|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the fiscal year ended May 31, 2019
|
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the transition period from to
|
|

|
Delaware
|
|
11-3146460
|
|
(State or other jurisdiction of
incorporation or organization)
|
|
(I.R.S. Employer
Identification No.)
|
|
14 Plaza Drive Latham, New York
|
|
12110
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
|
Title of each class
|
|
Name of each exchange on which registered
|
|
Common Stock, par value $.01 per share
|
|
NASDAQ Global Select Market
|
|
|
||
|
Large accelerated filer
x
|
|
Accelerated filer
¨
|
|
|
Non-accelerated filer
¨
|
|
Smaller reporting company
¨
|
|
|
Emerging growth company
¨
|
|||
|
Page
|
||
|
Part I:
|
||
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
Part II:
|
||
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
Part III:
|
||
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
Part IV:
|
||
|
Item 15.
|
||
|
Item 1.
|
Business.
|



|
•
|
Soft-Vu flush catheters are available in flush and selective varieties. Flush catheters are used in procedures where a high flow of contrast is required for “big picture” diagnostics. Anomalies discovered through a flush angiogram may require further investigation into a vessel of interest. Soft-Vu selective catheters are used to gain access to smaller or more distal vessels and advance the catheter or wire into the diseased section.
|
|
•
|
Accu-Vu sizing catheters feature radiopaque marker bands at the distal (farthest away) portion of the catheter to provide a highly accurate measurement of the patient’s anatomy. This enables precise measurement for interventional devices (stents, filters, etc.)
|
|
•
|
AngiOptic catheters have total catheter radiopacity, ensuring tip-to-hub visibility. This catheter is also constructed with a firm tip material that enhances stability during high-flow injections, providing excellent pushability.
|
|
•
|
Mariner catheters have a hydrophilic coating that, when combined with water, reduces friction. This makes insertion potentially easier and more comfortable for the patient, and can also be used for advancing through tortuous anatomy.
|


|
•
|
BioFlo
®
PICC
: Our BioFlo line is the only power injectable PICC available that incorporates Endexo Technology into the manufacturing and design of the catheter. Advanced features such as large lumen diameters allow the BioFlo
®
PICC to deliver the power injection flow rates required for contrast-enhanced Computed Tomography (CT) scans compatible with up to 325 psi CT injections.
|
|
•
|
BioFlo
®
Midline:
The BioFlo Midline Catheter is an effective solution to preserving a patient’s peripheral access. It provides a cost-effective alternative to multiple IV site rotations for patients who need short-term venous access.
|
|
•
|
Xcela PICC
: The Xcela
®
PICC line is designed to provide a high degree of safety, ease and confidence in patient care. Advanced features such as large lumen diameters allow the Xcela
®
PICC to deliver the power injection flow rates required for contrast-enhanced CTs compatible with up to 325 psi CT injections.
|
|
•
|
PASV
®
Valve Technology:
The PASV
®
Valve Technology is available in both BioFlo and Xcela lines and is designed to automatically resist backflow and reduce blood reflux that could lead to catheter-related complications.
|
|
•
|
BioFlo
®
Port
: Our BioFlo Port is the only port available that features a catheter with Endexo Technology. Advanced features of the BioFlo Port include multiple profile and catheter options, a large septum area for ease of access and the ability to administer contrast through a CT injection for purposes of imaging.
|
|
•
|
SmartPort
®
: The Smart Port power-injectable port with Vortex technology offers the ability for a clinician to access a vein for both the delivery of medications or fluids and for administering power-injected contrast to perform a (CT) scan. The ability to access a port for power-injected contrast studies eliminates the need for additional needle sticks in the patient’s arm and wrist veins. Once implanted, repeated access to the bloodstream can be accomplished with greater ease and less discomfort. Our Smart Port is available in mini and low-profiles to accommodate more patient anatomies.
|
|
•
|
Vortex
®
:
Our Vortex port technology line of ports is a clear-flow port technology that, we believe, revolutionized port design. With its rounded chamber, the Vortex port is designed to have no sludge-harboring corners or dead spaces. This product line consists of titanium, plastic and dual-lumen offerings.
|
|
•
|
PASV
®
Valve Technology:
The PASV
®
Valve Technology is designed to automatically resist backflow and reduce blood reflux that could lead to catheter-related complications.
|
|
•
|
LifeGuard
®
:
The LifeGuard Safety Infusion Set and The LifeGuard Vision are used to infuse our ports and complement our port and vascular access catheters. The needles’ low profile design is intended to allow clinicians to easily dress the site.
|
|
•
|
BioFlo
®
DuraMax
: Our BioFlo DuraMax is the only dialysis catheter with Endexo Technology. Advanced features of the BioFlo DuraMax dialysis catheter include large inner diameter lumens designed for long term patency, a proprietary guidewire lumen to facilitate catheter exchanges and Curved Tip Technology that allows the catheter to self-center in the Superior Vena Cava (SVC).
|
|
•
|
DuraMax
®
:
The DuraMax catheter is a stepped-tip catheter designed to improve ease of use, dialysis efficiency and overall patient outcomes.
|
|
Name
|
Age
|
Position
|
||
|
James C. Clemmer
|
55
|
President and Chief Executive Officer
|
||
|
Michael C. Greiner
|
46
|
Executive Vice President and Chief Financial Officer
|
||
|
Stephen A. Trowbridge
|
45
|
Senior Vice President and General Counsel
|
||
|
David D. Helsel
|
55
|
Senior Vice President Global Operations
|
||
|
Warren G. Nighan
|
50
|
Senior Vice President Quality and Regulatory Affairs
|
||
|
Benjamin H. Davis
|
54
|
Senior Vice President Business Development
|
||
|
Chad T. Campbell
|
49
|
Senior Vice President and General Manager, Vascular Access
|
||
|
Brent J. Boucher
|
52
|
Senior Vice President and General Manager, Oncology/Surgery
|
||
|
Robert A. Simpson
|
47
|
Senior Vice President and General Manager, Vascular Interventions and Therapies
|
||
|
Kim L. Seabury
|
52
|
Senior Vice President, Information Technology
|
||
|
Item 1A.
|
Risk Factors.
|
|
•
|
financial and other resources to devote to product acquisitions, research and development, marketing and manufacturing;
|
|
•
|
variety of products;
|
|
•
|
technical capabilities;
|
|
•
|
history of developing and introducing new products;
|
|
•
|
patent portfolios that may present an obstacle to our conduct of business;
|
|
•
|
name recognition; and
|
|
•
|
distribution networks and in-house sales forces.
|
|
•
|
recruit engineers;
|
|
•
|
timely and accurately identify new market trends;
|
|
•
|
accurately assess customer needs;
|
|
•
|
minimize the time and costs required to obtain regulatory clearance or approval;
|
|
•
|
adopt competitive pricing;
|
|
•
|
timely manufacture and deliver products;
|
|
•
|
accurately predict and control costs associated with the development, manufacturing and support of our products; and
|
|
•
|
anticipate and compete effectively with our competitors’ efforts.
|
|
•
|
Enrolled patients may have unforeseen adverse side effects;
|
|
•
|
New therapies may become the standard of care while we are conducting our clinical trials, which may require us to revise or amend our clinical trial protocols or terminate a clinical trial;
|
|
•
|
Fatalities may occur during a clinical trial due to medical problems that may or may not be related to clinical trial treatments;
|
|
•
|
Patient enrollment in the clinical trials may be insufficient or significantly delayed.
|
|
•
|
potential disruption of our business while we evaluate opportunities, complete acquisitions and develop and implement new business strategies to take advantage of these opportunities;
|
|
•
|
inability of our management to maximize our financial and strategic position by incorporating an acquired technology or business into our existing offerings;
|
|
•
|
our inability to achieve the cost savings and operating synergies anticipated in the acquisition, which would prevent us from achieving the positive earnings gains expected as a result of the acquisition;
|
|
•
|
diversion of management attention from ongoing business concerns to integration matters;
|
|
•
|
difficulty of maintaining uniform standards, controls, procedures and policies;
|
|
•
|
challenges in demonstrating to our customers that the acquisition will not result in adverse changes in customer service standards or business focus;
|
|
•
|
possible cash flow interruption or loss of revenue as a result of change of ownership transition matters;
|
|
•
|
difficulty of assimilating the operations and personnel of acquired businesses;
|
|
•
|
potential loss of key employees of acquired businesses, and the impairment of relationships with employees and customers as a result of changes in management; and
|
|
•
|
uncertainty as to the long-term success of any acquisitions we may make including the impact on contingent liabilities.
|
|
•
|
fluctuations in currency exchange rates;
|
|
•
|
healthcare reform legislation;
|
|
•
|
multiple non-U.S. regulatory requirement that are subject to change and could restrict our ability to manufacture and sell our products;
|
|
•
|
local product preferences and product requirements;
|
|
•
|
longer-term receivables than are typical in the U.S.;
|
|
•
|
trade protection measures and import or export licensing requirements;
|
|
•
|
less intellectual property protection in some countries outside the U.S. than exists in the U.S.;
|
|
•
|
different labor regulations and workforce instability;
|
|
•
|
political instability;
|
|
•
|
the potential payment of U.S. income taxes on earnings of certain foreign subsidiaries subject to U.S. taxation upon repatriation;
|
|
•
|
the expiration and non-renewal of foreign tax rulings;
|
|
•
|
potential negative consequences from changes in or interpretation of tax laws; and
|
|
•
|
economic instability and inflation, recession or interest rate fluctuations.
|
|
•
|
Our Board of Directors is authorized, without prior stockholder approval, to create and issue “blank check” preferred stock, with rights senior to those of our common stock;
|
|
•
|
Our Board of Directors is classified so that not all members of our Board of Directors are elected at one time, which may make it more difficult for a person who acquires control of a majority of our outstanding voting stock to replace our directors;
|
|
•
|
Advance notice is required for stockholders to nominate individuals to serve on our Board of Directors or for stockholders to submit proposals that can be acted upon at stockholder meetings;
|
|
•
|
Stockholder action by written consent is prohibited; and
|
|
•
|
Stockholders are not permitted to cumulatively vote for the election of directors.
|
|
•
|
controls on government-funded reimbursement for healthcare services and price controls on medical products and services providers;
|
|
•
|
challenges to the pricing of medical procedures or limits or prohibitions on reimbursement for specific devices and therapies through other means; and
|
|
•
|
the introduction of managed care systems in which healthcare providers contract to provide comprehensive healthcare for a fixed cost per person.
|
|
•
|
the level of sales of our products and services in our markets;
|
|
•
|
our ability to introduce new products or services and enhancements in a timely manner;
|
|
•
|
the demand for and acceptance of our products and services;
|
|
•
|
the success of our competition and the introduction of alternative products or services;
|
|
•
|
our ability to command favorable pricing for our products and services;
|
|
•
|
the growth of the market for our devices and services;
|
|
•
|
the expansion and rate of success of our direct sales force in the United States and internationally and our independent distributors internationally;
|
|
•
|
actions relating to ongoing FDA compliance;
|
|
•
|
our ability to integrate acquired assets or companies;
|
|
•
|
our ability to timely divest of assets that are covered by Transition Service Agreements;
|
|
•
|
the effect of intellectual property disputes;
|
|
•
|
the size and timing of orders from independent distributors or customers;
|
|
•
|
the attraction and retention of key personnel, particularly in sales and marketing, regulatory, manufacturing and research and development;
|
|
•
|
unanticipated delays or an inability to control costs;
|
|
•
|
general economic conditions as well as those specific to our customers and markets; and
|
|
•
|
seasonal fluctuations in revenue due to the elective nature of some procedures.
|
|
•
|
general economic, industry and market conditions;
|
|
•
|
actions by institutional or other large stockholders;
|
|
•
|
the depth and liquidity of the market for our common stock;
|
|
•
|
volume and timing of orders for our products;
|
|
•
|
developments generally affecting medical device companies;
|
|
•
|
the announcement of new products or product enhancements by us or our competitors;
|
|
•
|
changes in earnings estimates or recommendations by securities analysts;
|
|
•
|
investor perceptions of us and our business, including changes in market valuations of medical device companies; and
|
|
•
|
our results of operations and financial performance.
|
|
Item 1B.
|
Unresolved Staff Comments.
|
|
Item 2.
|
Properties.
|
|
Location
|
Purpose
|
Approx.
