|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ý
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
30-0645032
|
|
(State of other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification Number)
|
Charlottesville, VA 22902
(Address of principal executive offices, including zip code)
|
Large accelerated filer
o
|
Accelerated filer
o
|
|
Non-accelerated filer
o
(Do not check if a smaller reporting company)
|
Smaller reporting company
ý
|
|
Emerging growth company
o
|
|
|
Page
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ITEM 1.
|
FINANCIAL STATEMENTS
|
|
March 31, 2017
|
December 31, 2016
|
||||||
|
Assets
|
|
|
|||||
|
Current assets:
|
|
|
|||||
|
Cash and cash equivalents
|
$
|
12,212,025
|
|
$
|
1,552,852
|
|
|
|
Subscription receivable
|
8,280,935
|
|
—
|
|
|||
|
Prepaid expenses, deposits and other current assets
|
138,986
|
|
50,844
|
|
|||
|
Total current assets
|
20,631,946
|
|
1,603,696
|
|
|||
|
Property and equipment, net
|
79,524
|
|
79,755
|
|
|||
|
Intangible asset
|
8,639,000
|
|
8,639,000
|
|
|||
|
Goodwill
|
6,929,258
|
|
6,929,258
|
|
|||
|
Other assets
|
63,815
|
|
232,675
|
|
|||
|
Total assets
|
$
|
36,343,543
|
|
$
|
17,484,384
|
|
|
|
Liabilities, Convertible Preferred Stock and Stockholders’ Equity (Deficit)
|
|
|
|||||
|
Current liabilities:
|
|
|
|||||
|
Current portion of convertible debt
|
$
|
1,880,000
|
|
$
|
1,880,000
|
|
|
|
Accounts payable
|
557,629
|
|
1,684,158
|
|
|||
|
Accrued expenses and other current liabilities
|
1,016,263
|
|
874,264
|
|
|||
|
Common stock warrant liability
|
48,145,520
|
|
—
|
|
|||
|
Total current liabilities
|
51,599,412
|
|
4,438,422
|
|
|||
|
Convertible debt, net of current portion
|
550,000
|
|
550,000
|
|
|||
|
Deferred income taxes
|
3,279,363
|
|
3,279,363
|
|
|||
|
Other liabilities
|
33,307
|
|
31,915
|
|
|||
|
Total liabilities
|
55,462,082
|
|
8,299,700
|
|
|||
|
Commitments and Contingencies
|
|
|
|||||
|
Convertible preferred stock, $0.001 par value:
|
|
|
|
|
|||
|
Series A - 13,750,000 shares authorized, 12,376,329 issued and outstanding at March 31, 2017; No shares authorized, issued or outstanding at December 31, 2016 (liquidation value of $25,000,000 at March 31, 2017)
|
—
|
|
—
|
|
|||
|
Total convertible preferred stock
|
—
|
|
—
|
|
|||
|
Stockholders’ Equity (Deficit):
|
|
|
|||||
|
Common stock, $0.001 par value:
|
|
|
|||||
|
1,000,000,000 shares authorized; 10,345,637 shares issued and outstanding
|
10,346
|
|
10,346
|
|
|||
|
Additional paid-in capital
|
69,700,264
|
|
69,363,575
|
|
|||
|
Accumulated deficit
|
(88,829,149
|
)
|
(60,189,237
|
)
|
|||
|
Total stockholders' equity (deficit)
|
(19,118,539
|
)
|
9,184,684
|
|
|||
|
Total liabilities, convertible preferred stock and stockholders' equity (deficit)
|
$
|
36,343,543
|
|
$
|
17,484,384
|
|
|
|
|
Three Months Ended March 31,
|
||||||
|
|
2017
|
2016
|
|||||
|
Operating expenses:
|
|
|
|||||
|
Research and development
|
$
|
1,007,571
|
|
$
|
2,352,807
|
|
|
|
General and administrative
|
1,553,139
|
|
3,862,484
|
|
|||
|
Depreciation
|
6,603
|
|
7,853
|
|
|||
|
Loss from operations
|
2,567,313
|
|
6,223,144
|
|
