|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| COLORADO | 84-0916344 | |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |
|
8229 Boone Blvd., Suite 802
Vienna, Virginia
|
22182 | |
| (Address of principal executive offices) | (Zip Code) | |
| Large accelerated filer | o | Accelerated filer | o |
| Non-accelerated filer | o | Smaller reporting company | þ |
| (Do not check if a smaller reporting company) |
|
|
1)
|
Multikine® (Leukocyte Interleukin, Injection) investigational cancer therapy;
|
|
|
2)
|
LEAPS technology, with two products, pandemic flu treatment for hospitalized patients and CEL-2000, a rheumatoid arthritis treatment vaccine, in development.
|
|
●
|
Phase II clinical trials:
In the final Phase II clinical study, using the same dosages and Multikine treatment regimen as is being used in the Phase III study, head and neck cancer patients with locally advanced primary disease who received the investigational therapy Multikine as first-line therapy followed by surgery and radiotherapy were reported by the clinical investigators to have had a 63.2% overall survival (OS) rate at 3.5 years from surgery. This percentage OS was arrived at as follows: of the 22 subjects enrolled in this final Phase II study, the consent for the survival follow-up portion of the study was received from 19 subjects. One subject did not consent to the follow-up portion of the study. The other 2 subjects did not have squamous cell carcinoma of the oral cavity and were thus not evaluable per the protocol. The overall survival rate of subjects receiving the investigational therapy in this study was compared to the overall survival rate that was calculated based upon a review of 55 clinical trials conducted in the same cancer population with a total of 7294 subjects patients studied, which were reported in the peer reviewed scientific literature between 1987 and 2007. Review of this literature showed an approximate survival rate of 47.5% at 3.5 year from treatment. Therefore, the results of CEL-SCI’s final Phase II study were considered to be potentially favorable in terms of overall survival recognizing the limitations of this early-phase study. It should be noted that an earlier investigational therapy Multikine study appears to lend support to the overall survival findings described above – Feinmesser et al Arch Otolaryngol. Surg. 2003. However, no definitive conclusions can be drawn from these data about the potential efficacy or safety profile of this investigational therapy. Moreover, further research is required, and these results must be confirmed in the well-controlled Phase III clinical trial of this investigational therapy that is currently in progress. Subject to completion of that Phase III trial and FDA's review and acceptance of CEL-SCI's entire data set on this investigational therapy, CEL-SCI believes that these early-stage clinical trial results indicate the potential for this investigational therapy to become a treatment for advanced primary head and neck cancer.
|
|
●
|
Reported average of 50% reduction in tumor cells in Phase II trials:
The clinical investigators who administered the 3 week Multikine treatment regimen used in Phase II studies reported that, as was determined in a controlled pathology study, Multikine administration appeared to have caused, on average, the disappearance of about half of the cancer cells present at surgery (as determined by histopathology assessing the area of Stroma/Tumor (Mean+/- Standard Error of the Mean of the number of cells counted per filed)) even before the start of standard therapy with radiation and chemotherapy (Timar et al JCO 2005).
|
|
●
|
Reported 12% complete response in the final Phase II trial:
The clinical investigators who administered the 3 week Multikine investigational treatment regimen used in the Phase II study reported that, as was determined in a controlled pathology study, the tumor apparently was no longer present in approximately 12 % of patients (2 of 17 evaluable by pathology). This determination was made by three blinded pathologists from the surgical specimen after a 3 week treatment with Multikine (Timar et al JCO 2005).
|
|
●
|
Adverse events reported in clinical trials:
In clinical trials conducted to date with the Multikine investigational therapy, adverse events which have been reported by the clinical investigators as possibly or probably related to Multikine administration included pain at the injection site, local minor bleeding and edema at the injection site, diarrhea, headache, nausea, and constipation.
|
|
●
|
product design, development and manufacture;
|
|
●
|
product application and use
|
|
●
|
adverse drug experience;
|
|
●
|
product advertising and promotion;
|
|
●
|
product manufacturing, including good manufacturing practices
|
|
●
|
record keeping requirements;
|
|
●
|
registration and listing of CEL-SCI's establishments and products with the FDA, EMA and other state and national agencies;
|
|
●
|
product storage and shipping;
|
|
●
|
drug sampling and distribution requirements;
|
|
●
|
electronic record and signature requirements; and
|
|
●
|
labeling changes or modifications.
|
| Quarter Ending | High | Low | ||||||
| 12/31/08 | $ | 0.50 | $ | 0.18 | ||||
| 3/31/09 | $ | 0.40 | $ | 0.14 | ||||
| 6/30/09 | $ | 0.80 | $ | 0.20 | ||||
| 9/30/09 | $ | 2.10 | $ | 0.38 | ||||
| Quarter Ending | High | Low | ||||||
| 12/31/09 | $ | 1.79 | $ | 0.85 | ||||
| 3/31/10 | $ | 1.12 | $ | 0.50 | ||||
| 6/30/10 | $ | 0.76 | $ | 0.45 | ||||
| 9/30/10 | $ | 0.84 | $ | 0.43 | ||||
| 12/31/10 | $ | 1.05 | $ | 0.60 | ||||
| 3/31/11 | $ | 0.86 | $ | 0.51 | ||||
| 6/30/11 | $ | 0.74 | $ | 0.46 | ||||
| 9/30/11 | $ | 0.57 | $ | 0.35 | ||||
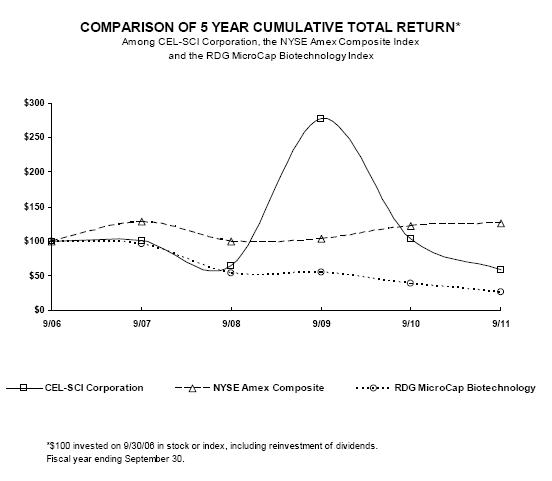
| 9/06 | 9/07 | 9/08 | 9/09 | 9/10 | 9/11 | |||||||||||||||||||
|
CEL-SCI Corporation
|
100.00 | 100.84 | 64.52 | 277.42 | 103.87 | 58.87 | ||||||||||||||||||
|
NYSE Amex Composite
|
100.00 | 127.85 | 99.27 | 103.93 | 122.61 | 125.42 | ||||||||||||||||||
|
RDG MicroCap Biotechnology
|
100.00 | 96.23 | 54.05 | 54.24 | 38.88 | 25.57 |
|
Statements of Operations
|
2011
|
2010
|
2009
|
2008
|
2007
|
|||||||||||||||
|
Rent and grant revenue and other
|
$ | 956,154 | $ | 153,300 | $ | 80,093 | $ | 5,065 | $ | 57,043 | ||||||||||
|
Operating expenses:
|
||||||||||||||||||||
|
Research and development
|
11,745,629 | 11,911,626 | 6,011,750 | 4,101,563 | 2,528,528 | |||||||||||||||
|
Depreciation and
amortization
|
531,316 | 516,117 | 417,205 | 215,060 | 176,186 | |||||||||||||||
|
General and administrative
|
6,664,883 | 6,285,810 | 5,671,595 | 5,200,735 | 6,704,538 | |||||||||||||||
|
Gain (loss) on derivative instruments
|
4,432,148 | 28,843,772 | (28,491,650 | ) | 1,799,393 | 868,182 | ||||||||||||||
|
Other expenses (3)
|
(12,000,000 | ) | - | - | - | - | ||||||||||||||
|
Interest income
|
164,163 | 362,236 | - | 483,252 | 562,973 | |||||||||||||||
|
Interest expense
|
(322,980 | ) | (162,326 | ) | (397,923 | ) | (473,767 | ) | (1,708,603 | ) | ||||||||||
|
Net income (loss)
|
(25,712,343 | ) | 10,483,429 | (40,910,030 | ) | (7,703,415 | ) | (9,629,657 | ) | |||||||||||
|
Modification of warrants
|
(1,068,369 | ) | (1,532,456 | ) | (490,728 | ) | (424,815 | ) | - | |||||||||||
|
Net income (loss) available to common shareholders
|
$ | (26,780,712 | ) | $ | 8,950,973 | $ | (41,400,758 | ) | $ | (8,128,230 | ) | $ | (9,629,657 | ) | ||||||
|
Net income (loss) per common share
|
||||||||||||||||||||
|
Basic
|
$ | (0.13 | ) | $ | 0.04 | $ | (0.31 | ) | $ | (0.07 | ) | $ | (0.10 | ) | ||||||
|
Diluted
|
$ | (0.15 | ) | $ | (0.06 | ) | $ | (0.31 | ) | $ | (0.07 | ) | $ | (0.10 | ) | |||||
|
Weighted average common
shares outstanding
|
||||||||||||||||||||
|
Basic
|
208,488,987 | 202,102,859 | 133,535,050 | 117,060,866 | 97,310,488 | |||||||||||||||
|
Diluted (1)
|
208,488,987 | 202,102,859 | 133,535,050 | 117,060,866 | 97,310,488 | |||||||||||||||
|
|
||||||||||||||||||||
|
2011
|
2010
|
2009
|
2008
|
2007
|
||||||||||||||||
|
Working capital
|
$ | 1,796,349 | $ | 25,799,304 | $ | 34,339,772 | $ | (2,492,555 | ) | $ | 10,257,568 | |||||||||
|
Total assets
|
18,625,440 | 37,804,985 | 46,027,598 | 14,683,672 | 20,730,802 | |||||||||||||||
|
Convertible note and derivative instruments - current (2)
|
5,068,552 | 424,286 | - | 3,018,697 | 782,732 | |||||||||||||||
|
Derivative instruments –
noncurrent (2)
|
2,192,521 | 6,521,765 | 35,113,970 | - | 4,831,252 | |||||||||||||||
|
Total liabilities
|
9,546,616 | 9,950,220 | 37,186,954 | 3,847,637 | 6,060,703 | |||||||||||||||
|
Stockholders' equity
|
9,078,824 | 27,854,765 | 8,840,644 | 10,836,035 | 14,670,099 | |||||||||||||||
| Quarter | Net income | Net income (loss) per share | ||||||||||
| (loss) | Basic | Diluted | ||||||||||
| 12/31/2009 | $ | 19,159,517 | $ | 0.10 | $ | 0.02 | ||||||
| 3/31/2010 | $ | (2,176,975 | ) | $ | (0.01 | ) | $ | (0.03 | ) | |||
| 6/30/2010 | $ | (601,124 | ) | $ | (0.00 | ) | $ | (0.02 | ) | |||
| 9/30/2010 | $ | (7,430,445 | ) | $ | (0.04 | ) | $ | (0.04 | ) | |||
| 12/31/2010 | $ | (6,250,952 | ) | $ | (0.03 | ) | $ | (0.03 | ) | |||
| 3/31/2011 | $ | (15,097,973 | ) | $ | (0.07 | ) | $ | (0.09 | ) | |||
| 6/30/2011 | $ | (3,114,255 | ) | $ | (0.01 | ) | $ | (0.02 | ) | |||
| 9/30/2011 | $ | (2,317,532 | ) | $ | (0.01 | ) | $ | (0.02 | ) | |||
| 2011 | 2010 | 2009 | 2008 | 2007 | ||||||||||||||||
| MULTIKINE | $ | 11,257,157 | $ | 10,868,046 | $ | 5,281,999 | $ | 3,765,258 | $ | 2,217,108 | ||||||||||
| LEAPS | 488,472 | 1,043,580 | 729,751 | 336,305 | 311,420 | |||||||||||||||
| TOTAL | $ | 11,745,629 | $ | 11,911,626 | $ | 6,011,750 | $ | 4,101,563 | $ | 2,528,528 | ||||||||||
| Years Ending September 30, | ||||||||||||||||||||||||||||
| Total | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 & thereafter | ||||||||||||||||||||||
| Operating Leases | $ | 33,346,809 | $ | 1,896,205 | $ | 1,855,890 | $ | 1,579,931 | $ | 1,572,839 | $ | 1,629,121 | $ | 24,812,823 | ||||||||||||||
| Employment Contracts | $ | 3,850,862 | $ | 1,282,878 | $ | 1,214,639 | $ | 464,004 | $ | 464,004 | $ | 425,337 | - | |||||||||||||||
| Convertible Notes | $ | 4,950,000 | $ | 4,950,000 | - | - | - | - | - | |||||||||||||||||||
| Interest on Notes | $ | 215,705 | $ | 215,705 | - | - | - | - | - | |||||||||||||||||||
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISKS
|
|
ITEM 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
| Name | Age | Position | ||
| Maximilian de Clara | 81 | Director and President | ||
| Geert R. Kersten, Esq. | 52 | Director, Chief Executive Officer and Treasurer | ||
| Patricia B. Prichep | 60 | Senior Vice President of Operations and Secretary | ||
| Dr. Eyal Talor | 55 | Chief Scientific Officer | ||
| Dr. Daniel H. Zimmerman | 70 | Senior Vice President of Research, Cellular Immunology | ||
| John Cipriano | 69 | Senior Vice President of Regulatory Affairs | ||
| Alexander G. Esterhazy | 69 | Director | ||
| Dr. C. Richard Kinsolving | 75 | Director | ||
| Dr. Peter R. Young | 66 | Director |
|
●
|
Market-driven.
