|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
DELAWARE
|
26-2025616
|
|
(State or other jurisdiction of
incorporation or organization)
|
(I.R.S. Employer
Identification No.)
|
|
245 First Street, Suite 1800
Cambridge, MA
|
02142
|
|
(Address of principal executive offices)
|
(Zip code)
|
|
Title of each class
|
Name of each exchange on which registered
|
|
Common Stock, par value $0.001 per share
|
Nasdaq Global Market
|
|
Large Accelerated filer
|
o
|
Accelerated filer
|
o
|
|
Non-accelerated filer
|
o
(Do not check if a smaller reporting company)
|
Smaller reporting company
|
x
|
|
Emerging growth company
|
x
|
||
|
|
|
Page
|
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
Item 15.
|
||
|
Item 16.
|
||
|
•
|
our expected future loss and accumulated deficit levels;
|
|
•
|
our projected financial position and estimated cash burn rate;
|
|
•
|
our estimates regarding expenses, future revenues, capital requirements and needs for, and ability to obtain, additional financing;
|
|
•
|
our ability to continue as a going concern;
|
|
•
|
our need to raise substantial additional capital to fund our operations;
|
|
•
|
the potential impairment of our goodwill and indefinite lived intangible assets;
|
|
•
|
the effect of recent changes in our senior management team on our business;
|
|
•
|
the success, cost and timing of our pre-clinical studies and clinical trials in the United States, Canada and in other foreign jurisdictions;
|
|
•
|
the potential that results of pre-clinical studies and clinical trials indicate our product candidates are unsafe or ineffective;
|
|
•
|
our dependence on third parties, including contract research organizations, or CROs, in the conduct of our pre-clinical studies and clinical trials;
|
|
•
|
the difficulties and expenses associated with obtaining and maintaining regulatory approval of our product candidates and companion diagnostics, if any, in the United States, Canada and in other foreign jurisdictions, and the labeling under any approval we may obtain;
|
|
•
|
our plans and ability to develop and commercialize our product candidates;
|
|
•
|
our ability to achieve certain future regulatory, development and commercialization milestones under our license agreement, which we refer to as the License Agreement, with F. Hoffmann-La Roche Ltd and Hoffmann La-Roche Inc., or collectively, Roche;
|
|
•
|
market acceptance of our product candidates, the size and growth of the potential markets for our product candidates, and our ability to serve those markets;
|
|
•
|
obtaining and maintaining intellectual property protection for our product candidates and our proprietary technology;
|
|
•
|
the successful development of our commercialization capabilities, including sales and marketing capabilities; and
|
|
•
|
the success of competing therapies and products that are or become available.
|
|
Item 1.
|
Business.
|
|
•
|
Deliver insufficient drug to tumors.
Existing ADCs utilize full-length antibodies, which, due to their large size, have a reduced ability to penetrate tumors, thereby reducing their efficacy.
|
|
•
|
Inability to kill a broad array of cancer cells within a tumor.
Subsets of cancer cells within tumors may have mechanisms to resist and not be responsive to the cytotoxic payloads, or small molecule chemotherapies, used in existing ADCs.
|
|
•
|
Off-target toxicities due to unstable chemical linkage between targeting antibody and cytotoxic payload.
Existing ADCs utilize chemical linkage strategies to join antibodies to small molecule cytotoxic payloads. While in the circulatory system, these chemical linkages can break and release free cytotoxic payloads in the circulation. These free small molecule cytotoxic payloads are not targeted and cannot discriminate between dividing cancer cells and non-cancerous cells, thus resulting in increased off-target toxicities.
|
|
•
|
Limited combination therapy potential.
The release of free cytotoxic payloads in the tumor region can result in toxicity to immune cells that attack tumors. This effect on anti-tumor immune cells may limit the potential utility of existing ADCs in combination therapies, including those employing immune checkpoint inhibitors.
|
|
•
|
Complex and challenging manufacturing process.
The multi-step manufacturing process of existing ADCs creates a non-homogeneous product that limits efficacy and drives greater costs than our manufacturing process.
|
|
•
|
Deliver a greater amount of drug to tumors.
Our TPTs are designed using smaller targeting proteins that have an increased ability to exit the circulatory system and have binding properties designed to enable deeper penetration into targeted tumors, and we believe this will increase efficacy.
|
|
•
|
Ability to kill a broader array of cancer cells within a tumor.
Our novel cytotoxic payloads consist of proteins rather than small molecule cytotoxic payloads. We believe the larger size of our cytotoxic protein payloads helps circumvent multi-drug resistance mechanisms that can make certain cancer cells resistant to small molecule cytotoxic payloads. By contrast to existing ADCs, which employ cytotoxic payloads that inhibit cellular replication and are effective at killing rapidly proliferating cancer cells, our cytotoxic protein payloads inhibit protein synthesis and are designed to kill not only rapidly proliferating, but also slowly growing cancer cells including tumor progenitor cells/cancer stem-like cells.
|
|
•
|
Increase safety due to a more stable linkage between targeting protein and cytotoxic payload.
Our single protein molecules are designed to remain intact until they reach the inside of the cancer cell and to not release free cytotoxins into the circulatory system, thereby minimizing off-target toxicity.
|
|
•
|
Promote a therapeutic immune response.
We believe that the potent TPT toxin-mediated killing of cancer cells in this immunologically active setting leads to the efficient presentation of cancer antigens to the immune system, thereby promoting an anti-tumor cellular immune response. Our locally-administered TPTs utilize an immunogenic cytotoxic payload that we believe promotes a heightened immune response in the local tumor environment.
|
|
•
|
Potential combination with checkpoint inhibitors.
We believe that the potential effect of checkpoint inhibitors, which are antibodies that promote the action of anti-tumor T-cells by blocking inhibitory ligand/receptor interactions that include PD-1 and PD-L1, may be enhanced when used in combination with other agents. We believe that, by mediating specific killing of tumor cells and promoting anti-tumor immune responses, our TPTs, while potentially effective on their own, may complement checkpoint inhibitors. In particular, we believe that our use of our cytotoxin payload ETA, which promotes an immune response in the local tumor environment, may facilitate the presentation of tumor cell surface antigens following the death of cancer cells, thereby providing a tumor immune response to enhance the action of checkpoint inhibitor therapies.
|
|
•
|
Utilize a simpler and more efficient manufacturing process.
Our proprietary recombinant one-step manufacturing process creates a homogeneous product that we believe will improve efficacy and result in lower manufacturing costs.
|
|
•
|
Rapidly advance Vicinium through clinical development and obtain regulatory approval.
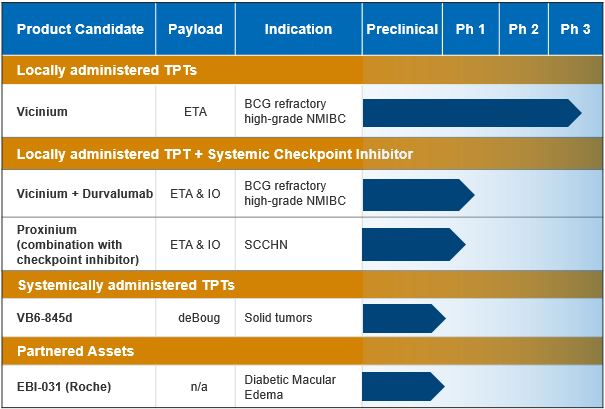
Based upon our September 2014 end of Phase 2 meeting with the FDA, in the third quarter of 2015, we, through our subsidiary Viventia, commenced an open-label, non-randomized Phase 3 clinical trial of Vicinium in subjects with high-grade NMIBC in the United States and Canada. In November 2016, the FDA issued draft guidance regarding appropriate clinical trial design for new drugs and biologics for BCG-unresponsive NMIBC, including the use of single-arm studies. The FDA finalized this guidance in February 2018 and retained many of the recommendations from the 2016 draft guidance regarding clinical trial design, including the use of single-arm studies. We believe that our Phase 3 clinical trial design is consistent with these aspects of the FDA’s guidance. We completed enrollment of this Phase 3 clinical trial in March 2018 and anticipate reporting topline three-month data in mid-2018 and topline twelve-month data in the second quarter of 2019. If this Phase 3 clinical trial is successful, we intend to pursue regulatory approval initially in the United States and Canada. Assuming that we receive positive data in our Phase 3 clinical trial, we intend to initiate discussions with the European Medicines Agency, or EMA, regarding a regulatory pathway for European Union, or E.U., approval.
|
|
•
|
Explore opportunities in combination therapies.
We plan to continue discussions with potential partners that utilize technologies whose mechanism of action could be complementary to our TPT platform. These technologies include, but are not limited to, checkpoint inhibitors, immune modulators and other immuno-oncology agents. In June 2017, we entered into a CRADA with the NCI for the development of Vicinium in combination with AstraZeneca’s immune checkpoint inhibitor, durvalumab, for the treatment of NMIBC. Under the terms of the CRADA, the NCI will conduct a Phase 1 clinical trial in subjects with high-grade NMIBC to evaluate the safety, efficacy and biological correlates of Vicinium in combination with durvalumab. This Phase 1 trial is open and actively recruiting subjects.
|
|
•
|
Advance Proxinium through clinical development and obtain regulatory approval.
We intend to initiate a Phase 1/2a clinical trial that will explore the potential of Proxinium in combination with a checkpoint inhibitor. We anticipate that the Phase 1/2a clinical trial will explore the potential for Proxinium, due to its potential immunogenic effect, to enhance checkpoint inhibitors in combination therapy for the treatment of EpCAM-expressing SCCHN.
|
|
•
|
Advance our systemically-administered product candidate, VB6-845d.
In April 2016, we submitted an IND to the FDA in preparation of initiating a Phase 1/2 clinical trial of VB6-845d in subjects with EpCAM-positive cancers in the United States. The IND was withdrawn in July 2016 after we received initial feedback from the FDA indicating that they had identified hold and non-hold deficiencies that needed to be addressed. In December 2016, we submitted a request for a pre-IND meeting to seek input on the manufacturing, nonclinical and clinical plans for VB6-845d prior to resubmitting an IND. In February 2017, the FDA provided guidance on our manufacturing and nonclinical plans for VB6-845d. Based on this guidance, we are performing additional studies to support an updated IND submission. We believe that the deBouganin payload in VB6-845d may enhance the action of checkpoint inhibitors as a result of the promotion of a local tumor immune response following the death of cancer cells.
|
|
•
|
Expand on the value of selected product candidates through strategic partnerships.
We may decide to selectively partner with pharmaceutical and biopharmaceutical companies when we believe that a partner could bring additional resources and expertise to maximize the value of one or more of our product candidates.
|
|
•
|
Leverage our TPT platform to develop additional product candidates.
We intend to develop additional product candidates based on our TPT platform. Depending on the strategic and financial merits, we may enter into partnerships and collaborations to support these development efforts.
|
|
•
|
Maximize the commercial value of our product candidates.
