|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
94-3267295
|
|
(State or other jurisdiction of
incorporation or organization)
|
(I.R.S. Employer
Identification Number)
|
|
Large accelerated filer
|
x
|
Accelerated filer
|
¨
|
|
Non-accelerated filer
|
o
(Do not check if a smaller reporting company)
|
Smaller reporting company
|
¨
|
|
Emerging growth company
|
¨
|
||
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act.
|
|||
|
PART I
|
||
|
ITEM 1.
|
||
|
ITEM 2.
|
||
|
ITEM 3.
|
||
|
ITEM 4.
|
||
|
PART II
|
||
|
ITEM 1.
|
||
|
ITEM 1A.
|
||
|
ITEM 2.
|
||
|
ITEM 3.
|
||
|
ITEM 4.
|
||
|
ITEM 5.
|
||
|
ITEM 6.
|
||
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
||||||||||||||
|
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Net revenues
|
$
|
490,259
|
|
$
|
356,482
|
|
$
|
927,183
|
|
$
|
666,823
|
|
|||
|
Cost of net revenues
|
124,677
|
|
85,565
|
|
234,193
|
|
160,281
|
|
|||||||
|
Gross profit
|
365,582
|
|
270,917
|
|
692,990
|
|
506,542
|
|
|||||||
|
Operating expenses:
|
|||||||||||||||
|
Selling, general and administrative
|
212,087
|
|
162,964
|
|
411,712
|
|
314,112
|
|
|||||||
|
Research and development
|
30,804
|
|
24,384
|
|
60,395
|
|
47,188
|
|
|||||||
|
Total operating expenses
|
242,891
|
|
187,348
|
|
472,107
|
|
361,300
|
|
|||||||
|
Income from operations
|
122,691
|
|
83,569
|
|
220,883
|
|
145,242
|
|
|||||||
|
Interest income
|
1,917
|
|
1,441
|
|
4,093
|
|
2,636
|
|
|||||||
|
Other income (expense), net
|
(7,099
|
)
|
1,771
|
|
(6,922
|
)
|
2,221
|
|
|||||||
|
Net income before provision for income taxes and equity in losses of investee
|
117,509
|
|
86,781
|
|
218,054
|
|
150,099
|
|
|||||||
|
Provision for income taxes
|
7,703
|
|
15,387
|
|
10,605
|
|
8,164
|
|
|||||||
|
Equity in losses of investee, net of tax
|
3,701
|
|
2,215
|
|
5,478
|
|
3,336
|
|
|||||||
|
Net income
|
$
|
106,105
|
|
$
|
69,179
|
|
$
|
201,971
|
|
$
|
138,599
|
|
|||
|
Net income per share:
|
|||||||||||||||
|
Basic
|
$
|
1.32
|
|
$
|
0.86
|
|
$
|
2.52
|
|
$
|
1.73
|
|
|||
|
Diluted
|
$
|
1.30
|
|
$
|
0.85
|
|
$
|
2.48
|
|
$
|
1.70
|
|
|||
|
Shares used in computing net income per share:
|
|||||||||||||||
|
Basic
|
80,216
|
|
80,188
|
|
80,127
|
|
80,047
|
|
|||||||
|
Diluted
|
81,471
|
|
81,631
|
|
81,575
|
|
81,668
|
|
|||||||
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
|||||||||||||||
|
|
2018
|
2017
|
2018
|
2017
|
||||||||||||
|
Net income
|
$
|
106,105
|
|
$
|
69,179
|
|
$
|
201,971
|
|
$
|
138,599
|
|
||||
|
Net change in foreign currency translation adjustment
|
759
|
|
1,199
|
|
(283
|
)
|
740
|
|
||||||||
|
Change in unrealized (losses) gains on investments, net of tax
|
(186
|
)
|
27
|
|
(57
|
)
|
42
|
|
||||||||
|
Other comprehensive income (loss)
|
573
|
|
1,226
|
|
(340
|
)
|
782
|
|
||||||||
|
Comprehensive income
|
$
|
106,678
|
|
$
|
70,405
|
|
$
|
201,631
|
|
$
|
139,381
|
|
||||
|
June 30,
2018 |
December 31,
2017 |
||||||
|
ASSETS
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
547,993
|
|
$
|
449,511
|
|
|
|
Marketable securities, short-term
|
164,629
|
|
272,031
|
|
|||
|
Accounts receivable, net of allowance for doubtful accounts of $2,729 and $5,814, respectively
|
374,371
|
|
324,189
|
|
|||
|
Inventories
|
47,252
|
|
31,688
|
|
|||
|
Prepaid expenses and other current assets
|
126,754
|
|
80,948
|
|
|||
|
Total current assets
|
1,260,999
|
|
1,158,367
|
|
|||
|
Marketable securities, long-term
|
8,061
|
|
39,948
|
|
|||
|
Property, plant and equipment, net
|
447,933
|
|
348,793
|
|
|||
|
Equity method investments
|
49,128
|
|
54,606
|
|
|||
|
Goodwill and intangible assets, net
|
85,307
|
|
89,068
|
|
|||
|
Deferred tax assets
|
45,859
|
|
49,334
|
|
|||
|
Other assets
|
19,302
|
|
43,893
|
|
|||
|
Total assets
|
$
|
1,916,589
|
|
$
|
1,784,009
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
48,744
|
|
$
|
36,776
|
|
|
|
Accrued liabilities
|
196,754
|
|
195,562
|
|
|||
|
Deferred revenues
|
321,148
|
|
267,713
|
|
|||
|
Total current liabilities
|
566,646
|
|
500,051
|
|
|||
|
Income tax payable
|
115,701
|
|
114,091
|
|
|||
|
Other long-term liabilities
|
15,283
|
|
15,579
|
|
|||
|
Total liabilities
|
697,630
|
|
629,721
|
|
|||
|
Commitments and contingencies (Notes 8 and 9)
|
|
|
|||||
|
Stockholders’ equity:
|
|||||||
|
Preferred stock, $0.0001 par value (5,000 shares authorized; none issued)
|
—
|
|
—
|
|
|||
|
Common stock, $0.0001 par value (200,000 shares authorized; 80,313 and 80,040 issued and outstanding, respectively)
|
8
|
|
8
|
|
|||
|
Additional paid-in capital
|
844,599
|
|
886,435
|
|
|||
|
Accumulated other comprehensive income (loss), net
|
911
|
|
571
|
|
|||
|
Retained earnings
|
373,441
|
|
267,274
|
|
|||
|
Total stockholders’ equity
|
1,218,959
|
|
1,154,288
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
1,916,589
|
|
$
|
1,784,009
|
|
|
|
|
Six Months Ended
June 30, |
||||||
|
|
2018
|
2017
|
|||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
|||||||
|
Net income
|
$
|
201,971
|
|
$
|
138,599
|
|
|
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
|||||||
|
Deferred taxes
|
3,667
|
|
6,145
|
|
|||
|
Depreciation and amortization
|
24,066
|
|
16,743
|
|
|||
|
Stock-based compensation
|
32,720
|
|
29,057
|
|
|||
|
Equity in losses of investee
|
5,478
|
|
3,336
|
|
|||
|
Other non-cash operating activities
|
4,543
|
|
5,837
|
|
|||
|
Changes in assets and liabilities, net of effects of acquisitions:
|
|||||||
|
Accounts receivable
|
(44,266
|
)
|
(52,038
|
)
|
|||
|
Inventories
|
(15,586
|
)
|
(8,616
|
)
|
|||
|
Prepaid expenses and other assets
|
(9,366
|
)
|
388
|
|
|||
|
Accounts payable
|
10,743
|
|
4,410
|
|
|||
|
Accrued and other long-term liabilities
|
(53,442
|
)
|
(14,802
|
)
|
|||
|
Long-term income tax payable
|
1,610
|
|
(551
|
)
|
|||
|
Deferred revenues
|
54,983
|
|
29,580
|
|
|||
|
Net cash provided by operating activities
|
217,121
|
|
158,088
|
|
|||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
|||||||
|
Acquisitions, net of cash acquired
|
—
|
|
(8,953
|
)
|
|||
|
Purchase of property, plant and equipment
|
(115,295
|
)
|
(78,045
|
)
|
|||
|
Purchase of marketable securities
|
(78,405
|
)
|
(212,226
|
)
|
|||
|
Proceeds from maturities of marketable securities
|
207,475
|
|
173,094
|
|
|||
|
Proceeds from sales of marketable securities
|
9,560
|
|
32,352
|
|
|||
|
Loan advances to equity investee
|
—
|
|
(15,000
|
)
|
|||
|
Loan repayment from equity investee