Compensation programs are structured to be competitive both in their design and in the total compensation that they offer.
|
|
●
|
Performance-based.
Certain officers have some portion of their incentive compensation linked to CEL-SCI’s performance. The application of performance measures as well as the form of the reward may vary depending on the employee’s position and responsibilities.
|
|
●
|
The salaries paid to employees are consistent with the employees’ duties and responsibilities.
|
|
●
|
Employees who have high impact relative to the expectations of their job duties and functions are rewarded.
|
|
●
|
CEL-SCI retains employees who have skills critical to its long term success.
|
|
●
|
Base Salary
|
|
●
|
Long-Term Incentives (stock options and/or grants of stock)
|
|
●
|
Benefits
|
|
●
|
Enhance the link between the creation of shareholder value and long-term executive incentive compensation;
|
|
●
|
Provide focus, motivation and retention incentive; and
|
|
●
|
Provide competitive levels of total compensation.
|
|
Restricted Stock
|
Option
|
All Other Annual
|
|||||||||||||||||||||||
|
|
Fiscal
|
Salary
|
Bonus
|
Awards
|
Awards
|
Compensation
|
|||||||||||||||||||
|
Name and Princi
pal Position
|
Year
|
(1) | (2) | (3) | (4) | (5) |
Total
|
||||||||||||||||||
| $ | $ | $ | $ | $ | $ | ||||||||||||||||||||
|
Maximilian de Clara,
|
2011
|
363,000 | -- | -- | 176,709 | 105,226 | 644,935 | ||||||||||||||||||
|
President
|
2010
|
363,000 | -- | -- | 107,424 | 102,186 | 572,610 | ||||||||||||||||||
|
2009
|
334,720 | -- | 205,000 | 531,236 | 83,274 | 1,154,230 | |||||||||||||||||||
|
Geert R. Kersten,
|
2011
|
464,005 | -- | 14,700 | 207,314 | 57,656 | 743,675 | ||||||||||||||||||
|
Chief Executive
Officer and
Treasurer
|
2010
|
454,009 | 220,995 | 11,025 | 128,909 | 55,309 | 870,247 | ||||||||||||||||||
|
|
2009
|
408,691 | -- | 5,000 | 1,735,284 | 34,892 | 2,183,867 | ||||||||||||||||||
|
|
|||||||||||||||||||||||||
|
Patricia B. Prichep
|
2011
|
204,013 | -- | 12,541 | 99,141 | 6,031 | 321,726 | ||||||||||||||||||
|
Senior Vice President
of Operations and
Secretary
|
2010
|
199,898 | -- | 11,790 | 64,455 | 6,027 | 282,170 | ||||||||||||||||||
|
|
2009
|
174,913 | -- | -- | 1,142,155 | 4,225 | 1,321,293 | ||||||||||||||||||
|
|
|||||||||||||||||||||||||
|
Eyal Talor, Ph.D.
|
2011
|
251,861 | -- | 9,600 | 100,362 | 6,031 | 367,854 | ||||||||||||||||||
|
Chief Scientific
Officer
|
2010
|
239,868 | -- | 15,623 | 64,455 | 6,027 | 325,973 | ||||||||||||||||||
|
|
2009
|
212,265 | -- | -- | 1,044,566 | 4,225 | 1,261,056 | ||||||||||||||||||
| Daniel Zimmerman, Ph.D. |
2011
|
193,260 | -- | 11,896 | 98,948 | 6,031 | 310,135 | ||||||||||||||||||
|
Senior Vice President of
Research. Cellular
Immunology (6)
|
2010
|
165,800 | -- | 9,233 | 64,455 | 5,027 | 244,515 | ||||||||||||||||||
|
|
2009
|
47,124 | -- | -- | -- | 875 | 47,999 | ||||||||||||||||||
|
|
|||||||||||||||||||||||||
|
John Cipriano
|
2011
|
178,870 | -- | -- | 91,815 | 31 | 270,716 | ||||||||||||||||||
|
Senior Vice President
of Regulatory Affairs (7)
|
2010
|
175,952 | -- | -- | 240,711 | 27 | 416,690 | ||||||||||||||||||
|
|
2009
|
48,594 | -- | -- | -- | 25 | 48,619 | ||||||||||||||||||
|
(1)
|
The dollar value of base salary (cash and non-cash) earned.
|
|
(2)
|
The dollar value of bonus (cash and non-cash) earned.
|
|
(3)
|
During the periods covered by the table, the value of the shares of restricted stock issued as compensation for services to the persons listed in the table. In the case of Mr. de Clara, during three years ended September 30, 2011, 2010, and 2009, $0, $0 and $200,000, respectively, were paid in restricted shares of CEL-SCI’s common stock which cannot be sold in the public market for a period of three years after the date of issuance. In the case of all other persons listed in the table, the shares were issued as CEL-SCI's contribution on behalf of the named officer to CEL-SCI's 401(k) retirement plan and restricted shares issued at the market price from the Stock Compensation Plan. The value of all stock awarded during the periods covered by the table are calculated according to ASC 718-10-30-3 which represented the grant date fair value.
|
|
(4)
|
The greatest part of the value in FY 2009 was derived from options awarded to employees who did not collect a salary, or reduced or deferred their salary between September 15, 2008 and June 30, 2009. For example, Mr. de Clara, Mr. Kersten and Ms. Prichep did not collect any salary between September 30, 2008 and June 30, 2009. The fair value of all stock options granted during the periods covered by the table are calculated on the grant date in accordance with ASC 718-10-30-3 which represented the grant date fair value
|
|
(5)
|
All other compensation received that CEL-SCI could not properly report in any other column of the table including annual contributions or other allocations to vested and unvested defined contribution plans, and the dollar value of any insurance premiums paid by, or on behalf of, CEL-SCI with respect to term life insurance for the benefit of the named executive officer, and the full dollar value of the remainder of the premiums paid by, or on behalf of, CEL-SCI and car allowances paid by CEL-SCI. Includes board of directors fees for Mr. de Clara and Mr. Kersten.
|
|
(6)
|
Dr. Zimmerman was CEL-SCI’s Senior Vice President of Research, Cellular Immunology between January 1996 and December 2008 and since November 2009.
|
|
(7)
|
Mr. Cipriano was CEL-SCI’s Senior Vice President of Regulatory Affairs between March 2004 and December 2008 and since October 2009.
|
|
(8)
|
In 2009, CEL-SCI made performance share awards to the senior management which entitles these employees to receive a specified number of options to purchase the Company's common stock provided that certain milestones are met. One third of the options can be exercised when the first 400 patients are enrolled in CEL-SCI's Phase III head and neck cancer clinical trial. One third of the options can be exercised when all of the patients have been enrolled in the Phase III clinical trial. One third of the options can be exercised when the Phase III trial is completed. The grant-date fair value of these options awarded to the senior management of the Company amounts to $3.3 million in total. A major consideration in the valuation of these options is the likelihood of the CEL-SCI reaching these milestones. CEL-SCI’s management has assumed the likelihood of these milestones being reached to be 100%.
|
|
Stock
Awards (1) |
Option
Awards (2) |
|||||||||||||||
|
Name
|
Paid in Cash
|
Total
|
||||||||||||||
|
Maximilian de Clara
|
$ | 40, 000 | $ | - | $ | 176,709 | $ | 216,709 | ||||||||
|
Geert Kersten
|
$ | 40, 000 | $ | - | $ | 207,314 | $ | 247,314 | ||||||||
|
Alexander Esterhazy
|
$ | 44, 000 | $ | - | $ | 91,815 | $ | 135,815 | ||||||||
|
C. Richard Kinsolving
|
$ | 44, 000 | $ | - | $ | 91,815 | $ | 135,815 | ||||||||
|
Peter R. Young
|
$ | 44, 000 | $ | - | $ | 91,815 | $ | 135,815 | ||||||||
|
(1)
|
The fair value of stock issued for services.
|
|
(2)
|
The fair value of options granted computed in accordance with ASC 718-10-30-3 on the date of grant which represents their grant date fair value.
|
|
|
Exercise
|
|||||||||
|
Grant
|
Options
|
Price Per
|
Expiration
|
|||||||
|
Name
|
Date
|
Granted
|
Share
|
Date
|
||||||
|
Maximilian de Clara
|
4/15/2011
|
250,000 | $ | 0.69 |
4/14/2021
|
|||||
|
Geert Kersten
|
4/15/2011
|
300,000 | $ | 0.69 |
4/14/2021
|
|||||
|
Patricia B. Prichep
|
4/15/2011
|
150,000 | $ | 0.69 |
4/14/2021
|
|||||
|
Eyal Talor, Ph.D.
|
4/15/2011
|
150,000 | $ | 0.69 |
4/14/2021
|
|||||
|
Daniel Zimmerman, Ph.D.
