|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the fiscal year ended December 31, 2017
|
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|

|
Delaware
|
33-0112644
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
|
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|
|
Common Stock, $0.0005 par value
|
The Nasdaq Global Select Market
|
|
|
Large accelerated filer
x
|
Accelerated filer
o
|
|
|
Non-accelerated filer
o
|
Smaller reporting company
o
|
|
|
(Do not check if a smaller reporting company)
|
Emerging growth company
o
|
|
|
|
|
Page
|
|
•
|
the anticipated amount, timing and accounting of revenues, contingent payments, milestone, royalty and other payments under licensing, collaboration or acquisition agreements, tax positions and contingencies, collectability of receivables, pre-approval inventory, cost of sales, research and development costs, compensation and other selling, general and administrative expenses, amortization of intangible assets, foreign currency exchange risk, estimated fair value of assets and liabilities and impairment assessments;
|
|
•
|
expectations, plans and prospects relating to sales, pricing, growth and launch of our marketed and pipeline products;
|
|
•
|
our plans to invest in emerging growth areas such as pain, ophthalmology, neuropsychiatry and acute neurology;
|
|
•
|
the potential impact of increased product competition in the markets in which we compete;
|
|
•
|
patent terms, patent term extensions, patent office actions and expected availability and period of regulatory exclusivity;
|
|
•
|
the costs and timing of potential clinical trials, filings and approvals, and the potential therapeutic scope of the development and commercialization of our and our collaborators’ pipeline products;
|
|
•
|
the drivers for growing our business, including our plans and intent to commit resources relating to business development opportunities and research and development programs;
|
|
•
|
the anticipated benefits and the potential costs and expenses related to our current or future initiatives to streamline our operations and reallocate resources;
|
|
•
|
our manufacturing capacity, use of third-party contract manufacturing organizations and plans and timing relating to the expansion of our manufacturing capabilities, including anticipated investments and activities in new manufacturing facilities;
|
|
•
|
the potential impact on our results of operations and liquidity of the United Kingdom's (U.K.) intent to voluntarily depart from the European Union (E.U.);
|
|
•
|
the impact of the continued uncertainty of the credit and economic conditions in certain countries in Europe and our collection of accounts receivable in such countries;
|
|
•
|
the potential impact of healthcare reform in the United States (U.S.) and measures being taken worldwide designed to reduce healthcare costs to constrain the overall level of government expenditures, including the impact of pricing actions and reduced reimbursement for our products;
|
|
•
|
the timing, outcome and impact of administrative, regulatory, legal and other proceedings related to our patents and other proprietary and intellectual property rights, tax audits, assessments and settlements, pricing matters, sales and promotional practices, product liability and other matters;
|
|
•
|
lease commitments, purchase obligations and the timing and satisfaction of other contractual obligations;
|
|
•
|
our ability to finance our operations and business initiatives and obtain funding for such activities;
|
|
•
|
the anticipated benefits, costs and tax treatment of the spin-off of our hemophilia business; and
|
|
•
|
the impact of new laws, including the Tax Cuts and Jobs Act of 2017, and accounting standards.
|
|
•
|
“Biogen,” the “company,” “we,” “us” and “our” refer to Biogen Inc. and its consolidated subsidiaries;
|
|
•
|
“RITUXAN” refers to both RITUXAN (the trade name for rituximab in the U.S., Canada and Japan) and MabThera (the trade name for rituximab outside the U.S., Canada and Japan); and
|
|
•
|
"ELOCTATE" refers to both ELOCTATE (the trade name for Antihemophilic Factor (recombinant), Fc Fusion Protein in the U.S., Canada and Japan) and ELOCTA (the trade name for Antihemophilic Factor (recombinant), Fc Fusion Protein in the E.U.).
|
|
•
|
maximizing the resilience of our MS core business;
|
|
•
|
accelerating efforts in SMA as a significant new growth opportunity;
|
|
•
|
developing and expanding our neuroscience portfolio;
|
|
•
|
focusing our capital allocation efforts to drive investment for future growth; and
|
|
•
|
creating a leaner and simpler operating model to streamline our operations and reallocate resources towards prioritized research and development and commercial value creation opportunities.
|
|
•
|
Michel Vounatsos, Chief Executive Officer;
|
|
•
|
Jeffrey Capello, Executive Vice President and Chief Financial Officer;
|
|
•
|
Ginger Gregory, Executive Vice President and Chief Human Resources Officer; and
|
|
•
|
Chirfi Guindo, Executive Vice President and Head of Global Marketing, Market Access and Customer Innovation.
|
|
•
|
In April 2017 we presented new real-world data evidence supporting TECFIDERA at the 69
th
annual meeting of the American Academy of Neurology (AAN) in Boston, MA.
|
|
•
|
In February 2017 the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion to update the TYSABRI E.U. label with pediatric information to remove the contraindication in pediatrics and to describe the results of the post-marketing meta-analysis of pediatric data. The label update entitles us to apply for a six-month extension to the E.U. patent Supplementary Protection Certificate.
|
|
•
|
In April 2017 we presented new real-world data from the TYSABRI Observational Program that confirmed the efficacy of TYSABRI and demonstrated that early and continued treatment leads to better clinical outcomes. These data were presented at the 69
th
annual meeting of AAN in Boston, MA.
|
|
•
|
In May 2017 the European Commission (EC) granted a standard marketing authorization for FAMPYRA for walking improvement in people with MS.
|
|
•
|
In July 2017 the EMA announced that it had provisionally restricted the use of ZINBRYTA to adult patients with highly active relapsing disease despite a full and adequate course of treatment with at least one DMT or with rapidly evolving severe relapsing MS who are unsuitable for treatment with other DMTs. These restrictions followed the initiation of an EMA review (referred to as an Article 20 Procedure) of ZINBRYTA following the report of a case of fatal fulminant liver failure, as well as four cases of serious liver injury.
|
|
•
|
In October 2017, as part of the Article 20 Procedure of ZINBRYTA, the EMA Pharmacovigilance Risk Assessment Committee (PRAC) completed its assessment and recommended a further set of restrictions on the use of ZINBRYTA by MS patients.
|
|
•
|
In November 2017 the CHMP adopted an opinion, confirming the PRAC's recommendations, for further restrictions to minimize the risk of serious liver injury with ZINBRYTA, including restriction of its use to adult patients with relapsing forms of MS who have had an inadequate response to at least two DMTs and for whom treatment with any other DMT is contraindicated or otherwise unsuitable. In January 2018 the EC adopted a final and legally-binding decision, which concluded the Article 20 Procedure, confirming the CHMP opinion. As a result of the CHMP's recommendation of these restrictions, we recorded net impairment charges related to intangible assets, inventory, property, plant and equipment and prepaid tax assets, totaling approximately
$190.8 million
. Offsetting these amounts was an unrecorded tax benefit related to certain ZINBRYTA related assets totaling approximately
$93.8 million
.
|
|
•
|
In October 2017 we initiated the Phase 2b clinical trial AFFINITY, designed to evaluate opicinumab as an investigational add-on therapy in people with relapsing MS. The trial follows the comprehensive review of SYNERGY, a Phase 2 trial, which identified a specific population that may be more likely to respond to treatment.
|
|
•
|
In October 2017 we presented data supporting opicinumab as a potential therapy to repair damage to the central nervous system caused by MS. These data were presented at the seventh Joint Meeting of the European Committee for Treatment and Research in MS and Americas Committee for Treatment and Research in MS (ECTRIMS-ACTRIMS).
|
|
•
|
In January 2017 we presented new data from the Phase 3 ENDEAR study of SPINRAZA, which demonstrated a statistically significant reduction in the risk of death or permanent ventilation in SPINRAZA-treated infants with SMA compared to untreated infants. The data were presented at the British Pediatric Neurology Association annual conference in Cambridge, U.K.
|
|
•
|
In April 2017 the CHMP of the EMA adopted a positive opinion recommending the granting of a marketing authorization in the E.U. for SPINRAZA to treat patients with SMA.
|
|
•
|
In April 2017 we presented Phase 3 end of study SPINRAZA data from CHERISH, which demonstrated a highly statistically significant and clinically meaningful improvement in motor function in children with later-onset (most likely to develop Type 2 or Type 3) SMA compared to untreated children. The overall findings continued to support the efficacy and favorable safety profile of SPINRAZA across a broad range of individuals with SMA.
|
|
•
|
In June 2017 the EC granted a marketing authorization for SPINRAZA for the treatment of 5q SMA in pediatric and adult patients in the E.U. SPINRAZA is the first approved treatment in the E.U. for SMA. SPINRAZA was reviewed under the EMA’s accelerated assessment program.
|
|
•
|
In June 2017 we presented robust efficacy and safety data from Phase 2 and Phase 3 SPINRAZA studies at the Cure SMA 2017 Annual SMA Conference in Orlando, FL. Data demonstrated motor function improvements in infants on permanent ventilation and no increase in the risk of adverse events in children with scoliosis.
|
|
•
|
In July 2017 the Japanese Ministry of Health, Labor and Welfare approved the use of SPINRAZA for the treatment of infantile SMA.
|
|
•
|
In September 2017 the Japanese Ministry of Health, Labor and Welfare approved the use of SPINRAZA for the treatment of pediatric and adult patients with SMA.
|
|
•
|
In October 2017 we presented new data at the 22
nd
International Congress of the World Muscle Society demonstrating that earlier initiation of treatment with SPINRAZA may improve motor function outcomes in infants and children with SMA. Results demonstrated the favorable efficacy and safety profile of SPINRAZA.
|
|
•
|
In October 2017 we and Ionis were awarded the 2017 Prix Galien USA Award for Best Biotechnology Product for SPINRAZA.
|
|
•
|
In November 2017 the end of study results from ENDEAR, the Phase 3 study of SPINRAZA, were published in
The New England Journal of Medicine
.
|
|
•
|
In March 2017 we presented data from research of aducanumab at the 13
th
International Conference on Alzheimer’s and Parkinson’s Diseases (AD/PD™) in Vienna, Austria.
|
|
•
|
In April 2017 we presented data from a Phase 1b study of aducanumab at the 69
th
annual meeting of the AAN in Boston, MA. This data was previously presented at the Clinical Trials on Alzheimer’s Disease (CTAD) meeting
|
|
•
|
In May 2017 we announced that we had amended the protocol of the Phase 3 trials of aducanumab. ApoE4 carriers that previously would be on a high dose of 6 mg/kg may now be titrated up to 10 mg/kg. This amendment is being reviewed by regulatory bodies and clinical study ethic independent review boards globally and may be implemented on a country by country basis. The change has already been incorporated in the U.S.
|
|
•
|
In July 2017 we presented a new post-hoc analysis of the Phase 1b PRIME study of aducanumab at the Alzheimer’s Association International Conference in London, U.K. Data presented included changes in the cognitive and functional subscores of the clinical dementia rating score. Aducanumab slowed decline on both the cognitive and functional assessments compared to placebo, and the results of all subgroups studied were consistent with the overall study population.
|
|
•
|
In August 2017 we announced results from a recently conducted analysis of the LTE of our ongoing Phase 1b study of aducanumab. The updated analyses include data from the placebo-controlled period and the LTE for patients treated with aducanumab up to 24 months in the titration cohort and up to 36 months in the fixed-dose cohorts. The results are consistent with previously reported analyses from this ongoing Phase 1b study and support the design of the ongoing Phase 3 studies of aducanumab for early AD.
|
|
•
|
In November 2017 we presented new data from the LTE of our ongoing Phase 1b study of aducanumab at the CTAD meeting in Boston, MA. The data included results from patients in the Phase 1b study who were treated with a gradually increased dose of aducanumab for up to 24 months and those who were treated with a fixed dose of 3, 6 or 10 mg/kg aducanumab for up to 36 months. The results are consistent with previously reported analyses from the Phase 1b study and support the design of the ongoing Phase 3 studies of aducanumab for early AD.
|
|
•
|
In December 2017 we announced that an Independent Data Monitoring Committee determined that BAN2401 did not meet the criteria for success based on a Bayesian analysis at 12 months as the primary endpoint in an 856-patient Phase 2 clinical study. Following the predefined study protocol, the blinded study will continue and a comprehensive final analysis will be conducted at 18 months seeking to demonstrate clinically significant results. The results of the final analysis are expected to be obtained during the second half of 2018.
|
|
•
|
In January 2017 we initiated a Phase 1 trial of BIIB076, an anti-tau monoclonal antibody, in healthy volunteers and participants with AD.
|
|
•
|
In June 2017 we dosed our first patient in our Phase 2 study of BIIB092 for PSP.
|
|
•
|
In October 2017 our collaboration partner Ionis announced the initiation of a Phase 1/2a clinical study of IONIS-MAPT
Rx
in patients with mild AD. IONIS-MAPT
Rx
is an antisense drug designed to selectively reduce the production of microtubule-associated protein tau (MAPT), or tau protein, in the brain. We have an option to develop and commercialize IONIS-MAPT
Rx
.
|
|
•
|
In March 2017 we presented data from research of BIIB054, our investigational treatment for Parkinson’s disease, at the 13
th
International Conference on Alzheimer’s and Parkinson’s Diseases (AD/PD™) in Vienna, Austria.
|
|
•
|
In July 2017 we completed enrollment in the Phase 1 study of BIIB054 in both healthy volunteers and patients with early onset Parkinson’s disease.
|
|
•
|
In January 2018 we dosed our first patient in our Phase 2 SPARK study of BIIB054 in Parkinson's disease.
|
|
•
|
In August 2017 we completed enrollment in the Phase 2b ACTION2 study evaluating the effects of natalizumab versus placebo on clinical measures of functional independence and activities of daily living in acute ischemic stroke patients.
|
|
•
|
In October 2017 we initiated the Phase 2 OPUS study evaluating the efficacy, safety and tolerability of natalizumab in drug-resistant focal epilepsy.
|
|
•
|
In June 2017 we presented real-world evidence from investigator-initiated studies supported by us demonstrating sustained efficacy and safety of, and high acceptance and adherence in patients initiating treatment with, BENEPALI. These data were presented at the Annual European Congress of Rheumatology (EULAR) in Madrid.
|
|
•
|
In June 2017 the CHMP of the EMA issued a positive opinion for IMRALDI, an adalimumab biosimilar candidate referencing HUMIRA.
|
|
•
|
In August 2017 the EC granted a marketing authorization for IMRALDI.
|
|
•
|
In March 2017 the FDA approved OCREVUS, a humanized anti-CD20 monoclonal antibody, for the treatment of relapsing MS (RMS) and primary progressive MS (PPMS).
|
|
•
|
In July 2017 OCREVUS was approved in Australia for the treatment of RMS and PPMS.
|
|
•
|
In September 2017 OCREVUS was approved in Switzerland for the treatment of RMS and PPMS.
|
|
•
|
In January 2018 the EC granted a marketing authorization for OCREVUS for the treatment of RMS and PPMS.
|
|
•
|
In March 2017 Roche announced that the FDA’s Oncologic Drugs Advisory Committee voted unanimously that the benefit-risk of rituximab/hyaluronidase for subcutaneous (under the skin) injection was favorable for the treatment of certain blood cancers. This new co-formulation includes the same monoclonal antibody as intravenous RITUXAN and hyaluronidase, a molecule that helps to deliver medicine under the skin.
|
|
•
|
In June 2017 the FDA approved RITUXAN HYCELA (rituximab and hyaluronidase human) for subcutaneous injection for the treatment of adults with previously untreated and relapsed or refractory follicular lymphoma, previously untreated diffuse large B-cell lymphoma and CLL. This new treatment includes the same monoclonal antibody as intravenous RITUXAN in combination with hyaluronidase human, an enzyme that helps to deliver rituximab under the skin.
|
|
•
|
In November 2017 the FDA approved GAZYVA in combination with chemotherapy, followed by GAZYVA alone in those who responded, for people with previously untreated advanced follicular lymphoma. The approval is based on results from the Phase 3 GALLIUM study, which showed superior progression-free survival for patients who received this GAZYVA-based regimen compared with those who received a RITUXAN-based regimen as an initial therapy.
|
|
•
|
In October 2017 we reported that BG00011 (STX-100) achieved proof of biology in a Phase 2a study in patients with idiopathic pulmonary fibrosis (IPF), a chronic irreversible and ultimately fatal disease characterized by a progressive decline in lung function. We plan to initiate a Phase 2b study for BG00011 in 2018.
|
|
Product
|
Indication
|
Collaborator
|
Major Markets
|
||

|
Relapsing forms of MS in the U.S.
Relapsing-remitting MS (RRMS) in the E.U.
|
None
|
U.S.
Canada
France
Germany
Italy
Spain
U.K.
|
||

|
Relapsing forms of MS
|
None
|
U.S.
France
Germany
Japan
Italy
Spain
U.K.
|
||

|
Relapsing forms of MS in the U.S.
RRMS in the E.U.
|
None
|
U.S.
France
Germany
Italy
Spain
U.K.
|
||

|
Relapsing forms of MS
Crohn's disease in the U.S.
|
None
|
U.S.
France
Germany
Italy
Spain
U.K.
|
||

|
Relapsing forms of MS
|
AbbVie Inc. (AbbVie)
|
U.S.
Germany
|
||

|
Walking ability for patients with MS
|
Acorda Therapeutics, Inc. (Acorda)
|
France
Germany
|
||
|
Product
|
Indication
|
Collaborator
|
Major Markets
|
||

|
SMA
|
Ionis
|
U.S.
France
Germany
Japan
Turkey
|
||
|
Product
|
Indication
|
Major Markets
|
|

|
Moderate to severe rheumatoid arthritis
Progressive psoriatic arthritis
Axial spondyloarthritis
Moderate to severe plaque psoriasis
|
Germany
Norway
Sweden
U.K.
|
|

|
Rheumatoid arthritis
Moderate to severe Crohn's disease Severe ulcerative colitis Severe ankylosing spondylitis Psoriatic arthritis Moderate to severe plaque psoriasis |
Germany
|
|
|
Product
|
Indication
|
Major Markets
|
|

|
Non-Hodgkin's lymphoma
CLL
Rheumatoid arthritis
Two forms of ANCA-associated vasculitis
|
U.S.
Canada
|
|

|
In combination with chlorambucil for previously untreated CLL
Follicular lymphoma
|
U.S.
|
|

|
RMS
PPMS
|
U.S.
Australia
Switzerland
|
|
|
Product
|
Indication
|
Collaborator
|
Major Markets
|
||

