(b)/15d-14(b) of the Securities Exchange Act of 1934 and 18 U.S.C. Section 1350
|
|
101**
|
Interactive Date Files Pursuant to Rule 405 of Regulation S-T.
|
|
*
|
The referenced exhibit is a management contract, compensatory plan or arrangement required to be filed as an exhibit to this Annual Report on Form 10-K pursuant to Item 15(c) of Form 10-K.
|
|
**
|
In accordance with Rule 405(a)(2) of Regulation S-T, the registrant will file its first Interactive Data Files that comply with paragraphs (d)(1) through (d)(4), (e)(1) and (e)(2) of Rule 405 of Regulation S-T as exhibits to an amendment to this Annual Report on Form 10-K to be filed no later than 30 days after the date hereof.
|
|
(1)
|
Incorporated by reference to Acacia Research Corporation's Current Report on Form 8-K filed on June 5, 2008 (SEC File No. 000-26068).
|
|
(2)
|
Incorporated by reference to Appendix A to Acacia Research Corporation's Definitive Proxy Statement on Schedule 14A filed on April 10, 2000 (SEC File No. 000-26068).
|
|
(3)
|
Incorporated by reference to Appendix A to Acacia Research Corporation's Definitive Proxy Statement on Schedule 14A filed on April 26, 1996 (SEC File No. 000-26068).
|
|
(4)
|
Incorporated by reference to Annex E to the Proxy Statement/Prospectus which formed part of Acacia Research Corporation's Registration Statement on Form S-4 (SEC File No. 333-87654) which became effective on November 8, 2002.
|
|
(5)
|
Incorporated by reference to Acacia Research Corporation's Registration Statement on Form S-8 (SEC File No. 333-144754) which became effective on July 20, 2007.
|
|
(6)
|
Incorporated by reference to Acacia Research Corporation's Quarterly Report on Form 10-Q for the period ended September 30, 2007, filed on November 2, 2007 (SEC File No. 000-26068).
|
|
(7)
|
Incorporated by reference to Acacia Research Corporation's Annual Report on Form 10‑K for the year ended December 31, 2001, filed on March 27, 2002 (SEC File No. 000‑26068).
|
|
(8)
|
Incorporated by reference to Acacia Research Corporation's Current Report on Form 8-K filed on September 19, 2005 (SEC File No. 000-26068).
|
|
(9)
|
Incorporated by reference to Acacia Research Corporation's Quarterly Report on Form 10-Q for the period ended March 31, 2006, filed on May 10, 2006 (SEC File No. 000‑26068).
|
|
(10)
|
Incorporated by reference to Acacia Research Corporation's Annual Report on Form 10-K for the year ended December 31, 2007, filed on March 14, 2008 (File No. 000-26068).
|
|
(11)
|
Incorporated by reference to Acacia Research Corporation's Current Report on Form 8-K filed on January 7, 2008 (File No. 000-26068).
|
|
(12)
|
Incorporated by reference to Acacia Research Corporation's Current Report on Form 8-K filed on April 2, 2008 (SEC File No. 000-26068).
|
|
(13)
|
Incorporated by reference to Acacia Research Corporation's Annual Report on Form 10-K for the year ended December 31, 2008, filed on February 26, 2009 (File No. 000-26068).
|
|
(14)
|
Incorporated by reference to Acacia Research Corporation's Annual Report on Form 10-K for the year ended December 31, 2009, filed on February 25, 2010, as amended on February 26, 2010 (File No. 000-26068)
|
|
(15)
|
Incorporated by reference to Acacia Research Corporation's Annual Report on Form 10-K for the year ended December 31, 2010, filed on February 28, 2011, as amended on March 24, 2011 (File No. 000-26068).
|
|
(16)
|
Incorporated by reference to Acacia Research Corporation's Current Report on Form 8-K/A filed on January 19, 2012 (File No. 000-26068). Portions of this exhibit have been omitted pursuant to a request for confidential treatment under Rule 24-b-2 of the Securities Exchange Act of 1934, as amended. The omitted material has been separately filed with the Securities and Exchange Commission.
|
|
(17)
|
Incorporated by reference to Acacia Research Corporation's Current Report on Form 8-K filed on February 16, 2012 (File No. 000-26068).
