|
(Mark One)
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
☐
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
77-0701774
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
|
|
820 S. Friendswood Drive, Suite 201, Friendswood, Texas
|
77546
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
|
Title of each class
|
Trading Symbol(s)
|
Name of each exchange on which registered
|
|
Common Stock, $0.001 par value per share
|
CSTL
|
The Nasdaq Global Market
|
|
Large accelerated filer
|
¨
|
Accelerated filer
|
¨
|
|
Non-accelerated filer
|
x
|
Smaller reporting company
|
x
|
|
Emerging growth company
|
x
|
||
|
Page
|
||
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A
|
||
|
Item 9B.
|
||
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
Item 15.
|
||
|
Item 16.
|
||
|
•
|
estimates of our addressable market, future revenue, expenses, capital requirements and our needs for additional financing;
|
|
•
|
expectations with respect to reimbursement for our products, including third-party payor reimbursement and coverage decisions;
|
|
•
|
anticipated cost, timing and success of our products in development, and our plans to research, develop and commercialize new tests;
|
|
•
|
our ability to obtain funding for our operations, including funding necessary to complete the expansion of our operations and development of our product candidates;
|
|
•
|
the implementation of our business model and strategic plans for our products, technologies and businesses;
|
|
•
|
our ability to manage and grow our business by expanding our sales to existing customers or introducing our products to new customers;
|
|
•
|
our ability to develop and maintain sales and marketing capabilities;
|
|
•
|
regulatory developments in the United States and foreign countries;
|
|
•
|
the performance of our third-party suppliers;
|
|
•
|
the success of competing diagnostic products that are or become available;
|
|
•
|
our ability to attract and retain key personnel;
|
|
•
|
our expectations regarding the period during which we qualify as an emerging growth company under the Jumpstart Our Business Startups Act, as amended, or the JOBS Act, enacted in April 2012; and
|
|
•
|
our expectations regarding our ability to obtain and maintain intellectual property protection for our products and our ability to operate our business without infringing on the intellectual property rights of others.
|

|
•
|
Development of our products required our machine learning expertise and our proprietary algorithm, which are complex and difficult to replicate.
We develop our products using our machine-learning expertise to analyze clinical specimens with associated long-term outcomes data to identify genomic patterns in tumor biology that we believe will accurately predict the risk of metastasis and recurrence. We then validate these genomic patterns, by refining and locking down algorithms to enable additional studies to validate the accuracy of our tests and subsequently document the clinically actionable changes made by physicians when they incorporate our test results into their treatment plan decisions.
|
|
•
|
We have demonstrated the ability to provide clinically actionable information despite the complex genomics of skin cancer.
In the diagnosis and prognosis of cancer, there is significant current interest in DNA driver mutations as being a predictor of the behavior of cancers. We believe that while the behavior of some cancers may be elucidated by DNA analysis and the response to certain targeted therapies, the majority of skin cancer behavior will best be understood at the gene expression level. Specifically, while DNA mutations of a specific gene are important for tumor
|
|
•
|
Our growing database of tumor samples and associated long-term outcomes data enables us to improve our current products and accelerate development of new products.
The development and validation of accurate tests is a complex process that requires access to tumor tissue specimens and long-term outcomes data. Such data is not readily available for skin cancer, which creates a barrier to rapid test development and validation. However, over the past ten years we created a sample bank comprised of over 55,000 samples, including 5,900 well-annotated samples that we have used in our clinical studies to date. We have been able to use this sample bank to expand the clinical use of our products, evaluate improvements in new proprietary genomic algorithm approaches and develop new products.
|
|
•
|
We have generated, and will continue to generate, robust clinical validity and utility data supporting the use of our products.
For example, DecisionDx-Melanoma has been studied in more than 5,700 patient samples, including 22 published studies since 2015. We also are making significant investments in further clinical studies to continue to support DecisionDx-Melanoma, DecisionDx-UM and our pipeline products. This growing set of data is significant in educating physicians and patients about the value of our products and supporting reimbursement of our products by third-party payors.
|
|
•
|
We have established relationships with physicians that allow us to optimize our interactions, increase adoption of our current products and identify areas of unmet clinical need to efficiently launch additional products.
We have published rigorous clinical data, which allows our sales and medical affairs representatives to have substantive, in depth dialogues with physicians. Through these established relationships we have been able to integrate our products into physicians’ workflows and identify further educational programs, which we believe drives adoption of our products. We can also leverage these relationships to identify areas of significant unmet medical need and efficiently launch additional skin cancer products.
|
|
•
|
We have experience in navigating the reimbursement landscape.
In the molecular diagnostics industry securing reimbursement for new tests is a long, complex and uncertain process. We have developed significant expertise in securing reimbursement for our products.
|
|
•
|
Expand adoption of our currently marketed products and educate physicians and their patients on the need for our products to make a more informed treatment plan decision.
We believe that cancer treatment plans will be most effective if decisions are personalized for each patient based on the biology of their specific tumor, instead of a one-size-fits-all approach. We will continue to educate physicians and their patients on the diagnostic discordance that leads to over- and under-treatment.
|
|
•
|
Continue to generate evidence supporting the clinical utility and validity of our products.
We have conducted extensive clinical utility and validity studies to support the adoption of, and reimbursement for, our products. In order to maintain our competitive advantage and increase sales of our products, we will continue to generate additional clinical data to support the use of our products.
|
|
•
|
Execute planned expansion of our commercial channel.
We plan to increase sales of our products by adding new physicians to our customer base as well as increasing orders by physicians already using our products. We increased the number of sales and medical affairs representatives in the first quarter of 2019 with a second expansion occurring in December 2019. We will evaluate the needs of our customers through 2020 and may make additional commercial investments to better support the educational needs of our customers with our currently marketed products as well as support the launch of additional products.
|
|
•
|
Expand coverage and reimbursement for our products.
We plan to increase dialogue with third-party payors to highlight our clinical utility and patient outcomes data. We believe these data will validate the benefit of our products for patients and will persuade more third-party payors to provide coverage and reimbursement. Additionally, we will continue to emphasize our ability to reduce overall cost to the healthcare system by appropriately classifying high-risk patients and removing the need for unnecessary invasive products for low-risk patients.
|
|
•
|
Utilize our development expertise and commercial channel insight to provide additional solutions.
We are continuing to develop products that address the challenges facing physicians, including genomic tests for patients with
|


|
•
|
Better Information for Physicians.
We provide physicians and their patients with a report that contains clinically actionable information to inform the treatment plan for each individual patient. Our reports are updated as new clinical data is generated that may enable additional clinical decisions to be made. Based on four studies that we have conducted on clinical actionability, based on our test reports, physicians changed a patient’s treatment in more than 50% of cases, indicating physician confidence in the evidence underlying our reports.
|
|
•
|
Better Patient Care.
The clinical evidence shows that our products are accurate predictors of a patient’s specific risk of metastasis or recurrence of their cancer based upon the gene expression profile of their tumor, independent of available clinical and pathology factors. Physicians use this information to identify patients who are likely to benefit from an escalation of care as well as those who may avoid unnecessary treatments, such as medical and surgical interventions.
|
|
•
|
Reduced Healthcare Costs for Payors.
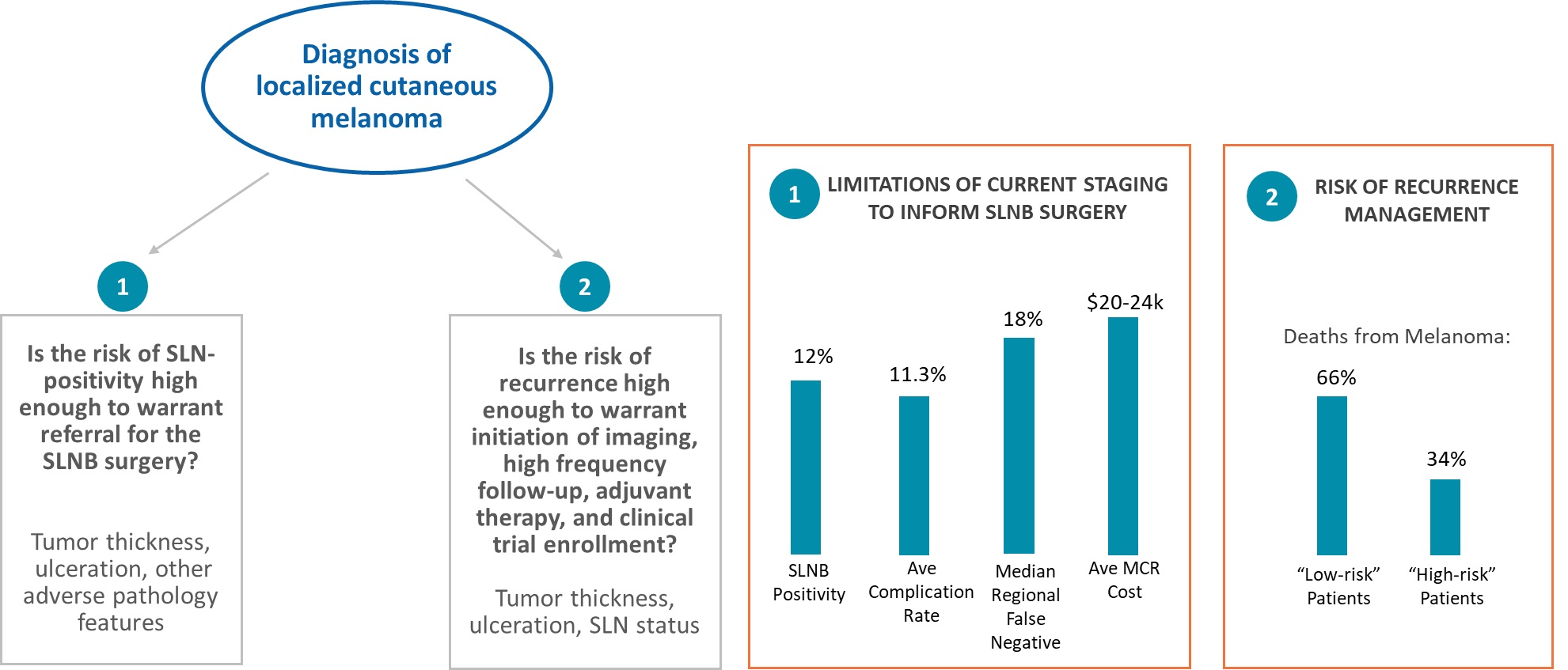
We believe our products have the potential to reduce overall healthcare costs by enabling physicians and their patients to avoid unnecessary medical and surgical interventions, including the SLNB surgery. As an example, without DecisionDx-Melanoma, 88% of patients who receive the SLNB surgery, which has an average in-patient reimbursed cost of $20,000 to $24,000, are found to be SLN-negative and remain classified as low risk. If all patients eligible for the SLNB surgery were tested and their test results were acted upon, we estimate the potential savings to the U.S. healthcare system could be up to $250 million, after considering the cost of DecisionDx-Melanoma.
|



|
Study
|
Design
|
# of Patients
|
% Change in Management
|
|
Berger et al. CMRO 2016
|
Prospectively tested cohort, multi-center. Retrospective pre test / post test management.
|
156
|
53%
|
|
Dillon et al. SKIN J Cutan Med 2018
|
Prospective, multi-center: pre test / post test management.
|
247
|
49%
|
|
Farberg et al. J Drugs Derm 2017
|
169 physician impact study: patient vignettes with pre test / post test management.
|
n/a
|
47-50%
|
|
Schuitevoerder et al. J Drugs Derm 2018
|
Prospectively tested cohort, single center. Retrospective pre test / post test management; and modeling of prospective cohort.
|
91
|
52%
|

|
Study
|
Peer-Reviewed Publications
(Methods)
|
Main Findings
|
|||
|
Clinical Validity
|
|||||
|
Guidance of sentinel lymph node biopsy decisions in patients with T1-T2 melanoma using gene expression profiling
|
Future Oncology, January 2019
(SLNB rate: multicenter;
prospective; n=1421
Survival analysis:
retrospective; n=690)
|
•
|
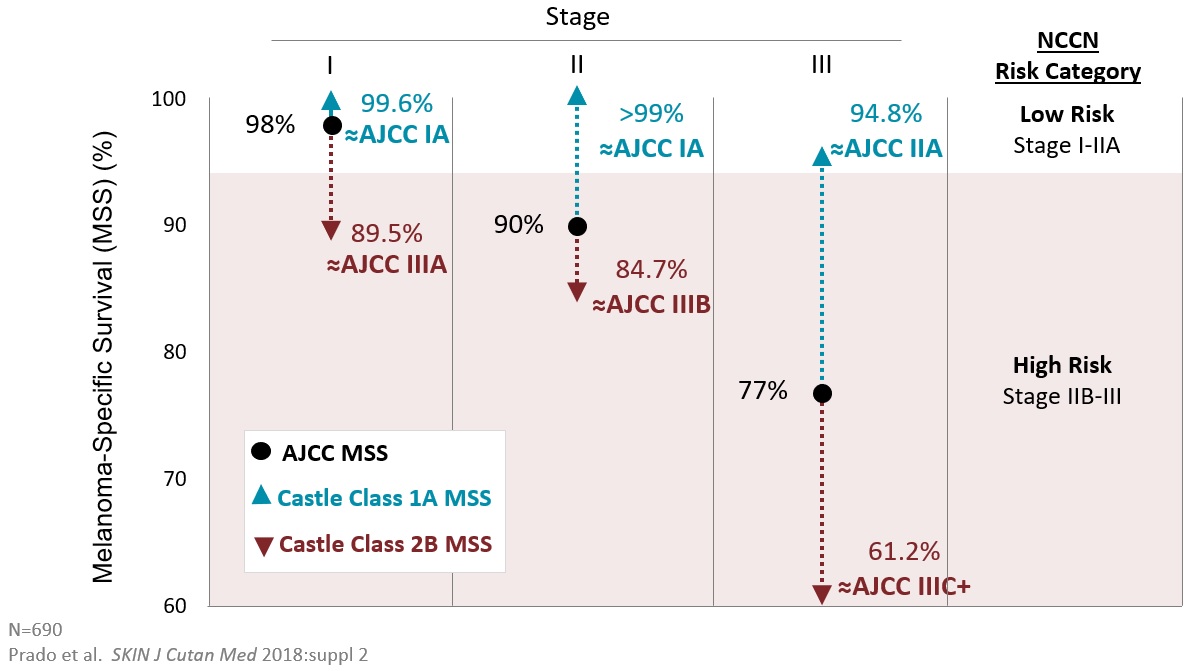
Patients with T1/T2 melanomas and a Class 1A result had a SLN positive rate <5% while Class 2B patients had a rate above 10%. This is clinically significant as national guidelines do not recommend a SLNB if the risk is <5% and do recommend it if the risk is >10%.
