|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended
December 31, 2018
|
|
☐
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _______________ to _______________
|
|
Amneal Pharmaceuticals, Inc.
|
||
|
(Exact name of registrant as specified in its charter)
|
||
|
Delaware
|
|
32-0546926
|
|
(State or other jurisdiction of incorporation or organization)
|
|
(I.R.S. Employer Identification No.)
|
|
400 Crossing Boulevard, Bridgewater, NJ
|
|
08807
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
|
(908) 947-3120
|
||
|
(Registrant’s telephone number, including area code)
|
||
|
Title of each class
|
|
Name of each exchange on which registered
|
|
Class A Common Stock, par value $0.01 per share
|
|
New York Stock Exchange
|
|
Large accelerated filer
|
☐
|
Accelerated filer
|
☐
|
|
Non-accelerated filer
|
x
|
Smaller reporting company
|
☐
|
|
Emerging growth company
|
☐
|
||
|
|
|
|
|
|
PART I.
|
|
|
|
|
Item 1.
|
|||
|
Item 1A.
|
|||
|
Item 1B.
|
|||
|
Item 2.
|
|||
|
Item 3.
|
|||
|
Item 4.
|
|||
|
|
|
|
|
|
PART II.
|
|
|
|
|
Item 5.
|
|||
|
Item 6.
|
|||
|
Item 7.
|
|||
|
Item 7A.
|
|||
|
Item 8.
|
|||
|
Item 9.
|
|||
|
Item 9A.
|
|||
|
Item 9B.
|
|||
|
|
|
|
|
|
PART III.
|
|
|
|
|
Item 10.
|
|||
|
Item 11.
|
|||
|
Item 12.
|
|||
|
Item 13.
|
|||
|
Item 14.
|
|||
|
|
|
|
|
|
PART IV.
|
|
|
|
|
Item 15.
|
|||
|
Item 16.
|
|
||
|
|
|
|
|
|
•
|
the impact of global economic conditions;
|
|
•
|
our ability to integrate the operations of Amneal Pharmaceuticals LLC and Impax Laboratories, LLC pursuant to the business combination completed on May 4, 2018, and our ability to realize the anticipated synergies and other benefits of the combination;
|
|
•
|
our ability to successfully develop, license, acquire and commercialize new products on a timely basis;
|
|
•
|
our ability to obtain exclusive marketing rights for our products;
|
|
•
|
the competition we face in the pharmaceutical industry from brand and generic drug product companies, and the impact of that competition on our ability to set prices;
|
|
•
|
our ability to manage our growth through acquisitions and otherwise;
|
|
•
|
our dependence on the sales of a limited number of products for a substantial portion of our total revenues;
|
|
•
|
the risk of product liability and other claims against us by consumers and other third parties;
|
|
•
|
risks related to changes in the regulatory environment, including United States federal and state laws related to healthcare fraud abuse and health information privacy and security and changes in such laws;
|
|
•
|
changes to FDA product approval requirements;
|
|
•
|
risks related to federal regulation of arrangements between manufacturers of branded and generic products;
|
|
•
|
the impact of healthcare reform and changes in coverage and reimbursement levels by governmental authorities and other third-party payers;
|
|
•
|
the continuing trend of consolidation of certain customer groups;
|
|
•
|
our reliance on certain licenses to proprietary technologies from time to time;
|
|
•
|
our dependence on third-party suppliers and distributors for raw materials for our products and certain finished goods;
|
|
•
|
our dependence on third-party agreements for a portion of our product offerings;
|
|
•
|
our ability to identify and make acquisitions of or investments in complementary businesses and products on advantageous terms;
|
|
•
|
legal, regulatory and legislative efforts by our brand competitors to deter competition from our generic alternatives;
|
|
•
|
the significant amount of resources we expend on research and development;
|
|
•
|
our substantial amount of indebtedness and our ability to generate sufficient cash to service our indebtedness in the future, and the impact of interest rate fluctuations on such indebtedness;
|
|
•
|
such other factors as may be set forth elsewhere in this Annual Report on Form 10-K, particularly in the section entitled
1A. Risk Factors
and our public filings with the SEC.
|
|
•
|
On May 4, 2018, we entered into a licensing agreement for the U.S. market with MabXience S.L. for its biosimilar candidate for Avastin® (bevacizumab).
|
|
•
|
On May 7, 2018, we acquired 98.0% of the outstanding equity interests in Gemini Laboratories, LLC ("Gemini"), a company with a portfolio of licensed and owned, niche and mature branded products.
|
|
•
|
On August 16, 2018, we entered into a 10-year license and supply agreement with Jerome Stevens Pharmaceuticals, Inc. ("JSP") for Levothyroxine sodium tablets with an effective date of March 22, 2019.
|
|
•
|
On August 31, 2018, we entered into a 5-year supply and distribution agreement with American Regent, Inc. for the only preservative-free generic alternative to Makena® (hydroxyprogesterone caproate injection, USP, 250mg/mL).
|
|
•
|
On November 9, 2018, we entered into transition agreement with Lannett Company to begin commercialization of Levothyroxine sodium tablets on December 1, 2018, ahead of the effective date of our agreement with JSP.
|
|
•
|
a generic pharmaceutical products manufacturer’s ability to rapidly develop and obtain regulatory approval for and supply commercial quantities of generic pharmaceutical products;
|
|
•
|
the introduction of other generic pharmaceutical manufacturers’ products in direct competition with our products;
|
|
•
|
the introduction of authorized generic pharmaceutical products in direct competition with our products;
|
|
•
|
consolidation among our customers and the formation of buyer consortia;
|
|
•
|
pricing pressures by competitors and customers;
|
|
•
|
product quality of our generic pharmaceutical competitors;
|
|
•
|
our and our competitors’ breadth of product offerings across its portfolio;
|
|
•
|
our ability and the ability of our generic pharmaceutical competitors to quickly enter the market after the expiration of patents or statutory exclusivity periods, limiting the extent and duration of profitability for our products;
|
|
•
|
the willingness of our customers to switch their source of supply of products among various generic pharmaceutical competitors;
|
|
•
|
the ability of our generic pharmaceutical competitors to identify and market niche products;
|
|
•
|
our and our competitors’ level of service (including maintenance of inventories for timely delivery) and reputation as a reliable developer and manufacturer of generic pharmaceutical products; and
|
|
•
|
product appearance and labeling for our products and those of our competitors.
|
|
•
|
Laboratory and clinical tests;
|
|
•
|
Submission of an Investigational New Drug (“IND”) application, which must become effective before clinical studies may begin;
|
|
•
|
Adequate and well-controlled human clinical studies to establish the safety and efficacy of the proposed product for its intended use;
|
|
•
|
Submission of a NDA containing the results of the preclinical tests and clinical studies establishing the safety and efficacy of the proposed product for its intended use, as well as extensive data addressing such matters such as manufacturing and quality assurance;
|
|
•
|
Scale-up to commercial manufacturing; and
|
|
•
|
FDA approval of a NDA.
|
|
•
|
Paragraph I: That the required patent information relating to the patent for the referenced branded pharmaceutical product has not been filed;
|
|
•
|
Paragraph II: That the patent for the referenced branded pharmaceutical product has expired;
|
|
•
|
Paragraph III: That the patent for the referenced branded pharmaceutical product will expire on a particular date; or
|
|
•
|
Paragraph IV: That the patent for the referenced branded pharmaceutical product is invalid and/or will not be infringed by the pharmaceutical product for which approval is being sought
|
|
•
|
our ability to develop products in a timely and cost-efficient manner and in compliance with regulatory requirements, including delays associated with the FDA listing and approval process and our ability to obtain required regulatory approvals in a timely manner, or at all, and maintain such approvals if obtained;
|
|
•
|
the success of our clinical testing process to ensure that new products are safe and effective or bioequivalent to the reference listed drug;
|
|
•
|
the risk that any of our products presently under development, if and when fully developed and tested, will not perform as expected;
|
|
•
|
the risk that legal action may be brought against our generic drug products by our branded drug product competitors, including patent infringement claims among others;
|
|
•
|
the availability, on commercially reasonable terms, of raw materials, including APIs and other key ingredients necessary to the development of our drug products; and
|
|
•
|
Our ability to scale-up manufacturing methods to successfully manufacture commercial quantities of drug product in compliance with regulatory requirements.
|
|
•
|
introduction of other generic drug manufacturers’ products in direct competition with our generic drug products;
|
|
•
|
introduction of authorized generic drug products in direct competition with our products, particularly during exclusivity periods;
|
|
•
|
the ability of generic drug product competitors to quickly enter the market after the expiration of patents or exclusivity periods, diminishing the amount and duration of significant profits;
|
|
•
|
consolidation among distribution outlets through mergers and acquisitions and the formation of buying groups;
|
|
•
|
the willingness of generic drug customers, including wholesale and retail customers, to switch among products of different pharmaceutical manufacturers;
|
|
•
|
pricing pressures by competitors and customers;
|
|
•
|
a company’s reputation as a manufacturer and distributor of quality products;
|
|
•
|
a company’s level of service (including maintaining sufficient inventory levels for timely deliveries);
|
|
•
|
product appearance and labeling; and
|
|
•
|
a company’s breadth of product offerings.
|
|
•
|
the availability of alternative products from our competitors;
|
|
•
|
the prices of our products relative to those of our competitors;
|
|
•
|
the timing of our market entry;
|
|
•
|
the ability to market our products effectively at the retail level;
|
|
•
|
the perception of patients and the healthcare community, including third-party payers, regarding the safety, efficacy and benefits of our drug products compared to those of competing products; and
|
|
•
|
the acceptance of our products by government and private formularies.
|
|
•
|
marketing an authorized generic version of a branded product at the same time that we introduce a generic equivalent of that product, directly or through agreement with a generic competitor;
|
|
•
|
filing "citizen’s petitions" with the FDA to thwart generic competition by causing delays of our product approvals;
|
|
•
|
using risk evaluation and mitigation strategies ("REMS"), related distribution restrictions or other means of limiting access to their branded products, to prevent us from obtaining product samples needed to conduct bioequivalence testing required for ANDA approval, thereby delaying or preventing us from obtaining FDA approval of a generic version of such branded products;
|
|
•
|
seeking to secure patent protection of certain "Elements to Assure Safe Use" of a REMS program, which are required medical interventions or other actions healthcare professionals need to execute prior to prescribing or dispensing the drug to the patient, in an attempt to thwart our ability to avoid infringement of the patents in question or secure approval;
|
|
•
|
seeking to establish regulatory and legal obstacles that would make it more difficult for us to demonstrate a generic product’s bioequivalence or "sameness" to the related branded product;
|
|
•
|
initiating legislative and administrative efforts in various states to limit the substitution of generic versions of branded pharmaceutical products for the corresponding branded products;
|
|
•
|
filing suits for patent infringement that automatically delay FDA approval of our generic products;
|
|
•
|
introducing "next-generation" products prior to the expiration of market exclusivity for their branded product, which often materially reduces the demand for the generic product for which we may be seeking FDA approval;
|
|
•
|
obtaining extensions of market exclusivity by conducting clinical trials of branded drugs in pediatric populations or by other methods as discussed below;
|
|
•
|
persuading the FDA to withdraw the approval of branded drugs for which the associated patents are about to expire, thus allowing the brand company to develop and launch new patented products serving as substitutes for the withdrawn products;
|
|
•
|
seeking to obtain new patents on drugs for which patent protection is about to expire;
|
|
•
|
filing patent applications that are more complex and costly to challenge;
|
|
•
|
seeking temporary restraining orders and injunctions against selling a generic equivalent of their branded product based on alleged misappropriation of trade secrets or breach of confidentiality obligations;
|
|
•
|
seeking temporary restraining orders and injunctions against us after we have received final FDA approval for a product for which we are attempting to launch at-risk prior to resolution of related patent litigation;
|
|
•
|
reducing the marketing of the branded product to healthcare providers, thereby reducing the branded drug’s commercial exposure and market size, which in turn adversely affects the market potential of the equivalent generic product; and
|
|
•
|
converting branded prescription drugs that are facing potential generic competition to over-the-counter products, thereby significantly impeding the growth of the generic prescription market for such drugs.