|
30,000
|
|
—
|
|
|||
|
Other investing activities
|
668
|
|
(2,940
|
)
|
|||
|
Net cash provided by (used in) investing activities
|
54,003
|
|
(111,718
|
)
|
|||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
|||||||
|
Proceeds from issuance of common stock
|
8,585
|
|
7,521
|
|
|||
|
Common stock repurchases
|
(100,000
|
)
|
(38,793
|
)
|
|||
|
Equity forward contract related to accelerated share repurchase
|
—
|
|
(15,000
|
)
|
|||
|
Employees’ taxes paid upon the vesting of restricted stock units
|
(79,330
|
)
|
(37,968
|
)
|
|||
|
Net cash used in financing activities
|
(170,745
|
)
|
(84,240
|
)
|
|||
|
Effect of foreign exchange rate changes on cash, cash equivalents, and restricted cash
|
(1,923
|
)
|
3,640
|
|
|||
|
Net increase (decrease) in cash, cash equivalents, and restricted cash
|
98,456
|
|
(34,230
|
)
|
|||
|
Cash, cash equivalents, and restricted cash at beginning of the period
|
450,125
|
|
393,019
|
|
|||
|
Cash, cash equivalents, and restricted cash at end of the period
|
$
|
548,581
|
|
$
|
358,789
|
|
|
|
SUPPLEMENTAL CASH FLOW INFORMATION:
|
|||||||
|
Accounts payable or accrued liabilities related to property, plant and equipment
|
20,854
|
|
20,291
|
|
|||
|
Conversion of convertible notes receivable into equity securities
|
4,862
|
|
—
|
|
|||
|
December 31, 2017
|
||||||||||||
|
As Previously Reported
|
Adjustment
|
As Adjusted
|
||||||||||
|
Asset Accounts:
|
||||||||||||
|
Accounts receivable, net
|
$
|
322,825
|
|
$
|
1,364
|
|
$
|
324,189
|
|
|||
|
Deferred tax assets
|
50,059
|
|
(725
|
)
|
49,334
|
|
||||||
|
Other assets
|
38,379
|
|
5,514
|
|
43,893
|
|
||||||
|
Liability and Stockholders’ Equity Accounts:
|
||||||||||||
|
Accrued liabilities
|
$
|
194,198
|
|
$
|
1,364
|
|
$
|
195,562
|
|
|||
|
Deferred revenues
|
266,842
|
|
871
|
|
267,713
|
|
||||||
|
Retained earnings
|
263,356
|
|
3,918
|
|
267,274
|
|
||||||
|
Six Months Ended
June 30, 2017 |
||||||||||||
|
As Previously Reported
|
Adjustment
|
As Adjusted
|
||||||||||
|
Cash Flows from Investing Activities
|
||||||||||||
|
Other investing activities
|
$
|
224
|
|
$
|
(3,164
|
)
|
$
|
(2,940
|
)
|
|||
|
Net cash used in investing activities
|
(108,554
|
)
|
(3,164
|
)
|
(111,718
|
)
|
||||||
|
Effect of foreign exchange rate changes on cash, cash equivalents, and restricted cash
|
3,613
|
|
27
|
|
3,640
|
|
||||||
|
Net decrease in cash, cash equivalents, and restricted cash
|
(31,093
|
)
|
(3,137
|
)
|
(34,230
|
)
|
||||||
|
Cash, cash equivalents, and restricted cash at beginning of the period
|
389,275
|
|
3,744
|
|
393,019
|
|
||||||
|
Cash, cash equivalents, and restricted cash at end of the period
|
$
|
358,182
|
|
$
|
607
|
|
$
|
358,789
|
|
|||
|
June 30, 2018
|
Amortized
Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Fair Value
|
||||||||||||
|
Commercial paper
|
$
|
32,656
|
|
$
|
—
|
|
$
|
—
|
|
$
|
32,656
|
|
||||
|
Corporate bonds
|
84,246
|
|
1
|
|
(205
|
)
|
84,042
|
|
||||||||
|
U.S. government agency bonds
|
14,013
|
|
—
|
|
(41
|
)
|
13,972
|
|
||||||||
|
U.S. government treasury bonds
|
32,953
|
|
3
|
|
(19
|
)
|
32,937
|
|
||||||||
|
Certificates of deposit
|
1,022
|
|
—
|
|
—
|
|
1,022
|
|
||||||||
|
Total marketable securities, short-term
|
$
|
164,890
|
|
$
|
4
|
|
$
|
(265
|
)
|
$
|
164,629
|
|
||||
|
June 30, 2018
|
Amortized
Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Fair Value
|
||||||||||||
|
U.S. government agency bonds
|
$
|
7,006
|
|
$
|
—
|
|
$
|
(74
|
)
|
$
|
6,932
|
|
||||
|
Corporate bonds
|
1,128
|
|
1
|
|
—
|
|
1,129
|
|
||||||||
|
Total marketable securities, long-term
|
$
|
8,134
|
|
$
|
1
|
|
$
|
(74
|
)
|
$
|
8,061
|
|
||||
|
December 31, 2017
|
Amortized
Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Fair Value
|
||||||||||||
|
Commercial paper
|
$
|
58,503
|
|
$
|
—
|
|
$
|
(1
|
)
|
$
|
58,502
|
|
||||
|
Corporate bonds
|
145,728
|
|
3
|
|
(174
|
)
|
145,557
|
|
||||||||
|
U.S. government agency bonds
|
3,013
|
|
—
|
|
(7
|
)
|
3,006
|
|
||||||||
|
U.S. government treasury bonds
|
60,650
|
|
—
|
|
(70
|
)
|
60,580
|
|
||||||||
|
Certificates of deposit
|
4,386
|
|
—
|
|
—
|
|
4,386
|
|
||||||||
|
Total marketable securities, short-term
|
$
|
272,280
|
|
$
|
3
|
|
$
|
(252
|
)
|
$
|
272,031
|
|
||||
|
December 31, 2017
|
Amortized
Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Fair Value
|
||||||||||||
|
U.S. government agency bonds
|
$
|
15,023
|
|
$
|
—
|
|
$
|
(68
|
)
|
$
|
14,955
|
|
||||
|
Corporate bonds
|
25,067
|
|
2
|
|
(76
|
)
|
24,993
|
|
||||||||
|
Total marketable securities, long-term
|
$
|
40,090
|
|
$
|
2
|
|
$
|
(144
|
)
|
$
|
39,948
|
|
||||
|
June 30,
2018
|
December 31,
2017
|
||||||
|
One year or less
|
$
|
164,629
|
|
$
|
272,031
|
|
|
|
Due in greater than one year
|
8,061
|
|
39,948
|
|
|||
|
Total available for sale short-term and long-term marketable securities
|
$
|
172,690
|
|
$
|
311,979
|
|
|
|
June 30,
2018
|
December 31,
2017
|
||||||
|
Equity securities under the equity method investment
1
|
$
|
49,128
|
|
$
|
54,606
|
|
|
|
Equity securities without readily determinable fair values
2
|
$
|
4,862
|
|
$
|
—
|
|
|
|
Description
|
Balance as of
June 30, 2018
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
Cash equivalents:
|
||||||||||||||||
|
Money market funds
|
$
|
390,103
|
|
$
|
390,103
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Short-term investments:
|
||||||||||||||||
|
Commercial paper
|
32,656
|
|
—
|
|
32,656
|
|
—
|
|
||||||||
|
Corporate bonds
|
84,042
|
|
—
|
|
84,042
|
|
—
|
|
||||||||
|
U.S. government agency bonds
|
13,972
|
|
—
|
|
13,972
|
|
—
|
|
||||||||
|
U.S. government treasury bonds
|
32,937
|
|
32,937
|
|
—
|
|
—
|
|
||||||||
|
Certificates of deposit
|
1,022
|
|
—
|
|
1,022
|
|
—
|
|
||||||||
|
Long-term investments:
|
||||||||||||||||
|
U.S. government agency bonds
|
6,932
|
|
—
|
|
6,932
|
|
—
|
|
||||||||
|
Corporate bonds
|
1,129
|
|
—
|
|
1,129
|
|
—
|
|
||||||||
|
Prepaid expenses and other current assets:
|
||||||||||||||||
|
Israeli funds
|
3,099
|
|
—
|
|
3,099
|
|
—
|
|
||||||||
|
$
|
565,892
|
|
$
|
423,040
|
|
$
|
142,852
|
|
$
|
—
|
|
|||||
|
Description
|
Balance as of
December 31, 2017
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
Cash equivalents:
|
||||||||||||||||
|
Money market funds
|
$
|
253,155
|
|
$
|
253,155
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Commercial paper
|
7,246
|
|
—
|
|
7,246
|
|
—
