|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ý
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
|
20-2463898
|
|
(State or Other Jurisdiction of
Incorporation or Organization)
|
|
(I.R.S. Employer
Identification No.)
|
|
5818 El Camino Real, Carlsbad,
California
|
|
92008
|
|
(Address of Principal Executive Offices)
|
|
(Zip Code)
|
|
Title of Each Class
|
|
Name of Each Exchange on Which Registered
|
|
Common Stock, par value $0.0001 per share
|
|
The NASDAQ Global Select Market
|
|
Large accelerated filer
¨
|
Accelerated filer
x
|
|
|
|
Non-accelerated filer
¨
|
(Do not check if a smaller reporting company)
|
Smaller reporting company
|
¨
|
|
|
|
Page
|
|
Business
|
||
|
Risk Factors
|
||
|
Unresolved Staff Comments
|
||
|
Properties
|
||
|
Legal Proceedings
|
||
|
Mine Safety Disclosures
|
||
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
||
|
Selected Financial Data
|
||
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
||
|
Quantitative and Qualitative Disclosures About Market Risk
|
||
|
Financial Statements and Supplementary Data
|
||
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
|
||
|
Controls and Procedures
|
||
|
Other Information
|
||
|
Directors, Executive Officers and Corporate Governance
|
||
|
Executive Compensation
|
||
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
||
|
Certain Relationships and Related Transactions, and Director Independence
|
||
|
Principal Accounting Fees and Services
|
||
|
Exhibits, Financial Statement Schedules
|
||
|
Item 1.
|
Business
|
|
•
|
Strategic Pillar #1: Deliver Advancements in our “Go-to-Market” Product Portfolio and our R&D Pipeline Strategy to Compete More Effectively.
|
|
•
|
Strategic Pillar #2: Transform our Manufacturing Operations and Physical Distribution
|
|
•
|
Strategic Pillar #3: Transform our Commercial Execution and Global Participation.
|
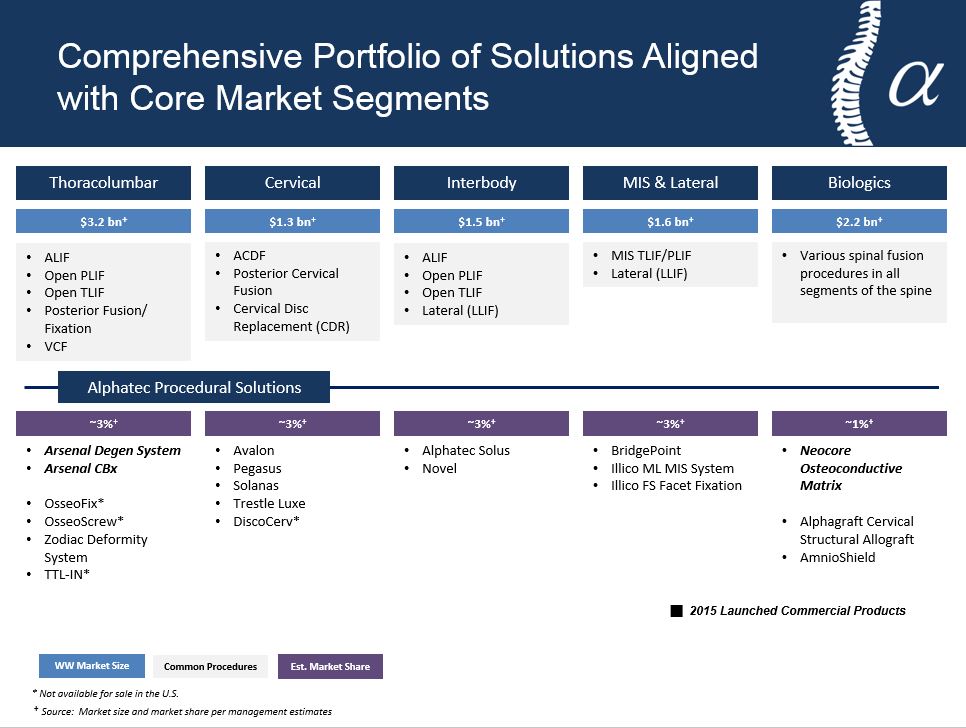
|
•
|
improved outcomes for spine pathology procedures;
|
|
•
|
ease of use, quality and reliability;
|
|
•
|
effective and efficient sales, marketing and distribution;
|
|
•
|
quality service and an educated and knowledgeable sales network;
|
|
•
|
technical leadership and superiority;
|
|
•
|
surgeon services, such as training and education;
|
|
•
|
responsiveness to the needs of surgeons;
|
|
•
|
acceptance by spine surgeons;
|
|
•
|
product price and qualification for reimbursement; and
|
|
•
|
speed to market.
|
|
•
|
product design and development;
|
|
•
|
product testing;
|
|
•
|
product manufacturing;
|
|
•
|
product labeling;
|
|
•
|
product storage;
|
|
•
|
premarket clearance or approval;
|
|
•
|
advertising and promotion;
|
|
•
|
product marketing, sales and distribution; and
|
|
•
|
post-market surveillance, including reporting deaths or serious injuries related to products and certain product malfunctions.
|
|
•
|
quality system regulations, which require manufacturers, including third-party contract manufacturers, to follow stringent design, testing, control, documentation, record maintenance and other quality assurance controls, during all aspects of the manufacturing process and to maintain and investigate complaints;
|
|
•
|
labeling regulations, and FDA prohibitions against the promotion of products for uncleared or unapproved “off-label” uses;
|
|
•
|
medical device reporting obligations, which require that manufacturers submit reports to the FDA of adverse events; and
|
|
•
|
other post-market surveillance requirements, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device.
|
|
•
|
warning letters;
|
|
•
|
fines, injunctions, and civil penalties;
|
|
•
|
recall or seizure of products;
|
|
•
|
operating restrictions, partial suspension or total shutdown of production;
|
|
•
|
refusal to grant 510(k) clearance or PMA approvals of new products; and
|
|
•
|
criminal prosecution.
|
|
•
|
more established relationships with spine surgeons;
|
|
•
|
more established distribution networks;
|
|
•
|
broader spine surgery product offerings;
|
|
•
|
stronger intellectual property portfolios;
|
|
•
|
greater financial and other resources for product research and development, sales and marketing, and patent litigation;
|
|
•
|
greater experience in, and resources for, launching, marketing, distributing and selling products;
|
|
•
|
significantly greater name recognition as well as more recognizable trademarks for products similar to the products that we sell;
|
|
•
|
more established relationships with healthcare providers and payors;
|
|
•
|
products supported by more extensive clinical data; and
|
|
•
|
greater experience in obtaining and maintaining FDA and other regulatory clearances or approvals for products and product enhancements.
|
|
•
|
changes in foreign medical reimbursement policies and programs;
|
|
•
|
changes in foreign regulatory requirements;
|
|
•
|
differing local product preferences and product requirements;
|
|
•
|
diminished protection of intellectual property in some countries outside of the U.S.;
|
|
•
|
differing payment cycles;
|
|
•
|
trade protection measures and import or export licensing requirements;
|
|
•
|
difficulty in staffing, training and managing foreign operations;
|
|
•
|
differing legal requirements and labor relations;
|
|
•
|
potentially negative consequences from changes in tax laws (including potentially taxes payable on earnings of foreign subsidiaries upon repatriation); and
|
|
•
|
political and economic instability.
|
|
•
|
the federal Anti-Kickback Statute, as well as state analogs, which constrains our marketing practices and those of our independent sales agents and distributors, educational programs, pricing policies, and relationships with healthcare providers by prohibiting, among other things, knowingly and willfully soliciting, receiving, offering or providing remuneration, intended to induce the purchase or recommendation of an item or service reimbursable under a federal (or state or commercial) healthcare program (such as the Medicare or Medicaid programs);
|
|
•
|
the federal ban, as well as state analogs, on physician self-referrals, which prohibits, subject to certain exceptions, physician referrals of Medicare and Medicaid patients to an entity providing certain “designated health services” if the physician or an immediate family member of the physician has any financial relationship with the entity;
|
|
•
|
federal false claims laws which prohibit, among other things, knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent;
|
|
•
|
HIPAA, and its implementing regulations, which created federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
|
|
•
|
the state and federal laws “sunshine” provisions that require detailed reporting and disclosures to CMS and made available on CMS's website starting in the fall of 2014, and applicable states of any payments or “transfer of value” made or distributed to prescribers and other health care providers, and for certain states prohibit some forms of these payments, require the adoption of marketing codes of conduct, and constrain their relationships with physicians and other referral sources;
|
|
•
|
state laws analogous to each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers, and state laws governing the privacy of certain health information, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts;
|
|
•
|
the Administrative Simplification provisions of HIPAA, specifically, privacy and security provisions including recent amendments under HITECH which impose stringent restrictions on uses and disclosures of protected health information such as for marketing or clinical research purposes and impose significant civil and criminal penalties for non-compliance and require the reporting of breaches to affected individuals, the government and in some cases the media in the event of a violation; and
|
|
•
|
a variety of state-imposed privacy and data security laws which require the protection of information beyond health information, such as employee information or any class of information combining name with state issued identification numbers, social security numbers, credit card, bank or other financial information and which require reporting to state officials in the event of breach or violation and which impose both civil and criminal penalties.
|
|
•
|
delay or prevent commercialization of products we develop;
|
|
•
|
require us to perform costly tests or studies;
|
|
•
|
diminish any competitive advantages that we might otherwise have obtained; and
|
|
•
|
reduce our ability to collect revenues.
|
|
•
|
properly identify and anticipate surgeon and patient needs;
|
|
•
|
develop new products or enhancements in a timely manner;
|
|
•
|
obtain the necessary regulatory approvals for new products or product enhancements;
|
|
•
|
provide adequate training to potential users of new products;
|
|
•
|
receive adequate reimbursement approval of third-party payors such as Medicaid, Medicare and private insurers; and
|
|
•
|
develop an effective marketing and distribution network.
|
|
•
|
the payments due in connection with the settlement of the Orthotec matter;
|
|
•
|
the revenues generated by sales of our products;
|
|
•
|
the costs associated with expanding our sales and marketing efforts;
|
|
•
|
the expenses that we incur from the manufacture of our products by third parties and that we incur from selling our products;
|
|
•
|
the costs of developing new products or technologies;
|
|
•
|
the cost of obtaining and maintaining FDA or other regulatory approval or clearance for our products and products in development;
|
|
•
|
the number and timing of acquisitions and other strategic transactions;
|
|
•
|
the costs and any payments we may make related to our pending litigation matters (in addition to the Orthotec matter);
|
|
•
|
the costs associated with increased capital expenditures; and
|
|
•
|
the costs associated with our employee retention programs and related benefits.