|
|
•
|
delays in patient enrollment, and variability in the number and types of patients available for clinical trials;
|
|
•
|
regulators or institutional review boards may not allow us to commence or continue a clinical trial;
|
|
•
|
our inability, or the inability of our partners, to manufacture or obtain from third parties materials sufficient to complete our clinical trials;
|
|
•
|
delays or failure in reaching agreement on acceptable clinical trial contracts or clinical trial protocols with prospective clinical trial sites;
|
|
•
|
risks associated with trial design, which may result in a failure of the trial to show statistically significant results even if the product candidate is effective;
|
|
•
|
difficulty in maintaining contact with patients after treatment commences, resulting in incomplete data;
|
|
•
|
poor effectiveness of product candidates during clinical trials;
|
|
•
|
safety issues, including adverse events associated with product candidates;
|
|
•
|
the failure of patients to complete clinical trials due to adverse side effects, dissatisfaction with the product candidate, or other reasons;
|
|
•
|
governmental or regulatory delays or changes in regulatory requirements, policy and guidelines; and
|
|
•
|
varying interpretation of data by the FDA or foreign regulatory authorities.
|
|
•
|
the number of new product introductions by us;
|
|
•
|
losses related to inventory write-offs;
|
|
•
|
marketing exclusivity, if any, which may be obtained on certain new products;
|
|
•
|
the level of competition in the marketplace for certain products;
|
|
•
|
our ability to create demand in the marketplace for our products;
|
|
•
|
availability of raw materials and finished products from suppliers;
|
|
•
|
our ability to manufacture products at our manufacturing facilities;
|
|
•
|
the scope and outcome of governmental regulatory actions;
|
|
•
|
our dependence on a small number of products for a significant portion of net revenue or income;
|
|
•
|
legal actions against our generic products brought by brand competitors, and legal challenges to our intellectual property rights by generic competitors;
|
|
•
|
price erosion and customer consolidation; and
|
|
•
|
significant payments (such as milestones) payable by us under collaboration, licensing, and development agreements to our partners before the related product has received FDA approval.
|
|
•
|
increase our vulnerability to adverse economic and industry conditions;
|
|
•
|
limit our ability to obtain additional financing for future working capital, capital expenditures, raw materials, strategic acquisitions and other general corporate requirements;
|
|
•
|
expose us to interest rate fluctuations because the interest on certain debt under the credit facilities is imposed at variable rates;
|
|
•
|
require us to dedicate a substantial portion of our cash flow from operations to payments on our debt, thereby reducing the availability of cash flow for operations and other purposes;
|
|
•
|
make it more difficult for us to satisfy our obligations to our lenders, resulting in possible defaults on and acceleration of such indebtedness;
|
|
•
|
limit our ability to refinance indebtedness or increase the associated costs;
|
|
•
|
require us to sell assets to reduce debt or influence the decision about whether to do so;
|
|
•
|
limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate or prevent us from carrying out capital spending that is necessary or important to our growth strategy and efforts to improve operating margins or our business; and
|
|
•
|
place us at a competitive disadvantage compared to any competitors that have less debt or comparable debt at more favorable interest rates and that, as a result, may be better positioned to withstand economic downturn.
|
|
•
|
our debt holders could declare all outstanding principal and interest to be due and payable;
|
|
•
|
the lenders under our credit agreements could terminate their commitments to lend us money; and
|
|
•
|
we could be forced into bankruptcy or liquidation.
|
|
•
|
incur additional indebtedness;
|
|
•
|
pay dividends or make other distributions or repurchase or redeem capital stock;
|
|
•
|
prepay, redeem or repurchase certain debt;
|
|
•
|
make loans and investments;
|
|
•
|
sell assets;
|
|
•
|
incur liens;
|
|
•
|
enter into transactions with affiliates;
|
|
•
|
alter the businesses conducted by us;
|
|
•
|
enter into agreements restricting subsidiaries’ ability to pay dividends; and
|
|
•
|
consolidate, merge or sell all or substantially all of our assets.
|
|
•
|
unable to raise additional debt or equity financing to operate during general economic or business downturns; or
|
|
•
|
our ability to obtain regulatory approvals for product candidates, and delays or failures to obtain such approvals;
|
|
•
|
the failure of any of our product candidates, if approved for marketing and commercialization, to achieve commercial success;
|
|
•
|
issues in manufacturing our approved products or product candidates;
|
|
•
|
the entry into, or termination of, key agreements, including key licensing or collaboration agreements;
|
|
•
|
the initiation of material developments in, or conclusion of, litigation to enforce or defend any of our intellectual property rights or defend against the intellectual property rights of others;
|
|
•
|
announcements by commercial partners or competitors of new commercial products, clinical progress (or the lack thereof), significant contracts, commercial relationships, or capital commitments;
|
|
•
|
adverse publicity relating to our markets, including with respect to other products and potential products in such markets;
|
|
•
|
the introduction of technological innovations or new therapies competing with our products or our potential products;
|
|
•
|
the loss of talented employees;
|
|
•
|
changes in estimates or recommendations by securities analysts, if any, who cover the Class A Common Stock regarding us, our business, our industry or our competitors, or the failure of analysts to regularly publish reports on us;
|
|
•
|
general and industry-specific economic conditions potentially affecting our research and development expenditures;
|
|
•
|
changes in the structure of health care payment systems;
|
|
•
|
period-to-period fluctuations in our financial results;
|
|
•
|
failure to meet or exceed financial and development projections we may provide to the public;
|
|
•
|
failure to meet or exceed the financial and development projections of the investment community;
|
|
•
|
the perception of the pharmaceutical industry by the public, legislators, regulators, and the investment community;
|
|
•
|
adverse regulatory decisions;
|
|
•
|
disputes or other developments relating to proprietary rights, including patents, litigation matters, and our ability to obtain patent protection for our technologies;
|
|
•
|
sales of the Class A Common Stock by us or our stockholders in the future; and
|
|
•
|
trading volume of the Class A Common Stock.
|
|
Property Address
|
Legal Status
|
Purpose
|
||
|
400 Crossing Boulevard, Bridgewater, New Jersey
|
Leased
|
Executive Office
|
||
|
118 Beaver Trail, Glasgow, Kentucky
|
Leased
|
Administrative, Distribution and Warehouse
|
||
|
40 Aberdeen Drive, Glasgow, Kentucky
|
Leased
|
Warehouse
|
||
|
19 Nichols Drive, Yaphank, New York
|
Leased
|
Warehouse
|
||
|
21 Colonial Drive, Piscataway, New Jersey
|
Leased
|
Warehouse
|
||
|
41-49 Colonial Drive, Piscataway, New Jersey
|
Leased
|
Manufacturing
|
||
|
1045 Centennial Ave, Piscataway, New Jersey
|
Leased
|
R&D, manufacturing
|
||
|
131 Chambersbrook Rd., Branchburg, New Jersey
|
Leased
|
Manufacturing
|
||
|
65 Readington, Branchburg, New Jersey
|
Leased
|
Manufacturing
|
||
|
1 New England Avenue, Piscataway, New Jersey
|
Leased
|
Manufacturing
|
||
|
19 Readington Road, Branchburg, New Jersey
|
Leased
|
Warehouse
|
||
|
1 Murray Road, East Hanover, New Jersey
|
Leased
|
Packaging
|
||
|
50 Horseblock Road, (Yaphank) Brookhaven, New York
|
Leased
|
Manufacturing, R&D, Quality and Regulatory
|
||
|
75 Adams, Hauppauge, New York
|
Leased
|
Manufacturing, R&D, Quality and Regulatory
|
||
|
Cahir Road, Cashel Co, Tipperary, Ireland
|
Owned
|
R&D, manufacturing
|
||
|
881/1 and 871, Near Hotel Karnavati, Vill Rajoda, Tal Bavla, Ahmedabad—380001, India
|
Owned
|
Oral Solids Manufacturing and R&D
|
||
|
Plot No 15-16-17, Pharmasez, Sarkhej Balva Highway NH No. 8A Village Matoda, India
|
Leased
|
Oral Solids and Injectables Manufacturing and R&D
|
||
|
Magnet Park, Corporate House No 18, Sarkhej Gandhinagar Highway, Thaltej, Ahmedabad, India
|
Leased
|
R&D (Injectables), Corporate Office
|
||
|
Plot No 99, Gallops Industrial Park, Village Rajoda, Bavla, Ahmedabad 382 220, India
|
Leased
|
Additional Warehouse for OSD
|
||
|
901-905, 906-910& 911 Iscon Elegance, S.G.Highway, Ahmedabad, India
|
Leased
|
Corporate Office
|
||
|
63, Silver Industrial Estate, B/H JP Cold Storage, Village-Moraiya, Tal-Sanand, Dist Ahmedabad, India
|
Leased
|
Warehouse
|
||
|
72, Silver Industrial Estate, B/H JP Cold Storage, Village-Moraiya, Tal-Sanand, Dist Ahmedabad, India
|
Leased
|
Warehouse
|
||
|
Plot S3, S4 & S5 -A, TSIIC,Sez, Jadcherla Telangana Mahabubnagar 509302, India
|
Leased
|
Oncology R&D and Manufacturing
|
||
|
Plot No 68 SY No 60,62&63 Ofe Bonamgi Revenue Village Parawada Mandal AP 008 Visakhapatam Apandhra Pradesh, 530001, India
|
Owned
|
API Manufacturing and R&D
|
||
|
Plot No Z/111/A Dahej Sez, Part II Dahej, Gujarat Bharuch-392110, India
|
Leased
|
API Manufacturing
|
||
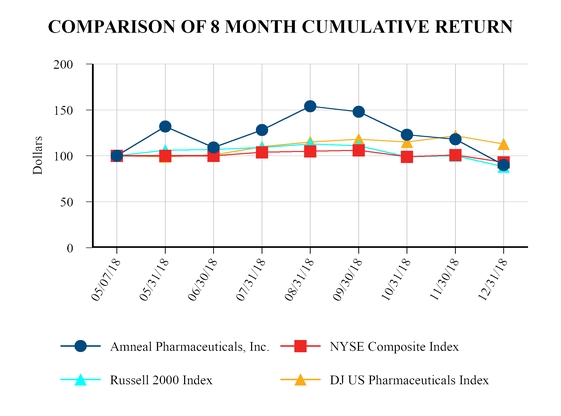
|
(In thousands, except per share data)
|
Years Ended December 31,
|
||||||||||||||||||
|
Statements of Operations Data:
|
2018
(1)(2)(3)
|
2017
|
2016
|
2015
|
2014
|
||||||||||||||
|
Net revenue
|
$
|
1,662,991
|
|
$
|
1,033,654
|
|
$
|
1,018,225
|
|
$
|
866,280
|
|
$
|
785,263
|
|
||||
|
Research and development and intellectual property legal development expenses
|
210,451
|
|
191,938
|
|
204,747
|
|
153,713
|
|
118,539
|
|
|||||||||
|
In-process research and development impairment charges
|
39,259
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Operating (loss) income
|
(19,673
|
)
|
245,103
|
|
284,881
|
|
236,158
|
|
218,575
|
|
|||||||||
|
Net (loss) income
|
(201,303
|
)
|
169,325
|
|
209,426
|
|
170,629
|
|
177,812
|
|
|||||||||
|
Net loss attributable to Amneal Pharmaceuticals, Inc.
|
$
|
(20,920
|
)
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Per share data:
|
|||||||||||||||||||
|
Net loss per share — basic and diluted
|
$
|
(0.16
|
)
|
||||||||||||||||
|
(In thousands)
|
As of December 31,
|
||||||||||||||||||
|
Balance Sheet Data:
|
2018
(1)(2)(3)
|
2017
|
2016
|
2015
|
2014
|
||||||||||||||
|
Cash and cash equivalents
|
$
|
213,394
|
|
$
|
74,166
|
|
$
|
27,367
|
|
$
|
61,087
|
|
$
|
117,522
|
|
||||
|
Working capital
|
732,794
|
|
475,050
|
|
501,041
|
|
365,454
|
|
325,989
|
|
|||||||||
|
Total assets
|
4,352,736
|
|
1,341,889
|
|
1,218,817
|
|
1,014,093
|
|
829,983
|
|
|||||||||
|
Long-term debt, net
|
2,630,598
|
|
1,355,274
|
|
1,119,268
|
|
911,043
|
|
711,914
|
|
|||||||||
|
Total liabilities
|
3,456,373
|
|
1,717,471
|
|
1,394,762
|
|
1,200,966
|
|
927,670
|
|
|||||||||
|
Total equity (deficit)
|
$
|
896,363
|
|
$
|
(375,582
|
)
|
$
|
(175,945
|
)
|
$
|
(186,873
|
)
|
$
|
(97,687
|
)
|
||||
|
•
|
$56 million
for restructuring charges related to the Combination, of which
$45 million
was for employee separation and
$11 million
was for asset-related charges. For more information, see
Note 6. Restructuring and Asset-Related Charges
.
|
|
•
|
$222 million
for acquisition, transaction-related and integration expenses related to the Combination, including
$35 million
for professional service fees (e.g. legal, investment banking and accounting), information technology systems conversions, and contract termination/renegotiation costs,
$159 million
for the accelerated vesting of certain of Amneal's profit participation units that occurred prior to the closing of the Combination, and
$28 million
for a transaction-related bonus. For more information, see
Note 7. Acquisition, Transaction-Related and Integration Expenses
.
|
|
•
|
$48 million
for impairment of intangible assets recognized in connection with the Combination, of which
$9 million
was recognized in cost of goods sold and
$39 million
was recognized for in-process research and development. For more information, see
Note 14. Goodwill and Intangible Assets
.
