|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
þ
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the fiscal year ended December 31, 2013
|
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
33-0112644
|
|
|
(State or other jurisdiction of
incorporation or organization)
|
(I.R.S. Employer
Identification No.)
|
|
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|
|
Common Stock, $0.0005 par value
|
The Nasdaq Global Select Market
|
|
|
Large accelerated filer
þ
|
|
Accelerated filer
¨
|
|
Non-accelerated filer
¨
|
|
Smaller reporting company
¨
|
|
|
|
(Do not check if a smaller reporting company)
|
|
|||
|
|
|
Page
|
|
•
|
the anticipated amount, timing and accounting of revenues, contingent payments, milestone, royalty and other payments under licensing, collaboration or acquisition agreements, tax positions and contingencies, doubtful accounts, pre-approval inventory, cost of sales, research and development costs, compensation and other expenses, amortization of intangible assets, and foreign currency forward contracts;
|
|
•
|
the anticipated timing of commercial launches of TECFIDERA in European countries;
|
|
•
|
anticipated regulatory filings and regulatory actions relating to, and commercial launch of, ELOCTATE and ALPROLIX;
|
|
•
|
additional anticipated commercial launches of FAMPYRA and the timing thereof;
|
|
•
|
patent terms, patent term extensions, patent office actions, and expected availability and period of data protection and market exclusivity rights;
|
|
•
|
the potential impact of increased product competition in the multiple sclerosis (MS) market, including competition from and growth of our own products and the possibility of future competition from biosimilars, generic versions or related prodrug derivatives;
|
|
•
|
the potential for increased competition between RITUXAN and GAZYVA in the oncology market;
|
|
•
|
our plans to develop further risk stratification protocols and therapies for TYSABRI and the impact of such protocols;
|
|
•
|
the timing, outcome and impact of administrative, regulatory, litigation and other proceedings related to patents and other proprietary and intellectual property rights, tax audits, assessments and settlements, product liability and other matters;
|
|
•
|
the impact of the commercial launch of TECFIDERA on sales and market share of our products;
|
|
•
|
the expected timing and financial impact of the final approval of the settlement of our dispute with the Italian National Medicines Agency relating to sales of TYSABRI;
|
|
•
|
the anticipated lifetime revenues of AVONEX and TYSABRI and amortization recorded in relation to their technology;
|
|
•
|
the costs, timing, potential approval and therapeutic scope of the development and commercialization of our pipeline products;
|
|
•
|
lease commitments and purchase obligations;
|
|
•
|
the potential impact of budget cuts and other measures in the U.S. and worldwide designed to reduce healthcare costs to constrain the overall level of government expenditures, including the impact of pricing actions in Europe and elsewhere;
|
|
•
|
the impact of the continued uncertainty and deterioration of the credit and economic conditions in certain countries in Europe and our collection of accounts receivable in such countries;
|
|
•
|
our ability to finance our operations and business initiatives and obtain funding for such activities;
|
|
•
|
the impact of new laws and accounting standards;
|
|
•
|
the expected timing of completion of our manufacturing obligation for Zevalin;
|
|
•
|
manufacturing capacity and our intent to utilize third party contract manufacturing organizations to provide manufacturing services for our small molecule products; and
|
|
•
|
the drivers for growing our business, including our plans to pursue business development and research opportunities, and competitive conditions.
|
|
Item 1.
|
Business
|
|
|
|
|
Product Revenues
to Biogen Idec (in millions)
|
|||||||||||||
|
Product
|
Indications
|
Development or
Marketing Collaborator
|
2013
|
2012
|
2011
|
|||||||||||
|
AVONEX (1)
|
Multiple sclerosis
|
None
|
$
|
3,005.5
|
|
$
|
2,913.1
|
|
$
|
2,686.6
|
|
|||||
|
TYSABRI (2)
|
Multiple sclerosis
Crohn’s disease
|
None
|
$
|
1,526.5
|
|
$
|
1,135.9
|
|
$
|
1,079.5
|
|
|||||
|
TECFIDERA (3)
|
Multiple sclerosis
|
None
|
$
|
876.1
|
|
$
|
—
|
|
$
|
—
|
|
|||||
|
FAMPYRA (4)
|
Multiple sclerosis
(walking ability)
|
Acorda Therapeutics
|
$
|
74.0
|
|
$
|
57.4
|
|
$
|
13.6
|
|
|||||
|
FUMADERM (5)
|
Psoriasis
|
None
|
$
|
60.2
|
|
$
|
59.7
|
|
$
|
54.7
|
|
|||||
|
|
|
|
Unconsolidated Joint Business
Revenues to Biogen Idec (in millions)
|
|||||||||||||
|
2013
|
2012
|
2011
|
||||||||||||||
|
RITUXAN (6)
|
Non-Hodgkin’s lymphoma
Rheumatoid arthritis
Chronic lymphocytic leukemia
ANCA-associated vasculitis
|
Genentech
(Roche Group)
|
$
|
1,126.0
|
|
$
|
1,137.9
|
|
$
|
996.6
|
|
|||||
|
(1)
|
AVONEX (interferon beta-1a), injection for intramuscular use, is indicated for the treatment of patients with relapsing forms of MS to slow the accumulation of physical disability and decrease the frequency of clinical exacerbations.
|
|
(2)
|
TYSABRI (natalizumab), injection for intravenous infusion, is indicated (1) as a monotherapy for the treatment of patients with relapsing forms of MS to delay the accumulation of physical disability and reduce the frequency of clinical exacerbations and (2) in the U.S. for inducing and maintaining clinical response and remission in adult patients with moderately to severely active Crohn's disease with evidence of inflammation who have had an inadequate response to, or are unable to tolerate, conventional Crohn's disease therapies and TNF inhibitors.
|
|
(3)
|
TECFIDERA (dimethyl fumarate), delayed release capsules for oral use, is indicated for the treatment of patients with relapsing forms of MS. TECFIDERA was approved by the U.S. Food and Drug Administration (FDA) in March 2013. In February 2014, the European Commission (EC) approved the use of TECFIDERA in the European Union (E.U.) as a first-line oral treatment for people with relapsing-remitting MS.
|
|
(4)
|
FAMPYRA (prolonged-release fampridine tablets) is indicated for the improvement of walking ability in adult patients with MS who have walking disability.
|
|
(5)
|
FUMADERM (fumaric acid esters), prolonged release tablets
,
is only approved in Germany and is indicated for the treatment of adult patients with moderate to severe plaque psoriasis for whom topical therapy is ineffective.
|
|
(6)
|
RITUXAN (rituximab), injection for intravenous infusion, is indicated for the treatment of (1)(a) relapsed or refractory, low-grade or follicular, CD20-positive, B-cell Non-Hodgkin's lymphoma (NHL) as a single agent, (b) previously untreated follicular, CD20-positive, B-cell NHL in combination with first line chemotherapy and, in patients achieving a complete or partial response to RITUXAN in combination with chemotherapy, as a single-agent maintenance therapy, (c) non-progressing (including stable disease), low-grade, CD20-positive, B-cell NHL, as a single agent, after first-line CVP chemotherapy, and (d) previously untreated diffuse large B-cell, CD20-positive NHL in combination with CHOP or
|
|
(In millions)
|
2013
|
2012
|
2011
|
||||||||||
|
Research and development
|
$
|
1,444.1
|
|
$
|
1,334.9
|
|
$
|
1,219.6
|
|
||||
|
Amortization of acquired intangible assets
|
$
|
342.9
|
|
$
|
202.2
|
|
$
|
208.6
|
|
||||
|
(Gain) loss on fair value remeasurement of contingent consideration
|
$
|
(0.5
|
)
|
$
|
27.2
|
|
$
|
36.1
|
|
||||
|
•
|
We previously collaborated with Elan on the development, manufacture and commercialization of TYSABRI. On April 2, 2013, we acquired full ownership of, and strategic, commercial and decision-making rights to, TYSABRI from Elan, for an upfront payment of
$3.25 billion
together with an agreement to make contingent payments to Elan. Upon the closing of the transaction, our collaboration agreement with Elan was terminated. For additional information related to this relationship, please read Note 2,
Acquisitions
and Note 20,
Collaborative and Other Relationships
to our consolidated financial statements included within this report.
|
|
•
|
In 2013, the FDA and the European Medicines Agency (EMA) approved updates to the TYSABRI product labels. In July 2013, the EMA approved an expanded indication statement for TYSABRI to include glatiramer acetate (GA) treatment failures in the definition of non-responders eligible for TYSABRI, and in December 2013, the FDA approved a modification to the indication statement in the U.S. product label for TYSABRI clarifying the intended use of TYSABRI for people living with relapsing forms of MS, as well as updates to certain safety information. In May 2013, we withdrew the variation in our application we submitted to the EMA requesting to expand the indication statement to allow first-line use of TYSABRI for people living with certain relapsing forms of MS who have tested negative for antibodies to the JC virus.
|
|
•
|
In 2013, we submitted an application for approval of TYSABRI in Japan.
|
|
•
|
The EC granted a conditional marketing authorization for FAMPYRA in the E.U. in July 2011. A conditional marketing authorization is renewable annually and is granted to a medicinal product with a positive benefit-risk assessment that fulfills an unmet medical need when the benefit to public health of immediate availability outweighs the risk inherent in the fact that additional data are still required. This marketing authorization was renewed as of July 2013. To meet the conditions of this marketing authorization, we will continue to provide additional data from on-going clinical studies regarding FAMPYRA's benefits and safety.
|
|
(In millions)
|
2013
|
2012
|
2011
|
|||||||||
|
Royalty revenues
|
$
|
185.7
|
|
$
|
168.7
|
|
$
|
158.5
|
|
|||
|
Corporate partner revenues
|
$
|
78.2
|
|
$
|
43.8
|
|
$
|
57.4
|
|
|||
|
Therapeutic Area
|
|
Product Candidate
|
|
Targeted Indications
|
Collaborator
|
|
Status
|
|
|
Neurology
|
PLEGRIDY (peginterferon beta-1a)
|
|
MS
|
None
|
|
U.S. BLA and EMA marketing authorisation application submitted and under regulatory review
|
||
|
Daclizumab High Yield Process (HYP)
|
|
MS
|
AbbVie Biotherapeutics
|
|
Phase 3
|
|||
|
|
TYSABRI
|
|
Secondary progressive MS
|
None
|
|
Phase 3
|
||
|
Stroke
|
None
|
Phase 2
|
||||||
|
|
Anti-LINGO
|
|
Optic Neuritis
|
None
|
|
Phase 2
|
||
|
|
MS
|
None
|
Phase 2
|
|||||
|
Neublastin
|
|
Neuropathic pain
|
None
|
|
Phase 2
|
|||
|
BIIB037
|
|
Alzheimer’s disease
|
None
|
|
Phase 1b
|
|||
|
|
ISIS - SMN
Rx
|
|
Spinal muscular atrophy
|
Isis Pharmaceuticals
|
|
Phase 1b/2a
|
||
|
BIIB061
|
MS
|
None
|
Phase 1
|
|||||
|
|
||||||||
|
Hemophilia
|
|
ALPROLIX [Coagulation Factor IX, Fc Fusion Protein (Recombinant)]
|
|
Hemophilia B
|
Swedish Orphan Biovitrum
|
|
U.S. BLA submitted and under regulatory review
|
|
|
|
ELOCTATE [(Antihemophilic Factor, Fc Fusion Protein (Recombinant)]
|
|
Hemophilia A
|
Swedish Orphan Biovitrum
|
|
U.S. BLA submitted and under regulatory review
|
||
|
|
||||||||
|
Immunology
|
|
STX-100
|
|
Idiopathic pulmonary fibrosis
|
None
|
|
Phase 2a
|
|
|
Anti-TWEAK
|
|
Lupus nephritis
|
None
|
|
Phase 2
|
|||
|
Anti-CD40 Ligand
|
General lupus
|
UCB, Inc.
|
Phase 1
|
|||||
|
|
||||||||
|
Other
|
|
GAZYVA (obinutuzumab)
|
|
Non-Hodgkin’s lymphoma
|
Genentech (Roche Group)
|
|
Phase 3
|
|
|
•
|
GOYA
: investigating the efficacy and safety of GAZYVA in combination with CHOP chemotherapy compared to RITUXAN with CHOP chemotherapy in previously untreated patients with CD20-positive diffuse large B-cell lymphoma.
|
|
•
|
GALLIUM
: investigating the efficacy and safety of GAZYVA in combination with chemotherapy followed by maintenance with GAZYVA compared to RITUXAN in combination with chemotherapy followed by maintenance with RITUXAN in previously untreated patients with indolent non-Hodgkin's lymphoma.
|
|
•
|
GADOLIN
: investigating the efficacy and safety of GAZYVA plus bendamustine compared with bendamustine alone in patients with RITUXAN-refractory, indolent non-Hodgkin's lymphoma.
|
|
•
|
U.S. patent no. 6,509,376, having claims to formulations of dimethyl fumarate for use in the treatment of autoimmune diseases including MS, expiring in 2019;
|
|
•
|
U.S. patent no. 7,320,999, having claims to a method of treating MS using dimethyl fumarate, expiring in 2020;
|
|
•
|
U.S. patent no. 7,619,001, having claims to a method of treating MS using dimethyl fumarate, monomethyl fumarate, or a combination thereof, expiring in 2018;
|
|
•
|
U.S. patent no. 7,803,840, having claims to a method of treating an autoimmune disease selected from autoimmune polyarthritis and MS using dimethyl fumarate, expiring in 2018;
|
|
•
|
U.S. patent no. 8,399,514, covering the dosing regimen of 240 mg of TECFIDERA administered twice a day, expiring in 2028; and
|
|
•
|
U.S. patent no. 8,524,773, having claims to a method of treating MS using monomethyl fumarate, expiring in 2018.
|
|
•
|
EP 1131065, directed to formulations of dimethyl fumarate and to uses thereof for treating autoimmune diseases, including MS, expiring in 2019; and
|
|
•
|
EP 2137537, the counterpart patent to our U.S. patent covering the dosing regimen of 240 mg of TECFIDERA administered twice a day, expiring in 2028.
|
|
•
|
COPAXONE (glatiramer acetate), which is marketed by Teva Pharmaceutical Industries Ltd. COPAXONE generated worldwide revenues of approximately $4.0 billion in 2012.
|
|
•
|
REBIF (interferon-beta-1a), which is marketed by Merck (and co-promoted with Pfizer Inc. in the U.S.). REBIF generated worldwide revenues of approximately $2.5 billion in 2012.
|
|
•
|
BETASERON/BETAFERON (interferon-beta-1b), which is marketed by the Bayer Group. BETASERON/BETAFERON generated worldwide revenues of approximately $1.6 billion in 2012.
|
|
•
|
EXTAVIA (interferon-beta-1b), which is marketed by Novartis AG. EXTAVIA generated worldwide revenues of approximately $159.0 million in 2012.
|
|
•
|
GILENYA (fingolimod), which is marketed by Novartis AG. GILENYA generated worldwide revenues of approximately $1.2 billion in 2012.
|
|
•
|
AUBAGIO (teriflunomide), which is marketed by Sanofi. AUBAGIO generated worldwide revenues of approximately $9.2 million in 2012.
|
|
•
|
TREANDA (bendamustine HCL) (marketed by Cephalon (Teva Pharmaceuticals)), which is indicated for CLL and for patients with indolent B-cell NHL that has progressed within 6 months of treatment with RITUXAN.
|
|
•
|
ARZERRA (ofatumumab) (marketed by GenMab in collaboration with GlaxoSmithKline), which is indicated for CLL patients refractory to both alemtuzumab and fludarabine.
|
|
•
|
Traditional therapies for RA, including disease-modifying anti-rheumatic drugs such as steroids, methotrexate and cyclosporine, and pain relievers such as acetaminophen.
|
|
•
|
TNF inhibitors, such as REMICADE (infliximab) and SIMPONI and SIMPONI ARIA (golimumab) (marketed by Johnson & Johnson), HUMIRA (adalimumab) (marketed by AbbVie, Inc.), ENBREL (etanercept) (marketed by Amgen, Inc. and Pfizer) and CIMZIA (certolizumab pegol) (marketed by UCB, S.A.).
|
|
•
|
ORENCIA (abatacept) (marketed by Bristol-Myers Squibb Company).
|
|
•
|
ACTEMRA (tocilizumab) (marketed by the Roche Group).
|
|
•
|
XELJANZ (tofacitinib) (marketed by Pfizer).
|
|
Item 1A.
|
Risk Factors
|
|
•
|
intense competition in the increasingly crowded MS market, including the possibility of future competition from generic versions of TECFIDERA or related prodrug derivatives or from off-label use by physicians of therapies indicated for other conditions to treat MS patients;
|
|
•
|
our significant reliance on third parties to manufacture TECFIDERA, including the risks these third parties may not be able to supply TECFIDERA in a timely and cost-effective manner or in compliance with applicable regulations or otherwise fail to have sufficient aggregate manufacturing capacity to satisfy demand;
|
|
•
|
our sales and marketing efforts may not result in product revenues that meet the investment community's expectations for TECFIDERA;
|
|
•
|
additional risks associated with our anticipated launches of TECFIDERA in the E.U., including the impact of delays and the effects of a slower rollout of TECFIDERA across European countries over an extended number of months, the impact of competitive oral MS therapies approved in the E.U. prior to TECFIDERA, and our ability to obtain appropriate pricing and reimbursement for TECFIDERA in countries throughout the E.U.;
|
|
•
|
damage to our sales and reputation, and physician and patient confidence in TECFIDERA relating to any adverse experiences or events that may occur with patients treated with TECFIDERA, including any PML cases that may develop in patients previously treated with TYSABRI that switch to therapy with TECFIDERA; and
|
|
•
|
the other risks related to commercialization of new products described throughout these “Risk Factors”.
|
|
•
|
the medical community's acceptance of the product and the confidence of patients in the product;
|
|
•
|
the effectiveness of our sales force and marketing efforts;
|
|
•
|
the size of the patient population and our ability to identify new patients;
|
|
•
|
pricing and the extent of reimbursement from third party payors;
|
|
•
|
the ability to obtain and maintain data or market exclusivity for our products in the relevant indication(s);
|
|
•
|
our ability to offer products that have convenient dosing and delivery methods;
|
|
•
|
the availability or introduction of competing treatments that are deemed more effective, safer, more convenient, or less expensive;
|
|
•
|
manufacturing the product in a timely and cost-effective manner; and
|
|
•
|
compliance with complex regulatory requirements.
|
|
•
|
the hemophilia treatment market is highly competitive, with current treatments marketed by companies that have substantially greater financial resources and marketing expertise, and we may have difficulty penetrating this highly competitive market unless our long-lasting blood clotting factor candidates are regarded as offering substantial benefits over current treatments;
|
|
•
|
we do not have marketing experience within the hemophilia treatment market or well-established relationships with the associated medical and scientific community;
|
|
•
|
filing of our planned marketing authorization applications with the EMA requires the submission of positive pediatric data from our ongoing global pediatric studies with our applications, and there can be no assurance that we will receive such positive data; and
|
|
•
|
several companies are working to develop additional treatments for hemophilia and may obtain marketing approval of their treatments before we do, which has the potential to bar our application with the EMA under operation of the EMA’s Orphan Medicines Regulation; and
|
|
•
|
other companies may introduce longer-lasting or more efficacious, safer, cheaper or more convenient treatments than our long-lasting blood clotting factor candidates.
|
|
•
|
Our RITUXAN revenues, as well as any future revenues related to GAZYVA, are dependent on the efforts of Genentech and the Roche Group. Their interests may not always be aligned with our interests and they may not market RITUXAN or GAZYVA in the same manner or to the same extent that we would, which could adversely affect our RITUXAN or GAZYVA revenues.
|
|
•
|
Under our collaboration agreement with Genentech, the successful development and commercialization of GAZYVA and certain other anti-CD20 products will decrease our percentage of the collaboration's co-promotion profits.
|
|
•
|
Any failure on the part of our collaborators to comply with applicable laws and regulatory requirements in the sale, marketing and maintenance of the market authorization of our products or to fulfill any responsibilities they may have to protect and enforce any intellectual property rights underlying our products could have an adverse effect on our revenues as well as involve us in possible legal proceedings.
|
|
•
|
Collaborations often require the parties to cooperate, and failure to do so effectively could have an adverse impact on product sales by our collaborators, and could adversely affect the clinical development or regulatory approvals of products under joint control.
|
|
•
|
The process of manufacturing biologics is extremely susceptible to product loss due to contamination, oxidation, equipment failure or improper installation or operation of equipment, or vendor or operator error. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects and other supply disruptions. If microbial, viral or other contaminations are discovered in our products or manufacturing facilities, we may need to close our manufacturing facilities for an extended period of time to investigate and remediate the contaminant.
