|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
þ
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
| CEL-SCI CORPORATION |
| (Exact name of registrant as specified in its charter) |
| COLORADO | 84-0916344 | |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
|
8229 Boone Blvd., Suite 802
Vienna, Virginia
|
22182 | |
| (Address of principal executive offices) | (Zip Code) |
|
Common Stock, $.01 par value
Series S Warrants
|
|
(Title of Class)
|
| Large accelerated filer | o | Accelerated filer | þ |
| Non-accelerated filer | o | Smaller reporting company | o |
| (Do not check if a smaller reporting company) |
|
|
1)
|
Multikine (Leukocyte Interleukin, Injection) investigational immunotherapy against cancer and Human Papilloma Virus (HPV);
|
|
|
2)
|
LEAPS technology, with two investigational therapies, LEAPS-H1N1-DC pandemic flu treatment for hospitalized patients and CEL-2000, a rheumatoid arthritis treatment vaccine.
|
|
●
|
In the final Phase II clinical study, using the same dosage and treatment regimen as is being used in the Phase III study, head and neck cancer patients with locally advanced primary disease who received the investigational therapy Multikine as first-line investigational therapy followed by surgery and radiotherapy were reported by the clinical investigators to have had a 63.2% overall survival (OS) rate at 3.5 years from surgery. This percentage OS was arrived at as follows: of the 22 subjects enrolled in this final Phase II study, the consent for the survival follow-up portion of the study was received from 19 subjects. One subject did not consent to the follow-up portion of the study. The other 2 subjects did not have squamous cell carcinoma of the oral cavity and were thus not evaluable per the protocol. The overall survival rate of subjects receiving the investigational therapy in this study was compared to the overall survival rate that was calculated based upon a review of 55 clinical trials conducted in the same cancer population (with a total of 7,294 patients studied), and reported in the peer reviewed scientific literature between 1987 and 2007. Review of this literature showed an approximate survival rate of 47.5% at 3.5 year from treatment. Therefore, the results of CEL-SCI's final Phase II study were considered to be potentially favorable in terms of overall survival recognizing the limitations of this early-phase study. It should be noted that an earlier investigational therapy Multikine study appears to lend support to the overall survival findings described above - Feinmesser et al Arch Otolaryngol. Surg. 2003. However, no definitive conclusions can be drawn from these data about the potential efficacy or safety profile of this investigational therapy. Moreover, further research is required, and these results must be confirmed in the well-controlled Phase III clinical trial of this investigational therapy that is currently in progress. Subject to completion of that Phase III trial and FDA's review and acceptance of CEL-SCI's entire data set on this investigational therapy, CEL-SCI believes that these early-stage clinical trial results indicate the potential for this investigational therapy to become a treatment for advanced primary head and neck cancer.
|
|
●
|
Reported average of 50% reduction in tumor cells in Phase II trials:
The clinical investigators who administered the three week Multikine treatment regimen used in Phase II studies reported that, as was determined in a controlled pathology study, Multikine administration appeared to have caused, on average, the disappearance of about half of the cancer cells present at surgery (as determined by histopathology assessing the area of Stroma/Tumor (Mean+/- Standard Error of the Mean of the number of cells counted per filed)) even before the start of standard therapy such as radiation and chemotherapy (Timar et al JCO 2005).
|
|
●
|
Reported 12% complete response in the final Phase II trial:
The clinical investigators who administered the three week Multikine investigational treatment regimen used in the final Phase II study reported that, as was determined in a controlled pathology study, the tumor apparently was no longer present (as determined by histopathology) in approximately 12 % of patients (2 of 17 evaluable by pathology). This determination was made by three pathologists blinded to the study from the surgical specimen after a three week treatment with Multikine (Timar et al JCO 2005).
|
|
●
|
Adverse events reported in clinical trials:
In clinical trials conducted to date with the Multikine investigational therapy, adverse events which have been reported by the clinical investigators as possibly or probably related to Multikine administration included pain at the injection site, local minor bleeding and edema at the injection site, diarrhea, headache, nausea, and constipation
.
|
|
●
|
product design, development and manufacture;
|
|
●
|
product application and use
|
|
●
|
adverse drug experience;
|
|
●
|
product advertising and promotion;
|
|
●
|
product manufacturing, including good manufacturing practices
|
|
●
|
record keeping requirements;
|
|
●
|
registration and listing of CEL-SCI's establishments and products with the FDA, EMA and other state and national agencies;
|
|
●
|
product storage and shipping;
|
|
●
|
drug sampling and distribution requirements;
|
|
●
|
electronic record and signature requirements; and
|
|
●
|
labeling changes or modifications.
