|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ý
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
|
71-0872999
|
|
(State or other Jurisdiction of
Incorporation or Organization)
|
|
(I.R.S. Employer
Identification No.)
|
|
200 Penobscot Drive,
Redwood City, California
|
|
94063
|
|
(Address of Principal Executive Offices)
|
|
(Zip Code)
|
|
Title of Each Class:
|
|
Name of Each Exchange on which Registered:
|
|
Common Stock, par value $0.0001 per share
|
|
The NASDAQ Global Select Market
|
|
Large accelerated filer
|
¨
|
Accelerated filer
|
ý
|
|
|
Non-accelerated filer
|
¨
|
Smaller reporting company
|
¨
|
|
|
(Do not check if a smaller reporting company)
|
||||
|
PART I
|
|||
|
Item 1
|
|||
|
Item 1A
|
|||
|
Item 1B
|
|||
|
Item 2
|
|||
|
Item 3
|
|||
|
Item 4
|
|||
|
PART II
|
|||
|
Item 5
|
|||
|
Item 6
|
|||
|
Item 7
|
|||
|
Item 7A
|
|||
|
Item 8
|
|||
|
Item 9
|
|||
|
Item 9A
|
|||
|
Item 9B
|
|||
|
PART III
|
|||
|
Item 10
|
|||
|
Item 11
|
|||
|
Item 12
|
|||
|
Item 13
|
|||
|
Item 14
|
|||
|
PART IV
|
|||
|
Item 15
|
|||
|
•
|
Licensing our CodeEvolver
®
protein engineering technology platform.
We intend to continue to pursue opportunities to license our CodeEvolver
®
protein engineering technology platform to third parties so they can create cost-saving protein catalyst solutions utilizing their own in-house protein engineering capability.
|
|
•
|
Growing our pharmaceutical protein catalysts business.
We intend to continue to pursue opportunities in the pharmaceutical market to use our protein catalysis products and services to reduce the costs for manufacturing small molecule drugs. We intend to increase the number of pharmaceutical customers and processes that utilize and benefit from our novel, cost-saving protein catalyst solutions.
|
|
•
|
Growing our fine chemicals protein catalysts business.
We intend to continue to pursue opportunities in the fine chemicals market to use protein catalysis products and services to reduce the costs for manufacturing in adjacent markets like food and food ingredients. We intend to increase the number of fine chemical customers and processes who utilize and benefit from our novel, cost-saving protein catalyst solutions.
|
|
•
|
Creating and advancing novel biotherapeutic drug candidates.
We intend to continue to pursue opportunities to apply our protein engineering capabilities to the creation and development of novel biotherapeutic drug candidates, both in partnership with customers and as proprietary Codexis drug candidates. We intend to continue to advance our own novel enzyme biotherapeutic candidate for the potential treatment of phenylketonuria ("PKU") disease. We have also invested in research and development in an effort to generate additional early stage novel biotherapeutic candidates.
|
|
•
|
Developing high-performance enzymes for use in diagnostic applications
. We intend to offer high-performance enzymes to customers using next generation sequencing (“NGS”) and polymerase chain reaction (“PCR/qPCR”) for
in vitro
molecular diagnosis applications.
|
|
|
Percentage of Total Revenues
For The Years Ended December 31,
|
|||||||
|
|
2016
|
2015
|
2014
|
|||||
|
Customers:
|
||||||||
|
Merck
|
47
|
%
|
29
|
%
|
24
|
%
|
||
|
GSK
|
22
|
%
|
20
|
%
|
17
|
%
|
||
|
Exela
|
*
|
|
12
|
%
|
21
|
%
|
||
|
•
|
our ability to achieve or maintain profitability;
|
|
•
|
our relationships with, and dependence on, collaborators in our principal markets;
|
|
•
|
our dependence on a limited number of customers;
|
|
•
|
our dependence on a limited number of products in our biocatalysis business;
|
|
•
|
our reliance on a limited number of contract manufacturers for large scale production of substantially all of our enzyme products;
|
|
•
|
our ability to develop and successfully commercialize new products for the biocatalysis market(s);
|
|
•
|
our ability to deploy our technology platform in the fine chemicals market;
|
|
•
|
the success of our customers’ pharmaceutical products in the market and the ability of such customers to obtain regulatory approvals for products and processes;
|
|
•
|
our ability to deploy our technology platform in the
in vitro
molecular diagnostics market;
|
|
•
|
our ability to compete if we do not adequately protect our proprietary technologies or if we lose some of our intellectual property rights;
|
|
•
|
our ability to avoid infringing the intellectual property rights of third parties;
|
|
•
|
our involvement in lawsuits to protect or enforce our patents or other intellectual property rights;
|
|
•
|
our ability to enforce our intellectual property rights throughout the world;
|
|
•
|
our dependence on, and the need to attract and retain, key management and other personnel;
|
|
•
|
our ability to prevent the theft or misappropriation of our biocatalysts, the genes that code for our biocatalysts, know-how or technologies;
|
|
•
|
our ability to protect our trade secrets and other proprietary information from disclosure by employees and others;
|
|
•
|
our ability to obtain substantial additional capital that may be necessary to expand our business;
|
|
•
|
our ability to find a partner for or otherwise advance our biotherapeutic program;
|
|
•
|
our customers’ ability to pay amounts owed to us in a timely manner;
|
|
•
|
our ability to avoid charges to earnings as a result of any impairment of goodwill, intangible assets or other long-lived assets;
|
|
•
|
our ability to implement and maintain effective internal control over financial reporting;
|
|
•
|
our dependency on information technology systems, infrastructure and data;
|
|
•
|
our ability to control and to improve product gross margins;
|
|
•
|
our ability to protect against risks associated with the international aspects of our business;
|
|
•
|
the cost of compliance with European Union chemical regulations;
|
|
•
|
our or our customers’ ability to obtain regulatory approval for the sale and manufacturing of food products using our enzymes;
|
|
•
|
potential advantages that our competitors and potential competitors may have in securing funding or developing products;
|
|
•
|
our ability to accurately report our financial results in a timely manner;
|
|
•
|
results of regulatory tax examinations;
|
|
•
|
business interruptions, such as earthquakes and other natural disasters;
|
|
•
|
public concerns about the ethical, legal and social ramifications of genetically engineered products and processes;
|
|
•
|
our ability to integrate our current business with any businesses that we may acquire in the future;
|
|
•
|
our ability to properly handle and dispose of hazardous materials in our business;
|
|
•
|
potential product liability claims; and
|
|
•
|
our ability to use our net operating loss carryforwards to offset future taxable income.
|
|
•
|
we do not achieve our research and development objectives under our collaboration agreements in a timely manner or at all;
|
|
•
|
we develop products and processes or enter into additional collaborations that conflict with the business objectives of our other collaborators;
|
|
•
|
our collaborators and/or our contract manufacturers do not receive the required regulatory and other approvals necessary for the commercialization of the applicable product;
|
|
•
|
we disagree with our collaborators as to rights to intellectual property that are developed during the collaboration, or their research programs or commercialization activities;
|
|
•
|
we are unable to manage multiple simultaneous collaborations;
|
|
•
|
our collaborators or licensees are unable or unwilling to implement or use the technology or products that we provide or license to them;
|
|
•
|
our collaborators become competitors of ours or enter into agreements with our competitors;
|
|
•
|
our collaborators become unable or less willing to expend their resources on research and development or commercialization efforts due to general market conditions, their financial condition or other circumstances beyond our control; or
|
|
•
|
our collaborators experience business difficulties, which could eliminate or impair their ability to effectively perform under our agreements.
|
|
•
|
customers in these markets may be reluctant to adopt new manufacturing processes that use our enzymes;
|
|
•
|
we may be unable to successfully develop the enzymes or manufacturing processes for our products in a timely and cost-effective manner, if at all;
|
|
•
|
we may face difficulties in transferring the developed technologies to our customers and the contract manufacturers that we may use for commercial scale production of intermediates and enzymes in these markets;
|
|
•
|
the contract manufacturers that we may use may be unable to scale their manufacturing operations to meet the demand for these products and we may be unable to secure additional manufacturing capacity;
|
|
•
|
customers may not be willing to purchase these products for these markets from us on favorable terms, if at all;
|
|
•
|
we may face product liability litigation, unexpected safety or efficacy concerns and product recalls or withdrawals;
|
|
•
|
changes in laws or regulations relating to the pharmaceutical industry or the industries into which we sell our fine chemicals products, including the food industry, could cause us to incur increased costs of compliance or otherwise harm our business;
|
|
•
|
our customers’ products may experience adverse events or face competition from new products, which would reduce demand for our products;
|
|
•
|
we may face pressure from existing or new competitive products; and
|
|
•
|
we may face pricing pressures from existing or new competitors, some of which may benefit from government subsidies or other incentives.
|
|
•
|
stop selling or using our products or technologies that use the subject intellectual property;
|
|
•
|
pay monetary damages or substantial royalties;
|
|
•
|
grant cross-licenses to third parties relating to our patents or proprietary rights;
|
|
•
|
obtain from the third party asserting its intellectual property rights a license to sell or use the relevant technology, which license may not be available on reasonable terms, or at all; or
|
|
•
|
redesign those products or processes that use any allegedly infringing technology, or relocate the operations relating to the allegedly infringing technology to another jurisdiction, which may result in significant cost or delay to us, could be technically infeasible or could prevent us from selling some of our products in the United States or other jurisdictions.
|
|
•
|
Our efforts to use CodeEvolver
®
protein engineering technology platform
to generate new lead biotherapeutic candidates may not be successful in creating candidates of value.
|
|
•
|
The successful development of biotherapeutic candidates is full of risk and uncertainty, requires long timelines and may lead to uncertain results, is highly regulated and may require expertise and capital resources we do not currently possess.
|
|
•
|
If we are not successful in obtaining a partner to assist us with the funding and development of our PKU program, we may not have sufficient funds or expertise to advance development of the program on our own.
