|
FORM 10-K
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE TRANSITION PERIOD FROM TO
|
|
Bionano Genomics, Inc.
|
||
|
(Exact name of Registrant as specified in its Charter)
|
||
|
Delaware
|
26-1756290
|
|
|
(State or other jurisdiction of
incorporation or organization)
|
(I.R.S. Employer
Identification No.) |
|
|
9540 Towne Centre Drive, Suite 100,
San Diego, CA
|
92121
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
|
Registrant’s telephone number, including area code: (858) 888-7600
|
||
|
Title of Each Class
|
Trading Symbol(s)
|
Name of Each Exchange on which Registered
|
|
Common Stock, $0.0001 par value
|
BNGO
|
The Nasdaq Stock Market, LLC
|
|
Warrants to purchase Common Stock
|
BNGOW
|
The Nasdaq Stock Market, LLC
|
|
Large accelerated filer
|
¨
|
Accelerated filer
|
¨
|
|
Non-accelerated filer
|
x
|
Smaller reporting company
|
x
|
|
Emerging growth company
|
x
|
||
|
Page
|
||
|
PART I
|
||
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
PART II
|
||
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
PART III
|
||
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
PART IV
|
||
|
Item 15.
|
||
|
Item 16.
|
||
|
•
|
the size and growth potential of the markets for our products, and our ability to serve those markets;
|
|
•
|
the rate and degree of market acceptance of our products;
|
|
•
|
ability to expand our sales organization to address effectively existing and new markets that we intend to target;
|
|
•
|
impact from future regulatory, judicial, and legislative changes or developments in the U.S. and foreign countries;
|
|
•
|
ability to compete effectively in a competitive industry;
|
|
•
|
the success of competing technologies that are or may become available;
|
|
•
|
the performance of our third-party contract sales organizations, suppliers and manufacturers;
|
|
•
|
our ability to attract and retain key scientific or management personnel;
|
|
•
|
the accuracy of our estimates regarding expenses, future revenues, reimbursement rates, capital requirements and needs for additional financing;
|
|
•
|
our ability to comply with the covenants and satisfy certain conditions of our debt facility;
|
|
•
|
our ability to obtain funding for our operations; and
|
|
•
|
our ability to attract collaborators and strategic partnerships;
|
|
•
|
We announced the adoption of our Saphyr system by PerkinElmer Genomics and the University of Iowa, including their development of assays using our optical mapping technology to expand their genetic tests assessing disease-associated chromosomal abnormalities.
|
|
•
|
We announced that Novogene added the Saphyr system to their repertoire of genomics data services.
|
|
•
|
We announced the adoption of our Saphyr system by Mayo Clinic for applications in neurodegenerative disease.
|
|
•
|
We announced the adoption of our Saphyr system by GeneDx, using our system for the clinical detection of genetic variants in muscular dystrophies, developmental and reproductive disorders.
|
|
•
|
Sequencing for Discovery Research.
In discovery research across patient cohorts, sequencing is primarily used to find single nucleotide variations responsible for disease or therapeutic response. Sequencing alone, however, is significantly limited due to its inability to reveal structural variations. Our Saphyr system has been expanding this market segment by complementing sequencing to expand the scope of genome variation that can be analyzed in a study and achieve a more comprehensive view of the genome.
|
|
•
|
Cytogenetics.
To provide a clinical diagnosis, cytogenetic tests detect known variations that are linked to specific diseases or therapeutic responses. The technologies used for detecting structural variations are expensive and involve cumbersome workflows with relatively limited ability to scale to higher volumes or more complex testing panels. Sequencers tend not to be used for cytogenetics due to their inability to reliably detect structural variations. Cytogenetics laboratories are beginning to adopt Saphyr as a more effective and efficient approach to finding the structural variations relevant to cytogenetics. For this segment, Saphyr is used alone to provide comprehensive detection of structural variations and enable diagnostic calls without the need for any sequencing or cytogenetic technology.
|
|
•
|
dystrophin gene variation – structural variation disrupting dystrophin production that is found in Duchenne Muscular Dystrophy;
|
|
•
|
9pminus variation – deletion found in a rare developmental syndrome in children;
|
|
•
|
TMPRSS2-ERG fusion – gene fusion found in prostate cancer;
|
|
•
|
EML4-ALK fusion – gene fusion found in lung cancer; and
|
|
•
|
BCR-ABL fusion (Philadelphia chromosome) – gene fusion found in leukemias such as chronic myelogenous leukemia, acute lymphoblastic leukemia and acute myelogenous leukemia.
|
|
•
|
Extremely long molecules for analysis.
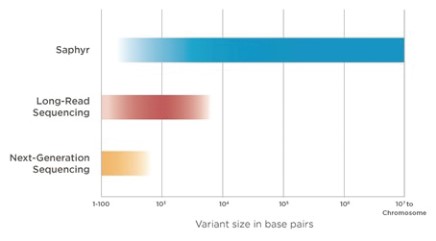
Structural accuracy can only come from analysis of extremely long chromosomal fragments. The Saphyr system is capable of analyzing single molecules that are on average approximately 250,000 base pairs long. Such fragments will contain enough unique sequence information that they are distinguishable from other fragments. These lengths are over 1,000 times longer than the average read length with Illumina systems and approximately 10 times longer than the average read lengths with Pacific Biosciences and Oxford Nanopore systems. Building a picture of the genome with massive building blocks overcomes the inherent challenge of genome complexity and is the key to Saphyr’s unprecedented sensitivity and specificity.
|
|
•
|
Proprietary nanotechnology for massively parallel linearization and analysis of long molecules with single molecule imaging.
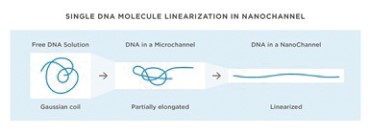
Analyzing these extremely long chromosomal fragments required invention. Molecules of this size are more like balls of yarn in a test tube and must be unraveled for meaningful analysis. We invented, patented, developed and commercialized nanochannel arrays to capture them from solution and unwind and linearize them for structural variation analysis. Each molecule is imaged separately, making it possible to deconvolute complex mixtures including haplotypes and heterogeneous tumors, as shown in the graphic below.
|
|
•
|
DNA labeling chemistry specifically for physical mapping.
The detailed analysis of sequence we use is also highly unique and novel. Instead of identifying the sequence of every base pair in these long fragments, we label and detect specific sequence patterns or motifs that occur universally across every genome with an average frequency of approximately one
|
|
•
|
Bioinformatic tools for structural variation analysis.
Finally, our approach includes a novel bioinformatics platform that we developed from the ground-up to take advantage of the unique benefits of our solution. It comprises proprietary algorithms for the construction of a structurally accurate physical map of the genome without using a reference genome in assignment of structure. Physical maps of a test subject are then compared in cross-mapping analysis that allows our system to detect genome wide structural variation, including the most complex balanced events. Our system can do so by comparing one physical map against a common reference, or against the maps of a mother and father in the case of an afflicted child with an undiagnosed disease for example, or against maps of normal blood when studying solid tumor cancers. This comparative approach uses our proprietary database of healthy individuals to filter out the non-disease causing structural variants found in the general healthy population.
|

|
•
|
Highly sensitive.
We believe Saphyr is the most sensitive structural variation detector currently on the market in that it can identify structural variations that no other system can.
|
|
•
|
Highly specific.
The structural variations found by Saphyr are found by direct observation rather than inference. Saphyr has a very low false positive rate, typically less than 2%.
|
|
•
|
Cost effective.
We expect the cost per sample to continue to decline to less than $300 per sample in 2020 and less than $100 per sample in 2021.
|
|
•
|
Fast.
Saphyr generates greater than 1,920 giga base pairs of information per day, on par with some of the faster short-read sequencers in the market. For highly sensitive structural variation detection, this allows Saphyr to process six human samples per day. We expect Saphyr’s throughput to increase to 12 per day by the end of 2020. Over this same period, we expect to continuously improve the automation of sample prep and bioinformatics to help drive efficiencies of workflow.
|




|
•
|
set up runs and monitor real-time data quality metrics remotely to flag potential sample quality issues early;
|
|
•
|
automatically start de novo assemblies and structural variation analysis when the desired amount of data has been collected;
|
|
•
|
detect variants with an allele fraction of 5%
|
|
•
|
visualize and manipulate maps and structural variants; and
|
|
•
|
analyze trios and clinical samples by filtering through uncommon variants to identify inherited and de novo variants, and export in a file format that is used consistently throughout the industry.
|
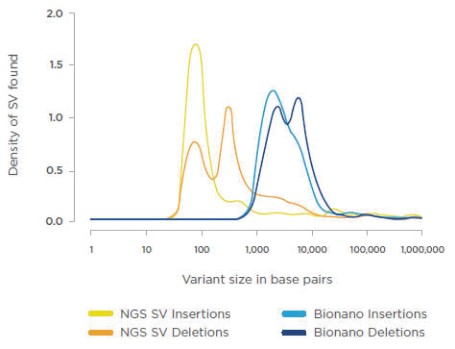
|
•
|
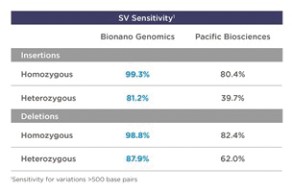
99% sensitivity for homozygous insertions/deletions larger than 500 base pairs;
|
|
•
|
95% sensitivity for heterozygous insertions/deletions larger than 500 base pairs;
|
|
•
|
95% sensitivity for balanced and unbalanced translocations larger than 50,000 base pairs;
|
|
•
|
99% sensitivity for inversions larger than 30,000 base pairs;
|
|
•
|
97% sensitivity for duplications larger than 30,000 base pairs; and
|
|
•
|
97% sensitivity for copy number variants larger than 500,000 base pairs.
|
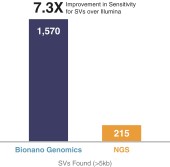
|
•
|
Highly differentiated technology platform enables researchers and clinicians to obtain information that cannot be had systematically and cost efficiently from traditional technologies.
Saphyr’s unique ability to systematically and cost efficiently see structural variations across the genome from 500 base pairs to tens of millions of base pairs is unique in the industry. We believe this greater insight will facilitate a paradigm shift in healthcare from an emphasis on treatment with
|
|
•
|
Validated solution recognized industry-wide.
We have deep and expanding scientific validation. Our system has been cited in hundreds of publications, and we believe our technology is becoming a vital tool in cutting-edge life sciences research.
|
|
•
|
Validated solution for genetic testing.
Our system has been validated in a CLIA lab setting at PerkinElmer to test for facioscapulohumeral muscular dystrophy (FSHD). PerkinElmer introduced the LDT to the market in 2020.
|
|
•
|
Strong installed base of premier customers.
We have shipped 104 Saphyr instruments globally, of which 84 have been installed. Our customers include some of the world’s most prominent clinical, translational research, basic research, academic and government institutions as well as leading pharmaceutical and diagnostic companies. Examples include Children’s National Health System, Boston Children’s Hospital, PerkinElmer, GeneDx, Mayo Clinic, DuPont Pioneer, Garvan Institute of Medical Research, Genentech, Icahn School of Medicine at Mount Sinai, McDonnell Genome Institute at Washington University, National Cancer Institute, National Institutes of Health, Pennsylvania State University and Salk Institute for Biological Studies.
|
|
•
|
Attractive business model with a growing, high-margin recurring revenue component.
As we continue to grow our installed base of Saphyr systems, optimize workflows and expand our structural variation detection capabilities, we expect to rapidly increase sales of our high-margin consumables. The successful integration of our technology into our customers’ projects provides ongoing sales of assays and consumables.
|
|
•
|
Industry-leading intellectual property portfolio.