|
|
•
|
We rely on third party suppliers and manufacturers for, among other things, manufacturing of RITUXAN and GAZYVA, the majority of our clinical and commercial requirements for TECFIDERA and other small molecule products and product candidates, raw materials and supplies for production of products we manufacture, our fill-finish operations, the majority of our final product storage, and a substantial portion of our packaging operations. In addition, due to the unique manner in which our products are manufactured, we rely on single source providers of several raw materials and manufacturing supplies. These third parties are independent entities subject to their own unique operational and financial risks that are outside of our control. These third parties may not perform their obligations in a timely and cost-effective manner or in compliance with applicable regulations, and they may be unable or unwilling to increase production capacity commensurate with demand for our existing or future products. Finding alternative providers could take a significant amount of time and involve significant expense due to the specialized nature of the services and the need to obtain regulatory approval of any significant changes to our suppliers or manufacturing methods. We cannot be certain that we could reach agreement with alternative providers or that the FDA or other regulatory authorities would approve our use of such alternatives.
|
|
•
|
We rely on our manufacturing facilities in Research Triangle Park, North Carolina (RTP) and Hillerød, Denmark for the production of TYSABRI and our manufacturing facilities in RTP and Cambridge, Massachusetts for the production of AVONEX. Our global bulk supply of TYSABRI and AVONEX depends on the uninterrupted and efficient operation of these facilities, which could be adversely affected by equipment failures, labor shortages, natural disasters, power failures and numerous other factors. If we are unable to meet demand for TYSABRI or AVONEX for any reason, we would need to rely on a limited number of qualified third party contract manufacturers.
|
|
•
|
We and our third party providers are generally required to maintain compliance with current Good Manufacturing Practices and other stringent requirements and are subject to inspections by the FDA and comparable agencies in other jurisdictions to confirm such compliance. Any delay, interruption or other issues that arise in the manufacture, fill-finish, packaging, or storage of our products as a result of a failure of our facilities or the facilities or operations of third parties to pass any regulatory agency inspection could significantly impair our ability to develop and commercialize our products. Significant noncompliance could also result in the imposition of monetary penalties or other civil or criminal sanctions and damage our reputation.
|
|
•
|
new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or decisions, related to health care availability, pricing or marketing practices, compliance with wage and hour laws and other employment practices, method of delivery, payment for health care products and services, compliance with health information and data privacy and security laws and regulations, tracking and reporting payments and other transfers of value made to physicians and teaching hospitals, and extensive anti-bribery and anti-corruption prohibitions;
|
|
•
|
changes in the FDA and foreign regulatory approval processes that may delay or prevent the approval of new products and result in lost market opportunity; and
|
|
•
|
changes in FDA and foreign regulations that may require additional safety monitoring, labeling changes, restrictions on product distribution or use, or other measures after the introduction of our products to market, which could increase our costs of doing business, adversely affect the future permitted uses of approved products, or otherwise adversely affect the market for our products.
|
|
•
|
the inability to obtain necessary foreign regulatory or pricing approvals of products in a timely manner;
|
|
•
|
collectability of accounts receivable;
|
|
•
|
fluctuations in currency exchange rates;
|
|
•
|
difficulties in staffing and managing international operations;
|
|
•
|
the imposition of governmental controls;
|
|
•
|
less favorable intellectual property or other applicable laws;
|
|
•
|
increasingly complex standards for complying with foreign laws and regulations that may differ substantially from country to country and may conflict with corresponding U.S. laws and regulations;
|
|
•
|
the emergence of far-reaching anti-bribery and anti-corruption legislation in the U.K., including passage of the U.K. Bribery Act 2010, and elsewhere and escalation of investigations and prosecutions pursuant to such laws;
|
|
•
|
compliance with complex import and export control laws;
|
|
•
|
restrictions on direct investments by foreign entities and trade restrictions;
|
|
•
|
greater political or economic instability; and
|
|
•
|
changes in tax laws and tariffs.
|
|
•
|
the cost of restructurings;
|
|
•
|
impairments with respect to investments, fixed assets and long-lived assets, including in-process research and development and other intangible assets;
|
|
•
|
inventory write-downs for failed quality specifications, charges for excess or obsolete inventory and charges for inventory write downs relating to product suspensions, expirations or recalls;
|
|
•
|
bad debt expenses and increased bad debt reserves;
|
|
•
|
outcomes of litigation and other legal proceedings, regulatory matters and tax matters;
|
|
•
|
milestone payments under license and collaboration agreements; and
|
|
•
|
payments in connection with acquisitions and other business development activity.
|
|
Item 1B.
|
Unresolved Staff Comments
|
|
Item 2.
|
Properties
|
|
•
|
729,000 square feet in Cambridge, Massachusetts, which is comprised of a 67,000 square foot biologics manufacturing facility and 662,000 square feet for our corporate headquarters, laboratory and additional office space;
|
|
•
|
357,000 square feet of office space in Weston, Massachusetts, of which 175,000 square feet has been subleased for a term which started in January 2014 and will continue through the remaining term of our lease agreement; and
|
|
•
|
46,000 square feet of warehouse space in Somerville, Massachusetts.
|
|
•
|
357,000 square feet of laboratory and office space;
|
|
•
|
175,000 square feet related to a large-scale biologics manufacturing facility;
|
|
•
|
105,000 square feet related to a biologics manufacturing facility;
|
|
•
|
60,000 square feet of warehouse space; and
|
|
•
|
43,000 square feet related to a large-scale purification facility.
|
|
•
|
140,000 square feet of warehouse, utilities and support space;
|
|
•
|
70,000 square feet related to a label and packaging facility;
|
|
•
|
50,000 square feet of administrative space; and
|
|
•
|
50,000 square feet related to a laboratory facility.
|
|
Item 3.
|
Legal Proceedings
|
|
Item 4.
|
Mine Safety Disclosures
|
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
|
|
Common Stock Price
|
||||||||||||||
|
|
2013
|
2012
|
|||||||||||||
|
|
High
|
Low
|
High
|
Low
|
|||||||||||
|
First Quarter
|
$
|
192.92
|
|
$
|
139.72
|
|
$
|
127.85
|
|
$
|
111.44
|
|
|||
|
Second Quarter
|
$
|
242.64
|
|
$
|
191.80
|
|
$
|
144.38
|
|
$
|
124.23
|
|
|||
|
Third Quarter
|
$
|
248.95
|
|
$
|
203.55
|
|
$
|
157.18
|
|
$
|
137.88
|
|
|||
|
Fourth Quarter
|
$
|
298.82
|
|
$
|
221.07
|
|
$
|
155.30
|
|
$
|
134.00
|
|
|||
|
Period
|
Total
Number of
Shares
Purchased
(#)
|
Average Price
Paid per
Share
($)
|
Total Number of
Shares Purchased
as Part of Publicly
Announced Programs
(#)
|
Maximum Number
of Shares That May
Yet Be Purchased
Under Our Programs
($ in millions)
|
|||||||
|
Oct-13
|
—
|
|
—
|
|
—
|
|
4,185,526
|
|
|||
|
Nov-13
|
—
|
|
—
|
|
—
|
|
4,185,526
|
|
|||
|
Dec-13
|
—
|
|
—
|
|
—
|
|
4,185,526
|
|
|||
|
Total
|
—
|
|
—
|
|
|||||||
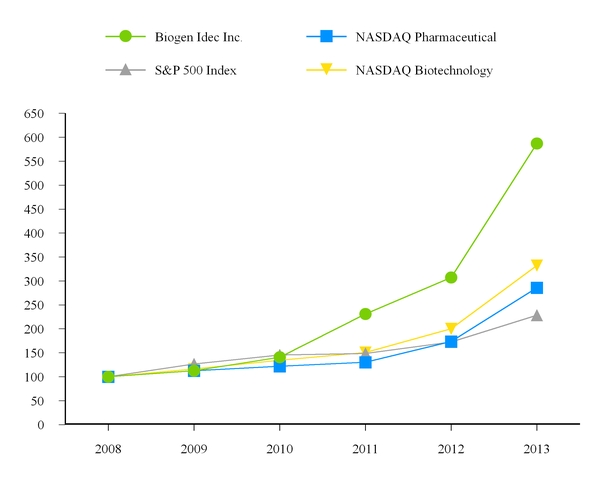
|
2008
|
2009
|
2010
|
2011
|
2012
|
2013
|
|||||||
|
Biogen Idec Inc.
|
100.00
|
|
112.32
|
|
140.77
|
|
231.05
|
|
307.31
|
|
586.96
|
|
|
NASDAQ Pharmaceutical
|
100.00
|
|
112.36
|
|
121.80
|
|
130.37
|
|
173.45
|
|
285.96
|
|
|
S&P 500 Index
|
100.00
|
|
126.46
|
|
145.51
|
|
148.59
|
|
172.37
|
|
228.19
|
|
|
NASDAQ Biotechnology
|
100.00
|
|
115.96
|
|
134.58
|
|
150.85
|
|
200.25
|
|
332.45
|
|
|
Item 6.
|
Selected Financial Data
|
|
|
For the Years Ended December 31,
|
||||||||||||||||||
|
|
2013
|
2012
|
2011
|
2010
|
2009
|
||||||||||||||
|
(In millions, except per share amounts)
|
(5) (7) (9) (10)
|
(7) (8)
|
(5) (6)
|
(3) (4)
|
(1) (2)
|
||||||||||||||
|
Results of Operations
|
|||||||||||||||||||
|
Product revenues
|
$
|
5,542.3
|
|
$
|
4,166.1
|
|
$
|
3,836.1
|
|
$
|
3,470.1
|
|
$
|
3,152.9
|
|
||||
|
Revenues from unconsolidated joint business
|
1,126.0
|
|
1,137.9
|
|
996.6
|
|
1,077.2
|
|
1,094.9
|
|
|||||||||
|
Other revenues
|
263.9
|
|
212.5
|
|
215.9
|
|
169.1
|
|
129.5
|
|
|||||||||
|
Total revenues
|
6,932.2
|
|
5,516.5
|
|
5,048.6
|
|
4,716.4
|
|
4,377.3
|
|
|||||||||
|
Cost and expenses:
|
|||||||||||||||||||
|
Cost of sales, excluding amortization of acquired intangible assets
|
857.7
|
|
545.5
|
|
466.8
|
|
400.3
|
|
382.1
|
|
|||||||||
|
Research and development
|
1,444.1
|
|
1,334.9
|
|
1,219.6
|
|
1,248.6
|
|
1,283.1
|
|
|||||||||
|
Selling, general and administrative
|
1,712.1
|
|
1,277.5
|
|
1,056.1
|
|
1,031.5
|
|
911.0
|
|
|||||||||
|
Amortization of acquired intangible assets
|
342.9
|
|
202.2
|
|
208.6
|
|
208.9
|
|
289.8
|
|
|||||||||
|
Collaboration profit sharing
|
85.4
|
|
317.9
|
|
317.8
|
|
258.1
|
|
215.9
|
|
|||||||||
|
(Gain) loss on fair value remeasurement of contingent consideration
|
(0.5
|
)
|
27.2
|
|
36.1
|
|
—
|
|
—
|
|
|||||||||
|
Restructuring charges
|
—
|
|
2.2
|
|
19.0
|
|
75.2
|
|
—
|
|
|||||||||
|
Acquired in-process research and development
|
—
|
|
—
|
|
—
|
|
245.0
|
|
—
|
|
|||||||||
|
Total cost and expenses
|
4,441.6
|
|
3,707.4
|
|
3,323.9
|
|
3,467.5
|
|
3,081.9
|
|
|||||||||
|
Gain on sale of rights
|
24.9
|
|
46.8
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Income from operations
|
2,515.5
|
|
1,855.9
|
|
1,724.7
|
|
1,248.9
|
|
1,295.4
|
|
|||||||||
|
Other income (expense), net
|
(34.9
|
)
|
(0.7
|
)
|
(13.5
|
)
|
(19.0
|
)
|
37.3
|
|
|||||||||
|
Income before income tax expense and equity in loss of investee, net of tax
|
2,480.6
|
|
1,855.1
|
|
1,711.2
|
|
1,229.9
|
|
1,332.7
|
|
|||||||||
|
Income tax expense
|
601.0
|
|
470.6
|
|
444.5
|
|
331.3
|
|
355.6
|
|
|||||||||
|
Equity in loss of investee, net of tax
|
17.2
|
|
4.5
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Net income
|
1,862.3
|
|
1,380.0
|
|
1,266.7
|
|
898.6
|
|
977.1
|
|
|||||||||
|
Net income (loss) attributable to noncontrolling interests, net of tax
|
—
|
|
—
|
|
32.3
|
|
(106.7
|
)
|
6.9
|
|
|||||||||
|
Net income attributable to Biogen Idec Inc.
|
$
|
1,862.3
|
|
$
|
1,380.0
|
|
$
|
1,234.4
|
|
$
|
1,005.2
|
|
$
|
970.1
|
|
||||
|
Diluted Earnings Per Share
|
|||||||||||||||||||
|
Diluted earnings per share attributable to Biogen Idec Inc.
|
$
|
7.81
|
|
$
|
5.76
|
|
$
|
5.04
|
|
$
|
3.94
|
|
$
|
3.35
|
|
||||
|
Weighted-average shares used in calculating diluted earnings per share attributable to Biogen Idec Inc.
|
238.3
|
|
239.7
|
|
245.0
|
|
254.9
|
|
289.5
|
|
|||||||||
|
Financial Condition
|
|||||||||||||||||||
|
Cash, cash equivalents and marketable securities
|
$
|
1,848.5
|
|
$
|
3,742.4
|
|
$
|
3,107.4
|
|
$
|
1,950.8
|
|
$
|
2,457.8
|
|
||||
|
Total assets
|
$
|
11,863.3
|
|
$
|
10,130.1
|
|
$
|
9,049.6
|
|
$
|
8,092.5
|
|
$
|
8,551.9
|
|
||||
|
Notes payable, line of credit and other financing arrangements, less current portion
|
$
|
592.4
|
|
$
|
687.4
|
|
$
|
1,060.8
|
|
$
|
1,066.4
|
|
$
|
1,080.2
|
|
||||
|
Total Biogen Idec Inc. shareholders’ equity
|
$
|
8,620.2
|
|
$
|
6,961.5
|
|
$
|
6,425.5
|
|
$
|
5,396.5
|
|
$
|
6,221.5
|
|
||||
|
(1)
|
Total cost and expenses includes the $110.0 million upfront payment made to Acorda Therapeutics, Inc. pursuant to our June 30, 2009 collaboration and license agreement to develop and commercialize products containing fampridine in markets outside the U.S.
|
|
(2)
|
Changes in tax law in certain state jurisdictions in which we operate and the resolution of multiple federal, state and foreign tax audits, including the effective settlement of several uncertain tax positions resulted in a $58.3 million reduction to our income tax expense.
|
|
(3)
|
Included in total cost and expenses is a charge to acquired in-process research and development of
$40.0 million
related to the achievement of a milestone by Biogen Idec Hemophilia, Inc. (formerly Syntonix Pharmaceuticals, Inc.).
|
|
(4)
|
Included in total cost and expenses is a charge to acquired in-process research and development of $205.0 million incurred in connection with the license agreement entered into with Knopp Neurosciences Inc. (Knopp), which we consolidated as we determined that we were the primary beneficiary of the entity. The $205.0 million charge was partially offset by an attribution of $145.0 million to the noncontrolling interest.
|
|
(5)
|
Our share of revenues from unconsolidated joint business reflects charges of
$50.0 million
in 2011 and
$49.7 million
in 2013 for damages and interest awarded to Hoechst in Genentech's arbitration with Hoechst for RITUXAN.
|
|
(6)
|
Biogen Idec Inc.’s shareholders’ equity reflects a reduction in additional paid in capital and noncontrolling interests totaling $187.3 million resulting from our purchase of the noncontrolling interest in our joint venture investments in Biogen Dompé SRL and Biogen Dompé Switzerland GmbH.
|
|
(7)
|
Gain on sale of rights relates to the sale of all of our rights, including rights to royalties, related to BENLYSTA.
|
|
(8)
|
Equity in loss of investee, net of tax relates to our ownership interest in Samsung Bioepis to develop, manufacture and market biosimilar pharmaceuticals.
|
|
(9)
|
Commencing in the second quarter of 2013, product revenues and total revenues includes 100% of net revenues related to sales of TYSABRI as a result of our acquisition of TYSABRI rights from Elan and net revenues related to sales of TECFIDERA, our new oral first-line treatment for people with relapsing forms of multiple sclerosis (MS), which was approved by the FDA and commenced commercial sales. In addition, upon the closing of our acquisition of TYSABRI rights, our collaboration agreement was terminated, and we no longer record collaboration profit sharing.
|
|
(10)
|
Included in net income is a $38.5 million benefit, net of ancillary federal and state income and non-income tax effects, related to years 2005 through 2012 as a result of receiving updated technical guidance from the IRS concerning our current and prior year filings and calculation of our U.S. federal manufacturing deduction and overall tax classification of our unconsolidated joint business.
|
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
|
|
For the Years Ended
December 31,
|
% Change
|
||||||||
|
|
2013 compared to 2012
|
|||||||||
|
(In millions, except per share amounts and percentages)
|
2013
(1) (2)
|
2012
|
||||||||
|
Total revenues
|
$
|
6,932.2
|
|
$
|
5,516.5
|
|
25.7
|
%
|
||
|
Income from operations
|
$
|
2,515.5
|
|
$
|
1,855.8
|
|
35.5
|
%
|
||
|
Net income attributable to Biogen Idec Inc.
|
$
|
1,862.3
|
|
$
|
1,380.0
|
|
34.9
|
%
|
||
|
Diluted earnings per share attributable to Biogen Idec Inc.
|
$
|
7.81
|
|
$
|
5.76
|
|
35.8
|
%
|
||
|
(1)
|
Commencing in the second quarter of 2013, product revenues and total revenues includes 100% of net revenues related to sales of TYSABRI as a result of our acquisition of TYSABRI rights from Elan and net revenues related to sales of TECFIDERA, our new oral first-line treatment for people with relapsing forms of MS, which was approved by the FDA and commenced commercial sales.
|
|
(2)
|
Our share of revenues from unconsolidated joint business reflects a charge of
$49.7 million
for damages and interest awarded to Hoechst in Genentech's arbitration with Hoechst for RITUXAN.
|
|
•
|
Worldwide AVONEX revenues totaled
$3,005.5 million
for
2013
, representing an increase of
3.2%
over
2012
.
|
|
•
|
Worldwide TYSABRI revenues totaled
$1,526.5 million
for
2013
, representing an increase of
34.4%
over
2012
. The increase in revenue is primarily due to 100% of net U.S. revenue being recognized starting in April 2013 as a result of our acquisition of TYSABRI rights.
|
|
•
|
Worldwide TECFIDERA revenues totaled
$876.1 million
for
2013
. Approximately $134.0 million of U.S. TECFIDERA revenues in 2013 represent inventory in the channel.
|
|
•
|
Our share of revenues from unconsolidated joint business totaled
$1,126.0 million
for
2013
, representing a decrease of
1.0%
from
2012
.
|
|
•
|
Total cost and expenses increased
19.8%
for
2013
compared to
2012
. This increase resulted from a
69.6%
increase in the amortization of acquired intangible assets, a
57.2%
increase in cost of sales, a
34.0%
increase in selling, general and administrative expense and an
8.2%
increase in research and development expense, partially offset by a
73.1%
decrease in collaboration profit sharing compared with the same period in
2012
.