|
|
Quarter Ending
|
High
|
Low
|
||||||
|
12/31/12
|
$ | 3.90 | $ | 2.60 | ||||
|
3/31/13
|
$ | 2.90 | $ | 2.10 | ||||
|
6/30/13
|
$ | 3.10 | $ | 2.00 | ||||
|
9/30/13
|
$ | 2.70 | $ | 1.60 | ||||
|
12/31/13
|
$ | 1.80 | $ | 0.53 | ||||
|
3/31/14
|
$ | 1.90 | $ | 0.59 | ||||
|
6/30/14
|
$ | 1.72 | $ | 0.98 | ||||
|
9/30/14
|
$ | 1.30 | $ | 0.75 | ||||
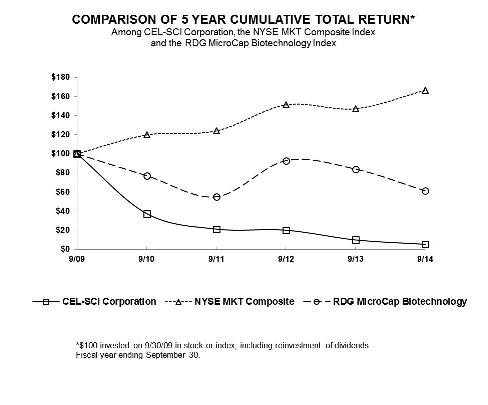
| 9/09 | 9/10 | 9/11 | 9/12 | 9/13 | 9/14 | |||||||||||||||||||
|
CEL-SCI Corporation
|
100.00 | 37.44 | 21.22 | 20.06 | 9.88 | 5.30 | ||||||||||||||||||
|
NYSE MKT Composite
|
100.00 | 119.74 | 123.95 | 151.16 | 147.16 | 166.46 | ||||||||||||||||||
|
RDG MicroCap Biotechnology
|
100.00 | 77.22 | 55.21 | 92.86 | 84.06 | 61.43 | ||||||||||||||||||
|
|
2014
|
2013
|
2012
|
2011
|
2010
|
|||||||||||||||
|
Grant income and other
|
$ | 264,033 | $ | 159,583 | $ | 254,610 | $ | 956,154 | $ | 153,300 | ||||||||||
|
Operating expenses:
|
||||||||||||||||||||
|
Research and development
|
17,000,145 | 12,681,049 | 10,368,695 | 11,745,629 | 11,911,626 | |||||||||||||||
|
Depreciation and
Amortization
|
231,752 | 364,124 | 533,468 | 531,316 | 516,117 | |||||||||||||||
|
General and administrative
|
10,606,248 | 6,982,686 | 6,595,287 | 6,664,883 | 6,285,810 | |||||||||||||||
|
Gain on derivative instruments
|
248,767 | 10,750,666 | 1,911,683 | 4,432,148 | 28,843,772 | |||||||||||||||
|
Other expenses (3)
|
- | - | - | (12,000,000 | ) | - | ||||||||||||||
|
Interest income
|
122,854 | 117,086 | 116,061 | 164,163 | 362,236 | |||||||||||||||
|
Interest expense
|
(163,774 | ) | (170,423 | ) | (262,214 | ) | (322,980 | ) | (162,326 | ) | ||||||||||
|
Net income (loss)
|
(27,366,265 | ) | (9,170,947 | ) | (15,477,310 | ) | (25,712,343 | ) | 10,483,429 | |||||||||||
|
Issuance of additional shares due to reset provision
|
(1,117,447 | ) | - | (250,000 | ) | - | - | |||||||||||||
|
Modification of warrants
|
- | (59,531 | ) | (325,620 | ) | (1,068,369 | ) | (1,532,456 | ) | |||||||||||
|
Inducement warrants
|
- | - | (1,593,000 | ) | - | - | ||||||||||||||
|
Net income (loss) available to common shareholders
|
$ | (28,483,712 | ) | $ | (9,230,478 | ) | $ | (17,645,930 | ) | $ | (26,780,712 | ) | $ | 8,950,973 | ||||||
|
Net income (loss) per common share
|
||||||||||||||||||||
|
Basic
|
$ | (0.48 | ) | $ | (0.30 | ) | $ | (0.70 | ) | $ | (1.28 | ) | $ | 0.44 | ||||||
|
Diluted
|
$ | (0.49 | ) | $ | (0.66 | ) | $ | (0.78 | ) | $ | (1.49 | ) | $ | (0.55 | ) | |||||
|
Weighted average common
shares outstanding
|
||||||||||||||||||||
|
Basic and Diluted (1)
|
58,804,622 | 30,279,442 | 25,183,654 | 20,848,899 | 20,210,286 | |||||||||||||||
|
|
2014
|
2013
|
2012
|
2011
|
2010
|
|||||||||||||||
|
Working capital (deficit)
|
$ | 8,496,076 | $ | (1,033,370 | ) | $ | 5,529,438 | $ | 1,796,349 | $ | 25,799,304 | |||||||||