|
|
•
|
acceptance of our products by surgeons, patients, hospitals and third-party payors;
|
|
•
|
demand and pricing of our products;
|
|
•
|
the mix of our products sold, because profit margins differ among our products;
|
|
•
|
timing of new product offerings, acquisitions, licenses or other significant events by us or our competitors;
|
|
•
|
our ability to grow and maintain a productive sales and marketing organization;
|
|
•
|
regulatory approvals and legislative changes affecting the products we may offer or those of our competitors;
|
|
•
|
the effect of competing technological and market developments;
|
|
•
|
levels of third-party reimbursement for our products;
|
|
•
|
interruption in the manufacturing or distribution of our products;
|
|
•
|
our ability to produce or obtain products of satisfactory quality or in sufficient quantities to meet demand; and
|
|
•
|
changes in our ability to obtain FDA, state and international approval or clearance for our products.
|
|
•
|
volume and timing of orders for our products;
|
|
•
|
quarterly variations in our or our competitors’ results of operations;
|
|
•
|
our announcement or our competitors’ announcements regarding new products, product enhancements, significant contracts, number of distributors, number of hospitals and surgeons using products, acquisitions, and collaborative or strategic investments;
|
|
•
|
announcements of technological or medical innovations for the treatment of spine pathology;
|
|
•
|
changes in earnings estimates or recommendations by securities analysts;
|
|
•
|
our ability to develop, obtain regulatory clearance or approval for, and market new and enhanced products on a timely basis;
|
|
•
|
changes in healthcare policy in the U.S. and internationally;
|
|
•
|
product liability claims or other litigation involving us;
|
|
•
|
sales of large blocks of our common stock, including sales by our executive officers, directors and significant stockholders;
|
|
•
|
changes in governmental regulations or in the status of our regulatory approvals, clearances or applications;
|
|
•
|
disputes or other developments with respect to intellectual property rights;
|
|
•
|
changes in the availability of third-party reimbursement in the U.S. or other countries;
|
|
•
|
changes in accounting principles; and
|
|
•
|
general market conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors.
|
|
•
|
delaying, deferring or preventing our change in control;
|
|
•
|
impeding a merger, consolidation, takeover or other business combination involving us;
|
|
•
|
causing us to enter into transactions or agreements that are not in the best interests of all of our stockholders; or
|
|
•
|
reducing our public float held by non-affiliates.
|
|
•
|
allow the authorized number of directors to be changed only by resolution of our Board of Directors;
|
|
•
|
allow vacancies on our Board of Directors to be filled only by resolution of our Board of Directors;
|
|
•
|
authorize our Board of Directors to issue, without stockholder approval, blank check preferred stock that, if issued, could operate as a “poison pill” to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that is not approved by our Board of Directors;
|
|
•
|
require that stockholder actions must be effected at a duly called stockholder meeting and prohibit stockholder action by written consent;
|
|
•
|
establish advance notice requirements for stockholder nominations to our Board of Directors and for stockholder proposals that can be acted on at stockholder meetings; and
|
|
•
|
limit who may call stockholder meetings.
|
|
•
|
our estimates regarding anticipated operating losses, future revenue, expenses, capital requirements, uses and sources of cash and liquidity, including our anticipated revenue growth and cost savings;
|
|
•
|
our ability to meet the financial covenants under our credit facilities, to obtain waivers from our lenders with respect to any noncompliance with our financial covenants, and to refinance our existing debt prior to the maturity of our credit facilities with our current or new lenders;
|
|
•
|
our ability to regain and maintain compliance with the continued listing requirements of The NASDAQ Global Select Market
|
|
•
|
our ability to ensure that we have effective disclosure controls and procedures and to remedy our material weakness in our internal control over financial reporting;
|
|
•
|
our ability to meet and potential liability from not meeting the payment obligations under the Orthotec settlement agreement;
|
|
•
|
our ability to regain and maintain compliance with the quality requirements of the FDA and similar regulatory authorities outside of the U.S., including our ability to resolve the deficiencies cited in the Warning Letter that we received from the FDA in July 2015 following the FDA's inspection of our manufacturing facilities;
|
|
•
|
our ability to market, improve, grow, commercialize and achieve market acceptance of any of our products or any product candidates that we are developing or may develop in the future;
|
|
•
|
our beliefs about the features, strengths and benefits of our products;
|
|
•
|
our ability to continue to enhance our product offerings, outsource our manufacturing operations and expand the commercialization of our products, and the effect of our strategy;
|
|
•
|
our expectations about the timing, costs and benefits of the restructuring and outsourcing of our manufacturing operations;
|
|
•
|
our beliefs about the ability of our supplier relationships and quality processes to fulfill our production requirements;
|
|
•
|
our ability to successfully integrate, and realize benefits from licenses and acquisitions;
|
|
•
|
our ability to successfully achieve and maintain regulatory clearance or approval for our products in applicable jurisdictions and in a timely manner;
|
|
•
|
the effect of any existing or future federal, state or international regulations on our ability to effectively conduct our business;
|
|
•
|
our estimates of market sizes and anticipated uses of our products;
|
|
•
|
our business strategy and our underlying assumptions about market data, demographic trends, reimbursement trends and pricing trends;
|
|
•
|
our ability to achieve profitability, and the potential need to raise additional funding;
|
|
•
|
our ability to maintain an adequate sales network for our products, including to attract and retain independent distributors;
|
|
•
|
our ability to enhance our U.S. and international sales and distributions networks and product penetration;
|
|
•
|
our ability to increase the use and promotion of our products by training and educating surgeons and our sales network;
|
|
•
|
our ability to attract and retain a qualified management team, as well as other qualified personnel and advisors;
|
|
•
|
our ability to enter into licensing and business combination agreements with third parties and to successfully integrate the acquired technology and/or businesses;
|
|
•
|
our management team’s ability to accommodate growth and manage a larger organization;
|
|
•
|
our ability to protect our intellectual property, and to not infringe upon the intellectual property of third parties;
|
|
•
|
the effects of the escalating cost of medical products and services and the effects of market demand, government regulation, third-party reimbursement policies and societal pressures on the worldwide healthcare industry and our business;
|
|
•
|
our ability to meet or exceed the industry standard in clinical and legal compliance and corporate governance programs;
|
|
•
|
our beliefs about our competitors and the principal competitive factors in our market and the effect of non-operative treatments on demand for our products;
|
|
•
|
potential liability resulting from litigation;
|
|
•
|
our beliefs about our employee relations;
|
|
•
|
potential liability resulting from a governmental review of our business practices;
|
|
•
|
our beliefs about the usefulness of the non-GAAP financial measures included in this Annual Report on Form 10-K;
|
|
•
|
our beliefs with respect to our critical accounting policies and the reasonableness of our estimates and assumptions; and
|
|
•
|
other factors discussed elsewhere in this Annual Report on Form 10-K or any document incorporated by reference herein or therein.
|
|
Item 1B.
|
Unresolved Staff Comments
|
|
Item 2.
|
Properties
|
|
Location
|
Use
|
Approximate Square
Footage
|
Lease Expiration
|
||||
|
Carlsbad, California
|
Corporate headquarters and product design
|
76,693
|
|
July 2021
|
|||
|
Carlsbad, California
|
Product design and distribution
|
73,480
|
|
January 2017
|
|||
|
Item 3.
|
Legal Proceedings
|
|
Item 4.
|
Mine Safety Disclosures
|
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
|
Year Ended December 31, 2015
|
High
|
Low
|
||||||
|
First quarter
|
$
|
1.54
|
|
$
|
1.28
|
|
||
|
Second quarter
|
1.48
|
|
1.28
|
|
||||
|
Third quarter
|
1.43
|
|
0.32
|
|
||||
|
Fourth quarter
|
0.45
|
|
0.18
|
|
||||
|
Year Ended December 31, 2014
|
High
|
Low
|
||||||
|
First quarter
|
$
|
2.53
|
|
$
|
1.16
|
|
||
|
Second quarter
|
1.70
|
|
1.20
|
|
||||
|
Third quarter
|
1.92
|
|
1.32
|
|
||||
|
Fourth quarter
|
1.70
|
|
1.23
|
|
||||
|
Item 6.
