|
UNITED STATES
|
|
|
X
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
OR
|
|
|
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
Commission file number: 0-30314
|
|
|
Portage Biotech Inc.
|
|
|
(Exact name of Registrant as specified in its charter)
|
|
|
Inapplicable
|
|
Page No.
|
||
|
Forward-looking statements
|
1
|
|
|
Foreign Private Issuer Status and Reporting currency
|
2
|
|
|
Part I
|
||
|
Item 1.
|
Identity of Directors, Senior Management and Advisors
|
2
|
|
Item 2.
|
Offer Statistics and Expected Timetable
|
2
|
|
Item 3.
|
Key Information
|
2
|
|
Item 4.
|
Information on the Company
|
9
|
|
Item 5.
|
Operating and Financial Review and Prospects
|
14
|
|
Item 6.
|
Directors, Senior Management and Employees
|
19
|
|
Item 7.
|
Major Shareholders and Related Party Transactions
|
24
|
|
Item 8.
|
Financial Information
|
25
|
|
Item 9.
|
The Offer and Listing
|
26
|
|
Item 10.
|
Additional Information
|
27
|
|
Item 11.
|
Quantitative and Qualitative Disclosures about Market Risk
|
39
|
|
Item 12.
|
Description of Securities Other than Equity Securities
|
40
|
|
Part II
|
||
|
Item 13.
|
Defaults, Dividend Arrearages and Delinquencies
|
40
|
|
Item 14.
|
Material Modifications to the Rights of Security Holders and Use of Proceeds
|
40
|
|
Item 15.
|
Controls and Procedures
|
41
|
|
Item 16.
|
Audit Committee, Code of Ethics, and Principal Accountant’s Fees and Services
|
42
|
|
Part III
|
||
|
Item 17.
|
Financial Statements
|
42
|
|
Item 18.
|
Financial Statements
|
43
|
|
Item 19.
|
Exhibits
|
43
|
|
·
our plans and ability to develop and commercialize product candidates and the timing of these development programs;
|
|
|
·
clinical development of our product candidates, including the results of current and future clinical trials;
|
|
|
·
the benefits and risks of our product candidates as compared to others;
|
|
|
·
our maintenance and establishment of intellectual property rights in our product candidates;
|
|
|
·
our need for additional financing and our estimates regarding our capital requirements and future revenues and profitability;
|
|
|
·
our estimates of the size of the potential markets for our product candidates;
|
|
|
·
our selection and licensing of product candidates;
|
|
|
Year ended March 31, 2014
|
Period from May 23, 2012 to March 31, 2013
|
|
|
Revenue
|
-
|
-
|
|
Loss before non-controlling interests
|
$(6,626,630)
|
$(29,486)
|
|
Non-controlling interests
|
$(321,683)
|
$ -
|
|
Net Loss attributable to shareholders
|
$(6,304,947)
|
$(29,486)
|
|
Net loss per share (1)
|
($0.04)
|
($0.00)
|
|
Working capital
|
$2,067,319
|
$474,009
|
|
Total assets
|
$5,263,413
|
$486,401
|
|
Capital stock
|
$7,256,715
|
$503,495
|
|
Warrants
|
$1,108,402
|
$ -
|
|
Stock option reserve
|
$362,440
|
$ -
|
|
Shareholders' equity
|
$2,393,124
|
$474,009
|
|
Weighted average number of shares outstanding
|
161,977,171
|
81,759,076
|
|
2014
|
June
|
May
|
April
|
March
|
February
|
January
|
|
High for period
|
$0.94
|
$0.92
|
$0.92
|
$0.91
|
$0.91
|
$.94
|
|
Low for period
|
$0.91
|
$0.91
|
$0.90
|
$0.89
|
$0.89
|
$0.89
|
|
Year Ended March 31,
|
|||||
|
2014
|
2013
|
2012
|
2011
|
2010
|
|
|
Average for the year
|
0.95
|
1.00
|
1.01
|
0.98
|
0.92
|
|
·
|
our inability to manufacture or obtain sufficient quantities of materials for use in clinical trials;
|
|
·
|
delays arising from our collaborative partnerships;
|
|
·
|
delays in obtaining regulatory approvals to commence a study, or government intervention to suspend or terminate a study;
|
|
·
|
delays, suspension, or termination of the clinical trials due to the institutional review board or independent ethics board responsible for overseeing the study to protect research subjects at a particular study site;
|
|
·
|
delays in identifying and reaching agreement on acceptable terms with prospective clinical trial sites;
|
|
·
|
slower than expected rates of patient recruitment and enrollment;
|
|
·
|
uncertain dosing issues;
|
|
·
|
inability or unwillingness of medical investigators to follow our clinical protocols;
|
|
·
|
variability in the number and types of subjects available for each study and resulting difficulties in identifying and enrolling subjects who meet trial eligibility criteria;
|
|
·
|
scheduling conflicts with participating clinicians and clinical institutions;
|
|
·
|
difficulty in maintaining contact with subjects after treatment, which results in incomplete data;
|
|
·
|
unforeseen safety issues or side effects;
|
|
·
|
lack of efficacy during the clinical trials;
|
|
·
|
our reliance on clinical research organizations to conduct clinical trials, which may not conduct those trials with good clinical or laboratory practices; or
|
|
·
|
other regulatory delays.
