|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ireland
|
|
98-1341933
|
|
State or other jurisdiction of incorporation or organization
|
|
(I.R.S. Employer Identification No.)
|
|
|
|
|
|
Block 10-1, Blanchardstown Corporate Park
Ballycoolin
Dublin 15, Ireland
|
|
Not Applicable
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
|
|
|
|
|
Registrant’s telephone number, including area code: +011-1-485-1200
|
||
|
|
|
|
|
American Depositary Shares*
Ordinary Shares**
|
|
NASDAQ Stock Market LLC
(NASDAQ Global Market)
|
|
Title of each class
|
|
Name of exchange on which registered
|
|
|
|
|
|
*
|
American Depositary Shares may be evidenced by American Depository Receipts. Each American Depositary Share represents one (1) Ordinary Share.
|
|
|
**
|
Nominal value $0.01 per share. Not for trading, but only in connection with the listing of American Depositary Shares.
|
|
|
|
Page #
|
|
|
|
|
|
|
|
|
|
|
|
||
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
|
|
|
|
|
|
|
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
|
|
|
|
|
|
|
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
|
|
|
|
|
|
|
|
Item 15.
|
||
|
|
|
|
|
•
|
our ability to fully pursue our business strategy is limited due to a decrease in our available liquid assets;
|
|
•
|
our recent restructuring plan may not be as effective as we anticipated and may have unintended negative impacts;
|
|
•
|
further restructuring actions, if needed, may require third-party consents that may not be granted;
|
|
•
|
the Chapter 11 bankruptcy filing by our subsidiary Avadel Specialty Pharmaceuticals LLC (“Specialty Pharma”) may have unexpected adverse results; and
|
|
•
|
a management-directed audit of the development program for our FT218 sodium oxybate product could result in changes that increase the cost of the program and further delay its completion; and
|
|
•
|
our three products Bloxiverz®,Vazculep® and Akovaz®, which are not patent protected, and have a small number of customers for such products, produce a majority of our revenues, and such products could face further competition resulting in a loss of market share and/or forcing us to further reduce our prices for those products;
|
|
•
|
we could fail to develop our current “unapproved marketed drug” (UMD) product candidate or future potential UMD product candidates, or competitors could develop such products and apply for FDA approval of such products before us;
|
|
•
|
we could experience failure or delay in completing the Phase 3 clinical trial for our FT218 product, and if the FDA ultimately approves such product, the approval may not include any period of market exclusivity;
|
|
•
|
Servicing our $143.75 million Exchangeable Senior Notes due 2023 may require a significant amount of cash, and we may not have sufficient cash or the ability to raise the funds necessary to settle exchanges of the 2023 Notes in cash, repay the Notes at maturity, or repurchase the 2023 Notes as required following a “fundamental change” event described in the indenture governing the 2023 Notes;
|
|
•
|
our products may not reach the commercial market or gain market acceptance;
|
|
•
|
we must invest substantial sums in research and development in order to remain competitive;
|
|
•
|
we depend on one or a limited number of providers to develop certain of our products and drug delivery technologies, to manufacture certain of our products and to provide certain raw materials used in our products;
|
|
•
|
our competitors may develop and market technologies or products that are more effective or safer than ours, or obtain regulatory approval and market such technologies or products before we do;
|
|
•
|
we face challenges in protecting intellectual property underlying our products and drug delivery technologies; and
|
|
•
|
we depend on key personnel to execute our business plan.
|
|
•
|
Akovaz®
(ephedrine sulfate injection, USP), an alpha- and beta-adrenergic agonist and a norepinephrine-releasing agent that is indicated for the treatment of clinically important hypotension occurring in the setting of anesthesia
.
|
|
•
|
Bloxiverz®
(neostigmine methylsulfate injection), a cholinesterase inhibitor, is indicated for the reversal of the effects of non-depolarizing neuromuscular blocking agents (NMBAs) after surgery.
|
|
•
|
Vazculep®
(phenylephrine hydrochloride injection), an alpha-1 adrenergic receptor agonist indicated for the treatment of clinically important hypotension resulting primarily from vasodilation in the setting of anesthesia.
|
|
•
|
Noctiva™
, a vasopressin analog indicated for the treatment of nocturia due to nocturnal polyuria in adults who awaken at least two times per night to void. Due to disappointing results after a substantial investment of resources after
Noctiva’s
commercial launch in March 2018
, Specialty Pharma,
the Avadel subsidiary responsible for the marketing and sale of
Noctiva,
made a voluntary filing for Chapter 11 bankruptcy protection on February 6, 2019. Although Specialty Pharma currently continues its marketing and sales efforts for this product, Avadel anticipates that Specialty Pharma will discontinue all activities with respect to
Noctiva
during 2019 as a result of the bankruptcy.
|
|
•
|
Bloxiverz®
(neostigmine methylsulfate injection),
Bloxiverz’s NDA was filed on July 31, 2012. Bloxiverz was approved by the FDA on May 31, 2013 and was launched in July 2013. Bloxiverz is a drug used intravenously in the operating room for the reversal of the effects of non-depolarizing neuromuscular blocking agents after surgery. Bloxiverz was the first FDA-approved version of neostigmine methylsulfate. Today, neostigmine is one of the two the most frequently used products for the reversal of the effects of other agents used for neuromuscular blocks. There are approximately 2.5 million vials sold annually in the U.S. In the future, sales of Bloxiverz are dependent upon the competitive market dynamics between Avadel and four other competitors in addition to any additional competitors who may obtain FDA approval of an abbreviated new drug application (ANDA) for a generic form of
Bloxiverz
.
|
|
•
|
Vazculep®
(phenylephrine hydrochloride injection)
On June 28, 2013, Avadel filed an NDA for Vazculep (phenylephrine hydrochloride injection). The product was approved by the FDA on June 27, 2014 and is indicated for the treatment of clinically important hypotension occurring in the setting of anesthesia. Avadel started shipping Vazculep (in 1mL single use vials, and 5mL and 10mL pharmacy bulk package vials) to wholesalers in October 2014. There are approximately 7 million vials sold annually in the U.S. Vazculep is the only FDA-approved version of phenylephrine hydrochloride to be available in all three vial sizes. Avadel competes against one other manufacturer who commercializes the 1mL single-dose vial. The volume of sales of Vazculep is dependent upon the competitive landscape in the marketplace, and potential for new competitors that may receive generic approvals in the future.
|
|
•
|
Akovaz® (ephedrine sulfate injection)
. On June 30, 2015, Avadel announced that our third NDA was accepted by the FDA; the FDA subsequently approved
Akovaz
on April 29, 2016. On August 12, 2016, Avadel launched Akovaz, into a market of approximately 7.5 million vials annually in the U.S. Avadel was the first approved formulation of ephedrine sulfate, an alpha- and beta- adrenergic agonist and a norepinephrine-releasing agent that is indicated for the treatment of
|
|
Proprietary Product Pipeline
|
||||||
|
Platform / Strategy
|
|
Drug/Product
|
|
Indication
|
|
Stage
|
|
Micropump®
|
|
Sodium oxybate
|
|
EDS / Cataplexy
|
|
Phase 3 trial ongoing
|
|
UMD #4
|
Sterile Injectable - Drug Undisclosed
|
Undisclosed
|
Development ongoing
|
|||
|
•
|
Flamel ceased to exist as a separate entity and the Company continued as the surviving entity and assumed all of the assets and liabilities of Flamel.
|
|
•
|
our authorized share capital is $5,500 divided into 500,000,000 ordinary shares with a nominal value of $0.01 each and 50,000,000 preferred shares with a nominal value of $0.01 each
|
|
•
|
all outstanding ordinary shares of Flamel, €0.122 nominal value per share, were canceled and exchanged on a one-for-one basis for newly issued ordinary shares of the Company, $0.01 nominal value per share. This change in nominal value of our outstanding shares resulted in our reclassifying $5,937 on our balance sheet from ordinary shares to additional paid-in capital
|
|
•
|
our Board of Directors is authorized to issue preferred shares on a non-pre-emptive basis, for a maximum period of five years, at which point such an authorization may be renewed by shareholders. The Board of Directors has discretion to dictate terms attached to the preferred shares, including voting, dividend, conversion rights, and priority relative to other classes of shares with respect to dividends and upon a liquidation.
|
|
•
|
all outstanding American Depositary Shares (ADSs) representing ordinary shares of Flamel were canceled and exchanged on a one-for-one basis for ADSs representing ordinary shares of the Company.
|
|
•
|
the FDA, the European Medicines Agency (“EMA”), the competent authority of an EU Member State or an Institutional Review Board (“IRB”), or an Ethics Committee (EU equivalent to IRB), or our partners may delay or halt applicable clinical trials;
|
|
•
|
we or our partners may face slower than expected rate of patient recruitment and enrollment in clinical trials, or may devote insufficient funding to the clinical trials;
|
|
•
|
our drug delivery technologies and drug products may be found to be ineffective or to cause harmful side effects, or may fail during any stage of pre-clinical testing or clinical trials;
|
|
•
|
we or our partners may find that certain products cannot be manufactured on a commercial scale and, therefore, may not be economical or feasible to produce; or
|
|
•
|
our products could fail to obtain regulatory approval or, if approved, could fail to achieve market acceptance, could fail to be included within the pricing and reimbursement schemes of the U.S. or EU Member States, or could be precluded from commercialization by proprietary rights of third parties.
|
|
•
|
the scope of regulatory approvals, including limitations or warnings in a product’s regulatory-approved labeling;
|
|
•
|
in the case of any new “unapproved-marketed-drug” product we may successfully pursue, whether and the extent to which the FDA removes competing products from the market;
|
|
•
|
demonstration of the clinical safety and efficacy of the product or technology;
|
|
•
|
the absence of evidence of undesirable side effects of the product or technology that delay or extend trials;
|
|
•
|
the lack of regulatory delays or other regulatory actions;
|
|
•
|
its cost-effectiveness and related access to payor coverage;
|
|
•
|
its potential advantage over alternative treatment methods;
|
|
•
|
the availability of third-party reimbursement; and
|
|
•
|
the marketing and distribution support it receives.
|
|
•
|
the jurisdictions in which profits are determined to be earned and taxed;
|
|
•
|
increases in expenses not deductible for tax purposes, including increases in the fair value of related party payables, write-offs of acquired in-process R&D and impairment of goodwill in connection with acquisitions;
|
|
•
|
changes in domestic or international tax laws or the interpretation of such tax laws;
|
|
•
|
adjustments to estimated taxes upon finalization of various tax returns;
|
|
•
|
changes in available tax credits;
|
|
•
|
changes in share-based compensation expense;
|
|
•
|
changes in the valuation of our deferred tax assets and liabilities;
|
|
•
|
the resolution of issues arising from tax audits with various tax authorities; and
|
|
•
|
the tax effects of purchase accounting for acquisitions that may cause fluctuations between reporting periods.
|
|
•
|
adverse drug experiences and other reporting requirements;
|
|
•
|
product promotion and marketing;
|
|
•
|
APIs and/or product manufacturing, including cGMP compliance;
|
|
•
|
record keeping;
|
|
•
|
distribution of drug samples;
|
|
•
|
required clinical trials and/or post-marketing studies;
|
|
•
|
authorization renewal procedures;
|
|
•
|
authorization variation procedures;
|
|
•
|
compliance with any required REMS;
|
|
•
|
updating safety and efficacy information;
|
|
•
|
processing of personal data;
|
|
•
|
use of electronic records and signatures; and
|
|
•
|
changes to product manufacturing or labeling.
|
|
•
|
obtaining regulatory approval to commence a trial;
|
|
•
|
reaching agreement on acceptable terms with prospective contract research organizations (“CROs”) and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
|
|
•
|
obtaining institutional review board or ethics committee approval at each site;
|
|
•
|
recruiting suitable patients to participate in a trial;
|
|
•
|
having patients complete a trial or return for post-treatment follow-up;
|
|
•
|
clinical sites dropping out of a trial;
|
|
•
|
adding new sites; or
|
|
•
|
manufacturing sufficient quantities of medicine candidates for use in clinical trials.
|
|
•
|
issue warning letters;
|
|
•
|
impose fines;
|
|
•
|
seize products or request or order recalls;
|
|
•
|
issue injunctions to stop future sales of products;
|
|
•
|
refuse to permit products to be imported into, or exported out of, the U.S. or the E.U.;
|
|
•
|
suspend or limit our production;
|
|
•
|
withdraw or vary approval of marketing applications;
|
|
•
|
order the competent authorities of EU Member States to withdraw or vary national authorization; and
|
|
•
|
initiate criminal prosecutions.
|
|
•
|
warning letters or untitled letters;
|
|
•
|
fines and civil penalties;
|
|
•
|
delays in clearing or approving, or refusal to clear or approve, products;
|
|
•
|
withdrawal, suspension or variation of approval of products; product recall or seizure;
|
|
•
|
orders to the competent authorities of EU Member States to withdraw or vary national authorization;
|
|
•
|
orders for physician notification or device repair, replacement or refund;
|
|
•
|
interruption of production;
|
|
•
|
operating restrictions;
|
|
•
|
injunctions; and
|
|
•
|
criminal prosecution.
|
|
•
|
fluctuations in our operating results;
|
|
•
|
announcements of technological partnerships, innovations or new products by us or our competitors;
|
|
•
|
actions with respect to the acquisition of new or complementary businesses;
|
|
•
|
governmental regulations;
|
|
•
|
developments in patent or other proprietary rights owned by us or others;
|
|
•
|
public concern as to the safety of drug delivery technologies developed by us or drugs developed by others using our platform;
|
|
•
|
the results of pre-clinical testing and clinical studies or trials by us or our competitors;
|
|
•
|
adverse events related to our products or products developed by pharmaceutical and biotechnology company partners that use our drug delivery technologies;
|
|
•
|
lack of efficacy of our products;
|
|
•
|
litigation;
|
|
•
|
decisions by our pharmaceutical and biotechnology company partners relating to the products incorporating our technologies;
|
|
•
|
the perception by the market of specialty pharma, biotechnology, and high technology companies generally;
|
|
•
|
general market conditions, including the impact of the current financial environment; and
|
|
•
|
the dilutive impact of any new equity or convertible debt securities we may issue or have issued.
