|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ý
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
95-3540776
|
|
|
(State or other jurisdiction of
incorporation or organization)
|
(I.R.S. Employer
Identification No.)
|
|
|
One Amgen Center Drive,
|
91320-1799
|
|
|
Thousand Oaks, California
|
(Zip Code)
|
|
|
(Address of principal executive offices)
|
||
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|
|
Common stock, $0.0001 par value
|
The NASDAQ Global Select Market
|
|
|
Large accelerated filer
x
|
Accelerated filer
¨
|
Non-accelerated filer
¨
|
Smaller reporting company
¨
|
|
(Do not check if a smaller reporting company)
|
|||
|
(A)
|
Excludes 805,131 shares of common stock held by directors and executive officers at June 30, 2014. Exclusion of shares held by any person should not be construed to indicate that such person possesses the power, directly or indirectly, to direct or cause the direction of the management or policies of the registrant, or that such person is controlled by or under common control with the registrant.
|
|
Page No.
|
||
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
Item 15.
|
||
|
Item 1.
|
BUSINESS
|
|
•
|
In August 2014, we announced that the phase 3 YUKAWA-2 (StudY of LDL-Cholesterol Reduction Using a Monoclonal PCSK9 Antibody in Japanese Patients With Advanced Cardiovascular Risk) study evaluating evolocumab in combination with statin therapy in Japanese patients with high cardiovascular risk and high cholesterol met its co-primary endpoints.
|
|
•
|
In September 2014, we announced that we submitted a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) for the treatment of high cholesterol.
|
|
•
|
In November 2014, we announced that the U.S. Food and Drug Administration (FDA) accepted for review our Biologics License Application (BLA) for evolocumab for the treatment of high cholesterol.
|
|
•
|
In August 2014, we announced that the FDA granted priority review designation for the treatment of chronic heart failure.
|
|
•
|
In January 2015, we announced a three-month extension of the Prescription Drug User Fee Act (PDUFA) target action date due to a request from the FDA for submission of additional existing clinical data, which has been submitted.
|
|
•
|
In April 2014, we announced the initiation of two phase 3 studies in patients with psoriatic arthritis.
|
|
•
|
In 2014, we and AstraZeneca Plc. (AstraZeneca) announced that all three phase 3 AMAGINE
™
trials evaluating brodalumab in patients with moderate-to-severe plaque psoriasis met all their primary endpoints.
|
|
•
|
In July 2014, we announced that a phase 3 study evaluating AMG 416 for the treatment of secondary hyperparathyroidism in patients with chronic kidney disease (CKD) receiving hemodialysis, met its primary and all secondary endpoints.
|
|
•
|
In August 2014, we announced that a second placebo-controlled phase 3 study evaluating AMG 416 for the treatment of secondary hyperparathyroidism in patients with CKD, receiving hemodialysis, met its primary and all secondary endpoints.
|
|
•
|
In October 2014, we announced that we submitted an MAA to the EMA for the treatment of adults with Philadelphia-negative relapsed/refractory B-precursor acute lymphoblastic leukemia (ALL), a rapidly progressing cancer of the blood and bone marrow.
|
|
•
|
In December 2014, we announced that the FDA has granted approval of BLINCYTO
™
for the treatment of patients with Philadelphia chromosome-negative (Ph-) relapsed or refractory B-cell precursor ALL. This indication is approved under accelerated approval and continued approval for this indication may be contingent upon verification of clinical benefit in subsequent trials. Commercial sales launched in December 2014.
|
|
•
|
In August 2014, we and our subsidiary Onyx Pharmaceuticals, Inc. (Onyx) announced that a planned interim analysis demonstrated that the phase 3 clinical trial ASPIRE (CArfilzomib, Lenalidomide, and DexamethaSone versus Lenalidomide and Dexamethasone for the treatment of PatIents with Relapsed Multiple MyEloma) met its primary endpoint of progression-free survival (PFS). While the data for overall survival, a secondary endpoint, are not yet mature, the analysis showed a trend in favor of
Kyprolis
®
in combination with REVLIMID
®
(lenalidomide) and low-dose dexamethasone
that did not reach statistical significance.
|
|
•
|
In August 2014, we and Onyx announced that the phase 3 clinical trial FOCUS (CarFilzOmib for AdvanCed Refractory MUltiple Myeloma European Study) did not meet its primary endpoint of improving overall survival.
|
|
•
|
In January 2015, we and Onyx announced the submission of a supplemental New Drug Application (sNDA) to the FDA and an MAA to the EMA for Kyprolis
®
to seek approval for the treatment of patients with relapsed multiple myeloma who have received at least one prior therapy. In the United States, the sNDA is designed to support the conversion of accelerated approval to full approval and expand the current approved indication. In the European Union (EU), Kyprolis
®
received orphan drug designation and the MAA has been granted accelerated assessment.
|
|
•
|
In December 2014, the FDA granted approval of the Neulasta
®
Delivery Kit, including the On-body Injector for Neulasta
®
.
|
|
•
|
In November 2014, we announced the termination of all Amgen-sponsored clinical studies of rilotumumab in advanced gastric cancer, including the phase 3 RILOMET-1 and RILOMET-2 studies.
|
|
•
|
In July 2014, we announced that we submitted a BLA in the United States for regionally and distantly metastatic melanoma.
|
|
•
|
In September 2014, we announced that we submitted an MAA to the EMA for the treatment of adults with regionally and distantly metastatic melanoma.
|
|
•
|
In January 2015, we announced a three-month extension of the PDUFA target action date for our BLA due to a request from the FDA for submission of additional existing manufacturing data, which has been submitted.
|
|
•
|
In November 2014, we announced the top-line secondary endpoint results of overall survival from the phase 3 TRINOVA-1 trial in women with recurrent platinum-resistant ovarian cancer. The study, which evaluated trebananib plus paclitaxel versus placebo plus paclitaxel, did not demonstrate a statistically significant improvement in overall survival. We have terminated the clinical development program in recurrent ovarian cancer.
|
|
•
|
In October 2014, we announced that the phase 3 study evaluating efficacy and safety of biosimilar candidate ABP 501 compared with Humira
®
(adalimumab) in patients with moderate-to-severe plaque psoriasis met its primary endpoint.
|
|
•
|
In February 2015, we announced that the phase 3 study evaluating efficacy and safety of biosimilar candidate ABP 501 compared with Humira
®
in patients with moderate-to-severe rheumatoid arthritis (RA) met its primary and key secondary endpoints.
|
|
•
|
In 2014, we completed facilities construction and entered the licensure process for a Next-Generation Biomanufacturing facility in Singapore. We believe, when licensed, this facility will enable us to increase our manufacturing productivity versus conventional alternatives at lower capital costs and operating expense.
|
|
•
|
During the second half of 2014, we announced a restructuring plan which will reduce staff by between 3,500 and 4,000 positions by the end of 2015. In addition, we will close our facilities in the states of Washington and Colorado, and will reduce the number of buildings at our headquarters in Thousand Oaks, California. The total pre-tax restructuring charges are expected to range between approximately $935 million and $1,035 million. As of December 31, 2014, $558 million of these charges have been incurred.
|
|
•
|
moderately to severely active RA,
|
|
•
|
chronic moderate-to-severe plaque psoriasis patients who are candidates for systemic therapy or phototherapy, and
|
|
•
|
active psoriatic arthritis.
|
|
Product
|
Territory
|
General Subject Matter
|
Expiration
|
|||
|
Neulasta
®
(pegfilgrastim)
|
U.S.
|
Pegylated G-CSF
|
10/20/2015
|
|||
|
Europe
|
Pegylated G-CSF
(1)
|
2/8/2015
|
||||
|
Enbrel
®
(etanercept)
|
U.S.
|
Methods of treating psoriasis
|
8/13/2019
|
|||
|
U.S.
|
Aqueous formulation and methods of treatment using the formulation
(2)
|
6/8/2023
|
||||
|
U.S.
|
Fusion protein, and pharmaceutical compositions
|
11/22/2028
|
||||
|
U.S.
|
DNA encoding fusion protein, and methods of making fusion protein
|
4/24/2029
|
||||
|
Prolia
®
/
XGEVA ® (denosumab) |
U.S.
|
RANKL antibodies; and methods of use
(3)
|
12/22/2017
|
|||
|
U.S.
|
Methods of treatment
|
6/25/2022
|
||||
|
U.S.
|
Nucleic acids encoding RANKL antibodies, and methods of producing RANKL antibodies
|
11/30/2023
|
||||
|
U.S.
|
RANKL antibodies including sequences
|
2/19/2025
|
||||
|
Europe
|
RANKL antibodies
(1)
|
12/22/2017
|
||||
|
Europe
|
Medical use of RANKL antibodies
(1)
|
4/15/2018
|
||||
|
Europe
|
RANKL antibodies including epitope binding
|
2/23/2021
|
||||
|
Europe
|
RANKL antibodies including sequences
(1)
|
6/25/2022
|
||||
|
EPOGEN
®
(epoetin alfa)
|
U.S.
|
Cells that make certain levels of erythropoietin
|
5/26/2015
|
|||
|
Aranesp
®
(darbepoetin alfa)
|
U.S.
|
Glycosylation analogs of erythropoietin proteins
|
5/15/2024
|
|||
|
Europe
|
Glycosylation analogs of erythropoietin proteins
(1)
|
8/16/2014
|
||||
|
Sensipar
®
/
Mimpara ® (cinacalcet) |
U.S.
|
Calcium receptor-active molecules including species
|
10/23/2015
|
|||
|
U.S.
|
Methods of treatment
|
12/14/2016
|
||||
|
U.S.
|
Calcium receptor-active molecules
|
3/8/2018
|
||||
|
Europe
|
Calcium receptor-active molecules
(1)
|
10/23/2015
|
||||
|
Vectibix
®
(panitumumab)
|
U.S.
|
Human monoclonal antibodies to epidermal growth factor receptor (EGFr)
|
4/8/2020
|
|||
|
Europe
|
Human monoclonal antibodies to EGFr
(1)
|
5/5/2018
|
||||
|
Nplate
®
(romiplostim)
|
U.S.
|
Thrombopoietic compounds
|
1/19/2022
|
|||
|
Europe
|
Thrombopoietic compounds
(1)
|
10/22/2019
|
||||
|
Kyprolis
®
(carfilzomib)
|
U.S.
|
Compositions, and methods of treatment
(4)
|
4/14/2025
|
|||
|
Europe
|
Compositions
|
8/8/2025
|
||||
|
BLINCYTO
™
(blinatumomab)
|
U.S.
|
Bifunctional polypeptides
(4)
|
4/21/2019
|
|||
|
U.S.
|
Method of administration
|
9/28/2027
|
||||
|
Europe
|
Bifunctional polypeptides
|
4/21/2019
|
||||
|
Europe
|
Method of administration
|
11/29/2026
|
||||
|
(1)
|
A European patent with this subject matter may also be entitled to supplemental protection in one or more countries in Europe and the length of any such extension will vary by country. For example, supplementary protection certificates have been issued related to the indicated products for patents in at least the following countries:
|
|
•
|
pegfilgrastim - France, Germany, Italy, Spain, and the United Kingdom, expiring in 2017
|
|
•
|
darbepoetin alfa - France, Germany, Italy, Spain, and the United Kingdom, expiring in 2016
|
|
•
|
denosumab - France, Italy and Spain
, expiring in
2025
|
|
•
|
cinacalcet - France, Germany, Italy, Spain, and the United Kingdom, expiring in 2019
|
|
•
|
panitumumab - France, Germany, Italy, Spain, and the United Kingdom, expiring in 2022
|
|
•
|
romiplostim - France, Italy, Spain, and the United Kingdom, expiring in 2024
|
|
(2)
|
This formulation patent relates to the currently approved liquid formulation of ENBREL, which formulation accounts for the majority of ENBREL sales in the United States. However, ENBREL is also sold as an alternative lyophilized formulation that requires reconstituting before it can be administered to the patient.
|
|
(3)
|
The U.S. Patent and Trademark Office has issued a Notice of Final Determination that a patent with this subject matter is eligible for patent term extension with an expiry of September 17, 2021.
|
|
(4)
|
A patent with this subject matter may be entitled to patent term extension in the United States.
|
|
Product
|
Territory
|
Competitor Marketed Product
|
Competitors
|
|||
|
Neulasta
®
/
NEUPOGEN
®
|
U.S.
|
Granix
®(1)
|
Teva Pharmaceutical Industries Ltd. (Teva)
|
|||
|
Europe
|
Lonquex
®(2)
|
Teva
|
||||
|
Europe
|
Filgrastim biosimilars
(3)
|
Various
|
||||
|
ENBREL
|
U.S. & Canada
|
REMICADE
®
|
Janssen Biotech, Inc. (Janssen)
(8)
/Merck & Company, Inc.
|
|||
|
U.S. & Canada
|
HUMIRA
®
|
AbbVie Inc.
|
||||
|
U.S. & Canada
|
STELARA
®(4)
|
Janssen
(8)
|
||||
|
XGEVA
®
|
U.S. & Europe
|
Zometa
®
|
Novartis AG (Novartis)
|
|||
|
U.S. & Europe
|
Zoledronate generics
|
Various
|
||||
|
Prolia
®
|
U.S. & Europe
|
Alendronate generics
|
Various
|
|||
|
U.S. & Europe
|
Raloxifene generics
|
Various
|
||||
|
U.S. & Europe
|
Zoledronate generics
|
Various
|
||||
|
EPOGEN
®
|
U.S.
|
MIRCERA
®(5)
|
F. Hoffmann-La Roche Ltd. (Roche)
|
|||
|
Aranesp
®
|
U.S.
|
PROCRIT
®(6)
|
Janssen
(8)
|
|||
|
Europe
|
EPREX
®
/ERYPO
®
|
Janssen-Cilag
(8)
|
||||
|
Europe
|
Epoetin alfa biosimilars
(3)
|
Various
|
||||
|
Europe
|
MIRCERA
®(5)
|
Roche
|
||||
|
Product
|
Territory
|
Competitor Marketed Product
|
Competitors
|
|||
|
Sensipar
®(7)
/
Mimpara
®
|
U.S. & Europe
|
Active Vitamin D analogs
|
Various
|
|||
|
Vectibix
®
|
U.S. & Europe
|
Erbitux
®
|
Eli Lilly/Bristol-Myers Squibb Company (BMS); Merck KGaA
|
|||
|
U.S. & Europe
|
Avastin
®
|
Genentech, Inc. (a Member of the Roche Group)
|
||||
|
Nplate
®
|
U.S. & Europe
|
Promacta
®
/Revolade
®
|
GlaxoSmithKline plc
|
|||
|
Kyprolis
®
|
U.S.
|
VELCADE
®
|
Millennium Pharmaceuticals, Inc.
(9)
|
|||
|
U.S.
|
REVLIMID
®
|
Celgene Corporation
|
||||
|
U.S.
|
POMALYST
®
|
Celgene Corporation
|
||||
|
(1)
|
Granix
®
launched at the end of 2013 and could have an impact over time on sales of NEUPOGEN
®
and, to a lesser extent, Neulasta
®
.
|
|
(2)
|
Lonquex
®
is a long-acting filgrastim product launched in Europe.
|
|
(3)
|
Approved via the EU biosimilar regulatory pathway.
|
|
(4)
|
Dermatology only.
|
|
(5)
|
MIRCERA
®
has been approved by the FDA for the treatment of anemia associated with chronic renal failure in patients on and not on dialysis. Roche began selling MIRCERA
®
in October 2014 in the United States under terms of a limited patent license obtained from Amgen in connection with the settlement of patent litigation. It competes with Aranesp
®
in the nephrology segment only.
|
|
(6)
|
PROCRIT
®
competes with Aranesp
®
in the supportive cancer care and pre-dialysis settings.
|
|
(7)
|
Teva and Barr Pharmaceuticals have received tentative approval from the FDA for generic versions of Sensipar
®
that could compete with Sensipar
®
in the future. There is an injunction prohibiting them from commercializing in the United States until expiration of the patents.
|
|
(8)
|
A subsidiary of Johnson & Johnson (J&J).
|
|
(9)
|
A wholly-owned subsidiary of Takeda Pharmaceutical Company Limited.
|
|
•
|
to manufacture Sensipar
®
/Mimpara
®
, except for certain fill and finish activities performed by us in Puerto Rico;
|
|
•
|
to supplement commercial bulk manufacturing, as needed, for ENBREL, Prolia
®
, XGEVA
®
and Vectibix
®
;
|
|
•
|
to supplement certain portions of fill and finish for ENBREL; and
|
|
•
|
to supplement formulation, fill and finish of Nplate
®
.
|
|
•
|
In phase 1, we conduct small clinical trials to investigate the safety and proper dose ranges of our product candidates in a small number of human subjects.
|
|
•
|
In phase 2, we conduct clinical trials to investigate side effect profiles and the efficacy of our product candidates in a larger number of patients who have the disease or condition under study.
|
|
•
|
In phase 3, we conduct clinical trials to investigate the safety and efficacy of our product candidates in a large number of patients who have the disease or condition under study.
|
|
Molecule
|
Disease/Condition
|
|
|
Phase 3 Programs
|
||
|
AMG 416
|
Secondary hyperparathyroidism in patients with CKD receiving dialysis
|
|
|
Aranesp
®
|
Myelodysplastic syndromes
|
|
|
BLINCYTO
™
|
ALL
|
|
|
Brodalumab
|
Psoriasis;
Psoriatic arthritis
|
|
|
Evolocumab
|
Dyslipidemia
|
|
|
Kyprolis
®
*
|
Multiple myeloma
|
|
|
Prolia
®
|
Glucocorticoid-induced osteoporosis
|
|
|
Romosozumab
|
Postmenopausal osteoporosis
Male osteoporosis
|
|
|
Talimogene laherparepvec
|
Metastatic melanoma
|
|
|
Trebananib
|
First-line ovarian cancer
|
|
|
Vectibix
®
|
Metastatic colorectal cancer (mCRC) (US only)
|
|
|
XGEVA
®
|
Delay or prevention of bone metastases in breast cancer;
Cancer-related bone damage in patients with multiple myeloma
|
|
|
Phase 2 Programs
|
||
|
AMG 139
|
Inflammatory diseases
|
|
|
AMG 157
|
Asthma
|
|
|
AMG 181
|
Inflammatory bowel diseases
|
|
|
AMG 334
|
Migraine
|
|
|
AMG 337
|
Gastric cancer
|
|
|
BLINCYTO
™
|
Diffuse Large B-Cell Lymphoma (DLBCL)
|
|
|
Brodalumab
|
Inflammatory diseases
|
|
|
Kyprolis
®
*
|
Small-cell lung cancer
|
|
|
Omecamtiv mecarbil
|
Heart failure
|
|
|
Oprozomib*
|
Hematologic malignancies
|
|
|
XGEVA
®
|
Metastatic non-small cell lung cancer (NSCLC)
|
|
|
Phase 1 Programs
|
||
|
AMG 172
|
Renal cell carcinoma
|
|
|
AMG 208
|
Various cancer types
|
|
|
AMG 211
|
Various cancer types
|
|
|
AMG 232
|
Various cancer types
|
|
|
AMG 282
|
Asthma
|
|
|
AMG 319
|
Hematologic malignancies
|
|
|
AMG 357
|
Autoimmune diseases
|
|
|
AMG 557
|
Systemic lupus erythematosus
|
|
|
AMG 581
|
Schizophrenia
|
|
|
AMG 595
|
Glioblastoma
|
|
|
AMG 780
|
Various cancer types
|
|
|
AMG 811
|
Systemic lupus erythematosus
|
|
|
AMG 820
|
Various cancer types
|
|
|
AMG 876
|
Type 2 diabetes
|
|
|
AMG 900
|
Various cancer types
|
|
|
Oprozomib*
|
Solid tumors
|
|
|
*
|
Being developed by Onyx, an Amgen subsidiary.