|
Moderate to severe plaque psoriasis
|
None
|
Germany
|
||
|
Product
|
Territory
|
Patent No.
|
General Subject Matter
|
Patent
Expiration
(1)
|
||||
|
TECFIDERA
|
U.S.
|
7,619,001
|
Methods of treatment
|
2018
|
||||
|
U.S.
|
7,803,840
|
Methods of treatment
|
2018
|
|||||
|
U.S.
|
8,399,514
|
Methods of treatment
|
2028
|
|||||
|
U.S.
|
8,524,773
|
Methods of treatment
|
2018
|
|||||
|
U.S.
|
6,509,376
|
Formulations of dialkyl fumarates for use in the treatment of autoimmune diseases
|
2019
|
|||||
|
U.S.
|
8,759,393
|
Formulations
|
2019
|
|||||
|
U.S.
|
7,320,999
|
Methods of treatment
|
2018
|
|||||
|
Europe
|
1131065
|
Formulations of dialkyl fumarates and their use for treating autoimmune diseases
|
2019
(2)
|
|||||
|
Europe
|
2137537
|
Methods of use
|
2028
(3)
|
|||||
|
AVONEX and PLEGRIDY
|
U.S.
|
7,588,755
|
Use of recombinant beta interferon for immunomodulation
|
2026
|
||||
|
PLEGRIDY
|
U.S.
|
7,446,173
|
Polymer conjugates of interferon beta-1a
|
2022
|
||||
|
U.S.
|
8,524,660
|
Methods of treatment
|
2023
|
|||||
|
U.S.
|
8,017,733
|
Polymer conjugates of interferon beta-1a
|
2027
|
|||||
|
Europe
|
1656952
|
Polymer conjugates of interferon-beta-1a and uses thereof
|
2019
|
|||||
|
Europe
|
1476181
|
Polymer conjugates of interferon-beta-1a and uses thereof
|
2023
(4)
|
|||||
|
TYSABRI
|
U.S.
|
6,602,503
|
Humanized recombinant antibodies; nucleic acids and host cells; processes for production; therapeutic compositions; methods of use
|
2020
|
||||
|
U.S.
|
7,807,167
|
Methods of treatment
|
2023
|
|||||
|
U.S.
|
9,493,567
|
Methods of treatment
|
2027
|
|||||
|
Europe
|
0804237
|
Humanized immunoglobulins; nucleic acids; pharmaceutical compositions; medical uses
|
2020
(5)
|
|||||
|
Europe
|
1485127
|
Methods of use
|
2023
|
|||||
|
FAMPYRA
|
Europe
|
1732548
|
Sustained-release aminopyridine compositions for increasing walking speed in patients with MS
|
2025
(6)
|
||||
|
Europe
|
23775536
|
Sustained-release aminopyridine compositions for treating MS
|
2025
(7)
|
|||||
|
ZINBRYTA
|
U.S.
|
8,454,965
|
Methods of treatment
|
2024
|
||||
|
U.S.
|
7,258,859
|
Methods of treatment
|
2024
|
|||||
|
U.S.
|
9,340,619
|
Daclizumab HYP compositions
|
2032
|
|||||
|
Europe
|
1539200
|
Anti-IL-2-receptor antibody for use in a method of treating a subject with MS
|
2023
|
|||||
|
SPINRAZA
|
U.S.
|
6,166,197
|
Oligomeric Compounds Having Pyrimidine Nucleotide(s)
|
2017
|
||||
|
U.S.
|
6,210,892
|
Alteration of Cellular Behavior By Antisense Modulation of MRNA Processing
|
2018
|
|||||
|
U.S.
|
7,101,993
|
Oligonucleotides Containing 2’-O-Modified Purines
|
2023
|
|||||
|
U.S.
|
7,838,657
|
SMA Treatment Via Targeting of SMN2 Splice Site Inhibitory Sequences
|
2027
|
|||||
|
U.S.
|
8,110,560
|
SMA Treatment Via Targeting of SMN2 Splice Site Inhibitory Sequences
|
2025
|
|||||
|
U.S.
|
8,361,977
|
Compositions And Methods For Modulation of SMN2 Splicing
|
2030
|
|||||
|
U.S.
|
8,980,853
|
Compositions And Methods For Modulation of SMN2 Splicing
|
2030
|
|||||
|
U.S.
|
9,717,750
|
Compositions and Methods For Modulation of SMN2 Splicing
|
2030
|
|||||
|
Europe
|
1910395
|
Compositions And Methods For Modulation of SMN2 Splicing
|
2026
|
|||||
|
Europe
|
2548560
|
Compositions And Methods For Modulation of SMN2 Splicing
|
2026
|
|||||
|
(1)
|
In addition to patent protection, certain of our products are entitled to regulatory exclusivity in the U.S. and the E.U. expected until the dates set forth below:
|
|
Product
|
Territory
|
Expected Expiration
|
||
|
TECFIDERA
|
U.S.
|
2018
|
||
|
E.U.
|
2024
|
|||
|
PLEGRIDY
|
U.S.
|
2026
|
||
|
E.U.
|
2024
|
|||
|
FAMPYRA
|
E.U.
|
2021
|
||
|
ZINBRYTA
|
U.S.
|
2028
|
||
|
E.U.
|
*
|
|||
|
SPINRAZA
|
U.S.
|
2023
|
||
|
E.U.
|
2027**
|
|||
|
(2)
|
This patent is subject to granted SPCs in certain European countries, which extended the patent term in those countries to 2024.
|
|
(3)
|
This patent was revoked in a European opposition. This decision is being appealed. The patent is subject to granted SPCs in certain European countries, which extended the patent term in those countries to 2029.
|
|
(4)
|
This patent is subject to granted SPCs in certain European countries, which extended the patent term in those countries to 2028.
|
|
(5)
|
Reflects SPCs granted in most European countries and pediatric extension in some countries.
|
|
(6)
|
This patent is subject to granted SPCs in certain European countries, which extended the patent term in those countries to 2026.
|
|
(7)
|
This patent is subject to granted SPCs in certain European countries, which extended the patent term in those countries to 2026.
|
|
Competing Product
|
Competitor
|
|
|
AUBAGIO (teriflunomide)
|
Sanofi
|
|
|
BETASERON/BETAFERON (interferon-beta-1b)
|
Bayer Group
|
|
|
COPAXONE
(glatiramer acetate)
|
Teva Pharmaceuticals Industries Ltd.
|
|
|
EXTAVIA
(interferon-beta-1b)
|
Novartis AG
|
|
|
GILENYA (fingolimod)
|
Novartis AG
|
|
|
GLATOPA (glatiramer acetate)
|
Sandoz, a division of Novartis AG
|
|
|
LEMTRADA (alemtuzumab)
|
Sanofi
|
|
|
OCREVUS (ocrelizumab)
|
Genentech
|
|
|
REBIF
(interferon-beta-1)
|
Merck KGaA (and co-promoted with Pfizer Inc. in the U.S.)
|
|
|
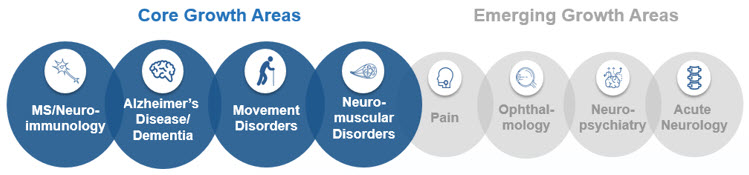
Core Growth Areas
|
MS and Neuroimmunology
|
BIIB098 (monomethly fumarate prodrug)* - MS
|
Phase 3
|
|||||
|
Opicinumab (anti-LINGO-1) - MS
|
Phase 2
|
|||||||
|
Alzheimer's Disease and Dementia
|
Aducanumab (Aβ mAb)* - Alzheimer's
|
Phase 3
|
||||||
|
Elenbecestat (E2609)* - Alzheimer's
|
Phase 3
|
|||||||
|
BAN2401 (Aβ mAb)* - Alzheimer's
|
Phase 2
|
|||||||
|
BIIB092 (anti-tau mAb) - Alzheimer's
|
Phase 1
|
|||||||
|
BIIB076 (anti-tau mAb)* - Alzheimer's
|
Phase 1
|
|||||||
|
BIIB080 (IONIS-MAPT
Rx
)* - Alzheimer's
|
Phase 1
|
|||||||
|
Parkinson's Disease and Movement Disorders
|
BIIB092 (anti-tau mAb) - PSP
|
Phase 2
|
||||||
|
BIIB054 (anti-
alpha
-synuclein mAb) - Parkinson's
|
Phase 2
|
|||||||
|
Neuromuscular Disease Including SMA and ALS
|
BIIB067 (IONIS-SOD1
Rx
)* - ALS
|
Phase 1
|
||||||
|
Emerging Growth Areas
|
Pain
|
BIIB074 (Vixotrigine) - Trigeminal Neuralgia
|
Phase 2
|
|||||
|
BIIB074 (Nav1.7) - PLSR
#
|
Phase 2
|
|||||||
|
Ophthalmology
|
BIIB087 (gene therapy)* - XLRS^
|
Phase 1/2
|
||||||
|
Acute Neurology
|
BIIB093 (glibenclamide IV) - LHI Stroke
|
Phase 2
|
||||||
|
Natalizumab - AI Stroke
|
Phase 2
|
|||||||
|
Natalizumab - Epilepsy
|
Phase 2
|
|||||||
|
Other
|
Dapirolzumab pegol (anti-CD40L)* - SLE
@
|
Phase 2
|
||||||
|
BG00011 (STX-100) - IPF
|
Phase 2
|
|||||||
|
BIIB059 (anti-BDCA2) - SLE
@
|
Phase 2
|
|||||||
|
•
|
Accelerated Approval
: The FDA may grant “accelerated approval” status to products that treat serious or life-threatening illnesses and that provide meaningful therapeutic benefits to patients over existing treatments. Under this pathway, the FDA may approve a product based on surrogate endpoints, or clinical endpoints other than survival or irreversible morbidity. When approval is based on surrogate endpoints or clinical endpoints other than survival or morbidity, the sponsor will be required to conduct additional post-approval clinical studies to verify and describe clinical benefit. Under the FDA's accelerated approval regulations, if the FDA concludes that a drug that has been shown to be effective can be safely used only if distribution or use is restricted, it may require certain post-marketing restrictions as necessary to assure safe use. In addition, for products approved under accelerated approval, sponsors may be required to submit all copies of their promotional materials, including advertisements, to the FDA at least 30 days prior to initial dissemination. The FDA may withdraw approval under accelerated approval after a hearing if, for instance, post-marketing studies fail to verify any clinical benefit, it becomes clear that restrictions on the distribution of the product are inadequate to ensure its safe use, or if a sponsor fails to comply with the conditions of the accelerated approval.
|
|
•
|
Fast Track Status
: The FDA may grant "fast track" status to products that treat a serious condition and have data demonstrating the potential to address an unmet medical need or a drug that has been designated as a qualified infectious disease product.
|
|
•
|
Breakthrough Therapy
: The FDA may grant “breakthrough therapy” status to drugs designed to treat, alone or in combination with another drug or drugs, a serious or life-threatening disease or condition and for which preliminary clinical evidence suggests a substantial improvement over existing therapies. Such drugs need not address an unmet need, but are nevertheless eligible for expedited review if they offer the potential for an improvement. Breakthrough therapy status entitles the sponsor to earlier and more frequent meetings with the
|
|
•
|
Priority Review
: Priority Review only applies to applications (original or efficacy supplement) for a drug that treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness of the treatment, diagnosis or prevention of serious conditions when compared to standard applications. Priority Review may also be granted for any supplement that proposes a labeling change due to studies completed in response to a written request from the FDA for pediatric studies, for an application for a drug that has been designated as a qualified infectious disease product, or any application or supplement for a drug submitted with a priority review voucher.
|
|
•
|
a national procedure, which requires an application to the competent authority of an E.U. country (if an application is to be made in more than one E.U. country, following approval in the first country, the applicant must submit applications in the other countries using the mutual recognition procedure);
|
|
•
|
a decentralized procedure, whereby applicants submit identical applications to several countries and receive simultaneous approval, if the medicine has not yet been authorized in any E.U. country; and
|
|
•
|
a mutual recognition procedure, where applicants that have a medicine authorized in one E.U. country can apply for mutual recognition of this authorization in other E.U. countries.
|
|
•
|
Medicaid
: Medicaid is a joint federal and state program that is administered by the states for low income and disabled beneficiaries. Under the Medicaid Drug Rebate Program, we are required to pay a rebate for each unit of product reimbursed by the state Medicaid programs. The amount of the rebate is established by law and is adjusted upward if average manufacture price (AMP) increases more than inflation (measured by the Consumer Price Index - Urban). The rebate amount is calculated each quarter based on our report of current AMP and best price for each of our products to the Centers for Medicare & Medicaid Services (CMS). The requirements for calculating AMP and best price are complex. We are required to report any revisions to AMP or best price previously reported within a certain period, which revisions could affect our rebate liability for prior quarters. In addition, if we fail to provide information timely or we are found to have knowingly submitted false information to the government, the statute governing the Medicaid Drug Rebate Program provides for civil monetary penalties.
|
|
•
|
Medicare
: Medicare is a federal program that is administered by the federal government. The program covers individuals age 65 and over as well as those with certain disabilities. Medicare Part B generally covers drugs that must be administered by physicians or other health care practitioners; are provided in connection with certain durable medical equipment; or are certain oral anti-cancer drugs and certain oral immunosuppressive drugs. Medicare Part B pays for such drugs under a payment methodology based on the average sales price (ASP) of the drugs. Manufacturers, including us, are required to provide ASP information to the CMS on a quarterly basis. The manufacturer-submitted information is used to calculate Medicare payment rates. If a manufacturer is found to have made a misrepresentation in the reporting of ASP, the governing statute provides for civil monetary penalties.
|
|
•
|
Medicare Part D provides coverage to enrolled Medicare patients for self-administered drugs (i.e., drugs that are not administered by a physician). Medicare Part D is administered by private prescription drug plans approved by the U.S. government. Each drug plan establishes its own Medicare Part D formulary for prescription drug coverage and pricing, which the drug plan may modify from time-to-time. The prescription drug plans negotiate pricing with manufacturers and pharmacies, and may condition formulary placement on the availability of manufacturer
|
|
•
|
Federal Agency Discounted Pricing
: Our products are subject to discounted pricing when purchased by federal agencies via the Federal Supply Schedule (FSS). FSS participation is required for our products to be covered and reimbursed by the Veterans Administration (VA), Department of Defense, Coast Guard and Public Health Service (PHS). Coverage under Medicaid, Medicare and the PHS pharmaceutical pricing program is also conditioned upon FSS participation. FSS pricing is intended not to exceed the price that we charge our most-favored non-federal customer for a product. In addition, prices for drugs purchased by the VA, Department of Defense (including drugs purchased by military personnel and dependents through the TriCare retail pharmacy program), Coast Guard and PHS are subject to a cap on pricing equal to 76% of the non-federal average manufacturer price (non-FAMP). An additional discount applies if non-FAMP increases more than inflation (measured by the Consumer Price Index - Urban). In addition, if we fail to provide information timely or we are found to have knowingly submitted false information to the government, the governing statute provides for civil monetary penalties.
|
|
•
|
340B Discounted Pricing
: To maintain coverage of our products under the Medicaid Drug Rebate Program and Medicare Part B, we are required to extend significant discounts to certain covered entities that purchase products under Section 340B of the PHS pharmaceutical pricing program. Purchasers eligible for discounts include hospitals that serve a disproportionate share of financially needy patients, community health clinics and other entities that receive certain types of grants under the PHSA. For all of our products, we must agree to charge a price that will not exceed the amount determined under statute (the “ceiling price”) when we sell outpatient drugs to these covered entities. In addition, we may, but are not required to, offer these covered entities a price lower than the 340B ceiling price. The 340B discount formula is based on AMP and is generally similar to the level of rebates calculated under the Medicaid Drug Rebate Program.
|
|
Facility
|
Drug Substance Manufactured
|
|
|
RTP, North Carolina
|
ALPROLIX
AVONEX
ELOCTATE
PLEGRIDY
TYSABRI
ZINBRYTA
Other*
|
|
|
Hillerød, Denmark
|
TYSABRI
Biosimilars
|
|
|
Officer
|
Current Position
|
Age
|
Year Joined Biogen
|
|||
|
Michel Vounatsos
|
Chief Executive Officer
|
56
|
2016
|
|||
|
Susan H. Alexander
|
Executive Vice President, Chief Legal, Corporate Services and Secretary
|
61
|
2006
|
|||
|
Jeffrey D. Capello
|
Executive Vice President and Chief Financial Officer
|
53
|
2017
|
|||
|
Gregory F. Covino
|
Vice President, Finance and Chief Accounting Officer
|
52
|
2012
|
|||
|
Michael D. Ehlers, M.D., Ph.D.
|
Executive Vice President, Research and Development
|
49
|
2016
|
|||
|
Ginger Gregory, Ph.D.
|
Executive Vice President and Chief Human Resources Officer
|
50
|
2017
|
|||
|
Chirfi Guindo
|
Executive Vice President and Head of Global Marketing, Market Access and Customer Innovation
|
52
|
2017
|
|||
|
Paul McKenzie, Ph.D.
|
Executive Vice President, Pharmaceutical Operations and Technology
|
52
|
2016
|
|||
|
Alfred W. Sandrock, Jr., M.D., Ph.D.
|
Executive Vice President and Chief Medical Officer
|
60
|
1998
|
|||
|
Michel Vounatsos
|
|
|
Experience
|
|
|
Mr. Vounatsos has served as our Chief Executive Officer since January 2017. Prior to that, from April 2016 to December 2016, Mr. Vounatsos served as our Executive Vice President and Chief Commercial Officer. Prior to joining Biogen, Mr. Vounatsos spent 20 years at Merck where he most recently served as President, Primary Care, Customer Business Line. In this role, he led Merck’s global primary care business unit, a role which encompassed Merck’s cardiology-metabolic, general medicine, women’s health and biosimilars groups and developed and instituted a strategic framework for enhancing the company’s relationships with key constituents, including the most significant providers, payors and retailers and the world’s largest governments. Mr. Vounatsos previously held leadership positions across Europe and in China for Merck. Prior to that, Mr. Vounatsos held management positions at Ciba-Geigy.
|
|
|
Education
|
|
|
l
|
Universite Victor Segalen, Bordeaux II, France, C.S.C.T. Certificate in Medicine
|
|
l
|
HEC School of Management - Paris, M.B.A.
|
|
Susan H. Alexander
|
|
|
Experience
|
|
|
Ms. Alexander has served as our Executive Vice President, Chief Legal, Corporate Services and Secretary since March 2017. Prior to that, from December 2011 to March 2017, Ms. Alexander served as our Executive Vice President, Chief Legal Officer and Secretary and from 2006 to December 2011, as our Executive Vice President, General Counsel and Corporate Secretary. From 2003 to January 2006, Ms. Alexander served as the Senior Vice President, General Counsel and Corporate Secretary of PAREXEL International Corporation, a biopharmaceutical services company. From 2001 to 2003, Ms. Alexander served as General Counsel of IONA Technologies, a software company. From 1995 to 2001, Ms. Alexander served as Counsel at Cabot Corporation, a specialty chemicals and performance materials company. Prior to that, Ms. Alexander was a partner at the law firms of Hinckley, Allen & Snyder and Fine & Ambrogne.
|
|
|
Public Company Boards
|
|
|
l
|
Board of Directors of Invacare Corporation, a medical and healthcare product company
|
|
Education
|
|
|
l
|
Wellesley College, B.A.
|
|
l
|
Boston University School of Law, J.D.
|
|
Jeffrey D. Capello
|
|
|
Experience
|
|
|
Mr. Capello has served as our Executive Vice President and Chief Financial Officer since December 2017. Prior to that, Mr. Capello served as the Chief Financial Officer of Beacon Health Options, Inc., a behavioral health company, with responsibility for finance, human resources, information technology, real estate and procurement, from October 2016 until November 2017. From July 2015 until September 2016, Mr. Capello was the founder and Chief Executive Officer of Monomoy Advisors which focuses on helping companies drive shareholder value. From July 2014 until June 2015, Mr. Capello served as the Executive Vice President and Chief Financial Officer of Ortho-Clinical Diagnostics, an in vitro diagnostics company that was acquired by the Carlyle Group from Johnson & Johnson, with responsibility for global finance and business development. Prior to his role at Ortho-Clinical Diagnostics, Mr. Capello served as Chief Financial Officer and Executive Vice President of Boston Scientific Corporation, a medical device company, from March 2010 to December 2013. At Boston Scientific, Mr. Capello was responsible for the worldwide management of Boston Scientific’s finance, information systems, business development and corporate strategy functions. Mr. Capello joined Boston Scientific in June 2008 and served as Senior Vice President and Chief Accounting Officer until March 2010. Prior to joining Boston Scientific, he was the Senior Vice President and Chief Financial Officer with responsibilities for global finance and business development at PerkinElmer, Inc., a life sciences tool company, from 2006 to 2008. Previously, he served as PerkinElmer’s Vice President of Finance, Corporate Controller, Treasurer and Chief Accounting Officer from 2001 to 2006. Prior to his tenure at PerkinElmer, Mr. Capello was a Partner at PricewaterhouseCoopers LLP, both in the United States and in the Netherlands.
|
|
|
Public Company Boards
|
|
|
l
|
OvaScience, Inc., a biotechnology company
|
|
l
|
Flex Pharma, Inc., a biotechnology company
|
|
Education
|
|
|
l
|
University of Vermont, B.S. in Business Administration
|
|
l
|
Harvard Business School, M.B.A.
|
|
Gregory F. Covino
|
|
|
Experience
|
|
|
Mr. Covino has served as our Vice President and Chief Accounting Officer since April 2012. From June 2017 to December 2017, Mr. Covino also served as our interim Principal Financial Officer. From March 2010 to April 2012, Mr. Covino served at Boston Scientific Corporation, a medical device company, as Vice President, Corporate Analysis and Control, having responsibility for the company's internal audit function, and as Vice President, Finance, International from February 2008 to March 2010, having responsibility for the financial activities of the company's international division. Prior to that, Mr. Covino held several finance positions at Hubbell Incorporated, an electrical products company, including Vice President, Chief Accounting Officer and Controller from 2002 to January 2008, Interim Chief Financial Officer from 2004 to 2005, and Director, Corporate Accounting from 1999 to 2002.
|
|
|
Education
|
|
|
l
|
Bryant University, B.S. in Business Administration
|
|
Michael D. Ehlers, M.D., Ph.D.
|
|
|
Experience
|
|
|
Dr. Ehlers has served as our Executive Vice President, Head of Research and Development since May 2016. Prior to joining Biogen, from August 2010 to April 2016, Dr. Ehlers served in leadership positions at Pfizer, Inc., a biopharmaceutical company, including Senior Vice President & Head BioTherapeutics R&D and Chief Scientific Officer, Neuroscience & Pain. Prior to that, Dr. Ehlers was the George Barth Geller Professor of Neurobiology and an Investigator of the Howard Hughes Medical Institute at Duke University Medical Center. He is the recipient of numerous awards including the Eppendorf & Science Prize in Neurobiology, the John J. Abel Award in Pharmacology, the Society for Neuroscience Young Investigator Award, a National Institute of Mental Health MERIT Award, the National Alliance for Schizophrenia and Depression Distinguished Investigator Award and the Massachusetts Medical Society Honored Business Leader Award. In 2013, Dr. Ehlers became the 11
th
recipient of the Thudichum Medal of the Biochemical Society of the United Kingdom. Past recipients include two Nobel laureates. Dr. Ehlers has authored over 100 scientific papers, has served on the Editorial Boards of Annual Reviews in Medicine, Annual Reviews in Pharmacology and Toxicology, the Journal of Neuroscience, the Journal of Biological Chemistry, the Journal of Molecular and Cellular Neuroscience and has sat on advisory committees of the National Institutes of Health.
|
|
|
Outside Affiliations
|
|
|
l
|
PhRMA Foundation Basic Pharmacology Advisory Committee
|
|
l
|
Janelia Research Institute Advisory Committee
|
|
l
|
McKnight Endowment Fund for Neuroscience Board
|
|
l
|
World Economic Forum Global Agenda Council on Brain Research
|
|
Education
|
|
|
l
|
California Institute of Technology, B.S. Chemistry
|
|
l
|
The Johns Hopkins University School of Medicine, M.D.
|
|
l
|
The Johns Hopkins University School of Medicine, Ph.D. Neuroscience
|
|
Ginger Gregory, Ph.D.
|
|
|
Experience
|
|
|
Dr. Gregory has served as our Executive Vice President and Chief Human Resources Officer since July 2017. Prior to joining Biogen, Dr. Gregory served as Executive Vice President and Chief Human Resources Officer at Shire PLC, a global specialty biopharmaceutical company, from February 2014 to April 2017. Prior to that, Dr. Gregory held executive-level human resources positions for several multinational companies across a variety of industries, including Dunkin’ Brands, where she served as Chief Human Resource Officer; Novartis, AG, where she was the division head of Human Resources for Novartis Vaccines and Diagnostics, Novartis Consumer Health and Novartis Institutes of BioMedical Research from 2005 to 2012; and Novo Nordisk, where she served as Senior Vice President, Corporate People & Organization at the company’s headquarters in Copenhagen, Denmark. Earlier in her career, she held a variety of human resources generalist and specialist positions at Bristol-Myers Squibb and served as a consultant with Booz Allen & Hamilton in the area of organization change and effectiveness.
|
|
|
Education
|
|
|
l
|
University of Massachusetts B.A., in Psychology
|
|
l
|
The George Washington University, Ph.D. Psychology
|
|
Chirfi Guindo
|
|
|
Experience
|
|
|
Mr. Guindo has served as our Executive Vice President and Head of Global Marketing, Market Access and Customer Innovation since November 2017. Prior to joining Biogen, Mr. Guindo spent 27 years in the global pharmaceutical industry and has held several leadership positions at Merck in Canada, the U.S., France, Africa and the Netherlands. He worked in several disciplines including Finance, Sales & Marketing, General Management and Global Strategy/Product Development in specialty, acute and hospital care. Most recently Mr. Guindo was Vice President and Managing Director and President and Managing Director of Merck Canada from October 2014 to November 2017. From January 2011 to October 2014, he was Vice President and General Manager, Global HIV Franchise at Merck & Co.
|
|
|
Education
|
|
|
l
|
Ecole Central de Paris (France), Engineering
|
|
l
|
Stern School of Business, New York University, M.B.A. in Finance/Economics
|
|
Paul McKenzie, Ph.D.
|
|
|
Experience
|
|
|
Dr. McKenzie has served as our Executive Vice President, Pharmaceutical Operations and Technology since July 2016. Prior to that, from February 2016 to June 2016, he served as our Senior Vice President for Global Biologics Manufacturing & Technical Operations. Prior to joining Biogen, since 2008, Dr. McKenzie held a number of positions of increasing responsibility at Johnson & Johnson (J&J), including Vice President of R&D for J&J’s Ethicon business where he led the manufacturing and technical operations team responsible for internal and external manufacturing of Janssen’s pharmaceutical portfolio. He also ran global Development for Janssen R&D, helping to manage pipeline activities from discovery through clinical development and commercialization. Prior to J&J, Dr. McKenzie also held various R&D and manufacturing positions at Bristol-Myers Squibb and Merck & Co.
|
|
|
Education
|
|
|
l
|
University of Pennsylvania, B.S. Chemical Engineering
|
|
l
|
Carnegie Mellon University, Ph.D. Chemical Engineering
|
|
Alfred W. Sandrock, Jr., M.D., Ph.D.
|
|
|
Experience
|
|
|
Dr. Sandrock has served as our Executive Vice President and Chief Medical Officer since October 2017. Prior to that, Dr. Sandrock served as our Executive Vice President, Chief Medical Officer Neurology and Neurodegeneration from October 2015 to October 2017, as our Chief Medical Officer and Group Senior Vice President from April 2013 to October 2015 and as our Chief Medical Officer and Senior Vice President of Development Sciences from February 2012 to April 2013. Prior to that, Dr. Sandrock held several senior executive positions since joining us in 1998, including Senior Vice President of Neurology Research and Development and Vice President of Clinical Development, Neurology.
|
|
|
Public Company Boards
|
|
|
l
|
Board of Directors of Neurocrine Biosciences, Inc., a life sciences company
|
|
Education
|
|
|
l
|
Stanford University, B.A. in Human Biology
|
|
l
|
Harvard Medical School, M.D.
|
|
l
|
Harvard University, Ph.D. in Neurobiology
|
|
l
|
Massachusetts General Hospital, internship in Medicine, residency and chief residency in Neurology, and clinical fellowship in Neuromuscular Disease and Clinical Neurophysiology (electromyography)
|
|
•
|
safety or efficacy issues;
|
|
•
|
the introduction or greater acceptance of competing products, including lower-priced competing products;
|
|
•
|
constraints and additional pressures on product pricing or price increases, including those resulting from governmental or regulatory requirements, increased competition or changes in, or implementation of, reimbursement policies and practices of payors and other third parties; or
|
|
•
|
adverse legal, administrative, regulatory or legislative developments.
|
|
•
|
our limited marketing experience within the SMA market, which may impact our ability to develop relationships with the associated medical and scientific community;
|
|
•
|
the lack of readiness of healthcare providers to treat patients with SMA;
|
|
•
|
the effectiveness of our commercial strategy for marketing SPINRAZA; and
|
|
•
|