|
|
•
|
the demand for our drug delivery technologies and products;
|
|
•
|
the level of product and price competition;
|
|
•
|
our ability to develop new partnerships and additional commercial applications for our products;
|
|
•
|
our ability to control our costs;
|
|
•
|
our ability to broaden our customer base;
|
|
•
|
the effectiveness of our marketing strategy;
|
|
•
|
our effective tax rate;
|
|
•
|
the effectiveness of our partners’ marketing strategy for products that use our technology; and
|
|
•
|
general economic conditions.
|
|
•
|
the development and acquisition of new products and drug delivery technologies;
|
|
•
|
the progress of our research and product development programs; and
|
|
•
|
the timing of, and amounts received from, future product sales, product development fees and licensing revenue and royalties.
|
|
•
|
permit our board of directors to issue preferred shares with such rights and preferences as they may designate, subject to applicable law;
|
|
•
|
impose advance notice requirements for shareholder proposals and director nominations to be considered at annual shareholder meetings; and
|
|
•
|
require the approval of a supermajority of the voting power of the shares of our share capital entitled to vote generally at a meeting of shareholders to amend or repeal certain provisions of our articles of association.
|
|
•
|
effect service of process within the U.S. against us and our non-U.S. resident directors and officers;
|
|
•
|
enforce United States court judgments based upon the civil liability provisions of the United States federal securities laws against us and our non-U.S. resident directors and officers in Ireland; or
|
|
•
|
bring an original action in an Irish court to enforce liabilities based upon the U.S. federal securities laws against us and our non-U.S. resident directors and officers.
|
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
|
|
|
2018 Price Range
|
2017 Price Range
|
|||||||||||||
|
|
High
|
Low
|
High
|
Low
|
|||||||||||
|
First quarter
|
$
|
11.70
|
|
$
|
6.76
|
|
$
|
12.30
|
|
$
|
8.87
|
|
|||
|
Second quarter
|
7.78
|
|
5.89
|
|
11.72
|
|
8.75
|
|
|||||||
|
Third quarter
|
7.14
|
|
4.08
|
|
11.18
|
|
8.14
|
|
|||||||
|
Fourth quarter
|
4.66
|
|
1.74
|
|
11.53
|
|
7.52
|
|
|||||||
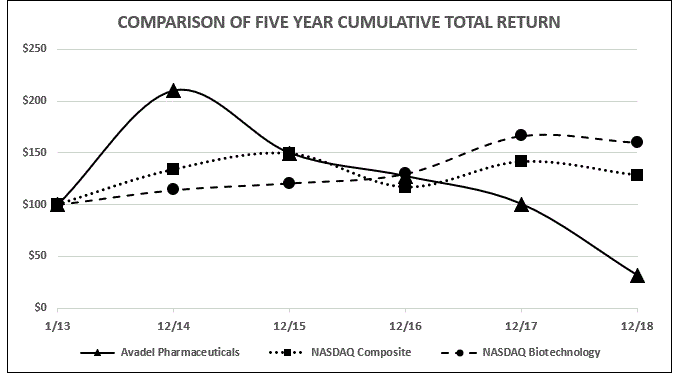
|
Statement of (Loss) Income Data:
|
2018
|
2017
|
2016
|
2015
|
2014
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Total revenues
|
$
|
103,269
|
|
$
|
173,245
|
|
$
|
150,246
|
|
$
|
173,009
|
|
$
|
14,975
|
|
|||||
|
Gross profit
(a)
|
85,753
|
|
156,944
|
|
136,998
|
|
161,599
|
|
11,592
|
|
||||||||||
|
Operating (loss) income
|
(104,926
|
)
|
89,505
|
|
(4,965
|
)
|
70,758
|
|
(93,657
|
)
|
||||||||||
|
Net (loss) income from continuing operations
|
(95,304
|
)
|
68,271
|
|
(41,276
|
)
|
41,798
|
|
(89,487
|
)
|
||||||||||
|
Net income from discontinued operations
|
—
|
|
—
|
|
—
|
|
—
|
|
4,018
|
|
||||||||||
|
Net (loss) income
|
(95,304
|
)
|
68,271
|
|
(41,276
|
)
|
41,798
|
|
(85,469
|
)
|
||||||||||
|
Net (loss) income per share - basic:
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
Continuing operations
|
(2.55
|
)
|
1.69
|
|
(1.00
|
)
|
1.03
|
|
(2.47
|
)
|
||||||||||
|
Discontinued operations
|
—
|
|
—
|
|
—
|
|
—
|
|
0.11
|
|
||||||||||
|
Net (loss) income per share - basic
|
(2.55
|
)
|
1.69
|
|
(1.00
|
)
|
1.03
|
|
(2.36
|
)
|
||||||||||
|
Net (loss) income per share - diluted:
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
Continuing operations
|
(2.55
|
)
|
1.63
|
|
(1.00
|
)
|
0.96
|
|
(2.47
|
)
|
||||||||||
|
Discontinued operations
|
—
|
|
—
|
|
—
|
|
—
|
|
0.11
|
|
||||||||||
|
Net (loss) income per share - diluted
|
(2.55
|
)
|
1.63
|
|
(1.00
|
)
|
0.96
|
|
(2.36
|
)
|
||||||||||
|
Balance Sheet Data:
|
2018
|
2017
|
2016
|
2015
|
2014
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Cash and cash equivalents
|
$
|
9,325
|
|
$
|
16,564
|
|
$
|
39,215
|
|
$
|
65,064
|
|
$
|
39,760
|
|
|||||
|
Marketable securities
|
90,590
|
|
77,511
|
|
114,980
|
|
79,738
|
|
53,074
|
|
||||||||||
|
Goodwill
|
18,491
|
|
18,491
|
|
18,491
|
|
18,491
|
|
18,491
|
|
||||||||||
|
Intangible assets, net
|
1,629
|
|
92,289
|
|
22,837
|
|
15,825
|
|
28,389
|
|
||||||||||
|
Total assets
|
190,300
|
|
253,277
|
|
245,482
|
|
215,081
|
|
174,382
|
|
||||||||||
|
Long-term debt (incl. current portion)
|
115,840
|
|
267
|
|
815
|
|
1,118
|
|
3,717
|
|
||||||||||
|
Long-term related party payable (incl. current portion)
|
28,840
|
|
98,925
|
|
169,347
|
|
122,693
|
|
114,750
|
|
||||||||||
|
2018:
|
March 31
|
June 30
|
September 30
|
December 31
|
|||||||||||
|
|
|
|
|
|
|
|
|
||||||||
|
Revenues
|
$
|
33,293
|
|
$
|
29,230
|
|
$
|
19,826
|
|
$
|
20,920
|
|
|||
|
Gross profit
(a)
|
26,701
|
|
25,718
|
|
16,706
|
|
16,628
|
|
|||||||
|
Operating loss
(b)
|
(12,625
|
)
|
(2,785
|
)
|
(14,095
|
)
|
(75,421
|
)
|
|||||||
|
Net loss
|
(12,236
|
)
|
(3,438
|
)
|
(15,771
|
)
|
(63,859
|
)
|
|||||||
|
Net loss per share - basic
|
(0.32
|
)
|
(0.09
|
)
|
(0.43
|
)
|
(1.72
|
)
|
|||||||
|
Net loss per share - diluted
|
(0.32
|
)
|
(0.09
|
)
|
(0.43
|
)
|
(1.72
|
)
|
|||||||
|
2017:
|
March 31
|
June 30
|
September 30
|
December 31
|
|||||||||||
|
|
|
|
|
|
|
|
|
||||||||
|
Revenues
|
$
|
52,507
|
|
$
|
46,311
|
|
$
|
39,675
|
|
$
|
34,752
|
|
|||
|
Gross profit
(a)
|
48,605
|
|
41,750
|
|
35,885
|
|
30,704
|
|
|||||||
|
Operating income (loss)
|
33,341
|
|
34,126
|
|
26,118
|
|
(4,080
|
)
|
|||||||
|
Net income (loss)
|
25,910
|
|
28,927
|
|
21,679
|
|
(8,245
|
)
|
|||||||
|
Net income (loss) per share - basic
|
0.63
|
|
0.70
|
|
0.54
|
|
(0.21
|
)
|
|||||||
|
Net income (loss) per share - diluted
|
0.61
|
|
0.68
|
|
0.52
|
|
(0.21
|
)
|
|||||||
|
•
|
Akovaz®
(ephedrine sulfate injection, USP), an alpha- and beta-adrenergic agonist and a norepinephrine-releasing agent that is indicated for the treatment of clinically important hypotension occurring in the setting of anesthesia
.
|
|
•
|
Bloxiverz®
(neostigmine methylsulfate injection), a cholinesterase inhibitor, is indicated for the reversal of the effects of non-depolarizing neuromuscular blocking agents (NMBAs) after surgery.
|
|
•
|
Vazculep®
(phenylephrine hydrochloride injection), an alpha-1 adrenergic receptor agonist indicated for the treatment of clinically important hypotension resulting primarily from vasodilation in the setting of anesthesia.
|
|
•
|
Noctiva™
, a vasopressin analog indicated for the treatment of nocturia due to nocturnal polyuria in adults who awaken at least two times per night to void. Due to disappointing results after a substantial investment of resources after
Noctiva’s
commercial launch in March 2018
, Specialty Pharma,
the Avadel subsidiary responsible for the marketing and sale of
Noctiva,
made a voluntary filing for Chapter 11 bankruptcy protection on February 6, 2019. Although Specialty Pharma currently continues its marketing and sales efforts for this product, Avadel anticipates that Specialty Pharma will discontinue all activities with respect to
Noctiva
during 2019 as a result of the bankruptcy.
|
|
•
|
Healthcare and Regulatory Reform
: Various health care reform laws in the U.S. may impact our ability to successfully commercialize our products and technologies. The success of our commercialization efforts may depend on the extent to which the government health administration authorities, the health insurance funds in the E.U. Member States, private health insurers and other third-party payers in the U.S. will reimburse consumers for the cost of healthcare products and services.
|
|
•
|
Competition and Technological Change:
Competition in the pharmaceutical and biotechnology industry continues to be intense and is expected to increase. We compete with academic laboratories, research institutions, universities, joint ventures, and other pharmaceutical and biotechnology companies, including other companies developing niche branded or generic specialty pharmaceutical products or drug delivery platforms. Furthermore, major technological changes can happen quickly in the pharmaceutical and biotechnology industries. Such rapid technological change, or the development by our competitors of technologically improved or differentiated products, could render our drug delivery platforms obsolete or noncompetitive.
|
|
•
|
Pricing Environment for Pharmaceuticals
: The pricing environment continues to be in the political spotlight in the U.S. As a result, the need to obtain and maintain appropriate pricing for our products may become more challenging due to, among other things, the attention being paid to healthcare cost containment and other austerity measures in the U.S. and worldwide.
|
|
•
|
Generics Playing a Larger Role in Healthcare
: Generic pharmaceutical products will continue to play a large role in the U.S. healthcare system. Specifically, we have seen, or likely will see, additional generic competition to our current and future products and we continue to expect generic competition in the future.
|
|
•
|
Access to and Cost of Capital
: The process of raising capital and associated cost of such capital for a company of our financial profile can be difficult and potentially expensive. If the need were to arise to raise additional capital, access to that capital may be difficult and/or expensive and, as a result, could create liquidity challenges for the Company.
|
|
•
|
Possible Net Loss from Operations in 2019:
In part because we expect sales of our hospital products to significantly decline from 2018’s levels and we will incur substantial expenses to further the clinical development of FT218, we likely will incur a net loss in 2019, the amount of which is not known to us at this time.
|
|
•
|
Revenue was
$103,269
for the year ended
December 31, 2018
compared to
$173,245
in the same period last year. This decrease was primarily the result of increased competition driving lower prices in our hospital injectables products as noted above in our discussion of
Key Business Trends and Highlights
. We experienced price declines across all of our hospital products and a unit volume decline with our Akovaz product due to additional competition.
|
|
•
|
Operating loss was
$104,926
for the year ended
December 31, 2018
compared to operating income of
$89,505
for the year ended
December 31, 2017
. The primary reasons for the decrease in operating income was due to a decrease in gross margin of
$71,191
driven by lower revenue as described above, impairment of the Noctiva intangible asset of
$66,087
and higher SG&A of
$41,499
primarily driven by sales and marketing costs related to Noctiva.
|
|
•
|
Net loss was
$95,304
for the year ended
December 31, 2018
compared to net income of
$68,271
in the same period last year.
|
|
•
|
Diluted net loss per share was
$2.55
for the year ended
December 31, 2018
compared to diluted net income per share of
$1.63
in the same period last year.
|
|
•
|
Cash and marketable securities increased
$5,840
to
$99,915
at
December 31, 2018
from
$94,075
at
December 31, 2017
. The increase primarily results from net proceeds from our February 2018 debt issuance of
$137,560
, partially offset by our use of cash in operating activities of
$82,716
, a milestone payment of
$20,000
for Noctiva and
$27,637
in cash used as a return to shareholders through our share buyback program.