Sq. Ft.
|
Property
Type
|
|||
|
Latham, NY
|
Corporate headquarters
|
55,000
|
|
Leased
|
||
|
Glens Falls, NY
|
Manufacturing
|
189,000
|
|
Owned
|
||
|
Queensbury, NY
|
Manufacturing and distribution
|
129,000
|
|
Owned
|
||
|
Marlborough, MA
|
Research & Development
|
31,000
|
|
Leased
|
||
|
Amsterdam, NL
|
Selling, Marketing & Administrative
|
8,100
|
|
Leased
|
||
|
Houston, TX
|
Selling, Marketing & Distribution
|
4,000
|
|
Leased
|
||
|
Item 3.
|
Legal Proceedings.
|
|
Item 4.
|
Mine Safety Disclosures.
|
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities.
|
|
|
Sale Price
|
||||||
|
|
High
|
Low
|
|||||
|
Year ended May 31, 2019
|
|||||||
|
Fourth Quarter
|
$
|
25.01
|
|
$
|
18.79
|
|
|
|
Third Quarter
|
$
|
23.46
|
|
$
|
18.90
|
|
|
|
Second Quarter
|
$
|
24.23
|
|
$
|
19.84
|
|
|
|
First Quarter
|
$
|
23.65
|
|
$
|
18.95
|
|
|
|
|
Sale Price
|
||||||
|
|
High
|
Low
|
|||||
|
Year ended May 31, 2018
|
|||||||
|
Fourth Quarter
|
$
|
21.04
|
|
$
|
16.13
|
|
|
|
Third Quarter
|
$
|
17.70
|
|
$
|
15.71
|
|
|
|
Second Quarter
|
$
|
18.76
|
|
$
|
16.29
|
|
|
|
First Quarter
|
$
|
17.12
|
|
$
|
15.12
|
|
|
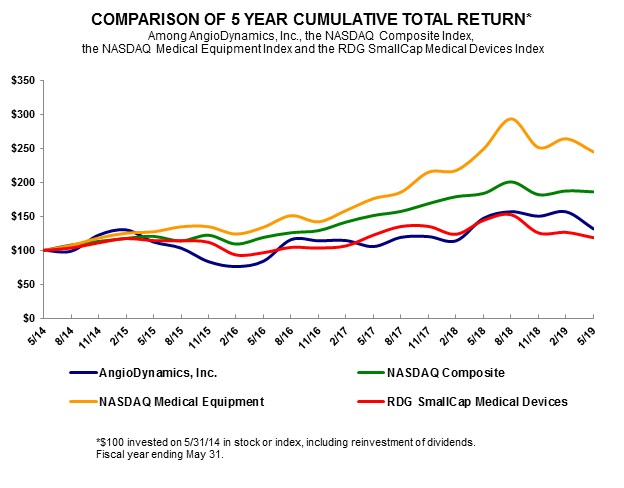
|
Item 6.
|
Selected Financial Data.
|
|
|
Year ended May 31,
|
||||||||||||||||||
|
(in thousands, except per share information)
|
2019
|
2018
|
2017
|
2016
|
2015
|
||||||||||||||
|
Consolidated Statements of Operations Data:
|
|||||||||||||||||||
|
Net sales
|
$
|
270,634
|
|
$
|
261,655
|
|
$
|
269,788
|
|
$
|
271,014
|
|
$
|
271,795
|
|
||||
|
Gross profit (exclusive of intangible amortization)
|
156,000
|
|
143,856
|
|
143,950
|
|
141,719
|
|
142,773
|
|
|||||||||
|
Operating expenses
|
|||||||||||||||||||
|
Research and development
|
28,258
|
|
24,338
|
|
24,148
|
|
23,932
|
|
25,473
|
|
|||||||||
|
Sales and marketing
|
76,829
|
|
73,109
|
|
74,865
|
|
79,789
|
|
78,397
|
|
|||||||||
|
General and administrative
|
34,902
|
|
30,991
|
|
31,133
|
|
30,314
|
|
29,769
|
|
|||||||||
|
Amortization of intangibles
|
17,056
|
|
13,906
|
|
14,567
|
|
15,235
|
|
16,624
|
|
|||||||||
|
Change in fair value of contingent consideration
|
(6,776
|
)
|
250
|
|
(15,261
|
)
|
948
|
|
(8,096
|
)
|
|||||||||
|
Acquisition, restructuring and other items, net
(1)
|
15,127
|
|
15,432
|
|
27,510
|
|
12,591
|
|
18,043
|
|
|||||||||
|
Medical device excise tax
|
—
|
|
—
|
|
(1,837
|
)
|
2,416
|
|
4,142
|
|
|||||||||
|
Total operating expenses
|
165,396
|
|
158,026
|
|
155,125
|
|
165,225
|
|
164,352
|
|
|||||||||
|
Operating loss
|
(9,396
|
)
|
(14,170
|
)
|
(11,175
|
)
|
(23,506
|
)
|
(21,579
|
)
|
|||||||||
|
Total other expenses, net
|
(5,306
|
)
|
(3,093
|
)
|
(3,120
|
)
|
(4,271
|
)
|
(4,682
|
)
|
|||||||||
|
Net loss from continuing operations
|
(11,146
|
)
|
(6,227
|
)
|
(7,052
|
)
|
(55,895
|
)
|
(14,991
|
)
|
|||||||||
|
Net income from discontinued operations
|
72,486
|
|
22,562
|
|
12,060
|
|
12,305
|
|
11,603
|
|
|||||||||
|
Net income (loss)
|
$
|
61,340
|
|
$
|
16,335
|
|
$
|
5,008
|
|
$
|
(43,590
|
)
|
$
|
(3,388
|
)
|
||||
|
Loss per share from continuing operations
|
|||||||||||||||||||
|
Basic
|
$
|
(0.30
|
)
|
$
|
(0.17
|
)
|
$
|
(0.19
|
)
|
$
|
(1.55
|
)
|
$
|
(0.42
|
)
|
||||
|
Diluted
|
$
|
(0.30
|
)
|
$
|
(0.17
|
)
|
$
|
(0.19
|
)
|
$
|
(1.55
|
)
|
$
|
(0.42
|
)
|
||||
|
Earnings per share from discontinued operations
|
|||||||||||||||||||
|
Basic
|
$
|
1.93
|
|
$
|
0.61
|
|
$
|
0.33
|
|
$
|
0.34
|
|
$
|
0.33
|
|
||||
|
Diluted
|
$
|
1.93
|
|
$
|
0.61
|
|
$
|
0.33
|
|
$
|
0.34
|
|
$
|
0.33
|
|
||||
|
Earnings (loss) per share from net income
|
|||||||||||||||||||
|
Basic
|
$
|
1.64
|
|
$
|
0.44
|
|
$
|
0.14
|
|
$
|
(1.21
|
)
|
$
|
(0.09
|
)
|
||||
|
Diluted
|
$
|
1.64
|
|
$
|
0.44
|
|
$
|
0.14
|
|
$
|
(1.21
|
)
|
$
|
(0.09
|
)
|
||||
|
|
As of May 31,
|
||||||||||||||||||
|
(in thousands)
|
2019
|
2018
|
2017
|
2016
|
2015
|
||||||||||||||
|
Consolidated Balance Sheet Data:
|
|||||||||||||||||||
|
Cash, cash equivalents and marketable securities
|
$
|
227,641
|
|
$
|
75,413
|
|
$
|
48,759
|
|
$
|
33,986
|
|
$
|
20,080
|
|
||||
|
Total assets
|
836,438
|
|
705,472
|
|
707,961
|
|
726,194
|
|
773,058
|
|
|||||||||
|
Long-term debt, including current portion
|
131,907
|
|
91,621
|
|
96,320
|
|
120,541
|
|
137,660
|
|
|||||||||
|
Contingent consideration, including current portion
|
13,486
|
|
3,261
|
|
12,761
|
|
38,275
|
|
47,384
|
|
|||||||||
|
Total long-term liabilities
|
148,321
|
|
105,576
|
|
121,418
|
|
152,239
|
|
167,444
|
|
|||||||||
|
Total stockholders’ equity
|
614,815
|
|
542,595
|
|
515,027
|
|
507,228
|
|
545,099
|
|
|||||||||
|
Item 7.
|
Management’s Discussion and Analysis of Financial Conditions and Results of Operations.
|
|
•
|
Revenue
increased
by
3.4%
to
$270.6 million
|
|
•
|
Gross margin as a percentage of sales
increased
by 260 bps to
57.6%
|
|
•
|
Operating loss decreased by
$4.8 million
to
$9.4 million
|
|
•
|
Cash flow from operations
decreased
by
$3.8 million
to
$37.4 million
|
|
•
|
Product development process.
The Company continued its robust product development process which is intended to improve the Company’s ability to bring new products to market.
|
|
•
|
Value Creation.
To create value and drive future growth, the Company plans to practice dispassionate portfolio optimization and continue to focus on areas of compelling unmet needs including those that are patient-centric and evidenced-based. This was evident through the BioSentry and RadiaDyne acquisitions noted above along with the divestiture of the Fluid Management business. In addition, the Company is pursuing targeted global expansion opportunities.
|
|
Year ended May 31,
|
|||||||||
|
(in thousands)
|
2019
|
2018
|
% Growth
|
||||||
|
Net Sales by Product Category
|
|||||||||
|
Vascular Interventions & Therapies
|
$
|
119,901
|
|
$
|
119,704
|
|
0%
|
||
|
Vascular Access
|
94,730
|
|
92,760
|
|
2%
|
||||
|
Oncology/Surgery
|
56,003
|
|
49,191
|
|
14%
|
||||
|
Total
|
$
|
270,634
|
|
$
|
261,655
|
|
3%
|
||
|
Net Sales by Geography
|
|||||||||
|
United States
|
$
|
216,957
|
|
$
|
213,727
|
|
2%
|
||
|
International
|
53,677
|
|
47,928
|
|
12%
|
||||
|
Total
|
$
|
270,634
|
|
$
|
261,655
|
|
3%
|
||
|
•
|
Total Vascular Interventions & Therapies sales increased
$0.2 million
primarily attributable to strong performance in AngioVac and Core Peripheral products. The Company continues to see strong case volumes in AngioVac, which increased 20% percent from the prior year due to increased adoption of the Company's unique technology. This was partially offset by decreases in Venous products due to reimbursement challenges and the termination of the Asclera distribution agreement.
|
|
•
|
U.S. Vascular Interventions & Therapies sales decreased $1.1 million due to decreased sales volume in Venous products and the termination of the Asclera distribution agreement. This was partially offset by increased case volume in AngioVac and increased sales volume of Core Peripheral products.
|
|
•
|
International Vascular Interventions & Therapies sales increased $1.3 million due to increased volume in Venous and Angiographic catheters in EMEA and China.
|
|
•
|
Total Vascular Access sales increased
$2.0 million
due to growth in BioFlo which increased $2.8 million year over year. The increase in sales is also due to the launch of the BIIM ultrasound product in fiscal year 2019 which had $0.5 million in sales. This was partially offset by a decline in non-BioFlo PICCs of $1.8 million. The Company's BioFlo portfolio now comprises 51% of overall Vascular Access sales, compared to 49% a year ago.
|
|
•
|
U.S. Vascular Access sales decreased by $1.2 million due to competitive pressures in the PICC product line. This was partially offset by growth in Midlines and BioFlo Dialysis and Ports which continue to gain traction in the marketplace.
|
|
•
|
International Vascular Access sales increased by $3.2 million as the Company continues to expand its global reach of its Vascular Access product offerings.
|
|
•
|
Total Oncology/Surgery sales increased
$6.8 million
year over year primarily due to $4.9 million in sales of BioSentry products and $4.8 million in sales of RadiaDyne products along with increased sales of NanoKnife disposables of $0.5 million. This was partially offset by decreased sales in Radiofrequency Ablation, Microwave and NanoKnife capital. Microwave sales were negatively impacted by the timing of the Company's prior year replacement shipments of $2.6 million which took place primarily in the first and second quarters of the prior year as a result of the market withdrawal of Acculis. NanoKnife capital decreased $0.9 million due to the timing of capital sales.
|
|
•
|
U.S. Oncology sales increased by $6.9 million primarily due to $4.8 million in sales of BioSentry products and $4.8 million in sales of RadiaDyne products. This was partially offset by a $1.5 million decrease in NanoKnife capital and disposable sales and a $0.6 million decrease in RadioFrequency Ablation and Microwave sales.
|
|
•
|
International Oncology sales decreased by $0.1 million due to decreased RadioFrequency Ablation and Microwave sales of $0.7 million, partially offset by increased NanoKnife capital and disposable sales of $0.8 million.
|
|
Year ended May 31,
|
|||||||||||
|
(in thousands)
|
2019
|
2018
|
% Change
|
||||||||
|
Gross profit (exclusive of intangible amortization)
|
$
|
156,000
|
|
$
|
143,856
|
|
8.4
|
%
|
|||
|
Gross profit % of sales
|
57.6
|
%
|
55.0
|
%
|
|||||||
|
Research and development
|
$
|
28,258
|
|
$
|
24,338
|
|
16.1
|
%
|
|||
|
% of sales
|
10.4
|
%
|
9.3
|
%
|
|||||||
|
Selling and marketing
|
$
|
76,829
|
|
$
|
73,109
|
|
5.1
|
%
|
|||
|
% of sales
|
28.4
|
%
|
27.9
|
%
|
|||||||
|
General and administrative
|
$
|
34,902
|
|
$
|
30,991
|
|
12.6
|
%
|
|||
|
% of sales
|
12.9
|
%
|
11.8
|
%
|
|||||||
|
•
|
Net productivity of $2.5 million, where plant consolidation contributed $3.3 million of favorability
partially offset by increased freight expense of $0.8 million.
|
|
•
|
Sales volume and mix positively contributed $1.5 million year over year.
|
|
•
|
Currency and pricing headwinds negatively impacting gross margin by $2.0 million year over year.
|
|
•
|
Sales of BioSentry and RadiaDyne products contributed $6.9 million to gross profit.
|
|
•
|
Prior year reserve of $1.7 million related to the discontinuation of our RadioFrequency Ablation product in Japan.
|
|
•
|
The expiration of a royalty agreement in fiscal year 2018 resulted in $1.5 million of favorability compared to the prior year.
|
|
•
|
New product development and clinical efforts related to the Company’s investment areas of NanoKnife, Thrombus Management and BioFlo increased $3.5 million.
|
|
•
|
Increased compensation and benefits of $0.5 million primarily as a result of increased variable compensation.
|
|
•
|
Compensation and benefits increase of approximately $3.7 million which is primarily attributed to increased headcount as a result of the BioSentry and RadiaDyne acquisitions along with higher variable compensation.
|
|
•
|
Compensation and benefits increase of approximately $2.8 million primarily as a result of increased variable compensation, salaries and benefits and stock based compensation.
|
|
•
|
Increased legal fees related to ongoing litigation that is within the normal course of business of $0.6 million.
|
|
•
|
Increased other expenses for technology investments of $0.3 million and lease expense of $0.2 million.