We maintain global development, marketing and commercialization rights for all of our TPT-based product candidates. If we obtain regulatory approval for Vicinium in high-grade NMIBC, we may build a North American specialty urology sales force to market the product in the United States and Canada or seek commercialization partners. Outside the United States and Canada, we will seek commercialization partners with urology expertise. If we obtain regulatory approval for
|


|
Dose
|
30 mg of Vicinium (in 50 mL of saline)
|
||
|
Estimated total enrollment
|
Approximately 134 subjects, including 77 CIS subjects whose disease is refractory to or relapsed within 6 months of the last dose of adequate BCG treatment
|
||
|
Primary endpoint
|
•
|
Complete response rate in subjects with CIS (with or without papillary disease) whose disease is refractory or relapsed in six months or less following adequate BCG treatment, which is defined as at least two courses of full dose BCG; and
|
|
|
•
|
DoR will be estimated (Kaplan-Meier Estimate) for those subjects with CIS whose disease is refractory to or relapsed within 6 months of the last dose of adequate BCG treatment (with or without papillary disease) who experience a complete response.
|
||
|
Secondary endpoints
|
•
|
Complete response rate and DoR in subjects with CIS whose disease is refractory to or relapsed within 6 months of the last dose of adequate BCG treatment (with or without papillary disease) whose disease is refractory or relapsed from six months to 11 months following adequate BCG treatment;
|
|
|
•
|
Complete response rate and DoR in all subjects with CIS (with or without papillary disease) following adequate BCG treatment;
|
||
|
•
|
Event-free survival, or EFS, in all subjects;
|
||
|
•
|
Complete response rate in subjects at three, six, nine, 12, 15, 18, 21, and 24 months in subjects with CIS whose disease is refractory to or relapsed within 6 months of the last dose of adequate BCG treatment;
|
||
|
•
|
Time to cystectomy in all subjects;
|
||
|
•
|
Time to disease recurrence in all subjects;
|
||
|
•
|
Progression-free survival, or PFS, in all subjects;
|
||
|
•
|
Overall survival, or OS, in all subjects; and
|
||
|
•
|
Safety and tolerability of Vicinium therapy in all subjects.
|
||
|
Exploratory endpoint
|
To evaluate biomarkers that may be associated with response or disease progression or treatment failure, which may include, for example, EpCAM status, tumor subtype morphology, furin levels in tumor cell endosomes, presence of a glycosaminoglycan coat, and presence of receptors that could impede a host anti-tumor immune response such as PD-L1.
|
||


|
Number of
|
Complete
|
Stable
|
Response of
|
|
|
evaluable subjects
|
response
|
Response
|
response
|
progression
|
|
16.................................
|
4 of the 16
|
6 of the 16
|
4 of the 16
|
2 of the 16
|
|
(25.0%)
|
(37.5%)
|
(25.0%)
|
(12.5%)
|
|


|
•
|
a United States, a New Zealand, Japan, Taiwan, China and a South Africa composition of matter patent covering isunakinra which expires in 2031;
|
|
•
|
composition-of-matter patent applications covering isunakinra in Australia, Brazil, Canada, China, Europe, Hong Kong, India, Israel, Korea, Mexico and Russia, which, if granted, are expected to expire in 2031;
|
|
•
|
patent applications covering the formulation of isunakinra filed in the United States, Australia, Canada, China, Europe, Hong Kong, Japan, New Zealand, Russia, and Singapore, which, if granted, are expected to expire in 2034;
|
|
•
|
a patent application covering the formulation of isunakinra in a blow fill seal container filed in Taiwan, which if granted, is expected to expire in 2035;
|
|
•
|
a provisional application directed to compositions and methods for increasing the retention of therapeutic agents in the eye which, if converted and granted, is expected to expire in 2038.
|
|
•
|
a provisional application directed to compositions and methods for increasing the retention of anti-VEGF therapeutic agents in the eye which, if converted and granted, is expected to expire in 2038; and
|
|
•
|
a provisional application directed to compositions and methods for increasing the retention of RGD therapeutic agents in the eye which, if converted and granted, is expected to expire in 2038
|
|
•
|
patent applications covering the IL-6 antagonistic anti-IL6 monoclonal antibodies and active fragments thereof, including IL-6 antibody EBI-029, filed in the United States, Australia, Brazil, Canada, China, Europe, Hong Kong, India, Israel, Japan, Korea, Mexico, New Zealand, Russia, Singapore, and South Africa, and, if granted, are expected to expire in 2033;
|
|
•
|
patent applications covering IL-6 antagonistic anti-IL6 monoclonal antibodies and active fragments thereof, including the IL-6 antibody EBI-031, having a pending PCT application and applications pending or to be filed in Algeria, Australia, Bahrain, Brazil, Canada, Chile, Colombia, Costa Rica, Egypt, Europe (to be filed), India, Israel, Korea, Malaysia, Mexico, Morocco, New Zealand, Oman, Philippines, Qatar, Russian Federation, Saudi Arabia, Singapore, South Africa, Thailand, Ukraine, United Arab Emirates, and Vietnam, and, if granted are expected to expire in 2035; and
|
|
•
|
a PCT Application and an Argentine application each corresponding to a United States provisional application covering the IL-6 antibody EBI-031 formulation, which if granted, are expected to expire in 2036.
|
|
•
|
NMIBC: Aadi, LLC (ABI-009), Altor Bioscience Corporation (ALT-801), Cold Genesys, Inc. (CG0070), Endo Pharmaceuticals Inc. (Valstar) (approved drug), FKD Therapies Oy (Instilidrin), Merck and other pharmaceutical companies (BCG) (approved drug), Eli Lilly and Company (Gemcitabine) and Telormedix SA (Vesimune);
|
|
•
|
SCCHN: Bristol-Myers Squibb Company (nivolumab)(approved drug), Eli Lilly and Company, and Merck (Erbitux, pembrolizumab) (approved drugs);
|
|
•
|
Multiple types of solid tumors: Amgen Inc. (Panitumumab) (approved drug), Bayer AG and Onyx Pharmaceuticals (Sorafenib) (approved drug), Bristol-Myers Squibb Company, Eli Lilly and Company, and Merck (Erbitux) (approved drug), F. Hoffmann-La Roche AG (Bevacizumab) (approved drug), Genentech, Inc. (Bevacizumab, Erlotinib and Trastuzumab) (approved drugs), Pfizer, Inc. (Sunitinib) and Trion Research GmbH (Removab); and
|
|
•
|
In addition to competition from alternative treatments, we may also face competition from products that are biosimilar to, and possibly interchangeable with, our product candidates. Biosimilar products are expected to become available over the coming years. Even if our product candidates achieve marketing approval, they may be priced at a significant premium over competitive biosimilar products if any have been approved by then and insurers or other third party payors may encourage or even require the use of lower priced biosimilar products. Even if our treatments receive market authorization, they may not be listed on the formularies of payors (public or private insurers) or reimbursed. This may impact the uptake of the drug as a treatment option for patients and/or the price at which the drug can be sold at. Further, if the drug is reimbursed it may be at a narrower indication than the full scope of market authorization.
|
|
•
|
completion of pre-clinical studies, animal studies and formulation studies, some in compliance with the FDA’s Good Laboratory Practices, or GLP, regulations, and the Animal Welfare Act administered and enforced by the U.S. Department of Agriculture;
|
|
•
|
submission to the FDA of an IND to support human clinical testing, which must become effective before human clinical trials may commence;
|
|
•
|
approval by an IRB before each trial may be initiated at each clinical site;
|
|
•
|
performance of adequate and well-controlled clinical trials under protocols submitted to FDA for review and approval by each IRB, conducted in accordance with federal regulations and with current Good Clinical Practices, or GCPs, to establish the safety, purity and potency of the biologic for each targeted indication;
|
|
•
|
submission of a BLA to the FDA;
|
|
•
|
satisfactory completion of an FDA Advisory Committee review, if applicable;
|
|
•
|
satisfactory completion of an FDA inspection of the manufacturing facilities at which the biologic is produced to assess compliance with current Good Manufacturing Practices, or cGMP, and to assure that the facilities, methods and controls are adequate; and
|
|
•
|
FDA review and approval of the BLA.
|
|
•
|
Phase 1.
Phase 1 involves the initial introduction of a product candidate into humans. Phase 1 clinical trials are typically conducted in healthy human subjects, but in some situations are conducted in subjects with the target disease or condition. These clinical trials are generally designed to evaluate the safety, metabolism, PK properties and pharmacologic actions of the product candidate in humans, the side effects associated with increasing doses and, if possible, to gain early evidence on effectiveness. During Phase 1 clinical trials, sufficient information about the product candidate’s PK properties and pharmacological effects may be obtained to inform and support the design of Phase 2 clinical trials. The total number of participants included in Phase 1 clinical trials varies, but is generally in the range of 20 to 80;
|
|
•
|
Phase 2.
Phase 2 includes the controlled clinical trials conducted to obtain initial evidence of effectiveness of the product candidate for a particular indication(s) in subjects with the target disease or condition, to determine dosage tolerance and optimal dosage, gather additional information on possible adverse side effects and safety risks associated with the product candidate. Phase 2 clinical trials are typically well-controlled, closely monitored, and conducted in a limited subject population, usually involving no more than several hundred participants; and
|
|
•
|
Phase 3.
Phase 3 clinical trials are controlled clinical trials conducted in an expanded subject population at geographically dispersed clinical trial sites. They are performed after preliminary evidence suggesting effectiveness of the product candidate has been obtained and are intended to further evaluate dosage, clinical effectiveness and safety, to establish the overall benefit-risk relationship of the product candidate and to provide an adequate basis for regulatory approval. Phase 3 clinical trials usually involve several hundred to several thousand participants. In most cases, the FDA requires two adequate and well controlled Phase 3 clinical trials to demonstrate the efficacy of the product candidate, although a single Phase 3 clinical trial with other confirmatory evidence may be sufficient in certain instances.
|
|
•
|
General IVDs;
|
|
•
|
IVDs for self-testing;
|
|
•
|
IVDs falling within the scope of Annex II, List A:
|
|
•
|
reagents and reagent products, including related calibrators and control materials, for determining the following blood groups: ABO system, rhesus (C, c, D, E, e), or anti-Kell; and
|
|
•
|
reagents and reagent products, including related calibrators and control materials, for the detection, confirmation and quantification in human specimens of markers of human immunodeficiency virus, or HIV, infection (HIV 1 and 2), human T-lymphotropic virus I and II, and hepatitis B, C and D.
|
|
•
|
IVDs falling within the scope of Annex II, List B:
|
|
•
|
reagents and reagent products, including related calibrators and control materials, for determining the following blood groups: anti-Duffy and anti-Kidd;
|
|
•
|
reagents and reagent products, including related calibrators and control materials, for determining irregular anti-erythrocyte antibodies;
|
|
•
|
reagents and reagent products, including related calibrators and control materials, for the detection and quantification in human samples of the following congenital infections: rubella, toxoplasmosis;
|
|
•
|
reagents and reagent products, including related calibrators and control materials, for diagnosing the following hereditary disease: phenylketonuria;
|
|
•
|
reagents and reagent products, including related calibrators and control materials, for determining the following human infections: cytomegalovirus, chlamydia;
|
|
•
|
reagents and reagent products, including related calibrators and control materials, for determining the following human leukocyte antigen tissue groups: DR, A, B;
|
|
•
|
reagents and reagent products, including related calibrators and control materials, for determining the following tumoral marker: prostate-specific antigen;
|
|
•
|
reagents and reagent products, including related calibrators, control materials and software, designed specifically for evaluating the risk of trisomy 21; and
|
|
•
|
the following device for self-diagnosis, including its related calibrators and control materials: device for the measurement of blood sugar.
|
|
•
|
The second applicant can establish in its application that its product, although similar to the orphan medicinal product already authorized, is safer, more effective or otherwise clinically superior;
|
|
•
|
The holder of the marketing authorization for the original orphan medicinal product consents to a second orphan medicinal product application; or
|
|
•
|
The holder of the marketing authorization for the original orphan medicinal product cannot supply enough orphan medicinal product.