|
|
||||||||
|
Corporate bonds
|
2,016
|
|
—
|
|
2,016
|
|
—
|
|
||||||||
|
Short-term investments:
|
||||||||||||||||
|
Commercial paper
|
58,502
|
|
—
|
|
58,502
|
|
—
|
|
||||||||
|
Corporate bonds
|
145,557
|
|
—
|
|
145,557
|
|
—
|
|
||||||||
|
U.S. government agency bonds
|
3,006
|
|
—
|
|
3,006
|
|
—
|
|
||||||||
|
U.S. government treasury bonds
|
60,580
|
|
60,580
|
|
—
|
|
—
|
|
||||||||
|
Certificates of deposit
|
4,386
|
|
—
|
|
4,386
|
|
—
|
|
||||||||
|
Long-term investments:
|
||||||||||||||||
|
U.S. government agency bonds
|
14,955
|
|
—
|
|
14,955
|
|
—
|
|
||||||||
|
Corporate bonds
|
24,993
|
|
—
|
|
24,993
|
|
—
|
|
||||||||
|
Prepaid expenses and other current assets:
|
||||||||||||||||
|
Israeli funds
|
3,075
|
|
—
|
|
3,075
|
|
—
|
|
||||||||
|
Short-term notes receivable
|
4,476
|
|
—
|
|
—
|
|
4,476
|
|
||||||||
|
$
|
581,947
|
|
$
|
313,735
|
|
$
|
263,736
|
|
$
|
4,476
|
|
|||||
|
June 30, 2018
|
|||||
|
Local Currency Amount
|
Notional Contract Amount (USD)
|
||||
|
Euro
|
€64,400
|
$
|
75,322
|
|
|
|
Chinese Yuan
|
¥150,000
|
22,574
|
|
||
|
Canadian Dollar
|
C$28,000
|
21,277
|
|
||
|
British Pound
|
£13,000
|
17,159
|
|
||
|
Japanese Yen
|
¥1,513,000
|
13,670
|
|
||
|
Brazilian Real
|
R$37,300
|
9,582
|
|
||
|
Australian Dollar
|
A$7,300
|
5,390
|
|
||
|
$
|
164,974
|
|
|||
|
June 30,
2018 |
December 31,
2017 |
||||||
|
Raw materials
|
$
|
22,976
|
|
$
|
12,721
|
|
|
|
Work in process
|
12,396
|
|
12,157
|
|
|||
|
Finished goods
|
11,880
|
|
6,810
|
|
|||
|
Total inventories
|
$
|
47,252
|
|
$
|
31,688
|
|
|
|
June 30,
2018 |
December 31,
2017 |
||||||
|
Capitalized commissions
|
$
|
7,947
|
|
$
|
5,515
|
|
|
|
Equity securities
|
4,862
|
|
—
|
|
|||
|
Security deposits
|
3,734
|
|
3,557
|
|
|||
|
Loan receivable from equity investee
|
—
|
|
30,000
|
|
|||
|
Other long-term assets
|
2,759
|
|
4,821
|
|
|||
|
Total other assets
|
$
|
19,302
|
|
$
|
43,893
|
|
|
|
June 30,
2018 |
December 31,
2017 |
||||||
|
Accrued payroll and benefits
|
$
|
87,697
|
|
$
|
103,004
|
|
|
|
Accrued expenses
|
36,776
|
|
27,318
|
|
|||
|
Accrued fixed assets
|
12,889
|
|
11,362
|
|
|||
|
Accrued customer credits
|
11,552
|
|
5,373
|
|
|||
|
Accrued income taxes
|
7,764
|
|
12,405
|
|
|||
|
Accrued warranty
|
7,286
|
|
5,929
|
|
|||
|
Accrued professional fees
|
6,876
|
|
6,316
|
|
|||
|
Accrued sales tax and value added tax
|
5,955
|
|
5,503
|
|
|||
|
Accrued sales return reserve
1
|
5,554
|
|
1,364
|
|
|||
|
Accrued sales rebate
|
3,772
|
|
11,209
|
|
|||
|
Other accrued liabilities
|
10,633
|
|
5,779
|
|
|||
|
Total accrued liabilities
|
$
|
196,754
|
|
$
|
195,562
|
|
|
|
Six Months Ended
June 30, |
|||||||
|
|
2018
|
2017
|
|||||
|
Balance at beginning of period
|
$
|
5,929
|
|
$
|
3,841
|
|
|
|
Charged to cost of revenues
|
5,728
|
|
3,690
|
|
|||
|
Actual warranty expenditures
|
(4,371
|
)
|
(2,503
|
)
|
|||
|
Balance at end of period
|
$
|
7,286
|
|
$
|
5,028
|
|
|
|
June 30,
2018 |
December 31,
2017 |
||||||
|
Deferred revenues - current
|
$
|
321,148
|
|
$
|
267,713
|
|
|
|
Deferred revenues - long-term
1
|
7,625
|
|
4,588
|
|
|||
|
Total
|
|||
|
Balance as of December 31, 2017
|
$
|
64,614
|
|
|
Adjustments
1
|
(434
|
)
|
|
|
Balance as of June 30, 2018
|
$
|
64,180
|
|
|
Weighted Average Amortization Period (in years)
|
Gross Carrying Amount as of
June 30, 2018 |
Accumulated
Amortization
|
Accumulated
Impairment Loss
|
Net Carrying
Value as of June 30, 2018 |
|||||||||||||
|
Trademarks
|
15
|
$
|
7,100
|
|
$
|
(1,838
|
)
|
$
|
(4,179
|
)
|
$
|
1,083
|
|
||||
|
Existing technology
|
13
|
12,600
|
|
(4,986
|
)
|
(4,328
|
)
|
3,286
|
|
||||||||
|
Customer relationships
|
11
|
33,500
|
|
(15,612
|
)
|
(10,751
|
)
|
7,137
|
|
||||||||
|
Reacquired rights
|
3
|
7,500
|
|
(2,894
|
)
|
—
|
|
4,606
|
|
||||||||
|
Patents
|
8
|
6,796
|
|
(1,919
|
)
|
—
|
|
4,877
|
|
||||||||
|
Other
|
2
|
618
|
|
(480
|
)
|
—
|
|
138
|
|
||||||||
|
Total intangible assets
|
$
|
68,114
|
|
$
|
(27,729
|
)
|
$
|
(19,258
|
)
|
$
|
21,127
|
|
|||||
|
Weighted Average Amortization Period (in years)
|
Gross Carrying
Amount as of
December 31, 2017
|
Accumulated
Amortization
|
Accumulated Impairment Loss
|
Net Carrying
Value as of
December 31, 2017
|
|||||||||||||
|
Trademarks
|
15
|
$
|
7,100
|
|
$
|
(1,769
|
)
|
$
|
(4,179
|
)
|
$
|
1,152
|
|
||||
|
Existing technology
|
13
|
12,600
|
|
(4,704
|
)
|
(4,328
|
)
|
3,568
|
|
||||||||
|
Customer relationships
|
11
|
33,500
|
|
(14,681
|
)
|
(10,751
|
)
|
8,068
|
|
||||||||
|
Reacquired rights
|
3
|
7,500
|
|
(1,356
|
)
|
—
|
|
6,144
|
|
||||||||
|
Patents
|
8
|
6,798
|
|
(1,504
|
)
|
—
|
|
5,294
|
|
||||||||
|
Other
|
2
|
618
|
|
(390
|
)
|
—
|
|
228
|
|
||||||||
|
Total intangible assets
|
$
|
68,116
|
|
$
|
(24,404
|
)
|
$
|
(19,258
|
)
|
$
|
24,454
|
|
|||||
|
Fiscal Year Ending December 31,
|
Amortization
|
|||
|
Remainder of 2018
|
$
|
3,149
|
|
|
|
2019
|
6,184
|
|
||
|
2020
|
3,855
|
|
||
|
2021
|
3,389
|
|
||
|
2022
|
2,116
|
|
||
|
Thereafter
|
2,434
|
|
||
|
Total
|
$
|
21,127
|
|
|
|
Fiscal Year Ending December 31,
|
Operating Leases
|
|||
|
Remainder of 2018
|
$
|
9,213
|
|
|
|
2019
|
17,350
|
|
||
|
2020
|
13,503
|
|
||
|
2021
|
11,827
|
|
||
|
2022
|
9,617
|
|
||
|
Thereafter
|
20,738
|
|
||
|
Total minimum future lease payments
|
$
|
82,248
|
|
|
|
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
|||||||||||||
|
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Cost of net revenues
|
$
|
900
|
|
$
|
768
|
|
$
|
1,781
|
|
$
|
1,693
|
|
|||
|
Selling, general and administrative
|
13,216
|
|
11,218
|
|
25,794
|
|
22,934
|
|
|||||||
|
Research and development
|
2,774
|
|
2,259
|
|
5,145
|
|
4,430
|
|
|||||||
|
Total stock-based compensation
|
$
|
16,890
|
|
$
|
14,245
|
|
$
|
32,720
|
|
$
|
29,057
|
|
|||
|
Number of Shares
Underlying
Stock Options
(in thousands)
|
Weighted
Average
Exercise
Price per Share
|
Weighted Average
Remaining
Contractual Term (in years)
|
Aggregate
Intrinsic
Value
(in thousands)
|
|||||||||
|
Outstanding as of December 31, 2017
|
75
|
|
$
|
11.36
|
|
|||||||
|
Exercised
|
(61
|
)
|
12.13
|
|
||||||||
|
Cancelled or expired
|
—
|
|
—
|
|
||||||||
|
Outstanding as of June 30, 2018
|
14
|
|
$
|
7.96
|
|
0.62
|
$
|
4,651
|
|
|||
|
Vested at June 30, 2018
|
14
|
|
$
|
7.96
|
|
0.62
|
$
|
4,651
|
|
|||
|
Exercisable at June 30, 2018
|
14
|
|
$
|
7.96
|
|
0.62
|
$
|
4,651
|
|
|||
|
Shares
Underlying RSUs
(in thousands)