|
Selected Financial Data
|
|
|
Year Ended December 31,
|
|||||||||||||||||||
|
|
2015
|
2014
|
2013
|
2012
|
2011
|
|||||||||||||||
|
|
(in thousands, except per share amounts)
|
|||||||||||||||||||
|
|
||||||||||||||||||||
|
Revenues
|
$
|
185,279
|
|
$
|
206,980
|
|
$
|
204,724
|
|
$
|
196,278
|
|
$
|
197,711
|
|
|||||
|
Operating (loss) income
|
(172,439
|
)
|
1,844
|
|
(73,433
|
)
|
(9,837
|
)
|
(24,516
|
)
|
||||||||||
|
Net loss
|
$
|
(178,676
|
)
|
$
|
(12,882
|
)
|
$
|
(82,227
|
)
|
$
|
(15,459
|
)
|
$
|
(22,181
|
)
|
|||||
|
Net loss per basic share
|
$
|
(1.79
|
)
|
$
|
(0.13
|
)
|
$
|
(0.85
|
)
|
$
|
(0.17
|
)
|
$
|
(0.25
|
)
|
|||||
|
Net loss per diluted share
|
$
|
(1.79
|
)
|
$
|
(0.16
|
)
|
$
|
(0.85
|
)
|
$
|
(0.17
|
)
|
$
|
(0.25
|
)
|
|||||
|
Weighted-average shares used in computing net loss per share:
|
||||||||||||||||||||
|
Shares used in calculating basic net loss per share
|
99,574
|
|
97,347
|
|
96,235
|
|
90,218
|
|
88,798
|
|
||||||||||
|
Shares used in calculating diluted net loss per share
|
99,574
|
|
97,735
|
|
96,235
|
|
90,218
|
|
88,798
|
|
||||||||||
|
|
As of December 31,
|
|||||||||||||||||||
|
|
2015
|
2014
|
2013
|
2012
|
2011
|
|||||||||||||||
|
|
(in thousands)
|
|||||||||||||||||||
|
|
||||||||||||||||||||
|
Cash
|
$
|
11,229
|
|
$
|
19,735
|
|
$
|
21,345
|
|
$
|
22,241
|
|
$
|
20,666
|
|
|||||
|
Working (deficit) capital
|
(23,542
|
)
|
49,511
|
|
34,026
|
|
65,264
|
|
59,292
|
|
||||||||||
|
Total assets
|
146,704
|
|
344,923
|
|
365,630
|
|
382,127
|
|
366,692
|
|
||||||||||
|
Total debt, including current portion
|
80,585
|
|
82,673
|
|
54,902
|
|
41,667
|
|
28,198
|
|
||||||||||
|
Redeemable preferred stock
|
23,603
|
|
23,603
|
|
23,603
|
|
23,603
|
|
23,603
|
|
||||||||||
|
Total stockholders’ (deficit) equity
|
(36,576
|
)
|
148,954
|
|
171,676
|
|
245,816
|
|
245,328
|
|
||||||||||
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
|
(in thousands)
|
|||||||||||
|
Revenues
|
$
|
185,279
|
|
$
|
206,980
|
|
$
|
204,724
|
|
|||
|
Cost of revenues
|
63,742
|
|
61,834
|
|
78,669
|
|
||||||
|
Amortization of acquired intangible assets
|
1,453
|
|
1,736
|
|
1,733
|
|
||||||
|
Gross profit
|
120,084
|
|
143,410
|
|
124,322
|
|
||||||
|
Operating expenses:
|
||||||||||||
|
Research and development
|
17,767
|
|
16,799
|
|
14,190
|
|
||||||
|
In-process research and development
|
274
|
|
527
|
|
—
|
|
||||||
|
Sales and marketing
|
70,856
|
|
77,179
|
|
76,960
|
|
||||||
|
General and administrative
|
34,867
|
|
43,381
|
|
47,949
|
|
||||||
|
Amortization of acquired intangible assets
|
2,400
|
|
2,974
|
|
3,009
|
|
||||||
|
Goodwill and intangible assets impairment
|
165,171
|
|
—
|
|
—
|
|
||||||
|
Restructuring expenses
|
1,188
|
|
706
|
|
9,665
|
|
||||||
|
Litigation settlement expenses
|
—
|
|
—
|
|
45,982
|
|
||||||
|
Total operating expenses
|
292,523
|
|
141,566
|
|
197,755
|
|
||||||
|
Operating (loss) income
|
(172,439
|
)
|
1,844
|
|
(73,433
|
)
|
||||||
|
Other income (expense):
|
||||||||||||
|
Interest income
|
53
|
|
10
|
|
6
|
|
||||||
|
Interest expense
|
(12,589
|
)
|
(13,616
|
)
|
(3,959
|
)
|
||||||
|
Other income (expense), net
|
6,980
|
|
(33
|
)
|
(1,662
|
)
|
||||||
|
Total other income (expense)
|
(5,556
|
)
|
(13,639
|
)
|
(5,615
|
)
|
||||||
|
Pretax net loss
|
(177,995
|
)
|
(11,795
|
)
|
(79,048
|
)
|
||||||
|
Income tax provision
|
681
|
|
1,087
|
|
3,179
|
|
||||||
|
Net loss
|
$
|
(178,676
|
)
|
$
|
(12,882
|
)
|
$
|
(82,227
|
)
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Net loss
|
$
|
(178,676
|
)
|
$
|
(12,882
|
)
|
$
|
(82,227
|
)
|
|||
|
Stock-based compensation
|
2,643
|
|
4,554
|
|
4,078
|
|
||||||
|
Depreciation
|
12,974
|
|
12,160
|
|
14,638
|
|
||||||
|
Amortization of intangible assets
|
2,204
|
|
1,515
|
|
6,898
|
|
||||||
|
Amortization of acquired intangible assets
|
3,853
|
|
4,710
|
|
4,741
|
|
||||||
|
Goodwill and intangible assets impairment
|
165,171
|
|
—
|
|
—
|
|
||||||
|
In-process research and development
|
274
|
|
527
|
|
—
|
|
||||||
|
Stock price guarantee
|
4,877
|
|
—
|
|
—
|
|
||||||
|
Interest expense, net
|
12,536
|
|
13,606
|
|
3,953
|
|
||||||
|
Income tax provision
|
681
|
|
1,087
|
|
3,179
|
|
||||||
|
Other (income) expense, net
|
(6,980
|
)
|
33
|
|
1,662
|
|
||||||
|
Restructuring and other expenses
|
1,188
|
|
742
|
|
18,603
|
|
||||||
|
Litigation expenses and trial costs
|
—
|
|
4,779
|
|
49,657
|
|
||||||
|
Adjusted EBITDA
|
$
|
20,745
|
|
$
|
30,831
|
|
$
|
25,182
|
|
|||
|
|
Payment Due by Year
|
|||||||||||||||||||||||||||
|
|
Total
|
2016
|
2017
|
2018
|
2019
|
2020
|
Thereafter
|
|||||||||||||||||||||
|
Amended Credit Facility with MidCap
(1)
|
$
|
56,799
|
|
$
|
56,799
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||||||
|
Facility Agreement with Deerfield
(1)
|
26,000
|
|
—
|
|
8,667
|
|
8,667
|
|
8,666
|
|
—
|
|
—
|
|
||||||||||||||
|
Interest expense
(1)
|
8,468
|
|
5,087
|
|
1,706
|
|
948
|
|
727
|
|
—
|
|
—
|
|
||||||||||||||
|
Note payable for software licenses
|
189
|
|
189
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Note payable for insurance premiums
|
1,599
|
|
1,599
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Capital lease obligations
|
1,382
|
|
877
|
|
437
|
|
68
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Operating lease obligations
(2)
|
3,570
|
|
2,268
|
|
823
|
|
304
|
|
170
|
|
5
|
|
—
|
|
||||||||||||||
|
Litigation settlement obligations
|
34,833
|
|
4,400
|
|
4,400
|
|
4,400
|
|
4,400
|
|
4,400
|
|
12,833
|
|
||||||||||||||
|
Guaranteed minimum royalty obligations
|
5,840
|
|
2,036
|
|
1,450
|
|
1,368
|
|
618
|
|
368
|
|
—
|
|
||||||||||||||
|
Stock price guarantee
(3)
|
4,877
|
|
—
|
|
2,185
|
|
2,195
|
|
497
|
|
—
|
|
—
|
|
||||||||||||||
|
New product development milestones
(4)
|
575
|
|
175
|
|
200
|
|
—
|
|
200
|
|
—
|
|
—
|
|
||||||||||||||
|
Total
|
$
|
144,132
|
|
$
|
73,430
|
|
$
|
19,868
|
|
$
|
17,950
|
|
$
|
15,278
|
|
$
|
4,773
|
|
$
|
12,833
|
|
|||||||
|
(1)
|
The amounts above are presented based on the contractual payment schedule in each of the respective agreements. However, the debt balance under the Amended Credit Facility and Facility Agreement was callable as of December 31, 2015 due to the events of default (See Note 1 of the notes to consolidated financial statements) and therefore, is presented as a current liability in the consolidated balance sheet as of December 31, 2015.
|
|
(2)
|
The amounts above do not reflect the commitments under the new Lease agreement that we entered into in January 2016 as disclosed in the "Real Property Leases" section below.
|
|
(3)
|
Based on our closing stock price as of December 31, 2015 of $0.30 per share. Actual cash obligation will vary depending on the price of our common stock on the settlement dates.
|
|
(4)
|
This commitment represents payments in cash, and is subject to attaining certain development milestones such as FDA approval, product design and functionality testing requirements, which we believe are reasonably likely to be achieved in
2016
through 2019.
|
|
•
|
a determination that the carrying value of such assets cannot be recovered through undiscounted cash flows;
|
|
•
|
loss of legal ownership or title to the assets;
|
|
•
|
significant changes in our strategic business objectives and utilization of the assets; or
|
|
•
|
the impact of significant negative industry or economic trends.
|
|
•
|
Estimated volatility is a measure of the amount by which our stock price is expected to fluctuate each year during the expected life of the award. Our estimated volatility through December 31,
2015
was based on our actual historical volatility. An increase in the estimated volatility would result in an increase to our stock-based compensation expense.
|
|
•
|
The expected term represents the period of time that awards granted are expected to be outstanding. Our estimated expected term through December 31,
2015
was calculated using a weighted-average term based on historical exercise patterns and the term from option grant date to exercise for the options granted within the specified date range. An increase in the expected term would result in an increase to our stock-based compensation expense.
|
|
•
|
The risk-free interest rate is based on the yield curve of a zero-coupon U.S. Treasury bond on the date the stock option award is granted with a maturity equal to the expected term of the stock option award. An increase in the risk-free interest rate would result in an increase to our stock-based compensation expense.
|
|
•
|
The assumed dividend yield is based on our expectation of not paying dividends in the foreseeable future.
|
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Cost of revenues
|
$
|
72
|
|
$
|
274
|
|
$
|
228
|
|
|||
|
Research and development
|
286
|
|
2,080
|
|
719
|
|
||||||
|
Sales and marketing
|
359
|
|
470
|
|
459
|
|
||||||
|
General and administrative
|
1,926
|
|
1,730
|
|
2,672
|
|
||||||
|
Total
|
$
|
2,643
|
|
$
|
4,554
|
|
$
|
4,078
|
|
|||
|
Effect on basic and diluted net loss per share
|
$
|
(0.03
|
)
|
$
|
(0.05
|
)
|
$
|
(0.04
|
)
|
|||
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
|
|
Item 8.
|
Financial Statements and Supplementary Data
|
|
Item 9.
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
|
|
Item 9A.
|
Controls and Procedures
|
|
/s/ Ernst & Young LLP
|
|
Item 9B.
|
Other Information
|
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
|
Item 11.
|
Executive Compensation
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
|
Item 14.
|
Principal Accounting Fees and Services
|
|
Item 15.
|
Exhibits, Financial Statement Schedules
|
|
|
Page
|
|
Exhibit
Number
|
|
Exhibit Description
|
|
Filed
with this
Report
|
|
Incorporated by
Reference herein
from Form or
Schedule
|
|
Filing
Date
|
|
SEC File/
Reg.
Number
|
|
3.1
|
|
Restated Certificate of Incorporation
|
|
|
Amendment No. 2 to
Form S-1
(Exhibit 3.2)
|
|
04/20/06
|
|
333-131609
|
|
|
3.2
|
|
Restated Bylaws
|
|
|
Amendment No. 5 to
Form S-1
(Exhibit 3.4)
|
|
05/26/06
|
|
333-131609
|
|
|
4.1
|
|
Form of Common Stock Certificate
|
|
|
Form 10-K
(Exhibit 4.1)
|
03/20/14
|
333-131609
|
|||
|
4.2
|
|
Corporate Governance Agreement, dated December 17, 2009, between the Company and certain shareholders of Scient’x Groupe S.A.S. and Scient’x S.A.
|
|
|
Form 8-K
(Exhibit 10.1)
|
|
12/22/09
|
|
000-52024
|
|
|
4.3
|
|
Registration Rights Agreement, dated March 26, 2010, by and among Alphatec Holdings, Inc. and the other signatories thereto
|
|
|
Form 8-K
(Exhibit 4.1)
|
|
03/31/10
|
|
000-52024
|
|
|
4.4
|
|
Warrant with Silicon Valley Bank as the Warrantholder, dated December 16, 2011
|
|
|
Form 10-K
(Exhibit 4.8)
|
|
03/05/12
|
|
000-52024
|
|
|
Exhibit
Number
|
|
Exhibit Description
|
|
Filed
with this
Report
|
|
Incorporated by
Reference herein
from Form or
Schedule
|
|
Filing
Date
|
|
SEC File/
Reg.