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2018
|
2017
|
2016
|
||||||||
|
Net revenue
|
$
|
1,662,991
|
|
$
|
1,033,654
|
|
$
|
1,018,225
|
|
||
|
Cost of goods sold
|
946,588
|
|
507,476
|
|
420,770
|
|
|||||
|
Gross profit
|
716,403
|
|
526,178
|
|
597,455
|
|
|||||
|
Selling, general and administrative
|
230,435
|
|
109,046
|
|
118,757
|
|
|||||
|
Research and development
|
194,190
|
|
171,420
|
|
179,019
|
|
|||||
|
In-process research and development impairment charges
|
39,259
|
|
—
|
|
—
|
|
|||||
|
Acquisition, transaction-related and integration expenses
|
221,818
|
|
9,403
|
|
70
|
|
|||||
|
Restructuring and asset-related charges
|
56,413
|
|
—
|
|
—
|
|
|||||
|
Legal settlement gains
|
(22,300
|
)
|
(29,312
|
)
|
(11,000
|
)
|
|||||
|
Intellectual property legal development expenses
|
16,261
|
|
20,518
|
|
25,728
|
|
|||||
|
Operating (loss) income
|
(19,673
|
)
|
245,103
|
|
284,881
|
|
|||||
|
Total other expense, net
|
(183,049
|
)
|
(73,780
|
)
|
(70,060
|
)
|
|||||
|
(Loss) income before income taxes
|
(202,722
|
)
|
171,323
|
|
214,821
|
|
|||||
|
(Benefit from) provision for income taxes
|
(1,419
|
)
|
1,998
|
|
5,395
|
|
|||||
|
Net (loss) income
|
$
|
(201,303
|
)
|
$
|
169,325
|
|
$
|
209,426
|
|
||
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Net revenue
|
$
|
1,439,031
|
|
$
|
1,033,654
|
|
$
|
1,018,225
|
|
||
|
Cost of goods sold
|
842,996
|
|
507,476
|
|
420,770
|
|
|||||
|
Gross profit
|
596,035
|
|
526,178
|
|
597,455
|
|
|||||
|
Selling, general and administrative
|
68,426
|
|
56,050
|
|
69,540
|
|
|||||
|
Research and development
|
183,412
|
|
171,420
|
|
179,019
|
|
|||||
|
In-process research and development impairment charges
|
39,259
|
|
—
|
|
—
|
|
|||||
|
Acquisition, transaction-related and integration expenses
|
114,622
|
|
—
|
|
—
|
|
|||||
|
Restructuring and asset-related charges
|
33,943
|
|
—
|
|
—
|
|
|||||
|
Legal settlement gains
|
(22,300
|
)
|
(29,312
|
)
|
(11,000
|
)
|
|||||
|
Intellectual property legal development expenses
|
15,772
|
|
20,518
|
|
25,728
|
|
|||||
|
Operating income
|
$
|
162,901
|
|
$
|
307,502
|
|
$
|
334,168
|
|
||
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Net revenue
|
$
|
223,960
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Cost of goods sold
|
103,592
|
|
—
|
|
—
|
|
|||||
|
Gross profit
|
120,368
|
|
—
|
|
—
|
|
|||||
|
Selling, general and administrative
|
49,465
|
|
—
|
|
—
|
|
|||||
|
Research and development
|
10,778
|
|
—
|
|
—
|
|
|||||
|
Restructuring and asset-related charges
|
4,076
|
|
—
|
|
—
|
|
|||||
|
Intellectual property legal development expenses
|
489
|
|
—
|
|
—
|
|
|||||
|
Operating income
|
$
|
55,560
|
|
$
|
—
|
|
$
|
—
|
|
||
|
|
Payments Due by Period
|
|||||||||||||||||||
|
Contractual Obligations
|
Total
|
Less
Than 1 Year |
1-3
Years |
3-5
Years |
More
Than 5 Years |
|||||||||||||||
|
Bank term loan and other
|
$
|
2,686,500
|
|
$
|
27,000
|
|
$
|
54,000
|
|
$
|
54,000
|
|
$
|
2,551,500
|
|
|||||
|
Interest payments on bank term loan
(1)
|
971,851
|
|
157,435
|
|
310,112
|
|
453,271
|
|
51,033
|
|
||||||||||
|
Operating lease obligations
(2)
|
97,561
|
|
25,885
|
|
23,176
|
|
20,372
|
|
28,128
|
|
||||||||||
|
Financing obligation - related party
(3)
|
134,566
|
|
5,474
|
|
10,948
|
|
10,948
|
|
107,196
|
|
||||||||||
|
Levothyroxine transition payment
|
3,816
|
|
3,816
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Open purchase order commitments
|
65,302
|
|
31,078
|
|
34,224
|
|
—
|
|
—
|
|
||||||||||
|
Total
|
$
|
3,959,596
|
|
$
|
250,688
|
|
$
|
432,460
|
|
$
|
538,591
|
|
$
|
2,737,857
|
|
|||||
|
Plan Category
|
Number of securities to
be issued upon exercise
of outstanding options,
warrants and rights
(a)
|
Weighted-average
exercise price of
outstanding options,
warrants and rights
(b)
|
Number of securities
remaining available for
future issuance under
equity compensation
plans (excluding
securities reflected in
column (a))
(c)
|
|
Equity compensation plans approved by security holders
|
7,145,205
(1)
|
17.73
(2)
|
18,292,841
|
|
Equity compensation plans not approved by security holders
|
—
|
—
|
—
|
|
Total
|
7,145,205
|
17.73
|
18,292,841
|
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Net revenue
|
$
|
1,662,991
|
|
$
|
1,033,654
|
|
$
|
1,018,225
|
|
||
|
Cost of goods sold
|
946,588
|
|
507,476
|
|
420,770
|
|
|||||
|
Gross profit
|
716,403
|
|
526,178
|
|
597,455
|
|
|||||
|
Selling, general and administrative
|
230,435
|
|
109,046
|
|
118,757
|
|
|||||
|
Research and development
|
194,190
|
|
171,420
|
|
179,019
|
|
|||||
|
In-process research and development impairment charges
|
39,259
|
|
—
|
|
—
|
|
|||||
|
Acquisition, transaction-related and integration expenses
|
221,818
|
|
9,403
|
|
70
|
|
|||||
|
Restructuring and asset-related charges
|
56,413
|
|
—
|
|
—
|
|
|||||
|
Legal settlement gains
|
(22,300
|
)
|
(29,312
|
)
|
(11,000
|
)
|
|||||
|
Intellectual property legal development expenses
|
16,261
|
|
20,518
|
|
25,728
|
|
|||||
|
Operating (loss) income
|
(19,673
|
)
|
245,103
|
|
284,881
|
|
|||||
|
Other (expense) income:
|
|||||||||||
|
Interest expense, net
|
(143,571
|
)
|
(71,061
|
)
|
(55,283
|
)
|
|||||
|
Foreign exchange (loss) gain
|
(19,701
|
)
|
29,092
|
|
(14,108
|
)
|
|||||
|
Loss on extinguishment of debt
|
(19,667
|
)
|
(2,532
|
)
|
—
|
|
|||||
|
Loss on sale of certain international businesses
|
(2,958
|
)
|
(29,232
|
)
|
—
|
|
|||||
|
Other income (expense)
|
2,848
|
|
(47
|
)
|
(669
|
)
|
|||||
|
Total other expense, net
|
(183,049
|
)
|
(73,780
|
)
|
(70,060
|
)
|
|||||
|
(Loss) income before income taxes
|
(202,722
|
)
|
171,323
|
|
214,821
|
|
|||||
|
(Benefit from) provision for income taxes
|
(1,419
|
)
|
1,998
|
|
5,395
|
|
|||||
|
Net (loss) income
|
(201,303
|
)
|
169,325
|
|
209,426
|
|
|||||
|
Less: Net loss (income) attributable to Amneal Pharmaceuticals LLC pre-Combination
|
148,806
|
|
(167,648
|
)
|
(207,378
|
)
|
|||||
|
Less: Net loss (income) attributable to non-controlling interests
|
32,753
|
|
(1,677
|
)
|
(2,048
|
)
|
|||||
|
Net loss attributable to Amneal Pharmaceuticals, Inc. before accretion of redeemable non-controlling interest
|
(19,744
|
)
|
—
|
|
—
|
|
|||||
|
Accretion of redeemable non-controlling interest
|
(1,176
|
)
|
—
|
|
—
|
|
|||||
|
Net loss attributable to Amneal Pharmaceuticals, Inc.
|
$
|
(20,920
|
)
|
$
|
—
|
|
$
|
—
|
|
||
|
Net loss per share attributable to Amneal Pharmaceuticals, Inc.'s common stockholders:
|
|||||||||||
|
Class A and Class B-1 basic and diluted
|
$
|
(0.16
|
)
|
|
|
||||||
|
Weighted-average common shares outstanding:
|
|||||||||||
|
Class A and Class B-1 basic and diluted
|
127,252
|
|
|
|
|||||||
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Net (loss) income
|
$
|
(201,303
|
)
|
|
$
|
169,325
|
|
$
|
209,426
|
|
|
|
Less: Net loss (income) attributable to Amneal Pharmaceuticals LLC pre-Combination
|
148,806
|
|
|
(167,648
|
)
|
(207,378
|
)
|
||||
|
Less: Net loss (income) attributable to non-controlling interests
|
32,753
|
|
|
(1,677
|
)
|
(2,048
|
)
|
||||
|
Net loss attributable to Amneal Pharmaceuticals, Inc. before accretion of redeemable non-controlling interest
|
(19,744
|
)
|
—
|
|
—
|
|
|||||
|
Accretion of redeemable non-controlling interest
|
(1,176
|
)
|
—
|
|
—
|
|
|||||
|
Net loss attributable to Amneal Pharmaceuticals, Inc.
|
(20,920
|
)
|
|
—
|
|
—
|
|
||||
|
Other comprehensive (loss) income:
|
|
|
|
|
|
||||||
|
Foreign currency translation adjustments
|
(3,952
|
)
|
|
(1,435
|
)
|
3,047
|
|
||||
|
Less: Other comprehensive (income) loss attributable to Amneal Pharmaceuticals LLC pre-Combination
|
(1,721
|
)
|
|
1,435
|
|
(3,047
|
)
|
||||
|
Less: Other comprehensive loss attributable to non-controlling interests
|
3,256
|
|
|
—
|
|
—
|
|
||||
|
Other comprehensive loss attributable to Amneal Pharmaceuticals, Inc.
|
(2,417
|
)
|
|
—
|
|
—
|
|
||||
|
Comprehensive loss attributable to Amneal Pharmaceuticals, Inc.
