|
FORM 20-F
|
|
o
|
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
|
||
|
Title of each class
|
Trading Symbol
|
|
Name of each exchange on which registered
|
|
|
American Depository Shares, each representing one ordinary share, nominal value
$0.000042
per share
|
AUTL
|
The Nasdaq Stock Market LLC
|
||
|
U.S. GAAP
x
|
International Financial Reporting Standards as issued
by the International Accounting Standards Board
o
|
Other
o
|
||
|
Page
|
||
|
PART I
|
||
|
ITEM 1.
|
IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
|
|
|
ITEM 2.
|
OFFER STATISTICS AND EXPECTED TIMETABLE
|
|
|
ITEM 3.
|
KEY INFORMATION
|
|
|
A. Selected financial data
|
||
|
B. Capitalization and indebtedness
|
||
|
C. Reasons for the offer and use of proceeds
|
||
|
D. Risk factors
|
||
|
ITEM 4.
|
INFORMATION ON THE COMPANY
|
|
|
A. History and development of the company
|
||
|
B. Business overview
|
||
|
C. Organizational structure
|
||
|
D. Property, plant and equipment
|
||
|
ITEM 4A.
|
UNRESOLVED STAFF COMMENTS
|
|
|
ITEM 5.
|
OPERATING AND FINANCIAL REVIEW AND PROSPECTS
|
|
|
A. Operating results
|
||
|
B. Liquidity and capital resources
|
||
|
C. Research and development, patents and licenses, etc.
|
||
|
D. Trend information
|
||
|
E. Off-balance sheet arrangements
|
||
|
F. Tabular disclosure of contractual obligations
|
||
|
G. Other disclosures
|
||
|
ITEM 6.
|
DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
|
|
|
A. Directors and senior management
|
||
|
B. Compensation
|
||
|
C. Board practices
|
||
|
D. Employees
|
||
|
E. Share ownership
|
||
|
ITEM 7.
|
MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
|
|
|
A. Major shareholders
|
||
|
B. Related party transactions
|
||
|
C. Interests of experts and counsel
|
||
|
ITEM 8.
|
FINANCIAL INFORMATION
|
|
|
A. Consolidated statements and other financial information
|
||
|
B. Significant changes
|
||
|
ITEM 9.
|
THE OFFER AND LISTING
|
|
|
A. Offer and listing details
|
||
|
B. Plan of distribution
|
||
|
C. Markets
|
||
|
D. Selling shareholders
|
||
|
E. Dilution
|
||
|
F. Expense of the issue
|
||
|
ITEM 10.
|
ADDITIONAL INFORMATION
|
|
|
A. Share capital
|
||
|
B. Memorandum and articles of association
|
||
|
C. Material contracts
|
||
|
D. Exchange controls
|
||
|
E. Taxation
|
||
|
F. Dividends and paying agents
|
||
|
G. Statement by experts
|
||
|
H. Documents on display
|
||
|
I. Subsidiary information
|
||
|
ITEM 11.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
|
|
ITEM 12.
|
DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
|
|
|
A. Debt securities
|
||
|
B. Warrants and rights
|
||
|
C. Other securities
|
||
|
D. American Depositary Shares
|
||
|
PART II
|
||
|
ITEM 13.
|
DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
|
|
|
ITEM 14.
|
MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
|
|
|
ITEM 15.
|
CONTROLS AND PROCEDURES
|
|
|
ITEM 16
|
[Reserved].
|
|
|
ITEM 16A
|
Audit committee financial expert
|
|
|
ITEM 16B
|
Code of ethics
|
|
|
ITEM 16C
|
Principal accountant fees and services
|
|
|
ITEM 16D
|
Exemptions from the listing standards for audit committees
|
|
|
ITEM 16E
|
Purchases of equity securities by the issuer and affiliated purchasers
|
|
|
ITEM 16F
|
Changes in registrant’s certifying accountant
|
|
|
ITEM 16G
|
Corporate governance
|
|
|
ITEM 16H
|
Mine safety disclosure
|
|
|
PART III
|
||
|
ITEM 17.
|
FINANCIAL STATEMENTS
|
|
|
ITEM 18.
|
FINANCIAL STATEMENTS
|
|
|
ITEM 19.
|
EXHIBITS
|
|
|
•
|
the development of our product candidates, including statements regarding the timing of initiation, completion and the outcome of clinical studies or trials and related preparatory work, the period during which the results of the trials will become available and our research and development programs;
|
|
•
|
our ability to advance our product candidates into, and successfully complete, clinical trials;
|
|
•
|
our ability to obtain and maintain regulatory approval of our product candidates in the indications for which we plan to develop them, and any related restrictions, limitations or warnings in the label of an approved drug or therapy;
|
|
•
|
our ability to license additional intellectual property relating to our product candidates from third parties and to comply with our existing license agreement;
|
|
•
|
our plans to research, develop, manufacture and commercialize our product candidates;
|
|
•
|
the timing of our regulatory filings for our product candidates, along with regulatory developments in the United States, European Union and other foreign countries;
|
|
•
|
the size and growth potential of the markets for our product candidates, if approved, and the rate and degree of market acceptance of our product candidates, including reimbursement that may be received from payors;
|
|
•
|
our ability to raise additional capital;
|
|
•
|
our commercialization, marketing and manufacturing capabilities and strategy;
|
|
•
|
our expectations regarding our ability to obtain and maintain intellectual property protection;
|
|
•
|
our ability to attract and retain qualified employees and key personnel;
|
|
•
|
our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately;
|
|
•
|
the scalability and commercial viability of our manufacturing methods and processes;
|
|
•
|
the success of competing therapies that are or may become available;
|
|
•
|
whether we are classified as a PFIC for current and future periods; and
|
|
•
|
our estimates regarding future expenses, revenues and needs for additional financing and the accuracy thereof.
|
|
Year Ended December 31,
|
Three Months Ended December 31,
|
Year Ended September 30,
|
||||||||||||||||||
|
2019
|
2018
|
2018
|
2017
|
2016
|
||||||||||||||||
|
(in thousands, except share and per share data)
|
||||||||||||||||||||
|
Comprehensive Loss Data:
|
||||||||||||||||||||
|
Grant income
|
$
|
2,908
|
|
$
|
296
|
|
$
|
1,407
|
|
$
|
1,693
|
|
$
|
1,212
|
|
|||||
|
Operating expenses:
|
||||||||||||||||||||
|
Research and development
|
(105,418
|
)
|
(17,713
|
)
|
(36,150
|
)
|
(16,012
|
)
|
(10,436
|
)
|
||||||||||
|
General and administrative
|
(39,452
|
)
|
(7,593
|
)
|
(22,790
|
)
|
(9,099
|
)
|
(5,152
|
)
|
||||||||||
|
Total operating expenses, net
|
(146,064
|
)
|
(25,010
|
)
|
(57,533
|
)
|
(23,418
|
)
|
(14,376
|
)
|
||||||||||
|
Other income (expense):
|
||||||||||||||||||||
|
Interest income
|
2,542
|
|
660
|
|
1,532
|
|
84
|
|
75
|
|
||||||||||
|
Other income (expense)
|
4,514
|
|
1,097
|
|
3,970
|
|
(46
|
)
|
(26
|
)
|
||||||||||
|
Total other income, net
|
7,056
|
|
1,757
|
|
5,502
|
|
38
|
|
49
|
|
||||||||||
|
Net loss before income tax
|
(139,008
|
)
|
(23,253
|
)
|
$
|
(52,031
|
)
|
(23,380
|
)
|
(14,327
|
)
|
|||||||||
|
Income tax benefit
|
15,159
|
|
2,605
|
|
7,280
|
|
3,653
|
|
1,777
|
|
||||||||||
|
Net loss attributable to ordinary shareholders
|
(123,849
|
)
|
(20,648
|
)
|
$
|
(44,751
|
)
|
$
|
(19,727
|
)
|
$
|
(12,550
|
)
|
|||||||
|
Other comprehensive income (loss):
|
||||||||||||||||||||
|
Foreign exchange translation adjustment
|
6,797
|
|
(5,568
|
)
|
(6,071
|
)
|
802
|
|
(2,942
|
)
|
||||||||||
|
Total comprehensive loss
|
(117,052
|
)
|
(26,216
|
)
|
(50,822
|
)
|
$
|
(18,925
|
)
|
$
|
(15,492
|
)
|
||||||||
|
Basic and diluted net loss per ordinary share
|
$
|
(2.88
|
)
|
$
|
(0.52
|
)
|
$
|
(1.42
|
)
|
$
|
(1.43
|
)
|
$
|
(1.26
|
)
|
|||||
|
As of December 31,
|
As of September 30,
|
|||||||||||||||||||
|
2019
|
2018
|
2018
|
2017
|
2016
|
||||||||||||||||
|
(in thousands)
|
||||||||||||||||||||
|
|
||||||||||||||||||||
|
Cash
|
$
|
210,643
|
|
$
|
217,450
|
|
$
|
247,089
|
|
$
|
137,070
|
|
$
|
28,059
|
|
|||||
|
Working capital
(1)
|
224,272
|
|
211,890
|
|
242,139
|
|
137,449
|
|
28,191
|
|
||||||||||
|
Net Assets
|
254,839
|
|
232,642
|
|
255,465
|
|
142,601
|
|
30,687
|
|
||||||||||
|
Total assets
|
303,533
|
|
254,210
|
|
273,205
|
|
148,662
|
|
34,180
|
|
||||||||||
|
Ordinary shares
|
2
|
|
2
|
|
2
|
|
1
|
|
—
|
|
||||||||||
|
Additional paid-in-capital
|
500,560
|
|
361,311
|
|
255,465
|
|
142,601
|
|
30,687
|
|
||||||||||
|
Ordinary Shares
|
44,983,006
|
|
40,145,617
|
|
40,146,182
|
|
29,962,742
|
|
13,921,544
|
|
||||||||||
|
D.
|
Risk factors.
|
|
•
|
continue our ongoing and planned research and development of our current programmed T cell product candidates for the treatment of hematological cancers and solid tumors;
|
|
•
|
initiate preclinical studies and clinical trials for any additional product candidates that we may pursue in the future, including our planned development of additional T cell therapies for the treatment of hematological cancers and solid tumors;
|
|
•
|
seek to discover and develop additional product candidates and further expand our clinical product pipeline;
|
|
•
|
seek regulatory approvals for any product candidates that successfully complete clinical trials;
|
|
•
|
continue to scale up internal and external manufacturing capacity with the aim of securing sufficient quantities to meet our capacity requirements for clinical trials and potential commercialization;
|
|
•
|
establish sales, marketing and distribution infrastructure to commercialize any product candidate for which we may obtain regulatory approval;
|
|
•
|
make required milestone and royalty payments to UCL Business plc, or UCLB, the technology-transfer company of University College London, or UCL, or other third parties, under license agreements pursuant to which we were granted some of our intellectual property rights;
|
|
•
|
develop, maintain, expand and protect our intellectual property portfolio;
|
|
•
|
acquire or in-license other product candidates and technologies;
|
|
•
|
hire additional clinical, quality control and manufacturing personnel;
|
|
•
|
add clinical, operational, financial and management information systems and personnel, including personnel to support our product development and planned future commercialization efforts;
|
|
•
|
expand our operations in the United States, Europe and other geographies; and
|
|
•
|
incur additional legal, accounting and other expenses associated with operating as a public company.
|
|
•
|
the progress, results and costs of laboratory testing, manufacturing, and preclinical and clinical development of our current and future product candidates;
|
|
•
|
the timing and amounts of any milestone or royalty payments we may be required to make under current or future license agreements;
|
|
•
|
the costs of leasing, building out, equipping, and operating the facilities necessary to research, develop, manufacture and commercialize our product candidates, as well as to support our continuing operations;
|
|
•
|
the costs of hiring additional clinical, quality control and manufacturing personnel;
|
|
•
|
the costs, timing and outcome of regulatory review of our product candidates;
|
|
•
|
the costs and timing of future commercialization activities, including product manufacturing, marketing, sales and distribution, for any of our product candidates for which we receive marketing approval;
|
|
•
|
the revenue, if any, received from commercial sales of our product candidates for which we receive marketing approval;
|
|
•
|
the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims; and
|
|
•
|
the costs of operating as a public company.
|
|
•
|
completing preclinical studies and receiving regulatory approvals or clearance for conducting clinical trials for our preclinical-stage programs;
|
|
•
|
obtaining positive results in our clinical trials demonstrating efficacy, safety, and durability of effect of our product candidates;
|
|
•
|
receiving approvals for commercialization of our product candidates from regulatory authorities;
|
|
•
|
manufacturing our product candidates at an acceptable cost; and
|
|
•
|
maintaining and growing an organization of scientists, medical professionals and business people who can develop and commercialize our products and technology.
|
|
•
|
obtaining regulatory approval for our product candidates, as the FDA, the EMA and other regulatory authorities have limited experience with programmed T cell therapies for cancer;
|
|
•
|
sourcing clinical and, if approved, commercial supplies of the materials used to manufacture our product candidates;
|
|
•
|
developing programming modules with the desired properties, while avoiding adverse reactions;
|
|
•
|
creating viral vectors capable of delivering multiple programming modules;
|
|
•
|
developing a reliable and consistent vector and cell manufacturing process;
|
|
•
|
establishing manufacturing capacity suitable for the manufacture of our product candidates in line with expanding enrollment in our clinical studies and our projected commercial requirements;
|
|
•
|
achieving cost efficiencies in the scale-up of our manufacturing capacity;
|
|
•
|
developing protocols for the safe administration of our product candidates;
|
|
•
|
educating medical personnel regarding our programmed T cell therapies and the potential side effect profile of each of our product candidates, such as potential adverse side effects related to cytokine release syndrome;
|
|
•
|
establishing integrated solutions in collaboration with specialty treatment centers in order to reduce the burdens and complex logistics commonly associated with the administration of T cell therapies;
|
|
•
|
establishing sales and marketing capabilities to successfully launch and commercialize our product candidates if and when we obtain any required regulatory approvals, and risks associated with gaining market acceptance of a novel therapy if we receive approval; and
|
|
•
|
obtaining coverage and adequate reimbursement from third-party payors for our novel and personalized therapies in connection with commercialization of any approved product candidates.
|
|
•
|
disagreement with the design, protocol or conduct of our clinical trials;
|
|
•
|
failure to demonstrate that a product candidate is safe and effective for its proposed indication;
|
|
•
|
failure of clinical trials to meet the level of statistical significance required for approval;
|
|
•
|
failure to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks;
|
|
•
|
disagreement with our interpretation of data from preclinical studies or clinical trials;
|
|
•
|
insufficiency of data collected from clinical trials of our product candidates to support the submission and filing of a Biologics License Application, or BLA, or other submission or to obtain regulatory approval;
|
|
•
|
failure to obtain approval of the manufacturing processes or our facilities;
|
|
•
|
changes in the approval policies or regulations that render our preclinical and clinical data insufficient for approval; or
|
|
•
|
lack of adequate funding to complete a clinical trial in a manner that is satisfactory to the applicable regulatory authority.