|
|
•
|
we experience scientific progress sooner than expected in our future discovery, research and development projects, if we expand the magnitude and scope of these activities, or if we modify our focus as a result of our discoveries;
|
|
•
|
we experience setbacks in our progress with pre-clinical studies and clinical trials are delayed;
|
|
•
|
we experience delays or unexpected increased costs in connection with obtaining regulatory approvals;
|
|
•
|
we are required to perform additional pre-clinical studies and clinical trials;
|
|
•
|
we experience unexpected or increased costs relating to preparing, filing, prosecuting, maintaining, defending and enforcing patent claims; or
|
|
•
|
we elect to develop, acquire or license new technologies and products.
|
|
·
|
to recognize or enforce against us judgments of U.S. courts based on certain civil liability provisions of U.S. securities laws; and
|
|
·
|
to impose liabilities against us, in original actions brought in the British Virgin Islands, based on certain civil liability provisions of U.S. securities laws that are penal in nature.
|
|
(A)
|
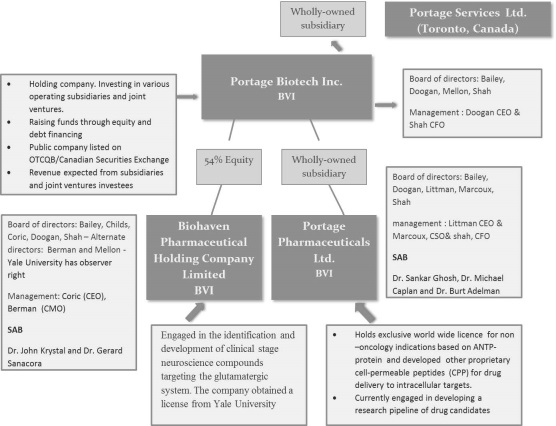
HISTORY AND DEVELOPMENT OF THE COMPANY
|
PPL has converted its previously filed provisional patent application for this delivery system to an international patent application that includes a variety of structures utilizing cargos that address important areas of medical need.
|
•
|
Has more than 30 years’ experience in the global pharmaceutical industry.
|
|
•
|
He joined Pfizer in 1982, where he held a number of senior positions in R&D in the USA, UK and Japan. He retired from Pfizer in 2007 as the Senior VP Head of World Development. Subsequently
|
|
•
|
Was interim CEO and CMO at Amarin.
|
|
•
|
Holds visiting professorships at Glasgow, Kitasato (Tokyo) and Cork Universities . He received his Medical degree from Glasgow University.
|
|
•
|
Senior financial executive with over 25 years of corporate finance,
|
|
•
|
Was senior manager with two of the largest accounting firms, Ernst & Young and Price Waterhouse Coopers
|
|
•
|
Worked in industry under various roles from an office manager to CEO, CFO of public companies.
|
|
|
Gregory Bailey M.D. – Chairman
|
|
•
|
Former director and financier of Medivation Inc. (MDVN: NASDAQ).
