|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|

|
Delaware
|
|
33-0112644
|
|
(State or other jurisdiction of
incorporation or organization)
|
|
(I.R.S. Employer
Identification No.)
|
|
Large accelerated filer
x
|
Accelerated filer
o
|
|
|
Non-accelerated filer
o
|
Smaller reporting company
o
|
|
|
(Do not check if a smaller reporting company)
|
Emerging growth company
o
|
|
|
|
|
Page
|
|
PART I —
FINANCIAL INFORMATION
|
||
|
Item 1.
|
Financial Statements (unaudited)
|
|
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
PART II —
OTHER INFORMATION
|
||
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 2.
|
||
|
Item 6.
|
||
|
•
|
the anticipated amount, timing and accounting of revenues, contingent payments, milestone, royalty and other payments under licensing, collaboration or acquisition agreements, tax positions and contingencies, collectability of receivables, pre-approval inventory, cost of sales, research and development costs, compensation and other selling, general and administrative expenses, amortization of intangible assets, foreign currency exchange risk, estimated fair value of assets and liabilities and impairment assessments;
|
|
•
|
expectations, plans and prospects relating to sales, pricing, growth and launch of our marketed and pipeline products;
|
|
•
|
the potential impact of increased product competition in the markets in which we compete;
|
|
•
|
the anticipated benefits, costs and tax treatment of the spin-off of our hemophilia business;
|
|
•
|
patent terms, patent term extensions, patent office actions and expected availability and period of regulatory exclusivity;
|
|
•
|
the costs and timing of potential clinical trials, filing and approvals, and the potential therapeutic scope of the development and commercialization of our and our collaborators’ pipeline products;
|
|
•
|
the drivers for growing our business, including our plans and intent to commit resources relating to business development opportunities and research and development programs;
|
|
•
|
our manufacturing capacity, use of third-party contract manufacturing organizations and plans and timing relating to the expansion of our manufacturing capabilities, including anticipated investments and activities in new manufacturing facilities;
|
|
•
|
the potential impact on our results of operations and liquidity of the United Kingdom's (U.K.'s) intent to voluntarily depart from the European Union (E.U.);
|
|
•
|
the impact of the continued uncertainty of the credit and economic conditions in certain countries in Europe and our collection of accounts receivable in such countries;
|
|
•
|
the potential impact of healthcare reform in the United States (U.S.) and measures being taken worldwide designed to reduce healthcare costs to limit the overall level of government expenditures, including the impact of pricing actions and reduced reimbursement for our products;
|
|
•
|
the timing, outcome and impact of administrative, regulatory, legal and other proceedings related to our patents and other proprietary and intellectual property rights, tax audits, assessments and settlements, pricing matters, sales and promotional practices, product liability and other matters;
|
|
•
|
lease commitments, purchase obligations and the timing and satisfaction of other contractual obligations;
|
|
•
|
our ability to finance our operations and business initiatives and obtain funding for such activities; and
|
|
•
|
the impact of new laws and accounting standards.
|
|
•
|
“Biogen,” the “company,” “we,” “us” and “our” refer to Biogen Inc. and its consolidated subsidiaries;
|
|
•
|
“RITUXAN” refers to both RITUXAN (the trade name for rituximab in the U.S., Canada and Japan) and MabThera (the trade name for rituximab outside the U.S., Canada and Japan);
|
|
•
|
"ELOCTATE" refers to both ELOCTATE (the trade name for Antihemophilic Factor (Recombinant), Fc Fusion Protein in the U.S., Canada and Japan) and ELOCTA (the trade name for Antihemophilic Factor (Recombinant), Fc Fusion Protein in the E.U.).
|
|
|
For the Three Months
Ended March 31, |
||||||
|
|
2017
|
2016
|
|||||
|
Revenues:
|
|||||||
|
Product, net
|
$
|
2,380.1
|
|
$
|
2,309.4
|
|
|
|
Revenues from anti-CD20 therapeutic programs
|
340.6
|
|
329.5
|
|
|||
|
Other
|
90.0
|
|
87.9
|
|
|||
|
Total revenues
|
2,810.7
|
|
2,726.8
|
|
|||
|
Cost and expenses:
|
|||||||
|
Cost of sales, excluding amortization of acquired intangible assets
|
384.6
|
|
313.0
|
|
|||
|
Research and development
|
423.4
|
|
437.3
|
|
|||
|
Selling, general and administrative
|
499.1
|
|
497.3
|
|
|||
|
Amortization of acquired intangible assets
|
448.5
|
|
88.8
|
|
|||
|
Collaboration profit (loss) sharing
|
20.8
|
|
—
|
|
|||
|
(Gain) loss on fair value remeasurement of contingent consideration
|
10.0
|
|
2.3
|
|
|||
|
Restructuring charges
|
—
|
|
9.7
|
|
|||
|
Total cost and expenses
|
1,786.4
|
|
1,348.4
|
|
|||
|
Income from operations
|
1,024.3
|
|
1,378.4
|
|
|||
|
Other income (expense), net
|
(37.6
|
)
|
(52.8
|
)
|
|||
|
Income before income tax expense and equity in loss of investee, net of tax
|
986.7
|
|
1,325.6
|
|
|||
|
Income tax expense
|
239.2
|
|
356.4
|
|
|||
|
Equity in loss of investee, net of tax
|
—
|
|
—
|
|
|||
|
Net income
|
747.5
|
|
969.2
|
|
|||
|
Net income (loss) attributable to noncontrolling interests, net of tax
|
(0.1
|
)
|
(1.7
|
)
|
|||
|
Net income attributable to Biogen Inc.
|
$
|
747.6
|
|
$
|
970.9
|
|
|
|
Net income per share:
|
|||||||
|
Basic earnings per share attributable to Biogen Inc.
|
$
|
3.47
|
|
$
|
4.44
|
|
|
|
Diluted earnings per share attributable to Biogen Inc.
|
$
|
3.46
|
|
$
|
4.43
|
|
|
|
Weighted-average shares used in calculating:
|
|||||||
|
Basic earnings per share attributable to Biogen Inc.
|
215.6
|
|
218.9
|
|
|||
|
Diluted earnings per share attributable to Biogen Inc.
|
215.9
|
|
219.3
|
|
|||
|
|
For the Three Months
Ended March 31, |
||||||
|
|
2017
|
2016
|
|||||
|
Net income attributable to Biogen Inc.
|
$
|
747.6
|
|
$
|
970.9
|
|
|
|
Other comprehensive income:
|
|||||||
|
Unrealized gains (losses) on securities available for sale, net of tax
|
(1.6
|
)
|
2.5
|
|
|||
|
Unrealized gains (losses) on cash flow hedges, net of tax
|
(23.8
|
)
|
(47.6
|
)
|
|||
|
Unrealized gains (losses) on pension benefit obligation, net of tax
|
0.1
|
|
0.2
|
|
|||
|
Currency translation adjustment
|
20.0
|
|
9.6
|
|
|||
|
Total other comprehensive income (loss), net of tax
|
(5.3
|
)
|
(35.3
|
)
|
|||
|
Comprehensive income attributable to Biogen Inc.