|
|
•
|
an annual, non-deductible fee on any entity that manufactures or imports specified branded prescription products and biological products;
|
|
•
|
an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program to 23.1% for innovator drugs and 13% for non-innovator drugs of the average manufacturer price;
|
|
•
|
a new methodology by which average manufacturer price is calculated and reported by manufacturers for products that are inhaled, infused, instilled, implanted or injected and not generally dispensed through retail community pharmacies;
|
|
•
|
expansion of healthcare fraud and abuse laws, including the civil False Claims Act and the federal Anti-Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance;
|
|
•
|
a new partial prescription drug benefit for Medicare recipients, or Medicare Part D, coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand products to eligible beneficiaries during their coverage gap period, as a condition for the manufacturers’ outpatient products to be covered under Medicare Part D;
|
|
•
|
extension of manufacturers’ Medicaid rebate liability from fee-for-service Medicaid utilization to include the utilization of Medicaid managed care organizations as well;
|
|
•
|
expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals, thereby potentially increasing manufacturers’ Medicaid rebate liability;
|
|
•
|
expansion of the entities eligible for discounts under the Public Health Service 340B pharmaceutical pricing program;
|
|
•
|
new requirements to report to CMS annually specifying financial arrangements with physicians and teaching hospitals, as defined in the Affordable Care Act and its implementing regulations, including reporting any ‘‘payments or other transfers of value’’ made or distributed to prescribers, teaching hospitals, and other healthcare providers and reporting any ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations during the preceding calendar year;
|
|
•
|
a new requirement to annually report product samples that manufacturers and distributors provide to physicians;
|
|
•
|
a mandatory non-deductible payment for employers with 50 or more full-time employees (or equivalents) who fail to provide certain minimum health insurance coverage for such employees and their dependents;
|
|
•
|
establishment of the Center for Medicare and Medicaid Innovation within CMS to test innovative payments and service delivery models;
|
|
•
|
a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; and
|
|
•
|
a mandatory nondeductible payment for employers with 50 or more full-time employees (or equivalents) who fail to provide certain minimum health insurance coverage for such employees and their dependents.
|
|
Item 1A.
|
Risk Factors
|
|
•
|
continue our Phase 3 clinical trial for Vicinium;
|
|
•
|
continue the research and pre-clinical and clinical development of our other product candidates;
|
|
•
|
seek and conduct combination trials of one or more of our product candidates;
|
|
•
|
seek to discover and develop additional product candidates;
|
|
•
|
in-license or acquire the rights to other products, product candidates or technologies;
|
|
•
|
seek marketing approvals for any product candidates that successfully complete clinical trials;
|
|
•
|
establish sales, marketing and distribution capabilities and scale up and validate external manufacturing capabilities to commercialize any product candidates for which we may obtain marketing approval;
|
|
•
|
maintain, expand and protect our intellectual property portfolio;
|
|
•
|
add equipment and physical infrastructure to support our research and development;
|
|
•
|
hire additional clinical, quality control, scientific and management personnel; and
|
|
•
|
expand our operational, financial and management systems and personnel.
|
|
•
|
we are required by the FDA, the EMA or Health Canada to perform studies or clinical trials in addition to those currently expected; or
|
|
•
|
if there are any delays in enrollment of subjects in, or completing our clinical trials or the development of any product candidates that we may develop.
|
|
•
|
successfully completing development activities, including clinical trial design and enrollment of a sufficient number of subjects in our clinical trials and completion of the necessary clinical trials;
|
|
•
|
completing and submitting BLAs to the FDA and obtaining regulatory approval for indications for which there is a commercial market;
|
|
•
|
completing and submitting applications to, and obtaining regulatory approval from, foreign regulatory authorities, including Health Canada and the European Commission;
|
|
•
|
establishing sales, marketing and distribution capabilities, either ourselves or through collaborations or other arrangements with third parties, to effectively market and sell our product candidates;
|
|
•
|
achieving an adequate level of market acceptance of our product candidates;
|
|
•
|
successfully commercializing any product candidates, if approved;
|
|
•
|
protecting our rights to our intellectual property portfolio;
|
|
•
|
ensuring the manufacture of commercial quantities of our product candidates;
|
|
•
|
finding suitable partners to help us develop certain of our product candidates and market, sell and/or distribute any of our products that receive regulatory approval in other markets; and
|
|
•
|
obtaining adequate pricing, coverage and reimbursement from third parties, including government and private payors.
|
|
•
|
the initiation, progress, timing, costs and results of clinical trials for our product candidates;
|
|
•
|
the scope, progress, results and costs of pre-clinical development and laboratory testing of our pre-clinical product candidates;
|
|
•
|
our ability to establish collaborations on favorable terms, if at all, particularly manufacturing, marketing and distribution arrangements for our product candidates;
|
|
•
|
the costs and timing of the implementation of commercial-scale manufacturing activities;
|
|
•
|
the costs and timing of establishing sales, marketing and distribution capabilities for any product candidates for which we may receive regulatory approval;
|
|
•
|
the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims;
|
|
•
|
our obligation to make milestone, royalty and other payments to third party licensors under our licensing agreements;
|
|
•
|
the extent to which we in-license or acquire rights to other products, product candidates or technologies;
|
|
•
|
the outcome, timing and cost of regulatory review by the FDA and comparable foreign regulatory authorities, including the potential for the FDA or comparable foreign regulatory authorities, including Health Canada or the EMA, to require that we perform more studies than those that we currently expect;
|
|
•
|
the effect of competing technological and market developments; and
|
|
•
|
the revenue, if any, received from commercial sales of any product candidates for which we receive regulatory approval.
|
|
•
|
receipt of marketing approvals from the FDA, Health Canada, the European Commission or comparable foreign regulatory authorities;
|
|
•
|
performance of our future collaborators, if any;
|
|
•
|
extent of any required post-marketing approval commitments to applicable regulatory authorities;
|
|
•
|
obtaining and maintaining patent, trade secret protection and regulatory exclusivity, both in the United States and internationally;
|
|
•
|
protection of our rights in our intellectual property portfolio;
|
|
•
|
launch of commercial sales, if and when marketing approval is received;
|
|
•
|
demonstration of an acceptable safety profile prior to and following any marketing approval;
|
|
•
|
marketplace acceptance, if and when approved, by patients, the medical community and third-party payors;
|
|
•
|
establishing and maintaining pricing sufficient to realize a meaningful return on our investment; and
|
|
•
|
competition with other therapies.
|
|
•
|
clinical trials of our product candidates may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials or abandon product development programs;
|
|
•
|
the number of subjects required for clinical trials of our product candidates may be larger than we anticipate, enrollment in these clinical trials may be slower or more challenging than we anticipate or subjects may drop out of these clinical trials at a higher rate than we anticipate;
|
|
•
|
our third-party contractors may fail to comply with regulatory requirements, including GCPs or meet their contractual obligations to us in a timely manner, or at all;
|
|
•
|
inspection of the clinical trial operations, trial sites or manufacturing facility by the FDA or other comparable foreign regulatory authorities such as Health Canada, or the competent authorities of the E.U. Member States, could result in findings of non-compliance and the imposition of a clinical suspension or termination;
|
|
•
|
regulators or IRBs may delay or not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site;
|
|
•
|
we may experience delays or fail to reach agreement with the FDA or a comparable foreign regulatory authority, including Health Canada, or the competent authorities of the E.U. Member States, on a trial design that we are able to execute;
|
|
•
|
we may be unable to identify and maintain a sufficient number of trial sites, many of which may already be engaged in other clinical trial programs, including for the same indications as our clinical trials;
|
|
•
|
we may experience delays in reaching, or fail to reach, agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites;
|
|
•
|
trial sites and investigators may deviate from clinical trial protocols or otherwise fail to conduct the trial in accordance with regulatory requirements, and investigators may drop out of the clinical trial;
|
|
•
|
trial sites may withdraw from our clinical trials, including as a result of changing standards of care or ineligibility of a site to participate in our clinical trials;
|
|
•
|
we may decide, or regulators or IRBs/Ethics Committees or other reviewing entities, including comparable foreign regulatory authorities such as Health Canada, or the competent authorities of the E.U. Member States, may require us to suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements including GCPs or a finding that the subjects are being exposed to unacceptable health risks;
|
|
•
|
the cost of clinical trials of our product candidates may be greater than we anticipate;
|
|
•
|
we may receive feedback from DSMBs or the FDA, or a comparable foreign regulatory authority, including Health Canada or the competent authorities of the E.U. Member States, that might require modification to the protocol for the clinical trial or performance of additional studies before clinical trials may continue;
|
|
•
|
as a clinical trial proceeds, or as the results of earlier stage studies or concurrent studies become available, we may determine that we need to modify the protocol and/or other aspects of the clinical trial before it may continue;
|
|
•
|
the FDA, a comparable foreign regulatory authority, including Health Canada, or the competent authorities of the E.U. Member States, or we may decide to, or a DSMB may recommend to, suspend or terminate clinical trials at any time for safety issues or for any other reason;
|
|
•
|
the supply or quality of our product candidates or other materials necessary to conduct clinical trials of our product candidates may be insufficient or inadequate;
|
|
•
|
our product candidates may have undesirable side effects or other unexpected characteristics, causing us or our investigators, regulators or IRBs to suspend or terminate the trials;
|
|
•
|
lack of adequate funding to continue a clinical trial, including the incurrence of unforeseen costs due to enrollment delays, requirements to conduct additional clinical trials or increased expenses associated with the services of our CROs and other third parties; and
|
|
•
|
changes in applicable laws, governmental regulations or administrative actions.
|
|
•
|
be delayed in obtaining marketing approval for our product candidates;
|
|
•
|
not obtain marketing approval at all;
|
|
•
|
obtain approval for indications or patient populations that are not as broad as intended or desired;
|
|
•
|
obtain approval with labeling that includes significant use or distribution restrictions or safety warnings, or is subject to a REMS;
|
|
•
|
be subject to additional post-marketing testing requirements; or
|
|
•
|
have the product removed from the market after obtaining marketing approval.
|
|
•
|
the severity of the disease under investigation;
|
|
•
|
the eligibility criteria for the clinical trial in question;
|
|
•
|
the size of the patient population for the disease;
|
|
•
|
the size of the subject population required for statistically significant analysis of the clinical trial’s primary endpoints;
|
|
•
|
the design of the clinical trial;
|
|
•
|
the clinicians' and subjects' perceived risks and benefits of the product candidate under study, including relative to alternative treatments;
|
|
•
|
the efforts to facilitate timely enrollment in clinical trials;
|
|
•
|
the subject referral practices of physicians;
|
|
•
|
any ongoing clinical trials conducted by competitors for the same indication;
|
|
•
|
the risk that subjects enrolled in clinical trials will drop out of the clinical trials before completion;
|
|
•
|
the ability to monitor subjects adequately during and after treatment; and
|
|
•
|
the proximity and availability of clinical trial sites for prospective subjects.
|
|
•
|
difficulty in establishing or managing relationships with CROs and physicians;
|
|
•
|
different or additional standards for the conduct of clinical trials;
|
|
•
|
absence in some countries of established groups with sufficient regulatory expertise for review of the protocols associated with our product candidates;
|
|
•
|
ensuring that clinical trial quality is sufficient to meet the standards of the FDA or other regulatory authorities;
|
|
•
|
our inability to locate qualified local consultants, physicians and partners; and
|
|
•
|
the potential burden of complying with a variety of foreign laws, medical standards and regulatory requirements, including the regulation of pharmaceutical and biotechnology products and treatments.
|
|
•
|
we may suspend or be forced to suspend marketing of our product candidates;
|
|
•
|
we may be obliged to conduct a product recall or product withdrawal;
|
|
•
|
regulatory authorities may suspend, vary, or withdraw their approvals of our product candidates;
|
|
•
|
regulatory authorities may order the seizure or recall of our product candidates;
|
|
•
|
regulatory authorities may require additional warnings on the label or a REMS that could diminish the usage or otherwise limit the commercial success of our product candidates;
|
|
•
|
we may be required to conduct post-marketing studies;
|
|
•
|
we could be sued and held liable for harm caused to subjects or patients;
|
|
•
|
we could be required to pay fines and face other administrative, civil and criminal penalties; and
|
|
•
|
our reputation may suffer.
