|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|

|
Delaware
|
|
33-0112644
|
|
(State or other jurisdiction of
incorporation or organization)
|
|
(I.R.S. Employer
Identification No.)
|
|
Large accelerated filer
x
|
Accelerated filer
o
|
|
|
Non-accelerated filer
o
|
Smaller reporting company
o
|
|
|
(Do not check if a smaller reporting company)
|
Emerging growth company
o
|
|
|
|
|
Page
|
|
PART I —
FINANCIAL INFORMATION
|
||
|
Item 1.
|
Financial Statements (unaudited)
|
|
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
PART II —
OTHER INFORMATION
|
||
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 2.
|
||
|
Item 6.
|
||
|
•
|
the anticipated amount, timing and accounting of revenues, contingent payments, milestone, royalty and other payments under licensing, collaboration or acquisition agreements, tax positions and contingencies, collectability of receivables, pre-approval inventory, cost of sales, research and development costs, compensation and other selling, general and administrative expenses, amortization of intangible assets, foreign currency exchange risk, estimated fair value of assets and liabilities and impairment assessments;
|
|
•
|
expectations, plans and prospects relating to sales, pricing, growth and launch of our marketed and pipeline products;
|
|
•
|
our plans and investments in our core and emerging growth areas;
|
|
•
|
the potential impact of increased product competition in the markets in which we compete;
|
|
•
|
patent terms, patent term extensions, patent office actions and expected availability and period of regulatory exclusivity;
|
|
•
|
the costs and timing of potential clinical trials, filings and approvals, and the potential therapeutic scope of the development and commercialization of our and our collaborators’ pipeline products;
|
|
•
|
the drivers for growing our business, including our plans and intent to commit resources relating to business development opportunities and research and development programs;
|
|
•
|
the anticipated benefits and the potential costs and expenses related to our current or future initiatives to streamline our operations and reallocate resources;
|
|
•
|
our manufacturing capacity, use of third-party contract manufacturing organizations and plans and timing relating to the expansion of our manufacturing capabilities, including anticipated investments and activities in new manufacturing facilities;
|
|
•
|
the potential impact on our results of operations and liquidity of the United Kingdom's (U.K.) intent to voluntarily depart from the European Union (E.U.);
|
|
•
|
the impact of the continued uncertainty of the credit and economic conditions in certain countries in Europe and our collection of accounts receivable in such countries;
|
|
•
|
the potential impact of healthcare reform in the United States (U.S.) and measures being taken worldwide designed to reduce healthcare costs to limit the overall level of government expenditures, including the impact of pricing actions and reduced reimbursement for our products;
|
|
•
|
the timing, outcome and impact of administrative, regulatory, legal and other proceedings related to our patents and other proprietary and intellectual property rights, tax audits, assessments and settlements, pricing matters, sales and promotional practices, product liability and other matters;
|
|
•
|
lease commitments, purchase obligations and the timing and satisfaction of other contractual obligations;
|
|
•
|
our ability to finance our operations and business initiatives and obtain funding for such activities;
|
|
•
|
adverse safety events involving our marketed products;
|
|
•
|
the anticipated timing to complete certain business development transactions;
|
|
•
|
the impact of new laws, including the Tax Cuts and Jobs Act of 2017, and accounting standards; and
|
|
•
|
the anticipated costs and tax treatment of the spin-off of our hemophilia business.
|
|
•
|
“Biogen,” the “company,” “we,” “us” and “our” refer to Biogen Inc. and its consolidated subsidiaries;
|
|
•
|
“RITUXAN” refers to both RITUXAN (the trade name for rituximab in the U.S., Canada and Japan) and MabThera (the trade name for rituximab outside the U.S., Canada and Japan); and
|
|
•
|
"ELOCTATE" refers to both ELOCTATE (the trade name for Antihemophilic Factor (recombinant), Fc Fusion Protein in the U.S., Canada and Japan) and ELOCTA (the trade name for Antihemophilic Factor (recombinant), Fc Fusion Protein in the E.U.).
|
|
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
|||||||||||||
|
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Revenues:
|
|||||||||||||||
|
Product, net
|
$
|
2,757.5
|
|
$
|
2,639.7
|
|
$
|
5,281.0
|
|
$
|
5,019.8
|
|
|||
|
Revenues from anti-CD20 therapeutic programs
|
490.4
|
|
397.1
|
|
933.6
|
|
737.7
|
|
|||||||
|
Other
|
108.6
|
|
41.6
|
|
273.0
|
|
131.6
|
|
|||||||
|
Total revenues
|
3,356.5
|
|
3,078.4
|
|
6,487.6
|
|
5,889.1
|
|
|||||||
|
Cost and expenses:
|
|||||||||||||||
|
Cost of sales, excluding amortization of acquired intangible assets
|
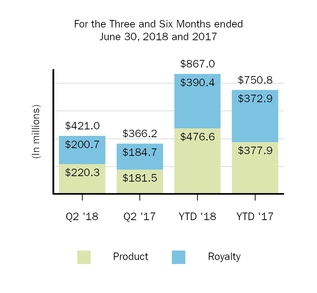
421.0
|
|
366.2
|
|
867.0
|
|
750.8
|
|
|||||||
|
Research and development
|
981.0
|
|
796.2
|
|
1,477.7
|
|
1,219.6
|
|
|||||||
|
Selling, general and administrative
|
516.2
|
|
429.8
|
|
1,017.5
|
|
928.5
|
|
|||||||
|
Amortization of acquired intangible assets
|
107.4
|
|
117.5
|
|
211.3
|
|
566.0
|
|
|||||||
|
Acquired in-process research and development
|
75.0
|
|
120.0
|
|
85.0
|
|
120.0
|
|
|||||||
|
Collaboration profit (loss) sharing
|
39.2
|
|
26.5
|
|
81.7
|
|
47.3
|
|
|||||||
|
Loss (gain) on fair value remeasurement of contingent consideration
|
1.9
|
|
21.2
|
|
(3.7
|
)
|
31.2
|
|
|||||||
|
Restructuring charges
|
1.6
|
|
—
|
|
3.2
|
|
—
|
|
|||||||
|
Total cost and expenses
|
2,143.3
|
|
1,877.4
|
|
3,739.7
|
|
3,663.4
|
|
|||||||
|
Income from operations
|
1,213.2
|
|
1,201.0
|
|
2,747.9
|
|
2,225.7
|
|
|||||||
|
Other income (expense), net
|
(34.5
|
)
|
(68.6
|
)
|
(75.5
|
)
|
(106.6
|
)
|
|||||||
|
Income before income tax expense and equity in loss of investee, net of tax
|
1,178.7
|
|
1,132.4
|
|
2,672.4
|
|
2,119.1
|
|
|||||||
|
Income tax expense
|
263.7
|
|
269.6
|
|
586.2
|
|
508.8
|
|
|||||||
|
Equity in loss of investee, net of tax
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Net income
|
915.0
|
|
862.8
|
|
2,086.2
|
|
1,610.3
|
|
|||||||
|
Net income (loss) attributable to noncontrolling interests, net of tax
|
48.4
|
|
—
|
|
46.7
|
|
(0.1
|
)
|
|||||||
|
Net income attributable to Biogen Inc.
|
$
|
866.6
|
|
$
|
862.8
|
|
$
|
2,039.5
|
|
$
|
1,610.4
|
|
|||
|
Net income per share:
|
|||||||||||||||
|
Basic earnings per share attributable to Biogen Inc.
|
$
|
4.18
|
|
$
|
4.07
|
|
$
|
9.75
|
|
$
|
7.53
|
|
|||
|
Diluted earnings per share attributable to Biogen Inc.
|
$
|
4.18
|
|
$
|
4.07
|
|
$
|
9.73
|
|
$
|
7.52
|
|
|||
|
Weighted-average shares used in calculating:
|
|||||||||||||||
|
Basic earnings per share attributable to Biogen Inc.
|
207.1
|
|
211.9
|
|
209.2
|
|
213.7
|
|
|||||||
|
Diluted earnings per share attributable to Biogen Inc.
|
207.3
|
|
212.2
|
|
209.5
|
|
214.0
|
|
|||||||
|
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
|||||||||||||
|
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Net income attributable to Biogen Inc.
|
$
|
866.6
|
|
$
|
862.8
|
|
$
|
2,039.5
|
|
$
|
1,610.4
|
|
|||
|
Other comprehensive income:
|
|||||||||||||||
|
Unrealized gains (losses) on securities available for sale, net of tax
|
0.6
|
|
7.2
|
|
(1.6
|
)
|
5.6
|
|
|||||||
|
Unrealized gains (losses) on cash flow hedges, net of tax
|
132.8
|
|
(103.0
|
)
|
103.8
|
|
(126.8
|
)
|
|||||||
|
Unrealized gains (losses) on pension benefit obligation, net of tax
|
0.9
|
|
(0.6
|
)
|
0.4
|
|
(0.5
|
)
|
|||||||
|
Currency translation adjustment, net of tax
|
(92.0
|
)
|
82.8
|
|
(47.3
|
)
|
102.8
|
|
|||||||
|
Total other comprehensive income (loss), net of tax
|
42.3
|
|
(13.6
|
)
|
55.3
|
|
(18.9
|
)
|
|||||||
|
Comprehensive income attributable to Biogen Inc.
