|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| AWARE, INC. | ||||||||
| (Exact Name of Registrant as Specified in Its Charter) | ||||||||
| Massachusetts | 04-2911026 | |||||||
| (State or Other Jurisdiction of | (I.R.S. Employer Identification No.) | |||||||
| Incorporation or Organization) | ||||||||
| 40 Middlesex Turnpike, Bedford, Massachusetts 01730 | ||||
|
(Address of Principal Executive Offices)
|
||||
| (Zip Code) | ||||
| (781) 276-4000 | ||||
| (Registrant’s Telephone Number, Including Area Code) | ||||
| Securities registered pursuant to Section 12(b) of the Act: | ||||
| Title of Each Class | Name of Each Exchange on Which Registered | ||||
| Common Stock, par value $.01 per share | The Nasdaq Global Market | ||||
| Large Accelerated Filer ☐ | Accelerated Filer ☒ | Non-Accelerated Filer ☐ | Smaller Reporting Company ☐ |
| PART I | ||||
|
Item 1.
|
Business
|
3
|
||
|
Item 1A.
|
Risk Factors
|
14
|
||
|
Item 1B.
|
Unresolved Staff Comments
|
20
|
||
|
Item 2.
|
Properties
|
20
|
||
|
Item 3.
|
Legal Proceedings
|
20
|
||
|
Item 4.
|
Mine Safety Disclosures
|
20
|
||
|
PART II
|
||||
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
21
|
||
|
Item 6.
|
Selected Financial Data
|
23
|
||
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results
of Operations
|
24
|
||
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
|
37
|
||
|
Item 8.
|
Financial Statements and Supplementary Data
|
38
|
||
|
Item 9.
|
Changes in and Disagreements with Accountants on Accounting and Financial
Disclosure
|
62
|
||
|
Item 9A.
|
Controls and Procedures
|
62
|
||
|
Item 9B.
|
Other Information
|
62
|
||
|
PART III
|
||||
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
63
|
||
|
Item 11.
|
Executive Compensation
|
63
|
||
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
63
|
||
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
63
|
||
|
Item 14.
|
Principal Accountant Fees and Services
|
63
|
||
|
PART IV
|
||||
|
Item 15.
|
Exhibits and Financial Statement Schedule
|
64
|
||
|
Signatures
|
66
|
|||
| 2 |
| 3 |
| 4 |
|
i)
|
Technology suppliers
– Within this category, there are suppliers that provide software and hardware technologies that enable biometrics systems.
|
|
Biometrics software products provide functionality that: i) captures and formats images; ii) processes and transports images, and iii) matches images. Companies that sell products in this category include: i) Aware; ii) Cognitec Systems GmbH (“Cognitec”); iii) Neurotechnology; iv) Iritech, Inc. (“Iritech”); v) Innovatrics s.r.o. (“Innovatrics”); vi) WCC Group B.V. (“WCC”); vii) Daon, Inc. (“Daon”); viii) M2Sys Technology (“M2Sys”); and ix) Agnitio S.L. (“Agnitio”).
|
|
Hardware companies that provide equipment used in biometrics systems include:
|
|
|
●
|
Biometrics-specific hardware vendors, such as: i) Cross Match Technologies, Inc. (“Cross Match”); ii) Iris ID Systems, Inc.; iii) Integrated Biometrics, LLC (“Integrated Biometrics”); and iv) Lumidigm, Inc. which was acquired by HID Global Corporation in 2014 (“HID/Lumidigm”); and
|
|
|
●
|
General purpose computer hardware companies, such as Hewlett-Packard Development Company, L.P. and Dell, Inc.
|
|
ii)
|
System integrators
– This category of suppliers includes companies that provide system integration services. System integrators purchase hardware and software from biometrics technology vendors, such as those listed immediately above. They then incorporate those components into customized biometrics systems that they build for their end-user customers. System integrators include large multinationals with a broad range of expertise and the capacity to execute very large projects, as well as smaller system integrators that have more focused expertise on a particular market sector, technology, or geography.
|
|
Examples of systems integrators include: i) Northrop Grumman Corporation; ii) Lockheed Martin Corporation; iii) Science Applications International Corporation; iv) Hewlett-Packard Enterprise Services; v) International Business Machines; vi) Fujitsu Limited; vii) Computer Science Corporation; vii) Accenture plc; vii) Raytheon Company; ix) Unisys Corporation; x) Unicom Government, Inc; and xi) Telos Corporation.
|
|
iii)
|
Fully integrated solutions vendors
– This category of suppliers includes companies that are fully integrated providers of biometric systems. Such companies combine their in-house hardware and software technologies with their systems integration services to deliver customized biometrics systems to their end-user customers. While these vendors may purchase some components from third parties, we believe such component purchases represent a minor portion of the total systems they deliver.
|
|
We believe there are three primary fully integrated solutions vendors. They are: i) MorphoTrak and MorphoTrust, divisions of the Safran Group Company (“Safran Morpho”); ii) 3M Cogent Inc. (“3M/Cogent”); and iii) NEC Corporation (“NEC”). We believe that these companies supply a large percentage of the government market.
|
|
|
●
|
User authentication for login and access to mobile devices, computers, networks, and software programs.
|
|
|
●
|
User authentication for financial transactions in the financial services industry.
|
|
|
●
|
User authentication for in-person or online purchases in the retail industry.
|
|
|
●
|
Authentication for physical access to secured buildings and perimeters.
|
| 5 |
|
|
●
|
Authentication of employees to access private patient information in the healthcare industry.
|
|
|
●
|
Authentication of test takers in the educational testing industry.
|
|
|
●
|
Identification of prospective customers in the financial services industry.
|
|
|
●
|
Identification of candidates for pre-employment screening and background checks.
|
|
|
●
|
Identification of patients in hospital and surgical settings.
|
|
|
●
|
Identification of undesirable customers in the gaming industry.
|
| 6 |
|
●
|
Hardware companies involved in the mobile segment of the commercial market
. These companies include: i) Apple, Inc. through its acquisition of AuthenTec, Inc.; ii) Synaptics, Inc., which is a supplier to Samsung
Electronics Co., Ltd; iii) Qualcomm Technologies, Inc. through its acquisition of UltraScan Corporation; and iv) Sonavation, Inc.
|
|
●
|
Hardware companies involved in the enterprise segment of the commercial market
. These companies include: i) DigitalPersona, Inc., which was acquired by Francisco Partners Management LLC and merged with Cross Match in 2014; ii) Integrated Biometrics; iii) HID/Lumidigm; iv) Fingerprint Cards AB; v) Suprema, Inc.; vi) Credence ID, LLC; vii) SecuGen Corporation; viii) Next Biometrics AS; and ix) Eyelock, Inc. (“Eyelock”).
|
|
●
|
Hardware companies focused on the access control segment of the enterprise commercial market
. These companies include: i) Honeywell International, Inc.; ii) HID/Lumidigm; iii) Tyco International Ltd.; iv) Lenel Systems International Inc.; v) Stanley Security Limited; vi) IrisGuard, Inc.; and vii) Aurora Biometrics, Inc.
|
|
|
1.
|
Enrollment, analysis, and processing of biometrics images and data.
|
|
|
2.
|
Integration of peripheral biometric capture devices.
|
|
|
3.
|
Centralized transaction workflow and processing.
|
|
|
4.
|
Matching of biometric images to verify identity or searching biometric databases; and
|
|
|
5.
|
Analysis and processing of text-based identity data.
|
|
1)
|
Software Development Kits
.
Our software development kits or (“SDKs”) consist of: i) multiple software libraries; ii) sample applications that show customers how to use the libraries; and iii) documentation. Customers use our SDKs to design and develop biometrics applications. Our SDK products may be categorized into three groups: i) Enrollment; ii) Search and Matching; and iii) Identity Data Management and Analytics. These products are described below.
|
|
SDK Group 1: Enrollment SDKs
. Our suite of enrollment SDKs performs a variety of functions that are critical to biometric enrollment, including image capture, image quality assurance, image formatting, and image compression. Our enrollment SDK products include:
|
|
|
●
|
Hardware abstraction, autocapture, and quality assurance products
, including: i) FastCapture with LiveScan API; ii) PreFace
TM
with Camera API; iii) IrisCheck
TM
with IrisCam API; iv) SequenceCheck
TM
; and v) Quality Check
TM
.
|
|
|
●
|
Biometric data formatting, validation and reading products
, including: i) NISTPack; ii) ICAOPack; and iii) PIVPack
TM
.
|
|
|
●
|
Fingerprint, facial, and iris image compression and decompression products,
including: i) Aware WSQ1000; and ii) Aware JPEG2000.
|
| 7 |
|
|
●
|
Biometric authentication products
, including AwareXM
TM
.
|
|
|
●
|
Fingerprint card scanning and printing products
, including: i) AccuScan
TM
; and ii) AccuPrint
TM
.
|
|
|
●
|
Mobile products for smartphones and tablets
, including: i) NISTPack Mobile; ii) WSQ1000 Mobile; iii) ICAOPack Mobile; iv) PIVPack
TM
Mobile; and v) AwareXM
TM
Mobile.
|
|
|
●
|
Application specific bundles
, including CaptureSuite
TM
which is used for the development of applications for the capture of live scan or card scan fingerprint images.
|
|
2)
|
BioComponents.