|
|
|
|
|
|
Increase / (Decrease)
|
||||||||||||||||||||||
|
|
Years Ended December 31,
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||||||||||||
|
Comparative Statements of (Loss) Income:
|
2018
|
2017
|
2016
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Product sales
|
$
|
101,423
|
|
$
|
172,841
|
|
$
|
147,222
|
|
$
|
(71,418
|
)
|
(41.3
|
)%
|
$
|
25,619
|
|
17.4
|
%
|
|||||||
|
License revenue
|
1,846
|
|
404
|
|
3,024
|
|
1,442
|
|
356.9
|
%
|
(2,620
|
)
|
(86.6
|
)%
|
||||||||||||
|
Total revenues
|
103,269
|
|
173,245
|
|
150,246
|
|
(69,976
|
)
|
(40.4
|
)%
|
22,999
|
|
15.3
|
%
|
||||||||||||
|
Operating expenses:
|
||||||||||||||||||||||||||
|
Cost of products
|
17,516
|
|
16,301
|
|
13,248
|
|
1,215
|
|
7.5
|
%
|
3,053
|
|
23.0
|
%
|
||||||||||||
|
Research and development expenses
|
39,329
|
|
33,418
|
|
34,611
|
|
5,911
|
|
17.7
|
%
|
(1,193
|
)
|
(3.4
|
)%
|
||||||||||||
|
Selling, general and administrative expenses
|
100,359
|
|
58,860
|
|
44,179
|
|
41,499
|
|
70.5
|
%
|
14,681
|
|
33.2
|
%
|
||||||||||||
|
Intangible asset amortization
|
6,619
|
|
3,659
|
|
13,888
|
|
2,960
|
|
80.9
|
%
|
(10,229
|
)
|
(73.7
|
)%
|
||||||||||||
|
(Gain) loss - changes in fair value of related party contingent consideration
|
(22,731
|
)
|
(31,040
|
)
|
49,285
|
|
8,309
|
|
26.8
|
%
|
(80,325
|
)
|
(163.0
|
)%
|
||||||||||||
|
Impairment of intangible asset
|
66,087
|
|
—
|
|
—
|
|
66,087
|
|
100.0
|
%
|
—
|
|
—
|
%
|
||||||||||||
|
Restructuring costs
|
1,016
|
|
2,542
|
|
—
|
|
(1,526
|
)
|
(60.0
|
)%
|
2,542
|
|
100.0
|
%
|
||||||||||||
|
Total operating expenses
|
208,195
|
|
83,740
|
|
155,211
|
|
124,455
|
|
148.6
|
%
|
(71,471
|
)
|
(46.0
|
)%
|
||||||||||||
|
Operating (loss) income
|
(104,926
|
)
|
|
89,505
|
|
|
(4,965
|
)
|
(194,431
|
)
|
(217.2
|
)%
|
94,470
|
|
1,902.7
|
%
|
||||||||||
|
Investment and other income, net
|
452
|
|
2,136
|
|
2,758
|
|
(1,684
|
)
|
(78.8
|
)%
|
(622
|
)
|
(22.6
|
)%
|
||||||||||||
|
Interest expense
|
(10,622
|
)
|
(1,052
|
)
|
(963
|
)
|
9,570
|
|
909.7
|
%
|
89
|
|
9.2
|
%
|
||||||||||||
|
Other income (expense) - changes in fair value of related party payable
|
1,899
|
|
2,071
|
|
(6,548
|
)
|
172
|
|
8.3
|
%
|
(8,619
|
)
|
(131.6
|
)%
|
||||||||||||
|
(Loss) income before income taxes
|
(113,197
|
)
|
92,660
|
|
(9,718
|
)
|
(205,857
|
)
|
(222.2
|
)%
|
102,378
|
|
1,053.5
|
%
|
||||||||||||
|
Income tax (benefit) provision
|
(17,893
|
)
|
24,389
|
|
31,558
|
|
(42,282
|
)
|
(173.4
|
)%
|
(7,169
|
)
|
(22.7
|
)%
|
||||||||||||
|
Net (loss) income
|
$
|
(95,304
|
)
|
$
|
68,271
|
|
$
|
(41,276
|
)
|
$
|
(163,575
|
)
|
(239.6
|
)%
|
$
|
109,547
|
|
265.4
|
%
|
|||||||
|
Net (loss) income per share - diluted
|
$
|
(2.55
|
)
|
$
|
1.63
|
|
$
|
(1.00
|
)
|
$
|
(4.18
|
)
|
(256.4
|
)%
|
$
|
2.63
|
|
263.0
|
%
|
|||||||
|
|
|
|
|
Increase / (Decrease)
|
||||||||||||||||||||||
|
|
Years Ended December 31,
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||||||||||||
|
Revenues
|
2018
|
2017
|
2016
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Bloxiverz
|
$
|
20,850
|
|
$
|
45,596
|
|
$
|
82,896
|
|
$
|
(24,746
|
)
|
(54.3
|
)%
|
$
|
(37,300
|
)
|
(45.0
|
)%
|
|||||||
|
Vazculep
|
42,916
|
|
38,187
|
|
39,796
|
|
4,729
|
|
12.4
|
%
|
(1,609
|
)
|
(4.0
|
)%
|
||||||||||||
|
Akovaz
|
33,759
|
|
80,617
|
|
16,831
|
|
(46,858
|
)
|
(58.1
|
)%
|
63,786
|
|
379.0
|
%
|
||||||||||||
|
Noctiva
|
1,204
|
|
—
|
|
—
|
|
1,204
|
|
100.0
|
%
|
—
|
|
n/a
|
|
||||||||||||
|
Other
|
2,694
|
|
8,441
|
|
7,699
|
|
(5,747
|
)
|
(68.1
|
)%
|
742
|
|
9.6
|
%
|
||||||||||||
|
Total product sales
|
101,423
|
|
172,841
|
|
147,222
|
|
(71,418
|
)
|
(41.3
|
)%
|
25,619
|
|
17.4
|
%
|
||||||||||||
|
License revenue
|
1,846
|
|
404
|
|
3,024
|
|
1,442
|
|
356.9
|
%
|
(2,620
|
)
|
(86.6
|
)%
|
||||||||||||
|
Total revenues
|
$
|
103,269
|
|
$
|
173,245
|
|
$
|
150,246
|
|
$
|
(69,976
|
)
|
(40.4
|
)%
|
$
|
22,999
|
|
15.3
|
%
|
|||||||
|
|
|
|
|
Increase / (Decrease)
|
||||||||||||||||||||||
|
|
Years Ended December 31,
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||||||||||||
|
Cost of Products
|
2018
|
2017
|
2016
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Cost of products
|
$
|
17,516
|
|
$
|
16,301
|
|
$
|
13,248
|
|
$
|
1,215
|
|
7.5
|
%
|
$
|
3,053
|
|
23.0
|
%
|
|||||||
|
Percentage of sales
|
17.0
|
%
|
9.4
|
%
|
8.8
|
%
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
Increase / (Decrease)
|
||||||||||||||||||||||
|
|
Years Ended December 31,
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||||||||||||
|
Research and Development Expenses
|
2018
|
2017
|
2016
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Research and development expenses
|
$
|
39,329
|
|
$
|
33,418
|
|
$
|
34,611
|
|
$
|
5,911
|
|
17.7
|
%
|
$
|
(1,193
|
)
|
(3.4
|
)%
|
|||||||
|
Percentage of sales
|
38.1
|
%
|
19.3
|
%
|
23.0
|
%
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
Increase / (Decrease)
|
||||||||||||||||||||||
|
|
Years Ended December 31,
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||||||||||||
|
Selling, General and Administrative Expenses
|
2018
|
2017
|
2016
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Selling, general and administrative expenses
|
$
|
100,359
|
|
$
|
58,860
|
|
$
|
44,179
|
|
$
|
41,499
|
|
70.5
|
%
|
$
|
14,681
|
|
33.2
|
%
|
|||||||
|
Percentage of sales
|
97.2
|
%
|
34.0
|
%
|
29.4
|
%
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
Increase / (Decrease)
|
||||||||||||||||||||||
|
|
Years Ended December 31,
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||||||||||||
|
Intangibles Asset Amortization
|
2018
|
2017
|
2016
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Intangible asset amortization
|
$
|
6,619
|
|
$
|
3,659
|
|
$
|
13,888
|
|
$
|
2,960
|
|
80.9
|
%
|
$
|
(10,229
|
)
|
(73.7
|
)%
|
|||||||
|
Percentage of sales
|
6.4
|
%
|
2.1
|
%
|
9.2
|
%
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
Increase / (Decrease)
|
||||||||||||||||||||||
|
|
Years Ended December 31,
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||||||||||||
|
Changes in Fair Value of Related Party Contingent Consideration
|
2018
|
2017
|
2016
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
(Gain) loss - changes in fair value of related party contingent consideration
|
$
|
(22,731
|
)
|
$
|
(31,040
|
)
|
$
|
49,285
|
|
$
|
8,309
|
|
26.8
|
%
|
$
|
(80,325
|
)
|
(163.0
|
)%
|
|||||||
|
Percentage of sales
|
(22.0
|
)%
|
(17.9
|
)%
|
32.8
|
%
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
Increase / (Decrease)
|
|||||||||||||||||||||
|
|
Years Ended December 31,
|
2018 vs. 2017
|
2017 vs. 2016
|
||||||||||||||||||||||
|
Impairment of Intangible Asset
|
2018
|
2017
|
2016
|
$
|
%
|
$
|
%
|
||||||||||||||||||
|
Impairment of intangible asset
|
$
|
66,087
|
|
$
|
—
|
|
$
|
—
|
|
$
|
66,087
|
|
100.0
|
%
|
$
|
—
|
|
n/a
|
|||||||
|
Percentage of sales
|
64.0
|
%
|
—
|
%
|
—
|
%
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
Increase / (Decrease)
|
||||||||||||||||||||||
|
|
Years Ended December 31,
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||||||||||||
|
Restructuring Costs
|
2018
|
2017
|
2016
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Restructuring costs
|
$
|
1,016
|
|
$
|
2,542
|
|
$
|
—
|
|
$
|
(1,526
|
)
|
(60.0
|
)%
|
$
|
2,542
|
|
100.0
|
%
|
|||||||
|
Percentage of sales
|
1.0
|
%
|
1.5
|
%
|
—
|
%
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
Increase / (Decrease)
|
||||||||||||||||||||||
|
|
Years Ended December 31,
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||||||||||||
|
Investment and Other Income, net
|
2018
|
2017
|
2016
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Investment and other income, net
|
$
|
452
|
|
$
|
2,136
|
|
$
|
2,758
|
|
$
|
(1,684
|
)
|
(78.8
|
)%
|
$
|
(622
|
)
|
(22.6
|
)%
|
|||||||
|
Percentage of sales
|
0.4
|
%
|
1.2
|
%
|
1.8
|
%
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
Increase / (Decrease)
|
||||||||||||||||||||||
|
|
Years Ended December 31,
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||||||||||||
|
Interest Expense
|
2018
|
2017
|
2016
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Interest expense
|
$
|
10,622
|
|
$
|
1,052
|
|
$
|
963
|
|
$
|
9,570
|
|
909.7
|
%
|
$
|
89
|
|
9.2
|
%
|
|||||||
|
Percentage of sales
|
(10.3
|
)%
|
(0.6
|
)%
|
(0.6
|
)%
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
Increase / (Decrease)
|
||||||||||||||||||||||
|
|
Years Ended December 31,
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||||||||||||
|
Other Income (Expense) - Changes in Fair Value of Related Party Payable:
|
2018
|
2017
|
2016
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Other income (expense) - changes in fair value of related party payable
|
$
|
1,899
|
|
$
|
2,071
|
|
$
|
(6,548
|
)
|
$
|
172
|
|
8.3
|
%
|
$
|
(8,619
|
)
|
(131.6
|
)%
|
|||||||
|
Percentage of sales
|
1.8
|
%
|
1.2
|
%
|
(4.4
|
)%
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
Increase / (Decrease)
|
||||||||||||||||||||||
|
|
Years Ended December 31,
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||||||||||||
|
Income Taxes:
|
2018
|
2017
|
2016
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Income tax (benefit) provision
|
$
|
(17,893
|
)
|
$
|
24,389
|
|
$
|
31,558
|
|
$
|
(42,282
|
)
|
(173.4
|
)%
|
$
|
(7,169
|
)
|
(22.7
|
)%
|
|||||||
|
Percentage of income (loss) before income taxes
|
15.8
|
%
|
26.3
|
%
|
(324.7
|
)%
|
|
|
|
|
|
|
|
|
||||||||||||
|
Reconciliation to Effective Income Tax Rate:
|
2018
|
2017
|
2016
|
|||||||||
|
Statutory tax rate
|
12.5
|
%
|
12.5
|
%
|
12.5
|
%
|
||||||
|
Differences in international tax rates
|
8.0
|
%
|
22.2
|
%
|
(31.9
|
)%
|
||||||
|
Nondeductible changes in fair value of contingent consideration
|
4.0
|
%
|
(11.6
|
)%
|
(165.0
|
)%
|
||||||
|
Income tax deferred charge
|
—
|
%
|
—
|
%
|
(9.7
|
)%
|
||||||
|
Change in valuation allowances
|
(5.3
|
)%
|
(0.7
|
)%
|
11.8
|
%
|
||||||
|
Nondeductible stock-based compensation
|
(1.3
|
)%
|
(0.4
|
)%
|
(14.8
|
)%
|
||||||
|
Cross border merger
|
—
|
%
|
0.3
|
%
|
(100.6
|
)%
|
||||||
|
Unrealized tax benefits
|
(1.3
|
)%
|
1.4
|
%
|
(15.2
|
)%
|
||||||
|
State and local taxes (net of federal)
|
(0.3
|
)%
|
0.3
|
%
|
(9.6
|
)%
|
||||||
|
Change in U.S. tax law
|
(0.2
|
)%
|
3.8
|
%
|
—
|
%
|
||||||
|
Nondeductible interest expense
|
(1.1
|
)%
|
—
|
%
|
—
|
%
|
||||||
|
Other
|
0.7
|
%
|
(1.5
|
)%
|
(2.3
|
)%
|
||||||
|
Effective income tax rate
|
15.7
|
%
|
26.3
|
%
|
(324.8
|
)%
|
||||||
|
Income tax (benefit) provision - at statutory tax rate
|
$
|
(14,149
|
)
|
$
|
11,582
|
|
$
|
(1,215
|
)
|
|||
|
Differences in international tax rates
|
(9,039
|
)
|
20,557
|
|
3,097
|
|
||||||
|