|
908.9
|
|
849.2
|
|
2,094.8
|
|
1,591.5
|
|
|||||||
|
Comprehensive income (loss) attributable to noncontrolling interests, net of tax
|
48.4
|
|
—
|
|
46.7
|
|
(0.1
|
)
|
|||||||
|
Comprehensive income
|
$
|
957.3
|
|
$
|
849.2
|
|
$
|
2,141.5
|
|
$
|
1,591.4
|
|
|||
|
As of June 30,
2018 |
As of December 31,
2017 |
||||||
|
ASSETS
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
1,250.2
|
|
$
|
1,573.8
|
|
|
|
Marketable securities
|
1,974.0
|
|
2,115.2
|
|
|||
|
Accounts receivable, net
|
1,951.0
|
|
1,787.0
|
|
|||
|
Due from anti-CD20 therapeutic programs
|
481.9
|
|
532.6
|
|
|||
|
Inventory
|
931.7
|
|
902.7
|
|
|||
|
Other current assets
|
843.3
|
|
962.0
|
|
|||
|
Total current assets
|
7,432.1
|
|
7,873.3
|
|
|||
|
Marketable securities
|
1,160.2
|
|
3,057.3
|
|
|||
|
Property, plant and equipment, net
|
3,409.0
|
|
3,182.4
|
|
|||
|
Intangible assets, net
|
3,661.3
|
|
3,879.6
|
|
|||
|
Goodwill
|
5,170.3
|
|
4,632.5
|
|
|||
|
Deferred tax assets
|
2,217.9
|
|
595.9
|
|
|||
|
Investments and other assets
|
902.1
|
|
431.6
|
|
|||
|
Total assets
|
$
|
23,952.9
|
|
$
|
23,652.6
|
|
|
|
LIABILITIES AND EQUITY
|
|||||||
|
Current liabilities:
|
|||||||
|
Current portion of notes payable
|
$
|
—
|
|
$
|
3.2
|
|
|
|
Taxes payable
|
223.0
|
|
68.2
|
|
|||
|
Accounts payable
|
295.7
|
|
395.5
|
|
|||
|
Accrued expenses and other
|
2,633.7
|
|
2,901.3
|
|
|||
|
Total current liabilities
|
3,152.4
|
|
3,368.2
|
|
|||
|
Notes payable
|
5,928.4
|
|
5,935.0
|
|
|||
|
Deferred tax liabilities
|
1,160.8
|
|
122.6
|
|
|||
|
Other long-term liabilities
|
1,457.6
|
|
1,628.7
|
|
|||
|
Total liabilities
|
11,699.2
|
|
11,054.5
|
|
|||
|
Commitments and contingencies
|
|
|
|
|
|||
|
Equity:
|
|||||||
|
Biogen Inc. shareholders’ equity:
|
|||||||
|
Preferred stock, par value $0.001 per share
|
—
|
|
—
|
|
|||
|
Common stock, par value $0.0005 per share
|
0.1
|
|
0.1
|
|
|||
|
Additional paid-in capital
|
—
|
|
97.8
|
|
|||
|
Accumulated other comprehensive loss
|
(261.6
|
)
|
(318.4
|
)
|
|||
|
Retained earnings
|
15,499.5
|
|
15,810.4
|
|
|||
|
Treasury stock, at cost
|
(2,977.1
|
)
|
(2,977.1
|
)
|
|||
|
Total Biogen Inc. shareholders’ equity
|
12,260.9
|
|
12,612.8
|
|
|||
|
Noncontrolling interests
|
(7.2
|
)
|
(14.7
|
)
|
|||
|
Total equity
|
12,253.7
|
|
12,598.1
|
|
|||
|
Total liabilities and equity
|
$
|
23,952.9
|
|
$
|
23,652.6
|
|
|
|
|
For the Six Months
Ended June 30, |
||||||
|
|
2018
|
2017
|
|||||
|
Cash flows from operating activities:
|
|||||||
|
Net income
|
$
|
2,086.2
|
|
$
|
1,610.3
|
|
|
|
Adjustments to reconcile net income to net cash flows from operating activities:
|
|||||||
|
Depreciation and amortization
|
340.4
|
|
694.6
|
|
|||
|
Acquired in-process research and development
|
85.0
|
|
120.0
|
|
|||
|
Share-based compensation
|
81.7
|
|
67.3
|
|
|||
|
Deferred income taxes
|
(57.4
|
)
|
(20.5
|
)
|
|||
|
Other
|
42.4
|
|
73.5
|
|
|||
|
Changes in operating assets and liabilities, net:
|
|||||||
|
Accounts receivable
|
(187.2
|
)
|
(301.2
|
)
|
|||
|
Inventory
|
(40.1
|
)
|
(85.3
|
)
|
|||
|
Accrued expenses and other current liabilities
|
13.3
|
|
(452.3
|
)
|
|||
|
Income tax assets and liabilities
|
183.4
|
|
(114.7
|
)
|
|||
|
Other changes in operating assets and liabilities, net
|
8.7
|
|
(187.3
|
)
|
|||
|
Net cash flows provided by operating activities
|
2,556.4
|
|
1,404.4
|
|
|||
|
Cash flows from investing activities:
|
|||||||
|
Proceeds from sales and maturities of marketable securities
|
6,802.7
|
|
3,584.5
|
|
|||
|
Purchases of marketable securities
|
(4,774.3
|
)
|
(2,536.0
|
)
|
|||
|
Contingent consideration paid related to Fumapharm AG acquisition
|
(900.0
|
)
|
(600.0
|
)
|
|||
|
Purchases of property, plant and equipment
|
(381.5
|
)
|
(407.7
|
)
|
|||
|
Acquired in-process research and development
|
(85.0
|
)
|
(120.0
|
)
|
|||
|
Acquisitions of intangible assets
|
(3.0
|
)
|
(860.3
|
)
|
|||
|
Purchase of Ionis Pharmaceuticals, Inc. stock
|
(463.7
|
)
|
—
|
|
|||
|
Other
|
2.9
|
|
(7.2
|
)
|
|||
|
Net cash flows provided by (used in) investing activities
|
198.1
|
|
(946.7
|
)
|
|||
|
Cash flows from financing activities:
|
|||||||
|
Purchases of treasury stock
|
(3,000.0
|
)
|
(1,365.4
|
)
|
|||
|
Payments related to issuance of stock for share-based compensation arrangements, net
|
(14.3
|
)
|
(17.8
|
)
|
|||
|
Repayment of borrowings
|
(3.2
|
)
|
—
|
|
|||
|
Net distribution to noncontrolling interest
|
(38.9
|
)
|
—
|
|
|||
|
Net cash contribution to Bioverativ Inc.
|
—
|
|
(302.7
|
)
|
|||
|
Other
|
(3.7
|
)
|
33.5
|
|
|||
|
Net cash flows used in financing activities
|
(3,060.1
|
)
|
(1,652.4
|
)
|
|||
|
Net increase (decrease) in cash and cash equivalents
|
(305.6
|
)
|
(1,194.7
|
)
|
|||
|
Effect of exchange rate changes on cash and cash equivalents
|
(18.0
|
)
|
37.8
|
|
|||
|
Cash and cash equivalents, beginning of the period
|
1,573.8
|
|
2,326.5
|
|
|||
|
Cash and cash equivalents, end of the period
|
$
|
1,250.2
|
|
$
|
1,169.6
|
|
|
|
(i)
|
our share of pre-tax profits and losses in the U.S. for RITUXAN and GAZYVA; and
|
|
(ii)
|
other revenues from anti-CD20 therapeutic programs, which primarily consist of our share of pre-tax co-promotion profits on RITUXAN in Canada and royalty revenues on sales of OCREVUS.
|
|
|
For the Three Months
Ended June 30, |
||||||||||||||||||||||
|
(In millions)
|
2018
|
2017
|
|||||||||||||||||||||
|
United
States
|
Rest of
World
|
Total
|
United
States
|
Rest of
World
|
Total
|
||||||||||||||||||
|
Multiple Sclerosis:
|
|||||||||||||||||||||||
|
TECFIDERA
|
$
|
825.8
|
|
$
|
261.0
|
|
$
|
1,086.8
|
|
$
|
875.0
|
|
$
|
235.6
|
|
$
|
1,110.6
|
|
|||||
|
Interferon*
|
444.7
|
|
180.8
|
|
625.5
|
|
501.7
|
|
188.9
|
|
690.6
|
|
|||||||||||
|
TYSABRI
|
265.5
|
|
201.7
|
|
467.2
|
|
289.4
|
|
206.6
|
|
496.0
|
|
|||||||||||
|
FAMPYRA
|
—
|
|
23.0
|
|
23.0
|
|
—
|
|
22.6
|
|
22.6
|
|
|||||||||||
|
ZINBRYTA
|
—
|
|
—
|
|
—
|
|
—
|
|
16.1
|
|
16.1
|
|
|||||||||||
|
Spinal Muscular Atrophy:
|
|
|
|||||||||||||||||||||
|
SPINRAZA
|
205.9
|
|
216.8
|
|
422.7
|
|
194.8
|
|
8.1
|
|
202.9
|
|
|||||||||||
|
Other Product Revenues:
|
|
|
|||||||||||||||||||||
|
FUMADERM
|
—
|
|
5.5
|
|
5.5
|
|
—
|
|
10.3
|
|
10.3
|
|
|||||||||||
|
BENEPALI
|
—
|
|
115.6
|
|
115.6
|
|
—
|
|
88.7
|
|
88.7
|
|
|||||||||||
|
FLIXABI
|
—
|
|
11.2
|
|
11.2
|
|
—
|
|
1.9
|
|
1.9
|
|
|||||||||||
|
Total product revenues
|
$
|
1,741.9
|
|
$
|
1,015.6
|
|
$
|
2,757.5
|
|
$
|
1,860.9
|
|
$
|
778.8
|
|
$
|
2,639.7
|
|
|||||
|
|
For the Six Months
Ended June 30, |
||||||||||||||||||||||
|
(In millions)
|
2018
|
2017
|
|||||||||||||||||||||
|
United
States
|
Rest of
World
|
Total
|
United
States
|
Rest of
World
|
Total
|
||||||||||||||||||
|
Multiple Sclerosis:
|
|||||||||||||||||||||||
|
TECFIDERA
|
$
|
1,554.7
|
|
$
|
519.0
|
|
$
|
2,073.7
|
|
$
|
1,626.1
|
|
$
|
442.7
|
|
$
|
2,068.8
|
|
|||||
|
Interferon*
|
816.0
|
|
359.8
|
|
1,175.8
|
|
966.5
|
|
372.4
|
|
1,338.9
|
|
|||||||||||
|
TYSABRI
|
515.2
|
|
414.1
|
|
929.3
|
|
594.9
|
|
446.1
|
|
1,041.0
|
|
|||||||||||
|
FAMPYRA
|
—
|
|
47.4
|
|
47.4
|
|
—
|
|
43.1
|
|
43.1
|
|
|||||||||||
|
ZINBRYTA
|
—
|
|
1.4
|
|
1.4
|
|
—
|
|
26.8
|
|
26.8
|
|
|||||||||||
|
Spinal Muscular Atrophy:
|
|||||||||||||||||||||||
|
SPINRAZA
|
393.9
|
|
392.7
|
|
786.6
|
|
241.2
|
|
9.1
|
|
250.3
|
|
|||||||||||
|
Hemophilia:
|
|||||||||||||||||||||||
|
ELOCTATE
|
—
|
|
—
|
|
—
|
|
42.2
|
|
6.2
|
|
48.4
|
|
|||||||||||
|
ALPROLIX
|
—
|
|
—
|
|
—
|
|
21.0
|
|
5.0
|
|
26.0
|
|
|||||||||||
|
Other Product Revenues:
|
|||||||||||||||||||||||
|
FUMADERM
|
—
|
|
12.5
|
|
12.5
|
|
—
|
|
20.0
|
|
20.0
|
|
|||||||||||
|
BENEPALI
|
—
|
|
236.5
|
|
236.5
|
|
—
|
|
154.0
|
|
154.0
|
|
|||||||||||
|
FLIXABI
|
—
|
|
17.8
|
|
17.8
|
|
—
|
|
2.5
|
|
2.5
|
|
|||||||||||
|
Total product revenues
|
$
|
3,279.8
|
|
$
|
2,001.2
|
|
$
|
5,281.0
|
|
$
|
3,491.9
|
|
$
|
1,527.9
|
|
$
|
5,019.8
|
|
|||||
|
(In millions)
|
Discounts
|
Contractual
Adjustments
|
Returns
|
Total
|
|||||||||||
|
Balance, as of December 31, 2017
|
$
|
109.6
|
|
$
|
606.0
|
|
$
|
46.0
|
|
$
|
761.6
|
|
|||
|
Current provisions relating to sales in current year
|
333.2
|
|
1,288.0
|
|
11.4
|
|
1,632.6
|
|
|||||||
|
Adjustments relating to prior years
|
(0.3
|
)
|