|
|
Phase 3
|
clinical trials investigate the safety and efficacy of a product candidate in a large number of patients who have the disease or condition under study.
|
|
Phase 2
|
clinical trials investigate side effect profiles and efficacy of a product candidate in a large number of patients who have the disease or condition under study.
|
|
Phase 1
|
clinical trials investigate safety and proper dose ranges of a product candidate in a small number of human subjects.
|
|
Molecule
|
Disease / Condition
|
Program Change
|
||
|
Brodalumab
|
Psoriatic arthritis
|
Advanced to phase 3
|
||
|
Prolia
®
|
Male osteoporosis (EU only)
|
Approved by EMA
|
||
|
Rilotumumab
|
Gastric cancer
|
Terminated
|
||
|
Romosozumab
|
Male osteoporosis
|
Advanced to phase 3
|
||
|
Sensipar
®
/ Mimpara
®
|
Post renal transplant
|
Concluded - No longer pursuing indication
|
||
|
Molecule
|
Territory
|
General Subject Matter
|
Estimated Expiration*
|
|||
|
Brodalumab
|
U.S.
|
Polynucleotides and polypeptides
|
2027
|
|||
|
Europe
|
Polynucleotides and polypeptides
|
2027
|
||||
|
Evolocumab
|
U.S.
|
Polypeptides
|
2029
|
|||
|
Romosozumab
|
U.S.
|
Polypeptides
|
2026
|
|||
|
Europe
|
Polypeptides
|
2026
|
||||
|
Talimogene laherparepvec
|
U.S.
|
Modified HSV-1 compounds and strains
|
2021
|
|||
|
Europe
|
Modified HSV-1 compounds and strains
|
2021
|
||||
|
Trebananib
|
U.S.
|
Polynucleotides and polypeptides
|
2025
|
|||
|
Europe
|
Polynucleotides and polypeptides
|
2022
|
||||
|
AMG 416
|
U.S.
|
Compound
|
2030
|
|||
|
*
|
Patent expiration estimates are based on issued patents, which may be challenged, invalidated, or circumvented by competitors. The patent expiration estimates do not include any term adjustments, extensions or supplemental protection certificates that may be obtained in the future and extend these dates. Corresponding patent applications are pending in other jurisdictions. Additional patents may be filed or issued and may provide additional exclusivity for the product candidate or its use.
|
|
Biosimilar
|
Status
|
|
|
adalimumab (HUMIRA
®
)
|
Phase 3 psoriasis study met primary endpoint
Phase 3 RA study met primary and key secondary endpoints
|
|
|
trastuzumab (Herceptin
®
)
|
Phase 3 breast cancer
|
|
|
bevacizumab (Avastin
®
)
|
Phase 3 NSCLC
|
|
|
infliximab (REMICADE
®
)
|
Phase 1
|
|
|
Item 1A.
|
RISK FACTORS
|
|
•
|
requirement of risk management activities or other regulatory agency compliance actions related to the promotion and sale of our products;
|
|
•
|
revocation of approval for our products from the market completely, or within particular therapeutic areas or patient types;
|
|
•
|
the product candidate did not demonstrate acceptable clinical trial results even though it demonstrated positive preclinical trial results, for reasons that could include changes in the standard of care of medicine;
|
|
•
|
the product candidate was not effective or more effective than currently available therapies in treating a specified condition or illness;
|
|
•
|
the biosimilar product candidate fails to demonstrate the requisite biosimilarity to the applicable reference product, or is otherwise determined to be unacceptable for purposes of safety or efficacy, to gain approval;
|
|
•
|
other parties have or may have proprietary rights relating to our product candidate, such as patent rights, and will not let us sell it on reasonable terms, or at all;
|
|
•
|
we and certain of our licensees, partners or independent investigators may fail to effectively conduct clinical development or clinical manufacturing activities; and
|
|
•
|
render the product candidate commercially unfeasible or limit our ability to market existing products completely or in certain therapeutic areas.
|
|
•
|
failure to comply with our quality standards which results in quality and product failures, product contamination and/or recall;
|
|
•
|
a material shortage, contamination, recall and/or restrictions on the use of certain biologically derived substances or other raw materials;
|
|
Item 1B.
|
UNRESOLVED STAFF COMMENTS
|
|
Item 2.
|
PROPERTIES
|
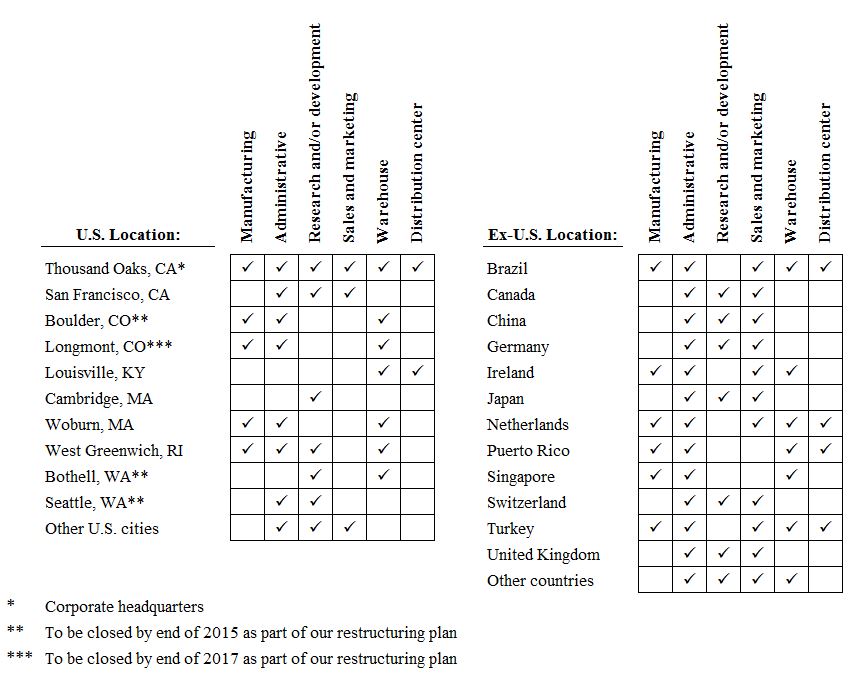
|
Item 3.
|
LEGAL PROCEEDINGS
|
|
Item 4.
|
MINE SAFETY DISCLOSURES
|
|
Item 5.
|
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
Year ended December 31, 2014
|
High
|
Low
|
||||||
|
Fourth quarter
|
$
|
171.64
|
|
$
|
130.45
|
|
||
|
Third quarter
|
144.01
|
|
115.39
|
|
||||
|
Second quarter
|
126.07
|
|
110.29
|
|
||||
|
First quarter
|
127.47
|
|
113.48
|
|
||||
|
Year ended December 31, 2013
|
||||||||
|
Fourth quarter
|
$
|
118.69
|
|
$
|
106.28
|
|
||
|
Third quarter
|
117.52
|
|
95.81
|
|
||||
|
Second quarter
|
113.42
|
|
94.60
|
|
||||
|
First quarter
|
102.51
|
|
82.08
|
|
||||
|
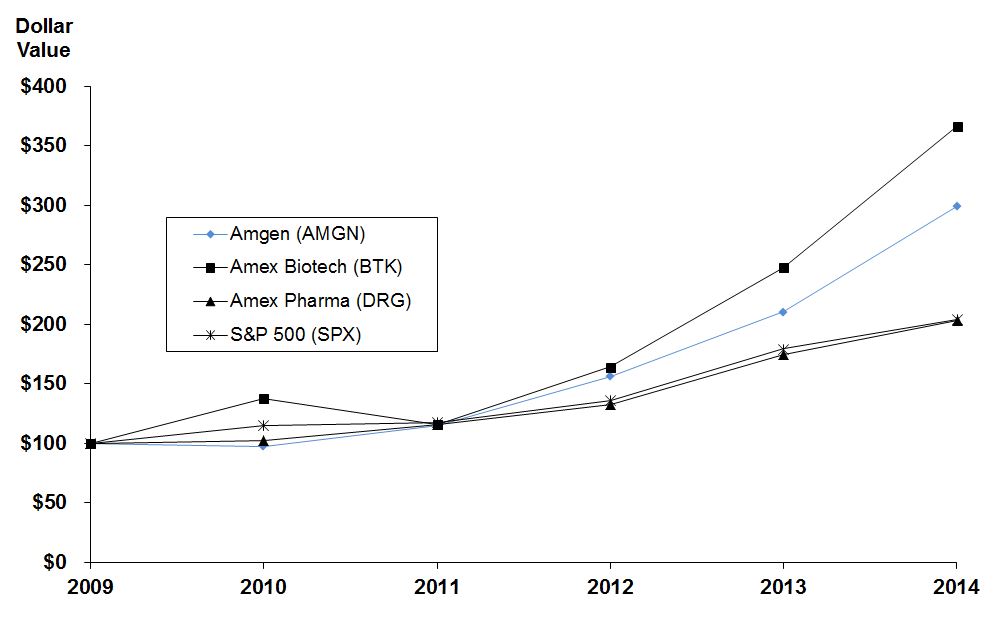
Amgen vs. Amex Biotech, Amex Pharmaceutical and S&P 500 Indices
|
|
Comparison of Five-Year Cumulative Total Return
|
|
Value of Investment of $100 on December 31, 2009
|
|
12/31/2009
|
12/31/2010
|
12/31/2011
|
12/31/2012
|
12/31/2013
|
12/31/2014
|
||||||||||||
|
Amgen (AMGN)
|
100.00
|
|
97.05
|
|
114.74
|
|
156.35
|
|
210.32
|
|
299.33
|
|
|||||
|
Amex Biotech (BTK)
|
100.00
|
|
137.73
|
|
115.91
|
|
164.13
|
|
247.47
|
|
366.04
|
|
|||||
|
Amex Pharmaceutical (DRG)
|
100.00
|
|
102.51
|
|
115.75
|
|
133.00
|
|
174.52
|
|
203.45
|
|
|||||
|
S&P 500 (SPX)
|
100.00
|
|
114.82
|
|
117.23
|
|
135.75
|
|
179.25
|
|
203.77
|
|
|||||
|
Total
number of
shares
purchased
|
Average
price paid
per share
(1)
|
Total number
of shares
purchased as
publicly
announced
program
|
Maximum dollar
value that may
yet be purchased
under the
program
(2)
|
|||||||||||
|
January 1 - September 30
|
—
|
|
$
|
—
|
|
—
|
|
$
|
1,559,838,541
|
|
||||
|
October 1 - October 31
|
—
|
|
—
|
|
—
|
|
4,000,000,000
|
|
||||||
|
November 1 - November 30
|
223,000
|
|
162.67
|
|
223,000
|
|
3,963,725,678
|
|
||||||
|
December 1 - December 31
|
714,209
|
|
163.77
|
|
714,209
|
|
3,846,756,797
|
|
||||||
|
January 1 - December 31
|
937,209
|
|
$
|
163.51
|
|
937,209
|
|
|||||||
|
(1)
|
Average price paid per share includes related expenses.
|
|
(2)
|
In October 2014, our Board of Directors authorized an increase that resulted in a total of $4.0 billion available under the stock repurchase program.
|
|
Item 6.
|
SELECTED FINANCIAL DATA
|
|
|
Years ended December 31,
|
||||||||||||||||||
|
Consolidated Statement of Income Data:
|
2014
|
2013
|
2012
|
2011
|
2010
|
||||||||||||||
|
|
(In millions, except per share data)
|
||||||||||||||||||
|
Revenues:
|
|||||||||||||||||||
|
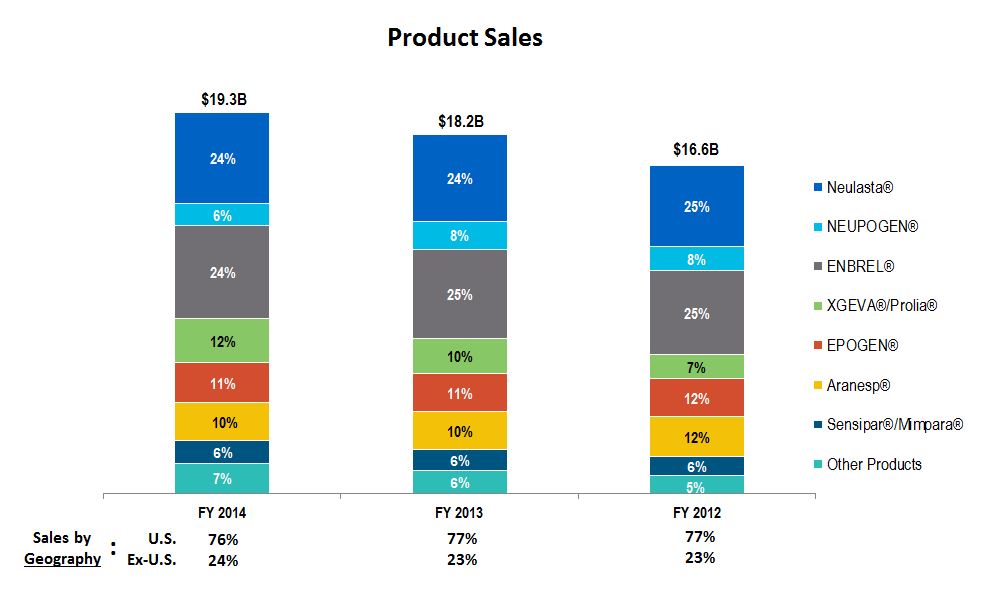
Product sales
|
$
|
19,327
|
|
$
|
18,192
|
|
$
|
16,639
|
|
$
|
15,295
|
|
$
|
14,660
|
|
||||
|
Other revenues
|
736
|
|
484
|
|
626
|
|
287
|
|
393
|
|
|||||||||
|
Total revenues
|
20,063
|
|
18,676
|
|
17,265
|
|
15,582
|
|
15,053
|
|
|||||||||
|
Operating expenses:
|
|||||||||||||||||||
|
Cost of sales
|
4,422
|
|
3,346
|
|
3,199
|
|
2,708
|
|
2,501
|
|
|||||||||
|
Research and development
|
4,297
|
|
4,083
|
|
3,380
|
|
3,167
|
|
2,894
|
|
|||||||||
|
Selling, general and administrative
|
4,699
|
|
5,184
|
|
4,814
|
|
4,499
|
|
3,996
|
|
|||||||||
|
Other
(1)
|
454
|
|
196
|
|
295
|
|
896
|
|
117
|
|
|||||||||
|
Net income
|
5,158
|
|
5,081
|
|
4,345
|
|
3,683
|
|
4,627
|
|
|||||||||
|
Diluted earnings per share
|
6.70
|
|
6.64
|
|
5.52
|
|
4.04
|
|
4.79
|
|
|||||||||
|
Dividends paid per share
|
2.44
|
|
1.88
|
|
1.44
|
|
0.56
|
|
—
|
|
|||||||||
|
|
As of December 31,
|
||||||||||||||||||
|
Consolidated Balance Sheet Data:
|
2014
|
2013
|
2012
|
2011
|
2010
|
||||||||||||||
|
|
(In millions)
|
||||||||||||||||||
|
Total assets
|
$
|
69,009
|
|
$
|
66,125
|
|
$
|
54,298
|
|
$
|
48,871
|
|
$
|
43,486
|
|
||||
|
Total debt
(2)
|
30,715
|
|
32,128
|
|
26,529
|
|
21,428
|
|
13,362
|
|
|||||||||
|
Total stockholders’ equity
(3)
|
25,778
|
|
22,096
|
|
19,060
|
|
19,029
|
|
23,944
|
|
|||||||||
|
(1)
|
In 2011, we recorded a $780 million legal settlement charge ($705 million, net of tax) in connection with an agreement in principle to settle allegations related to our sales and marketing practices.
|
|
(2)
|
See Part IV—Note 14, Financing arrangements, to the Consolidated Financial Statements for discussion of our financing arrangements. In addition, in 2011 and 2010, we issued $10.5 billion and $2.5 billion, respectively, aggregate principal amount of notes. In 2011, we repaid our 0.125% Convertible Notes of $2.5 billion. No debt was due or repaid in 2010.
|
|
(3)
|
Throughout the five years ended December 31, 2014, we had a stock repurchase program authorized by the Board of Directors through which we repurchased
$0.2 billion
,
$0.8 billion
,
$4.7 billion
, $8.3 billion and $3.8 billion, respectively, of Amgen common stock.
|
|
Item 7.
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
|
Clinical Program
|
Lead Indication
|
Milestone
|
||
|
Repatha
™
|
Dyslipidemia
|
US submission
|
||
|
EU submission
|
||||
|
Corlanor
®
|
Chronic heart failure
|
US submission
|
||
|
Kyprolis
®*
|
Multiple myeloma
|
Phase 3 ASPIRE data
|
||
|
Talimogene laherparepvec
|
Metastatic melanoma
|
US submission
|
||
|
EU submission
|
||||
|
BLINCYTO
™
|
Relapsed/refractory ALL
|
US approval
|
||
|
EU submission
|
||||
|
Brodalumab
**
|
Moderate-to-severe plaque psoriasis
|
Phase 3 data
|
||
|
AMG 416
|
Secondary hyperparathyroidism
|
Phase 3 data
|
||
|
AMG 334
|
Migraine prophylaxis
|
Phase 2b data (episodic)
|
||
|
Year ended December 31,
|
Year ended December 31,
|
|||||||||
|
2014
|
Change
|
2013
|
||||||||
|
Product sales:
|
||||||||||
|
U.S.
|
$
|
14,732
|
|
5
|
%
|
$
|
14,045
|
|
||
|
ROW
|
4,595
|
|
11
|
%
|
4,147
|
|
||||
|
Total product sales
|
19,327
|
|
6
|
%
|
18,192
|
|
||||
|
Other revenues
|
736
|
|
52
|
%
|
484
|
|
||||
|
Total revenues
|
$
|
20,063
|
|
7
|
%
|
$
|
18,676
|
|
||
|
Operating expenses
|
$
|
13,872
|
|
8
|
%
|
$
|
12,809
|
|
||
|
Operating income
|
$
|
6,191
|
|
6
|
%
|
$
|
5,867
|
|
||
|
Net income
|
$
|
5,158
|
|
2
|
%
|
$
|
5,081
|
|
||
|
Diluted EPS
|
$
|
6.70
|
|
1
|
%
|
$
|
6.64
|
|
||
|
Diluted shares
|
770
|
|
1
|
%
|
765
|
|
||||
|
Year ended December 31,
|
Year ended December 31,
|
Year ended December 31,
|
|||||||||||||||
|
2014
|
Change
|
2013
|
Change
|
2012
|
|||||||||||||
|
Neulasta
®
/NEUPOGEN
®
|
$
|
5,755
|
|
(1
|
)%
|
$
|
5,790
|
|
8
|
%
|
$
|
5,352
|
|
||||
|
ENBREL
|
4,688
|
|
3
|
%
|
4,551
|
|
7
|
%
|
4,236
|
|
|||||||
|
XGEVA
®
|
1,221
|
|
20
|
%
|
1,019
|
|
36
|
%
|
748
|
|
|||||||
|
Prolia
®
|
1,030
|
|
38
|
%
|
744
|
|
58
|
%
|
472
|
|
|||||||
|
EPOGEN
®
|
2,031
|
|
4
|
%
|
1,953
|
|
1
|
%
|
1,941
|
|
|||||||
|
Aranesp
®
|
1,930
|
|
1
|
%
|
1,911
|
|
(6
|
)%
|
2,040
|
|
|||||||
|
Sensipar
®
/Mimpara
®
|
1,158
|
|
6
|
%
|
1,089
|
|
15
|
%
|
950
|
|
|||||||
|
Other products
|
1,514
|
|
33
|
%
|
1,135
|
|
26
|
%
|
900
|
|
|||||||
|
Total product sales
|
$
|
19,327
|
|
6
|
%
|
$
|
18,192
|
|
9
|
%
|
$
|
16,639
|
|
||||
|
Total U.S.
