|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Delaware
|
52-0845822
|
|
|
(State or other jurisdiction of
|
(I.R.S. Employer Identification
|
|
|
incorporation or organization)
|
Number)
|
|
1617 JFK Boulevard Philadelphia, Pennsylvania
|
19103
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
Page
|
||||
|
PART I
|
||||
|
Item 1.
|
Business
|
1
|
||
|
Item 1A.
|
Risk Factors
|
18
|
||
|
Item 1B.
|
Unresolved Staff Comments
|
29
|
||
|
Item 2.
|
Properties
|
29
|
||
|
Item 3.
|
Legal Proceedings
|
30
|
||
|
PART II
|
||||
|
Item 5.
|
Market for the Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
30 | ||
|
Item 6.
|
Selected Financial Data
|
32
|
||
|
Item 7.
|
Management's Discussion and Analysis of Financial
Condition and Results of Operations
|
33
|
||
|
Item 7A.
|
Quantitative and Qualitative Disclosures About
Market Risk
|
47
|
||
|
Item 8.
|
Financial Statements and Supplementary Data
|
47
|
||
|
Item 9.
|
Changes In and Disagreements with Accountants on
Accounting and Financial Disclosure
|
47 | ||
|
Item 9A.
|
Controls and Procedures
|
48
|
||
|
Item 9B.
|
Other Information
|
50
|
||
|
PART III
|
||||
|
Item 10.
|
Directors and Executive Officers and Corporate Governance
|
51
|
||
|
Item 11.
|
Executive Compensation
|
57
|
||
|
Item 12.
|
Security Ownership of Certain Beneficial Owners
and Management and Related Stockholder Matters
|
82
|
||
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
85 | ||
|
|
||||
|
Item 14.
|
Principal Accountant Fees and Services
|
86
|
||
|
PART IV
|
||||
|
Item 15.
|
Exhibits and Financial Statement Schedules
|
87
|
|
1.
|
There is no diagnostic laboratory test or biomarker for CFS;
|
|
2.
|
Fatigue and other symptoms of CFS are common to many illnesses;
|
|
3.
|
CFS is an invisible illness and many patients don't look sick;
|
|
4.
|
The illness has a pattern of remission and relapse;
|
|
5.
|
Symptoms vary from person to person in type, number and severity.
|
|
(dollars in thousands)
|
||||||||||||||||||||
|
Year Ended December 31, 2010
|
||||||||||||||||||||
|
Costs and Expenses
|
||||||||||||||||||||
|
Ampligen®
NDA
|
Alferon N
Injection®
|
Alferon
®
LDO
|
Other
|
Total
|
||||||||||||||||
|
Production/cost of goods sold
|
$ | - | $ | 1,341 | $ | - | $ | - | $ | 1,341 | ||||||||||
|
Research and development
|
2,787 | - | 4,658 | 168 | 7,613 | |||||||||||||||
|
General and administrative
|
2,356 | 1,133 | 3,937 | 142 | 7,568 | |||||||||||||||
|
Total
|
$ | 5,143 | $ | 2,474 | $ | 8,595 | $ | 310 | $ | 16,522 | ||||||||||
|
(dollars in thousands)
|
||||||||||||||||||||
|
Year Ended December 31, 2009
|
||||||||||||||||||||
|
Costs and Expenses
|
||||||||||||||||||||
|
Ampligen®
NDA
|
Alferon N
Injection®
|
Alferon
®
LDO
|
Other
|
Total
|
||||||||||||||||
|
Production/cost of goods sold
|
$ | - | $ | 584 | $ | - | $ | - | $ | 584 | ||||||||||
|
Research and development
|
5,026 | - | 1,784 | 185 | 6,995 | |||||||||||||||
|
General and administrative
|
3,844 | 447 | 1,364 | 141 | 5,796 | |||||||||||||||
|
Total
|
$ | 8,870 | $ | 1,031 | $ | 3,148 | $ | 326 | $ | 13,375 | ||||||||||
|
(dollars in thousands)
|
||||||||||||||||||||
|
Year Ended December 31, 2008
|
||||||||||||||||||||
|
Costs and Expenses
|
||||||||||||||||||||
|
Ampligen®
NDA
|
Alferon N
Injection®
|
Alferon
®
LDO
|
Other
|
Total
|
||||||||||||||||
|
Production/cost of goods sold
|
$ | - | $ | 798 | $ | - | $ | - | $ | 798 | ||||||||||
|
Research and development
|
5,491 | - | - | 309 | 5,800 | |||||||||||||||
|
General and administrative
|
5,392 | 783 | - | 303 | 6,478 | |||||||||||||||
|
Total
|
$ | 10,883 | $ | 1,581 | $ | - | $ | 612 | $ | 13,076 | ||||||||||
|
(in thousands)
|
||||||||||||
|
2008
|
2009
|
2010
|
||||||||||
|
Ampligen
®
New Drug Application for the treatment of Chronic Fatigue Syndrome
|
$ | 5,491 | $ | 5,026 | $ | 2,787 | ||||||
|
Alferon
®
LDO for influenza
|
- | 1,784 | 4,658 | |||||||||
|
Alferon N Injection® for influenza
|
- | - | 168 | |||||||||
|
Other projects
|
309 | 185 | - | |||||||||
|
Total research and development
|
$ | 5,800 | $ | 6,995 | $ | 7,613 | ||||||
|
·
|
announcements of the results of clinical trials by us or our competitors;
|
|
·
|
announcement of legal actions against us and/or settlements or verdicts adverse to us;
|
|
·
|
adverse reactions to products;
|
|
·
|
governmental approvals, delays in expected governmental approvals or withdrawals of any prior governmental approvals or public or regulatory agency comments regarding the safety or effectiveness of our products, or the adequacy of the procedures, facilities or controls employed in the manufacture of our products;
|
|
·
|
changes in U.S. or foreign regulatory policy during the period of product development;
|
|
·
|
developments in patent or other proprietary rights, including any third party challenges of our intellectual property rights;
|
|
·
|
announcements of technological innovations by us or our competitors;
|
|
·
|
announcements of new products or new contracts by us or our competitors;
|
|
·
|
actual or anticipated variations in our operating results due to the level of development expenses and other factors;
|
|
·
|
changes in financial estimates by securities analysts and whether our earnings meet or exceed the estimates;
|
|
·
|
conditions and trends in the pharmaceutical and other industries;
|
|
·
|
new accounting standards;
|
|
·
|
overall investment market fluctuation;
|
|
·
|
restatement of financial results; and
|
|
·
|
occurrence of any of the risks described in these "Risk Factors".
|
|
COMMON STOCK
|
High
|
Low
|
||||||
|
Time Period:
|
||||||||
|
January 1, 2010 through March 31, 2010
|
0.84 | 0.56 | ||||||
|
April 1, 2010 through June 30, 2010
|
0.87 | 0.44 | ||||||
|
July 1, 2010 through September 30, 2010
|
0.62 | 0.44 | ||||||
|
October 1, 2010 through December 31, 2010
|
0.57 | 0.46 | ||||||
|
January 1, 2009 through March 31, 2009
|
0.84 | 0.26 | ||||||
|
April 1, 2009 through June 30, 2009
|
4.54 | 0.44 | ||||||
|
July 1, 2009 through September 30, 2009
|
3.58 | 1.86 | ||||||
|
October 1, 2009 through December 31, 2009
|
2.16 | 0.54 | ||||||
|
Plan Category
|
Number of
Securities to be
issued upon
exercise of
outstanding
options,
warrants and
rights
|
Weighted-average
Exercise price of
Outstanding
options, warrants
and rights
|
Number of
securities
Remaining
available for
future issuance
under equity
compensation
plans
(excluding
securities
reflected in
column) (a)
|
|||||||||
|
(a)
|
(b)
|
(c)
|
||||||||||
|
Equity compensation plans approved by security holders:
|
10,332,912 | $ | 2.28 | 3,411,560 | ||||||||
|
Equity compensation plans not approved by security holders:
|
10,983,246 | $ | 1.61 | - | ||||||||
|
Total
|
21,316,158 | $ | 1.93 | 3,411,560 | ||||||||
|
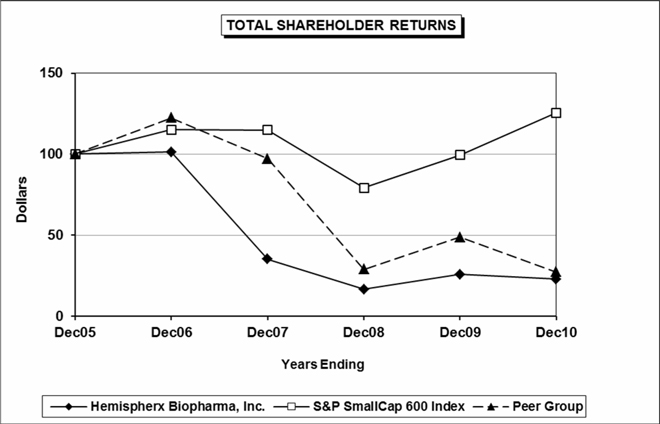
Total Return To Shareholders
|
||||||||||||||||||||
|
(Includes reinvestment of dividends)
|
||||||||||||||||||||
|
ANNUAL RETURN PERCENTAGE
|
||||||||||||||||||||
| Years Ending | ||||||||||||||||||||
|
Company Name / Index
|
Dec06
|
Dec07
|
Dec08
|
Dec09
|
Dec10
|
|||||||||||||||
|
Hemispherx Biopharma, Inc.
|
1.38 | -65.45 | -52.63 | 55.56 | -11.88 | |||||||||||||||
|
S&P SmallCap 600 Index
|
15.12 | -0.30 | -31.07 | 25.57 | 26.31 | |||||||||||||||
|
Peer Group
|
22.33 | -20.65 | -70.17 | 67.87 | -44.07 | |||||||||||||||
|
INDEXED RETURNS
|
||||||||||||||||||||||||
|
Base
|
Years Ending
|
|||||||||||||||||||||||
|
Period
|
||||||||||||||||||||||||
|
Company Name / Index
|
Dec05
|
Dec06
|
Dec07
|
Dec08
|
Dec09
|
Dec10
|
||||||||||||||||||
|
Hemispherx Biopharma, Inc.
|
100 | 101.38 | 35.02 | 16.59 | 25.81 | 22.74 | ||||||||||||||||||
|
S&P SmallCap 600 Index
|
100 | 115.12 | 114.78 | 79.11 | 99.34 | 125.47 | ||||||||||||||||||
|
Peer Group
|
100 | 122.33 | 97.08 | 28.95 | 48.60 | 27.18 | ||||||||||||||||||
|
Peer Group Companies
|
||||||||||||||||||||||||
|
CARDIUM THERAPEUTICS INC
|
||||||||||||||||||||||||
|
CYTRX CORP
|
||||||||||||||||||||||||
|
GENVEC INC
|
||||||||||||||||||||||||
|
OXIGENE INC
|
||||||||||||||||||||||||
|
REGENERX BIOPHARMACEUTICALS
|
||||||||||||||||||||||||
|
Year Ended
December 31
|
2006
|
2007
|
2008
|
2009
|
2010
|
|||||||||||||||
| Statement of Operations Data: | ||||||||||||||||||||
|
Revenues and License fee Income
|
$ | 933 | $ | 1,059 | $ | 265 | $ | 111 | $ | 135 | ||||||||||
|
Total Costs and Expenses
(1)
|
19,627 | 20,348 | 13,076 | 13,375 | 16,522 | |||||||||||||||
|
Interest Expense and Financing Costs
(2)
|
1,259 | 396 | - | 241 | 11 | |||||||||||||||
|
Redeemable warrants valuation adjustment
|
- | - | - | (6,258 | ) | (879 | ) | |||||||||||||
|
Net loss
|
(19,399 | ) | (18,139 | ) | (12,219 | ) | (7,180 | ) | (13,136 | ) | ||||||||||
|
Deemed Dividend
|
- | - | - | - | - | |||||||||||||||
|
Net loss
applicable to common stockholders
|
(19,399 | ) | (18,139 | ) | (12,219 | ) | (7,180 | ) | (13,136 | ) | ||||||||||
|
Basic and diluted net loss per share
|
$ | (0.31 | ) | $ | (0.25 | ) | $ | (0.16 | ) | $ | (0.07 | ) | $ | (0.10 | ) | |||||
|
Shares used in computing basic and diluted net loss per share
|
61,815,358 | 71,839,782 | 75,142,075 | 109,514,401 | 134,018,243 | |||||||||||||||
|
Balance Sheet Data:
|
||||||||||||||||||||
|
Working Capital
|
$ | 16,559 | $ | 14,412 | $ | 5,646 | $ | 55,789 | $ | 33,842 | ||||||||||
|
Total Assets
|
31,431 | 23,142 | 13,211 | 64,994 | 51,680 | |||||||||||||||
|
Debt, net of discount
|
3,871 | - | - | - | - | |||||||||||||||
|
Stockholders’ Equity
|
24,751 | 20,955 | 11,544 | 58,695 | 45,947 | |||||||||||||||
|
Cash Flow Data:
|
||||||||||||||||||||
|
Cash used in operating activities
|
(13,747 | ) | (15,112 | ) | (9,358 | ) | (9,297 | ) | (11,886 | ) | ||||||||||
|
Capital expenditures
|
$ | (1,351 | ) | $ | (212 | ) | $ | (73 | ) | $ | (332 | ) | $ | (729 | ) | |||||
|
(1)
|
General and Administrative expenses include stock compensation expense of $2,483, $2,291, $573, $826 and $740 for the years ended December 31, 2006, 2007, 2008, 2009 and 2010, respectively.
|
|
(2)
|
For information concerning our financing see Note 7 to our consolidated financial statements for the year ended December 31, 2010 contained herein.
|
|
2009
|
2010
|
||||
|
Underlying price per share
|
$ 0.56 - $2.54 | $ 0.47-$0.74 | |||
|
Exercise price per share
|
$ 1.10 – $1.65 | $ 1.31-$1.65 | |||
|
Risk-free interest rate
|
0.19% - 2.67% | 0.83%-2.36% | |||
|
Expected holding period
|
0.122-5.50 years
|
3.38-4.63 years
|
|||
|
Expected volatility
|
94.99%–226.46% | 112.16%-122.02% | |||
|
Expected dividend yield
|
None
|
None
|
|
a.
|
The Company only has one product that is FDA approved;
|
|
b.
|
The Company will have to perform additional clinical trials for FDA approval of its flagship product;
|
|
c.
|
Industry and market conditions continue to include a global market recession, adding risk to any transaction;
|
|
d.
|
Available capital for a potential buyer in a cash transaction continues to be limited;
|
|
e.
|
The nature of a life sciences company is heavily dependent on future funding and high fixed costs, including Research & Development;
|
|
f.
|
According to Forbes.com, of approximately 17,000 public companies, fewer than 30 went private in 2008 and less than 100 were completed in 2007, representing 0.18% and 0.6%, respectively. This would be further reduced based on the nature of a life sciences company and the potential lack of revenues, cash flows and the Company’s funding needs; and
|
|
g.
|
The Company's Rights Agreement makes it less attractive to a potential buyer.
|
|
Range of Probability
|
Probability
|
|
|
Low
|
0.5%
|
|
|
Medium
|
1.0%
|
|
|
High
|
5.0%
|
|
1)
|
increased Research and Development costs in 2010 of approximately $618,000 or 9% as compared to the same period in 2009;
|
|
2)
|
increased Production/Cost of Goods Sold in 2010 of approximately $757,000 or 130%; and
|
|
3)
|
increased General and Administrative expenses of approximately $1,772,000 or 31% as compared to the same period in 2009; which increases were offset by
|
|
4)
|
an increase in interest and other income in 2010 of approximately $2,316,000 or 3,457% as compared to the same period in 2009;
|
|
5)
|
a decrease in non-cash financing costs of $241,000 in 2010 as compared to the same period in 2009 primarily due to the issuance of Common Stock Purchase Warrants in 2009 as part of the February 2009 Standby Financing Agreement; and
|
|
6)
|
an adjustment at December 31, 2009 to record the change in fair value for a Liability related to certain redeemable warrants issued in May 2009. This Liability was recorded in May 2009, adjusted and revalued to $3,684,000 at December 31, 2009, resulting in a related non-cash gain of $6,258,000 in 2009. The value of this Liability at December 31, 2010 was $2,805,000. The adjustment needed at December 31, 2010 to revalue the liability resulted in a related non-cash gain of $879,000 at December 31, 2010.