|
|
Year ended May 31,
|
||||||||||||
|
(in thousands)
|
2019
|
2018
|
$ Change
|
|||||||||
|
Amortization of intangibles
|
$
|
17,056
|
|
$
|
13,906
|
|
$
|
3,150
|
|
|||
|
Change in fair value of contingent consideration
|
$
|
(6,776
|
)
|
$
|
250
|
|
$
|
(7,026
|
)
|
|||
|
Acquisition, restructuring and other items, net
|
$
|
15,127
|
|
$
|
15,432
|
|
$
|
(305
|
)
|
|||
|
Other expense
|
$
|
(5,306
|
)
|
$
|
(3,093
|
)
|
$
|
(2,213
|
)
|
|||
|
•
|
The change in amortization expense from the prior year is due to intangible asset additions as a result of the BioSentry and RadiaDyne acquisitions. The BioSentry acquisition increased intangible assets by $26.0 million and resulted in additional amortization expense of $1.5 million. The RadiaDyne acquisition increased intangible assets by $25.6 million and resulted in additional amortization expense of $1.7 million
|
|
•
|
The increase from the prior year is due to contingent considerations that were recorded as part of the BioSentry and RadiaDyne acquisitions of $2.8 million and $22.3 million, respectively. In the fourth quarter, adjustments to the sales projections for RadiaDyne products resulted in an $8.4 million gain. The remaining change in the fair value in contingent consideration is the result of amortization of the present value discount of $1.6 million. In addition, in the second quarter of fiscal year 2018, the final minimum payment was made on the AngioVac product contingent consideration and a $2.1 million payment was made on the Microsulis contingent consideration during the first quarter of fiscal 2019. Only one minimum payment is remaining on the Microsulis contingent consideration.
|
|
•
|
M&A expense of $4.0 million was incurred in fiscal year 2019 compared to $1.7 million in the prior year.
|
|
•
|
Legal expense, related to litigation that is outside of the normal course of business, of $7.8 million was recorded in fiscal year 2019 compared to $8.4 million in fiscal year 2018. Included in the
$7.8 million
, is a $3.4 million settlement received for the Biolitec litigation and a $2.5 million accrual for the settlement of the Merz contract termination.
|
|
•
|
For the year ended 2018, the Company incurred $4.7 million of expense which consisted of $1.4 million of severance, $2.9 million of costs to move the product lines and $0.2 million in contract termination expenses related to the plant consolidation that was announced in the third quarter of fiscal year 2017. The plant consolidation was completed in the fourth quarter of fiscal year 2018; therefore, only $0.3 million of expense was incurred for the year ended 2019.
|
|
•
|
The increase in other expenses from the prior year of $2.2 million is due to increased interest expense of $1.9 million primarily due to the draw on the Revolving Facility during fiscal year 2019. In addition, foreign currency fluctuations increased $0.7 million. These increases were partially offset by other income of $0.4 million.
|
|
For year ended May 31,
|
||||||||
|
(in thousands)
|
2019
|
2018
|
||||||
|
Income tax expense (benefit)
|
$
|
(3,556
|
)
|
$
|
(11,036
|
)
|
||
|
Effective tax rate including discrete items
|
24
|
%
|
64
|
%
|
||||
|
For year ended May 31,
|
||||||||
|
(in thousands)
|
2019
|
2018
|
||||||
|
Income from discontinued operations before gain on sale of Fluid Management
|
$
|
27,579
|
|
$
|
24,728
|
|
||
|
Gain on sale of Fluid Management
|
46,592
|
|
—
|
|
||||
|
Income from discontinued operations before income taxes
|
74,171
|
|
24,728
|
|
||||
|
Provision for income taxes
|
1,685
|
|
2,166
|
|
||||
|
Income from discontinued operations
|
$
|
72,486
|
|
$
|
22,562
|
|
||
|
Year ended May 31,
|
|||||||||
|
(in thousands)
|
2018
|
2017
|
% Growth
|
||||||
|
Net Sales by Product Category
|
|||||||||
|
Vascular Interventions & Therapies
|
$
|
119,704
|
|
$
|
128,747
|
|
(7)%
|
||
|
Vascular Access
|
92,760
|
|
96,481
|
|
(4)%
|
||||
|
Oncology/Surgery
|
49,191
|
|
44,560
|
|
10%
|
||||
|
Total
|
$
|
261,655
|
|
$
|
269,788
|
|
(3)%
|
||
|
Net Sales by Geography
|
|||||||||
|
United States
|
$
|
213,727
|
|
$
|
223,816
|
|
(5)%
|
||
|
International
|
47,928
|
|
45,972
|
|
4%
|
||||
|
Total
|
$
|
261,655
|
|
$
|
269,788
|
|
(3)%
|
||
|
•
|
Total Vascular Interventions & Therapies sales decreased
$9.0 million
primarily attributable to decreased sales volume of Venous by $9.3 million and Angiographic and Core products of $1.6 million. The decrease in Venous is primarily attributed to our largest customer discontinuing their exclusive use of our EVLT product. This decreased sales volume was offset by an increase of volume of AngioVac of $0.9 million.
|
|
•
|
U.S. Vascular Interventions & Therapies sales decreased $8.0 million primarily due to decreased sales volume of Venous of $9.1 million and Angiographic and Core products of $0.9 million. This decreased sales volume was offset by an increase in AngioVac of $1.0 million and $0.9 million in increased freight and rebate revenue.
|
|
•
|
International Vascular Interventions & Therapies sales decreased $1.0 million.
|
|
•
|
Total Vascular Access sales decreased
$3.7 million
primarily in our non-BioFlo businesses. Our BioFlo product lines, other than BioFlo PICCs, increased $2.4 million. This was partially offset by decreased sales of BioFlo PICCs of $2.5 million and non-BioFlo products of $3.1 million. BioFlo product lines comprise 49% of our overall Vascular Access sales, compared to 47% a year ago.
|
|
•
|
U.S. Vascular Access sales declined by $3.8 million due to softness across the portfolio offset by Midline, BioFlo dialysis and ports which continued to gain traction in the marketplace.
|
|
•
|
International Vascular Access sales increased $0.1 million.
|
|
•
|
Total Oncology/Surgery sales increased
$4.6 million
year over year primarily due to increased sales of the Solero Microwave generators of $1.3 million, Solero Microwave probes of $6.4 million and other sales of $0.7 million. $5.2 million of the increased probe sales were a result of the market withdrawal of Acculis, which was replaced by Solero probes in the beginning of fiscal year 2018 upon the approval of the device. This was partially offset by decreased Radiofrequency Ablation sales of $3.4 million and NanoKnife disposable sales of $0.6 million. The decrease in Radiofrequency Ablation sales was primarily due to the discontinuation of the product in Japan.
|
|
•
|
U.S. Oncology/Surgery increased by $0.6 million, driven primarily through increased Microwave sales of $3.4 million. This increase was partially offset by decreases in Radiofrequency Ablation of $1.7 million and NanoKnife of $1.4 million.
|
|
•
|
International Oncology/Surgery sales increased $4.0 million year over year as a result of increased NanoKnife capital and disposable sales of $1.1 million and Solero Microwave capital and disposable sales of $4.3 million. This increase was partially offset by a $1.7 million decrease in RadioFrequency Ablation.
|
|
Year ended May 31,
|
|||||||||||
|
(in thousands)
|
2018
|
2017
|
% Change
|
||||||||
|
Gross profit
|
$
|
143,856
|
|
$
|
143,950
|
|
(0.1
|
)%
|
|||
|
Gross profit % of sales
|
55.0
|
%
|
53.4
|
%
|
|||||||
|
Research and development
|
$
|
24,338
|
|
$
|
24,148
|
|
0.8
|
%
|
|||
|
% of sales
|
9.3
|
%
|
9.0
|
%
|
|||||||
|
Selling and marketing
|
$
|
73,109
|
|
$
|
74,865
|
|
(2.3
|
)%
|
|||
|
% of sales
|
27.9
|
%
|
27.7
|
%
|
|||||||
|
General and administrative
|
$
|
30,991
|
|
$
|
31,133
|
|
(0.5
|
)%
|
|||
|
% of sales
|
11.8
|
%
|
11.5
|
%
|
|||||||
|
•
|
The expiration of a royalty agreement of approximately $4.8 million.
|
|
•
|
$2.3 million in deferred revenue was recognized in fiscal year 2018 related to the Acculis probe recall announced in the fourth quarter of fiscal year 2017 which was offset by additional expense of $3.2 million that was incurred as a result of the market withdrawal of Microwave generators.
|
|
•
|
In the second quarter of fiscal year 2018, the Company decided to discontinue selling our RadioFrequency Ablation product in Japan which resulted in a $1.7 million inventory provision.
|
|
•
|
Currency tailwinds netted with slight negative price driving $0.5 million favorable gross margin impact.
|
|
•
|
Volume softness and mix of approximately $4.5 million partially offset by net productivity of $1.7 million.
|
|
•
|
Increased headcount in the R&D department compared to the prior year resulted in $0.6 million of additional expense. These increases were partially offset by less project spend of $0.4 million.
|
|
•
|
Compensation and benefits decrease of approximately $2.7 million was primarily the result of decreased variable compensation of $2.3 million and open headcount of $0.5 million.
|
|
•
|
Open headcount resulted in increased travel of $0.5 million and recruiting expense of $0.3 million.
|
|
•
|
Lower consulting spend of $0.7 million was partially offset by increased trade show and meeting expense of $0.5 million and increased samples expense related to the Solero launch of $0.2 million.
|
|
•
|
Compensation and benefits increase of approximately $1.9 million was primarily the result of increased headcount year over year which was partially offset by a decrease in variable compensation expense of $0.5 million.
|
|
•
|
These increases were offset by lower depreciation expense of $0.8 million, a decrease in bad debt expense of $0.3 million and a decrease in recruiting expenses of $0.4 million.
|
|
Year ended May 31,
|
||||||||||||
|
(in thousands)
|
2018
|
2017
|
$ Change
|
|||||||||
|
Amortization of intangibles
|
$
|
13,906
|
|
$
|
14,567
|
|
$
|
(661
|
)
|
|||
|
Change in fair value of contingent consideration
|
$
|
250
|
|
$
|
(15,261
|
)
|
$
|
15,511
|
|
|||
|
Acquisition, restructuring and other items, net
|
$
|
15,432
|
|
$
|
27,510
|
|
$
|
(12,078
|
)
|
|||
|
Medical device excise tax
|
$
|
—
|
|
$
|
(1,837
|
)
|
$
|
1,837
|
|
|||
|
Other expense
|
$
|
(3,093
|
)
|
$
|
(3,120
|
)
|
$
|
27
|
|
|||
|
•
|
The decrease of $0.7 million is primarily related to intangible assets that became fully amortized in the prior year.
|
|
•
|
In fiscal year 2017, a gain of $13.4 million was taken on the AngioVac product as a result of decreases in future sales projections that eliminated any payments above minimums and a gain of $3.1 million on the TiLo product as the milestone was determined to not be achieved. The normal amortization of the present value discount on the contingent liabilities was approximately $0.1 million for the first two quarters of fiscal year 2018. For the last two quarters of fiscal year 2018, amortization is now less than $0.1 million per quarter as the final minimum payment was made on AngioVac in the second quarter of fiscal year 2018
|
|
•
|
In fiscal year 2018, there was $4.7 million of expense related to the plant consolidation that was announced in the third quarter of fiscal year 2017. The expense consisted mainly of severance of $1.4 million, costs to move the product lines including equipment transfer expenses, accelerated depreciation for assets that will not be transferred, product validation and other start-up costs of $2.9 million and $0.2 million in contract termination expenses.
|
|
•
|
In fiscal year 2017, there was $1.3 million of expense related to the plant consolidation that was announced in the third quarter of fiscal year 2017. The expense consisted of severance of $0.8 million and start-up costs to move the product lines including equipment transfer expenses and accelerated depreciation for assets that will not be transferred of $0.5 million.
|
|
•
|
In fiscal year 2017, there was a $2.0 million write-off of Embomedics due to termination of the agreement and a $3.6 million write-off related to the decision to discontinue our investment in the TiLo product.
|
|
•
|
A litigation settlement accrual of $12.5 million was recorded in the fourth quarter of fiscal year 2017 for the agreement that was reached with the DOJ.
|
|
•
|
Legal expenses, related to litigation that is outside of the normal course of business, of $10.1 million were recorded in the current year compared to $7.0 million in the prior year.
|
|
•
|
The Medical Device Excise Tax was suspended on January 1, 2016 and the suspension was upheld in January 2018. In fiscal year 2017, there was a $1.8 million refund from the Internal Revenue Service related to prior medical device taxes paid.
|
|
Year ended May 31,
|
||||||||
|
(in thousands)
|
2018
|
2017
|
||||||
|
Income tax expense (benefit)
|
$
|
(11,036
|
)
|
$
|
(7,243
|
)
|
||
|
Effective tax rate including discrete items
|
64
|
%
|
51
|
%
|
||||
|
For year ended May 31,
|
||||||||
|
(in thousands)
|
2018
|
2017
|
||||||
|
Income from discontinued operations before income taxes
|
24,728
|
|
24,142
|
|
||||
|
Provision for income taxes
|
2,166
|
|
12,082
|
|
||||
|
Income from discontinued operations
|
$
|
22,562
|
|
$
|
12,060
|
|
||
|
Year ended May 31,
|
|||||||||||
|
(in thousands)
|
2019
|
2018
|
2017
|
||||||||
|
Cash provided by (used in):
|
|||||||||||
|
Operating activities
|
$
|
37,440
|
|
$
|
41,287
|
|
$
|
55,745
|
|
||
|
Investing activities
|
82,554
|
|
(3,656
|
)
|
(2,551
|
)
|
|||||
|
Financing activities
|
33,931
|
|
(11,551
|
)
|
(37,983
|
)
|
|||||
|
Effect of exchange rate changes on cash and cash equivalents
|
(380
|
)
|
472
|
|
—
|
|
|||||
|
Net change in cash and cash equivalents
|
$
|
153,545
|
|
$
|
26,552
|
|
$
|
15,211
|
|
||
|
•
|
Net income was driven by the sale of the Fluid Management business. Net loss from continuing operations was driven by higher operating expenses in research and development, selling and marketing and general administrative as well as costs related to our acquisition and restructuring activities. The loss was partially offset by increased sales and improved gross profit.