|
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
Product Revenues:
|
|||||||||||||||||
|
United States
|
$
|
3,581.0
|
|
$
|
2,176.8
|
|
$
|
1,954.8
|
|
64.5
|
%
|
11.4
|
%
|
||||
|
Rest of world
|
1,961.3
|
|
1,989.3
|
|
1,881.3
|
|
(1.4
|
)%
|
5.7
|
%
|
|||||||
|
Total product revenues
|
5,542.3
|
|
4,166.1
|
|
3,836.1
|
|
33.0
|
%
|
8.6
|
%
|
|||||||
|
Unconsolidated joint business revenues
|
1,126.0
|
|
1,137.9
|
|
996.6
|
|
(1.0
|
)%
|
14.2
|
%
|
|||||||
|
Other revenues
|
263.9
|
|
212.5
|
|
215.9
|
|
24.2
|
%
|
(1.6
|
)%
|
|||||||
|
Total revenues
|
$
|
6,932.2
|
|
$
|
5,516.5
|
|
$
|
5,048.6
|
|
25.7
|
%
|
9.3
|
%
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
AVONEX
|
$
|
3,005.5
|
|
$
|
2,913.1
|
|
$
|
2,686.6
|
|
3.2
|
%
|
8.4
|
%
|
||||
|
TYSABRI
|
1,526.5
|
|
1,135.9
|
|
1,079.5
|
|
34.4
|
%
|
5.2
|
%
|
|||||||
|
TECFIDERA
|
876.1
|
|
—
|
|
—
|
|
**
|
|
**
|
|
|||||||
|
Other product revenues
|
134.2
|
|
117.1
|
|
70.0
|
|
14.6
|
%
|
67.3
|
%
|
|||||||
|
Total product revenues
|
$
|
5,542.3
|
|
$
|
4,166.1
|
|
$
|
3,836.1
|
|
33.0
|
%
|
8.6
|
%
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
United States
|
$
|
1,902.4
|
|
$
|
1,793.7
|
|
$
|
1,628.3
|
|
6.1
|
%
|
10.2
|
%
|
||||
|
Rest of world
|
1,103.1
|
|
1,119.4
|
|
1,058.3
|
|
(1.5
|
)%
|
5.8
|
%
|
|||||||
|
Total AVONEX revenues
|
$
|
3,005.5
|
|
$
|
2,913.1
|
|
$
|
2,686.6
|
|
3.2
|
%
|
8.4
|
%
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
United States
|
$
|
814.2
|
|
$
|
383.1
|
|
$
|
326.5
|
|
112.5
|
%
|
17.3
|
%
|
||||
|
Rest of world
|
712.3
|
|
752.8
|
|
753.0
|
|
(5.4
|
)%
|
—
|
%
|
|||||||
|
Total TYSABRI revenues
|
$
|
1,526.5
|
|
$
|
1,135.9
|
|
$
|
1,079.5
|
|
34.4
|
%
|
5.2
|
%
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||
|
United States
|
$
|
864.4
|
|
$
|
—
|
|
$
|
—
|
|
**
|
**
|
||||
|
Rest of world
|
11.7
|
|
—
|
|
—
|
|
**
|
**
|
|||||||
|
Total TECFIDERA revenues
|
$
|
876.1
|
|
$
|
—
|
|
$
|
—
|
|
**
|
**
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
FAMPYRA
|
$
|
74.0
|
|
$
|
57.4
|
|
$
|
13.6
|
|
28.9
|
%
|
322.1
|
%
|
||||
|
FUMADERM
|
60.2
|
|
59.7
|
|
54.7
|
|
0.8
|
%
|
9.1
|
%
|
|||||||
|
Other
|
—
|
|
—
|
|
1.7
|
|
**
|
|
(100.0
|
)%
|
|||||||
|
Total other product revenues
|
$
|
134.2
|
|
$
|
117.1
|
|
$
|
70.0
|
|
14.6
|
%
|
67.3
|
%
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
Biogen Idec's share of profits in the U.S. for RITUXAN and GAZYVA (1)
|
$
|
1,085.2
|
|
$
|
1,031.7
|
|
$
|
872.7
|
|
5.2
|
%
|
18.2
|
%
|
||||
|
Reimbursement of selling and development expenses in the U.S. for RITUXAN
|
2.1
|
|
1.6
|
|
6.1
|
|
31.3
|
%
|
(73.8
|
)%
|
|||||||
|
Revenue on sales in the rest of world for RITUXAN
|
38.7
|
|
104.6
|
|
117.8
|
|
(63.0
|
)%
|
(11.2
|
)%
|
|||||||
|
Total unconsolidated joint business revenues
|
$
|
1,126.0
|
|
$
|
1,137.9
|
|
$
|
996.6
|
|
(1.0
|
)%
|
14.2
|
%
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
Product revenues, net
|
$
|
3,425.8
|
|
$
|
3,131.8
|
|
$
|
2,924.5
|
|
9.4
|
%
|
7.1
|
%
|
||||
|
Cost and expenses
|
615.9
|
|
543.7
|
|
730.8
|
|
13.3
|
%
|
(25.6
|
)%
|
|||||||
|
Pre-tax profits in the U.S. for RITUXAN and GAZYVA
|
$
|
2,809.9
|
|
$
|
2,588.1
|
|
$
|
2,193.7
|
|
8.6
|
%
|
18.0
|
%
|
||||
|
Biogen Idec's share of pre-tax profits in the U.S. for RITUXAN and GAZYVA
|
$
|
1,085.2
|
|
$
|
1,031.7
|
|
$
|
872.7
|
|
5.2
|
%
|
18.2
|
%
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
Royalty revenues
|
$
|
185.7
|
|
$
|
168.7
|
|
$
|
158.5
|
|
10.1
|
%
|
6.4
|
%
|
||||
|
Corporate partner revenues
|
78.2
|
|
43.8
|
|
57.4
|
|
78.5
|
%
|
(23.7
|
)%
|
|||||||
|
Total other revenues
|
$
|
263.9
|
|
$
|
212.5
|
|
$
|
215.9
|
|
24.2
|
%
|
(1.6
|
)%
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
Discounts
|
$
|
235.6
|
|
$
|
96.2
|
|
$
|
84.3
|
|
144.9
|
%
|
14.1
|
%
|
||||
|
Contractual adjustments
|
835.0
|
|
529.5
|
|
358.1
|
|
57.7
|
%
|
47.9
|
%
|
|||||||
|
Returns
|
24.0
|
|
21.9
|
|
14.8
|
|
9.6
|
%
|
48.0
|
%
|
|||||||
|
Total allowances
|
$
|
1,094.6
|
|
$
|
647.6
|
|
$
|
457.2
|
|
69.0
|
%
|
41.6
|
%
|
||||
|
Gross product revenues
|
$
|
6,636.9
|
|
$
|
4,813.7
|
|
$
|
4,293.3
|
|
37.9
|
%
|
12.1
|
%
|
||||
|
Percent of gross product revenues
|
16.5
|
%
|
13.5
|
%
|
10.6
|
%
|
|||||||||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
Cost of sales, excluding amortization of acquired intangible assets
|
$
|
857.7
|
|
$
|
545.5
|
|
$
|
466.8
|
|
57.2
|
%
|
16.9
|
%
|
||||
|
Research and development
|
1,444.1
|
|
1,334.9
|
|
1,219.6
|
|
8.2
|
%
|
9.5
|
%
|
|||||||
|
Selling, general and administrative
|
1,712.1
|
|
1,277.5
|
|
1,056.1
|
|
34.0
|
%
|
21.0
|
%
|
|||||||
|
Amortization of acquired intangible assets
|
342.9
|
|
202.2
|
|
208.6
|
|
69.6
|
%
|
(3.1
|
)%
|
|||||||
|
Collaboration profit sharing
|
85.4
|
|
317.9
|
|
317.8
|
|
(73.1
|
)%
|
—
|
%
|
|||||||
|
(Gain) loss on fair value remeasurement of contingent consideration
|
(0.5
|
)
|
27.2
|
|
36.1
|
|
(102.0
|
)%
|
(24.6
|
)%
|
|||||||
|
Restructuring charges
|
—
|
|
2.2
|
|
19.0
|
|
(100.0
|
)%
|
(88.3
|
)%
|
|||||||
|
Total cost and expenses
|
$
|
4,441.6
|
|
$
|
3,707.4
|
|
$
|
3,323.9
|
|
19.8
|
%
|
11.5
|
%
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
Product cost of sales
|
$
|
427.6
|
|
$
|
365.9
|
|
$
|
307.3
|
|
16.9
|
%
|
19.1
|
%
|
||||
|
Royalty cost of sales
|
430.1
|
|
179.6
|
|
159.5
|
|
139.5
|
%
|
12.6
|
%
|
|||||||
|
Total cost of sales
|
$
|
857.7
|
|
$
|
545.5
|
|
$
|
466.8
|
|
57.2
|
%
|
16.9
|
%
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
Marketed products
|
$
|
252.1
|
|
$
|
128.2
|
|
$
|
111.0
|
|
96.6
|
%
|
15.5
|
%
|
||||
|
Late stage programs
|
272.8
|
|
467.0
|
|
428.1
|
|
(41.6
|
)%
|
9.1
|
%
|
|||||||
|
Early stage programs
|
130.8
|
|
90.7
|
|
72.5
|
|
44.2
|
%
|
25.1
|
%
|
|||||||
|
Research and discovery
|
97.6
|
|
94.6
|
|
97.3
|
|
3.2
|
%
|
(2.8
|
)%
|
|||||||
|
Other research and development costs
|
552.7
|
|
479.0
|
|
465.6
|
|
15.4
|
%
|
2.9
|
%
|
|||||||
|
Milestone and upfront payments
|
138.1
|
|
75.4
|
|
45.1
|
|
83.2
|
%
|
67.2
|
%
|
|||||||
|
Total research and development
|
$
|
1,444.1
|
|
$
|
1,334.9
|
|
$
|
1,219.6
|
|
8.2
|
%
|
9.5
|
%
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
Selling, general and administrative
|
$
|
1,712.1
|
|
$
|
1,277.5
|
|
$
|
1,056.1
|
|
34.0
|
%
|
21.0
|
%
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
Amortization of acquired intangible assets
|
$
|
342.9
|
|
$
|
202.2
|
|
$
|
208.6
|
|
69.6
|
%
|
(3.1
|
)%
|
||||
|
(In millions)
|
As of December 31, 2013
|
||
|
2014
|
$
|
426.3
|
|
|
2015
|
336.4
|
|
|
|
2016
|
322.7
|
|
|
|
2017
|
327.5
|
|
|
|
2018
|
330.2
|
|
|
|
Total
|
$
|
1,743.1
|
|
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
Collaboration profit sharing
|
$
|
85.4
|
|
$
|
317.9
|
|
$
|
317.8
|
|
(73.1
|
)%
|
—
|
%
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
(Gain) loss on fair value remeasurement of contingent consideration
|
$
|
(0.5
|
)
|
$
|
27.2
|
|
$
|
36.1
|
|
(102.0
|
)%
|
(24.6
|
)%
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
Restructuring charges
|
$
|
—
|
|
$
|
2.2
|
|
$
|
19.0
|
|
(100.0
|
)%
|
(88.3
|
)%
|
||||
|
•
|
We out-licensed or terminated certain research and development programs, including those in oncology and cardiovascular medicine, that were no longer a strategic fit for us.
|
|
•
|
We completed a 13% reduction in workforce spanning our sales, research and development, and administrative functions.
|
|
•
|
We vacated and recognized the sale of the San Diego, California facility as well as consolidated certain of our Massachusetts facilities.
|
|
|
For the Years Ended
December 31,
|
% Change
|
||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
|||||||||||||
|
Gain on sale of rights
|
$
|
24.9
|
|
$
|
46.8
|
|
$
|
—
|
|
(46.8
|
)%
|
**
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
|||||||||||||
|
Other income (expense), net
|
$
|
(34.9
|
)
|
$
|
(0.7
|
)
|
$
|
(13.5
|
)
|
**
|
(94.5
|
)%
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
Effective tax rate on pre-tax income
|
24.2
|
%
|
25.4
|
%
|
26.0
|
%
|
(4.7
|
)%
|
(2.3
|
)%
|
|||||||
|
Income tax expense
|
$
|
601.0
|
|
$
|
470.6
|
|
$
|
444.5
|
|
27.7
|
%
|
5.9
|
%
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
|||||||||||||
|
Equity in loss of investee, net of tax
|
$
|
17.2
|
|
$
|
4.5
|
|
$
|
—
|
|
281.2
|
%
|
**
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
|||||||||||||
|
Net income attributable to noncontrolling interests, net of tax
|
$
|
—
|
|
$
|
—
|
|
$
|
32.3
|
|
**
|
(100.0
|
)%
|
||||
|
|
As of December 31,
|
% Change
|
||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2013 compared to 2012
|
|||||||
|
Financial assets:
|
||||||||||
|
Cash and cash equivalents
|
$
|
602.6
|
|
$
|
570.7
|
|
5.6
|
%
|
||
|
Marketable securities — current
|
620.2
|
|
1,135.0
|
|
(45.4
|
)%
|
||||
|
Marketable securities — non-current
|
625.8
|
|
2,036.7
|
|
(69.3
|
)%
|
||||
|
Total cash, cash equivalents and marketable securities
|
$
|
1,848.5
|
|
$
|
3,742.4
|
|
(50.6
|
)%
|
||
|
Borrowings:
|
||||||||||
|
Current portion of notes payable and line of credit
|
$
|
3.5
|
|
$
|
453.4
|
|
(99.2
|
)%
|
||
|
Notes payable and other financing arrangements
|
592.4
|
|
687.4
|
|
(13.8
|
)%
|
||||
|
Total borrowings
|
$
|
595.9
|
|
$
|
1,140.8
|
|
(47.8
|
)%
|
||
|
Working Capital:
|
||||||||||
|
Current assets
|
$
|
3,184.9
|
|
$
|
3,244.3
|
|
(1.8
|
)%
|
||
|
Current liabilities
|
(1,758.3
|
)
|
(1,657.4
|
)
|
6.1
|
%
|
||||
|
Total working capital
|
$
|
1,426.6
|
|
$
|
1,586.9
|
|
(10.1
|
)%
|
||
|
•
|
$3.25 billion
used for our acquisition of TYSABRI rights from Elan;
|
|
•
|
$643.2 million
in total payments for income taxes;
|
|
•
|
$450.0 million
used for the repayment of principal of our
6.0%
Senior Notes;
|
|
•
|
$400.3 million
used for share repurchases;
|
|
•
|
$246.3 million
used for purchases of property, plant and equipment; and
|
|
•
|
$100.0 million
upfront payment made to Isis pursuant to our collaboration agreement dated September 2013.
|
|
•
|
$133.2 million in cash collections on accounts receivable balances in Spain and Portugal as part of new programs to resolve outstanding amounts long overdue;
|
|
•
|
$67.5 million
in proceeds from the issuance of stock for share-based compensation arrangements;
|
|
•
|
$46.8 million
in proceeds from the sale of our royalty and other rights to BENLYSTA;
|
|
•
|
$984.7 million
used for share repurchases;
|
|
•
|
$526.6 million
in total payments for income taxes;
|
|
•
|
$254.5 million
used for purchases of property, plant and equipment;
|
|
•
|
$72.4 million
of net cash paid for the acquisition of Stromedix, Inc.;
|
|
•
|
$71.0 million
in upfront payments made to Isis, recognized as research and development expense, pursuant to our collaboration agreements dated January, June, and December 2012; and
|
|
•
|
$32.1 million
in contributions made to Samsung Bioepis.
|
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2013 compared to 2012
|
2012 compared to 2011
|
|||||||||||||||
|
(In millions, except percentages)
|
2013
|
2012
|
2011
|
||||||||||||||
|
Net cash flows provided by operating activities
|
$
|
2,345.1
|
|
$
|
1,879.9
|
|
$
|
1,727.7
|
|
24.7
|
%
|
8.8
|
%
|
||||
|
Net cash flows used in by investing activities
|
$
|
(1,604.7
|
)
|
$
|
(950.3
|
)
|
$
|
(1,650.3
|
)
|
68.9
|
%
|
(42.4
|
)%
|
||||
|
Net cash flows used in financing activities
|
$
|
(716.5
|
)
|
$
|
(877.5
|
)
|
$
|
(319.9
|
)
|
(18.3
|
)%
|
174.3
|
%
|
||||
|
•
|
Non-cash operating items such as depreciation and amortization, impairment charges and share-based compensation charges;
|
|
•
|
Changes in operating assets and liabilities which reflect timing differences between the receipt and payment of cash associated with transactions and when they are recognized in results of operations; and
|
|
•
|
Changes associated with the fair value of contingent milestones associated with our acquisitions of businesses and payments related to collaborations.
|
|
|
Payments Due by Period
|
||||||||||||||||||
|
(In millions)
|
Total
|
Less than
1 Year
|
1 to 3
Years
|
3 to 5
Years
|
After
5 Years
|
||||||||||||||
|
Non-cancellable operating leases (1), (2)
|
$
|
611.7
|
|
$
|
66.8
|
|
$
|
109.6
|
|
$
|
92.4
|
|
$
|
342.9
|
|
||||
|
Notes payable (3)
|
723.4
|
|
41.3
|
|
82.0
|
|
81.6
|
|
518.5
|
|
|||||||||
|
Purchase and other obligations (4)
|
188.7
|
|
165.2
|
|
22.1
|
|
1.4
|
|
—
|
|
|||||||||
|
Defined benefit obligation
|
42.6
|
|
—
|
|
—
|
|
—
|
|
42.6
|
|
|||||||||
|
Total contractual obligations
|
$
|
1,566.4
|
|
$
|
273.3
|
|
$
|
213.7
|
|
$
|
175.4
|
|
$
|
904.0
|
|
||||
|
(1)
|
We lease properties and equipment for use in our operations. In addition to rent, the leases may require us to pay additional amounts for taxes, insurance, maintenance and other operating expenses. Amounts reflected within the table, detail future minimum rental commitments under non-cancelable operating leases as of December 31 for each of the periods presented.
|
|
(2)
|
Includes future minimum rental commitments related to leases executed for two office buildings in Cambridge, Massachusetts, which completed construction in July and November 2013, net of sublease income expected to be received for the vacated portion of our Weston, MA facility. For additional information related to our leases, please read Note 11,
Property, Plant and Equipment
to our consolidated financial statements included in this report.
|
|
(3)
|
Notes payable includes principal and interest payments.
|
|
(4)
|
Purchase and other obligations include our obligations of approximately
$23.5 million
related to the fair value of net liabilities on derivative contracts, approximately $5.0 million related to fixed obligations for the purchase of natural gas and approximately $24.7 million related to obligations for communication services.
|
|
Cumulative Sales Level
|
||||||||||||||||||||
|
Prior 12 Month Sales
|
$500M
|
$1.0B
|
$2.0B
|
$3.0B
|
Each additional $1.0B up to $20.0B
|
|||||||||||||||
|
Payment Amount (In millions)
|
||||||||||||||||||||
|
< $500 million
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||||
|
$500 million - $1.0 billion
|
22.0
|
|
25.0
|
|
50.0
|
|
50.0
|
|
50.0
|
|
||||||||||
|
$1.0 billion - $1.5 billion
|
—
|
|
50.0
|
|
100.0
|
|
100.0
|
|
100.0
|
|
||||||||||
|
$1.5 billion - $2.0 billion
|
—
|
|
—
|
|
150.0
|
|
150.0
|
|
150.0
|
|
||||||||||
|
$2.0 billion - $2.5 billion
|
—
|
|
—
|
|
200.0
|
|
200.0
|
|
200.0
|
|
||||||||||
|
$2.5 billion - $3.0 billion
|
—
|
|
—
|
|
—
|
|
250.0
|
|
250.0
|
|
||||||||||
|
> $3.0 billion
|
—
|
|
—
|
|
—
|
|
—
|
|
300.0
|
|
||||||||||
|
•
|
estimating the timing of and expected costs to complete the in-process projects;
|
|
•
|
projecting regulatory approvals;
|
|
•
|
estimating future cash flows from product sales resulting from completed products and in process projects; and
|
|
•
|
developing appropriate discount rates and probability rates by project.
|
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
|
|
Item 8.
|
Financial Statements and Supplementary Data
|
|
Item 9.
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
|
|
Item 9A.
|
Controls and Procedures
|
|
•
|
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect our transactions and dispositions of our assets;
|
|
•
|
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
|
|
•
|
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
|
|
Item 9B.
|
Other Information
|
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
|
Item 11.
|
Executive Compensation
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
|
Item 14.
|
Principal Accountant Fees and Services
|
|
Item 15.
|
Exhibits and Financial Statement Schedules
|
|
a.
|
(1)
Consolidated Financial Statements:
|
|
Financial Statements
|
|
Page Number
|
|
Consolidated Statements of Income
|
|
F-2
|
|
Consolidated Statements of Comprehensive Income
|
F-3
|
|
|
Consolidated Balance Sheets
|
|
F-4
|
|
Consolidated Statements of Cash Flows
|
|
F-5
|
|
Consolidated Statements of Equity
|
|
F-6
|
|
Notes to Consolidated Financial Statements
|
|
F-9
|
|
Report of Independent Registered Public Accounting Firm
|
|
F-69
|
|
BIOGEN IDEC INC.
|
|
|
By:
|
/
S
/ G
EORGE
A. S
CANGOS
|
|
George A. Scangos
|
|
|
Chief Executive Officer
|
|
|
Name
|
|
Capacity
|
|
Date
|
|
/
S
/ G
EORGE
A. S
CANGOS
|
|
Director and Chief Executive Officer (principal executive officer)
|
|
February 6, 2014
|
|
George A. Scangos
|
||||
|
/
S
/ P
AUL
J. C
LANCY
|
|
Executive Vice President, Finance and Chief Financial Officer (principal financial officer)
|
|
February 6, 2014
|
|
Paul J. Clancy
|
||||
|
/
S
/ G
REGORY
F. C
OVINO
|
Vice President, Finance, Chief Accounting Officer (principal accounting officer)
|
February 6, 2014
|
||
|
Gregory F. Covino
|
||||
|
/
S
/ W
ILLIAM
D. Y
OUNG
|
|
Director and Chairman of the Board of Directors
|
|
February 6, 2014
|
|
William D. Young
|
||||
|
/
S
/
A
LEXANDER
J
.