|
|
1)
|
Increased Research and Development costs in 2009 of approximately $1,195,000 or 21% as compared to the same period in 2008.
|
|
2)
|
Sales of Alferon N Injection® for 2008 of approximately $173,000 compared to no sales recorded in 2009.
|
|
3)
|
Decreased interest and other income in 2009 of approximately $525,000 or 89% as compared to the same period in 2008.
|
|
4)
|
Increased non-cash financing costs of $241,000 in 2009 in the form of Common Stock Commitment Warrants issued as a result of the February 2009 implementation of the Standby Financing Agreement. No agreement of this type was in effect during 2008.
|
|
5)
|
Decreased Production/Cost of Goods Sold in 2009 of approximately $214,000 or 27% and decreased General and Administrative expenses of approximately $682,000 or 11% as compared to the same period in 2008.
|
|
6)
|
An adjustment at December 31, 2009 to record the change in fair value for a Liability related to the Warrants issued in May 2009. This Liability was recorded in May 2009, adjusted and revalued to $3,684,000 at December 31, 2009, resulting in a related non-cash gain of $6,258,000.
|
|
(dollars in thousands)
|
||||||||||||||||
|
Obligations Expiring by Period
|
||||||||||||||||
|
Contractual Cash
Obligations
|
||||||||||||||||
|
Total
|
2011
|
2012
|
2013
|
|||||||||||||
|
Operating Leases
|
$ | 265 | $ | 198 | 67 | $ | -0- | |||||||||
|
Total
|
$ | 265 | $ | 198 | $ | 67 | $ | -0- | ||||||||
|
/s/ McGladrey & Pullen, LLP
|
|
|
Blue Bell, Pennsylvania
|
|
|
March 28, 2011
|
|
Name
|
Age
|
Position
|
|
William A. Carter, M.D.
|
73
|
Chairman of the Board, Chief Executive Officer and Chief Science Officer
|
|
Thomas K. Equels
|
58
|
Executive Vice Chairman of the Board (effective June 1, 2010), Secretary and General Counsel
|
|
Richard C. Piani
|
84
|
Lead Director
|
|
William M. Mitchell, M.D., Ph.D.
|
76
|
Director
|
|
Iraj Eqhbal Kiani, N.D., Ph.D
.
|
65
|
Director
|
|
Charles T. Bernhardt, CPA
|
49
|
Chief Financial Officer and Chief Accounting Officer
|
|
David R. Strayer, M.D.
|
65
|
Chief Medical Officer and Medical Director, Regulatory Affairs
|
|
Robert Dickey IV
|
55
|
Senior Vice President
|
|
Wayne Springate
|
40
|
Vice President of Operations
|
|
Russel Lander, Ph.D.
|
61
|
Vice President of Process and Quality Assurance
|
|
Ralph C. Cavalli, Ph.D.
|
53
|
Vice President of Quality Control (effective April 15, 2010)
|
|
·
|
Leadership Experience – Chairman and CEO of Hemispherx;
|
|
·
|
Industry Experience - Knowledge of new and existing technologies, particularly as they relate to anti-viral and immune therapies;
|
|
·
|
Scientific, Legal or Regulatory Experience - M.D., co-inventor of Ampligen®, leading innovator in the development of interferon-based drugs and expertise in patent development; and
|
|
·
|
Finance Experience – Extensive knowledge of financial markets and successfully completed numerous financing efforts on behalf of Hemispherx.
|
|
·
|
Leadership Experience – President, Managing Director of Equels Law Firm;
|
|
·
|
Industry Experience –legal counsel to Hemispherx; and
|
|
·
|
Scientific, Legal or Regulatory Experience - Law degree with over 25 years as a practicing attorney specializing in litigation.
|
|
·
|
Leadership Experience – Chairman of Industrielle du Batiment-Morin, Chairman and CEO of Societe "La Cellophane";
|
|
·
|
Industry Experience - Rhone-Poulenc (now Sanofi Aventis);
|
|
·
|
Scientific, Legal or Regulatory Experience – Law degree, delegate for Industry to the City of Science and Industry; and
|
|
·
|
Finance Experience – over 40 years of diverse international business experience.
|
|
·
|
Leadership Experience – Professor at Vanderbilt University School of Medicine. He is a member of the Board of Directors for Chronix Biomedical and is Chairman of its Medical Advisory Board. Additionally, he has served on multiple governmental review committees of the National Institutes of Health, Centers for Disease Control and Prevention and for the European Union, including key roles as Chairman;
|
|
·
|
Academic and Industry Experience – Well published medical researcher with extensive investigative experience on virus and immunology issues relevant to the scientific business of Hemispherx along with being a Director of an entrepreneurial diagnostic company (Chronix Biomedical) that is involved in next generation DNA sequencing for medical diagnostics; and
|
|
·
|
Scientific, Legal or Regulatory Experience - M.D., Ph.D. and professor at a top ranked school of medicine, and inventor of record on numerous U.S. and international patents who is experienced in regulatory affairs through filings with the FDA.
|
|
·
|
Leadership Experience – former Mayor and Governor of Yasoi in Iran;
|
|
·
|
Industry Experience – Broad international network and contacts within the field of immunology;
|
|
·
|
Scientific, Legal or Regulatory Experience – N.D. and Ph.D. with trading company management experience; and
|
|
·
|
Finance Experience – over 30 years of international business experience.
|
|
·
|
Dr. William A. Carter, Chief Executive Officer (“CEO”) and Chief Science Officer (“CSO”);
|
|
·
|
Charles T. Bernhardt, Chief Financial Officer (“CFO”) & Chief Accounting Officer (“CAO”);
|
|
·
|
Thomas K. Equels, General Counsel
and Litigation Counsel
(effective June 1, 2010);
|
|
·
|
Dr. David Strayer, Chief Medical Officer (“CMO”) and Medical Director;
|
|
·
|
Robert Dickey, IV, Senior Vice President (“S.V.P.”);
|
|
·
|
Russel J. Lander,
Vice President (“V.P.”) of Process and Quality Assurance; and
|
|
·
|
Wayne S. Springate, Vice President (“V.P.”) of Operations.
|
|
Compensation Committee
|
• |
Fulfills the Board of Directors' responsibilities relating to compensation of Hemispherx’ NEO, other non-officer Executives and non-Executives.
|
|
| • |
Oversees implementation and administration of Hemispherx’ compensation and employee benefits programs, including incentive compensation and equity compensation plans.
|
||
| • |
Reviews and approves Hemispherx’ goals and objectives and, in light of these, evaluates each NEO's performance and sets his annual base salary, annual incentive opportunity, long-term incentive opportunity and any special/supplemental benefits or payments.
|
||
| • |
Reviews and approves compensation for all other non-officer Executives of Hemispherx including annual base salary, annual incentive opportunity, long-term incentive opportunity and any special/supplemental benefits or payments.
|
||
| • |
In consultation with the CEO and CFO, reviews the talent development process within Hemispherx to ensure it is effectively managed and sufficient to undertake successful succession planning.
|
||
| • |
Reviews and approves employment agreements, severance arrangements, issuances of equity compensation and change in control agreements.
|
||
|
Chairman and CEO
|
• |
Presents to the Compensation Committee the overall performance evaluation of, and compensation recommendations for, each of the NEO and other non-officer Executives.
|
|
CFO and Director of
|
• |
Reports directly or indirectly to the Chief Executive Officer.
|
|
|
Human Resources
|
|||
|
|
• |
Assists the Compensation Committee with the data for competitive pay and benchmarking purposes.
|
|
| • |
Reviews relevant market data and advises the Compensation Committee on interpretation of information, including cost of living statistics, within the framework of Hemispherx.
|
||
| • |
Informs the Compensation Committee of regulatory developments and how these may affect Hemispherx’ compensation program.
|
|
·
|
Base salary (impacted by cost of living adjustments);
|
|
·
|
Variable compensation consisting of a cash bonus based upon individual and corporate performance;
|
|
·
|
Long-term bonus incentive programs consisting of the Goal Achievement Program and Employee Bonus Pool Program; and
|
|
·
|
Stock option grants with exercise prices set at the fair market value at the time of grant.
|
|
·
|
Dr. William Carter, Chairman & CEO (bonus opportunity up to 25%);
|
|
·
|
Thomas Equels, General Counsel, Litigation Counsel, Secretary and Executive Vice Chairman of the Board (bonus opportunity up to 25%);
|
|
·
|
Charles Bernhardt, Chief Financial Officer and Chief Accounting Officer (bonus opportunity up to 25%); and
|
|
·
|
Wayne Springate, V.P. of Operations (bonus opportunity up to 20%).
|
|
1.
|
The Company-wide goals & objectives along with individual performance goals for each NEO used to determine annual bonuses for the fiscal year;
|
|
2.
|
How each goal individually or in totality was weighted, if applicable, to the extent that any of the performance goals were quantitative and/or quantitative measurable;
|
|
3.
|
The threshold, target, and maximum levels of achievement of each performance goal, if applicable;
|
|
4.
|
The intended relationship between the level of achievement of Company-wide performance goals and the amount of bonus to be awarded;
|
|
5.
|
The intended relationship between the level of achievement of each NEO’s individual performance goals and the amount of bonus to be awarded;
|
|
6.
|
The evaluation by the Committee of the level of achievement by each NEO of the Company-wide and individual performance goals applicable to him individually;
|
|
7.
|
If applicable, whether the Committee reviewed any report(s) from compensation consultant(s);
|
|
8.
|
How this level of achievement translated into the actual bonuses awarded for the 2010 fiscal year;
|
|
9.
|
The adequate disclosure of the percentage of base salary awarded in the form of an incentive bonus to each NEO as a result of their or the Company’s performance; and
|
|
10.
|
If applicable, how the Company’s compensation policies and practices relate to the Company’s risk management.
|
|
A.
|
Continued productive interaction with the FDA concerning issues necessary for approval of Ampligen® for CFS;
|
|
B.
|
FDA approval of Ampligen® for CFS and/or confirmatory clinical study;
|
|
C.
|
Continued productive interaction with the FDA concerning issues necessary for approval of Ampligen® for CFS and/or confirmatory clinical;
|
|
D.
|
The start of confirmatory clinical trial for CFS as necessary towards obtaining FDA approval;
|
|
E.
|
A country by country strategic plan for Ampligen® to be submitted and approved by the Board;
|
|
F.
|
An overall strategic plan for marketing and partners for Ampligen® and Alferon® to be submitted to the Board;
|
|
G.
|
An overall strategic plans for the marketing and partners for Alferon® to be submitted to the Board;
|
|
H.
|
Continued development of microbiological enhancement of vaccines requiring Ampligen®;
|
|
I.
|
Success in the protection of Company Intellectual Property;
|
|
J.
|
Continued development of Alferon® LDO;
|
|
K.
|
Continued development of Ampligen® for flu;
|
|
L.
|
Maintaining the overall financial strength of the Company and operations consistent with the budget; and
|
|
M.
|
Implementation of research & development partnerships.
|
|
(i)
|
Dr. William Carter, Chairman & CEO: Goals “A”, “C”, “E”, “F”, “G”, “H”, “I”, “J”, “K”, “L” and “M”;
|
|
(ii)
|
Thomas Equels, General Counsel and Litigation Counsel : Goals “E”, “F”, “G”, “I”, “L” and “M”;
|
|
(iii)
|
Charles Bernhardt, CFO & CAO: Goals “E”, “F”, “G”, “I”, “J”, “L” and “M”;
|
|
(iv)
|
Dr. David Strayer, Medical Director: Goals “A”, “C”, “E”, “F”, “G”, “H”, “I”, “J”, and “K”;
|
|
(v)
|
Russel Lander, V.P of Process and Quality Assurance: “A”, “C”, “G”, “I”, “J”, and “K”; and
|
|
(vi)
|
Wayne Springate, V.P. of Operations: Goals “E”, “F”, “G”, “H”, “I”, “J”, “K”, and “L”.
|
|
1.
|
Dr. William Carter, related to his development of strategic plans for the Company, obtaining product testing, marketing and sales partnership agreements, issuance of new U.S. Patents for Ampligen® and spearheading the successful raising of new capital through the At The Market stock program;
|
|
2.
|
Charles Bernhardt, related to his outstanding service as Chief Financial Officer in completing SEC filings, controlling the Company’s cash burn rate and enforcing budgetary requirements; and
|
|
3.
|
Thomas Equels, related to his work and efforts towards settling the various class action suits and judgment against Johannesburg Consolidated Investments (“JCI”) and former JCI officers R.B. Kebble and H.C. Buitendag.
|
|
·
|
Stock options align the interests of Executives and employees with those of the stockholders, support a pay-for-performance culture, foster employee stock ownership, and focus the management team on increasing value for the stockholders;
|
|
·
|
Stock options are performance based. All the value received by the recipient of a stock option is based on the growth of the stock price; and
|
|
·
|
Stock options help to provide a balance to the overall executive compensation program as base salary and our discretionary annual bonus program focus on short-term compensation.
|
|
·
|
William A. Carter, CEO and CSO for 500,000 shares with immediate vesting; and
|
|
·
|
Thomas K. Equels, General Counsel and Litigation Counsel for 300,000 shares with immediate vesting.
|
|
·
|
Health, vision and dental insurance;
|
|
·
|
Life insurance;
|
|
·
|
Short and long-term disability insurance; and
|
|
·
|
401(k) with company match of up to 6% of employee’s contribution.
|
|
·
|
Automobile allowance;
|
|
·
|
Reimbursement of home office and phone expenses; and
|
|
·
|
Supplementary life and disability insurance policies.
|
|
·
|
Reimbursement of home office and phone expenses; and
|
|
·
|
Supplementary life and disability insurance policies.
|
|
·
|
William A. Carter, Chairman of the Board & Chief Executive Officer;
|
|
·
|
Thomas K. Equels, Executive Vice Chairman of the Board, Secretary and General (effective June 1, 2010);
|
|
·
|
Charles T. Bernhardt, Chief Financial Officer and Chief Accounting Officer (effective December 3, 2010); and
|
|
·
|
Wayne Springate, Vice President of Operations.