our ability to maintain a positive reputation among patients, healthcare providers and others in the SMA community, which may be impacted by pricing and reimbursement decisions relating to SPINRAZA.
|
|
•
|
the introduction of more efficacious, safer, less expensive or more convenient alternatives to our MS products, including our own products and products of our collaborators;
|
|
•
|
the introduction of lower-cost biosimilars, follow-on products or generic versions of branded MS products sold by our competitors, and the possibility of future competition from generic versions or prodrugs of existing therapeutics or from off-label use by physicians of therapies indicated for other conditions to treat MS patients;
|
|
•
|
patient dynamics, including the size of the patient population and our ability to attract new patients to our therapies;
|
|
•
|
damage to physician and patient confidence in any of our MS products or to our sales and reputation as a result of label changes or adverse experiences or events that may occur with patients treated with our MS products;
|
|
•
|
inability to obtain appropriate pricing and reimbursement for our MS products compared to our competitors in key international markets; or
|
|
•
|
our ability to obtain and maintain patent, data or market exclusivity for our MS products.
|
|
•
|
changes in, and implementation of, federal, state or foreign government regulations or private third-party payors’ reimbursement policies;
|
|
•
|
pressure by employers on private health insurance plans to reduce costs;
|
|
•
|
consolidation and increasing assertiveness of payors, including managed care organizations, health insurers, pharmacy benefit managers, government health administration authorities, private health insurers and other organizations, seeking price discounts or rebates in connection with the placement of our products on their formularies and, in some cases, the imposition of restrictions on access or coverage of particular drugs or pricing determined based on perceived value; and
|
|
•
|
our value-based contracting pilot program pursuant to which we aim to tie the pricing of our products to their clinical values by either aligning price to patient outcomes or adjusting price for patients who discontinue therapy for any reason, including efficacy or tolerability concerns.
|
|
•
|
Risk of Product Loss.
The manufacturing process for our products is extremely susceptible to product loss due to contamination, oxidation, equipment failure or improper installation or operation of equipment or vendor or operator error. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects and other supply disruptions. If microbial, viral or other contaminations are discovered in our products or manufacturing facilities, we may need to close our manufacturing facilities for an extended period of time to investigate and remediate the contaminant.
|
|
•
|
Risks of Reliance on Third Parties and Single Source Providers.
We rely on third-party suppliers and manufacturers for many aspects of our manufacturing process for our products and product candidates. In some cases, due to the unique manner in which our products are manufactured, we rely on single source providers of raw materials and manufacturing supplies. These third parties are independent entities subject to their own unique operational and financial risks that are outside of our control. These third parties may not perform their obligations in a timely and cost-effective manner or in compliance with applicable regulations, and they may be unable or unwilling to increase production capacity commensurate with demand for our existing or future products. Finding alternative providers could take a significant amount of time and involve significant expense due to the specialized nature of the services and the need to obtain regulatory approval of any significant changes to our suppliers or manufacturing methods. We cannot be certain that we could reach agreement with alternative providers or that the FDA or other regulatory authorities would approve our use of such alternatives.
|
|
•
|
Global Bulk Supply Risks.
We rely on our principal manufacturing facilities for the production of drug substance for our large molecule products and product candidates. Our global bulk supply of these products and product candidates depends on the uninterrupted and efficient operation of these facilities, which could be adversely affected by equipment failures, labor shortages, natural disasters, power failures and numerous other factors.
|
|
•
|
Risks Relating to Compliance with cGMP.
We and our third-party providers are generally required to maintain compliance with cGMP and other stringent requirements and are subject to inspections by the FDA and comparable agencies in other jurisdictions to confirm such compliance. Any delay, interruption or other issues that arise in the manufacture, fill-finish, packaging or storage of our products as a result of a failure of our facilities or the facilities or operations of third parties to pass any regulatory agency inspection could significantly impair our ability to develop and commercialize our products. Significant noncompliance could also result in the imposition of monetary penalties or other civil or criminal sanctions and damage our reputation.
|
|
•
|
we may be unable to control the resources our collaborators or third parties devote to our programs or products;
|
|
•
|
disputes may arise under the agreement, including with respect to the achievement and payment of milestones or ownership of rights to technology developed with our collaborators or other third parties, and the underlying contract with our collaborators or other third parties may fail to provide significant protection or may fail to be effectively enforced if the collaborators or third parties fail to perform;
|
|
•
|
the interests of our collaborators or third parties may not always be aligned with our interests, and such parties may not pursue regulatory approvals or market a product in the same manner or to the same extent that we would, which could adversely affect our revenues;
|
|
•
|
third-party relationships and collaborations often require the parties to cooperate, and failure to do so effectively could adversely affect product sales, or the clinical development or regulatory approvals of products under joint control or could result in termination of the research, development or commercialization of product candidates or result in litigation or arbitration; and
|
|
•
|
any failure on the part of our collaborators or other third parties to comply with applicable laws and regulatory requirements in the marketing, sale and maintenance of the marketing authorization of our products or to fulfill any responsibilities our collaborators or other third parties may have to protect and enforce any intellectual property rights underlying our products could have an adverse effect on our revenues as well as involve us in possible legal proceedings.
|
|
•
|
new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or judicial decisions, related to health care availability, pricing or marketing practices, compliance with wage and hour laws and other employment practices, method of delivery, payment for health care products and services, compliance with health information and data privacy and security laws and regulations, tracking and reporting payments and other transfers of value made to physicians and teaching hospitals, extensive anti-bribery and anti-corruption prohibitions, product serialization and labeling requirements and used product take-back requirements;
|
|
•
|
changes in the FDA and foreign regulatory approval processes that may delay or prevent the approval of new products and result in lost market opportunity;
|
|
•
|
the hiring freeze implemented by the federal government in 2017, including at the FDA, could impact the review and potential approval of new products, which may adversely affect our business and financial condition;
|
|
•
|
requirements that provide for increased transparency of clinical trial results and quality data, such as the EMA’s clinical transparency policy, which could impact our ability to protect trade secrets and competitively-sensitive information contained in approval applications or could be misinterpreted leading to reputational damage, misperception or legal action which could harm our business; and
|
|
•
|
changes in FDA and foreign regulations that may require additional safety monitoring, labeling changes, restrictions on product distribution or use, or other measures after the introduction of our products to market, which could increase our costs of doing business, adversely affect the future permitted uses of approved products or otherwise adversely affect the market for our products.
|
|
•
|
the inability to obtain necessary foreign regulatory or pricing approvals of products in a timely manner;
|
|
•
|
uncertainties regarding the collectability of accounts receivable;
|
|
•
|
fluctuations in foreign currency exchange rates that may adversely impact our revenues and net income;
|
|
•
|
difficulties in staffing and managing international operations;
|
|
•
|
the imposition of governmental controls;
|
|
•
|
less favorable intellectual property or other applicable laws;
|
|
•
|
increasingly complex standards for complying with foreign laws and regulations that may differ substantially from country to country and may conflict with corresponding U.S. laws and regulations;
|
|
•
|
the far-reaching anti-bribery and anti-corruption legislation in the U.K., including the Bribery Act, and elsewhere and escalation of investigations and prosecutions pursuant to such laws;
|
|
•
|
the effects of the implementation of the U.K.’s decision to voluntarily depart from the E.U., known as Brexit;
|
|
•
|
compliance with complex import and export control laws;
|
|
•
|
restrictions on direct investments by foreign entities and trade restrictions;
|
|
•
|
greater political or economic instability; and
|
|
•
|
changes in tax laws and tariffs.
|
|
•
|
the cost of restructurings or other initiatives to streamline our operations and reallocate resources;
|
|
•
|
impairments with respect to investments, fixed assets and long-lived assets, including in-process R&D and other intangible assets;
|
|
•
|
inventory write-downs for failed quality specifications, charges for excess or obsolete inventory and charges for inventory write downs relating to product suspensions, expirations or recalls;
|
|
•
|
changes in the fair value of contingent consideration;
|
|
•
|
bad debt expenses and increased bad debt reserves;
|
|
•
|
outcomes of litigation and other legal or administrative proceedings, regulatory matters and tax matters;
|
|
•
|
milestone payments under license and collaboration agreements; and
|
|
•
|
payments in connection with acquisitions and other business development activities.
|
|
•
|
Reliance on Third Parties.
We are dependent on the efforts of Samsung Bioepis and other third parties over whom we have limited or no control in the development and manufacturing of biosimilars products. If Samsung Bioepis or such other third parties fail to perform successfully, we may not realize the anticipated benefits of our investment in Samsung Bioepis;
|
|
•
|
Regulatory Compliance.
Biosimilar products may face regulatory hurdles or delays due to the evolving and uncertain regulatory and commercial pathway of biosimilars products in certain jurisdictions;
|
|
•
|
Intellectual Property and Regulatory Challenges.
Biosimilar products may face extensive patent clearances, patent infringement litigation, injunctions or regulatory challenges, which could prevent the commercial launch of a product or delay it for many years;
|
|
•
|
Failure to Gain Market and Patient Acceptance.
Market success of biosimilar products will be adversely affected if patients, physicians and/or payors do not accept biosimilar products as safe and efficacious products offering a more competitive price or other benefit over existing therapies;
|
|
•
|
Ability to Provide Adequate Supply.
Manufacturing biosimilars is complex. If we encounter any manufacturing or supply chain difficulties, we may be unable to meet higher than anticipated demand; and
|
|
•
|
Competitive Challenges.
Biosimilar products face significant competition, including from innovator products and from biosimilar products offered by other companies. In some jurisdictions, local tendering processes may restrict biosimilar products from being marketed and sold in those jurisdictions. The number of competitors in a jurisdiction, the timing of approval and the ability to market biosimilar products successfully in a timely and cost-effective matter are additional factors that may impact our success and/or the success of Samsung Bioepis in this business area.
|
|
•
|
increase our vulnerability to general adverse economic and industry conditions;
|
|
•
|
limit our ability to access capital markets and incur additional debt in the future;
|
|
•
|
require us to dedicate a substantial portion of our cash flow from operations to payments on our indebtedness, thereby reducing the availability of our cash flow for other purposes, including business development efforts, research and development and mergers and acquisitions; and
|
|
•
|
limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate, thereby placing us at a competitive disadvantage compared to our competitors that have less debt.
|
|
•
|
800,000 square feet in Cambridge, MA, which is comprised of offices for our corporate headquarters, and other administrative and development functions and laboratories, of which 242,000 square feet is subleased by multiple companies for general office space, laboratories and manufacturing facilities; and
|
|
•
|
357,000 square feet of office space in Weston, MA, of which 174,000 square feet has been subleased through the remaining term of our lease agreement.
|
|
•
|
357,000 square feet of laboratory and office space;
|
|
•
|
188,000 square feet related to an oral solid dose manufacturing facility;
|
|
•
|
175,000 square feet related to a large-scale biologics manufacturing facility;
|
|
•
|
105,000 square feet related to a small-scale biologics manufacturing facility;
|
|
•
|
84,000 square feet of warehouse space and utilities;
|
|
•
|
70,000 square feet related to a parenteral fill-finish facility; and
|
|
•
|
43,000 square feet related to a large-scale purification facility.
|
|
•
|
139,000 square feet of warehouse, utilities and support space;
|
|
•
|
70,000 square feet related to a label and packaging facility;
|
|
•
|
50,000 square feet related to a laboratory facility; and
|
|
•
|
47,000 square feet of administrative space.
|
|
|
Common Stock Price
|
||||||||||||||
|
|
2017
|
2016
|
|||||||||||||
|
|
High
|
Low
|
High
|
Low
|
|||||||||||
|
First Quarter
|
$
|
298.00
|
|
$
|
254.15
|
|
$
|
301.02
|
|
$
|
242.07
|
|
|||
|
Second Quarter
|
$
|
291.90
|
|
$
|
244.28
|
|
$
|
292.69
|
|
$
|
223.02
|
|
|||
|
Third Quarter
|
$
|
330.00
|
|
$
|
269.50
|
|
$
|
333.65
|
|
$
|
240.07
|
|
|||
|
Fourth Quarter
|
$
|
348.84
|
|
$
|
301.81
|
|
$
|
329.83
|
|
$
|
268.00
|
|
|||
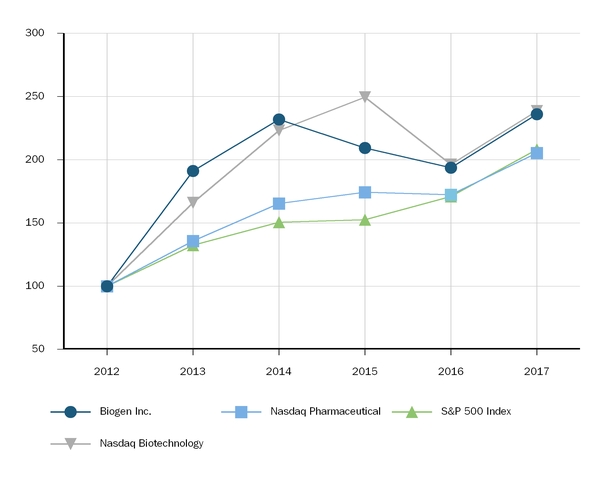
|
2012
|
2013
|
2014
|
2015
|
2016
|
2017
|
|||||||
|
Biogen Inc.
|
100.00
|
|
191.00
|
|
231.91
|
|
209.30
|
|
193.74
|
|
235.96
|
|
|
Nasdaq Pharmaceutical
|
100.00
|
|
135.68
|
|
165.29
|
|
174.27
|
|
172.37
|
|
205.33
|
|
|
S&P 500 Index
|
100.00
|
|
132.39
|
|
150.51
|
|
152.59
|
|
170.84
|
|
208.14
|
|
|
Nasdaq Biotechnology
|
100.00
|
|
166.02
|
|
223.13
|
|
249.39
|
|
196.15
|
|
238.64
|
|
|
Our results of operations are summarized as follows:
|
|||||||||||||||||||
|
|
For the Years Ended December 31,
|
||||||||||||||||||
|
|
2017
|
2016
|
2015
|
2014
|
2013
|
||||||||||||||
|
(In millions, except per share amounts)
|
(a) (b) (c) (d) (e)
|
(c) (e)
|
(e) (f)
|
(g)
|
|||||||||||||||
|
Results of Operations (1)
|
|||||||||||||||||||
|
Product revenues, net
(2)
|
$
|
10,354.7
|
|
$
|
9,817.9
|
|
$
|
9,188.5
|
|
$
|
8,203.4
|
|
$
|
5,542.3
|
|
||||
|
Revenues from anti-CD20 therapeutic programs
|
1,559.2
|
|
1,314.5
|
|
1,339.2
|
|
1,195.4
|
|
1,126.0
|
|
|||||||||
|
Other revenues
|
360.0
|
|
316.4
|
|
236.1
|
|
304.5
|
|
263.9
|
|
|||||||||
|
Total revenues
|
12,273.9
|
|
11,448.8
|
|
10,763.8
|
|
9,703.3
|
|
6,932.2
|
|
|||||||||
|
Total cost and expenses
|
6,929.7
|
|
6,298.4
|
|
5,872.8
|
|
5,747.7
|
|
4,441.6
|
|
|||||||||
|
Gain on sale of rights
|
—
|
|
—
|
|
—
|
|
16.8
|
|
24.9
|
|
|||||||||
|
Income from operations
|
5,344.2
|
|
5,150.4
|
|
4,891.0
|
|
3,972.4
|
|
2,515.5
|
|
|||||||||
|
Other income (expense), net
|
(215.4
|
)
|
(217.4
|
)
|
(123.7
|
)
|
(25.8
|
)
|
(34.9
|
)
|
|||||||||
|
Income before income tax expense and equity in loss of investee, net of tax
|
5,128.8
|
|
4,933.0
|
|
4,767.3
|
|
3,946.6
|
|
2,480.6
|
|
|||||||||
|
Income tax expense
|
2,458.7
|
|
1,237.3
|
|
1,161.6
|
|
989.9
|
|
601.0
|
|
|||||||||
|
Equity in loss of investee, net of tax
|
—
|
|
—
|
|
12.5
|
|
15.1
|
|
17.2
|
|
|||||||||
|
Net income
|
2,670.1
|
|
3,695.7
|
|
3,593.2
|
|
2,941.6
|
|
1,862.3
|
|
|||||||||
|
Net income (loss) attributable to noncontrolling interests, net of tax
|
131.0
|
|
(7.1
|
)
|
46.2
|
|
6.8
|
|
—
|
|
|||||||||
|
Net income attributable to Biogen Inc.
|
$
|
2,539.1
|
|
$
|
3,702.8
|
|
$
|
3,547.0
|
|
$
|
2,934.8
|
|
$
|
1,862.3
|
|
||||
|
Diluted Earnings Per Share
|
|||||||||||||||||||
|
Diluted earnings per share attributable to Biogen Inc.
|
$
|
11.92
|
|
$
|
16.93
|
|
$
|
15.34
|
|
$
|
12.37
|
|
$
|
7.81
|
|
||||
|
Weighted-average shares used in calculating diluted earnings per share attributable to Biogen Inc.
|
213.0
|
|
218.8
|
|
231.2
|
|
237.2
|
|
238.3
|
|
|||||||||
|
Our financial condition is summarized as follows:
|
|||||||||||||||||||
|
As of December 31,
|
|||||||||||||||||||
|
2017
|
2016
|
2015
|
2014
|
2013
|
|||||||||||||||
|
(In millions)
|
|||||||||||||||||||
|
Financial Condition (1)
|
|||||||||||||||||||
|
Cash, cash equivalents and marketable securities
|
$
|
6,746.3
|
|
$
|
7,724.5
|
|
$
|
6,188.9
|
|
$
|
3,316.0
|
|
$
|
1,848.5
|
|
||||
|
Total assets
|
$
|
23,652.6
|
|
$
|
22,876.8
|
|
$
|
19,504.8
|
|
$
|
14,314.7
|
|
$
|
11,863.3
|
|
||||
|
Notes payable and other financing arrangements, less current portion
(3)
|
$
|
5,935.0
|
|
$
|
6,512.7
|
|
$
|
6,521.5
|
|
$
|
580.3
|
|
$
|
592.4
|
|
||||
|
Total Biogen Inc. shareholders’ equity
(4)
|
$
|
12,612.8
|
|
$
|
12,140.1
|
|
$
|
9,372.8
|
|
$
|
10,809.0
|
|
$
|
8,620.2
|
|
||||
|
(1)
|
On February 1, 2017, we completed the spin-off of our hemophilia business, Bioverativ, as an independent, publicly traded company. Our consolidated results of operations and financial position reflect the financial results of our hemophilia business for all periods through January 31, 2017. For additional information on the spin-off of our hemophilia business, please read Note 3,
Hemophilia Spin-Off
, to our consolidated financial statements included in this report.
|
|
(2)
|
Product revenues, net reflect the impact of the following product launches:
|
|
•
|
Commercial sales of SPINRAZA in the U.S. began in the fourth quarter of 2016 and in rest of world markets in the first quarter of 2017.
|
|
•
|
Under our collaboration agreement with AbbVie, we began to recognize revenues on sales of ZINBRYTA to third parties in the E.U. in the third quarter of 2016.
|
|
•
|
Under our commercial agreement with Samsung Bioepis, we began to recognize revenues on sales of BENEPALI and FLIXABI to third parties in the E.U. in the first quarter of 2016 and third quarter of 2016, respectively.
|
|
•
|
Commercial sales of ALPROLIX commenced in the second quarter of 2014 and commercial sales of ELOCTATE and PLEGRIDY commenced in the third quarter of 2014.
|
|
•
|
Commercial sales of TECFIDERA began in April 2013.
|
|
(3)
|
Notes payable and other financing arrangements reflects:
|
|
•
|
Our 2017 repayment of our 6.875% notes that were issued in 2008 with an aggregate principal amount of $550.0 million, and
|
|
•
|
The issuance of our senior unsecured notes for an aggregate principal amount of $6.0 billion in September 2015.
|
|
(4)
|
Total Biogen Inc. shareholders' equity reflects the repurchase of approximately 29.9 million shares of our common stock at a cost of approximately $8.7 billion between 2013 and 2017:
|
|
•
|
During 2017 we repurchased and retired approximately 3.7 million shares of our common stock at a cost of $1.0 billion under our 2016 Share Repurchase Program.
|
|
•
|
During 2017 we repurchased approximately 1.2 million shares of our common stock at a cost of $365.4 million under our 2011 Share Repurchase Program.
|
|
•
|
During 2016 we repurchased and retired approximately 3.3 million shares of our common stock at a cost of $1.0 billion under our 2016 Share Repurchase Program.
|
|
•
|
During 2015 we repurchased and retired approximately 16.8 million shares of our common stock at a cost of $5.0 billion under a program authorized by our Board of Directors in May 2015 for the repurchase of up to $5.0 billion of our common stock (2015 Share Repurchase Program).
|
|
•
|
During 2014 and 2013 we repurchased approximately 2.9 million and 2.0 million shares, respectively, of our common stock at a cost of approximately $1.3 billion under our 2011 Share Repurchase Program.
|
|
(a)
|
Total cost and expenses for the year ended December 31, 2017, includes a pre-tax charge to acquired in-process research and development of $120.0 million for an upfront payment made to Remedy upon closing of our asset purchase transaction for BIIB093.
|
|
(b)
|
Net income (loss) attributable to noncontrolling interests, net of tax for the year ended December 31, 2017, includes a pre-tax charge of $150.0 million for a payment to Neurimmune in exchange for a 15% reduction in royalty rates payable on products developed under the agreement, including on potential commercial sales of aducanumab.
|
|
(c)
|
Total cost and expenses for the year ended December 31, 2016, includes a pre-tax charge of $454.8 million related to our January 2017 settlement and license agreement with Forward Pharma.
|
|
(d)
|
Income tax expense for the year ended December 31, 2017, includes
$1,173.6 million
related to our current estimate of the provisions of the 2017 Tax Act, including a
$989.6 million
expense under the Transition Toll Tax. For additional information on the 2017 Tax Act, please read Note 17,
Income Taxes
, to our consolidated financial statements included in this report.
|
|
(e)
|
Total cost and expenses for the years ended December 31, 2017, 2016 and 2015, include restructuring charges of $0.9 million, $33.1 million and $93.4 million, respectively. In addition, total cost and expenses for the year ended December 31, 2016, also include charges to cost of sales totaling $52.4 million of expenses incurred as a result of our determination to cease manufacturing and vacate our small-scale biologics facility in Cambridge, MA as well as close and vacate our warehouse in Somerville, MA. Total cost and expenses for the years ended December 31, 2017 and 2016, also includes $19.2 million and $18.1 million, respectively, of costs incurred directly related to the spin-off of our hemophilia business into an independent, publicly traded company.
|
|
(f)
|
Net income attributable to Biogen Inc. for the year ended December 31, 2015, includes a pre-tax charge to noncontrolling interest of $60.0 million for a milestone payment due to Neurimmune upon the enrollment of the first patient in a Phase 3 trial for aducanumab.
|
|
(g)
|
Commencing in the second quarter of 2013 product and total revenues include 100% of net revenues related to sales of TYSABRI as a result of our acquisition of all remaining rights to TYSABRI from Elan Pharma International, Ltd (Elan), an affiliate of Elan Corporation, plc. Upon closing of this transaction, our collaboration agreement was terminated.
|
|
•
|
maximizing the resilience of our MS core business;
|
|
•
|
accelerating efforts in SMA as a significant new growth opportunity;
|
|
•
|
developing and expanding our neuroscience portfolio;
|
|
•
|
focusing our capital allocation efforts to drive investment for future growth; and
|
|
•
|
creating a leaner and simpler operating model to streamline our operations and reallocate resources towards prioritized research and development and commercial value creation opportunities.
|
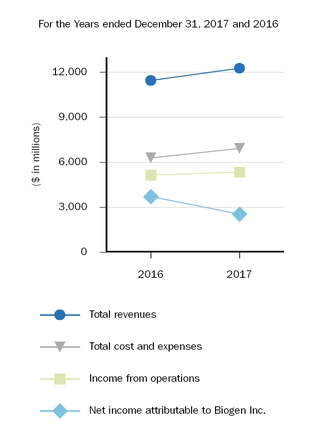
|
•
|
Total revenues were
$12,273.9 million
for
2017
, representing an increase of
7.2%
over the same period in
2016
.
|
|
•
|
Product revenues, net totaled
$10,354.7 million
for
2017
, representing an increase of
5.5%
over the same period in
2016
. This increase was primarily driven by revenues from SPINRAZA, TECFIDERA and BENEPALI, partially offset by the elimination of worldwide ALPROLIX and ELOCTATE revenues resulting from the spin-off of our hemophilia business on February 1, 2017 and a net decrease in total Interferon sales.
|
|
•
|
Revenues from anti-CD20 therapeutic programs totaled
$1,559.2 million
for
2017
, representing an increase of
18.6%
over the same period in
2016
. This increase was primarily driven by royalty revenues on sales of OCREVUS and Biogen's share of pre-tax profits on RITUXAN.
|
|
•
|
Other revenues totaled
$360.0 million
for
2017
, representing an increase of
13.8%
from the same period in
2016
. This increase was primarily driven by an increase in other royalty and corporate revenues.
|
|
•
|
Total cost and expenses totaled
$6,929.7 million
for
2017
, representing an increase of
10.0%
, compared to the same period in
2016
. This increase was primarily driven by
$444.2 million
of amortization and impairment charges related to our U.S. and rest of world licenses to Forward Pharma's intellectual property, including Forward Pharma's intellectual property related to TECFIDERA, a
14.2%
increase in research and development primarily related to higher milestone and upfront expenses, a
10.2%
increase in cost of goods sold, a $120.0 million pre-tax charge to acquired in-process research and development for an upfront payment made to Remedy upon the closing of the asset purchase transaction for BIIB093 and an increase in collaboration profit sharing. These increases were partially offset by a $454.8 million litigation settlement charge in the prior year.
|
|
•
|
We generated
$4,551.0 million
of net cash flows from operations for
2017
, which were primarily driven by earnings.
|
|
•
|
Cash, cash equivalents and marketable securities totaled approximately
$6,746.3 million
as of
December 31, 2017
.
|
|
•
|
We repurchased approximately 4.9 million shares of common stock at a cost of $1.4 billion during 2017 under our share repurchase programs.
|
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2017 compared to 2016
|
2016 compared to 2015
|
|||||||||||||||
|
(In millions, except percentages)
|
2017
|
2016
|
2015
|
||||||||||||||
|
Product Revenues:
|
|||||||||||||||||
|
United States
|
$
|
7,017.1
|
|
$
|
7,050.4
|
|
$
|
6,545.8
|
|
(0.5
|
)%
|
7.7
|
%
|
||||
|
Rest of world
|
3,337.6
|
|
2,767.5
|
|
2,642.7
|
|
20.6
|
%
|
4.7
|
%
|
|||||||
|
Total product revenues
|
10,354.7
|
|
9,817.9
|
|
9,188.5
|
|
5.5
|
%
|
6.8
|
%
|
|||||||
|
Revenues from anti-CD20 therapeutic programs
|
1,559.2
|
|
1,314.5
|
|
1,339.2
|
|
18.6
|
%
|
(1.8
|
)%
|
|||||||
|
Other revenues
|
360.0
|
|
316.4
|
|
236.1
|
|
13.8
|
%
|
34.0
|
%
|
|||||||
|
Total revenues
|
$
|
12,273.9
|
|
$
|
11,448.8
|
|
$
|
10,763.8
|
|
7.2
|
%
|
6.4
|
%
|
||||
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2017 compared to 2016
|
2016 compared to 2015
|
|||||||||||||||
|
(In millions, except percentages)
|
2017
|
2016
|
2015
|
||||||||||||||
|
Multiple Sclerosis:
|
|||||||||||||||||
|
TECFIDERA
|
$
|
4,214.0
|
|
$
|
3,968.1
|
|
$
|
3,638.4
|
|
6.2
|
%
|
9.1
|
%
|
||||
|
Interferon*
|
2,645.8
|
|
2,795.2
|
|
2,968.7
|
|
(5.3
|
)%
|
(5.8
|
)%
|
|||||||
|
TYSABRI
|
1,973.1
|
|
1,963.8
|
|
1,886.1
|
|
0.5
|
%
|
4.1
|
%
|
|||||||
|
FAMPYRA
|
91.6
|
|
84.9
|
|
89.7
|
|
7.9
|
%
|
(5.4
|
)%
|
|||||||
|
ZINBRYTA
|
52.7
|
|
7.8
|
|
—
|
|
**
|
|
**
|
|
|||||||
|
Spinal Muscular Atrophy:
|
|||||||||||||||||
|
SPINRAZA
|
883.7
|
|
4.6
|
|
—
|
|
**
|
|
**
|
|
|||||||
|
Hemophilia:
|
|||||||||||||||||
|
ELOCTATE
|
48.4
|
|
513.2
|
|
319.7
|
|
(90.6
|
)%
|
60.5
|
%
|
|||||||
|
ALPROLIX
|
26.0
|
|
333.7
|
|
234.5
|
|
(92.2
|
)%
|
42.3
|
%
|
|||||||
|
Other product revenues:
|
|||||||||||||||||
|
FUMADERM
|
39.6
|
|
45.9
|
|
51.4
|
|
(13.7
|
)%
|
(10.7
|
)%
|
|||||||
|
BENEPALI
|
370.8
|
|
100.6
|
|
—
|
|
**
|
|
**
|
|
|||||||
|
FLIXABI
|
9.0
|
|
0.1
|
|
—
|
|
**
|
|
**
|
|
|||||||
|
Total product revenues
|
$
|
10,354.7
|
|
$
|
9,817.9
|
|
$
|
9,188.5
|
|
5.5
|
%
|
6.8
|
%
|
||||
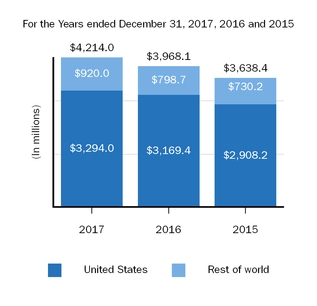
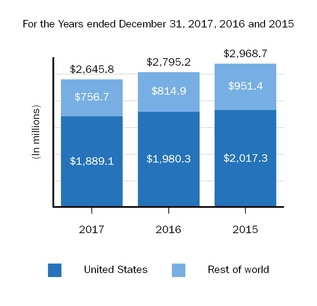
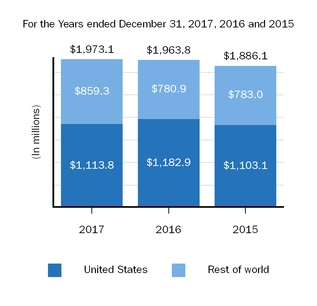
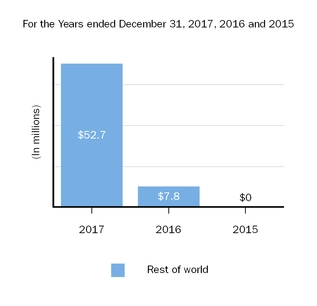