|
|
•
|
To obtain regulatory approval to market our product candidate, preclinical studies and costly and lengthy clinical trials are required, and the results of the studies and trials are highly uncertain. A failure of one or more pre-clinical or clinical trials can occur at any stage, and many companies that have believed their drug candidates performed satisfactorily in pre-clinical and clinical testing have nonetheless failed to obtain marketing approval of their product candidates.
|
|
•
|
We do not have experience in drug development or regulatory matters related to drug development. As a result, we rely or will rely on third parties to conduct our pre-clinical studies, assist us with drug manufacturing and formulation and perform other tasks for us. If these third parties do not successfully carry out their responsibilities or comply with regulatory requirements, we may receive lower quality products or services, suffer reputational harm and not be able to obtain regulatory approval for our product candidate.
|
|
•
|
The results of animal studies of our product candidate may not be predictive of future study results.
|
|
•
|
If we begin clinical trials for our product candidate, we may find it difficult to enroll patients in our clinical trials given the limited number of patients that have PKU. Any enrollment difficulties could delay clinical trials and any potential product approval.
|
|
•
|
Drug development is a highly regulated process. In particular, the regulatory approval process of the FDA and comparable foreign authorities is lengthy, time consuming and inherently unpredictable. If we are ultimately unable to obtain regulatory approval for our product candidate, our business will be harmed.
|
|
•
|
We will be exposed to potential product liability risks through the testing of experimental therapeutics in humans, which may expose us to substantial uninsured liabilities.
|
|
•
|
Third parties may develop intellectual property that could limit our ability to develop, market and commercialize our PKU product candidate, if approved.
|
|
•
|
Changes in methods of treatment of disease, such as gene therapy, could cause us to stop development of our product candidate or reduce or eliminate potential demand for our product candidate, if approved.
|
|
•
|
changes in or interpretations of foreign regulations that may adversely affect our ability to sell our products, repatriate profits to the United States or operate our foreign-located facilities;
|
|
•
|
the imposition of tariffs;
|
|
•
|
the imposition of limitations on, or increase of, withholding and other taxes on remittances and other payments by foreign subsidiaries or joint ventures;
|
|
•
|
the imposition of limitations on genetically-engineered products or processes and the production or sale of those products or processes in foreign countries;
|
|
•
|
currency exchange rate fluctuations;
|
|
•
|
uncertainties relating to foreign laws, regulations and legal proceedings including tax, import/export, anti-corruption and exchange control laws;
|
|
•
|
the availability of government subsidies or other incentives that benefit competitors in their local markets that are not available to us;
|
|
•
|
increased demands on our limited resources created by our operations may constrain the capabilities of our administrative and operational resources and restrict our ability to attract, train, manage and retain qualified management, technicians, scientists and other personnel;
|
|
•
|
economic or political instability in foreign countries;
|
|
•
|
difficulties associated with staffing and managing foreign operations; and
|
|
•
|
the need to comply with a variety of United States and foreign laws applicable to the conduct of international business, including import and export control laws and anti-corruption laws.
|
|
•
|
public attitudes about the safety and environmental hazards of, and ethical concerns over, genetic research and genetically engineered products and processes, which could influence public acceptance of our technologies, products and processes;
|
|
•
|
public attitudes regarding, and potential changes to laws governing ownership of genetic material, which could harm our intellectual property rights with respect to our genetic material and discourage collaborators from supporting, developing, or commercializing our products, processes and technologies; and
|
|
•
|
governmental reaction to negative publicity concerning genetically modified organisms, which could result in greater government regulation of genetic research and derivative products. The subject of genetically modified organisms has received negative publicity, which has aroused public debate. This adverse publicity could lead to greater regulation and trade restrictions on imports of genetically altered products. The protein catalysts that we develop have significantly enhanced characteristics compared to those found in naturally occurring enzymes or microbes. While we produce our biocatalysts only for use in a controlled industrial environment, the release of such biocatalysts into uncontrolled environments could have unintended consequences. Any adverse effect resulting from such a release could have a material adverse effect on our business and financial condition, and we may have exposure to liability for any resulting harm.
|
|
•
|
issue additional equity securities, which would dilute our current stockholders;
|
|
•
|
incur substantial debt to fund the acquisitions;
|
|
•
|
use our cash to fund the acquisitions; or
|
|
•
|
assume significant liabilities including litigation risk.
|
|
•
|
actual or anticipated fluctuations in our financial condition and operating results;
|
|
•
|
the position of our cash, cash equivalents and marketable securities;
|
|
•
|
actual or anticipated changes in our growth rate relative to our competitors;
|
|
•
|
actual or anticipated fluctuations in our competitors’ operating results or changes in their growth rate;
|
|
•
|
announcements of technological innovations by us, our collaborators or our competitors;
|
|
•
|
announcements by us, our collaborators or our competitors of significant acquisitions or dispositions, strategic partnerships, joint ventures or capital commitments;
|
|
•
|
additions or losses of one or more significant pharmaceutical products;
|
|
•
|
announcements or developments regarding pharmaceutical products manufactured using our protein catalysts and intermediates;
|
|
•
|
the entry into, modification or termination of collaborative arrangements;
|
|
•
|
additions or losses of customers;
|
|
•
|
additions or departures of key management or scientific personnel;
|
|
•
|
competition from existing products or new products that may emerge;
|
|
•
|
issuance of new or updated research reports by securities or industry analysts;
|
|
•
|
fluctuations in the valuation of companies perceived by investors to be comparable to us;
|
|
•
|
disputes or other developments related to proprietary rights, including patent litigation and our ability to obtain patent protection for our technologies;
|
|
•
|
contractual disputes or litigation with our partners, customers or suppliers;
|
|
•
|
announcement or expectation of additional financing efforts;
|
|
•
|
sales of our common stock by us, our insiders or our other stockholders;
|
|
•
|
share price and volume fluctuations attributable to inconsistent trading volume levels of our shares;
|
|
•
|
general market conditions in our industry; and
|
|
•
|
general economic and market conditions, including the recent financial crisis.
|
|
Fiscal 2016
|
High
|
Low
|
|||||
|
First Quarter
|
$
|
4.50
|
|
$
|
2.93
|
|
|
|
Second Quarter
|
4.34
|
|
3.00
|
|
|||
|
Third Quarter
|
4.63
|
|
3.87
|
|
|||
|
Fourth Quarter
|
5.25
|
|
4.31
|
|
|||
|
Fiscal 2015
|
High
|
Low
|
|||||
|
First Quarter
|
$
|
4.59
|
|
$
|
2.50
|
|
|
|
Second Quarter
|
5.65
|
|
3.62
|
|
|||
|
Third Quarter
|
4.62
|
|
3.02
|
|
|||
|
Fourth Quarter
|
4.50
|
|
3.02
|
|
|||
|
December 31,
|
||||||||||||||||||||||||||
|
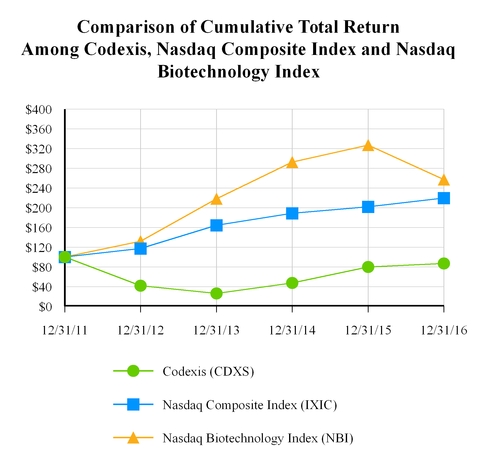
$100 investment in stock or index
|
Ticker
|
2011
|
2012
|
2013
|
2014
|
2015
|
2016
|
|||||||||||||||||||
|
Codexis, Inc.