|
|
|
ACACIA RESEARCH CORPORATION
|
|
||
|
|
|
|
|
|
|
Dated:
|
February 29, 2012
|
By:
|
/s/ Paul R. Ryan
|
|
|
|
|
Paul R. Ryan
|
|
|
|
|
|
Chairman of the Board
and Chief Executive Officer
(Authorized Signatory)
|
|
|
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
|
|
/s/
|
Paul R. Ryan
|
|
Chairman of the Board and
|
|
February 29, 2012
|
|
|
Paul R. Ryan
|
|
Chief Executive Officer
|
|
|
|
|
|
|
(Principal Executive Officer)
|
|
|
|
|
|
|
|
|
|
|
/s/
|
Robert L. Harris, II
|
|
Director and President
|
|
February 29, 2012
|
|
|
Robert L. Harris, II
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/
|
Clayton J. Haynes
|
|
Chief Financial Officer and Treasurer
|
|
February 29, 2012
|
|
|
Clayton J. Haynes
|
|
(Principal Financial and Accounting Officer)
|
|
|
|
|
|
|
|
|
|
|
/s/
|
Fred A. de Boom
|
|
Director
|
|
February 29, 2012
|
|
|
Fred A. de Boom
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/
|
Edward W. Frykman
|
|
Director
|
|
February 29, 2012
|
|
|
Edward W. Frykman
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/
|
G. Louis Graziadio, III
|
|
Director
|
|
February 29, 2012
|
|
|
G. Louis Graziadio, III
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/
|
William S. Anderson
|
|
Director
|
|
February 29, 2012
|
|
|
William S. Anderson
|
|
|
|
|
|
|
December 31,
|
December 31,
|
||||||
|
|
2011
|
2010
|
||||||
|
ASSETS
|
|
|
||||||
|
Current assets:
|
|
|
||||||
|
Cash and cash equivalents
|
$
|
314,733
|
|
$
|
102,515
|
|
||
|
Short-term investments
|
6,597
|
|
—
|
|
||||
|
Accounts receivable
|
2,915
|
|
7,987
|
|
||||
|
Prepaid expenses and other current assets
|
803
|
|
1,679
|
|
||||
|
Total current assets
|
325,048
|
|
112,181
|
|
||||
|
Property and equipment, net of accumulated depreciation and amortization
|
220
|
|
135
|
|
||||
|
Patents, net of accumulated amortization
|
25,188
|
|
19,803
|
|
||||
|
Investments - noncurrent
|
1,956
|
|
2,001
|
|
||||
|
Other assets
|
465
|
|
664
|
|
||||
|
|
$
|
352,877
|
|
$
|
134,784
|
|
||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
|
|
|
|
||||
|
Current liabilities:
|
|
|
|
|
||||
|
Accounts payable and accrued expenses
|
$
|
6,625
|
|
$
|
7,099
|
|
||
|
Royalties and contingent legal fees payable
|
23,508
|
|
12,760
|
|
||||
|
Total current liabilities
|
30,133
|
|
19,859
|
|
||||
|
Other liabilities
|
632
|
|
1,072
|
|
||||
|
Total liabilities
|
30,765
|
|
20,931
|
|
||||
|
Commitments and contingencies (Note 12)
|
|
|
|
|
||||
|
Stockholders' equity:
|
|
|
|
|
||||
|
Preferred stock, par value $0.001 per share; 10,000,000 shares authorized; no shares issued or outstanding
|
—
|
|
—
|
|
||||
|
Common stock, par value $0.001 per share; 100,000,000 shares authorized; 42,928,001 and 36,029,068 shares issued and outstanding as of December 31, 2011 and 2010, respectively
|
43
|
|
36
|
|
||||
|
Additional paid-in capital
|
386,821
|
|
197,026
|
|
||||
|
Accumulated comprehensive loss
|
(1,830
|
)
|
—
|
|
||||
|
Accumulated deficit
|
(65,085
|
)
|
(86,191
|
)
|
||||
|
Total Acacia Research Corporation stockholders' equity
|
319,949
|
|
110,871
|
|
||||
|
Noncontrolling interests in operating subsidiaries
|
2,163
|
|
2,982
|
|
||||
|
Total stockholders' equity
|
322,112
|
|
113,853
|
|
||||
|
|
$
|
352,877
|
|
$
|
134,784
|
|
||
|
|
2011
|
2010
|
2009
|
|||||||||
|
Revenues
|
$
|
172,256
|
|
$
|
131,829
|
|
$
|
67,340
|
|
|||
|
Operating costs and expenses:
|
|
|
|
|
|
|
||||||
|
Cost of revenues:
|
|
|
|
|
|
|
||||||
|
Inventor royalties
|
43,727
|
|
25,292
|
|
15,673
|
|
||||||
|
Contingent legal fees
|
40,281
|
|
19,906
|
|
15,945
|
|
||||||
|
Litigation and licensing expenses - patents
|
13,005
|
|
13,891
|
|
14,055
|
|
||||||
|
Amortization of patents
|
9,745
|
|
6,931
|
|
4,634
|
|
||||||
|
Verdict insurance proceeds
|
(12,451
|
)
|
—
|
|
—
|
|
||||||
|
Verdict insurance proceeds related costs
|
7,661
|
|
—
|
|
—
|
|
||||||
|
Marketing, general and administrative expenses (including non-cash stock compensation expense of $13,579 in 2011, $7,121 in 2010, and $7,065 in 2009)
|
35,693
|
|
25,067
|
|
21,070
|
|
||||||
|
Research, consulting and other expenses - business development
|
4,338
|
|
2,121
|
|
1,689
|
|
||||||
|
Total operating costs and expenses
|
141,999
|
|
93,208
|
|
73,066
|
|
||||||
|
Operating income (loss)
|