Our BioComponents products allow customers to develop biometric enrollment applications more quickly than if they purchased our SDKs. Each product in the group includes a user interface and one or more software libraries that perform a discrete set of functions, such as automated image capture, quality assurance, and capture hardware integration. BioComponents comprise modular, independent, self-contained software components that can operate either independently or in concert with one another. Specific BioComponents products and the functions they perform are:
|
|
|
●
|
BiographicComponent enables highly configurable data entry of biographic and textual information.
|
|
|
●
|
FingerprintComponent is used to capture, verify image quality, and compress fingerprint images.
|
|
|
●
|
FaceComponent is used to capture, verify image quality, and manipulate facial images.
|
|
|
●
|
IrisComponent is used to capture, segment, and verify image quality of iris images.
|
|
|
●
|
TravelDocComponent is used to authenticate travel documents, such as passports and driver’s licenses.
|
|
|
●
|
ScanningComponent is used to scan forms such as inked fingerprint cards.
|
|
|
●
|
PrintingComponent is used for printing FBI-quality fingerprint images on cards and forms.
|
|
|
●
|
SignatureComponent is used to collect handwritten signature images from an electronic signature pad.
|
|
|
●
|
PackagingComponent allows access to the data sets from the other components.
|
|
3)
|
Biometric applications.
Our products in this category combine user interfaces with multiple Aware software libraries and/or BioComponents to create more complete applications that operate on client workstations or mobile devices. Our application products and the functions they perform are:
|
|
|
●
|
Universal Registration Client (“URC
TM
”).
URC is a configurable Windows-based application that performs a variety of biometric data capture, analysis, matching, formatting, and hardware abstraction functions.
|
|
|
●
|
URC Mobile
. URC Mobile is a software application for performing biometric enrollment, identification, and screening on mobile biometric devices, such as those used by military personnel in the field.
|
|
|
●
|
FormScannerSE and FormScannerMB
. These are two independent applications for scanning and processing of inked fingerprint cards.
|
| 8 |
|
|
●
|
FormScannerSWFT
. This product is a version of FormScannerSE that is preconfigured for use in compliance with the “Secure Web Fingerprint Transmission” program of the U.S. Department of Defense.
|
|
|
●
|
Forensic Workbench
. Forensic Workbench is a software application for the categorization, processing, and standards-compliant formatting of biometric images and demographic data.
|
|
|
●
|
Sequence Workbench
. Sequence Workbench is a software application for the detection and assisted repair of fingerprint records containing sequence errors.
|
|
|
●
|
WebEnroll
. WebEnroll provides a reference application that uses BioComponents for browser-based enrollment of biographic data, fingerprints, facial images, and iris images.
|
|
4)
|
Biometric Services Platform (BioSP
). Our Biometric Services Platform, or BioSP product, is a service-oriented application used to enable biometric data processing and management. BioSP is suited for applications that require the collection of biometrics throughout a distributed network. BioSP is designed to be modular, programmable, scalable, and secure. It is used to manage transaction workflow, including messaging, submissions, responses, and logging.
|
|
|
●
|
automated biometric image and data analysis, processing, formatting, quality assurance, and reporting.
|
|
|
●
|
web services in support of a scalable, secure, service-oriented architecture.
|
|
|
●
|
integration of biometric functions with other enterprise systems such as identity management, access management, card management, and AFIS/ABIS.
|
|
|
●
|
1-to-1 and 1-to-many biometric matching for verification, identification, and duplicate checking.
|
|
|
●
|
centralized system administration and user management.
|
|
|
●
|
advanced reporting capabilities for fast troubleshooting of biometric capture problems.
|
|
|
●
|
centralized configuration, distribution, and management of enrollment client software.
|
|
|
●
|
support for fingerprint, facial, iris, and palm modalities.
|
| 9 |
|
|
i)
|
Systems integrator channel – we sell to systems integrators that incorporate our software products into biometrics systems that are delivered primarily to government end users.
|
|
|
ii)
|
OEM channel – we sell to hardware and software solution providers that incorporate our software products into their products.
|
|
|
iii)
|
Direct channel – we also sell directly to government, and, to a lesser degree, to commercial customers.
|
|
|
i)
|
Product strategy
– Our product strategy is to offer more complete biometrics solutions. We believe this strategy will allow us to us to sell more software and services into biometrics projects. We recognized the need to make this transition several years ago and began developing products to enable this strategy.
|
| 10 |
|
|
●
|
Product line expansion - Our aim is to expand our product portfolio by adding new products and increasing the functionality of existing products using our internal engineering teams. This means we may add resources to our engineering staff. To the extent we are unable to develop critical new technologies internally, we may purchase or license such technologies from third parties. Alternatively, we may also acquire biometrics companies provided we believe the acquisition cost is reasonable relative to the estimated future revenue and profits the acquired company may produce.
|
|
|
●
|
Engineering services – We believe that services are an important element of our strategy to sell complete solutions. We intend to have adequate engineering resources on hand to ensure that we can staff projects with the technical expertise we need to win new projects.
|
|
|
ii)
|
Market strategy –
Our market strategy is to continue to focus on our legacy government biometrics market and expand into new commercial biometrics markets. Historically our revenue has been primarily derived from the government biometrics market and we intend to continue our focus there.
|
|
|
iii)
|
Sales strategy
– While the United States remains a large market, we believe there are attractive growth opportunities in international markets. We intend to continue our focus on international markets and pursue opportunities there through additions to our sales staff, as well as through the use of third party sales agents.
|
| 11 |
| 12 |
| 13 |
|
●
|
the lack or reduction of government funding and the political, budgetary and purchasing constraints of government customers who purchase products and services directly or indirectly from us;
|
|
●
|
the terms of customer contracts that affect the timing of revenue recognition;
|
|
●
|
the size and timing of our receipt of customer orders;
|
|
●
|
significant fluctuations in demand for our products and services;
|
|
●
|
the loss of a key customer or one of its key customers;
|
|
●
|
new competitors, or the introduction of enhanced solutions from new or existing competitors;
|
|
●
|
competitive pressures on selling prices;
|
|
●
|
cancellations, delays or contract amendments by government customers;
|
|
●
|
higher than expected costs, asset write-offs, and other one-time financial charges; and
|
|
●
|
general economic trends and other factors
|
|
●
|
changes in fiscal policies or decreases in available government funding,
|
|
●
|
changes in government funding priorities;
|
|
●
|
changes in government programs or applicable requirements;
|
|
●
|
the adoption of new laws or regulations or changes to existing laws or regulations;
|
|
●
|
changes in political or social attitudes with respect to security and defense issues;
|
|
●
|
changes in audit policies and procedures of government entities;
|
|
●
|
potential delays or changes in the government appropriations process; and
|
|
●
|
delays in the payment of our invoices by government payment offices.
|
| 14 |
|
●
|
a reduction in sales efforts by our partners;
|
|
●
|
the failure of our partners to win government awards in which our products are used;
|
|
●
|
a reduction in technical capabilities or financial viability of our partners;
|
|
●
|
a misalignment of interest between us and them;
|
|
●
|
the termination of our relationship with a major systems integrator or OEM; or
|
|
●
|
any adverse effect on a partner’s business related to competition, pricing and other factors.
|
|
●
|
the cost, performance and reliability of our products and services and the products and services offered by our competitors;
|
|
●
|
the continued growth in demand for biometrics solutions within the government and law enforcement markets as well as the development and growth of demand for biometric solutions in markets outside of government and law enforcement;
|
|
●
|
customers’ perceptions regarding the benefits of biometrics solutions;
|
|
●
|
public perceptions regarding the intrusiveness of these solutions and the manner in which organizations use the biometric information collected;
|
|
●
|
public perceptions regarding the confidentiality of private information;
|
|
●
|
proposed or enacted legislation related to privacy of information;
|
|
●
|
customers’ satisfaction with biometrics solutions; and
|
|
●
|
marketing efforts and publicity regarding biometrics solutions.
