|
☐
|
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
☐
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2018
|
|
☐
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
☐
|
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
for the transition period from __________ to ___________
|
|
|
|
|
|
|
|
|
|
|
|
|
|
☐
Large Accelerated filer
|
☐
Accelerated filer
|
☒
Non-accelerated filer
|
☒
Emerging growth company
|
|
☒
US GAAP
|
☐
International Financial Reporting
Standards as issued by the International Accounting Standards Board
|
☐
Other
|
|
|
|
Page
|
|
|
|
3 | ||
|
|
3 | ||
|
|
3 | ||
|
A.
|
|
Selected financial data
|
3 |
|
B.
|
|
Capitalization and indebtedness
|
4 |
|
C.
|
|
Reasons for the offer and use of proceeds
|
4 |
|
D.
|
|
Risk factors
|
4 |
|
|
|
|
|
|
|
33 | ||
|
A.
|
|
History and Development of the Company
|
33 |
|
B.
|
|
Business Overview
|
33 |
|
C.
|
|
Organizational Structure
|
54 |
|
D.
|
|
Property, Plants and Equipment
|
54 |
|
|
|
|
|
|
|
54 | ||
|
|
55 | ||
|
A.
|
|
Operating Results
|
56 |
|
B.
|
|
Liquidity and Capital Resources
|
60 |
|
C.
|
|
Research and development, patents and licenses, etc.
|
64 |
|
D.
|
|
Trend Information
|
65 |
|
E.
|
|
Off-balance Sheet Arrangements
|
65 |
|
F.
|
|
Tabular Disclosure of Contractual Obligations.
|
65 |
|
|
|
|
|
|
|
65 | ||
|
A.
|
|
Directors and senior management
|
65 |
|
B.
|
|
Compensation
|
68 |
|
C.
|
|
Board Practices
|
71 |
|
D.
|
|
Employees
|
81 |
|
E.
|
|
Share Ownership
|
81 |
|
|
|
|
|
|
|
85 | ||
|
A.
|
|
Major shareholders
|
85 |
|
B.
|
|
Related party transactions
|
86 |
|
C.
|
|
Interests of experts and counsel
|
88 |
|
|
|
|
|
|
|
88 | ||
|
A.
|
|
Statements and Other Financial Information
|
88 |
|
B.
|
|
Significant Changes
|
89 |
|
|
|
|
|
|
|
89 | ||
|
A.
|
|
Offer and listing details
|
89 |
|
B.
|
|
Plan of distribution
|
89 |
|
C.
|
|
Markets
|
89 |
|
D.
|
|
Selling shareholders
|
89 |
|
E.
|
|
Dilution
|
89 |
|
F.
|
|
Expenses of the issue
|
90 |
|
|
|
|
|
|
|
90 | ||
|
A.
|
|
Share capital
|
90 |
|
B.
|
|
Memorandum and articles of association
|
90 |
|
C.
|
|
Material contracts
|
94 |
|
D.
|
|
Exchange controls
|
94 |
|
E.
|
|
Taxation
|
94 |
|
F.
|
|
Dividends and paying agents
|
105 |
|
G.
|
|
Statement by experts
|
105 |
|
H.
|
|
Documents on display
|
105 |
|
I.
|
|
Subsidiary Information
|
106 |
|
|
106 | ||
|
|
106 | ||
|
|
|
|
|
|
|
106 | ||
|
|
106 | ||
|
|
107 | ||
|
|
107 | ||
|
|
|
|
|
|
|
107 | ||
|
|
|
|
|
|
|
107 | ||
|
|
|
|
|
|
|
107 | ||
|
|
|
|
|
|
|
108 | ||
|
|
|
|
|
|
|
108 | ||
|
|
|
|
|
|
|
108 | ||
|
|
|
|
|
|
|
108 | ||
|
|
|
|
|
|
|
109 | ||
|
|
|
|
|
|
|
109 | ||
|
|
|
|
|
|
|
109 | ||
|
|
|
|
|
|
|
110 | ||
|
|
|
|
|
| 112 | |||
| ● |
our history of losses and our ability to continue as a going concern;
|
| ● |
our needs for additional capital to fund our operations and our inability to obtain additional capital on acceptable terms, or at all;
|
| ● |
the initiation, timing, progress and results of our clinical trials and other product development efforts;
|
| ● |
our reliance on one product or product line;
|
| ● |
the clinical development, commercialization and market acceptance of our C-Scan system;
|
| ● |
our ability to receive de novo classification and other regulatory approvals for our C-Scan system;
|
| ● |
our ability to successfully complete clinical trials;
|
| ● |
our reliance on single-source suppliers;
|
| ● |
our reliance on third parties
, such as for purposes of our clinical trials and clinical development and the marketing and distribution of our C-Scan system;
|
| ● |
our ability to achieve reimbursement and coverage from government and private third-party payors;
|
| ● |
the implementation of our business model and strategic plans for our business;
|
| ● |
the scope of protection we are able to establish and maintain for intellectual property rights covering our C-Scan system and our ability to operate our business without infringing the intellectual property rights of others;
|
| ● |
competitive companies, technologies and our industry;
|
| ● |
statements as to the impact of the political and security situation in Israel on our business; and
|
| ● |
those factors referred to in “Item 3. Key Information – D. Risk Factors,” “Item 4. Information on the Company,” and “Item 5. Operating and Financial Review and Prospects”, as well as in this Annual Report generally.
|
|
A.
|
Directors and Senior Management
|
|
B.
|
Advisers
|
|
C.
|
Auditors
|
|
A.
|
Selected financial data
|
|
|
Year Ended December 31,
|
|||||||||||||||||||
|
|
2018
|
2017
|
2016
|
2015
|
2014
|
|||||||||||||||
|
|
(US$ in thousands, except per share data)
|
|||||||||||||||||||
|
Operating expenses
(1)
|
||||||||||||||||||||
|
Research and development expenses, net
(2)
|
$
|
7,618
|
$
|
6,837
|
$
|
5,491
|
$
|
5,837
|
$
|
2,832
|
||||||||||
|
General and administrative expenses
|
3,445
|
3,164
|
3,571
|
6,626
|
1,703
|
|||||||||||||||
|
Operating loss
|
11,063
|
10,001
|
9,062
|
12,463
|
4,535
|
|||||||||||||||
|
Finance income, net
|
473
|
236
|
244
|
173
|
3,925
|
|||||||||||||||
|
Loss before income tax
|
10,590
|
9,765
|
8,818
|
12,290
|
610
|
|||||||||||||||
|
Taxes on income
|
(1
|
)
|
6
|
8
|
-
|
-
|
||||||||||||||
|
Net loss
|
$
|
10,589
|
$
|
9,771
|
$
|
8,826
|
$
|
12,290
|
$
|
610
|
||||||||||
|
Comprehensive loss:
|
||||||||||||||||||||
|
Net loss
|
10,589
|
9,771
|
8,826
|
12,290
|
610
|
|||||||||||||||
|
Change in fair value of cash flow hedge
|
13
|
-
|
-
|
-
|
-
|
|||||||||||||||
|
Comprehensive loss
|
10,602
|
9,771
|
8,826
|
12,290
|
610
|
|||||||||||||||
|
Net loss per ordinary share of NIS 2.40 par value, basic and diluted
(3)
|
$
|
2.61
|
$
|
6.72
|
$
|
7.31
|
$
|
12.67
|
$
|
14.12
|
||||||||||
|
Weighted average number of ordinary shares outstanding – basic and diluted (in thousands)
(3)
|
4,058
|
1,455
|
1,208
|
993
|
182
|
|||||||||||||||
|
|
As of December 31,
|
|||||||||||||||||||
|
|
2018
|
2017
|
2016
|
2015
|
2014
|
|||||||||||||||
|
|
(US$ in thousands, except per share data)
|
|||||||||||||||||||
|
Cash and cash equivalents
|
$
|
8,572
|
$
|
6,997
|
$
|
11,639
|
$
|
9,392
|
$
|
1,075
|
||||||||||
|
Working capital
(4)
|
$
|
12,763
|
$
|
5,841
|
$
|
10,514
|
$
|
12,856
|
$
|
(1,622
|
)
|
|||||||||
|
Total assets
|
15,436
|
7,906
|
12,295
|
15,298
|
2,985
|
|||||||||||||||
|
Capital stock
|
76,344
|
58,617
|
53,348
|
46,763
|
20,999
|
|||||||||||||||
|
Total shareholders’ equity (deficiency)
|
$
|
13,030
|
$
|
5,905
|
$
|
10,407
|
$
|
12,648
|
$
|
(826
|
)
|
|||||||||
|
(1)
|
Operating expenses include share-based compensation expense in the total amount of $0.7 million, $1.2 million, $3.7 million and $0.3 million for the years ended December 31, 2017, 2016, 2015 and 2014, respectively and a negative share-based compensation expense of $65,000 as a result of forfeitures of awards for the year ended December 31, 2018. For additional information, see Item 5B “Operating and Financial Review and Prospects—Liquidity and Capital Resources—Application of Critical Accounting Policies and Estimates-Share-based compensation.”
|
|
(2)
|
Research and development expenses, net is presented net of the amount of grants received from the Israel Innovation Authority, or IIA, of the Ministry of Economy and Industry (formerly the Office of the Chief Scientist, or OCS, of the Ministry of Economy and Industry), and the Israel-United States Binational Industrial Research and Development Foundation, or the BIRD Foundation. The effect of the participation by the IIA and the BIRD Foundation totaled $0.2 million, $1.1 million, $0.3 million and $0.6 million for the years ended December 31, 2018, 2017, 2016, 2015 and 2014, respectively. See Item 5A “Operating and Financial Review and Prospects—Operating Results - Financial Operations Overview—Research and Development, Expenses, Net” for more information.
|
|
(3)
|
Basic and diluted loss per ordinary share is computed based on the basic and diluted weighted average number of ordinary shares outstanding during each period. For purposes of these calculations, the following ordinary shares were deemed to be outstanding: (i) 8,315 ordinary shares that were issuable to Guy Neev upon exercise of options, referred to as the Neev Options, which options were exercised immediately prior to the consummation of our initial public offering on February 24, 2015; (ii) 32,174 ordinary shares that were issuable under warrants that were subject to automatic exercise, for no consideration (unless the holder thereof objected to such exercise), upon the exercise by Guy Neev of the Neev Options, of which warrants to purchase 7,724, 56 and 17,149 ordinary shares were exercised during the years ended December 31, 2018, 2016 and 2015, respectively. No such warrants were exercised during the year ended December 31, 2017; (iii) since October 14, 2014, 221,556 ordinary shares issuable upon the exercise of outstanding warrants with an exercise price of NIS 2.40 per share, of which warrants to purchase 22,501, 9,912, 43,739 and 129,797 ordinary shares were exercised during the years ended December 31, 2018, 2017, 2016 and 2015, respectively, and warrants to purchase 5,192, 243, 761 and 2,022 ordinary shares expired during the years ended December 31, 2018, 2017, 2016 and 2015, respectively; (iv) since August 11, 2016, 209,524 ordinary shares issuable upon the exercise of outstanding pre-funded warrants with an exercise price of NIS 2.40 per share, of which pre-funded warrants to purchase 24,167 and 185,357 ordinary shares were exercised during the years ended December 31, 2017 and 2016, respectively; and (v) since May 8, 2018, 450,909 ordinary shares issuable upon the exercise of outstanding pre-funded warrants with an exercise price of $0.01 per share; these pre-funded warrants were fully exercised between May 8, 2018 and May 17, 2018. For additional information, see Note 9 to our Consolidated Financial Statements for the year ended December 31, 2018 included elsewhere in this Annual Report.
|
|
(4)
|
Working capital is defined as total current assets minus total current liabilities.
|
|
B.
|
Capitalization and Indebtedness
|
|
C.
|
Reasons for the Offer and Use of Proceeds
|
|
D.
|
Risk factors
|
|
|
•
|
we may not have adequate financial or other resources to complete the development of our product, demonstrate adequate clinical results, attain required regulatory approvals and licensures, and begin the commercialization efforts for our C-Scan system;
|
|
|
•
|
we may fail to obtain or maintain required regulatory approvals and licensures for our C-Scan system in our target markets or may face adverse regulatory or legal actions relating to our system even if regulatory approval is obtained;
|
|
|
•
|
we may not demonstrate adequate clinical safety and clinical effectiveness results to support regulatory body approval or market acceptance and adoption;
|
|
|
•
|
we may not be able to scale up the manufacture of our C-Scan system to commercial quantities at an adequate quality or at an acceptable cost;
|
|
|
•
|
we may not be able to establish adequate sales, marketing and distribution channels;
|
|
|
•
|
healthcare professionals and patients may not accept our C-Scan system;
|
|
|
•
|
we may not be aware of possible complications from the continued use of our C-Scan system because we have limited clinical experience with respect to the actual use of our C-Scan system;
|
|
|
•
|
other technological breakthroughs in CRC screening, treatment and prevention may reduce the demand for our C-Scan system;
|
|
|
•
|
changes in the market for CRC screening, new alliances between existing market participants and the entrance of new market participants may interfere with our market penetration efforts;
|
|
|
•
|
government and private third-party payors may not agree to provide coding, coverage and payment adequate to reimburse healthcare providers and patients for any or all of the purchase price of our C-Scan system, which may adversely affect healthcare providers’ and patients’ willingness to purchase our C-Scan system;
|
|
|
•
|
uncertainty as to market demand may result in inefficient pricing of our C-Scan system;
|
|
|
•
|
we may not be able to adequately protect our intellectual property or may face third-party claims of intellectual property infringement; and
|
|
|
•
|
we are dependent upon the results of ongoing clinical studies relating to our C-Scan system and the products of our competitors.
|
|
|
•
|
market acceptance of a new product, including healthcare professionals’ and patients’ preferences;
|
|
|
•
|
market acceptance of the clinical safety and performance of our C-Scan system;
|
|
|
•
|
development of similarly cost-effective products by our competitors;
|
|
|
•
|
development delays of our C-Scan system;
|
|
|
•
|
technological innovations in CRC screening, treatment and prevention;
|
|
|
•
|
adverse medical side effects suffered by patients using our C-Scan system, whether actually resulting from the use of our C-Scan system or not;
|
|
|
•
|
changes in regulatory policies toward CRC screening or imaging technologies;
|
|
|
•
|
changes in regulatory approval, clearance requirements and licensure for our product;
|
|
|
•
|
third-party claims of intellectual property infringement;
|
|
|
•
|
budget constraints and the availability of reimbursement or insurance coverage from third-party payors for our C-Scan system;
|
|
|
•
|
increases in market acceptance of other technologies;
|
|
|
•
|
adverse responses from certain of our competitors to the offering of our C-Scan system;
|
|
|
•
|
licensure and perceived risk of manufacturing and using a product containing a radioactive source; and
|
|
|
•
|
the shelf life of our C-Scan Cap.
|
|
|
•
|
there is sufficient long-term clinical and health-economic evidence to convince them to alter their existing screening methods and device recommendations;
|
|
|
•
|
there are recommendations from other prominent physicians, educators and/or associations that our C-Scan system is safe and effective;
|
|
|
•
|
we obtain favorable data from clinical and health-economic studies for our C-Scan system;
|
|
|
•
|
reimbursement or insurance coverage from government and private third-party payors is available;
|
|
|
•
|
healthcare professionals obtain required approvals and licensures for the handling, storage, dispensing, and disposal of our C-Scan system; and
|
|
|
•
|
healthcare professionals become familiar with the complexities of our C-Scan system.
|
|
|
•
|
foreign certification, registration and other regulatory requirements;
|
|
|
•
|
customs clearance and shipping delays;
|
|
|
•
|
import and export controls;
|
|
|
•
|
trade restrictions;
|
|
|
•
|
multiple and possibly overlapping tax structures;
|
|
|
•
|
difficulty forecasting the results of our international operations and managing our inventory due to our reliance on third-party distributors;
|
|
|
•
|
differing laws and regulations, business and clinical practices, licensures, government and private third-party payor reimbursement policies and patient preferences;
|
|
|
•
|
differing standards of intellectual property protection among countries;
|
|
|
•
|
difficulties in staffing and managing our international operations;
|
|
|
•
|
difficulties in penetrating markets in which our competitors’ products are more established and achieving a competitive sale price for our product;
|
|
|
•
|
currency exchange rate fluctuations and foreign currency exchange controls and tax rates; and
|
|
|
•
|
political and economic instability, war or acts of terrorism.