We have developed a global patent portfolio that includes 65 issued patents across 14 patent families and an exclusively licensed portfolio of patents and applications from Princeton University, which includes 34 patents across two families. This global patent portfolio has filing dates ranging from 2001 to 2019. We have robust intellectual property protection surrounding our devices, systems, and methods for macromolecular analysis. Our ideation stems from our highly active research programs and results in our patent portfolio continually expanding at a significant pace.
|
|
•
|
Highly experienced senior management team.
We are led by a dedicated and highly experienced senior management team with significant industry experience and proven ability to develop novel solutions. Each of the members of our senior management has more than 20 years of relevant experience.
|
|
•
|
Drive adoption of Saphyr in discovery research and cytogenetics markets.
Saphyr has the potential to significantly expand the life science research market and genomics-based diagnostics market because of its unrivaled sensitivity, by enabling researchers to perform studies on structural variations that they were previously unable to perform. We believe Saphyr has the capability to enable the development of a new category of diagnostic tests and tools.
|
|
•
|
Support the publication of findings with Saphyr by our customers beyond the more than 280 papers published to date.
The annual number of publications featuring data generated by Saphyr and its predecessor system has steadily increased since 2010 when the first publication appeared. Recently, the overall number of these publications has grown significantly. For example, of the more than 280 papers published to date, approximately 80 were published in 2019 alone and 213 since 2017, the year Saphyr was launched. We will continue to support and foster our customer base to help grow the number of publications featuring our systems’ data. We believe that these publications are impactful as our customers’ studies cover structural variations in areas of high unmet medical need, such as rare and undiagnosed pediatric diseases, muscular diseases, developmental delays and disorders, prostate cancer and leukemia.
|
|
•
|
Expand gross margins through economies of scale and growing sales of consumables.
Our overall gross margin has historically been driven by our instrument gross margin as the sales of our instruments have constituted the significant majority of our total revenues to date. However, our instrument gross margin is significantly lower than our consumables gross margin. We expect our overall gross margin to expand in 2020 and beyond as:
|
|
◦
|
We further negotiate with silicon fabrication manufacturers for better contract pricing of our consumables. As our manufacturing lot volumes increase, we expect to have lower costs of goods sold. This is driven by the pass along of some of the economies of scale of contract manufacturers that mainly operate in the ultra-high-volume silicon computer chip industry.
|
|
◦
|
Consumables sales continue to represent the fastest growing component of overall revenues. As consumables growth continues to outpace instrument growth, we expect the proportion of our product mix which is higher gross margin to increase, thereby expanding our overall gross margin.
|
|
•
|
Continue to innovate our products and technologies.
We designed Saphyr to accommodate performance enhancements without the need for replacement of the entire instrument. For example, hardware upgrades and new consumables are made available to purchase by customers. We intend for these performance enhancements to be delivered on a regular basis. In addition, we periodically make available software upgrades to customers through download at no charge. We expect to continue developing and refining our technologies to improve the ease of use of our Saphyr system and enable our existing installed systems to meaningfully increase sample throughput and sensitivity and specificity of structural variation detection.
|
|
•
|
Partner with industry-leading companies and laboratories to accelerate adoption in clinical markets.
Establish additional collaborations with customers to help drive validating studies. Expand partnership efforts with clinical diagnostic companies to commercialize LDTs in the U.S. as well as LDTs and approved tests outside the U.S.
|
|
Commercial Focus
|
Number of Issued and Pending Patents
|
|
|
Nanochannel devices and systems
|
76
|
|
|
Methods of macromolecule analysis using nanochannel arrays
|
71
|
|
|
Methods of genetic detection and copy number analysis
|
28
|
|
|
Method of genomic sequence and epigenomic analysis.
|
49
|
|
|
Biomolecule isolation and processing for use in nanochannel analysis
|
3
|
|
|
Method of optimizing nanochannel analysis
|
6
|
|
|
Next-generation products
|
10
|
|
|
•
|
compliance with QSRs, which require manufacturers to follow stringent design, testing, control, documentation, record maintenance, including maintenance of complaint and related investigation files, and other quality assurance controls during the manufacturing process;
|
|
•
|
reporting of device malfunctions, serious injuries or deaths;
|
|
•
|
registration of the establishments where the devices are produced;
|
|
•
|
labeling regulations, which prohibit the promotion of products for uncleared or unapproved uses; and
|
|
•
|
medical device reporting obligations, which require that manufacturers investigate and report to the FDA adverse events, including deaths, or serious injuries that may have been or were caused by a medical device and malfunctions in the device that would likely cause or contribute to a death or serious injury if it were to recur.
|
|
•
|
adoption of our systems and related products;
|
|
•
|
the timing of customer orders to purchase our systems;
|
|
•
|
the rate of utilization of consumables by our customers;
|
|
•
|
receipt and timing of revenue for services provided by out data solutions service;
|
|
•
|
the timing of the introduction of new systems, products, system and product enhancements and services; and
|
|
•
|
the receipt and timing of revenue from our distribution and marketing arrangements.
|
|
•
|
expand our sales and marketing efforts to further commercialize our products;
|
|
•
|
expand our research and development efforts to improve our existing products and develop and launch new products, particularly if any of our products are deemed by the U.S. Food and Drug Administration, or FDA, to be medical devices or otherwise subject to additional regulation by the FDA;
|
|
•
|
seek FDA approval to market our existing products or new products utilized for diagnostic purposes;
|
|
•
|
lease a larger facility or build out our existing facility as we continue to grow our employee headcount;
|
|
•
|
hire additional personnel;
|
|
•
|
enter into collaboration arrangements, if any, or in-license other products and technologies;
|
|
•
|
add operational, financial and management information systems; and
|
|
•
|
incur increased costs as a result of continued operation as a public company.
|
|
•
|
market acceptance of our products;
|
|
•
|
the cost and timing of establishing additional sales, marketing and distribution capabilities;
|
|
•
|
the cost of our research and development activities;
|
|
•
|
the success of our existing distribution and marketing arrangements and our ability to enter into additional arrangements in the future; and
|
|
•
|
the effect of competing technological and market developments.
|
|
•
|
our ability to attract, retain and manage the sales, marketing and service personnel necessary to expand market acceptance for our technology;
|
|
•
|
the time and cost of maintaining and growing a specialized sales, marketing and service force; and
|
|
•
|
our sales, marketing and service force may be unable to execute successful commercial activities.
|
|
•
|
changes in government programs that provide funding to research institutions and companies;
|
|
•
|
macroeconomic conditions and the political climate;
|
|
•
|
changes in the regulatory environment;
|
|
•
|
differences in budgetary cycles; and
|
|
•
|
market acceptance of relatively new technologies, such as ours.
|
|
•
|
correctly identify customer needs and preferences and predict future needs and preferences;
|
|
•
|
allocate our research and development funding to products with higher growth prospects;
|
|
•
|
anticipate and respond to our competitors’ development of new products and technological innovations;
|
|
•
|
innovate and develop new technologies and applications, and acquire or obtain rights to third-party technologies that may have valuable applications in the markets we serve;
|
|
•
|
successfully commercialize new technologies in a timely manner, price them competitively and manufacture and deliver sufficient volumes of new products of appropriate quality on time; and
|
|
•
|
customers' willingness to adopt new technologies.
|
|
•
|
required compliance with existing and changing foreign regulatory requirements and laws;
|
|
•
|
difficulties and costs of staffing and managing foreign operations;
|
|
•
|
difficulties protecting or procuring intellectual property rights;
|
|
•
|
required compliance with anti-bribery laws, such as the U.S. Foreign Corrupt Practices Act, data privacy requirements, labor laws and anti-competition regulations;
|
|
•
|
export or import restrictions;
|
|
•
|
laws and business practices favoring local companies;
|
|
•
|
longer payment cycles and difficulties in enforcing agreements and collecting receivables through certain foreign legal systems;
|
|
•
|
political and economic instability; and
|
|
•
|
potentially adverse tax consequences, tariffs, customs charges, bureaucratic requirements and other trade barriers.
|
|
•
|
greater name and brand recognition;
|
|
•
|
substantially greater financial and human resources;
|
|
•
|
broader product lines;
|
|
•
|
larger sales forces and more established distributor networks;
|
|
•
|
substantial intellectual property portfolios;
|
|
•
|
larger and more established customer bases and relationships; and
|
|
•
|
better established, larger scale, and lower cost manufacturing capabilities.
|
|
•
|
cost of instruments and consumables;
|
|
•
|
accuracy, including sensitivity and specificity, and reproducibility of results;
|
|
•
|
reputation among customers;
|
|
•
|
innovation in product offerings;
|
|
•
|
flexibility and ease of use; and
|
|
•
|
compatibility with existing laboratory processes, tools and methods.
|
|
•
|
disruption in our relationships with customers, distributors or suppliers as a result of such a transaction;
|
|
•
|
unanticipated liabilities related to acquired companies;
|
|
•
|
difficulties integrating acquired personnel, technologies and operations into our existing business;
|
|
•
|
diversion of management time and focus from operating our business to acquisition integration challenges;
|
|
•
|
increases in our expenses and reductions in our cash available for operations and other uses; and
|
|
•
|
possible write-offs or impairment charges relating to acquired businesses.
|
|
•
|
we or our licensors might not have been the first to make the inventions claimed or disclosed by our pending patent applications or issued patents;
|
|
•
|
we or our licensors might not have been the first to file patent applications for these inventions. To determine the priority of these inventions, we may have to participate in interference proceedings or derivation proceedings declared by the U.S. Patent and Trademark Office, or the USPTO, which could result in substantial cost to us, and could possibly result in a loss or narrowing of patent rights. No assurance can be given that our patent applications or granted patents (or those of our licensors) will have priority over any other patent or patent application involved in such a proceeding, or will be held valid as an outcome of the proceeding;
|
|
•
|
other parties may independently develop similar or alternative products and technologies or duplicate any of our products and technologies, which can potentially impact our market share, revenue, and goodwill, regardless of whether intellectual property rights are successfully enforced against these other parties;
|
|
•
|
it is possible that our owned or licensed pending patent applications will not result in granted patents, and even if such pending patent applications issue as patents, they may not provide intellectual property protection of commercially viable products or product features, may not provide us with any competitive advantages, or may be challenged and invalidated by third parties, patent offices, and/or the courts;
|
|
•
|
we may be unaware of or unfamiliar with prior art and/or interpretations of prior art that could potentially impact the validity or scope of our patents or pending patent applications, or patent applications that we intend to file;
|
|
•
|
we take efforts and enter into agreements with employees, consultants, collaborators, and advisors to confirm ownership and chain of title in intellectual property rights. However, an inventorship or ownership dispute could arise that may permit one or more third parties to practice or enforce our intellectual property rights, including possible efforts to enforce rights against us;
|
|
•
|
we may elect not to maintain or pursue intellectual property rights that, at some point in time, may be considered relevant to or enforceable against a competitor;
|
|
•
|
we may not develop additional proprietary products and technologies that are patentable, or we may develop additional proprietary products and technologies that are not patentable;
|
|
•
|
the patents or other intellectual property rights of others may have an adverse effect on our business; and
|
|
•
|
we apply for patents relating to our products and technologies and uses thereof, as we deem appropriate. However, we or our representatives or their agents may fail to apply for patents on important products and technologies in a timely fashion or at all, or we or our representatives or their agents may fail to apply for patents in potentially relevant jurisdictions.
|
|
•
|
royalty payments;
|
|
•
|
annual maintenance fees;
|
|
•
|
using commercially reasonable efforts to develop and sell a product using the licensed technology and developing a market for such product;
|
|
•
|
paying and/or reimbursing fees related to prosecution, maintenance and enforcement of patent rights; and
|
|
•
|
providing certain reports.
|
|
•
|
the scope of rights granted under the license agreement and other interpretation-related issues;
|
|
•
|
whether and the extent to which our technology and processes infringe any intellectual property of the licensor that is not subject to the licensing agreement;
|
|
•
|
whether to take action to enforce any intellectual property rights against an allegedly infringing product or process of a third party;
|
|
•
|
our right to sublicense patent and other rights to third parties;
|
|
•
|
our diligence obligations with respect to the use of licensed technology in relation to our development and commercialization of our products, and what activities satisfy those diligence obligations; and
|
|
•
|
the ownership of inventions and know-how, such as intellectual property resulting from the joint creation or use of intellectual property by our licensors and us and our partners.
