|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the fiscal year ended December 31, 2013
|
Commission file number 0-16093
|
|
New York
|
16-0977505
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
|
525 French Road, Utica, New York
|
13502
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
|
|
Page
|
|
|
|
|
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
|
Part II
|
|
|
|
|
|
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
|
|
|
|
|
Part III
|
|
|
|
|
|
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
|
|
|
|
|
Part IV
|
|
|
|
|
|
|
Item 15.
|
||
|
|
|
|
|
|
||
|
Item 1.
|
Business
|
|
•
|
general economic and business conditions;
|
|
•
|
changes in foreign exchange and interest rates;
|
|
•
|
cyclical customer purchasing patterns due to budgetary and other constraints;
|
|
•
|
changes in customer preferences;
|
|
•
|
competition;
|
|
•
|
changes in technology;
|
|
•
|
the introduction and acceptance of new products;
|
|
•
|
the ability to evaluate, finance and integrate acquired businesses, products and companies;
|
|
•
|
changes in business strategy;
|
|
•
|
the availability and cost of materials;
|
|
•
|
the possibility that United States or foreign regulatory and/or administrative agencies may initiate enforcement actions against us or our distributors;
|
|
•
|
future levels of indebtedness and capital spending;
|
|
•
|
quality of our management and business abilities and the judgment of our personnel;
|
|
•
|
the availability, terms and deployment of capital;
|
|
•
|
the risk of litigation, especially patent litigation as well as the cost associated with patent and other litigation;
|
|
•
|
the risk of a lack of allograft tissues due to reduced donations of such tissues or due to tissues not meeting the appropriate high standards for screening and/or processing of such tissues;
|
|
•
|
changes in regulatory requirements; and
|
|
•
|
various other factors referenced in this Form 10-K.
|
|
•
|
Favorable Demographics
. The number of surgical procedures performed is increasing and we believe the long-term demographic trend of the aging of the population will lead to continued growth in surgical procedures, and technological advancements, which result in safer and less invasive (or non-invasive) surgical procedures. Additionally, as people are living longer, more active lives, they are engaging in contact sports and activities such as running, skiing, rollerblading, golf and tennis which result in injuries with greater frequency and at an earlier age than ever before. Sales of surgical products aggregated to approximately 90% of our total net revenues in
2013
. See “Products.”
|
|
•
|
Continued Pressure to Reduce Health Care Costs
. In response to rising health care costs, managed care companies and other third-party payers have placed pressures on health care providers to reduce costs. As a result, health care providers have focused on the high cost areas such as surgery. To reduce costs, health care providers use minimally invasive techniques, which generally reduce patient trauma, recovery time and ultimately the length of hospitalization. Approximately 50% of our products are designed for use in minimally invasive surgical procedures. See “Products.” Health care providers are also increasingly purchasing single-use, disposable products, which reduce the costs associated with sterilizing surgical instruments and products following surgery. The single-use nature of disposable products lowers the risk of incorrectly sterilized instruments spreading infection into the patient and increasing the cost of post-operative care. Approximately
80%
of our sales are derived from single-use disposable products.
|
|
•
|
Increased Global Medical Spending
. We believe that foreign markets offer significant growth opportunities for our products. We currently distribute our products through our own sales subsidiaries or through local dealers in over 100 foreign countries.
|
|
•
|
Brand Recognition.
Our products are marketed under leading brand names, including CONMED
®
, CONMED Linvatec
®
and Hall Surgical
®
. These brand names are recognized by physicians and healthcare professionals for quality and service. It is our belief that brand recognition facilitates increased demand for our products in the marketplace, enables us to build upon the brand’s associated reputation for quality and service, and allows us to realize increased market acceptance of new branded products.
|
|
•
|
Breadth of Product Offering.
The breadth of our product lines in our key product areas enables us to meet a wide range of customer requirements and preferences. This has enhanced our ability to market our products to surgeons, hospitals, surgery centers, GPOs, IHNs and other customers, particularly as institutions seek to reduce costs and minimize the number of suppliers.
|
|
•
|
Successful Integration of Acquisitions.
We seek to build growth platforms around our core markets through focused acquisitions of complementary businesses and product lines. These acquisitions have enabled us to diversify our product portfolio, expand our sales and marketing capabilities and strengthen our presence in key geographical markets.
|
|
•
|
Strategic Marketing and Distribution Channels.
We market our products domestically through five focused sales force groups consisting of approximately
275
employee sales representatives and
215
sales professionals employed by independent sales agent groups. Our dedicated sales professionals are highly knowledgeable in the applications and procedures for the products they sell. Our sales representatives foster close professional relationships with physicians, surgeons, hospitals, outpatient surgery centers and physicians’ offices. Additionally, we maintain a global presence through sales subsidiaries and branches located in key international markets. We directly service hospital customers located in these markets through an employee-based international sales force of approximately
185
sales representatives. We also maintain distributor relationships domestically and in numerous countries worldwide. See “Marketing.”
|
|
•
|
Operational Improvements and Manufacturing.
We are focused on continuously improving our supply chain effectiveness, strengthening our manufacturing processes and optimizing our plant network to increase operational efficiencies within the organization. Substantially all of our products are manufactured and assembled from components we produce. Our strategy has historically been to vertically integrate our manufacturing facilities in order to develop a competitive advantage. This integration provides us with cost efficient and flexible manufacturing operations which permit us to allocate capital more efficiently. Additionally, we attempt to exploit commercial synergies between operations, such as the procurement of common raw materials and components used in production.
|
|
•
|
Technological Leadership.
Research and development efforts are closely aligned with our key business objectives, namely developing and improving products and processes, applying innovative technology to the manufacture of products for new global markets and reducing the cost of producing core products. These efforts are evidenced by recent product introductions, such as the Y-Knot® Flex System for instability repairs, Y-Knot® RC anchors for rotator cuffs, the D4000 Resection System, the IM8000 2DHD Camera System, Hall 50™ Powered Instrument System, GS2000 50L Insufflator, EntriPort line of trocars, new D-Flex probes, and DetachaTip® III Multi-Use Endosurgery Instruments.
|
|
•
|
Introduction of New Products and Product Enhancements.
We continually pursue organic growth through the development of new products and enhancements to existing products. We seek to develop new technologies which improve the durability, performance and usability of existing products. In addition to our internal research and development efforts, we receive new ideas for products and technologies, particularly in procedure-specific areas, from surgeons, inventors and other healthcare professionals.
|
|
•
|
Pursue Strategic Acquisitions
. We pursue strategic acquisitions, distribution and similar arrangements in existing and new growth markets to achieve increased operating efficiencies, geographic diversification and market penetration. Targeted companies have historically included those with proven technologies and established brand names which provide potential sales, marketing and manufacturing synergies.
|
|
•
|
Realize Manufacturing and Operating Efficiencies.
We continually review our production systems for opportunities to reduce operating costs, consolidate product lines or identical process flows, reduce inventory requirements and optimize existing processes. Our vertically integrated manufacturing facilities allow for further opportunities to reduce overhead, increase operating efficiencies and capacity utilization.
|
|
•
|
Geographic Diversification.
We believe that significant growth opportunities exist for our surgical products outside the United States. Principal foreign markets for our products include Europe, Latin America and Asia/Pacific Rim. Critical elements of our future sales growth in these markets include leveraging our existing relationships with foreign surgeons, hospitals, third-party payers and foreign distributors, maintaining an appropriate presence in emerging market countries and continually evaluating our routes-to-market.
|
|
•
|
Active Participation In The Medical Community.
We believe that excellent working relationships with physicians and others in the medical industry enable us to gain an understanding of new therapeutic and diagnostic alternatives, trends and emerging opportunities. Active participation allows us to quickly respond to the changing needs of physicians and patients. In addition, we are an active sponsor of medical education both in the United States and internationally, offering new and innovative surgical techniques as well as other medical education materials for use with our products.
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2011
|
2012
|
2013
|
||||||||
|
Orthopedic surgery
|
51
|
%
|
54
|
%
|
54
|
%
|
|||||
|
General surgery
|
40
|
%
|
37
|
%
|
37
|
%
|
|||||
|
Surgical visualization
|
9
|
%
|
9
|
%
|
9
|
%
|
|||||
|
Consolidated net sales
|
100
|
%
|
100
|
%
|
100
|
%
|
|||||
|
Net Sales (in thousands)
|
$
|
725,077
|
|
$
|
767,140
|
|
$
|
762,704
|
|
||
|
•
|
imposition of limitations on conversions of foreign currencies into dollars or remittance of dividends and other payments by international subsidiaries;
|
|
•
|
imposition or increase of withholding and other taxes on remittances and other payments by international subsidiaries;
|
|
•
|
trade barriers;
|
|
•
|
political risks, including political instability;
|
|
•
|
reliance on third parties to distribute our products;
|
|
•
|
hyperinflation in certain foreign countries; and
|
|
•
|
imposition or increase of investment and other restrictions by foreign governments.
|
|
•
|
fines or other enforcement actions;
|
|
•
|
recall or seizure of products;
|
|
•
|
total or partial suspension of production;
|
|
•
|
loss of certification;
|
|
•
|
withdrawal of existing product approvals or clearances;
|
|
•
|
refusal to approve or clear new applications or notices;
|
|
•
|
increased quality control costs; or
|
|
•
|
criminal prosecution.
|
|
•
|
capital constraints;
|
|
•
|
research and development delays;
|
|
•
|
delays in securing regulatory approvals; or
|
|
•
|
changes in the competitive landscape, including the emergence of alternative products or solutions which reduce or eliminate the markets for pending products.
|
|
•
|
our ability to develop and introduce new products and product enhancements in the time frames we currently estimate;
|
|
•
|
our ability to successfully implement new technologies;
|
|
•
|
the market’s readiness to accept new products;
|
|
•
|
having adequate financial and technological resources for future product development and promotion;
|
|
•
|
the efficacy of our products; and
|
|
•
|
the prices of our products compared to the prices of our competitors’ products.
|
|
•
|
a portion of our cash flow from operations must be dedicated to debt service and will not be available for operations, capital expenditures, acquisitions, dividends and other purposes;
|
|
•
|
our ability to obtain additional financing in the future for working capital, capital expenditures, acquisitions or general corporate purposes may be limited or impaired, or may be at higher interest rates;
|
|
•
|
we may be at a competitive disadvantage when compared to competitors that are less leveraged;
|
|
•
|
we may be hindered in our ability to adjust rapidly to market conditions;
|
|
•
|
our degree of leverage could make us more vulnerable in the event of a downturn in general economic conditions or other adverse circumstances applicable to us; and
|
|
•
|
our interest expense could increase if interest rates in general increase because a portion of our borrowings, including our borrowings under our credit agreement, are and will continue to be at variable rates of interest.
|
|
•
|
pending patent applications will result in issued patents;
|
|
•
|
patents issued to or licensed by us will not be challenged by competitors;
|
|
•
|
our patents will be found to be valid or sufficiently broad to protect our technology or provide us with a competitive advantage; or
|
|
•
|
we will be successful in defending against pending or future patent infringement claims asserted against our products.