|
||
|
•
|
Melanoma-specific survival (MSS) was 99.6% for patients with Class 1A, T1/T2 tumors who would avoid a SLNB.
|
||||
|
Prospective validation of the prognostic 31-gene expression profiling test in primary cutaneous melanoma
|
Cancer Medicine, March 2019 (single center; prospective; n=159 stage I-III melanomas)
|
•
|
Median follow-up of 45 months for recurrence-free cases.
|
||
|
•
|
DecisionDx-Melanoma Class 1 was an independent predictor of recurrence (p=0.0001) and the most significant predictor of recurrence with a hazard ratio of 9.2. DecisionDx-Melanoma Class 1 was also an independent predictor of recurrence (p=0.009) and the most significant predictor of distant metastasis with a hazard ratio of 19.0.
|
||||
|
•
|
NPV for Class 1 for distant metastasis free survival was 99%.
|
||||
|
•
|
Of 29 recurrences, 10 (34%) occurred in SLN positive cases while 23 (79%) occurred in Class 2. Of the 10 recurrences in SLN positive cases, 9 were Class 2.
|
||||
|
Early outcome of a 31-GEP test in 86 AJCC stage IB-II melanoma patients. A prospective multicentre cohort study
|
Journal of the European Academy of Dermatology and Venereology, February 2019 (Multicenter; prospective; n=86 stage IB-II melanomas)
|
•
|
DecisionDx-Melanoma Class 1 was an independent predictor of recurrence (p=0.01) and the most significant predictor of recurrence with a hazard ratio of 18.82. AJCC stage and age were not independent of DecisionDx-Melanoma Class 1.
|
||
|
•
|
NPV for Class 1 for RFS was 100%.
|
||||
|
•
|
All recurrences occurred in Class 2 patients.
|
||||
|
Identification of patients at risk for metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria
|
Journal of the American Academy of Dermatology, January 2019
(Multicenter; archival; n=690 stage I-III melanomas)
|
•
|
DecisionDx-Melanoma Class 1A was an independent predictor of RFS, DMFS and MSS in the entire cohort and the most significant predictor with hazard ratios of 2.92, 2.89 and 9.02 for RFS, DMFS and MSS, respectively.
|
||
|
•
|
Subpopulation analysis of patients with Stage I-IIA melanoma showed that DecisionDx-Melanoma Class 1A was the only independent predictor of RFS, DMFS and MSS for all three endpoints compared to tumor thickness, ulceration status, and mitotic rate. Tumor thickness was an independent predictor for RFS but DecisionDx-Melanoma Class 1A was 499% greater than tumor thickness for this endpoint.
|
||||
|
•
|
Subpopulation analysis of patients with melanomas <1.0mm showed that DecisionDx-Melanoma Class 1A was an independent predictor or RFS and the most significant predictor with a hazard ratio of 9.34 which was over 200% greater than SLNB status.
|
||||
|
•
|
NPV for Class 1A for MSS was 99%.
|
||||
|
Estimation of Prognosis in Invasive Cutaneous Melanoma: An Independent Study of the Accuracy of a GEP Profile Test
|
Dermatologic Surgery,
December 2018 (Independent; single center; prospective; n=256 stage I/II melanomas)
|
•
|
Patients with a DecisionDx-Melanoma Class 2 result were 22 times more likely to metastasize compared to a Class 1 result. Multi-variate statistical analysis for independence was not reported.
|
||
|
•
|
NPV for Class 1 for recurrence was 99%.
|
||||
|
Interim analysis of survival in a prospective, multi-center registry cohort of cutaneous melanoma patients tested with a prognostic 31-GEP test
|
Journal of Hematology and Oncology, August 2017 (Multicenter; prospective; n=322 stage I-III melanomas)
|
•
|
DecisionDx-Melanoma Class 1 was an independent predictor of RFS and the most significant predictor with a hazard ratio of 7.15 and 290% greater than the nearest predictor, SLNB.
|
||
|
•
|
NPV for Class 1 was 98-99% for RFS, DMFS and OS.
|
||||
|
•
|
Of 12 distant metastatic events, 10 occurred in the Class 2 group compared to 6 in the SLN positive group.
|
||||
|
Study
|
Peer-Reviewed Publications
(Methods)
|
Main Findings
|
|||
|
Development of a prognostic genetic signature to predict the metastatic risk associated with cutaneous melanoma
|
Clinical Cancer Research, January 2015
(Multicenter; archival; n=268 stage I-IV melanomas)
|
•
|
DecisionDx-Melanoma Class 1 was an independent predictor of disease-free survival, DFS, and the most significant predictor with a hazard ratio of 9.55 compared to 5.40 for AJCC stage.
|
||
|
•
|
5-year DFS rate for Class 1 (97%) was significantly better than for Class 2 (31%; p<0.0001) and 98% for patients with Stage I or II melanomas.
|
||||
|
•
|
SLN positivity rate was 2% in patients with a Class 1 result.
|
||||
|
Identification of high-risk cutaneous melanoma tumors is improved when combining the online American Joint Committee on Cancer Individualized Melanoma Patient Outcome Prediction Tool with a 31-GEP based classification
|
Journal of the American Academy of Dermatology, May 2016
(Multicenter; archival; n=205 stage I-II melanomas)
|
•
|
DecisionDx-Melanoma Class 1 was an independent predictor of RFS, DMFS and OS compared to the AJCC Individualized Melanoma Patient Outcome Prediction Tool and was 163% or greater than AJCC for all outcomes.
|
||
|
•
|
Adding the DecisionDx-Melanoma Class 1 result to AJCC staging improved sensitivity for identifying recurrence, distant metastasis or death by up to 22% compared to AJCC staging alone.
|
||||
|
•
|
21% of cases had discordant risk prediction from DecisionDx-Melanoma Class 1 and AJCC tools, with the DecisionDx-Melanoma providing the more accurate prognosis for the majority of cases
|
||||
|
Gene expression profiling for molecular staging of cutaneous melanoma in patients undergoing sentinel lymph node biopsy
|
Journal of the American Academy of Dermatology, May 2015
(Multicenter; archival; n=217 stage I-III melanomas all of whom underwent SLNB)
|
•
|
DecisionDx-Melanoma Class 1 was an independent predictor of RFS, DMFS and OS with hazard ratios of 4.9, 3.9 and 4.7, respectively, and was 185% - 392% greater than SLNB.
|
||
|
•
|
DecisionDx-Melanoma Class 1 NPV for distant metastasis was 82% compared to 67% for SLNB.
|
||||
|
•
|
DecisionDx-Melanoma Class 1 sensitivity was 85%, 84% and 85% compared to SLNB sensitivity of 35%, 38% and 29% for the endpoints of DFS, DMFS and OS.
|
||||
|
Performance of a prognostic 31-GEP in an independent cohort of 523 cutaneous melanoma patients
|
BMC Cancer, February 2018
(Multicenter; archival; n=523 stage I-III melanomas)
|
•
|
DecisionDx-Melanoma Class 1A was an independent predictor of RFS and DMFS with hazard ratios of 3.8 and 5.3, respectively, and was between 146% and 408% greater than SLNB and tumor thickness.
|
||
|
•
|
NPV for MSS for Class 1A patients was 100% for Stage I, 100% for Stage II, 94% for Stage IIIA and 91% for Stage IIIB-C patients.
|
||||
|
Performance of a 31-GEP test in cutaneous melanomas of the head and neck
|
Head & Neck, January 2019
(Multicenter; archival; n=157 melanomas of the head and neck region)
|
•
|
DecisionDx-Melanoma Class 1 was an independent predictor of RFS, DMFS, OS and MSS with hazard ratios of 2.8, 2.8, 4.1 and 6.8, respectively compared to AJCC stage.
|
||
|
•
|
NPV for Class 1A for MSS was 98%.
|
||||
|
Clinical Utility
|
|||||
|
Prospective, Multicenter Clinical Impact Evaluation of a 31-GEP Test for Management of Melanoma Patients
|
SKIN: The Journal of Cutaneous Medicine, March 2018
(Multicenter, prospective; pre-test post-test methodology; n=247 patients)
|
•
|
Post-test management plans changed by 49% compared to pre-test plans; 85% of Class 2 patients and 36% of Class 1 patients having a change in management (p<0.001).
|
||
|
•
|
Significant changes for imaging (p<0.001), request for laboratory work (p=0.04) and frequency of office visits (p<0.001).
|
||||
|
Clinical impact of a 31-GEP test for cutaneous melanoma in 156 prospectively and consecutively tested patients
|
Current Medical Research and Opinion, September 2016 (Multicenter; retrospective chart review, pre-test post-test methodology; n=156 patients)
|
•
|
Post-test management plans were recorded in 53% of patients, with 77% of Class 2 patients and 37% of Class 1 patients (p<0.0001) having a change in management.
|
||
|
•
|
94% of patient management changes were concordant with the risk indicated by the GEP test result (p<0.0001).
|
||||
|
Impact of a 31-gene Expression Profiling Test for Cutaneous Melanoma on Dermatologists’ Clinical Management Decisions
|
Journal of Drugs in Dermatology, May 2017 (Intended use pre-test post-test vignette methodology; n=170 participating dermatologists)
|
•
|
Risk appropriate management recommendations for implementing SLNB and imaging were more likely to be made following incorporation of DecisionDx-Melanoma test results (p<0.05).
|
||
|
•
|
Dermatologists changed their tumor thickness inflection point for implementing SLNB, oncology referral and imaging from 1.0mm to 0.7mm following a Class 2 DecisionDx-Melanoma test result.
|
||||
|
Study
|
Peer-Reviewed Publications
(Methods)
|
Main Findings
|
|||
|
Impact of Genetic Expression Profile on Decision-Making in Clinically Node Negative Melanoma Patients After Surgical staging
|
Journal of Drugs in Dermatology, February 2018 (Single center, prospective study at Oregon Health and Science Center; n=91 patients)
|
•
|
DecisionDx-Melanoma test results were significantly associated with management of patients with Stage I or II melanoma by Dermatology (most often Class 1) or Surgical Oncology (most often Class 2) (p<0.05).
|
||
|
•
|
Decision-tree model derived from the treatment and clinical data found that DecisionDx-Melanoma class result accounted for 52% of management changes whereas AJCC stage accounted for 48%.
|
||||
|
Factors affecting dermatologists’ use of a 31-gene expression profiling test as an adjunct for predicting metastatic risk in cutaneous melanoma
|
Journal of Drugs in Dermatology, May,2018
(Intended use pre-test post-test vignette methodology; n=181 participating dermatologists)
|
•
|
The DecisionDx-Melanoma result had a significant impact on the likelihood that SLNB would be recommended for a patient with a T1b tumor.
|
||
|
•
|
The presence of ulceration increased the proportion of respondents who would recommend the test (p<0.001).
|
||||
|
Management decisions made by physician assistants and nurse practitioners in cutaneous malignant melanoma patients: impact of a 31-GEP test
|
Journal of Drugs in Dermatology, November 2018
(Intended use pre-test post-test vignette methodology; n=164 participating nurse practitioners and physician assistants)
|
•
|
DecisionDx-Melanoma Class 1 results led to a significant decrease in the proportion of PA/NPs who would recommend SLNB, imaging or quarterly follow-up intervals, while Class 2 results led to significant increases in each (p<0.01 for 5 of 6 patient vignettes included in clinical impact survey).
|
||
|
Establishing an evidence-based decision point for clinical use of the 31-gene expression profile test in cutaneous melanoma
|
SKIN: The Journal of Cutaneous Medicine, July 2019 (Multi-center, multi-cohort, multiple endpoint, prospective and archival methodology; n=1,037, 437, 8,944 and 160 patients)
|
•
|
Evidence-based analysis of four datasets demonstrated that a Breslow thickness of ≥ 0.3 is an appropriate cut-point for the second use of DecisionDx-Melanoma.
|
||
|
•
|
A fitted Loess regression curve of cumulative recurrence rates plotted at 0.1mm Breslow thickness showed significant separation occurred between 0.2 and 0.3mm for both RFS and DMFS rates for tumors < 0.3mm versus ≥ 0.3 and 1.0mm (p < 0.0001 for RFS and p = 0.0008 for DMFS). Frequency of non-Class 1A melanomas from a large dermatopathology practice was 16% in 437 consecutively tested melanomas between 0.3 and 1.0mm and 11% for 8,944 consecutively tested melanomas from May 2018 through April 2019.
|
||||
|
•
|
Analysis of two multi-center clinical use studies found a clinically significant and relevant change in management in 25% of patients tested in the 0.3 to 1.0mm group.
|
||||
|
Level of Evidence Review for a Gene Expression Profile Test for Cutaneous Melanoma
|
Am J Clin Dermatol 2019 (Level of evidence review of 7 studies using AJCC, NCCN and AAD criteria. n.b. two 2019 prospective were not included due to study censor date)
|
•
|
Level of evidence ranking using AJCC criteria was I/II.
|
||
|
•
|
Level of evidence ranking using NCCN criteria was I-IIIB (n.b. in December 2019, NCCN updated guidelines showing that DecisionDx-Melanoma met level IIB).
|
||||
|
•
|
Level of evidence ranking using AAD criteria was IIA.
|
||||
|
Appropriate Use Criteria for the Integration of Diagnostic and Prognostic Gene Expression Profile Assays into the Management of Cutaneous Malignant Melanoma: An Expert Panel Consensus-Based Modified Delphi Process Assessment
|
SKIN: The Journal of Cutaneous Medicine, September 2019 (Systematic literature review (censor date 2018) by expert consensus panel using Strength of Recommendation Taxonomy (SORT) methodology followed by modified Delphi technique for ACE recommendations)
|
•
|
Eight clinical use recommendation received SORT strength ranking of A or B, including use in SLN biopsy eligible patients with T1 or T2 melanomas and guiding subsequent treatment recommendation in patients with T1, T2 and T3 melanomas.
|
||
|
Integrating Skin Cancer-
Related Technologies into
Clinical Practice
|
Dermatol Clinics 2017 (using Oxford Centre for Evidence-Based Medicine criteria, the eleven member Melanoma Evolving Diagnostic Technologies Integration Group reviewed 49 articles published between Jan 1, 2012 and June 15, 2015 and using a modified Delphi technique developed an algorithm to effectively integrate technologies into melanoma diagnosis and early management)
|
•
|
Algorithm recommended the use of DecisionDx-Melanoma to guide SLNB discussions and subsequent follow-up management.