|
|
•
|
seek to obtain licenses that may not be available on commercially reasonable terms, if at all;
|
|
•
|
abandon any product alleged or held to infringe, or redesign our products or processes to avoid potential assertion of infringement;
|
|
•
|
pay substantial damages including, in exceptional cases, treble damages and attorneys’ fees, which we may have to pay if a court decides that the product or proprietary technology at issue infringes upon or violates the third-party’s rights;
|
|
•
|
pay substantial royalties or fees or grant cross-licenses to our technology; or
|
|
•
|
defend litigation or administrative proceedings that may be costly whether we win or lose, and which could result in a substantial diversion of our financial and management resources.
|
|
•
|
others may be able to develop and/or use technology that is similar to our technology or aspects of our technology but that does not cover the claims of any our patents or patents that may issue from our patent applications or those we license;
|
|
•
|
we or the licensor of our licensed-in patents might not have been the first to make the inventions disclosed and/or claimed in a pending patent application that we own or license;
|
|
•
|
we or the licensor of our licensed-in patents might not have been the first to file patent applications disclosing and/or claiming an invention;
|
|
•
|
others may independently develop similar or alternative technologies without infringing our or our licensors’ intellectual property rights;
|
|
•
|
pending patent applications that we own or license may not lead to issued patents or may not result in the claims that we want (for example, as to the scope of issued claims, if any);
|
|
•
|
patents, if issued, that we own or license may not provide us with any competitive advantages, or may be held invalid or unenforceable, as a result of legal challenges by our competitors or other third parties;
|
|
•
|
third parties may compete with us in jurisdictions where we do not pursue and obtain patent protection;
|
|
•
|
we may not be able to obtain and/or maintain necessary or useful licenses on reasonable terms or at all;
|
|
•
|
third parties may assert an ownership interest in our intellectual property and, if successful, such disputes may preclude us from exercising exclusive rights over that intellectual property;
|
|
•
|
we may not be able to maintain the confidentiality of our trade secrets or other proprietary information;
|
|
•
|
we may not develop or in-license additional proprietary technologies that are patentable; and
|
|
•
|
the patents or other intellectual property of others may have an adverse effect on our business.
|
|
•
|
our commercial progress in marketing and selling our systems, including sales and revenue trends;
|
|
•
|
changes in laws or regulations applicable our systems;
|
|
•
|
adverse developments related to our laboratory facilities;
|
|
•
|
increased competition in the diagnostics services industry;
|
|
•
|
the failure of our customers to obtain and/or maintain coverage and adequate reimbursement for their services using our systems;
|
|
•
|
adverse developments concerning our manufacturers and suppliers;
|
|
•
|
our inability to establish future collaborations;
|
|
•
|
additions or departures of key scientific or management personnel;
|
|
•
|
introduction of new testing services offered by us or our competitors;
|
|
•
|
announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by us or our competitors;
|
|
•
|
our ability to effectively manage our growth;
|
|
•
|
the size and growth, if any, of our targeted markets;
|
|
•
|
actual or anticipated variations in quarterly operating results;
|
|
•
|
our cash position;
|
|
•
|
our failure to meet the estimates and projections of the investment community or that we may otherwise provide to the public;
|
|
•
|
publication of research reports about us or our industry or positive or negative recommendations or withdrawal of research coverage by securities analysts;
|
|
•
|
changes in the market valuations of similar companies;
|
|
•
|
overall performance of the equity markets;
|
|
•
|
issuances of debt or equity securities;
|
|
•
|
sales of our securities by us or our stockholders in the future;
|
|
•
|
trading volume of our securities;
|
|
•
|
changes in accounting practices;
|
|
•
|
ineffectiveness of our internal controls;
|
|
•
|
disputes or other developments relating to proprietary rights, including our ability to adequately protect our technologies;
|
|
•
|
significant lawsuits, including patent or stockholder litigation;
|
|
•
|
general political and economic conditions; and
|
|
•
|
other events or factors, many of which are beyond our control.
|
|
•
|
a board of directors divided into three classes serving staggered three-year terms, such that not all members of the board will be elected at one time;
|
|
•
|
a prohibition on stockholder action through written consent, which requires that all stockholder actions be taken at a meeting of our stockholders;
|
|
•
|
a requirement that special meetings of stockholders be called only by the chairman of the board of directors, the chief executive officer, the president or by a majority of the total number of authorized directors;
|
|
•
|
advance notice requirements for stockholder proposals and nominations for election to our board of directors;
|
|
•
|
a requirement that no member of our board of directors may be removed from office by our stockholders except for cause and, in addition to any other vote required by law, upon the approval of not less than two-thirds of all outstanding shares of our voting stock then entitled to vote in the election of directors;
|
|
•
|
a requirement of approval of not less than two-thirds of all outstanding shares of our voting stock to amend any bylaws by stockholder action or to amend specific provisions of our certificate of incorporation; and
|
|
•
|
the authority of the board of directors to issue preferred stock on terms determined by the board of directors without stockholder approval and which preferred stock may include rights superior to the rights of the holders of common stock.
|
|
•
|
expand our sales and marketing efforts to further commercialize our products;
|
|
•
|
continue research and development efforts to improve our existing products;
|
|
•
|
hire additional personnel;
|
|
•
|
enter into collaboration arrangements, if any;
|
|
•
|
add operational, financial and management information systems; and
|
|
•
|
incur increased costs as a result of operating as a public company.
|
|
Year Ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Product revenue
|
$
|
9,474,444
|
|
$
|
11,463,173
|
|
|
|
Service and other revenue
|
655,064
|
|
537,562
|
|
|||
|
Total
|
$
|
10,129,508
|
|
$
|
12,000,735
|
|
|
|
Year Ended December 31,
|
|||||||||||||
|
2019
|
2018
|
||||||||||||
|
$
|
%
|
$
|
%
|
||||||||||
|
North America
|
$
|
5,030,267
|
|
50
|
%
|
$
|
4,594,814
|
|
38
|
%
|
|||
|
EMEIA
|
3,627,602
|
|
36
|
%
|
3,954,693
|
|
33
|
%
|
|||||
|
Asia Pacific
|
1,471,639
|
|
14
|
%
|
3,451,228
|
|
29
|
%
|
|||||
|
Total
|
$
|
10,129,508
|
|
100
|
%
|
$
|
12,000,735
|
|
100
|
%
|
|||
|
Year Ended December 31,
|
Period-to-Period Change
|
|||||||||||||
|
2019
|
2018
|
$
|
%
|
|||||||||||
|
Product revenue
|
$
|
9,474,444
|
|
$
|
11,463,173
|
|
$
|
(1,988,729
|
)
|
(17
|
)%
|
|||
|
Service and other revenue
|
655,064
|
|
537,562
|
|
117,502
|
|
22
|
%
|
||||||
|
Total revenue
|
10,129,508
|
|
12,000,735
|
|
(1,871,227
|
)
|
(16
|
)%
|
||||||
|
Cost of product revenue
|
6,495,693
|
|
8,562,042
|
|
(2,066,349
|
)
|
(24
|
)%
|
||||||
|
Cost of service and other revenue
|
272,454
|
|
149,284
|
|
123,170
|
|
83
|
%
|
||||||
|
Total cost of revenue
|
6,768,147
|
|
8,711,326
|
|
(1,943,179
|
)
|
(22
|
)%
|
||||||
|
Research and development
|
9,080,891
|
|
9,484,163
|
|
(403,272
|
)
|
(4
|
)%
|
||||||
|
Selling, general and administrative
|
20,155,376
|
|
14,220,331
|
|
5,935,045
|
|
42
|
%
|
||||||
|
Total operating expenses
|
29,236,267
|
|
23,704,494
|
|
5,531,773
|
|
23
|
%
|
||||||
|
Loss from operations
|
(25,874,906
|
)
|
(20,415,085
|
)
|
(5,459,821
|
)
|
27
|
%
|
||||||
|
Interest expense
|
(2,286,196
|
)
|
(1,381,024
|
)
|
(905,172
|
)
|
66
|
%
|
||||||
|
Change in fair value of preferred stock warrants and expirations
|
—
|
|
3,991,081
|
|
(3,991,081
|
)
|
(100
|
)%
|
||||||
|
Loss on debt extinguishment
|
(1,333,496
|
)
|
(342,164
|
)
|
(991,332
|
)
|
290
|
%
|
||||||
|
Other expense
|
(299,424
|
)
|
(333,689
|
)
|
34,265
|
|
(10
|
)%
|
||||||
|
Loss before income taxes
|
(29,794,022
|
)
|
(18,480,881
|
)
|
(11,313,141
|
)
|
61
|
%
|
||||||
|
Provision for income taxes
|
(21,048
|
)
|
(15,511
|
)
|
(5,537
|
)
|
36
|
%
|
||||||
|
Net loss
|
$
|
(29,815,070
|
)
|
$
|
(18,496,392
|
)
|
$
|
(11,318,678
|
)
|
61
|
%
|
|||
|
Year Ended December 31,
|
Period-to-Period Change
|
|||||||||||||
|
2019
|
2018
|
$
|
%
|
|||||||||||
|
Instrument revenue
|
$
|
6,762,463
|
|
$
|
8,441,325
|
|
$
|
(1,678,862
|
)
|
(20
|
)%
|
|||
|
Consumable revenue
|
2,711,981
|
|
3,021,848
|
|
(309,867
|
)
|
(10
|
)%
|
||||||
|
Product revenue
|
9,474,444
|
|
11,463,173
|
|
(1,988,729
|
)
|
(17
|
)%
|
||||||
|
Services and other revenue
|
655,064
|
|
537,562
|
|
117,502
|
|
22
|
%
|
||||||
|
Total revenue
|
$
|
10,129,508
|
|
$
|
12,000,735
|
|
$
|
(1,871,227
|
)
|
(16
|
)%
|
|||
|
Year Ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Net cash provided by (used in):
|
|||||||
|
Operating activities
|
$
|
(29,529,720
|
)
|
$
|
(19,943,847
|
)
|
|
|
Investing activities
|
(61,056
|
)
|
(331,716
|
)
|
|||
|
Financing activities
|
30,379,420
|
|
35,776,395
|
|
|||
|
Year Ended December 31,
|
|||||||||||||
|
2019
|
2018
|
||||||||||||
|
$
|
%
|
$
|
%
|
||||||||||
|
North America
|
$
|
5,030,267
|
|
50
|
%
|
$
|
4,594,814
|
|
38
|
%
|
|||
|
EMEIA
|
3,627,602
|
|
36
|
%
|
3,954,693
|
|
33
|
%
|
|||||
|
Asia Pacific
|
1,471,639
|
|
14
|
%
|
3,451,228
|
|
29
|
%
|
|||||
|
Total
|
$
|
10,129,508
|
|
100
|
%
|
$
|
12,000,735
|
|
100
|
%
|
|||
|
•
|
we will present only two years of audited consolidated financial statements, plus unaudited consolidated condensed financial statements for any interim period, and related management’s discussion and analysis of financial condition and results of operations in our initial registration statement;
|
|
•
|
we will avail ourselves of the exemption from the requirement to obtain an attestation and report from our auditors on the assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act;
|
|
•
|
we will avail ourselves of the extended transition periods available to emerging growth companies under the JOBS Act for complying with new or revised accounting standards; and
|
|
•
|
we will provide less extensive disclosure about our executive compensation arrangements.