|
$
|
(23,337
|
)
|
|
$
|
—
|
|
$
|
—
|
|
|
|
December 31, 2018
|
December 31, 2017
|
||||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
213,394
|
|
$
|
74,166
|
|
|
|
Restricted cash
|
5,385
|
|
3,756
|
|
|||
|
Trade accounts receivable, net
|
481,495
|
|
351,367
|
|
|||
|
Inventories
|
457,219
|
|
284,038
|
|
|||
|
Prepaid expenses and other current assets
|
128,321
|
|
42,396
|
|
|||
|
Related party receivables
|
830
|
|
16,210
|
|
|||
|
Total current assets
|
1,286,644
|
|
771,933
|
|
|||
|
Property, plant and equipment, net
|
544,146
|
|
486,758
|
|
|||
|
Goodwill
|
426,226
|
|
26,444
|
|
|||
|
Intangible assets, net
|
1,654,969
|
|
44,599
|
|
|||
|
Deferred tax asset, net
|
373,159
|
|
898
|
|
|||
|
Other assets
|
67,592
|
|
11,257
|
|
|||
|
Total assets
|
$
|
4,352,736
|
|
$
|
1,341,889
|
|
|
|
Liabilities and Stockholders' Equity (Members' Deficit)
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable and accrued expenses
|
$
|
514,440
|
|
$
|
194,779
|
|
|
|
Current portion of long-term debt, net
|
21,449
|
|
89,171
|
|
|||
|
Current portion of financing obligation - related party
|
266
|
|
311
|
|
|||
|
Related party payables
|
17,695
|
|
12,622
|
|
|||
|
Total current liabilities
|
553,850
|
|
296,883
|
|
|||
|
Long-term debt, net
|
2,630,598
|
|
1,355,274
|
|
|||
|
Financing obligations - related party
|
39,083
|
|
39,987
|
|
|||
|
Deferred income taxes
|
1,178
|
|
2,491
|
|
|||
|
Liabilities under tax receivable agreement
|
192,884
|
|
—
|
|
|||
|
Other long-term liabilities
|
38,780
|
|
7,793
|
|
|||
|
Related party payable - long term
|
—
|
|
15,043
|
|
|||
|
Total long-term liabilities
|
2,902,523
|
|
1,420,588
|
|
|||
|
Commitments and contingencies (Notes 5 & 18)
|
|
|
|
|
|||
|
Stockholders' equity (members' deficit):
|
|||||||
|
Members' equity, 189,000 units authorized, issued and outstanding at December 31, 2017
|
—
|
|
2,716
|
|
|||
|
Members' accumulated deficit
|
—
|
|
(382,785
|
)
|
|||
|
Preferred stock, $0.01 par value, 2,000 shares authorized; none issued and outstanding at December 31, 2018
|
—
|
|
—
|
|
|||
|
Class A common stock, $0.01 par value, 900,000 shares authorized; 115,047 shares issued and outstanding at December 31, 2018
|
1,151
|
|
—
|
|
|||
|
Class B common stock, $0.01 par value, 300,000 shares authorized; 171,261 shares issued and outstanding at December 31, 2018
|
1,713
|
|
—
|
|
|||
|
Class B-1 common stock, $0.01 par value, 18,000 shares authorized; 12,329 shares issued and outstanding at December 31, 2018
|
123
|
|
—
|
|
|||
|
Additional paid-in capital
|
530,438
|
|
8,562
|
|
|||
|
Stockholders' accumulated deficit
|
(20,920
|
)
|
—
|
|
|||
|
Accumulated other comprehensive loss
|
(7,755
|
)
|
(14,232
|
)
|
|||
|
Total Amneal Pharmaceuticals, Inc. stockholders' equity (members' deficit)
|
504,750
|
|
(385,739
|
)
|
|||
|
Non-controlling interests
|
391,613
|
|
10,157
|
|
|||
|
Total stockholders' equity (members' deficit)
|
896,363
|
|
(375,582
|
)
|
|||
|
Total liabilities and stockholders' equity (members’ deficit)
|
$
|
4,352,736
|
|
$
|
1,341,889
|
|
|
|
|
|
Class A Common Stock
|
Class B Common Stock
|
Class B-1 Common Stock
|
Additional Paid-in Capital
|
|
Accumulated Other Comprehensive Loss
|
Non-Controlling Interests
|
Total Equity
|
Redeemable Non-Controlling Interest
|
|||||||||||||||||||||||||||||||
|
Members' Equity
|
Members' Accumulated Deficit
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Stockholders' Accumulated Deficit
|
|||||||||||||||||||||||||||||||||
|
Balance at January 1, 2016
|
$
|
2,675
|
|
$
|
(181,974
|
)
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
(15,844
|
)
|
$
|
8,270
|
|
$
|
(186,873
|
)
|
$
|
—
|
|
||
|
Net income
|
—
|
|
207,378
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,048
|
|
209,426
|
|
—
|
|
|||||||||||||
|
Dividend to non-controlling interest
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(973
|
)
|
(973
|
)
|
—
|
|
|||||||||||||
|
Distributions to members
|
—
|
|
(200,615
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(200,615
|
)
|
—
|
|
|||||||||||||
|
Foreign currency translation adjustment
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
3,047
|
|
—
|
|
3,047
|
|
—
|
|
|||||||||||||
|
Return of capital
|
—
|
|
43
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
43
|
|
—
|
|
|||||||||||||
|
Balance at December 31, 2016
|
2,675
|
|
(175,168
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(12,797
|
)
|
9,345
|
|
(175,945
|
)
|
—
|
|
|||||||||||||
|
Net income
|
—
|
|
167,648
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,677
|
|
169,325
|
|
—
|
|
|||||||||||||
|
Dividend to non-controlling interest
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(865
|
)
|
(865
|
)
|
—
|
|
|||||||||||||
|
Capital contribution
|
41
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
8,562
|
|
—
|
|
—
|
|
—
|
|
8,603
|
|
—
|
|
|||||||||||||
|
Distributions to members
|
—
|
|
(375,265
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(375,265
|
)
|
—
|
|
|||||||||||||
|
Foreign currency translation adjustment
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(1,435
|
)
|
—
|
|
(1,435
|
)
|
—
|
|
|||||||||||||
|
Balance at December 31, 2017
|
$
|
2,716
|
|
$
|
(382,785
|
)
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
$
|
8,562
|
|
$
|
—
|
|
$
|
(14,232
|
)
|
$
|
10,157
|
|
$
|
(375,582
|
)
|
$
|
—
|
|
||
|
Class A Common Stock
|
Class B Common Stock
|
Class B-1 Common Stock
|
Additional Paid-in Capital
|
Stockholders' Accumulated Deficit
|
Accumulated Other Comprehensive Loss
|
Non-Controlling Interests
|
Total Equity
|
Redeemable Non-Controlling Interest
|
||||||||||||||||||||||||||||||||
|
Members' Equity
|
Members' Accumulated Deficit
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||||||||||
|
Balance at January 1, 2018
|
$
|
2,716
|
|
$
|
(382,785
|
)
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
$
|
8,562
|
|
$
|
—
|
|
$
|
(14,232
|
)
|
$
|
10,157
|
|
$
|
(375,582
|
)
|
$
|
—
|
|
|
|
Period Prior to the Combination
|
|
|||||||||||||||||||||||||||||||||||||||
|
Net (loss) income
|
—
|
|
(148,806
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
97
|
|
(148,709
|
)
|
—
|
|
||||||||||||
|
Cumulative-effective adjustment from adoption of ASU 2014-09 (Topic 606)
|
—
|
|
4,977
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
4,977
|
|
—
|
|
||||||||||||
|
Capital contribution from non-controlling interest
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
360
|
|
360
|
|
—
|
|
||||||||||||
|
Distributions to members
|
—
|
|
(182,998
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(8,562
|
)
|
—
|
|
—
|
|
—
|
|
(191,560
|
)
|
—
|
|
||||||||||||
|
PPU expense
|
158,757
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
158,757
|
|
—
|
|
||||||||||||||
|
Foreign currency translation adjustment
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,721
|
|
—
|
|
1,721
|
|
—
|
|
||||||||||||
|
Capital contribution by Amneal Holdings for employee bonuses
|
27,742
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
27,742
|
|
—
|
|
||||||||||||
|
Period Subsequent to the Combination
|
||||||||||||||||||||||||||||||||||||||||
|
Effect of the Combination
|
(189,215
|
)
|
709,612
|
|
73,289
|
|
733
|
|
224,996
|
|
2,250
|
|
—
|
|
—
|
|
330,678
|
|
—
|
|
9,437
|
|
626,737
|
|
1,490,232
|
|
—
|
|
||||||||||||
|
Redemption of Class B Common Stock for PIPE
|
—
|
|
—
|
|
34,520
|
|
345
|
|
(46,849
|
)
|
(468
|
)
|
12,329
|
|
123
|
|
165,180
|
|
—
|
|
(1,965
|
)
|
(130,501
|
)
|
32,714
|
|
—
|
|
||||||||||||
|
Redemption of Class B Common Stock for distribution to PPU Holders
|
—
|
|
—
|
|
6,886
|
|
69
|
|
(6,886
|
)
|
(69
|
)
|
—
|
|
—
|
|
24,293
|
|
—
|
|
(289
|
)
|
(19,181
|
)
|
4,823
|
|
—
|
|
||||||||||||
|
Net (loss) income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(19,744
|
)
|
—
|
|
(32,917
|
)
|
(52,661
|
)
|
67
|
|
||||||||||||
|
Foreign currency translation adjustment
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(2,417
|
)
|
(3,256
|
)
|
(5,673
|
)
|
—
|
|
||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
8,840
|
|
—
|
|
—
|
|
—
|
|
8,840
|
|
—
|
|
||||||||||||
|
Exercise of stock options
|
—
|
|
—
|
|
352
|
|
4
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,184
|
|
—
|
|
(10
|
)
|
1,619
|
|
3,797
|
|
—
|
|
||||||||||||
|
Reclassification of redeemable non-controlling interest
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(1,176
|
)
|
—
|
|
(10,532
|
)
|
(11,708
|
)
|
11,708
|
|
||||||||||||
|
Non-controlling interests from acquisition of Gemini
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,518
|
|
2,518
|
|
—
|
|
||||||||||||
|
Acquisition of redeemable non-controlling interest
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(11,775
|
)
|
||||||||||||
|
Acquisition of non-controlling interests
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(920
|
)
|
—
|
|
—
|
|
(2,565
|
)
|
(3,485
|
)
|
—
|
|
||||||||||||
|
Tax distribution
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(48,955
|
)
|
(48,955
|
)
|
—
|
|
||||||||||||
|
Other
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
183
|
|
—
|
|
—
|
|
(1,968
|
)
|
(1,785
|
)
|
—
|
|
||||||||||||
|
Balance at December 31, 2018
|
$
|
—
|
|
$
|
—
|
|
115,047
|
|
$
|
1,151
|
|
171,261
|
|
$
|
1,713
|
|
12,329
|
|
$
|
123
|
|
$
|
530,438
|
|
$
|
(20,920
|
)
|
$
|
(7,755
|
)
|
$
|
391,613
|
|
$
|
896,363
|
|
$
|
—
|
|
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2018
|
2017
|
2016
|
||||||||
|
Cash flows from operating activities:
|
|
|
|||||||||
|
Net (loss) income
|
$
|
(201,303
|
)
|
$