|
|
Name & Principal
Position
|
Year
|
Salary /
Fees
|
Bonus
|
Stock
Awards
(5)
|
Option
Awards
(5)
|
Non-Equity
Incentive Plan
Compensation
|
Change
in
Pension
Valued
and
NQDC
Earnings
($)
|
All Other
Compensation
|
Total
|
|||||||||||||||||||||||||
|
William A. Carter
|
2010
|
$ | 951,837 | $ | 200,000 | (9) | $ | 405,083 | (17) | $ | 253,721 | (1) | $ | -0- | — | $ | 100,699 | (10) | $ | 1,911,340 | ||||||||||||||
|
Chief Executive
|
2009
|
$ | 554,105 | $ | 482,072 | (6)(7) | $ | 188,311 | (17) | $ | -0- | $ | -0- | — | $ | 76,896 | (10) | $ | 1,301,384 | |||||||||||||||
|
Officer (1)
|
2008
|
$ | 664,624 | $ | -0- | $ | -0- | $ | 316,571 | (18) | $ | -0- | — | $ | 106,094 | (10) | $ | 1,087,289 | ||||||||||||||||
|
Thomas K. Equels
|
2010
|
$ | 398,333 | $ | 250,000 | (8)(9) | $ | -0- | $ | 140,528 | (2) | $ | -0- | — | $ | 39,973 | (11) | $ | 828,834 | |||||||||||||||
|
General Counsel (2)
|
2009
|
$ | -0- | $ | -0- | $ | -0- | $ | -0- | $ | -0- | — | $ | -0- | $ | -0- | ||||||||||||||||||
|
2008
|
$ | -0- | $ | -0- | $ | -0- | $ | -0- | $ | -0- | — | $ | -0- | $ | -0- | |||||||||||||||||||
|
Charles T. Bernhardt
|
2010
|
$ | 194,133 | $ | 50,000 | (9) | $ | 117,296 | (17) | $ | 37,301 | (3) | $ | -0- | — | $ | 24,273 | (12) | $ | 423,003 | ||||||||||||||
|
Chief Financial Officer (3)
|
2009
|
$ | 134,662 | $ | 44,000 | (7) | $ | 45,334 | (17) | $ | -0- | $ | -0- | — | $ | 9,380 | (12) | $ | 233,376 | |||||||||||||||
|
2008
|
$ | -0- | $ | -0- | $ | -0- | $ | -0- | $ | -0- | — | $ | 26,000 | (12) | $ | 26,000 | ||||||||||||||||||
|
Robert Dickey (4)
|
2010
|
$ | 302,500 | $ | -0- | $ | -0- | $ | -0- | $ | -0- | — | $ | 8,232 | (13) | $ | 310,732 | |||||||||||||||||
|
Sr. Vice President
|
2009
|
$ | 152,131 | $ | -0- | $ | -0- | $ | 252,312 | (4) | $ | -0- | — | $ | 4,824 | (13) | $ | 409,267 | ||||||||||||||||
|
2008
|
$ | -0- | $ | -0- | $ | -0- | $ | -0- | $ | -0- | — | $ | -0- | $ | -0- | |||||||||||||||||||
|
David Strayer
|
2010
|
$ | 243,685 | $ | 48,737 | (9) | $ | 132,587 | (17) | $ | -0- | $ | -0- | — | $ | 13,227 | (14) | $ | 438,236 | |||||||||||||||
|
Medical Director
|
2009
|
$ | 167,484 | $ | 194,306 | (6)(7) | $ | 53,054 | (17) | $ | -0- | $ | -0- | — | $ | 3,229 | (14) | $ | 418,073 | |||||||||||||||
|
2008
|
$ | 201,389 | $ | -0- | $ | -0- | $ | 16,168 | (18) | $ | -0- | — | $ | -0- | $ | 217,557 | ||||||||||||||||||
|
Russel Lander
|
2010
|
$ | 215,380 | $ | 43,076 | (9) | $ | -0- | $ | -0- | $ | -0- | — | $ | 18,632 | (15) | $ | 277,088 | ||||||||||||||||
|
Vice President
|
2009
|
$ | 171,596 | $ | 39,160 | (7) | $ | 118,912 | (17) | $ | -0- | $ | -0- | — | $ | 9,648 | (15) | $ | 339,316 | |||||||||||||||
|
2008
|
$ | 178,000 | $ | -0- | $ | -0- | $ | -0- | $ | -0- | — | $ | 8,929 | (15) | $ | 186,929 | ||||||||||||||||||
|
Wayne Springate
|
2010
|
$ | 181,580 | $ | 36,300 | (9) | $ | 109,777 | (17) | $ | -0- | $ | -0- | — | $ | 16,507 | (16) | $ | 344,114 | |||||||||||||||
|
V.P., Operations
|
2009
|
$ | 126,250 | $ | 33,000 | (7) | $ | 42,500 | (17) | $ | -0- | $ | -0- | — | $ | 3,229 | (16) | $ | 204,979 | |||||||||||||||
|
2008
|
$ | 150,000 | $ | -0- | $ | -0- | $ | -0- | $ | -0- | — | $ | 7,354 | (16) | $ | 157,354 | ||||||||||||||||||
|
(1)
|
Dr. Carter renewed his Employment Agreements on June 11, 2010, which was amended on July 15, 2010, that granted him the Options to purchase 500,000 shares of Hemispherx common stock as an element of his Employment Agreement.
|
|
(2)
|
Mr. Equels transitioned from the role of external to internal General Counsel and Litigation Counsel effective June 1, 2010 with an Employment Agreement of June 11, 2010, which was amended on July 15, 2010, that granted him the Options to purchase 300,000 shares of Hemispherx common stock as an element of his Employment Agreement.
|
|
(3)
|
Mr. Bernhardt transitioned from the role of a contract consultant in 4
th
Quarter 2008 to Chief Financial Officer effective January 1, 2009. He entered into an Employment Agreement on December 6, 2010 and was granted the Option to purchase 100,000 shares of Hemispherx common stock as an element of his Employment Agreement.
|
|
(4)
|
Mr. Dickey joined Hemispherx effective June 11, 2009 and was granted the Options to purchase 150,000 shares of Hemispherx common stock as an element of his Employment Agreement.
|
|
(5)
|
The value was obtained using the Black-Scholes pricing model for stock based compensation in accordance with FASB ASC 718 (formerly SFAS 123R). See Note 2(j) Equity based compensation in the financial statements.
|
|
(6)
|
On May 20, 2009, our Board of Directors awarded bonuses of $300,000 to Dr. William Carter, and $150,000 to Dr. David Strayer in recognition for their accomplishment of 2008 corporate goals and objectives.
|
|
(7)
|
On February 8, 2009, our Board of Directors awarded bonuses to certain NEO and senior, non-officer Executives in recognition for their achievement towards of 2009 Company-wide and individual goals.
|
|
(8)
|
On December 6, 2010, our Board of Directors awarded an extraordinary bonus of $150,000 to Mr. Equels related to his service as external legal counsel from 2008 through May 2010.
|
|
(9)
|
On December 22, 2010, our Board of Directors awarded bonuses to certain NEO and senior, non-officer Executives in recognition for their achievement towards of 2009 Company-wide and individual goals.
|
|
(10)
|
Dr. Carter’s All Other Compensation Consists of:
|
|
2008
|
2009
|
2010
|
||||||||||
|
Life and Disability Insurance
|
$ | 66,411 | $ | 38,679 | $ | 64,707 | ||||||
|
Healthcare Insurance
|
28,586 | 28,586 | 24,139 | |||||||||
|
Company Car Expenses
|
11,097 | 9,631 | 11,853 | |||||||||
|
401(k) matching funds
|
-0- | -0- | -0- | |||||||||
| $ | 106,094 | $ | 76,896 | $ | 100,699 | |||||||
|
(11)
|
Mr. Equels’ All Other Compensation consists of:
|
|
2008
|
2009
|
2010
|
||||||||||
|
Life and Disability Insurance
|
$ | -0- | $ | -0- | $ | 34,140 | ||||||
|
Healthcare Insurance
|
-0- | -0- | 5,833 | |||||||||
|
401(k) matching funds
|
-0- | -0- | -0- | |||||||||
| $ | -0- | $ | -0- | $ | 39,973 | |||||||
|
(12)
|
Mr. Bernhardt’s All Other Compensation consists of:
|
|
2008
|
2009
|
2010
|
||||||||||
|
Life and Disability Insurance
|
$ | -0- | $ | -0- | $ | -0- | ||||||
|
Healthcare Insurance
|
-0- | 9,380 | 9,985 | |||||||||
|
Company Common Stock
|
26,000 | -0- | -0- | |||||||||
|
401(k) matching funds
|
-0- | -0- | 14,288 | |||||||||
| $ | 26,000 | $ | 9,380 | $ | 24,273 | |||||||
|
(13)
|
Mr. Dickey’s All Other Compensation consists of:
|
|
2008
|
2009
|
2010
|
||||||||||
|
Life and Disability Insurance
|
$ | -0- | $ | -0- | $ | -0- | ||||||
|
Healthcare Insurance
|
-0- | 4,824 | 8,232 | |||||||||
|
401(k) matching funds
|
-0- | -0- | -0- | |||||||||
| $ | -0- | $ | 4,824 | $ | 8,232 | |||||||
|
(14)
|
Dr. Strayer’s All Other Compensation consists of:
|
|
2008
|
2009
|
2010
|
||||||||||
|
Life and Disability Insurance
|
$ | -0- | $ | -0- | $ | -0- | ||||||
|
Healthcare Insurance
|
-0- | 3,229 | 3,727 | |||||||||
|
401(k) matching funds
|
-0- | -0- | 9,500 | |||||||||
| $ | -0- | $ | 3,229 | $ | 13,227 | |||||||
|
(15)
|
Dr. Lander’s All Other Compensation consists of:
|
|
2008
|
2009
|
2010
|
||||||||||
|
Life and Disability Insurance
|
$ | -0- | $ | -0- | $ | -0- | ||||||
|
Healthcare Insurance
|
8,929 | 9,648 | 3,360 | |||||||||
|
401(k) matching funds
|
-0- | -0- | 15,272 | |||||||||
| $ | 8,929 | $ | 9,648 | $ | 18,632 | |||||||
|
(16)
|
Mr. Springate’s All Other Compensation consists of:
|
|
2008
|
2009
|
2010
|
||||||||||
|
Life and Disability Insurance
|
$ | -0- | $ | -0- | $ | -0- | ||||||
|
Healthcare Insurance
|
3,229 | 3,229 | 3,637 | |||||||||
|
401(k) matching funds
|
4,125 | -0- | 12,870 | |||||||||
| $ | 7,354 | $ | 3,229 | $ | 16,507 | |||||||
|
(17)
|
Hemispherx’ “Employee Wage Or Hours Reduction Program” allowed an individual to elected a 50% reduction in salary/fees which would them to be eligible for an incentive award of three times the value of Stock based on the average NYSE Amex closing value of the stock during the respective months of January through May, 2009. The value was obtained using the Black-Scholes pricing model for stock based compensation in accordance with FASB ASC 718 (formerly SFAS 123R).
|
|
(18)
|
Issue of options for options previously granted that expired unexercised.
|
|
Name
|
Grant Date
(3)(5)
|
Estimated Future Payouts Under
Non-Equity Incentive Plan
Awards(1)
|
Estimated Future Payouts
Under Equity Incentive Plan
Awards
|
All
Other
Stock
Awards:
Number
of
Shares
of Stock
or Units
(#)
|
All Other
Option
Awards:
Number of
Securities
of
Underlying
Options
(#)(3)
|
Exercise
or Base
Price of
Option
Awards
($/Sh)
|
Grant Date
Fair Value
of Stock
and
Option
Awards
($)
|
||||||||||||||||||||||||||||||||||||
|
Threshold
($)
|
Target
($)
|
Maximum
($)
|
Threshold
($)
|
Target
($)(4)
|
Maximum
($)
|
||||||||||||||||||||||||||||||||||||||
|
William A. Carter,
|
— | 164,803 | 206,004 | — | — | — | — | — | $ | — | — | ||||||||||||||||||||||||||||||||
|
Chief Executive Officer
|
06/11/10
|
186,505 | 500,000 | 0.66 | 253,721 | ||||||||||||||||||||||||||||||||||||||
|
Thomas K. Equels,
|
— | 82,402 | 103,002 | — | — | — | — | — | $ | — | — | ||||||||||||||||||||||||||||||||
|
General Counsel
|
06/11/10
|
111,903 | 300,000 | 0.66 | 140,528 | ||||||||||||||||||||||||||||||||||||||
|
Charles T. Bernhardt,
|
— | 41,203 | 51,504 | — | — | — | — | — | $ | — | — | ||||||||||||||||||||||||||||||||
|
Chief Financial Officer
|
12/06/10
|
25,000 | (2) | 25,000 | (2) | 100,000 | 0.55 | 37,301 | |||||||||||||||||||||||||||||||||||
|
Robert Dickey,
|
N/A | — | 60,500 | 75,625 | — | — | — | — | — | $ | — | — | |||||||||||||||||||||||||||||||
|
Senior Vice President
|
$ | ||||||||||||||||||||||||||||||||||||||||||
|
David Strayer,
|
N/A | — | 50,199 | 62,749 | — | — | — | — | — | $ | — | — | |||||||||||||||||||||||||||||||
|
Medical Director
|
|||||||||||||||||||||||||||||||||||||||||||
|
Russel Lander,
|
N/A | — | 44,371 | 55,464 | — | — | — | — | — | $ | — | — | |||||||||||||||||||||||||||||||
|
Vice President
|
|||||||||||||||||||||||||||||||||||||||||||
|
Wayne Springate,
|
N/A | — | 37,392 | 46,740 | — | — | — | — | — | $ | — | — | |||||||||||||||||||||||||||||||
|
Vice President
|
|||||||||||||||||||||||||||||||||||||||||||
|
(1)
|
For 2010 or 2011, the Compensation Committee did not establish or estimate possible future payouts to the NEO under a Cash Bonus Plan. All Bonuses are at the discretion of the Compensation Committee. Utilizing existing Employment Agreements as a benchmark and the respective employees’ Base Salary at December 31, 2010, the “Target” was estimated at 20% of the Base Salary and “Maximum” estimated at 25% of Base Salary. Details regarding all of which reported as Non-Equity Incentive Plan Compensation in the 2010 is reported in the Summary Compensation Table above.
|
|
(2)
|
Consists of an extraordinary bonus granted Mr. Bernhardt on March 3, 2011.
|
|
(3)
|
Consists of stock options granted during 2010 under our 2009 Equity Incentive Plan. The stock options have a ten-year term and an exercise price equal to 110% of the closing market price of the our common stock on the date of grant. The value was obtained using the Black-Scholes pricing model for stock based compensation in accordance with FASB ASC 718 (formerly SFAS 123R).
|
|
(4)
|
Consists of stock options contractually required per the employee’s respective Employment Agreement to be granted during 2011 under our 2009 Equity Incentive Plan. The stock options have a ten-year term and an exercise price equal to 110% of the closing market price of the our common stock on the date of grant. For the purpose of this schedule, a NYSE Amex closing price at December 31, 2010 of $0.49 was assumed with an estimated exercise price of $0.54. The value was obtained using the Black-Scholes pricing model for stock based compensation in accordance with FASB ASC 718 (formerly SFAS 123R).
|
|
(5)
|
N/A represents Not Applicable.
