|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

|
DELAWARE
|
95-4405754
|
|
(State or other jurisdiction of
|
(I.R.S. Employer
|
|
incorporation organization)
|
Identification No.)
|
|
|
|
|
520 NEWPORT CENTER DRIVE, 12TH FLOOR
|
|
|
NEWPORT BEACH, CA
|
92660
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|
Common Stock, $0.001 par value
|
The NASDAQ Stock Market, LLC
|
|
Large accelerated filer
o
|
Accelerated filer
x
|
||
|
Non-accelerated filer
o
(Do not check if a smaller reporting company)
|
Smaller reporting company
o
|
||
|
Emerging growth company
o
|
|||
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
o
|
|||
|
|
Page
|
|
|
PART I
|
||
|
|
|
|
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
|
|
|
|
|
|
|
|
PART II
|
||
|
|
|
|
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
|
|
|
|
|
|
|
|
PART III
|
||
|
|
|
|
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
|
|
|
|
|
|
|
|
PART IV
|
||
|
|
|
|
|
Item 15.
|
||
|
•
|
our corporate code of conduct, our code of conduct for our board of directors and our fraud policy;
|
|
•
|
our insider trading policy;
|
|
•
|
charters for our audit committee, nominating and corporate governance committee, disclosure committee and compensation committee; and
|
|
•
|
applicable dividend related tax forms.
|
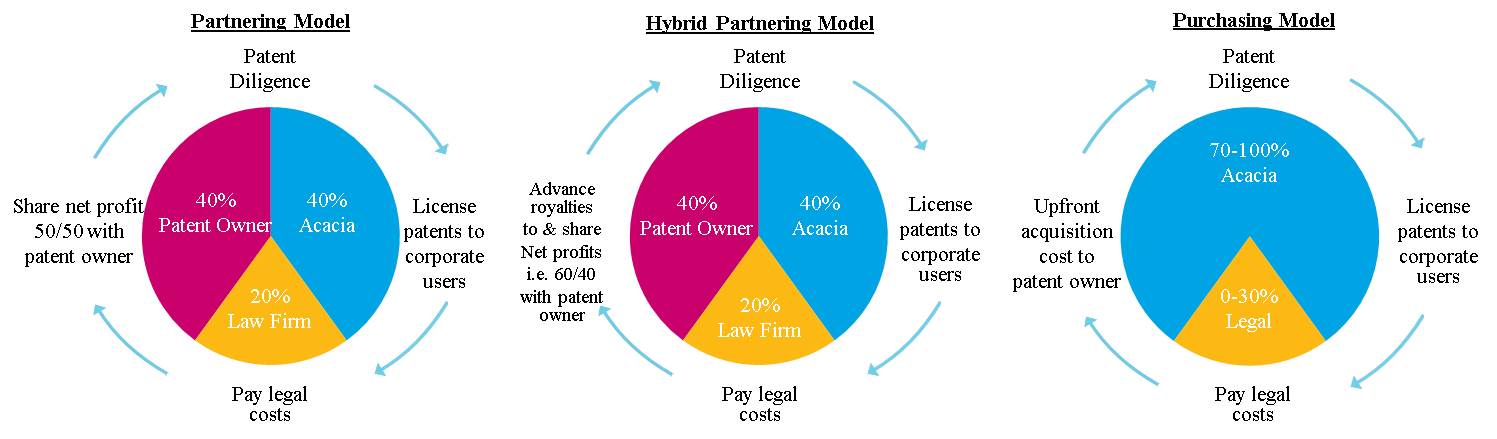
|
•
|
Patent Discovery
- Discover potentially valuable patents or patent portfolios.
|
|
•
|
Assessment of Economic Value -
Work internally and with external experts to evaluate the use of the patented invention(s) in the relevant marketplace and assess a patents or patent portfolios’ expected economic value.
|
|
•
|
Licensing and Enforcement
-
License those users wanting to utilize the patented invention with authorization. For unauthorized users of the patented invention, enter into license negotiations and, if necessary, litigation to monetize the patent based on its assessed value.
|
|
•
|
our inability to enter into a definitive agreement with respect to any potential patent portfolio investment, or if we are able to enter into such agreement, our inability to consummate the potential investment transaction;
|
|
•
|
difficulty integrating the operations, technology and personnel of the acquired entity;
|
|
•
|
our inability to achieve the anticipated financial and other benefits of the specific patent portfolio investment;
|
|
•
|
our inability to retain key personnel from the acquired company, if necessary;
|
|
•
|
difficulty in maintaining controls, procedures and policies during the transition and integration process;
|
|
•
|
diversion of our management’s attention from other business concerns; and
|
|
•
|
failure of our due diligence process to identify significant issues, including issues with respect to patented technologies and patent portfolios, and other legal and financial contingencies.
|
|
•
|
Section 203 of the Delaware General Corporation Law, which prohibits a merger with a 15%-or-greater stockholder, such as a party that has completed a successful tender offer, until three years after that party became a 15%-or-greater stockholder;
|
|
•
|
amendment of our bylaws by the stockholders requires a two-thirds approval of the outstanding shares;
|
|
•
|
the authorization in our certificate of incorporation of undesignated preferred stock, which could be issued without stockholder approval in a manner designed to prevent or discourage a takeover;
|
|
•
|
provisions in our bylaws eliminating stockholders’ rights to call a special meeting of stockholders, which could make it more difficult for stockholders to wage a proxy contest for control of our board of directors or to vote to repeal any of the anti-takeover provisions contained in our certificate of incorporation and bylaws; and
|
|
•
|
the division of our board of directors into three classes with staggered terms for each class, which could make it more difficult for an outsider to gain control of our board of directors.
|
|
•
|
the dollar amount of agreements executed in each period, which is primarily driven by the nature and characteristics of the technology being licensed and the magnitude of infringement associated with a specific licensee;
|
|
•
|
the specific terms and conditions of agreements executed in each period and the periods of infringement contemplated by the respective payments;
|
|
•
|
fluctuations in the total number of agreements executed;
|
|
•
|
fluctuations in the sales results or other royalty-per-unit activities of our licensees that impact the calculation of license fees due;
|
|
•
|
the timing of the receipt of periodic license fee payments and/or reports from licensees;
|
|
•
|
fluctuations in the net number of active licensees period to period;
|
|
•
|
costs related to investments, alliances, licenses and other efforts to expand our operations;
|
|
•
|
the timing of payments under the terms of any customer or license agreements into which our operating subsidiaries may enter;
|
|
•
|
we may elect to account for equity investments in companies where our investment gives us the ability to exercise significant influence over the operating and financial policies of the investee at fair value, which may result in significant fluctuations in operating results (unrealized gains and losses) each period based on fluctuations in the stock price of our investments and the requirement to mark such investments to market at each balance sheet date;
|
|
•
|
expenses related to, and the timing and results of, patent filings and other enforcement proceedings relating to intellectual property rights, as more fully described in this section; and
|
|
•
|
new litigation or developments in current litigation and the unpredictability of litigation results or settlements or appeals.
|
|
•
|
announcements of developments in our patent enforcement actions;
|
|
•
|
developments or disputes concerning our patents;
|
|
•
|
our or our competitors’ technological innovations;
|
|
•
|
developments in relationships with licensees;
|
|
•
|
variations in our quarterly operating results;
|
|
•
|
our failure to meet or exceed securities analysts’ expectations of our financial results;
|
|
•
|
a change in financial estimates or securities analysts’ recommendations;
|
|
•
|
changes in management’s or securities analysts’ estimates of our financial performance;
|
|
•
|
changes in market valuations of similar companies;
|
|
•
|
concerns about sovereign debt of the United States and the European Union;
|
|
•
|
announcements by us or our competitors of significant contracts, investments, partnerships, joint ventures, capital commitments, new technologies, or patents; and
|
|
•
|
failure to complete significant transactions.
|
|
|
2017
|
2016
|
||||||||||||||
|
|
Fourth
Quarter
|
Third
Quarter
|
Second
Quarter
|
First
Quarter
|
Fourth
Quarter
|
Third
Quarter
|
Second
Quarter
|
First
Quarter
|
||||||||
|
High
|
$4.75
|
$5.50
|
$5.75
|
$7.20
|
$7.68
|
$7.25
|
$5.64
|
$4.30
|
||||||||
|
Low
|
$3.80
|
$2.90
|
$3.70
|
$5.00
|
$5.55
|
$4.20
|
$3.75
|
$2.82
|
||||||||
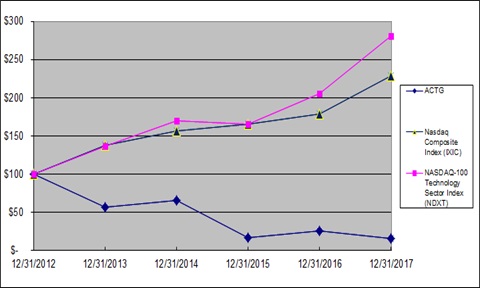
|
|
2013
|
2014
|
2015
|
2016
|
2017
|
|||||
|
Acacia Research Corporation common stock
|
$57
|
$66
|
$17
|
$25
|
$16
|
|||||
|
Nasdaq Composite Index (IXIC)
|
$138
|
$157
|
$166
|
$178
|
$229
|
|||||
|
NASDAQ-100 Technology Sector Index (NDXT)
|
$137
|
$170
|
$166
|
$206
|
$281
|
|||||
|
|
For the Years Ended December 31,
|
|||||||||||||||||||
|
|
2017
|
2016
|
2015
|
2014
|
2013
|
|||||||||||||||
|
Revenues
|
$
|
65,402
|
|
$
|
152,699
|
|
$
|
125,037
|
|
$
|
130,876
|
|
$
|
130,556
|
|
|||||
|
Inventor royalties and contingent legal fees expense
|
21,634
|
|
49,204
|
|
34,631
|
|
44,233
|
|
54,508
|
|
||||||||||
|
Litigation and licensing expenses - patents
|
18,219
|
|
27,858
|
|
39,373
|
|
37,614
|
|
39,335
|
|
||||||||||
|
Amortization of patents
|
22,154
|
|
34,208
|
|
53,067
|
|
53,745
|
|
49,039
|
|
||||||||||
|
General and administrative expenses (excluding non-cash stock compensation expense)
|
17,145
|
|
23,857
|
|
27,128
|
|
30,439
|
|
31,335
|
|
||||||||||
|
Non-cash stock compensation expense (included in G&A in the statements of operations)
|
8,885
|
|
9,062
|
|
11,048
|
|
18,115
|
|
27,894
|
|
||||||||||
|
Other expenses - business development
|
1,189
|
|
3,079
|
|
3,391
|
|
3,840
|
|
3,251
|
|
||||||||||
|
Impairment of patent-related intangible assets
|
2,248
|
|
42,340
|
|
74,731
|
|
3,497
|
|
4,619
|
|
||||||||||
|
Impairment of goodwill
|
—
|
|
—
|
|
30,149
|
|
—
|
|
—
|
|
||||||||||
|
Other
|
1,200
|
|
500
|
|
4,141
|
|
1,548
|
|
3,506
|
|
||||||||||
|
Operating loss
|
(27,272
|
)
|
(37,409
|
)
|
(152,622
|
)
|
(62,155
|
)
|
(82,931
|
)
|
||||||||||
|
Other income (expense)
|
51,911
|
|
798
|
|
(56
|
)
|
(595
|
)
|
2,131
|
|
||||||||||
|
Income (loss) before (provision for) benefit from income taxes
|
24,639
|
|
(36,611
|
)
|
(152,678
|
)
|
(62,750
|
)
|
(80,800
|
)
|
||||||||||
|
(Provision for) benefit from income taxes
|
(2,955
|
)
|
(18,188
|
)
|
(4,800
|
)
|
(3,912
|
)
|
21,958
|
|
||||||||||
|
Net income (loss) including noncontrolling interests in subsidiaries
|
$
|
21,684
|
|
$
|
(54,799
|
)
|
$
|
(157,478
|
)
|
$
|
(66,662
|
)
|
$
|
(58,842
|
)
|
|||||
|
Net income (loss) attributable to Acacia Research Corporation
|
$
|
22,180
|
|
$
|
(54,067
|
)
|
$
|
(160,036
|
)
|
$
|
(66,029
|
)
|
$
|
(56,434
|
)
|
|||||
|
Diluted income (loss) per common share
|
$
|
0.44
|
|
$
|
(1.08
|
)
|
$
|
(3.25
|
)
|
$
|
(1.37
|
)
|
$
|
(1.18
|
)
|
|||||
|
Cash dividends declared per common share
|
$
|
—
|
|
$
|
—
|
|
$
|
0.50
|
|
$
|
0.50
|
|
$
|
0.375
|
|
|||||
|
|
At December 31,
|
|||||||||||||||||||
|
|
2017
|
2016
|
2015
|
2014
|
2013
|
|||||||||||||||
|
Cash and cash equivalents, restricted cash and investments
|
$
|
136,604
|
|
$
|
158,495
|
|
$
|
145,948
|
|
$
|
193,024
|
|
$
|
256,702
|
|
|||||
|
Investment at fair value
|
104,754
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Patents, net of accumulated amortization
|
61,917
|
|
86,319
|
|
162,642
|
|
286,636
|
|
288,432
|
|
||||||||||
|
Goodwill
|
—
|
|
—
|
|
—
|
|
30,149
|
|
30,149
|
|
||||||||||
|
Total assets
|
308,768
|
|
296,003
|
|
347,901
|
|
536,348
|
|
593,393
|
|
||||||||||
|
Total liabilities
|
13,109
|
|
28,560
|
|
33,746
|
|
47,300
|
|
31,195
|
|
||||||||||
|
Noncontrolling interests in operating subsidiaries
|
1,358
|
|
1,854
|
|
3,944
|
|
5,491
|
|
6,488
|
|
||||||||||
|
Acacia Research Corporation stockholders’ equity
|
294,301
|
|
265,589
|
|
310,211
|
|
483,557
|
|
555,710
|
|
||||||||||
|
•
|
Investments at fair value.
