|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Delaware
|
|
22-0790350
|
|
(State or other jurisdiction of
incorporation or organization)
|
|
(
I.R.S
Employer
Identification No.)
|
|
Title of each class
|
|
Name of each exchange on which registered
|
|
Common Stock, $0.10 Par Value
|
|
New York Stock Exchange
|
|
1.000% Notes due 2025
|
New York Stock Exchange
|
|
|
1.750% Notes due 2035
|
New York Stock Exchange
|
|
|
Title of each class
|
|
$2 Convertible Preferred Stock, $1 Par Value
|
|
Large accelerated filer
x
|
Accelerated filer
¨
|
Non-accelerated filer
¨
|
Smaller reporting company
¨
|
Emerging growth company
¨
|
||||
|
*
|
Indicates brand names of products which are trademarks not owned by BMS. Specific trademark ownership information is included in the Exhibit Index at the end of this
2018
Form 10-K.
|
|
Item 1.
|
BUSINESS.
|
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
United States
|
56
|
%
|
55
|
%
|
55
|
%
|
||||||
|
Europe
|
25
|
%
|
24
|
%
|
22
|
%
|
||||||
|
Rest of the World
|
19
|
%
|
21
|
%
|
23
|
%
|
||||||
|
Total Revenues
|
$
|
22,561
|
|
$
|
20,776
|
|
$
|
19,427
|
|
|||
|
Opdivo
|
Opdivo
(nivolumab), a biological product, is a fully human monoclonal antibody that binds to the PD-1 on T and NKT cells.
Opdivo
has received approvals for several anti-cancer indications including bladder, blood, colon, head and neck, kidney, liver, lung, melanoma and stomach. The
Opdivo
+
Yervoy
regimen also is approved in multiple markets for the treatment of melanoma, RCC, and CRC. There are several ongoing potentially registrational studies for
Opdivo
across other tumor types and disease areas, in monotherapy and in combination with
Yervoy
and various anti-cancer agents.
|
|
Eliquis
|
Eliquis
(apixaban) is an oral Factor Xa inhibitor, targeted at stroke prevention in adult patients with NVAF and the prevention and treatment of VTE disorders.
|
|
Orencia
|
Orencia
(abatacept), a biological product, is a fusion protein indicated for adult patients with moderately to severely active RA and PSA and is also indicated for reducing signs and symptoms in certain pediatric patients with moderately to severely active polyarticular JIA.
|
|
Sprycel
|
Sprycel
(dasatinib) is an oral inhibitor of multiple tyrosine kinase indicated for the first-line treatment of patients with Philadelphia chromosome-positive CML in chronic phase, the treatment of adults with chronic, accelerated, or myeloid or lymphoid blast phase CML with resistance or intolerance to prior therapy, including
Gleevec*
(imatinib mesylate) and the treatment of children and adolescents aged 1 year to 18 years with chronic phase Philadelphia chromosome-positive CML.
|
|
Yervoy
|
Yervoy
(ipilimumab), a biological product, is a monoclonal antibody for the treatment of patients with unresectable or metastatic melanoma.
|
|
Empliciti
|
Empliciti
(elotuzumab), a biological product, is a humanized monoclonal antibody for the treatment of multiple myeloma.
|
|
Baraclude
|
Baraclude
(entecavir) is an oral antiviral agent for the treatment of chronic hepatitis B.
|
|
Reyataz Franchise
|
The
Reyataz (
atazanavir
sulfate
) Franchise
includes
Reyataz
- a protease inhibitor for the treatment of HIV and
Evotaz
(atazanavir 300 mg and cobicistat 150 mg) - a combination therapy containing
Reyataz
and
Tybost
* (cobicistat).
|
|
Sustiva Franchise
|
The
Sustiva (
efavirenz
) Franchise
is a non-nucleoside reverse transcriptase inhibitor for the treatment of HIV, which includes
Sustiva
, an antiretroviral drug, and bulk efavirenz, which is also included in the combination therapy,
Atripla*
.
|
|
Hepatitis C Franchise
|
Daklinza
(daclatasvir) is an NS5A replication complex inhibitor.
|
|
|
Estimated LOE
|
||||
|
U.S.
|
EU
(a)
|
Japan
|
|||
|
Prioritized Brands
|
|||||
|
Opdivo (nivolumab)
|
2028
|
2030
|
2031
|
||
|
Eliquis (apixaban)
|
2026
|
2026
|
2026
|
||
|
Orencia (abatacept)
(b)
|
2021
|
2021
|
2019
|
||
|
Sprycel (dasatinib)
|
2020
(c)
|
^^
|
2021
|
||
|
Yervoy (ipilimumab)
|
2025
|
2026
|
2025
|
||
|
Empliciti (elotuzumab)
|
2029
|
2029
|
2029
|
||
|
Established Brands
|
|||||
|
Reyataz (atazanavir sulfate) Franchise
|
Expired
|
2019
|
2019
|
||
|
Hepatitis C Franchise
(d)
|
2028
|
2027
|
2028
|
||
|
^^
|
In December 2018, the EPO's Opposition Division upheld the validity of the patent directed to the use of dasatinib to treat CML, which expires in 2024. Refer to “Item 8. Financial Statements and Supplementary Data—Note
18
. Legal Proceedings and Contingencies” for more information.
|
|
(a)
|
In EU countries where there is no granted PTR, the LOE is based on the COM patent or RDP expiry which is 2026 for
Opdivo
, 2022 for
Eliquis
, 2020 for
Yervoy,
and 2026 for
Empliciti
.
|
|
(b)
|
BMS is not aware of an
Orencia
biosimilar on the market in the U.S., EU or Japan. For the U.S. and the EU, estimated LOE dates are based on method of use patents that expires in 2021. Formulation and additional patents expire in 2026 and beyond.
|
|
(c)
|
In 2013, BMS entered into a settlement agreement with Apotex regarding a patent infringement suit covering the monohydrate form of dasatinib whereby Apotex can launch its generic dasatinib monohydrate aNDA product in September 2024, or earlier in certain circumstances.
|
|
(d)
|
Hepatitis C Franchise relates to products containing daclatasvir. The LOE dates in the U.S. and EU do not reflect pending PTRs.
|
|
PHASE I
|
PHASE II
|
PHASE III
|
APPROVED INDICATIONS
|
|||||
|
OPDIVO
ª
--Solid Tumors & Hematologic Malignancies
OPDIVO
ª
+
YERVOY
ª
--Solid Tumors
Relatlimab
ª
^
--Solid Tumors & Hematologic Malignancies
NLRP3 Agonist^
--Solid Tumors
Anti-TIM-3^
--Solid Tumors
HuMax-IL8^
--Solid Tumors
EP4
ª
Antagonist^
--Solid Tumors
CD80/αCD3 Oncolytic Virus^
--Solid Tumors
Anti-CTLA-4 Probody^
--Solid Tumors
Anti-ICOS^
--Solid Tumors
Anti-CTLA-4 NF^
--Solid Tumors
Anti-TIGIT^
--Solid Tumors
Anti-CD73^
--Solid Tumors
BET Inhibitor
--Solid Tumors
Ulocuplumab
--Hematologic Malignancies
|
OPDIVO
ª
--1L CRC
--Non-Hodgkin Lymphoma (Diffuse Large B-cell Lymphoma)
--Non-Hodgkin Lymphoma (Follicular Lymphoma)
--Ovarian
#
--Pan Tumor TMB High
--Pediatric
--Primary Testicular Lymphoma
OPDIVO
ª
^
--Solid Tumors
OPDIVO
ª
+ YERVOY
ª
--Prostate
OPDIVO
ª
+ YERVOY
ª
^
--Solid Tumors
Relatlimab
ª
+ OPDIVO
ª
^
--Solid Tumors
IDO
+ OPDIVO
ª
^
--Solid Tumors
NKTR-214
ª
+ OPDIVO
ª
^
--Solid Tumors
CCR2/5 Dual Antagonist^
--Solid Tumors
Cabiralizumab
ª
^
--Solid Tumors
|
OPDIVO
ª
--1L Glioblastoma
--1L HCC
--1L Head & Neck
--1L Head & Neck Locally Advanced
--2L Esophageal
--Adjuvant Bladder
--Adjuvant Esophageal/Gastroesophageal
--Adjuvant Gastric
--Adjuvant HCC
--Adjuvant RCC
--NSCLC Neoadjuvant
--Refractory Hodgkin Lymphoma
--Unresectable NSCLC
OPDIVO
ª
+ YERVOY
ª
--1L Bladder
--1L Esophageal
--1L Gastric
--1L Head & Neck
--1L Mesothelioma
--1L NSCLC
--1L SCLC
--Adjuvant Melanoma
--Adjuvant RCC
--NSCLC EGFR mutant
OPDIVO
ª
+ YERVOY
ª
+ Cabozantinib
ª
--Metastatic RCC
OPDIVO
ª
+ EMPLICITI
ª
--Multiple Myeloma
OPDIVO
ª
+
IDO
--1L Metastatic Melanoma
--Neoadjuvant Muscle-Invasive Bladder Cancer
OPDIVO
ª
+ NKTR-214
ª
--1L Melanoma
--1L RCC
#
Relatlimab
ª
+ OPDIVO
ª
--1L Melanoma
EMPLICITI
ª
--1L Multiple Myeloma
Revlimid*
Combo
|
OPDIVO
ª
--1L BRAF wild-type Metastatic Melanoma
--Adjuvant Melanoma
--Advanced Hodgkin Lymphoma
--Melanoma across BRAF status
--Mesothelioma
--Previously treated advanced RCC
--Previously treated Gastric cancer (JPN)
--Previously treated HCC
--Previously treated Metastatic Head & Neck
--Previously treated Metastatic Melanoma
--Previously treated Metastatic MSI-High CRC
--Previously treated Metastatic Non-squamous NSCLC
--Previously treated Metastatic Squamous NSCLC
--Previously treated Metastatic SCLC
--Previously treated Metastatic Urothelial
OPDIVO
ª
+ YERVOY
ª
--1L RCC
--BRAF wild-type Metastatic Melanoma
--Melanoma across BRAF status
--Previously treated Metastatic MSI-High CRC
YERVOY
ª
--Adjuvant Melanoma
--Adolescent Metastatic Melanoma
--Metastatic Melanoma
EMPLICITI
ª
--Relapsed/Refractory Multiple Myeloma
Pomalyst*
Combo
--Relapsed/Refractory Multiple Myeloma
Revlimid
* Combo
SPRYCEL
ª
--1L CML
--Pediatric
--Refractory CML
|
|||||
|
Note: Above pipeline excludes clinical collaborations
|
||||||||
|
ª
Development Partnership:
OPDIVO, YERVOY
, Relatlimab, EP4:
Ono (our collaboration with Ono also includes other early stage compounds);
EMPLICITI
:
AbbVie;
NKTR-214:
Nektar;
Cabiralizumab:
Five Prime;
Cabozantinib:
Exelixis
|
||||||||
|
^ Trial(s) exploring various combinations
|
||||||||
|
# Partner-run study
|
||||||||
|
PHASE I
|
PHASE II
|
PHASE III
|
APPROVED INDICATIONS
|
|||||
|
RORγT
--Autoimmune Disease
S1P1 Agonist
--Autoimmune Disease
BTK Max
--RA
TYK2 Inhibitor (2)
--Autoimmune Disease
TLR 7/8 Antagonist
--Autoimmune Disease
|
TYK2 Inhibitor (1)
--Autoimmune Diseases
BTK Inhibitor
--RA
|
ORENCIA
--Idiopathic Inflammatory Myopathy
--Sjögren’s Disease
TYK2 Inhibitor (1)
--Psoriasis
NULOJIX
--Switch from Calcineurin Inhibitor Renal Transplant
|
ORENCIA
--Early RA
--JIA Intravenous
--JIA Subcutaneous
--Psoriatic Arthritis
--RA Auto injector
--RA Intravenous
--RA Subcutaneous
NULOJIX
--De Novo Renal Transplant
|
|||||
|
PHASE I
|
PHASE II
|
PHASE III
|
APPROVED INDICATIONS
|
|||||
|
FPR-2 Agonist
--Heart Failure
APJ Agonist
--Heart Failure
|
Nitroxyl Donor
--Heart Failure
Factor XIa Inhibitor
ª
--Thrombosis
ELIQUIS
ª
--Pediatric Heart Disease
|
ELIQUIS
ª
--Pediatric Venous Thromboembolism Prevention
|
ELIQUIS
ª
--Stroke Prevention in Atrial Fibrillation
--Venous Thromboembolism Prevention Orthopedic Surgery
--Venous Thromboembolism Treatment
|
|||||
|
PHASE I
|
PHASE II
|
|||||||
|
LPA1 Antagonist
--Fibrosis
|
HSP47
ª
--Fibrosis
Pegbelfermin (PEG-FGF21)
--Non-alcoholic Steatohepatitis
|
|||||||
|
Note: Above pipeline excludes clinical collaborations
|
||||||||
|
ª
Development Partnership:
ELIQUIS:
Pfizer;
Factor XIa Inhibitor:
Janssen;
HSP47:
Nitto Denko
|
||||||||
|
Tumor
|
Study Details
|
Tumor
|
Study Details
|
||
|
Non-Small Cell Lung Cancer
|
CM-227 - Opdivo + Yervoy (1
st
line) Part 1a
|
Bladder Cancer
|
CM-901 - Opdivo + Chemo (1
st
line)
|
||
|
CM-227 - Opdivo + Yervoy (1
st
line) Part 1b
|
CM-274 - Opdivo (Adjuvant)
|
||||
|
CM-227 - Opdivo + Chemo (1
st
line) Part 2
|
Esophageal Cancer
|
CM-648 - Opdivo + Yervoy +/- Chemo (1
st
line)
|
|||
|
CM-9LA - Opdivo + Yervoy + Chemo (1
st
line)
|
CM-577 - Opdivo (Adjuvant)
|
||||
|
CM-722 - Opdivo + Yervoy (EGFR T790M Mutant)
|
Renal Cancer
|
CM-9ER - Opdivo + Chemo (1
st
line)
|
|||
|
CM-816 - Opdivo + Chemo (Neoadjuvant)
|
Glioblastoma
|
CM-548 - Opdivo + Chemo (1
st
line Methylated)
|
|||
|
Hepatocellular Carcinoma
|
CM-459 - Opdivo (1
st
line)
|
CM-498 - Opdivo + Chemo (1
st
line Un-methylated)
|
|||
|
Head and Neck Cancer
|
CM-651 - Opdivo + Yervoy (1
st
line)
|
Mesothelioma
|
CM-743 - Opdivo + Yervoy (1
st
line)
|
||
|
CM-714 - Opdivo + Yervoy (1
st
line)
|
Melanoma
|
CM-915 - Opdivo +/- Yervoy (Adjuvant)
|
|||
|
Phase II
|
Phase III
|
|
Item 1A.
|
RISK FACTORS.
|
|
Item 1B.
|
UNRESOLVED STAFF COMMENTS.
|
|
Item 2.
|
PROPERTIES.
|
|
Manufacturing
|
R&D
|
||||
|
United States
|
4
|
|
5
|
|
|
|
Europe
|
3
|
|
2
|
|
|
|
Total
|
7
|
|
7
|
|
|
|
Item 3.
|
LEGAL PROCEEDINGS.
|
|
Item 4.
|
MINE SAFETY DISCLOSURES.
|
|
Name and Current Position
|
Age
|
Employment History for the Past 5 Years
|
|||
|
Giovanni Caforio, M.D.
Chairman of the Board and Chief Executive Officer
Member of the Leadership Team
|
54
|
|
2011 to 2013 – President, U.S. Pharmaceuticals
2013 to 2014 – Executive Vice President and Chief Commercial Officer
2014 to 2015 – Chief Operating Officer and Director of the Company
2015 to 2017 – Chief Executive Officer and Director of the Company
2017 to present – Chairman of the Board and Chief Executive Officer
|
||
|
Charles A. Bancroft
Chief Financial Officer and Executive Vice President, Global Business Operations
Member of the Leadership Team
|
59
|
|
2011 to 2016 – Chief Financial Officer and Executive Vice President, Global Services
2016 to present – Chief Financial Officer and Executive Vice President, Global Business Operations |
||
|
Paul Biondi
Senior Vice President, Strategy and Business Development
Member of the Leadership Team
|
49
|
|
2010 to 2015 – Senior Vice President, R&D Operations
2015 to 2018 – Head of Business Development
2018 to present – Senior Vice President and Head of Strategy & Business Development
|
||
|
Christopher Boerner, Ph.D.
Executive Vice President, Chief Commercial Officer Member of the Leadership Team |
48
|
|
2012 to 2014 – Senior Vice President, Commercial, Seattle Genetics
2014 to 2015 – Executive Vice President, Seattle Genetics
2015 to 2017 – President and Head of U.S. Commercial
2017 to 2018 – President and Head, International Markets
2018 to present – Executive Vice President and Chief Commercial Officer
|
||
|
Adam Dubow
Senior Vice President, Chief Compliance and Ethics Officer Member of the Leadership Team |
52
|
|
2013 to 2015 – Vice President and Assistant General Counsel, China, Japan and Intercon Region and EMAC Region
2015 to 2018 – Vice President and Associate General Counsel, Research and Development
2018 to present – Senior Vice President, Chief Compliance and Ethics Officer
|
||
|
John E. Elicker
Senior Vice President, Corporate Affairs and Investor Relations
Member of the Leadership Team
|
59
|
|
2012 to 2017 – Senior Vice President, Public Affairs and Investor Relations
2017 to present – Senior Vice President, Corporate Affairs and Investor Relations
|
||
|
Ann Powell Judge
Senior Vice President, Chief Human Resources Officer
Member of the Leadership Team
|
53
|
|
2009 to 2013 – Chief Human Resources Officer, Shire Pharmaceuticals
2013 to 2016 – Senior Vice President, Global Human Resources
2016 to present – Senior Vice President, Chief Human Resources Officer
|
||
|
Sandra Leung
Executive Vice President, General Counsel
Member of the Leadership Team
|
58
|
|
2007 to 2014 – General Counsel and Corporate Secretary
2014 to 2015 – Executive Vice President, General Counsel and Corporate Secretary
2015 to present – Executive Vice President, General Counsel
|
||
|
Thomas J. Lynch., M.D.
