|
|
|
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) or (g) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
OR
|
||
|
|
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2019
|
|
OR
|
||
|
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
OR
|
||
|
|
|
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from __________ to __________
|
|
|
Title of each class
|
Trading Symbol(s)
|
|
Name of each exchange on which registered
|
|
|
|
|
|
|
|
SIX Swiss Exchange
|
|
|
|
|
||||||
|
Large Accelerated Filer
|
☐
|
Accelerated Filer
|
☐
|
|
☒
|
Emerging Growth Company
|
|
|
U.S. GAAP
|
☐
|
|
☒
|
Other
|
☐
|
|
as issued by the International Accounting Standards Board
|
|||||
|
INDEX
|
Page
|
|
|
Introduction and Use of Certain Terms
|
||
|
Market Information
|
||
|
Special Note About Forward-Looking Statements
|
||
|
PART I
|
||
|
Item 1.
|
Identity of Directors, Senior Management and Advisers
|
|
|
Item 2.
|
Offer Statistics and Expected Timetable
|
|
|
Item 3.
|
Key Information
|
|
|
Item 4.
|
Information on the Company
|
|
|
Item 4A.
|
Unresolved Staff Comments
|
|
|
Item 5.
|
Operating and Financial Review and Prospects
|
|
|
Item 6.
|
Directors, Senior Management and Employees
|
|
|
Item 7.
|
Major Shareholders and Related Party Transactions
|
|
|
Item 8.
|
Financial Information
|
|
|
Item 9.
|
The Offer and Listing
|
|
|
Item 10.
|
Additional Information
|
|
|
Item 11.
|
Quantitative and Qualitative Disclosures About Market Risk
|
|
|
Item 12.
|
Description of Securities Other than Equity Securities
|
|
|
PART II
|
||
|
Item 13.
|
Defaults, Dividend Arrearages and Delinquencies
|
|
|
Item 14.
|
Material Modifications to the Rights of Security Holders and Use of Proceeds
|
|
|
Item 15.
|
Controls and Procedures
|
|
|
Item 16A.
|
Audit Committee and Financial Expert
|
|
|
Item 16B.
|
Code of Ethics
|
|
|
Item 16C.
|
Principal Accountant Fees and Services
|
|
|
Item 16D.
|
Exemptions from the Listing Standards for Audit Committees
|
|
|
Item 16E.
|
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
|
|
|
Item 16F.
|
Change in Registrant's Certifying Accountant
|
|
|
Item 16G.
|
Corporate Governance
|
|
|
Item 16H.
|
Mine Safety Disclosure
|
|
|
PART III
|
||
|
Item 17.
|
Financial Statements
|
|
|
Item 18.
|
Financial Statements
|
|
|
Item 19.
|
Exhibits
|
|
|
Consolidated Financial Statements of Alcon Inc.
|
||
|
▪
|
the commercial success of its products and its ability to maintain and strengthen its position in its markets;
|
|
▪
|
the success of its research and development efforts, including its ability to innovate to compete effectively;
|
|
▪
|
its success in completing and integrating strategic acquisitions;
|
|
▪
|
pricing pressure from changes in third party payor coverage and reimbursement methodologies;
|
|
▪
|
global economic, financial, legal, tax, political, and social change;
|
|
▪
|
ongoing industry consolidation;
|
|
▪
|
its ability to properly educate and train healthcare providers on its products;
|
|
▪
|
changes in inventory levels or buying patterns of its customers;
|
|
▪
|
disruption in its global supply chain or important facilities;
|
|
▪
|
ability to service its debt obligations;
|
|
▪
|
the uncertainty as to what interest rate benchmark will replace LIBOR;
|
|
▪
|
the need for additional financing through the issuance of debt or equity;
|
|
▪
|
its reliance on outsourcing key business functions;
|
|
▪
|
its ability to protect its intellectual property;
|
|
▪
|
the impact on unauthorized importation of its products from countries with lower prices to countries with higher prices;
|
|
▪
|
the effects of litigation, including product liability lawsuits, and governmental investigations;
|
|
▪
|
its ability to comply with all laws to which it may be subject;
|
|
▪
|
effect of product recalls or voluntary market withdrawals;
|
|
▪
|
data breaches or other disruptions of its information technology systems;
|
|
▪
|
the implementation of its enterprise resource planning system;
|
|
▪
|
its ability to attract and retain qualified personnel;
|
|
▪
|
the accuracy of its accounting estimates and assumptions, including pension plan obligations, the carrying value of intangible assets, and our separation and transformation programs cost;
|
|
▪
|
the ability to obtain regulatory clearance and approval of its products as well as compliance with any post-approval obligations, including quality control of its manufacturing;
|
|
▪
|
legislative and regulatory reform;
|
|
▪
|
the ability of Alcon Pharmaceuticals Ltd. to comply with its investment tax incentive agreement with the Swiss State Secretariat for Economic Affairs in Switzerland and the Canton of Fribourg, Switzerland;
|
|
▪
|
the impact of environmental, social, and governance matters;
|
|
▪
|
its ability to operate as a stand-alone company;
|
|
▪
|
whether the transitional services Novartis has agreed to provide Alcon are sufficient;
|
|
▪
|
the impact of being listed on two stock exchanges;
|
|
▪
|
the ability to declare and pay dividends;
|
|
▪
|
the different rights afforded to its shareholders as a Swiss corporation compared to a US corporation; and
|
|
▪
|
the effect of maintaining or losing its foreign private issuer status under US securities laws.
|
|
ITEM 1.
|
IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
|
|
1.A.
|
DIRECTORS AND SENIOR MANAGEMENT
|
|
1.B.
|
ADVISERS
|
|
1.C.
|
AUDITORS
|
|
ITEM 2.
|
OFFER STATISTICS AND EXPECTED TIMETABLE
|
|
ITEM 3.
|
KEY INFORMATION
|
|
3.A.
|
SELECTED FINANCIAL DATA
|
|
($ millions except (loss)/earnings per share)
|
2019
|
|
2018
|
|
2017
|
|
2016
|
|
2015
|
|
|||||
|
Net sales to third parties
|
7,362
|
|
7,149
|
|
6,785
|
|
6,589
|
|
6,751
|
|
|||||
|
Net sales and other revenues
|
7,508
|
|
7,153
|
|
6,792
|
|
6,596
|
|
6,776
|
|
|||||
|
Operating (loss)/income
|
(187
|
)
|
(248
|
)
|
(77
|
)
|
10
|
|
417
|
|
|||||
|
Interest expense
|
(113
|
)
|
(24
|
)
|
(27
|
)
|
(31
|
)
|
(18
|
)
|
|||||
|
Other financial income & expense
|
(32
|
)
|
(28
|
)
|
(23
|
)
|
(92
|
)
|
(48
|
)
|
|||||
|
(Loss)/income before taxes
|
(332
|
)
|
(300
|
)
|
(127
|
)
|
(113
|
)
|
351
|
|
|||||
|
Taxes
|
(324
|
)
|
73
|
|
383
|
|
(57
|
)
|
(43
|
)
|
|||||
|
Net (loss)/income
|
(656
|
)
|
(227
|
)
|
256
|
|
(170
|
)
|
308
|
|
|||||
|
(Loss)/earnings per share
|
|||||||||||||||
|
Basic
|
(1.34
|
)
|
(0.46
|
)
|
0.52
|
|
(0.35
|
)
|
0.63
|
|
|||||
|
Diluted
|
(1.34
|
)
|
(0.46
|
)
|
0.52
|
|
(0.35
|
)
|
0.63
|
|
|||||
|
Weighted average number of shares outstanding (millions)
(1)
|
|||||||||||||||
|
Basic
|
488.2
|
|
488.2
|
|
488.2
|
|
488.2
|
|
488.2
|
|
|||||
|
Diluted
|
488.2
|
|
488.2
|
|
488.2
|
|
488.2
|
|
488.2
|
|
|||||
|
(1)
|
F
or periods prior to the Spin-off, the denominator for basic and diluted earnings per share was calculated using the
488.2 million
shares of common stock distributed in the Spin-off.
|
|
|
At December 31,
|
|||||||||||||
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
2016
|
|
2015
|
|
||||
|
|
|
|
|
|
|
|
|
|||||||
|
Cash and cash equivalents
|
822
|
|
227
|
|
172
|
|
162
|
|
285
|
|
||||
|
Inventories
|
1,505
|
|
1,440
|
|
1,303
|
|
1,207
|
|
1,149
|
|
||||
|
Other current assets
|
1,909
|
|
1,732
|
|
1,812
|
|
1,650
|
|
1,540
|
|
||||
|
Non-current assets
|
23,419
|
|
23,663
|
|
24,101
|
|
24,721
|
|
25,228
|
|
||||
|
Total assets
|
27,655
|
|
27,062
|
|
27,388
|
|
27,740
|
|
28,202
|
|
||||
|
Trade payables
|
833
|
|
663
|
|
615
|
|
516
|
|
493
|
|
||||
|
Other current liabilities
|
1,467
|
|
1,230
|
|
1,163
|
|
1,149
|
|
1,150
|
|
||||
|
Non-current liabilities
|
6,052
|
|
2,530
|
|
2,581
|
|
3,063
|
|
2,922
|
|
||||
|
Total liabilities
|
8,352
|
|
4,423
|
|
4,359
|
|
4,728
|
|
4,565
|
|
||||
|
Equity
|
19,303
|
|
22,639
|
|
23,029
|
|
23,012
|
|
23,637
|
|
||||
|
Total equity and liabilities
|
27,655
|
|
27,062
|
|
27,388
|
|
27,740
|
|
28,202
|
|
||||
|
Net assets
|
19,303
|
|
22,639
|
|
23,029
|
|
23,012
|
|
23,637
|
|
||||
|
Outstanding share capital
|
20
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||
|
Total outstanding shares (millions)
(1)
|
488.3
|
|
488.2
|
|
488.2
|
|
488.2
|
|
488.2
|
|
||||
|
(1)
|
F
or periods prior to the Spin-off, the shares outstanding represent the
488.2 million
shares of common stock distributed in the Spin-off.
|
|
($ per CHF)
|
Average
(1)
|
|
|
Year ended December 31, 2015
|
1.04
|
|
|
Year ended December 31, 2016
|
1.01
|
|
|
Year ended December 31, 2017
|
1.02
|
|
|
Year ended December 31, 2018
|
1.02
|
|
|
Year ended December 31, 2019
|
1.01
|
|
|
($ per CHF)
|
Low
(2)
|
|
High
(2)
|
|
|
|
January 2019
|
1.00
|
1.01
|
|||
|
February 2019
|
1.00
|
1.01
|
|||
|
March 2019
|
1.00
|
1.01
|
|||
|
April 2019
|
0.98
|
0.98
|
|||
|
May 2019
|
0.99
|
1.00
|
|||
|
June 2019
|
1.02
|
1.03
|
|||
|
July 2019
|
1.01
|
1.01
|
|||
|
August 2019
|
1.01
|
1.01
|
|||
|
September 2019
|
1.00
|
|
1.01
|
|
|
|
October 2019
|
1.01
|
|
1.01
|
|
|
|
November 2019
|
1.00
|
|
1.00
|
|
|
|
December 2019
|
1.03
|
|
1.04
|
|
|
|
January 2020
|
1.00
|
|
1.03
|
|
|
|
February 2020 (through February 21, 2020)
|
1.02
|
|
1.04
|
|
|
|
3.B.
|
|
|
3.C.
|
REASONS FOR THE OFFER AND USE OF PROCEEDS
|
|
3.D.
|
RISK FACTORS
|
|
▪
|
disruptive product technology;
|
|
▪
|
alternative treatment modalities;
|
|
▪
|
breadth of product lines and product services;
|
|
▪
|
ability to identify new market trends;
|
|
▪
|
acceptance of equipment and other products by ophthalmic surgeons;
|
|
▪
|
customer and clinical support;
|
|
▪
|
regulatory status and speed to market;
|
|
▪
|
price;
|
|
▪
|
product quality, reliability and performance;
|
|
▪
|
capacity to recruit engineers, scientists and other qualified associates;
|
|
▪
|
digital initiatives that change business models;
|
|
▪
|
reimbursement approval from governmental payors and private healthcare insurance providers; and
|
|
▪
|
reputation for technical leadership.
|
|
▪
|
make it difficult for us to satisfy our obligations, including making interest payments on our debt obligations;
|
|
▪
|
require us to dedicate a portion of our cash flows to payments on our debt, reducing our ability to use our cash flows to fund capital expenditures, BD&L or other strategic transactions, working capital and other general operational requirements, or to pay dividends to our shareholders;
|
|
▪
|
limit our flexibility to plan for and react to changes in our business;
|
|
▪
|
negatively impact our credit rating and increase the cost of servicing our debt;
|
|
▪
|
place us at a competitive disadvantage relative to some of our competitors that have less debt than us;
|
|
▪
|
increase our vulnerability to general adverse economic and industry conditions, including changes in interest rates or a downturn in our business or the economy; and
|
|
▪
|
make it difficult to refinance our existing debt or incur new debt on terms that we would consider to be commercially reasonable, if at all.
|
|
▪
|
finance unanticipated working capital requirements or refinance our existing indebtedness;
|
|
▪
|
develop or enhance our infrastructure and our existing products and services;
|
|
▪
|
engage in mergers and acquisitions or strategic BD&L transactions;
|
|
▪
|
fund strategic relationships; and
|
|
▪
|
respond to competitive pressures.
|
|
▪
|
warning letters or untitled letters issued by the FDA;
|
|
▪
|
fines, civil penalties, in rem forfeiture proceedings, injunctions, consent decrees and criminal prosecution;
|
|
▪
|
detention of imported products;
|
|
▪
|
delays in approving, or refusal to approve, our products;
|
|
▪
|
withdrawal or suspension of approval of our products or those of our third-party suppliers by the FDA or other regulatory bodies;
|
|
▪
|
product recall or seizure;
|
|
▪
|
operating restrictions or interruption of production; and
|
|
▪
|
inability to export to certain countries.
|
|
▪
|
the non-Swiss court had jurisdiction pursuant to the Swiss Federal Act on Private International Law;
|
|
▪
|
the judgment of such non-Swiss court has become final and non-appealable;
|
|
▪
|
the judgment does not contravene Swiss public policy;
|
|
▪
|
the court procedures and the service of documents leading to the judgment were in accordance with the due process of law; and
|
|
▪
|
no proceeding involving the same position and the same subject matter was first brought in Switzerland, or adjudicated in Switzerland, or was earlier adjudicated in a third state and this decision is recognizable in Switzerland.
|
|
ITEM 4.
|
INFORMATION ON THE COMPANY
|
|
4.A.
|
HISTORY AND DEVELOPMENT OF THE COMPANY
|
|
▪
|
Series 2026 Notes - $0.5 billion due in 2026 issued at 99.5%, 2.750% interest is payable twice per year in March and September, beginning in March 2020 (“2026 Notes”).
|
|
▪
|
Series 2029 Notes - $1.0 billion due in 2029 issued at 99.6%, 3.000% interest is payable twice per year in March and September, beginning March 2020 (“2029 Notes”).
|
|
▪
|
Series 2049 Notes - $0.5 billion due in 2049 issued at 99.8%, 3.800% interest is payable twice per year in March and September, beginning March 2020 (“2049 Notes”).
|
|
4.B.
|
BUSINESS OVERVIEW
|

|
•
|
Aging population with growing eye care needs
: A growing aging population continues to drive the increased prevalence of eye care conditions worldwide, as the number of persons aged 60 years or over is expected to more than double by 2050, rising from 962 million globally in 2017 to 2.1 billion in 2050.
|
|
•
|
Innovation improving the quality of eye care
: Technology innovation in eye care is driving an increased variety of products that more effectively treat eye conditions. Given the importance of vision correction and preservation, which can provide a high return on healthcare spend, the resulting better patient outcomes are leading to increased coverage and reimbursement opportunities from governmental and private third-party payers, expanding patient access to such eye care products.
|
|
•
|
Increasing wealth and growth from emerging economies
: It is estimated that between 2015 and 2030, the middle class population in emerging markets will grow by approximately 1.5 billion people, from 2.0 billion to 3.5 billion; this major demographic shift is generating a large, new customer base with increased access to eye care products and services along with the resources to pay for them. The expansion of training opportunities for eye care professionals in emerging markets is also leading to increased patient awareness and access to premium eye care products and surgical procedures, facilitating their growth.
|
|
•
|
Increasing prevalence of myopia, progressive myopia and digital eye strain
: It is estimated that by 2050, half of the world's population (nearly five billion people) will be myopic. Further, the modern work environment, along with leisure preferences, have increased the number of hours people spend in front of a screen, adversely impacting vision and increasing the risk of progressive myopia and digital eye strain.
|
|
•
|
Global growth of cataract and vitreoretinal procedures, driven by an aging population;
|
|
•
|
Increased access to care, for example, in emerging markets and other markets outside the US where the cataract surgery rate is 3.2 procedures per 1,000 people as compared to 12.7 in the US;
|
|
•
|
Higher uptake of premium patient-pay technologies, for example AT-IOL penetration is only 7% outside the US versus 14% in the US;
|
|
•
|
Increased adoption of advanced technologies, for example, improved diagnostic instruments, surgical options for glaucoma management, and the growing use of phacoemulsification during cataract removal, which is utilized in less than 50% of cases in emerging markets versus over 95% in the US; and
|
|
•
|
Eye disease as a comorbidity linked to the global prevalence of diabetes, which has nearly doubled from 4.7% in 1980 to 8.5% in 2014, combined with improving diagnostics capabilities and new product innovations, driving uptake of premium procedures.
|
|
•
|
Continued modality shift to daily disposable lenses from reusable lenses and the resulting sales premium (an increase of 2
-
3x sales per patient, after customary rebates and discounts) associated with daily disposable wearers as compared to users of reusable lenses;
|
|
•
|
Advancements in specialty lenses combined with increasing demand for toric, multifocal and cosmetic lenses, which command an approximately 15
-
30% pricing premium over spherical lenses, allowing patients to continue wearing contact lenses as they become older and helping to expand the market;
|
|
•
|
A significant population of approximately 194 million undiagnosed dry eye patients, with an additional 42 million self-diagnosed dry eye patients using unsuitable products for treatment, and advances in diagnostics and ocular health treatments, facilitating the increase in patient awareness of dry eye and treatment;
|
|
•
|
Growing access and consumption of vision care products in emerging markets such as Asia, which had an estimated single-digit contact lens penetration as compared to double digits in the developed world; and
|
|
•
|
Increasing consumer access through the expansion of distribution models, including internet sales and other direct-to-consumer channels.
|
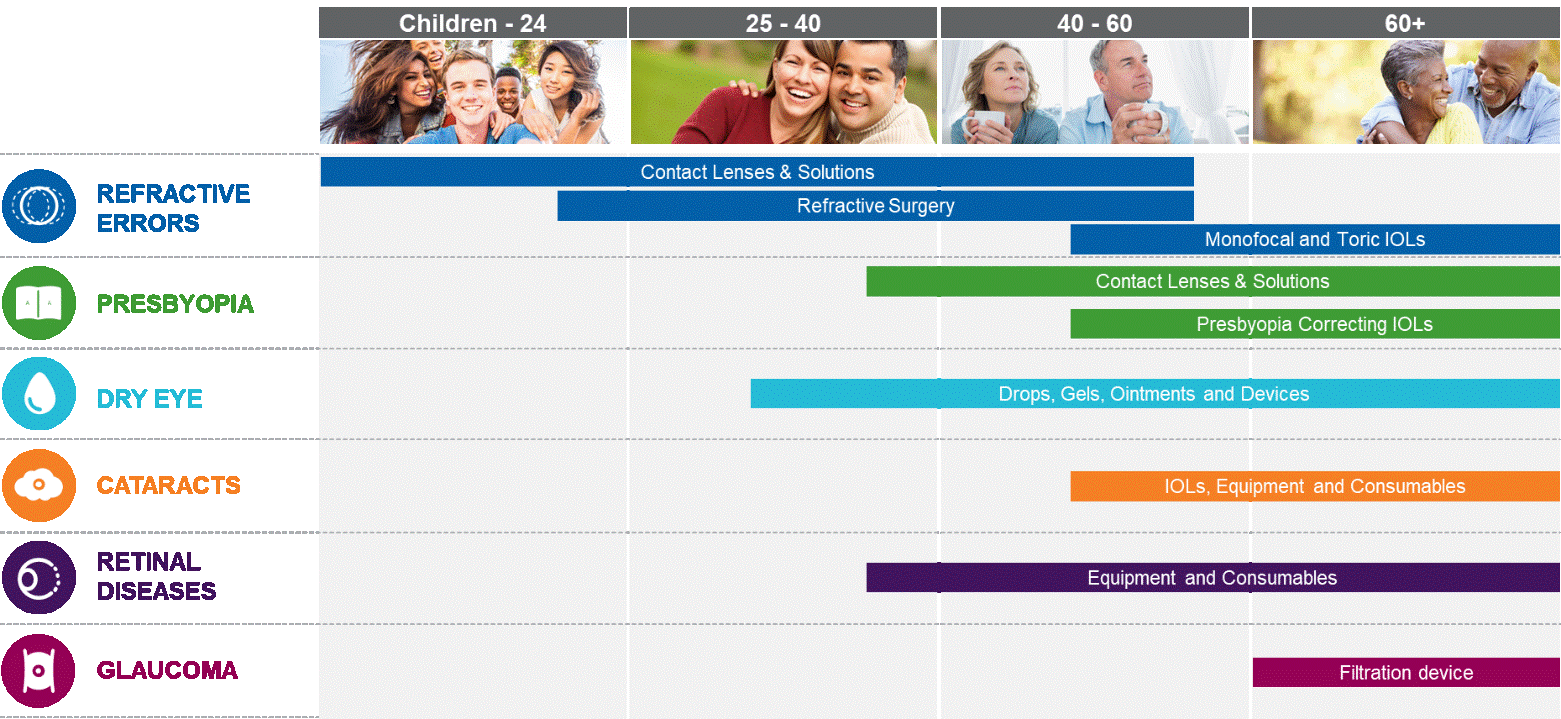


|
•
|
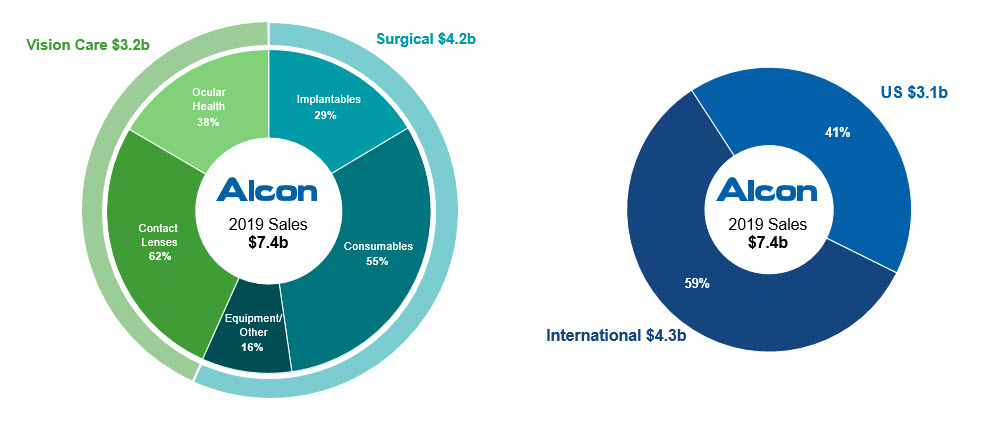
Global leader in highly attractive markets with the most complete brand portfolio.
With
$7.4 billion
in net sales in the year ended December 31, 2019, we are the leader in an attractive eye care market, which is supported by favorable population megatrends and is expected to grow at approximately 4% to 5% per year from 2019 to 2024. Our Surgical business is the market leader in sales of ophthalmic equipment used in the operating room and is supported by the largest installed base of equipment worldwide, which we use to cross-promote our surgical consumables and IOLs. In our Vision Care business, our extensive portfolio of contact lens and ocular health products includes well-recognized brands such as
Dailies
,
Systane
and
Opti-Free
. We believe our global leadership position and extensive brand portfolio allow us to benefit and build on the robust fundamentals driving growth in our markets.
|
|
•
|
Innovation-focused with market leading development capabilities and investment.
We have made one of the largest commitments to research and development in the eye care market, with proven R&D capabilities in the areas of optical design, material and surface chemistry, automation and equipment platforms. Currently, we employ over 1,200 individuals dedicated to our research and development efforts, including physicians, doctors of optometry and PhDs. In addition, we actively seek opportunities to collaborate with third parties on advanced technologies to support our eye care business.
|
|
•
|
Global scale and reach supported by high-quality manufacturing network.
We have an extensive global commercial footprint that provides us with the scale and reach to support future growth, maximize the potential of new launches, enter new geographies efficiently and to take advantage of the large, dynamic and growing surgical and vision care markets. Our commercial footprint, which includes operations in over 74 countries, reaches consumers and patients in over 140 countries and is supported by over 3,000 sales force associates, 18 state-of-the-art manufacturing facilities employing our proprietary technologies and know-how, and our extensive global regulatory capability. Our extensive sales and distribution network, supported by our market leadership position and focus on innovation and customer experience, enhances our ability to expand our geographic reach and extend our product offerings through the launch of new and innovative products worldwide.
|
|
•
|
Outstanding customer relationships and a trusted reputation for customer service, training and education.
We believe that maintaining the highest levels of service excellence in our customer experience is a critical success factor in our industry. In our Vision Care business, we regularly meet with eye care practitioners to gain feedback and insights on our products and consumers' needs. We also provide training support at our approximately 30 state-of-the-art interactive training centers around the world, as well as through numerous digital and event-based training programs that we provide for practitioners, clinical support staff, students, residents, patients and consumers. In each of our businesses, we have built and maintained our relationships with key stakeholders to establish our trusted reputation in the industry.
|
|
•
|
World leading expertise in eye care led by a first-class management team.
Our expertise in eye care is driven by our more than 70-year history in the industry and is supported by a high-quality workforce of more than 20,000 associates. We believe our institutional knowledge provides a competitive advantage because our associates' industry expertise, relationships with our customers and understanding of the development, manufacture and sale of our products helps us to better identify new customer needs, assess markets for entry and identify promising technologies. In addition, we believe the diverse experience of our management team in running complex businesses allows them to add significant value to our company. In particular, we benefit from having a management team with an extensive background in the medical device industry. Led by David J. Endicott, our Chief Executive Officer, our management team's deep knowledge of eye care has allowed us to build a more nimble medical device culture within Alcon and created excitement among our workforce for our mission.
|
|
•
|
Maximize the potential of our near-term portfolio by growing key products.
In Surgical, we plan to build on our leading position in the IOL market through the launch of new AT-IOLs, where premium pricing drives market value. In addition, we expect improved diagnostics and new optical designs will address historical barriers to AT-IOL adoption to further grow this patient-pay market. We will also continue to invest behind our presbyopia-correcting products (e.g.
Panoptix
), and will continue to invest in our vitreoretinal equipment and consumables, where we also see meaningful opportunities for near-term growth. In Vision Care, we intend to maintain and grow our leading position in most of our product categories through increased eye care professional and consumer education, supported by continuous production innovation. We intend to expand our position in the daily disposable category behind our
DAILIES TOTAL1 and PRECISION1
family of products. We also aim to expand the dry eye product market by leveraging our well-recognized
Systane
family of eye drops and increasing investment in dry eye education and awareness, where we see a significant unmet need and an opportunity for robust market growth.
|
|
•
|
Accelerate innovation and deliver the next wave of technologies.
We are committed to accelerating innovation by continuing to be one of the market leaders in investment in ophthalmic research and development. The R&D activities of our Surgical business are focused on expanding our AT-IOL portfolio to further improve surgical and refractive outcomes, including through the use of advanced optics, light adjustable materials, accommodating lenses and modular platforms. We are also developing next-generation lasers, robotics and other equipment for cataract, vitreoretinal and laser-refractive surgery, as well as improved visualization equipment. In our Vision Care business, our focus is on developing and launching new contact lens materials, coatings and designs to extend our product lines and improve patient comfort, as well as on new products to expand our portfolio of dry eye diagnostic and treatment, presbyopia and ocular health products. Finally, we expect to continue to supplement our internal innovation investments by identifying and executing on attractive acquisition, licensing and collaboration opportunities with leading academic institutions and early-stage companies.
|
|
•
|
Capture opportunities to expand markets and pursue adjacencies.
We believe there is a significant opportunity for growth in markets around the world due to under-penetration of both premium surgical devices, such as AT-IOLs, and of our Vision Care portfolio. We intend to facilitate this growth by continued investment in promotion and
|
|
•
|
Support new business models to expand customer experience.
In Surgical, we intend to continue to identify new business models that benefit healthcare providers and improve access to leading Alcon products and technologies. For example, we are pursuing value-based business models that reward improved patient outcomes, as well as models that contract the entire procedure versus individual products. In Vision Care, where e-commerce entries have created some disruption of traditional sales channels, we believe that digital technology can address pain points experienced in existing paths to purchase. We intend to continue investing and innovating in digital capabilities to develop new business models in response to channel shifts and the increase in direct-to-consumer influence.
|
|
•
|
Leverage infrastructure to improve operating efficiencies and margin profile over time.
With the significant organizational and infrastructure investments we have made over the last several years, we believe we have established a stable foundation that will allow us to continue to enhance the productivity of our commercial resources and meaningfully improve our core operating income margins over time. Further, we intend to improve the mix of our products, implement further supply chain efficiency initiatives and support new lower-cost manufacturing platforms to drive future operating profit and cash flows.
|
|
Cataract
|

|
AcrySof
family of IOLs, including:
AcrySof
IQ monofocal IOLs
|

|
UltraSert
pre-loaded IOL delivery system with the
AcrySof
IQ monofocal IOL
|
|
|
AcrySof
IQ Toric astigmatism-correcting IOLs
AcrySof
IQ
ReSTOR
presbyopia-correcting IOLs
AcrySof
IQ
ReSTOR
Toric presbyopia- and astigmatism-correcting IOLs
AcrySof
IQ
PanOptix
presbyopia-correcting IOLs
AcrySof
IQ
PanOptix
Toric presbyopia- and astigmatism-correcting IOLs
|
||