|
|
|
•
|
we may not be able to demonstrate to FDA’s satisfaction that our products are safe and effective for their intended use;
|
|
|
•
|
the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval;
|
|
|
•
|
in the case of a PMA submission, that the manufacturing process or facilities we use may not meet applicable requirements; and
|
|
|
•
|
changes in FDA’s 510(k) clearance, de novo reclassification, or PMA approval processes and policies, or the adoption of new regulations may require additional data.
|
|
|
•
|
patients do not enroll in the clinical trial at the rate we expect;
|
|
|
•
|
patients do not comply with trial protocols;
|
|
|
•
|
patient follow-up is not at the rate we expect;
|
|
|
•
|
undetected capsule retention in patients;
|
|
|
•
|
patients experience adverse side effects, including related to excessive radiation exposure as a result of capsule malfunction or break down;
|
|
|
•
|
patient death during a clinical trial, even though their death may be unrelated to our product;
|
|
|
•
|
FDA, institutional review boards, or IRBs, or other regulatory authorities do not approve a clinical trial protocol or a clinical trial, or place a clinical trial on hold;
|
|
|
•
|
IRBs, Ethics Committees and third-party clinical investigators may delay or reject our trial protocol and Informed Consent Form;
|
|
|
•
|
third-party clinical investigators decline to participate in a study or trial or do not perform a study or trial on our anticipated schedule or consistent with the investigator agreements, study or trial protocol, good clinical practices or other FDA or IRBs, Ethics Committees, or any other applicable requirements;
|
|
|
•
|
third-party organizations do not perform data collection, monitoring and analysis in a timely or accurate manner or consistent with the study or trial protocol or investigational or statistical plans;
|
|
|
•
|
regulatory inspections of our studies, trials or manufacturing facilities may require us to, among other things, undertake corrective action or suspend or terminate our studies or clinical trials;
|
|
|
•
|
changes in governmental regulations or administrative actions;
|
|
|
•
|
we may not be able to develop our C-Scan system at the rate or to the stage we desire:
|
|
|
•
|
the interim or final results of the study or clinical trial are inconclusive or unfavorable as to safety or efficacy; and
|
|
|
•
|
a regulatory agency or our Notified Body concludes that our trial design is or was inadequate to demonstrate safety and efficacy.
|
|
|
•
|
untitled letters, warning letters, fines, injunctions, corporate integrity agreements, consent decrees and civil penalties;
|
|
|
•
|
unanticipated expenditures to address or defend such actions;
|
|
|
•
|
customer notifications for repair, replacement or refunds;
|
|
|
•
|
recall, detention or seizure of our products;
|
|
|
•
|
operating restrictions or partial suspension or total shutdown of production;
|
|
|
•
|
refusing or delaying our requests for 510(k) clearance or premarket approval of new products or modified products;
|
|
|
•
|
operating restrictions;
|
|
|
•
|
withdrawing 510(k) clearances on PMA approvals that have already been granted;
|
|
|
•
|
suspension or withdrawal of our CE Certificates;
|
|
|
•
|
refusal to grant export approval for our products; or
|
|
|
•
|
criminal prosecution.
|
|
|
•
|
pending and future patent applications may not result in the issuance of patents or, if issued, may not be issued in a form that will be advantageous to us;
|
|
|
•
|
our issued patents may be challenged, invalidated or legally circumvented by third parties;
|
|
|
•
|
our patents may not be upheld as valid and enforceable or prevent the development of competitive products;
|
|
|
•
|
the eligibility of certain inventions related to diagnostic medicine, more specifically diagnostic methods and processes, for patent protection in the United States has been limited recently which may affect our ability to enforce our issued patents in the United States or may make it difficult to obtain broad patent protection going forward in the United States;
|
|
|
•
|
for a variety of reasons, we may decide not to file for patent protection on various improvements or additional features; and
|
|
|
•
|
intellectual property protection and/or enforcement may be unavailable or limited in some countries where laws or law enforcement practices may not protect our proprietary rights to the same extent as the laws of the United States, the European Union, Canada or Israel.
|
|
|
•
|
the agreements may be breached, may not provide the scope of protection we believe they provide or may be determined to be unenforceable;
|
|
|
•
|
we may have inadequate remedies for any breach;
|
|
|
•
|
proprietary information could be disclosed to our competitors; or
|
|
|
•
|
others may independently develop substantially equivalent or superior proprietary information and techniques or otherwise gain access to our trade secrets or disclose such technologies.
|
|
|
•
|
we may not be able to develop our C-Scan system at the rate or to the stage we desire;
|
|
|
•
|
inability to obtain the approvals necessary to commence further clinical trials;
|
|
|
•
|
unsatisfactory results of clinical trials;
|
|
|
•
|
announcements of regulatory approval or the failure to obtain it, or specific label indications or patient populations for its use, or changes or delays in the regulatory review process;
|
|
|
•
|
any intellectual property infringement actions in which we may become involved;
|
|
|
•
|
announcements concerning our competitors or the medical device industry in general;
|
|
|
•
|
achievement of expected product sales and profitability or our failure to meet expectations;
|
|
|
•
|
our commencement of, or involvement in, litigation;
|
|
|
•
|
any major changes in our board of directors or management;
|
|
|
•
|
legislation in the United States relating to the sale or pricing of medical device;
|
|
|
•
|
future substantial sales of our ordinary shares;
|
|
|
•
|
changes in earnings estimates or recommendations by securities analysts, if our ordinary shares are covered by analysts; or
|
|
|
•
|
the trading volume of our ordinary shares.
|
|
A.
|
History and Development of the Company
|
|
B.
|
Business Overview
|
|
|
•
|
eliminating the need for fasting and prior bowel preparation, which would differentiate our system from every other currently available structural screening exam;
|
|
|
•
|
providing patients with a procedure that requires them to swallow our C-Scan Cap and small amounts of a contrast agent, thereby minimizing any disruption to their normal activities;
|
|
|
•
|
eliminating the need to sedate patients;
|
|
|
•
|
obviating the requirement for the insufflation (the forcing of air into the gastrointestinal tract) of patients; and
|
|
|
•
|
providing digital reporting, storage and remote consulting capabilities.
|
|
|
•
|
seeking to obtain regulatory approvals for the sale of our C-Scan system initially in the United States and Japan following receipt of CE marking for the marketing and sale of our C-Scan system in the European Union;
|
|
|
•
|
in Europe and Japan, we intend to offer our C-Scan system as an imaging and screening tool for the general population. In the United States, we may choose to first obtain regulatory clearance/approval for our C-Scan system in a screening sub-population, and after we have conducted more extensive clinical studies in the United States, we would anticipate applying to the FDA for the use of our C-Scan system as a primary screening tool;
|
|
|
•
|
obtaining government and private third-party reimbursement for our technology;
|
|
|
•
|
improving and enhancing our existing technology portfolio and developing new technologies; and
|
|
|
•
|
successfully marketing our product to establish a large customer base.
|
|
|
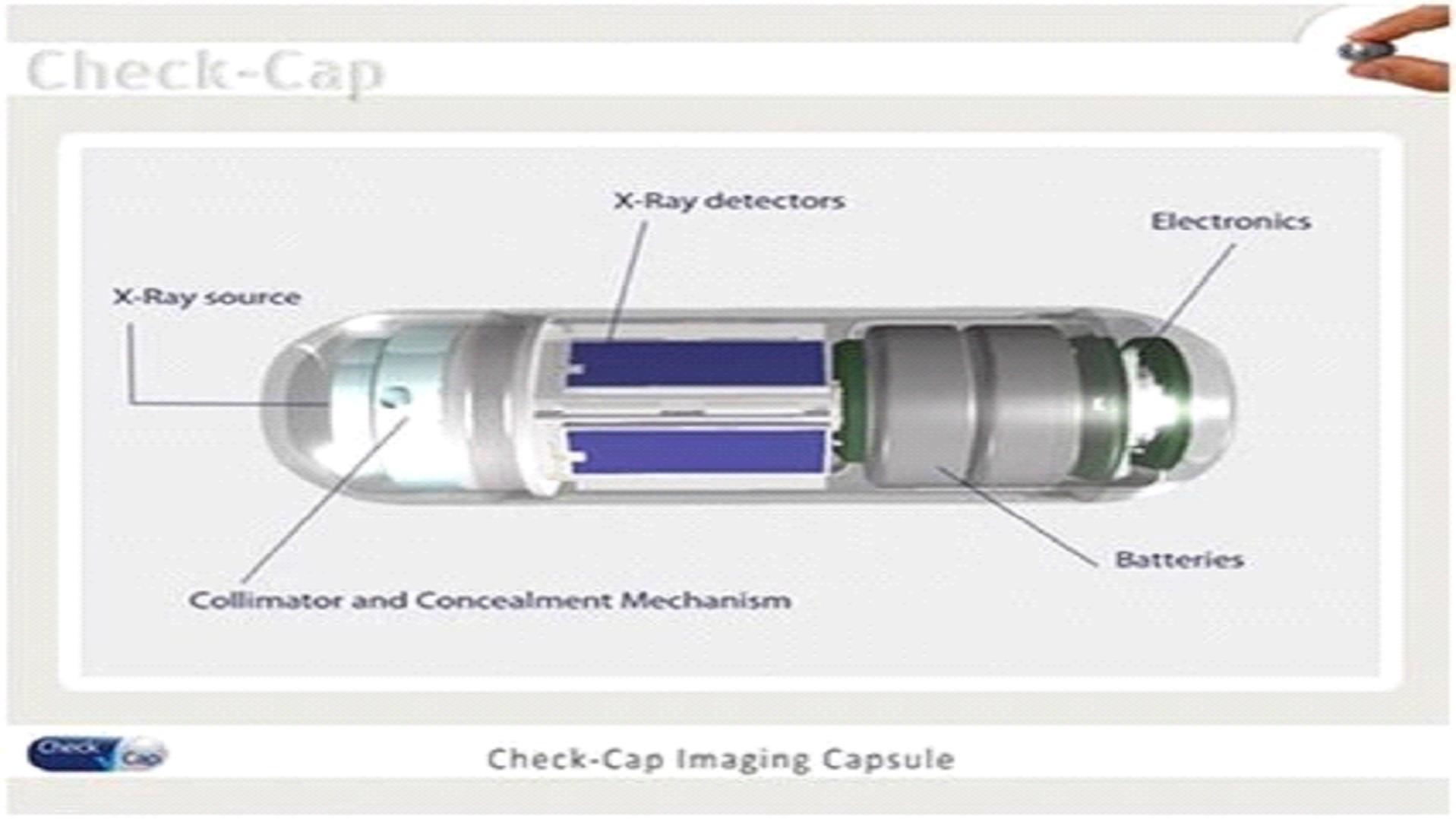
•
|
X-ray Source – Including radioactive material sealed in a cylindrical housing.
|
|
|
•
|
Collimator – Radiation shield around the source, which absorbs most of the radiation. Several radial holes enable emission of radiation in defined directions.
|
|
|
•
|
X-ray Sensor – Comprised of several solid state X-ray detectors for measuring the scattered radiation intensity.
|
|
|
•
|
Tilt Sensor – Indication of capsule motion (3D acceleration).
|
|
|
•
|
Rotation Motor – For rotating the collimator and X-ray Source.
|
|
|
•
|
Compass sensor – Indication of true north (reference coordinate system).
|
|
|
•
|
Pressure sensor – indicating the hydrostatic pressure inside the colon.
|
|
|
•
|
Source Concealment Mechanism – Conceals the source inside the radiation shield.
|
|
|
•
|
R-T – Radio frequency transceiver device to communicate with the receiver.
|
|
|
•
|
Batteries – Electrical power supply for the capsule.
|
|
|
•
|
Memory – Data storage. The capsule should be able to store up to an hour of measured data.
|
|
|
•
|
C-Scan Track Coil – Transmits a continuous electromagnetic field utilized by an external localization system to track 3D position.
|
|
|
•
|
Sticker Housings – Biocompatible and water-resistant stickers and housing integrating all functional components, attached to the patient’s back, enabling five days of continuous operation.
|
|
|
•
|
Recorder – Consists of receiver electronics embedded software and nonvolatile memory.
|
|
|
•
|
Antennas – Radio frequency antennas are embedded into the sticker housings and used to communicate with the capsule.
|
|
|
•
|
Activation/Deactivation Circuit – Used to activate/deactivate the C-Scan Track through a specialized protocol.
|
|
|
•
|
UI Indicators – Provides user with vocal and vibration indication as required.
|
|
|
•
|
PCB – Electronics’ printed circuit boards.
|
|
|
•
|
Microcontroller – Runs embedded software, logic that manages the C-Scan Track and SCA.
|
|
|
•
|
RF Transceivers – Several transceivers used to communicate with the capsule.
|
|
|
•
|
TILT/Compass Sensors – To determine the patient’s body movements.
|
|
|
•
|
Batteries – Electrical power supply for the C-Scan Track.
|
|
|
•
|
Memory – Non-volatile data storage to store data acquired by the system.
|

|
|
•
|
Communication Driver Software – to communicate with the C-Scan Track and retrieve collected data following procedure completion.
|
|
|
•
|
Data Processing Software – to process and reconstruct clinical data into a 3D structure.
|
|
|
•
|
Data Display and Management Software – includes the following functions:
|
|
|
○
|
3D visualization and 2D maps of the reconstructed colon surface.
|
|
|
○
|
Annotation tools.
|
|
|
○
|
Registration of patient and capsule data and management of the patient database.
|
|
|
○
|
Report – to enable generation of clinical results report out.
|
 Image for illustration purpose only
Image for illustration purpose only


|
|
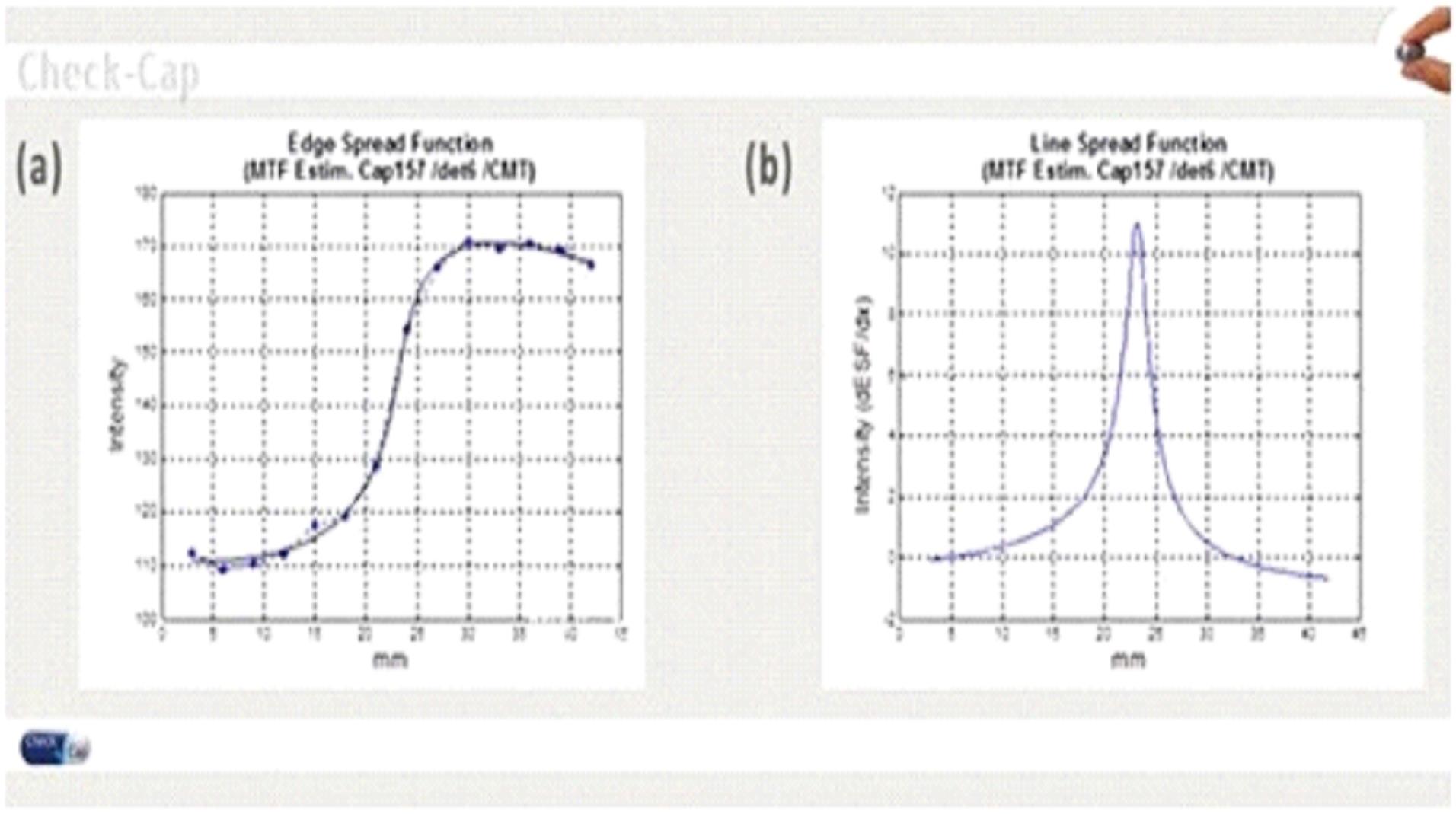
•
|
The number of photons hitting the detector per time frame.