|
CDXS
|
$
|
100.00
|
|
$
|
41.70
|
|
$
|
26.42
|
|
$
|
47.55
|
|
$
|
79.81
|
|
$
|
86.79
|
|
|||||||
|
Nasdaq Composite Index
|
IXIC
|
$
|
100.00
|
|
$
|
117.45
|
|
$
|
164.57
|
|
$
|
188.84
|
|
$
|
201.98
|
|
$
|
219.89
|
|
|||||||
|
Nasdaq Biotechnology Index
|
NBI
|
$
|
100.00
|
|
$
|
131.91
|
|
$
|
218.45
|
|
$
|
292.93
|
|
$
|
327.40
|
|
$
|
257.51
|
|
|||||||
|
|
Years Ended December 31,
|
||||||||||||||||||
|
|
2016
|
2015
|
2014
|
2013
|
2012
|
||||||||||||||
|
|
(In Thousands, Except Per Share Amounts)
|
||||||||||||||||||
|
Consolidated Statements of Operations Data:
|
|||||||||||||||||||
|
Revenues:
|
|||||||||||||||||||
|
Product sales
|
$
|
15,321
|
|
$
|
11,376
|
|
$
|
13,064
|
|
$
|
20,423
|
|
$
|
35,924
|
|
||||
|
Research and development revenues
|
31,316
|
|
25,599
|
|
14,945
|
|
6,868
|
|
49,977
|
|
|||||||||
|
Revenue sharing arrangement
|
2,200
|
|
4,829
|
|
7,298
|
|
4,631
|
|
150
|
|
|||||||||
|
Government awards
|
—
|
|
—
|
|
—
|
|
—
|
|
2,247
|
|
|||||||||
|
Total revenues
|
48,837
|
|
41,804
|
|
35,307
|
|
31,922
|
|
88,298
|
|
|||||||||
|
Costs and operating expenses:
|
|||||||||||||||||||
|
Cost of product sales
|
9,753
|
|
6,586
|
|
9,726
|
|
14,554
|
|
30,647
|
|
|||||||||
|
Research and development
|
22,229
|
|
20,673
|
|
22,755
|
|
31,606
|
|
56,785
|
|
|||||||||
|
Selling, general and administrative
|
25,419
|
|
22,315
|
|
21,937
|
|
26,908
|
|
31,379
|
|
|||||||||
|
Total costs and operating expenses
|
57,401
|
|
49,574
|
|
54,418
|
|
73,068
|
|
118,811
|
|
|||||||||
|
Loss from operations
|
(8,564
|
)
|
(7,770
|
)
|
(19,111
|
)
|
(41,146
|
)
|
(30,513
|
)
|
|||||||||
|
Interest income
|
60
|
|
19
|
|
18
|
|
60
|
|
252
|
|
|||||||||
|
Other expense
|
(94
|
)
|
(168
|
)
|
(234
|
)
|
(304
|
)
|
(326
|
)
|
|||||||||
|
Loss before income taxes
|
(8,598
|
)
|
(7,919
|
)
|
(19,327
|
)
|
(41,390
|
)
|
(30,587
|
)
|
|||||||||
|
Provision for (benefit from) income taxes
|
(40
|
)
|
(338
|
)
|
(256
|
)
|
(87
|
)
|
270
|
|
|||||||||
|
Net loss
|
$
|
(8,558
|
)
|
$
|
(7,581
|
)
|
$
|
(19,071
|
)
|
$
|
(41,303
|
)
|
$
|
(30,857
|
)
|
||||
|
Net loss per share, basic and diluted
|
$
|
(0.21
|
)
|
$
|
(0.19
|
)
|
$
|
(0.50
|
)
|
$
|
(1.08
|
)
|
$
|
(0.84
|
)
|
||||
|
Weighted average common shares used in computing net loss per share, basic and diluted
|
40,629
|
|
39,438
|
|
38,209
|
|
38,231
|
|
36,768
|
|
|||||||||
|
|
December 31,
|
||||||||||||||||||
|
|
2016
|
2015
|
2014
|
2013
|
2012
|
||||||||||||||
|
Consolidated Balance Sheets Data:
|
(In Thousands)
|
||||||||||||||||||
|
Cash, cash equivalents and short-term investments
|
$
|
19,240
|
|
$
|
23,273
|
|
$
|
26,487
|
|
$
|
25,135
|
|
$
|
45,527
|
|
||||
|
Working capital
|
14,860
|
|
17,998
|
|
19,272
|
|
24,582
|
|
43,486
|
|
|||||||||
|
Total assets
|
35,648
|
|
44,647
|
|
48,122
|
|
58,840
|
|
99,965
|
|
|||||||||
|
Total liabilities
|
16,549
|
|
21,768
|
|
21,811
|
|
17,357
|
|
21,525
|
|
|||||||||
|
Total stockholders’ equity
|
19,099
|
|
22,879
|
|
26,311
|
|
41,483
|
|
78,440
|
|
|||||||||
|
|
Years Ended December 31,
|
% of Total Revenues
|
||||||||||||||||||
|
|
2016
|
2015
|
2014
|
2016
|
2015
|
2014
|
||||||||||||||
|
Revenues:
|
||||||||||||||||||||
|
Product sales
|
$
|
15,321
|
|
$
|
11,376
|
|
$
|
13,064
|
|
31
|
%
|
27
|
%
|
37
|
%
|
|||||
|
Research and development revenues
|
31,316
|
|
25,599
|
|
14,945
|
|
64
|
%
|
61
|
%
|
42
|
%
|
||||||||
|
Revenue sharing arrangement
|
2,200
|
|
4,829
|
|
7,298
|
|
5
|
%
|
12
|
%
|
21
|
%
|
||||||||
|
Total revenues
|
48,837
|
|
41,804
|
|
35,307
|
|
100
|
%
|
100
|
%
|
100
|
%
|
||||||||
|
Costs and operating expenses:
|
||||||||||||||||||||
|
Cost of product sales
|
9,753
|
|
6,586
|
|
9,726
|
|
20
|
%
|
16
|
%
|
28
|
%
|
||||||||
|
Research and development
|
22,229
|
|
20,673
|
|
22,755
|
|
46
|
%
|
49
|
%
|
64
|
%
|
||||||||
|
Selling, general and administrative
|
25,419
|
|
22,315
|
|
21,937
|
|
52
|
%
|
53
|
%
|
62
|
%
|
||||||||
|
Total costs and operating expenses
|
57,401
|
|
49,574
|
|
54,418
|
|
118
|
%
|
118
|
%
|
154
|
%
|
||||||||
|
Loss from operations
|
(8,564
|
)
|
(7,770
|
)
|
(19,111
|
)
|
(18
|
)%
|
(19
|
)%
|
(54
|
)%
|
||||||||
|
Interest income
|
60
|
|
19
|
|
18
|
|
—
|
%
|
—
|
%
|
—
|
%
|
||||||||
|
Other expense
|
(94
|
)
|
(168
|
)
|
(234
|
)
|
—
|
%
|
—
|
%
|
(1
|
)%
|
||||||||
|
Loss before income taxes
|
(8,598
|
)
|
(7,919
|
)
|
(19,327
|
)
|
(18
|
)%
|
(19
|
)%
|
(55
|
)%
|
||||||||
|
Benefit from income taxes
|
(40
|
)
|
(338
|
)
|
(256
|
)
|
—
|
%
|
(1
|
)%
|
(1
|
)%
|
||||||||
|
Net loss
|
$
|
(8,558
|
)
|
$
|
(7,581
|
)
|
$
|
(19,071
|
)
|
(18
|
)%
|
(18
|
)%
|
(54
|
)%
|
|||||
|
•
|
Product sales consist of sales of enzymes, chemical intermediates, and Codex
®
Biocatalyst Panels and Kits.
|
|
•
|
Research and development revenues include license, technology access and exclusivity fees, research services fees for full time employee (“FTE”), milestone payments, royalties, and optimization and screening fees.
|
|
•
|
Revenue sharing arrangement is recognized based upon receipt of information regarding the sales of licensed products by Exela.
|
|
Change
|
|||||||||||||||||||||||||
|
|
Years Ended December 31,
|
2016
|
2015
|
||||||||||||||||||||||
|
(In Thousands)
|
2016
|
2015
|
2014
|
$
|
%
|
$
|
%
|
||||||||||||||||||
|
Product sales
|
$
|
15,321
|
|
$
|
11,376
|
|
$
|
13,064
|
|
$
|
3,945
|
|
35
|
%
|
$
|
(1,688
|
)
|
(13
|
)%
|
||||||
|
Research and development revenues
|
31,316
|
|
25,599
|
|
14,945
|
|
5,717
|
|
22
|
%
|
10,654
|
|
71
|
%
|
|||||||||||
|
Revenue sharing arrangement
|
2,200
|
|
4,829
|
|
7,298
|
|
(2,629
|
)
|
(54
|
)%
|
(2,469
|
)
|
(34
|
)%
|
|||||||||||
|
Total revenues
|
$
|
48,837
|
|
$
|
41,804
|
|
$
|
35,307
|
|
$
|
7,033
|
|
17
|
%
|
$
|
6,497
|
|
18
|
%
|
||||||
|
Change
|
|||||||||||||||||||||||||
|
|
Years Ended December 31,
|
2016
|
2015
|
||||||||||||||||||||||
|
(In Thousands)
|
2016
|
2015
|
2014
|
$
|
%
|
$
|
%
|
||||||||||||||||||
|
Cost of product sales
|
$
|
9,753
|
|
$
|
6,586
|
|
$
|
9,726
|
|
$
|
3,167
|
|
48
|
%
|
$
|
(3,140
|
)
|
(32
|
)%
|
||||||
|
Research and development
|
22,229
|
|
20,673
|
|
22,755
|
|
1,556
|
|
8
|
%
|
(2,082
|
)
|
(9
|
)%
|
|||||||||||
|
Selling, general and administrative
|
25,419
|
|
22,315
|
|
21,937
|
|
3,104
|
|
14
|
%
|
378
|
|
2
|
%
|
|||||||||||
|
Total operating expenses
|
$
|
57,401
|
|
$
|
49,574
|
|
$
|
54,418
|
|
$
|
7,827
|
|
16
|
%
|
$
|
(4,844
|
)
|
(9
|
)%
|
||||||
|
Change
|
|||||||||||||||||||||||||
|
|
Years Ended December 31,
|
2016
|
2015
|
||||||||||||||||||||||
|
(In Thousands)
|
2016
|
2015
|
2014
|
$
|
%
|
$
|
%
|
||||||||||||||||||
|
Interest income
|
$
|
60
|
|
$
|
19
|
|
$
|
18
|
|
$
|
41
|
|
216
|
%
|
$
|
1
|
|
6
|
%
|
||||||
|
Other expense
|
(94
|
)
|
(168
|
)
|
(234
|
)
|
(74
|
)
|
(44
|
)%
|
(66
|
)
|
(28
|
)%
|
|||||||||||
|
Total other income (expense), net
|
$
|
(34
|
)
|
$
|
(149
|
)
|
$
|
(216
|
)
|
$
|
(115
|
)
|
(77
|
)%
|
$
|
(67
|
)
|
(31
|
)%
|
||||||
|
Change
|
||||||||||||||||||||||||||
|
|
Years Ended December 31,
|
2016
|
2015
|
|||||||||||||||||||||||
|
(In Thousands)
|
2016
|
2015
|
2014
|
$
|
%
|
$
|
%
|
|||||||||||||||||||
|
Benefit from income taxes
|
$
|
(40
|
)
|
$
|
(338
|
)
|
$
|
(256
|
)
|
$
|
(298
|
)
|
(88
|
)%
|
$
|
82
|
|
32
|
%
|
|||||||
|
|
December 31,
|
||||||||||
|
(In Thousands)
|
2016
|
2015
|
2014
|
||||||||
|
Cash and cash equivalents
|
$
|
19,240
|
|
$
|
23,273
|
|
$
|
26,487
|
|
||
|
Working capital
|
14,860
|
|
17,998
|
|
19,272
|
|
|||||
|
|
Years Ended December 31,
|
||||||||||
|
(In Thousands)
|
2016
|
2015
|
2014
|
||||||||
|
Net cash (used in) provided by operating activities
|
$
|
(2,701
|
)
|
$
|
(433
|
)
|
$
|
321
|
|
||
|
Net cash (used in) provided by investing activities
|
(842
|
)
|
(1,257
|
)
|
4,647
|
|
|||||
|
Net cash used in financing activities
|
(490
|
)
|
(1,524
|
)
|
(611
|
)
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
$
|
(4,033
|
)
|
$
|
(3,214
|
)
|
$
|
4,357
|
|
||
|
Contractual Obligations
|
Total
|
Less than 1 year
|
1 to 3 years
|
4 to 5 years
|
More than 5 years
|
||||||||||||||
|
Operating leases obligations
(1)
|
$
|
10,785
|
|
$
|
3,077
|
|
$
|
6,465
|
|
$
|
1,202
|
|
$
|
41
|
|
||||
|
Purchase obligations
(2)
|
1,800
|
|
—
|
|
—
|
|
—
|
|
1,800
|
|
|||||||||
|
Total
(3)
|
$
|
12,585
|
|
$
|
3,077
|
|
$
|
6,465
|
|
$
|
1,202
|
|
$
|
1,841
|
|
||||
|
(1)
|
Represents future minimum lease payments under non-cancelable operating leases in effect as of
December 31, 2016
for our facilities in Redwood City, California. The minimum lease payments above do not include common area maintenance charges or real estate taxes. In addition, amounts have not been reduced by future minimum sublease rentals of
$1.8 million
to be received under non-cancellable subleases.