Number
|
|
4.5
|
Form of Warrant to Purchase Common Stock issued to
each of Deerfield Private Design Fund II, L.P., Deerfield Private Design International II, L.P., Deerfield Special Situations Fund, L.P. and Deerfield Special Situations International Master Fund, L.P. (collectively, “
Deerfield
”) on each of March 17, 2014 and November 21, 2014.
|
Form 8-K
(Exhibit 4.1)
|
03/19/14
|
000-52024
|
||||||
|
4.6
|
Registration Rights Agreement, dated March 17, 2014, by and among Alphatec Holdings, Inc., Deerfield Private Design Fund II, L.P., Deerfield Private Design International II, L.P., Deerfield Special Situations Fund, L.P. and Deerfield Special Situations International Master Fund, L.P.
|
Form 8-K
(Exhibit 4.2)
|
03/19/14
|
000-52024
|
||||||
|
|
Real Property Lease Agreements
|
|||||||||
|
10.1
|
|
Standard Industrial Lease (Net) by and between Alphatec Holdings, Inc. and H.G. Fenton Property Company, dated as of January 30, 2008
|
|
|
Form 10-Q
(Exhibit 10.2)
|
|
05/12/08
|
|
000-52024
|
|
|
10.2
|
|
Lease Agreement by and between Alphatec Holdings, Inc. and Fenton Property Company., dated as of January 21, 2016
|
|
X
|
|
|||||
|
|
Loan Agreements
|
|
|
|
|
|||||
|
10.3†
|
|
Amended and Restated Credit, Security and Guaranty Agreement dated August 30, 2013 by and among Alphatec Holdings, Inc., Alphatec Spine, Inc., Alphatec International LLC, Alphatec Pacific, Inc. and MidCap Funding IV, LLC
|
|
|
Form 10-Q/A (Exhibit 10.1)
|
10/21/15
|
000-52024
|
|||
|
10.4†
|
First Amendment to Amended and Restated Credit, Security and Guaranty Agreement, dated March 17, 2014, with MidCap Funding IV, LLC as Administrative Agent and lender and other lenders from time to time a party thereto
|
Form 8-K/A
(Exhibit 10.3)
|
10/21/15
|
000-52024
|
||||||
|
10.5†
|
Second Amendment to the Amended and Restated Credit, Security and Guaranty Agreement, dated July 10, 2015, with MidCap Funding IV Trust, as a lender and other lenders from time to time a party thereto
|
Form 10-Q (Exhibit 10.1)
|
11/03/15
|
000-52024
|
||||||
|
10.6
|
Amended and Restated Term Loan Note, dated July 10, 2015, with MidCap Funding IV Trust
|
Form 10-Q (Exhibit 10.3)
|
11/03/15
|
000-52024
|
||||||
|
10.7†
|
Facility Agreement, dated March 17, 2014, by and among Alphatec Holdings, Inc., Deerfield Private Design Fund II, L.P., Deerfield Private Design International II, L.P., Deerfield Special Situations Fund, L.P., and Deerfield Special Situations International Master Fund, L.P.
|
Form 8-K/A (Exhibit 10.1)
|
10/21/15
|
000-52024
|
||||||
|
Exhibit
Number
|
|
Exhibit Description
|
|
Filed
with this
Report
|
|
Incorporated by
Reference herein
from Form or
Schedule
|
|
Filing
Date
|
|
SEC File/
Reg.
Number
|
|
10.8
|
First Amendment to the Facility Agreement, dated July 10, 2015, by and among Alphatec Holdings, Inc., Deerfield Private Design Fund II, L.P., Deerfield Private Design International II, L.P., and Deerfield Special Situations Fund, L.P.
|
Form 10-Q (Exhibit 10.2)
|
10/03/15
|
000-52024
|
||||||
|
10.9
|
Guaranty and Security Agreement, dated March 17, 2014 by and among Alphatec Holdings, Inc., Alphatec Spine, Inc., Alphatec International LLC, Alphatec Pacific, Inc., Deerfield Private Design Fund II, L.P., Deerfield Private Design International II, L.P., Deerfield Special Situations Fund, L.P., and Deerfield Special Situations International Master Fund, L.P.
|
Form 8-K (Exhibit 10.2)
|
03/19/14
|
000-52024
|
||||||
|
Agreements with Respect to Collaborations, Licenses, Research and Development
|
||||||||||
|
10.10†
|
Supply Agreement by and between Alphatec Spine, Inc. and Invibio, Inc., dated as of October 18, 2004 and amended by Letter of Amendment in respect of the Supply Agreement, dated as of December 13, 2004
|
|
|
Amendment No. 4 to
Form S-1
(Exhibit 10.29)
|
|
05/15/06
|
|
333-131609
|
||
|
10.11†
|
Letter Amendment between Alphatec Spine, Inc. and Invibio, Inc., dated November 24, 2010
|
Form 10-Q
(Exhibit 10.3) |
05/06/11
|
000-52024
|
||||||
|
10.12†
|
Exclusive License Agreement by and between Alphatec Spine, Inc. and Stout Medical Group, LP, dated as of September 11, 2007
|
Form 10-Q
(Exhibit 10.2)
|
11/09/07
|
000-52024
|
||||||
|
10.13†
|
First Amendment to the Exclusive License Agreement, effective March 31, 2009 between Alphatec Spine, Inc. and Stout Medical Group LP
|
Form 10-Q
(Exhibit 10.4)
|
05/05/09
|
000-52024
|
||||||
|
10.14†
|
Amendment to the Exclusive License Agreement dated August 1, 2014 between Alphatec Spine, Inc. and Stout Medical Group, L.P.
|
Form 10-Q
(Exhibit 10.
|
10/30/14
|
000-52024
|
||||||
|
10.15†
|
Collaboration Agreement by and among Alphatec Spine, Inc., Elite Medical Holdings, LLC and Pac 3 Surgical Products, LLC, dated as of October 22, 2013
|
Form 10-K
(Exhibit 10.26)
|
03/20/14
|
333-18790
|
||||||
|
10.16
|
First Amendment to the Collaboration Agreement by and among Alphatec Spine, Inc., Elite Medical Holdings, LLC and Pac 3 Surgical Products, LLC, dated November 2, 2015
|
X
|
||||||||
|
Agreements with Officers and Directors
|
||||||||||
|
10.17*
|
Employment Agreement by and among Alphatec Spine, Inc., Alphatec Holdings, Inc. and Michael O’Neill, dated October 11, 2010
|
Form 10-Q
(Exhibit 10.2) |
11/08/10
|
000-52024
|
||||||
|
10.18*
|
Employment Agreement, dated February 26, 2012, by and among Alphatec Holdings, Inc., Alphatec Spine, Inc, and Leslie Cross
|
Form 10-Q
(Exhibit 10.1) |
05/08/12
|
000-52024
|
||||||
|
Exhibit
Number
|
|
Exhibit Description
|
|
Filed
with this
Report
|
|
Incorporated by
Reference herein
from Form or
Schedule
|
|
Filing
Date
|
|
SEC File/
Reg.
Number
|
|
10.19*
|
Amendment to the Employment Agreement by and among Les Cross, Alphatec Holdings, Inc. and Alphatec Spine, Inc., dated May 1, 2014
|
Form 10-K (Exhibit 10.23)
|
02/27/15
|
000-52024
|
||||||
|
10.20*
|
Employment Agreement by and between Alphatec Spine, Inc. and Mitsuo Asai, dated February 17, 2014
|
Form 10-Q
(Exhibit 10.5) |
05/01/14
|
000-52024
|
||||||
|
10.21*
|
Amended and Restated Employment Agreement by and among Alphatec Holdings, Inc., Alphatec Spine, Inc. and Ebun S. Garner, Esq., dated July 17, 2006
|
Form 10-K
(Exhibit 10.20) |
03/07/08
|
000-52024
|
||||||
|
10.22*
|
Employment Agreement by and among James M. Corbett, Alphatec Holdings, Inc. and Alphatec Spine, Inc., dated April 25, 2014
|
Form 10-Q
(Exhibit 10.1)
|
07/31/14
|
000-52024
|
||||||
|
10.23*
|
Employment Agreement by and among Michael Plunkett, Alphatec Spine, Inc., and Alphatec Holdings, Inc., dated February 17, 2014
|
Form 10-Q
(Exhibit 10.4)
|
05/01/14
|
000-52024
|
||||||
|
10.24*
|
Form of Indemnification Agreement entered into with each of the Company’s non-employee directors
|
Form 10-Q
(Exhibit 10.5) |
05/05/09
|
000-52024
|
||||||
|
10.25*
|
Vesting Acceleration Agreement by and between James Glynn and Alphatec Holdings, Inc., dated November 2, 2015
|
X
|
||||||||
|
|
Equity Compensation Plans
|
|||||||||
|
10.26*
|
|
Amended and Restated 2005 Employee, Director and Consultant Stock Plan
|
|
|
Form S-8
(Exhibit 99.1)
|
|
03/23/13
|
|
333-187190
|
|
|
10.27*
|
Amendment to the Amended and Restated 2005 Employee, Director and Consultant Stock Plan
|
Schedule 14A (Appendix B)
|
06/11/13
|
000-52024
|
||||||
|
10.28*
|
Amendment to the Alphatec Holdings, Inc. Amended and Restated 2005 Employee, Director and Consultant Stock Plan
|
Form 10-Q
(Exhibit 10.1)
|
10/30/14
|
000-52024
|
||||||
|
10.29*
|
|
Form of Non-Qualified Stock Option Agreement issued under the Amended and Restated 2005 Stock Plan
|
|
|
Form 10-K
(Exhibit 10.40) |
|
03/05/13
|
|
000-52024
|
|
|
10.30*
|
|
Form of Incentive Stock Option Agreement issued under the Amended and Restated 2005 Stock Plan
|
|
|
Form 10-K
(Exhibit 10.41) |
|
03/05/13
|
|
000-52024
|
|
|
10.31*
|
|
Form of Restricted Stock Agreement issued under the Amended and Restated 2005 Stock Plan
|
|
|
Form 10-K
(Exhibit 10.42) |
|
03/05/14
|
|
000-52024
|
|
|
10.32*
|
Form of Performance-Based Restricted Unit Agreement issued under the Amended and Restated 2005 Employee, Director and Consultant Stock Plan, as amended.
|
Form 10-Q
(Exhibit 10.2)
|
10/30/14
|
000-52024
|
||||||
|
10.33*
|
Amended 2007 Employee Stock Purchase Plan
|
Schedule 14A (Appendix C)
|
06/11/13
|
000-52024
|
||||||
|
Exhibit
Number
|
|
Exhibit Description
|
|
Filed
with this
Report
|
|
Incorporated by
Reference herein
from Form or
Schedule
|
|
Filing
Date
|
|
SEC File/
Reg.