|
|
•
|
the patient eligibility criteria defined in the protocol;
|
|
•
|
the number of patients with the disease or condition being studied;
|
|
•
|
the perceived risks and benefits of the product candidate in the trial;
|
|
•
|
clinicians’ and patients’ perceptions as to the potential advantages of the product candidate being studied in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating or drugs that may be used off-label for these indications;
|
|
•
|
the size and nature of the patient population required for analysis of the trial’s primary endpoints;
|
|
•
|
the proximity of patients to study sites;
|
|
•
|
the design of the clinical trial;
|
|
•
|
our ability to recruit clinical trial investigators with the appropriate competencies and experience;
|
|
•
|
competing clinical trials for similar therapies or other new therapeutics not involving T cell-based immunotherapy;
|
|
•
|
our ability to obtain and maintain patient consents; and
|
|
•
|
the risk that patients enrolled in clinical trials will drop out of the clinical trials before completion of their treatment.
|
|
•
|
the FDA, the EMA or other comparable regulatory authority may disagree as to the number, design or implementation of our clinical trials, or may not interpret the results from clinical trials as we do;
|
|
•
|
regulators or institutional review boards may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site;
|
|
•
|
we may not reach agreement on acceptable terms with prospective clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different clinical trial sites;
|
|
•
|
clinical trials of our product candidates may produce negative or inconclusive results;
|
|
•
|
we may decide, or regulators may require us, to conduct additional clinical trials or abandon product development programs;
|
|
•
|
the number of patients required for clinical trials of our product candidates may be larger than we anticipate, enrollment in these clinical trials may be slower than we anticipate, participants may drop out of these clinical trials at a higher rate than we anticipate or we may fail to recruit suitable patients to participate in a trial;
|
|
•
|
our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all;
|
|
•
|
regulators may issue a clinical hold, or regulators or institutional review boards may require that we or our investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks;
|
|
•
|
the cost of clinical trials of our product candidates may be greater than we anticipate;
|
|
•
|
the FDA, the EMA or other comparable regulatory authorities may fail to approve our manufacturing processes or facilities;
|
|
•
|
the supply or quality of our product candidates or other materials necessary to conduct clinical trials of our product candidates may be insufficient or inadequate;
|
|
•
|
our product candidates may have undesirable side effects or other unexpected characteristics, particularly given their novel, first-in-human application, such as cytokine-induced toxicity and T cell aplasia, causing us or our investigators, regulators or institutional review boards to suspend or terminate the clinical trials; and
|
|
•
|
the approval policies or regulations of the FDA, the EMA or other comparable regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval.
|
|
•
|
the trial or trials required to verify the predicted clinical benefit of our product candidates fail to verify such benefit or do not demonstrate sufficient clinical benefit to justify the risks associated with the drug;
|
|
•
|
other evidence demonstrates that our product candidates are not shown to be safe or effective under the conditions of use;
|
|
•
|
we fail to conduct any required post-approval trial of our product candidates with due diligence; or
|
|
•
|
we disseminate false or misleading promotional materials relating to the relevant product candidate.
|
|
•
|
economic weakness, including inflation, or political instability in particular non-U.S. economies and markets;
|
|
•
|
differing and changing regulatory requirements for product approvals;
|
|
•
|
differing jurisdictions could present different issues for securing, maintaining or obtaining freedom to operate in such jurisdictions;
|
|
•
|
potentially reduced protection for intellectual property rights;
|
|
•
|
difficulties in compliance with different, complex and changing laws, regulations and court systems of multiple jurisdictions and compliance with a wide variety of foreign laws, treaties and regulations;
|
|
•
|
changes in non-U.S. regulations and customs, tariffs and trade barriers;
|
|
•
|
changes in non-U.S. currency exchange rates of the pound sterling, U.S. dollar, euro and currency controls;
|
|
•
|
changes in a specific country’s or region’s political or economic environment, including the implications of the United Kingdom's withdrawal from the European Union;
|
|
•
|
trade protection measures, import or export licensing requirements or other restrictive actions by governments;
|
|
•
|
differing reimbursement regimes and price controls in certain non-U.S. markets;
|
|
•
|
negative consequences from changes in tax laws;
|
|
•
|
compliance with tax, employment, immigration and labor laws for employees living or traveling abroad, including, for example, the variable tax treatment in different jurisdictions of options granted under our share option schemes or equity incentive plans;
|
|
•
|
workforce uncertainty in countries where labor unrest is more common than in the United States;
|
|
•
|
litigation or administrative actions resulting from claims against us by current or former employees or consultants individually or as part of class actions, including claims of wrongful terminations, discrimination, misclassification or other violations of labor law or other alleged conduct;
|
|
•
|
difficulties associated with staffing and managing international operations, including differing labor relations;
|
|
•
|
production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and
|
|
•
|
business interruptions resulting from geo-political actions, including war and terrorism, natural disasters--including earthquakes, typhoons, floods and fires--or health epidemics, such as the recent outbreak of the Coronavirus first identified in Wuhan, Hubei Province, China.
|
|
•
|
identifying, recruiting, integrating, maintaining and motivating additional employees;
|
|
•
|
managing our internal development efforts effectively, including the clinical, FDA and EMA review processes for our product candidates; and
|
|
•
|
improving our operational, financial and management controls, reporting systems and procedures.
|
|
•
|
increased operating expenses and cash requirements;
|
|
•
|
the assumption of additional indebtedness or contingent liabilities;
|
|
•
|
assimilation of operations, intellectual property and products of an acquired company, including difficulties associated with integrating new personnel;
|
|
•
|
the diversion of our management’s attention from our existing programs and initiatives in pursuing such a strategic partnership, merger or acquisition;
|
|
•
|
retention of key employees, the loss of key personnel and uncertainties in our ability to maintain key business relationships;
|
|
•
|
risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing products or product candidates and regulatory approvals; and
|
|
•
|
our inability to generate revenue from acquired technology sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs.
|
|
•
|
the scope of rights granted under the license agreement and other interpretation-related issues;
|
|
•
|
whether and the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement;
|
|
•
|
our rights to third parties;
|
|
•
|
our diligence obligations with respect to the use of the licensed technology in relation to our development and commercialization of our product candidates, and what activities satisfy those diligence obligations;
|
|
•
|
the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us;
|
|
•
|
our right to transfer or assign the license; and
|
|
•
|
the effects of termination.
|
|
•
|
litigation involving patients taking our products;
|
|
•
|
restrictions on such products, manufacturers or manufacturing processes;
|
|
•
|
restrictions on the labeling or marketing of a product;
|
|
•
|
restrictions on product distribution or use;
|
|
•
|
requirements to conduct post-marketing studies or clinical trials;
|
|
•
|
warning or untitled letters;
|
|
•
|
withdrawal of the products from the market;
|
|
•
|
refusal to approve pending applications or supplements to approved applications that we submit;
|
|
•
|
recall of products;
|
|
•
|
fines, restitution or disgorgement of profits or revenues;
|
|
•
|
suspension or withdrawal of marketing approvals;
|
|
•
|
suspension of any ongoing clinical trials;
|
|
•
|
damage to relationships with any potential collaborators;
|
|
•
|
unfavorable press coverage and damage to our reputation;
|
|
•
|
refusal to permit the import or export of our products;
|
|
•
|
product seizure; or
|
|
•
|
injunctions or the imposition of civil or criminal penalties.
|
|
•
|
the U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, lease, order or recommendation of, any good, facility, item or service, for which payment may be made, in whole or in part, under federal and state healthcare programs such as Medicare and Medicaid. The term ‘‘remuneration’’ has been broadly interpreted to include anything of value. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other hand. Although there are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, the exceptions and safe harbors are drawn narrowly, and practices that involve remuneration that are alleged to be intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the U.S. federal Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all its facts and circumstances. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the U.S. federal Anti-Kickback Statute has been violated;
|
|
•
|
U.S. federal civil and criminal false claims laws, including the U.S. federal False Claims Act, which can be enforced though civil whistleblower or qui tam actions, and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government. Pharmaceutical and other healthcare companies have been prosecuted under these laws for, among other things, allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. In addition, certain marketing practices, including off-label promotion, may also violate false claims laws. Further, pharmaceutical manufacturers can be held liable under the U.S. federal False Claims Act even when they do not submit claims directly to government payors if they are deemed to “cause” the submission of false or fraudulent claims;
|
|
•
|
the U.S. federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of whether the payor is public or private, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters;
|
|
•
|
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, which impose obligations on “covered entities,” including certain healthcare providers, health plans, and healthcare clearinghouses, as well as their respective “business associates” that create, receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity, with respect to safeguarding the privacy, security and transmission of individually identifiable health information. Additionally, HITECH also created four new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in U.S. federal courts to enforce HIPAA and seek attorneys’ fees and costs associated with pursuing federal civil actions;
|
|
•
|
the FDCA, which prohibits, among other things, the adulteration or misbranding of drugs, biologics and medical devices;
|
|
•
|
the U.S. federal Physician Payments Sunshine Act, created under Section 6002 of Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, the ACA, and its implementing regulations, created annual reporting requirements for certain manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain
|
|
•
|
analogous state laws and regulations and foreign laws, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; state and foreign laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or to adopt compliance programs as prescribed by state laws and regulations, or that otherwise restrict payments that may be made to healthcare providers; state and foreign laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; state and local laws that require the registration of pharmaceutical sales representatives; and state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts; and
|
|
•
|
similar healthcare laws and regulations in the European Union and other jurisdictions, including reporting requirements detailing interactions with and payments to healthcare providers and laws governing the privacy and security of certain protected information, such as GDPR, which imposes obligations and restrictions on the collection and use of personal data relating to individuals located in the European Union (including health data).
|
|
•
|
the clinical indications for which our product candidates are approved;
|
|
•
|
physicians, hospitals, cancer treatment centers, and patients considering our product candidates as a safe and effective treatment;
|
|
•
|
hospitals and cancer treatment centers establishing the infrastructure required for the administration of redirected T cell therapies;
|
|
•
|
the potential and perceived advantages of our product candidates over alternative treatments;
|
|
•
|
the prevalence and severity of any side effects;
|
|
•
|
product labeling or product insert requirements of the FDA, the EMA or other regulatory authorities;
|
|
•
|
limitations or warnings contained in the labeling approved by the FDA or the EMA;
|
|
•
|
the timing of market introduction of our product candidates as well as competitive products;
|
|
•
|
the cost of treatment in relation to alternative treatments;
|
|
•
|
the amount of upfront costs or training required for physicians to administer our product candidates;
|
|
•
|
the availability of coverage, adequate reimbursement, and pricing by third-party payors and government authorities;
|
|
•
|
the willingness of patients to pay out-of-pocket in the absence of comprehensive coverage and adequate reimbursement by third-party payors and government authorities;
|
|
•
|
relative convenience and ease of administration, including as compared to alternative treatments and competitive therapies; and
|
|
•
|
the effectiveness of our sales and marketing efforts and distribution support.
|
|
•
|
reduced resources of our management to pursue our business strategy;
|
|
•
|
decreased demand for any product candidates or products that we may develop;
|
|
•
|
injury to our reputation and significant negative media attention;
|
|
•
|
withdrawal of clinical trial participants;
|
|
•
|
initiation of investigations by regulators;
|
|
•
|
product recalls, withdrawals or labeling, marketing or promotional restrictions;
|
|
•
|
significant costs to defend the resulting litigation;
|
|
•
|
substantial monetary awards paid to clinical trial participants or patients;
|
|
•
|
loss of revenue; and
|
|
•
|
the inability to commercialize any products that we may develop.
|
|
•
|
the commencement, enrollment or results of our planned and future clinical trials;
|
|
•
|
positive or negative results from, or delays in, testing and clinical trials by us, collaborators or competitors;
|
|
•
|
the loss of any of our key scientific or management personnel;
|
|
•
|
regulatory or legal developments in the United States, United Kingdom and other countries;
|
|
•
|
the success of competitive products or technologies;
|
|
•
|
adverse actions taken by regulatory agencies with respect to our clinical trials or manufacturers;
|
|
•
|
changes or developments in laws or regulations applicable to our product candidates and preclinical program;
|
|
•
|
changes to our relationships with collaborators, manufacturers or suppliers;
|
|
•
|
concerns regarding the safety of our product candidates or programmed T cells in general;
|
|
•
|
announcements concerning our competitors or the pharmaceutical industry in general;
|
|
•
|
actual or anticipated fluctuations in our operating results;
|
|
•
|
changes in financial estimates or recommendations by securities analysts;
|
|
•
|
potential acquisitions, financing, collaborations or other corporate transactions;
|
|
•
|
the results of our efforts to discover, develop, acquire or in-license additional product candidates;
|
|
•
|
the trading volume of our ADSs on The Nasdaq Global Select Market;
|
|
•
|
sales of our ADSs or ordinary shares by us, members of our senior management and directors or our shareholders or the anticipation that such sales may occur in the future;
|
|
•
|
general economic, political, and market conditions and overall fluctuations in the financial markets in the United States or the United Kingdom;
|
|
•
|
price and volume fluctuations of the listed securities comparable companies and, in particular, those that operate in the biopharmaceutical industry;
|
|
•
|
investors’ general perception of us and our business; and
|
|
•
|
other events and factors, many of which are beyond our control.
|
|
A.
|
History and development of the company.