|
|
•
|
the perceived quality, efficacy and safety of our product candidates;
|
|
•
|
clinical indications for which our product candidates are approved;
|
|
•
|
availability of alternative effective treatments for the disease indications of our product candidates are intended to treat and the relative risks, benefits and costs of those treatments;
|
|
•
|
acceptance by physicians, major operators of cancer clinics and patients of our product candidates as safe and effective treatments;
|
|
•
|
the success of our physician education programs;
|
|
•
|
potential and perceived advantages of our product candidates over alternative treatments;
|
|
•
|
safety of our product candidates seen in a broader patient group, including their use outside the approved indications should physicians choose to prescribe them for such uses;
|
|
•
|
prevalence and severity of any side effects;
|
|
•
|
any new or unexpected results from additional clinical trials or further analysis of clinical data of completed clinical trials by us or our competitors;
|
|
•
|
product labeling or patient information requirements imposed by the FDA or other foreign regulatory authorities, including Health Canada and the EMA;
|
|
•
|
timing of market introduction of our product candidates as well as competitive products;
|
|
•
|
the pricing of our treatments, particularly in relation to alternative treatments, and willingness and ability of patients to pay for our product candidates;
|
|
•
|
availability of coverage and adequate reimbursement and pricing by third-party payors and government authorities;
|
|
•
|
maintaining compliance with all applicable regulatory requirements;
|
|
•
|
relative convenience and ease of administration; and
|
|
•
|
effectiveness of our sales, marketing and distribution efforts and operations.
|
|
•
|
our inability to recruit, train and retain adequate numbers of effective sales and marketing personnel;
|
|
•
|
the inability of sales personnel to obtain access to physicians or persuade adequate numbers of physicians to prescribe our products;
|
|
•
|
the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and
|
|
•
|
unforeseen costs and expenses associated with creating an independent sales and marketing organization.
|
|
•
|
decreased demand for any product candidates or products that we develop;
|
|
•
|
injury to our reputation and significant negative media attention;
|
|
•
|
withdrawal of clinical trial subjects;
|
|
•
|
significant costs to defend the related litigation;
|
|
•
|
substantial monetary awards to trial subjects or patients;
|
|
•
|
loss of revenue;
|
|
•
|
reduced time and attention of our management to pursue our business strategy; and
|
|
•
|
the inability to commercialize any products that we develop.
|
|
•
|
collaborators or licensees have significant discretion in determining the amount and timing of efforts and resources that they will apply to these collaborations or licenses;
|
|
•
|
collaborators or licensees may not perform their obligations as expected;
|
|
•
|
collaborators or licensees may not pursue development and commercialization of our product candidates that receive marketing approval or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in the collaborators’ or licensees' strategic focus or available funding, or external factors, such as an acquisition, that divert resources or create competing priorities;
|
|
•
|
collaborators or licensees may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing;
|
|
•
|
collaborators or licensees could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates if the collaborators or licensees believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours;
|
|
•
|
product candidates discovered under the collaboration or license with us may be viewed by our collaborators or licensees as competitive with their own product candidates or products, which may cause collaborators or licensees to cease to devote resources to the commercialization of our product candidates;
|
|
•
|
a collaborator or licensee with marketing and distribution rights to one or more of our product candidates that achieve regulatory approval may not commit sufficient resources to the marketing and distribution of such product or products;
|
|
•
|
disagreements with collaborators or licensees, including disagreements over proprietary rights, contract interpretation or the preferred course of development, might cause delays or termination of the research,
|
|
•
|
collaborators or licensees may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation;
|
|
•
|
collaborators or licensees may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability; and
|
|
•
|
collaborations or licenses may be terminated for the convenience of the collaborator or licensee and, if terminated, we could be required to raise additional capital to pursue further development or commercialization of the applicable product candidates.
|
|
•
|
The development of commercial-scale manufacturing capabilities may require our third-party manufacturer to invest substantial additional funds and hire and retain technical personnel who have the necessary manufacturing experience. Our third-party manufacturer may fail to devote sufficient time and resources to develop the capabilities to manufacture our product candidates.
|
|
•
|
Because of the complex nature of our product candidates, our third party manufacturer, or other third parties we rely on, may encounter difficulties in achieving the volume of production needed to satisfy commercial demand, may not be able to achieve such volume at an acceptable cost, may experience technical issues that impact comparability, quality, or compliance with applicable regulations governing the manufacture of biological products, and may experience shortages of qualified personnel to adequately staff production operations.
|
|
•
|
Our third-party manufacturer could default on its agreement with us to meet our requirements for commercialization of our product candidates, or it may terminate or decide not to renew its agreement with us, based on its own business priorities, at a time that is costly or damaging to us. If our third-party manufacturer were to terminate our arrangement or fail to meet our commercial manufacturing demands, we may be delayed in our ability to obtain and maintain regulatory approval of our product candidates or, if approved, commercialize our product candidates.
|
|
•
|
It may be difficult or impossible for us to find a replacement manufacturer on acceptable terms quickly, or at all. Identifying alternate manufacturers may be difficult because the number of potential manufacturers that have the necessary expertise to produce biologics is limited. Additionally, the FDA must approve any alternative manufacturer before we may use the alternative manufacturer to produce commercial supply of a product candidate, if approved.
|
|
•
|
others may be able to make product candidates that are the same as or similar to our product candidates but that are not covered by the claims of the patents that we own or have licensed;
|
|
•
|
biosimilar product manufacturers may develop, seek approval for, and launch biosimilar versions of our products, which could be significantly less costly to bring to market and priced significantly lower than our products;
|
|
•
|
we or our licensors might not have been the first inventor to file patent applications covering certain of our inventions;
|
|
•
|
others may design around our intellectual property rights or independently develop similar or alternative technologies or duplicate any of our technologies without infringing or misappropriating our intellectual property rights;
|
|
•
|
it is possible that our pending patent applications will not lead to issued patents with claims that cover our products or even issued patents;
|
|
•
|
issued patents that we own or have exclusively licensed may not provide us with any competitive advantages, or may be held invalid or unenforceable as a result of legal challenges;
|
|
•
|
our competitors might conduct research and development activities in the United States and other countries that provide a safe harbor from patent infringement claims for certain research and development activities, as well as in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets;
|
|
•
|
we may not develop additional proprietary technologies or product candidates that are patentable; and
|
|
•
|
the intellectual property rights of others may have an adverse effect on our business.
|
|
•
|
The second applicant can establish in its application that its product, although similar to the orphan medicinal product already authorized, is safer, more effective or otherwise clinically superior;
|
|
•
|
The holder of the marketing authorization for the original orphan medicinal product consents to a second orphan medicinal product application; or
|
|
•
|
The holder of the marketing authorization for the original orphan medicinal product cannot supply enough orphan medicinal product.
|
|
•
|
the United States Congress could amend the BPCIA to significantly shorten this exclusivity period as has been previously proposed; and
|
|
•
|
a potential competitor could seek and obtain approval of its own BLA during our exclusivity period instead of seeking approval of a biosimilar version.
|
|
•
|
litigation involving patients taking our products;
|
|
•
|
restrictions on such products, manufacturers or manufacturing processes;
|
|
•
|
restrictions on the labeling or marketing of a product;
|
|
•
|
restrictions on product distribution or use;
|
|
•
|
requirements to conduct post-marketing studies or clinical trials;
|
|
•
|
warning letters or untitled letters;
|
|
•
|
withdrawal of the products from the market;
|
|
•
|
refusal to approve pending applications or supplements to approved applications that we submit;
|
|
•
|
recall of products;
|
|
•
|
fines, restitution or disgorgement of profits or revenues;
|
|
•
|
suspension or withdrawal of marketing approvals;
|
|
•
|
damage to relationships with any potential collaborators;
|
|
•
|
unfavorable press coverage and damage to our reputation;
|
|
•
|
refusal to permit the import or export of our products;
|
|
•
|
product seizure or detention; or
|
|
•
|
injunctions or the imposition of civil or criminal penalties.
|
|
•
|
the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation or arranging of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid;
|
|
•
|
the federal False Claims Act imposes criminal and civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities for, among other things, knowingly presenting, or causing to be presented, false or fraudulent claims for payment by a federal healthcare program or making a false statement or record material to payment of a false claim or avoiding, decreasing or concealing an obligation to pay money to the federal government, with potential liability including mandatory treble damages and significant per-claim penalties, set at $10,781 to $21,563 per false claim for violations occurring after November 2, 2015;
|
|
•
|
HIPAA, which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statement or entry relating to the delivery of or payment for healthcare benefits, items or services;
|
|
•
|
the federal Physician Payments Sunshine Act requires applicable manufacturers of covered products to report payments and other transfers of value to physicians and teaching hospitals;
|
|
•
|
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, and their respective implementing regulations, which imposes obligations, including mandatory contractual terms, on covered healthcare providers, health plans and healthcare clearinghouses, as well as their business associates, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; and
|
|
•
|
analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws and transparency statutes, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; state and foreign laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; state and foreign laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; state laws that prohibit certain marketing-related activities, including the provision of gifts, meals, or other items to certain health care providers, and restrict the ability of manufacturers to offer co-pay support to patients for certain prescription drugs; state laws that require identification or licensing of sales representatives; and state and foreign laws governing the privacy, security, collection, use and disclosure of health information, including state data breach notification laws, state health information privacy laws, and federal and state consumer protection laws (e.g., Section 5 of the FTC Act), many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
|
|
•
|
an annual, non-deductible fee on any entity that manufactures or imports specified branded prescription products and biologic agents;
|
|
•
|
an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program;
|
|
•
|
a new methodology by which average manufacturer price is calculated and reported by manufacturers for products that are inhaled, infused, instilled, implanted or injected and not generally dispensed through retail community pharmacies;
|
|
•
|
expansion of healthcare fraud and abuse laws, including the civil False Claims Act and the federal Anti-Kickback Statute, new government investigative powers and enhanced penalties for noncompliance;
|
|
•
|
a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand products to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient products to be covered under Medicare Part D;
|
|
•
|
extension of manufacturers’ Medicaid rebate liability from fee-for-service Medicaid utilization to include the utilization of Medicaid managed care organizations as well;
|
|
•
|
expansion of eligibility criteria for Medicaid programs;
|
|
•
|
expansion of the entities eligible for discounts under the Public Health Service 340B pharmaceutical pricing program;
|
|
•
|
new requirements to report certain financial arrangements with physicians and teaching hospitals;
|
|
•
|
a new requirement to annually report product samples that manufacturers and distributors provide to physicians;
|
|
•
|
a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research;
|
|
•
|
a new IPAB which has authority to recommend certain changes to the Medicare program to reduce expenditures by the program that could result in reduced payments for prescription products; and
|
|
•
|
establishment of the Center for Medicare and Medicaid Innovation within CMS to test innovative payment and service delivery models.
|
|
•
|
managing our clinical trials effectively;
|
|
•
|
identifying, recruiting, maintaining, motivating and integrating additional employees;
|
|
•
|
managing our internal development efforts effectively while complying with our contractual obligations to licensors, licensees, contractors and other third parties;
|
|
•
|
improving our managerial, development, operational and finance systems; and
|
|
•
|
expanding our facilities.
|
|
•
|
delay, defer or prevent a change in control;
|
|
•
|
entrench our management and the board of directors; or
|
|
•
|
delay or prevent a merger, consolidation, takeover or other business combination involving us on terms that other stockholders may desire.
|
|
•
|
establish a classified board of directors such that only one of three classes of directors is elected each year;
|
|
•
|
allow the authorized number of our directors to be changed only by resolution of our board of directors;
|
|
•
|
limit the manner in which stockholders can remove directors from our board of directors;
|
|
•
|
establish advance notice requirements for stockholder proposals that can be acted on at stockholder meetings and nominations to our board of directors;
|
|
•
|
require that stockholder actions must be effected at a duly called stockholder meeting and prohibit actions by our stockholders by written consent;
|
|
•
|
limit who may call stockholder meetings;
|
|
•
|
authorize our board of directors to issue preferred stock without stockholder approval, which could be used to institute a “poison pill” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors; and
|
|
•
|
require the approval of the holders of at least 75% of the votes that all our stockholders would be entitled to cast to amend or repeal specified provisions of our certificate of incorporation or bylaws.