|
|
Location
|
Square Feet
|
Own or Lease
|
Lease Expiration
|
||||
|
Utica, NY
|
500,000
|
|
Own
|
—
|
|||
|
Largo, FL
|
278,000
|
|
Own
|
—
|
|||
|
Centennial, CO
|
87,500
|
|
Own
|
—
|
|||
|
Chihuahua, Mexico
|
207,720
|
|
Lease
|
September 2019
|
|||
|
Lithia Springs, GA
|
188,400
|
|
Lease
|
December 2019
|
|||
|
Brussels, Belgium
|
45,531
|
|
Lease
|
June 2015
|
|||
|
Mississauga, Canada
|
22,378
|
|
Lease
|
December 2016
|
|||
|
Westborough, MA
|
18,210
|
|
Lease
|
September 2015
|
|||
|
Frenchs Forest, Australia
|
16,909
|
|
Lease
|
July 2015
|
|||
|
Seoul, Korea
|
15,554
|
|
Lease
|
January 2017
|
|||
|
Anaheim, CA
|
14,037
|
|
Lease
|
September 2015
|
|||
|
Frankfurt, Germany
|
13,606
|
|
Lease
|
March 2023
|
|||
|
Milan, Italy
|
13,024
|
|
Lease
|
March 2017
|
|||
|
Westborough, MA
|
10,230
|
|
Lease
|
April 2016
|
|||
|
Swindon, Wiltshire, UK
|
8,562
|
|
Lease
|
December 2015
|
|||
|
Askim, Sweden
|
8,353
|
|
Lease
|
May 2016
|
|||
|
Rungis Cedex, France
|
7,406
|
|
Lease
|
December 2016
|
|||
|
Montreal, Canada
|
7,232
|
|
Lease
|
March 2016
|
|||
|
Copenhagen, Denmark
|
5,899
|
|
Lease
|
April 2014
|
|||
|
Shepshed, Leicestershire, UK
|
5,770
|
|
Lease
|
October 2015
|
|||
|
Barcelona, Spain
|
5,382
|
|
Lease
|
December 2018
|
|||
|
Edison, NJ
|
4,015
|
|
Lease
|
December 2014
|
|||
|
New York, NY
|
3,473
|
|
Lease
|
September 2022
|
|||
|
Beijing, China
|
3,456
|
|
Lease
|
June 2014
|
|||
|
Warsaw, Poland
|
3,222
|
|
Lease
|
February 2018
|
|||
|
Espoo, Finland
|
3,078
|
|
Lease
|
Open Ended
|
|||
|
San Mateo, CA
|
3,068
|
|
Lease
|
December 2015
|
|||
|
Shanghai, China
|
2,269
|
|
Lease
|
February 2015
|
|||
|
Innsbruck, Austria
|
1,820
|
|
Lease
|
June 2020
|
|||
|
|
2012
|
||||||
|
Period
|
High
|
Low
|
|||||
|
First Quarter
|
$
|
30.47
|
|
$
|
26.00
|
|
|
|
Second Quarter
|
30.42
|
|
26.03
|
|
|||
|
Third Quarter
|
29.25
|
|
25.85
|
|
|||
|
Fourth Quarter
|
29.33
|
|
25.71
|
|
|||
|
|
2013
|
||||||
|
Period
|
High
|
Low
|
|||||
|
First Quarter
|
$
|
34.29
|
|
$
|
28.03
|
|
|
|
Second Quarter
|
34.04
|
|
30.42
|
|
|||
|
Third Quarter
|
33.96
|
|
31.07
|
|
|||
|
Fourth Quarter
|
42.50
|
|
33.25
|
|
|||
|
Period
|
(a) Total Number of Shares Purchased
|
(b) Average Price Paid per Share
1
|
(c) Total Number of Shares Purchased as Part of Publicly Announced Program
2
|
(d) Approximate Dollar Value of Shares That May Yet Be Purchased Under the Program
|
||||||||||
|
October 1, 2013 to
|
||||||||||||||
|
October 31, 2013
|
—
|
|
$
|
—
|
|
|
|
$
|
60,128,968
|
|
||||
|
November 1, 2013 to
|
||||||||||||||
|
November 30, 2013
|
—
|
|
$
|
—
|
|
—
|
|
60,128,968
|
|
|||||
|
December 1, 2013 to
|
||||||||||||||
|
December 31, 2013
|
146,905
|
|
$
|
39.67
|
|
146,905
|
|
54,301,615
|
|
|||||
|
Total
|
146,905
|
|
146,905
|
|
||||||||||
|
Equity Compensation Plan Information
|
|||||||
|
Plan category
|
Number of securities to be issued upon exercise of outstanding options, warrants and rights
(a)
|
Weighted-average exercise price of outstanding options, warrants and rights
(b)
|
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a))
(c)
|
||||
|
Equity compensation plans approved by security holders
|
1,606,739
|
|
|
$25.55
|
|
1,145,915
|
|
|
Equity compensation plans not approved by security holders
|
—
|
|
—
|
|
—
|
|
|
|
Total
|
1,606,739
|
|
|
$25.55
|
|
1,145,915
|
|
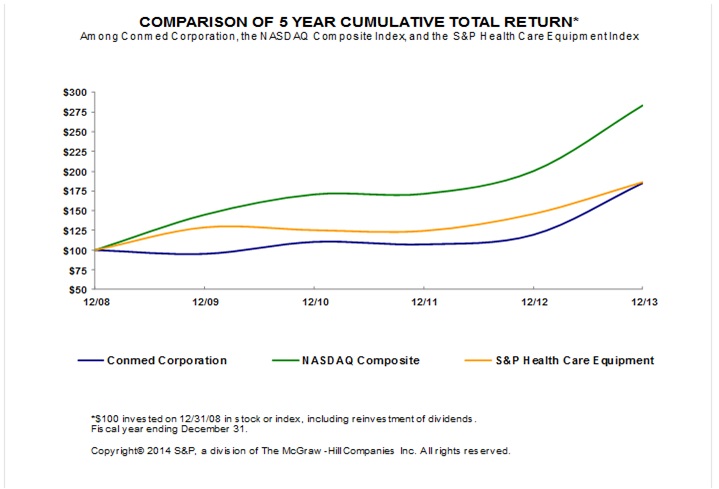
|
Years Ended December 31,
|
||||||||||||||||||||
|
2009
|
2010
|
2011
|
2012
|
2013
|
||||||||||||||||
|
|
(in thousands, except per share data)
|
|||||||||||||||||||
|
Statements of Operations Data (1):
|
|
|
|
|
||||||||||||||||
|
Net sales
|
$
|
694,739
|
|
$
|
713,723
|
|
$
|
725,077
|
|
$
|
767,140
|
|
$
|
762,704
|
|
|||||
|
Cost of sales (2)
|
357,407
|
|
348,339
|
|
350,143
|
|
361,297
|
|
350,287
|
|
||||||||||
|
Gross profit
|
337,332
|
|
365,384
|
|
374,934
|
|
405,843
|
|
412,417
|
|
||||||||||
|
Selling and administrative
|
266,310
|
|
276,463
|
|
276,615
|
|
302,469
|
|
310,730
|
|
||||||||||
|
Research and development
|
31,837
|
|
29,652
|
|
28,651
|
|
28,214
|
|
25,831
|
|
||||||||||
|
Impairment of goodwill (3)
|
—
|
|
—
|
|
60,302
|
|
—
|
|
—
|
|
||||||||||
|
Medical device excise tax
|
—
|
|
—
|
|
—
|
|
—
|
|
5,949
|
|
||||||||||
|
Other expense (4)
|
10,916
|
|
2,176
|
|
1,092
|
|
9,950
|
|
13,399
|
|
||||||||||
|
Income from operations
|
28,269
|
|
57,093
|
|
8,274
|
|
65,210
|
|
56,508
|
|
||||||||||
|
(Gain) loss on early extinguishment of debt (5)
|
(1,083
|
)
|
79
|
|
—
|
|
—
|
|
263
|
|
||||||||||
|
Amortization of debt discount
|
4,111
|
|
4,244
|
|
3,903
|
|
—
|
|
—
|
|
||||||||||
|
Interest expense
|
7,086
|
|
7,113
|
|
6,676
|
|
5,730
|
|
5,613
|
|
||||||||||
|
Income (loss) before income taxes
|
18,155
|
|
45,657
|
|
(2,305
|
)
|
59,480
|
|
50,632
|
|
||||||||||
|
Provision (benefit) for income taxes
|
6,018
|
|
15,311
|
|
(3,057
|
)
|
18,999
|
|
14,693
|
|
||||||||||
|
Net income
|
$
|
12,137
|
|
$
|
30,346
|
|
$
|
752
|
|
$
|
40,481
|
|
$
|
35,939
|
|
|||||
|
Per Share Data
|
|
|
|
|
|
|
|
|
||||||||||||
|
Basic earnings per share
|
$
|
0.42
|
|
$
|
1.06
|
|
$
|
.03
|
|
$
|
1.43
|
|
$
|
1.30
|
|
|||||
|
Diluted earnings per share
|
$
|
0.42
|
|
$
|
1.05
|
|
$
|
.03
|
|
$
|
1.41
|
|
$
|
1.28
|
|
|||||
|
Dividends per share of common stock
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
0.60
|
|
$
|
0.65
|
|
|||||
|
Weighted Average Number of Common Shares In Calculating:
|
|
|
|
|
|
|
|
|
||||||||||||
|
Basic earnings per share
|
29,074
|
|
28,715
|
|
28,246
|
|
28,301
|
|
27,722
|
|
||||||||||
|
Diluted earnings per share
|
29,142
|
|
28,911
|
|
28,633
|
|
28,653
|
|
28,114
|
|
||||||||||
|
Other Financial Data:
|
|
|
|
|
|
|
|
|
||||||||||||
|
Depreciation and amortization
|
$
|
41,283
|
|
$
|
41,807
|
|
$
|
42,687
|
|
$
|
46,616
|
|
$
|
47,867
|
|
|||||
|
Capital expenditures
|
21,444
|
|
14,732
|
|
17,552
|
|
21,532
|
|
18,445
|
|
||||||||||
|
Balance Sheet Data (at period end):
|
|
|
|
|
|
|
|
|
||||||||||||
|
Cash and cash equivalents
|
$
|
10,098
|
|
$
|
12,417
|
|
$
|
26,048
|
|
$
|
23,720
|
|
$
|
54,443
|
|
|||||
|
Total assets
|
958,413
|
|
985,773
|
|
935,594
|
|
1,078,849
|
|
1,090,508
|
|
||||||||||
|
Long-term obligations
|
302,791
|
|
219,344
|
|
231,339
|
|
346,637
|
|
372,924
|
|
||||||||||
|
Total shareholders’ equity
|
576,515
|
|
586,563
|
|
573,071
|
|
606,998
|
|
606,319
|
|
||||||||||
|
(1)
|
Results of operations of acquired businesses have been recorded in the financial statements since the date of acquisition.
|
|
(2)
|
In
2009
,
2010
,
2011
,
2012
and
2013
, we incurred charges related to the restructuring of certain of our operations of
$12.7 million
,
$2.4 million
,
$3.5 million
,
$7.1 million
and
$6.5 million
, respectively; in
2010
and
2013
we incurred charges of
$2.5 million
and
$2.1 million
, respectively, related to the termination of a product offering. See additional discussion in Note 15 to the Consolidated Financial Statements.
|
|
(3)
|
During 2011, we recorded a
$60.3 million
charge for the impairment of goodwill related to the legacy CONMED Patient Care reporting unit. Refer to Note 4 to the Consolidated Financial Statements for further details.