|
Clareon
monofocal IOL with the automated, disposable
AutonoMe
pre-loaded IOL delivery system
|
|
|
Cataract Refractive Suite by Alcon, including:
|
||

|
Centurion
vision system
LenSx
femtosecond laser
|
|

|
LuxOR
ophthalmic microscope
ORA
System for intra-operative measurements and guidance
Verion
imaged guided system
|
|
|
Surgical Procedure Packs
|

|
Custom Pak
surgical procedure packs
|
|
Vitreoretinal
|

|
Constellation
vision system
|

|
Grieshaber
DSP and
MIVS
instrumentation
|
|

|
Purepoint
laser
|
|
|
Ultravit
high speed vitrectomy probes
|
|
|
NGENUITY
3D visualization system
|
|
|
Refractive
|

|
WaveLight
EX500 excimer laser for LASIK and other refractive correction procedures
|
|
WaveLight Topolyzer
VARIO diagnostic device for measurement and planning before refractive surgery
|
||
|
WaveLight
FS200 femtosecond laser for refractive surgery
|
||
|
Glaucoma
|

|
EX-PRESS
glaucoma filtration device
|
|
Contact Lenses
|

|
DAILIES TOTAL1
|

|
PRECISION1
|
|

|
DAILIES AquaComfort PLUS
|
|

|
Air Optix
family of silicone hydrogel contact lenses (including
Air Optix plus HydraGlyde
and
Air Optix Colors
lenses)
|
|

|
||

|
FreshLook
family of color contact lenses
|
|
|
Ocular Health
|

|
Clear Care
family of hydrogen peroxide contact lens care solution (
AOSEPT
PLUS
outside of North America)
|