|
$
|
14,732
|
|
5
|
%
|
$
|
14,045
|
|
10
|
%
|
$
|
12,815
|
|
||||
|
Total ROW
|
4,595
|
|
11
|
%
|
4,147
|
|
8
|
%
|
3,824
|
|
|||||||
|
Total product sales
|
$
|
19,327
|
|
6
|
%
|
$
|
18,192
|
|
9
|
%
|
$
|
16,639
|
|
||||
|
Year ended December 31,
|
Year ended December 31,
|
Year ended December 31,
|
|||||||||||||||
|
2014
|
Change
|
2013
|
Change
|
2012
|
|||||||||||||
|
Neulasta
®
— U.S.
|
$
|
3,649
|
|
4
|
%
|
$
|
3,499
|
|
9
|
%
|
$
|
3,207
|
|
||||
|
Neulasta
®
— ROW
|
947
|
|
6
|
%
|
893
|
|
1
|
%
|
885
|
|
|||||||
|
Total Neulasta
®
|
4,596
|
|
5
|
%
|
4,392
|
|
7
|
%
|
4,092
|
|
|||||||
|
NEUPOGEN
®
— U.S.
|
839
|
|
(28
|
)%
|
1,169
|
|
16
|
%
|
1,007
|
|
|||||||
|
NEUPOGEN
®
— ROW
|
320
|
|
40
|
%
|
229
|
|
(9
|
)%
|
253
|
|
|||||||
|
Total NEUPOGEN
®
|
1,159
|
|
(17
|
)%
|
1,398
|
|
11
|
%
|
1,260
|
|
|||||||
|
Total Neulasta
®
/NEUPOGEN
®
|
$
|
5,755
|
|
(1
|
)%
|
$
|
5,790
|
|
8
|
%
|
$
|
5,352
|
|
||||
|
Year ended December 31,
|
Year ended December 31,
|
Year ended December 31,
|
|||||||||||||||
|
2014
|
Change
|
2013
|
Change
|
2012
|
|||||||||||||
|
ENBREL — U.S.
|
$
|
4,404
|
|
3
|
%
|
$
|
4,256
|
|
7
|
%
|
$
|
3,967
|
|
||||
|
ENBREL — Canada
|
284
|
|
(4
|
)%
|
295
|
|
10
|
%
|
269
|
|
|||||||
|
Total ENBREL
|
$
|
4,688
|
|
3
|
%
|
$
|
4,551
|
|
7
|
%
|
$
|
4,236
|
|
||||
|
Year ended December 31,
|
Year ended December 31,
|
Year ended December 31,
|
|||||||||||||||
|
2014
|
Change
|
2013
|
Change
|
2012
|
|||||||||||||
|
XGEVA
®
— U.S.
|
$
|
857
|
|
12
|
%
|
$
|
764
|
|
19
|
%
|
$
|
644
|
|
||||
|
XGEVA
®
— ROW
|
364
|
|
43
|
%
|
255
|
|
*
|
|
104
|
|
|||||||
|
Total XGEVA
®
|
1,221
|
|
20
|
%
|
1,019
|
|
36
|
%
|
748
|
|
|||||||
|
Prolia
®
— U.S.
|
625
|
|
35
|
%
|
462
|
|
58
|
%
|
292
|
|
|||||||
|
Prolia
®
— ROW
|
405
|
|
44
|
%
|
282
|
|
57
|
%
|
180
|
|
|||||||
|
Total Prolia
®
|
1,030
|
|
38
|
%
|
744
|
|
58
|
%
|
472
|
|
|||||||
|
Total XGEVA
®
/Prolia
®
|
$
|
2,251
|
|
28
|
%
|
$
|
1,763
|
|
45
|
%
|
$
|
1,220
|
|
||||
|
Year ended December 31,
|
Year ended December 31,
|
Year ended December 31,
|
|||||||||||||||
|
2014
|
Change
|
2013
|
Change
|
2012
|
|||||||||||||
|
EPOGEN
®
— U.S.
|
$
|
2,031
|
|
4
|
%
|
$
|
1,953
|
|
1
|
%
|
$
|
1,941
|
|
||||
|
Year ended December 31,
|
Year ended December 31,
|
Year ended December 31,
|
|||||||||||||||
|
2014
|
Change
|
2013
|
Change
|
2012
|
|||||||||||||
|
Aranesp
®
— U.S.
|
$
|
794
|
|
6
|
%
|
$
|
747
|
|
(4
|
)%
|
$
|
782
|
|
||||
|
Aranesp
®
— ROW
|
1,136
|
|
(2
|
)%
|
1,164
|
|
(7
|
)%
|
1,258
|
|
|||||||
|
Total Aranesp
®
|
$
|
1,930
|
|
1
|
%
|
$
|
1,911
|
|
(6
|
)%
|
$
|
2,040
|
|
||||
|
Year ended December 31,
|
Year ended December 31,
|
Year ended December 31,
|
|||||||||||||||
|
2014
|
Change
|
2013
|
Change
|
2012
|
|||||||||||||
|
Sensipar
®
— U.S.
|
$
|
796
|
|
5
|
%
|
$
|
757
|
|
18
|
%
|
$
|
639
|
|
||||
|
Sensipar
®
/Mimpara
®
— ROW
|
362
|
|
9
|
%
|
332
|
|
7
|
%
|
311
|
|
|||||||
|
Total Sensipar
®
/Mimpara
®
|
$
|
1,158
|
|
6
|
%
|
$
|
1,089
|
|
15
|
%
|
$
|
950
|
|
||||
|
Year ended December 31,
|
Year ended December 31,
|
Year ended December 31,
|
|||||||||||||||
|
2014
|
Change
|
2013
|
Change
|
2012
|
|||||||||||||
|
Vectibix
®
— U.S.
|
$
|
168
|
|
33
|
%
|
$
|
126
|
|
3
|
%
|
$
|
122
|
|
||||
|
Vectibix
®
— ROW
|
337
|
|
28
|
%
|
263
|
|
11
|
%
|
237
|
|
|||||||
|
Nplate
®
— U.S.
|
260
|
|
8
|
%
|
241
|
|
13
|
%
|
214
|
|
|||||||
|
Nplate
®
— ROW
|
209
|
|
12
|
%
|
186
|
|
21
|
%
|
154
|
|
|||||||
|
Kyprolis
®
— U.S.
|
306
|
|
*
|
|
71
|
|
N/A
|
|
—
|
|
|||||||
|
Kyprolis
®
— ROW
|
25
|
|
*
|
|
2
|
|
N/A
|
|
—
|
|
|||||||
|
BLINCYTO
™
— U.S.
|
3
|
|
N/A
|
|
—
|
|
N/A
|
|
—
|
|
|||||||
|
Other — ROW
|
206
|
|
(16
|
)%
|
246
|
|
42
|
%
|
173
|
|
|||||||
|
Total other product sales
|
$
|
1,514
|
|
33
|
%
|
$
|
1,135
|
|
26
|
%
|
$
|
900
|
|
||||
|
Total U.S. — other products
|
$
|
737
|
|
68
|
%
|
$
|
438
|
|
30
|
%
|
$
|
336
|
|
||||
|
Total ROW — other products
|
777
|
|
11
|
%
|
697
|
|
24
|
%
|
564
|
|
|||||||
|
Total other product sales
|
$
|
1,514
|
|
33
|
%
|
$
|
1,135
|
|
26
|
%
|
$
|
900
|
|
||||
|
Year ended December 31,
|
Year ended December 31,
|
Year ended December 31,
|
|||||||||||||||
|
2014
|
Change
|
2013
|
Change
|
2012
|
|||||||||||||
|
Operating expenses:
|
|||||||||||||||||
|
Cost of sales
|
$
|
4,422
|
|
32
|
%
|
$
|
3,346
|
|
5
|
%
|
$
|
3,199
|
|
||||
|
% of product sales
|
22.9
|
%
|
18.4
|
%
|
19.2
|
%
|
|||||||||||
|
% of total revenues
|
22.0
|
%
|
17.9
|
%
|
18.5
|
%
|
|||||||||||
|
Research and development
|
$
|
4,297
|
|
5
|
%
|
$
|
4,083
|
|
21
|
%
|
$
|
3,380
|
|
||||
|
% of product sales
|
22.2
|
%
|
22.4
|
%
|
20.3
|
%
|
|||||||||||
|
% of total revenues
|
21.4
|
%
|
21.9
|
%
|
19.6
|
%
|
|||||||||||
|
Selling, general and administrative
|
$
|
4,699
|
|
(9
|
)%
|
$
|
5,184
|
|
8
|
%
|
$
|
4,814
|
|
||||
|
% of product sales
|
24.3
|
%
|
28.5
|
%
|
28.9
|
%
|
|||||||||||
|
% of total revenues
|
23.4
|
%
|
27.8
|
%
|
27.9
|
%
|
|||||||||||
|
Other
|
$
|
454
|
|
*
|
|
$
|
196
|
|
(34
|
)%
|
$
|
295
|
|
||||
|
Category
|
Description
|
|
|
DRTS
|
R&D expenses incurred in activities substantially in support of early research through the completion of phase 1 clinical trials. These activities encompass our DRTS functions, including drug discovery, toxicology, pharmacokinetics and drug metabolism, and process development.
|
|
|
Later stage clinical programs
|
R&D expenses incurred in or related to phase 2 and phase 3 clinical programs intended to result in registration of a new product or a new indication for an existing product in the United States or the EU.
|
|
|
Marketed products
|
R&D expenses incurred in support of the Company’s marketed products that are authorized to be sold in the United States or the EU. Includes clinical trials designed to gather information on product safety (certain of which may be required by regulatory authorities) and their product characteristics after regulatory approval has been obtained, as well as the costs of obtaining regulatory approval of a product in a new market after approval in either the United States or the EU has been obtained.
|
|
|
Years ended December 31,
|
|||||||||||
|
2014
|
2013
|
2012
|
|||||||||
|
DRTS
|
$
|
1,212
|
|
$
|
1,233
|
|
$
|
1,137
|
|
||
|
Later stage clinical programs
|
2,287
|
|
1,950
|
|
1,285
|
|
|||||
|
Marketed products
|
798
|
|
900
|
|
958
|
|
|||||
|
Total R&D expense
|
$
|
4,297
|
|
$
|
4,083
|
|
$
|
3,380
|
|
||
|
Years ended December 31,
|
|||||||||||
|
2014
|
2013
|
2012
|
|||||||||
|
Interest expense, net
|
$
|
1,071
|
|
$
|
1,022
|
|
$
|
1,053
|
|
||
|
Interest and other income, net
|
$
|
465
|
|
$
|
420
|
|
$
|
485
|
|
||
|
Provision for income taxes
|
$
|
427
|
|
$
|
184
|
|
$
|
664
|
|
||
|
Effective tax rate
|
7.6
|
%
|
3.5
|
%
|
13.3
|
%
|
|||||
|
December 31,
|
|||||||
|
2014
|
2013
|
||||||
|
Cash, cash equivalents and marketable securities
|
$
|
27,026
|
|
$
|
19,401
|
|
|
|
Restricted investments
|
—
|
|
3,412
|
|
|||
|
Total cash, cash equivalents, marketable securities and restricted investments
|
$
|
27,026
|
|
$
|
22,813
|
|
|
|
Total assets
|
69,009
|
|
66,125
|
|
|||
|
Current portion of long-term debt
|
500
|
|
2,505
|
|
|||
|
Long-term debt
|
30,215
|
|
29,623
|
|
|||
|
Stockholders’ equity
|
25,778
|
|
22,096
|
|
|||
|
Years ended December 31,
|
|||||||||||
|
2014
|
2013
|
2012
|
|||||||||
|
Net cash provided by operating activities
|
$
|
8,555
|
|
$
|
6,291
|
|
$
|
5,882
|
|
||
|
Net cash used in investing activities
|
(5,752
|
)
|
(8,469
|
)
|
(9,990
|
)
|
|||||
|
Net cash (used in) provided by financing activities
|
(2,877
|
)
|
2,726
|
|
419
|
|
|||||
|
|
Payments due by period as of December 31, 2014
|
||||||||||||||||||
|
|
|
Year
|
Years
|
Years
|
Years
|
||||||||||||||
|
Contractual obligations
|
Total
|
1
|
2 and 3
|
4 and 5
|
6 and beyond
|
||||||||||||||
|
Long-term debt obligations
(1) (2) (3) (4)
|
$
|
48,262
|
|
$
|
1,611
|
|
$
|
8,985
|
|
$
|
9,579
|
|
$
|
28,087
|
|
||||
|
Operating lease obligations
|
1,034
|
|
135
|
|
323
|
|
282
|
|
294
|
|
|||||||||
|
Purchase obligations
(5)
|
3,398
|
|
1,377
|
|
811
|
|
401
|
|
809
|
|
|||||||||
|
UTBs
(6)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Total contractual obligations
|
$
|
52,694
|
|
$
|
3,123
|
|
$
|
10,119
|
|
$
|
10,262
|
|
$
|
29,190
|
|
||||
|
(1)
|
Long-term debt obligations include future interest payments which are included in our financing arrangements at the fixed contractual coupon rates. To achieve a desired mix of fixed and floating interest rate debt, we enter into interest rate swap contracts that effectively convert a fixed rate interest coupon for certain of our debt issuances to a floating LIBOR-based coupon over the life of the respective note. We used an interest rate forward curve at December 31, 2014, in computing net amounts to be paid or received under our interest rate swap contracts which resulted in an aggregate net increase in future interest payments of $272 million. See Part IV—Note 14, Financing arrangements,
to the Consolidated Financial Statements for further discussion of our interest swap contracts.
|
|
(2)
|
Long-term debt obligations include future interest payments under our Term Loan at LIBOR-based variable rates of interest. We used an interest rate forward curve at December 31, 2014, in computing interest payments on this debt obligation. See Part IV—Note 14, Financing arrangements,
to the Consolidated Financial Statements for further discussion of this debt obligation.
|
|
(3)
|
Long-term debt obligations include contractual interest payments and principal repayment of our foreign denominated debt obligations. In order to hedge our exposure to foreign currency exchange rate risk associated with certain of our pound sterling and euro denominated long-term debt, we entered into cross-currency swap contracts that effectively convert interest payments and principal repayment on this debt from euros/pounds sterling to U.S. dollars. For purposes of this table, we used the contracted exchange rates in the cross-currency swap contracts to compute the net amounts of future interest payments and principal repayments on this debt. See Part IV—Note 17, Derivative instruments, to the Consolidated Financial Statements for further discussion of our cross-currency swap contracts.
|
|
(4)
|
Interest payments and the repayment of principal on our 4.375% 2018 euro Notes were translated into U.S. dollars at the foreign currency exchange rate in effect at December 31, 2014. See Part IV—Note 14, Financing arrangements, to the Consolidated Financial Statements for further discussion of our long-term debt obligations.
|
|
(5)
|
Purchase obligations relate primarily to: (i) R&D commitments (including those related to clinical trials) for new and existing products; (ii) capital expenditures; (iii) open purchase orders for the acquisition of goods and services in the ordinary course of business; and (iv) a $225 million payment due to the former shareholders of Proteolix, Inc. in settlement of contingent consideration assumed in the acquisition of Onyx (see Note 16, Fair value measurement to the Consolidated Financial Statements). Our obligation to pay certain of these amounts may be reduced based on certain future events.
|
|
(6)
|
Liabilities for UTBs (net of foreign tax credits and federal tax benefit of state taxes) and related accrued interest and penalties totaling approximately $1.7 billion at
December 31, 2014
, are not included in the table above because, due to their nature, there is a high degree of uncertainty regarding the timing of future cash outflows and other events that extinguish these liabilities.
|
|
Rebates
|
Chargebacks
|
Other deductions
|
Total
|
||||||||||||
|
Balance as of January 1, 2012
|
$
|
1,047
|
|
$
|
199
|
|
$
|
80
|
|
$
|
1,326
|
|
|||
|
Amounts charged against product sales
|
1,480
|
|
2,709
|
|
659
|
|
4,848
|
|
|||||||
|
Payments
|
(1,680
|
)
|
(2,741
|
)
|
(624
|
)
|
(5,045
|
)
|
|||||||
|
Balance as of December 31, 2012
|
847
|
|
167
|
|
115
|
|
1,129
|
|
|||||||
|
Amounts charged against product sales
|
1,784
|
|
3,008
|
|
669
|
|
5,461
|
|
|||||||
|
Payments
|
(1,736
|
)
|
(2,924
|
)
|
(682
|
)
|
(5,342
|
)
|
|||||||
|
Balance as of December 31, 2013
|
895
|
|
251
|
|
102
|
|
1,248
|
|
|||||||
|
Amounts charged against product sales
|
2,499
|
|
3,399
|
|
688
|
|
6,586
|
|
|||||||
|
Payments
|
(2,274
|
)
|
(3,454
|
)
|
(727
|
)
|
(6,455
|
)
|
|||||||
|
Balance as of December 31, 2014
|
$
|
1,120
|
|
$
|
196
|
|
$
|
63
|
|
$
|
1,379
|
|
|||
|
•
|
determining the timing and expected costs to complete in-process projects taking into account the stage of completion at the acquisition date;
|
|
•
|
projecting the probability and timing of obtaining marketing approval from the FDA and other regulatory agencies for product candidates;
|
|
•
|
estimating the timing of and future net cash flows from product sales resulting from completed products and in-process projects; and
|
|
•
|
developing appropriate discount rates to calculate the present values of the cash flows.
|
|
Item 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
|
Item 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
|
Item 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES
|
|
Item 9A.
|
CONTROLS AND PROCEDURES
|
|
Item 9B.
|
OTHER INFORMATION
|
|
Item 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE OF THE REGISTRANT
|
|
Item 11.
|
EXECUTIVE COMPENSATION
|
|
Item 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
|
(a)
|
(b)
|
(c)
|
||||||||
|
Plan Category
|
Number of Securities to be Issued Upon Exercise of Outstanding Options and Rights
|
Weighted Average Exercise Price Outstanding Options and Rights
|
Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a))
|
|||||||
|
Equity compensation plans approved by Amgen security holders:
|
||||||||||
|
Amended and Restated 2009 Equity Incentive Plan
(1)
|
16,045,767
|
|
$
|
57.37
|
|
51,960,037
|
|
|||
|
Amended and Restated 1991 Equity Incentive Plan
(2)
|
1,201,310
|
|
$
|
48.50
|
|
—
|
|
|||
|
Amended and Restated Employee Stock Purchase Plan
|
—
|
|
—
|
|
5,205,930
|
|
||||
|
Total Approved Plans
|
17,247,077
|
|
$
|
54.76
|
|
57,165,967
|
|
|||
|
Equity compensation plans not approved by Amgen security holders:
|
||||||||||
|
Amended and Restated 1999 Equity Incentive Plan
(3)
|
123,406
|
|
$
|
46.12
|
|
—
|
|
|||
|
Amended and Restated 1999 Incentive Stock Plan
(4)
|
10,077
|
|
$
|
51.75
|
|
—
|
|
|||
|
Amended and Restated Assumed Avidia Incentive Equity Plan
(5)
|
1,328
|
|
$
|
1.91
|
|
—
|
|
|||
|
Amgen Profit Sharing Plan for Employees in Ireland
(6)
|
—
|
|
—
|
|
143,220
|
|
||||
|
Total Unapproved Plans
|
134,811
|
|
$
|
46.10
|
|
143,220
|
|
|||
|
Total All Plans
|
17,381,888
|
|
$
|
54.48
|
|
57,309,187
|
|
|||
|
(1)
|
The Amended and Restated 2009 Equity Incentive Plan employs a fungible share counting formula for determining the number of shares available for issuance under the plan. In accordance with this formula, each option or stock appreciation right counts as one share, while each restricted stock unit, performance unit or dividend equivalent counts as 1.9 shares. The number under column (a) represents the actual number of shares issuable under our outstanding awards without giving effect to the fungible share counting formula. The number under column (c) represents the number of shares available for issuance under this plan based on each such available share counting as one share. Commencing with the grants made in April 2012, RSUs and performance units accrue dividend equivalents that are payable in shares only to the extent and when the underlying RSUs vest or underlying performance units have been earned and the related shares are issued to the grantee. The performance units granted under this plan are earned based on the accomplishment of specified performance goals at the end of their respective three-year performance periods; the number of performance units granted represent target performance and the maximum number of units that could be earned based on our performance is 150% of the performance units granted.
|
|
(2)
|
This plan has terminated as to future grants. The number under column (a) with respect to this plan includes 28,583 shares issuable upon the vesting of outstanding RSUs (including 1,768 related dividend equivalents), which are not included in calculating the weighted average exercise price in column (b).
|
|
(3)
|
This plan has terminated as to future grants. This plan was originally assumed pursuant to the terms of the merger agreement between Amgen and Immunex which was approved by our stockholders in May 2002. This plan was previously approved by Immunex’s shareholders.
|
|
(4)
|
This plan has terminated as to future grants. This plan was originally assumed by Amgen in connection with the merger of Abgenix with and into Amgen Fremont Inc., a wholly owned subsidiary of Amgen, on April 1, 2006. The number under column (a) with respect to this plan includes 57 shares issuable upon the vesting of outstanding RSUs, which are not included in calculating the weighted average exercise price in column (b).
|
|
(5)
|
This plan has terminated as to future grants. This plan was originally assumed by Amgen in connection with the merger of Avidia, Inc. with and into Amgen Mountain View Inc., a wholly owned subsidiary of Amgen, on October 24, 2006.
|
|
(6)
|
The Amgen Profit Sharing Plan for Employees in Ireland (the Profit Sharing Plan) was approved by the Board of Directors on July 28, 2011. The Profit Sharing Plan permits eligible employees of the Company’s subsidiaries located in Ireland, which participate in the Profit Sharing Plan, to apply a portion of their qualifying bonus and salary to the purchase the Company’s Common Stock on the open market at the market price by a third-party trustee as described in the Profit Sharing Plan.
|
|
Item 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
|
|
Item 14.