D
ENNER
|
|
Director
|
|
February 6, 2014
|
|
Alexander J. Denner
|
||||
|
/
S
/ C
AROLINE
D. D
ORSA
|
|
Director
|
|
February 6, 2014
|
|
Caroline D. Dorsa
|
||||
|
/
S
/ N
ANCY
L. L
EAMING
|
|
Director
|
|
February 6, 2014
|
|
Nancy L. Leaming
|
||||
|
/
S
/ R
ICHARD
C
.
M
ULLIGAN
|
|
Director
|
|
February 6, 2014
|
|
Richard C. Mulligan
|
||||
|
/
S
/ R
OBERT
W. P
ANGIA
|
|
Director
|
|
February 6, 2014
|
|
Robert W. Pangia
|
||||
|
/
S
/ S
TELIOS
P
APADOPOULOS
|
|
Director
|
|
February 6, 2014
|
|
Stelios Papadopoulos
|
||||
|
/
S
/ B
RIAN
S. P
OSNER
|
Director
|
February 6, 2014
|
||
|
Brian S. Posner
|
||||
|
/
S
/ E
RIC
K. R
OWINSKY
|
Director
|
February 6, 2014
|
||
|
Eric K. Rowinsky
|
||||
|
/
S
/ L
YNN
S
CHENK
|
Director
|
February 6, 2014
|
||
|
Lynn Schenk
|
||||
|
/
S
/ S
TEPHEN
A. S
HERWIN
|
Director
|
February 6, 2014
|
||
|
Stephen A. Sherwin
|
||||
|
|
|
Page Number
|
|
Consolidated Statements of Income
|
|
F-2
|
|
Consolidated Statements of Comprehensive Income
|
F-3
|
|
|
Consolidated Balance Sheets
|
|
F-4
|
|
Consolidated Statements of Cash Flows
|
|
F-5
|
|
Consolidated Statements of Equity
|
|
F-6
|
|
Notes to Consolidated Financial Statements
|
|
F-9
|
|
Report of Independent Registered Public Accounting Firm
|
|
F-69
|
|
|
For the Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
Revenues:
|
|||||||||||
|
Product, net
|
$
|
5,542,331
|
|
$
|
4,166,074
|
|
$
|
3,836,117
|
|
||
|
Unconsolidated joint business
|
1,126,017
|
|
1,137,923
|
|
996,597
|
|
|||||
|
Other
|
263,851
|
|
212,464
|
|
215,920
|
|
|||||
|
Total revenues
|
6,932,199
|
|
5,516,461
|
|
5,048,634
|
|
|||||
|
Cost and expenses:
|
|||||||||||
|
Cost of sales, excluding amortization of acquired intangible assets
|
857,726
|
|
545,494
|
|
466,780
|
|
|||||
|
Research and development
|
1,444,053
|
|
1,334,919
|
|
1,219,602
|
|
|||||
|
Selling, general and administrative
|
1,712,051
|
|
1,277,465
|
|
1,056,133
|
|
|||||
|
Amortization of acquired intangible assets
|
342,948
|
|
202,204
|
|
208,566
|
|
|||||
|
Collaboration profit sharing
|
85,357
|
|
317,895
|
|
317,771
|
|
|||||
|
(Gain) loss on fair value remeasurement of contingent consideration
|
(547
|
)
|
27,202
|
|
36,065
|
|
|||||
|
Restructuring charges
|
—
|
|
2,225
|
|
19,026
|
|
|||||
|
Total cost and expenses
|
4,441,588
|
|
3,707,404
|
|
3,323,943
|
|
|||||
|
Gain on sale of rights
|
24,898
|
|
46,792
|
|
—
|
|
|||||
|
Income from operations
|
2,515,509
|
|
1,855,849
|
|
1,724,691
|
|
|||||
|
Other income (expense), net
|
(34,930
|
)
|
(744
|
)
|
(13,477
|
)
|
|||||
|
Income before income tax expense and equity in loss of investee, net of tax
|
2,480,579
|
|
1,855,105
|
|
1,711,214
|
|
|||||
|
Income tax expense
|
601,014
|
|
470,554
|
|
444,528
|
|
|||||
|
Equity in loss of investee, net of tax
|
17,224
|
|
4,518
|
|
—
|
|
|||||
|
Net income
|
1,862,341
|
|
1,380,033
|
|
1,266,686
|
|
|||||
|
Net income attributable to noncontrolling interests, net of tax
|
—
|
|
—
|
|
32,258
|
|
|||||
|
Net income attributable to Biogen Idec Inc.
|
$
|
1,862,341
|
|
$
|
1,380,033
|
|
$
|
1,234,428
|
|
||
|
Net income per share:
|
|||||||||||
|
Basic earnings per share attributable to Biogen Idec Inc.
|
$
|
7.86
|
|
$
|
5.80
|
|
$
|
5.09
|
|
||
|
Diluted earnings per share attributable to Biogen Idec Inc.
|
$
|
7.81
|
|
$
|
5.76
|
|
$
|
5.04
|
|
||
|
Weighted-average shares used in calculating:
|
|||||||||||
|
Basic earnings per share attributable to Biogen Idec Inc.
|
236,919
|
|
237,938
|
|
242,395
|
|
|||||
|
Diluted earnings per share attributable to Biogen Idec Inc.
|
238,308
|
|
239,740
|
|
245,033
|
|
|||||
|
|
For the Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
Net income attributable to Biogen Idec Inc.
|
$
|
1,862,341
|
|
$
|
1,380,033
|
|
$
|
1,234,428
|
|
||
|
Other comprehensive income:
|
|||||||||||
|
Unrealized gains (losses) on securities available for sale:
|
|||||||||||
|
Unrealized gains (losses) recognized during the period, net of tax of $6,394, $2,940 and $133
|
11,770
|
|
5,080
|
|
(224
|
)
|
|||||
|
Less: reclassification adjustment for (gains) losses included in net income, net of tax of $5,576, $486 and $7,155
|
(10,355
|
)
|
(903
|
)
|
(12,184
|
)
|
|||||
|
Unrealized gains (losses) on securities available for sale, net of tax of $818, $2,454 and $7,288
|
1,415
|
|
4,177
|
|
(12,408
|
)
|
|||||
|
Unrealized gains (losses) on foreign currency forward contracts:
|
|||||||||||
|
Unrealized gains (losses) recognized during the period, net of tax of $1,721, $1,396 and $3,647
|
(26,679
|
)
|
(11,808
|
)
|
32,830
|
|
|||||
|
Less: reclassification adjustment for (gains) losses included in net income, net of tax of $533, $3,360 and $1,268
|
13,716
|
|
(31,713
|
)
|
9,767
|
|
|||||
|
Unrealized gains (losses) on foreign currency forward contracts, net of tax of $1,187, $4,756 and $4,915
|
(12,963
|
)
|
(43,521
|
)
|
42,597
|
|
|||||
|
Unrealized gains (losses) on pension benefit obligation
|
2,096
|
|
(12,656
|
)
|
(9,280
|
)
|
|||||
|
Currency translation adjustment
|
37,012
|
|
23,230
|
|
(25,834
|
)
|
|||||
|
Total other comprehensive income (loss), net of tax
|
27,560
|
|
(28,770
|
)
|
(4,925
|
)
|
|||||
|
Comprehensive income attributable to Biogen Idec Inc.
|
1,889,901
|
|
1,351,263
|
|
1,229,503
|
|
|||||
|
Comprehensive income attributable to noncontrolling interests, net of tax
|
—
|
|
65
|
|
37,161
|
|
|||||
|
Comprehensive income
|
$
|
1,889,901
|
|
$
|
1,351,328
|
|
$
|
1,266,664
|
|
||
|
|
As of December 31,
|
||||||
|
|
2013
|
2012
|
|||||
|
ASSETS
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
602,562
|
|
$
|
570,721
|
|
|
|
Marketable securities
|
620,167
|
|
1,134,989
|
|
|||
|
Accounts receivable, net
|
824,406
|
|
686,848
|
|
|||
|
Due from unconsolidated joint business, net
|
252,662
|
|
268,395
|
|
|||
|
Inventory
|
659,003
|
|
447,373
|
|
|||
|
Other current assets
|
226,134
|
|
136,011
|
|
|||
|
Total current assets
|
3,184,934
|
|
3,244,337
|
|
|||
|
Marketable securities
|
625,772
|
|
2,036,658
|
|
|||
|
Property, plant and equipment, net
|
1,750,710
|
|
1,742,226
|
|
|||
|
Intangible assets, net
|
4,474,653
|
|
1,631,547
|
|
|||
|
Goodwill
|
1,232,916
|
|
1,201,296
|
|
|||
|
Investments and other assets
|
594,350
|
|
274,054
|
|
|||
|
Total assets
|
$
|
11,863,335
|
|
$
|
10,130,118
|
|
|
|
LIABILITIES AND EQUITY
|
|||||||
|
Current liabilities:
|
|||||||
|
Current portion of notes payable and line of credit
|
$
|
3,494
|
|
$
|
453,379
|
|
|
|
Taxes payable
|
179,685
|
|
20,066
|
|
|||
|
Accounts payable
|
219,913
|
|
203,999
|
|
|||
|
Accrued expenses and other
|
1,355,187
|
|
979,945
|
|
|||
|
Total current liabilities
|
1,758,279
|
|
1,657,389
|
|
|||
|
Notes payable and other financing arrangements
|
592,433
|
|
687,396
|
|
|||
|
Long-term deferred tax liability
|
232,554
|
|
217,272
|
|
|||
|
Other long-term liabilities
|
659,231
|
|
604,266
|
|
|||
|
Total liabilities
|
3,242,497
|
|
3,166,323
|
|
|||
|
Commitments and contingencies
|
|
|
|
|
|||
|
Equity:
|
|||||||
|
Biogen Idec Inc. shareholders’ equity
|
|||||||
|
Preferred stock, par value $0.001 per share
|
—
|
|
—
|
|
|||
|
Common stock, par value $0.0005 per share
|
128
|
|
127
|
|
|||
|
Additional paid-in capital
|
4,023,651
|
|
3,854,525
|
|
|||
|
Accumulated other comprehensive loss
|
(27,745
|
)
|
(55,305
|
)
|
|||
|
Retained earnings
|
6,349,135
|
|
4,486,794
|
|
|||
|
Treasury stock, at cost; 19,641 shares and 17,655 shares, respectively
|
(1,724,927
|
)
|
(1,324,618
|
)
|
|||
|
Total Biogen Idec Inc. shareholders’ equity
|
8,620,242
|
|
6,961,523
|
|
|||
|
Noncontrolling interests
|
596
|
|
2,272
|
|
|||
|
Total equity
|
8,620,838
|
|
6,963,795
|
|
|||
|
Total liabilities and equity
|
$
|
11,863,335
|
|
$
|
10,130,118
|
|
|
|
|
For the Years Ended December 31,
|
||||||||||
|
|
2013
|
2012
|
2011
|
||||||||
|
Cash flows from operating activities:
|
|||||||||||
|
Net income
|
$
|
1,862,341
|
|
$
|
1,380,033
|
|
$
|
1,266,686
|
|
||
|
Adjustments to reconcile net income to net cash flows from operating activities:
|
|||||||||||
|
Depreciation and amortization of property, plant and equipment, and intangible assets
|
531,740
|
|
365,648
|
|
358,933
|
|
|||||
|
Share-based compensation
|
136,293
|
|
118,566
|
|
113,005
|
|
|||||
|
Deferred income taxes
|
(245,077
|
)
|
(116,900
|
)
|
153,576
|
|
|||||
|
Other
|
(27,612
|
)
|
28,822
|
|
20,153
|
|
|||||
|
Changes in operating assets and liabilities, net:
|
|||||||||||
|
Accounts receivable
|
(126,753
|
)
|
3,571
|
|
(73,374
|
)
|
|||||
|
Inventory
|
(243,960
|
)
|
(140,309
|
)
|
(59,219
|
)
|
|||||
|
Other assets
|
(160,188
|
)
|
(27,347
|
)
|
(43,241
|
)
|
|||||
|
Accrued expenses and other current liabilities
|
284,049
|
|
273,372
|
|
33,722
|
|
|||||
|
Other liabilities and taxes payable
|
318,512
|
|
34,112
|
|
(36,235
|
)
|
|||||
|
Other
|
15,733
|
|
(39,671
|
)
|
(6,265
|
)
|
|||||
|
Net cash flows provided by operating activities
|
2,345,078
|
|
1,879,897
|
|
1,727,741
|
|
|||||
|
Cash flows from investing activities:
|
|||||||||||
|
Proceeds from sales and maturities of marketable securities
|
5,190,052
|
|
2,749,558
|
|
2,276,720
|
|
|||||
|
Purchases of marketable securities
|
(3,278,091
|
)
|
(3,334,434
|
)
|
(3,696,995
|
)
|
|||||
|
Acquisition of TYSABRI rights
|
(3,262,719
|
)
|
—
|
|
—
|
|
|||||
|
Acquisitions of businesses and variable interest entities, net of cash acquired
|
(15,000
|
)
|
(72,401
|
)
|
(5,000
|
)
|
|||||
|
Purchases of property, plant and equipment
|
(246,281
|
)
|
(254,548
|
)
|
(208,020
|
)
|
|||||
|
Other
|
7,371
|
|
(38,517
|
)
|
(16,999
|
)
|
|||||
|
Net cash flows used in investing activities
|
(1,604,668
|
)
|
(950,342
|
)
|
(1,650,294
|
)
|
|||||
|
Cash flows from financing activities:
|
|||||||||||
|
Purchase of treasury stock
|
(400,309
|
)
|
(984,715
|
)
|
(497,975
|
)
|
|||||
|
Proceeds from issuance of stock for share-based compensation arrangements
|
66,770
|
|
67,493
|
|
314,650
|
|
|||||
|
Excess tax benefit from share-based compensation
|
73,467
|
|
54,738
|
|
50,586
|
|
|||||
|
Acquisition of noncontrolling interests
|
—
|
|
—
|
|
(148,264
|
)
|
|||||
|
Repayments of borrowings
|
(452,340
|
)
|
(2,428
|
)
|
(11,459
|
)
|
|||||
|
Other
|
(4,116
|
)
|
(12,566
|
)
|
(27,400
|
)
|
|||||
|
Net cash flows used in financing activities
|
(716,528
|
)
|
(877,478
|
)
|
(319,862
|
)
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
23,882
|
|
52,077
|
|
(242,415
|
)
|
|||||
|
Effect of exchange rate changes on cash and cash equivalents
|
7,959
|
|
4,102
|
|
(2,641
|
)
|
|||||
|
Cash and cash equivalents, beginning of the year
|
570,721
|
|
514,542
|
|
759,598
|
|
|||||
|
Cash and cash equivalents, end of the year
|
$
|
602,562
|
|
$
|
570,721
|
|
$
|
514,542
|
|
||
|
|
Preferred stock
|
Common stock
|
Additional
paid-in
capital
|
Accumulated
other
comprehensive
loss
|
Retained
earnings
|
Treasury stock
|
Total
Biogen Idec Inc.
shareholders’
equity
|
Noncontrolling
interests
|
Total
equity
|
|||||||||||||||||||||||||||||||||||
|
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
||||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2012
|
—
|
|
$
|
—
|
|
254,237
|
|
$
|
127
|
|
$
|
3,854,525
|
|
$
|
(55,305
|
)
|
$
|
4,486,794
|
|
(17,655
|
)
|
$
|
(1,324,618
|
)
|
$
|
6,961,523
|
|
$
|
2,272
|
|
$
|
6,963,795
|
|
|||||||||||
|
Net income
|
1,862,341
|
|
1,862,341
|
|
—
|
|
1,862,341
|
|
||||||||||||||||||||||||||||||||||||
|
Other comprehensive income, net of tax
|
27,560
|
|
27,560
|
|
—
|
|
27,560
|
|
||||||||||||||||||||||||||||||||||||
|
Deconsolidation of noncontrolling interests
|
—
|
|
(1,676
|
)
|
(1,676
|
)
|
||||||||||||||||||||||||||||||||||||||
|
Repurchase of common stock for Treasury pursuant to the 2011 share repurchase plan, at cost
|
(1,986
|
)
|
(400,309
|
)
|
(400,309
|
)
|
(400,309
|
)
|
||||||||||||||||||||||||||||||||||||
|
Issuance of common stock under stock option and stock purchase plans
|
767
|
|
—
|
|
66,770
|
|
66,770
|
|
66,770
|
|
||||||||||||||||||||||||||||||||||
|
Issuance of common stock under stock award plan
|
969
|
|
1
|
|
(89,747
|
)
|
(89,746
|
)
|
(89,746
|
)
|
||||||||||||||||||||||||||||||||||
|
Compensation expense related to share-based payments
|
146,210
|
|
146,210
|
|
146,210
|
|
||||||||||||||||||||||||||||||||||||||
|
Tax benefit from share-based payments
|
45,893
|
|
45,893
|
|
45,893
|
|
||||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2013
|
—
|
|
$
|
—
|
|
255,973
|
|
$
|
128
|
|
$
|
4,023,651
|
|
$
|
(27,745
|
)
|
$
|
6,349,135
|
|
(19,641
|
)
|
$
|
(1,724,927
|
)
|
$
|
8,620,242
|
|
$
|
596
|
|
$
|
8,620,838
|
|
|||||||||||
|
|
Preferred stock
|
Common stock
|
Additional
paid-in
capital
|
Accumulated
other
comprehensive
loss
|
Retained
earnings
|
Treasury stock
|
Total
Biogen Idec Inc.
shareholders’
equity
|
Noncontrolling
interests
|
Total
equity
|
|||||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2011
|
—
|
|
$
|
—
|
|
255,633
|
|
$
|
128
|
|
$
|
4,185,048
|
|
$
|
(26,535
|
)
|
$
|
3,106,761
|
|
(13,518
|
)
|
$
|
(839,903
|
)
|
$
|
6,425,499
|
|
$
|
1,488
|
|
$
|
6,426,987
|
|
|||||||||||
|
Net income
|
1,380,033
|
|
1,380,033
|
|
—
|
|
1,380,033
|
|
||||||||||||||||||||||||||||||||||||
|
Other comprehensive income, net of tax
|
(28,770
|
)
|
(28,770
|
)
|
65
|
|
(28,705
|
)
|
||||||||||||||||||||||||||||||||||||
|
Distributions to noncontrolling interests
|
—
|
|
1,199
|
|
1,199
|
|
||||||||||||||||||||||||||||||||||||||
|
Capital contributions from noncontrolling interests
|
—
|
|
73
|
|
73
|
|
||||||||||||||||||||||||||||||||||||||
|
Deconsolidation of noncontrolling interests
|
(3
|
)
|
(3
|
)
|
(553
|
)
|
(556
|
)
|
||||||||||||||||||||||||||||||||||||
|
Repurchase of common stock for Treasury pursuant to the 2011 share repurchase plan, at cost
|
(7,811
|
)
|
(984,715
|
)
|
(984,715
|
)
|
(984,715
|
)
|
||||||||||||||||||||||||||||||||||||
|
Retirement of common stock pursuant to the 2011 share repurchase plan, at cost
|
(3,674
|
)
|
(2
|
)
|
(499,998
|
)
|
3,674
|
|
500,000
|
|
—
|
|
—
|
|
||||||||||||||||||||||||||||||
|
Issuance of common stock under stock option and stock purchase plans
|
1,039
|
|
—
|
|
67,493
|
|
67,493
|
|
67,493
|
|
||||||||||||||||||||||||||||||||||
|
Issuance of common stock under stock award plan
|
1,239
|
|
1
|
|
(71,358
|
)
|
(71,357
|
)
|
(71,357
|
)
|
||||||||||||||||||||||||||||||||||
|
Compensation expense related to share-based payments
|
123,956
|
|
123,956
|
|
123,956
|
|
||||||||||||||||||||||||||||||||||||||
|
Tax benefit from share-based payments
|
49,387
|
|
49,387
|
|
49,387
|
|
||||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2012
|
—
|
|
$
|
—
|
|
254,237
|
|
$
|
127
|
|
$
|
3,854,525
|
|
$
|
(55,305
|
)
|
$
|
4,486,794
|
|
(17,655
|
)
|
$
|
(1,324,618
|
)
|
$
|
6,961,523
|
|
$
|
2,272
|
|
$
|
6,963,795
|
|
|||||||||||
|
Preferred stock
|
Common stock
|
Additional
paid-in
capital
|
Accumulated
other
comprehensive
loss
|
Retained
earnings
|
Treasury stock
|
Total
Biogen Idec Inc.