|
| 15 |
|
●
|
Diversified technology providers that offer integrated biometrics solutions to governments, law enforcement agencies and other organizations. This group of competitors includes companies such as Safran Morpho, 3M/Cogent, and NEC.
|
|
●
|
Component providers that offer biometrics software and hardware components for fingerprint, facial, and iris biometric identification. This group of competitors includes companies such as Cognitec; Neurotechnology; Iritech; Innovatrics; WCC; Daon; and M2Sys.
|
| 16 |
| 17 |
|
●
|
reduced demand for our products;
|
|
●
|
increased risk of order cancellations or delays;
|
|
●
|
increased pressure on the prices for our products;
|
|
●
|
greater difficulty in collecting accounts receivable; and
|
|
●
|
risks to our liquidity, including the possibility that we might not have access to our cash when needed.
|
| 18 |
|
●
|
quarterly variations in operating results;
|
|
●
|
announcements of technological innovations or new products by us or our competitors,
|
|
●
|
changes in customer relationships, such as the loss of a key customer;
|
|
●
|
recruitment or departure of key personnel;
|
|
●
|
corporate actions we may initiate, such as acquisitions, stock sales or repurchases, dividend declarations, or corporate reorganizations; and
|
|
●
|
other events or factors.
|
| 19 |
| 20 |
|
First
|
Second
|
Third
|
Fourth
|
|||||||||||||
|
Quarter
|
Quarter
|
Quarter
|
Quarter
|
|||||||||||||
|
2014
|
||||||||||||||||
|
High
|
$ | 6.70 | $ | 6.70 | $ | 6.79 | $ | 5.02 | ||||||||
|
Low
|
5.27 | 5.02 | 3.50 | 3.46 | ||||||||||||
|
2013
|
||||||||||||||||
|
High
|
$ | 6.25 | $ | 5.65 | $ | 5.64 | $ | 6.14 | ||||||||
|
Low
|
4.58 | 4.50 | 4.80 | 5.06 | ||||||||||||
| 21 |
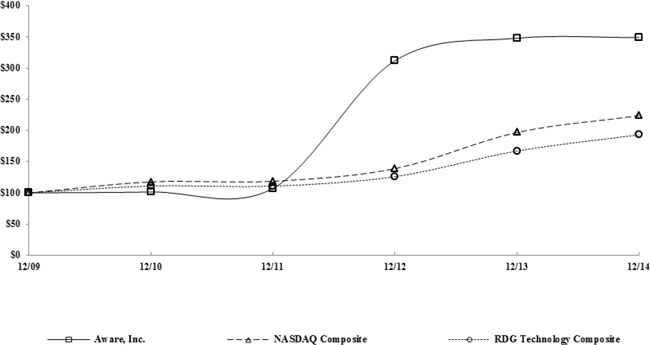
|
*$100 invested on 12/31/09 in stock or index, including reinvestment of dividends.
Fiscal year ending December 31.
|
|
Value of Investment ($)
|
||||||||||||||||||||||||
|
12/31/09
|
12/31/10
|
12/31/11
|
12/31/12
|
12/31/13
|
12/31/14
|
|||||||||||||||||||
|
Aware, Inc.
|
$ | 100.00 | $ | 101.43 | $ | 107.14 | $ | 312.06 | $ | 347.94 | $ | 349.02 | ||||||||||||
|
Nasdaq Composite Index
|
100.00 | 117.61 | 118.70 | 139.00 | 196.83 | 223.74 | ||||||||||||||||||
|
RDG Technology Composite Index
|
100.00 | 111.01 | 110.85 | 126.07 | 167.16 | 193.22 | ||||||||||||||||||
| 22 |
|
Year ended December 31,
|
2014
|
2013
|
2012
|
2011
|
2010
|
|||||||||||||||
|
(in thousands, except per share data)
|
||||||||||||||||||||
|
Statements of Comprehensive Income Data
|
||||||||||||||||||||
|
Revenue
|
$ | 23,720 | $ | 19,357 | $ | 17,304 | $ | 16,199 | $ | 12,975 | ||||||||||
|
Patent related income
|
2,127 | 780 | 87,515 | - | - | |||||||||||||||
|
Operating income (loss)
|
7,089 | 5,318 | 92,558 | 3,500 | (360 | ) | ||||||||||||||
|
Income from continuing operations
|
4,583 | 3,752 | 72,383 | 3,581 | 154 | |||||||||||||||
|
Income (loss) from discontinued operations, net of income taxes
|
- | (1,156 | ) | (76 | ) | (1,014 | ) | 26 | ||||||||||||
|
Net income
|
4,583 | 2,596 | 72,307 | 2,567 | 180 | |||||||||||||||
|
Net income per share – basic
|
$ | 0.20 | $ | 0.12 | $ | 3.32 | $ | 0.12 | $ | 0.01 | ||||||||||
|
Net income per share – diluted
|
$ | 0.20 | $ | 0.11 | $ | 3.28 | $ | 0.12 | $ | 0.01 | ||||||||||
|
Balance Sheet Data
|
||||||||||||||||||||
|
Cash and cash equivalents
|
$ | 43,985 | $ | 72,660 | $ | 71,074 | $ | 46,577 | $ | 39,949 | ||||||||||
|
Working capital
|
44,745 | 75,760 | 73,358 | 48,069 | 43,818 | |||||||||||||||
|
Total assets
|
55,893 | 89,329 | 85,854 | 57,851 | 53,400 | |||||||||||||||
|
Total liabilities
|
3,504 | 4,179 | 3,958 | 3,276 | 3,517 | |||||||||||||||
|
Total stockholders’ equity
|
52,389 | 85,150 | 81,896 | 54,575 | 49,883 | |||||||||||||||
| 23 |
|
Year ended December 31,
|
||||||||||||
|
Revenue:
|
2014
|
2013
|
2012
|
|||||||||
|
Software licenses
|
36 | % | 43 | % | 55 | % | ||||||
|
Software maintenance
|
18 | 20 | 18 | |||||||||
|
Services
|
22 | 16 | 15 | |||||||||
|
Hardware
|
21 | 16 | - | |||||||||
|
Royalties
|
3 | 5 | 12 | |||||||||
|
Total revenue
|
100 | 100 | 100 | |||||||||
|
Costs and expenses:
|
||||||||||||
|
Cost of hardware
|
15 | 12 | - | |||||||||
|
Cost of services
|
10 | 8 | 9 | |||||||||
|
Research and development
|
23 | 21 | 20 | |||||||||
|
Selling and marketing
|
16 | 18 | 20 | |||||||||
|
General and administrative
|
15 | 18 | 22 | |||||||||
|
Total costs and expenses
|
79 | 77 | 71 | |||||||||
|
Patent related income
|
9 | 4 | 506 | |||||||||
|
Operating income
|
30 | 27 | 535 | |||||||||
|
Other income (expense)
|
- | - | - | |||||||||
|
Interest income
|
- | 2 | 1 | |||||||||
|
Income from continuing operations before income taxes
|
30 | 29 | 536 | |||||||||
|
Provision for income taxes
|
11 | 10 | 118 | |||||||||
|
Income from continuing operations
|
19 | 19 | 418 | |||||||||
|
Loss from discontinued operations, net of income taxes
|
- | (6 | ) | - | ||||||||
|
Net income
|
19 | % | 13 | % | 418 | % | ||||||
| 24 |
| 25 |
| 26 |
|
Years ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Research and development expense
|
$ | 5,505 | $ | 4,085 | $ | 3,489 | ||||||
|
Cost of services
|
2,359 | 1,503 | 1,542 | |||||||||
|
Total engineering costs
|
$ | 7,864 | $ | 5,588 | $ | 5,031 | ||||||
| 27 |
Research and development expense increased 35% from $4.1 million in 2013 to $5.5 million in 2014. As a percentage of total revenue, research and development expense increased from 21% in 2013 to 23% in 2014
| 28 |
|
|
●
|
The claims in one of the patents we assigned were rejected by the United States Patent Office (“USPTO”) in May 2013. The USPTO’s Patent Trial & Appeal Board (“PTAB”) affirmed the USPTO decision in June 2014. The PTAB decision was appealed to the Federal Circuit. In December 2014, the Federal Circuit remanded the appeal back to the PTAB for further consideration.
|
|
|
●
|
Notwithstanding the outcome at the PTAB, we do not know whether any patent monetization efforts by the third party will be successful.