|
742.3
|
|
935.6
|
|
|||
|
Comprehensive income (loss) attributable to noncontrolling interests, net of tax
|
(0.1
|
)
|
(1.7
|
)
|
|||
|
Comprehensive income
|
$
|
742.2
|
|
$
|
933.9
|
|
|
|
As of March 31,
2017 |
As of December 31,
2016 |
||||||
|
ASSETS
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
924.0
|
|
$
|
2,326.5
|
|
|
|
Marketable securities
|
1,956.2
|
|
2,568.6
|
|
|||
|
Accounts receivable, net
|
1,501.5
|
|
1,441.6
|
|
|||
|
Due from anti-CD20 therapeutic programs
|
324.5
|
|
300.6
|
|
|||
|
Inventory
|
921.6
|
|
1,001.6
|
|
|||
|
Other current assets
|
1,231.9
|
|
1,093.3
|
|
|||
|
Total current assets
|
6,859.7
|
|
8,732.2
|
|
|||
|
Marketable securities
|
2,825.2
|
|
2,829.4
|
|
|||
|
Property, plant and equipment, net
|
2,610.9
|
|
2,501.8
|
|
|||
|
Intangible assets, net
|
4,103.9
|
|
3,808.3
|
|
|||
|
Goodwill
|
3,611.7
|
|
3,669.3
|
|
|||
|
Investments and other assets
|
1,184.5
|
|
1,335.8
|
|
|||
|
Total assets
|
$
|
21,195.9
|
|
$
|
22,876.8
|
|
|
|
LIABILITIES AND EQUITY
|
|||||||
|
Current liabilities:
|
|||||||
|
Current portion of notes payable and other financing arrangements
|
$
|
561.5
|
|
$
|
4.7
|
|
|
|
Taxes payable
|
70.0
|
|
231.9
|
|
|||
|
Accounts payable
|
316.4
|
|
279.8
|
|
|||
|
Accrued expenses and other
|
2,044.6
|
|
2,903.5
|
|
|||
|
Total current liabilities
|
2,992.5
|
|
3,419.9
|
|
|||
|
Notes payable and other financing arrangements
|
5,952.7
|
|
6,512.7
|
|
|||
|
Deferred tax liability
|
94.5
|
|
93.1
|
|
|||
|
Other long-term liabilities
|
688.8
|
|
722.5
|
|
|||
|
Total liabilities
|
9,728.5
|
|
10,748.2
|
|
|||
|
Commitments and contingencies
|
|
|
|
|
|||
|
Equity:
|
|||||||
|
Biogen Inc. shareholders’ equity
|
|||||||
|
Preferred stock, par value $0.001 per share
|
—
|
|
—
|
|
|||
|
Common stock, par value $0.0005 per share
|
0.1
|
|
0.1
|
|
|||
|
Additional paid-in capital
|
—
|
|
—
|
|
|||
|
Accumulated other comprehensive loss
|
(325.2
|
)
|
(319.9
|
)
|
|||
|
Retained earnings
|
14,781.2
|
|
15,071.6
|
|
|||
|
Treasury stock, at cost
|
(2,977.1
|
)
|
(2,611.7
|
)
|
|||
|
Total Biogen Inc. shareholders’ equity
|
11,479.0
|
|
12,140.1
|
|
|||
|
Noncontrolling interests
|
(11.6
|
)
|
(11.5
|
)
|
|||
|
Total equity
|
11,467.4
|
|
12,128.6
|
|
|||
|
Total liabilities and equity
|
$
|
21,195.9
|
|
$
|
22,876.8
|
|
|
|
|
For the Three Months
Ended March 31, |
||||||
|
|
2017
|
2016
|
|||||
|
Cash flows from operating activities:
|
|||||||
|
Net income
|
$
|
747.5
|
|
$
|
969.2
|
|
|
|
Adjustments to reconcile net income to net cash flows from operating activities:
|
|||||||
|
Depreciation and amortization
|
512.0
|
|
149.7
|
|
|||
|
Share-based compensation
|
37.0
|
|
45.3
|
|
|||
|
Deferred income taxes
|
76.1
|
|
8.9
|
|
|||
|
Other
|
18.1
|
|
(11.9
|
)
|
|||
|
Changes in operating assets and liabilities, net:
|
|||||||
|
Accounts receivable
|
(195.7
|
)
|
(160.0
|
)
|
|||
|
Inventory
|
(46.2
|
)
|
(88.6
|
)
|
|||
|
Accrued expenses and other current liabilities
|
(684.2
|
)
|
(136.7
|
)
|
|||
|
Income tax assets and liabilities
|
(195.1
|
)
|
276.8
|
|
|||
|
Other changes in operating assets and liabilities, net
|
(34.3
|
)
|
(36.9
|
)
|
|||
|
Net cash flows provided by operating activities
|
235.2
|
|
1,015.8
|
|
|||
|
Cash flows from investing activities:
|
|||||||
|
Proceeds from sales and maturities of marketable securities
|
1,884.3
|
|
1,181.1
|
|
|||
|
Purchases of marketable securities
|
(1,256.7
|
)
|
(1,914.4
|
)
|
|||
|
Contingent consideration related to Fumapharm AG acquisition
|
(300.0
|
)
|
(300.0
|
)
|
|||
|
Purchases of property, plant and equipment
|
(210.0
|
)
|
(126.9
|
)
|
|||
|
Acquisitions of intangible assets
|
(855.2
|
)
|
(28.6
|
)
|
|||
|
Other
|
(6.6
|
)
|
(14.4
|
)
|
|||
|
Net cash flows used in investing activities
|
(744.2
|
)
|
(1,203.2
|
)
|
|||
|
Cash flows from financing activities:
|
|||||||
|
Purchases of treasury stock
|
(583.6
|
)
|
—
|
|
|||
|
Payments related to issuance of stock for share-based compensation arrangements, net
|
(25.5
|
)
|
(29.6
|
)
|
|||
|
Net cash contribution to Bioverativ
|
(302.7
|
)
|
—
|
|
|||
|
Other
|
10.4
|
|
24.6
|
|
|||
|
Net cash flows used in financing activities
|
(901.4
|
)
|
(5.0
|
)
|
|||
|
Net decrease in cash and cash equivalents
|
(1,410.4
|
)
|
(192.4
|
)
|
|||
|
Effect of exchange rate changes on cash and cash equivalents
|
7.9
|
|
15.1
|
|
|||
|
Cash and cash equivalents, beginning of the period
|
2,326.5
|
|
1,308.0
|
|
|||
|
Cash and cash equivalents, end of the period
|
$
|
924.0
|
|
$
|
1,130.7
|
|
|
|
•
|
ASU No. 2016-06, Derivatives and Hedging (Topic 815): Contingent Put and Call Options in Debt Instruments.
|
|
•
|
ASU No. 2016-07, Investments - Equity Method and Joint Ventures (Topic 323): Simplifying the Transition to the Equity Method of Accounting.
|
|
•
|
ASU No. 2016-09, Compensation - Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting.