|
4/15/2011
|
150,000 | $ | 0.69 |
4/14/2021
|
|||||
|
John Cipriano
|
4/15/2011
|
150,000 | $ | 0.69 |
4/14/2021
|
|||||
|
Shares
|
||||||||||||
|
Date of
|
Acquired On
|
Value
|
||||||||||
|
Name
|
Exercise
|
Exercise
|
Realized
|
|||||||||
|
None
|
||||||||||||
|
Shares Underlying Unexercised
|
|||||||||||||
|
Options Which are:
|
Exercise
|
Expiration
|
|||||||||||
|
Name
|
Exercisable
|
Unexercisable
|
Price
|
Date
|
|||||||||
|
Maximilian de Clara
|
23,333 | 2.87 |
07/31/13
|
||||||||||
| 95,000 | (1) | 1.94 |
08/31/13
|
||||||||||
| 70,000 | 1.05 |
09/25/12
|
|||||||||||
| 56,666 | 1.05 |
05/01/13
|
|||||||||||
| 50,000 | 1.05 |
05/01/13
|
|||||||||||
| 50,000 | 1.05 |
04/12/12
|
|||||||||||
| 60,000 | 1.05 |
04/19/13
|
|||||||||||
| 60,000 | 1.38 |
03/22/14
|
|||||||||||
| 75,000 | 0.54 |
03/14/12
|
|||||||||||
| 50,000 | 0.61 |
09/02/14
|
|||||||||||
| 50,000 | 0.48 |
09/21/15
|
|||||||||||
| 100,000 | 0.58 |
09/12/16
|
|||||||||||
| 200,000 | 0.63 |
09/13/17
|
|||||||||||
| 200,000 | 0.62 |
03/04/18
|
|||||||||||
| 1,436,250 | (2) | 0.25 |
04/23/19
|
||||||||||
| 166,667 | 0.38 |
07/20/19
|
|||||||||||
| 83,334 | 0.48 |
07/20/20
|
|||||||||||
| 2,826,250 | |||||||||||||
| 500,000 | (3) | 0.38 |
07/06/19
|
||||||||||
| 83,333 | 0.38 |
07/20/19
|
|||||||||||
| 166,666 | 0.48 |
07/20/20
|
|||||||||||
| 250,000 | 0.69 |
04/14/21
|
|||||||||||
| 999,999 | |||||||||||||
|
Geert R. Kersten
|
50,000 | 1.05 |
11/01/13
|
||||||||||
| 14,000 | 1.05 |
10/31/13
|
|||||||||||
| 50,000 | 1.05 |
07/31/13
|
|||||||||||
| 224,000 | (1) | 1.05 |
06/10/13
|
||||||||||
| 50,000 | 1.05 |
09/25/12
|
|
Shares Underlying Unexercised
|
||||||||||||
|
Options Which are:
|
Exercise
|
Expiration
|
||||||||||
|
Name
|
Exercisable
|
Unexercisable
|
Price
|
Date
|
||||||||
|
Geert Kersten (cont’d)
|
150,000 | 1.05 |
05/01/13
|
|||||||||
| 50,000 | 1.05 |
05/01/13
|
||||||||||
| 50,000 | 1.05 |
04/12/12
|
||||||||||
| 95,000 | (1) | 1.94 |
08/31/13
|
|||||||||
| 60,000 | 1.05 |
04/19/13
|
||||||||||
| 60,000 | 1.38 |
03/22/14
|
||||||||||
| 560,000 | (1) | 1.05 |
10/16/13
|
|||||||||
| 105,000 | 0.54 |
03/14/12
|
||||||||||
| 1,890,000 | 0.22 |
04/01/13
|
||||||||||
| 50,000 | 0.61 |
09/02/14
|
||||||||||
| 50,000 | 0.48 |
09/21/15
|
|||||||||||
| 200,000 | 0.58 |
09/12/16
|
|||||||||||
| 200,000 | 0.63 |
09/13/17
|
|||||||||||
| 200,000 | 0.62 |
03/04/18
|
|||||||||||
| 1,838,609 | (2) | 0.25 |
04/23/19
|
||||||||||
| 200,000 | 0.38 |
07/20/19
|
|||||||||||
| 100,000 | 0.48 |
07/20/20
|
|||||||||||
| 6,246,609 | |||||||||||||
| 4,000,000 | (3) | 0.38 |
07/06/19
|
||||||||||
| 100,000 | 0.38 |
07/20/19
|
|||||||||||
| 200,000 | 0.48 |
07/20/20
|
|||||||||||
| 300,000 | 0.69 |
04/14/21
|
|||||||||||
| 4,600,000 | |||||||||||||
|
Patricia B. Prichep
|
6,000 | 1.05 |
12/01/13
|
||||||||||
| 10,000 | 1.05 |
11/30/13
|
|||||||||||
| 9,500 | 1.05 |
07/31/13
|
|||||||||||
| 3,000 | 1.05 |
12/31/12
|
|||||||||||
| 35,000 | 1.05 |
03/01/13
|
|||||||||||
| 17,000 | 1.05 |
12/01/13
|
|||||||||||
| 15,000 | 1.05 |
04/12/12
|
|||||||||||
| 47,500 | (1) | 1.94 |
08/31/13
|
||||||||||
| 23,000 | 1.05 |
02/02/13
|
|||||||||||
| 25,000 | 1.18 |
12/08/13
|
|||||||||||
| 30,000 | 1.00 |
12/03/14
|
|||||||||||
| 200,000 | (1) | 1.05 |
10/16/13
|
||||||||||
| 10,500 | 0.54 |
03/14/12
|
|||||||||||
| 50,000 | 0.33 |
04/26/12
|
|||||||||||
| 243,000 | 0.22 |
04/01/13
|
|||||||||||
| 337,000 | 0.22 |
04/01/13
|
|||||||||||
| 50,000 | 0.61 |
09/02/14
|
|||||||||||
| 30,000 | 0.48 |
09/21/15
|
|||||||||||
| 90,000 | 0.58 |
09/12/16
|
|||||||||||
| 100,000 | 0.63 |
09/13/17
|
|||||||||||
| 100,000 | 0.62 |
03/04/18
|
|||||||||||
| 717,096 | (2) | 0.25 |
04/23/19
|
||||||||||
| 100,000 | 0.38 |
07/20/19
|
|
Shares underlying unexercised
|
|||||||||||||
|
Option which are:
|
Exercise
|
Expiration
|
|||||||||||
|
Name
|
Exercisable
|
Unexercisable
|
Price
|
Date
|
|||||||||
|
Patricia B. Prichep
|
50,000 | 0.48 |
07/20/20
|
||||||||||
| (Cont’d) | 2,298,596 |
|
|||||||||||
| 3,000,000 | (3) | 0.38 |
07/06/19
|
||||||||||
| 50,000 | 0.38 |
07/20/19
|
|||||||||||
| 100,000 | 0.48 |
07/20/20
|
|||||||||||
| 150,000 | 0.69 |
04/14/21
|
|||||||||||
| 3,300,000 | |||||||||||||
|
Eyal Talor, Ph.D.
|
15,500 | 1.05 |
07/31/13
|
||||||||||
| 16,666 | 1.05 |
03/16/13
|
|||||||||||
| 15,000 | 1.05 |
08/03/13
|
|||||||||||
| 10,000 | (1) | 1.94 |
08/31/13
|
||||||||||
| 20,000 | 1.05 |
08/02/12
|
|||||||||||
| 25,000 | 1.76 |
11/10/13
|
|||||||||||
| 35,000 | 1.00 |
12/03/14
|
|||||||||||
| 160,000 | (1) | 1.05 |
10/16/13
|
||||||||||
| 50,000 | 0.33 |
04/26/12
|
|||||||||||
| 374,166 | 0.22 |
04/01/13
|
|||||||||||
| 50,000 | 0.61 |
09/02/14
|
|||||||||||
| 30,000 | 0.48 |
09/21/15
|
|||||||||||
| 80,000 | 0.58 |
09/12/16
|
|||||||||||
| 100,000 | 0.63 |
09/13/17
|
|||||||||||
| 100,000 | 0.62 |
03/04/18
|
|||||||||||
| 240,820 | (2) | 0.25 |
04/23/19
|
||||||||||
| 100,000 | 0.38 |
07/20/19
|
|||||||||||
| 50,000 | 0.48 |
07/20/20
|
|||||||||||
| 1,472,152 | |||||||||||||
| 3,000,000 | (3) | 0.38 |
07/06/19
|
||||||||||
| 50,000 | 0.38 |
07/20/19
|
|||||||||||
| 100,000 | 0.48 |
07/20/20
|
|||||||||||
| 150,000 | 0.69 |
04/14/21
|
|||||||||||
| 3,300,000 | |||||||||||||
|
Daniel Zimmerman, Ph.D.
|
12,000 | 1.05 |
12/31/13
|
||||||||||
| 7,000 | 1.05 |
06/19/13
|
|||||||||||
| 15,000 | 1.05 |
02/19/13
|
|||||||||||
| 30,000 | (1) | 1.94 |
08/31/13
|
||||||||||
| 20,000 | 1.05 |
02/02/13
|
|||||||||||
| 20,000 | 1.85 |
01/26/14
|
|||||||||||
| 120,000 | (1) | 1.05 |
10/16/13
|
||||||||||
| 41,000 | 0.54 |
03/14/12
|
|||||||||||
| 50,000 | 0.33 |
04/16/12
|
|||||||||||
| 392,000 | 0.22 |
04/01/13
|
|||||||||||
| 50,000 | 0.61 |
09/02/14
|
|||||||||||
| 30,000 | 0.48 |
09/21/15
|
|||||||||||
|
Shares
Underlying U
nexercised
|
Options Which are:
|
Exercise
|
Expiration
|
||||||||||
|
Name
|
Exercisable
|
Unexercisable
|
Price
|
Date
|
|||||||||
|
Daniel Zimmerman, Ph.D.
|
60,000 | 0.58 |
09/12/16
|
||||||||||
|
(cont’d)
|
75,000 | 0.63 |
09/13/17
|
||||||||||
| 75,000 | 0.62 |
03/04/18
|
|||||||||||
| 200,000 | (4) | 0.38 |
07/15/14
|
||||||||||
| 50,000 | 0.48 |
07/20/20
|
|||||||||||
| 1,247,000 | |||||||||||||
| 100,000 | 0.48 |
07/20/20
|
|||||||||||
| 150,000 | 0.69 |
04/14/21
|
|||||||||||
| 250,000 | |||||||||||||
|
John Cipriano
|
20,000 | 0.61 |
09/02/14
|
||||||||||
| 30,000 | 0.48 |
09/21/15
|
|||||||||||
| 60,000 | 0.58 |
09/12/16
|
|||||||||||
| 75,000 | 0.63 |
09/13/17
|
|||||||||||
| 75,000 | 0.62 |
03/04/18
|
|||||||||||
| 66,667 | 1.93 |
09/30/19
|
|||||||||||
| 50,000 | 0.48 |
07/20/20
|
|||||||||||
| 376,667 | |||||||||||||
| 33,333 | 1.93 |
09/30/19
|
|||||||||||
| 100,000 | 0.48 |
07/20/20
|
|||||||||||
| 150,000 | 0.69 |
04/14/21
|
|||||||||||
| 283,333 | |||||||||||||
|
(1)
|
Options purchased by Employee through the Salary Reduction Plan.
|
|
(2)
|
Options awarded to employees who did not collect a salary, or reduced or deferred their salary between September 15, 2008 and June 30, 2009. For example, Mr. de Clara, Mr. Kersten and Ms. Prichep did not collect any salary between September 30, 2008 and June 30, 2009.
|
|
(3)
|
Long-term performance options: The Board of Directors has identified the successful Phase III clinical trial for Multikine to be the most important corporate event to create shareholder value. Therefore, one third of the options can be exercised when the first 400 patients are enrolled in CEL-SCI's Phase III head and neck cancer clinical trial. One third of the options can be exercised when all of the patients have been enrolled in the Phase III clinical trial. One third of the options can be exercised when the Phase III trial is completed. The grant-date fair value of these options awarded to the senior management of the Company amounts to $3.3 million in total. A major consideration in the valuation of these options is the likelihood of the CEL-SCI reaching these milestones. CEL-SCI’s management has assumed the likelihood of these milestones being reached to be 100%.of these options is the likelihood of the CEL-SCI reaching these milestones.
|
|
(4)
|
Options awarded to employee during the period that he was a consultant to CEL-SCI.
|
|
Total
|
Shares
|
|||||||||||||||
|
Shares
|
Reserved for
|
Shares
|
Remaining
|
|||||||||||||
|
Reserved
|
Outstanding
|
Issued as
|
Options/Shares
|
|||||||||||||
|
Name of Plan
|
Under Plans
|
Options
|
Stock Bonus
|
Under Plans
|
||||||||||||
|
Incentive Stock Option Plans
|
19,100,000 | 11,168,041 | N/A | 7,320,225 | ||||||||||||
|
Non-Qualified Stock Option Plans
|
35,760,000 | 23,461,240 | N/A | 8,690,510 | ||||||||||||
|
Stock Bonus Plans
|
13,940,000 | N/A | 7,766,340 | 6,171,372 | ||||||||||||
|
Stock Compensation Plan
|
11,500,000 | N/A | 5,386,531 | 6,113,469 | ||||||||||||
|
Number of Securities
|
||||||||||||
|
Number
|
Remaining Available
|
|||||||||||
|
of Securities
|
For Future Issuance
|
|||||||||||
|
to be Issued
|
Weighted-Average
|
Under Equity
|
||||||||||
|
Upon Exercise
|
Exercise Price of
|
Compensation Plans,
|
||||||||||
|
of Outstanding
|
of Outstanding
|
Excluding Securities
|
||||||||||
|
Plan category
|
Options (a)
|
Options
|
Reflected in Column (a)
|
|||||||||
|
Incentive Stock
|
11,168,041 | $ | 0.42 | 7,320,225 | ||||||||
|
Option Plans
|
||||||||||||
|
Non-Qualified Stock
|
23,461,240 | $ | 0.51 | 8,690,510 | ||||||||
|
Option Plans
|
||||||||||||
| Name and Address | Number of Shares (1) | Percent of Class (3) | ||||||
|
Maximilian de Clara
|
6,557,023 | 2.8 | % | |||||
|
Bergstrasse 79
|
||||||||
|
6078 Lungern,
|
||||||||
|
Obwalden, Switzerland
|
||||||||
|
Geert R. Kersten
|
9,308,921 | 3.9 | % | |||||
|
8229 Boone Blvd., Suite 802
|
||||||||
|
Vienna, VA 22182
|
||||||||
|
Patricia B. Prichep
|
3,029,149 | 1.3 | % | |||||
|
8229 Boone Blvd., Suite 802
|
||||||||
|
Vienna, VA 22182
|
||||||||
|
Eyal Talor, Ph.D.