|
||
|
Analytical Validity
|
|||||
|
Analytic validity of DecisionDx-Melanoma, a proprietary GEP test, for determining metastatic risk in melanoma patients
|
Diagnostic Pathology, February 2018
(Inter-assay, inter-instrument, and inter-observer analysis; technical reliability in clinically tested melanoma specimens)
|
•
|
Inter-assay concordance on 168 specimens was 99% and matched probability scores were significantly correlated (R2 = 0.96); inter-instrument concordance was 95% and matched probability scores were correlated (R2=0.99; p < 0.001).
|
||
|
•
|
A technical success rate of 98% was achieved for the test in 7,023 clinical cases.
|
||||



|
Study
|
Peer-Reviewed Publications
(Methods)
|
Main Findings
|
|||
|
Clinical Validity
|
|||||
|
Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma
|
Ophthalmology, August 2012 (Multicenter, prospective, n=446)
|
•
|
DecisionDx-UM Class 1 was the only significant predictor of MFS (p<0.0001) in multivariate analysis, compared to patient age, ciliary body involvement, tumor thickness, tumor diameter, cell type, and chromosome 3 status.
|
||
|
•
|
NPV for Class 1 for MFS was 99%
|
||||
|
•
|
MFS rates as 50-months were 97% for Class 1 and 20% for Class 2 patients (p<0.0001).
|
||||
|
Do largest basal tumor diameter and the American Joint Committee on Cancer’s cancer staging influence prognostication by gene expression profiling in choroidal melanoma
|
American Journal of Ophthalmology, November 2018
(Two-centers, retrospective, consecutively tested patients; n=293)
|
•
|
NPV for Class 1A for MFS was 99%.
|
||
|
•
|
Three-year MFS rates were 99% for Class 1A, 90% for Class 1B, and 60% for Class 2 patients.
|
||||
|
•
|
DecisionDx-UM was a significant predictor of metastasis in multivariate analysis (p=0.001) with increasing largest basal diameter.
|
||||
|
Clinical performance and management outcomes with DecisionDx-UM in a prospective multi-center study
|
Journal of Oncology, June 2016
(Multicenter, prospective; n=70)
|
•
|
NPV for Class 1 for MFS was 95%
|
||
|
•
|
Three-year MFS rates were 100% for Class 1 and 63% for Class 2 patients (p=0.003).
|
||||
|
•
|
DecisionDx-UM was the most significant predictor of metastasis in multivariate analysis (p=0.016) compared to age, largest basal diameter, ciliary body involvement, and tumor thickness.
|
||||
|
Uveal melanoma: molecular pattern, clinical features, and radiation response
|
American Journal of Ophthalmology, August 2012
(Single-center, retrospective; n=197)
|
•
|
5-year disease specific survival, or DFS, rates were 93% for Class 1 patients and 38% for Class 2 patients (p<0.0001).
|
||
|
•
|
DecisionDx-UM Class 1 was the only significant predictor of DFS (p<0.0001) in multivariate analysis compared with cell type, age of patient at radiation, and pretreatment ultrasound measurement.
|
||||
|
•
|
DecisionDx-UM Class 1 was the only significant predictor disease-specific mortality (p<0.0001) in multivariate analysis with cell type, age of patient at radiation, and pretreatment ultrasound measurement.
|
||||
|
An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma
|
Journal of Molecular Diagnostics, July 2010
(Single-center, prospective (outcomes); n=172)
|
•
|
MFS rates were significantly different between Class 1 and Class 2 patients (p<0.0001).
|
||
|
Study
|
Peer-Reviewed Publications
(Methods)
|
Main Findings
|
|||
|
Uveal melanoma: from lesion size and cell type to molecular class
|
Canadian Journal of Ophthalmology, June 2012
(Independent, meta-analysis)
|
•
|
DecisionDx-UM had the strongest predictive value for UM metastasis and mortality.
|
||
|
Gene expression profiling and PRAME status versus tumor-node-metastasis staging for prognostication in uveal melanoma
|
American Journal of Ophthalmology, November 2018
(Single-center, retrospective consecutively tested patients; n=240)
|
•
|
DecisionDx-UM was the most significant predictor of metastasis in multivariate analysis (p<0.0001) with PRAME status, TNM stage, and gender.
|
||
|
•
|
DecisionDx-UM was the most significant predictor of metastasis in multivariate analysis (p<0.0001) with PRAME status, largest basal diameter, tumor thickness, ciliary body involvement, and gender.
|
||||
|
Prognostic implications of tumor diameter in association with gene expression profile for uveal melanoma
|
JAMA Ophthalmology, July 2016
(Independent, retrospective, two centers, n=580 patients)
|
•
|
NPV for Class 1 of MSS was 96%.
|
||
|
•
|
DecisionDx-UM was the most significant predictor of metastasis (p<0.001) in multivariate analysis compared to age, sex, tumor thickness, largest basal diameter, ciliary body involvement, and cell type.
|
||||
|
Driver mutations in uveal melanoma: Associations with gene expression profile and patient outcomes
|
JAMA Ophthalmology, July 2016
(Independent, retrospective, single center, n=81 patients)
|
•
|
DecisionDx-UM was the most significant predictor of metastasis (p<0.001) in multivariate analysis compared to age, sex, ciliary body involvement, cell type, extraocular extension, largest basal diameter, tumor thickness, and mutational status.
|
||
|
•
|
DecisionDx-UM was the most significant predictor of melanoma-specific death (p<0.001) compared to age, sex, ciliary body involvement, cell type, extraocular extension, largest basal diameter, tumor thickness, and mutational status.
|
||||
|
Independent prognostic significance of gene expression profile class and largest basal diameter of posterior uveal melanomas
|
American Journal of Ophthalmology, February 2016
(Single-center, prospective; n=299)
|
•
|
DecisionDx-UM was the most significant prognostic factor for UM mortality (p=0.0019) in multivariate analysis.
|
||
|
•
|
NPV for Class 1 for DFS was 95%.
|
||||
|
•
|
Five-year disease-specific survival rates were 92% for Class 1 patients and 55% for Class 2 patients (p=0.005).
|
||||
|
Sufficiency of FNAB aspirates of posterior uveal melanoma for cytologic versus GEP classification in 159 patients, and relative prognostic significance of these classifications
|
Graefes Archives in Clinical Experimental Ophthalmology, January 2014
(Prospective; n=159)
|
•
|
NPV for Class 1 for DFS was 95%.
|
||
|
•
|
Five-year disease-specific survival rates were 92% for Class 1 patients and 55% for Class patients (p=0.005).
|
||||
|
Clinical Utility
|
|||||
|
Clinical performance and management outcomes with the DecisionDx-UM GEP test in a prospective multi-center study
|
Journal of Oncology, June 2016
(Multi-center, prospective; n=70)
|
•
|
81% of Class 1 patients were prescribed low-intensity surveillance schedules (annual metastatic screening) compared to 0% of Class 2 patients.
|
||
|
•
|
100% of Class 2 patients were prescribed high intensity surveillance schedules (quarterly-biannual metastatic screening) compared to 19% of Class 1 patients (p<0.001).
|
||||
|
Current clinical practice: differential Management of Uveal Melanoma in the Era of Molecular Tumor Analyses
|
Clinical Ophthalmology, December 2014
(Multi-center, chart review, pre-test post-test methodology; n=88)
|
•
|
100% of Medicare-eligible Class 1 patients had low-intensity surveillance schedules.
|
||
|
•
|
100% of Medicare-eligible Class 2 patients had high-intensity surveillance schedules (p<0.0001).
|
||||
|
Risk-stratified systemic surveillance in uveal melanoma
|
British Journal of Opthalmology, January 2019
(Single-center, chart review; n= 107 consecutively diagnosed patients; 68 with GEP testing)
|
•
|
100% of metastases occurred in the Class 2 group.
|
||
|
•
|
The only significant predictor of intensity of the surveillance protocol was high risk Class 2 (p<0.01 for all analyses).
|
||||
|
Study
|
Peer-Reviewed Publications
(Methods)
|
Main Findings
|
|||
|
Analytical Validity
|
|||||
|
Gene Expression Profiling in Uveal Melanoma: Technical Reliability and Correlation and Correlation of Molecular Class with Pathologic Characteristics. Diagnostic Pathology
|
Diagnostic Pathology, August 2017
(Technical reliability studies and technical success in clinical testing (n=5,516))
|
•
|
Intra-assay, inter-assay (short-term and long-term), and inter-laboratory concordance experiments demonstrated 100% Class concordance and a high degree of correlation between discriminant scores.
|
||
|
•
|
Inter-instrument/operator concordance was 96% between Class 1 and 2.
|
||||
|
•
|
96.3% technical success was achieved on fine-needle aspiration biopsy (FNAB) and formalin-fixed paraffin-embedded (FFPE) specimens in clinical testing.
|
||||
|
An Accurate, Clinically Feasible Multi-Gene Expression Assay for Predicting Metastasis in Uveal Melanoma
|
Journal of Molecular Diagnostics, July 2010
(Technical reliability studies)
|
•
|
Technical success was 95% in 609 samples.
|
||
|
•
|
DecisionDx-UM was successful in 50/51 FNABs that had insufficient quantity for cytology.
|
||||
|
•
|
100% Class concordance in tissue from FNABs and matched fresh-frozen tumor slices.
|
||||
|
•
|
31/32 (97%) Class concordance in multiple tumor sections from 7 tumors.
|
||||


|
•
|
Proprietary, disciplined approach to genomic analysis including the use of proprietary deep learning, machine learning and other techniques to identify and optimize gene selection and algorithmic approaches to answer the clinically important questions with accuracy tests. This involves the ability to design and efficiently conduct the right clinical studies at the right time;
|
|
•
|
Research and development investments to document the quality, quantity, consistency and strength of the clinical validity data, the impact our products have on clinical use, and demonstration of net health outcome improvement that reduce health system costs;
|
|
•
|
Maintaining a strong reputation with the treating physician by providing consistent, transparent, clinically relevant information that will improve the appropriate management of their patients;
|
|
•
|
Ease of use in accessing our products, reimbursement support for the physician and their patient and laboratory reports that clearly communicate the clinically relevant data points;
|
|
•
|
Demonstrated ability to work with, and secure coverage and reimbursement from, governmental and commercial payors;
|
|
•
|
Ability to efficiently commercialize pipeline products to the same customer base as our current products.
|
|
Commercial Focus
|
Number of Issued Patents
and Pending Patent
Applications
|
||
|
Methods for predicting risk of metastasis in cutaneous melanoma
|
16
|
|
|
|
Compositions and methods for detecting cancer metastasis
|
4
|
|
|
|
Methods of diagnosing and treating patients with cutaneous squamous cell carcinoma
|
2
|
|
|
|
Method for predicting risk of metastasis
|
2
|
|
|
|
Method of predicting risk for recurrence for soft tissue sarcoma
|
1
|
|
|
|
•
|
the device may not be shown safe or effective to the FDA’s satisfaction;
|
|
•
|
the data from pre-clinical studies and/or clinical trials may be found unreliable or insufficient to support approval;
|
|
•
|
the manufacturing process or facilities may not meet applicable requirements; and
|
|
•
|
changes in FDA approval policies or adoption of new regulations may require additional data.
|
|
•
|
denial of payment for the services provided in violation of the prohibition;
|
|
•
|
refunds of amounts collected by an entity in violation of the Stark Law;
|
|
•
|
a civil penalty for each bill or claim for a service arising out of the prohibited referral;
|
|
•
|
the imposition of up to three times the amounts for each item or service wrongfully claimed;
|
|
•
|
possible exclusion from federal healthcare programs, including Medicare and Medicaid; and
|
|
•
|
a civil penalty for each arrangement or scheme that the parties know (or should know) has the principal purpose of circumventing the Stark Law’s prohibition.
|
|
•
|
increase our sales and marketing efforts for DecisionDx-Melanoma and address competitive developments;
|
|
•
|
fund ongoing development of our pipeline products, including for SCC and suspicious pigmented lesions, in addition to other programs in development;
|
|
•
|
expand our laboratory testing facility and related testing capacity;
|
|
•
|
expand our technologies into other types of skin cancer management and detection products;
|
|
•
|
acquire, license or invest in technologies;
|
|
•
|
acquire or invest in complementary businesses or assets; and
|
|
•
|
finance capital expenditures and general and administrative expenses.
|
|
•
|
our ability to achieve revenue growth;
|
|
•
|
our rate of progress in establishing payor coverage and reimbursement arrangements with third-party payors;
|
|
•
|
our rate of progress in, and cost of the sales, marketing, coverage and reimbursement activities associated with, establishing adoption of DecisionDx-Melanoma, among our other products;
|
|
•
|
the cost of expanding our laboratory operations and offerings, including our sales, marketing, coverage and reimbursement efforts;
|
|
•
|
our rate of progress in, and cost of research and development activities associated with, diagnostic products in research and early development;
|
|
•
|
the potential cost of, and delays in, the development of new products as a result of changes in regulatory oversight applicable to our products; and
|
|
•
|
the effect of competing technological and market developments.
|
|
•
|
differences between the billing rates and reimbursement rates for our products;
|
|
•
|
compliance with complex federal and state regulations related to billing government healthcare programs, including Medicare, Medicaid and TRICARE;
|
|
•
|
risk of government audits related to billing;
|
|
•
|
disputes among payors as to which party is responsible for payment;
|
|
•
|
differences in coverage and information and billing requirements among payors, including the need for prior authorization and/or advanced notification;
|
|
•
|
the effect of patient co-payments or co-insurance and our ability to collect such payments from patients;
|
|
•
|
changes to billing codes used for our products;
|
|
•
|
changes to requirements related to our current or future clinical studies, including our registry studies, which can affect eligibility for payment;
|
|
•
|
ongoing monitoring provisions of LCDs for our products, which can affect the circumstances under which a claim would be considered medically necessary;
|
|
•
|
incorrect or missing billing information; and
|
|
•
|
the resources required to manage the billing and claims appeals process.