|
|
(In millions)
|
|||
|
Assets
|
|||
|
Cash
|
$
|
302.7
|
|
|
Accounts receivable
|
144.7
|
|
|
|
Inventory
|
116.1
|
|
|
|
Property, plant and equipment, net
|
20.2
|
|
|
|
Intangible assets, net
|
56.8
|
|
|
|
Goodwill
|
314.1
|
|
|
|
Other, net
|
53.7
|
|
|
|
Assets transferred, net
|
$
|
1,008.3
|
|
|
Liabilities
|
|||
|
Accrued expenses and other current liabilities
|
$
|
87.8
|
|
|
Other long-term liabilities
|
67.7
|
|
|
|
Liabilities transferred, net
|
$
|
155.5
|
|
|
(In millions)
|
Discounts
|
Contractual
Adjustments
|
Returns
|
Total
|
|||||||||||
|
Balance, as of December 31, 2016
|
$
|
71.6
|
|
$
|
482.7
|
|
$
|
51.2
|
|
$
|
605.5
|
|
|||
|
Current provisions relating to sales in current year
|
131.3
|
|
568.2
|
|
7.6
|
|
707.1
|
|
|||||||
|
Adjustments relating to prior years
|
0.7
|
|
0.8
|
|
(6.3
|
)
|
(4.8
|
)
|
|||||||
|
Payments/credits relating to sales in current year
|
(62.3
|
)
|
(231.7
|
)
|
—
|
|
(294.0
|
)
|
|||||||
|
Payments/credits relating to sales in prior years
|
(64.4
|
)
|
(267.5
|
)
|
(5.4
|
)
|
(337.3
|
)
|
|||||||
|
Balance, as of March 31, 2017
|
$
|
76.9
|
|
$
|
552.5
|
|
$
|
47.1
|
|
$
|
676.5
|
|
|||
|
(In millions)
|
As of
March 31, 2017 |
As of
December 31, 2016 |
|||||
|
Reduction of accounts receivable
|
$
|
171.2
|
|
$
|
166.9
|
|
|
|
Component of accrued expenses and other
|
505.3
|
|
438.6
|
|
|||
|
Total reserves
|
$
|
676.5
|
|
$
|
605.5
|
|
|
|
(In millions)
|
As of
March 31, 2017 |
As of
December 31, 2016 |
|||||
|
Raw materials
|
$
|
158.2
|
|
$
|
170.4
|
|
|
|
Work in process
|
644.4
|
|
698.7
|
|
|||
|
Finished goods
|
156.8
|
|
170.3
|
|
|||
|
Total inventory
|
$
|
959.4
|
|
$
|
1,039.4
|
|
|
|
Balance Sheet Classification:
|
|||||||
|
Inventory
|
$
|
921.6
|
|
$
|
1,001.6
|
|
|
|
Investments and other assets
|
37.8
|
|
37.8
|
|
|||
|
Total inventory
|
$
|
959.4
|
|
$
|
1,039.4
|
|
|
|
|
|
As of March 31, 2017
|
As of December 31, 2016
|
||||||||||||||||||||||
|
(In millions)
|
Estimated
Life
|
Cost
|
Accumulated
Amortization
|
Net
|
Cost
|
Accumulated
Amortization
|
Net
|
||||||||||||||||||
|
Out-licensed patents
|
13-23 years
|
$
|
543.3
|
|
$
|
(526.6
|
)
|
$
|
16.7
|
|
$
|
543.3
|
|
$
|
(523.6
|
)
|
$
|
19.7
|
|
||||||
|
Developed
technology
|
15-23 years
|
3,005.3
|
|
(2,648.1
|
)
|
357.2
|
|
3,005.3
|
|
(2,634.3
|
)
|
371.0
|
|
||||||||||||
|
In-process research and development
|
Indefinite until commercialization
|
653.7
|
|
—
|
|
653.7
|
|
648.0
|
|
—
|
|
648.0
|
|
||||||||||||
|
Trademarks and
tradenames
|
Indefinite
|
64.0
|
|
—
|
|
64.0
|
|
64.0
|
|
—
|
|
64.0
|
|
||||||||||||
|
Acquired and in-licensed rights
and patents
|
4-18 years
|
3,887.1
|
|
(874.8
|
)
|
3,012.3
|
|
3,481.7
|
|
(776.1
|
)
|
2,705.6
|
|
||||||||||||
|
Total intangible assets
|
$
|
8,153.4
|
|
$
|
(4,049.5
|
)
|
$
|
4,103.9
|
|
$
|
7,742.3
|
|
$
|
(3,934.0
|
)
|
$
|
3,808.3
|
|
|||||||
|
(In millions)
|
As of
March 31, 2017 |
||
|
2017 (remaining nine months)
|
$
|
346.5
|
|
|
2018
|
425.5
|
|
|
|
2019
|
412.4
|
|
|
|
2020
|
378.5
|
|
|
|
2021
|
239.2
|
|
|
|
2022
|
216.7
|
|
|
|
(In millions)
|
As of
March 31, 2017 |
||
|
Goodwill, beginning of period
|
$
|
3,669.3
|
|
|
Elimination of goodwill allocated to our hemophilia business
|
(314.1
|
)
|
|
|
Increase to goodwill
|
254.7
|
|
|
|
Other
|
1.8
|
|
|
|
Goodwill, end of period
|
$
|
3,611.7
|
|
|
As of March 31, 2017 (In millions)
|
Total
|
Quoted Prices
in Active
Markets
(Level 1)
|
Significant Other
Observable Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
|||||||||||
|
Assets:
|
|||||||||||||||
|
Cash equivalents
|
$
|
645.1
|
|
$
|
—
|
|
$
|
645.1
|
|
$
|
—
|
|
|||
|
Marketable debt securities:
|
|||||||||||||||
|
Corporate debt securities
|
2,500.7
|
|
—
|
|
2,500.7
|
|
—
|
|
|||||||
|
Government securities
|
1,738.2
|
|
—
|
|
1,738.2
|
|
—
|
|
|||||||
|
Mortgage and other asset backed securities
|
542.5
|
|
—
|
|
542.5
|
|
—
|
|
|||||||
|
Marketable equity securities
|
18.7
|
|
18.7
|
|
—
|
|
—
|
|
|||||||
|
Derivative contracts
|
44.5
|
|
—
|
|
44.5
|
|
—
|
|
|||||||
|
Plan assets for deferred compensation
|
31.0
|
|
—
|
|
31.0
|
|
—
|
|
|||||||
|
Total
|
$
|
5,520.7
|
|
$
|
18.7
|
|
$
|
5,502.0
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Derivative contracts
|
$
|
13.6
|
|
$
|
—
|
|
$
|
13.6
|
|
$
|
—
|
|
|||
|
Contingent consideration obligations
|
470.9
|
|
—
|
|
—
|
|
470.9
|
|
|||||||
|
Total
|
$
|
484.5
|
|
$
|
—
|
|
$
|
13.6
|
|
$
|
470.9
|
|
|||
|
As of December 31, 2016 (In millions)
|
Total
|
Quoted Prices
in Active
Markets
(Level 1)
|
Significant Other
Observable Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
|||||||||||
|
Assets:
|
|||||||||||||||
|
Cash equivalents
|
$
|
2,039.6
|
|
$
|
—
|
|
$
|
2,039.6
|
|
$
|
—
|
|
|||
|
Marketable debt securities:
|
|||||||||||||||
|
Corporate debt securities
|
2,663.8
|
|
—
|
|
2,663.8
|
|
—
|
|
|||||||
|
Government securities
|
2,172.5
|
|
—
|
|
2,172.5
|
|
—
|
|
|||||||
|
Mortgage and other asset backed securities
|
561.7
|
|
—
|
|
561.7
|
|
—
|
|
|||||||
|
Marketable equity securities
|
24.9
|
|
24.9
|
|
—
|
|
—
|
|
|||||||
|
Derivative contracts
|
61.0
|
|
—
|
|
61.0
|
|
—
|
|
|||||||
|
Plan assets for deferred compensation
|
34.5
|
|
—
|
|
34.5
|
|
—
|
|
|||||||
|
Total
|
$
|
7,558.0
|
|
$
|
24.9
|
|
$
|
7,533.1
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Derivative contracts
|
$
|
13.6
|
|
$
|
—
|
|
$
|
13.6
|
|
$
|
—
|
|
|||
|
Contingent consideration obligations
|
467.6
|
|
—
|
|
—
|
|
467.6
|
|
|||||||
|
Total
|
$
|
481.2
|
|
$
|
—
|
|
$
|
13.6
|
|
$
|
467.6
|
|
|||
|
|
As of March 31, 2017
|
As of December 31, 2016
|
|||||||||||||
|
(In millions)
|
Fair
Value
|
Carrying
Value
|
Fair
Value
|
Carrying
Value
|
|||||||||||
|
Notes payable to Fumedica AG
|
$
|
6.3
|
|
$
|
6.3
|
|
$
|
6.1
|
|
$
|
6.0
|
|
|||
|
6.875% Senior Notes due March 1, 2018
|
575.7
|
|
556.7
|
|
583.7
|