|
|
For the Years Ended
December 31,
|
||||||||||
|
|
|||||||||||
|
(In millions)
|
2017
|
2016
|
2015
|
||||||||
|
Product revenues, net
|
$
|
4,206.9
|
|
$
|
3,941.8
|
|
$
|
3,847.9
|
|
||
|
Cost and expenses
|
755.2
|
|
744.5
|
|
673.7
|
|
|||||
|
Pre-tax profits in the U.S.
|
$
|
3,451.7
|
|
$
|
3,197.3
|
|
$
|
3,174.2
|
|
||
|
Biogen's share of pre-tax profits
|
$
|
1,316.4
|
|
$
|
1,249.5
|
|
$
|
1,269.8
|
|
||
|
For The Years
Ended December 31,
|
% Change
|
||||||||||||||||
|
2017 compared to 2016
|
2016 compared to 2015
|
||||||||||||||||
|
(In millions, except percentages)
|
2017
|
2016
|
2015
|
||||||||||||||
|
Revenues from collaborative and other relationships
|
$
|
36.5
|
|
$
|
39.3
|
|
$
|
69.1
|
|
(7.1
|
)%
|
(43.1
|
)%
|
||||
|
Other royalty and corporate revenues
|
323.5
|
|
277.1
|
|
167.0
|
|
16.7
|
%
|
65.9
|
%
|
|||||||
|
Total other revenues
|
$
|
360.0
|
|
$
|
316.4
|
|
$
|
236.1
|
|
13.8
|
%
|
34.0
|
%
|
||||