|
|
|
●
|
We sold a portion of our patent portfolio pertaining to wireless technology to an unaffiliated third party for $75.0 million. The proceeds from the sale were reduced by $3.8 million of transaction costs, which consisted primarily of fees from the law firm that assisted us in the sale. We recorded a gain of $71.2 million on the sale.
|
| 29 |
|
|
●
|
We sold a portion of our patent portfolio pertaining to DSL semiconductor intellectual property technology for $16.0 million. The proceeds from the sale were reduced by $0.8 million of transaction costs, which also consisted primarily of fees from the law firm that assisted us in the sale. We recorded a gain of $15.2 million on the sale.
|
| 30 |
|
Years ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Revenue
|
$ | - | $ | 3,216 | $ | 5,431 | ||||||
|
Expenses
|
- | 5,120 | 5,558 | |||||||||
|
Loss before income taxes
|
- | (1,904 | ) | (127 | ) | |||||||
|
Income tax benefit
|
- | (748 | ) | (51 | ) | |||||||
|
Loss from discontinued operations
|
$ | - | ($ | 1,156 | ) | ($ | 76 | ) | ||||
| 31 |
|
|
●
|
Cash provided by on-going operations of $6.7 million was the result of: i) operating income before patent related income of $5.0 million; ii) interest income and other income of $0.3 million; iii) adjustments for non-cash items related to depreciation and stock-based compensation of $0.5 million and $0.3 million, respectively; and iv) changes in working capital components that increased cash by $0.6 million.
|
|
|
●
|
Cash provided by on-going operations of $6.7 million was reduced by $22.0 million of income tax items that were incurred as a result of our patent sales in 2012. The $22.0 million of income tax items comprises: i) $7.6 million of tax payments; and ii) $14.4 million of tax expense that is reduced by a tax benefit from excess stock-based compensation that must be presented as cash flows from financing activities under generally accepted accounting principles as opposed to an item that benefits cash flows from operating activities.
|
| 32 |
| 33 |
| 34 |
|
Payments Due By Period
|
||||||||||||||||||||
|
Contractual Obligations
|
Total
|
Less than
1 year
|
1-3 years
|
3-5 years
|
More than
5 years
|
|||||||||||||||
|
Purchase orders
|
$ | 52 | $ | 52 | $ | - | $ | - | $ | - | ||||||||||
|
Total
|
$ | 52 | $ | 52 | $ | - | $ | - | $ | - | ||||||||||
|
o
|
When software licenses and maintenance contracts are sold together, we recognize software license revenue upon delivery, provided we have vendor specific objective evidence (“VSOE”) for the fair value of the maintenance contract fee, and we recognize the fair value of maintenance contract revenue ratably over the related contract period.
|
|
o
|
When software engineering services and software licenses are sold together, the total fee is generally recognized by applying contract accounting. We have adopted the percentage-of-completion method of contract accounting, and we primarily use an input method (i.e., labor hours) to determine our completion percentage.
|
|
o
|
When we sell software licenses, software maintenance, and software services together, revenue is recognized as follows: i) maintenance revenue is separated from the other two elements and is recognized ratably over the related contract period; provided we have VSOE for the fair value of the maintenance element; and ii) the total fee from the software license and engineering service elements is recognized by applying the contract accounting method described in the previous paragraph.
|
| 35 |
|
o
|
We also have a multiple element arrangement with one customer that involves the delivery of hardware, software maintenance, and software services. We determined that these elements qualified as separate units of accounting under ASC 605, Revenue Recognition, because they have value to the customer on a standalone basis. Accordingly, we recognize hardware revenue upon delivery and acceptance by the customer, maintenance revenue ratably over the related contract period, and service revenue as services are delivered.
|
| 36 |
|
1.
|
Cash and cash equivalents.
As of December 31, 2014, our cash and cash equivalents of $44.0 million were primarily invested in money market funds. The money market funds were invested in high quality, short term financial instruments. Due to the nature, short duration, and professional management of these funds, we do not expect that a general increase in interest rates would result in any material loss.
|
|
2.
|
Investments.
As of December 31, 2014, our investments of $1.4 million were invested in high yield bonds with three corporate debt issuers, which mature in 2017 and 2018. While we are exposed to default risk, the high current yield of these bonds largely mitigates interest rate risk. Therefore, due to the high current yield and the two to five year life of these instruments, we do not believe that a general increase in interest rates would result in any material loss.
|
| 37 |
| 38 |
|
December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$ | 43,985 | $ | 72,660 | ||||
|
Accounts receivable (less allowance for doubtful accounts of $20 at December 31, 2014 and 2013)
|
3,619 | 4,582 | ||||||
|
Inventories
|
2 | 1,601 | ||||||
|
Deferred tax assets
|
168 | 383 | ||||||
|
Prepaid expenses and other current assets
|
401 | 695 | ||||||
|
Total current assets
|
48,175 | 79,921 | ||||||
|
Property and equipment, net
|
5,289 | 5,582 | ||||||
|
Investments
|
1,428 | 2,754 | ||||||
|
Long term deferred tax assets
|
804 | 762 | ||||||
|
Other assets
|
197 | 310 | ||||||
|
Total assets
|
$ | 55,893 | $ | 89,329 | ||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$ | 258 | $ | 1,516 | ||||
|
Accrued expenses
|
108 | 108 | ||||||
|
Accrued compensation
|
585 | 571 | ||||||
|
Accrued professional fees
|
127 | 118 | ||||||
|
Deferred revenue
|
2,352 | 1,848 | ||||||
|
Total current liabilities
|
3,430 | 4,161 | ||||||
|
Long-term deferred revenue
|
74 | 18 | ||||||
|
Commitments and contingent liabilities (Note 9)
|
||||||||
|
Stockholders’ equity:
|
||||||||
|
Preferred stock, $1.00 par value; 1,000,000 shares authorized,
none outstanding
|
- | - | ||||||
|
Common stock, $.01 par value; shares authorized,
70,000,000 in 2014 and 2013; issued
and outstanding 22,808,761 in 2014 and 22,574,251 in 2013
|
228 | 226 | ||||||
|
Additional paid-in capital
|
103,756 | 101,293 | ||||||
|
Accumulated other comprehensive loss
|
(29 | ) | (125 | ) | ||||
|
Accumulated deficit
|
(51,566 | ) | (16,244 | ) | ||||
|
Total stockholders’ equity
|
52,389 | 85,150 | ||||||
|
Total liabilities and stockholders’ equity
|
$ | 55,893 | $ | 89,329 | ||||
| 39 |
|
Years ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Revenue:
|
||||||||||||
|
Software licenses
|
$ | 8,537 | $ | 8,241 | $ | 9,525 | ||||||
|
Software maintenance
|
4,351 | 3,866 | 3,038 | |||||||||
|
Services
|
5,173 | 3,148 | 2,571 | |||||||||
|
Hardware
|
4,933 | 3,182 | - | |||||||||
|
Royalties
|
726 | 920 | 2,170 | |||||||||
|
Total revenue
|
23,720 | 19,357 | 17,304 | |||||||||
|
Costs and expenses:
|
||||||||||||
|
Cost of hardware
|
3,485 | 2,365 | - | |||||||||
|
Cost of services
|
2,359 | 1,503 | 1,542 | |||||||||
|
Research and development
|
5,505 | 4,085 | 3,489 | |||||||||
|
Selling and marketing
|
3,741 | 3,344 | 3,370 | |||||||||
|
General and administrative
|
3,668 | 3,522 | 3,860 | |||||||||
|
Total costs and expenses
|
18,758 | 14,819 | 12,261 | |||||||||
|
Patent related income
|
2,127 | 780 | 87,515 | |||||||||
|
Operating income
|
7,089 | 5,318 | 92,558 | |||||||||
|
Other income (expense)
|
(59 | ) | 23 | 85 | ||||||||
|
Interest income
|
225 | 328 | 227 | |||||||||
|
Income from continuing operations before income taxes
|
7,255 | 5,669 | 92,870 | |||||||||