|
|
(4)
|
Other expense includes the following:
|
|
|
2009
|
2010
|
2011
|
2012
|
2013
|
|||||||||||||||
|
New plant/facility consolidation
|
$
|
2,726
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||||
|
Net pension gain
|
(1,882
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Product recall
|
5,992
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Administrative consolidation costs
|
4,080
|
|
2,176
|
|
792
|
|
6,497
|
|
8,750
|
|
||||||||||
|
Costs associated with purchase of a distributor
|
—
|
|
—
|
|
300
|
|
704
|
|
—
|
|
||||||||||
|
Costs associated with legal arbitration and patent dispute
|
—
|
|
—
|
|
—
|
|
1,555
|
|
3,206
|
|
||||||||||
|
Pension settlement expense
|
—
|
|
—
|
|
—
|
|
—
|
|
1,443
|
|
||||||||||
|
Costs associated with purchase of a business
|
—
|
|
—
|
|
—
|
|
1,194
|
|
—
|
|
||||||||||
|
Other expense
|
$
|
10,916
|
|
$
|
2,176
|
|
$
|
1,092
|
|
$
|
9,950
|
|
$
|
13,399
|
|
|||||
|
(5)
|
Includes in 2010 and 2013, a charge of
$0.1 million
and
$0.3 million
, respectively, related to a loss on the early extinguishment of debt. Includes in
2009
, a gain of
$1.1 million
on the early extinguishment of debt. See additional discussion in Note 5 to the Consolidated Financial Statements.
|
|
|
2011
|
2012
|
2013
|
|||||
|
Orthopedic surgery
|
51
|
%
|
54
|
%
|
54
|
%
|
||
|
General surgery
|
40
|
|
37
|
|
37
|
|
||
|
Surgical visualization
|
9
|
|
9
|
|
9
|
|
||
|
Consolidated net sales
|
100
|
%
|
100
|
%
|
100
|
%
|
||
|
•
|
Sales to customers are evidenced by firm purchase orders. Title and the risks and rewards of ownership are transferred to the customer when product is shipped under our stated shipping terms. Payment by the customer is due under fixed payment terms and collectability is reasonably assured.
|
|
•
|
We place certain of our capital equipment with customers on a loaned basis in return for commitments to purchase related single-use products over time periods generally ranging from one to three years. In these circumstances, no revenue is recognized upon capital equipment shipment as the equipment is loaned and subject to return if certain minimum single-use purchases are not met. Revenue is recognized upon the sale and shipment of the related single-use products. The cost of the equipment is amortized over its estimated useful life.
|
|
•
|
We recognize revenues related to the promotion and marketing of sports medicine allograft tissue in accordance with the contractual terms of our agreement with Musculoskeletal Transplant Foundation (“MTF”) on a net basis as our role is limited to that of an agent earning a commission or fee. MTF records revenue when the tissue is shipped to the customer. Our services are completed at this time and net revenues for the “Service Fee” for our promotional and marketing efforts are then recognized based on a percentage of the net amounts billed by MTF to its customers. The timing of revenue recognition is determined through review of the net billings made by MTF each month. Our net commission Service Fee is based on the contractual terms of our agreement and is currently 50%. This percentage can vary over the term of the agreement but is contractually determinable. Our Service Fee revenues are recorded net of amortization of the acquired assets, which are being expensed over the expected useful life of 25 years.
|
|
•
|
Product returns are only accepted at the discretion of the Company and in accordance with our “Returned Goods Policy”. Historically the level of product returns has not been significant. We accrue for sales returns, rebates and allowances based upon an analysis of historical customer returns and credits, rebates, discounts and current market conditions.
|
|
•
|
Our terms of sale to customers generally do not include any obligations to perform future services. Limited warranties are provided for capital equipment sales and provisions for warranty are provided at the time of product sale based upon an analysis of historical data.
|
|
•
|
Amounts billed to customers related to shipping and handling have been included in net sales. Shipping and handling costs included in selling and administrative expense were
$13.0 million
,
$12.8 million
and
$12.6 million
for
2011
,
2012
and
2013
, respectively.
|
|
•
|
We sell to a diversified base of customers around the world and, therefore, believe there is no material concentration of credit risk.
|
|
•
|
We assess the risk of loss on accounts receivable and adjust the allowance for doubtful accounts based on this risk assessment. Historically, losses on accounts receivable have not been material. Management believes that the allowance for doubtful accounts of
$1.4 million
at
December 31, 2013
is adequate to provide for probable losses resulting from accounts receivable.
|
|
|
Year Ended December 31,
|
|||||||
|
|
2011
|
2012
|
2013
|
|||||
|
Net sales
|
100.0
|
%
|
100.0
|
%
|
100.0
|
%
|
||
|
Cost of sales
|
48.3
|
|
47.1
|
|
45.9
|
|
||
|
Gross margin
|
51.7
|
|
52.9
|
|
54.1
|
|
||
|
Selling and administrative expense
|
38.1
|
|
39.4
|
|
40.7
|
|
||
|
Research and development expense
|
4.0
|
|
3.7
|
|
3.4
|
|
||
|
Impairment of goodwill
|
8.3
|
|
—
|
|
—
|
|
||
|
Medical device excise tax
|
—
|
|
—
|
|
0.8
|
|
||
|
Other expense
|
0.2
|
|
1.3
|
|
1.8
|
|
||
|
Income from operations
|
1.1
|
|
8.5
|
|
7.4
|
|
||
|
Loss on early extinguishment of debt
|
—
|
|
—
|
|
0.0
|
|
||
|
Amortization of debt discount
|
0.5
|
|
—
|
|
—
|
|
||
|
Interest expense
|
0.9
|
|
0.7
|
|
0.7
|
|
||
|
Income (loss) before income taxes
|
(0.3
|
)
|
7.8
|
|
6.7
|
|
||
|
Provision (benefit) for income taxes
|
(0.4
|
)
|
2.5
|
|
1.9
|
|
||
|
Net income
|
0.1
|
%
|
5.3
|
%
|
4.8
|
%
|
||
|
•
|
Orthopedic surgery sales decreased
$3.7 million
(
-0.9%
) in
2013
to
$410.2 million
from
$413.9 million
in
2012
mainly due to lower sales in our resection product offerings and large bone burs and blades. In local currency, excluding the effects of the hedging program, sales increased
0.1%
.
|
|
•
|
General surgery sales remained relatively flat with a
$0.1 million
(
0.0%
) increase in
2013
to
$286.7 million
from
$286.6 million
in
2012
mainly due to increased sales in our endomechanical, gastrointestinal and pulmonary product offerings offset by decreased sales in our advanced energy and patient monitoring product offerings. In local currency, excluding the effects of the hedging program, sales increased
0.5%
.
|
|
•
|
Surgical visualization sales decreased
$0.8 million
(
-1.2%
) in
2013
to
$65.8 million
from
$66.6 million
in
2012
mainly due to lower video system product sales. In local currency, excluding the effects of the hedging program, sales decreased
-0.9%
.
|
|
•
|
Orthopedic surgery sales increased
$42.7 million
(
11.5%
) in
2012
to
$413.9 million
from
$371.2 million
in
2011
mainly due to the distribution agreement with MTF, increased sales of our procedure specific, large bone burs and blades and small bone handpiece product offerings. In local currency, excluding the effects of the hedging program sales increased
11.4%
.
|
|
•
|
General surgery sales decreased
$0.8 million
(
-0.3%
) in
2012
to
$286.6 million
from
$287.4 million
in
2011
mainly due to lower sales in our patient monitoring products and advanced energy products offset by increases in our gastrointestinal and pulmonary products. In local currency, excluding the effects of the hedging program, sales decreased
-0.4%
.
|
|
•
|
Surgical visualization sales remained relatively flat, with a
$0.1 million
(
0.2%
) increase in
2012
to
$66.6 million
from
$66.5 million
in
2011
due to higher video systems sales. In local currency, excluding the effects of the hedging program, sales increased
0.7%
.
|
|
|
Payments Due by Period
|
||||||||||||||||||
|
Total
|
Less than
1 Year
|
1-3
Years
|
3-5
Years
|
More than
5 Years
|
|||||||||||||||
|
Long-term debt
|
$
|
215,575
|
|
$
|
1,140
|
|
$
|
2,573
|
|
$
|
211,026
|
|
$
|
836
|
|
||||
|
Contingent consideration
|
50,000
|
|
16,667
|
|
33,333
|
|
—
|
|
—
|
|
|||||||||
|
Purchase obligations
|
40,130
|
|
39,996
|
|
134
|
|
—
|
|
—
|
|
|||||||||
|
Operating lease obligations
|
28,529
|
|
6,723
|
|
9,926
|
|
6,874
|
|
5,006
|
|
|||||||||
|
Total contractual obligations
|
$
|
334,234
|
|
$
|
64,526
|
|
$
|
45,966
|
|
$
|
217,900
|
|
$
|
5,842
|
|
||||
|
Index to Financial Statements
|
||
|
|
|
|
|
(a)(1)
|
List of Financial Statements
|
Page in Form 10-K
|
|
|
|
|
|
|
Management’s Report on Internal Control Over Financial Reporting
|
40
|
|
|
|
|
|
|
Report of Independent Registered Public Accounting Firm
|
41
|
|
|
|
|
|
|
Consolidated Balance Sheets at December 31, 2012 and 2013
|
42
|
|
|
|
|
|
|
Consolidated Statements of Comprehensive Income for the Years Ended December 31, 2011, 2012 and 2013
|
43
|
|
|
|
|
|
|
Consolidated Statements of Shareholders’ Equity for the Years Ended December 31, 2011, 2012 and 2013
|
44
|
|
|
|
|
|
|
Consolidated Statements of Cash Flows for the Years Ended December 31, 2011, 2012 and 2013
|
46
|
|
|
|
|
|
|
Notes to Consolidated Financial Statements
|
48
|
|
|
|
|
|
(2)
|
List of Financial Statement Schedules
|
|
|
|
|
|
|
|
Valuation and Qualifying Accounts (Schedule II)
|
75
|
|
|
|
|
|
|
All other schedules have been omitted because they are not applicable, or the required information is shown in the financial statements or notes thereto.
|
|
|
|
|
|
|
(3)
|
List of Exhibits
|
|
|
|
|
|
|
|
The exhibits listed on the accompanying Exhibit Index on page 37 below are filed as part of this Form 10-K.
|
|
|
|
|
|
|
|
|
|
|
CONMED CORPORATION
|
|
|
|
By: /s/ Joseph J. Corasanti
|
|
Joseph J. Corasanti
|
|
(President and Chief
|
|
Executive Officer)
|
|
|
|
Date:
|
|
February 24, 2014
|
|
Signature
|
Title
|
Date
|
||
|
|
|
|
||
|
/s/ EUGENE R. CORASANTI
|
Chairman of the Board
|
|
||
|
Eugene R. Corasanti
|
of Directors
|
February 24, 2014
|
||
|
|
|
|
||
|
/s/ JOSEPH J. CORASANTI
|
President, Chief Executive
|
|
||
|
Joseph J. Corasanti
|
Officer and Director
|
February 24, 2014
|
||
|
|
|
|
||
|
/s/ ROBERT D. SHALLISH, JR.
|
Executive Vice President-Finance
|
|
||
|
Robert D. Shallish, Jr.
|
and Chief Financial Officer (Principal Financial Officer)
|
February 24, 2014
|
||
|
|
|
|
||
|
/s/ LUKE A. POMILIO
|
Executive Vice President-
|
|
||
|
Luke A. Pomilio
|
Controller and Corporate General Manager (Principal Accounting Officer)
|
February 24, 2014
|
||
|
|
|
|
||
|
/s/ BRIAN CONCANNON
|
||||
|
Brian Concannon
|
Director
|
February 24, 2014
|
||
|
/s/ BRUCE F. DANIELS
|
|
|
||
|
Bruce F. Daniels
|
Director
|
February 24, 2014
|
||
|
|
|
|
||
|
/s/ JO ANN GOLDEN
|
|
|
||
|
Jo Ann Golden
|
Director
|
February 24, 2014
|
||
|
|
|
|
||
|
/s/ DIRK M. KUYPER
|
||||
|
Dirk M. Kuyper
|
Director
|
February 24, 2014
|
||
|
/s/ STEPHEN M. MANDIA
|
|
|
||
|
Stephen M. Mandia
|
Director
|
February 24, 2014
|
||
|
|
|
|
||
|
/s/ STUART J. SCHWARTZ
|
|
|
||
|
Stuart J. Schwartz
|
Director
|
February 24, 2014
|
||
|
|
|
|
||
|
/s/ MARK E. TRYNISKI
|
|
|
||
|
Mark E. Tryniski
|
Director
|
February 24, 2014
|
||
|
Exhibit No.