|
Total assets
|
$ | 19,230,434 | $ | 10,838,572 | $ | 16,067,450 | $ | 18,625,440 | $ | 37,804,985 | ||||||||||
|
Convertible note and derivative instruments - current (2)
|
$ | 18,105 | - | - | $ | 5,068,552 | $ | 424,286 | ||||||||||||
|
Derivative instruments –
noncurrent (2)
|
$ | 5,487,141 | $ | 433,024 | $ | 6,983,690 | $ | 2,192,521 | $ | 6,521,765 | ||||||||||
|
Total liabilities
|
$ | 8,787,034 | $ | 4,138,482 | $ | 9,040,018 | $ | 9,546,616 | $ | 9,950,220 | ||||||||||
|
Stockholders' equity
|
$ | 10,443,400 | $ | 6,700,090 | $ | 7,027,432 | $ | 9,078,824 | $ | 27,854,765 | ||||||||||
| (1) | The calculation of diluted earnings per share for the years ended September 30, 2014, 2013, 2012, and 2011excluded potentially dilutive shares because their effect would have been anti-dilutive. |
| (2) | Included in total liabilities. |
| (3) | The $12 million other expense in 2011 was the cost of the lawsuit settlement. The detailed terms of the lawsuit settlement and the related agreements and documents were filed as exhibits to CEL-SCI’s report on Form 10-Q for the three months ended March 31, 2011 . |
| Net loss per share | ||||||||||||
|
Quarter
|
Net loss | Basic | Diluted | |||||||||
|
12/31/2013
|
$ | (5,451,865 | ) | $ | (0.11 | ) | $ | (0.15 | ) | |||
|
3/31/2014
|
$ | (13,365,580 | ) | $ | (0.24 | ) | $ | (0.24 | ) | |||
|
6/30/2014
|
$ | (2,444,480 | ) | $ | (0.04 | ) | $ | (0.11 | ) | |||
|
9/30/2014
|
$ | (7,221,787 | ) | $ | (0.11 | ) | $ | (0.13 | ) | |||
|
12/31/2012
|
$ | (2,310,246 | ) | $ | (0.08 | ) | $ | (0.18 | ) | |||
|
3/31/2013
|
$ | (713,371 | ) | $ | (0.02 | ) | $ | (0.14 | ) | |||
|
6/30/2013
|
$ | (4,507,004 | ) | $ | (0.15 | ) | $ | (0.18 | ) | |||
|
9/30/2013
|
$ | (1,699,857 | ) | $ | (0.05 | ) | $ | (0.16 | ) | |||
| 2014 | 2013 | 2012 | 2011 | 2010 | ||||||||||||||||
|
MULTIKINE
|
$ | 16,625,367 | $ | 12,303,564 | $ | 9,977,617 | $ | 11,257,157 | $ | 10,868,046 | ||||||||||
|
LEAPS
|
374,778 | 377,485 | 391,078 | 488,472 | 1,043,580 | |||||||||||||||
|
TOTAL
|
$ | 17,000,145 | $ | 12,681,049 | $ | 10,368,695 | $ | 11,745,629 | $ | 11,911,626 | ||||||||||
|
Years Ending September 30,
|
||||||||||||||||||||||||||||
|
Total
|
2015
|
2016
|
2017
|
2018
|
2019
|
2020 & thereafter
|
||||||||||||||||||||||
|
Operating Leases
|
$ | 28,427,429 | $ | 1,785,873 | $ | 1,769,497 | $ | 1,746,328 | $ | 1,746,802 | $ | 1,808,302 | $ | 19,570,627 | ||||||||||||||
|
Related Party Note & Interest
|
1,228,263 | 1,228,263 | - | - | - | - | - | |||||||||||||||||||||
|
Total Contractual Obligations
|
$ | 29,655,692 | $ | 3,014,136 | $ | 1,769,497 | $ | 1,746,328 | $ | 1,746,802 | $ | 1,808,302 | $ | 19,570,627 | ||||||||||||||
| Name | Age | Position | ||
|
Maximilian de Clara
|
84 |
Director and President
|
||
|
Geert R. Kersten, Esq.