|
|
•
|
our ability to increase awareness of our products through successful clinical utility and validity studies;
|
|
•
|
the rate of adoption of our products by physicians and other healthcare providers;
|
|
•
|
our ability to achieve guideline inclusion for our products;
|
|
•
|
the timeliness with which we can provide our clinical reports to the ordering physician;
|
|
•
|
the timing and scope of any regulatory approval for our products, if such approvals become required, and maintaining ongoing compliance with regulatory requirements;
|
|
•
|
our ability to obtain and maintain positive coverage decisions for our products from government and commercial payors;
|
|
•
|
our ability to obtain and maintain adequate reimbursement from third-party payors, including Medicare, Medicare Advantage plans, United Healthcare and BlueCross BlueShield plans, which accounted for an aggregate of approximately 90% and 83% of our total revenue for the years ended December 31, 2019 and 2018, respectively;
|
|
•
|
the impact of our investments in research and development and commercial growth;
|
|
•
|
negative publicity regarding our or our competitors’ products resulting from scientific publications, or defects or errors in the products; and
|
|
•
|
our ability to further validate our products through clinical research and accompanying publications.
|
|
•
|
federal and state laws applicable to test ordering, documentation of tests ordered, billing practices and claims payment and/or regulatory agencies enforcing those laws and regulations;
|
|
•
|
federal and state fraud and abuse laws;
|
|
•
|
federal and state laboratory anti-mark-up laws;
|
|
•
|
coverage and reimbursement levels by Medicare, Medicaid, other governmental payors and private insurers;
|
|
•
|
restrictions on coverage of and reimbursement for tests;
|
|
•
|
federal and state laws governing laboratory testing, including CLIA, and state licensing laws;
|
|
•
|
federal and state laws and enforcement policies governing the development, use and distribution of diagnostic medical devices, including LDTs;
|
|
•
|
federal, state and local laws governing the handling and disposal of medical and hazardous waste;
|
|
•
|
federal and state Occupational Safety and Health Administration rules and regulations; and
|
|
•
|
the Health Insurance Portability and Accountability Act of 1996, or HIPAA, and similar state data privacy laws.
|
|
•
|
the federal Anti-Kickback Statute, or the AKS, which prohibits, among other things, any person or entity from knowingly and willfully soliciting, receiving, offering or paying any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of an item or service reimbursable, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs. The term ‘‘remuneration’’ has been broadly interpreted to include anything of value, such as specimen collection materials or test kits. There are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, however these are drawn narrowly. Additionally, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. Violations are subject to civil and criminal fines and monetary penalties of up to $100,000 for each violation, plus up to three times the remuneration involved, imprisonment of up to ten years and exclusion from government healthcare programs. In addition, the ACA codified case law that a claim including items or services resulting from a violation of the AKS constitutes a false or fraudulent claim for purposes of the federal False Claims Act, or the FCA;
|
|
•
|
the Stark Law, which prohibits a physician from making a referral for certain designated health services covered by the Medicare or Medicaid program, including laboratory and pathology services, if the physician or an immediate family member of the physician has a financial relationship with the entity providing the designated health services and prohibits that entity from billing, presenting or causing to be presented a claim for the designated health services furnished pursuant to the prohibited referral, unless an exception applies. Sanctions for violating the Stark Law include denial of payment, civil monetary penalties and exclusion from the federal health care programs. Failure to refund amounts received as a result of a prohibited referral on a timely basis may constitute a false or fraudulent claim and may result in civil penalties and additional penalties under the FCA;
|
|
•
|
federal civil and criminal false claims laws and civil monetary penalty laws, such as the FCA, which can be enforced by private citizens through civil qui tam actions, prohibits individuals or entities from, among other things, knowingly presenting, or causing to be presented through distribution of template medical necessity language or other coverage and reimbursement information, false, fictitious or fraudulent claims for payment or approval by the federal government, including federal health care programs, such as Medicare and Medicaid, and knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim, or knowingly making a false statement to improperly avoid, decrease or conceal an obligation to pay money to the federal government. In addition, a claim including items or services resulting from a violation of the AKS constitutes a false or fraudulent
|
|
•
|
HIPAA, which, among other things, imposes criminal liability for executing or attempting to execute a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation, in connection with the delivery of or payment for healthcare benefits, items or services. Like the AKS, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation;
|
|
•
|
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their implementing regulations, which imposes privacy, security and breach reporting obligations with respect to individually identifiable health information upon entities subject to the law, such as health plans, healthcare clearinghouses and certain healthcare providers, known as covered entities, and their respective business associates, individuals or entities that perform services for them that involve individually identifiable health information. Failure to comply with the HIPAA privacy and security standards can result in civil monetary penalties, and, in certain circumstances, criminal penalties. HITECH also created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in U.S. federal courts to enforce HIPAA and seek attorneys’ fees and costs associated with pursuing federal civil actions;
|
|
•
|
state laws that prohibit other specified practices, such as billing physicians for tests that they order or providing tests at no or discounted cost to induce physician or patient adoption; insurance fraud laws; waiving coinsurance, copayments, deductibles, and other amounts owed by patients; billing a state Medicaid program at a price that is higher than what is charged to one or more other third-party payors employing, exercising control over or splitting professional fees with licensed professionals in violation of state laws prohibiting fee splitting or the corporate practice of medicine and other professions; and
|
|
•
|
federal and state consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers;
|
|
•
|
the federal transparency requirements under the Physician Payments Sunshine Act, created under the ACA, which requires, among other things, certain manufacturers of drugs, devices, biologics and medical supplies reimbursed under Medicare, Medicaid, or the Children’s Health Insurance Program to annually report to CMS information related to payments and other transfers of value provided to physicians, certain other healthcare professionals, and teaching hospitals and physician ownership and investment interests, including such ownership and investment interests held by a physician’s immediate family members. Failure to submit required information may result in civil monetary penalties for all payments, transfers of value or ownership or investment interests that are not timely, accurately, and completely reported in an annual submission, and may result in liability under other federal laws or regulations. We believe that we are exempt from these reporting requirements. We cannot assure you, however, that our regulators, principally the federal government, will agree with our determination, and a determination that we have violated these laws and regulations, or a public announcement that we are being investigated for possible violations, could adversely affect our business;
|
|
•
|
the prohibition on reassignment of Medicare claims, which, subject to certain exceptions, precludes the reassignment of Medicare claims to any other part;
|
|
•
|
state and foreign law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, that may impose similar or more prohibitive restrictions, and may apply to items or services reimbursed by any non-governmental third-party payors, including private insurers; and
|
|
•
|
federal, state and foreign laws that govern the privacy and security of health information or personally identifiable information in certain circumstances, including state health information privacy and data breach notification laws which govern the collection, use, disclosure, and protection of health-related and other personal information, many of which differ from each other in significant ways and often are not pre-empted by HIPAA, thus complicating compliance efforts.
|
|
•
|
others may be able to develop and/or practice technology that is similar to our technology or aspects of our technology, but that are not covered by the claims of the patents that we own or control, assuming such patents have issued or do issue;
|
|
•
|
we or our licensors or any future strategic partners might not have been the first to conceive or reduce to practice the inventions covered by the issued patents or pending patent applications that we own or have exclusively licensed;
|
|
•
|
we or our licensors or any future strategic partners might not have been the first to file patent applications covering certain of our inventions;
|
|
•
|
others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights;
|
|
•
|
it is possible that our pending patent applications will not lead to issued patents;
|
|
•
|
issued patents that we own or have exclusively licensed may not provide us with any competitive advantage, or may be held invalid or unenforceable, as a result of legal challenges by our competitors;
|
|
•
|
our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive tests for sale in our major commercial markets;
|
|
•
|
third parties performing manufacturing or testing for us using our products or technologies could use the intellectual property of others without obtaining a proper license;
|
|
•
|
parties may assert an ownership interest in our intellectual property and, if successful, such disputes may preclude us from exercising exclusive rights over that intellectual property;
|
|
•
|
we may not develop or in-license additional proprietary technologies that are patentable;
|
|
•
|
we may not be able to obtain and maintain necessary licenses on commercially reasonable terms, or at all; and
|
|
•
|
the patents of others may have an adverse effect on our business.
|
|
•
|
we may initiate litigation or other proceedings against third parties seeking to invalidate the patents held by those third parties or to obtain a judgment that our products or technologies do not infringe those third parties’ patents;
|
|
•
|
we may participate at substantial cost in International Trade Commission proceedings to abate importation of products that would compete unfairly with our products or technologies;
|
|
•
|
if a competitor files patent applications that claim technology also claimed by us or our licensors, we or our licensors may be required to participate in interference, derivation or opposition proceedings to determine the priority of invention, which could jeopardize our patent rights and potentially provide a third party with a dominant patent position;
|
|
•
|
if third parties initiate litigation claiming that our products or technologies infringe their patent or other intellectual property rights, we will need to defend against such proceedings;
|
|
•
|
if third parties initiate litigation or other proceedings seeking to invalidate patents owned by or licensed to us or to obtain a declaratory judgment that their products, services, or technologies do not infringe our patents or patents licensed to us, we will need to defend against such proceedings;
|
|
•
|
we may be subject to ownership disputes relating to intellectual property, including disputes arising from conflicting obligations of consultants or others who are involved in developing our products and technologies; and
|
|
•
|
if a license to necessary technology is terminated, the licensor may initiate litigation claiming that our products or technologies infringe or misappropriate its patent or other intellectual property rights and/or that we breached our obligations under the license agreement, and we would need to defend against such proceedings.
|
|
•
|
incur substantial monetary liability for infringement or other violations of intellectual property rights, which we may have to pay if a court decides that the diagnostic test or technology at issue infringes or violates the third party’s rights, and if the court finds that the infringement was willful, we could be ordered to pay treble damages and the third party’s attorneys’ fees;
|
|
•
|
stop manufacturing, offering for sale, selling, using, importing, exporting or licensing the diagnostic test or technology incorporating the allegedly infringing technology or stop incorporating the allegedly infringing technology into such test or technology;
|
|
•
|
obtain from the owner of the infringed intellectual property right a license, which may require us to pay substantial upfront fees or royalties to sell or use the relevant technology and which may not be available on commercially reasonable terms, or at all;
|
|
•
|
redesign our products and technologies so they do not infringe or violate the third party’s intellectual property rights, which may not be possible or may require substantial monetary expenditures and time;
|
|
•
|
enter into cross-licenses with applicable third party, which could weaken our overall intellectual property position;
|
|
•
|
lose the opportunity to license our technology to others or to collect royalty payments based upon successful protection and assertion of our intellectual property against others;
|
|
•
|
find alternative suppliers for non-infringing technologies, which could be costly and create significant delay; or
|
|
•
|
relinquish rights associated with one or more of our patent claims, if our claims are held invalid or otherwise unenforceable.
|
|
•
|
the scope of rights granted under the license agreement and other interpretation-related issues;
|
|
•
|
the extent to which our products, technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement;
|
|
•
|
the sublicensing of patent and other rights under our collaborative development relationships;
|
|
•
|
our diligence obligations under the license agreement and what activities satisfy those diligence obligations;
|
|
•
|
the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and
|
|
•
|
the priority of invention of patented technology.
|
|
•
|
decreased demand for our current tests any tests that we may develop, and the inability to commercialize such tests;
|
|
•
|
injury to our reputation and significant negative media attention;
|
|
•
|
reluctance of experts willing to conduct our clinical studies;
|
|
•
|
initiation of investigations by regulators;
|
|
•
|
significant costs to defend the related litigation and diversion of management’s time and our resources;
|
|
•
|
substantial monetary awards to study subjects or patients;
|
|
•
|
product recalls, withdrawals or labeling, or marketing or promotional restrictions; and
|
|
•
|
loss of revenue.
|
|
•
|
multiple, conflicting and changing laws and regulations such as privacy regulations, tax laws, export and import restrictions, economic sanctions and embargoes, employment laws, regulatory requirements and other governmental approvals, permits and licenses;
|
|
•
|
limits in our ability to penetrate international markets if we are not able to perform tests locally;
|
|
•
|
logistics and regulations associated with shipping tissue samples, including infrastructure conditions and transportation delays;
|
|
•
|
difficulties in staffing and managing foreign operations;
|
|
•
|
failure to obtain regulatory approvals for the commercialization of our products in various countries;
|
|
•
|
complexities and difficulties in obtaining intellectual property protection and enforcing our intellectual property;
|
|
•
|
complexities associated with managing multiple payor reimbursement regimes, government payors, or patient self-pay systems;
|
|
•
|
financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our products and exposure to foreign currency exchange rate fluctuations;
|
|
•
|
natural disasters, political and economic instability, including wars, terrorism, and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions; and
|
|
•
|
regulatory and compliance risks that relate to maintaining accurate information and control over sales and distributors’ activities that may fall within the purview of the U.S. Foreign Corrupt Practices Act, or FCPA, its books and records provisions, or its anti-bribery provisions.
|
|
•
|
our operating performance and the performance of other similar companies;
|
|
•
|
our success in marketing and selling our products;
|
|
•
|
reimbursement determinations by third-party payors and reimbursement rates for our products;
|
|
•
|
changes in our projected operating results that we provide to the public, our failure to meet these projections or changes in recommendations by securities analysts that elect to follow our common stock;
|
|
•
|
regulatory or legal developments in the United States and other countries;
|
|
•
|
the level of expenses related to product development and clinical studies for our products;
|
|
•
|
our ability to achieve product development goals in the timeframe we announce;
|
|
•
|
announcements of clinical study results, regulatory developments, acquisitions, strategic alliances or significant agreements by us or by our competitors;
|
|
•
|
the success or failure of our efforts to acquire, license or develop additional tests;
|
|
•
|
recruitment or departure of key personnel;
|
|
•
|
the economy as a whole and market conditions in our industry;
|
|
•
|
trading activity by a limited number of stockholders who together beneficially own a significant percentage of our outstanding common stock;
|
|
•
|
the size of our market float; and
|
|
•
|
any other factors discussed in this Annual Report on Form 10-K.
|
|
•
|
being permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced ‘‘Management’s Discussion and Analysis of Financial Condition and Results of Operations’’ disclosure;
|
|
•
|
not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting;
|
|
•
|
not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements;
|
|
•
|
reduced disclosure obligations regarding executive compensation; and
|
|
•
|
not being required to hold a non-binding advisory vote on executive compensation or obtain stockholder approval of any golden parachute payments not previously approved.