7.4
|
|
2.6
|
|
9.7
|
|
|||||||
|
Payments/credits relating to sales in current year
|
(222.3
|
)
|
(731.9
|
)
|
—
|
|
(954.2
|
)
|
|||||||
|
Payments/credits relating to sales in prior years
|
(101.0
|
)
|
(469.6
|
)
|
(13.5
|
)
|
(584.1
|
)
|
|||||||
|
Balance, as of June 30, 2018
|
$
|
119.2
|
|
$
|
699.9
|
|
$
|
46.5
|
|
$
|
865.6
|
|
|||
|
(In millions)
|
As of
June 30, 2018 |
As of
December 31, 2017 |
|||||
|
Reduction of accounts receivable
|
$
|
162.8
|
|
$
|
189.6
|
|
|
|
Component of accrued expenses and other
|
702.8
|
|
572.0
|
|
|||
|
Total reserves
|
$
|
865.6
|
|
$
|
761.6
|
|
|
|
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
|||||||||||||
|
(In millions)
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Biogen’s share of pre-tax profits in the U.S. for RITUXAN and GAZYVA
|
$
|
359.0
|
|
$
|
347.5
|
|
$
|
708.6
|
|
$
|
671.0
|
|
|||
|
Other revenues from anti-CD20 therapeutic programs
|
131.4
|
|
49.6
|
|
225.0
|
|
66.7
|
|
|||||||
|
Total revenues from anti-CD20 therapeutic programs
|
$
|
490.4
|
|
$
|
397.1
|
|
$
|
933.6
|
|
$
|
737.7
|
|
|||
|
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
|||||||||||||
|
(In millions)
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Revenues from collaborative and other relationships:
|
|||||||||||||||
|
AbbVie
|
$
|
(2.5
|
)
|
$
|
(3.9
|
)
|
$
|
(7.2
|
)
|
$
|
(9.8
|
)
|
|||
|
Samsung Bioepis and other
|
14.7
|
|
12.9
|
|
32.6
|
|
25.1
|
|
|||||||
|
Other royalty and corporate revenues:
|
|||||||||||||||
|
Royalty
|
17.3
|
|
11.8
|
|
27.9
|
|
37.3
|
|
|||||||
|
Other corporate
|
79.1
|
|
20.8
|
|
219.7
|
|
79.0
|
|
|||||||
|
Total other revenues
|
$
|
108.6
|
|
$
|
41.6
|
|
$
|
273.0
|
|
$
|
131.6
|
|
|||
|
(In millions)
|
As of
June 30, 2018 |
As of
December 31, 2017 |
|||||
|
Raw materials
|
$
|
176.1
|
|
$
|
162.4
|
|
|
|
Work in process
|
610.0
|
|
605.7
|
|
|||
|
Finished goods
|
158.5
|
|
157.4
|
|
|||
|
Total inventory
|
$
|
944.6
|
|
$
|
925.5
|
|
|
|
Balance Sheet Classification:
|
|||||||
|
Inventory
|
$
|
931.7
|
|
$
|
902.7
|
|
|
|
Investments and other assets
|
12.9
|
|
22.8
|
|
|||
|
Total inventory
|
$
|
944.6
|
|
$
|
925.5
|
|
|
|
|
|
As of June 30, 2018
|
As of December 31, 2017
|
||||||||||||||||||||||
|
(In millions)
|
Estimated
Life
|
Cost
|
Accumulated
Amortization
|
Net
|
Cost
|
Accumulated
Amortization
|
Net
|
||||||||||||||||||
|
Out-licensed patents
|
13-23 years
|
$
|
543.3
|
|
$
|
(539.9
|
)
|
$
|
3.4
|
|
$
|
543.3
|
|
$
|
(535.6
|
)
|
$
|
7.7
|
|
||||||
|
Developed
technology
|
15-23 years
|
3,005.3
|
|
(2,713.1
|
)
|
292.2
|
|
3,005.3
|
|
(2,689.0
|
)
|
316.3
|
|
||||||||||||
|
In-process research and development
|
Indefinite until commercialization
|
670.5
|
|
—
|
|
670.5
|
|
680.6
|
|
—
|
|
680.6
|
|
||||||||||||
|
Trademarks and
tradenames
|
Indefinite
|
64.0
|
|
—
|
|
64.0
|
|
64.0
|
|
—
|
|
64.0
|
|
||||||||||||
|
Acquired and in-licensed rights
and patents
|
4-18 years
|
3,974.2
|
|
(1,343.0
|
)
|
2,631.2
|
|
3,971.4
|
|
(1,160.4
|
)
|
2,811.0
|
|
||||||||||||
|
Total intangible assets
|
$
|
8,257.3
|
|
$
|
(4,596.0
|
)
|
$
|
3,661.3
|
|
$
|
8,264.6
|
|
$
|
(4,385.0
|
)
|
$
|
3,879.6
|
|
|||||||
|
(In millions)
|
As of
June 30, 2018 |
||
|
2018 (remaining six months)
|
$
|
215.8
|
|
|
2019
|
402.6
|
|
|
|
2020
|
380.9
|
|
|
|
2021
|
255.0
|
|
|
|
2022
|
242.3
|
|
|
|
2023
|
211.2
|
|
|
|
(In millions)
|
As of
June 30, 2018 |
||
|
Goodwill, beginning of period
|
$
|
4,632.5
|
|
|
Increase to goodwill
|
540.9
|
|
|
|
Other
|
(3.1
|
)
|
|
|
Goodwill, end of period
|
$
|
5,170.3
|
|
|
As of June 30, 2018 (In millions)
|
Total
|
Quoted Prices
in Active
Markets
(Level 1)
|
Significant Other
Observable Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
|||||||||||
|
Assets:
|
|||||||||||||||
|
Cash equivalents
|
$
|
732.2
|
|
$
|
—
|
|
$
|
732.2
|
|
$
|
—
|
|
|||
|
Marketable debt securities:
|
|||||||||||||||
|
Corporate debt securities
|
2,045.5
|
|
—
|
|
2,045.5
|
|
—
|
|
|||||||
|
Government securities
|
828.7
|
|
—
|
|
828.7
|
|
—
|
|
|||||||
|
Mortgage and other asset backed securities
|
260.0
|
|
—
|
|
260.0
|
|
—
|
|
|||||||
|
Marketable equity securities
|
489.7
|
|
70.1
|
|
419.6
|
|
—
|
|
|||||||
|
Derivative contracts
|
40.0
|
|
—
|
|
40.0
|
|
—
|
|
|||||||
|
Plan assets for deferred compensation
|
28.2
|
|
—
|
|
28.2
|
|
—
|
|
|||||||
|
Total
|
$
|
4,424.3
|
|
$
|
70.1
|
|
$
|
4,354.2
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Derivative contracts
|
$
|
43.5
|
|
$
|
—
|
|
$
|
43.5
|
|
$
|
—
|
|
|||
|
Contingent consideration obligations
|
499.9
|
|
—
|
|
—
|
|
499.9
|
|
|||||||
|
Total
|
$
|
543.4
|
|
$
|
—
|
|
$
|
43.5
|
|
$
|
499.9
|
|
|||
|
As of December 31, 2017 (In millions)
|
Total
|
Quoted Prices
in Active
Markets
(Level 1)
|
Significant Other
Observable Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
|||||||||||
|
Assets:
|
|||||||||||||||
|
Cash equivalents
|
$
|
1,229.4
|
|
$
|
—
|
|
$
|
1,229.4
|
|
$
|
—
|
|
|||
|
Marketable debt securities:
|
|||||||||||||||
|
Corporate debt securities
|
2,609.8
|
|
—
|
|
2,609.8
|
|
—
|
|
|||||||
|
Government securities
|
1,919.3
|
|
—
|
|
1,919.3
|
|
—
|
|
|||||||
|
Mortgage and other asset backed securities
|
643.4
|
|
—
|
|
643.4
|
|
—
|
|
|||||||
|
Marketable equity securities
|
11.8
|
|
11.8
|
|
—
|
|
—
|
|
|||||||
|
Derivative contracts
|
2.7
|
|
—
|
|
2.7
|
|
—
|
|
|||||||
|
Plan assets for deferred compensation
|
28.5
|
|
—
|
|
28.5
|
|
—
|
|
|||||||
|
Total
|
$
|
6,444.9
|
|
$
|
11.8
|
|
$
|
6,433.1
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Derivative contracts
|
$
|
111.3
|
|
$
|
—
|
|
$
|
111.3
|
|
$
|
—
|
|
|||
|
Contingent consideration obligations
|
523.6
|
|
—
|
|
—
|
|
523.6
|
|
|||||||
|
Total
|
$
|
634.9
|
|
$
|
—
|
|
$
|
111.3
|
|
$
|
523.6
|
|
|||
|
|
As of June 30, 2018
|
As of December 31, 2017
|
|||||||||||||
|
(In millions)
|
Fair
Value
|
Carrying
Value
|
Fair
Value
|
Carrying
Value
|
|||||||||||
|
Notes payable to Fumedica AG
|
$
|
—
|
|
$
|
—
|
|
$
|
3.2
|
|
$
|
3.2
|
|
|||
|
2.900% Senior Notes due September 15, 2020
|
1,491.0
|
|
1,474.3
|
|
1,517.7
|
|
1,482.4
|
|
|||||||
|
3.625% Senior Notes due September 15, 2022
|
999.6
|
|
994.9
|
|
1,032.9
|
|
994.3
|
|
|||||||
|
4.050% Senior Notes due September 15, 2025
|
1,757.9
|
|
1,737.0
|
|
1,851.9
|
|
1,736.3
|
|
|||||||
|
5.200% Senior Notes due September 15, 2045
|
1,854.5
|
|
1,722.2
|
|
2,077.6
|
|
1,722.0
|
|
|||||||
|
Total
|
$
|
6,103.0
|
|
$
|
5,928.4
|
|
$
|
6,483.3
|
|
$
|
5,938.2
|
|
|||
|
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
|||||||||||||
|
(In millions)
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Fair value, beginning of period
|
$
|
498.0
|
|
$
|
470.9
|
|
$
|
523.6
|
|
$
|
467.6
|
|
|||
|
Changes in fair value
|
1.9
|
|
21.2
|
|
(3.7
|
)
|
31.2
|
|
|||||||
|
Payments
|
—
|
|
—
|
|
(20.0
|
)
|
(6.7
|
)
|
|||||||
|
Fair value, end of period
|
$
|
499.9
|
|
$
|
492.1
|
|
$
|
499.9
|
|
$
|
492.1
|
|
|||
|
(In millions)
|
As of
June 30, 2018 |
As of
December 31, 2017 |
|||||
|
Commercial paper
|
$
|
125.2
|
|
$
|
30.5
|
|
|
|
Overnight reverse repurchase agreements
|
23.9
|
|
3.6
|
|
|||
|
Money market funds
|
541.7
|
|
948.0
|
|
|||
|
Short-term debt securities
|
41.4
|
|
247.3
|
|
|||
|
Total
|
$
|
732.2
|
|
$
|
1,229.4
|
|
|
|
As of June 30, 2018 (In millions)
|
Fair
Value
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Amortized
Cost
|
|||||||||||
|
Corporate debt securities
|
|||||||||||||||
|
Current
|
$
|
1,484.2
|
|
$
|
0.1
|
|
$
|
(0.7
|
)
|
$
|
1,484.8
|
|
|||
|
Non-current
|
561.3
|
|
0.4
|
|
(0.6
|
)
|
561.5
|
|
|||||||
|
Government securities
|
|||||||||||||||
|
Current
|
489.6
|
|
—
|
|
(0.1
|
)
|
489.7
|
|
|||||||
|
Non-current
|
339.1
|
|
0.1
|
|
(0.6
|
)
|
339.6
|
|
|||||||
|
Mortgage and other asset backed securities
|
|||||||||||||||
|
Current
|
0.2
|
|
—
|
|
—
|
|
0.2
|
|
|||||||
|
Non-current
|
259.8
|
|
0.1
|
|
(0.8
|
)
|
260.5
|
|
|||||||
|
Total marketable debt securities
|
$
|
3,134.2
|
|
$
|
0.7
|
|
$
|
(2.8
|
)
|
$
|
3,136.3
|
|
|||
|
Marketable equity securities, non-current
|
$
|
489.7
|
|
$
|
42.5
|
|
$
|
(49.4
|
)
|
$
|
496.6
|
|
|||
|
As of December 31, 2017 (In millions)
|
Fair
Value
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Amortized
Cost
|