|||
|
Other expense:
|
|||||||
|
Interest expense, net
|
55,719
|
|
21
|
|
|||
|
Change in fair value of warrant liabilities (Note 10)
|
12,919,674
|
|
—
|
|
|||
|
Warrant related expenses (Note 7)
|
10,225,846
|
|
—
|
|
|||
|
Other financing expenses
|
2,870,226
|
|
—
|
|
|||
|
Net loss
|
$
|
(28,638,778
|
)
|
$
|
(6,223,165
|
)
|
|
|
Series A cumulative preferred dividends
|
(58,845
|
)
|
—
|
|
|||
|
Net loss attributable to common stockholders
|
$
|
(28,697,623
|
)
|
$
|
(6,223,165
|
)
|
|
|
Per share information:
|
|
|
|||||
|
Net loss per share of common stock, basic and diluted
|
$
|
(2.78
|
)
|
$
|
(0.62
|
)
|
|
|
Weighted average shares outstanding, basic and diluted
|
10,337,726
|
|
9,996,381
|
|
|||
|
Convertible Preferred Stock
|
Stockholders' Equity (Deficit)
|
|||||||||||||||||||||||||
|
|
Series A
|
Common Stock
|
Additional
Paid-in
Capital
|
Accumulated
Deficit
|
Total
Stockholders'
Equity (Deficit)
|
|||||||||||||||||||||
|
|
Shares
|
Amount
|
Shares
|
Amount
|
||||||||||||||||||||||
|
Balance at January 1, 2017
|
—
|
|
$
|
—
|
|
10,345,637
|
|
$
|
10,346
|
|
$
|
69,363,575
|
|
$
|
(60,189,237
|
)
|
$
|
9,184,684
|
|
|||||||
|
Cumulative effect of change in accounting principle
(a)
|
—
|
|
—
|
|
—
|
|
—
|
|
1,134
|
|
(1,134
|
)
|
—
|
|
||||||||||||
|
Sale of Series A convertible preferred stock and common stock warrants
|
12,376,329
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||
|
Series A cumulative preferred dividend
|
—
|
|
—
|
|
—
|
|
—
|
|
(58,845
|
)
|
—
|
|
(58,845
|
)
|
||||||||||||
|
Beneficial conversion feature for accrued interest of convertible debt
|
—
|
|
—
|
|
—
|
|
—
|
|
28,017
|
|
—
|
|
28,017
|
|
||||||||||||
|
Stock-based compensation expense
|
—
|
|
—
|
|
—
|
|
—
|
|
366,383
|
|
—
|
|
366,383
|
|
||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(28,638,778
|
)
|
(28,638,778
|
)
|
||||||||||||
|
Balance at March 31, 2017
|
12,376,329
|
|
$
|
—
|
|
10,345,637
|
|
$
|
10,346
|
|
$
|
69,700,264
|
|
$
|
(88,829,149
|
)
|
$
|
(19,118,539
|
)
|
|||||||
|
|
Three Months Ended March 31,
|
||||||
|
|
2017
|
2016
|
|||||
|
Operating activities:
|
|
|
|||||
|
Net loss
|
$
|
(28,638,778
|
)
|
$
|
(6,223,165
|
)
|
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|||||
|
Depreciation
|
6,603
|
|
7,853
|
|
|||
|
Stock-based compensation expense
|
366,383
|
|
393,471
|
|
|||
|
Common stock issued for advisory services
|
—
|
|
487,500
|
|
|||
|
Warrant related expense, change in fair value, and other financing expenses
|
26,015,746
|
|
—
|
|
|||
|
Non-cash interest expense
|
57,185
|
|
3,383
|
|
|||
|
Changes in operating assets and liabilities:
|
|
|
|||||
|
Prepaid expenses, deposits and other assets
|
(99,737
|
)
|
(97,058
|
)
|
|||
|
Accounts payable, accrued expenses and other liabilities
|
(1,123,303
|
)
|
797,827
|
|
|||
|
Net cash used in operating activities
|
(3,415,901
|
)
|
(4,630,189
|
)
|
|||
|
Cash flows (used in) provided by investing activities:
|
|
|
|||||
|
Purchases of property and equipment
|
(6,372
|
)
|
(1,994
|
)
|
|||
|
Cash received in reverse merger transaction
|
—
|
|
8,500,602
|
|
|||
|
Net cash (used in) provided by investing activities