Executive Vice President and Chief Scientific Officer
Member of the Leadership Team
|
58
|
|
2017 to present – Executive Vice President and Chief Scientific Officer
|
||
|
Karen Santiago
Senior Vice President and Corporate Controller |
48
|
|
2012 to 2015 – Vice President Finance, Global Manufacturing and Supply
2015 to 2016 – Vice President Finance, U.S. Commercial and Global Capability Hub
2016 to 2018 – Lead, Enabling Functions and Finance Transformation
2018 to present – Senior Vice President and Corporate Controller
|
||
|
Louis S. Schmukler
Senior Vice President and President, Global Product Development and Supply
Member of the Leadership Team
|
63
|
|
2011 to 2017 – President, Global Product Development and Supply
2017 to present – Senior Vice President and President, Global Product Development and Supply
|
||
|
Paul von Autenried
Senior Vice President, Chief Information Officer
Member of the Leadership Team
|
57
|
|
2012 to 2016 – Senior Vice President, Enterprise Services and Chief Information Officer
2016 to present – Senior Vice President, Chief Information Officer
|
||
|
Item 5.
|
MARKET FOR THE REGISTRANT’S COMMON STOCK AND OTHER STOCKHOLDER MATTERS.
|
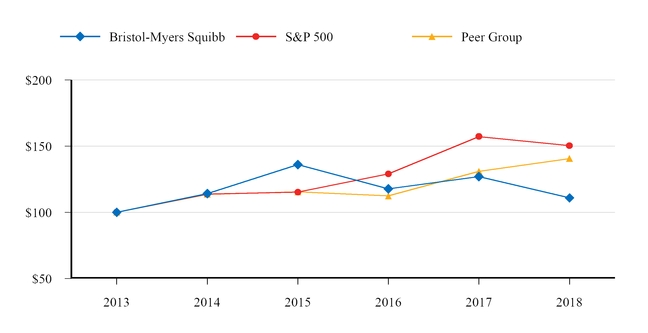
|
2013
|
2014
|
2015
|
2016
|
2017
|
2018
|
||||||||||||||||||
|
Bristol-Myers Squibb
|
$
|
100.00
|
|
$
|
114.06
|
|
$
|
136.04
|
|
$
|
117.73
|
|
$
|
126.95
|
|
$
|
110.82
|
|
|||||
|
S&P 500
|
100.00
|
|
113.69
|
|
115.26
|
|
129.05
|
|
157.22
|
|
150.33
|
|
|||||||||||
|
Peer Group
|
100.00
|
|
113.55
|
|
115.40
|
|
112.35
|
|
130.89
|
|
140.60
|
|
|||||||||||
|
Period
|
Total Number of
Shares Purchased
(a)
|
Average Price
Paid
per Share
(a)
|
Total Number of Shares
Purchased as Part of
Publicly Announced Programs
(b)
|
Approximate Dollar Value
of Shares that May Yet Be
Purchased Under the
Programs
(b)
|
|||||||||
|
Dollars in Millions, Except Per Share Data
|
|
|
|
|
|||||||||
|
October 1 to 31, 2018
|
7,987
|
|
$
|
62.01
|
|
—
|
|
$
|
1,348
|
|
|||
|
November 1 to 30, 2018
|
13,978
|
|
52.52
|
|
—
|
|
1,348
|
|
|||||
|
December 1 to 31, 2018
|
16,110
|
|
53.02
|
|
—
|
|
1,348
|
|
|||||
|
Three months ended December 31, 2018
|
38,075
|
|
—
|
|
|||||||||
|
(a)
|
Includes shares repurchased as part of publicly announced programs and shares of common stock surrendered to the Company to satisfy tax withholding obligations in connection with the vesting of awards under our long-term incentive program.
|
|
(b)
|
In May 2010, the Board of Directors authorized the repurchase of up to $3.0 billion of common stock and in June 2012 increased its authorization for the repurchase of common stock by an additional $3.0 billion. In October 2016, the Board of Directors approved a new share repurchase program authorizing the repurchase of an additional $3.0 billion of common stock. The stock repurchase program does not have an expiration date.
|
|
Item 6.
|
SELECTED FINANCIAL DATA.
|
|
Amounts in Millions, except per share data
|
2018
|
2017
|
2016
|
2015
|
2014
|
|||||||||||||||
|
Income Statement Data:
|
||||||||||||||||||||
|
Total Revenues
|
$
|
22,561
|
|
$
|
20,776
|
|
$
|
19,427
|
|
$
|
16,560
|
|
$
|
15,879
|
|
|||||
|
Net Earnings
|
4,947
|
|
975
|
|
4,507
|
|
1,631
|
|
2,029
|
|
||||||||||
|
Net Earnings/(Loss) Attributable to:
|
||||||||||||||||||||
|
Noncontrolling Interest
|
27
|
|
(32
|
)
|
50
|
|
66
|
|
25
|
|
||||||||||
|
BMS
|
4,920
|
|
1,007
|
|
4,457
|
|
1,565
|
|
2,004
|
|
||||||||||
|
Net Earnings per Common Share Attributable to BMS:
|
||||||||||||||||||||
|
Basic
|
$
|
3.01
|
|
$
|
0.61
|
|
$
|
2.67
|
|
$
|
0.94
|
|
$
|
1.21
|
|
|||||
|
Diluted
|
3.01
|
|
0.61
|
|
2.65
|
|
0.93
|
|
1.20
|
|
||||||||||
|
Average common shares outstanding:
|
||||||||||||||||||||
|
Basic
|
1,633
|
|
1,645
|
|
1,671
|
|
1,667
|
|
1,657
|
|
||||||||||
|
Diluted
|
1,637
|
|
1,652
|
|
1,680
|
|
1,679
|
|
1,670
|
|
||||||||||
|
Cash dividends paid on BMS common and preferred stock
|
$
|
2,613
|
|
$
|
2,577
|
|
$
|
2,547
|
|
$
|
2,477
|
|
$
|
2,398
|
|
|||||
|
Cash dividends declared per common share
|
$
|
1.61
|
|
$
|
1.57
|
|
$
|
1.53
|
|
$
|
1.49
|
|
$
|
1.45
|
|
|||||
|
Financial Position Data at December 31:
|
||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
6,911
|
|
$
|
5,421
|
|
$
|
4,237
|
|
$
|
2,385
|
|
$
|
5,571
|
|
|||||
|
Marketable securities
(a)
|
3,748
|
|
3,871
|
|
4,832
|
|
6,545
|
|
6,272
|
|
||||||||||
|
Total Assets
|
34,986
|
|
33,551
|
|
33,707
|
|
31,748
|
|
33,749
|
|
||||||||||
|
Long-term debt
(a)
|
6,895
|
|
6,975
|
|
6,465
|
|
6,550
|
|
7,242
|
|
||||||||||
|
Equity
|
14,127
|
|
11,847
|
|
16,347
|
|
14,424
|
|
14,983
|
|
||||||||||
|
(a)
|
Includes current and non-current portion.
|
|
Item 7.
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
|
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions, except per share data
|
2018
|
2017
|
2016
|
|||||||||
|
Total Revenues
|
$
|
22,561
|
|
$
|
20,776
|
|
$
|
19,427
|
|
|||
|
Diluted Earnings Per Share
|
||||||||||||
|
GAAP
|
$
|
3.01
|
|
$
|
0.61
|
|
$
|
2.65
|
|
|||
|
Non-GAAP
|
3.98
|
|
3.01
|
|
2.83
|
|
||||||
|
Product
|
Date
|
Approval
|
|
Opdivo
|
August 2018
|
Approval in Japan for patients with MPM which has progressed after chemotherapy.
|
|
August 2018
|
Approval in Japan for adjuvant treatment of melanoma.
|
|
|
August 2018
|
FDA approval as the first and only IO treatment option for patients with metastatic SCLC whose cancer has progressed after platinum-based chemotherapy and at least one other line of therapy.
|
|
|
July 2018
|
EC approval for the adjuvant treatment of adult patients with melanoma with involvement of lymph nodes or metastatic disease who have undergone complete resection.
|
|
|
June 2018
|
Approval in China for the treatment of locally advanced or metastatic NSCLC after prior platinum-based chemotherapy in adult patients without EGFR or ALK genomic tumor aberrations.
|
|
|
Opdivo+Yervoy
|
August 2018
|
Approval in Japan of
Opdivo
plus low-dose
Yervoy
for the treatment of unresectable or metastatic RCC.
|
|
July 2018
|
FDA approval of
Opdivo
plus low-dose
Yervoy
for the treatment of adult and pediatric patients 12 years and older with MSI-H or dMMR mCRC that has progressed following treatment with fluoropyrimidine, oxaliplatin and irinotecan.
|
|
|
May 2018
|
Approval in Japan of
Opdivo+Yervoy
combination for previously untreated patients with unresectable melanoma.
|
|
|
April 2018
|
FDA approval of
Opdivo+Yervoy
combination for previously untreated patients with intermediate and poor-risk advanced RCC.
|
|
|
Orencia
|
February 2018
|
Approval in Japan for an intravenously administered treatment of moderate to severe polyarticular JIA in patients two years of age and older.
|
|
Empliciti
|
November 2018
|
FDA approval of
Empliciti
injection for intravenous use in combination with pomalidomide and dexamethasone for the treatment of adult patients with multiple myeloma who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor.
|
|
Sprycel
|
December 2018
|
FDA expanded the indication for
Sprycel
to include the treatment of pediatric patients one year of age and older with newly diagnosed Philadelphia chromosome-positive ALL in combination with chemotherapy.
|
|
July 2018
|
EC expanded the indication for
Sprycel
to include the treatment of children and adolescents aged 1 year to 18 years with chronic phase Philadelphia chromosome-positive CML and to include a powder for oral suspension.
|
|
|
Yervoy
|
January 2018
|
EC approval of advanced (unresectable or metastatic) melanoma in pediatric patients 12 years of age and older.
|
|
|
Year Ended December 31,
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||||||||||
|
|
Total Revenues
|
Analysis of % Change
|
Analysis of % Change
|
|||||||||||||||||||||
|
|
|
|
|
Total
|
Foreign
|
Total
|
Foreign
|
|||||||||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
Change
|
Exchange
(b)
|
Change
|
Exchange
(b)
|
|||||||||||||||||
|
United States
|
$
|
12,586
|
|
$
|
11,358
|
|
$
|
10,720
|
|
11
|
%
|
—
|
|
6
|
%
|
—
|
|
|||||||
|
Europe
|
5,658
|
|
4,988
|
|
4,215
|
|
13
|
%
|
3
|
%
|
18
|
%
|
1
|
%
|
||||||||||
|
Rest of the World
|
3,733
|
|
3,877
|
|
3,964
|
|
(4
|
)%
|
(2
|
)%
|
(2
|
)%
|
—
|
|
||||||||||
|
Other
(a)
|
584
|
|
553
|
|
528
|
|
6
|
%
|
N/A
|
|
5
|
%
|
N/A
|
|
||||||||||
|
Total
|
$
|
22,561
|
|
$
|
20,776
|
|
$
|
19,427
|
|
9
|
%
|
1
|
%
|
7
|
%
|
—
|
|
|||||||
|
(a)
|
Other revenues include royalties and alliance-related revenues for products not sold by our regional commercial organizations.
|
|
(b)
|
Foreign exchange impacts were derived by applying the prior period average currency rates to the current period sales.
|
|
Dollars in Millions
|
Charge-Backs and Cash Discounts
|
Medicaid and Medicare Rebates
|
Other Rebates, Returns, Discounts and Adjustments
|
Total
|
||||||||||||
|
Balance at January 1, 2017
|
$
|
126
|
|
$
|
520
|
|
$
|
1,160
|
|
$
|
1,806
|
|
||||
|
Provision related to sale made in:
|
||||||||||||||||
|
Current period
|
2,087
|
|
2,090
|
|
2,135
|
|
6,312
|
|
||||||||
|
Prior period
|
(3
|
)
|
(4
|
)
|
(64
|
)
|
(71
|
)
|
||||||||
|
Payments and returns
|
(2,004
|
)
|
(1,810
|
)
|
(2,107
|
)
|
(5,921
|
)
|
||||||||
|
Foreign currency translation and other
|
3
|
|
—
|
|
104
|
|
107
|
|
||||||||
|
Balance at December 31, 2017
|
$
|
209
|
|
$
|
796
|
|
$
|
1,228
|
|
$
|
2,233
|
|
||||
|
Provision related to sale made in:
|
||||||||||||||||
|
Current period
|
2,738
|
|
3,258
|
|
2,693
|
|
8,689
|
|
||||||||
|
Prior period
|
(3
|
)
|
(33
|
)
|
(60
|
)
|
(96
|
)
|
||||||||
|
Payments and returns
|
(2,695
|
)
|
(2,960
|
)
|
(2,424
|
)
|
(8,079
|
)
|
||||||||
|
Assets/related liabilities held-for-sale
|
—
|
|
—
|
|
(28
|
)
|
(28
|
)
|
||||||||
|
Foreign currency translation and other
|
(4
|
)
|
—
|
|
(53
|
)
|
(57
|
)
|
||||||||
|
Balance at December 31, 2018
|
$
|
245
|
|
$
|
1,061
|
|
$
|
1,356
|
|
$
|
2,662
|
|
||||
|
|
Year Ended December 31,
|
% Change
|
||||||||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||
|
Gross product sales
|
$
|
30,174
|
|
$
|
25,499
|
|
$
|
22,364
|
|
18
|
%
|
14
|
%
|
|||||
|
GTN Adjustments
|
||||||||||||||||||
|
Charge-backs and cash discounts
|
(2,735
|
)
|
(2,084
|
)
|
(1,582
|
)
|
31
|
%
|
32
|
%
|
||||||||
|
Medicaid and Medicare rebates
|
(3,225
|
)
|
(2,086
|
)
|
(1,382
|
)
|
55
|
%
|
51
|
%
|
||||||||
|
Other rebates, returns, discounts and adjustments
|
(2,633
|
)
|
(2,071
|
)
|
(1,698
|
)
|
27
|
%
|
22
|
%
|
||||||||
|
Total GTN Adjustments
|
(8,593
|
)
|
(6,241
|
)
|
(4,662
|
)
|
38
|
%
|
34
|
%
|
||||||||
|
Net product sales
|
$
|
21,581
|
|
$
|
19,258
|
|
$
|
17,702
|
|
12
|
%
|
9
|
%
|
|||||
|
GTN adjustments percentage
|
28
|
%
|
24
|
%
|
21
|
%
|
4
|
%
|
3
|
%
|
||||||||
|
U.S.
|
36
|
%
|
31
|
%
|
26
|
%
|
5
|
%
|
5
|
%
|
||||||||
|
Non-U.S.
|
13
|
%
|
13
|
%
|
13
|
%
|
—
|
|
—
|
|
||||||||
|
|
Year Ended December 31,
|
% Change
|
||||||||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||
|
Prioritized Brands
|
||||||||||||||||||
|
Opdivo
|
$
|
6,735
|
|
$
|
4,948
|
|
$
|
3,774
|
|
36
|
%
|
31
|
%
|
|||||
|
U.S.
|
4,239
|
|
3,102
|
|
2,664
|
|
37
|
%
|
16
|
%
|
||||||||
|
Non-U.S.
|
2,496
|
|
1,846
|
|
1,110
|
|
35
|
%
|
66
|
%
|
||||||||
|
Eliquis
|
6,438
|
|
4,872
|
|
3,343
|
|
32
|
%
|
46
|
%
|
||||||||
|
U.S.
|
3,760
|
|
2,887
|
|
1,963
|
|
30
|
%
|
47
|
%
|
||||||||
|
Non-U.S.
|
2,678
|
|
1,985
|
|
1,380
|
|
35
|
%
|
44
|
%
|
||||||||
|
Orencia
|
2,710
|
|
2,479
|
|
2,265
|
|
9
|
%
|
9
|
%
|
||||||||
|
U.S.
|
1,875
|
|
1,704
|
|
1,532
|
|
10
|
%
|
11
|
%
|
||||||||
|
Non-U.S.
|
835
|
|
775
|
|
733
|
|
8
|
%
|
6
|
%
|
||||||||
|
Sprycel
|
2,000
|
|
2,005
|
|
1,824
|
|
—
|
|
10
|
%
|
||||||||
|
U.S.
|
1,091
|
|
1,105
|
|
969
|
|
(1
|
)%
|
14
|
%
|
||||||||
|
Non-U.S.
|
909
|
|
900
|
|
855
|
|
1
|
%
|
5
|
%
|
||||||||
|
Yervoy
|
1,330
|
|
1,244
|
|
1,053
|
|
7
|
%
|
18
|
%
|
||||||||
|
U.S.
|
941
|
|
908
|
|
802
|
|
4
|
%
|
13
|
%
|
||||||||
|
Non-U.S.
|
389
|
|
336
|
|
251
|
|
16
|
%
|
34
|
%
|
||||||||
|
Empliciti
|
247
|
|
231
|
|
150
|
|
7
|
%
|
54
|
%
|
||||||||
|
U.S.
|
164
|
|
151
|
|
133
|
|
9
|
%
|
14
|
%
|
||||||||
|
Non-U.S.
|
83
|
|
80
|
|
17
|
|
4
|
%
|
**
|
|
||||||||
|
Established Brands
|
||||||||||||||||||
|
Baraclude
|
744
|
|
1,052
|
|
1,192
|
|
(29
|
)%
|
(12
|
)%
|
||||||||
|
U.S.
|
32
|
|
53
|
|
66
|
|
(40
|
)%
|
(20
|
)%
|
||||||||
|
Non-U.S.
|
712
|
|
999
|
|
1,126
|
|
(29
|
)%
|
(11
|
)%
|
||||||||
|
Reyataz Franchise
|
427
|
|
698
|
|
912
|
|
(39
|
)%
|
(23
|
)%
|
||||||||
|
U.S.
|
157
|
|
327
|
|
484
|
|
(52
|
)%
|
(32
|
)%
|
||||||||
|
Non-U.S.
|
270
|
|
371
|
|
428
|
|
(27
|
)%
|
(13
|
)%
|
||||||||
|
Sustiva Franchise
|
283
|
|
729
|
|
1,065
|
|
(61
|
)%
|
(32
|
)%
|
||||||||
|
U.S.
|
27
|
|
622
|
|
901
|
|
(96
|
)%
|
(31
|
)%
|
||||||||
|
Non-U.S.
|
256
|
|
107
|
|
164
|
|
**
|
|
(35
|
)%
|
||||||||
|
Hepatitis C Franchise
|
17
|
|
406
|
|
1,578
|
|
(96
|
)%
|
(74
|
)%
|
||||||||
|
U.S.
|
(16
|
)
|
109
|
|
827
|
|
**
|
|
(87
|
)%
|
||||||||
|
Non-U.S.
|
33
|
|
297
|
|
751
|
|
(89
|
)%
|
(60
|
)%
|
||||||||
|
Other Brands
|
1,630
|
|
2,112
|
|
2,271
|
|
(23
|
)%
|
(7
|
)%
|
||||||||
|
U.S.
|
316
|
|
390
|
|
379
|
|
(19
|
)%
|
3
|
%
|
||||||||
|
Non-U.S.
|
1,314
|
|
1,722
|
|
1,892
|
|
(24
|
)%
|
(9
|
)%
|
||||||||
|
Total Revenues
|
22,561
|
|
20,776
|
|
19,427
|
|
9
|
%
|
7
|
%
|
||||||||
|
U.S.
|
12,586
|
|
11,358
|
|
10,720
|
|
11
|
%
|
6
|
%
|
||||||||
|
Non-U.S.
|
9,975
|
|
9,418
|
|
8,707
|
|
6
|
%
|
8
|
%
|
||||||||
|
**
|
Change in excess of 100%
|
|
•
|
U.S. revenues increased in both periods due to higher demand. The higher growth rate in 2018 was primarily due to the approvals for the treatment of adjuvant melanoma, liver cancer and the
Opdivo+Yervoy
combination for kidney cancer, which is partially offset by the decline in lung cancer indication.
|
|
•
|
International revenues increased in both periods due to higher demand as a result of approvals for additional indications and launches in new countries. The lower growth rate in 2018 was primarily due to additional competition for
Opdivo
in the NSCLC indication.
|
|
•
|
U.S. revenues increased in both periods due to market share gains partially offset by lower average net selling prices.
|
|
•
|
International revenues increased in both periods due to higher demand attributed to market share gains and growth of the novel oral anticoagulants market.
|
|
•
|
U.S. revenues increased in both periods due to higher demand and higher average net selling prices.
|
|
•
|
International revenues increased in both periods due to higher demand. We may experience additional competition in Europe from biosimilars of competitor products in future periods.
|
|
•
|
U.S. revenues decreased in 2018 due to inventory workdown offset by higher average net selling prices. U.S. revenues increased in 2017 due to higher demand and higher average net selling prices.
|
|
•
|
International revenues remained unchanged in 2018. International revenues increased in 2017 due to higher demand. We may experience a decline in European revenues in the event that generic datasinib product enters the market.
|
|
•
|
U.S. revenues increased in both periods due to higher demand. Revenue growth rate in 2018 decreased due to lower demand resulting from other IO products being used in the adjuvant treatment of patients with melanoma, including
Opdivo
.
|
|
•
|
International revenues increased in both periods due to higher demand primarily in Europe following the approval of the
Opdivo+Yervoy
combination therapy for melanoma.
|
|
•
|
International revenues decreased in both periods due to lower demand resulting from increased competition.
|
|
•
|
The LOE for
Reyataz
in the U.S. occurred in December 2017, as a result revenues will continue to decline.
|
|
•
|
International revenues decreased in both periods due to lower demand resulting from increased competition.
|
|
•
|
The LOE for
Sustiva
in the U.S. occurred in December 2017. Gilead terminated BMS's participation in the U.S. and Canada joint venture following the launch of a generic version of
Sustiva
in the U.S. As a result, BMS's share of
Atripla
* revenues will further decline during the next two years. Refer to “Item 8. Financial Statements—Note 3. Alliances” for further discussion.
|
|
•
|
International revenues for 2018 include $
204 million
of U.S.