|

|
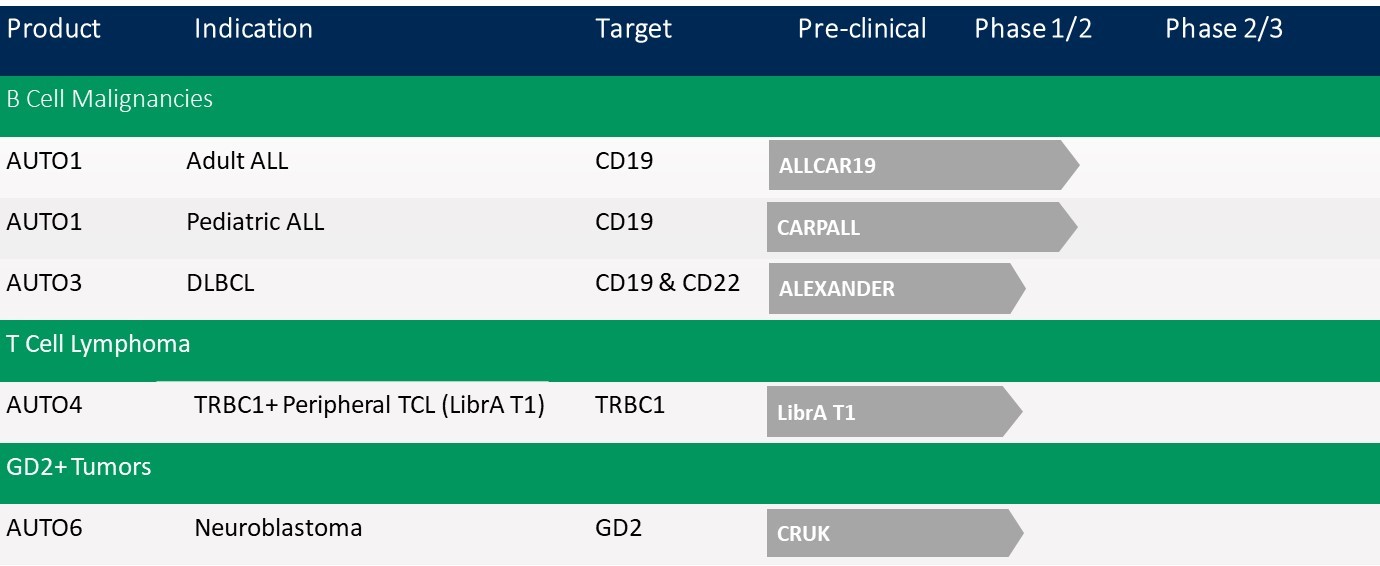
AUTO1:
|
a CD19-targeting programmed T cell therapy with a CD19 binder designed to improve the efficacy and safety profile of AUTO1 compared to the first generation CAR-T products approved for commercial sale. In December 2019, we announced updated data that support the anti-leukemia activity of AUTO1 in the absence of severe cytokine release syndrome, or severe CRS, in two ongoing Phase 1 trials of both pediatric and adult patients with relapsed or refractory acute B lymphocytic leukemia (ALL). We expect to initiate a Phase 2 clinical trial of AUTO1 for the treatment of adult ALL in 2020. This trial may potentially be a registrational trial. In addition, we will pursue a pediatric label through a pediatric investigational program. Further, we will also commence in 2020 a Phase 1 study in pediatric relapsed or refractory acute B lymphocytic leukemia with our next generation product candidate, AUTO1NG.
|
|
AUTO3:
|
the first dual-targeting, bicistronic, or having two chimeric antigen receptors within one vector, programmed T cell therapy for the treatment of relapsed or refractory diffuse large B-cell lymphoma, or r/r DLBCL, and pediatric ALL, independently targeting B-lymphocyte antigens CD19 and CD22.
|
|
AUTO4:
|
a programmed T cell therapy for the treatment of peripheral T-cell lymphoma targeting TRBC1. We initiated a Phase 1/2 clinical trial in the fourth quarter of 2018 and we expect to report Phase 1 interim data in the fourth quarter of 2020.
|
|
AUTO6:
|
a programmed T cell therapy targeting GD2 in development for the treatment of neuroblastoma. A Phase 1 clinical trial with AUTO6 is being sponsored and conducted by Cancer Research UK, or CRUK, and preliminary data has shown initial anti-tumor activity in this solid tumor indication. We are developing a next-generation product candidate, which we refer to as AUTO6NG, incorporating additional programming modules designed to improve the efficacy, safety and persistence of AUTO6. We presented preclinical data of AUTO6NG in November 2019. We expect to initiate the first of two planned Phase 1/2 clinical trials of AUTO6NG in the second half of 2020.
|
|
•
|
completion of preclinical laboratory tests and animal studies according to Good Laboratory Practices, or GLPs, and applicable requirements for the humane use of laboratory animals or other applicable regulations;
|
|
•
|
submission to the FDA of an Investigational New Drug Application, or IND, which must become effective before human clinical trials may begin;
|
|
•
|
performance of adequate and well-controlled human clinical trials according to the FDA’s regulations commonly referred to as Good Clinical Practices, or GCPs, and any additional requirements for the protection of human research subjects and their health information, to establish the safety and efficacy of the proposed biological product for its intended use;
|
|
•
|
preparation and submission to the FDA of a Biologics License Application, or BLA, for marketing approval that includes substantive evidence of safety, purity, and potency from results of nonclinical testing and clinical trials;
|
|
•
|
satisfactory completion of one or more FDA inspections of the manufacturing facility or facilities where the biological product is produced to assess compliance with cGMP to assure that the facilities, methods and controls used in product manufacture are adequate to preserve the biological product’s identity, strength, quality and purity and, if applicable, the FDA’s current Good Tissue Practices, or GTPs, for the use of human cellular and tissue products;
|
|
•
|
potential FDA audit of the nonclinical study and clinical trial sites that generated the data in support of the BLA;
|
|
•
|
payment of user fees for FDA review of the BLA; and
|
|
•
|
FDA acceptance, review and approval, or licensure, of the BLA, which might include review by an advisory committee, a panel typically consisting of independent clinicians and other experts who provide recommendations as to whether the application should be approved and under what conditions.
|
|
•
|
Phase 1
. The biological product is initially introduced into healthy human subjects and tested for safety. In the case of some products for severe or life-threatening diseases, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients with the target disease or condition.
|
|
•
|
Phase 2
. The biological product is evaluated in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance, optimal dosage and dosing schedule.
|
|
•
|
Phase 3
. Clinical trials are undertaken to further evaluate dosage, clinical efficacy, potency, and safety in an expanded patient population, generally at geographically dispersed clinical trial sites. These clinical trials are intended to generate enough
|
|
•
|
an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drug agents or biologic agents, which is apportioned among these entities according to their market share in certain government healthcare programs;
|
|
•
|
an increase in the rebates a manufacturer must pay under the Medicaid Drug Rebate Program to 23.1% and 13% of the average manufacturer price for branded and generic drugs, respectively;
|
|
•
|
a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% (70% commencing January 1, 2019) point-of-sale discounts to negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D;
|
|
•
|
extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations, unless the drug is subject to discounts under the 340B drug discount program;
|
|
•
|
a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected;
|
|
•
|
expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for certain individuals with income at or below 133% of the federal poverty level, thereby potentially increasing manufacturers’ Medicaid rebate liability;
|
|
•
|
expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program;
|
|
•
|
new requirements under the federal Physician Payments Sunshine Act for drug manufacturers to report information related to payments and other transfers of value made to physicians and teaching hospitals as well as ownership or investment interests held by physicians and their immediate family members;
|
|
•
|
a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research;
|
|
•
|
establishment of a Center for Medicare and Medicaid Innovation at CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending; and
|
|
•
|
a licensure framework for follow on biologic products.
|
|
•
|
the applicant must complete an identified program of studies within a time period specified by the competent authority, the results of which form the basis of a reassessment of the benefit/risk profile;
|
|
•
|
the medicinal product in question may be supplied on medical prescription only and may in certain cases be administered only under strict medical supervision, possibly in a hospital and in the case of a radiopharmaceutical, by an authorized person; and
|
|
•
|
the package leaflet and any medical information must draw the attention of the medical practitioner to the fact that the particulars available concerning the medicinal product in question are as yet inadequate in certain specified respects.
|
|
A.
|
Operating results
.
|
|
•
|
expenses incurred under agreements with contract research organizations, or CROs, as well as investigative sites and consultants that conduct our clinical trials, preclinical studies and other scientific development services;
|
|
•
|
manufacturing scale-up expenses and the cost of acquiring and manufacturing preclinical and clinical trial materials;
|
|
•
|
employee-related expenses, including salaries, related benefits, travel and share-based compensation expense for employees engaged in research and development functions;
|
|
•
|
expenses incurred for outsourced professional scientific development services;
|
|
•
|
costs for laboratory materials and supplies used to support our research activities;
|
|
•
|
allocated facilities costs, depreciation and other expenses, which include rent and utilities; and
|
|
•
|
upfront, milestone and management fees for maintaining licenses under our third-party licensing agreements.
|
|
Year Ended December 31,
|
2019 - 2018
|
||||||||||
|
(unaudited)
|
|||||||||||
|
2019
|
2018
|
Change
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Direct research and development expenses by program:
|
|||||||||||
|
B cell malignancies (AUTO1 & AUTO3)
|
$
|
15,346
|
|
5,436
|
|
$
|
9,910
|
|
|||
|
T cell lymphoma (AUTO4 & AUTO 5)
|
1,495
|
|
1,234
|
|
261
|
|
|||||
|
Multiple myeloma (AUTO2 & AUTO8)
|
846
|
|
2,586
|
|
(1,740
|
)
|
|||||
|
Solid Tumors (AUTO6 & AUTO7)
|
2,027
|
|
178
|
|
1,849
|
|
|||||
|
Total direct research and development expense
|
19,714
|
|
9,434
|
|
10,280
|
|
|||||
|
Research and discovery and unallocated costs:
|
|||||||||||
|
Personnel related (including share-based compensation)
|
54,187
|
|
21,453
|
|
32,734
|
|
|||||
|
Indirect research and development expense
|
31,517
|
|
17,412
|
|
14,105
|
|
|||||
|
Total research and development expenses
|
$
|
105,418
|
|
48,299
|
|
$
|
57,119
|
|
|||
|
•
|
the scope, progress, outcome and costs of our clinical trials and other research and development activities, including establishing an appropriate safety profile with IND-directed studies;
|
|
•
|
successful patient enrollment in, and the initiation and completion of, clinical trials;
|
|
•
|
the timing, receipt and terms of any marketing approvals from applicable regulatory authorities;
|
|
•
|
establishing commercial manufacturing capabilities or making arrangements with third-party manufacturers;
|
|
•
|
development and timely delivery of commercial-grade drug formulations that can be used in our clinical trials and for commercial manufacturing;
|
|
•
|
obtaining, maintaining, defending and enforcing patent claims and other intellectual property rights;
|
|
•
|
significant and changing government regulation;
|
|
•
|
launching commercial sales of our product candidates, if and when approved, whether alone or in collaboration with others;
|
|
•
|
maintaining a continued acceptable safety profile of the product candidates following approval; and
|
|
•
|
significant competition and rapidly changing technologies within the biopharmaceutical industry.
|
|
Year Ended
|
|||||||||||
|
(unaudited)
|
|||||||||||
|
December 31, 2019
|
December 31, 2018
|
Change
|
|||||||||
|
Grant income
|
$
|
2,908
|
|
$
|
1,472
|
|
$
|
1,436
|
|
||
|
Operating expenses:
|
|||||||||||
|
Research and development
|
(105,418
|
)
|
(48,299
|
)
|
(57,119
|
)
|
|||||
|
General and administrative
|
(39,452
|
)
|
(27,299
|
)
|
(12,153
|
)
|
|||||
|
Loss on impairment of leasehold improvements
|
(4,102
|
)
|
—
|
|
(4,102
|
)
|
|||||
|
Total operating expenses, net
|
(146,064
|
)
|
(74,126
|
)
|
(71,938
|
)
|
|||||
|
Other income (expense):
|
|||||||||||
|
Interest income
|
2,542
|
|
2,011
|
|
531
|
|
|||||
|
Other income (expense)
|
4,514
|
|
5,752
|
|
(1,238
|
)
|
|||||
|
Total other income, net
|
7,056
|
|
7,763
|
|
(707
|
)
|
|||||
|
Net loss before income tax
|
(139,008
|
)
|
(66,363
|
)
|
(72,645
|
)
|
|||||
|
Income tax benefit
|
15,159
|
|
8,488
|
|
6,671
|
|
|||||
|
Net loss attributable to ordinary shareholders
|
$
|
(123,849
|
)
|
$
|
(57,875
|
)
|
$
|
(65,974
|
)
|
||
|
Year Ended December 31,
|
|||||||
|
(unaudited)
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Net cash used in operating activities
|
$
|
(101,484
|
)
|
$
|
(42,525
|
)
|
|
|
Net cash used in investing activities
|
(18,668
|
)
|
(13,189
|
)
|
|||
|
Net cash provided by financing activities
|
108,863
|
|
156,487
|
|
|||
|
Effect of exchange rate changes on cash
|
5,164
|
|
(12,202
|
)
|
|||
|
Net (decrease) increase in cash
|
$
|
(6,125
|
)
|
$
|
88,571
|
|
|
|
December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
(in thousands)
|
|||||||
|
Total cash held
|
$
|
210,643
|
|
$
|
217,450
|
|
|
|
U.S. dollars
|
$
|
29,800
|
|
$
|
49,000
|
|
|
|
British sterling
|
£
|
136,900
|
|
£
|
132,100
|
|
|
|
•
|
seek regulatory approvals for any product candidates that successfully complete preclinical and clinical trials;
|
|
•
|
establish a sales, marketing and distribution infrastructure in anticipation of commercializing of any product candidates for which we may obtain marketing approval and intend to commercialize on our own or jointly;
|
|
•
|
hire additional clinical, medical, and development personnel;
|
|
•
|
expand our infrastructure and facilities to accommodate our growing employee base; and
|
|
•
|
maintain, expand and protect our intellectual property portfolio.
|
|
•
|
the scope, progress, outcome and costs of our clinical trials and other research and development activities;
|
|
•
|
the costs, timing, receipt and terms of any marketing approvals from applicable regulatory authorities;
|
|
•
|
the costs of future activities, including product sales, medical affairs, marketing, manufacturing and distribution, for any of our product candidates for which we receive marketing approval;
|
|
•
|
the revenue, if any, received from commercial sale of our products, should any of our product candidates receive marketing approval;
|
|
•
|
the costs and timing of hiring new employees to support our continued growth;
|
|
•
|
the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims; and
|
|
•
|
the extent to which we in-license or acquire additional product candidates or technologies.
|
|
C.
|
Research and development, patents and licenses, etc.
|
|
Payments Due by Period
|
|||||||||||||||||||||
|
Total
|
Less than 1 Year
|
1 to 3
Years |
4 to 5 Years
|
More than
5 Years |
|||||||||||||||||
|
Operating lease obligations
(1)
|
$
|
34,435
|
|
$
|
4,486
|
|
$
|
9,499
|
|
$
|
8,228
|
|
$
|
12,222
|
|
||||||
|
(1) Amounts in the table reflect minimum payments due for our leases of office, laboratory and manufacturing space and payments required to reimburse the landlord for leasehold improvements related to operating leases.
|
|||||||||||||||||||||
|
A.
|
Directors and senior management.
|
|
NAME
|
AGE
|
POSITION(S)
|
||
|
Senior Management:
|
||||
|
Christian Itin, Ph.D.
|
55
|
Chief Executive Officer and Chairman of the Board of Directors
|
||
|
Andrew Oakley
|
57
|
Senior Vice President, Chief Financial Officer
|
||
|
Martin Pulé, MBBS
|
47
|
Senior Vice President, Founder, Chief Scientific Officer and Director
|
||
|
Muhammad Al-Hajj, Ph.D.
|
49
|
Senior Vice President, Translational Sciences
|
||
|
Vijay Peddareddigari, M.D.
|
48
|
Senior Vice President, Chief Medical Officer
|
||
|
Christopher Vann
|
55
|
Senior Vice President, Chief Operating Officer
|
||
|
Matthias Alder
|
54
|
Senior Vice President, Chief Business Officer and Company Secretary
|
||
|
David Brochu
|
63
|
Senior Vice President, Product Delivery
|
||
|
Adam Hacker
|
50
|
Senior Vice President for Regulatory Affairs and Quality
|
||
|
Non-Executive Directors:
|
||||
|
Joseph Anderson, Ph.D.
|
60
|
Director
|
||
|
Linda Bain
|
49
|
Director
|
||
|
John Berriman
|
71
|
Director
|
||
|
Cynthia Butitta
|
65
|
Director
|
||
|
Kapil Dhingra, M.D.
|
60
|
Director
|
||
|
Martin Murphy, Ph.D.
|
51
|
Director
|
||
|
Name
|
Salary/Fees
|
Annual Bonus
|
Pension
Benefit
|
All Other
Compensation
|
Total
|
||||||||||
|
Christian Itin, Ph.D.