30,257
|
|
38,621
|
|
(5,726
|
)
|
||||||
|
Interest and investment income
|
96
|
|
135
|
|
302
|
|
||||||
|
Income (loss) from operations before provision for income taxes
|
30,353
|
|
38,756
|
|
(5,424
|
)
|
||||||
|
Provision for income taxes
|
(8,708
|
)
|
(1,740
|
)
|
(209
|
)
|
||||||
|
Net income (loss) including noncontrolling interests in operating subsidiaries
|
21,645
|
|
37,016
|
|
(5,633
|
)
|
||||||
|
Net income attributable to noncontrolling interests in operating subsidiaries
|
(539
|
)
|
(2,965
|
)
|
(5,657
|
)
|
||||||
|
Net income (loss) attributable to Acacia Research Corporation
|
$
|
21,106
|
|
$
|
34,051
|
|
$
|
(11,290
|
)
|
|||
|
Net income (loss) per common share attributable to Acacia Research Corporation:
|
|
|
|
|
|
|
||||||
|
Basic income (loss) per share
|
$
|
0.53
|
|
$
|
1.05
|
|
$
|
(0.38
|
)
|
|||
|
Diluted income (loss) per share
|
$
|
0.51
|
|
$
|
0.97
|
|
$
|
(0.38
|
)
|
|||
|
Weighted-average shares:
|
||||||||||||
|
Weighted-average number of shares outstanding, basic
|
39,743,433
|
|
32,306,322
|
|
29,914,801
|
|
||||||
|
Weighted-average number of shares outstanding, diluted
|
41,258,297
|
|
35,081,611
|
|
29,914,801
|
|
||||||
|
|
Common Shares
|
Common Stock
|
Additional Paid-in Capital
|
Other Comprehensive Loss
|
Accumulated Deficit
|
Noncontrolling Interests in Operating Subsidiaries
|
Total
|
||||||||||||||||||||
|
Balance at December 31, 2008
|
30,884,994
|
|
$
|
31
|
|
$
|
167,468
|
|
$
|
—
|
|
$
|
(108,952
|
)
|
$
|
—
|
|
$
|
58,547
|
|
|||||||
|
2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
Net loss attributable to Acacia Research Corporation
|
—
|
|
—
|
|
—
|
|
—
|
|
(11,290
|
)
|
—
|
|
(11,290
|
)
|
|||||||||||||
|
Stock options exercised
|
94,700
|
|
—
|
|
247
|
|
—
|
|
—
|
|
—
|
|
247
|
|
|||||||||||||
|
Repurchased restricted common stock
|
(174,628
|
)
|
—
|
|
(1,107
|
)
|
—
|
|
—
|
|
—
|
|
(1,107
|
)
|
|||||||||||||
|
Compensation expense relating to stock options and restricted stock awards
|
1,107,000
|
|
1
|
|
7,064
|
|
—
|
|
—
|
|
—
|
|
7,065
|
|
|||||||||||||
|
Net income attributable to noncontrolling interests in operating subsidiary
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
5,657
|
|
5,657
|
|
|||||||||||||
|
Distributions to noncontrolling interests in operating subsidiary
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(3,150
|
)
|
(3,150
|
)
|
|||||||||||||
|
Balance at December 31, 2009
|
31,912,066
|
|
32
|
|
173,672
|
|
—
|
|
(120,242
|
)
|
2,507
|
|
55,969
|
|
|||||||||||||
|
2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
Net income attributable to Acacia Research Corporation
|
—
|
|
—
|
|
—
|
|
—
|
|
34,051
|
|
—
|
|
34,051
|
|
|||||||||||||
|
Stock options exercised
|
2,852,002
|
|
3
|
|
15,065
|
|
—
|
|
—
|
|
—
|
|
15,068
|
|
|||||||||||||
|
Compensation expense relating to stock options and restricted stock awards
|
1,265,000
|
|
1
|
|
7,120
|
|
—
|
|
—
|
|
—
|
|
7,121
|
|
|||||||||||||
|
Excess tax benefits from stock-based compensation
|
—
|
|
—
|
|
1,302
|
|
—
|
|
—
|
|
—
|
|
1,302
|
|
|||||||||||||
|
Net income attributable to noncontrolling interests in operating subsidiaries
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,965
|
|
2,965
|
|
|||||||||||||
|
Contributions from noncontrolling interests in operating subsidiary, net
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,317
|
|
2,317
|
|
|||||||||||||
|
Distributions to noncontrolling interests in operating subsidiary
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(4,807
|
)
|
(4,807
|
)
|
|||||||||||||
|
Issuance costs
|
—
|
|
—
|
|
(133
|
)
|
—
|
|
—
|
|
—
|
|
(133
|
)
|
|||||||||||||
|
Balance at December 31, 2010
|
36,029,068
|
|
36
|
|
197,026
|
|
—
|
|
(86,191
|
)
|
2,982
|
|
113,853
|
|
|||||||||||||
|
2011
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
Net income attributable to Acacia Research Corporation
|
—
|
|
—
|
|
—
|
|
—
|
|
21,106
|
|
—
|
|
21,106
|
|
|||||||||||||
|
Sale of common stock, net of issuance costs of $5,896
|
5,750,000
|
|
6
|
|
175,223
|
|
—
|
|
—
|
|
—
|
|
175,229
|
|
|||||||||||||
|
Stock options exercised
|
87,068
|
|
—
|
|
411
|
|
—
|
|
—
|
|
—
|
|
411
|
|
|||||||||||||
|
Compensation expense relating to stock options and restricted stock awards
|
1,061,865
|
|
1
|
|
13,578
|
|
—
|
|
—
|
|
—