|
169,325
|
|
$
|
209,426
|
|
||
|
Adjustments to reconcile net (loss) income to net cash provided by operating activities:
|
|
|
|||||||||
|
Depreciation and amortization
|
137,403
|
|
45,936
|
|
33,016
|
|
|||||
|
Unrealized foreign currency loss (gain)
|
18,582
|
|
(30,823
|
)
|
12,162
|
|
|||||
|
Amortization of debt issuance costs
|
5,859
|
|
4,585
|
|
3,055
|
|
|||||
|
Loss on extinguishment of debt
|
19,667
|
|
2,532
|
|
—
|
|
|||||
|
Loss on sale of certain international businesses
|
2,958
|
|
29,232
|
|
—
|
|
|||||
|
Intangible asset impairment charges
|
47,928
|
|
—
|
|
—
|
|
|||||
|
Non-cash restructuring and asset-related charges
|
11,295
|
|
—
|
|
—
|
|
|||||
|
Deferred tax (benefit) provision
|
(9,439
|
)
|
742
|
|
121
|
|
|||||
|
Stock-based compensation and PPU expense
|
167,597
|
|
—
|
|
—
|
|
|||||
|
Inventory provision
|
44,539
|
|
3,771
|
|
9,235
|
|
|||||
|
Other operating charges and credits, net
|
(1,866
|
)
|
9,935
|
|
197
|
|
|||||
|
Changes in assets and liabilities:
|
|
|
|||||||||
|
Trade accounts receivable, net
|
89,084
|
|
35,255
|
|
(122,482
|
)
|
|||||
|
Inventories
|
(42,875
|
)
|
(31,826
|
)
|
(42,587
|
)
|
|||||
|
Prepaid expenses, other current assets and other assets
|
19,198
|
|
(25,305
|
)
|
2,042
|
|
|||||
|
Related party receivables
|
10,928
|
|
(5,485
|
)
|
307
|
|
|||||
|
Accounts payable, accrued expenses and other liabilities
|
(55,212
|
)
|
18,105
|
|
6,265
|
|
|||||
|
Related party payables
|
(14,113
|
)
|
8,208
|
|
4,303
|
|
|||||
|
Net cash provided by operating activities
|
250,230
|
|
234,187
|
|
115,060
|
|
|||||
|
Cash flows from investing activities:
|
|
|
|||||||||
|
Purchases of property, plant and equipment
|
(83,088
|
)
|
(94,771
|
)
|
(122,756
|
)
|
|||||
|
Acquisition of product rights and licenses
|
(14,000
|
)
|
(19,500
|
)
|
(1,850
|
)
|
|||||
|
Acquisitions, net of cash acquired
|
(324,634
|
)
|
—
|
|
—
|
|
|||||
|
Proceeds from sales of property, plant and equipment
|
25,344
|
|
—
|
|
—
|
|
|||||
|
Proceeds from sale of certain international businesses, net of cash sold
|
—
|
|
15,717
|
|
—
|
|
|||||
|
Net cash used in investing activities
|
(396,378
|
)
|
(98,554
|
)
|
(124,606
|
)
|
|||||
|
Cash flows from financing activities:
|
|
|
|||||||||
|
Payments of deferred financing costs and debt extinguishment costs
|
(54,955
|
)
|
(5,026
|
)
|
(6,506
|
)
|
|||||
|
Proceeds from issuance of debt
|
1,325,383
|
|
250,000
|
|
225,000
|
|
|||||
|
Payments of principal on debt and capital leases
|
(617,051
|
)
|
(13,625
|
)
|
(11,137
|
)
|
|||||
|
Net (payments) borrowings on revolving credit line
|
(75,000
|
)
|
50,000
|
|
(25,000
|
)
|
|||||
|
Payments of principal on financing obligation - related party
|
(243
|
)
|
(274
|
)
|
(259
|
)
|
|||||
|
Proceeds from exercise of stock options
|
3,797
|
|
—
|
|
—
|
|
|||||
|
Equity contributions
|
27,742
|
|
40
|
|
(5
|
)
|
|||||
|
Capital contribution from (dividend to) non-controlling interest
|
360
|
|
(865
|
)
|
(973
|
)
|
|||||
|
Acquisition of redeemable non-controlling interest
|
(11,775
|
)
|
—
|
|
—
|
|
|||||
|
Tax distribution to non-controlling interest
|
(35,543
|
)
|
—
|
|
—
|
|
|||||
|
Distributions to members
|
(182,998
|
)
|
(375,265
|
)
|
(200,615
|
)
|
|||||
|
Repayment of related party notes
|
(92,042
|
)
|
—
|
|
—
|
|
|||||
|
Net cash provided by (used in) financing activities
|
287,675
|
|
(95,015
|
)
|
(19,495
|
)
|
|||||
|
Effect of foreign exchange rate on cash
|
(670
|
)
|
(242
|
)
|
1,481
|
|
|||||
|
Net increase (decrease) in cash, cash equivalents, and restricted cash
|
140,857
|
|
40,376
|
|
(27,560
|
)
|
|||||
|
Cash, cash equivalents, and restricted cash - beginning of period
|
77,922
|
|
37,546
|
|
65,106
|
|
|||||
|
Cash, cash equivalents, and restricted cash - end of period
|
$
|
218,779
|
|
$
|
77,922
|
|
$
|
37,546
|
|
||
|
Cash and cash equivalents - end of period
|
$
|
213,394
|
|
$
|
74,166
|
|
$
|
27,367
|
|
||
|
Restricted cash - end of period
|
5,385
|
|
3,756
|
|
10,179
|
|
|||||
|
Cash, cash equivalents, and restricted cash - end of period
|
$
|
218,779
|
|
$
|
77,922
|
|
$
|
37,546
|
|
||
|
Supplemental disclosure of cash flow information:
|
|||||||||||
|
Cash paid for interest
|
$
|
131,505
|
|
$
|
65,086
|
|
$
|
50,569
|
|
||
|
Cash received (paid), net for income taxes
|
$
|
34,952
|
|
$
|
(5,780
|
)
|
$
|
(4,922
|
)
|
||
|
Supplemental disclosure of non-cash investing and financing activity:
|
|||||||||||
|
Acquisition of non-controlling interest
|
$
|
3,485
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Tax distribution to non-controlling interest
|
13,412
|
|
—
|
|
—
|
|
|||||
|
Distribution to members
|
8,562
|
|
—
|
|
—
|
|
|||||
|
Receivable from the sale of certain international businesses
|
—
|
|
1,936
|
|
—
|
|
|||||
|
Note payable resulting from the Ireland building purchase
|
—
|
|
14,758
|
|
—
|
|
|||||
|
Transaction costs paid by Amneal Holdings
|
$
|
—
|
|
$
|
8,561
|
|
$
|
—
|
|
||
|
Contract Charge-backs and Sales Volume Allowances
|
Cash Discount Allowances
|
Accrued Returns Allowance
|
Accrued Medicaid and Commercial Rebates
|
|||||||||||||
|
Balance at January 1, 2016
|
$
|
330,811
|
|
$
|
14,894
|
|
$
|
32,124
|
|
$
|
14,385
|
|
||||
|
Provision related to sales recorded in the period
|
2,182,606
|
|
70,662
|
|
31,741
|
|
17,181
|
|
||||||||
|
Credits/payments issued during the period
|
(2,146,569
|
)
|
(67,118
|
)
|
(17,670
|
)
|
(23,509
|
)
|
||||||||
|
Balance at December 31, 2016
|
366,848
|
|
18,438
|
|
46,195
|
|
8,057
|
|
||||||||
|
Provision related to sales recorded in the period
|
2,489,681
|
|
79,837
|
|
24,571
|
|
25,982
|
|
||||||||
|
Credits/payments issued during the period
|
(2,402,826
|
)
|
(77,867
|
)
|
(25,591
|
)
|
(21,128
|
)
|
||||||||
|
Balance at December 31, 2017
|
453,703
|
|
20,408
|
|
45,175
|
|
12,911
|
|
||||||||
|
Liabilities assumed from acquisitions
|
|
222,970
|
|
|
11,781
|
|
|
102,502
|
|
|
51,618
|
|
||||
|
Provision related to sales recorded in the period
|
3,463,983
|
|
117,010
|
|
85,996
|
|
104,664
|
|
||||||||
|
Credits/payments issued during the period
|
(3,311,060
|
)
|
(113,042
|
)
|
(79,170
|
)
|
(94,991
|
)
|
||||||||
|
Balance at December 31, 2018
|
$
|
829,596
|
|
$
|
36,157
|
|
$
|
154,503
|
|
$
|
74,202
|
|
||||
|
Asset Classification
|
Estimated Useful Life
|
|
|
Buildings
|
30 years
|
|
|
Computer equipment
|
5 years
|
|
|
Furniture and fixtures
|
7 years
|
|
|
Leasehold improvements
|
Shorter of asset's useful life or remaining life of lease
|
|
|
Machinery and equipment
|
7 years
|
|
|
Vehicles
|
5 years
|
|
|
Fully diluted Impax share number
(1)
|
73,288,792
|
|
||
|
Closing quoted market price of an Impax common share on May 4, 2018
|
$
|
18.30
|
|
|
|
Equity consideration - subtotal
|
$
|
1,341,185
|
|
|
|
Add: Fair value of Impax stock options as of May 4, 2018
(2)
|
22,610
|
|
||
|
Total equity consideration
|
1,363,795
|
|
||
|
Add: Extinguishment of certain Impax obligations, including accrued and unpaid interest
|
320,290
|
|
||
|
Less: Cash acquired
|
(37,907
|
)
|
||
|
Purchase price, net of cash acquired
|
$
|
1,646,178
|
|
|
|
(1)
Represents shares of Impax Common Stock issued and outstanding immediately prior to the Combination.
|
||||
|
(2)
Represents the fair value of 3.0 million fully vested Impax stock options valued using the Black-Scholes options pricing model.
|
||||
|
Preliminary Fair Values
As of December 31, 2018 |
||||
|
Trade accounts receivable, net
|
$
|
211,762
|
|
|
|
Inventories
|
183,088
|
|
||
|
Prepaid expenses and other current assets
|
91,430
|
|
||
|
Property, plant and equipment
|
87,472
|
|
||
|
Goodwill
|
399,988
|
|
||
|
Intangible assets
|
1,574,929
|
|
||
|
Other
|
55,790
|
|
||
|
Total assets acquired
|
2,604,459
|
|
||
|
Accounts payable
|
47,912
|
|
||
|
Accrued expenses and other current liabilities
|
277,176
|
|
||
|
Long-term debt
|
599,400
|
|
||
|
Other long-term liabilities
|
33,793
|
|
||
|
Total liabilities assumed
|
958,281
|
|
||
|
Net assets acquired
|
$
|
1,646,178
|
|
|
|
Preliminary Fair Values
|
Weighted-Average Useful Life (Years)
|
|||||
|
Marketed product rights
|
$
|
1,045,617
|
|
12.9
|
||
|
Preliminary Fair Values
As of December 31, 2018 |
||||
|
Trade accounts receivable, net
|
$
|
8,158
|
|
|
|
Inventories
|
1,851
|
|
||
|
Prepaid expenses and other current assets
|
3,795
|
|
||
|
Property, plant and equipment, net
|
11
|
|
||
|
Goodwill
|
1,500
|
|
||
|
Intangible assets
|
142,740
|
|
||
|
Other
|
324
|
|
||
|
Total assets acquired
|
158,379
|
|
||
|
Accounts payable
|
1,764
|
|
||
|
Accrued expenses and other current liabilities
|
14,644
|
|
||
|
License liability
|
20,000
|
|
||
|
Total liabilities assumed
|
36,408
|
|
||
|
Net assets acquired
|
$
|
121,971
|
|
|
|
Preliminary Fair Values
|
Weighted-Average Useful Life
|
|||||
|
Product rights for licensed / developed technology
|
$
|
110,350
|
|
10 years
|
||
|
Product rights for developed technologies
|
5,500
|
|
9 years
|
|||
|
Product rights for out-licensed generics royalty agreement
|
390
|
|
2 years
|
|||
|
$
|
116,240
|
|
||||
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Net revenue
|
$
|
1,839,083
|
|
$
|
1,809,441
|
|
$
|
1,842,654
|
|
||
|
Net loss
|
(163,915
|
)
|
(340,223
|
)
|
(535,087
|
)
|
|||||
|
Net loss attributable to Amneal Pharmaceuticals, Inc.
|
$
|
(30,270
|
)
|
$
|
(109,920
|
)
|
$
|
(110,638
|
)
|
||
|
•
|
Adjustments to costs of goods sold related to the inventory acquired; and
|
|
•
|
Adjustments to selling, general and administrative expense related to transaction costs directly attributable to the transactions.