|
(6,372
|
)
|
8,498,608
|
|
|||
|
Cash flows provided by financing activities:
|
|
|
|||||
|
Proceeds from the sale of Series A convertible preferred stock, net
|
14,269,095
|
|
—
|
|
|||
|
Payment of offering costs
|
(187,649
|
)
|
—
|
|
|||
|
Net cash provided by financing activities
|
14,081,446
|
|
—
|
|
|||
|
Net increase in cash and cash equivalents
|
10,659,173
|
|
3,868,419
|
|
|||
|
Cash and cash equivalents at beginning of period
|
1,552,852
|
|
1,997,192
|
|
|||
|
Cash and cash equivalents at end of period
|
$
|
12,212,025
|
|
$
|
5,865,611
|
|
|
|
Supplemental disclosure of non-cash investing and financing activities:
|
|
|
|||||
|
Reclassification of deferred offering costs upon completion of private placement
|
$
|
180,456
|
|
$
|
—
|
|
|
|
Offering costs in accounts payable and accrued expenses
|
$
|
178,258
|
|
$
|
—
|
|
|
|
Series A cumulative preferred dividends
|
$
|
(58,845
|
)
|
$
|
—
|
|
|
|
Issuance of subscription receivable upon sale of Series A convertible preferred stock
|
$
|
(8,280,935
|
)
|
$
|
—
|
|
|
|
Conversion of convertible notes and related accrued interest into common stock
|
$
|
—
|
|
$
|
711,495
|
|
|
|
Consideration in connection with RestorGenex Corporation merger transaction
|
$
|
—
|
|
$
|
21,261,000
|
|
|
|
1.
|
Organization and Description of Business
|
|
2.
|
Liquidity
|
|
3.
|
Basis of Presentation and Summary of Significant Accounting Policies
|
|
|
March 31,
|
||||
|
|
2017
|
2016
|
|||
|
Convertible debt
|
766,351
|
|
428,134
|
|
|
|
Convertible preferred stock
|
12,376,329
|
|
—
|
|
|
|
Common stock warrants
|
14,016,608
|
|
477,688
|
|
|
|
Stock options
|
2,304,132
|
|
1,796,360
|
|
|
|
Unvested restricted stock awards
|
7,665
|
|
13,802
|
|
|
|
|
29,471,085
|
|
2,715,984
|
|
|
|
4.
|
Acquisition
|
|
Fair value of RestorGenex shares outstanding
|
$
|
19,546,000
|
|
|
Estimated fair value of RestorGenex stock options outstanding
|
1,321,000
|
|
|
|
Estimated fair value of RestorGenex warrants outstanding
|
384,000
|
|
|
|
CVRs – RES-440 product candidate
|
10,000
|
|
|
|
Total purchase price
|
$
|
21,261,000
|
|
|
Cash and cash equivalents
|
$
|
8,500,602
|
|
|
Prepaid expenses and other assets
|
195,200
|
|
|
|
Property and equipment
|
57,531
|
|
|
|
Intangible assets
|
9,600,000
|
|
|
|
Goodwill
|
6,929,258
|
|
|
|
Accrued liabilities
|
(377,432
|
)
|
|
|
Deferred tax liability
|
(3,644,159
|
)
|
|
|
Net assets acquired
|
$
|
21,261,000
|
|
|
|
Three Months Ended March 31, 2016
|
|||
|
Net revenues
|
$
|
—
|
|
|
|
Net loss
|
(4,655,944
|
)
|
||
|
Basic and diluted loss per share
|
$
|
0.47
|
|
|
|
5.
|
Other Accrued Expenses and Liabilities
|
|
|
March 31, 2017
|
December 31, 2016
|
|||||
|
Accrued interest payable
|
$
|
57,173
|
|
$
|
29,359
|
|
|
|
Accrued Series A dividends
|
58,845
|
|
—
|
|
|||
|
Accrued payroll and payroll related expenses
|
135,072
|
|
399,740
|
|
|||
|
Accrued professional fees
|
185,163
|
|
72,855
|
|
|||
|
Accrued clinical studies expenses
|
346,861
|
|
220,978
|
|
|||
|
Other accrued expenses
|
233,149
|
|
151,332
|
|
|||
|
Total
|
$
|
1,016,263
|
|
$
|
874,264
|
|
|
|
6.