|
|
13,579
|
|
|||||||||||||
|
Excess tax benefits from stock-based compensation
|
—
|
|
—
|
|
583
|
|
—
|
|
—
|
|
—
|
|
583
|
|
|||||||||||||
|
Net income attributable to noncontrolling interests in operating subsidiaries
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
539
|
|
539
|
|
|||||||||||||
|
Contributions from noncontrolling interests in operating subsidiary, net
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,539
|
|
1,539
|
|
|||||||||||||
|
Distributions to noncontrolling interests in operating subsidiary
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(2,897
|
)
|
(2,897
|
)
|
|||||||||||||
|
Unrealized loss on short-term investments
|
—
|
|
—
|
|
—
|
|
(1,830
|
)
|
—
|
|
—
|
|
(1,830
|
)
|
|||||||||||||
|
Balance at December 31, 2011
|
42,928,001
|
|
$
|
43
|
|
$
|
386,821
|
|
$
|
(1,830
|
)
|
$
|
(65,085
|
)
|
$
|
2,163
|
|
$
|
322,112
|
|
|||||||
|
|
2011
|
2010
|
2009
|
|||||||||
|
Cash flows from operating activities:
|
|
|
|
|||||||||
|
Net income (loss) including noncontrolling interests in operating subsidiaries
|
$
|
21,645
|
|
$
|
37,016
|
|
$
|
(5,633
|
)
|
|||
|
Adjustments to reconcile net income (loss) including noncontrolling interests in operating subsidiaries to net cash provided by operating activities:
|
|
|
|
|
|
|
||||||
|
Depreciation and amortization
|
9,850
|
|
7,017
|
|
4,759
|
|
||||||
|
Non-cash stock compensation
|
13,579
|
|
7,121
|
|
7,065
|
|
||||||
|
(Gain) loss on investments
|
(15
|
)
|
(32
|
)
|
47
|
|
||||||
|
Changes in assets and liabilities:
|
|
|
|
|
|
|
||||||
|
Accounts receivable
|
5,072
|
|
(2,877
|
)
|
2,326
|
|
||||||
|
Prepaid expenses and other assets
|
1,075
|
|
(757
|
)
|
(106
|
)
|
||||||
|
Accounts payable and accrued expenses
|
(1,364
|
)
|
(1,414
|
)
|
4,836
|
|
||||||
|
Royalties and contingent legal fees payable
|
10,748
|
|
358
|
|
1,632
|
|
||||||
|
Deferred revenues
|
—
|
|
(1,510
|
)
|
1,192
|
|
||||||
|
Net cash provided by operating activities
|
60,590
|
|
44,922
|
|
16,118
|
|
||||||
|
Cash flows from investing activities:
|
|
|
|
|
|
|
||||||
|
Purchase of property and equipment
|
(190
|
)
|
(58
|
)
|
(67
|
)
|
||||||
|
Purchase of available-for-sale investments
|
(8,427
|
)
|
—
|
|
—
|
|
||||||
|
Sale of available-for-sale investments
|
60
|
|
184
|
|
1,040
|
|
||||||
|
Patent acquisition costs
|
(14,680
|
)
|
(8,224
|
)
|
(9,625
|
)
|
||||||
|
Net cash used in investing activities
|
(23,237
|
)
|
(8,098
|
)
|
(8,652
|
)
|
||||||
|
Cash flows from financing activities:
|
|
|
|
|
|
|
||||||
|
Proceeds from sale of common stock, net of issuance costs
|
175,229
|
|
—
|
|
—
|
|
||||||
|
Distributions to noncontrolling interests in operating subsidiary
|
(2,897
|
)
|
(4,807
|
)
|
(3,150
|
)
|
||||||
|
Contributions from noncontrolling interests in operating subsidiary, net of issuance costs
|
1,539
|
|
2,393
|
|
—
|
|
||||||
|
Repurchased restricted common stock
|
—
|
|
—
|
|
(1,107
|
)
|
||||||
|
Proceeds from the exercise of stock options
|
411
|
|
15,068
|
|
247
|
|
||||||
|
Excess tax benefits from stock-based compensation
|
583
|
|
1,302
|
|
—
|
|
||||||
|
Net cash provided by (used in) financing activities
|
174,865
|
|
13,956
|
|
(4,010
|
)
|
||||||
|
Increase in cash and cash equivalents
|
212,218
|
|
50,780
|
|
3,456
|
|
||||||
|
Cash and cash equivalents, beginning
|
102,515
|
|
51,735
|
|
48,279
|
|
||||||
|
Cash and cash equivalents, ending
|
$
|
314,733
|
|
$
|
102,515
|
|
$
|
51,735
|
|
|||
|
|
2011
|
|
2010
|
|
2009
|
||||||
|
|
|
||||||||||
|
Net income attributable to noncontrolling interests
(1)
|
$
|
—
|
|
$
|
(3,191
|
)
|
$
|
(5,657
|
)
|
||
|
Net (income) loss attributable to noncontrolling interests - Acacia IP Fund
|
(539
|
)
|
226
|
|
—
|
|
|||||
|
Total net income attributable to noncontrolling interests
|
$
|
(539
|
)
|
$
|
(2,965
|
)
|
$
|
(5,657
|
)
|
||
|
|
●
|
Level 1 -
|
Observable Inputs: Quoted prices in active markets for identical investments;
|
|
|
●
|
Level 2 -
|
Pricing Models with Significant Observable Inputs: Other significant observable inputs, including quoted prices for similar investments, interest rates, credit risk, etc.; and
|
|
|
●
|
Level 3 -
|
Unobservable Inputs: Significant unobservable inputs, including the entity’s own assumptions in determining the fair value of investments.