|
|
|
•
|
The angular spread of the photon beam coming out of the capsule collimator.
|


|
|
•
|
our technology has been tested on a limited basis and therefore we cannot assure the product’s clinical value;
|
|
|
•
|
following the receipt of CE Mark of conformity to protection
standards for sale of the
C-Scan system in the European Union, we may need to obtain additional regulatory approvals in certain local jurisdictions in the European Union before we can commence marketing and sale of our C-Scan system and will need to obtain the requisite regulatory approvals in the United States, Japan and other markets where we plan to focus our commercialization efforts;
|
|
|
•
|
we need to raise an amount of capital sufficient to complete the development of our technology, obtain the requisite regulatory approvals and commercialize our current and future products;
|
|
|
•
|
we need to obtain reimbursement coverage from third-party payors for procedures using our C-Scan system;
|
|
|
•
|
we need to scale-up our manufacturing capabilities of our C-Scan system in commercial quantities at an adequate quality and at an acceptable cost; and
|
|
|
•
|
we need to establish and expand our user base while competing against other sellers of capsule endoscopy systems as well as other current and future CRC screening technologies and methods.
|
|
|
•
|
product design and development;
|
|
|
•
|
product testing;
|
|
|
•
|
validation and verifications;
|
|
|
•
|
product manufacturing;
|
|
|
•
|
product labeling;
|
|
|
•
|
product storage, shipping and handling;
|
|
|
•
|
premarket clearance or approval;
|
|
|
•
|
advertising and promotion;
|
|
|
•
|
product marketing, sales and distribution; and
|
|
|
•
|
post-market surveillance reporting death or serious injuries and medical device reporting.
|
|
|
•
|
Class I devices, which are subject to only general controls (
e.g.
, labeling, medical devices reporting, and prohibitions against adulteration and misbranding) and, in some cases, to the 510(k) premarket clearance requirements;
|
|
|
•
|
Class II devices, generally requiring 510(k) premarket clearance before they may be commercially marketed in the United States; and
|
|
|
•
|
Class III devices, consisting of devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a predicate device, generally requiring the submission of a PMA approval supported by clinical trial data.
|
|
|
•
|
product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action;
|
|
|
•
|
Quality System Regulation, or QSR, and current good manufacturing practices, or cGMP, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process;
|
|
|
•
|
labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label use or indication;
|
|
|
•
|
clearance of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use of one of our cleared devices;
|
|
|
•
|
approval of product modifications that affect the safety or effectiveness of one of our approved devices;
|
|
|
•
|
medical device reporting regulations, which require that manufacturers comply with FDA requirements to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of the device or a similar device were to recur;
|
|
|
•
|
post-approval restrictions or conditions, including post-approval study commitments;
|
|
|
•
|
post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device;
|
|
|
•
|
FDA’s recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations;
|
|
|
•
|
regulations pertaining to voluntary recalls; and
|
|
|
•
|
notices of corrections or removals.
|
|
|
•
|
untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties;
|
|
|
•
|
unanticipated expenditures to address or defend such actions;
|
|
|
•
|
customer notifications for repair, replacement, refunds;
|
|
|
•
|
recall, detention or seizure of our products;
|
|
|
•
|
operating restrictions or partial suspension or total shutdown of production;
|
|
|
•
|
refusing or delaying our requests for 510(k) clearance or premarket approval of new products or modified products;
|
|
|
•
|
operating restrictions;
|
|
|
•
|
withdrawing 510(k) clearances on PMA approvals that have already been granted;
|
|
|
•
|
refusal to grant export approval for our products; or
|
|
|
•
|
criminal prosecution.
|
|
|
•
|
The federal Anti-Kickback Statute, which prohibits, among other things, knowingly or willingly offering, paying, soliciting or receiving remuneration, directly or indirectly, in cash or in kind, to induce or reward the purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any health care items or service for which payment may be made, in whole or in part, by federal healthcare programs such as Medicare and Medicaid. This statute has been interpreted to apply to arrangements between medical device manufacturers on one hand and prescribers, purchasers and formulary managers on the other. Further, PPACA, among other things, clarified that a person or entity needs not to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it. Although there are a number of statutory exemptions and regulatory safe harbors to the federal Anti-Kickback Statute protecting certain common business arrangements and activities from prosecution or regulatory sanctions, the exemptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exemption or safe harbor may be subject to scrutiny;
|
|
|
•
|
The federal civil False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment of government funds or knowingly making, using or causing to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to pay money to the federal government. In addition, PPACA amended the Social Security Act to provide that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. Many medical device manufacturers and other healthcare companies have been investigated and have reached substantial financial settlements with the federal government under the civil False Claims Act for a variety of alleged improper marketing activities, including providing free product to customers with the expectation that the customers would bill federal programs for the product; providing consulting fees, grants, free travel, and other benefits to physicians to induce them to use the company’s products. In addition, in recent years the government has pursued civil False Claims Act cases against a number of manufacturers for causing false claims to be submitted as a result of the marketing of their products for unapproved, and thus non-reimbursable, uses. Device manufacturers also are subject to other federal false claim laws, including, among others, federal criminal healthcare fraud and false statement statutes that extend to non-government health benefit programs;
|
|
|
•
|
Analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to items or services reimbursed under Medicaid and other state programs or, in several states, apply regardless of the payor. Several states now require medical device manufacturers to report expenses relating to the marketing and promotion or require them to implement compliance programs or marketing codes. For example, California, Connecticut and Nevada mandate the implementation of corporate compliance programs, while Massachusetts and Vermont impose more detailed restrictions on device manufacturers' marketing practices and tracking and reporting of gifts, compensation and other remuneration to healthcare providers;
|
|
|
•
|
The federal Foreign Corrupt Practices Act of 1997 and other similar anti-bribery laws in other jurisdictions generally prohibit companies and their intermediaries from providing money or anything of value to officials of foreign governments, foreign political parties, or international organizations with the intent to obtain or retain business or seek a business advantage. Recently, there has been a substantial increase in anti-bribery law enforcement activity by U.S. regulators, with more frequent and aggressive investigations and enforcement proceedings by both the Department of Justice and the U.S. Securities and Exchange Commission. Violations of these laws can result in the imposition of substantial fines, interruptions of business, loss of supplier, vendor or other third-party relationships, termination of necessary licenses and permits, and other legal or equitable sanctions. Other internal or government investigations or legal or regulatory proceedings, including lawsuits brought by private litigants, may also follow as a consequence; and
|
|
|
•
|
The federal Physician Payment Sunshine Act, being implemented as the Open Payments Program, requires manufacturers of “covered products” (drugs, devices, biologics, or medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program) to track and publicly report payments and other transfers of value that they provide to U.S. physicians and teaching hospitals, as well as any ownership interests that U.S. physicians hold in applicable manufacturer. Applicable manufacturers must submit a report to the Centers for Medicare & Medicaid Services, or CMS, by the 90th day of each calendar year disclosing payments and transfers of value made in the preceding calendar year.
|
|
|
•
|
No. 1 type license for marketing – Specially controlled medical devices (Class III, IV)
|
|
|
•
|
No. 2 type license for marketing – Controlled medical devices (Class II)
|
|
|
•
|
No. 3 type license for marketing – General medical devices (Class I)
|
|
C.
|
Organizational Structure
|
|
D.
|
Property, Plants and Equipment
|
|
A.
|
Operating Results
|
|
|
•
|
employee-related expenses for research and development staff, including salaries, benefits and related expenses, including share-based compensation and travel expenses;
|
|
|
•
|
payments made to third-party contract research organizations, contract manufacturers, investigative sites and consultants;
|
|
|
•
|
manufacturing development costs;
|
|
|
•
|
costs associated with preclinical and clinical activities and regulatory operations;
|
|
|
•
|
facilities, depreciation and other expenses, which include direct and allocated expenses for rent and maintenance of facilities; and
|
|
|
•
|
costs associated with obtaining and maintaining patents.
|
|
|
Year Ended December 31,
|
|||||||
|
|
2018
|
2017
|
||||||
|
|
(US$ in thousands, except
per
share data)
|
|||||||
|
Research and development expenses, net
|
$
|
7,618
|
$
|
6,837
|
||||
|
General and administrative expenses
|
3,445
|
3,164
|
||||||
|
Operating loss
|
11,063
|
10,001
|
||||||
|
Finance income, net
|
473
|
236
|
||||||
|
Loss before income tax
|
10,590
|
9,765
|
||||||
|
Taxes on income
|
(1
|
)
|
6
|
|||||
|
Net loss
|
$
|
10,589
|
$
|
9,771
|
||||
|
|
2018
|
2017
|
Change
|
|||||||||
|
|
(US$ in thousands)
|
|||||||||||
|
Salaries and related expenses
|
$
|
4,410
|
$
|
4,656
|
$
|
(246
|
)
|
|||||
|
Share-based compensation
|
234
|
116
|
118
|
|||||||||
|
Materials
|
1,508
|
614
|
894
|
|||||||||
|
Subcontractors and consultants
|
311
|
456
|
(145
|
)
|
||||||||
|
Depreciation
|
138
|
147
|
(9
|
)
|
||||||||
|
Cost for registration of patents
|
126
|
157
|
(31
|
)
|
||||||||
|
Other research and development expenses
|
1,099
|
893
|
206
|
|||||||||
|
|
7,826
|
7,039
|
787
|
|||||||||
|
Less participation of the IIA (formerly the OCS) and the BIRD Foundation
|
(208
|
)
|
(202
|
)
|
(6
|
)
|
||||||
|
Total research and development expenses, net
|
$
|
7,618
|
$
|
6,837
|
$
|
781
|
||||||
|
|
2018
|
2017
|
Change
|
|||||||||
|
|
(US$ in thousands)
|
|||||||||||
|
Salaries and related expenses
|
$
|
1,839
|
$
|
1,395
|
$
|
444
|
||||||
|
Share-based compensation
|
(299
|
)
|
610
|
(909
|
)
|
|||||||
|
Professional services
|
833
|
414
|
419
|
|||||||||
|
Office rent and maintenance
|
163
|
161
|
2
|
|||||||||
|
Depreciation
|
10
|
10
|
-
|
|||||||||
|
Other general and administrative expenses
|
899
|
574
|
325
|
|||||||||
|
Total general and administrative expenses
|
$
|
3,445
|
$
|
3,164
|
$
|
281
|
||||||
|
|
• |
For the year ended December 31, 2018, we recorded $243,000 of interest income on short-term deposits as compared to $66,000, for the year ended December 31, 2017, an increase of $177,000 resulting from a higher average short-term deposits balance in 2018 as a result of our May 2018 underwritten public offering and an increase in interest rates on these short-term deposits.
|
|
|
• |
For the year ended December 31, 2018, we recorded finance income of $255,000 as a result of changes in provision for royalties, primarily to Check–Cap LLC unitholders, as compared to finance income of $82,000 for the year ended December 31, 2017, an increase of $173,000.
|
|
|
• |
For the year ended December 31, 2018, we recorded $27,000 finance expense as a result of exchange rate differences as compared to finance income of $95,000 for the year ended December 31, 2017, a decrease of $122,000.
|
|
|
Year Ended December 31,
|
|||||||
|
|
2017
|
2016
|
||||||
|
|
(US$ in thousands, except
per share data)
|
|||||||
|
Research and development expenses, net
|
$
|
6,837
|
$
|
5,491
|
||||
|
General and administrative expenses
|
3,164
|
3,571
|
||||||
|
Operating loss
|
10,001
|
9,062
|
||||||
|
Finance income, net
|
236
|
244
|
||||||
|
Loss before income tax
|
9,765
|
8,818
|
||||||
|
Taxes on income
|
6
|
8
|
||||||
|
Net loss
|
$
|
9,771
|
$
|
8,826
|
||||
|
|
2017
|
2016
|
Change
|
|||||||||
|
|
(US$ in thousands)
|
|||||||||||
|
Salaries and related expenses
|
$
|
4,656
|
$
|
4,683
|
$
|
(27
|
)
|
|||||
|
Share-based compensation
|
116
|
234
|
(118
|
)
|
||||||||
|
Materials
|
614
|
596
|
18
|
|||||||||
|
Subcontractors and consultants
|
456
|
320
|
136
|
|||||||||
|
Depreciation
|
147
|
121
|
26
|
|||||||||
|
Cost for registration of patents
|
157
|
150
|
7
|
|||||||||
|
Other research and development expenses
|
893
|
511
|
382
|
|||||||||
|
|
7,039
|
6,615
|
424
|
|||||||||
|
Less participation of the IIA (formerly the OCS) and the BIRD Foundation
|
(202
|
)
|
(1,124
|
)
|
922
|
|||||||
|
Total research and development expenses, net
|
$
|
6,837
|
$
|
5,491
|
$
|
1,346
|
||||||
|
|
2017
|
2016
|
Change
|
|||||||||
|
|
(US$ in thousands)
|
|||||||||||
|
Salaries and related expenses
|
$
|
1,395
|
$
|
1,411
|
$
|
(16
|
)
|
|||||
|
Share-based compensation
|
610
|
975
|
(365
|
)
|
||||||||
|
Professional services
|
414
|
354
|
60
|
|||||||||
|
Office rent and maintenance
|
161
|
144
|
17
|
|||||||||
|
Depreciation
|
10
|
9
|
1
|
|||||||||
|
Other general and administrative expenses
|
574
|
678
|
(104
|
)
|
||||||||
|
Total general and administrative expenses
|
$
|
3,164
|
$
|
3,571
|
$
|
(407
|
)
|
|||||
|
|
• |
For the year ended December 31, 2017, we recorded $66,000 of interest income on short-term deposits as compared to $139,000, for the year ended December 31, 2016, a decrease of $73,000 resulting from a lower average short-term deposits balance in 2017.
|
|
|
• |
For the year ended December 31, 2017, we recorded finance income of $82,000 as a result of changes in provision for royalties, primarily to Check–Cap LLC unitholders, as compared to a finance income of $56,000 in the year ended December 31, 2016, a decrease of $26,000.
|
|
|
• |
For the year ended December 31, 2017, we recorded $95,000 finance income as a result of exchange rate differences as compared to $56,000, for the year ended December 31, 2016, an increase of $39,000.
|
|
B.
|
Liquidity and Capital Resources
|
|
|
Year Ended December 31,
|
|||||||||||
|
|
2018
|
2017
|
2016
|
|||||||||
|
|
(US$ in thousands)
|
|||||||||||
|
Net cash used in operating activities
|
$
|
(10,034
|
)
|
$
|
(9,150
|
)
|
$
|
(7,923
|
)
|
|||
|
Net cash provided by (used in) investing activities
|
$
|
(5,723
|
)
|
$
|
(231
|
)
|
$
|
4,645
|
||||
|
Net cash provided by financing activities
|
$
|
17,762
|
$
|
4,575
|
$
|
5,424
|
||||||
|
|
•
|
completion of the clinical development of our C-Scan system;
|
|
|
•
|
conducting clinical trials in Europe, the United States and other territories for purposes of regulatory approval and post-marketing validation;
|
|
|
•
|
development of future generations of our C-Scan system and future products;
|
|
|
•
|
FDA and additional regulatory filing activities in countries we intend to commercialize our system; and
|
|
|
•
|
patent maintenance fees.
|
| • |
Fair Value of our Ordinary Shares.