|
|
Description
|
|
|
|
|
|
3.1
|
-
|
Amended and Restated By-Laws, as adopted by the Board of Directors on April 29, 2011 (Incorporated by reference to the Company’s Current Report on Form 10-Q filed with the Securities and Exchange Commission on May 2, 2011).
|
|
|
|
|
|
3.2
|
-
|
1999 Amendment to Certificate of Incorporation and Restated Certificate of Incorporation of CONMED Corporation (Incorporated by reference to Exhibit 3.2 of the Company’s Annual Report on Form 10-K for the year ended December 31, 1999).
|
|
|
|
|
|
4.1
|
-
|
See Exhibit 3.1.
|
|
|
|
|
|
4.2
|
-
|
See Exhibit 3.2.
|
|
4.3
|
-
|
Guarantee and Collateral Agreement, dated August 28, 2002, made by CONMED Corporation and certain of its subsidiaries in favor of JP Morgan Chase Bank (Incorporated by reference to Exhibit 10.2 of the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2002).
|
|
|
|
|
|
4.4
|
-
|
First Amendment to Guarantee and Collateral Agreement, dated June 30, 2003, made by CONMED Corporation and certain of its subsidiaries in favor of JP Morgan Chase Bank and the several banks and other financial institutions or entities from time to time parties thereto (Incorporated by reference to Exhibit 10.2 of the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2003).
|
|
|
|
|
|
4.5
|
-
|
Second Amendment to Guarantee and Collateral Agreement, dated April 13, 2006, made by CONMED Corporation and certain of its subsidiaries in favor of JP Morgan Chase Bank and the several banks and other financial institutions or entities from time to time parties thereto (Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on April 19, 2006).
|
|
4.6
|
-
|
Third Amendment to Guarantee and Collateral Agreement, dated as of January 17, 2013, made by CONMED Corporation and certain of its subsidiaries in favor of JP Morgan Chase Bank (Incorporated by reference to Exhibit 4.6 of the Company's Annual Report on Form 10-K for the year ended December 31, 2012).
|
|
|
|
|
|
10.1+
|
-
|
Employment Agreement between the Company and Eugene R. Corasanti, dated October 31, 2006 (Incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on November 2, 2006).
|
|
10.2+
|
-
|
Amended and Restated Employment Agreement, dated October 30, 2009, by and between CONMED Corporation and Joseph J. Corasanti, Esq. (Incorporated by reference to the Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2009).
|
|
|
|
|
|
10.3
|
-
|
Amended and Restated Employee Stock Option Plan (including form of Stock Option Agreement) (Incorporated by reference to Exhibit 10.6 of the Company’s Annual Report on Form 10-K for the year ended December 31, 1996).
|
|
|
|
|
|
10.4
|
-
|
Stock Option Plan for Non-Employee Directors of CONMED Corporation (Incorporated by reference to Exhibit 10.5 of the Company’s Annual Report on Form 10-K for the year ended December 31, 1996).
|
|
|
|
|
|
10.5
|
-
|
Amendment to Stock Option Plan for Non-employee Directors of CONMED Corporation (Incorporated by reference to the Company’s Definitive Proxy Statement for the 2002 Annual Meeting filed with the Securities and Exchange Commission on April 17, 2002).
|
|
|
|
|
|
10.6
|
-
|
Amended and Restated 1999 Long Term Incentive Plan (Incorporated by reference to Exhibit 4.3 of the Company’s Registration Statement on Form S-8 on November 3, 2009).
|
|
|
||
|
10.7
|
-
|
2002 Employee Stock Purchase Plan (Incorporated by reference to the Company’s Definitive Proxy Statement for the 2002 Annual Meeting filed with the Securities and Exchange Commission on April 17, 2002).
|
|
|
|
|
|
10.8
|
-
|
Amendment to CONMED Corporation 2002 Employee Stock Purchase Plan (Incorporated by reference to Exhibit 10.11 of the Company’s Annual Report on Form 10-K for the year ended December 31, 2005).
|
|
|
||
|
10.9
|
-
|
2006 Stock Incentive Plan (Incorporated by reference to Exhibit 4.3 of the Company’s Registration Statement on Form S-8 on August 8, 2006).
|
|
|
|
|
|
10.10
|
-
|
Amended and Restated 2007 Non-Employee Director Equity Compensation Plan of CONMED Corporation (Incorporated by reference to Exhibit 4.3 of the Company’s Registration Statement on Form S-8 on August 3, 2010).
|
|
10.11
|
-
|
Amended and Restated Long Term Incentive Plan (Incorporated by reference to Exhibit 4.3 of the Company’s Registration Statement on Form S-8 on July 27, 2012).
|
|
10.12
|
-
|
Amended and Restated Credit Agreement, dated January 17, 2013, among CONMED Corporation, JP Morgan Chase Bank and the several banks and other financial institutions or entities from time to time parties thereto (Incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on January 18, 2013).
|
|
|
||
|
10.13
|
-
|
Change in Control Severance Agreement for Joseph J. Corasanti (Incorporated by reference to Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2008).
|
|
|
||
|
10.14
|
-
|
Change in Control Severance Agreement for Robert D. Shallish, Jr. (Incorporated by reference to Exhibit 10.2 of the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2008).
|
|
|
|
|
|
10.15
|
-
|
Change in Control Severance Agreement for Daniel S. Jonas (Incorporated by reference to Exhibit 10.4 of the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2008).
|
|
|
||
|
10.16
|
-
|
Change in Control Severance Agreement for Luke A. Pomilio (Incorporated by reference to Exhibit 10.5 of the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2008).
|
|
|
|
|
|
10.17
|
-
|
Executive Severance Agreement for Joseph G. Darling (Incorporated by reference to Exhibit 10.28 of the Company's Annual Report on Form 10-K for the year ended December 31, 2008).
|
|
|
||
|
10.18
|
-
|
Change in Control Severance Agreement for Joseph G. Darling (Incorporated by reference to Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2010).
|
|
10.19
|
-
|
Sports Medicine Joint Development and Distribution Agreement by and between Musculoskeletal Transplant Foundation, Inc. and CONMED Corporation dated as of January 3, 2012 (Incorporated by reference to Exhibit 10.1 of the Company's Current Report on Form 8-K dated January 3, 2012).
|
|
|
|
|
|
14
|
-
|
Code of Ethics. The CONMED code of ethics may be accessed via the Company’s website at http://www.CONMED.com/conmed_investor_template.php
|
|
21*
|
-
|
Subsidiaries of the Registrant.
|
|
|
|
|
|
23*
|
-
|
Consent of Independent Registered Public Accounting Firm.
|
|
|
|
|
|
31.1*
|
-
|
Certification of Joseph J. Corasanti pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities Exchange Act, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
|
|
|
31.2*
|
-
|
Certification of Robert D. Shallish, Jr. pursuant to Rule 13a-14(a) and Rule 15d-14(a) of the Securities Exchange Act, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
|
|
|
32.1*
|
-
|
Certifications of Joseph J. Corasanti and Robert D. Shallish, Jr. pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
101*
|
The following materials from CONMED Corporation's Annual Report on Form 10-K for the year ended December 31, 2013 formatted in XBRL (Extensible Business Reporting Language): (i) Consolidated Statements of Comprehensive Income for the three years ended December 31, 2013, (ii) Consolidated Balance Sheets at December 31, 2013 and 2012, (iii) Consolidated Statements of Shareholders' Equity for the three years ended December 31, 2013 (iv) Consolidated Statements of Cash Flows for the three years ended December 31, 2013, (v) Notes to the Consolidated Financial Statements for the year ended December 31, 2013 and (vi) Schedule II - Valuation and Qualifying Accounts. In accordance with Rule 406T of Regulation S-T, the XBRL related information in Exhibit 101 to this Annual Report on Form 10-K shall not be deemed to be “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liability of that section, and shall not be part of any registration statement or other document filed under the Securities Act or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
|
|
|
|
|
|
|
|
*
|
Filed herewith
|
|
+
|
Management contract or compensatory plan or arrangement.