Nondeductible changes in fair value of contingent consideration
|
(4,559
|
)
|
(10,779
|
)
|
16,036
|
|
||||||
|
Income tax deferred charge
|
—
|
|
—
|
|
938
|
|
||||||
|
Change in valuation allowances
|
5,998
|
|
(610
|
)
|
(1,143
|
)
|
||||||
|
Nondeductible stock-based compensation
|
1,499
|
|
(375
|
)
|
1,436
|
|
||||||
|
Cross-border merger
|
—
|
|
265
|
|
9,773
|
|
||||||
|
Unrecognized tax benefits
|
1,440
|
|
1,296
|
|
1,475
|
|
||||||
|
State and local taxes (net of federal)
|
299
|
|
252
|
|
934
|
|
||||||
|
Change in U.S. tax law
|
274
|
|
3,513
|
|
—
|
|
||||||
|
Nondeductible interest expense
|
1,269
|
|
—
|
|
—
|
|
||||||
|
Other
|
(925
|
)
|
(1,312
|
)
|
227
|
|
||||||
|
Income tax (benefit) provision - at effective income tax rate
|
$
|
(17,893
|
)
|
$
|
24,389
|
|
$
|
31,558
|
|
|||
|
|
|
|
|
Increase / (Decrease)
|
|||||||||||||||||||||
|
|
Years Ended December 31,
|
2018 vs. 2017
|
2017 vs. 2016
|
||||||||||||||||||||||
|
Net Cash Provided By (Used In):
|
2018
|
2017
|
2016
|
$
|
%
|
$
|
%
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
Operating activities
|
$
|
(82,716
|
)
|
$
|
16,662
|
|
$
|
18,901
|
|
$
|
(99,378
|
)
|
(596.4
|
)%
|
$
|
(2,239
|
)
|
(11.8
|
)%
|
||||||
|
Investing activities
|
(36,981
|
)
|
(15,698
|
)
|
(36,630
|
)
|
(21,283
|
)
|
(135.6
|
)%
|
20,932
|
|
57.1
|
%
|
|||||||||||
|
Financing activities
|
112,659
|
|
(23,318
|
)
|
(7,954
|
)
|
135,977
|
|
583.1
|
%
|
(15,364
|
)
|
(193.2
|
)%
|
|||||||||||
|
Purchase Commitments:
|
Balance
|
|||
|
2019
|
$
|
10,754
|
|
|
|
2020
|
5,948
|
|
||
|
2021
|
4,880
|
|
||
|
2022
|
4,880
|
|
||
|
2023
|
220
|
|
||
|
Thereafter
|
—
|
|
||
|
Total
|
$
|
26,682
|
|
|
|
Lease Commitment:
|
Balance
|
|||
|
2019
|
$
|
1,191
|
|
|
|
2020
|
1,208
|
|
||
|
2021
|
1,008
|
|
||
|
2022
|
767
|
|
||
|
2023
|
695
|
|
||
|
Thereafter
|
967
|
|
||
|
Total
|
$
|
5,836
|
|
|
|
|
Payments Due by Period
|
|||||||||||||||||||
|
Contractual Obligations:
|
Total
|
Less than
1 Year |
1 to 3
Years |
3 to 5
Years |
More than
5 Years |
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Long-term debt and interest
|
$
|
173,009
|
|
$
|
6,575
|
|
$
|
12,981
|
|
$
|
153,453
|
|
$
|
—
|
|
|||||
|
Long-term related party payable
(undiscounted) |
51,284
|
|
9,439
|
|
8,713
|
|
7,250
|
|
25,882
|
|
||||||||||
|
Purchase commitments
|
26,682
|
|
10,754
|
|
10,828
|
|
5,100
|
|
—
|
|
||||||||||
|
Operating leases
|
5,836
|
|
1,191
|
|
2,217
|
|
1,461
|
|
967
|
|
||||||||||
|
Total contractual cash obligations
|
$
|
256,811
|
|
$
|
27,959
|
|
$
|
34,739
|
|
$
|
167,264
|
|
$
|
26,849
|
|
|||||
|
|
Years ended December 31,
|
|||||||||||
|
|
2018
|
2017
|
2016
|
|||||||||
|
Revenues:
|
|
|
|
|
|
|
||||||
|
Product sales
|
$
|
101,423
|
|
$
|
172,841
|
|
$
|
147,222
|
|
|||
|
License revenue
|
1,846
|
|
404
|
|
3,024
|
|
||||||
|
Total revenues
|
103,269
|
|
|
173,245
|
|
|
150,246
|
|
||||
|
Operating expenses:
|
|
|
|
|
|
|
||||||
|
Cost of products
|
17,516
|
|
16,301
|
|
13,248
|
|
||||||
|
Research and development expenses
|
39,329
|
|
33,418
|
|
34,611
|
|
||||||
|
Selling, general and administrative expenses
|
100,359
|
|
58,860
|
|
44,179
|
|
||||||
|
Intangible asset amortization
|
6,619
|
|
3,659
|
|
13,888
|
|
||||||
|
(Gain) loss - changes in fair value of related party contingent consideration
|
(22,731
|
)
|
(31,040
|
)
|
49,285
|
|
||||||
|
Impairment of intangible asset
|
66,087
|
|
—
|
|
—
|
|
||||||
|
Restructuring costs
|
1,016
|
|
2,542
|
|
—
|
|
||||||
|
Total operating expenses
|
208,195
|
|
|
83,740
|
|
|
155,211
|
|
||||
|
Operating (loss) income
|
(104,926
|
)
|
|
89,505
|
|
|
(4,965
|
)
|
||||
|
Investment and other income, net
|
452
|
|
2,136
|
|
2,758
|
|
||||||
|
Interest expense
|
(10,622
|
)
|
(1,052
|
)
|
(963
|
)
|
||||||
|
Other income (expense) - changes in fair value of related party payable
|
1,899
|
|
2,071
|
|
(6,548
|
)
|
||||||
|
(Loss) income before income taxes
|
(113,197
|
)
|
|
92,660
|
|
|
(9,718
|
)
|
||||
|
Income tax (benefit) provision
|
(17,893
|
)
|
24,389
|
|
31,558
|
|
||||||
|
Net (loss) income
|
$
|
(95,304
|
)
|
|
$
|
68,271
|
|
|
$
|
(41,276
|
)
|
|
|
Net (loss) income per share - basic
|
$
|
(2.55
|
)
|
|
$
|
1.69
|
|
|
$
|
(1.00
|
)
|
|
|
Net (loss) income per share - diluted
|
$
|
(2.55
|
)
|
|
$
|
1.63
|
|
|
$
|
(1.00
|
)
|
|
|
Weighted average number of shares outstanding - basic
|
37,325
|
|
40,465
|
|
41,248
|
|
||||||
|
Weighted average number of shares outstanding - diluted
|
37,325
|
|
41,765
|
|
41,248
|
|
||||||
|
|
Years ended December 31,
|
|||||||||||
|
|
2018
|
2017
|
2016
|
|||||||||
|
Net (loss) income
|
$
|
(95,304
|
)
|
$
|
68,271
|
|
$
|
(41,276
|
)
|
|||
|
Other comprehensive income (loss), net of tax:
|
|
|
|
|
|
|
||||||
|
Foreign currency translation (loss) gain
|
(419
|
)
|
134
|
|
(1,024
|
)
|
||||||
|
Net other comprehensive income, net of ($18), $28, $16 tax, respectively
|
269
|
|
165
|
|
116
|
|
||||||
|
Total other comprehensive (loss) income, net of tax
|
(150
|
)
|
|
299
|
|
|
(908
|
)
|
||||
|
Total comprehensive (loss) income
|
$
|
(95,454
|
)
|
|
$
|
68,570
|
|
|
$
|
(42,184
|
)
|
|
|
|
December 31,
|
|||||||
|
|
2018
|
2017
|
||||||
|
ASSETS
|
|
|
|
|
||||
|
Current assets:
|
|
|
|
|
||||
|
Cash and cash equivalents
|
$
|
9,325
|
|
$
|
16,564
|
|
||
|
Marketable securities
|
90,590
|
|
77,511
|
|
||||
|
Accounts receivable
|
11,330
|
|
14,785
|
|
||||
|
Inventories, net
|
4,770
|
|
6,157
|
|
||||
|
Prepaid expenses and other current assets
|
8,836
|
|
8,958
|
|
||||
|
Total current assets
|
124,851
|
|
|
123,975
|
|
|||
|
Property and equipment, net
|
1,911
|
|
3,001
|
|
||||
|
Goodwill
|
18,491
|
|
18,491
|
|
||||
|
Intangible assets, net
|
1,629
|
|
92,289
|
|
||||
|
Research and development tax credit receivable
|
7,272
|
|
5,272
|
|
||||
|
Other non-current assets
|
36,146
|
|
10,249
|
|
||||
|
Total assets
|
$
|
190,300
|
|
|
$
|
253,277
|
|
|
|
LIABILITIES AND SHAREHOLDERS’ EQUITY
|
|
|
|
|
||||
|
Current liabilities:
|
|
|
|
|
||||
|
Current portion of long-term debt
|
$
|
106
|
|
$
|
111
|
|
||
|
Current portion of long-term related party payable
|
9,439
|
|
25,007
|
|
||||
|
Accounts payable
|
3,503
|
|
7,477
|
|
||||
|
Deferred revenue
|
114
|
|
2,007
|
|
||||
|
Accrued expenses
|
21,695
|
|
50,926
|
|
||||
|
Income taxes
|
73
|
|
414
|
|
||||
|
Other current liabilities
|
3,453
|
|
597
|
|
||||
|
Total current liabilities
|
38,383
|
|
|
86,539
|
|
|||
|
Long-term debt, less current portion
|
115,734
|
|
156
|
|
||||
|
Long-term related party payable, less current portion
|
19,401
|
|
73,918
|
|
||||
|
Other non-current liabilities
|
14,002
|
|
7,084
|
|
||||
|
Total liabilities
|
187,520
|
|
|
167,697
|
|
|||
|
Shareholders’ equity:
|
|
|
|
|
||||
|
Preferred shares, nominal value of $0.01 per share; 50,000 shares authorized; none issued or outstanding at December 31, 2018 and December 31, 2017, respectively
|
—
|
|
—
|
|
||||
|
Ordinary shares, nominal value of $0.01 per share; 500,000 shares authorized; 42,720 issued and 37,313 outstanding at December 31, 2018, and 41,463 issued and 39,346 outstanding at December 31, 2017
|
427
|
|
414
|
|
||||
|
Treasury shares, at cost, 5,407 and 2,117 shares held at December 31, 2018 and December 31, 2017, respectively
|
(49,998
|
)
|
(22,361
|
)
|
||||
|
Additional paid-in capital
|
433,756
|
|
393,478
|
|
||||
|
Accumulated deficit
|
(357,989
|
)
|
(262,685
|
)
|
||||
|
Accumulated other comprehensive loss
|
(23,416
|
)
|
(23,266
|
)
|
||||
|
Total shareholders’ equity
|
2,780
|
|
|
85,580
|
|
|||
|
Total liabilities and shareholders’ equity
|
$
|
190,300
|
|
|
$
|
253,277
|
|
|
|
|
Ordinary shares
|
Additional
|
Accumulated
|
Accumulated
other
comprehensive
|
Treasury Shares
|
Total
shareholders’
|
||||||||||||||||||||||||
|
|
Shares
|
Amount
|
paid-in capital
|
deficit
|
(loss) income
|
Shares
|
Amount
|
equity
|
||||||||||||||||||||||
|
Balance, December 31, 2015
|
41,241
|
|
|
$
|
6,331
|
|
|
$
|
363,984
|
|
|
$
|
(278,524
|
)
|
|
$
|
(22,657
|
)
|
|
—
|
|
$
|
—
|
|
$
|
69,134
|
|
|||
|
Net loss
|
—
|
|
—
|
|
—
|
|
(41,276
|
)
|
—
|
|
—
|
|
—
|
|
(41,276
|
)
|
||||||||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(908
|
)
|
—
|
|
—
|
|
(908
|
)
|
||||||||||||||
|
Subscription of warrants
|
—
|
|
—
|
|
326
|
|
—
|
|
—
|
|
—
|
|
—
|
|
326
|
|
||||||||||||||
|
Exercise of stock options or warrants
|
15
|
|
2
|
|
112
|
|
—
|
|
—
|
|
—
|
|
—
|
|
114
|
|
||||||||||||||
|
Vesting of restricted shares
|
115
|
|
18
|
|
(18
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Stock-based compensation expense
|
—
|
|
—
|
|
14,679
|
|
—
|
|
—
|
|
—
|
|
—
|
|
14,679
|
|
||||||||||||||
|
Cross-border merger nominal value adjustment
|
—
|
|
(5,937
|
)
|
5,937
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Balance, December 31, 2016
|
41,371
|
|
|
414
|
|
|
385,020
|
|
|
(319,800
|
)
|
|
(23,565
|
)
|
|
—
|
|
—
|
|
42,069
|
|
|||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
68,271
|
|
—
|
|
—
|
|
—
|
|
68,271
|
|
||||||||||||||
|
Other comprehensive income
|
—
|
|
—
|
|
—
|
|
—
|
|
299
|
|
—
|
|
—
|
|
299
|
|
||||||||||||||
|
Exercise of stock options
|
69
|
|
—
|
|
396
|
|
—
|
|
—
|
|
—
|
|
—
|
|
396
|
|
||||||||||||||
|
Vesting of restricted shares
|
23
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Stock-based compensation expense
|
—
|
|
—
|
|
8,062
|
|
—
|
|
—
|
|
—
|
|
—
|
|
8,062
|
|
||||||||||||||
|
Share repurchases
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,117
|
|
(22,361
|
)
|
(22,361
|
)
|
||||||||||||||
|
Adjustment to accumulated deficit (see
Note 12: Income Taxes)
|
—
|
|
—
|
|
—
|
|
(11,156
|
)
|
—
|
|
—
|
|
—
|
|
(11,156
|
)
|
||||||||||||||
|
Balance, December 31, 2017
|
41,463
|
|
|
414
|
|
|
393,478
|
|
|
(262,685
|
)
|
|
(23,266
|
)
|
|
2,117
|
|
(22,361
|
)
|
85,580
|
|
|||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
(95,304
|
)
|
—
|
|
—
|
|
—
|
|
(95,304
|
)
|
||||||||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(150
|
)
|
—
|
|
—
|
|
(150
|
)
|
||||||||||||||
|
Exercise of stock options
|
82
|
|
1
|
|
534
|
|
—
|
|
—
|
|
—
|
|
—
|
|
535
|
|
||||||||||||||
|
Exercise of warrants
|
603
|
|
6
|
|
2,905
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,911
|
|
||||||||||||||
|
Expiration of warrants
|
—
|
|
—
|
|
2,167
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,167
|
|
||||||||||||||
|
Vesting of restricted shares
|
547
|
|
6
|
|
(6
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Employee share purchase plan share issuance
|
25
|
|
—
|
|
127
|
|
—
|
|
—
|
|
—
|
|
—
|
|
127
|
|
||||||||||||||
|
Stock-based compensation expense
|
—
|
|
—
|
|
7,852
|
|
—
|
|
—
|
|
—
|
|
—
|
|
7,852
|
|
||||||||||||||
|
Equity component of 2023 Notes
|
—
|
|
—
|
|
26,699
|
|
—
|