|
|
•
|
Co-founder, of Ascent Healthcare Solutions: VirnetX Inc internet security (VHC: AMEX) and Duramedic Inc. a medical products company.
|
|
•
|
Has Medical Doctorate from the University of Western Ontario.
|
|
|
Jim Mellon – Director
|
|
•
|
Director of multiple public companies: In the biopharma sector Miraculins, Plethora Solutions, and the Summit Corporation.
|
|
•
|
Chairman of AIM listed Port Erin Biopharma Investments, a fund specialising in biopharma investments
|
|
•
|
The author of the best-selling book “Cracking the Code.
|
|
•
|
Other listed company directorships include chairman of Manx Financial Group and Speymill, co-chairman of both Regent Pacific Group and West African Mining Corporation, and a board member of Brazilian Gold Corporation, Charlemagne Capital and Condor Resources.
|
|
|
Bruce H. Littman,
MD – CEO
|
|
•
|
Former Pfizer VP Global Translational Medicine
|
|
•
|
Over 30 years pharmaceutical company and academic research experience
|
|
|
Frank W. Marcoux
, Ph.D. - CSO
|
|
•
|
Former Pfizer VP Quantitative and Innovative Medicine WW Development and former VP Biology Discipline WW Discovery
|
|
•
|
Over 25 years pharmaceutical company and academic research
|
|
|
Vlad Coric, MD
– Director
|
|
•
|
Has over 14 years of clinical trial experience as the Chief of Inpatient Services at the Yale Clinical Neuroscience Research Unit.
|
|
•
|
An Associate Clinical Professor of Psychiatry at the Yale
|
|
•
|
A co-inventor of Yale intellectual property related to the use of glutamate modulating agents
|
|
•
|
Earned his medical degree at Wake Forest University School of Medicine, and received his BS from University of Connecticut in Physiology and Neurobiology.
|
|
•
|
Has over 45 peer-reviewed journal and book publications.
|
|
|
Robert Berman , MD -
CMO
|
|
•
|
Almost 30 years of neuroscience research
|
|
•
|
13 years of clinical development experience (Pfizer and Bristol-Myers Squibb)
|
|
•
|
Professor of Psychiatry (Adjunct), Yale School of Medicine
|
|
•
|
Over 60 peer-reviewed publications – including first clinical trial with ketamine in patients with depression and leading the registrational program to obtain the first indication for a neuroleptic in the adjunctive treatment of major depressive disorder
|
|
•
|
BA, Molecular Biophysics and Biochemistry, Yale University
|
|
•
|
M.D., Mount Sinai School of Medicine
|
|
•
|
Chairman of Psychiatry and Professor, Yale School of Medicine.
|
|
•
|
Expert in the areas of psychopharmacology, glutamatergic neurotransmission, alcoholism, schizophrenia, and post-traumatic stress disorders.
|
|
•
|
Professor of Psychiatry and Director of the Yale Depression Research Clinic
|
|
•
|
Expert in elucidating the pathophysiological mechanisms associated with mood and other neuropsychiatric disorders.
|
|
•
|
Director, MGH Clinical Research Program (CRP), Executive Vice Chair for the MGH Department of Psychiatry, Executive Director, MGH Clinical Trials Network and Institute, Director, and Slater Family Professor of Psychiatry at Harvard Medical School
|
|
•
|
Expert in affective disorders and clinical trial design – with over 600 original articles
|
|
|
(D) PROPERTY PLANTS AND EQUIPMENT
|
|
Year ended
March 31,2014
|
May 23, 2012 to
March 31, 2013
|
||
|
in 000' US $
|
in 000' US $
|
||
|
Expenses
|
(6,627)
|
(29)
|
|
|
(6,627)
|
(29)
|
||
|
Non-controlling interests
|
(322)
|
-
|
|
|
Net loss attributable to shareholders
|
(6,305)
|
(29)
|
|
|
Deficit at end of year
|
(6,334)
|
(29)
|
|
a.
|
The assets and liabilities of PPL at their pre-acquisition carrying amounts as at March 31, 2014 and expenses for the year ended on that date
|
|
b.