|
|
|
|
2012
|
2013
|
|||||
|
ASSETS
|
|
|
|||||
|
Current assets:
|
|
|
|||||
|
Cash and cash equivalents
|
$
|
23,720
|
|
$
|
54,443
|
|
|
|
Accounts receivable, less allowance for doubtful
|
|
|
|
|
|||
|
accounts of $1,203 in 2012 and $1,384 in 2013
|
139,124
|
|
140,426
|
|
|||
|
Inventories
|
156,228
|
|
143,211
|
|
|||
|
Income taxes receivable
|
2,897
|
|
3,805
|
|
|||
|
Deferred income taxes
|
11,931
|
|
13,202
|
|
|||
|
Prepaid expenses and other current assets
|
14,993
|
|
17,045
|
|
|||
|
Total current assets
|
348,893
|
|
372,132
|
|
|||
|
Property, plant and equipment, net
|
139,041
|
|
138,985
|
|
|||
|
Deferred income taxes
|
1,057
|
|
1,183
|
|
|||
|
Goodwill
|
248,502
|
|
248,428
|
|
|||
|
Other intangible assets, net
|
334,185
|
|
319,440
|
|
|||
|
Other assets
|
7,171
|
|
10,340
|
|
|||
|
Total assets
|
$
|
1,078,849
|
|
$
|
1,090,508
|
|
|
|
LIABILITIES AND SHAREHOLDERS' EQUITY
|
|
|
|
|
|||
|
Current liabilities:
|
|
|
|
|
|||
|
Current portion of long-term debt
|
$
|
1,050
|
|
$
|
1,140
|
|
|
|
Accounts payable
|
23,622
|
|
27,448
|
|
|||
|
Accrued compensation and benefits
|
33,511
|
|
33,426
|
|
|||
|
Income taxes payable
|
2,706
|
|
2,116
|
|
|||
|
Other current liabilities
|
64,325
|
|
47,135
|
|
|||
|
Total current liabilities
|
125,214
|
|
111,265
|
|
|||
|
Long-term debt
|
160,802
|
|
214,435
|
|
|||
|
Deferred income taxes
|
99,199
|
|
113,199
|
|
|||
|
Other long-term liabilities
|
86,636
|
|
45,290
|
|
|||
|
Total liabilities
|
471,851
|
|
484,189
|
|
|||
|
Commitments and contingencies
|
|
|
|
|
|||
|
Shareholders' equity:
|
|
|
|
|
|||
|
Preferred stock, par value $.01 per share; authorized
|
|
|
|
|
|||
|
500,000 shares, none issued or outstanding
|
—
|
|
—
|
|
|||
|
Common stock, par value $.01 per share; 100,000,000
|
|
|
|
|
|||
|
authorized; 31,299,194 issued in 2012 and 2013, respectively
|
313
|
|
313
|
|
|||
|
Paid-in capital
|
324,322
|
|
326,436
|
|
|||
|
Retained earnings
|
377,907
|
|
395,889
|
|
|||
|
Accumulated other comprehensive loss
|
(27,581
|
)
|
(17,572
|
)
|
|||
|
Less: Treasury stock, at cost;
|
|
|
|
|
|||
|
2,925,801 and 3,718,332 shares in
|
|
|
|
|
|||
|
2012 and 2013, respectively
|
(67,963
|
)
|
(98,747
|
)
|
|||
|
Total shareholders' equity
|
606,998
|
|
606,319
|
|
|||
|
Total liabilities and shareholders' equity
|
$
|
1,078,849
|
|
$
|
1,090,508
|
|
|
|
2011
|
2012
|
2013
|
|||||||||
|
Net sales
|
$
|
725,077
|
|
$
|
767,140
|
|
$
|
762,704
|
|
||
|
Cost of sales
|
350,143
|
|
361,297
|
|
350,287
|
|
|||||
|
Gross profit
|
374,934
|
|
405,843
|
|
412,417
|
|
|||||
|
Selling and administrative expense
|
276,615
|
|
302,469
|
|
310,730
|
|
|||||
|
Research and development expense
|
28,651
|
|
28,214
|
|
25,831
|
|
|||||
|
Impairment of goodwill
|
60,302
|
|
—
|
|
—
|
|
|||||
|
Medical device excise tax
|
—
|
|
—
|
|
5,949
|
|
|||||
|
Other expense
|
1,092
|
|
9,950
|
|
13,399
|
|
|||||
|
|
366,660
|
|
340,633
|
|
355,909
|
|
|||||
|
Income from operations
|
8,274
|
|
65,210
|
|
56,508
|
|
|||||
|
Loss on early extinguishment of debt
|
—
|
|
—
|
|
263
|
|
|||||
|
Amortization of debt discount
|
3,903
|
|
—
|
|
—
|
|
|||||
|
Interest expense
|
6,676
|
|
5,730
|
|
5,613
|
|
|||||
|
Income (loss) before income taxes
|
(2,305
|
)
|
59,480
|
|
50,632
|
|
|||||
|
Provision (benefit) for income taxes
|
(3,057
|
)
|
18,999
|
|
14,693
|
|
|||||
|
Net income
|
$
|
752
|
|
$
|
40,481
|
|
$
|
35,939
|
|
||
|
Per share data:
|
|
|
|
|
|
|
|||||
|
Basic
|
$
|
0.03
|
|
$
|
1.43
|
|
$
|
1.30
|
|
||
|
Diluted
|
$
|
0.03
|
|
$
|
1.41
|
|
$
|
1.28
|
|
||
|
Dividends per share of common stock
|
$
|
—
|
|
$
|
0.60
|
|
$
|
0.65
|
|
||
|
Other comprehensive income (loss), before tax:
|
|||||||||||
|
Foreign currency translation adjustments
|
$
|
(1,937
|
)
|
$
|
1,995
|
|
$
|
(1,193
|
)
|
||
|
Pension liability
|
(20,250
|
)
|
1,387
|
|
18,175
|
|
|||||
|
Cash flow hedging gain (loss)
|
6,690
|
|
(6,507
|
)
|
(404
|
)
|
|||||
|
Other comprehensive income (loss), before tax
|
(14,745
|
)
|
37,356
|
|
52,517
|
|
|||||
|
Provision (benefit) for income taxes related to items of other comprehensive income
|
(5,010
|
)
|
(1,892
|
)
|
6,569
|
|
|||||
|
Comprehensive income (loss)
|
$
|
(9,735
|
)
|
$
|
39,248
|
|
$
|
45,948
|
|
||
|
|
Common Stock
|
Paid-in
Capital
|
Retained
Earnings
|
Accumulated
Other
Comprehensive
Loss
|
Treasury
Stock
|
Shareholders’
Equity
|
||||||||||||||||||||
|
|
Shares
|
Amount
|
||||||||||||||||||||||||
|
Balance at December 31, 2010
|
31,299
|
|
$
|
313
|
|
$
|
319,406
|
|
$
|
354,020
|
|
$
|
(15,861
|
)
|
$
|
(71,315
|
)
|
$
|
586,563
|
|
||||||
|
Common stock issued
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
under employee plans
|
|
|
|
|
(3,849
|
)
|
(333
|
)
|
|
|
9,009
|
|
4,827
|
|
||||||||||||
|
Repurchase of treasury stock
|
(15,021
|
)
|
(15,021
|
)
|
||||||||||||||||||||||
|
Tax benefit arising from
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
common stock issued
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
under employee plans
|
|
|
|
|
1,197
|
|
|
|
|
|
|
|
1,197
|
|
||||||||||||
|
Stock-based compensation
|
|
|
|
|
5,240
|
|
|
|
|
|
|
|
5,240
|
|
||||||||||||
|
Comprehensive income (loss):
|
||||||||||||||||||||||||||
|
Foreign currency
translation adjustments
|
(1,937
|
)
|
||||||||||||||||||||||||
|
Pension liability (net of income tax benefit of $7,482)
|
(12,768
|
)
|
||||||||||||||||||||||||
|
Cash flow hedging gain (net of income tax expense of $2,472)
|
4,218
|
|
||||||||||||||||||||||||
|
Net income
|
752
|
|
||||||||||||||||||||||||
|
Total comprehensive
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
income (loss)
|
|
|
|
|
|
|
(9,735
|
)
|
||||||||||||||||||
|
Balance at December 31, 2011
|
31,299
|
|
$
|
313
|
|
$
|
321,994
|
|
$
|
354,439
|
|
$
|
(26,348
|
)
|
$
|
(77,327
|
)
|
$
|
573,071
|
|
||||||
|
Common stock issued
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
under employee plans
|
|
|
|
|
(4,377
|
)
|
|
|
|
|
13,287
|
|
8,910
|
|
||||||||||||
|
Repurchase of treasury
|
||||||||||||||||||||||||||
|
stock
|
|
|
|
|
|
|
(3,923
|
)
|
(3,923
|
)
|
||||||||||||||||
|
Tax benefit arising from
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
common stock issued
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
under employee plans
|
|
|
|
|
1,052
|
|
|
|
|
|
|
|
1,052
|
|
||||||||||||
|
Stock based compensation
|
|
|
|
|
5,653
|
|
|
|
|
|
|
|
5,653
|
|
||||||||||||
|
Dividend on common stock
|
(17,013
|
)
|
(17,013
|
)
|
||||||||||||||||||||||
|
|
Common Stock
|
Paid-in
Capital
|
Retained
Earnings
|
Accumulated
Other
Comprehensive
Loss
|
Treasury
Stock
|
Shareholders’
Equity
|
||||||||||||||||||||
|
Shares
|
Amount
|
|||||||||||||||||||||||||
|
Comprehensive income (loss):
|
||||||||||||||||||||||||||
|
Foreign currency
translation adjustments
|
1,995
|
|
||||||||||||||||||||||||
|
Pension liability (net of income tax expense of $512)
|
875
|
|
||||||||||||||||||||||||
|
Cash flow hedging loss (net of income tax benefit of $2,404)
|
(4,103
|
)
|
||||||||||||||||||||||||
|
Net income
|
40,481
|
|
||||||||||||||||||||||||
|
Total comprehensive
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
income (loss)
|
|
|
|
|
|
|
|
|
39,248
|
|
||||||||||||||||
|
Balance at December 31, 2012
|
31,299
|
|
$
|
313
|
|
$
|
324,322
|
|
$
|
377,907
|
|
$
|
(27,581
|
)
|
$
|
(67,963
|
)
|
$
|
606,998
|
|
||||||
|
Common stock issued
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
under employee plans
|
|
|
|
|
(4,576
|
)
|
|
|
|
|
19,772
|
|
15,196
|
|
||||||||||||
|
Repurchase of treasury
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
stock
|
|
|
|
|
|
|
|
|
|
|
(50,556
|
)
|
(50,556
|
)
|
||||||||||||
|
Tax benefit arising from
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
common stock issued
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
under employee plans
|
|
|
|
|
1,097
|
|
|
|
|
|
|
|
1,097
|
|
||||||||||||
|
Stock based compensation
|
|
|
|
|
5,593
|
|
|
|
|
|
|
|
5,593
|
|
||||||||||||
|
Dividends on common stock
|
(17,957
|
)
|
(17,957
|
)
|
||||||||||||||||||||||
|
Comprehensive income (loss):
|
|
|
||||||||||||||||||||||||
|
Foreign currency
translation adjustments
|
(1,193
|
)
|
||||||||||||||||||||||||
|
Pension liability (net of income tax expense of $6,718)
|
11,457
|
|
||||||||||||||||||||||||
|
Cash flow hedging loss (net of income tax benefit of $149)
|
(255
|
)
|
||||||||||||||||||||||||
|
Net income
|
35,939
|
|
||||||||||||||||||||||||
|
Total comprehensive
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
income (loss)
|
|
|
|
|
|
|
|
|
|
|
|
|
45,948
|
|
||||||||||||
|
Balance at December 31, 2013
|
31,299
|
|
$
|
313
|
|
$
|
326,436
|
|
$
|
395,889
|
|
$
|
(17,572
|
)
|
$
|
(98,747
|
)
|
$
|
606,319
|
|
||||||
|
2011
|
2012
|
2013
|
|||||||||
|
Cash flows from operating activities:
|
|
|
|
||||||||
|
Net income
|
$
|
752
|
|
$
|
40,481
|
|
$
|
35,939
|
|
||
|
Adjustments to reconcile net income
|
|
|
|
|
|
|
|||||
|
to net cash provided by operating activities:
|
|
|
|
|
|
|
|||||
|
Depreciation
|
18,519
|
|
18,635
|
|
18,653
|
|
|||||
|
Amortization of debt discount
|
3,903
|
|
—
|
|
—
|
|
|||||
|
Amortization, all other
|
20,265
|
|
27,981
|
|
29,214
|
|
|||||
|
Stock-based compensation
|
5,240
|
|
5,653
|
|
5,593
|
|
|||||
|
Deferred income taxes
|
(13,098
|
)
|
12,946
|
|
7,218
|
|
|||||
|
Income tax benefit of stock
|
|
|
|
|
|
|
|||||
|
option exercises
|
1,197
|
|
1,052
|
|
1,097
|
|
|||||
|
Excess tax benefit from stock
|
|
|
|
|
|
|
|||||
|
option exercises
|
(1,363
|
)
|
(1,206
|
)
|
(1,518
|
)
|
|||||
|
Loss on early extinguishment of debt
|
—
|
|
—
|
|
263
|
|
|||||
|
Impairment of goodwill
|
60,302
|
|
—
|
|
—
|
|
|||||
|
Increase (decrease) in cash flows from changes in assets and
|
|
|
|
|
|
|
|||||
|
liabilities, net of effects from acquisitions:
|
|
|
|
|
|
|
|||||
|
Accounts receivable
|
8,464
|
|
1,687
|
|
(798
|
)
|
|||||
|
Inventories
|
(7,850
|
)
|
3,810
|
|
(1,817
|
)
|
|||||
|
Accounts payable
|
2,649
|
|
259
|
|
4,223
|
|
|||||
|
Income taxes
|
4,838
|
|
(6,497
|
)
|
(1,098
|
)
|
|||||
|
Accrued compensation and benefits
|
1,673
|
|
767
|
|
(71
|
)
|
|||||
|
Other assets
|
(4,243
|
)
|
(1,210
|
)
|
(5,222
|
)
|
|||||
|
Other liabilities
|
1,745
|
|
(9,159
|
)
|
(10,727
|
)
|
|||||
|
|
102,241
|
|
54,718
|
|
45,010
|
|
|||||
|
Net cash provided by operating activities
|
102,993
|
|
95,199
|
|
80,949
|
|
|||||
|
Cash flows from investing activities:
|
|
|
|
|
|
|
|||||
|
Payments related to business acquisitions and distribution agreements,
|
|
|
|
|
|
|
|||||
|
net of cash acquired
|
(4,191
|
)
|
(86,253
|
)
|
—
|
|
|||||
|
Proceeds from sale of property
|
—
|
|
1,836
|
|
—
|
|
|||||
|
Purchases of property, plant and equipment
|
(17,552
|
)
|
(21,532
|
)
|
(18,445
|
)
|
|||||
|
Net cash used in investing activities
|
(21,743
|
)
|
(105,949
|
)
|
(18,445
|
)
|
|||||
|
Cash flows from financing activities:
|
|
|
|
|
|
|
|||||
|
Net proceeds from common stock issued
|
|
|
|
|
|
|
|||||
|
under employee plans
|
6,117
|
|
10,165
|
|
17,264
|
|
|||||
|
Repurchase of common stock
|
(15,021
|
)
|
(3,923
|
)
|
(50,556
|
)
|
|||||
|
Excess tax benefit from stock option exercises
|
1,363
|
|
1,206
|
|
1,518
|
|
|||||
|
Payments on senior credit agreement
|
(1,350
|
)
|
(53,588
|
)
|
—
|
|
|||||
|
Proceeds of senior credit agreement
|
58,000
|
|
73,000
|
|
55,000
|
|
|||||
|
Payments related to distribution agreement
|
—
|
|
—
|
|
(34,000
|
)
|
|||||
|
Payments on mortgage notes
|
(894
|
)
|
(969
|
)
|
(1,050
|
)
|
|||||
|
Payments on senior subordinated notes
|
(111,766
|
)
|
(100
|
)
|
(227
|
)
|
|||||
|
Payments related to issuance of debt
|
—
|
|
—
|
|
(1,725
|
)
|
|||||
|
Dividends paid on common stock
|
—
|
|
(12,862
|
)
|
(16,696
|
)
|
|||||
|
Other, net
|
(3,148
|
)
|
(1,576
|
)
|
(824
|
)
|
|||||
|
Net cash provided by (used in) financing activities
|
(66,699
|
)
|
11,353
|
|
(31,296
|
)
|
|||||
|
Effect of exchange rate changes
|
|
|
|
|
|
|
|||||
|
on cash and cash equivalents
|
(920
|
)
|
(2,931
|
)
|
(485
|
)
|
|||||
|
Net increase (decrease) in cash
|
|
|
|
|
|
|
|||||
|
and cash equivalents
|
13,631
|
|
(2,328
|
)
|
30,723
|
|
|||||
|
Cash and cash equivalents at beginning of year
|
12,417
|
|
26,048
|
|
23,720
|
|
|||||
|
Cash and cash equivalents at end of year
|
$
|
26,048
|
|
$
|
23,720
|
|
$
|
54,443
|
|
||
|
Non-cash financing activities:
|
|||||||||||
|
Dividends payable
|
$
|
—
|
|
$
|
4,256
|
|
$
|
5,545
|
|
||
|
Supplemental disclosures of cash flow information:
|
|
|
|
|
|
|
|||||
|
Cash paid during the year for:
|
|
|
|
|
|
|
|||||
|
Interest
|
$
|
5,797
|
|
$
|
5,038
|
|
$
|
5,143
|
|
||
|
Income taxes
|
4,760
|
|
10,953
|
|
6,837
|
|
|||||
|
|
Building and improvements
|
12 to 40 years
|
|
|
Leasehold improvements
|
Shorter of life of asset or life of lease
|
|
|
Machinery and equipment
|
2 to 15 years
|
|
•
|
Sales to customers are evidenced by firm purchase orders. Title and the risks and rewards of ownership are transferred to the customer when product is shipped under our stated shipping terms. Payment by the customer is due under fixed payment terms and collectability is reasonably assured.