Following our initial public offering, the fair value of our ordinary shares is determined based on the trading price of our ordinary shares on the Nasdaq Capital Market.
|
| • |
Expected Volatility.
In the year ended December 31, 2018, we estimated the
expected volatility
for our ordinary shares
based upon actual historical stock price movements of the share price
of our ordinary shares on the Nasdaq Capital Market
over the most recent periods ending on the grant date, equal to the expected term of the options.
In the years ended December 31, 2017 and 2016, due to the lack of history of trading information of our shares, we estimated the expected share price volatility for our ordinary shares by considering the historic price volatility for industry peers based on price observations over a period equivalent to the expected term of the share option grants. Industry peers consist of public companies in the medical device and healthcare industries.
|
| • |
Expected Term.
The expected term of options granted represents the period of time that options granted are expected to be outstanding, and is determined based on the simplified method in accordance with ASC No. 718-10-S99-1 (SAB No. 110), as adequate historical experience is not available to provide a reasonable estimate. ASU 2016-09, Compensation-Stock Compensation (Topic 718) permits forfeitures to be accounted for when they occur.
|
| • |
Risk-Free Rate.
The risk-free interest rate is based on the yield from U.S. Treasury zero-coupon bonds with a term equivalent to the contractual life of the options.
|
| • |
Expected
Dividend
Yield.
We have never declared or paid any cash dividends and do not presently plan to pay cash dividends in the foreseeable future. Consequently, we used an expected dividend yield of zero.
|
|
Parameters
|
|
Year 2018 Grants
|
|
|
Year 2017 Grants
|
|
|
Year 2016 Grants
|
|
|
|
Expected volatility (in %)
|
|
104-108
|
|
|
58-60
|
|
|
59-60
|
|
|
|
Expected term (in years)
|
|
5.5-7
|
|
|
5.5-7
|
|
|
5.5-7
|
|
|
|
Risk free interest rate (in %)
|
|
2.67-3.15
|
|
|
1.9-2.2
|
|
|
1.2-2.1
|
|
|
|
Anticipated rate of dividends (in %)
|
|
0
|
|
|
0
|
|
|
0
|
|
|
|
Share Price
|
|
$3.24-$9.07
|
|
|
$15.96-$26.40
|
|
|
$26.64-$38.40
|
|
|
|
C.
|
Research and development, patents and licenses, etc.
|
|
D.
|
Trend Information
|
|
E.
|
Off-balance Sheet Arrangements
|
|
F.
|
Tabular Disclosure of Contractual Obligations
|
|
|
Payments due by period
|
|||||||||||||||||||
|
|
(US$ in thousands)
|
|||||||||||||||||||
|
|
Total
|
Less than 1
year
|
1-3 years
|
4-5 years
|
More than 5
years
|
|||||||||||||||
|
Operating lease obligations
(1)
:
|
$
|
446
|
180
|
233
|
33
|
0
|
||||||||||||||
|
|
||||||||||||||||||||
|
Other long-term liabilities reflected on the Statements of Financial Position:
|
||||||||||||||||||||
|
Royalties to ASIC designer
(2)
|
$
|
120
|
0
|
0
|
120
|
0
|
||||||||||||||
|
Reimbursement liability to Check-Cap LLC unitholders
(3)
|
$
|
65
|
0
|
0
|
30
|
35
|
||||||||||||||
|
Total
|
$
|
631
|
180
|
233
|
183
|
35
|
||||||||||||||
|
(1)
|
Operating lease obligations consist of payments pursuant to a lease agreement for office facilities as well as lease agreements for vehicles, which generally run for a period of three years.
|
|
(2)
|
See Item 5B “Operating and Financial Review and Prospects—Liquidity and Capital Resources—Application of Critical Accounting Policies and Estimates—Royalties provision—Provision for royalties to an ASIC designer.”
|
|
(3)
|
See Item 5B “Operating and Financial Review and Prospects—Liquidity and Capital Resources—Application of Critical Accounting Policies and Estimates—Royalties provision—
Reimbursement liability to Check-Cap LLC unitholders
.”
|
|
A.
|
Directors and senior management
|
|
Name
|
|
Age
|
|
Position(s)
|
|
|
Alex Ovadia
|
|
57
|
|
Chief Executive Officer
|
|
|
Mira Rosenzweig
(1)
|
47
|
Incoming Chief Financial Officer
|
|||
|
Yoav Kimchy
|
|
58
|
|
Chief Technology Officer
|
|
|
Boaz Shpigelman
|
|
47
|
|
Vice President, Research and Development
|
|
|
Steven Hanley (2)(3)
|
|
51
|
|
Chairman of the Board of Directors
|
|
|
Clara Ezed (4)(5)
|
|
47
|
|
Director
|
|
|
Mary Jo Gorman (2)(3)(4)(5)
|
|
59
|
|
Director
|
|
|
XiangQian (XQ) Lin
|
|
35
|
|
Director
|
|
|
Yuval Yanai (2)(3)(4)(5)
|
|
66
|
|
Director
|
|
|
(1)
|
On February 28, 2019, we appointed Mr. Kochav as our Interim Chief Financial Officer. On March 18, 2019, we announced that Mira Rosenzweig will serve as our Chief Financial Officer, effective as of April 28, 2019. Concurrently with Ms. Rosenzweig’s appointment as our Chief Financial Officer, Mr. Kochav will discontinue serving as our Interim Chief Financial Officer.
|
|
(2)
|
Member of our Nominating Committee.
|
|
(3)
|
Member of our Financing Committee.
|
|
|
|
|
(4)
|
Member of our Compensation Committee.
|
|
(5)
|
Member of our Audit Committee.
|
|
B.
|
Compensation of Directors and Executive Officers
|
|
Salary Cost
(1)
|
Bonus
(2)
|
Share-Based
Compensation (3) |
Total
|
|||||||||||||
|
Name and Principal Position
|
US$
|
|||||||||||||||
|
Alex Ovadia - Chief Executive Officer (since February 2018); former Chief Operations Officer, Israeli Site Manager and Vice President, Research and Development (4)
|
367,641
|
113,998
|
21,272
|
502,912
|
||||||||||||
|
Lior Torem – Former Chief Financial Officer and Vice President of Operations (5)
|
316,136
|
90,483
|
51,029
|
457,649
|
||||||||||||
|
Yoav Kimchy -
Chief Technology Officer
|
331,844
|
90,235
|
48,214
|
470,293
|
||||||||||||
|
Boaz Shpigelman -Vice President, Research and Development (since March 2018); former
director of development
(6)
|
206,243
|
45,683
|
36,204
|
288,130
|
||||||||||||
|
Israel Hershko – Director of Quality Assurance and Regulatory Affairs (7)
|
171,649
|
13,682
|
16,159
|
201,490
|
||||||||||||
|
(1)
|
“Salary Cost” includes the Covered Executive’s gross salary plus payment of social benefits made by us on behalf of such Covered Executive. Such benefits may include, to the extent applicable to the Covered Executive, payments, contributions and/or allocations for savings funds, education funds, pension, severance, risk insurances, payments for social security and tax gross-up payments, vacation, car, medical insurances and benefits, convalescence or recreation pay and other benefits and perquisites consistent with our policies.
|
|
(2)
|
Represents bonuses for 2018 awarded to the Covered Executives, consistent with our Compensation Policy.
|
|
(3)
|
Represents the share-based compensation expenses recorded in our consolidated financial statements for the year ended December 31, 2018 based on the fair value of the grant date of the equity awards, in accordance with accounting guidance for equity-based compensation.
|
|
(4)
|
Mr. Ovadia was appointed as our Chief Executive Officer on February 26, 2018.
|
|
(5)
|
Mr. Torem ceased to serve as our Chief Financial Officer and Vice President of Operations on February 14, 2019.
|
|
(6)
|
Boaz Shpigelman was appointed as our Vice President, Research and Development in March 2018. Mr. Shpigelman served as our
Director
of
Development
from August 2012 to March 2018.
|
|
(7)
|
Israel Hershko was appointed as our Director of Quality and Regulation Director on September 2017. Mr. Hershko is not considered an executive officer, and is included in the table above solely to satisfy disclosure requirements under the Israeli Companies Law.
|
|
Name
|
Date of
Grant (1) |
Security Type
|
Purchase
Price (in NIS) |
Number of Shares
Underlying Award
|
|
Expiration Date
|
|
Total Benefit
(in US$) |
|
Benefit
recognized in 2018 (in US$) |
|
||||
|
Lior
Torem
|
July 30, 2018
|
Options
|
$3.92
|
29,632
|
|
July 30, 2028
|
|
77,472
|
|
21,734
|
|
||||
|
RSUs
|
-
|
12,699
|
|
-
|
|
41,146
|
|
11,543
|
|
||||||
|
Yoav
Kimchy
|
July 30, 2018
|
Options
|
$3.92
|
29,632
|
|
July 30, 2028
|
|
77,472
|
|
21,734
|
|
||||
|
RSUs
|
-
|
12,699
|
|
-
|
|
41,146
|
|
11,543
|
|
||||||
|
Boaz
Shpigelman
|
July 30, 2018
|
Options
|
$3.92
|
25,928
|
|
July 30, 2028
|
|
67,788
|
|
19,017
|
|
||||
|
RSUs
|
-
|
11,112
|
|
-
|
|
36,002
|
|
10,100
|
|
||||||
|
Israel
Hershko
|
July 30, 2018
|
Options
|
$3.92
|
11,112
|
|
July 30, 2028
|
|
29,052
|
|
8,150
|
|
||||
|
RSUs
|
-
|
4,762
|
|
-
|
|
15,430
|
|
4,329
|
|
|
__________________________
|
|
|
(1)
|
All options and RSUs granted to
the Covered Executives
in fiscal year 2018 vest over a three-year period
commencing on their date of grant,
such that
33.33% of the award shall vest on the first anniversary of the date of grant and an additional 8.33% will vest at the end of each subsequent three-month period thereafter, subject to each executive officer’s continuing service in such capacity on each applicable vesting date
.
|
|
C.
|
Board Practices
|
|
|
·
|
oversight of our independent registered public accounting firm and recommending the engagement, compensation or termination of engagement of our independent registered public accounting firm to the board of directors or shareholders for their approval, as applicable, in accordance with the requirements of the Israeli Companies Law;
|
|
|
·
|
recommending the engagement or termination of the person filling the office of our internal auditor; and
|
|
|
·
|
recommending the terms of audit and non-audit services provided by the independent registered public accounting firm for pre-approval by our board of directors or shareholders for their approval, as applicable, in accordance with the requirements of the Israeli Companies Law.
|
|
|
·
|
determining whether there are deficiencies in the business management practices of our company, including in consultation with our internal auditor or the independent auditor, and making recommendations to the board of directors to improve such practices;
|
|
|
·
|
determining whether to approve certain related party transactions (including transactions in which an office holder has a personal interest) and whether such transaction is extraordinary or material under Israeli Companies Law (see “— Approval of Related Party Transactions under Israeli Law”);
|
|
|
·
|
determining whether a competitive process must be implemented for the approval of certain transactions with controlling shareholders or its relative or in which a controlling shareholder has a personal interest (whether or not the transaction is an extraordinary transaction), under the supervision of the audit committee or other party determined by the audit committee and in accordance with standards to be determined by the audit committee, or whether a different process determined by the audit committee should be implemented for the approval of such transactions;
|
|
|
·
|
determining the process for the approval of certain transactions with controlling shareholders or in which a controlling shareholder has a personal interest that the audit committee has determined are not extraordinary transactions but are not immaterial transactions;
|
|
|
·
|
where the board of directors approves the working plan of the internal auditor, to examine such working plan before its submission to the board of directors and proposing amendments thereto;
|
|
|
·
|
examining our internal controls and internal auditor’s performance, including whether the internal auditor has sufficient resources and tools to dispose of its responsibilities;
|
|
|
·
|
examining the scope of our auditor’s work and compensation and submitting a recommendation with respect thereto to our board of directors or shareholders, depending on which of them is considering the compensation of our auditor; and
|
|
|
·
|
establishing procedures for the handling of employees’ complaints as to the management of our business and the protection to be provided to such employees.
|
|
|
·
|
recommending to the board of directors for its approval (i) a compensation policy; (ii) whether a compensation policy should continue in effect, if the then-current policy has a term of greater than three years (approval of either a new compensation policy or the continuation of an existing compensation policy must in any case occur every three years); and (iii) periodic updates to the compensation policy. See “— Compensation Committee and Compensation Policy.” In addition, the compensation committee is required to periodically examine the implementation of the compensation policy;
|
|
|
·
|
the approval of the terms of employment and service of office holders (including determining whether the compensation terms of a candidate for chief executive officer of the company need not be brought to approval of the shareholders); and
|
|
|
·
|
reviewing and approving grants of options and other incentive awards to persons other than office holders to the extent such authority is delegated by our board of directors, subject to the limitations on such delegation as provided in the Israeli Companies Law.
|
|
|
•
|
the knowledge, skills, expertise, professional experience and accomplishments of the relevant office holder;
|
|
|
•
|
the office holder’s roles and responsibilities and prior compensation agreements with him or her;
|
|
|
•
|
the ratio of the cost of the offered terms to the cost of compensation of the other employees of the company (including any employees employed through manpower companies), specifically to the cost of the average and median salaries of such employees and the impact of the disparities between them upon work relationships in the company;
|
|
|
•
|
with respect to variable compensation - the possibility of reducing variable compensation at the discretion of the board of directors, and the possibility of setting a limit on the exercise value of non-cash variable equity-based compensation; and
|
|
|
•
|
with respect to severance compensation, the period of employment or service of the office holder, the terms of his or her compensation during such period, the company’s performance during such period, the person’s contribution towards the company’s achievement of its goals and the maximization of its profits, and the circumstances under which the person is leaving the company.
|
|
|
•
|
the link between variable compensation (e.g., bonuses) and long-term performance and measurable criteria (i.e., variable compensation must be determined based on long-term performance and measurable criteria). Only “non-material” portion of variable compensation may be determined based on criteria that is not measurable, taking into account office holders’ contribution to the company;
|
|
|
•
|
the ratio of variable to fixed compensation, and the ceiling for the value of variable compensation, which is determined at the time of payment, except that the ceiling for equity-based compensation is determined at the time of grant;
|
|
|
•
|
the conditions under which an office holder would be required to repay compensation paid to him or her if it was later shown that the data upon which such compensation was based was inaccurate and was required to be restated in the company’s financial statements;
|
|
|
•
|
the minimum holding or vesting period for variable, equity-based compensation, while taking into account long-term objectives; and
|
|
|
•
|
maximum limits for severance compensation.
|
|
|
•
|
a person (or a relative of a person) who holds more than 5% of the company’s outstanding shares or voting rights;
|
|
|
•
|
a person (or a relative of a person) who has the power to appoint a director or the general manager of the company;
|
|
|
•
|
an office holder, within the meaning of the Israeli Companies Law (including a director and the general manager) of the company (or a relative thereof); or
|
|
|
•
|
a member of the company’s independent accounting firm, or anyone on his or her behalf.
|
|
|
•
|
information on the advisability of a given action brought for his or her approval or performed by virtue of his or her position; and
|
|
|
•
|
all other important information pertaining to any such action.
|
|
|
•
|
refrain from any conflict of interest between the performance of his or her duties to the company and his or her other duties or personal affairs;
|
|
|
•
|
refrain from any activity that is competitive with the company;
|
|
|
•
|
refrain from exploiting any business opportunity of the company to receive a personal gain for himself or herself or others; and
|
|
|
•
|
disclose to the company any information or documents relating to the company’s affairs which the office holder received as a result of his or her position as an office holder.
|
|
|
•
|
a majority of the shares held by all shareholders who do not have a personal interest in the transaction and who are present and voting on the matter approves the transaction, excluding abstentions; or
|
|
|
•
|
the shares voted against the transaction by shareholders who have no personal interest in the transaction and who are present and voting at the meeting do not exceed 2% of the voting rights in the company.