|
|
•
|
Working capital was negatively impacted by increased inventory on hand of $1.4 million. Days sales outstanding ("DSO") increased as a result of increased sales during the fourth quarter. This had a $3.2 million negative impact on working capital. The Company continued to optimize days payables outstanding ("DPO"), which resulted in a $5.2 million favorable impact on working capital.
|
|
•
|
Net income was driven by higher gross margin, lower operating expenses in selling and marketing, general and administrative and acquisition, restructuring and other items, net. Partially offsetting the positive cash impact was a $9.3 million non-cash discrete tax benefit as a result of the Tax Reform Act and the revaluation of the Company's deferred tax assets and liabilities to reflect the lower statutory rate.
|
|
•
|
The Company continues to focus on optimizing days sales outstanding ("DSO") which contributed $5.0 million to working capital improvement. Working capital was also positively impacted by decreased inventory on hand of $5.7 million. Even though the Company continued to optimize days payables outstanding ("DPO"), the decrease in raw material purchases at year end negatively impacted working capital from accounts payable and accrued liabilities.
|
|
•
|
Net income was driven by higher gross margins, lower sales and marketing expenses as well as the medical device tax refund. Also impacting net income, were non-cash items which consisted of $15.3 million of contingent consideration gains, $2.0 million in the write-off of the Embomedics investment and $3.6 million in intangible write-offs related to TiLo.
|
|
•
|
With regards to working capital, the Company focused on optimizing both DSO and DPO which contributed to $15.2 million of working capital improvement. With respect to inventory, the $2.4 million reserve for Acculis inventory partially offset the inventory build related to the plant consolidation.
|
|
•
|
$3.1 million in fixed asset additions, primarily for maintenance of equipment.
|
|
•
|
$37.0 million cash payment to acquire the BioSentry product from SSC and a $47.9 million cash payment to acquire RadiaDyne as described in Note 2 to the financial statements.
|
|
•
|
$169.2 million in cash proceeds as a result of the Divestiture described in Note 3 to the financial statements.
|
|
•
|
$1.3 million in proceeds from the sale of an auction rate security.
|
|
•
|
$2.4 million in fixed asset additions.
|
|
•
|
In the third quarter, we entered into a distribution and license agreement where we recorded the upfront license fee of $1.3 million as an intangible asset that will be amortized over thirty-six months.
|
|
•
|
$3.0 million in fixed asset additions.
|
|
•
|
$0.5 million in proceeds from an auction rate security that was called during fiscal year 2017.
|
|
•
|
$55.0 million draw on the Revolving Facility as a result of the RadiaDyne acquisition described in Note 2 to the financial statements.
|
|
•
|
$5.0 million repayment on the Term Loan in both the current year and prior year. This is consistent with the required amortization payment on the Term Loan. There was also a $10.0 million repayment on the Revolving Facility in the third quarter of fiscal year 2019.
|
|
•
|
$2.0 million of proceeds from stock option and ESPP activity.
|
|
•
|
$8.1 million payment on earn-out liabilities.
|
|
•
|
$5.0 million in repayments on long-term debt, consistent with the required amortization payment on the Term Loan.
|
|
•
|
$2.9 million of proceeds from stock option and ESPP activity.
|
|
•
|
$9.5 million payment on earn-out liabilities.
|
|
•
|
Net $23.9 million in repayments on long-term debt after the proceeds from the Credit Agreement and repayment of the old credit agreement.
|
|
•
|
$1.3 million in deferred financing fees related to the new credit agreement.
|
|
•
|
$10.7 million of proceeds from stock option and ESPP. The large increase is related to the exercise of stock based awards from executive management turnover that took place in fiscal year 2017.
|
|
•
|
$9.9 million payment on earn-out liabilities.
|
|
•
|
$13.6 million from the repurchase of common shares in fiscal year 2017.
|
|
|
Cash payments due by period as of May 31, 2019
|
||||||||||||||||||
|
(in thousands)
|
Total
|
Less than
One Year
|
1-3 Years
|
3-5 Years
|
After 5
Years
|
||||||||||||||
|
Contractual Obligations:
|
|||||||||||||||||||
|
Long term debt and interest
|
$
|
145,032
|
|
$
|
12,797
|
|
$
|
132,235
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Operating leases
(1)
|
12,749
|
|
2,920
|
|
6,602
|
|
3,227
|
|
—
|
|
|||||||||
|
Purchase obligations
(1)
|
407
|
|
407
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Acquisition-related future obligations
(2)
|
15,208
|
|
5,208
|
|
10,000
|
|
—
|
|
—
|
|
|||||||||
|
Indemnification holdback
|
5,000
|
|
5,000
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Royalties
|
55,300
|
|
3,800
|
|
11,400
|
|
11,400
|
|
28,700
|
|
|||||||||
|
Litigation matters
(3)
|
2,700
|
|
2,700
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
$
|
236,396
|
|
$
|
32,832
|
|
$
|
160,237
|
|
$
|
14,627
|
|
$
|
28,700
|
|
|||||
|
Item 7A.
|
Quantitative and Qualitative Disclosures about Market Risk.
|
|
Item 8.
|
Financial Statements and Supplementary Data.
|
|
Item 9.
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
|
|
Item 9A.
|
Controls and Procedures.
|
|
•
|
Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets;
|
|
•
|
Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States, and that our receipts and expenditures are being made only in accordance with authorizations of our management and members of our board of directors; and
|
|
•
|
Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
|
|
Item 9B.
|
Other Information.
|
|
Item 10.
|
Directors, Executive Officers and Corporate Governance.
|
|
Item 11.
|
Executive Compensation.
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
|
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence.
|
|
Item 14.
|
Principal Accounting Fees and Services.
|
|
Item 15.
|
Exhibits, Financial Statement Schedules.
|
|
|
|||
|
|
Year ended May 31,
|
||||||||||
|
|
2019
|
2018
|
2017
|
||||||||
|
Net sales
|
$
|
270,634
|
|
$
|
261,655
|
|
$
|
269,788
|
|
||
|
Cost of sales (exclusive of intangible amortization)
|
114,634
|
|
117,799
|
|
125,838
|
|
|||||
|
Gross profit
|
156,000
|
|
143,856
|
|
143,950
|
|
|||||
|
Operating expenses
|
|||||||||||
|
Research and development
|
28,258
|
|
24,338
|
|
24,148
|
|
|||||
|
Sales and marketing
|
76,829
|
|
73,109
|
|
74,865
|
|
|||||
|
General and administrative
|
34,902
|
|
30,991
|
|
31,133
|
|
|||||
|
Amortization of intangibles
|
17,056
|
|
13,906
|
|
14,567
|
|
|||||
|
Change in fair value of contingent consideration
|
(6,776
|
)
|
250
|
|
(15,261
|
)
|
|||||
|
Acquisition, restructuring and other items, net
|
15,127
|
|
15,432
|
|
27,510
|
|
|||||
|
Medical device excise tax
|
—
|
|
—
|
|
(1,837
|
)
|
|||||
|
Total operating expenses
|
165,396
|
|
158,026
|
|
155,125
|
|
|||||
|
Operating loss
|
(9,396
|
)
|
(14,170
|
)
|
(11,175
|
)
|
|||||
|
Other expenses
|
|||||||||||
|
Interest expense, net
|
(5,099
|
)
|
(3,062
|
)
|
(2,839
|
)
|
|||||
|
Other expense
|
(207
|
)
|
(31
|
)
|
(281
|
)
|
|||||
|
Total other expenses, net
|
(5,306
|
)
|
(3,093
|
)
|
(3,120
|
)
|
|||||
|
Loss from continuing operations before income tax benefit
|
(14,702
|
)
|
(17,263
|
)
|
(14,295
|
)
|
|||||
|
Income tax benefit
|
(3,556
|
)
|
(11,036
|
)
|
(7,243
|
)
|
|||||
|
Net loss from continuing operations
|
(11,146
|
)
|
(6,227
|
)
|
(7,052
|
)
|
|||||
|
Income from discontinued operations, net of income tax
|
72,486
|
|
22,562
|
|
12,060
|
|
|||||
|
Net income
|
$
|
61,340
|
|
$
|
16,335
|
|
$
|
5,008
|
|
||
|
Loss per share from continuing operations
|
|||||||||||
|
Basic
|
$
|
(0.30
|
)
|
$
|
(0.17
|
)
|
$
|
(0.19
|
)
|
||
|
Diluted
|
$
|
(0.30
|
)
|
$
|
(0.17
|
)
|
$
|
(0.19
|
)
|
||
|
Earnings per share from discontinued operations
|
|
||||||||||
|
Basic
|
$
|
1.93
|
|
$
|
0.61
|
|
$
|
0.33
|
|
||
|
Diluted
|
$
|
1.93
|
|
$
|
0.61
|
|
$
|
0.33
|
|
||
|
Earnings per share from net income
|
|
||||||||||
|
Basic
|
$
|
1.64
|
|
$
|
0.44
|
|
$
|
0.14
|
|
||
|
Diluted
|
$
|
1.64
|
|
$
|
0.44
|
|
$
|
0.14
|
|
||
|
Weighted average shares outstanding
|
|||||||||||
|
Basic
|
37,485
|
|
37,075
|
|
36,617
|
|
|||||
|
Diluted
|
37,485
|
|
37,075
|
|
36,617
|
|
|||||
|
|
Year ended May 31,
|
||||||||||
|
|
2019
|
2018
|
2017
|
||||||||
|
Net income
|
$
|
61,340
|
|
$
|
16,335
|
|
$
|
5,008
|
|
||
|
Other comprehensive income (loss), before tax:
|
|||||||||||
|
Unrealized gain on marketable securities
|
33
|
|
102
|
|
12
|
|
|||||
|
Reclassification adjustment for gains included in net income
|
(116
|
)
|
—
|
|
—
|
|
|||||
|
Foreign currency translation gain (loss)
|
(317
|
)
|
270
|
|
(545
|
)
|
|||||
|
Other comprehensive income (loss), before tax
|
(400
|
)
|
372
|
|
(533
|
)
|
|||||
|
Income tax benefit (expense) related to items of other comprehensive income (loss)
|
—
|
|
—
|
|
—
|
|
|||||
|
Other comprehensive income (loss), net of tax
|
(400
|
)
|
372
|
|
(533
|
)
|
|||||
|
Total comprehensive income, net of tax
|
$
|
60,940
|
|
$
|
16,707
|
|
$
|
4,475
|
|
||
|
May 31, 2019
|
May 31, 2018
|
||||||
|
Assets
|
|||||||
|
Current Assets
|
|||||||
|
Cash and cash equivalents
|
$
|
227,641
|
|
$
|
74,096
|
|
|
|
Marketable securities, at fair value
|
—
|
|
1,317
|
|
|||
|
Accounts receivable, net of allowances of $1,906 and $2,466, respectively
|
43,577
|
|
39,401
|
|
|||
|
Inventories
|
40,071
|
|
39,274
|
|
|||
|
Prepaid expenses and other
|
4,003
|
|
4,302
|
|
|||
|
Current assets held for sale
|
—
|
|
9,642
|
|
|||
|
Total current assets
|
315,292
|
|
168,032
|
|
|||
|
Property, plant and equipment, net
|
24,258
|
|
25,715
|
|
|||
|
Other assets
|
3,835
|
|
3,417
|
|
|||
|
Intangible assets, net
|
145,387
|
|
112,547
|
|
|||
|
Goodwill
|
347,666
|
|
285,944
|
|
|||
|
Non-current assets held for sale
|
—
|
|
109,817
|
|
|||
|
Total Assets
|
$
|
836,438
|
|
$
|
705,472
|
|
|
|
Liabilities and Stockholders' Equity
|
|||||||
|
Current Liabilities
|
|||||||
|
Accounts payable
|
$
|
22,829
|
|
$
|
15,775
|
|
|
|
Accrued liabilities
|
38,338
|
|
34,426
|
|
|||
|
Current portion of long-term debt
|
7,500
|
|
5,000
|
|
|||
|
Current portion of contingent consideration
|
4,635
|
|
2,100
|
|
|||