|
|
•
|
We place certain of our capital equipment with customers on a loaned basis in return for commitments to purchase related single-use products over time periods generally ranging from one to three years. In these circumstances, no revenue is recognized upon capital equipment shipment as the equipment is loaned and subject to return if certain minimum single-use purchases are not met. Revenue is recognized upon the sale and shipment of the related single-use products. The cost of the equipment is amortized over its estimated useful life.
|
|
•
|
We recognize revenues related to the promotion and marketing of sports medicine allograft tissue in accordance with the contractual terms of our agreement with Musculoskeletal Transplant Foundation (“MTF”) on a net basis as our role is limited to that of an agent earning a commission or fee. MTF records revenue when the tissue is shipped to the customer. Our services are completed at this time and net revenues for the “Service Fee” for our promotional and marketing efforts are then recognized based on a percentage of the net amounts billed by MTF to its customers. The timing of revenue recognition is determined through review of the net billings made by MTF each month. Our net commission Service Fee is based on the contractual terms of our agreement and is currently
50%
. This percentage can vary over the term of the agreement but is contractually determinable. Our Service Fee revenues are recorded net of amortization of the acquired assets, which are being expensed over the expected useful life of
25
years.
|
|
•
|
Product returns are only accepted at the discretion of the Company and in accordance with our “Returned Goods Policy”. Historically the level of product returns has not been significant. We accrue for sales returns, rebates and allowances based upon an analysis of historical customer returns and credits, rebates, discounts and current market conditions.
|
|
•
|
Our terms of sale to customers generally do not include any obligations to perform future services. Limited warranties are provided for capital equipment sales and provisions for warranty are provided at the time of product sale based upon an analysis of historical data.
|
|
•
|
Amounts billed to customers related to shipping and handling have been included in net sales. Shipping and handling costs included in selling and administrative expense were
$13.0 million
,
$12.8 million
and
$12.6 million
for
2011
,
2012
and
2013
, respectively.
|
|
•
|
We sell to a diversified base of customers around the world and, therefore, believe there is no material concentration of credit risk.
|
|
•
|
We assess the risk of loss on accounts receivable and adjust the allowance for doubtful accounts based on this risk assessment. Historically, losses on accounts receivable have not been material. Management believes that the allowance for doubtful accounts of
$1.4 million
at
December 31, 2013
is adequate to provide for probable losses resulting from accounts receivable.
|
|
|
2011
|
2012
|
2013
|
||||||||
|
Net income
|
$
|
752
|
|
$
|
40,481
|
|
$
|
35,939
|
|
||
|
Basic-weighted average shares outstanding
|
28,246
|
|
28,301
|
|
27,722
|
|
|||||
|
Effect of dilutive potential securities
|
387
|
|
352
|
|
392
|
|
|||||
|
Diluted-weighted average shares outstanding
|
28,633
|
|
28,653
|
|
28,114
|
|
|||||
|
Basic EPS
|
$
|
0.03
|
|
$
|
1.43
|
|
$
|
1.30
|
|
||
|
Diluted EPS
|
$
|
0.03
|
|
$
|
1.41
|
|
$
|
1.28
|
|
||
|
Cash Flow
Hedging
Gain (Loss)
a
|
Pension
Liability
a
|
Cumulative
Translation
Adjustments
|
Accumulated
Other
Comprehensive
Loss
|
||||||||||||
|
Balance, December 31, 2012
|
$
|
(1,130
|
)
|
$
|
(30,375
|
)
|
$
|
3,924
|
|
$
|
(27,581
|
)
|
|||
|
Other comprehensive income
|
|||||||||||||||
|
before reclassifications
|
(158
|
)
|
8,618
|
|
(1,193
|
)
|
7,267
|
|
|||||||
|
Amounts reclassified from accumulated other comprehensive income before tax
b
|
(153
|
)
|
4,502
|
|
—
|
|
4,349
|
|
|||||||
|
Tax expense (benefit)
|
56
|
|
(1,663
|
)
|
—
|
|
(1,607
|
)
|
|||||||
|
Total amounts reclassified from other accumulated comprehensive income
|
(97
|
)
|
2,839
|
|
—
|
|
2,742
|
|
|||||||
|
Net current-period other comprehensive income
|
(255
|
)
|
11,457
|
|
(1,193
|
)
|
10,009
|
|
|||||||
|
Balance, December 31, 2013
|
$
|
(1,385
|
)
|
$
|
(18,918
|
)
|
$
|
2,731
|
|
$
|
(17,572
|
)
|
|||
|
|
2012
|
2013
|
|||||
|
Raw materials
|
$
|
45,115
|
|
$
|
39,029
|
|
|
|
Work in process
|
14,229
|
|
14,736
|
|
|||
|
Finished goods
|
96,884
|
|
89,446
|
|
|||
|
|
$
|
156,228
|
|
$
|
143,211
|
|
|
|
|
2012
|
2013
|
|||||
|
Land
|
$
|
4,243
|
|
$
|
4,243
|
|
|
|
Building and improvements
|
92,775
|
|
95,397
|
|
|||
|
Machinery and equipment
|
176,102
|
|
180,064
|
|
|||
|
Construction in progress
|
5,508
|
|
8,750
|
|
|||
|
|
278,628
|
|
288,454
|
|
|||
|
Less: Accumulated depreciation
|
(139,587
|
)
|
(149,469
|
)
|
|||
|
|
$
|
139,041
|
|
$
|
138,985
|
|
|
|
2014
|
$
|
6,723
|
|
|
2015
|
5,782
|
|
|
|
2016
|
4,144
|
|
|
|
2017
|
3,492
|
|
|
|
2018
|
3,382
|
|
|
|
Thereafter
|
5,006
|
|
|
|
|
2012
|
2013
|
|||||
|
Balance as of January 1,
|
$
|
234,815
|
|
$
|
248,502
|
|
|
|
Goodwill resulting from business acquisitions
|
13,702
|
|
—
|
|
|||
|
Foreign currency translation
|
(15
|
)
|
(74
|
)
|
|||
|
Balance as of December 31,
|
$
|
248,502
|
|
$
|
248,428
|
|
|
|
|
December 31, 2012
|
December 31, 2013
|
|||||||||||||
|
Gross
Carrying
Amount
|
Accumulated
Amortization
|
Gross
Carrying
Amount
|
Accumulated
Amortization
|
||||||||||||
|
Amortized intangible assets:
|
|
|
|
|
|||||||||||
|
Customer relationships
|
$
|
135,690
|
|
$
|
(50,083
|
)
|
$
|
135,690
|
|
$
|
(54,982
|
)
|
|||
|
|
|
|
|
|
|
|
|
||||||||
|
Promotional, marketing & distribution rights
|
149,376
|
|
(6,000
|
)
|
149,376
|
|
(12,000
|
)
|
|||||||
|
Patents and other intangible assets
|
56,212
|
|
(37,554
|
)
|
53,903
|
|
(39,091
|
)
|
|||||||
|
|
|
|
|
|
|
|
|
||||||||
|
Unamortized intangible assets
:
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
||||||||
|
Trademarks and tradenames
|
86,544
|
|
—
|
|
86,544
|
|
—
|
|
|||||||
|
|
$
|
427,822
|
|
$
|
(93,637
|
)
|
$
|
425,513
|
|
$
|
(106,073
|
)
|
|||
|
Amortization included in expense
|
Amortization recorded as a reduction of revenue
|
Total
|
|||||||||
|
2013
|
$
|
7,721
|
|
$
|
6,000
|
|
$
|
13,721
|
|
||
|
2014
|
7,031
|
|
6,000
|
|
$
|
13,031
|
|
||||
|
2015
|
6,642
|
|
6,000
|
|
$
|
12,642
|
|
||||
|
2016
|
6,539
|
|
6,000
|
|
$
|
12,539
|
|
||||
|
2017
|
6,527
|
|
6,000
|
|
$
|
12,527
|
|
||||
|
2018
|
6,470
|
|
6,000
|
|
$
|
12,470
|
|
||||
|
|
2012
|
2013
|
|||||
|
Revolving line of credit
|
$
|
153,000
|
|
$
|
208,000
|
|
|
|
|
|
||||||
|
2.50% convertible senior subordinated notes
|
227
|
|
—
|
|
|||
|
|
|
|
|
||||
|
Mortgage notes
|
8,625
|
|
7,575
|
|
|||
|
Total long-term debt
|
161,852
|
|
215,575
|
|
|||
|
Less: Current portion
|
1,050
|
|
1,140
|
|
|||
|
|
$
|
160,802
|
|
$
|
214,435
|
|
|
|
2014
|
$
|
1,140
|
|
|
2015
|
1,234
|
|
|
|
2016
|
1,339
|
|
|
|
2017
|
1,452
|
|
|
|
2018
|
209,574
|
|
|
|
Thereafter
|
836
|
|
|
|
|
2011
|
2012
|
2013
|
||||||||
|
Current tax expense (benefit):
|
|
|
|
||||||||
|
Federal
|
$
|
3,021
|
|
$
|
503
|
|
$
|
(2,274
|
)
|
||
|
State
|
1,596
|
|
374
|
|
502
|
|
|||||
|
Foreign
|
5,424
|
|
5,176
|
|
9,247
|
|
|||||
|
|
10,041
|
|
6,053
|
|
7,475
|
|
|||||
|