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
|
Item 15.
|
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
|
|
(a)1.
|
Index to Financial Statements
|
|
|
Page
number
|
|
Report of Independent Registered Public Accounting Firm
|
|
|
Consolidated Statements of Income for each of the three years in the period ended December 31, 2014
|
|
|
Consolidated Statements of Comprehensive Income for each of the three years in the period ended December 31, 2014
|
|
|
Consolidated Balance Sheets at December 31, 2014 and 2013
|
|
|
Consolidated Statements of Stockholders’ Equity for each of the three years in the period ended December 31, 2014
|
|
|
Consolidated Statements of Cash Flows for each of the three years in the period ended December 31, 2014
|
|
|
Notes to Consolidated Financial Statements
|
|
|
(a)2.
|
Index to Financial Statement Schedules
|
|
|
Page
number
|
|
II. Valuation and Qualifying Accounts
|
|
|
(a)3.
|
Exhibits
|
|
Exhibit No.
|
Description
|
|
|
3.1
|
Restated Certificate of Incorporation of Amgen Inc. (As Restated March 6, 2013.) (Filed as an exhibit to Form 10-Q for the quarter ended March 31, 2013 on May 3, 2013 and incorporated herein by reference.)
|
|
|
3.2
|
Amended and Restated Bylaws of Amgen Inc. (As Amended and Restated March 6, 2013). (Filed as an exhibit to Form 8-K on March 6, 2013 and incorporated herein by reference.)
|
|
|
3.3
|
First Amendment to the Amended and Restated Bylaws of Amgen Inc. (As Amended and Restated March 6, 2013). (Filed as an exhibit to Form 8-K on October 16, 2013 and incorporated herein by reference.)
|
|
|
4.1
|
Form of stock certificate for the common stock, par value $.0001 of the Company. (Filed as an exhibit to Form 10-Q for the quarter ended March 31, 1997 on May 13, 1997 and incorporated herein by reference.)
|
|
|
4.2
|
Form of Indenture, dated January 1, 1992. (Filed as an exhibit to Form S-3 Registration Statement filed on December 19, 1991 and incorporated herein by reference.)
|
|
|
4.3
|
Agreement of Resignation, Appointment and Acceptance dated February 15, 2008. (Filed as an exhibit to Form 10-K for the year ended December 31, 2007 on February 28, 2008 and incorporated herein by reference.)
|
|
|
4.4
|
First Supplemental Indenture, dated February 26, 1997. (Filed as an exhibit to Form 8-K on March 14, 1997 and incorporated herein by reference.)
|
|
|
4.5
|
8-1/8% Debentures due April 1, 2097. (Filed as an exhibit to Form 8-K on April 8, 1997 and incorporated herein by reference.)
|
|
|
Exhibit No.
|
Description
|
|
|
4.6
|
Officer's Certificate of Amgen Inc., dated January 1, 1992, as supplemented by the First Supplemental Indenture, dated February 26, 1997, establishing a series of securities entitled “8 1/8% Debentures due April 1, 2097.” (Filed as an exhibit to Form 8-K on April 8, 1997 and incorporated herein by reference.)
|
|
|
4.7
|
Indenture, dated August 4, 2003. (Filed as an exhibit to Form S-3 Registration Statement on August 4, 2003 and incorporated herein by reference.)
|
|
|
4.8
|
Corporate Commercial Paper - Master Note between and among Amgen Inc., as Issuer, Cede & Co., as Nominee of The Depository Trust Company, and Citibank, N.A., as Paying Agent. (Filed as an exhibit to Form 10-Q for the quarter ended March 31, 1998 on May 13, 1998 and incorporated herein by reference.)
|
|
|
4.9
|
Officers' Certificate of Amgen Inc., dated May 30, 2007, including forms of the Company's Senior Floating Rate Notes due 2008, 5.85% Senior Notes due 2017 and 6.375% Senior Notes due 2037. (Filed as an exhibit to Form 8-K on May 30, 2007 and incorporated herein by reference.)
|
|
|
4.10
|
Officers' Certificate of Amgen Inc., dated May 23, 2008, including forms of the Company's 6.15% Senior Notes due 2018 and 6.90% Senior Notes due 2038. (Filed as exhibit to Form 8-K on May 23, 2009 and incorporated herein by reference.)
|
|
|
4.11
|
Officers' Certificate of Amgen Inc., dated January 16, 2009, including forms of the Company's 5.70% Senior Notes due 2019 and 6.40% Senior Notes due 2039. (Filed as exhibit to Form 8-K on January 16, 2009 and incorporated herein by reference.)
|
|
|
4.12
|
Officers' Certificate of Amgen Inc., dated March 12, 2010, including forms of the Company's 4.50% Senior Notes due 2020 and 5.75% Senior Notes due 2040. (Filed as exhibit to Form 8-K on March 15, 2010 and incorporated herein by reference.)
|
|
|
4.13
|
Officers' Certificate of Amgen Inc., dated September 16, 2010, including forms of the Company's 3.45% Senior Notes due 2020 and 4.95% Senior Notes due 2041. (Filed as an exhibit to Form 8-K on September 17, 2010 and incorporated herein by reference.)
|
|
|
4.14
|
Officers' Certificate of Amgen Inc., dated June 30, 2011, including forms of the Company's 2.30% Senior Notes due 2016, 4.10% Senior Notes due 2021 and 5.65% Senior Notes due 2042. (Filed as an exhibit to Form 8-K on June 30, 2011 and incorporated herein by reference.)
|
|
|
4.15
|
Officers' Certificate of Amgen Inc., dated November 10, 2011, including forms of the Company's 1.875% Senior Notes due 2014, 2.50% Senior Notes due 2016, 3.875% Senior Notes due 2021 and 5.15% Senior Notes due 2041. (Filed as an exhibit to Form 8-K on November 10, 2011 and incorporated herein by reference.)
|
|
|
4.16
|
Officers' Certificate of Amgen Inc., dated December 5, 2011, including forms of the Company's 4.375% Senior Notes due 2018 and 5.50% Senior Notes due 2026. (Filed as an exhibit to Form 8-K on December 5, 2011 and incorporated herein by reference.)
|
|
|
4.17
|
Officers' Certificate of Amgen Inc., dated May 15, 2012, including forms of the Company's 2.125% Senior Notes due 2017, 3.625% Senior Notes due 2022 and 5.375% Senior Notes due 2043. (Filed as an exhibit to Form 8-K on May 15, 2012 and incorporated herein by reference.)
|
|
|
4.18
|
Officers' Certificate of Amgen Inc., dated September 13, 2012, including forms of the Company's 2.125% Senior Notes due 2019 and 4.000% Senior Notes due 2029. (Filed as an exhibit to Form 8-K on September 13, 2012 and incorporated herein by reference.)
|
|
|
4.19
|
Indenture, dated May 22, 2014, between Amgen Inc. and The Bank of New York Mellon Trust Company, N.A., as Trustee. (Filed as an exhibit to Form 8-K on May 22, 2014 and incorporated herein by reference.)
|
|
|
4.20
|
Officers' Certificate of Amgen Inc., dated May 22, 2014, including forms of the Company's Senior Floating Rate Notes due 2017, Senior Floating Rate Notes due 2019, 1.250% Senior Notes due 2017, 2.200% Senior Notes due 2019 and 3.625% Senior Notes due 2024. (Filed as an exhibit to Form 8-K on May 22, 2014 and incorporated herein by reference.)
|
|
|
10.1+
|
Amgen Inc. Amended and Restated 2009 Equity Incentive Plan. (Filed as Appendix C to the Definitive Proxy Statement on Schedule 14A on April 8, 2013 and incorporated herein by reference.)
|
|
|
10.2+
|
Form of Stock Option Agreement for the Amgen Inc. Amended and Restated 2009 Equity Incentive Plan. (As Amended on March 6, 2013.) (Filed as an exhibit to Form 10-Q for the quarter ended March 31, 2013 on May 3, 2013 and incorporated herein by reference.)
|
|
|
Exhibit No.
|
Description
|
|
|
10.3+*
|
Form of Restricted Stock Unit Agreement for the Amgen Inc. Amended and Restated 2009 Equity Incentive Plan. (As Amended on December 17, 2014.)
|
|
|
10.4+
|
Amgen Inc. 2009 Performance Award Program. (As Amended on December 13, 2013.) (Filed as an exhibit to Form 10-K for the year ended December 31, 2013 on February 24, 2014 and incorporated herein by reference.)
|
|
|
10.5+*
|
Form of Performance Unit Agreement for the Amgen Inc. 2009 Performance Award Program. (As Amended on December 17, 2014.)
|
|
|
10.6+
|
Amgen Inc. 2009 Director Equity Incentive Program. (As Amended on March 6, 2013.) (Filed as an exhibit to Form 10-Q for the quarter ended March 31, 2013 on May 3, 2013 and incorporated herein by reference.)
|
|
|
10.7+
|
Form of Grant of Non-Qualified Stock Option Agreement for the Amgen Inc. 2009 Director Equity Incentive Program. (Filed as an exhibit to Form 8-K on May 8, 2009 and incorporated herein by reference.)
|
|
|
10.8+
|
Form of Restricted Stock Unit Agreement for the Amgen Inc. 2009 Director Equity Incentive Program. (As Amended on March 6, 2013.) (Filed as an exhibit to Form 10-Q for the quarter ended March 31, 2013 on May 3, 2013 and incorporated herein by reference.)
|
|
|
10.9+
|
Amgen Inc. Supplemental Retirement Plan. (As Amended and Restated effective October 16, 2013.) (Filed as an exhibit to Form 10-K for the year ended December 31, 2013 on February 24, 2014 and incorporated herein by reference.)
|
|
|
10.10+
|
Amended and Restated Amgen Change of Control Severance Plan. (As Amended and Restated effective December 9, 2010 and subsequently amended effective March 2, 2011.) (Filed as an exhibit to Form 10-Q for the quarter ended March 31, 2011 on May 10, 2011 and incorporated herein by reference.)
|
|
|
10.11+
|
Amgen Inc. Executive Incentive Plan. (As Amended and Restated effective January 1, 2009.) (Filed as an exhibit to Form 10-Q for the quarter ended September 30, 2008 on November 7, 2008 and incorporated herein by reference.)
|
|
|
10.12+
|
First Amendment to the Amgen Inc. Executive Incentive Plan, effective December 13, 2012. (Filed as an exhibit to Form 10-K for the year ended December 31, 2012 on February 27, 2013 and incorporated herein by reference.)
|
|
|
10.13+
|
Amgen Inc. Executive Nonqualified Retirement Plan. (As Amended and Restated effective January 1, 2009.) (Filed as an exhibit to Form 10-Q for the quarter ended September 30, 2008 on November 7, 2008 and incorporated herein by reference.)
|
|
|
10.14+
|
First Amendment to the Amgen Inc. Executive Nonqualified Retirement Plan, effective July 21, 2010. (Filed as an exhibit to Form 10-Q for the quarter ended June 30, 2010 on August 9, 2010 and incorporated herein by reference.)
|
|
|
10.15+
|
Amgen Nonqualified Deferred Compensation Plan. (As Amended and Restated effective October 16, 2013.) (Filed as an exhibit to Form 10-K for the year ended December 31, 2013 on February 24, 2014 and incorporated herein by reference.)
|
|
|
10.16+
|
Agreement between Amgen Inc. and Mr. Anthony C. Hooper, dated October 12, 2011. (Filed as an exhibit to Form 10-K for the year ended December 31, 2011 on February 29, 2012 and incorporated herein by reference.)
|
|
|
10.17+
|
Agreement and General Release of Claims, entered into January 9, 2014, by and between Amgen Inc. and Jonathan M. Peacock. (Filed as an exhibit to Form 10-K for the year ended December 31, 2013 on February 24, 2014 and incorporated herein by reference.)
|
|
|
10.18+
|
Agreement between Amgen Inc. and David W. Meline, effective July 21, 2014. (Filed as an exhibit to Form 10-Q for the quarter ended September 30, 2014 on October 29, 2014 and incorporated herein by reference.)
|
|
|
10.19
|
Shareholders' Agreement, dated May 11, 1984, among Amgen, Kirin Brewery Company, Limited and Kirin-Amgen, Inc. (Filed as an exhibit to Form 10-K for the year ended December 31, 2000 on March 7, 2001 and incorporated herein by reference.)
|
|
|
10.20
|
Amendment No. 1 dated March 19, 1985, Amendment No. 2 dated July 29, 1985 (effective July 1, 1985), and Amendment No. 3, dated December 19, 1985, to the Shareholders' Agreement dated May 11, 1984. (Filed as an exhibit to Form 10-Q for the quarter ended June 30, 2000 on August 1, 2000 and incorporated herein by reference.)
|
|
|
Exhibit No.
|
Description
|
|
|
10.21
|
Amendment No. 4 dated October 16, 1986 (effective July 1, 1986), Amendment No. 5 dated December 6, 1986 (effective July 1, 1986), Amendment No. 6 dated June 1, 1987, Amendment No. 7 dated July 17, 1987 (effective April 1, 1987), Amendment No. 8 dated May 28, 1993 (effective November 13, 1990), Amendment No. 9 dated December 9, 1994 (effective June 14, 1994), Amendment No. 10 effective March 1, 1996, and Amendment No. 11 effective March 20, 2000 to the Shareholders' Agreement, dated May 11, 1984. (Filed as exhibits to Form 10-K for the year ended December 31, 2000 on March 7, 2001 and incorporated herein by reference.)
|
|
|
10.22
|
Amendment No. 12 to the Shareholders' Agreement, dated January 31, 2001. (Filed as an exhibit to Form 10-Q for the quarter ended June 30, 2005 on August 8, 2005 and incorporated herein by reference.)
|
|
|
10.23
|
Amendment No. 13 to the Shareholders' Agreement, dated June 28, 2007 (portions of the exhibit have been omitted pursuant to a request for confidential treatment). (Filed as an exhibit to Form 10-Q for the quarter ended June 30, 2007 on August 9, 2007 and incorporated herein by reference.)
|
|
|
10.24
|
Amendment No. 14 to the Shareholders' Agreement, dated March 26, 2014. (Filed as an exhibit to Form 10-Q for the quarter ended March 31, 2014 on April 30, 2014 and incorporated herein by reference.)
|
|
|
10.25
|
Assignment and License Agreement, dated October 16, 1986 (effective July 1, 1986), between Amgen and Kirin-Amgen, Inc. (Filed as an exhibit to Form 10-K for the year ended December 31, 2000 on March 7, 2001 and incorporated herein by reference.)
|
|
|
10.26
|
G-CSF United States License Agreement, dated June 1, 1987 (effective July 1, 1986), Amendment No. 1, dated October 20, 1988, and Amendment No. 2, dated October 17, 1991 (effective November 13, 1990), between Kirin-Amgen, Inc. and Amgen Inc. (Filed as exhibits to Form 10-K for the year ended December 31, 2000 on March 7, 2001 and incorporated herein by reference.)
|
|
|
10.27
|
G-CSF European License Agreement, dated December 30, 1986, between Kirin-Amgen and Amgen, Amendment No. 1 to Kirin-Amgen, Inc. / Amgen G-CSF European License Agreement, dated June 1, 1987, Amendment No. 2 to Kirin-Amgen, Inc. / Amgen G-CSF European License Agreement, dated March 15, 1998, Amendment No. 3 to Kirin-Amgen, Inc. / Amgen G-CSF European License Agreement, dated October 20, 1988, and Amendment No. 4 to Kirin-Amgen, Inc. / Amgen G-CSF European License Agreement, dated December 29, 1989, between Kirin-Amgen, Inc. and Amgen Inc. (Filed as exhibits to Form 10-K for the year ended December 31, 2000 on March 7, 2001 and incorporated herein by reference.)
|
|
|
10.28
|
Amended and Restated Promotion Agreement, dated December 16, 2001, by and among Immunex Corporation, American Home Products Corporation and Amgen Inc. (portions of the exhibit have been omitted pursuant to a request for confidential treatment). (Filed as an exhibit to Amendment No. 1 to Form S-4 Registration Statement on March 22, 2002 and incorporated herein by reference.)
|
|
|
10.29
|
Description of Amendment No. 1 to Amended and Restated Promotion Agreement, effective July 8, 2003, among Wyeth, Amgen Inc. and Immunex Corporation (portions of the exhibit have been omitted pursuant to a request for confidential treatment). (Filed as an exhibit to Form 10-K for the year ended December 31, 2003 on March 11, 2004 and incorporated herein by reference.)
|
|
|
10.30
|
Description of Amendment No. 2 to Amended and Restated Promotion Agreement, effective April 20, 2004, by and among Wyeth, Amgen Inc. and Immunex Corporation. (Filed as an exhibit to Amendment No. 1 to Form S-4 Registration Statement on June 29, 2004 and incorporated herein by reference.)
|
|
|
10.31
|
Amendment No. 3 to Amended and Restated Promotion Agreement, effective January 1, 2005, by and among Wyeth, Amgen Inc. and Immunex Corporation (portions of the exhibit have been omitted pursuant to a request for confidential treatment). (Filed as an exhibit to Form 10-Q for the quarter ended March 31, 2005 on May 4, 2005 and incorporated herein by reference.)
|
|
|
10.32
|
Amended and Restated Credit Agreement, dated July 30, 2014, among Amgen Inc., the Banks therein named, Citibank, N.A., as administrative agent, and JPMorgan Chase Bank, N.A., as syndication agent (Filed as an exhibit to Form 8-K on July 30, 2014 and incorporated herein by reference.)