|
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Employee separation charges
(1)
|
$
|
45,118
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Asset-related charges
(2)
|
11,295
|
|
—
|
|
—
|
|
|||||
|
Total restructuring and asset-related charges
|
$
|
56,413
|
|
$
|
—
|
|
$
|
—
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2018
|
2017
|
2016
|
||||||||
|
Generics
|
$
|
33,943
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Specialty
|
4,076
|
|
—
|
|
—
|
|
|||||
|
Corporate
|
18,394
|
|
—
|
|
—
|
|
|||||
|
Total restructuring and asset-related charges
|
$
|
56,413
|
|
$
|
—
|
|
$
|
—
|
|
||
|
|
Employee Separation
|
||
|
Balance at December 31, 2017
|
$
|
—
|
|
|
Liabilities assumed in Impax acquisition
|
2,199
|
|
|
|
Charges to income
|
48,246
|
|
|
|
Change in estimated liability
|
(3,128
|
)
|
|
|
Payments
|
(25,205
|
)
|
|
|
Balance at December 31, 2018
|
$
|
22,112
|
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2018
|
2017
|
2016
|
||||||||
|
Acquisition, transaction-related and integration expenses
(1)
|
$
|
35,319
|
|
$
|
9,403
|
|
$
|
70
|
|
||
|
Profit participation units
(2)
|
158,757
|
|
—
|
|
—
|
|
|||||
|
Transaction-related bonus
(3)
|
27,742
|
|
—
|
|
—
|
|
|||||
|
Total
|
$
|
221,818
|
|
$
|
9,403
|
|
$
|
70
|
|
||
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
United States
|
$
|
(138,484
|
)
|
$
|
275,235
|
|
$
|
334,750
|
|
||
|
International
|
(64,238
|
)
|
(103,912
|
)
|
(119,929
|
)
|
|||||
|
Total (loss) income before income taxes
|
$
|
(202,722
|
)
|
$
|
171,323
|
|
$
|
214,821
|
|
||
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Current:
|
|||||||||||
|
Domestic
|
$
|
2,299
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Foreign
|
5,721
|
|
1,256
|
|
5,274
|
|
|||||
|
Total current income tax
|
8,020
|
|
1,256
|
|
5,274
|
|
|||||
|
Deferred:
|
|||||||||||
|
Domestic
|
(2,967
|
)
|
—
|
|
—
|
|
|||||
|
Foreign
|
(6,472
|
)
|
742
|
|
121
|
|
|||||
|
Total deferred income tax
|
(9,439
|
)
|
742
|
|
121
|
|
|||||
|
Total (benefit from) provision for income tax
|
$
|
(1,419
|
)
|
$
|
1,998
|
|
$
|
5,395
|
|
||
|
Years Ended December 31,
|
||||||||
|
2018
|
2017
|
2016
|
||||||
|
Federal income tax at the statutory rate
|
21.0
|
%
|
—
|
%
|
—
|
%
|
||
|
State income tax, net of federal benefit
|
(1.1
|
)%
|
—
|
%
|
—
|
%
|
||
|
Losses for which no benefit has been recognized
|
(12.3
|
)%
|
10.6
|
%
|
8.2
|
%
|
||
|
Foreign rate differential
|
(6.3
|
)%
|
(6.5
|
)%
|
(5.4
|
)%
|
||
|
Other
|
(0.6
|
)%
|
(2.9
|
)%
|
(0.3
|
)%
|
||
|
Effective income tax rate
|
0.7
|
%
|
1.2
|
%
|
2.5
|
%
|
||
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Balance at the beginning of the period
|
$
|
41,617
|
|
$
|
42,231
|
|
$
|
22,567
|
|
||
|
(Decreases) increases due to net operating losses and temporary differences
|
(382
|
)
|
23,286
|
|
19,664
|
|
|||||
|
Divestitures
|
—
|
|
(23,900
|
)
|
—
|
|
|||||
|
Balance at the end of the period
|
$
|
41,235
|
|
$
|
41,617
|
|
$
|
42,231
|
|
||
|
December 31, 2018
|
December 31, 2017
|
||||||
|
Deferred tax assets:
|
|||||||
|
Partnership interest in Amneal
|
$
|
240,044
|
|
$
|
—
|
|
|
|
Projected imputed interest on TRA
|
9,838
|
|
—
|
|
|||
|
Net operating loss carryforward
|
107,942
|
|
34,889
|
|
|||
|
IRC Section 163(j) interest carryforward
|
33,789
|
|
—
|
|
|||
|
Capitalized costs
|
900
|
|
949
|
|
|||
|
Accrued expenses
|
4,298
|
|
985
|
|
|||
|
Intangible assets
|
1,553
|
|
122
|
|
|||
|
Tax credits and other
|
16,030
|
|
6,366
|
|
|||
|
Total deferred tax assets
|
414,394
|
|
43,311
|
|
|||
|
Valuation allowance
|
(41,235
|
)
|
(41,617
|
)
|
|||
|
Net deferred tax assets
|
373,159
|
|
1,694
|
|
|||
|
Deferred tax liabilities:
|
|||||||
|
Fixed assets
|
—
|
|
(3,287
|
)
|
|||
|
Intangible assets
|
(1,178
|
)
|
—
|
|
|||
|
Total deferred tax liabilities
|
(1,178
|
)
|
(3,287
|
)
|
|||
|
Net deferred tax assets (liabilities)
|
$
|
371,981
|
|
$
|
(1,593
|
)
|
|
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Unrecognized tax benefits at the beginning of the period
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Gross change for current period positions
|
182
|
|
—
|
|
—
|
|
|||||
|
Gross change for prior period positions
|
2,346
|
|
—
|
|
—
|
|
|||||
|
Gross change due to Combination
|
5,208
|
|
—
|
|
—
|
|
|||||
|
Decrease due to expiration of statutes of limitations
|
(530
|
)
|
—
|
|
—
|
|
|||||
|
Decrease due to settlements and payments
|
—
|
|
—
|
|
—
|
|
|||||
|
Unrecognized tax benefits at the end of the period
|
$
|
7,206
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Years Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Numerator:
|
|||||||||||
|
Net loss attributable to Amneal Pharmaceuticals, Inc.
|
$
|
(20,920
|
)
|
$
|
—
|
|
$
|
—
|
|
||
|
Denominator:
|
|||||||||||
|
Weighted-average shares of Class A Common Stock and Class B-1 Common Stock outstanding-basic and diluted
|
127,252
|
|
|||||||||
|
Net loss per share attributable to Amneal Pharmaceuticals, Inc.'s common stockholders:
|
|||||||||||
|
Class A and Class B-1 basic and diluted
|
$(0.16)
|
||||||||||
|
Years Ended December 31,
|
||||||||
|
2018
|
2017
|
2016
|
||||||
|
Stock options
(1)
|
5,815
|
|
|
—
|
|
—
|
|
|
|
Restricted stock units
(1)
|
1,331
|
|
|
—
|
|
—
|
|
|
|
Shares of Class B Common Stock
(2)
|
171,261
|
|
|
—
|
|
—
|
|
|
|
December 31, 2018
|
December 31, 2017
|
||||||
|
Gross accounts receivable
|
$
|
1,349,588
|
|
$
|
827,302
|
|
|
|
Allowance for doubtful accounts
|
(2,340
|
)
|
(1,824
|
)
|
|||
|
Contract charge-backs and sales volume allowances
|
(829,596
|
)
|
(453,703
|
)
|
|||
|
Cash discount allowances
|
(36,157
|
)
|
(20,408
|
)
|
|||
|
Subtotal
|
(868,093
|
)
|
(475,935
|
)
|
|||
|
Trade accounts receivable, net
|
$
|
481,495
|
|
$
|
351,367
|
|
|
|
|
December 31, 2018
|
December 31, 2017
|
|||||
|
Raw materials
|
$
|
181,654
|
|
$
|
140,051
|
|
|
|
Work in process
|
54,152
|
|
38,146
|
|
|||
|
Finished goods
|
221,413
|
|
105,841
|
|
|||
|
Total inventories
|
$
|
457,219
|
|
$
|
284,038
|
|
|
|
December 31, 2018
|
December 31, 2017
|
||||||
|
Deposits and advances
|
$
|
2,142
|
|
$
|
1,851
|
|
|
|
Prepaid insurance
|
6,094
|
|
3,154
|
|
|||
|
Prepaid regulatory fees
|
4,924
|
|
5,926
|
|
|||
|
Levothyroxine transition contract asset
(1)
|
36,393
|
|
—
|
|
|||
|
Income tax receivable
|
29,625
|
|
—
|
|
|||
|
Other current receivables
|
16,979
|
|
15,150
|
|
|||
|
Other prepaid assets
|
32,164
|
|
16,315
|
|
|||
|
Total prepaid expenses and other current assets
|
$
|
128,321
|
|
$
|
42,396
|
|
|
|
December 31, 2018
|
December 31, 2017
|
||||||
|
Land
|
$
|
1,572
|
|
$
|
5,275
|
|
|
|
Buildings
|
233,185
|
|
227,864
|
|
|||
|
Leasehold improvements
|
98,399
|
|
70,354
|
|
|||
|
Machinery and equipment
|
334,351
|
|
260,637
|
|
|||
|
Furniture and fixtures
|
10,779
|
|
18,415
|
|
|||
|
Vehicles
|
1,506
|
|
1,517
|
|
|||
|
Computer equipment
|
33,019
|
|
26,831
|
|
|||
|
Construction-in-progress
|
40,771
|
|
32,235
|
|
|||
|
Total property, plant, and equipment
|
753,582
|
|
643,128
|
|
|||
|
Less: Accumulated depreciation
|
(209,436
|
)
|
(156,370
|
)
|
|||
|
Property, plant, and equipment, net
|
$
|
544,146
|
|
$
|
486,758
|
|
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2018
|
2017
|
2016
|
||||||||
|
Depreciation
|
$
|
64,417
|
|
$
|
41,962
|
|
$
|
29,314
|
|
||
|
|
December 31, 2018
|
December 31, 2017
|
|||||
|
Balance, beginning of period
|
$
|
26,444
|
|
$
|
28,441
|
|
|
|
Goodwill acquired during the period
|
401,488
|
|
—
|
|
|||
|
Goodwill divested during the period
|
—
|
|
(3,895
|
)
|
|||
|
Currency translation
|
(1,706
|
)
|
1,898
|
|
|||
|
Balance, end of period
|
$
|
426,226
|
|
$
|
26,444
|
|
|
|
December 31, 2018
|
December 31, 2017
|
||||||||||||||||||||||||
|
Weighted-Average Amortization Period (in years)
|
Cost
|
Accumulated Amortization
|
Net
|
Cost
|
Accumulated Amortization
|
Net
|
|||||||||||||||||||
|
Amortizing intangible assets:
|
|||||||||||||||||||||||||
|
Product rights
|
12.4
|
$
|
1,282,011
|
|
$
|
(88,081
|
)
|
$
|
1,193,930
|
|
$
|
49,700
|
|
$
|
(17,210
|
)
|
$
|
32,490
|
|
||||||
|
Customer relationships
|
14.4
|
7,005
|
|
(1,955
|
)
|
5,050
|
|
7,421
|
|
(1,072
|
)
|
6,349
|
|
||||||||||||
|
Other intangible assets
|
12.5
|
$
|
5,620
|
|
$
|
(1,561
|
)
|
$
|
4,059
|
|
$
|
5,775
|
|
$
|
(1,165
|
)
|
$
|
4,610
|
|
||||||
|
Total
|
|
$
|
1,294,636
|
|
$
|
(91,597
|
)
|
$
|
1,203,039
|
|
$
|
62,896
|
|
$
|
(19,447
|
)
|
$
|
43,449
|
|
||||||
|
In-process research and development
|
|
451,930
|
|
—
|
|
451,930
|
|
1,150
|
|
—
|
|
1,150
|
|
||||||||||||
|
Total intangible assets
|
$
|
1,746,566
|
|
$
|
(91,597
|
)
|
$
|
1,654,969
|
|
$
|
64,046
|
|
$
|
(19,447
|
)
|
$
|
44,599
|
|
|||||||
|
|
Years Ended December 31,
|
||||||||||
|
|
2018
|
2017
|
2016
|
||||||||
|
Amortization
|
$
|
72,986
|
|
$
|
3,974
|
|
$
|
3,702
|
|
||
|
Future Amortization
|
||||
|
2019
|
$
|
123,497
|
|
|
|
2020
|
130,154
|
|
||
|
2021
|
146,843
|
|
||
|
2022
|
149,053
|
|
||
|
2023
|