Our equity investment in Veritone is recorded at fair value at each balance sheet date, with changes in fair value reflected in the statements of operations. Results for the year ended
December 31, 2017
included a net unrealized gain (included in other income (expense) in our consolidated statements of operations and in the table above) on our equity investment in Veritone totaling
$49.5 million
, comprised of an unrealized gain on conversion of our Veritone loans to equity of
$2.7 million
and an unrealized gain on the exercise of our Primary Warrant of
$4.6 million
, both as of May 2017, and an unrealized gain related to the change in fair value of our equity investment in Veritone through
December 31, 2017
of
$42.2 million
. Refer to Note
7
to the consolidated financial statements elsewhere herein for additional information regarding the impact of our equity investment in Veritone.
|
|
•
|
Litigation and licensing expenses - patents
. Litigation and licensing expenses-patents fluctuate from period to period based on patent enforcement and prosecution activity associated with ongoing licensing and enforcement programs and the timing of the commencement of new licensing and enforcement programs in each period. The trend of declining litigation and licensing expenses-patents reflects an overall decrease in portfolio related enforcement activities over the applicable periods.
Refer to “
Investments in Patent Portfolios” below for additional information regarding the impact of portfolio acquisition trends on licensing and enforcement activities and current and future licensing and enforcement related revenues.
|
|
•
|
Non-cash stock compensation expense.
In February 2017, AIP Operation LLC, or AIP, an indirect subsidiary of ours, adopted a Profits Interests Plan, or the Profits Interests Plan, that provides for the grant of AIP membership interests to certain members of management and the Board of Directors of Acacia Research Corporation as compensation for services rendered. The membership interests are represented by units, or the Units, reserved for the issuance of awards under the Profits Interests Plan. As of
December 31, 2017
, AIP holds the Veritone 10% Warrant described at Note
10
. The fair value of the Units totaled
$3.0 million
as of
December 31, 2017
and is classified as a liability in our consolidated balance sheet, with the corresponding compensation charge included in non-cash general and administrative expenses in the statement of operations for the year ended December 31, 2017.
|
|
•
|
Impairment of patent related intangible assets.
The impairment charges for the periods presented reflect the impact of reductions in expected estimated future net cash flows for certain portfolios due to adverse legal outcomes, conclusion of the related licensing and enforcement programs and /or certain patent portfolios that management determined it would no longer allocate resources to in future periods. The impairment charges consisted of the excess of the asset’s carrying value over its estimated fair value as of the applicable measurement date.
|
|
•
|
Goodwill.
We conducted an annual goodwill impairment test as of December 31, 2015. Based upon the difference between the implied fair value of goodwill and the historical carrying value of goodwill, due primarily to the sustained decline in the Company’s stock price and adverse litigation outcomes in the fourth quarter of 2015, we recognized a goodwill impairment charge totaling $30.1 million. Refer to “Critical Accounting Policies” below for additional information.
|
|
|
2017
|
|
2016
|
|
2015
|
||||||
|
|
|
|
|||||||||
|
Revenues (in thousands)
|
$
|
65,402
|
|
|
$
|
152,699
|
|
$
|
125,037
|
|
|
|
New agreements executed
|
20
|
|
|
39
|
|
63
|
|
||||
|
Licensing and enforcement programs generating revenues - during the respective period
|
13
|
|
|
28
|
|
30
|
|
||||
|
Licensing and enforcement programs with initial revenues
|
1
|
|
|
7
|
|
4
|
|
||||
|
New patent portfolios
|
1
|
|
|
2
|
|
3
|
|
||||
|
Year end cash, cash equivalents and short-term investments*
|
$
|
136,604
|
|
$
|
158,495
|
|
$
|
145,948
|
|
||
|
•
|
the dollar amount of agreements executed each period, which can be driven by the nature and characteristics of the technology or technologies being licensed and the magnitude of infringement associated with a specific licensee;
|
|
•
|
the specific terms and conditions of agreements executed each period including the nature and characteristics of rights granted, and the periods of infringement or term of use contemplated by the respective payments;
|
|
•
|
fluctuations in the total number of agreements executed each period;
|
|
•
|
the number of, timing, results and uncertainties associated with patent licensing negotiations, mediations, patent infringement actions, trial dates and other enforcement proceedings relating to our patent licensing and enforcement programs;
|
|
•
|
the relative maturity of licensing programs during the applicable periods;
|
|
•
|
other external factors, including the periodic status or results of ongoing negotiations, the status or results of ongoing litigations and appeals, actual or perceived shifts in the regulatory environment, impact of unrelated patent related judicial proceedings and other macroeconomic factors;
|
|
•
|
historically, based on the merits and strength of our operating subsidiary’s patent infringement claims and other factors, many prospective licensees have elected to settle significant patent infringement cases and pay reasonable license fees for the use of our patented technology, as those patent infringement cases approached a court determined trial date; and
|
|
•
|
fluctuations in overall patent portfolio related enforcement activities which are impacted by the portfolio intake challenges discussed above.
|
|
Fiscal Year
|
% Change
|
||||||||||||||||
|
|
2017
|
|
2016
|
2015
|
|
2017 vs. 2016
|
|
2016 vs. 2015
|
|||||||||
|
|
|
|
|
|
|||||||||||||
|
Revenues
|
$
|
65,402
|
|
|
$
|
152,699
|
|
$
|
125,037
|
|
|
(57
|
)%
|
|
22
|
%
|
|
|
Inventor royalties and contingent legal fees
|
21,634
|
|
49,204
|
|
34,631
|
|
(56
|
)%
|
|
42
|
%
|
||||||
|
Litigation and licensing expenses - patents
|
18,219
|
|
27,858
|
|
39,373
|
|
(35
|
)%
|
(29
|
)%
|
|||||||
|
Amortization expense
|
22,154
|
|
34,208
|
|
53,067
|
|
(35
|
)%
|
|
(36
|
)%
|
||||||
|
Impairment of patent-related intangible assets
|
2,248
|
|
42,340
|
|
74,731
|
|
(95
|
)%
|
|
(43
|
)%
|
||||||
|
Impairment of goodwill
|
—
|
|
—
|
|
30,149
|
|
—
|
%
|
|
(100
|
)%
|
||||||
|
Other operating costs and expenses
(1)
|
28,419
|
|
36,498
|
|
45,708
|
|
|
(22
|
)%
|
|
(20
|
)%
|
|||||
|
Operating loss
|
(27,272
|
)
|
(37,409
|
)
|
(152,622
|
)
|
(27
|
)%
|
|
(75
|
)%
|
||||||
|
Total other income (expense)
|
51,911
|
|
798
|
|
(56
|
)
|
*
|
|
|
*
|
|
||||||
|
Provision for income taxes
|
(2,955
|
)
|
(18,188
|
)
|
(4,800
|
)
|
(84
|
)%
|
279
|
%
|
|||||||
|
Net (income) loss attributable to noncontrolling interests in subsidiaries
|
496
|
|
|
732
|
|
(2,558
|
)
|
|
(32
|
)%
|
|
(129
|
)%
|
||||
|
Net income (loss) attributable to Acacia Research Corporation
|
22,180
|
|
|
(54,067
|
)
|
(160,036
|
)
|
|
(141
|
)%
|
|
(66
|
)%
|
||||
|
•
|
Revenues decreased
$87.3 million
, or
57%
to
$65.4 million
,
due primarily to a decrease in the number of agreements executed and a decrease in average revenue per
agreement. Refer to “
Investments in Patent Portfolios”
below for additional information regarding the impact of portfolio acquisition trends on current and future licensing and enforcement related revenues.
|
|
•
|
Income before provision for income taxes was
$24.6 million
for fiscal year 2017, as compared to a loss before provision for income taxes of
$36.6 million
for fiscal year 2016. The net change was primarily comprised of the change in revenues described above, a net
$49.5 million
unrealized gain on our equity investment in Veritone, a
$3.0 million
non-cash stock compensation charge for our Veritone related profits interest units and a net decrease in operating expenses, as follows:
|
|
•
|
Inventor royalties and contingent legal fees, on a combined basis, decreased
$27.6 million
, or
56%
, relatively consistent with the
57%
decrease in revenues in fiscal year 2017. Contingent legal fees decreased
$9.8 million
, or
37%
, due to an increase in average contingent legal fee rates for the portfolios generating revenues in fiscal year 2017. Inventor royalties decreased
$17.8 million
, or
78%
, primarily due to lower average inventor royalty rates for the portfolios generating revenues during fiscal year 2017.
|
|
•
|
Litigation and licensing expenses-patents decreased
$9.6 million
, or
35%
, to
$18.2 million
,
due primarily to a net decrease in litigation support and third-party technical consulting expenses associated with ongoing licensing and enforcement programs and an overall decrease in portfolio related enforcement activities.
Refer to “
Investments in
|
|
•
|
Amortization expense decreased
$12.1 million
, or
35%
, to
$22.2 million
, due to a decrease in scheduled amortization resulting from patent portfolio impairment charges previously recorded in the second and fourth quarters of 2016, and no new patent portfolio acquisition costs incurred during fiscal year 2017.
|
|
•
|
Impairment of patent-related intangible asset charges decreased
$40.1 million
, or
95%
, to
$2.2 million
. Impairment charges reflect the impact of reductions in expected estimated future net cash flows for certain patent portfolios and/or the impairment of certain portfolios that management determined it would no longer allocate resources to in future periods.
|
|
•
|
General and administrative expenses decreased
$6.9 million
, or
21%
, to
$26.0 million
,
due primarily to a reduction in personnel costs in connection with headcount reductions in 2016 and 2017, a decrease in variable performance based compensation costs consistent with the decrease in revenues for the periods and a decrease in corporate, general and administrative costs.
|
|
•
|
Results for fiscal year 2017 included a net unrealized gain on our investment in Veritone totaling
$49.5 million
(included in other income (expense)), comprised of an unrealized gain on conversion of our Veritone loans to equity of
$2.7 million
and an unrealized gain on the exercise of our Primary Warrant of
$4.6 million
, both as of May 2017, and an unrealized gain related to the change in fair value of our equity investment in Veritone through
December 31, 2017
of
$42.2 million
.
|
|
•
|
Tax expense for fiscal years 2017 and 2016 primarily reflects the impact of state taxes and foreign withholding taxes incurred on revenue agreements executed with third-party licensees domiciled in foreign jurisdictions. Results for fiscal year 2017 included a significant unrealized gain on our investment in Veritone, which created a related deferred tax liability. The future anticipated reversal of this deferred tax liability provides for a source of taxable income that allows for the realizability of existing deferred tax assets that have been reduced by a valuation allowance for the periods presented. The effective tax rate reflects both the recognition of the deferred tax liability and the reversal of valuation allowance. See below for additional information.
|
|
•
|
Revenues increased $27.7 million, or 22% to $152.7 million for fiscal year
2016, due to an increase in average revenue per agreement, which was partially offset by a decrease in the number of agreements executed.
|
|
•
|
Inventor royalties and contingent legal fees, on a combined basis, increased $14.6 million, or 42%, due primarily to the
22%
increase in revenues in fiscal year 2016, and a 4% increase in average contingent legal fee rates for the portfolios generating revenues in fiscal year 2016, as compared to the portfolios generating revenues in fiscal year 2015.
|
|
•
|
Litigation and licensing expenses-patents decreased $11.5 million, or 29%, to $27.9 million,
due primarily to a net decrease in litigation support and third-party technical consulting expenses associated with patent trials and ongoing licensing and enforcement programs.
|
|
•
|
Amortization expense decreased $18.9 million, or 36%, to $34.2 million, due to a decrease in scheduled amortization on existing patent portfolios resulting from various patent portfolio impairment charges previously recorded in the fourth quarter of 2015 and second quarter of 2016.
|
|
•
|
Impairment of patent-related intangible asset charges decreased $32.4 million, or 43%, to $42.3 million. Impairment charges reflect the impact of reductions in expected estimated future net cash flows for certain patent portfolios and certain patent portfolios that management determined it would no longer allocate resources to in future periods. The impairment charges consisted of the excess of the asset’s carrying value over its estimated fair value as of the applicable measurement date.