|
1,851,022 | 0.8 | % | |||||
|
8229 Boone Blvd., Suite 802
|
||||||||
|
Vienna, VA 22182
|
||||||||
|
Daniel H. Zimmerman, Ph.D.
|
1,518,522 | 0.7 | % | |||||
|
8229 Boone Blvd., Suite 802
|
||||||||
|
Vienna, VA 22182
|
||||||||
|
John Cipriano
|
559,360 | 0.2 | % | |||||
|
8229 Boone Blvd., Suite 802
|
||||||||
|
Vienna, VA 22182
|
||||||||
|
Alexander G. Esterhazy
|
920,489 | 0.4 | % | |||||
|
20 Chemin du Pre-Poiset
|
||||||||
|
CH- 1253 Vandoeuvres
|
||||||||
|
Geneve, Switzerland
|
||||||||
|
C. Richard Kinsolving, Ph.D.
|
1,096,247 | 0.5 | % | |||||
|
P.O. Box 20193
|
||||||||
|
Bradenton, FL 34204-0193
|
||||||||
|
Peter R. Young, Ph.D.
|
937,757 | 0.4 | % | |||||
|
2500 Marketplace Drive, Unit 431
|
||||||||
|
Waco, TX 76711
|
||||||||
|
All Officers and Directors
|
25,778,490 | 10.3 | % | |||||
|
as a Group (9 persons)
|
||||||||
|
(1)
|
Includes shares issuable prior to February 28, 2012 upon the exercise of options or warrants granted to the following persons:
|
|
Options or Warrants Exercisable
|
||||
|
Name
|
Prior to February 28, 2012
|
|||
|
Maximilian de Clara
|
6,205,789 | |||
|
Geert R. Kersten
|
5,933,009 | |||
|
Patricia B. Prichep
|
2,202,296 | |||
|
Eyal Talor, Ph.D.
|
1,402,719 | |||
|
Daniel Zimmerman
|
1,184,000 | |||
|
John Cipriano
|
372,667 | |||
|
Alexander G. Esterhazy
|
687,332 | |||
|
C. Richard Kinsolving, Ph.D.
|
794,000 | |||
|
Peter R. Young, Ph.D.
|
689,999 | |||
|
(2)
|
Amount includes shares held in trust for the benefit of Mr. Kersten's minor children. Geert R. Kersten is the stepson of Maximilian de Clara.
|
|
(3)
|
Amount includes shares referred to in (1) above but excludes shares which may be issued upon the exercise or conversion of other options, warrants and other convertible securities previously issued by CEL-SCI.
|
|
Year Ended
September 30,
|
||||||||
|
2011
|
2010
|
|||||||
|
Audit Fees
|
$ | 237,835 | $ | 232,725 | ||||
|
Audit-Related Fees
|
-- | -- | ||||||
|
Tax Fees
|
-- | -- | ||||||
|
All Other Fees
|
$ | 4,370 | $ | 44,126 | ||||
| Exhibits | ||||
| 3(a) | Articles of Incorporation | Incorporated by reference to Exhibit 3(a) of CEL-SCI's combined Registration Statement on Form S-1 and Post-Effective Amendment ("Registration Statement"), Registration Nos. 2-85547-D and 33-7531. | ||
| 3(b) | Amended Articles | Incorporated by reference to Exhibit 3(a) of CEL-SCI's Registration Statement on Form S-1, Registration Nos. 2-85547-D and 33-7531. | ||
| 3(c) | Amended Articles (Name change only) | Filed as Exhibit 3(c) to CEL-SCI's Registration Statement on Form S-1 Registration Statement (No. 33-34878). | ||
| 3(d) | Bylaws | Incorporated by reference to Exhibit 3(b) of CEL-SCI's Registration Statement on Form S-1, Registration Nos. 2-85547-D and 33-7531. | ||
| 4 | Shareholders Rights Agreement | Incorporated by reference to Exhibit 4 of CEL-SCI’S report on Form 8-K dated November 7, 2007. | ||
| 10(d) | Employment Agreement with Maximilian de Clara | Incorporated by reference to Exhibit 10(d) of CEL-SCI’s report on Form 8-K (dated April 21, 2005) and Exhibit 10(d) to CEL-SCI’s report on Form 8-K dated September 8, 2006. | ||
| 10(f) | Distribution and Royalty Agreement with Eastern Biotech | Incorporated by reference to Exhibit 10(x) to Amendment No. 2 to CEL-SCI’s Registration statement on Form S-3 (Commission File No. 333-106879). | ||
| 10(g) | Securities Purchase Agreement (together with schedule required by Instruction 2 to Item 601 of Regulation S-K) pertaining to Series K notes and warrants, together with The exhibits to the Securities Purchase Agreement. | Incorporated by reference to Exhibit 10 to CEL-SCI’s report on Form 8-K dated August 4, 2006. | ||
| 10(h) | Subscription Agreement (together with Schedule required by Instruction 2 to Item 601 of Regulation S-K) pertaining to April 2007 sale of 20,000,000 shares of CEL-SCI’s common stock, 10,000,000 Series L warrants and 10,000,000 Series M Warrants. | Incorporated by reference to Exhibit 10 of CEL-SCI’s report on Form 8-K dated April 18, 2007. |
| 10(i) | Warrant Adjustment Agreement with Laksya Ventures | Incorporated by reference to Exhibit 10(i) of CEL-SCI’s report on Form 8-K dated August 3, 2010. | ||
| 10(j) | Employment Agreement with Patricia Prichep | Incorporated by reference to Exhibit 10(j) of CEL-SCI’s report on Form 8-K dated August 30, 2010. | ||
| 10(k) | Employment Agreement with Eyal Taylor | Incorporated by reference to Exhibit 10(k) of CEL-SCI’s report on Form 8-K dated August 30, 2010. | ||
| 10(l) | Amendment to Employment Agreement with Maximilian de Clara | Incorporated by reference to Exhibit 10(l) of CEL-SCI’s report on Form 8-K dated August 30, 2010. | ||
| 10(m) | Amendment to Development Supply and Distribution Agreement with Orient Europharma. (part of Exhibit 10(m) has been omitted pursuant to a request for confidential treatment). | Incorporated by reference to Exhibit 10(m) filed with CEL-SCI’s 10-K report for the year ended September 30, 2010. | ||
| 10(n) | Licensing Agreement with Teva Pharmaceutical Industries Ltd. (parts of Exhibit 10(n) have been omitted pursuantto a request for confidential treatment.) | Incorporated by reference to Exhibit 10(n) filed with CEL-SCI’s 10-K report for the year ended September 30, 2010. | ||
| 10(o) | Lease Agreement (parts of Exhibit 10(o) have been omitted pursuant to a request for confidential treatment). | Incorporated by reference to Exhibit 10(o) filed with CEL-SCI’s 10-K report for the year ended September 30, 2010. | ||
| 10(p) | Loan Agreements with Maximilian de Clara | Incorporated by reference to Exhibit 10(p) filed with CEL-SCI’s 10-K report for the year ended September 30, 2010. | ||
| 10(q) | Licensing Agreement with Byron Biopharma | Incorporated by reference to Exhibit 10(i) of CEL-SCI’s report on Form 8-K dated March 27, 2009. | ||
| 10(r) | At Market Issuance Sales Agreement with McNicoll, Lewis & Vlak LLC | Incorporated by reference to Exhibit 10(r) filed with CEL-SCI’s 10-K report for the year ended September 30, 2010. |
| 10(z) | Development, Supply and Distribution Agreement with Orient Europharma | Incorporated by reference to Exhibit 10(z) filed with CEL-SCI’s report on Form 10-K for the year ended September 30, 2003. | ||
| 10(aa) | Securities Purchase Agreement and form of the Series F warrants, which is and exhibit to the Securities Purchase Agreement. | Incorporated by reference to Exhibit 10(aa) of CEL-SCI’s report on Form 8-K dated October 3, 2011. | ||
| 10(bb) | Employment Agreement with Geert Kersten | Incorporated by reference to Exhibit 10(bb) to CEL-SCI’s report on Form 8-K dated September 1, 2011. | ||
| 23.1 | Consent of BDO USA, LLP | |||
| 31.1 | Rule 13a-14(a) Certifications | |||
| 31.2 | Rule 13a-14(a) Certifications | |||
| 32.1 | Section 1350 Certifications |
| PAGE | |
| REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM | F-3 |
| CONSOLIDATED FINANCIAL STATEMENTS FOR THE YEARS ENDED SEPTEMBER 30, 2011, 2010, AND 2009: | |
| Consolidated Balance Sheets | F-5 |
| Consolidated Statements of Operations | F-6 |
| Consolidated Statements of Stockholders' Equity | F-7 |
| Consolidated Statements of Cash Flows | F-8 |
| Notes to Consolidated Financial Statements | F-11 |
|
CEL-SCI CORPORATION
|
||||||||
|
CONSOLIDATED BALANCE SHEETS
|
||||||||
|
SEPTEMBER 30, 2011 AND 2010
|
||||||||
|
ASSETS
|
2011
|
2010
|
||||||
|
CURRENT ASSETS:
|
||||||||
|
Cash and cash equivalents
|
$ | 4,260,594 | $ | 26,568,243 | ||||
|
Receivables
|
457,337 | - | ||||||
|
Prepaid expenses
|
2,028,531 | 298,719 | ||||||
|
Inventory used for R&D and manufacturing
|
1,571,182 | 1,476,234 | ||||||
|
Deferred rent - current portion
|
703,274 | 751,338 | ||||||
|
Total current assets
|
9,020,918 | 29,094,534 | ||||||
|
|
||||||||
|
RESEARCH AND OFFICE EQUIPMENT AND
LEASEHOLD IMPROVEMENTS-- less accumulated depreciation and amortization of $3,034,018 and $2,626,759
|
1,032,881 | 1,264,831 | ||||||
|
PATENT COSTS--less accumulated
amortization of $1,287,323 and $1,205,690
|
414,158 | 356,079 | ||||||
|
RESTRICTED CASH
|
- | 21,357 | ||||||
|
DEFERRED RENT - net of current portion
|
6,486,566 | 7,068,184 | ||||||
|
DEPOSITS
|
1,670,917 | - | ||||||
|
TOTAL ASSETS
|
$ | 18,625,440 | $ | 37,804,985 | ||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
CURRENT LIABILITIES:
|
||||||||
|
Accounts payable
|
738,951 | 1,497,383 | ||||||
|
Accrued expenses
|
290,220 | 223,696 | ||||||
|
Due to employees
|
22,789 | 45,808 | ||||||
|
Related party loan
|
1,104,057 | 1,104,057 | ||||||
|
Convertible note
|
4,999,000 | - | ||||||
|
Derivative instruments - current portion
|
69,552 | 424,286 | ||||||
|
Total current liabilities
|
7,224,569 | 3,295,230 | ||||||
|
Derivative instruments - net of current portion
|
2,192,521 | 6,521,765 | ||||||
|
Deferred revenue
|
125,000 | 125,000 | ||||||
|
Deferred rent
|
4,526 | 8,225 | ||||||
|
Total liabilities
|
9,546,616 | 9,950,220 | ||||||
|
COMMITMENTS AND CONTINGENCIES
|
||||||||
|
STOCKHOLDERS' EQUITY
|
||||||||
|
Preferred stock, $.01 par value--authorized
|
||||||||
|
200,000 shares, issued and outstanding, -0-
|
- | - | ||||||
|
Common stock, $.01 par value--authorized
|
||||||||
|
450,000,000 shares; issued and
|
||||||||
|
outstanding, 214,723,023 and 204,868,853
|
||||||||
|
shares at September 30, 2011 and 2010,
|
||||||||
|
respectively
|
2,147,230 | 2,048,689 | ||||||
|
Additional paid-in capital
|
194,443,905 | 187,606,044 | ||||||
|