|
Provision for income taxes
|
2,672 | 1,917 | 20,487 | |||||||||
|
Income from continuing operations
|
4,583 | 3,752 | 72,383 | |||||||||
|
Loss from discontinued operations, net of income taxes
|
- | (1,156 | ) | (76 | ) | |||||||
|
Net income
|
$ | 4,583 | $ | 2,596 | $ | 72,307 | ||||||
|
Basic net income per share:
|
||||||||||||
|
Basic net income per share from continuing operations
|
$ | 0.20 | $ | 0.17 | $ | 3.32 | ||||||
|
Basic net loss per share from discontinued operations
|
- | (0.05 | ) | (0.00 | ) | |||||||
|
Basic net income per share
|
$ | 0.20 | $ | 0.12 | $ | 3.32 | ||||||
|
Diluted net income per share:
|
||||||||||||
|
Diluted net income per share from continuing operations
|
$ | 0.20 | $ | 0.16 | $ | 3.28 | ||||||
|
Diluted net loss per share from discontinued operations
|
- | (0.05 | ) | (0.00 | ) | |||||||
|
Diluted net income per share
|
$ | 0.20 | $ | 0.11 | $ | 3.28 | ||||||
|
Weighted-average shares - basic
|
22,703 | 22,543 | 21,814 | |||||||||
|
Weighted-average shares - diluted
|
22,787 | 22,641 | 22,071 | |||||||||
|
Comprehensive income:
|
||||||||||||
|
Net income
|
$ | 4,583 | $ | 2,596 | $ | 72,307 | ||||||
|
Other comprehensive income (net of tax):
|
||||||||||||
|
Unrealized gain/(loss) on available for sale securities
|
96 | (75 | ) | (30 | ) | |||||||
|
Comprehensive income
|
$ | 4,679 | $ | 2,521 | $ | 72,277 | ||||||
| 40 |
|
Years ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Cash flows from operating activities:
|
||||||||||||
|
Net income
|
$ | 4,583 | $ | 2,596 | $ | 72,307 | ||||||
|
Adjustments to reconcile net income to net cash
provided by (used in) operating activities:
|
||||||||||||
|
Depreciation and amortization
|
541 | 458 | 453 | |||||||||
|
Stock-based compensation
|
915 | 662 | 321 | |||||||||
|
Gain on sale of patent assets
|
(2,127 | ) | - | (86,394 | ) | |||||||
|
Amortization of premium (discount) on investments
|
(3 | ) | 15 | (18 | ) | |||||||
|
(Gain)/loss on sale of investments
|
59 | (23 | ) | (85 | ) | |||||||
|
Deferred tax benefit (expense) on other comprehensive
income
|
(66 | ) | 81 | - | ||||||||
|
Loss on disposal of property and equipment
|
- | 28 | - | |||||||||
|
Provision for doubtful accounts
|
- | - | 4 | |||||||||
|
Increase (decrease) from changes in assets and liabilities:
|
||||||||||||
|
Accounts receivable
|
963 | (1,125 | ) | 85 | ||||||||
|
Receivable from patent arrangement
|
- | 1,121 | (1,121 | ) | ||||||||
|
Inventories
|
1,599 | (1,601 | ) | 547 | ||||||||
|
Prepaid expenses and other current assets
|
294 | (167 | ) | (315 | ) | |||||||
|
Deferred tax assets
|
173 | 563 | (1,760 | ) | ||||||||
|
Accounts payable
|
(1,258 | ) | 1,188 | (71 | ) | |||||||
|
Accrued expenses, compensation and professional
|
23 | (310 | ) | 9 | ||||||||
|
Deferred revenue
|
560 | (657 | ) | 744 | ||||||||
|
Net cash provided by (used in) operating activities
|
6,256 | 2,829 | (15,294 | ) | ||||||||
|
Cash flows from investing activities:
|
||||||||||||
|
Purchases of property and equipment
|
(135 | ) | (160 | ) | (116 | ) | ||||||
|
Proceeds from sale of property and equipment
|
- | 24 | - | |||||||||
|
Purchases of investments
|
- | (2,008 | ) | (2,065 | ) | |||||||
|
Sales of investments
|
1,432 | 1,117 | 855 | |||||||||
|
Proceeds from sale of patent assets, net
|
2,127 | - | 86,394 | |||||||||
|
Purchase of other assets
|
- | (338 | ) | - | ||||||||
|
Net cash provided by (used in) investing activities
|
3,424 | (1,365 | ) | 85,068 | ||||||||
|
Cash flows from financing activities:
|
||||||||||||
|
Proceeds from issuance of common stock
|
508 | 65 | 6,526 | |||||||||
|
Payment of dividends
|
(39,905 | ) | - | (66,024 | ) | |||||||
|
Excess tax benefits from stock-based compensation
|
1,247 | 128 | 14,395 | |||||||||
|
Payments made for taxes of employees who surrendered
shares related to unrestricted stock
|
(205 | ) | (71 | ) | (174 | ) | ||||||
|
Repurchase of common stock
|
- | - | - | |||||||||
|
Net cash provided by (used in) financing activities
|
(38,355 | ) | 122 | (45,277 | ) | |||||||
|
Increase/(decrease) in cash and cash equivalents
|
(28,675 | ) | 1,586 | 24,497 | ||||||||
|
Cash and cash equivalents, beginning of year
|
72,660 | 71,074 | 46,577 | |||||||||
|
Cash and cash equivalents, end of year
|
$ | 43,985 | $ | 72,660 | $ | 71,074 | ||||||
|
Supplemental disclosure:
|
||||||||||||
|
Cash paid for income taxes
|
$ | 1,018 | $ | 542 | $ | 7,954 | ||||||
| 41 |
|
Additional
|
Accumulated
Other
|
Total
|
||||||||||||||||||||||
|
Common Stock
|
Paid-In
|
Comprehensive
|
(Accumulated
|
Stockholders’
|
||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Loss
|
Deficit)
|
Equity
|
|||||||||||||||||||
|
Balance at December 31, 2011
|
20,623 | $ | 206 | $ | 79,512 | ($ | 20 | ) | ($ | 25,123 | ) | $ | 54,575 | |||||||||||
|
Exercise of common stock options
|
1,764 | 18 | 6,481 | 6,499 | ||||||||||||||||||||
|
Issuance of unrestricted stock
|
151 | 1 | (1 | ) | - | |||||||||||||||||||
|
Shares surrendered by employees to
pay taxes related to unrestricted stock
|
(33 | ) | - | (174 | ) | (174 | ) | |||||||||||||||||
|
Issuance of common stock under
employee stock purchase plan
|
5 | - | 27 | 27 | ||||||||||||||||||||
|
Stock-based compensation expense
|
321 | 321 | ||||||||||||||||||||||
|
Tax benefits from stock-based awards
|
14,395 | 14,395 | ||||||||||||||||||||||
|
Dividend payment
|
(66,024 | ) | (66,024 | ) | ||||||||||||||||||||
|
Accumulated other comprehensive loss:
|
||||||||||||||||||||||||
|
Unrealized loss on securities
|
(30 | ) | (30 | ) | ||||||||||||||||||||
|
Net income
|
72,307 | 72,307 | ||||||||||||||||||||||
|
Balance at December 31, 2012
|
22,510 | 225 | 100,561 | (50 | ) | (18,840 | ) | 81,896 | ||||||||||||||||
|
Exercise of common stock options
|
6 | - | 26 | 26 | ||||||||||||||||||||
|
Issuance of unrestricted stock
|
65 | 1 | (1 | ) | - | |||||||||||||||||||
|
Shares surrendered by employees to
pay taxes related to unrestricted stock
|
(14 | ) | - | (71 | ) | (71 | ) | |||||||||||||||||
|
Issuance of common stock under
employee stock purchase plan
|
7 | - | 40 | 40 | ||||||||||||||||||||
|
Stock-based compensation expense
|
662 | 662 | ||||||||||||||||||||||
|
Tax benefits from stock-based awards
|
128 | 128 | ||||||||||||||||||||||
|
Deferred tax asset write-off
|
(52 | ) | (52 | ) | ||||||||||||||||||||
|
Accumulated other comprehensive loss:
|
||||||||||||||||||||||||
|
Unrealized loss on securities
|
(156 | ) | (156 | ) | ||||||||||||||||||||
|
Deferred tax benefit on unrealized loss
|
81 | 81 | ||||||||||||||||||||||
|
Net income
|
2,596 | 2,596 | ||||||||||||||||||||||
|
Balance at December 31, 2013
|
22,574 | 226 | 101,293 | (125 | ) | (16,244 | ) | 85,150 | ||||||||||||||||
|
Exercise of common stock options
|
118 | 1 | 468 | 469 | ||||||||||||||||||||
|
Issuance of unrestricted stock
|
141 | 1 | (1 | ) | - | |||||||||||||||||||
|
Shares surrendered by employees to
pay taxes related to unrestricted stock
|
(32 | ) | - | (205 | ) | (205 | ) | |||||||||||||||||
|
Issuance of common stock under
employee stock purchase plan
|
8 | - | 39 | 39 | ||||||||||||||||||||
|
Stock-based compensation expense
|
915 | 915 | ||||||||||||||||||||||
|
Tax benefits from stock-based awards
|
1,247 | 1,247 | ||||||||||||||||||||||
|
Dividend payment
|
(39,905 | ) | (39,905 | ) | ||||||||||||||||||||
|
Accumulated other comprehensive loss:
|
||||||||||||||||||||||||
|
Unrealized gain on securities
|
162 | 162 | ||||||||||||||||||||||
|
Deferred tax expense on unrealized gain
|
(66 | ) | (66 | ) | ||||||||||||||||||||
|
Net income
|
4,583 | 4,583 | ||||||||||||||||||||||
|
Balance at December 31, 2014
|
22,809 | $ | 228 | $ | 103,756 | ($ | 29 | ) | ($ | 51,566 | ) | $ | 52,389 | |||||||||||
| 42 |
|
1.