|
55 |
Director, Chief Executive Officer and Treasurer
|
||
|
Patricia B. Prichep
|
63 |
Senior Vice President of Operations and Corporate Secretary
|
||
|
Dr. Eyal Talor
|
58 |
Chief Scientific Officer
|
||
|
Dr. Daniel H. Zimmerman
|
73 |
Senior Vice President of Research, Cellular Immunology
|
||
|
John Cipriano
|
72 |
Senior Vice President of Regulatory Affairs
|
||
|
Alexander G. Esterhazy
|
72 |
Director
|
||
|
Dr. C. Richard Kinsolving
|
78 |
Director
|
||
|
Dr. Peter R. Young
|
69 | Director |
|
●
|
Market-driven.
Compensation programs are structured to be competitive both in their design and in the total compensation that they offer.
|
|
●
|
Performance-based.
Certain officers have some portion of their incentive compensation linked to CEL-SCI’s performance. The application of performance measures as well as the form of the reward may vary depending on the employee’s position and responsibilities.
|
|
●
|
The salaries paid to employees are consistent with the employees’ duties and responsibilities.
|
|
●
|
Employees who have high impact relative to the expectations of their job duties and functions are rewarded.
|
|
●
|
CEL-SCI retains employees who have skills critical to its long term success.
|
|
●
|
Base Salary
|
|
●
|
Long-Term Incentives (stock options and/or grants of stock)
|
|
●
|
Benefits
|
|
●
|
Enhance the link between the creation of shareholder value and long-term executive incentive compensation;
|
|
●
|
Provide focus, motivation and retention incentive; and
|
|
●
|
Provide competitive levels of total compensation.
|
|
Fiscal
|
Salary
|
Bonus
|
Restric
ted Stock
Awards
|
Option
Awards
|
All Other
Annual
Compen
sation
|
|||||||||||||||||||||
|
Name and
Principal Position
|
Year
|
(1) | (2) | (3) | (4) | (5) |
Total
|
|||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||
|
Maximilian de Clara,
|
2014
|
393,250 | -- | -- | 298,648 | 73,183 | 765,081 | |||||||||||||||||||
|
President
|
2013 | 332,750 | -- | -- | 306,863 | 40,000 | 679,613 | |||||||||||||||||||
|
|
2012 | 363,000 | -- | -- | 200,863 | 102,591 | 666,454 | |||||||||||||||||||
|
Geert R. Kersten,
|
2014
|
584,621 | -- | 3,236,526 | 82,917 | 57,581 | 3,961,645 | |||||||||||||||||||
|
Chief Executive
|
2013
|
439,093 | -- | 15,225 | 1,516,692 | 53,514 | 2,024,524 | |||||||||||||||||||
|
Officer and
|
2012
|
477,924 | -- | 14,925 | 332,027 | 56,935 | 881,811 | |||||||||||||||||||
|
Treasurer
|
||||||||||||||||||||||||||
|
Patricia B. Prichep
|
2014
|
247,852 | -- | 1,735,938 | 55,278 | 6,531 | 2,045,599 | |||||||||||||||||||
|
Senior Vice President
|
2013
|
202,253 | -- | 13,941 | 485,634 | 5,531 | 707,359 | |||||||||||||||||||
|
of Operations and
|
2012
|
210,133 | -- | 12,968 | 156,715 | 6,031 | 385,847 | |||||||||||||||||||
|
Secretary
|
||||||||||||||||||||||||||
|
Eyal Talor, Ph.D.