shareholders’
equity
|
Noncontrolling
interests
|
Total
equity
|
||||||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2010
|
8
|
|
$
|
—
|
|
248,200
|
|
$
|
124
|
|
$
|
3,895,103
|
|
$
|
(21,610
|
)
|
$
|
1,872,481
|
|
(7,662
|
)
|
$
|
(349,592
|
)
|
$
|
5,396,506
|
|
$
|
52,937
|
|
$
|
5,449,443
|
|
|||||||||||
|
Net income
|
1,234,428
|
|
1,234,428
|
|
32,258
|
|
1,266,686
|
|
||||||||||||||||||||||||||||||||||||
|
Other comprehensive income, net of tax
|
(4,925
|
)
|
(4,925
|
)
|
4,903
|
|
(22
|
)
|
||||||||||||||||||||||||||||||||||||
|
Distributions to noncontrolling interests
|
(148
|
)
|
(148
|
)
|
(26,914
|
)
|
(27,062
|
)
|
||||||||||||||||||||||||||||||||||||
|
Acquisitions of noncontrolling interests
|
(125,641
|
)
|
(125,641
|
)
|
(61,696
|
)
|
(187,337
|
)
|
||||||||||||||||||||||||||||||||||||
|
Repurchase of common stock for Treasury pursuant to the 2011 share repurchase plan, at cost
|
(6,018
|
)
|
(497,975
|
)
|
(497,975
|
)
|
(497,975
|
)
|
||||||||||||||||||||||||||||||||||||
|
Issuance of common stock under stock option and stock purchase plans
|
5,458
|
|
3
|
|
306,982
|
|
162
|
|
7,664
|
|
314,649
|
|
314,649
|
|
||||||||||||||||||||||||||||||
|
Issuance of common stock under stock award plan
|
1,482
|
|
1
|
|
(50,954
|
)
|
(50,953
|
)
|
(50,953
|
)
|
||||||||||||||||||||||||||||||||||
|
Conversion of preferred stock
|
(8
|
)
|
493
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||||||||||||||||
|
Compensation expense related to share-based payments
|
117,347
|
|
117,347
|
|
117,347
|
|
||||||||||||||||||||||||||||||||||||||
|
Recharacterization of share-based awards from equity to cash-settled due to restructuring
|
(8,172
|
)
|
(8,172
|
)
|
(8,172
|
)
|
||||||||||||||||||||||||||||||||||||||
|
Tax benefit from share-based payments
|
50,383
|
|
50,383
|
|
50,383
|
|
||||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2011
|
—
|
|
$
|
—
|
|
255,633
|
|
$
|
128
|
|
$
|
4,185,048
|
|
$
|
(26,535
|
)
|
$
|
3,106,761
|
|
(13,518
|
)
|
$
|
(839,903
|
)
|
$
|
6,425,499
|
|
$
|
1,488
|
|
$
|
6,426,987
|
|
|||||||||||
|
1.
|
Summary of Significant Accounting Policies
|
|
•
|
Medicaid rebates relate to our estimated obligations to states under established reimbursement arrangements. Rebate accruals are recorded in the same period the related revenue is recognized resulting in a reduction of product revenue and the establishment of a liability which is included in other current liabilities. Our liability for Medicaid rebates consists of estimates for claims that a state will make for the current quarter, claims for prior quarters that have been estimated for which an invoice has not been received, invoices received for claims from the prior quarters that have not been paid, and an estimate of potential claims that will be made for inventory that exists in the distribution channel at period end.
|
|
•
|
Governmental rebates or chargebacks, including VA and PHS discounts, represent our estimated obligations resulting from contractual commitments to sell products to qualified healthcare providers at prices lower than the list prices we charge to wholesalers which provide those products. The wholesaler charges us for the difference between what the wholesaler pays for the products and the ultimate selling price to the qualified healthcare providers. Rebate and chargeback reserves are established in the same period as the related revenue is recognized resulting in a reduction in product revenue and accounts receivable. Chargeback amounts are generally determined at the time of resale to the qualified healthcare provider from the wholesaler, and we generally issue credits for such amounts within a few weeks of the wholesaler notifying us about the resale. Our reserves for VA, PHS and chargebacks consists of amounts that we expect to issue for inventory that exists at the wholesalers that we expect will be sold to qualified healthcare providers and chargebacks that wholesalers have claimed for which we have not issued a credit.
|
|
•
|
Managed care rebates represent our estimated obligations to third parties, primarily pharmacy benefit managers. Rebate accruals are recorded in the same period the related revenue is recognized resulting in a reduction of product revenue and the establishment of a liability which is included in accrued expenses and other current liabilities. These rebates result from performance-based goals that are primarily based on attaining contractually specified sales volumes and growth and price increase limit allowances (price protection). The calculation of the accrual for these rebates is based on an estimate of the customer’s buying patterns and the resulting applicable contractual rebate rate(s) to be earned over a contractual period.
|
|
•
|
Other governmental rebates or applicable allowances primarily relate to mandatory rebates and discounts in markets where government-sponsored healthcare systems are the primary payors for healthcare.
|
|
•
|
Level 1
— Fair values are determined utilizing quoted prices (unadjusted) in active markets for identical assets or liabilities that we have the ability to access;
|
|
•
|
Level 2
— Fair values are determined by utilizing quoted prices for identical or similar assets and liabilities in active markets or other market observable inputs such as interest rates, yield curves and foreign currency spot rates; and
|
|
•
|
Level 3
— Prices or valuations that require inputs that are both significant to the fair value measurement and unobservable.
|
|
Asset Category
|
Useful Lives
|
|
Land
|
Not depreciated
|
|
Buildings
|
15 to 40 years
|
|
Leasehold Improvements
|
Lesser of the useful life or the term of the respective lease
|
|
Furniture and Fixtures
|
5 to 7 years
|
|
Machinery and Equipment
|
5 to 20 years
|
|
Computer Software and Hardware
|
3 to 5 years
|
|
2.
|
Acquisitions
|
|
3.
|
Gain on Sale of Rights
|
|
4.
|
Accounts Receivable
|
|
|
As of December 31, 2013
|
||||||||||
|
(In millions)
|
Current
Balance Included
within Accounts
Receivable, net
|
Non-Current
Balance Included
within Investments
and Other Assets
|
Total
|
||||||||
|
Spain
|
$
|
113.3
|
|
$
|
6.8
|
|
$
|
120.1
|
|
||
|
Italy
|
$
|
76.1
|
|
$
|
2.4
|
|
$
|
78.5
|
|
||
|
Portugal
|
$
|
10.4
|
|
$
|
8.2
|
|
$
|
18.6
|
|
||
|
|
As of December 31, 2012
|
||||||||||
|
(In millions)
|
Current
Balance Included
within Accounts
Receivable, net
|
Non-Current
Balance Included
within Investments
and Other Assets
|
Total
|
||||||||
|
Spain
|
$
|
78.9
|
|
$
|
—
|
|
$
|
78.9
|
|
||
|
Italy
|
$
|
94.4
|
|
$
|
10.2
|
|
$
|
104.6
|
|
||
|
Portugal
|
$
|
16.6
|
|
$
|
7.4
|
|
$
|
24.0
|
|
||
|
(In millions)
|
Discounts
|
Contractual
Adjustments
|
Returns
|
Total
|
|||||||||||
|
2013
|
|||||||||||||||
|
Beginning balance
|
$
|
14.3
|
|
$
|
196.0
|
|
$
|
26.8
|
|
$
|
237.1
|
|
|||
|
Current provisions relating to sales in current year
|
236.3
|
|
851.4
|
|
22.9
|
|
1,110.6
|
|
|||||||
|
Adjustments relating to prior years
|
(0.7
|
)
|
(16.4
|
)
|
1.1
|
|
(16.0
|
)
|
|||||||
|
Payments/returns relating to sales in current year
|
(189.7
|
)
|
(560.4
|
)
|
—
|
|
(750.1
|
)
|
|||||||
|
Payments/returns relating to sales in prior years
|
(13.2
|
)
|
(135.0
|
)
|
(17.1
|
)
|
(165.3
|
)
|
|||||||
|
Ending balance
|
$
|
47.0
|
|
$
|
335.6
|
|
$
|
33.7
|
|
$
|
416.3
|
|
|||
|
(In millions)
|
Discounts
|
Contractual
Adjustments
|
Returns
|
Total
|
|||||||||||
|
2012
|
|||||||||||||||
|
Beginning balance
|
$
|
11.9
|
|
$
|
120.0
|
|
$
|
23.7
|
|
$
|
155.6
|
|
|||
|
Current provisions relating to sales in current year
|
96.5
|
|
534.2
|
|
22.0
|
|
652.7
|
|
|||||||
|
Adjustments relating to prior years
|
(0.3
|
)
|
(4.7
|
)
|
(0.1
|
)
|
(5.1
|
)
|
|||||||
|
Payments/returns relating to sales in current year
|
(83.6
|
)
|
(363.2
|
)
|
(4.3
|
)
|
(451.1
|
)
|
|||||||
|
Payments/returns relating to sales in prior years
|
(10.2
|
)
|
(90.3
|
)
|
(14.5
|
)
|
(115.0
|
)
|
|||||||
|
Ending balance
|
$
|
14.3
|
|
$
|
196.0
|
|
$
|
26.8
|
|
$
|
237.1
|
|
|||
|
(In millions)
|
Discounts
|
Contractual
Adjustments
|
Returns
|
Total
|
|||||||||||
|
2011
|
|||||||||||||||
|
Beginning balance
|
$
|
13.9
|
|
$
|
107.0
|
|
$
|
21.1
|
|
$
|
142.0
|
|
|||
|
Current provisions relating to sales in current year
|
84.3
|
|
372.1
|
|
15.7
|
|
472.1
|
|
|||||||
|
Adjustments relating to prior years
|
—
|
|
(14.0
|
)
|
(0.9
|
)
|
(14.9
|
)
|
|||||||
|
Payments/returns relating to sales in current year
|
(73.3
|
)
|
(277.0
|
)
|
(0.4
|
)
|
(350.7
|
)
|
|||||||
|
Payments/returns relating to sales in prior years
|
(13.0
|
)
|
(68.1
|
)
|
(11.8
|
)
|
(92.9
|
)
|
|||||||
|
Ending balance
|
$
|
11.9
|
|
$
|
120.0
|
|
$
|
23.7
|
|
$
|
155.6
|
|
|||
|
|
As of December 31,
|
||||||
|
(In millions)
|
2013
|
2012
|
|||||
|
Reduction of accounts receivable
|
$
|
151.4
|
|
$
|
46.1
|
|
|
|
Component of accrued expenses and other
|
264.9
|
|
191.0
|
|
|||
|
Total reserves
|
$
|
416.3
|
|
$
|
237.1
|
|
|
|
6.
|
Inventory
|
|
|
As of December 31,
|
||||||
|
(In millions)
|
2013
|
2012
|
|||||
|
Raw materials
|
$
|
115.0
|
|
$
|
101.8
|
|
|
|
Work in process
|
435.4
|
|
244.9
|
|
|||
|
Finished goods
|
108.6
|
|
100.7
|
|
|||
|
Total inventory
|
$
|
659.0
|
|
$
|
447.4
|
|
|
|
|
As of December 31,
|
||||||
|
(In millions)
|
2013
|
2012
|
|||||
|
AVONEX
|
$
|
176.9
|
|
$
|
144.0
|
|
|
|
TYSABRI
|
170.9
|
|
114.8
|
|
|||
|
TECFIDERA
|
33.6
|
|
—
|
|
|||
|
Other
|
162.6
|
|
86.8
|
|
|||
|
Total finished goods and work in process
|
544.0
|
|
345.6
|
|
|||
|
Raw materials
|
115.0
|
|
101.8
|
|
|||
|
Total inventory
|
$
|
659.0
|
|
$
|
447.4
|
|
|
|
7.
|
Intangible Assets and Goodwill
|
|
|
|
As of December 31, 2013
|
As of December 31, 2012
|
||||||||||||||||||||||
|
(In millions)
|
Estimated Life
|
Cost
|
Accumulated
Amortization
|
Net
|
Cost
|
Accumulated
Amortization
|
Net
|
||||||||||||||||||
|
Out-licensed patents
|
13-23 years
|
$
|
578.0
|
|
$
|
(450.8
|
)
|
$
|
127.2
|
|
$
|
578.0
|
|
$
|
(421.0
|
)
|
$
|
157.0
|
|
||||||
|
Developed technology
|
15-23 years
|
3,005.3
|
|
(2,165.4
|
)
|
839.9
|
|
3,005.3
|
|
(1,965.7
|
)
|
1,039.6
|
|
||||||||||||
|
In-process research and development
|
Indefinite until commercialization
|
327.4
|
|
—
|
|
327.4
|
|
330.1
|
|
—
|
|
330.1
|
|
||||||||||||
|
Trademarks and tradenames
|
Indefinite
|
64.0
|
|
—
|
|
64.0
|
|
64.0
|
|
—
|
|
64.0
|
|
||||||||||||
|
Acquired and in-licensed rights and patents
|
6-17 years
|
3,240.0
|
|
(123.8
|
)
|
3,116.2
|
|
53.7
|
|
(12.9
|
)
|
40.8
|
|
||||||||||||
|
Total intangible assets
|
$
|
7,214.7
|
|
$
|
(2,740.0
|
)
|
$
|
4,474.7
|
|
$
|
4,031.1
|
|
$
|
(2,399.6
|
)
|
$
|
1,631.5
|
|
|||||||
|
(In millions)
|
As of December 31, 2013
|
||
|
2014
|
$
|
426.3
|
|
|
2015
|
336.4
|
|
|
|
2016
|
322.7
|
|
|
|
2017
|
327.5
|
|
|
|
2018
|
330.2
|
|
|
|
Total
|
$
|
1,743.1
|
|
|
|
As of December 31,
|
||||||
|
(In millions)
|
2013
|
2012
|
|||||
|
Goodwill, beginning of year
|
$
|
1,201.3
|
|
$
|
1,146.3
|
|
|
|
Increase to goodwill
|
35.7
|
|
48.2
|
|
|||
|
Other
|
(4.1
|
)
|
6.8
|
|
|||
|
Goodwill, end of year
|
$
|
1,232.9
|
|
$
|
1,201.3
|
|
|
|
8.
|
Fair Value Measurements
|
|
(In millions)
|
As of
December 31, 2013 |
Quoted
Prices in
Active
Markets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
|||||||||||
|
Assets:
|
|||||||||||||||
|
Cash equivalents
|
$
|
424.7
|
|
$
|
—
|
|
$
|
424.7
|
|
$
|
—
|
|
|||
|
Marketable debt securities:
|
|||||||||||||||
|
Corporate debt securities
|
439.8
|
|
—
|
|
439.8
|
|
—
|
|
|||||||
|
Government securities
|
674.7
|
|
—
|
|
674.7
|
|
—
|
|
|||||||
|
Mortgage and other asset backed securities
|
131.4
|
|
—
|
|
131.4
|
|
—
|
|
|||||||
|
Marketable equity securities
|
11.2
|
|
11.2
|
|
—
|
|
—
|
|
|||||||
|
Venture capital investments
|
21.9
|
|
—
|
|
—
|
|
21.9
|
|
|||||||
|
Derivative contracts
|
3.8
|
|
—
|
|
3.8
|
|
—
|
|
|||||||
|
Plan assets for deferred compensation
|
22.7
|
|
—
|
|
22.7
|
|
—
|
|
|||||||
|
Total
|
$
|
1,730.2
|
|
$
|
11.2
|
|
$
|
1,697.1
|
|
$
|
21.9
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Derivative contracts
|
$
|
23.5
|
|
$
|
—
|
|
$
|
23.5
|
|
$
|
—
|
|
|||
|
Contingent consideration obligations
|
280.9
|
|
—
|
|
—
|
|
280.9
|
|
|||||||
|
Total
|
$
|
304.4
|
|
$
|
—
|
|
$
|
23.5
|
|
$
|
280.9
|
|
|||
|
(In millions)
|
As of
December 31, 2012 |
Quoted
Prices
in Active
Markets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
|||||||||||
|
Assets:
|
|||||||||||||||
|
Cash equivalents
|
$
|
439.4
|
|
$
|
—
|
|
$
|
439.4
|
|
$
|
—
|
|
|||
|
Marketable debt securities:
|
|||||||||||||||
|
Corporate debt securities
|
1,001.0
|
|
—
|
|
1,001.0
|
|
—
|
|
|||||||
|
Government securities
|
1,657.8
|
|
—
|
|
1,657.8
|
|
—
|
|
|||||||
|
Mortgage and other asset backed securities
|
512.9
|
|
—
|
|
512.9
|
|
—
|
|
|||||||
|
Marketable equity securities
|
9.0
|
|
9.0
|
|
—
|
|
—
|
|
|||||||
|
Venture capital investments
|
20.3
|
|
—
|
|
—
|
|
20.3
|
|
|||||||
|
Derivative contracts
|
1.8
|
|
—
|
|
1.8
|
|
—
|
|
|||||||
|
Plan assets for deferred compensation
|
14.3
|
|
—
|
|
14.3
|
|
—
|
|
|||||||
|
Total
|
$
|
3,656.5
|
|
$
|
9.0
|
|
$
|
3,627.2
|
|
$
|
20.3
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Derivative contracts
|
$
|
14.4
|
|
$
|
—
|
|
$
|
14.4
|
|
$
|
—
|
|
|||
|
Contingent consideration obligations
|
293.9
|
|
—
|
|
—
|
|
293.9
|
|
|||||||
|
Total
|
$
|
308.3
|
|
$
|
—
|
|
$
|
14.4
|
|
$
|
293.9
|
|
|||
|
|
As of December 31,
|
||||||
|
(In millions)
|
2013
|
2012
|
|||||
|
Fair value, beginning of year
|
$
|
20.3
|
|
$
|
23.5
|
|
|
|
Unrealized gains included in earnings
|
10.5
|
|
5.4
|
|
|||
|
Unrealized losses included in earnings
|
(6.3
|
)
|
(9.2
|
)
|
|||
|
Purchases
|
0.7
|
|
0.6
|
|
|||
|
Settlements
|
(3.3
|
)
|
—
|
|
|||
|
Fair value, end of year
|
$
|
21.9
|
|
$
|
20.3
|
|
|
|
|
As of December 31,
|
||||||
|
(In millions)
|
2013
|
2012
|
|||||
|
Notes payable to Fumedica
|
$
|
17.5
|
|
$
|
20.0
|
|
|
|
Credit Facility
|
—
|
|
—
|
|
|||
|
6.0% Senior Notes due March 1, 2013
|
—
|
|
453.7
|
|
|||
|
6.875% Senior Notes due March 1, 2018
|
647.9
|
|
681.6
|
|
|||
|
Total
|
$
|
665.4
|
|
$
|
1,155.3
|
|
|
|
|
As of December 31,
|
||||||
|
(In millions)
|
2013
|
2012
|
|||||
|
Fair value, beginning of year
|
$
|
293.9
|
|
$
|
151.0
|
|
|
|
Additions
|
—
|
|
122.2
|
|
|||
|
Changes in fair value
|
(0.5
|
)
|
27.2
|
|
|||
|
Payments
|
(12.5
|
)
|
(6.5
|
)
|
|||
|
Fair value, end of year
|
$
|
280.9
|
|
$
|
293.9
|
|
|
|
As of December 31, 2013 (In millions)
|
Fair
Value
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Amortized
Cost
|
|||||||||||
|
Available-for-sale:
|
|||||||||||||||
|
Corporate debt securities
|
|||||||||||||||
|
Current
|
$
|
100.7
|
|
$
|
—
|
|
$
|
—
|
|
$
|
100.7
|
|
|||
|
Non-current
|
339.1
|
|
0.4
|
|
(0.1
|
)
|
338.8
|
|
|||||||
|
Government securities
|
|||||||||||||||
|
Current
|
519.5
|
|
—
|
|
—
|
|
519.5
|
|
|||||||
|
Non-current
|
155.2
|
|
—
|
|
(0.1
|
)
|
155.3
|
|
|||||||
|
Mortgage and other asset backed securities
|
|||||||||||||||
|
Current
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Non-current
|
131.4
|
|
—
|
|
(0.1
|
)
|
131.5
|
|
|||||||
|
Total marketable debt securities
|
$
|
1,245.9
|
|
$
|
0.4
|
|
$
|
(0.3
|
)
|
$
|
1,245.8
|
|
|||
|
Marketable equity securities, non-current
|
$
|
11.2
|
|
$
|
8.7
|
|
$
|
—
|
|
$
|
2.5
|
|
|||
|
As of December 31, 2012 (In millions)
|
Fair
Value
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Amortized
Cost
|
|||||||||||
|
Available-for-sale:
|
|||||||||||||||
|
Corporate debt securities
|
|||||||||||||||
|
Current
|
$
|
346.9
|
|
$
|
0.3
|
|
$
|
—
|
|
$
|
346.6
|
|
|||
|
Non-current
|
654.1
|
|
2.8
|
|
(0.6
|
)
|
651.9
|
|
|||||||
|
Government securities
|
|||||||||||||||
|
Current
|
783.4
|
|
0.3
|
|
—
|
|
783.1
|
|
|||||||
|
Non-current
|
874.4
|
|
0.8
|
|
—
|
|
873.6
|
|
|||||||
|
Mortgage and other asset backed securities
|
|||||||||||||||
|
Current
|
4.8
|
|
—
|
|
—
|
|
4.8
|
|
|||||||
|
Non-current
|
508.1
|
|
1.4
|
|
(1.3
|
)
|
508.0
|
|
|||||||
|
Total marketable debt securities
|
$
|
3,171.7
|
|
$
|
5.6
|
|
$
|
(1.9
|
)
|
$
|
3,168.0
|
|
|||
|
Marketable equity securities, non-current
|
$
|
9.0
|
|
$
|
3.0
|
|
$
|
—
|
|
$
|
6.0
|
|
|||
|
|
As of December 31,
|
||||||
|
(In millions)
|
2013
|
2012
|
|||||
|
Commercial paper
|
$
|
1.2
|
|
$
|
40.7
|
|
|
|
Overnight reverse repurchase agreements
|
22.4
|
|
67.4
|
|
|||
|
Short-term debt securities
|
401.1
|
|
331.3
|
|
|||
|
Total
|
$
|
424.7
|
|
$
|
439.4
|
|
|
|
|
As of December 31, 2013
|
As of December 31, 2012
|
|||||||||||||
|
(In millions)
|
Estimated
Fair Value
|
Amortized
Cost
|
Estimated
Fair Value
|
Amortized
Cost
|
|||||||||||
|
Due in one year or less
|
$
|
620.2
|
|
$
|
620.2
|
|
$
|
1,135.0
|
|
$
|
1,134.5
|
|
|||
|
Due after one year through five years
|
573.1
|
|
572.9
|
|
1,744.3
|
|
1,741.2
|
|
|||||||
|
Due after five years
|
52.6
|
|
52.7
|
|
292.4
|
|
292.3
|
|
|||||||
|
Total available-for-sale securities
|
$
|
1,245.9
|
|
$
|
1,245.8
|
|
$
|
3,171.7
|
|
$
|
3,168.0
|
|
|||
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2013
|
2012
|
2011
|
||||||||
|
Proceeds from maturities and sales
|
$
|
5,190.1
|
|
$
|
2,749.6
|
|
$
|
2,276.7
|
|
||
|
Realized gains
|
$
|
6.6
|
|
$
|
2.1
|
|
$
|
3.9
|
|
||
|
Realized losses
|
$
|
2.1
|
|
$
|
3.5
|
|
$
|
2.3
|
|
||
|
10.