|||||||||||
|
Corporate debt securities
|
|||||||||||||||
|
Current
|
$
|
1,039.3
|
|
$
|
—
|
|
$
|
(0.2
|
)
|
$
|
1,039.5
|
|
|||
|
Non-current
|
1,570.5
|
|
0.9
|
|
—
|
|
1,569.6
|
|
|||||||
|
Government securities
|
|||||||||||||||
|
Current
|
1,075.1
|
|
0.1
|
|
(0.7
|
)
|
1,075.7
|
|
|||||||
|
Non-current
|
844.2
|
|
0.2
|
|
(1.1
|
)
|
845.1
|
|
|||||||
|
Mortgage and other asset backed securities
|
|||||||||||||||
|
Current
|
0.8
|
|
—
|
|
—
|
|
0.8
|
|
|||||||
|
Non-current
|
642.6
|
|
1.1
|
|
(0.8
|
)
|
642.3
|
|
|||||||
|
Total marketable debt securities
|
$
|
5,172.5
|
|
$
|
2.3
|
|
$
|
(2.8
|
)
|
$
|
5,173.0
|
|
|||
|
Marketable equity securities, non-current
|
$
|
11.8
|
|
$
|
1.8
|
|
$
|
(4.4
|
)
|
$
|
14.4
|
|
|||
|
|
As of June 30, 2018
|
As of December 31, 2017
|
|||||||||||||
|
(In millions)
|
Estimated
Fair Value
|
Amortized
Cost
|
Estimated
Fair Value
|
Amortized
Cost
|
|||||||||||
|
Due in one year or less
|
$
|
1,974.0
|
|
$
|
1,974.7
|
|
$
|
2,115.2
|
|
$
|
2,116.0
|
|
|||
|
Due after one year through five years
|
1,063.5
|
|
1,064.5
|
|
2,730.0
|
|
2,730.0
|
|
|||||||
|
Due after five years
|
96.7
|
|
97.1
|
|
327.3
|
|
327.0
|
|
|||||||
|
Total available-for-sale securities
|
$
|
3,134.2
|
|
$
|
3,136.3
|
|
$
|
5,172.5
|
|
$
|
5,173.0
|
|
|||
|
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
|||||||||||||
|
(In millions)
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Proceeds from maturities and sales
|
$
|
2,733.7
|
|
$
|
1,700.2
|
|
$
|
6,802.7
|
|
$
|
3,584.5
|
|
|||
|
Realized gains
|
$
|
0.8
|
|
$
|
1.2
|
|
$
|
2.6
|
|
$
|
2.4
|
|
|||
|
Realized losses
|
$
|
(0.8
|
)
|
$
|
(1.3
|
)
|
$
|
(10.2
|
)
|
$
|
(3.2
|
)
|
|||
|
|
Notional Amount
|
||||||
|
Foreign Currency: (In millions)
|
As of
June 30, 2018 |
As of
December 31, 2017 |
|||||
|
Euro
|
$
|
1,553.3
|
|
$
|
1,875.6
|
|
|
|
British pound
|
72.5
|
|
150.9
|
|
|||
|
Canadian dollar
|
45.8
|
|
83.5
|
|
|||
|
Swiss franc
|
36.8
|
|
88.7
|
|
|||
|
Total foreign currency forward contracts
|
$
|
1,708.4
|
|
$
|
2,198.7
|
|
|
|
For the Three Months Ended June 30, 2018
|
For the Six Months Ended June 30, 2018
|
|||||||||||||||||||||
|
Net Gains/(Losses)
Reclassified from AOCI into Operating Income (in millions)
|
Net Gains/(Losses)
Recognized in Operating Income (in millions)
|
Net Gains/(Losses)
Reclassified from AOCI into Operating Income (in millions)
|
Net Gains/(Losses)
Recognized in Operating Income (in millions)
|
|||||||||||||||||||
|
Location
|
2018
|
Location
|
2018
|
Location
|
2018
|
Location
|
2018
|
|||||||||||||||
|
Revenues
|
$
|
(10.4
|
)
|
Revenues
|
$
|
7.9
|
|
Revenues
|
$
|
(43.3
|
)
|
Revenues
|
$
|
7.0
|
|
|||||||
|
Operating expenses
|
$
|
(0.4
|
)
|
Operating expenses
|
$
|
(0.1
|
)
|
Operating expenses
|
$
|
0.9
|
|
Operating expenses
|
$
|
(0.4
|
)
|
|||||||
|
For the Three Months Ended June 30, 2017
|
For the Six Months Ended June 30, 2017
|
|||||||||||||||||||||
|
Net Gains/(Losses)
Reclassified from AOCI into Operating Income (in millions)
|
Net Gains/(Losses)
Recognized Directly into Net Income (in millions)
|
Net Gains/(Losses)
Reclassified from AOCI into
Operating Income (in millions)
|
Net Gains/(Losses)
Recognized Directly into Net
Income (in millions)
|
|||||||||||||||||||
|
Location
|
2017
|
Location
|
2017
|
Location
|
2017
|
Location
|
2017
|
|||||||||||||||
|
Revenues
|
$
|
(3.0
|
)
|
Other income (expense)
|
$
|
2.0
|
|
Revenue
|
$
|
3.7
|
|
Other income (expense)
|
$
|
6.0
|
|
|||||||
|
Operating expenses
|
$
|
0.3
|
|
Other income (expense)
|
$
|
(0.1
|
)
|
Operating expenses
|
$
|
0.2
|
|
Other income (expense)
|
$
|
(0.3
|
)
|
|||||||
|
Fair Value
|
||||
|
(In millions)
|
Balance Sheet Location
|
As of June 30, 2018
|
||
|
Hedging Instruments:
|
||||
|
Asset derivatives
|
Other current assets
|
$
|
27.2
|
|
|
Investments and other assets
|
$
|
8.8
|
|
|
|
Liability derivatives
|
Accrued expenses and other
|
$
|
22.2
|
|
|
Other long-term liabilities
|
$
|
19.6
|
|
|
|
Other Derivatives:
|
||||
|
Asset derivatives
|
Other current assets
|
$
|
4.0
|
|
|
Liability derivatives
|
Accrued expenses and other
|
$
|
1.7
|
|
|
Fair Value
|
||||
|
(In millions)
|
Balance Sheet Location
|
As of December 31, 2017
|
||
|
Hedging Instruments:
|
||||
|
Asset derivatives
|
Other current assets
|
$
|
0.7
|
|
|
Investments and other assets
|
$
|
0.2
|
|
|
|
Liability derivatives
|
Accrued expenses and other
|
$
|
84.7
|
|
|
Other long-term liabilities
|
$
|
23.6
|
|
|
|
Other Derivatives:
|
||||
|
Asset derivatives
|
Other current assets
|
$
|
1.8
|
|
|
Liability derivatives
|
Accrued expenses and other
|
$
|
3.0
|
|
|
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
|||||||||||||
|
(In millions)
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
NCI, beginning of period
|
$
|
(16.2
|
)
|
$
|
(11.6
|
)
|
$
|
(14.7
|
)
|
$
|
(11.5
|
)
|
|||
|
Net income (loss) attributable to NCI, net of tax
|
48.4
|
|
—
|
|
46.7
|
|
(0.1
|
)
|
|||||||
|
Capital contribution by noncontrolling interest
|
11.1
|
|
—
|
|
11.1
|
|
—
|
|
|||||||
|
Distribution to noncontrolling interest
|
(50.0
|
)
|
—
|
|
(50.0
|
)
|
—
|
|
|||||||
|
Translation adjustment and other
|
(0.5
|
)
|
—
|
|
(0.3
|
)
|
—
|
|
|||||||
|
NCI, end of period
|
$
|
(7.2
|
)
|
$
|
(11.6
|
)
|
$
|
(7.2
|
)
|
$
|
(11.6
|
)
|
|||
|
(In millions)
|
Unrealized Gains (Losses) on Securities Available for Sale, Net of Tax
|
Unrealized Gains (Losses) on Cash Flow Hedges, Net of Tax
|
Unfunded Status of Postretirement Benefit Plans, Net of Tax
|
Translation Adjustments
|
Total
|
||||||||||||||
|
Balance, December 31, 2017
|
$
|
(1.6
|
)
|
$
|
(104.5
|
)
|
$
|
(36.8
|
)
|
$
|
(175.5
|
)
|
$
|
(318.4
|
)
|
||||
|
Amount reclassified, net of tax, upon adoption of ASU 2016-01
|
1.5
|
|
—
|
|
—
|
|
—
|
|
1.5
|
|
|||||||||
|
Balance, January 1, 2018
|
(0.1
|
)
|
(104.5
|
)
|
(36.8
|
)
|
(175.5
|
)
|
(316.9
|
)
|
|||||||||
|
Other comprehensive income (loss) before reclassifications
|
(7.6
|
)
|
61.7
|
|
0.4
|
|
(47.3
|
)
|
7.2
|
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive income (loss)
|
6.0
|
|
42.1
|
|
—
|
|
—
|
|
48.1
|
|
|||||||||
|
Net current period other comprehensive income (loss)
|
(1.6
|
)
|
103.8
|
|
0.4
|
|
(47.3
|
)
|
55.3
|
|
|||||||||
|
Balance, June 30, 2018
|
$
|
(1.7
|
)
|
$
|
(0.7
|
)
|
$
|
(36.4
|
)
|
$
|
(222.8
|
)
|
$
|
(261.6
|
)
|
||||
|
(In millions)
|
Unrealized Gains (Losses) on Securities Available for Sale, Net of Tax
|
Unrealized Gains (Losses) on Cash Flow Hedges, Net of Tax
|
Unfunded Status of Postretirement Benefit Plans, Net of Tax
|
Translation Adjustments
|
Total
|
||||||||||||||
|
Balance, December 31, 2016
|
$
|
(10.8
|
)
|
$
|
57.8
|
|
$
|
(32.7
|
)
|
$
|
(334.2
|
)
|
$
|
(319.9
|
)
|
||||
|
Other comprehensive income (loss) before reclassifications
|
5.1
|
|
(122.8
|
)
|
(0.5
|
)
|
102.8
|
|
(15.4
|
)
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive income (loss)
|
0.5
|
|
(4.0
|
)
|
—
|
|
—
|
|
(3.5
|
)
|
|||||||||
|
Net current period other comprehensive income (loss)
|
5.6
|
|
(126.8
|
)
|
(0.5
|
)
|
102.8
|
|
(18.9
|
)
|
|||||||||
|
Balance, June 30, 2017
|
$
|
(5.2
|
)
|
$
|
(69.0
|
)
|
$
|
(33.2
|
)
|
$
|
(231.4
|
)
|
$
|
(338.8
|
)
|
||||
|
(In millions)
|
Income Statement Location
|
Amounts Reclassified from Accumulated Other Comprehensive Income
|
||||||||||||||
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
|||||||||||||||
|
2018
|
2017
|
2018
|
2017
|
|||||||||||||
|
Gains (losses) on securities available for sale
|
Other income (expense)
|
$
|
—
|
|
$
|
(0.1
|
)
|
$
|
(7.6
|
)
|
$
|
(0.8
|
)
|
|||
|
Income tax benefit (expense)
|
—
|
|
—
|
|
1.6
|
|
0.3
|
|
||||||||
|
Gains (losses) on cash flow hedges
|
Revenues
|
(10.4
|
)
|
(3.0
|
)
|
(43.3
|
)
|
3.7
|
|
|||||||
|
Operating expenses
|
(0.4
|
)
|
0.3
|
|
0.9
|
|
0.2
|
|
||||||||
|
Other income (expense)
|
—
|
|
—
|
|
0.1
|
|
0.1
|
|
||||||||
|
Income tax benefit (expense)
|
0.1
|
|
—
|
|
0.2
|
|
—
|
|
||||||||
|
Total reclassifications, net of tax
|
$
|
(10.7
|
)
|
$
|
(2.8
|
)
|
$
|
(48.1
|
)
|
$
|
3.5
|
|
||||
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
||||||||||||||
|
(In millions)
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Numerator:
|
|||||||||||||||
|
Net income attributable to Biogen Inc.