|
Weighted Average Grant Date Fair Value
|
Weighted
Remaining
Contractual Term (in years)
|
Aggregate
Intrinsic
Value
(in thousands)
|
|||||||||
|
Nonvested as of December 31, 2017
|
1,341
|
|
$
|
82.30
|
|
|||||||
|
Granted
|
214
|
|
256.85
|
|
||||||||
|
Vested and released
|
(491
|
)
|
71.93
|
|
||||||||
|
Forfeited
|
(65
|
)
|
98.99
|
|
||||||||
|
Nonvested as of June 30, 2018
|
999
|
|
$
|
123.73
|
|
1.44
|
$
|
341,829
|
|
|||
|
Number of Shares
Underlying MSUs
(in thousands)
|
Weighted Average Grant Date Fair Value
|
Weighted Average
Remaining
Contractual Term (in years)
|
Aggregate
Intrinsic
Value
(in thousands)
|
|||||||||
|
Nonvested as of December 31, 2017
|
428
|
|
$
|
78.53
|
|
|||||||
|
Granted
|
208
|
|
261.86
|
|
||||||||
|
Vested and released
|
(312
|
)
|
62.41
|
|
||||||||
|
Forfeited
|
—
|
|
—
|
|
||||||||
|
Nonvested as of June 30, 2018
|
324
|
|
$
|
211.91
|
|
1.66
|
$
|
110,922
|
|
|||
|
|
Six Months Ended
June 30, |
||||||
|
|
2018
|
2017
|
|||||
|
Expected term (in years)
|
1.3
|
|
1.2
|
|
|||
|
Expected volatility
|
35.7
|
%
|
26.1
|
%
|
|||
|
Risk-free interest rate
|
1.9
|
%
|
0.9
|
%
|
|||
|
Expected dividends
|
—
|
|
—
|
|
|||
|
Weighted average fair value at grant date
|
$
|
78.38
|
|
$
|
26.09
|
|
|
|
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
|||||||||||||
|
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Numerator:
|
|||||||||||||||
|
Net income
|
$
|
106,105
|
|
$
|
69,179
|
|
$
|
201,971
|
|
$
|
138,599
|
|
|||
|
Denominator:
|
|||||||||||||||
|
Weighted-average common shares outstanding, basic
|
80,216
|
|
80,188
|
|
80,127
|
|
80,047
|
|
|||||||
|
Dilutive effect of potential common stock
|
1,255
|
|
1,443
|
|
1,448
|
|
1,621
|
|
|||||||
|
Total shares, diluted
|
81,471
|
|
81,631
|
|
81,575
|
|
81,668
|
|
|||||||
|
Net income per share, basic
|
$
|
1.32
|
|
$
|
0.86
|
|
$
|
2.52
|
|
$
|
1.73
|
|
|||
|
Net income per share, diluted
|
$
|
1.30
|
|
$
|
0.85
|
|
$
|
2.48
|
|
$
|
1.70
|
|
|||
|
•
|
Our Clear Aligner segment consists of Comprehensive Products, Non-Comprehensive Products and Non-Case revenues as defined below:
|
|
•
|
Comprehensive Products include our Invisalign Full, Teen and Assist products.
|
|
•
|
Non-Comprehensive Products include our Invisalign Express, Invisalign Lite, Invisalign i7 and Invisalign Go products in addition to revenues from the sale of aligners to SDC under our supply agreement.
|
|
•
|
Non-Case includes our Vivera retainers along with our training and ancillary products for treating malocclusion.
|
|
•
|
Our Scanner segment consists of intraoral scanning systems and additional services available with the intraoral scanners that provide digital alternatives to the traditional cast models. This segment includes our iTero scanner and OrthoCAD services.
|
|
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
|||||||||||||
|
Net revenues
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Clear Aligner
|
$
|
433,241
|
|
$
|
321,036
|
|
$
|
818,746
|
|
$
|
603,435
|
|
|||
|
Scanner
|
57,018
|
|
35,446
|
|
108,437
|
|
63,388
|
|
|||||||
|
Total net revenues
|
$
|
490,259
|
|
$
|
356,482
|
|
$
|
927,183
|
|
$
|
666,823
|
|
|||
|
Gross profit
|
|||||||||||||||
|
Clear Aligner
|
$
|
331,612
|
|
$
|
250,814
|
|
$
|
628,588
|
|
$
|
470,761
|
|
|||
|
Scanner
|
33,970
|
|
20,103
|
|
64,402
|
|
35,781
|
|
|||||||
|
Total gross profit
|
$
|
365,582
|
|
$
|
270,917
|
|
$
|
692,990
|
|
$
|
506,542
|
|
|||
|
Income from operations
|
|||||||||||||||
|
Clear Aligner
|
$
|
190,287
|
|
$
|
133,916
|
|
$
|
351,741
|
|
$
|
248,650
|
|
|||
|
Scanner
|
17,670
|
|
8,795
|
|
33,752
|
|
14,799
|
|
|||||||
|
Unallocated corporate expenses
|
(85,266
|
)
|
(59,142
|
)
|
(164,610
|
)
|
(118,207
|
)
|
|||||||
|
Total income from operations
|
$
|
122,691
|
|
$
|
83,569
|
|
$
|
220,883
|
|
$
|
145,242
|
|
|||
|
Depreciation and amortization
|
|||||||||||||||
|
Clear Aligner
|
$
|
6,759
|
|
$
|
5,600
|
|
$
|
13,143
|
|
$
|
9,963
|
|
|||
|
Scanner
|
1,169
|
|
1,082
|
|
2,273
|
|
2,119
|
|
|||||||
|
Unallocated corporate depreciation and amortization
|
4,704
|
|
2,194
|
|
8,650
|
|
4,661
|
|
|||||||
|
Total depreciation and amortization
|
$
|
12,632
|
|
$
|
8,876
|
|
$
|
24,066
|
|
$
|
16,743
|
|
|||
|
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
|||||||||||||
|
2018
|
2017
|
2018
|
2017
|
||||||||||||
|
Total segment income from operations
|
$
|
207,957
|
|
$
|
142,711
|
|
$
|
385,493
|
|
$
|
263,449
|
|
|||
|
Unallocated corporate expenses
|
(85,266
|
)
|
(59,142
|
)
|
(164,610
|
)
|
(118,207
|
)
|
|||||||
|
Total income from operations
|
122,691
|
|
83,569
|
|
220,883
|
|
145,242
|
|
|||||||
|
Interest income
|
1,917
|
|
1,441
|
|
4,093
|
|
2,636
|
|
|||||||
|
Other income (expense), net
|
(7,099
|
)
|
1,771
|
|
(6,922
|
)
|
2,221
|
|
|||||||
|
Net income before provision for income taxes and equity in losses of investee
|
$
|
117,509
|
|
$
|
86,781
|
|
$
|
218,054
|
|
$
|
150,099
|
|
|||
|
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
|||||||||||||
|
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Net revenues
1
:
|
|||||||||||||||
|
United States
|
$
|
254,020
|
|
$
|
207,021
|
|
$
|
491,123
|
|
$
|
390,294
|
|
|||
|
The Netherlands
|
156,428
|
|
109,532
|
|
295,959
|
|
209,331
|
|
|||||||
|
Other International
|
79,811
|
|
39,929
|
|
140,101
|
|
67,198
|
|
|||||||
|
Total net revenues
|
$
|
490,259
|
|
$
|
356,482
|
|
$
|
927,183
|
|
$
|
666,823
|
|
|||
|
|
June 30,
2018 |
December 31, 2017
|
|||||
|
Long-lived assets
2
:
|
|||||||
|
The Netherlands
|
$
|
190,130
|
|
$
|
143,673
|
|
|
|
United States
|
129,837
|
|
128,171
|
|
|||
|
Costa Rica
|
61,609
|
|
30,738
|
|
|||
|
Mexico
|
31,289
|
|
25,090
|
|
|||
|
China
|
16,393
|
|
5,480
|
|
|||
|
Other International
|
18,675
|
|
15,641
|
|
|||
|
Total long-lived assets
|
$
|
447,933
|
|
$
|
348,793
|
|
|
|
•
|
New Invisalign Product Portfolio and Pricing
. In July 2018, we launched a new expanded Invisalign product portfolio which includes new options and greater flexibility to treat a broader range of patients. The new Invisalign product portfolio offers doctors more choices by extending desirable features across the entire portfolio and creating new Invisalign treatment packages, as well as new options to treat young patients with early mixed dentition (with a mixture of primary/baby and permanent teeth). The new end-to-end Invisalign product portfolio will include clear aligner product offerings for almost every patient age group and case complexity to make it easier for our doctors to tailor treatment planning to the needs of each patient. Pricing and availability for the new Invisalign product offerings and the associated terms and conditions will vary by region.