|
Derivative Instruments
|
|
|
Notional Amount
As of December 31,
|
||||||
|
Foreign Currency: (In millions)
|
2013
|
2012
|
|||||
|
Euro
|
$
|
636.3
|
|
$
|
492.2
|
|
|
|
Canadian dollar
|
34.0
|
|
31.8
|
|
|||
|
British pound sterling
|
72.3
|
|
—
|
|
|||
|
Total foreign currency forward contracts
|
$
|
742.6
|
|
$
|
524.0
|
|
|
|
For the Years Ended December 31,
|
||||||||||||||||||||||||||
|
Net Gains/(Losses)
Reclassified from AOCI into Net Income
(Effective Portion)
|
Net Gains/(Losses)
Recognized into Net Income
(Ineffective Portion)
|
|||||||||||||||||||||||||
|
Location
|
2013
|
2012
|
2011
|
Location
|
2013
|
2012
|
2011
|
|||||||||||||||||||
|
Revenue
|
$
|
(13.2
|
)
|
$
|
35.1
|
|
$
|
(36.9
|
)
|
Other income (expense)
|
$
|
(0.2
|
)
|
$
|
4.8
|
|
$
|
(3.9
|
)
|
|||||||
|
(In millions)
|
Balance Sheet Location
|
Fair Value
As of December 31, 2013 |
||
|
Hedging Instruments:
|
||||
|
Asset derivatives
|
Other current assets
|
$
|
0.6
|
|
|
Liability derivatives
|
Accrued expenses and other
|
$
|
23.4
|
|
|
Other Derivatives:
|
||||
|
Asset derivatives
|
Other current assets
|
$
|
3.2
|
|
|
Liability derivatives
|
Accrued expenses and other
|
$
|
0.1
|
|
|
(In millions)
|
Balance Sheet Location
|
Fair Value
As of December 31, 2012 |
||
|
Hedging Instruments:
|
||||
|
Asset derivatives
|
Other current assets
|
$
|
0.6
|
|
|
Liability derivatives
|
Accrued expenses and other
|
$
|
11.5
|
|
|
Other Derivatives:
|
||||
|
Asset derivatives
|
Other current assets
|
$
|
1.2
|
|
|
Liability derivatives
|
Accrued expenses and other
|
$
|
2.9
|
|
|
11.
|
Property, Plant and Equipment
|
|
|
As of December 31,
|
||||||
|
(In millions)
|
2013
|
2012
|
|||||
|
Land
|
$
|
59.7
|
|
$
|
55.7
|
|
|
|
Buildings
|
961.5
|
|
902.5
|
|
|||
|
Leasehold improvements
|
139.6
|
|
107.3
|
|
|||
|
Machinery and equipment
|
944.5
|
|
882.0
|
|
|||
|
Computer software and hardware
|
559.2
|
|
476.6
|
|
|||
|
Furniture and fixtures
|
60.3
|
|
46.9
|
|
|||
|
Construction in progress
|
144.2
|
|
212.3
|
|
|||
|
Total cost
|
2,869.0
|
|
2,683.3
|
|
|||
|
Less: accumulated depreciation
|
(1,118.3
|
)
|
(941.1
|
)
|
|||
|
Total property, plant and equipment, net
|
$
|
1,750.7
|
|
$
|
1,742.2
|
|
|
|
12.
|
Indebtedness
|
|
|
As of December 31,
|
||||||
|
(In millions)
|
2013
|
2012
|
|||||
|
Current portion:
|
|||||||
|
6.0% Senior notes due March 1, 2013
|
$
|
—
|
|
$
|
450.0
|
|
|
|
Note payable to Fumedica
|
3.5
|
|
3.4
|
|
|||
|
Credit facility
|
—
|
|
—
|
|
|||
|
Current portion of notes payable and line of credit
|
$
|
3.5
|
|
$
|
453.4
|
|
|
|
Non-current portion:
|
|||||||
|
6.875% Senior notes due March 1, 2018
|
580.1
|
|
586.4
|
|
|||
|
Note payable to Fumedica
|
12.3
|
|
14.5
|
|
|||
|
Financing arrangement for the construction of the Cambridge facilities
|
—
|
|
86.5
|
|
|||
|
Non-current portion of notes payable and other financing arrangements
|
$
|
592.4
|
|
$
|
687.4
|
|
|
|
(In millions)
|
As of December 31, 2013
|
||
|
2014
|
$
|
3.5
|
|
|
2015
|
3.6
|
|
|
|
2016
|
3.6
|
|
|
|
2017
|
3.6
|
|
|
|
2018
|
553.6
|
|
|
|
2019 and thereafter
|
—
|
|
|
|
Total
|
$
|
567.9
|
|
|
13.
|
Equity
|
|
|
As of December 31, 2013
|
As of December 31, 2012
|
|||||||||||||||
|
(In thousands)
|
Authorized
|
Issued
|
Outstanding
|
Authorized
|
Issued
|
Outstanding
|
|||||||||||
|
Series A
|
1,750
|
|
—
|
|
—
|
|
1,750
|
|
—
|
|
—
|
|
|||||
|
Series X junior participating
|
1,000
|
|
—
|
|
—
|
|
1,000
|
|
—
|
|
—
|
|
|||||
|
Undesignated
|
5,250
|
|
—
|
|
—
|
|
5,250
|
|
—
|
|
—
|
|
|||||
|
Total preferred stock
|
8,000
|
|
—
|
|
—
|
|
8,000
|
|
—
|
|
—
|
|
|||||
|
|
As of December 31, 2013
|
As of December 31, 2012
|
|||||||||||||||
|
(In thousands)
|
Authorized
|
Issued
|
Outstanding
|
Authorized
|
Issued
|
Outstanding
|
|||||||||||
|
Common stock
|
1,000,000
|
|
255,973
|
|
236,332
|
|
1,000,000
|
|
254,237
|
|
236,582
|
|
|||||
|
14.
|
Accumulated Other Comprehensive Income (Loss)
|
|
(In millions)
|
Unrealized Gains (Losses) on Securities Available for Sale
|
Unrealized Gains (Losses) on Foreign Currency Forward Contracts
|
Unfunded Status of Postretirement Benefit Plans
|
Translation Adjustments
|
Total
|
||||||||||||||
|
Balance, December 31, 2012
|
$
|
4.2
|
|
$
|
(10.7
|
)
|
$
|
(21.7
|
)
|
$
|
(27.1
|
)
|
$
|
(55.3
|
)
|
||||
|
Other comprehensive income (loss) before reclassifications
|
11.8
|
|
(26.7
|
)
|
2.1
|
|
37.1
|
|
24.3
|
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive income (loss)
|
(10.4
|
)
|
13.7
|
|
—
|
|
—
|
|
3.3
|
|
|||||||||
|
Net current period other comprehensive income (loss)
|
1.4
|
|
(13.0
|
)
|
2.1
|
|
37.1
|
|
27.6
|
|
|||||||||
|
Balance, December 31, 2013
|
$
|
5.6
|
|
$
|
(23.7
|
)
|
$
|
(19.6
|
)
|
$
|
10.0
|
|
$
|
(27.7
|
)
|
||||
|
(In millions)
|
Unrealized Gains (Losses) on Securities Available for Sale
|
Unrealized Gains (Losses) on Foreign Currency Forward Contracts
|
Unfunded Status of Postretirement Benefit Plans
|
Translation Adjustments
|
Total
|
||||||||||||||
|
Balance, December 31, 2011
|
$
|
—
|
|
$
|
32.8
|
|
$
|
(9.0
|
)
|
$
|
(50.3
|
)
|
$
|
(26.5
|
)
|
||||
|
Other comprehensive income (loss) before reclassifications
|
5.1
|
|
(11.8
|
)
|
(12.7
|
)
|
23.2
|
|
3.8
|
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive income (loss)
|
(0.9
|
)
|
(31.7
|
)
|
—
|
|
—
|
|
(32.6
|
)
|
|||||||||
|
Net current period other comprehensive income (loss)
|
4.2
|
|
(43.5
|
)
|
(12.7
|
)
|
23.2
|
|
(28.8
|
)
|
|||||||||
|
Balance, December 31, 2012
|
$
|
4.2
|
|
$
|
(10.7
|
)
|
$
|
(21.7
|
)
|
$
|
(27.1
|
)
|
$
|
(55.3
|
)
|
||||
|
(In millions)
|
Unrealized Gains (Losses) on Securities Available for Sale
|
Unrealized Gains (Losses) on Foreign Currency Forward Contracts
|
Unfunded Status of Postretirement Benefit Plans
|
Translation Adjustments
|
Total
|
||||||||||||||
|
Balance, December 31, 2010
|
$
|
12.4
|
|
$
|
(9.8
|
)
|
$
|
0.2
|
|
$
|
(24.4
|
)
|
$
|
(21.6
|
)
|
||||
|
Other comprehensive income (loss) before reclassifications
|
(0.2
|
)
|
32.8
|
|
(9.2
|
)
|
(25.9
|
)
|
(2.5
|
)
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive income (loss)
|
(12.2
|
)
|
9.8
|
|
—
|
|
—
|
|
(2.4
|
)
|
|||||||||
|
Net current period other comprehensive income (loss)
|
(12.4
|
)
|
42.6
|
|
(9.2
|
)
|
(25.9
|
)
|
(4.9
|
)
|
|||||||||
|
Balance, December 31, 2011
|
$
|
—
|
|
$
|
32.8
|
|
$
|
(9.0
|
)
|
$
|
(50.3
|
)
|
$
|
(26.5
|
)
|
||||
|
(In millions)
|
Income Statement Location
|
Amounts Reclassified from
Accumulated Other Comprehensive Income
|
||||||||||
|
For the Years Ended December 31,
|
||||||||||||
|
2013
|
2012
|
2011
|
||||||||||
|
Gains (losses) on securities available for sale
|
Other income (expense)
|
$
|
15.9
|
|
$
|
1.4
|
|
$
|
19.4
|
|
||
|
Income tax benefit (expense)
|
(5.5
|
)
|
(0.5
|
)
|
(7.2
|
)
|
||||||
|
Gains (losses) on foreign currency forward contracts
|
Revenues
|
(13.2
|
)
|
35.1
|
|
(11.1
|
)
|
|||||
|
Income tax benefit (expense)
|
(0.5
|
)
|
(3.4
|
)
|
1.3
|
|
||||||
|
Total reclassifications, net of tax
|
$
|
(3.3
|
)
|
$
|
32.6
|
|
$
|
2.4
|
|
|||
|
15.
|
Earnings per Share
|
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2013
|
2012
|
2011
|
||||||||
|
Numerator:
|
|||||||||||
|
Net income attributable to Biogen Idec Inc.
|
$
|
1,862.3
|
|
$
|
1,380.0
|
|
$
|
1,234.4
|
|
||
|
Adjustment for net income allocable to preferred stock
|
—
|
|
—
|
|
(0.5
|
)
|
|||||
|
Net income used in calculating basic and diluted earnings per share
|
$
|
1,862.3
|
|
$
|
1,380.0
|
|
$
|
1,233.9
|
|
||
|
Denominator:
|
|||||||||||
|
Weighted average number of common shares outstanding
|
236.9
|
|
237.9
|
|
242.4
|
|
|||||
|
Effect of dilutive securities:
|
|||||||||||
|
Stock options and employee stock purchase plan
|
0.3
|
|
0.5
|
|
1.0
|
|
|||||
|
Time-vested restricted stock units
|
0.8
|
|
1.0
|
|
1.3
|
|
|||||
|
Market stock units
|
0.3
|
|
0.3
|
|
0.3
|
|
|||||
|
Dilutive potential common shares
|
1.4
|
|
1.8
|
|
2.6
|
|
|||||
|
Shares used in calculating diluted earnings per share
|
238.3
|
|
239.7
|
|
245.0
|
|
|||||
|
16.
|
Share-based Payments
|
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2013
|
2012
|
2011
|
||||||||
|
Research and development
|
$
|
95.6
|
|
$
|
74.7
|
|
$
|
62.0
|
|
||
|
Selling, general and administrative
|
160.3
|
|
109.6
|
|
88.7
|
|
|||||
|
Restructuring charges
|
—
|
|
—
|
|
(0.6
|
)
|
|||||
|
Subtotal
|
255.9
|
|
184.3
|
|
150.1
|
|
|||||
|
Capitalized share-based compensation costs
|
(9.8
|
)
|
(5.4
|
)
|
(4.5
|
)
|
|||||
|
Share-based compensation expense included in total cost and expenses
|
246.1
|
|
178.9
|
|
145.6
|
|
|||||
|
Income tax effect
|
(73.3
|
)
|
(53.4
|
)
|
(44.6
|
)
|
|||||
|
Share-based compensation expense included in net income attributable to Biogen Idec Inc.
|
$
|
172.8
|
|
$
|
125.5
|
|
$
|
101.0
|
|
||
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2013
|
2012
|
2011
|
||||||||
|
Stock options
|
$
|
0.6
|
|
$
|
2.3
|
|
$
|
5.9
|
|
||
|
Market stock units
|
32.8
|
|
23.3
|
|
14.6
|
|
|||||
|
Time-vested restricted stock units
|
103.5
|
|
93.0
|
|
89.6
|
|
|||||
|
Performance-vested restricted stock units settled in shares
|
—
|
|
0.1
|
|
1.0
|
|
|||||
|
Cash settled performance shares
|
109.8
|
|
60.4
|
|
32.7
|
|
|||||
|
Employee stock purchase plan
|
9.2
|
|
5.2
|
|
6.3
|
|
|||||
|
Subtotal
|
255.9
|
|
184.3
|
|
150.1
|
|
|||||
|
Capitalized share-based compensation costs
|
(9.8
|
)
|
(5.4
|
)
|
(4.5
|
)
|
|||||
|
Share-based compensation expense included in total cost and expenses
|
$
|
246.1
|
|
$
|
178.9
|
|
$
|
145.6
|
|
||
|
Shares
|
Weighted
Average
Exercise
Price
|
|||||
|
Outstanding at December 31, 2012
|
907,000
|
|
$
|
54.48
|
|
|
|
Granted
|
—
|
|
$
|
—
|
|
|
|
Exercised
|
(523,000
|
)
|
$
|
53.73
|
|
|
|
Cancelled
|
—
|
|
$
|
—
|
|
|
|
Outstanding at December 31, 2013
|
384,000
|
|
$
|
55.49
|
|
|
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2013
|
2012
|
2011
|
||||||||
|
Tax benefit realized for stock options
|
$
|
29.4
|
|
$
|
20.9
|
|
$
|
47.5
|
|
||
|
Cash received from the exercise of stock options
|
$
|
28.1
|
|
$
|
38.8
|
|
$
|
291.9
|
|
||
|
Shares
|
Weighted
Average
Grant Date
Fair Value
|
|||||
|
Unvested at December 31, 2012
|
606,000
|
|
$
|
94.73
|
|
|
|
Granted (a)
|
271,000
|
|
$
|
193.45
|
|
|
|
Vested
|
(296,000
|
)
|
$
|
85.12
|
|
|
|
Forfeited
|
(31,000
|
)
|
$
|
128.39
|
|
|
|
Unvested at December 31, 2013
|
550,000
|
|
$
|
128.04
|
|
|
|
(a)
|
MSUs granted in 2013 include approximately
18,000
and
39,000
MSUs issued in 2013 based upon the attainment of performance criteria set for 2012 and 2011, respectively, in relation to shares granted in those years. The remainder of MSUs granted during 2013 include awards granted in conjunction with our annual awards made in February 2013 and MSUs granted in conjunction with the hiring of employees. These grants reflect the target number of shares eligible to be earned at the time of grant.
|
|
|
For the Years Ended December 31,
|
||
|
|
2013
|
2012
|
|
|
Expected dividend yield
|
—%
|
—%
|
|
|
Range of expected stock price volatility
|
21.7% - 25.7%
|
29.6% - 34.0%
|
|
|
Range of risk-free interest rates
|
0.1% - 0.7%
|
0.2% - 0.6%
|
|
|
60 calendar day average stock price on grant date
|
$150.33 - $240.14
|
$113.83 - $149.79
|
|
|
Weighted-average per share grant date fair value
|
$193.45
|
$134.95
|
|
|
Shares
|
||
|
Unvested at December 31, 2012
|
592,000
|
|
|
Granted (a)
|
273,000
|
|
|
Vested
|
(317,000
|
)
|
|
Forfeited
|
(34,000
|
)
|
|
Unvested at December 31, 2013
|
514,000
|
|
|
(a)
|
CSPSs granted in 2013 include approximately
76,000
CSPSs issued in 2013 based upon the attainment of performance criteria set for 2012 in relation to shares granted in 2012. The remainder of the CSPSs granted in 2013 include awards granted in conjunction with our annual awards made in February 2013 and CSPSs granted in conjunction with the hiring of employees. These grants reflect the target number of shares eligible to be earned at the time of grant.