|
$
|
866.6
|
|
$
|
862.8
|
|
$
|
2,039.5
|
|
$
|
1,610.4
|
|
|||
|
Denominator:
|
|||||||||||||||
|
Weighted-average number of common shares outstanding
|
207.1
|
|
211.9
|
|
209.2
|
|
213.7
|
|
|||||||
|
Effect of dilutive securities:
|
|||||||||||||||
|
Stock options and employee stock purchase plan
|
—
|
|
0.1
|
|
—
|
|
—
|
|
|||||||
|
Time-vested restricted stock units
|
0.1
|
|
0.1
|
|
0.2
|
|
0.2
|
|
|||||||
|
Market stock units
|
0.1
|
|
0.1
|
|
0.1
|
|
0.1
|
|
|||||||
|
Performance stock units settled in stock
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||
|
Dilutive potential common shares
|
0.2
|
|
0.3
|
|
0.3
|
|
0.3
|
|
|||||||
|
Shares used in calculating diluted earnings per share
|
207.3
|
|
212.2
|
|
209.5
|
|
214.0
|
|
|||||||
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
||||||||||||||
|
(In millions)
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Research and development
|
$
|
17.6
|
|
$
|
17.5
|
|
$
|
39.5
|
|
$
|
36.2
|
|
|||
|
Selling, general and administrative
|
25.2
|
|
18.9
|
|
53.7
|
|
48.3
|
|
|||||||
|
Subtotal
|
42.8
|
|
36.4
|
|
93.2
|
|
84.5
|
|
|||||||
|
Capitalized share-based compensation costs
|
(2.8
|
)
|
(2.4
|
)
|
(6.2
|
)
|
(5.1
|
)
|
|||||||
|
Share-based compensation expense included in total cost and expenses
|
40.0
|
|
34.0
|
|
87.0
|
|
79.4
|
|
|||||||
|
Income tax effect
|
(6.5
|
)
|
(8.5
|
)
|
(14.1
|
)
|
(20.9
|
)
|
|||||||
|
Share-based compensation expense included in net income attributable to Biogen Inc.
|
$
|
33.5
|
|
$
|
25.5
|
|
$
|
72.9
|
|
$
|
58.5
|
|
|||
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
||||||||||||||
|
(In millions)
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Market stock units
|
$
|
7.1
|
|
$
|
3.0
|
|
$
|
13.2
|
|
$
|
12.6
|
|
|||
|
Time-vested restricted stock units
|
30.6
|
|
27.6
|
|
66.7
|
|
54.5
|
|
|||||||
|
Cash settled performance units
|
(0.7
|
)
|
2.5
|
|
3.6
|
|
6.0
|
|
|||||||
|
Performance units
|
2.0
|
|
1.2
|
|
1.2
|
|
5.7
|
|
|||||||
|
Performance stock units settled in stock
|
1.4
|
|
—
|
|
2.1
|
|
—
|
|
|||||||
|
Performance stock units settled in cash
|
0.3
|
|
—
|
|
0.4
|
|
—
|
|
|||||||
|
Employee stock purchase plan
|
2.1
|
|
2.1
|
|
6.0
|
|
5.7
|
|
|||||||
|
Subtotal
|
42.8
|
|
36.4
|
|
93.2
|
|
84.5
|
|
|||||||
|
Capitalized share-based compensation costs
|
(2.8
|
)
|
(2.4
|
)
|
(6.2
|
)
|
(5.1
|
)
|
|||||||
|
Share-based compensation expense included in total cost and expenses
|
$
|
40.0
|
|
$
|
34.0
|
|
$
|
87.0
|
|
$
|
79.4
|
|
|||
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
||||||||||
|
|
2018
|
2017
|
2018
|
2017
|
|||||||
|
Statutory rate
|
21.0
|
%
|
35.0
|
%
|
21.0
|
%
|
35.0
|
%
|
|||
|
State taxes
|
0.6
|
|
0.8
|
|
0.8
|
|
0.4
|
|
|||
|
Taxes on foreign earnings
|
—
|
|
(11.7
|
)
|
(0.4
|
)
|
(11.4
|
)
|
|||
|
Credits and net operating loss utilization
|
(0.7
|
)
|
(0.9
|
)
|
(0.8
|
)
|
(0.8
|
)
|
|||
|
Purchased intangible assets
|
0.6
|
|
1.4
|
|
0.6
|
|
1.4
|
|
|||
|
Manufacturing deduction
|
—
|
|
(2.1
|
)
|
—
|
|
(2.1
|
)
|
|||
|
Other permanent items
|
0.4
|
|
0.7
|
|
0.4
|
|
0.7
|
|
|||
|
Other
|
0.5
|
|
0.6
|
|
0.3
|
|
0.8
|
|
|||
|
Effective tax rate
|
22.4
|
%
|
23.8
|
%
|
21.9
|
%
|
24.0
|
%
|
|||
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
||||||||||||||
|
(In millions)
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Interest income
|
$
|
28.7
|
|
$
|
16.9
|
|
$
|
55.4
|
|
$
|
33.6
|
|
|||
|
Interest expense
|
(51.7
|
)
|
(63.6
|
)
|
(102.2
|
)
|
(127.0
|
)
|
|||||||
|
Gain (loss) on investments, net
|
5.3
|
|
(13.4
|
)
|
(9.1
|
)
|
(11.0
|
)
|
|||||||
|
Foreign exchange gains (losses), net
|
(13.0
|
)
|
(2.3
|
)
|
(14.0
|
)
|
1.7
|
|
|||||||
|
Other, net
|
(3.8
|
)
|
(6.2
|
)
|
(5.6
|
)
|
(3.9
|
)
|
|||||||
|
Total other income (expense), net
|
$
|
(34.5
|
)
|
$
|
(68.6
|
)
|
$
|
(75.5
|
)
|
$
|
(106.6
|
)
|
|||
|
(In millions)
|
As of
June 30, 2018 |
As of
December 31, 2017 |
|||||
|
Revenue-related reserves for discounts and allowances
|
$
|
702.8
|
|
$
|
572.0
|
|
|
|
Current portion of contingent consideration obligations
|
529.4
|
|
844.6
|
|
|||
|
Royalties and licensing fees
|
218.2
|
|
206.7
|
|
|||
|
Employee compensation and benefits
|
209.8
|
|
297.7
|
|
|||
|
Construction in progress
|
179.3
|
|
159.7
|
|
|||
|
Collaboration expenses
|
156.1
|
|
183.7
|
|
|||
|
Other
|
638.1
|
|
636.9
|
|
|||
|
Total accrued expenses and other
|
$
|
2,633.7
|
|
$
|
2,901.3
|
|
|
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
||||||||||||||
|
(In millions)
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Total development expense incurred by the collaboration related to the advancement of BAN2401 and E2609
|
$
|
54.6
|
|
$
|
29.5
|
|
$
|
111.1
|
|
$
|
66.7
|
|
|||
|
Biogen's share of BAN2401 and E2609 development expense reflected in research and development expense in our condensed consolidated statements of income
|
$
|
27.3
|
|
$
|
14.7
|
|
$
|
55.5
|
|
$
|
33.3
|
|
|||
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
||||||||||||||
|
(In millions)
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Total development expense incurred by the collaboration related to the advancement of aducanumab
|
$
|
76.5
|
|
$
|
74.6
|
|
$
|
140.0
|
|
$
|
128.9
|
|
|||
|
Biogen's share of aducanumab development expense reflected in research and development expense in our condensed consolidated statements of income
|
$
|
65.0
|
|
$
|
74.6
|
|
$
|
128.5
|
|
$
|
128.9
|
|
|||
|
Total sales and marketing expense incurred by the collaboration related to the advancement of aducanumab
|
$
|
14.0
|
|
$
|
4.8
|
|
$
|
21.2
|
|
$
|
9.8
|
|
|||
|
Biogen's share of aducanumab sales and marketing expense reflected in selling, general and administrative expense our condensed consolidated statements of income
|
$
|
9.2
|
|
$
|
4.8
|
|
$
|
13.9
|
|
$
|
9.8
|
|
|||
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
||||||||||||||
|
(In millions)
|
2018
|
2017
|
2018
|
2017
|
|||||||||||
|
Biogen's share of pre-tax profits in the U.S. for RITUXAN and GAZYVA
|
$
|
359.0
|
|
$
|
347.5
|
|
$
|
708.6
|
|
$
|
671.0
|
|
|||
|
Other revenues from anti-CD20 therapeutic programs
|
131.4
|
|
49.6
|
|
225.0
|
|
66.7
|
|
|||||||
|
Total revenues from anti-CD20 therapeutic programs
|
$
|
490.4
|
|
$
|
397.1
|
|
$
|
933.6
|
|
$
|
737.7
|
|
|||
|
•
|
Total revenues were
$3,356.5 million
for the
second
quarter of
2018
, representing an increase of 9.0% over the same period in
2017
.
|
|
•
|
Product revenues, net totaled
$2,757.5 million
for the
second
quarter of
2018
, representing an increase of 4.5% over the same period in
2017
. This increase was primarily due to revenues from SPINRAZA and BENEPALI and the positive impact of comparative foreign currency exchange gains, net of hedge of $48.7 million. This increase was partially offset by a decrease in our MS product revenues due to a decrease in unit sales volumes and pricing reductions in certain European countries.
|
|
•
|
Revenues from anti-CD20 therapeutic programs totaled
$490.4 million
for the
second
quarter of
2018
, representing an increase of 23.5% over the same period in
2017
. This increase was primarily due to royalty revenues on sales of OCREVUS.
|
|
•
|
Other revenues totaled
$108.6 million
for the
second
quarter of
2018
, representing an increase of 161.1% over the same period in
2017
. This increase was primarily due to higher contract manufacturing revenues related to the volume of shipments of drug product and drug substance production provided to our strategic partners.
|
|
•
|
Total cost and expenses totaled
$2,143.3 million
for the
second
quarter of
2018
, representing an increase of
14.2%
over the same period in
2017
. This increase was primarily due to a
23.2%
increase in research and development, a
20.1%
increase in selling, general and administrative expenses and a
15.0%
increase in cost of sales.
|
|
◦
|
The increase in research and development was primarily due to the $482.6 million net charge recognized upon the closing of our collaboration with Ionis Pharmaceuticals, Inc. (Ionis), partially offset by charges recognized in the prior year comparative period totaling $360.0 million related to our exclusive license agreement with Bristol-Myers Squibb Company (BMS).
|
|
◦
|
The increase in selling, general and administrative expenses was primarily due to an increase in spending on sales and marketing activities in support of our marketed products, including SPINRAZA.