|
|
Pages
|
|
|
December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
17,311,373
|
|
$
|
16,522,729
|
|
|
|
Accounts receivable, net
|
6,333,963
|
|
4,514,333
|
|
|||
|
Inventory
|
3,443,559
|
|
1,068,557
|
|
|||
|
Prepaid expenses and other current assets
|
1,169,346
|
|
919,500
|
|
|||
|
Total current assets
|
28,258,241
|
|
23,025,119
|
|
|||
|
Property and equipment, net
|
1,949,625
|
|
1,777,302
|
|
|||
|
Total assets
|
$
|
30,207,866
|
|
$
|
24,802,421
|
|
|
|
Liabilities and stockholders’ equity
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
2,699,153
|
|
$
|
1,351,736
|
|
|
|
Accrued expenses
|
3,225,431
|
|
2,900,129
|
|
|||
|
Contract liabilities
|
357,492
|
|
270,998
|
|
|||
|
Current portion of long-term debt
|
20,084,945
|
|
—
|
|
|||
|
Total current liabilities
|
26,367,021
|
|
4,522,863
|
|
|||
|
Long-term debt, net of current portion
|
—
|
|
9,029,374
|
|
|||
|
Long-term contract liabilities
|
182,648
|
|
304,467
|
|
|||
|
Other non-current liabilities
|
44,479
|
|
808,366
|
|
|||
|
Total liabilities
|
26,594,148
|
|
14,665,070
|
|
|||
|
Commitments and contingencies (Note 9)
|
|
|
|
|
|||
|
Stockholders’ equity:
|
|
|
|||||
|
Common stock, $0.0001 par value, 200,000,000 shares authorized at December 31, 2019 and December 31, 2018; 34,274,469 and 10,055,072 shares issued and outstanding at December 31, 2019 and December 31, 2018, respectively
|
3,427
|
|
1,004
|
|
|||
|
Preferred stock, $0.0001 par value; 10,000,000 shares authorized; no shares issued and outstanding as of December 31, 2019 and 2018
|
—
|
|
—
|
|
|||
|
Additional paid-in capital
|
106,187,789
|
|
82,898,775
|
|
|||
|
Accumulated deficit
|
(102,577,498
|
)
|
(72,762,428
|
)
|
|||
|
Total stockholders’ equity
|
3,613,718
|
|
10,137,351
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
30,207,866
|
|
$
|
24,802,421
|
|
|
|
Year Ended
December 31, |
|||||||
|
2019
|
2018
|
||||||
|
Revenue:
|
|||||||
|
Product revenue
|
$
|
9,474,444
|
|
$
|
11,463,173
|
|
|
|
Service and other revenue
|
655,064
|
|
537,562
|
|
|||
|
Total revenue
|
10,129,508
|
|
12,000,735
|
|
|||
|
Cost of revenue:
|
|||||||
|
Cost of product revenue
|
6,495,693
|
|
8,562,042
|
|
|||
|
Cost of service and other revenue
|
272,454
|
|
149,284
|
|
|||
|
Total cost of revenue
|
6,768,147
|
|
8,711,326
|
|
|||
|
Operating expenses:
|
|||||||
|
Research and development
|
9,080,891
|
|
9,484,163
|
|
|||
|
Selling, general and administrative
|
20,155,376
|
|
14,220,331
|
|
|||
|
Total operating expenses
|
29,236,267
|
|
23,704,494
|
|
|||
|
Loss from operations
|
(25,874,906
|
)
|
(20,415,085
|
)
|
|||
|
Other income (expense)
|
|||||||
|
Interest expense
|
(2,286,196
|
)
|
(1,381,024
|
)
|
|||
|
Change in fair value of preferred stock warrants and expirations
|
—
|
|
3,991,081
|
|
|||
|
Loss on debt extinguishment
|
(1,333,496
|
)
|
(342,164
|
)
|
|||
|
Other expenses
|
(299,424
|
)
|
(333,689
|
)
|
|||
|
Total other income (expenses)
|
(3,919,116
|
)
|
1,934,204
|
|
|||
|
Loss before income taxes
|
(29,794,022
|
)
|
(18,480,881
|
)
|
|||
|
Provision for income taxes
|
(21,048
|
)
|
(15,511
|
)
|
|||
|
Net loss
|
$
|
(29,815,070
|
)
|
$
|
(18,496,392
|
)
|
|
|
Net loss per share, basic and diluted
|
$
|
(1.99
|
)
|
$
|
(2.61
|
)
|
|
|
Weighted-average common shares outstanding, basic and diluted
|
14,977,901
|
|
7,077,126
|
|
|||
|
Series A
|
Series B
|
Series B-1
|
Series C
|
Series D
|
Series D-1
|
Additional
Paid-in
Capital
|
Accumulated
Deficit
|
Total
Stockholders' Equity
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Convertible Preferred
Stock |
Convertible Preferred
Stock |
Convertible Preferred
Stock |
Convertible Preferred
Stock |
Convertible Preferred
Stock |
Convertible Preferred
Stock |
Common Stock
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Balance at January 1, 2018
|
345,587
|
|
$
|
61,847
|
|
8,058,170
|
|
$
|
842,845
|
|
3,437,950
|
|
$
|
359,593
|
|
23,357,047
|
|
$
|
5,547,841
|
|
20,652,486
|
|
$
|
4,838,379
|
|
66,141,257
|
|
$
|
31,359,632
|
|
77,257
|
|
$
|
8
|
|
$
|
4,038,817
|
|
$
|
(54,266,036
|
)
|
$
|
(50,227,211
|
)
|
|||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(18,496,392
|
)
|
(18,496,392
|
)
|
|||||||||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,193,873
|
|
—
|
|
1,193,873
|
|
|||||||||||||||||||||||||||
|
Stock option exercises
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
2,062
|
|
—
|
|
3,499
|
|
—
|
|
3,499
|
|
|||||||||||||||||||||||||||
|
IPO, net of offering costs
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
3,864,000
|
|
386
|
|
19,389,592
|
|
—
|
|
19,389,978
|
|
|||||||||||||||||||||||||||
|
Conversion of preferred stock upon IPO
|
(345,587
|
)
|
(61,847
|
)
|
(8,058,170
|
)
|
(842,845
|
)
|
(3,437,950
|
)
|
(359,593
|
)
|
(23,357,047
|
)
|
(5,547,841
|
)
|
(20,652,486
|
)
|
(4,838,379
|
)
|
(66,141,257
|
)
|
(31,359,632
|
)
|
2,850,280
|
|
285
|
|
43,009,852
|
|
—
|
|
43,010,137
|
|
|||||||||||||||||||||||||||
|
Conversion of convertible note upon IPO
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
3,239,294
|
|
323
|
|
14,898,004
|
|
—
|
|
14,898,327
|
|
|||||||||||||||||||||||||||
|
Conversion of preferred stock warrants into common stock warrants
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
84,676
|
|
—
|
|
84,676
|
|
|||||||||||||||||||||||||||
|
Issue warrants for services
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
165,000
|
|
—
|
|
165,000
|
|
|||||||||||||||||||||||||||
|
Issuance of common stock for Employee Stock Purchase Plan
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
22,179
|
|
2
|
|
115,462
|
|
—
|
|
115,464
|
|
|||||||||||||||||||||||||||
|
December 31, 2018
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
10,055,072
|
|
$
|
1,004
|
|
$
|
82,898,775
|
|
$
|
(72,762,428
|
)
|
$
|
10,137,351
|
|
|||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(29,815,070
|
)
|
(29,815,070
|
)
|
|||||||||||||||||||||||||||
|
Issue common stock, net of issuance costs
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
11,829,388
|
|
1,183
|
|
10,958,352
|
|
—
|
|
10,959,535
|
|
|||||||||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,346,023
|
|
—
|
|
1,346,023
|
|
|||||||||||||||||||||||||||
|
Stock option Exercises
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
50,665
|
|
6
|
|
65,858
|
|
—
|
|
65,864
|
|
|||||||||||||||||||||||||||
|
Issue stock for covenant waiver
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
572,917
|
|
57
|
|
504,110
|
|
—
|
|
504,167
|
|
|||||||||||||||||||||||||||
|
Reduce warrant exercise price for covenant waiver
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
45,787
|
|
—
|
|
45,787
|
|
|||||||||||||||||||||||||||
|
Issuance of common stock for Employee Stock Purchase Plan
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
87,969
|
|
9
|
|
141,697
|
|
—
|
|
141,706
|
|
|||||||||||||||||||||||||||
|
Issue common stock for debt
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
201,789
|
|
—
|
|
201,789
|
|
|||||||||||||||||||||||||||
|
Issue warrants for debt
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
629,830
|
|
—
|
|
629,830
|
|
|||||||||||||||||||||||||||
|
Stock warrant exercises
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
11,678,458
|
|
1,168
|
|
9,395,568
|
|
—
|
|
9,396,736
|
|
|||||||||||||||||||||||||||
|
December 31, 2019
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
34,274,469
|
|
$
|
3,427
|
|
$
|
106,187,789
|
|
$
|
(102,577,498
|
)
|
$
|
3,613,718
|
|
|||||||||||||||||
|
Year Ended
December 31, |
|||||||
|
2019
|
2018
|
||||||
|
Operating activities:
|
|||||||
|
Net loss
|
$
|
(29,815,070
|
)
|
$
|
(18,496,392
|
)
|
|
|
Adjustments to reconcile net loss to cash used in operating activities:
|
|||||||
|
Depreciation and amortization expense
|
1,127,850
|
|
1,320,521
|
|
|||
|
Change in fair value of preferred stock warrants and expirations
|
—
|
|
(3,991,081
|
)
|
|||
|
Non-cash interest
|
883,269
|
|
750,474
|
|
|||
|
Stock-based compensation
|
1,346,023
|
|
1,193,873
|
|
|||
|
Provision for bad debt expense
|
554,867
|
|
(262,000
|
)
|
|||
|
Inventory impairment
|
—
|
|
1,287,000
|
|
|||
|
Loss on debt extinguishment
|
1,333,496
|
|
342,164
|
|
|||
|
(Gain) loss on disposal of fixed assets
|
11,918
|
|
—
|
|
|||
|
Fair value of warrants issued for services
|
—
|
|
165,000
|
|
|||
|
Other items
|
—
|
|
115,464
|
|
|||
|
Changes in operating assets and liabilities:
|
|||||||
|
Accounts receivable
|
(2,374,497
|
)
|
(900,119
|
)
|
|||
|
Inventory
|
(3,641,017
|
)
|
(418,984
|
)
|
|||
|
Prepaid expenses and other current assets
|
(245,046
|
)
|
152,012
|
|
|||
|
Accounts payable
|
1,362,397
|
|
(954,377
|
)
|
|||
|
Accrued expenses and contract liabilities
|
(73,910
|
)
|
(247,402
|
)
|
|||
|
Net cash used in operating activities
|
(29,529,720
|
)
|
(19,943,847
|
)
|
|||
|
Investing activities:
|
|||||||
|
Purchases of property and equipment
|
(61,056
|
)
|
(331,716
|
)
|
|||
|
Net cash used in investing activities
|
(61,056
|
)
|
(331,716
|
)
|
|||
|
Financing activities:
|
|||||||
|
Proceeds from issuance of debt, net of issuance costs
|
19,134,424
|
|
23,830,489
|
|
|||
|
Proceeds from borrowing from line of credit
|
5,113,072
|
|
—
|
|
|||
|
Repayments of borrowing from line of credit
|
(3,615,117
|
)
|
—
|
|
|||
|
Repayment of long-term debt
|
(10,812,000
|
)
|
(7,447,571
|
)
|
|||
|
Proceeds from sale of common stock, net of offering costs
|
19,556,464
|
|
19,389,978
|
|
|||
|
Proceeds from sale of common stock under employee stock purchase plan
|
141,706
|
|
—
|
|
|||
|
Proceeds from warrant and option exercises
|
860,871
|
|
3,499
|
|
|||
|
Net cash provided by financing activities
|
30,379,420
|
|
35,776,395
|
|
|||
|
Net increase in cash and cash equivalents
|
788,644
|
|
15,500,832
|
|
|||
|
Cash and cash equivalents at beginning of period
|
16,522,729
|
|
1,021,897
|
|
|||
|
Cash and cash equivalents at end of period
|
$
|
17,311,373
|
|
$
|
16,522,729
|
|
|
|
Supplemental disclosure of non-cash financing and investing activity:
|
|||||||
|
Transfer of instruments and servers from inventory into property and equipment
|
$
|
1,266,015
|
|
$
|
—
|
|
|
|
Transfer of instruments and servers from property and equipment into inventory
|
$
|
—
|
|
$
|
242,831
|
|
|
|
Conversion of convertible note into common stock
|
$
|
—
|
|
$
|
14,898,327
|
|
|
|
Fair value of stock and warrants issued in conjunction with debt
|
$
|
831,619
|
|
$
|
176,813
|
|
|
|
Issue stock for covenant waiver
|
$
|
504,167
|
|
$
|
—
|
|
|
|
Reduce warrant exercise price for covenant waiver
|
$
|
45,787
|
|
$
|
—
|
|
|
|
Final payment fee due in connection with the repayment of debt classified within other long-term liabilities
|
$
|
—
|
|
$
|
400,000
|
|
|
|
Conversion of preferred stock warrants into common stock and common stock warrants
|
$
|
—
|
|
$
|
84,676
|
|
|
|
Property and equipment costs incurred but not paid included in accounts payable and accrued expenses
|
$
|
—
|
|
$
|
3,150
|
|
|
|
Supplemental disclosure of cash flow information
|
|||||||
|
Interest paid
|
$
|
1,277,184
|
|
$
|
700,353
|
|
|
|
•
|
The conversion of all outstanding shares of convertible preferred stock into an aggregate
2,850,280
shares of common stock.