|
|
Option Awards
|
Stock Awards
|
|||||||||||||||||||||||||||||||||||
|
Name
|
Number of
Securities
Underlying
Unexercised
Options (#)
Exercisable
|
Number of
Securities
Underlying
Unexercised
Options (#)
Unexercisable
|
Equity
Incentive Plan
Awards:
Number of
Securities
Underlying
Unexercised
Unearned
Options (#)
|
Option
Exercise
Price
($)
|
Option
Expiration
Date
|
Number of
Shares or
Units of
Stock
That Have
Not
Vested (#)
|
Market Value
of Shares or
Units of Stock
That Have Not
Vested ($)
|
Equity
Incentive
Plan
Awards:
Number
of
Unearned
Shares,
Units or
Other
Rights
That
Have Not
Vested (#)
|
Equity
Incentive
Plan Awards:
Market or
Payout Value
of Unearned
Shares, Units
or Other
Rights that
Have Not
Vested (#)
|
|||||||||||||||||||||||||||
|
William A. Carter
|
1,450,000 | 0 | 0 | 2.20 | ||||||||||||||||||||||||||||||||
|
Chief Executive Officer
|
1,000,000 | 0 | 0 | 2.00 |
09/9/17
|
|||||||||||||||||||||||||||||||
| 190,000 | 0 | 0 | 4.00 |
02/18/18
|
||||||||||||||||||||||||||||||||
| 73,728 | 0 | 0 | 2.71 |
12/12/20
|
||||||||||||||||||||||||||||||||
| 10,000 | 0 | 0 | 4.03 |
01/3/11
|
||||||||||||||||||||||||||||||||
| 167,000 | 0 | 0 | 2.60 |
09/7/14
|
||||||||||||||||||||||||||||||||
| 153,000 | 0 | 0 | 2.60 |
012/7/14
|
||||||||||||||||||||||||||||||||
| 100,000 | 0 | 0 | 1.75 |
04/26/15
|
||||||||||||||||||||||||||||||||
| 465,000 | 0 | 0 | 1.86 |
06/30/15
|
||||||||||||||||||||||||||||||||
| 70,000 | 0 | 0 | 2.87 |
12/9/15
|
||||||||||||||||||||||||||||||||
| 300,000 | 0 | 0 | 2.38 |
01/1/16
|
||||||||||||||||||||||||||||||||
| 10,000 | 0 | 0 | 2.61 |
12/8/15
|
||||||||||||||||||||||||||||||||
| 376,650 | 0 | 0 | 3.78 |
02/22/16
|
||||||||||||||||||||||||||||||||
| 1,400,000 | 0 | 0 | 3.50 |
09/30/17
|
||||||||||||||||||||||||||||||||
| 500,000 | 0 | 0 | 0.66 |
06/11/20
|
||||||||||||||||||||||||||||||||
|
Thomas K. Equels
|
300,000 | 0 | 0 | 0.660 |
06/11/20
|
|||||||||||||||||||||||||||||||
|
General Counsel
|
||||||||||||||||||||||||||||||||||||
|
Charles Bernhardt
|
100,000 | 0 | 0 | 0.55 |
12/06/19
|
|||||||||||||||||||||||||||||||
|
Chief Financial Officer
|
||||||||||||||||||||||||||||||||||||
|
Robert Dickey,
|
56,250 | 93,750 | 0 | 2.55 |
06/11/19
|
|||||||||||||||||||||||||||||||
|
Sr. Vice President
|
||||||||||||||||||||||||||||||||||||
|
David Strayer,
|
50,000 | 0 | 0 | 2.00 |
09/09/17
|
|||||||||||||||||||||||||||||||
|
Medical
Director
|
50,000 | 0 | 0 | 4.00 |
02/28/18
|
|||||||||||||||||||||||||||||||
|
|
10,000 | 0 | 0 | 4.03 |
01/03/11
|
|||||||||||||||||||||||||||||||
| 20,000 | 0 | 0 | 2.37 |
01/23/17
|
||||||||||||||||||||||||||||||||
| 10,000 | 0 | 0 | 1.90 |
12/07/14
|
||||||||||||||||||||||||||||||||
| 10,000 | 0 | 0 | 2.61 |
12/08/15
|
||||||||||||||||||||||||||||||||
| 15,000 | 0 | 0 | 2.20 |
11/20/16
|
||||||||||||||||||||||||||||||||
| 25,000 | 0 | 0 | 1.30 |
12/06/17
|
||||||||||||||||||||||||||||||||
|
Russel Lander
|
150,000 | 0 | 0 | 1.30 |
12/06/14
|
|||||||||||||||||||||||||||||||
|
Vice President
|
||||||||||||||||||||||||||||||||||||
|
Wayne Springate,
|
1,812 | 0 | 0 | 1.90 |
12/07/14
|
|||||||||||||||||||||||||||||||
|
Vice President
|
2,088 | 0 | 0 | 2.61 |
12/08/15
|
|||||||||||||||||||||||||||||||
| 5,000 | 0 | 0 | 2.20 |
11/20/16
|
||||||||||||||||||||||||||||||||
| 20,000 | 0 | 0 | 1.78 |
04/30/17
|
||||||||||||||||||||||||||||||||
| 20,000 | 0 | 0 | 1.30 |
12/06/17
|
||||||||||||||||||||||||||||||||
|
Option Awards
|
Stock Awards
|
|||||||||||||||
|
Name and Principal Position
|
Number of Shares
Acquired on Exercise (#)
|
Value Realized on
Exercise ($)
|
Number of Shares
Acquired on Vesting (#)
|
Value Realized
on Vesting ($)
|
||||||||||||
|
William A. Carter,
|
— | — | — | — | ||||||||||||
|
Chief Executive Officer
|
||||||||||||||||
|
Thomas K. Equels, ,
|
— | — | — | — | ||||||||||||
|
General Counsel
|
||||||||||||||||
|
Charles T. Bernhardt,
|
— | — | — | — | ||||||||||||
|
Chief Financial Officer
|
||||||||||||||||
|
Robert Dickey,
|
— | — | — | — | ||||||||||||
|
Senior Vice President
|
||||||||||||||||
|
David Strayer,
|
— | — | — | — | ||||||||||||
|
Medical Director
|
||||||||||||||||
|
Russel Lander,
|
— | — | — | — | ||||||||||||
|
Vice President
|
||||||||||||||||
|
Wayne Springate,
|
— | — | — | — | ||||||||||||
|
VP, Operations
|
||||||||||||||||
|
Name
|
Event
|
Cash
Severance
($)
|
Value of
Stock
Awards
That
Will
Become
Vested (1) ($)
|
Continuation
of
Medical
Benefits
(2) ($)
|
Additional
Life
Insurance
(3) ($)
|
Total
($)
|
||||||||||||||||
|
William A. Carter
|
Involuntary (no cause)
|
4,120,080 | 932,525 | 106,301 | 337,928 | 5,496,832 | ||||||||||||||||
|
Chief Executive Officer
|
Termination (for cause)
|
— | — | — | — | — | ||||||||||||||||
|
Death or disability
|
824,016 | 186,505 | 21,260 | 67,586 | 1,099,367 | |||||||||||||||||
|
Termination by employee or retirement
|
824,016 | 186,505 | 21,260 | 67,586 | 1,099,367 | |||||||||||||||||
|
Thomas K. Equels
|
Involuntary (no cause)
|
2,060,040 | 559,515 | 29,163 | 170,700 | 2,819,418 | ||||||||||||||||
|
General Counsel
|
Termination (for cause)
|
— | — | — | — | — | ||||||||||||||||
|
Death or disability
|
412,008 | 111,903 | 5,833 | 34,140 | 563,884 | |||||||||||||||||
|
Termination by employee or retirement
|
412,008 | 111,903 | 5,833 | 34,140 | 563,844 | |||||||||||||||||
|
Charles T. Bernhardt
|
Involuntary (no cause)
|
206,015 | — | 6,662 | 3,323 | 216,000 | ||||||||||||||||
|
Chief Financial Officer
|
Termination (for cause)
|
— | — | — | — | — | ||||||||||||||||
|
Death or disability
|
206,015 | — | 6,662 | 3,323 | 216,000 | |||||||||||||||||
|
Termination by employee or retirement
|
206,015 | — | 6,662 | 3,323 | 216,000 | |||||||||||||||||
|
Robert Dickey
|
Involuntary (no cause)
|
25,208 | — | 567 | 119 | 25,894 | ||||||||||||||||
|
Senior Vice President
|
Termination (for cause)
|
25,208 | — | 567 | 119 | 25,894 | ||||||||||||||||
|
Death or disability
|
— | — | — | — | — | |||||||||||||||||
|
Termination by employee or retirement
|
25,208 | — | 567 | 119 | 25,894 | |||||||||||||||||
|
David Strayer
|
Involuntary (no cause)
|
— | — | — | — | — | ||||||||||||||||
|
Medical Director
|
Termination (for cause)
|
— | — | — | — | — | ||||||||||||||||
|
Death or disability
|
— | — | — | — | — | |||||||||||||||||
|
Termination by employee or retirement
|
— | — | — | — | — | |||||||||||||||||
|
Russel Lander
|
Involuntary (no cause)
|
— | — | — | — | — | ||||||||||||||||
|
Vice President
|
Termination (for cause)
|
— | — | — | — | — | ||||||||||||||||
|
Death or disability
|
— | — | — | — | — | |||||||||||||||||
|
Termination by employee or retirement
|
— | — | — | — | ||||||||||||||||||
|
Wayne Springate
|
Involuntary (no cause)
|
67,820 | — | 124 | 1,088 | 69,032 | ||||||||||||||||
|
Vice President
|
Termination (for cause)
|
— | — | — | — | — | ||||||||||||||||
|
Death or disability
|
— | — | — | — | — | |||||||||||||||||
|
Termination by employee or retirement
|
— | — | — | — | — | |||||||||||||||||
|
(1)
|
Consists of stock options contractually required per the employee’s respective Employment Agreement to be granted during each calendar year of the term under our 2009 Equity Incentive Plan. The stock options have a ten-year term and an exercise price equal to 110% of the closing market price of the our common stock on the date of grant. For the purpose of this schedule, a NYSE Amex closing price of $0.49 was assumed with an estimated exercise price of $0.54. The value was obtained using the Black-Scholes pricing model for stock based compensation in accordance with FASB ASC 718 (formerly SFAS 123R).
|
|
(2)
|
This amount reflects the current premium incremental cost to us for continuation of elected benefits to the extent required under an applicable agreement.
|
|
(3)
|
The life insurance benefit represents life insurance paid for by us including the standard coverage.
|
|
Name
|
Aggregate
Severance Pay
($)
|
PVSU
Acceleration
(4) ($)
|
Early
Vesting
of
Restricted
Stock (5) ($)
|
Early
Vesting
of Stock
Options
and SARs
(5) ($)
|
Acceleration
and
Vesting of
Supplemental
Award (6) ($)
|
Welfare
Benefits
Continuation
(7) ($)
|
Outplacement
Assistance
($)
|
Parachute
Tax
Gross-up
Payment
($)
|
Total
($)
|
|||||||||||||||||||||||||||
|
William
A. Carter
|
4,120,128 | (1) | -0- | -0- | -0- | 1,492,040 | 886,766 | (1)(8) | -0- | -0- | 6,498,934 | |||||||||||||||||||||||||
|
Thomas K. Equels
|
3,296,064 | (1) | -0- | -0- | -0- | 895,224 | 495,781 | (1)(8) | -0- | -0- | 4,687,069 | |||||||||||||||||||||||||
|
Charles T. Bernhardt
|
824,064 | (1) | -0- | -0- | -0- | -0- | 127,940 | (1)(8) | -0- | -0- | 952,004 | |||||||||||||||||||||||||
|
Robert Dickey
|
25,208 | (2) | -0- | -0- | -0- | (5) | -0- | 47 | (2) | -0- | -0- | 60,225 | ||||||||||||||||||||||||
|
David Strayer
|
-0- | -0- | -0- | -0- | -0- | -0- | -0- | -0- | -0- | |||||||||||||||||||||||||||
|
Russel Lander
|
-0- | -0- | -0- | -0- | -0- | -0- | -0- | -0- | -0- | |||||||||||||||||||||||||||
|
Wayne Springate
|
67,820 | (3) | -0- | -0- | -0- | -0- | 41 | (3) | -0- | -0- | 67,861 | |||||||||||||||||||||||||
|
(1)
|
This amount represents the base salary or benefits for remaining term of the NEO’s employment agreement plus a three year extension in the occurrence of termination from a change in control.
|
|
(2)
|
This amount represents the one-twelfth of base salary or benefits for the NEO.
|
|
(3)
|
This amount represents the four-twelfth of base salary or benefits for the NEO.
|
|
(4)
|
This amount represents the payout of all outstanding performance-vesting share units (“PVSU”) awards on a change in control at the target payout level with each award then pro-rated based on the time elapsed for the applicable three-year performance period.
|
|
(5)
|
This amount is the intrinsic value [fair market value on December 31, 2010 ($0.49 per share) minus the per share exercise price] of all unvested stock options for each NEO, including Stock Appreciation Rights (“SAR”). Any option with an exercise price of greater than fair market value was assumed to be cancelled for no consideration and, therefore, had no intrinsic value.
|
|
(6)
|
This amount represents the options to be issued annually for remaining term of the NEO’s employment agreement plus a three year extension in the occurrence of termination from a change in control. The calculation was based on a NYSE Amex closing price for December 31, 2010 of $0.49 with an estimated exercise price of $0.54. The value was obtained using the Black-Scholes pricing model for stock based compensation in accordance with FASB ASC 718 (formerly SFAS 123R).
|
|
(7)
|
This amount represents the employer-paid portion of the premiums for medical, dental and life insurance coverage.
|
|
(8)
|
This amount also includes the estimated cost of Company matching 401(k) contributions of $22,000 per year.
|
|
|
·
|
Any person or entity other than Hemispherx, any of our current directors or officers or a trustee or fiduciary holding our securities, becomes the beneficial owner of more than 50% of the combined voting power of our outstanding securities;
|
|
|
·
|
An acquisition, sale, merger or other transaction that results in a change in ownership of more than 50% of the combined voting power of our stock or the sale/transfer of more than 75% of our assets;
|
|
|
·
|
A change in the majority of our Board of Directors over a two-year period that is not approved by at least two-thirds of the directors then in office who were directors at the beginning of the period; or
|
|
|
·
|
Execution of an agreement with Hemispherx, which if consummated, would result in any of the above events.
|
|
|
·
|
Significantly reducing or diminishing the nature or scope of the executive’s authority or duties;
|
|
|
·
|
Materially reducing the executive’s annual salary or incentive compensation opportunities;
|
|
|
·
|
Changing the executive’s office location so that he must commute more than 50 miles, as compared to his commute as of the date of the agreement;
|
|
|
·
|
Failing to provide substantially similar fringe benefits, or substitute benefits that were substantially similar taken as a whole, to the benefits provided as of the date of the agreement; or
|
|
|
·
|
Failing to obtain a satisfactory agreement from any successor to Hemispherx to assume and agree to perform the obligations under the agreement.
|
|
|
·
|
Fails to give us written notice of his intention to claim constructive termination and the basis for that claim at least 10 days in advance of the effective date of the executive’s resignation; or
|
|
|
·
|
We cure the circumstances giving rise to the constructive termination before the effective date of the executive’s resignation.
|
|
Name and
Title
|
Fees
Earned
or Paid
in
Cash
($)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-
Equity
Incentive
Plan
Compen-
sation
($)
|
Change in
pension
Value
and
Nonqualified
Deferred
Compensation
Earnings ($)
|
All Other
Compen-
sation
($)
|
Total
($)
|
|||||||||||||||||||||
|
W. Carter, Chairman & Chief Executive Officer
|
165,000 | 0 | 253,721 | (1) | 0 | 0 | 1,492,619 | (2) | 1,911,340 | |||||||||||||||||||
|
T. Equels, Executive Vice Chairman, Secretary & General Counsel
|
165,000 | 0 | 140,528 | (3) | 0 | 0 | 523,306 | (4) | 828,837 | |||||||||||||||||||
|
W. Mitchell, Director
|
165,000 | 0 | 0 | 0 | 0 | 0 | 165,000 | |||||||||||||||||||||
|
R. Piani, Director
|
165,000 | 0 | 0 | 0 | 0 | 0 | 165,000 | |||||||||||||||||||||
|
I. Kiani, Director
|
165,000 | 0 | 0 | 0 | 0 | 0 | 165,000 | |||||||||||||||||||||
|
|
(1)
|
Ten year Option to purchase 500,000 shares at $0.66 per share awarded consistent with Employment Agreement of June 11, 2010. The value was obtained using the Black-Scholes pricing model for stock based compensation in accordance with FASB ASC 718 (formerly SFAS 123R).
|
|
|
(2)
|
Chief Executive Officer salary and Consultant fees paid consistent with Engagement Agreements in effect during 2010 along the year-end performance bonus for 2010.
|
|
|
(3)
|
Ten year Option to purchase 300,000 shares at $0.66 per share awarded consistent with Employment Agreement of June 11, 2010. The value was obtained using the Black-Scholes pricing model for stock based compensation in accordance with FASB ASC 718 (formerly SFAS 123R).
|
|
|
(4)
|
General Counsel salary and Consultant fees paid consistent with Engagement Agreements in effect June 1, 2010 along the performance bonus in 2010 of $150,000 for services prior to employment and year-end performance bonus for 2010.