|
|
◦
|
The increase in cost of sales was primarily due to higher contract manufacturing shipments of drug product and drug substance production provided to our strategic partners and increased royalties payable to Ionis on higher sales of SPINRAZA, partially offset by lower sales on our MS products.
|
|
•
|
Net income attributable to Biogen Inc. was favorably impacted by a decrease in our effective tax rate to 22.4% for the
second
quarter of
2018
, from 23.8% for the same period in 2017, primarily due to the favorable impact resulting from the enactment of the Tax Cuts and Jobs Act of 2017 (2017 Tax Act) in December 2017.
|
|
•
|
Cash, cash equivalents and marketable securities totaled approximately
$4.4 billion
as of
June 30, 2018
.
|
|
•
|
We repurchased approximately
9.6 million
shares of our common stock at a cost of
$2.75 billion
during the
second
quarter of
2018
under the share repurchase program authorized by our Board of Directors in July 2016, which allowed us to repurchase up to $5.0 billion of our common stock (2016 Share Repurchase Program). Our 2016 Share Repurchase Program was completed as of June 30, 2018.
|
|
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
|||||||||||||||||||||||||
|
(In millions, except percentages)
|
2018
|
2017
|
2018
|
2017
|
|||||||||||||||||||||||
|
Product revenues:
|
|||||||||||||||||||||||||||
|
United States
|
$
|
1,741.9
|
|
51.9
|
%
|
$
|
1,860.9
|
|
60.5
|
%
|
$
|
3,279.8
|
|
50.6
|
%
|
$
|
3,491.9
|
|
59.3
|
%
|
|||||||
|
Rest of world
|
1,015.6
|
|
30.3
|
%
|
778.8
|
|
25.2
|
%
|
2,001.2
|
|
30.8
|
%
|
1,527.9
|
|
26.0
|
%
|
|||||||||||
|
Total product revenues
|
2,757.5
|
|
82.2
|
%
|
2,639.7
|
|
85.7
|
%
|
5,281.0
|
|
81.4
|
%
|
5,019.8
|
|
85.3
|
%
|
|||||||||||
|
Revenues from anti-CD20 therapeutic programs
|
490.4
|
|
14.6
|
%
|
397.1
|
|
12.9
|
%
|
933.6
|
|
14.4
|
%
|
737.7
|
|
12.5
|
%
|
|||||||||||
|
Other revenues
|
108.6
|
|
3.2
|
%
|
41.6
|
|
1.4
|
%
|
273.0
|
|
4.2
|
%
|
131.6
|
|
2.2
|
%
|
|||||||||||
|
Total revenues
|
$
|
3,356.5
|
|
100.0
|
%
|
$
|
3,078.4
|
|
100.0
|
%
|
$
|
6,487.6
|
|
100.0
|
%
|
$
|
5,889.1
|
|
100.0
|
%
|
|||||||
|
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
|||||||||||||||||||||||||
|
(In millions, except percentages)
|
2018
|
2017
|
2018
|
2017
|
|||||||||||||||||||||||
|
Multiple Sclerosis:
|
|||||||||||||||||||||||||||
|
TECFIDERA
|
$
|
1,086.8
|
|
39.4
|
%
|
$
|
1,110.6
|
|
42.1
|
%
|
$
|
2,073.7
|
|
39.3
|
%
|
$
|
2,068.8
|
|
41.2
|
%
|
|||||||
|
Interferon*
|
625.5
|
|
22.7
|
%
|
690.6
|
|
26.2
|
%
|
1,175.8
|
|
22.3
|
%
|
1,338.9
|
|
26.7
|
%
|
|||||||||||
|
TYSABRI
|
467.2
|
|
16.9
|
%
|
496.0
|
|
18.8
|
%
|
929.3
|
|
17.6
|
%
|
1,041.0
|
|
20.7
|
%
|
|||||||||||
|
FAMPYRA
|
23.0
|
|
0.8
|
%
|
22.6
|
|
0.8
|
%
|
47.4
|
|
0.9
|
%
|
43.1
|
|
0.9
|
%
|
|||||||||||
|
ZINBRYTA
|
—
|
|
—
|
%
|
16.1
|
|
0.6
|
%
|
1.4
|
|
—
|
%
|
26.8
|
|
0.5
|
%
|
|||||||||||
|
Spinal Muscular Atrophy:
|
|||||||||||||||||||||||||||
|
SPINRAZA
|
422.7
|
|
15.3
|
%
|
202.9
|
|
7.7
|
%
|
786.6
|
|
14.9
|
%
|
250.3
|
|
5.0
|
%
|
|||||||||||
|
Hemophilia:
|
|
|
|
|
|||||||||||||||||||||||
|
ELOCTATE
|
—
|
|
—
|
%
|
—
|
|
—
|
%
|
—
|
|
—
|
%
|
48.4
|
|
1.0
|
%
|
|||||||||||
|
ALPROLIX
|
—
|
|
—
|
%
|
—
|
|
—
|
%
|
—
|
|
—
|
%
|
26.0
|
|
0.5
|
%
|
|||||||||||
|
Other Product Revenues:
|
|||||||||||||||||||||||||||
|
FUMADERM
|
5.5
|
|
0.2
|
%
|
10.3
|
|
0.4
|
%
|
12.5
|
|
0.2
|
%
|
20.0
|
|
0.4
|
%
|
|||||||||||
|
BENEPALI
|
115.6
|
|
4.2
|
%
|
88.7
|
|
3.4
|
%
|
236.5
|
|
4.5
|
%
|
154.0
|
|
3.1
|
%
|
|||||||||||
|
FLIXABI
|
11.2
|
|
0.4
|
%
|
1.9
|
|
—
|
%
|
17.8
|
|
0.3
|
%
|
2.5
|
|
—
|
%
|
|||||||||||
|
Total product revenues
|
$
|
2,757.5
|
|
100.0
|
%
|
$
|
2,639.7
|
|
100.0
|
%
|
$
|
5,281.0
|
|
100.0
|
%
|
$
|
5,019.8
|
|
100.0
|
%
|
|||||||
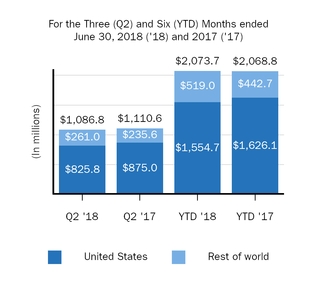
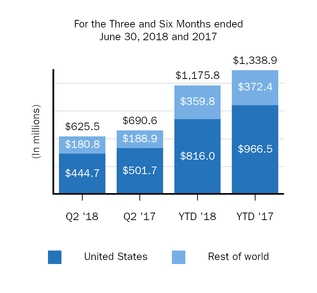
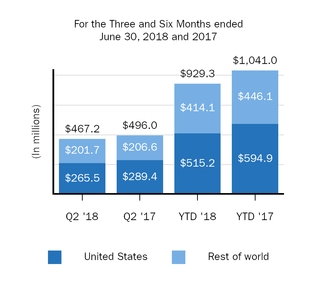

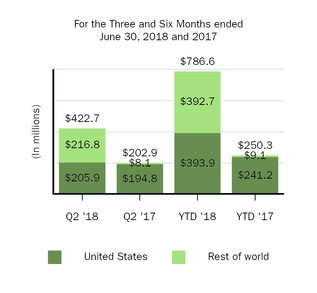
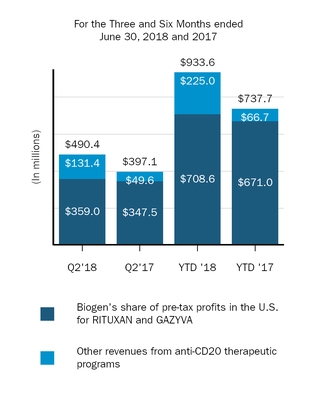
|
|
For the Three Months
Ended June 30, |
||||||
|
(In millions)
|
2018
|
2017
|
|||||
|
Product revenues, net
|
$
|
1,141.9
|
|
$
|
1,083.8
|
|
|
|
Cost and expenses
|
189.2
|
|
193.6
|
|
|||
|
Pre-tax profits in the U.S.
|
952.7
|
|
890.2
|
|
|||
|
Biogen's share of pre-tax profits
|
$
|
359.0
|
|
$
|
347.5
|
|
|
|
|
For the Six Months
Ended June 30, |
||||||
|
(In millions)
|
2018
|
2017
|
|||||
|
Product revenues, net
|
$
|
2,238.1
|
|
$
|
2,124.8
|
|
|
|
Cost and expenses
|
342.9
|
|
394.9
|
|
|||
|
Pre-tax profits in the U.S.