Atripla
* royalty revenue.
|
|
•
|
U.S. and international revenues decreased in both periods due to lower demand resulting from increased competition.
|
|
•
|
International revenues decreased in 2018 primarily due to lower
Plavix*
royalties as a result of the adoption of amended revenue guidance, the expiration of rights to
Abilify*
in Canada, lower diabetes product supply sales and continued generic erosion. The revenue decrease in 2017 was due to out-licensing and divestiture of certain other brands and continued generic erosion.
|
|
|
|
|
|
% Change
|
||||||||||||||
|
Dollar in Millions
|
2018
|
2017
|
2016
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||
|
Cost of products sold
|
$
|
6,547
|
|
$
|
6,094
|
|
$
|
4,969
|
|
7
|
%
|
23
|
%
|
|||||
|
Marketing, selling and administrative
|
4,551
|
|
4,751
|
|
4,979
|
|
(4
|
)%
|
(5
|
)%
|
||||||||
|
Research and development
|
6,345
|
|
6,482
|
|
5,012
|
|
(2
|
)%
|
29
|
%
|
||||||||
|
Other income (net)
|
(850
|
)
|
(1,682
|
)
|
(1,448
|
)
|
(49
|
)%
|
16
|
%
|
||||||||
|
Total Expenses
|
$
|
16,593
|
|
$
|
15,645
|
|
$
|
13,512
|
|
6
|
%
|
16
|
%
|
|||||
|
•
|
Cost of products sold increased in 2018 due to higher royalties and
profit sharing of
$905 million
resulting primarily from higher
Eliquis
sales partially offset by product cost improvements, a $146 million impairment charge in 2017 to reduce the carrying value of the small molecule active pharmaceutical ingredient manufacturing operations in Swords, Ireland, and lower inventory charges.
|
|
•
|
Cost of products sold increased in 2017 due to higher royalties and profit sharing of
$753 million
resulting primarily from higher
Eliquis
sales and a
$146 million
impairment charge as discussed above. The remaining increase was primarily due to higher sales volume, inventory charges, manufacturing startup costs and foreign currency.
|
|
•
|
Marketing, selling and administrative expenses decreased in 2018 due to lower advertising, promotion and marketing expenses, lower costs attributed to transformation initiatives and lower branded prescription drug fee, partially offset by higher BMS foundation grants.
|
|
•
|
Marketing, selling and administrative expenses decreased in 2017 due to lower advertising, promotion and sales-force expenses supporting
Daklinza
and other established brands and lower BMS foundation grants.
|
|
•
|
Research and development expense decreased in 2018 due to lower site exit costs and IPRD impairment charges, partially offset by expansion of
Opdivo
and other IO development programs, including NKTR-214.
|
|
•
|
Research and development expense increased in 2017 due to higher license and asset acquisition charges, site exit charges, IPRD impairment charges and expansion of
Opdivo
and other IO development programs.
|
|
|
Year Ended December 31,
|
|||||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||||
|
Nektar
|
$
|
1,050
|
|
(a)
|
$
|
—
|
|
$
|
—
|
|
||||
|
Cormorant
|
60
|
|
(b)
|
—
|
|
35
|
|
(a)
|
||||||
|
IFM
|
25
|
|
(b)
|
311
|
|
(a)
|
—
|
|
||||||
|
CytomX
|
—
|
|
200
|
|
(a)
|
25
|
|
(a)
|
||||||
|
Halozyme
|
—
|
|
|
105
|
|
(a)
|
—
|
|
||||||
|
Flexus
|
—
|
|
|
324
|
|
(b)
|
100
|
|
(b)
|
|||||
|
Cardioxyl
|
—
|
|
100
|
|
(b)
|
—
|
|
|||||||
|
PsiOxus
|
—
|
|
50
|
|
(a)
|
—
|
|
|||||||
|
Ono
|
—
|
|
40
|
|
(a)
|
—
|
|
|||||||
|
Padlock
|
—
|
|
—
|
|
139
|
|
(a)
|
|||||||
|
Nitto Denko
|
—
|
|
—
|
|
100
|
|
(a)
|
|||||||
|
Other
|
—
|
|
—
|
|
40
|
|
||||||||
|
License and asset acquisition charges
|
1,135
|
|
1,130
|
|
439
|
|
||||||||
|
F-Star
|
—
|
|
75
|
|
—
|
|
||||||||
|
Other
|
—
|
|
—
|
|
13
|
|
||||||||
|
IPRD impairments
|
—
|
|
75
|
|
13
|
|
||||||||
|
Site exit costs
|
79
|
|
383
|
|
83
|
|
||||||||
|
Research and development significant charges
|
$
|
1,214
|
|
$
|
1,588
|
|
$
|
535
|
|
|||||
|
(a)
|
Upfront payment
|
|
(b)
|
Milestone payment
|
|
•
|
License and asset acquisition charges resulted from strategic transactions to acquire or license certain investigational oncology, cardiovascular, immunoscience and fibrotic disease compounds (or options to acquire or license) as disclosed in “—Acquisitions, Divestitures, Licensing and Collaboration Arrangements.”
|
|
•
|
IPRD impairment charges includes the discontinued development of an investigational compound which was part of our alliance with F-Star in 2017.
|
|
•
|
Site exit costs resulted from the expected exit of R&D sites in the U.S. through 2020 primarily due to the reduction in the estimated useful lives of the related assets and an impairment charge in 2017 to reduce the carrying value of an R&D facility in Wallingford, Connecticut.
|
|
•
|
Other income (net) decreased in 2018 primarily due to losses on equity investments related to Nektar and a patent infringement settlement in 2017 partially offset by lower restructuring and debt redemption charges.
|
|
•
|
Other income (net) increased in 2017 primarily due to a patent infringement settlement and out-licensing income partially offset by lower divestiture gains and related service fees and higher restructuring and debt redemption charges.
|
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Interest expense
|
$
|
183
|
|
$
|
196
|
|
$
|
167
|
|
|||
|
Investment income
|
(173
|
)
|
(126
|
)
|
(97
|
)
|
||||||
|
Loss/(gain) on equity investments
|
512
|
|
(23
|
)
|
37
|
|
||||||
|
Provision for restructuring
|
131
|
|
293
|
|
109
|
|
||||||
|
Litigation and other settlements
|
76
|
|
(487
|
)
|
47
|
|
||||||
|
Equity in net income of affiliates
|
(93
|
)
|
(75
|
)
|
(77
|
)
|
||||||
|
Divestiture gains
|
(178
|
)
|
(164
|
)
|
(576
|
)
|
||||||
|
Royalties and licensing income
|
(1,353
|
)
|
(1,351
|
)
|
(719
|
)
|
||||||
|
Transition and other service fees
|
(12
|
)
|
(37
|
)
|
(238
|
)
|
||||||
|
Pension and postretirement
|
(27
|
)
|
(1
|
)
|
(72
|
)
|
||||||
|
Intangible asset impairment
|
64
|
|
—
|
|
15
|
|
||||||
|
Loss on debt redemption
|
—
|
|
109
|
|
—
|
|
||||||
|
Other
|
20
|
|
(16
|
)
|
(44
|
)
|
||||||
|
Other income (net)
|
$
|
(850
|
)
|
$
|
(1,682
|
)
|
$
|
(1,448
|
)
|
|||
|
•
|
Loss/(gain) on equity investments includes a fair value adjustment of
$534 million
related to the Company's equity investment in Nektar in 2018.
|
|
•
|
Restructuring charges relate to changes to the Company's operating model to drive continued success in the near- and long-term through a more focused investment in commercial opportunities for key brands and markets, a competitive and more agile R&D organization that can accelerate the pipeline, streamline operations and realign manufacturing capabilities that broaden biologics capabilities to reflect the current and future portfolio as well as streamline and simplify our small-molecule supply network. The new operating model is expected to enable the Company to deliver the strategic, financial and operational flexibility necessary to invest in the highest priorities across the Company. Aggregate restructuring charges of
$268 million
and
$826 million
have been incurred in 2018 and 2017, respectively, for all actions including accelerated depreciation and impairment charges resulting from early site exits.
|
|
•
|
Litigation and other settlements include
$481 million
for BMS's share of a patent-infringement settlement related to Merck's PD-1 antibody
Keytruda*
in 2017 and
$70 million
related to intellectual property and product liability settlements in 2018, including
$42 million
recognized subsequent to the Company's earnings release for the fourth quarter of 2018.
|
|
•
|
Divestiture gains includes divestiture of multiple mature global product lines in oncology and infectious therapy in 2018, additional contingent consideration for the diabetes business in 2017 and certain OTC brands and investigational HIV medicines businesses in 2016.
|
|
•
|
Royalties and licensing income includes
Keytruda*
royalties in 2018 and 2017, upfront licensing fees from Biogen and Roche in connection with the out-licensing of certain investigational genetically defined disease compounds in 2017 and contingent consideration from the
Erbitux*
and diabetes business divestitures in 2018, 2017 and 2016, including the transfer of certain royalty rights pertaining to diabetes product sales. A
$50 million
fee for amending a royalty rate and
$25 million
sales-based milestone was also included in 2018.
|
|
•
|
Transition and other service fees included fees resulting from the divestiture of the diabetes and investigational HIV medicines businesses in 2017 and 2016.
|
|
•
|
Pension and postretirement includes the interest cost, expected return on plan assets and amortization components of the net periodic benefit cost (credit) as well as net charges for settlements, curtailments and special termination benefits of
$121 million
in 2018,
$162 million
in 2017 and
$92 million
in 2016.
|
|
•
|
Intangible asset impairment includes
$64 million
in 2018 for an out-licensed asset obtained in the 2010 acquisition of ZymoGenetics, Inc., which did not meet its primary endpoint in a Phase II clinical study.
|
|
•
|
A debt redemption loss of
$109 million
resulted from the early redemption of certain long-term debt obligations in 2017.
|
|
Dollars in Millions
|
2018
|
2017
|
2016
|
||||||||
|
Earnings Before Income Taxes
|
$
|
5,968
|
|
$
|
5,131
|
|
$
|
5,915
|
|
||
|
Provision for Income Taxes
|
1,021
|
|
4,156
|
|
1,408
|
|
|||||
|
Effective Tax Rate
|
17.1
|
%
|
81.0
|
%
|
23.8
|
%
|
|||||
|
Impact of Specified Items
|
—
|
|
60.0
|
%
|
1.8
|
%
|
|||||
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Impairment charges
|
$
|
17
|
|
$
|
146
|
|
$
|
—
|
|
|||
|
Accelerated depreciation and other shutdown costs
|
41
|
|
3
|
|
21
|
|
||||||
|
Cost of products sold
|
58
|
|
149
|
|
21
|
|
||||||
|
Marketing, selling and administrative
|
2
|
|
1
|
|
—
|
|
||||||
|
License and asset acquisition charges
|
1,135
|
|
1,130
|
|
439
|
|
||||||
|
IPRD impairments
|
—
|
|
75
|
|
13
|
|
||||||
|
Site exit costs
|
79
|
|
383
|
|
83
|
|
||||||
|
Research and development
|
1,214
|
|
1,588
|
|
535
|
|
||||||
|
Loss/(gain) on equity investments
(a)
|
512
|
|
—
|
|
—
|
|
||||||
|
Provision for restructuring
|
131
|
|
293
|
|
109
|
|
||||||
|
Litigation and other settlements
|
70
|
|
(481
|
)
|
40
|
|
||||||
|
Divestiture gains
|
(177
|
)
|
(126
|
)
|
(559
|
)
|
||||||
|
Royalties and licensing income
|
(75
|
)
|
(497
|
)
|
(10
|
)
|
||||||
|
Pension and postretirement
|
121
|
|
162
|
|
91
|
|
||||||
|
Intangible asset impairment
|
64
|
|
—
|
|
15
|
|
||||||
|
Loss on debt redemption
|
—
|
|
109
|
|
—
|
|
||||||
|
Other income (net)
|
646
|
|
(540
|
)
|
(314
|
)
|
||||||
|
Increase to pretax income
|
1,920
|
|
1,198
|
|
242
|
|
||||||
|
Income taxes on items above
|
(268
|
)
|
(87
|
)
|
51
|
|
||||||
|
Income taxes attributed to U.S. tax reform
|
(56
|
)
|
2,911
|
|
—
|
|
||||||
|
Income taxes
|
(324
|
)
|
2,824
|
|
51
|
|
||||||
|
Increase to net earnings
|
1,596
|
|
4,022
|
|
293
|
|
||||||
|
Noncontrolling interest
|
—
|
|
(59
|
)
|
—
|
|
||||||
|
Increase to net earnings used for Diluted Non-GAAP EPS calculation
|
$
|
1,596
|
|
$
|
3,963
|
|
$
|
293
|
|
|||
|
(a)
|
Specified items included these amounts upon adoption of amended guidance for the recognition and measurement of financial assets and liabilities in 2018.
|
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions, except per share data
|
2018
|
2017
|
2016
|
|||||||||
|
Net Earnings Attributable to BMS used for Diluted EPS Calculation — GAAP
|
$
|
4,920
|
|
$
|
1,007
|
|
$
|
4,457
|
|
|||
|
Specified Items
|
1,596
|
|
3,963
|
|
293
|
|
||||||
|
Net Earnings Attributable to BMS used for Diluted EPS Calculation — Non-GAAP
|
$
|
6,516
|
|
$
|
4,970
|
|
$
|
4,750
|
|
|||
|
Average Common Shares Outstanding — Diluted
|
1,637
|
|
1,652
|
|
1,680
|
|
||||||
|
Diluted EPS Attributable to BMS — GAAP
|
$
|
3.01
|
|
$
|
0.61
|
|
$
|
2.65
|
|
|||
|
Diluted EPS Attributable to Specified Items
|
0.97
|
|
2.40
|
|
0.18
|
|
||||||
|
Diluted EPS Attributable to BMS — Non-GAAP
|
$
|
3.98
|
|
$
|
3.01
|
|
$
|
2.83
|
|
|||
|
Dollars in Millions
|
2018
|
2017
|
||||||
|
Cash and cash equivalents
|
$
|
6,911
|
|
$
|
5,421
|
|
||
|
Marketable securities — current
|
1,973
|
|
1,391
|
|
||||
|
Marketable securities — non-current
|
1,775
|
|
2,480
|
|
||||
|
Total cash, cash equivalents and marketable securities
|
10,659
|
|
9,292
|
|
||||
|
Short-term debt obligations
|
(1,703
|
)
|
(987
|
)
|
||||
|
Long-term debt
|
(5,646
|
)
|
(6,975
|
)
|
||||
|
Net cash position
|
$
|
3,310
|
|
$
|
1,330
|
|
||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Cash flow provided by/(used in):
|
||||||||||||
|
Operating activities
|
$
|
5,940
|
|
$
|
5,275
|
|
$
|
3,058
|
|
|||
|
Investing activities
|
(874
|
)
|
(66
|
)
|
1,480
|
|
||||||
|
Financing activities
|
(3,535
|
)
|
(4,077
|
)
|
(2,653
|
)
|
||||||
|
•
|
Higher cash collections and timing of payments in the ordinary course of business of approximately
$2.2 billion
.
|
|
•
|
Higher R&D licensing and collaboration payments of approximately
$600 million
primarily due to the Nektar transaction in 2018;
|
|
•
|
Lower litigation settlement proceeds of approximately
$500 million
primarily due to the Merck settlement in 2017; and
|
|
•
|
Lower out-license proceeds of approximately
$400 million
primarily due to the Biogen and Roche transactions in 2017.
|
|
•
|
Higher cash collections and timing of payments in the ordinary course of business of approximately
$400 million
;
|
|
•
|
Lower income tax payments of approximately
$1.5 billion
;
|
|
•
|
Litigation settlement proceeds of approximately
$500 million
primarily due to the Merck settlement; and
|
|
•
|
Out-licensing proceeds of
$500 million
primarily due to the Biogen and Roche transactions.
|
|
•
|
Higher R&D licensing payments of approximately
$400 million
primarily due to the CytomX, Halozyme and Nitto Denko transactions; and
|
|
•
|
Higher contributions to pension plans of approximately
$300 million
.
|
|
•
|
Lower net sales and maturities of marketable securities with maturities greater than 90 days of approximately
$900 million
; and
|
|
•
|
Higher net acquisition and other payments of approximately
$500 million
primarily due to the purchase of
8.3 million
shares of Nektar common stock in 2018.
|
|
•
|
Higher business divestiture proceeds of approximately
$500 million
primarily due to the divestiture of manufacturing operations in Swords, Ireland and certain mature brands.
|
|
•
|
Lower net sales of marketable securities with maturities greater than 90 days of approximately
$700 million
;
|
|
•
|
Lower business divestiture proceeds of approximately
$600 million
primarily due to certain OTC brands and investigational HIV medicines businesses in 2016; and
|
|
•
|
Higher asset acquisition payments of approximately
$300 million
primarily due to the acquisition of IFM in 2017.
|
|
•
|
Lower repurchases of common stock of
$2.1 billion
primarily due to the accelerated share repurchase agreements in 2017.
|
|
•
|
Lower net borrowings of
$1.5 billion
primarily due to the issuance of long-term debt used to repurchase common stock in 2017.
|
|
•
|
Higher repurchase of common stock of
$2.2 billion
primarily due to the accelerated share repurchase agreements.
|
|
•
|
Higher net borrowing activity of
$900 million
primarily to fund the repurchase of common stock.
|
|
|
Obligations Expiring by Period
|
|||||||||||||||||||||||||||
|
Dollars in Millions
|
Total
|
2019
|
2020
|
2021
|
2022
|
2023
|
Later Years
|
|||||||||||||||||||||
|
Short-term borrowings
|
$
|
454
|
|
$
|
454
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||||||
|
Long-term debt
|
6,776
|
|
1,250
|
|
—
|
|
—
|
|
750
|
|
817
|
|
3,959
|
|
||||||||||||||
|
Interest on long-term debt
(a)
|
2,832
|
|
192
|
|
183
|
|
183
|
|
183
|
|
167
|
|
1,924
|
|
||||||||||||||
|
Operating leases
|
663
|
|
122
|
|
92
|
|
77
|
|
69
|
|
61
|
|
242
|
|
||||||||||||||
|
Purchase obligations
|
3,074
|
|
1,087
|
|
620
|
|
430
|
|
353
|
|
291
|
|
293
|
|
||||||||||||||
|
Uncertain tax positions
(b)
|
72
|
|
72
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Deemed repatriation transition tax
|
2,119
|
|
79
|
|
101
|
|
196
|
|
196
|
|
299
|
|
1,248
|
|
||||||||||||||
|
Total
(c)
|
$
|
15,990
|
|
$
|
3,256
|
|
$
|
996
|
|
$
|
886
|
|
$
|
1,551
|
|
$
|
1,635
|
|
$
|
7,666
|
|
|||||||
|
(a)
|
Includes estimated future interest payments and periodic cash settlements of derivatives.
|
|
(b)
|
Includes only short-term uncertain tax benefits because of uncertainties regarding the timing of resolution.
|
|
(c)
|
Excludes pension and other liabilities because of uncertainties regarding the timing of resolution.
|
|
Product
|
Indication
|
Date
|
Developments
|
|
Opdivo
|
Melanoma
|
August 2018
|
Approval in Japan for treatment of adjuvant melanoma.
|
|
July 2018
|
EC approval for the adjuvant treatment of adult patients with involvement of lymph nodes or metastatic disease who have undergone complete resection.
|
||
|
June 2018
|
Announced results from the Phase III CheckMate-238 trial evaluating
Opdivo
versus
Yervoy
in patients with stage IIIB/C or stage IV melanoma who are at high risk of recurrence following complete surgical resection demonstrated statistically longer recurrence-free survival for
Opdivo
, the primary endpoint of the study, versus
Yervoy
at a minimum follow-up of 24 months across key subgroups, including disease stages and BRAF mutation status.
|
||
|
Multiple Myeloma
|
June/August 2018
|
Announced in June 2018 that the FDA lifted a partial clinical hold placed on CheckMate-602, a randomized, open-label Phase III study evaluating the addition of
Opdivo
to pomalidomide and dexamethasone in patients with relapsed or refractory multiple myeloma. The decision follows consultation with the FDA and agreement on amendments to the study protocol. In August 2018, the Company discontinued further enrollment of this study following a futility analysis.
|
|
|
NSCLC
|
June 2018
|
Approval in China for the treatment of locally advanced or metastatic NSCLC after prior platinum-based chemotherapy in adult patients without EGFR or ALK genomic tumor aberrations.
|
|
|
April 2018
|
Announced that the pivotal, randomized Phase III CheckMate-078 trial evaluating
Opdivo
versus docetaxel in a predominantly Chinese population with previously treated advanced NSCLC demonstrated superior overall survival benefit in the primary endpoint regardless of PD-L1 expression or tumor histology.
|
||
|
SCCHN
|
January 2019
|
Acceptance in China of sBLA filing for patients who had previously been treated for metastatic or recurrent SCCHN.
|
|
|
April 2018
|
Announced two-year overall survival data from CheckMate-141, a Phase III study, evaluating
Opdivo
compared with investigator’s choice chemotherapy (cetuximab, docetaxel or methotrexate) in patients with recurrent or metastatic SCCHN after failure on platinum-based therapy.
|
||
|
SCLC
|
October 2018
|
Announced topline results from the Phase III CheckMate-331 study did not meet its primary endpoint of overall survival with
Opdivo
versus chemotherapy in patients with previously treated relapsed SCLC.
|
|
|
August 2018
|
FDA approval as the first and only IO treatment option for patients with metastatic SCLC whose cancer has progressed after platinum-based chemotherapy and at least one other line of therapy.
|
||
|
Various
|
August 2018
|
Approval in Japan for patients with MPM which has progressed after chemotherapy.
|
|
|
August 2018
|
Approval in Japan of an every 2 week/30 minute infusion dose and administration schedule for
Opdivo
in six indications.