|
|
•
|
The automatic adjustment of certain preferred stock warrants into common stock warrants; the entire
$84,676
balance of preferred stock warrant liability was reclassified as additional paid-in-capital. In addition, the Company issued warrants to the IPO underwriters to purchase up to
115,920
shares of its common stock at fair value of
$0.4 million
.
|
|
•
|
The conversion of an aggregate of
$14.9 million
of outstanding convertible promissory notes and accrued interest into an aggregate of
3,239,294
shares of common stock.
|
|
December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Materials and supplies
|
$
|
950,846
|
|
$
|
161,468
|
|
|
|
Finished Goods
|
2,492,713
|
|
907,089
|
|
|||
|
$
|
3,443,559
|
|
$
|
1,068,557
|
|
||
|
Year Ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Instrument revenue
|
$
|
6,762,463
|
|
$
|
8,441,325
|
|
|
|
Consumable revenue
|
2,711,981
|
|
3,021,848
|
|
|||
|
Product revenue
|
9,474,444
|
|
11,463,173
|
|
|||
|
Services and other revenue
|
655,064
|
|
537,562
|
|
|||
|
Total revenue
|
$
|
10,129,508
|
|
$
|
12,000,735
|
|
|
|
Year Ended December 31,
|
|||||||||||||
|
2019
|
2018
|
||||||||||||
|
$
|
%
|
$
|
%
|
||||||||||
|
North America
|
$
|
5,030,267
|
|
50
|
%
|
$
|
4,594,814
|
|
38
|
%
|
|||
|
EMEIA
|
3,627,602
|
|
36
|
%
|
3,954,693
|
|
33
|
%
|
|||||
|
Asia Pacific
|
1,471,639
|
|
14
|
%
|
3,451,228
|
|
29
|
%
|
|||||
|
Total
|
$
|
10,129,508
|
|
100
|
%
|
$
|
12,000,735
|
|
100
|
%
|
|||
|
Year Ended December 31,
|
|||||
|
2019
|
2018
|
||||
|
Common stock options
|
1,742,912
|
|
1,282,847
|
|
|
|
Common warrants
|
24,026,550
|
|
4,062,507
|
|
|
|
Total
|
25,769,462
|
|
5,345,354
|
|
|
|
December 31,
2019 |
December 31,
2018 |
||||||
|
Prepayment to supplier
|
$
|
409,851
|
|
$
|
74,685
|
|
|
|
Prepaid insurance
|
302,433
|
|
460,684
|
|
|||
|
Other current assets
|
457,062
|
|
384,131
|
|
|||
|
Total
|
$
|
1,169,346
|
|
$
|
919,500
|
|
|
|
December 31,
2019 |
December 31,
2018 |
||||||
|
Computer and office equipment
|
$
|
476,402
|
|
$
|
476,402
|
|
|
|
Lab equipment
|
4,623,714
|
|
4,437,794
|
|
|||
|
Service equipment placed at customer sites
|
1,247,328
|
|
149,823
|
|
|||
|
Leasehold improvements
|
1,875,647
|
|
1,875,647
|
|
|||
|
8,223,091
|
|
6,939,666
|
|
||||
|
Less accumulated depreciation and amortization
|
(6,273,466
|
)
|
(5,162,364
|
)
|
|||
|
$
|
1,949,625
|
|
$
|
1,777,302
|
|
||
|
December 31,
2019 |
December 31,
2018 |
||||||
|
Compensation expenses
|
$
|
1,805,357
|
|
$
|
1,832,630
|
|
|
|
Deferred rent
|
266,282
|
|
268,319
|
|
|||
|
Goods received not invoiced
|
191,721
|
|
—
|
|
|||
|
Professional fees and royalties
|
213,514
|
|
195,854
|
|
|||
|
Employee expense reimbursements
|
187,516
|
|
133,697
|
|
|||
|
Interest
|
125,743
|
|
—
|
|
|||
|
Other
|
435,298
|
|
469,629
|
|
|||
|
Total
|
$
|
3,225,431
|
|
$
|
2,900,129
|
|
|
|
December 31,
2019 |
December 31,
2018 |
||||||
|
Innovatus LSA
|
$
|
20,473,436
|
|
$
|
—
|
|
|
|
MidCap Financial CSA
|
—
|
|
10,000,000
|
|
|||
|
Revolver
|
1,497,955
|
|
—
|
|
|||
|
Total principal
|
21,971,391
|
|
10,000,000
|
|
|||
|
Less: unamortized debt issuance costs
|
(1,886,446
|
)
|
(970,626
|
)
|
|||
|
Total carrying value of debt
|
$
|
20,084,945
|
|
$
|
9,029,374
|
|
|
|
December 31,
2019 |
|||
|
2020
|
$
|
5,000,000
|
|
|
2021
|
—
|
|
|
|
2022
|
5,435,837
|
|
|
|
2023
|
7,926,346
|
|
|
|
2024
|
2,111,253
|
|
|
|
Total
|
$
|
20,473,436
|
|
|
•
|
the lowest sale price of the Company’s common stock on the purchase date; or
|
|
•
|
the average of the three lowest closing sale prices for the Company’s common stock during the
ten
consecutive trading days ending on the trading day immediately preceding the purchase date.
|
|
Shares of Stock under Warrants
|
Weighted-
Average
Exercise
Price
|
Weighted-
Average
Remaining
Contractual
Term
|
Aggregate
Intrinsic
Value
|
|||||||||
|
Outstanding at January 1, 2019
|
4,062,507
|
|
$
|
6.32
|
|
4.67
|
||||||
|
Granted
|
32,023,503
|
|
$
|
0.57
|
|
4.84
|
||||||
|
Exercised
|
(11,678,458
|
)
|
$
|
0.07
|
|
$
|
10,457,345
|
|
||||
|
Canceled
|
(1,502
|
)
|
$
|
59.90
|
|
|||||||
|
Outstanding at December 31, 2019
|
24,406,050
|
|
$
|
1.76
|
|
4.82
|
$
|
7,932,689
|
|
|||
|
Shares of Stock under Stock Options
|
Weighted-
Average Exercise Price |
Weighted-
Average Remaining Contractual Term |
Aggregate
Intrinsic Value |
||||||||
|
Outstanding at January 1, 2018
|
436,341
|
$
|
5.14
|
|
9.0
|
|
|
||||
|
Granted
|
886,023
|
$
|
7.69
|
|
|
|
|||||
|
Exercised
|
(2,062)
|
$
|
1.70
|
|
$
|
6,046
|
|
||||
|
Canceled
|
(37,455)
|
$
|
5.20
|
|
|
|
|||||
|
Outstanding at December 31, 2018
|
1,282,847
|
$
|
6.90
|
|
9.2
|
|
|
||||
|
Granted
|
716,960
|
$
|
3.78
|
|
|
|
|||||
|
Exercised
|
(50,665)
|
$
|
1.30
|
|
$
|
151,819
|
|
||||
|
Canceled
|
(206,230)
|
$
|
7.20
|
|
|
|
|||||
|
Outstanding at December 31, 2019
|
1,742,912
|
$
|
5.73
|
|
8.2
|
$
|
4,356
|
|
|||
|
Vested and expected to vest at December 31, 2019
|
1,709,588
|
$
|
5.81
|
|
8.2
|
$
|
4,356
|
|
|||
|
Vested and exercisable at December 31, 2019
|
817,026
|
$
|
6.33
|
|
7.4
|
$
|
—
|
|
|||
|
Year Ended
December 31, |
|||||||
|
2019
|
2018
|
||||||
|
Research and development
|
$
|
240,692
|
|
$
|
260,840
|
|
|
|
General and administrative
|
1,105,331
|
|
933,033
|
|
|||
|
Total stock-based compensation expense
|
$
|
1,346,023
|
|
$
|
1,193,873
|
|
|
|
Year Ended December 31,
|
|||
|
2019
|
2018
|
||
|
Risk-free interest rate
|
2.4%
|
2.9%
|
|
|
Expected volatility
|
66.7%
|
62.1%
|
|
|
Expected term (in years)
|
5.1
|
5.2
|
|
|
Expected dividend yield
|
0.0%
|
0.0%
|
|
|
Year Ending December 31,
|
Gross Payments
|
Scheduled Sublease Payments
|
Net Payments
|
||||||||
|
2020
|
$
|
902,412
|
|
$
|
(464,334
|
)
|
$
|
438,078
|
|
||
|
2021
|
611,061
|
|
—
|
|
611,061
|
|
|||||
|
2022
|
638,740
|
|
—
|
|
638,740
|
|
|||||
|
2023
|
666,411
|
|
—
|
|
666,411
|
|
|||||
|
2024
|
696,388
|
|
—
|
|
696,388
|
|
|||||
|
2025 and thereafter
|
728,671
|
|
—
|
|
728,671
|
|
|||||
|
Total minimum lease payments
|
$
|
4,243,683
|
|
$
|
(464,334
|
)
|
$
|
3,779,349
|
|
||
|
December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Deferred tax assets:
|
|||||||
|
Net operating loss carryforwards
|
$
|
41,780,806
|
|
$
|
35,104,728
|
|
|
|
Research and development credits
|
5,007,178
|
|
4,577,582
|
|
|||
|
Other
|
1,406,893
|
|
1,725,577
|
|
|||
|
Total
|
48,194,877
|
|
41,407,887
|
|
|||
|
Less: valuation allowance
|
(48,194,877
|
)
|
(41,407,887
|
)
|
|||
|
Deferred tax assets, net of valuation allowance
|
$
|
—
|
|
$
|
—
|
|
|
|
Years Ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Current:
|
|||||||
|
Foreign
|
$
|
19,492
|
|
$
|
13,955
|
|
|
|
State and local
|
1,556
|
|
1,556
|
|
|||
|
Income tax provision
|
$
|
21,048
|
|
$
|
15,511
|
|
|
|
Years Ended December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Domestic
|
$
|
(29,851,428
|
)
|
$
|
(18,515,638
|
)
|
|
|
Foreign
|
57,406
|
|
34,757
|
|
|||
|
Loss before provision for income taxes
|
$
|
(29,794,022
|
)
|
$
|
(18,480,881
|
)
|
|
|
December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Income taxes at statutory rate
|
$
|
(6,256,953
|
)
|
$
|
(3,880,985
|
)
|
|
|
State income taxes, net of federal benefits
|
(529,351
|
)
|
(476,179
|
)
|
|||
|
Change in valuation allowance
|
6,786,990
|
|
5,617,471
|
|
|||
|
Other permanent differences
|
449,958
|
|
95,852
|
|
|||
|
Research credits
|
(429,596
|
)
|
(502,511
|
)
|
|||
|
Change in fair value of warrants
|
—
|
|
(838,137
|
)
|
|||
|
Income tax expense
|
$
|
21,048
|
|
$
|
15,511
|
|
|
|
December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
Balance at beginning of the year
|
$
|
3,389,136
|
|
$
|
3,018,563
|
|
|
|
Increase related to current year positions
|
319,026
|
|
370,573
|
|
|||
|
Balance at the end of the year
|
$
|
3,708,162
|
|
$
|
3,389,136
|
|
|
|
Three months ended
|
||||||||||||||||
|
March 31,
|
June 30,
|
September 30,
|
December 31,
|
|||||||||||||
|
2019
|
||||||||||||||||
|
Revenue
|
$
|
1,852,746
|
|
$
|
2,174,635
|
|
$
|
3,312,997
|
|
$
|
2,789,130
|
|
||||
|
Cost of Revenue
|
$
|
1,147,042
|
|
$
|
1,555,246
|
|
$
|
2,375,100
|
|
$
|
1,690,759
|
|
||||
|
Operating Expenses
|
$
|
6,890,713
|
|
$
|
7,463,697
|
|
$
|
6,622,993
|
|
$
|
8,258,864
|
|
||||
|
Net Loss
|
$
|
(7,851,743
|
)
|
$
|
(7,664,566
|
)
|
$
|
(6,398,166
|
)
|
$
|
(7,900,595
|
)
|
||||
|
2018
|
||||||||||||||||
|
Revenue
|
$
|
1,768,485
|
|
$
|
3,390,009
|
|
$
|
2,828,704
|
|
$
|
4,013,537
|
|
||||
|
Cost of Revenue
|
$
|
841,449
|
|
$
|
1,813,430
|
|
$
|
3,068,332
|
|
$
|
2,988,115
|
|
||||
|
Operating Expenses
|
$
|
5,262,497
|
|
$
|
5,588,800
|
|
$
|
5,729,212
|
|
$
|
7,123,985
|
|
||||
|
Net Loss
|
$
|
(3,847,362
|
)
|
$
|
(3,311,476
|
)
|
$
|
(4,926,097
|
)
|
$
|
(6,411,457
|
)
|
||||
|
Name
|
Age
|
Position
|
||
|
Executive Officers:
|
||||
|
R. Erik Holmlin, Ph.D.
|
52
|
President, Chief Executive Officer and Director
|
||
|
Warren Robinson
|
50
|
Chief Commercial Officer
|
||
|
Mark Oldakowski
|
46
|
Chief Operating Officer
|
||
|
Non-Employee Directors:
|
||||
|
David L. Barker, Ph.D.
(1)(2)
|
78
|
Chairman, Director
|
||
|
Albert Luderer, Ph.D.
(2)(3)
|
71
|
Director
|
||
|
Christopher Twomey
(2)
|
60
|
Director
|
||
|
Junfeng Wang
(1)(3)
|
45
|
Director
|
||
|
Kristiina Vuori, M.D., Ph.D.
(1)(3)
|
52
|
Director
|
||
|
(1)
|
Member of the compensation committee.
|
|
(2)
|
Member of the audit committee.
|
|
(3)
|
Member of the nominating and corporate governance committee.
|
|
•
|
helping our board of directors oversee our corporate accounting and financial reporting processes;
|
|
•
|
managing the selection, engagement, qualifications, independence and performance of a qualified firm to serve as the independent registered public accounting firm to audit our financial statements;
|
|
•
|
discussing the scope and results of the audit with the independent registered public accounting firm, and reviewing, with management and the independent accountants, our interim and year-end operating results;
|
|
•
|
developing procedures for employees to submit concerns anonymously about questionable accounting or audit matters;
|
|
•
|
reviewing related person transactions;
|
|
•
|
obtaining and reviewing a report by the independent registered public accounting firm at least annually, that describes our internal quality control procedures, any material issues with such procedures, and any steps taken to deal with such issues when required by applicable law; and
|
|
•
|
approving, or, as permitted, pre-approving, audit and permissible non-audit services to be performed by the independent registered public accounting firm.
|
|
•
|
reviewing and approving the compensation of our chief executive officer, other executive officers and senior management;
|
|
•
|
reviewing and recommending to our board of directors the compensation paid to our directors;
|
|
•
|
reviewing and approving the compensation arrangements with our executive officers and other senior management;
|
|
•
|
administering our equity incentive plans and other benefit programs;
|
|
•
|
reviewing, adopting, amending and terminating, incentive compensation and equity plans, severance agreements, profit sharing plans, bonus plans, change-of-control protections and any other compensatory arrangements for our executive officers and other senior management;
|
|
•
|
reviewing, evaluating and recommending to our board of directors succession plans for our executive officers; and
|
|
•
|
reviewing and establishing general policies relating to compensation and benefits of our employees, including our overall compensation philosophy.
|
|
•
|
identifying and evaluating candidates, including the nomination of incumbent directors for reelection and nominees recommended by stockholders, to serve on our board of directors;
|
|
•
|
considering and making recommendations to our board of directors regarding the composition and chairmanship of the committees of our board of directors;
|
|
•
|
instituting plans or programs for the continuing education of our board of directors and orientation of new directors;
|
|
•
|
developing and making recommendations to our board of directors regarding corporate governance guidelines and matters; and
|
|
•
|
overseeing periodic evaluations of the board of directors’ performance, including committees of the board of directors and management.
|
|
•
|
R. Erik Holmlin, Ph.D., our Chief Executive Officer;
|
|
•
|
Mike Ward, our former Chief Financial Officer
(1)
;
|
|
•
|
Warren Robinson, our Chief Commercial Officer; and
|
|
•
|
Mark Oldakowski, our Chief Operating Officer.
|
|
Name and Principal Position
|
Year
|
Salary
($) |
Bonus
($) |
Option
Awards (1) ($) |
Non-Equity
Incentive Plan Compensation (2) ($) |
All Other
Compensation (3) ($) |
Total
($) |
|||||||||||||
|
R. Erik Holmlin, Ph.D.
|
2019
|
403,636
|
|
—
|
|
351,002
|
|
132,392
|
|
11,560
|
|
898,590
|
|
|||||||
|
Chief Executive Officer
|
2018
|
389,986
|
|
—
|
|
1,993,316
|
|
132,595
|
|
11,360
|
|
2,527,257
|
|
|||||||
|
Mike Ward
|
2019
|
289,874
|
|
—
|
|
145,242
|
|
—
|
|
11,560
|
|
446,676
|
|
|||||||
|
Former Chief Financial Officer
|
2018
|
289,061
|
|
—
|
|
463,014
|
|
78,241
|
|
11,360
|
|
841,676
|
|
|||||||
|
Warren Robinson
|
2019
|
305,325
|
|
—
|
|
145,242
|
|
72,667
|
|
11,560
|
|
534,794
|
|
|||||||
|
Chief Commercial Officer
|
2018
|
295,000
|
|
—
|
|
502,253
|
|
88,795
|
|
11,360
|
|
897,408
|
|
|||||||
|
Mark Oldakowski
|
2019
|
305,325
|
|
—
|
|
145,242
|
|
84,727
|
|
11,560
|
|
546,854
|
|
|||||||
|
Chief Operating Officer
|
2018
|
295,000
|
|
—
|
|
258,710
|
|
47,354
|
|
11,360
|
|
612,424
|
|
|||||||
|
(1)
|
In accordance with SEC rules, this column reflects the aggregate grant date fair value of stock options granted to our named executive officers during fiscal years ended December 31, 2018 and December 31, 2019 under our 2018 Plan, as determined in accordance with the provisions of FASB ASC Topic 718. The valuation assumptions used in calculating their fair value of the stock options are included in Note 8 to our financial statements included elsewhere in this Annual Report. These amounts do not reflect the actual economic value that may be realized by the named executive officer upon the vesting of the stock options, the exercise of the stock options, or the sale of the common stock underlying such stock options.
|
|
(2)
|
Amounts reported represent bonuses earned for 2018 and paid in 2019 and earned in 2019 and paid in 2020 at the discretion of our board of directors.
|
|
(3)
|
Amounts for 2018 reflect $11,000 for 401(k) matching contributions and $360 for life insurance premiums and amounts for 2019 reflect $11,200 for 401(k) matching contributions and $360 for life insurance premiums.
|
|
(4)
|
Mr. Ward terminated services with us effective December 6, 2019.
|
|
NAME
|
2019 BASE
SALARY ($) |
||
|
R. Erik Holmlin, Ph.D.
|
403,636
|
|
|
|
Mike Ward
|
308,493
|
|
|
|
Warren Robinson
|
305,325
|
|
|
|
Mark Oldakowski
|
305,325
|
|
|
|
Option Awards
(1)
|
||||||||||||||
|
Name
|
Grant
Date |
Number of
Securities Underlying Unexercised Options Exercisable |
Number of
Securities Underlying Unexercised Options Unexercisable |
Option
Exercise Price Per Share (2) |
Option
Expiration Date |
|||||||||
|
R. Erik Holmlin, Ph.D
(6)
|
3/1/2019
(3)
|
27,187
|
|
117,813
|
|
$
|
4.25
|
|
2/28/2029
|
|||||
|
10/1/2018
(4)
|
138,9559
|
|
117,581
|
|
$
|
7.77
|
|
9/30/2028
|
||||||
|
2/7/2017
(5)
|
96,243
|
|
57,744
|
|
$
|
1.30
|
|
2/6/2027
|
||||||
|
1/29/2015
|
7,284
|
|
—
|
|
$
|
64.22
|
|
1/28/2025
|
||||||
|
6/20/2012
|
1,115
|
|
—
|
|
$
|
68.50
|
|
6/19/2022
|
||||||
|
5/16/2011
|
2,992
|
|
—
|
|
$
|
42.82
|
|
5/15/2021
|
||||||
|
Mike Ward
(6)
|
3/1/2019
|
11,250
|
|
—
|
|
$
|
4.25
|
|
2/28/2029
|
|||||
|
10/01/2018
|
32,227
|
|
—
|
|
$
|
7.77
|
|
9/30/2028
|
||||||
|
2/7/2017
|
21,388
|
|
—
|
|
$
|
1.30
|
|
2/6/2027
|
||||||
|
1/29/2015
|
889
|
|
—
|
|
$
|
64.20
|
|
1/28/2025
|
||||||
|
4/21/2014
|
612
|
|
—
|
|
$
|
81.34
|
|
4/20/2024
|
||||||
|
Warren Robinson
|
3/1/2019
(3)
|
11,250
|
|
48,750
|
|
$
|
4.25
|
|
2/28/2029
|
|||||
|
10/1/2018
(4)
|
35,013
|
|
29,627
|
|
$
|
7.77
|
|
9/30/2028
|
||||||
|
2/7/2017
(5)
|
27,269
|
|
1,817
|
|
$
|
1.30
|
|
2/6/2027
|
||||||
|
11/9/2015
|
1,168
|
|
—
|
|
$
|
64.22
|
|
11/8/2025
|
||||||
|
Mark Oldakowski
|
3/1/2019
(3)
|
11,250
|
|
48,750
|
|
$
|
4.25
|
|
2/28/2029
|
|||||
|
10/1/2018
(4)
|
32,277
|
|
27,318
|
|
$
|
7.77
|
|
9/30/2028
|
||||||
|
2/7/2017
(5)
|
32,081
|
|
2,138
|
|
$
|
1.30
|
|
2/6/2027
|
||||||
|
1/29/2015
|
541
|
|
—
|
|
$
|
64.22
|
|
1/28/2025
|
||||||
|
1/27/2014
|
1,086
|
|
—
|
|
$
|
64.22
|
|
1/26/2024
|
||||||
|
(1)
|
Option awards were granted under the 2006 Plan and 2018 Plan.