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
|
|
|
·
|
Each person, individually or as a group, known to us to be deemed the beneficial owners of five percent or more of our issued and outstanding common stock;
|
|
|
·
|
Each of our directors and the Named Executives; and
|
|
|
·
|
All of our officers and directors as a group.
|
|
Name and Address of
Beneficial Owner
|
Shares Beneficially
Owned
|
% Of Shares
Beneficially Owned
|
||||||
|
William A. Carter, M.D.
|
7,637,159 | (1)(2) | 5.65 | % | ||||
|
Thomas K. Equels
|
1,728,622 | (3) | 1.28 | % | ||||
|
Richard C. Piani
97 Rue Jeans-Jaures
Levaillois-Perret, France 92300
|
757,420 | (4) | * | |||||
|
William M. Mitchell, M.D.
Vanderbilt University
Department of Pathology
Medical Center North
21
st
and Garland
Nashville, TN 37232
|
616,025 | (5) | * | |||||
|
Iraj Eqhbal Kiani, N.D., Ph.D.
Orange County Immune Institute
18800 Delaware Street
Huntingdon Beach, CA 92648
|
323,271 | (6) | * | |||||
|
Charles T. Bernhardt CPA
|
277,420 | (7) | * | |||||
|
David R. Strayer, M.D.
|
410,932 | (8) | * | |||||
|
Wayne Springate
|
152,421 | (9) | * | |||||
|
Robert Dickey, IV
|
152,500 | (10) | * | |||||
|
Russel Lander, Ph.D.
|
168,073 | (11) | * | |||||
|
Ralph C. Cavalli, Ph.D.
|
20,000 | (12) | * | |||||
|
All directors and executive officers as a group (11 persons)
|
12,243,843 | 9.06 | % | |||||
|
(1)
|
Dr. Carter is our Chairman and Chief Executive Officer. He owns 889,570 shares of common stock and beneficially owns 6,746,574 shares issuable or issued upon exercise of:
|
|
Date
|
Exercise
|
Number
|
Expiration
|
||||||||||
|
Plan
|
Issued
|
Price
|
Of Shares
|
Date
|
|||||||||
|
Options
|
|||||||||||||
|
|
2009
|
12/22/10
|
$ | 2.71 | 73,728 |
12/22/20
|
|||||||
|
2004
|
09/08/04
|
$ | 2.60 | 167,000 |
09/07/14
|
||||||||
|
2004
|
12/07/04
|
$ | 2.60 | 153,000 |
12/07/14
|
||||||||
|
2004
|
04/26/05
|
$ | 1.75 | 100,000 |
04/26/15
|
||||||||
|
2004
|
07/01/05
|
$ | 1.86 | 465,000 |
06/30/15
|
||||||||
|
2004
|
12/09/05
|
$ | 2.61 | 10,000 |
12/08/15
|
||||||||
|
2004
|
12/09/05
|
$ | 2.87 | 70,000 |
12/09/15
|
||||||||
|
2004
|
01/01/06
|
$ | 2.38 | 300,000 |
01/01/16
|
||||||||
|
2004
|
02/22/06
|
$ | 3.78 | 376,650 |
02/22/16
|
||||||||
|
2004
|
09/10/07
|
$ | 2.00 | 1,000,000 |
09/09/17
|
||||||||
|
2004
|
10/01/07
|
$ | 3.50 | 1,400,000 |
09/30/17
|
||||||||
|
2004
|
02/18/08
|
$ | 4.00 | 190,000 |
02/18/18
|
||||||||
|
2007
|
09/17/08
|
$ | 2.20 | 1,450,000 |
09/17/18
|
||||||||
|
2009
|
06/11/10
|
$ | 0.66 | 500,000 |
06/11/20
|
||||||||
|
Total Options
|
6,255,378 | ||||||||||||
|
Warrants
|
|||||||||||||
|
Total Warrants
|
2009
|
02/1/09
|
$ | 0.51 | 491,196 |
02/01/19
|
|||||||
|
(2)
|
Dr. Kovari is the spouse of Dr. Carter and accordingly all shares owned by each are deemed to be beneficially owned by the other. She owns 1,015 shares of common stock.
|
|
(3)
|
Mr. Equels is Executive Vice Chairman of our Board of Directors, Secretary and General Counsel who owns 937,426 shares of common stock and beneficially owns 791,196 shares issuable or issued upon exercise of:
|
|
Date
|
Exercise
|
Number
|
Expiration
|
||||||||||
|
Options
|
Plan
|
Issued
|
Price
|
Of Shares
|
Date
|
||||||||
|
Total Options
|
2009
|
06/11/10
|
$ | 0.66 | 300,000 |
06/11/20
|
|||||||
|
Date
|
Exercise
|
Number
|
Expiration
|
||||||||||
|
Warrants
|
Plan
|
Issued
|
Price
|
Of Shares
|
Date
|
||||||||
|
Total Warrants
|
2009
|
02/1/09
|
$ | 0.51 | 491,196 |
02/01/19
|
|||||||
|
(4)
|
Mr. Piani is a member of our Board of Directors who owns 432,812 shares of common stock and beneficially owns 324,608 shares issuable upon exercise of:
|
|
Date
|
Exercise
|
Number
|
Expiration
|
||||||||||
|
Options
|
Plan
|
Issued
|
Price
|
Of Shares
|
Date
|
||||||||
|
2004
|
09/08/04
|
$ | 2.60 | 54,608 |
09/07/14
|
||||||||
|
2004
|
04/26/05
|
$ | 1.75 | 100,000 |
04/26/15
|
||||||||
|
2004
|
02/24/06
|
$ | 3.86 | 50,000 |
02/24/16
|
||||||||
|
2004
|
09/10/07
|
$ | 2.00 | 100,000 |
09/09/17
|
||||||||
|
2004
|
02/18/08
|
$ | 4.00 | 20,000 |
02/18/18
|
||||||||
|
Total Options
|
324,608 | ||||||||||||
|
(5)
|
Dr. Mitchell is a member of our Board of Directors that owns 304,025 shares of common stock and beneficially owns 312,000 shares issuable upon exercise of:
|
|
Date
|
Exercise
|
Number
|
Expiration
|
||||||||||
|
Options
|
Plan
|
Issued
|
Price
|
Of Shares
|
Date
|
||||||||
|
2004
|
09/08/04
|
$ | 2.60 | 50,000 |
09/07/14
|
||||||||
|
2004
|
04/26/05
|
$ | 1.75 | 100,000 |
04/26/15
|
||||||||
|
2004
|
02/24/06
|
$ | 3.86 | 50,000 |
02/24/16
|
||||||||
|
2004
|
09/10/07
|
$ | 2.00 | 100,000 |
09/09/17
|
||||||||
|
2004
|
09/17/08
|
$ | 6.00 | 12,000 |
09/17/18
|
||||||||
|
Total Options
|
312,000 | ||||||||||||
|
(6)
|
Dr. Kiani is a member of our Board of Directors who owns 246,271 shares of common stock and beneficially owns 77,000 shares issuable upon exercise of:
|
|
Date
|
Exercise
|
Number
|
Expiration
|
||||||||||
|
Options
|
Plan
|
Issued
|
Price
|
Of Shares
|
Date
|
||||||||
|
2004
|
04/26/05
|
$ | 1.75 | 15,000 |
04/26/15
|
||||||||
|
2004
|
06/02/05
|
$ | 1.63 | 12,000 |
06/30/15
|
||||||||
|
2004
|
02/24/06
|
$ | 3.86 | 50,000 |
02/24/16
|
||||||||
|
Total Options
|
77,000 | ||||||||||||
|
(7)
|
Charles T. Bernhardt is our Chief Financial Officer and owns 177,420 shares of common stock and beneficially owns 100,000 shares issuable upon exercise of:
|
|
Date
|
Exercise
|
Number
|
Expiration
|
||||||||||
|
Options
|
Plan
|
Issued
|
Price
|
Of Shares
|
Date
|
||||||||
|
Total Options
|
2009
|
12/06/09
|
$ | 0.55 | 100,000 |
12/06/19
|
|||||||
|
(8)
|
Dr. Strayer is our Medical Director that has ownership of 230,932 shares of common stock and beneficially owns 180,000 shares issuable upon exercise of:
|
|
Date
|
Exercise
|
Number
|
Expiration
|
||||||||||
|
Plan
|
Issued
|
Price
|
Of Shares
|
Date
|
|||||||||
|
Options
|
|||||||||||||
|
2004
|
12/07/04
|
$ | 1.90 | 10,000 |
12/07/14
|
||||||||
|
2004
|
12/09/05
|
$ | 2.61 | 10,000 |
12/08/15
|
||||||||
|
2004
|
11/20/06
|
$ | 2.20 | 15,000 |
11/20/16
|
||||||||
|
2004
|
01/23/07
|
$ | 2.37 | 20,000 |
01/23/17
|
||||||||
|
2004
|
09/10/07
|
$ | 2.00 | 50,000 |
09/09/17
|
||||||||
|
2004
|
12/06/07
|
$ | 1.30 | 25,000 |
12/06/17
|
||||||||
|
2004
|
02/18/08
|
$ | 4.00 | 50,000 |
09/18/18
|
||||||||
|
Total Options
|
180,000 | ||||||||||||
|
(9)
|
Mr. Springate is our Vice President of Operations who owns 103,521 shares of common stock and beneficially owns 48,900 shares issuable upon exercise of:
|
|
Date
|
Exercise
|
Number
|
Expiration
|
||||||||||
|
Plan
|
Issued
|
Price
|
Of Shares
|
Date
|
|||||||||
|
Options
|
|||||||||||||
|
2004
|
12/07/04
|
$ | 1.90 | 1,812 |
12/07/14
|
||||||||
|
2004
|
12/09/05
|
$ | 2.61 | 2,088 |
12/08/15
|
||||||||
|
2004
|
11/20/06
|
$ | 2.20 | 5,000 |
11/20/16
|
||||||||
|
2004
|
05/01/07
|
$ | 1.78 | 20,000 |
09/09/17
|
||||||||
|
2004
|
12/06/07
|
$ | 1.30 | 20,000 |
12/06/17
|
||||||||
|
Total Options
|
48,900 | ||||||||||||
|
(10)
|
Mr. Dickey is our Senior Vice President and owns 2,500 shares of common stock and beneficially owns 150,000 shares issuable upon exercise of:
|
|
Date
|
Exercise
|
Number
|
Expiration
|
||||||||||
|
Options
|
Plan
|
Issued
|
Price
|
Of Shares
|
Date
|
||||||||
|
Total Options
|
2009
|
07/01/09
|
$ | 2.81 | 150,000 |
07/01/19
|
|||||||
|
(11)
|
Dr. Lander is our Vice President of Quality Assurance who owns 153,073 shares of common stock and beneficially owns 15,000 shares issuable upon exercise of:
|
|
Date
|
Exercise
|
Number
|
Expiration
|
||||||||||
|
Options
|
Plan
|
Issued
|
Price
|
Of Shares
|
Date
|
||||||||
|
Total Options
|
2004
|
12/06/07
|
$ | 1.30 | 15,000 |
12/06/17
|
|||||||
|
(12)
|
Dr. Ralph C. Cavalli is our Vice President of Quality Control who beneficially owns 20,000 shares issuable upon exercise of:
|
|
Date
|
Exercise
|
Number
|
Expiration
|
||||||||||
|
Options
|
Plan
|
Issued
|
Price
|
Of Shares
|
Date
|
||||||||
|
Total Options
|
2009
|
06/11/10
|
$ | 0.66 | 20,000 |
06/11/20
|
|||||||
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence.
|
|
ITEM 14.
|
Principal Accountant Fees and Services.
|
|
Amount ($)
|
||||||||
|
Description of Fees:
|
2010* | 2009* | ||||||
|
Audit Fees
|
$ | 270,000 | $ | 413,000 | ||||
|
Audit-Related Fees
|
34,000 | -0- | ||||||
|
Tax Fees
|
-0- | -0- | ||||||
|
All Other Fees
|
-0- | -0- | ||||||
|
Total
|
$ | 304,000 | $ | 413,000 | ||||
|
(a)
|
Financial Statements and Schedules - See index to financial statements on page F-1 of this Annual Report.
|
|
(b)
|
Exhibits - See exhibit index below.
|
|
Exhibit
|
||
|
No.
|
Description
|
|
|
1.1
|
Engagement Letter between the Company and Rodman & Renshaw, LLC. (1)
|
|
|
1.2
|
May 28, 2010 Equity Distribution Agreement with Maxim Group LLC (18)
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation of the Company, as amended, along with Certificates of Designations.
|
|
|
3.2
|
Amended and Restated By-laws of Registrant. (2)
|
|
|
4.1
|
Specimen certificate representing our Common Stock.
|
|
|
4.2
|
Rights Agreement, dated as of November 19, 2002, between the Company and Continental Stock Transfer & Trust Company. The Right Agreement includes the Form of Certificate of Designation, Preferences and Rights of the Series A Junior Participating Preferred Stock, the Form of Rights Certificate and the Summary of the Right to Purchase Preferred Stock.(3)
|
|
|
4.3
|
Form of Commitment Warrant issued in February 2009 under the Standby Financing Agreement. (11)
|
|
|
4.4
|
Form of Indenture filed with Universal shelf registration statement. (4)
|
|
|
4.5
|
Form of Series I common stock purchase warrant pursuant to May 10, 2009 Securities Purchase Agreement. (1)
|
|
|
4.6
|
Form of Series II common stock purchase warrant pursuant to May 10, 2009 Securities Purchase Agreement. (1)
|
|
|
4.7
|
Form of common stock purchase warrant pursuant to May 18, 2009 Securities Purchase Agreement. (5)
|
|
|
10.1
|
Form of Confidentiality, Invention and Non-Compete Agreement.
|
|
|
10.2
|
Form of Clinical Research Agreement.
|
|
|
10.5
|
Change in control agreement with Dr. William A. Carter. (6)
|
|
|
10.6
|
Change in control agreement with Dr. William A. Carter. (6)
|
|
|
10.7
|
Supply Agreement with Hollister-Stier Laboratories LLC. (7)
|
|
|
10.8
|
Biken Activating Agreement. (8)
|
|
|
10.9
|
Biken Material Evaluation Agreement. (8)
|
|
|
10.10
|
Common Stock Purchase Agreement, dated July 2, 2008, by and among the Company and Fusion Capital. (9)
|
|
|
10.11
|
Registration Rights Agreement, dated July 2, 2008, by and among the Company and Fusion Capital. (9)
|
|
|
10.12
|
Amendment to Common Stock Purchase Agreement, dated July 23, 2008, by and among the Company and Fusion Capital. (10)
|
|
|
10.13
|
Employee Wage Or Hours Reduction Program. (11)
|
|
|
10.14
|
Standby Financing Agreement.(11)
|
|
|
10.15
|
Employment Agreement with Charles T. Bernhardt, CPA. (15)
|
|
|
10.16
|
Goal Achievement Incentive Award Program. (12)
|
|
|
10.17
|
Form of Securities Purchase Agreement entered into on May 10, 2009. (1)
|
|
|
10.18
|
Form of Securities Purchase Agreement entered into on May 18, 2009. (5)
|
|
|
10.19
|
Amended and Restated Employment Agreement with Robert Dickey IV, dated September 1, 2010. (14)
|
|
|
10.20
|
Amendment to Supply Agreement with Hollister-Stier Laboratories LLC dated February 25, 2010. (16)
|
|
|
10.21
|
August 2009 Material Evaluation Agreement with Biken. (16)
|
|
|
10.22
|
Amended and Restated
Employment Agreement of Dr. William A. Carter dated June 11, 2010 (14)
|
|
|
10.23
|
Amended and Restated Engagement Agreement of Dr. William A. Carter dated July 15, 2010. (13)
|
|
|
10.24
|
Amended Employment Agreement with Thomas K. Equels dated July 15, 2010. (14)
|
| 10.25 |
Amended Adviser’s Agreement with The Sage Group Inc. dated July 15, 2010. (14)
|
|
|
10.26
|
Employment Agreement with Charles T. Bernhardt dated December 3, 2010. (15)
|
|
|
10.27
|
Employment Agreement with Wayne Springate dated January 1, 2007. (16)
|
|
|
21
|
Subsidiaries of the Registrant. (16)
|
|
|
23.1
|
McGladrey & Pullen, LLP consent. *
|
|
|
31.1
|
Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 from the Company's Chief Executive Officer. *
|
|
|
31.2
|
Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 from the Company's Chief Financial Officer. *
|
|
|
32.1
|
Certification pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 from the Company's Chief Executive Officer. *
|
|
|
32.2
|
Certification pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 from the Company's Chief Financial Officer. *
|
|
By:
|
/s/ William A. Carter
|
|
William A. Carter, M.D.