|
||
|
June 2018
|
Announced preliminary data from the ongoing PIVOT Phase I/II Study, which is evaluating the combination of
Opdivo
with Nektar's investigational medicine, NKTR-214. The preliminary results presented at the 2018 American Society of Clinical Oncology reported safety, efficacy and biomarker data for patients enrolled in the Phase I dose-escalation stage of the study and for the first patients consecutively enrolled in select dose expansion cohorts in Phase II.
|
||
|
April 2018
|
EC approval of an every four-week (Q4W)
Opdivo
dosing schedule of 480 mg infused over 60 minutes as an option for patients with advanced melanoma and previously treated RCC as well as the approval of a two-week
Opdivo
dosing option of 240 mg infused over 30 minutes to replace weight-based dosing for all six approved monotherapy indications in the EU.
|
||
|
March 2018
|
FDA approval of the Company's sBLA to update
Opdivo
dosing to include 480 mg infused every four weeks for a majority of approved indications as well as a shorter 30 minute infusion across all approved indications.
|
||
|
Product
|
Indication
|
Date
|
Developments
|
|
Opdivo+Yervoy
|
CRC
|
October 2018
|
Announced new data from a cohort of the CheckMate-142 study in which
Opdiv
o plus low-dose
Yervoy
demonstrated durable clinical benefit as a first-line treatment in patients with MSI-H or dMMR mCRC.
|
|
July 2018
|
FDA approval of
Opdivo
plus low-dose
Yervoy
for the treatment of adult and pediatric patients 12 years and older with MSI-H or dMMR mCRC that has progressed following treatment with a fluoropyrimidine, oxaliplatin and irinotecan.
|
||
|
mCRPC
|
February 2019
|
Announced results from an interim analysis of the Phase II CheckMate-650 trial evaluating
Opdivo+Yervoy
in patients with mCRPC showed that among 32 asymptomatic or minimally symptomatic patients whose disease had progressed after second-generation hormone therapy and who had not received chemotherapy (cohort 1), with a median follow-up of 11.9 months, the objective response rate was 25%. Additionally, among 30 patients whose disease progressed after taxane-based chemotherapy (cohort 2), with a median follow-up of 13.5 months, the objective response rate was 10%.
|
|
|
Melanoma
|
October 2018
|
Announced four-year data from the Phase III CheckMate-067 clinical trial which continues to demonstrate durable, long-term survival benefits with the first-line combination of
Opdivo+Yervoy
, versus
Yervoy
alone, in patients with advanced melanoma.
|
|
|
May 2018
|
Approval in Japan of
Opdivo+Yervoy
combination for chemotherapy-naive patients with unresectable melanoma.
|
||
|
mUC
|
October 2018
|
Announced follow-up data evaluating
Opdivo
monotherapy and
Opdivo
in combination with
Yervoy
in patients with platinum-pretreated mUC. Results from the Phase I/II CheckMate-032 trial showed that patients who received the combination of
Opdiv
o1 mg/kg plus
Yervoy
3 mg/kg experienced a higher objective response rate compared to those who received
Opdivo
3 mg/kg plus
Yervoy
1 mg/kg or
Opdivo
alone.
|
|
|
NSCLC
|
January 2019
|
Announced voluntary withdrawal of the Company's sBLA for the
Opdivo
plus low-dose
Yervoy
for treatment of first-line advanced NSCLC in patients with TMB greater than or equal to 10 mutations per megabase as data from CheckMate-227, Part 1a, will not be available within the PDUFA goal date of May 20, 2019.
|
|
|
October 2018
|
Announced updates regarding regulatory actions by the CHMP in the EU for the ongoing review of its applications for an indication in metastatic first-line NSCLC with
Opdivo
plus low-dose
Yervoy
in patients with TMB greater than or equal to 10 mutations per megabase. The CHMP requested additional information from CheckMate-227, including an overall survival analysis of
Opdivo+Yervoy
in patients who have TMB less than 10 mutations per megabase.
|
||
|
June 2018
|
Announced results from a part of the Phase III CheckMate-227 trial that evaluated
Opdivo
plus low-dose
Yervoy
and
Opdiv
o plus chemotherapy versus chemotherapy in patients with first-line NSCLC with PD-L1 expression <1%, across squamous and non-squamous tumor histologies extended progression-free survival.
|
||
|
May 2018
|
Announced the EMA validated a type II variation application for treatment in adult patients with first-line metastatic NSCLC who have TMB greater than or equal to 10 mutations per megabase.
|
||
|
RCC
|
February 2019
|
Announced new results from the Phase III CheckMate-214 study, showing that therapy with
Opdivo
plus low-dose
Yervoy
continued to demonstrate long-term survival benefits in patients with previously untreated advanced or metastatic RCC.
|
|
|
January 2019
|
Announced the EC approval of
Opdiv
o plus low-dose
Yervo
y for previously untreated patients with intermediate and poor-risk advanced RCC.
|
||
|
August 2018
|
Approval in Japan of
Opdivo
plus low-dose
Yervoy
for the treatment of unresectable or metastatic RCC.
|
||
|
June 2018
|
Announced patient-reported outcomes data from the Phase III CheckMate-214 trial in intermediate- and poor-risk patients with advanced RCC treated with
Opdivo
plus low-dose
Yervoy
versus sunitinib over a two-year follow-up period reported significant and sustained health-related quality of life improvements.
|
||
|
April 2018
|
FDA approval of
Opdivo+Yervoy
combination for previously untreated patients with intermediate and poor-risk advanced RCC.
|
||
|
SCLC
|
November 2018
|
Announced patient-reported outcomes from the Phase III CheckMate-451 study did not meet its primary endpoint of overall survival with
Opdivo+Yervoy
versus placebo as a maintenance therapy in patients with extensive-stage SCLC after completion of first-line platinum-based chemotherapy.
|
|
|
Eliquis
|
NVAF
|
November 2018
|
Announced findings from the largest real-world data analysis of NVAF patient populations aged 80 and older receiving direct oral anticoagulants showing that
Eliquis
is associated with lower rates of stroke or systemic embolism and major bleeding than rivaroxaban or dabigatran.
|
|
Product
|
Indication
|
Date
|
Developments
|
|
Orencia
|
JIA
|
January 2019
|
Received a positive CHMP opinion for polyarticular JIA via subcutaneous injection in pediatric patients down to two years of age.
|
|
Empliciti
|
RRMM
|
November 2018
|
FDA approval of
Empliciti
injection for intravenous use in combination with pomalidomide and dexamethasone for the treatment of adult patients with multiple myeloma who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor.
|
|
September 2018
|
Announced the EMA has validated the Company's type II variation application for
Empliciti
in combination with pomalidomide and low-dose dexamethasone for the treatment of adult patients with multiple myeloma who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor (PI), and have demonstrated disease progression on the last therapy.
|
||
|
Sprycel
|
ALL
|
February 2019
|
Announced EC approval of
Sprycel
, in both tablet and powder for oral suspension formulations, in combination with chemotherapy for the treatment of pediatric patients with newly diagnosed Philadelphia chromosome-positive ALL.
|
|
December 2018
|
FDA expanded the indication for
Sprycel
to include the treatment of pediatric patients one year of age and older with newly diagnosed Philadelphia chromosome-positive ALL in combination with chemotherapy.
|
||
|
CML
|
July 2018
|
EC expanded the indication for
Sprycel
to include the treatment of children and adolescents aged 1 year to 18 years with chronic phase Philadelphia chromosome positive CML and to include a powder for oral suspension.
|
|
|
Yervoy
|
Melanoma
|
January 2018
|
EC approval of advanced (unresectable or metastatic) melanoma in pediatric patients 12 years of age and older.
|
|
TYK2 Inhibitor
|
Psoriasis
|
September 2018
|
Announced results from a Phase II study of BMS-986165, an oral, selective TYK2 inhibitor which delivered significant skin clearance in patients with moderate to severe plaque psoriasis.
|
|
Item 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
|
|
Item 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
|
|
|
Year Ended December 31,
|
|||||||||||
|
EARNINGS
|
2018
|
2017
|
2016
|
|||||||||
|
Net product sales
|
$
|
21,581
|
|
$
|
19,258
|
|
$
|
17,702
|
|
|||
|
Alliance and other revenues
|
980
|
|
1,518
|
|
1,725
|
|
||||||
|
Total Revenues
|
22,561
|
|
20,776
|
|
19,427
|
|
||||||
|
Cost of products sold
|
6,547
|
|
6,094
|
|
4,969
|
|
||||||
|
Marketing, selling and administrative
|
4,551
|
|
4,751
|
|
4,979
|
|
||||||
|
Research and development
|
6,345
|
|
6,482
|
|
5,012
|
|
||||||
|
Other income (net)
|
(850
|
)
|
(1,682
|
)
|
(1,448
|
)
|
||||||
|
Total Expenses
|
16,593
|
|
15,645
|
|
13,512
|
|
||||||
|
Earnings Before Income Taxes
|
5,968
|
|
5,131
|
|
5,915
|
|
||||||
|
Provision for Income Taxes
|
1,021
|
|
4,156
|
|
1,408
|
|
||||||
|
Net Earnings
|
4,947
|
|
975
|
|
4,507
|
|
||||||
|
Noncontrolling Interest
|
27
|
|
(32
|
)
|
50
|
|
||||||
|
Net Earnings Attributable to BMS
|
$
|
4,920
|
|
$
|
1,007
|
|
$
|
4,457
|
|
|||
|
Earnings per Common Share
|
||||||||||||
|
Basic
|
$
|
3.01
|
|
$
|
0.61
|
|
$
|
2.67
|
|
|||
|
Diluted
|
3.01
|
|
0.61
|
|
2.65
|
|
||||||
|
|
Year Ended December 31,
|
|||||||||||
|
COMPREHENSIVE INCOME
|
2018
|
2017
|
2016
|
|||||||||
|
Net Earnings
|
$
|
4,947
|
|
$
|
975
|
|
$
|
4,507
|
|
|||
|
Other Comprehensive (Loss)/Income, net of taxes and reclassifications to earnings:
|
||||||||||||
|
Derivatives qualifying as cash flow hedges
|
70
|
|
(57
|
)
|
4
|
|
||||||
|
Pension and postretirement benefits
|
53
|
|
214
|
|
(17
|
)
|
||||||
|
Available-for-sale securities
|
(25
|
)
|
39
|
|
16
|
|
||||||
|
Foreign currency translation
|
(254
|
)
|
18
|
|
(38
|
)
|
||||||
|
Total Other Comprehensive (Loss)/Income
|
(156
|
)
|
214
|
|
(35
|
)
|
||||||
|
Comprehensive Income
|
4,791
|
|
1,189
|
|
4,472
|
|
||||||
|
Comprehensive Income/(Loss) Attributable to Noncontrolling Interest
|
27
|
|
(32
|
)
|
50
|
|
||||||
|
Comprehensive Income Attributable to BMS
|
$
|
4,764
|
|
$
|
1,221
|
|
$
|
4,422
|
|
|||
|
|
December 31,
|
|||||||
|
ASSETS
|
2018
|
2017
|
||||||
|
Current Assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
6,911
|
|
$
|
5,421
|
|
||
|
Marketable securities
|
1,973
|
|
1,391
|
|
||||
|
Receivables
|
5,965
|
|
6,300
|
|
||||
|
Inventories
|
1,195
|
|
1,166
|
|
||||
|
Prepaid expenses and other
|
1,116
|
|
576
|
|
||||
|
Total Current Assets
|
17,160
|
|
14,854
|
|
||||
|
Property, plant and equipment
|
5,027
|
|
5,001
|
|
||||
|
Goodwill
|
6,538
|
|
6,863
|
|
||||
|
Other intangible assets
|
1,091
|
|
1,210
|
|
||||
|
Deferred income taxes
|
1,371
|
|
1,610
|
|
||||
|
Marketable securities
|
1,775
|
|
2,480
|
|
||||
|
Other assets
|
2,024
|
|
1,533
|
|
||||
|
Total Assets
|
$
|
34,986
|
|
$
|
33,551
|
|
||
|
LIABILITIES
|
||||||||
|
Current Liabilities:
|
||||||||
|
Short-term debt obligations
|
$
|
1,703
|
|
$
|
987
|
|
||
|
Accounts payable
|
1,892
|
|
2,248
|
|
||||
|
Accrued liabilities
|
6,489
|
|
6,014
|
|
||||
|
Deferred income
|
172
|
|
83
|
|
||||
|
Income taxes payable
|
398
|
|
231
|
|
||||
|
Total Current Liabilities
|
10,654
|
|
9,563
|
|
||||
|
Deferred income
|
468
|
|
454
|
|
||||
|
Income taxes payable
|
3,043
|
|
3,548
|
|
||||
|
Pension and other liabilities
|
1,048
|
|
1,164
|
|
||||
|
Long-term debt
|
5,646
|
|
6,975
|
|
||||
|
Total Liabilities
|
20,859
|
|
21,704
|
|
||||
|
Commitments and contingencies
|
|
|
||||||
|
EQUITY
|
||||||||
|
Bristol-Myers Squibb Company Shareholders’ Equity:
|
||||||||
|
Preferred stock, $2 convertible series, par value $1 per share: Authorized 10 million shares; issued and outstanding 3,590 in 2018 and 4,070 in 2017, liquidation value of $50 per share
|
—
|
|
—
|
|
||||
|
Common stock, par value of $0.10 per share: Authorized 4.5 billion shares; 2.2 billion issued in both 2018 and 2017
|
221
|
|
221
|
|
||||
|
Capital in excess of par value of stock
|
2,081
|
|
1,898
|
|
||||
|
Accumulated other comprehensive loss
|
(2,762
|
)
|
(2,289
|
)
|
||||
|
Retained earnings
|
34,065
|
|
31,160
|
|
||||
|
Less cost of treasury stock — 576 million common shares in 2018 and 575 million common shares in 2017
|
(19,574
|
)
|
(19,249
|
)
|
||||
|
Total Bristol-Myers Squibb Company Shareholders' Equity
|
14,031
|
|
11,741
|
|
||||
|
Noncontrolling interest
|
96
|
|
106
|
|
||||
|
Total Equity
|
14,127
|
|
11,847
|
|
||||
|
Total Liabilities and Equity
|
$
|
34,986
|
|
$
|
33,551
|
|
||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2018
|
2017
|
2016
|
|||||||||
|
Cash Flows From Operating Activities:
|
||||||||||||
|
Net earnings
|
$
|
4,947
|
|
$
|
975
|
|
$
|
4,507
|
|
|||
|
Adjustments to reconcile net earnings to net cash provided by operating activities:
|
||||||||||||
|
Depreciation and amortization, net
|
637
|
|
789
|
|
382
|
|
||||||
|
Deferred income taxes
|
86
|
|
1,010
|
|
(204
|
)
|
||||||
|
Stock-based compensation
|
221
|
|
199
|
|
205
|
|
||||||
|
Impairment charges
|
126
|
|
327
|
|
63
|
|
||||||
|
Pension settlements and amortization
|
186
|
|
236
|
|
169
|
|
||||||
|
Divestiture gains and royalties
|
(992
|
)
|
(706
|
)
|
(1,187
|
)
|
||||||
|
Asset acquisition charges
|
85
|
|
760
|
|
274
|
|
||||||
|
Loss/(gain) on equity investments
|
512
|
|
(23
|
)
|
37
|
|
||||||
|
Other adjustments
|
(44
|
)
|
120
|
|
(36
|
)
|
||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Receivables
|
(429
|
)
|
(431
|
)
|
(803
|
)
|
||||||
|
Inventories
|
(216
|
)
|
(29
|
)
|
(152
|
)
|
||||||
|
Accounts payable
|
(59
|
)
|
320
|
|
104
|
|
||||||
|
Deferred income
|
84
|
|
(642
|
)
|
(64
|
)
|
||||||
|
Income taxes payable
|
162
|
|
2,597
|
|
(453
|
)
|
||||||
|
Other
|
634
|
|
(227
|
)
|
216
|
|
||||||
|
Net Cash Provided by Operating Activities
|
5,940
|
|
5,275
|
|
3,058
|
|
||||||
|
Cash Flows From Investing Activities:
|
||||||||||||
|
Sale and maturities of marketable securities
|
2,379
|
|
6,412
|
|
4,809
|
|
||||||
|
Purchase of marketable securities
|
(2,305
|
)
|
(5,437
|
)
|
(3,089
|
)
|
||||||
|
Capital expenditures
|
(951
|
)
|
(1,055
|
)
|
(1,215
|
)
|
||||||
|
Divestiture and other proceeds
|
1,249
|
|
722
|
|
1,334
|
|
||||||
|
Acquisition and other payments
|
(1,246
|
)
|
(708
|
)
|
(359
|
)
|
||||||
|
Net Cash (Used in)/Provided by Investing Activities
|
(874
|
)
|
(66
|
)
|
1,480
|
|
||||||
|
Cash Flows From Financing Activities:
|
||||||||||||
|
Short-term debt obligations, net
|
(543
|
)
|
727
|
|
125
|
|
||||||
|
Issuance of long-term debt
|
—
|
|
1,488
|
|
—
|
|
||||||
|
Repayment of long-term debt
|
(5
|
)
|
(1,224
|
)
|
(15
|
)
|
||||||
|
Repurchase of common stock
|
(320
|
)
|
(2,469
|
)
|
(231
|
)
|
||||||
|
Dividends
|
(2,613
|
)
|
(2,577
|
)
|
(2,547
|
)
|
||||||
|
Other
|
(54
|
)
|
(22
|
)
|
15
|
|
||||||
|
Net Cash Used in Financing Activities
|
(3,535
|
)
|
(4,077
|
)
|
(2,653
|
)
|
||||||
|
Effect of Exchange Rates on Cash and Cash Equivalents
|
(41
|
)
|
52
|
|
(33
|
)
|
||||||
|
Increase in Cash and Cash Equivalents
|
1,490
|
|
1,184
|
|
1,852
|
|
||||||
|
Cash and Cash Equivalents at Beginning of Year
|
5,421
|
|
4,237
|
|
2,385
|
|
||||||
|
Cash and Cash Equivalents at End of Year
|
$
|
6,911
|
|
$
|
5,421
|
|
$
|
4,237
|
|
|||
|
Year Ended December 31,
|
||||||||||||||||
|
2017
|
2016
|
|||||||||||||||
|
Dollars in Millions
|
As Reported
|
As Adjusted
|
As Reported
|
As Adjusted
|
||||||||||||
|
Cost of products sold
|
$
|
6,066
|
|
$
|
6,094
|
|
$
|
4,946
|
|
$
|
4,969
|
|
||||
|
Marketing, selling and administrative
|
4,687
|
|
4,751
|
|
4,911
|
|
4,979
|
|
||||||||
|
Research and development
|
6,411
|
|
6,482
|
|
4,940
|
|
5,012
|
|
||||||||
|
Other income (net)
|
(1,519
|
)
|
(1,682
|
)
|
(1,285
|
)
|
(1,448
|
)
|
||||||||
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Net product sales
|
$
|
21,581
|
|
$
|
19,258
|
|
$
|
17,702
|
|
|||
|
Alliance revenues
|
647
|
|
962
|
|
1,252
|
|
||||||
|
Other revenues
|
333
|
|
556
|
|
473
|
|
||||||
|
Total Revenues
|
$
|
22,561
|
|
$
|
20,776
|
|
$
|
19,427
|
|
|||
|
|
2018
|
2017
|
2016
|
||||||
|
McKesson Corporation
|
25
|
%
|
24
|
%
|
22
|
%
|
|||
|
AmerisourceBergen Corporation
|
20
|
%
|
18
|
%
|
18
|
%
|
|||
|
Cardinal Health, Inc.
|
17
|
%
|
15
|
%
|
14
|
%
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Gross product sales
|
$
|
30,174
|
|
$
|
25,499
|
|
$
|
22,364
|
|
|||
|
GTN adjustments
(a)
|
||||||||||||
|
Charge-backs and cash discounts
|
(2,735
|
)
|
(2,084
|
)
|
(1,582
|
)
|
||||||
|
Medicaid and Medicare rebates
|
(3,225
|
)
|
(2,086
|
)
|
(1,382
|
)
|
||||||
|
Other rebates, returns, discounts and adjustments
|
(2,633
|
)
|
(2,071
|
)
|
(1,698
|
)
|
||||||
|
Total GTN adjustments
|
(8,593
|
)
|
(6,241
|
)
|
(4,662
|
)
|
||||||
|
Net product sales
|
$
|
21,581
|
|
$
|
19,258
|
|
$
|
17,702
|
|
|||
|
(a)
|
Includes adjustments for provisions for product sales made in prior periods resulting from changes in estimates of
$96 million
,
$71 million
and
$155 million
for the years ended December 31, 2018, 2017 and 2016, respectively.