|
|
|
•
|
an amendment to the company’s articles of association;
|
|
|
•
|
an increase of the company’s authorized share capital;
|
|
|
•
|
a merger; and
|
|
|
•
|
the approval of related party transactions and acts of office holders that require shareholder approval.
|
|
|
•
|
a financial liability imposed on him or her in favor of another person pursuant to a judgment, including a settlement or arbitrator’s award approved by a court. However, if an undertaking to indemnify an office holder with respect to such liability is provided in advance, then such an undertaking must be limited to events which, in the opinion of the board of directors, can be foreseen based on the company’s activities when the undertaking to indemnify is given, and to an amount or according to criteria determined by the board of directors as reasonable under the circumstances, and such undertaking shall detail the abovementioned foreseen events and amount or criteria;
|
|
|
•
|
reasonable litigation expenses, including attorneys’ fees, incurred by the office holder (1) as a result of an investigation or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (i) no indictment was filed against such office holder as a result of such investigation or proceeding; and (ii) no financial liability was imposed upon him or her as a substitute for the criminal proceeding as a result of such investigation or proceeding or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent; and (2) in connection with a monetary sanction;
|
|
|
•
|
reasonable litigation expenses, including attorneys’ fees, incurred by the office holder or imposed by a court in proceedings instituted against him or her by the company, on its behalf, or by a third party, or in connection with criminal proceedings in which the office holder was acquitted, or as a result of a conviction for an offense that does not require proof of criminal intent; and
|
|
|
•
|
expenses, including reasonable litigation expenses and legal fees, incurred by an office holder in relation to an administrative proceeding instituted against such office holder, or certain compensation payments made to an injured party imposed on an office holder by an administrative proceeding, pursuant to certain provisions of the Israeli Securities Law.
|
|
|
•
|
a breach of the duty of loyalty to the company, provided that the office holder acted in good faith and had a reasonable basis to believe that the act would not harm the company;
|
|
|
•
|
a breach of the duty of care to the company or to a third party, to the extent such a breach arises out of the negligent conduct of the office holder;
|
|
|
•
|
a financial liability imposed on the office holder in favor of a third party; and
|
|
|
•
|
expenses, including reasonable litigation expenses and legal fees, incurred by an office holder in relation to an administrative proceeding instituted against such office holder or certain compensation payments to an injured party imposed on an office holder by an administrative proceeding, pursuant to certain provisions of the Securities Law.
|
|
|
•
|
a breach of the duty of loyalty, except for indemnification and insurance for a breach of the duty of loyalty to the company to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice the company;
|
|
|
•
|
a breach of the duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent conduct of the office holder;
|
|
|
•
|
an act or omission committed with intent to derive illegal personal benefit; or
|
|
|
•
|
a fine, monetary sanction or forfeit levied against the office holder.
|
|
D.
|
Employees
|
|
E.
|
Share Ownership
|
|
|
·
|
To determine whether and to what extent awards are to be granted to participants under the 2015 Plan and to select the eligible recipients of awards under the 2015 Plan;
|
|
|
·
|
To approve forms of agreement for use under the 2015 Plan;
|
|
|
·
|
To determine the terms and conditions of any award under the 2015 Plan, including the exercise price, the time or times and the extent to which the awards may be exercised (which may be based on performance criteria), any vesting acceleration or waiver of forfeiture restrictions, and any restriction or limitation regarding any award or the ordinary shares relating thereto, based in each case on such factors as the Administrator, at its sole discretion, shall determine;
|
|
|
·
|
To determine the fair market value of the shares covered by each award;
|
|
|
·
|
To make an election as to the type of Section 102 Option;
|
|
|
·
|
To prescribe, amend and rescind rules and regulations relating to the 2015 Plan, including rules and regulations relating to sub-plans established for the purpose of qualifying for preferred tax treatment under foreign tax laws;
|
|
|
·
|
To authorize conversion or substitution under the 2015 Plan of any or all awards and to cancel or suspend awards, as necessary, provided the material interests of the participants are not harmed; and
|
|
|
·
|
To construe and interpret the terms of the 2015 Plan and awards granted pursuant to the 2015 Plan;
|
|
|
·
|
To alter, revise or otherwise adjust the terms of the 2015 Plan and the award agreement, as may be required pursuant to any applicable laws of local or foreign jurisdictions.
|
|
A.
|
Major shareholders
|
|
|
Ordinary Shares
Beneficially Owned
|
|||||||
|
Name of Beneficial Owner
|
Number
|
Percent
|
||||||
|
5% or Greater Shareholders
|
||||||||
|
Barna Capital Group Ltd.(1)
|
778,011
|
9.4
|
%
|
|||||
|
Intracoastal Capital LLC (2)
|
701,201
|
8.0
|
%
|
|||||
|
Donald Norman (3)
|
623,600
|
7.6
|
%
|
|||||
|
Smotrich SARL-SPF(4)
|
555,000
|
6.7
|
%
|
|||||
|
Directors and Executive Officers
|
||||||||
|
Alex Ovadia
|
*
|
*
|
||||||
|
Niv Kochav
|
-
|
-
|
||||||
|
Yoav Kimchy (5)
|
64,431
|
*
|
||||||
|
Boaz Shpigelman
|
*
|
*
|
||||||
|
Steven Hanley
|
*
|
*
|
||||||
|
Clara Ezed
|
*
|
*
|
||||||
|
Mary Jo Gorman
|
*
|
*
|
||||||
|
XiangQian (XQ) Lin
(6)
|
51,282
|
*
|
||||||
|
Yuval Yanai
|
*
|
*
|
||||||
|
All director and executive officers as a group (9 persons)
|
152,426
|
1.9
|
%
|
|||||
|
(1)
|
Based solely upon, and qualified in its entirety with reference to, Schedule 13G filed with the SEC on February 15, 2019.
|
|
(2)
|
Based solely upon, and qualified in its entirety with reference to, Schedule 13G filed with the SEC on February 12, 2019. According to the Schedule 13G, (i) Mitchell P. Kopin, an individual who is a citizen of the United States of America (“Mr. Kopin”), (ii) Daniel B. Asher, an individual who is a citizen of the United States of America (“Mr. Asher”) and (iii) Intracoastal Capital LLC, a Delaware limited liability company (“Intracoastal” and together with Mr. Kopin and Mr. Asher, collectively the “Reporting Persons”) have shared voting power over these ordinary shares. The Schedule 13G states such 701201 ordinary shares are comprised of (i) 216,705 ordinary shares held by Intracoastal and (ii) 484,496 ordinary shares issuable upon exercise of Series D warrants issued in connection with the February 2019 registered direct offering. According to the Schedule 13G, the
foregoing excludes 241,364 ordinary shares issuable upon exercise of Series D warrants issued in connection with the February 2019 registered direct offering because the warrant contains a blocker provision under which the holder thereof does not have the right to exercise the warrants to the extent (but only to the extent) that such exercise would result in beneficial ownership by the holder thereof, together with the holder’s affiliates, and any other persons acting as a group together with the holder or any of the holder’s affiliates, of more than 4.99% of the ordinary shares. Without such blocker provision, each of the Reporting Persons may have been deemed to have beneficial ownership of 942,565 ordinary shares.
|
|
(3)
|
Based solely
upon
a Non-Objecting Beneficial Owner list with a record date of March 15, 2019 obtained by the Company from Broadridge Financial Solutions, Inc. As of
such date
, the Company does not have information as to (i) whether beneficial ownership of any of these shares has been disclaimed, (ii) who holds voting and dispositive power over these shares, (iii) whether any other ordinary shares are beneficially owned, or (iv) whether these shares continued to be beneficially owned by such person and in such amounts since March 15, 2019.
|
|
(4)
|
Based solely upon, and qualified in its entirety with reference to, Schedule 13G filed with the SEC on February 13, 2019. Based on the Schedule 13G, Mr. Alejandro Weinstein, Chalet les Ecureuils Ouest, Rte. de Zires 17, 3963 Crans-Montana, CH, is the ultimate beneficial owner of these ordinary shares and as such has the power to direct the receipt of dividends from, or the proceeds from the sale of, such securities.
|
|
(5)
|
Includes: (i) 27,173 ordinary shares directly held by Yoav Kimchy
,
(ii) 10,520 ordinary shares subject to options held by Yoav Kimchy currently exercisable or exercisable within 60 days of March 21, 2019
,
(iii) 26,644 ordinary shares directly held by Sigalit Kimchy, the wife of Yoav Kimchy
,
and (iv) 94 ordinary shares subject to options held by Sigalit Kimchy currently exercisable or exercisable within 60 days of March 21, 2019. Yoav Kimchy and Sigalit Kimchy have joint beneficial ownership over the shares beneficially held by them.
|
|
(6)
|
Includes: (i) 14,429 outstanding ordinary shares
,
(ii) 9,074 ordinary shares subject to options currently exercisable or exercisable within 60 days of March 21, 2019
,
(iii) 6,945 ordinary shares issuable upon exercise of the Series A Warrants that are currently exercisable
,
and (iv) 20,834 ordinary shares issuable upon exercise of Long Term Incentive Warrants that are currently exercisable.
|
|
B.
|
Related Party Transactions
|
|
C.
|
Interests of Experts and Counsel
|
|
A.
|
Consolidated Statements and Other Financial Information.
|
|
B.
|
Significant Changes
|
|
A.
|
Offer and Listing Details
|
|
B.
|
Plan of Distribution
|
|
C.
|
Markets for Ordinary Shares
|
|
D.
|
Selling Shareholders
|
|
E.
|
Dilution
|
|
F.
|
Expenses of the Issue
|
|
A.
|
Share Capital
|
|
B.
|
Memorandum and Articles of Association
|
|
|
·
|
amendments to our articles of association;
|
|
|
·
|
appointment, terms of service and termination of service of our auditors;
|
|
|
·
|
appointment of external directors
;
|
|
|
·
|
approval of certain related party transactions;
|
|
|
·
|
increases or reductions of our authorized share capital;
|
|
|
·
|
mergers; and
|
|
|
·
|
the exercise of our board of director’s powers by a general meeting, if our board of directors is unable to exercise its powers and the exercise of any of its powers is essential for our proper management.
|
|
C.
|
Material Contracts
|
|
D.
|
Exchange controls
|
|
E.
|
Taxation
|
|
|
·
|
amortization over an eight-year period of the cost of purchased know-how and patents and rights to use a patent and know-how which are used for the development or advancement of the Industrial Enterprise;
|
|
|
·
|
under limited conditions, an election to file consolidated tax returns with related Israeli Industrial Companies; and
|
|
|
·
|
expenses related to a public offering are deductible in equal amounts over three years.
|
|
|
•
|
an individual citizen or resident of the United States;
|
|
|
•
|
a corporation (or other entity treated as a corporation) that is created or organized (or treated as created or organized) in or under the laws of the United States, any state thereof or the District of Columbia;
|
|
|
•
|
an estate whose income is includible in gross income for U.S. federal income tax purposes regardless of its source; or
|
|
|
•
|
a trust if (i) a U.S. court can exercise primary supervision over the trust’s administration and one or more U.S. persons are authorized to control all substantial decisions of the trust; or (ii) it has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
|
|
|
•
|
financial institutions or financial services entities;
|
|
|
•
|
broker-dealers;
|
|
|
•
|
persons that are subject to the mark-to-market accounting rules under Section 475 of the Code;
|
|
|
•
|
tax-exempt entities;
|
|
|
•
|
governments or agencies or instrumentalities thereof;
|
|
|
•
|
insurance companies;
|
|
|
•
|
regulated investment companies;
|
|
|
•
|
real estate investment trusts;
|
|
|
•
|
certain expatriates or former long-term residents of the United States;
|
|
|
•
|
persons that actually or constructively own 5% or more of our shares;
|
|
|
•
|
except as specifically discussed herein in respect of the Long Term Incentive Warrants, persons that acquired our securities pursuant to an exercise of employee options, in connection with employee incentive plans or otherwise as compensation;
|
|
|
•
|
persons that hold our securities as part of a straddle, constructive sale, hedging, conversion or other integrated transaction;
|
|
|
•
|
persons whose functional currency is not the U.S. dollar;
|
|
|
•
|
passive foreign investment companies; or
|
|
|
•
|
controlled foreign corporations.
|
|
|
•
|
any gain recognized by the U.S. Holder on the sale or other disposition of its ordinary shares or Series A Warrants, Series C Warrants or Series D Warrants; and
|
|
|
•
|
any “excess distribution” made to the U.S. Holder (generally, any distributions to such U.S. Holder during a taxable year of the U.S. Holder that are greater than 125% of the average annual distributions received by such U.S. Holder in respect of the ordinary shares during the three preceding taxable years of such U.S. Holder or, if shorter, such U.S. Holder’s holding period for the ordinary shares).
|
|
|
•
|
the U.S. Holder’s gain or excess distribution will be allocated ratably over the U.S. Holder’s holding period for the ordinary shares or Series A Warrants, Series C Warrants or Series D Warrants;
|
|
|
•
|
the amount allocated to the U.S. Holder’s taxable year in which the U.S. Holder recognized the gain or received the excess distribution or to the period in the U.S. Holder’s holding period before the first day of our first taxable year in which we qualified as a PFIC will be taxed as ordinary income;
|
|
|
•
|
the amount allocated to other taxable years (or portions thereof) of the U.S. Holder and included in its holding period will be taxed at the highest ordinary tax rate in effect for that year and applicable to the U.S. Holder; and
|
|
|
•
|
the interest charge generally applicable to underpayments of tax will be imposed in respect of the tax attributable to each such other taxable year of the U.S. Holder.
|
|
|
•
|
fails to provide an accurate taxpayer identification number;
|
|
|
•
|
is notified by the IRS that backup withholding is required; or
|
|
|
•
|
in certain circumstances, fails to comply with applicable certification requirements.
|
|
F.
|
Dividends and paying agents
|
|
G.
|
Statement by experts
|
|
H.
|
Documents on display
|
|
I.
|
Subsidiary Information
|
|
|
2018
|
2017
|
||||||
|
Audit Fees
(1)
|
$
|
60,000
|
$
|
60,000
|
||||
|
Audit-Related Fees
(2)
|
$
|
45,000
|
$
|
4,000
|
||||
|
Other Fees
(3)
|
$
|
-
|
$
|
25,481
|
||||
|
Total
|
$
|
105,000
|
$
|
89,481
|
||||
|
(1)
|
The audit fees for the years ended December 31, 2018 and 2017 were for professional services rendered for the audits of our financial statements, consents and in connection with certain of our filings with the U.S. Securities and Exchange Commission.
|
|
(2)
|
Audit-related fees for the year ended December 31, 2018,
are for services rendered by our auditors in connection with our May 2018 underwritten public offering.
Audit-related fees for the year ended December 31, 2017 are for services rendered by our auditors in connection with determining the number of Long-Term Incentive Warrants that were vested at February 24, 2016 and February 24, 2017.
|
|
(3)
|
Other fees for the year ended December 31, 2017 were for services related to the application for an additional grant from the IIA (formerly the OCS).
|
|
|
•
|
Nomination of our directors
. Israeli law and our amended articles of association do not require director nominations to be made by a nominating committee of our board of directors consisting solely of independent directors, as required under the Listing Rules of the Nasdaq Stock Market. We rely on the exemption available to foreign private issuers under the Nasdaq Listing Rules and follow Israeli law and practice with regard to the process of nominating directors, in accordance with which directors are recommended by our board of directors for election by our shareholders (other than directors elected by our board of directors to fill a vacancy). In October 2015, our Board of Directors established a non-independent Nominating Committee, whose role is to select and recommend to the Board of Directors for selection, director nominees, while considering the appropriate size and composition of the Board of Directors, the requirements of applicable law regarding service as a member of our Board of Directors and the criteria for the selection of new members of the Board of Directors.
|
|
|
•
|
Compensation of officers
. We follow Israeli law and practice with respect to the approval of officer compensation. While our compensation committee currently complies with the provisions of the Nasdaq Listing Rules relating to composition requirements and Israeli law generally requires that the compensation of the chief executive officer and all other executive officers be approved, or recommended to the board for approval, by the compensation committee (and in certain instances, shareholder approval is required), Israeli law includes relief from compensation committee approval in certain instances. For details regarding the approvals required under the Israeli Companies Law and regulation promulgated thereunder for the approval of compensation of the chief executive officer, all other executive officers and directors, see Item 6C “Directors, Senior Management and Employees— Board Practices — Approval of Related Party Transactions under Israeli Law — Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions”).
|
|
|
•
|
Shareholder approval.