Executive Director
|
£
|
390,000
|
|
£
|
160,875
|
|
£
|
1,997
|
|
£
|
3,426,497
|
|
£
|
3,979,369
|
|
|
Joseph Anderson, Ph.D.
Non-Executive Director
|
£
|
39,000
|
|
£
|
—
|
|
£
|
—
|
|
£
|
342,420
|
|
£
|
381,420
|
|
|
Linda Bain
Non-Executive Director
|
£
|
45,000
|
|
£
|
—
|
|
£
|
—
|
|
£
|
465,986
|
|
£
|
510,986
|
|
|
John Berriman
Non-Executive Director
|
£
|
51,000
|
|
£
|
—
|
|
£
|
—
|
|
£
|
389,720
|
|
£
|
440,720
|
|
|
Cynthia Butitta
Non-Executive Director
|
£
|
40,500
|
|
£
|
—
|
|
£
|
—
|
|
£
|
420,630
|
|
£
|
461,130
|
|
|
Kapil Dhingra, M.D.
Non-Executive Director
|
£
|
36,000
|
|
£
|
—
|
|
£
|
—
|
|
£
|
389,720
|
|
£
|
425,720
|
|
|
Martin Murphy, Ph.D.
Non-Executive Director
|
£
|
34,500
|
|
£
|
—
|
|
£
|
—
|
|
£
|
342,421
|
|
£
|
376,921
|
|
|
Annual Cash Retainer
(
£)
|
||
|
Annual retainer
|
30,000
|
|
|
Additional retainer for lead independent director
|
12,000
|
|
|
Additional retainer for audit committee chair
|
12,000
|
|
|
Additional retainer for audit committee member
|
6,000
|
|
|
Additional retainer for compensation committee chair
|
9,000
|
|
|
Additional retainer for compensation committee member
|
4,500
|
|
|
Additional retainer for nominating and governance committee chair
|
6,000
|
|
|
Additional retainer for nominating and governance committee member
|
3,000
|
|
|
Additional retainer for research and development committee chair (effective 1 Jan 2020)
|
12,000
|
|
|
Name
|
Ordinary Share Underlying Option
|
Exercise Price
|
Grant
Date
|
Expiration
Date
|
|||
|
Senior Management
|
|||||||
|
Christian Itin, Ph.D.
|
300,000
|
|
$
|
13.00
|
|
12/12/2019
|
12/12/2029
|
|
Andrew Oakley
|
90,000
|
|
12.65
|
|
12/13/2019
|
12/13/2029
|
|
|
Martin Pulé, MBBS
|
90,000
|
|
12.65
|
|
12/13/2019
|
12/13/2029
|
|
|
Muhammad Al-Hajj, Ph.D.
|
80,000
|
|
12.65
|
|
12/13/2019
|
12/13/2029
|
|
|
Vijay Peddareddigari, M.D.
|
90,000
|
|
12.65
|
|
12/13/2019
|
12/13/2029
|
|
|
Christopher Vann
|
110,000
|
|
12.65
|
|
12/13/2019
|
12/13/2029
|
|
|
Matthias Alder
|
90,000
|
|
12.65
|
|
12/13/2019
|
12/13/2029
|
|
|
Adam Hacker
|
100,000
|
|
12.65
|
|
12/13/2019
|
12/13/2029
|
|
|
David Brochu
|
35,000
|
|
24.70
|
|
3/15/2019
|
3/15/2029
|
|
|
David Brochu
|
100,000
|
|
12.35
|
|
10/7/2019
|
10/7/2029
|
|
|
David Brochu
|
50,000
|
|
12.65
|
|
12/13/2019
|
12/13/2029
|
|
|
Non-Executive Directors
|
|
|
|
|
|||
|
Joseph Anderson, Ph.D.
|
25,000
|
|
30.24
|
|
3/28/2019
|
3/28/2029
|
|
|
Linda Bain
|
25,000
|
|
30.24
|
|
3/28/2019
|
3/28/2029
|
|
|
John Berriman
|
25,000
|
|
30.24
|
|
3/28/2019
|
3/28/2029
|
|
|
Cynthia Butitta
|
25,000
|
|
30.24
|
|
3/28/2019
|
3/28/2029
|
|
|
Kapil Dhingra, M.D.
|
25,000
|
|
30.24
|
|
3/28/2019
|
3/28/2029
|
|
|
Martin Murphy, Ph.D.
|
25,000
|
|
30.24
|
|
3/28/2019
|
3/28/2029
|
|
|
•
|
Class I, which consists of Joseph Anderson and Martin Murphy, whose terms will expire at our 2022 annual general meeting;
|
|
•
|
Class II, which consists of John Berriman and Kapil Dhingra, whose terms will expire at our 2020 annual general meeting; and
|
|
•
|
Class III, which consists of Christian Itin, Cynthia Butitta and Linda Bain, whose terms will expire at our 2021 annual general meeting.
|
|
•
|
recommending the appointment of the independent auditor to shareholders for approval at the general meeting of shareholders;
|
|
•
|
the appointment, compensation, retention and oversight of any accounting firm engaged for the purpose of preparing or issuing an audit report or performing other audit services;
|
|
•
|
pre-approving the audit services and non-audit services to be provided by our independent auditor before the auditor is engaged to render such services;
|
|
•
|
evaluating the independent auditor’s qualifications, performance and independence, and presenting its conclusions to the full board of directors on at least an annual basis;
|
|
•
|
reviewing and discussing with management and our independent registered public accounting firm our financial statements and our financial reporting process; and
|
|
•
|
reviewing, approving or ratifying any related party transactions.
|
|
•
|
identifying, reviewing, overseeing and proposing policies relevant to the compensation and benefits of our directors and senior management;
|
|
•
|
evaluating the performance of senior management in light of such policies and reporting to the board; and
|
|
•
|
overseeing and administering our share option plan, equity incentive plan and other benefit plans in operation from time to time.
|
|
•
|
drawing up selection criteria and appointment procedures for directors;
|
|
•
|
recommending nominees for appointment to our board of directors and its corresponding committees; and
|
|
•
|
assessing the functioning of individual members of our board of directors and management and reporting the results of such assessment to the full board of directors.
|
|
•
|
overseeing the Company's scientific, technical, research and development strategy, and the implementation thereof;
|
|
•
|
advising our board of directors and management regarding program prioritization, clinical development strategy, regulatory strategy and interactions, intellectual property, product manufacture and supply, and related matters; and
|
|
•
|
reviewing and assessing business development opportunities related to research collaborations, licensing or strategic transactions.
|
|
At December 31,
|
At September 30,
|
||||||
|
2019
|
2018
|
2018
|
2017
|
||||
|
Function:
|
|||||||
|
Administrative
|
43
|
38
|
33
|
14
|
|||
|
Research and development
|
247
|
162
|
133
|
86
|
|||
|
Total
|
290
|
200
|
166
|
100
|
|||
|
Geography:
|
|||||||
|
United Kingdom
|
251
|
177
|
153
|
99
|
|||
|
Germany and Switzerland
|
6
|
3
|
2
|
1
|
|||
|
United States
|
33
|
20
|
11
|
-
|
|||
|
•
|
each beneficial owner of 5% or more of our outstanding ordinary shares;
|
|
•
|
each of our directors and each member of our senior management; and
|
|
•
|
all of our directors and senior management as a group.
|
|
NAME OF BENEFICIAL OWNER
|
Number of Ordinary Shares Beneficially Owned (#)
|
Percent of Ordinary Shares Beneficially Owned (%)
|
||||
|
5% or Greater Shareholders:
|
||||||
|
Syncona Portfolio Limited (1)
|
15,955,734
|
|
30.6
|
%
|
||
|
PPF Capital Partners Fund B.V. (2)
|
10,955,724
|
|
21.0
|
%
|
||
|
Arix Bioscience Holdings Limited (3)
|
3,369,868
|
|
6.5
|
%
|
||
|
Senior Management and Directors:
|
||||||
|
Christian Itin, Ph.D. (4)
|
1,234,689
|
|
2.4
|
%
|
||
|
Andrew Oakley (5)
|
144,235
|
|
*
|
|
||
|
Martin Pulé, MBBS (6)
|
751,600
|
|
1.4
|
%
|
||
|
Muhammad Al-Hajj, Ph.D. (7)
|
103,221
|
|
*
|
|
||
|
Vijay Peddareddigari, M.D. (8)
|
236,556
|
|
*
|
|
||
|
Christopher Vann (9)
|
194,477
|
|
*
|
|
||
|
Matthias Alder (10)
|
207,854
|
|
*
|
|
||
|
Adam Hacker (11)
|
55,996
|
|
*
|
|
||
|
David Brochu
|
0
|
|
*
|
|
||
|
Joseph Anderson, Ph.D. (12)
|
3,394,868
|
|
6.5
|
%
|
||
|
Linda Bain (13)
|
38,736
|
|
*
|
|
||
|
John Berriman (14)
|
169,506
|
|
*
|
|
||
|
Cynthia Butitta (15)
|
48,547
|
|
*
|
|
||
|
Kapil Dhingra, M.D. (16)
|
106,712
|
|
*
|
|
||
|
Martin Murphy, Ph.D. (17)
|
15,980,734
|
|
30.6
|
%
|
||
|
All directors and senior management as a group (14 persons) (18)
|
22,667,731
|
|
43.4
|
%
|
||
|
* Represents beneficial ownership of less than one percent.
|
||||||
|
(1) The information shown is based, in part, upon disclosures filed on a Schedule 13G/A on February 11, 2020 by Syncona Portfolio Limited. The number reported consists of (i) 12,180,333 ordinary shares and (ii) 3,775,401 ADSs. Syncona Portfolio Limited is a wholly owned subsidiary of Syncona Holdings Limited, which, in turn, is a wholly controlled subsidiary of Syncona Limited, a publicly-listed company. Each of Syncona Holdings Limited and Syncona Limited may be deemed to have voting and dispositive power over the securities held by Syncona Portfolio Limited. Investment and voting decisions with respect to these securities are made by Syncona Portfolio Limited acting upon the recommendation of an investment committee of Syncona Investment Management Limited, also a subsidiary of Syncona Holdings Limited. The members of this investment committee consist of Nigel Keen, Martin Murphy and Chris Hollowood. The address for Syncona Portfolio Limited is Arnold House, St Julian's Avenue, St Peter Port, Guernsey GY1 3RD, Channel Islands.
|
||||||
|
(2) The information shown is based, in part, upon disclosures filed on a Schedule 13D on January 24, 2020 by PPF Capital Partners Fund B.V., PPF Group N.V. and Petr Kellner. The number reported consists of 10,955,724 ADSs. The principal shareholder of PPF Capital Partners Fund B.V. is PPF Group N.V., which is ultimately beneficially owned by Petr Kellner. The address of the principal office of each of PPF Group and PPF Capital is Strawinskylaan 933, 1077XX Amsterdam, The Netherlands. The address of the principal office of Petr Kellner is c/o PPF a.s., Evropská 2690/17, P.O. Box 177, 160 41 Prague 6, Czech Republic.
|
||||||
|
(3) The information shown is based, in part, upon disclosures filed on a Schedule 13D on July 7, 2018 by Arix Bioscience plc and Arix Bioscience Holdings Limited. The number reported consists of (i) 2,736,535 ordinary shares and (ii) 633,333 ADSs. Investment and voting decisions with respect to these securities are made by Arix Bioscience Holdings Limited acting upon the recommendation of an investment committee. The members of this investment committee consist of Joseph Anderson, Johnathan Tobin, Mark Chin, Daniel O'Connell and Edward Rayner. The address for Arix Bioscience Holdings Limited is 20 Berkeley Square, London, W1J 6EQ, United Kingdom.
|
||||||
|
(4) Consists of (i) 1,066,009 ordinary shares issuable upon conversion of restricted ordinary shares and (ii) 168,680 ordinary shares underlying options that are vested and exercisable within 60 days of January 31, 2020.
|
||||||
|
(5) Consists of ordinary shares underlying options that are vested and exercisable within 60 days of January 31, 2020.
|
||||||
|
(6) Consists of (i) 538,677 ordinary shares, (ii) 160,064 ordinary shares issuable upon conversion of restricted ordinary shares, and (iii) 52,859 ordinary shares underlying options that are vested and exercisable within 60 days of January 31, 2020.
|
||||||
|
(7) Consists of ordinary shares underlying options that are vested and exercisable within 60 days of January 31, 2020.
|
||||||
|
(8) Consists of (i) 45,435 ordinary shares issuable upon conversion of restricted ordinary shares and (ii) 191,121 ordinary shares underlying options that are vested and exercisable within 60 days of January 31, 2020.
|
||||||
|
(9) Consists of (i) 112,211 ordinary shares issuable upon conversion of restricted ordinary shares and (ii) 82,266 ordinary shares underlying options that are vested and exercisable within 60 days of January 31, 2020.
|
||||||
|
(10) Consists of (i) 125,588 ordinary shares issuable upon conversion of restricted ordinary shares and (ii) 82,266 ordinary shares underlying options that are vested and exercisable within 60 days of January 31, 2020.
|
||||||
|
(11) Consists of ordinary shares underlying options that are vested and exercisable within 60 days of January 31, 2020.
|
||||||
|
(12) Consists of (i) shares set forth in footnote (3) above. Dr. Anderson is the chief executive officer of Arix Bioscience plc, the parent company of Arix Bioscience Holdings Limited and (ii) 25,000 ordinary shares underlying options that are vested and exercisable within 60 days of January 31, 2020.