|
Total current liabilities
|
73,302
|
|
57,301
|
|
|||
|
Long-term debt, net of current portion
|
124,407
|
|
86,621
|
|
|||
|
Deferred income taxes
|
14,542
|
|
17,173
|
|
|||
|
Contingent consideration, net of current portion
|
8,851
|
|
1,161
|
|
|||
|
Other long-term liabilities
|
521
|
|
621
|
|
|||
|
Total Liabilities
|
221,623
|
|
162,877
|
|
|||
|
Commitments and Contingencies (Note 17)
|
|
|
|||||
|
Stockholders’ Equity
|
|||||||
|
Preferred stock, par value $.01 per share, 5,000,000 shares authorized; no shares issued and outstanding
|
—
|
|
—
|
|
|||
|
Common stock, par value $.01 per share, 75,000,000 shares authorized; 37,984,382 and 37,594,493 shares issued and 37,614,382 and 37,224,493 shares outstanding at May 31, 2019 and 2018, respectively
|
372
|
|
370
|
|
|||
|
Additional paid-in capital
|
555,040
|
|
543,762
|
|
|||
|
Retained earnings
|
66,469
|
|
5,129
|
|
|||
|
Treasury stock, 370,000 shares, at cost at May 31, 2019 and 2018, respectively
|
(5,714
|
)
|
(5,714
|
)
|
|||
|
Accumulated other comprehensive loss
|
(1,352
|
)
|
(952
|
)
|
|||
|
Total Stockholders' Equity
|
614,815
|
|
542,595
|
|
|||
|
Total Liabilities and Stockholders' Equity
|
$
|
836,438
|
|
$
|
705,472
|
|
|
|
Common Stock
|
Additional
paid in
capital
|
Retained
earnings (accumulated deficit)
|
Accumulated
other
comprehensive
loss
|
Treasury Stock
|
Total
|
||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
||||||||||||||||||||||||||
|
Balance at May 31, 2016
|
36,420,403
|
|
$
|
363
|
|
$
|
525,775
|
|
$
|
(16,015
|
)
|
$
|
(791
|
)
|
(142,305
|
)
|
$
|
(2,104
|
)
|
$
|
507,228
|
|
|||||||
|
Net income
|
5,008
|
|
5,008
|
|
|||||||||||||||||||||||||
|
Exercise of stock options
|
751,062
|
|
7
|
|
9,858
|
|
9,865
|
|
|||||||||||||||||||||
|
Issuance/cancellation of restricted stock units
|
158,341
|
|
1
|
|
(587
|
)
|
(586
|
)
|
|||||||||||||||||||||
|
Issuance of performance share units
|
23,405
|
|
|
|
—
|
|
|||||||||||||||||||||||
|
Purchase of common stock under Employee Stock Purchase Plan
|
129,185
|
|
1
|
|
1,418
|
|
1,419
|
|
|||||||||||||||||||||
|
Stock-based compensation
|
6,183
|
|
6,183
|
|
|||||||||||||||||||||||||
|
Treasury stock retirement
|
(642,305
|
)
|
(2
|
)
|
(9,942
|
)
|
642,305
|
|
9,944
|
|
—
|
|
|||||||||||||||||
|
Common stock repurchased
|
370,000
|
|
(3
|
)
|
|
(870
|
)
|
(13,554
|
)
|
(13,557
|
)
|
||||||||||||||||||
|
Other comprehensive loss, net of tax
|
(533
|
)
|
(533
|
)
|
|||||||||||||||||||||||||
|
Balance at May 31, 2017
|
37,210,091
|
|
$
|
367
|
|
$
|
532,705
|
|
$
|
(11,007
|
)
|
$
|
(1,324
|
)
|
(370,000
|
)
|
$
|
(5,714
|
)
|
$
|
515,027
|
|
|||||||
|
Adjustment for ASU 2016-09
|
199
|
|
(199
|
)
|
—
|
|
|||||||||||||||||||||||
|
Net income
|
16,335
|
|
16,335
|
|
|||||||||||||||||||||||||
|
Exercise of stock options
|
148,937
|
|
1
|
|
1,916
|
|
1,917
|
|
|||||||||||||||||||||
|
Issuance/cancellation of restricted stock units
|
145,522
|
|
1
|
|
(232
|
)
|
(231
|
)
|
|||||||||||||||||||||
|
Issuance of performance share units
|
|
—
|
|
||||||||||||||||||||||||||
|
Purchase of common stock under Employee Stock Purchase Plan
|
89,943
|
|
1
|
|
1,262
|
|
1,263
|
|
|||||||||||||||||||||
|
Stock-based compensation
|
7,912
|
|
7,912
|
|
|||||||||||||||||||||||||
|
Other comprehensive income, net of tax
|
372
|
|
372
|
|
|||||||||||||||||||||||||
|
Balance at May 31, 2018
|
37,594,493
|
|
$
|
370
|
|
$
|
543,762
|
|
$
|
5,129
|
|
$
|
(952
|
)
|
(370,000
|
)
|
$
|
(5,714
|
)
|
$
|
542,595
|
|
|||||||
|
Net income
|
61,340
|
|
61,340
|
|
|||||||||||||||||||||||||
|
Exercise of stock options
|
134,253
|
|
1
|
|
1,525
|
|
1,526
|
|
|||||||||||||||||||||
|
Issuance/cancellation of restricted stock units
|
177,538
|
|
(667
|
)
|
(667
|
)
|
|||||||||||||||||||||||
|
Issuance of performance share units
|
5,235
|
|
—
|
|
|||||||||||||||||||||||||
|
Purchase of common stock under Employee Stock Purchase Plan
|
72,863
|
|
1
|
|
1,171
|
|
1,172
|
|
|||||||||||||||||||||
|
Stock-based compensation
|
9,249
|
|
9,249
|
|
|||||||||||||||||||||||||
|
Other comprehensive loss, net of tax
|
(400
|
)
|
(400
|
)
|
|||||||||||||||||||||||||
|
Balance at May 31, 2019
|
37,984,382
|
|
$
|
372
|
|
$
|
555,040
|
|
$
|
66,469
|
|
$
|
(1,352
|
)
|
(370,000
|
)
|
$
|
(5,714
|
)
|
$
|
614,815
|
|
|||||||
|
|
Year ended May 31,
|
||||||||||
|
|
2019
|
2018
|
2017
|
||||||||
|
Cash flows from operating activities:
|
|||||||||||
|
Net income
|
$
|
61,340
|
|
$
|
16,335
|
|
$
|
5,008
|
|
||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
|||||||||||
|
Depreciation and amortization
|
25,880
|
|
23,163
|
|
24,811
|
|
|||||
|
Stock based compensation
|
9,249
|
|
7,912
|
|
6,183
|
|
|||||
|
Gain on disposition
|
(46,592
|
)
|
—
|
|
—
|
|
|||||
|
Transaction costs for disposition
|
(4,030
|
)
|
—
|
|
—
|
|
|||||
|
Change in fair value of contingent consideration
|
(6,776
|
)
|
250
|
|
(15,261
|
)
|
|||||
|
Deferred income tax provision
|
(2,655
|
)
|
(8,947
|
)
|
4,428
|
|
|||||
|
Changes in accounts receivable allowances
|
(202
|
)
|
179
|
|
(313
|
)
|
|||||
|
Fixed and intangible asset impairments and disposals
|
2,495
|
|
540
|
|
3,930
|
|
|||||
|
Write-off of other assets
|
—
|
|
—
|
|
2,685
|
|
|||||
|
Other
|
(5
|
)
|
(605
|
)
|
(586
|
)
|
|||||
|
Changes in operating assets and liabilities:
|
|||||||||||
|
Accounts receivable
|
(3,177
|
)
|
5,044
|
|
8,479
|
|
|||||
|
Inventories
|
(1,428
|
)
|
5,740
|
|
687
|
|
|||||
|
Prepaid expenses and other
|
(1,871
|
)
|
(1,231
|
)
|
(3,520
|
)
|
|||||
|
Accounts payable, accrued and other liabilities
|
5,212
|
|
(7,093
|
)
|
19,214
|
|
|||||
|
Net cash provided by operating activities
|
37,440
|
|
41,287
|
|
55,745
|
|
|||||
|
Cash flows from investing activities:
|
|||||||||||
|
Additions to property, plant and equipment
|
(3,118
|
)
|
(2,391
|
)
|
(3,001
|
)
|
|||||
|
Proceeds from disposition of discontinued operations
|
169,242
|
|
—
|
|
—
|
|
|||||
|
Acquisition of businesses, net of cash acquired
|
(84,920
|
)
|
—
|
|
—
|
|
|||||
|
Acquisition of intangibles
|
—
|
|
(1,265
|
)
|
—
|
|
|||||
|
Proceeds from sale or maturity of marketable securities
|
1,350
|
|
—
|
|
450
|
|
|||||
|
Net cash provided by (used in) investing activities
|
82,554
|
|
(3,656
|
)
|
(2,551
|
)
|
|||||
|
Cash flows from financing activities:
|
|||||||||||
|
Proceeds from issuance of and borrowings on long-term debt
|
—
|
|
—
|
|
116,471
|
|
|||||
|
Repayment of long-term debt
|
(15,000
|
)
|
(5,000
|
)
|
(140,381
|
)
|
|||||
|
Proceeds from borrowings on revolving credit facility
|
55,000
|
|
—
|
|
—
|
|
|||||
|
Deferred financing costs on long-term debt
|
—
|
|
—
|
|
(1,364
|
)
|
|||||
|
Payment of acquisition related contingent consideration
|
(8,100
|
)
|
(9,500
|
)
|
(9,850
|
)
|
|||||
|
Repurchase of common stock
|
—
|
|
—
|
|
(13,557
|
)
|
|||||
|
Proceeds from exercise of stock options and employee stock purchase plan
|
2,031
|
|
2,949
|
|
10,698
|
|
|||||
|
Net cash provided by (used in) financing activities
|
33,931
|
|
(11,551
|
)
|
(37,983
|
)
|
|||||
|
Effect of exchange rate changes on cash and cash equivalents
|
(380
|
)
|
472
|
|
—
|
|
|||||
|
Increase in cash and cash equivalents
|
153,545
|
|
26,552
|
|
15,211
|
|
|||||
|
Cash and cash equivalents at beginning of year
|
74,096
|
|
47,544
|
|
32,333
|
|
|||||
|
Cash and cash equivalents at end of year
|
$
|
227,641
|
|
$
|
74,096
|
|
$
|
47,544
|
|
||
|
|
Year ended May 31,
|
||||||||||
|
|
2019
|
2018
|
2017
|
||||||||
|
Supplemental disclosure of non-cash investing and financing activities:
|
|||||||||||
|
Increase (decrease) in accounts payable for purchases of fixed assets
|
$
|
(114
|
)
|
$
|
56
|
|
$
|
26
|
|
||
|
Fair value of contingent consideration for acquisitions
|
25,100
|
|
—
|
|
—
|
|
|||||
|
Fair value of acquisition consideration included in accrued expenses
|
4,650
|
|
—
|
|
—
|
|
|||||
|
Cash paid (received) during the year for:
|
|||||||||||
|
Interest
|
$
|
5,115
|
|
$
|
3,190
|
|
$
|
2,969
|
|
||
|
Income taxes
|
426
|
|
36
|
|
(102
|
)
|
|||||
|
Estimated useful lives
|
||
|
Building and building improvements
|
39 years
|
|
|
Machinery and equipment
|
5 to 8 years
|
|
|
Computer software and equipment
|
3 to 5 years
|
|
|
Recently Issued Accounting Pronouncements - Adopted
|
|||
|
Standard
|
Description
|
Date Adopted
|
Effect on the Consolidated Financial Statements
|
|
ASU No. 2014-09,
Revenue from Contracts with Customers (ASU 2014-09)
|
This ASU provides a single, comprehensive accounting model for revenues arising from contracts with customers that supersedes most of the existing revenue recognition guidance, including industry-specific guidance. Under this model, revenue is recognized at an amount that an entity expects to be entitled to upon transferring control of goods or services to a customer, as opposed to when risks and rewards transfer to a customer under existing revenue recognition guidance.
|
June 1, 2018
|
See Note 4, "Revenue from Contracts with Customers" for the required disclosures related to the impact of adopting this standard.
The adoption of this standard did not have a material impact on the Company’s consolidated balance sheets and statements of operations.
|
|
ASU No. 2016-15,
Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments (ASU 2016-15)
|
This ASU identifies how certain cash receipts and cash payments are presented and classified in the Statement of Cash Flows under Topic 230.
|
June 1, 2018
|
This adoption did not have an impact on the Company's financial statements.
|
|
Recently Issued Accounting Pronouncements - Not Yet Applicable or Adopted
|
|||
|
Standard
|
Description
|
Effective Date
|
Effect on the Consolidated Financial Statements
|
|
ASU 2016-02,
Leases (Topic 842)
|
This ASU increases transparency and comparability among organizations by recognizing lease assets and liabilities on the balance sheet and disclosing key information about leasing arrangements. For leases with a term of twelve months or less, a lessee is permitted to make an accounting policy election by class of underlying asset not to recognize lease assets and liabilities.
|
June 1, 2019
|
On June 1, 2019, the Company adopted ASC 842 and elected the optional transition method to apply the standard as of the effective date and therefore, the Company will not apply the standard to the comparative periods presented in the consolidated financial statements.