|
|
Furniture and fixtures
|
3 to 5 years
|
|
Computer hardware and software
|
3 to 5 years
|
|
Leasehold improvements
|
2 to 5 years (Lesser of lease term or useful life of improvement)
|
|
|
2011
|
|
2010
|
|
2009
|
||||
|
|
|
|
|||||||
|
Weighted-average common shares outstanding - basic
|
39,743,433
|
|
|
32,306,322
|
|
|
29,914,801
|
|
|
|
Dilutive effect of Equity-based Incentive Awards
|
1,514,864
|
|
|
2,775,289
|
|
|
—
|
|
|
|
Weighted-average common shares outstanding - diluted
|
41,258,297
|
|
|
35,081,611
|
|
|
29,914,801
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Anti-dilutive Equity-based Incentive Awards excluded from the computation of diluted income (loss) per share
|
77,760
|
|
|
14,768
|
|
|
5,144,960
|
|
|
|
|
2011
|
2010
|
||||||
|
Furniture and fixtures
|
$
|
344
|
|
$
|
373
|
|
||
|
Computer hardware and software
|
454
|
|
478
|
|
||||
|
Leasehold improvements
|
165
|
|
154
|
|
||||
|
|
963
|
|
1,005
|
|
||||
|
Less: accumulated depreciation and amortization
|
(743
|
)
|
(870
|
)
|
||||
|
|
$
|
220
|
|
$
|
135
|
|
||
|
|
2011
|
2010
|
||||||
|
Accounts payable
|
$
|
354
|
|
$
|
359
|
|
||
|
Payroll and other employee benefits
|
909
|
|
941
|
|
||||
|
Accrued vacation
|
693
|
|
589
|
|
||||
|
Accrued legal expenses - patent
|
1,765
|
|
2,535
|
|
||||
|
Accrued consulting and other professional fees
|
1,286
|
|
1,786
|
|
||||
|
Accrued patent acquisition related payments
|
900
|
|
300
|
|
||||
|
Accrued taxes payable
|
583
|
|
198
|
|
||||
|
Other accrued liabilities
|
135
|
|
391
|
|
||||
|
|
$
|
6,625
|
|
$
|
7,099
|
|
||
|
|
2011
|
2010
|
||||||
|
Gross carrying amount - patents
|
$
|
61,519
|
|
$
|
51,001
|
|
||
|
Accumulated amortization - patents
|
(36,331
|
)
|
(31,198
|
)
|
||||
|
Patents, net
|
$
|
25,188
|
|
$
|
19,803
|
|
||
|
|
2011
|
2010
|
||||||
|
Auction rate securities:
|
|
|
||||||
|
Beginning balance as of January 1
|
$
|
2,001
|
|
$
|
2,152
|
|
||
|
Total gains or (losses) (realized or unrealized):
|
|
|
|
|
||||
|
Recognized (losses) included in earnings
|
—
|
|
—
|
|
||||
|
Recognized gains included in earnings
|
15
|
|
49
|
|
||||
|
Settlements
|
(60
|
)
|
(200
|
)
|
||||
|
Ending balance as of December 31
|
$
|
1,956
|
|
$
|
2,001
|
|
||
|
|
2011
|
2010
|
2009
|
|||||||||
|
Current:
|
|
|
|
|||||||||
|
Federal
|
$
|
179
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
State taxes
|
943
|
|
1,473
|
|
209
|
|
||||||
|
Foreign taxes
|
7,586
|
|
267
|
|
—
|
|
||||||
|
|
$
|
8,708
|
|
$
|
1,740
|
|
$
|
209
|
|
|||
|
|
2011
|
2010
|
||||||
|
Deferred tax assets:
|
|
|
||||||
|
Net operating loss and capital loss carryforwards and credits
|
$
|
7,626
|
|
$
|
13,988
|
|
||
|
Amortization and depreciation
|
6,252
|
|
5,702
|
|
||||
|
Stock compensation
|
2,665
|
|
1,595
|
|
||||
|
Basis of investments in affiliates
|
1,375
|
|
1,344
|
|
||||
|
Accrued liabilities and other
|
471
|
|
550
|
|
||||
|
Unrealized loss on short-term investments
|
746
|
|
—
|
|
||||
|
State taxes
|
124
|
|
5
|
|
||||
|
Intangibles
|
—
|
|
100
|
|
||||
|
Other
|
278
|
|
—
|
|
||||
|
Total deferred tax assets
|
19,537
|
|
23,284
|
|
||||
|
Less: valuation allowance
|
(19,537
|
)
|
(23,284
|
)
|
||||
|
Net deferred taxes
|
$
|
—
|
|
$
|
—
|
|
||
|
|
2011
|
2010
|
2009
|
||||||
|
Statutory federal tax rate
|
35
|
%
|
34
|
%
|
34
|
%
|
|||
|
State income and foreign taxes, net of federal tax effect
|
27
|
%
|
5
|
%
|
(4
|
)%
|
|||
|
Foreign tax credit
|
(25
|
)%
|
—
|
|
—
|
|
|||
|
Noncontrolling interests in operating subsidiaries
|
(1
|
)%
|
(3
|
)%
|
40
|
%
|
|||
|
Equity compensation
|
—
|
|
(1
|
)%
|
(1
|
)%
|
|||
|
Non deductible permanent items
|
4
|
%
|
—
|
|
—
|
|
|||
|
Expired net operating loss carryforwards
|
1
|
%
|
1
|
%
|
—
|
|
|||
|
Valuation allowance
|
(12
|
)%
|
(32
|
)%
|
(73
|
)%
|
|||
|
|
29
|
%
|
4
|
%
|
(4
|
)%
|
|||
|
•
|
Discretionary Option Grant Program
. Under the discretionary option grant program, Acacia's compensation committee may grant (1) non-statutory options to purchase shares of common stock to eligible individuals in the employ or service of Acacia or its subsidiaries (including employees, non-employee board members and consultants) at an exercise price not less than 85% of the fair market value of those shares on the grant date, and (2) incentive stock options to purchase shares of common stock to eligible employees at an exercise price not less than 100% of the fair market value of those shares on the grant date (not less than 110% of fair market value if such employee actually or constructively owns more than 10% of Acacia's voting stock or the voting stock of any of its subsidiaries).
|
|
•
|
Stock Issuance Program
. Under the stock issuance program, eligible individuals may be issued shares of common stock directly, upon the attainment of performance milestones or the completion of a specified period of service or as a bonus for past services. Under this program, the purchase price for the shares shall not be less than 100% of the fair market value of the shares on the date of issuance, and payment may be in the form of cash or past services rendered.
|
|
•
|
Automatic Option Grant Program (2002 Plan only)
. Commencing in fiscal 2008, each non-employee director will receive restricted stock units for the number of shares determined by dividing the annual retainer by the closing price of Acacia's common stock on the grant date, provided that such individual has served as a non-employee director for at least 6 months. In addition, as of May 2007, each new non-employee director will receive restricted stock units for the number of shares determined by dividing the annual board of directors retainer by the closing price of Acacia's common stock on the commencement date. Restricted stock units vest in a series of twelve quarterly installments over the three year period following the grant date, subject to immediate acceleration upon a change in control. Acacia will deliver shares corresponding to the vested restricted stock units within thirty (30) days after the first to occur of the following events: (i) the fifth (5th) anniversary of the grant date; or (ii) termination of the non-employee director's service as a member of the Company's Board of Directors. The non-employee directors do not have any rights, benefits or entitlements with respect to any shares unless and until the shares have been delivered.