Deferred income tax expense (benefit)
|
(13,098
|
)
|
12,946
|
|
7,218
|
|
|||||
|
Provision (benefit) for income taxes
|
$
|
(3,057
|
)
|
$
|
18,999
|
|
$
|
14,693
|
|
||
|
|
2011
|
2012
|
2013
|
|||||
|
Tax provision (benefit) at statutory rate based
|
|
|
|
|
|
|
||
|
on income before income taxes
|
(35.0
|
)%
|
35.0
|
%
|
35.0
|
%
|
||
|
|
|
|
|
|
|
|||
|
State income taxes, net of federal tax benefit
|
22.7
|
|
1.5
|
|
1.8
|
|
||
|
|
|
|
|
|
|
|||
|
Stock-based compensation
|
(1.6
|
)
|
(0.2
|
)
|
(0.5
|
)
|
||
|
|
|
|
|
|
|
|||
|
Foreign income taxes
|
1.4
|
|
(4.0
|
)
|
(3.1
|
)
|
||
|
Impact of repatriation of foreign earnings
|
(57.5
|
)
|
—
|
|
—
|
|
||
|
|
|
|
|
|
|
|||
|
Research & development credit
|
(32.3
|
)
|
—
|
|
(2.8
|
)
|
||
|
|
|
|
|
|
|
|||
|
Settlement of taxing authority examinations
|
(6.5
|
)
|
(0.8
|
)
|
(2.0
|
)
|
||
|
European permanent deduction
|
—
|
|
—
|
|
(2.4
|
)
|
||
|
|
|
|
|
|
|
|||
|
Non deductible/non-taxable items
|
(13.3
|
)
|
1.3
|
|
2.9
|
|
||
|
|
|
|
|
|
|
|||
|
Other, net
|
(10.5
|
)
|
(0.9
|
)
|
0.1
|
|
||
|
|
(132.6
|
)%
|
31.9
|
%
|
29.0
|
%
|
||
|
|
2012
|
2013
|
|||||
|
Assets:
|
|
|
|||||
|
Inventory
|
$
|
4,370
|
|
$
|
3,445
|
|
|
|
Net operating losses
|
7,716
|
|
6,450
|
|
|||
|
Capitalized research and development
|
2,730
|
|
2,286
|
|
|||
|
Deferred compensation
|
2,905
|
|
3,025
|
|
|||
|
Accounts receivable
|
2,759
|
|
2,642
|
|
|||
|
Employee benefits
|
5,725
|
|
5,601
|
|
|||
|
Accrued pension
|
8,081
|
|
(173
|
)
|
|||
|
Research and development credit
|
3,378
|
|
5,027
|
|
|||
|
Other
|
4,335
|
|
4,365
|
|
|||
|
Foreign tax credit
|
—
|
|
332
|
|
|||
|
|
41,999
|
|
33,000
|
|
|||
|
Liabilities:
|
|
|
|
|
|||
|
Goodwill and intangible assets
|
111,770
|
|
114,075
|
|
|||
|
Depreciation
|
13,070
|
|
13,486
|
|
|||
|
State taxes
|
2,992
|
|
3,914
|
|
|||
|
Contingent interest
|
378
|
|
339
|
|
|||
|
|
128,210
|
|
131,814
|
|
|||
|
Net liability
|
$
|
(86,211
|
)
|
$
|
(98,814
|
)
|
|
|
|
2011
|
2012
|
2013
|
||||||||
|
U.S. income (loss)
|
$
|
(20,521
|
)
|
$
|
33,121
|
|
$
|
20,106
|
|
||
|
Foreign income
|
18,216
|
|
26,359
|
|
30,526
|
|
|||||
|
Total income (loss)
|
$
|
(2,305
|
)
|
$
|
59,480
|
|
$
|
50,632
|
|
||
|
|
2011
|
2012
|
2013
|
||||||||
|
Balance as of January 1,
|
$
|
1,330
|
|
$
|
2,343
|
|
$
|
1,587
|
|
||
|
Increases for positions taken in prior periods
|
283
|
|
30
|
|
70
|
|
|||||
|
Increases for positions taken in current periods
|
789
|
|
1,129
|
|
1,132
|
|
|||||
|
Decreases in unrecorded tax positions related to settlement with the taxing authorities
|
—
|
|
(1,857
|
)
|
(1,010
|
)
|
|||||
|
Decreases in unrecorded tax positions related to lapse of statute of limitations
|
(59
|
)
|
(58
|
)
|
(90
|
)
|
|||||
|
Balance as of December 31,
|
$
|
2,343
|
|
$
|
1,587
|
|
$
|
1,689
|
|
||
|
2011
|
2012
|
2013
|
|||||||||
|
Fair value of options & SARs
|
$
|
10.43
|
|
$
|
7.38
|
|
$
|
9.77
|
|
||
|
Expected stock price volatility
|
35.52
|
%
|
35.84
|
%
|
35.88
|
%
|
|||||
|
Risk-free interest rate
|
1.59
|
%
|
0.62
|
%
|
1.04
|
%
|
|||||
|
Expected annual dividend yield
|
—
|
%
|
2.00
|
%
|
1.79
|
%
|
|||||
|
Expected life of options & SARs (years)
|
6.3
|
|
6.4
|
|
6.3
|
|
|||||
|
Number
of
Shares
(in 000’s)
|
Weighted-
Average
Exercise
Price
|
|||||
|
Outstanding at December 31, 2012
|
1,769
|
|
$
|
25.35
|
|
|
|
Granted
|
164
|
|
$
|
32.94
|
|
|
|
Forfeited
|
(9
|
)
|
$
|
25.96
|
|
|
|
Exercised
|
(794
|
)
|
$
|
26.62
|
|
|
|
Outstanding at December 31, 2013
|
1,130
|
|
$
|
25.55
|
|
|
|
Exercisable at December 31, 2013
|
659
|
|
$
|
24.40
|
|
|
|
Number
of
Shares
(in 000’s)
|
Weighted-
Average
Grant-Date
Fair Value
|
|||||
|
Outstanding at December 31, 2012
|
521
|
|
$
|
24.25
|
|
|
|
Granted
|
211
|
|
$
|
33.02
|
|
|
|
Vested
|
(168
|
)
|
$
|
23.76
|
|
|
|
Forfeited
|
(88
|
)
|
$
|
30.55
|
|
|
|
Outstanding at December 31, 2013
|
476
|
|
$
|
27.14
|
|
|
|
|
2011
|
2012
|
2013
|
||||||||
|
Orthopedic surgery
|
$
|
371,245
|
|
$
|
413,891
|
|
$
|
410,171
|
|
||
|
General surgery
|
287,350
|
|
286,606
|
|
286,747
|
|
|||||
|
Surgical visualization
|
66,482
|
|
66,643
|
|
65,786
|
|
|||||
|
Consolidated net sales
|
$
|
725,077
|
|
$
|
767,140
|
|
$
|
762,704
|
|
||
|
|
2011
|
2012
|
2013
|
||||||||
|
United States
|
$
|
364,588
|
|
$
|
382,256
|
|
$
|
375,473
|
|
||
|
Canada
|
65,794
|
|
73,746
|
|
73,457
|
|
|||||
|
United Kingdom
|
32,106
|
|
31,653
|
|
28,471
|
|
|||||
|
Japan
|
34,178
|
|
33,997
|
|
36,705
|
|
|||||
|
Australia
|
40,122
|
|
40,835
|
|
38,752
|
|
|||||
|
All other countries
|
188,289
|
|
204,653
|
|
209,846
|
|
|||||
|
Total
|
$
|
725,077
|
|
$
|
767,140
|
|
$
|
762,704
|
|
||
|
|
2012
|
2013
|
|||||
|
Accumulated Benefit Obligation
|
$
|
85,363
|
|
$
|
75,946
|
|
|
|
Change in benefit obligation
|
|
|
|
|
|||
|
Projected benefit obligation at beginning of year
|
$
|
82,289
|
|
$
|
85,363
|
|
|
|
Service cost
|
277
|
|
253
|
|
|||
|
Interest cost
|
3,429
|
|
3,315
|
|
|||
|
Actuarial (gain) loss
|
2,790
|
|
(8,082
|
)
|
|||
|
Benefits paid
|
(1,059
|
)
|
(1,250
|
)
|
|||
|
Settlement
|
(2,363
|
)
|
(3,653
|
)
|
|||
|
Projected benefit obligation at end of year
|
$
|
85,363
|
|
$
|
75,946
|
|
|
|
Change in plan assets
|
|
|
|
|
|||
|
Fair value of plan assets at beginning of year
|
$
|
51,822
|
|
$
|
62,763
|
|
|
|
Actual gain on plan assets
|
5,866
|
|
11,082
|
|
|||
|
Employer contributions
|
8,497
|
|
7,500
|
|
|||
|
Benefits paid
|
(1,059
|
)
|
(1,250
|
)
|
|||
|
Settlement
|
(2,363
|
)
|
(3,653
|
)
|
|||
|
Fair value of plan assets at end of year
|
$
|
62,763
|
|
$
|
76,442
|
|
|
|
Funded status
|
$
|
(22,600
|
)
|
$
|
496
|
|
|
|
|
2012
|
2013
|
|||||
|
Other assets/(Other long-term liabilities)
|
$
|
(22,600
|
)
|
$
|
496
|
|
|
|
Accumulated other comprehensive loss
|
(48,176
|
)
|
(30,001
|
)
|
|||
|
|
2012
|
2013
|
|||
|
Discount rate
|
3.90
|
%
|
4.75
|
%
|
|
|
Expected return on plan assets
|
8.00
|
%
|
8.00
|
%
|
|
|
Current year actuarial gain
|
$
|
13,673
|
|
|
Settlement loss/amortization of actuarial loss
|
4,502
|
|
|
|
Total recognized in other comprehensive loss
|
$
|
18,175
|
|
|
|
2011
|
2012
|
2013
|
|||||||||
|
Service cost
|
$
|
281
|
|
$
|
277
|
|
$
|
253
|
|
|||
|
Interest cost on projected benefit obligation
|
3,519
|
|
3,429
|
|
3,315
|
|
||||||
|
Return on plan assets
|
(4,378
|
)
|
(4,566
|
)
|
(5,491
|
)
|
||||||
|
Amortization of loss
|
1,578
|
|
2,876
|
|
3,059
|
|
||||||
|
Settlement expense
|
—
|
|
—
|
|
1,443
|
|
||||||
|
Net periodic pension cost
|
$
|
1,000
|
|
$
|
2,016
|
|
$
|
2,579
|
|
|||
|
|
2011
|
2012
|
2013
|
|||||
|
Discount rate
|
5.41
|
%
|
4.30
|
%
|
3.90
|
%
|
||
|
Expected return on plan assets
|
8.00
|
%
|
8.00
|
%
|
8.00
|
%
|
||
|
Percentage of Pension
Plan Assets
|
Target
Allocation
|
|||||||
|
|
2012
|
2013
|
2013
|
|||||
|
Equity securities
|
76
|
%
|
79
|
%
|
75
|
%
|
||
|
Debt securities
|
24
|
|
21
|
|
25
|
|
||
|
Total
|
100
|
%
|
100
|
%
|
100
|
%
|
||
|
|
2012
|
2013
|
|||||
|
Common Stock
|
$
|
25,124
|
|
$
|
31,412
|
|
|
|
Money Market Fund
|
5,209
|
|
7,018
|
|
|||
|
Mutual Funds
|
22,810
|
|
28,726
|
|
|||
|
Fixed Income Securities
|
9,620
|
|
9,286
|
|
|||
|
Total Assets at Fair Value
|
$
|
62,763
|
|
$
|
76,442
|
|
|
|
Common Stock:
|
Common stock is valued at the closing price reported on the common stock’s respective stock exchange and is classified within level 1 of the valuation hierarchy.