|
2014
|
283,283 | -- | 1,731,290 | 55,278 | 6,031 | 2,075,882 | |||||||||||||||||||
|
Chief Scientific
|
2013
|
272,388 | -- | 9,600 | 460,255 | 6,031 | 748,274 | |||||||||||||||||||
|
Officer
|
2012
|
259,417 | -- | 9,600 | 140,564 | 6,031 | 415,612 | |||||||||||||||||||
|
Daniel Zimmerman,
|
2014
|
213,231 | -- | 13,274 | 227,319 | 6,031 | 459,855 | |||||||||||||||||||
|
Ph.D. Senior Vice
|
2013
|
205,030 | -- | 12,989 | 87,911 | 6,031 | 311,961 | |||||||||||||||||||
|
President of
|
2012
|
199,058 | -- | 12,303 | 115,354 | 6,031 | 332,746 | |||||||||||||||||||
|
Research. Cellular
|
||||||||||||||||||||||||||
|
Immunology
|
||||||||||||||||||||||||||
|
John Cipriano
|
2014
|
197,354 | -- | 888,614 | 41,549 | 31 | 1,127,458 | |||||||||||||||||||
|
Senior Vice President
|
2013
|
189,763 | -- | -- | 47,968 | 31 | 237,762 | |||||||||||||||||||
|
of Regulatory Affairs
|
2012
|
184,236 | -- | -- | 76,515 | 31 | 260,782 | |||||||||||||||||||
|
(1)
|
The dollar value of base salary (cash and non-cash) earned.
|
|
(2)
|
The dollar value of bonus (cash and non-cash) earned.
|
|
(3)
|
The fair value of the shares of restricted stock issued during the periods covered by the table is shown as compensation for services to the persons listed in the table. In the case of all persons listed in the table, the shares were issued as CEL-SCI's contribution on behalf of the named officer who participates in CEL-SCI's 401(k) retirement plan and, by far the largest part, restricted shares issued from the 2014 Incentive Stock Bonus Plan that was voted on and passed by the shareholders at the annual meeting on July 22, 2014. These shares are not vested and are held in escrow. The shares will only be earned upon the achievement of certain milestones leading to the commercialization of the Company’s Multikine technology, or specified increases in the market price of the Company’s stock. If the performance or market criteria are not met as specified in the Incentive Stock Bonus Plan, all or a portion of the awarded shares will be forfeited. The value of all stock awarded during the periods covered by the table are calculated according to ASC 718-10-30-3 which represented the grant date fair value.
|
|
(4)
|
The fair value of all stock options granted during the periods covered by the table are calculated on the grant date in accordance with ASC 718-10-30-3 which represented the grant date fair value
.
|
|
(5)
|
All other compensation received that CEL-SCI could not properly report in any other column of the table including the dollar value of any insurance premiums paid by, or on behalf of, CEL-SCI with respect to term life insurance for the benefit of the named executive officer and car allowances paid by CEL-SCI. Includes board of directors fees for Mr. de Clara and Mr. Kersten.
|
| Name | Paid in Cash | Stock Awards (1) | Option Awards (2) | Total | ||||||||||||
|
Maximilian de Clara
|
$ | 40,000 | $ | - | $ | 298,648 | $ | 338,648 | ||||||||
|
Geert Kersten
|
$ | 40,000 | $ | 3,236,526 | $ | 82,917 | $ | 3,359,443 | ||||||||
|
Alexander Esterhazy
|
$ | 45,000 | $ | - | $ | 134,389 | $ | 179,389 | ||||||||
|
C. Richard Kinsolving
|
$ | 45,000 | $ | - | $ | 134,389 | $ | 179,389 | ||||||||
|
Peter R. Young
|
$ | 50,000 | $ | - | $ | 134,389 | $ | 184,389 | ||||||||
|
(1)
|
The fair value of stock issued for services. This stock is not vested, is held in escrow and will only be released to Mr. Kersten upon the achievement of major shareholder approved Company milestones.
|
|
(2)
|
The fair value of options granted computed in accordance with ASC 718-10-30-3 on the date of grant which represents their grant date fair value.