|
The assets and liabilities of Bontan as at March 31, 2014 and expenses from June 4, 2013 to March 31, 2014.
|
|
c.
|
Share capital representing the total number of shares issued by the Company.
|
|
d.
|
Value of the share capital was computed by adding to the value of the share capital of PPL on the date of acquisition, June 4, 2013, the fair value of Bontan as allocated to shares issued on the date of acquisition, and adjusted to any exercise or issuance of shares, warrants and options during the year ended March 31, 2014.
|
|
e.
|
Comparative figures are those of PPL.
|
|
Cash
|
$3,006,593
|
|
Office equipment and furniture
|
5,286
|
|
Other assets
|
153,963
|
|
Liabilities
|
(296,027)
|
|
Fair value of consideration
|
2,869,815
|
|
Year ended March 31, 2014
|
May 23, 2012 to March 31, 2013
|
|
|
Acquisition related costs
|
3,839
|
-
|
|
Consulting fees
|
1,162
|
-
|
|
Research & development
|
$ 1,136
|
$ 27
|
|
Professional fees
|
336
|
-
|
|
Other costs
|
154
|
2
|
|
$ 6,627
|
$ 29
|
|
Year ended March 31, 2014
|
May 23, 2012 to March 31, 2013
|
|
|
in 000$
|
||
|
licenses fee
|
26
|
|
|
patent registration (a)
|
29
|
|
|
Consulting fee ( c)
|
365
|
27
|
|
fee paid by Biohaven under a service contract (b)
|
500
|
|
|
Other outside services - lab tseting, peptide production etc.
|
215
|
|
|
$ 1,135
|
$ 27
|
|
|
(a)
|
Company’s subsidiary PPL paid the license fee to a non related entity in respect of ANTP license under License Agreement dated January 25, 2013.
|
|
(b)
|
Biohaven has signed a Master Service Agreement on January 31, 2014, as subsequently amended in April 2014, with Biohaven Pharmaceuticals Inc, a private Delaware incorporated research and development company (“BPI”). BPI is owned by non-controlling shareholders of Biohaven and is engaged by Biohaven to conduct, on behalf of Biohaven, research and development services relating to identification and development of clinical stage neuroscience compounds targeting the glutamatergic system.
|
|
(c)
|
Consulting fee includes fees totaling to approximately $306,000 paid to the CEO and CSO of PPL . Fee includes value of the vested options of approximately $57,000 and balance in cash.
|
|
|
Working Capital
|
|
|
Operating cash flow
|
|
|
Investing cash flows
|
|
|
Financing cash flows
|
|
(a)
|
Cargo Peptides and Uses for Antennapedia Homeodomain-based Protein Biological Drugs – new provisional patent for Antennapedia structures and indications.
|
|
(b)
|
Structure, Manufacturing and uses of Human-derived Cell-Permeable Peptides Conjugated with Special Biologically Active Cargo Peptides – Converted 2013 provisional patent into an international patent for our own proprietary human-derived cell permeable peptides to maintain June 11, 2013 priority date with addition of more specific examples with supporting animal data, new specific structures, indications and manufacturing details.
|
|
Name, Province/State and Country of Residence and Present Position with Portage (1)
|
Date became Director/Officer
|
Principal Occupation Last five years
|
|
Dr. Gregory Bailey (2)
London, UK
Chairman of the Board of Director
|
June 4, 2013
|
See brief biography below
|
|
Dr. Declan Doogan
Stonington, CT, USA
Chief Executive Officer and Director
|
June 4, 2013
|
See brief biography below
|
|
Mr. Jim Mellon (2) (3)
Isle of Man
Director
|
June 4, 2013
|
See brief biography below
|
|
Mr. Kam Shah (2)
Ontario, Canada
Director and Chief Financial Officer
|
January 3, 1999
|
May 17, 2004 – June 4, 2013 – Chief Executive Officer of Bontan,
March 9, 2010 till date – Sole director, CEO/CFO of ZD Ventures Corporation
|
|
(1)
|
Neither age nor date of birth of directors or executive officers is required to be reported in our home country nor otherwise publicly disclosed.
|
|
(2)
|
Member of the Audit and Compensation Committee. Mr. Jim Mellon is the Chair of this Committee.