We will seek shareholder approval for all corporate actions requiring such approval under the requirements of the Israeli Companies Law, rather than seeking approval for corporate actions in accordance with Nasdaq Listing Rule 5635. In particular, under the Nasdaq Listing Rule, shareholder approval is generally required for: (i) an acquisition of shares/assets of another company that involves the issuance of 20% or more of the acquirer’s shares or voting rights or if a director, officer or 5% shareholder has greater than a 5% interest (or such persons collectively have a 10% or greater interest) in the target company or the assets to be acquired or the consideration to be received and the present or potential issuance of ordinary shares, or securities convertible into or exercisable for ordinary shares, could result in an increase in outstanding common shares or voting power of 5% or more; (ii) the issuance of shares leading to a change of control; (iii) adoption/amendment of a stock option or purchase plan or other equity compensation arrangements, pursuant to which stock may be acquired by officers, directors, employees or consultants (with certain limited exception); and (iv) issuances of 20% or more of the shares or voting rights (including securities convertible into, or exercisable for, equity) of a listed company via a private placement (and/or via sales by directors/officers/5% shareholders) if such equity is issued (or sold) at below the greater of the book or market value of shares. We will seek shareholder approval for all actions requiring such under the Israeli Companies Law. Under the Israeli Companies Law, the adoption of, and material changes to, equity-based compensation plans generally require the approval of the board of directors. For details regarding the approvals required under the Israeli Companies Law for the approval of compensation of the chief executive officer, all other executive officers and directors, see “Item 6C “Directors, Senior Management and Employees — Board Practices -Approval of Related Party Transactions under Israeli Law — Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions.” For details regarding the approvals required under the Israeli Companies Law for the approval of transactions with and compensation of controlling shareholders, see “Item 6C “Directors, Senior Management and Employees — Board Practices -Approval of Related Party Transactions under Israeli Law — Disclosure of Personal Interests of Controlling Shareholders and Approval of Certain Transactions. ” For details regarding the approvals required under the Israeli Companies Law for certain acquisitions of our shares and mergers, see Item 10B. “Memorandum and Articles of Association— Acquisitions under Israeli Law.”
|
|
|
•
|
Quorum requirement.
Under our amended and restated articles of association and as permitted under the Israeli Companies Law, a quorum for any meeting of shareholders shall be the presence of at least two shareholders present in person, by proxy or by a written ballot, who hold at least 25% of the voting power of our shares (or if a higher percentage is required by law, such higher percentage) instead of 33 1/3% of the issued share capital required under the Nasdaq Listing Rules. If the meeting was adjourned for lack of a quorum, at the adjourned meeting, at least two shareholders present in person or by proxy shall constitute a quorum, unless the meeting of shareholders was convened at the demand of shareholders, in which case, the quorum shall be the presence of one or more shareholders holding at least 5% of our issued share capital and at least one percent of the voting power of our shares, or one or more shareholders with at least 5% of the voting power of our shares.
|
|
|
|
|
|
101.INS
|
XBRL Instant Document
|
|
|
|
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
|
|
|
|
|
|
101.CAL
|
XBLR Taxonomy Extension Calculation Linkbase Document
|
|
|
|
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase
|
|
|
|
|
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase
|
|
|
|
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase
|
|
(1)
|
Incorporated by reference to the Registration Statement on Form F-1 of the Registrant (File No. 333-201250).
|
|
(2)
|
Incorporated by reference to the Form 6-K filed by the Registrant with the Securities Exchange Commission on July 6, 2015.
|
|
(3)
|
Incorporated by reference to the Form 6-K/A filed by the Registrant with the Securities and Exchange Commission on May 24, 2017.
|
|
(4)
|
Incorporated by reference to the Form 6-K filed by the Registrant with the Securities and Exchange Commission on August 12, 2016.
|
|
(5)
|
Incorporated by reference to the Form 6-K filed by the Registrant with the Securities and Exchange Commission on June 2, 2017.
|
|
(6)
|
Incorporated by reference to the Form 6-K filed by the Registrant with the Securities and Exchange Commission on November 22, 2017.
|
|
(7)
|
Incorporated by reference to the Form 20-F filed by the Registrant with the Securities and Exchange Commission on April 4, 2018.
|
|
(8)
|
Incorporated by reference to the Form 6-K filed by the Registrant with the Securities and Exchange Commission on June 24, 2015.
|
|
(9)
|
Incorporated by reference to the Registration Statement on Form F-1/A by the Registrant with the Securities and Exchange Commission on April 25, 2018.
|
|
(10)
|
Incorporated by reference to the Form 6-K filed by the Registrant with the Securities and Exchange Commission on May 4, 2018.
|
|
(11)
|
Incorporated by reference to the Form 6-K filed by the Registrant with the Securities and Exchange Commission on February 6, 2019.
|
|
(12)
|
Incorporated by reference to the Annual Report on Form 20-F filed by the Registrant with the Securities and Exchange Commission on March 15, 2016.
|
|
|
CHECK-CAP LTD.
|
|
|
|
|
|
|
Date: March 28, 2019
|
By:
|
/s/ Alex Ovadia
|
|
|
Name:
|
Alex Ovadia
|
|
|
Title:
|
Chief Executive Officer
(Principal Executive Officer)
|
|
|
|
|
|
|
|
|
|
|
By:
|
/s/ Niv Kochav
|
|
|
Name:
|
Niv Kochav
|
|
|
Title:
|
Interim Chief Financial Officer
|
|
|
|
(Principal Financial Officer
|
|
|
|
and Principal Accounting Officer)
|
|
|
Page
|
|
|
|
|
F-3
|
|
|
|
|
|
F-4
|
|
|
|
|
|
F-5
|
|
|
|
|
|
F-6
|
|
|
|
|
|
F-7
|
|
|
|
|
|
F-8 - F-31
|

|
|
December 31,
|
|||||||||||
|
|
Note
|
2 0 1 8
|
2 0 1 7
|
|||||||||
|
Assets
|
||||||||||||
|
Current assets
|
||||||||||||
|
Cash and cash equivalents
|
8,572
|
6,997
|
||||||||||
|
Restricted cash
|
2
|
350
|
-
|
|||||||||
|
Short-term bank deposit
|
5,643
|
-
|
||||||||||
|
Prepaid expenses and other current assets
|
3
|
419
|
406
|
|||||||||
|
Total current assets
|
14,984
|
7,403
|
||||||||||
|
|
||||||||||||
|
Non-current assets
|
||||||||||||
|
Property and equipment, net
|
4
|
452
|
503
|
|||||||||
|
Total non-current assets
|
452
|
503
|
||||||||||
|
Total assets
|
15,436
|
7,906
|
||||||||||
|
|
||||||||||||
|
Liabilities and shareholders' equity
|
||||||||||||
|
Current liabilities
|
||||||||||||
|
Accounts payable and accruals
|
||||||||||||
|
Trade
|
1,113
|
608
|
||||||||||
|
Other
|
214
|
347
|
||||||||||
|
Other current liabilities
|
35
|
5
|
||||||||||
|
Employees and payroll accruals
|
5
|
859
|
602
|
|||||||||
|
Total current liabilities
|
2,221
|
1,562
|
||||||||||
|
|
||||||||||||
|
Non-current liabilities
|
||||||||||||
|
Royalties provision
|
7A
|
|
185
|
439
|
||||||||
|
Total non-current liabilities
|
185
|
439
|
||||||||||
|
|
||||||||||||
|
Shareholders' equity
|
9
|
|||||||||||
|
Share capital
|
3,456
|
974
|
||||||||||
|
Additional paid-in capital
|
72,888
|
57,643
|
||||||||||
|
Accumulated other comprehensive loss
|
(13
|
)
|
-
|
|||||||||
|
Accumulated deficit
|
(63,301
|
)
|
(52,712
|
)
|
||||||||
|
Total shareholders' equity
|
13,030
|
5,905
|
||||||||||
|
|
||||||||||||
|
Total liabilities and shareholders' equity
|
15,436
|
7,906
|
||||||||||
|
|
Year ended December 31,
|
|||||||||||||||
|
|
Note
|
2 0 1 8
|
2 0 1 7
|
2 0 1 6
|
||||||||||||
|
|
||||||||||||||||
|
Research and development expenses, net
|
11
|
7,618
|
6,837
|
5,491
|
||||||||||||
|
General and administrative expenses
|
12
|
3,445
|
3,164
|
3,571
|
||||||||||||
|
Operating loss
|
11,063
|
10,001
|
9,062
|
|||||||||||||
|
|
||||||||||||||||
|
Financial income, net
|
13
|
473
|
236
|
244
|
||||||||||||
|
|
||||||||||||||||
|
Loss before income tax
|
10,590
|
9,765
|
8,818
|
|||||||||||||
|
Taxes on income
|
(1
|
)
|
6
|
8
|
||||||||||||
|
Net loss
|
10,589
|
9,771
|
8,826
|
|||||||||||||
|
Comprehensive loss:
|
||||||||||||||||
|
Net loss
|
10,589
|
9,771
|
8,826
|
|||||||||||||
|
Change in fair value of cash flow hedge
|
13
|
-
|
-
|
|||||||||||||
|
Comprehensive loss
|
10,602
|
9,771
|
8,826
|
|||||||||||||
|
Loss per share:
|
||||||||||||||||
|
Net loss per ordinary share (in USD) basic and diluted
|
2.61
|
6.72
|
7.31
|
|||||||||||||
|
|
||||||||||||||||
|
Weighted average number of ordinary shares outstanding - basic and diluted (in thousands)
|
14
|
4,058
|
1,455
|
1,208
|
||||||||||||
|
|
Number
|
Amount
|
Additional
paid-in capital
|
Other comprehensive loss
|
Accumulated
deficit
|
Total Shareholders’ equity
|
||||||||||||||||||
|
Balance as of December 31, 2015
|
984,292
|
$
|
599
|
$
|
46,164
|
$
|
-
|
$
|
(34,115
|
)
|
$
|
12,648
|
||||||||||||
|
Changes during 2016:
|
||||||||||||||||||||||||
|
Issuance of ordinary shares and pre-funded warrants in August 2016 registered direct offering, net of issuance expenses in an amount of $615 (2)
|
53,635
|
32
|
5,227
|
-
|
-
|
5,259
|
||||||||||||||||||
|
Exercise of warrants into ordinary shares
|
229,153
|
140
|
(23
|
)
|
-
|
-
|
117
|
|||||||||||||||||
|
Share-based compensation
|
-
|
-
|
1,209
|
-
|
-
|
1,209
|
||||||||||||||||||
|
Net loss
|
-
|
-
|
-
|
-
|
(8,826
|
(8,826
|
)
|
|||||||||||||||||
|
Balance as of December 31, 2016
|
1,267,080
|
$
|
771
|
$
|
52,577
|
$
|
-
|
$
|
(42,941
|
)
|
$
|
10,407
|
||||||||||||
|
Changes during 2017:
|
||||||||||||||||||||||||
|
Issuance of ordinary shares and warrants in June 2017 registered direct offering, net of issuance expenses in an amount of $399 (3)
|
112,460
|
67
|
2,282
|
-
|
-
|
2,349
|
||||||||||||||||||
|
Issuance of ordinary shares and warrants in November 2017 registered direct offering, net of issuance expenses in an amount of $378 (3)
|
189,387
|
114
|
2,066
|
-
|
-
|
2,180
|
||||||||||||||||||
|
Exercise of warrants into ordinary shares
|
35,474
|
22
|
(8
|
)
|
-
|
-
|
14
|
|||||||||||||||||
|
Share-based compensation
|
1,033
|
-
|
726
|
-
|
726
|
|||||||||||||||||||
|
Net loss
|
-
|
-
|
-
|
-
|
(9,771
|
)
|
(9,771
|
)
|
||||||||||||||||
|
Balance as of December 31, 2017
|
1,605,434
|
$
|
974
|
$
|
57,643
|
$
|
-
|
$
|
(52,712
|
)
|
$
|
5,905
|
||||||||||||
|
Changes during 2018:
|
||||||||||||||||||||||||
|
Issuance of ordinary shares in May 2018 in the Public Offering, net of issuance expenses in an amount of $2,381 (4)
|
3,669,129
|
2,444
|
15,343
|
-
|
-
|
17,787
|
||||||||||||||||||
|
Exercise of warrants into ordinary shares
|
56,121
|
38
|
(33
|
)
|
-
|
-
|
5
|
|||||||||||||||||
|
Share-based compensation
|
-
|
-
|
(65
|
)
|
-
|
-
|
(65
|
)
|
||||||||||||||||
|
Change in fair values of cash flow hedge
|
-
|
-
|
-
|
(13
|
)
|
-
|
(13
|
)
|
||||||||||||||||
|
Net loss
|
-
|
-
|
-
|
-
|
(10,589
|
)
|
(10,589
|
)
|
||||||||||||||||
|
Balance as of December 31, 2018
|
5,330,684
|
3,456
|
72,888
|
(13
|
)
|
(63,301
|
)
|
13,030
|
||||||||||||||||
|
|
Year ended December 31,
|
|||||||||||
|
|
2 0 1 8
|
2 0 1 7
|
2 0 1 6
|
|||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
||||||||||||
|
Net loss
|
(10,589
|
)
|
(9,771
|
)
|
(8,826
|
)
|
||||||
|
Adjustments required to reconcile net loss to net cash used in operating activities:
|
||||||||||||
|
Depreciation and amortization
|
147
|
157
|
130
|
|||||||||
|
Share-based compensation
|
(65
|
)
|
726
|
1,209
|
||||||||
|
Financial Expenses
(income), net
|
67
|
(165
|
)
|
(56
|
)
|
|||||||
|
|
||||||||||||
|
Changes in assets and liabilities items:
|
||||||||||||
|
Decrease (increase) in prepaid and other current assets
and non-current assets
|
(13
|
)
|
(164
|
)
|
438
|
|||||||
|
Increase (decrease) in trade accounts payable, accruals and other current liabilities
|
416
|
275
|
(252
|
)
|
||||||||
|
Increase (decrease) in employees and payroll accruals
|
258
|
(126
|
)
|
(510
|
)
|
|||||||
|
Decrease in royalties provision
|
(255
|
)
|
(82
|
)
|
(56
|
)
|
||||||
|
Net cash used in operating activities
|
(10,034
|
)
|
(9,150
|
)
|
(7,923
|
)
|
||||||
|
|
||||||||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES
|
.
|
|||||||||||
|
Purchase of property and equipment
|
(94
|
)
|
(231
|
)
|
(166
|
)
|
||||||
|
Proceeds from (Investment in) short-term bank deposit
|
(5,629
|
)
|
-
|
4,811
|
||||||||
|
Net cash provided by (used in) investing activities
|
(5,723
|
)
|
(231
|
)
|
4,645
|
|||||||
|
|
||||||||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES
|
||||||||||||
|
Exercise of warrants into ordinary shares
|
-
|
196
|
117
|
|||||||||
|
Issuance of ordinary shares in the RD Offerings, net of issuance expenses
|
(30
|
)
|
4,379
|
5,307
|
||||||||
|
Issuance of ordinary shares in the 2018 Public Offering
|
17,792
|
-
|
-
|
|||||||||
|
Net cash provided by financing activities
|
17,762
|
4,575
|
5,424
|
|||||||||
|
|
||||||||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
(80
|
)
|
164
|
55
|
||||||||
|
|
||||||||||||
|
Net increase (decrease) in cash, cash equivalents and restricted cash
|
1,925
|
(4,642
|
)
|
2,201
|
||||||||
|
|
||||||||||||
|
Cash, cash equivalents and restricted cash at the beginning of the year
|
6,997
|
11,639
|
9,438
|
*
|
||||||||
|
|
||||||||||||
|
Cash, cash equivalents and restricted cash at the end of the year
|
8,922
|
6,997
|
11,639
|
|||||||||
|
|
Year ended December 31,
|
|||||||||||
|
|
2 0 1 8
|
2 0 1 7
|
2 0 1 6
|
|||||||||
|
Supplemental disclosure of non-cash flow information
|
||||||||||||
|
Cashless exercise of warrants to purchase ordinary shares into ordinary shares
|
33
|
8
|
23
|
|||||||||
|
Purchase of property and equipment
|
3
|
15
|
8
|
|||||||||
|
Issuance expenses*
|
-
|
30
|
47
|
|||||||||
|
Supplemental disclosure of cash flow information
|
||||||||||||
|
Cash paid for income taxes
|
5
|
3
|
8
|
|||||||||
| A. |
General
|
| (1) |
Check Cap Ltd. (together with its wholly-owned subsidiary, the “Company") was incorporated under the laws of the State of Israel. The registered address of its offices is 29 Abba Hushi Ave, Isfiya 3009000, Israel.