|
Opti-Free
family of multi-purpose disinfecting contact lens care solution
|
|

|
Genteal
family of artificial tears
|
|

|
Systane
family of artificial tears and related dry eye products
|
|

|
Tears Naturale
family of lubricant eye drops
|
|

|
Systane iLux
Thermal Pulsation System
|
|
|
•
|
disruptive product technology;
|
|
•
|
alternative treatment modalities;
|
|
•
|
breadth of product lines and product services;
|
|
•
|
ability to identify new market trends;
|
|
•
|
acceptance by ophthalmic surgeons;
|
|
•
|
customer and clinical support;
|
|
•
|
regulatory status and speed to market;
|
|
•
|
price;
|
|
•
|
product quality, reliability and performance;
|
|
•
|
capacity to recruit engineers, scientists and other qualified associates;
|
|
•
|
digital initiatives that change business models;
|
|
•
|
reimbursement approval from governmental payers and private healthcare insurance providers; and
|
|
•
|
reputation for technical leadership.
|
|
4.C.
|
ORGANIZATIONAL STRUCTURE
|
|
Name
|
Country of formation
|
|
% of equity interest
|
|
|
Alcon Pharmaceuticals Ltd.
|
Switzerland
|
|
100
|
|
|
Alcon Vision, LLC
|
United States
|
|
100
|
|
|
Alcon Laboratories, Inc.
|
United States
|
|
100
|
|
|
4.D.
|
PROPERTY, PLANTS AND EQUIPMENT
|
|
Location
|
Size of Site
(in m
2
)
|
Major Activity
|
|||
|
Fort Worth, Texas
|
315,200
|
|
Production, research and development for Surgical and Vision Care businesses
|
||
|
Johns Creek, Georgia
|
84,100
|
|
Production, research and development for Vision Care business
|
||
|
Grosswallstadt, Germany
|
82,300
|
|
Production, research and development for Vision Care business
|
||
|
Johor, Malaysia
|
43,900
|
|
Production for Vision Care business
|
||
|
Irvine, California
|
40,800
|
|
Production, research and development for Surgical business
|
||
|
Houston, Texas
|
37,400
|
|
Production for Surgical business
|
||
|
Batam, Indonesia
|
35,000
|
|
Production for Vision Care business
|
||
|
Singapore
|
35,000
|
|
Production for Vision Care business
|
||
|
Huntington, West Virginia
|
27,500
|
|
Production for Surgical business
|
||
|
Sinking Spring, Pennsylvania
|
21,800
|
|
Production for Surgical business
|
||
|
Cork, Ireland
|
13,600
|
|
Production for Surgical business
|
||
|
Puurs, Belgium
|
8,000
|
|
Production for Surgical business
|
||
|
Schaffhausen, Switzerland
|
4,100
|
|
Production for Surgical business
|
||
|
ITEM 4A.
|
UNRESOLVED STAFF COMMENTS
|
|
5.A.
|
OPERATING RESULTS
|
|
•
|
For certain of the periods covered by our Consolidated Financial Statements, our business was operated within legal entities which hosted portions of other Novartis businesses. In addition, in all the periods presented, our Consolidated Financial Statements include the ophthalmic over-the-counter products and a small portfolio of surgical diagnostics medications, the management and reporting of which was transferred to Alcon from the Innovative Medicines Division of Novartis effective as of January 1, 2018.
|
|
•
|
For periods prior to the Spin-off, income taxes attributable to the Alcon Division were determined using the separate return approach, under which current and deferred income taxes were calculated as if a separate tax return had been prepared in each tax jurisdiction. In various tax jurisdictions, Alcon and Novartis businesses operated within the same legal entity and certain Alcon subsidiaries were part of a Novartis tax group. This required an assumption that the subsidiaries and operations of Alcon in those tax jurisdictions operated on a standalone basis and constitute separate taxable entities. Actual outcomes and results could differ from these separate tax return estimates, including those estimates and assumptions related to realization of tax benefits within these Novartis tax groups.
|
|
•
|
For periods prior to the Spin-off, our Consolidated Financial Statements also include an allocation and charges of expenses related to certain Novartis functions. However, the allocations and charges may not be indicative of the actual expense that would have been incurred had we operated as an independent, publicly traded company during those periods. For example, historically, our business has been charged with a significant portion of appropriate administrative costs, such as those related to services Alcon has received from Novartis across the following service domains: human resources operations, real estate and facility services, including site security and executive protection, procurement, information technology, commercial and medical support services and financial reporting and accounting operations, and these have been reflected in our Consolidated Financial Statements based on historical allocations and charges. Accordingly, these overhead costs were affected by the historical arrangements that existed between the historical reporting units of the Alcon Division and Novartis and typically did not include a profit margin.
|
|
•
|
For periods prior to the Spin-off, our Consolidated Financial Statements also include an allocation from Novartis of certain corporate related general and administrative expenses that we would have incurred as a publicly traded company. These include costs associated with corporate governance, including board of directors, corporate responsibility and other corporate functions, such as tax, corporate governance and listed company compliance, investor relations, internal audit, treasury and communications functions. The allocation of these additional expenses may not be indicative of the actual expense that would have been incurred had we operated as an independent, publicly traded company for those periods.
|
|
•
|
On August 28, 2018, we announced our immediate, voluntary market withdrawal of our
CyPass
micro-stent surgical glaucoma product from the global market. Our Consolidated Financial Statements include the sales of
CyPass
micro-stent products from and after the launch of the product in 2016 until our withdrawal of the product from the market in August 2018. As a result, in the year ended December 31, 2018, we recognized a one-time pre-tax charge of $282 million (after tax $206 million). This consisted of $11 million for the costs associated with the market withdrawal and $337 million for the impairment of the
CyPass
intangible assets. These charges were partially offset by the $66 million gain for the reduction in the related contingent consideration liability.
|
|
•
|
Fix the foundation (2016–2017)
: The initial phase of our growth plan in 2016 and 2017 focused on fixing the foundation of Alcon by investing in promotion, capital and systems, reinvigorating the innovation pipeline, and strengthening our customer relationships. Improving the culture at Alcon has also been a top priority, and the organization has responded with significant morale improvement. Strong results have followed, including sales returning to growth.
|
|
•
|
Execute the growth plan (2018–2020)
: We began the second phase of our growth plan in 2018, with a focus on superior execution, further investing in high-potential products and market segments and accelerating our product development cycle. We have begun to transform our company by cultivating a more nimble and agile culture. In our surgical business, we intend to continue to expand and grow the premium IOL market with our AT-IOL offerings and our
PanOptix
brand of presbyopia correcting IOLs ("PC-IOLs"). We also plan to expand our vitreoretinal business, in part through enhancing technology penetration in key markets and by accelerating conversion from optical to digital surgery. In our vision care business, we intend to grow our
DAILIES TOTAL1
family of products and expand the presbyopia category through increased consumer awareness, lens comfort and quality. We also plan to continue the global roll-out of our
Systane
COMPLETE product and grow consumer demand with investments in direct-to-consumer marketing.
|
|
•
|
Deliver leading-edge solutions (2021 and beyond)
: Following the completion of the second phase of our growth plan, the third phase will focus on accelerating innovation, capturing opportunities to expand markets and pursue adjacencies and developing new business models to improve access to our leading product portfolio.
|
|
•
|
the amount and timing of projected cash flows;
|
|
•
|
the timing and probability of regulatory and commercial success;
|
|
•
|
the royalty rate for the Alcon brand name;
|
|
•
|
the terminal growth rate; and
|
|
•
|
the discount rate.
|
|
•
|
the amount and timing of projected cash flows;
|
|
•
|
long-term sales forecasts;
|
|
•
|
the timing and probability of regulatory and commercial success; and
|
|
•
|
the appropriate discount rate.
|
|
2019 compared to 2018
|
2018 compared to 2017
|
||||||||||||||||||
|
Change %
|
Change %
|
||||||||||||||||||
|
($ millions unless indicated otherwise)
|
2019
|
|
2018
|
|
$
|
|
cc
(1)
|
|
2017
|
|
$
|
cc
(1)
|
|
||||||
|
|
|||||||||||||||||||
|
Net sales to third parties
|
7,362
|
|
7,149
|
|
3
|
|
5
|
|
6,785
|
|
5
|
5
|
|
||||||
|
Gross profit
|
3,662
|
|
3,192
|
|
15
|
|
19
|
|
3,204
|
|
—
|
(1
|
)
|
||||||
|
Operating (loss)
|
(187
|
)
|
(248
|
)
|
25
|
|
54
|
|
(77
|
)
|
nm
|
nm
|
|
||||||
|
Operating margin (%)
|
(2.5
|
)
|
(3.5
|
)
|
(1.1
|
)
|
|||||||||||||
|
Net (loss)/income
|
(656
|
)
|
(227
|
)
|
(189
|
)
|
(163
|
)
|
256
|
|
nm
|
nm
|
|
||||||
|
Basic and diluted (loss)/earnings per share ($)
(2)
|
(1.34
|
)
|
(0.46
|
)
|
(191
|
)
|
(163
|
)
|
0.52
|
|
nm
|
nm
|
|
||||||
|
Core results
(1)
|
|||||||||||||||||||
|
Core operating income
|
1,265
|
|
1,212
|
|
4
|
|
11
|
|
1,086
|
|
12
|
12
|
|
||||||
|
Core operating margin %
|
17.2
|
|
17.0
|
|
16.0
|
|
|||||||||||||
|
Core net income
|
925
|
|
974
|
|
(5
|
)
|
1
|
|
908
|
|
7
|
8
|
|
||||||
|
Core basic earnings per share ($)
(2)
|
1.89
|
|
2.00
|
|
(6
|
)
|
1
|
|
1.86
|
|
8
|
8
|
|
||||||
|
Core diluted earnings per share ($)
(3)
|
1.89
|
|
2.00
|
|
(6
|
)
|
1
|
|
1.86
|
|
8
|
8
|
|
||||||
|
(1)
|
Core results and constant currencies (cc) as presented in this table are non-IFRS measures. Alcon uses certain non-IFRS metrics when measuring performance, including when measuring current period results against prior periods. Refer to "Item
5.A. Operating Results
—
Non-IFRS measures as defined by the Company
" section for additional information and reconciliation tables.
|
|
(2)
|
Calculated using
488.2
million shares for both current and prior year periods.
|
|
(3)
|
Calculated using
490.1
million weighted average diluted shares for the year ended December 31, 2019, and
488.2
million shares for the prior year periods.
|
|
2019 compared to 2018
|
2018 compared to 2017
|
|||||||||||||||
|
Change %
|
Change %
|
|||||||||||||||
|
($ millions unless indicated otherwise)
|
2019
|
|
2018
|
|
$
|
cc
(1)
|
2017
|
|
$
|
cc
(1)
|
||||||
|
|
||||||||||||||||
|
Surgical
|
|
|
|
|
|
|
|
|
||||||||
|
Implantables
|
1,210
|
|
1,136
|
|
7
|
9
|
1,045
|
|
9
|
9
|
||||||
|
Consumables
|
2,304
|
|
2,227
|
|
3
|
6
|
2,104
|
|
6
|
5
|
||||||
|
Equipment/other
|
660
|
|
636
|
|
4
|
6
|
584
|
|
9
|
9
|
||||||
|
Total Surgical
|
4,174
|
|
3,999
|
|
4
|
7
|
3,733
|
|
7
|
7
|
||||||
|
Vision Care
|
||||||||||||||||
|
Contact lenses
|
1,969
|
|
1,928
|
|
2
|
4
|
1,836
|
|
5
|
4
|
||||||
|
Ocular health
|
1,219
|
|
1,222
|
|
—
|
2
|
1,216
|
|
—
|
1
|
||||||
|
Total Vision Care
|
3,188
|
|
3,150
|
|
1
|
3
|
3,052
|
|
3
|
3
|
||||||
|
Net sales to third parties
|
7,362
|
|
7,149
|
|
3
|
5
|
6,785
|
|
5
|
5
|
||||||
|
(1)
|
Constant currencies is a non-IFRS measure. Refer to "Item
5.A. Operating Results
—
Non-IFRS measures as defined by the Company
" section for additional information.
|
|
2019 compared to 2018
|
2018 compared to 2017
|
|||||||||||||||
|
Change %
|
Change %
|
|||||||||||||||
|
($ millions unless indicated otherwise)
|
2019
|
|
2018
|
|
$
|
|
cc
(1)
|
|
2017
|
|
$
|
|
cc
(1)
|
|
||
|
Gross profit
|
3,662
|
|
3,192
|
|
15
|
|
19
|
|
3,204
|
|
—
|
|
(1
|
)
|
||
|
Selling, general & administration
|
(2,847
|
)
|
(2,801
|
)
|
(2
|
)
|
(4
|
)
|
(2,596
|
)
|
(8
|
)
|
(7
|
)
|
||
|
Research & development
|
(656
|
)
|
(587
|
)
|
(12
|
)
|
(12
|
)
|
(584
|
)
|
(1
|
)
|
—
|
|
||
|
Other income
|
55
|
|
47
|
|
17
|
|
19
|
|
47
|
|
—
|
|
1
|
|
||
|
Other expense
|
(401
|
)
|
(99
|
)
|
nm
|
|
nm
|
|
(148
|
)
|
33
|
|
33
|
|
||
|
Operating (loss)
|
(187
|
)
|
(248
|
)
|
25
|
|
54
|
|
(77
|
)
|
nm
|
|
nm
|
|
||
|
Operating margin (%)
|
(2.5
|
)
|
(3.5
|
)
|
(1.1
|
)
|
||||||||||
|
Core results
(1)
|
||||||||||||||||
|
Core gross profit
|
4,663
|
|
4,541
|
|
3
|
|
6
|
|
4,211
|
|
8
|
|
8
|
|
||
|
Core operating income
|
1,265
|
|
1,212
|
|
4
|
|
11
|
|
1,086
|
|
12
|
|
12
|
|
||
|
Core operating margin (%)
|
17.2
|
|
17.0
|
|
16.0
|
|
||||||||||
|
(1)
|
Core results and constant currencies are non-IFRS measures. Refer to "Item
5.A. Operating Results
—
Non-IFRS measures as defined by the Company
" section for additional information and reconciliation tables.
|
|
2019 compared to 2018
|
2018 compared to 2017
|
|||||||||||||||
|
($ millions unless indicated otherwise)
|
Change %
|
Change %
|
||||||||||||||
|
2019
|
|
2018
|
|
$
|
|
cc
(2)
|
|
2017
|
|
$
|
|
cc
(2)
|
|
|||
|
Surgical segment contribution
|
923
|
|
813
|
|
14
|
|
19
|
|
691
|
|
18
|
|
18
|
|
||
|
As % of net sales
|
22.1
|
|
20.3
|
|
|
|
|
|
18.5
|
|
||||||
|
Vision Care segment contribution
|
563
|
|
594
|
|
(5
|
)
|
(1
|
)
|
625
|
|
(5
|
)
|
(5
|
)
|
||
|
As % of net sales
|
17.7
|
|
18.9
|
|
|
|
|
|
20.5
|
|
||||||
|
Not allocated to segments
|
(1,673
|
)
|
(1,655
|
)
|
(1
|
)
|
(1
|
)
|
(1,393
|
)
|
(19
|
)
|
(18
|
)
|
||
|
Operating (loss)
|
(187
|
)
|
(248
|
)
|
25
|
|
54
|
|
(77
|
)
|
nm
|
|
nm
|
|
||
|
Core results
(2)
|
||||||||||||||||
|
Core Surgical segment contribution
|
957
|
|
846
|
|
13
|
|
19
|
|
701
|
|
21
|
|
21
|
|
||
|
As % of net sales
|
22.9
|
|
21.2
|
|
|
|
|
|
18.8
|
|
||||||
|
Core Vision Care segment contribution
|
580
|
|
600
|
|
(3
|
)
|
1
|
|
625
|
|
(4
|
)
|
(3
|
)
|
||
|
As % of net sales
|
18.2
|
|
19.0
|
|
|
|
|
|
20.5
|
|
||||||
|
Core not allocated to segments
|
(272
|
)
|
(234
|
)
|
(16
|
)
|
(17
|
)
|
(240
|
)
|
3
|
|
3
|
|
||
|
Core operating income
|
1,265
|
|
1,212
|
|
4
|
|
11
|
|
1,086
|
|
12
|
|
12
|
|
||
|
(1)
|
For additional information
regarding segment contribution please refer to Note
5
to the Consolidated Financial Statements.
|
|
(2)
|
Core results and constant currencies are non-IFRS measures. Refer to "Item
5.A. Operating Results
—
Non-IFRS measures as defined by the Company
" section for additional information and reconciliation tables.
|
|
2019 compared to 2018
|
2018 compared to 2017
|
|||||||||||||||
|
Change %
|
Change %
|
|||||||||||||||
|
($ millions unless indicated otherwise)
|
2019
|
|
2018
|
|
$
|
|
cc
(1)
|
|
2017
|
|
$
|
|
cc
(1)
|
|
||
|
Operating (loss)
|
(187
|
)
|
(248
|
)
|
25
|
|
54
|
|
(77
|
)
|
nm
|
|
nm
|
|
||
|
Interest expense
|
(113
|
)
|
(24
|
)
|
nm
|
|
nm
|
|
(27
|
)
|
11
|
|
(2
|
)
|
||
|
Other financial income & expense
|
(32
|
)
|
(28
|
)
|
(14
|
)
|
(15
|
)
|
(23
|
)
|
(22
|
)
|
(29
|
)
|
||
|
(Loss) before taxes
|
(332
|
)
|
(300
|
)
|
(11
|
)
|
13
|
|
(127
|
)
|
(136
|
)
|
(129
|
)
|
||
|
Taxes
|
(324
|
)
|
73
|
|
nm
|
|
nm
|
|
383
|
|
(81
|
)
|
(81
|
)
|
||
|
Net (Loss)/income
|
(656
|
)
|
(227
|
)
|
(189
|
)
|
(163
|
)
|
256
|
|
nm
|
|
nm
|
|
||
|
Basic and diluted (loss)/earnings per share ($)
|
(1.34
|
)
|
(0.46
|
)
|
(191
|
)
|
(163
|
)
|
0.52
|
|
nm
|
|
nm
|
|
||
|
Core results
(1)
|
||||||||||||||||
|
Core taxes
|
(195
|
)
|
(186
|
)
|
(5
|
)
|
(12
|
)
|
(128
|
)
|
(45
|
)
|
(45
|
)
|
||
|
Core net income
|
925
|
|
974
|
|
(5
|
)
|
1
|
|
908
|
|
7
|
|
8
|
|
||
|
Core basic earnings per share ($)
|
1.89
|
|
2.00
|
|
(6
|
)
|
1
|
|
1.86
|
|
8
|
|
8
|
|
||
|
Core diluted earnings per share ($)
|
1.89
|
|
2.00
|
|
(6
|
)
|
1
|
|
1.86
|
|
8
|
|
8
|
|
||
|
(1)
|
Core results and constant currencies are non-IFRS measures. Refer to "Item
5.A. Operating Results
—
Non-IFRS measures as defined by the Company
" section for additional information and reconciliation tables.
|
|
|
Average for year
|
As of December 31
|
|||||||||||
|
($ per unit unless indicated otherwise)
|
2019
|
2018
|
Change %
|
|
2019
|
2018
|
Change %
|
|
|||||
|
AUD
|
0.695
|
0.748
|
(7
|
)
|
0.701
|
0.707
|
(1
|
)
|
|||||
|
BRL
|
0.254
|
0.275
|
(8
|
)
|
0.249
|
0.258
|
(3
|
)
|
|||||
|
CAD
|
0.754
|
0.772
|
(2
|
)
|
0.767
|
0.735
|
4
|
|
|||||
|
CHF
|
1.006
|
1.023
|
(2
|
)
|
1.032
|
1.014
|
2
|
|
|||||
|
CNY
|
0.145
|
0.151
|
(4
|
)
|
0.144
|
0.145
|
(1
|
)
|
|||||
|
EUR
|
1.120
|
1.181
|
(5
|
)
|
1.121
|
1.144
|
(2
|
)
|
|||||
|
GBP
|
1.277
|
1.336
|
(4
|
)
|
1.313
|
1.274
|
3
|
|
|||||
|
JPY (100)
|
0.917
|
0.906
|
1
|
|
0.920
|
0.907
|
1
|
|
|||||
|
RUB (100)
|
1.546
|
1.600
|
(3
|
)
|
1.613
|
1.437
|
12
|
|
|||||
|
|
Average for year
|
As of December 31
|
|||||||||||
|
($ per unit unless indicated otherwise)
|
2018
|
2017
|
Change %
|
|
2018
|
2017
|
Change %
|
|
|||||
|
AUD
|
0.748
|
0.766
|
(2
|
)
|
0.707
|
0.779
|
(9
|
)
|
|||||
|
BRL
|
0.275
|
0.313
|
(12
|
)
|
0.258
|
0.302
|
(15
|
)
|
|||||
|
CAD
|
0.772
|
0.771
|
—
|
|
0.735
|
0.797
|
(8
|
)
|
|||||
|
CHF
|
1.023
|
1.016
|
1
|
|
1.014
|
1.024
|
(1
|
)
|
|||||
|
CNY
|
0.151
|
0.148
|
2
|
|
0.145
|
0.154
|
(6
|
)
|
|||||
|
EUR
|
1.181
|
1.129
|
5
|
|
1.144
|
1.195
|
(4
|
)
|
|||||
|
GBP
|
1.336
|
1.288
|
4
|
|
1.274
|
1.347
|
(5
|
)
|
|||||
|
JPY (100)
|
0.906
|
0.892
|
2
|
|
0.907
|
0.888
|
2
|
|
|||||
|
RUB (100)
|
1.600
|
1.715
|
(7
|
)
|
1.437
|
1.734
|
(17
|
)
|
|||||
|
2019 compared to 2018
|
2018 compared to 2017
|
|||||||||||||||
|
Change %
|
Percentage point currency impact
|
|
Change %
|
Percentage point currency impact
|
|
|||||||||||
|
$
|
|
cc
(1)
|
|
$
|
|
cc
(1)
|
||||||||||
|
Net sales to third parties
|
3
|
|
5
|
|
(2
|
)
|
5
|
|
5
|
—
|
|
|||||
|
Gross profit
|
15
|
|
19
|
|
(4
|
)
|
—
|
|
(1)
|
1
|
|
|||||
|
Operating (loss)
|
25
|
|
54
|
|
(29
|
)
|
nm
|
|
nm
|
nm
|
|
|||||
|
Net (loss)/income
|
(189
|
)
|
(163
|
)
|
(26
|
)
|
nm
|
|
nm
|
nm
|
|
|||||
|
Basic and diluted (loss)/earnings per share
|
(191
|
)
|
(163
|
)
|
(28
|
)
|
nm
|
|
nm
|
nm
|
|
|||||
|
Core results
(1)
|
||||||||||||||||
|
Core operating income
|
4
|
|
11
|
|
(7
|
)
|
12
|
|
12
|
—
|
|
|||||
|
Core net income
|
(5
|
)
|
1
|
|
(6
|
)
|
7
|
|
8
|
(1
|
)
|
|||||
|
Core basic earnings per share
|
(6
|
)
|
1
|
|
(7
|
)
|
8
|
|
8
|
—
|
|
|||||
|
Core diluted earnings per share
|
(6
|
)
|
1
|
|
(7
|
)
|
8
|
|
8
|
—
|
|
|||||
|
(1)
|
Core results and constant currencies (cc) as presented in this table are non-IFRS measures. Alcon uses certain non-IFRS metrics when measuring performance, including when measuring current period results against prior periods. Refer to "Item
5.A. Operating Results
—
Non-IFRS measures as defined by the Company
" section for additional information.
|
|
•
|
the impact of translating the income statements of consolidated entities from their non-US dollar functional currencies to the US dollar; and
|
|
•
|
the impact of exchange rate movements on the major transactions of consolidated entities performed in currencies other than their functional currency.
|
|
($ millions)
|
IFRS results
|
|
Amortization of intangible assets
(1)
|
|
Separation costs
(2)
|
|
Transformation Costs
(3)
|
|
Legal items
(4)
|
|
Other items
(5)
|
|
Core results
|
|
|||||||
|
Surgical segment contribution
|
923
|
|
—
|
|
7
|
|
—
|
|
—
|
|
27
|
|
957
|
|
|||||||
|
Vision Care segment contribution
|
563
|
|
—
|
|
16
|
|
—
|
|
—
|
|
1
|
|
580
|
|
|||||||
|
Not allocated to segments
|
(1,673
|
)
|
1,040
|
|
214
|
|
52
|
|
32
|
|
63
|
|
(272
|
)
|
|||||||
|
Total operating (loss)/income
|
(187
|
)
|
1,040
|
|
237
|
|
52
|
|
32
|
|
91
|
|
1,265
|
|
|||||||
|
(1)
|
Includes recurring amortization for all intangible assets other than software.
|
|
(2)
|
Separation costs are expected to be incurred over the two to three-year period following the completion of the Spin-off from Novartis and primarily include costs related to IT and third party consulting fees.
|
|
(3)
|
Transformation costs, primarily related to restructuring and third party consulting fees, for the multi-year transformation program.
|
|
(4)
|
Includes legal settlement costs and certain external legal fees.
|
|
(5)
|
Surgical segment contribution includes
$85 million
for the amortization of option rights, manufacturing sites consolidation activities, post marketing study following a product's voluntary market withdrawal expenses, integration of recent acquisitions, and spin readiness costs and other items, partially offset by
$58 million
in fair value adjustments to contingent consideration liabilities. Vision Care segment contribution includes
$18 million
in spin readiness costs and the integration of recent acquisitions, partially offset by
$17 million
in fair value adjustments to contingent consideration liabilities. Not allocated to segments primarily includes spin readiness costs and fair value adjustments of a financial asset.
|
|
($ millions)
|
IFRS results
|
|
Amortization of intangible assets
(1)
|
|
Impairments
(2)
|
|
Restructuring items
(3)
|
|
Legal items
(4)
|
|
Other items
(5)
|
|
Core results
|
|
||||||
|
Surgical segment contribution
|
813
|
|
—
|
|
—
|
|
—
|
|
—
|
|
33
|
|
846
|
|
||||||
|
Vision Care segment contribution
|
594
|
|
—
|
|
—
|
|
—
|
|
—
|
|
6
|
|
600
|
|
||||||
|
Not allocated to segments
|
(1,655
|
)
|
1,007
|
|
378
|
|
9
|
|
28
|
|
(1
|
)
|
(234
|
)
|
||||||
|
Total operating (loss)/income
|
(248
|
)
|
1,007
|
|
378
|
|
9
|
|
28
|
|
38
|
|
1,212
|
|
||||||
|
(1)
|
Includes recurring amortization for all intangible assets other than software.
|
|
(2)
|
Includes impairment charges related to intangible assets.
|
|
(3)
|
Includes restructuring income and charges and related items. Certain amounts previously reported under "restructuring items" in the 2018 Form 20-F have been reclassified to "other items" to conform with presentation in the current year.
|
|
(4)
|
Includes legal costs related to an investigation.
|
|
(5)
|
Surgical segment contribution includes
$99 million
for the amortization of option rights and charges and reversal of charges related to a product's voluntary market withdrawal, spin readiness costs, and other items, partially offset by a
$66 million
fair value adjustment to a contingent consideration liability due to a product's voluntary market withdrawal. Vision Care segment contribution includes spin readiness costs and other items. Not allocated to segments includes
$21 million
in fair value adjustments of a financial asset and other items, partially offset by
$20 million
spin readiness costs. Certain amounts previously reported under "restructuring items" in the 2018 Form 20-F have been reclassified to "other items" to conform with presentation in the current year.
|
|
($ millions)
|
IFRS results
|
|
Amortization of intangible assets
(1)
|
|
Impairments
(2)
|
|
Restructuring items
(3)
|
|
Legal items
(4)
|
|
Other items
(5)
|
|
Core results
|
|
||||||
|
Surgical segment contribution
|
691
|
|
—
|
|
29
|
|
—
|
|
—
|
|
(19
|
)
|
701
|
|
||||||
|
Vision Care segment contribution
|
625
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
625
|
|
||||||
|
Not allocated to segments
|
(1,393
|
)
|
1,017
|
|
57
|
|
30
|
|
61
|
|
(12
|
)
|
(240
|
)
|
||||||
|
Total operating (loss)/income
|
(77
|
)
|
1,017
|
|
86
|
|
30
|
|
61
|
|
(31
|
)
|
1,086
|
|
||||||
|
(1)
|
Includes recurring amortization for all intangible assets other than software.
|
|
(2)
|
Includes impairment charges related to intangible and financial assets.
|
|
(3)
|
Includes restructuring income and charges and related items.
|
|
(4)
|
Includes an increase to a legal settlement provision and legal costs related to an investigation.
|
|
(5)
|
Includes fair value adjustments to contingent consideration liabilities, a gain from a Swiss pension plan amendment and the partial reversal of a prior period charge.
|
|
($ millions except (loss)/earnings per share)
|
IFRS Results
|
|
Amortization of certain intangible assets
(1)
|
|
Separation costs
(2)
|
|
Transformation Costs
(3)
|
|
Legal items
(4)
|
|
Other items
(5)
|
|
Core Results
|
|
|
|
Gross profit
|
3,662
|
|
1,007
|
|
10
|
|
—
|
|
—
|
|
(16
|
)
|
4,663
|
|
|
|
Operating (loss)/income
|
(187
|
)
|
1,040
|
|
237
|
|
52
|
|
32
|
|
91
|
|
1,265
|
|
|
|
(Loss)/income before taxes
|
(332
|
)
|
1,040
|
|
237
|
|
52
|
|
32
|
|
91
|
|
1,120
|
|
|
|
Taxes
(6)
|
(324
|
)
|
(140
|
)
|
(54
|
)
|
(7
|
)
|
(8
|
)
|
338
|
|
(195
|
)
|
|
|
Net (loss)/income
|
(656
|
)
|
900
|
|
183
|
|
45
|
|
24
|
|
429
|
|
925
|
|
|
|
Basic (loss)/earnings per share
|
(1.34
|
)
|
|
|
|
|
|
|
|
|
|
|
1.89
|
|
|
|
Diluted (loss)/earnings per share
|
(1.34
|
)
|
|
|
|
|
|
|
|
|
|
|
1.89
|
|
|
|
Basic - weighted average shares outstanding
(7)
|
488.2
|
|
|
|
|
|
|
488.2
|
|
||||||
|
Diluted - weighted average shares outstanding
(7)
|
488.2
|
|
|
|
|
|
|
490.1
|
|
||||||
|
Adjustments to arrive at core operating income
|
|||||||||||||||
|
Selling, general & administration
|
(2,847
|
)
|
—
|
|
30
|
|
—
|
|
—
|
|
15
|
|
(2,802
|
)
|
|
|
Research & development
|
(656
|
)
|
33
|
|
4
|
|
—
|
|
—
|
|
35
|
|
(584
|
)
|
|
|
Other income
|
55
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(9
|
)
|
46
|
|
|
|
Other expense
|
(401
|
)
|
—
|
|
193
|
|
52
|
|
32
|
|
66
|
|
(58
|
)
|
|
|
(1)
|
Includes recurring amortization for all intangible assets other than software.
|
|
(2)
|
Separation costs are expected to be incurred over the two to three-year period following the completion of the Spin-off from Novartis and primarily include costs related to IT and third party consulting fees.
|
|
(3)
|
Transformation costs, primarily related to restructuring and third party consulting fees, for the multi-year transformation program.
|
|
(4)
|
Includes legal settlement costs and certain external legal fees.
|
|
(5)
|
Gross Profit includes
$37 million
in fair value adjustments of contingent consideration liabilities, partially offset by
$21 million
in spin readiness costs, manufacturing sites consolidation activities, and integration of recent acquisitions. Selling, general & administration primarily includes spin readiness costs and the integration of recent acquisitions. Research & development includes
$73 million
for the amortization of option rights, post-marketing study following a product's voluntary market withdrawal, and the integration of recent acquisitions, partially offset by
$38 million
in fair value adjustments for contingent consideration liabilities. Other income primarily includes a realized gain on a financial asset. Other expense primarily includes spin readiness costs, fair value adjustments of a financial asset and other items.
|
|
(6)
|
Total tax adjustments of
$129 million
include tax associated with operating income core adjustments and discrete tax items. Tax associated with operating income core adjustments of
$1.5 billion
totaled
$215 million
with an average tax rate of
14.8%
.
|
|
(7)
|
Core basic earnings per share is calculated using the weighted-average shares of common stock outstanding during the period. Core diluted earnings per share also contemplate dilutive shares associated with unvested equity-based awards as described in Note
8
to the Consolidated Financial Statements.
|
|
($ millions except (loss)/earnings per share)
|
IFRS Results
|
|
Amortization of certain intangible assets
(1)
|
|
Impairments
(2)
|
|
Restructuring items
(3)
|
|
Legal items
(4)
|
|
Other items
(5)
|
|
Core Results
|
|
|
Gross profit
|
3,192
|
|
996
|
|
376
|
|
—
|
|
—
|
|
(23
|
)
|
4,541
|
|
|
Operating (loss)/income
|
(248
|
)
|
1,007
|
|
378
|
|
9
|
|
28
|
|
38
|
|
1,212
|
|
|
(Loss)/income before taxes
|
(300
|
)
|
1,007
|
|
378
|
|
9
|
|
28
|
|
38
|
|
1,160
|
|
|
Taxes
(6)
|
73
|
|
|
|
|
|
|
|
|
|
|
|
(186
|
)
|
|
Net (loss)/income
|
(227
|
)
|
|
|
|
|
|
|
|
|
|
|
974
|
|
|
Basic (loss)/earnings per share
|
(0.46
|
)
|
|
|
|
|
|
|
|
|
|
|
2.00
|
|
|
Diluted (loss)/earnings per share
|
(0.46
|
)
|
|
|
|
|
|
|
|
|
|
|
2.00
|
|
|
Basic - weighted average shares outstanding
(7)
|
488.2
|
|
|
|
|
|
|
|
|
|
|
|
488.2
|
|
|
Diluted - weighted average shares outstanding
(7)
|
488.2
|
|
|
|
|
|
|
|
|
|
|
|
488.2
|
|
|
Adjustments to arrive at core operating income
|
||||||||||||||
|
Selling, general & administration
|
(2,801
|
)
|
—
|
|
2
|
|
—
|
|
—
|
|
13
|
|
(2,786
|
)
|
|
Research & development
|
(587
|
)
|
11
|
|
—
|
|
—
|
|
—
|
|
47
|
|
(529
|
)
|
|
Other income
|
47
|
|
—
|
|
—
|
|
(4
|
)
|
—
|
|
(19
|
)
|
24
|
|
|
Other expense
|
(99
|
)
|
—
|
|
—
|
|
13
|
|
28
|
|
20
|
|
(38
|
)
|
|
(1)
|
Includes recurring amortization for all intangible assets other than software.
|
|
(2)
|
Includes impairment charges related to intangible assets.
|
|
(3)
|
Includes restructuring income and charges and related items. Certain amounts previously reported under "restructuring items" in the 2018 Form 20-F have been reclassified to "other items" to conform with presentation in the current year.
|
|
(4)
|
Includes legal costs related to an investigation.
|
|
(5)
|
Gross profit, selling, general & administration and research & development include charges and reversal of charges related to a product’s voluntary market withdrawal. Research & development also includes amortization of option rights and a fair value adjustment of a contingent consideration liability. Other income includes fair value adjustments on a financial asset. Other expense includes spin-readiness costs and other items. Certain amounts previously reported under "restructuring items" in the 2018 Form 20-F have been reclassified to "other items" to conform with presentation in the current year.
|
|
(6)
|
Total tax adjustments of $259 million included tax associated with operating income adjustments and discrete tax items. Tax associated with operating income adjustments of $1.5 billion totaled $237 million with average tax rate of 16.2%. Core tax adjustments for discrete items totaled $22 million, including a net out of period income tax benefit of $55 million partially offset by net changes in uncertain tax positions of $33 million.
|
|
(7)
|
For periods prior to the Spin-off, the denominator for both core basic and diluted earnings per share was calculated using the shares of common stock distributed in the Spin-off.
|
|
($ millions except earnings per share)
|
IFRS
Results |
|
Amortization
of certain intangible
assets
(1)
|
|
Impairments
(2)
|
|
Restructuring
items
(3)
|
|
Legal
items
(4)
|
|
Other
items
(5)
|
|
Core
Results |
|
|
Gross profit
|
3,204
|
|
1,007
|
|
—
|
|
—
|
|
—
|
|
—
|
|
4,211
|
|
|
Operating (loss)/income
|
(77
|
)
|
1,017
|
|
86
|
|
30
|
|
61
|
|
(31
|
)
|
1,086
|
|
|
(Loss)/income before taxes
|
(127
|
)
|
1,017
|
|
86
|
|
30
|
|
61
|
|
(31
|
)
|
1,036
|
|
|
Taxes
(6)
|
383
|
|
(128
|
)
|
||||||||||
|
Net income
|
256
|
|
908
|
|
||||||||||
|
Basic earnings per share
|
0.52
|
|
1.86
|
|
||||||||||
|
Diluted earnings per share
|
0.52
|
|
1.86
|
|
||||||||||
|
Basic - weighted average shares outstanding
(7)
|
488.2
|
|
488.2
|
|
||||||||||
|
Diluted - weighted average shares outstanding
(7)
|
488.2
|
|
488.2
|
|
||||||||||
|
Adjustments to arrive at core operating income
|
||||||||||||||
|
Research & development
|
(584
|
)
|
10
|
|
86
|
|
—
|
|
—
|
|
(18
|
)
|
(506
|
)
|
|
Other income
|
47
|
|
—
|
|
—
|
|
(4
|
)
|
—
|
|
(13
|
)
|
30
|
|
|
Other expense
|
(148
|
)
|
—
|
|
—
|
|
34
|
|
61
|
|
—
|
|
(53
|
)
|
|
(1)
|
Includes recurring amortization for all intangible assets other than software.
|
|
(2)
|
Includes impairment charges related to intangible and financial assets.
|
|
(3)
|
Includes restructuring income and charges and related items.
|
|
(4)
|
Includes an increase to a legal settlement provision and legal costs related to an investigation.
|
|
(5)
|
Research & development includes fair value adjustments to contingent consideration liabilities; other income includes a gain from a Swiss pension plan amendment and the partial reversal of a prior period charge.
|
|
(6)
|
The required revaluation of the deferred tax assets and liabilities and a portion of current tax payables to the newly enacted tax rate at the date of enactment of the US enacted tax reform legislation (Tax Cuts and Jobs Act), resulted in a net tax income of $413 million that has been adjusted out of core taxes. Due to these factors and the differing effective tax rates in the various jurisdictions, the tax on the total adjustments of $1.2 billion to arrive at the core results before tax amounts to $98 million, excluding the tax income from US tax reform. The average tax rate on these adjustments is 8.4%.
|
|
(7)
|
For periods prior to the Spin-off, the denominator for both core basic and diluted earnings per share was calculated using the shares of common stock distributed in the Spin-off.
|
|
5.B.
|
LIQ
UI
DITY AND CAPITAL RESOURCES
|
|
($ millions)
|
2019
|
|
2018
|
|
|
|
Net cash flows from operating activities
|
920
|
|
1,140
|
|
|
|
Net cash flows used in investing activities
|
(1,011
|
)
|
(1,001
|
)
|
|
|
Net cash flows from/(used in) financing activities
|
659
|
|
(78
|
)
|
|
|
Effect of exchange rate changes on cash and cash equivalents
|
27
|
|
(6
|
)
|
|
|
Net change in cash and cash equivalents
|
595
|
|
55
|
|
|
|
Change in derivative financial instrument assets
|
1
|
|
—
|
|
|
|
Change in current and non-current financial debts
|
(3,432
|
)
|
18
|
|
|
|
Change in other financial liabilities to former parent
|
67
|
|
(21
|
)
|
|
|
Change in other financial receivables from former parent
|
(39
|
)
|
(26
|
)
|
|
|
Change in net (debt)
(1)
|
(2,808
|
)
|
26
|
|
|
|
Net liquidity at January 1
|
152
|
|
126
|
|
|
|
Net (debt)/liquidity at December 31
(1)
|
(2,656
|
)
|
152
|
|
|
|
(1)
|
The balances previously reported in "Financial debts" for a finance lease obligation have been reclassified from "Financial debts" to "Non-current lease liabilities". This reclassification resulted in an increase in Net liquidity as of January 1, 2019 and January 1, 2018 of
$89 million
and
$84 million
, respectively.
|
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
||
|
Net cash flows from operating activities
|
920
|
|
1,140
|
|
1,218
|
|
||
|
Purchase of property, plant & equipment
|
(553
|
)
|
(524
|
)
|
(415
|
)
|
||
|
Proceeds from sales of property, plant & equipment
|
—
|
|
—
|
|
1
|
|
||
|
Free cash flow
|
367
|
|
616
|
|
804
|
|
||
|
($ millions)
|
2019
|
|
2018
|
|
|
Not overdue
|
1,135
|
|
1,018
|
|
|
Past due for not more than one month
|
118
|
|
118
|
|
|
Past due for more than one month but less than three months
|
81
|
|
70
|
|
|
Past due for more than three months but less than six months
|
47
|
|
34
|
|
|
Past due for more than six months but less than one year
|
21
|
|
20
|
|
|
Past due for more than one year
|
36
|
|
47
|
|
|
Provisions for doubtful trade receivables
|
(48
|
)
|
(54
|
)
|
|
Total trade receivables, net
|
1,390
|
|
1,253
|
|
|
($ millions)
|
2019
|
|
2018
|
|
|
|
Current financial debt
|
(261
|
)
|
(47
|
)
|
|
|
Other financial liabilities to former parent
|
—
|
|
(67
|
)
|
|
|
Other financial receivables from former parent
|
—
|
|
39
|
|
|
|
Non-current financial debt
|
(3,218
|
)
|
—
|
|
|
|
Total financial debt
|
(3,479
|
)
|
(75
|
)
|
|
|
Less liquidity:
|
|||||
|
Cash and cash equivalents
|
822
|
|
227
|
|
|
|
Derivative financial instruments
|
1
|
|
—
|
|
|
|
Total liquidity
|
823
|
|
227
|
|
|
|
Net (debt)/liquidity
|
(2,656
|
)
|
152
|
|
|
|
(1)
|
The balance previously reported in "Financial debts" for a finance lease obligation has been reclassified from "Financial debts" to "Non-current lease liabilities". This reclassification resulted in an increase in Net (debt)/liquidity of
$89 million
as of December 31, 2018.
|
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
||
|
Net (loss)/income
|
(656
|
)
|
(227
|
)
|
256
|
|
||
|
Taxes
|
324
|
|
(73
|
)
|
(383
|
)
|
||
|
Depreciation of property, plant & equipment
|
267
|
|
239
|
|
215
|
|
||
|
Depreciation on right-of-use assets
|
66
|
|
—
|
|
—
|
|
||
|
Amortization of intangible assets
|
1,084
|
|
1,019
|
|
1,033
|
|
||
|
Impairments of property, plant & equipment, and intangible assets
|
8
|
|
380
|
|
57
|
|
||
|
Interest expense
|
113
|
|
24
|
|
27
|
|
||
|
Other financial income & expense
|
32
|
|
28
|
|
23
|
|
||
|
EBITDA
|
1,238
|
|
1,390
|
|
1,228
|
|
||
|
Liquidity (%)
(1)
|
Financial debts (%)
(2)
|
||||||||||
|
2019
|
|
2018
|
|
2019
|
|
2018
|
|
||||
|
USD
|
63
|
|
35
|
|
80
|
|
5
|
|
|||
|
EUR
|
6
|
|
41
|
|
11
|
|
3
|
|
|||
|
CHF
|
1
|
|
8
|
|
—
|
|
—
|
|
|||
|
JPY
|
—
|
|
—
|
|
5
|
|
—
|
|
|||
|
Other
|
30
|
|
16
|
|
4
|
|
92
|
|
|||
|
Total
|
100
|
|
100
|
|
100
|
|
100
|
|
|||
|
(1)
|
Liquidity includes cash and cash equivalents and time deposits.
|
|
(2)
|
Financial debt includes non-current and current financial debts. The balances previously reported in "Financial debts" for a finance lease obligation have been reclassified from "Financial debts" to "Non-current lease liabilities". This reclassification has also been reflected in the computation of financial debts by currency.
|
|
5.C.
|
RESEARCH AND DEVELOPMENT, PATENTS AND LICENSES, ETC.
|
|
5.D.
|
TREND INFORMATION
|
|
5.E.
|
OFF-BALANCE SHEET ARRANGEMENTS
|
|
5.F.
|
AGGREGATE CONTRACTUAL OBLIGATIONS
|
|
|
Payments due by period
|
|||||||||||||
|
($ millions)
|
Total
|
|
1 year
|
|
2 - 3 years
|
|
4 - 5 years
|
|
After 5 years
|
|
||||
|
Financial debt
|
3,508
|
|
261
|
|
55
|
|
1,192
|
|
2,000
|
|
||||
|
Interest on financial debt
|