|
|
•
|
the success of competitive products or technologies;
|
|
•
|
results of clinical trials of Vicinium or any other product candidate that we may develop;
|
|
•
|
results of clinical trials of product candidates of our competitors;
|
|
•
|
regulatory or legal developments in the United States and other countries;
|
|
•
|
developments or disputes concerning patent applications, issued patents or other proprietary rights;
|
|
•
|
the recruitment or departure of key scientific or management personnel;
|
|
•
|
the level of expenses related to any of our product candidates or clinical development programs;
|
|
•
|
the results of our efforts to discover, develop, acquire or in-license additional products, product candidates or technologies for the treatment of ophthalmic diseases, the costs of commercializing any such products and the costs of development of any such product candidates or technologies;
|
|
•
|
actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts;
|
|
•
|
variations in our financial results or those of companies that are perceived to be similar to us;
|
|
•
|
changes in the structure of healthcare payment systems;
|
|
•
|
market conditions in the pharmaceutical and biotechnology sectors;
|
|
•
|
general economic, industry and market conditions; and
|
|
•
|
the other factors described in this “Risk Factors” section.
|
|
•
|
not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting;
|
|
•
|
not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements;
|
|
•
|
reduced disclosure obligations regarding executive compensation; and
|
|
•
|
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
|
|
Item 1B.
|
Unresolved Staff Comments.
|
|
Item 2.
|
Properties.
|
|
Item 3.
|
Legal Proceedings.
|
|
Item 4.
|
Mine Safety Disclosures.
|
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
|
|
Market Price
|
|||||||
|
High
|
Low
|
||||||
|
First quarter 2016
|
$
|
3.00
|
|
$
|
0.25
|
|
|
|
Second quarter 2016
|
$
|
3.80
|
|
$
|
0.31
|
|
|
|
Third quarter 2016
|
$
|
5.97
|
|
$
|
1.58
|
|
|
|
Fourth quarter 2016
|
$
|
3.23
|
|
$
|
1.32
|
|
|
|
First quarter 2017
|
$
|
2.50
|
|
$
|
1.80
|
|
|
|
Second quarter 2017
|
$
|
2.54
|
|
$
|
1.31
|
|
|
|
Third quarter 2017
|
$
|
1.84
|
|
$
|
0.90
|
|
|
|
Fourth quarter 2017
|
$
|
1.70
|
|
$
|
0.62
|
|
|
|
Item 6.
|
Selected Financial Data.
|
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations.
|
|
•
|
the scope, initiation, progress, timing, costs and results of pre-clinical development and laboratory testing and clinical trials for our product candidates;
|
|
•
|
our ability to establish collaborations on favorable terms, if at all, particularly manufacturing, marketing and distribution arrangements for our product candidates;
|
|
•
|
the costs and timing of the implementation of commercial-scale manufacturing activities;
|
|
•
|
the costs and timing of establishing sales, marketing and distribution capabilities for our product candidates for which we may receive regulatory approval;
|
|
•
|
the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims;
|
|
•
|
our obligation to make milestone, royalty and other payments to third-party licensors under our licensing agreements;
|
|
•
|
the extent to which we in-license or acquire rights to other products, product candidates or technologies;
|
|
•
|
the outcome, timing and cost of regulatory review by the FDA and comparable foreign regulatory authorities, including the potential for the FDA or comparable foreign regulatory authorities, including Health Canada, to require that we perform more studies or clinical trials than those that we currently expect;
|
|
•
|
our ability to achieve certain future regulatory, development and commercialization milestones under the License Agreement with Roche;
|
|
•
|
the effect of competing technological and market developments; and
|
|
•
|
the revenue, if any, received from commercial sales of any product candidates for which we receive regulatory approval.
|
|
•
|
employee-related expenses, including salaries, benefits, travel and stock-based compensation expense;
|
|
•
|
expenses incurred under agreements with CROs, and investigative sites that conduct our clinical trials;
|
|
•
|
expenses associated with developing manufacturing capabilities and manufacturing clinical study materials;
|
|
•
|
facilities, depreciation, and other expenses, which include direct and allocated expenses for rent and maintenance of facilities, insurance, and other supplies; and
|
|
•
|
expenses associated with pre-clinical and regulatory activities.
|
|
•
|
the scope, progress, outcome and costs of our clinical trials and other research and development activities;
|
|
•
|
the efficacy and potential advantages of our product candidates compared to alternative treatments, including any standard of care;
|
|
•
|
the market acceptance of our product candidates;
|
|
•
|
the cost and timing of the implementation of commercial-scale manufacturing of our product candidates;
|
|
•
|
obtaining, maintaining, defending and enforcing patent claims and other intellectual property rights;
|
|
•
|
significant and changing government regulation; and
|
|
•
|
the timing, receipt and terms of any marketing approvals.
|
|
Year ended December 31,
|
|||||||||||
|
2017
|
2016*
|
2015
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Programs:
|
|||||||||||
|
Vicinium (1)
|
$
|
6,974
|
|
$
|
1,564
|
|
$
|
—
|
|
||
|
Proxinium (2)
|
—
|
|
—
|
|
—
|
|
|||||
|
VB6-845d (2)
|
—
|
|
—
|
|
—
|
|
|||||
|
EBI-031 (3)
|
—
|
|
2,996
|
|
5,384
|
|
|||||
|
Isunakinra/EBI-005 (4)
|
—
|
|
1,653
|
|
14,455
|
|
|||||
|
Total direct program expenses
|
6,974
|
|
6,213
|
|
19,839
|
|
|||||
|
Personnel and other expenses:
|
|||||||||||
|
Employee and contractor-related expenses
|
3,871
|
|
5,863
|
|
4,762
|
|
|||||
|
Platform-related lab expenses
|
455
|
|
479
|
|
620
|
|
|||||
|
Facility expenses
|
398
|
|
561
|
|
536
|
|
|||||
|
Other expenses
|
812
|
|
363
|
|
579
|
|
|||||
|
Total personnel and other expenses
|
5,536
|
|
7,266
|
|
6,497
|
|
|||||
|
Total research and development expenses
|
$
|
12,510
|
|
$
|
13,479
|
|
$
|
26,336
|
|
||
|
•
|
the delivered item or items have stand-alone value to the customer; and
|
|
•
|
delivery or performance of the undelivered item(s) is considered probable and substantially in our control, and the arrangement includes a general right of return relative to the delivered item(s).
|
|
•
|
it can only be achieved based in whole or in part on either our performance or the occurrence of a specific outcome resulting from our performance;
|
|
•
|
there is substantive uncertainty at the date an arrangement is entered into that the event will be achieved; and
|
|
•
|
it would result in additional payments being due to us.
|
|
Year Ended December 31,
|
|||||
|
2017
|
2016
|
2015
|
|||
|
Risk-free interest rate
|
1.88 - 2.04%
|
1.23 - 2.38%
|
1.42 - 1.92%
|
||
|
Expected dividend yield
|
—%
|
—%
|
—%
|
||
|
Expected term (in years)
|
5.3 - 6
|
5.5 - 6
|
5.75 - 6
|
||
|
Expected volatility
|
75.4 - 86.66%
|
71.44 - 92.09%
|
69.06 - 74.11%
|
||
|
Year ended
December 31,
|
|||||||||||
|
2017
|
2016
|
Change
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Revenue:
|
|||||||||||
|
Collaboration revenue
|
$
|
—
|
|
$
|
406
|
|
$
|
(406
|
)
|
||
|
License revenue
|
425
|
|
29,575
|
|
(29,150
|
)
|
|||||
|
Total revenue
|
425
|
|
29,981
|
|
(29,556
|
)
|
|||||
|
Operating expenses:
|
|||||||||||
|
Research and development
|
12,510
|
|
13,479
|
|
(969
|
)
|
|||||
|
General and administrative
|
8,070
|
|
14,736
|
|
(6,666
|
)
|
|||||
|
Loss (gain) from change in fair value of contingent consideration
|
9,100
|
|
(1,100
|
)
|
10,200
|
|
|||||
|
Total operating expenses
|
29,680
|
|
27,115
|
|
2,565
|
|
|||||
|
(Loss) income from operations
|
(29,255
|
)
|
2,866
|
|
(32,121
|
)
|
|||||
|
Other income (expense), net
|
226
|
|
(970
|
)
|
1,196
|
|
|||||
|
Net (loss) income before income taxes
|
(29,029
|
)
|
1,896
|
|
(30,925
|
)
|
|||||
|
Provision for income taxes
|
—
|
|
5
|
|
(5
|
)
|
|||||
|
Net (loss) income and comprehensive (loss) income
|
$
|
(29,029
|
)
|
$
|
1,891
|
|
$
|
(30,920
|
)
|
||
|
Year ended
December 31,
|
|||||||||||
|
2016
|
2015
|
Change
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Revenue:
|
|||||||||||
|
Collaboration revenue
|
$
|
406
|
|
$
|
490
|
|
$
|
(84
|
)
|
||
|
License revenue
|
29,575
|
|
500
|
|
29,075
|
|
|||||
|
Total revenue
|
29,981
|
|
990
|
|
28,991
|
|
|||||
|
Operating expenses:
|
|||||||||||
|
Research and development
|
13,479
|
|
26,336
|
|
(12,857
|
)
|
|||||
|
General and administrative
|
14,736
|
|
9,850
|
|
4,886
|
|
|||||
|
Gain from change in fair value of contingent consideration
|
(1,100
|
)
|
—
|
|
(1,100
|
)
|
|||||
|
Total operating expenses
|
27,115
|
|
36,186
|
|
(9,071
|
)
|
|||||
|
Income (loss) from operations
|
2,866
|
|
(35,196
|
)
|
38,062
|
|
|||||
|
Other income (expense), net
|
(970
|
)
|
1,744
|
|
(2,714
|
)
|
|||||
|
Net income (loss) before income taxes
|
1,896
|
|
(33,452
|
)
|
35,348
|
|
|||||
|
Provision for income taxes
|
5
|
|
$
|
—
|
|
5
|
|
||||
|
Net income (loss) and comprehensive income (loss)
|
$
|
1,891
|
|
$
|
(33,452
|
)
|
$
|
35,343
|
|
||
|
Year ended December 31,
|
|||||||||||
|
2017
|
2016
|
2015
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Net cash provided by (used in):
|
|||||||||||
|
Operating activities
|
$
|
(17,765
|
)
|
$
|
2,622
|
|
$
|
(34,529
|
)
|
||
|
Investing activities
|
98
|
|
461
|
|
(287
|
)
|
|||||
|
Financing activities
|
7,005
|
|
(13,820
|
)
|
16,836
|
|
|||||
|
Net (decrease) increase in cash and cash equivalents
|
$
|
(10,662
|
)
|
$
|
(10,737
|
)
|
$
|
(17,980
|
)
|
||
|
•
|
continue our Phase 3 clinical trial for Vicinium;
|
|
•
|
continue the research and pre-clinical and clinical development of our other product candidates;
|
|
•
|
seek to discover and develop additional product candidates;
|
|
•
|
in-license or acquire the rights to other products, product candidates or technologies;
|
|
•
|
seek marketing approvals for any product candidates that successfully complete clinical trials;
|
|
•
|
establish sales, marketing and distribution capabilities and scale up and validate external manufacturing capabilities to commercialize any product candidates for which we may obtain marketing approval;
|
|
•
|
maintain, expand and protect our intellectual property portfolio;
|
|
•
|
add equipment and physical infrastructure to support our research and development;
|
|
•
|
hire additional clinical, quality control, scientific and management personnel; and
|
|
•
|
expand our operational, financial and management systems and personnel.