|
—
|
|
—
|
|
—
|
|
26,699
|
|
||||||||||||||
|
Share repurchases
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
3,290
|
|
(27,637
|
)
|
(27,637
|
)
|
||||||||||||||
|
Balance, December 31, 2018
|
42,720
|
|
$
|
427
|
|
$
|
433,756
|
|
$
|
(357,989
|
)
|
$
|
(23,416
|
)
|
5,407
|
|
$
|
(49,998
|
)
|
$
|
2,780
|
|
||||||||
|
|
Years ended December 31,
|
|||||||||||
|
|
2018
|
2017
|
2016
|
|||||||||
|
Cash flows from operating activities:
|
|
|
|
|
|
|
||||||
|
Net (loss) income
|
$
|
(95,304
|
)
|
$
|
68,271
|
|
$
|
(41,276
|
)
|
|||
|
Adjustments to reconcile net (loss) income to net cash provided by operating activities:
|
|
|
|
|
|
|
||||||
|
Depreciation and amortization
|
7,430
|
|
4,883
|
|
14,489
|
|
||||||
|
Impairment of intangible asset
|
66,087
|
|
—
|
|
—
|
|
||||||
|
Amortization of premiums on marketable securities
|
2,823
|
|
732
|
|
918
|
|
||||||
|
Remeasurement of related party acquisition-related contingent consideration
|
(22,731
|
)
|
(31,040
|
)
|
49,285
|
|
||||||
|
Remeasurement of related party financing-related contingent consideration
|
(1,899
|
)
|
(2,071
|
)
|
6,548
|
|
||||||
|
Amortization of debt discount and debt issuance costs
|
4,830
|
|
—
|
|
—
|
|
||||||
|
Change in deferred tax and income tax deferred charge
|
(19,152
|
)
|
3,556
|
|
(4,000
|
)
|
||||||
|
Stock-based compensation expense
|
7,852
|
|
8,072
|
|
14,679
|
|
||||||
|
Other adjustments
|
1,365
|
|
(968
|
)
|
(331
|
)
|
||||||
|
Net changes in assets and liabilities
|
|
|
|
|
|
|
||||||
|
Accounts receivable
|
3,452
|
|
3,054
|
|
(10,050
|
)
|
||||||
|
Inventories, net
|
711
|
|
(2,899
|
)
|
1,831
|
|
||||||
|
Prepaid expenses and other current assets
|
3,577
|
|
(3,741
|
)
|
3,412
|
|
||||||
|
Research and development tax credit receivable
|
(2,545
|
)
|
(3,141
|
)
|
397
|
|
||||||
|
Accounts payable & other current liabilities
|
(2,032
|
)
|
595
|
|
(434
|
)
|
||||||
|
Deferred revenue
|
(1,892
|
)
|
(216
|
)
|
(2,923
|
)
|
||||||
|
Accrued expenses
|
(10,640
|
)
|
13,187
|
|
6,764
|
|
||||||
|
Accrued income taxes
|
(341
|
)
|
(786
|
)
|
1,778
|
|
||||||
|
Earn-out payments for related party contingent consideration in excess of acquisition-date fair value
|
(19,468
|
)
|
(31,636
|
)
|
(20,252
|
)
|
||||||
|
Royalty payments for related party payable in excess of original fair value
|
(2,838
|
)
|
(4,429
|
)
|
(2,469
|
)
|
||||||
|
Other assets and liabilities
|
(2,001
|
)
|
(4,761
|
)
|
535
|
|
||||||
|
Net cash (used in) provided by operating activities
|
(82,716
|
)
|
16,662
|
|
18,901
|
|
||||||
|
Cash flows from investing activities:
|
|
|
|
|
|
|
||||||
|
Purchases of property and equipment
|
(178
|
)
|
(591
|
)
|
(1,201
|
)
|
||||||
|
Acquisitions of businesses, including cash acquired and other adjustments
|
—
|
|
—
|
|
628
|
|
||||||
|
Purchase of intangible assets
|
(20,000
|
)
|
(53,111
|
)
|
—
|
|
||||||
|
Proceeds from sales of marketable securities
|
359,507
|
|
189,009
|
|
71,546
|
|
||||||
|
Purchases of marketable securities
|
(376,310
|
)
|
(151,005
|
)
|
(107,603
|
)
|
||||||
|
Net cash used in investing activities
|
(36,981
|
)
|
(15,698
|
)
|
(36,630
|
)
|
||||||
|
Cash flows from financing activities:
|
|
|
|
|
|
|
||||||
|
Proceeds from debt issuance
|
143,750
|
|
—
|
|
—
|
|
||||||
|
Payments for debt issuance costs
|
(6,190
|
)
|
—
|
|
—
|
|
||||||
|
Earn-out payments for related party contingent consideration
|
(645
|
)
|
(1,246
|
)
|
(6,892
|
)
|
||||||
|
Royalty payments for related party payable
|
—
|
|
—
|
|
(1,225
|
)
|
||||||
|
Exercise of warrants
|
2,911
|
|
—
|
|
—
|
|
||||||
|
Proceeds from issuance of ordinary shares and warrants
|
577
|
|
404
|
|
440
|
|
||||||
|
Share repurchases
|
(27,637
|
)
|
(22,361
|
)
|
—
|
|
||||||
|
Other financing activities, net
|
(107
|
)
|
(115
|
)
|
(277
|
)
|
||||||
|
Net cash provided by (used in) financing activities
|
112,659
|
|
(23,318
|
)
|
(7,954
|
)
|
||||||
|
Effect of foreign currency exchange rate changes on cash and cash equivalents
|
(201
|
)
|
(297
|
)
|
(166
|
)
|
||||||
|
Net change in cash and cash equivalents
|
(7,239
|
)
|
(22,651
|
)
|
(25,849
|
)
|
||||||
|
Cash and cash equivalents at January 1
|
16,564
|
|
39,215
|
|
65,064
|
|
||||||
|
Cash and cash equivalents at December 31
|
$
|
9,325
|
|
$
|
16,564
|
|
$
|
39,215
|
|
|||
|
Supplemental disclosures of cash flow information:
|
|
|
|
|
|
|
||||||
|
Income tax paid
|
$
|
776
|
|
$
|
19,143
|
|
$
|
27,180
|
|
|||
|
Interest paid
|
3,359
|
|
1,050
|
|
788
|
|
||||||
|
•
|
Akovaz®
(ephedrine sulfate injection, USP), an alpha- and beta-adrenergic agonist and a norepinephrine-releasing agent that is indicated for the treatment of clinically important hypotension occurring in the setting of anesthesia
|
|
•
|
Bloxiverz®
(neostigmine methylsulfate injection), a cholinesterase inhibitor, is indicated for the reversal of the effects of non-depolarizing neuromuscular blocking agents (NMBAs) after surgery.
|
|
•
|
Vazculep®
(phenylephrine hydrochloride injection), an alpha-1 adrenergic receptor agonist indicated for the treatment of clinically important hypotension resulting primarily from vasodilation in the setting of anesthesia.
|
|
•
|
Noctiva™
, a vasopressin analog indicated for the treatment of nocturia due to nocturnal polyuria in adults who awaken at least two times per night to void. Due to disappointing results after a substantial investment of resources after
Noctiva’s
commercial launch in March 2018
, Avadel Specialty Pharmaceuticals LLC, (“Specialty Pharma”),
the Avadel subsidiary responsible for the marketing and sale of
Noctiva,
made a voluntary filing for Chapter 11 bankruptcy protection on February 6, 2019. Although Specialty Pharma currently continues its marketing and sales efforts for this product, Avadel anticipates that Specialty Pharma will discontinue all activities with respect to
Noctiva
during 2019 as a result of the bankruptcy.
|
|
•
|
Flamel ceased to exist as a separate entity and the Company continued as the surviving entity and assumed all of the assets and liabilities of Flamel.
|
|
•
|
our authorized share capital is
$5,500
divided into
500,000
ordinary shares with a nominal value of
$0.01
each and
50,000
preferred shares with a nominal value of
$0.01
each
|
|
◦
|
all outstanding ordinary shares of Flamel,
€0.122
nominal value per share, were canceled and exchanged on a one-for-one basis for newly issued ordinary shares of the Company,
$0.01
nominal value per share. This change in nominal value of our outstanding shares resulted in our reclassifying
$5,937
on our balance sheet from ordinary shares to additional paid-in capital
|
|
◦
|
our Board of Directors is authorized to issue preferred shares on a non-pre-emptive basis, for a maximum period of five years, at which point such an authorization may be renewed by shareholders. The Board of Directors has discretion to dictate terms attached to the preferred shares, including voting, dividend, conversion rights, and priority relative to other classes of shares with respect to dividends and upon a liquidation.
|
|
•
|
all outstanding American Depositary Shares (ADSs) representing ordinary shares of Flamel were canceled and exchanged on a one-for-one basis for ADSs representing ordinary shares of the Company.
|
|
Laboratory equipment
|
4-8 years
|
|
Software, office and computer equipment
|
3 years
|
|
Leasehold improvements, furniture, fixtures and fittings
|
5-10 years
|
|
•
|
Income approach, which is based on the present value of a future stream of net cash flows.
|
|
•
|
Market approach, which is based on market prices and other information from market transactions involving identical or comparable assets or liabilities.
|
|
•
|
Level 1 - Quoted prices for identical assets or liabilities in active markets.
|
|
•
|
Level 2 - Quoted prices for similar assets or liabilities in active markets, or quoted prices for identical or similar assets or liabilities in markets that are not active, or inputs other than quoted prices that are directly or indirectly observable, or inputs that are derived principally from, or corroborated by, observable market data by correlation or other means.
|
|
•
|
Level 3 - Unobservable inputs that reflect estimates and assumptions.
|
|
As of December 31, 2018
|
As of December 31, 2017
|
|||||||||||||||||||||||
|
Fair Value Measurements:
|
Level 1
|
Level 2
|
Level 3
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||||||||
|
Marketable securities (see
Note 5)
|
||||||||||||||||||||||||
|
Equity securities
|
$
|
9,145
|
|
$
|
—
|
|
$
|
—
|
|
$
|
468
|
|
$
|
—
|
|
$
|
—
|
|
||||||
|
Money market funds
|
52,996
|
|
—
|
|
—
|
|
44,481
|
|
—
|
|
—
|
|
||||||||||||
|
Corporate bonds
|
—
|
|
6,339
|
|
—
|
|
—
|
|
9,262
|
|
—
|
|
||||||||||||
|
Government securities - U.S.
|
—
|
|
12,701
|
|
—
|
|
—
|
|
19,050
|
|
—
|
|
||||||||||||
|
Other fixed-income securities
|
—
|
|
9,409
|
|
—
|
|
—
|
|
4,250
|
|
—
|
|
||||||||||||
|
Total assets
|
$
|
62,141
|
|
|
$
|
28,449
|
|
|
$
|
—
|
|
|
$
|
44,949
|
|
|
$
|
32,562
|
|
|
$
|
—
|
|
|
|
Related party payable (see
Note 11)
|
—
|
|
—
|
|
28,840
|
|
—
|
|
—
|
|
98,925
|
|
||||||||||||
|
Total liabilities
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
28,840
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
98,925
|
|
|
|
2018
|
||||||||||||||||
|
Marketable Securities:
|
Adjusted Cost
|
Unrealized Gains
|
Unrealized Losses
|
Fair Value
|
||||||||||||
|
Equity securities
|
$
|
10,101
|
|
$
|
—
|
|
$
|
(956
|
)
|
$
|
9,145
|
|
||||
|
Money market funds
|
52,733
|
|
316
|
|
(53
|
)
|
52,996
|
|
||||||||
|
Corporate bonds
|
6,411
|
|
7
|
|
(79
|
)
|
6,339
|
|
||||||||
|
Government securities - U.S.
|
12,714
|
|
66
|
|
(79
|
)
|
12,701
|
|
||||||||
|
Other fixed-income securities
|
9,400
|
|
22
|
|
(13
|
)
|
9,409
|
|
||||||||
|
Total
|
$
|
91,359
|
|
|
$
|
411
|
|
|
$
|
(1,180
|
)
|
|
$
|
90,590
|
|
|
|
2017
|
||||||||||||||||
|
Marketable Securities:
|
Adjusted Cost
|
Unrealized Gains
|
Unrealized Losses
|
Fair Value
|
||||||||||||
|
Equity securities
|
$
|
443
|
|
$
|
31
|
|
$
|
(6
|
)
|
$
|
468
|
|
||||
|
Money market funds
|
44,525
|
|
—
|
|
(44
|
)
|
44,481
|
|
||||||||
|
Corporate bonds
|
9,285
|
|
1
|
|
(24
|
)
|
9,262
|
|
||||||||
|
Government securities - U.S.
|
19,080
|
|
—
|
|
(30
|
)
|
19,050
|
|
||||||||
|
Other fixed-income securities
|
4,259
|
|
—
|
|
(9
|
)
|
4,250
|
|
||||||||
|
Total
|
$
|
77,592
|
|
|
$
|
32
|
|
|
$
|
(113
|
)
|
|
$
|
77,511
|
|
|
|
Maturities
|
||||||||||||||||||||
|
Marketable Debt Securities:
|
Less than 1 Year
|
1-5 Years
|
5-10 Years
|
Greater than 10 Years
|
Total
|
|||||||||||||||
|
Corporate bonds
|
$
|
1,511
|
|
$
|
4,828
|
|
$
|
—
|
|
$
|
—
|
|
$
|
6,339
|
|
|||||
|
Government securities - U.S.