|
|
(2)
|
Represents a purchase commitment from a manufacture and supply agreement that resulted in a total commitment up to
$1.8 million
, with payment to be made in December 2022 or after.
|
|
(3)
|
Excludes
$0.7 million
of uncertain tax liabilities for which we cannot make a reasonably reliable estimate of the period of cash settlement.
|
|
|
December 31,
|
||||||
|
|
2016
|
2015
|
|||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
19,240
|
|
$
|
23,273
|
|
|
|
Accounts receivable, net of allowances of $421 at December 31, 2016 and 2015
|
5,924
|
|
7,329
|
|
|||
|
Inventories
|
825
|
|
992
|
|
|||
|
Prepaid expenses and other assets, current
|
1,238
|
|
1,245
|
|
|||
|
Total current assets
|
27,227
|
|
32,839
|
|
|||
|
Restricted cash
|
1,624
|
|
787
|
|
|||
|
Marketable securities
|
1,142
|
|
1,549
|
|
|||
|
Property and equipment, net
|
2,155
|
|
3,109
|
|
|||
|
Intangible assets, net
|
—
|
|
2,812
|
|
|||
|
Goodwill
|
3,241
|
|
3,241
|
|
|||
|
Other assets, non-current
|
259
|
|
310
|
|
|||
|
Total assets
|
$
|
35,648
|
|
$
|
44,647
|
|
|
|
Liabilities and Stockholders’ Equity
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
4,232
|
|
$
|
3,399
|
|
|
|
Accrued compensation
|
4,314
|
|
3,331
|
|
|||
|
Other accrued liabilities
|
2,111
|
|
2,013
|
|
|||
|
Deferred revenues
|
1,710
|
|
6,098
|
|
|||
|
Total current liabilities
|
12,367
|
|
14,841
|
|
|||
|
Deferred revenues, net of current portion
|
1,066
|
|
3,120
|
|
|||
|
Lease incentive obligation, net of current portion
|
885
|
|
1,310
|
|
|||
|
Other liabilities
|
2,231
|
|
2,497
|
|
|||
|
Total liabilities
|
16,549
|
|
21,768
|
|
|||
|
Commitments and contingencies (Note 13)
|
|
|
|||||
|
Stockholders’ equity:
|
|||||||
|
Preferred stock, $0.0001 par value per share; 5,000 shares authorized, none issued and outstanding
|
—
|
|
—
|
|
|||
|
Common stock, $0.0001 par value per share; 100,000 shares authorized; 41,255 and 40,343 shares issued and outstanding at December 31, 2016 and December 31, 2015, respectively
|
4
|
|
4
|
|
|||
|
Additional paid-in capital
|
311,164
|
|
305,981
|
|
|||
|
Accumulated other comprehensive income
|
—
|
|
405
|
|
|||
|
Accumulated deficit
|
(292,069
|
)
|
(283,511
|
)
|
|||
|
Total stockholders’ equity
|
19,099
|
|
22,879
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
35,648
|
|
$
|
44,647
|
|
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Revenues:
|
|||||||||||
|
Product sales
|
$
|
15,321
|
|
$
|
11,376
|
|
$
|
13,064
|
|
||
|
Research and development revenues
|
31,316
|
|
25,599
|
|
14,945
|
|
|||||
|
Revenue sharing arrangement
|
2,200
|
|
4,829
|
|
7,298
|
|
|||||
|
Total revenues
|
48,837
|
|
41,804
|
|
35,307
|
|
|||||
|
Costs and operating expenses:
|
|||||||||||
|
Cost of product sales
|
9,753
|
|
6,586
|
|
9,726
|
|
|||||
|
Research and development
|
22,229
|
|
20,673
|
|
22,755
|
|
|||||
|
Selling, general and administrative
|
25,419
|
|
22,315
|
|
21,937
|
|
|||||
|
Total costs and operating expenses
|
57,401
|
|
49,574
|
|
54,418
|
|
|||||
|
Loss from operations
|
(8,564
|
)
|
(7,770
|
)
|
(19,111
|
)
|
|||||
|
Interest income
|
60
|
|
19
|
|
18
|
|
|||||
|
Other expense
|
(94
|
)
|
(168
|
)
|
(234
|
)
|
|||||
|
Loss before income taxes
|
(8,598
|
)
|
(7,919
|
)
|
(19,327
|
)
|
|||||
|
Benefit from income taxes
|
(40
|
)
|
(338
|
)
|
(256
|
)
|
|||||
|
Net loss
|
$
|
(8,558
|
)
|
$
|
(7,581
|
)
|
$
|
(19,071
|
)
|
||
|
Net loss per share, basic and diluted
|
$
|
(0.21
|
)
|
$
|
(0.19
|
)
|
$
|
(0.50
|
)
|
||
|
Weighted average common shares used in computing net loss per share, basic and diluted
|
40,629
|
|
39,438
|
|
38,209
|
|
|||||
|
|
Years Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Net loss
|
$
|
(8,558
|
)
|
$
|
(7,581
|
)
|
$
|
(19,071
|
)
|
||
|
Other comprehensive income (loss):
|
|||||||||||
|
Unrealized gain (loss) on marketable securities, net of tax
(1)
|
(405
|
)
|
547
|
|
(110
|
)
|
|||||
|
Other comprehensive income (loss)
|
(405
|
)
|
547
|
|
(110
|
)
|
|||||
|
Total comprehensive loss
|
$
|
(8,963
|
)
|
$
|
(7,034
|
)
|
$
|
(19,181
|
)
|
||
|
(1)
|
Net of benefit from income taxes of
$0
,
$314
, and
$0
in
2016
,
2015
and
2014
, respectively.
|
|
|
Common Stock
|
Additional
Paid-in
Capital
|
Accumulated
Other
Comprehensive
Income (Loss)
|
Accumulated
Deficit
|
Total
Stockholders’ Equity |
||||||||||||||||||
|
|
Shares
|
Amount
|
|||||||||||||||||||||
|
December 31, 2013
|
38,351
|
|
$
|
4
|
|
$
|
298,370
|
|
$
|
(32
|
)
|
$
|
(256,859
|
)
|
$
|
41,483
|
|
||||||
|
Exercise of stock options
|
146
|
|
—
|
|
195
|
|
—
|
|
—
|
|
195
|
|
|||||||||||
|
Cancellation of shares
|
(456
|
)
|
—
|
|
(806
|
)
|
—
|
|
—
|
|
(806
|
)
|
|||||||||||
|
Release of stock awards
|
1,522
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Employee stock-based compensation
|
—
|
|
—
|
|
4,608
|
|
—
|
|
—
|
|
4,608
|
|
|||||||||||
|
Non-employee stock-based compensation
|
—
|
|
—
|
|
12
|
|
—
|
|
—
|
|
12
|
|
|||||||||||
|
Total comprehensive loss
|
—
|
|
—
|
|
—
|
|
(110
|
)
|
(19,071
|
)
|
(19,181
|
)
|
|||||||||||
|
December 31, 2014
|
39,563
|
|
4
|
|
302,379
|
|
(142
|
)
|
(275,930
|
)
|
26,311
|
|
|||||||||||
|
Exercise of stock options
|
172
|
|
—
|
|
289
|
|
—
|
|
—
|
|
289
|
|
|||||||||||
|
Cancellation of shares
|
(444
|
)
|
—
|
|
(1,813
|
)
|
—
|
|
—
|
|
(1,813
|
)
|
|||||||||||
|
Release of stock awards
|
1,052
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Employee stock-based compensation
|
—
|
|
—
|
|
5,122
|
|
—
|
|
—
|
|
5,122
|
|
|||||||||||
|
Non-employee stock-based compensation
|
—
|
|
—
|
|
4
|
|
—
|
|
—
|
|
4
|
|
|||||||||||
|
Total comprehensive loss
|
—
|
|
—
|
|
—
|
|
547
|
|
(7,581
|
)
|
(7,034
|
)
|
|||||||||||
|
December 31, 2015
|
40,343
|
|
4
|
|
305,981
|
|
405
|
|
(283,511
|
)
|
22,879
|
|
|||||||||||
|
Exercise of stock options
|
398
|
|
—
|
|
1,034
|
|
—
|
|
—
|
|
1,034
|
|
|||||||||||
|
Cancellation of shares
|
(397
|
)
|
—
|
|
(1,524
|
)
|
—
|
|
—
|
|
(1,524
|
)
|
|||||||||||
|
Release of stock awards
|
911
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
Employee stock-based compensation
|
—
|
|
—
|
|
5,673
|
|
—
|
|
—
|
|
5,673
|
|
|||||||||||
|
Total comprehensive loss
|
—
|
|
—
|
|
—
|
|
(405
|
)
|
(8,558
|
)
|
(8,963
|
)
|
|||||||||||
|
December 31, 2016
|
41,255
|
|
$
|
4
|
|
$
|
311,164
|
|
$
|
—
|
|
$
|
(292,069
|
)
|
$
|
19,099
|
|
||||||
|
|
Years Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Operating activities:
|
|||||||||||
|
Net loss
|
$
|
(8,558
|
)
|
$
|
(7,581
|
)
|
$
|
(19,071
|
)
|
||
|
Adjustments to reconcile net loss to net cash provided by (used in) operating activities:
|
|||||||||||
|
Amortization of intangible assets
|