1,083
|
|
94
|
|
168
|
|
168
|
|
653
|
|
||||
|
Leases
|
449
|
|
73
|
|
109
|
|
67
|
|
200
|
|
||||
|
Pensions and other post-employment benefit plans
|
573
|
|
62
|
|
99
|
|
110
|
|
302
|
|
||||
|
Property, plant & equipment purchase commitments
|
212
|
|
194
|
|
18
|
|
—
|
|
—
|
|
||||
|
Research & development potential milestone commitments
|
181
|
|
28
|
|
45
|
|
37
|
|
71
|
|
||||
|
Other purchase commitments
|
169
|
|
42
|
|
68
|
|
49
|
|
10
|
|
||||
|
Total contractual cash obligations
|
6,175
|
|
754
|
|
562
|
|
1,623
|
|
3,236
|
|
||||
|
ITEM 6.
|
DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
|
|
6.A.
|
DIRECTORS AND SENIOR MANAGEMENT
|
|
6.B.
|
COMPENSATION
|
|
•
|
Attracting exceptional executive talent to lead the Company, and retaining and motivating them over the long term through a mix of fixed and variable compensation elements;
|
|
•
|
Designing and structuring programs that appropriately incentivize executives to achieve short and long-term strategic business objectives established by our Board of Directors ("Board"); and
|
|
•
|
Aligning the interests of Alcon executives with those of shareholders.
|
Chair of the Compensation, Governance and Nomination Committee
|
Annual Base Salary
|
Short-Term Incentive
(annual incentive) |
Long-Term Incentive
|
Benefits
|
|
|
Purpose
|
In line with global pay practices, reflects responsibilities, experience and skills
|
Rewards annual performance against key objectives
|
Rewards long-term value creation in line with Alcon’s strategy and business priorities
|
Retirement savings and insurances in line with local market practices and benefits associated with global mobility and international relocation
|
|
Payment
|
Cash
|
Cash and equity
|
Equity (Performance Stock Units)
|
Cash or in-kind, contributions to retirement savings and insurance policies
|
|
Performance
period |
—
|
One year
|
Three year cliff vesting
|
—
|
|
Performance
measures |
—
|
Three financial performance measures and individual performance rating
|
Four equally weighted performance measures including financial, external and innovation metrics
|
—
|
|
Payout range
|
0%-200% of the individual target award
|
0%-200% of the number of Performance Stock Units granted
|
||
|
Basis
|
Fixed
|
Variable
|
Variable
|
Fixed and variable
|
|
Compensation
|
Fixed compensation
|
Variable compensation
|
Additional
compensation |
Totals
|
||||||||
|
From January 1, 2019 to December 31, 2019
|
Annual
base salary |
Pension and
insurance benefits |
2019 short-term
incentive |
2019-2021 long-term
incentive awards |
Other
benefits |
Total
compensation |
||||||
|
USD
|
Amount
in cash |
Total
amount |
Cash
amount |
RSU
1
value at grant |
PSU
2
target
value at grant |
Amount
|
Amount
|
|||||
|
David J. Endicott, CEO
|
1,134,358
|
279,851
|
745,380
|
745,380
|
2,738,036
|
1,177,487
|
6,820,492
|
|||||
|
Other ECA members
|
3,541,122
|
960,531
|
2,459,312
|
1,053,990
|
7,821,030
|
3,396,392
|
19,232,377
|
|||||
|
Totals in USD
3
|
4,675,480
|
1,240,382
|
3,204,692
|
1,799,370
|
10,559,066
|
4,573,879
|
26,052,869
|
|||||
|
Totals in CHF
4
|
4,647,132
|
1,232,862
|
3,185,262
|
1,788,460
|
10,495,046
|
4,546,148
|
25,894,910
|
|||||
|
1
|
Restricted Stock Units
|
|
2
|
Performance Stock Units
|
|
3
|
Includes the CEO and six other ECA members post Spin-off date, and the CEO and five other ECA members pre Spin-off date.
|
|
4
|
The amounts were converted at the rate of 1.0 CHF : 1.0061 USD.
|
|
Fee for the period
from April 9, 2019 to the 2020 AGM |
||
|
Board function
|
USD
1
|
CHF
|
|
Annual base fee:
|
||
|
Board Chair
|
955,795
|
950 000
|
|
Board member base fee (Board retainer fee)
|
201,220
|
200 000
|
|
Additional fees:
|
||
|
Vice Chair
|
40,244
|
40 000
|
|
Chair of the Audit and Risk Committee
|
70,427
|
70 000
|
|
Chair of the Compensation, Governance and Nomination Committee
|
50,305
|
50 000
|
|
Chair of the Innovation Committee
|
50,305
|
50 000
|
|
Member of the Audit and Risk Committee
|
35,214
|
35 000
|
|
Member of the Compensation, Governance and Nomination Committee
|
25,153
|
25 000
|
|
Member of the Innovation Committee
|
25,153
|
25 000
|
|
1
|
The Board fees are paid in Swiss Francs, converted at the rate of 1.0 CHF : 1.0061 USD.
|
|
Payment in
cash |
Payment in
shares |
Number of
shares |
Other
payments |
Total fees
|
|
|
Total fees paid in 2019
1
in USD
|
953,725
|
866,688
|
14,512
|
102,440
|
1,922,853
|
|
Total fees paid in 2019 in CHF
2
|
947,943
|
861,433
|
14,512
|
101,819
|
1,911,195
|
|
1
|
Represents compensation for nine out of ten members of the Board as David J. Endicott does not receive additional compensation for his service as a member of the Board.
|
|
2
|
The payments in cash were made in Swiss Francs (CHF) for consistency they are reported in USD as all compensation in this report. The amounts were converted at the rate of 1.0 CHF : 1.0061 USD. All amounts are before deduction of the social security contributions and income tax due by the Board member.
|
|
•
|
Substantially the same overall structure of ECA compensation as compared to 2019 (base pay, STI, LTI and benefits);
|
|
•
|
STI to be delivered in cash;
|
|
•
|
An additional profitability funding mechanism added to the 2019 STI metrics;
|
|
•
|
LTI metrics unchanged compared to 2019;
|
|
•
|
An increase to the CEO’s LTI award at target to align total compensation closer to the median of the blended peer group;
|
|
•
|
Broadly no significant change to the other ECA member’s compensation except slight adjustments;
|
|
•
|
Continuation of robust share ownership requirements; and
|
|
•
|
No material changes to benefits provisions.
|
|
•
|
Board Chair fee unchanged compared to 2019;
|
|
•
|
Same mix of fees payable in cash and shares as in 2019, including the option for a higher percentage of shares; and
|
|
•
|
Establishing fees for the new Governance and Nomination Committee’s Chair and members.
|
|
What we do
|
What we don’t do
|
|
•
Provide a majority of executive pay in variable, rather than fixed, compensation in order to ensure pay for performance
|
•
No severance agreements
|
|
•
Tie 100% of Short-Term and Long-Term Incentive awards to appropriately ambitious performance metrics
|
•
No single-trigger change in control payments
|
|
•
Follow best practices in executive compensation design
|
•
No change in control related excise tax gross ups
|
|
•
Prohibit hedging, pledging, and short sales of Company stock by executive officers and Directors
|
•
No termination notice period in excess of twelve months
|
|
•
Have robust share ownership requirements to reinforce alignment between executives and shareholders
|
•
No stock option awards
|
|
•
Include forfeiture and claw-back provisions for all variable compensation payments
|
•
No active defined benefit pension plans
|
|
•
Ensure that STI and LTI plans have target and maximum payout limits
|
•
No compensation guarantees
|
|
•
Award all equity grants at market value
|
|
|
•
Conduct ongoing investor outreach
|
|
|
•
|
Paying for performance and the execution of the Alcon strategy;
|
|
•
|
Pursuing value for shareholders over the long-term;
|
|
•
|
Creating alignment in the interests of executives and shareholders; and
|
|
•
|
Motivating and retaining executives for the long-term.
|
|
Leadership level
|
Share ownership requirement
|
|
David J. Endicott, CEO
|
5 times annual base salary
|
|
Other members of the ECA
|
3 times annual base salary
|
|
Members of the ELT (Executive Leadership Team)
|
2 times annual base salary
|
|
Authority levels in ECA compensation
|
CEO
|
CGNC
|
Board
|
AGM
|
|
ECA compensation policy and principles
|
R
|
A
|
||
|
CEO compensation and benefits
|
R
|
A
|
||
|
Other ECA member compensation and benefits
|
R
|
A
|
||
|
CEO performance targets and assessment of achievements
|
R
|
A
|
||
|
Other ECA members' performance targets and assessment of achievements
|
R
|
A
|
||
|
Share ownership requirements for the CEO and other members of the ECA
|
R
|
A
|
||
|
Maximum aggregate ECA compensation
|
R
|
P
|
A
1
|
|
|
Incentive plan design and rules
|
R
|
P
|
A
|
|
|
Compensation report of the Company
|
R
|
P
|
A
2
|
|
|
1
|
binding vote
|
|
2
|
advisory vote
|
|
•
|
fixed and variable compensation elements;
|
|
•
|
short-term and long-term incentive compensation; and
|
|
•
|
Company and individual performance.
|
|
Annual Base Salary
|
Annual base salary is set and reviewed considering:
•
Market value of the role
•
Benchmark information of peer companies
•
Market median within the peer companies
•
Executive’s role, performance, experience and potential
•
Increases in line with inflation and market
•
Business performance and the external environment
|
|
Short-Term Incentive
|
The Short-Term Incentive (STI) is designed and delivers awards based on:
Target value
•
Annual base salary ("ABS") x STI target (% of ABS) = STI target value in USD/CHF
Performance measurement
•
Measurement of financial performance (Business Performance Factor “BPF”) and individual performance (Individual Performance Factor “IPF”) (see the description of the STI below for more information)
Payout
•
Performance period: 1 year
•
Range 0%-200% of the target value
•
Payout formula: STI target value x IPF x BPF = STI payout
•
Paid in the first quarter of the following year
•
Delivered in cash and in Restricted Stock Units ("RSUs"), RSUs vest after 3 years
|
|
Long-Term Incentive
|
The Long-Term Incentive (LTI) is designed and delivers awards based on:
Target value
•
Annual Base Salary (ABS) x LTI target (% of ABS) = Target value in USD/CHF
Target award
•
Target value divided by the Alcon share price at grant date = number of Performance Stock Units ("PSUs") at target
•
Granted at the onset of the performance period
Performance measurement
•
Measurement of metrics (see the description of the LTI below for more information)
Payout
•
Performance period: 3 years
•
Range 0%-200% of the target number of PSUs
•
Payout formula: Target number of PSUs x LTI payout factor = number of PSUs vested
•
Cliff vesting of PSUs (e.g., all PSUs vest at the end of the performance period, subject to performance conditions)
•
Conversion of vested PSUs to Alcon shares
•
Payout delivered in unrestricted Alcon shares
•
Paid in the first quarter of the year following the performance period
•
PSUs carry dividend equivalents payable in shares at the end of the performance period based on the number of PSU vested
|
|
CEO
|
Other ECA members (excl. CEO)
|
|
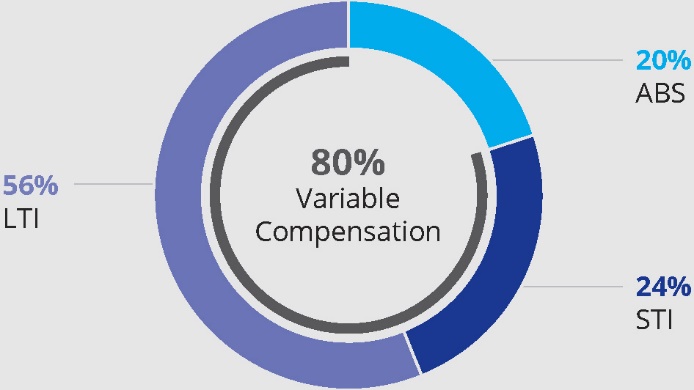
|
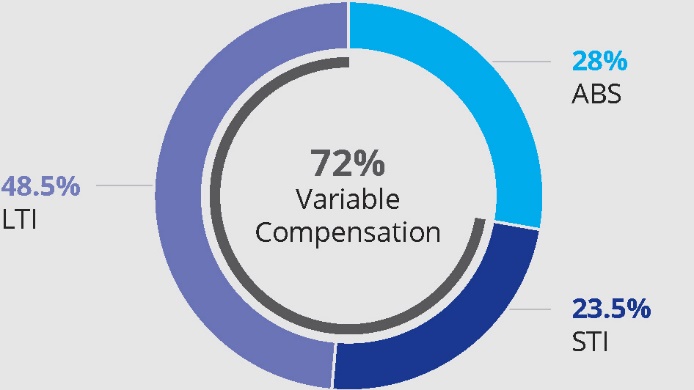
|
|
CEO ratios and average ratios of other ECA members are based on values 2019 of ABS, target STI and target 2019-2021 LTI (annualized)
Graphics exclude retirement savings and insurance benefits as well as any other benefits
|
STI payout opportunity as a % of annual base salary
|
at target*
|
|
at maximum*
|
|
|
David J. Endicott, CEO
|
120
|
%
|
240
|
%
|
|
Other members of the ECA (average)
|
80
|
%
|
160
|
%
|
|
Financial metrics
1
|
Non-financial metric
|
|||||||||||||
|
Metric
|
Group Net Sales
|
Core Operating Income
|
Free Cash Flow
|
Individual performance
|
||||||||||
|
Definition
|
Measures the Company's top line performance
|
Measures the Company’s operating income
|
Measures the Company’s capacity to realize cash
|
Measures the achievement of individual objectives and individual values and behaviors
|
||||||||||
|
Rationale
|
Fosters the Company’s top line performance
|
Recognizes the primary indicator of Company performance and profitability
|
Indicates the cash realized from operating activities
|
Considers individual contribution to the Company’s results
|
||||||||||
|
Weighting
|
40%
|
40%
|
20%
|
100%
|
||||||||||
|
Performance factors
|
BPF (total weightings of financial metrics 100%)
|
IPF
|
||||||||||||
|
Payout formula
|
||||||||||||||
|
ABS
|
X
|
STI
Target |
X
|
BPF
|
X
|
IPF
|
=
|
STI
Payout |
||||||
|
BPF maximum 150% x IPF maximum 150% = maximum 225% (capped at 200%)
|
||||||||||||||
|
Payout range
|
0-200%
|
|||||||||||||
|
1
|
Financial achievements are measured in constant currencies to reflect operational performance.
|
|
LTI payout opportunity as a % of annual base salary
|
Below threshold
|
|
at target
|
|
at maximum
1
|
|
|
David J. Endicott, CEO
|
0
|
%
|
280
|
%
|
560
|
%
|
|
Other members of the ECA (average)
|
0
|
%
|
167
|
%
|
334
|
%
|
|
1
|
The maximum number of units that may be awarded is limited to 200% of the target number of units granted.
|
|
Performance metrics
|
||||||||||||||||||
|
Metric
|
Group Net Sales CAGR
1,2
|
Core EPS CAGR
2
|
Share of Peers
3
|
Innovation scorecard
4
|
||||||||||||||
|
Definition
|
Measures the Company's top
Line performance |
Measure of the profitability by the earnings per share
|
A set of measures to compare the Company to the market shares of competitors
|
Measure of key product pipeline and achievement of milestones
|
||||||||||||||
|
Rationale
|
Fosters the Company’s top line performance
|
Aligns ECA with shareholders by measuring earnings per share
|
Indicates relative competitive position against peers in terms of market share
|
Delivery of future products and key future growth drivers
|
||||||||||||||
|
Weighting
|
25%
|
25%
|
25%
|
25%
|
||||||||||||||
|
Payout formula
|
||||||||||||||||||
|
Metric
1 25% |
+
|
Metric
2 25% |
+
|
Metric
3 25% |
+
|
Metric
4 25% |
||||||||||||
|
ABS
|
X
|
LTI
Target |
X
|
Addition of weighted metrics
= Performance Factor |
=
|
Payout/Number of PSUs
|
||||||||||||
|
Weighted achievements of metrics = additive payout factor maximum 200% (cap)
|
||||||||||||||||||
|
Payout range
|
0-200%
|
|||||||||||||||||
|
1
|
CAGR means Compound Annual Growth Rate
|
|
2
|
Financial achievements are measured in constant currencies to reflect operational performance.
|
|
3
|
Metric “Share of peers” measures Alcon’s market share of key products in the Surgical and Vision Care segments against a peer group of competitors.
|
|
4
|
The innovation scorecard for 2019-2021 includes 10 milestones: one sales-related; one related to the cost of a development program; and eight related to the timeline of achievements. Each milestone is tied to a key internal development project. The LTI payout for the innovation metric will depend upon the number of milestones achieved within the relevant performance period. The milestones established are approved by the Board’s Innovation Committee.
|
|
Retirement savings
and insurance contributions |
Retirement and insurance benefits plan contributions provided in line with local market practice (most governed by legal provisions)
Employer-paid
•
Contributions to retirement savings plan
•
Insurance premiums for disability and survivor benefits
•
Health insurance (only in the US)
•
Contributions to mandatory social security systems
|
|
Other benefits
|
•
Expense and representation allowance in line with Swiss market practice (covering small expenses)
•
Mandatory allowances for children and education (only in Switzerland)
•
Car allowance
•
Employer-paid international benefits (e.g. relocation cost, cost of living adjustments, settling in allowance, international health insurance, housing, schooling/education fees) in line with Alcon’s global mobility policies
|
|
Compensation
|
Fixed compensation
|
Variable compensation
|
Additional
compensation |
Totals
|
|||||||||
|
From January 1, 2019 to December 31, 2019
|
Annual
base salary 1 |
Pension
and insurance 2 |
2019
short-term
incentive 3, 4 |
2019-2021
long-term
incentive 5-9 |
Other
benefits 10 |
Total
compensation 11 |
|||||||
|
Amount
in cash |
Amount/
value |
Amount
in cash |
RSU value
at grant |
PSU target
value FMV at grant |
Amount/
value |
Total
amount |
|||||||
|
David J. Endicott, CEO
12
|
1,134,358
|
279,851
|
745,380
|
745,380
|
2,738,036
|
1,177,487
|
6,820,492
|
||||||
|
Aggregate amount of 6 other ECA members
13
|
3,541,122
|
960,531
|
2,459,312
|
1,053,990
|
7,821,030
|
3,396,392
|
19,232,377
|
||||||
|
Totals in USD
14
|
4,675,480
|
1,240,382
|
3,204,692
|
1,799,370
|
10,559,066
|
4,573,879
|
26,052,869
|
||||||
|
Totals in CHF
14
|
4,647,132
|
1,232,862
|
3,185,262
|
1,788,460
|
10,495,046
|
4,546,148
|
25,894,910
|
||||||
|
1
|
The Annual Base Salaries of the six designated ECA members pre Spin-off date (including the CEO) and the seven active ECA members post Spin-off date (including the CEO) are based on their individual compensation arrangements pre and post Spin-off.
|
|
2
|
The retirement pension and insurance benefits are the actual contributions paid to benefit plans for the period from January 1 to December 31, 2019. It also includes the amount of USD 71,994 for mandatory contributions paid by Alcon to governmental social security systems for all ECA members, which provide the ECA members with the right to the maximum future insured government pension benefit. The aforementioned amount is a portion of a total amount of contributions of USD 622,142 paid by Alcon to the social security systems.
|
|
3
|
The STI award disclosed is the amount earned for the performance year 2019. It will be paid in March 2020. Fifty percent of the value of the STI award of the CEO will be paid in cash, and fifty percent in RSUs. For other ECA members, seventy percent of the value of the STI award will be paid in cash, and thirty percent in RSUs. RSUs are subject to a vesting period of 3 years. The deferred portions are shown at the value that will be delivered in RSUs based on the underlying Alcon share at the closing price on the future grant date in March 2020.
|
|
4
|
The aggregate Short-Term Incentive awards in cash disclosed for this period includes the STI award at target value of Alcon's former CFO who stepped down from the function when Alcon was still a division of Novartis on April 8, 2019. This individual did not join Alcon as an independent company and remained with the Novartis organization.
|
|
5
|
The amounts of the 2019-2021 LTI awards represent the total value of the target number of PSUs granted to the then designated members of the future ECA on January 22, 2019. The value of the PSUs is based on the closing price of the underlying Novartis share on the date of grant of USD 88.32 or CHF 88.14 respectively. The amount of the LTI awards disclosed includes also the award made to Alcon's former CFO who stepped down from the function when Alcon was still a division of Novartis on April 8, 2019, prorated for the period from the onset of the performance period 2019 through to April 8, 2019.
|
|
6
|
The amount includes the value of the target number of PSUs of the 2019-2021 LTI award granted to the seventh ECA member on January 22, 2019, pro-rated for the period from April 9, 2019 to the end of the performance period in 2021. The value of the PSUs is based on the underlying Novartis share price as described above.
|
|
7
|
The amount includes the value of the target number of PSUs of the 2019-2021 LTI award granted to the new incumbent of the CFO role on April 10, 2019, prorated to his period of service as acting member of the ECA within the performance period 2019-2021. The value of the PSUs is based on the closing price of the underlying Alcon share on the date of grant of CHF 58.05.
|
|
8
|
The amount includes the total value of the target PSUs of additional 2019-2021 LTI awards granted to the members of the ECA (excluding the CFO) on April 10, 2019 for increasing their pre Spin-off LTI awards to the new target LTI award levels effective from Spin-off date. The value of the PSUs is based on the closing price of the underlying Alcon share on the date of grant of USD 58.04 and CHF 58.05 respectively.
|
|
9
|
The amount includes further the value of the target number of PSUs of the special LTI award granted to the new incumbent of the CFO role on April 10, 2019, subject to the same performance conditions as the 2019-2021 LTI awards. The value of the PSUs is based on the closing price of the underlying Alcon share on the date of grant of CHF 58.05.
|
|
10
|
The amounts of other benefits include the Company-paid benefits, values of benefits in kind, payments made, and payments or values promised to ECA members for the relevant period in 2019. They include mostly benefits for relocation to the new Alcon headquarters in Switzerland (e.g. relocation support, housing, schooling, tax and social security equalization, benefit equalization, other international relocation benefits). The amounts of other benefits also includes cost to the Company for transferring the relevant ECA members to Switzerland such as immigration cost, search of housing, pre-visit to the location and other costs related to relocation.
|
|
11
|
The vesting and forfeiture of Novartis shares and their replacement by Alcon shares under the equity restoration plan did not provide additional values earned, paid or granted and therefore no value is included in the total compensation. The restoration of equity awards is outlined below in section "Alcon Equity Restoration Plan."
|
|
12
|
The total compensation of the CEO from January 1, 2019 to December 31, 2019 includes his compensation as designated CEO from January 1, 2019 to April 8, 2019 under the Novartis compensation structure and terms.
|
|
13
|
The compensation of the six other members of the ECA from January 1, 2019 to December 31, 2019 includes (i) the compensation of five designated members of the ECA from January 1, 2019 to April 8, 2019 under the Novartis compensation structure and terms, and (ii) the compensation of six active ECA members from April 9, 2019 to December 31, 2019 under Alcon's compensation terms.
|
|
14
|
Payments to ECA members were made in CHF and/or USD. The amounts were converted at the rate of 1.0 CHF : 1.0061 USD.
|
|
CEO
|
Other ECA members (excl. CEO)
|
|
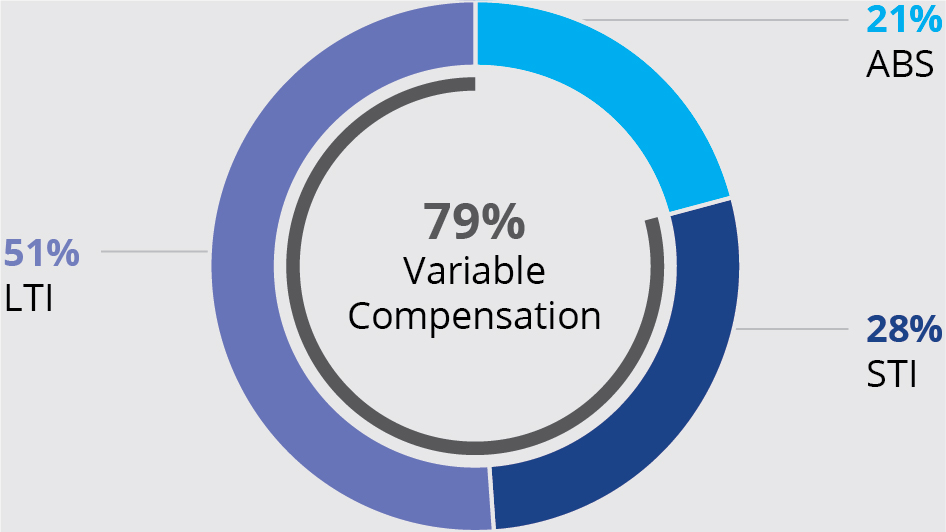
|
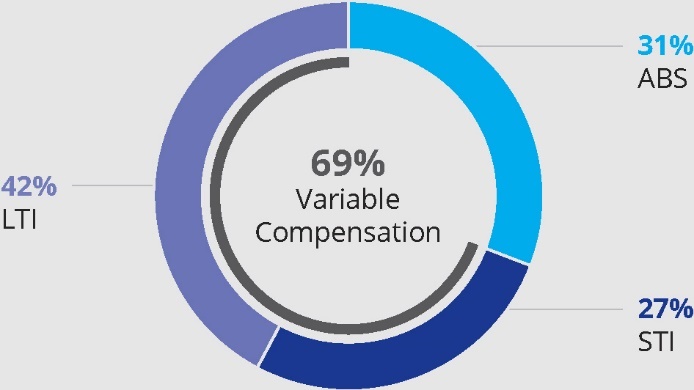
|
|
|
Number of units granted to
|
PSUs
(target number) |
|
David J. Endicott, CEO
|
24,740
|
|
Other ECA members
1
|
32,086
|
|
Total
|
56,826
|
|
1
|
Includes the number of PSUs granted to the Alcon's former CFO who stepped down from the function when Alcon was still a division of Novartis, prorated from January 1 to April 8, 2019, and the number of PSUs granted to the seventh ECA member, prorated from April 9, 2019 to the end of the performance period in 2021.
|
|
Number of units granted to
|
Deferred Share Plan
RSUs based on the 2019 STI 1 |
PSUs based on the 2019-2021 LTI
target Award 2, 3 |
|
David J. Endicott, CEO
|
na
|
9,317
|
|
Other ECA members
|
na
|
85,086
|
|
Total
|
na
|
94,403
|
|
1
|
Number of RSUs that will be granted in 2020 based on a percentage of the 2019 STI delivered in Alcon equity is not available at the time of editing this 2019 Compensation Report (na) as the number of shares is dependent on the stock price when the STI award is paid in March 2020. The value that will be granted is set out in exhibit 16.
|
|
2
|
Number of PSUs granted to the new CFO of a prorated LTI award for the performance period 2019-2021, and of a special LTI award subject to the same the performance period and conditions.
|
|
3
|
Number of PSU granted to the ECA members (excluding the CFO) for increasing their pre Spin-off target LTI award to the new target award level effective from Spin-off.
|
|
Number of units granted to
|
Alcon equity units granted as
Refill awards 1 |
Alcon equity units
granted as
Keep Whole awards
2
|
|
David, J. Endicott, CEO
|
124,062
|
23,639
|
|
Other ECA members
|
222,966
|
39,764
|
|
Total
|
347,028
|
63,403
|
|
1
|
Number of Alcon shares granted to replace the forfeited value of Novartis share-based instruments.
|
|
2
|
Number of Alcon shares granted to compensate for the dividend in kind based on Novartis unvested PSUs and RSUs.
|
|
Number of units
|
Vested shares
|
Unvested RSUs
|
Unvested target PSUs
|
Total
|
|
David J. Endicott
|
25,346
|
69,798
|
82,187
|
177,331
|
|
Laurent Attias
|
0
|
24,855
|
22,435
|
47,290
|
|
Ian Bell
|
0
|
36,432
|
27,836
|
64,268
|
|
Leon Sergio Duplan Fraustro
|
4,183
|
29,393
|
26,595
|
60,171
|
|
Rajkumar Narayanan
|
0
|
21,293
|
19,380
|
40,673
|
|
Michael Onuscheck
|
6,424
|
36,524
|
35,877
|
78,825
|
|
Tim C. Stonesifer
|
0
|
0
|
61,672
|
61,672
|
|
Total
|
35,953
|
218,295
|
275,982
|
530,230
|
|
Fee for the period from
April 9, 2019 to the 2020 AGM
|
||
|
Board function
|
USD
1
|
CHF
|
|
Annual base fee:
|
||
|
Board Chair
|
955,795
|
950 000
|
|
Board member base fee (Board retainer fee)
|
201,220
|
200 000
|
|
Additional fees:
|
||
|
Vice Chair
|
40,244
|
40 000
|
|
Chair of the Audit and Risk Committee
|
70,427
|
70 000
|
|
Chair of the Compensation, Governance and Nomination Committee
|
50,305
|
50 000
|
|
Chair of the Innovation Committee
|
50,305
|
50 000
|
|
Member of the Audit and Risk Committee
|
35,214
|
35 000
|
|
Member of the Compensation, Governance and Nomination Committee
|
25,153
|
25 000
|
|
Member of the Innovation Committee
|
25,153
|
25 000
|
|
One-off fee (on-boarding fee)
2
|
10,061
|
10 000
|
|
1
|
Converted into USD at a rate of CHF 1.0 = USD 1.0061
|
|
2
|
Fee for services to prepare the Spin-off (on-boarding fee)
|
|
•
|
Fifty percent of the total fees is paid in shares on a mandatory basis in two installments: September 2019 and March 2020
|
|
•
|
Fifty percent of the total fees is paid in cash in four installments: June, September, and December 2019 and March 2020
|
|
•
|
Each board member may elect to receive up to one hundred percent of their fees in shares
|
|
•
|
The fees are paid in Swiss Francs
|
|
•
|
The shares delivered are unrestricted (free shares) listed at the SIX Swiss Exchange
|
|
•
|
The members of the Board are subject to share ownership requirements (see below)
|
|
•
|
Board members bear the full cost of their own social security contributions
|
|
•
|
Board members do not receive variable compensation, in line with their focus on corporate strategy, supervision and governance. Their payment in shares is in unrestricted shares. They do not receive share options or other share-based instruments.
|
|
Board level
|
Share ownership requirement
|
|
Board Chair
|
1 times annual base fee, within 4 years
|
|
Other Board members
|
1 times annual base fee, within 4 years
|
|
Authority levels in Board compensation
|
CGNC
|
Board
|
AGM
|
|
Board compensation policy and principles
|
P
|
A
|
|
|
Board Chair compensation
|
P
|
A
|
|
|
Other Board member compensation
|
P
|
A
|
|
|
Share ownership requirements for Board members
|
P
|
A
|
|
|
Maximum aggregate compensation of the Board members
|
R
|
P
|
A
1
|
|
Compensation Report of the company
|
R
|
P
|
A
2
|
|
1
|
binding vote
|
|
2
|
advisory vote
|
|
Board members, functions
9
|
Payment in
cash 1,2 |
Payment in
shares 3 |
Number of
shares 4 |
Other
payments 5 |
|
Total
fees 2019 |
Fee payable March 2020
6
|
Total fees
for term
7
|
|
F. Michael Ball
Board Chair
|
418,206
|
179,166
|
3,000
|
—
|
|
597,372
|
358,423
|
955,795
|
|
Lynn D. Bleil
Member ARC and IC
|
83,685
|
73,518
|
1,231
|
—
|
|
157,203
|
114,444
|
271,647
|
|
Arthur B. Cummings
Member IC
|
112,486
|
39,058
|
654
|
89,243
|
|
240,787
|
84,890
|
325,677
|
|
Thomas H. Glanzmann
Chair IC, member CGNC
|
16,474
|
131,926
|
2,209
|
4,399
|
|
152,799
|
138,339
|
291,138
|
|
D. Keith Grossman
Vice Chair, member CGNC, IC
|
137,711
|
54,706
|
916
|
—
|
|
192,417
|
109,413
|
301,830
|
|
Scott H. Maw
Chair ARC
|
44,058
|
101,826
|
1,705
|
—
|
|
145,884
|
135,824
|
281,708
|
|
Karen J. May
Chair CGNC, member ARC
|
45,930
|
107,500
|
1,800
|
—
|
|
153,430
|
143,369
|
296,799
|
|
Ines Pöschel
Member CGNC
|
77,980
|
67,904
|
1,137
|
4,399
|
|
150,283
|
90,549
|
240,832
|
|
Dieter P. Spälti
Member ARC
|
17,195
|
111,084
|
1,860
|
4,399
|
|
132,678
|
118,217
|
250,895
|
|
Total fees paid in 2019 in USD
|
953,725
|
866,688
|
14,512
|
102,440
|
|
1,922,853
|
1,293,468
|
3,216,321
|
|
Total fees paid in 2019 in CHF
8
|
947,943
|
861,433
|
14,512
|
101,819
|
|
1,911,195
|
1,285,626
|
3,196,820
|
|
1
|
The amounts include the USD 10,061 (CHF 10,000) on-boarding fee paid in March 2019.
|
|
2
|
The amounts represent the fees paid in cash or the value of tax and, if applicable, social security withheld upon the allocation of shares to be paid in cash to the applicable authorities.
|
|
3
|
The amounts in USD represent the converted value in CHF based on the Alcon shares granted on September 11, 2019 at the closing price of CHF 59.36 per share on the date of grant. The shares granted are listed at the SIX Swiss Exchange.
|
|
4
|
The number of shares reported were delivered to each Board member in the first installment of shares in September 2019. The second and final installment in shares for the services from the Spin-off date April 9, 2019 to the 2020 AGM will be delivered in March 2020.
|
|
5
|
Includes (i) an amount of USD 17,596 for mandatory employer contributions for all Board members paid by Alcon to governmental social security systems, which provides a right to the maximum future insured government pension benefit for the relevant Board members (this amount is a part out of total employer contributions of USD 47,826 to the governmental social security systems) and (ii) USD 84,844 paid to Dr. Cummings (or his related entities) for consulting services, including assistance with clinical trials that Dr. Cummings, as an ophthalmologist, provided to Alcon (these services were unrelated to Dr. Cummings' board service).
|
|
6
|
Fees payable in March 2020, the final installment of the total fees payable for service from the Spin-off to the 2020 AGM, which includes both shares and cash portions.
|
|
7
|
Total fees that will be paid for the Board members' term of office from the Spin-off to the 2020 AGM.
|
|
8
|
The payments in cash were made in Swiss Francs (CHF). For consistency they are reported in USD as all compensation in this 2019 Compensation Report. The amounts in CHF were converted to USD at the exchange rate of 1.0 CHF : 1.0061 USD. All amounts are before deductions of social security contributions and income tax paid by the Board members.
|
|
9
|
Board Committees: “ARC” Audit and Risk Committee; “CGNC” Compensation, Governance and Nomination Committee; “IC” Innovation Committee.
|
|
Board member
|
Total shares
|
|
F. Michael Ball
|
13,202
|
|
D. Keith Grossman
|
916
|
|
Lynn D. Bleil
|
1,231
|
|
Arthur B. Cummings
|
787
|
|
Thomas H. Glanzmann
|
2,473
|
|
Scott H. Maw
|
1,705
|
|
Karen J. May
|
1,800
|
|
Ines Pöschel
|
1,679
|
|
Dieter P. Spälti
|
8,860
|
|
Total
|
32,653
|
|
•
|
Ensures a broadly competitive level of remuneration appropriate to each executives’ scale of responsibility and individual performance
|
|
•
|
Attracts, retains and motivates a world-class executive team to drive performance
|
|
•
|
Supports long-term value creation for shareholders
|
|
•
|
Considers the geographic and industry-specific nature of our talent pool and the medical device industry
|
|
•
|
Aligns the compensation program for the senior executives with the broader management and employee population
|
|
•
|
Fully embraces Swiss governance expectations and follows principles of simplicity and transparency
|
|
Pay for performance
|
•
Programs are designed to compensate short-term performance and long-term success
•
Rewards are achieved if financial and non-financial performance metrics are met
|
|
Alignment with shareholders
|
•
A significant part of compensation is delivered in Alcon equity
•
Executives are expected to hold a meaningful level of Alcon shares
|
|
Market competitiveness
|
•
Overall compensation is competitive with other companies in the medical device and other industries in which Alcon competes for talent
•
Total opportunity is targeted at market median
|
|
Motivation and retention
|
•
Compensation is designed to attract, retain and motivate executives to achieve Company objectives
•
Compensation is reviewed periodically to ensure competitiveness and alignment to key strategic objectives
|
|
Global Peer Group
|
|
|
•
Agilent Technologies Inc.
•
Align Technology Inc.
•
Allergan plc
•
Bausch Health Companies Inc.
•
Baxter International Inc.
•
Becton Dickinson & Company
•
Biogen Inc.
•
Boston Scientific Corporation
•
Dentsply Sirona Inc.
•
Edwards Lifesciences Corporation
|
•
EssilorLuxottica
•
Fresenius Medical Care
•
Givaudan
•
Lonza Group
•
Merck KGaA
•
Smith & Nephew
•
Stryker Corporation
•
The Cooper Companies Inc.
•
UCB
•
Zimmer Biomet Holding Inc.
|
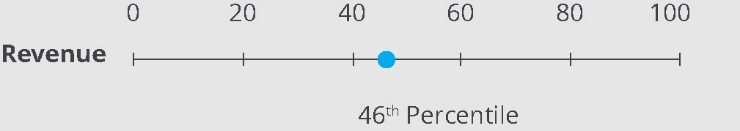
|
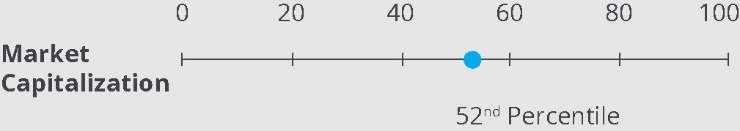
|
|
•
|
The overall structure of ECA compensation including annual base salary, variable compensation elements STI and LTI, and benefits will remain unchanged in 2020;
|
|
•
|
Slight adjustments will be made to some ECA member’s total target compensation but overall it will broadly remain unchanged;
|
|
•
|
The 2020 STI payouts will be delivered in cash to align it with peer group compensation practices;
|
|
•
|
The LTI award target percent of the CEO will be increased, to align his total compensation with the median of the peer group;
|
|
•
|
An additional profitability funding mechanism will be added to the current STI metrics to align the measurements better with company performance;
|
|
•
|
The performance metrics of the 2019-2021 LTI cycle will also be used for the performance measurement of the 2020-2022 LTI cycle (group net sales CAGR; Core EPS CAGR; Share of Peers; and Innovation);
|
|
•
|
The robust share ownership requirements will continue to apply; and
|
|
•
|
There will be no material change to benefit provisions.
|
|
•
|
The overall framework of Board compensation from Spin-off date in 2019 to the 2020 AGM will be carried forward to the term from the 2020 AGM to 2021 AGM;
|
|
•
|
The Board Chair fee will remain unchanged;
|
|
•
|
The payment of fifty percent in shares (mandatory) and a voluntary election of a higher percentage in shares will continue; and
|
|
•
|
As a result of the split of the CGNC into two separate committees, fees for an additional Board committee Chair and members will be added to the Board compensation framework.
|
|
•
|
The aggregate amount of compensation payable to non-executive members of the Board for their term of office from the 2020 AGM to the 2021 AGM;
|
|
•
|
The aggregate amount of compensation payable to ECA members in the financial year 2021.
|
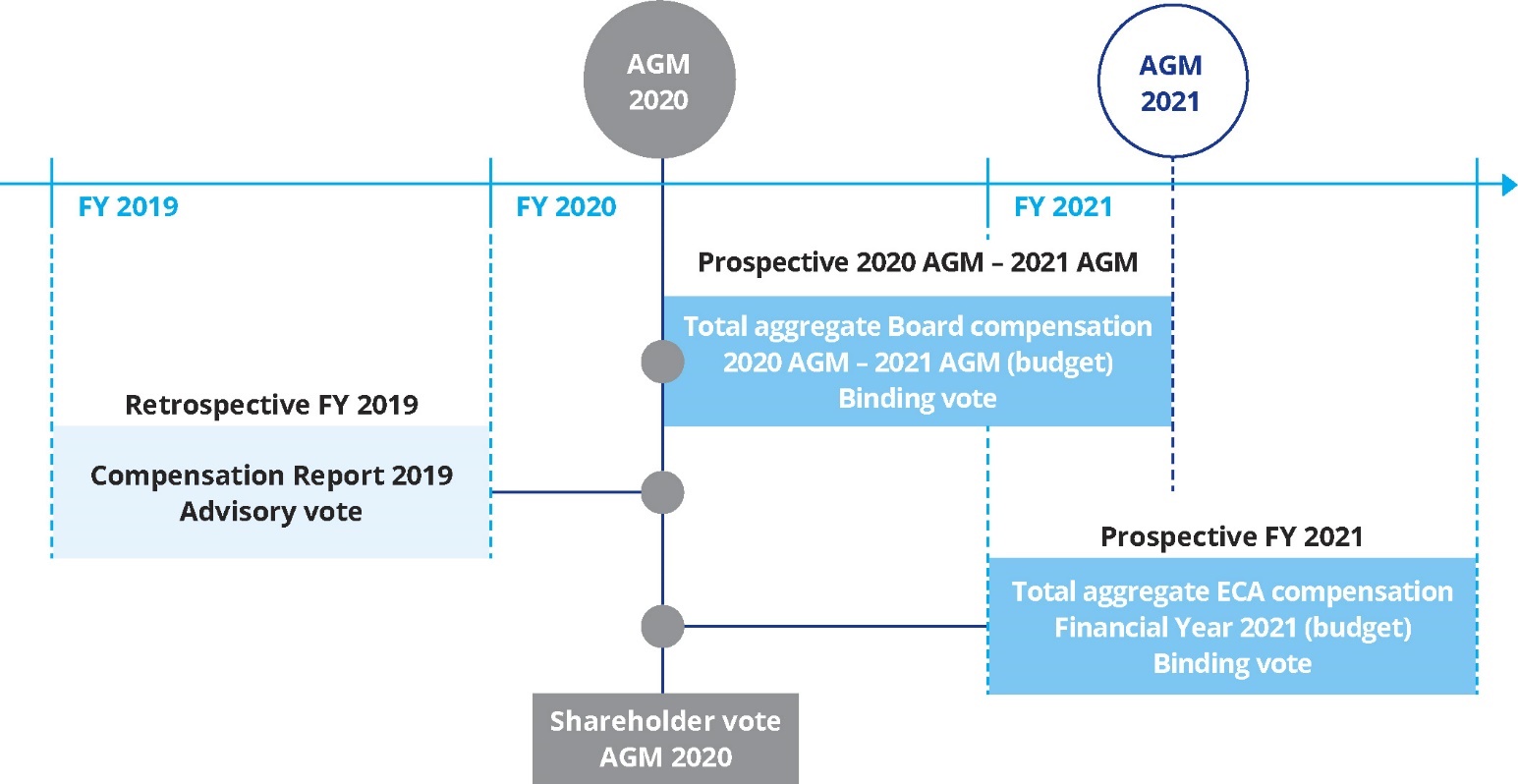
|
6.C.
|
BOARD PRACTICE
|
|
Country of Organization/ Entity Name
|
Equity Interest
|
Principal Place of Business
|
Share Capital
|
|
Japan
|
|||
|
Alcon Japan Ltd.
|
100%
|
Tokyo
|
JPY 500,000,000
|
|
Switzerland
|
|||
|
Alcon Pharmaceuticals Ltd.
|
100%
|
Fribourg
|
CHF 200,000
|
|
United States
|
|||
|
Alcon Finance Corporation
|
100%
|
Fort Worth, TX
|
USD 1
|
|
Alcon Laboratories, Inc.
|
100%
|
Fort Worth, TX
|
USD 1
|
|
Alcon Research, LLC
|
100%
|
Fort Worth, TX
|
USD 12.5
|
|
Alcon Vision, LLC
|
100%
|
Fort Worth, TX
|
USD 1,000
|
|
Holder
|
Number of Shares
|
Percentage
|
|
|
Chase Nominee Ltd., London (UK)
|
84,771,429
|
17.24%
|
|
|
Cede & Co (DTC nominee), New York, NY (USA)
|
82,425,818
|
16.76%
|
|
|
Holder
|
Number of shares and voting rights as per SIX Threshold Notification
|
Percentage as per SIX Threshold Notification
1
|
Number of shares beneficially owned as per SEC Notification
2
|
Percentage as per SEC Notification
3
|
|
|
T. Rowe Price Associates, Inc. 100 East Pratt Street, Baltimore, MD 21202
|
26,641,206
4
|
5.45 %
|
49,485,411
5
|
10.1 %
|
|
|
The Capital Group Companies, Inc.
333 South Hope Street, Los Angeles, CA 90071
|
25,357,346
6
|
5.19 %
|
31,824,542
7
|
6.5 %
|
|
|
BlackRock, Inc. c/o BlackRock Investment Management (UK) Limited 12 Throgmorton Ave, London, EC2N 2DL, UK
|
24,679,231
8
|
5.06 %
|
--
|
--
|
|
|
1
|
Percentages indicated in this column have been established based on the share capital of the Company registered with the commercial register of the Canton of Fribourg on the date on which the respective disclosure obligation pursuant to the FMIA was triggered. Furthermore, according to the FMIA, these shareholders are required to notify Alcon and the SIX Swiss Exchange only at the time they reach, exceed or fall below any of the thresholds set forth in the FMIA; therefore, their shareholding as of December 31, 2019 may differ from the figures indicated as per the contents of the relevant SIX Threshold Notifications.
|
|
2
|
In general, under SEC rules, "beneficial ownership", for the purposes of this column, refers to shares that an entity had the power to vote or the power to dispose of, and shares that such entity or individual had the right to acquire within 60 days after December 31, 2019.
|
|
3
|
Percentage ownership is calculated by dividing the number of shares reported as beneficially owned by such entity by the 488,349,066 shares of our common stock outstanding as of January 31, 2020.
|
|
4
|
Based solely on a SIX Threshold Notification dated May 1, 2019.
|
|
5
|
Based solely on a Statement on Schedule 13G filed on January 10, 2020. Such filing indicates that T. Rowe Price Associates, Inc. has sole voting power with respect to 17,419,268 shares and sole dispositive power with respect to 49,485,111 shares.
|
|
6
|
Based solely on a SIX Threshold Notification dated October 25, 2019.
|
|
7
|
Based solely on a Statement on Schedule 13G filed on February 14, 2020. Such filing indicates that The Capital Group Companies, Inc. has sole voting power with respect to 31,808,983 shares and sole dispositive power with respect to 31,824,542 shares.
|
|
8
|
Based solely on a SIX Threshold Notification dated November 9, 2019. This figure does not include its derivative position.
|