Accumulated deficit
|
(187,512,311 | ) | (161,799,968 | ) | ||||
|
Total stockholders' equity
|
9,078,824 | 27,854,765 | ||||||
|
TOTAL LIABILITIES AND
STOCKHOLDERS' EQUITY
|
$ | 18,625,440 | $ | 37,804,985 | ||||
|
CEL-SCI CORPORATION
|
||||||||||||
|
|
||||||||||||
|
YEARS ENDED SEPTEMBER 30, 2011, 2010 AND 2009
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
GRANT INCOME AND OTHER
|
$ | 956,154 | $ | 153,300 | $ | 80,093 | ||||||
|
OPERATING EXPENSES:
|
||||||||||||
|
Research and development (excluding
|
||||||||||||
|
R&D depreciation of $438,738, $434,030
|
||||||||||||
|
and $329,866 respectively, included below)
|
11,745,629 | 11,911,626 | 6,011,750 | |||||||||
|
Depreciation and amortization
|
531,316 | 516,117 | 417,205 | |||||||||
|
General & administrative
|
6,664,883 | 6,285,810 | 5,671,595 | |||||||||
|
Total operating expenses
|
18,941,828 | 18,713,553 | 12,100,550 | |||||||||
|
OPERATING LOSS
|
(17,985,674 | ) | (18,560,253 | ) | (12,020,457 | ) | ||||||
|
OTHER EXPENSES
|
(12,000,000 | ) | - | - | ||||||||
|
GAIN (LOSS) ON DERIVATIVE INSTRUMENTS
|
4,432,148 | 28,843,772 | (28,491,650 | ) | ||||||||
|
INTEREST INCOME
|
164,163 | 362,236 | - | |||||||||
|
INTEREST EXPENSE
|
(322,980 | ) | (162,326 | ) | (397,923 | ) | ||||||
|
NET (LOSS) INCOME
|
(25,712,343 | ) | 10,483,429 | (40,910,030 | ) | |||||||
|
MODIFICATIONS OF WARRANTS
|
(1,068,369 | ) | (1,532,456 | ) | (490,728 | ) | ||||||
|
NET (LOSS) INCOME AVAILABLE TO COMMON SHAREHOLDERS
|
$ | (26,780,712 | ) | $ | 8,950,973 | $ | (41,400,758 | ) | ||||
|
NET (LOSS) INCOME PER COMMON SHARE
|
||||||||||||
|
BASIC
|
$ | (0.13 | ) | $ | 0.04 | $ | (0.31 | ) | ||||
|
DILUTED
|
$ | (0.15 | ) | $ | (0.06 | ) | $ | (0.31 | ) | |||
|
WEIGHTED AVERAGE COMMON SHARES OUTSTANDING
|
||||||||||||
|
BASIC
|
208,488,987 | 202,102,859 | 133,535,050 | |||||||||
|
DILUTED
|
208,488,987 | 202,102,859 | 133,535,050 | |||||||||
|
CEL-SCI CORPORATION
|
||||||||||||||||||||
|
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
|
||||||||||||||||||||
|
YEARS ENDED SEPTEMBER 30, 2011, 2010 AND 2009
|
||||||||||||||||||||
|
Additional
|
||||||||||||||||||||
|
Common
|
Stock
|
Paid-In
|
Accumulated
|
|||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Deficit
|
Total
|
||||||||||||||||
|
BALANCE, SEPTEMBER 30, 2008
|
120,796,094 | $ | 1,207,961 | $ | 134,324,370 | $ | (124,696,296 | ) | $ | 10,836,035 | ||||||||||
|
Sale of common stock
|
45,451,547 | 454,515 | 31,788,201 | 32,242,716 | ||||||||||||||||
|
401(k) contributions paid
|
||||||||||||||||||||
|
in common stock
|
91,766 | 917 | 56,912 | 57,829 | ||||||||||||||||
|
Exercise of stock options
|
15,659,116 | 156,591 | 8,524,663 | 8,681,254 | ||||||||||||||||
|
Stock issued to nonemployees
|
||||||||||||||||||||
|
for service
|
3,316,438 | 33,164 | 1,528,179 | 1,561,343 | ||||||||||||||||
|
Stock issued to employees
|
1,324,385 | 13,244 | 672,614 | 685,858 | ||||||||||||||||
|
Stock issued for principal payments
|
||||||||||||||||||||
|
on Series K notes
|
972,753 | 9,728 | 275,272 | 285,000 | ||||||||||||||||
|
Stock issued for interest on Series K notes
|
177,403 | 1,774 | 41,111 | 42,885 | ||||||||||||||||
|
Issuance of stock options and warrants
|
||||||||||||||||||||
|
to nonemployees
|
449,641 | 449,641 | ||||||||||||||||||
|
Loss on conversion of convertible stock
|
2,145,754 | 2,145,754 | ||||||||||||||||||
|
Issuance of warrants for short term loan
|
65,796 | 65,796 | ||||||||||||||||||
|
Modification of options
|
6,142 | 6,142 | ||||||||||||||||||
|
Employee option cost
|
1,699,448 | 1,699,448 | ||||||||||||||||||
|
Premium on loan from shareholder
|
489,776 | 489,776 | ||||||||||||||||||
|
Conversion of convertible debt
|
||||||||||||||||||||
|
into common stock
|
3,015,852 | 30,159 | 1,176,182 | 1,206,341 | ||||||||||||||||
|
Cost of derivative liabilities
|
(8,632,217 | ) | (8,632,217 | ) | ||||||||||||||||
|
Financing costs
|
(2,072,927 | ) | (2,072,927 | ) | ||||||||||||||||
|
Dividends
|
1,166,667 | 11,667 | 479,061 | (490,728 | ) | - | ||||||||||||||
|
Net loss
|
(40,910,030 | ) | (40,910,030 | ) | ||||||||||||||||
|
BALANCE, SEPTEMBER 30, 2009
|
191,972,021 | 1,919,720 | 173,017,978 | (166,097,054 | ) | 8,840,644 | ||||||||||||||
|
401(k) contributions paid
|
||||||||||||||||||||
|
in common stock
|
182,233 | 1,822 | 110,503 | 112,325 | ||||||||||||||||
|
Exercise of warrants and stock options
|
12,249,441 | 122,495 | 6,186,379 | 6,308,874 | ||||||||||||||||
|
Stock issued to nonemployees
|
||||||||||||||||||||
|
for service
|
465,158 | 4,652 | 1,236,374 | 1,241,026 | ||||||||||||||||
|
Exercise of derivative liabilities
|
5,510,490 | 5,510,490 | ||||||||||||||||||
|
Modification of stock options and warrants
|
227,921 | 227,921 | ||||||||||||||||||
|
Employee option cost
|
1,316,399 | 1,316,399 | ||||||||||||||||||
|
Adoption of ASC 815-40
|
(6,186,343 | ) | (6,186,343 | ) | ||||||||||||||||
|
Net income
|
10,483,429 | 10,483,429 | ||||||||||||||||||
|
BALANCE, SEPTEMBER 30, 2010
|
204,868,853 | 2,048,689 | 187,606,044 | (161,799,968 | ) | 27,854,765 | ||||||||||||||
|
Sale of stock
|
7,424,982 | 74,250 | 3,862,034 | 3,936,284 | ||||||||||||||||
|
401(k) contributions paid
|
||||||||||||||||||||
|
in common stock
|
294,309 | 2,943 | 147,922 | 150,865 | ||||||||||||||||
|
Exercise of warrants and stock options
|
1,786,599 | 17,866 | 661,722 | 679,588 | ||||||||||||||||
|
Stock issued to nonemployees
|
||||||||||||||||||||
|
for service
|
348,280 | 3,482 | 210,641 | 214,123 | ||||||||||||||||
|
Dismissal of liability for overpayment
|
81,395 | 81,395 | ||||||||||||||||||
|
Exercise of derivative liabilities
|
202,830 | 202,830 | ||||||||||||||||||
|
Modification of stock options and warrants
|
135,988 | 135,988 | ||||||||||||||||||
|
Employee option cost
|
1,535,329 | 1,535,329 | ||||||||||||||||||
|
Net loss
|
(25,712,343 | ) | (25,712,343 | ) | ||||||||||||||||
|
BALANCE, SEPTEMBER 30, 2011
|
214,723,023 | $ | 2,147,230 | $ | 194,443,905 | $ | (187,512,311 | ) | $ | 9,078,824 | ||||||||||
|
CEL-SCI CORPORATION
|
||||||||||||
|
|
||||||||||||
|
YEARS ENDED SEPTEMBER 30, 2011, 2010 AND 2009
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||||||||||
|
Net (loss) income
|
$ | (25,712,343 | ) | $ | 10,483,429 | $ | (40,910,030 | ) | ||||
|
Adjustments to reconcile net (loss) income to
|
||||||||||||
|
net cash used in operating activities:
|
||||||||||||
|
Depreciation and amortization
|
531,316 | 516,117 | 417,205 | |||||||||
|
Issuance of stock options and warrants to
|
||||||||||||
|
nonemployees for services
|
- | - | 449,641 | |||||||||
|
Issuance of convertible notes and preferred stock in legal settlement
|
9,000,000 | - | - | |||||||||
|
Issuance of common stock for services
|
214,123 | 1,241,026 | 1,561,343 | |||||||||
|
Premium on loan
|
- | - | 341,454 | |||||||||
|
Loan premium adjustment
|
- | - | 489,776 | |||||||||
|
Amortization of loan premium
|
- | (3,282 | ) | (338,172 | ) | |||||||
|
Modification of stock options and warrants
|
135,988 | 227,921 | 6,142 | |||||||||
|
Issuance of stock to employees
|
- | - | 685,858 | |||||||||
|
Loss on conversion of convertible notes
|
- | - | 2,145,754 | |||||||||
|
Employee option cost
|
1,535,329 | 1,316,399 | 1,699,448 | |||||||||
|
Common stock contributed to 401(k) plan
|
150,865 | 112,325 | 57,829 | |||||||||
|
Warrants issued in consideration for loan
|
- | - | 65,796 | |||||||||
|
Impairment loss on abandonment of patents
|
9,016 | 13,877 | 138,525 | |||||||||
|
Loss on retired equipment
|
2,828 | 2,323 | 270 | |||||||||
|
Deferred rent
|
(3,699 | ) | (6,080 | ) | 7,688 | |||||||
|
Amortization of discount on
|
||||||||||||
|
convertible note
|
- | - | 193,980 | |||||||||
|
(Gain)/loss on derivative instruments
|
(4,432,148 | ) | (28,843,772 | ) | 25,514,667 | |||||||
|
Change in assets and liabilities:
|
||||||||||||
|
(Increase)/decrease in deposits
|
(1,670,917 | ) | 1,585,064 | 4,764 | ||||||||
|
Increase in receivables
|
(457,337 | ) | - | - | ||||||||
|
Decrease in deferred rent
|
629,682 | 955,842 | 622,350 | |||||||||
|
Increase in prepaid expenses
|
(1,729,812 | ) | (258,747 | ) | (12,763 | ) | ||||||
|
Increase in inventory used for
|
||||||||||||
|
R&D and manufacturing
|
(94,948 | ) | (1,076,760 | ) | (4,304 | ) | ||||||
|
(Decrease)/increase in accounts payable
|
(788,254 | ) | 693,799 | 343,208 | ||||||||
|
Increase/(decrease) in accrued expenses
|
147,919 | 125,031 | (14,514 | ) | ||||||||
|
Decrease in accrued interest on convertible debt
|
- | - | (2,674 | ) | ||||||||
|
Increase in deferred revenue
|
- | 125,000 | - | |||||||||
|
(Decrease)/increase in due to employees
|
(23,019 | ) | (3,719 | ) | 13,450 | |||||||
|
(Decrease)/increase in deposits held
|
- | (10,000 | ) | 10,000 | ||||||||
|
Net cash used in operating activities
|
(22,555,411 | ) | (12,804,207 | ) | (6,513,309 | ) | ||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||||||
|
Additional investment in manufacturing facility
|
- | (32,059 | ) | (505,225 | ) | |||||||
|
Decrease in restricted cash
|
21,357 | 47,195 | 919,100 | |||||||||
|
Sale of investments in available-for-sale securities
|
- | - | 200,000 | |||||||||
|
Purchases of equipment
|
(216,761 | ) | (493,736 | ) | (191,868 | ) | ||||||
|
Expenditures for patent costs
|
(122,706 | ) | (25,340 | ) | (53,290 | ) | ||||||
|
Net cash (used in) provided by investing activities
|
(318,110 | ) | (503,940 | ) | 368,717 | |||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