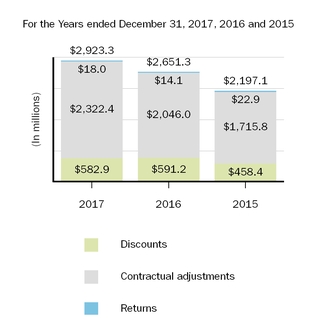
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2017 compared to 2016
|
2016 compared to 2015
|
|||||||||||||||
|
(In millions, except percentages)
|
2017
|
2016
|
2015
|
||||||||||||||
|
Cost of sales, excluding amortization of acquired intangible assets
|
$
|
1,630.0
|
|
$
|
1,478.7
|
|
$
|
1,240.4
|
|
10.2
|
%
|
19.2
|
%
|
||||
|
Research and development
|
2,253.6
|
|
1,973.3
|
|
2,012.8
|
|
14.2
|
%
|
(2.0
|
)%
|
|||||||
|
Selling, general and administrative
|
1,935.5
|
|
1,947.9
|
|
2,113.1
|
|
(0.6
|
)%
|
(7.8
|
)%
|
|||||||
|
Amortization of acquired intangible assets
|
814.7
|
|
385.6
|
|
382.6
|
|
111.3
|
%
|
0.8
|
%
|
|||||||
|
Acquired in-process research and development
|
120.0
|
|
—
|
|
—
|
|
**
|
|
**
|
|
|||||||
|
Collaboration profit sharing
|
112.3
|
|
10.2
|
|
—
|
|
**
|
|
**
|
|
|||||||
|
Loss (gain) on fair value remeasurement of contingent consideration
|
62.7
|
|
14.8
|
|
30.5
|
|
323.6
|
%
|
(51.5
|
)%
|
|||||||
|
Restructuring charges
|
0.9
|
|
33.1
|
|
93.4
|
|
(97.3
|
)%
|
(64.6
|
)%
|
|||||||
|
TECFIDERA litigation settlement charge
|
—
|
|
454.8
|
|
—
|
|
(100.0
|
)%
|
**
|
|
|||||||
|
Total cost and expenses
|
$
|
6,929.7
|
|
$
|
6,298.4
|
|
$
|
5,872.8
|
|
10.0
|
%
|
7.2
|
%
|
||||
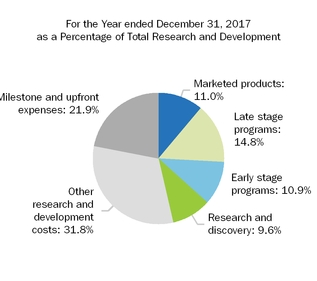
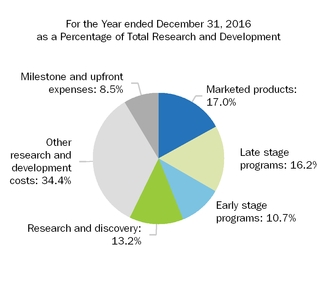
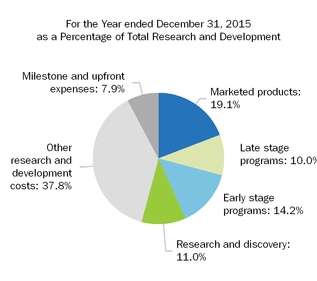
|
•
|
$300.0 million upfront payment made to BMS upon entering into our agreement to exclusively license BIIB092;
|
|
•
|
$60.0 million developmental milestone payment due to the former shareholders of iPierian, Inc. (iPierian), which became payable upon dosing of the first patient in the Phase 2 PSP study for BIIB092;
|
|
•
|
$28.0 million upfront payment made to Alkermes upon entering into our agreement to exclusively license BIIB098, representing our share of BIIB098 development costs already incurred in 2017;
|
|
•
|
$50.0 million accrual based upon the expected continuation of our agreement with Alkermes to develop and exclusively license BIIB098; and
|
|
•
|
$25.0 million upfront payment recognized upon entering into a new collaboration agreement with Ionis to identify new ASO drug candidates for the treatment of SMA.
|
|
•
|
$75.0 million license fee paid to Ionis as we exercised our option to develop and commercialize SPINRAZA from Ionis;
|
|
•
|
$50.0 million milestone payment to Eisai related to the initiation of a Phase 3 trial for E2609; and
|
|
•
|
$20.0 million upfront payment recognized upon entering into a collaboration and alliance agreement with UPenn.
|
|
•
|
$60.0 million recognized upon entering into our collaboration with Mitsubishi Tanabe Pharma Corporation;
|
|
•
|
$48.1 million recognized upon entering into our collaboration with AGTC;
|
|
•
|
$30.0 million in milestones recognized in relation to our collaboration agreements with Ionis; and
|
|
•
|
$16.0 million paid to AbbVie related to milestones for the development of ZINBRYTA as a result of filing with the FDA and EMA during 2015.
|
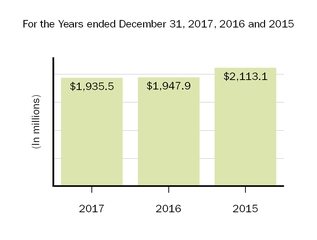
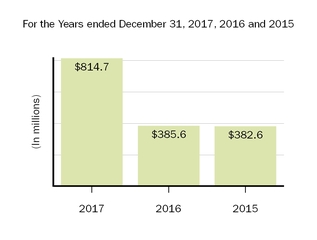
|
(In millions)
|
As of December 31, 2017
|
||
|
2018
|
$
|
423.5
|
|
|
2019
|
401.8
|
|
|
|
2020
|
381.6
|
|
|
|
2021
|
254.3
|
|
|
|
2022
|
242.3
|
|
|


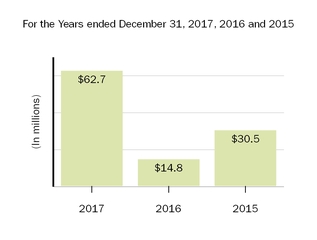
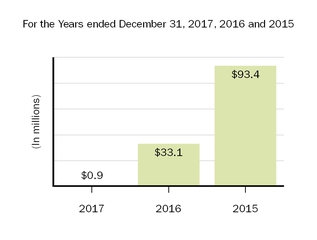
|
•
|
maximizing the resilience of our MS core business;
|
|
•
|
accelerating efforts in SMA as a significant new growth opportunity;
|
|
•
|
developing and expanding our neuroscience portfolio;
|
|
•
|
focusing our capital allocation efforts to drive investment for future growth; and
|
|
•
|
creating a leaner and simpler operating model to streamline our operations and reallocate resources towards prioritized research and development and commercial value creation opportunities.
|
|
(In millions)
|
Workforce
Reduction
|
Pipeline
Programs
|
Total
|
||||||||
|
Restructuring reserve as of December 31, 2015
|
$
|
33.7
|
|
$
|
3.6
|
|
$
|
37.3
|
|
||
|
Expense
|
4.9
|
|
5.4
|
|
10.3
|
|
|||||
|
Payment
|
(31.2
|
)
|
(9.0
|
)
|
(40.2
|
)
|
|||||
|
Adjustments to previous estimates, net
|
(5.2
|
)
|
2.9
|
|
(2.3
|
)
|
|||||
|
Restructuring reserve as of December 31, 2016
|
$
|
2.2
|
|
$
|
2.9
|
|
$
|
5.1
|
|
||
|
Payment
|
(1.7
|
)
|
(2.9
|
)
|
(4.6
|
)
|
|||||
|
Restructuring reserve as of December 31, 2017
|
$
|
0.5
|
|
$
|
—
|
|
$
|
0.5
|
|
||