2,812
|
|
3,374
|
|
3,374
|
|
|||||
|
Depreciation and amortization of property and equipment
|
1,734
|
|
2,035
|
|
3,311
|
|
|||||
|
Stock-based compensation
|
5,673
|
|
5,126
|
|
4,620
|
|
|||||
|
Accretion of premium on marketable securities
|
—
|
|
—
|
|
2
|
|
|||||
|
Loss (gain) on disposal of property and equipment
|
(42
|
)
|
32
|
|
24
|
|
|||||
|
Impairment of property and equipment
|
—
|
|
—
|
|
1,841
|
|
|||||
|
Gain on sale of Hungarian subsidiary
|
—
|
|
—
|
|
(760
|
)
|
|||||
|
Loss on disposal and exchange of assets held for sale, net
|
—
|
|
—
|
|
87
|
|
|||||
|
Change in fair value of assets held for sale
|
—
|
|
—
|
|
698
|
|
|||||
|
Income tax benefit related to marketable securities
|
—
|
|
(314
|
)
|
—
|
|
|||||
|
Changes in operating assets and liabilities:
|
|||||||||||
|
Accounts receivable
|
1,405
|
|
(3,459
|
)
|
1,587
|
|
|||||
|
Inventories
|
167
|
|
403
|
|
92
|
|
|||||
|
Prepaid expenses and other current assets
|
7
|
|
10
|
|
(339
|
)
|
|||||
|
Restricted cash
|
(841
|
)
|
—
|
|
—
|
|
|||||
|
Other assets
|
52
|
|
(16
|
)
|
(78
|
)
|
|||||
|
Accounts payable
|
942
|
|
(1,274
|
)
|
713
|
|
|||||
|
Accrued compensation
|
983
|
|
385
|
|
(530
|
)
|
|||||
|
Other accrued liabilities
|
(593
|
)
|
(1,062
|
)
|
555
|
|
|||||
|
Deferred revenues
|
(6,442
|
)
|
1,908
|
|
4,195
|
|
|||||
|
Net cash (used in) provided by operating activities
|
(2,701
|
)
|
(433
|
)
|
321
|
|
|||||
|
Investing activities:
|
|||||||||||
|
Purchase of property and equipment
|
(888
|
)
|
(1,199
|
)
|
(302
|
)
|
|||||
|
Proceeds from disposal of property and equipment
|
42
|
|
18
|
|
167
|
|
|||||
|
Proceeds from sale of Hungarian subsidiary
|
—
|
|
—
|
|
1,500
|
|
|||||
|
Proceeds from sale of assets held for sale
|
—
|
|
—
|
|
282
|
|
|||||
|
Proceeds from sale of marketable securities
|
—
|
|
—
|
|
3,000
|
|
|||||
|
Change in restricted cash
|
4
|
|
(76
|
)
|
—
|
|
|||||
|
Net cash (used in) provided by investing activities
|
(842
|
)
|
(1,257
|
)
|
4,647
|
|
|||||
|
Financing activities:
|
|||||||||||
|
Proceeds from exercises of stock options
|
1,034
|
|
289
|
|
195
|
|
|||||
|
Proceeds from issuance of common stock, net of issuance costs
|
—
|
|
—
|
|
9
|
|
|||||
|
Taxes paid related to net share settlement of equity awards
|
(1,524
|
)
|
(1,813
|
)
|
(815
|
)
|
|||||
|
Net cash used in financing activities
|
(490
|
)
|
(1,524
|
)
|
(611
|
)
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
(4,033
|
)
|
(3,214
|
)
|
4,357
|
|
|||||
|
Cash and cash equivalents at the beginning of the year
|
23,273
|
|
26,487
|
|
22,130
|
|
|||||
|
Cash and cash equivalents at the end of the year
|
$
|
19,240
|
|
$
|
23,273
|
|
$
|
26,487
|
|
||
|
Supplemental cash flow disclosures:
|
|||||||||||
|
Cash paid for income taxes
|
$
|
5
|
|
$
|
8
|
|
$
|
15
|
|
||
|
Equipment in property and equipment transferred from assets held for sale
|
$
|
—
|
|
$
|
—
|
|
$
|
(333
|
)
|
||
|
•
|
Level 1: Inputs that are unadjusted, quoted prices in active markets for identical assets or liabilities at the measurement date.
|
|
•
|
Level 2: Inputs that are either directly or indirectly observable for the asset or liability through correlation with market data at the measurement date and for the duration of the instrument’s anticipated life.
|
|
•
|
Level 3: Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities and which reflect management’s best estimate of what market participants would use in pricing the asset or liability at the measurement date.
|
|
Asset classification
|
Estimated useful life
|
|
|
Laboratory equipment
|
5 years
|
|
|
Computer equipment and software
|
3 to 5 years
|
|
|
Office equipment and furniture
|
5 years
|
|
|
Leasehold improvements
|
Lesser of useful life or lease term
|
|
|
|
Years Ended December 31,
|
|||||||
|
|
2016
|
2015
|
2014
|
|||||
|
Shares issuable under Equity Incentive Plan
|
5,567
|
|
5,932
|
|
6,193
|
|
||
|
Shares issuable upon the conversion of warrants
|
73
|
|
75
|
|
75
|
|
||
|
Total anti-dilutive securities
|
5,640
|
|
6,007
|
|
6,268
|
|
||
|
|
December 31, 2016
|
||||||||||||||||
|
|
Adjusted Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Estimated
Fair Value
|
Average
Contractual
Maturities
|
||||||||||||
|
|
|
(in days)
|
|||||||||||||||
|
Money market funds
|
$
|
11,172
|
|
$
|
—
|
|
$
|
—
|
|
$
|
11,172
|
|
n/a
|
||||
|
Common shares of CO
2
Solutions
|
563
|
|
579
|
|
—
|
|
1,142
|
|
n/a
|
||||||||
|
Total
|
$
|
11,735
|
|
$
|
579
|
|
$
|
—
|
|
$
|
12,314
|
|
|||||
|
|
December 31, 2015
|
||||||||||||||||
|
|
Adjusted Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Estimated
Fair Value
|
Average
Contractual
Maturities
|
||||||||||||
|
|
(in days)
|
||||||||||||||||
|
Money market funds
|
$
|
11,120
|
|
$
|
—
|
|
$
|
—
|
|
$
|
11,120
|
|
n/a
|
||||
|
Common shares of CO2 Solutions
|
563
|
|
986
|
|
—
|
|
1,549
|
|
n/a
|
||||||||
|
Total
|
$
|
11,683
|
|
$
|
986
|
|
$
|
—
|
|
$
|
12,669
|
|
|||||
|
|
December 31, 2016
|
||||||||||||||
|
Financial Assets
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||||
|
Money market funds
|
$
|
11,172
|
|
$
|
—
|
|
$
|
—
|
|
$
|
11,172
|
|
|||
|
Common shares of CO
2
Solutions
(1)
|
—
|
|
1,142
|
|
—
|
|
1,142
|
|
|||||||
|
Total
|
$
|
11,172
|
|
$
|
1,142
|
|
$
|
—
|
|
$
|
12,314
|
|
|||
|
|
December 31, 2015
|
||||||||||||||
|
Financial Assets
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||||
|
Money market funds
|
$
|
11,120
|
|
|
$
|
—
|
|
$
|
—
|
|
|
$
|
11,120
|
|
|
|
Common shares of CO
2
Solutions
(1)
|
—
|
|
|
1,549
|
|
—
|
|
1,549
|
|
||||||
|
Total
|
$
|
11,120
|
|
$
|
1,549
|
|
$
|
—
|
|
$
|
12,669
|
|
|||
|
(1)
|
We calculated the fair value of our investment in
10,000,000
common shares of CO
2
Solutions using the market value of common shares as determined by trading on the TSX Venture Exchange.
|
|
December 31,
|
||||||||||||
|
2016
|
2015
|
2014
|
||||||||||
|
Allowance - beginning of period
|
$
|
(421
|
)
|
$
|
(428
|
)
|
$
|
(460
|
)
|
|||
|
Provision for bad debts
|
—
|
|
—
|
|
(11
|
)
|
||||||
|
Recoveries from bad debts
|
—
|
|
7
|
|
—
|
|
||||||
|
Write-offs and other
|
—
|
|
—
|
|
43
|
|
||||||
|
Allowance - end of period
|
$
|
(421
|
)
|
$
|
(421
|
)
|
$
|
(428
|
)
|
|||
|
|
December 31,
|
||||||
|
|
2016
|
2015
|
|||||
|
Raw materials
(1)
|
$
|
118
|
|
$
|
262
|
|
|
|
Work in process
(2)
|
59
|
|
—
|
|
|||
|
Finished goods
(2)
|
648
|
|
730
|
|
|||
|
Total
|
$
|
825
|
|
$
|
992
|
|
|
|
(1)
|
Raw materials include active pharmaceutical ingredients and other raw materials.
|
|
(2)
|
Work-in-process and finished goods include third party manufacturing costs and labor and indirect costs we incur in the production process.