||||||||||||
|
Proceeds from issuance of common stock
|
3,936,284 | - | 32,242,716 | |||||||||
|
Proceeds from exercise of warrants and stock options
|
679,588 | 6,308,874 | 8,681,254 | |||||||||
|
Proceeds from short-term loan
|
- | - | 3,104,057 | |||||||||
|
Repayment of short-term loan
|
- | - | (2,200,000 | ) | ||||||||
|
Payments for repurchase of preferred stock
|
(4,050,000 | ) | - | (754,250 | ) | |||||||
|
Costs for equity related transactions
|
- | - | (2,072,927 | ) | ||||||||
|
Net cash provided by financing activities
|
565,872 | 6,308,874 | 39,000,850 | |||||||||
|
NET (DECREASE) INCREASE
IN CASH AND CASH EQUIVALENTS
|
(22,307,649 | ) | (6,999,273 | ) | 32,856,258 | |||||||
|
CASH AND CASH EQUIVALENTS, BEGINNING OF YEAR
|
26,568,243 | 33,567,516 | 711,258 | |||||||||
|
CASH AND CASH EQUIVALENTS, END OF YEAR
|
$ | 4,260,594 | $ | 26,568,243 | $ | 33,567,516 | ||||||
|
CEL-SCI CORPORATION
|
||||||||||||
|
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
||||||||||||
|
YEARS ENDED SEPTEMBER 30, 2011, 2010 AND 2009
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
CONVERSION OF CONVERTIBLE DEBT INTO COMMON STOCK:
|
||||||||||||
|
Decrease in convertible debt
|
$ | - | $ | - | $ | 1,206,341 | ||||||
|
Increase in common stock
|
- | - | (30,159 | ) | ||||||||
|
Increase in additional paid-in capital
|
- | - | (1,176,182 | ) | ||||||||
| $ | - | $ | - | $ | - | |||||||
|
CONVERSION OF INTEREST ON CONVERTIBLE DEBT INTO COMMON STOCK:
|
||||||||||||
|
Decrease in accrued liabilities
|
$ | - | $ | - | $ | 42,885 | ||||||
|
Increase in common stock
|
- | - | (1,774 | ) | ||||||||
|
Increase in additional paid-in capital
|
- | - | (41,111 | ) | ||||||||
| $ | - | $ | - | $ | - | |||||||
|
PAYMENT OF CONVERTIBLE DEBT PRINCIPAL WITH
COMMON STOCK:
|
||||||||||||
|
Decrease in convertible debt
|
$ | - | $ | - | $ | 285,000 | ||||||
|
Increase in common stock
|
- | - | (9,728 | ) | ||||||||
|
Increase in additional paid-in capital
|
- | - | (275,272 | ) | ||||||||
| $ | - | $ | - | $ | - | |||||||
|
ISSUANCE OF WARRANTS:
|
||||||||||||
|
Increase in derivative liabilities
|
$ | - | $ | - | $ | (8,877,217 | ) | |||||
|
Increase in discount on notes payable
|
- | - | 245,000 | |||||||||
|
Decrease in additional paid-in capital
|
- | - | 8,632,217 | |||||||||
| $ | - | $ | - | $ | - | |||||||
|
EXERCISE OF DERIVATIVE LIABILITIES:
|
||||||||||||
|
Decrease in derivative liabilities
|
$ | 202,830 | $ | 5,510,490 | $ | - | ||||||
|
Increase in additional paid-in capital
|
(202,830 | ) | (5,510,490 | ) | - | |||||||
| $ | - | $ | - | $ | - | |||||||
|
MODIFICATION OF WARRANTS:
|
||||||||||||
|
Increase in additional paid-in capital
|
$ | - | $ | (1,532,456 | ) | $ | (24,061 | ) | ||||
|
Decrease in additional paid-in capital
|
- | 1,532,456 | 24,061 | |||||||||
| $ | - | $ | - | $ | - | |||||||
|
CEL-SCI CORPORATION
|
||||||||||||
|
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
||||||||||||
|
YEARS ENDED SEPTEMBER 30, 2011, 2010 AND 2009
|
||||||||||||
|
2011
|
2010
|
2009
|
||||||||||
|
PATENT COSTS INCLUDED IN ACCOUNTS PAYABLE:
|
||||||||||||
|
Increase in patent costs
|
$ | 28,531 | $ | - | $ | 7,285 | ||||||
|
Increase in accounts payable
|
(28,531 | ) | - | (7,285 | ) | |||||||
| $ | - | $ | - | $ | - | |||||||
|
EQUIPMENT COSTS INCLUDED IN ACCOUNTS PAYABLE:
|
||||||||||||
|
Increase in research and office equipment
|
$ | 1,291 | $ | 10,436 | $ | 15,147 | ||||||
|
Increase in accounts payable
|
(1,291 | ) | (10,436 | ) | (15,147 | ) | ||||||
| $ | - | $ | - | $ | - | |||||||
|
WARRANTS ISSUED FOR LOAN:
|
||||||||||||
|
Increase in debt discount
|
$ | - | $ | - | $ | 65,796 | ||||||
|
Increase in additional paid-in capital
|
- | - | (65,796 | ) | ||||||||
| $ | - | $ | - | |||||||||
|
STOCK MODIFICATION RECORDED AS DIVIDEND
|
||||||||||||
|
Increase in common stock
|
$ | - | $ | - | $ | (11,667 | ) | |||||
|
Increase additional paid-in capital
|
- | - | (479,061 | ) | ||||||||
|
Increase accumulated deficit
|
- | - | 490,728 | |||||||||
| $ | - | $ | - | $ | - | |||||||
|
ADOPTION OF ASC 815-40
|
||||||||||||
|
Increase in derivative liabilities
|
$ | - | $ | (6,186,343 | ) | $ | - | |||||
|
Increase in accumulated deficit
|
- | 6,186,343 | - | |||||||||
| $ | - | $ | - | $ | - | |||||||
|
DISMISSAL OF LIABILITY FOR OVERPAYMENT
|
||||||||||||
|
Decrease in accrued expenses
|
$ | 81,395 | $ | - | $ | - | ||||||
|
Increase in additional paid-in capital
|
(81,395 | ) | - | - | ||||||||
| $ | - | $ | - | $ | - | |||||||
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOWS
|
||||||||||||
|
INFORMATION:
|
||||||||||||
|
Cash expenditure for interest expense
|
$ | 195,980 | $ | 162,326 | $ | 115,559 | ||||||
|
a.
|
Principles of Consolidation
-- The consolidated financial statements include the accounts of the Company and its wholly owned subsidiary, Viral Technologies, Inc. (VTI). All significant intercompany transactions have been eliminated upon consolidation.
|
|
b.
|
Cash and Cash Equivalents
-- For purposes of the statements of cash flows, cash and cash equivalents consists principally of unrestricted cash on deposit and short-term money market funds. The Company considers all highly liquid investments with a maturity when purchased of less than three months, as cash and cash equivalents.
|
|
c.
|
Restricted Cash
-- The restricted cash as of September 30, 2010 was money held in escrow pursuant to the lease agreement for the manufacturing facility. The restrictions on the cash were released during the year ended September 30, 2011.
|
|
d.
|
Prepaid Expenses and Inventory
--
Prepaid expenses are payments for services to be rendered over a long period and are expensed over the time period for which the service is rendered. Inventory consists of manufacturing production advances and bulk purchases of laboratory supplies to be consumed in the manufacturing of the Company's product for clinical studies.
|
|
e.
|
Deposits
--
The deposit on September 30, 2011 was for the manufacturing facility required by the lease agreement.
|
|
f.
|
Research and Office Equipment and Leasehold Improvements
-- Research and office equipment is recorded at cost and depreciated using the straight-line method over estimated useful lives of five to seven years. Leasehold improvements are depreciated over the shorter of the estimated useful life of the asset or the term of the lease. Repairs and maintenance which do not extend the life of the asset are expensed when incurred. The fixed assets are reviewed on a quarterly basis to determine if any of the assets are impaired. Depreciation expense for the years ended September 30, 2011, 2010 and 2009 totaled $447,174, $437,629, and $330,820, respectively. During the years ended September 30, 2011, 2010 and 2009, equipment with a net book value of $2,828, $2,323 and $270, respectively, was retired.
|
|
g.
|
Patents
-- Patent expenditures are capitalized and amortized using the straight-line method over the shorter of the expected useful life or the legal life of the patent (17 years). In the event changes in technology or other circumstances impair the value or life of the patent, appropriate adjustment in the asset value and period of amortization is made. An impairment loss is recognized when estimated future undiscounted cash flows expected to result from the use of the asset, and from disposition, is less than the carrying value of the asset. The amount of the impairment loss is the difference between the estimated fair value of the asset and its carrying value. During the years ended September 30, 2011, 2010 and 2009, the Company recorded patent impairment charges of $9,016, $13,877, and $138,525, respectively, for the net book value of patents abandoned during the year. These amounts are included in general and administrative expenses. Amortization expense for the years ended September 30, 2011, 2010 and 2009 totaled $84,142, $78,488, and $86,385, respectively. The total estimated future amortization is as follows:
|
|
Year ending September 30,
|
||||
| 2012 | $ | 86,409 | ||
| 2013 | 86,409 | |||
| 2014 | 86,409 | |||
| 2015 | 45,231 | |||
| 2016 | 8,896 | |||
| Thereafter | 100,804 | |||
| $ | 414,158 | |||
|
h.
|
Deferred Rent
--
Interest on the deferred rent is calculated at 3% on the funds deposited on the manufacturing facility and is included in the deferred rent. This interest income will be used to offset future rent. On September 30, 2011, the Company has included in deferred rent the following: 1) deposit on the manufacturing facility ($3,150,000); 2) the fair value of the warrants issued to lessor ($1,403,654); 3) additional investment ($2,995,541); 4) deposit on the cost of the leasehold improvements for the manufacturing facility ($1,786,591),; and 5) accrued interest on deposit ($287,668), less amortization of $2,433,614
.
|
|
|
On September 30, 2010, the Company has included in deferred rent the following: 1) deposit on the manufacturing facility ($3,150,000); 2) the fair value of the warrants issued to lessor ($1,403,654); 3) additional investment ($2,976,396; 4) deposit on the cost of the leasehold improvements for the manufacturing facility ($1,786,591); and 5) accrued interest on deposit ($186,194), less amortization of $1,683,313 .
|
|
i
.
|
Deferred Rent (liability)
--
The deferred rent (liability) is amortized on a straight-line basis over the term of the lease with the offset going against rent expense
.
|
|
j
.