|
|
•
|
the scope, initiation, progress, timing, costs and results of pre-clinical development and laboratory testing of our pre-clinical product candidates;
|
|
•
|
our ability to establish collaborations on favorable terms, if at all, particularly manufacturing, marketing and distribution arrangements for our product candidates;
|
|
•
|
the costs and timing of the implementation of commercial-scale manufacturing activities;
|
|
•
|
the costs and timing of establishing sales, marketing and distribution capabilities for any product candidates for which we may receive regulatory approval;
|
|
•
|
the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims;
|
|
•
|
our obligation to make milestone, royalty and other payments to third party licensors under our licensing agreements;
|
|
•
|
the extent to which we in-license or acquire rights to other products, product candidates or technologies;
|
|
•
|
the outcome, timing and cost of regulatory review by the FDA and comparable foreign regulatory authorities, including the potential for the FDA or comparable foreign regulatory authorities, including Health Canada, to require that we perform more studies or clinical trials than those that we currently expect;
|
|
•
|
our ability to achieve certain future regulatory, development and commercialization milestones under the License Agreement with Roche;
|
|
•
|
the effect of competing technological and market developments; and
|
|
•
|
the revenue, if any, received from commercial sales of any product candidates for which we receive regulatory approval.
|
|
Total
|
Less than 1 Year
|
1 to 3 Years
|
3 to 5 Years
|
More than 5 Years
|
|||||||||||||||
|
(in thousands)
|
|||||||||||||||||||
|
Operating lease obligations (1)
|
$
|
1,017
|
|
$
|
429
|
|
$
|
588
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
License maintenance fees (2)
|
1,175
|
|
185
|
|
555
|
|
435
|
|
—
|
|
|||||||||
|
Total fixed contractual obligations
|
$
|
2,192
|
|
$
|
614
|
|
$
|
1,143
|
|
$
|
435
|
|
$
|
—
|
|
||||
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk.
|
|
Item 8.
|
Financial Statements and Supplementary Data.
|
|
Item 9.
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
|
|
Item 9A.
|
Controls and Procedures.
|
|
Item 9B.
|
Other Information.
|
|
Item 10.
|
Directors, Executive Officers and Corporate Governance.
|
|
Item 11.
|
Executive Compensation.
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
|
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence.
|
|
Item 14.
|
Principal Accountant Fees and Services.
|
|
Item 15.
|
Exhibits and Financial Statement Schedules.
|
|
Exhibit
No.
|
Description
|
|
|
2.1
|
||
|
3.1
|
|
|
|
3.2
|
|
|
|
4.1
|
|
|
|
4.2
|
|
|
|
4.3
|
||
|
4.4
|
||
|
4.5
|
||
|
4.6
|
||
|
4.7
|
||
|
10.1+
|
|
|
|
10.2+
|
|
|
|
10.3+
|
|
|
|
10.4+
|
|
|
|
10.5+
|
|
|
|
10.6+
|
|
|
|
10.7+
|
|
|
|
10.8+
|
|
|
|
10.9
|
||
|
10.10+
|
||
|
10.11+
|
|
|
|
10.12+
|
|
|
|
10.13†
|
|
|
|
10.14†
|
|
|
|
10.15†
|
|
|
|
10.16
|
||
|
10.17
|
||
|
10.18+
|
||
|
10.19+
|
||
|
10.20+
|
||
|
10.21+
|
||
|
10.22+
|
||
|
10.23+
|
||
|
10.24+
|
||
|
10.25
|
||
|
10.26+
|
||
|
10.27+
|
||
|
10.28+
|
|
|
|
10.29+
|
||
|
10.30
|
||
|
21.1*
|
||
|
23.1*
|
|
|
|
31.1*
|
|
|
|
31.2*
|
|
|
|
32.1*
|
|
|
|
101.INS*
|
|
XBRL Instance Document
|
|
101.SCH*
|
|
XBRL Taxonomy Extension Schema Document
|
|
101.CAL*
|
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
101.DEF*
|
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
101.LAB*
|
|
XBRL Taxonomy Extension Label Linkbase Document
|
|
101.PRE*
|
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
*
|
Filed herewith.
|
|
+
|
This exhibit is a compensatory plan or arrangement in which our executive officers or directors participate.
|
|
†
|
Confidential treatment requested as to portions of the exhibit. Confidential materials omitted and filed separately with the Securities and Exchange Commission.
|
|
Item 16.
|
Form 10-K Summary.
|
|
ELEVEN BIOTHERAPEUTICS, INC.
|
||
|
By:
|
|
/s/ Stephen A. Hurly
|
|
|
Stephen A. Hurly
|
|
|
|
President and Chief Executive Officer
|
|
|
/s/ Stephen A. Hurly
|
Director, President and Chief Executive Officer (Principal Executive Officer)
|
April 2, 2018
|
|
|
Stephen A. Hurly
|
|
||
|
/s/ Richard F. Fitzgerald
|
|
Chief Financial Officer (Principal Financial and Accounting Officer)
|
April 2, 2018
|
|
Richard F. Fitzgerald
|
|||
|
/s/ Wendy L. Dixon, Ph.D.
|
|
Chair of the Board of Directors
|
April 2, 2018
|
|
Wendy L. Dixon, Ph.D.
|
|||
|
/s/ Abbie C. Celniker
|
|
Director
|
April 2, 2018
|
|
Abbie C. Celniker, Ph.D.
|
|||
|
/s/ Paul G. Chaney
|
|
Director
|
April 2, 2018
|
|
Paul G. Chaney
|
|||
|
/s/ Leslie Dan, B.Sc. Phm,. M.B.A., C.M.
|
|
Director
|
April 2, 2018
|
|
Leslie Dan, B.Sc. Phm,. M.B.A., C.M.
|
|||
|
/s/ Jay S. Duker, M.D.
|
|
Director
|
April 2, 2018
|
|
Jay S. Duker, M.D.
|
|||
|
/s/ Barry J. Gertz, M.D., Ph.D.
|
|
Director
|
April 2, 2018
|
|
Barry J. Gertz, M.D., Ph.D.
|
|||
|
/s/ Jane V. Henderson
|
|
Director
|
April 2, 2018
|
|
Jane V. Henderson
|
|||
|
/s/ Daniel S. Lynch
|
|
Director
|
April 2, 2018
|
|
Daniel S. Lynch
|
|||
|
Consolidated Statements of Operations and Comprehensive
(Loss) Income
|
|
|
December 31,
|
|||||||
|
2017
|
2016
|
||||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
14,680
|
|
$
|
25,342
|
|
|
|
Prepaid expenses and other current assets
|
301
|
|
585
|
|
|||
|
Total current assets
|
14,981
|
|
25,927
|
|
|||
|
Property and equipment, net
|
522
|
|
796
|
|
|||
|
Restricted cash
|
10
|
|
10
|
|
|||
|
Intangible assets
|
46,400
|
|
60,500
|
|
|||
|
Goodwill
|
13,064
|
|
16,864
|
|
|||
|
Other assets
|
120
|
|
—
|
|
|||
|
Total assets
|
$
|
75,097
|
|
$
|
104,097
|
|
|
|
Liabilities and stockholders’ equity
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
907
|
|
$
|
1,667
|
|
|
|
Accrued expenses
|
3,813
|
|
1,774
|
|
|||
|
Deferred revenue, current portion
|
—
|
|
425
|
|
|||
|
Due to related party
|
—
|
|
|
114
|
|
||
|
Total current liabilities
|
4,720
|
|
3,980
|
|
|||
|
Other liabilities
|
215
|
|
—
|
|
|||
|
Warrant liability
|
—
|
|
5
|
|
|||
|
Deferred tax liability
|
12,528
|
|
16,335
|
|
|||
|
Contingent consideration
|
39,600
|
|
45,100
|
|
|||
|
Commitments and contingencies (Note 9)
|
|
|
|||||
|
Stockholders’ equity:
|
|||||||
|
Preferred stock, $0.001 par value per share; 5,000,000 shares authorized at December 31, 2017 and 2016 and no shares issued and outstanding at December 31, 2017 and 2016
|
—
|
|
—
|
|
|||
|
Common stock, $0.001 par value per share; 200,000,000 shares authorized at December 31, 2017 and 2016 and 34,702,565 and 24,531,964 shares issued and outstanding at December 31, 2017 and 2016, respectively
|
35
|
|
25
|
|
|||
|
Additional paid-in capital
|
170,330
|
|
161,963
|
|
|||
|
Accumulated deficit
|
(152,331
|
)
|
(123,311
|
)
|
|||
|
Total stockholders’ equity
|
18,034
|
|
38,677
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
75,097
|
|
$
|
104,097
|
|
|
|
Year Ended December 31,
|
|||||||||||
|
2017
|
2016
|
2015
|
|||||||||
|
Revenue:
|
|||||||||||
|
Collaboration revenue
|
$
|
—
|
|
$
|
406
|
|
$
|
490
|
|
||
|
License revenue
|
425
|
|
29,575
|
|
500
|
|
|||||
|
Total revenue
|
425
|
|
29,981
|
|
990
|
|
|||||
|
Operating expenses:
|
|||||||||||
|
Research and development
|
12,510
|
|
13,479
|
|
26,336
|
|
|||||
|
General and administrative
|
8,070
|
|
14,736
|
|
9,850
|
|
|||||
|
Loss (gain) from change in fair value of contingent consideration
|
9,100
|
|
(1,100
|
)
|
—
|
|
|||||
|
Total operating expenses
|
29,680
|
|
27,115
|
|
36,186
|
|
|||||
|
(Loss) income from operations
|
(29,255
|
)
|
2,866
|
|
(35,196
|
)
|
|||||
|
Other income (expense):
|
|||||||||||
|
Other income (expense), net
|
226
|
|
(723
|
)
|
3,139
|
|
|||||
|
Interest expense
|
—
|
|
(247
|
)
|
(1,395
|
)
|
|||||
|
Total other income (expense), net
|
226
|
|
(970
|
)
|
1,744
|
|
|||||
|
Net (loss) income before income taxes
|
(29,029
|
)
|
1,896
|
|
(33,452
|
)
|
|||||
|
Provision for income taxes
|
—
|
|
5
|
|
—
|
|
|||||
|
Net (loss) income and comprehensive (loss) income
|
$
|
(29,029
|
)
|
$
|
1,891
|
|
$
|
(33,452
|
)
|
||
|
Net (loss) income per share applicable to common stockholders—basic
|
$
|
(1.11
|
)
|
$
|
0.09
|
|
$
|
(1.76
|
)
|
||
|
Weighted-average number of common shares used in net (loss) income per share applicable to common stockholders—basic
|
26,105
|
|
21,083
|
|
18,993
|
|
|||||
|
Net (loss) income per share applicable to common stockholders-diluted
|
$
|
(1.11
|
)
|
$
|
0.09
|
|
$
|
(1.76
|
)
|
||
|
Weighted-average number of common shares used in net (loss) income per share applicable to common stockholders—diluted