|
558.5
|
|
|||||||
|
2.900% Senior Notes due September 15, 2020
|
1,528.1
|
|
1,483.2
|
|
1,521.5
|
|
1,485.3
|
|
|||||||
|
3.625% Senior Notes due September 15, 2022
|
1,032.4
|
|
993.5
|
|
1,026.6
|
|
993.2
|
|
|||||||
|
4.050% Senior Notes due September 15, 2025
|
1,812.9
|
|
1,735.2
|
|
1,796.0
|
|
1,734.8
|
|
|||||||
|
5.200% Senior Notes due September 15, 2045
|
1,890.0
|
|
1,721.6
|
|
1,874.5
|
|
1,721.5
|
|
|||||||
|
Total
|
$
|
6,845.4
|
|
$
|
6,496.5
|
|
$
|
6,808.4
|
|
$
|
6,499.3
|
|
|||
|
|
For the Three Months
Ended March 31, |
||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Fair value, beginning of period
|
$
|
467.6
|
|
$
|
506.0
|
|
|
|
Additions
|
—
|
|
—
|
|
|||
|
Changes in fair value
|
10.0
|
|
2.3
|
|
|||
|
Payments
|
(6.7
|
)
|
—
|
|
|||
|
Fair value, end of period
|
$
|
470.9
|
|
$
|
508.3
|
|
|
|
(In millions)
|
As of
March 31, 2017 |
As of
December 31, 2016 |
|||||
|
Commercial paper
|
$
|
71.9
|
|
$
|
31.0
|
|
|
|
Money market funds
|
519.2
|
|
741.7
|
|
|||
|
Short-term debt securities
|
54.0
|
|
1,266.9
|
|
|||
|
Total
|
$
|
645.1
|
|
$
|
2,039.6
|
|
|
|
As of March 31, 2017 (In millions)
|
Fair
Value
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Amortized
Cost
|
|||||||||||
|
Corporate debt securities
|
|||||||||||||||
|
Current
|
$
|
1,288.5
|
|
$
|
0.1
|
|
$
|
(0.8
|
)
|
$
|
1,289.2
|
|
|||
|
Non-current
|
1,212.2
|
|
2.1
|
|
(3.3
|
)
|
1,213.4
|
|
|||||||
|
Government securities
|
|||||||||||||||
|
Current
|
664.4
|
|
0.2
|
|
(0.4
|
)
|
664.6
|
|
|||||||
|
Non-current
|
1,073.8
|
|
0.8
|
|
(3.0
|
)
|
1,076.0
|
|
|||||||
|
Mortgage and other asset backed securities
|
|||||||||||||||
|
Current
|
3.3
|
|
—
|
|
—
|
|
3.3
|
|
|||||||
|
Non-current
|
539.2
|
|
1.1
|
|
(1.4
|
)
|
539.5
|
|
|||||||
|
Total marketable debt securities
|
$
|
4,781.4
|
|
$
|
4.3
|
|
$
|
(8.9
|
)
|
$
|
4,786.0
|
|
|||
|
Marketable equity securities, non-current
|
$
|
18.7
|
|
$
|
—
|
|
$
|
(14.9
|
)
|
$
|
33.6
|
|
|||
|
As of December 31, 2016 (In millions)
|
Fair
Value
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Amortized
Cost
|
|||||||||||
|
Corporate debt securities
|
|||||||||||||||
|
Current
|
$
|
1,408.6
|
|
$
|
0.2
|
|
$
|
(0.6
|
)
|
$
|
1,409.0
|
|
|||
|
Non-current
|
1,255.2
|
|
1.2
|
|
(4.7
|
)
|
1,258.7
|
|
|||||||
|
Government securities
|
|||||||||||||||
|
Current
|
1,156.0
|
|
0.2
|
|
(0.3
|
)
|
1,156.1
|
|
|||||||
|
Non-current
|
1,016.5
|
|
0.5
|
|
(3.4
|
)
|
1,019.4
|
|
|||||||
|
Mortgage and other asset backed securities
|
|||||||||||||||
|
Current
|
4.0
|
|
—
|
|
—
|
|
4.0
|
|
|||||||
|
Non-current
|
557.7
|
|
0.8
|
|
(2.2
|
)
|
559.1
|
|
|||||||
|
Total marketable debt securities
|
$
|
5,398.0
|
|
$
|
2.9
|
|
$
|
(11.2
|
)
|
$
|
5,406.3
|
|
|||
|
Marketable equity securities, non-current
|
$
|
24.9
|
|
$
|
0.7
|
|
$
|
(9.3
|
)
|
$
|
33.5
|
|
|||
|
|
As of March 31, 2017
|
As of December 31, 2016
|
|||||||||||||
|
(In millions)
|
Estimated
Fair Value
|
Amortized
Cost
|
Estimated
Fair Value
|
Amortized
Cost
|
|||||||||||
|
Due in one year or less
|
$
|
1,956.2
|
|
$
|
1,957.1
|
|
$
|
2,568.6
|
|
$
|
2,569.1
|
|
|||
|
Due after one year through five years
|
2,561.9
|
|
2,565.4
|
|
2,552.6
|
|
2,559.7
|
|
|||||||
|
Due after five years
|
263.3
|
|
263.5
|
|
276.8
|
|
277.5
|
|
|||||||
|
Total available-for-sale securities
|
$
|
4,781.4
|
|
$
|
4,786.0
|
|
$
|
5,398.0
|
|
$
|
5,406.3
|
|
|||
|
|
For the Three Months
Ended March 31, |
||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Proceeds from maturities and sales
|
$
|
1,884.3
|
|
$
|
1,181.1
|
|
|
|
Realized gains
|
$
|
1.2
|
|
$
|
0.4
|
|
|
|
Realized losses
|
$
|
(1.9
|
)
|
$
|
(0.4
|
)
|
|
|
|
Notional Amount
|
||||||
|
Foreign Currency: (In millions)
|
As of
March 31, 2017 |
As of
December 31, 2016 |
|||||
|
Euro
|
$
|
1,215.7
|
|
$
|
871.7
|
|
|
|
British pound
|
113.1
|
|
—
|
|
|||
|
Canadian dollar
|
59.6
|
|
—
|
|
|||
|
Swiss franc
|
58.2
|
|
—
|
|
|||
|
Total foreign currency forward contracts
|
$
|
1,446.6
|
|
$
|
871.7
|
|
|
|
For the Three Months Ended March 31,
|
||||||||||||||||||
|
Net Gains/(Losses)
Reclassified from AOCI into Operating Income
(Effective Portion)
|
Net Gains/(Losses)
Recognized into Net Income
(Ineffective Portion)
|
|||||||||||||||||
|
Location
|
2017
|
2016
|
Location
|
2017
|
2016
|
|||||||||||||
|
Revenue
|
$
|
6.7
|
|
$
|
8.8
|
|
Other income (expense)
|
$
|
4.0
|
|
$
|
1.9
|
|
|||||
|
Operating expenses
|
$
|
(0.1
|
)
|
$
|
(0.1
|
)
|
Other income (expense)
|
$
|
(0.2
|
)
|
$
|
(0.3
|
)
|
|||||
|
Fair Value
|
||||
|
(In millions)
|
Balance Sheet Location
|
As of March 31, 2017
|
||
|
Hedging Instruments:
|
||||
|
Asset derivatives
|
Other current assets
|
$
|
38.3
|
|
|
Investments and other assets
|
$
|
3.3
|
|
|
|
Liability derivatives
|
Accrued expenses and other
|
$
|
4.2
|
|
|
Other long-term liabilities
|
$
|
7.7
|
|
|
|
Other Derivatives:
|
||||
|
Asset derivatives
|
Other current assets
|
$
|
2.9
|
|
|
Liability derivatives
|
Accrued expenses and other
|
$
|
1.7
|
|
|
Fair Value
|
||||
|
(In millions)
|
Balance Sheet Location
|
As of December 31, 2016
|
||
|
Hedging Instruments:
|
||||
|
Asset derivatives
|
Other current assets
|
$
|
50.4
|
|
|
Investments and other assets
|
$
|
6.6
|
|
|
|
Liability derivatives
|
Other long-term liabilities
|
$
|
4.6
|
|
|
Other Derivatives:
|
||||
|
Asset derivatives
|
Other current assets
|
$
|
4.0
|
|
|
Liability derivatives
|
Accrued expenses and other
|
$
|
9.0
|
|
|
|
For the Three Months
Ended March 31, |
||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
NCI, beginning of period
|
$
|
(11.5
|
)
|
$
|
2.1
|
|
|
|
Net income (loss) attributable to NCI, net of tax
|
(0.1
|
)
|
(1.7
|
)
|
|||
|
Fair value of net assets and liabilities acquired and assigned to NCI
|
—
|
|
0.9
|
|
|||
|
NCI, end of period
|
$
|
(11.6
|
)
|
$
|
1.3
|
|
|
|
(In millions)
|