|
|
|
|
Weighted-Average
|
|
||||||||||
|
|
Options
|
Exercise
Price
|
Remaining
Contractual Term
|
Aggregate
Intrinsic Value
|
|||||||||
|
Outstanding at December 31, 2010
|
521,000
|
|
$
|
5.41
|
|
|
|
||||||
|
Exercised
|
(87,000
|
)
|
$
|
4.73
|
|
|
|
||||||
|
Outstanding at December 31, 2011
|
434,000
|
|
$
|
5.55
|
|
2.8 years
|
$
|
13,442,000
|
|
||||
|
Vested
|
434,000
|
|
$
|
5.55
|
|
2.8 years
|
$
|
13,442,000
|
|
||||
|
Exercisable at December 31, 2011
|
434,000
|
|
$
|
5.55
|
|
2.8 years
|
$
|
13,442,000
|
|
||||
|
|
Nonvested
Restricted Shares
|
Weighted
Average Grant Date Fair Value
|
|||||
|
Nonvested restricted stock at December 31, 2010
|
1,623,000
|
|
$
|
6.85
|
|
||
|
Granted
|
1,107,000
|
|
$
|
29.24
|
|
||
|
Vested
|
(1,180,000
|
)
|
$
|
9.36
|
|
||
|
Canceled
|
(45,000
|
)
|
$
|
17.72
|
|
||
|
Nonvested restricted stock at December 31, 2011
|
1,505,000
|
|
$
|
21.01
|
|
||
|
|
Restricted
Stock Units
|
Weighted
Average Grant Date Fair Value
|
|||||
|
Nonvested restricted stock units outstanding at December 31, 2010
|
38,000
|
|
$
|
8.12
|
|
||
|
Granted
|
15,000
|
|
$
|
24.87
|
|
||
|
Vested
|
(27,000
|
)
|
$
|
9.41
|
|
||
|
Nonvested restricted stock units outstanding at December 31, 2011
|
26,000
|
|
$
|
16.57
|
|
||
|
Vested restricted stock units outstanding at December 31, 2011
|
72,000
|
|
$
|
7.56
|
|
||
|
Year
|
|
||
|
2012
|
$
|
675,000
|
|
|
2013
|
761,000
|
|
|
|
2014
|
690,000
|
|
|
|
2015
|
718,000
|
|
|
|
2016
|
366,000
|
|
|
|
Total minimum lease payments
|
$
|
3,210,000
|
|
|
|
Quarter Ended
|
|||||||||||||||||||||||||||||||
|
|
Mar. 31,
|
Jun. 30,
|
Sep. 30,
|
Dec. 31,
|
Mar. 31,
|
Jun. 30,
|
Sep. 30,
|
Dec. 31,
|
||||||||||||||||||||||||
|
|
2011
|
2011
|
2011
|
2011
|
2010
|
2010
|
2010
|
2010
|
||||||||||||||||||||||||
|
|
(Unaudited, In thousands, except share and per share information)
|
|||||||||||||||||||||||||||||||
|
Revenues
|
$
|
61,130
|
|
$
|
39,746
|
|
$
|
50,585
|
|
$
|
20,795
|
|
$
|
39,772
|
|
$
|
15,006
|
|
$
|
63,949
|
|
$
|
13,102
|
|
||||||||
|
Operating costs and expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
Cost of revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
Inventor royalties
|
13,089
|
|
8,588
|
|
15,592
|
|
6,458
|
|
3,911
|
|
2,877
|
|
14,508
|
|
3,996
|
|
||||||||||||||||
|
Contingent legal fees
|
9,367
|
|
13,039
|
|
12,328
|
|
5,547
|
|
4,407
|
|
3,465
|
|
9,739
|
|
2,295
|
|
||||||||||||||||
|
Litigation and licensing expenses - patents
|
3,538
|
|
3,761
|
|
3,501
|
|
2,205
|
|
3,696
|
|
4,433
|
|
2,835
|
|
2,927
|
|
||||||||||||||||
|
Amortization of patents
|
3,772
|
|
2,600
|
|
1,946
|
|
1,427
|
|
1,703
|
|
1,876
|
|
1,963
|
|
1,389
|
|
||||||||||||||||
|
Verdict insurance proceeds
|
—
|
|
—
|
|
(12,451
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||
|
Verdict insurance proceeds related costs
|
—
|
|
—
|
|
7,661
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||
|
Marketing, general and administrative expenses (including non-cash stock compensation expense)
|
9,981
|
|
8,302
|
|
8,748
|
|
8,662
|
|
6,332
|
|
6,056
|
|
6,388
|
|
6,291
|
|
||||||||||||||||
|
Research, consulting and other expenses - business development
|
708
|
|
1,335
|
|
850
|
|
1,445
|
|
372
|
|
453
|
|
461
|
|
835
|
|
||||||||||||||||
|
Total operating costs and expenses
|
40,455
|
|
37,625
|
|
38,175
|
|
25,744
|
|
20,421
|
|
19,160
|
|
35,894
|
|
17,733
|
|
||||||||||||||||
|
Operating income (loss)
|
20,675
|
|
2,121
|
|
12,410
|
|
(4,949
|
)
|
19,351
|
|
(4,154
|
)
|
28,055
|
|
(4,631
|
)
|
||||||||||||||||
|
Other income (expense)
|
29
|
|
24
|
|
25
|
|
18
|
|
19
|
|
20
|
|
44
|
|
52
|
|
||||||||||||||||
|
Income (loss) before provision for income taxes
|
20,704
|
|
2,145
|
|
12,435
|
|
(4,931
|
)
|
19,370
|
|
(4,134
|
)
|
28,099
|
|
(4,579
|
)
|
||||||||||||||||
|
(Provision) benefit for income taxes
|
(7,148
|
)
|
(306
|
)
|
(1,889
|
)
|
635
|
|
(321
|
)
|
13
|
|
(317
|
)
|
(1,115
|
)
|
||||||||||||||||
|
Net income (loss) including noncontrolling interests
|
13,556
|
|
1,839
|
|
10,546
|
|
(4,296
|
)
|
19,049
|
|
(4,121
|
)
|
27,782
|
|
(5,694
|
)
|
||||||||||||||||
|
Net (income) loss attributable to noncontrolling interests
|
(1,203
|
)
|
300
|
|
257
|
|
107
|
|
(537
|
)
|
255
|
|
(3,107
|
)
|
424
|
|
||||||||||||||||
|
Net income (loss) attributable to Acacia Research Corporation
|
$
|
12,353
|
|
$
|
2,139
|
|
$
|
10,803
|
|
$
|
(4,189
|
)
|
$
|
18,512
|
|
$
|
(3,866
|
)
|
$
|
24,675
|
|
$
|
(5,270
|
)
|
||||||||
|
Net income (loss) per common share attributable to Acacia Research Corporation:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
Basic income (loss) per share
|
$
|
0.35
|
|
$
|
0.05
|
|
$
|
0.26
|
|
$
|
(0.10
|
)
|
$
|
0.60
|
|
$
|
(0.12
|
)
|
$
|
0.75
|
|
$