|
26,105
|
|
21,733
|
|
18,993
|
|
|||||
|
|
Common Stock
|
Additional
Paid-in
Capital
|
Accumulated
Deficit
|
Stockholders’
Equity
|
||||||||||||||
|
|
Shares
|
Amount
|
||||||||||||||||
|
(in thousands, except share data)
|
||||||||||||||||||
|
Balance at December 31, 2014
|
17,933,260
|
|
$
|
18
|
|
$
|
128,558
|
|
$
|
(91,750
|
)
|
$
|
36,826
|
|
||||
|
Issuance of common stock, net of issuance costs of $819
|
1,446,781
|
|
2
|
|
12,648
|
|
—
|
|
12,650
|
|
||||||||
|
Exercise of stock options and vesting of restricted stock awards
|
239,083
|
|
—
|
|
63
|
|
—
|
|
63
|
|
||||||||
|
Issuance of common stock warrants in connection with notes payable
|
—
|
|
—
|
|
328
|
|
—
|
|
328
|
|
||||||||
|
Stock-based compensation expense
|
—
|
|
—
|
|
2,529
|
|
—
|
|
2,529
|
|
||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
(33,452
|
)
|
(33,452
|
)
|
||||||||
|
Balance at December 31, 2015
|
19,619,124
|
|
20
|
|
144,126
|
|
(125,202
|
)
|
18,944
|
|
||||||||
|
Exercise of stock options and vesting of restricted stock awards
|
810,538
|
|
1
|
|
268
|
|
—
|
|
269
|
|
||||||||
|
Issuance of common stock pursuant to the ESPP
|
88,871
|
|
—
|
|
35
|
|
—
|
|
35
|
|
||||||||
|
Issuance of common stock in connection with the acquisition of Viventia
|
4,013,431
|
|
4
|
|
13,521
|
|
—
|
|
13,525
|
|
||||||||
|
Stock-based compensation expense
|
—
|
|
—
|
|
4,013
|
|
—
|
|
4,013
|
|
||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
1,891
|
|
1,891
|
|
||||||||
|
Balance at December 31, 2016
|
24,531,964
|
|
25
|
|
161,963
|
|
(123,311
|
)
|
38,677
|
|
||||||||
|
Cumulative effect of adoption of ASU 2016-09
|
(9
|
)
|
9
|
|
—
|
|
||||||||||||
|
Exercise of stock options and vesting of restricted stock awards
|
161,453
|
|
—
|
|
40
|
|
—
|
|
40
|
|
||||||||
|
Issuance of common stock pursuant to the ESPP
|
9,148
|
|
—
|
|
12
|
|
—
|
|
12
|
|
||||||||
|
Issuance of common stock and common stock warrants, net of issuance costs of $1 million
|
5,525,000
|
|
6
|
|
6,902
|
|
—
|
|
6,908
|
|
||||||||
|
Exercise of pre-funded common stock warrants
|
4,475,000
|
|
|
4
|
|
|
41
|
|
|
—
|
|
|
45
|
|
||||
|
Stock-based compensation expense
|
—
|
|
—
|
|
1,381
|
|
|
|
1,381
|
|
||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
(29,029
|
)
|
(29,029
|
)
|
||||||||
|
Balance at December 31, 2017
|
34,702,565
|
|
$
|
35
|
|
$
|
170,330
|
|
$
|
(152,331
|
)
|
$
|
18,034
|
|
||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Operating activities
|
|||||||||||
|
Net (loss) income
|
$
|
(29,029
|
)
|
$
|
1,891
|
|
$
|
(33,452
|
)
|
||
|
Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activities:
|
|||||||||||
|
Depreciation and amortization
|
285
|
|
178
|
|
366
|
|
|||||
|
Non-cash interest expense
|
—
|
|
26
|
|
108
|
|
|||||
|
Stock-based compensation expense
|
1,381
|
|
4,013
|
|
2,529
|
|
|||||
|
Change in fair value of warrant liability
|
(5
|
)
|
(110
|
)
|
(3,104
|
)
|
|||||
|
Loss (gain) from change in fair value of contingent consideration
|
9,100
|
|
(1,100
|
)
|
—
|
|
|||||
|
Loss on extinguishment of debt
|
—
|
|
221
|
|
—
|
|
|||||
|
Gain on sale of equipment
|
(108
|
)
|
(24
|
)
|
—
|
|
|||||
|
Changes in operating assets and liabilities:
|
|||||||||||
|
Prepaid expenses and other assets
|
164
|
|
800
|
|
110
|
|
|||||
|
Restricted cash
|
—
|
|
84
|
|
—
|
|
|||||
|
Accounts payable
|
(760
|
)
|
(742
|
)
|
(1,212
|
)
|
|||||
|
Accrued expenses and other liabilities
|
1,746
|
|
(1,936
|
)
|
226
|
|
|||||
|
Deferred revenue
|
(425
|
)
|
19
|
|
(100
|
)
|
|||||
|
Due to related party
|
(114
|
)
|
(698
|
)
|
—
|
|
|||||
|
Net cash (used in) provided by operating activities
|
(17,765
|
)
|
2,622
|
|
(34,529
|
)
|
|||||
|
Investing activities
|
|||||||||||
|
Cash acquired in acquisition
|
—
|
|
136
|
|
—
|
|
|||||
|
Net sales (purchases) of property and equipment
|
98
|
|
325
|
|
(287
|
)
|
|||||
|
Net cash provided by (used in) investing activities
|
98
|
|
461
|
|
(287
|
)
|
|||||
|
Financing activities
|
|||||||||||
|
Proceeds from issuance of notes payable, net of debt issuance costs
|
—
|
|
—
|
|
5,000
|
|
|||||
|
Payments on equipment financing and notes payable
|
—
|
|
(14,124
|
)
|
(877
|
)
|
|||||
|
Proceeds from issuance of common stock and common stock warrants, net of issuance costs
|
6,908
|
|
—
|
|
12,650
|
|
|||||
|
Proceeds from exercise of common stock options and common stock warrants
|
85
|
|
269
|
|
63
|
|
|||||
|
Proceeds from sale of common stock pursuant to ESPP
|
12
|
|
35
|
|
—
|
|
|||||
|
Net cash provided by (used in) financing activities
|
7,005
|
|
(13,820
|
)
|
16,836
|
|
|||||
|
Net decrease in cash and cash equivalents
|
(10,662
|
)
|
(10,737
|
)
|
(17,980
|
)
|
|||||
|
Cash and cash equivalents at beginning of period
|
25,342
|
|
36,079
|
|
54,059
|
|
|||||
|
Cash and cash equivalents at end of period
|
$
|
14,680
|
|
$
|
25,342
|
|
$
|
36,079
|
|
||
|
Supplemental non-cash investing and financing activities
|
|||||||||||
|
Common stock issued in connection with the acquisition (Note 3)
|
—
|
|
13,525
|
|
—
|
|
|||||
|
Fair value of assets acquired and liabilities assumed in the acquisition (Note 3):
|
|||||||||||
|
Fair value of assets acquired in the acquisition, excluding cash
|
$
|
—
|
|
$
|
79,366
|
|
$
|
—
|
|
||
|
Fair value of liabilities assumed in the acquisition
|
$
|
—
|
|
$
|
19,777
|
|
$
|
—
|
|
||
|
Adjustment to fair value of assets acquired and liabilities assumed during provisional period (Note 3)
|
$
|
14,600
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Issuance of warrants to purchase common stock
|
$
|
2,679
|
|
$
|
—
|
|
$
|
328
|
|
||
|
Supplemental cash flow information
|
|||||||||||
|
Cash paid for interest
|
$
|
—
|
|
$
|
663
|
|
$
|
930
|
|
||
|
•
|
the delivered item or items have stand-alone value to the customer; and
|
|
•
|
delivery or performance of the undelivered item(s) is considered probable and substantially in the control of the Company, and the arrangement includes a general right of return relative to the delivered item(s).
|
|
•
|
it can only be achieved based in whole or in part on either the Company’s performance or the occurrence of a specific outcome resulting from the Company’s performance;
|
|
•
|
there is substantive uncertainty at the date an arrangement is entered into that the event will be achieved; and
|
|
•
|
it would result in additional payments being due to the Company.
|
|
Year Ended December 31,
|
|||||||||||
|
2017
|
2016
|
2015
|
|||||||||
|
Research and development expense
|
$
|
404
|
|
$
|
1,455
|
|
$
|
1,032
|
|
||
|
General and administrative expense
|
977
|
|
2,558
|
|
1,497
|
|
|||||
|
$
|
1,381
|
|
$
|
4,013
|
|
$
|
2,529
|
|
|||
|
Description
|
December 31, 2017
|
Active
Markets
(Level 1)
|
Observable
Inputs
(Level 2)
|
Unobservable
Inputs
(Level 3)
|
|||||||||||
|
Assets:
|
|||||||||||||||
|
Cash and cash equivalents
|
$
|
14,680
|
|
$
|
14,680
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Restricted cash
|
10
|
|
10
|
|
—
|
|
—
|
|
|||||||
|
Total assets
|
$
|
14,690
|
|
$
|
14,690
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Contingent consideration
|
39,600
|
|
—
|
|
—
|
|
39,600
|
|
|||||||
|
Total liabilities
|
$
|
39,600
|
|
$
|
—
|
|
$
|
—
|
|
$
|
39,600
|
|
|||
|
Description
|
December 31, 2016
|
Active
Markets
(Level 1)
|
Observable
Inputs
(Level 2)
|
Unobservable
Inputs
(Level 3)
|
|||||||||||
|
Assets:
|
|||||||||||||||
|
Cash and cash equivalents
|
$
|
25,342
|
|
$
|
25,342
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Restricted cash
|
10
|
|
10
|
|
—
|
|
—
|
|
|||||||
|
Total assets
|
$
|
25,352
|
|
$
|
25,352
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|||||||||||||||
|
Warrant liability
|
$
|
5
|
|
$
|
—
|
|
$
|
—
|
|
$
|
5
|
|
|||
|
Contingent consideration
|
45,100
|
|
—
|
|
—
|
|
45,100
|
|
|||||||
|
Total liabilities
|
$
|
45,105
|
|
$
|
—
|
|
$
|
—
|
|
$
|
45,105
|
|
|||
|
Beginning balance, January 1, 2017
|
$
|
5
|
|
|
Change in fair value of common stock warrants included in other income (expense)
|
(5
|
)
|
|
|
Ending balance, December 31, 2017
|
$
|
—
|
|
|
Beginning balance, January 1, 2017
|
$
|
45,100
|
|
|
Provisional purchase price accounting adjustment (Note 3)
|
(14,600
|
)
|
|
|
Loss from change in fair value of contingent consideration
|
9,100
|
|
|
|
Ending balance, December 31, 2017
|
$
|
39,600
|
|
|
Year ended December 31,
|
||||||||
|
|
2017
|
2016
|
2015
|
|||||
|
Stock options
|
—
|
|
650,109
|
|
—
|
|
||
|
—
|
|
650,109
|
|
|
—
|
|
||
|
Year ended December 31,
|
||||||||
|
2017
|
2016
|
2015
|
||||||
|
Stock options
|
2,695,796
|
|
1,374,359
|
|
1,803,574
|
|
||
|
Unvested restricted stock