|
|
•
|
permit our board of directors to issue up to 10,000,000 shares of preferred stock, with any rights, preferences and privileges as they may designate (including the right to approve an acquisition or other change in our control);
|
|
•
|
provide that the authorized number of directors may be changed only by resolution of the board of directors;
|
|
•
|
provide that the board of directors or any individual director may only be removed with cause and the affirmative vote of the holders of at least 66-2/3% of the voting power of all of our then outstanding common stock;
|
|
•
|
provide that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum;
|
|
•
|
divide our board of directors into three classes;
|
|
•
|
require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and not be taken by written consent;
|
|
•
|
provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide notice in writing in a timely manner and also specify requirements as to the form and content of a stockholder’s notice;
|
|
•
|
do not provide for cumulative voting rights (therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose);
|
|
•
|
provide that special meetings of our stockholders may be called only by the chairman of the board, our Chief Executive Officer or by the board of directors pursuant to a resolution adopted by a majority of the total number of authorized directors;
|
|
•
|
provide that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware (or, if and only if the Court of Chancery of the State of Delaware lacks subject matter jurisdiction, any state court located within the State of Delaware or, if and only if all such state courts lack subject matter jurisdiction, the federal district court for the District of Delaware) will be the sole and exclusive forum for the following types of actions or proceedings under Delaware statutory or common law: (i) any derivative action or proceeding brought on our behalf; (ii) any action or proceeding asserting a claim of breach of a fiduciary duty owed by any of our current or former directors, officers or other employees to us or our stockholders; (iii) any action or proceeding asserting a claim against us or any of our current or former directors, officers or other employees, arising out of or pursuant to any provision of the Delaware General Corporation Law, our certificate of incorporation or our bylaws; (iv) any action or proceeding to interpret, apply, enforce or determine the validity of our certificate of incorporation or our bylaws; (v) any action or proceeding as to which the Delaware General Corporation Law confers jurisdiction to the Court of Chancery of the State of Delaware; and (vi) any action asserting a claim against us or any of our directors, officers or other employees governed by the internal affairs doctrine, in all cases to the fullest extent permitted by law and subject to the court’s having personal jurisdiction over the indispensable parties named as defendants; provided these provisions of our amended and restated certificate of incorporation and amended and restated bylaws will not apply to suits brought to enforce a duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction; and
|
|
•
|
provide that unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act, subject to and contingent upon a final adjudication in the State of Delaware of the enforceability of such exclusive forum provision.
|
|
|
DecisionDx-
Melanoma
|
DecisionDx-UM
|
Total
|
|||||
|
Q1 2019
|
3,232
|
|
360
|
|
3,592
|
|
||
|
Q2 2019
|
3,691
|
|
376
|
|
4,067
|
|
||
|
Q3 2019
|
4,126
|
|
356
|
|
4,482
|
|
||
|
Q4 2019
|
4,480
|
|
434
|
|
4,914
|
|
||
|
For the year ended December 31, 2019
|
15,529
|
|
1,526
|
|
17,055
|
|
||
|
Q1 2018
|
2,727
|
|
322
|
|
3,049
|
|
||
|
Q2 2018
|
2,899
|
|
382
|
|
3,281
|
|
||
|
Q3 2018
|
3,136
|
|
324
|
|
3,460
|
|
||
|
Q4 2018
|
3,270
|
|
385
|
|
3,655
|
|
||
|
For the year ended December 31, 2018
|
12,032
|
|
1,413
|
|
13,445
|
|
||
|
•
|
execute clinical studies to generate evidence supporting our current and future product candidates;
|
|
•
|
execute our commercialization strategy for our current and future products;
|
|
•
|
continue our ongoing and planned development of new products;
|
|
•
|
seek to discover and develop additional product candidates;
|
|
•
|
hire additional scientific and research and development staff; and
|
|
•
|
add additional operational, financial and management information systems and personnel.
|
|
•
|
Report volume.
We believe that the number of reports we deliver to physicians is an important indicator of growth of adoption among the healthcare provider community. Our revenue and costs are affected by the volume of testing and mix of customers. Our performance depends on our ability to retain and broaden adoption with existing prescribing physicians, as well as attract new physicians.
|
|
•
|
Reimbursement.
We believe that expanding reimbursement is an important indicator of the value of our products. Payors require extensive evidence of clinical utility, clinical validity, patient outcomes and health economic benefits in order to provide reimbursement for diagnostic products. Our revenue depends on our ability to demonstrate the value of our products to these payors.
|
|
•
|
Gross margin.
We believe that our gross margin is an important indicator of the operating performance of our business. Higher gross margins reflect the average selling price of our tests, as well as the operating efficiency of our laboratory operations.
|
|
•
|
New product development.
A significant aspect of our business is our investment in research and development activities, including activities related to the development of new products. In addition to the development of new product candidates, we believe these studies are critical to gaining physician adoption of new products and driving favorable coverage decisions by payors for such products.
|
|
|
Years Ended December 31,
|
Increase (Decrease)
|
||||||||||||
|
|
2019
|
2018
|
||||||||||||
|
Net revenues
|
$
|
51,865
|
|
$
|
22,786
|
|
$
|
29,079
|
|
127.6
|
%
|
|||
|
Cost of sales
|
7,310
|
|
5,297
|
|
2,013
|
|
38.0
|
%
|
||||||
|
Gross margin
|
44,555
|
|
17,489
|
|
27,066
|
|
154.8
|
%
|
||||||
|
Operating expenses:
|
||||||||||||||
|
Research and development
|
7,385
|
|
4,854
|
|
2,531
|
|
52.1
|
%
|
||||||
|
Selling, general and administrative
|
29,842
|
|
16,471
|
|
13,371
|
|
81.2
|
%
|
||||||
|
Total operating expenses
|
37,227
|
|
21,325
|
|
15,902
|
|
74.6
|
%
|
||||||
|
Operating income (loss)
|
7,328
|
|
(3,836
|
)
|
11,164
|
|
291.0
|
%
|
||||||
|
Interest income
|
312
|
|
24
|
|
288
|
|
1,200.0
|
%
|
||||||
|
Interest expense
|
(4,571
|
)
|
(2,274
|
)
|
(2,297
|
)
|
(101.0
|
)%
|
||||||
|
Gain on extinguishment of debt
|
5,213
|
|
—
|
|
5,213
|
|
N/A
|
|
||||||
|
Other expense, net
|
(2,933
|
)
|
(272
|
)
|
(2,661
|
)
|
(978.3
|
)%
|
||||||
|
Income (loss) before income taxes
|
5,349
|
|
(6,358
|
)
|
11,707
|
|
184.1
|
%
|
||||||
|
Income tax expense
|
72
|
|
9
|
|
63
|
|
700.0
|
%
|
||||||
|
Net income (loss)
|
$
|
5,277
|
|
$
|
(6,367
|
)
|
$
|
11,644
|
|
182.9
|
%
|
|||
|
•
|
successful commencement and completion of clinical study protocols;
|
|
•
|
successful identification and acquisition of tissue samples;
|
|
•
|
the development and validation of genomic classifiers; and
|
|
•
|
acceptance of new genomic tests by physicians, patients and third-party payors.
|
|
Years Ended December 31,
|
|||||||
|
|
2019
|
2018
|
|||||
|
Term debt
|
$
|
26,688
|
|
$
|
21,350
|
|
|
|
Revolving line of credit
|
—
|
|
5,000
|
|
|||
|
Total principal amount
|
26,688
|
|
26,350
|
|
|||
|
Unamortized discount and issuance costs
|
(1,566
|
)
|
(1,850
|
)
|
|||
|
Total long-term debt
|
25,122
|
|
24,500
|
|
|||
|
Less: Current portion of long-term debt
|
(5,833
|
)
|
—
|
|
|||
|
Long-term debt, less current portion
|
$
|
19,289
|
|
$
|
24,500
|
|
|
|
|
Years Ended December 31,
|
|||||||
|
|
2019
|
2018
|
||||||
|
Net cash provided by (used in) operating activities
|
$
|
7,015
|
|
$
|
(12,295
|
)
|
||
|
Net cash used in investing activities
|
(937
|
)
|
(277
|
)
|
||||
|
Net cash provided by financing activities
|
88,288
|
|
15,839
|
|
||||
|
Net increase in cash and cash equivalents
|
$
|
94,366
|
|
$
|
3,267
|
|
||
|
•
|
Expected term
. The expected term is the period of time that granted options are expected to be outstanding. For stock options, we have set the expected term using the simplified method based on the weighted average of both the period to vesting and the period to maturity for each option, as we have concluded that our stock option exercise history does not provide a reasonable basis upon which to estimate the expected term. For the ESPP, the expected term is the period of time from the offering date to the purchase date.
|
|
•
|
Expected volatility
. Because of the limited period of time our stock has been traded in an active market, we calculate volatility by using the historical stock prices of a group of similar companies looking back over the estimated life of the option or the ESPP purchase right and averaging the volatilities of these companies.
|
|
•
|
Risk-free interest rate
. We base the risk-free interest rate used in the Black-Scholes valuation model on the market yield in effect at the time of option grant and at the offering date for ESPP provided from the Federal Reserve Board’s Statistical Releases and historical publications from the Treasury constant maturities rates for the equivalent remaining terms.
|
|
•
|
Dividend yield
. We have not paid, and do not have plans to pay, cash dividends. Therefore, we use an expected dividend yield of zero in the Black-Scholes option valuation model.
|
|
Years Ended December 31,
|
||||
|
2019
|
2018
|
|||
|
Average expected term
|
6 years
|
6 years
|
||
|
Expected stock price volatility
|
56.74% - 60.17%
|
57.77% - 58.29%
|
||
|
Risk-free interest rate
|
1.63% - 2.47%
|
2.77% - 3.13%
|
||
|
Dividend yield
|
—%
|
—%
|
||
|
Years Ended December 31,
|
||||
|
2019
|
2018
|
|||
|
Average expected term
|
1.2 years
|
Not applicable
|
||
|
Expected stock price volatility
|
61.12% - 68.89%
|
Not applicable
|
||
|
Risk-free interest rate
|
1.56% - 1.80%
|
Not applicable
|
||
|
Dividend yield
|
—%
|
Not applicable
|
||
|
•
|
Hired a full-time director of SEC reporting and technical accounting, a certified public accountant with an active license, with public company reporting experience to provide oversight and technical expertise with respect to
|
|
•
|
Began enhancement of our information technology tools to improve the efficiency of our processes with respect to revenue recognition under ASC 606;
|
|
•
|
Engaged a third-party consultant to assist us with formalizing our internal control documentation and implementation of enhancements to our internal control over financial reporting; and
|
|
•
|
Designed and implemented a new process control and review procedure with respect to manual journal entries.
|
|
Exhibit Number
|
Description of document
|
|
|
3.1
|
||
|
3.2
|
||
|
4.1*
|
||
|
4.2
|
||
|
4.3
|
||
|
4.4
|
||
|
4.5
|
||
|
4.6
|
||
|
4.7
|
||
|
4.8
|
||
|
4.9
|
||
|
4.10
|
||
|
10.1+
|
||
|
10.2+
|
||
|
Exhibit Number
|
Description of document
|
|
|
10.3+
|
||
|
10.4+
|
||
|
10.5+
|
||
|
10.6+
|
||
|
10.7+
|
||
|
10.8+
|
||
|
10.9+
|
||
|
10.10+
|
||
|
10.11+
|
||
|
10.12+
|
||
|
10.13+
|
||
|
10.14
|
||
|
10.15*
|
||
|
10.16
|
||
|
10.17
|
||
|
10.18
|
||
|
10.19
|
||
|
10.20#
|
||
|
10.21#
|
||
|
Exhibit Number
|
Description of document
|
|
|
10.22#
|
||
|
10.23+
|
||
|
23.1*
|
||
|
31.1*
|
||
|
31.2*
|
||
|
32.1**
|
||
|
32.2**
|
||
|
101.INS*
|
XBRL Instance Document.
|
|
|
101.SCH*
|
XBRL Taxonomy Extension Schema Document.
|
|
|
101.CAL*
|
XBRL Taxonomy Extension Calculation Linkbase Document.
|
|
|
101.DEF*
|
XBRL Taxonomy Extension Definition Linkbase Document.
|
|
|
101.LAB*
|
XBRL Taxonomy Extension Label Linkbase Document.
|
|
|
101.PRE*
|
XBRL Taxonomy Extension Presentation Linkbase Document.
|
|
|
*
|
Filed herewith
|
|
**
|
Furnished herewith.
|
|
+
|
Indicates management contract or compensatory plan.
|
|
#
|
Certain portions of this exhibit (indicated by “[***]”) have been omitted as we have determined (i) the omitted information is not material and (ii) the omitted information would likely cause harm to us if publicly disclosed.
|
|
|
CASTLE BIOSCIENCES, INC.
|
|||
|
|
|
|
||
|
By:
|
/s/ Derek J. Maetzold
|
|||
|
|
Derek J. Maetzold
President and Chief Executive Officer
(Principal Executive Officer)
|
|||
|
SIGNATURE
|
TITLE
|
DATE
|
||
|
/s/ Derek J. Maetzold
|
President, Chief Executive Officer and Director
|
March 10, 2020
|
||
|
(Derek J. Maetzold)
|
(
Principal Executive Officer
)
|
|||
|
/s/ Frank Stokes
|
Chief Financial Officer
|
March 10, 2020
|
||
|
(Frank Stokes)
|
(
Principal Financial and Accounting Officer
)
|
|||
|
/s/ Daniel M. Bradbury
|
Chairman of the Board of Directors
|
March 10, 2020
|
||
|
(Daniel M. Bradbury)
|
||||
|
/s/ Bonnie H. Anderson
|
Member of the Board of Directors
|
March 10, 2020
|
||
|
(Bonnie H. Anderson)
|
||||
|
/s/ Mara G. Aspinall
|
Member of the Board of Directors
|
March 10, 2020
|
||
|
(Mara G. Aspinall)
|
||||
|
/s/ G. Bradley Cole
|
Member of the Board of Directors
|
March 10, 2020
|
||
|
(G. Bradley Cole)
|
||||
|
/s/ Joseph C. Cook III
|
Member of the Board of Directors
|
March 10, 2020
|
||
|
(Joseph C. Cook III)
|
||||
|
/s/ David Kabakoff, Ph.D.
|
Member of the Board of Directors
|
March 10, 2020
|
||
|
(David Kabakoff, Ph.D.)
|
||||
|
CASTLE BIOSCIENCES, INC.