|
| (2) |
Check-Cap Ltd has a wholly-owned subsidiary, Check-Cap U.S. Inc., incorporated under the laws of the State of Delaware on May 15, 2015.
|
| (3) |
The Company is a clinical-stage medical diagnostics company developing C-Scan®, the first capsule-based system for preparation-free colorectal cancer screening (the "C-Scan system"). Utilizing innovative ultra-low dose X-ray and wireless communication technologies, the capsule generates information on the contours of the inside of the colon as it passes naturally. This information is used to create a 3D map of the colon, which allows physicians to look for polyps and other abnormalities. Designed to improve the patient experience and increase the willingness of individuals to participate in recommended colorectal cancer screening, the C-Scan system removes many frequently-cited barriers, such as laxative bowel preparation, invasiveness and sedation.
|
| (4) |
As described in Notes 9C(2)(b) and 9C(2)(d), on February 24, 2015 the Company consummated an Initial Public Offering in the United States (U.S.) (the "IPO") concurrently with a Private Placement (the "Private Placement").
|
|
The consolidated financial statements of the Company as of and for the year ended December 31, 2018 include the financial statements of the Company and its wholly-owned U.S. subsidiary.
|
| B. |
Going concern and management plans
|
| C. |
Reverse share splits
|
| A. |
Use of estimates in preparation of financial statements
|
| B. |
Principles of consolidation
|
| C. |
Financial statements in U.S dollars
|
| D. |
Cash and cash equivalents
|
| E. |
Short-term bank deposit
|
| F. |
Cash flow hedges
|
| F |
Cash flow hedges (cont.)
|
| G. |
Property and equipment
|
|
|
Length of useful life
|
|
Depreciation rate
|
|
|
|
Years
|
|
%
|
|
|
|
|
|
|
|
|
Office furniture and equipment
|
10-14
|
|
7-10
|
|
|
Laboratory equipment
|
3-7
|
|
15-33
|
|
|
Computers and auxiliary equipment
|
3
|
|
33
|
|
| H. |
Impairment of long-lived assets
|
| I. |
Research and development costs
|
| J. |
Contingent liabilities
|
| K. |
Share-based compensation
|
| L. |
Income taxes
|
| M. |
Fair value of financial instruments
|
| N. |
Comprehensive
loss
|
| O. |
Restricted Cash
|
|
|
2 0 1 8
|
2 0 1 7
|
2 0 1 6 | |||||||||
|
|
||||||||||||
|
Cash and Cash equivalents
|
8,572
|
6,997
|
11,639
|
|||||||||
|
Restricted cash included current assets
|
350
|
-
|
-
|
|||||||||
|
Total cash, cash equivalents, and restricted cash shown in the statement of cash flows
|
8,922
|
6,997 | 11,639 | |||||||||
| P. |
New accounting pronouncements
|
|
|
December 31,
|
|||||||
|
|
2 0 1 8
|
2 0 1 7
|
||||||
|
|
||||||||
|
Government institutions
|
178
|
89
|
||||||
|
Prepaid expenses
|
206
|
261
|
||||||
|
Deposits
|
34
|
55
|
||||||
|
Other assets
|
1
|
1
|
||||||
|
|
419
|
406
|
||||||
|
|
December 31,
|
|||||||
|
|
2 0 1 8
|
2 0 1 7
|
||||||
|
Cost:
|
||||||||
|
Office furniture and equipment
|
186
|
157
|
||||||
|
Laboratory equipment
|
644
|
626
|
||||||
|
Computers and auxiliary equipment
|
388
|
339
|
||||||
|
|
1,218
|
1,122
|
||||||
|
Accumulated depreciation
|
766
|
619
|
||||||
|
Property and equipment, net
|
452
|
503
|
||||||
| A. |
Composition:
|
|
|
December 31,
|
|||||||
|
|
2 0 1 8
|
2 0 1 7
|
||||||
|
Short-term employee benefits:
|
||||||||
|
Benefits for vacation and recreation pay
|
162
|
235
|
||||||
|
Liability for payroll, bonuses and wages
|
697
|
367
|
||||||
|
|
859
|
602
|
||||||
| B. |
Post-employment benefits
|
| C. |
Short-term employee benefits
|
| (1) |
Paid vacation days
|
| (2) |
Related parties
|
| A. |
The Company
|
| 1. |
Corporate tax rates in Israel
|
| 2. |
The Law for the Encouragement of Capital Investments, 1959 (the "Investments Law")
|
| a) |
Reduced tax rates
|
| b) |
Conditions for entitlement to the benefits
|
| c) |
Amendment of the Law for the Encouragement of Capital Investments, 1959
|
| 2. |
The Law for the Encouragement of Capital Investments, 1959 (the "Investments Law") (Cont.)
|
| c) |
Amendment of the Law for the Encouragement of Capital Investments, 1959 (Cont.)
|
| 3. |
The Company recorded $6 and $8 current taxes for the years ended December 31, 2017 and 2016. The Company did not record current taxes for the year ended December 31, 2018, since it had no taxable income during this year.
|
| B. |
Check-Cap U.S. Inc.
|
| C. |
Deferred income taxes
|
|
|
December 31,
|
|||||||
|
|
2 0 1 8
|
2 0 1 7
|
||||||
|
|
||||||||
|
Carry forward tax losses
|
12,525
|
13,083
|
||||||
|
Less valuation allowance
|
(12,525
|
)
|
(13,083
|
)
|
||||
|
|
-
|
-
|
||||||
| D. |
Reconciliation of the theoretical tax expense to actual tax expense
|
| A. |
Royalties provision
|
| 1. |
Royalties to an ASIC designer
|
| 2. |
Reimbursement liability to Predecessor Entity's unit holders
|
|
|
December 31,
|
|||||||
|
|
2 0 1 8
|
2 0 1 7
|
||||||
|
|
||||||||
|
Royalties to an ASIC designer
|
120
|
127
|
||||||
|
Reimbursement liability to Predecessor Entity's unit holders
|
65
|
312
|
||||||
|
|
185
|
439
|
||||||
| B. |
Commitments
|
| (1) |
Royalties
|
| (2) |
Rental agreement
|
| B. |
Commitments (Cont.)
|
| (3) |
Vehicle lease and maintenance agreements
|
|
Lease agreement for vehicles
|
22
|
|||
|
Lease agreement for office facilities lease
|
13
|
|||
|
|
35
|
| C. |
Legal
|
|
December 31, 2018
|
||||||||||||
|
Fair value measurements using input type
|
||||||||||||
|
Level 1
|
Level 2
|
Total
|
||||||||||
|
Cash equivalents:
|
||||||||||||
|
Money market funds
|
$
|
4,307
|
$
|
4,307
|
||||||||
|
Foreign currency derivative instruments
|
$
|
(15
|
)
|
$
|
(15
|
)
|
||||||
|
Total financial assets (Liabilities)
|
$
|
4,307
|
$
|
(15
|
)
|
$
|
4,292
|
|||||
| A. |
All share and per share amounts in the financial statements have been adjusted to reflect the Reverse Share Split.
|
| B. |
Ordinary shares
|
| 1. |
The ordinary shares provide their owners with rights to receive dividends in cash and shares, and rights to participate at the time of distributing liquidation dividends. Additionally, the ordinary shareholders have the right to vote at shareholder meetings in a manner that each share provides one voting right to its holder.
|
| 2. |
Changes in ordinary share capital
|
| a) |
On May 11, 2010, the Company issued, free of charge, to all of its shareholders (except for certain ordinary shareholders), warrants to purchase an aggregate of 32,174 ordinary shares (hereafter- "Anti-dilution Warrants"). The Anti-dilution Warrants were issued in order to prevent the dilution of the holdings of such Company shareholders due to certain options granted to the Company's CEO (hereafter- "CEO options"). The Anti-dilution Warrants were subject to automatic exercise, without consideration (unless the holder thereof objected to such exercise), upon the exercise by the Company's CEO of the CEO Options. The fair value of the Anti-dilution Warrants on the grant date was immaterial. Anti-dilution warrants to purchase 7,724 and 56 ordinary shares were exercised during the years ended December 31, 2018 and 2016, respectively. No such warrants were exercised during the year ended December 31, 2017. As of December 31, 2018, 7,246 Anti-dilution Warrants were outstanding.
|
| b) |
On February 24, 2015, the Company consummated an IPO in the U.S. of 166,667 units at a public offering price of $72 per unit, before underwriting discounts and offering expenses. Each unit consisted of one ordinary share and one-half of a Series A Warrant to purchase one ordinary share. Each unit was issued with one and one-half non-transferrable Long Term Incentive Warrants. Each whole Series A Warrant entitles the holder to purchase one ordinary share at an exercise price of $90. Upon vesting, each Long Term Incentive Warrant entitles the holder to purchase one ordinary share at an exercise price of $82.80.
|
| c) |
Immediately prior to the consummation of the IPO, certain members of the Company's management exercised options to purchase 25,624 ordinary shares granted to them under the 2006 Unit Option Plan.
|
| B. |
Ordinary shares (Cont.)
|
| 2. |
Changes in ordinary share capital (Cont.)
|
| d) |
On August 20, 2014, the Company entered into a certain credit line agreement, pursuant to which it obtained a credit line in an aggregate principal amount of $12 million from certain lenders and existing shareholders (the "Lenders"). The credit line amount was deposited in an escrow account at the closing, which was consummated on October 14, 2014. The Company issued to each Lender at closing a warrant (collectively, the "Credit Line Warrants"), to purchase a number of the Company's ordinary shares constituting 2% of its share capital on a fully diluted basis (assuming conversion of all of the Company's convertible securities into ordinary shares at a 1:1 conversion rate) as of the closing for each $1 million (or portion thereof) extended by such Lender. The Company issued Credit Line Warrants ("CLA Warrants") to purchase in the aggregate 221,556 of its ordinary shares. The CLA Warrants are exercisable for a period of ten years at an exercise price of NIS 2.40 per share, and may be exercised on a net issuance basis.
|
| e) |
During the years ended December 31, 2018, 2017 and 2016, certain Private Placement investors exercised CLA Warrants to purchase an aggregate 22,501, 9,912 and 34,508 ordinary shares, respectively, on a cashless basis, which resulted in the expiration of 5,192, 243, and 761 CLA Warrants, respectively. As of December 31, 2018 and 2017, 7,389 and 35,082 CLA Warrants were outstanding, respectively.
|
| f) |
Upon the closing of the IPO, the Company issued warrants to purchase 8,334 ordinary shares at an exercise price of $90 to the IPO lead underwriter and warrants to purchase 1,250 ordinary shares at an exercise price of $60.72 to the Company's U.S. legal counsel.
|
| g) |
On June 24, 2015, the Company entered into Amendment No. 1 to the Warrant Agreement, dated June 24, 2015, between the Company and American Stock Transfer & Trust Company LLC, as Warrant Agent, to extend the Registration Due Date to the date which is 180 days following the date of closing of the Company's initial public offering (i.e., August 23, 2015) in order to allow the shareholders who were the original purchasers of IPO Units additional time to become the direct registered owners of the ordinary shares underlying the IPO Units. As of December 31, 2018, and December 31, 2017, 378,047 Long Term Incentive Warrants were outstanding.
|
| B. |
Ordinary shares (Cont.)
|
| 2. |
Changes in ordinary share capital (Cont.)
|
| h) |
On August 11, 2016, the Company consummated a registered direct offering of 53,635 ordinary shares at a price of $22.80 per share and pre-funded warrants to purchase 209,524 ordinary shares at a purchase price of $22.20 per pre-funded warrant. The pre-funded warrants have an exercise price of $0.60 per share, subject to certain adjustments and will expire on August 11, 2023, unless otherwise extended in accordance with the terms of the pre-funded warrants. The Company received gross proceeds from the August registered direct offering of approximately $5.9 million (including proceeds from the exercise of 47,917 pre-funded warrants at the closing of the offering).
|
| i) |
On June 2, 2017, the Company consummated a registered direct offering of 112,460 ordinary shares at a price of $24.00 per share and a simultaneous private placement of one-year warrants to purchase 112,460 ordinary shares at an exercise price of $25.50 per share immediately exercisable. The Company received gross proceeds from the June registered direct offering of approximately $2.69 million. As of December 31, 2017, all the warrants issued in this offering were outstanding.
|
| j) |
On November 22, 2017, the Company consummated a registered direct offering of 189,387 ordinary shares at a price of $13.20 per share and a simultaneous private placement of five-year warrants to purchase 142,042 ordinary shares at an exercise price of $15 per share immediately exercisable. The Company received gross proceeds from the November registered direct offering of approximately $2.5 million.
|
| k) |
On May 8, 2018, the Company consummated an underwritten public offering (the “2018 Public Offering”) of 2,738,472 units (the “Units”), at a public offering price of $5.5 per unit, and 450,909 pre-funded units (the “Pre-funded Units”), at a public offering price of $5.49 per Pre-funded Unit. Each Unit consisted of one ordinary share of the Company and one Series C warrant to purchase one ordinary share of the Company. Each Pre-funded Unit consisted of one pre-funded warrant to purchase one ordinary share and one Series C Warrant to purchase one ordinary share. The exercise price of each pre-funded warrant included in the pre-funded unit was $0.01 per share. The Series C warrants have an exercise price of $
5.50 per share, are exercisable immediately and will expire five years from the date of issuance
.
|
| A. |
General
|
| 1. |
In connection with the transfer of all of the business operations and substantially all of the assets of Check-Cap LLC to the Company in 2009, the Company assumed the Check-Cap LLC 2006 Unit Option Plan (hereafter: the "2006 Plan"). According to the 2006 Plan, the Company is authorized to grant options to purchase ordinary shares of the Company to employees, directors and consultants of the Company. The options granted according to the 2006 Plan are generally exercisable for 10 years from the grant date unless otherwise determined by the Company's Board of Directors, vest over a period to be determined by the Company's Board of Directors, and have an exercise price to be determined by the Company's Board of Directors.
|
| 2. |
On January 15, 2015, the Board of Directors resolved to increase the number of ordinary shares by 4% of the Company's fully-diluted share capital (including the option pool) immediately following the consummation of the IPO. As a result, the number of the Company's ordinary shares reserved for issuance under the 2006 Plan increased by 80,646.
|
| 3. |
On April 6, 2015, the Company's Board of Directors resolved to increase the number of ordinary shares of the Company's reserved for issuance under the 2006 Plan by 143,212 shares.
|
| 4. |
On August 13, 2015, the shareholders approved and adopted the Check-Cap Ltd. 2015 Equity Incentive Plan (the "2015 Israeli Plan") and the Check-Ltd. 2015 United States Sub-Plan to Check-Cap Ltd. 2015 Equity Incentive Plan (the "2015 U.S. Sub-Plan" and together with the 2015 Israeli Plan, the "2015 Plan"). As of such date, the Company ceased to grant options under the 2006 Plan. All of the remaining shares authorized but unissued under the 2006 Plan were rolled over to the 2015 Plan.
|
| 5. |
On July 30, 2018, the Company's Board of Directors resolved to increase the number of ordinary shares of the Company's reserved for issuance under the 2015 Plan by 870,261 shares to 1,205,594 shares.