|
|
|
December 31,
|
||||||
|
|
2016
|
2015
|
|||||
|
Laboratory equipment
(1)
|
$
|
18,849
|
|
$
|
20,503
|
|
|
|
Leasehold improvements
|
10,395
|
|
10,369
|
|
|||
|
Computer equipment and software
|
3,267
|
|
3,271
|
|
|||
|
Office equipment and furniture
|
1,171
|
|
1,178
|
|
|||
|
Construction in progress
(2)
|
124
|
|
3
|
|
|||
|
Property and equipment
|
33,806
|
|
35,324
|
|
|||
|
Less: accumulated depreciation and amortization
|
(31,651
|
)
|
(32,215
|
)
|
|||
|
Property and equipment, net
|
$
|
2,155
|
|
$
|
3,109
|
|
|
|
(1)
|
Fully depreciated laboratory equipment with a cost of
$2.3 million
was retired during 2016.
|
|
(2)
|
Construction in progress includes equipment received but not yet placed into service pending installation.
|
|
|
December 31, 2016
|
December 31, 2015
|
|
||||||||||||||||||||||
|
|
Gross
Carrying
Amount
|
Accumulated
Amortization
|
Net
Carrying
Amount
|
Gross
Carrying
Amount
|
Accumulated
Amortization
|
Net
Carrying
Amount
|
Weighted-
Average
Amortization
Period
|
||||||||||||||||||
|
|
|
|
|
|
(in years)
|
||||||||||||||||||||
|
Developed and core technology
|
$
|
1,534
|
|
$
|
(1,534
|
)
|
$
|
—
|
|
$
|
1,534
|
|
$
|
(1,534
|
)
|
$
|
—
|
|
5
|
||||||
|
Maxygen intellectual property
|
20,244
|
|
(20,244
|
)
|
—
|
|
20,244
|
|
(17,432
|
)
|
2,812
|
|
6
|
||||||||||||
|
Total
|
$
|
21,778
|
|
$
|
(21,778
|
)
|
$
|
—
|
|
$
|
21,778
|
|
$
|
(18,966
|
)
|
$
|
2,812
|
|
|||||||
|
|
December 31,
|
||||||
|
|
2016
|
2015
|
|||||
|
Accrued purchase
(1)
|
$
|
67
|
|
$
|
430
|
|
|
|
Accrued professional and outside service fees
|
746
|
|
498
|
|
|||
|
Deferred rent
|
168
|
|
143
|
|
|||
|
Lease incentive obligation
|
425
|
|
425
|
|
|||
|
Other
|
705
|
|
517
|
|
|||
|
Total
|
$
|
2,111
|
|
$
|
2,013
|
|
|
|
(1)
|
Amount represents products and services received but have not been billed as of December 31, 2016 and 2015.
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Research and development
|
$
|
1,033
|
|
$
|
935
|
|
$
|
953
|
|
||
|
Selling, general and administrative
|
4,640
|
|
4,191
|
|
3,667
|
|
|||||
|
$
|
5,673
|
|
$
|
5,126
|
|
$
|
4,620
|
|
|||
|
|
Years Ended December 31,
|
|||||||
|
|
2016
|
2015
|
2014
|
|||||
|
Expected life (years)
|
5.3
|
|
6.1
|
|
6.0
|
|
||
|
Volatility
|
64.2
|
%
|
66.1
|
%
|
65.0
|
%
|
||
|
Risk-free interest rate
|
1.3
|
%
|
1.7
|
%
|
1.9
|
%
|
||
|
Expected dividend yield
(1)
|
0.0
|
%
|
0.0
|
%
|
0.0
|
%
|
||
|
(1)
|
We do not currently pay dividends, and thus the dividend rate variable in the Black-Scholes-Merton option-pricing model is
zero
.
|
|
Number
of Shares |
Weighted
Average Exercise Price Per Share |
Weighted
Average Remaining Contractual Term |
Aggregate Intrinsic
Value |
|||||||||
|
(in thousands)
|
(in years)
|
(in thousands)
|
||||||||||
|
Balance at January 1, 2016
|
3,918
|
|
$
|
4.49
|
|
|||||||
|
Granted
|
971
|
|
$
|
4.16
|
|
|||||||
|
Exercised
|
(398
|
)
|
$
|
2.60
|
|
|||||||
|
Forfeited/Expired
|
(601
|
)
|
$
|
5.76
|
|
|||||||
|
Outstanding at December 31, 2016
|
3,890
|
|
$
|
4.40
|
|
6.54
|
$
|
4,494
|
|
|||
|
Exercisable at December 31, 2016
|
2,477
|
|
$
|
4.84
|
|
5.31
|
$
|
3,138
|
|
|||
|
Vested and expected to vest at December 31, 2016
|
3,693
|
|
$
|
4.42
|
|
6.40
|
$
|
4,381
|
|
|||
|
Number
of Shares |
Weighted Average
Grant Date Fair Value Per Share |
|||||
|
(in thousands)
|
||||||
|
Non-vested balance at January 1, 2016
|
480
|
|
$
|
3.29
|
|
|
|
Granted
|
185
|
|
$
|
4.21
|
|
|
|
Vested
|
(435
|
)
|
$
|
3.40
|
|
|
|
Forfeited/Expired
|
—
|
|
$
|
—
|
|
|
|
Non-vested balance at December 31, 2016
|
230
|
|
$
|
3.82
|
|
|
|
Number
of Shares |
Weighted Average
Grant Date Fair Value Per Share |
|||||
|
(in thousands)
|
||||||
|
Non-vested balance at January 1, 2016
|
545
|
|
$
|
3.15
|
|
|
|
Granted
|
330
|
|
$
|
4.10
|
|
|
|
Vested
|
(243
|
)
|
$
|
3.11
|
|
|
|
Forfeited/Expired
|
(15
|
)
|
$
|
2.74
|
|
|
|
Non-vested balance at December 31, 2016
|
617
|
|
$
|
3.69
|
|
|
|
Number
of Shares |
Weighted Average
Grant Date Fair Value Per Share |
|||||
|
(in thousands)
|
||||||
|
Non-vested balance at January 1, 2016
|
989
|
|
$
|
2.94
|
|
|
|
Granted
|
629
|
|
$
|
4.10
|
|
|
|
Vested
|
(482
|
)
|
$
|
2.89
|
|
|
|
Forfeited/Expired
|
(305
|
)
|
$
|
2.82
|
|
|
|
Non-vested balance at December 31, 2016
|
831
|
|
$
|
3.88
|
|
|
|
Issue Date
|
Shares Subject
to warrants
|
Exercise Price
per Share
|
Expiration
|
||||||
|
September 28, 2007
|
72,727
|
|
$
|
8.25
|
|
September 28, 2017
|
|||
|
|
Years Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
United States
|
$
|
(8,174
|
)
|
$
|
(7,641
|
)
|
$
|
(20,980
|
)
|
||
|
Foreign
|
(424
|
)
|
(278
|
)
|
1,653
|
|
|||||
|
Loss before provision for income taxes
|
$
|
(8,598
|
)
|
$
|
(7,919
|
)
|
$
|
(19,327
|
)
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Current provision (benefit):
|
|||||||||||
|
Federal
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
State
|
5
|
|
5
|
|
5
|
|
|||||
|
Foreign
|
(14
|
)
|
(13
|
)
|
(371
|
)
|
|||||
|
Total current provision (benefit)
|
(9
|
)
|
(8
|
)
|
(366
|
)
|
|||||
|
Deferred provision (benefit):
|
|||||||||||
|
Federal
|
—
|
|
(293
|
)
|
—
|
|
|||||
|
State
|
—
|
|
(21
|
)
|
—
|
|
|||||
|
Foreign
|
(31
|
)
|
(16
|
)
|
110
|
|
|||||
|
Total deferred provision (benefit)
|
(31
|
)
|
(330
|
)
|
110
|
|
|||||
|
Total benefit from income taxes
|
$
|
(40
|
)
|
$
|
(338
|
)
|
$
|
(256
|
)
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Tax benefit at federal statutory rate
|
$
|
(2,924
|
)
|
$
|
(2,693
|
)
|
$
|
(6,571
|
)
|
||
|
State taxes
|
127
|
|
1,126
|
|
249
|
|
|||||
|
Research and development credits
|
(161
|
)
|
(85
|
)
|
(57
|
)
|
|||||
|
Foreign operations taxed at different rates
|
30
|
|
31
|
|
447
|
|
|||||
|
Stock-based compensation
|
327
|
|
77
|
|
(2
|
)
|
|||||
|
Other nondeductible items
|
660
|
|
(43
|
)
|
(364
|
)
|
|||||
|
Change in valuation allowance
|
1,901
|
|
1,249
|
|
6,042
|
|
|||||
|
Benefit from income taxes
|
$
|
(40
|
)
|
$
|
(338
|
)
|
$
|
(256
|
)
|
||
|
|
December 31,
|
||||||
|
|
2016
|
2015
|
|||||
|
Deferred tax assets:
|
|||||||
|
Net operating losses
|
$
|
72,588
|
|
$
|
70,005
|
|
|
|
Credits
|
5,016
|
|
4,671
|
|
|||
|
Deferred revenues
|
1,025
|
|
3,357
|
|
|||
|
Stock-based compensation
|
3,750
|
|
3,460
|
|
|||
|
Reserves and accruals
|
2,952
|
|
2,713
|
|
|||
|
Depreciation
|
2,516
|
|
2,377
|
|
|||
|
Intangible assets
|
5,536
|
|
5,127
|
|
|||
|
Capital losses
|
933
|
|
933
|
|
|||
|
Unrealized gain/loss
|
277
|
|
126
|
|
|||
|
Other assets
|
110
|
|
98
|
|
|||
|
Total deferred tax assets:
|
94,703
|
|
92,867
|
|
|||
|
Deferred tax liabilities:
|
|||||||
|
Other
|
(103
|
)
|
(199
|
)
|
|||
|
Total deferred tax liabilities:
|
(103
|
)
|
(199
|
)
|
|||
|
Valuation allowance
|
(94,663
|
)
|
(92,762
|
)
|
|||
|
Net deferred tax liabilities
|
$
|
(63
|
)
|
$
|
(94
|
)
|
|
|
|
December 31, 2016
|
||||
|
|
Amount
|
Expiration
Years
|
|||
|
Net operating losses, federal
|
$
|
213,061
|
|
2022-2036
|
|
|
Net operating losses, state
|
120,234
|
|
2017-2036
|
||
|
Tax credits, federal
|
5,634
|
|
2022-2036
|
||
|
Tax credits, state
|
7,048
|
|
Do not expire
|
||
|
Net operating losses, foreign
|
627
|
|
Various
|
||
|
Tax credits, foreign
|
10
|
|
Various
|
||
|
Rollforward Table (at Gross): As of
|
|||||||||||
|
|
December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Balance at beginning of year
|
$
|
8,152
|
|
$
|
7,838
|
|
$
|
8,306
|
|
||
|
Additions based on tax positions related to current year
|
459
|
|
368
|
|
346
|
|
|||||
|
Additions to tax provision of prior years
|
—
|
|
—
|
|
—
|
|
|||||
|
Reductions to tax provision of prior years
|
(45
|
)
|
(54
|
)
|
(814
|
)
|
|||||
|
Lapse of the applicable statute of limitations
|
—
|
|
—
|
|
—
|
|
|||||
|
Balance at end of year
|
$
|
8,566
|
|
$
|
8,152
|
|
$
|
7,838
|
|
||
|
|
Lease Payments
|
||
|
Years ending December 31,
|
|||
|
2017
|
$
|
3,077
|
|
|
2018
|
3,185
|
|
|
|
2019
|
3,280
|
|
|
|
2020
|
712
|
|
|
|
2021
|
490
|
|
|
|
Thereafter
|
41
|
|
|
|
Total minimum payments
(1)
|
$
|
10,785
|
|
|
(1)
|
Minimum payments have not been reduced by future minimum sublease rentals of
$1.8 million
to be received under non-cancellable subleases.