|
NATURE OF BUSINESS
|
|
Fair Value Measurement at December 31, 2014 Using:
|
||||||||||||
|
Quoted Prices in
Active Markets for
Identical Assets
|
Significant Other
Observable Inputs |
Significant Unobservable
Inputs |
||||||||||
|
(Level 1)
|
(Level 2)
|
(Level 3)
|
||||||||||
|
Corporate debt securities
|
$ | 1,428 | $ | - | $ | - | ||||||
|
Money market funds (included in cash and cash equivalents)
|
34,339 | |||||||||||
|
Total
|
$ | 35,767 | $ | - | $ | - | ||||||
| 43 |
|
Fair Value Measurement at December 31, 2013 Using:
|
||||||||||||
|
Quoted Prices in
Active Markets for
Identical Assets
|
Significant Other
Observable Inputs |
Significant Unobservable
Inputs |
||||||||||
|
(Level 1)
|
(Level 2)
|
(Level 3)
|
||||||||||
|
Corporate debt securities
|
$ | 2,754 | $ | - | $ | - | ||||||
|
Money market funds (included in cash and cash equivalents)
|
68,556 | |||||||||||
|
Total
|
$ | 71,310 | $ | - | $ | - | ||||||
|
Building
|
30 years
|
|
Building improvements
|
5 to 20 years
|
|
Furniture and fixtures
|
5 years
|
|
Computer, office & manufacturing equipment
|
3 years
|
|
Purchased software
|
3 years
|
| 44 |
| 45 |
|
|
●
|
Software licenses and software maintenance
. When software licenses and software maintenance contracts are sold together, we recognize software license revenue upon delivery, provided we have vendor specific objective evidence (“VSOE”) for the fair value of the maintenance contract fee, and we recognize the fair value of maintenance contract revenue ratably over the related contract period. Under ASC 985-605, the residual method is the appropriate manner in which to allocate arrangement consideration to the license when VSOE exists for all undelivered elements (e.g., PCS), but not for the delivered element (e.g., the license).
|
|
|
●
|
Software licenses and services.
When software licenses and software engineering services are sold together, the total fee is generally recognized by applying contract accounting. We have adopted the percentage-of-completion method of contract accounting, and we generally use an input method (i.e., labor hours) to determine our completion percentage. The software license portion of the arrangement is classified as software license revenue and the engineering services portion is classified as services revenue in our consolidated statements of income and comprehensive income.
|
|
|
●
|
Software licenses, software maintenance and services.
When we sell software licenses, software maintenance and software services together, revenue is recognized as follows: i) software maintenance revenue is separated from the other two elements and is recognized ratably over the related software maintenance contract period; provided we have VSOE for the fair value of the maintenance element; and ii) the total fee from the software license and engineering service elements is recognized by applying the contract accounting method described in the previous paragraph.
|
| 46 |
|
2014
|
2013
|
||||||||
|
Customer A
|
18 | % | 60 | % | |||||
|
Customer B
|
15 | % | - | % | |||||
|
Customer C
|
11 | % | - | % | |||||
| 47 |
|
3.
|
PATENT RELATED INCOME
|
| 48 |
|
|
●
|
The claims in one of the patents we assigned were rejected by the United States Patent Office (“USPTO”) in May 2013. The USPTO’s Patent Trial & Appeal Board (“PTAB”) affirmed the USPTO decision in June 2014. The PTAB decision was appealed to the Federal Circuit. In December 2014, the Federal Circuit remanded the appeal back to the PTAB for further consideration.
|
|
|
●
|
Notwithstanding the outcome at the PTAB, we do not know whether any patent monetization efforts by the third party will be successful.
|
|
|
●
|
We sold a portion of our patent portfolio pertaining to wireless technology to an unaffiliated third party for $75.0 million. The proceeds from the sale were reduced by $3.8 million of transaction costs, which consisted primarily of fees from the law firm that assisted us in the sale. We recorded a gain of $71.2 million on the sale.
|
|
|
●
|
We sold a portion of our patent portfolio pertaining to DSL semiconductor intellectual property technology for $16.0 million. The proceeds from the sale were reduced by $0.8 million of transaction costs, which also consisted primarily of fees from the law firm that assisted us in the sale. We recorded a gain of $15.2 million on the sale.
|
|
4.
|
DISCONTINUED OPERATIONS
|
|
Years ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Revenue
|
$ | - | $ | 3,216 | $ | 5,431 | ||||||
|
Expenses
|
- | 5,120 | 5,558 | |||||||||
|
Loss before income taxes
|
- | (1,904 | ) | (127 | ) | |||||||
|
Income tax benefit
|
- | (748 | ) | (51 | ) | |||||||
|
Loss from discontinued operations
|
$ | - | ($ | 1,156 | ) | ($ | 76 | ) | ||||
| 49 |
|
5.
|
INVENTORIES
|
|
2014
|
2013
|
|||||||
|
Raw materials
|
$ | 2 | $ | 1,584 | ||||
|
Finished goods
|
- | 17 | ||||||
|
Total
|
$ | 2 | $ | 1,601 | ||||
|
6.