|
|
|
10.33
|
Collaboration and License Agreement between Amgen Inc. and Celltech R&D Limited dated May 10, 2002 (portions of the exhibit have been omitted pursuant to a request for confidential treatment) and Amendment No. 1, effective June 9, 2003, to Collaboration and License Agreement between Amgen Inc. and Celltech R&D Limited (portions of the exhibit have been omitted pursuant to a request for confidential treatment). (Filed as an exhibit to Form 10-K/A for the year ended December 31, 2012 on July 31, 2013 and incorporated herein by reference.)
|
|
|
Exhibit No.
|
Description
|
|
|
10.34
|
Sourcing and Supply Agreement, dated November 15, 2011, by and between Amgen USA Inc, a wholly owned subsidiary of Amgen Inc., and DaVita Inc. (portions of the exhibit have been omitted pursuant to a request for confidential treatment). (Filed as an exhibit to Form 10-K for the year ended December 31, 2011 on February 29, 2012 and incorporated herein by reference.)
|
|
|
10.35
|
Amendment Number 1 to Sourcing and Supply Agreement, effective January 1, 2013, by and between Amgen USA Inc., a wholly owned subsidiary of Amgen Inc., and DaVita Healthcare Partners Inc. f/k/a DaVita Inc. (portions of the exhibit have been omitted pursuant to a request for confidential treatment). (Filed as an exhibit to Form 10-K for the year ended December 31, 2012 on February 27, 2013 and incorporated herein by reference.)
|
|
|
10.36
|
Collaboration Agreement dated March 30, 2012 by and between Amgen Inc. and AstraZeneca Collaboration Ventures, LLC, a wholly owned subsidiary of AstraZeneca Pharmaceuticals LP (portions of the exhibit have been omitted pursuant to a request for confidential treatment). (Filed as an exhibit to Form 10-Q for the quarter ended March 31, 2012 on May 8, 2012 and incorporated herein by reference.)
|
|
|
10.37*
|
Amendment No. 1 to Collaboration Agreement, dated October 1, 2014, by and among Amgen Inc., AstraZeneca Collaboration Ventures, LLC and AstraZeneca Pharmaceuticals LP (portions of the exhibit have been omitted pursuant to a request for confidential treatment).
|
|
|
10.38
|
Collaboration Agreement, dated April 22, 1994, by and between Bayer Corporation (formerly Miles, Inc.) and Onyx Pharmaceuticals, Inc. (Filed as an exhibit to Form 10-Q for the quarter ended March 31, 2011 by Onyx Pharmaceuticals, Inc. on May 10, 2011 and incorporated herein by reference.)
|
|
|
10.39
|
Amendment to Collaboration Agreement, dated April 24, 1996, by and between Bayer Corporation and Onyx Pharmaceuticals, Inc. (Filed as an exhibit to Form 10-Q for the quarter ended March 31, 2006 by Onyx Pharmaceuticals, Inc. on May 10, 2006 and incorporated herein by reference.)
|
|
|
10.40
|
Amendment to Collaboration Agreement, dated February 1, 1999, by and between Bayer Corporation and Onyx Pharmaceuticals, Inc. (Filed as an exhibit to Form 10-Q for the quarter ended March 31, 2006 by Onyx Pharmaceuticals, Inc. on May 10, 2006 and incorporated herein by reference.)
|
|
|
10.41
|
United States Co-Promotion Agreement, dated March 6, 2006, by and between Bayer Pharmaceuticals Corporation and Onyx Pharmaceuticals, Inc. (Filed as an exhibit to Form 10-Q for the quarter ended March 31, 2006 by Onyx Pharmaceuticals, Inc. on May 10, 2006 and incorporated herein by reference.)
|
|
|
10.42
|
Settlement Agreement and Release, dated October 11, 2011, by and between Bayer Corporation, Bayer AG, Bayer HealthCare LLC and Bayer Pharma AG and Onyx Pharmaceuticals, Inc. (Filed as an exhibit to Form 10-K for the year ended December 31, 2011 by Onyx Pharmaceuticals, Inc. on February 27, 2012 and incorporated herein by reference.)
|
|
|
10.43
|
Fourth Amendment to Collaboration Agreement, dated October 11, 2011, by and between Bayer Corporation and Onyx Pharmaceuticals, Inc. (Filed as an exhibit to Form 10-K for the year ended December 31, 2011 by Onyx Pharmaceuticals, Inc. on February 27, 2012 and incorporated herein by reference.)
|
|
|
10.44
|
Commitment Letter, dated August 24, 2013, among Amgen Inc., Bank of America, N.A., Merrill Lynch, Pierce, Fenner & Smith Incorporated, JPMorgan Chase Bank, N.A., J.P. Morgan Securities LLC and Barclays Bank PLC. (Filed as an exhibit to Form 8-K on August 26, 2013 and incorporated herein by reference.)
|
|
|
10.45
|
Master Repurchase Agreement, dated August 24, 2013, between Amgen Inc. and Bank of America, N.A. (Filed as an exhibit to Form 8-K on August 26, 2013 and incorporated herein by reference.)
|
|
|
10.46
|
Master Repurchase Agreement, dated October 28, 2013, between Amgen Inc. and SMBC Repo Pass-Thru Trust, 2013-1. (Filed as an exhibit to Form 10-Q for the quarter ended September 30, 2013 on October 29, 2013 and incorporated herein by reference.)
|
|
|
10.47
|
Master Repurchase Agreement, dated October 29, 2013, between Amgen Inc. and HSBC Bank USA, N.A. (Filed as an exhibit to Form 10-Q for the quarter ended September 30, 2013 on October 29, 2013 and incorporated herein by reference.)
|
|
|
10.48
|
Term Loan Facility Credit Agreement, dated September 20, 2013, among Amgen Inc., the Banks therein named, Bank of America, N.A., as Administrative Agent, and Barclays Bank PLC and JPMorgan Chase Bank, N.A., as Syndication Agents. (Filed as an exhibit to Form 8-K on September 20, 2013 and incorporated herein by reference.)
|
|
|
21*
|
Subsidiaries of the Company.
|
|
|
Exhibit No.
|
Description
|
|
|
23
|
Consent of the Independent Registered Public Accounting Firm. The consent is set forth on page 74 of this Annual Report on Form 10-K.
|
|
|
24
|
Power of Attorney. The Power of Attorney is set forth on page 75 of this Annual Report on Form 10-K.
|
|
|
31*
|
Rule 13a-14(a) Certifications.
|
|
|
32**
|
Section 1350 Certifications.
|
|
|
101.INS*
|
XBRL Instance Document.
|
|
|
101.SCH*
|
XBRL Taxonomy Extension Schema Document.
|
|
|
101.CAL*
|
XBRL Taxonomy Extension Calculation Linkbase Document.
|
|
|
101.DEF*
|
XBRL Taxonomy Extension Definition Linkbase Document.
|
|
|
101.LAB*
|
XBRL Taxonomy Extension Label Linkbase Document.
|
|
|
101.PRE*
|
XBRL Taxonomy Extension Presentation Linkbase Document.
|
|
|
AMGEN INC.
|
||||
|
(Registrant)
|
||||
|
Date:
|
February 19, 2015
|
By:
|
|
/
S
/ DAVID W. MELINE
|
|
|
David W. Meline
|
|||
|
|
Executive Vice President and Chief Financial Officer
|
|||
|
|
||||
|
•
|
Registration Statement (Form S-8 No. 333-159377) pertaining to the Amgen Inc. 2009 Equity Incentive Plan;
|
|
•
|
Registration Statement (Form S-8 No. 33-39183) pertaining to the Amended and Restated Employee Stock Purchase Plan;
|
|
•
|
Registration Statements (Form S-8 No. 33-39104, as amended by Form S-8 No. 333-144581) pertaining to the Amended and Restated Amgen Retirement and Savings Plan (formerly known as the Amgen Retirement and Savings Plan);
|
|
•
|
Registration Statements (Form S-8 Nos. 33-42072 and 333-144579) pertaining to the Amgen Inc. Amended and Restated 1991 Equity Incentive Plan;
|
|
•
|
Registration Statements (Form S-8 Nos. 33-47605 and 333-144580) pertaining to the Retirement and Savings Plan for Amgen Manufacturing, Limited (formerly known as the Retirement and Savings Plan for Amgen Manufacturing, Inc.);
|
|
•
|
Registration Statements (Form S-8 Nos. 333-81284 and 333-177868) pertaining to the Amgen Nonqualified Deferred Compensation Plan;
|
|
•
|
Registration Statements (Form S-8 No. 333-92424 and Amendment No. 1 thereto) pertaining to the Amgen Inc. Amended and Restated 1999 Equity Incentive Plan (formerly known as the Immunex Corporation 1999 Stock Option Plan);
|
|
•
|
Registration Statements (Form S-8 Nos. 333-132932 and 333-133002) pertaining to the Amgen Inc. Amended and Restated 1999 Incentive Stock Plan (formerly known as Abgenix, Inc. 1999 Nonstatutory Stock Option Plan, as amended and restated);
|
|
•
|
Registration Statement (Form S-8 No. 333-138325) pertaining to the Amgen Inc. Amended and Restated Assumed Avidia Equity Incentive Plan (formerly known as the Avidia, Inc. Amended and Restated 2003 Equity Incentive Plan);
|
|
•
|
Registration Statement (Form S-3 No. 333-194103) relating to debt securities, common stock, preferred stock, warrants to purchase debt securities, common stock, preferred stock or depositary shares, rights to purchase common stock or preferred stock, securities purchase contracts, securities purchase units and depositary shares of Amgen Inc. and in the related Prospectus; and
|
|
•
|
Registration Statement (Form S-8 No. 333-176240) pertaining to the Amgen Profit Sharing Plan for Employees in Ireland;
|
|
Signature
|
Title
|
Date
|
||
|
/S/ ROBERT A. BRADWAY
|
Chairman of the Board, Chief Executive Officer
and President, and Director (Principal Executive Officer) |
2/19/2015
|
||
|
Robert A. Bradway
|
||||
|
/S/ DAVID W. MELINE
|
Executive Vice President and
Chief Financial Officer
(Principal Financial and Accounting Officer)
|
2/19/2015
|
||
|
David W. Meline
|
||||
|
/S/ DAVID BALTIMORE
|
Director
|
2/19/2015
|
||
|
David Baltimore
|
||||
|
/S/ FRANK J. BIONDI, JR.
|
Director
|
2/19/2015
|
||
|
Frank J. Biondi, Jr.
|
||||
|
/S/ FRANÇOIS DE CARBONNEL
|
Director
|
2/19/2015
|
||
|
François de Carbonnel
|
||||
|
/S/ VANCE D. COFFMAN
|
Director
|
2/19/2015
|
||
|
Vance D. Coffman
|
||||
|
/S/ ROBERT A. ECKERT
|
Director
|
2/19/2015
|
||
|
Robert A. Eckert
|
||||
|
/S/ GREG C. GARLAND
|
Director
|
2/19/2015
|
||
|
Greg C. Garland
|
||||
|
/S/ REBECCA M. HENDERSON
|
Director
|
2/19/2015
|
||
|
Rebecca M. Henderson
|
||||
|
/S/ FRANK C. HERRINGER
|
Director
|
2/19/2015
|
||
|
Frank C. Herringer
|
||||
|
/S/ TYLER JACKS
|
Director
|
2/19/2015
|
||
|
Tyler Jacks
|
||||
|
/S/ JUDITH C. PELHAM
|
Director
|
2/19/2015
|
||
|
Judith C. Pelham
|
||||
|
/S/ RONALD D. SUGAR
|
Director
|
2/19/2015
|
||
|
Ronald D. Sugar
|
||||
|
/S/ R. SANDERS WILLIAMS
|
Director
|
2/19/2015
|
||
|
R. Sanders Williams
|
||||
|
2014
|
2013
|
2012
|
|||||||||
|
Revenues:
|
|||||||||||
|
Product sales
|
$
|
19,327
|
|
$
|
18,192
|
|
$
|
16,639
|
|
||
|
Other revenues
|
736
|
|
484
|
|
626
|
|
|||||
|
Total revenues
|
20,063
|
|
18,676
|
|
17,265
|
|
|||||
|
Operating expenses:
|
|||||||||||
|
Cost of sales
|
4,422
|
|
3,346
|
|
3,199
|
|
|||||
|
Research and development
|
4,297
|
|
4,083
|
|
3,380
|
|
|||||
|
Selling, general and administrative
|
4,699
|
|
5,184
|
|
4,814
|
|
|||||
|
Other
|
454
|
|
196
|
|
295
|
|
|||||
|
Total operating expenses
|
13,872
|
|
12,809
|
|
11,688
|
|
|||||
|
Operating income
|
6,191
|
|
5,867
|
|
5,577
|
|
|||||
|
Interest expense, net
|
1,071
|
|
1,022
|
|
1,053
|
|
|||||
|
Interest and other income, net
|
465
|
|
420
|
|
485
|
|
|||||
|
Income before income taxes
|
5,585
|
|
5,265
|
|
5,009
|
|
|||||
|
Provision for income taxes
|
427
|
|
184
|
|
664
|
|
|||||
|
Net income
|
$
|
5,158
|
|
$
|
5,081
|
|
$
|
4,345
|
|
||
|
Earnings per share:
|
|||||||||||
|
Basic
|
$
|
6.80
|
|
$
|
6.75
|
|
$
|
5.61
|
|
||
|
Diluted
|
$
|
6.70
|
|
$
|
6.64
|
|
$
|
5.52
|
|
||
|
Shares used in the calculation of earnings per share:
|
|||||||||||
|
Basic
|
759
|
|
753
|
|
775
|
|
|||||
|
Diluted
|
770
|
|
765
|
|
787
|
|
|||||
|
2014
|
2013
|
2012
|
|||||||||
|
Net income
|
$
|
5,158
|
|
$
|
5,081
|
|
$
|
4,345
|
|
||
|
Other comprehensive income (loss), net of reclassification adjustments and taxes:
|
|
|
|
|
|
|
|||||
|
Foreign currency translation losses
|
(196
|
)
|
(80
|
)
|
(9
|
)
|
|||||
|
Effective portion of cash flow hedges
|
323
|
|
2
|
|
(78
|
)
|
|||||
|
Net unrealized gains (losses) on available-for-sale securities
|
24
|
|
(226
|
)
|
63
|
|
|||||
|
Other
|
2
|
|
(3
|
)
|
(1
|
)
|
|||||
|
Other comprehensive income (loss), net of tax
|
153
|
|
(307
|
)
|
(25
|
)
|
|||||
|
Comprehensive income
|
$
|
5,311
|
|
$
|
4,774
|
|
$
|
4,320
|
|
||
|
2014
|
2013
|
||||||
|
ASSETS
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
3,731
|
|
$
|
3,805
|
|
|
|
Marketable securities
|
23,295
|
|
15,596
|
|
|||
|
Trade receivables, net
|
2,546
|
|
2,697
|
|
|||
|
Inventories
|
2,647
|
|
3,019
|
|
|||
|
Other current assets
|
2,494
|
|
2,250
|
|
|||
|
Total current assets
|
34,713
|
|
27,367
|
|
|||
|
Property, plant and equipment, net
|
5,223
|
|
5,349
|
|
|||
|
Intangible assets, net
|
12,693
|
|
13,262
|
|
|||
|
Goodwill
|
14,788
|
|
14,968
|
|
|||
|
Restricted investments
|
—
|
|
3,412
|
|
|||
|
Other assets
|
1,592
|
|
1,767
|
|
|||
|
Total assets
|
$
|
69,009
|
|
$
|
66,125
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
1,212
|
|
$
|
787
|
|
|
|
Accrued liabilities
|
5,296
|
|
4,655
|
|
|||
|
Current portion of long-term debt
|
500
|
|
2,505
|
|
|||
|
Total current liabilities
|
7,008
|
|
7,947
|
|
|||
|
Long-term debt
|
30,215
|
|
29,623
|
|
|||
|
Long-term deferred tax liability
|
3,461
|
|
3,498
|
|
|||
|
Other noncurrent liabilities
|
2,547
|
|
2,961
|
|
|||
|
Contingencies and commitments
|
|
|
|
|
|||
|
Stockholders’ equity:
|
|||||||
|
Common stock and additional paid-in capital; $0.0001 par value; 2,750.0 shares authorized; outstanding — 760.4 shares in 2014 and 754.6 shares in 2013
|
30,410
|
|
29,891
|
|
|||
|
Accumulated deficit
|
(4,624
|
)
|
(7,634
|
)
|
|||
|
Accumulated other comprehensive loss
|
(8
|
)
|
(161
|
)
|
|||
|
Total stockholders’ equity
|
25,778
|
|
22,096
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
69,009
|
|
$
|
66,125
|
|
|
|
Number
of shares
of common
stock
|
Common
stock and
additional
paid-in capital
|
Accumulated
deficit
|
Accumulated
other
comprehensive
income (loss)
|
Total
|
||||||||||||||
|
Balance at December 31, 2011
|
795.6
|
|
$
|
27,777
|
|
$
|
(8,919
|
)
|
$
|
171
|
|
$
|
19,029
|
|
||||
|
Net income
|
—
|
|
—
|
|
4,345
|
|
—
|
|
4,345
|
|
||||||||
|
Other comprehensive loss, net of tax
|
—
|
|
—
|
|
—
|
|
(25
|
)
|
(25
|
)
|
||||||||
|
Dividends
|
—
|
|
—
|
|
(1,187
|
)
|
—
|
|
(1,187
|
)
|
||||||||
|
Issuance of common stock in connection with the Company’s equity award programs
|
23.0
|
|
1,288
|
|
—
|
|
—
|
|
1,288
|
|
||||||||
|
Stock-based compensation
|
—
|
|
359
|
|
—
|
|
—
|
|
359
|
|
||||||||
|
Tax impact related to employee stock-based compensation
|
—
|
|
(87
|
)
|
—
|
|
—
|
|
(87
|
)
|
||||||||
|
Repurchases of common stock
|
(62.3
|
)
|
—
|
|
(4,662
|
)
|
—
|
|
(4,662
|
)
|
||||||||
|
Balance at December 31, 2012
|
756.3
|
|
29,337
|
|
(10,423
|
)
|
146
|
|
19,060
|
|
||||||||
|
Net income
|
—
|
|
—
|
|
5,081
|
|
—
|
|
5,081
|
|
||||||||
|
Other comprehensive loss, net of tax
|
—
|
|
—
|
|
—
|
|
(307
|
)
|
(307
|
)
|