|
|
•
|
In the fourth quarter of fiscal 2015, we performed an impairment analysis of goodwill. Based upon the difference between the implied fair value of goodwill and the historical carrying value of goodwill, due primarily to the sustained decline in the Company’s stock price and adverse litigation outcomes occurring in the fourth quarter of 2015, we recognized a goodwill impairment charge totaling $30.1 million in the fourth quarter of 2015.
|
|
•
|
General and administrative expenses decreased $5.3 million, or 14%, to $32.9 million,
due primarily to a net decrease in personnel costs in connection with the net reduction in headcount during 2016 and 2015 and a net decrease in non-cash stock compensation expense.
|
|
•
|
Fiscal year 2016 and 2015 operating expenses included expenses for court ordered attorney fees totaling $500,000 and $4.1 million, respectively.
|
|
•
|
Tax expense for the periods presented reflects foreign taxes withheld on revenue agreements with licensees in foreign jurisdictions and other state taxes, and the impact of full valuation allowances recorded for net operating loss (2015 only) and foreign tax credit related tax assets generated during the periods. As such, no tax benefit was recognized for net operating loss and foreign tax credit related tax benefits generated during the applicable periods presented.
|
|
•
|
360 Degree View Technology
(3)
|
•
|
Oil and Gas Drilling technology
(2)
|
|
|
•
|
3G & 4G Cellular Air Interface and Infrastructure technology
(3)
|
•
|
Oil and Gas Production technology
(3)
|
|
|
•
|
4G Wireless technology
(2)(3)
|
•
|
Online Auction Guarantee technology
(1)(2)(3)
|
|
|
•
|
Audio Communications Fraud Detection technology
(2)(3)
|
•
|
Optical Networking technology
(1)(2)(3)
|
|
|
•
|
Automotive Safety, Navigation and Diagnostics technology
(3)
|
•
|
Optimized Microprocessor Operation technology
(3)
|
|
|
•
|
Bone Wedge technology
(1)(2)(3)
|
•
|
Reflective and Radiant Barrier Insulation technology
(2)(3)
|
|
|
•
|
Broadband Communications technology
(2)(3)
|
•
|
Semiconductor 3D Die Stacking technology
(2)
|
|
|
•
|
Cardiology and Vascular Device technology
(1)(2)(3)
|
•
|
Semiconductor Memory Circuit and Manufacturing Processes technology
(2)
|
|
|
•
|
Diamond and Gemstone Grading technology
(2)
|
•
|
Semiconductor and Memory-Related technology
(1)
|
|
|
•
|
DisplayPort and MIPI DSI technology
(1)(2)(3)
|
•
|
Semiconductor Testing technology
(3)
|
|
|
•
|
DRAM and Flash Memory technology
(2)
|
•
|
Shared Memory for Multimedia Processing
(1)(2)(3)
|
|
|
•
|
Electronic Access Control technology
(1)(3)
|
•
|
Speech codes used in wireless and wireline systems technology
(1)(2)(3)
|
|
|
•
|
Electronic spreadsheet, data analysis and software development technology
(2)
|
•
|
Spinning and Jousting Toy Game technology
(3)
|
|
|
•
|
Enhanced Mobile Communications technology
(3)
|
•
|
Super Resolutions Microscopy technology
(1)(2)(3)
|
|
|
•
|
Flash Memory technology
(2)
|
•
|
Surgical Access technology
(3)
|
|
|
•
|
Gas Modulation Control Systems technology
(2)(3)
|
•
|
Suture Anchors technology
(3)
|
|
|
•
|
High Speed Circuit Interconnect and Display Control technology
(2) (3)
|
•
|
Telematics technology
(2)(3)
|
|
|
•
|
Improved Lighting technology
(3)
|
•
|
Unicondylar Knee Replacement technology
(3)
|
|
|
•
|
Innovative Display technology
(1)(3)
|
•
|
Variable Data Printing technology
(2)
|
|
|
•
|
Intercarrier SMS technology
(3)
|
•
|
Video Analytics for Security technology
(3)
|
|
|
•
|
Interstitial and Pop-Up Internet Advertising technology
(2)(3)
|
•
|
Video Conferencing technology
(1)
|
|
|
•
|
Knee Replacement technology
(2)
|
•
|
Voice-Over-IP technology
(3)
|
|
|
•
|
Lighting Ballast technology
(2)
|
•
|
Wireless Data Synchronization & Data Transfer technology
(3)
|
|
|
•
|
Location Based Services technology
(3)
|
•
|
Wireless Infrastructure and User Equipment technology
(1)(2)(3)
|
|
|
•
|
Messaging technology
(3)
|
•
|
Wireless Location Based Services technology
(3)
|
|
|
•
|
Microprocessor and Memory technology
(2)(3)
|
•
|
Wireless Monitoring technology
(3)
|
|
|
•
|
Mobile Computer Synchronization technology
(3)
|
|||
|
(1)
|
Licensing and enforcement program generating revenue in 2017.
|
|
(2)
|
Licensing and enforcement program generating revenue in 2016.
|
|
(3)
|
Licensing and enforcement program generating revenue in 2015.
|
|
•
|
Increases in patent-related legal expenses associated with patent infringement litigation, including, but not limited to, increases in costs billed by outside legal counsel for discovery, depositions, economic analyses, damages assessments, expert witnesses and other consultants, re-exam and i
nter partes review costs,
case-related audio/video presentations and other litigation support and administrative costs could increase our operating costs and decrease our profit generating opportunities;
|
|
•
|
Our patented technologies and enforcement actions are complex and, as a result, we may be required to appeal adverse decisions by trial courts in order to successfully enforce our patents. Moreover, such appeals may not be successful;
|
|
•
|
New legislation, regulations or rules related to enforcement actions, including any fee or cost shifting provisions, could significantly increase our operating costs and decrease our profit generating opportunities.
Increased focus on the growing number of patent-related lawsuits may result in legislative changes which increase our costs and related risks of asserting patent enforcement actions. For instance, the United States House of Representatives passed a bill that would require non-practicing entities that bring patent infringement lawsuits to pay legal costs of the defendants, if the lawsuits are unsuccessful and certain standards are not met;
|
|
•
|
Courts may rule that our subsidiaries have violated certain statutory, regulatory, federal, local or governing rules or standards by pursuing such enforcement actions, which may expose us and our operating subsidiaries to material liabilities, which could harm our operating results and our financial position;
|
|
•
|
The complexity of negotiations and potential magnitude of exposure for potential infringers associated with higher quality patent portfolios may lead to increased intervals of time between the filing of litigation and potential revenue events (i.e. markman dates, trial dates), which may lead to increased legal expenses, consistent with the higher revenue potential of such portfolios; and
|
|
•
|
Fluctuations in overall patent portfolio related enforcement activities which are impacted by the portfolio intake challenges discussed above could harm our operating results and our financial position.
|
|
•
|
revenue recognition;
|
|
•
|
stock-based compensation expense, including valuation of profits interests;
|
|
•
|
valuation of long-lived and intangible assets including goodwill;
|
|
•
|
valuation of investments; and
|
|
•
|
accounting for income taxes.
|
|
•
|
In December 2015, we announced that our subsidiary Adaptix, Inc. received a jury verdict in its case against Alcatel Lucent USA, Inc., and others. The jury returned a verdict that the asserted claims of the applicable patent at issue were invalid and non-infringed. The Adaptix trial loss resulted in a reduction in estimated cash flows for the Adaptix portfolio expected to be realized from future licensing and enforcement activities, leading to impairment charges on the portfolio in the fourth quarter of 2015.
|
|
•
|
Management considered the impact of the fourth quarter 2015 adverse trial outcomes on our estimates of future cash flows that could be realized from future licensing and enforcement activities for other patent portfolios. Estimates of future cash flows for certain portfolios were reduced in part, in connection with our assessment of probabilities of realization given the recent adverse trial outcomes.
|
|
•
|
Patent impairment charges include the carrying value of other patent portfolios for which, in the fourth quarter of 2015, we experienced adverse litigation or trial outcomes, leading to a reduction in or elimination of expected future cash flows. In addition, headcount reductions and internal staff optimization efforts led to changes with respect to which patent portfolios we intend to allocate licensing and enforcement resources to in future periods. As such, certain portfolio programs were selected for termination due to a decision to no longer pursue or allocate resources, resulting in a write-off of any remaining carrying value in the fourth quarter of 2015.
|
|
•
|
significant consistent gradual decline in the our stock price for a sustained period;
|
|
•
|
significant underperformance relative to expected historical or projected future operating results;
|
|
•
|
significant changes in the manner of use of assets or the strategy for our overall business;
|
|
•
|
significant negative industry or economic trends; and
|
|
•
|
significant adverse changes in legal factors or in the business climate, including adverse regulatory actions or assessments.
|
|
•
|
Adverse legal outcomes and changes in legal factors
. In December 2015, we announced that our subsidiary Adaptix, Inc. received a jury verdict in its case against Alcatel Lucent USA, et al., deciding that the claims of the applicable patent in suit were invalid and non-infringed. This adverse legal outcome and others in the fourth quarter of 2015 resulted in changes in estimates of realization related to litigation outcomes in future periods for certain patent portfolios.
|
|
•
|
Consistent gradual decline in the Company’s stock price
: Historically, our stock price has been volatile, and the volatility continued during fiscal 2015, declining from $16.72 as of January 2, 2015, to $4.29 as of December 31, 2015, a 74% decline. In addition, subsequent to December 31, 2015, our stock price volatility has continued, trending downward to $3.16 as of February 29, 2016. In the fourth quarter of 2015, given the continued decline in stock price up through December 31, 2015, and the impact of the December 2015 adverse trial outcomes noted above, the gradual consistent decline in our stock price was deemed to be sustained, and hence indicative of a reduction in the estimated fair value of our company, as reflected in our lower overall market capitalization.
|
|
•
|
Changes in Company Management and Resource Allocations
. In connection with certain resource allocation changes within the organization due to changes in our management in the fourth quarter of 2015, headcount reductions and internal staff optimization efforts occurred, which led to changes with respect to estimates of which patent portfolios we intend to continue to allocate licensing and enforcement resources to in future periods. As such, certain patent portfolio programs were selected for termination due to our decision to no longer allocate resources to those programs. In addition, we made changes in estimates regarding the best and highest use of certain patent portfolios, resulting in reductions in estimated future cash flows.
|
|
•
|
At December 31, 2015, the initial qualitative assessment included consideration of the factors described above, resulting in a conclusion that as of December 31, 2015, the consistent gradual decline in our stock price was sustained. We also considered the impact of the December 2015 adverse trial outcomes on our stock price and related estimates of fair value for remaining portfolio opportunities. Based on our assessment of these factors, we determined that it was more likely than not that goodwill was impaired, constituting a triggering event requiring a goodwill impairment test as of December 31, 2015.
|
|
•
|
We conducted the first step of the goodwill impairment test for our single reporting unit as of December 31, 2015. We utilized the market capitalization plus cost synergies approach to estimate the fair value of the Company. The estimated market capitalization was determined by multiplying our stock price and the common shares outstanding as of December 31, 2015. Management also considered a control premium in its estimate of fair value for our single reporting unit. The cost synergies were estimated based on the cost savings which could be achieved if the Company was acquired by a competitor in the same operating business.
|
|
•
|
Based on the analysis utilizing the market capitalization plus cost synergies approach, the estimated fair value of the reporting unit of $252 million was below its carrying value of $344.3 million as of December 31, 2015, and therefore, goodwill was determined to be more likely than not, impaired.
|
|
•
|
The purpose of step 2 of the analysis was to determine the estimated fair value of the assets and liabilities of our reporting unit, in order to determine the implied fair value of goodwill for the reporting unit. The excess, if any, of the fair value of a reporting unit over the amounts assigned to its assets and liabilities is the implied fair value of goodwill. Based upon the analysis performed, the fair value of our reporting unit did not exceed the amounts assigned to our reporting unit assets and liabilities, resulting in a difference between the implied fair value of goodwill of zero and the historical carrying value of goodwill. As a result, we recognized a goodwill impairment charge totaling $30.1 million in the fourth quarter of 2015.