|
|
|
Chief Executive Officer
|
|
/s/ William A. Carter
|
Chairman of the Board, Chief
|
March 28, 2011
|
||
|
William A. Carter, M.D.
|
Executive Officer and
|
|||
|
Director
|
||||
|
/s/ Richard Piani
|
Director
|
March 28, 2011
|
||
|
Richard Piani
|
||||
|
/s/ Charles T. Bernhardt
|
Chief Financial Officer and
|
March 28, 2011
|
||
|
Charles T. Bernhardt CPA
|
Chief Accounting Officer
|
|||
|
/s/ Thomas K. Equels
|
Director, Secretary and
|
March 28, 2011
|
||
|
Thomas Equels
|
General Counsel
|
|||
|
/s/ William Mitchell
|
Director
|
March 28, 2011
|
||
|
William Mitchell, M.D., Ph.D.
|
||||
|
/s/ Iraj E. Kiani
|
Director
|
March 28, 2011
|
||
|
Iraj E. Kiani, N.D., Ph.D.
|
|
|
|
Page
|
||
|
Report of Independent Registered Public Accounting Firm
|
F-2
|
|
|
Consolidated Balance Sheets at December 31, 2009 and 2010
|
F-3
|
|
|
Consolidated Statements of Operations for each of the years in the three-year period ended December 31, 2010
|
F-4
|
|
|
Consolidated Statements of Changes in Stockholders' Equity and Comprehensive Loss for each of the years in the three-year period ended December 31, 2010
|
F-5
|
|
|
Consolidated Statements of Cash Flows for each of the years in the three-year period ended December 31, 2010
|
F-6
|
|
|
Notes to Consolidated Financial Statements
|
F-8
|
|
|
Schedule II – Valuation and Qualifying Accounts for each of the years in the three year period ended December 31, 2010
|
F-37
|
|
/s/ McGladrey & Pullen, LLP
|
|
Blue Bell, Pennsylvania
|
|
March 28, 2011
|
|
2009
|
2010
|
|||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents (Notes 2 and 16)
|
$ | 58,072 | $ | 2,920 | ||||
|
Marketable securities maturing in less than one year(Note 4)
|
- | 32,689 | ||||||
|
Inventories (Note 3)
|
- | 787 | ||||||
|
Prepaid expenses and other current assets
|
332 | 278 | ||||||
|
Total current assets
|
58,404 | 36,674 | ||||||
|
Property and equipment, net (Note 2)
|
4,704 | 4,876 | ||||||
|
Patent and trademark rights, net (Notes 2 & 5)
|
830 | 794 | ||||||
|
Investment
|
35 | 35 | ||||||
|
Marketable securities maturing in one year or more(Note 4)
|
- | 8,778 | ||||||
|
Construction in progress (Note 2)
|
135 | 485 | ||||||
|
Other assets(Note 3)
|
886 | 38 | ||||||
|
Total assets
|
$ | 64,994 | $ | 51,680 | ||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$ | 1,294 | $ | 1,328 | ||||
|
Accrued expenses (Note 6)
|
1,321 | 1,443 | ||||||
|
Current portion of capital lease (Note 18)
|
- | 61 | ||||||
|
Total current liabilities
|
2,615 | 2,832 | ||||||
|
Long-term liabilities:
|
||||||||
|
Long-term portion of capital lease (Note 18)
|
- | 96 | ||||||
|
Redeemable warrants (Note 17)
|
3,684 | 2,805 | ||||||
|
Total liabilities
|
6,299 | 5,733 | ||||||
|
Commitments and contingencies (Notes 9,11, 12, 13, 14 & 18)
|
||||||||
|
Stockholders’ equity (Note 7):
|
||||||||
|
Preferred stock, par value $0.01 per share, authorized 5,000,000; issued and outstanding; none
|
- | - | ||||||
|
Common stock, par value $0.001 per share, authorized 200,000,000 shares; issued and outstanding 132,787,447 and 135,241,609, respectively
|
133 | 135 | ||||||
|
Additional paid-in capital
|
263,151 | 264,511 | ||||||
|
Unrealized loss
|
- | (974 | ) | |||||
|
Accumulated deficit
|
(204,589 | ) | (217,725 | ) | ||||
|
Total stockholders’ equity
|
58,695 | 45,947 | ||||||
|
Total liabilities and stockholders’ equity
|
$ | 64,994 | $ | 51,680 | ||||
|
Years ended December 31,
|
||||||||||||
|
2008
|
2009
|
2010
|
||||||||||
|
Revenues:
|
||||||||||||
|
Sales of product, net
|
$ | 173 | $ | - | $ | - | ||||||
|
Clinical treatment programs
|
92 | 111 | 135 | |||||||||
|
Total Revenues
|
265 | 111 | 135 | |||||||||
|
Costs and Expenses:
|
||||||||||||
|
Production/cost of goods sold
|
798 | 584 | 1,341 | |||||||||
|
Research and development
|
5,800 | 6,995 | 7,613 | |||||||||
|
General and administrative
|
6,478 | 5,796 | 7,568 | |||||||||
|
Total Costs and Expenses
|
13,076 | 13,375 | 16,522 | |||||||||
|
Operating loss
|
(12,811 | ) | (13,264 | ) | (16,387 | ) | ||||||
|
Interest and other income
|
592 | 67 | 2,383 | |||||||||
|
Interest expense
|
- | - | (11 | ) | ||||||||
|
Financing costs from standby financing agreement
|
- | (241 | ) | - | ||||||||
|
Redeemable warrants valuation adjustment (Note 17)
|
- | 6,258 | 879 | |||||||||
|
Net loss
|
$ | (12,219 | ) | $ | (7,180 | ) | $ | (13,136 | ) | |||
|
Basic and diluted loss per share
|
$ | (.16 | ) | $ | (.07 | ) | $ | (.10 | ) | |||
|
Weighted average shares outstanding Basic and Diluted
|
75,142,075 | 109,514,401 | 134,018,243 | |||||||||
|
Common
Stock
Share
s
|
Common
Stock .001
Par Value
|
Additional
Paid-in
Capital
|
Accumulated
Other
Comprehensive
Income (Loss)
|
Accumulated
Deficit
|
Total
Stockholders
Equity
|
|||||||||||||||||||
|
Balance December 31, 2007
|
73,760,446 | $ | 74 | $ | 206,078 | $ | (7 | ) | $ | (185,190 | ) | $ | 20,955 | |||||||||||
|
Shares issued for:
|
||||||||||||||||||||||||
|
Private placement, net of issuance costs
|
1,211,122 | 1 | 269 | - | - | 270 | ||||||||||||||||||
|
Settlement of accounts payable
|
3,779,427 | 4 | 1,954 | - | - | 1,958 | ||||||||||||||||||
|
Stock based compensation
|
- | - | 573 | - | - | 573 | ||||||||||||||||||
|
Net comprehensive loss
|
- | - | - | 7 | (12,219 | ) | (12,212 | ) | ||||||||||||||||
|
Balance December 31, 2008
|
78,750,995 | 79 | 208,874 | - | (197,409 | ) | 11,544 | |||||||||||||||||
|
Shares issued for:
|
||||||||||||||||||||||||
|
Warrants exercised
|
5,589,790 | 6 | 6,133 | - | - | 6,139 | ||||||||||||||||||
|
Options exercised
|
293,831 | - | 130 | - | - | 130 | ||||||||||||||||||
|
Private placement, net of issuance costs
|
45,591,304 | 46 | 55,524 | - | - | 55,570 | ||||||||||||||||||
|
Settlement of accounts payable
|
1,925,408 | 2 | 1,365 | - | - | 1,367 | ||||||||||||||||||
|
Stock based compensation
|
636,119 | - | 826 | - | - | 826 | ||||||||||||||||||
|
Standby Finance- finance costs
|
- | - | 241 | - | - | 241 | ||||||||||||||||||
|
Redeemable warrants valuation adjustment
|
- | - | (9,942 | ) | - | - | (9,942 | ) | ||||||||||||||||
|
Net comprehensive loss
|
- | - | - | - | (7,180 | ) | (7,180 | ) | ||||||||||||||||
|
Balance December 31, 2009
|
132,787,447 | 133 | 263,151 | - | (204,589 | ) | 58,695 | |||||||||||||||||
|
Shares issued for:
|
||||||||||||||||||||||||
|
Settlement of accounts payable
|
498,867 | - | 329 | - | - | 329 | ||||||||||||||||||
|
Shares sold at the market
|
520,000 | - | 292 | - | - | 292 | ||||||||||||||||||
|
Stock based compensation
|
1,435,295 | 2 | 739 | - | - | 741 | ||||||||||||||||||
|
Net comprehensive loss
|
- | - | - | (974 | ) | (13,136 | ) | (14,110 | ) | |||||||||||||||
|
Balance December 31, 2010
|
135,241,609 | $ | 135 | $ | 264,511 | $ | (974 | ) | $ | (217,725 | ) | $ | 45,947 | |||||||||||
|
Years ended December 31
|
||||||||||||
|
2008
|
2009
|
2010
|
||||||||||
|
Cash flows from operating activities:
|
||||||||||||
|
Net loss
|
$ | (12,219 | ) | $ | (7,180 | ) | $ | (13,136 | ) | |||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||||||
|
Depreciation of property and equipment
|
342 | 359 | 407 | |||||||||
|
Amortization of patent, trademark rights, and royalty interest
|
374 | 381 | 373 | |||||||||
|
Finance costs amortization and for Standby financing
|
- | 241 | - | |||||||||
|
Redeemable warrants valuation adjustment
|
- | (6,258 | ) | (879 | ) | |||||||
|
Stock option and warrant compensation and service expense
|
573 | 826 | 740 | |||||||||
|
Gain on disposal of equipment
|
- | (83 | ) | - | ||||||||
|
Inventory reserve
|
(65 | ) | - | - | ||||||||
|
Changes in assets and liabilities:
|
||||||||||||
|
Inventories
|
(288 | ) | - | 77 | ||||||||
|
Accounts and other receivables
|
77 | - | - | |||||||||
|
Assets held for sale
|
450 | - | - | |||||||||
|
Prepaid expenses and other current assets
|
(184 | ) | 93 | 54 | ||||||||
|
Other assets
|
- | (5 | ) | (7 | ) | |||||||
|
Accounts payable
|
1,702 | 1,884 | 362 | |||||||||
|
Accrued expenses
|
(120 | ) | 45 | 123 | ||||||||
|
Net cash used in operating activities
|
(9,358 | ) | (9,297 | ) | (11,886 | ) | ||||||
|
Cash flows from investing activities:
|
||||||||||||
|
Purchases of property and equipment and construction in progress, net
|
(73 | ) | (332 | ) | (729 | ) | ||||||
|
Additions to patent and trademark rights
|
(142 | ) | (242 | ) | (337 | ) | ||||||
|
Capital lease deposit
|
- | - | (9 | ) | ||||||||
|
Maturities of short term investments
|
3,951 | - | 7,448 | |||||||||
|
Purchase of short term investments
|
- | - | (49,889 | ) | ||||||||
|
Net cash (used in) provided by investing activities
|
$ | 3,736 | $ | (574 | ) | $ | (43,516 | ) | ||||
|
Years ended December 31,
|
||||||||||||
|
2008
|
2009
|
2010
|
||||||||||
|
Cash flows from financing activities:
|
||||||||||||
|
Proceeds from issuance of common stock, net
|
$ | 270 | $ | 55,570 | $ | 293 | ||||||
|
Payments on capital leases
|
- | - | (43 | ) | ||||||||
|
Proceeds from exercise of stock Warrants and options
|
- | 6,254 | - | |||||||||
|
Net cash provided by financing activities
|
270 | 61,824 | 250 | |||||||||
|
Net (decrease) increase in cash and cash equivalents
|
(5,352 | ) | 51,953 | (55,152 | ) | |||||||
|
Cash and cash equivalents at beginning of year
|
1,471 | 6,119 | 58,072 | |||||||||
|
Cash and cash equivalents at end of year
|
$ | 6,119 | $ | 58,072 | $ | 2,920 | ||||||
|
Supplemental disclosures of cash flow information:
|
||||||||||||
|
Issuance of common stock for accounts payable and accrued expenses
|
$ | 1,958 | $ | 1,382 | $ | 329 | ||||||
|
Equipment acquired by capital leases
|
$ | - | $ | - | $ | 200 | ||||||
|
Unrealized losses on investments
|
$ | 7 | $ | - | $ | (974 | ) | |||||
|
Redeemable warrants valuation adjustment
|
$ | - | $ | (6,258 | ) | $ | (879 | ) | ||||
|
(b) Property and Equipment
|
(in thousands)
|
|||||||
|
December 31,
|
||||||||
|
2009
|
2010
|
|||||||
|
Land, buildings and improvements
|
$ | 4,139 | $ | 4,193 | ||||
|
Furniture, fixtures, and equipment
|
2,629 | 3,154 | ||||||
|
Leasehold improvements
|
85 | 85 | ||||||
|
Total property and equipment
|
6,853 | 7,432 | ||||||
|
Less accumulated depreciation and amortization
|
2,149 | 2,556 | ||||||
|
Property and equipment, net
|
$ | 4,704 | $ | 4,876 | ||||
|
December 31,
|
||||||||||||
|
2008
|
2009
|
2010
|
||||||||||
|
Risk-free interest rate
|
2.52 – 3.74 | % | 1.76 - 2.69 | % | 1.02 - 2.06 | % | ||||||
|
Expected dividend yield
|
- | - | - | |||||||||
|
Expected lives
|
2.5 - 5 yrs.
|
2 - 5 yrs.
|
5 yrs.