BALANCE SHEETS
(in thousands, except share and per share data)
|
|||||||
|
December 31,
|
|||||||
|
2019
|
2018
|
||||||
|
ASSETS
|
|||||||
|
Current Assets
|
|
|
|
|
|||
|
Cash and cash equivalents
|
$
|
98,845
|
|
$
|
4,479
|
|
|
|
Accounts receivable, net
|
14,648
|
|
12,090
|
|
|||
|
Inventory
|
1,237
|
|
882
|
|
|||
|
Prepaid expenses and other current assets
|
1,951
|
|
675
|
|
|||
|
Total current assets
|
116,681
|
|
18,126
|
|
|||
|
Long-term accounts receivable, net
|
870
|
|
2,532
|
|
|||
|
Property and equipment, net
|
2,060
|
|
1,529
|
|
|||
|
Intangible assets, net
|
—
|
|
4
|
|
|||
|
Other assets – long-term
|
135
|
|
214
|
|
|||
|
Total assets
|
$
|
119,746
|
|
$
|
22,405
|
|
|
|
LIABILITIES, CONVERTIBLE PREFERRED STOCK AND
STOCKHOLDERS’ EQUITY (DEFICIT)
|
|||||||
|
Current Liabilities
|
|||||||
|
Accounts payable
|
$
|
1,865
|
|
$
|
1,451
|
|
|
|
Accrued compensation
|
5,779
|
|
4,571
|
|
|||
|
Other accrued liabilities
|
1,812
|
|
715
|
|
|||
|
Current portion of long-term debt
|
5,833
|
|
—
|
|
|||
|
Total current liabilities
|
15,289
|
|
6,737
|
|
|||
|
Long-term debt
|
19,289
|
|
24,500
|
|
|||
|
Preferred stock warrant liability
|
—
|
|
1,194
|
|
|||
|
Deferred rent liability
|
55
|
|
44
|
|
|||
|
Total liabilities
|
34,633
|
|
32,475
|
|
|||
|
Commitments and Contingencies (Note 11)
|
|
|
|
|
|||
|
Convertible Preferred Stock
|
|||||||
|
Convertible preferred stock Series C, $0.001 par value, zero and 503,056 shares authorized as of December 31, 2019 and 2018, respectively; zero and 503,056 shares issued and outstanding as of December 31, 2019 and 2018, respectively; $0 and $2,417 aggregate liquidation preference as of December 31, 2019 and 2018, respectively.
|
—
|
|
1,501
|
|
|||
|
Redeemable convertible preferred stock Series A, B, D, E-1, E-2, E-2A, E-3 and F, $0.001 par value, zero and 9,640,493 shares authorized as of December 31, 2019 and 2018, respectively; zero and 9,456,775 shares issued and outstanding as of December 31, 2019 and 2018, respectively; $0 and $57,570 aggregate liquidation preference as of December 31, 2019 and 2018, respectively.
|
—
|
|
44,995
|
|
|||
|
Stockholders’ Equity (Deficit)
|
|||||||
|
Common stock, $0.001 par value; 200,000,000 and 15,102,000 shares authorized as of December 31, 2019 and 2018, respectively; 17,130,907 and 1,916,224 shares issued and outstanding as of December 31, 2019 and 2018, respectively.
|
17
|
|
2
|
|
|||
|
Preferred stock, $0.001 par value; 10,000,000 and zero shares authorized as of December 31, 2019 and 2018, respectively; no shares issued and outstanding as of December 31, 2019 and 2018.
|
—
|
|
—
|
|
|||
|
Additional paid-in capital
|
137,308
|
|
921
|
|
|||
|
Accumulated deficit
|
(52,212
|
)
|
(57,489
|
)
|
|||
|
Total stockholders’ equity (deficit)
|
85,113
|
|
(56,566
|
)
|
|||
|
Total liabilities, convertible preferred stock and stockholders’ equity (deficit)
|
$
|
119,746
|
|
$
|
22,405
|
|
|
|
CASTLE BIOSCIENCES, INC.
STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME (LOSS)
(in thousands, except per share data)
|
||||||||
|
Years Ended December 31,
|
||||||||
|
2019
|
2018
|
|||||||
|
NET REVENUES
|
$
|
51,865
|
|
$
|
22,786
|
|
||
|
COST OF SALES
|
7,310
|
|
5,297
|
|
||||
|
Gross margin
|
44,555
|
|
17,489
|
|
||||
|
OPERATING EXPENSES
|
||||||||
|
Research and development
|
7,385
|
|
4,854
|
|
||||
|
Selling, general and administrative
|
29,842
|
|
16,471
|
|
||||
|
Total operating expenses
|
37,227
|
|
21,325
|
|
||||
|
Operating income (loss)
|
7,328
|
|
(3,836
|
)
|
||||
|
Interest income
|
312
|
|
24
|
|
||||
|
Interest expense
|
(4,571
|
)
|
(2,274
|
)
|
||||
|
Gain on extinguishment of debt (Note 7)
|
5,213
|
|
—
|
|
||||
|
Other expense, net
|
(2,933
|
)
|
(272
|
)
|
||||
|
Income (loss) before income taxes
|
5,349
|
|
(6,358
|
)
|
||||
|
Income tax expense
|
72
|
|
9
|
|
||||
|
Net income (loss) and comprehensive income (loss)
|
5,277
|
|
(6,367
|
)
|
||||
|
Convertible preferred stock cumulative dividends
|
2,156
|
|
3,577
|
|
||||
|
Accretion of redeemable convertible preferred stock to redemption value
|
130
|
|
219
|
|
||||
|
Net income (loss) and comprehensive income (loss) attributable to common stockholders
|
$
|
2,991
|
|
$
|
(10,163
|
)
|
||
|
Earnings (loss) per share attributable to common stockholders:
|
||||||||
|
Basic
|
$
|
0.35
|
|
$
|
(5.33
|
)
|
||
|
Diluted
|
$
|
(0.21
|
)
|
$
|
(5.33
|
)
|
||
|
Weighted-average shares outstanding:
|
||||||||
|
Basic
|
8,584
|
|
1,906
|
|
||||
|
Diluted
|
8,658
|
|
1,906
|
|
||||
|
CASTLE BIOSCIENCES, INC.
STATEMENTS OF CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY (DEFICIT)
(in thousands, except shares)
|
||||||||||||||||||||||||||||||||||||||||
|
Convertible
Preferred
Stock Series C
|
Redeemable
Convertible
Preferred Stock
Series A, B, D, E-1,
E-2, E-2A, E-3 and F
|
Common Stock
|
Preferred Stock
|
Additional
Paid-in
Capital
|
Accumulated
Deficit
|
Total
Stockholders’
Equity (Deficit)
|
||||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||||||||||
|
BALANCE, JANUARY 1, 2018
|
503,056
|
|
$
|
1,501
|
|
7,611,010
|
|
$
|
34,538
|
|
1,896,469
|
|
$
|
2
|
|
—
|
|
$
|
—
|
|
$
|
809
|
|
$
|
(51,122
|
)
|
$
|
(50,311
|
)
|
|||||||||||
|
Stock compensation expense
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
294
|
|
—
|
|
294
|
|
||||||||||||||||||
|
Exercise of common stock options
|
—
|
|
—
|
|
—
|
|
—
|
|
19,755
|
|
—
|
|
—
|
|
—
|
|
38
|
|
—
|
|
38
|
|
||||||||||||||||||
|
Issuance of Series F redeemable convertible preferred stock
|
—
|
|
—
|
|
1,809,564
|
|
9,990
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||
|
Accretion of redeemable convertible preferred stock to redemption value:
|
||||||||||||||||||||||||||||||||||||||||
|
Series E-1
|
—
|
|
—
|
|
—
|
|
4
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(4
|
)
|
—
|
|
(4
|
)
|
||||||||||||||||||
|
Series E-2A
|
—
|
|
—
|
|
—
|
|
1
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(1
|
)
|
—
|
|
(1
|
)
|
||||||||||||||||||
|
Series E-3
|
—
|
|
—
|
|
—
|
|
12
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(12
|
)
|
—
|
|
(12
|
)
|
||||||||||||||||||
|
Series F
|
—
|
|
—
|
|
—
|
|
203
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(203
|
)
|
—
|
|
(203
|
)
|
||||||||||||||||||
|
Exercise of redeemable convertible preferred stock warrants:
|
||||||||||||||||||||||||||||||||||||||||
|
Series E-1
|
—
|
|
—
|
|
3,250
|
|
15
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||
|
Series F
|
—
|
|
—
|
|
32,951
|
|
232
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(6,367
|
)
|
(6,367
|
)
|
||||||||||||||||||
|
BALANCE, DECEMBER 31, 2018
|
503,056
|
|
$
|
1,501
|
|
9,456,775
|
|
$
|
44,995
|
|
1,916,224
|
|
$
|
2
|
|
—
|
|
$
|
—
|
|
$
|
921
|
|
$
|
(57,489
|
)
|
$
|
(56,566
|
)
|
|||||||||||
|
Stock compensation expense
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,249
|
|
—
|
|
1,249
|
|
||||||||||||||||||
|
Exercise of common stock options
|
—
|
|
—
|
|
—
|
|
—
|
|
693,140
|
|
1
|
|
—
|
|
—
|
|
1,174
|
|
—
|
|
1,175
|
|
||||||||||||||||||
|
Exercise of common stock warrants
|
—
|
|
—
|
|
—
|
|
—
|
|
51,238
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||
|
Accretion of redeemable convertible preferred stock to redemption value:
|
||||||||||||||||||||||||||||||||||||||||
|
Series E-1
|
—
|
|
—
|
|
—
|
|
2
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(2
|
)
|
—
|
|
(2
|
)
|
||||||||||||||||||
|
Series E-3
|
—
|
|
—
|
|
—
|
|
7
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(7
|
)
|
—
|
|
(7
|
)
|
||||||||||||||||||
|
Series F
|
—
|
|
—
|
|
—
|
|
121
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(121
|
)
|
—
|
|
(121
|
)
|
||||||||||||||||||
|
Exercise of redeemable convertible preferred stock warrants:
|
||||||||||||||||||||||||||||||||||||||||
|
Series E-1
|
—
|
|
—
|
|
12,999
|
|
107
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||
|
Series F
|
—
|
|
—
|
|
1,054
|
|
10
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||||
|
Recognition of beneficial conversion feature on convertible promissory notes
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
8,378
|
|
—
|
|
8,378
|
|
||||||||||||||||||
|
Extinguishment of beneficial conversion feature on convertible promissory notes
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(15,265
|
)
|
—
|
|
(15,265
|
)
|
||||||||||||||||||
|
CASTLE BIOSCIENCES, INC.
STATEMENTS OF CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY (DEFICIT) (Continued)
(in thousands, except shares)
|
||||||||||||||||||||||||||||||||||||||||
|
Convertible
Preferred
Stock Series C
|
Redeemable
Convertible
Preferred Stock
Series A, B, D, E-1,
E-2, E-2A, E-3 and F
|
Common Stock
|
Preferred Stock
|
Additional
Paid-in
Capital
|
Accumulated
Deficit
|
Total
Stockholders’
Equity (Deficit)
|
||||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||||||||||
|
Initial public offering of common stock, net of underwriting discounts, commissions and offering costs
|
—
|
|
—
|
|
—
|
|
—
|
|
4,600,000
|
|
5
|
|
—
|
|
—
|
|
65,926
|
|
—
|
|
65,931
|
|
||||||||||||||||||
|
Conversion of convertible promissory notes
|
—
|
|
—
|
|
—
|
|
—
|
|
1,661,106
|
|
1
|
|
—
|
|
—
|
|
26,576
|
|
—
|
|
26,577
|
|
||||||||||||||||||
|
Conversion of convertible preferred stock
|
(503,056
|
)
|
(1,501
|
)
|
(9,470,828
|
)
|
(45,242
|
)
|
8,181,992
|
|
8
|
|
—
|
|
—
|
|
46,735
|
|
—
|
|
46,743
|
|
||||||||||||||||||
|
Reclassification of preferred stock warrant liability and net exercise of certain warrants in connection with initial public offering
|
—
|
|
—
|
|
—
|
|
—
|
|
27,207
|
|
—
|
|
—
|
|
—
|
|
1,744
|
|
—
|
|
1,744
|
|
||||||||||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
5,277
|
|
5,277
|
|
||||||||||||||||||
|
BALANCE, DECEMBER 31, 2019
|
—
|
|
$
|
—
|
|
—
|
|
$
|
—
|
|
17,130,907
|
|
$
|
17
|
|
—
|
|
$
|
—
|
|
$
|
137,308
|
|
$
|
(52,212
|
)
|
$
|
85,113
|
|
|||||||||||
|
CASTLE BIOSCIENCES, INC.
STATEMENTS OF CASH FLOWS
(in thousands)
|
|||||||
|
|
Years Ended December 31,
|
||||||
|
|
2019
|
2018
|
|||||
|
OPERATING ACTIVITIES
|
|
|
|
||||
|
Net income (loss)
|
$
|
5,277
|
|
$
|
(6,367
|
)
|
|
|
Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities:
|
|||||||
|
Depreciation
|
354
|
|
287
|
|
|||
|
Stock compensation expense
|
1,249
|
|
294
|
|
|||
|
Amortization of intangibles
|
4
|
|
36
|
|
|||
|
Amortization of debt discounts and issuance costs
|
1,925
|
|
566
|
|
|||
|
Other non-cash interest
|
442
|
|
—
|
|
|||
|
Gain on extinguishment of debt
|
(5,213
|
)
|
—
|
|
|||
|
Change in fair value of preferred stock warrant liability
|
619
|
|
272
|
|
|||
|
Change in fair value of embedded derivative
|
237
|
|
—
|
|
|||
|
Change in fair value of convertible promissory note accounted for under the fair value option
|
2,077
|
|
—
|
|
|||
|
Other
|
—
|
|
(24
|
)
|
|||
|
Change in operating assets and liabilities:
|
|||||||
|
Accounts receivable
|
(896
|
)
|
(8,408
|
)
|
|||
|
Prepaid expenses and other current assets
|
(1,276
|
)
|
(160
|
)
|
|||
|
Inventory
|
(355
|
)
|
(578
|
)
|
|||
|
Other assets
|
(85
|
)
|
14
|
|
|||
|
Accounts payable
|
557
|
|
197
|
|
|||
|
Accrued compensation
|
1,208
|
|
1,347
|
|
|||
|
Other accrued liabilities
|
879
|
|
201
|
|
|||
|
Deferred rent liability
|
12
|
|
28
|
|
|||
|
Net cash provided by (used in) operating activities
|
7,015
|
|
(12,295
|
)
|
|||
|
INVESTING ACTIVITES
|
|||||||
|
Purchases of property and equipment
|
(937
|
)
|
(277
|
)
|
|||
|
Net cash used in investing activities
|
(937
|
)
|
(277
|
)
|
|||
|
FINANCING ACTIVITIES
|
|||||||
|
Proceeds from initial public offering of common stock, net of underwriting discounts, commissions and issuance costs
|
65,931
|
|
—
|
|
|||
|
Proceeds from issuance of preferred stock and preferred stock warrants (including exercised warrants)
|
49
|
|
10,383
|
|
|||
|
Proceeds from issuance of term debt and preferred stock warrants, net of issuance costs
|
—
|
|
4,418
|
|
|||
|
Proceeds from issuance of convertible promissory notes (including $4,756 from related parties), net of issuance costs
|
11,695
|
|
—
|
|
|||
|
Proceeds from issuance of convertible promissory note and common stock warrant, net of issuance costs
|
9,236
|
|
—
|
|
|||
|
Proceeds from issuance of term debt, net of issuance costs
|
1,776
|
|
—
|
|
|||
|
Proceeds from line of credit
|
—
|
|
1,000
|
|
|||
|
Repayments on line of credit
|
(1,791
|
)
|
—
|
|
|||
|
Proceeds from exercise of common stock options
|
1,174
|
|
38
|
|
|||
|
Proceeds from contributions to the employee stock purchase plan
|
218
|
|
—
|
|
|||
|
Net cash provided by financing activities
|
88,288
|
|
15,839
|
|
|||
|
NET CHANGE IN CASH AND CASH EQUIVALENTS
|
94,366
|
|
3,267
|
|
|||
|
Beginning of period
|
4,479
|
|
1,212
|
|
|||
|
End of period
|
$
|
98,845
|
|
$
|
4,479
|
|
|
|
CASTLE BIOSCIENCES, INC.