|
|
Veritone Common Stock
|
Veritone Warrants
|
|||||||
|
IPO Date
|
December 31, 2017
|
IPO Date
|
December 31, 2017
|
|||||
|
Estimated DLOM applied
|
5.7%
|
5%
|
5.7%
|
10%
|
||||
|
Volatility assumptions
|
35%
|
37%
|
35%
|
72% - 87%
|
||||
|
Term assumptions
|
6 months
|
2 months
|
6 months
|
5 months
|
||||
|
2017 vs. 2016
|
2016 vs. 2015
|
|||||||||||||||||||||||||
|
|
2017
|
|
2016
|
|
2015
|
$ Change
|
% Change
|
$ Change
|
% Change
|
|||||||||||||||||
|
(in thousands, except percentage change values and number of agreements)
|
||||||||||||||||||||||||||
|
Revenues
|
$
|
65,402
|
|
|
$
|
152,699
|
|
$
|
125,037
|
|
$
|
(87,297
|
)
|
(57
|
)%
|
$
|
27,662
|
|
22
|
%
|
||||||
|
New revenue agreements executed
|
20
|
|
|
39
|
|
63
|
|
|||||||||||||||||||
|
Average revenue per agreement
|
$
|
3,270
|
|
$
|
3,915
|
|
$
|
1,985
|
|
|||||||||||||||||
|
2017 vs. 2016
|
2016 vs. 2015
|
|||||||
|
(in thousands)
|
||||||||
|
Decrease in number of agreements executed
|
$
|
(74,392
|
)
|
$
|
(47,633
|
)
|
||
|
Increase (decrease) in average revenue per agreement executed
|
(12,905
|
)
|
75,295
|
|
||||
|
Total
|
$
|
(87,297
|
)
|
$
|
27,662
|
|
||
|
2017 vs. 2016
|
2016 vs. 2015
|
|||||||||||||||||||||||||
|
|
2017
|
|
2016
|
|
2015
|
$ Change
|
% Change
|
$ Change
|
% Change
|
|||||||||||||||||
|
(in thousands, except percentages)
|
||||||||||||||||||||||||||
|
Net income (loss) attributable to Acacia Research Corporation
|
$
|
22,180
|
|
$
|
(54,067
|
)
|
$
|
(160,036
|
)
|
$
|
76,247
|
|
(141
|
)%
|
$
|
105,969
|
|
(66
|
)%
|
|||||||
|
2017 vs. 2016
|
%
|
2016 vs. 2015
|
%
|
||||||||||
|
(in thousands, except percentage values)
|
|||||||||||||
|
Increase (decrease) in revenues
|
$
|
(87,297
|
)
|
(114
|
)%
|
$
|
27,662
|
|
26
|
%
|
|||
|
(Increase) decrease in inventor royalties and contingent legal fees combined
|
27,570
|
|
36
|
%
|
(14,573
|
)
|
(14
|
)%
|
|||||
|
Decrease in general and administrative expenses
|
6,889
|
|
9
|
%
|
5,257
|
|
5
|
%
|
|||||
|
Decrease in litigation and licensing expenses
|
9,639
|
|
13
|
%
|
11,515
|
|
11
|
%
|
|||||
|
Decrease in patent amortization expenses
|
12,054
|
|
16
|
%
|
18,859
|
|
18
|
%
|
|||||
|
Decrease in impairment of patent-related intangible assets
|
40,092
|
|
53
|
%
|
32,391
|
|
31
|
%
|
|||||
|
Decrease in impairment for goodwill
|
—
|
|
—
|
%
|
30,149
|
|
28
|
%
|
|||||
|
Change in provision for income taxes
|
15,233
|
|
20
|
%
|
(13,388
|
)
|
(13
|
)%
|
|||||
|
Unrealized gain and change in fair value of investment
|
49,526
|
|
65
|
%
|
—
|
|
—
|
%
|
|||||
|
Other
|
2,541
|
|
2
|
%
|
8,097
|
|
8
|
%
|
|||||
|
Net change in net income (loss)
|
$
|
76,247
|
|
100
|
%
|
$
|
105,969
|
|
100
|
%
|
|||
|
2017 vs. 2016
|
2016 vs. 2015
|
||||||||||||||||||||||||
|
|
2017
|
2016
|
|
2015
|
$ Change
|
% Change
|
$ Change
|
% Change
|
|||||||||||||||||
|
(in thousands, except percentages)
|
|||||||||||||||||||||||||
|
Inventor royalties
|
$
|
4,952
|
|
$
|
22,730
|
|
$
|
18,462
|
|
$
|
(17,778
|
)
|
(78
|
)%
|
$
|
4,268
|
|
23
|
%
|
||||||
|
Contingent legal fees
|
16,682
|
|
26,474
|
|
16,169
|
|
(9,792
|
)
|
(37
|
)%
|
10,305
|
|
64
|
%
|
|||||||||||
|
Litigation and licensing expenses - patents
|
18,219
|
|
27,858
|
|
39,373
|
|
(9,639
|
)
|
(35
|
)%
|
(11,515
|
)
|
(29
|
)%
|
|||||||||||
|
Amortization of patents
|
22,154
|
|
34,208
|
|
53,067
|
|
(12,054
|
)
|
(35
|
)%
|
(18,859
|
)
|
(36
|
)%
|
|||||||||||
|
2017 vs. 2016
|
% of Prior Period Balance
|
2016 vs. 2015
|
% of Prior Period Balance
|
||||||||||
|
Inventor Royalties:
|
(in thousands, except percentage change values)
|
||||||||||||
|
Increase (decrease) in inventor royalty rates
|
$
|
(4,345
|
)
|
(19
|
)%
|
$
|
11,518
|
|
62
|
%
|
|||
|
Increase (decrease) in total revenues
|
(30,254
|
)
|
(133
|
)%
|
4,729
|
|
26
|
%
|
|||||
|
Decrease (increase) in revenues without inventor royalty obligations
|
16,821
|
|
74
|
%
|
(11,979
|
)
|
(65
|
)%
|
|||||
|
Total change - inventor royalties expense
|
$
|
(17,778
|
)
|
(78
|
)%
|
$
|
4,268
|
|
23
|
%
|
|||
|
2017 vs. 2016
|
% of Prior Period Balance
|
2016 vs. 2015
|
% of Prior Period Balance
|
||||||||||
|
Contingent Legal Fees:
|
(in thousands, except percentage change values)
|
||||||||||||
|
Increase in contingent legal fee rates
|
$
|
5,359
|
|
20
|
%
|
$
|
6,850
|
|
43
|
%
|
|||
|
Increase (decrease) in total revenues
|
(15,832
|
)
|
(60
|
)%
|
3,719
|
|
23
|
%
|
|||||
|
Decrease (increase) in revenues without contingent legal fee obligations
|
681
|
|
3
|
%
|
(264
|
)
|
(2
|
)%
|
|||||
|
Total change - contingent legal fees
|
$
|
(9,792
|
)
|
(37
|
)%
|
$
|
10,305
|
|
64
|
%
|
|||
|
2017 vs. 2016
|
2016 vs. 2015
|
||||||
|
(in thousands)
|
|||||||
|
Scheduled amortization related to patent portfolios owned or controlled as of the end of the prior year
|
$
|
(11,829
|
)
|
$
|
(18,704
|
)
|
|
|
Accelerated amortization related to recovery of upfront advances
|
(225
|
)
|
225
|
|
|||
|
Patent portfolio dispositions
|
—
|
|
(380
|
)
|
|||
|
Total change in patent amortization expense
|
$
|
(12,054
|
)
|
$
|
(18,859
|
)
|
|
|
2017 vs. 2016
|
2016 vs. 2015
|
||||||||||||||||||||||||
|
|
2017
|
2016
|
|
2015
|
$ Change
|
% Change
|
$ Change
|
% Change
|
|||||||||||||||||
|
(in thousands, except percentages)
|
|||||||||||||||||||||||||
|
Impairment of patent-related intangible assets
|
$
|
2,248
|
|
$
|
42,340
|
|
$
|
74,731
|
|
$
|
(40,092
|
)
|
(95
|
)%
|
$
|
(32,391
|
)
|
(43
|
)%
|
||||||
|
Impairment of goodwill
|
—
|
|
—
|
|
30,149
|
|
—
|
|
—
|
%
|
(30,149
|
)
|
(100
|
)%
|
|||||||||||
|
2017 vs. 2016
|
2016 vs. 2015
|
|||||||||||||||||||||||||
|
|
2017
|
|
2016
|
|
2015
|
$ Change
|
% Change
|
$ Change
|
% Change
|
|||||||||||||||||
|
(in thousands, except percentages)
|
||||||||||||||||||||||||||
|
General and administrative
|
$
|
17,145
|
|
$
|
23,857
|
|
$
|
27,128
|
|
$
|
(6,712
|
)
|
(28
|
)%
|
$
|
(3,271
|
)
|
(12
|
)%
|
|||||||
|
Non-cash stock compensation expense - G&A
|
5,844
|
|
9,062
|
|
11,048
|
|
(3,218
|
)
|
(36
|
)%
|
(1,986
|
)
|
(18
|
)%
|
||||||||||||
|
Non-cash stock compensation expense - Veritone Profits Interests
|
3,041
|
|
—
|
|
—
|
|
3,041
|
|
100
|
%
|
—
|
|
—
|
%
|
||||||||||||
|
Total general and administrative expenses
|
$
|
26,030
|
|
$
|
32,919
|
|
$
|
38,176
|
|
$
|
(6,889
|
)
|
(21
|
)%
|
$
|
(5,257
|
)
|
(14
|
)%
|
|||||||
|
Other expenses - business development
|
$
|
1,189
|
|
$
|
3,079
|
|
$
|
3,391
|
|
$
|
(1,890
|
)
|
(61
|
)%
|
$
|
(312
|
)
|
(9
|
)%
|
|||||||
|
2017 vs. 2016
|
2016 vs. 2015
|
||||||
|
(in thousands)
|
|||||||
|
Net change in personnel costs due to reductions in headcount
|
$
|
(3,162
|
)
|
$
|
(5,841
|
)
|
|
|
Variable performance-based compensation costs
|
(2,580
|
)
|
1,839
|
|
|||
|
Corporate, general and administrative costs
|
(1,821
|
)
|
1,594
|
|
|||
|
Non-cash stock compensation expense - general and administrative
(1)
|
(3,218
|
)
|
(1,986
|
)
|
|||
|
Non-cash stock compensation expense - Veritone related profits interests
(1)
|
3,041
|
|
—
|
|
|||
|
Employee severance costs
|
851
|
|
(863
|
)
|
|||
|
Total change in general and administrative expenses
|
$
|
(6,889
|
)
|
|
$
|
(5,257
|
)
|
|
|
2017
|
|
2016
|
|
2015
|
||||||
|
Provision for income taxes (in thousands)
|
$
|
(2,955
|
)
|
|
$
|
(18,188
|
)
|
$
|
(4,800
|
)
|
|
|
Effective tax rate
|
(12
|
)%
|
50
|
%
|
3
|
%
|
|||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Net cash provided by (used in):
|
|
|
|
|||||||||
|
Operating activities
|
$
|
24,478
|
|
$
|
34,061
|
|
$
|
(9,949
|
)
|
|||
|
Investing activities
|
(16,114
|
)
|
(40,630
|
)
|
39,307
|
|
||||||
|
Financing activities
|
700
|
|
(1,114
|
)
|
(28,601
|
)
|
||||||
|
$
|
9,064
|
|
$
|
(7,683
|
)
|
$
|
757
|
|
||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Investment in Investees
(1)
|
$
|
(31,514
|
)
|
$
|
—
|
|
$
|
—
|
|
|||
|
Advances to Investee
(1)
|
(4,000
|
)
|
(20,000
|
)
|
—
|
|
||||||
|
Purchases of property and equipment
|
(2
|
)
|
(4
|
)
|
(8
|
)
|
||||||
|
Net sale (purchase) of available-for-sale investments
|
19,402
|
|
(19,401
|
)
|
58,819
|
|
||||||
|
Patent portfolio investment costs
|
—
|
|
(1,225
|
)
|
(19,504
|
)
|
||||||
|
Net cash provided by (used in) investing activities
|
$
|
(16,114
|
)
|
$
|
(40,630
|
)
|
$
|
39,307
|
|
|||
|
(1) - Refer to Note 7 in the accompany consolidated financial statements
|
||||||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Dividends paid to stockholders
|
$
|
—
|
|
$
|
—
|
|
$
|
(25,434
|
)
|
|||
|
Distributions to noncontrolling interests - Acacia IP Fund
|
—
|
|
(1,358
|
)
|
(4,105
|
)
|
||||||
|
Proceeds from the exercise of stock options
|
745
|
|
326
|
|
938
|
|
||||||
|
Repurchases of common stock
|
(45
|
)
|
(82
|
)
|
—
|
|
||||||
|
Net cash provided by financing activities
|
$
|
700
|
|
$
|
(1,114
|
)
|
$
|
(28,601
|
)
|
|||
|
|
Payments Due by Period (In thousands)
|
||||||||||||||||||
|
Contractual Obligations
|
Total
|
|
Less than 1 year
|
|
1-3 years
|
|
3-5 years
|
More than 5 years
|
|||||||||||
|
Operating leases, net of guaranteed sublease income
|
$
|
2,598
|
|
|
$
|
1,213
|
|
|
$
|
1,385
|
|
|
$
|
—
|
|
$
|
—
|
|
|
|
Total contractual obligations
|
$
|
2,598
|
|
|
$
|
1,213
|
|
|
$
|
1,385
|
|
|
$
|
—
|
|
$
|
—
|
|
|
|
(a)
|
The following documents are filed as part of this report.
|
|
(1) Financial Statements
|
Page
|
|
|
|
|
|
|
Acacia Research Corporation Consolidated Financial Statements
|
|
|
|
|
|
|
|
(2) Financial Statement Schedules
|
|
|
|
|
|
|
|
Financial statement schedules are omitted because they are not applicable or the required information is shown in the Financial Statements or the Notes thereto.
|
||
|
|
|
|
|
(3) Exhibits
|
|
|
|
|
|
|
|
Refer to Item 15(b) below.