|
1,895.2
|
|
1,729.9
|
|
|||
|
Biogen's share of pre-tax profits
|
$
|
708.6
|
|
$
|
671.0
|
|
|
|
|
For the Three Months
Ended June 30, |
For the Six Months
Ended June 30, |
|||||||||||||||||||||||||
|
(In millions, except percentages)
|
2018
|
2017
|
2018
|
2017
|
|||||||||||||||||||||||
|
Revenues from collaborative and other relationships
|
$
|
12.2
|
|
11.2
|
%
|
$
|
9.0
|
|
21.6
|
%
|
$
|
25.4
|
|
9.3
|
%
|
$
|
15.3
|
|
11.6
|
%
|
|||||||
|
Other royalty and corporate revenues
|
96.4
|
|
88.8
|
%
|
32.6
|
|
78.4
|
%
|
247.6
|
|
90.7
|
%
|
116.3
|
|
88.4
|
%
|
|||||||||||
|
Total other revenues
|
$
|
108.6
|
|
100.0
|
%
|
$
|
41.6
|
|
100.0
|
%
|
$
|
273.0
|
|
100.0
|
%
|
$
|
131.6
|
|
100.0
|
%
|
|||||||
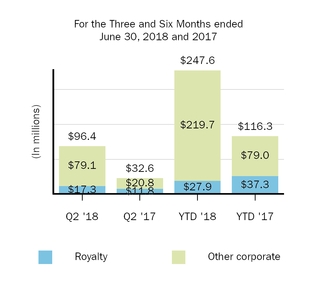
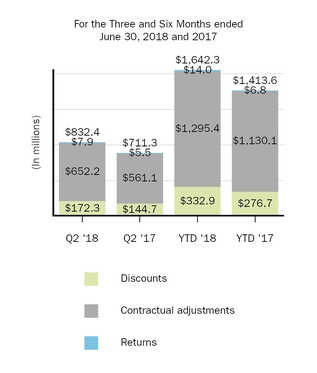
|
|
For the Three Months
Ended June 30, |
|
For the Six Months
Ended June 30, |
||||||||||||||||||
|
(In millions, except percentages)
|
2018
|
2017
|
Change %
|
|
2018
|
2017
|
Change %
|
||||||||||||||
|
Cost of sales, excluding amortization of acquired intangible assets
|
$
|
421.0
|
|
$
|
366.2
|
|
15.0
|
%
|
$
|
867.0
|
|
$
|
750.8
|
|
15.5
|
%
|
|||||
|
Research and development
|
981.0
|
|
796.2
|
|
23.2
|
%
|
1,477.7
|
|
1,219.6
|
|
21.2
|
%
|
|||||||||
|
Selling, general and administrative
|
516.2
|
|
429.8
|
|
20.1
|
%
|
1,017.5
|
|
928.5
|
|
9.6
|
%
|
|||||||||
|
Amortization of acquired intangible assets
|
107.4
|
|
117.5
|
|
(8.6
|
)%
|
211.3
|
|
566.0
|
|
(62.7
|
)%
|
|||||||||
|
Acquired in-process research and development
|
75.0
|
|
120.0
|
|
(37.5
|
)%
|
85.0
|
|
120.0
|
|
(29.2
|
)%
|
|||||||||
|
Collaboration profit (loss) sharing
|
39.2
|
|
26.5
|
|
47.9
|
%
|
81.7
|
|
47.3
|
|
72.7
|
%
|
|||||||||
|
Loss (gain) on fair value remeasurement of contingent consideration
|
1.9
|
|
21.2
|
|
(91.0
|
)%
|
(3.7
|
)
|
31.2
|
|
(111.9
|
)%
|
|||||||||
|
Restructuring charges
|
1.6
|
|
—
|
|
**
|
|
3.2
|
|
—
|
|
**
|
|
|||||||||
|
Total cost and expenses
|
$
|
2,143.3
|
|
$
|
1,877.4
|
|
14.2
|
%
|
$
|
3,739.7
|
|
$
|
3,663.4
|
|
2.1
|
%
|
|||||
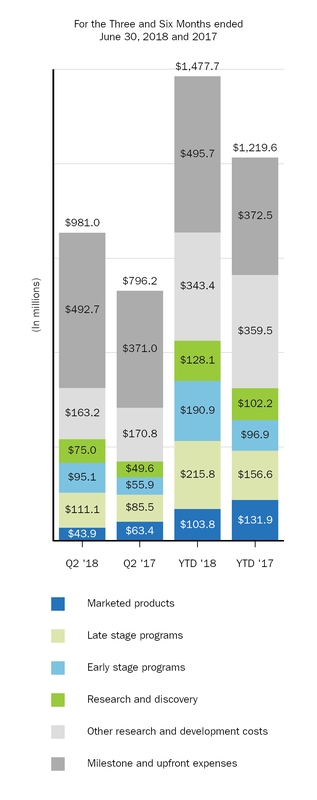
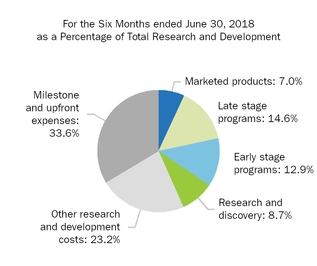
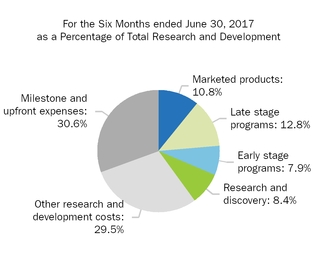
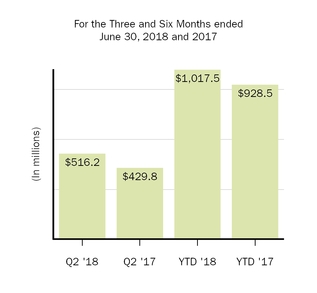
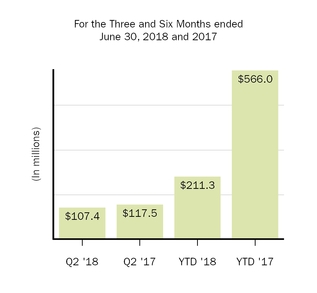

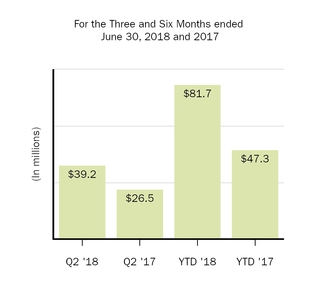
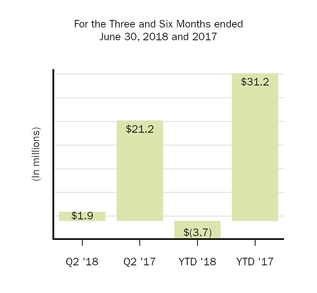
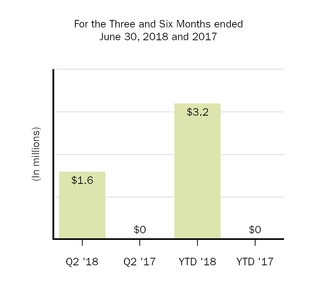
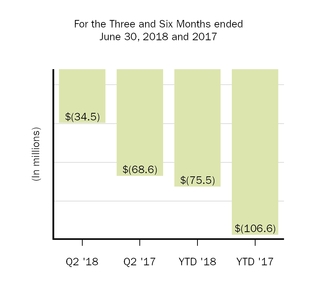
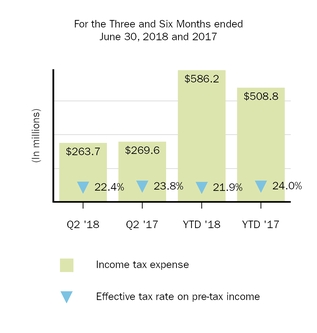
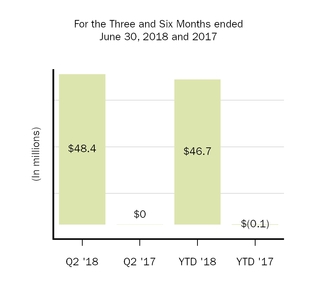
|
(In millions, except percentages)
|
As of
June 30, 2018 |
As of
December 31, 2017 |
Change %
|
|||||||
|
Financial assets:
|
||||||||||
|
Cash and cash equivalents
|
$
|
1,250.2
|
|
$
|
1,573.8
|
|
(20.6
|
)%
|
||
|
Marketable securities — current
|
1,974.0
|
|
2,115.2
|
|
(6.7
|
)%
|
||||
|
Marketable securities — non-current
|
1,160.2
|
|
3,057.3
|
|
(62.1
|
)%
|
||||
|
Total cash, cash equivalents and marketable securities
|
$
|
4,384.4
|
|
$
|
6,746.3
|
|
(35.0
|
)%
|
||
|
Borrowings:
|
||||||||||
|
Current portion of notes payable
|
$
|
—
|
|
$
|
3.2
|
|
(100.0
|
)%
|
||
|
Notes payable
|
5,928.4
|
|
5,935.0
|
|
(0.1
|
)%
|
||||
|
Total borrowings
|
$
|
5,928.4
|
|
$
|
5,938.2
|
|
(0.2
|
)%
|
||
|
Working capital:
|
||||||||||
|
Current assets
|
$
|
7,432.1
|
|
$
|
7,873.3
|
|
(5.6
|
)%
|
||
|
Current liabilities
|
(3,152.4
|
)
|
(3,368.2
|
)
|
(6.4
|
)%
|
||||
|
Total working capital
|
$
|
4,279.7
|
|
$
|
4,505.1
|
|
(5.0
|
)%
|
||
|
•
|
$2.6 billion
in net cash flows provided by operating activities, net of
;
|
|
◦
|
$375.0 million upfront payment
made to Ionis upon the closing of our new ten-year collaboration and
a $162.1 million charge reflecting the premium paid for the purchase of Ionis' common stock
; and
|
|
◦
|
$472.0 million in total payments for income taxes;
|
|
•
|
$3.0 billion used for share repurchases;
|
|
•
|
$900.0 million
in contingent payments made to former shareholders of Fumapharm AG and holders of their rights;
|
|
•
|
$462.9 million payment made to Ionis reflecting the fair value of the common stock purchased upon the closing of our new ten-year collaboration;
|
|
•
|
$381.5 million
used for purchases of property, plant and equipment; and
|
|
•
|
$85.0 million in upfront payments made for the BIIB100 and BIIB104 acquisitions.
|
|
•
|
$1.4 billion
in net cash flows provided by operating activities, net of:
|
|
◦
|
$654.8 million in total payments for income taxes;
|
|
◦
|
$454.8 million payment made to Forward Pharma for the litigation settlement charge that was accrued as of December 31, 2016; and
|
|
◦
|
$300.0 million upfront payment to BMS;
|
|
•
|
$1.4 billion
used for share repurchases;
|
|
•
|
$795.2 million payment made to Forward Pharma to license Forward Pharma's intellectual property, including Forward Pharma's intellectual property related to TECFIDERA;
|
|
•
|
$600.0 million
in contingent payments made to former shareholders of Fumapharm AG and holders of their rights;
|
|
•
|
$407.7 million
used for purchases of property, plant and equipment;
|
|
•
|
$302.7 million net cash contribution made in connection with the spin-off of our hemophilia business; and
|
|
•
|
$180.0 million in upfront and milestone payments made to Remedy and Ionis.
|
|
•
|
$1.5 billion aggregate principal amount of 2.90% Senior Notes due September 15, 2020;
|
|
•
|
$1.0 billion aggregate principal amount of 3.625% Senior Notes due September 15, 2022;
|
|
•
|
$1.75 billion aggregate principal amount of 4.05% Senior Notes due September 15, 2025; and
|
|
•
|
$1.75 billion aggregate principal amount of 5.20% Senior Notes due September 15, 2045.
|
|
|
For the Six Months
Ended June 30, |
|||||||||
|
(In millions, except percentages)
|
2018
|
2017
|
% Change
|
|||||||
|
Net cash flows provided by operating activities
|
$
|
2,556.4
|
|
$
|
1,404.4
|
|
82.0
|
%
|
||
|
Net cash flows provided by (used in) investing activities
|
$
|
198.1
|
|
$
|
(946.7
|
)
|
(120.9
|
)%
|
||
|
Net cash flows used in financing activities
|
$
|
(3,060.1
|
)
|
$
|
(1,652.4
|
)
|
85.2
|
%
|
||
|
•
|
Non-cash operating items such as depreciation and amortization, impairment charges, acquired in-process research and development and share-based compensation;
|
|
•
|
Changes in operating assets and liabilities which reflect timing differences between the receipt and payment of cash associated with transactions and when they are recognized in results of operations; and
|
|
•
|
Changes associated with the fair value of contingent payments associated with our acquisitions of businesses and payments related to collaborations.
|
|
•
|
safety or efficacy issues;
|
|
•
|
the introduction or greater acceptance of competing products, including lower-priced competing products;
|
|
•
|
limitations and additional pressures on product pricing or price increases, including those resulting from governmental or regulatory requirements, increased competition or changes in, or implementation of, reimbursement policies and practices of payors and other third parties; or
|
|
•
|
adverse legal, administrative, regulatory or legislative developments.
|
|
•
|
our limited marketing experience within the SMA market, which may impact our ability to develop relationships with the associated medical and scientific community;
|
|
•
|
the lack of readiness of healthcare providers to treat patients with SMA;
|
|
•
|
the effectiveness of our commercial strategy for marketing SPINRAZA; and
|
|
•
|
our ability to maintain a positive reputation among patients, healthcare providers and others in the SMA community, which may be impacted by pricing and reimbursement decisions relating to SPINRAZA.
|
|
•
|
the introduction of more efficacious, safer, less expensive or more convenient alternatives to our MS products, including our own products and products of our collaborators;
|
|
•
|
the introduction of lower-cost biosimilars, follow-on products, generic versions of branded MS products, prodrugs or products approved under other alternative regulatory pathways, or the off-label use by physicians of therapies indicated for other conditions to treat MS patients;
|
|
•
|
patient dynamics, including the size of the patient population and our ability to attract new patients to our therapies;
|
|
•
|
damage to physician and patient confidence in any of our MS products or to our sales and reputation as a result of label changes or adverse experiences or events that may occur with patients treated with our MS products;
|
|
•
|
inability to obtain appropriate pricing and reimbursement for our MS products compared to our competitors in key international markets; or
|
|
•
|
our ability to obtain and maintain patent, data or market exclusivity for our MS products.
|
|
•
|
changes in, and implementation of, federal, state or foreign government regulations or private third-party payors' reimbursement policies;
|
|
•
|
pressure by employers on private health insurance plans to reduce costs;
|
|
•
|
consolidation and increasing assertiveness of payors, including managed care organizations, health insurers, pharmacy benefit managers, government health administration authorities, private health insurers and other organizations, seeking price discounts or rebates in connection with the placement of our products on their formularies and, in some cases, the imposition of restrictions on access or coverage of particular drugs or pricing determined based on perceived value; and
|
|
•
|
our value-based contracting pilot program pursuant to which we aim to tie the pricing of our products to their clinical values by either aligning price to patient outcomes or adjusting price for patients who discontinue therapy for any reason including efficacy or tolerability concerns.
|
|
•
|
Risk of Product Loss.