|
|
|
Percentage of Total Revenues
For The Years Ended December 31,
|
|||||||
|
|
2016
|
2015
|
2014
|
|||||
|
Merck
|
47
|
%
|
29
|
%
|
24
|
%
|
||
|
GSK
|
22
|
%
|
20
|
%
|
17
|
%
|
||
|
Exela
|
*
|
|
12
|
%
|
21
|
%
|
||
|
|
Percentage of Accounts Receivables
As Of December 31,
|
||||
|
|
2016
|
2015
|
|||
|
Customer A
(1)
|
54
|
%
|
12
|
%
|
|
|
Customer B
|
16
|
%
|
*
|
|
|
|
Customer C
|
—
|
%
|
22
|
%
|
|
|
Customer D
(2)
|
—
|
%
|
40
|
%
|
|
|
(1)
|
This customer also contributed 10% or more of our net revenue in
2016
,
2015
and
2014
.
|
|
(2)
|
This represents a
$3.1 million
final settlement relating to past-due payments and settlement of future payments associated with our royalty business with a non-core customer as of December 31, 2015. We collected the full amount in February 2016.
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Revenues
|
|||||||||||
|
United States
|
$
|
21,309
|
|
$
|
24,795
|
|
$
|
16,136
|
|
||
|
Europe
|
17,138
|
|
14,151
|
|
15,067
|
|
|||||
|
Asia
|
|||||||||||
|
India
|
3,578
|
|
1,026
|
|
919
|
|
|||||
|
Singapore
|
3,836
|
|
963
|
|
1,435
|
|
|||||
|
Others
|
1,159
|
|
864
|
|
1,637
|
|
|||||
|
Others
|
1,817
|
|
5
|
|
113
|
|
|||||
|
Total
|
$
|
48,837
|
|
$
|
41,804
|
|
$
|
35,307
|
|
||
|
|
December 31,
|
||||||
|
|
2016
|
2015
|
|||||
|
Long-lived assets
|
|||||||
|
United States
|
$
|
2,414
|
|
$
|
6,231
|
|
|
|
|
Quarter Ended
(1)
|
||||||||||||||||||||||||||||||
|
|
December 31, 2016
(3)
|
September 30,
2016 |
June 30,
2016 |
March 31,
2016 |
December 31,
2015 |
September 30,
2015 |
June 30,
2015 |
March 31,
2015 |
|||||||||||||||||||||||
|
Revenues:
|
|||||||||||||||||||||||||||||||
|
Product sales
|
$
|
4,249
|
|
$
|
4,052
|
|
$
|
3,280
|
|
$
|
3,740
|
|
$
|
4,462
|
|
$
|
1,818
|
|
$
|
2,020
|
|
$
|
3,076
|
|
|||||||
|
Research and development revenues
|
5,345
|
|
10,373
|
|
12,064
|
|
3,534
|
|
6,352
|
|
14,517
|
|
2,533
|
|
2,197
|
|
|||||||||||||||
|
Revenue sharing arrangement
|
375
|
|
445
|
|
658
|
|
722
|
|
773
|
|
1,066
|
|
1,465
|
|
1,525
|
|
|||||||||||||||
|
Total revenues
|
$
|
9,969
|
|
$
|
14,870
|
|
16,002
|
|
7,996
|
|
$
|
11,587
|
|
$
|
17,401
|
|
$
|
6,018
|
|
$
|
6,798
|
|
|||||||||
|
Costs and operating expenses:
|
|||||||||||||||||||||||||||||||
|
Cost of product sales
|
$
|
2,287
|
|
$
|
2,756
|
|
$
|
2,221
|
|
$
|
2,489
|
|
$
|
2,578
|
|
$
|
1,302
|
|
$
|
1,250
|
|
$
|
1,456
|
|
|||||||
|
Research and development
|
5,964
|
|
5,467
|
|
5,112
|
|
5,686
|
|
5,216
|
|
4,994
|
|
5,170
|
|
5,293
|
|
|||||||||||||||
|
Selling, general and administrative
|
6,968
|
|
5,229
|
|
6,420
|
|
6,802
|
|
6,026
|
|
5,415
|
|
5,296
|
|
5,578
|
|
|||||||||||||||
|
Total costs and operating expenses
|
$
|
15,219
|
|
$
|
13,452
|
|
$
|
13,753
|
|
$
|
14,977
|
|
$
|
13,820
|
|
$
|
11,711
|
|
$
|
11,716
|
|
$
|
12,327
|
|
|||||||
|
Income (loss) before income taxes
|
$
|
(5,285
|
)
|
$
|
1,437
|
|
$
|
2,213
|
|
$
|
(6,963
|
)
|
$
|
(2,247
|
)
|
$
|
5,668
|
|
$
|
(5,790
|
)
|
$
|
(5,550
|
)
|
|||||||
|
Net income (loss)
|
$
|
(5,260
|
)
|
$
|
1,437
|
|
$
|
2,239
|
|
$
|
(6,974
|
)
|
$
|
(2,053
|
)
|
$
|
5,394
|
|
$
|
(5,360
|
)
|
$
|
(5,562
|
)
|
|||||||
|
Net income (loss) per share, basic
|
$
|
(0.13
|
)
|
$
|
0.04
|
|
$
|
0.06
|
|
$
|
(0.17
|
)
|
$
|
(0.05
|
)
|
$
|
0.14
|
|
$
|
(0.14
|
)
|
$
|
(0.14
|
)
|
|||||||
|
Net income (loss) per share, diluted
|
$
|
(0.13
|
)
|
$
|
0.03
|
|
$
|
0.05
|
|
$
|
(0.17
|
)
|
$
|
(0.05
|
)
|
$
|
0.13
|
|
$
|
(0.14
|
)
|
$
|
(0.14
|
)
|
|||||||
|
Weighted average common shares used in computing net loss per share, basic
(2)
|
41,002
|
|
40,940
|
|
40,495
|
|
40,072
|
|
39,840
|
|
39,767
|
|
39,301
|
|
38,779
|
|
|||||||||||||||
|
Weighted average common shares used in computing net loss per share, diluted
(2)
|
41,002
|
|
42,134
|
|
41,568
|
|
40,072
|
|
39,840
|
|
40,970
|
|
39,301
|
|
38,779
|
|
|||||||||||||||
|
(1)
|
The 2016 and 2015 amounts were computed independently for each quarter, and the sum of the quarters may not total the annual amounts.
|
|
|
(2)
|
The full year net loss per share of common stock, basic and diluted, may not equal the sum of the quarters due to weighting of outstanding shares.
|
|
|
(3)
|
PSUs and cash bonus awards are granted to certain employees and are subject to our performance in achieving pre-determined criteria approved by our board of directors. Based on the actual achievement of the annual goals, we updated the calculation of the annual expense in the fourth quarter which resulted in an additional true-up charge of approximately $0.3 million, primarily in operating expense.
|
|
|
1.
|
Financial Statements: See “Index to Consolidated Financial Statements” in Part II, Item 8 of this Annual Report on Form 10-K
|
|
2.
|
Exhibits: The exhibits listed in the accompanying index to exhibits are filed or incorporated by reference as part of this Annual Report on Form 10-K.
|
|
CODEXIS, INC.
|
||
|
Date: March 9, 2017
|
By:
|
/s/ John J. Nicols
|
|
President and Chief Executive Officer
|
||
|
(Principal Executive Officer)
|
||
|
SIGNATURE
|
TITLE
|
|
DATE
|
|
|
/s/ John J. Nicols
|
President, Chief Executive Officer and Director (Principal Executive Officer)
|
|
Date: March 9, 2017
|
|
|
John J. Nicols
|
||||
|
/s/ Gordon T. B. Sangster
|
Senior Vice President and Chief Financial Officer (Principal Financial and Accounting Officer)
|
|
Date: March 9, 2017
|
|
|
Gordon T. B. Sangster
|
||||
|
/s/ Bernard J. Kelley
|
Chairman of the Board of Directors
|
|
Date: March 9, 2017
|
|
|
Bernard J. Kelley
|
||||
|
/s/ Thomas R. Baruch
|
Chairman Emeritus, Director
|
|
Date: March 9, 2017
|
|
|
Thomas R. Baruch
|
||||
|
/s/ Pam P. Cheng
|
Director
|
|
Date: March 9, 2017
|
|
|
Pam P. Cheng
|
||||
|
/s/ Byron L. Dorgan
|
Director
|
|
Date: March 9, 2017
|
|
|
Byron L. Dorgan
|
||||
|
/s/ Kathleen S. Glaub
|
Director
|
|
Date: March 9, 2017
|
|
|
Kathleen S. Glaub
|
||||
|
/s/ David V. Smith
|
Director
|
Date: March 9, 2017
|
||
|
David V. Smith
|
||||
|
/s/ Dennis P. Wolf
|
Director
|
|
Date: March 9, 2017
|
|
|
Dennis P. Wolf
|
||||
|
/s/ Patrick Y. Yang
|
Director
|
|
Date: March 9, 2017
|
|
|
Patrick Y. Yang
|
||||
|
Exhibit
No.