|
|
|
|
(b)
|
Exhibits. The following exhibits are either filed herewith or incorporated herein by reference:
|
|
Exhibit
Number
|
Description
|
|
|
|
|
2.1
|
|
|
3.1
|
|
|
3.2
|
|
|
4.1
|
|
|
10.1*
|
|
|
10.2*
|
|
|
10.3*
|
|
|
10.4*
|
|
|
10.5*
|
|
|
10.6*
|
|
|
10.7*
|
|
|
10.8
|
|
|
10.9
|
|
|
10.10
|
|
|
10.11*
|
|
|
10.12
|
|
|
10.13
|
|
|
10.15*
|
|
|
10.16*
|
|
|
10.17*
|
|
|
10.18
|
|
|
10.19
|
|
|
10.20*
|
|
|
10.21*
|
|
|
10.22*
|
|
|
10.23*
|
|
|
10.24*
|
|
|
10.25*
|
|
|
10.26*
|
|
|
10.27*
|
|
|
10.28*
|
|
|
10.29
|
|
|
10.30
|
|
|
10.31
|
|
|
10.32
|
|
|
10.33
|
|
|
10.34
|
|
|
21.1
|
|
|
23.1
|
|
|
24.1
|
|
|
31.1†
|
|
|
31.2†
|
|
|
32.1
|
|
|
32.2
|
|
|
101
|
Interactive Date Files Pursuant to Rule 405 of Regulation S-T.
|
|
*
|
The referenced exhibit is a management contract, compensatory plan or arrangement required to be filed as an exhibit to this Annual Report on Form 10-K pursuant to Item 15(c) of Form 10-K.
|
|
†
|
The certifications attached as Exhibits 32.1 and 32.2 that accompany this Annual Report on Form 10-K are not deemed filed with the SEC and are not to be incorporated by reference into any filing of Acacia Research Corporation under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Annual Report on Form 10-K, regardless of any general incorporation language contained in any filing.
|
|
(1)
|
Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on June 5, 2008 (File No. 000-26068).
|
|
(2)
|
Incorporated by reference to Appendix A to Acacia Research Corporation’s Definitive Proxy Statement on Schedule 14A filed on April 20, 2000 (File No. 000-26068).
|
|
(3)
|
Incorporated by reference to Appendix A to Acacia Research Corporation’s Definitive Proxy Statement on Schedule 14A filed on April 26, 1996 (File No. 000-26068).
|
|
(4)
|
Incorporated by reference to Annex E to the Proxy Statement/Prospectus which formed part of Acacia Research Corporation’s Registration Statement on Form S-4 (File No. 333-87654) which became effective on November 8, 2002.
|
|
(5)
|
Incorporated by reference to Acacia Research Corporation’s Registration Statement on Form S-8 (File No. 333-144754) which became effective on July 20, 2007.
|
|
(6)
|
Incorporated by reference to Acacia Research Corporation’s Quarterly Report on Form 10-Q for the period ended September 30, 2007, filed on November 2, 2007 (File No. 000-26068).
|
|
(7)
|
Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10‑K for the year ended December 31, 2001, filed on March 27, 2002 (File No. 000‑26068).
|
|
(8)
|
Incorporated by reference to Acacia Research Corporation’s Quarterly Report on Form 10-Q for the period ended June 30, 2012, filed on July 30, 2012 (File No. 000-26068).
|
|
(9)
|
Incorporated by reference to Acacia Research Corporation’s Quarterly Report on Form 10-Q for the period ended March 31, 2006, filed on May 10, 2006 (File No. 000‑26068).
|
|
(10)
|
Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2007, filed on March 14, 2008 (File No. 000-26068).
|
|
(11)
|
Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on April 2, 2008 (File No. 000-26068).
|
|
(12)
|
Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2008, filed on February 26, 2009 (File No. 000-26068).
|
|
(13)
|
Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2009, filed on February 26, 2010, as amended on March 1, 2010 (File No. 000-26068).
|
|
(14)
|
Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2010, filed on February 28, 2011, as amended on March 24, 2011 (File No. 000-26068).
|
|
(15)
|
Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K/A filed on January 19, 2012 (File No. 000-26068). Portions of this exhibit have been omitted pursuant to a request for confidential treatment under Rule 24-b-2 of the Securities Exchange Act of 1934, as amended. The omitted material has been separately filed with the Securities and Exchange Commission.
|
|
(16)
|
Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on February 16, 2012 (File No. 000-26068).
|
|
(17)
|
Incorporated by reference to Appendix A to Acacia Research Corporation’s Definitive Proxy Statement on Schedule 14A filed on April 24, 2013 (File No. 000-26068).
|
|
(18)
|
Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on May 22, 2013 (File No. 000-26068).
|
|
(19)
|
Incorporated by reference to Acacia Research Corporation’s Quarterly Report on Form 10-Q for the period ended September 30, 2015, filed on November 9, 2015 (File No. 000-26068).
|
|
(20)
|
Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on March 28, 2016 (File No. 001-37721).
|
|
(21)
|
Incorporated by reference to Acacia Research Corporation’s Quarterly Report on Form 10-Q for the period ended June 30, 2016, filed on August 9, 2016 (File No. 001-37721).
|
|
(22)
|
Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on March 4, 2016 (File No. 000-26068).
|
|
(23)
|
Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on March 21, 2016 (File No. 000-26068).
|
|
(24)
|
Incorporated by reference to Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2016, filed on March 10, 2017 (File No. 001-37721).
|
|
(25)
|
Incorporated by reference to Acacia Research Corporation’s Quarterly Report on Form 10-Q for the period ended September 30, 2017, filed on November 7, 2017 (File No. 001-37721).
|
|
(26)
|
Incorporated by reference to Acacia Research Corporation’s Quarterly Report on Form 10-Q for the period ended March 31, 2017, filed on May 10, 2017 (File No. 001-37721).
|
|
(27)
|
Incorporated by reference to Acacia Research Corporation’s Current Report on Form 8-K filed on March 16, 2017 (File No. 001-37721).
|
|
|
ACACIA RESEARCH CORPORATION
|
|
||
|
|
|
|
|
|
|
Dated:
|
March 7, 2018
|
By:
|
/s/ Robert Stewart
|
|
|
|
|
Robert Stewart
|
|
|
|
|
|
President
(Authorized Signatory)
|
|
|
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
|
|
/s/
|
Robert Stewart
|
|
President
|
|
March 7, 2018
|
|
|
Robert Stewart
|
|
(Principal Executive Officer)
|
|
|
|
|
|
|
|
|
|
|
/s/
|
Clayton J. Haynes
|
|
Chief Financial Officer and Treasurer
|
|
March 7, 2018
|
|
|
Clayton J. Haynes
|
|
(Principal Financial and Accounting Officer)
|
|
|
|
/s/
|
Fred A. de Boom
|
|
Director
|
|
March 7, 2018
|
|
|
Fred A. de Boom
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/
|
Edward W. Frykman
|
|
Director
|
|
March 7, 2018
|
|
|
Edward W. Frykman
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/
|
G. Louis Graziadio, III
|
|
Executive Chairman and Director
|
|
March 7, 2018
|
|
|
G. Louis Graziadio, III
|
|
|
|
|
|
|
|
|
|
|
|
|
/s/
|
William S. Anderson
|
|
Director
|
|
March 7, 2018
|
|
|
William S. Anderson
|
|
|
|
|
|
/s/
|
Frank E. Walsh, III
|
|
Director
|
|
March 7, 2018
|
|
|
Frank E. Walsh, III
|
|
|
|
|
|
/s/
|
James F. Sanders
|
|
Director
|
|
March 7, 2018
|
|
|
James F. Sanders
|
|
|
|
|
|
|
2017
|
2016
|
||||||
|
ASSETS
|
|
|
||||||
|
Current assets:
|
|
|
||||||
|
Cash and cash equivalents
|
$
|
136,604
|
|
$
|
127,540
|
|
||
|
Restricted cash
|
—
|
|
11,512
|
|
||||
|
Short-term investments
|
—
|
|
19,443
|
|
||||
|
Accounts receivable
|
153
|
|
26,750
|
|
||||
|
Prepaid expenses and other current assets
|
2,938
|
|
3,245
|
|
||||
|
Total current assets
|
139,695
|
|
188,490
|
|
||||
|
Investment at fair value
(1)
|
104,754
|
|
—
|
|
||||
|
Investment - equity method
(1)
|
2,195
|
|
—
|
|
||||
|
Loan receivable and accrued interest
(1)
|
—
|
|
18,616
|
|
||||
|
Investment in warrants and shares
(1)
|
—
|
|
1,960
|
|
||||
|
Patents, net of accumulated amortization
|
61,917
|
|
86,319
|
|
||||
|
Other non-current assets
|
207
|
|
618
|
|
||||
|
|
$
|
308,768
|
|
$
|
296,003
|
|
||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|
|
|
|
||||
|
Current liabilities:
|
|
|
|
|
||||
|
Accounts payable and accrued expenses
|
$
|
7,956
|
|
$
|
14,283
|
|
||
|
Royalties and contingent legal fees payable
|
1,601
|
|
13,908
|
|
||||
|
Total current liabilities
|
9,557
|
|
28,191
|
|
||||
|
Other liabilities
|
3,552
|
|
369
|
|
||||
|
Total liabilities
|
13,109
|
|
28,560
|
|
||||
|
Commitments and contingencies (Note 11)
|
|
|
|
|
||||
|
Stockholders’ equity:
|
|
|
|
|
||||
|
Preferred stock, par value $0.001 per share; 10,000,000 shares authorized; no shares issued or outstanding
|
—
|
|
—
|
|
||||
|
Common stock, par value $0.001 per share; 100,000,000 shares authorized; 50,639,926 shares issued and outstanding as of December 31, 2017 and 50,476,042 shares issued and outstanding as of December 31, 2016
|
51
|
|
50
|
|
||||
|
Treasury stock, at cost, 1,729,408 shares as of December 31, 2017 and 2016
|
(34,640
|
)
|
(34,640
|
)
|
||||
|
Additional paid-in capital
|
648,996
|
|
642,453
|
|
||||
|
Accumulated comprehensive loss
|
(88
|
)
|
(76
|
)
|
||||
|
Accumulated deficit
|
(320,018
|
)
|
(342,198
|
)
|
||||
|
Total Acacia Research Corporation stockholders’ equity
|
294,301
|
|
265,589
|
|
||||
|
Noncontrolling interests in operating subsidiaries
|
1,358
|
|
1,854
|
|
||||
|
Total stockholders’ equity
|
295,659
|
|
267,443
|
|
||||
|
|
$
|
308,768
|
|
$
|
296,003
|
|
||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Revenues
|
$
|
65,402
|
|
$
|
152,699
|
|
$
|
125,037
|
|
|||
|
Operating costs and expenses:
|
|
|
|
|
|
|
||||||
|
Cost of revenues:
|
|
|
|
|
|
|
||||||
|
Inventor royalties
|
4,952
|
|
22,730
|
|
18,462
|
|
||||||
|
Contingent legal fees
|
16,682
|
|
26,474
|
|
16,169
|
|
||||||
|
Litigation and licensing expenses - patents
|
18,219
|
|
27,858
|
|
39,373
|
|
||||||
|
Amortization of patents
|
22,154
|
|
34,208
|
|
53,067
|
|
||||||
|
General and administrative expenses (including non-cash stock compensation expense of $8,885 in 2017, $9,062 in 2016 and $11,048 in 2015)
|
26,030
|
|
32,919
|
|
38,176
|
|
||||||
|
Other expenses - business development
|
1,189
|
|
3,079
|
|
3,391
|
|
||||||
|
Impairment of patent-related intangible assets
|
2,248
|
|
42,340
|
|
74,731
|
|
||||||
|
Impairment of goodwill
|
—
|
|
—
|
|
30,149
|
|
||||||
|
Other
|
1,200
|
|
500
|
|
4,141
|
|
||||||
|
Total operating costs and expenses
|
92,674
|
|
190,108
|
|
277,659
|
|
||||||
|
Operating loss