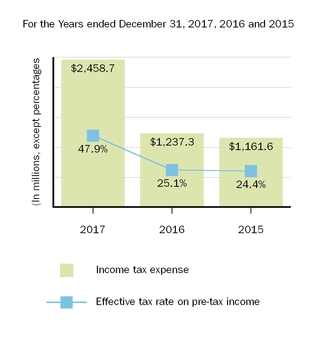
|
|
As of December 31,
|
% Change
|
||||||||
|
(In millions, except percentages)
|
2017
|
2016
|
2017 compared to 2016
|
|||||||
|
Financial assets:
|
||||||||||
|
Cash and cash equivalents
|
$
|
1,573.8
|
|
$
|
2,326.5
|
|
(32.4
|
)%
|
||
|
Marketable securities — current
|
2,115.2
|
|
2,568.6
|
|
(17.7
|
)%
|
||||
|
Marketable securities — non-current
|
3,057.3
|
|
2,829.4
|
|
8.1
|
%
|
||||
|
Total cash, cash equivalents and marketable securities
|
$
|
6,746.3
|
|
$
|
7,724.5
|
|
(12.7
|
)%
|
||
|
Borrowings:
|
||||||||||
|
Current portion of notes payable and other financing arrangements
|
$
|
3.2
|
|
$
|
4.7
|
|
(31.9
|
)%
|
||
|
Notes payable and other financing arrangements
|
5,935.0
|
|
6,512.7
|
|
(8.9
|
)%
|
||||
|
Total borrowings
|
$
|
5,938.2
|
|
$
|
6,517.4
|
|
(8.9
|
)%
|
||
|
Working Capital:
|
||||||||||
|
Current assets
|
$
|
7,873.3
|
|
$
|
8,732.2
|
|
(9.8
|
)%
|
||
|
Current liabilities
|
(3,368.2
|
)
|
(3,419.9
|
)
|
(1.5
|
)%
|
||||
|
Total working capital
|
$
|
4,505.1
|
|
$
|
5,312.3
|
|
(15.2
|
)%
|
||
|
•
|
$4.6 billion
in net cash flows provided by operating activities, net of:
|
|
◦
|
$1.1 billion
in total net payments for income taxes;
|
|
◦
|
$463.0 million in upfront and milestone payments to BMS, iPierian, Eisai, Alkermes and Ionis; and
|
|
◦
|
$454.8 million payment made to Forward Pharma for the litigation settlement charge that was accrued as of December 31, 2016;
|
|
•
|
$1.4 billion
used for share repurchases;
|
|
•
|
$1.2 billion
in contingent payments made to former shareholders of Fumapharm AG and holders of their rights;
|
|
•
|
$867.4 million
used for purchases of property, plant and equipment;
|
|
•
|
$795.2 million payment made to Forward Pharma to license Forward Pharma's intellectual property, including Forward Pharma's intellectual property related to TECFIDERA;
|
|
•
|
$557.7 million payment made for the redemption of our
6.875%
Senior Notes due March 1, 2018 prior to their maturity;
|
|
•
|
$302.7 million net cash contribution made in connection with the spin-off of our hemophilia business;
|
|
•
|
$295.0 million in upfront and milestone payments made to Remedy, Ionis and Samsung Bioepis; and
|
|
•
|
$132.4 million payment, net of tax, made to Neurimmune in exchange for a 15% reduction in royalty rates payable on products developed under the agreement, including on potential commercial sales of aducanumab.
|
|
•
|
$4.6 billion
in net cash flows provided by operating activities, net of:
|
|
◦
|
$1.6 billion
in total net payments for income taxes;
|
|
◦
|
$75.0 million license fee payment made to Ionis; and
|
|
◦
|
$20.0 million upfront payment to UPenn;
|
|
•
|
$1.2 billion in contingent payments made to former shareholders of Fumapharm AG and holders of their rights;
|
|
•
|
$1.0 billion
used for share repurchases;
|
|
•
|
$616.1 million
used for purchases of property, plant and equipment; and
|
|
•
|
$82.0 million in milestone payments made to Samsung Bioepis and AbbVie.
|
|
•
|
$1.5 billion
aggregate principal amount of
2.90%
Senior Notes due September 15, 2020, valued at
99.792%
of par;
|
|
•
|
$1.0 billion
aggregate principal amount of
3.625%
Senior Notes due September 15, 2022, valued at
99.920%
of par;
|
|
•
|
$1.75 billion
aggregate principal amount of
4.05%
Senior Notes due September 15, 2025, valued at
99.764%
of par; and
|
|
•
|
$1.75 billion
aggregate principal amount of
5.20%
Senior Notes due September 15, 2045, valued at
99.294%
of par.
|
|
|
For the Years Ended
December 31,
|
% Change
|
|||||||||||||||
|
|
2017 compared to 2016
|
2016 compared to 2015
|
|||||||||||||||
|
(In millions, except percentages)
|
2017
|
2016
|
2015
|
||||||||||||||
|
Net cash flows provided by operating activities
|
$
|
4,551.0
|
|
$
|
4,587.2
|
|
$
|
3,919.4
|
|
(0.8
|
)%
|
17.0
|
%
|
||||
|
Net cash flows used in by investing activities
|
$
|
(2,963.1
|
)
|
$
|
(2,484.8
|
)
|
$
|
(4,553.6
|
)
|
19.2
|
%
|
(45.4
|
)%
|
||||
|
Net cash flows (used in) provided by financing activities
|
$
|
(2,380.0
|
)
|
$
|
(1,052.6
|
)
|
$
|
783.1
|
|
126.1
|
%
|
(234.4
|
)%
|
||||
|
•
|
Non-cash operating items such as depreciation and amortization, impairment charges, acquired in-process research and development and share-based compensation;
|
|
•
|
Changes in operating assets and liabilities which reflect timing differences between the receipt and payment of cash associated with transactions and when they are recognized in results of operations; and
|
|
•
|
Changes associated with the fair value of contingent payments associated with our acquisitions of businesses and payments related to collaborations.
|
|
•
|
the
$795.2 million payment made to Forward Pharma to license Forward Pharma's intellectual property, including Forward Pharma's intellectual property related to TECFIDERA;
|
|
•
|
an increase in purchases of property, plant and equipment primarily related to the construction of our Solothurn, Switzerland facility;
|
|
•
|
$175.0 million in milestone payments made to Ionis and Samsung Bioepis; and
|
|
•
|
the $120.0 million payment made to Remedy for the purchase of BIIB093.
|
|
|
Payments Due by Period
|
||||||||||||||||||
|
(In millions)
|
Total
|
Less than
1 Year
|
1 to 3
Years
|
3 to 5
Years
|
After
5 Years
|
||||||||||||||
|
Non-cancellable operating leases (1), (2)
|
$
|
428.5
|
|
$
|
48.3
|
|
$
|
92.1
|
|
$
|
88.3
|
|
$
|
199.8
|
|
||||
|
Long-term debt obligations (3)
|
9,430.0
|
|
244.8
|
|
1,983.3
|
|
1,396.3
|
|
5,805.6
|
|
|||||||||
|
Purchase and other obligations (4)
|
1,657.1
|
|
637.3
|
|
344.9
|
|
234.6
|
|
440.3
|
|
|||||||||
|
Defined benefit obligation
|
91.8
|
|
—
|
|
—
|
|
—
|
|
91.8
|
|
|||||||||
|
Total contractual obligations
|
$
|
11,607.4
|
|
$
|
930.4
|
|
$
|
2,420.3
|
|
$
|
1,719.2
|
|
$
|
6,537.5
|
|
||||
|
(1)
|
We lease properties and equipment for use in our operations. Amounts reflected within the table above detail future minimum rental commitments under non-cancelable operating leases as of December 31 for each of the periods presented. In addition to the minimum rental commitments, these leases may require us to pay additional amounts for taxes, insurance, maintenance and other operating expenses.
|
|
(2)
|
Obligations are presented net of sublease income expected to be received for the vacated small-scale biologics manufacturing facility in Cambridge, MA, the vacated portion of our Weston, MA facility and other facilities throughout the world.
|
|
(3)
|
Long-term debt obligations are primarily related to our Senior Notes, including principal and interest payments.
|
|
(4)
|
Purchase and other obligations primarily includes our obligations to purchase direct materials,
$989.6 million
related to our current estimate of the impact of the 2017 Tax Act,
$270.0 million
in contractual commitments for the construction of our large-scale biologics manufacturing facility in Solothurn, Switzerland and
$111.3 million
related to the fair value of net liabilities on derivative contracts.
|
|
•
|
estimating the timing of and expected costs to complete the in-process projects;
|
|
•
|
projecting regulatory approvals;
|
|
•
|
estimating future cash flows from product sales resulting from completed products and in process projects; and
|
|
•
|
developing appropriate discount rates and probability rates by project.
|
|
•
|
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect our transactions and dispositions of our assets;
|
|
•
|
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
|
|
•
|
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
|
|
Financial Statements
|
Page Number
|
|
|
Consolidated Statements of Income
|
F-2
|
|
|
Consolidated Statements of Comprehensive Income
|
F-3
|
|
|
Consolidated Balance Sheets
|
F-4
|
|
|
Consolidated Statements of Cash Flows
|
F-5
|
|
|
Consolidated Statements of Equity
|
F-6
|
|
|
Notes to Consolidated Financial Statements
|
F-9
|
|
|
Report of Independent Registered Public Accounting Firm
|
F-77
|
|
|
Exhibit No.
|
|
Description
|
|
2.1†
|
||
|
2.2
|
||
|
3.1
|
|
|
|
3.2
|
||
|
3.3
|
|
|
|
4.1
|
|
|
|
4.2
|
||
|
4.3
|
||
|
10.1
|
|
|
|
10.2†
|
|
|
|
10.3†
|
|
|
|
10.4
|
||
|
10.5*
|
|
|
|
10.6*
|
|
|
|
10.7*
|
||
|
10.8*
|
|
|
|
10.9*
|
|
|
|
10.10*+
|
|
|
|
10.11*+
|
||
|
10.12*
|
|
|
|
10.13*
|
|
|
|
Exhibit No.
|
|
Description
|
|
10.14*
|
|
|
|
10.15*
|
|
|
|
10.16*
|
|
|
|
10.17*
|
|
|
|
10.18*
|
|
|
|
10.19*
|
|
|
|
10.20*
|
|
|
|
10.21*
|
|
|
|
10.22*
|
|
|
|
10.23*
|
|
|
|
10.24*
|
|
|
|
10.25*
|
|
|
|
10.26*
|
|
|
|
10.27*
|
||
|
10.28*+
|
||
|
10.29*
|
||
|
10.30*
|
||
|
10.31*+
|
||
|
10.32*+
|
||
|
10.33*+
|
||
|
10.34*
|
||
|
10.35*
|
||
|
10.36*
|
||
|
10.37*
|
||
|
Exhibit No.
|
|
Description
|
|
10.38*
|
||
|
21+
|
|
|
|
23+
|
|
|
|
31.1+
|
|
|
|
31.2+
|
|
|
|
32.1++
|
|
|
|
101++
|
|
The following materials from Biogen Inc.’s Annual Report on Form 10-K for the year ended December 31, 2017, formatted in XBRL (Extensible Business Reporting Language): (i) the Consolidated Statements of Income, (ii) the Consolidated Statements of Comprehensive Income, (iii) the Consolidated Balance Sheets, (iv) the Consolidated Statements of Cash Flows, (v) the Consolidated Statements of Equity and (vi) Notes to Consolidated Financial Statements.
|
|
^
|
References to “our” filings mean filings made by Biogen Inc. and filings made by IDEC Pharmaceuticals Corporation prior to the merger with Biogen, Inc. Unless otherwise indicated exhibits were previously filed with the Securities and Exchange Commission under Commission File Number 0-19311 and are incorporated herein by reference.
|
|
*
|
Management contract or compensatory plan or arrangement.
|
|
†
|
Confidential treatment has been granted or requested with respect to portions of this exhibit.
|
|
+
|
Filed herewith.
|
|
+
|
+
|
Furnished herewith.
|
|
BIOGEN INC.
|
|
|
By:
|
/
S
/ M
ICHEL
V
OUNATSOS
|
|
Michel Vounatsos
|
|
|
Chief Executive Officer
|
|
|
Name
|
|
Capacity
|
|
Date
|
|
/
S
/ M
ICHEL
V
OUNATSOS
|
|
Director and Chief Executive Officer (principal executive officer)
|
|
February 1, 2018
|
|
Michel Vounatsos
|
||||
|
/
S
/ Jeffrey D. Capello
|
|
Executive Vice President and Chief Financial Officer (principal financial officer)
|
|
February 1, 2018
|
|
Jeffrey D. Capello
|
||||
|
/
S
/ G
REGORY
F. C
OVINO
|
Vice President, Finance, Chief Accounting Officer (principal accounting officer)
|
February 1, 2018
|
||
|
Gregory F. Covino
|
||||
|
/
S
/ S
TELIOS
P
APADOPOULOS
|
|
Director and Chairman of the Board of Directors
|
|
February 1, 2018
|
|
Stelios Papadopoulos
|
||||
|
/
S
/
A
LEXANDER
J
.
D
ENNER
|
|
Director
|
|
February 1, 2018
|
|
Alexander J. Denner
|
||||
|
/
S
/ C
AROLINE
D. D
ORSA
|
|
Director
|
|
February 1, 2018
|
|
Caroline D. Dorsa
|
||||
|
/
S
/ N
ANCY
L. L
EAMING
|
|
Director
|
|
February 1, 2018
|
|
Nancy L. Leaming
|
||||
|
/
S
/ R
ICHARD
C
.
M
ULLIGAN
|
|
Director
|
|
February 1, 2018
|
|
Richard C. Mulligan
|
||||
|
/
S
/ R
OBERT
W. P
ANGIA
|
|
Director
|
|
February 1, 2018
|
|
Robert W. Pangia
|
||||
|
/
S
/ B
RIAN
S. P
OSNER
|
Director
|
February 1, 2018
|
||
|
Brian S. Posner
|
||||
|
/
S
/ E
RIC
K. R
OWINSKY
|
Director
|
February 1, 2018
|
||
|
Eric K. Rowinsky
|
||||
|
/
S
/ L
YNN
S
CHENK
|
Director
|
February 1, 2018
|
||
|
Lynn Schenk
|
||||
|
/
S
/ S
TEPHEN
A. S
HERWIN
|
Director
|
February 1, 2018
|
||
|
Stephen A. Sherwin
|
||||
|
|
|
Page Number
|
|
Consolidated Statements of Income
|
|
F-2
|
|
Consolidated Statements of Comprehensive Income
|
F-3
|
|
|
Consolidated Balance Sheets
|
|
F-4
|
|
Consolidated Statements of Cash Flows
|
|
F-5
|
|
Consolidated Statements of Equity
|
|
F-6
|
|
Notes to Consolidated Financial Statements
|
|
F-9
|
|
Report of Independent Registered Public Accounting Firm
|
|
F-77
|
|
|
For the Years Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Revenues:
|
|||||||||||
|
Product, net
|
$
|
10,354.7
|
|
$
|
9,817.9
|
|
$
|
9,188.5
|
|
||
|
Revenues from anti-CD20 therapeutic programs
|
1,559.2
|
|
1,314.5
|
|
1,339.2
|
|
|||||
|
Other
|
360.0
|
|
316.4
|
|
236.1
|
|
|||||
|
Total revenues
|
12,273.9
|
|
11,448.8
|
|
10,763.8
|
|
|||||
|
Cost and expenses:
|
|||||||||||
|
Cost of sales, excluding amortization of acquired intangible assets
|
1,630.0
|
|
1,478.7
|
|
1,240.4
|
|
|||||
|
Research and development
|
2,253.6
|
|
1,973.3
|
|
2,012.8
|
|
|||||
|
Selling, general and administrative
|
1,935.5
|
|
1,947.9
|
|
2,113.1
|
|
|||||
|
Amortization of acquired intangible assets
|
814.7
|
|
385.6
|
|
382.6
|
|
|||||
|
Acquired in-process research and development
|
120.0
|
|
—
|
|
—
|
|
|||||
|
Collaboration profit (loss) sharing
|
112.3
|
|
10.2
|
|
—
|
|
|||||
|
Loss (gain) on fair value remeasurement of contingent consideration
|
62.7
|
|
14.8
|
|
30.5
|
|
|||||
|
Restructuring charges
|
0.9
|
|
33.1
|
|
93.4
|
|
|||||
|
TECFIDERA litigation settlement charge
|
—
|
|
454.8
|
|
—
|
|
|||||
|
Total cost and expenses
|
6,929.7
|
|
6,298.4
|
|
5,872.8
|
|
|||||
|
Income from operations
|
5,344.2
|
|
5,150.4
|
|
4,891.0
|
|
|||||
|
Other income (expense), net
|
(215.4
|
)
|
(217.4
|
)
|
(123.7
|
)
|
|||||
|
Income before income tax expense and equity in loss of investee, net of tax
|
5,128.8
|
|
4,933.0
|
|
4,767.3
|
|
|||||
|
Income tax expense
|
2,458.7
|
|
1,237.3
|
|
1,161.6
|
|
|||||
|
Equity in loss of investee, net of tax
|
—
|
|
—
|
|
12.5
|
|
|||||
|
Net income
|
2,670.1
|
|
3,695.7
|
|
3,593.2
|
|
|||||
|
Net income (loss) attributable to noncontrolling interests, net of tax
|
131.0
|
|
(7.1
|
)
|
46.2
|
|
|||||
|
Net income attributable to Biogen Inc.
|
$
|
2,539.1
|
|
$
|
3,702.8
|
|
$
|
3,547.0
|
|
||
|
Net income per share:
|
|||||||||||
|
Basic earnings per share attributable to Biogen Inc.
|
$
|
11.94
|
|
$
|
16.96
|
|
$
|
15.38
|
|
||
|
Diluted earnings per share attributable to Biogen Inc.
|
$
|
11.92
|
|
$
|
16.93
|
|
$
|
15.34
|
|
||
|
Weighted-average shares used in calculating:
|
|||||||||||
|
Basic earnings per share attributable to Biogen Inc.
|
212.6
|
|
218.4
|
|
230.7
|
|
|||||
|
Diluted earnings per share attributable to Biogen Inc.
|
213.0
|
|
218.8
|
|
231.2
|
|
|||||
|
|
For the Years Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Net income attributable to Biogen Inc.
|
$
|
2,539.1
|
|
$
|
3,702.8
|
|
$
|
3,547.0
|
|
||
|
Other comprehensive income:
|
|||||||||||
|
Unrealized gains (losses) on securities available for sale:
|
|||||||||||
|
Unrealized gains (losses) recognized during the period, net of tax
|
(3.5
|
)
|
(10.6
|
)
|
(1.7
|
)
|
|||||
|
Less: reclassification adjustment for (gains) losses included in net income, net of tax
|
12.7
|
|
0.6
|
|
1.3
|
|
|||||
|
Unrealized gains (losses) on securities available for sale, net of tax
|
9.2
|
|
(10.0
|
)
|
(0.4
|
)
|
|||||
|
Unrealized gains (losses) on cash flow hedges:
|
|||||||||||
|
Unrealized gains (losses) recognized during the period, net of tax
|
(193.8
|
)
|
51.6
|
|
110.8
|
|
|||||
|
Less: reclassification adjustment for (gains) losses included in net income, net of tax
|
31.5
|
|
(4.0
|
)
|
(172.3
|
)
|
|||||
|
Unrealized gains (losses) on cash flow hedges, net of tax
|
(162.3
|
)
|
47.6
|
|
(61.5
|
)
|
|||||
|
Unrealized gains (losses) on pension benefit obligation, net of tax
|
(4.1
|
)
|
5.1
|
|
(6.2
|
)
|
|||||
|
Currency translation adjustment
|
158.7
|
|
(138.6
|
)
|
(96.4
|
)
|
|||||
|
Total other comprehensive income (loss), net of tax
|
1.5
|
|
(95.9
|
)
|
(164.5
|
)
|
|||||
|
Comprehensive income attributable to Biogen Inc.
|
2,540.6
|
|
3,606.9
|
|
3,382.5
|
|
|||||
|
Comprehensive income (loss) attributable to noncontrolling interests, net of tax
|
131.0
|
|
(7.1
|
)
|
46.2
|
|
|||||
|
Comprehensive income
|
$
|
2,671.6
|
|
$
|
3,599.8
|
|
$
|
3,428.7
|
|
||
|
|
As of December 31,
|
||||||
|
|
2017
|
2016
|
|||||
|
ASSETS
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
1,573.8
|
|
$
|
2,326.5
|
|
|
|
Marketable securities
|
2,115.2
|
|
2,568.6
|
|
|||
|
Accounts receivable, net
|
1,787.0
|
|
1,441.6
|
|
|||
|
Due from anti-CD20 therapeutic programs
|
532.6
|
|
300.6
|
|
|||
|
Inventory
|
902.7
|
|
1,001.6
|
|
|||
|
Other current assets
|
962.0
|
|
1,093.3
|
|
|||
|
Total current assets
|
7,873.3
|
|
8,732.2
|
|
|||
|
Marketable securities
|
3,057.3
|
|
2,829.4
|
|
|||
|
Property, plant and equipment, net
|
3,182.4
|
|
2,501.8
|
|
|||
|
Intangible assets, net
|
3,879.6
|
|
3,808.3
|
|
|||
|
Goodwill
|
4,632.5
|
|
3,669.3
|
|
|||
|
Investments and other assets
|
1,027.5
|
|
1,335.8
|
|
|||
|
Total assets
|
$
|
23,652.6
|
|
$
|
22,876.8
|
|
|
|
LIABILITIES AND EQUITY
|
|||||||
|
Current liabilities:
|
|||||||
|
Current portion of notes payable and other financing arrangements
|
$
|
3.2
|
|
$
|
4.7
|
|
|
|
Taxes payable
|
68.2
|
|
231.9
|
|
|||
|
Accounts payable
|
395.5
|
|
279.8
|
|
|||
|
Accrued expenses and other
|
2,901.3
|
|
2,903.5
|
|
|||
|
Total current liabilities
|
3,368.2
|
|
3,419.9
|
|
|||
|
Notes payable and other financing arrangements
|
5,935.0
|
|
6,512.7
|
|
|||
|
Deferred tax liability
|
122.6
|
|
93.1
|
|
|||
|
Other long-term liabilities
|
1,628.7
|
|
722.5
|
|
|||
|
Total liabilities
|
11,054.5
|
|
10,748.2
|
|
|||
|
Commitments and contingencies
|
|
|
|
|
|||
|
Equity:
|
|||||||
|
Biogen Inc. shareholders’ equity
|
|||||||
|
Preferred stock, par value $0.001 per share
|
—
|
|
—
|
|
|||
|
Common stock, par value $0.0005 per share
|
0.1
|
|
0.1
|
|
|||
|
Additional paid-in capital
|
97.8
|
|
—
|
|
|||
|
Accumulated other comprehensive loss
|
(318.4
|
)
|
(319.9
|
)
|
|||
|
Retained earnings
|
15,810.4
|
|
15,071.6
|
|
|||
|
Treasury stock, at cost; 23.8 million and 22.6 million shares, respectively
|
(2,977.1
|
)
|
(2,611.7
|
)
|
|||
|
Total Biogen Inc. shareholders’ equity
|
12,612.8
|
|
12,140.1
|
|
|||
|
Noncontrolling interests
|
(14.7
|
)
|
(11.5
|
)
|
|||
|
Total equity
|
12,598.1
|
|
12,128.6
|
|
|||
|
Total liabilities and equity
|
$
|
23,652.6
|
|
$
|
22,876.8
|
|
|
|
|
For the Years Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Cash flows from operating activities:
|
|||||||||||
|
Net income
|
$
|
2,670.1
|
|
$
|
3,695.7
|
|
$
|
3,593.2
|
|
||
|
Adjustments to reconcile net income to net cash flows from operating activities:
|
|||||||||||
|
Depreciation and amortization
|
1,081.0
|
|
682.7
|
|
600.4
|
|
|||||
|
Acquired in-process research and development
|
120.0
|
|
—
|
|
—
|
|
|||||
|
Share-based compensation
|
128.0
|
|
154.8
|
|
161.4
|
|
|||||
|
Deferred income taxes
|
91.7
|
|
(175.0
|
)
|
(145.6
|
)
|
|||||
|
Contingent consideration
|
62.7
|
|
14.8
|
|
30.5
|
|
|||||
|
Other
|
162.1
|
|
89.0
|
|
129.9
|
|
|||||
|
Changes in operating assets and liabilities, net:
|
|||||||||||
|
Accounts receivable
|
(435.6
|
)
|
(241.4
|
)
|
29.0
|
|
|||||
|
Due from anti-CD20 therapeutic programs
|
(232.0
|
)
|
13.9
|
|
(31.1
|
)
|
|||||
|
Inventory
|
(94.5
|
)
|
(165.6
|
)
|
(174.4
|
)
|
|||||
|
Other assets
|
(76.6
|
)
|
59.1
|
|
(127.0
|
)
|
|||||
|
Accrued expenses and other current liabilities
|
(227.4
|
)
|
622.3
|
|
199.3
|
|
|||||
|
Income tax assets and liabilities
|
1,303.9
|
|
(232.6
|
)
|
(429.4
|
)
|
|||||
|
Other liabilities
|
(2.4
|
)
|
69.5
|
|
83.2
|
|
|||||
|
Net cash flows provided by operating activities
|
4,551.0
|
|
4,587.2
|
|
3,919.4
|
|
|||||
|
Cash flows from investing activities:
|
|||||||||||
|
Proceeds from sales and maturities of marketable securities
|
5,565.9
|
|
7,378.9
|
|
4,063.0
|
|
|||||
|
Purchases of marketable securities
|
(5,355.2
|
)
|
(7,913.2
|
)
|
(6,864.9
|
)
|
|||||
|
Contingent consideration related to Fumapharm AG acquisition
|
(1,200.0
|
)
|
(1,200.0
|
)
|
(850.0
|
)
|
|||||
|
Acquired in-process research and development
|
(120.0
|
)
|
—
|
|
—
|
|
|||||
|
Acquisitions of businesses, net of cash acquired
|
—
|
|
—
|
|
(198.8
|
)
|
|||||
|
Purchases of property, plant and equipment
|
(867.4
|
)
|
(616.1
|
)
|
(643.0
|
)
|
|||||
|
Acquisitions of intangible assets
|
(975.4
|
)
|
(111.6
|
)
|
(15.4
|
)
|
|||||
|
Other
|
(11.0
|
)
|
(22.8
|
)
|
(44.5
|
)
|
|||||
|
Net cash flows used in investing activities
|
(2,963.1
|
)
|
(2,484.8
|
)
|
(4,553.6
|
)
|
|||||
|
Cash flows from financing activities:
|
|||||||||||
|
Purchases of treasury stock
|
(1,365.4
|
)
|
(1,000.0
|
)
|
(5,000.0
|
)
|
|||||
|
Payments related to issuance of stock for share-based compensation arrangements, net
|
(5.3
|
)
|
(8.5
|
)
|
(70.9
|
)
|
|||||
|
Net distribution to noncontrolling interest
|
(134.1
|
)
|
—
|
|
(56.1
|
)
|
|||||
|
Proceeds from borrowings
|
—
|
|
—
|
|
5,930.5
|
|
|||||
|
Repayments of borrowings
|
(560.9
|
)
|
(2.7
|
)
|
(2.1
|
)
|
|||||
|
Net cash contribution to Bioverativ, Inc.
|
(302.7
|
)
|
—
|
|
—
|
|
|||||
|
Contingent consideration payments
|
(3.0
|
)
|
(38.6
|
)
|
(13.1
|
)
|
|||||
|
Other
|
(8.6
|
)
|
(2.8
|
)
|
(5.2
|
)
|
|||||
|
Net cash flows provided by (used in) financing activities
|
(2,380.0
|
)
|
(1,052.6
|
)
|
783.1
|
|
|||||
|
Net increase in cash and cash equivalents
|
(792.1
|
)
|
1,049.8
|
|
148.9
|
|
|||||
|
Effect of exchange rate changes on cash and cash equivalents
|
39.4
|
|
(31.3
|
)
|
(45.8
|
)
|
|||||
|
Cash and cash equivalents, beginning of the year
|
2,326.5
|
|
1,308.0
|
|
1,204.9
|
|
|||||
|
Cash and cash equivalents, end of the year
|
$
|
1,573.8
|
|
$
|
2,326.5
|
|
$
|
1,308.0
|
|
||
|
|
Preferred stock
|
Common stock
|
Additional
paid-in
capital
|
Accumulated
other
comprehensive
loss
|
Retained
earnings
|
Treasury stock
|
Total
Biogen Inc.
shareholders’
equity
|
Noncontrolling
interests
|
Total
equity
|
|||||||||||||||||||||||||||||||||||
|
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
||||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2016
|
—
|
|
$
|
—
|
|
238.5
|
|
$
|
0.1
|
|
$
|
—
|
|
$
|
(319.9
|
)
|
$
|
15,071.6
|
|
(22.6
|
)
|
$
|
(2,611.7
|
)
|
$
|
12,140.1
|
|
$
|
(11.5
|
)
|
$
|
12,128.6
|
|
|||||||||||
|
Net income
|
2,539.1
|
|
2,539.1
|
|
131.0
|
|
2,670.1
|
|
||||||||||||||||||||||||||||||||||||
|
Other comprehensive income (loss), net of tax
|
1.5
|
|
1.5
|
|
—
|
|
1.5
|
|
||||||||||||||||||||||||||||||||||||
|
Capital contribution by noncontrolling interest
|
—
|
|
15.8
|
|
15.8
|
|
||||||||||||||||||||||||||||||||||||||
|
Distribution to noncontrolling interest
|
—
|
|
(150.0
|
)
|
(150.0
|
)
|
||||||||||||||||||||||||||||||||||||||
|
Repurchase of common stock pursuant to the 2016 Share Repurchase Program, at cost
|
(3.7
|
)
|
(1,000.0
|
)
|
(1,000.0
|
)
|
(1,000.0
|
)
|
||||||||||||||||||||||||||||||||||||
|
Retirement of common stock pursuant to the 2016 Share Repurchase Program, at cost
|
(3.7
|
)
|
—
|
|
(36.0
|
)
|
(964.0
|
)
|
3.7
|
|
1,000.0
|
|
—
|
|
—
|
|
||||||||||||||||||||||||||||
|
Repurchase of common stock pursuant to the 2011 Share Repurchase Program, at cost
|
(1.2
|
)
|
(365.4
|
)
|
(365.4
|
)
|
(365.4
|
)
|
||||||||||||||||||||||||||||||||||||
|
Issuance of common stock under stock option and stock purchase plans
|
0.2
|
|
—
|
|
40.5
|
|
—
|
|
40.5
|
|
40.5
|
|
||||||||||||||||||||||||||||||||
|
Issuance of common stock under stock award plan
|
0.3
|
|
—
|
|
(44.8
|
)
|
(1.0
|
)
|
(45.8
|
)
|
(45.8
|
)
|
||||||||||||||||||||||||||||||||
|
Compensation related to share-based payments
|
138.1
|
|
138.1
|
|
138.1
|
|
||||||||||||||||||||||||||||||||||||||
|
Hemophilia spin-off adjustment
|
(852.8
|
)
|
(852.8
|
)
|
(852.8
|
)
|
||||||||||||||||||||||||||||||||||||||
|
Tax benefit
|
17.5
|
|
17.5
|
|
17.5
|
|
||||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2017
|
—
|
|
$
|
—
|
|
235.3
|
|
$
|
0.1
|
|
$
|
97.8
|
|
$
|
(318.4
|
)
|
$
|
15,810.4
|
|
(23.8
|
)
|
$
|
(2,977.1
|
)
|
$
|
12,612.8
|
|
$
|
(14.7
|
)
|
$
|
12,598.1
|
|
|||||||||||
|
|
Preferred stock
|
Common stock
|
Additional
paid-in
capital
|
Accumulated
other
comprehensive
loss
|
Retained
earnings
|
Treasury stock
|
Total
Biogen Inc.
shareholders’
equity
|
Noncontrolling
interests
|
Total
equity
|
|||||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2015
|
—
|
|
$
|
—
|
|
241.2
|
|
$
|
0.1
|
|
$
|
—
|
|
$
|
(224.0
|
)
|
$
|
12,208.4
|
|
(22.6
|
)
|
$
|
(2,611.7
|
)
|
$
|
9,372.8
|
|
$
|
2.1
|
|
$
|
9,374.9
|
|
|||||||||||
|
Net income
|
3,702.8
|
|
3,702.8
|
|
(7.1
|
)
|
3,695.7
|
|
||||||||||||||||||||||||||||||||||||
|
Other comprehensive income (loss), net of tax
|
(95.9
|
)
|
(95.9
|
)
|
0.1
|
|
(95.8
|
)
|
||||||||||||||||||||||||||||||||||||
|
Acquisition of noncontrolling interest
|
—
|
|
(0.6
|
)
|
(0.6
|
)
|
||||||||||||||||||||||||||||||||||||||
|
Capital contribution by noncontrolling interest
|
—
|
|
1.5
|
|
1.5
|
|
||||||||||||||||||||||||||||||||||||||
|
Deconsolidation of noncontrolling interest
|
—
|
|
(7.5
|
)
|
(7.5
|
)
|
||||||||||||||||||||||||||||||||||||||
|
Repurchase of common stock pursuant to the 2016 Share Repurchase Program, at cost
|
(3.3
|
)
|
(1,000.0
|
)
|
(1,000.0
|
)
|
(1,000.0
|
)
|
||||||||||||||||||||||||||||||||||||
|