|
|
(3)
|
Independent directors
|
|
ANNUAL COMPENSATION
|
LONG-TERM COMPENSATION
|
||||||||
|
Awards
|
Payouts
|
||||||||
|
Name and principal position
|
Year
|
Fee (3)
|
Bonus
|
Other annual compensation(6)
|
Securities under options/SARs Granted (1) & (4)
|
Shares or units subject to resale restrictions (4)
|
LTIP (2) payouts
|
all other compensation (5)
|
Total compensation
|
|
($)
|
($)
|
($)
|
$
|
($)
|
($)
|
($)
|
($)
|
||
|
Declan Doogan
|
|||||||||
|
CEO
|
2014
|
|
135,743
|
270,000
|
405,743
|
||||
|
CEO
|
2013
|
-
|
-
|
-
|
-
|
||||
|
Kam Shah
|
|||||||||
|
CFO
|
2014
|
253,458
|
67,871
|
-
|
321,329
|
||||
|
Gregory Bailey
|
|||||||||
|
Chairman/Business development
|
2014
|
135,743
|
270,000
|
405,743
|
|||||
|
Chairman/business development
|
2013
|
-
|
-
|
-
|
|||||
|
James Mellon
|
|||||||||
|
Independent director
|
2014
|
-
|
54,297
|
54,297
|
|||||
|
1.
|
“SAR” means stock appreciation rights. The Company never issued any SARs
|
|
2.
|
“LTIP” means long term incentive plan.
|
|
3.
|
Fee includes issuance of 1 million shares to Mr. Shah valued at $151,000.
|
|
4.
|
Consists of 1.5 million restricted shares each to Dr. Doogan and Dr. Bailey valued at $270,000 each for services rendered. Restrictive legend can only be removed by either filing a registration statement or seeking exemption under Rule 144 of the Securities Act.
|
|
5.
|
Total of 2.9 million options were issued to the four key executives. One million each to Dr. Doogan and Dr. Bailey, 500,000 to Mr. Shah and 400,000 to Mr. Mellon.
.
These options are valid for five years and are convertible into equal number of common shares of the Company at an exercise price of $0.20 per common share. The Options were registered with the US Securities and Exchange Commission on December 19, 2013 and will vest in equal instalment over the twelve months ending December 31, 2014.
|
|
·
|
reviewing the quarterly and annual consolidated financial statements and management discussion and analyses;
|
|
·
|
meeting at least annually with our external auditor;
|
|
·
|
reviewing the adequacy of the system of internal controls in consultation with the chief executive and financial officer;
|
|
·
|
reviewing any relevant accounting and financial matters including reviewing our public disclosure of information extracted or derived from our financial statements;
|
|
·
|
establishing procedures for the receipt, retention and treatment of complaints received by us regarding accounting, internal controls or auditing matters and the confidential, anonymous submission by employees of concerns regarding questionable accounting or auditing matters;
|
|
·
|
pre-approving all non-audit services and recommending the appointment of external auditors; and
|
|
·
|
reviewing and approving our hiring policies regarding personnel of our present and former external auditor
|
|
·
|
Reviewing and approving all employee and consultants contracts, bonuses and other compensation matters
|
|
Common Shares
Beneficially Owned
|
Options and Warrants Exercisable
for Common Shares
|
|||||||||
|
Name
|
Number
|
Percentage *
|
Number
|
Exercise price - in US$
|
Expiry date(s)
|
|||||
|
Kam Shah
|
2,359,131
|
1.31%
|
200,000
|
O
|
0.35
|
18-Aug-15
|
||||
|
500,000
|
O
|
0.20
|
12-Dec-18
|
|||||||
|
Declan Doogan
|
27,711,068
|
15.33%
|
22,908,149
|
W
|
0.29
|
06- June- 15
|
||||
|
1,000,000
|
O
|
0.20
|
12-Dec-18
|
|||||||
|
Greg Bailey
|
27,711,068
|
15.33%
|
22,908,149
|
W
|
0.29
|
06- June- 15
|
||||
|
1,000,000
|
O
|
0.20
|
12-Dec-18
|
|||||||
|
James Mellon
|
26,211,068
|
14.50%
|
22,908,149
|
0.29
|
06- June- 15
|
|||||
|
400,000
|
O
|
0.20
|
12-Dec-18
|
|||||||
|
Name of Beneficial Owner
|
No. of Shares
|
Percentage of Shares
|
|
Declan Doogan
|
51,202,548 (1)
|
20%
|
|
Greg Bailey
|
51,202,548 (1)
|
20%
|
|
James Mellon
|