|
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Prioritized Brands
|
||||||||||||
|
Opdivo
|
$
|
6,735
|
|
$
|
4,948
|
|
$
|
3,774
|
|
|||
|
Eliquis
|
6,438
|
|
4,872
|
|
3,343
|
|
||||||
|
Orencia
|
2,710
|
|
2,479
|
|
2,265
|
|
||||||
|
Sprycel
|
2,000
|
|
2,005
|
|
1,824
|
|
||||||
|
Yervoy
|
1,330
|
|
1,244
|
|
1,053
|
|
||||||
|
Empliciti
|
247
|
|
231
|
|
150
|
|
||||||
|
Established Brands
|
||||||||||||
|
Baraclude
|
744
|
|
1,052
|
|
1,192
|
|
||||||
|
Reyataz Franchise
|
427
|
|
698
|
|
912
|
|
||||||
|
Sustiva Franchise
|
283
|
|
729
|
|
1,065
|
|
||||||
|
Hepatitis C Franchise
|
17
|
|
406
|
|
1,578
|
|
||||||
|
Other Brands
|
1,630
|
|
2,112
|
|
2,271
|
|
||||||
|
Total Revenues
|
$
|
22,561
|
|
$
|
20,776
|
|
$
|
19,427
|
|
|||
|
United States
|
$
|
12,586
|
|
$
|
11,358
|
|
$
|
10,720
|
|
|||
|
Europe
|
5,658
|
|
4,988
|
|
4,215
|
|
||||||
|
Rest of World
|
3,733
|
|
3,877
|
|
3,964
|
|
||||||
|
Other
(a)
|
584
|
|
553
|
|
528
|
|
||||||
|
Total Revenues
|
$
|
22,561
|
|
$
|
20,776
|
|
$
|
19,427
|
|
|||
|
(a)
|
Other revenues included royalties and alliance-related revenues for products not sold by our regional commercial organizations.
|
|
Dollars in Millions
|
December 31, 2018
|
January 1, 2018
|
||||||
|
Prepaid expenses and other
|
$
|
35
|
|
$
|
349
|
|
||
|
Other assets
|
19
|
|
32
|
|
||||
|
Total Contract Assets
|
$
|
54
|
|
$
|
381
|
|
||
|
•
|
When BMS is the principal in the end customer sale, 100% of product sales are included in Net product sales. When BMS's alliance partner is the principal in the end customer sale, BMS's contractual share of the third-party sales and/or royalty income are included in Alliance revenues as the sale of commercial products are considered part of BMS's ongoing major or central operations. Refer to “—Note
2
. Revenue” for information regarding recognition criteria.
|
|
•
|
Amounts payable to BMS by alliance partners (who are the principal in the end customer sale) for supply of commercial products are included in Alliance revenues as the sale of commercial products are considered part of BMS's ongoing major or central operations.
|
|
•
|
Profit sharing, royalties and other sales-based fees payable by BMS to alliance partners are included in Cost of products sold as incurred.
|
|
•
|
Cost reimbursements between the parties are recognized as incurred and included in Cost of products sold; Marketing, selling and administrative expenses; or Research and development expenses, based on the underlying nature of the related activities subject to reimbursement.
|
|
•
|
Upfront and contingent development and approval milestones payable to BMS by alliance partners for investigational compounds and commercial products are deferred and amortized over the expected period of BMS's development and co-promotion obligation through the market exclusivity period or the periods in which the related compounds or products are expected to contribute to future cash flows. The amortization is presented consistent with the nature of the payment under the arrangement. For example, amounts received for investigational compounds are presented in Other income (net) as the activities being performed at that time are not related to the sale of commercial products included in BMS’s ongoing major or central operations; amounts received for commercial products are presented in alliance revenue as the sale of commercial products are considered part of BMS’s ongoing major or central operations.
|
|
•
|
Upfront and contingent approval milestones payable by BMS to alliance partners for commercial products are capitalized and amortized over the shorter of the contractual term or the periods in which the related products are expected to contribute to future cash flows. The amortization is included in Cost of products sold.
|
|
•
|
Upfront and contingent milestones payable by BMS to alliance partners prior to regulatory approval are expensed as incurred and included in Research and development expense.
|
|
•
|
Royalties and other contingent consideration payable to BMS by alliance partners related to the divestiture of such businesses are included in Other income (net) when earned.
|
|
•
|
All payments between BMS and its alliance partners are presented in Cash Flows From Operating Activities, except as otherwise described below.
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
||||||||
|
Revenues from alliances:
|
|||||||||||
|
Net product sales
|
$
|
8,359
|
|
$
|
6,917
|
|
$
|
5,530
|
|
||
|
Alliance revenues
|
647
|
|
962
|
|
1,252
|
|
|||||
|
Total Revenues
|
$
|
9,006
|
|
$
|
7,879
|
|
$
|
6,782
|
|
||
|
Payments to/(from) alliance partners:
|
|||||||||||
|
Cost of products sold
|
$
|
3,439
|
|
$
|
2,718
|
|
$
|
2,126
|
|
||
|
Marketing, selling and administrative
|
(104
|
)
|
(62
|
)
|
(30
|
)
|
|||||
|
Research and development
|
1,044
|
|
(28
|
)
|
(9
|
)
|
|||||
|
Other income (net)
|
(67
|
)
|
(46
|
)
|
(42
|
)
|
|||||
|
Selected Alliance Balance Sheet Information:
|
December 31,
|
|||||||
|
Dollars in Millions
|
2018
|
2017
|
||||||
|
Receivables – from alliance partners
|
$
|
395
|
|
$
|
322
|
|
||
|
Accounts payable – to alliance partners
|
904
|
|
875
|
|
||||
|
Deferred income from alliances
(a)
|
491
|
|
467
|
|
||||
|
(a)
|
Includes unamortized upfront and milestone payments.
|
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Revenues from Pfizer alliance:
|
||||||||||||
|
Net product sales
|
$
|
6,329
|
|
$
|
4,808
|
|
$
|
3,306
|
|
|||
|
Alliance revenues
|
109
|
|
64
|
|
37
|
|
||||||
|
Total Revenues
|
$
|
6,438
|
|
$
|
4,872
|
|
$
|
3,343
|
|
|||
|
Payments to/(from) Pfizer:
|
||||||||||||
|
Cost of products sold – Profit sharing
|
$
|
3,078
|
|
$
|
2,314
|
|
$
|
1,595
|
|
|||
|
Other income (net) – Amortization of deferred income
|
(55
|
)
|
(55
|
)
|
(55
|
)
|
||||||
|
Selected Alliance Balance Sheet Information:
|
December 31,
|
|||||||
|
Dollars in Millions
|
2018
|
2017
|
||||||
|
Receivables
|
$
|
220
|
|
$
|
193
|
|
||
|
Accounts payable
|
786
|
|
625
|
|
||||
|
Deferred income
|
$
|
410
|
|
$
|
466
|
|
||
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Revenues from Otsuka alliances:
|
||||||||||||
|
Net product sales – Oncology territory
|
$
|
1,705
|
|
$
|
1,699
|
|
$
|
1,544
|
|
|||
|
Payments to Otsuka:
|
||||||||||||
|
Cost of products sold – Oncology fee
|
$
|
297
|
|
$
|
299
|
|
$
|
304
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Revenues from Ono alliances:
|
||||||||||||
|
Net product sales
|
$
|
165
|
|
$
|
145
|
|
$
|
147
|
|
|||
|
Alliance revenues
|
294
|
|
268
|
|
280
|
|
||||||
|
Total Revenues
|
$
|
459
|
|
$
|
413
|
|
$
|
427
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Revenues from Gilead alliances:
|
||||||||||||
|
Alliance revenues
|
$
|
253
|
|
$
|
623
|
|
$
|
934
|
|
|||
|
Equity in net loss of affiliates
|
$
|
2
|
|
$
|
13
|
|
$
|
12
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Revenues from AbbVie alliance:
|
||||||||||||
|
Net product sales
|
$
|
162
|
|
$
|
150
|
|
$
|
132
|
|
|||
|
Payments to AbbVie:
|
||||||||||||
|
Cost of products sold – Profit sharing
|
$
|
44
|
|
$
|
41
|
|
$
|
34
|
|
|||
|
Dollars in Millions
|
Year
|
Upfront Payment
|
R&D Expense
|
Deferred Tax Assets
(a)
|
Contingent Consideration
|
|||||||||||||
|
IFM
(b)
|
2017
|
$
|
325
|
|
$
|
311
|
|
$
|
14
|
|
$
|
2,020
|
|
|||||
|
Cormorant
|
2016
|
35
|
|
35
|
|
—
|
|
485
|
|
|||||||||
|
Padlock
|
2016
|
150
|
|
139
|
|
11
|
|
453
|
|
|||||||||
|
(a)
|
Relates to net operating loss and tax credit carryforwards.
|
|
(b)
|
Includes
$25 million
for certain negotiation rights to collaborate, license or acquire an NLRP3 antagonist program from a newly formed entity established by the former shareholders of IFM.
|
|
Proceeds
(a)
|
Divestiture Gains
|
Royalty Income
|
|||||||||||||||||||||||||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
2018
|
2017
|
2016
|
2018
|
2017
|
2016
|
||||||||||||||||||||||||||
|
Diabetes Business
|
$
|
579
|
|
$
|
405
|
|
$
|
333
|
|
$
|
—
|
|
$
|
(126
|
)
|
$
|
—
|
|
$
|
(661
|
)
|
$
|
(329
|
)
|
$
|
(361
|
)
|
||||||||
|
Erbitux*
Business
|
216
|
|
218
|
|
252
|
|
—
|
|
—
|
|
—
|
|
(145
|
)
|
(224
|
)
|
(246
|
)
|
|||||||||||||||||
|
Manufacturing Operations
|
160
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||
|
Plavix*
and
Avapro*
/
Avalide*
|
80
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||
|
Investigational HIV Business
|
—
|
|
—
|
|
387
|
|
—
|
|
(11
|
)
|
(272
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||
|
OTC Business
|
—
|
|
—
|
|
317
|
|
—
|
|
—
|
|
(277
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||
|
Mature Brands and Other
|
212
|
|
28
|
|
28
|
|
(178
|
)
|
(24
|
)
|
(15
|
)
|
(8
|
)
|
(4
|
)
|
(11
|
)
|
|||||||||||||||||
|
$
|
1,247
|
|
$
|
651
|
|
$
|
1,317
|
|
$
|
(178
|
)
|
$
|
(161
|
)
|
$
|
(564
|
)
|
$
|
(814
|
)
|
$
|
(557
|
)
|
$
|
(618
|
)
|
|||||||||
|
(a)
|
Includes royalties received subsequent to the related sale of the asset or business.
|
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Interest expense
|
$
|
183
|
|
$
|
196
|
|
$
|
167
|
|
|||
|
Investment income
|
(173
|
)
|
(126
|
)
|
(97
|
)
|
||||||
|
Loss/(gain) on equity investments
|
512
|
|
(23
|
)
|
37
|
|
||||||
|
Provision for restructuring
|
131
|
|
293
|
|
109
|
|
||||||
|
Litigation and other settlements
|
76
|
|
(487
|
)
|
47
|
|
||||||
|
Equity in net income of affiliates
|
(93
|
)
|
(75
|
)
|
(77
|
)
|
||||||
|
Divestiture gains
|
(178
|
)
|
(164
|
)
|
(576
|
)
|
||||||
|
Royalties and licensing income
|
(1,353
|
)
|
(1,351
|
)
|
(719
|
)
|
||||||
|
Transition and other service fees
|
(12
|
)
|
(37
|
)
|
(238
|
)
|
||||||
|
Pension and postretirement
|
(27
|
)
|
(1
|
)
|
(72
|
)
|
||||||
|
Intangible asset impairment
|
64
|
|
—
|
|
15
|
|
||||||
|
Loss on debt redemption
|
—
|
|
109
|
|
—
|
|
||||||
|
Other
|
20
|
|
(16
|
)
|
(44
|
)
|
||||||
|
Other income (net)
|
$
|
(850
|
)
|
$
|
(1,682
|
)
|
$
|
(1,448
|
)
|
|||
|
•
|
Loss/(gain) on equity investments includes a fair value adjustment of
$534 million
related to the Company's equity investment in Nektar in 2018.
|
|
•
|
Litigation and other settlements include
$481 million
for BMS's share of a patent-infringement settlement related to Merck's PD-1 antibody
Keytruda*
in 2017.
|
|
•
|
Royalties and licensing income includes royalties resulting from business divestitures, intellectual property legal settlements and upfront licensing fees including
$470 million
from Biogen and Roche in 2017.
|
|
•
|
Transition and other service fees were primarily related to the divestiture of the diabetes and investigational HIV medicines businesses in 2016.
|
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Employee termination costs
|
$
|
87
|
|
$
|
267
|
|
$
|
97
|
|
|||
|
Other termination costs
|
44
|
|
26
|
|
12
|
|
||||||
|
Provision for restructuring
|
131
|
|
293
|
|
109
|
|
||||||
|
Accelerated depreciation
|
113
|
|
289
|
|
72
|
|
||||||
|
Asset impairments
|
16
|
|
241
|
|
13
|
|
||||||
|
Other shutdown costs
|
8
|
|
3
|
|
19
|
|
||||||
|
Total charges
|
$
|
268
|
|
$
|
826
|
|
$
|
213
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Cost of products sold
|
$
|
57
|
|
$
|
149
|
|
$
|
21
|
|
|||
|
Marketing, selling and administrative
|
1
|
|
1
|
|
—
|
|
||||||
|
Research and development
|
79
|
|
383
|
|
83
|
|
||||||
|
Other income (net)
|
131
|
|
293
|
|
109
|
|
||||||
|
Total charges
|
$
|
268
|
|
$
|
826
|
|
$
|
213
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Liability at January 1
|
$
|
186
|
|
$
|
114
|
|
$
|
125
|
|
|||
|
Charges
|
148
|
|
319
|
|
116
|
|
||||||
|
Change in estimates
|
(17
|
)
|
(26
|
)
|
(7
|
)
|
||||||
|
Provision for restructuring
|
131
|
|
293
|
|
109
|
|
||||||
|
Foreign currency translation and other
|
1
|
|
18
|
|
—
|
|
||||||
|
Payments
|
(219
|
)
|
(239
|
)
|
(120
|
)
|
||||||
|
Liability at December 31
|
$
|
99
|
|
$
|
186
|
|
$
|
114
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Current:
|
||||||||||||
|
U.S.
|
$
|
485
|
|
$
|
2,782
|
|
$
|
1,144
|
|
|||
|
Non-U.S.
|
450
|
|
364
|
|
468
|
|
||||||
|
Total Current
|
935
|
|
3,146
|
|
1,612
|
|
||||||
|
Deferred:
|
||||||||||||
|
U.S.
|
29
|
|
1,063
|
|
(101
|
)
|
||||||
|
Non-U.S.
|
57
|
|
(53
|
)
|
(103
|
)
|
||||||
|
Total Deferred
|
86
|
|
1,010
|
|
(204
|
)
|
||||||
|
Total Provision
|
$
|
1,021
|
|
$
|
4,156
|
|
$
|
1,408
|
|
|||
|
% of Earnings Before Income Taxes
|
||||||||||||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||||||||||
|
Earnings before income taxes:
|
||||||||||||||||||||
|
U.S.
|
$
|
2,338
|
|
$
|
2,280
|
|
$
|
3,100
|
|
|||||||||||
|
Non-U.S.
|
3,630
|
|
2,851
|
|
2,815
|
|
||||||||||||||
|
Total
|
$
|
5,968
|
|
$
|
5,131
|
|
$
|
5,915
|
|
|||||||||||
|
U.S. statutory rate
|
1,253
|
|
21.0
|
%
|
1,796
|
|
35.0
|
%
|
2,070
|
|
35.0
|
%
|
||||||||
|
Deemed repatriation transition tax
|
(56
|
)
|
(0.9
|
)%
|
2,611
|
|
50.9
|
%
|
—
|
|
—
|
|
||||||||
|
Deferred tax remeasurement
|
—
|
|
—
|
|
285
|
|
5.6
|
%
|
—
|
|
—
|
|
||||||||
|
Global intangible low taxed income (GILTI)
|
94
|
|
1.6
|
%
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||
|
Foreign tax effect of certain operations in Ireland, Puerto Rico and Switzerland
|
(202
|
)
|
(3.4
|
)%
|
(561
|
)
|
(10.9
|
)%
|
(442
|
)
|
(7.5
|
)%
|
||||||||
|
U.S. Federal valuation allowance
|
119
|
|
2.0
|
%
|
—
|
|
—
|
|
(29
|
)
|
(0.5
|
)%
|
||||||||
|
U.S. Federal, state and foreign contingent tax matters
|
(55
|
)
|
(0.9
|
)%
|
72
|
|
1.4
|
%
|
87
|
|
1.5
|
%
|
||||||||
|
U.S. Federal research based credits
|
(138
|
)
|
(2.3
|
)%
|
(144
|
)
|
(2.8
|
)%
|
(144
|
)
|
(2.4
|
)%
|
||||||||
|
Goodwill allocated to divestitures
|
—
|
|
—
|
|
4
|
|
0.1
|
%
|
34
|
|
0.6
|
%
|
||||||||
|
U.S. Branded Prescription Drug Fee
|
21
|
|
0.3
|
%
|
52
|
|
1.0
|
%
|
52
|
|
0.9
|
%
|
||||||||
|
Non-deductible R&D charges
|
17
|
|
0.3
|
%
|
266
|
|
5.2
|
%
|
100
|
|
1.7
|
%
|
||||||||
|
Puerto Rico excise tax
|
(152
|
)
|
(2.6
|
)%
|
(131
|
)
|
(2.6
|
)%
|
(131
|
)
|
(2.2
|
)%
|
||||||||
|
Domestic manufacturing deduction
|
—
|
|
—
|
|
(78
|
)
|
(1.5
|
)%
|
(122
|
)
|
(2.1
|
)%
|
||||||||
|
State and local taxes (net of valuation allowance)
|
67
|
|
1.1
|
%
|
77
|
|
1.5
|
%
|
23
|
|
0.4
|
%
|
||||||||
|
Foreign and other
|
53
|
|
0.9
|
%
|
(93
|
)
|
(1.9
|
)%
|
(90
|
)
|
(1.6
|
)%
|
||||||||
|
$
|
1,021
|
|
17.1
|
%
|
$
|
4,156
|
|
81.0
|
%
|
$
|
1,408
|
|
23.8
|
%
|
||||||
|
|
December 31,
|
|||||||
|
Dollars in Millions
|
2018
|
2017
|
||||||
|
Deferred tax assets
|
||||||||
|
Foreign net operating loss carryforwards
|
$
|
2,978
|
|
$
|
2,872
|
|
||
|
State net operating loss and credit carryforwards
|
121
|
|
143
|
|
||||
|
U.S. Federal net operating loss and credit carryforwards
|
67
|
|
99
|
|
||||
|
Deferred income
|
188
|
|
212
|
|
||||
|
Milestone payments and license fees
|
552
|
|
386
|
|
||||
|
Pension and postretirement benefits
|
26
|
|
131
|
|
||||
|
Intercompany profit and other inventory items
|
670
|
|
651
|
|
||||
|
Other foreign deferred tax assets
|
327
|
|
312
|
|
||||
|
Share-based compensation
|
54
|
|
60
|
|
||||
|
Other
|
352
|
|
280
|
|
||||
|
Total deferred tax assets
|
5,335
|
|
5,146
|
|
||||
|
Valuation allowance
|
(3,193
|
)
|
(2,827
|
)
|
||||
|
Deferred tax assets net of valuation allowance
|
2,142
|
|
2,319
|
|
||||
|
Deferred tax liabilities
|
||||||||
|
Depreciation
|
(61
|
)
|
(11
|
)
|
||||
|
Acquired intangible assets
|
(220
|
)
|
(216
|
)
|
||||
|
Goodwill and other
|
(533
|
)
|
(527
|
)
|
||||
|
Total deferred tax liabilities
|
(814
|
)
|
(754
|
)
|
||||
|
Deferred tax assets, net
|
$
|
1,328
|
|
$
|
1,565
|
|
||
|
Recognized as:
|
||||||||
|
Deferred income taxes – non-current
|
$
|
1,371
|
|
$
|
1,610
|
|
||
|
Income taxes payable – non-current
|
(18
|
)
|
(45
|
)
|
||||
|
Liabilities related to assets held-for-sale
|
(25
|
)
|
—
|
|
||||
|
Total
|
$
|
1,328
|
|
$
|
1,565
|
|
||
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Balance at beginning of year
|
$
|
2,827
|
|
$
|
3,078
|
|
$
|
3,534
|
|
|||
|
Provision
|
458
|
|
50
|
|
39
|
|
||||||
|
Utilization
|
(43
|
)
|
(335
|
)
|
(355
|
)
|
||||||
|
Foreign currency translation
|
(48
|
)
|
341
|
|
(142
|
)
|
||||||
|
Acquisitions
|
—
|
|
2
|
|
2
|
|
||||||
|
Non U.S. rate change
|
(1
|
)
|
(309
|
)
|
—
|
|
||||||
|
Balance at end of year
|
$
|
3,193
|
|
$
|
2,827
|
|
$
|
3,078
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Balance at beginning of year
|
$
|
1,155
|
|
$
|
995
|
|
$
|
944
|
|
|||
|
Gross additions to tax positions related to current year
|
48
|
|
173
|
|
49
|
|
||||||
|
Gross additions to tax positions related to prior years
|
21
|
|
30
|
|
49
|
|
||||||
|
Gross additions to tax positions assumed in acquisitions
|
—
|
|
—
|
|
1
|
|
||||||
|
Gross reductions to tax positions related to prior years
|
(106
|
)
|
(22
|
)
|
(22
|
)
|
||||||
|
Settlements
|
2
|
|
(20
|
)
|
(13
|
)
|
||||||
|
Reductions to tax positions related to lapse of statute
|
(119
|
)
|
(13
|
)
|
(4
|
)
|
||||||
|
Cumulative translation adjustment
|
(6
|
)
|
12
|
|
(9
|
)
|
||||||
|
Balance at end of year
|
$
|
995
|
|
$
|
1,155
|
|
$
|
995
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Unrecognized tax benefits that if recognized would impact the effective tax rate
|
$
|
853
|
|
$
|
1,002
|
|
$
|
854
|
|
|||
|
Accrued interest
|
167
|
|
148
|
|
112
|
|
||||||
|
Accrued penalties
|
11
|
|
15
|
|
17
|
|
||||||
|
U.S.