|
(27,272
|
)
|
(37,409
|
)
|
(152,622
|
)
|
||||||
|
Other income (expense):
|
||||||||||||
|
Gain on conversion of loans and accrued interest
(1)
|
2,671
|
|
—
|
|
—
|
|
||||||
|
Gain on exercise of Primary Warrant
(1)
|
4,616
|
|
—
|
|
—
|
|
||||||
|
Change in fair value of investment, net
(1)
|
42,239
|
|
—
|
|
—
|
|
||||||
|
Equity in losses of investee
(1)
|
(220
|
)
|
—
|
|
—
|
|
||||||
|
Other income
|
1,000
|
|
—
|
|
—
|
|
||||||
|
Interest income
|
1,605
|
|
798
|
|
(56
|
)
|
||||||
|
Total other income (expense)
|
51,911
|
|
798
|
|
(56
|
)
|
||||||
|
Income (loss) from operations before provision for income taxes
|
24,639
|
|
(36,611
|
)
|
(152,678
|
)
|
||||||
|
Provision for income taxes
|
(2,955
|
)
|
(18,188
|
)
|
(4,800
|
)
|
||||||
|
Net income (loss) including noncontrolling interests in subsidiaries
|
21,684
|
|
(54,799
|
)
|
(157,478
|
)
|
||||||
|
Net (income) loss attributable to noncontrolling interests in subsidiaries
|
496
|
|
732
|
|
(2,558
|
)
|
||||||
|
Net income (loss) attributable to Acacia Research Corporation
|
$
|
22,180
|
|
$
|
(54,067
|
)
|
$
|
(160,036
|
)
|
|||
|
Net income (loss) attributable to common stockholders - basic and diluted
|
$
|
22,147
|
|
$
|
(54,067
|
)
|
$
|
(160,730
|
)
|
|||
|
Basic and diluted income (loss) per common share
|
$
|
0.44
|
|
$
|
(1.08
|
)
|
$
|
(3.25
|
)
|
|||
|
Weighted-average number of shares outstanding, basic
|
50,495,119
|
|
50,075,847
|
|
49,505,817
|
|
||||||
|
Weighted-average number of shares outstanding, diluted
|
50,692,012
|
|
50,075,847
|
|
49,505,817
|
|
||||||
|
Cash dividends declared per common share
|
$
|
—
|
|
$
|
—
|
|
$
|
0.50
|
|
|||
|
2017
|
2016
|
2015
|
|||||||||
|
Net income (loss) including noncontrolling interests in subsidiaries
|
$
|
21,684
|
|
$
|
(54,799
|
)
|
$
|
(157,478
|
)
|
||
|
Other comprehensive income (loss):
|
|||||||||||
|
Unrealized gain (loss) on short-term investments, net of tax of $0
|
(40
|
)
|
40
|
|
(356
|
)
|
|||||
|
Unrealized gain (loss) on foreign currency translation, net of tax of $0
|
58
|
|
77
|
|
(123
|
)
|
|||||
|
Add: reclassification adjustment for (gains) losses included in net income (loss)
|
(30
|
)
|
22
|
|
617
|
|
|||||
|
Total other comprehensive income (loss)
|
21,672
|
|
(54,660
|
)
|
(157,340
|
)
|
|||||
|
Comprehensive income (loss) attributable to noncontrolling interests
|
496
|
|
732
|
|
(2,558
|
)
|
|||||
|
Comprehensive income (loss) attributable to Acacia Research Corporation
|
$
|
22,168
|
|
|
$
|
(53,928
|
)
|
$
|
(159,898
|
)
|
|
|
|
|||||||||||||||||||||||||||||||
|
|
Common Shares
|
Common Stock
|
Treasury Stock
|
Additional Paid-in Capital
|
Accumulated Comprehensive Income (Loss)
|
Accumulated Deficit
|
Noncontrolling Interests in Operating Subsidiaries
|
Total
|
|||||||||||||||||||||||
|
Balance at December 31, 2014
|
50,065,382
|
|
$
|
50
|
|
$
|
(34,640
|
)
|
$
|
646,595
|
|
$
|
(353
|
)
|
$
|
(128,095
|
)
|
$
|
5,491
|
|
$
|
489,048
|
|
||||||||
|
Net loss attributable to Acacia Research Corporation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(160,036
|
)
|
—
|
|
(160,036
|
)
|
|||||||||||||||
|
Dividends paid to stockholders
|
—
|
|
—
|
|
—
|
|
(25,434
|
)
|
—
|
|
—
|
|
—
|
|
(25,434
|
)
|
|||||||||||||||
|
Stock options exercised
|
135,000
|
|
—
|
|
—
|
|
938
|
|
—
|
|
—
|
|
—
|
|
938
|
|
|||||||||||||||
|
Compensation expense for share-based awards, net of forfeitures
|
450,857
|
|
1
|
|
—
|
|
11,047
|
|
—
|
|
—
|
|
—
|
|
11,048
|
|
|||||||||||||||
|
Net income attributable to noncontrolling interests in subsidiaries
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,558
|
|
2,558
|
|
|||||||||||||||
|
Distributions to noncontrolling interests in operating subsidiary
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(4,105
|
)
|
(4,105
|
)
|
|||||||||||||||
|
Unrealized loss on foreign currency translation
|
—
|
|
—
|
|
—
|
|
—
|
|
(123
|
)
|
—
|
|
—
|
|
(123
|
)
|
|||||||||||||||
|
Unrealized gain on short-term investments
|
—
|
|
—
|
|
—
|
|
—
|
|
261
|
|
—
|
|
—
|
|
261
|
|
|||||||||||||||
|
Balance at December 31, 2015
|
50,651,239
|
|
51
|
|
(34,640
|
)
|
633,146
|
|
(215
|
)
|
(288,131
|
)
|
3,944
|
|
314,155
|
|
|||||||||||||||
|
Net loss attributable to Acacia Research Corporation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(54,067
|
)
|
—
|
|
(54,067
|
)
|
|||||||||||||||
|
Stock options exercised
|
100,992
|
|
—
|
|
—
|
|
326
|
|
—
|
|
—
|
|
—
|
|
326
|
|
|||||||||||||||
|
Compensation expense for share-based awards, net of forfeitures
|
(262,660
|
)
|
(1
|
)
|
—
|
|
9,063
|
|
—
|
|
—
|
|
—
|
|
9,062
|
|
|||||||||||||||
|
Repurchase of restricted common stock
|
(13,529
|
)
|
—
|
|
—
|
|
(82
|
)
|
—
|
|
—
|
|
—
|
|
(82
|
)
|
|||||||||||||||
|
Net loss attributable to noncontrolling interests in subsidiaries
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(732
|
)
|
(732
|
)
|
|||||||||||||||
|
Distributions to noncontrolling interests in operating subsidiary
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(1,358
|
)
|
(1,358
|
)
|
|||||||||||||||
|
Unrealized gain on short-term investments
|
—
|
|
—
|
|
—
|
|
—
|
|
40
|
|
—
|
|
—
|
|
40
|
|
|||||||||||||||
|
Unrealized gain on foreign currency translation
|
—
|
|
—
|
|
—
|
|
—
|
|
99
|
|
—
|
|
—
|
|
99
|
|
|||||||||||||||
|
Balance at December 31, 2016
|
50,476,042
|
|
50
|
|
(34,640
|
)
|
642,453
|
|
(76
|
)
|
(342,198
|
)
|
1,854
|
|
267,443
|
|
|||||||||||||||
|
Net income attributable to Acacia Research Corporation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
22,180
|
|
—
|
|
22,180
|
|
|||||||||||||||
|
Stock options exercised
|
207,863
|
|
1
|
|
—
|
|
744
|
|
—
|
|
—
|
|
—
|
|
745
|
|
|||||||||||||||
|
Compensation expense for share-based awards, net of forfeitures
|
(35,310
|
)
|
—
|
|
—
|
|
5,844
|
|
—
|
|
—
|
|
—
|
|
5,844
|
|
|||||||||||||||
|
Repurchase of restricted common stock
|
(8,669
|
)
|
—
|
|
—
|
|
(45
|
)
|
—
|
|
—
|
|
—
|
|
(45
|
)
|
|||||||||||||||
|
Net loss attributable to noncontrolling interests in subsidiaries
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(496
|
)
|
(496
|
)
|
|||||||||||||||
|
Unrealized gain on foreign currency translation
|
—
|
|
—
|
|
—
|
|
—
|
|
28
|
|
—
|
|
—
|
|
28
|
|
|||||||||||||||
|
Unrealized loss on short-term investments
|
—
|
|
—
|
|
—
|
|
—
|
|
(40
|
)
|
—
|
|
—
|
|
(40
|
)
|
|||||||||||||||
|
Balance at December 31, 2017
|
50,639,926
|
|
$
|
51
|
|
$
|
(34,640
|
)
|
$
|
648,996
|
|
$
|
(88
|
)
|
$
|
(320,018
|
)
|
$
|
1,358
|
|
$
|
295,659
|
|
||||||||
|
|
2017
|
2016
|
2015
|
|||||||||
|
Cash flows from operating activities:
|
|
|
|
|||||||||
|
Net income (loss) including noncontrolling interests in subsidiaries
|
$
|
21,684
|
|
$
|
(54,799
|
)
|
$
|
(157,478
|
)
|
|||
|
Adjustments to reconcile net income (loss) including noncontrolling interests in subsidiaries to net cash provided by (used in) operating activities:
|
|
|
|
|
|
|
||||||
|
Gain on conversion of loans and accrued interest
|
(2,671
|
)
|
—
|
|
—
|
|
||||||
|
Gain on exercise of Primary Warrant
|
(4,616
|
)
|
—
|
|
—
|
|
||||||
|
Change in fair value of investment, net
|
(42,239
|
)
|
—
|
|
—
|
|
||||||
|
Depreciation and amortization
|
22,243
|
|
34,355
|
|
53,289
|
|
||||||
|
Non-cash stock compensation
|
8,885
|
|
9,062
|
|
11,048
|
|
||||||
|
Impairment of patent-related intangible assets
|
2,248
|
|
42,340
|
|
74,731
|
|
||||||
|
Impairment of goodwill
|
—
|
|
—
|
|
30,149
|
|
||||||
|
Other
|
(374
|
)
|
(477
|
)
|
(109
|
)
|
||||||
|
Changes in assets and liabilities:
|
|
|
|
|
|
|
||||||
|
Restricted cash
|
11,512
|
|
(787
|
)
|
(10,725
|
)
|
||||||
|
Accounts receivable
|
26,597
|
|
6,750
|
|
(13,332
|
)
|
||||||
|
Prepaid expenses and other assets
|
(135
|
)
|
1,593
|
|
(619
|
)
|
||||||
|
Accounts payable and accrued expenses / patent costs
|
(6,349
|
)
|
(3,006
|
)
|
2,570
|
|
||||||
|
Royalties and contingent legal fees payable
|
(12,307
|
)
|
(970
|
)
|
527
|
|
||||||
|
Net cash provided by (used in) operating activities
|
24,478
|
|
34,061
|
|
(9,949
|
)
|
||||||
|
Cash flows from investing activities:
|
|
|
|
|
|
|
||||||
|
Investment in Investees
|
(31,514
|
)
|
—
|
|
—
|
|
||||||
|
Advances to Investee (Note 7)
|
(4,000
|
)
|
(20,000
|
)
|
—
|
|
||||||
|
Purchases of property and equipment
|
|
(2
|
)
|
|
(4
|
)
|
|
(8
|
)
|
|||
|
Purchases of short-term investments
|
|
(448,388
|
)
|
|
(62,633
|
)
|
|
(23,296
|
)
|
|||
|
Sales and maturities of short-term investments
|
|
467,790
|
|
|
43,232
|
|
|
82,115
|
|
|||
|
Patent portfolio investment costs
|
|
—
|
|
(1,225
|
)
|
(19,504
|
)
|
|||||
|
Net cash provided by (used in) investing activities
|
|
(16,114
|
)
|
|
(40,630
|
)
|
|
39,307
|
|
|||
|
Cash flows from financing activities:
|
|
|
|
|
|
|
||||||
|
Dividends paid to stockholders
|
—
|
|
—
|
|
(25,434
|
)
|
||||||
|
Distributions to noncontrolling interests in operating subsidiary
|
—
|
|
|
(1,358
|
)
|
|
(4,105
|
)
|
||||
|
Proceeds from the exercise of stock options
|
745
|
|
|
326
|
|
|
938
|
|
||||
|
Repurchases of restricted common stock
|
(45
|
)
|
|
(82
|
)
|
|
—
|
|
||||
|
Net cash provided by (used in) financing activities
|
700
|
|
(1,114
|
)
|
(28,601
|
)
|
||||||
|
Increase (decrease) in cash and cash equivalents
|
9,064
|
|
(7,683
|
)
|
757
|
|
||||||
|
Cash and cash equivalents, beginning
|
127,540
|
|
135,223
|
|
134,466
|
|
||||||
|
Cash and cash equivalents, ending
|
$
|
136,604
|
|
$
|
127,540
|
|
$
|
135,223
|
|
|||
|
Supplemental schedule of noncash investing activities:
|
||||||||||||
|
Patent portfolio investment costs included in accrued expenses / costs
|
$
|
—
|
|
$
|
—
|
|
$
|
1,000
|
|
|||
|
(i)
|
Level 1
-
Observable Inputs
: Quoted prices in active markets for identical investments;
|
|
(ii)
|
Level 2
-
Pricing Models with Significant Observable Inputs
: Other significant observable inputs, including quoted prices for similar investments, interest rates, credit risk, etc.; and
|
|
(iii)
|
Level 3
-
Unobservable Inputs
: Significant unobservable inputs, including the entity’s own assumptions in determining the fair value of investments.