Retirement of common stock pursuant to the 2016 Share Repurchase Program, at cost
|
(3.3
|
)
|
—
|
|
(164.9
|
)
|
(835.1
|
)
|
3.3
|
|
1,000.0
|
|
—
|
|
—
|
|
||||||||||||||||||||||||||||
|
Issuance of common stock under stock option and stock purchase plans
|
0.2
|
|
—
|
|
43.7
|
|
43.7
|
|
43.7
|
|
||||||||||||||||||||||||||||||||||
|
Issuance of common stock under stock award plan
|
0.4
|
|
—
|
|
(47.6
|
)
|
(4.5
|
)
|
(52.1
|
)
|
(52.1
|
)
|
||||||||||||||||||||||||||||||||
|
Compensation related to share-based payments
|
169.4
|
|
169.4
|
|
169.4
|
|
||||||||||||||||||||||||||||||||||||||
|
Tax benefit from share-based payments
|
(0.6
|
)
|
(0.6
|
)
|
(0.6
|
)
|
||||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2016
|
—
|
|
$
|
—
|
|
238.5
|
|
$
|
0.1
|
|
$
|
—
|
|
$
|
(319.9
|
)
|
$
|
15,071.6
|
|
(22.6
|
)
|
$
|
(2,611.7
|
)
|
$
|
12,140.1
|
|
$
|
(11.5
|
)
|
$
|
12,128.6
|
|
|||||||||||
|
Preferred stock
|
Common stock
|
Additional
paid-in
capital
|
Accumulated
other
comprehensive
loss
|
Retained
earnings
|
Treasury stock
|
Total
Biogen Inc.
shareholders’
equity
|
Noncontrolling
interests
|
Total
equity
|
||||||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2014
|
—
|
|
$
|
—
|
|
257.1
|
|
$
|
0.1
|
|
$
|
4,196.2
|
|
$
|
(59.5
|
)
|
$
|
9,283.9
|
|
(22.6
|
)
|
$
|
(2,611.7
|
)
|
$
|
10,809.0
|
|
$
|
5.0
|
|
$
|
10,814.0
|
|
|||||||||||
|
Net income
|
3,547.0
|
|
3,547.0
|
|
46.2
|
|
3,593.2
|
|
||||||||||||||||||||||||||||||||||||
|
Other comprehensive income (loss), net of tax
|
(164.5
|
)
|
(164.5
|
)
|
—
|
|
(164.5
|
)
|
||||||||||||||||||||||||||||||||||||
|
Distribution to noncontrolling interests
|
—
|
|
(60.0
|
)
|
(60.0
|
)
|
||||||||||||||||||||||||||||||||||||||
|
Acquisition of noncontrolling interests
|
—
|
|
10.9
|
|
10.9
|
|
||||||||||||||||||||||||||||||||||||||
|
Repurchase of common stock pursuant to the 2015 Share Repurchase Program, at cost
|
(16.8
|
)
|
(5,000.0
|
)
|
(5,000.0
|
)
|
(5,000.0
|
)
|
||||||||||||||||||||||||||||||||||||
|
Retirement of common stock pursuant to the 2015 Share Repurchase Program, at cost
|
(16.8
|
)
|
—
|
|
(4,377.5
|
)
|
(622.5
|
)
|
16.8
|
|
5,000.0
|
|
—
|
|
—
|
|
||||||||||||||||||||||||||||
|
Issuance of common stock under stock option and stock purchase plans
|
0.3
|
|
—
|
|
54.2
|
|
54.2
|
|
54.2
|
|
||||||||||||||||||||||||||||||||||
|
Issuance of common stock under stock award plan
|
0.6
|
|
—
|
|
(125.1
|
)
|
(125.1
|
)
|
(125.1
|
)
|
||||||||||||||||||||||||||||||||||
|
Compensation related to share-based payments
|
183.2
|
|
183.2
|
|
183.2
|
|
||||||||||||||||||||||||||||||||||||||
|
Tax benefit from share-based payments
|
69.0
|
|
69.0
|
|
69.0
|
|
||||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2015
|
—
|
|
$
|
—
|
|
241.2
|
|
$
|
0.1
|
|
$
|
—
|
|
$
|
(224.0
|
)
|
$
|
12,208.4
|
|
(22.6
|
)
|
$
|
(2,611.7
|
)
|
$
|
9,372.8
|
|
$
|
2.1
|
|
$
|
9,374.9
|
|
|||||||||||
|
•
|
Medicaid rebates relate to our estimated obligations to states under established reimbursement arrangements. Rebate accruals are recorded in the same period the related revenue is recognized, resulting in a reduction of product revenue and the establishment of a liability which is included in other current liabilities. Our liability for Medicaid rebates consists of estimates for claims that a state will make for the current quarter, claims for prior quarters that have been estimated for which an invoice has not been received, invoices received for claims from the prior quarters that have not been paid, and an estimate of potential claims that will be made for inventory that exists in the distribution channel at period end.
|
|
•
|
Governmental rebates or chargebacks, including VA and PHS discounts, represent our estimated obligations resulting from contractual commitments to sell products to qualified healthcare providers at prices lower than the list prices we charge to wholesalers which provide those products. The wholesaler charges us for the
|
|
•
|
Managed care rebates represent our estimated obligations to third parties, primarily pharmacy benefit managers. Rebate accruals are recorded in the same period the related revenue is recognized, resulting in a reduction of product revenue and the establishment of a liability which is included in accrued expenses and other current liabilities. These rebates result from performance-based goals, formulary position and price increase limit allowances (price protection). The calculation of the accrual for these rebates is based on an estimate of the customer’s buying patterns and the resulting applicable contractual rebate rate(s) to be earned over a contractual period.
|
|
•
|
Copay assistance represents financial assistance to qualified patients, assisting them with prescription drug co-payments required by insurance. The calculation of the accrual for copay is based on an estimate of claims and the cost per claim that we expect to receive associated with inventory that exists in the distribution channel at period end.
|
|
•
|
Other governmental rebates, non-US pharmaceutical taxes or applicable allowances primarily relate to mandatory rebates and discounts in international markets where government-sponsored healthcare systems are the primary payors for healthcare.
|
|
(i)
|
our share of pre-tax profits and losses in the United States (U.S.) for RITUXAN and GAZYVA;
|
|
(ii)
|
reimbursement of our selling and development expenses in the U.S. for RITUXAN; and
|
|
(iii)
|
other revenues from anti-CD20 therapeutic programs, which primarily consist of our share of pre-tax co-promotion profits on RITUXAN in Canada and royalty revenues on sales of OCREVUS.
|
|
•
|
Level 1
— Fair values are determined utilizing quoted prices (unadjusted) in active markets for identical assets or liabilities that we have the ability to access;
|
|
•
|
Level 2
— Fair values are determined by utilizing quoted prices for identical or similar assets and liabilities in active markets or other market observable inputs such as interest rates, yield curves and foreign currency spot rates; and
|
|
•
|
Level 3
— Prices or valuations that require inputs that are both significant to the fair value measurement and unobservable.
|
|
Asset Category
|
Useful Lives
|
|
Land
|
Not depreciated
|
|
Buildings
|
15 to 40 years
|
|
Leasehold Improvements
|
Lesser of the useful life or the term of the respective lease
|
|
Furniture and Fixtures
|
5 to 7 years
|
|
Machinery and Equipment
|
5 to 20 years
|
|
Computer Software and Hardware
|
3 to 5 years
|
|
(In millions)
|
|||
|
Assets
|
|||
|
Cash
|
$
|
302.7
|
|
|
Accounts receivable
|
144.7
|
|
|
|
Inventory
|
116.1
|
|
|
|
Property, plant and equipment, net
|
20.2
|
|
|
|
Intangible assets, net
|
56.8
|
|
|
|
Goodwill
|
314.1
|
|
|
|
Other, net
|
53.7
|
|
|
|
Assets transferred, net
|
$
|
1,008.3
|
|
|
Liabilities
|
|||
|
Accrued expenses and other current liabilities
|
$
|
87.8
|
|
|
Other long-term liabilities
|
67.7
|
|
|
|
Liabilities transferred, net
|
$
|
155.5
|
|
|
•
|
maximizing the resilience of our MS core business;
|
|
•
|
accelerating efforts in SMA as a significant new growth opportunity;
|
|
•
|
developing and expanding our neuroscience portfolio;
|
|
•
|
focusing our capital allocation efforts to drive investment for future growth; and
|
|
•
|
creating a leaner and simpler operating model to streamline our operations and reallocate resources towards prioritized research and development and commercial value creation opportunities.
|
|
(In millions)
|
Workforce
Reduction
|
Pipeline
Programs
|
Total
|
||||||||
|
Restructuring reserve as of December 31, 2015
|
$
|
33.7
|
|
$
|
3.6
|
|
$
|
37.3
|
|
||
|
Expense
|
4.9
|
|
5.4
|
|
10.3
|
|
|||||
|
Payments
|
(31.2
|
)
|
(9.0
|
)
|
(40.2
|
)
|
|||||
|
Adjustments to previous estimates, net
|
(5.2
|
)
|
2.9
|
|
(2.3
|
)
|
|||||
|
Restructuring reserve as of December 31, 2016
|
$
|
2.2
|
|
$
|
2.9
|
|
$
|
5.1
|
|
||
|
Payments
|
(1.7
|
)
|
(2.9
|
)
|
(4.6
|
)
|
|||||
|
Restructuring reserve as of December 31, 2017
|
$
|
0.5
|
|
$
|
—
|
|
$
|
0.5
|
|
||
|
(In millions)
|
Discounts
|
Contractual
Adjustments
|
Returns
|
Total
|
|||||||||||
|
2017
|
|||||||||||||||
|
Beginning balance
|
$
|
71.6
|
|
$
|
482.7
|
|
$
|
51.2
|
|
$
|
605.5
|
|
|||
|
Current provisions relating to sales in current year
|
583.0
|
|
2,307.4
|
|
26.9
|
|
2,917.3
|
|
|||||||
|
Adjustments relating to prior years
|
(0.1
|
)
|
15.0
|
|
(8.9
|
)
|
6.0
|
|
|||||||
|
Payments/returns relating to sales in current year
|
(475.8
|
)
|
(1,756.9
|
)
|
(0.1
|
)
|
(2,232.8
|
)
|
|||||||
|
Payments/returns relating to sales in prior years
|
(69.1
|
)
|
(442.2
|
)
|
(23.1
|
)
|
(534.4
|
)
|
|||||||
|
Ending balance
|
$
|
109.6
|
|
$
|
606.0
|
|
$
|
46.0
|
|
$
|
761.6
|
|
|||
|
(In millions)
|
Discounts
|
Contractual
Adjustments
|
Returns
|
Total
|
|||||||||||
|
2016
|
|||||||||||||||
|
Beginning balance
|
$
|
56.1
|
|
$
|
548.7
|
|
$
|
57.9
|
|
$
|
662.7
|
|
|||
|
Current provisions relating to sales in current year
|
592.6
|
|
2,044.5
|
|
30.9
|
|
2,668.0
|
|
|||||||
|
Adjustments relating to prior years
|
(1.4
|
)
|
1.5
|
|
(16.8
|
)
|
(16.7
|
)
|
|||||||
|
Payments/returns relating to sales in current year
|
(522.5
|
)
|
(1,576.0
|
)
|
(1.0
|
)
|
(2,099.5
|
)
|
|||||||
|
Payments/returns relating to sales in prior years
|
(53.2
|
)
|
(536.0
|
)
|
(19.8
|
)
|
(609.0
|
)
|
|||||||
|
Ending balance
|
$
|
71.6
|
|
$
|
482.7
|
|
$
|
51.2
|
|
$
|
605.5
|
|
|||
|
(In millions)
|
Discounts
|
Contractual
Adjustments
|
Returns
|
Total
|
|||||||||||
|
2015
|
|||||||||||||||
|
Beginning balance
|
$
|
47.6
|
|
$
|
387.1
|
|
$
|
49.1
|
|
$
|
483.8
|
|
|||
|
Current provisions relating to sales in current year
|
459.7
|
|
1,732.1
|
|
37.6
|
|
2,229.4
|
|
|||||||
|
Adjustments relating to prior years
|
(1.3
|
)
|
(16.3
|
)
|
(14.7
|
)
|
(32.3
|
)
|
|||||||
|
Payments/returns relating to sales in current year
|
(405.9
|
)
|
(1,258.1
|
)
|
(2.6
|
)
|
(1,666.6
|
)
|
|||||||
|
Payments/returns relating to sales in prior years
|
(44.0
|
)
|
(296.1
|
)
|
(11.5
|
)
|
(351.6
|
)
|
|||||||
|
Ending balance
|
$
|
56.1
|
|
$
|
548.7
|
|
$
|
57.9
|
|
$
|
662.7
|
|
|||
|
|
As of December 31,
|
||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Reduction of accounts receivable
|
$
|
189.6
|
|
$
|
166.9
|
|
|
|
Component of accrued expenses and other
|
572.0
|
|
438.6
|
|
|||
|
Total revenue-related reserves
|
$
|
761.6
|
|
$
|
605.5
|
|
|
|
|
As of December 31,
|
||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Raw materials
|
$
|
162.4
|
|
$
|
170.4
|
|
|
|
Work in process
|
605.7
|
|
698.7
|
|
|||
|
Finished goods
|
157.4
|
|
170.3
|
|
|||
|
Total inventory
|
$
|
925.5
|
|
$
|
1,039.4
|
|
|
|
Balance Sheet Classification:
|
|||||||
|
Inventory
|
$
|
902.7
|
|
$
|
1,001.6
|
|
|
|
Investments and other assets
|
22.8
|
|
37.8
|
|
|||
|
Total inventory
|
$
|
925.5
|
|
$
|
1,039.4
|
|
|
|
|
|
As of December 31, 2017
|
As of December 31, 2016
|
||||||||||||||||||||||
|
(In millions)
|
Estimated Life
|
Cost
|
Accumulated
Amortization
|
Net
|
Cost
|
Accumulated
Amortization
|
Net
|
||||||||||||||||||
|
Out-licensed patents
|
13-23 years
|
$
|
543.3
|
|
$
|
(535.6
|
)
|
$
|
7.7
|
|
$
|
543.3
|
|
$
|
(523.6
|
)
|
$
|
19.7
|
|
||||||
|
Developed technology
|
15-23 years
|
3,005.3
|
|
(2,689.0
|
)
|
316.3
|
|
3,005.3
|
|
(2,634.3
|
)
|
371.0
|
|
||||||||||||
|
In-process research and development
|
Indefinite until commercialization
|
680.6
|
|
—
|
|
680.6
|
|
648.0
|
|
—
|
|
648.0
|
|
||||||||||||
|
Trademarks and trade names
|
Indefinite
|
64.0
|
|
—
|
|
64.0
|
|
64.0
|
|
—
|
|
64.0
|
|
||||||||||||
|
Acquired and in-licensed rights and patents
|
4-18 years
|
3,971.4
|
|
(1,160.4
|
)
|
2,811.0
|
|
3,481.7
|
|
(776.1
|
)
|
2,705.6
|
|
||||||||||||
|
Total intangible assets
|
$
|
8,264.6
|
|
$
|
(4,385.0
|
)
|
$
|
3,879.6
|
|
$
|
7,742.3
|
|
$
|
(3,934.0
|
)
|
$
|
3,808.3
|
|
|||||||
|
(In millions)
|
As of December 31, 2017
|
||
|
2018
|
$
|
423.5
|
|
|
2019
|
401.8
|
|
|
|
2020
|
381.6
|
|
|
|
2021
|
254.3
|
|
|
|
2022
|
242.3
|
|
|
|
|
As of December 31,
|
||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Goodwill, beginning of year
|
$
|
3,669.3
|
|
$
|
2,663.8
|
|
|
|
Elimination of goodwill allocated to our hemophilia business
|
(314.1
|
)
|
—
|
|
|||
|
Increase to goodwill
|
1,267.3
|
|
1,026.9
|
|
|||
|
Other
|
10.0
|
|
(21.4
|
)
|
|||
|
Goodwill, end of year
|
$
|
4,632.5
|
|
$
|
3,669.3
|
|
|
|
(In millions)
|
As of
December 31, 2017 |
Quoted
Prices in
Active
Markets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
|||||||||||
|
Assets:
|
|||||||||||||||
|
Cash equivalents
|
$
|
1,229.4
|
|
$
|
—
|
|
$
|
1,229.4
|
|
$
|
—
|
|
|||
|
Marketable debt securities:
|
|||||||||||||||
|
Corporate debt securities
|
2,609.8
|
|
—
|
|
2,609.8
|
|
—
|
|
|||||||
|
Government securities
|
1,919.3
|
|
—
|
|
1,919.3
|
|
—
|
|
|||||||
|
Mortgage and other asset backed securities
|
643.4
|
|
—
|
|
643.4
|
|
—
|
|
|||||||
|
Marketable equity securities
|
11.8
|
|
11.8
|
|
—
|
|
—
|
|
|||||||
|
Derivative contracts
|
2.7
|
|
—
|
|
2.7
|
|
—
|
|
|||||||
|
Plan assets for deferred compensation
|
28.5
|
|
—
|
|
28.5
|
|
—
|
|
|||||||
|
Total
|
$
|
6,444.9
|
|
$
|
11.8
|
|
$
|
6,433.1
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Derivative contracts
|
$
|
111.3
|
|
$
|
—
|
|
$
|
111.3
|
|
$
|
—
|
|
|||
|
Contingent consideration obligations
|
523.6
|
|
—
|
|
—
|
|
523.6
|
|
|||||||
|
Total
|
$
|
634.9
|
|
$
|
—
|
|
$
|
111.3
|
|
$
|
523.6
|
|
|||
|
(In millions)
|
As of
December 31, 2016 |
Quoted
Prices
in Active
Markets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
|||||||||||
|
Assets:
|
|||||||||||||||
|
Cash equivalents
|
$
|
2,039.6
|
|
$
|
—
|
|
$
|
2,039.6
|
|
$
|
—
|
|
|||
|
Marketable debt securities:
|
|||||||||||||||
|
Corporate debt securities
|
2,663.8
|
|
—
|
|
2,663.8
|
|
—
|
|
|||||||
|
Government securities
|
2,172.5
|
|
—
|
|
2,172.5
|
|
—
|
|
|||||||
|
Mortgage and other asset backed securities
|
561.7
|
|
—
|
|
561.7
|
|
—
|
|
|||||||
|
Marketable equity securities
|
24.9
|
|
24.9
|
|
—
|
|
—
|
|
|||||||
|
Derivative contracts
|
61.0
|
|
—
|
|
61.0
|
|
—
|
|
|||||||
|
Plan assets for deferred compensation
|
34.5
|
|
—
|
|
34.5
|
|
—
|
|
|||||||
|
Total
|
$
|
7,558.0
|
|
$
|
24.9
|
|
$
|
7,533.1
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Derivative contracts
|
$
|
13.6
|
|
$
|
—
|
|
$
|
13.6
|
|
$
|
—
|
|
|||
|
Contingent consideration obligations
|
467.6
|
|
—
|
|
—
|
|
467.6
|
|
|||||||
|
Total
|
$
|
481.2
|
|
$
|
—
|
|
$
|
13.6
|
|
$
|
467.6
|
|
|||
|
|
As of December 31,
|
||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Notes payable to Fumedica
|
$
|
3.2
|
|
$
|
6.1
|
|
|
|
6.875% Senior Notes due March 1, 2018
|
—
|
|
583.7
|
|
|||
|
2.900% Senior Notes due September 15, 2020
|
1,517.7
|
|
1,521.5
|
|
|||
|
3.625% Senior Notes due September 15, 2022
|
1,032.9
|
|
1,026.6
|
|
|||
|
4.050% Senior Notes due September 15, 2025
|
1,851.9
|
|
1,796.0
|
|
|||
|
5.200% Senior Notes due September 15, 2045
|
2,077.6
|
|
1,874.5
|
|
|||
|
Total
|
$
|
6,483.3
|
|
$
|
6,808.4
|
|
|
|
|
As of December 31,
|
||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Fair value, beginning of year
|
$
|
467.6
|
|
$
|
506.0
|
|
|
|
Changes in fair value
|
62.7
|
|
14.8
|
|
|||
|
Payments and other
|
(6.7
|
)
|
(53.2
|
)
|
|||
|
Fair value, end of year
|
$
|
523.6
|
|
$
|
467.6
|
|
|
|
|
As of December 31,
|
||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Commercial paper
|
$
|
30.5
|
|
$
|
31.0
|
|
|
|
Overnight reverse repurchase agreements
|
3.6
|
|
—
|
|
|||
|
Money market funds
|
948.0
|
|
741.7
|
|
|||
|
Short-term debt securities
|
247.3
|
|
1,266.9
|
|
|||
|
Total
|
$
|
1,229.4
|
|
$
|
2,039.6
|
|
|
|
As of December 31, 2017 (In millions)
|
Fair
Value
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Amortized
Cost
|
|||||||||||
|
Corporate debt securities
|
|||||||||||||||
|
Current
|
$
|
1,039.3
|
|
$
|
—
|
|
$
|
(0.2
|
)
|
$
|
1,039.5
|
|
|||
|
Non-current
|
1,570.5
|
|
0.9
|
|
—
|
|
1,569.6
|
|
|||||||
|
Government securities
|
|||||||||||||||
|
Current
|
1,075.1
|
|
0.1
|
|
(0.7
|
)
|
1,075.7
|
|
|||||||
|
Non-current
|
844.2
|
|
0.2
|
|
(1.1
|
)
|
845.1
|
|
|||||||
|
Mortgage and other asset backed securities
|
|||||||||||||||
|
Current
|
0.8
|
|
—
|
|
—
|
|
0.8
|
|
|||||||
|
Non-current
|
642.6
|
|
1.1
|
|
(0.8
|
)
|
642.3
|
|
|||||||
|
Total marketable debt securities
|
$
|
5,172.5
|
|
$
|
2.3
|
|
$
|
(2.8
|
)
|
$
|
5,173.0
|
|
|||
|
Marketable equity securities, non-current
|
$
|
11.8
|
|
$
|
1.8
|
|
$
|
(4.4
|
)
|
$
|
14.4
|
|
|||
|
As of December 31, 2016 (In millions)
|
Fair
Value
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Amortized
Cost
|
|||||||||||
|
Corporate debt securities
|
|||||||||||||||
|
Current
|
$
|
1,408.6
|
|
$
|
0.2
|
|
$
|
(0.6
|
)
|
$
|
1,409.0
|
|
|||
|
Non-current
|
1,255.2
|
|
1.2
|
|
(4.7
|
)
|
1,258.7
|
|
|||||||
|
Government securities
|
|||||||||||||||
|
Current
|
1,156.0
|
|
0.2
|
|
(0.3
|
)
|
1,156.1
|
|
|||||||
|
Non-current
|
1,016.5
|
|
0.5
|
|
(3.4
|
)
|
1,019.4
|
|
|||||||
|
Mortgage and other asset backed securities
|
|||||||||||||||
|
Current
|
4.0
|
|
—
|
|
—
|
|
4.0
|
|
|||||||
|
Non-current
|
557.7
|
|
0.8
|
|
(2.2
|
)
|
559.1
|
|
|||||||
|
Total marketable debt securities
|
$
|
5,398.0
|
|
$
|
2.9
|
|
$
|
(11.2
|
)
|
$
|
5,406.3
|
|
|||
|
Marketable equity securities, non-current
|
$
|
24.9
|
|
$
|
0.7
|
|
$
|
(9.3
|
)
|
$
|
33.5
|
|
|||
|
|
As of December 31, 2017
|
As of December 31, 2016
|
|||||||||||||
|
(In millions)
|
Estimated
Fair Value
|
Amortized
Cost
|
Estimated
Fair Value
|
Amortized
Cost
|
|||||||||||
|
Due in one year or less
|
$
|
2,115.2
|
|
$
|
2,116.0
|
|
$
|
2,568.6
|
|
$
|
2,569.1
|
|
|||
|
Due after one year through five years
|
2,730.0
|
|
2,730.0
|
|
2,552.6
|
|
2,559.7
|
|
|||||||
|
Due after five years
|
327.3
|
|
327.0
|
|
276.8
|
|
277.5
|
|
|||||||
|
Total available-for-sale securities
|
$
|
5,172.5
|
|
$
|
5,173.0
|
|
$
|
5,398.0
|
|
$
|
5,406.3
|
|
|||
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2017
|
2016
|
2015
|
||||||||
|
Proceeds from maturities and sales
|
$
|
5,565.9
|
|
$
|
7,378.9
|
|
$
|
4,063.0
|
|
||
|
Realized gains
|
$
|
3.0
|
|
$
|
3.3
|
|
$
|
1.5
|
|
||
|
Realized losses
|
$
|
22.4
|
|
$
|
4.3
|
|
$
|
3.5
|
|
||
|
|
Notional Amount
As of December 31,
|
||||||
|
Foreign Currency: (In millions)
|
2017
|
2016
|
|||||
|
Euro
|
$
|
1,875.6
|
|
$
|
871.7
|
|
|
|
British pound sterling
|
150.9
|
|
—
|
|
|||
|
Swiss francs
|
88.7
|
|
—
|
|
|||
|
Canadian dollar
|
83.5
|
|
—
|
|
|||
|
Total foreign currency forward contracts
|
$
|
2,198.7
|
|
$
|
871.7
|
|
|
|
For the Years Ended December 31,
|
||||||||||||||||||||||||||
|
Net Gains/(Losses)
Reclassified from AOCI into Operating Income
(Effective Portion) (in millions)
|
Net Gains/(Losses)
Recognized into Net Income
(Ineffective Portion) (in millions)
|
|||||||||||||||||||||||||
|
Location
|
2017
|
2016
|
2015
|
Location
|
2017
|
2016
|
2015
|
|||||||||||||||||||
|
Revenues
|
$
|
(32.5
|
)
|
$
|
5.3
|
|
$
|
173.2
|
|
Other income (expense)
|
$
|
8.9
|
|
$
|
2.9
|
|
$
|
4.9
|
|
|||||||
|
Operating expenses
|
$
|
0.6
|
|
$
|
(1.5
|
)
|
$
|
—
|
|
Other income (expense)
|
$
|
(0.2
|
)
|
$
|
0.1
|
|
$
|
—
|
|
|||||||
|
(In millions)
|
Balance Sheet Location
|
Fair Value
As of December 31, 2017 |
||
|
Hedging Instruments:
|
||||
|
Asset derivatives
|
Other current assets
|
$
|
0.7
|
|
|
Investments and other assets
|
$
|
0.2
|
|
|
|
Liability derivatives
|
Accrued expenses and other
|
$
|
84.7
|
|
|
Other long-term liabilities
|
23.6
|
|
||
|
Other Derivatives:
|
||||
|
Asset derivatives
|
Other current assets
|
$
|
1.8
|
|
|
Liability derivatives
|
Accrued expenses and other
|
$
|
3.0
|
|
|
(In millions)
|
Balance Sheet Location
|
Fair Value
As of December 31, 2016 |
||
|
Hedging Instruments:
|
||||
|
Asset derivatives
|
Other current assets
|
$
|
50.4
|
|
|
Investments and other assets
|
$
|
6.6
|
|
|
|
Liability derivatives
|
Other long-term liabilities
|
$
|
4.6
|
|
|
Other Derivatives:
|
||||
|
Asset derivatives
|
Other current assets
|
$
|
4.0
|
|
|
Liability derivatives
|
Accrued expenses and other
|
$
|
9.0
|
|
|
|
As of December 31,
|
||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Land
|
$
|
141.2
|
|
$
|
137.8
|
|
|
|
Buildings
|
1,213.6
|
|
1,107.8
|
|
|||
|
Leasehold improvements
|
80.6
|
|
123.7
|
|
|||
|
Machinery and equipment
|
1,207.7
|
|
1,105.8
|
|
|||
|
Computer software and hardware
|
767.1
|
|
746.8
|
|
|||
|
Furniture and fixtures
|
55.3
|
|
60.6
|
|
|||
|
Construction in progress
|
1,276.0
|
|
658.6
|
|
|||
|
Total cost
|
4,741.5
|
|
3,941.1
|
|
|||
|
Less: accumulated depreciation
|
(1,559.1
|
)
|
(1,439.3
|
)
|
|||
|
Total property, plant and equipment, net
|
$
|
3,182.4
|
|
$
|
2,501.8
|
|
|
|
|
As of December 31,
|
||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Current portion:
|
|||||||
|
Notes payable to Fumedica
|
$
|
3.2
|
|
$
|
3.0
|
|
|
|
Financing arrangement for the purchase of the RTP facility
|
—
|
|
1.7
|
|
|||
|
Current portion of notes payable and other financing arrangements
|
$
|
3.2
|
|
$
|
4.7
|
|
|
|
Non-current portion:
|
|||||||
|
6.875% Senior Notes due March 1, 2018
|
$
|
—
|
|
$
|
558.5
|
|
|
|
2.900% Senior Notes due September 15, 2020
|
1,482.4
|
|
1,485.3
|
|
|||
|
3.625% Senior Notes due September 15, 2022
|
994.3
|
|
993.2
|
|
|||
|
4.050% Senior Notes due September 15, 2025
|
1,736.3
|
|
1,734.8
|
|
|||
|
5.200% Senior Notes due September 15, 2045
|
1,722.0
|
|
1,721.5
|
|
|||
|
Notes payable to Fumedica
|
—
|
|
3.0
|
|
|||
|
Financing arrangement for the purchase of the RTP facility
|
—
|
|
16.4
|
|
|||
|
Non-current portion of notes payable and other financing arrangements
|
$
|
5,935.0
|
|
$
|
6,512.7
|
|
|
|
•
|
$1.5 billion
aggregate principal amount of
2.90%
Senior Notes due September 15, 2020, valued at
99.792%
of par;
|
|
•
|
$1.0 billion
aggregate principal amount of
3.625%
Senior Notes due September 15, 2022, valued at
99.920%
of par;
|
|
•
|
$1.75 billion
aggregate principal amount of
4.05%
Senior Notes due September 15, 2025, valued at
99.764%
of par; and
|
|
•
|
$1.75 billion
aggregate principal amount of
5.20%
Senior Notes due September 15, 2045, valued at
99.294%
of par.
|
|
(In millions)
|
As of December 31, 2017
|
||
|
2018
|
$
|
3.2
|
|
|
2019
|
—
|
|
|
|
2020
|
1,500.0
|
|
|
|
2021
|
—
|
|
|
|
2022
|
1,000.0
|
|
|
|
2023 and thereafter
|
3,500.0
|
|
|
|
Total
|
$
|
6,003.2
|
|
|
|
As of December 31, 2017
|
As of December 31, 2016
|
|||||||||||||||
|
(In millions)
|
Authorized
|
Issued
|
Outstanding
|
Authorized
|
Issued
|
Outstanding
|
|||||||||||
|
Common stock
|
1,000.0
|
|
235.3
|
|
211.5
|
|
1,000.0
|
|
238.5
|
|
215.9
|
|
|||||
|
(In millions)
|
Unrealized Gains (Losses) on Securities Available for Sale, net of tax
|
Unrealized Gains (Losses) on Cash Flow Hedges, net of tax
|
Unfunded Status of Postretirement Benefit Plans, net of tax
|
Translation Adjustments
|
Total
|
||||||||||||||
|
Balance, December 31, 2016
|
$
|
(10.8
|
)
|
$
|
57.8
|
|
$
|
(32.7
|
)
|
$
|
(334.2
|
)
|
$
|
(319.9
|
)
|
||||
|
Other comprehensive income (loss) before reclassifications
|
(3.5
|
)
|
(193.8
|
)
|
(4.1
|
)
|
158.7
|
|
(42.7
|
)
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive income (loss)
|
12.7
|
|
31.5
|
|
—
|
|
—
|
|
44.2
|
|
|||||||||
|
Net current period other comprehensive income (loss)
|
9.2
|
|
(162.3
|
)
|
(4.1
|
)
|
158.7
|
|
1.5
|
|
|||||||||
|
Balance, December 31, 2017
|
$
|
(1.6
|
)
|
$
|
(104.5
|
)
|
$
|
(36.8
|
)
|
$
|
(175.5
|
)
|
$
|
(318.4
|
)
|
||||
|
(In millions)
|
Unrealized Gains (Losses) on Securities Available for Sale, net of tax
|
Unrealized Gains (Losses) on Cash Flow Hedges, net of tax
|
Unfunded Status of Postretirement Benefit Plans, net of tax
|
Translation Adjustments
|
Total
|
||||||||||||||
|
Balance, December 31, 2015
|
$
|
(0.8
|
)
|
$
|
10.2
|
|
$
|
(37.8
|
)
|
$
|
(195.6
|
)
|
$
|
(224.0
|
)
|
||||
|
Other comprehensive income (loss) before reclassifications
|
(10.6
|
)
|
51.6
|
|
5.1
|
|
(138.6
|
)
|
(92.5
|
)
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive income (loss)
|
0.6
|
|
(4.0
|
)
|
—
|
|
—
|
|
(3.4
|
)
|
|||||||||
|
Net current period other comprehensive income (loss)
|
(10.0
|
)
|
47.6
|
|
5.1
|
|
(138.6
|
)
|
(95.9
|
)
|
|||||||||
|
Balance, December 31, 2016
|
$
|
(10.8
|
)
|
$
|
57.8
|
|
$
|
(32.7
|
)
|
$
|
(334.2
|
)
|
$
|
(319.9
|
)
|
||||
|
(In millions)
|
Unrealized Gains (Losses) on Securities Available for Sale, net of tax
|
Unrealized Gains (Losses) on Cash Flow Hedges, net of tax
|
Unfunded Status of Postretirement Benefit Plans, net of tax
|
Translation Adjustments
|
Total
|
||||||||||||||
|
Balance, December 31, 2014
|
$
|
(0.4
|
)
|
$
|
71.7
|
|
$
|
(31.6
|
)
|
$
|
(99.2
|
)
|
$
|
(59.5
|
)
|
||||
|
Other comprehensive income (loss) before reclassifications
|
(1.7
|
)
|
110.8