49,352,548 (2)
|
20%
|
|
(1)
|
Includes 23,491,480 shares issuable upon exercise of warrants and vested options
|
|
(2)
|
Includes 23,141,480 shares issuable upon exercise of warrants and vested options
|
|
Fiscal year ended March 31
2014
|
High
(US$)
0.42
|
Low
(US$)
0.06
|
|
2013
|
0.16
|
0.01
|
|
2012
|
0.18
|
0.02
|
|
2011
2010
|
0.40
0.45
|
0.07
0.06
|
|
Fiscal Quarter ended
|
High
|
Low
|
|
In US$
|
In US$
|
|
|
June 30, 2014
|
0.12
|
0.09
|
|
March 31, 2014
|
0.23
|
0.08
|
|
December 31, 2013
|
0.30
|
0.16
|
|
September 30, 2013
|
0.38
|
0.22
|
|
June 30, 2013
|
0.42
|
0.15
|
|
March 31, 2013
|
0.16
|
0.07
|
|
December 31, 2012
|
0.11
|
0.04
|
|
September 31, 2012
|
0.06
|
0.01
|
|
June 30, 2012
|
0.04
|
0.02
|
|
Month
|
High
|
Low
|
|
In US$
|
In US$
|
|
|
June 2014
|
0.11
|
0.09
|
|
May 2014
|
0.11
|
0.09
|
|
April 2014
|
0.
12
|
0.
09
|
|
March 2014
|
0.17
|
0.
06
|
|
February 2014
|
0.19
|
0.13
|
|
January 2014
|
0.
24
|
0.
13
|
|
·
|
all checks, not being less than three in total number, for any sums payable in cash to the holder of such shares have remained uncashed for a period of 12 years;
|
|
·
|
we have not during that time or before the expiry of the three-month period referred to in the following point received any indication of the existence of the shareholder or person entitled to such shares by death, bankruptcy or operation of law; and
|
|
·
|
upon expiration of the twelve-year period, we have caused an advertisement to be published in newspapers, giving notice of our intention to sell these shares, and a period of three months or such shorter period has elapsed since the date of such advertisement.
|
|
o
|
consolidate and divide all or any of our unissued authorized shares into shares of a larger amount than our existing shares;
|
|
o
|
sub-divide our existing ordinary shares, or any of them into shares of smaller amount than is fixed by our memorandum of association, subject nevertheless to the provisions of the BVI Act;
|
|
o
|
•cancel any ordinary shares that, at the date of the passing of the resolution, have not been taken or agreed to be taken by any person; or
|
|
o
|
create new classes of shares with preferences to be determined by the board of directors at the time of authorization, although any such new classes of shares may only be created with prior shareholder approval.
|
|
•
|
vote on a matter relating to the transaction;
|
|
•
|
attend a meeting of directors at which a matter relating to the transaction arises and be included among the directors present at the meeting for the purposes of a quorum; and
|
|
•
|
sign a document on behalf of the company, or do any other thing in his capacity as a director, that relates to the transaction.
|
|
•
|
banks and other financial institutions;
|
|
•
|
insurance companies;
|
|
•
|
regulated investment companies;
|
|
•
|
real estate investment trusts;
|
|
•
|
dealers and traders in securities that use mark-to-market accounting for U.S. federal income tax purposes;
|
|
•
|
U.S. Holders holding Class A ordinary shares as part of a hedging transaction, straddle, conversion transaction or other integrated transaction;
|
|
•
|
U.S. Holders whose functional currency for U.S. federal income tax purposes is not the U.S. dollar;
|
|
•
|
U.S. Holders liable for the alternative minimum tax;
|
|
•
|
tax-exempt organizations or entities, including an "individual retirement account" or "Roth IRA" as defined in Section 408 or 408A of the Code, respectively;
|
|
•
|
U.S. Holders that received the Class A ordinary shares as compensation for the performance of services;
|
|
•
|
U.S. Holders holding Class A ordinary shares that own or are deemed to own 10% or more of the voting shares of the Company; or
|
|
•
|
former citizens and residents of the United States subject to tax as expatriates.