STATEMENTS OF CASH FLOWS (Continued)
(in thousands)
|
|||||||
|
|
Years Ended December 31,
|
||||||
|
|
2019
|
2018
|
|||||
|
SUPPLEMENTAL DISCLOSURE OF CASH PAID (REFUNDED) FOR:
|
|||||||
|
Interest
|
$
|
2,206
|
|
$
|
1,635
|
|
|
|
Income taxes
|
$
|
(150
|
)
|
$
|
160
|
|
|
|
DISCLOSURE OF NON-CASH INVESTING AND FINANCING
ACTIVITIES:
|
|||||||
|
Accrued capital expenditures
|
$
|
7
|
|
$
|
58
|
|
|
|
Initial public offering costs incurred but not paid
|
$
|
—
|
|
$
|
91
|
|
|
|
Issuance of common stock upon conversion of convertible preferred stock
|
$
|
46,743
|
|
$
|
—
|
|
|
|
Conversion of preferred stock warrants to common stock warrants
|
$
|
1,745
|
|
$
|
—
|
|
|
|
Issuance of common stock upon conversion of convertible promissory notes
|
$
|
26,578
|
|
$
|
—
|
|
|
|
Cashless exercise of common stock warrants
|
$
|
455
|
|
$
|
—
|
|
|
|
|
Percentage of Revenues
|
Percentage of
Accounts Receivable
(current)
|
Percentage of
Accounts Receivable
(non-current)
|
||||||||||||||
|
|
Year Ended December 31,
|
As of December 31,
|
As of December 31,
|
||||||||||||||
|
|
2019
|
2018
|
2019
|
2018
|
2019
|
2018
|
|||||||||||
|
Medicare
|
49
|
%
|
36
|
%
|
7
|
%
|
54
|
%
|
—
|
%
|
—
|
%
|
|||||
|
Medicare Advantage plans
|
29
|
%
|
17
|
%
|
41
|
%
|
9
|
%
|
18
|
%
|
18
|
%
|
|||||
|
United Healthcare
|
6
|
%
|
12
|
%
|
9
|
%
|
7
|
%
|
—
|
%
|
15
|
%
|
|||||
|
BlueCross BlueShield plans
|
6
|
%
|
18
|
%
|
25
|
%
|
18
|
%
|
46
|
%
|
41
|
%
|
|||||
|
|
Years Ended December 31,
|
|||||||
|
|
2019
|
2018
|
||||||
|
|
||||||||
|
Numerator:
|
||||||||
|
Net income (loss) attributable to common stockholders
|
$
|
2,991
|
|
$
|
(10,163
|
)
|
||
|
Assumed conversion of convertible promissory notes
(1)
:
|
|
|
|
|
||||
|
Subtract: Extinguishment gain
|
(5,213
|
)
|
—
|
|
||||
|
Add: Interest expense and change in fair value of embedded derivative
|
420
|
|
—
|
|
||||
|
Numerator for diluted loss per share
|
$
|
(1,802
|
)
|
$
|
(10,163
|
)
|
||
|
Denominator:
|
|
|
|
|
||||
|
Weighted-average common shares outstanding, basic
|
8,584
|
|
1,906
|
|
||||
|
Assumed conversion of convertible promissory notes
(1)
|
74
|
|
—
|
|
||||
|
Weighted-average common shares outstanding, diluted
|
8,658
|
|
1,906
|
|
||||
|
Earnings (loss) per share attributable to common stockholders:
|
||||||||
|
Basic
|
$
|
0.35
|
|
$
|
(5.33
|
)
|
||
|
Diluted
|
$
|
(0.21
|
)
|
$
|
(5.33
|
)
|
||
|
(1)
|
For the year ended December 31, 2019
, reflects the assumed conversion of the Q1 2019 Notes (as defined in Note 7) into shares of common stock beginning July 1, 2019, in accordance with the requirements in ASC 260,
Earnings per Share
, for contingently issuable shares due to the contingency not being met until the third quarter of 2019.
The July 2019 Note (as defined in Note 7), was excluded from the computation of diluted earnings (loss) per share prior to its conversion on July 29, 2019 in connection with the IPO, as disclosed below.
|
|
Years Ended December 31,
|
||||||
|
2019
|
2018
|
|||||
|
Convertible preferred stock
|
4,679
|
|
7,845
|
|
||
|
Convertible promissory note
(1)
|
33
|
|
—
|
|
||
|
Stock options
|
1,820
|
|
1,429
|
|
||
|
Common stock warrants
|
41
|
|
—
|
|
||
|
Preferred stock warrants
|
80
|
|
132
|
|
||
|
Employee stock purchase plan
|
24
|
|
—
|
|
||
|
Total
|
6,677
|
|
9,406
|
|
||
|
(1)
|
Associated with the July 2019 Note.
|
|
As of December 31,
|
|||||||
|
|
2019
|
2018
|
|||||
|
Lab equipment
|
$
|
1,563
|
|
$
|
1,153
|
|
|
|
Computer equipment
|
887
|
|
629
|
|
|||
|
Leasehold improvements
|
635
|
|
554
|
|
|||
|
Furniture and fixtures
|
178
|
|
102
|
|
|||
|
Total
|
3,263
|
|
2,438
|
|
|||
|
Less accumulated depreciation
|
(1,203
|
)
|
(909
|
)
|
|||
|
Property and equipment, net
|
$
|
2,060
|
|
$
|
1,529
|
|
|
|
Years Ended December 31,
|
|||||||
|
|
2019
|
2018
|
|||||
|
Cost of sales
|
$
|
277
|
|
$
|
233
|
|
|
|
Research and development
|
7
|
|
4
|
|
|||
|
Selling, general and administrative
|
70
|
|
50
|
|
|||
|
Total
|
$
|
354
|
|
$
|
287
|
|
|
|
As of December 31,
|
|||||||||||
|
|
2019
|
2018
|
|||||||||
|
|
Value
|
Weighted-Average Remaining Life (Years)
|
Value
|
Weighted-Average Remaining Life (Years)
|
|||||||
|
Licenses
|
$
|
275
|
|
0
|
$
|
275
|
|
0
|
|||
|
Accumulated amortization
|
(275
|
)
|
(271
|
)
|
|||||||
|
Intangible assets, net
|
$
|
—
|
|
$
|
4
|
|
|||||
|
As of December 31,
|
|||||||
|
|
2019
|
2018
|
|||||
|
Accrued state income taxes
|
$
|
79
|
|
$
|
9
|
|
|
|
Accrued interest
|
184
|
|
185
|
|
|||
|
Accrued royalties
|
169
|
|
240
|
|
|||
|
Accrued service fees
|
1,162
|
|
281
|
|
|||
|
Employee stock purchase plan contributions
|
218
|
|
—
|
|
|||
|
Total
|
$
|
1,812
|
|
$
|
715
|
|
|
|
At Original Issuance
(1)
|
||||
|
Liability component:
|
||||
|
Principal (including $4,756 with related parties)
|
$
|
11,770
|
|
|
|
Unamortized issuance costs
|
(75
|
)
|
||
|
Unamortized discount from beneficial conversion feature
|
(8,378
|
)
|
||
|
Unamortized discount from embedded derivative
|
(2,816
|
)
|
||
|
Net carrying amount of the liability component
|
$
|
501
|
|
|
|
Embedded derivative liability
|
$
|
2,816
|
|
|
|
Equity Component:
|
||||
|
Carrying value of beneficial conversion feature recorded in additional paid-in capital
|
$
|
8,378
|
|
|
|
Gain (Loss) Recognized in Net Income
|
|||||
|
Statement of Operations and Comprehensive Income (Loss) Location
|
Year Ended
December 31, 2019
|
||||
|
Derivatives Not Classified as Hedging Instruments
|
|||||
|
Embedded derivative in convertible promissory notes
|
Other expense, net
|
$
|
(237
|
)
|
|
|
As of December 31,
|
|||||||
|
|
2019
|
2018
|
|||||
|
Term debt
|
$
|
26,688
|
|
$
|
21,350
|
|
|
|
Revolving line of credit
|
—
|
|
5,000
|
|
|||
|
Total principal amount
|
26,688
|
|
26,350
|
|
|||
|
Unamortized discount and issuance costs
|
(1,566
|
)
|
(1,850
|
)
|
|||
|
Total long-term debt
|
25,122
|
|
24,500
|
|
|||
|
Less: Current portion of long-term debt
|
(5,833
|
)
|
—
|
|
|||
|
Total long-term debt, less current portion
|
$
|
19,289
|
|
$
|
24,500
|
|
|
|
Years Ending December 31,
|
||||
|
2020
|
$
|
5,833
|
|
|
|
2021
|
10,000
|
|
||
|
2022
|
10,855
|
|
||
|
2023
|
—
|
|
||
|
2024
|
—
|
|
||
|
Total
|
$
|
26,688
|
|
|
|
Years Ending December 31,
|
||||
|
2020
|
$
|
754
|
|
|
|
2021
|
995
|
|
||
|
2022
|
993
|
|
||
|
2023
|
1,029
|
|
||
|
2024
|
999
|
|
||
|
Thereafter
|
1,625
|
|
||
|
$
|
6,395
|
|
||
|
|
As of December 31, 2018
|
||||||||||||||
|
|
Quoted Prices in Active Markets for Identical Items (Level 1)
|
Significant Other Observable Inputs (Level 2)
|
Significant Unobservable Inputs (Level 3)
|
Total
|
|||||||||||
|
Liabilities:
|
|
|
|
|
|
|
|
|
|||||||
|
Preferred stock warrants
|
$
|
—
|
|
$
|
—
|
|
$
|
1,194
|
|
$
|
1,194
|
|
|||
|
|
Preferred stock warrant liability
|
Embedded derivative liability in the Q1 2019 Notes
|
The
July 2019 Note
|
||||||||
|
Balance, January 1, 2018
|
$
|
525
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Issuance of warrants
|
632
|
|
—
|
|
—
|
|
|||||
|
Change in fair value included in net loss
|
272
|
|
—
|
|
—
|
|
|||||
|
Exercised warrants
|
(235
|
)
|
—
|
|
—
|
|
|||||
|
Balance, December 31, 2018
|
$
|
1,194
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Issuance of convertible promissory notes
|
—
|
|
2,816
|
|
9,236
|
|
|||||
|
Change in fair value included in net income
|
619
|
|
237
|
|
2,077
|
|
|||||
|
Extinguishment of convertible promissory notes and exercise of warrants
|
(68
|
)
|
(3,053
|
)
|
(11,313
|
)
|
|||||
|
Reclassification of preferred stock warrant liability to stockholders’ equity
|
(1,745
|
)
|
—
|
|
—
|
|
|||||
|
Balance, December 31, 2019
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
|
December 31, 2018
|
|
|
Average expected life (years)
|
9
|
|
|
Expected stock price volatility
|
64%
|
|
|
Risk-free interest rate
|
2.51% - 2.69%
|
|
|
Dividend yield
|
—%
|
|
|
As of December 31, 2018
|
|||||||||||||||||||||||||
|
Shares Authorized
|
Shares Issued and Outstanding
|
Original Issue Price per Share
|
Original Issue Value
|
Accumulated and Undeclared Dividends
|
Aggregate Liquidation Preference
|
Carrying Value
|
|||||||||||||||||||
|
Series C
|
503,056
|
|
503,056
|
|
|
$3.4800
|
|
$
|
1,751
|
|
$
|
667
|
|
$
|
2,417
|
|
$
|
1,501
|
|
||||||
|
As of December 31, 2018
|
|||||||||||||||||||||||||
|
Shares Authorized
|
Shares Issued and Outstanding
|
Original Issue Price per Share
|
Original Issue Value
|
Accumulated and Undeclared Dividends
|
Aggregate Liquidation Preference
|
Carrying Value
|
|||||||||||||||||||
|
Series A
|
533,711
|
|
533,711
|
|
|
$2.1400
|
|
$
|
1,142
|
|
$
|
713
|
|
$
|
1,856
|
|
$
|
1,142
|
|
||||||
|
Series B
|
816,654
|
|
816,654
|
|
|
$2.2500
|
|
1,837
|
|
983
|
|
2,820
|
|
1,932
|
|
||||||||||
|
Series D
|
756,416
|
|
756,416
|
|
|
$3.7700
|
|
2,852
|
|
1,340
|
|
4,191
|
|
2,852
|
|
||||||||||
|
Series E-1
|
842,641
|
|
829,642
|
|
|
$3.7700
|
|
3,128
|
|
1,095
|
|
4,223
|
|
3,099
|
|
||||||||||
|
Series E-2
|
949,725
|
|
934,433
|
|
|
$4.5776
|
|
4,277
|
|
1,503
|
|
5,780
|
|
4,277
|
|
||||||||||
|
Series E-2A
|
27,306
|
|
27,306
|
|
|
$4.5776
|
|
125
|
|
39
|
|
164
|
|
125
|
|
||||||||||
|
Series E-3
|
830,554
|
|
824,000
|
|
|
$5.3405
|
|
4,401
|
|
1,364
|
|
5,764
|
|
4,394
|
|
||||||||||
|
Series F
|
4,883,486
|
|
4,734,613
|
|
|
$5.8208
|
|
27,559
|
|
5,212
|
|
32,772
|
|
27,174
|
|
||||||||||
|
Total
|
9,640,493
|
|
9,456,775
|
|
$
|
45,321
|
|
$
|
12,249
|
|
$
|
57,570
|
|
$
|
44,995
|
|
|||||||||
|
Convertible Preferred Stock Class
|
Annual Dividend Rate
|
|||
|
Series F
|
$
|
0.46570
|
|
|
|
Series E-3
|
$
|
0.42720
|
|
|
|
Series E-2A
|
$
|
0.36620
|
|
|
|
Series E-2
|
$
|
0.36620
|
|
|
|
Series E-1
|
$
|
0.30160
|
|
|
|
Series D
|
$
|