|
Convertible Debt
|
|
Note
|
Issue
Date
|
Maturity
Date
|
Conversion
Price
|
Interest
Rate
|
Total
Principal
|
||||||||||
|
2016 Convertible Notes
|
9/27/2016
|
9/27/2017
|
$
|
3.50
|
|
6.00
|
%
|
$
|
1,880,000
|
|
|||||
|
Series B Note
|
3/15/2011
|
6/30/2018
|
$
|
2.74
|
|
1.00
|
%
|
550,000
|
|
||||||
|
Total principal amount
|
|
|
|
|
$
|
2,430,000
|
|
||||||||
|
Less current portion of convertible notes
|
(1,880,000
|
)
|
|||||||||||||
|
Convertible notes, net of current portion
|
$
|
550,000
|
|
||||||||||||
|
7.
|
Convertible Preferred Stock and Common Stock Warrants
|
|
Outstanding
|
Range of exercise price per share
|
|||
|
Common stock warrants issued prior to Merger
|
460,721
|
|
$20.00 - $750.00
|
|
|
Common stock warrants issued in Series A
|
13,555,887
|
|
$2.22
|
|
|
14,016,608
|
|
|||
|
8.
|
Stock-Based Compensation
|
|
|
Three Months Ended March 31,
|
||||||
|
|
2017
|
2016
|
|||||
|
Research and development
|
$
|
43,322
|
|
$
|
242,277
|
|
|
|
General and administrative
|
323,061
|
|
151,194
|
|
|||
|
Total stock-based compensation expense
|
$
|
366,383
|
|
$
|
393,471
|
|
|
|
|
Number of
Options
|
Weighted
average
exercise price
per share
|
Weighted
average
remaining
contractual life
(in years)
|
|||||
|
Balance at January 1, 2017
|
2,207,409
|
|
$
|
8.09
|
|
|
||
|
Granted
|
98,184
|
|
2.33
|
|
|
|||
|
Expired
|
(1,461
|
)
|
15.00
|
|
||||
|
Outstanding at March 31, 2017
|
2,304,132
|
|
$
|
7.84
|
|
7.6
|
||
|
Exercisable at March 31, 2017
|
1,548,216
|
|
$
|
9.04
|
|
6.9
|
||
|
Expected term (in years)
|
5.77
|
|
|
Risk-free interest rate
|
2.1
|
%
|
|
Expected volatility
|
125.2
|
%
|
|
Dividend yield
|
—
|
%
|
|
9.
|
Commitments and Contingencies
|
|
|
Rental Commitments
|
||
|
2017
|
$
|
73,989
|
|
|
2018
|
112,354
|
|
|
|
2019
|
114,409
|
|
|
|
2020
|
116,464
|
|
|
|
2021
|
118,519
|
|
|
|
Thereafter
|
39,735
|
|
|
|
Total
|
$
|
575,470
|
|
|
10.
|
Fair Value Measurements
|
|
|
March 31, 2017
|
||||||||||
|
|
(Level 1)
|
(Level 2)
|
(Level 3)
|
||||||||
|
Assets
|
|
|
|
||||||||
|
Cash and cash equivalents
|
$
|
12,212,025
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Liabilities
|
|||||||||||
|
Common stock warrant liability
|
$
|
—
|
|
$
|
—
|
|
$
|
48,145,520
|
|
||
|
|
December 31, 2016
|
||||||||||
|
|
(Level 1)
|
(Level 2)
|
(Level 3)
|
||||||||
|
Assets
|
|
|
|
||||||||
|
Cash and cash equivalents
|
$
|
1,552,852
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Liabilities
|
|||||||||||
|
Common stock warrant liability
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
|
Common Stock Warrant Liability
|
||
|
Balance at December 31, 2016
|
$
|
—
|
|
|
Issued in connection with the Series A convertible preferred stock
|
35,225,846
|
|
|
|
Change in fair value
|
12,919,674
|
|
|
|
Balance at March 31, 2017
|
$
|
48,145,520
|
|
|
Stock price
|
$
|
2.38
|
|
|
Exercise price
|
$
|
2.22
|
|
|
Expected term (in years)
|
5
|
|
|
|
Risk-free interest rate
|
2.1
|
%
|
|
|
Expected volatility
|
127.0
|
%
|
|
|
Dividend yield
|
—
|
|
|
|
ITEM 2.