|
2008 to 2012, 2015 to 2018
|
|
|
Canada
|
2009 to 2018
|
|
|
France
|
2015 to 2018
|
|
|
Germany
|
2008 to 2018
|
|
|
Italy
|
2017 to 2018
|
|
|
Mexico
|
2013 to 2018
|
|
|
|
Year Ended December 31,
|
|||||||||||
|
Amounts in Millions, Except Per Share Data
|
2018
|
2017
|
2016
|
|||||||||
|
Net Earnings Attributable to BMS used for Basic and Diluted EPS Calculation
|
$
|
4,920
|
|
$
|
1,007
|
|
$
|
4,457
|
|
|||
|
Weighted-average common shares outstanding - basic
|
1,633
|
|
1,645
|
|
1,671
|
|
||||||
|
Incremental shares attributable to share-based compensation plans
|
4
|
|
7
|
|
9
|
|
||||||
|
Weighted-average common shares outstanding - diluted
|
1,637
|
|
1,652
|
|
1,680
|
|
||||||
|
Earnings per share - basic
|
$
|
3.01
|
|
$
|
0.61
|
|
$
|
2.67
|
|
|||
|
Earnings per share - diluted
|
3.01
|
|
0.61
|
|
2.65
|
|
||||||
|
December 31, 2018
|
December 31, 2017
|
|||||||||||||||
|
Dollars in Millions
|
Level 1
|
Level 2
|
Level 1
|
Level 2
|
||||||||||||
|
Cash and cash equivalents - Money market and other securities
|
$
|
—
|
|
$
|
6,173
|
|
$
|
—
|
|
$
|
4,728
|
|
||||
|
Marketable securities:
|
||||||||||||||||
|
Certificates of deposit
|
—
|
|
971
|
|
—
|
|
141
|
|
||||||||
|
Commercial paper
|
—
|
|
273
|
|
—
|
|
50
|
|
||||||||
|
Corporate debt securities
|
—
|
|
2,379
|
|
—
|
|
3,548
|
|
||||||||
|
Equity investments
|
—
|
|
125
|
|
—
|
|
132
|
|
||||||||
|
Derivative assets
|
—
|
|
44
|
|
—
|
|
13
|
|
||||||||
|
Equity investments
|
88
|
|
266
|
|
67
|
|
—
|
|
||||||||
|
Derivative liabilities
|
—
|
|
(31
|
)
|
—
|
|
(52
|
)
|
||||||||
|
December 31, 2018
|
December 31, 2017
|
||||||||||||||||||||||||||||||
|
Dollars in Millions
|
Amortized
Cost |
Gross Unrealized
|
Fair Value
|
Amortized
Cost |
Gross Unrealized
|
Fair Value
|
|||||||||||||||||||||||||
|
Gains
|
Losses
|
Gains
|
Losses
|
||||||||||||||||||||||||||||
|
Certificates of deposit
|
$
|
971
|
|
$
|
—
|
|
$
|
—
|
|
$
|
971
|
|
$
|
141
|
|
$
|
—
|
|
$
|
—
|
|
$
|
141
|
|
|||||||
|
Commercial paper
|
273
|
|
—
|
|
—
|
|
273
|
|
50
|
|
—
|
|
—
|
|
50
|
|
|||||||||||||||
|
Corporate debt securities
|
2,416
|
|
—
|
|
(37
|
)
|
2,379
|
|
3,555
|
|
3
|
|
(10
|
)
|
3,548
|
|
|||||||||||||||
|
Equity investments
(a)
|
—
|
|
—
|
|
—
|
|
—
|
|
31
|
|
37
|
|
(1
|
)
|
67
|
|
|||||||||||||||
|
$
|
3,660
|
|
$
|
—
|
|
$
|
(37
|
)
|
$
|
3,623
|
|
$
|
3,777
|
|
$
|
40
|
|
$
|
(11
|
)
|
$
|
3,806
|
|
||||||||
|
Equity investments
(b)
|
479
|
|
132
|
|
|||||||||||||||||||||||||||
|
Total
|
$
|
4,102
|
|
$
|
3,938
|
|
|||||||||||||||||||||||||
|
Dollars in Millions
|
December 31,
2018 |
December 31,
2017 |
|||||
|
Current marketable securities
|
$
|
1,973
|
|
$
|
1,391
|
|
|
|
Non-current marketable securities
(c)
|
1,775
|
|
2,480
|
|
|||
|
Other assets
(a)
|
354
|
|
67
|
|
|||
|
Total
|
$
|
4,102
|
|
$
|
3,938
|
|
|
|
(a)
|
Includes equity investments with readily determinable fair values not measured using the fair value option as of
December 31, 2017
.
|
|
(b)
|
Includes equity and fixed income funds measured using the fair value option at
December 31, 2017
. Refer to “
—
Note.
1
Accounting Policies and Recently Issued Accounting Standards
” for more information.
|
|
(c)
|
All non-current marketable securities mature within five years as of
December 31, 2018
and
December 31, 2017
.
|
|
Year Ended December 31,
|
||||
|
Dollars in Millions
|
2018
|
|||
|
Net loss recognized
|
$
|
(530
|
)
|
|
|
Less: Net gain recognized for equity investments sold
|
7
|
|
||
|
Net unrealized loss on equity investments held
|
$
|
(537
|
)
|
|
|
|
December 31, 2018
|
December 31, 2017
|
|||||||||||||||||||||||||||||
|
Asset
(a)
|
Liability
(b)
|
Asset
(a)
|
Liability
(b)
|
||||||||||||||||||||||||||||
|
Dollars in Millions
|
Notional
|
Fair Value
|
Notional
|
Fair Value
|
Notional
|
Fair Value
|
Notional
|
Fair Value
|
|||||||||||||||||||||||
|
Derivatives designated as hedging instruments:
|
|||||||||||||||||||||||||||||||
|
Interest rate swap contracts
|
$
|
—
|
|
$
|
—
|
|
$
|
755
|
|
$
|
(10
|
)
|
$
|
—
|
|
$
|
—
|
|
$
|
755
|
|
$
|
(6
|
)
|
|||||||
|
Cross-currency interest rate swap contracts
|
50
|
|
—
|
|
250
|
|
(5
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||
|
Foreign currency forward contracts
|
1,503
|
|
44
|
|
496
|
|
(10
|
)
|
944
|
|
12
|
|
489
|
|
(9
|
)
|
|||||||||||||||
|
Derivatives not designated as hedging instruments:
|
|||||||||||||||||||||||||||||||
|
Foreign currency forward contracts
|
54
|
|
—
|
|
600
|
|
(6
|
)
|
206
|
|
1
|
|
1,369
|
|
(37
|
)
|
|||||||||||||||
|
(a)
|
Included in prepaid expenses and other and other assets.
|
|
(b)
|
Included in accrued liabilities and pension and other liabilities.
|
|
Year Ended December 31,
|
|||||||||||||||||||||||
|
2018
|
2017
|
2016
|
|||||||||||||||||||||
|
Dollars in Millions
|
Cost of products sold
|
Other income (net)
|
Cost of products sold
|
Other income (net)
|
Cost of products sold
|
Other income (net)
|
|||||||||||||||||
|
Interest rate swap contracts
|
$
|
—
|
|
$
|
23
|
|
$
|
—
|
|
$
|
31
|
|
$
|
—
|
|
$
|
36
|
|
|||||
|
Cross-currency interest rate swap contracts
|
—
|
|
8
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Foreign currency forward contracts
|
4
|
|
14
|
|
12
|
|
(52
|
)
|
(20
|
)
|
(36
|
)
|
|||||||||||
|
Year Ended December 31,
|
||||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Derivatives qualifying as cash flow hedges
|
||||||||||||
|
Foreign currency forward contracts gain/(loss):
|
||||||||||||
|
Recognized in Other Comprehensive (Loss)/Income
(a)
|
$
|
86
|
|
$
|
(108
|
)
|
$
|
6
|
|
|||
|
Reclassified to Cost of products sold
|
(4
|
)
|
(12
|
)
|
20
|
|
||||||
|
Reclassified to Other income (net)
|
—
|
|
36
|
|
(8
|
)
|
||||||
|
Derivatives qualifying as net investment hedges
|
||||||||||||
|
Cross-currency interest rate swap contracts loss:
|
||||||||||||
|
Recognized in Other Comprehensive (Loss)/Income
|
(5
|
)
|
—
|
|
—
|
|
||||||
|
Non-derivatives qualifying as net investment hedges
|
||||||||||||
|
Non U.S. dollar borrowings gain/(loss):
|
||||||||||||
|
Recognized in Other Comprehensive (Loss)/Income
|
45
|
|
(134
|
)
|
48
|
|
||||||
|
(a)
|
The amount is expected to be reclassified into earnings in the next 12 months.
|
|
December 31,
|
|||||||
|
Dollars in Millions
|
2018
|
2017
|
|||||
|
Commercial paper
|
$
|
—
|
|
$
|
299
|
|
|
|
Non-U.S. short-term borrowings
|
320
|
|
512
|
|
|||
|
Current portion of long-term debt
|
1,249
|
|
—
|
|
|||
|
Other
|
134
|
|
176
|
|
|||
|
Total
|
$
|
1,703
|
|
$
|
987
|
|
|
|
|
December 31,
|
|||||||
|
Dollars in Millions
|
2018
|
2017
|
||||||
|
Principal Value:
|
||||||||
|
1.750% Notes due 2019
|
$
|
500
|
|
$
|
500
|
|
||
|
1.600% Notes due 2019
|
750
|
|
750
|
|
||||
|
2.000% Notes due 2022
|
750
|
|
750
|
|
||||
|
7.150% Notes due 2023
|
302
|
|
302
|
|
||||
|
3.250% Notes due 2023
|
500
|
|
500
|
|
||||
|
1.000% Euro Notes due 2025
|
655
|
|
682
|
|
||||
|
6.800% Notes due 2026
|
256
|
|
256
|
|
||||
|
3.250% Notes due 2027
|
750
|
|
750
|
|
||||
|
1.750% Euro Notes due 2035
|
655
|
|
682
|
|
||||
|
5.875% Notes due 2036
|
287
|
|
287
|
|
||||
|
6.125% Notes due 2038
|
226
|
|
230
|
|
||||
|
3.250% Notes due 2042
|
500
|
|
500
|
|
||||
|
4.500% Notes due 2044
|
500
|
|
500
|
|
||||
|
6.875% Notes due 2097
|
87
|
|
87
|
|
||||
|
0.13% - 5.75% Other - maturing 2019 - 2024
|
58
|
|
59
|
|
||||
|
Subtotal
|
6,776
|
|
6,835
|
|
||||
|
Adjustments to Principal Value:
|
||||||||
|
Fair value of interest rate swap contracts
|
(10
|
)
|
(6
|
)
|
||||
|
Unamortized basis adjustment from swap terminations
|
201
|
|
227
|
|
||||
|
Unamortized bond discounts and issuance costs
|
(72
|
)
|
(81
|
)
|
||||
|
Total
|
$
|
6,895
|
|
$
|
6,975
|
|
||
|
Current portion of long-term debt
|
$
|
1,249
|
|
$
|
—
|
|
||
|
Long-term debt
|
5,646
|
|
6,975
|
|
||||
|
Dollars in Millions
|
2017
|
||
|
Principal Value:
|
|||
|
1.600% Notes due 2019
|
$
|
750
|
|
|
3.250% Notes due 2027
|
750
|
|
|
|
Total
|
$
|
1,500
|
|
|
Proceeds net of discount and deferred loan issuance costs
|
$
|
1,488
|
|
|
Forward starting interest rate swap contracts terminated:
|
|||
|
Notional amount
|
$
|
750
|
|
|
Realized gain
|
6
|
|
|
|
Unrealized loss
|
(2
|
)
|
|
|
Dollars in Millions
|
2017
|
||
|
Principal amount
|
$
|
337
|
|
|
Carrying value
|
366
|
|
|
|
Debt redemption price
|
474
|
|
|
|
Loss on debt redemption
(a)
|
109
|
|
|
|
(a)
|
Including acceleration of debt issuance costs, gain on previously terminated interest rate swap contracts and other related fees.
|
|
|
December 31,
|
|||||||
|
Dollars in Millions
|
2018
|
2017
|
||||||
|
Trade receivables
|
$
|
4,914
|
|
$
|
4,599
|
|
||
|
Less charge-backs and cash discounts
|
(245
|
)
|
(209
|
)
|
||||
|
Less bad debt allowances
|
(33
|
)
|
(43
|
)
|
||||
|
Net trade receivables
|
4,636
|
|
4,347
|
|
||||
|
Alliance receivables
|
395
|
|
322
|
|
||||
|
Prepaid and refundable income taxes
|
218
|
|
691
|
|
||||
|
Royalties, VAT and other
|
716
|
|
940
|
|
||||
|
Receivables
|
$
|
5,965
|
|
$
|
6,300
|
|
||
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Balance at beginning of year
|
$
|
252
|
|
$
|
174
|
|
$
|
122
|
|
|||
|
Provision
|
2,739
|
|
2,090
|
|
1,613
|
|
||||||
|
Utilization
|
(2,707
|
)
|
(2,015
|
)
|
(1,561
|
)
|
||||||
|
Other
|
(6
|
)
|
3
|
|
—
|
|
||||||
|
Balance at end of year
|
$
|
278
|
|
$
|
252
|
|
$
|
174
|
|
|||
|
December 31,
|
||||||||
|
Dollars in Millions
|
2018
|
2017
|
||||||
|
Finished goods
|
$
|
396
|
|
$
|
384
|
|
||
|
Work in process
|
1,026
|
|
931
|
|
||||
|
Raw and packaging materials
|
202
|
|
273
|
|
||||
|
Inventories
|
$
|
1,624
|
|
$
|
1,588
|
|
||
|
Inventories
|
$
|
1,195
|
|
$
|
1,166
|
|
||
|
Other assets
|
429
|
|
422
|
|
||||
|
|
December 31,
|
|||||||
|
Dollars in Millions
|
2018
|
2017
|
||||||
|
Land
|
$
|
104
|
|
$
|
100
|
|
||
|
Buildings
|
5,231
|
|
4,848
|
|
||||
|
Machinery, equipment and fixtures
|
2,962
|
|
3,059
|
|
||||
|
Construction in progress
|
548
|
|
980
|
|
||||
|
Gross property, plant and equipment
|
8,845
|
|
8,987
|
|
||||
|
Less accumulated depreciation
|
(3,818
|
)
|
(3,986
|
)
|
||||
|
Property, plant and equipment
|
$
|
5,027
|
|
$
|
5,001
|
|
||
|
United States
|
$
|
3,772
|
|
$
|
3,617
|
|
||
|
Europe
|
1,140
|
|
1,266
|
|
||||
|
Rest of the World
|
115
|
|
118
|
|
||||
|
Total
|
$
|
5,027
|
|
$
|
5,001
|
|
||
|
December 31,
|
||||||||||
|
Dollars in Millions
|
Estimated
Useful Lives
|
2018
|
2017
|
|||||||
|
Goodwill
|
$
|
6,538
|
|
$
|
6,863
|
|
||||
|
Other intangible assets:
|
||||||||||
|
Licenses
|
5 – 15 years
|
$
|
510
|
|
|
$
|
567
|
|
||
|
Developed technology rights
|
9 – 15 years
|
2,357
|
|
|
2,357
|
|
||||
|
Capitalized software
|
3 – 10 years
|
1,156
|
|
|
1,381
|
|
||||
|
IPRD
|
32
|
|
|
32
|
|
|||||
|
Gross other intangible assets
|
4,055
|
|
|
4,337
|
|
|||||
|
Less accumulated amortization
|
(2,964
|
)
|
|
(3,127
|
)
|
|||||
|
Total other intangible assets
|
$
|
1,091
|
|
|
$
|
1,210
|
|
|||
|
|
December 31,
|
|||||||
|
Dollars in Millions
|
2018
|
2017
|
||||||
|
Rebates and returns
|
$
|
2,417
|
|
$
|
2,024
|
|
||
|
Employee compensation and benefits
|
848
|
|
869
|
|
||||
|
Research and development
|
805
|
|
783
|
|
||||
|
Dividends
|
669
|
|
654
|
|
||||
|
Royalties
|
391
|
|
285
|
|
||||
|
Branded Prescription Drug Fee
|
188
|
|
303
|
|
||||
|
Liabilities related to assets held-for-sale
|
152
|
|
—
|
|
||||
|
Litigation and other settlements
|
118
|
|
|
38
|
|
|||
|
Restructuring
|
85
|
|
155
|
|
||||
|
Pension and postretirement benefits
|
35
|
|
40
|
|
||||
|
Other
|
781
|
|
863
|
|
||||
|
Accrued liabilities
|
$
|
6,489
|
|
$
|
6,014
|
|
||
|
|
Common Stock
|
Capital in Excess
of Par Value
of Stock
|
Accumulated Other Comprehensive Loss
|
Retained
Earnings
|
Treasury Stock
|
Noncontrolling
Interest
|
|||||||||||||||||||||||
|
Dollars and Shares in Millions
|
Shares
|
Par Value
|
Shares
|
Cost
|
|||||||||||||||||||||||||
|
Balance at January 1, 2016
|
2,208
|
|
$
|
221
|
|
$
|
1,459
|
|
$
|
(2,468
|
)
|
$
|
31,613
|
|
539
|
|
$
|
(16,559
|
)
|
$
|
158
|
|
|||||||
|
Net earnings
|
—
|
|
—
|
|
—
|
|
—
|
|
4,457
|
|
—
|
|
—
|
|
50
|
|
|||||||||||||
|
Other Comprehensive (Loss)/Income
|
—
|
|
—
|
|
—
|
|
(35
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Cash dividends declared
(c)
|
—
|
|
—
|
|
—
|
|
—
|
|
(2,557
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Stock repurchase program
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
4
|
|
(231
|
)
|
—
|
|
|||||||||||||
|
Stock compensation
|
—
|
|
—
|
|
266
|
|
—
|
|
—
|
|
(7
|
)
|
11
|
|
—
|
|
|||||||||||||
|
Distributions
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(38
|
)
|
|||||||||||||
|
Balance at December 31, 2016
|
2,208
|
|
221
|
|
1,725
|
|
(2,503
|
)
|
33,513
|
|
536
|
|
(16,779
|
)
|
170
|
|
|||||||||||||
|
Accounting change - cumulative effect
(a)
|
—
|
|
—
|
|
—
|
|
—
|
|
(787
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Adjusted balance at January 1, 2017
|
2,208
|
|
221
|
|
1,725
|
|
(2,503
|
)
|
32,726
|
|
536
|
|
(16,779
|
)
|
170
|
|
|||||||||||||
|
Net earnings
|
—
|
|
—
|
|
—
|
|
—
|
|
1,007
|
|
—
|
|
—
|
|
27
|
|
|||||||||||||
|
Other Comprehensive (Loss)/Income
|
—
|
|
—
|
|
—
|
|
214
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Cash dividends declared
(c)
|
—
|
|
—
|
|
—
|
|
—
|
|
(2,573
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Stock repurchase program
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
44
|
|
(2,477
|
)
|
—
|
|
|||||||||||||
|
Stock compensation
|
—
|
|
—
|
|
173
|
|
—
|
|
—
|
|
(5
|
)
|
7
|
|
—
|
|
|||||||||||||
|
Variable interest entity
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(59
|
)
|
|||||||||||||
|
Distributions
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(32
|
)
|
|||||||||||||
|
Balance at December 31, 2017
|
2,208
|
|
221
|
|
1,898
|
|
(2,289
|
)
|
31,160
|
|
575
|
|
(19,249
|
)
|
106
|
|
|||||||||||||
|
Accounting change - cumulative effect
(b)
|
—
|
|
—
|
|
—
|
|
(34
|
)
|
332
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Adjusted balance at January 1, 2018
|
2,208
|
|
221
|
|
1,898
|
|
(2,323
|
)
|
31,492
|
|
575
|
|
(19,249
|
)
|
106
|
|
|||||||||||||
|
Net earnings
|
—
|
|
—
|
|
—
|
|
—
|
|
4,920
|
|
—
|
|
—
|
|
27
|
|
|||||||||||||
|
Other Comprehensive (Loss)/Income
|
—
|
|
—
|
|
—
|
|
(156
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Cash dividends declared
(c)
|
—
|
|
—
|
|
—
|
|
—
|
|
(2,630
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Stock repurchase program
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
5
|
|
(313
|
)
|
—
|
|
|||||||||||||
|
Stock compensation
|
—
|
|
—
|
|
183
|
|
—
|
|
—
|
|
(4
|
)
|
(12
|
)
|
—
|
|
|||||||||||||
|
Adoption of ASU 2018-02
(b)
|
—
|
|
—
|
|
—
|
|
(283
|
)
|
283
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Distributions
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(37
|
)
|
|||||||||||||
|
Balance at December 31, 2018
|
2,208
|
|
$
|
221
|
|
$
|
2,081
|
|
$
|
(2,762
|
)
|
$
|
34,065
|
|
576
|
|
$
|
(19,574
|
)
|
$
|
96
|
|
|||||||
|
(a)
|
Cumulative effect resulting from adoption of ASU 2016-16.