|
||||||
|
(13) Consists of ordinary shares underlying options that are vested and exercisable within 60 days of January 31, 2020.
|
||||||
|
(14) Consists of (i) 62,794 ordinary shares and (ii) 73,537 ordinary shares issuable upon conversion of restricted ordinary shares, and (iii) 33,175 ordinary shares underlying options that are vested and exercisable within 60 days of January 31, 2020.
|
||||||
|
(15) Consists of ordinary shares underlying options that are vested and exercisable within 60 days of January 31, 2020.
|
||||||
|
(16) Consists of (i) 73,537 ordinary shares issuable upon conversion of restricted ordinary shares and (ii) 33,175 ordinary shares underlying options that are vested and exercisable within 60 days of January 31, 2020.
|
||||||
|
(17) Consists of (i) the shares set forth in footnote (1) above. Dr. Murphy is the chief executive officer of Syncona Investment Management Limited (both Syncona Investment Management Limited and Syncona Portfolio Limited are subsidiaries of Syncona Limited) and (ii) and 25,000 ordinary shares underlying options that are vested and exercisable within 60 days of January 31, 2020.
|
||||||
|
(18) Consists of (i) 19,927,073 ordinary shares, (ii) 1,656,381 ordinary shares issuable upon conversion of restricted ordinary shares and (ii) 1,084,277 ordinary shares underlying options that are vested and exercisable within 60 days of January 31, 2020.
|
||||||
|
•
|
enterprise that directly or indirectly controls or is controlled by or is under common control with us;
|
|
•
|
enterprise over which we have a significant influence or which has significant influence over us;
|
|
•
|
individual owning, directly or indirectly, an interest in our voting power that gives them significant influence over us, and close members of any such individual’s family;
|
|
•
|
persons having authority or responsibility for planning, directing or controlling our activities, including directors and senior management and close members of such individuals’ families; or
|
|
•
|
enterprise in which a substantial interest in our voting power is owned, directly or indirectly, by any person described above or over which such a person is able to exercise significant influence, including enterprises owned by our directors or major shareholders and enterprises that have a member of key management in common with us.
|
|
•
|
person who is, or at any time since the beginning of our last fiscal year was, a director or member of senior management of us or a nominee to become a director of us;
|
|
•
|
security holder known by us to be the beneficial owner of more than 5% of any class of our voting securities;
|
|
•
|
immediate family member of any of the foregoing; and
|
|
•
|
firm, corporation or other entity in which any of the foregoing persons is an executive, partner or principal or similar control position or in which such person has a 5% or greater beneficial ownership interest.
|
|
A.
|
Offer and listing details.
|
|
(i)
|
it is for a share which is fully paid up;
|
|
(ii)
|
it is for a share upon which the company has no lien;
|
|
(iii)
|
it is only for one class of share;
|
|
(iv)
|
it is in favor of a single transferee or no more than four joint transferees;
|
|
(v)
|
it is duly stamped or is duly certificated or otherwise shown to the satisfaction of the board of directors to be exempt from stamp duty; and
|
|
(vi)
|
it is delivered for registration to the registered office of the company (or such other place as the board of directors may determine), accompanied (except in the case of a transfer by a person to whom the company is not required by law to issue a certificate and to whom a certificate has not been issued or in the case of a renunciation) by the certificate for the shares to which it relates and such other evidence as the board of directors may reasonably require to prove the title of the transferor (or person renouncing) and the due execution of the transfer or renunciation by him or, if the transfer or renunciation is executed by some other person on his behalf, the authority of that person to do so.
|
|
(i)
|
to the extent permitted by the Companies Act, the matter in question shall have been proposed by any director for consideration in the same way that any other matter may be proposed to the directors under the provisions of the articles of association;
|
|
(ii)
|
any requirement as to the quorum for consideration of the relevant matter is met without counting the conflicted director and any other conflicted director; and
|
|
(iii)
|
the matter is agreed to without the conflicted director voting or would be agreed to if the conflicted director’s and any other interested director’s vote is not counted.
|
|
(a)
|
borrow money;
|
|
(b)
|
indemnify and guarantee;
|
|
(c)
|
mortgage or charge the assets of the company;
|
|
(d)
|
create and issue debentures and other securities; and
|
|
(e)
|
give security either outright or as collateral security for any debt, liability or obligation of the company or of any third party.
|
|
(i)
|
The Takeover Code does not apply to the company. However if the company were to become subject to the Takeover Code in the future, the following provisions will apply. Under Rule 9 of the Takeover Code, where:
|
|
a.
|
any person, together with persons acting in concert with him, acquires, whether by a series of transactions over a period of time or not, an interest in shares which (taken together with shares in which he is already interested, and in which persons acting in concert with him are interested) carry 30% or more of the voting rights of a company; or
|
|
b.
|
any person who, together with persons acting in concert with him, is interested in shares which in the aggregate carry not less than 30% of the voting rights of a company but does not hold shares carrying more than 50% of such voting rights and such person, or any person acting in concert with him, acquires an interest in any other shares which increases the percentage of shares carrying voting rights in which he is interested;
|
|
(ii)
|
An offer under Rule 9 of the Takeover Code must be in cash and at the highest price paid for any interest in the shares by the person required to make an offer or any person acting in concert with him during the 12 months prior to the announcement of the offer.
|
|
(iii)
|
Under the Takeover Code, a “concert party” arises where persons acting together pursuant to an agreement or understanding (whether formal or informal and whether or not in writing) actively cooperate, through the acquisition by them of an interest in shares in a company, to obtain or consolidate control of the company. “Control” means holding, or aggregate holdings, of an interest in shares carrying 30% or more of the voting rights of the company, irrespective of whether the holding or holdings give
de facto
control.
|
|
(i)
|
Under sections 979 to 982 of the Companies Act, if an offeror were to acquire, or unconditionally contract to acquire, not less than 90% in value of the ordinary shares of the company and 90% of the voting rights carried by the ordinary shares of the company, it could then compulsorily acquire the remaining 10%. It would do so by sending a notice to outstanding shareholders telling them that it will compulsorily acquire their shares, provided that no such notice may be served after the end of: (a) the period of three months beginning with the day after the last day on which the offer can be accepted; or (b) if earlier, and the offer is not one to which section 943(1) of the Companies Act applies, the period of six months beginning with the date of the offer.
|
|
(ii)
|
Six weeks following service of the notice, the offeror must send a copy of it to the company together with the consideration for the ordinary shares to which the notice relates, and an instrument of transfer executed on behalf of the outstanding shareholder(s) by a person appointed by the offeror.
|
|
(iii)
|
The company will hold the consideration on trust for the outstanding shareholders.
|
|
(i)
|
Sections 983 to 985 of the Companies Act also give minority shareholders in the company a right to be bought out in certain circumstances by an offeror who has made a takeover offer. If a takeover offer relating to all the ordinary shares of the company is made at any time before the end of the period within which the offer could be accepted and the offeror held or had agreed to acquire not less than 90% of the ordinary shares, any holder of shares to which the offer related who had not accepted the offer could by a written communication to the offeror require it to acquire those shares. The offeror is required to give any shareholder notice of his right to be bought out within one month of that right arising. The offeror may impose a time limit on the rights of minority shareholders to be bought out, but that period cannot end less than three months after the end of the acceptance period, or, if longer a period of three months from the date of the notice.
|
|
(ii)
|
If a shareholder exercises his rights, the offeror is bound to acquire those shares on the terms of the offer or on such other terms as may be agreed.
|
|
ENGLAND
|
DELAWARE
|
|
|
Number of Directors
|
Under the Companies Act, a public limited company must have at least two directors and the number of directors may be fixed by or in the manner provided in a company’s articles of association.
|
Under Delaware law, a corporation must have at least one director and the number of directors shall be fixed by or in the manner provided in the bylaws.
|
|
ENGLAND
|
DELAWARE
|
|
|
Removal of Directors
|
Under the Companies Act, shareholders may remove a director without cause by an ordinary resolution (which is passed by a simple majority of those voting in person or by proxy at a general meeting) irrespective of any provisions of any service contract the director has with the company, provided 28 clear days’ notice of the resolution has been given to the company and its shareholders. On receipt of notice of an intended resolution to remove a director, the company must forthwith send a copy of the notice to the director concerned. Certain other procedural requirements under the Companies Act must also be followed, such as allowing the director to make representations against his or her removal either at the meeting or in writing.
|
Under Delaware law, any director or the entire board of directors may be removed, with or without cause, by the holders of a majority of the shares then entitled to vote at an election of directors, except (i) unless the certificate of incorporation provides otherwise, in the case of a corporation whose board of directors is classified, stockholders may effect such removal only for cause, or (ii) in the case of a corporation having cumulative voting, if less than the entire board of directors is to be removed, no director may be removed without cause if the votes cast against his removal would be sufficient to elect him if then cumulatively voted at an election of the entire board of directors, or, if there are classes of directors, at an election of the class of directors of which he is a part.
|
|
ENGLAND
|
DELAWARE
|
|
|
Vacancies of Board of Directors
|
Under English law, the procedure by which directors, other than a company’s initial directors, are appointed is generally set out in a company’s articles of association, provided that where two or more persons are appointed as directors of a public limited company by resolution of the shareholders, resolutions appointing each director must be voted on individually.
|
Under Delaware law, vacancies and newly created directorships may be filled by a majority of the directors then in office (even though less than a quorum) or by a sole remaining director unless (i) otherwise provided in the certificate of incorporation or bylaws of the corporation or (ii) the certificate of incorporation directs that a particular class of stock is to elect such director, in which case a majority of the other directors elected by such class, or a sole remaining director elected by such class, will fill such vacancy.
|
|
ENGLAND
|
DELAWARE
|
|
|
Annual General Meeting
|
Under the Companies Act, a public limited company must hold an annual general meeting in each six-month period following the company’s annual accounting reference date.
|
Under Delaware law, the annual meeting of stockholders shall be held at such place, on such date and at such time as may be designated from time to time by the board of directors or as provided in the certificate of incorporation or by the bylaws.
|
|
ENGLAND
|
DELAWARE
|
|
|
General Meeting
|
Under the Companies Act, a general meeting of the shareholders of a public limited company may be called by the directors.
Shareholders holding at least 5% of the paid-up capital of the company carrying voting rights at general meetings (excluding any paid up capital held as treasury shares) can require the directors to call a general meeting and, if the directors fail to do so within a certain period, may themselves convene a general meeting.
|
Under Delaware law, special meetings of the stockholders may be called by the board of directors or by such person or persons as may be authorized by the certificate of incorporation or by the bylaws.
|
|
ENGLAND
|
DELAWARE
|
|
|
Notice of General Meeting
|
Under the Companies Act, at least 21 days’ notice must be given for an annual general meeting and any resolutions to be proposed at the meeting. Subject to a company’s articles of association providing for a longer period, at least 14 days’ notice is required for any other general meeting of a public limited company. In addition, certain matters, such as the removal of directors or auditors, require special notice, which is 28 days’ notice. The shareholders of a company may in all cases consent to a shorter notice period, the proportion of shareholders’ consent required being 100% of those entitled to attend and vote in the case of an annual general meeting and, in the case of any other general meeting, a majority in number of the members having a right to attend and vote at the meeting, being a majority who together hold not less than 95% in nominal value of the shares giving a right to attend and vote at the meeting.
|
Under Delaware law, unless otherwise provided in the certificate of incorporation or bylaws, written notice of any meeting of the stockholders must be given to each stockholder entitled to vote at the meeting not less than ten nor more than 60 days before the date of the meeting and shall specify the place, date, hour and purpose or purposes of the meeting.
|
|
ENGLAND
|
DELAWARE
|
|
|
Proxy
|
Under the Companies Act, at any meeting of shareholders, a shareholder may designate another person to attend, speak and vote at the meeting on their behalf by proxy.
|
Under Delaware law, at any meeting of stockholders, a stockholder may designate another person to act for such stockholder by proxy, but no such proxy shall be voted or acted upon after three years from its date, unless the proxy provides for a longer period. A director of a Delaware corporation may not issue a proxy representing the director’s voting rights as a director.
|
|
ENGLAND
|
DELAWARE
|
|
|
Preemptive Rights
|
Under the Companies Act, “equity securities,” being (i) shares in the company other than shares that, with respect to dividends and capital, carry a right to participate only up to a specified amount in a distribution, referred to as “ordinary shares,” or (ii) rights to subscribe for, or to convert securities into, ordinary shares, proposed to be allotted for cash must be offered first to the existing equity shareholders in the company in proportion to the respective nominal value of their holdings, unless an exception applies or a special resolution to the contrary has been passed by shareholders in a general meeting or the articles of association provide otherwise in each case in accordance with the provisions of the Companies Act.
|
Under Delaware law, shareholders have no preemptive rights to subscribe to additional issues of stock or to any security convertible into such stock unless, and except to the extent that, such rights are expressly provided for in the certificate of incorporation.
|
|
ENGLAND
|
DELAWARE
|
|
|
Authority to Allot
|
Under the Companies Act, the directors of a company must not allot shares or grant rights to subscribe for or convert any security into shares unless an exception applies or an ordinary resolution to the contrary has been passed by shareholders in a general meeting or the articles of association provide otherwise, in each case in accordance with the provisions of the Companies Act.
|
Under Delaware law, if the corporation’s charter or certificate of incorporation so provides, the board of directors has the power to authorize the issuance of stock. The board may authorize capital stock to be issued for consideration consisting of cash, any tangible or intangible property or any benefit to the corporation or any combination thereof. It may determine the amount of such consideration by approving a formula. In the absence of actual fraud in the transaction, the judgment of the directors as to the value of such consideration is conclusive.
|
|
ENGLAND
|
DELAWARE
|
|
|
Liability of Directors and Officers
|
Under the Companies Act, any provision, whether contained in a company’s articles of association or any contract or otherwise, that purports to exempt a director of a company, to any extent, from any liability that would otherwise attach to him in connection with any negligence, default, breach of duty or breach of trust in relation to the company, is void. Any provision by which a company directly or indirectly provides an indemnity, to any extent, for a director of the company or of an associated company against any liability attaching to him in connection with any negligence, default, breach of duty or breach of trust in relation to the company of which he is a director is also void except as permitted by the Companies Act, which provides exceptions for the company to (i) purchase and maintain insurance against such liability; (ii) provide a “qualifying third party indemnity,” or an indemnity against liability incurred by the director to a person other than the company or an associated company or criminal proceedings in which he is convicted; and (iii) provide a “qualifying pension scheme indemnity,” or an indemnity against liability incurred in connection with the company’s activities as trustee of an occupational pension plan.
|
Under Delaware law, a corporation’s certificate of incorporation may include a provision eliminating or limiting the personal liability of a director to the corporation and its stockholders for damages arising from a breach of fiduciary duty as a director. However, no provision can limit the liability of a director for:
any breach of the director’s duty of loyalty to the corporation or its stockholders;
acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law;
intentional or negligent payment of unlawful
|
|
ENGLAND
|
DELAWARE
|
|
|
Voting Rights
|
Under English law, unless a poll is demanded by the shareholders of a company or is required by the chairman of the meeting or the company’s articles of association, shareholders shall vote on all resolutions on a show of hands. Under the Companies Act, a poll may be demanded by (i) not fewer than five shareholders having the right to vote on the resolution; (ii) any shareholder(s) representing not less than 10% of the total voting rights of all the shareholders having the right to vote on the resolution (excluding any voting rights attaching to treasury shares); or (iii) any shareholder(s) holding shares in the company conferring a right to vote on the resolution (excluding any voting rights attaching to treasury shares) being shares on which an aggregate sum has been paid up equal to not less than 10% of the total sum paid up on all the shares conferring that right. A company’s articles of association may provide more extensive rights for shareholders to call a poll.