The Company has elected the transition package of three practical expedients permitted within the standard, which eliminates the requirements to reassess prior conclusions about lease identification, lease classification, and initial direct costs. Further, the Company has elected a short-term lease exception policy, permitting the Company to not apply the recognition requirements of this standard to short-term leases (i.e., leases with terms of 12 months or less) and an accounting policy to account for lease and non-lease components as a single component for certain classes of assets. While the Company is finalizing its evaluation of the impact of the adoption of ASC 842 on its consolidated financial statements and related disclosures, the Company expects to recognize on its balance sheet right-of-use assets ranging from $4.5 to $5.5 million, in aggregate, and lease liabilities ranging from $5.0 to $6.0 million, in aggregate, which are primarily related to the Company’s facilities operating leases (Note 17). The difference between the right-of-use assets and lease liabilities is primarily attributed to the elimination of deferred rent. The adoption of ASC 842 is also expected to impact the Company’s consolidated financial statement disclosures. The Company does not anticipate the adoption of ASC 842 will have a material impact to the Consolidated Statements of Operations and Statements of Cashflows or to require a cumulative-effect adjustment to the opening balance of retained earnings. |
|
Preliminary allocation
|
Adjustments
(1)
|
Revised allocation
|
||||||||||
|
(in thousands)
|
||||||||||||
|
Accounts receivable
|
$
|
900
|
|
$
|
—
|
|
$
|
900
|
|
|||
|
Inventory
|
732
|
|
—
|
|
732
|
|
||||||
|
Prepaid and other current assets
|
98
|
|
—
|
|
98
|
|
||||||
|
Property, plant and equipment
|
133
|
|
—
|
|
133
|
|
||||||
|
Intangible assets:
|
||||||||||||
|
RadiaDyne trademark
|
400
|
|
—
|
|
400
|
|
||||||
|
OARtrac trademark
|
200
|
|
—
|
|
200
|
|
||||||
|
RadiaDyne legacy product technology
|
1,500
|
|
—
|
|
1,500
|
|
||||||
|
OARtrac product technology
|
16,300
|
|
2,600
|
|
18,900
|
|
||||||
|
RadiaDyne customer relationships
|
3,700
|
|
900
|
|
4,600
|
|
||||||
|
Goodwill
|
51,482
|
|
(3,500
|
)
|
47,982
|
|
||||||
|
Total assets acquired
|
$
|
75,445
|
|
$
|
—
|
|
$
|
75,445
|
|
|||
|
Liabilities assumed
|
||||||||||||
|
Accounts payable
|
$
|
352
|
|
$
|
—
|
|
$
|
352
|
|
|||
|
Accrued expenses
|
106
|
|
—
|
|
106
|
|
||||||
|
Total liabilities assumed
|
$
|
458
|
|
$
|
—
|
|
$
|
458
|
|
|||
|
Net assets acquired
|
$
|
74,987
|
|
|
$
|
—
|
|
$
|
74,987
|
|
||
|
|
Preliminary allocation
|
Adjustments
(1)
|
Revised allocation
|
|||||||||
|
(in thousands)
|
||||||||||||
|
Inventory
|
$
|
50
|
|
$
|
—
|
|
$
|
50
|
|
|||
|
Property, plant and equipment
|
10
|
|
—
|
|
10
|
|
||||||
|
Intangible assets:
|
||||||||||||
|
BioSentry trademark
|
1,700
|
|
800
|
|
2,500
|
|
||||||
|
BioSentry product technology
|
13,800
|
|
7,100
|
|
20,900
|
|
||||||
|
Customer relationships
|
2,500
|
|
100
|
|
2,600
|
|
||||||
|
Goodwill
|
21,740
|
|
(8,000
|
)
|
13,740
|
|
||||||
|
Net assets acquired
|
$
|
39,800
|
|
$
|
—
|
|
$
|
39,800
|
|
|||
|
Year ended May 31,
|
|||||||||||
|
(in thousands)
|
2019
|
2018
|
2017
|
||||||||
|
Net sales
|
$
|
88,850
|
|
$
|
82,630
|
|
$
|
79,855
|
|
||
|
Cost of sales (exclusive of amortization)
|
52,978
|
|
49,611
|
|
47,636
|
|
|||||
|
Gross profit
|
35,872
|
|
33,019
|
|
32,219
|
|
|||||
|
Operating expenses
|
|||||||||||
|
Research and development
|
1,177
|
|
1,121
|
|
1,121
|
|
|||||
|
Sales and marketing
|
4,129
|
|
4,167
|
|
3,954
|
|
|||||
|
General and administrative
|
271
|
|
274
|
|
273
|
|
|||||
|
Amortization of intangibles
|
2,716
|
|
2,729
|
|
2,729
|
|
|||||
|
Total operating expenses
|
8,293
|
|
8,291
|
|
8,077
|
|
|||||
|
Operating income
|
27,579
|
|
24,728
|
|
24,142
|
|
|||||
|
|
|
|
|
|
|
||||||
|
Gain on divestiture
|
46,592
|
|
—
|
|
—
|
|
|||||
|
Income from discontinued operations before income taxes
|
74,171
|
|
24,728
|
|
24,142
|
|
|||||
|
Income tax expense
|
(1,685
|
)
|
(2,166
|
)
|
(12,082
|
)
|
|||||
|
Income from discontinued operations
|
$
|
72,486
|
|
$
|
22,562
|
|
$
|
12,060
|
|
||
|
(in thousands)
|
|||
|
Proceeds received from Divestiture
|
$
|
169,242
|
|
|
Working capital adjustment
|
(612
|
)
|
|
|
Fluid Management assets:
|
|||
|
Inventories
|
11,029
|
|
|
|
Property, plant and equipment, net
|
16,624
|
|
|
|
Intangible assets, net
|
15,047
|
|
|
|
Goodwill
|
75,308
|
|
|
|
Total Fluid Management assets
|
118,008
|
|
|
|
Transaction costs for Divestiture
(1)
|
4,030
|
|
|
|
Gain on sale of the Fluid Management business before income taxes
|
$
|
46,592
|
|
|
(in thousands)
|
May 31, 2018
|
||
|
Inventories
|
$
|
9,642
|
|
|
Total current assets held for sale
|
9,642
|
|
|
|
Property, plant and equipment, net
|
16,746
|
|
|
|
Intangible assets, net
|
17,763
|
|
|
|
Goodwill
|
75,308
|
|
|
|
Total non-current assets held for sale
|
109,817
|
|
|
|
Total assets held for sale
|
$
|
119,459
|
|
|
2019
|
2018
|
2017
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Net cash provided by operating activities
|
$
|
2,245
|
|
$
|
5,581
|
|
$
|
3,968
|
|
||
|
Net cash provided by investing activities
|
982
|
|
1,146
|
|
1,361
|
|
|||||
|
Year ended May 31, 2019
|
|||||||||||
|
(in thousands)
|
United States
|
International
|
Total
|
||||||||
|
Net sales
|
|||||||||||
|
Vascular Interventions & Therapies
|
$
|
106,767
|
|
$
|
13,134
|
|
$
|
119,901
|
|
||
|
Vascular Access
|
79,611
|
|
15,119
|
|
$
|
94,730
|
|
||||
|
Oncology
|
30,579
|
|
25,424
|
|
$
|
56,003
|
|
||||
|
Total
|
$
|
216,957
|
|
$
|
53,677
|
|
$
|
270,634
|
|
||
|
May 31, 2019
|
May 31, 2018
|
||||||
|
(in thousands)
|
|||||||
|
Receivables
|
$
|
43,577
|
|
$
|
39,401
|
|
|
|
Contract assets
|
$
|
—
|
|
$
|
—
|
|
|
|
Contract liabilities
|
$
|
681
|
|
$
|
1,203
|
|
|
|
•
|
Level 1 - Inputs to the valuation methodology are quoted market prices for identical assets or liabilities.
|
|
•
|
Level 2 - Inputs to the valuation methodology are other observable inputs, including quoted market prices for similar assets or liabilities and market-corroborated inputs.
|
|
•
|
Level 3 - Inputs to the valuation methodology are unobservable inputs based on management’s best estimate of inputs market participants would use in pricing the asset or liability at the measurement date, including assumptions about risk.
|
|
|
Fair Value Measurements using inputs considered as:
|
||||||||||||||
|
(in thousands)
|
Level 1
|
Level 2
|
Level 3
|
Fair Value at
May 31, 2019
|
|||||||||||
|
Financial Liabilities
|
|||||||||||||||
|
Contingent liability for acquisition earn outs
|
$
|
—
|
|
$
|
—
|
|
$
|
13,486
|
|
$
|
13,486
|
|
|||
|
Total Financial Liabilities
|
$
|
—
|
|
$
|
—
|
|
$
|
13,486
|
|
$
|
13,486
|
|
|||
|
Fair Value Measurements using inputs considered as:
|
|||||||||||||||
|
(in thousands)
|
Level 1
|
Level 2
|
Level 3
|
Fair Value at
May 31, 2018
|
|||||||||||
|
Financial Assets
|
|||||||||||||||
|
Short-term investments
(1)
|
$
|
2,100
|
|
$
|
—
|
|
$
|
—
|
|
$
|
2,100
|
|
|||
|
Marketable Securities
|
—
|
|
—
|
|
1,317
|
|
1,317
|
|
|||||||
|
Total Financial Assets
|
$
|
2,100
|
|
$
|
—
|
|
$
|
1,317
|
|
$
|
3,417
|
|
|||
|
Financial Liabilities
|
|||||||||||||||
|
Contingent liability for acquisition earn out
|
$
|
—
|
|
$
|
—
|
|
$
|
3,261
|
|
$
|
3,261
|
|
|||
|
Total Financial Liabilities
|
$
|
—
|
|
$
|
—
|
|
$
|
3,261
|
|
$
|
3,261
|
|
|||
|
|
Financial Assets
|
Financial Liabilities
|
|||||
|
(in thousands)
|
Fair Value Measurements
Using Significant Unobservable Inputs (Level 3) |
Fair Value Measurements
Using Significant Unobservable Inputs (Level 3) |
|||||
|
Balance at May 31, 2018
|
$
|
1,317
|
|
$
|
3,261
|
|
|
|
Contingent consideration liabilities recorded as the result of acquisitions (Note 2)
|
—
|
|
25,101
|
|
|||
|
Change in present value of contingent consideration
(1)
|
—
|
|
(6,776
|
)
|
|||
|
Fair market value adjustments
|
33
|
|
—
|
|
|||
|
Proceeds from sale of marketable securities
|
(1,350
|
)
|
—
|
|
|||
|
Contingent consideration payments
|
—
|
|
(8,100
|
)
|
|||
|
Balance at May 31, 2019
|
$
|
—
|
|
$
|
13,486
|
|
|
|
Financial Assets
|
Financial Liabilities
|
||||||
|
(in thousands)
|
Fair Value Measurements
Using Significant Unobservable Inputs (Level 3) |
Fair Value Measurements
Using Significant Unobservable Inputs (Level 3) |
|||||
|
Balance at May 31, 2017
|
$
|
1,215
|
|
$
|
12,761
|
|
|
|
Change in fair value of contingent consideration
(1)
|
—
|
|
250
|
|
|||
|
Fair market value adjustments
|
102
|
|
—
|
|
|||
|
Contingent consideration payments
|
—
|
|
(9,750
|
)
|
|||
|
Balance at May 31, 2018
|
$
|
1,317
|
|
$
|
3,261
|
|
|
|
(in thousands)
|
Fair Value
|
Valuation Technique
|
Unobservable Input
|
Range
|
|||||
|
Revenue based payments
|
$
|
10,058
|
|
Discounted cash flow
|
Discount rate
|
4% - 5%
|
|||
|
Probability of payment
|
66% - 100%
|
||||||||
|
Projected fiscal year of payment
|
2020 - 2023
|
||||||||
|
Technical milestones
|
3,428
|
|
Estimated probability
|
Estimated probability
|
90%
|
||||
|
Projected year of payment
|
2020
|
||||||||
|
$
|
13,486
|
|
|||||||
|
Amortized
Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Fair
Value
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Available-for-sales securities:
|
|||||||||||||||
|
New York State government agency obligations
|
$
|
1,350
|
|
$
|
—
|
|
$
|
(33
|
)
|
$
|
1,317
|
|
|||
|
$
|
1,350
|
|
$
|
—
|
|
$
|
(33
|
)
|
$
|
1,317
|
|
||||
|
May 31, 2019
|
May 31, 2018
|
||||||
|
(in thousands)
|
|||||||
|
Raw materials
|
$
|
16,045
|
|
$
|
15,247
|
|
|
|
Work in process
|
6,786
|
|
6,656
|
|
|||
|
Finished goods
|
17,240
|
|
17,371
|
|
|||
|
Total
|
$
|
40,071
|
|
$
|
39,274
|
|
|
|
May 31, 2019
|
May 31, 2018
|
||||||
|
(in thousands)
|
|||||||
|
Software licenses
|
$
|
1,131
|
|
$
|
1,043
|
|
|
|
License fees
|
145
|
|
143
|
|
|||
|
Trade shows
|
213
|
|
223
|
|
|||
|
Rent
|
199
|
|
134
|
|
|||
|
Other prepaid taxes
|
112
|
|
254
|
|
|||
|
Other
|
2,203
|
|
2,505
|
|
|||
|
Total
|
$
|
4,003
|
|
$
|
4,302
|
|
|
|
May 31, 2019
|
May 31, 2018
|
||||||
|
(in thousands)
|
|||||||
|
Building and building improvements
|
$
|
24,535
|
|
$
|
24,509
|
|
|
|
Machinery and equipment
|
14,029
|
|
13,308
|
|
|||
|
Computer software and equipment
|
24,980
|
|
24,459
|
|
|||
|
Construction in progress
|
1,239
|
|
1,666
|
|
|||
|
64,783
|
|
63,942
|
|
||||
|
Less accumulated depreciation and amortization
|
(41,608
|
)
|
(39,290
|
)
|
|||
|
23,175
|
|
24,652
|
|
||||
|
Land and land improvements
|
1,083
|
|
1,063
|
|
|||
|
$
|
24,258
|
|
$
|
25,715
|
|
||
|
(in thousands)
|
|||
|
Goodwill balance at May 31, 2018, before adjustment to conform goodwill to current presentation
|
$
|
361,252
|
|
|
Goodwill associated with the Fluid Management divestiture (Note 3)
|
(75,308
|
)
|
|
|
Goodwill balance at May 31, 2018
|
285,944
|
|
|
|
Additions for BioSentry acquisition (Note 2)
|
13,740
|
|
|
|
Additions for RadiaDyne acquisition (Note 2)
|
47,982
|
|
|
|
Goodwill balance at May 31, 2019
|
$
|
347,666
|
|
|
|
May 31, 2019
|
||||||||||
|
Gross carrying
value
|
Accumulated
amortization
|
Net carrying
value
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Product technologies
|
$
|
182,971
|
|
$
|
(75,412
|
)
|
$
|
107,559
|
|
||
|
Customer relationships
|
60,166
|
|
(25,950
|
)
|
34,216
|
|
|||||
|
Trademarks
|
9,300
|
|
(6,404
|
)
|
2,896
|
|
|||||
|
Licenses
|
5,752
|
|
(5,036
|
)
|
716
|
|
|||||
|
$
|
258,189
|
|
$
|
(112,802
|
)
|
$
|
145,387
|
|
|||
|
May 31, 2018
|
|||||||||||
|
Gross carrying
value
|
Accumulated
amortization
|
Net carrying
value
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Product technologies
|
$
|
141,675
|
|
$
|
(64,153
|
)
|
$
|
77,522
|
|
||
|
Customer relationships
|
55,028
|
|
(22,794
|
)
|
32,234
|
|
|||||
|
Trademarks
|
6,200
|
|
(5,642
|
)
|
558
|
|
|||||
|
Licenses
|
5,752
|
|
(4,357
|
)
|
1,395
|
|
|||||
|
Distributor relationships
|
1,250
|
|
(412
|
)
|
838
|
|
|||||
|
$
|
209,905
|
|
$
|
(97,358
|
)
|
$
|
112,547
|
|
|||
|
(in thousands)
|
|||
|
2020
|
$
|
15,248
|
|
|
2021
|
14,021
|
|
|
|
2022
|
13,405
|
|
|
|
2023
|
13,368
|
|
|
|
2024
|
11,812
|
|
|
|
2025 and thereafter
|
77,533
|
|
|
|
$
|
145,387
|
|
|
|
Year ended May 31,
|
|||||||||||
|
2019
|
2018
|
2017
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Loss from continuing operations before tax expense:
|
|
|
|
||||||||
|
U.S.