|
|
(2)
|
All of the option awards were granted with a per share exercise price equal to the fair market value of one share of our common stock on the date of grant, as determined in good faith by our board of directors.
|
|
(3)
|
Each option award vests as follows: The shares subject to the option vest monthly over 48 months beginning on the one-month anniversary of the vesting commencement date, such that the option shall be fully vested and exercisable on the four-year anniversary of the vesting commencement date.
|
|
(4)
|
Each option award vests as follows: 25% of the shares subject to the option vest on the date of grant and the balance of the shares vest in a series of 36 successive equal monthly installments thereafter, provided in each case that the holder is then providing services to us in accordance with the terms of the 2018 Plan.
|
|
(5)
|
Each option award vests as follows: 25% of the shares subject to the option are fully vested and 6.25% of the shares subject to the option vest at the end of each three month anniversary of the vesting commencement date, subject to single trigger acceleration of vesting in connection with a change of control, provided in each case that the holder is then providing services to us in accordance with the terms of the 2006 Plan.
|
|
(6)
|
All outstanding options under the 2006 option plan, held by Dr. Holmlin and Mr. Ward were amended by our board of directors in August 2018 to suspend the vesting until such time as the price of our common stock is at least $12.00 per share for 90 consecutive trading days, at which point the suspension will automatically and immediately lapse and the awards will vest to the extent they otherwise would have vested pursuant to their terms and notwithstanding the suspension and will continue to vest thereafter under their original vesting schedules. In addition, the suspension will lapse as to the awards held by Dr. Holmlin or Mr. Ward upon Dr. Holmlin's or Mr. Ward`s respective death, disability or upon a change in control of the Company, as such terms are defined in the 2018 Plan.
|
|
•
|
arrange for the assumption, continuation, or substitution of a stock award by a successor corporation;
|
|
•
|
arrange for the assignment of any reacquisition or repurchase rights held by us to a successor corporation;
|
|
•
|
accelerate the vesting, in whole or in part, of the stock award and provide for its termination if not exercised (if applicable) at or before the effective time of the transaction;
|
|
•
|
arrange for the lapse, in whole or in part, of any reacquisition or repurchase rights held by us;
|
|
•
|
cancel or arrange for the cancellation of the stock award, to the extent not vested or not exercised before the effective time of the transaction, in exchange for a cash payment, if any, as determined by the board; or
|
|
•
|
make a payment, in the form determined by our board of directors, equal to the excess, if any, of (A) the value of the property the participant would have received on exercise of the award immediately before the effective time of the transaction, over (B) any exercise price payable by the participant in connection with the exercise.
|
|
•
|
an annual cash retainer of $30,000;
|
|
•
|
an additional annual cash retainer of $20,000 for service as chairman of the board of directors;
|
|
•
|
an additional annual cash retainer of $15,000, $10,000 and $10,000 for service as chair of the audit committee, compensation committee and the nominating and corporate governance committee, respectively;
|
|
•
|
an additional annual cash retainer of $7,500, $5,000 and $5,000 for service as a member of the audit committee, compensation committee and the nominating and corporate governance committee, respectively (not applicable to committee chairs);
|
|
•
|
an initial option grant to purchase common stock with an aggregate Black-Scholes option value of $50,000 on the date of each such non-employee director’s appointment to our board of directors; and
|
|
•
|
an annual option grant to purchase common stock with an aggregate Black-Scholes option value of $35,000 on the date of each of our annual stockholder meetings.
|
|
NAME
|
FEES EARNED OR PAID IN
CASH
|
OPTION AWARDS ($)
(1)
|
TOTAL ($)
|
|||||||||
|
David L. Barker, Ph.D.
|
$
|
67,500
|
|
$
|
35,000
|
|
$
|
102,500
|
|
|||
|
Darren Cai, Ph.D.
(2)
|
$
|
22,458
|
|
$
|
35,000
|
|
$
|
57,458
|
|
|||
|
Albert Luderer, Ph.D.
|
$
|
47,500
|
|
$
|
35,000
|
|
$
|
82,500
|
|
|||
|
Christopher Twomey
|
$
|
45,000
|
|
$
|
35,000
|
|
$
|
80,000
|
|
|||
|
Junfeng Wang
|
$
|
40,000
|
|
$
|
35,000
|
|
$
|
75,000
|
|
|||
|
Kristiina Vuori, M.D., Ph.D.
(3)
|
$
|
16,978
|
|
$
|
50,000
|
|
$
|
66,978
|
|
|||
|
Quan Zhou
(4)
|
$
|
32,569
|
|
$
|
35,000
|
|
$
|
67,569
|
|
|||
|
(1)
|
The amounts reported reflect the aggregate grant date fair value of each equity award granted to our non-employee directors during the fiscal year ended December 31, 2019, as determined in accordance with the provisions of FASB ASC Topic 718. The valuation assumptions used in calculating these amounts are included in Note 8 to our financial statements included elsewhere in this Annual Report. As required by SEC rules, the amounts shown exclude the impact of estimated forfeitures related to service-based vesting conditions. These amounts do not reflect the actual economic value that will be realized by our non-employee directors upon the vesting of the stock options, the exercise of the stock options, or the sale of the common stock underlying such stock options. As of December 31, 2019, the aggregate number of shares outstanding under all options to purchase our common stock held by our non-employee directors were: Dr. Barker, 45,813; Dr. Cai, 6,572; Dr. Luderer 41,608; Mr. Twomey 32,427; Mr. Wang, 32,427; Dr. Vuori 30,274, and Mr. Zhou, 15,044.
|
|
(2)
|
Dr. Cai resigned from our board of directors in August 2019.
|
|
(3)
|
Dr. Vuori was appointed to our board of directors in May 2019.
|
|
(4)
|
Mr. Zhou resigned from our board of directors in December 2019.
|
|
•
|
each person, or group of affiliated persons, known by us to beneficially own more than 5% of our common stock;
|
|
•
|
each of our directors;
|
|
•
|
each of our named executive officers; and
|
|
•
|
all of our current executive officers and directors as a group.
|
|
Name of Beneficial Owner
|
Shares Owned Directly
|
Options Exercisable within 60 Days of 12/31/2019
|
Warrants
|
Number of
Shares Beneficially Owned (1) |
%
(2)
|
||||||||||
|
Greater than 5% Stockholders
|
|||||||||||||||
|
LC Fund VI, L.P. and Affiliates
(3)
|
2,906,915
|
|
|
|
—
|
|
2,906,915
|
|
8.5
|
%
|
|||||
|
Wealth Strategy Holding Limited
(4)
|
975,000
|
|
975,000
|
|
1,950,000
|
|
5.5
|
%
|
|||||||
|
Hudson Bay Capital Management LP
(5)
|
—
|
|
—
|
|
2,020,000
|
|
2,020,000
|
|
5.6
|
%
|
|||||
|
Entities affiliated with Innovatus Life Sciences GP, LP(6)
|
1,354,421
|
|
—
|
|
536,987
|
|
1,891,408
|
|
5.4
|
%
|
|||||
|
Directors and Named Executive Officers
|
**
|
|
**
|
|
**
|
|
**
|
|
|||||||
|
David L. Barker, Ph.D.
|
3,894
|
|
32,666
|
|
3,894
|
|
40,454
|
|
*
|
|
|||||
|
R. Erik Holmlin, Ph.D.
|
3,026
|
|
29,511
|
|
1,630
|
|
34,167
|
|
*
|
|
|||||
|
Albert Luderer, Ph.D
|
—
|
|
28,461
|
|
—
|
|
28,461
|
|
*
|
|
|||||
|
Mark Oldakowski
|
9,736
|
|
78,356
|
|
815
|
|
88,907
|
|
*
|
|
|||||
|
Warren Robinson
|
3,932
|
|
81,710
|
|
—
|
|
85,642
|
|
*
|
|
|||||
|
Christopher Twomey
|
19,500
|
|
19,280
|
|
19,500
|
|
58,280
|
|
*
|
|
|||||
|
Junfeng Wang
(3)
|
2,906,915
|
|
19,280
|
|
—
|
|
2,926,195
|
|
8.5
|
%
|
|||||
|
Mike Ward
|
—
|
|
66,416
|
|
—
|
|
66,416
|
|
*
|
|
|||||
|
Kristiina Vuori, M.D., Ph.D.
|
—
|
|
6,727
|
|
—
|
|
6,727
|
|
*
|
|
|||||
|
All directors and executive officers as a group (9 persons)
|
2,947,003
|
|
362,407
|
|
25,839
|
|
3,335,249
|
|
9.6
|
%
|
|||||
|
Shares outstanding as of 12/31/2019
|
—
|
|
—
|
|
—
|
|
34,274,469
|
|
|||||||
|
*
|
Represents beneficial ownership of less than 1%.
|
|
(1)
|
Beneficial ownership is determined in accordance with SEC rules. In computing the beneficial ownership we have included shares for which the named person has sole or shared power over voting or investment decisions. The number of shares of common stock beneficially owned includes common stock which the named person has the right to acquire, through option exercise or otherwise, within 60 days after December 31, 2019.
|
|
(2)
|
For each named person, the percentage ownership includes common stock that the person has the right to acquire within 60 days after December 31, 2019, as described in Footnote 1. However, such shares are not deemed outstanding with respect to the calculation of ownership percentage for any other person. In some cases, beneficial ownership calculations for five percent or greater stockholders are based solely on publicly-filed Schedules 13D or 13G, which five percent or greater stockholders are required to file with the SEC, and which generally set forth ownership interests as of December 31, 2019 unless otherwise provided.
|
|
(3)
|
Based solely on a Schedule 13G filed with the SEC by the reporting persons on June 7, 2019, the indicated ownership consists of (i) 426,900 shares of common stock held by LC Fund VI, L.P., (ii) 20,656 shares of common stock held by LC Parallel Fund VI, L.P., (iii) 1,325,359 shares of common stock held by LC Healthcare Fund I, L.P., and (iv) 1,134,000 shares of common stock held by Rosy Shine Limited and (v) 19,280 shares of common stock subject to options exercisable as of February 29, 2020 held by Junfeng Wang. Each of LC Fund VI, L.P., LC Parallel Fund VI, L.P., and LC Healthcare Fund I, L.P., collectively referred to as the LC Funds, are ultimately controlled and managed by Legend Capital, a limited liability Chinese company. Legend Capital is ultimately controlled by a management team consisting of three key individuals, Linan Zhu, Hao Chen, and Nengguang Wang. In addition, Junfeng Wang and Quan Zhou are Managing Directors of Legend Capital. Each of these individual managers of Legend
|
|
(4)
|
Based solely on a Schedule 13G filed with the SEC by the reporting person on December 14, 2018, the indicated ownership consists of 975,000 shares of common stock and 975,000 shares of common stock issuable upon the exercise of warrants held by Wealth Strategy Holding Limited. According to the Schedule 13G, Mr. Kung Hung Ka may be deemed to have sole voting and dispositive power with respect to the shares held by Wealth Strategy Holding Limited. The Schedule 13G/A filed by the reporting person provides information as of August 21, 2018 and, consequently, the beneficial ownership of the reporting person may have changed between August 21, 2018 and December 31, 2019. The address for Wealth Strategy Holding Limited listed in the Schedule 13G is Level 12, International Commerce Centre, 1 Austin Road West, Kowloon, Hong Kong.