|
F. Michael Ball, Chairman
F. Michael Ball held the position of Chief Executive Officer of the Alcon Division and served as a member of the Novartis Executive Committee from February 1, 2016 until June 30, 2018. He previously served as Chief Executive Officer of Hospira, Inc. from 2011 to 2015. Prior to that, Mr. Ball held a number of senior leadership positions at Allergan, Inc., including President from 2006 to 2011. Before joining Allergan, Inc. in 1995, he held roles of increasing responsibility in marketing and sales at Syntex Corporation and Eli Lilly & Co. He has served on the board of the ICO Foundation since January 2016. Mr. Ball served on the board of directors of several organizations, including Kythera Biopharmaceuticals Inc., Hospira, Inc., IntraLase Corp., AdvaMed and sTec, Inc. He began his career in the healthcare industry in 1981.
He holds a Bachelor of Science and a Master of Business Administration from Queen’s University in Canada.
|
|
Age:
64
Nationality:
American
Year of initial
appointment: 2019
Expiration of current
term of office: 2020 |
|

|
Lynn D. Bleil
Lynn D. Bleil has been a member of the boards of directors of Stericycle, Inc. since 2015 (where she chairs the Nominating & Governance Committee), Sonova Holding AG since 2016, and Amicus Therapeutics, Inc. since 2018. Ms. Bleil has also served on the advisory boards of private healthcare companies, including Navigen Pharmaceuticals and Halo Neuroscience since 2016. She is a former member of the board of directors of DST Systems Inc and Auspex Pharmaceuticals (until their sale to SS&C Technologies) and Teva Pharmaceuticals. She also has served as vice chair of the governing board of Intermountain’s Park City Hospital since 2014. From 1985 through 2013, Ms. Bleil was a Senior Partner at McKinsey & Company where she led the West Coast healthcare practice and advised CEOs and boards of directors in the healthcare and life sciences industry.
Ms. Bleil holds a Bachelor of Science in Chemical Engineering from Princeton University, U.S., and a Master of Business Administration from the Stanford Graduate School of Business, U.S.
|
|
Age:
56
Nationality:
American
Year of initial
appointment: 2019
Expiration of current
term of office: 2020 |
|

|
Arthur Cummings, M.D.
Arthur Cummings, M.D., has been Consultant Ophthalmologist at Beacon Hospital, since 2007, and Owner and Medical Director at Wellington Eye Clinic, since 1998, both in Dublin, Ireland. Also, he has been Owner of Arthur Cummings Eye Clinic Ltd. since 2014 and a member of the board of directors of Beacon Audiology Ltd. since 2015.
Dr. Cummings holds a Bachelor of Science in Medicine and Surgery (MB. ChB.), and a Master of Medicine in Ophthalmology (M. Med) from the University of Pretoria, South Africa. Dr. Cummings is a Fellow of the College of Surgeons in South Africa (FCS SA) in Ophthalmology, and a Fellow of the Royal College of Surgeons of Edinburgh (FRCSEd) in Ophthalmology.
|
|
Age:
57
Nationality:
Irish and South African
Year of initial
appointment: 2019
Expiration of current
term of office: 2020 |
|

|
David J. Endicott
David J. Endicott is the Chief Executive Officer of the Alcon Group. He joined the Alcon Division, when still operating under the Novartis group, in July 2016 as President, Commercial and Innovation, and Chief Operating Officer. Prior to joining the Alcon Division in 2016, Mr. Endicott was President of Hospira Infusion Systems, a Pfizer company. Before joining Hospira, Mr. Endicott served as an officer and executive committee member of Allergan, Inc. where he spent more than 25 years of his career in leadership roles across Europe, Asia and Latin America, as well as the U.S. Mr. Endicott served on the board of directors of Zeltiq, Inc. and Orexigen Therapeutics, Inc. He currently serves on the board of AdvaMed.
He holds an undergraduate degree in Chemistry from Whitman College and a Master’s degree in Business Administration from the University of Southern California, both in the United States.
|
|
Age:
54
Nationality:
American
Year of initial
appointment: 2019
Expiration of current
term of office: 2020 |
|

|
Thomas Glanzmann
Thomas Glanzmann is the Founder and has been a Partner at Medtech Ventures Partners since 2016. He has been a member of the board of directors of Grifols S.A. since 2006, including serving as Vice Chairman since 2017, and a member of the healthcare advisory board of Madison Dearborn Partners, LLC since 2011. He is also Chairman of Glanzmann Enterprises AG. He was President and Chief Executive Officer of Gambro AB from 2006 to 2011, and Chief Executive Officer and Managing Director of HemoCue AB from 2005 to 2006. Mr. Glanzmann was Senior Advisor to the Executive Chairman and Acting Managing Director of the World Economic Forum from 2004 to 2005. From 1988 to 2004, Mr. Glanzmann worked in various positions at Baxter International Inc., including President of Baxter Bioscience, Chief Executive Officer of Immuno International Co., Ltd. and President of Europe Biotech Group. In 2004, he was a Senior Vice President and Corporate Officer of Baxter AG.
He holds a Bachelor of Science in Political Science from Dartmouth College, U.S., a Master of Business Administration from the IMD Business School, Switzerland and a Board of Directors Certification from the UCLA Anderson School of Management, U.S.
|
|
Age:
61
Nationality:
Swiss
Year of initial
appointment: 2019
Expiration of current
term of office: 2020 |
|

|
D. Keith Grossman
D. Keith Grossman has been the Chairman, Chief Executive Officer, and President of Nevro, Inc. since March 2019. He has also been Chairman of the board of directors of Outset Medical, Inc. since 2014 and a member of the board of directors of ViewRay, Inc. since 2018. He was President and Chief Executive Officer of Thoratec Corporation from 1996 to 2006 and from 2014 to 2015, and was a member of the board of directors from 1996 to 2015. Mr. Grossman was Chief Executive Officer and a member of the board of directors at Conceptus, Inc. from 2011 to 2013. He was Managing Director and Senior Advisor at TPG Capital, L.P. from 2007 to 2011. Mr. Grossman also served as a member of the board of directors of Zeltiq, Inc., as Lead Director, from 2013 to 2017, of Intuitive Surgical, Inc. from 2004 to 2010 and of Kyphon Inc. in 2007, and served on a number of private boards of directors.
Mr. Grossman holds a Bachelor of Science in Animal Sciences from The Ohio State University, U.S., and Master of Business Administration in Finance from Pepperdine Graziadio Business School at Pepperdine University, U.S.
|
|
Age:
59
Nationality:
American
Year of initial
appointment: 2019
Expiration of current
term of office: 2020 |
|

|
Scott Maw
Scott Maw has been managing director of WestRiver Group since September 2019. Previously, he was Executive Vice President and Chief Financial Officer at Starbucks Corporation from 2014 until the end of 2018. He was also Senior Vice President in Corporate Finance at Starbucks Corporation from 2012 to 2013, and Senior Vice President and Global Controller from 2011 to 2012. Since 2016, he has been a member of the board of directors of Avista Corporation, and since 2019, a member of the board of directors of Chipotle Mexican Grill Inc. Mr. Maw is also member of the board of trustees of Gonzaga University. From 2010 to 2011, he was Senior Vice President and Chief Financial Officer of SeaBright Holdings, Inc. From 2008 to 2010, he was Senior Vice President and Chief Financial Officer of the Consumer Bank at JP Morgan Chase and Company. Prior to this, Mr. Maw held leadership positions in finance at Washington Mutual, Inc. from 2003 to 2008, and GE Capital from 1994 to 2004.
Mr. Maw holds a Bachelor of Business Administration in Accounting from Gonzaga University,
|
|
Age:
52
Nationality:
American
Year of initial
appointment: 2019
Expiration of current
term of office: 2020 |
|

|
Karen May
Karen May has been a member of the board of directors of Ace Hardware Corporation, where she is Chair of the Audit Committee, since 2017. Previously, Ms. May was on the board of directors of MB Financial, Inc., where she served as Chair of the Compensation Committee until 2019. From 2012 to 2018, she was Executive Vice President and Chief Human Resources Officer at Mondelez International, Inc. (name changed from Kraft Foods, Inc. after the spin-off of selected Kraft North American businesses in 2012). From 2005 to 2012, Ms. May was the Executive Vice President and Chief Human Resources Officer of Kraft Foods, Inc. Between 1990 and 2005, she held various positions in Human Resources and Finance at Baxter International Inc., including Corporate Vice President and Chief Human Resources Officer and Vice President, International Finance. Prior to Baxter International Inc., Ms. May was a Certified Public Accountant in the audit practice of Price Waterhouse.
Ms. May holds a Bachelor of Science in Accounting from the University of Illinois, U.S., and was a licensed Certified Public Accountant in the U.S. from 1980 to 1990.
|
|
Age:
61
Nationality:
American
Year of initial
appointment: 2019
Expiration of current
term of office: 2020 |
|

|
Ines Pöschel
Ines Pöschel has been a Partner at Kellerhals Carrard Zurich KIG since 2007. She has been a member of the board of directors of Implenia AG since 2016 and Graubündner Kantonalbank since 2018, and serves on the board of directors of the non-listed Swiss companies of Reichle Holding, Wirz Partner Holding and Bioengineering Holding. Ms. Pöschel is also a member of the Swiss Federal expert commission for commercial register. From 2002 to 2007, Ms. Pöschel was a Senior Associate at Bär & Karrer AG. She was a Senior Manager at Andersen Legal LLC from 1999 to 2002.
Ms. Pöschel has a Master in Law from the University of Zurich, Switzerland, and passed the Swiss Bar Exam in 1996.
|
|
Age:
51
Nationality:
Swiss
Year of initial
appointment: 2019
Expiration of current
term of office: 2020 |
|

|
Dieter Spälti, Ph.D.
Dieter Spälti has been Chief Executive Officer and a member of the board of directors at Spectrum Value Management Ltd., Switzerland since 2006. He was Managing Partner from 2002 to 2006. He has been a member of the board of directors at LafargeHolcim Ltd. since 2003. He has also been a member of the board of directors at SCI (Schweizerische Cement Industrie AG) since 2003. Dr. Spälti has been Chairman of the board of directors at Dorsay Development Corporation, Canada, since 2003. He has also served as Vice Chairman of the board of directors at Grand Resort Bad Ragaz AG, Switzerland, since 2005 and Vice Chairman of the board of directors at IHAG Holding AG, Switzerland, since 2002. Dr. Spälti served, or continues to serve, on the board of directors of various non-listed Swiss and international companies that are controlled by the same beneficial owner. Dr. Spälti was a Partner at McKinsey and Company from 1993 to 2001.
He holds a Ph.D. in Law from the University of Zurich, Switzerland.
|
|
Age:
58
Nationality:
Swiss
Year of initial
appointment: 2019
Expiration of current
term of office: 2020 |
|
|
Name
|
Audit and Risk Committee
|
Compensation, Governance and
Nomination Committee |
Innovation Committee
|
|
F. Michael Ball
|
|||
|
Lynn D. Bleil
|
Member
|
Member
|
|
|
Arthur Cummings
|
Member
|
||
|
David J. Endicott
|
|||
|
Thomas Glanzmann
|
Member
|
Chair
|
|
|
D. Keith Grossman
|
Member
|
Member
|
|
|
Scott Maw
|
Chair
|
||
|
Karen May
|
Member
|
Chair
|
|
|
Ines Pöschel
|
Member
|
||
|
Dieter Spälti
|
Member
|
||
|
•
|
Supervising external auditors, and selecting and nominating external auditors for election at the Annual General Meeting of shareholders
|
|
•
|
Overseeing internal auditors
|
|
•
|
Overseeing accounting policies, financial controls, and compliance with accounting and internal control standards
|
|
•
|
Approving quarterly financial statements and financial results releases
|
|
•
|
Overseeing internal control and compliance processes and procedures
|
|
•
|
Overseeing compliance with laws, and external and internal regulations
|
|
•
|
Ensuring that Alcon has implemented an appropriate and effective risk management system and process
|
|
•
|
Ensuring that all necessary steps are taken to foster a culture of risk-adjusted decision-making without constraining reasonable risk-taking and innovation
|
|
•
|
Approving guidelines and reviewing policies and processes
|
|
•
|
Reviewing with management, internal auditors and external auditors the identification, prioritization and management of risks; the accountabilities and roles of the functions involved in risk management; the risk portfolio; and the related actions implemented by management.
|
|
•
|
Designing, reviewing and recommending corporate governance principles to the Alcon Board
|
|
•
|
Identifying candidates for election as Directors
|
|
•
|
Assessing existing Directors and recommending to the Alcon Board whether they should stand for re-election
|
|
•
|
Preparing and reviewing the succession plan for the Chief Executive Officer of Alcon
|
|
•
|
Developing and reviewing an onboarding program for new Directors, and an ongoing education plan for existing Directors
|
|
•
|
Reviewing on a regular basis the Articles of Incorporation with a view to reinforcing shareholder rights
|
|
•
|
Reviewing on a regular basis the composition and size of the Alcon Board and its committees
|
|
•
|
Reviewing annually the independence status of each Director
|
|
•
|
Reviewing directorships and agreements of Directors for conflicts of interest, and dealing with conflicts of interest
|
|
•
|
Overseeing Alcon strategy and governance on corporate responsibility
|
|
•
|
Designing, reviewing and recommending to the Alcon Board compensation policies and programs
|
|
•
|
Advising the Alcon Board on the compensation of Directors and the Chief Executive Officer of Alcon
|
|
•
|
Determining the compensation of ECA members
|
|
•
|
Preparing the annual compensation report and submitting it to the Alcon Board for approval.
|
|
•
|
Providing counsel and know-how to the Alcon Board and management in the area of technology, application of technology and new business models
|
|
•
|
Assisting the Alcon Board with oversight and evaluation of management’s development and implementation of Alcon technology and innovation strategies and its alignment with Alcon overall strategy and objectives
|
|
•
|
Informing the Alcon Board on a periodic basis about emerging scientific trends, research and development programs and opportunities and activities critical to the success of the Alcon product development pipeline
|
|
•
|
Advising the Alcon Board on scientific, technological and research development matters
|
|
•
|
Reviewing and discussing significant emerging science and technology issues and trends
|
|
•
|
Reviewing such other matters in relation to Alcon research and development, technology and innovation programs as the committee may, in its own discretion, deem desirable in connection with its responsibilities
|
|
Board of Directors
|
Audit and Risk
Committee |
Compensation,
Governance and Nomination Committee |
Innovation
Committee |
|
|
Number of meetings
1
|
6
|
6
|
6
|
3
|
|
Approximate average duration
2
|
6 hrs 35 min
|
2 hrs 20 min
|
1 h 50 min
|
2 hrs
|
|
Overall attendance
|
98%
|
96%
|
100%
|
100%
|
|
Meeting attendance
|
Board of Directors
|
Audit and Risk
Committee |
Compensation,
Governance and Nomination Committee |
Innovation
Committee |
|
Number of Meetings
6 |
Number of Meetings
6 |
Number of Meetings
6 |
Number of Meetings
3 |
|
|
F. Michael Ball
|
6
|
|||
|
Lynn D. Bleil
|
5
|
6
|
3
|
|
|
Arthur Cummings
|
6
|
3
|
||
|
David J. Endicott
|
6
|
|||
|
Thomas Glanzmann
|
6
|
6
|
3
|
|
|
D. Keith Grossman
|
6
|
6
|
3
|
|
|
Scott Maw
|
6
|
6
|
||
|
Karen May
|
6
|
6
|
6
|
|
|
Ines Pöschel
|
6
|
6
|
||
|
Dieter Spälti
|
6
|
5
|
||
|
1
|
The
number of meetings includes physical meetings as well as meetings held through videoconference or conference call, but excludes any meetings prior to April 9, 2019, the effective date of the current Board of Directors' appointment.
|
|
2
|
The approximate average duration does not include dinners, lunches and breaks. Meetings held through videoconference or conference calls had in principle a shorter duration than physically held meetings.
|
|
•
|
The Enterprise Risk Management program and risk assessment reports
|
|
•
|
The Compliance Program
|
|
•
|
The Internal Audit function
|
|
•
|
Manufacturing and Technical Operations
|
|
•
|
Research & Development and product pipeline
|
|
•
|
Commercial strategies and product launches
|

|
David J. Endicott, Chief Executive Officer
Please refer to the biography set forth under "Board of Directors".
|
|
Age:
54
Nationality:
American |
|

|
Tim C. Stonesifer, Chief Financial Officer
Mr. Stonesifer has been the Chief Financial Officer of Alcon since April 2019. Prior to joining Alcon, he served as Executive Vice President and Chief Financial Officer at Hewlett Packard Enterprise. He had served in that role from November 2015 through September 2018. Prior to that role, Mr. Stonesifer acted as Senior Vice President and Chief Financial Officer, Enterprise Group at HP Co. from February 2014 to November 2015.
Before joining HP Co., he served as Chief Financial Officer of General Motors’ International Operations from May 2011 to January 2014. Previously, he served as Chief Financial Officer of Alegco Scotsman, a storage company, from June 2010 to May 2011.
Prior to that, Mr. Stonesifer served as Chief Financial Officer of Sabic Innovative Plastics (formerly GE Plastics) from August 2007 to June 2010 after having served in various other positions at General Electric since joining the company in 1989.
Mr. Stonesifer holds a Bachelor of Arts in Economics from the University of Michigan in the U.S.
|
|
Age:
52
Nationality:
American |
|

|
Laurent Attias, Head Corporate Development, Strategy, Business Development and Licensing (BD&L) and Mergers and Acquisitions (M&A)
Laurent Attias is Head of Corporate Development, Strategy, BD&L and M&A of Alcon. In this role, Mr. Attias leads the development of long-term strategic plans for the Surgical and Vision Care franchises of Alcon. He is also responsible for the Alcon’s BD&L, M&A, partnerships and alliance activities.
Mr. Attias joined Alcon in March 1994. During his more than 25 years with Alcon, Mr. Attias progressed through the Sales and Marketing organizations by defining key strategic directions for Surgical and Pharmaceutical flagship brands. Starting in 2002, Mr. Attias held the position of Vice President, Refractive Sales and Marketing, where he helped define Alcon’s participation in the laser refractive market.
Mr. Attias moved to Europe in 2009 to assume the role of Vice President, Central & Eastern Europe, Italy and Greece. In 2010, Mr. Attias was promoted to President, EMEA. Previously, Mr. Attias served as Vice President/General Manager of Alcon Canada, an international relocation role he assumed in 2007.
Mr. Attias holds both a Bachelor of Business Administration in Marketing and a Master of Business Administration from Texas Christian University in the U.S.
|
|
Age:
52
Nationality:
American and French |
|

|
Ian Bell, President International
Ian Bell is the President-International of Alcon, overseeing the Europe, Russia, Middle East and Africa, Asia Pacific, Japan and Latin America and Caribbean markets. He joined Alcon in March 2016 as President of Europe, Middle East and Africa (“EMEA”). Mr. Bell brings more than 20 years of experience in the medical device and pharmaceutical industries. Mr. Bell joined Alcon from Hospira, where he served as Corporate Vice President and President of the EMEA region.
Prior to his work at Hospira, Mr. Bell was Corporate Vice President and President of Allergan, Inc.’s Asia Pacific region, based in Singapore, from 2008 to 2014. Mr. Bell joined Allergan, Inc. in 2005 as Vice President and Managing Director of its neurosciences division for the EMEA region.
Mr. Bell began his career at GlaxoSmithKline, where he held roles of increasing responsibility and scope in sales, marketing and strategy for more than 10 years.
Mr. Bell was awarded the degree of Bachelor of Arts with honors in Economics from the University of York in the United Kingdom.
|
|
Age:
49
Nationality:
British |
|

|
Leon Sergio Duplan Fraustro, President North America
Sergio Duplan is President-North America of Alcon, overseeing the United States and Canada markets. He leads about 3,000 associates across these two unique markets and the Surgical and Vision Care franchises of Alcon. He is a board member of The Alcon Foundation.
Mr. Duplan began his career with Novartis in 2004, as Vice President of Sales in General Medicines, in Mexico. In 2006, he was promoted to Head of Marketing and Sales for Latin America, General Medicines, Pharma. In 2008, he became Country Pharma Organization Head and Country President of Novartis Mexico. Mr. Duplan joined Alcon in August 2012.
Prior to his current role, Mr. Duplan was President of Latin America and Canada for Alcon for three years. He was appointed to his current role in August 2015.
Prior to joining Novartis, Mr. Duplan held several positions of increasing responsibility in Sales, Finance and Country Management at Procter & Gamble and Eli Lilly & Co.
Mr. Duplan holds a Bachelor degree in Industrial Engineering from Universidad Iberoamericana in Mexico and a Master of Business Administration from The Wharton School at the University of Pennsylvania in the U.S.
|
|
Age:
52
Nationality:
American and Mexican |
|

|
Michael Onuscheck, President Global Businesses and Innovation
Michael Onuscheck is the President-Global Businesses and Innovation of Alcon. Mr. Onuscheck joined Alcon in January 2015, as President and General Manager of the Global Surgical franchise. He joined Alcon from Boston Scientific, where he spent 10 years in leadership positions of increasing responsibility. Prior to joining Alcon, Mr. Onuscheck most recently held the position of President of Boston Scientific, overseeing the company’s business operations in Europe and Russia. He previously served as Senior Vice President and President of Boston Scientific’s Neuromodulation division, with responsibility for research and development, manufacturing, marketing, sales, clinical research and customer service.
Prior to joining Boston Scientific, Mr. Onuscheck held a variety of management positions at Medtronic in spinal reconstructive surgery and stereotactic image guided surgery, and various sales and marketing positions for Pfizer.
Mr. Onuscheck earned his degree in Business Administration and Psychology from Washington and Jefferson College in the U.S.
|
|
Age:
53
Nationality:
American |
|