|
|
Level 1
|
Level 2
|
Level 3
|
|||||||||
|
Assets as of December 31, 2017:
|
|
||||||||||
|
Investment at fair value (Note 7)
(1)
|
$
|
—
|
|
$
|
—
|
|
$
|
104,754
|
|
||
|
Assets as of December 31, 2016:
|
|||||||||||
|
Short-term investments
(1)
|
$
|
19,443
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Investment at Fair Value
|
|||||||||||
|
Common Stock
|
Warrants
|
Total
|
|||||||||
|
Opening balance as of January 1, 2017
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Total gains and losses included in earnings for the period
(1)
|
|||||||||||
|
Gain on conversion of loans and accrued interest
|
2,671
|
|
—
|
|
2,671
|
|
|||||
|
Gain on exercise of Primary Warrant
|
—
|
|
4,616
|
|
4,616
|
|
|||||
|
Change in fair value of investment, net
|
33,922
|
|
8,317
|
|
42,239
|
|
|||||
|
Purchases, issues, sales and settlements
|
|
|
|
|
|||||||
|
Purchases and issues
(2)
|
54,202
|
|
1,026
|
|
55,228
|
|
|||||
|
Total recurring fair value measurements
(1)
|
$
|
90,795
|
|
$
|
13,959
|
|
$
|
104,754
|
|
||
|
Furniture and fixtures
|
3 to 5 years
|
|
Computer hardware and software
|
3 to 5 years
|
|
Leasehold improvements
|
2 to 5 years (Lesser of lease term or useful life of improvement)
|
|
For the Years Ended
|
|||
|
December 31, 2017
|
December 31, 2016
|
||
|
Risk-free interest rate
|
1.77%
|
1.1%
|
|
|
Term
|
4.37
|
3.06
|
|
|
Volatility
|
51%
|
53%
|
|
|
Dividend yield
|
—%
|
—%
|
|
|
|
2017
|
|
2016
|
2015
|
||||||||
|
Numerator (in thousands):
|
||||||||||||
|
Basic and Diluted
|
||||||||||||
|
Net income (loss) attributable to Acacia Research Corporation
|
$
|
22,180
|
|
$
|
(54,067
|
)
|
$
|
(160,036
|
)
|
|||
|
Undistributed earnings allocated to participating securities
|
(33
|
)
|
—
|
|
—
|
|
||||||
|
Total dividends declared / paid
|
—
|
|
—
|
|
(25,434
|
)
|
||||||
|
Dividends attributable to common stockholders
|
—
|
|
—
|
|
24,740
|
|
||||||
|
Net income (loss) attributable to common stockholders – basic and diluted
|
$
|
22,147
|
|
$
|
(54,067
|
)
|
$
|
(160,730
|
)
|
|||
|
Denominator:
|
||||||||||||
|
Weighted-average shares used in computing net loss per share attributable to common stockholders – basic
|
50,495,119
|
|
50,075,847
|
|
49,505,817
|
|
||||||
|
Effect of potentially dilutive securities:
|
||||||||||||
|
Common stock options and restricted stock units
|
196,893
|
|
—
|
|
—
|
|
||||||
|
Weighted-average shares used in computing net income (loss) per share attributable to common stockholders – diluted
|
50,692,012
|
|
50,075,847
|
|
49,505,817
|
|
||||||
|
Basic and diluted net loss per common share
|
$
|
0.44
|
|
$
|
(1.08
|
)
|
$
|
(3.25
|
)
|
|||
|
Anti-dilutive equity-based incentive awards excluded from the computation of diluted loss per share
|
4,425,187
|
|
3,682,532
|
|
71,468
|
|
||||||
|
December 31, 2016
|
|||||||||||||||
|
Security Type
|
Cost
|
Gross Unrealized Gains
|
Gross Unrealized Losses
|
Fair Value
|
|||||||||||
|
U.S. government fixed income securities
|
$
|
19,403
|
|
$
|
40
|
|
$
|
—
|
|
$
|
19,443
|
|
|||
|
|
2017
|
2016
|
||||||
|
Payroll and other employee benefits
|
$
|
465
|
|
$
|
1,593
|
|
||
|
Accrued vacation
|
294
|
|
533
|
|
||||
|
Accrued legal expenses - patent
|
5,479
|
|
6,564
|
|
||||
|
Foreign taxes payable
|
15
|
|
3,150
|
|
||||
|
Accrued consulting and other professional fees
|
1,364
|
|
1,967
|
|
||||
|
Other accrued liabilities
|
339
|
|
476
|
|
||||
|
|
$
|
7,956
|
|
$
|
14,283
|
|
||
|
|
2017
|
2016
|
||||||
|
Gross carrying amount - patents
|
$
|
444,137
|
|
$
|
444,362
|
|
||
|
Accumulated amortization - patents
(1)
|
(382,220
|
)
|
(358,043
|
)
|
||||
|
Patents, net
|
$
|
61,917
|
|
$
|
86,319
|
|
||
|
•
|
significant consistent gradual decline in the Company’s stock price for a sustained period;
|
|
•
|
significant underperformance relative to expected historical or projected future operating results;
|
|
•
|
significant changes in the manner of use of assets or the strategy for the Company’s overall business;
|
|
•
|
significant negative industry or economic trends; and
|
|
•
|
significant adverse changes in legal factors or in the business climate, including adverse regulatory actions or assessments.
|
|
•
|
Adverse legal outcomes and changes in legal factors.
In December 2015, Acacia announced that its subsidiary Adaptix, Inc. received a jury verdict in its case against Alcatel Lucent USA, et al., deciding that the claims of the applicable patents in suit were invalid and non-infringed. This adverse legal outcome and others in the fourth quarter of 2015 resulted in changes in estimates of realization related to litigation outcomes in future periods for certain patent portfolios.
|
|
•
|
Significant consistent gradual decline in the Company’s stock price.
Historically, the Company’s stock price had been volatile, and the volatility continued during fiscal 2015, declining from
$16.72
as of January 2, 2015, to
$4.29
as of December 31, 2015, a 74% decline. In addition, subsequent to December 31, 2015, the Company’s stock price volatility has continued, trending downward. In the fourth quarter of 2015, given the continued decline in stock price up through December 31, 2015, and the impact of the December 2015 adverse trial outcomes noted above, the gradual consistent decline in the Company’s stock price was deemed to be sustained, and hence indicative of a reduction in the estimated fair value of the Company, as reflected in its lower overall market capitalization.
|
|
•
|
Changes in Company Management and Resource Allocations.
In connection with certain resource allocation changes within the organization given a change in management in the fourth quarter of 2015, headcount reductions and internal staff optimization efforts occurred, which led to changes with respect to estimates of which patent portfolios the Company intends to continue to allocate licensing and enforcement resources to in future periods. As such, certain patent portfolio programs were selected for termination due to a decision to no longer allocate resources. In addition, changes in estimates regarding the best and highest use of certain patent portfolios were made, resulting in reductions in estimated future cash flows.
|
|
•
|
At December 31, 2015, the initial qualitative assessment included consideration of the factors described above, resulting in a conclusion that as of December 31, 2015, the consistent gradual decline in the Company’s stock price was sustained. The Company also considered the impact of the December 2015 adverse trial outcomes on the Company’s stock price and related estimates of fair value for remaining portfolio opportunities. Based on the Company’s assessment of these factors, the Company determined that it was more likely than not that goodwill was impaired, constituting a triggering event requiring a goodwill impairment test as of December 31, 2015.
|
|
•
|
The Company conducted the first step of the goodwill impairment test for its single reporting unit as of December 31, 2015. The Company utilized the market capitalization plus cost synergies approach to estimate the fair value of the Company. The estimated market capitalization was determined by multiplying the Company’s stock price and the common shares outstanding as of December 31, 2015. Management also considered a control premium in its
|
|
•
|
Based on the analysis utilizing the market capitalization plus cost synergies approach, the estimated fair value of the reporting unit of
$252 million
was below its carrying value of
$344.3 million
as of December 31, 2015, and therefore, goodwill was determined to be more likely than not, impaired.
|
|
•
|
The purpose of step 2 of the analysis was to determine the estimated fair value of the assets and liabilities of the Company’s reporting unit, in order to determine the implied fair value of goodwill for the reporting unit. The excess, if any, of the fair value of a reporting unit over the amounts assigned to its assets and liabilities is the implied fair value of goodwill. Based upon the analysis performed, the fair value of the Company’s single reporting unit did not exceed the amounts assigned to its reporting unit assets and liabilities, resulting in a difference between the implied fair value of goodwill of zero and the historical carrying value of goodwill. As a result, the Company recognized a goodwill impairment charge totaling
$30.1 million
in the fourth quarter of 2015.
|
|
Veritone Common Stock
|
Veritone Warrants
|
||||||||||
|
IPO Date
|
December 31, 2017
|
IPO Date
|
December 31, 2017
|
||||||||
|
Estimated DLOM applied
|
5.7%
|
5%
|
5.7%
|
10%
|
|||||||
|
Volatility assumptions
|
35%
|
37%
|
35%
|
72
|
%
|
-
|
87%
|
||||
|
Term assumptions
|
6 months
|
2 months
|
6 months
|
5 months
|
|||||||
|
2017
|
||||
|
Gain on conversion of loans and accrued interest
(1)
|
$
|
2,671
|
|
|
|
Gain on exercise of warrant
(2)
|
4,616
|
|
||
|
Change in fair value of investment, warrants
|
8,317
|
|
||
|
Change in fair value of investment, common stock
|
33,922
|
|
||
|
Net unrealized gain on investment at fair value
|
$
|
49,526
|
|
|
|
|
Nine Months Ended
September 30, 2017
|
|||
|
(Unaudited)
|
||||
|
Revenues
|
$
|
10,914
|
|
|
|
Gross profit
|
10,090
|
|
||
|
Operating expenses
|
44,024
|
|
||
|
Other income (expense), net
|
(12,872
|
)
|
||
|
Net loss attributable to common stockholders
|
(51,281
|
)
|
||
|
Net loss per share attributable to common stockholders - basic and diluted
|
$
|
(5.94
|
)
|
|
|
|
September 30,
2017 |
|||
|
Current assets
|
$
|
78,509
|
|
|
|
Noncurrent assets
|
1,173
|
|
||
|
Total Assets
|
$
|
79,682
|
|
|
|
Current liabilities
|
$
|
31,836
|
|
|
|
Noncurrent liabilities
|
14
|
|
||
|
Total liabilities
|
31,850
|
|
||
|
Preferred stock
|
—
|
|
||
|
Total stockholders’ equity (deficit)
|
47,832
|
|
||
|
Total liabilities, preferred stock and stockholders’ equity
|
$
|
79,682
|
|
|
|
|
2017
|
2016
|
2015
|
|||||||||
|
Current:
|
|
|
|
|||||||||
|
Federal
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
State
|
90
|
|
262
|
|
379
|
|
||||||
|
Foreign
|
2,865
|
|
17,926
|
|
4,421
|
|
||||||
|
Total current
|
2,955
|
|
18,188
|
|
4,800
|
|
||||||
|
Deferred:
|
||||||||||||
|
Federal
|
—
|
|
—
|
|
—
|
|
||||||
|
State
|
—
|
|
—
|
|
—
|
|
||||||
|
Total deferred
|
—
|
|
—
|
|
—
|
|
||||||
|
Provision for income taxes
|
$
|
2,955
|
|
$
|
18,188
|
|
$
|
4,800
|
|
|||
|
|
2017
|
2016
|
||||||
|
Deferred tax assets:
|
|
|
||||||
|
Net operating loss and capital loss carryforwards and credits
|
$
|
90,871
|
|
$
|
83,323
|
|
||
|
Stock compensation
|
2,635
|
|
2,416
|
|
||||
|
Fixed assets and intangibles
|
6,197
|
|
14,343
|
|
||||
|
Basis of investments in affiliates
|
984
|
|
2,195
|
|
||||
|
Accrued liabilities and other
|
167
|
|
422
|
|
||||
|
State taxes
|
35
|
|
90
|
|
||||
|
Total deferred tax assets
|
100,889
|
|
102,789
|
|
||||
|
Valuation allowance
|
(90,278
|
)
|
(102,627
|
)
|
||||
|
Total deferred tax assets, net of valuation allowance
|
10,611
|
|
162
|
|
||||
|
Deferred tax liabilities:
|
||||||||
|
Unrealized gain on investments held at fair value
|
(10,587
|
)
|
—
|
|
||||
|
Other
|
(24
|
)
|
(162
|
)
|
||||
|
Total deferred tax liabilities
|
(10,611
|
)
|
(162
|
)
|
||||
|
Net deferred tax assets (liabilities)
|
$
|
—
|
|
$
|
—
|
|
||
|
|
2017
|
2016
|
2015
|
||||||
|
Statutory federal tax rate - (benefit) expense
|
35
|
%
|
(35
|
)%
|
(35
|
)%
|
|||
|
State income and foreign taxes, net of federal tax effect
|
8
|
%
|
50
|
%
|
3
|
%
|
|||
|
Foreign tax credit
|
—
|
%
|
(49
|
)%
|
(3
|
)%
|
|||
|
Noncontrolling interests in operating subsidiaries
|
1
|
%
|
1
|
%
|
(1
|
)%
|
|||
|
Goodwill
|
—
|
%
|
—
|
%
|
7
|
%
|
|||
|
Nondeductible permanent items
|
3
|
%
|
—
|
%
|
—
|
%
|
|||
|
Expired capital loss carryforwards
|
—
|
%
|
—
|
%
|
1
|
%
|
|||
|
Change in tax rate
|
102
|
%
|
—
|
%
|
—
|
%
|
|||
|
Valuation allowance
|
(137
|
)%
|
83
|
%
|
31
|
%
|
|||
|
|
12
|
%
|
50
|
%
|
3
|
%
|
|||
|
•
|
Discretionary Option Grant Program
. Under the discretionary option grant program, Acacia’s compensation committee may grant (1) non-statutory options to purchase shares of common stock to eligible individuals in the employ or service of Acacia or its subsidiaries (including employees, non-employee board members and consultants) at an exercise price not less than
85%
of the fair market value of those shares on the grant date, and (2) incentive stock options to purchase shares of common stock to eligible employees at an exercise price not less than
100%
of the fair market value of those shares on the grant date (not less than
110%
of fair market value if such employee actually or constructively owns more than
10%
of Acacia’s voting stock or the voting stock of any of its subsidiaries).