The manufacturing process for our products is extremely susceptible to product loss due to contamination, oxidation, equipment failure or improper installation or operation of equipment or vendor or operator error. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects and other supply disruptions. If microbial, viral or other contaminations are discovered in our products or manufacturing facilities, we may need to close our manufacturing facilities for an extended period of time to investigate and remediate the contaminant.
|
|
•
|
Risks of Reliance on Third Parties and Single Source Providers.
We rely on third-party suppliers and manufacturers for many aspects of our manufacturing process for our products and product candidates. In some cases, due to the unique manner in which our products are manufactured, we rely on single source providers of raw materials and manufacturing supplies. These third parties are independent entities subject to their own unique operational and financial risks that are outside of our control. These third parties may not perform their obligations in a timely and cost-effective manner or in compliance with applicable regulations, and they may be unable or unwilling to increase production capacity commensurate with demand for our existing or future products. Finding alternative providers could take a significant amount of time and involve significant expense due to the specialized nature of the services and the need to obtain regulatory approval of any significant changes to our suppliers or manufacturing methods. We cannot be certain that we could reach agreement with alternative providers or that the FDA or other regulatory authorities would approve our use of such alternatives.
|
|
•
|
Global Bulk Supply Risks.
We rely on our principal manufacturing facilities for the production of drug substance for our large molecule products and product candidates. Our global bulk supply of these products and product candidates depends on the uninterrupted and efficient operation of these facilities, which could be adversely affected by equipment failures, labor shortages, natural disasters, power failures and numerous other factors.
|
|
•
|
Risks Relating to Compliance with current Good Manufacturing Practices (cGMP).
We and our third-party providers are generally required to maintain compliance with cGMP and other stringent requirements and are subject to inspections by the FDA and comparable agencies in other jurisdictions to confirm such compliance. Any delay, interruption or other issues that arise in the manufacture, fill-finish, packaging or storage of our products as a result of a failure of our facilities or the facilities or operations of third parties to pass any regulatory agency inspection could significantly impair our ability to develop and commercialize our products. Significant noncompliance could also result in the imposition of monetary penalties or other civil or criminal sanctions and damage our reputation.
|
|
•
|
we may be unable to control the resources our collaborators or third parties devote to our programs or products;
|
|
•
|
disputes may arise under the agreement, including with respect to the achievement and payment of milestones or ownership of rights to technology developed with our collaborators or other third parties, and the underlying contract with our collaborators or other third parties may fail to provide significant protection or may fail to be effectively enforced if the collaborators or third parties fail to perform;
|
|
•
|
the interests of our collaborators or third parties may not always be aligned with our interests, and such parties may not pursue regulatory approvals or market a product in the same manner or to the same extent that we would, which could adversely affect our revenues;
|
|
•
|
third-party relationships and collaborations often require the parties to cooperate, and failure to do so effectively could adversely affect product sales, or the clinical development or regulatory approvals of products under joint control or could result in termination of the research, development or commercialization of product candidates or result in litigation or arbitration;
|
|
•
|
any failure on the part of our collaborators or other third parties to comply with applicable laws and regulatory requirements in the marketing, sale and maintenance of the marketing authorization of our products or to fulfill any responsibilities our collaborators or other third parties may have to protect and enforce any intellectual property rights underlying our products could have an adverse effect on our revenues as well as involve us in possible legal proceedings; and
|
|
•
|
any improper conduct or actions on the part of our collaborators or other third parties could subject us to civil or criminal investigations, and monetary and injunctive penalties, and could adversely impact our ability to conduct business, our operating results and reputation.
|
|
•
|
new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or judicial decisions, related to health care availability, pricing or marketing practices, compliance with wage and hour laws and other employment practices, method of delivery, payment for health care products and services, compliance with health information and data privacy and security laws and regulations, tracking and reporting payments and other transfers of value made to physicians and teaching hospitals, extensive anti-bribery and anti-corruption prohibitions, product serialization and labeling requirements and used product take-back requirements;
|
|
•
|
changes in the FDA and foreign regulatory approval processes that may delay or prevent the approval of new products and result in lost market opportunity;
|
|
•
|
the hiring freeze implemented by the federal government in 2017, including at the FDA, could impact the review and potential approval of new products, which may adversely affect our business and financial condition;
|
|
•
|
requirements that provide for increased transparency of clinical trial results and quality data, such as the EMA's clinical transparency policy, which could impact our ability to protect trade secrets and competitively-sensitive information contained in approval applications or could be misinterpreted leading to reputational damage, misperception or legal action, which could harm our business; and
|
|
•
|
changes in FDA and foreign regulations that may require additional safety monitoring, labeling changes, restrictions on product distribution or use or other measures after the introduction of our products to market, which could increase our costs of doing business, adversely affect the future permitted uses of approved products or otherwise adversely affect the market for our products.
|
|
•
|
the inability to obtain necessary foreign regulatory or pricing approvals of products in a timely manner;
|
|
•
|
uncertainties regarding the collectability of accounts receivable;
|
|
•
|
fluctuations in foreign currency exchange rates that may adversely impact our revenues and net income;
|
|
•
|
difficulties in staffing and managing international operations;
|
|
•
|
the imposition of governmental controls;
|
|
•
|
less favorable intellectual property or other applicable laws;
|
|
•
|
increasingly complex standards for complying with foreign laws and regulations that may differ substantially from country to country and may conflict with corresponding U.S. laws and regulations;
|
|
•
|
the far-reaching anti-bribery and anti-corruption legislation in the U.K., including the U.K. Bribery Act 2010, and elsewhere and escalation of investigations and prosecutions pursuant to such laws;
|
|
•
|
the effects of the implementation of the U.K.'s decision to voluntarily depart from the E.U., known as Brexit;
|
|
•
|
compliance with complex import and export control laws;
|
|
•
|
restrictions on direct investments by foreign entities and trade restrictions;
|
|
•
|
greater political or economic instability; and
|
|
•
|
changes in tax laws and tariffs.
|
|
•
|
the cost of restructurings or other initiatives to streamline our operations and reallocate resources;
|
|
•
|
impairments with respect to investments, fixed assets and long-lived assets, including in-process R&D and other intangible assets;
|
|
•
|
inventory write-downs for failed quality specifications, charges for excess or obsolete inventory and charges for inventory write downs relating to product suspensions, expirations or recalls;
|
|
•
|
changes in the fair value of contingent consideration;
|
|
•
|
bad debt expenses and increased bad debt reserves;
|
|
•
|
outcomes of litigation and other legal or administrative proceedings, regulatory matters and tax matters;
|
|
•
|
milestone payments under license and collaboration agreements; and
|
|
•
|
payments in connection with acquisitions and other business development activities.
|
|
•
|
Reliance on Third Parties.
We are dependent on the efforts of Samsung Bioepis and other third parties over whom we have limited or no control in the development and manufacturing of biosimilars products. If Samsung Bioepis or such other third parties fail to perform successfully, we may not realize the anticipated benefits of our investment in Samsung Bioepis;
|
|
•
|
Regulatory Compliance.
Biosimilar products may face regulatory hurdles or delays due to the evolving and uncertain regulatory and commercial pathway of biosimilars products in certain jurisdictions;
|
|
•
|
Intellectual Property and Regulatory Challenges.
Biosimilar products may face extensive patent clearances, patent infringement litigation, injunctions or regulatory challenges, which could prevent the commercial launch of a product or delay it for many years;
|
|
•
|
Failure to Gain Market and Patient Acceptance.
Market success of biosimilar products will be adversely affected if patients, physicians and/or payors do not accept biosimilar products as safe and efficacious products offering a more competitive price or other benefit over existing therapies;
|
|
•
|
Ability to Provide Adequate Supply.
Manufacturing biosimilars is complex. If we encounter any manufacturing or supply chain difficulties, we may be unable to meet higher than anticipated demand; and
|
|
•
|
Competitive Challenges.
Biosimilar products face significant competition, including from innovator products and from biosimilar products offered by other companies. In some jurisdictions, local tendering processes may restrict biosimilar products from being marketed and sold in those jurisdictions. The number of competitors in a jurisdiction, the timing of approval and the ability to market biosimilar products successfully in a timely and cost-effective matter are additional factors that may impact our success and/or the success of Samsung Bioepis in this business area.
|
|
•
|
increase our vulnerability to general adverse economic and industry conditions;
|
|
•
|
limit our ability to access capital markets and incur additional debt in the future;
|
|
•
|
require us to dedicate a substantial portion of our cash flow from operations to payments on our indebtedness, thereby reducing the availability of our cash flow for other purposes, including business development efforts, research and development and mergers and acquisitions; and
|
|
•
|
limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate, thereby placing us at a competitive disadvantage compared to our competitors that have less debt.
|
|
Period
|
Total Number of
Shares Purchased
(#)
|
Average Price
Paid per Share
($)
|
Total Number of
Shares Purchased
as Part of Publicly
Announced Programs
(#)
|
Maximum
Approximate Dollar Value
of Shares That May Yet Be
Purchased Under
Our Programs ($ in millions)
|
|||||||||
|
April 2018
|
658,041
|
|
$
|
272.25
|
|
658,041
|
|
$
|
2,570.9
|
|
|||
|
May 2018
|
5,327,384
|
|
$
|
277.48
|
|
5,327,384
|
|
$
|
1,092.6
|
|
|||
|
June 2018
|
3,642,173
|
|
$
|
299.99
|
|
3,642,173
|
|
$
|
—
|
|
|||
|
Total
|
9,627,598
|
|
$
|
285.64
|
|
||||||||
|
Exhibit
Number
|
|
Description of Exhibit
|
|
31.1+
|
|
|
|
31.2+
|
|
|
|
32.1++
|
|
|
|
101++
|
|
The following materials from Biogen Inc.’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2018, formatted in XBRL (Extensible Business Reporting Language): (i) the Condensed Consolidated Statements of Income, (ii) the Condensed Consolidated Statements of Comprehensive Income, (iii) the Condensed Consolidated Balance Sheets, (iv) the Condensed Consolidated Statements of Cash Flows, and (v) Notes to Condensed Consolidated Financial Statements.
|
|
BIOGEN INC.
|
|
/s/ Jeffrey D. Capello
|
|
Jeffrey D. Capello
|
|
Executive Vice President,
|
|
Chief Financial Officer and
|
|
Chief Accounting Officer
|
|
(principal financial officer)
|