|
$
|
(15,593
|
)
|
$
|
(18,454
|
)
|
$
|
(15,317
|
)
|
||
|
Non-U.S.
|
891
|
|
1,191
|
|
1,022
|
|
|||||
|
$
|
(14,702
|
)
|
$
|
(17,263
|
)
|
$
|
(14,295
|
)
|
|||
|
Year ended May 31,
|
|||||||||||
|
(in thousands)
|
2019
|
2018
|
2017
|
||||||||
|
Current
|
|
|
|
||||||||
|
Federal
|
$
|
—
|
|
$
|
(148
|
)
|
$
|
—
|
|
||
|
State and local
|
128
|
|
152
|
|
141
|
|
|||||
|
Non U.S.
|
289
|
|
73
|
|
270
|
|
|||||
|
417
|
|
77
|
|
411
|
|
||||||
|
Deferred
|
(3,973
|
)
|
(11,113
|
)
|
(7,654
|
)
|
|||||
|
Income tax benefit
|
$
|
(3,556
|
)
|
$
|
(11,036
|
)
|
$
|
(7,243
|
)
|
||
|
(in thousands)
|
May 31, 2019
|
May 31, 2018
|
|||||
|
Deferred tax assets
|
|
|
|||||
|
Net operating loss carryforward
|
$
|
18,955
|
|
$
|
33,880
|
|
|
|
Stock-based compensation
|
3,395
|
|
2,421
|
|
|||
|
Federal and state R&D tax credit carryforward
|
4,259
|
|
3,644
|
|
|||
|
Inventories
|
971
|
|
1,550
|
|
|||
|
Expenses incurred not currently deductible
|
1,337
|
|
1,714
|
|
|||
|
Accrued liabilities
|
139
|
|
277
|
|
|||
|
Gross deferred tax asset
|
29,056
|
|
43,486
|
|
|||
|
Deferred tax liabilities
|
|
|
|||||
|
Excess tax over book depreciation and amortization
|
31,878
|
|
34,044
|
|
|||
|
31,878
|
|
34,044
|
|
||||
|
Valuation Allowance
|
(11,688
|
)
|
(26,607
|
)
|
|||
|
Net deferred tax liability
(1)
|
$
|
(14,510
|
)
|
$
|
(17,165
|
)
|
|
|
Year ended May 31,
|
|||||||||||
|
(in thousands)
|
2019
|
2018
|
2017
|
||||||||
|
Income tax benefit at federal statutory tax rate of 21.0%, 28.6% and 35.0%, respectively
|
$
|
(3,087
|
)
|
$
|
(4,941
|
)
|
$
|
(5,003
|
)
|
||
|
Effect of graduated tax rates
|
—
|
|
—
|
|
143
|
|
|||||
|
State income taxes, net of Federal tax benefit
|
(177
|
)
|
120
|
|
(505
|
)
|
|||||
|
Impact of Non-U.S. operations
|
76
|
|
(288
|
)
|
403
|
|
|||||
|
Research and development tax credit
|
(936
|
)
|
(951
|
)
|
(403
|
)
|
|||||
|
Impact of tax reform
|
—
|
|
12,860
|
|
—
|
|
|||||
|
Meals and entertainment
|
190
|
|
242
|
|
266
|
|
|||||
|
Non-deductible interest on contingent payments
|
—
|
|
—
|
|
174
|
|
|||||
|
Non-taxable gain on revaluation of contingent consideration liability
|
—
|
|
—
|
|
(5,576
|
)
|
|||||
|
Change in valuation allowance
|
175
|
|
(18,526
|
)
|
2,749
|
|
|||||
|
Effect of elimination of stock compensation APIC pool
|
—
|
|
—
|
|
1,380
|
|
|||||
|
IPR&D intangible write-off
|
—
|
|
—
|
|
(1,224
|
)
|
|||||
|
Other
|
203
|
|
448
|
|
353
|
|
|||||
|
Income tax benefit
|
$
|
(3,556
|
)
|
$
|
(11,036
|
)
|
$
|
(7,243
|
)
|
||
|
Year ended May 31,
|
|||||||||||
|
(in thousands)
|
2019
|
2018
|
2017
|
||||||||
|
Unrecognized tax benefits balance at June 1
|
$
|
464
|
|
$
|
899
|
|
$
|
899
|
|
||
|
Decrease in gross amounts of tax positions related to prior years due to U.S. tax reform
|
—
|
|
(287
|
)
|
—
|
|
|||||
|
Decrease due to lapse in statute of limitations
|
—
|
|
(148
|
)
|
—
|
|
|||||
|
Unrecognized tax benefits balance at May 31
|
$
|
464
|
|
$
|
464
|
|
$
|
899
|
|
||
|
May 31, 2019
|
May 31, 2018
|
||||||
|
(in thousands)
|
|||||||
|
Payroll and related expenses
|
$
|
14,987
|
|
$
|
10,235
|
|
|
|
Royalties
|
2,088
|
|
1,537
|
|
|||
|
Accrued severance
|
504
|
|
1,940
|
|
|||
|
Sales and franchise taxes
|
807
|
|
683
|
|
|||
|
Outside services
|
3,514
|
|
2,396
|
|
|||
|
Litigation matters (Note 17)
|
2,700
|
|
12,500
|
|
|||
|
Indemnification holdback
|
4,807
|
|
—
|
|
|||
|
Other
|
8,931
|
|
5,135
|
|
|||
|
Total
|
$
|
38,338
|
|
$
|
34,426
|
|
|
|
(in thousands)
|
|||
|
2020
|
7,500
|
|
|
|
2021
|
11,250
|
|
|
|
2022
|
68,750
|
|
|
|
Total term loan
|
87,500
|
|
|
|
Revolving facility
|
45,000
|
|
|
|
Total debt
|
132,500
|
|
|
|
Less: Unamortized debt issuance costs
|
(593
|
)
|
|
|
Total
|
131,907
|
|
|
|
Less: Current portion of long-term debt
|
(7,500
|
)
|
|
|
Total long-term debt, net of current portion
|
$
|
124,407
|
|
|
Shares
|
Weighted-
average
exercise
price
|
Weighted
average
remaining
contractual
life
|
Aggregate
intrinsic
value (in
thousands)
|
|||||||||
|
Outstanding at beginning of year - June 1, 2018
|
1,696,413
|
|
$
|
15.42
|
|
|||||||
|
Granted
|
391,264
|
|
$
|
20.71
|
|
|||||||
|
Exercised
|
(184,168
|
)
|
$
|
14.32
|
|
|||||||
|
Forfeited
|
(143,196
|
)
|
$
|
17.52
|
|
|||||||
|
Expired
|
—
|
|
$
|
—
|
|
|||||||
|
Outstanding at end of year - May 31, 2019
|
1,760,313
|
|
$
|
16.54
|
|
5.76
|
$
|
4,672
|
|
|||
|
Options exercisable at year-end
|
831,924
|
|
$
|
15.09
|
|
3.90
|
$
|
3,092
|
|
|||
|
Options expected to vest in future periods
|
928,389
|
|
$
|
17.83
|
|
7.43
|
$
|
1,579
|
|
|||
|
Restricted Stock Units
|
Weighted Average
Grant-Date Fair Value
|
|||||
|
Non-vested at beginning of year, June 1, 2018
|
474,502
|
|
$
|
16.23
|
|
|
|
Granted
|
244,405
|
|
$
|
20.87
|
|
|
|
Vested
|
(210,865
|
)
|
$
|
21.69
|
|
|
|
Canceled
|
(77,106
|
)
|
$
|
17.98
|
|
|
|
Non-vested at end of year, May 31, 2019
|
430,936
|
|
$
|
18.60
|
|
|
|
Performance Unit Awards
|
Weighted Average
Grant-Date Fair Value
|
|||||
|
Non-vested at beginning of year, June 1, 2018
|
451,856
|
|
$
|
19.75
|
|
|
|
Granted
|
126,356
|
|
$
|
28.62
|
|
|
|
Vested
|
(7,803
|
)
|
$
|
20.50
|
|
|
|
Canceled
|
(31,804
|
)
|
$
|
24.03
|
|
|
|
Non-vested at end of year, May 31, 2019
|
538,605
|
|
$
|
21.55
|
|
|
|
Year ended May 31,
|
||||||||||||
|
(in thousands)
|
2019
|
2018
|
2017
|
|||||||||
|
Cost of sales
|
$
|
461
|
|
$
|
119
|
|
$
|
299
|
|
|||
|
Research and development
|
724
|
|
554
|
|
314
|
|
||||||
|
Sales and marketing
|
1,952
|
|
1,778
|
|
1,762
|
|
||||||
|
General and administrative
|
6,112
|
|
5,461
|
|
4,026
|
|
||||||
|
Acquisition, restructuring and other items, net
|
—
|
|
—
|
|
(218
|
)
|
||||||
|
$
|
9,249
|
|
$
|
7,912
|
|
$
|
6,183
|
|
||||
|
Year ended May 31,
|
||||||||
|
2019
|
2018
|
2017
|
||||||
|
Basic
|
37,484,573
|
|
37,074,797
|
|
36,616,859
|
|
||
|
Effect of dilutive securities
|
—
|
|
—
|
|
—
|
|
||
|
Diluted
|
37,484,573
|
|
37,074,797
|
|
36,616,859
|
|
||
|
Securities excluded as their inclusion would be anti-dilutive
|
2,200,318
|
|
1,077,256
|
|
1,058,790
|
|
||
|
(in thousands)
|
|||
|
2020
|
$
|
2,920
|
|
|
2021
|
2,338
|
|
|
|
2022
|
2,133
|
|
|
|
2023
|
2,131
|
|
|
|
2024 and thereafter
|
3,227
|
|
|
|
$
|
12,749
|
|
|
|
(in thousands)
|
Total
|
2020
|
2021
|
2022
|
2023
|
2024 and thereafter
|
|||||||||||||||||
|
Purchase obligations
(1)
|
$
|
407
|
|
$
|
407
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||||
|
Royalties
|
55,300
|
|
3,800
|
|
3,800
|
|
3,800
|
|
3,800
|
|
40,100
|
|
|||||||||||
|
$
|
55,707
|
|
$
|
4,207
|
|
$
|
3,800
|
|
$
|
3,800
|
|
$
|
3,800
|
|
$
|
40,100
|
|
||||||
|
|
Year ended May 31,
|
||||||||||
|
(in thousands)
|
2019
|
2018
|
2017
|
||||||||
|
Net sales by Product Category
|
|||||||||||
|
Vascular Interventions & Therapies
|
$
|
119,901
|
|
$
|
119,704
|
|
$
|
128,747
|
|
||
|
Vascular Access
|
94,730
|
|
92,760
|
|
96,481
|
|
|||||
|
Oncology/Surgery
|
56,003
|
|
49,191
|
|
44,560
|
|
|||||
|
Total
|
$
|
270,634
|
|
$
|
261,655
|
|
$
|
269,788
|
|
||
|
|
Year ended May 31,
|
||||||||||
|
(in thousands)
|
2019
|
2018
|
2017
|
||||||||
|
Net sales by Geography
|
|||||||||||
|
United States
|
$
|
216,957
|
|
$
|
213,727
|
|
$
|
223,816
|
|
||
|
International
|
53,677
|
|
47,928
|
|
45,972
|
|
|||||
|
Total
|
$
|
270,634
|
|
$
|
261,655
|
|
$
|
269,788
|
|
||
|
Year ended May 31,
|
|||||||||||
|
(in thousands)
|
2019
|
2018
|
2017
|
||||||||
|
Legal
(1)
|
$
|
7,802
|
|
$
|
8,407
|
|
$
|
19,480
|
|
||
|
Mergers and acquisitions
(2)
|
4,030
|
|
1,660
|
|
—
|
|
|||||
|
Intangible and other asset impairment
|
1,704
|
|
—
|
|
5,604
|
|
|||||
|
Restructuring
|
289
|
|
4,674
|
|
1,348
|
|
|||||
|
Other
|
1,302
|
|
691
|
|
1,078
|
|
|||||
|
Total
|
$
|
15,127
|
|
$
|
15,432
|
|
$
|
27,510
|
|
||
|
Contract
|
||||||||||||||||||||||||
|
Termination
|
Plant
|
Regulatory
|
Cancellation
|
Other
|
||||||||||||||||||||
|
Benefits
|
Consolidation
|
Filings
|
Costs
|
Costs
|
Total
|
|||||||||||||||||||
|
(in thousands)
|
||||||||||||||||||||||||
|
Balance at May 31, 2016
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||||
|
Charges
|
851
|
|
494
|
|
—
|
|
—
|
|
3
|
|
1,348
|
|
||||||||||||
|
Non-cash adjustments
|
—
|
|
(108
|
)
|
—
|
|
—
|
|
—
|
|
(108
|
)
|
||||||||||||
|
Cash payments
|
—
|
|
(275
|
)
|
—
|
|
—
|
|
(3
|
)
|
(278
|
)
|
||||||||||||
|
Balance at May 31, 2017
|
$
|
851
|
|
$
|
111
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
962
|
|
||||||
|
Charges
|
1,440
|
|
2,892
|
|
68
|
|
200
|
|
74
|
|
4,674
|
|
||||||||||||
|