|
|
•
|
a citizen or individual resident of the United States;
|
|
•
|
a corporation, or other entity taxable as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States, any state thereof or the District of Columbia;
|
|
•
|
an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or
|
|
•
|
a trust that (1) is subject to the primary supervision of a U.S. court and one or more U.S. persons that have the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable Treasury regulations to be treated as a U.S. person.
|
|
•
|
at least 75% of its gross income is "passive income"; or
|
|
•
|
at least 50% of the average quarterly value of its total gross assets (which may be determined, in part, by the market value of our ordinary shares, which is subject to change) is attributable to assets that produce "passive income" or are held for the production of passive income.
|
|
a)
|
Fair value of financial instruments
|
|
|
.
|
|
b)
|
Credit risk
|
|
a.
|
Cash– Cash is held with a major international financial institution in Canada and a major law firm in the USA and therefore the risk of loss is minimal.
|
|
b.
|
Other receivable – The Company is not exposed to major credit risk attributable to customers. A significant portion of this amount is prepaid to BPI under a master service agreement.
|
|
c)
|
Liquidity risk
|
|
March 31,
|
2014
|
2013
|
|
Audit fee
|
$45,000
|
-
|
|
Other services
|
2,413
|
-
|
|
(a)
|
Financial Statements
|
|
Description of Document
|
Page No.
|
|
Cover Sheet
|
|
|
Index
|
F1
|
|
Report of Independent Registered Public Accounting Firm
|
F2
|
|
Consolidated Statements of Financial Position
|
F3
|
|
Consolidated Statements of Operations and Comprehensive Loss
|
F4
|
|
Consolidated Statement of Shareholders Equity
Consolidated Statements of Cash Flows
|
F5-6
F7
|
|
Notes to Consolidated Financial Statements
|
F8-21
|
|
|
1.1
|
Certificate of Continuance -
Incorporated herein by reference
to Exhibit 3.1 to Form 6-K filed on August 1, 2013.
|
|
|
1.2
|
Memorandum and Articles of Association -
Incorporated herein by reference
to Exhibit 99.2 to Form 6-K filed on August 1, 2013.
|
|
|
4(c)1
|
Consulting Agreement dated April 1, 2005 with Kam Shah
Incorporated herein by reference
to Exhibit 4 (c) 1 to the Company’s Annual Report on Form 20-F for fiscal 2005 filed on September 28, 2005.
|
|
|
4(c) 2
|
Letter of April 1, 2010 extending consulting Agreement of Mr. Kam Shah to March 31, 2015.
Incorporated herein by reference
to Exhibit 4 (c) 2 to the Company’s registration statement on Form F-1 Amendment No. 2 filed on June 17, 2010.
|
|
|
4(c) (iv).1
|
2011 Consultant stock compensation plan -
Incorporated herein by reference
to Form S-8 filed on April 21, 2011.
|
|
|
4(c) (iv).2
|
2013 Stock option plan -
Incorporated herein by reference
to Form S-8 filed on December 19, 2013.
|
|
|
11.1
|
Charter of audit and compensation committee regarding compensation matters
|
|
|
11.2
|
Charter of audit and compensation committee regarding audit matters
|
|
|
11.3
|
Code of conduct
|
|
|
12.1
|
Certifications of Chief Executive Officer Pursuant to Rule 13a-14(a) or 15d-14(a) under the Securities Exchange Act of 1934, as amended.
|
|
|
12.2
|
Certifications of Chief Financial Officer Pursuant to Rule 13a-14(a) or 15d-14(a) under the Securities Exchange Act of 1934, as amended.
|
|
|
13.1
|
Certification of Chief Executive Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
13.2
|
Certification of Chief Financial Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|