|
(0.16
|
)
|
||||||||
|
Diluted income (loss) per share
|
$
|
0.34
|
|
$
|
0.05
|
|
$
|
0.25
|
|
$
|
(0.10
|
)
|
$
|
0.55
|
|
$
|
(0.12
|
)
|
$
|
0.70
|
|
$
|
(0.16
|
)
|
||||||||
|
Weighted-average number of shares outstanding, basic
|
35,182,811
|
|
40,994,082
|
|
41,292,819
|
|
41,418,470
|
|
30,847,403
|
|
31,664,869
|
|
32,794,553
|
|
33,879,777
|
|
||||||||||||||||
|
Weighted-average number of shares outstanding, diluted
|
36,448,005
|
|
42,453,782
|
|
42,857,880
|
|
41,418,470
|
|
33,411,093
|
|
31,664,869
|
|
35,105,354
|
|
33,879,777
|
|
||||||||||||||||
|
Exhibit
Number
|
Description
|
|
|
|
|
2.1
|
Agreement and Plan of Merger, dated November 22, 2011, by and among Acacia Research Group LLC, Apollo Patent Corp., Adaptix, Inc., and Baker Communications Fund II (QP), L.P., solely in its capacity as representative for the shareholders of Adaptix, Inc.(16)
|
|
3.1
|
Amended and Restated Certificate of Incorporation (1)
|
|
3.2
|
Amended and Restated Bylaws (10)
|
|
3.2.1
|
Amendment to Amended and Restated Bylaws (11)
|
|
10.1*
|
Acacia Research Corporation 1996 Stock Option Plan, as amended (2)
|
|
10.2*
|
Form of Option Agreement constituting the Acacia Research Corporation 1996 Executive Stock Bonus Plan (3)
|
|
10.3*
|
2002 Acacia Technologies Stock Incentive Plan (4)
|
|
10.4*
|
2007 Acacia Technologies Stock Incentive Plan (5)
|
|
10.5*
|
Form of Acacia Technologies Stock Option Agreement for the 2007 Acacia Technologies Stock Incentive Plan (6)
|
|
10.6*
|
Form of Acacia Technologies Stock Issuance Agreement for the 2002 Acacia Technologies Stock Incentive Plan (6)
|
|
10.7*
|
Form of Acacia Technologies Stock Issuance Agreement for the 2007 Acacia Technologies Stock Incentive Plan (6)
|
|
10.8
|
Office Space Lease dated January 28, 2002, between Acacia Research Corporation and The Irvine Company (7)
|
|
10.10
|
Form of Indemnification Agreement (8)
|
|
10.11
|
Form of Subscription Agreement between Acacia Research Corporation and certain investors (9)
|
|
10.12
|
Third Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (9)
|
|
10.19*
|
Employment Agreement, dated January 28, 2005, by and between Acacia Technologies Services Corporation, and Dooyong Lee, as amended (10)
|
|
10.19.1*
|
Amendment to Employment Agreement, dated December 17, 2008, by and between Acacia Technologies, LLC and Dooyong Lee (14)
|
|
10.20*
|
Employment Agreement, dated April 12, 2004, by and between Acacia Media Technologies Corporation and Edward Treska (10)
|
|
10.20.1*
|
Addendum, dated March 31, 2008, to Employment Agreement by and between Acacia Media Technologies Corporation and Edward Treska (12)
|
|
10.21
|
Fourth Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (10)
|
|
10.22
|
Fifth Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (10)
|
|
10.23*
|
Employment Agreement, dated March 31, 2008, by and between Acacia Technologies, LLC and Paul Ryan (13)
|
|
10.23.1*
|
Amendment to Employment Agreement, dated December 17, 2008, by and between Acacia Technologies, LLC and Paul Ryan (13)
|
|
10.24*
|
Employment Agreement, dated March 31, 2008, by and between Acacia Technologies, LLC and Robert L. Harris (12)
|
|
10.24.1*
|
Amendment to Employment Agreement, dated December 17, 2008, by and between Acacia Technologies, LLC and Robert L. Harris (13)
|
|
10.25*
|
Amended Employment Agreement, dated March 31, 2008, by and between Acacia Technologies, LLC and Clayton J. Haynes (12)
|
|
10.25.1*
|
Amendment to Amended Employment Agreement, dated December 17, 2008, by and between Acacia Technologies, LLC and Clayton J. Haynes (13)
|
|
10.26*
|
Acacia Research Corporation Amended and Restated Executive Severance Policy (13)
|
|
10.27
|
Sixth Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (15)
|
|
10.28
|
Form of Purchase Agreement (17)
|
|
18.1
|
Preferability Letter dated February 25, 2010 from Grant Thornton LLP, independent registered public accounting firm, regarding change in accounting principle (14)
|
|
21.1
|
List of Subsidiaries
|
|
23.1
|
Consent of Independent Registered Public Accounting Firm
|
|
24.1
|
Power of Attorney (included in the signature page hereto)
|
|
31.1
|
Certification of Chief Executive Officer Pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934
|
|
31.2
|
Certification of Chief Financial Officer Pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934
|
|
32.1
|
Certification of Chief Executive Officer Pursuant to Rule 13a-14(b)/15d-14(b) of the Securities Exchange Act of 1934 and 18 U.S.C. Section 1350
|
|
32.2
|
Certification of Chief Financial Officer Pursuant to Rule 13a-14(b)/15d-14(b) of the Securities Exchange Act of 1934 and 18 U.S.C. Section 1350
|
|
101**
|
Interactive Date Files Pursuant to Rule 405 of Regulation S-T.