Under English law, an ordinary resolution is passed on a show of hands if it is approved by a simple majority (more than 50%) of the votes cast by shareholders present (in person or by proxy) and entitled to vote. If a poll is demanded, an ordinary resolution is passed if it is approved by holders representing a simple majority of the total voting rights of shareholders present, in person or by proxy, who, being entitled to vote, vote on the resolution. Special resolutions require the affirmative vote of not less than 75% of the votes cast by shareholders present, in person or by proxy, at the meeting.
|
Delaware law provides that, unless otherwise provided in the certificate of incorporation, each stockholder is entitled to one vote for each share of capital stock held by such stockholder.
|
|
ENGLAND
|
DELAWARE
|
|
|
Shareholder Vote on Certain Transactions
|
The Companies Act provides for schemes of arrangement, which are arrangements or compromises between a company and any class of shareholders or creditors and used in certain types of reconstructions, amalgamations, capital reorganizations or takeovers. These arrangements require:
the approval at a shareholders’ or creditors’ meeting convened by order of the court, of a majority in number of shareholders or creditors representing 75% in value of the capital held by, or debt owed to, the class of shareholders or creditors, or class thereof present and voting, either in person or by proxy; and
the approval of the court.
|
Generally, under Delaware law, unless the certificate of incorporation provides for the vote of a larger portion of the stock, completion of a merger, consolidation, sale, lease or exchange of all or substantially all of a corporation’s assets or dissolution requires:
the approval of the board of directors; and
the approval by the vote of the holders of a majority of the outstanding stock or, if the certificate of incorporation provides for more or less than one vote per share, a majority of the votes of the outstanding stock of the corporation entitled to vote on the matter.
|
|
ENGLAND
|
DELAWARE
|
|
|
Standard of Conduct for Directors
|
Under English law, a director owes various statutory and fiduciary duties to the company, including:
to act in the way he considers, in good faith, would be most likely to promote the success of the company for the benefit of its members as a whole;
to avoid a situation in which he has, or can have, a direct or indirect interest that conflicts, or possibly conflicts, with the interests of the company;
to act in accordance with the company’s constitution and only exercise his powers for the purposes for which they are conferred;
to exercise independent judgment;
to exercise reasonable care, skill and diligence;
not to accept benefits from a third party conferred by reason of his being a director or doing, or not doing, anything as a director; and
to declare any interest that he has, whether directly or indirectly, in a proposed or existing transaction or arrangement with the company.
|
Delaware law does not contain specific provisions setting forth the standard of conduct of a director. The scope of the fiduciary duties of directors is generally determined by the courts of the State of Delaware. In general, directors have a duty to act without self-interest, on a well-informed basis and in a manner they reasonably believe to be in the best interest of the stockholders.
Directors of a Delaware corporation owe fiduciary duties of care and loyalty to the corporation and to its shareholders. The duty of care generally requires that a director acts in good faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself of all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he reasonably believes to be in the best interests of the corporation. He must not use his corporate position for personal gain or advantage. In general, but subject to certain exceptions, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary duties. Delaware courts have also imposed a heightened standard of conduct upon directors of a Delaware corporation who take any action designed to defeat a threatened change in control of the corporation.
In addition, under Delaware law, when the board of directors of a Delaware corporation approves the sale or break-up of a corporation, the board of directors may, in certain circumstances, have a duty to obtain the highest value reasonably available to the shareholders.
|
|
ENGLAND
|
DELAWARE
|
|
|
Shareholder Litigation
|
Under English law, generally, the company, rather than its shareholders, is the proper claimant in an action in respect of a wrong done to the company or where there is an irregularity in the company’s internal management. Notwithstanding this general position, the Companies Act provides that (i) a court may allow a shareholder to bring a derivative claim (that is, an action in respect of and on behalf of the company) in respect of a cause of action arising from a director’s negligence, default, breach of duty or breach of trust and (ii) a shareholder may bring a claim for a court order where the company’s affairs have been or are being conducted in a manner that is unfairly prejudicial to some of its shareholders.
|
Under Delaware law, a stockholder may initiate a derivative action to enforce a right of a corporation if the corporation fails to enforce the right itself. The complaint must:
state that the plaintiff was a stockholder at the time of the transaction of which the plaintiff complains or that the plaintiff’s shares thereafter devolved on the plaintiff by operation of law; and
allege with particularity the efforts made by the plaintiff to obtain the action the plaintiff desires from the directors and the reasons for the plaintiff’s failure to obtain the action; or
state the reasons for not making the effort.
Additionally, the plaintiff must remain a stockholder through the duration of the derivative suit. The action will not be dismissed or compromised without the approval of the Delaware Court of Chancery.
|
|
•
|
banks, insurance companies, and certain other financial institutions;
|
|
•
|
U.S. expatriates and certain former citizens or long-term residents of the United States;
|
|
•
|
dealers or traders in securities who use a mark-to-market method of tax accounting;
|
|
•
|
persons holding ADSs as part of a hedging transaction, “straddle,” wash sale, conversion transaction or integrated transaction or persons entering into a constructive sale with respect to ADSs;
|
|
•
|
persons whose “functional currency” for U.S. federal income tax purposes is not the U.S. dollar;
|
|
•
|
brokers, dealers or traders in securities, commodities or currencies;
|
|
•
|
tax-exempt entities or government organizations;
|
|
•
|
S corporations, partnerships, or other entities or arrangements classified as partnerships for U.S. federal income tax purposes (and investors therein);
|
|
•
|
regulated investment companies or real estate investment trusts;
|
|
•
|
persons who acquired ADSs pursuant to the exercise of any employee share option or otherwise as compensation;
|
|
•
|
persons that own or are deemed to own 10 percent or more of our shares including shares represented by ADSs (by vote or value); and
|
|
•
|
persons holding our ADSs in connection with a trade or business, permanent establishment, or fixed base outside the United States.
|
|
(i)
|
a citizen or individual resident of the United States;
|
|
(ii)
|
a corporation, or other entity taxable as a corporation, created or organized in or under the laws of the United States, any state therein or the District of Columbia;
|
|
(iii)
|
an estate the income of which is subject to U.S. federal income taxation regardless of its source; or
|
|
(iv)
|
a trust if (1) a U.S. court is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions of the trust or (2) the trust has a valid election to be treated as a U.S. person under applicable U.S. Treasury Regulations.
|
|
•
|
at least 75% of its gross income is passive income (such as interest income); or
|
|
•
|
at least 50% of its gross assets (determined on the basis of a quarterly average) is attributable to assets that produce passive income or are held for the production of passive income.
|
|
•
|
the excess distribution or gain will be allocated ratably over a U.S. Holder’s holding period for the ADSs;
|
|
•
|
the amount allocated to the current taxable year, and any taxable year prior to the first taxable year in which we became a PFIC, will be treated as ordinary income; and
|
|
•
|
the amount allocated to each other year will be subject to the highest tax rate in effect for that year and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year.
|
|
•
|
persons who are connected with the company;
|
|
•
|
financial institutions;
|
|
•
|
insurance companies;
|
|
•
|
charities or tax-exempt organizations;
|
|
•
|
collective investment schemes;
|
|
•
|
pension schemes;
|
|
•
|
market makers, intermediaries, brokers or dealers in securities;
|
|
•
|
persons who have (or are deemed to have) acquired their ADSs by virtue of an office or employment or who are or have been officers or employees of the company or any of its affiliates; and
|
|
•
|
individuals who are subject to U.K. taxation on a remittance basis.
|
|
SERVICE
|
FEE
|
|
|
Issuance of ADSs (e.g., an issuance of ADS upon a deposit of ordinary shares or upon a change in the ADS(s)-to-ordinary shares ratio), excluding ADS issuances as a result of distributions of ordinary shares
|
Up to $0.05 per ADS issued
|
|
|
Cancellation of ADSs (e.g., a cancellation of ADSs for delivery of deposited property or upon a change in the ADS(s)-to-ordinary shares ratio, or for any other reason)
|
Up to $0.05 per ADS canceled
|
|
|
Distribution of cash dividends or other cash distributions (e.g., upon a sale of rights and other entitlements)
|
Up to $0.05 per ADS held
|
|
|
Distribution of ADSs pursuant to (i) share dividends or other free share distributions, or (ii) exercise of rights to purchase additional ADSs
|
Up to $0.05 per ADS held
|
|
|
Distribution of securities other than ADSs or rights to purchase additional ADSs (e.g., upon a spin-off)
|
Up to $0.05 per ADS held
|
|
|
ADS Services
|
Up to $0.05 per ADS held on
the applicable record date(s) established by the depositary |
|
|
•
|
taxes (including applicable interest and penalties) and other governmental charges;
|
|
•
|
the registration fees as may from time to time be in effect for the registration of ordinary shares on the share register and applicable to transfers of ordinary shares to or from the name of the custodian, the depositary or any nominees upon the making of deposits and withdrawals, respectively;
|
|
•
|
certain cable, telex and facsimile transmission and delivery expenses;
|
|
•
|
the expenses and charges incurred by the depositary in the conversion of foreign currency;
|
|
•
|
the fees and expenses incurred by the depositary in connection with compliance with exchange control regulations and other regulatory requirements applicable to ordinary shares, ADSs and ADRs; and
|
|
•
|
the fees and expenses incurred by the depositary, the custodian or any nominee in connection with the servicing or delivery of deposited property.
|
|
A.
|
Disclosure Controls and Procedures.
|
|
Year Ended December 31,
|
Three Months Ended December 31,
|
Year Ended September 30,
|
|||||||||||||
|
2019
|
2018
|
2018
|
2017
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Audit fees
|
$
|
518
|
|
$
|
184
|
|
$
|
283
|
|
$
|
380
|
|
|||
|
Audit-related fees
|
114
|
|
193
|
|
392
|
|
—
|
|
|||||||
|
Total
|
$
|
632
|
|
$
|
377
|
|
$
|
675
|
|
$
|
380
|
|
|||
|
▪
|
Exemption from filing quarterly reports on Form 10-Q containing unaudited financial and other specified information or current reports on Form 8-K upon the occurrence of specified significant events.
|
|
▪
|
Exemption from Section 16 rules requiring insiders to file public reports of their share ownership and trading activities and liability for insiders who profit from trades in a short period of time, which will provide less data in this regard than shareholders of U.S. companies that are subject to the Exchange Act.
|
|
▪
|
Exemption from the requirement that our board have a compensation committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities.
|
|
▪
|
Exemption from the requirement to have independent director oversight of director nominations.
|
|
▪
|
We do not intend to follow Nasdaq Rule 5620(c) regarding quorum requirements applicable to meetings of shareholders. Such quorum requirements are not required under English law. In accordance with generally accepted business practice, our Articles of Association provide alternative quorum requirements that are generally applicable to meetings of shareholders.
|
|
▪
|
We do not intend to follow Nasdaq Rule 5605(b)(2), which requires that independent directors regularly meet in executive sessions where only independent directors are present. Our independent directors may choose to meet in executive sessions at their discretion.
|
|
INCORPORATED BY REFERENCE
|
||||||||||
|
EXHIBIT
NUMBER
|
DESCRIPTION OF EXHIBIT
|
SCHEDULE/
FORM
|
FILE NUMBER
|
EXHIBIT
|
FILE
DATE
|
|||||
|
Articles of Association of Autolus Therapeutics plc.
|
Form F-1/A
|
333-224720
|
3.1
|
6/19/18
|
||||||
|
Deposit Agreement by and among the registrant, Citibank, N.A., as the depositary bank and the holders and beneficial owners of American Depositary Shares issued thereunder.
|
Form F-6/A
|
333-224837
|
99.(a)
|
6/19/18
|
||||||
|
Form of American Depositary Receipt (included in exhibit 2.1).
|
Form F-6/A
|
333-224837
|
99.(a)
|
6/19/18
|
||||||
|
Autolus Therapeutics plc, Registration Rights Agreement, dated as June 26, 2018
|
||||||||||
|
License Agreement, dated as of September 25, 2014 by and between the registrant and UCL Business plc, as amended on March 2, 2016 and March 28, 2018.
|
Form F-1/A
|
333-224720
|
10.1
|
5/10/18
|
||||||
|
Supply Agreement, dated as of March 23, 2018, by and between the registrant and Miltenyi Biotec GmbH.
|
Form F-1/A
|
333-224720
|
10.2
|
6/8/18
|
||||||
|
Autolus Therapeutics plc 2018 Equity Incentive Plan.
|
Form F-1/A
|
333-224720
|
10.3
|
6/19/18
|
||||||
|
Non-employee Sub Plan to the Autolus Therapeutics plc 2018 Equity Incentive Plan.
|
Form F-1/A
|
333-224720
|
10.4
|
6/19/18
|
||||||
|
Management Incentive Compensation Plan.
|
Form F-1/A
|
333-224720
|
10.5
|
6/8/18
|
||||||
|
Form of Deed of Indemnity between the registrant and each of its members of senior management and directors.
|
Form F-1/A
|
333-224720
|
10.6
|
6/8/18
|
||||||
|
Description of Securities
|
||||||||||
|
Subsidiaries of the registrant.
|
||||||||||
|
Certification of Principal Executive Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
||||||||||
|
Certification of Principal Financial Officer Pursuant to Rules 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934, as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
||||||||||
|
Certification of Principal Executive Officer and Principal Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
||||||||||
|
15.1
*
|
Consent of Ernst & Young LLP.
|
|||||||||
|
101.INS*
|
XBRL Instance Document
|
|||||||||
|
101.SCH*
|
XBRL Taxonomy Extension Schema Document
|
|||||||||
|
101.CAL*
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|||||||||
|
101.DEF*
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|||||||||
|
101.LAB*
|
XBRL Taxonomy Extension Label Linkbase Document
|
|||||||||
|
101.PRE*
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|||||||||
|
+
|
Indicates management contract or compensatory plan.