Unrealized Gains (Losses) on Securities Available for Sale, Net of Tax
|
Unrealized Gains (Losses) on Cash Flow Hedges, Net of Tax
|
Unfunded Status of Postretirement Benefit Plans, Net of Tax
|
Translation Adjustments
|
Total
|
||||||||||||||
|
Balance, as of December 31, 2016
|
$
|
(10.8
|
)
|
$
|
57.8
|
|
$
|
(32.7
|
)
|
$
|
(334.2
|
)
|
$
|
(319.9
|
)
|
||||
|
Other comprehensive income (loss) before reclassifications
|
(2.0
|
)
|
(17.1
|
)
|
0.1
|
|
20.0
|
|
1.0
|
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive income (loss)
|
0.4
|
|
(6.7
|
)
|
—
|
|
—
|
|
(6.3
|
)
|
|||||||||
|
Net current period other comprehensive income (loss)
|
(1.6
|
)
|
(23.8
|
)
|
0.1
|
|
20.0
|
|
(5.3
|
)
|
|||||||||
|
Balance, as of March 31, 2017
|
$
|
(12.4
|
)
|
$
|
34.0
|
|
$
|
(32.6
|
)
|
$
|
(314.2
|
)
|
$
|
(325.2
|
)
|
||||
|
(In millions)
|
Unrealized Gains (Losses) on Securities Available for Sale, Net of Tax
|
Unrealized Gains (Losses) on Cash Flow Hedges, Net of Tax
|
Unfunded Status of Postretirement Benefit Plans, Net of Tax
|
Translation Adjustments
|
Total
|
||||||||||||||
|
Balance, as of December 31, 2015
|
$
|
(0.8
|
)
|
$
|
10.2
|
|
$
|
(37.8
|
)
|
$
|
(195.6
|
)
|
$
|
(224.0
|
)
|
||||
|
Other comprehensive income (loss) before reclassifications
|
2.5
|
|
(38.9
|
)
|
0.2
|
|
9.6
|
|
(26.6
|
)
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive income (loss)
|
—
|
|
(8.7
|
)
|
—
|
|
—
|
|
(8.7
|
)
|
|||||||||
|
Net current period other comprehensive income (loss)
|
2.5
|
|
(47.6
|
)
|
0.2
|
|
9.6
|
|
(35.3
|
)
|
|||||||||
|
Balance, as of March 31, 2016
|
$
|
1.7
|
|
$
|
(37.4
|
)
|
$
|
(37.6
|
)
|
$
|
(186.0
|
)
|
$
|
(259.3
|
)
|
||||
|
(In millions)
|
Income Statement Location
|
Amounts Reclassified from Accumulated Other Comprehensive Income
|
||||||
|
For the Three Months
Ended March 31, |
||||||||
|
2017
|
2016
|
|||||||
|
Gains (losses) on securities available for sale
|
Other income (expense)
|
$
|
(0.7
|
)
|
$
|
—
|
|
|
|
Income tax benefit (expense)
|
0.3
|
|
—
|
|
||||
|
Gains (losses) on cash flow hedges
|
Revenues
|
6.7
|
|
8.8
|
|
|||
|
Operating expenses
|
(0.1
|
)
|
(0.1
|
)
|
||||
|
Other income (expense)
|
0.1
|
|
0.1
|
|
||||
|
Income tax benefit (expense)
|
—
|
|
(0.1
|
)
|
||||
|
Total reclassifications, net of tax
|
$
|
6.3
|
|
$
|
8.7
|
|
||
|
For the Three Months
Ended March 31, |
|||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Numerator:
|
|||||||
|
Net income attributable to Biogen Inc.
|
$
|
747.6
|
|
$
|
970.9
|
|
|
|
Denominator:
|
|||||||
|
Weighted-average number of common shares outstanding
|
215.6
|
|
218.9
|
|
|||
|
Effect of dilutive securities:
|
|||||||
|
Stock options and employee stock purchase plan
|
—
|
|
0.1
|
|
|||
|
Time-vested restricted stock units
|
0.2
|
|
0.2
|
|
|||
|
Market stock units
|
0.1
|
|
0.1
|
|
|||
|
Dilutive potential common shares
|
0.3
|
|
0.4
|
|
|||
|
Shares used in calculating diluted earnings per share
|
215.9
|
|
219.3
|
|
|||
|
For the Three Months
Ended March 31, |
|||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Research and development
|
$
|
18.7
|
|
$
|
21.4
|
|
|
|
Selling, general and administrative
|
29.4
|
|
34.7
|
|
|||
|
Restructuring charges
|
—
|
|
(1.8
|
)
|
|||
|
Subtotal
|
48.1
|
|
54.3
|
|
|||
|
Capitalized share-based compensation costs
|
(2.7
|
)
|
(3.1
|
)
|
|||
|
Share-based compensation expense included in total cost and expenses
|
45.4
|
|
51.2
|
|
|||
|
Income tax effect
|
(12.4
|
)
|
(15.2
|
)
|
|||
|
Share-based compensation expense included in net income attributable to Biogen Inc.
|
$
|
33.0
|
|
$
|
36.0
|
|
|
|
For the Three Months
Ended March 31, |
|||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Market stock units
|
$
|
9.6
|
|
$
|
13.4
|
|
|
|
Time-vested restricted stock units
|
26.9
|
|
30.1
|
|
|||
|
Cash settled performance units
|
3.5
|
|
0.2
|
|
|||
|
Performance units
|
4.5
|
|
6.9
|
|
|||
|
Employee stock purchase plan
|
3.6
|
|
3.7
|
|
|||
|
Subtotal
|
48.1
|
|
54.3
|
|
|||
|
Capitalized share-based compensation costs
|
(2.7
|
)
|
(3.1
|
)
|
|||
|
Share-based compensation expense included in total cost and expenses
|
$
|
45.4
|
|
$
|
51.2
|
|
|
|
For the Three Months
Ended March 31, |
|||||
|
|
2017
|
2016
|
|||
|
Statutory rate
|
35.0
|
%
|
35.0
|
%
|
|
|
State taxes
|
0.1
|
|
1.0
|
|
|
|
Taxes on foreign earnings
|
(11.2
|
)
|
(8.1
|
)
|
|
|
Credits and net operating loss utilization
|
(0.7
|
)
|
(1.2
|
)
|
|
|
Purchased intangible assets
|
1.4
|
|
1.1
|
|
|
|
Manufacturing deduction
|
(2.1
|
)
|
(1.8
|
)
|
|
|
Other permanent items
|
0.7
|
|
0.6
|
|
|
|
Other
|
1.0
|
|
0.3
|
|
|
|
Effective tax rate
|
24.2
|
%
|
26.9
|
%
|
|
|
For the Three Months
Ended March 31, |
|||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Interest income
|
$
|
16.7
|
|
$
|
11.2
|
|
|
|
Interest expense
|
(63.4
|
)
|
(63.3
|
)
|
|||
|
Gain (loss) on investments, net
|
2.4
|
|
1.6
|
|
|||
|
Foreign exchange gains (losses), net
|
4.0
|
|
2.1
|
|
|||
|
Other, net
|
2.7
|
|
(4.4
|
)
|
|||
|
Total other income (expense), net
|
$
|
(37.6
|
)
|
$
|
(52.8
|
)
|
|
|
(In millions)
|
As of
March 31, 2017 |
As of
December 31, 2016 |
|||||
|
Current portion of contingent consideration obligations and milestones
|
$
|
516.8
|
|
$
|
580.8
|
|
|
|
Revenue-related reserves for discounts and allowances
|
505.3
|
|
438.6
|
|
|||
|
Royalties and licensing fees
|
181.0
|
|
195.8
|
|
|||
|
Employee compensation and benefits
|
160.5
|
|
282.9
|
|
|||
|
Construction in progress
|
116.2
|
|
134.0
|
|
|||
|
Collaboration expenses
|
89.8
|
|
130.9
|
|
|||
|
Accrued TECFIDERA litigation settlement and license charges
|
—
|
|
454.8
|
|
|||
|
Other
|
475.0
|
|
685.7
|
|
|||
|
Total accrued expenses and other
|
$
|
2,044.6
|
|
$
|
2,903.5
|
|
|

|
•
|
Total revenues were
$2,810.7 million
for the
first
quarter of
2017
, representing an increase of 3.1% over the same period in
2016
.