||||||||
|
Dividends
|
—
|
|
—
|
|
(1,521
|
)
|
—
|
|
(1,521
|
)
|
||||||||
|
Issuance of common stock in connection with the Company’s equity award programs
|
7.4
|
|
296
|
|
—
|
|
—
|
|
296
|
|
||||||||
|
Stock-based compensation
|
—
|
|
400
|
|
—
|
|
—
|
|
400
|
|
||||||||
|
Settlement of conversion value of convertible debt in excess of principal
|
—
|
|
(99
|
)
|
—
|
|
—
|
|
(99
|
)
|
||||||||
|
Settlement of convertible note hedge
|
—
|
|
99
|
|
—
|
|
—
|
|
99
|
|
||||||||
|
Settlement of warrants
|
—
|
|
(100
|
)
|
—
|
|
—
|
|
(100
|
)
|
||||||||
|
Tax impact related to employee stock-based compensation
|
—
|
|
(42
|
)
|
—
|
|
—
|
|
(42
|
)
|
||||||||
|
Repurchases of common stock
|
(9.1
|
)
|
—
|
|
(771
|
)
|
—
|
|
(771
|
)
|
||||||||
|
Balance at December 31, 2013
|
754.6
|
|
29,891
|
|
(7,634
|
)
|
(161
|
)
|
22,096
|
|
||||||||
|
Net income
|
—
|
|
—
|
|
5,158
|
|
—
|
|
5,158
|
|
||||||||
|
Other comprehensive income, net of tax
|
—
|
|
—
|
|
—
|
|
153
|
|
153
|
|
||||||||
|
Dividends
|
—
|
|
—
|
|
(1,995
|
)
|
—
|
|
(1,995
|
)
|
||||||||
|
Issuance of common stock in connection with the Company’s equity award programs
|
6.7
|
|
186
|
|
—
|
|
—
|
|
186
|
|
||||||||
|
Stock-based compensation
|
—
|
|
404
|
|
—
|
|
—
|
|
404
|
|
||||||||
|
Tax impact related to employee stock-based compensation
|
—
|
|
(71
|
)
|
—
|
|
—
|
|
(71
|
)
|
||||||||
|
Repurchases of common stock
|
(0.9
|
)
|
—
|
|
(153
|
)
|
—
|
|
(153
|
)
|
||||||||
|
Balance at December 31, 2014
|
760.4
|
|
$
|
30,410
|
|
$
|
(4,624
|
)
|
$
|
(8
|
)
|
$
|
25,778
|
|
||||
|
2014
|
2013
|
2012
|
|||||||||
|
Cash flows from operating activities:
|
|||||||||||
|
Net income
|
$
|
5,158
|
|
$
|
5,081
|
|
$
|
4,345
|
|
||
|
Depreciation and amortization
|
2,092
|
|
1,286
|
|
1,088
|
|
|||||
|
Stock-based compensation expense
|
408
|
|
403
|
|
362
|
|
|||||
|
Deferred income taxes
|
(108
|
)
|
(189
|
)
|
28
|
|
|||||
|
Property, plant and equipment impairments
|
97
|
|
19
|
|
178
|
|
|||||
|
Other items, net
|
(213
|
)
|
84
|
|
(74
|
)
|
|||||
|
Changes in operating assets and liabilities, net of acquisitions:
|
|||||||||||
|
Trade receivables, net
|
136
|
|
(38
|
)
|
348
|
|
|||||
|
Inventories
|
327
|
|
(7
|
)
|
(150
|
)
|
|||||
|
Other assets
|
(1
|
)
|
(59
|
)
|
124
|
|
|||||
|
Accounts payable
|
405
|
|
(184
|
)
|
161
|
|
|||||
|
Accrued income taxes
|
(103
|
)
|
(326
|
)
|
87
|
|
|||||
|
Legal reserve
|
—
|
|
—
|
|
(780
|
)
|
|||||
|
Other liabilities
|
357
|
|
221
|
|
165
|
|
|||||
|
Net cash provided by operating activities
|
8,555
|
|
6,291
|
|
5,882
|
|
|||||
|
Cash flows from investing activities:
|
|||||||||||
|
Purchases of property, plant and equipment
|
(718
|
)
|
(693
|
)
|
(689
|
)
|
|||||
|
Cash paid for acquisitions, net of cash acquired
|
(165
|
)
|
(9,434
|
)
|
(2,390
|
)
|
|||||
|
Purchases of intangible assets
|
(285
|
)
|
—
|
|
(25
|
)
|
|||||
|
Purchases of marketable securities
|
(25,878
|
)
|
(21,965
|
)
|
(26,241
|
)
|
|||||
|
Proceeds from sales of marketable securities
|
16,697
|
|
19,123
|
|
17,372
|
|
|||||
|
Proceeds from maturities of marketable securities
|
4,199
|
|
5,090
|
|
1,994
|
|
|||||
|
Change in restricted investments, net
|
533
|
|
(520
|
)
|
—
|
|
|||||
|
Other
|
(135
|
)
|
(70
|
)
|
(11
|
)
|
|||||
|
Net cash used in investing activities
|
(5,752
|
)
|
(8,469
|
)
|
(9,990
|
)
|
|||||
|
Cash flows from financing activities:
|
|||||||||||
|
Net proceeds from issuance of debt
|
4,476
|
|
8,054
|
|
4,933
|
|
|||||
|
Repayment of debt
|
(5,605
|
)
|
(3,371
|
)
|
(123
|
)
|
|||||
|
Repurchases of common stock
|
(138
|
)
|
(832
|
)
|
(4,607
|
)
|
|||||
|
Dividends paid
|
(1,851
|
)
|
(1,415
|
)
|
(1,118
|
)
|
|||||
|
Net proceeds from issuance of common stock in connection with the Company's equity award programs
|
186
|
|
296
|
|
1,288
|
|
|||||
|
Other
|
55
|
|
(6
|
)
|
46
|
|
|||||
|
Net cash (used in) provided by financing activities
|
(2,877
|
)
|
2,726
|
|
419
|
|
|||||
|
(Decrease) increase in cash and cash equivalents
|
(74
|
)
|
548
|
|
(3,689
|
)
|
|||||
|
Cash and cash equivalents at beginning of period
|
3,805
|
|
3,257
|
|
6,946
|
|
|||||
|
Cash and cash equivalents at end of period
|
$
|
3,731
|
|
$
|
3,805
|
|
$
|
3,257
|
|
||
|
During the year ended December 31, 2014
|
||||||||||||||||||||
|
Separation Costs
|
Asset Impairments
|
Accelerated Depreciation
|
Other
|
Total
|
||||||||||||||||
|
Cost of sales
|
$
|
—
|
|
$
|
81
|
|
$
|
23
|
|
$
|
—
|
|
$
|
104
|
|
|||||
|
Research and development
|
—
|
|
—
|
|
28
|
|
21
|
|
49
|
|
||||||||||
|
Selling, general and administrative
|
—
|
|
—
|
|
4
|
|
5
|
|
9
|
|
||||||||||
|
Other
|
377
|
|
6
|
|
—
|
|
13
|
|
396
|
|
||||||||||
|
Total
|
$
|
377
|
|
$
|
87
|
|
$
|
55
|
|
$
|
39
|
|
$
|
558
|
|
|||||
|
During the year ended December 31, 2014
|
|||||||||||
|
Separation Costs
|
Other
|
Total
|
|||||||||
|
Restructuring liabilities as of January 1, 2014
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Expense
|
353
|
|
32
|
|
385
|
|
|||||
|
Payments
|
(132
|
)
|
(9
|
)
|
(141
|
)
|
|||||
|
Restructuring liabilities as of December 31, 2014
|
$
|
221
|
|
$
|
23
|
|
$
|
244
|
|
||
|
•
|
In 2012, we determined that certain manufacturing facilities located in Boulder, Colorado, were no longer needed and accordingly, they were abandoned during the fourth quarter. This resulted in the write-off of the carrying value of the facility, which aggregated
$118 million
, during the year ended December 31, 2012. The amount is included in Cost of sales in the Consolidated Statement of Income.
|
|
•
|
During the years ended December 31, 2013 and 2012, we recorded certain charges aggregating approximately
$71 million
and
$175 million
, respectively, which are included in Other operating expenses in the Consolidated Statements of Income. The expenses are primarily severance-related. The 2012 charges also included expenses associated with abandoning leased facilities.
|
|
Total consideration transferred
|
$
|
9,517
|
|
|
Compensation expense
|
197
|
|
|
|
Total cash paid
|
$
|
9,714
|
|
|
Cash and cash equivalents
|
$
|
319
|
|
|
Marketable securities
|
337
|
|
|
|
Inventories
|
170
|
|
|
|
Indefinite-lived intangible assets - IPR&D
|
1,180
|
|
|
|
Finite-lived intangible assets - Developed product technology rights
|
6,190
|
|
|
|
Finite-lived intangible assets - Licensing rights
|
2,792
|
|
|
|
Goodwill
|
2,402
|
|
|
|
Convertible debt
|
(742
|
)
|
|
|
Assumed contingent consideration
|
(261
|
)
|
|
|
Deferred income taxes, net
|
(3,011
|
)
|
|
|
Other assets (liabilities), net
|
141
|
|
|
|
Total consideration
|
$
|
9,517
|
|
|
Years ended December 31,
|
|||||||
|
2013
|
2012
|
||||||
|
Pro forma net revenues
|
$
|
19,141
|
|
$
|
17,616
|
|
|
|
Pro forma net income
|
4,848
|
|
3,700
|
|
|||
|
Total consideration transferred
|
$
|
497
|
|
|
Settlement of preexisting relationship at fair value
|
(99
|
)
|
|
|
Total consideration transferred to acquire the Product Rights
|
$
|
398
|
|
|
Finite-lived intangible assets - Marketing-related rights
|
$
|
363
|
|
|
Finite-lived intangible assets - Developed product technology rights
|
11
|
|
|
|
Goodwill
|
3
|
|
|
|
Other assets
|
21
|
|
|
|
Total consideration
|
$
|
398
|
|
|
deCODE
|
KAI
|
MN
|
Micromet
|
|||||||||||||
|
IPR&D
|
$
|
—
|
|
$
|
240
|
|
$
|
—
|
|
$
|
570
|
|
||||
|
Developed product technology rights
|
—
|
|
—
|
|
81
|
|
—
|
|
||||||||
|
R&D technology rights
|
465
|
|
—
|
|
—
|
|
350
|
|
||||||||
|
Marketing-related rights
|
—
|
|
—
|
|
82
|
|
—
|
|
||||||||
|
Deferred income taxes, net
|
(37
|
)
|
(59
|
)
|
(45
|
)
|
(191
|
)
|
||||||||
|
Other assets (liabilities), net
|
(29
|
)
|
26
|
|
179
|
|
170
|
|
||||||||
|
Goodwill
|
—
|
|
125
|
|
380
|
|
247
|
|
||||||||
|
Total consideration
|
$
|
399
|
|
$
|
332
|
|
$
|
677
|
|
|
$
|
1,146
|
|
|||
|
Years ended December 31,
|
|||||||||||
|
2014
|
2013
|
2012
|
|||||||||
|
RSUs
|
$
|
219
|
|
$
|
206
|
|
$
|
186
|
|
||
|
Performance units
|
171
|
|
163
|
|
117
|
|
|||||
|
Stock options
|
18
|
|
34
|
|
59
|
|
|||||
|
Total stock-based compensation expense, pretax
|
408
|
|
403
|
|
362
|
|
|||||
|
Tax benefit from stock-based compensation expense
|
(152
|
)
|
(149
|
)
|
(134
|
)
|
|||||
|
Total stock-based compensation expense, net of tax
|
$
|
256
|
|
$
|
254
|
|
$
|
228
|
|
||
|
During the year ended December 31, 2014
|
||||||
|
Units
(in millions)
|
Weighted-average
grant date
fair value
|
|||||
|
Balance nonvested at December 31, 2013
|
8.8
|
|
$
|
76.75
|
|
|
|
Granted
|
2.3
|
|
$
|
115.63
|
|
|
|
Vested
|
(3.0
|
)
|
$
|
63.36
|
|
|
|
Forfeited
|
(1.0
|
)
|
$
|
90.77
|
|
|
|
Balance nonvested at December 31, 2014
|
7.1
|
|
$
|
92.88
|
|
|
|
During the year ended December 31, 2014
|
||||||||||||
|
Options
(in millions)
|
Weighted-
average
exercise price
|
Weighted-
average
remaining
contractual
life (years)
|
Aggregate
intrinsic
value
(in millions)
|
|||||||||
|
Balance unexercised at December 31, 2013
|
7.4
|
|
$
|
54.91
|
|
|||||||
|
Granted
|
—
|
|
$
|
—
|
|
|||||||
|
Exercised
|
(3.1
|
)
|
$
|
55.42
|
|
|||||||
|
Expired/forfeited
|
(0.2
|
)
|
$
|
56.18
|
|
|||||||
|
Balance unexercised at December 31, 2014
|
4.1
|
|
$
|
54.48
|
|
4.2
|
$
|
432
|
|
|||
|
Vested or expected to vest at December 31, 2014
|
4.1
|
|
$
|
54.48
|
|
4.2
|
$
|
432
|
|
|||
|
Exercisable at December 31, 2014
|
3.5
|
|
$
|
54.45
|
|
3.9
|
$
|
371
|
|
|||
|
Years ended December 31,
|
|||||||||||
|
2014
|
2013
|
2012
|
|||||||||
|
Closing price of our common stock on grant date
|
$
|
112.43
|
|
$
|
92.03
|
|
$
|
68.75
|
|
||
|
Volatility
|
23.8
|
%
|
21.0
|
%
|
22.9
|
%
|
|||||
|
Risk-free interest rate
|
0.8
|
%
|
0.4
|
%
|
0.5
|
%
|
|||||
|
Fair value of unit
|
$
|
104.47
|
|
$
|
102.73
|
|
$
|
78.21
|
|
||
|
Years ended December 31,
|
|||||||||||
|
2014
|
2013
|
2012
|
|||||||||
|
Current provision:
|
|||||||||||
|
Federal
|
$
|
251
|
|
$
|
54
|
|
$
|
438
|
|
||
|
State
|
58
|
|
26
|
|
47
|
|
|||||
|
Foreign
|
194
|
|
191
|
|
158
|
|
|||||
|
Total current provision
|
503
|
|
271
|
|
643
|
|
|||||
|
Deferred provision (benefit):
|
|||||||||||
|
Federal
|
(22
|
)
|
(86
|
)
|
83
|
|
|||||
|
State
|
(4
|
)
|
19
|
|
(43
|
)
|
|||||
|
Foreign
|
(50
|
)
|
(20
|
)
|
(19
|
)
|
|||||
|
Total deferred provision (benefit)
|
(76
|
)
|
(87
|
)
|
21
|
|
|||||
|
Total provision
|
$
|
427
|
|
$
|
184
|
|
$
|
664
|
|
||
|
December 31,
|
|||||||
|
2014
|
2013
|
||||||
|
Deferred income tax assets:
|
|||||||
|
NOL and credit carryforwards
|
$
|
588
|
|
$
|
1,017
|
|
|
|
Expense accruals
|
730
|
|
697
|
|
|||
|
Expenses capitalized for tax
|
221
|
|
196
|
|
|||
|
Stock-based compensation
|
206
|
|
211
|
|
|||
|
Other
|
191
|
|
144
|
|
|||
|
Total deferred income tax assets
|
1,936
|
|
2,265
|
|
|||
|
Valuation allowance
|
(336
|
)
|
(314
|
)
|
|||
|
Net deferred income tax assets
|
1,600
|
|
1,951
|
|
|||
|
Deferred income tax liabilities:
|
|||||||
|
Acquired intangibles
|
(4,089
|
)
|
(4,430
|
)
|
|||
|
Other
|
(232
|
)
|
(263
|
)
|
|||
|
Total deferred income tax liabilities
|
(4,321
|
)
|
(4,693
|
)
|
|||
|
Total deferred income taxes, net
|
$
|
(2,721
|
)
|
$
|
(2,742
|
)
|
|
|
During the years ended December 31,
|
|||||||||||
|
2014
|
2013
|
2012
|
|||||||||
|
Balance at beginning of year
|
$
|
1,415
|
|
$
|
1,200
|
|
$
|
975
|
|
||
|
Additions based on tax positions related to the current year
|
379
|
|
335
|
|
300
|
|
|||||
|
Additions based on tax positions related to prior years
|
37
|
|
96
|
|
5
|
|
|||||
|
Reductions for tax positions of prior years
|
(45
|
)
|
(192
|
)
|
(50
|
)
|
|||||
|
Reductions for expiration of statute of limitations
|
(12
|
)
|
—
|
|
—
|
|
|||||
|
Settlements
|
(2
|
)
|
(24
|
)
|
(30
|
)
|
|||||
|
Balance at end of year
|
$
|
1,772
|
|
$
|
1,415
|
|
$
|
1,200
|
|
||
|
Years ended December 31,
|
||||||||
|
2014
|
2013
|
2012
|
||||||
|
Federal statutory tax rate
|
35.0
|
%
|
35.0
|
%
|
35.0
|
%
|
||
|
Foreign earnings, including earnings invested indefinitely
|
(22.4
|
)%
|
(21.3
|
)%
|
(17.8
|
)%
|
||
|
Credits, Puerto Rico Excise Tax
|
(4.4
|
)%
|
(4.7
|
)%
|
(5.2
|
)%
|
||
|
Credits, primarily federal R&D
|
(1.5
|
)%
|
(3.0
|
)%
|
0.0
|
%
|
||
|
State taxes
|
0.7
|
%
|
0.8
|
%
|
0.6
|
%
|
||
|
Audit settlements (federal, state, foreign)
|
0.0
|
%
|
(3.7
|
)%
|
0.3
|
%
|
||
|
Other, net
|
0.2
|
%
|
0.4
|
%
|
0.4
|
%
|
||
|
Effective tax rate
|
7.6
|
%
|
3.5
|
%
|
13.3
|
%
|
||
|
Years ended December 31,
|
|||||||||||
|
2014
|
2013
|
2012
|
|||||||||
|
Income (Numerator):
|
|||||||||||
|
Net income for basic and diluted EPS
|
$
|
5,158
|
|
$
|
5,081
|
|
$
|
4,345
|
|
||
|
Shares (Denominator):
|
|||||||||||
|
Weighted-average shares for basic EPS
|
759
|
|
753
|
|
775
|
|
|||||
|
Effect of dilutive securities
|
11
|
|
12
|
|
12
|
|
|||||
|
Weighted-average shares for diluted EPS
|
770
|
|
765
|
|
787
|
|
|||||
|
Basic EPS
|
$
|
6.80
|
|
$
|
6.75
|
|
$
|
5.61
|
|
||
|
Diluted EPS
|
$
|
6.70
|
|
$
|
6.64
|
|
$
|
5.52
|
|
||
|
Type of security as of December 31, 2014
|
Amortized
cost
|
Gross
unrealized
gains
|
Gross
unrealized
losses
|
Estimated
fair value
|
|||||||||||
|
U.S. Treasury securities
|
$
|
3,632
|
|
$
|
22
|
|
$
|
(8
|
)
|
$
|
3,646
|
|
|||
|
Other government-related debt securities:
|
|||||||||||||||
|
U.S.
|
530
|
|
1
|
|
(3
|
)
|
528
|
|
|||||||
|
Foreign and other
|
1,572
|
|
21
|
|
(24
|
)
|
1,569
|
|
|||||||
|
Corporate debt securities:
|
|||||||||||||||
|
Financial
|
6,036
|
|
21
|
|
(16
|
)
|
6,041
|
|
|||||||
|
Industrial
|
6,394
|
|
23
|
|
(66
|
)
|
6,351
|
|
|||||||
|
Other
|
650
|
|
3
|
|
(4
|
)
|
649
|
|
|||||||
|
Residential mortgage-backed securities
|
1,708
|
|
4
|
|
(10
|
)
|
1,702
|
|
|||||||
|
Other mortgage- and asset-backed securities
|
1,837
|
|
—
|
|
(41
|
)
|
1,796
|
|
|||||||
|
Money market mutual funds
|
3,004
|
|
—
|
|
—
|
|
3,004
|
|
|||||||
|
Other short-term interest-bearing securities
|
1,302
|
|
—
|
|
—
|
|
1,302
|
|
|||||||
|
Total interest-bearing securities
|
26,665
|
|
95
|
|
(172
|
)
|
26,588
|
|
|||||||
|
Equity securities
|
98
|
|
48
|
|
(2
|
)
|
144
|
|
|||||||
|
Total available-for-sale investments
|
$
|
26,763
|
|
$
|
143
|
|
$
|
(174
|
)
|
$
|
26,732
|
|
|||
|
Type of security as of December 31, 2013
|
Amortized
cost
|
Gross
unrealized
gains
|
Gross
unrealized
losses
|
Estimated
fair value
|
|||||||||||
|
U.S. Treasury securities
|
$
|
4,737
|
|
$
|
2
|
|
$
|
(9
|
)
|
$
|
4,730
|
|
|||
|
Other government-related debt securities:
|
|||||||||||||||
|
U.S.