|
Derivative Instruments
-- The Company entered into financing arrangements that consisted of freestanding derivative instruments or were hybrid instruments that contained embedded derivative features. The Company accounted for this arrangement in accordance with Codification 815-10-50, “
Accounting for Derivative Instruments and Hedging Activities
”, “
Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company's Own Stock
”. In accordance with accounting principles generally accepted in the United States (“GAAP”), derivative instruments and hybrid instruments are recognized as either assets or liabilities in the Company’s balance sheet and are measured at fair value with gains or losses recognized in earnings or other comprehensive income depending on the nature of the derivative or hybrid instruments. Embedded derivatives that are not clearly and closely related to the host contract are bifurcated and recognized at fair value with changes in fair value recognized as either a gain or loss in earnings if they can be reliably measured. When the fair value of embedded derivative features cannot be reliably measured, the Company measures and reports the entire hybrid instrument at fair value with changes in fair value recognized as either a gain or loss in earnings. The Company determined the fair value of derivative instruments and hybrid instruments based on available market data using appropriate valuation models, giving consideration to all of the rights and obligations of each instrument and precluding the use of “blockage” discounts or premiums in determining the fair value of a large block of financial instruments. Fair value under these conditions does not necessarily represent fair value determined using valuation standards that give consideration to blockage discounts and other factors that may be considered by market participants in establishing fair value.
|
|
k.
|
Research and Development Grant Revenues
-- The Company received a $733,437 grant in November 2010 under The Patient Protection and Affordable Care Act of 2010 (PPACA). The grant was related to three of the Company’s projects, including the Phase III trial of Multikine. The PPACA provides small and mid-sized biotech, pharmaceutical and medical device companies with up to a 50% tax credit for investments in qualified therapeutic discoveries for tax years 2009 and 2010 for all qualified “therapeutic discovery projects”. The Company recognizes revenue as the expenses are incurred. During the fiscal year ended September 30, 2011, the Company has earned $733,437 in PPACA grants and has receivables of $161,297 for grant money earned but not yet received. This payment was received in November 2011.
|
|
l.
|
Research and Development Costs
-- Research and development expenditures are expensed as incurred. Total research and development costs, excluding depreciation, were $11,745,629, $11,911,626, and $6,011,750, respectively, for the years ended September 30, 2011, 2010 and 2009.
|
|
m.
|
Net (Loss) Income Per Common Share
--
Net (loss) income per common share is computed by dividing the net (loss) income by the weighted average number of common shares outstanding during the period. Potentially dilutive common stock equivalents, including convertible preferred stock, convertible debt and options to purchase common stock, are included in the calculation of diluted net (loss) income per share unless the result is antidilutive.
|
|
n.
|
Concentration of Credit Risk
-- Financial instruments, which potentially subject the Company to concentrations of credit risk, consist of cash and cash equivalents. The Company maintains its cash and cash equivalents with high quality financial institutions. At times, these accounts may exceed federally insured limits. The Company has not experienced any losses in such bank accounts. The Company believes it is not exposed to significant credit risk related to cash and cash equivalents. All of our non-interest bearing cash balances were fully insured at September 30, 2011 due to a temporary federal program in effect from December 31, 2010 through December 31, 2012. Under the program, there is no limit to the amount of insurance for eligible accounts. Beginning 2013, insurance coverage will revert to $250,000 per depositor at each financial institution, and our non-interest bearing cash balances may again exceed federally insured limits. Interest-bearing amounts on deposit in excess of federally insured limits at September 30, 2011 approximated $3,666,000.
|
|
o.
|
Income Taxes
-- The Company uses the asset and liability method of accounting for income taxes. Under the asset and liability method, deferred tax assets and liabilities are recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating and tax loss carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. The Company records a valuation allowance to reduce the deferred tax assets to the amount that is more likely than not to be recognized.
|
|
p.
|
Use of Estimates
-- The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Accounting for derivatives is based upon valuations of derivative instruments determined using various valuation techniques including the Black-Scholes and binomial pricing methodologies. The Company considers such valuations to be significant estimates.
|
|
q.
|
Recent Accounting Pronouncements
-- In January 2010, the FASB issued ASU 2010-06, “
Fair Value Measurements and Disclosures”,
which requires new disclosures for transfers in and out of Level 1 and Level 2 and activity in Level 3 of the fair value hierarchy. ASU 2010-06 requires separate disclosure of the amounts of significant transfers in and out of Level 1 and Level 2 fair value measurements and a description of the reasons for the transfers. In the reconciliation for fair value measurements using Level 3 inputs, a reporting entity should present separately information about purchases, sales, issuances and settlements. ASU 2010-06 is effective for new disclosures and clarification of existing disclosures for interim and annual periods beginning after December 15, 2009 except for disclosures about purchases, sales, issuances and settlements in the Level 3 activity rollforward, which are effective for fiscal years beginning after December 15, 2010, and for interim periods within those fiscal years. The adoption of ASU 2010-06 did not have a material impact on its financial statements.
|
|
|
In May 2011, the FASB Issued Accounting Standards Update (ASU) No. 2011-04,
“Fair Value Measurement (Topic 820) – Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs”,
which is effective for interim and annual periods beginning after December 15, 2011. The ASU is mainly the result of
the joint efforts by the FASB and the International Accounting Standards Board to develop a
single, converged fair value framework on how to measure fair value and common disclosure
requirements for fair value measurements. The ASU amends various fair value guidance such as
requiring the highest-and-best-use and valuation-premise concepts only to measuring the fair
value of nonfinancial assets and prohibits the use of blockage factors and control premiums when
measuring fair value. In addition, the ASU expands disclosure requirements particularly for Level 3
inputs and requires disclosure of the level in the fair value hierarchy of items that are not
measured at fair value in the statement of financial position but whose fair value must be
disclosed
.
The Company does not believe that this amendment will have a material impact on its financial statements
|
|
r.
|
Stock-Based Compensation
-- Compensation cost for all stock-based awards is measured at fair value as of the grant date. The fair value of our stock options is calculated using the Black-Scholes option pricing model. The Black-Scholes model requires various highly judgmental assumptions including volatility, forfeiture rates and expected option life. If any of the assumptions used in the Black-Scholes model change significantly, stock-based compensation expense for new awards may differ materially in the future from that recorded in the current period.
The fair value of each option grant was estimated on the date of grant using the Black-Scholes option-pricing model with the following assumptions:
|
|
2011
|
2010
|
2009
|
|||
|
Expected stock price volatility
|
96.5-97%
|
98.6-104.5%
|
79.5-80.2%
|
||
|
Risk-free interest rate
|
2.97-3.68%
|
2.54-4.01%
|
2.82-3.72%
|
||
|
Expected life of options
|
9.62-9.63 Years
|
9.63-10 Years
|
10 Years
|
||
|
|
|||||
|
Expected dividend yield
|
-
|
-
|
-
|
| Outstanding | Exercisable | |||||||||||||||||||||||||||||||
| Number of Shares | Weighted Average Exercise Price | Weighted Ave Remaining Contractual Term (Years) | Aggregate Intrinsic Value | Number of Shares | Weighted Average Exercise Price | Weighted Ave Remaining Contractual Term (Years) | Aggregate Intrinsic Value | |||||||||||||||||||||||||
|
Outstanding at October 1, 2010
|
21,720,414 | $ | 0.49 | 7.06 | $ | 4,365,776 | 8,906,473 | $ | 0.60 | 4.34 | $ | 1,291,973 | ||||||||||||||||||||
|
Vested
|
1,059,721 | $ | 0.53 | |||||||||||||||||||||||||||||
|
Granted
|
1,779,594 | $ | 0.69 | 9.58 | $ | - | ||||||||||||||||||||||||||
|
Exercised
|
(29,268 | ) | $ | 0.45 | 5.01 | $ | 10,944 | (29,268 | ) | $ | 0.45 | 5.01 | 10,944 | |||||||||||||||||||
|
Forfeited
|
(2,000 | ) | $ | 0.61 | ||||||||||||||||||||||||||||
|
Expired
|
(7,500 | ) | $ | 1.22 | (7,500 | ) | $ | 1.22 | ||||||||||||||||||||||||
| Outstanding at September 30, 2011 | 23,461,240 | $ | 0.51 | 6.35 | $ | 471,789 | 9,929,426 | $ | 0.62 | 3.93 | $ | 364,539 | ||||||||||||||||||||
| Number of Shares |
Weighted
Average
Grant Date
Fair Value
|
|||||||
|
Unvested at September 30, 2010
|
12,813,941 | $ | 0.40 | |||||
|
Vested
|
(1,059,721 | ) | ||||||
|
Granted
|
1,779,594 | $ | 0.61 | |||||
|
Forfeited
|
(2,000 | ) | ||||||
|
Unvested at September 30, 2011
|
13,531,814 | $ | 0.37 | |||||
| Outstanding | Exercisable | |||||||||||||||||||||||||||||||
|
Number of
Shares
|
Weighted Average
Exercise Price
|
Weighted Ave
Remaining Contractual
Term (Years)
|
Aggregate Intrinsic
Value
|
Number of
Shares
|
Weighted
Average Exercise
Price
|
Weighted
Ave Remaining
Contractual Term
(Years)
|
Aggregate Intrinsic
Value
|
|||||||||||||||||||||||||
|
Outstanding at October 1, 2010
|
10,593,041 | $ | 0.40 | 6.65 | $ | 3,101,582 | 8,893,042 | $ | 0.38 | 6.02 | $ | 2,794,276 | ||||||||||||||||||||
|
Vested
|
716,667 | $ | 0.53 | |||||||||||||||||||||||||||||
|
Granted
|
600,000 | $ | 0.69 | 9.58 | $ | - | ||||||||||||||||||||||||||
|
Expired
|
(25,000 | ) | $ | 1.39 | (25,000 | ) | $ | 1.39 | ||||||||||||||||||||||||
|
Outstanding at September 30, 2011
|
11,168,041 | $ | 0.42 | 5.83 | $ | 1,019,989 | 9,584,709 | $ | 0.38 | 5.29 | $ | 1,017,489 | ||||||||||||||||||||
| Number of Shares |
Weighted Average
Grant Date Fair Value
|
|||||||
|
Unvested at September 30, 2010
|
1,699,999 | $ | 0.54 | |||||
|
Vested
|
(716,667 | ) | ||||||
|
Granted
|
600,000 | $ | 0.61 | |||||
|
Forfeited
|
- | |||||||
|
Unvested at September 30, 2011
|
1,583,332 | $ | 0.54 | |||||
|
Warrant
|
Issue
Date
|
Shares Issuable upon Exercise of
Warrant
|
Exercise
Price
|
Expiration Date
|
Reference
|
|||||||||
|
Series K
|
8/4/06
|
2,318,396 | $ | 0.40 |
2/4/12
|
1 | ||||||||
|
Series N
|
8/18/08
|
3,890,782 | 0.40 |
8/18/14
|
1 | |||||||||
|
Series A
|
6/24/09
|
1,303,472 | 0.50 |
12/24/14
|
1 | |||||||||
|
C. Schleuning (Series A)
|
7/8/09
|
167,500 | 0.50 |
01/08/15
|
1 | |||||||||
|
Series B
|
9/4/09
|
500,000 | 0.68 |
9/4/14
|
1 | |||||||||
|
Series C
|
8/20/09 – 8/26/09
|
4,634,886 | 0.55 |
2/20/15
|
1 | |||||||||
|
Series E
|
9/21/09
|
714,286 | 1.75 |
8/12/14
|
1 | |||||||||
|
Series L
|
4/18/07
|
951,669 | 0.75 |
4/17/12
|
2 | |||||||||
|
Series L (repriced)
|
4/18/07
|
1,000,000 | 0.56 |
4/17/13
|
2 | |||||||||
|
Series M
|
4/18/07
|
1,221,668 | 2.00 |
4/17/12
|
2 | |||||||||
|
Series M (modified)
|
4/18/07
|
6,000,000 | 0.60 |
7/31/14
|
2 | |||||||||
|
Series O
|
3/6/09
|
6,500,000 | 0.25 |
3/6/16
|
3 | |||||||||
|
Private Investors
|
5/30/03- 6/30/09
|
8,609,375 | 0.47 – 1.25 |
5/30/13 - 7/18/14
|
4 | |||||||||
|
Warrants held by
Officer and Director
|
6/24/09- 7/6/09
|
3,497,539 | 0.40 – 0.50 |
12/24/14 – 1/6/15
|
5 | |||||||||
|
Consultants
|
5/22/03 – 7/16/09
|
837,500 | 0.28 – 2.00 |
8/23/12 - 10/14/15
|
6 | |||||||||
|
1.