|
|
•
|
Product revenues, net totaled
$2,380.1 million
for the
first
quarter of
2017
, representing an increase of 3.1% over the same period in
2016
. This increase was driven by a 14.3% increase in worldwide TYSABRI revenues and revenues from BENEPALI and SPINRAZA. Product revenues, net for the
first
quarter of
2017
, compared to the same period in
2016
, were negatively impacted by a decrease in worldwide ALPROLIX and ELOCTATE revenues resulting from the spin-off of our hemophilia business on February 1, 2017.
|
|
•
|
Revenues from anti-CD20 therapeutic programs totaled
$340.6 million
for the
first
quarter of
2017
, representing an increase of 3.4% over the same period in
2016
.
|
|
•
|
Other revenues totaled
$90.0 million
for the
first
quarter of
2017
, representing an increase of 2.4% over the same period in
2016
.
|
|
•
|
Total cost and expenses totaled
$1,786.4 million
for the
first
quarter of
2017
, representing an increase of
32.5%
over the same period in
2016
. This increase was primarily driven by the $353.6 million impairment and amortization charges recorded in relation to
our intellectual property related to TECFIDERA
and a
22.9%
increase in cost of sales.
|
|
|
For the Three Months
Ended March 31, |
||||||||||||
|
(In millions, except percentages)
|
2017
|
2016
|
|||||||||||
|
Product revenues:
|
|||||||||||||
|
United States
|
$
|
1,631.0
|
|
58.0
|
%
|
$
|
1,663.3
|
|
61.0
|
%
|
|||
|
Rest of world
|
749.1
|
|
26.7
|
%
|
646.1
|
|
23.7
|
%
|
|||||
|
Total product revenues
|
2,380.1
|
|
84.7
|
%
|
2,309.4
|
|
84.7
|
%
|
|||||
|
Revenues from anti-CD20 therapeutic programs
|
340.6
|
|
12.1
|
%
|
329.5
|
|
12.1
|
%
|
|||||
|
Other revenues
|
90.0
|
|
3.2
|
%
|
87.9
|
|
3.2
|
%
|
|||||
|
Total revenues
|
$
|
2,810.7
|
|
100.0
|
%
|
$
|
2,726.8
|
|
100.0
|
%
|
|||
|
|
For the Three Months
Ended March 31, |
||||||||||||
|
(In millions, except percentages)
|
2017
|
2016
|
|||||||||||
|
Multiple Sclerosis:
|
|||||||||||||
|
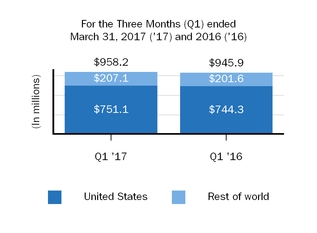
TECFIDERA
|
$
|
958.2
|
|
40.3
|
%
|
$
|
945.9
|
|
41.0
|
%
|
|||
|
Interferon*
|
648.3
|
|
27.2
|
%
|
670.4
|
|
29.0
|
%
|
|||||
|
TYSABRI
|
545.0
|
|
23.0
|
%
|
477.0
|
|
20.6
|
%
|
|||||
|
FAMPYRA
|
20.5
|
|
0.9
|
%
|
20.2
|
|
0.9
|
%
|
|||||
|
ZINBRYTA
|
10.7
|
|
0.4
|
%
|
—
|
|
—
|
%
|
|||||
|
Hemophilia:
|
|
|
|||||||||||
|
ELOCTATE
|
48.4
|
|
2.0
|
%
|
107.7
|
|
4.7
|
%
|
|||||
|
ALPROLIX
|
26.0
|
|
1.1
|
%
|
75.0
|
|
3.2
|
%
|
|||||
|
Spinal Muscular Atrophy:
|
|||||||||||||
|
SPINRAZA
|
47.4
|
|
2.0
|
%
|
—
|
|
—
|
%
|
|||||
|
Other Product Revenues:
|
|||||||||||||
|
FUMADERM
|
9.7
|
|
0.4
|
%
|
11.4
|
|
0.5
|
%
|
|||||
|
BENEPALI
|
65.3
|
|
2.7
|
%
|
1.8
|
|
0.1
|
%
|
|||||
|
FLIXABI
|
0.6
|
|
—
|
%
|
—
|
|
—
|
%
|
|||||
|
Total product revenues
|
$
|
2,380.1
|
|
100.0
|
%
|
$
|
2,309.4
|
|
100.0
|
%
|
|||






|
|
For the Three Months
Ended March 31, |
||||||
|
(In millions)
|
2017
|
2016
|
|||||
|
Product revenues, net
|
$
|
1,041.0
|
|
$
|
974.7
|
|
|
|
Cost and expenses
|
201.3
|
|
175.1
|
|
|||
|
Pre-tax profits in the U.S.
|
839.7
|
|
799.6
|
|
|||
|
Biogen's share of pre-tax profits
|
$
|
323.5
|
|
$
|
312.7
|
|
|
|
|
For the Three Months
Ended March 31, |
||||||||||||
|
(In millions, except percentages)
|
2017
|
2016
|
|||||||||||
|
Revenues from collaborative and other relationships
|
$
|
6.3
|
|
7.0
|
%
|
$
|
11.5
|
|
13.1
|
%
|
|||
|
Other royalty and corporate revenues
|
83.7
|
|
93.0
|
%
|
76.4
|
|
86.9
|
%
|
|||||
|
Total other revenues
|
$
|
90.0
|
|
100.0
|
%
|
$
|
87.9
|
|
100.0
|
%
|
|||


|
|
For the Three Months
Ended March 31, |
|||||||||
|
(In millions, except percentages)
|
2017
|
2016
|
Change %
|
|||||||
|
Cost of sales, excluding amortization of acquired intangible assets
|
$
|
384.6
|
|
$
|
313.0
|
|
22.9
|
%
|
||
|
Research and development
|
423.4
|
|
437.3
|
|
(3.2
|
)%
|
||||
|
Selling, general and administrative
|
499.1
|
|
497.3
|
|
0.4
|
%
|
||||
|
Amortization of acquired intangible assets
|
448.5
|
|
88.8
|
|
405.1
|
%
|
||||
|
Collaboration profit (loss) sharing
|
20.8
|
|
—
|
|
**
|
|
||||
|
(Gain) loss on fair value remeasurement of contingent consideration
|
10.0
|
|
2.3
|
|
334.8
|
%
|
||||
|
Restructuring charges
|
—
|
|
9.7
|
|
(100.0
|
)%
|
||||
|
Total cost and expenses
|
$
|
1,786.4
|
|
$
|
1,348.4
|
|
32.5
|
%
|
||









|
(In millions, except percentages)
|
As of
March 31, 2017 |
As of
December 31, 2016 |
Change %
|
|||||||
|
Financial assets:
|
||||||||||
|
Cash and cash equivalents
|
$
|
924.0
|
|
$
|
2,326.5
|
|
(60.3
|
)%
|
||
|
Marketable securities — current
|
1,956.2
|
|
2,568.6
|
|
(23.8
|
)%
|
||||
|
Marketable securities — non-current
|
2,825.2
|
|
2,829.4
|
|
(0.1
|
)%
|
||||
|
Total cash, cash equivalents and marketable securities
|
$
|
5,705.4
|
|
$
|
7,724.5
|
|
(26.1
|
)%
|
||
|
Borrowings:
|
||||||||||
|
Current portion of notes payable and other financing arrangements
|
$
|
561.5
|
|
$
|
4.7
|
|
**
|
|
||
|
Notes payable and other financing arrangements
|
5,952.7
|
|
6,512.7
|
|
(8.6
|
)%
|
||||
|
Total borrowings
|
$
|
6,514.2
|
|
$
|
6,517.4
|
|
—
|
%
|
||
|
Working capital:
|
||||||||||
|
Current assets
|
$
|
6,859.7
|
|
$
|
8,732.2
|
|
(21.4
|
)%
|
||
|
Current liabilities
|
(2,992.5
|
)
|
(3,419.9
|
)
|
(12.5
|
)%
|
||||
|
Total working capital
|
$
|
3,867.2
|
|
$
|
5,312.3
|
|
(27.2
|
)%
|
||
|
•
|
$235.2 million
in net cash flows provided by operating activities, including:
|
|
◦
|
$454.8 million payment made to Forward Pharma for the litigation and settlement charges that were accrued as of December 31, 2016; and
|
|
◦
|
$360.0 million in total payments for income taxes;
|
|
•
|
$795.2 million payment made to Forward Pharma to license intellectual property related to TECFIDERA
;
|
|
•
|
$583.6 million
used for share repurchases;
|
|
•
|
$302.7 million net cash contribution made to Bioverativ;
|
|
•
|
$300.0 million
in contingent payments made to former shareholders of Fumapharm AG and holders of their rights; and
|
|
•
|
$210.0 million
used for purchases of property, plant and equipment.