|
1,087
|
|
—
|
|
(8
|
)
|
1,079
|
|
|||||||
|
Foreign and other
|
1,574
|
|
13
|
|
(41
|
)
|
1,546
|
|
|||||||
|
Corporate debt securities:
|
|||||||||||||||
|
Financial
|
3,667
|
|
28
|
|
(19
|
)
|
3,676
|
|
|||||||
|
Industrial
|
3,745
|
|
36
|
|
(21
|
)
|
3,760
|
|
|||||||
|
Other
|
388
|
|
4
|
|
(2
|
)
|
390
|
|
|||||||
|
Residential mortgage-backed securities
|
1,478
|
|
3
|
|
(21
|
)
|
1,460
|
|
|||||||
|
Other mortgage- and asset-backed securities
|
1,555
|
|
1
|
|
(45
|
)
|
1,511
|
|
|||||||
|
Money market mutual funds
|
3,366
|
|
—
|
|
—
|
|
3,366
|
|
|||||||
|
Other short-term interest-bearing securities
|
750
|
|
—
|
|
—
|
|
750
|
|
|||||||
|
Total interest-bearing securities
|
22,347
|
|
87
|
|
(166
|
)
|
22,268
|
|
|||||||
|
Equity securities
|
85
|
|
10
|
|
—
|
|
95
|
|
|||||||
|
Total available-for-sale investments
|
$
|
22,432
|
|
$
|
97
|
|
$
|
(166
|
)
|
$
|
22,363
|
|
|||
|
December 31,
|
|||||||
|
Classification in the Consolidated Balance Sheets
|
2014
|
2013
|
|||||
|
Cash and cash equivalents
|
$
|
3,293
|
|
$
|
3,266
|
|
|
|
Marketable securities
|
23,295
|
|
15,596
|
|
|||
|
Other assets — noncurrent
|
144
|
|
95
|
|
|||
|
Restricted investments
|
—
|
|
3,406
|
|
|||
|
Total available-for-sale investments
|
$
|
26,732
|
|
$
|
22,363
|
|
|
|
December 31,
|
|||||||
|
Contractual maturity
|
2014
|
2013
|
|||||
|
Maturing in one year or less
|
$
|
4,936
|
|
$
|
6,799
|
|
|
|
Maturing after one year through three years
|
6,829
|
|
4,785
|
|
|||
|
Maturing after three years through five years
|
7,840
|
|
6,057
|
|
|||
|
Maturing after five years through ten years
|
3,267
|
|
1,656
|
|
|||
|
Maturing after ten years
|
218
|
|
—
|
|
|||
|
Mortgage- and asset-backed securities
|
3,498
|
|
2,971
|
|
|||
|
Total interest-bearing securities
|
$
|
26,588
|
|
$
|
22,268
|
|
|
|
Less than 12 months
|
12 months or greater
|
|||||||||||||||
|
Type of security as of December 31, 2014
|
Fair value
|
Unrealized losses
|
Fair value
|
Unrealized losses
|
||||||||||||
|
U.S. Treasury securities
|
$
|
1,770
|
|
$
|
(7
|
)
|
$
|
171
|
|
$
|
(1
|
)
|
||||
|
Other government-related debt securities:
|
||||||||||||||||
|
U.S.
|
160
|
|
—
|
|
178
|
|
(3
|
)
|
||||||||
|
Foreign and other
|
514
|
|
(14
|
)
|
159
|
|
(10
|
)
|
||||||||
|
Corporate debt securities:
|
||||||||||||||||
|
Financial
|
3,150
|
|
(14
|
)
|
158
|
|
(2
|
)
|
||||||||
|
Industrial
|
3,931
|
|
(62
|
)
|
222
|
|
(4
|
)
|
||||||||
|
Other
|
354
|
|
(4
|
)
|
5
|
|
—
|
|
||||||||
|
Residential mortgage-backed securities
|
614
|
|
(4
|
)
|
413
|
|
(6
|
)
|
||||||||
|
Other mortgage- and asset-backed securities
|
1,071
|
|
(8
|
)
|
561
|
|
(33
|
)
|
||||||||
|
Equity securities
|
78
|
|
(2
|
)
|
—
|
|
—
|
|
||||||||
|
Total
|
$
|
11,642
|
|
$
|
(115
|
)
|
$
|
1,867
|
|
$
|
(59
|
)
|
||||
|
Less than 12 months
|
12 months or greater
|
|||||||||||||||
|
Type of security as of December 31, 2013
|
Fair value
|
Unrealized losses
|
Fair value
|
Unrealized losses
|
||||||||||||
|
U.S. Treasury securities
|
$
|
2,362
|
|
$
|
(9
|
)
|
$
|
—
|
|
$
|
—
|
|
||||
|
Other government-related debt securities:
|
||||||||||||||||
|
U.S.
|
789
|
|
(8
|
)
|
—
|
|
—
|
|
||||||||
|
Foreign and other
|
986
|
|
(38
|
)
|
39
|
|
(3
|
)
|
||||||||
|
Corporate debt securities:
|
||||||||||||||||
|
Financial
|
1,781
|
|
(19
|
)
|
—
|
|
—
|
|
||||||||
|
Industrial
|
1,543
|
|
(21
|
)
|
1
|
|
—
|
|
||||||||
|
Other
|
182
|
|
(2
|
)
|
—
|
|
—
|
|
||||||||
|
Residential mortgage-backed securities
|
794
|
|
(14
|
)
|
257
|
|
(7
|
)
|
||||||||
|
Other mortgage- and asset-backed securities
|
982
|
|
(29
|
)
|
313
|
|
(16
|
)
|
||||||||
|
Total
|
$
|
9,419
|
|
$
|
(140
|
)
|
$
|
610
|
|
$
|
(26
|
)
|
||||
|
December 31,
|
|||||||
|
2014
|
2013
|
||||||
|
Raw materials
|
$
|
198
|
|
$
|
217
|
|
|
|
Work in process
|
1,551
|
|
2,064
|
|
|||
|
Finished goods
|
898
|
|
738
|
|
|||
|
Total inventories
|
$
|
2,647
|
|
$
|
3,019
|
|
|
|
December 31,
|
||||||||||
|
Useful life (in years)
|
2014
|
2013
|
||||||||
|
Land
|
—
|
|
$
|
398
|
|
$
|
408
|
|
||
|
Buildings and improvements
|
10-40
|
|
3,612
|
|
3,467
|
|
||||
|
Manufacturing equipment
|
8-12
|
|
1,711
|
|
2,024
|
|
||||
|
Laboratory equipment
|
8-12
|
|
1,240
|
|
1,165
|
|
||||
|
Other
|
3-15
|
|
4,112
|
|
4,107
|
|
||||
|
Construction in progress
|
—
|
|
1,183
|
|
1,120
|
|
||||
|
Property, plant and equipment, gross
|
12,256
|
|
12,291
|
|
||||||
|
Less accumulated depreciation and amortization
|
(7,033
|
)
|
(6,942
|
)
|
||||||
|
Property, plant and equipment, net
|
$
|
5,223
|
|
$
|
5,349
|
|
||||
|
During the years ended December 31,
|
|||||||
|
2014
|
2013
|
||||||
|
Beginning balance
|
$
|
14,968
|
|
$
|
12,662
|
|
|
|
Goodwill related to acquisitions of businesses
(1)
|
(114
|
)
|
2,397
|
|
|||
|
Currency translation and other adjustments
|
(66
|
)
|
(91
|
)
|
|||
|
Ending balance
|
$
|
14,788
|
|
$
|
14,968
|
|
|
|
(1)
|
Composed of goodwill recognized on the acquisition dates of business combinations and subsequent adjustments to these amounts resulting from changes to the acquisition date fair values of net assets acquired in the business combinations recorded during their respective measurement periods.
|
|
December 31,
|
|||||||||||||||||||||||
|
|
2014
|
2013
|
|||||||||||||||||||||
|
|
Gross
carrying
amount
|
Accumulated
amortization
|
Intangible
assets, net
|
Gross
carrying
amount
|
Accumulated
amortization
|
Intangible
assets, net
|
|||||||||||||||||
|
Finite-lived intangible assets:
|
|||||||||||||||||||||||
|
Developed product technology rights
|
$
|
10,826
|
|
$
|
(4,155
|
)
|
$
|
6,671
|
|
$
|
10,130
|
|
$
|
(3,347
|
)
|
$
|
6,783
|
|
|||||
|
Licensing rights
|
3,236
|
|
(696
|
)
|
2,540
|
|
3,241
|
|
(366
|
)
|
2,875
|
|
|||||||||||
|
R&D technology rights
|
1,167
|
|
(569
|
)
|
598
|
|
1,207
|
|
(496
|
)
|
711
|
|
|||||||||||
|
Marketing-related rights
|
1,241
|
|
(512
|
)
|
729
|
|
619
|
|
(366
|
)
|
253
|
|
|||||||||||
|
Total finite-lived intangible assets
|
16,470
|
|
(5,932
|
)
|
10,538
|
|
15,197
|
|
(4,575
|
)
|
10,622
|
|
|||||||||||
|
Indefinite-lived intangible assets:
|
|||||||||||||||||||||||
|
IPR&D
|
2,155
|
|
—
|
|
2,155
|
|
2,640
|
|
—
|
|
2,640
|
|
|||||||||||
|
Total identifiable intangible assets
|
$
|
18,625
|
|
$
|
(5,932
|
)
|
$
|
12,693
|
|
$
|
17,837
|
|
$
|
(4,575
|
)
|
$
|
13,262
|
|
|||||
|
December 31,
|
|||||||
|
2014
|
2013
|
||||||
|
Sales deductions
|
$
|
1,379
|
|
$
|
1,248
|
|
|
|
Employee compensation and benefits
|
920
|
|
1,003
|
|
|||
|
Clinical development costs
|
445
|
|
522
|
|
|||
|
Dividends payable
|
601
|
|
460
|
|
|||
|
Sales returns reserve
|
361
|
|
295
|
|
|||
|
Other
|
1,590
|
|
1,127
|
|
|||
|
Total accrued liabilities
|
$
|
5,296
|
|
$
|
4,655
|
|
|
|
December 31,
|
|||||||
|
2014
|
2013
|
||||||
|
1.875% notes due 2014 (1.875% 2014 Notes)
|
$
|
—
|
|
$
|
1,000
|
|
|
|
4.85% notes due 2014 (4.85% 2014 Notes)
|
—
|
|
1,000
|
|
|||
|
2.30% notes due 2016 (2.30% 2016 Notes)
|
749
|
|
749
|
|
|||
|
2.50% notes due 2016 (2.50% 2016 Notes)
|
1,000
|
|
999
|
|
|||
|
Floating Rate Notes due 2017
|
600
|
|
—
|
|
|||
|
1.25% notes due 2017 (1.25% 2017 Notes)
|
849
|
|
—
|
|
|||
|
2.125% notes due 2017 (2.125% 2017 Notes)
|
1,249
|
|
1,248
|
|
|||
|
5.85% notes due 2017 (5.85% 2017 Notes)
|
1,100
|
|
1,099
|
|
|||
|
6.15% notes due 2018 (6.15% 2018 Notes)
|
500
|
|
500
|
|
|||
|
Master Repurchase Agreement obligation due 2018
|
—
|
|
3,100
|
|
|||
|
Term Loan due 2018
|
4,375
|
|
4,875
|
|
|||
|
4.375% euro-denominated notes due 2018 (4.375% 2018 euro Notes)
|
668
|
|
751
|
|
|||
|
Floating Rate Notes due 2019
|
250
|
|
—
|
|
|||
|
2.20% notes due 2019 (2.20% 2019 Notes)
|
1,398
|
|
—
|
|
|||
|
5.70% notes due 2019 (5.70% 2019 Notes)
|
999
|
|
999
|
|
|||
|
2.125% euro-denominated notes due 2019 (2.125% 2019 euro Notes)
|
814
|
|
925
|
|
|||
|
4.50% notes due 2020 (4.50% 2020 Notes)
|
300
|
|
300
|
|
|||
|
3.45% notes due 2020 (3.45% 2020 Notes)
|
898
|
|
898
|
|
|||
|
4.10% notes due 2021 (4.10% 2021 Notes)
|
998
|
|
998
|
|
|||
|
3.875% notes due 2021 (3.875% 2021 Notes)
|
1,747
|
|
1,746
|
|
|||
|
3.625% notes due 2022 (3.625% 2022 Notes)
|
747
|
|
747
|
|
|||
|
3.625% notes due 2024 (3.625% 2024 Notes)
|
1,398
|
|
—
|
|
|||
|
5.50% pound-sterling-denominated notes due 2026 (5.50% 2026 pound sterling Notes)
|
735
|
|
781
|
|
|||
|
4.00% pound-sterling-denominated notes due 2029 (4.00% 2029 pound sterling Notes)
|
1,076
|
|
1,144
|
|
|||
|
6.375% notes due 2037 (6.375% 2037 Notes)
|
899
|
|
899
|
|
|||
|
6.90% notes due 2038 (6.90% 2038 Notes)
|
499
|
|
499
|
|
|||
|
6.40% notes due 2039 (6.40% 2039 Notes)
|
996
|
|
996
|
|
|||
|
5.75% notes due 2040 (5.75% 2040 Notes)
|
697
|
|
697
|
|
|||
|
4.95% notes due 2041 (4.95% 2041 Notes)
|
596
|
|
596
|
|
|||
|
5.15% notes due 2041 (5.15% 2041 Notes)
|
2,233
|
|
2,233
|
|
|||
|
5.65% notes due 2042 (5.65% 2042 Notes)
|
1,245
|
|
1,244
|
|
|||
|
5.375% notes due 2043 (5.375% 2043 Notes)
|
1,000
|
|
1,000
|
|
|||
|
Other notes
|
100
|
|
105
|
|
|||
|
Total debt
|
30,715
|
|
32,128
|
|
|||
|
Less current portion
|
(500
|
)
|
(2,505
|
)
|
|||
|
Total noncurrent debt
|
$
|
30,215
|
|
$
|
29,623
|
|
|
|
•
|
In 2014, we issued
$4.5 billion
aggregate principal amount of notes, comprised of the Floating Rate Notes due 2017, the
1.25%
2017 Notes, the Floating Rate Notes due 2019, the
2.20%
2019 Notes and the
3.625%
2024 Notes. The Floating Rate Notes due in 2017 and 2019 bear interest equal to three-month London Interbank Offered Rates (LIBOR) plus
0.38%
and three-month LIBOR plus
0.60%
, respectively, and are not subject to redemption at our option. The fixed rate notes that were issued may be redeemed at any time at our option, in whole or in part, at the principal amount of the notes being redeemed plus accrued and unpaid interest and, except as discussed below, a make-whole amount, as defined. The
2.20%
2019 Notes and
3.625%
2024 Notes may be redeemed without payment of a make-whole amount if they are redeemed on or after one month or three months, respectively, prior to their maturity dates. In the event of a change-in-control triggering event, as defined, we may be required to purchase all or a portion of the notes at a price equal to
101%
of the principal amount of the notes plus accrued and unpaid interest.
|
|
•
|
In 2013, we issued
$8.1 billion
of debt in connection with the acquisition of Onyx, comprised of obligations under a Master Repurchase Agreement and a Term Loan.
|
|
•
|
In 2012, we issued
$5.0 billion
aggregate principal amount of notes, comprised of the
2.125%
2017 Notes, the
2.125%
2019 euro Notes (
€675 million
aggregate principal amount), the
3.625%
2022 Notes, the
4.00%
2029 pound sterling Notes (
£700 million
aggregate principal amount) and the
5.375%
2043 Notes.
|
|
Originating during year ended December 31, 2014
|
|||||
|
Notes
|
Effective interest rate
|
Notional amount
|
|||
|
1.25% 2017 Notes
|
LIBOR + 0.4%
|
$
|
850
|
|
|
|
2.20% 2019 Notes
|
LIBOR + 0.6%
|
1,400
|
|
||
|
$
|
2,250
|
|
|||
|
Originating during year ended December 31, 2013
|
|||||
|
Notes
|
Effective interest rate
|
Notional amount
|
|||
|
3.45% 2020 Notes
|
LIBOR + 1.1%
|
$
|
900
|
|
|
|
4.10% 2021 Notes
|
LIBOR + 1.7%
|
1,000
|
|
||
|
3.875% 2021 Notes
|
LIBOR + 2.0%
|
1,750
|
|
||
|
3.625% 2022 Notes
|
LIBOR + 1.6%
|
750
|
|
||
|
$
|
4,400
|
|
|||
|
Maturity date
|
Amount
|
||
|
2015
|
$
|
500
|
|
|
2016
|
2,250
|
|
|
|
2017
|
4,300
|
|
|
|
2018
|
4,045
|
|
|
|
2019
|
3,467
|
|
|
|
Thereafter
|
16,230
|
|
|
|
Total
|
$
|
30,792
|
|
|
During the years ended December 31,
|
||||||||||||||||||||
|
2014
|
2013
|
2012
|
||||||||||||||||||
|
Shares
|
Dollars
|
Shares
|
Dollars
|
Shares
|
Dollars
|
|||||||||||||||
|
First quarter
|
—
|
|
$
|
—
|
|
9.1
|
|
$
|
771
|
|
21.0
|
|
$
|
1,429
|
|
|||||
|
Second quarter
|
—
|
|
—
|
|
—
|
|
—
|
|
17.4
|
|
1,203
|
|
||||||||
|
Third quarter
|
—
|
|
—
|
|
—
|
|
—
|
|
9.7
|
|
797
|
|
||||||||
|
Fourth quarter
|
0.9
|
|
153
|
|
—
|
|
—
|
|
14.2
|
|
1,233
|
|
||||||||
|
Total stock repurchases
|
0.9
|
|
$
|
153
|
|
9.1
|
|
$
|
771
|
|
62.3
|
|
$
|
4,662
|
|
|||||
|
Foreign
currency
translation
|
Cash flow
hedges
|
Available-for-sale
securities
|
Other
|
AOCI
|
|||||||||||||||
|
Balance as of December 31, 2011
|
$
|
21
|
|
$
|
43
|
|
$
|
120
|
|
$
|
(13
|
)
|
$
|
171
|
|
||||
|
Foreign currency translation adjustments
|
(13
|
)
|
—
|
|
—
|
|
—
|
|
(13
|
)
|
|||||||||
|
Unrealized gains (losses)
|
—
|
|
15
|
|
233
|
|
(1
|
)
|
247
|
|
|||||||||
|
Reclassification adjustments to income
|
—
|
|
(134
|
)
|
(132
|
)
|
—
|
|
(266
|
)
|
|||||||||
|
Income taxes
|
4
|
|
41
|
|
(38
|
)
|
—
|
|
7
|
|
|||||||||
|
Balance as of December 31, 2012
|
12
|
|
(35
|
)
|
183
|
|
(14
|
)
|
146
|
|
|||||||||
|
Foreign currency translation adjustments
|
(71
|
)
|
—
|
|
—
|
|
—
|
|
(71
|
)
|
|||||||||
|
Unrealized gains (losses)
|
—
|
|
88
|
|
(284
|
)
|
(1
|
)
|
(197
|
)
|
|||||||||
|
Reclassification adjustments to income
|
—
|
|
(85
|
)
|
(75
|
)
|
—
|
|
(160
|
)
|
|||||||||
|
Other
|
—
|
|
—
|
|
—
|
|
(2
|
)
|
(2
|
)
|
|||||||||
|
Income taxes
|
(9
|
)
|
(1
|
)
|
133
|
|
—
|
|
123
|
|
|||||||||
|
Balance as of December 31, 2013
|
(68
|
)
|
(33
|
)
|
(43
|
)
|
(17
|
)
|
(161
|
)
|
|||||||||
|
Foreign currency translation adjustments
|
(218
|
)
|
—
|
|
—
|
|
—
|
|
(218
|
)
|
|||||||||
|
Unrealized gains
|
—
|
|
298
|
|
37
|
|
1
|
|
336
|
|
|||||||||
|
Reclassification adjustments to income
|
—
|
|
203
|
|
1
|
|
—
|
|
204
|
|
|||||||||
|
Other
|
—
|
|
—
|
|
—
|
|
1
|
|
1
|
|
|||||||||
|
Income taxes
|
22
|
|
(178
|
)
|
(14
|
)
|
—
|
|
(170
|
)
|
|||||||||
|
Balance as of December 31, 2014
|
$
|
(264
|
)
|
$
|
290
|
|
$
|
(19
|
)
|
$
|
(15
|
)
|
$
|
(8
|
)
|
||||
|
Amounts reclassified out of AOCI
|
||||||||||
|
Components of AOCI
|
Year ended December 31, 2014
|
Year ended December 31, 2013
|
Line item affected in the Statements of Income
|
|||||||
|
Cash flow hedges:
|
||||||||||
|
Foreign currency contract gains
|
$
|
28
|
|
$
|
4
|
|
Product sales
|
|||
|
Cross-currency swap contract (losses) gains
|
(230
|
)
|
82
|
|
Interest and other income, net
|
|||||
|
Forward interest rate contract losses
|
(1
|
)
|
(1
|
)
|
Interest expense, net
|
|||||
|
(203
|
)
|
85
|
|
Total before income tax
|
||||||
|
74
|
|
(33
|
)
|
Tax benefit (expense)
|
||||||
|
$
|
(129
|
)
|
$
|
52
|
|
Net of taxes
|
||||
|
Available-for-sale securities:
|
||||||||||
|
Net realized (losses) gains
|
$
|
(1
|
)
|
$
|
75
|
|
Interest and other income, net
|
|||
|
—
|
|
(28
|
)
|
Tax expense
|
||||||
|
$
|
(1
|
)
|
$
|
47
|
|
Net of taxes
|
||||
|
Level 1
|
—
|
Valuations based on unadjusted quoted prices in active markets for identical assets or liabilities that the Company has the ability to access
|
|
Level 2
|
—
|
Valuations for which all significant inputs are observable, either directly or indirectly, other than level 1 inputs
|
|
Level 3
|
—
|
Valuations based on inputs that are unobservable and significant to the overall fair value measurement
|
|
Fair value measurement as of December 31, 2014, using:
|
Quoted prices in
active markets for
identical assets
(Level 1)
|
Significant other
observable
inputs
(Level 2)
|
Significant
unobservable
inputs
(Level 3)
|
Total
|
||||||||||||
|
Assets:
|
||||||||||||||||
|
Available-for-sale investments:
|
||||||||||||||||
|
U.S. Treasury securities
|
$
|
3,646
|
|
$
|
—
|
|
$
|
—
|
|
$
|
3,646
|
|
||||
|
Other government-related debt securities:
|
||||||||||||||||
|
U.S.