Number
|
|
10.34*
|
|
Summary of the Alphatec Holdings, Inc. 2015 Discretionary Bonus Plan
|
|
|
Form 10-Q
(Exhibit 10.1)
|
|
05/01/15
|
|
000-52024
|
|
|
Settlement Agreements
|
||||||||||
|
10.35
|
Settlement and Release Agreement, dated as of August 13, 2014, by and among Alphatec Holdings, Inc. and its direct and indirect subsidiaries and affiliates, Orthotec, LLC, Patrick Bertranou and the other parties named therein
|
Form 10-Q
(Exhibit 10.3)
|
10/30/14
|
000-52024
|
||||||
|
21.1
|
|
Subsidiaries of the Registrant and Wholly Owned Subsidiaries of the Registrant's Subsidiaries
|
|
X
|
|
|
|
|||
|
23.1
|
|
Consent of Independent Registered Public Accounting Firm
|
|
X
|
|
|
|
|||
|
31.1
|
|
Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
X
|
|
|
|
|||
|
31.2
|
|
Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
X
|
|
|
|
|||
|
32
|
|
Certification pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
|
X
|
|
|
|
|||
|
101.1
|
|
XBRL Instance Document**
|
|
|
|
|
||||
|
101.2
|
|
XBRL Taxonomy Extension Schema Document**
|
|
|
|
|
||||
|
101.3
|
|
XBRL Taxonomy Extension Calculation Linkbase Document**
|
|
|
|
|
||||
|
101.4
|
|
XBRL Taxonomy Extension Definition Linkbase Document**
|
|
|
|
|
||||
|
101.5
|
|
XBRL Taxonomy Extension Label Linkbase Document**
|
|
|
|
|
||||
|
101.6
|
|
XBRL Taxonomy Extension Presentation Linkbase Document**
|
|
|
|
|
||||
|
(*)
|
Management contract or compensatory plan or arrangement.
|
|
(†)
|
Confidential treatment has been granted by the Securities and Exchange Commission as to certain portions.
|
|
(**)
|
Confidential treatment is being requested as to certain portions of this exhibit.
|
|
|
|
ALPHATEC HOLDINGS, INC.
|
|||
|
Dated:
|
March 15, 2016
|
|
By:
|
|
/
S
/ JAMES M. CORBETT
|
|
|
Name:
|
|
James M. Corbett
|
||
|
|
Title:
|
|
President and Chief Executive Officer
|
||
|
|
|
(principal executive officer)
|
|||
|
Signature
|
|
Title
|
|
Date
|
|
/S/ JAMES M. CORBETT
|
|
President and Chief Executive Officer and Director (principal executive officer)
|
|
March 15, 2016
|
|
James M. Corbett
|
||||
|
/S/ MICHAEL O’NEILL
|
|
Chief Financial Officer, Vice President and Treasurer (principal financial officer and principal accounting officer)
|
|
March 15, 2016
|
|
Michael O’Neill
|
||||
|
/S/ LESLIE H. CROSS
|
Chairman of the Board of Directors
|
March 15, 2016
|
||
|
Leslie H. Cross
|
||||
|
/S/ MORTIMER BERKOWITZ III
|
|
Chairman of the Executive Committee of the Board of Directors
|
|
March 15, 2016
|
|
Mortimer Berkowitz III
|
||||
|
/S/ TOM C. DAVIS
|
|
Director
|
|
March 15, 2016
|
|
Tom C. Davis
|
||||
|
/S/ SIRI S. MARSHALL
|
|
Director
|
|
March 15, 2016
|
|
Siri S. Marshall
|
||||
|
/S/ R. IAN MOLSON
|
|
Director
|
|
March 15, 2016
|
|
R. Ian Molson
|
||||
|
/S/ STEPHEN E. O’NEIL
|
|
Director
|
|
March 15, 2016
|
|
Stephen E. O’Neil
|
||||
|
/S/ DONALD A. WILLIAMS
|
|
Director
|
|
March 15, 2016
|
|
Donald A. Williams
|
||||
|
|
Page
|
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash
|
$
|
11,229
|
|
$
|
19,735
|
|
|
|
Restricted cash
|
2,350
|
|
4,400
|
|
|||
|
Accounts receivable, net
|
38,319
|
|
40,440
|
|
|||
|
Inventories, net
|
44,908
|
|
41,747
|
|
|||
|
Prepaid expenses and other current assets
|
5,052
|
|
5,466
|
|
|||
|
Deferred income tax assets
|
—
|
|
1,324
|
|
|||
|
Total current assets
|
101,858
|
|
113,112
|
|
|||
|
Property and equipment, net
|
21,945
|
|
26,040
|
|
|||
|
Goodwill
|
—
|
|
171,333
|
|
|||
|
Intangibles, net
|
21,616
|
|
30,259
|
|
|||
|
Other assets
|
1,285
|
|
4,179
|
|
|||
|
Total assets
|
$
|
146,704
|
|
$
|
344,923
|
|
|
|
Liabilities and Stockholders’ (Deficit) Equity
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
14,169
|
|
$
|
10,130
|
|
|
|
Accrued expenses
|
29,791
|
|
35,393
|
|
|||
|
Deferred revenue
|
648
|
|
1,300
|
|
|||
|
Common stock warrant liabilities
|
687
|
|
8,702
|
|
|||
|
Current portion of long-term debt
|
80,105
|
|
8,076
|
|
|||
|
Total current liabilities
|
125,400
|
|
63,601
|
|
|||
|
Long-term debt, less current portion
|
480
|
|
74,597
|
|
|||
|
Other long-term liabilities
|
33,797
|
|
32,220
|
|
|||
|
Deferred income tax liabilities
|
—
|
|
1,948
|
|
|||
|
Redeemable preferred stock, $0.0001 par value; 20,000 authorized at December 31, 2015 and 2014; 3,319 shares issued and outstanding at both December 31, 2015 and 2014
|
23,603
|
|
23,603
|
|
|||
|
Commitments and contingencies
|
|
|
|||||
|
Stockholders’ (deficit) equity:
|
|||||||
|
Common stock, $0.0001 par value; 200,000 authorized; 102,158 and 99,856 shares issued and outstanding at December 31, 2015 and 2014, respectively
|
10
|
|
10
|
|
|||
|
Treasury stock, 19 shares
|
(97
|
)
|
(97
|
)
|
|||
|
Additional paid-in capital
|
416,939
|
|
413,921
|
|
|||
|
Shareholder note receivable
|
(5,000
|
)
|
(5,000
|
)
|
|||
|
Accumulated other comprehensive loss
|
(21,188
|
)
|
(11,316
|
)
|
|||
|
Accumulated deficit
|
(427,240
|
)
|
(248,564
|
)
|
|||
|
Total stockholders’ (deficit) equity
|
(36,576
|
)
|
148,954
|
|
|||
|
Total liabilities and stockholders’ (deficit) equity
|
$
|
146,704
|
|
$
|
344,923
|
|
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Revenues
|
$
|
185,279
|
|
$
|
206,980
|
|
$
|
204,724
|
|
||
|
Cost of revenues
|
63,742
|
|
61,834
|
|
78,669
|
|
|||||
|
Amortization of acquired intangible assets
|
1,453
|
|
1,736
|
|
1,733
|
|
|||||
|
Gross profit
|
120,084
|
|
143,410
|
|
124,322
|
|
|||||
|
Operating expenses:
|
|||||||||||
|
Research and development
|
17,767
|
|
16,799
|
|
14,190
|
|
|||||
|
In-process research and development
|
274
|
|
527
|
|
—
|
|
|||||
|
Sales and marketing
|
70,856
|
|
77,179
|
|
76,960
|
|
|||||
|
General and administrative
|
34,867
|
|
43,381
|
|
47,949
|
|
|||||
|
Amortization of acquired intangible assets
|
2,400
|
|
2,974
|
|
3,009
|
|
|||||
|
Goodwill and intangible assets impairment
|
165,171
|
|
—
|
|
—
|
|
|||||
|
Restructuring expenses
|
1,188
|
|
706
|
|
9,665
|
|
|||||
|
Litigation settlement expenses
|
—
|
|
—
|
|
45,982
|
|
|||||
|
Total operating expenses
|
292,523
|
|
141,566
|
|
197,755
|
|
|||||
|
Operating (loss) income
|
(172,439
|
)
|
1,844
|
|
(73,433
|
)
|
|||||
|
Other income (expense):
|
|||||||||||
|
Interest income
|
53
|
|
10
|
|
6
|
|
|||||
|
Interest expense
|
(12,589
|
)
|
(13,616
|
)
|
(3,959
|
)
|
|||||
|
Other income (expense), net
|
6,980
|
|
(33
|
)
|
(1,662
|
)
|
|||||
|
Total other income (expense)
|
(5,556
|
)
|
(13,639
|
)
|
(5,615
|
)
|
|||||
|
Loss before income taxes
|
(177,995
|
)
|
(11,795
|
)
|
(79,048
|
)
|
|||||
|
Income tax provision
|
681
|
|
1,087
|
|
3,179
|
|
|||||
|
Net loss
|
$
|
(178,676
|
)
|
$
|
(12,882
|
)
|
$
|
(82,227
|
)
|
||
|
Net loss per basic share
|
$
|
(1.79
|
)
|
$
|
(0.13
|
)
|
$
|
(0.85
|
)
|
||
|
Net loss per diluted share
|
$
|
(1.79
|
)
|
$
|
(0.16
|
)
|
$
|
(0.85
|
)
|
||
|
Shares used in calculating basic net loss per share
|
99,574
|
|
97,347
|
|
96,235
|
|
|||||
|
Shares used in calculating diluted net loss per share
|
99,574
|
|
97,735
|
|
96,235
|
|
|||||
|
|
Year Ended
December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Net loss
|
$
|
(178,676
|
)
|
$
|
(12,882
|
)
|
$
|
(82,227
|
)
|
||
|
Foreign currency translation adjustments
|
(9,872
|
)
|
(15,193
|
)
|
3,765
|
|
|||||
|
Comprehensive loss
|
$
|
(188,548
|
)
|
$
|
(28,075
|
)
|
$
|
(78,462
|
)
|
||
|
|
Common stock
|
Additional
paid-in
capital
|
Shareholder
note receivable |
Treasury
stock
|
Accumulated
other
comprehensive
income (loss)
|
Accumulated
deficit
|
Total
stockholders’
(deficit) equity
|
|||||||||||||||||||||||
|
|
Shares
|
Amount
|
||||||||||||||||||||||||||||
|
Balance at December 31, 2012
|
96,703
|
|
$
|
10
|
|
$
|
399,246
|
|
$
|
—
|
|
$
|
(97
|
)
|
$
|
112
|
|
$
|
(153,455
|
)
|
$
|
245,816
|
|
|||||||
|
Stock-based compensation
|
—
|
|
—
|
|
2,590
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,590
|
|
||||||||||||||
|
Exercise of stock options
|
6
|
|
—
|
|
8
|
|
—
|
|
—
|
|
—
|
|
—
|
|
8
|
|
||||||||||||||
|
Repurchase and/or forfeiture of common stock
|
(142
|
)
|
—
|
|
(172
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(172
|
)
|
||||||||||||||
|
Shares issued for consulting services
|
354
|
|
—
|
|
1,488
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,488
|
|
||||||||||||||
|
Issuance of common stock in connection with license agreements
|
130
|
|
—
|
|
250
|
|
—
|
|
—
|
|
—
|
|
—
|
|
250
|
|
||||||||||||||
|
Forfeiture of common stock in connection with business acquisition
|
(328
|
)
|
—