|
|
#
|
Confidential treatment has been granted as to portions of the exhibit (indicated by asterisks). Confidential materials omitted and filed separately with the Securities and Exchange Commission.
|
|
*
|
Filed herewith.
|
|
**
|
Furnished herewith.
|
|
AUTOLUS THERAPEUTICS PLC
|
|||
|
Date: March 3, 2020
|
By:
|
/s/ Christian Itin
|
|
|
Christian Itin
|
|||
|
Chief Executive Officer
|
|||
|
Consolidated Balance Sheets as of December 31, 2019 and 2018
|
|
|
Consolidated Statements of Operations and Comprehensive Loss for the year ended December 31, 2019, the three months ended December 31, 2018, and the years ended September 30, 2018 and 2017
|
|
|
Consolidated Statements of Shareholders’ Equity for the year ended December 31, 2019, the three months ended December 31, 2018, and the years ended September 30, 2018 and 2017
|
|
|
Consolidated Statements of Cash Flows for the year ended December 31, 2019, the three months ended December 31, 2018, and the years ended September 30, 2018 and 2017
|
|
|
December 31,
|
||||||||
|
2019
|
2018
|
|||||||
|
Assets
|
||||||||
|
Current assets:
|
||||||||
|
Cash
|
$
|
210,643
|
|
$
|
217,450
|
|
||
|
Restricted cash
|
787
|
|
105
|
|
||||
|
Prepaid expenses and other current assets
|
37,826
|
|
15,411
|
|
||||
|
Total current assets
|
249,256
|
|
232,966
|
|
||||
|
Non-current assets:
|
||||||||
|
Property and equipment, net
|
28,164
|
|
19,968
|
|
||||
|
Right of use asset, net
|
23,409
|
|
—
|
|
||||
|
Long-term deposits
|
2,040
|
|
1,276
|
|
||||
|
Deferred tax asset
|
410
|
|
—
|
|
||||
|
Intangible assets, net
|
254
|
|
—
|
|
||||
|
Total assets
|
$
|
303,533
|
|
$
|
254,210
|
|
||
|
Liabilities and shareholders' equity
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
1,075
|
|
2,022
|
|
||||
|
Accrued expenses and other liabilities
|
21,398
|
|
19,054
|
|
||||
|
Lease liability
|
2,511
|
|
—
|
|
||||
|
Total current liabilities
|
24,984
|
|
21,076
|
|
||||
|
Non-current liabilities:
|
||||||||
|
Lease liability
|
23,710
|
|
—
|
|
||||
|
Long-term lease incentive obligation
|
—
|
|
207
|
|
||||
|
Other long-term payables
|
—
|
|
285
|
|
||||
|
Total liabilities
|
48,694
|
|
21,568
|
|
||||
|
Shareholders' equity:
|
||||||||
|
Ordinary shares, $0.000042 par value; 200,000,000 shares authorized at December 31, 2019 and 2018, 44,983,006 and 40,145,617 shares issued and outstanding at December 31, 2019 and 2018
|
2
|
|
2
|
|
||||
|
Deferred shares, £0.00001 par value; 34,425 shares authorized, issued and outstanding at December 31, 2019 and 2018
|
—
|
|
—
|
|
||||
|
Deferred B shares, £0.00099 par value; 88,893,548 shares authorized, issued and outstanding at December 31, 2019 and 2018
|
118
|
|
118
|
|
||||
|
Deferred C shares, £0.000008 par value; 1 share authorized, issued and outstanding at December 31, 2019 and 2018
|
—
|
|
—
|
|
||||
|
Additional paid-in capital
|
500,560
|
|
361,311
|
|
||||
|
Accumulated other comprehensive loss
|
(8,691
|
)
|
(15,488
|
)
|
||||
|
Accumulated deficit
|
(237,150
|
)
|
(113,301
|
)
|
||||
|
Total shareholders' equity
|
254,839
|
|
232,642
|
|
||||
|
Total liabilities and shareholders' equity
|
$
|
303,533
|
|
$
|
254,210
|
|
||
|
For the Year Ended December 31,
|
For the three-months ended December 31,
|
For the Year Ended September 30,
|
|||||||||||||
|
2019
|
2018
|
2018
|
2017
|
||||||||||||
|
Grant income
|
$
|
2,908
|
|
$
|
296
|
|
$
|
1,407
|
|
$
|
1,693
|
|
|||
|
Operating expenses:
|
|||||||||||||||
|
Research and development
|
(105,418
|
)
|
(17,713
|
)
|
(36,150
|
)
|
(16,012
|
)
|
|||||||
|
General and administrative
|
(39,452
|
)
|
(7,593
|
)
|
(22,790
|
)
|
(9,099
|
)
|
|||||||
|
Loss on impairment of leasehold improvements
|
(4,102
|
)
|
—
|
|
—
|
|
—
|
|
|||||||
|
Total operating expenses, net
|
(146,064
|
)
|
(25,010
|
)
|
(57,533
|
)
|
(23,418
|
)
|
|||||||
|
Other income (expense):
|
|||||||||||||||
|
Interest income
|
2,542
|
|
660
|
|
1,532
|
|
84
|
|
|||||||
|
Other income (expense)
|
4,514
|
|
1,097
|
|
3,970
|
|
(46
|
)
|
|||||||
|
Total other income, net
|
7,056
|
|
1,757
|
|
5,502
|
|
38
|
|
|||||||
|
Net loss before income tax
|
(139,008
|
)
|
(23,253
|
)
|
(52,031
|
)
|
(23,380
|
)
|
|||||||
|
Income tax benefit
|
15,159
|
|
2,605
|
|
7,280
|
|
3,653
|
|
|||||||
|
Net loss attributable to ordinary shareholders
|
(123,849
|
)
|
(20,648
|
)
|
(44,751
|
)
|
(19,727
|
)
|
|||||||
|
Other comprehensive (loss) income:
|
|||||||||||||||
|
Foreign currency exchange translation adjustment
|
6,797
|
|
(5,568
|
)
|
(6,071
|
)
|
802
|
|
|||||||
|
Total comprehensive loss
|
(117,052
|
)
|
(26,216
|
)
|
(50,822
|
)
|
(18,925
|
)
|
|||||||
|
Basic and diluted net loss per ordinary share
|
$
|
(2.88
|
)
|
$
|
(0.52
|
)
|
$
|
(1.42
|
)
|
$
|
(1.43
|
)
|
|||
|
Weighted-average basic and diluted ordinary shares
|
43,065,542
|
|
39,366,634
|
31,557,034
|
|
13,783,222
|
|
||||||||
|
Ordinary shares
|
Deferred Shares
|
Deferred B shares
|
Deferred C Shares
|
||||||||||||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Additional Paid in Capital
|
Accumulated other comprehensive loss
|
Accumulated deficit
|
Total
|
||||||||||||||||||||||||||||||||||
|
Balance at September 30, 2016
|
13,921,544
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
$
|
63,513
|
|
$
|
(4,651
|
)
|
$
|
(28,175
|
)
|
$
|
30,687
|
|
|||||||||||||
|
Issuance of ordinary shares, net of issuance costs
|
16,041,198
|
|
1
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
127,685
|
|
—
|
|
—
|
|
127,686
|
|
|||||||||||||||||||||
|
Share-based compensation expense
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
3,153
|
|
—
|
|
—
|
|
3,153
|
|
|||||||||||||||||||||
|
Unrealized loss on foreign currency translation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
802
|
|
—
|
|
802
|
|
|||||||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(19,727
|
)
|
$
|
(19,727
|
)
|
||||||||||||||||||||
|
Balance at September 30, 2017
|
29,962,742
|
|
$
|
1
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
$
|
194,351
|
|
$
|
(3,849
|
)
|
$
|
(47,902
|
)
|
$
|
142,601
|
|
|||||||||||||
|
Issuance of ordinary shares, net of issuance costs
|
10,183,440
|
|
1
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
156,802
|
|
—
|
|
156,803
|
|
|||||||||||||||||||||||||
|
Issuance of deferred shares
|
—
|
|
34,425
|
|
—
|
|
88,893,548
|
|
118
|
|
1
|
|
—
|
|
—
|
|
—
|
|
—
|
|
118
|
|
|||||||||||||||||||||||
|
Share-based compensation expense
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
6765
|
|
—
|
|
—
|
|
6,765
|
|
|||||||||||||||||||||||
|
Unrealized loss on foreign currency translation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(6,071
|
)
|
—
|
|
(6,071
|
)
|
|||||||||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(44,751
|
)
|
$
|
(44,751
|
)
|
||||||||||||||||||||||
|
Balance at September 30, 2018
|
40,146,182
|
|
$
|
2
|
|
34,425
|
|
$
|
—
|
|
88,893,548
|
|
$
|
118
|
|
1
|
|
$
|
—
|
|
$
|
357,918
|
|
$
|
(9,920
|
)
|
$
|
(92,653
|
)
|
$
|
255,465
|
|
|||||||||||||
|
Share-based compensation expense
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
3,393
|
|
—
|
|
—
|
|
3,393
|
|
|||||||||||||||||||||
|
Restricted shares - forfeited
|
(565
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||||||
|
Unrealized gain on foreign currency translation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(5,568
|
)
|
—
|
|
(5,568
|
)
|
|||||||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(20,648
|
)
|
$
|
(20,648
|
)
|
||||||||||||||||||||
|
Balance at December 31, 2018
|
40,145,617
|
|
$
|
2
|
|
34,425
|
|
$
|
—
|
|
88,893,548
|
|
$
|
118
|
|
1
|
|
$
|
—
|
|
$
|
361,311
|
|
$
|
(15,488
|
)
|
$
|
(113,301
|
)
|
$
|
232,642
|
|
|||||||||||||
|
Issuance of ordinary shares, net of issuance costs
|
4,830,000
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
108,815
|
|
—
|
|
—
|
|
108,815
|
|
|||||||||||||||||||||
|
Share-based compensation expense
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
30,386
|
|
—
|
|
—
|
|
30,386
|
|
|||||||||||||||||||||
|
Restricted shares - forfeited
|
(4,335
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||||||
|
Exercise of stock options
|
11,724
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
48
|
|
—
|
|
—
|
|
48
|
|
|||||||||||||||||||||
|
Unrealized gain on foreign currency translation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
6,797
|
|
—
|
|
6,797
|
|
|||||||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(123,849
|
)
|
(123,849
|
)
|
|||||||||||||||||||||
|
Balance at December 31, 2019
|
44,983,006
|
|
$
|
2
|
|
34,425
|
|
—
|
|
88,893,548
|
|
$
|
118
|
|
$
|
1
|
|
—
|
|
$
|
500,560
|
|
$
|
(8,691
|
)
|
$
|
(237,150
|
)
|
$
|
254,839
|
|
||||||||||||||
|
Year Ended December 31,
|
Three Months Ended December 31,
|
Year Ended September 30,
|
|||||||||||||
|
2019
|
2018
|
2018
|
2017
|
||||||||||||
|
Cash flows from operating activities:
|
|||||||||||||||
|
Net loss
|
$
|
(123,849
|
)
|
$
|
(20,648
|
)
|
$
|
(44,751
|
)
|
$
|
(19,727
|
)
|
|||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|||||||||||||||
|
Depreciation
|
4,611
|
|
645
|
|
1,709
|
|
1,009
|
|
|||||||
|
Loss on disposal of fixed assets and intangible assets
|
43
|
|
394
|
|
8
|
|
—
|
|
|||||||
|
Non-cash share-based compensation (net of amount capitalized)
|
30,212
|
|
3,393
|
|
6,765
|
|
3,153
|
|
|||||||
|
Loss on impairment of leasehold improvements
|
4,102
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Deferred income tax
|
(399
|
)
|
—
|
|
—
|
|
—
|
|
|||||||
|
Changes in operating assets and liabilities
|
|||||||||||||||
|
Prepaid expenses and other current assets
|
(21,008
|
)
|
(3,521
|
)
|
(7,132
|
)
|
(2,317
|
)
|
|||||||
|
Long-term deposits
|
(702
|
)
|
(1,285
|
)
|
—
|
|
—
|
|
|||||||
|
Right of use asset, net
|
2,551
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Accounts payable
|
(1,451
|
)
|
(723
|
)
|
838
|
|
434
|
|
|||||||
|
Accrued expenses and other liabilities
|
4,958
|
|
1,960
|
|
11,026
|
|
1,088
|
|
|||||||
|
Lease liabilities
|
(552
|
)
|
—
|
|
—
|
|
—
|
|
|||||||
|
Net cash used in operating activities
|
(101,484
|
)
|
(19,785
|
)
|
(31,537
|
)
|
(16,360
|
)
|
|||||||
|
Cash flows from investing activities:
|
|||||||||||||||
|
Purchases of property and equipment
|
(18,341
|
)
|
(4,422
|
)
|
(9,119
|
)
|
(2,876
|
)
|
|||||||
|
Purchase of intangible assets
|
(327
|
)
|
—
|
|
(412
|
)
|
—
|
|
|||||||
|
Net cash used in investing activities
|
(18,668
|
)
|
(4,422
|
)
|
(9,531
|
)
|
(2,876
|
)
|
|||||||
|
Cash flows from financing activities:
|
|||||||||||||||
|
Proceeds of issuance of ordinary shares, net of issuance costs
|
108,863
|
|
—
|
|
156,920
|
|
127,686
|
|
|||||||
|
Net cash provided by financing activities
|
108,863
|
|
—
|
|
156,920
|
|
127,686
|
|
|||||||
|
Effect of exchange rate changes on cash
|
5,164
|
|
(5,327
|
)
|
(5,833
|
)
|
561
|
|
|||||||
|
Net increase in cash
|
(6,125
|
)
|
(29,534
|
)
|
110,019
|
|
109,011
|
|
|||||||
|
Cash and restricted cash, beginning of period
|
217,555
|
|
247,089
|
|
137,070
|
|
28,059
|
|
|||||||
|
Cash and restricted cash, end of period
|
$
|
211,430
|
|
$
|
217,555
|
|
$
|
247,089
|
|
$
|
137,070