127,249
|
|
||
|
Thereafter
|
526,243
|
|
||
|
Total
|
$
|
1,203,039
|
|
|
|
|
December 31, 2018
|
December 31, 2017
|
|||||
|
Accounts payable
|
$
|
114,846
|
|
$
|
70,013
|
|
|
|
Accrued returns allowance
|
154,503
|
|
45,175
|
|
|||
|
Accrued compensation
|
77,066
|
|
23,954
|
|
|||
|
Accrued Medicaid and commercial rebates
|
74,202
|
|
12,911
|
|
|||
|
Accrued royalties
|
23,639
|
|
2,970
|
|
|||
|
Estimated Teva and Allergan chargebacks and rebates
(1)
|
13,277
|
|
—
|
|
|||
|
Medicaid reimbursement accrual
|
15,000
|
|
15,000
|
|
|||
|
Accrued professional fees
|
4,555
|
|
938
|
|
|||
|
Accrued other
|
37,352
|
|
23,818
|
|
|||
|
Total accounts payable and accrued expenses
|
$
|
514,440
|
|
$
|
194,779
|
|
|
|
|
December 31, 2018
|
|
December 31, 2017
|
||||
|
Senior Secured Credit Facility – Term Loan due May 2025
|
$
|
2,685,876
|
|
|
$
|
—
|
|
|
Senior Credit Facility – Term Loan
|
—
|
|
1,378,160
|
|
|||
|
Senior Credit Facility – Revolver
|
—
|
|
75,000
|
|
|||
|
Other
|
624
|
|
—
|
|
|||
|
Total debt
|
2,686,500
|
|
|
1,453,160
|
|
||
|
Less: debt issuance costs
|
(34,453
|
)
|
|
(8,715)
|
|
||
|
Total debt, net of debt issuance costs
|
2,652,047
|
|
|
1,444,445
|
|
||
|
Less: current portion of long-term debt
|
(21,449)
|
|
|
(89,171)
|
|
||
|
Total long-term debt, net
|
$
|
2,630,598
|
|
|
$
|
1,355,274
|
|
|
Fair Value Measurement Based on
|
||||||||||||||||
|
Total
|
Quoted Prices in Active Markets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant Unobservable
Inputs
(Level 3)
|
|||||||||||||
|
Assets
|
||||||||||||||||
|
Deferred Compensation Plan asset
(1)
|
$
|
40,101
|
|
$
|
—
|
|
$
|
40,101
|
|
$
|
—
|
|
||||
|
Liabilities
|
||||||||||||||||
|
Deferred Compensation Plan liabilities
(1)
|
$
|
27,978
|
|
$
|
—
|
|
$
|
27,978
|
|
$
|
—
|
|
||||
|
|
|
Operating Leases
|
||
|
2019
|
|
$
|
25,885
|
|
|
2020
|
|
12,071
|
|
|
|
2021
|
|
11,105
|
|
|
|
2022
|
|
10,329
|
|
|
|
2023
|
10,043
|
|
||
|
Thereafter
|
|
28,128
|
|
|
|
Total
|
|
$
|
97,561
|
|
|
Stock Options
|
Number of
Shares Under Option |
Weighted-
Average Exercise Price per Share |
Weighted-
Average Remaining Contractual Life |
Aggregate
Intrinsic Value (in millions) |
||||||||
|
Outstanding at December 31, 2017
|
—
|
|
$
|
—
|
|
|||||||
|
Conversion of Impax stock options outstanding on May 4, 2018
|
3,002,669
|
|
18.90
|
|
||||||||
|
Options granted
|
3,555,808
|
|
16.64
|
|
||||||||
|
Options exercised
|
(351,668
|
)
|
10.80
|
|
||||||||
|
Options forfeited
|
(392,228
|
)
|
23.02
|
|
||||||||
|
Outstanding at December 31, 2018
|
5,814,581
|
|
$
|
17.73
|
|
8.0
|
$
|
2.6
|
|
|||
|
Options exercisable at December 31, 2018
|
2,438,046
|
|
$
|
19.37
|
|
6.0
|
$
|
2.6
|
|
|||
|
Restricted Stock Units
|
Number of
Restricted Stock Units |
Weighted-
Average Grant Date Fair Value |
Weighted-
Average Remaining Years |
Aggregate
Intrinsic Value (in millions) |
||||||||
|
Non-vested at December 31, 2017
|
—
|
|
$
|
—
|
|
|||||||
|
Granted
|
1,421,814
|
|
17.28
|
|
||||||||
|
Vested
|
—
|
|
—
|
|
||||||||
|
Forfeited
|
(91,190
|
)
|
19.19
|
|
||||||||
|
Non-vested at December 31, 2018
|
1,330,624
|
|
$
|
17.15
|
|
3.3
|
$
|
18.0
|
|
|||
|
|
December 31, 2018
|
|
Volatility
|
46.5%
|
|
Risk-free interest rate
|
2.9%
|
|
Dividend yield
|
—%
|
|
Weighted-average expected life (years)
|
6.25
|
|
Weighted average grant date fair value
|
$8.14
|
|
|
Year Ended December 31,
|
||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Cost of goods sold
|
$
|
921
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Selling, general and administrative
|
6,923
|
|
—
|
|
—
|
|
|||||
|
Research and development
|
996
|
|
—
|
|
—
|
|
|||||
|
Total
|
$
|
8,840
|
|
$
|
—
|
|
$
|
—
|
|
||
|
|
|
Payments Due
|
||
|
2019
|
|
$
|
5,474
|
|
|
2020
|
|
5,474
|
|
|
|
2021
|
|
5,474
|
|
|
|
2022
|
|
5,474
|
|
|
|
2023
|
5,474
|
|
||
|
Thereafter
|
|
107,196
|
|
|
|
Total
|
|
$
|
134,566
|
|
|
Year Ended December 31, 2018
|
Generics
|
Specialty
|
Corporate and Other
|
Total
Company |
||||||||||||
|
Net revenue
|
$
|
1,439,031
|
|
$
|
223,960
|
|
$
|
—
|
|
$
|
1,662,991
|
|
||||
|
Cost of goods sold
|
842,996
|
|
103,592
|
|
—
|
|
946,588
|
|
||||||||
|
Gross profit
|
596,035
|
|
120,368
|
|
—
|
|
716,403
|
|
||||||||
|
Selling, general and administrative
|
68,426
|
|
49,465
|
|
112,544
|
|
230,435
|
|
||||||||
|
Research and development
|
183,412
|
|
10,778
|
|
—
|
|
194,190
|
|
||||||||
|
In-process research and development impairment charges
|
39,259
|
|
—
|
|
—
|
|
39,259
|
|
||||||||
|
Acquisition, transaction-related and integration expenses
|
114,622
|
|
—
|
|
107,196
|
|
221,818
|
|
||||||||
|
Restructuring and asset-related charges
|
33,943
|
|
4,076
|
|
18,394
|
|
56,413
|
|
||||||||
|
Intellectual property legal development expenses
|
15,772
|
|
489
|
|
—
|
|
16,261
|
|
||||||||
|
Legal settlement gains
|
(22,300
|
)
|
—
|
|
—
|
|
(22,300
|
)
|
||||||||
|
Operating income (loss)
|
$
|
162,901
|
|
$
|
55,560
|
|
$
|
(238,134
|
)
|
$
|
(19,673
|
)
|
||||
|
Year Ended December 31, 2017
|
Generics
|
Specialty
|
Corporate
and Other |
Total
Company |
||||||||||||
|
Net revenue
|
$
|
1,033,654
|
|
$
|
—
|
|
$
|
—
|
|
$
|
1,033,654
|
|
||||
|
Cost of goods sold
|
507,476
|
|
—
|
|
—
|
|
507,476
|
|
||||||||
|
Gross profit
|
526,178
|
|
—
|
|
—
|
|
526,178
|
|
||||||||
|
Selling, general and administrative
|
56,050
|
|
—
|
|
52,996
|
|
109,046
|
|
||||||||
|
Research and development
|
171,420
|
|
—
|
|
—
|
|
171,420
|
|
||||||||
|
Intellectual property legal development expenses
|
20,518
|
|
—
|
|
—
|
|
20,518
|
|
||||||||
|
Legal settlement gains
|
(29,312
|
)
|
—
|
|
—
|
|
(29,312
|
)
|
||||||||
|
Acquisition and transaction-related expenses
|
—
|
|
—
|
|
9,403
|
|
9,403
|
|
||||||||
|
Operating income (loss)
|
$
|
307,502
|
|
$
|
—
|
|
$
|
(62,399
|
)
|
$
|
245,103
|
|
||||
|
Year Ended December 31, 2016
|
Generics
|
Specialty
|
Corporate
and Other |
Total
Company |
||||||||||||
|
Net revenue
|
$
|
1,018,225
|
|
$
|
—
|
|
$
|
—
|
|
$
|
1,018,225
|
|
||||
|
Cost of goods sold
|
420,770
|
|
—
|
|
—
|
|
420,770
|
|
||||||||
|
Gross profit
|
597,455
|
|
—
|
|
—
|
|
597,455
|
|
||||||||
|
Selling, general and administrative
|
69,540
|
|
—
|
|
49,217
|
|
118,757
|
|
||||||||
|
Research and development
|
179,019
|
|
—
|
|
—
|
|
179,019
|
|
||||||||
|
Intellectual property legal development expenses
|
25,728
|
|
—
|
|
—
|
|
25,728
|
|
||||||||
|
Legal settlement gains
|
(11,000
|
)
|
—
|
|
—
|
|
(11,000
|
)
|
||||||||
|
Acquisition and transaction-related expenses
|
—
|
|
—
|
|
70
|
|
70
|
|
||||||||
|
Operating income (loss)
|
$
|
334,168
|
|
$
|
—
|
|
$
|
(49,287
|
)
|
$
|
284,881
|
|
||||
|
Segment
|
Product Family
|
Year Ended December 31, 2018
|
||||||
|
$
|
%
|
|||||||
|
Generics
|
Yuvafem-Estradiol
|
$
|
130,920
|
|
8%
|
|||
|
Generics
|
Diclofenac Sodium Gel
|
103,131
|
|
6%
|
||||
|
Specialty
|
Rytary® family
|
95,541
|
|
6%
|
||||
|
Generics
|
Aspirin; Dipyridamole ER Capsul
|
78,541
|
|
5%
|
||||
|
Generics
|
Epinephrine Auto-Injector family (generic Adrenaclick®)
|
$
|
67,529
|
|
4%
|
|||
|
Segment
|
Product Family
|
Year Ended December 31, 2017
|
||||||
|
$
|
%
|
|||||||
|
Generics
|
Yuvafem-Estradiol
|
$
|
130,480
|
|
13%
|
|||
|
Generics
|
Diclofenac Sodium Gel
|
94,395
|
|
9%
|
||||
|
Generics
|
Aspirin; Dipyridamole ER Capsul
|
79,674
|
|
8%
|
||||
|
Generics
|
Oseltamivir
|
37,240
|
|
4%
|
||||
|
Generics
|
Ranitidine
|
$
|
31,283
|
|
3%
|
|||
|
Segment
|
Product Family
|
Year Ended December 31, 2016
|
||||||
|
$
|
%
|
|||||||
|
Generics
|
Lidocaine
|
$
|
121,832
|
|
12%
|
|||
|
Generics
|
Diclofenac Sodium Gel
|
71,672
|
|
7%
|
||||
|
Generics
|
Yuvafem-Estradiol
|
53,025
|
|
5%
|
||||
|
Generics
|
Metaxalone
|
33,698
|
|
3%
|
||||
|
Generics
|
Metformin ER
|
$
|
33,420
|
|
3%
|
|||
|
|
Quarters Ended
|
|||||||||||||||
|
2018
(1) (2)
|
March 31
|
June 30
|
September 30
|
December 31
|
||||||||||||
|
|
||||||||||||||||
|
Net revenue
|
$
|
275,189
|
|
$
|
413,787
|
|
$
|
476,487
|
|
$
|
497,528
|
|
||||
|
Gross profit
|
144,595
|
|
178,295
|
|
200,105
|
|
193,408
|
|
||||||||
|
Net income (loss)
|
51,652
|
|
(250,090
|
)
|
17,465
|
|
(20,330
|
)
|
||||||||
|
Net (loss) income attributable to Amneal Pharmaceuticals, Inc.
|
—
|
|
(19,104
|
)
|
6,952
|
|
(8,768
|
)
|
||||||||
|
Net income (loss) per share attributable to Amneal Pharmaceuticals, Inc.'s common stockholders:
|
|
|
|
|
||||||||||||
|
Class A and Class B-1 basic
|
—
|
|
(0.15
|
)
|
0.05
|
|
(0.07
|
)
|
||||||||
|
Class A and Class B-1 diluted
|
$
|
—
|
|
$
|
(0.15
|
)
|
$
|
0.05
|
|
$
|
(0.07
|
)
|
||||
|
Quarters Ended
|
||||||||||||||||
|
2017
(2)
|
March 31
|
June 30
|
September 30
|
December 31
|
||||||||||||
|
|
||||||||||||||||
|
Net revenue
|
$
|
225,681
|
|
$
|
259,871
|
|
$
|
254,733
|
|
$
|
293,369
|
|
||||
|
Gross profit
|
116,016
|
|
123,733
|
|
135,013
|
|
151,416
|
|
||||||||
|
Net income
|
42,261
|
|
37,748
|
|
27,122
|
|
62,194
|
|
||||||||
|
Net income attributable to Amneal Pharmaceuticals, Inc.