|
|
•
|
New Invisalign Products and Feature Enhancements
. Product innovation drives greater treatment predictability, clinical applicability and ease of use for our customers which supports adoption of Invisalign treatment in their practices. Our focus is to develop solutions and features to treat a wide range of cases from simple to complex.
|
|
◦
|
In March 2017, we announced Invisalign Teen with mandibular advancement, the first clear aligner solution for Class II correction in growing tween and teen patients. This offering combines the benefits of the most advanced clear aligner system in the world with features for moving the lower jaw forward while simultaneously aligning the teeth. Invisalign Teen with mandibular advancement is available in Canada and in select Europe, Middle East and Africa (“EMEA”), Asia Pacific (“APAC”) and Latin America (“LATAM”) countries. Invisalign Teen with mandibular advancement is pending 510(k) clearance and is not yet available in the United States (“U.S.”).
|
|
◦
|
Beginning July 2018, Invisalign First clear aligners are commercially available to Invisalign-trained doctors in the U.S., Canada, Australia, New Zealand, Japan, and the EMEA region. Invisalign First clear aligners, a treatment option designed with features specifically for younger patients with early mixed dentition. Phase 1 treatment is early interceptive orthodontic treatment for young patients, traditionally done through arch expanders, or partial
|
|
◦
|
In April 2018, we announced a new Invisalign Go product with more user-friendly iTero digital chairside experience and greater flexibility to treat a wider range of mild to moderate cases, such as crowded or gap teeth that require teeth straightening prior to restorative treatments. Invisalign Go also incorporates new data-driven clinical protocols for predictable tooth movement and automated case assessments that leverages our Invisalign patients treated to date. These improvements make it easier for dentists to tailor their treatment plans to the individual needs of each patient.
|
|
•
|
New iTero Products and Technology Innovation.
The iTero scanner is an important component to customer experience and is central to a digital approach as well as overall customer utilization of Invisalign.
|
|
◦
|
In April 2018, we expanded the iTero Element portfolio with the launch of the iTero Element 2 and the iTero Element Flex scanners. These additions build on the existing high precision, full-color imaging and fast scan times of the iTero Element portfolio while streamlining orthodontic and restorative workflows. The next-generation iTero Element 2 is designed for greater performance with 2X faster start-up and 25% faster scan processing time compared to the iTero Element. The new iTero Element Flex wand-only configuration is a portable scanner for easy transport from office to office. iTero Element 2 and iTero Element Flex scanners are available in Canada, the U.S., the majority of European countries, including France, Germany, Italy, Spain, and the United Kingdom as well as select APAC markets. The existing iTero Element scanner will continue to be available in all markets.
|
|
◦
|
On April 25, 2018, we announced that we received market approval for the iTero Element intra-oral scanner from the China Food and Drug Administration, and we began offering this scanner in China. The iTero Element scanner launch in China not only supports growth of our base Invisalign clear aligner business but also represents a major milestone for digital dentistry in China. As we continue to expand the markets into which we sell our intra-oral scanners, we expect continued growth for the foreseeable future due to the size of the market opportunity and our relatively low market penetration of these regions.
|
|
•
|
Invisalign Adoption.
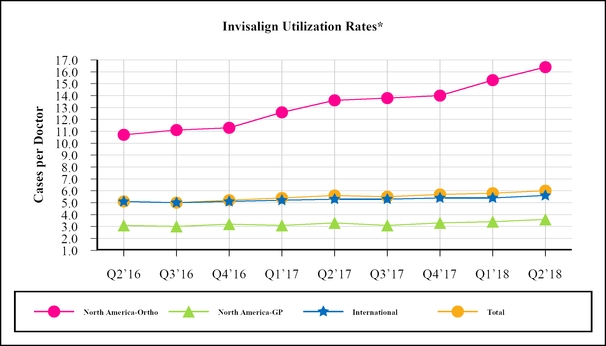
Our goal is to establish Invisalign as the treatment of choice for treating malocclusion ultimately driving increased product adoption and frequency of use by dental professionals, also known as “utilization rates.” Our quarterly utilization rates for the last 9 quarters are as follows:
|
|
◦
|
Total utilization in the second quarter of 2018 increased to 6.0 cases per doctor compared to 5.6 in the second quarter of 2017.
|
|
▪
|
North America:
Utilization among our North American orthodontist customers reached an all time high in the second quarter of 2018 at 16.4 cases per doctor. Compared to 13.6 cases per doctor utilized in the second quarter of 2017, the increase in North American orthodontist utilization in the second quarter of 2018 reflects improvements in product and technology which continues to strengthen our doctors’ clinical confidence such that they now utilize Invisalign more often and on more complex cases, including their teenage patients.
|
|
▪
|
International:
International doctor utilization was 5.6 cases per doctor in the second quarter of 2018 compared to 5.3 in the second quarter of 2017. The increase in International utilization reflects growth in both the EMEA and APAC regions due to increasing adoption of the product due in part to its ability to treat more complex cases.
|
|
•
|
Number of New Invisalign Doctors Trained.
We continue to expand our Invisalign customer base through the training of new doctors. In 2017, Invisalign growth was driven primarily by increased utilization across all regions as well as by the continued expansion of our customer base as we trained a total of 16,500 new Invisalign doctors, of which 62% were trained in the International region. During the six months ended June 30, 2018, we trained 9,345 new Invisalign doctors of which 3,400 were trained in the Americas region and 5,945 in the International region.
|
|
•
|
International Invisalign Growth.
We continue to focus our efforts towards increasing Invisalign clear aligner adoption by dental professionals in our direct EMEA and APAC markets. On a year-over-year basis, our International Invisalign
|
|
•
|
Establish Regional Order Acquisition, Treatment Planning and Manufacturing Operations.