|
|
(6.2
|
)
|
(96.4
|
)
|
6.5
|
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive income (loss)
|
1.3
|
|
(172.3
|
)
|
—
|
|
—
|
|
(171.0
|
)
|
|||||||||
|
Net current period other comprehensive income (loss)
|
(0.4
|
)
|
(61.5
|
)
|
(6.2
|
)
|
(96.4
|
)
|
(164.5
|
)
|
|||||||||
|
Balance, December 31, 2015
|
$
|
(0.8
|
)
|
$
|
10.2
|
|
$
|
(37.8
|
)
|
$
|
(195.6
|
)
|
$
|
(224.0
|
)
|
||||
|
(In millions)
|
Income Statement Location
|
Amounts Reclassified from
Accumulated Other Comprehensive Income
|
||||||||||
|
For the Years Ended December 31,
|
||||||||||||
|
2017
|
2016
|
2015
|
||||||||||
|
Gains (losses) on securities available for sale
|
Other income (expense)
|
$
|
(19.5
|
)
|
$
|
(0.9
|
)
|
$
|
(2.0
|
)
|
||
|
Income tax benefit (expense)
|
6.8
|
|
0.3
|
|
0.7
|
|
||||||
|
Gains (losses) on cash flow hedges
|
Revenues
|
(32.5
|
)
|
5.3
|
|
173.2
|
|
|||||
|
Operating expenses
|
0.6
|
|
(1.5
|
)
|
—
|
|
||||||
|
Other income (expense)
|
0.3
|
|
0.2
|
|
(0.1
|
)
|
||||||
|
Income tax benefit (expense)
|
0.1
|
|
—
|
|
(0.8
|
)
|
||||||
|
Total reclassifications, net of tax
|
$
|
(44.2
|
)
|
$
|
3.4
|
|
$
|
171.0
|
|
|||
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2017
|
2016
|
2015
|
||||||||
|
Numerator:
|
|||||||||||
|
Net income attributable to Biogen Inc.
|
$
|
2,539.1
|
|
$
|
3,702.8
|
|
$
|
3,547.0
|
|
||
|
Denominator:
|
|||||||||||
|
Weighted average number of common shares outstanding
|
212.6
|
|
218.4
|
|
230.7
|
|
|||||
|
Effect of dilutive securities:
|
|||||||||||
|
Stock options and employee stock purchase plan
|
0.1
|
|
0.1
|
|
0.1
|
|
|||||
|
Time-vested restricted stock units
|
0.2
|
|
0.2
|
|
0.3
|
|
|||||
|
Market stock units
|
0.1
|
|
0.1
|
|
0.1
|
|
|||||
|
Dilutive potential common shares
|
0.4
|
|
0.4
|
|
0.5
|
|
|||||
|
Shares used in calculating diluted earnings per share
|
213.0
|
|
218.8
|
|
231.2
|
|
|||||
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2017
|
2016
|
2015
|
||||||||
|
Research and development
|
$
|
74.0
|
|
$
|
84.5
|
|
$
|
88.6
|
|
||
|
Selling, general and administrative
|
95.7
|
|
121.7
|
|
127.3
|
|
|||||
|
Restructuring charges
|
—
|
|
(1.8
|
)
|
(8.6
|
)
|
|||||
|
Subtotal
|
169.7
|
|
204.4
|
|
207.3
|
|
|||||
|
Capitalized share-based compensation costs
|
(9.6
|
)
|
(14.6
|
)
|
(11.0
|
)
|
|||||
|
Share-based compensation expense included in total cost and expenses
|
160.1
|
|
189.8
|
|
196.3
|
|
|||||
|
Income tax effect
|
(42.8
|
)
|
(54.0
|
)
|
(55.8
|
)
|
|||||
|
Share-based compensation expense included in net income attributable to Biogen Inc.
|
$
|
117.3
|
|
$
|
135.8
|
|
$
|
140.5
|
|
||
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2017
|
2016
|
2015
|
||||||||
|
Market stock units
|
$
|
22.4
|
|
$
|
38.4
|
|
$
|
38.1
|
|
||
|
Time-vested restricted stock units
|
107.3
|
|
120.0
|
|
119.0
|
|
|||||
|
Cash settled performance units
|
18.4
|
|
16.3
|
|
22.4
|
|
|||||
|
Performance units
|
12.3
|
|
18.6
|
|
13.9
|
|
|||||
|
Employee stock purchase plan
|
9.3
|
|
11.1
|
|
13.9
|
|
|||||
|
Subtotal
|
169.7
|
|
204.4
|
|
207.3
|
|
|||||
|
Capitalized share-based compensation costs
|
(9.6
|
)
|
(14.6
|
)
|
(11.0
|
)
|
|||||
|
Share-based compensation expense included in total cost and expenses
|
$
|
160.1
|
|
$
|
189.8
|
|
$
|
196.3
|
|
||
|
Shares
|
Weighted
Average
Exercise
Price
|
|||||
|
Outstanding at December 31, 2016
|
66,000
|
|
$
|
54.06
|
|
|
|
Hemophilia spin-off adjustment
|
—
|
|
$
|
—
|
|
|
|
Granted
|
—
|
|
$
|
—
|
|
|
|
Exercised
|
(14,000
|
)
|
$
|
50.89
|
|
|
|
Cancelled
|
(10,000
|
)
|
$
|
55.11
|
|
|
|
Outstanding at December 31, 2017
|
42,000
|
|
$
|
53.83
|
|
|
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2017
|
2016
|
2015
|
||||||||
|
Tax benefit realized for stock options
|
$
|
3.4
|
|
$
|
4.0
|
|
$
|
11.9
|
|
||
|
Cash received from the exercise of stock options
|
$
|
0.7
|
|
$
|
2.2
|
|
$
|
6.3
|
|
||
|
Shares
|
Weighted
Average
Grant Date
Fair Value
|
|||||
|
Unvested at December 31, 2016
|
230,000
|
|
$
|
355.60
|
|
|
|
Hemophilia spin-off adjustment
|
4,000
|
|
$
|
—
|
|
|
|
Granted (a)
|
94,000
|
|
$
|
382.59
|
|
|
|
Vested
|
(112,000
|
)
|
$
|
311.17
|
|
|
|
Forfeited
|
(45,000
|
)
|
$
|
372.35
|
|
|
|
Unvested at December 31, 2017
|
171,000
|
|
$
|
370.83
|
|
|
|
(a)
|
MSUs granted in 2017 include approximately
9,000
MSUs issued in 2017 based upon the attainment of performance criteria set for 2013, in relation to awards granted in that year. MSUs granted during 2017 also include awards granted in conjunction with our annual awards made in February 2017 and MSUs granted in conjunction with the hiring of employees. These grants reflect the target number of shares eligible to be earned at the time of grant. MSUs granted in 2017 reflect an adjustment based upon the final performance multiplier in relation to shares granted in 2016, 2015 and 2014.
|
|
|
For the Years Ended December 31,
|
||||
|
|
2017
|
2016
|
2015
|
||
|
Expected dividend yield
|
—%
|
—%
|
—%
|
||
|
Range of expected stock price volatility
|
33.0% - 35.6%
|
38.2% - 40.7%
|
31.0% - 33.2%
|
||
|
Range of risk-free interest rates
|
0.9% - 1.6%
|
0.6% - 0.9%
|
0.2% - 1.0%
|
||
|
30 calendar day average stock price on grant date
|
$263.18 - $267.88
|
$260.67 - $304.86
|
$277.35 - $426.27
|
||
|
Weighted-average per share grant date fair value
|
$382.59
|
$328.03
|
$493.43
|
||
|
Shares
|
||
|
Unvested at December 31, 2016
|
122,000
|
|
|
Hemophilia spin-off adjustment
|
3,000
|
|
|
Granted (a)
|
83,000
|
|
|
Vested
|
(69,000
|
)
|
|
Forfeited
|
(34,000
|
)
|
|
Unvested at December 31, 2017
|
105,000
|
|
|
(a)
|
CSPUs granted in 2017 include awards granted in conjunction with our annual awards made in February 2017 and CSPUs granted in conjunction with the hiring of employees. These grants reflect the target number of shares eligible to be earned at the time of grant. CSPUs granted in 2017 also include CSPUs issued in 2017 based upon the attainment of performance criteria set for 2016 in relation to shares granted in 2016.
|
|
Shares
|
||
|
Unvested at December 31, 2016
|
110,000
|
|
|
Hemophilia spin-off adjustment
|
3,000
|
|
|
Granted (a)
|
40,000
|
|
|
Vested
|
(43,000
|
)
|
|
Forfeited
|
(19,000
|
)
|
|
Unvested at December 31, 2017
|
91,000
|
|
|
(a)
|
PUs granted in 2017 include awards granted in conjunction with our annual awards made in February 2017 and PUs granted in conjunction with the hiring of employees. These grants reflect the target number of shares eligible to be earned at the time of grant.
|
|
Shares
|
Weighted
Average
Grant Date
Fair Value
|
|||||
|
Unvested at December 31, 2016
|
888,000
|
|
$
|
303.49
|
|
|
|
Hemophilia spin-off adjustment
|
12,000
|
|
$
|
—
|
|
|
|
Granted (a)
|
464,000
|
|
$
|
293.41
|
|
|
|
Vested
|
(350,000
|
)
|
$
|
308.04
|
|
|
|
Forfeited
|
(182,000
|
)
|
$
|
292.57
|
|
|
|
Unvested at December 31, 2017
|
832,000
|
|
$
|
291.85
|
|
|
|
(a)
|
RSUs granted in 2017 primarily represent RSUs granted in conjunction with our annual awards made in February 2017 and awards made in conjunction with the hiring of new employees. RSUs granted in 2017 also include approximately
11,000
RSUs granted to our Board of Directors.
|
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions, except share amounts)
|
2017
|
2016
|
2015
|
||||||||
|
Shares issued under the 2015 ESPP
|
167,000
|
|
190,000
|
|
78,000
|
|
|||||
|
Shares issued under the 1995 ESPP
|
—
|
|
—
|
|
98,000
|
|
|||||
|
Cash received under the 2015 ESPP
|
$
|
39.8
|
|
$
|
41.5
|
|
$
|
19.3
|
|
||
|
Cash received under the 1995 ESPP
|
$
|
—
|
|
$
|
—
|
|
$
|
30.0
|
|
||
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2017
|
2016
|
2015
|
||||||||
|
Income before income taxes (benefit):
|
|||||||||||
|
Domestic
|
$
|
3,540.4
|
|
$
|
3,655.4
|
|
$
|
3,386.7
|
|
||
|
Foreign
|
1,588.4
|
|
1,277.6
|
|
1,380.6
|
|
|||||
|
Total
|
$
|
5,128.8
|
|
$
|
4,933.0
|
|
$
|
4,767.3
|
|
||
|
Income tax expense (benefit):
|
|||||||||||
|
Current:
|
|||||||||||
|
Federal
|
$
|
2,201.4
|
|
$
|
1,304.3
|
|
$
|
1,214.1
|
|
||
|
State
|
57.0
|
|
55.1
|
|
38.6
|
|
|||||
|
Foreign
|
108.6
|
|
52.9
|
|
54.5
|
|
|||||
|
Total
|
2,367.0
|
|
1,412.3
|
|
1,307.2
|
|
|||||
|
Deferred:
|
|||||||||||
|
Federal
|
$
|
241.0
|
|
$
|
(125.6
|
)
|
$
|
(129.6
|
)
|
||
|
State
|
9.9
|
|
(3.8
|
)
|
(1.9
|
)
|
|||||
|
Foreign
|
(159.2
|
)
|
(45.6
|
)
|
(14.1
|
)
|
|||||
|
Total
|
91.7
|
|
(175.0
|
)
|
(145.6
|
)
|
|||||
|
Total income tax expense
|
$
|
2,458.7
|
|
$
|
1,237.3
|
|
$
|
1,161.6
|
|
||
|
|
As of December 31,
|
||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Deferred tax assets:
|
|||||||
|
Tax credits
|
$
|
60.0
|
|
$
|
201.1
|
|
|
|
Inventory, other reserves and accruals
|
147.8
|
|
250.6
|
|
|||
|
Intangibles, net
|
378.8
|
|
459.8
|
|
|||
|
Net operating loss
|
209.8
|
|
65.9
|
|
|||
|
Share-based compensation
|
26.9
|
|
61.5
|
|
|||
|
Other
|
25.1
|
|
49.0
|
|
|||
|
Valuation allowance
|
(16.6
|
)
|
(16.1
|
)
|
|||
|
Total deferred tax assets
|
$
|
831.8
|
|
$
|
1,071.8
|
|
|
|
Deferred tax liabilities:
|
|||||||
|
Purchased intangible assets
|
$
|
(250.7
|
)
|
$
|
(376.6
|
)
|
|
|
Depreciation, amortization and other
|
(107.9
|
)
|
(113.5
|
)
|
|||
|
Total deferred tax liabilities
|
$
|
(358.6
|
)
|
$
|
(490.1
|
)
|
|
|
|
For the Years Ended December 31,
|
|||||||
|
|
2017
|
2016
|
2015
|
|||||
|
Statutory rate
|
35.0
|
%
|
35.0
|
%
|
35.0
|
%
|
||
|
State taxes
|
0.8
|
|
0.9
|
|
0.5
|
|
||
|
Taxes on foreign earnings
|
(11.1
|
)
|
(9.6
|
)
|
(10.0
|
)
|
||
|
Credits and net operating loss utilization
|
(0.8
|
)
|
(1.4
|
)
|
(1.3
|
)
|
||
|
Purchased intangible assets
|
1.4
|
|
1.2
|
|
1.0
|
|
||
|
Manufacturing deduction
|
(1.9
|
)
|
(1.9
|
)
|
(1.8
|
)
|
||
|
2017 Tax Act
|
22.9
|
|
—
|
|
—
|
|
||
|
Impairment of ZINBRYTA related tax assets
|
0.9
|
|
—
|
|
—
|
|
||
|
Other permanent items
|
0.7
|
|
0.5
|
|
0.7
|
|
||
|
Other
|
—
|
|
0.4
|
|
0.3
|
|
||
|
Effective tax rate
|
47.9
|
%
|
25.1
|
%
|
24.4
|
%
|
||
|
(In millions)
|
2017
|
2016
|
2015
|
||||||||
|
Balance at January 1,
|
$
|
32.4
|
|
$
|
67.9
|
|
$
|
131.5
|
|
||
|
Additions based on tax positions related to the current period
|
5.7
|
|
7.2
|
|
10.5
|
|
|||||
|
Additions for tax positions of prior periods
|
7.3
|
|
36.3
|
|
19.5
|
|
|||||
|
Reductions for tax positions of prior periods
|
(21.8
|
)
|
(13.3
|
)
|
(49.9
|
)
|
|||||
|
Statute expirations
|
(1.4
|
)
|
(1.4
|
)
|
(1.2
|
)
|
|||||
|
Settlement refund (payment)
|
44.6
|
|
(64.3
|
)
|
(42.5
|
)
|
|||||
|
Balance at December 31,
|
$
|
66.8
|
|
$
|
32.4
|
|
$
|
67.9
|
|
||
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2017
|
2016
|
2015
|
||||||||
|
Cash paid during the year for:
|
|||||||||||
|
Interest
|
$
|
281.7
|
|
$
|
281.2
|
|
$
|
39.1
|
|
||
|
Income taxes
|
$
|
1,066.4
|
|
$
|
1,642.2
|
|
$
|
1,674.8
|
|
||
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2017
|
2016
|
2015
|
||||||||
|
Interest income
|
$
|
78.5
|
|
$
|
63.4
|
|
$
|
22.1
|
|
||
|
Interest expense
|
(250.8
|
)
|
(260.0
|
)
|
(95.5
|
)
|
|||||
|
Gain (loss) on investments, net
|
(36.3
|
)
|
6.0
|
|
(3.8
|
)
|
|||||
|
Foreign exchange gains (losses), net
|
6.3
|
|
(9.8
|
)
|
(32.7
|
)
|
|||||
|
Other, net
|
(13.1
|
)
|
(17.0
|
)
|
(13.8
|
)
|
|||||
|
Total other income (expense), net
|
$
|
(215.4
|
)
|
$
|
(217.4
|
)
|
$
|
(123.7
|
)
|
||
|
|
As of December 31,
|
||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Current portion of contingent consideration obligations
|
$
|
844.6
|
|
$
|
580.8
|
|
|
|
Revenue-related reserves for discounts and allowances
|
572.0
|
|
438.6
|
|
|||
|
Employee compensation and benefits
|
297.7
|
|
282.9
|
|
|||
|
Royalties and licensing fees
|
206.7
|
|
195.8
|
|
|||
|
Collaboration expenses
|
183.7
|
|
130.9
|
|
|||
|
Construction in progress
|
159.7
|
|
134.0
|
|
|||
|
Accrued TECFIDERA litigation settlement charge
|
—
|
|
454.8
|
|
|||
|
Other
|
636.9
|
|
685.7
|
|
|||
|
Total accrued expenses and other
|
$
|
2,901.3
|
|
$
|
2,903.5
|
|
|
|
Until GAZYVA First Non-CLL FDA Approval
|
40.0
|
%
|
|
After GAZYVA First Non-CLL FDA Approval until First GAZYVA Threshold Date
|
39.0
|
%
|
|
After First GAZYVA Threshold Date until Second GAZYVA Threshold Date
|
37.5
|
%
|
|
After Second GAZYVA Threshold Date
|
35.0
|
%
|
|
Until First GAZYVA Threshold Date
|
39.0
|
%
|
|
After First GAZYVA Threshold Date until Second GAZYVA Threshold Date
|
37.5
|
%
|
|
After Second GAZYVA Threshold Date
|
35.0
|
%
|
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2017
|
2016
|
2015
|
||||||||
|
Biogen's share of pre-tax profits in the U.S. for RITUXAN and GAZYVA, including the reimbursement of selling and development expenses
|
$
|
1,316.4
|
|
$
|
1,249.5
|
|
$
|
1,269.8
|
|
||
|
Other revenues from anti-CD20 therapeutic programs
|
242.8
|
|
65.0
|
|
69.4
|
|
|||||
|
Total revenues from anti-CD20 therapeutic programs
|
$
|
1,559.2
|
|
$
|
1,314.5
|
|
$
|
1,339.2
|
|
||
|
|
For the Year Ended December 31,
|
||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Product revenues, net
|
$
|
53.1
|
|
$
|
6.1
|
|
|
|
Costs and expenses
|
92.6
|
|
50.0
|
|
|||
|
Co-promotion losses in the U.S.
|
$
|
39.5
|
|
$
|
43.9
|
|
|
|
Biogen's share of co-promotion losses in the U.S.
|
$
|
16.9
|
|
$
|
21.9
|
|
|
|
|
For the Years Ended December 31,
|
||||||||||
|
(In millions)
|
2017
|
2016
|
2015
|
||||||||
|
Total development expense incurred by the collaboration in development of BAN2401 and E2609
|
$
|
146.2
|
|
$
|
95.1
|
|
$
|
84.1
|
|
||
|
Biogen's share of BAN2401 and E2609 development expense reflected in our consolidated statements of income, excluding upfront and milestone payments
|
$
|
74.3
|
|
$
|
50.5
|
|
$
|
40.4
|
|
||
|
(In millions)
|
2018
|
2019
|
2020
|
2021
|
2022
|
Thereafter
|
Total
|
||||||||||||||||||||
|
Minimum lease payments
|
$
|
72.6
|
|
$
|
72.3
|
|
$
|
68.4
|
|
$
|
66.9
|
|
$
|
65.7
|
|
$
|
271.1
|
|
$
|
617.0
|
|
||||||
|
Less: income from subleases (1)
|
(24.3
|
)
|
(24.7
|
)
|
(23.9
|
)
|
(22.3
|
)
|
(22.0
|
)
|
(71.3
|
)
|
(188.5
|
)
|
|||||||||||||
|
Net minimum lease payments
|
$
|
48.3
|
|
$
|
47.6
|
|
$
|
44.5
|
|
$
|
44.6
|
|
$
|
43.7
|
|
$
|
199.8
|
|
$
|
428.5
|
|
||||||
|
(1)
|
Represents sublease income expected to be received for the vacated manufacturing facility in Cambridge, MA, the vacated portion of our Weston, MA facility and other facilities throughout the world.
|
|
|
For the Years Ended December 31,
|
||||||||||||||||||||||||||||||||||
|
|
2017
|
2016
|
2015
|
||||||||||||||||||||||||||||||||
|
(In millions)
|
United
States
|
Rest of
World
|
Total
|
United
States
|
Rest of
World
|
Total
|
United
States
|
Rest of
World
|
Total
|
||||||||||||||||||||||||||
|
Multiple Sclerosis (MS):
|
|||||||||||||||||||||||||||||||||||
|
TECFIDERA
|
$
|
3,294.0
|
|
$
|
920.0
|
|
$
|
4,214.0
|
|
$
|
3,169.4
|
|
$
|
798.7
|
|
$
|
3,968.1
|
|
$
|
2,908.2
|
|
$
|
730.2
|
|
$
|
3,638.4
|
|
||||||||
|
AVONEX
|
1,593.6
|
|
557.9
|
|
2,151.5
|
|
1,675.3
|
|
638.2
|
|
2,313.5
|
|
1,790.2
|
|
840.0
|
|
2,630.2
|
|
|||||||||||||||||
|
PLEGRIDY
|
295.5
|
|
198.8
|
|
494.3
|
|
305.0
|
|
176.7
|
|
481.7
|
|
227.1
|
|
111.4
|
|
338.5
|
|
|||||||||||||||||
|
TYSABRI
|
1,113.8
|
|
859.3
|
|
1,973.1
|
|
1,182.9
|
|
780.9
|
|
1,963.8
|
|
1,103.1
|
|
783.0
|
|
1,886.1
|
|
|||||||||||||||||
|
FAMPYRA
|
—
|
|
91.6
|
|
91.6
|
|
—
|
|
84.9
|
|
84.9
|
|
—
|
|
89.7
|
|
89.7
|
|
|||||||||||||||||
|
ZINBRYTA
|
—
|
|
52.7
|
|
52.7
|
|
—
|
|
7.8
|
|
7.8
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||
|
Spinal Muscular Atrophy:
|
|||||||||||||||||||||||||||||||||||
|
SPINRAZA
|
657.0
|
|
226.7
|
|
883.7
|
|
4.6
|
|
—
|
|
4.6
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||
|
Hemophilia:
|
|||||||||||||||||||||||||||||||||||
|
ELOCTATE
|
42.2
|
|
6.2
|
|
48.4
|
|
445.2
|
|
68.0
|
|
513.2
|
|
308.3
|
|
11.4
|
|
319.7
|
|
|||||||||||||||||
|
ALPROLIX
|
21.0
|
|
5.0
|
|
26.0
|
|
268.0
|
|
65.7
|
|
333.7
|
|
208.9
|
|
25.6
|
|
234.5
|
|
|||||||||||||||||
|
Other product revenues:
|
|||||||||||||||||||||||||||||||||||
|
FUMADERM
|
—
|
|
39.6
|
|
39.6
|
|
—
|
|
45.9
|
|
45.9
|
|
—
|
|
51.4
|
|
51.4
|
|
|||||||||||||||||
|
BENEPALI
|
—
|
|
370.8
|
|
370.8
|
|
—
|
|
100.6
|
|
100.6
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||
|
FLIXABI
|
—
|
|
9.0
|
|
9.0
|
|
—
|
|
0.1
|
|
0.1
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||
|
Total product revenues
|
$
|
7,017.1
|
|
$
|
3,337.6
|
|
$
|
10,354.7
|
|
$
|
7,050.4
|
|
$
|
2,767.5
|
|
$
|
9,817.9
|
|
$
|
6,545.8
|
|
$
|
2,642.7
|
|
$
|
9,188.5
|
|
||||||||
|
December 31, 2017 (In millions)
|
U.S.
|
Europe
|
Asia
|
Other
|
Total
|
||||||||||||||
|
Product revenues from external customers
|
$
|
7,017.1
|
|
$
|
2,844.8
|
|
$
|
160.1
|
|
$
|
332.7
|
|
$
|
10,354.7
|
|
||||
|
Revenues from anti-CD20 therapeutic programs
|
$
|
1,475.6
|
|
$
|
0.6
|
|
$
|
—
|
|
$
|
83.0
|
|
$
|
1,559.2
|
|
||||
|
Other revenues from external customers
|
$
|
249.5
|
|
$
|
67.8
|
|
$
|
42.7
|
|
$
|
—
|
|
$
|
360.0
|
|
||||
|
Long-lived assets
|
$
|
1,226.9
|
|
$
|
1,948.2
|
|
$
|
5.2
|
|
$
|
2.1
|
|
$
|
3,182.4
|
|
||||
|
December 31, 2016 (In millions)
|
U.S.
|
Europe
|
Asia
|
Other
|
Total
|
||||||||||||||
|
Product revenues from external customers
|
$
|
7,050.4
|
|
$
|
2,237.2
|
|
$
|
217.3
|
|
$
|
313.0
|
|
$
|
9,817.9
|
|
||||
|
Revenues from anti-CD20 therapeutic programs
|
$
|
1,249.5
|
|
$
|
1.9
|
|
$
|
—
|
|
$
|
63.1
|
|
$
|
1,314.5
|
|
||||
|
Other revenues from external customers
|
$
|
224.7
|
|
$
|
71.5
|
|
$
|
20.2
|
|
$
|
—
|
|
$
|
316.4
|
|
||||
|
Long-lived assets
|
$
|
1,272.3
|
|
$
|
1,221.1
|
|
$
|
7.0
|
|
$
|
1.4
|
|
$
|
2,501.8
|
|
||||
|
December 31, 2015 (In millions)
|
U.S.
|
Europe
|
Asia
|
Other
|
Total
|
||||||||||||||
|
Product revenues from external customers
|
$
|
6,545.8
|
|
$
|
2,165.7
|
|
$
|
143.7
|
|
$
|
333.3
|
|
$
|
9,188.5
|
|
||||
|
Revenues from anti-CD20 therapeutic programs
|
$
|
1,269.8
|
|
$
|
3.5
|
|
$
|
—
|
|
$
|
65.9
|
|
$
|
1,339.2
|
|
||||
|
Other revenues from external customers
|
$
|
142.0
|
|
$
|
31.2
|
|
$
|
62.9
|
|
$
|
—
|
|
$
|
236.1
|
|
||||
|
Long-lived assets
|
$
|
1,296.5
|
|
$
|
881.7
|
|
$
|
7.7
|
|
$
|
1.7
|
|
$
|
2,187.6
|
|
||||
|
(In millions, except per share amounts)
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
Total
Year
|
||||||||||||||
|
2017
|
(a)
|
(a) (b) (c) (d)
|
(a)
|
(a) (e) (f) (g) (h)
|
|||||||||||||||
|
Product revenues, net
|
$
|
2,380.1
|
|
$
|
2,639.7
|
|
$
|
2,622.5
|
|
$
|
2,712.4
|
|
$
|
10,354.7
|
|
||||
|
Revenues from anti-CD20 therapeutic programs
|
$
|
340.6
|
|
$
|
397.1
|
|
$
|
406.5
|
|
$
|
415.0
|
|
$
|
1,559.2
|
|
||||
|
Other revenues
|
$
|
90.0
|
|
$
|
41.6
|
|
$
|
48.8
|
|
$
|
179.6
|
|
$
|
360.0
|
|
||||
|
Total revenues
|
$
|
2,810.7
|
|
$
|
3,078.4
|
|
$
|
3,077.8
|
|
$
|
3,307.0
|
|
$
|
12,273.9
|
|
||||
|
Gross profit (1)
|
$
|
2,426.1
|
|
$
|
2,712.2
|
|
$
|
2,707.8
|
|
$
|
2,797.8
|
|
$
|
10,643.9
|
|
||||
|
Net income
|
$
|
747.5
|
|
$
|
862.8
|
|
$
|
1,226.1
|
|
$
|
(166.3
|
)
|
$
|
2,670.1
|
|
||||
|
Net income attributable to Biogen Inc.
|
$
|
747.6
|
|
$
|
862.8
|
|
$
|
1,226.1
|
|
$
|
(297.4
|
)
|
$
|
2,539.1
|
|
||||
|
Net income per share:
|
|||||||||||||||||||
|
Basic earnings per share attributable to Biogen Inc.
|
$
|
3.47
|
|
$
|
4.07
|
|
$
|
5.80
|
|
$
|
(1.41
|
)
|
$
|
11.94
|
|
||||
|
Diluted earnings per share attributable to Biogen Inc.
|
$
|
3.46
|
|
$
|
4.07
|
|
$
|
5.79
|
|
$
|
(1.40
|
)
|
$
|
11.92
|
|
||||
|
Weighted-average shares used in calculating:
|
|||||||||||||||||||
|
Basic earnings per share attributable to Biogen Inc.
|
215.6
|
|
211.9
|
|
211.4
|
|
211.5
|
|
212.6
|
|
|||||||||
|
Diluted earnings per share attributable to Biogen Inc.
|
215.9
|
|
212.2
|
|
211.8
|
|
212.0
|
|
213.0
|
|
|||||||||
|
(In millions, except per share amounts)
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
Total
Year
|
||||||||||||||
|
2016
|
(i)
|
(i) (j)
|
(i) (k) (l)
|
|
|||||||||||||||
|
Product revenues, net
|
$
|
2,309.4
|
|
$
|
2,466.0
|
|
$
|
2,539.6
|
|
$
|
2,502.9
|
|
$
|
9,817.9
|
|
||||
|
Revenues from anti-CD20 therapeutic programs
|
$
|
329.5
|
|
$
|
349.2
|
|
$
|
317.6
|
|
$
|
318.2
|
|
$
|
1,314.5
|
|
||||
|
Other revenues
|
$
|
87.9
|
|
$
|
79.0
|
|
$
|
98.6
|
|
$
|
50.9
|
|
$
|
316.4
|
|
||||
|
Total revenues
|
$
|
2,726.8
|
|
$
|
2,894.2
|
|
$
|
2,955.8
|
|
$
|
2,872.0
|
|
$
|
11,448.8
|
|
||||
|
Gross profit (1)
|
$
|
2,413.8
|
|
$
|
2,523.9
|
|
$
|
2,538.9
|
|
$
|
2,493.5
|
|
$
|
9,970.1
|
|
||||
|
Net income
|
$
|
969.2
|
|
$
|
1,048.4
|
|
$
|
1,030.2
|
|
$
|
647.9
|
|
$
|
3,695.7
|
|
||||
|
Net income attributable to Biogen Inc.
|
$
|
970.9
|
|
$
|
1,049.8
|
|
$
|
1,032.9
|
|
$
|
649.2
|
|
$
|
3,702.8
|
|
||||
|
Net income per share:
|
|||||||||||||||||||
|
Basic earnings per share attributable to Biogen Inc.
|
$
|
4.44
|
|
$
|
4.79
|
|
$
|
4.72
|
|
$
|
3.00
|
|
$
|
16.96
|
|
||||
|
Diluted earnings per share attributable to Biogen Inc.
|
$
|
4.43
|
|
$
|
4.79
|
|
$
|
4.71
|
|
$
|
2.99
|
|
$
|
16.93
|
|
||||
|
Weighted-average shares used in calculating:
|
|||||||||||||||||||
|
Basic earnings per share attributable to Biogen Inc.
|
218.9
|
|
219.1
|
|
218.9
|
|
216.6
|
|
218.4
|
|
|||||||||
|
Diluted earnings per share attributable to Biogen Inc.
|
219.3
|
|
219.4
|
|
219.4
|
|
217.0
|
|
218.8
|
|
|||||||||
|
(a)
|
Net income and net income attributable to Biogen Inc. for the first, second, third and fourth quarters of 2017 include a pre-tax charge of
$353.6 million
,
$29.4 million
,
$30.4 million
and
$30.8 million
, respectively, related to our U.S. and rest of world licenses to Forward Pharma's intellectual property, including Forward Pharma's intellectual property related to TECFIDERA.
|
|
(b)
|
Net income and net income attributable to Biogen Inc. for the second quarter of 2017 includes a pre-tax charge to research and development expense of
$300.0 million
for an upfront payment to BMS upon the closing of our agreement to exclusively license BIIB092.
|
|
(c)
|
Net income and net income attributable to Biogen Inc. for the second quarter of 2017 includes a pre-tax charge to acquired in-process research and development of
$120.0 million
for an upfront payment to Remedy upon closing of the asset purchase transaction.
|
|
(d)
|
Net income and net income attributable to Biogen Inc. for the second quarter of 2017 includes a pre-tax charge to research and development expense of
$60.0 million
for a developmental milestone that became payable to the former shareholders of iPierian upon dosing of the first patient in the Phase 2 PSP study for BIIB092.
|
|
(e)
|
Net income attributable to Biogen Inc., for the fourth quarter of 2017, includes a pre-tax charge to noncontrolling interest of
$150.0 million
for a payment to Neurimmune in exchange for a
15%
reduction in royalty rates payable on potential commercial sales of aducanumab.
|
|
(f)
|
Net income and net income attributable to Biogen Inc. for the fourth quarter of 2017 includes pre-tax charges to research and development expense of
$28.0 million
and
$50.0 million
for an upfront payment and a continuation payment, respectively, to Alkermes.
|
|
(g)
|
Net income and net income attributable to Biogen Inc. for the fourth quarter of 2017 includes a pre-tax charge to research and development expense of
$25.0 million
for an upfront payment to Ionis upon entering into a new collaboration agreement to identify new antisense-oligonucleotide drug candidates for the treatment of SMA.
|
|
(h)
|
Net income and net income attributable to Biogen Inc. for the fourth quarter of 2017 includes
$1,173.6 million
related to the provisions of the 2017 Tax Act, including a
$989.6 million
expense under the Transition Toll Tax.
|
|
(i)
|
Net income and net income attributable to Biogen Inc. for the second, third and fourth quarters of 2016 includes additional pre-tax depreciation expense totaling
$15.8 million
,
$15.7 million
and
$14.0 million
, respectively, as part of our decision to cease manufacturing and vacate our small-scale biologics manufacturing facility in Cambridge, MA as well as close and vacate our warehouse space in Somerville, MA.
|
|
(j)
|
Net income and net income attributable to Biogen Inc. for the third quarter of 2016 includes a pre-tax charge to research and development expense of
$75.0 million
for a license fee paid to Ionis as we exercised our option to develop and commercialize SPINRAZA.
|
|
(k)
|
Net income and net income attributable to Biogen Inc. for the fourth quarter of 2016 includes a pre-tax charge to research and development expense of
$50.0 million
for a milestone payment due to Eisai related to the initiation of a Phase 3 trial for E2609.
|
|
(l)
|
Net income and net income attributable to Biogen Inc. for the fourth quarter of 2016 includes a pre-tax charge of
$454.8 million
related to our January 2017 settlement and license agreement with Forward Pharma.
|