|
|
(5)
|
Based solely on a Schedule 13G filed with the SEC by the reporting person January 31, 2020, the indicated ownership consists of 2,020,000 shares of common stock issuable upon the exercise of warrants held by Hudson Bay Master Fund Ltd. According to the Schedule 13G, Hudson Bay Capital Management LP acts as investment manager to Hudson Bay Master Fund Ltd., and Mr. Sander Gerber, a United States citizen, serves as managing member of Hudson Bay Capital GP LLC, which is a general partner of Hudson Bay Capital Management LP. Hudson Bay Capital Management LP and Mr. Sander Gerber may be deemed to have shared voting and dispositive power with respect to the shares held by Hudson Bay Master Fund Ltd. The address for Hudson Bay Capital Management LP and Mr. Sander Gerber listed in the Schedule 13G is 777 Third Avenue, 30th Floor, New York, NY 10017.
|
|
(6)
|
The indicated ownership consists of (i) 839,781 shares of common stock and 332,948 shares of common stock issuable upon the exercise of warrants held by Innovatus Life Sciences Lending Fund I, LP, or Innovatus, and (ii) 514,640 shares of common stock and 204,039 shares of common stock issuable upon the exercise of warrants held by Innovatus Life Sciences Offshore Fund I-A, LP., or Innovatus Offshore. Innovatus Life Sciences GP, LP, or Innovatus GP, is the general partner of Innovatus Life Sciences Lending Fund I, LP, or Innovatus. Innovatus Capital Partners LLC, or Innovatus Manager, serves as investment manager to each of Innovatus and Innovatus Life Sciences Offshore Fund I-A, LP, or Innovatus Offshore. Innovatus Life Sciences Offshore GP, LP, or Innovatus Offshore GP, is the general partner of Innovatus Offshore. Each of Innovatus GP, Innovatus, Innovatus Manager, Innovatus Offshore GP and Innovatus Offshore are collectively referred to as the Innovatus Entities. Innovatus Flagship Parent GP, LLC, or Innovatus Holdings, is the parent company of Innovatus GP and Innovatus Offshore Parent GP, LLC, or Innovatus Offshore Holdings. David Schiff, a citizen of the United States, is the principal shareholder of each of Innovatus Holdings and Innovatus Offshore Holdings, collectively referred to as the Parent Entities, and could be deemed to exercise voting and investment power over the Parent Entities and the Innovatus Entities. The address of the principal place of business for each of the Parent Entities and David Schiff is 777 Third Avenue, 25th Floor New York, NY 10017.
|
|
Plan Category
|
Number of securities to be issued upon exercise of outstanding options, warrants, and rights
|
Weighted average exercise price of outstanding options, warrants, and rights
|
Number of securities remaining available for future issuance under equity compensation plans
|
|||||||
|
Equity compensation plans approved by stockholders
|
||||||||||
|
Amended and Restated 2006 Equity Compensation Plan
|
323,735
|
|
$
|
4.70
|
|
—
|
|
|||
|
2018 Equity Incentive Plan
|
1,419,177
|
|
$
|
5.96
|
|
196,731
|
|
|||
|
2018 Employee Stock Purchase Plan
|
165,402
|
|
||||||||
|
Equity compensation plans not approved by stockholders
|
||||||||||
|
None
|
||||||||||
|
Total
|
1,742,912
|
|
$
|
5.73
|
|
362,133
|
|
|||
|
•
|
the amounts involved exceeded or will exceed the lesser of (a) $120,000 or (b) 1% of our total assets at December 31, 2018 or 2019; and
|
|
•
|
any of our directors, executive officers or holders of more than 5% of our capital stock, or any member of the immediate family of, or person sharing the household with, the foregoing persons, had or will have a direct or indirect material interest.
|
|
Name of Participant
|
Shares of Series D-1
Convertible Preferred Stock |
Aggregate
Purchase Price |
||||
|
Full Succeed International Limited
|
10,416,667
|
$
|
5,000,000
|
|
||
|
LC Healthcare Fund I, L.P.
|
6,472,371
|
$
|
3,106,738
|
|
||
|
Praise Alliance International Limited
|
6,250,000
|
$
|
3,000,000
|
|
||
|
Entities affiliated with Domain Partners VIII, L.P.
(1)
|
3,710,247
|
$
|
1,780,919
|
|
||
|
Han Cao, Ph.D.
|
104,167
|
$
|
50,000
|
|
||
|
(1)
|
Includes (i) $1,767,801 in cash from Domain Partners VIII, L.P., and (ii) $13,117 in cash from DP VIII Associates, L.P.
|
|
Name of Participant
|
Total Principal
Amount |
|||
|
Entities affiliated with LC Fund VI, L.P.
(1)
|
$
|
8,460,000
|
|
|
|
Entities affiliated with Domain Partners VIII, L.P.
(2)
|
$
|
1,500,000
|
|
|
|
(1)
|
Includes (i) $3,460,000 in cash from LC Healthcare Fund I, L.P.; and (ii) $5,000,000 cash from Rosy Shine Limited.
|
|
(2)
|
Includes (i) $1,488,952 in cash from Domain Partners VIII, L.P., and (ii) $11,048 in cash from DP VIII Associates, L.P.
|
|
2019
|
2018
|
||||||
|
Audit fees
(1)
|
$
|
615,000
|
|
$
|
1,020,000
|
|
|
|
Audit-related fees
(2)
|
46,000
|
|
49,000
|
|
|||
|
Tax fees
(3)
|
19,700
|
|
20,000
|
|
|||
|
All other fees
(4)
|
—
|
|
2,000
|
|
|||
|
Total
|
$
|
680,700
|
|
$
|
1,091,000
|
|
|
|
(1)
|
Audit fees consist of fees billed for professional services rendered for the audit of the consolidated annual financial statements of the Company, review of the interim condensed consolidated financial statements included in quarterly reports, review of the SEC-filings associated with the Company's IPO in 2018, and services that are normally provided by Deloitte & Touche, LLP in connection with statutory and regulatory filings or engagements.
|
|
(2)
|
Audit-related fees consist of fees billed for assurance and related services that are reasonably related to the performance of the audit or review of the consolidated financial statements of the Company and are not reported under "Audit fees." For the fiscal years ended December 31, 2019 and 2018, these fees primarily related to miscellaneous professional services.
|
|
(3)
|
Tax fees consist of fees billed for professional services rendered for tax compliance, advice and planning. For the fiscal years ended December 31, 2019 and 2018, these services included assistance regarding federal and state tax compliance and consultations regarding various income tax issues.
|
|
(4)
|
All other fees for the fiscal year ended December 31, 2018 related to software subscription services.
|
|
(a)
|
List the following documents filed as a part of the report:
|
|
(1)
|
Financial statements
|
|
(2)
|
Financial statement schedule.
|
|
(3)
|
Exhibits
|
|
Exhibit
Number
|
Description
|
|
|
3.1
(1)
|
||
|
3.2
(1)
|
||
|
4.1
(2)
|
||
|
4.2
(2)
|
||
|
4.3
(2)
|
||
|
4.4
(2)
|
||
|
4.5
(2)
|
||
|
4.6
(2)
|
||
|
4.7
(2)
|
||
|
4.8
(2)
|
||
|
4.9
(3)
|
||
|
4.10
(4)
|
||
|
4.11
(4)
|
||
|
4.12
(4)
|
||
|
4.13
(5)
|
||
|
4.14
(5)
|
||
|
4.15
|
Warrant Amendment Agreement, dated October 30, 2019, by and between the Company and Innovatus.
|
|
|
4.16
|
||
|
10.1
(2)
|
||
|
10.2+
(2)
|
||
|
10.3+
(2)
|
||
|
10.4+
(6)
|
||
|
10.5+
(2)
|
||
|
10.6+
(6)
|
||
|
10.7+
(2)
|
||
|
10.8+
(2)
|
||
|
10.9+
(2)
|
||
|
10.10+
(2)
|
||
|
Exhibit
Number
|
Description
|
|
|
10.11+
(2)
|
||
|
10.12+
(2)
|
||
|
10.13+
(2)
|
||
|
10.14+
(2)
|
||
|
10.15
(2)
|
||
|
10.16
(2)
|
||
|
10.17
(2)
|
||
|
10.18
(2)
|
||
|
10.19
(2)
|
||
|
10.20
(2)
|
||
|
10.21
(2)
|
||
|
10.22
(2)
|
||
|
10.23#
(2)
|
||
|
10.24#
(2)
|
||
|
10.25#
(2)
|
||
|
10.26#
(2)
|
||
|
10.27#
(2)
|
||
|
10.28#
(2)
|
||
|
10.29#
(2)
|
||
|
10.30#
(2)
|
||
|
10.31#
(2)
|
||
|
10.32#
(2)
|
||
|
10.33#
(2)
|
||
|
10.34#
(2)
|
||
|
10.35#
(2)
|
||
|
10.36
(2)
|
||
|
10.37
(2)
|
||
|
10.38
(2)
|
||
|
10.39#
(2)
|
||
|
10.40
(2)
|
||
|
10.41
(2)
|
||
|
10.42
(2)
|
||
|
10.43
(2)
|
||
|
10.44
(2)
|
||
|
Exhibit
Number
|
Description
|
|
|
10.45
(3)
|
||
|
10.46
(4)
|
||
|
10.47
(4)
|
||
|
10.48
(7)
|
||
|
10.49
|
||
|
10.50
(4)
|
||
|
22.1
(2)
|
||
|
23.1
|
||
|
24.1
|
Power of Attorney (included on signature page)
|
|
|
31.1*
|
||
|
32.1*
|
||
|
101.INS
|
XBRL Instance Document.
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document.
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document.
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document.
|
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document.
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document.
|
|
|
(1)
|
Incorporated by reference to the Company’s Current Report on Form 8-K, filed with the SEC on August 24, 2018.
|
|
|
(2)
|
Incorporated by reference to the Company’s Registration Statement on Form S-1 (File No. 333-225970), as amended.
|
|
|
(3)
|
Incorporated by reference to the Company’s Current Report on Form 8-K, filed with the SEC on November 21, 2018.
|
|
|
(4)
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, filed with the SEC on March 14, 2019.
|
|
|
(5)
|
Incorporated by reference to the Registrant’s Registration Statement on Form S-1 (File No. 333-233828), as amended.
|
|
|
(6)
|
Incorporated by reference to the Registrant’s Registration Statement on Form S-8 (File No. 333-227073).
|
|
|
(7)
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q, filed with the SEC on August 8, 2019.
|
|
|
+
|
Indicates management contract or compensatory plan.
|
|
|
#
|
Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the SEC.
|
|
|
*
|
This certification is deemed not filed for purpose of section 18 of the Exchange Act or otherwise subject to the liability of that section, nor shall it be deemed incorporated by reference into any filing under the Securities Act or the Exchange Act.
|
|
|
Company Name
|
||
|
Date: March 10, 2020
|
By:
|
/s/ R. Erik Holmlin, Ph.D.
|
|
R. Erik Holmlin, Ph.D.
|
||
|
President and Chief Executive Officer
|
||
|
Signature
|
Title
|
Date
|
||
|
/s/ R. Erik Holmlin, Ph.D.
|
Chief Executive Officer and Director
(Principal Executive and Financial Officer) |
March 10, 2020
|
||
|
R. Erik Holmlin, Ph.D.
|
||||
|
/s/ Mark Adamchak
|
Controller
(Principal Accounting Officer) |
March 10, 2020
|
||
|
Mark Adamchak
|
||||
|
/s/ David L. Barker, Ph.D.
|
Director
|
March 10, 2020
|
||
|
David L. Barker, Ph.D.
|
||||
|
/s/ Albert A. Luderer, Ph.D.
|
Director
|
March 10, 2020
|
||
|
Albert A. Luderer, Ph.D.
|
||||
|
/s/ Junfeng Wang
|
Director
|
March 10, 2020
|
||
|
Junfeng Wang
|
||||
|
/s/ Christopher Twomey
|
Director
|
March 10, 2020
|
||
|
Christopher Twomey
|
||||
|
/s/ Kristiina Vuori, M.D., Ph.D.
|
Director
|
March 10, 2020
|
||
|
Kristiina Vuori, M.D., Ph.D.
|
||||