|
771
|
|
11,145
|
|
281
|
|
504
|
|
12,701
|
|
||||||||||
|
Other fixed-income securities
|
—
|
|
9,409
|
|
—
|
|
—
|
|
9,409
|
|
||||||||||
|
Total
|
$
|
2,282
|
|
|
$
|
25,382
|
|
|
$
|
281
|
|
|
$
|
504
|
|
|
$
|
28,449
|
|
|
|
Inventory:
|
2018
|
2017
|
||||||
|
Finished goods
|
$
|
4,270
|
|
$
|
4,774
|
|
||
|
Raw materials
|
500
|
|
1,383
|
|
||||
|
Total
|
$
|
4,770
|
|
$
|
6,157
|
|
||
|
Property and Equipment, net:
|
2018
|
2017
|
||||||
|
Laboratory equipment
|
$
|
8,864
|
|
$
|
10,135
|
|
||
|
Software, office and computer equipment
|
2,487
|
|
3,115
|
|
||||
|
Furniture, fixtures and fittings
|
3,715
|
|
4,779
|
|
||||
|
Less - accumulated depreciation
|
(13,155
|
)
|
(15,028
|
)
|
||||
|
Total
|
$
|
1,911
|
|
$
|
3,001
|
|
||
|
•
|
$15,000
long-term liability to Deerfield CSF. Under the terms of the acquisition agreement, the Company will pay
$1,050
annually for
five
years with a final payment in January 2021 of
$15,000
.
|
|
•
|
an estimate of $
6,659
in contingent consideration to Deerfield CSF. Under the terms of the acquisition agreement, the Company shall pay quarterly a
15%
royalty on the net sales of certain FSC products, up to
$12,500
for a period not exceeding
ten
years.
|
|
Assigned Fair Value:
|
Amount
|
|||
|
|
|
|||
|
Accounts receivable
|
$
|
142
|
|
|
|
Inventories
|
1,135
|
|
||
|
Prepaid expenses and other current assets
|
1,712
|
|
||
|
Intangible assets:
|
|
|
||
|
Acquired product marketing rights
|
16,600
|
|
||
|
Acquired developed technology
|
4,300
|
|
||
|
Deferred tax assets
|
853
|
|
||
|
Other assets
|
277
|
|
||
|
Accounts payable and other liabilities
|
(3,827
|
)
|
||
|
Total
|
$
|
21,192
|
|
|
|
Pro Forma Net Revenue and Income (Loss):
|
2016
|
|||
|
Net revenue
|
$
|
150,721
|
|
|
|
Net loss
|
(42,290
|
)
|
||
|
|
2018
|
2017
|
||||||||||||||||||||||||||
|
Goodwill and Intangible Assets:
|
Gross
Value
|
Accumulated
Amortization
|
Impairment
|
Net Carrying Amount
|
Gross
Value
|
Accumulated
Amortization
|
Net Carrying Amount
|
|||||||||||||||||||||
|
Amortizable intangible assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
Acquired developed technology - Noctiva
|
$
|
73,111
|
|
$
|
(7,024
|
)
|
$
|
(66,087
|
)
|
$
|
—
|
|
$
|
73,111
|
|
$
|
(1,401
|
)
|
$
|
71,710
|
|
|||||||
|
Acquired developed technology - Vazculep
|
12,061
|
|
(10,432
|
)
|
—
|
|
1,629
|
|
12,061
|
|
(9,616
|
)
|
2,445
|
|
||||||||||||||
|
Acquired product marketing rights
(1)
|
—
|
|
—
|
|
—
|
|
—
|
|
16,600
|
|
(2,132
|
)
|
14,468
|
|
||||||||||||||
|
Acquired developed technology
(1)
|
—
|
|
—
|
|
—
|
|
—
|
|
4,300
|
|
(634
|
)
|
3,666
|
|
||||||||||||||
|
Total amortizable intangible assets
|
$
|
85,172
|
|
|
$
|
(17,456
|
)
|
$
|
(66,087
|
)
|
|
$
|
1,629
|
|
|
$
|
106,072
|
|
|
$
|
(13,783
|
)
|
|
$
|
92,289
|
|
||
|
Unamortizable intangible assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
Goodwill
|
$
|
18,491
|
|
$
|
—
|
|
$
|
—
|
|
$
|
18,491
|
|
$
|
18,491
|
|
$
|
—
|
|
$
|
18,491
|
|
|||||||
|
Total unamortizable intangible assets
|
$
|
18,491
|
|
$
|
—
|
|
$
|
—
|
|
$
|
18,491
|
|
$
|
18,491
|
|
$
|
—
|
|
$
|
18,491
|
|
|||||||
|
Estimated Amortization Expense:
|
Amount
|
|||
|
2019
|
$
|
815
|
|
|
|
2020
|
814
|
|
||
|
2021
|
—
|
|
||
|
2022
|
—
|
|
||
|
2023
|
—
|
|
||
|
December 31, 2018
|
December 31, 2017
|
|||||||
|
Principal amount of 4.50% exchangeable senior notes due 2023
|
$
|
143,750
|
|
$
|
—
|
|
||
|
Less: unamortized debt discount and issuance costs, net
|
(28,059
|
)
|
—
|
|
||||
|
Net carrying amount of liability component
|
115,691
|
|
—
|
|
||||
|
Other debt
|
149
|
|
267
|
|
||||
|
Subtotal
|
115,840
|
|
267
|
|
||||
|
Less: current maturities
|
(106
|
)
|
(111
|
)
|
||||
|
Long-term debt
|
$
|
115,734
|
|
$
|
156
|
|
||
|
Equity component:
|
||||||||
|
Equity component of exchangeable notes, net of issuance costs
|
$
|
(26,699
|
)
|
$
|
—
|
|
||
|
•
|
Prior to the close of business on the business day immediately preceding August 1, 2022, a holder of the 2023 Notes may surrender all or any portion of its 2023 Notes for exchange at any time during the
five
business day period immediately after any
five
consecutive trading day period (the “Measurement Period”) in which the trading price per $1 principal amount of 2023 Notes, as determined following a request by a holder of the 2023 Notes, for each trading day of the measurement period was less than 98% of the product of the last reported sale price of the ADSs and the exchange rate on each such trading day.
|
|
•
|
If a transaction or event that constitutes a fundamental change or a make-whole fundamental change occurs prior to the close of business on the business day immediately preceding August 1, 2022, regardless of whether a holder of the 2023 Notes has the right to require the Company to repurchase the 2023 Notes, or if Avadel is a party to a merger event that
|
|
•
|
Prior to the close of business on the business day immediately preceding August 1, 2022, a holder of the 2023 Notes may surrender all or any portion of its 2023 Notes for exchange at any time during any calendar quarter commencing after the calendar quarter ending on June 30, 2018 (and only during such calendar quarter), if the last reported sale price of the ADSs for at least
20
trading days (whether or not consecutive) during the period of
30
consecutive trading days ending on, and including, the last trading day of the immediately preceding calendar quarter is greater than or equal to
130%
of the exchange price on each applicable trading day.
|
|
•
|
If the Company calls the 2023 Notes for redemption pursuant to Article 16 to the Indenture prior to the close of business on the business day immediately preceding August 1, 2022, then a holder of the 2023 Notes may surrender all or any portion of its 2023 Notes for exchange at any time prior to the close of business on the second business day prior to the redemption date, even if the 2023 Notes are not otherwise exchangeable at such time. After that time, the right to exchange shall expire, unless the Company defaults in the payment of the redemption price, in which case a holder of the 2023 Notes may exchange its 2023 Notes until the redemption price has been paid or duly provided for.
|
|
|
|
Activity during the Twelve Months Ended December 31, 2018
|
|
|||||||||||||||||||||||||
|
|
|
|
Changes in Fair Value of
Related Party Payable
|
|
||||||||||||||||||||||||
|
Balance,
December 31, 2017
|
Payments to
Related Parties
|
Operating (Gain)
Expense
|
Other
Income
|
Expiration of Warrants
|
Disposal
|
Balance,
December 31, 2018 |
||||||||||||||||||||||
|
Acquisition-related contingent consideration:
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
|
Warrants - Éclat Pharmaceuticals
(a)
|
$
|
2,479
|
|
$
|
—
|
|
$
|
(312
|
)
|
$
|
—
|
|
$
|
(2,167
|
)
|
$
|
—
|
|
$
|
—
|
|
|||||||
|
Earn-out payments - Éclat Pharmaceuticals
(b)
|
67,744
|
|
(19,468
|
)
|
(22,661
|
)
|
—
|
|
—
|
|
—
|
|
25,615
|
|
||||||||||||||
|
Royalty agreement - FSC
(c)
|
5,740
|
|
(645
|
)
|
242
|
|
—
|
|
—
|
|
(5,337
|
)
|
—
|
|
||||||||||||||
|
Financing-related:
|
|
|
|
|
|
|
|
|
||||||||||||||||||||
|
Royalty agreement - Deerfield
(d)
|
5,392
|
|
(1,922
|
)
|
—
|
|
(1,286
|
)
|
—
|
|
—
|
|
2,184
|
|
||||||||||||||
|
Royalty agreement - Broadfin
(e)
|
2,570
|
|
(916
|
)
|
—
|
|
(613
|
)
|
—
|
|
—
|
|
1,041
|
|
||||||||||||||
|
Long-term liability - FSC
(f)
|
15,000
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(15,000
|
)
|
—
|
|
||||||||||||||
|
Total related party payable
|
98,925
|
|
$
|
(22,951
|
)
|
$
|
(22,731
|
)
|
$
|
(1,899
|
)
|
$
|
(2,167
|
)
|
$
|
(20,337
|
)
|
28,840
|
|
|||||||||
|
Less: Current portion
|
(25,007
|
)
|
|
|
|
|
|
|
(9,439
|
)
|
||||||||||||||||||
|
Total long-term related party payable
|
$
|
73,918
|
|
|
|
|
|
|
|
$
|
19,401
|
|
||||||||||||||||
|
(a)
|
As part of the consideration for the Company’s acquisition of Éclat Pharmaceuticals, LLC on March 13, 2012, the Company issued
two
warrants to a related party with a
six
-year term which allow for the purchase of a combined total of
3,300
ordinary shares of Avadel. One warrant was exercisable for
2,200
ordinary shares at an exercise price of
$7.44
per share, and the other warrant was exercisable for
1,100
ordinary shares at an exercise price of
$11.00
per share. On February 23, 2018, the related party exercised in full the warrant for
2,200
ordinary shares. On March 12, 2018, the remaining warrant for
1,100
ordinary shares expired worthless.
|
|
Assumptions for the Warrant Valuation:
|
2017
|
||||
|
Stock price
|
$
|
8.20
|
|
||
|
Weighted average exercise price per share
|
8.63
|
|
|||
|
Expected term (years)
|
0.25
|
|
|||
|
Expected volatility
|
37.90
|
%
|
|||
|
Risk-free interest rate
|
1.39
|
%
|
|||
|
Expected dividend yield
|
—
|
|
|||
|
(b)
|
In March 2012, the Company acquired all of the membership interests of Éclat from Breaking Stick Holdings, L.L.C. (“Breaking Stick”, formerly Éclat Holdings), an affiliate of Deerfield. Breaking Stick is majority owned by Deerfield, with a minority interest owned by certain current and former employees. As part of the consideration, the Company committed to provide quarterly earn-out payments equal to
20%
of any gross profit generated by certain Éclat products. These payments will continue in perpetuity, to the extent gross profit of the related products also continue in perpetuity.
|
|
(c)
|
In February 2016, the Company acquired all of the membership interests of FSC from Deerfield. The consideration for this transaction in part included a commitment to pay quarterly a
15%
royalty on the net sales of certain FSC products, up to
$12,500
for a period not exceeding
ten
years. This obligation was assumed by the buyer as part of the disposition of the pediatrics products on February 16, 2018.
See Note
16
: Divestiture of the Pediatric Assets.
|
|
(d)
|
As part of a February 2013 debt financing transaction conducted with Deerfield, the Company received cash of
$2,600
in exchange for entering into a royalty agreement whereby the Company shall pay quarterly a
1.75%
royalty on the net sales of certain Éclat products until December 31, 2024. In connection with such debt financing transaction, the Company granted Deerfield a security interest in the product registration rights of the Eclat products.
|
|
(e)
|
As part of a December 2013 debt financing transaction conducted with Broadfin Healthcare Master Fund, a related party and current shareholder, the Company received cash of
$2,200
in exchange for entering into a royalty agreement whereby the Company shall pay quarterly a
0.834%
royalty on the net sales of certain Éclat products until December 31, 2024.
|
|
(f)
|
In February 2016, the Company acquired all of the membership interests of FSC from Deerfield. The consideration for this transaction in part consisted of payments totaling
$1,050
annually for
five
years with a final payment in January 2021 of
$15,000
. Substantially all of FSC’s, and its subsidiaries, assets were pledged as collateral under this agreement. This obligation was assumed by the buyer as part of the disposition of the pediatrics products on February 16, 2018.