|
|
*
|
The referenced exhibit is a management contract, compensatory plan or arrangement required to be filed as an exhibit to this Annual Report on Form 10-K pursuant to Item 15(c) of Form 10-K.
|
|
**
|
In accordance with Rule 405(a)(2) of Regulation S-T, the registrant will file its first Interactive Data Files that comply with paragraphs (d)(1) through (d)(4), (e)(1) and (e)(2) of Rule 405 of Regulation S-T as exhibits to an amendment to this Annual Report on Form 10-K to be filed no later than 30 days after the date hereof.
|
|
(1)
|
Incorporated by reference to Acacia Research Corporation's Current Report on Form 8-K filed on June 5, 2008 (SEC File No. 000-26068).
|
|
(2)
|
Incorporated by reference to Appendix A to Acacia Research Corporation's Definitive Proxy Statement on Schedule 14A filed on April 10, 2000 (SEC File No. 000-26068).
|
|
(3)
|
Incorporated by reference to Appendix A to Acacia Research Corporation's Definitive Proxy Statement on Schedule 14A filed on April 26, 1996 (SEC File No. 000-26068).
|
|
(4)
|
Incorporated by reference to Annex E to the Proxy Statement/Prospectus which formed part of Acacia Research Corporation's Registration Statement on Form S-4 (SEC File No. 333-87654) which became effective on November 8, 2002.
|
|
(5)
|
Incorporated by reference to Acacia Research Corporation's Registration Statement on Form S-8 (SEC File No. 333-144754) which became effective on July 20, 2007.
|
|
(6)
|
Incorporated by reference to Acacia Research Corporation's Quarterly Report on Form 10-Q for the period ended September 30, 2007, filed on November 2, 2007 (SEC File No. 000-26068).
|
|
(7)
|
Incorporated by reference to Acacia Research Corporation's Annual Report on Form 10‑K for the year ended December 31, 2001, filed on March 27, 2002 (SEC File No. 000‑26068).
|
|
(8)
|
Incorporated by reference to Acacia Research Corporation's Current Report on Form 8-K filed on September 19, 2005 (SEC File No. 000-26068).
|
|
(9)
|
Incorporated by reference to Acacia Research Corporation's Quarterly Report on Form 10-Q for the period ended March 31, 2006, filed on May 10, 2006 (SEC File No. 000‑26068).
|
|
(10)
|
Incorporated by reference to Acacia Research Corporation's Annual Report on Form 10-K for the year ended December 31, 2007, filed on March 14, 2008 (File No. 000-26068).
|
|
(11)
|
Incorporated by reference to Acacia Research Corporation's Current Report on Form 8-K filed on January 7, 2008 (File No. 000-26068).
|
|
(12)
|
Incorporated by reference to Acacia Research Corporation's Current Report on Form 8-K filed on April 2, 2008 (SEC File No. 000-26068).
|
|
(13)
|
Incorporated by reference to Acacia Research Corporation's Annual Report on Form 10-K for the year ended December 31, 2008, filed on February 26, 2009 (File No. 000-26068).
|
|
(14)
|
Incorporated by reference to Acacia Research Corporation's Annual Report on Form 10-K for the year ended December 31, 2009, filed on February 25, 2010, as amended on February 26, 2010 (File No. 000-26068)
|
|
(15)
|
Incorporated by reference to Acacia Research Corporation's Annual Report on Form 10-K for the year ended December 31, 2010, filed on February 28, 2011, as amended on March 24, 2011 (File No. 000-26068).
|
|
(16)
|
Incorporated by reference to Acacia Research Corporation's Current Report on Form 8-K/A filed on January 19, 2012 (File No. 000-26068). Portions of this exhibit have been omitted pursuant to a request for confidential treatment under Rule 24-b-2 of the Securities Exchange Act of 1934, as amended. The omitted material has been separately filed with the Securities and Exchange Commission.
|
|
(17)
|
Incorporated by reference to Acacia Research Corporation's Current Report on Form 8-K filed on February 16, 2012 (File No. 000-26068).
|