|
|
(561
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(561
|
)
|
||||||||||||||
|
Issuance of common stock for employee stock purchase plan
|
500
|
|
—
|
|
719
|
|
—
|
|
—
|
|
—
|
|
—
|
|
719
|
|
||||||||||||||
|
Issuance of common stock for restricted share awards granted to employees
|
376
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Foreign currency translation adjustments
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
3,765
|
|
—
|
|
3,765
|
|
||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(82,227
|
)
|
(82,227
|
)
|
||||||||||||||
|
Balance at December 31, 2013
|
97,599
|
|
10
|
|
403,568
|
|
—
|
|
(97
|
)
|
3,877
|
|
(235,682
|
)
|
171,676
|
|
||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
2,690
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,690
|
|
||||||||||||||
|
Exercise of stock options
|
21
|
|
—
|
|
29
|
|
—
|
|
—
|
|
—
|
|
—
|
|
29
|
|
||||||||||||||
|
Repurchase and/or forfeiture of common stock
|
(266
|
)
|
—
|
|
(3
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(3
|
)
|
||||||||||||||
|
Shares issued for consulting services
|
1,327
|
|
—
|
|
1,864
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,864
|
|
||||||||||||||
|
Issuance of common stock for employee stock purchase plan
|
608
|
|
—
|
|
671
|
|
—
|
|
—
|
|
—
|
|
—
|
|
671
|
|
||||||||||||||
|
Issuance of common stock for restricted share awards granted to employees
|
493
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Shareholder note receivable
|
—
|
|
—
|
|
5,000
|
|
(5,000
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Issuance of common stock for acquired technology
|
74
|
|
—
|
|
102
|
|
—
|
|
—
|
|
—
|
|
—
|
|
102
|
|
||||||||||||||
|
Foreign currency translation adjustments
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(15,193
|
)
|
—
|
|
(15,193
|
)
|
||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(12,882
|
)
|
(12,882
|
)
|
||||||||||||||
|
Balance at December 31, 2014
|
99,856
|
|
10
|
|
413,921
|
|
(5,000
|
)
|
(97
|
)
|
(11,316
|
)
|
(248,564
|
)
|
148,954
|
|
||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
2,562
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,562
|
|
||||||||||||||
|
Exercise of stock options
|
5
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Repurchase and/or forfeiture of common stock
|
(261
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Shares issued for consulting services
|
1,325
|
|
—
|
|
81
|
|
—
|
|
—
|
|
—
|
|
—
|
|
81
|
|
||||||||||||||
|
Issuance of common stock for employee stock purchase plan
|
868
|
|
—
|
|
375
|
|
—
|
|
—
|
|
—
|
|
—
|
|
375
|
|
||||||||||||||
|
Issuance of common stock for restricted share awards granted to employees
|
292
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Issuance of common stock for acquired technology
|
73
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Foreign currency translation adjustments
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(9,872
|
)
|
—
|
|
(9,872
|
)
|
||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(178,676
|
)
|
(178,676
|
)
|
||||||||||||||
|
Balance at December 31, 2015
|
102,158
|
|
$
|
10
|
|
$
|
416,939
|
|
$
|
(5,000
|
)
|
$
|
(97
|
)
|
$
|
(21,188
|
)
|
$
|
(427,240
|
)
|
$
|
(36,576
|
)
|
|||||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Operating activities:
|
|||||||||||
|
Net loss
|
$
|
(178,676
|
)
|
$
|
(12,882
|
)
|
$
|
(82,227
|
)
|
||
|
Adjustments to reconcile net loss to net cash provided by (used in) operating activities:
|
|||||||||||
|
Depreciation and amortization
|
19,031
|
|
18,385
|
|
26,277
|
|
|||||
|
Goodwill and intangible assets impairment
|
165,171
|
|
—
|
|
—
|
|
|||||
|
Stock-based compensation
|
2,643
|
|
4,554
|
|
4,078
|
|
|||||
|
Interest expense related to amortization of debt discount and debt issuance costs
|
4,695
|
|
6,700
|
|
368
|
|
|||||
|
In-process research and development
|
98
|
|
102
|
|
—
|
|
|||||
|
Provision for doubtful accounts
|
584
|
|
522
|
|
404
|
|
|||||
|
Provision for excess and obsolete inventory
|
2,156
|
|
3,539
|
|
11,652
|
|
|||||
|
Deferred income tax (benefit) provision
|
(333
|
)
|
251
|
|
816
|
|
|||||
|
Other non-cash items
|
(4,363
|
)
|
1,913
|
|
1,464
|
|
|||||
|
Changes in operating assets and liabilities:
|
|||||||||||
|
Restricted cash
|
4,400
|
|
(6,750
|
)
|
—
|
|
|||||
|
Accounts receivable
|
1,197
|
|
(1,028
|
)
|
(1,940
|
)
|
|||||
|
Inventories
|
(5,456
|
)
|
(4,348
|
)
|
(4,407
|
)
|
|||||
|
Prepaid expenses and other current assets
|
2,472
|
|
4,863
|
|
450
|
|
|||||
|
Other assets
|
(6
|
)
|
(276
|
)
|
64
|
|
|||||
|
Accounts payable
|
3,209
|
|
(1,042
|
)
|
(3,853
|
)
|
|||||
|
Accrued expenses and other
|
(6,365
|
)
|
(35,130
|
)
|
55,171
|
|
|||||
|
Deferred revenue
|
(333
|
)
|
356
|
|
(510
|
)
|
|||||
|
Net cash provided by (used in) operating activities
|
10,124
|
|
(20,271
|
)
|
7,807
|
|
|||||
|
Investing activities:
|
|||||||||||
|
Purchases of property and equipment
|
(12,247
|
)
|
(11,300
|
)
|
(14,352
|
)
|
|||||
|
Purchase of intangible assets
|
—
|
|
—
|
|
(750
|
)
|
|||||
|
Cash paid for acquisitions
|
—
|
|
—
|
|
(4,000
|
)
|
|||||
|
Cash received from sale of assets
|
—
|
|
300
|
|
—
|
|
|||||
|
Net cash used in investing activities
|
(12,247
|
)
|
(11,000
|
)
|
(19,102
|
)
|
|||||
|
Financing activities:
|
|||||||||||
|
Exercise of stock options
|
375
|
|
26
|
|
8
|
|
|||||
|
Borrowings under lines of credit
|
141,583
|
|
163,067
|
|
154,622
|
|
|||||
|
Repayments under lines of credit
|
(144,567
|
)
|
(156,106
|
)
|
(168,855
|
)
|
|||||
|
Principal payments on capital lease obligations
|
(747
|
)
|
(766
|
)
|
(434
|
)
|
|||||
|
Proceeds from issuance of notes payable
|
5,000
|
|
30,350
|
|
28,000
|
|
|||||
|
Principal payments on notes payable
|
(8,176
|
)
|
(5,837
|
)
|
(2,654
|
)
|
|||||
|
Net cash (used in) provided by financing activities
|
(6,532
|
)
|
30,734
|
|
10,687
|
|
|||||
|
Effect of exchange rate changes on cash
|
149
|
|
(1,073
|
)
|
(288
|
)
|
|||||
|
Net decrease in cash
|
(8,506
|
)
|
(1,610
|
)
|
(896
|
)
|
|||||
|
Cash at beginning of period
|
19,735
|
|
21,345
|
|
22,241
|
|
|||||
|
Cash at end of period
|
$
|
11,229
|
|
$
|
19,735
|
|
$
|
21,345
|
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Supplemental disclosure of cash flow information:
|
|||||||||||
|
Cash paid for interest
|
$
|
7,627
|
|
$
|
5,885
|
|
$
|
3,973
|
|
||
|
Cash paid for income taxes
|
$
|
621
|
|
$
|
565
|
|
$
|
1,780
|
|
||
|
Purchases of property and equipment in accounts payable
|
$
|
2,323
|
|
$
|
1,638
|
|
$
|
1,513
|
|
||
|
Purchase of property and equipment through capital leases
|
$
|
243
|
|
$
|
1,212
|
|
$
|
—
|
|
||
|
Non-cash purchases of license agreements
|
$
|
—
|
|
$
|
—
|
|
$
|
250
|
|
||
|
Non-cash debt discount
|
$
|
—
|
|
$
|
650
|
|
$
|
—
|
|
||
|
Initial fair value of warrant liability
|
$
|
—
|
|
$
|
11,280
|
|
$
|
—
|
|
||
|
Level 1:
|
Observable inputs such as quoted prices in active markets;
|
|
Level 2:
|
Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and
|
|
Level 3:
|
Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions.
|
|
|
Common Stock Warrant Liabilities
|
||
|
Balance at December 31, 2013
|
$
|
—
|
|
|
Issuance
|
11,280
|
|
|
|
Changes in fair value
|
(2,578
|
)
|
|
|
Balance at December 31, 2014
|
8,702
|
|
|
|
Issuance
|
—
|
|
|
|
Changes in fair value
|
(8,015
|
)
|
|
|
Balance at December 31, 2015
|
$
|
687
|
|
|
•
|
Estimated volatility is a measure of the amount by which the Company’s common stock price is expected to fluctuate each year during the expected life of the award. The Company’s estimated volatility through December 31,
2015
was based on a weighted-average volatility of its actual historical volatility over a period equal to the expected remaining life of the awards.
|
|
•
|
The expected term represents the period of time that awards granted are expected to be outstanding. Through December 31,
2015
, the Company calculated the expected term using a weighted-average term based on historical exercise patterns and the term from option date to full exercise for the options granted within the specified date range.
|
|
•
|
The risk-free interest rate is based on the yield curve of a zero-coupon U.S. Treasury bond on the date the stock option award is granted with a maturity equal to the expected term of the stock option award.
|
|
•
|
The assumed dividend yield is based on the Company’s expectation of not paying dividends in the foreseeable future.