|
|
Shares
|
Weighted
Average
Grant Date
Fair Value
|
|||||
|
Unvested at December 31, 2012
|
2,187,000
|
|
$
|
90.37
|
|
|
|
Granted (a)
|
758,000
|
|
$
|
176.53
|
|
|
|
Vested
|
(1,181,000
|
)
|
$
|
79.17
|
|
|
|
Forfeited
|
(104,000
|
)
|
$
|
119.42
|
|
|
|
Unvested at December 31, 2013
|
1,660,000
|
|
$
|
135.95
|
|
|
|
(a)
|
RSUs granted in 2013 primarily represent RSUs granted in conjunction with our annual awards made in February 2013 and awards made in conjunction with the hiring of new employees. RSUs granted in 2013 also include approximately
16,000
RSUs granted to our Board of Directors.
|
|
Shares
|
Weighted
Average
Grant Date
Fair Value
|
|||||
|
Unvested at December 31, 2012
|
930
|
|
$
|
53.64
|
|
|
|
Granted
|
—
|
|
$
|
—
|
|
|
|
Vested
|
(930
|
)
|
$
|
53.64
|
|
|
|
Forfeited
|
—
|
|
$
|
—
|
|
|
|
Unvested at December 31, 2013
|
—
|
|
$
|
—
|
|
|
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions, except share amounts)
|
2013
|
2012
|
2011
|
||||||||
|
Shares issued under ESPP
|
245,000
|
|
274,000
|
|
434,000
|
|
|||||
|
Cash received under ESPP
|
$
|
38.7
|
|
$
|
28.7
|
|
$
|
22.8
|
|
||
|
17.
|
Income Taxes
|
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2013
|
2012
|
2011
|
||||||||
|
Income before income taxes (benefit):
|
|||||||||||
|
Domestic
|
$
|
1,953.0
|
|
$
|
1,398.0
|
|
$
|
1,408.9
|
|
||
|
Foreign
|
527.6
|
|
457.1
|
|
302.3
|
|
|||||
|
Total
|
$
|
2,480.6
|
|
$
|
1,855.1
|
|
$
|
1,711.2
|
|
||
|
Income tax expense (benefit):
|
|||||||||||
|
Current:
|
|||||||||||
|
Federal
|
$
|
700.9
|
|
$
|
507.9
|
|
$
|
231.7
|
|
||
|
State
|
98.4
|
|
35.6
|
|
15.1
|
|
|||||
|
Foreign
|
46.8
|
|
44.0
|
|
44.1
|
|
|||||
|
Total
|
846.1
|
|
587.5
|
|
290.9
|
|
|||||
|
Deferred:
|
|||||||||||
|
Federal
|
$
|
(200.6
|
)
|
$
|
(133.0
|
)
|
$
|
160.9
|
|
||
|
State
|
(35.9
|
)
|
(13.0
|
)
|
(8.1
|
)
|
|||||
|
Foreign
|
(8.6
|
)
|
29.1
|
|
0.8
|
|
|||||
|
Total
|
(245.1
|
)
|
(116.9
|
)
|
153.6
|
|
|||||
|
Total income tax expense
|
$
|
601.0
|
|
$
|
470.6
|
|
$
|
444.5
|
|
||
|
|
As of December 31,
|
||||||
|
(In millions)
|
2013
|
2012
|
|||||
|
Deferred tax assets:
|
|||||||
|
Tax credits
|
$
|
64.1
|
|
$
|
69.3
|
|
|
|
Inventory, other reserves, and accruals
|
169.6
|
|
118.3
|
|
|||
|
Capitalized costs
|
7.9
|
|
7.6
|
|
|||
|
Intangibles, net
|
124.2
|
|
84.5
|
|
|||
|
Net operating loss
|
14.7
|
|
37.5
|
|
|||
|
Share-based compensation
|
85.0
|
|
58.6
|
|
|||
|
Other
|
76.9
|
|
57.8
|
|
|||
|
Valuation allowance
|
(1.5
|
)
|
(12.3
|
)
|
|||
|
Total deferred tax assets
|
$
|
540.9
|
|
$
|
421.3
|
|
|
|
Deferred tax liabilities:
|
|||||||
|
Purchased intangible assets
|
$
|
(550.8
|
)
|
$
|
(411.3
|
)
|
|
|
Unrealized gain on investments and cumulative translation adjustment
|
(3.2
|
)
|
(1.2
|
)
|
|||
|
Inventory
|
(24.9
|
)
|
(50.8
|
)
|
|||
|
Depreciation, amortization and other
|
(112.6
|
)
|
(146.4
|
)
|
|||
|
Total deferred tax liabilities
|
$
|
(691.5
|
)
|
$
|
(609.7
|
)
|
|
|
|
For the Years Ended December 31,
|
|||||||
|
|
2013
|
2012
|
2011
|
|||||
|
Statutory rate
|
35.0
|
%
|
35.0
|
%
|
35.0
|
%
|
||
|
State taxes
|
3.1
|
|
0.9
|
|
1.7
|
|
||
|
Taxes on foreign earnings
|
(6.7
|
)
|
(6.2
|
)
|
(5.9
|
)
|
||
|
Credits and net operating loss utilization
|
(2.6
|
)
|
(3.5
|
)
|
(4.4
|
)
|
||
|
Purchased intangible assets
|
1.5
|
|
1.2
|
|
1.3
|
|
||
|
Manufacturing deduction
|
(6.6
|
)
|
(2.1
|
)
|
(1.5
|
)
|
||
|
Other permanent items
|
0.8
|
|
(0.4
|
)
|
0.3
|
|
||
|
Contingent consideration
|
—
|
|
0.5
|
|
0.7
|
|
||
|
Other
|
(0.3
|
)
|
—
|
|
(1.2
|
)
|
||
|
Effective tax rate
|
24.2
|
%
|
25.4
|
%
|
26.0
|
%
|
||
|
(In millions)
|
2013
|
2012
|
2011
|
||||||||
|
Balance at January 1,
|
$
|
125.9
|
|
$
|
64.4
|
|
$
|
121.5
|
|
||
|
Additions based on tax positions related to the current period
|
11.9
|
|
13.0
|
|
2.2
|
|
|||||
|
Additions for tax positions of prior periods
|
71.7
|
|
69.8
|
|
48.6
|
|
|||||
|
Reductions for tax positions of prior periods
|
(92.1
|
)
|
(18.6
|
)
|
(75.8
|
)
|
|||||
|
Statute expirations
|
(1.9
|
)
|
(1.9
|
)
|
(2.3
|
)
|
|||||
|
Settlements
|
(5.4
|
)
|
(0.8
|
)
|
(29.8
|
)
|
|||||
|
Balance at December 31,
|
$
|
110.1
|
|
$
|
125.9
|
|
$
|
64.4
|
|
||
|
18.
|
Other Consolidated Financial Statement Detail
|
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2013
|
2012
|
2011
|
||||||||
|
Cash paid during the year for:
|
|||||||||||
|
Interest
|
$
|
53.6
|
|
$
|
65.4
|
|
$
|
66.7
|
|
||
|
Income taxes
|
$
|
643.2
|
|
$
|
526.6
|
|
$
|
332.7
|
|
||
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2013
|
2012
|
2011
|
||||||||
|
Interest income
|
$
|
8.2
|
|
$
|
29.5
|
|
$
|
19.2
|
|
||
|
Interest expense
|
(31.9
|
)
|
(36.5
|
)
|
(33.0
|
)
|
|||||
|
Impairments on investments
|
(2.8
|
)
|
(5.5
|
)
|
(9.9
|
)
|
|||||
|
Gain (loss) on investments, net
|
21.7
|
|
10.6
|
|
18.8
|
|
|||||
|
Foreign exchange gains (losses), net
|
(15.2
|
)
|
(2.5
|
)
|
(6.3
|
)
|
|||||
|
Other, net
|
(14.9
|
)
|
3.7
|
|
(2.3
|
)
|
|||||
|
Total other income (expense), net
|
$
|
(34.9
|
)
|
$
|
(0.7
|
)
|
$
|
(13.5
|
)
|
||
|
|
As of December 31,
|
||||||
|
(In millions)
|
2013
|
2012
|
|||||
|
Employee compensation and benefits
|
$
|
343.4
|
|
$
|
248.5
|
|
|
|
Revenue-related rebates
|
264.9
|
|
191.0
|
|
|||
|
Deferred revenue
|
172.7
|
|
148.0
|
|
|||
|
Royalties and licensing fees
|
160.7
|
|
45.2
|
|
|||
|
Clinical development expenses
|
55.2
|
|
51.6
|
|
|||
|
Current portion of contingent consideration obligations
|
29.0
|
|
22.4
|
|
|||
|
Construction in progress accrual
|
25.0
|
|
12.3
|
|
|||
|
Collaboration expenses
|
18.7
|
|
37.4
|
|
|||
|
Other
|
285.6
|
|
223.5
|
|
|||
|
Total accrued expenses and other
|
$
|
1,355.2
|
|
$
|
979.9
|
|
|
|
19.
|
Investments in Variable Interest Entities
|
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2013
|
2012
|
2011
|
||||||||
|
Milestone payments made to Knopp
|
$
|
—
|
|
$
|
—
|
|
$
|
10.0
|
|
||
|
Biogen Idec’s share of expense reflected within our consolidated statements of income
|
$
|
—
|
|
$
|
113.0
|
|
$
|
54.8
|
|
||
|
Collaboration expense attributed to noncontrolling interests, net of tax
|
$
|
—
|
|
$
|
—
|
|
$
|
8.6
|
|
||
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2013
|
2012
|
2011
|
||||||||
|
Milestone payments made to Neurimmune
|
$
|
—
|
|
$
|
—
|
|
$
|
15.0
|
|
||
|
Biogen Idec’s share of expense reflected within our consolidated statements of income
|
$
|
27.2
|
|
$
|
13.3
|
|
$
|
24.2
|
|
||
|
Collaboration expense attributed to noncontrolling interests, net of tax
|
$
|
—
|
|
$
|
—
|
|
$
|
14.7
|
|
||
|
(In millions)
|
As of December 31, 2013
|
||
|
Total expense incurred by Biogen Idec
|
$
|
115.7
|
|
|
Estimate of additional amounts to be incurred by us in development of the lead compound
|
$
|
800.9
|
|
|
20.
|
Collaborative and Other Relationships
|
|
Until GAZYVA First Non-CLL FDA Approval
|
40.0
|
%
|
|
After GAZYVA First Non-CLL FDA Approval until First GAZYVA Threshold Date
|
39.0
|
%
|
|
After First GAZYVA Threshold Date until Second GAZYVA Threshold Date
|
37.5
|
%
|
|
After Second GAZYVA Threshold Date
|
35.0
|
%
|
|
Until First GAZYVA Threshold Date
|
39.0
|
%
|
|
After First GAZYVA Threshold Date until Second GAZYVA Threshold Date
|
37.5
|
%
|
|
After Second GAZYVA Threshold Date
|
35.0
|
%
|
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2013
|
2012
|
2011
|
||||||||
|
Biogen Idec’s share of pre-tax profits in the U.S. for RITUXAN and GAZYVA (1)
|
$
|
1,085.2
|
|
$
|
1,031.7
|
|
$
|
872.7
|
|
||
|
Reimbursement of selling and development expenses in the U.S. for RITUXAN
|
2.1
|
|
1.6
|
|
6.1
|
|
|||||
|
Revenue on sales in the rest of world for RITUXAN
|
38.7
|
|
104.6
|
|
117.8
|
|
|||||
|
Total unconsolidated joint business revenues
|
$
|
1,126.0
|
|
$
|
1,137.9
|
|
$
|
996.6
|
|
||
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2013
|
2012
|
2011
|
||||||||
|
Upfront and milestones payments made to Acorda (1)
|
$
|
—
|
|
$
|
—
|
|
$
|
25.0
|
|
||
|
Total cost of sales related to royalties and commercial supply of FAMPYRA reflected within our statements of income
|
$
|
24.3
|
|
$
|
20.2
|
|
$
|
6.5
|
|
||
|
|
|
|
Rates should Sobi exercise
its option right
(3)
|
||||
|
Royalty and Net Revenue Share Rates:
|
Method
|
Rate prior to 1st
commercial sale in
the Sobi Territory:
|
Base Rate following
1st commercial sale in
the Sobi Territory:
|
Rate during the
Reimbursement
Period:
|
|||
|
Sobi rate to Biogen Idec on net sales in the Sobi Territory
|
Royalty
|
N/A
|
10 to 12%
|
Base Rate
plus 5%
|
|||
|
Biogen Idec rate to Sobi on net sales in the Biogen Idec North America Territory
|
Royalty
|
2%
|
10 to 12%
|
Base Rate
less 5%
|
|||
|
Biogen Idec rate to Sobi on net sales in the Biogen Idec Direct Territory
|
Royalty
|
2%
|
15 to 17%
|
Base Rate
less 5%
|
|||
|
Biogen Idec rate to Sobi on net revenue
(1)
from the Biogen Idec Distributor Territory
(2)
|
Net
Revenue
Share
|
10%
|
50%
|
Base Rate
less 15%
|
|||
|
(1)
|
Net revenue represents Biogen Idec’s pre-tax receipts from third-party distributors, less expenses incurred by Biogen Idec in the conduct of commercialization activities supporting the distributor activities.
|
|
(2)
|
The Biogen Idec Distributor Territory represents Biogen Idec territories where sales are derived utilizing a third-party distributor.
|
|
(3)
|
A credit will be issued to Sobi against its reimbursement of the Opt-in Consideration in an amount equal to the difference in the rate paid by Biogen Idec to Sobi on sales in the Biogen Idec territories for certain periods prior to the first commercial sale in the Sobi Territory versus the rate that otherwise would have been payable on such sales.
|
|
|
For the Years Ended
December 31,
|
||||||||||
|
(In millions)
|
2013
|
2012
|
2011
|
||||||||
|
Total development expense incurred by the collaboration
|
$
|
133.4
|
|
$
|
128.0
|
|
$
|
105.2
|
|
||
|
Biogen Idec’s share of development expense reflected within our consolidated statements of income
|
$
|
71.0
|
|
$
|
65.6
|
|
$
|
54.2
|
|
||
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2013
|
2012
|
2011
|
||||||||
|
Total expense incurred by the collaboration
|
$
|
1.6
|
|
$
|
18.8
|
|
$
|
1.1
|
|
||
|
Total expense reflected within our consolidated statements of income, excluding upfront and milestone payments
|
$
|
1.6
|
|
$
|
14.2
|
|
$
|
0.9
|
|
||
|
21.
|
Litigation
|
|
22.
|
Commitments and Contingencies
|
|
(In millions)
|
2014
|
2015
|
2016
|
2017
|
2018
|
Thereafter
|
Total
|
||||||||||||||||||||
|
Minimum lease payments (1)
|
$
|
66.8
|
|
$
|
64.2
|
|
$
|
57.0
|
|
$
|
54.9
|
|
$
|
49.8
|
|
$
|
384.4
|
|
$
|
677.1
|
|
||||||
|
Less: income from subleases
|
—
|
|
(5.6
|
)
|
(6.0
|
)
|
(6.0
|
)
|
(6.3
|
)
|
(41.5
|
)
|
(65.4
|
)
|
|||||||||||||
|
Net minimum lease payments
|
$
|
66.8
|
|
$
|
58.6
|
|
$
|
51.0
|
|
$
|
48.9
|
|
$
|
43.5
|
|
$
|
342.9
|
|
$
|
611.7
|
|
||||||
|
(1)
|
Includes future minimum rental commitments related to leases executed for two office buildings in Cambridge, Massachusetts, which completed construction in July and November 2013. The leases both have
15
year terms and we have options to extend the term of each lease for two additional five-year terms. Future minimum rental commitments under these leases will total approximately
$340.0 million
over the initial 15 year lease terms.
|
|
Cumulative Sales Level
|
||||||||||||||||||||
|
Prior 12 Month Sales
|
$500M
|
$1.0B
|
$2.0B
|
$3.0B
|
Each additional $1.0B up to $20.0B
|
|||||||||||||||
|
Payment Amount (In millions)
|
||||||||||||||||||||
|
< $500 million
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||||
|
$500 million - $1.0 billion
|
22.0
|
|
25.0
|
|
50.0
|
|
50.0
|
|
50.0
|
|
||||||||||
|
$1.0 billion - $1.5 billion
|
—
|
|
50.0
|
|
100.0
|
|
100.0
|
|
100.0
|
|
||||||||||
|
$1.5 billion - $2.0 billion
|
—
|
|
—
|
|
150.0
|
|
150.0
|
|
150.0
|
|
||||||||||
|
$2.0 billion - $2.5 billion
|
—
|
|
—
|
|
200.0
|
|
200.0
|
|
200.0
|
|
||||||||||
|
$2.5 billion - $3.0 billion
|
—
|
|
—
|
|
—
|
|
250.0
|
|
250.0
|
|
||||||||||
|
> $3.0 billion
|
—
|
|
—
|
|
—
|
|
—
|
|
300.0
|
|
||||||||||
|
23.
|
Guarantees
|
|
24.
|
Employee Benefit Plans
|
|
25.
|
Segment Information
|
|
|
For the Years Ended December 31,
|
||||||||||||||||||||||||||||||||||
|
|
2013
|
2012
|
2011
|
||||||||||||||||||||||||||||||||
|
(In millions)
|
United
States
|
Rest of
World
|
Total
|
United
States
|
Rest of
World
|
Total
|
United
States
|
Rest of
World
|
Total
|
||||||||||||||||||||||||||
|
AVONEX
|
$
|
1,902.4
|
|
$
|
1,103.1
|
|
$
|
3,005.5
|
|
$
|
1,793.7
|
|
$
|
1,119.4
|
|
$
|
2,913.1
|
|
$
|
1,628.3
|
|
$
|
1,058.3
|
|
$
|
2,686.6
|
|
||||||||
|
TYSABRI
|
814.2
|
|
712.3
|
|
1,526.5
|
|
383.1
|
|
752.8
|
|
1,135.9
|
|
326.5
|
|
753.0
|
|
1,079.5
|
|
|||||||||||||||||
|
TECFIDERA
|
864.4
|
|
11.7
|
|
876.1
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||
|
Other
|
—
|
|
134.2
|
|
134.2
|
|
—
|
|
117.1
|
|
117.1
|
|
—
|
|
70.0
|
|
70.0
|
|
|||||||||||||||||
|
Total product revenues
|
$
|
3,581.0
|
|
$
|
1,961.3
|
|
$
|
5,542.3
|
|
$
|
2,176.8
|
|
$
|
1,989.3
|
|
$
|
4,166.1
|
|
$
|
1,954.8
|
|
$
|
1,881.3
|
|
$
|
3,836.1
|
|
||||||||
|
December 31, 2013 (In millions)
|
U.S.
|
Europe
(1)
|
Germany
|
Asia
|
Other
|
Total
|
|||||||||||||||||
|
Product revenues from external customers
|
$
|
3,581.0
|
|
$
|
1,170.2
|
|
$
|
417.7
|
|
$
|
93.2
|
|
$
|
280.2
|
|
$
|
5,542.3
|
|
|||||
|
Revenues from unconsolidated joint business
|
$
|
1,087.3
|
|
$
|
1.6
|
|
$
|
—
|
|
$
|
3.2
|
|
$
|
33.9
|
|
$
|
1,126.0
|
|
|||||
|
Other revenues from external customers
|
$
|
193.5
|
|
$
|
26.1
|
|
$
|
1.2
|
|
$
|
43.1
|
|
$
|
—
|
|
$
|
263.9
|
|
|||||
|
Long-lived assets
|
$
|
984.4
|
|
$
|
758.3
|
|
$
|
2.5
|
|
$
|
2.1
|
|
$
|
3.3
|
|
$
|
1,750.7
|
|
|||||
|
December 31, 2012 (In millions)
|
U.S.
|
Europe
(1)
|
Germany
|
Asia
|
Other
|
Total
|
|||||||||||||||||
|
Product revenues from external customers
|
$
|
2,176.8
|
|
$
|
1,216.2
|
|
$
|
409.2
|
|
$
|
93.2
|
|
$
|
270.7
|
|
$
|
4,166.1
|
|
|||||
|
Revenues from unconsolidated joint business
|
$
|
1,033.3
|
|
$
|
14.3
|
|
$
|
—
|
|
$
|
27.5
|
|
$
|
62.8
|
|
$
|
1,137.9
|
|
|||||
|
Other revenues from external customers
|
$
|
170.2
|
|
$
|
27.9
|
|
$
|
1.1
|
|
$
|
13.3
|
|
$
|
—
|
|
$
|
212.5
|
|
|||||
|
Long-lived assets
|
$
|
996.6
|
|
$
|
738.6
|
|
$
|
1.9
|
|
$
|
2.9
|
|
$
|
2.2
|
|
$
|
1,742.2
|
|
|||||
|
December 31, 2011 (In millions)
|
U.S.