We will continue to establish and expand additional order acquisition, treatment planning and manufacturing operations closer to our international customers in order to improve our operational efficiency and to provide doctors confidence in using Invisalign clear aligners to treat more patients and more often. In July 2018, we moved into new facilities in Costa Rica in order to support our expanding treatment planning as well as other support functions. In 2018, we expect to open a treatment planning facility in Madrid, Spain, a manufacturing facility in Ziyang, China as well as another new facility in Costa Rica to support treatment planning and administrative activities (Refer
to
Item 1A Risk Factors - “As we continue to grow, we are subject to growth related risks, including risks related to excess or constrained capacity at our existing facilities.”
for information on related risk factors).
|
|
•
|
Expenses.
We expect expenses to increase in fiscal year 2018 due in part to:
|
|
◦
|
Investments in international expansion in new country markets;
|
|
◦
|
Product and technology innovation to enhance product efficiency and operational productivity;
|
|
◦
|
Investments in manufacturing capacity and facilities to enhance our regional capabilities;
|
|
◦
|
Increases in legal expenses primarily related to the continued protection of our intellectual property rights, including our patents; and
|
|
◦
|
Increases in sales, marketing, advertising and customer support resources.
|
|
•
|
Stock Repurchases:
|
|
◦
|
April 2016 Repurchase Program.
In February 2018, we repurchased $100.0 million of our common stock on the open market. In July 2018, we repurchased $100.0 million of our common stock on the open market, completing the April 2016 Repurchase Program.
|
|
◦
|
May 2018 Repurchase Program.
In May 2018, we announced that our Board of Directors had authorized a plan to repurchase up to $600.0 million of our common stock. As of
June 30, 2018
, we have $600.0 million remaining under the May 2018 Repurchase Program (
Refer to Note 11 “Common Stock Repurchase Programs” of the Notes to Condensed Consolidated Financial Statements
for details on our stock repurchase programs).
|
|
•
|
U.S. Tax Cuts and Jobs Act.
The U.S. Tax Cuts and Jobs Act (the “TCJA”) was enacted into law on December 22, 2017 and impacted our effective tax rate. The TCJA made significant changes to the Internal Revenue Code, including, but not limited to, a corporate tax rate decrease from 35% to 21% effective for tax years beginning after December 31, 2017, the transition of U.S. international taxation from a worldwide tax system to a territorial system, and a one-time transition tax on the mandatory deemed repatriation of cumulative foreign earnings. We recorded a provisional one-time transition tax liability of $73.9 million in the fourth quarter of 2017 and an additional $0.5 million during the six months ended June 30, 2018. Additional work is necessary for a more detailed analysis of our historical foreign earnings as well as potential correlative adjustments. Any subsequent adjustment to these amounts will be recorded to provision from income taxes throughout the fiscal year of 2018 when the analysis is complete.
|
|
•
|
SmileDirectClub.
On April 5, 2018, SDC Financial LLC, SmileDirectClub LLC, and the Members of SDC Financial LLC other than Align (collectively, the "SDC Entities") initiated proceedings that seek, among other forms of relief, to preliminarily and permanently enjoin all activities related to the Invisalign store pilot project, require Align to close the
|
|
•
|
Our Clear Aligner segment consists of Comprehensive Products, Non-Comprehensive Products and Non-Case revenues as defined below:
|
|
•
|
Comprehensive Products include our Invisalign Full, Teen and Assist products.
|
|
•
|
Non-Comprehensive Products include our Invisalign Express, Invisalign Lite, Invisalign i7 and Invisalign Go products in addition to revenues from the sale of aligners to SmileDirectClub (“SDC”) under our supply agreement.
|
|
•
|
Non-Case includes our Vivera retainers along with our training and ancillary products for treating malocclusion.
|
|
•
|
Our Scanner segment consists of intraoral scanning systems and additional services available with the intraoral scanners that provide digital alternatives to the traditional cast models. This segment includes our iTero scanner and OrthoCAD services.
|
|
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
|||||||||||||||||||||||||||
|
Net Revenues
|
2018
|
2017
|
Net
Change
|
%
Change
|
2018
|
2017
|
Net
Change
|
%
Change
|
|||||||||||||||||||||
|
Clear Aligner revenues:
|
|||||||||||||||||||||||||||||
|
Americas
|
$
|
234.0
|
|
$
|
188.6
|
|
$
|
45.4
|
|
24.1
|
%
|
$
|
443.6
|
|
$
|
354.2
|
|
$
|
89.4
|
|
25.2
|
%
|
|||||||
|
International
|
173.0
|
|
111.8
|
|
61.1
|
|
54.7
|
%
|
324.7
|
|
210.7
|
|
114.0
|
|
54.1
|
%
|
|||||||||||||
|
Non-case
|
26.2
|
|
20.6
|
|
5.6
|
|
27.4
|
%
|
50.4
|
|
38.5
|
|
11.9
|
|
31.0
|
%
|
|||||||||||||
|
Total Clear Aligner net revenues
|
$
|
433.2
|
|
$
|
321.0
|
|
$
|
112.2
|
|
35.0
|
%
|
$
|
818.7
|
|
$
|
603.4
|
|
$
|
215.3
|
|
35.7
|
%
|
|||||||
|
Scanner net revenues
|
57.0
|
|
35.4
|
|
21.6
|
|
60.9
|
%
|
108.4
|
|
63.4
|
|
45.0
|
|
71.1
|
%
|
|||||||||||||
|
Total net revenues
|
$
|
490.3
|
|
$
|
356.5
|
|
$
|
133.8
|
|
37.5
|
%
|
$
|
927.2
|
|
$
|
666.8
|
|
$
|
260.4
|
|
39.0
|
%
|
|||||||
|
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
|||||||||||||||||||||
|
Region
|
2018
|
2017
|
Net
Change
|
%
Change
|
2018
|
2017
|
Net
Change
|
%
Change
|
|||||||||||||||
|
Americas
|
198.0
|
|
156.6
|
|
41.4
|
|
26.4
|
%
|
374.5
|
|
294.3
|
|
80.2
|
|
27.3
|
%
|
|||||||
|
International
|
121.3
|
|
83.4
|
|
37.8
|
|
45.4
|
%
|
226.8
|
|
157.0
|
|
69.8
|
|
44.4
|
%
|
|||||||
|
Total case volume
|
319.2
|
|
240.0
|
|
79.2
|
|
33.0
|
%
|
601.3
|
|
451.3
|
|
150.0
|
|
33.2
|
%
|
|||||||
|
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
|||||||||||||||||||||
|
|
2018
|
2017
|
Change
|
2018
|
2017
|
Change
|
|||||||||||||||||
|
Clear Aligner
|
|||||||||||||||||||||||
|
Cost of net revenues
|
$
|
101.6
|
|
$
|
70.2
|
|
$
|
31.4
|
|
$
|
190.2
|
|
$
|
132.7
|
|
$
|
57.5
|
|
|||||
|
% of net segment revenues
|
23.5
|
%
|
21.9
|
%
|
23.2
|
%
|
22.0
|
%
|
|||||||||||||||
|
Gross profit
|
$
|
331.6
|
|
$
|
250.8
|
|
$
|
80.8
|
|
$
|
628.6
|
|
$
|
470.8
|
|
$
|
157.8
|
|
|||||
|
Gross margin %
|
76.5
|
%
|
78.1
|
%
|
76.8
|
%
|
78.0
|
%
|
|||||||||||||||
|
Scanner
|
|||||||||||||||||||||||
|
Cost of net revenues
|
$
|
23.0
|
|
$
|
15.3
|
|
$
|
7.7
|
|
$
|
44.0
|
|
$
|
27.6
|
|
$
|
16.4
|
|
|||||
|
% of net segment revenues
|
40.4
|
%
|
43.3
|
%
|
40.6
|
%
|
43.6
|
%
|
|||||||||||||||
|
Gross profit
|
$
|
34.0
|
|
$
|
20.1
|
|
$
|
13.9
|
|
$
|
64.4
|
|
$
|
35.8
|
|
$
|
28.6
|
|
|||||
|
Gross margin %
|
59.6
|
%
|
56.7
|
%
|
59.4
|
%
|
56.4
|
%
|
|||||||||||||||
|
Total cost of net revenues
|
$
|
124.7
|
|
$
|
85.6
|
|
$
|
39.1
|
|
$
|
234.2
|
|
$
|
160.3
|
|
$
|
73.9
|
|
|||||
|
% of net revenues
|
25.4
|
%
|
24.0
|
%
|
25.3
|
%
|
24.0
|
%
|
|||||||||||||||
|
Gross profit
|
$
|