|
| B. |
Details of share-based grants made by the Company
|
|
Grant date
|
No. of options
|
Expiration date
|
Exercise price
|
Fair value on grant date
|
Share based expenses (1)
$ in thousands
|
Vesting terms
|
|||||||||||||||
|
February 29, 2016
|
1,642
|
March 1, 2026
|
$
|
28.44
|
$
|
24.12
|
$
|
40
|
(2
|
)
|
|||||||||||
|
May 4, 2016
|
7,608
|
May 4, 2026
|
$
|
34.68
|
$
|
17.40
|
$
|
132
|
(2
|
)
|
|||||||||||
|
August 4, 2016
|
404
|
August 4, 2026
|
$
|
15.36
|
$
|
20.40
|
$
|
8
|
(2
|
)
|
|||||||||||
|
October 31, 2016
|
202
|
October 31, 2026
|
$
|
23.64
|
$
|
15.84
|
$
|
3
|
(2
|
)
|
|||||||||||
|
February 27, 2017
|
1,964
|
February 27, 2027
|
$
|
27.96
|
$
|
14.40
|
$
|
28
|
(2
|
)
|
|||||||||||
|
May 9, 2017
|
1,580
|
May 9, 2027
|
$
|
25.86
|
$
|
12.96
|
$
|
20
|
(2
|
)
|
|||||||||||
|
June 22, 2017 (4)
|
5,041
|
June 22, 2027
|
$
|
22.32
|
$
|
12.60
|
$
|
64
|
(3
|
)
|
|||||||||||
|
August 3, 2017
|
404
|
August 3, 2027
|
$
|
22.36
|
$
|
12.36
|
$
|
5
|
(2
|
)
|
|||||||||||
|
November 2, 2017
|
853
|
November 2, 2027
|
$
|
20.76
|
$
|
8.16
|
$
|
7
|
(2
|
)
|
|||||||||||
|
February 13, 2018
|
4,584
|
February 13, 2028
|
$
|
10.44
|
$
|
4.95
|
$
|
23
|
(2
|
)
|
|||||||||||
|
July 30, 2018 (6)
|
231,819
|
July 30, 2028
|
$
|
3.92
|
$
|
2.61
|
$
|
605
|
(5
|
)
|
|||||||||||
|
September 20, 2018 (4)
|
37,039
|
September 20, 2028
|
$
|
3.92
|
$
|
2.96
|
$
|
110
|
(5
|
)
|
|||||||||||
|
November 1, 2018
|
44,450
|
November 1, 2028
|
$
|
3.81
|
$
|
3.23
|
$
|
144
|
(5
|
)
|
|||||||||||
| B. |
Details of share-based grants made by the Company (Cont.)
|
|
Grant date
|
No. of RSUs and PSUs
|
Expiration date
|
Fair value on grant date
|
Share based expenses (1)
|
Vesting terms
|
||||||||||||
|
February 27, 2017
|
7,457
|
February 27, 2027
|
$
|
2.20
|
$
|
197
|
(2
|
)
|
|||||||||
|
June 22, 2017 (7)
|
17,448
|
June 22, 2027
|
$
|
1.88
|
$
|
393
|
(7
|
)
|
|||||||||
|
August 3, 2017 (8)
|
24,951
|
August 3, 2027
|
$
|
1.84
|
$
|
551
|
(8
|
)
|
|||||||||
|
July 30, 2018 (6)
|
79,844
|
July 30, 2028
|
$
|
3.24
|
$
|
251
|
(5
|
)
|
|||||||||
|
September 20, 2018 (4)
|
15,875
|
September 20, 2028
|
$
|
3.65
|
$
|
58
|
(5
|
)
|
|||||||||
| 1. |
Share based expenses are based on their fair value on grant date. The amount is charged to statement of operations over the vesting periods.
|
| 2. |
The options vest over a period of four years commencing on the date of grant, such that 25% of the options vested on the first anniversary of the date of grant and thereafter, the remaining options vest in quarterly installments.
|
| 3. |
The options vest over a period of three years commencing on the date of grant in quarterly installments.
|
| 4. |
Options or RSUs granted to certain members of the Company's Board of Directors.
|
| 5. |
The options vest over a period of three years commencing on the date of grant, such that one third of the options vested on the first anniversary of the date of grant and thereafter, the remaining options vest in quarterly installments.
|
| 6. |
Of the 231,819 options and 79,844 RSUs, 122,232 options and 50,796 RSUs, respectively, were issued to certain of the Company’s officers. The remaining options and RSUs were issued to employees and consultants.
|
| 7. |
On June 22, 2017, the Company's shareholders approved the award of 11,302 and 6,146 RSUs to the Mr. Densel, who served as the Company’s CEO at such time, and to certain members of the Company's Board of Directors, respectively. The terms of the RSUs awarded to the Company’s former CEO provided that they shall vest over a period of four years commencing on the date of grant, such that 25% of the RSUs shall vest on the first anniversary of the date of grant and thereafter, the remaining RSUs will vest in quarterly installments. The RSUs granted to certain members of the Company's Board of Directors shall vest over a period of three years commencing on the date of grant in quarterly installments. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $255 and $138 for the RSUs granted to the Company’s CEO and to certain members of the Company's Board of Directors, respectively. These amounts are charged to statement of operations over the vesting periods.
|
| 8. |
On August 3, 2017, the Company's Board of Directors approved the award of 24,951 Performance Stock Units (“PSUs”) to certain of the Company's employees. The PSUs shall vest based on four pre-determined milestones, of which the first milestone (15%) in 2017, the second and third milestones in 2018 (22.5% each) and the forth milestone in 2019 (40%). The compensation expense was based on the fair value on the grant date, and was estimated at approximately $551. No expenses were recorded during the years ended December 31, 2018 and 2017 as the Company did not achieve the pre-determined milestones for these years, and estimates that it will not meet the remaining pre-determined milestone for year 2019.
|
| 9. |
On February 26, 2018, upon the termination of the employment of Mr. Densel, the Company’s former CEO, options to purchase 49,965 ordinary shares and 11,302 RSUs were forfeited.
|
| C. |
Options Fair Value
|
|
|
For the year ended December 31,
|
|||||||||||
|
|
2 0 1 8
|
2 0 1 7
|
2 0 1 6
|
|||||||||
|
Expected volatility (1)
|
104%-108
|
%
|
58%-60
|
%
|
59%-60
|
%
|
||||||
|
Risk-free rate
|
2.67-3.15
|
%
|
1.9%-2.2
|
%
|
1.2%-2.1
|
%
|
||||||
|
Dividend yield
|
0
|
%
|
0
|
%
|
0
|
%
|
||||||
|
Expected term (in years)
|
5.5-7
|
5.5-7
|
5.5-7
|
|||||||||
|
Share price
|
$
|
3.24 - $9.07
|
$
|
15.96-$26.40
|
$
|
26.64-$38.40
|
||||||
| (1) |
In the year ended December 31, 2018, expected volatility was calculated based upon actual historical stock price movements over the most recent periods ending on the grant date, equal to the expected term of the options. In the years ended December 31, 2017 and 2016, due to lack of history of trading, expected volatility was calculated based on certain peer companies that the Company considered to be comparable.
|
| D. |
Effect of share-based compensation transactions on the Company's statements of operations
|
|
|
For the year ended December 31,
|
|||||||||||
|
|
2 0 1 8
|
2 0 1 7
|
2 0 1 6
|
|||||||||
|
|
||||||||||||
|
Research and development, net
|
234
|
116
|
234
|
|||||||||
|
General and administrative, net*
|
(299
|
)
|
610
|
975
|
||||||||
|
Total
|
(65
|
)
|
726
|
1,209
|
||||||||
| E. |
A summary of the Company's option activity related to options granted to employees, service providers and directors, and related information under the 2006 Plan and the 2015 Plan is as follows:
|
|
|
Year ended December 31, 2018
|
|||||||||||||||
|
|
Number
|
Weighted average of exercise price
(in $)
|
Weighted average remaining contractual life
(in years)
|
Aggregate
intrinsic value
($ in thousands)
|
||||||||||||
|
Options outstanding at beginning of year
|
208,222
|
46.32
|
7.11
|
(2
|
)
|
|||||||||||
|
Options granted
|
318,094
|
4.00
|
||||||||||||||
|
Options forfeited
|
(103,532
|
)
|
45.88
|
|||||||||||||
|
Options outstanding at end of year
|
422,784
|
15.54
|
8.60
|
(2
|
)
|
|||||||||||
|
|
||||||||||||||||
|
Options exercisable at end of year
|
101,040
|
46.18
|
4.61
|
(2
|
)
|
|||||||||||
|
|
Year ended December 31, 2017
|
|||||||||||||||
|
|
Number
|
Weighted average of exercise price
(in $)
|
Weighted average remaining contractual life
(in years)
|
Aggregate
intrinsic value
($ in thousands)
|
||||||||||||
|
Options outstanding at beginning of year
|
207,769
|
47.04
|
7.39
|
(2
|
)
|
|||||||||||
|
Options granted
|
9,842
|
23.88
|
||||||||||||||
|
Options forfeited
|
(9,389
|
)
|
39.24
|
|||||||||||||
|
Options outstanding at end of year
|
208,222
|
46.32
|
7.11
|
(2
|
)
|
|||||||||||
|
|
||||||||||||||||
|
Options exercisable at end of year
|
134,550
|
48.00
|
5.99
|
(2
|
)
|
|||||||||||
|
Year ended December 31, 2016
|
||||||||||||||||
|
|
Number of options
|
Weighted average of exercise price
(in $)
|
Weighted average remaining contractual life
(in years)
|
Aggregate
intrinsic value
($ in thousands)
|
||||||||||||
|
Outstanding at the beginning of the year
|
230,958
|
48.12
|
7.99
|
(2
|
)
|
|||||||||||
|
Granted
|
9,856
|
32.64
|
||||||||||||||
|
Exercised
|
-
|
-
|
||||||||||||||
|
Forfeited
|
(33,045
|
)
|
50.52
|
|||||||||||||
|
Outstanding at the end of the year
|
207,769
|
47.04
|
7.39
|
(2
|
)
|
|||||||||||
|
|
||||||||||||||||
|
Exercisable at the end of the year
|
106,725
|
46.80
|
6.34
|
(2
|
)
|
|||||||||||
| 1. |
The weighted average grant date fair values of options granted during the years ended December 31, 2018, 2017 and 2016 were $2.77, $12.48 and $18.60, respectively.
|
| 2. |
The aforementioned options are out of the money.
|
| 3. |
As of December 31, 2018, 2017 and 2016, there were $701, $346 and $766 of total unrecognized compensation cost related to non-vested share-based compensation arrangements granted under the 2006 Plan and the 2015 Plan, respectively. This cost is expected to be recognized over a period of up to 4 years.
|
|
Year ended December 31
|
||||||||||||
|
2018
|
2017
|
2016
|
||||||||||
|
Number of RSUs
|
||||||||||||
|
Unvested at beginning of year
|
44,473
|
-
|
-
|
|||||||||
|
Granted
|
95,719
|
49,856
|
-
|
|||||||||
|
Vested
|
(4,818
|
)
|
(1,033
|
)
|
-
|
|||||||
|
Forfeited
|
(25,905
|
)
|
(4,350
|
)
|
-
|
|||||||
|
Unvested at end of year
|
109,469
|
44,473
|
-
|
|||||||||
| 1. |
The weighted average grant date fair values of RSUs awarded during the years ended December 31, 2018 and 2017 were $3.31 and $1.91. There were no RSU awards in the year ended December 31, 2016.
|
| 2. |
As of December 31, 2018 and 2017, there were $263 and $287 unrecognized compensation cost related to non-vested share-based compensation arrangements granted under the 2015 Plan, respectively. This cost is expected to be recognized over a period of up to 4 years.
|
|
|
For the year ended December 31,
|
|||||||||||
|
|
2 0 1 8
|
2 0 1 7
|
2 0 1 6
|
|||||||||
|
|
||||||||||||
|
Salaries and related expenses
|
4,410
|
4,656
|
4,683
|
|||||||||
|
Share-based compensation
|
234
|
116
|
234
|
|||||||||
|
Materials
|
1,508
|
614
|
596
|
|||||||||
|
Subcontractors and consultants
|
311
|
456
|
320
|
|||||||||
|
Depreciation
|
138
|
147
|
121
|
|||||||||
|
Cost for registration of patents
|
126
|
157
|
150
|
|||||||||
|
Others
|
1,099
|
893
|
511
|
|||||||||
|
|
7,826
|
7,039
|
6,615
|
|||||||||
|
Less participation of the IIA and BIRD Foundation
|
(208
|
)
|
(202
|
)
|
(1,124
|
)
|
||||||
|
Total research and development, net
|
7,618
|
6,837
|
5,491
|
|||||||||
|
|
For the year ended December 31,
|
|||||||||||
|
|
2 0 1 8
|
2 0 1 7
|
2 0 1 6
|
|||||||||
|
|
||||||||||||
|
Salaries and related expenses
|
1,839
|
1,395
|
1,411
|
|||||||||
|
Share-based compensation, net
|
(299
|
)
|
610
|
975
|
||||||||
|
Professional services
|
833
|
414
|
354
|
|||||||||
|
Office rent and maintenance
|
163
|
161
|
144
|
|||||||||
|
Depreciation
|
10
|
10
|
9
|
|||||||||
|
Others
|
899
|
574
|
678
|
|||||||||
|
Total general and administrative
|
3,445
|
3,164
|
3,571
|
|||||||||
|
|
For the year ended December 31,
|
|||||||||||
|
|
2 0 1 8
|
2 0 1 7
|
2 0 1 6
|
|||||||||
|
|
||||||||||||
|
Interest income on short-term deposits
|
243
|
66
|
139
|
|||||||||
|
Bank fees and interest expenses
|
(7
|
)
|
(7
|
)
|
(7
|
)
|
||||||
|
Changes in provision for royalties
|
255
|
82
|
56
|
|||||||||
|
Exchange rate differences
|
(27
|
)
|
95
|
56
|
||||||||
|
Changes in derivatives fair value
|
9
|
-
|
-
|
|||||||||
|
Total financing income
|
473
|
236
|
244
|
|||||||||
|
|
For the year ended December 31,
|
|||||||||||
|
|
2 0 1 8
|
2 0 1 7
|
2 0 1 6
|
|||||||||
|
|
||||||||||||
|
Net loss
|
10,589
|
9,771
|
8,826
|
|||||||||
|
Shares used in computing net loss per ordinary share, basic and diluted (in thousands)
|
4,058
|
1,455
|
1,208
|
|||||||||
|
Net loss per ordinary share, basic and diluted
|
2.61
|
6.72
|
7.31
|
|||||||||
|
|
For the year ended December 31,
|
|||||||||||
|
|
2 0 1 8
|
2 0 1 7
|
2 0 1 6
|
|||||||||
|
|
(number)
|
|||||||||||
|
Warrants and share options
|
3,457
|
914
|
849
|
|||||||||
|
|
7,515
|
2,369
|
2,057
|
|||||||||
| A. |
Compensation to the non-executive directors:
|
|
|
For the year ended December 31,
|
|||||||||||
|
|
2 0 1 8
|
2 0 1 7
|
2 0 1 6
|
|||||||||
|
|
||||||||||||
|
Fees, including reimbursement of expenses
|
340
|
274
|
238
|
|||||||||
|
Share-based compensation
|
113
|
179
|
252
|
|||||||||
|
|
453
|
453
|
490
|
|||||||||
|
B
.
|
Transactions with related parties:
|
|
|
For the year ended December 31,
|
|||||||||||
|
|
2 0 1 8
|
2 0 1 7
|
2 0 1 6
|
|||||||||
|
|
||||||||||||
|
Consulting fees, including share-based compensation and reimbursement of expenses (1) (2)
|
47
|
57
|
67
|
|||||||||
|
Key man life insurance premium (3)
|
-
|
1
|
12
|
|||||||||
|
|
47
|
58
|
79
|
|||||||||
| 1. |
On July 1, 2005, the Company entered into an agreement with Hadar Kimchy according to which Hadar Kimchy provided marketing communication and graphical design services to the Company in consideration for a monthly fee of NIS 10,260 ($3). On August 1, 2014, the monthly fee was increased to NIS 13,680 ($4). Such services were provided to the Company by Sigalit Kimchy, who is employed by Hadar Kimchy. On April 4, 2016, the agreement was terminated.
|
| 2. |
In connection with the asset transfer agreement entered into with the Predecessor Entity in May 2009, the Company assumed the former obligation of the Predecessor Entity to distribute any proceeds it collects on the $1 million key man life insurance policy with respect to Yoav Kimchy, the Company's chief technology officer and a former director, to the former holders of the Series A preferred units in an amount equal to their respective capital contributed to the Predecessor Entity, less any amounts previously distributed to them, plus any accrued and unpaid dividends due to them as of the date of distribution. On November 16, 2016, the Company cancelled the key man life insurance policy with respect to Yoav Kimchy.
|