|
|
(b)
|
Refer to “—Note
1
. Accounting Policies and Recently Issued Accounting Standards” for additional information.
|
|
(c)
|
Cash dividends declared per common share were
$1.61
,
$1.57
and
$1.53
in 2018, 2017 and 2016, respectively.
|
|
|
Year Ended December 31,
|
||||||||||||||||||||||||||||||||||
|
2018
|
2017
|
2016
|
|||||||||||||||||||||||||||||||||
|
Dollars in Millions
|
Pretax
|
Tax
|
After Tax
|
Pretax
|
Tax
|
After Tax
|
Pretax
|
Tax
|
After Tax
|
||||||||||||||||||||||||||
|
Derivatives qualifying as cash flow hedges:
|
|||||||||||||||||||||||||||||||||||
|
Unrealized gains/(losses)
|
$
|
86
|
|
$
|
(9
|
)
|
$
|
77
|
|
$
|
(101
|
)
|
$
|
33
|
|
$
|
(68
|
)
|
$
|
(5
|
)
|
$
|
—
|
|
$
|
(5
|
)
|
||||||||
|
Reclassified to net earnings
(a)
|
(4
|
)
|
(3
|
)
|
(7
|
)
|
19
|
|
(8
|
)
|
11
|
|
12
|
|
(3
|
)
|
9
|
|
|||||||||||||||||
|
Derivatives qualifying as cash flow hedges
|
82
|
|
(12
|
)
|
70
|
|
(82
|
)
|
25
|
|
(57
|
)
|
7
|
|
(3
|
)
|
4
|
|
|||||||||||||||||
|
Pension and postretirement benefits:
|
|||||||||||||||||||||||||||||||||||
|
Actuarial (losses)/gains
|
(89
|
)
|
(3
|
)
|
(92
|
)
|
47
|
|
11
|
|
58
|
|
(126
|
)
|
(3
|
)
|
(129
|
)
|
|||||||||||||||||
|
Amortization
(b)
|
65
|
|
(13
|
)
|
52
|
|
77
|
|
(31
|
)
|
46
|
|
78
|
|
(25
|
)
|
53
|
|
|||||||||||||||||
|
Settlements
(b)
|
121
|
|
(28
|
)
|
93
|
|
167
|
|
(57
|
)
|
110
|
|
91
|
|
(32
|
)
|
59
|
|
|||||||||||||||||
|
Pension and postretirement benefits
|
97
|
|
(44
|
)
|
53
|
|
291
|
|
(77
|
)
|
214
|
|
43
|
|
(60
|
)
|
(17
|
)
|
|||||||||||||||||
|
Available-for-sale securities:
|
|||||||||||||||||||||||||||||||||||
|
Unrealized (losses)/gains
|
(30
|
)
|
5
|
|
(25
|
)
|
38
|
|
6
|
|
44
|
|
(12
|
)
|
(1
|
)
|
(13
|
)
|
|||||||||||||||||
|
Realized (gains)/losses
(b)
|
—
|
|
—
|
|
—
|
|
(7
|
)
|
2
|
|
(5
|
)
|
29
|
|
—
|
|
29
|
|
|||||||||||||||||
|
Available-for-sale securities
|
(30
|
)
|
5
|
|
(25
|
)
|
31
|
|
8
|
|
39
|
|
17
|
|
(1
|
)
|
16
|
|
|||||||||||||||||
|
Foreign currency translation
|
(245
|
)
|
(9
|
)
|
(254
|
)
|
(20
|
)
|
38
|
|
18
|
|
(33
|
)
|
(5
|
)
|
(38
|
)
|
|||||||||||||||||
|
Total Other Comprehensive (Loss)/Income
|
$
|
(96
|
)
|
$
|
(60
|
)
|
$
|
(156
|
)
|
$
|
220
|
|
$
|
(6
|
)
|
$
|
214
|
|
$
|
34
|
|
$
|
(69
|
)
|
$
|
(35
|
)
|
||||||||
|
(a)
|
Included in Cost of products sold.
|
|
(b)
|
Included in Other income (net).
|
|
|
December 31,
|
|||||||
|
Dollars in Millions
|
2018
|
2017
|
||||||
|
Derivatives qualifying as cash flow hedges
|
$
|
51
|
|
$
|
(19
|
)
|
||
|
Pension and postretirement benefits
|
(2,102
|
)
|
(1,883
|
)
|
||||
|
Available-for-sale securities
|
(30
|
)
|
32
|
|
||||
|
Foreign currency translation
|
(681
|
)
|
(419
|
)
|
||||
|
Accumulated other comprehensive loss
|
$
|
(2,762
|
)
|
$
|
(2,289
|
)
|
||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Service cost — benefits earned during the year
|
$
|
26
|
|
$
|
25
|
|
$
|
24
|
|
|||
|
Interest cost on projected benefit obligation
|
193
|
|
188
|
|
192
|
|
||||||
|
Expected return on plan assets
|
(386
|
)
|
(411
|
)
|
(418
|
)
|
||||||
|
Amortization of prior service credits
|
(4
|
)
|
(4
|
)
|
(3
|
)
|
||||||
|
Amortization of net actuarial loss
|
74
|
|
82
|
|
84
|
|
||||||
|
Settlements and curtailments
|
121
|
|
159
|
|
91
|
|
||||||
|
Special termination benefits
|
—
|
|
3
|
|
1
|
|
||||||
|
Net periodic benefit cost/(credit)
|
$
|
24
|
|
$
|
42
|
|
$
|
(29
|
)
|
|||
|
Dollars in Millions
|
2018
|
2017
|
||||||
|
Benefit obligations at beginning of year
|
$
|
6,749
|
|
$
|
6,440
|
|
||
|
Service cost—benefits earned during the year
|
26
|
|
25
|
|
||||
|
Interest cost
|
193
|
|
188
|
|
||||
|
Settlements and Curtailments
|
(278
|
)
|
(330
|
)
|
||||
|
Actuarial (gains)/losses
|
(523
|
)
|
368
|
|
||||
|
Benefits paid
|
(123
|
)
|
(121
|
)
|
||||
|
Foreign currency and other
|
(78
|
)
|
179
|
|
||||
|
Benefit obligations at end of year
|
$
|
5,966
|
|
$
|
6,749
|
|
||
|
Fair value of plan assets at beginning of year
|
$
|
6,749
|
|
$
|
5,831
|
|
||
|
Actual return on plan assets
|
(203
|
)
|
804
|
|
||||
|
Employer contributions
|
71
|
|
396
|
|
||||
|
Settlements
|
(276
|
)
|
(330
|
)
|
||||
|
Benefits paid
|
(123
|
)
|
(121
|
)
|
||||
|
Foreign currency and other
|
(89
|
)
|
169
|
|
||||
|
Fair value of plan assets at end of year
|
$
|
6,129
|
|
$
|
6,749
|
|
||
|
Funded status
|
$
|
163
|
|
$
|
—
|
|
||
|
Assets/(Liabilities) recognized:
|
||||||||
|
Other assets
|
$
|
622
|
|
$
|
487
|
|
||
|
Accrued liabilities
|
(32
|
)
|
(31
|
)
|
||||
|
Pension and other liabilities
|
(427
|
)
|
(456
|
)
|
||||
|
Funded status
|
$
|
163
|
|
$
|
—
|
|
||
|
Recognized in Accumulated other comprehensive loss:
|
||||||||
|
Net actuarial losses
|
$
|
2,717
|
|
$
|
2,849
|
|
||
|
Prior service credit
|
(30
|
)
|
(36
|
)
|
||||
|
Total
|
$
|
2,687
|
|
$
|
2,813
|
|
||
|
Dollars in Millions
|
2018
|
2017
|
||||||
|
Pension plans with projected benefit obligations in excess of plan assets:
|
||||||||
|
Projected benefit obligation
|
$
|
1,275
|
|
$
|
1,166
|
|
||
|
Fair value of plan assets
|
817
|
|
678
|
|
||||
|
Pension plans with accumulated benefit obligations in excess of plan assets
:
|
||||||||
|
Accumulated benefit obligation
|
$
|
1,181
|
|
$
|
1,008
|
|
||
|
Fair value of plan assets
|
757
|
|
550
|
|
||||
|
|
2018
|
2017
|
||||
|
Discount rate
|
3.5
|
%
|
3.1
|
%
|
||
|
Rate of compensation increase
|
0.5
|
%
|
0.5
|
%
|
||
|
|
2018
|
2017
|
2016
|
||||||
|
Discount rate
|
3.1
|
%
|
3.5
|
%
|
3.8
|
%
|
|||
|
Expected long-term return on plan assets
|
6.2
|
%
|
7.0
|
%
|
7.2
|
%
|
|||
|
Rate of compensation increase
|
0.5
|
%
|
0.5
|
%
|
0.5
|
%
|
|||
|
2018
|
2017
|
2016
|
|||||||
|
10 years
|
10.4
|
%
|
6.8
|
%
|
6.1
|
%
|
|||
|
15 years
|
7.8
|
%
|
9.3
|
%
|
7.1
|
%
|
|||
|
20 years
|
7.1
|
%
|
7.5
|
%
|
7.7
|
%
|
|||
|
|
December 31, 2018
|
December 31, 2017
|
||||||||||||||||||||||||||||||
|
Dollars in Millions
|
Level 1
|
Level 2
|
Level 3
|
Total
|
Level 1
|
Level 2
|
Level 3
|
Total
|
||||||||||||||||||||||||
|
Plan Assets
|
||||||||||||||||||||||||||||||||
|
Equity securities
|
$
|
124
|
|
$
|
—
|
|
$
|
—
|
|
$
|
124
|
|
$
|
799
|
|
$
|
—
|
|
$
|
—
|
|
$
|
799
|
|
||||||||
|
Equity funds
|
2
|
|
475
|
|
—
|
|
477
|
|
160
|
|
1,358
|
|
—
|
|
1,518
|
|
||||||||||||||||
|
Fixed income funds
|
—
|
|
606
|
|
—
|
|
606
|
|
—
|
|
724
|
|
—
|
|
724
|
|
||||||||||||||||
|
Corporate debt securities
|
—
|
|
3,865
|
|
—
|
|
3,865
|
|
—
|
|
1,919
|
|
—
|
|
1,919
|
|
||||||||||||||||
|
U.S. Treasury and agency securities
|
—
|
|
553
|
|
—
|
|
553
|
|
—
|
|
729
|
|
—
|
|
729
|
|
||||||||||||||||
|
Short-term investment funds
|
—
|
|
55
|
|
—
|
|
55
|
|
—
|
|
135
|
|
—
|
|
135
|
|
||||||||||||||||
|
Insurance contracts
|
—
|
|
—
|
|
134
|
|
134
|
|
—
|
|
—
|
|
138
|
|
138
|
|
||||||||||||||||
|
Cash and cash equivalents
|
311
|
|
—
|
|
—
|
|
311
|
|
214
|
|
—
|
|
—
|
|
214
|
|
||||||||||||||||
|
Other
|
—
|
|
105
|
|
19
|
|
124
|
|
—
|
|
92
|
|
13
|
|
105
|
|
||||||||||||||||
|
Plan assets subject to leveling
|
$
|
437
|
|
$
|
5,659
|
|
$
|
153
|
|
$
|
6,249
|
|
$
|
1,173
|
|
$
|
4,957
|
|
$
|
151
|
|
$
|
6,281
|
|
||||||||
|
Plan assets measured at NAV as a practical expedient
|
||||||||||||||||||||||||||||||||
|
Equity funds
|
$
|
—
|
|
$
|
488
|
|
||||||||||||||||||||||||||
|
Venture capital and limited partnerships
|
121
|
|
154
|
|
||||||||||||||||||||||||||||
|
Other
|
91
|
|
191
|
|
||||||||||||||||||||||||||||
|
Total plan assets measured at NAV as a practical expedient
|
212
|
|
833
|
|
||||||||||||||||||||||||||||
|
Net plan assets
|
$
|
6,461
|
|
$
|
7,114
|
|
||||||||||||||||||||||||||
|
|
Year Ended December 31,
|
|||||||||||
|
Dollars in Millions
|
2018
|
2017
|
2016
|
|||||||||
|
Restricted stock units
|
$
|
102
|
|
$
|
95
|
|
$
|
89
|
|
|||
|
Market share units
|
38
|
|
35
|
|
37
|
|
||||||
|
Performance share units
|
81
|
|
69
|
|
79
|
|
||||||
|
Total stock-based compensation expense
|
$
|
221
|
|
$
|
199
|
|
$
|
205
|
|
|||
|
Income tax benefit
|
$
|
41
|
|
$
|
59
|
|
$
|
69
|
|
|||
|
|
Stock Options
|
Restricted Stock Units
|
Market Share Units
|
Performance Share Units
|
||||||||||||||||||||||||
|
Number of
Options Outstanding
|
Weighted-
Average
Exercise Price of Shares
|
Number
of
Nonvested Awards
|
Weighted-
Average
Grant-Date Fair Value
|
Number
of
Nonvested Awards
|
Weighted-
Average
Grant-Date Fair Value
|
Number
of
Nonvested Awards
|
Weighted-
Average
Grant-Date Fair Value
|
|||||||||||||||||||||
|
Shares in Millions
|
||||||||||||||||||||||||||||
|
Balance at January 1, 2018
|
3.8
|
|
$
|
19.04
|
|
4.9
|
|
$
|
56.85
|
|
1.5
|
|
$
|
62.25
|
|
3.5
|
|
$
|
62.57
|
|
||||||||
|
Granted
|
—
|
|
—
|
|
2.4
|
|
61.40
|
|
0.7
|
|
72.33
|
|
1.1
|
|
67.60
|
|
||||||||||||
|
Released/Exercised
|
(2.1
|
)
|
20.22
|
|
(1.7
|
)
|
56.95
|
|
(0.6
|
)
|
61.70
|
|
(1.6
|
)
|
64.84
|
|
||||||||||||
|
Adjustments for actual payout
|
—
|
|
—
|
|
—
|
|
—
|
|
0.1
|
|
59.29
|
|
0.1
|
|
64.84
|
|
||||||||||||
|
Forfeited/Canceled
|
—
|
|
—
|
|
(0.6
|
)
|
58.85
|
|
(0.2
|
)
|
66.08
|
|
(0.3
|
)
|
63.12
|
|
||||||||||||
|
Balance at December 31, 2018
|
1.7
|
|
17.51
|
|
5.0
|
|
58.83
|
|
1.5
|
|
66.76
|
|
2.8
|
|
63.28
|
|
||||||||||||
|
Vested or expected to vest
|
1.7
|
|
17.51
|
|
4.4
|
|
58.85
|
|
1.3
|
|
66.67
|
|
3.3
|
|
63.10
|
|
||||||||||||
|
Restricted
|
Market
|
Performance
|
||||||||||
|
Dollars in Millions
|
Stock Units
|
Share Units
|
Share Units
|
|||||||||
|
Unrecognized compensation cost
|
$
|
212
|
|
$
|
43
|
|
$
|
85
|
|
|||
|
Expected weighted-average period in years of compensation cost to be recognized
|
2.7
|
|
2.7
|
|
1.7
|
|
||||||
|
Amounts in Millions, except per share data
|
2018
|
2017
|
2016
|
|||||||||
|
Weighted-average grant date fair value (per share):
|
||||||||||||
|
Restricted stock units
|
$
|
61.40
|
|
$
|
54.39
|
|
$
|
60.56
|
|
|||
|
Market share units
|
72.33
|
|
60.14
|
|
65.26
|
|
||||||
|
Performance share units
|
67.60
|
|
57.91
|
|
64.87
|
|
||||||
|
Fair value of awards that vested:
|
||||||||||||
|
Restricted stock units
|
$
|
98
|
|
$
|
91
|
|
$
|
81
|
|
|||
|
Market share units
|
40
|
|
33
|
|
50
|
|
||||||
|
Performance share units
|
103
|
|
84
|
|
93
|
|
||||||
|
Total intrinsic value of stock options exercised
|
$
|
89
|
|
$
|
84
|
|
$
|
158
|
|
|||
|
Number
Outstanding and Exercisable (in millions)
|
Weighted-Average
Remaining Contractual
Life (in years)
|
Weighted-Average
Exercise Price
Per Share
|
Aggregate
Intrinsic Value
(in millions)
|
||||||||||
|
Options Outstanding and Exercisable
|
1.7
|
|
0.2
|
$
|
17.51
|
|
$
|
57
|
|
||||
|
Dollars in Millions, except per share data
|
First Quarter
|
Second Quarter
|
Third Quarter
|
Fourth Quarter
|
Year
|
|||||||||||||||
|
2018
|
||||||||||||||||||||
|
Total Revenues
|
$
|
5,193
|
|
$
|
5,704
|
|
$
|
5,691
|
|
$
|
5,973
|
|
$
|
22,561
|
|
|||||
|
Gross Margin
|
3,609
|
|
4,079
|
|
4,043
|
|
4,283
|
|
16,014
|
|
||||||||||
|
Net Earnings
|
1,495
|
|
382
|
|
1,912
|
|
1,158
|
|
4,947
|
|
||||||||||
|
Net Earnings/(Loss) Attributable to:
|
||||||||||||||||||||
|
Noncontrolling Interest
|
9
|
|
9
|
|
11
|
|
(2
|
)
|
27
|
|
||||||||||
|
BMS
|
1,486
|
|
373
|
|
1,901
|
|
1,160
|
|
4,920
|
|
||||||||||
|
Earnings per Share - Basic
(a)
|
$
|
0.91
|
|
$
|
0.23
|
|
$
|
1.16
|
|
$
|
0.71
|
|
$
|
3.01
|
|
|||||
|
Earnings per Share - Diluted
(a)
|
0.91
|
|
0.23
|
|
1.16
|
|
0.71
|
|
3.01
|
|
||||||||||
|
Cash dividends declared per common share
|
$
|
0.40
|
|
$
|
0.40
|
|
$
|
0.40
|
|
$
|
0.41
|
|
$
|
1.61
|
|
|||||
|
Cash and cash equivalents
|
$
|
5,342
|
|
$
|
4,999
|
|
$
|
5,408
|
|
$
|
6,911
|
|
$
|
6,911
|
|
|||||
|
Marketable securities
(b)
|
3,680
|
|
3,193
|
|
3,439
|
|
3,748
|
|
3,748
|
|
||||||||||
|
Total Assets
|
33,083
|
|
32,641
|
|
33,734
|
|
34,986
|
|
34,986
|
|
||||||||||
|
Long-term debt
(c)
|
5,775
|
|
5,671
|
|
5,687
|
|
6,895
|
|
6,895
|
|
||||||||||
|
Equity
|
12,906
|
|
12,418
|
|
13,750
|
|
14,127
|
|
14,127
|
|
||||||||||
|
Dollars in Millions, except per share data
|
First Quarter
|
Second Quarter
|
Third Quarter
|
Fourth Quarter
|
Year
|
|||||||||||||||
|
2017
|
|
|
|
|
|
|||||||||||||||
|
Total Revenues
|
$
|
4,929
|
|
$
|
5,144
|
|
$
|
5,254
|
|
$
|
5,449
|
|
$
|
20,776
|
|
|||||
|
Gross Margin
|
3,664
|
|
3,575
|
|
3,675
|
|
3,768
|
|
14,682
|
|
||||||||||
|
Net Earnings
|
1,526
|
|
922
|
|
856
|
|
(2,329
|
)
|
975
|
|
||||||||||
|
Net Earnings/(Loss) Attributable to:
|
||||||||||||||||||||
|
Noncontrolling Interest
|
(48
|
)
|
6
|
|
11
|
|
(1
|
)
|
(32
|
)
|
||||||||||
|
BMS
|
1,574
|
|
916
|
|
845
|
|
(2,328
|
)
|
1,007
|
|
||||||||||
|
Earnings/(Loss) per Share - Basic
(a)
|
$
|
0.95
|
|
$
|
0.56
|
|
$
|
0.52
|
|
$
|
(1.42
|
)
|
$
|
0.61
|
|
|||||
|
Earnings/(Loss) per Share - Diluted
(a)
|
0.94
|
|
0.56
|
|
0.51
|
|
(1.42
|
)
|
0.61
|
|
||||||||||
|
Cash dividends declared per common share
|
$
|
0.39
|
|
$
|
0.39
|
|
$
|
0.39
|
|
$
|
0.40
|
|
$
|
1.57
|
|
|||||
|
Cash and cash equivalents
|
$
|
3,910
|
|
$
|
3,470
|
|
$
|
4,644
|
|
$
|
5,421
|
|
$
|
5,421
|
|
|||||
|
Marketable securities
(b)
|
4,884
|
|
5,615
|
|
5,004
|
|
3,871
|
|
3,871
|
|
||||||||||
|
Total Assets
|
32,937
|
|
33,409
|
|
33,977
|
|
33,551
|
|
33,551
|
|
||||||||||
|
Long-term debt
(c)
|
7,237
|
|
6,911
|
|
6,982
|
|
6,975
|
|
6,975
|
|
||||||||||
|
Equity
|
14,535
|
|
14,821
|
|
14,914
|
|
11,847
|
|
11,847
|
|
||||||||||
|
(a)
|
Earnings per share for the quarters may not add to the amounts for the year, as each period is computed on a discrete basis.