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
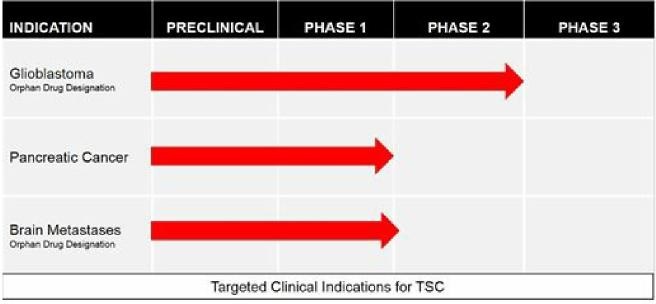
|
|
Three Months Ended March 31, 2017
|
|
|||||||||
|
|
2017
|
2016
|
Change
|
||||||||
|
Operating expenses:
|
|
|
|
||||||||
|
Research and development
|
$
|
1,007,571
|
|
$
|
2,352,807
|
|
$
|
(1,345,236
|
)
|
||
|
General and administrative
|
1,553,139
|
|
3,862,484
|
|
(2,309,345
|
)
|
|||||
|
Depreciation
|
6,603
|
|
7,853
|
|
(1,250
|
)
|
|||||
|
Loss from operations
|
2,567,313
|
|
6,223,144
|
|
(3,655,831
|
)
|
|||||
|
Other expense:
|
|
|
|||||||||
|
Interest expense, net
|
55,719
|
|
21
|
|
55,698
|
|
|||||
|
Change in fair value of warrant liabilities
|
12,919,674
|
|
—
|
|
12,919,674
|
|
|||||
|
Warrant related expenses
|
10,225,846
|
|
—
|
|
10,225,846
|
|
|||||
|
Other financing expenses
|
2,870,226
|
|
—
|
|
2,870,226
|
|
|||||
|
Net loss
|
$
|
(28,638,778
|
)
|
$
|
(6,223,165
|
)
|
$
|
(22,415,613
|
)
|
||
|
Series A cumulative preferred dividends
|
(58,845
|
)
|
—
|
|
(58,845
|
)
|
|||||
|
Net loss attributable to common stockholders
|
$
|
(28,697,623
|
)
|
$
|
(6,223,165
|
)
|
$
|
(22,474,458
|
)
|
||
|
|
Three Months Ended March 31,
|
||||||
|
Net cash (used in) provided by:
|
2017
|
2016
|
|||||
|
Operating activities
|
$
|
(3,415,901
|
)
|
$
|
(4,630,189
|
)
|
|
|
Investing activities
|
(6,372
|
)
|
8,498,608
|
|
|||
|
Financing activities
|
14,081,446
|
|
—
|
|
|||
|
Net increase in cash and cash equivalents
|
$
|
10,659,173
|
|
$
|
3,868,419
|
|
|
|
|
●
|
our ability to obtain additional financing;
|
|
|
●
|
our estimates regarding expenses, future revenues, capital requirements and needs for additional financing;
|
|
|
●
|
the success and timing of our preclinical studies and clinical trials;
|
|
|
●
|
the difficulties in obtaining and maintaining regulatory approval of our products and product candidates, and the labeling under any approval we may obtain;
|
|
|
●
|
our plans and ability to develop and commercialize our product candidates;
|
|
|
●
|
our failure to recruit or retain key scientific or management personnel or to retain our executive officers;
|
|
|
●
|
the accuracy of our estimates of the size and characteristics of the potential markets for our product candidates and our ability to serve those markets;
|
|
|
●
|
regulatory developments in the United States and foreign countries;
|
|
|
●
|
the rate and degree of market acceptance of any of our product candidates;
|
|
|
●
|
obtaining and maintaining intellectual property protection for our product candidates and our proprietary technology;
|
|
|
●
|
our ability to operate our business without infringing the intellectual property rights of others;
|
|
|
●
|
future legislation regarding the healthcare system;
|
|
|
●
|
the success of competing products that are or may become available; and
|
|
|
●
|
the performance of third parties, including contract research organizations, collaborators and manufacturers.