365.6
|
|
$
|
270.9
|
|
$
|
94.7
|
|
$
|
693.0
|
|
$
|
506.5
|
|
$
|
186.4
|
|
|||||
|
Gross margin %
|
74.6
|
%
|
76.0
|
%
|
74.7
|
%
|
76.0
|
%
|
|||||||||||||||
|
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
|||||||||||||||||||||
|
|
2018
|
2017
|
Change
|
2018
|
2017
|
Change
|
|||||||||||||||||
|
Selling, general and administrative
|
$
|
212.1
|
|
$
|
163.0
|
|
$
|
49.1
|
|
$
|
411.7
|
|
$
|
314.1
|
|
$
|
97.6
|
|
|||||
|
% of net revenues
|
43.3
|
%
|
45.7
|
%
|
44.4
|
%
|
47.1
|
%
|
|||||||||||||||
|
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
|||||||||||||||||||||
|
|
2018
|
2017
|
Change
|
2018
|
2017
|
Change
|
|||||||||||||||||
|
Research and development
|
$
|
30.8
|
|
$
|
24.4
|
|
$
|
6.4
|
|
$
|
60.4
|
|
$
|
47.2
|
|
$
|
13.2
|
|
|||||
|
% of net revenues
|
6.3
|
%
|
6.8
|
%
|
6.5
|
%
|
7.1
|
%
|
|||||||||||||||
|
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
|||||||||||||||||||||
|
|
2018
|
2017
|
Change
|
2018
|
2017
|
Change
|
|||||||||||||||||
|
Clear Aligner
|
|||||||||||||||||||||||
|
Income from operations
|
$
|
190.3
|
|
$
|
133.9
|
|
$
|
56.4
|
|
$
|
351.7
|
|
$
|
248.7
|
|
$
|
103.1
|
|
|||||
|
Operating margin %
|
43.9
|
%
|
41.7
|
%
|
43.0
|
%
|
41.2
|
%
|
|||||||||||||||
|
Scanner
|
|||||||||||||||||||||||
|
Income from operations
|
$
|
17.7
|
|
$
|
8.8
|
|
$
|
8.9
|
|
$
|
33.8
|
|
$
|
14.8
|
|
$
|
19.0
|
|
|||||
|
Operating margin %
|
31.0
|
%
|
24.8
|
%
|
31.1
|
%
|
23.3
|
%
|
|||||||||||||||
|
Total income from operations
1
|
$
|
122.7
|
|
$
|
83.6
|
|
$
|
39.1
|
|
$
|
220.9
|
|
$
|
145.2
|
|
$
|
75.6
|
|
|||||
|
Operating margin %
|
25.0
|
%
|
23.4
|
%
|
23.8
|
%
|
21.8
|
%
|
|||||||||||||||
|
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
|||||||||||||||||||||
|
|
2018
|
2017
|
Change
|
2018
|
2017
|
Change
|
|||||||||||||||||
|
Interest income
|
$
|
1.9
|
|
$
|
1.4
|
|
$
|
0.5
|
|
$
|
4.1
|
|
$
|
2.6
|
|
$
|
1.5
|
|
|||||
|
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
|||||||||||||||||||||
|
|
2018
|
2017
|
Change
|
2018
|
2017
|
Change
|
|||||||||||||||||
|
Other income (expense), net
|
$
|
(7.1
|
)
|
$
|
1.8
|
|
$
|
(8.9
|
)
|
$
|
(6.9
|
)
|
$
|
2.2
|
|
$
|
(9.1
|
)
|
|||||
|
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
|||||||||||||||||||||
|
|
2018
|
2017
|
Change
|
2018
|
2017
|
Change
|
|||||||||||||||||
|
Equity in losses of investee, net of tax
|
$
|
3.7
|
|
$
|
2.2
|
|
$
|
1.5
|
|
$
|
5.5
|
|
$
|
3.3
|
|
$
|
2.1
|
|
|||||
|
|
Three Months Ended
June 30, |
Six Months Ended
June 30, |
|||||||||||||||||||||
|
|
2018
|
2017
|
Change
|
2018
|
2017
|
Change
|
|||||||||||||||||
|
Provision for income taxes
|
$
|
7.7
|
|
$
|
15.4
|
|
$
|
(7.7
|
)
|
$
|
10.6
|
|
$
|
8.2
|
|
$
|
2.4
|
|
|||||
|
Effective tax rates
|
6.6
|
%
|
17.7
|
%
|
4.9
|
%
|
5.4
|
%
|
|||||||||||||||
|
June 30,
|
December 31,
|
|||||||
|
2018
|
2017
|
|||||||
|
Cash and cash equivalents
|
$
|
547,993
|
|
$
|
449,511
|
|
||
|
Marketable securities, short-term
|
164,629
|
|
272,031
|
|
||||
|
Marketable securities, long-term
|
8,061
|
|
39,948
|
|
||||
|
Total
|
$
|
720,683
|
|
$
|
761,490
|
|
||
|
|
Six Months Ended
June 30, |
|||||||
|
|
2018
|
2017
|
||||||
|
Net cash flow provided by (used in):
|
||||||||
|
Operating activities
|
$
|
217,121
|
|
$
|
158,088
|
|
||
|
Investing activities
|
54,003
|
|
(111,718
|
)
|
||||
|
Financing activities
|
(170,745
|
)
|
(84,240
|
)
|
||||
|
Effect of exchange rate changes on cash, cash equivalents, and restricted cash
|
(1,923
|
)
|
3,640
|
|
||||
|
Net increase (decrease) in cash, cash equivalents, and restricted cash
|
$
|
98,456
|
|
$
|
(34,230
|
)
|
||
|
•
|
Stock-based compensation was
$32.7 million
related to equity incentive compensation granted to employees and directors;
|
|
•
|
Depreciation and amortization of
$24.1 million
related to our property, plant and equipment and intangible assets; and
|
|
•
|
Equity in losses of investee of $5.5 million.
|
|
•
|
Increase of
$55.0 million
in deferred revenues corresponding to the increase in case shipments;
|
|
•
|
Decrease of
$53.4 million
in accrued and other long-term liabilities due to timing of payments and activities; and
|
|
•
|
Increase of
$44.3 million
in accounts receivable which is primarily a result of the increase in net revenues.
|
|
•
|
difficulties in hiring and retaining employees generally, as well as difficulties in hiring and retaining employees with the necessary skills to perform the more technical aspects of our operations;
|
|
•
|
difficulties in managing international operations, including any travel restrictions to or from our facilities;
|
|
•
|
fluctuations in currency exchange rates;
|
|
•
|
import and export controls, license requirements and restrictions;
|
|
•
|
controlling production volume and quality of the manufacturing process;
|
|
•
|
political, social and economic instability, including increased levels of violence in Juarez, Mexico or the Middle East. We cannot predict the effect on us of any future armed conflict, political instability or violence in these regions. In addition, some of our employees in Israel are obligated to perform annual reserve duty in the Israeli military and are subject to being called for additional active duty under emergency circumstances. We cannot predict the full impact of these conditions on us in the future, particularly if emergency circumstances or an escalation in the political situation occurs. If many of our employees are called for active duty, our operations in Israel and our business may not be able to function at full capacity;
|
|
•
|
acts of terrorism and acts of war;
|
|
•
|
general geopolitical instability and the responses to it, such as the possibility of additional sanctions against Russia which continue to bring uncertainty to this region;
|
|
•
|
interruptions and limitations in telecommunication services;
|
|
•
|
product or material transportation delays or disruption, including as a result of customs clearance, increased levels of violence, acts of terrorism, acts of war or health epidemics restricting travel to and from our international locations or as a result of natural disasters, such as earthquakes or volcanic eruptions;
|
|
•
|
burdens of complying with a wide variety of local country and regional laws, including the risks associated with the Foreign Corrupt Practices Act and local anti-bribery compliance;
|
|
•
|
trade restrictions and changes in tariffs; and
|
|
•
|
potential adverse tax consequences.
|
|
•
|
local political and economic instability;
|
|
•
|
the engagement of activities by our employees, contractors, partners and agents, especially in countries with developing economies, that are prohibited by international and local trade and labor laws and other laws prohibiting corrupt payments to government officials, including the Foreign Corrupt Practices Act, the United Kingdom (“UK”) Bribery Act of 2010 and export control laws, in spite of our policies and procedures designed to ensure compliance with these laws;
|
|
•
|
fluctuations in currency exchange rates; and
|
|
•
|
increased expense of developing, testing and making localized versions of our products.