|
4,430
|
|
22,150
|
|
41,657
|
|
||
|
Restricted stock units
|
—
|
|
3,333
|
|
150,932
|
|
||
|
Common stock warrants
|
10,055,000
|
|
926,840
|
|
926,840
|
|
||
|
12,755,226
|
|
2,326,682
|
|
|
2,923,003
|
|
||
|
Preliminary Fair Value of Consideration as of December 31, 2016
|
Adjustment
|
Final Fair Value of Consideration
|
|||||||||
|
Shares Issued
|
$
|
13,525
|
|
$
|
—
|
|
$
|
13,525
|
|
||
|
Contingent Consideration
|
46,200
|
|
(14,600
|
)
|
31,600
|
|
|||||
|
$
|
59,725
|
|
$
|
(14,600
|
)
|
$
|
45,125
|
|
|||
|
Preliminary Allocation as of December 31, 2016
|
Adjustment
|
Final Allocation
|
|||||||||
|
Cash and cash equivalents
|
$
|
136
|
|
$
|
—
|
|
$
|
136
|
|
||
|
Prepaid expenses and other assets
|
1,162
|
|
—
|
|
1,162
|
|
|||||
|
Property and equipment
|
867
|
|
—
|
|
867
|
|
|||||
|
In-process research and development assets (all markets)
|
60,500
|
|
(14,100
|
)
|
46,400
|
|
|||||
|
Goodwill
|
16,864
|
|
(3,800
|
)
|
13,064
|
|
|||||
|
Accounts payable
|
(1,163
|
)
|
—
|
|
(1,163
|
)
|
|||||
|
Accrued expenses
|
(1,494
|
)
|
(507
|
)
|
(2,001
|
)
|
|||||
|
Other liabilities
|
(812
|
)
|
—
|
|
(812
|
)
|
|||||
|
Deferred tax liability
|
(16,335
|
)
|
3,807
|
|
(12,528
|
)
|
|||||
|
$
|
59,725
|
|
$
|
(14,600
|
)
|
$
|
45,125
|
|
|||
|
Year Ended December 31,
|
|||||||
|
|
2016
|
2015
|
|||||
|
Revenue
|
$
|
29,981
|
|
$
|
990
|
|
|
|
Net loss
|
(3,026
|
)
|
(47,483
|
)
|
|||
|
Estimated Useful
Life (Years)
|
December 31,
|
||||||||
|
2017
|
2016
|
||||||||
|
Lab equipment
|
5
|
$
|
443
|
|
$
|
457
|
|
||
|
Furniture and fixtures
|
4
|
16
|
|
16
|
|
||||
|
Computer equipment
|
3
|
73
|
|
73
|
|
||||
|
Software
|
3
|
28
|
|
28
|
|
||||
|
Leasehold improvements
|
Lesser of useful life
or remaining lease term |
293
|
|
293
|
|
||||
|
853
|
|
867
|
|
||||||
|
Less accumulated depreciation and amortization
|
(331
|
)
|
(71
|
)
|
|||||
|
Total property and equipment, net
|
$
|
522
|
|
$
|
796
|
|
|||
|
|
December 31,
|
||||||
|
|
2017
|
2016
|
|||||
|
Development costs
|
$
|
2,581
|
|
$
|
852
|
|
|
|
Employee compensation
|
735
|
|
352
|
|
|||
|
Professional fees
|
463
|
|
413
|
|
|||
|
Other
|
34
|
|
|
157
|
|
||
|
$
|
3,813
|
|
$
|
1,774
|
|
||
|
2018
|
$
|
428,806
|
|
|
2019
|
336,000
|
|
|
|
2020
|
252,000
|
|
|
|
$
|
1,016,806
|
|
|
|
|
As of December 31,
|
||||
|
|
2017
|
2016
|
|||
|
Unvested restricted stock
|
4,430
|
|
22,150
|
|
|
|
Restricted stock units
|
—
|
|
3,333
|
|
|
|
Options to purchase common stock
|
3,879,535
|
|
3,112,771
|
|
|
|
Warrants to purchase common stock
|
10,055,000
|
|
926,840
|
|
|
|
Employee stock purchase plan
|
59,461
|
|
68,609
|
|
|
|
13,998,426
|
|
|
4,133,703
|
|
|
|
December 31,
2016
|
|||
|
Risk-free interest rate
|
0.85
|
%
|
|
|
Expected dividend yield
|
—
|
%
|
|
|
Expected term (in years)
|
0.92
|
|
|
|
Expected volatility
|
83.39
|
%
|
|
|
Shares
|
Weighted-Average
Exercise Price
|
Remaining
Contractual Life
(in years)
|
Aggregate
Intrinsic
Value
(in thousands)
|
||||||||
|
Outstanding at December 31, 2016
|
2,024,468
|
|
$
|
4.41
|
|
8.73
|
$
|
841
|
|
||
|
Granted
|
1,303,025
|
|
1.64
|
|
|||||||
|
Exercised
|
(140,400
|
)
|
0.28
|
|
|||||||
|
Cancelled or forfeited
|
(491,297
|
)
|
5.10
|
|
|||||||
|
Outstanding at December 31, 2017
|
2,695,796
|
|
$
|
3.16
|
|
8.55
|
$
|
146
|
|
||
|
Exercisable at December 31, 2017
|
1,267,285
|
|
$
|
4.35
|
|
7.56
|
$
|
146
|
|
||
|
Vested and expected to vest at December 31, 2017 (1)
|
2,275,796
|
|
$
|
3.45
|
|
8.33
|
$
|
146
|
|
||
|
Restricted
Stock
|
Weighted-Average
Grant Date
Fair Value
|
|||||
|
Unvested at December 31, 2016
|
22,150
|
|
$
|
11.43
|
|
|
|
Vested
|
(17,720
|
)
|
11.43
|
|
||
|
Unvested at December 31, 2017
|
4,430
|
|
$
|
11.43
|
|
|
|
Restricted
Stock Units
|
Weighted-Average
Grant Date
Fair Value
|
|||||
|
Unvested at December 31, 2016
|
3,333
|
|
$
|
4.09
|
|
|
|
Vested
|
(3,333
|
)
|
4.09
|
|
||
|
Unvested at December 31, 2017
|
—
|
|
$
|
—
|
|
|
|
Year Ended December 31,
|
|||||
|
2017
|
2016
|
2015
|
|||
|
Risk-free interest rate
|
1.88-2.04%
|
1.23-2.38%
|
1.42-1.92%
|
||
|
Expected dividend yield
|
—%
|
—%
|
—%
|
||
|
Expected term (in years)
|
5.3-6
|
5.5-6
|
5.75-6
|
||
|
Expected volatility
|
75.4-86.66%
|
71.44-73.42%
|
69.06-74.11%
|
||
|
Year Ended December 31,
|
|||||
|
2017
|
2016
|
2015
|
|||
|
Risk-free interest rate
|
1.50-2.32%
|
1.08-2.38%
|
1.19-2.26%
|
||
|
Expected dividend yield
|
—%
|
—%
|
—%
|
||
|
Expected option life (years)
|
10
|
10
|
10
|
||
|
Expected stock price volatility
|
74.4-84.31%
|
69.92-92.09%
|
67.24-92.40%
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Pre-tax income (loss):
|
|||||||||||
|
U.S.
|
$
|
(9,246
|
)
|
$
|
3,981
|
|
$
|
(33,452
|
)
|
||
|
Canada
|
(19,783
|
)
|
(2,085
|
)
|
—
|
|
|||||
|
Total pre-tax income (loss)
|
$
|
(29,029
|
)
|
$
|
1,896
|
|
$
|
(33,452
|
)
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2017
|
2016
|
2015
|
||||||||
|
Current tax provision:
|
|||||||||||
|
Federal
|
$
|
—
|
|
$
|
2
|
|
$
|
—
|
|
||
|
State
|
—
|
|
—
|
|
—
|
|
|||||
|
Foreign
|
—
|
|
—
|
|
—
|
|
|||||
|
Total current provision
|
—
|
|
2
|
|
—
|
|
|||||
|
Deferred tax provision:
|
|||||||||||
|
Federal
|
—
|
|
3
|
|
—
|
|
|||||
|
State
|
—
|
|
—
|
|
—
|
|
|||||
|
Foreign
|
—
|
|
—
|
|
—
|
|
|||||
|
Total deferred provision
|
—
|
|
3
|
|
—
|
|
|||||
|
Total tax provision
|
$
|
—
|
|
$
|
5
|
|
$
|
—
|
|
||
|
|
Year Ended December 31,
|
|||||||
|
|
2017
|
2016
|
2015
|
|||||
|
Income tax benefit computed at federal statutory tax rate
|
34.0
|
%
|
34.0
|
%
|
34.0
|
%
|
||
|
Impact of foreign rate differential
|
(2.6
|
)
|
7.7
|
|
—
|
|
||
|
State taxes, net of federal benefit
|
1.4
|
|
18.8
|
|
5.6
|
|
||
|
Net operating loss write off
|
—
|
|
14.4
|
|
—
|
|
||
|
Stock option cancellations
|
(0.8
|
)
|
49.6
|
|
—
|
|
||
|
Transaction costs
|
—
|
|
33.6
|
|
—
|
|
||
|
Contingent consideration
|
(10.7
|
)
|
(15.7
|
)
|
—
|
|
||
|
General business credits and other credits
|
0.8
|
|
(25.0
|
)
|
1.8
|
|
||
|
Permanent differences
|
0.3
|
|
5.3
|
|
2.4
|
|
||
|
Change in valuation allowance
|
28.2
|
|
(122.4
|
)
|
(43.8
|
)
|
||
|
Federal statutory rate change
|
(50.6
|
)
|
—
|
|
—
|
|
||
|
Total
|
—
|
%
|
0.3
|
%
|
—
|
%
|
||
|
December 31,
|
|||||||
|
2017
|
2016
|
||||||
|
Deferred tax assets:
|
|||||||
|
Net operating loss carryforwards
|
$
|
37,070
|
|
$
|
45,488
|
|
|
|
Research and development credit carryforwards
|
3,690
|
|
3,355
|
|
|||
|
Accruals and other
|
2,263
|
|
2,079
|
|
|||
|
Capitalized license and organization costs
|
—
|
|
61
|
|
|||
|
Capitalized start-up costs
|
150
|
|
246
|
|
|||
|
Other
|
38
|
|
—
|
|
|||
|
Total gross deferred tax asset
|
43,211
|
|
51,229
|
|
|||
|
Deferred tax liabilities:
|
|||||||
|
IPR&D
|
(12,528
|
)
|
(16,335
|
)
|
|||
|
Property and equipment
|
(107
|
)
|
(189
|
)
|
|||
|
Total gross deferred tax liabilities
|
(12,635
|
)
|
(16,524
|
)
|
|||
|
Valuation allowance
|
(43,104
|
)
|
(51,040
|
)
|
|||
|
Net deferred tax liability
|
$
|
(12,528
|
)
|
$
|
(16,335
|
)
|
|
|
Balance as of January 1, 2017
|
$
|
31
|
|
|
|
Charges
|
170
|
|
||
|
Payments
|
(90
|
)
|
||
|
Balance as of December 31, 2017
|
$
|
111
|
|
|
|
2017
|
|||||||||||||||||||
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
Total
|
|||||||||||||||
|
(in thousands, except per share data)
|
|||||||||||||||||||
|
Total revenue
|
$
|
425
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
425
|
|
||||
|
Total operating expenses
|
6,587
|
|
7,350
|
|
9,150
|
|
$
|
6,593
|
|
29,680
|
|
||||||||
|
Loss from operations
|
(6,162
|
)
|
(7,350
|
)
|
(9,150
|
)
|
$
|
(6,593
|
)
|
(29,255
|
)
|
||||||||
|
Net loss
|
(6,061
|
)
|
(7,316
|
)
|
(9,105
|
)
|
$
|
(6,547
|
)
|
$
|
(29,029
|
)
|
|||||||
|
Net loss per share—basic and diluted
|
$
|
(0.25
|
)
|
$
|
(0.30
|
)
|
$
|
(0.37
|
)
|
$
|
(0.22
|
)
|
$
|
(1.11
|
)
|
||||
|
2016
|
|||||||||||||||||||
|
First
Quarter
|
Second
Quarter
|
Third
Quarter *
|
Fourth
Quarter
|
Total
|
|||||||||||||||
|
(in thousands, except per share data)
|
|||||||||||||||||||
|
Total revenue
|
$
|
229
|
|
$
|
277
|
|
$
|
28,650
|
|
$
|
825
|
|
$
|
29,981
|
|
||||
|
Total operating expenses
|
6,779
|
|
6,769
|
|
9,120
|
|
4,447
|
|
27,115
|
|
|||||||||
|
Income (loss) from operations
|
(6,550
|
)
|
(6,492
|
)
|
19,530
|
|
(3,622
|
)
|
2,866
|
|
|||||||||
|
Net income (loss)
|
(7,574
|
)
|
(6,491
|
)
|
19,487
|
|
(3,531
|
)
|
1,891
|
|
|||||||||
|
Net income (loss) per share—basic
|
$
|
(0.39
|
)
|
$
|
(0.33
|
)
|
$
|
0.95
|
|
$
|
(0.15
|
)
|
$
|
0.09
|
|
||||
|
Net income (loss) per share—diluted
|
$
|
(0.39
|
)
|
$
|
(0.33
|
)
|
$
|
0.91
|
|
$
|
(0.15
|
)
|
$
|
0.09
|
|
||||