|
|
ITEM 3.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
|
ITEM 4.
|
CONTROLS AND PROCEDURES
|
|
ITEM 1.
|
LEGAL PROCEEDINGS
|
|
ITEM 1A.
|
RISK FACTORS
|
|
ITEM 2.
|
UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
|
|
ITEM 3.
|
DEFAULTS UPON SENIOR SECURITIES
|
|
ITEM 4.
|
MINE SAFETY DISCLOSURES
|
|
ITEM 5.
|
OTHER INFORMATION
|
|
ITEM 6.
|
EXHIBITS
|
|
|
|
DIFFUSION PHARMACEUTICALS INC.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
By:
|
/s/ David G. Kalergis
|
|
|
|
|
David G. Kalergis
|
|
|
|
|
Chairman and Chief Executive Officer
|
|
|
|
|
(Principal Executive Officer)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
By:
|
/s/ Ben L. Shealy
|
|
|
|
|
Ben L. Shealy
|
|
|
|
|
Senior Vice President, Finance, Treasurer and Secretary
|
|
|
|
|
(Principal Financial Officer)
|
|
|
Exhibit
No.
|
Description
|
Method of Filing
|
|
3.1
|
Certificate of Incorporation of Diffusion Pharmaceuticals Inc., as amended
|
Incorporated by reference to Exhibit 3.1 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2016, as amended
|
|
3.2
|
Certificate of Designation of Preferences, Rights and Limitations of the Series A Convertible Preferred Stock of Diffusion Pharmaceuticals Inc.
|
Incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on March 15, 2017
|
|
4.1
|
Form of Warrant issued to Investors in the 2017 Series A Private Placement by Diffusion Pharmaceuticals Inc.
|
Incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on March 15, 2017
|
|
4.2
|
Form of Registration Rights Agreement entered into by and among the Company and Investors in the 2017 Series A Private Placement
|
Filed herewith
|
|
10.1
|
Form of 2017 Series A Private Placement Subscription Agreement
|
Incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on March 15, 2017
|
|
10.2
|
Placement Agency Agreement, dated January 27, 2017, by and between Diffusion Pharmaceuticals Inc. and Maxim Merchant Capital, a division of Maxim Group LLC, with respect to the Series A Private Placement
|
Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on March 15, 2017
|
|
10.3
|
Amendment to the Placement Agency Agreement, dated March 14, 2017, by and between Diffusion Pharmaceuticals Inc. and Maxim Merchant Capital, a division of Maxim Group LLC, with respect to the Series A Private Placement
|
Incorporated by reference to Exhibit 10.17 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2016, as amended
|
|
10.4
|
Lease Agreement, dated March 31, 2017, by and between Diffusion Pharmaceuticals Inc. and One Carlton LLC
|
Filed herewith
|
|
31.1
|
Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 and SEC Rule 13a-14(a)
|
Filed herewith
|
|
31.2
|
Certification of principal financial officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 and SEC Rule 13a-14(a)
|
Filed herewith
|
|
32.1
|
Certification of Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
Furnished herewith
|
|
32.2
|
Certification of principal financial officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
Furnished herewith
|
|
101
|
The following materials from Diffusion’s quarterly report on Form 10-Q for the quarter ended March 31, 2017, formatted in XBRL (Extensible Business Reporting Language): (i) the Unaudited Condensed Consolidated Balance Sheets, (ii) the Unaudited Condensed Consolidated Statements of Operations, (iii) the Unaudited Condensed Consolidated Statement of Changes in Stockholders’ Equity (Deficit), (iv) the Unaudited Condensed Consolidated Statements of Cash Flows, and (v) Notes to Unaudited Condensed Consolidated Financial Statements
|
Filed herewith
|