|
|
•
|
Stock Issuance Program
. Under the stock issuance program, eligible individuals may be issued shares of common stock directly, upon the attainment of performance milestones or the completion of a specified period of service or as a bonus for past services. Under this program, the purchase price for the shares shall not be less than
100%
of the fair market value of the shares on the date of issuance, and payment may be in the form of cash or past services rendered. The eligible individuals shall have full stockholder rights with respect to any shares of Common Stock issued to them under the Stock Issuance Program, whether or not their interest in those shares is vested. Accordingly, the eligible individuals shall have the right to vote such shares and to receive any regular cash dividends paid on such shares.
|
|
•
|
Automatic Option Grant Program
. Each non-employee director will receive restricted stock units or stock options for the number of shares determined by dividing the annual retainer by the grant date fair value of Acacia’s common stock on the grant date. In addition, each new non-employee director will receive restricted stock units or stock options for the number of shares determined by dividing the annual board of directors retainer by the grant date fair value of Acacia’s common stock on the commencement date. Restricted stock units and stock options vest in a series of twelve quarterly installments over the three year period following the grant date, subject to immediate acceleration upon a change in control. Acacia will deliver the unrestricted shares corresponding to the vested restricted stock units within thirty (30) days after the first to occur of the following events: (i) the fifth (5th) anniversary of the grant date; or (ii) termination of the non-employee director’s service as a member of the Company’s Board of Directors. The non-employee directors do not have any rights, benefits or entitlements with respect to any shares unless and until the shares have been delivered.
|
|
2017
|
2016
|
|||||||||||||
|
Shares
|
Aggregate fair value (in thousands)
|
Shares
|
Aggregate fair value (in thousands)
|
|||||||||||
|
Restricted stock awards with performance-based vesting conditions
|
—
|
|
$
|
—
|
|
138,000
|
|
$
|
431
|
|
||||
|
Stock options with time-based service vesting conditions
|
1,368,000
|
|
2,930
|
|
3,434,000
|
|
5,704
|
|
||||||
|
Stock options with market-based vesting conditions
|
—
|
|
—
|
|
2,250,000
|
|
5,530
|
|
||||||
|
Stock options with performance-based vesting conditions
|
—
|
|
—
|
|
200,000
|
|
487
|
|
||||||
|
Total incentive awards granted
|
1,368,000
|
|
$
|
2,930
|
|
6,022,000
|
|
$
|
12,152
|
|
||||
|
|
|
Weighted-Average
|
|
||||||||||
|
|
Options
|
Exercise
Price
|
Remaining
Contractual Term
|
Aggregate
Intrinsic Value
|
|||||||||
|
Outstanding at December 31, 2016
|
5,596,000
|
|
$
|
4.93
|
|
|
|
||||||
|
Granted
|
1,368,000
|
|
$
|
5.52
|
|
||||||||
|
Exercised
|
(208,000
|
)
|
$
|
3.57
|
|
|
|
||||||
|
Expired/forfeited
|
(926,000
|
)
|
$
|
4.90
|
|
||||||||
|
Outstanding at December 31, 2017
|
5,830,000
|
|
$
|
5.13
|
|
5.8 years
|
$
|
856,000
|
|
||||
|
Vested
|
1,959,000
|
|
$
|
4.84
|
|
5.8 years
|
$
|
434,000
|
|
||||
|
Exercisable at December 31, 2017
|
1,959,000
|
|
$
|
4.84
|
|
5.8 years
|
$
|
434,000
|
|
||||
|
|
Nonvested
Restricted Shares
|
Weighted
Average Grant Date Fair Value
|
|||||
|
Nonvested restricted stock at December 31, 2016
|
333,000
|
|
$
|
8.9
|
|
||
|
Granted
|
—
|
|
$
|
—
|
|
||
|
Vested
|
(120,000
|
)
|
$
|
12.95
|
|
||
|
Canceled
|
(90,000
|
)
|
$
|
9.10
|
|
||
|
Nonvested restricted stock at December 31, 2017
|
123,000
|
|
$
|
4.77
|
|
||
|
|
Restricted
Stock Units
|
Weighted
Average Grant Date Fair Value
|
|||||
|
Nonvested restricted stock units outstanding at December 31, 2016
|
14,000
|
|
$
|
16.27
|
|
||
|
Vested
|
(12,000
|
)
|
$
|
16.18
|
|
||
|
Nonvested restricted stock units outstanding at December 31, 2017
|
2,000
|
|
$
|
16.72
|
|
||
|
Vested restricted stock units outstanding at December 31, 2017
|
60,000
|
|
$
|
15.38
|
|
||
|
2017
|
2016
|
2015
|
||||||||||
|
Restricted stock awards with time-based service conditions
|
$
|
1,025
|
|
$
|
4,071
|
|
$
|
10,575
|
|
|||
|
Restricted stock unit awards with time-based service conditions
|
161
|
|
320
|
|
473
|
|
||||||
|
Restricted stock awards with performance-based vesting conditions
|
121
|
|
197
|
|
—
|
|
||||||
|
Stock options with time-based service vesting conditions
|
2,165
|
|
1,316
|
|
—
|
|
||||||
|
Stock options with market-based vesting conditions
|
2,372
|
|
3,158
|
|
—
|
|
||||||
|
Stock options with performance-based vesting conditions
|
—
|
|
—
|
|
—
|
|
||||||
|
Profits interests units
|
3,041
|
|
—
|
|
—
|
|
||||||
|
Total compensation expense
|
$
|
8,885
|
|
$
|
9,062
|
|
$
|
11,048
|
|
|||
|
Years ending December 31,
|
|
||
|
2018
|
$
|
1,213
|
|
|
2019
|
1,369
|
|
|
|
2020
|
16
|
|
|
|
Total minimum lease payments
|
$
|
2,598
|
|
|
|
2017
|
2016
|
||||||
|
Cash and other assets
|
$
|
986
|
|
$
|
1,118
|
|
||
|
Investments - noncurrent
|
1,905
|
|
2,933
|
|
||||
|
Total assets
|
$
|
2,891
|
|
$
|
4,051
|
|
||
|
Accrued expenses and contributions
|
$
|
2,567
|
|
$
|
2,394
|
|
||
|
Net assets
|
$
|
324
|
|
$
|
1,657
|
|
||
|
|
2017
|
2016
|
||||||
|
Revenues
|
$
|
—
|
|
$
|
16
|
|
||
|
Operating expenses
|
390
|
|
572
|
|
||||
|
Loss from operations
|
(390
|
)
|
(556
|
)
|
||||
|
Net loss in equity method investments
|
(943
|
)
|
(1,013
|
)
|
||||
|
Net loss
|
$
|
(1,333
|
)
|
$
|
(1,569
|
)
|
||
|
|
Quarter Ended
|
|||||||||||||||||||||||||||||||
|
|
Mar. 31,
|
Jun. 30,
|
Sept. 30,
|
Dec. 31,
|
Mar. 31,
|
Jun. 30,
|
Sept. 30,
|
Dec. 31,
|
||||||||||||||||||||||||
|
|
2017
|
2017
|
2017
|
2017
|
2016
|
2016
|
2016
|
2016
|
||||||||||||||||||||||||
|
|
(Unaudited, in thousands, except share and per share information)
|
|||||||||||||||||||||||||||||||
|
Revenues
|
$
|
8,854
|
|
$
|
16,457
|
|
$
|
36,633
|
|
$
|
3,458
|
|
$
|
24,721
|
|
$
|
41,351
|
|
$
|
64,658
|
|
$
|
21,969
|
|
||||||||
|
Operating costs and expenses:
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||
|
Cost of revenues:
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||
|
Inventor royalties
|
666
|
|
4,273
|
|
—
|
|
13
|
|
1,573
|
|
—
|
|
17,844
|
|
3,313
|
|
||||||||||||||||
|
Contingent legal fees
|
627
|
|
3,236
|
|
12,173
|
|
646
|
|
4,109
|
|
10,418
|
|
7,709
|
|
4,238
|
|
||||||||||||||||
|
Litigation and licensing expenses - patents
|
6,386
|
|
4,134
|
|
4,073
|
|
3,626
|
|
7,723
|
|
7,324
|
|
7,348
|
|
5,463
|
|
||||||||||||||||
|
Amortization of patents
|
5,515
|
|
5,571
|
|
5,625
|
|
5,443
|
|
10,760
|
|
10,759
|
|
6,467
|
|
6,222
|
|
||||||||||||||||
|
General and administrative expenses (including non-cash stock compensation expense)
|
6,916
|
|
6,734
|
|
12,715
|
|
(335
|
)
|
7,994
|
|
7,535
|
|
8,334
|
|
9,056
|
|
||||||||||||||||
|
Other expenses - business development
|
320
|
|
433
|
|
241
|
|
195
|
|
522
|
|
1,334
|
|
666
|
|
557
|
|
||||||||||||||||
|
Impairment of patent-related intangible assets
|
—
|
|
—
|
|
2,248
|
|
—
|
|
—
|
|
40,165
|
|
—
|
|
2,175
|
|
||||||||||||||||
|
Other
|
—
|
|
—
|
|
—
|
|
1,200
|
|
1,742
|
|
(1,242
|
)
|
—
|
|
—
|
|
||||||||||||||||
|
Total operating costs and expenses
|
20,430
|
|
24,381
|
|
37,075
|
|
10,788
|
|
34,423
|
|
76,293
|
|
48,368
|
|
31,024
|
|
||||||||||||||||
|
Operating income (loss)
|
(11,576
|
)
|
(7,924
|
)
|
(442
|
)
|
(7,330
|
)
|
(9,702
|
)
|
(34,942
|
)
|
16,290
|
|
(9,055
|
)
|
||||||||||||||||
|
Total other income (expense)
|
696
|
|
(4,862
|
)
|
159,027
|
|
(102,950
|
)
|
(3
|
)
|
(52
|
)
|
261
|
|
592
|
|
||||||||||||||||
|
Income (loss) before (provision for) benefit from income taxes
|
(10,880
|
)
|
(12,786
|
)
|
158,585
|
|
(110,280
|
)
|
(9,705
|
)
|
(34,994
|
)
|
16,551
|
|
(8,463
|
)
|
||||||||||||||||
|
Provision for income taxes
|
(1,241
|
)
|
(1,478
|
)
|
(216
|
)
|
(20
|
)
|
(192
|
)
|
(5,927
|
)
|
(9,655
|
)
|
(2,414
|
)
|
||||||||||||||||
|
Net income (loss) including noncontrolling interests
|
(12,121
|
)
|
(14,264
|
)
|
158,369
|
|
(110,300
|
)
|
(9,897
|
)
|
(40,921
|
)
|
6,896
|
|
(10,877
|
)
|
||||||||||||||||
|
Net (income) loss attributable to noncontrolling interests in subsidiaries
|
291
|
|
12
|
|
96
|
|
97
|
|
(68
|
)
|
348
|
|
186
|
|
266
|
|
||||||||||||||||
|
Net income (loss) attributable to Acacia Research Corporation
|
$
|
(11,830
|
)
|
$
|
(14,252
|
)
|
$
|
158,465
|
|
$
|
(110,203
|
)
|
$
|
(9,965
|
)
|
$
|
(40,573
|
)
|
$
|
7,082
|
|
$
|
(10,611
|
)
|
||||||||
|
Net income (loss) per common share attributable to Acacia Research Corporation:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
Basic and diluted income (loss) per share
|
$
|
(0.24
|
)
|
$
|
(0.28
|
)
|
$
|
3.13
|
|
$
|
(2.18
|
)
|
$
|
(0.20
|
)
|
$
|
(0.81
|
)
|
$
|
0.14
|
|
$
|
(0.21
|
)
|
||||||||
|
Weighted-average number of shares outstanding, basic
|
50,333,056
|
|
50,499,948
|
|
50,554,234
|
|
50,590,460
|
|
49,925,550
|
|
50,015,869
|
|
50,124,302
|
|
50,237,784
|
|
||||||||||||||||
|
Weighted-average number of shares outstanding, diluted
|
50,333,056
|
|
50,499,948
|
|
50,599,974
|
|
50,590,460
|
|
49,925,550
|
|
50,015,869
|
|
50,618,757
|
|
50,237,784
|
|
||||||||||||||||