|
PROPERTY AND EQUIPMENT
|
|
2014
|
2013
|
|||||||
|
Land
|
$ | 1,056 | $ | 1,056 | ||||
|
Building and improvements
|
9,060 | 9,060 | ||||||
|
Computer equipment
|
614 | 557 | ||||||
|
Purchased software
|
78 | 88 | ||||||
|
Furniture and fixtures
|
778 | 778 | ||||||
|
Office equipment
|
191 | 191 | ||||||
|
Total
|
11,777 | 11,730 | ||||||
|
Less accumulated depreciation and amortization
|
(6,488 | ) | (6,148 | ) | ||||
|
Property and equipment, net
|
$ | 5,289 | $ | 5,582 | ||||
| 50 |
|
Year ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Current:
|
||||||||||||
|
Federal
|
$ | 1,976 | $ | 1,000 | $ | 20,654 | ||||||
|
State
|
589 | 273 | 1,594 | |||||||||
| 2,565 | 1,273 | 22,248 | ||||||||||
|
Deferred:
|
||||||||||||
|
Federal
|
93 | 471 | (1,354 | ) | ||||||||
|
State
|
14 | 173 | (407 | ) | ||||||||
| 107 | 644 | (1,761 | ) | |||||||||
|
Total provision for income taxes
|
$ | 2,672 | $ | 1,917 | $ | 20,487 | ||||||
|
Year ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Federal statutory rate
|
34 | % | 34 | % | 35 | % | ||||||
|
State rate, net of federal benefit
|
5 | 5 | 5 | |||||||||
|
Tax credits
|
(1 | ) | (2 | ) | - | |||||||
|
Permanent adjustments
|
(1 | ) | (1 | ) | - | |||||||
|
Change in valuation allowance
|
- | - | (18 | ) | ||||||||
|
Prior year adjustment
|
- | (2 | ) | - | ||||||||
|
Effective tax rate
|
37 | % | 34 | % | 22 | % | ||||||
| 51 |
|
2014
|
2013
|
|||||||
|
Depreciation
|
$ | 374 | $ | 418 | ||||
|
Stock compensation
|
303 | 199 | ||||||
|
Capitalized research and development costs
|
96 | 364 | ||||||
|
Other
|
199 | 164 | ||||||
|
Total
|
972 | 1,145 | ||||||
|
Less valuation allowance
|
(- | ) | (- | ) | ||||
|
Deferred tax assets, net
|
$ | 972 | $ | 1,145 | ||||
|
Uncertain tax positions at December 31, 2011
|
$ | - | ||
|
Increase due to positions taken in prior periods
|
2,945 | |||
|
Uncertain tax positions at December 31, 2012
|
$ | 2,945 | ||
|
Increase due to positions taken in prior periods
|
- | |||
|
Uncertain tax positions at December 31, 2013
|
$ | 2,945 | ||
|
Increase due to positions taken in prior periods
|
- | |||
|
Uncertain tax positions at December 31, 2014
|
$ | 2,945 |
| 52 |
|
Years ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Cost of services
|
$ | 44 | $ | 34 | $ | 16 | ||||||
|
Research and development
|
106 | 81 | 51 | |||||||||
|
Selling and marketing
|
17 | 14 | 126 | |||||||||
|
General and administrative
|
748 | 533 | 83 | |||||||||
|
Loss from discontinued operations
|
- | - | 45 | |||||||||
|
Stock-based compensation expense
|
$ | 915 | $ | 662 | $ | 321 | ||||||
|
Year Ended
December 31, 2012
|
||||
|
Expected term (1)
|
5 years
|
|||
|
Expected volatility factor (2)
|
58-63 | % | ||
|
Risk-free interest rate (3)
|
0.92 | % | ||
|
Expected annual dividend yield
|
n/a | |||
| 53 |
| 54 |
|
2014
|
2013
|
2012
|
||||||||||||||||||||||
|
Shares
|
Weighted
Average
Exercise
Price
|
Shares
|
Weighted
Average
Exercise
Price
|
Shares
|
Weighted
Average
Exercise
Price
|
|||||||||||||||||||
|
Outstanding at beginning of year
|
1,004,989 | $ | 5.68 | 1,063,025 | $ | 5.63 | 2,835,952 | $ | 4.42 | |||||||||||||||
|
Granted
|
- | - | - | - | 50,000 | 4.60 | ||||||||||||||||||
|
Exercised
|
(117,783 | ) | 3.98 | (6,163 | ) | 4.27 | (1,779,616 | ) | 3.71 | |||||||||||||||
|
Forfeited or cancelled
|
(781,004 | ) | 6.07 | (51,873 | ) | 4.83 | (43,311 | ) | 4.33 | |||||||||||||||
|
Outstanding at end of year
|
106,202 | $ | 4.71 | 1,004,989 | $ | 5.68 | 1,063,025 | $ | 5.63 | |||||||||||||||
|
Exercisable at year end
|
104,117 | $ | 4.69 | 986,237 | $ | 5.70 | 1,027,525 | $ | 5.66 | |||||||||||||||
|
Options Outstanding
|
Options Exercisable
|
|||||||||||||||||||
|
Exercise Price
Range
|
Number
|
Weighted
Average
Exercise
Price
|
Weighted Average
Remaining
Contractual
Term (in years)
|
Number
|
Weighted
Average
Exercise
Price
|
|||||||||||||||
|
$2 to $3
|
18,000 | $ | 2.52 | 4.39 | 18,000 | $ | 2.52 | |||||||||||||
|
$3 to $4
|
14,168 | 3.57 | 2.82 | 14,168 | 3.57 | |||||||||||||||
|
$4 to $5
|
33,200 | 4.64 | 2.93 | 33,200 | 4.64 | |||||||||||||||
|
$6 to $7
|
40,834 | 6.14 | 1.27 | 38,749 | 6.14 | |||||||||||||||
| 106,202 | $ | 4.71 | 2.52 | 104,117 | $ | 4.69 | ||||||||||||||
| 55 |
|
9.
|
COMMITMENTS AND CONTINGENT LIABILITIES
|
| 56 |
|
Year ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
United States
|
$ | 18,168 | $ | 13,909 | $ | 12,158 | ||||||
|
Rest of world
|
5,552 | 5,448 | 5,146 | |||||||||
| $ | 23,720 | $ | 19,357 | $ | 17,304 | |||||||
|
Year ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Biometrics
|
$ | 21,436 | $ | 17,085 | $ | 13,493 | ||||||
|
Imaging
|
1,558 | 1,352 | 1,641 | |||||||||
|
DSL royalties
|
726 | 920 | 2,170 | |||||||||
| $ | 23,720 | $ | 19,357 | $ | 17,304 | |||||||
|
Year ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Customer A
|
24 | % | 21 | % | 5 | % | ||||||
|
Customer B
|
10 | % | 3 | % | - | % | ||||||
|
11.
|
EMPLOYEE BENEFIT PLAN
|
| 57 |
|
12.
|
NET INCOME PER SHARE
|
|
Year ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Net income:
|
||||||||||||
|
Income from continuing operations
|
$ | 4,583 | $ | 3,752 | $ | 72,383 | ||||||
|
Loss from discontinued operations
|
- | (1,156 | ) | (76 | ) | |||||||
|
Net income
|
$ | 4,583 | $ | 2,596 | $ | 72,307 | ||||||
|
Shares outstanding
:
|
||||||||||||
|
Weighted-average common shares outstanding
|
22,703 | 22,543 | 21,814 | |||||||||
|
Additional dilutive common stock equivalents
|
84 | 98 | 257 | |||||||||
|
Diluted shares outstanding
|
22,787 | 22,641 | 22,071 | |||||||||
|
Basic net income per share
:
|
||||||||||||
|
Basic net income per share from continuing operations
|
$ | 0.20 | $ | 0.17 | $ | 3.32 | ||||||
|
Basic net loss per share from discontinued operations
|
- | (0.05 | ) | (0.00 | ) | |||||||
|
Basic net income per share
|
$ | 0.20 | $ | 0.12 | $ | 3.32 | ||||||
|
Diluted net income per share
:
|
||||||||||||
|
Diluted net income per share from continuing operations
|
$ | 0.20 | $ | 0.16 | $ | 3.28 | ||||||
|
Diluted net loss per share from discontinued operations
|
- | (0.05 | ) | (0.00 | ) | |||||||
|
Diluted net income per share
|
$ | 0.20 | $ | 0.11 | $ | 3.28 | ||||||
|
December 31,
|
Increase/
|
Reclassification
|
December 31,
|
||||||||||||||
|
2013
|
Decrease
|
Adjustments
|
2014
|
||||||||||||||
|
Unrealized losses on available for sale securities
|
($ | 206 | ) | $ | 60 | $ | 59 | ($ | 87 | ) | |||||||
|
Unrealized gains on available for sale securities
|
- | 43 | - | 43 | |||||||||||||
|
Net unrealized gains (losses) on available for sale securities
|
(206 | ) | 103 | 59 |
(a)
|
(44 | ) | ||||||||||
|
Income tax benefit (expense) on other comprehensive loss
|
81 | (46 | ) | (20 | ) | 15 | |||||||||||
|
Total accumulated other comprehensive loss, net of taxes
|
($ | 125 | ) | $ | 57 | $ | 39 | ($ | 29 | ) | |||||||
|
(a)
|
– Classified in other expense.