|
|
•
|
$1,015.8 million
in net cash flows provided by operating activities, including:
|
|
◦
|
$97.2 million in total payments for income taxes;
|
|
•
|
$300.0 million
in contingent payments made to former shareholders of Fumapharm AG and holders of their rights; and
|
|
•
|
$126.9 million
used for purchases of property, plant and equipment.
|
|
•
|
$550.0 million aggregate principal amount of 6.875% Senior Notes due March 1, 2018;
|
|
•
|
$1.5 billion aggregate principal amount of 2.90% Senior Notes due September 15, 2020;
|
|
•
|
$1.0 billion aggregate principal amount of 3.625% Senior Notes due September 15, 2022;
|
|
•
|
$1.75 billion aggregate principal amount of 4.05% Senior Notes due September 15, 2025; and
|
|
•
|
$1.75 billion aggregate principal amount of 5.20% Senior Notes due September 15, 2045.
|
|
|
For the Three Months
Ended March 31, |
||||||||||
|
(In millions, except percentages)
|
2017
|
2016
|
% Change
|
||||||||
|
Net cash flows provided by operating activities
|
$
|
235.2
|
|
$
|
1,015.8
|
|
(76.8
|
)%
|
|||
|
Net cash flows used in investing activities
|
$
|
(744.2
|
)
|
$
|
(1,203.2
|
)
|
(38.1
|
)%
|
|||
|
Net cash flows used in financing activities
|
$
|
(901.4
|
)
|
$
|
(5.0
|
)
|
17,928.0
|
%
|
|||
|
•
|
Non-cash operating items such as depreciation and amortization, impairment charges and share-based compensation charges;
|
|
•
|
Changes in operating assets and liabilities which reflect timing differences between the receipt and payment of cash associated with transactions and when they are recognized in results of operations; and
|
|
•
|
Changes associated with the fair value of contingent payments associated with our acquisitions of businesses and payments related to collaborations.
|
|
•
|
safety or efficacy issues;
|
|
•
|
the introduction or greater acceptance of competing products, including lower-priced competing products;
|
|
•
|
constraints and additional pressures on product pricing or price increases, including those resulting from governmental or regulatory requirements, increased competition, or changes in, or, implementation of, reimbursement policies and practices of payors and other third parties; or
|
|
•
|
adverse legal, administrative, regulatory or legislative developments.
|
|
•
|
our limited marketing experience within the SMA market, which may impact our ability to develop relationships with the associated medical and scientific community;
|
|
•
|
the lack of readiness of healthcare providers to treat patients with SMA;
|
|
•
|
the effectiveness of our commercial strategy for marketing SPINRAZA; and
|
|
•
|
our ability to maintain a positive reputation among patients, healthcare providers and others in the SMA community, which may be impacted by pricing and reimbursement decisions relating to SPINRAZA.
|
|
•
|
the introduction of more efficacious, safer, less expensive or more convenient alternatives to our MS products, including our own products and products of our collaborators;
|
|
•
|
the introduction of lower-cost biosimilars, follow-on products or generic versions of branded MS products sold by our competitors, and the possibility of future competition from generic versions or prodrugs of existing therapeutics or from off-label use by physicians of therapies indicated for other conditions to treat MS patients;
|
|
•
|
patient dynamics, including the size of the patient population and our ability to attract new patients to our therapies;
|
|
•
|
damage to physician and patient confidence in any of our MS products or to our sales and reputation as a result of label changes or adverse experiences or events that may occur with patients treated with our MS products;
|
|
•
|
inability to obtain appropriate pricing and reimbursement for our MS products compared to our competitors in key international markets; or
|
|
•
|
our ability to obtain and maintain patent, data or market exclusivity for our MS products.
|
|
•
|
changes in, and implementation of, federal, state or foreign government regulations or private third-party payors' reimbursement policies;
|
|
•
|
pressure by employers on private health insurance plans to reduce costs; and
|
|
•
|
consolidation and increasing assertiveness of payors, including managed care organizations, health insurers, pharmacy benefit managers, government health administration authorities, private health insurers and other organizations, seeking price discounts or rebates in connection with the placement of our products on their formularies and, in some cases, the imposition of restrictions on access or coverage of particular drugs or pricing determined based on perceived value.
|
|
•
|
Risk of Product Loss.
The manufacturing process for our products is extremely susceptible to product loss due to contamination, oxidation, equipment failure or improper installation or operation of equipment, or vendor or operator error. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects and other supply disruptions. If microbial, viral or other contaminations are discovered in our products or manufacturing facilities, we may need to close our manufacturing facilities for an extended period of time to investigate and remediate the contaminant.
|
|
•
|
Risks of Reliance on Third Parties and Single Source Providers.
We rely on third-party suppliers and manufacturers for many aspects of our manufacturing process for our products and product candidates. In some cases, due to the unique manner in which our products are manufactured, we rely on single source providers of raw materials and manufacturing supplies. These third parties are independent entities subject to their own unique operational and financial risks that are outside of our control. These third parties may not perform their obligations in a timely and cost-effective manner or in compliance with applicable regulations, and they may be unable or unwilling to increase production capacity commensurate with demand for our existing or future products. Finding alternative providers could take a significant amount of time and involve significant expense due to the specialized nature of the services and the need to obtain regulatory approval of any significant changes to our suppliers or manufacturing methods. We cannot be certain that we could reach agreement with alternative providers or that the FDA or other regulatory authorities would approve our use of such alternatives.
|
|
•
|
Global Bulk Supply Risks.
We rely on our principal manufacturing facilities for the production of drug substance for our large molecule products and product candidates. Our global bulk supply of these products and product candidates depends on the uninterrupted and efficient operation of these facilities, which could be adversely affected by equipment failures, labor shortages, natural disasters, power failures and numerous other factors.
|
|
•
|
Risks Relating to Compliance with current Good Manufacturing Practices (cGMP).
We and our third-party providers are generally required to maintain compliance with cGMP and other stringent requirements and are subject to inspections by the FDA and comparable agencies in other jurisdictions to confirm such compliance. Any delay, interruption or other issues that arise in the manufacture, fill-finish, packaging or storage of our products as a result of a failure of our facilities or the facilities or operations of third parties to pass any regulatory agency inspection could significantly impair our ability to develop and commercialize our products. Significant noncompliance could also result in the imposition of monetary penalties or other civil or criminal sanctions and damage our reputation.
|
|
•
|
we may be unable to control the resources our collaborators or third parties devote to our programs or products;
|
|
•
|
disputes may arise under the agreement, including with respect to the achievement and payment of milestones or ownership of rights to technology developed with our collaborators or other third parties, and the underlying contract with our collaborators or other third parties may fail to provide significant protection or may fail to be effectively enforced if the collaborators or third parties fail to perform;
|
|
•
|
the interests of our collaborators or third parties may not always be aligned with our interests, and such parties may not pursue regulatory approvals or market a product in the same manner or to the same extent that we would, which could adversely affect our revenues;
|
|
•
|
third-party relationships and collaborations often require the parties to cooperate, and failure to do so effectively could adversely affect product sales, or the clinical development or regulatory approvals of products under joint control or could result in termination of the research, development or commercialization of product candidates or result in litigation or arbitration; and
|
|
•
|
any failure on the part of our collaborators or other third parties to comply with applicable laws and regulatory requirements in the marketing, sale and maintenance of the marketing authorization of our products or to fulfill any responsibilities our collaborators or other third parties may have to protect and enforce any intellectual property rights underlying our products could have an adverse effect on our revenues as well as involve us in possible legal proceedings.