|
|
(b)
|
Marketable securities includes current and non-current assets.
|
|
(c)
|
Long-term debt includes the current portion.
|
|
Dollars in Millions
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
Year
|
|||||||||||||||
|
Cost of products sold
(a)
|
$
|
13
|
|
$
|
14
|
|
$
|
13
|
|
$
|
18
|
|
$
|
58
|
|
|||||
|
Marketing, selling and administrative
|
1
|
|
—
|
|
—
|
|
1
|
|
2
|
|
||||||||||
|
License and asset acquisition charges
|
60
|
|
1,075
|
|
—
|
|
—
|
|
1,135
|
|
||||||||||
|
Site exit costs
|
20
|
|
19
|
|
18
|
|
22
|
|
79
|
|
||||||||||
|
Research and development
|
80
|
|
1,094
|
|
18
|
|
22
|
|
1,214
|
|
||||||||||
|
Loss/(gain) on equity investments
|
(15
|
)
|
356
|
|
(97
|
)
|
268
|
|
512
|
|
||||||||||
|
Provision for restructuring
|
20
|
|
37
|
|
45
|
|
29
|
|
131
|
|
||||||||||
|
Litigation and other settlements
|
—
|
|
—
|
|
—
|
|
70
|
|
70
|
|
||||||||||
|
Divestiture gains
|
(43
|
)
|
(25
|
)
|
(108
|
)
|
(1
|
)
|
(177
|
)
|
||||||||||
|
Royalties and licensing income
|
(50
|
)
|
(25
|
)
|
—
|
|
—
|
|
(75
|
)
|
||||||||||
|
Pension and postretirement
|
31
|
|
37
|
|
27
|
|
26
|
|
121
|
|
||||||||||
|
Intangible asset impairment
|
64
|
|
—
|
|
—
|
|
—
|
|
64
|
|
||||||||||
|
Other income (net)
|
7
|
|
380
|
|
(133
|
)
|
392
|
|
646
|
|
||||||||||
|
Increase/(decrease) to pretax income
|
101
|
|
1,488
|
|
(102
|
)
|
433
|
|
1,920
|
|
||||||||||
|
Income taxes on items above
|
(8
|
)
|
(218
|
)
|
1
|
|
(43
|
)
|
(268
|
)
|
||||||||||
|
Income taxes attributed to U.S. tax reform
|
(32
|
)
|
3
|
|
(20
|
)
|
(7
|
)
|
(56
|
)
|
||||||||||
|
Income taxes
|
(40
|
)
|
(215
|
)
|
(19
|
)
|
(50
|
)
|
(324
|
)
|
||||||||||
|
Increase/(decrease) to net earnings
|
$
|
61
|
|
$
|
1,273
|
|
$
|
(121
|
)
|
$
|
383
|
|
$
|
1,596
|
|
|||||
|
Dollars in Millions
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
Year
|
|||||||||||||||
|
Cost of products sold
(a)
|
$
|
—
|
|
$
|
130
|
|
$
|
1
|
|
$
|
18
|
|
$
|
149
|
|
|||||
|
Marketing, selling and administrative
|
—
|
|
—
|
|
—
|
|
1
|
|
1
|
|
||||||||||
|
License and asset acquisition charges
|
50
|
|
393
|
|
310
|
|
377
|
|
1,130
|
|
||||||||||
|
IPRD impairments
|
75
|
|
—
|
|
—
|
|
—
|
|
75
|
|
||||||||||
|
Site exit costs
|
72
|
|
96
|
|
64
|
|
151
|
|
383
|
|
||||||||||
|
Research and development
|
197
|
|
489
|
|
374
|
|
528
|
|
1,588
|
|
||||||||||
|
Provision for restructuring
|
164
|
|
15
|
|
28
|
|
86
|
|
293
|
|
||||||||||
|
Litigation and other settlements
|
(481
|
)
|
—
|
|
—
|
|
—
|
|
(481
|
)
|
||||||||||
|
Divestiture gains
|
(100
|
)
|
—
|
|
—
|
|
(26
|
)
|
(126
|
)
|
||||||||||
|
Royalties and licensing income
|
—
|
|
(497
|
)
|
—
|
|
—
|
|
(497
|
)
|
||||||||||
|
Pension and postretirement
|
33
|
|
36
|
|
22
|
|
71
|
|
162
|
|
||||||||||
|
Loss on debt redemption
|
—
|
|
109
|
|
—
|
|
—
|
|
109
|
|
||||||||||
|
Other income (net)
|
(384
|
)
|
(337
|
)
|
50
|
|
131
|
|
(540
|
)
|
||||||||||
|
Increase/(decrease) to pretax income
|
(187
|
)
|
282
|
|
425
|
|
678
|
|
1,198
|
|
||||||||||
|
Income taxes on items above
|
72
|
|
20
|
|
(41
|
)
|
(138
|
)
|
(87
|
)
|
||||||||||
|
Income taxes attributed to U.S. tax reform
|
—
|
|
—
|
|
—
|
|
2,911
|
|
2,911
|
|
||||||||||
|
Income taxes
|
72
|
|
20
|
|
(41
|
)
|
2,773
|
|
2,824
|
|
||||||||||
|
Increase/(decrease) to net earnings
|
(115
|
)
|
302
|
|
384
|
|
3,451
|
|
4,022
|
|
||||||||||
|
Noncontrolling interest
|
(59
|
)
|
—
|
|
—
|
|
—
|
|
(59
|
)
|
||||||||||
|
Increase/(decrease) to net earnings attributable to BMS
|
$
|
(174
|
)
|
$
|
302
|
|
$
|
384
|
|
$
|
3,451
|
|
$
|
3,963
|
|
|||||
|
(a)
|
Specified items in Cost of products sold are accelerated depreciation, asset impairment and other shutdown costs.
|
|
Item 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
|
|
Item 9A.
|
CONTROLS AND PROCEDURES.
|
|
Item 9B.
|
OTHER INFORMATION.
|
|
Item 10.
|
DIRECTORS AND EXECUTIVE OFFICERS OF THE REGISTRANT.
|
|
(a)
|
Reference is made to our
2019
Proxy Statement with respect to our Directors, which is incorporated herein by reference and made a part hereof in response to the information required by Item 10.
|
|
(b)
|
The information required by Item 10 with respect to our Executive Officers has been included in Part IA of this
2018
Form 10-K in reliance on General Instruction G of Form 10-K and Instruction 3 to Item 401(b) of Regulation S-K, which is incorporated herein by reference and made a part hereof in response to the information required by Item 10.
|
|
Item 11.
|
EXECUTIVE COMPENSATION.
|
|
Item 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
|
|
Item 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS.
|
|
Item 14.
|
AUDITOR FEES.
|
|
Item 15.
|
EXHIBITS and FINANCIAL STATEMENT SCHEDULE.
|
|
(a)
|
|
|
|
|
Page
Number
|
|
1.
|
Consolidated Financial Statements
|
|
|
Consolidated Statements of Earnings
and Comprehensive Income
|
||
|
2.
|
Financial Statement Schedules
|
|
|
All other schedules not included with this additional financial data are omitted because they are not applicable or the required information is included in the financial statements or notes thereto.
|
||
|
3.
|
Exhibits
|
|
|
(b)
|
|
|
Item 16.
|
FORM 10-K SUMMARY.
|
|
BRISTOL-MYERS SQUIBB COMPANY
(Registrant)
|
||
|
By
|
|
/s/ GIOVANNI CAFORIO
|
|
|
|
Giovanni Caforio
|
|
|
|
Chairman of the Board and Chief Executive Officer
|
|
Date: February 25, 2019
|
||
|
Signature
|
|
Title
|
|
Date
|
|
/s/ GIOVANNI CAFORIO, M.D.
|
|
Chairman of the Board and Chief Executive Officer
|
|
February 25, 2019
|
|
(Giovanni Caforio, M.D.)
|
|
(Principal Executive Officer)
|
|
|
|
/s/ CHARLES BANCROFT
|
|
Chief Financial Officer
|
|
February 25, 2019
|
|
(Charles Bancroft)
|
|
(Principal Financial Officer)
|
|
|
|
/s/ KAREN SANTIAGO
|
|
Senior Vice President and Corporate Controller
|
|
February 25, 2019
|
|
(Karen Santiago)
|
|
(Principal Accounting Officer)
|
|
|
|
/s/ PETER J. ARDUINI
|
|
Director
|
|
February 25, 2019
|
|
(Peter J. Arduini)
|
|
|
||
|
/s/ ROBERT BERTOLINI
|
Director
|
February 25, 2019
|
||
|
(Robert Bertolini)
|
||||
|
/s/ MATTHEW W. EMMENS
|
Director
|
February 25, 2019
|
||
|
(Matthew W. Emmens)
|
||||
|
/s/ MICHAEL GROBSTEIN
|
|
Director
|
|
February 25, 2019
|
|
(Michael Grobstein)
|
|
|
||
|
/s/ ALAN J. LACY
|
|
Director
|
|
February 25, 2019
|
|
(Alan J. Lacy)
|
|
|
||
|
/s/ DINESH C. PALIWAL
|
Director
|
|
February 25, 2019
|
|
|
(Dinesh C. Paliwal)
|
||||
|
/s/ THEODORE R. SAMUELS
|
Director
|
February 25, 2019
|
||
|
(Theodore R. Samuels)
|
||||
|
/s/ VICKI L. SATO, PH.D.
|
|
Director
|
|
February 25, 2019
|
|
(Vicki L. Sato, Ph.D.)
|
|
|
||
|
/s/ GERALD L. STORCH
|
|
Director
|
|
February 25, 2019
|
|
(Gerald L. Storch)
|
|
|
||
|
/s/ KAREN H. VOUSDEN, PH.D.
|
|
Director
|
|
February 25, 2019
|
|
(Karen H. Vousden, Ph.D.)
|
|
|
||
|
2018 Form 10-K
|
Annual Report on Form 10-K for the fiscal year ended December 31, 2018
|
LOE
|
loss of exclusivity
|
|
AbbVie
|
AbbVie Inc.
|
MAA
|
Marketing Authorization Application
|
|
ALL
|
acute lymphoblastic leukemia
|
MCOs
|
Managed Care Organizations
|
|
Amgen
|
Amgen Inc.
|
mCRC
|
metastatic colorectal cancer
|
|
Amylin
|
Amylin Pharmaceuticals, Inc.
|
mCRPC
|
metastatic castration-resistant prostate cancer
|
|
aNDA
|
abbreviated New Drug Application
|
MDL
|
multi-district litigation
|
|
ASEAN
|
Association of Southeast Asian Nations
|
Mead Johnson
|
Mead Johnson Nutrition Company
|
|
AstraZeneca
|
AstraZeneca PLC
|
Merck
|
Merck & Co., Inc.
|
|
Biogen
|
Biogen, Inc.
|
MF
|
myelofibrosis
|
|
BLA
|
Biologics License Application
|
MPM
|
malignant pleural mesothelioma
|
|
Cardioxyl
|
Cardioxyl Pharmaceuticals, Inc.
|
MSI-H
|
high microsatellite instability
|
|
CERCLA
|
U.S. Comprehensive Environmental Response, Compensation and Liability Act
|
mUC
|
metastatic urothelial carcinoma
|
|
Celgene
|
Celgene Corporation
|
NAV
|
net asset value
|
|
cGMP
|
current Good Manufacturing Practices
|
Nektar
|
Nektar Therapeutics
|
|
CHMP
|
Committee for Medicinal Products for Human Use
|
NDA
|
New Drug Application
|
|
CML
|
chronic myeloid leukemia
|
Nitto Denko
|
Nitto Denko Corporation
|
|
Cormorant
|
Cormorant Pharmaceuticals
|
NKT
|
natural killer T
|
|
CPPIB
|
CPPIB Credit Europe S.A.R.L., a Luxembourg private limited liability company
|
Novartis
|
Novartis Pharmaceutical Corporation
|
|
CRC
|
colorectal cancer
|
NSCLC
|
non-small cell lung cancer
|
|
CytomX
|
CytomX Therapeutics, Inc.
|
NVAF
|
non-valvular atrial fibrillation
|
|
dMMR
|
DNA mismatch repair deficient
|
OIG
|
Office of Inspector General of the U.S. Department of Health and Human Services
|
|
DSA
|
Distribution Services Agreement
|
Ono
|
Ono Pharmaceutical Co., Ltd.
|
|
EC
|
European Commission
|
OTC
|
Over-the-counter
|
|
EMA
|
European Medicines Agency
|
Otsuka
|
Otsuka Pharmaceutical Co., Ltd.
|
|
EPO
|
European Patent Office
|
PAD
|
Protein/Peptidyl Arginine Deiminase
|
|
EPS
|
earnings per share
|
Padlock
|
Padlock Therapeutics, Inc.
|
|
ERISA
|
Employee Retirement Income Security Act of 1974
|
PBMs
|
Pharmacy Benefit Managers
|
|
EU
|
European Union
|
PD-1
|
programmed death receptor-1
|
|
FASB
|
Financial Accounting Standards Board
|
PDMA
|
Prescription Drug Marketing Act
|
|
FCPA
|
Foreign Corrupt Practices Act
|
Pfizer
|
Pfizer, Inc.
|
|
FDA
|
U.S. Food and Drug Administration
|
PhRMA Code
|
Pharmaceutical Research and Manufacturers of America’s Professional Practices Code
|
|
Five Prime
|
Five Prime Therapeutics, Inc.
|
Promedior
|
Promedior, Inc.
|
|
Flexus
|
Flexus Biosciences, Inc.
|
PRP
|
potentially responsible party
|
|
F-Star
|
F-Star Alpha Ltd.
|
PSA
|
prostate-specific antigen
|
|
GAAP
|
U.S. generally accepted accounting principles
|
PsiOxus
|
PsiOxus Therapeutics, Ltd.
|
|
Gilead
|
Gilead Sciences, Inc.
|
R&D
|
Research and Development
|
|
GlaxoSmithKline
|
GlaxoSmithKline PLC
|
RA
|
rheumatoid arthritis
|
|
GTN
|
gross-to-net
|
RCC
|
renal cell carcinoma
|
|
Halozyme
|
Halozyme Therapeutics, Inc.
|
RDP
|
regulatory data protection
|
|
HCC
|
Hepatocellular carcinoma
|
Reckitt
|
Reckitt Benckiser Group plc
|
|
HIV
|
human immunodeficiency virus
|
Roche
|
Roche Holding AG
|
|
HR 3590
|
The Patient Protection and Affordable Care Act
|
Sanofi
|
Sanofi S.A.
|
|
IFM
|
IFM Therapeutics, Inc.
|
sBLA
|
supplemental Biologics License Application
|
|
ImClone
|
ImClone Systems Incorporated
|
SCCHN
|
squamous cell carcinoma of the head and neck
|
|
IO
|
Immuno-Oncology
|
SCLC
|
small cell lung cancer
|
|
IPF
|
idiopathic pulmonary fibrosis
|
SEC
|
U.S. Securities and Exchange Commission
|
|
iPierian
|
iPierian, Inc.
|
SK Biotek
|
SK Biotek Co., Ltd.
|
|
IPRD
|
in-process research and development
|
the 2012 Plan
|
The 2012 Stock Award and Incentive Plan
|
|
Janssen
|
Janssen Pharmaceuticals, Inc.
|
U.S.
|
United States
|
|
JIA
|
Juvenile Idiopathic Arthritis
|
UK
|
United Kingdom
|
|
LIBOR
|
London Interbank Offered Rate
|
VTE
|
venous thromboembolic
|
|
Lilly
|
Eli Lilly and Company
|
WTO
|
World Trade Organization
|
|
Exhibit No.
|
Description
|
Page No
|
||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
4a.
|
Letter of Agreement dated March 28, 1984 (incorporated herein by reference to Exhibit 4 to the Form 10-K for the fiscal year ended December 31, 1983).
|
‡
|
||
|
4b.
|
Indenture, dated as of June 1, 1993, between Bristol-Myers Squibb Company and JPMorgan Chase Bank (as successor trustee to The Chase Manhattan Bank (National Association)) (incorporated herein by reference to Exhibit 4.1 to the Form 8-K dated May 27, 1993 and filed on June 3, 1993).
|
‡
|
||
|
4c.
|
Form of 7.15% Debenture due 2023 of Bristol-Myers Squibb Company (incorporated herein by reference to Exhibit 4.2 to the Form 8-K dated May 27, 1993 and filed on June 3, 1993).
|
‡
|
||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡
|
||||
|
‡‡10pp.
|
Squibb Corporation Deferral Plan for Fees of Outside Directors, as amended (as adopted, incorporated herein by reference to Exhibit 10e Squibb Corporation 1991 Form 10-K for the fiscal year ended December 31, 1987, File No. 1-5514; as amended effective December 31, 1991 incorporated herein by reference to Exhibit 10m to the Form 10-K for the fiscal year ended December 31, 1992).
|
‡
|
||
|
101.
|
The following financial statements from the Bristol-Myers Squibb Company Annual Report on Form 10-K for the years ended December 31, 2018, 2017 and 2016, formatted in Extensible Business Reporting Language (XBRL): (i) consolidated statements of earnings, (ii) consolidated statements of comprehensive income, (iii) consolidated balance sheets, (iv) consolidated statements of cash flows, and (v) the notes to the consolidated financial statements.
|
|||
|
†
|
Confidential treatment has been granted for certain portions which are omitted in the copy of the exhibit electronically filed with the Commission.
|
|
*
|
Indicates, in this 2018 Form 10-K, brand names of products, which are registered trademarks not solely owned by the Company or its subsidiaries.
Abilify
is a trademark of Otsuka Pharmaceutical Co., Ltd.;
Atripla, Truvada
and
Tybost
are
trademarks of Gilead Sciences, Inc.;
Avapro/Avalide
(known in the EU as
Aprovel/Karvea
) and
Plavix
are trademarks of Sanofi;
Byetta
is a trademark of Amylin Pharmaceuticals, LLC;
ENHANZE
is a trademark of Halozyme, Inc.;
Erbitux
is a trademark of ImClone LLC;
Farxiga
and
Onglyza
are trademarks of AstraZeneca AB;
Gleevec
is a trademark of Novartis AG;
Keytruda
is a trademark of Merck Sharp & Dohme Corp.;
Pomalyst
and
Revlimid
are trademarks of Celgene Corporation; and
Prostvac
is a trademark of BN ImmunoTherapeutics Inc. and/or one of its affiliates. Brand names of products that are in all italicized letters, without an asterisk, are registered trademarks of BMS and/or one of its subsidiaries.
|