|
Description
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation of Codexis, Inc. filed with the Secretary of the State of the State of Delaware on April 27, 2010 and effective as of April 27, 2010 (incorporated by reference to Exhibit 3.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2010, filed on May 28, 2010).
|
|
|
3.2
|
Certificate of Designations of Series A Junior Participating Preferred Stock of Codexis, Inc., filed with the Secretary of State of the State of Delaware on September 4, 2012 (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K, filed on September 4, 2012).
|
|
|
3.3
|
Amended and Restated Bylaws of Codexis, Inc. effective as of April 27, 2010 (incorporated by reference to Exhibit 3.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2010, filed on May 28, 2010).
|
|
|
4.1
|
Form of the Registrant’s Common Stock Certificate (incorporated by reference to Exhibit 4.1 to the Company’s Quarterly Report for the quarter ended June 30, 2012, filed on August 9, 2012).
|
|
|
4.2*
|
Form of Warrant to purchase shares of Series D preferred stock issued in connection with the Bridge Loan Agreement dated as of May 25, 2006.
|
|
|
4.3*
|
Form of Warrant to purchase shares of Series D preferred stock issued in connection with the Loan and Security Agreement dated as of September 28, 2007.
|
|
|
4.4*
|
Warrant to purchase shares of Common Stock issued to Alexandria Equities, LLC.
|
|
|
10.1A*
|
Lease Agreement by and between the Company and Metropolitan Life Insurance Company dated as of February 1, 2004.
|
|
|
10.1B*
|
Amendment to Lease Agreement by and between the Company and Metropolitan Life Insurance Company dated as of June 1, 2004.
|
|
|
10.1C*
|
Amendment to Lease Agreement by and between the Company and Metropolitan Life Insurance Company dated as of March 9, 2007.
|
|
|
10.1D*
|
Amendment to Lease Agreement by and between the Company and Metropolitan Life Insurance Company dated as of March 31, 2008.
|
|
|
10.1E
|
Fourth Amendment to Lease Agreement by and between the Company and Metropolitan Life Insurance Company dated as of September 17, 2010 (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2010, filed on November 4, 2010).
|
|
|
10.1F
|
Fifth Amendment to Lease Agreement by and between the Company and Metropolitan Life Insurance Company dated as of March 16, 2011 (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, filed on May 6, 2011).
|
|
|
10.1G
|
Sixth Amendment to Lease by and between the Company and Metropolitan Life Insurance Company dated as of September 27, 2012 (incorporated by reference to Exhibit 10.6 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2012, filed on November 7, 2012).
|
|
|
10.1H
|
Seventh Amendment to Lease by and between the Company and Metropolitan Life Insurance Company dated as of October 17, 2016 (incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2016, filed on November 8, 2016).
|
|
|
10.2+*
|
Codexis, Inc. 2002 Stock Plan, as amended, and Form of Stock Option Agreement.
|
|
|
10.3+*
|
Codexis, Inc. 2010 Equity Incentive Award Plan and Form of Stock Option Agreement.
|
|
|
10.4*
|
Form of Indemnification Agreement between the Company and each of its directors, officers and certain employees.
|
|
|
Exhibit
No.
|
Description
|
|
|
10.5A+*
|
Form of Change of Control Severance Agreement between the Company and certain of its officers.
|
|
|
10.5B+
|
Form of Amended and Restated Change of Control Severance Agreement by and between Codexis, Inc. and certain of its officers (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed on October 13, 2016).
|
|
|
10.6
|
Asset Purchase Agreement, dated October 28, 2010, by and among the Company, Codexis Mayflower Holdings, LLC and Maxygen, Inc. (incorporated by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K, filed on October 28, 2010).
|
|
|
10.7A†
|
Manufacture and Supply Agreement, dated May 16, 2011, by and between the Company and Lactosan GmbH & Co. KG (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2011, filed on August 3, 2011).
|
|
|
10.7B
|
Amendment No. 1 to the Manufacture and Supply Agreement by and between the Company and Lactosan GmbH & Co. KG dated as of March 9, 2012 (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2012, filed on May 10, 2012).
|
|
|
10.8A+
|
Employment Agreement by and between the Company and John Nicols effective as of May 28, 2012 (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2012, filed on August 9, 2012).
|
|
|
10.8B+
|
John Nicols Stock Option Grant Notice and Stock Option Agreement dated June 13, 2012 between John J. Nicols and the Company (incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2012, filed on August 9, 2012).
|
|
|
10.8C+
|
John Nicols Restricted Stock Grant Notice and Restricted Stock Agreement dated June 13, 2012 between John J. Nicols and the Company (incorporated by reference to Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2012, filed on August 9, 2012).
|
|
|
10.8D+
|
Amendment to Employment Agreement between the Company and John Nicols, dated April 21, 2016 (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2016).
|
|
|
10.9A†
|
Sitagliptin Catalyst Supply Agreement by and between Merck Sharp and Dohme Corp. and the Company dated as of February 1, 2012 (incorporated by reference to Exhibit 10.25 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2012, filed on April 2, 2013).
|
|
|
10.9B†
|
Amendment to Sitagliptin Catalyst Supply Agreement between Merck Sharp and Dohme Corp. and the Company dated as of October 1, 2013 (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2013, filed on November 12, 2013).
|
|
|
10.9C
|
Amendment No. 2 to Sitagliptin Catalyst Supply Agreement between Merck Sharp and Dohme Corp. and the Company dated as of February 25, 2015 (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2015, filed on May 7, 2015).
|
|
|
10.9D
|
Amendment No. 3 to Sitagliptin Catalysts Supply Agreement between Merck Sharp and Dohme Corp. and the Company dated as of December 17, 2015.
|
|
|
10.9E
|
Amendment No. 4 to Sitagliptin Catalysts Supply Agreement, effective as of January 1, 2016, by and between the Company and Merck Sharp and Dohme Corp. (incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2016, filed on November 8, 2016).
|
|
|
10.10A†
|
License Agreement by and between Exela PharmSci, Inc. and the Company effective as of September 18, 2007 (incorporated by reference to Exhibit 10.26A to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2012, filed on April 2, 2013).
|
|
|
10.10B†
|
Amendment No. 1 to the License Agreement between Exela PharmSci, Inc. and the Company effective as of December 28, 2009 (incorporated by reference to Exhibit 10.26B to the Company’s Annual Report on Form 10-K for the year ended December 31, 2012, filed on April 2, 2013) .
|
|
|
Exhibit
No.
|
Description
|
|
|
10.11†
|
Platform Technology Transfer, Collaboration and License Agreement by and between the Company and GlaxoSmithKline Intellectual Property Limited, effective as of July 10, 2014 (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 31, 2014, filed on November 6, 2014).
|
|
|
10.12+
|
Offer Letter Agreement by and between the Company and Gordon Sangster effective as of July 11, 2014 (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 31, 2014, filed on November 6, 2014).
|
|
|
10.13†
|
Platform Technology Transfer and License Agreement by and between the Company and Merck Sharp & Dohme Corp., dated as of August 3, 2015 (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 31, 2015, filed on November 6, 2015).
|
|
|
10.14+
|
Offer Letter, dated as of October 12, 2016, by and between the Company and Michael Aldridge (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2016).
|
|
|
12.1
|
Statement Regarding the Computation of Ratio of Earnings to Fixed Charges
|
|
|
21.1
|
List of Subsidiaries.
|
|
|
23.1
|
Consent of BDO USA, LLP, independent registered public accounting firm
|
|
|
24.1
|
Power of Attorney (see signature page to this Annual Report on Form 10-K).
|
|
|
31.1
|
Certification of Principal Executive Officer Required Under Rule 13a-14(a) and 15d-14(a) of the Securities Exchange Act of 1934, as amended.
|
|
|
31.2
|
Certification of Principal Financial Officer Required Under Rule 13a-14(a) and 15d-14(a) of the Securities Exchange Act of 1934, as amended.
|
|
|
32.1**
|
Certification of Principal Executive Officer and Principal Financial Officer Required Under Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, and 18 U.S.C. §1350.
|
|
|
101
|
The following materials from Registrant’s Annual Report on Form 10-K for the fiscal year ended December 31, 2016, formatted in Extensible Business Reporting Language (XBRL) includes: (i) Consolidated Balance Sheets at December 31, 2016 and December 31, 2015, (ii) Consolidated Statements of Income for the years ended December 31, 2016, December 31, 2015 and December 31, 2014, (iii) Consolidated Statements of Comprehensive Income for the years ended December 31, 2016, December 31, 2015 and December 31, 2014, (iv) Consolidated Statements of Cash Flows for the years ended December 31, 2016, December 31, 2015 and December 31, 2014, (v) Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2016, December 31, 2015 and December 31, 2014 and (vi) Notes to Consolidated Financial Statements.
|
|
|
+
|
Indicates a management contract or compensatory plan or arrangement.
|
|
†
|
Confidential treatment has been granted for certain information contained in this exhibit. Such information has been omitted and filed separately with the Securities and Exchange Commission.
|
|
*
|
Filed as exhibits to the registrant’s Registration Statement on Form S-1 (File No. 333-164044), effective April 21, 2010, and incorporated herein by reference.
|
|
**
|
Pursuant to Item 601(b)(32) of Regulation S-K this exhibit is furnished rather than filed with this report.
|