|
|
|
Year Ended December 31,
|
|||||||
|
|
2015
|
2014
|
2013
|
|||||
|
Risk-free interest rate
|
1.6-1.8%
|
|
1.8-1.9%
|
|
1.1-1.8%
|
|
||
|
Expected dividend yield
|
—
|
|
—
|
|
—
|
|
||
|
Weighted average expected life (years)
|
5.4-5.5
|
|
5.4-5.5
|
|
5.3-5.5
|
|
||
|
Volatility
|
59-68%
|
|
60-71%
|
|
75-76%
|
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Cost of revenues
|
$
|
72
|
|
$
|
274
|
|
$
|
228
|
|
||
|
Research and development
|
286
|
|
2,080
|
|
719
|
|
|||||
|
Sales and marketing
|
359
|
|
470
|
|
459
|
|
|||||
|
General and administrative
|
1,926
|
|
1,730
|
|
2,672
|
|
|||||
|
Total
|
$
|
2,643
|
|
$
|
4,554
|
|
$
|
4,078
|
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Numerator:
|
|||||||||||
|
Net loss for basic earnings per share
|
$
|
(178,676
|
)
|
$
|
(12,882
|
)
|
$
|
(82,227
|
)
|
||
|
Decrease in fair value of warrants
|
—
|
|
(2,578
|
)
|
—
|
|
|||||
|
Diluted net loss attributable to common stockholders
|
$
|
(178,676
|
)
|
$
|
(15,460
|
)
|
$
|
(82,227
|
)
|
||
|
Denominator:
|
|||||||||||
|
Weighted average common shares outstanding
|
100,385
|
|
98,138
|
|
97,111
|
|
|||||
|
Weighted average unvested common shares subject to repurchase
|
(811
|
)
|
(791
|
)
|
(876
|
)
|
|||||
|
Weighted average common shares outstanding—basic
|
99,574
|
|
97,347
|
|
96,235
|
|
|||||
|
Effect of dilutive securities:
|
|||||||||||
|
Conversion of preferred stock
|
—
|
|
—
|
|
—
|
|
|||||
|
Options
|
—
|
|
—
|
|
—
|
|
|||||
|
Warrants
|
—
|
|
388
|
|
—
|
|
|||||
|
Weighted average common shares outstanding—diluted
|
99,574
|
|
97,735
|
|
96,235
|
|
|||||
|
Net loss per share:
|
|||||||||||
|
Basic
|
$
|
(1.79
|
)
|
$
|
(0.13
|
)
|
$
|
(0.85
|
)
|
||
|
Diluted
|
$
|
(1.79
|
)
|
$
|
(0.16
|
)
|
$
|
(0.85
|
)
|
||
|
|
Year Ended December 31,
|
|||||||
|
|
2015
|
2014
|
2013
|
|||||
|
Options to purchase common stock
|
7,941
|
|
7,057
|
|
4,597
|
|
||
|
Warrants to purchase common stock
|
11,544
|
|
725
|
|
594
|
|
||
|
Unvested restricted stock awards
|
811
|
|
791
|
|
876
|
|
||
|
20,296
|
|
8,573
|
|
6,067
|
|
|||
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Accounts receivable
|
$
|
39,380
|
|
$
|
41,233
|
|
|
|
Less allowance for doubtful accounts
|
(1,061
|
)
|
(793
|
)
|
|||
|
Accounts receivables, net
|
$
|
38,319
|
|
$
|
40,440
|
|
|
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Raw materials
|
$
|
7,237
|
|
$
|
5,020
|
|
|
|
Work-in-process
|
1,908
|
|
1,032
|
|
|||
|
Finished goods
|
55,393
|
|
57,020
|
|
|||
|
64,538
|
|
63,072
|
|
||||
|
Less reserve for excess and obsolete finished goods
|
(19,630
|
)
|
(21,325
|
)
|
|||
|
Inventories, net
|
$
|
44,908
|
|
$
|
41,747
|
|
|
|
|
Useful lives
(in years)
|
December 31,
|
|||||||
|
2015
|
2014
|
||||||||
|
Surgical instruments
|
4
|
$
|
65,723
|
|
$
|
62,872
|
|
||
|
Machinery and equipment
|
7
|
15,520
|
|
15,382
|
|
||||
|
Computer equipment
|
3
|
3,984
|
|
3,180
|
|
||||
|
Office furniture and equipment
|
5
|
3,746
|
|
3,789
|
|
||||
|
Leasehold improvements
|
various
|
3,856
|
|
3,841
|
|
||||
|
Building
|
39
|
65
|
|
65
|
|
||||
|
Land
|
n/a
|
9
|
|
9
|
|
||||
|
Construction in progress
|
n/a
|
354
|
|
1,320
|
|
||||
|
93,257
|
|
90,458
|
|
||||||
|
Less accumulated depreciation and amortization
|
(71,312
|
)
|
(64,418
|
)
|
|||||
|
Property and equipment, net
|
$
|
21,945
|
|
$
|
26,040
|
|
|||
|
|
Remaining Avg. Useful lives (in years)
|
December 31,
|
|||||||
|
2015
|
2014
|
||||||||
|
Developed product technology
|
1
|
$
|
21,633
|
|
$
|
22,526
|
|
||
|
Distribution rights
|
4
|
2,100
|
|
2,095
|
|
||||
|
Intellectual property
|
—
|
1,004
|
|
1,004
|
|
||||
|
License agreements
|
1
|
16,714
|
|
16,716
|
|
||||
|
Core technology
|
4
|
4,086
|
|
4,554
|
|
||||
|
Trademarks and trade names
|
2
|
3,245
|
|
3,559
|
|
||||
|
Customer-related
|
9
|
19,169
|
|
20,493
|
|
||||
|
Distribution network
|
5
|
4,027
|
|
4,027
|
|
||||
|
Physician education programs
|
—
|
2,513
|
|
2,802
|
|
||||
|
Supply agreement
|
—
|
225
|
|
225
|
|
||||
|
74,716
|
|
78,001
|
|
||||||
|
Less accumulated amortization
|
(53,100
|
)
|
(47,742
|
)
|
|||||
|
Intangible assets, net
|
$
|
21,616
|
|
$
|
30,259
|
|
|||
|
Year Ending December 31,
|
|
||
|
2016
|
$
|
4,001
|
|
|
2017
|
3,995
|
|
|
|
2018
|
2,844
|
|
|
|
2019
|
2,410
|
|
|
|
2020
|
1,811
|
|
|
|
Thereafter
|
6,555
|
|
|
|
|
|||
|
Total
|
$
|
21,616
|
|
|
|
|||
|
|
|||||||
|
|
2015
|
2014
|
|||||
|
Balance at January 1
|
$
|
171,333
|
|
$
|
183,004
|
|
|
|
Impairment charge
|
(164,266
|
)
|
—
|
|
|||
|
Effect of foreign exchange rate on goodwill
|
(7,067
|
)
|
(11,671
|
)
|
|||
|
Balance at December 31
|
$
|
—
|
|
$
|
171,333
|
|
|
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Commissions and sales milestones
|
$
|
5,920
|
|
$
|
6,259
|
|
|
|
Payroll and payroll related
|
5,577
|
|
8,291
|
|
|||
|
Litigation settlements
|
4,400
|
|
7,393
|
|
|||
|
Accrued professional fees
|
2,203
|
|
2,342
|
|
|||
|
Royalties
|
1,578
|
|
2,129
|
|
|||
|
Restructuring and severance accruals
|
1,358
|
|
849
|
|
|||
|
Accrued taxes
|
1,074
|
|
1,344
|
|
|||
|
Accrued interest
|
999
|
|
946
|
|
|||
|
Other
|
6,682
|
|
5,840
|
|
|||
|
Total accrued expenses
|
$
|
29,791
|
|
$
|
35,393
|
|
|
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Amended Credit Facility with MidCap
|
$
|
56,799
|
|
$
|
60,390
|
|
|
|
Facility Agreement with Deerfield
|
26,000
|
|
26,000
|
|
|||
|
Note payable related to software license purchases
|
189
|
|
250
|
|
|||
|
Financing agreements for premiums on insurance policies
|
1,599
|
|
1,580
|
|
|||
|
Total
|
84,587
|
|
88,220
|
|
|||
|
Add: capital leases (See Note 6)
|
1,277
|
|
1,784
|
|
|||
|
Less: debt discount
|
(5,279
|
)
|
(7,331
|
)
|
|||
|
Total
|
80,585
|
|
82,673
|
|
|||
|
Less: current portion of long-term debt
|
(80,105
|
)
|
(8,076
|
)
|
|||
|
Total long-term debt, net of current portion
|
$
|
480
|
|
$
|
74,597
|
|
|
|
Year Ending December 31,
|
|
||
|
2016
(1)
|
$
|
58,587
|
|
|
2017
(1)
|
8,667
|
|
|
|
2018
(1)
|
8,667
|
|
|
|
2019
(1)
|
8,666
|
|
|
|
|
|||
|
Total
|
84,587
|
|
|
|
Add: capital lease principal payments
|
1,277
|
|
|
|
Less: debt discount
|
(5,279
|
)
|
|
|
|
|||
|
Total
|
80,585
|
|
|
|
Less: current portion of long-term debt
(1)
|
(80,105
|
)
|
|
|
|
|||
|
Long-term debt, net of current portion
|
$
|
480
|
|
|
|
|||
|
Year ending December 31,
|
Operating
|
Capital
|
|||||
|
2016
|
$
|
2,268
|
|
$
|
877
|
|
|
|
2017
|
823
|
|
437
|
|
|||
|
2018
|
304
|
|
68
|
|
|||
|
2019
|
170
|
|
—
|
|
|||
|
2020
|
5
|
|
—
|
|
|||
|
Thereafter
|
—
|
|
—
|
|
|||
|
$
|
3,570
|
|
1,382
|
|
|||
|
Less: amount representing interest
|
(105
|
)
|
|||||
|
Present value of minimum lease payments
|
1,277
|
|
|||||
|
Current portion of capital leases
|
(797
|
)
|
|||||
|
Capital leases, less current portion
|
$
|
480
|
|
||||
|
December 31, 2015
|
||
|
Risk-free interest rate
|
1.3
|
%
|
|
Dividend yield
|
—
|
%
|
|
Expected volatility
|
70
|
%
|
|
Expected life (years)
|
4.3
|
|
|
Shares
|
Weighted
average
exercise
price
|
Weighted
average
remaining
contractual
term
(in years)
|
Aggregate
intrinsic
value
|
||||||||||
|
Outstanding at December 31, 2014
|
8,267
|
|
$
|
2.08
|
|
7.35
|
|
$
|
71
|
|
|||
|
Granted
|
457
|
|
$
|
1.28
|
|
—
|
|
—
|
|
||||
|
Exercised
|
(5
|
)
|
$
|
—
|
|
—
|
|
—
|
|
||||
|
Forfeited
|
(1,081
|
)
|
$
|
2.10
|
|
—
|
|
—
|
|
||||
|
Outstanding at December 31, 2015
|
7,638
|
|
$
|
2.03
|
|
6.36
|
|
$
|
—
|
|
|||
|