|
|
•
|
correctly identify customer needs and preferences and predict future needs and preferences;
|
|
•
|
include functionality and features that address customer requirements;
|
|
•
|
ensure compatibility of our computer operating systems and hardware configurations with those of our customers;
|
|
•
|
allocate our research and development funding to products with higher growth prospects;
|
|
•
|
anticipate and respond to our competitors’ development of new products and technological innovations;
|
|
•
|
differentiate our offerings from our competitors’ offerings;
|
|
•
|
innovate and develop new technologies and applications;
|
|
•
|
the availability of third-party reimbursement of procedures using our products;
|
|
•
|
obtain adequate intellectual property rights; and
|
|
•
|
encourage customers to adopt new technologies.
|
|
•
|
limited visibility into and difficulty predicting from quarter to quarter, the level of activity in our customers’ practices including limited visibility into the number of aligners purchased by SmileDirectClub, LLC (“SDC”) under the supply agreement;
|
|
•
|
weakness in consumer spending as a result of a slowdown in the global, U.S. or other economies;
|
|
•
|
changes in relationships with our distributors;
|
|
•
|
changes in the timing of receipt of Invisalign case product orders during a given quarter which, given our cycle time and the delay between case receipts and case shipments, could have an impact on which quarter revenue can be recognized;
|
|
•
|
fluctuations in currency exchange rates against the U.S. dollar;
|
|
•
|
changes in product mix;
|
|
•
|
our inability to scale production of our iTero Element scanner to meet customer demand;
|
|
•
|
if participation in our customer rebate or discount programs increases, our average selling price will be adversely affected;
|
|
•
|
seasonal fluctuations in the number of doctors in their offices and their availability to take appointments;
|
|
•
|
success of or changes to our marketing programs from quarter to quarter;
|
|
•
|
our reliance on our contract manufacturers for the production of sub-assemblies for our intraoral scanners;
|
|
•
|
timing of industry tradeshows;
|
|
•
|
changes in the timing of when revenue is recognized, including as a result of the introduction of new products or promotions, modifications to our terms and conditions or as a result of changes to critical accounting estimates or new accounting pronouncements;
|
|
•
|
changes to our effective tax rate;
|
|
•
|
unanticipated delays in production caused by insufficient capacity or availability of raw materials;
|
|
•
|
any disruptions in the manufacturing process, including unexpected turnover in the labor force or the introduction of new production processes, power outages or natural or other disasters beyond our control;
|
|
•
|
the development and marketing of directly competitive products by existing and new competitors;
|
|
•
|
disruptions to our business as a result of our agreement to manufacture clear aligners for SDC, including market acceptance of the SDC business model and product, possible adverse customer reaction and negative publicity about us and our products;
|
|
•
|
impairments in the value of our investments in SDC and other privately held companies could be material;
|
|
•
|
major changes in available technology or the preferences of customers may cause our current product offerings to become less competitive or obsolete;
|
|
•
|
aggressive price competition from competitors;
|
|
•
|
costs and expenditures in connection with litigation;
|
|
•
|
the timing of new product introductions by us and our competitors, as well as customer order deferrals in anticipation of enhancements or new products;
|
|
•
|
unanticipated delays in our receipt of patient records made through an intraoral scanner for any reason;
|
|
•
|
disruptions to our business due to political, economic or other social instability, including the impact of an epidemic any of which results in changes in consumer spending habits, consumers unable or unwilling to visit the orthodontist or general practitioners office, as well as any impact on workforce absenteeism;
|
|
•
|
inaccurate forecasting of net revenues, production and other operating costs,
|
|
•
|
investments in research and development to develop new products and enhancements;
|
|
•
|
changes in accounting standards, policies and estimates including changes made by our equity investee; and
|
|
•
|
our ability to successfully hedge against a portion of our foreign currency-denominated assets and liabilities.
|
|
•
|
agreements with distributors may terminate prematurely due to disagreements or may result in litigation between the partners;
|
|
•
|
we may not be able to renew existing distributor agreements on acceptable terms;
|
|
•
|
our distributors may not devote sufficient resources to the sale of products;
|
|
•
|
our distributors may be unsuccessful in marketing our products;
|
|
•
|
our existing relationships with distributors may preclude us from entering into additional future arrangements with other distributors; and
|
|
•
|
we may not be able to negotiate future distributor agreements on acceptable terms.
|
|
•
|
product design, development, manufacturing and testing;
|
|
•
|
product labeling;
|
|
•
|
product storage;
|
|
•
|
pre-market clearance or approval;
|
|
•
|
complaint handling and corrective actions;
|
|
•
|
advertising and promotion; and
|
|
•
|
product sales and distribution.
|
|
•
|
warning letters, fines, injunctions, consent decrees and civil penalties;
|
|
•
|
repair, replacement, refunds, recall or seizure of our products;
|
|
•
|
operating restrictions or partial suspension or total shutdown of production;
|
|
•
|
refusing our requests for 510(k) clearance or pre-market approval of new products, new intended uses, or modifications to existing products;
|
|
•
|
withdrawing clearance or pre-market approvals that have already been granted; and
|
|
•
|
criminal prosecution.
|
|
•
|
storage, transmission and disclosure of medical information and healthcare records;
|
|
•
|
prohibitions against the offer, payment or receipt of remuneration to induce referrals to entities providing healthcare services or goods or to induce the order, purchase or recommendation of our products; and
|
|
•
|
the marketing and advertising of our products.
|
|
•
|
quarterly variations in our results of operations and liquidity;
|
|
•
|
changes in recommendations by the investment community or in their estimates of our net revenues or operating results;
|
|
•
|
speculation in the press or investment community concerning our business and results of operations;
|
|
•
|
strategic actions by our competitors, such as product announcements or acquisitions;
|
|
•
|
announcements of technological innovations or new products by us, our customers or competitors; and
|
|
•
|
general economic market conditions.
|
|
Period
|
Total Number of Shares Repurchased
|
Average Price Paid per Share
|
Total Number of Shares Repurchased as Part of Publicly Announced Program
|
Approximate Dollar Value of Shares that May Yet Be Repurchased Under the Programs
1
|
||||||||||
|
April 1, 2018 through April 30, 2018
|
—
|
|
$
|
—
|
|
—
|
|
$
|
100,000,000
|
|
||||
|
May 1, 2018 through May 31, 2018
|
—
|
|
$
|
—
|
|
—
|
|
$
|
700,000,000
|
|
||||
|
June 1, 2018 through June 30, 2018
|
—
|
|
$
|
—
|
|
—
|
|
$
|
700,000,000
|
|
||||
|
◦
|
April 2016 Repurchase Program.
In February 2018, we repurchased $100.0 million of our common stock on the open market. In July 2018, we repurchased $100.0 million of our common stock on the open market, completing the April 2016 Repurchase Program.
|
|
◦
|
May 2018 Repurchase Program.
In May 2018, we announced that our Board of Directors had authorized a plan to repurchase up to $600.0 million of our common stock. As of
June 30, 2018
, we have $600.0 million remaining under the May 2018 Repurchase Program (
Refer to Note 11 “Common Stock Repurchase Programs” of the Notes to Condensed Consolidated Financial Statements
for details on our stock repurchase programs).
|
|
ITEM 6.
|
EXHIBITS
|
|
Exhibit
Number
|
Description
|
Filing
|
Date
|
Exhibit
Number
|
Filed here with
|
|||||
|
10.1
|
Form 8-K
|
6/25/2018
|
10.1
|
|||||||
|
31.1
|
*
|
|||||||||
|
31.2
|
*
|
|||||||||
|
32.1
|
*
|
|||||||||
|
101.INS
|
XBRL Instance Document
|
*
|
||||||||
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
*
|
||||||||
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
*
|
||||||||
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
*
|
||||||||
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document
|
*
|
||||||||
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
*
|
||||||||
|
|
ALIGN TECHNOLOGY, INC.
|
|
|
August 2, 2018
|
By:
|
/s/ JOSEPH M. HOGAN
|
|
Joseph M. Hogan
President and Chief Executive Officer
|
||
|
By:
|
/s/ JOHN F. MORICI
|
|
|
John F. Morici
Chief Financial Officer and Senior Vice President, Global Finance
|
||