|
Rajkumar Narayanan, Operational Strategy and Chief Transformation Officer
Mr. Narayanan is the Senior Vice President Operational Strategy and Chief Transformation Officer of Alcon and is responsible for leading the development and implementation of Alcon’s Transformation program. He has over 25 years’ experience in pharmaceutical / medical devices businesses. He joined Alcon in June 2017 as President Asia Pacific Region and moved into his current role in April 2019.
Mr. Narayanan joined Alcon from Allergan Inc., where he worked for 22 years in roles of increasing responsibility, initially in the Finance function and subsequently in the commercial organization. He was Senior Vice President Asia Pacific Region between 2015-2017. Prior to this role, he was Vice President and Managing Director of the Medical Aesthetic Franchise for Europe Africa and Middle East from 2011-2014. He served as Vice-President, Greater China & Japan between 2008-2011. Between 1995 and 2007, Mr. Narayanan was a part of Allergan’s Finance function in a number of Country, Region and Corporate Finance roles. Mr. Narayanan started his career with Hindustan Unilever India in 1987 and worked in a number of roles in the Finance function.
Mr. Narayanan holds a Bachelor of Science degree in Accounting and Finance from Mumbai University. He is also Chartered Accountant and Cost and Works Accountant from India.
|
|
Age:
55
Nationality:
American |
|
|
($ millions)
|
Year ended
December 31, 2019 |
Year ended
December 31, 2018 |
|
Audit fees
|
11.7
|
7.0
|
|
Audit related fees
|
0.2
|
0.5
|
|
Tax fees
|
–
|
–
|
|
All other fees
|
–
|
–
|
|
Total
|
11.9
|
7.5
|
|
Topic
|
Website
|
|
Investor relations
|
https://www.alcon.com/about-us#investors
|
|
Media releases
|
https://www.alcon.com/about-us#media-releases
|
|
Leadership
|
https://www.alcon.com/about-us#leadership
|
|
Governance
|
https://investor.alcon.com/governance/governance/default.aspx
|
|
Financials
|
https://investor.alcon.com/financials/quarterly-results/default.aspx
|
|
6.D.
|
EMPLOYEES
|
|
|
For the year ended December 31,
|
|||||||
|
|
2019
|
|
2018
(1)
|
|
2017
(1)
|
|
||
|
Marketing & Sales
|
7,301
|
|
7,162
|
|
6,595
|
|
||
|
Production & Supply
|
11,026
|
|
10,655
|
|
10,218
|
|
||
|
Research & Development
|
1,695
|
|
1,431
|
|
1,356
|
|
||
|
General & Administration
|
2,120
|
|
1,133
|
|
961
|
|
||
|
Total full-time equivalent employees
|
22,142
|
|
20,381
|
|
19,130
|
|
||
|
(1)
|
Alcon historically received certain services from NBS, the shared service organization of Novartis. The corresponding full time equivalents providing such services were part of NBS and have therefore not been included in the table above for 2018 and 2017.
|
|
6.E.
|
SHARE OWNERSHIP
|
|
ITEM 7.
|
MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
|
|
7.A.
|
MAJOR SHAREHOLDERS
|
|
7.B.
|
RELATED PARTY TRANSACTIONS
|
|
7.C.
|
INTERESTS OF EXPERTS AND COUNSEL
|
|
ITEM 8.
|
FINANCIAL INFORMATION
|
|
8.A.
|
CONSOLIDATED STATEMENTS AND OTHER FINANCIAL INFORMATION
|
|
8.B.
|
SIGNIFICANT CHANGES
|
|
ITEM 9.
|
THE OFFER AND LISTING
|
|
9.A.
|
OFFER AND LISTING DETAILS
|
|
9.B.
|
PLAN OF DISTRIBUTION
|
|
9.C.
|
MARKETS
|
|
9.D.
|
SELLING SHAREHOLDERS
|
|
9.E.
|
DILUTION
|
|
9.F.
|
EXPENSES OF THE ISSUE
|
|
ITEM 10.
|
ADDITIONAL INFORMATION
|
|
10.A.
|
SHARE CAPITAL
|
|
10.B.
|
MEMORANDUM AND ARTICLES OF ASSOCIATION
|
|
10.C.
|
MATERIAL CONTRACTS
|
|
▪
|
tax matters agreement;
|
|
▪
|
employee matters agreement;
|
|
▪
|
manufacturing and supply agreements;
|
|
▪
|
transitional services agreement; and
|
|
▪
|
certain IP arrangements.
|
|
▪
|
Holders of unvested awards in the form of restricted Novartis shares received the dividend in‑kind resulting from the Spin‑off.
|
|
▪
|
Holders of unvested RSUs and PSUs did not receive the dividend in‑kind resulting from the Spin‑off, and such awards were treated as described in the section entitled “Item 6. Directors, Senior Management and Employees—6.B. Compensation—Section 3—ECA Compensation 2019—Section 3.6—Alcon Equity Restoration Plan”.
|
|
▪
|
Subject to certain exceptions, Novartis agreed that each member of the Novartis Group will not, for a period of two years following the Spin‑off, directly or indirectly: (i) solicit or induce certain senior Alcon employees to become employed or engaged by any member of the Novartis Group; or (ii) knowingly induce or encourage such employees to no longer be employed or engaged by Alcon.
|
|
▪
|
Subject to certain exceptions, Novartis agreed that it would not, and would undertake to procure that each member of the Novartis Group would not, for a period of two years following the Spin‑off, employ or engage certain senior Alcon employees.
|
|
•
|
Novartis transferred to us: (i) all intellectual property rights owned by the Novartis Group and used exclusively within the Alcon Division; and (ii) certain intellectual property rights owned by the Novartis Group used within both the Alcon Division and the other businesses of Novartis including, but not limited to, the Alcon brand; and
|
|
•
|
We transferred to Novartis: (i) all intellectual property rights owned by Alcon and used exclusively within the Novartis businesses; and (ii) certain intellectual property rights owned by the Alcon group used within both the Alcon Division and the other businesses of Novartis.
|
|
10.D.
|
EXCHANGE CONTROLS
|
|
10.E.
|
TAXATION
|
|
10.F.
|
DIVIDENDS AND PAYING AGENTS
|
|
10.G.
|
STATEMENTS BY EXPERTS
|
|
10.H.
|
DOCUMENTS ON DISPLAY
|
|
10.I.
|
SUBSIDIARY INFORMATION
|
|
ITEM 11.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
|
ITEM 12.
|
DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
|
|
12.A.
|
DEBT SECURITIES
|
|
12.B.
|
WARRANTS AND RIGHTS
|
|
12.C.
|
OTHER SECURITIES
|
|
12.D.
|
AMERICAN DEPOSITARY SHARES
|
|
ITEM 13.
|
DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
|
|
ITEM 14.
|
MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
|
|
ITEM 15.
|
CONTROLS AND PROCEDURES
|
|
ITEM 16A.
|
AUDIT COMMITTEE AND FINANCIAL EXPERT
|
|
ITEM 16B.
|
CODE OF ETHICS
|
|
ITEM 16C.
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
|
ITEM 16D.
|
EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
|
|
ITEM 16E.
|
PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
|
|
Period
|
Total Number of
Shares Purchased
|
Average Price
Paid per Share
(USD)
|
Total Number
of Shares
Purchased
as part of
Publicly
Announced
Plans or
Programs
|
Maximum Number (or Approximate
Dollar Value) of Shares that may yet be
Purchased Under the Plans or Programs
|
||
|
January 1-31
|
—
|
—
|
—
|
—
|
||
|
February 1-28
|
—
|
—
|
—
|
—
|
||
|
March 1-31
|
—
|
—
|
—
|
—
|
||
|
April 1-30
|
—
|
—
|
—
|
—
|
||
|
May 1-31
|
—
|
—
|
—
|
—
|
||
|
June 1-30
|
—
|
—
|
—
|
—
|
||
|
July 1-31
|
—
|
—
|
—
|
—
|
||
|
August 1-31
|
—
|
—
|
—
|
—
|
||
|
September 1-30
|
—
|
—
|
—
|
—
|
||
|
October 1-31
|
—
|
—
|
—
|
—
|
||
|
November 1-30
|
20,000
|
|
56.65
|
|
—
|
—
|
|
December 1-31
|
7,000
|
|
56.03
|
|
—
|
—
|
|
Total
|
27,000
|
|
56.49
|
|
—
|
—
|
|
ITEM 16F.
|
CHANGE IN REGISTRANT'S CERTIFYING ACCOUNTANT
|
|
ITEM 16G.
|
CORPORATE GOVERNANCE
|
|
ITEM 16H.
|
MINE SAFETY DISCLOSURE
|
|
ITEM 17.
|
FINANCIAL STATEMENTS
|
|
ITEM 18.
|
FINANCIAL STATEMENTS
|
|
ITEM 19.
|
EXHIBITS
|
|
Exhibit
Number
|
Description
|
||
|
1.1
|
|
||
|
1.2
|
|
||
|
2.1
|
|
||
|
2.2
|
|
The total amount of long-term debt securities authorized under any instrument does not exceed 10% of the total assets of Alcon and its subsidiaries on a consolidated basis. We hereby agree to furnish to the SEC, upon its request, a copy of any instrument defining the rights of holders of long-term debt of Alcon or of its subsidiaries for which consolidated or unconsolidated financial statements are required to be filed.
|
|
|
4.1
|
|
||
|
4.2
|
|
||
|
4.3
|
|
||
|
4.4
|
|
||
|
4.5
|
|
||
|
4.6
|
|
||
|
4.7
|
|
||
|
4.8
|
|
||
|
4.9
|
|
||
|
4.10
|
|
||
|
4.11
|
|
||
|
4.12
|
|
||
|
4.13
|
|
||
|
4.14
|
|
||
|
4.15
|
|
||
|
4.16
|
|
||
|
8.1
|
|
For a list of all principal subsidiaries of Alcon Inc., see "Item 18. Financial Statements-Note 28. Alcon subsidiaries".
|
|
|
12.1
|
|
||
|
12.2
|
|
||
|
13.1
|
|
||
|
13.2
|
|
||
|
15.1
|
|
||
|
15.2
|
|
||
|
15.3
|
|
||
|
101.SCH
|
|
Inline XBRL Taxonomy Extension Schema
|
|
|
101.CAL
|
|
Inline XBRL Taxonomy Extension Calculation
|
|
|
101.DEF
|
|
Inline XBRL Taxonomy Extension Definition
|
|
|
101.LAB
|
|
Inline XBRL Taxonomy Extension Label
|
|
|
101.PRE
|
|
Inline XBRL Taxonomy Extension Presentation
|
|
|
104
|
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101)
|
|
|
Alcon Inc.
|
|||
|
By:
|
/s/ David J. Endicott
|
||
|
|
Name:
|
David J. Endicott
|
|
|
|
Title:
|
Authorized Representative
|
|
|
By:
|
/s/ Timothy C. Stonesifer
|
||
|
|
Name:
|
Timothy C. Stonesifer
|
|
|
|
Title:
|
Authorized Representative
|
|
|
Audited Consolidated Financial Statements
|
|
|
Consolidated Income Statements
|
|
|
Consolidated Statements of Comprehensive (Loss)/Income
|
|
|
Consolidated Balance Sheets
|
|
|
Consolidated Statements of Changes in Equity
|
|
|
Consolidated Statements of Cash Flows
|
|
|
Notes to Consolidated Financial Statements
|
|
|
Report of Independent Registered Public Accounting Firm
|
|
|
Report of Predecessor Independent Registered Public Accounting Firm
|
|
|
($ millions except (loss)/earnings per share)
|
Note
|
2019
|
|
2018
|
|
2017
|
|
|||
|
Net sales to third parties
|
5
|
|
|
|
|
|
|
|||
|
Sales to former parent
|
25
|
|
|
|
|
|
|
|||
|
Other revenues
|
5
|
|
|
|
|
|
|
|||
|
Net sales and other revenues
|
|
|
|
|
|
|
||||
|
Cost of net sales
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
Cost of other revenues
|
(
|
)
|
|
|
|
|
||||
|
Gross profit
|
|
|
|
|
|
|
||||
|
Selling, general & administration
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
Research & development
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
Other income
|
|
|
|
|
|
|
||||
|
Other expense
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
Operating (loss)
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
Interest expense
|
6
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
Other financial income & expense
|
6
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
(Loss) before taxes
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
Taxes
|
7
|
(
|
)
|
|
|
|
|
|||
|
Net (loss)/income
|
(
|
)
|
(
|
)
|
|
|
||||
|
(Loss)/earnings per share
|
||||||||||
|
Basic
|
8
|
(
|
)
|
(
|
)
|
|
|
|||
|
Diluted
|
8
|
(
|
)
|
(
|
)
|
|
|
|||
|
Weighted average number of shares outstanding (millions)
(1)
|
||||||||||
|
Basic
|
8
|
|
|
|
|
|
|
|||
|
Diluted
|
8
|
|
|
|
|
|
|
|||
|
(1)
|
For periods prior to the Spin-off, the denominator for basic and diluted earnings per share was calculated using
|
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
||
|
Net (loss)/income
|
(
|
)
|
(
|
)
|
|
|
||
|
Other comprehensive income to be eventually recycled into the consolidated income statement:
|
||||||||
|
Fair value adjustments on marketable securities, net of taxes
(1)
|
|
|
|
|
|
|
||
|
Currency translation effects
|
(
|
)
|
(
|
)
|
|
|
||
|
Total of items to eventually recycle
|
(
|
)
|
(
|
)
|
|
|
||
|
Other comprehensive income never to be recycled into the consolidated income statement:
|
||||||||
|
Actuarial (losses)/gains from defined benefit plans, net of taxes
(2)
|
(
|
)
|
|
|
|
|
||
|
Fair value adjustments on equity securities, net of taxes
(3)
|
(
|
)
|
(
|
)
|
|
|
||
|
Total of items never to be recycled
|
(
|
)
|
(
|
)
|
|
|
||
|
Total comprehensive (loss)/income
|
(
|
)
|
(
|
)
|
|
|
||
|
(1)
|
|
|
(2)
|
Amounts are net of tax benefit of
$
|
|
(3)
|
Amount is net of tax benefit of
$
|
|
($ millions)
|
Note
|
2019
|
|
2018
|
|
||
|
Assets
|
|
|
|
||||
|
Non-current assets
|
|
|
|
||||
|
Property, plant & equipment
(1)(2)
|
9
|
|
|
|
|
||
|
Right-of-use assets
(1)(2)
|
16
|
|
|
|
|
||
|
Goodwill
|
10
|
|
|
|
|
||
|
Intangible assets other than goodwill
|
10
|
|
|
|
|
||
|
Deferred tax assets
|
11
|
|
|
|
|
||
|
Financial assets
|
12
|
|
|
|
|
||
|
Other non-current assets
|
12
|
|
|
|
|
||
|
Total non-current assets
|
|
|
|
|
|||
|
Current assets
|
|||||||
|
Inventories
|
13
|
|
|
|
|
||
|
Trade receivables
|
14
|
|
|
|
|
||
|
Receivables from former parent
|
25
|
|
|
|
|
||
|
Income tax receivables
|
|
|
|
|
|||
|
Other financial receivables from former parent
|
25
|
|
|
|
|
||
|
Cash and cash equivalents
|
18
|
|
|
|
|
||
|
Other current assets
|
15
|
|
|
|
|
||
|
Total current assets
|
|
|
|
|
|||
|
Total assets
|
|
|
|
|
|||
|
Equity and liabilities
|
|||||||
|
Equity
|
|||||||
|
Invested capital
|
|
|
|
|
|||
|
Share capital
|
8.1
|
|
|
|
|
||
|
Reserves
|
|
|
|
|
|||
|
Total equity
|
|
|
|
|
|||
|
Liabilities
|
|||||||
|
Non-current liabilities
|
|||||||
|
Financial debts
(1)(2)
|
17
|
|
|
|
|
||
|
Lease liabilities
(1)(2)
|
16
|
|
|
|
|
||
|
Deferred tax liabilities
|
11
|
|
|
|
|
||
|
Provisions and other non-current liabilities
|
19
|
|
|
|
|
||
|
Total non-current liabilities
|
|
|
|
|
|||
|
Current liabilities
|
|||||||
|
Trade payables
|
|
|
|
|
|||
|
Payables to former parent
|
25
|
|
|
|
|
||
|
Financial debts
|
17
|
|
|
|
|
||
|
Lease liabilities
|
16
|
|
|
|
|
||
|
Other financial liabilities to former parent
|
25
|
|
|
|
|
||
|
Current income tax liabilities
|
|
|
|
|
|||
|
Provisions and other current liabilities
|
20
|
|
|
|
|
||
|
Total current liabilities
|
|
|
|
|
|||
|
Total liabilities
|
|
|
|
|
|||
|
Total equity and liabilities
|
|
|
|
|
|||
|
(1)
|
Alcon adopted IFRS 16,
Leases
as of January 1, 2019 using the modified retrospective approach as described in Notes
3
and
16
to these Consolidated Financial Statements. Under the modified retrospective approach, comparative information was not restated.
|
|
(2)
|
The December 31, 2018 balances previously reported for a finance lease liability and corresponding asset of
$
|
|
($ millions)
|
Share Capital
|
|
Other Reserves
|
|
Former parent net investment
(1)
|
|
Fair value adjustments on marketable securities
|
|
Fair value adjustments on equity securities
|
|
Actuarial (losses)/gains from defined benefit plans
|
|
Cumulative currency translation effects
|
|
Total value adjustments
(2)
|
|
Equity
(1)
|
|
|
Balance as of January 1, 2017
|
|
|
|
|
|
|
|
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
|
|
|
Net income
|
|
|
|
|
||||||||||||||
|
Other comprehensive income
|
|
|
—
|
|
|
|
|
|
|
|
|
|
||||||
|
Total comprehensive income
|
—
|
|
—
|
|
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
Movements of financing provided to former parent, net
|
(
|
)
|
(
|
)
|
||||||||||||||
|
Other transactions with former parent
|
(
|
)
|
(
|
)
|
||||||||||||||
|
Total Other movements
|
—
|
|
—
|
|
(
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(
|
)
|
|
Balance as of December 31, 2017, as previously reported
|
|
|
|
|
|
|
|
|
|
|
(
|
)
|
|
|
|
|
|
|
|
Impact of change in accounting policies
(3)
|
|
|
(
|
)
|
—
|
|
—
|
|
—
|
|
(
|
)
|
—
|
|
||||
|
Restated balance as of January 1, 2018
|
|
|
|
|
|
|
|
|
|
|
(
|
)
|
|
|
|
|
|
|
|
Net (loss)
|
(
|
)
|
(
|
)
|
||||||||||||||
|
Other comprehensive (loss)
|
—
|
|
—
|
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
Total comprehensive (loss)
|
—
|
|
—
|
|
(
|
)
|
—
|
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
|
Movements of financing provided to former parent, net
|
(
|
)
|
(
|
)
|
||||||||||||||
|
Other transactions with former parent
|
|
|
|
|
||||||||||||||
|
Other movements
(4)
|
|
|
|
|
||||||||||||||
|
Total Other movements
|
—
|
|
—
|
|
(
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(
|
)
|
|
Balance as of December 31, 2018
|
|
|
|
|
|
|
|
|
(
|
)
|
(
|
)
|
|
|
(
|
)
|
|
|
|
Net (loss)
|
(
|
)
|
(
|
)
|
|
|
|
|
|
(
|
)
|
|||||||
|
Other comprehensive (loss)
|
—
|
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Total comprehensive (loss)
|
—
|
|
(
|
)
|
(
|
)
|
—
|
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|
Movements of financing provided to former parent, net
|
(
|
)
|
(
|
)
|
||||||||||||||
|
Other transactions with former parent
|
(
|
)
|
(
|
)
|
||||||||||||||
|
Reclassification of deferred equity-compensation
|
(
|
)
|
(
|
)
|
||||||||||||||
|
Distribution by former parent of share capital
|
|
|
|
|
(
|
)
|
|
|
||||||||||
|
Equity-based compensation
|
|
|
—
|
|
|
|
||||||||||||
|
Other movements
(4)
|
|
|
|
|
|
|
||||||||||||
|
Total Other movements
|
|
|
|
|
(
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(
|
)
|
|
Balance as of December 31, 2019
|
|
|
|
|
|
|
|
|
(
|
)
|
(
|
)
|
|
|
(
|
)
|
|
|
|
(1)
|
|
|
(2)
|
"Total value adjustments" recorded through Comprehensive Income are presented net of the corresponding tax effects.
|
|
(3)
|
|
|
(4)
|
Activity relates to hyperinflationary accounting (see Note
3
to the Consolidated Financial Statements).
|
|
($ millions)
|
Note
|
2019
|
|
2018
|
|
2017
|
|
|||
|
Net (loss)/income
|
(
|
)
|
(
|
)
|
|
|
||||
|
Adjustments to reconcile net (loss)/income to net cash flows from operating activities
|
|
|
|
|||||||
|
Depreciation, amortization, impairments and fair value adjustments
|
21.1
|
|
|
|
|
|
|
|||
|
Equity-based compensation expense
|
|
|
|
|
|
|
||||
|
Non-cash change in provisions and other non-current liabilities
|
|
(
|
)
|
(
|
)
|
|
|
|||
|
Losses on disposal and other adjustments on property, plant & equipment and other non-current assets, net
|
|
|
|
|
|
|
|
|||
|
Interest expense
|
|
|
|
|
|
|
|
|||
|
Other financial income & expense
|
|
|
|
|
|
|
|
|||
|
Taxes
|
|
|
|
(
|
)
|
(
|
)
|
|||
|
Interest received
|
|
|
|
|
|
|
|
|||
|
Interest paid
|
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
Other financial payments
|
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
Taxes paid
|
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
Net cash flows before working capital changes and net payments out of provisions and other non-current liabilities
|
|
|
|
|
|
|
||||
|
Net payments out of provisions and other cash movements in non-current liabilities
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
Change in net current assets and other operating cash flow items
|
21.2
|
(
|
)
|
|
|
|
|
|||
|
Net cash flows from operating activities
|
|
|
|
|
|
|
||||
|
Purchase of property, plant & equipment
|
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
Proceeds from sales of property, plant & equipment
|
|
|
|
|
|
|
|
|||
|
Purchase of intangible assets
|
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
Purchase of financial assets
|
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
Proceeds from sales of financial assets
|
|
|
|
|
|
|
|
|||
|
Purchase of other non-current assets
|
|
(
|
)
|
|
|
(
|
)
|
|||
|
Acquisitions of businesses, net
|
21.3
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
Net cash flows used in investing activities
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
Movements of financing provided to former parent, net
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
Proceeds from non-current financial debts, net of issuance costs
|
21.4
|
|
|
|
|
|
|
|||
|
Proceeds from Bridge Facility, net of issuance costs
|
21.4
|
|
|
|
|
|
|
|||
|
Repayment of non-current financial debts
|
21.4
|
(
|
)
|
|
|
|
|
|||
|
Repayment of Bridge Facility
|
21.4
|
(
|
)
|
|
|
|
|
|||
|
Change in current financial debts
|
21.4
|
|
|
(
|
)
|
(
|
)
|
|||
|
Lease payments
|
(
|
)
|
|
|
|
|
||||
|
Change in other financial receivables from former parent
|
21.4
|
|
|
|
|
(
|
)
|
|||
|
Change in other financial liabilities to former parent
|
21.4
|
(
|
)
|
|
|
|
|
|||
|
Other financing cash flows
|
(
|
)
|
|
|
|
|
||||
|
Net cash flows from/(used in) financing activities
|
|
|
(
|
)
|
(
|
)
|
||||
|
Effect of exchange rate changes on cash and cash equivalents
|
|
|
(
|
)
|
|
|
||||
|
Net change in cash and cash equivalents
|
|
|
|
|
|
|
||||
|
Cash and cash equivalents at January 1
|
|
|
|
|
|
|
||||
|
Cash and cash equivalents at December 31
|
|
|
|
|
|
|
||||
|
•
|
Currency translation adjustments of the Novartis Group multi-divisional subsidiaries were allocated between Alcon and the Novartis retained businesses by applying allocation keys based on net assets of each respective business.
|
|
•
|
Other transactions with Novartis Group as shown on the Consolidated Statements of Changes in Equity represents the movements in Invested capital resulting from the preparation of the financial statements in accordance with the basis of preparation described in this Note.
|
|
•
|
Movements of financing provided to Novartis Group as shown on the Consolidated Statements of Changes in Equity and on the Consolidated Statements of Cash Flows primarily represent the net contributions from Alcon to Novartis Group.
|
|
•
|
Alcon received services from Novartis Business Services (“NBS”), the shared service organization of Novartis Group, across the following service domains: human resources operations, real estate and facility services, including site security and executive protection, procurement, information technology, commercial and medical support services and financial reporting and accounting operations. The financial statements include the appropriate costs related to the services rendered, without profit margin, in accordance with the historical arrangements that existed between Novartis and the Alcon business prior to the Spin-off. Refer to Note
25
to these Consolidated Financial Statements for additional disclosures.
|
|
•
|
Certain Novartis corporate general and administrative functions costs, in the areas of corporate governance, including board of directors, corporate responsibility and other corporate functions, such as tax, corporate governance and listed company compliance, investor relations, internal audit, treasury, communications functions and the net interest on the net defined benefit liability were not charged or allocated to the Alcon business in the past. The financial statements include a reasonable allocation of these Novartis corporate general and administrative functions costs and net interest on the net defined
|
|
•
|
Income, expense and cash flows using for each month the average exchange rate with the USD values for each month being aggregated during the year.
|
|
•
|
Balance sheets using year-end exchange rates.
|
|
•
|
Resulting exchange rate differences are recognized in other comprehensive income.
|
|
|
Useful life
|
|
Buildings
|
20 to 40 years
|
|
Machinery and other equipment
|
|
|
Machinery and equipment
|
7 to 20 years
|
|
Furniture and vehicles
|
5 to 10 years
|
|
Computer hardware
|
3 to 7 years
|
|
•
|
fair values of the assets transferred;
|
|
•
|
liabilities incurred to the former owners of the acquired business;
|
|
•
|
equity interests issued by the Company;
|
|
•
|
fair value of an asset or liability resulting from a contingent consideration arrangement; and
|
|
•
|
fair value of any pre-existing equity interest in the subsidiary.
|
|
|
Useful life
|
Income statement location for
amortization and impairment charges
|
|
Currently marketed products
|
5 to 20 years
|
"Cost of net sales"
|
|
Marketing know-how
|
25 years
|
"Cost of net sales"
|
|
Technologies
|
10 to 20 years
|
"Cost of net sales" or "Research and Development"
|
|
Other (including software)
|
3 to 10 years
|
In the respective functional expense
|
|
Alcon brand name
|
Not amortized, indefinite useful life
|
"Other expense"
|
|
•
|
Amount and timing of projected future cash flows;
|
|
•
|
Long-term sales forecasts for periods of up to 25 years including sales growth rates;
|
|
•
|
Royalty rate for the Alcon brand name;
|
|
•
|
Terminal growth rate; and
|
|
•
|
Discount rate.
|
|
•
|
Future tax rate;
|
|
•
|
Actions of competitors (launch of competing products, marketing initiatives, etc.); and
|
|
•
|
Outcome of R&D activities and forecast of related costs (future product developments).
|
|
•
|
Surgical equipment revenue from outright cash sales and installment sales arrangements is recognized at the point in time when control is transferred to the customer. Current portion of long-term receivables from customers and long-term receivables from customers for installment sales arrangements are recorded in "Other current assets" (see "Current portion of long-term receivables from customers" in Note
15
of these Consolidated Financial Statements) and "Financial assets" (see "Long-term receivables
|
|
•
|
In addition to cash and installment sales, revenue is recognized under finance and operating lease arrangements. Leases in which Alcon transfers substantially all the risks and rewards incidental to ownership to the customer are treated as finance lease arrangements. Revenue from finance lease arrangements is recognized at amounts equal to the fair value of the equipment, which approximates the present value of the minimum lease payments under the arrangements. As interest rates embedded in lease arrangements are approximately market rates, revenue under finance lease arrangements is comparable to revenue for outright sales. Finance income for arrangements longer than twelve months is deferred and subsequently recognized based on a pattern that approximates the use of the effective interest method and recorded in "Other income". Operating lease revenue for equipment rentals is recognized on a straight-line basis over the lease term in "Net sales to third parties".
|
|
•
|
Rebates and discounts granted to government agencies, wholesalers, retail pharmacies and other customers are provisioned and recorded as a deduction from revenue at the time the related revenues are recorded or when the incentives are offered. They are calculated on the basis of historical experience and the specific terms in the individual agreements.
|
|
•
|
Cash discounts are offered to customers to encourage prompt payment and are provisioned and recorded as revenue deductions at the time the related sales are recorded.
|
|
•
|
Sales returns provisions are recognized and recorded as revenue deductions when there is historical experience of Alcon agreeing to customer returns and Alcon can reasonably estimate expected future returns. In doing so, the estimated rate of return is applied, determined based on historical experience of customer returns and considering any other relevant factors. This is applied to the amounts invoiced, also considering the amount of returned products to be destroyed versus products that can be placed back in inventory for resale. Where shipments are made on a re-sale or return basis, without sufficient historical experience for estimating sales returns, revenue is only recorded when there is evidence of consumption or when the right of return has expired.
|
|
|
Surgical
|
Vision Care
|
Company
|
||||||||||||||
|
($ millions)
|
2019
|
|
2018
|
|
2019
|
|
2018
|
|
2019
|
|
2018
|
|
|||||
|
Net sales to third parties
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Sales to former parent
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Other revenues
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Net sales and other revenues
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Segment contribution
(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Amortization of intangible assets
|
(
|
)
|
(
|
)
|
|||||||||||||
|
Impairment charges on intangible assets
|
|
|
(
|
)
|
|||||||||||||
|
General & administration (corporate)
|
(
|
)
|
(
|
)
|
|||||||||||||
|
Other (expense)/income, net
|
(
|
)
|
(
|
)
|
|||||||||||||
|
Operating (loss)
|
(
|
)
|
(
|
)
|
|||||||||||||
|
Interest expense
|
(
|
)
|
(
|
)
|
|||||||||||||
|
Other financial income & expense
|
(
|
)
|
(
|
)
|
|||||||||||||
|
(Loss) before taxes
|
(
|
)
|
(
|
)
|
|||||||||||||
|
Surgical
|
Vision Care
|
Not allocated
|
Total
|
||||||||||||||||||||
|
($ millions)
|
2019
|
|
2018
|
|
2019
|
|
2018
|
|
2019
|
|
2018
|
|
2019
|
|
2018
|
|
|||||||
|