|
|
|
|
|
Money Market Fund:
|
These investments are public investment vehicles valued using $1 for the Net Asset Value (NAV). The money market fund is classified within level 2 of the valuation hierarchy.
|
|
|
|
|
Mutual Funds:
|
These investments are public investment vehicles valued using the NAV provided by the administrator of the fund. The NAV is based on the value of the underlying assets owned by the fund, minus its liabilities, and then divided by the number of shares outstanding. The NAV is a quoted price in an active market and is classified within level 1 of the valuation hierarchy.
|
|
|
|
|
Fixed Income Securities:
|
Valued at the closing price reported on the active market on which the individual securities are traded and are classified within level 1 of the valuation hierarchy.
|
|
December 31, 2012
|
Level 1
|
Level 2
|
Total
|
||||||||
|
Common Stock
|
$
|
25,124
|
|
$
|
—
|
|
$
|
25,124
|
|
||
|
Money Market Fund
|
—
|
|
5,209
|
|
5,209
|
|
|||||
|
Mutual Funds
|
22,810
|
|
—
|
|
22,810
|
|
|||||
|
Fixed Income Securities
|
9,620
|
|
—
|
|
9,620
|
|
|||||
|
$
|
57,554
|
|
$
|
5,209
|
|
$
|
62,763
|
|
|||
|
December 31, 2013
|
Level 1
|
Level 2
|
Total
|
||||||||
|
Common Stock
|
$
|
31,412
|
|
$
|
—
|
|
$
|
31,412
|
|
||
|
Money Market Fund
|
—
|
|
7,018
|
|
7,018
|
|
|||||
|
Mutual Funds
|
28,726
|
|
—
|
|
28,726
|
|
|||||
|
Fixed Income Securities
|
9,286
|
|
—
|
|
9,286
|
|
|||||
|
$
|
69,424
|
|
$
|
7,018
|
|
$
|
76,442
|
|
|||
|
2014
|
|
$5,332
|
|
|
2015
|
3,140
|
|
|
|
2016
|
3,100
|
|
|
|
2017
|
3,351
|
|
|
|
2018
|
3,973
|
|
|
|
2019-2023
|
23,943
|
|
|
|
|
2011
|
2012
|
2013
|
||||||||
|
Administrative consolidation costs
|
$
|
792
|
|
$
|
6,497
|
|
$
|
8,750
|
|
||
|
Costs associated with purchase of a distributor
|
300
|
|
704
|
|
—
|
|
|||||
|
Costs associated with legal arbitration and patent dispute
|
—
|
|
1,555
|
|
3,206
|
|
|||||
|
Pension settlement expense
|
—
|
|
—
|
|
1,443
|
|
|||||
|
Costs associated with purchase of a business
|
—
|
|
1,194
|
|
—
|
|
|||||
|
Other expense
|
$
|
1,092
|
|
$
|
9,950
|
|
$
|
13,399
|
|
||
|
|
2011
|
2012
|
2013
|
||||||||
|
Balance as of January 1,
|
$
|
3,363
|
|
$
|
3,618
|
|
$
|
3,636
|
|
||
|
Provision for warranties
|
4,344
|
|
4,163
|
|
3,061
|
|
|||||
|
Claims made
|
(4,089
|
)
|
(4,145
|
)
|
(4,275
|
)
|
|||||
|
Balance as of December 31,
|
$
|
3,618
|
|
$
|
3,636
|
|
$
|
2,422
|
|
||
|
December 31, 2012
|
Asset
Balance Sheet
Location
|
Fair
Value
|
Liabilities
Balance Sheet
Location
|
Fair
Value
|
Net
Fair
Value
|
||||||||||
|
Derivatives designated as hedged instruments:
|
|
|
|
|
|||||||||||
|
Foreign exchange contracts
|
Other current liabilities
|
$
|
(457
|
)
|
Other current liabilities
|
$
|
2,249
|
|
$
|
1,792
|
|
||||
|
Derivatives not designated as hedging instruments:
|
|
|
|
|
|||||||||||
|
Foreign exchange contracts
|
Other current liabilities
|
—
|
|
Other current liabilities
|
150
|
|
150
|
|
|||||||
|
Total derivatives
|
|
$
|
(457
|
)
|
|
$
|
2,399
|
|
$
|
1,942
|
|
||||
|
December 31, 2013
|
Asset
Balance Sheet Location |
Fair
Value |
Liabilities
Balance Sheet Location |
Fair
Value |
Net
Fair Value |
||||||||||
|
Derivatives designated as hedged instruments:
|
|
|
|
|
|
||||||||||
|
Foreign exchange contracts
|
Other current liabilities
|
$
|
(975
|
)
|
Other current liabilities
|
$
|
3,172
|
|
$
|
2,197
|
|
||||
|
Derivatives not designated as hedging instruments:
|
|
|
|
|
|
|
|
|
|||||||
|
Foreign exchange contracts
|
Other current liabilities
|
(52
|
)
|
Other current liabilities
|
78
|
|
26
|
|
|||||||
|
Total derivatives
|
|
$
|
(1,027
|
)
|
|
$
|
3,250
|
|
$
|
2,223
|
|
||||
|
|
2011
|
2012
|
2013
|
||||||||
|
Facility consolidation costs
|
$
|
3,467
|
|
$
|
7,052
|
|
$
|
6,489
|
|
||
|
Termination of a product offering
|
—
|
|
—
|
|
2,137
|
|
|||||
|
Restructuring costs included in cost of sales
|
$
|
3,467
|
|
$
|
7,052
|
|
$
|
8,626
|
|
||
|
|
|||||||||||
|
Restructuring costs included in other expense
|
$
|
792
|
|
$
|
6,497
|
|
$
|
8,750
|
|
||
|
Balance as of January 1, 2013
|
$
|
4,120
|
|
||
|
Expenses incurred
|
3,895
|
|
|||
|
Payments made
|
(4,887
|
)
|
|||
|
Balance, December 31, 2013
|
$
|
3,128
|
|
||
|
Cash
|
$
|
390
|
|
|
|
Accounts receivable
|
1,349
|
|
||
|
Inventory
|
2,562
|
|
||
|
Prepaid expenses and other current assets
|
151
|
|
||
|
Deferred income taxes
|
7,492
|
|
||
|
Property, plant & equipment, net
|
117
|
|
||
|
Customer relationships
|
1,725
|
|
||
|
Patents
|
1,100
|
|
||
|
Goodwill
|
13,702
|
|
||
|
Total assets acquired
|
28,588
|
|
||
|
Accounts payable
|
1,324
|
|
||
|
Other liabilities
|
4,736
|
|
||
|
Total liabilities assumed
|
6,060
|
|
||
|
Net assets acquired
|
$
|
22,528
|
|
|
|
2011
|
2012
|
||||||
|
Net sales
|
$
|
735,857
|
|
$
|
774,239
|
|
|
|
Net income
|
(2,176
|
)
|
38,018
|
|
|||
|
Earnings per share:
|
|||||||
|
Basic
|
$
|
(0.08
|
)
|
$
|
1.34
|
|
|
|
Diluted
|
(0.08
|
)
|
1.33
|
|
|||
|
|
Three Months Ended
|
||||||||||||||
|
|
March
|
June
|
September
|
December
|
|||||||||||
|
2012
|
|
|
|
|
|||||||||||
|
Net sales
|
$
|
194,316
|
|
$
|
189,695
|
|
$
|
181,885
|
|
$
|
201,244
|
|
|||
|
Gross profit
|
100,911
|
|
99,732
|
|
97,913
|
|
107,287
|
|
|||||||
|
Net income
|
9,968
|
|
10,296
|
|
9,320
|
|
10,897
|
|
|||||||
|
EPS:
|
|
|
|
|
|
|
|
|
|||||||
|
Basic
|
.36
|
|
.36
|
|
.33
|
|
.38
|
|
|||||||
|
Diluted
|
.35
|
|
.36
|
|
.32
|
|
.38
|
|
|||||||
|
|
Three Months Ended
|
||||||||||||||
|
|
March
|
June
|
September
|
December
|
|||||||||||
|
2013
|
|
|
|
|
|||||||||||
|
Net sales
|
$
|
187,014
|
|
$
|
192,993
|
|
$
|
179,255
|
|
$
|
203,442
|
|
|||
|
Gross profit
|
102,682
|
|
102,916
|
|
95,424
|
|
111,395
|
|
|||||||
|
Net income
|
10,492
|
|
9,533
|
|
5,687
|
|
10,227
|
|
|||||||
|
EPS:
|
|
|
|
|
|
|
|
|
|||||||
|
Basic
|
.37
|
|
.35
|
|
.21
|
|
.37
|
|
|||||||
|
Diluted
|
.37
|
|
.34
|
|
.20
|
|
.36
|
|
|||||||
|
|
|
Column C
|
|
|
||||||||||||||||
|
|
|
Additions
|
|
|
||||||||||||||||
|
|
Column B
|
|
|
|
|
|||||||||||||||
|
|
Balance at
Beginning of
Period
|
Charged to
Costs and
Expenses
|
Charged to
Other
Accounts
|
|
Column E
|
|||||||||||||||
|
Column A
|
Column D
|
Balance at End
of Period
|
||||||||||||||||||
|
Description
|
Deductions
|
|||||||||||||||||||
|
2013
|
|
|
|
|
|
|||||||||||||||
|
Allowance for bad debts
|
$
|
1,203
|
|
$
|
421
|
|
$
|
—
|
|
$
|
(240
|
)
|
$
|
1,384
|
|
|||||
|
Sales returns and
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
allowance
|
3,609
|
|
398
|
|
—
|
|
(909
|
)
|
3,098
|
|
||||||||||
|
Deferred tax asset
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
valuation allowance
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
2012
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
Allowance for bad debts
|
$
|
1,183
|
|
$
|
530
|
|
$
|
—
|
|
$
|
(510
|
)
|
$
|
1,203
|
|
|||||
|
Sales returns and
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
allowance
|
4,097
|
|
317
|
|
—
|
|
(805
|
)
|
3,609
|
|
||||||||||
|
Deferred tax asset
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
valuation allowance
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
2011
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
Allowance for bad debts
|
$
|
1,066
|
|
$
|
3,935
|
|
$
|
—
|
|
$
|
(3,818
|
)
|
$
|
1,183
|
|
|||||
|
Sales returns and
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
allowance
|
3,980
|
|
291
|
|
—
|
|
(174
|
)
|
4,097
|
|
||||||||||
|
Deferred tax asset
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
valuation allowance
|
226
|
|
—
|
|
—
|
|
(226
|
)
|
—
|
|
||||||||||