0.30160
|
|
|
|
Series C
|
$
|
0.27840
|
|
|
|
Series B
|
$
|
0.18000
|
|
|
|
Series A
|
$
|
0.17120
|
|
|
|
As of December 31, 2018
|
||||||||||||||||||||
|
Warrants
|
Year of First Issuance
|
Shares
|
Exercise Price
|
Year of Expiration
|
Exercised
|
Expired
|
Outstanding
|
|||||||||||||
|
Series A Preferred Stock
|
2008
|
108,057
|
|
|
$2.1400
|
|
2015
|
(96,375
|
)
|
(11,682
|
)
|
—
|
|
|||||||
|
Series B Preferred Stock
|
2010
|
86,667
|
|
|
$2.2500
|
|
2017
|
(86,667
|
)
|
—
|
|
—
|
|
|||||||
|
Series E-1 Preferred Stock
|
2014
|
16,249
|
|
|
$3.7700
|
|
2024
|
(3,250
|
)
|
—
|
|
12,999
|
|
|||||||
|
Series E-2 Preferred Stock
|
2014
|
15,292
|
|
|
$4.5776
|
|
2024
|
—
|
|
—
|
|
15,292
|
|
|||||||
|
Series E-3 Preferred Stock
|
2015
|
6,554
|
|
|
$5.3405
|
|
2024
|
—
|
|
—
|
|
6,554
|
|
|||||||
|
Series F Preferred Stock
|
2016
|
103,090
|
|
|
$5.8208
|
|
2026-2028
|
—
|
|
—
|
|
103,090
|
|
|||||||
|
Series F Preferred Stock
|
2018
|
67,233
|
|
|
$0.0100
|
|
2023
|
(32,951
|
)
|
—
|
|
34,282
|
|
|||||||
|
Total
|
403,142
|
|
(219,243
|
)
|
(11,682
|
)
|
172,217
|
|
||||||||||||
|
As of December 31, 2019
|
|||||||
|
Expiration date
|
Number of shares
|
Exercise price
|
|||||
|
July 12, 2026
|
209,243
|
|
$
|
0.001
|
|
||
|
March 31, 2027
|
26,428
|
|
$
|
7.10
|
|
||
|
November 30, 2028
|
8,809
|
|
$
|
7.10
|
|
||
|
Total
|
244,480
|
|
|||||
|
|
|
|
Weighted-Average
|
|
|||||||||||
|
|
Shares
Available for
Grant
|
Stock Options
Outstanding
|
Exercise
Price
|
Remaining
Contractual
Term (Years)
|
Aggregate
Intrinsic
Value
(in thousands)
|
||||||||||
|
Balance as of December 31, 2017
|
551,918
|
|
1,175,987
|
|
$
|
1.79
|
|
||||||||
|
Additional options authorized
|
410,172
|
|
—
|
|
—
|
|
|||||||||
|
Granted
|
(580,402
|
)
|
580,402
|
|
2.39
|
|
|||||||||
|
Exercised
|
—
|
|
(19,755
|
)
|
1.91
|
|
|||||||||
|
Forfeited/Cancelled
|
77,038
|
|
(77,038
|
)
|
1.82
|
|
|||||||||
|
Balance as of December 31, 2018
|
458,726
|
|
1,659,596
|
|
$
|
1.99
|
|
||||||||
|
Additional options authorized
|
2,075,898
|
|
—
|
|
—
|
|
|||||||||
|
Granted
|
(1,711,325
|
)
|
1,711,325
|
|
19.41
|
|
|||||||||
|
Exercised
|
—
|
|
(693,127
|
)
|
1.70
|
|
|||||||||
|
Forfeited/Cancelled
|
39,828
|
|
(39,828
|
)
|
6.36
|
|
|||||||||
|
Balance as of December 31, 2019
|
863,127
|
|
2,637,966
|
|
$
|
13.30
|
|
8.64
|
$
|
55,578
|
|
||||
|
Exercisable at December 31, 2019
(1
)
|
590,104
|
|
$
|
2.20
|
|
6.11
|
$
|
18,984
|
|
||||||
|
(1)
|
Vested and exercisable options. Additionally, outstanding unvested options to purchase an aggregate of
126,044
shares of common stock with a weighted-average exercise price of
$2.39
per share may be exercised prior to vesting as of
December 31, 2019
under early-exercise provisions. In the event of such exercise, the shares obtained upon exercise would be restricted and subject to forfeiture prior to vesting. No such early exercises have occurred as of
December 31, 2019
.
|
|
•
|
Expected term
. The expected term is the period of time that granted options are expected to be outstanding. For stock options, the Company has set the expected term using the simplified method based on the weighted average of both the period to vesting and the period to maturity for each option, as the Company has concluded that its stock option exercise history does not provide a reasonable basis upon which to estimate the expected term. For the ESPP, the expected term is the period of time from the offering date to the purchase date.
|
|
•
|
Expected volatility
. Because of the limited period of time the Company’s stock has been traded in an active market, the Company calculates volatility by using the historical stock price volatility of a group of similar companies looking back over the estimated life of the option or the ESPP purchase right and averaging the volatilities of these companies.
|
|
•
|
Risk-free interest rate
. The Company bases the risk-free interest rate used in the Black-Scholes valuation model on the market yield in effect at the time of option grant and at the offering date for the ESPP, provided from the Federal Reserve Board’s Statistical Releases and historical publications from the Treasury constant maturities rates for the equivalent remaining terms.
|
|
•
|
Dividend yield
. The Company has not paid, and does not have plans to pay, cash dividends. Therefore, the Company use an expected dividend yield of zero in the Black-Scholes option valuation model.
|
|
|
Years Ended December 31,
|
||
|
2019
|
2018
|
||
|
Average expected term (years)
|
6
|
6
|
|
|
Expected stock price volatility
|
56.74% - 60.17%
|
57.77% - 58.29%
|
|
|
Risk-free interest rate
|
1.63% - 2.47%
|
2.77% - 3.13%
|
|
|
Dividend yield
|
—%
|
—%
|
|
|
|
Years Ended December 31,
|
||
|
2019
|
2018
|
||
|
Average expected term (years)
|
1.2
|
Not applicable
|
|
|
Expected stock price volatility
|
61.12% - 68.89%
|
Not applicable
|
|
|
Risk-free interest rate
|
1.56% - 1.80%
|
Not applicable
|
|
|
Dividend yield
|
—%
|
Not applicable
|
|
|
|
Years Ended December 31,
|
||||||
|
|
2019
|
2018
|
|||||
|
Cost of sales
|
$
|
170
|
|
$
|
24
|
|
|
|
Research and development
|
165
|
|
56
|
|
|||
|
Selling, general and administrative
|
914
|
|
214
|
|
|||
|
Total stock-based compensation expense
|
$
|
1,249
|
|
$
|
294
|
|
|
|
Years Ended December 31,
|
||||||||
|
2019
|
2018
|
|||||||
|
Current tax expense
|
||||||||
|
U.S. Federal
|
$
|
—
|
|
$
|
—
|
|
||
|
State and local
|
72
|
|
9
|
|
||||
|
Total current
|
72
|
|
9
|
|
||||
|
Deferred tax expense
|
||||||||
|
U.S. Federal
|
—
|
|
—
|
|
||||
|
State and local
|
—
|
|
—
|
|
||||
|
Total deferred
|
—
|
|
—
|
|
||||
|
Total income tax expense
|
$
|
72
|
|
$
|
9
|
|
||
|
Years Ended December 31,
|
||||||||
|
2019
|
2018
|
|||||||
|
Pre-tax income (loss)
|
$
|
5,349
|
|
$
|
(6,358
|
)
|
||
|
U.S. federal taxes at statutory rate
|
1,123
|
|
(1,335
|
)
|
||||
|
State income taxes
|
958
|
|
(1,174
|
)
|
||||
|
Mark-to-market losses
|
566
|
|
57
|
|
||||
|
Non-deductible meals
|
224
|
|
119
|
|
||||
|
Convertible debt interest
|
95
|
|
—
|
|
||||
|
Research and development (“R&D”) tax credit
|
(251
|
)
|
—
|
|
||||
|
Change in valuation allowance
|
(2,747
|
)
|
2,278
|
|
||||
|
Stock-based compensation
|
65
|
|
53
|
|
||||
|
Other
|
39
|
|
11
|
|
||||
|
Total income tax expense
|
$
|
72
|
|
$
|
9
|
|
||
|
As of December 31,
|
||||||||
|
2019
|
2018
|
|||||||
|
Deferred tax assets:
|
||||||||
|
Net operating loss (“NOL”) carryforwards
|
$
|
14,077
|
|
$
|
16,184
|
|
||
|
Accounts payable
|
456
|
|
390
|
|
||||
|
Accrued liabilities
|
1,817
|
|
1,432
|
|
||||
|
R&D tax credit
|
993
|
|
715
|
|
||||
|
Intangible assets
|
50
|
|
61
|
|
||||
|
Stock-based compensation
|
127
|
|
12
|
|
||||
|
Charitable contributions
|
2
|
|
4
|
|
||||
|
Total deferred tax assets
|
17,522
|
|
18,798
|
|
||||
|
Less valuation allowance
|
(12,420
|
)
|
(14,375
|
)
|
||||
|
Deferred tax assets, net
|
$
|
5,102
|
|
$
|
4,423
|
|
||
|
Deferred tax liabilities:
|
||||||||
|
Accounts receivable
|
$
|
(3,796
|
)
|
$
|
(3,249
|
)
|
||
|
Prepaid expenses
|
(757
|
)
|
(378
|
)
|
||||
|
Property and equipment
|
(246
|
)
|
(131
|
)
|
||||
|
Section 481(a) adjustment (cash to accrual)
|
(303
|
)
|
(665
|
)
|
||||
|
Total deferred tax liabilities
|
(5,102
|
)
|
(4,423
|
)
|
||||
|
Net deferred tax asset (liability)
|
$
|
—
|
|
$
|
—
|
|
||
|
Year Ended December 31, 2019
|
||||||||||||||||||||
|
First Quarter
|
Second Quarter
|
Third Quarter
|
Fourth Quarter
|
Total Year
|
||||||||||||||||
|
Net revenues
|
$
|
8,717
|
|
$
|
10,739
|
|
$
|
14,774
|
|
$
|
17,635
|
|
$
|
51,865
|
|
|||||
|
Gross margin
|
$
|
7,119
|
|
$
|
8,746
|
|
$
|
13,066
|
|
$
|
15,624
|
|
$
|
44,555
|
|
|||||
|
Net income (loss)
(1)
|
$
|
(1,358
|
)
|
$
|
(1,269
|
)
|
$
|
5,849
|
|
$
|
2,055
|
|
$
|
5,277
|
|
|||||
|
Net income (loss) attributable to common shareholders
(1)
|
$
|
(2,342
|
)
|
$
|
(2,265
|
)
|
$
|
5,543
|
|
$
|
2,055
|
|
$
|
2,991
|
|
|||||
|
Earnings (loss) per share
(3)
:
|
||||||||||||||||||||
|
Basic
|
$
|
(1.22
|
)
|
$
|
(1.05
|
)
|
$
|
0.43
|
|
$
|
0.12
|
|
$
|
0.35
|
|
|||||
|
Diluted
|
$
|
(1.22
|
)
|
$
|
(1.05
|
)
|
$
|
0.05
|
|
$
|
0.11
|
|
$
|
(0.21
|
)
|
|||||
|
Year Ended December 31, 2018
|
||||||||||||||||||||
|
First Quarter
|
Second Quarter
|
Third Quarter
|
Fourth Quarter
|
Total Year
|
||||||||||||||||
|
Net revenues
(2)
|
$
|
3,659
|
|
$
|
3,979
|
|
$
|
3,712
|
|
$
|
11,436
|
|
$
|
22,786
|
|
|||||
|
Gross margin
(2)
|
$
|
2,406
|
|
$
|
2,652
|
|
$
|
2,361
|
|
$
|
10,070
|
|
$
|
17,489
|
|
|||||
|
Net income (loss)
(2)
|
$
|
(3,630
|
)
|
$
|
(3,155
|
)
|
$
|
(3,450
|
)
|
$
|
3,868
|
|
$
|
(6,367
|
)
|
|||||
|
Net income (loss) attributable to common shareholders
(2)
|
$
|
(4,489
|
)
|
$
|
(4,080
|
)
|
$
|
(4,456
|
)
|
$
|
2,862
|
|
$
|
(10,163
|
)
|
|||||
|
Earnings (loss) per share
(3)
:
|
||||||||||||||||||||
|
Basic
|
$
|
(2.37
|
)
|
$
|
(2.15
|
)
|
$
|
(2.33
|
)
|
$
|
1.49
|
|
$
|
(5.33
|
)
|
|||||
|
Diluted
|
$
|
(2.37
|
)
|
$
|
(2.15
|
)
|
$
|
(2.33
|
)
|
$
|
0.38
|
|
$
|
(5.33
|
)
|
|||||
|
(2)
|
For the fourth quarter of 2018, includes revenue recognized of (in thousands)
$5,191
for tests delivered during the first three quarters of 2018 for which the requirements to release the constraint on variable consideration were not met until the fourth quarter of 2018 in connection with the issuance of the LCD for DecisionDx-Melanoma and establishment of a Medicare reimbursement rate.
|
|
(3)
|
Basic and diluted earnings (loss) per share are computed independently for each quarterly and annual period presented. Therefore, t
he sum of the quarterly basic and diluted earnings (loss) per share amounts may not equal annual basic and diluted earnings (loss) per share.
|