Options vested and exercisable at December 31, 2015
|
5,170
|
|
$
|
2.25
|
|
5.63
|
|
$
|
—
|
|
|||
|
Options vested and expected to vest at December 31, 2015
|
7,304
|
|
$
|
2.05
|
|
6.25
|
|
$
|
—
|
|
|||
|
Shares
|
Weighted
average
grant
date fair
value
|
Weighted
average
remaining
recognition
period
(in years)
|
||||||
|
Unvested at December 31, 2014
|
690
|
|
$
|
1.60
|
|
1.83
|
||
|
Awarded
|
291
|
|
$
|
1.36
|
|
|||
|
Vested
|
(243
|
)
|
$
|
1.44
|
|
|||
|
Forfeited
|
(5
|
)
|
$
|
1.80
|
|
|||
|
Unvested at December 31, 2015
|
733
|
|
$
|
1.55
|
|
0.88
|
||
|
Shares
|
Weighted
average
grant
date fair
value
|
Weighted
average
remaining
recognition
period
(in years)
|
||||||
|
Unvested at December 31, 2014
|
854
|
|
$
|
1.42
|
|
2.00
|
||
|
Awarded
|
1,854
|
|
$
|
1.34
|
|
|||
|
Vested
|
—
|
|
$
|
—
|
|
|||
|
Forfeited
|
(338
|
)
|
$
|
1.36
|
|
|||
|
Unvested at December 31, 2015
|
2,370
|
|
$
|
1.37
|
|
1.67
|
||
|
|
December 31, 2015
|
|
|
Stock options outstanding
|
7,638
|
|
|
Awards outstanding
|
733
|
|
|
Performance restricted stock units outstanding
|
2,370
|
|
|
Warrants outstanding
|
11,544
|
|
|
Authorized for future grant under 2005 Plan
|
3,840
|
|
|
|
||
|
26,125
|
|
|
|
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
U.S. Domestic
|
$
|
(90,342
|
)
|
$
|
(8,106
|
)
|
$
|
(9,264
|
)
|
||
|
Foreign
|
(87,653
|
)
|
(3,689
|
)
|
(69,784
|
)
|
|||||
|
Pretax loss from operations
|
$
|
(177,995
|
)
|
$
|
(11,795
|
)
|
$
|
(79,048
|
)
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Current income tax expense (benefit):
|
|||||||||||
|
Federal
|
$
|
221
|
|
$
|
—
|
|
$
|
(21
|
)
|
||
|
State
|
149
|
|
145
|
|
186
|
|
|||||
|
Foreign
|
634
|
|
526
|
|
2,525
|
|
|||||
|
Total current
|
1,004
|
|
671
|
|
2,690
|
|
|||||
|
Deferred income tax (benefit) expense:
|
|||||||||||
|
Federal
|
(1,363
|
)
|
238
|
|
229
|
|
|||||
|
State
|
(154
|
)
|
24
|
|
15
|
|
|||||
|
Foreign
|
1,194
|
|
154
|
|
245
|
|
|||||
|
Total deferred
|
(323
|
)
|
416
|
|
489
|
|
|||||
|
Total income tax expense
|
$
|
681
|
|
$
|
1,087
|
|
$
|
3,179
|
|
||
|
|
December 31,
|
|||||||
|
|
2015
|
2014
|
2013
|
|||||
|
Federal statutory rate
|
(35.0
|
)%
|
(35.0
|
)%
|
(35.0
|
)%
|
||
|
Adjustments for tax effects of:
|
||||||||
|
State taxes, net
|
(0.3
|
)
|
(1.1
|
)
|
(0.1
|
)
|
||
|
Stock-based compensation
|
0.3
|
|
6.2
|
|
0.5
|
|
||
|
Foreign taxes
|
0.2
|
|
3.4
|
|
1.1
|
|
||
|
Tax credits
|
(0.3
|
)
|
(3.3
|
)
|
(0.4
|
)
|
||
|
Deemed foreign dividend
|
0.1
|
|
—
|
|
—
|
|
||
|
Fair market value adjustments
|
(1.6
|
)
|
(7.6
|
)
|
—
|
|
||
|
Intercompany debt forgiveness and other permanent adjustments
|
0.6
|
|
3.1
|
|
9.5
|
|
||
|
Goodwill impairment
|
29.1
|
|
—
|
|
—
|
|
||
|
Tax rate adjustment
|
0.4
|
|
0.4
|
|
0.2
|
|
||
|
Uncertain tax positions
|
(0.1
|
)
|
5.3
|
|
2.7
|
|
||
|
Other
|
3.1
|
|
0.2
|
|
(0.4
|
)
|
||
|
Valuation allowance
|
3.8
|
|
37.5
|
|
25.9
|
|
||
|
Effective income tax rate
|
0.3
|
%
|
9.1
|
%
|
4.0
|
%
|
||
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Deferred tax assets:
|
|||||||
|
Allowances and reserves
|
$
|
955
|
|
$
|
818
|
|
|
|
Accrued expenses
|
2,331
|
|
3,674
|
|
|||
|
Inventory reserves
|
9,631
|
|
8,532
|
|
|||
|
Net operating loss carryforwards
|
43,427
|
|
41,965
|
|
|||
|
Property and equipment
|
2,420
|
|
1,976
|
|
|||
|
Stock-based compensation
|
2,377
|
|
2,168
|
|
|||
|
Legal settlement
|
11,806
|
|
1,204
|
|
|||
|
Goodwill
|
3,362
|
|
—
|
|
|||
|
Income tax credit carryforwards
|
3,235
|
|
2,218
|
|
|||
|
Total deferred tax assets
|
79,544
|
|
62,555
|
|
|||
|
Valuation allowance
|
(63,612
|
)
|
(58,781
|
)
|
|||
|
Total deferred tax assets, net of valuation allowance
|
15,932
|
|
3,774
|
|
|||
|
Deferred tax liabilities:
|
|||||||
|
Investment in foreign partnership
|
15,467
|
|
—
|
|
|||
|
Intangible assets
|
465
|
|
2,881
|
|
|||
|
Goodwill
|
—
|
|
1,518
|
|
|||
|
Total deferred tax liabilities
|
15,932
|
|
4,399
|
|
|||
|
Net deferred tax assets (liabilities)
|
$
|
—
|
|
$
|
(625
|
)
|
|
|
|
Year ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Unrecognized tax benefit at the beginning of the year
|
8,861
|
|
7,835
|
|
5,897
|
|
|||||
|
Additions based on tax positions related to the current year
|
859
|
|
1,050
|
|
1,664
|
|
|||||
|
Additions based on tax positions related to the prior year
|
1,144
|
|
391
|
|
221
|
|
|||||
|
Reductions as a result of lapse of applicable statute of limitations
|
(76
|
)
|
(40
|
)
|
(20
|
)
|
|||||
|
Reductions as a result of foreign exchange rates and other
|
(429
|
)
|
(375
|
)
|
73
|
|
|||||
|
Unrecognized tax benefits at the end of the year
|
$
|
10,359
|
|
$
|
8,861
|
|
$
|
7,835
|
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
United States
|
$
|
114,578
|
|
$
|
137,060
|
|
$
|
134,951
|
|
||
|
International
|
70,701
|
|
69,920
|
|
69,773
|
|
|||||
|
Total consolidated revenues
|
$
|
185,279
|
|
$
|
206,980
|
|
$
|
204,724
|
|
||
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
United States
|
$
|
97,967
|
|
$
|
200,978
|
|
|
|
International
|
48,737
|
|
143,945
|
|
|||
|
Total consolidated assets
|
$
|
146,704
|
|
$
|
344,923
|
|
|
|
|
Year ended December 31, 2015
|
||||||||||||||
|
|
1st
Quarter
|
2nd
Quarter
|
3rd
Quarter
|
4th
Quarter
|
|||||||||||
|
Selected quarterly financial data:
|
|||||||||||||||
|
Revenue
|
$
|
48,647
|
|
$
|
46,633
|
|
$
|
42,996
|
|
$
|
47,003
|
|
|||
|
Gross profit
|
32,943
|
|
27,527
|
|
28,479
|
|
31,135
|
|
|||||||
|
Total operating expenses
|
31,801
|
|
30,354
|
|
193,427
|
|
36,941
|
|
|||||||
|
Net loss
|
(4,561
|
)
|
(3,947
|
)
|
(160,265
|
)
|
(9,903
|
)
|
|||||||
|
Net loss per basic share (1)
|
(0.05
|
)
|
(0.04
|
)
|
(1.61
|
)
|
(0.10
|
)
|
|||||||
|
Net loss per diluted share (1)
|
(0.05
|
)
|
(0.04
|
)
|
(1.61
|
)
|
(0.10
|
)
|
|||||||
|
|
Year ended December 31, 2014
|
||||||||||||||
|
|
1st
Quarter
|
2nd
Quarter
|
3rd
Quarter
|
4th
Quarter
|
|||||||||||
|
Selected quarterly financial data:
|
|||||||||||||||
|
Revenue
|
$
|
49,173
|
|
$
|
53,167
|
|
$
|
51,013
|
|
$
|
53,627
|
|
|||
|
Gross profit
|
33,294
|
|
36,120
|
|
36,306
|
|
37,690
|
|
|||||||
|
Total operating expenses
|
37,996
|
|
34,279
|
|
34,574
|
|
34,717
|
|
|||||||
|
Net loss
|
(6,673
|
)
|
(2,895
|
)
|
(3,041
|
)
|
(273
|
)
|
|||||||
|
Net loss per basic share (1)
|
(0.07
|
)
|
(0.03
|
)
|
(0.03
|
)
|
0.00
|
|
|||||||
|
Net loss per diluted share (1)
|
(0.07
|
)
|
(0.03
|
)
|
(0.04
|
)
|
(0.03
|
)
|
|||||||
|
(1)
|
Basic and diluted net loss per share is computed independently for each of the quarters presented. Therefore, the sum of the quarterly per share amounts will not necessarily equal the total for the year.
|
|
Allowance
for
Doubtful
Accounts (1)
|
Reserve for
Excess and
Obsolete
Inventories (2)
|
||||||
|
|
(In thousands)
|
||||||
|
Balance at December 31, 2012
|
$
|
1,074
|
|
$
|
17,222
|
|
|
|
Provision
|
404
|
|
11,652
|
|
|||
|
Write-offs and recoveries, net
|
(430
|
)
|
(4,928
|
)
|
|||
|
Balance at December 31, 2013
|
1,048
|
|
23,946
|
|
|||
|
Provision
|
522
|
|
3,539
|
|
|||
|
Write-offs and recoveries, net
|
(777
|
)
|
(6,160
|
)
|
|||
|
Balance at December 31, 2014
|
793
|
|
21,325
|
|
|||
|
Provision
|
584
|
|
2,159
|
|
|||
|
Write-offs and recoveries, net
|
(315
|
)
|
(3,854
|
)
|
|||
|
Balance at December 31, 2015
|
$
|
1,062
|
|
$
|
19,630
|
|
|
|
(1)
|
The provision is included in selling expenses.
|
|
(2)
|
The provision is included in cost of revenues.
|