See Note
16
: Divestiture of the Pediatric Assets.
|
|
Related Party Payable:
|
Balance
|
|||
|
Balance at December 31, 2015
|
$
|
122,693
|
|
|
|
Additions
(2)
|
21,659
|
|
||
|
Payments of related party payable
|
(30,838
|
)
|
||
|
Fair value adjustments
(1)
|
55,833
|
|
||
|
Balance at December 31, 2016
|
169,347
|
|
||
|
Payments of related party payable
|
(37,311
|
)
|
||
|
Fair value adjustments
(1)
|
(33,111
|
)
|
||
|
Balance at December 31, 2017
|
98,925
|
|
||
|
Payments of related party payable
|
(22,951
|
)
|
||
|
Fair value adjustments
(1)
|
(24,630
|
)
|
||
|
Expiration of warrants
|
(2,167
|
)
|
||
|
Disposition of the pediatrics assets
|
(20,337
|
)
|
||
|
Balance at December 31, 2018
|
$
|
28,840
|
|
|
|
(Loss) Income Before Income Taxes:
|
2018
|
2017
|
2016
|
|||||||||
|
Ireland
|
$
|
(42,604
|
)
|
$
|
(3,123
|
)
|
$
|
(22,866
|
)
|
|||
|
United States
|
(70,340
|
)
|
92,754
|
|
32,786
|
|
||||||
|
France
|
(253
|
)
|
3,029
|
|
(19,638
|
)
|
||||||
|
Total (loss) income before income taxes
|
$
|
(113,197
|
)
|
$
|
92,660
|
|
$
|
(9,718
|
)
|
|||
|
Income Tax (Benefit) Provision:
|
2018
|
2017
|
2016
|
|||||||||
|
Current:
|
|
|
|
|
|
|
||||||
|
United States - Federal
|
$
|
—
|
|
$
|
18,064
|
|
$
|
30,738
|
|
|||
|
United States - State
|
330
|
|
331
|
|
1,081
|
|
||||||
|
France
|
—
|
|
265
|
|
5,267
|
|
||||||
|
Total current
|
330
|
|
18,660
|
|
37,086
|
|
||||||
|
Deferred:
|
|
|
|
|
|
|
||||||
|
United States - Federal
|
(19,503
|
)
|
4,686
|
|
(6,443
|
)
|
||||||
|
United States - State
|
1,280
|
|
1,043
|
|
(23
|
)
|
||||||
|
France
|
—
|
|
—
|
|
938
|
|
||||||
|
Total deferred
|
(18,223
|
)
|
5,729
|
|
(5,528
|
)
|
||||||
|
Income tax (benefit) provision
|
$
|
(17,893
|
)
|
$
|
24,389
|
|
$
|
31,558
|
|
|||
|
Reconciliation to Effective Income Tax Rate:
|
2018
|
2017
|
2016
|
|||||||||
|
Statutory tax rate
|
12.5
|
%
|
12.5
|
%
|
12.5
|
%
|
||||||
|
Differences in international tax rates
|
8.0
|
%
|
22.2
|
%
|
(31.9
|
)%
|
||||||
|
Nondeductible changes in fair value of contingent consideration
|
4.0
|
%
|
(11.6
|
)%
|
(165.0
|
)%
|
||||||
|
Income tax deferred charge
|
—
|
%
|
—
|
%
|
(9.7
|
)%
|
||||||
|
Change in valuation allowances
|
(5.3
|
)%
|
(0.7
|
)%
|
11.8
|
%
|
||||||
|
Nondeductible stock-based compensation
|
(1.3
|
)%
|
(0.4
|
)%
|
(14.8
|
)%
|
||||||
|
Cross border merger
|
—
|
%
|
0.3
|
%
|
(100.6
|
)%
|
||||||
|
Unrealized tax benefits
|
(1.3
|
)%
|
1.4
|
%
|
(15.2
|
)%
|
||||||
|
State and local taxes (net of federal)
|
(0.3
|
)%
|
0.3
|
%
|
(9.6
|
)%
|
||||||
|
Change in U.S. tax law
|
(0.2
|
)%
|
3.8
|
%
|
—
|
%
|
||||||
|
Nondeductible interest expense
|
(1.1
|
)%
|
—
|
%
|
—
|
%
|
||||||
|
Other
|
0.7
|
%
|
(1.5
|
)%
|
(2.3
|
)%
|
||||||
|
Effective income tax rate
|
15.7
|
%
|
|
26.3
|
%
|
|
(324.8
|
)%
|
||||
|
Income tax (benefit) provision - at statutory tax rate
|
$
|
(14,149
|
)
|
$
|
11,582
|
|
$
|
(1,215
|
)
|
|||
|
Differences in international tax rates
|
(9,039
|
)
|
20,557
|
|
3,097
|
|
||||||
|
Nondeductible changes in fair value of contingent consideration
|
(4,559
|
)
|
(10,779
|
)
|
16,036
|
|
||||||
|
Income tax deferred charge
|
—
|
|
—
|
|
938
|
|
||||||
|
Change in valuation allowances
|
5,998
|
|
(610
|
)
|
(1,143
|
)
|
||||||
|
Nondeductible stock-based compensation
|
1,499
|
|
(375
|
)
|
1,436
|
|
||||||
|
Cross-border merger
|
—
|
|
265
|
|
9,773
|
|
||||||
|
Unrecognized tax benefits
|
1,440
|
|
1,296
|
|
1,475
|
|
||||||
|
State and local taxes (net of federal)
|
299
|
|
252
|
|
934
|
|
||||||
|
Change in U.S. tax law
|
274
|
|
3,513
|
|
—
|
|
||||||
|
Nondeductible interest expense
|
1,269
|
|
—
|
|
—
|
|
||||||
|
Other
|
(925
|
)
|
(1,312
|
)
|
227
|
|
||||||
|
Income tax (benefit) provision - at effective income tax rate
|
$
|
(17,893
|
)
|
$
|
24,389
|
|
$
|
31,558
|
|
|||
|
Unrecognized Tax Benefit Activity
|
2018
|
2017
|
2016
|
|||||||||
|
Balance at January 1:
|
$
|
3,954
|
|
$
|
1,686
|
|
$
|
448
|
|
|||
|
Additions based on tax positions related to the current year
|
1,087
|
|
2,268
|
|
1,578
|
|
||||||
|
Increases (decreases) for tax positions of prior years
|
274
|
|
—
|
|
(340
|
)
|
||||||
|
Balance at December 31:
|
$
|
5,315
|
|
$
|
3,954
|
|
$
|
1,686
|
|
|||
|
Net Deferred Tax Assets and Liabilities:
|
2018
|
2017
|
||||||
|
Deferred tax assets:
|
|
|
|
|
||||
|
Net operating loss carryforwards
|
$
|
19,510
|
|
$
|
9,831
|
|
||
|
Amortization
|
20,642
|
|
7,563
|
|
||||
|
Stock based compensation
|
4,587
|
|
4,375
|
|
||||
|
Fair value royalty agreements
|
—
|
|
635
|
|
||||
|
Fair value contingent consideration
|
384
|
|
870
|
|
||||
|
Other
|
479
|
|
406
|
|
||||
|
Gross deferred tax assets
|
45,602
|
|
23,680
|
|
||||
|
Deferred tax liabilities:
|
|
|
|
|
||||
|
Amortization
|
(308
|
)
|
(2,419
|
)
|
||||
|
Accounts receivable
|
(661
|
)
|
(936
|
)
|
||||
|
Prepaid expenses
|
(405
|
)
|
(1,094
|
)
|
||||
|
Gross deferred tax liabilities
|
(1,374
|
)
|
|
(4,449
|
)
|
|||
|
Less: valuation allowances
|
(21,199
|
)
|
(15,354
|
)
|
||||
|
Net deferred tax assets
|
$
|
23,029
|
|
$
|
3,877
|
|
||
|
Retirement Benefit Obligation Assumptions:
|
2018
|
2017
|
2016
|
||||||
|
Compensation rate increase
|
2.75
|
%
|
3.00
|
%
|
3.00
|
%
|
|||
|
Discount rate
|
1.50
|
%
|
1.25
|
%
|
1.31
|
%
|
|||
|
Employee turn-over
|
Actuarial standard and average of the last 5 years
|
||||||||
|
Average age of retirement
|
60 to 65 years actuarial standard based on age and professional status
|
||||||||
|
Retirement Benefit Obligation Activity:
|
2018
|
2017
|
||||||
|
Retirement indemnity benefit obligation, beginning of year
|
$
|
1,303
|
|
$
|
2,431
|
|
||
|
Service cost
|
93
|
|
132
|
|
||||
|
Interest cost
|
17
|
|
21
|
|
||||
|
Plan amendment
|
—
|
|
(829
|
)
|
||||
|
Benefits paid
|
(12
|
)
|
—
|
|
||||
|
Curtailment gain
|
(148
|
)
|
(717
|
)
|
||||
|
Actuarial loss
|
(178
|
)
|
(25
|
)
|
||||
|
Exchange rate changes
|
(51
|
)
|
290
|
|
||||
|
Retirement indemnity benefit obligation, end of year
|
$
|
1,024
|
|
$
|
1,303
|
|
||
|
Future Retirement Indemnity Benefit Obligation:
|
Balance
|
|||
|
|
|
|||
|
2019
|
$
|
—
|
|
|
|
2020
|
—
|
|
||
|
2021
|
—
|
|
||
|
2022
|
17
|
|
||
|
2023
|
—
|
|
||
|
Next five years
|
158
|
|
||
|
Total
|
$
|
175
|
|
|
|
Prepaid Expenses and Other Current Assets:
|
2018
|
2017
|
||||||
|
|
|
|
|
|||||
|
Valued-added tax recoverable
|
$
|
1,378
|
|
$
|
1,206
|
|
||
|
Prepaid and other expenses
|
2,145
|
|
7,106
|
|
||||
|
Guarantee from Armistice (see
Note 16
)
|
534
|
|
—
|
|
||||
|
Income tax receivable
|
921
|
|
518
|
|
||||
|
Research and development tax credit receivable
|
283
|
|
—
|
|
||||
|
Short-term deposit
|
3,350
|
|
—
|
|
||||
|
Other
|
225
|
|
128
|
|
||||
|
Total
|
$
|
8,836
|
|
$
|
8,958
|
|
||
|
Other Non-Current Assets:
|
2018
|
2017
|
||||||
|
Deferred tax assets
|
$
|
23,029
|
|
$
|
3,877
|
|
||
|
Long-term deposits
|
1,477
|
|
3,350
|
|
||||
|
Guarantee from Armistice (see
Note 16
)
|
5,697
|
|
—
|
|
||||
|
Right of use assets at contract manufacturing organizations
|
5,894
|
|
2,909
|
|
||||
|
Other
|
49
|
|
113
|
|
||||
|
Total
|
$
|
36,146
|
|
$
|
10,249
|
|
||
|
Accrued Expenses:
|
2018
|
2017
|
||||||
|
|
|
|
|
|||||
|
Accrued compensation
|
$
|
3,971
|
|
$
|
3,157
|
|
||
|
Accrued social charges
|
1,009
|
|
1,204
|
|
||||
|
Accrued restructuring (see
Note 17
)
|
879
|
|
1,000
|
|
||||
|
Customer allowances
|
6,541
|
|
10,613
|
|
||||
|
Accrued ELAA payment
|
—
|
|
20,000
|
|
||||
|
Accrued contract research organization charges
|
1,000
|
|
156
|
|
||||
|
Accrued contract manufacturing organization costs
|
2,028
|
|
2,327
|
|
||||
|
Accrued contract sales organization and marketing costs
|
3,469
|
|
7,641
|
|
||||
|
Other
|
2,798
|
|
4,828
|
|
||||
|
Total
|
$
|
21,695
|
|
|
$
|
50,926
|
|
|
|
Other Non-Current Liabilities:
|
2018
|
2017
|
||||||
|
|
|
|||||||
|
Provision for retirement indemnity
|
$
|
1,024
|
|
$
|
1,303
|
|
||
|
Customer allowances
|
1,352
|
|
1,636
|
|
||||
|
Unrecognized tax benefits
|
5,315
|
|
3,954
|
|
||||
|
Guarantee to Deerfield (see
Note 16
)
|
5,717
|
|
—
|
|
||||
|
Other
|
594
|
|
191
|
|
||||
|
Total
|
$
|
14,002
|
|
$
|
7,084
|
|
||
|
Purchase Commitments:
|
Balance
|
|||
|
2019
|
$
|
10,754
|
|
|
|
2020
|
5,948
|
|
||
|
2021
|
4,880
|
|
||
|
2022
|
4,880
|
|
||
|
2023
|
220
|
|
||
|
Thereafter
|
—
|
|
||
|
Total
|
$
|
26,682
|
|
|
|
Lease Commitment:
|
Balance
|
|||
|
2019
|
$
|
1,191
|
|
|
|
2020
|
1,208
|
|
||
|
2021
|
1,008
|
|
||
|
2022
|
767
|
|
||
|
2023
|
695
|
|
||
|
Thereafter
|
967
|
|
||
|
Total
|
$
|
5,836
|
|
|
|
|
Payments Due by Period
|
|||||||||||||||||||
|
Contractual Obligations:
|
Total
|
Less than
1 Year |
1 to 3
Years |
3 to 5
Years |
More than
5 Years |
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Long-term debt and interest
|
$
|
173,009
|
|
$
|
6,575
|
|
$
|
12,981
|
|
$
|
153,453
|
|
$
|
—
|
|
|||||
|
Long-term related party payable
(undiscounted) |
51,284
|
|
9,439
|
|
8,713
|
|
7,250
|
|
25,882
|
|
||||||||||
|
Purchase commitments
|
26,682
|
|
10,754
|
|
10,828
|
|
5,100
|
|
—
|
|
||||||||||
|
Operating leases
|
5,836
|
|
1,191
|
|
2,217
|
|
1,461
|
|
967
|
|
||||||||||
|
Total contractual cash obligations
|
$
|
256,811
|
|
$
|
27,959
|
|
$
|
34,739
|
|
$
|
167,264
|
|
$
|
26,849
|
|
|||||
|
•
|
Avadel Ireland will provide Cerecor with four product formulations utilizing Avadel Ireland’s LiquiTime™ technology, and will complete pilot bioequivalence studies for such product formulations within 18 months;
|
|
•
|
Cerecor will reimburse Avadel Ireland for development costs of the four LiquiTime™ products in excess of
$1,000
in the aggregate;
|
|
•
|
Upon transfer of the four product formulations, Cerecor will assume all remaining development costs and responsibilities for the product development, clinical studies, NDA applications and associated filing fees; and
|
|
•
|
Upon regulatory approval and commercial launch of any LiquiTime™ products, Cerecor will pay Avadel Ireland quarterly royalties based on a percentage of net sales of any such products in the mid-single digit range.