|
Europe
(1)
|
Germany
|
Asia
|
Other
|
Total
|
|||||||||||||||||
|
Product revenues from external customers
|
$
|
1,954.8
|
|
$
|
1,163.3
|
|
$
|
377.5
|
|
$
|
88.7
|
|
$
|
251.8
|
|
$
|
3,836.1
|
|
|||||
|
Revenues from unconsolidated joint business
|
$
|
878.8
|
|
$
|
29.9
|
|
$
|
—
|
|
$
|
30.7
|
|
$
|
57.2
|
|
$
|
996.6
|
|
|||||
|
Other revenues from external customers
|
$
|
187.0
|
|
$
|
28.3
|
|
$
|
0.6
|
|
$
|
—
|
|
$
|
—
|
|
$
|
215.9
|
|
|||||
|
Long-lived assets
|
$
|
1,012.5
|
|
$
|
816.6
|
|
$
|
1.6
|
|
$
|
5.3
|
|
$
|
2.4
|
|
$
|
1,838.4
|
|
|||||
|
(1)
|
Represents amounts related to Europe less those attributable to Germany.
|
|
26.
|
Quarterly Financial Data (Unaudited)
|
|
(In millions, except per share amounts)
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
Total
Year
|
||||||||||||||
|
2013
|
(a)
|
(b)
|
(b) (c)
|
(b)
|
|||||||||||||||
|
Product revenues
|
$
|
1,095.8
|
|
$
|
1,385.9
|
|
$
|
1,453.6
|
|
$
|
1,607.1
|
|
$
|
5,542.3
|
|
||||
|
Unconsolidated joint business revenues
|
$
|
264.6
|
|
$
|
288.8
|
|
$
|
303.2
|
|
$
|
269.4
|
|
$
|
1,126.0
|
|
||||
|
Other revenues
|
$
|
54.7
|
|
$
|
48.8
|
|
$
|
71.0
|
|
$
|
89.4
|
|
$
|
263.9
|
|
||||
|
Total revenues
|
$
|
1,415.1
|
|
$
|
1,723.5
|
|
$
|
1,827.8
|
|
$
|
1,965.9
|
|
$
|
6,932.2
|
|
||||
|
Gross profit
|
$
|
1,281.4
|
|
$
|
1,492.8
|
|
$
|
1,593.1
|
|
$
|
1,707.3
|
|
$
|
6,074.5
|
|
||||
|
Net income
|
$
|
426.7
|
|
$
|
490.7
|
|
$
|
487.6
|
|
$
|
457.3
|
|
$
|
1,862.3
|
|
||||
|
Net income attributable to Biogen Idec Inc.
|
$
|
426.7
|
|
$
|
490.7
|
|
$
|
487.6
|
|
$
|
457.3
|
|
$
|
1,862.3
|
|
||||
|
Basic earnings per share attributable to Biogen Idec Inc.
|
$
|
1.80
|
|
$
|
2.07
|
|
$
|
2.06
|
|
$
|
1.94
|
|
$
|
7.86
|
|
||||
|
Diluted earnings per share attributable to Biogen Idec Inc.
|
$
|
1.79
|
|
$
|
2.06
|
|
$
|
2.05
|
|
$
|
1.92
|
|
$
|
7.81
|
|
||||
|
(In millions, except per share amounts)
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
Total
Year
|
||||||||||||||
|
2012
|
(d)
|
|
(e) (f)
|
|
|||||||||||||||
|
Product revenues
|
$
|
975.4
|
|
$
|
1,076.8
|
|
$
|
1,039.1
|
|
$
|
1,074.7
|
|
$
|
4,166.1
|
|
||||
|
Unconsolidated joint business revenues
|
$
|
284.6
|
|
$
|
284.6
|
|
$
|
287.8
|
|
$
|
280.9
|
|
$
|
1,137.9
|
|
||||
|
Other revenues
|
$
|
32.0
|
|
$
|
59.6
|
|
$
|
58.6
|
|
$
|
62.3
|
|
$
|
212.5
|
|
||||
|
Total revenues
|
$
|
1,292.0
|
|
$
|
1,421.0
|
|
$
|
1,385.5
|
|
$
|
1,417.9
|
|
$
|
5,516.5
|
|
||||
|
Gross profit
|
$
|
1,158.8
|
|
$
|
1,281.8
|
|
$
|
1,246.2
|
|
$
|
1,284.1
|
|
$
|
4,971.0
|
|
||||
|
Net income
|
$
|
302.4
|
|
$
|
387.1
|
|
$
|
398.4
|
|
$
|
292.1
|
|
$
|
1,380.0
|
|
||||
|
Net income attributable to Biogen Idec Inc.
|
$
|
302.7
|
|
$
|
386.8
|
|
$
|
398.4
|
|
$
|
292.1
|
|
$
|
1,380.0
|
|
||||
|
Basic earnings per share attributable to Biogen Idec Inc.
|
$
|
1.26
|
|
$
|
1.62
|
|
$
|
1.68
|
|
$
|
1.24
|
|
$
|
5.80
|
|
||||
|
Diluted earnings per share attributable to Biogen Idec Inc.
|
$
|
1.25
|
|
$
|
1.61
|
|
$
|
1.67
|
|
$
|
1.23
|
|
$
|
5.76
|
|
||||
|
(a)
|
Our share of revenues from unconsolidated joint business reflects a charge of
$41.5 million
for damages and interest awarded to Hoechst in Genentech's arbitration with Hoechst for RITUXAN.
|
|
(b)
|
Product revenues and total revenues for the second, third and fourth quarters of 2013 includes 100% of net revenues related to sales of TYSABRI as a result of our acquisition of TYSABRI rights from Elan on April 2, 2013 and net revenues related to sales of TECFIDERA, our new oral first-line treatment for people with relapsing forms of multiple sclerosis (MS), which was approved by the FDA in March 2013 and commenced commercial sales in April 2013.
|
|
(c)
|
Net income and net income attributable to Biogen Idec Inc., for the third quarter of 2013, includes a charge to research and development expense of
$75.0 million
related to an upfront payment made in connection with our collaboration agreement entered into with Isis.
|
|
(d)
|
Net income and net income attributable to Biogen Idec Inc. for the first quarter of 2012 includes a charge to research and development expense of
$29.0 million
related to an upfront payment made in connection with our development agreement entered into with Isis.
|
|
(e)
|
Net income and net income attributable to Biogen Idec Inc. for the fourth quarter of 2012 includes the correction of an error that had accumulated over several prior years in our deferred tax accounting for capitalized interest which resulted in an expense of
$29.0 million
.
|
|
(f)
|
Net income and net income attributable to Biogen Idec Inc. for the fourth quarter of 2012 includes a charge to research and development expense of
$30.0 million
related to an upfront payment made in connection with our development agreement entered into with Isis.
|
|
Exhibit No.
|
|
Description
|
|
2.1†
|
Asset Purchase Agreement among Biogen Idec International Holding Ltd., Elan Pharma International Limited and Elan Pharmaceuticals, Inc., dated as of February 5, 2013. Filed as Exhibit 2.1 to our Current Report on Form 8-K/A filed on February 12, 2013.
|
|
|
3.1
|
|
Amended and Restated Certificate of Incorporation, as amended. Filed as Exhibit 3.1 to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2012.
|
|
3.2
|
|
Second Amended and Restated Bylaws, as amended. Filed as Exhibit 3.1 to our Quarterly Report on Form 10-Q for the quarter ended September 30, 2012.
|
|
4.1
|
|
Reference is made to Exhibit 3.1 for a description of the rights, preferences and privileges of our Series A Preferred Stock and Series X Junior Participating Preferred Stock.
|
|
4.2
|
|
Indenture between Biogen Idec and The Bank of New York Trust Company, N.A. dated as of February 26, 2008. Filed as Exhibit 4.1 to our Registration Statement on Form S-3 (File No. 333-149379).
|
|
4.3
|
|
First Supplemental Indenture between Biogen Idec and The Bank of New York Trust Company, N.A. dated as of March 4, 2008. Filed as Exhibit 4.1 to our Current Report on Form 8-K filed on March 4, 2008.
|
|
10.1
|
|
Credit Agreement among Biogen Idec, Bank of America, N.A. as administrative agent, swing line lender and L/C issuer and the other lenders party thereto. Filed as Exhibit 10.1 to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2013.
|
|
10.2†
|
|
Expression Technology Agreement between Biogen Idec and Genentech. Inc. dated March 16, 1995. Filed as an exhibit to Biogen Idec’s Quarterly Report on Form 10-Q for the quarter ended March 31, 1995.
|
|
10.3
|
|
Letter Agreement between Biogen Idec and Genentech, Inc. dated May 21, 1996. Filed as Exhibit 10.1 to our Current Report on Form 8-K filed on June 6, 1996.
|
|
10.4†
|
|
Second Amended and Restated Collaboration Agreement between Biogen Idec and Genentech, Inc. dated as of October 18, 2010. Filed as Exhibit 10.5 to our Annual Report on Form 10-K for the year ended December 31, 2010.
|
|
10.5†
|
|
Letter agreement regarding GA101 financial terms between Biogen Idec and Genentech, Inc. dated October 18, 2010. Filed as Exhibit 10.6 to our Annual Report on Form 10-K for the year ended December 31, 2010.
|
|
10.6†
|
|
ANTEGREN (now TYSABRI) Development and Marketing Collaboration Agreement between Biogen Idec and Elan Pharma International Limited dated August 15, 2000. Filed as Exhibit 10.48 to Biogen, Inc.’s Annual Report on Form 10-K for the year ended December 31, 2002 (File No. 0-12042) and incorporated herein by reference.
|
|
10.7*
|
|
Biogen Idec Inc. 2008 Omnibus Equity Plan. Filed as Appendix A to our Definitive Proxy Statement on Schedule 14A filed on May 8, 2008.
|
|
10.8*
|
|
Amendment to Biogen Idec Inc. 2008 Omnibus Equity Plan dated October 13, 2008. Filed as Exhibit 10.19 to our Annual Report on Form 10-K for the year ended December 31, 2008.
|
|
10.9*
|
|
Form of restricted stock unit award agreement under the Biogen Idec Inc. 2008 Omnibus Equity Plan. Filed as Exhibit 10.1 to our Current Report on Form 8-K filed on August 1, 2008.
|
|
10.10*
|
|
Form of nonqualified stock option award agreement under the Biogen Idec Inc. 2008 Omnibus Equity Plan. Filed as Exhibit 10.2 to our Current Report on Form 8-K filed on August 1, 2008.
|
|
10.11*
|
|
Form of cash-settled performance shares award agreement under the Biogen Idec Inc. 2008 Omnibus Equity Plan. Filed as Exhibit 10.1 to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2010.
|
|
10.12+
|
Form of performance shares award agreement under the Biogen Idec Inc. 2008 Omnibus Equity Plan.
|
|
|
10.13*
|
|
Form of market stock unit award agreement under the Biogen Idec Inc. 2008 Omnibus Equity Plan. Filed as Exhibit 10.2 to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2010.
|
|
10.14*
|
|
Biogen Idec Inc. 2006 Non-Employee Directors Equity Plan, as amended. Filed as Appendix A to our Definitive Proxy Statement on Schedule 14A filed on April 28, 2010.
|
|
10.15*
|
|
Amendment to Biogen Idec Inc. 2006 Non-Employee Directors Equity Plan dated June 1, 2011. Filed as Exhibit 10.4 to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2011.
|
|
10.16*
|
|
Biogen Idec Inc. 2005 Omnibus Equity Plan. Filed as Appendix A to our Definitive Proxy Statement on Schedule 14A filed on April 15, 2005.
|
|
Exhibit No.
|
|
Description
|
|
10.17*
|
|
Amendment No. 1 to the Biogen Idec Inc. 2005 Omnibus Equity Plan dated April 4, 2006. Filed as Exhibit 10.1 to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2007.
|
|
10.18*
|
|
Amendment No. 2 to the Biogen Idec Inc. 2005 Omnibus Equity Plan dated February 12, 2007. Filed as Exhibit 10.2 to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2007.
|
|
10.19*
|
|
Amendment to the Biogen Idec Inc. 2005 Omnibus Equity Plan dated April 18, 2008. Filed as Exhibit 10.7 to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2008.
|
|
10.20*
|
|
Amendment to Biogen Idec Inc. 2005 Omnibus Equity Plan dated October 13, 2008. Filed as Exhibit 10.30 to our Annual Report on Form 10-K for the year ended December 31, 2008.
|
|
10.21*
|
|
Biogen Idec Inc. 2003 Omnibus Equity Plan. Filed as Exhibit 10.73 to our Current Report on Form 8-K filed on November 12, 2003.
|
|
10.22*
|
|
Amendment to Biogen Idec Inc. 2003 Omnibus Equity Plan. Filed as Exhibit 10.1 to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2005.
|
|
10.23*
|
|
Amendment to Biogen Idec Inc. 2003 Omnibus Equity Plan dated April 18, 2008. Filed as Exhibit 10.6 to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2008.
|
|
10.24*
|
|
Amendment to Biogen Idec Inc. 2003 Omnibus Equity Plan dated October 13, 2008. Filed as Exhibit 10.34 to our Annual Report on Form 10-K for the year ended December 31, 2008.
|
|
10.25*
|
|
Biogen Idec Inc. 1995 Employee Stock Purchase Plan as amended and restated effective April 6, 2005. Filed as Appendix B to our Definitive Proxy Statement on Schedule 14A filed on April 15, 2005.
|
|
10.26*
|
|
IDEC Pharmaceuticals Corporation 1993 Non-Employee Directors Stock Option Plan, as amended and restated through February 19, 2003. Filed as Appendix B to our Definitive Proxy Statement on Schedule 14A filed on April 11, 2003.
|
|
10.27*
|
|
Amendment to IDEC Pharmaceuticals Corporation 1993 Non-Employee Directors Stock Option Plan dated April 18, 2008. Filed as Exhibit 10.5 to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2008.
|
|
10.28*
|
|
Amendment to IDEC Pharmaceuticals Corporation 1993 Non-Employee Directors Stock Option Plan dated June 1, 2011. Filed as Exhibit 10.3 to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2011.
|
|
10.29*
|
|
IDEC Pharmaceuticals Corporation 1988 Stock Option Plan, as amended and restated through February 19, 2003. Filed as Appendix A to our Definitive Proxy Statement on Schedule 14A filed on April 11, 2003.
|
|
10.30*
|
|
Amendment to the IDEC Pharmaceuticals Corporation 1988 Stock Option Plan dated April 16, 2004. Filed as Exhibit 10.1 to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2004.
|
|
10.31*
|
|
Amendment to IDEC Pharmaceuticals Corporation 1988 Stock Option Plan dated April 18, 2008. Filed as Exhibit 10.4 to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2008.
|
|
10.32*
|
|
Biogen, Inc. 1985 Non-Qualified Stock Option Plan, as amended and restated through April 11, 2003. Filed as Exhibit 10.22 to our Annual Report on Form 10-K for the year ended December 31, 2007.
|
|
10.33*
|
|
Amendment to Biogen, Inc. 1985 Non-Qualified Stock Option Plan dated April 18, 2008. Filed as Exhibit 10.2 to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2008.
|
|
10.34*
|
|
Amendment to Biogen, Inc. 1985 Non-Qualified Stock Option Plan dated October 13, 2008. Filed as Exhibit 10.45 to our Annual Report on Form 10-K for the year ended December 31, 2008.
|
|
10.35*
|
|
Biogen Idec Inc. 2008 Performance-Based Management Incentive Plan. Filed as Appendix B to Biogen Idec’s Definitive Proxy Statement on Schedule 14A filed on May 8, 2008.
|
|
10.36*
|
|
Voluntary Executive Supplemental Savings Plan, as amended and restated effective January 1, 2004. Filed as Exhibit 10.13 to our Annual Report on Form 10-K for the year ended December 31, 2003.
|
|
10.37*
|
|
Supplemental Savings Plan, as amended and restated effective January 1, 2012. Filed as Exhibit 10.39 to our Annual Report on Form 10-K for the year ended December 31, 2011.
|
|
10.38*
|
|
Voluntary Board of Directors Savings Plan, as amended and restated effective January 1, 2012. Filed as Exhibit 10.40 to our Annual Report on Form 10-K for the year ended December 31, 2011.
|
|
10.39*+
|
|
Biogen Idec Inc. Executive Severance Policy — U.S. Executive Vice President, as amended effective January 1, 2014.
|
|
10.40*+
|
|
Biogen Idec Inc. Executive Severance Policy — International Executive Vice President, as amended effective January 1, 2014.
|
|
Exhibit No.
|
|
Description
|
|
10.41*
|
|
Biogen Idec Inc. Executive Severance Policy — U.S. Senior Vice President, as amended effective October 13, 2008. Filed as Exhibit 10.53 to our Annual Report on Form 10-K for the year ended December 31, 2008.
|
|
10.42*
|
|
Biogen Idec Inc. Executive Severance Policy — International Senior Vice President, as amended effective October 13, 2008. Filed as Exhibit 10.54 to our Annual Report on Form 10-K for the year ended December 31, 2008.
|
|
10.43*
|
|
Annual Retainer Summary for Board of Directors. Filed as Exhibit 10.2 to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2011.
|
|
10.44*
|
|
Form of indemnification agreement for directors and executive officers. Filed as Exhibit 10.1 to our Current Report on Form 8-K filed on June 7, 2011.
|
|
10.45*
|
|
Employment Agreement between Biogen Idec and George A. Scangos amended as of August 23, 2013. Filed as Exhibit 10.1 to our Current Report on Form 8-K filed on August 26, 2013.
|
|
10.46*
|
|
Letter regarding employment arrangement of Paul J. Clancy dated August 17, 2007. Filed as Exhibit 10.49 to our Annual Report on Form 10-K for the year ended December 31, 2007.
|
|
10.47*
|
|
Letter regarding employment arrangement of Douglas E. Williams dated December 7, 2010. Filed as Exhibit 10.57 to our Annual Report on Form 10-K for the year ended December 31, 2011.
|
|
10.48*
|
Letter regarding employment arrangement of Steven H. Holtzman dated November 19, 2010. Filed as Exhibit 10.58 to our Annual Report on Form 10-K for the year ended December 31, 2011.
|
|
|
10.49*
|
|
Letter regarding employment arrangement of Kenneth DiPietro dated December 12, 2011. Filed as Exhibit 10.49 to our Annual Report on Form 10-K for the year ended December 31, 2012.
|
|
10.50*
|
Letter regarding employment arrangement of Alfred Sandrock. Filed as Exhibit 10.1 to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2013.
|
|
|
10.51*+
|
Letter agreement regarding separation arrangement of Raymond Pawlicki dated November 5, 2013.
|
|
|
21
|
|
Subsidiaries. Filed as Exhibit 21 to our annual report on Form 10-K for the year ended December 31, 2012.
|
|
23+
|
|
Consent of PricewaterhouseCoopers LLP, an Independent Registered Public Accounting Firm.
|
|
31.1+
|
|
Certification of the Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
31.2+
|
|
Certification of the Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
32.1++
|
|
Certification of the Chief Executive Officer and the Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
101++
|
|
The following materials from Biogen Idec Inc.’s Annual Report on Form 10-K for the year ended December 31, 2013, formatted in XBRL (Extensible Business Reporting Language): (i) the Consolidated Statements of Income, (ii) the Consolidated Statements of Comprehensive Income, (iii) the Consolidated Balance Sheets, (iv) the Consolidated Statements of Cash Flows, (v) the Consolidated Statements of Equity and (vi) Notes to Consolidated Financial Statements.
|
|
^
|
References to “our” filings mean filings made by Biogen Idec Inc. and filings made by IDEC Pharmaceuticals Corporation prior to the merger with Biogen, Inc. Unless otherwise indicated, exhibits were previously filed with the Securities and Exchange Commission under Commission File Number 0-19311 and are incorporated herein by reference.
|
|
*
|
Management contract or compensatory plan or arrangement.
|
|
†
|
Confidential treatment has been granted or requested with respect to portions of this exhibit.
|
|
+
|
Filed herewith.
|
|
+
|
+
|
Furnished herewith.
|