|
|||||||||
|
Expected volatility
|
73.84 – 79.2 | % | 86.78-137.47 | % | 106.28%-110.01 | % | ||||||
|
Weighted average fair value of options and warrants issued in the years 2008, 2008 and 2010 respectively
|
$ | 473,954 | $ | 536,378 | $ | 674,281 | ||||||
|
Number of
Options
|
Weighted
Average
Exercise
Price
|
Weighted
Average
Remaining
Contracted
Term
(Years)
|
Aggregate
Intrinsic
Value
|
|||||||||||||
|
Outstanding January 1, 2008
|
4,626,089 | $ | 2.66 | 8.25 | - | |||||||||||
|
Options Granted
|
1,655,000 | 2.42 | 9.69 | - | ||||||||||||
|
Options Forfeited
|
(22,481 | ) | 2.13 | - | - | |||||||||||
|
Outstanding December 31, 2008
|
6,258,608 | $ | 2.60 | 7.92 | - | |||||||||||
|
Options granted
|
- | - | - | - | ||||||||||||
|
Options forfeited
|
(29,856 | ) | 2.24 | 5.75 | - | |||||||||||
|
Outstanding December 31, 2009
|
6,228,752 | $ | 2.60 | 6.95 | - | |||||||||||
|
Options granted
|
993,728 | .80 | 9.42 | - | ||||||||||||
|
Options forfeited
|
- | - | - | - | ||||||||||||
|
Outstanding December 31, 2010
|
7,222,480 | $ | 2.35 | 6.21 | - | |||||||||||
|
Exercisable December 31, 2010
|
7,171,928 | $ | 2.35 | 6.23 | - | |||||||||||
|
Number of
Options
|
Weighted
Average
Exercise
Price
|
Average
Remaining
Contracted
Term
(Years)
|
Aggregate
Intrinsic
Value
|
|||||||||||||
|
Outstanding January 1, 2008
|
166,673 | $ | 1.59 | 7.18 | - | |||||||||||
|
Options granted
|
-0- | -0- | -0- | - | ||||||||||||
|
Options vested
|
(73,420 | ) | 1.68 | 8.58 | - | |||||||||||
|
Options forfeited
|
(16,399 | ) | 2.00 | 6.18 | - | |||||||||||
|
Outstanding December 31, 2008
|
76,944 | $ | 1.41 | 3.89 | - | |||||||||||
|
Options granted
|
- | - | - | - | ||||||||||||
|
Options vested
|
(38,611 | ) | 1.28 | 7.92 | - | |||||||||||
|
Options forfeited
|
- | - | - | - | ||||||||||||
|
Outstanding December 31, 2009
|
38,333 | $ | 1.54 | 8.00 | - | |||||||||||
|
Options granted
|
20,000 | .66 | 9.50 | - | ||||||||||||
|
Options vested
|
(7,778 | ) | .66 | 9.50 | - | |||||||||||
|
Options forfeited
|
- | - | - | - | ||||||||||||
|
Outstanding December 31, 2010
|
50,555 | $ | 1.33 | 7.60 | - | |||||||||||
|
Number of
Options
|
Weighted
Average
Exercise
Price
|
Weighted
Average
Remaining
Contracted
Term
(Years)
|
Aggregate
Intrinsic
Value
|
|||||||||||||
|
Outstanding January 1, 2008
|
1,935,482 | $ | 2.43 | 8.05 | - | |||||||||||
|
Options granted
|
482,000 | 2.02 | 6.72 | - | ||||||||||||
|
Options forfeited
|
- | - | - | - | ||||||||||||
|
Outstanding December 31, 2008
|
2,417,482 | $ | 2.35 | 6.98 | - | |||||||||||
|
Options granted
|
361,250 | 2.12 | 7.00 | - | ||||||||||||
|
Options exercised
|
(293,831 | ) | 1.56 | 7.93 | - | |||||||||||
|
Options forfeited
|
(251,469 | ) | 2.14 | 7.43 | - | |||||||||||
|
Outstanding December 31, 2009
|
2,233,432 | $ | 2.44 | 5.73 | - | |||||||||||
|
Options granted
|
625,000 | .55 | 9.52 | - | ||||||||||||
|
Options exercised
|
- | - | - | - | ||||||||||||
|
Options forfeited
|
(10,000 | ) | 2.46 | - | - | |||||||||||
|
Outstanding December 31, 2010
|
2,848,432 | $ | 2.03 | 5.80 | - | |||||||||||
|
Exercisable December 31, 2010
|
2,746,348 | $ | 2.00 | 6.05 | - | |||||||||||
|
Number of
Options
|
Weighted
Average
Exercise
Price
|
Weighted
Average
Remaining
Contracted
Term
(Years)
|
Aggregate
Intrinsic
Value
|
|||||||||||||
|
Outstanding December 31, 2007
|
40,000 | $ | 1.50 | 9.30 | - | |||||||||||
|
Options granted
|
- | - | - | - | ||||||||||||
|
Options vested
|
(13,333 | ) | (1.64 | ) | 6.91 | - | ||||||||||
|
Outstanding December 31, 2008
|
26,667 | $ | 1.43 | 9.00 | - | |||||||||||
|
Options granted
|
131,250 | 2.81 | 3.42 | - | ||||||||||||
|
Options vested
|
(18,333 | ) | 1.79 | 7.45 | - | |||||||||||
|
Outstanding December 31, 2009
|
139.584 | $ | 2.68 | 3.76 | - | |||||||||||
|
Options granted
|
- | - | - | - | ||||||||||||
|
Options vested
|
(37,500 | ) | 2.81 | 2.5 | - | |||||||||||
|
Outstanding December 31, 2010
|
102,084 | $ | 2.63 | 3.54 | - | |||||||||||
|
Inventories consist of the following:
|
(in thousands)
|
|||||||
|
December 31,
|
||||||||
|
2009
|
2010
|
|||||||
|
Raw materials and work in process
|
$ | - | $ | - | ||||
|
Transfer from Other Assets
|
- | 864 | ||||||
|
Production
|
- | 373 | ||||||
|
Spoilage
|
- | (450 | ) | |||||
|
Finished goods, net of reserves of $250,000 and $250,000 at December 31, 2009 and 2010
|
- | - | ||||||
| $ | - | $ | 787 | |||||
|
Other assets consist of the following:
|
(in thousands)
|
|||||||
|
December 31,
|
||||||||
|
2009
|
2010
|
|||||||
|
Inventory work in process
|
$ | 864 | $ | - | ||||
|
Office Security Deposit
|
15 | 15 | ||||||
|
Deposits on Capital Leases
|
- | 9 | ||||||
|
Other Deposit
|
- | 7 | ||||||
|
Internet Domain Names
|
7 | 7 | ||||||
| $ | 886 | $ | 38 | |||||
|
December 31, 2010
(in thousands)
|
||||||||||||||
|
Name Of Security
|
Cost
|
Market
Value
|
Unrealized
Gain
(Loss)
|
Maturity
Date
|
||||||||||
|
Marketable Securities with maturity periods less than one year:
|
||||||||||||||
|
Safra National Bank
|
$ | 250 | $ | 250 | $ | - |
1/1/2011
|
|||||||
|
GE Money Bank
|
250 | 250 | - |
1/14/2011
|
||||||||||
|
Goldman Sachs
|
1,062 | 1,002 | (60 | ) |
1/15/2011
|
|||||||||
|
Oracle Corporation
|
785 | 751 | (34 | ) |
1/15/2011
|
|||||||||
|
World Financial Capital
|
300 | 300 | - |
1/28/2011
|
||||||||||
|
Cisco Systems
|
786 | 755 | (31 | ) |
2/22/2011
|
|||||||||
|
IBM Corporation
|
784 | 758 | (26 | ) |
3/22/2011
|
|||||||||
|
Bank of America
|
500 | 500 | - |
4/21/2011
|
||||||||||
|
Merrick Bank
|
250 | 250 | - |
4/21/2011
|
||||||||||
|
Discover Bank
|
250 | 250 | - |
6/23/2011
|
||||||||||
|
General Dynamics
|
762 | 756 | (6 | ) |
7/15/2011
|
|||||||||
|
Wells Fargo
|
1,081 | 1,033 | (48 | ) |
8/1/2011
|
|||||||||
|
Bank of America
|
1,066 | 1,026 | (40 | ) |
8/15/2011
|
|||||||||
|
Shell International
|
755 | 755 | - |
9/22/2011
|
||||||||||
|
Wachovia Bank
|
274 | 267 | (7 | ) |
9/28/2011
|
|||||||||
|
Plainscapital Bank
|
251 | 251 | - |
10/31/2011
|
||||||||||
|
Bank One Corp.
|
1,070 | 1,044 | (26 | ) |
11/15/2011
|
|||||||||
|
Merck & Co.
|
818 | 781 | (37 | ) |
11/15/2011
|
|||||||||
|
PIMCO
|
22,200 | 21,710 | (490 | ) |
NA
|
|||||||||
|
Total Marketable Securities with maturity periods less than one year:
|
$ | 33,494 | $ | 32,689 | $ | (805 | ) | |||||||
|
Morgan Stanley
|
$ | 1,077 | $ | 1,045 | $ | (32 | ) |
1/9/2012
|
||||||
|
Wright Expert Financial Services
|
250 | 251 | 1 |
4/26/2012
|
||||||||||
|
Citibank NA
|
250 | 251 | 1 |
4/30/2012
|
||||||||||
|
GE Capital
|
104 | 103 | (1 | ) |
5/29/2012
|
|||||||||
|
Sallie Mae Bank
|
104 | 103 | (1 | ) |
5/29/2012
|
|||||||||
|
Bank of Northern Miami
|
250 | 251 | 1 |
7/30/2012
|
||||||||||
|
Merrill Lynch
|
1,089 | 1,057 | (32 | ) |
8/15/2012
|
|||||||||
|
Merrill Lynch
|
811 | 793 | (18 | ) |
8/15/2012
|
|||||||||
|
Wells Fargo
|
1,083 | 1,054 | (29 | ) |
9/1/2012
|
|||||||||
|
Israel Discount Bank
|
250 | 250 | - |
9/11/2012
|
||||||||||
|
Allstate
|
115 | 113 | (2 | ) |
9/16/2012
|
|||||||||
|
Park Sterling Bank
|
250 | 251 | 1 |
10/16/2012
|
||||||||||
|
Columbus Bank & Trust Company
|
250 | 252 | 2 |
10/22/2012
|
||||||||||
|
World's Foremost Bank
|
100 | 104 | 4 |
1/28/2013
|
||||||||||
|
Merrill Lynch
|
543 | 525 | (18 | ) |
2/5/2013
|
|||||||||
|
World’s Foremost Bank
|
104 | 104 | - |
3/4/2013
|
||||||||||
|
Goldman Sachs
|
251 | 251 | - |
6/17/2013
|
||||||||||
|
Royal Bank of Scotland
|
1,034 | 1,010 | (24 | ) |
8/23/2013
|
|||||||||
|
Royal Bank of Scotland
|
1,032 | 1,010 | (22 | ) |
8/23/2013
|
|||||||||
|
Total Marketable Securities with maturity periods greater than one year:
|
$ | 8,947 | $ | 8,778 | $ | (169 | ) | |||||||
|
Total Marketable Securities
|
$ | 42,441 | $ | 41,467 | $ | (974 | ) |
|
(in thousands)
|
||||||||
|
December 31,
|
||||||||
|
2009
|
2010
|
|||||||
|
Compensation
|
$ | 716 | $ | 995 | ||||
|
Professional fees
|
421 | 207 | ||||||
|
Other expenses
|
71 | 128 | ||||||
|
Other liability
|
113 | 113 | ||||||
| $ | 1,321 | $ | 1,443 | |||||
|
2008
|
2009
|
2010
|
||||||||||||||||||||||||||||||||||
|
Shares
|
Option
Price
|
Weighted
Average
Exercise
Price
|
Shares
|
Option
Price
|
Weighted
Average
Exercise
Price
|
Shares
|
Option
Price
|
Weighted
Average
Exercise
Price
|
||||||||||||||||||||||||||||
|
Outstanding, beginning of year
|
345,728 | $ | 2.71-4.03 | $ | 3.01 | 345,728 | $ | 2.71-4.03 | $ | 3.01 | 335,728 | $ | 2.71-4.03 | $ | 2.98 | |||||||||||||||||||||
|
Granted
|
- | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
|
Canceled
|
- | - | - | (10,000 | ) | $ | 4.03 | $ | 4.03 | (73,728 | ) | $ | 2.71 | - | ||||||||||||||||||||||
|
Exercised
|
- | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
|
Outstanding, end of year
|
345,728 | $ | 2.71-4.03 | $ | 3.01 | 335,728 | $ | 2.71-4.03 | $ | 2.98 | 262,000 | $ | 2.75-4.03 | $ | 3.05 | |||||||||||||||||||||
|
Exercisable
|
345,728 | $ | 2.71-4.03 | $ | 3.01 | 335,728 | $ | 2.71-4.03 | $ | 2.98 | 262,000 | $ | 2.75-4.03 | $ | 3.05 | |||||||||||||||||||||
|
Weighted average remaining contractual life (years)
|
4.86
yrs.
|
- | - |
3.86
yrs.
|
- | - |
2.86
yrs.
|
- | - | |||||||||||||||||||||||||||
|
Exercised in current and prior years
|
(27,215 | ) | - | - | (27,215 | ) | - | - | (27,215 | ) | - | - | ||||||||||||||||||||||||
|
Available for future grants
|
- | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
|
2008
|
2009
|
2010
|
||||||||||||||||||||||||||||||||||
|
Shares
|
Option
Price
|
Weighted
Average
Exercise
Price
|
Shares
|
Option
Price
|
Weighted
Average
Exercise
Price
|
Shares
|
Option
Price
|
Weighted
Average
Exercise
Price
|
||||||||||||||||||||||||||||
|
Outstanding beginning at year
|
6,556,476 | $ | 1.30-3.86 | $ | 2.59 | 7,226,090 | $ | 0.68-6.00 | $ | 2.59 | 6,650,934 | $ | 1.30-6.00 | $ | 2.66 | |||||||||||||||||||||
|
Granted
|
687,000 | $ | 0.68-6.00 | $ | 2.61 | - | - | - | - | - | - | |||||||||||||||||||||||||
|
Canceled
|
(17,386 | ) | $ | 1.30– 2.61 | $ | 2.00 | (281,325 | ) | $ | 0.68– 2.20 | $ | 1.86 | (10,000 | ) | 2.46 | - | ||||||||||||||||||||
|
Exercised
|
- | - | - | (293,831 | ) | $ | 0.68-2.20 | $ | 1.56 | - | - | - | ||||||||||||||||||||||||
|
Outstanding end of year
|
7,226,090 | $ | 0.68-6.00 | $ | 2.59 | 6,650,934 | $ | 1.30-6.00 | $ | 2.66 | 6,640,934 | $ | 1.30-6.00 | $ | 2.66 | |||||||||||||||||||||
|
Exercisable
|
7,122,479 | $ | 0.68-6.00 | $ | 2.61 | 6,604,267 | $ | 1.30-6.00 | $ | 2.66 | 6,594,267 | $ | 1.30-6.00 | $ | 2.66 | |||||||||||||||||||||
|
Weighted average remaining contractual life (years)
|
7-8 yrs.
|
- | - |
6-7 yrs.
|
- | - |
5-6 yrs.