|
Derivative Liabilities
|
|
September 30, 2011
|
September 30, 2010
|
|||||||
|
Series K warrants
|
$ | 69,552 | $ | 1,002,502 | ||||
|
2009 financings warrants
|
||||||||
|
(Series A thru E)
|
1,375,458 | 4,037,066 | ||||||
|
2008 warrants reclassified from equity
|
||||||||
|
to derivative liabilities on
|
||||||||
|
October 1, 2009 (Series N)
|
817,063 | 1,906,483 | ||||||
|
Convertible notes issued in settlement (Note 11)
|
4,999,000 |
-
|
||||||
|
Total derivative liabilities
|
$ | 7,261,073 | $ | 6,946,051 | ||||
|
2.
|
Series L and M Warrants
|
|
Original
Warrants
|
Extended
Warrants
|
||
|
Expected stock risk volatility
|
104.00%
|
97.40%
|
|
|
Risk-free interest rate
|
0.61%
|
1.04%
|
|
|
Expected life of warrant
|
1.51 Year
|
3.51 Years
|
|
4.
|
Private Investor Warrants
|
|
Original
Warrants
|
Extended
Warrants
|
||
| Expected stock risk volatility |
104.0%
|
97.8-103.7%
|
|
|
Risk-free interest rate
|
0.15%
|
1.04%
|
|
|
Expected life of warrant
|
0.02-0.50 Year
|
3.02-3.50 Years
|
|
5.
|
Warrants held by Officer and Director
|
|
6.
|
Options held by Consultants
|
| 2011 | 2010 | |||||||
|
Research equipment
|
$ | 3,823,816 | $ | 3,647,684 | ||||
|
Furniture and equipment
|
116,173 | 116,996 | ||||||
|
Leasehold improvements
|
126,910 | 126,910 | ||||||
| 4,066,899 | 3,891,590 | |||||||
|
Less: Accumulated depreciation and amortization
|
(3,034,018 | ) | (2,626,759 | ) | ||||
|
Net research and office equipment
|
$ | 1,032,881 | $ | 1,264,831 |
| 2011 | 2010 | |||||||
|
Net operating loss
|
$ | 51,381,945 | $ | 45,940,445 | ||||
|
|
||||||||
|
R&D credit
|
2,340,614 | 2,340,614 | ||||||
|
Stock-based compensation
|
1,597,790 | 1,243,647 | ||||||
|
Vacation and other
|
190,522 | 83,593 | ||||||
|
Deferred rent
|
991,091 | 970,224 | ||||||
|
Litigation liability
|
1,842,297 | - | ||||||
|
Total deferred tax assets
|
58,344,259 | 50,578,523 | ||||||
|
Derivative gain
|
(3,639,050 | ) | (2,133,259 | ) | ||||
|
Depreciation
|
(76,841 | ) | (80,026 | ) | ||||
|
Total deferred tax liabilities
|
(3,715,891 | ) | (2,213,285 | ) | ||||
|
Valuation allowance
|
(54,628,368 | ) | (48,365,238 | ) | ||||
|
Net deferred tax asset
|
$ | - | $ | - |
| 2011 | 2010 | 2009 | ||||||||||
|
Federal Rate
|
34.00 | % | 34.0 | % | 34.0 | % | ||||||
|
State tax rate, net of federal benefit
|
3.22 | % | 5.91 | % | 3.96 | % | ||||||
| State tax rate change | 12.06 | % | 0 | % | 0 | % | ||||||
|
R&D credit
|
0 | % | 0 | % | 2.01 | % | ||||||
|
R&D credit true-up
|
0 | % | 0 | % | (0.40 | %) | ||||||
|
Other adjustments
|
(0.04 | %) | 0 | % | 0 | % | ||||||
| Nondeductible expenses | (0.48 | %) | 0.02 | % | (0 | %) | ||||||
| Valuation allowance | (24.64 | %) | (39.93 | %) | (39.57 | %) | ||||||
|
Effective tax rate
|
0.00 | % | 0.00 | % | 0.00 | % |
|
2012
|
$ | 1,896,205 | ||
|
2013
|
1,855,890 | |||
|
2014
|
1,579,931 | |||
|
2015
|
1,572,839 | |||
|
2016
|
1,629,121 | |||
|
2017 and thereafter
|
24,812,823 | |||
|
Total minimum lease payments:
|
$ | 33,346,809 |
|
o
|
Level 1 – Observable inputs such as quoted prices in active markets for identical assets or liabilities
|
|
o
|
Level 2 – Inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active and amounts derived from valuation models where all significant inputs are observable in active markets
|
|
o
|
Level 3 – Unobservable inputs that reflect management’s assumptions
|
|
Quoted Prices in
Active Markets for
Identical Assets or
Liabilities (Level 1
)
|
Significant
Other
Observable
Inputs (Level 2)
|
Significant
Unobservable
Inputs (Level 3)
|
Total | |||||||||||||
| Derivative Instruments | $ | - | $ | - | $ | 7,261,073 | $ | 7,261,073 | ||||||||
|
Quoted Prices in
Active Markets for
Identical Assets or
Liabilities (Level 1
)
|
Significant
Other
Observable
Inputs (Level 2)
|
Significant
Unobservable
Inputs (Level 3)
|
Total | |||||||||||||
| Derivative Instruments | $ | - | $ | - | $ | 6,946,051 | $ | 6,946,051 | ||||||||
|
2011
|
2010
|
|||||||
|
Beginning balance
|
$ | 6,946,051 | $ | 35,113,970 | ||||
|
Issuances
|
9,000,000 | 6,186,343 | ||||||
|
Settlements
|
(4,252,830 | ) | (5,510,490 | ) | ||||
| Realized and unrealized gains recorded in Earnings | ( 4,432,148 | ) | (28,843,772 | ) | ||||
|
Ending balance
|
$ | 7,261,073 | $ | 6,946,051 | ||||
|
Net income (loss) – available to
|
2011 | 2010 | 2009 | |||||||||
|
common
shareholders-basic
|
$ | (26,780,712 | ) | $ | 8,950,973 | $ | (41,400,758 | ) | ||||
|
Less: Conversion of derivative
|
||||||||||||
|
instruments
|
(4,199,256 | ) | (20,130,098 | ) | - | |||||||
|
Net income (loss) - diluted
|
$ | (30,979,968 | ) | $ | (11,179,125 | ) | $ | (41,400,758 | ) | |||
|
Weighted average number of
|
||||||||||||
|
shares - basic and diluted
|
208,488,987 | 202,102,859 | 133,535,050 | |||||||||
|
Earnings per share - basic
|
$ | (0.13 | ) | $ | 0.04 | $ | (0.31 | ) | ||||
|
Earnings per share - diluted
|
$ | (0.15 | ) | $ | (0.06 | ) | $ | (0.31 | ) | |||
|
Fiscal 2011
|
||||||||||||||||||||
| Three months ended | Three months ended | Three months ended | Three months Ended | Year Ended | ||||||||||||||||
|
December 31
2010
|
March 31,
2011
|
June 30,
2011
|
September
30,
2011
|
September 30,
2011
|
||||||||||||||||
|
Revenue
|
$ | 662,818 | $ | 43,815 | $ | 77,403 | $ | 172,118 | $ | 956,154 | ||||||||||
|
Operating expenses
|
4,978,852 | 5,140,811 | 4,923,147 | 3,899,018 | 18,941,828 | |||||||||||||||
|
Non operating income (expenses)
|
11,477 | 5,305 | (31,822 | ) | (143,777 | ) | (158,817 | ) | ||||||||||||
|
Other expenses
|
- | (12,000,000 | ) | - | - | (12,000,000 | ) | |||||||||||||
|
Gain/(loss) on derivative instruments
|
(1,946,395 | ) | 3,062,087 | 1,763,311 | 1,553,145 | 4,432,148 | ||||||||||||||
|
Net loss
|
(6,250,952 | ) | (14,029,604 | ) | (3,114,255 | ) | (2,317,532 | ) | (25,712,343 | ) | ||||||||||
|
Modification of warrants
|
- | (1,068,369 | ) | - | - | (1,068,369 | ) | |||||||||||||
|
Net loss available to
|
||||||||||||||||||||
|
common shareholders
|
$ | (6,250,952 | ) | $ | (15,097,973 | ) | $ | (3,114,255 | ) | $ | (2,317,532, | ) | $ | (26,780,712 | ) | |||||
|
Net loss per share-basic
|
$ | (0.03 | ) | $ | (0.07 | ) | $ | (0.01 | ) | $ | (0.01 | ) | $ | (0.13 | ) | |||||
|
Net loss per share-diluted
|
$ | (0.03 | ) | $ | (0.09 | ) | $ | (0.02 | ) | $ | (0.02 | ) | $ | (0.15 | ) | |||||
|
Weighted average shares-basic
|
205,112,418 | 207,089,841 | 208,402,408 | 213,319,921 | 208,488,987 | |||||||||||||||
|
Weighted average shares-diluted
|
205,112,418 | 207,089,841 | 208,402,408 | 213,319,921 | 208,488,987 | |||||||||||||||
|
Three months
Ended
|
Three months
ended
|
Three months
ended
|
Three months
ended
|
Year Ended
|
||||||||||||||||
|
December 31,
2009
|
March 31,
2010
|
June 30,
2010
|
September
30, 2010
|
September
30, 2010
|
||||||||||||||||
|
Revenue
|
$ | 30,000 | $ | 30,600 | $ | 30,900 | $ | 61,800 | $ | 153,300 | ||||||||||
|
Operating expenses
|
4,282,849 | 5,350,958 | 3,424,959 | 5,654,787 | 18,713,553 | |||||||||||||||
|
Non operating expenses (income)
|
(72,099 | ) | (56,167 | ) | (38,423 | ) | (33,221 | ) | (199,910 | ) | ||||||||||
|
Gain/loss on derivative instruments
|
23,340,267 | 4,519,672 | 2,754,512 | (1,770,679 | ) | 28,843,772 | ||||||||||||||
|
Modification of warrants
|
- | (1,432,456 | ) | - | (100,000 | ) | (1,532,456 | ) | ||||||||||||
|
Net income (loss) available to
|
||||||||||||||||||||
|
common shareholders
|
$ | 19,159,517 | $ | (2,176,975 | ) | $ | (601,124 | ) | $ | (7,430,445 | ) | $ | 8,950,973 | |||||||
|
Net loss per share-basic
|
$ | 0.10 | $ | (0.01 | ) | $ | 0.00 | $ | (0.04 | ) | $ | 0.04 | ||||||||
|
Net loss per share-diluted
|
$ | 0.02 | $ | (0.03 | ) | $ | (0.02 | ) | $ | (0.04 | ) | $ | (0.06 | ) | ||||||
|
Weighted average shares-basic
|
194,959,814 | 204,173,750 | 204,592,051 | 204,757,898 | 202,102,859 | |||||||||||||||
|
Weighted average shares-diluted
|
256,198,162 | 204,173,750 | 204,592,051 | 204,757,898 | 202,102,859 | |||||||||||||||
| CEL-SCI CORPORATION | |||
|
|
By:
|
/s/ Maximilian de Clara | |
| Maximilian de Clara, President | |||
| Title | |||
|
Signature
|
Title
|
Date
|
||
|
/s/ Maximilian de Clara
|
Director
|
23-Dec-11
|
||
|
Maximilian de Clara
|
||||
|
/s/ Geert Kersten
|
Chief Executive, Principal
|
|||
|
Geert R. Kersten
|
Accounting, Principal Financial
|
|||
|
Officer and a Director
|
23-Dec-11
|
|||
|
/s/ Alexander Esterhazy
|
Director
|
23-Dec-11
|
||
|
Alexander G. Esterhazy
|
||||
|
/s/ C. Richard Kinsolving
|
Director
|
23-Dec-11
|
||
|
Dr. C. Richard Kinsolving
|
||||
|
/s/ Peter Young
|
Director
|
23-Dec-11
|
||
|
Dr. Peter R. Young
|