|
|
•
|
new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or decisions, related to health care availability, pricing or marketing practices, compliance with wage and hour laws and other employment practices, method of delivery, payment for health care products and services, compliance with health information and data privacy and security laws and regulations, tracking and reporting payments and other transfers of value made to physicians and teaching hospitals, extensive anti-bribery and anti-corruption prohibitions, product serialization and labeling requirements and used product take-back requirements;
|
|
•
|
changes in the FDA and foreign regulatory approval processes that may delay or prevent the approval of new products and result in lost market opportunity;
|
|
•
|
the hiring freeze implemented by the federal government in 2017, including at the FDA, could impact the review and potential approval of new products, which may adversely affect our business and financial condition;
|
|
•
|
requirements that provide for increased transparency of clinical trial results and quality data, such as the European Medicines Agency's clinical transparency policy, which could impact our ability to protect trade secrets and competitively-sensitive information contained in approval applications or could be misinterpreted leading to reputational damage, misperception or legal action, which could harm our business; and
|
|
•
|
changes in FDA and foreign regulations that may require additional safety monitoring, labeling changes, restrictions on product distribution or use, or other measures after the introduction of our products to market, which could increase our costs of doing business, adversely affect the future permitted uses of approved products or otherwise adversely affect the market for our products.
|
|
•
|
increase our vulnerability to general adverse economic and industry conditions;
|
|
•
|
limit our ability to access capital markets and incur additional debt in the future;
|
|
•
|
require us to dedicate a substantial portion of our cash flow from operations to payments on our indebtedness, thereby reducing the availability of our cash flow for other purposes, including business development efforts, research and development and mergers and acquisitions; and
|
|
•
|
limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate, thereby placing us at a competitive disadvantage compared to our competitors that have less debt.
|
|
•
|
the inability to obtain necessary foreign regulatory or pricing approvals of products in a timely manner;
|
|
•
|
uncertainties regarding the collectability of accounts receivable;
|
|
•
|
fluctuations in foreign currency exchange rates, in particular the recent strength of the U.S. dollar versus foreign currencies, which has adversely impacted our revenues and net income;
|
|
•
|
difficulties in staffing and managing international operations;
|
|
•
|
the imposition of governmental controls;
|
|
•
|
less favorable intellectual property or other applicable laws;
|
|
•
|
increasingly complex standards for complying with foreign laws and regulations that may differ substantially from country to country and may conflict with corresponding U.S. laws and regulations;
|
|
•
|
the far-reaching anti-bribery and anti-corruption legislation in the U.K., including the U.K. Bribery Act 2010, and elsewhere and escalation of investigations and prosecutions pursuant to such laws;
|
|
•
|
compliance with complex import and export control laws;
|
|
•
|
restrictions on direct investments by foreign entities and trade restrictions;
|
|
•
|
greater political or economic instability; and
|
|
•
|
changes in tax laws and tariffs.
|
|
•
|
the cost of restructurings;
|
|
•
|
impairments with respect to investments, fixed assets and long-lived assets, including in-process R&D and other intangible assets;
|
|
•
|
inventory write-downs for failed quality specifications, charges for excess or obsolete inventory and charges for inventory write downs relating to product suspensions, expirations or recalls;
|
|
•
|
changes in the fair value of contingent consideration;
|
|
•
|
bad debt expenses and increased bad debt reserves;
|
|
•
|
outcomes of litigation and other legal or administrative proceedings, regulatory matters and tax matters;
|
|
•
|
milestone payments under license and collaboration agreements; and
|
|
•
|
payments in connection with acquisitions and other business development activities.
|
|
•
|
Reliance on Third Parties.
We are dependent on the efforts of Samsung Bioepis and other third parties over whom we have limited or no control in the development and manufacturing of biosimilars products. If Samsung Bioepis or such other third parties fail to perform successfully, we may not realize the anticipated benefits of our investment in Samsung Bioepis;
|
|
•
|
Regulatory Compliance.
Biosimilar products may face regulatory hurdles or delays due to the evolving and uncertain regulatory and commercial pathway of biosimilars products in certain jurisdictions;
|
|
•
|
Intellectual Property and Regulatory Challenges.
Biosimilar products may face extensive patent clearances, patent infringement litigation, injunctions or regulatory challenges, which could prevent the commercial launch of a product or delay it for many years;
|
|
•
|
Failure to Gain Market and Patient Acceptance.
Market success of biosimilar products will be adversely affected if patients, physicians and/or payers do not accept biosimilar products as safe and efficacious products offering a more competitive price or other benefit over existing therapies;
|
|
•
|
Ability to Provide Adequate Supply.
Manufacturing biosimlars is complex. If we encounter any manufacturing or supply chain difficulties, we may be unable to meet higher than anticipated demand; and
|
|
•
|
Competitive Challenges.
Biosimilar products face significant competition, including from innovator products and from biosimilar products offered by other companies. In some jurisdictions, local tendering processes may restrict biosimilar products from being marketed and sold in those jurisdictions. The number of competitors in a jurisdiction, the timing of approval and the ability to market biosimilar products successfully in a timely and cost-effective matter are additional factors that may impact our success and/or the success of Samsung Bioepis in this business area.
|
|
Period
|
Total Number of
Shares Purchased
(#)
|
Average Price
Paid per Share
($)
|
Total Number of
Shares Purchased
as Part of Publicly
Announced Programs
(#)
|
Maximum
Approximate Dollar Value
of Shares That May Yet Be
Purchased Under
Our Programs ($ in millions)
|
||||||||
|
January 2017
|
—
|
|
—
|
|
—
|
|
$
|
4,000.0
|
|
|||
|
February 2017
|
—
|
|
—
|
|
—
|
|
$
|
4,000.0
|
|
|||
|
March 2017
|
775,000
|
|
281.57
|
|
775,000
|
|
$
|
3,781.8
|
|
|||
|
Total
|
775,000
|
|
281.57
|
|
||||||||
|
Period
|
Total Number of
Shares Purchased
(#)
|
Average Price
Paid per Share
($)
|
Total Number of
Shares Purchased
as Part of Publicly
Announced Programs
(#)
|
Maximum
Number of Shares
That May Yet Be
Purchased Under
Our Programs (#)
|
|||||||
|
January 2017
|
—
|
|
—
|
|
—
|
|
1,264,156
|
|
|||
|
February 2017
|
600,000
|
|
286.02
|
|
600,000
|
|
664,156
|
|
|||
|
March 2017
|
664,156
|
|
291.72
|
|
664,156
|
|
—
|
|
|||
|
Total
|
1,264,156
|
|
289.02
|
|
|||||||
|
BIOGEN INC.
|
|
/s/ Paul J. Clancy
|
|
Paul J. Clancy
|
|
Executive Vice President and
|
|
Chief Financial Officer
|
|
(principal financial officer)
|
|
Exhibit
Number
|
|
Description of Exhibit
|
|
2.1
|
Separation Agreement between Biogen Inc. and Bioverativ Inc. Filed as Exhibit 2.1 to our Current Report on Form 8-K filed February 2, 2017.
|
|
|
10.1
|
Settlement and License Agreement, dated January 17, 2017, between Biogen Swiss Manufacturing GmbH, Biogen International Holding Ltd, Forward Pharma A/S and the other parties thereto. Filed as Exhibit 10.1 to our Current Report on Form 8-K filed on February 1, 2017.
|
|
|
31.1+
|
|
Certification of the Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
31.2+
|
|
Certification of the Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
32.1++
|
|
Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
101++
|
|
The following materials from Biogen Inc.’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2017, formatted in XBRL (Extensible Business Reporting Language): (i) the Condensed Consolidated Statements of Income, (ii) the Condensed Consolidated Statements of Comprehensive Income, (iii) the Condensed Consolidated Balance Sheets, (iv) the Condensed Consolidated Statements of Cash Flows, and (v) Notes to Condensed Consolidated Financial Statements.
|