|
|
|||
|
Supplemental cash flow information
|
|||||||||||||||
|
Cash paid for taxes
|
$
|
1,535
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Supplemental non-cash flow information
|
|||||||||||||||
|
Property and equipment purchases included in accounts payable or accrued
|
$
|
2,818
|
|
$
|
3,343
|
|
$
|
328
|
|
$
|
—
|
|
|||
|
Reduction in right of use asset
|
$
|
1,919
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Capitalized share-based compensation
|
$
|
174
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Reconciliation of cash and restricted cash reported within the consolidated balance sheets
|
|||||||||||||||
|
Cash
|
$
|
210,643
|
|
$
|
217,450
|
|
$
|
246,984
|
|
$
|
137,070
|
|
|||
|
Short-term restricted cash
|
787
|
|
105
|
|
105
|
|
—
|
|
|||||||
|
Total cash and restricted cash
|
$
|
211,430
|
|
$
|
217,555
|
|
$
|
247,089
|
|
$
|
137,070
|
|
|||
|
Year Ended December 31,
|
Three Months Ended December 31,
|
Years Ended September 30,
|
||||||||||
|
2019
|
2018
|
2018
|
2017
|
|||||||||
|
Unvested restricted shares and units
|
814,744
|
|
708,834
|
|
815,632
|
|
1,358,317
|
|
||||
|
Incentive share options
|
5,963,239
|
|
3,711,274
|
|
2,065,481
|
|
570,309
|
|
||||
|
Total
|
6,777,983
|
|
4,420,108
|
|
2,881,113
|
|
1,928,626
|
|
||||
|
December 31,
|
||||||||
|
2019
|
2018
|
|||||||
|
Research and development claims receivable
|
$
|
27,567
|
|
$
|
10,272
|
|
||
|
Prepayments
|
7,023
|
|
2,069
|
|
||||
|
VAT receivable
|
1,928
|
|
1,973
|
|
||||
|
Other asset
|
279
|
|
—
|
|
||||
|
Grant income receivable
|
547
|
|
661
|
|
||||
|
Other receivable
|
482
|
|
436
|
|
||||
|
Total prepaid expenses and other current assets
|
$
|
37,826
|
|
$
|
15,411
|
|
||
|
December 31,
|
||||||||
|
2019
|
2018
|
|||||||
|
Lab equipment
|
$
|
18,214
|
|
$
|
10,771
|
|
||
|
Office equipment
|
2,211
|
|
1,610
|
|
||||
|
Furniture and fixtures
|
1,301
|
|
599
|
|
||||
|
Leasehold improvements
|
10,316
|
|
2,076
|
|
||||
|
Assets under construction
|
4,687
|
|
8,620
|
|
||||
|
Less: accumulated depreciation
|
(8,565
|
)
|
(3,708
|
)
|
||||
|
Total property and equipment, net
|
$
|
28,164
|
|
$
|
19,968
|
|
||
|
December 31, 2019
|
||||||||||||||
|
Estimated Life
(years) |
Cost
|
Accumulated Amortization
|
Net
|
|||||||||||
|
Software license
|
3
|
$
|
336
|
|
$
|
(82
|
)
|
$
|
254
|
|
||||
|
December 31,
|
||||||||
|
2019
|
2018
|
|||||||
|
Compensation and benefits
|
$
|
6,568
|
|
$
|
3,296
|
|
||
|
Research and development costs
|
10,449
|
|
9,362
|
|
||||
|
UCLB milestone
|
663
|
|
638
|
|
||||
|
Professional fees
|
2,611
|
|
4,130
|
|
||||
|
Deferred rent
|
0
|
|
561
|
|
||||
|
Corporate tax
|
391
|
|
620
|
|
||||
|
Other liabilities
|
716
|
|
447
|
|
||||
|
Total accrued expenses and other liabilities
|
$
|
21,398
|
|
$
|
19,054
|
|
||
|
December 31,
|
September 30,
|
|||||||||||
|
2019
|
2018
|
2018
|
2017
|
|||||||||
|
Series A preferred shares
|
—
|
|
—
|
|
—
|
|
24,490,705
|
|
||||
|
Class B ordinary shares
|
—
|
|
—
|
|
—
|
|
3,375,196
|
|
||||
|
Class C ordinary shares
|
—
|
|
—
|
|
—
|
|
2,096,840
|
|
||||
|
Ordinary Shares
|
44,983,006
|
|
40,145,617
|
|
40,146,182
|
|
—
|
|
||||
|
Deferred shares
|
34,425
|
|
34,425
|
|
34,425
|
|
—
|
|
||||
|
Deferred B shares
|
88,893,548
|
|
88,893,548
|
|
88,893,548
|
|
—
|
|
||||
|
Deferred C share
|
1
|
|
1
|
|
1
|
|
—
|
|
||||
|
Total Ordinary and Deferred Shares
|
133,910,980
|
|
129,073,591
|
|
129,074,156
|
|
29,962,741
|
|
||||
|
Year Ended December 31,
|
Three Months Ended December 31,
|
Year Ended September 30,
|
|||||
|
2019
|
2018
|
2018
|
2017
|
||||
|
Expected option life (years)
|
5.27 to 6.08
|
6.08
|
6.08
|
6.08
|
|||
|
Risk-free interest rate
|
1.39% to 2.66%
|
2.70% to 3.09%
|
2.61% to 3.00%
|
1.91% to 2.05%
|
|||
|
Expected volatility
|
72.30% to 76.22%
|
69.52% to 71.26%
|
68.15% to 72.99%
|
68.61% to 68.93%
|
|||
|
Expected dividend yield
|
—%
|
—%
|
—%
|
—%
|
|||
|
Number of
Options |
Weighted-
Average Exercise Price |
Weighted-
Average Remaining Contractual Term (Years) |
Aggregate
Intrinsic Value |
|||||||||||
|
Outstanding as of December 31, 2018
|
3,711,274
|
|
$
|
19.25
|
|
9.47
|
|
$
|
51,464
|
|
||||
|
Granted
|
2,438,145
|
|
15.22
|
|
—
|
|
—
|
|
||||||
|
Exercised
|
(11,724
|
)
|
3.94
|
|
—
|
|
$
|
198
|
|
|||||
|
Forfeited
|
(201,456
|
)
|
19.72
|
|
—
|
|
—
|
|
||||||
|
Outstanding as of December 31, 2019
|
5,936,239
|
|
$
|
17.71
|
|
9.02
|
|
$
|
11,873
|
|
||||
|
Exercisable as of December 31, 2019
|
1,466,625
|
|
$
|
16.34
|
|
8.63
|
|
$
|
6,124
|
|
||||
|
Vested and expected to vest as of December 31, 2019
|
5,936,239
|
|
$
|
17.71
|
|
9.02
|
|
$
|
11,873
|
|
||||
|
March 2,
2016 |
April 26,
2017 |
September 25,
2017 |
March 31, 2018
|
May 31, 2018
|
|||||||||||
|
Expected term
|
2.8 years
|
|
1.2 years
|
|
0.8 years
|
|
1.8 years
|
|
1.8 years
|
|
|||||
|
Risk-free interest rate
|
1.0
|
%
|
1.0
|
%
|
1.3
|
%
|
2.1
|
%
|
2.1
|
%
|
|||||
|
Expected volatility
|
73.2
|
%
|
76.6
|
%
|
71.0
|
%
|
71
|
%
|
71
|
%
|
|||||
|
Expected dividend yield
|
0.0
|
%
|
0.0
|
%
|
0.0
|
%
|
0.0
|
%
|
0.0
|
%
|
|||||
|
Number of
restricted shares |
Weighted average
grant date fair value |
||||||
|
Unvested and outstanding at December 31, 2018
|
708,834
|
|
$
|
4.10
|
|
||
|
Granted
|
0
|
|
0.00
|
|
|||
|
Vested
|
(389,755
|
)
|
4.31
|
|
|||
|
Forfeited
|
(4,335
|
)
|
4.43
|
|
|||
|
Unvested and outstanding at December 31, 2019
|
314,744
|
|
9.05
|
|
|||
|
Number of
restricted units |
Weighted average
grant date fair value |
||||||
|
Unvested and outstanding at December 31, 2018
|
—
|
|
$
|
—
|
|
||
|
Granted
|
500,000
|
|
12.09
|
|
|||
|
Vested
|
—
|
|
—
|
|
|||
|
Forfeited
|
—
|
|
—
|
|
|||
|
Unvested and outstanding at December 31, 2019
|
500,000
|
|
12.09
|
|
|||
|
Year Ended December 31,
|
Three Months December 31,
|
Years Ended September 30,
|
|||||||||||||
|
2019
|
2018
|
2018
|
2017
|
||||||||||||
|
Research and development
|
$
|
17,761
|
|
$
|
1,906
|
|
$
|
3,116
|
|
$
|
1,145
|
|
|||
|
General and administrative
|
12,451
|
|
1,487
|
|
3,649
|
|
2,008
|
|
|||||||
|
Capitalized to fixed assets
|
174
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Total share-based compensation
|
$
|
30,386
|
|
$
|
3,393
|
|
$
|
6,765
|
|
$
|
3,153
|
|
|||
|
Year ended December 31,
|
Three months ended December 31,
|
Year ended September 30,
|
||||||||||||||
|
2019
|
2018
|
2018
|
2017
|
|||||||||||||
|
Net loss before taxes
|
$
|
(139,008
|
)
|
$
|
(23,253
|
)
|
$
|
(52,031
|
)
|
$
|
(23,380
|
)
|
||||
|
U.K. statutory tax rate
|
19.0
|
%
|
19.0
|
%
|
19.0
|
%
|
19.5
|
%
|
||||||||
|
Income tax benefit at U.K. statutory tax rate
|
(26,411
|
)
|
(4,423
|
)
|
(9,886
|
)
|
(4,559
|
)
|
||||||||
|
Tax incentives / credits
|
(16,312
|
)
|
(3,234
|
)
|
(7,296
|
)
|
(3,702
|
)
|
||||||||
|
Non-deductible expenses
|
4,048
|
|
127
|
|
1,553
|
|
609
|
|
||||||||
|
Adjustments in respect of prior years
|
96
|
|
265
|
|
(13
|
)
|
13
|
|
||||||||
|
Operating losses
|
21,643
|
|
3,605
|
|
7,317
|
|
3,754
|
|
||||||||
|
Tax on property, plant, equipment and intangibles
|
267
|
|
140
|
|
233
|
|
113
|
|
||||||||
|
Other, net
|
1,510
|
|
915
|
|
812
|
|
119
|
|
||||||||
|
Total income tax benefit
|
$
|
(15,159
|
)
|
$
|
(2,605
|
)
|
$
|
(7,280
|
)
|
$
|
(3,653
|
)
|
||||
|
Current income tax benefit
|
(14,749
|
)
|
(2,605
|
)
|
(7,280
|
)
|
(3,653
|
)
|
||||||||
|
Deferred income tax benefit
|
(410
|
)
|
—
|
|
—
|
|
—
|
|
||||||||
|
Effective rate of income tax
|
10.9
|
%
|
11.2
|
%
|
14.0
|
%
|
15.6
|
%
|
||||||||
|
December 31,
|
||||||||
|
2019
|
2018
|
|||||||
|
Deferred tax assets:
|
||||||||
|
Other differences
|
$
|
1,522
|
|
$
|
2,870
|
|
||
|
Tax losses
|
19,624
|
|
7,851
|
|
||||
|
Fixed assets
|
896
|
|
621
|
|
||||
|
Total deferred tax assets
|
22,042
|
|
11,342
|
|
||||
|
Valuation allowances
|
(21,632
|
)
|
(11,342
|
)
|
||||
|
Net deferred tax asset (liability)
|
$
|
410
|
|
$
|
—
|
|
||
|
•
|
As the Company's leases do not provide an implicit rate, it estimated the incremental borrowing rate for each lease based on a yield curve analysis, utilizing the interest rate derived from the fair value analysis of its existing leases and adjusting it for factors that appropriately reflect the profile of secured borrowing over the lease term. For leases existing as of the adoption date, the Company has utilized its incremental borrowing rate based on the remaining lease term as of the adoption date. For leases that commenced after the adoption date, the Company determined the incremental borrowing rate based on the lease term as determined at the commencement date of the lease.
|
|
•
|
The expected lease terms include both contractual lease periods and, when applicable, cancelable option periods where failure to exercise such options would result in an economic penalty.
|
|
•
|
Since the Company elected to account for each lease component and its associated non-lease components as a single combined lease component, all contract consideration was allocated to the combined lease component.
|
|
Leases (in thousands)
|
Balance Sheet Classification
|
As of December 31, 2019
|
||||
|
Assets
|
||||||
|
Operating lease assets
|
Operating right-of-use assets
|
$
|
23,409
|
|
||
|
Liabilities
|
||||||
|
Current
|
||||||
|
Operating lease liabilities
|
Current liabilities: Operating lease liabilities
|
$
|
2,511
|
|
||
|
Noncurrent
|
||||||
|
Operating lease liabilities
|
Non-current liabilities: Operating lease liabilities
|
$
|
23,710
|
|
||
|
Total lease liabilities
|
$
|
26,221
|
|
|||
|
Lease cost (in thousands)
|
Statement of Operations Classification
|
Year Ended December 31, 2019
|
||||
|
Operating lease cost
|
Operating expenses: research and development
|
$
|
3,156
|
|
||
|
Variable lease cost
|
Operating expenses: research and development
|
1,222
|
|
|||
|
Operating lease cost
|
Operating expenses: general and administrative
|
1,241
|
|
|||
|
Variable lease cost
|
Operating expenses: general and administrative
|
250
|
|
|||
|
Short term lease costs
|
270
|
|
||||
|
Total lease cost
|
$
|
6,139
|
|
|||
|
Other information (in thousands)
|
December 31, 2019
|
|||
|
Cash paid for amounts included in the measurement of lease liabilities
|
||||
|
Operating cash flows from operating leases
|
$
|
2,415
|
|
|
|
Right-of-use assets obtained in exchange for new operating lease liabilities
|
$
|
26,124
|
|
|
|
Weighted-average remaining lease term — operating leases (years)
|
7.7
|
|
||
|
Weighted-average discount rate — operating leases
|
6.8
|
%
|
||
|
Operating Leases
|
|||
|
Maturity of lease liabilities for the years ending December 31,
|
(in thousands)
|
||
|
2020
|
4,232
|
|
|
|
2021
|
5,039
|
|
|
|
2022
|
4,460
|
|
|
|
2023
|
4,359
|
|
|
|
2024
|
3,869
|
|
|
|
Thereafter
|
12,222
|
|
|
|
Total lease payments
|
34,181
|
|
|
|
Less: imputed interest
|
(7,943
|
)
|
|
|
Less: foreign exchange (gain)/loss
|
(17
|
)
|
|
|
Present value of lease liabilities
|
26,221
|
|
|
|
Year ending December 31,
|
||||
|
2019
|
$
|
2,244
|
|
|
|
2020
|
2,332
|
|
||
|
2021
|
1,934
|
|
||
|
2022
|
1,324
|
|
||
|
2023
|
1,196
|
|
||
|
Thereafter
|
1,121
|
|
||
|
Total
|
$
|
10,151
|
|
|