Non-cash adjustments
|
—
|
|
(276
|
)
|
—
|
|
—
|
|
—
|
|
(276
|
)
|
||||||||||||
|
Cash payments
|
(1,453
|
)
|
(2,706
|
)
|
(56
|
)
|
—
|
|
(74
|
)
|
(4,289
|
)
|
||||||||||||
|
Balance at May 31, 2018
|
$
|
838
|
|
$
|
21
|
|
$
|
12
|
|
$
|
200
|
|
$
|
—
|
|
$
|
1,071
|
|
||||||
|
Charges
|
—
|
|
244
|
|
44
|
|
—
|
|
—
|
|
288
|
|
||||||||||||
|
Non-cash adjustments
|
—
|
|
—
|
|
—
|
|
(9
|
)
|
—
|
|
(9
|
)
|
||||||||||||
|
Cash payments
|
(838
|
)
|
(265
|
)
|
(44
|
)
|
(191
|
)
|
—
|
|
(1,338
|
)
|
||||||||||||
|
Balance at May 31, 2019
|
$
|
—
|
|
$
|
—
|
|
$
|
12
|
|
$
|
—
|
|
$
|
—
|
|
$
|
12
|
|
||||||
|
(in thousands)
|
Foreign currency translation gain (loss)
|
Unrealized gain (loss) on marketable securities
|
Total
|
|||||||||
|
Balance at May 31, 2017
|
$
|
(1,305
|
)
|
$
|
(19
|
)
|
$
|
(1,324
|
)
|
|||
|
Other comprehensive income before reclassifications, net of tax
|
270
|
|
102
|
|
372
|
|
||||||
|
Amounts reclassified from accumulated other comprehensive income
|
—
|
|
—
|
|
—
|
|
||||||
|
Net other comprehensive income
|
$
|
270
|
|
$
|
102
|
|
$
|
372
|
|
|||
|
Balance at May 31, 2018
|
$
|
(1,035
|
)
|
$
|
83
|
|
$
|
(952
|
)
|
|||
|
Other comprehensive income (loss) before reclassifications, net of tax
|
(317
|
)
|
33
|
|
(284
|
)
|
||||||
|
Amounts reclassified from accumulated other comprehensive income
|
—
|
|
(116
|
)
|
(116
|
)
|
||||||
|
Net other comprehensive loss
|
$
|
(317
|
)
|
$
|
(83
|
)
|
$
|
(400
|
)
|
|||
|
Balance at May 31, 2019
|
$
|
(1,352
|
)
|
$
|
—
|
|
$
|
(1,352
|
)
|
|||
|
|
2019
|
||||||||||||||
|
|
First
quarter
|
Second
quarter
|
Third
quarter
|
Fourth
quarter
|
|||||||||||
|
(in thousands, except per share data)
|
|||||||||||||||
|
Net sales
|
$
|
63,943
|
|
$
|
69,985
|
|
$
|
65,524
|
|
$
|
71,182
|
|
|||
|
Gross profit
|
35,953
|
|
40,552
|
|
38,163
|
|
41,332
|
|
|||||||
|
Net income (loss) from continuing operations
|
(5,704
|
)
|
(3,587
|
)
|
(4,609
|
)
|
2,753
|
|
|||||||
|
Net income from discontinued operations
|
5,235
|
|
5,727
|
|
5,405
|
|
56,120
|
|
|||||||
|
Net income (loss)
(1)
|
$
|
(469
|
)
|
$
|
2,140
|
|
$
|
796
|
|
$
|
58,873
|
|
|||
|
Loss per share from continuing operations
|
|||||||||||||||
|
Basic
|
$
|
(0.15
|
)
|
$
|
(0.10
|
)
|
$
|
(0.12
|
)
|
$
|
0.07
|
|
|||
|
Diluted
|
$
|
(0.15
|
)
|
$
|
(0.10
|
)
|
$
|
(0.12
|
)
|
$
|
0.07
|
|
|||
|
Earnings per share from discontinued operations
|
|||||||||||||||
|
Basic
|
$
|
0.14
|
|
$
|
0.15
|
|
$
|
0.14
|
|
$
|
1.49
|
|
|||
|
Diluted
|
$
|
0.14
|
|
$
|
0.15
|
|
$
|
0.14
|
|
$
|
1.47
|
|
|||
|
Earnings per share net income
|
|||||||||||||||
|
Basic
|
$
|
(0.01
|
)
|
$
|
0.06
|
|
$
|
0.02
|
|
$
|
1.57
|
|
|||
|
Diluted
|
$
|
(0.01
|
)
|
$
|
0.06
|
|
$
|
0.02
|
|
$
|
1.54
|
|
|||
|
|
2018
|
||||||||||||||
|
|
First
quarter
|
Second
quarter
|
Third
quarter
|
Fourth
quarter
|
|||||||||||
|
(in thousands, except per share data)
|
|||||||||||||||
|
Net sales
|
$
|
64,909
|
|
$
|
66,617
|
|
$
|
63,936
|
|
$
|
66,193
|
|
|||
|
Gross profit
|
33,042
|
|
34,699
|
|
37,496
|
|
38,619
|
|
|||||||
|
Net income (loss) from continuing operations
|
(5,008
|
)
|
(4,413
|
)
|
9,903
|
|
(6,709
|
)
|
|||||||
|
Net income from discontinued operations
|
4,973
|
|
4,662
|
|
4,116
|
|
8,811
|
|
|||||||
|
Net income (loss)
(2)
|
$
|
(35
|
)
|
$
|
249
|
|
$
|
14,019
|
|
$
|
2,102
|
|
|||
|
Loss per share from continuing operations
|
|||||||||||||||
|
Basic
|
$
|
(0.14
|
)
|
$
|
(0.12
|
)
|
$
|
0.27
|
|
$
|
(0.18
|
)
|
|||
|
Diluted
|
$
|
(0.14
|
)
|
$
|
(0.12
|
)
|
$
|
0.26
|
|
$
|
(0.18
|
)
|
|||
|
Earnings per share from discontinued operations
|
|||||||||||||||
|
Basic
|
$
|
0.13
|
|
$
|
0.13
|
|
$
|
0.11
|
|
$
|
0.24
|
|
|||
|
Diluted
|
$
|
0.13
|
|
$
|
0.13
|
|
$
|
0.11
|
|
$
|
0.24
|
|
|||
|
Earnings per share net income
|
|||||||||||||||
|
Basic
|
$
|
0.00
|
|
$
|
0.01
|
|
$
|
0.38
|
|
$
|
0.06
|
|
|||
|
Diluted
|
$
|
0.00
|
|
$
|
0.01
|
|
$
|
0.37
|
|
$
|
0.06
|
|
|||
|
SCHEDULE II -VALUATION AND QUALIFYING ACCOUNTS
|
|||||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Column A
|
Column B
|
Column C
|
Column D
|
Column E
|
|||||||||||
|
Description
|
Balance at
Beginning
of Year
|
Additions -
Charged to
costs and
expenses
|
Deductions
|
Balance at
End of Period
|
|||||||||||
|
Year Ended May 31, 2017
|
|||||||||||||||
|
Allowance for deferred tax asset
|
$
|
42,209
|
|
$
|
6,139
|
|
$
|
—
|
|
$
|
48,348
|
|
|||
|
Allowance for sales returns and doubtful accounts
|
$
|
4,372
|
|
$
|
(291
|
)
|
$
|
(1,136
|
)
|
$
|
2,945
|
|
|||
|
Year Ended May 31, 2018
|
|||||||||||||||
|
Allowance for deferred tax asset
|
$
|
48,348
|
|
$
|
—
|
|
$
|
(21,741
|
)
|
$
|
26,607
|
|
|||
|
Allowance for sales returns and doubtful accounts
|
$
|
2,945
|
|
$
|
608
|
|
$
|
(1,087
|
)
|
$
|
2,466
|
|
|||
|
Year Ended May 31, 2019
|
|||||||||||||||
|
Allowance for deferred tax asset
|
$
|
26,607
|
|
$
|
—
|
|
$
|
(14,919
|
)
|
$
|
11,688
|
|
|||
|
Allowance for sales returns and doubtful accounts
|
$
|
2,466
|
|
$
|
393
|
|
$
|
(953
|
)
|
$
|
1,906
|
|
|||
|
Incorporated by Reference
|
||||
|
Exhibit Number
|
Description of Exhibits
|
Form
|
Exhibit
|
Filing Date
|
|
2.2
|
8-K
|
2.1
|
October 12, 2012
|
|
|
2.3
|
8-K
|
2.1
|
April 18, 2019
|
|
|
3.1.1
|
10-Q
|
3.1
|
October 7, 2005
|
|
|
3.1.2
|
10-K
|
3.1.2
|
August 10, 2015
|
|
|
3.2
|
8-K
|
10.1
|
October 21, 2015
|
|
|
10.1
|
|
8-K
|
10.1
|
November 10, 2016
|
|
10.1.1
|
10-K
|
10.1.1
|
July 23, 2018
|
|
|
10.1.2
|
8-K
|
10.1
|
June 6, 2019
|
|
|
10.1.3
|
DEF 14A
|
August 30, 2018
|
||
|
10.1.5
|
|
10-Q
|
10.1
|
October 5, 2016
|
|
10.1.6
|
|
10-Q
|
10.1
|
September 29, 2017
|
|
10.1.7
|
|
10-K
|
10.1.7
|
July 23, 2018
|
|
10.2
|
DEF 14A
|
August 30, 2018
|
||
|
10.3
|
10-Q
|
10.1
|
October 12, 2004
|
|
|
10.3.1
|
10-K
|
10.3.1
|
July 23, 2018
|
|
|
10.4.3
|
10-Q
|
10.2
|
October 5, 2016
|
|
|
10.4.4
|
10-Q
|
10.2
|
September 29, 2017
|
|
|
10.4.5
|
10-K
|
10.4.5
|
July 23, 2018
|
|
|
10.5
|
8-K
|
10.3
|
May 12, 2005
|
|
|
10.6
|
S-1
|
10.2
|
May 3, 2000
|
|
|
Incorporated by Reference
|
||||
|
Exhibit Number
|
Description of Exhibits
|
Form
|
Exhibit
|
Filing Date
|
|
10.7
|
S-1
|
10.11
|
February 13, 1998
|
|
|
10.8
|
S-1/A
|
10.3
|
June 14, 2000
|
|
|
10.9
|
S-8
|
99.2
|
July 8, 2005
|
|
|
10.10
|
S-8
|
99.1
|
July 8, 2005
|
|
|
10.11
|
8-K
|
10.1
|
May 12, 2006
|
|
|
10.11.1
|
8-K
|
10.1
|
April 6, 2016
|
|
|
10.11.2
|
8-K
|
10.1
|
July 25, 2016
|
|
|
10.12
|
8-K
|
10.2
|
April 6, 2016
|
|
|
10.12.1
|
8-K
|
10.1
|
October 31, 2007
|
|
|
10.13
|
10-K/A
|
10.13
|
January 12, 2015
|
|
|
10.13.1
|
10-Q
|
10.3
|
October 5, 2016
|
|
|
10.14
|
8-K
|
10.3
|
April 6, 2016
|
|
|
10.15
|
8-K
|
10.4
|
April 6, 2016
|
|
|
10.16
|
8-K
|
10.5
|
April 6, 2016
|
|
|
10.17
|
8-K
|
10.6
|
April 6, 2016
|
|
|
10.18
|
8-K
|
10.1
|
April 27, 2016
|
|
|
14
|
8-K
|
14
|
May 21, 2006
|
|
|
21
|
||||
|
23
|
||||
|
31.1
|
||||
|
31.2
|
||||
|
32.1
|
||||
|
32.2
|
||||
|
101.INS
|
XBRL Instance Document
|
|||
|
101.SCH
|
XBRL Schema Document
|
|||
|
101.CAL
|
XBRL Calculation Linkbase Documents
|
|||
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|||
|
101.LAB
|
XBRL Labels Linkbase Documents
|
|||
|
101.PRE
|
XBRL Presentation Linkbase Documents
|
|||
|
|
ANGIODYNAMICS, INC.
|
||||
|
Date:
|
|
July 25, 2019
|
By:
|
|
/
S
/ H
OWARD
W. D
ONNELLY
|
|
|
|
Howard W. Donnelly,
Chairman of the Board, Director
|
|||
|
Date:
|
|
July 25, 2019
|
/
S
/ H
OWARD
W. D
ONNELLY
|
|
|
|
|
Howard W. Donnelly,
|
|
|
|
|
Chairman of the Board, Director
|
|
Date:
|
|
July 25, 2019
|
/
S
/ JAMES C. CLEMMER
|
|
|
|
|
James C. Clemmer,
|
|
|
|
|
President, Chief Executive Officer
(Principal Executive Officer)
|
|
Date:
|
|
July 25, 2019
|
/
S
/ MICHAEL C. GREINER
|
|
|
|
|
Michael C. Greiner
|
|
|
|
|
Executive Vice President, Chief Financial Officer, (Principal Financial and Accounting Officer)
|
|
Date:
|
|
July 25, 2019
|
/
S
/ W
ESLEY
E. J
OHNSON
, J
R
.
|
|
|
|
|
Wesley E. Johnson, Jr.,
|
|
|
|
|
Director
|
|
Date:
|
|
July 25, 2019
|
/
S
/ J
EFFREY
G. G
OLD
|
|
|
|
|
Jeffrey G. Gold,
|
|
|
|
|
Director
|
|
Date:
|
|
July 25, 2019
|
/
S
/ D
ENNIS
S. M
ETENY
|
|
|
|
|
Dennis S. Meteny,
|
|
|
|
|
Director
|
|
Date:
|
|
July 25, 2019
|
/
S
/ K
EVIN
J. G
OULD
|
|
|
|
|
Kevin J. Gould,
|
|
|
|
|
Director
|
|
Date:
|
|
July 25, 2019
|
/
S
/ JAN S. REED
|
|
|
|
|
Jan S. Reed,
|
|
|
|
|
Director
|
|
Date:
|
|
July 25, 2019
|
/
S
/ EILEEN O. AUEN
|
|
|
|
|
Eileen O. Auen,
|
|
|
|
|
Director
|
|
Date:
|
|
July 25, 2019
|
|
|
|
|
|
Karen A. Licitra,
|
|
|
|
|
Director
|