|
- | - | |||||||||||||||||||||||||||
|
Available for future grants
|
18,081 | - | - | 5.575 | - | - | 10,019 | - | - | |||||||||||||||||||||||||||
|
December 31, 2008
|
December 31, 2009
|
December 31, 2010
|
||||||||||||||||||||||||||||||||||
|
Shares
|
Option
Price
|
Weighted
Average
Exercise
Price
|
Shares
|
Option
Price
|
Weighted
Average
Exercise
Price
|
Shares
|
Option
Price
|
Weighted
Average
Exercise
Price
|
||||||||||||||||||||||||||||
|
Outstanding beginning at year
|
- | - | - | 1,450,000 | $ | 2.20 | $ | 2.20 | 1,530,000 | $ | 0.72-3.05 | $ | 2.19 | |||||||||||||||||||||||
|
Granted
|
1,450,000 | $ | 2.20 | $ | 2.20 | 80,000 | $ | 0.72-3.05 | $ | 1.96 | 20,000 | $ | 0.89 | $ | 0.89 | |||||||||||||||||||||
|
Canceled
|
- | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
|
Exercised
|
- | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
|
Outstanding end of year
|
1,450,000 | $ | 2.20 | $ | 2.20 | 1,530,000 | $ | 0.72-3.05 | $ | 2.19 | 1,550,000 | $ | 0.72 –3.05 | $ | 2.17 | |||||||||||||||||||||
|
Exercisable
|
1,450,000 | $ | 2.20 | $ | 2.20 | 1,530,000 | $ | 0.72-3.05 | $ | 2.19 | 1,550,000 | $ | 0.72-3.05 | $ | 2.17 | |||||||||||||||||||||
|
Remaining contractual life
|
9 years
|
8.1 years
|
7.81 years
|
|||||||||||||||||||||||||||||||||
|
Available for future grants
|
3,914,813 | 107,225 | 19,626 | |||||||||||||||||||||||||||||||||
|
December 31,2009
|
December 31, 2010
|
|||||||||||||||||||||||
|
Shares
|
Option
Price
|
Weighted
Average
Exercise
Price
|
Shares
|
Option
Price
|
Weighted
Average
Exercise
Price
|
|||||||||||||||||||
|
Outstanding beginning at year
|
- | 281,250 | $ | 1.42-2.81 | $ | 2.16 | ||||||||||||||||||
|
Granted
|
281,250 | $ | 1.42-2.81 | $ | 2.16 | 1,598,728 | $ | 0.52-2.71 | $ | 0.70 | ||||||||||||||
|
Outstanding and Exercisable at end of year
|
281,250 | $ | 1.42-2.81 | $ | 2.16 | 1,879,978 | $ | 0.52-2.81 | $ | 0.92 | ||||||||||||||
|
Remaining contractual life
|
9.5 years
|
8.1 years
|
||||||||||||||||||||||
|
Available for future grants
|
13,642,525 | 11,618,085 | ||||||||||||||||||||||
|
December 31, 2008
|
December 31, 2009
|
December 31, 2010
|
||||||||||||||||||||||||||||||||||
|
Shares
|
Option
Price
|
Weighted
Average
Exercise
Price
|
Shares
|
Option
Price
|
Weighted
Average
Exercise
Price
|
Shares
|
Option
Price
|
Weighted
Average
Exercise
Price
|
||||||||||||||||||||||||||||
|
Outstanding beginning at year
|
7,262.771 | $ | 1.32-6.00 | $ | 2.96 | 5,266,187 | $ | 0.35-4.25 | $ | 3.13 | 11,008,246 | $ | 0.51-3.60 | $ | 1.44 | |||||||||||||||||||||
|
Granted
|
20,000 | $ | 0.35-0.80 | $ | 0.68 | 15,821,080 | $ | 0.51-1.65 | $ | 1.44 | - | - | - | |||||||||||||||||||||||
|
Canceled
|
(2,016,584 | ) | $ | 2.20-6.00 | $ | 2.53 | (3,347,777 | ) | $ | 2.08-4.25 | $ | 3.37 | (25,000 | ) | $ | 2.50 | $ | 2.50 | ||||||||||||||||||
|
Exercised
|
- | - | - | (6,753,244 | ) | $ | 0.35-3.33 | $ | 1.56 | - | - | - | ||||||||||||||||||||||||
|
Outstanding end of year
|
5,266,187 | $ | 0.35-4.25 | $ | 3.13 | 11,008,246 | $ | 0.51-3.60 | $ | 1.44 | 10,983.246 | $ | 0.51 –3.60 | $ | 1.61 | |||||||||||||||||||||
|
Exercisable
|
5,266,187 | $ | 0.35-4.25 | $ | 3.13 | 11,008,246 | $ | 0.51-3.60 | $ | 1.44 | 10,983,246 | $ | 0.51-3.60 | $ | 1.61 | |||||||||||||||||||||
|
Weighted average remaining contractual life
|
1 years
|
4.5 years
|
3.9 years
|
|||||||||||||||||||||||||||||||||
|
Years exercisable
|
2009-2018 | - | - | 2010-2019 | - | - | 2011-2019 | - | - | |||||||||||||||||||||||||||
|
|
o
|
Peterson waived his right to receive payment for the additional twelve month period as provided for in the Engagement Agreement;
|
|
|
o
|
On the occurrence of a “Change In Control, the Company shall pay to Peterson three times the amount of compensation paid to Peterson by the Company for calendar year 2008. A “Change In Control” shall be deemed to have occurred as set forth the Engagement Agreement Regarding Change In Control made as of March 11, 2005 between the Company and Peterson, with the definition of “Change In Control” as therein set forth;
|
|
|
o
|
Upon executing a “Financial Transaction”, the Company shall pay to Peterson one (1) percent (the “Peterson One Per Cent Fee”) of the cash to be received by the Company from each Financial Transaction. Provided, however, the Peterson One Per Cent Fee shall in no event exceed in the aggregate two times the amount of compensation paid to Peterson by the Company for calendar year 2008. A “Financial Transaction” shall be any agreements entered into by the Company in which the Company is to receive cash from such third parties. A Financial Transaction does not include agreements whereby the Company receives cash as a result of (i) the Company only being reimbursed for expenses, not including expenses for prior research conducted by the Company, incurred by the Company, (ii) an agreement in which the only economic benefit to the Company is a loan or loans to the Company, (iii) any transactions with Fusion pursuant to the July 2, 2008, Common Stock Purchase Agreement between the Company and Fusion;
|
|
|
o
|
For a period of thirty six (36) months following the Effective Date of December 31, 2008, subject to earlier termination by Peterson in his sole discretion, the Company shall engage Peterson as a part time advisor to the Company’s Chief Executive Officer and shall pay to Peterson for such services (“Advisory Services”) the sum of four thousand dollars ($4,000) per month, payable monthly with the first monthly payment being due and payable one month after the Effective Date;
|
|
|
o
|
Peterson is to receive Options to purchase 20,000 shares of the Company’s common stock at the end of each calendar quarter following the Effective Date. Peterson may terminate the Advisory Services at any time;
|
|
|
o
|
This Agreement shall terminate upon Peterson having received full payment for a change in control or upon receiving the maximum one percent fee. The Agreement provides for a “gross-up” payment to make Peterson whole for any Federal taxes imposed as a result of change of control or one percent payments to him.
|
|
(in thousands)
|
||||
|
Year Ending December 31,
|
||||
|
2011
|
$ | 198 | ||
|
2012
|
67 | |||
|
Total minimum lease payments
|
$ | 265 | ||
|
(in thousands)
|
||||||||
|
Deferred tax assets:
|
December 31,
|
|||||||
|
2009
|
2010
|
|||||||
|
Net operating losses
|
$ | 31,664 | $ | 34,514 | ||||
|
Amortization & depreciation
|
(1,018 | ) | (1,095 | ) | ||||
|
Research and development costs
|
1,924 | 2,477 | ||||||
|
Stock compensation
|
281 | 252 | ||||||
|
Total
|
32,851 | 36,148 | ||||||
|
Less: Valuation allowance
|
(32,851 | ) | (36,148 | ) | ||||
|
Balance
|
$ | -0- | $ | -0- | ||||
|
(a)
|
Hemispherx Biopharma, Inc. v. Johannesburg Consolidated Investments, et al.,U.S. District Court for the Southern District of Florida, Case No. 04-10129-CIV.
|
|
(b)
|
Hemispherx Biopharma, Inc. v. MidSouth Capital, Inc., Adam Cabibi, And Robert L. Rosenstein v. Hemispherx Biopharma, Inc. and The Sage Group, Inc.,
Civil Action No. 1:09-CV-03110-CAP.
|
|
(c)
|
Cato Capital, LLC v. Hemispherx Biopharma, Inc., U.S. District Court for the District of Delaware, Case No. 09-549-GMS.
|
|
(d)
|
In re Hemispherx Biopharma, Inc. Litigation, U. S. District Court for the Eastern District of Pennsylvania, Civil Action No. 09-5262.
|
|
|
·
|
the Action should be finally certified as a class action suit;
|
|
|
·
|
the proposed Settlement is fair, reasonable, adequate and should be approved;
|
|
|
·
|
the Released Claims against Hemispherx should be dismissed;
|
|
|
·
|
the proposed Plan of Allocation for the proceeds of the Settlement should be approved by the Court;
|
|
|
·
|
the application of the Lead Counsel for an award of attorney fees and reimbursement of litigation expenses should be approved;
|
|
|
·
|
to determine the amount of reimbursement of cost and expenses for representation of the suit;
|
|
|
·
|
any other matters as the Court deem appropriate.
|
|
(e)
|
In re Hanna v. Carter Litigation, U. S. District Court for the Eastern District of Pennsylvania, Civil Action No. 2:09-cv-06160-PD.
|
|
(f)
|
Jeffrey Bastedo v. Hemispherx Biopharma, Inc., Delaware Chancery Court, Case No. 5748-VCP.
|
|
(g)
|
Summation.
|
|
2009
|
2010
|
|||
|
Underlying price per share
|
$0.56 - $2.54
|
$0.47-$0.74
|
||
|
Exercise price per share
|
$1.10 – $1.65
|
$1.31-$1.65
|
||
|
Risk-free interest rate
|
0.19% - 2.67%
|
0.83%-2.36%
|
||
|
Expected holding period
|
0.122-5.50 yrs.
|
3.38-4.63 yrs.
|
||
|
Expected volatility
|
94.99%–226.46%
|
112.16%-122.02%
|
||
|
Expected dividend yield
|
None
|
None
|
|
|
a.
|
The Company only has one product that is FDA approved;
|
|
|
b.
|
The Company will have to perform additional clinical trials for FDA approval of its flagship product;
|
|
|
c.
|
Industry and market conditions continue to include a global market recession, adding risk to any transaction;
|
|
|
d.
|
Available capital for a potential buyer in a cash transaction continues to be limited;
|
|
e.
|
The nature of a life sciences company is heavily dependent on future funding and high fixed costs, including Research & Development;
|
|
|
f.
|
According to Forbes.com, of approximately 17,000 public companies, fewer than 30 went private in 2008 and less than 100 were completed in 2007, representing 0.18% and 0.6%, respectively. This would be further reduced based on the nature of a life sciences company and the potential lack of revenues, cash flows and the Company’s funding needs; and
|
|
|
g.
|
The Company's Rights Agreement makes it less attractive to a potential buyer.
|
|
Range of Probability
|
Probability
|
|||
|
Low
|
0.5 | % | ||
|
Medium
|
1.0 | % | ||
|
High
|
5.0 | % | ||
|
|
·
|
Level 1 – Quoted prices are available in active markets for identical assets or liabilities at the reporting date.
|
|
|
·
|
Level 2 – Observable inputs other than Level 1 prices such as quote prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
|
|
|
·
|
Level 3 – Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. Level 3 assets and liabilities include financial instruments whose value is determined using pricing models, discounted cash flow methodologies, or other valuation techniques, as well as instruments for which the determination of fair value requires significant management judgment or estimation. As of December 31, 2009 and 2010, the Company has classified the warrants with cash settlement features as Level 3. Management evaluates a variety of inputs and then estimates fair value based on those inputs. As discussed above, the Company utilized the Monte Carlo Simulation Model in valuing these warrants.
|
|
2010
|
||||||||||||||||
|
Total
|
Level 1
|
Level 2
|
Level 3
|
|||||||||||||
|
Assets
|
||||||||||||||||
|
Marketable Securities
|
$ | 41,467,000 | $ | 33,257,000 | $ | 8,210,000 | $ | - | ||||||||
|
Liabilities
|
||||||||||||||||
|
Warrants
|
2,805,000 | - | - | 2,805,000 | ||||||||||||
|
Total
|
$ | 44,272,000 | $ | 33,257,000 | $ | 8,210,000 | $ | 2,805,000 | ||||||||
|
2009
|
||||||||||||||||
|
Total
|
Level 1
|
Level 2
|
Level 3
|
|||||||||||||
|
Assets
|
||||||||||||||||
|
Marketable Securities
|
$ | - | $ | - | $ | - | $ | - | ||||||||
|
Liabilities
|
||||||||||||||||
|
Warrants
|
3,684,000 | - | - | 3,684,000 | ||||||||||||
|
Total
|
$ | 3,684,000 | $ | - | $ | - | $ | 3,684,000 | ||||||||
|
Fair Value of Redeemable
Warrants
(in thousands)
|
||||||||
|
2009
|
2010
|
|||||||
|
Balance at January 1
|
$ | 3,684 | ||||||
|
Fair value adjustment at March 31
|
1,336 | |||||||
|
Balance March 31
|
5,020 | |||||||
|
Value at issuance
|
$ | 17,359 | ||||||
|
Less: value of warrants exercised in May and June 2009
|
(3,742 | ) | ||||||
|
Fair value adjustment at June 30
|
7,185 | (2,260 | ) | |||||
|
Balance at June 3,
|
20,803 | 2,760 | ||||||
|
Fair value adjustment at September 30
|
(4,951 | ) | 583 | |||||
|
Balance at September 30
|
15,852 | 3,343 | ||||||
|
Fair value adjustment at December 31
|
(12,168 | ) | (538 | ) | ||||
|
Balance at December 31
|
$ | 3,684 | $ | 2,805 | ||||
|
(in thousands)
|
||||
|
Asset
|
||||
|
Balance at
|
||||
|
December 31, 2010
|
||||
|
Leased Equipment included with property and equipment
|
$ | 200 | ||
|
Less: accumulated depreciation
|
(20 | ) | ||
| $ | 180 | |||
|
2011
|
$ | 83 | ||
|
2012
|
50 | |||
|
2013
|
38 | |||
|
2014
|
27 | |||
|
2015
|
15 | |||
|
Total lease payments remaining
|
213 | |||
|
Less: amount representing interest
|
(56 | ) | ||
|
Present value of remaining minimum lease payments
|
157 | |||
|
Less: current obligations under lease obligations
|
(61 | ) | ||
|
Long-term capital lease obligations
|
$ | 96 |
|
|
March 31,
2010
|
June 30,
2010
|
September 30,
2010
|
December 31,
2010
|
Total
|
|||||||||||||||
|
Revenues
|
$ | 32 | $ | 41 | $ | 35 | $ | 27 | $ | 135 | ||||||||||
|
Costs and expenses
|
(4,105 | ) | (3,810 | ) | (3,727 | ) | (4,880 | ) | (16,522 | ) | ||||||||||
|
Interest & other
|
||||||||||||||||||||
|
Income (expense)
|
29 | 93 | 438 | 1,812 | 2,372 | |||||||||||||||
|
Redeemable warrants
|
||||||||||||||||||||
|
valuation adjustment
|
(1,336 | ) | 2,260 | (584 | ) | 539 | 879 | |||||||||||||
|
Net loss
|
$ | (5,380 | ) | $ | (1,416 | ) | $ | (3,838 | ) | $ | (2,502 | ) | $ | (13,136 | ) | |||||
|
Basic and diluted loss per share
|
$ | (.04 | ) | $ | (.01 | ) | $ | (.02 | ) | $ | $(.02 | ) | $ | (.10 | ) | |||||
|
|
March 31,
2009
|
June 30,
2009
|
September 30,
2009
|
December 31,
2009
|
Total
|
|||||||||||||||
|
Revenues
|
$ | 29 | $ | 17 | $ | 25 | $ | 40 | $ | 111 | ||||||||||
|
Costs and expenses
|
(2,882 | ) | (3,996 | ) | (2,483 | ) | (4,014 | ) | (13,375 | ) | ||||||||||
|
Finance costs
|
(241 | ) | - | - | - | (241 | ) | |||||||||||||
|
Interest & other
|
||||||||||||||||||||
|
Income (expense)
|
7 | 109 | 23 | (72 | ) | 67 | ||||||||||||||
|
Redeemable warrants
|
||||||||||||||||||||
|
valuation adjustment
|
- | (10,861 | ) | 4,950 | 12,169 | 6,258 | ||||||||||||||
|
Net loss
|
$ | (3,087 | ) | $ | (14,731 | ) | $ | 2,515 | $ | 8,123 | $ | (7,180 | ) | |||||||
|
Basic and diluted loss per share
|
$ | (.04 | ) | $ | (.15 | ) | $ | .02 | $ | .06 | $ | (.07 | ) | |||||||
|
Description
|
Balance
at
beginning
of
period
|
Charge
to
expense
|
Write-
offs
|
Balance
at end
of
period
|
||||||||||||
|
Year Ended December 31, 2008 Reserve for inventory
|
$ | 350 | $ | - | $ | (64 | ) | $ | 286 | |||||||
|
Year Ended December 31, 2009 Reserve for inventory
|
$ | 286 | $ | - | $ | (4 | ) | $ | 282 | |||||||
|
Year Ended December 31, 2010 Reserve for inventory
|
$ | 282 | $ | - | $ | (33 | ) | $ | 249 | |||||||