Depreciation of property, plant & equipment
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|
|
|
|
(
|
)
|
(
|
)
|
|||||||
|
Depreciation of right-of-use assets
|
(
|
)
|
|
|
(
|
)
|
|
|
|
|
|
|
(
|
)
|
|
|
|||||||
|
Impairment charges on property, plant & equipment, net
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|
|
|
|
(
|
)
|
(
|
)
|
|||||||
|
Equity-based compensation
(2)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||
|
(1)
|
The segment contribution corresponds to Net sales and Other revenues less Cost of net sales, Cost of other revenues, Selling, general & administration and Research & development attributable to segments, excluding amortization and impairments on intangible assets.
|
|
(2)
|
Equity-based compensation not allocated to segments in 2018 reflects an estimate of the allocation for corporate functions in the historical period based on 2019 actual percentages.
|
|
|
Surgical
|
Vision Care
|
Company
|
||||||||||||||
|
($ millions)
|
2018
|
|
2017
|
|
2018
|
|
2017
|
|
2018
|
|
2017
|
|
|||||
|
Net sales to third parties
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Sales to former parent
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Other revenues
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Net sales and other revenues
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Segment contribution
(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Amortization of intangible assets
|
(
|
)
|
(
|
)
|
|||||||||||||
|
Impairment charges on intangible assets
|
(
|
)
|
(
|
)
|
|||||||||||||
|
General & administration (corporate)
|
(
|
)
|
(
|
)
|
|||||||||||||
|
Other (expense)/income, net
|
(
|
)
|
(
|
)
|
|||||||||||||
|
Operating (loss)
|
(
|
)
|
(
|
)
|
|||||||||||||
|
Interest expense
|
(
|
)
|
(
|
)
|
|||||||||||||
|
Other financial income and expense
|
(
|
)
|
(
|
)
|
|||||||||||||
|
(Loss) before taxes
|
(
|
)
|
(
|
)
|
|||||||||||||
|
Surgical
|
Vision Care
|
Not allocated
|
Company
|
||||||||||||||||||||
|
($ millions)
|
2018
|
|
2017
|
|
2018
|
|
2017
|
|
2018
|
|
2017
|
|
2018
|
|
2017
|
|
|||||||
|
Depreciation of property, plant & equipment
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|
|
|
|
(
|
)
|
(
|
)
|
|||||||
|
Impairment charges on property, plant & equipment, net
|
(
|
)
|
|
|
(
|
)
|
|
|
|
|
|
|
(
|
)
|
|
|
|||||||
|
Equity-based compensation
(2)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||
|
(1)
|
The segment contribution corresponds to Net sales and Other revenues less Cost of net sales, Cost of other revenues, Selling, general & administration and Research & development attributable to segments, excluding amortization and impairments on intangible assets.
|
|
(2)
|
Equity-based compensation not allocated to segments in 2018 and 2017 reflects an estimate of the allocation for corporate functions in the historical periods based on 2019 actual percentages.
|
|
Surgical
|
Vision Care
|
Not allocated
(1)
|
Total
|
||||||||||||||||||||
|
($ millions)
|
2019
|
|
2018
|
|
2019
|
|
2018
|
|
2019
|
|
2018
|
|
2019
|
|
2018
|
|
|||||||
|
Goodwill
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
Intangible assets other than goodwill
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
||
|
|
||||||||
|
Surgical
|
|
|
|
|
|
|
||
|
Implantables
|
|
|
|
|
|
|
||
|
Consumables
|
|
|
|
|
|
|
||
|
Equipment/other
|
|
|
|
|
|
|
||
|
Total Surgical
|
|
|
|
|
|
|
||
|
Vision Care
|
||||||||
|
Contact lenses
|
|
|
|
|
|
|
||
|
Ocular health
|
|
|
|
|
|
|
||
|
Total Vision Care
|
|
|
|
|
|
|
||
|
Net sales to third parties
|
|
|
|
|
|
|
||
|
|
Net sales
(1)
|
Total of selected
non-current assets
(2)
|
|||||||||||||||||||||||||||
|
($ millions unless indicated otherwise)
|
2019 |
2018 |
2017 |
2019
|
2018
|
||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
Country
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
United States
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|||||||||
|
International
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|||||||||
|
thereof:
|
|||||||||||||||||||||||||||||
|
Switzerland (country of domicile)
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|||||||||
|
Japan
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|||||||||
|
China
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|||||||||
|
Other
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|||||||||
|
Company total
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|||||||||
|
(1)
|
Net sales from operations by location of third-party customer.
|
|
(2)
|
Includes property, plant & equipment, right-of-use assets, goodwill and other intangible assets.
|
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
||
|
Interest expense on financial debts
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
Interest expense from discounting long-term liabilities
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
Interest expense on lease liabilities
(1)
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
Total interest expense
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
(1)
|
For the years ended
December 31, 2018
and
2017
, interest expense on finance leases was included in "Interest expense on lease liabilities".
|
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
||
|
Interest income
|
|
|
|
|
|
|
||
|
Loss on extinguishment of financial debt
|
(
|
)
|
|
|
|
|
||
|
Other financial expense
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
Monetary loss from hyperinflation accounting
|
(
|
)
|
(
|
)
|
|
|
||
|
Currency result, net
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
Total other financial income & expense
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
||
|
Switzerland
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
Foreign
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
Total (loss) before taxes
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
||
|
Switzerland
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
Foreign
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
Current income tax expense
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
Switzerland
|
(
|
)
|
|
|
|
|
||
|
Foreign
|
|
|
|
|
|
|
||
|
Deferred tax (expense)/income
|
(
|
)
|
|
|
|
|
||
|
Total income tax (expense)/income
|
(
|
)
|
|
|
|
|
||
|
|
2019
|
2018
|
2017
|
||||||||||||||
|
|
$ m
|
|
%
|
|
$ m
|
|
%
|
|
$ m
|
|
%
|
|
|||||
|
Applicable tax rate
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|||||
|
Effect of disallowed expenditures
|
(
|
)
|
(
|
)%
|
(
|
)
|
(
|
)%
|
(
|
)
|
(
|
)%
|
|||||
|
Effect of share based compensation
|
(
|
)
|
(
|
)%
|
(
|
)
|
(
|
)%
|
(
|
)
|
(
|
)%
|
|||||
|
Effect of income taxed at reduced rates
|
|
|
|
%
|
|
|
|
%
|
|
|
|
|
|||||
|
Effect of tax credits and allowances
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|||||
|
Effect of adjustments to contingent consideration liabilities
|
|
|
|
%
|
|
|
|
%
|
(
|
)
|
(
|
)%
|
|||||
|
Effect of option payments
|
(
|
)
|
(
|
)%
|
(
|
)
|
(
|
)%
|
(
|
)
|
(
|
)%
|
|||||
|
Effect of liquidation of a subsidiary
|
|
|
|
|
|
|
|
|
(
|
)
|
(
|
)%
|
|||||
|
Effect of tax benefits expiring in 2017
(1)
|
|
|
|
|
|
|
|
|
(
|
)
|
(
|
)%
|
|||||
|
Effect of tax rate changes
(2)
|
(
|
)
|
(
|
)%
|
(
|
)
|
(
|
)%
|
|
|
|
|
|||||
|
Effect of changes in uncertain tax positions
|
|
|
|
%
|
(
|
)
|
(
|
)%
|
(
|
)
|
(
|
)%
|
|||||
|
Effect of other items
|
(
|
)
|
(
|
)%
|
(
|
)
|
(
|
)%
|
(
|
)
|
(
|
)%
|
|||||
|
Effect of prior year items
(3)
|
(
|
)
|
(
|
)%
|
|
|
|
%
|
|
|
|
%
|
|||||
|
Effect of tax rate change on current and deferred tax assets and liabilities from US tax reform
(4)
|
|
|
|
%
|
|
|
|
%
|
|
|
|
%
|
|||||
|
Effective tax rate
|
(
|
)
|
(
|
)%
|
|
|
|
%
|
|
|
|
%
|
|||||
|
(1)
|
Effect of tax benefits expiring in
2017
relates to a Swiss subsidiary that was not subject to income tax through the end of calendar year 2017.
|
|
(2)
|
Effect of tax rate changes in 2019 relates primarily to (i) the adoption of the Swiss Tax Reform which has resulted in a non-cash tax increase in the tax expense of
$
|
|
(3)
|
In 2019, the prior year items relate to changes in certain estimates which resulted in a
$
|
|
(4)
|
Effect of tax rate change on US current and deferred tax assets and liabilities in 2017 relate to the enactment of the Tax Cuts and Jobs Act by the US, which reduced the corporate tax rate from 35% to 21% effective January 1, 2018. This required a re-measurement of the deferred tax balances and a portion of the current tax payables.
|
|
($ millions)
|
Land
|
|
Buildings
|
|
Construction
in progress |
|
Machinery &
other equipment |
|
Total
|
|
||||
|
Cost
|
|
|
|
|
|
|
|
|
|
|
||||
|
January 1, 2019
|
|
|
|
|
|
|
|
|
|
|
||||
|
Additions
(1)
|
|
|
|
|
|
|
|
|
||||||
|
Impact of business combinations
|
|
|
|
|
||||||||||
|
Disposals and derecognitions
(2)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||
|
Transfers with former parent
|
|
|
|
|
|
|
|
|
||||||
|
Reclassifications for assets placed in service
|
|
|
|
|
(
|
)
|
|
|
|
|
||||
|
Other reclassifications
|
(
|
)
|
(
|
)
|
||||||||||
|
Currency translation effects
|
|
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
||||
|
December 31, 2019
|
|
|
|
|
|
|
|
|
|
|
||||
|
Accumulated depreciation
|
||||||||||||||
|
January 1, 2019
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
Depreciation charge
|
|
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
||||
|
Impairment charge
|
|
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
Disposals and derecognitions
(2)
|
|
|
|
|
|
|
||||||||
|
Transfers with former parent
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||||
|
Other reclassifications
|
|
|
|
|
||||||||||
|
Currency translation effects
|
|
|
|
|
|
|
|
|
|
|
||||
|
December 31, 2019
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
Net book value at December 31, 2019
|
|
|
|
|
|
|
|
|
|
|
||||
|
(1)
|
Includes
$
|
|
(2)
|
Derecognition of assets that are no longer used and are not considered to have a significant disposal value or other alternative use.
|
|
($ millions)
|
Land
|
|
Buildings
|
|
Construction
in progress |
|
Machinery &
other equipment |
|
Total
|
|
||||
|
Cost
|
|
|
|
|
|
|
|
|
|
|
||||
|
January 1, 2018
|
|
|
|
|
|
|
|
|
|
|
||||
|
Additions
|
|
|
|
|
|
|
|
|
||||||
|
Impact of business combinations
|
|
|
|
|
|
|
|
|
|
|
||||
|
Disposals and derecognitions
(1)
|
|
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
||||
|
Reclassifications and transfers with former parent
|
|
|
|
|
(
|
)
|
|
|
|
|
||||
|
Reclassification to right-of-use assets
(2)
|
(
|
)
|
(
|
)
|
||||||||||
|
Currency translation effects
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
December 31, 2018
|
|
|
|
|
|
|
|
|
|
|
||||
|
Accumulated depreciation
|
|
|
|
|
|
|
|
|
|
|
||||
|
January 1, 2018
|
(
|
)
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
||||
|
Depreciation charge
|
|
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
||||
|
Impairment charge
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||||
|
Disposals and derecognitions
(1)
|
|
|
|
|
|
|
|
|
|
|
||||
|
Transfers with former parent
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
Reclassification to right-of-use assets
(2)
|
|
|
|
|
||||||||||
|
Currency translation effects
|
|
|
|
|
|
|
|
|
|
|
||||
|
December 31, 2018
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
Net book value at December 31, 2018
|
|
|
|
|
|
|
|
|
|
|
||||
|
(1)
|
Derecognition of assets that are no longer used and are not considered to have a significant disposal value or other alternative use.
|
|
(2)
|
The December 31, 2018 balance previously reported for a finance lease asset of
$
|
|
|
Intangible assets other than goodwill
|
||||||||||||||||||||||
|
($ millions)
|
Goodwill
|
|
Alcon
brand name |
|
Acquired
research & development |
|
Technologies
|
|
Currently
marketed products |
|
Marketing
know-how |
|
Other
intangible assets (including software) |
|
Total
|
|
|||||||
|
Cost
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
January 1, 2019
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
Impact of business combinations
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
Additions
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
Reclassifications
|
—
|
|
—
|
|
(
|
)
|
—
|
|
—
|
|
—
|
|
|
|
|
|
|||||||
|
Disposals and derecognitions
(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
(
|
)
|
(
|
)
|
|||||||
|
December 31, 2019
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
Accumulated amortization
|
|||||||||||||||||||||||
|
January 1, 2019
|
|
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||
|
Amortization charge
|
|
|
|
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||
|
Accumulated amortization on disposals and derecognitions
(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
December 31, 2019
|
|
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||
|
Net book value at December 31, 2019
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
(1)
|
Derecognitions of assets that are no longer used or being developed and are not considered to have a significant disposal value or other alternative use.
|
|
|
Intangible assets other than goodwill
|
||||||||||||||||||||||
|
($ millions)
|
Goodwill
|
|
Alcon
brand name |
|
Acquired
research & development |
|
Technologies
|
|
Currently
marketed products |
|
Marketing
know-how |
|
Other
intangible assets (including software) |
|
Total
|
|
|||||||
|
Surgical
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Vision Care
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Not allocated to segment
(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
Net book value at December 31, 2019
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
(As a percentage)
|
Surgical
|
Vision Care
|
|
|
Terminal growth rate
|
|
|
|
|
Discount rate (post-tax)
|
|
|
|
|
|
Intangible assets other than goodwill
|
||||||||||||||||||||||
|
($ millions)
|
Goodwill
|
|
Alcon
brand name |
|
Acquired
research & development |
|
Technologies
|
|
Currently
marketed products |
|
Marketing
know-how |
|
Other
intangible assets (including software) |
|
Total
|
|
|||||||
|
Cost
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
January 1, 2018
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
Impact of business combinations
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
Additions
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
Disposals and derecognitions
(1)
|
|
|
|
|
(
|
)
|
|
|
|
|
|
|
(
|
)
|
(
|
)
|
|||||||
|
December 31, 2018
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
Accumulated amortization
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
January 1, 2018
|
|
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||
|
Amortization charge
|
|
|
|
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||
|
Accumulated amortization on disposals and derecognitions
(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
Impairment charge
|
|
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
|
|
|
|
(
|
)
|
|||||||
|
December 31, 2018
|
|
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||||||
|
Net book value at December 31, 2018
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
(1)
|
Derecognitions of assets that are no longer used or being developed and are not considered to have a significant disposal value or other alternative use.
|
|
|
Intangible assets other than goodwill
|
||||||||||||||||||||||
|
($ millions)
|
Goodwill
|
|
Alcon
brand name |
|
Acquired
research & development |
|
Technologies
|
|
Currently
marketed products |
|
Marketing
know-how |
|
Other
intangible assets (including software) |
|
Total
|
|
|||||||
|
Surgical
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
Vision Care
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
Not allocated to segment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
December 31, 2018
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
($ millions)
|
2019
|
|
2018
|
|
|
|
Surgical
|
|
|
(
|
)
|
|
|
Vision Care
|
|
|
|
|
|
|
Total
|
|
|
(
|
)
|
|
|
($ millions)
|
Property,
plant & equipment |
|
Intangible
assets |
|
Pensions and
other benefit obligations of associates |
|
Inventories
|
|
Tax loss
carry- forwards |
|
Other
assets, provision and accruals |
|
Total
|
|
||||||
|
Gross deferred tax assets at December 31, 2018
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Gross deferred tax liabilities at December 31, 2018
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
||||||
|
Net deferred tax balance at December 31, 2018
|
(
|
)
|
(
|
)
|
|
|
|
|
|
|
|
|
(
|
)
|
||||||
|
At December 31, 2018
|
(
|
)
|
(
|
)
|
|
|
|
|
|
|
|
|
(
|
)
|
||||||
|
(Charged)/credited to income
|
(
|
)
|
(
|
)
|
|
|
|
|
|
|
(
|
)
|
(
|
)
|
||||||
|
Credited to equity
|
|
|
|
|
||||||||||||||||
|
Credited to other comprehensive income
|
|
|
|
|
|
|
||||||||||||||
|
Impact of business combinations
|
(
|
)
|
|
|
(
|
)
|
||||||||||||||
|
Other movements
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
|
|
|
|
||||||
|
Net deferred tax balance at December 31, 2019
|
(
|
)
|
(
|
)
|
|
|
|
|
|
|
|
|
(
|
)
|
||||||
|
Gross deferred tax assets at December 31, 2019
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Gross deferred tax liabilities at December 31, 2019
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
||||||
|
Net deferred tax balance at December 31, 2019
|
(
|
)
|
(
|
)
|
|
|
|
|
|
|
|
|
(
|
)
|
||||||
|
($ millions)
|
At December 31, 2019
|
|
|
Deferred tax assets
|
|
|
|
Deferred tax liabilities
|
(
|
)
|
|
Net deferred tax balance
|
(
|
)
|
|
($ millions)
|
Property,
plant &
equipment
|
|
Intangible
assets
|
|
Pensions and
other benefit
obligations of
associates
|
|
Inventories
|
|
Tax loss
carry-
forwards
|
|
Other
assets,
provisions
and
accruals
|
|
Total
|
|
||||||
|
Gross deferred tax assets at January 1, 2018
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
Gross deferred tax liabilities at January 1, 2018
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
||||||||
|
Net deferred tax balance at January 1, 2018
|
(
|
)
|
(
|
)
|
|
|
|
|
|
|
|
|
(
|
)
|
||||||
|
At January 1, 2018
|
(
|
)
|
(
|
)
|
|
|
|
|
|
|
|
|
(
|
)
|
||||||
|
Credited/(charged) to income
|
(
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
Charged to equity
|
(
|
)
|
(
|
)
|
||||||||||||||||
|
Charged to other comprehensive income
|
(
|
)
|
(
|
)
|
||||||||||||||||
|
Impact of business combinations
|
(
|
)
|
|
|
(
|
)
|
||||||||||||||
|
Other movements
|
(
|
)
|
(
|
)
|
|
|
(
|
)
|
|
|
||||||||||
|
Net deferred tax balance at December 31, 2018
|
(
|
)
|
(
|
)
|
|
|
|
|
|
|
|
|
(
|
)
|
||||||
|
Gross deferred tax assets at December 31, 2018
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
Gross deferred tax liabilities at December 31, 2018
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
||||||
|
Net deferred tax balance at December 31, 2018
|
(
|
)
|
(
|
)
|
|
|
|
|
|
|
|
|
(
|
)
|
||||||
|
($ millions)
|
At December 31, 2018
|
|
|
Deferred tax assets
|
|
|
|
Deferred tax liabilities
|
(
|
)
|
|
Net deferred tax balance
|
(
|
)
|
|
($ billions)
|
At December 31, 2019
|
|
At December 31, 2018
|
|
|
|
Deferred tax assets
|
|
|
|
|
|
|
Deferred tax liabilities
|
|
|
|
|
|
|
($ millions)
|
2019
|
|
2018
|
|
|
|
Long-term financial investments measured at FVOCI
|
|
|
|
|
|
|
Long-term financial investments measured at FVPL
|
|
|
|
|
|
|
Long-term receivables from customers
|
|
|
|
|
|
|
Minimum lease payments from finance lease agreements
|
|
|
|
|
|
|
Long-term loans, advances, and security deposits
|
|
|
|
|
|
|
Total financial assets
|
|
|
|
|
|
|
2019
|
2018
|
||||||||||||||||||||||||||||
|
($ millions)
|
Total
future payments |
|
Unearned
interest income |
|
Present
value |
|
Provision
|
|
Net
book value |
|
Total
future payments |
|
Unearned
interest income |
|
Present
value |
|
Provision
|
|
Net
book value |
|
|||||||||
|
Not later than one year
(1)
|
|
|
(
|
)
|
|
|
(
|
)
|
|
|
|
|
(
|
)
|
|
|
(
|
)
|
|
|
|||||||||
|
Between one and five years
|
|
|
(
|
)
|
|
|
(
|
)
|
|
|
|
|
(
|
)
|
|
|
(
|
)
|
|
|
|||||||||
|
Later than five years
|
|
|
(
|
)
|
|
|
(
|
)
|
|
|
|
|
(
|
)
|
|
|
(
|
)
|
|
|
|||||||||
|
Total
|
|
|
(
|
)
|
|
|
(
|
)
|
|
|
|
|
(
|
)
|
|
|
(
|
)
|
|
|
|||||||||
|
($ millions)
|
2019
|
|
2018
|
|
|
|
Deferred compensation plans
|
|
|
|
|
|
|
Prepaid post-employment benefit plans
|
|
|
|
|
|
|
Other non-current assets
|
|
|
|
|
|
|
Total other non-current assets
|
|
|
|
|
|
|
($ millions)
|
2019
|
|
2018
|
|
||
|
Raw material, consumables
|
|
|
|
|
||
|
Work in progress
|
|
|
|
|
||
|
Finished products
|
|
|
|
|
||
|
Total inventories
|
|
|
|
|
||
|
($ millions)
|
2019
|
|
2018
|
|
|
|
Total gross trade receivables
|
|
|
|
|
|
|
Provisions for doubtful trade receivables
|
(
|
)
|
(
|
)
|
|
|
Total trade receivables, net
|
|
|
|
|
|
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
||
|
January 1
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
Transfers with former parent
|
|
|
|
|
|
|
||
|
Provisions for doubtful trade receivables charged to the consolidated income statement
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
Utilization of provisions for doubtful trade receivables
|
|
|
|
|
|
|
||
|
Reversal of provisions for doubtful trade receivables
|
|
|
|
|
|
|
||
|
Currency translation effects
|
|
|
|
|
(
|
)
|
||
|
December 31
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
($ millions)
|
2019
|
|
2018
|
|
|
|
Not overdue
|
|
|
|
|
|
|
Past due for not more than one month
|
|
|
|
|
|
|
Past due for more than one month but less than three months
|
|
|
|
|
|
|
Past due for more than three months but less than six months
|
|
|
|
|
|
|
Past due for more than six months but less than one year
|
|
|
|
|
|
|
Past due for more than one year
|
|
|
|
|
|
|
Provisions for doubtful trade receivables
|
(
|
)
|
(
|
)
|
|
|
Total trade receivables, net
|
|
|
|
|
|
|
($ millions)
|
2019
|
|
2018
|
|
|
|
Total balance of gross trade receivables from closely monitored countries
|
|
|
|
|
|
|
Past due for more than one year
|
|
|
|
|
|
|
Provisions for doubtful trade receivables
|
(
|
)
|
(
|
)
|
|
|
($ millions)
|
2019
|
|
2018
|
|
|
|
US dollar (USD)
|
|
|
|
|
|
|
Euro (EUR)
|
|
|
|
|
|
|
Japanese yen (JPY)
|
|
|
|
|
|
|
Chinese yuan (CNY)
|
|
|
|
|
|
|
Indian rupee (INR)
|
|
|
|
|
|
|
Canadian dollar (CAD)
|
|
|
|
|
|
|
Australian dollar (AUD)
|
|
|
|
|
|
|
British pound (GBP)
|
|
|
|
|
|
|
Russian ruble (RUB)
|
|
|
|
|
|
|
South Korean won (KRW)
|
|
|
|
|
|
|
Other currencies
|
|
|
|
|
|
|
Total trade receivables, net
|
|
|
|
|
|
|
($ millions)
|
2019
|
|
2018
|
|
|
|
Current portion of long-term financial investments measured at FVPL
|
|
|
|
|
|
|
Current portion of long-term receivables from customers
|
|
|
|
|
|
|
Current portion of minimum lease payments from finance lease agreements
|
|
|
|
|
|
|
Prepaid expenses
|
|
|
|
|
|
|
Other receivables, security deposits and current assets
|
|
|
|
|
|
|
Derivative financial instruments
|
|
|
|
|
|
|
VAT receivable
|
|
|
|
|
|
|
Total other current assets
|
|
|
|
|
|
|
•
|
contracts previously identified as leases by applying IAS 17,
Leases
and IFRIC 4,
Determining whether an Arrangement contains a Lease
, have not been re-assessed under IFRS 16,
|
|
•
|
leases with a remaining lease term less than
twelve months
from the date of adoption and leases of low-value assets have not been recognized as right-of-use assets and lease liabilities,
|
|
•
|
measurement of right-of-use assets at the date of adoption excluded the initial direct costs, and
|
|
•
|
use of hindsight in determining the lease term for contracts containing options to extend or terminate the lease.
|
|
($ millions)
|
December 31, 2019
|
|
January 1, 2019
|
|
|
|
Land
|
|
|
|
|
|
|
Buildings
|
|
|
|
|
|
|
Machinery & equipment and other assets
|
|
|
|
|
|
|
Total right-of-use assets
(1)
|
|
|
|
|
|
|
(1)
|
Right-of-use assets, related to operating leases at the date of implementation of IFRS 16, were higher than the lease liabilities at the date of implementation of IFRS 16 by
$
|
|
($ millions)
|
2019
|
|
|
Land
|
|
|
|
Buildings
|
|
|
|
Machinery & equipment and other assets
|
|
|
|
Total
|
|
|
|
($ millions)
|
||
|
Operating lease commitments as of December 31, 2018
|
|
|
|
Effect of discounting
|
(
|
)
|
|
Operating leases discounted using the incremental borrowing rate
(1)
|
|
|
|
Finance lease liabilities recognized as at December 31, 2018
|
|
|
|
Recognition exemption for short term and low-value leases
|
(
|
)
|
|
Lease liabilities as of January 1, 2019
|
|
|
|
(1)
|
|
|
($ millions)
|
Lease liabilities undiscounted
|
|
|
Not later than one year
|
|
|
|
Between one and five years
|
|
|
|
Later than five years
|
|
|
|
Total lease liabilities undiscounted
|
|
|
|
($ millions)
|
Lease liabilities
|
|
|
Not later than one year
|
|
|
|
Between one and five years
|
|
|
|
Later than five years
|
|
|
|
Total lease liabilities
|
|
|
|
($ millions)
|
2019
|
|
|
Interest expense on lease liabilities
|
|
|
|
Expense on short-term and low value leases
|
|
|
|
Total cash outflows for leases
|
|
|
|
Thereof:
|
||
|
Lease liability payments
(1)
|
|
|
|
Interest payments
(2)
|
|
|
|
Short-term and low value lease payments
(2)
|
|
|
|
(1)
|
Reported as cash outflows from financing activities net of lease incentives received
|
|
(2)
|
Included within total net cash flows from operating activities
|
|
($ millions)
|
2018
|
|
|
Not later than one year
|
|
|
|
Between one and five years
|
|
|
|
Later than five years
|
|
|
|
Total operational lease commitments
|
|
|
|
($ millions)
|
2018
|
|
|
Not later than one year
|
|
|
|
Between one and five years
|
|
|
|
Later than five years
|
|
|
|
Total minimum lease liabilities
|
|
|
|
Less future finance charges
|
(
|
)
|
|
Present value of minimum lease payments
|
|
|
|
($ millions)
|
2019
|
|
2018
|
|
|
Non-current financial debts
|
||||
|
Facility B
|
|
|
|
|
|
Facility C
|
|
|
|
|
|
Local facilities (Japan)
|
|
|
|
|
|
Series 2026 notes
|
|
|
|
|
|
Series 2029 notes
|
|
|
|
|
|
Series 2049 notes
|
|
|
|
|
|
Revolving facility
|
|
|
|
|
|
Total non-current financial debts
|
|
|
|
|
|
Current financial debts
|
||||
|
Local facilities:
|
||||
|
Japan
|
|
|
|
|
|
All others
|
|
|
|
|
|
Other short-term financial debts
|
|
|
|
|
|
Derivatives
|
|
|
|
|
|
Total current financial debts
|
|
|
|
|
|
Total financial debts
|
|
|
|
|
|
•
|
Series 2026 Notes -
$
|
|
•
|
Series 2029 Notes -
$
|
|
•
|
Series 2049 Notes -
$
|
|
($ millions)
|
Nominal amount - Current and non-current financial debt
|
|
Derivatives
|
|
Total
|
|
||
|
Not later than one year
|
|
|
|
|
|
|
||
|
Between one and five years
|
|
|
|
|
|
|
||
|
Later than five years
|
|
|
|
|
|
|
||
|
Total cash flows
|
|
|
|
|
|
|
||
|
Unamortized debt discount and issuance costs
|
(
|
)
|
|
|
(
|
)
|
||
|
Total carrying value
|
|
|
|
|
|
|
||
|
($ millions)
|
Interest
|
|
|
Not later than one year
|
|
|
|
Between one and five years
|
|
|
|
Later than five years
|
|
|
|
Total cash flows
|
|
|
|
($ millions)
|
Note
|
2019
|
|
2018
|
|
||
|
Cash and cash equivalents
|
|||||||
|
Cash in current accounts
|
|
|
|
|
|||
|
Cash held in time deposits and money market funds
|
|
|
|
|
|||
|
Total Cash and cash equivalents
|
|
|
|
|
|||
|
Financial assets - measured at fair value through other comprehensive income ("FVOCI")
|
|||||||
|
Long-term financial investments
|
12
|
|
|
|
|
||
|
Total financial assets - measured at FVOCI
|
|
|
|
|
|||
|
Financial assets - measured at amortized costs
(1)
|
|||||||
|
Trade receivables
|
14
|
|
|
|
|
||
|
Receivables from former parent
|
25
|
|
|
|
|
||
|
Income tax receivables
|
|
|
|
|
|
||
|
Other financial receivables from former parent
|
25
|
|
|
|
|
||
|
Other current assets (excluding prepaid expenses and other current assets measured at FVPL)
|
15
|
|
|
|
|
||
|