|
|
Restructuring Obligation:
|
2018
|
2017
|
||||||
|
Balance of restructuring accrual at January 1,
|
$
|
1,000
|
|
$
|
—
|
|
||
|
Charges for employee severance, benefits and other
|
1,164
|
|
3,259
|
|
||||
|
Payments
|
(1,261
|
)
|
(2,600
|
)
|
||||
|
Foreign currency impact
|
(24
|
)
|
341
|
|
||||
|
Balance of restructuring accrual at December 31,
|
$
|
879
|
|
$
|
1,000
|
|
||
|
Stock-based Compensation Expense:
|
2018
|
2017
|
2016
|
|||||||||
|
Research and development
|
$
|
880
|
|
$
|
672
|
|
$
|
3,523
|
|
|||
|
Selling, general and administrative
|
6,972
|
|
7,400
|
|
11,156
|
|
||||||
|
Total stock-based compensation expense
|
$
|
7,852
|
|
$
|
8,072
|
|
$
|
14,679
|
|
|||
|
Stock Option and Warrant Assumptions:
|
2018
|
2017
|
2016
|
||||||
|
Stock option grants:
|
|
|
|
|
|
|
|||
|
Expected term (years)
|
6.25
|
|
6.25
|
|
6.25
|
|
|||
|
Expected volatility
|
56.59
|
%
|
58.82
|
%
|
58.39
|
%
|
|||
|
Risk-free interest rate
|
2.68
|
%
|
2.20
|
%
|
2.04
|
%
|
|||
|
Expected dividend yield
|
—
|
|
—
|
|
—
|
|
|||
|
Warrant grants:
|
|
|
|
|
|
|
|||
|
Expected term (years)
|
—
|
|
0
|
|
2.50
|
|
|||
|
Expected volatility
|
—
|
%
|
—
|
%
|
60.57
|
%
|
|||
|
Risk-free interest rate
|
—
|
%
|
—
|
%
|
0.82
|
%
|
|||
|
Expected dividend yield
|
—
|
|
—
|
|
—
|
|
|||
|
Stock Option Activity and Other Data:
|
Number of Stock
Options
|
Weighted Average
Exercise Price per Share
|
Weighted Average
Remaining
Contractual Life
|
Aggregate
Intrinsic Value
|
|||||||||
|
Stock options outstanding, January 1, 2018
|
5,041
|
|
$
|
11.34
|
|
|
|
|
|||||
|
Granted
|
138
|
|
6.67
|
|
|
|
|
||||||
|
Exercised
|
(82
|
)
|
6.52
|
|
|
|
|
||||||
|
Forfeited
|
(428
|
)
|
10.04
|
|
|
|
|
||||||
|
Expired
|
(68
|
)
|
12.41
|
|
|||||||||
|
Stock options outstanding, December 31, 2018
|
4,601
|
|
$
|
11.39
|
|
7.25 years
|
$
|
—
|
|
||||
|
Stock options exercisable, December 31, 2018
|
3,005
|
|
$
|
11.99
|
|
6.66 years
|
$
|
—
|
|
||||
|
Warrant Activity and Other Data:
|
Number of
Warrants
|
Weighted Average Exercise Price per Share
|
Weighted Average Remaining
Contractual Life
|
Aggregate Intrinsic
Value
|
|||||||||
|
Warrants outstanding, January 1, 2018
|
894
|
|
$
|
16.77
|
|
|
|
|
|||||
|
Granted
|
—
|
|
—
|
|
|
|
|
||||||
|
Exercised
|
—
|
|
—
|
|
|
|
|
||||||
|
Forfeited
|
—
|
|
—
|
|
|
|
|
||||||
|
Expired
|
(298
|
)
|
14.87
|
|
|||||||||
|
Warrants outstanding, December 31, 2018
|
596
|
|
$
|
17.72
|
|
1.03 years
|
$
|
—
|
|
||||
|
Warrants exercisable, December 31, 2018
|
596
|
|
$
|
17.72
|
|
1.03 years
|
$
|
—
|
|
||||
|
Restricted Share Activity and Other Data:
|
Number of Restricted Share Awards
|
Weighted Average Grant Date
Fair Value
|
|||||
|
Non-vested restricted share awards outstanding, January 1, 2018
|
819
|
|
$
|
11.51
|
|
||
|
Granted
|
279
|
|
5.87
|
|
|||
|
Vested
|
(548
|
)
|
12.78
|
|
|||
|
Forfeited
|
(59
|
)
|
8.95
|
|
|||
|
Non-vested restricted share awards outstanding, December 31, 2018
|
491
|
|
$
|
7.20
|
|
||
|
Net (Loss) Income Per Share:
|
2018
|
2017
|
2016
|
|||||||||
|
Net (loss) income
|
$
|
(95,304
|
)
|
$
|
68,271
|
|
$
|
(41,276
|
)
|
|||
|
Weighted average shares:
|
|
|
|
|
|
|
||||||
|
Basic shares
|
37,325
|
|
40,465
|
|
41,248
|
|
||||||
|
Effect of dilutive securities—employee and director equity awards outstanding and 2023 Notes
|
—
|
|
|
1,300
|
|
|
—
|
|
||||
|
Diluted shares
|
37,325
|
|
41,765
|
|
41,248
|
|
||||||
|
Net (loss) income per share - basic
|
$
|
(2.55
|
)
|
$
|
1.69
|
|
$
|
(1.00
|
)
|
|||
|
Net (loss) income per share - diluted
|
$
|
(2.55
|
)
|
$
|
1.63
|
|
$
|
(1.00
|
)
|
|||
|
Accumulated Other Comprehensive (Loss) Income:
|
2018
|
2017
|
2016
|
|||||||||
|
Foreign currency translation adjustment:
|
|
|
|
|
||||||||
|
Beginning balance
|
$
|
(23,202
|
)
|
$
|
(23,336
|
)
|
$
|
(22,312
|
)
|
|||
|
Net other comprehensive (loss) income
|
(419
|
)
|
134
|
|
(1,024
|
)
|
||||||
|
Balance at December 31,
|
(23,621
|
)
|
|
(23,202
|
)
|
|
(23,336
|
)
|
||||
|
Unrealized gain (loss) on marketable securities, net
|
|
|
|
|
||||||||
|
Beginning balance
|
(64
|
)
|
(229
|
)
|
(345
|
)
|
||||||
|
Net other comprehensive income, net of ($18), $28, $16, tax, respectively
|
269
|
|
165
|
|
116
|
|
||||||
|
Balance at December 31,
|
205
|
|
|
(64
|
)
|
|
(229
|
)
|
||||
|
Accumulated other comprehensive loss at December 31,
|
$
|
(23,416
|
)
|
|
$
|
(23,266
|
)
|
|
$
|
(23,565
|
)
|
|
|
Revenue by Product:
|
2018
|
2017
|
2016
|
|||||||||
|
Bloxiverz
|
$
|
20,850
|
|
$
|
45,596
|
|
$
|
82,896
|
|
|||
|
Vazculep
|
42,916
|
|
38,187
|
|
39,796
|
|
||||||
|
Akovaz
|
33,759
|
|
80,617
|
|
16,831
|
|
||||||
|
Noctiva
|
1,204
|
|
—
|
|
—
|
|
||||||
|
Other
|
2,694
|
|
8,441
|
|
7,699
|
|
||||||
|
Total product sales
|
101,423
|
|
172,841
|
|
147,222
|
|
||||||
|
License revenue
|
1,846
|
|
404
|
|
3,024
|
|
||||||
|
Total revenues
|
$
|
103,269
|
|
$
|
173,245
|
|
$
|
150,246
|
|
|||
|
Revenue by Significant Customer:
|
2018
|
2017
|
2016
|
|||||||||
|
Customer A
|
$
|
26,794
|
|
$
|
44,762
|
|
$
|
51,648
|
|
|||
|
Customer B
|
25,413
|
|
37,965
|
|
39,359
|
|
||||||
|
Customer C
|
18,620
|
|
25,691
|
|
30,916
|
|
||||||
|
Customer D
|
9,653
|
|
53,342
|
|
17,728
|
|
||||||
|
Others
|
20,943
|
|
11,081
|
|
7,571
|
|
||||||
|
Total product sales
|
101,423
|
|
172,841
|
|
147,222
|
|
||||||
|
License revenue
|
1,846
|
|
404
|
|
3,024
|
|
||||||
|
Total revenues
|
$
|
103,269
|
|
$
|
173,245
|
|
$
|
150,246
|
|
|||
|
Revenue by Geographic Region:
|
2018
|
2017
|
2016
|
|||||||||
|
|
|
|
|
|
|
|||||||
|
United States
|
$
|
101,423
|
|
$
|
172,841
|
|
$
|
147,283
|
|
|||
|
Ireland
|
1,846
|
|
404
|
|
2,963
|
|
||||||
|
Total revenues
|
$
|
103,269
|
|
$
|
173,245
|
|
$
|
150,246
|
|
|||
|
Long-lived Assets by Geographic Region:
|
2018
|
2017
|
2016
|
|||||||||
|
|
|
|
|
|
|
|||||||
|
United States
|
$
|
27,761
|
|
$
|
116,536
|
|
$
|
42,021
|
|
|||
|
France
|
1,365
|
|
2,257
|
|
2,524
|
|
||||||
|
Ireland
|
6,028
|
|
1,360
|
|
202
|
|
||||||
|
Total
|
$
|
35,154
|
|
$
|
120,153
|
|
$
|
44,747
|
|
|||
|
(a)
|
Documents filed as part of this report:
|
|
1.
|
Financial Statements
|
|
2
.
|
Financial Statement Schedules
|
|
Deferred Tax Asset Valuation Allowance:
|
Balance,
Beginning of Period
|
Additions
(a)
|
Deductions
(b)
|
Other Changes
(c)
|
Balance,
End of Period
|
|||||||||||||||
|
2018
|
$
|
15,354
|
|
$
|
6,089
|
|
$
|
(75
|
)
|
$
|
(169
|
)
|
$
|
21,199
|
|
|||||
|
2017
|
$
|
7,599
|
|
$
|
391
|
|
$
|
(664
|
)
|
$
|
8,028
|
|
$
|
15,354
|
|
|||||
|
2016
|
$
|
45,516
|
|
$
|
6,873
|
|
$
|
(42,417
|
)
|
$
|
(2,373
|
)
|
$
|
7,599
|
|
|||||
|
a.
|
Additions to the deferred tax asset valuation allowance relate to movements on certain French, Irish and U.S. deferred tax assets where we continue to maintain a valuation allowance until sufficient positive evidence exists to support reversal.
|
|
b.
|
Deductions to the deferred tax asset valuation allowance include movements relating to utilization and removal of net operating losses and tax credit carryforwards, release in valuation allowance and other movements including adjustments following finalization of tax returns.
|
|
c.
|
Other changes to the deferred tax asset valuation allowance including currency translation adjustments recorded directly in equity and account method changes.
|
|
Exhibit Number
|
Exhibit Description
|
|
|
3.1
|
||
|
4.1
|
||
|
4.2
|
||
|
4.3
|
||
|
4.4
|
||
|
4.5
|
||
|
10.1
|
||
|
10.2*
|
||
|
10.3
|
||
|
10.4
|
||
|
10.5*
|
||
|
10.6*
|
||
|
10.7
|
||
|
10.8*
|
||
|
10.9
|
||
|
10.10
|
||
|
10.11
|
||
|
10.12
|
||
|
10.13*
|
||
|
10.14*
|
||
|
10.15
|
||
|
10.16
|
||
|
10.17
|
||
|
10.18‡
|
||
|
10.19‡
|
||
|
10.20
|
||
|
10.21‡
|
||
|
10.22‡
|
||
|
10.23‡
|
||
|
10.24‡
|
||
|
10.25‡
|
||
|
10.26‡
|
||
|
10.27
|
||
|
10.28‡
|
||
|
10.29‡
|
||
|
10.30‡
|
||
|
10.31
‡
|
||
|
10.32
‡
|
||
|
10.33‡
|
||
|
10.34‡
|
||
|
10.35‡
|
||
|
10.36‡
|
||
|
10.37‡
|
||
|
10.38*
|
||
|
10.39*
|
||
|
10.40*
|
||
|
10.41
|
||
|
10.42
|
||
|
10.43*
|
||
|
10.44*
|
||
|
10.45*
|
||
|
10.46*
|
||
|
10.47
|
||
|
10.48‡
|
||
|
10.49
|
||
|
14.1
|
||
|
14.2
|
||
|
21.1
|
||
|
23.1
|
||
|
31.1
|
||
|
31.2
|
||
|
32.1
|
||
|
32.2
|
||
|
101.INS
|
XBRL Instant Document
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
|
101.LAB
|
XBRL Taxonomy Extension Labels Linkbase Document
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
|
|
|
Avadel Pharmaceuticals PLC
|
|
|
|
|
|
Dated: March 15, 2019
|
By:
|
/s/ Gregory J. Divis
|
|
|
|
Name: Gregory J. Divis
|
|
|
|
Title: Interim Chief Executive Officer
|
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/ Gregory J. Divis
|
|
Interim Chief Executive Office (Principal Executive Officer)
|
|
March 15, 2019
|
|
Gregory J. Divis
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Michael F. Kanan
|
|
Chief Financial Officer (Principal Financial Officer)
|
|
March 15, 2019
|
|
Michael F. Kanan
|
|
|
|
|
|
|
|
|
|
|
|
/s/ David P. Gusky
|
|
Corporate Controller (Principal Accounting Officer)
|
|
March 15, 2019
|
|
David P. Gusky
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Geoffrey M. Glass
|
|
Non-Executive Chairman of the Board and Director
|
|
March 15, 2019
|
|
Geoffrey M. Glass
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Dr. Eric J. Ende
|
|
Director
|
|
March 15, 2019
|
|
Dr. Eric J. Ende
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Kevin Kotler
|
|
Director
|
|
March 15, 2019
|
|
Kevin Kotler
|
|
|
|
|
|
/s/ Linda S. Palczuk
|
|
Director
|
|
March 15, 2019
|
|
Linda S. Palczuk
|
|
|
|
|
|
/s/ Craig R. Stapleton
|
|
Director
|
|
March 15, 2019
|
|
Craig R. Stapleton
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Peter Thornton
|
|
Director
|
|
March 15, 2019
|
|
Peter Thornton
|
|
|
|
|