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||
|
Net income per share attributable to Amneal Pharmaceuticals, Inc.'s common stockholders:
|
|
|
|
|
||||||||||||
|
Class A and Class B-1 basic
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||
|
Class A and Class B-1 diluted
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Exhibit No.
|
Description of Document
|
|
|
Business Combination Agreement, dated as of October 17, 2017, by and among Amneal Pharmaceuticals LLC, Impax Laboratories, Inc., Atlas Holdings, Inc. and K2 Merger Sub Corporation (incorporated by reference to Exhibit 2.1 to the Company’s Registration Statement on Form S-1 filed on May 7, 2018).
|
||
|
Amendment No. 1, dated as of November 21, 2017, to the Business Combination Agreement, dated as of as of October 17, 2017, by and among Amneal Pharmaceuticals LLC, Impax Laboratories, Inc., Atlas Holdings, Inc. and K2 Merger Sub Corporation (incorporated by reference to Exhibit 2.2 to the Company’s Registration Statement on Form S-1 filed on May 7, 2018).
|
||
|
Amendment No. 2, dated as of December 16, 2017, to the Business Combination Agreement, dated as of as of October 17, 2017, as amended by Amendment No. 1 dated as of November 21, 2017 by and among Amneal Pharmaceuticals LLC, Impax Laboratories, Inc., Atlas Holdings, Inc. and K2 Merger Sub Corporation (incorporated by reference to Exhibit 2.3 to the Company’s Registration Statement on Form S-1 filed on May 7, 2018).
|
||
|
Purchase and Sale Agreement, dated as of May 7, 2018, by and between Amneal Pharmaceuticals LLC, Gemini Laboratories, LLC, the parties signatory thereto and the Sellers’ Representative (incorporated by reference to Exhibit 2.2 to the Company’s Current Report on Form 8-K filed on May 7, 2018).
|
||
|
Amended and Restated Certificate of Incorporation of Amneal Pharmaceuticals, Inc. adopted as of May 4, 2018 (incorporated by reference to Exhibit 3.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2018 filed on August 9, 2018).
|
||
|
Amended and Restated Bylaws of Amneal Pharmaceuticals, Inc. adopted as of May 4, 2018 (incorporated by reference to Exhibit 3.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2018 filed on August 9, 2018)
|
||
|
Second Supplemental Indenture dated as of May 4, 2018 to the Indenture dated as of June 30, 2015 by and between Impax Laboratories, LLC and Wilmington Trust, N.A. (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on May 7, 2018).
|
||
|
Term Loan Credit Agreement, dated as of May 4, 2018, by and among Amneal Pharmaceuticals LLC, as the borrower, JP Morgan Chase Bank, N.A., as administrative agent and collateral agent, and the lenders and other parties party thereto (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on May 7, 2018).
|
||
|
Revolving Credit Agreement, dated as of May 4, 2018, by and among Amneal Pharmaceuticals LLC, as the borrower, the other loan parties from time to time, JP Morgan Chase Bank, N.A., as administrative agent and collateral agent and the lenders and other parties party thereto (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on May 7, 2018).
|
||
|
Term Loan Guarantee and Collateral Agreement, dated as of May 4, 2018, by and among the loan parties from time to time party thereto and JPMorgan Chase Bank, N.A., as administrative agent and collateral agent (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on May 7, 2018).
|
||
|
Revolving Loan Guarantee and Collateral Agreement, dated as of May 4, 2018, by and among the loan parties from time to time party thereto and JPMorgan Chase Bank, N.A., as administrative agent and collateral agent (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K filed on May 7, 2018).
|
||
|
Third Amended and Restated Limited Liability Company Agreement of Amneal Pharmaceuticals LLC adopted as of May 4, 2018 (incorporated by reference to Exhibit 10.5 to the Company’s Current Report on Form 8-K filed on May 7, 2018).
|
||
|
Amendment No. 1 to Third Amended and Restated Limited Liability Company Agreement of Amneal Pharmaceuticals LLC, dated as of February 14, 2019, with effect as of May 4, 2018.*
|
||
|
Tax Receivable Agreement, dated as of May 4, 2018, by and among Amneal Pharmaceuticals, Inc., Amneal Pharmaceuticals LLC and the Members of Amneal Pharmaceuticals LLC from time to time party thereto (incorporated by reference to Exhibit 10.6 to the Company’s Current Report on Form 8-K filed on May 7, 2018).
|
||
|
Form of Indemnification and Advancement Agreement for the directors and officers of the Company (incorporated by reference to Exhibit 10.7 to the Company’s Current Report on Form 8-K filed on May 7, 2018). †
|
||
|
Amneal Pharmaceuticals, Inc. 2018 Incentive Award Plan (incorporated by reference to Exhibit 10.8 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2018 filed on August 9, 2018)†
|
||
|
Form of Amneal Pharmaceuticals, Inc. 2018 Incentive Award Plan Stock Option Grant Notice and Stock Option Agreement (incorporated by reference to Exhibit 10.9 to the Company’s Current Report on Form 8-K filed on May 7, 2018). †
|
||
|
Form of Amneal Pharmaceuticals, Inc. 2018 Incentive Award Plan Restricted Stock Unit Grant Notice and Restricted Stock Unit Agreement (incorporated by reference to Exhibit 10.10 to the Company’s Current Report on Form 8-K filed on May 7, 2018). †
|
||
|
Form of Amneal Pharmaceuticals, Inc. 2018 Incentive Award Plan Performance Restricted Stock Unit Grant Notice and Performance Restricted Stock Unit Agreement.* †
|
||
|
Amneal Pharmaceuticals, Inc. Non-Employee Director Compensation Policy (incorporated by reference to Exhibit 10.11 to the Company’s Current Report on Form 8-K filed on May 7, 2018). †
|
||
|
Employment Agreement, dated May 4, 2018, by and between Amneal Pharmaceuticals, Inc. and Paul M. Bisaro (incorporated by reference to Exhibit 10.12 to the Company’s Current Report on Form 8-K filed on May 12, 2018). †
|
||
|
Employment Agreement, dated December 12, 2012, by and among Impax Laboratories, Inc. and Bryan M. Reasons (incorporated by reference to Exhibit 10.11 to the Company's Registration Statement on Form S-1 filed on May 7, 2018).†
|
||
|
Amendment to Employment Agreement, dated April 1, 2014, by and between Impax Laboratories, Inc. and Bryan M. Reasons (incorporated by reference to Exhibit 10.12 to the Company's Registration Statement on Form S-1 filed on May 7, 2018).†
|
||
|
Employment Agreement, dated January 24, 2018, by and among Amneal Pharmaceuticals LLC, Amneal Holdings, LLC and Andrew Boyer (incorporated by reference to Exhibit 10.10 to the Company's Registration Statement on Form S-1 filed on May 7, 2018).†
|
||
|
Employment Agreement, dated December 16, 2017, by and among Amneal Pharmaceuticals LLC, Atlas Holdings, Inc. and Robert A. Stewart (incorporated by reference to Exhibit 10.7 to the Company's Registration Statement on Form S-1 filed on May 7, 2018).†
|
||
|
Employment Agreement, dated January 21, 2019, by and between Amneal Pharmaceuticals LLC, Amneal Pharmaceuticals, Inc., and Todd P. Branning (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on January 24, 2019). †
|
||
|
Separation Agreement, dated February 5, 2019, by and between Sheldon Hirt and Amneal Pharmaceuticals, Inc.* †
|
||
|
Separation Agreement, dated February 28, 2019, by and between Bryan Reasons and Amneal Pharmaceuticals, Inc.* †
|
||
|
Unsecured Promissory Note, dated as of May 7, 2018, issued by Amneal Pharmaceuticals LLC to the Sellers (as defined therein) (incorporated by reference to Exhibit 10.13 to the Company’s Current Report on Form 8-K filed on May 12, 2018).
|
||
|
Amneal Pharmaceuticals LLC Severance Plan and Summary Plan Description (incorporated by reference to Exhibit 10.14 to the Company’s Current Report on Form 8-K filed on May 12, 2018). †
|
||
|
Impax Laboratories, Inc. Executive Non-Qualified Deferred Compensation Plan, amended and restated effective January 1, 2008 (incorporated by reference to Exhibit 10.13 to the Company's Registration Statement on Form S-1 filed on May 7, 2018).†
|
||
|
Amendment to Impax Laboratories, Inc. Executive Non-Qualified Deferred Compensation Plan, effective as of January 1, 2009 (incorporated by reference to Exhibit 10.14 to the Company's Registration Statement on Form S-1 filed on May 7, 2018).†
|
||
|
Subsidiaries of the registrant.
|
||
|
Consent of Independent Registered Public Accounting Firm.
|
||
|
Certification of the Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.*
|
||
|
Certification of the Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.*
|
||
|
Certification of the Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.* **
|
||
|
Certification of the Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.* **
|
||
|
101
|
The following materials from the Company’s Annual Report on Form 10-K for the year ended December 31, 2018, formatted in XBRL (eXtensible Business Reporting Language): (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Operations, (iii) Consolidated Statements of Comprehensive Loss, (iv) Consolidated Statements of Changes in Stockholders’ Equity
/
Members’ Deficit, (v) Consolidated Statements of Cash Flows, and (vi) Notes to Consolidated Financial Statements.
|
|
|
Date: March 1, 2019
|
Amneal Pharmaceuticals, Inc.
|
|
|
By:
|
/s/ Todd P. Branning
|
|
|
Todd P. Branning
|
||
|
Senior Vice President and Chief Financial Officer
(Principal Financial and Accounting Officer)
|
||
|
Signature
|
Title
|
Date
|
||
|
/s/ Robert A. Stewart
|
President, Chief Executive Officer and Director
|
March 1, 2019
|
||
|
Robert A. Stewart
|
(Principal Executive Officer)
|
|
||
|
|
|
|
||
|
/s/ Todd P. Branning
|
Senior Vice President and
|
March 1, 2019
|
||
|
Todd P. Branning
|
Chief Financial Officer
|
|
||
|
|
(Principal Financial and Accounting Officer)
|
|
||
|
|
|
|||
|
/s/ Paul M. Bisaro
|
Executive Chairman and Director
|
March 1, 2019
|
||
|
Paul M. Bisaro
|
|
|
||
|
|
|
|
||
|
/s/ Chirag Patel
|
Co-Chairman of the Board of Directors
|
March 1, 2019
|
||
|
Chirag Patel
|
|
|
||
|
|
|
|
||
|
/s/ Chintu Patel
|
Co-Chairman of the Board of Directors
|
March 1, 2019
|
||
|
Chintu Patel
|
|
|
||
|
|
|
|
||
|
/s/ Robert L. Burr
|
Director
|
March 1, 2019
|
||
|
Robert L. Burr
|
|
|
||
|
|
|
|
||
|
/s/ Emily Peterson Alva
|
Director
|
March 1, 2019
|
||
|
Emily Peterson Alva
|
|
|
||
|
|
|
|
||
|
/s/ J. Kevin Buchi
|
Director
|
March 1, 2019
|
||
|
J. Kevin Buchi
|
|
|
||
|
|
|
|
||
|
/s/ Jean Selden Greene
|
Director
|
March 1, 2019
|
||
|
Jean Selden Greene
|
|
|
||
|
|
|
|
||
|
/s/ Ted Nark
|
Director
|
March 1, 2019
|
||
|
Ted Nark
|
|
|
||
|
|
|
|
||
|
/s/ Gautam Patel
|
Director
|
March 1, 2019
|
||
|
Gautam Patel
|
|
|
||
|
|
|
|
||
|
/s/ Dharmendra Rama
|
Director
|
March 1, 2019
|
||
|
Dharmendra Rama
|
|
|
||
|
|
|
|
||
|
/s/ Peter R. Terreri
|
Director
|
March 1, 2019
|
||
|
Peter R. Terreri
|
|
|
||
|
|
|
|
||
|
/s/ Janet S. Vergis
|
Director
|
March 1, 2019
|
||
|
Janet S. Vergis
|
|
|
||