Long-term receivables from customers
|
12
|
|
|
|
|
||
|
Non-current minimum lease payments from finance lease agreements
|
12
|
|
|
|
|
||
|
Long-term loans, advances, and security deposits
|
12
|
|
|
|
|
||
|
Total financial assets - measured at amortized costs
|
|
|
|
|
|||
|
Financial assets - measured at fair value through profit and loss ("FVPL")
|
|||||||
|
Current portion of long-term financial investments
|
15
|
|
|
|
|
||
|
Derivative fInancial instruments
|
15
|
|
|
|
|
||
|
Long-term financial investments
|
12
|
|
|
|
|
||
|
Total financial assets - measured at FVPL
|
|
|
|
|
|||
|
Total financial assets
|
|
|
|
|
|||
|
Financial liabilities - measured at amortized cost or cost
(1)
|
|||||||
|
Current financial liabilities
|
|||||||
|
Financial debts
|
17
|
|
|
|
|
||
|
Lease liabilities
|
16
|
|
|
|
|
||
|
Trade payables
|
|
|
|
|
|||
|
Payables to former parent
|
25
|
|
|
|
|
||
|
Other financial liabilities to former parent
|
25
|
|
|
|
|
||
|
Total current financial liabilities - measured at amortized cost or cost
|
|
|
|
|
|||
|
Non-current financial liabilities
|
|||||||
|
Financial debts
|
17
|
|
|
|
|
||
|
Lease liabilities
|
16
|
|
|
|
|
||
|
Total non-current financial liabilities - measured at amortized cost or cost
|
|
|
|
|
|||
|
Total financial liabilities - measured at amortized cost or cost
|
|
|
|
|
|||
|
Financial liabilities - measured at FVPL
|
|||||||
|
Contingent consideration liabilities
|
19/20
|
|
|
|
|
||
|
Derivative financial instruments
|
17
|
|
|
|
|
||
|
Total financial liabilities - measured at FVPL
|
|
|
|
|
|||
|
Total financial liabilities
|
|
|
|
|
|||
|
Net financial assets and financial liabilities
|
(
|
)
|
|
|
|||
|
(1)
|
The carrying amount is a reasonable approximation of fair value, with the exception of the Series 2026, 2029 and 2049 notes recorded in Non-current financial debts with a fair value of
$
|
|
|
December 31, 2019
|
|||||||||||||
|
($ millions)
|
Level 1
|
|
Level 2
|
|
Level 3
|
|
Valued at amortized cost or cost
|
|
Total
|
|
||||
|
Non-current financial assets
|
||||||||||||||
|
Long-term financial investments measured at FVOCI
|
|
|
|
|
|
|
—
|
|
|
|
||||
|
Long-term financial investments measured at FVPL
|
|
|
|
|
|
|
—
|
|
|
|
||||
|
Long-term receivables from customers
|
|
|
|
|
|
|
|
|
|
|
||||
|
Non-current minimum lease payments from finance lease agreements
|
|
|
|
|
|
|
|
|
|
|
||||
|
Long-term loans, advances, and security deposits
|
|
|
|
|
|
|
|
|
|
|
||||
|
Total non-current financial assets
|
|
|
|
|
|
|
|
|
|
|
||||
|
Current financial assets
|
||||||||||||||
|
Money market funds
|
|
|
|
|
|
|
—
|
|
|
|
||||
|
Current portion of long-term financial investments measured at FVPL
(1)
|
|
|
|
|
|
|
—
|
|
|
|
||||
|
Current portion of long-term receivables from customers
(1)
|
|
|
|
|
|
|
|
|
|
|
||||
|
Current portion of minimum lease payments from finance lease agreements
(1)
|
|
|
|
|
|
|
|
|
|
|
||||
|
Other receivables, security deposits and current assets
(1)
|
|
|
|
|
|
|
|
|
|
|
||||
|
VAT receivables
(1)
|
|
|
|
|
|
|
|
|
|
|
||||
|
Derivative financial instruments
(1)
|
|
|
|
|
|
|
—
|
|
|
|
||||
|
Total current financial assets
|
|
|
|
|
|
|
|
|
|
|
||||
|
Total financial assets at fair value and amortized cost or cost
|
|
|
|
|
|
|
|
|
|
|
||||
|
Financial liabilities
|
||||||||||||||
|
Contingent consideration liabilities
|
|
|
|
|
(
|
)
|
—
|
|
(
|
)
|
||||
|
Non-current financial debt
|
|
|
|
|
|
|
(
|
)
|
(
|
)
|
||||
|
Current financial debt
|
|
|
|
|
|
|
(
|
)
|
(
|
)
|
||||
|
Derivative financial instruments
|
|
|
(
|
)
|
|
|
—
|
|
(
|
)
|
||||
|
Total financial liabilities at fair value and amortized cost
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
(1)
|
Recorded in Other current assets.
|
|
December 31, 2018
|
||||||||||||||
|
($ millions)
|
Level 1
|
|
Level 2
|
|
Level 3
|
|
Valued at amortized cost or cost
|
|
Total
|
|
||||
|
Non-current financial assets
|
||||||||||||||
|
Long-term financial investments measured at FVOCI
|
|
|
|
|
|
|
—
|
|
|
|
||||
|
Long-term financial investments measured at FVPL
|
|
|
|
|
|
|
—
|
|
|
|
||||
|
Long-term receivables from customers
|
|
|
|
|
|
|
|
|
|
|
||||
|
Non-current minimum lease payments from finance lease agreements
|
|
|
|
|
|
|
|
|
|
|
||||
|
Long-term loans, advances, and security deposits
|
|
|
|
|
|
|
|
|
|
|
||||
|
Total non-current financial assets
|
|
|
|
|
|
|
|
|
|
|
||||
|
Current financial assets
(1)
|
||||||||||||||
|
Current portion of long-term financial investments measured at FVPL
|
|
|
|
|
|
|
—
|
|
|
|
||||
|
Current portion of long-term receivables from customers
|
|
|
|
|
|
|
|
|
|
|
||||
|
Current portion of minimum lease payments from finance lease agreements
|
|
|
|
|
|
|
|
|
|
|
||||
|
Other receivables, security deposits and current assets
|
|
|
|
|
|
|
|
|
|
|
||||
|
VAT receivables
|
|
|
|
|
|
|
|
|
|
|
||||
|
Derivative financial instruments
|
|
|
|
|
|
|
—
|
|
|
|
||||
|
Total current financial assets
|
|
|
|
|
|
|
|
|
|
|
||||
|
Total financial assets at fair value and amortized cost or cost
|
|
|
|
|
|
|
|
|
|
|
||||
|
Financial liabilities
|
||||||||||||||
|
Contingent consideration liabilities
|
|
|
|
|
(
|
)
|
—
|
|
(
|
)
|
||||
|
Non-current financial debt
|
|
|
|
|
|
|
|
|
|
|
||||
|
Current financial debt
|
|
|
|
|
|
|
(
|
)
|
(
|
)
|
||||
|
Derivative financial instruments
|
|
|
|
|
|
|
—
|
|
|
|
||||
|
Total financial liabilities at fair value and amortized cost
|
|
|
|
|
(
|
)
|
(
|
)
|
(
|
)
|
||||
|
(1)
|
Current financial assets referenced in the above table are recorded in Other current assets.
|
|
Long-term financial investments measured
at FVOCI |
Financial investments
measured at FVPL |
||||||||||
|
($ millions)
|
2019
|
|
2018
|
|
2019
|
|
2018
|
|
|||
|
Balance as of January 1
(1)
|
|
|
|
|
|
|
|
|
|||
|
Additions
|
|
|
|
|
|
|
|
|
|||
|
Cash receipts and payments
|
|
|
|
|
(
|
)
|
(
|
)
|
|||
|
Gains/(losses) recognized in consolidated statements of comprehensive (loss)/income
|
(
|
)
|
(
|
)
|
|
|
|
|
|||
|
Unrealized gains/(losses) in consolidated income statements
|
|
|
|
|
(
|
)
|
|
|
|||
|
Amortization
|
|
|
|
|
(
|
)
|
(
|
)
|
|||
|
Reclassification
|
|
|
|
|
|
|
|
|
|||
|
Balance as of December 31
|
|
|
|
|
|
|
|
|
|||
|
(1)
|
January 1, 2018 balances reflected in this table are as adjusted for adoption of IFRS 9,
Financial Instruments
.
|
|
Contingent consideration liabilities
|
|||||
|
($ millions)
|
2019
|
|
2018
|
|
|
|
Balance as of January 1
|
(
|
)
|
(
|
)
|
|
|
Additions
|
(
|
)
|
(
|
)
|
|
|
Accretion for passage of time
|
(
|
)
|
(
|
)
|
|
|
Adjustments for changes in assumptions
|
|
|
|
|
|
|
Payments
|
|
|
|
|
|
|
Balance as of December 31
|
(
|
)
|
(
|
)
|
|
|
($ millions)
|
2019
|
|
2018
|
|
|
|
Accrued liability for employee benefits:
|
|
|
|
|
|
|
Defined benefit pension plans
(1)
|
|
|
|
|
|
|
Other long-term employee benefits and deferred compensation
|
|
|
|
|
|
|
Other post-employment benefits
(1)
|
|
|
|
|
|
|
Provisions for product liabilities, governmental investigations and other legal matters
|
|
|
|
|
|
|
Contingent consideration
(2)
|
|
|
|
|
|
|
Other non-current liabilities
|
|
|
|
|
|
|
Total provisions and other non-current liabilities
|
|
|
|
|
|
|
(1)
|
Note
23
to these Consolidated Financial Statements provides additional disclosures related to post-employment benefits.
|
|
(2)
|
Note
18
to these Consolidated Financial Statements provides additional disclosures related to contingent consideration.
|
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
||
|
January 1
|
|
|
|
|
|
|
||
|
Additions to provisions
|
|
|
|
|
|
|
||
|
Cash payments
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
Releases of provisions
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
December 31
|
|
|
|
|
|
|
||
|
Less current portion
|
|
|
(
|
)
|
(
|
)
|
||
|
Non-current provisions for product liabilities, governmental investigations and other legal matters at December 31
|
|
|
|
|
|
|
||
|
($ millions)
|
2019
|
|
2018
|
|
|
Taxes other than income taxes
|
|
|
|
|
|
Restructuring provisions
|
|
|
|
|
|
Accrued expenses for goods and services received but not invoiced
|
|
|
|
|
|
Accruals for royalties
|
|
|
|
|
|
Accruals for deductions from revenue
|
|
|
|
|
|
Accruals for compensation and benefits including social security
|
|
|
|
|
|
Deferred income
|
|
|
|
|
|
Provisions for product liabilities, governmental investigations and other legal matters
(1)
|
|
|
|
|
|
Accrued share-based payments
|
|
|
|
|
|
Accrued interest on financial debts
|
|
|
|
|
|
Contingent considerations
(2)
|
|
|
|
|
|
Other payables
|
|
|
|
|
|
Total provisions and other current liabilities
|
|
|
|
|
|
(1)
|
Note
19
to these Consolidated Financial Statements provides additional disclosures related to legal provisions.
|
|
(2)
|
Note
18
to these Consolidated Financial Statements provides additional disclosures related to contingent consideration.
|
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
|
|
January 1
|
|
|
|
|
|
|
|
|
Additions
|
|
|
|
|
|
|
|
|
Payments/utilizations
|
(
|
)
|
(
|
)
|
(
|
)
|
|
|
Changes in offset against gross trade receivables
|
|
|
|
|
|
|
|
|
Currency translation effects
|
|
|
(
|
)
|
|
|
|
|
December 31
|
|
|
|
|
|
|
|
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
|
|
January 1
|
|
|
|
|
|
|
|
|
Additions
|
|
|
|
|
|
|
|
|
Cash payments
|
(
|
)
|
(
|
)
|
(
|
)
|
|
|
Releases
|
(
|
)
|
(
|
)
|
(
|
)
|
|
|
Currency translation effects
|
|
|
|
|
|
|
|
|
December 31
|
|
|
|
|
|
|
|
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
||
|
Property, plant & equipment
|
|
|
|
|
|
|
||
|
Right-of-use assets
|
|
|
|
|
|
|
||
|
Intangible assets
|
|
|
|
|
|
|
||
|
Financial assets
|
|
|
(
|
)
|
|
|
||
|
Total
|
|
|
|
|
|
|
||
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
||
|
(Increase) in inventories
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
(Increase)/decrease in trade receivables
|
(
|
)
|
|
|
(
|
)
|
||
|
Increase in trade payables
|
|
|
|
|
|
|
||
|
Net change in other current assets
|
(
|
)
|
|
|
|
|
||
|
Net change in other current liabilities
|
|
|
|
|
|
|
||
|
Total
|
(
|
)
|
|
|
|
|
||
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
|||
|
Net assets recognized as a result of business combinations
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
Payables contingent consideration
|
|
|
|
|
|
|
|||
|
Other payments
|
|
|
(
|
)
|
|||||
|
Cash flows
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
Financial Assets
|
Financial Liabilities
|
|||||||||||||
|
($ millions)
|
Other financial receivables from former parent
|
|
Non-current financial debts
|
|
Current financial debts
|
|
Other financial liabilities to former parent
|
|
Total
|
|
||||
|
January 1, 2019
|
(
|
)
|
|
|
|
|
|
|
|
|
||||
|
Proceeds from non-current financial debts, net of issuance costs
|
|
|
|
|
|
|
|
|
|
|
||||
|
Repayment of non-current financial debts
|
|
|
(
|
)
|
|
|
(
|
)
|
||||||
|
Proceeds from Bridge Facility, net of issuance costs
|
|
|
|
|
||||||||||
|
Repayment of Bridge Facility
|
(
|
)
|
(
|
)
|
||||||||||
|
Change in current financial debts
|
|
|
|
|
|
|
|
|
|
|
||||
|
Non-cash changes in derivatives and other fair value adjustments
|
|
|
|
|
|
|
|
|
|
|
||||
|
Change in other financial receivables from former parent
|
|
|
|
|
|
|
|
|
|
|
||||
|
Change in other financial liabilities to former parent
|
|
|
|
|
|
|
(
|
)
|
(
|
)
|
||||
|
Currency translation effects
|
|
|
|
|
(
|
)
|
|
|
(
|
)
|
||||
|
December 31, 2019
|
|
|
|
|
|
|
|
|
|
|
||||
|
Financial Assets
|
Financial Liabilities
|
|||||||||||||
|
($ millions)
|
Other financial receivables from former parent
|
|
Non-current financial debts
|
|
Current financial debts
|
|
Other financial liabilities to former parent
|
|
Total
|
|
||||
|
January 1, 2018
|
(
|
)
|
|
|
|
|
|
|
|
|
||||
|
Change in current financial debts
|
(
|
)
|
(
|
)
|
||||||||||
|
Change in other financial receivables from former parent
|
|
|
||||||||||||
|
Change in other financial liabilities to former parent
|
|
|
|
|
||||||||||
|
Non-cash change in finance lease obligation
|
|
|
|
|
||||||||||
|
Currency translation effects
|
(
|
)
|
(
|
)
|
||||||||||
|
Reclassification from non-current financial debts to lease liabilities
|
(
|
)
|
(
|
)
|
||||||||||
|
December 31, 2018
|
(
|
)
|
|
|
|
|
|
|
|
|
||||
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
||
|
Property, plant & equipment
|
|
|
|
|
|
|
||
|
Currently marketed products
|
|
|
|
|
|
|
||
|
Acquired research & development
|
|
|
|
|
|
|
||
|
Deferred tax assets
|
|
|
|
|
|
|
||
|
Inventories
|
|
|
|
|
|
|
||
|
Trade receivables and other current assets
|
|
|
|
|
|
|
||
|
Cash and cash equivalents
|
|
|
|
|
|
|
||
|
Deferred tax liabilities
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
Trade payables and other liabilities
|
(
|
)
|
(
|
)
|
|
|
||
|
Net identifiable assets acquired
|
|
|
|
|
|
|
||
|
Acquired liquidity
|
(
|
)
|
(
|
)
|
(
|
)
|
||
|
Goodwill
|
|
|
|
|
|
|
||
|
Net assets recognized as a result of business combinations
|
|
|
|
|
|
|
||
|
|
Pension plans
|
Other post-employment
benefit plans
|
|||||||||
|
($ millions)
|
2019
|
|
2018
|
|
2019
|
|
2018
|
|
|||
|
Benefit obligation at January 1
|
|
|
|
|
|
|
|
|
|||
|
Current service cost
|
|
|
|
|
|
|
|
|
|||
|
Interest cost
|
|
|
|
|
|
|
|
|
|||
|
Past service costs and settlements
|
|
|
|
|
|
|
|
|
|||
|
Administrative expenses
|
|
|
|
|
|
|
|
|
|||
|
Remeasurement losses/(gains) arising from changes in financial assumptions
|
|
|
(
|
)
|
|
|
(
|
)
|
|||
|
Remeasurement losses/(gains) arising from changes in demographic assumptions
|
|
|
|
|
(
|
)
|
|
|
|||
|
Experience-related remeasurement (gains)/losses
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
|||
|
Currency translation effects
|
|
|
(
|
)
|
|
|
|
|
|||
|
Benefit payments
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
Contributions of associates
|
|
|
|
|
|
|
|
|
|||
|
Effect of acquisitions, divestments or transfers
|
(
|
)
|
|
|
|
|
|
|
|||
|
Benefit obligation at December 31
|
|
|
|
|
|
|
|
|
|||
|
Fair value of plan assets at January 1
|
|
|
|
|
|
|
|
|
|||
|
Interest income
|
|
|
|
|
|
|
|
|
|||
|
Return on plan assets excluding interest income
|
|
|
(
|
)
|
|
|
(
|
)
|
|||
|
Currency translation effects
|
|
|
(
|
)
|
|
|
|
|
|||
|
Employer contributions
|
|
|
|
|
(
|
)
|
(
|
)
|
|||
|
Contributions of associates
|
|
|
|
|
|
|
|
|
|||
|
Settlements
|
|
|
(
|
)
|
|
|
|
|
|||
|
Benefit payments
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
Effect of acquisitions, divestments or transfers
|
(
|
)
|
(
|
)
|
|
|
|
|
|||
|
Fair value of plan assets at December 31
|
|
|
|
|
|
|
|
|
|||
|
Funded status
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
Limitation on recognition of fund surplus at January 1
|
(
|
)
|
(
|
)
|
|
|
|
|
|||
|
Change in limitation on recognition of fund surplus (including exchange rate differences)
|
(
|
)
|
|
|
|
|
|
|
|||
|
Limitation on recognition of fund surplus at December 31
|
(
|
)
|
(
|
)
|
|
|
|
|
|||
|
Net liability in the balance sheet at December 31
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
|
Pension plans
|
Other post-employment
benefit plans
|
|||||||||
|
($ millions)
|
2019
|
|
2018
|
|
2019
|
|
2018
|
|
|||
|
Net liability at January 1
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
Current service cost
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
Net interest expense
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
Administrative expenses
|
(
|
)
|
(
|
)
|
|
|
|
|
|||
|
Past service costs and settlements
|
(
|
)
|
(
|
)
|
|
|
|
|
|||
|
Remeasurements
|
(
|
)
|
|
|
(
|
)
|
|
|
|||
|
Currency translation effects
|
|
|
|
|
|
|
|
|
|||
|
Employer contributions
|
|
|
|
|
(
|
)
|
(
|
)
|
|||
|
Effect of acquisitions, divestments or transfers
|
|
|
(
|
)
|
|
|
|
|
|||
|
Change in limitation on recognition of fund surplus
|
(
|
)
|
|
|
|
|
|
|
|||
|
Net liability at December 31
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
Amounts recognized in the balance sheet
|
|||||||||||
|
Prepaid benefit cost
|
|
|
|
|
|
|
|
|
|||
|
Accrued benefit liability
|
(
|
)
|
(
|
)
|
(
|
)
|
(
|
)
|
|||
|
|
2019
|
||||||||||||||||
|
($ millions)
|
Switzerland
|
|
United
States |
|
Germany
|
|
United
Kingdom |
|
Rest of
the world |
|
Total
|
|
|||||
|
Benefit obligation at December 31
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Thereof: unfunded plans
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Thereof: unfunded portion of funded plans
(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
By type of member
|
|||||||||||||||||
|
Active
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Deferred pensioners
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Pensioners
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Fair value of plan assets at December 31
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Funded status
|
(
|
)
|
(
|
)
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
|||||
|
|
2018
|
||||||||||||||||
|
($ millions)
|
Switzerland
|
|
United
States |
|
Germany
|
|
United
Kingdom |
|
Rest of
the world |
|
Total
|
|
|||||
|
Benefit obligation at December 31
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Thereof: unfunded plans
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Thereof: unfunded portion of funded plans
(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
By type of member
|
|||||||||||||||||
|
Active
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Deferred pensioners
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Pensioners
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Fair value of plan assets at December 31
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
Funded status
|
(
|
)
|
(
|
)
|
(
|
)
|
|
|
(
|
)
|
(
|
)
|
|||||
|
|
Pension plans
|
Other post-employment
benefit plans |
|||||||||
|
|
2019
|
|
2018
|
|
2019
|
|
2018
|
|
|||
|
Discount rate
|
|
%
|
|
%
|
|
%
|
|
%
|
|||
|
Expected rate of pension increase
|
|
%
|
|
%
|
|
|
|
|
|||
|
Expected rate of salary increase
|
|
%
|
|
%
|
|
|
|
|
|||
|
Interest on savings account
|
|
%
|
|
%
|
|
|
|
|
|||
|
Current average life expectancy for a 65-year-old male (in years)
|
|
|
|
|
|
|
|
|
|||
|
Current average life expectancy for a 65-year-old female (in years)
|
|
|
|
|
|
|
|
|
|||
|
($ millions)
|
Change in 2019 year-end
|
|
|
25 basis point increase in discount rate
|
(
|
)
|
|
25 basis point decrease in discount rate
|
|
|
|
1 year increase in life expectancy
|
|
|
|
25 basis point increase in rate of pension increase
|
|
|
|
25 basis point decrease in rate of pension increase
|
(
|
)
|
|
25 basis point increase of interest on savings account
|
|
|
|
25 basis point decrease of interest on savings account
|
(
|
)
|
|
25 basis point increase in rate of salary increase
|
|
|
|
25 basis point decrease in rate of salary increase
|
(
|
)
|
|
2019
|
|
2018
|
|
2017
|
|
|||
|
Healthcare cost trend rate assumed for next year
|
|
%
|
|
%
|
|
%
|
||
|
Rate to which the cost trend rate is assumed to decline
|
|
%
|
|
%
|
|
%
|
||
|
Year that the rate reaches the ultimate trend rate
|
2028
|
|
2028
|
|
2025
|
|
||
|
Pension plans
|
|||||||
|
(as a percentage)
|
Long-term
target minimum |
Long-term
target maximum |
2019
|
2018
|
|||
|
Equity securities
|
|
|
|
|
|||
|
Debt securities
|
|
|
|
|
|||
|
Real estate
|
|
|
|
|
|||
|
Alternative investments
|
|
|
|
|
|||
|
Cash and other investments
|
|
|
|
|
|||
|
Total
|
|
|
|||||
|
($ millions)
|
Pension plans
|
|
Other
post-employment benefit plans |
|
|
|
Employer contributions
|
|
|
|
|
|
|
2020 (estimated)
|
|
|
|
|
|
|
Expected future benefit payments
|
|
|
|
|
|
|
2020
|
|
|
|
|
|
|
2021
|
|
|
|
|
|
|
2022
|
|
|
|
|
|
|
2023
|
|
|
|
|
|
|
2024
|
|
|
|
|
|
|
2025-2029
|
|
|
|
|
|
|
|
2019
|
|||||||
|
Number of
shares in thousand |
|
Weighted average
fair value at grant date in $ |
|
Fair value in
$ thousand |
|
|||
|
Replacement awards issued at Spin-off
(1)
|
|
|
n/a
|
|
|
|
||
|
Granted
|
||||||||
|
Restricted awards
|
|
|
|
|
|
|
||
|
Performance awards
|
|
|
|
|
|
|
||
|
Vested
(1)
|
(
|
)
|
n/a
|
|
(
|
)
|
||
|
Forfeited
(1)
|
(
|
)
|
n/a
|
|
(
|
)
|
||
|
Unvested shares at December 31
|
|
|
|
|
|
|
||
|
(1)
|
Based on estimated fair value per share at the time of Spin-off.
|
|
(thousands)
|
2019
|
|
|
Long-term Incentive Plan
|
|
|
|
Deferred Bonus Stock Plan
|
|
|
|
Swiss Employee Share Ownership Plan
|
|
|
|
Other share savings plans
|
|
|
|
Authorized as of December 31, 2019
|
|
|
|
|
2018
|
|||||||
|
|
Number of
shares in thousand |
|
Weighted average fair value at grant
date in $
|
|
Fair value in
$ thousand |
|
||
|
Unvested shares at January 1
|
|
|
|
|
|
|
||
|
Granted
|
||||||||
|
Annual incentive
|
|
|
|
|
|
|
||
|
Share savings plans
|
|
|
|
|
|
|
||
|
Select North America
|
|
|
|
|
|
|
||
|
Select outside North America
|
|
|
|
|
|
|
||
|
Long-Term Performance Plan
|
|
|
|
|
|
|
||
|
Long-Term Relative Performance Plan
|
|
|
|
|
|
|
||
|
Other share awards
|
|
|
|
|
|
|
||
|
Vested
|
(
|
)
|
|
|
(
|
)
|
||
|
Forfeited
|
(
|
)
|
|
|
(
|
)
|
||
|
Unvested shares at December 31
|
|
|
|
|
|
|
||
|
|
2018
|
|||||||
|
|
Options
(millions) |
|
Weighted average
exercise price ($) |
|
Weighted average
intrinsic value ($) |
|
||
|
Options outstanding at January 1
|
|
|
|
|
|
|
||
|
Sold or exercised
|
(
|
)
|
|
|
|
|
||
|
Outstanding at December 31
|
|
|
|
|
|
|
||
|
Exercisable at December 31
|
|
|
|
|
|
|
||
|
|
Options outstanding
|
||||||
|
Range of exercise prices($)
|
Number
outstanding (thousand) |
|
Average remaining
contractual life (years) |
Weighted average
exercise price ($) |
|
||
|
45 - 55
|
|
|
|
|
|
||
|
56 - 66
|
|
|
|
|
|
||
|
Total
|
|
|
|
|
|
||
|
|
2018
|
|||||||
|
|
ADR
options
(millions) |
|
Weighted average
exercise price ($) |
|
Weighted average
intrinsic value ($) |
|
||
|
Options outstanding at January 1
|
|
|
|
|
|
|
||
|
Sold or exercised
|
(
|
)
|
|
|
|
|
||
|
Outstanding at December 31
|
|
|
|
|
|
|
||
|
Excercisable at December 31
|
|
|
|
|
|
|
||
|
|
ADR options outstanding
|
||||||
|
Range of exercise prices ($)
|
Number
outstanding (thousand) |
|
Average remaining
contractual life (years) |
Weighted average
exercise price
($)
|
|
||
|
45 - 55
|
|
|
|
|
|
||
|
56 - 66
|
|
|
|
|
|
||
|
Total
|
|
|
|
|
|
||
|
($ millions)
|
2019
(1)
|
|
2018
|
|
2017
|
|
|
Sales to former parent
|
|
|
|
|
|
|
|
Contract manufacturing revenues from former parent
|
|
|
|
|
|
|
|
Purchases from former parent
|
|
|
|
|
|
|
|
($ millions)
|
December 31, 2018
(1)
|
|
|
Trade and other receivables from former parent
|
|
|
|
Trade and other payables to former parent
|
|
|
|
Other financial receivables from former parent
|
|
|
|
Other financial liabilities to former parent
|
|
|
|
(1)
|
Activity presented strictly relates to the period during which Novartis was a related party (up to April 9, 2019).
|
|
($ millions)
|
2019
|
|
2018
|
|
2017
|
|
|
Cash and other compensation
|
|
|
|
|
|
|
|
Post-employment benefits
|
|
|
|
|
|
|
|
Equity-based compensation
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
|
|
|
($ millions)
|
2019
|
|
|
2020
|
|
|
|
2021
|
|
|
|
2022
|
|
|
|
2023
|
|
|
|
2024
|
|
|
|
Thereafter
|
|
|
|
Total
|
|
|
|
|
|
||
|
Country of organization/Entity name
|
Place of business
|
Equity
interest |
|
|
Argentina
|
|||
|
Alcon Laboratorios Argentina S.A.
|
Buenos Aires
|
|
%
|
|
Australia
|
|||
|
Alcon Laboratories (Australia) Pty Ltd
|
Frenchs Forest, NSW
|
|
%
|
|
Austria
|
|||
|
Alcon Ophthalmika GmbH
|
Wein
|
|
%
|
|
Belgium
|
|||
|
Alcon Laboratories Belgium BVBA
|
Puurs
|
|
%
|
|
N.V. Alcon S.A.
|
Vilvoorde
|
|
%
|
|
Canada
|
|||
|
Alcon Canada Inc.
|
Mississauga, Ontario
|
|
%
|
|
Chile
|
|||
|
Alcon Laboratorios Chile Ltd.
|
Santiago de Chile
|
|
%
|
|
China
|
|||
|
Alcon (China) Ophthalmic Product Co., Ltd.
|
Beijing
|
|
%
|
|
Alcon Hong Kong Limited
|
Hong Kong
|
|
%
|
|
Colombia
|
|||
|
Laboratorios Alcon de Colombia S.A.
|
Santafé de Bogotá
|
|
%
|
|
Czech Republic
|
|||
|
Alcon Pharmaceuticals (Czech Republic) s.r.o.
|
Prague
|
|
%
|
|
Denmark
|
|||
|
Alcon Nordic A/S
|
Copenhagen
|
|
%
|
|
Dominican Republic
|
|||
|
Alcon Dominicana, SRL
|
Santo Domingo
|
|
%
|
|
Ecuador
|
|||
|
AlconLab Ecuador S.A.
|
Quito
|
|
%
|
|
France
|
|||
|
Laboratoires Alcon S.A.S.
|
Rueil-Malmaison
|
|
%
|
|
Germany
|
|||
|
Alcon Pharma GmbH
|
Freiburg im Breisgau
|
|
%
|
|
CIBA Vision GmbH
|
Grosswallstadt
|
|
%
|
|
WaveLight GmbH
|
Erlangen
|
|
%
|
|
Greece
|
|||
|
Alcon Laboratories Hellas- Single Member Commercial and Industrial S.A.C.I.
|
Maroussi, Athens
|
|
%
|
|
Hungary
|
|||
|
Alcon Hungary Pharmaceuticals Trading Limited Liability Company
|
Budapest
|
|
%
|
|
India
|
|||
|
Alcon Laboratories (India) Private Limited
|
Bangalore
|
|
%
|
|
Indonesia
|
|||
|
PT. CIBA Vision Batam
|
Batam
|
|
%
|
|
Ireland
|
|||
|
Alcon Laboratories Ireland Limited
|
Cork City
|
|
%
|
|
Israel
|
|||
|
Optonol Ltd.
|
Neve-Ilan
|
|
%
|
|
|
|
||
|
Country of organization/Entity name
|
Place of business
|
Equity
interest |
|
|
Italy
|
|||
|
Alcon Italia S.p.A.
|
Milano
|
|
%
|
|
Japan
|
|||
|
Alcon Japan Ltd.
|
Tokyo
|
|
%
|
|
Malaysia
|
|||
|
Alcon Laboratories (Malaysia) Sdn. Bhd.
|
Petaling Jaya
|
|
%
|
|
CIBA Vision Johor Sdn. Bhd.
|
Kuala Lumpur
|
|
%
|
|
Mexico
|
|||
|
Alcon Laboratorios, S.A. de C.V.
|
Ciudad de Mexico
|
|
%
|
|
Morocco
|
|||
|
Alcon Maroc SARL D´Associé Unique
|
Casablanca
|
|
%
|
|
Netherlands
|
|||
|
Alcon Nederland B.V.
|
Arnhem
|
|
%
|
|
New Zealand
|
|||
|
Alcon Laboratories (New Zealand) Ltd.
|
Auckland
|
|
%
|
|
Panama
|
|||
|
Alcon Centroamerica S.A.
|
Panama City
|
|
%
|
|
Peru
|
|||
|
Alcon Pharmaceutical del Peru S.A.
|
Lima
|
|
%
|
|
Philippines
|
|||
|
Alcon Laboratories (Philippines), Inc.
|
Manila
|
|
%
|
|
Poland
|
|||
|
Alcon Polska Sp. z o.o.
|
Warszawa
|
|
%
|
|
Portugal
|
|||
|
Alcon Portugal-Produtos e Equipamentos Oftalmológicos Lda.
|
Porto Salvo
|
|
%
|
|
Puerto Rico
|
|||
|
Alcon (Puerto Rico), Inc.
|
Cataño, PR
|
|
%
|
|
Romania
|
|||
|
Alcon Romania S.R.L.
|
Bucharest
|
|
%
|
|
Russian Federation
|
|||
|
Alcon Farmacevtika LLC
|
Moscow
|
|
%
|
|
Singapore
|
|||
|
Alcon Pte Ltd
|
Singapore
|
|
%
|
|
Alcon Singapore Manufacturing Pte Ltd
|
Singapore
|
|
%
|
|
CIBA Vision Asian Manufacturing and Logistics Pte Ltd.
|
Singapore
|
|
%
|
|
South Africa
|
|||
|
Alcon Laboratories (South Africa) (Pty) Ltd.
|
Midrand
|
|
%
|
|
South Korea
|
|||
|
Alcon Korea Ltd.
|
Seoul
|
|
%
|
|
Spain
|
|||
|
Alcon Healthcare S.A.
|
Barcelona
|
|
%
|
|
Switzerland
|
|||
|
Alcon Inc.
|
Fribourg
|
|
%
|
|
Alcon Grieshaber AG
|
Schaffhausen
|
|
%
|
|
Alcon Management SA
|
Vernier
|
|
%
|
|
Alcon Pharmaceuticals Ltd.
|
Fribourg
|
|
%
|
|
Alcon Services AG
|
Fribourg
|
|
%
|
|
Alcon Switzerland SA
|
Risch
|
|
%
|
|
Thailand
|
|||
|
Alcon Laboratories (Thailand) Limited
|
Bangkok
|
|
%
|
|
Turkey
|
|||
|
Alcon Laboratuvarlari Ticaret A.S.
|
Istanbul
|
|
%
|
|
|
|
||
|
Country of organization/Entity name
|
Place of business
|
Equity
interest |
|
|
Ukraine
|
|||
|
Alcon Ukraine LLC
|
Kiev
|
|
%
|
|
United Kingdom
|
|||
|
Alcon Eye Care UK Limited
|
Frimley/Camberley
|
|
%
|
|
United States of America
|
|||
|
Alcon Finance Corporation
|
Wilminton, DE
|
|
%
|
|
Alcon Laboratories, Inc.
|
Wilminton, DE
|
|
%
|
|
Alcon RefractiveHorizons, LLC
|
Fort Worth, TX
|
|
%
|
|
Alcon Research, LLC
|
Fort Worth, TX
|
|
%
|
|
Alcon Vision, LLC
|
Fort Worth, TX
|
|
%
|
|
CIBA Vision, LLC
|
Duluth, GA
|
|
%
|
|
WaveLight, Inc.
|
Sterling, VA
|
|
%
|
|
ClarVista Medical, Inc.
|
Aliso Viejo, CA
|
|
%
|
|
PowerVision, Inc.
|
Fort Worth, TX
|
|
%
|
|
Tear Film Innovations, Inc.
|
Fort Worth, TX
|
|
%
|
|
TrueVision Systems, Inc.
|
Fort Worth, TX
|
|
%
|
|
Alcon Lensx, Inc.
|
Fort Worth, TX
|
|
%
|
|
Brazil
(1)
|
|
Novartis Biociências S.A.
|
|
Mexico
|
|
Novartis Farmacéutica, S.A. de C.V.
|