|
—
|
|
528
|
|
—
|
|
528
|
|
||||||||
|
Foreign and other
|
—
|
|
1,569
|
|
—
|
|
1,569
|
|
||||||||
|
Corporate debt securities:
|
|
|
|
|
||||||||||||
|
Financial
|
—
|
|
6,041
|
|
—
|
|
6,041
|
|
||||||||
|
Industrial
|
—
|
|
6,351
|
|
—
|
|
6,351
|
|
||||||||
|
Other
|
—
|
|
649
|
|
—
|
|
649
|
|
||||||||
|
Residential mortgage-backed securities
|
—
|
|
1,702
|
|
—
|
|
1,702
|
|
||||||||
|
Other mortgage- and asset-backed securities
|
—
|
|
1,796
|
|
—
|
|
1,796
|
|
||||||||
|
Money market mutual funds
|
3,004
|
|
—
|
|
—
|
|
3,004
|
|
||||||||
|
Other short-term interest bearing securities
|
—
|
|
1,302
|
|
—
|
|
1,302
|
|
||||||||
|
Equity securities
|
144
|
|
—
|
|
—
|
|
144
|
|
||||||||
|
Derivatives:
|
|
|
|
|
||||||||||||
|
Foreign currency contracts
|
—
|
|
360
|
|
—
|
|
360
|
|
||||||||
|
Cross-currency swap contracts
|
—
|
|
32
|
|
—
|
|
32
|
|
||||||||
|
Interest rate swap contracts
|
—
|
|
46
|
|
—
|
|
46
|
|
||||||||
|
Total assets
|
$
|
6,794
|
|
$
|
20,376
|
|
$
|
—
|
|
$
|
27,170
|
|
||||
|
Liabilities:
|
||||||||||||||||
|
Derivatives:
|
||||||||||||||||
|
Foreign currency contracts
|
$
|
—
|
|
$
|
4
|
|
$
|
—
|
|
$
|
4
|
|
||||
|
Cross-currency swap contracts
|
—
|
|
12
|
|
—
|
|
12
|
|
||||||||
|
Interest rate swap contracts
|
—
|
|
26
|
|
—
|
|
26
|
|
||||||||
|
Contingent consideration obligations in connection with a business combination
|
—
|
|
—
|
|
215
|
|
215
|
|
||||||||
|
Total liabilities
|
$
|
—
|
|
$
|
42
|
|
$
|
215
|
|
$
|
257
|
|
||||
|
Fair value measurement as of December 31, 2013, using:
|
Quoted prices in
active markets for
identical assets
(Level 1)
|
Significant other
observable
inputs
(Level 2)
|
Significant
unobservable
inputs
(Level 3)
|
Total
|
||||||||||||
|
Assets:
|
||||||||||||||||
|
Available-for-sale investments:
|
||||||||||||||||
|
U.S. Treasury securities
|
$
|
4,730
|
|
$
|
—
|
|
$
|
—
|
|
$
|
4,730
|
|
||||
|
Other government-related debt securities:
|
||||||||||||||||
|
U.S.
|
—
|
|
1,079
|
|
—
|
|
1,079
|
|
||||||||
|
Foreign and other
|
—
|
|
1,546
|
|
—
|
|
1,546
|
|
||||||||
|
Corporate debt securities:
|
|
|
|
|
||||||||||||
|
Financial
|
—
|
|
3,676
|
|
—
|
|
3,676
|
|
||||||||
|
Industrial
|
—
|
|
3,760
|
|
—
|
|
3,760
|
|
||||||||
|
Other
|
—
|
|
390
|
|
—
|
|
390
|
|
||||||||
|
Residential mortgage-backed securities
|
—
|
|
1,460
|
|
—
|
|
1,460
|
|
||||||||
|
Other mortgage- and asset-backed securities
|
—
|
|
1,511
|
|
—
|
|
1,511
|
|
||||||||
|
Money market mutual funds
|
3,366
|
|
—
|
|
—
|
|
3,366
|
|
||||||||
|
Other short-term interest-bearing securities
|
—
|
|
750
|
|
—
|
|
750
|
|
||||||||
|
Equity securities
|
95
|
|
—
|
|
—
|
|
95
|
|
||||||||
|
Derivatives:
|
|
|
|
|
||||||||||||
|
Foreign currency contracts
|
—
|
|
53
|
|
—
|
|
53
|
|
||||||||
|
Cross-currency swap contracts
|
—
|
|
193
|
|
—
|
|
193
|
|
||||||||
|
Total assets
|
$
|
8,191
|
|
$
|
14,418
|
|
$
|
—
|
|
$
|
22,609
|
|
||||
|
Liabilities:
|
||||||||||||||||
|
Derivatives:
|
||||||||||||||||
|
Foreign currency contracts
|
$
|
—
|
|
$
|
107
|
|
$
|
—
|
|
$
|
107
|
|
||||
|
Cross-currency swap contracts
|
—
|
|
4
|
|
—
|
|
4
|
|
||||||||
|
Interest rate swap contracts
|
—
|
|
161
|
|
—
|
|
161
|
|
||||||||
|
Contingent consideration obligations in connection with business combinations
|
—
|
|
—
|
|
595
|
|
595
|
|
||||||||
|
Total liabilities
|
$
|
—
|
|
$
|
272
|
|
$
|
595
|
|
$
|
867
|
|
||||
|
During the years ended December 31,
|
|||||||
|
2014
|
2013
|
||||||
|
Beginning balance
|
$
|
595
|
|
$
|
221
|
|
|
|
Additions from Onyx acquisition
|
—
|
|
261
|
|
|||
|
Net changes in valuation
|
(30
|
)
|
113
|
|
|||
|
Agreement with former Proteolix, Inc. shareholders
|
(225
|
)
|
—
|
|
|||
|
Payment to former BioVex Group, Inc. shareholders
|
(125
|
)
|
—
|
|
|||
|
Ending balance
|
$
|
215
|
|
$
|
595
|
|
|
|
Foreign currency
|
U.S. dollars
|
|||||||||||||
|
Hedged notes
|
Notional amount
|
Interest rate
|
Notional amount
|
Interest rate
|
||||||||||
|
2.125% 2019 euro Notes
|
€
|
675
|
|
2.125
|
%
|
$
|
864
|
|
2.6
|
%
|
||||
|
5.50% 2026 pound sterling Notes
|
£
|
475
|
|
5.50
|
%
|
$
|
747
|
|
6.0
|
%
|
||||
|
4.00% 2029 pound sterling Notes
|
£
|
700
|
|
4.00
|
%
|
$
|
1,111
|
|
4.5
|
%
|
||||
|
Years ended December 31,
|
|||||||||||
|
Derivatives in cash flow hedging relationships
|
2014
|
2013
|
2012
|
||||||||
|
Foreign currency contracts
|
$
|
452
|
|
$
|
(44
|
)
|
$
|
(63
|
)
|
||
|
Cross-currency swap contracts
|
(154
|
)
|
132
|
|
85
|
|
|||||
|
Forward interest rate contracts
|
—
|
|
—
|
|
(7
|
)
|
|||||
|
Total
|
$
|
298
|
|
$
|
88
|
|
$
|
15
|
|
||
|
Years ended December 31,
|
||||||||||||||
|
Derivatives in cash flow hedging relationships
|
Statements of Income location
|
2014
|
2013
|
2012
|
||||||||||
|
Foreign currency contracts
|
Product sales
|
$
|
28
|
|
$
|
4
|
|
$
|
74
|
|
||||
|
Cross-currency swap contracts
|
Interest and other income, net
|
(230
|
)
|
82
|
|
61
|
|
|||||||
|
Forward interest rate contracts
|
Interest expense, net
|
(1
|
)
|
(1
|
)
|
(1
|
)
|
|||||||
|
Total
|
$
|
(203
|
)
|
$
|
85
|
|
$
|
134
|
|
|||||
|
Years ended December 31,
|
||||||||||||||
|
Derivatives not designated as hedging instruments
|
Statements of Income location
|
2014
|
2013
|
2012
|
||||||||||
|
Foreign currency contracts
|
Interest and other income, net
|
$
|
(10
|
)
|
$
|
15
|
|
$
|
19
|
|
||||
|
|
Derivative assets
|
Derivative liabilities
|
|||||||||
|
December 31, 2014
|
Balance Sheet location
|
Fair value
|
Balance Sheet location
|
Fair value
|
|||||||
|
Derivatives designated as hedging instruments:
|
|||||||||||
|
Cross-currency swap contracts
|
Other current assets/ Other noncurrent assets
|
$
|
32
|
|
Accrued liabilities/ Other noncurrent liabilities
|
$
|
12
|
|
|||
|
Foreign currency contracts
|
Other current assets/ Other noncurrent assets
|
356
|
|
Accrued liabilities/ Other noncurrent liabilities
|
—
|
|
|||||
|
Interest rate swap contracts
|
Other current assets/ Other noncurrent assets
|
46
|
|
Accrued liabilities/ Other noncurrent liabilities
|
26
|
|
|||||
|
Total derivatives designated as hedging instruments
|
434
|
|
38
|
|
|||||||
|
Derivatives not designated as hedging instruments:
|
|||||||||||
|
Foreign currency contracts
|
Other current assets
|
4
|
|
Accrued liabilities
|
4
|
|
|||||
|
Total derivatives not designated as hedging instruments
|
4
|
|
4
|
|
|||||||
|
Total derivatives
|
$
|
438
|
|
$
|
42
|
|
|||||
|
|
Derivative assets
|
Derivative liabilities
|
|||||||||
|
December 31, 2013
|
Balance Sheet location
|
Fair value
|
Balance Sheet location
|
Fair value
|
|||||||
|
Derivatives designated as hedging instruments:
|
|||||||||||
|
Cross-currency swap contracts
|
Other current assets/ Other noncurrent assets
|
$
|
193
|
|
Accrued liabilities/ Other noncurrent liabilities
|
$
|
4
|
|
|||
|
Foreign currency contracts
|
Other current assets/ Other noncurrent assets
|
53
|
|
Accrued liabilities/ Other noncurrent liabilities
|
104
|
|
|||||
|
Interest rate swap contracts
|
Other current assets/ Other noncurrent assets
|
—
|
|
Accrued liabilities/ Other noncurrent liabilities
|
161
|
|
|||||
|
Total derivatives designated as hedging instruments
|
246
|
|
269
|
|
|||||||
|
Derivatives not designated as hedging instruments:
|
|||||||||||
|
Foreign currency contracts
|
Other current assets
|
—
|
|
Accrued liabilities
|
3
|
|
|||||
|
Total derivatives not designated as hedging instruments
|
—
|
|
3
|
|
|||||||
|
Total derivatives
|
$
|
246
|
|
$
|
272
|
|
|||||
|
2015
|
$
|
135
|
|
|
2016
|
168
|
|
|
|
2017
|
155
|
|
|
|
2018
|
143
|
|
|
|
2019
|
139
|
|
|
|
Thereafter
|
294
|
|
|
|
Total minimum operating lease commitments
|
$
|
1,034
|
|
|
Years ended December 31,
|
|||||||||||
|
2014
|
2013
|
2012
|
|||||||||
|
Product sales:
|
|||||||||||
|
Neulasta
®
|
$
|
4,596
|
|
$
|
4,392
|
|
$
|
4,092
|
|
||
|
NEUPOGEN
®
|
1,159
|
|
1,398
|
|
1,260
|
|
|||||
|
ENBREL
|
4,688
|
|
4,551
|
|
4,236
|
|
|||||
|
XGEVA
®
|
1,221
|
|
1,019
|
|
748
|
|
|||||
|
Prolia
®
|
1,030
|
|
744
|
|
472
|
|
|||||
|
EPOGEN
®
|
2,031
|
|
1,953
|
|
1,941
|
|
|||||
|
Aranesp
®
|
1,930
|
|
1,911
|
|
2,040
|
|
|||||
|
Sensipar
®
/Mimpara
®
|
1,158
|
|
1,089
|
|
950
|
|
|||||
|
Vectibix
®
|
505
|
|
389
|
|
359
|
|
|||||
|
Nplate
®
|
469
|
|
427
|
|
368
|
|
|||||
|
Kyprolis
®
|
331
|
|
73
|
|
—
|
|
|||||
|
BLINCYTO
™
|
3
|
|
—
|
|
—
|
|
|||||
|
Other
|
206
|
|
246
|
|
173
|
|
|||||
|
Total product sales
|
19,327
|
|
18,192
|
|
16,639
|
|
|||||
|
Other revenues
|
736
|
|
484
|
|
626
|
|
|||||
|
Total revenues
|
$
|
20,063
|
|
$
|
18,676
|
|
$
|
17,265
|
|
||
|
|
Years ended December 31,
|
||||||||||
|
|
2014
|
2013
|
2012
|
||||||||
|
Revenues:
|
|||||||||||
|
United States
|
$
|
15,396
|
|
$
|
14,480
|
|
$
|
13,415
|
|
||
|
Rest of the world (ROW)
|
4,667
|
|
4,196
|
|
3,850
|
|
|||||
|
Total revenues
|
$
|
20,063
|
|
$
|
18,676
|
|
$
|
17,265
|
|
||
|
|
December 31,
|
||||||
|
|
2014
|
2013
|
|||||
|
Long-lived assets:
|
|||||||
|
United States
|
$
|
2,544
|
|
$
|
2,772
|
|
|
|
Puerto Rico
|
1,771
|
|
1,822
|
|
|||
|
ROW
|
908
|
|
755
|
|
|||
|
Total long-lived assets
|
$
|
5,223
|
|
$
|
5,349
|
|
|
|
Years ended December 31,
|
|||||||||||
|
2014
|
2013
|
2012
|
|||||||||
|
AmerisourceBergen Corporation:
|
|||||||||||
|
Gross product sales
|
$
|
9,142
|
|
$
|
8,527
|
|
$
|
7,556
|
|
||
|
% of total gross revenues
|
34
|
%
|
35
|
%
|
34
|
%
|
|||||
|
% of U.S. gross product sales
|
43
|
%
|
44
|
%
|
43
|
%
|
|||||
|
McKesson Corporation:
|
|||||||||||
|
Gross product sales
|
$
|
8,011
|
|
$
|
6,440
|
|
$
|
5,898
|
|
||
|
% of total gross revenues
|
30
|
%
|
27
|
%
|
27
|
%
|
|||||
|
% of U.S. gross product sales
|
35
|
%
|
32
|
%
|
32
|
%
|
|||||
|
Cardinal Health, Inc.:
|
|||||||||||
|
Gross product sales
|
$
|
3,407
|
|
$
|
3,209
|
|
$
|
3,245
|
|
||
|
% of total gross revenues
|
13
|
%
|
13
|
%
|
15
|
%
|
|||||
|
% of U.S. gross product sales
|
16
|
%
|
17
|
%
|
19
|
%
|
|||||
|
2014 Quarters ended
|
|||||||||||||||
|
(In millions, except per share data)
|
December 31
|
September 30
|
June 30
|
March 31
|
|||||||||||
|
Product sales
|
$
|
5,174
|
|
$
|
4,848
|
|
$
|
4,949
|
|
$
|
4,356
|
|
|||
|
Gross profit from product sales
|
3,991
|
|
3,780
|
|
3,868
|
|
3,266
|
|
|||||||
|
Net income
|
1,294
|
|
1,244
|
|
1,547
|
|
1,073
|
|
|||||||
|
Earnings per share:
|
|||||||||||||||
|
Basic
|
$
|
1.70
|
|
$
|
1.63
|
|
$
|
2.04
|
|
$
|
1.42
|
|
|||
|
Diluted
|
$
|
1.68
|
|
$
|
1.61
|
|
$
|
2.01
|
|
$
|
1.40
|
|
|||
|
2013 Quarters ended
|
|||||||||||||||
|
(In millions, except per share data)
|
December 31
|
September 30
|
June 30
|
March 31
|
|||||||||||
|
Product sales
|
$
|
4,799
|
|
$
|
4,647
|
|
$
|
4,595
|
|
$
|
4,151
|
|
|||
|
Gross profit from product sales
|
3,770
|
|
3,859
|
|
3,810
|
|
3,407
|
|
|||||||
|
Net income
|
1,021
|
|
1,368
|
|
1,258
|
|
1,434
|
|
|||||||
|
Earnings per share:
|
|||||||||||||||
|
Basic
|
$
|
1.35
|
|
$
|
1.81
|
|
$
|
1.67
|
|
$
|
1.91
|
|
|||
|
Diluted
|
$
|
1.33
|
|
$
|
1.79
|
|
$
|
1.65
|
|
$
|
1.88
|
|
|||
|
Allowance for doubtful accounts
|
Balance at
beginning
of period
|
Additions
charged to
costs and
expenses
|
Other
additions
|
Deductions
|
Balance
at end
of
period
|
||||||||||||||
|
Year ended December 31, 2014
|
$
|
59
|
|
$
|
3
|
|
$
|
—
|
|
$
|
12
|
|
$
|
50
|
|
||||
|
Year ended December 31, 2013
|
$
|
61
|
|
$
|
5
|
|
$
|
—
|
|
$
|
7
|
|
$
|
59
|
|
||||
|
Year ended December 31, 2012
|
$
|
54
|
|
$
|
7
|
|
$
|
—
|
|
$
|
—
|
|
$
|
61
|
|
||||