|
| 58 |
|
2014 Quarters Ended
|
||||||||||||||||
|
March 31
|
June 30
|
September 30
|
December 31
|
|||||||||||||
|
Revenue
|
$ | 6,617 | $ | 6,767 | $ | 6,027 | $ | 4,309 | ||||||||
|
Operating income
|
1,311 | 1,382 | 4,155 | 243 | ||||||||||||
|
Income from continuing operations
|
880 | 865 | 2,599 | 239 | ||||||||||||
|
Income from discontinued operations
|
- | - | - | - | ||||||||||||
|
Net income
|
880 | 865 | 2,599 | 239 | ||||||||||||
|
Net income per share – basic
|
$ | 0.04 | $ | 0.04 | $ | 0.11 | $ | 0.01 | ||||||||
|
Net income per share – diluted
|
$ | 0.04 | $ | 0.04 | $ | 0.11 | $ | 0.01 | ||||||||
|
2013 Quarters Ended
|
||||||||||||||||
|
March 31
|
June 30
|
September 30
|
December 31
|
|||||||||||||
|
Revenue
|
$ | 4,979 | $ | 4,427 | $ | 4,314 | $ | 5,637 | ||||||||
|
Operating income
|
2,982 | 543 | 1,039 | 754 | ||||||||||||
|
Income from continuing operations
|
1,972 | 465 | 790 | 525 | ||||||||||||
|
Income (loss) from discontinued
operations
|
(115 | ) | (158 | ) | (1,943 | ) | 1,060 | |||||||||
|
Net income (loss)
|
1,857 | 307 | (1,153 | ) | 1,585 | |||||||||||
|
Net income (loss) per share – basic
|
$ | 0.08 | $ | 0.01 | $ | (0.05 | ) | $ | 0.07 | |||||||
|
Net income (loss) per share – diluted
|
$ | 0.08 | $ | 0.01 | $ | (0.05 | ) | $ | 0.07 | |||||||
| 59 |
| 60 |
|
Col. A
|
Col. B
|
Col. C(1)
|
Col. C(2)
|
Col. D
|
Col. E
|
|||||||||||||||
|
Additions
|
||||||||||||||||||||
|
Balance at
|
Charged to
|
Charged
|
Deductions
|
Balance
|
||||||||||||||||
|
Beginning
|
Costs and
|
to Other
|
Charged to
|
at End
|
||||||||||||||||
|
of Period
|
Expenses
|
Accounts
|
Reserves
|
of Period
|
||||||||||||||||
|
Allowance for doubtful accounts receivable:
|
||||||||||||||||||||
|
2014
|
$ | 20 | $ | - | $ | - | $ | - | $ | 20 | ||||||||||
|
2013
|
$ | 20 | $ | - | $ | - | $ | - | $ | 20 | ||||||||||
|
2012
|
$ | 30 | $ | - | $ | - | $ | 10 | $ | 20 | ||||||||||
|
Inventory reserves:
|
||||||||||||||||||||
|
2014
|
$ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
|
2013
|
$ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
|
2012
|
$ | 1,403 | $ | 126 | $ | - | $ | 1,529 | $ | - | ||||||||||
|
Warranty reserves:
|
||||||||||||||||||||
|
2014
|
$ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
|
2013
|
$ | 10 | $ | - | $ | - | $ | 10 | $ | - | ||||||||||
|
2012
|
$ | - | $ | 12 | $ | - | $ | 2 | $ | 10 | ||||||||||
|
Deferred tax asset valuation allowance:
|
||||||||||||||||||||
|
2014
|
$ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
|
2013
|
$ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
|
2012
|
$ | 40,476 | $ | - | $ | - | $ | 40,476 | $ | - | ||||||||||
| 61 |
| 62 |
| 63 |
|
Page
|
|||
|
(1) Consolidated Balance Sheets as of December 31, 2014 and 2013
|
39 | ||
|
Consolidated Statements of Income and Comprehensive Income for each of the three
years in the period ended December 31, 2014
|
40 | ||
|
Consolidated Statements of Cash Flows for each of the
three years in the period ended December 31, 2014
|
41 | ||
|
Consolidated Statements of Stockholders’ Equity for each of
the three years in the period ended December 31, 2014
|
42 | ||
|
Notes to Consolidated Financial Statements
|
43 | ||
|
(2) Schedule II - Valuation and Qualifying Accounts
|
61 | ||
| (3) Exhibits: |
|
Exhibit No.
|
Description of Exhibit
|
||
|
3.1
|
Amended and Restated Articles of Organization, as amended (filed as Exhibit 3.1 to the Company’s Form 10-K for the year ended December 31, 2008 and incorporated herein by reference).
|
||
|
3.2
|
Amended and Restated By-Laws (filed as Exhibit 3.1 to the Company’s Form 8-K filed with the Securities and Exchange Commission on December 10, 2007 and incorporated herein by reference).
|
||
|
10.1*
|
1996 Stock Option Plan, as amended and restated (filed as Annex A to the Company’s Definitive Proxy Statement filed with the Securities and Exchange Commission on April 11, 2000 and incorporated herein by reference).
|
||
|
10.2*
|
1996 Employee Stock Purchase Plan, as amended and restated (filed as Exhibit 99.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on November 29, 2005 and incorporated herein by reference).
|
||
|
10.3*
|
Form of Indemnification Agreement for Directors and Officers of Aware, Inc. (filed as Exhibit 10.1 to the Company’s Form 8-K filed with the Securities and Exchange Commission on February 22, 2011 and incorporated herein by reference).
|
||
|
10.4*
|
2001 Nonqualified Stock Plan (filed as Exhibit 99(d)(4) to the Company’s Schedule TO filed with the Securities and Exchange Commission on March 3, 2003 and incorporated herein by reference).
|
||
| 10.5* | Form of Nonqualified Stock Option Agreement under the 2001 Nonqualified Stock Plan for options granted to executive officers and directors prior to May 21, 2008 (filed as Exhibit 10.6 to Company’s Form 10-K for the year ended December 31, 2006 and incorporated herein by reference). | ||
|
10.6*
|
Form of Nonqualified Stock Option Agreement under the 2001 Nonqualified Stock Plan for options granted to executive officers and directors from and after May 21, 2008 (filed as Exhibit 10.8 to Company’s Form 8-K on May 22, 2008 and incorporated herein by reference)
|
||
|
10.7*
|
Form of Unrestricted Stock Award for outside directors of Aware under the 2001 Nonqualified Stock Plan (filed as Exhibit 10.1 to Company’s Form 8-K filed with the Securities and Exchange Commission on July 28, 2010 and incorporated herein by reference).
|
||
|
10.8*
|
Form of Unrestricted Stock Award for officers of Aware under the 2001 Nonqualified Stock Plan (filed as Exhibit 10.2 to Company’s Form 8-K filed with the Securities and Exchange Commission on July 28, 2010 and incorporated herein by reference).
|
||
|
10.9
|
Asset Purchase Agreement by and between Aware, Inc. and Lantiq Broadband Holdco, Inc. and Lantiq Deutschland GmbH dated October 14, 2009 (filed as Exhibit 10.8 to Company’s Form 10-K for the year ended December 31, 2009 and incorporated herein by reference).
|
||
| 64 |
|
10.12*
|
Form of Unrestricted Stock Award for executive officers and directors of Aware, Inc. under the 2001 Nonqualified Plan (filed as Exhibit 10.1 to the Company’s Form 8-K filed with the Securities and Exchange Commission on April 4, 2013 and incorporated herein by reference).
|
|
|
21.1
|
Subsidiaries of Registrant.
|
|
|
23.1
|
Consent of Independent Registered Public Accounting Firm.
|
|
|
31.1
|
Certification of co-Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
31.2
|
Certification of co-Chief Executive Officer and Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
32.1
|
Certification of Chief Executive Officer and Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
101**
|
The following financial statements from Aware, Inc.’s Annual Report on Form 10-K for the year ended December 31, 2014, formatted in XBRL (eXtensible Business Reporting Language), as follows: (i) Consolidated Balance Sheets as of December 31, 2014 and December 31, 2013; (ii) Consolidated Statements of Income and Comprehensive Income for the Years Ended December 31, 2014, December 31, 2013 and December 31, 2012; (iii) Consolidated Statements of Cash Flows for the Years Ended December 31, 2014, December 31, 2013 and December 31, 2012; (iv) Consolidated Statements of Stockholders’ Equity for the Years Ended December 31, 2014, December 31, 2013 and December 31, 2012; and (v) Notes to Consolidated Financial Statements.
|
| 65 |
| AWARE, INC. | ||
|
|
By:
|
/s/ Kevin T. Russell |
| Kevin T. Russell | ||
| co-Chief Executive Officer & co-President | ||
| General Counsel | ||
|
|
By:
|
/s/ Richard P. Moberg |
| Richard P. Moberg | ||
| co-Chief Executive Officer & co-President | ||
| Chief Financial Officer (Principal Financial and Accounting Officer) | ||
| Date: February 13, 2015 | ||
|
Signature
|
Title
|
|
||||
|
/s/ Kevin T. Russell
|
co-Chief Executive Officer, co-President,
|
|||||
|
Kevin T. Russell
|
General Counsel & Director
|
|||||
|
(co-Principal Executive Officer)
|
||||||
|
/s/ Richard P. Moberg
|
co-Chief Executive Officer, co-President,
|
|||||
|
Richard P. Moberg
|
Chief Financial Officer & Director
|
|||||
|
(co-Principal Executive Officer)
|
||||||
|
(Principal Financial and Accounting Officer)
|
||||||
|
/s/ John S. Stafford, Jr.
|
Chairman of the Board & Director
|
|||||
|
John S. Stafford, Jr.
|
||||||
|
/s/ John S. Stafford, III
|
Director
|
|||||
|
John S. Stafford, III
|
||||||
|
/s/ Adrian F. Kruse
|
Director
|
|||||
|
Adrian F. Kruse
|
||||||
|
/s/ Brian D. Connolly
|
Director
|
|||||
|
Brian D. Connolly
|
||||||
|
/s/ Brent P. Johnstone
|
Director
|
|||||
|
Brent P. Johnstone
|
||||||
| 66 |