|
| Name | Grant Date | Options Granted | Price Per Share | Expiration Date | |||||||
|
Maximilian de Clara
|
2/26/2014
|
75,000 | $ | 1.09 |
2/25/2024
|
||||||
|
Maximilian de Clara
|
8/6/2014
|
100,000 | $ | 1.10 |
8/5/2024
|
||||||
|
Maximilian de Clara
|
8/26/2014
|
150,000 | $ | 1.08 |
8/25/2024
|
||||||
|
Geert Kersten
|
2/26/2014
|
90,000 | $ | 1.09 |
2/25/2024
|
||||||
|
Patricia Prichep
|
2/26/2014
|
60,000 | $ | 1.09 |
2/25/2024
|
||||||
|
Eyal Talor
|
2/26/2014
|
60,000 | $ | 1.09 |
2/25/2024
|
||||||
|
Daniel Zimmerman
|
2/26/2014
|
45,000 | $ | 1.09 |
2/25/2024
|
||||||
|
Daniel Zimmerman
|
8/6/2014
|
200,000 | $ | 1.10 |
8/5/2024
|
||||||
|
John Cipriano
|
2/26/2014
|
45,000 | $ | 1.09 |
2/25/2024
|
||||||
|
Alexander Esterhazy
|
2/26/2014
|
45,000 | $ | 1.09 |
2/25/2024
|
||||||
|
Alexander Esterhazy
|
8/6/2014
|
100,000 | $ | 1.10 |
8/5/2024
|
||||||
|
C. Richard Kinsolving
|
2/26/2014
|
45,000 | $ | 1.09 |
2/25/2024
|
||||||
|
C. Richard Kinsolving
|
8/6/2014
|
100,000 | $ | 1.10 |
8/5/2024
|
||||||
|
Peter Young
|
2/26/2014
|
45,000 | $ | 1.09 |
2/25/2024
|
||||||
|
Peter Young
|
8/6/2014
|
100,000 | $ | 1.10 |
8/5/2024
|
||||||
| Employee | Total Options | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term (Years) | |||||||||||
|
Date of
|
Shares Acquired
|
Value
|
||||||||||||
|
Name
|
Exercise
|
On Exercise
|
Realized
|
|||||||||||
|
Shares underlying unexercised
|
|||||||||||||
|
Option which are:
|
Exercise
|
Expiration
|
|||||||||||
|
Name
|
Exercisable
|
Unexercisable
|
Price
|
Date
|
|||||||||
|
Maximilian de Clara
|
5,000 | 4.80 |
09/21/15
|
||||||||||
| 10,000 | 5.80 |
09/12/16
|
|||||||||||
| 20,000 | 6.30 |
09/13/17
|
|||||||||||
| 20,000 | 6.20 |
03/04/18
|
|||||||||||
| 143,625 | (1) | 2.50 |
04/23/19
|
||||||||||
| 25,000 | 3.80 |
07/20/19
|
|||||||||||
| 25,000 | 4.80 |
07/20/20
|
|||||||||||
| 25,000 | 6.90 |
04/14/21
|
|||||||||||
| 47,200 | 3.20 |
12/01/16
|
|||||||||||
| 25,000 | 3.90 |
05/17/22
|
|||||||||||
| 20,000 | 2.80 |
12/17/22
|
|||||||||||
| 12,500 | 2.10 |
06/30/23
|
|||||||||||
| 100,000 | 1.10 |
08/05/24
|
|||||||||||
| 150,000 | 1.08 |
08/25/24
|
|||||||||||
| ---------- | |||||||||||||
| 628,325 | |||||||||||||
| 50,000 | (2) | 3.80 |
07/06/19
|
||||||||||
| 12,500 | 3.90 |
05/17/22
|
|||||||||||
| 80,000 | 2.80 |
12/17/22
|
|||||||||||
| 25,000 | 2.10 |
06/30/23
|
|||||||||||
| 75,000 | 1.09 |
02/25/24
|
|||||||||||
| ---------- | |||||||||||||
| 242,500 | |||||||||||||
|
Geert R. Kersten
|
5,000 | 4.80 |
09/21/15
|
||||||||||
| 20,000 | 5.80 |
09/12/16
|
|||||||||||
| 20,000 | 6.30 |
09/13/17
|
|||||||||||
| 20,000 | 6.20 |
03/04/18
|
|||||||||||
| 183,861 | (1) | 2.50 |
04/23/19
|
||||||||||
| 30,000 | 3.80 |
07/20/19
|
|||||||||||
| 30,000 | 4.80 |
07/20/20
|
|||||||||||
| 30,000 | 6.90 |
04/14/21
|
|||||||||||
| 125,440 | 3.20 |
12/01/16
|
|||||||||||
| 30,000 | 3.90 |
05/17/22
|
|||||||||||
| 189,000 | 2.80 |
12/17/17
|
|||||||||||
| 57,070 | 2.80 |
12/17/22
|
|||||||||||
| 15,000 | 2.10 |
06/30/23
|
|||||||||||
| ---------- | |||||||||||||
| 755,371 | |||||||||||||
| 400,000 | (2) | 3.80 |
07/06/19
|
||||||||||
| 15,000 | 3.90 |
05/17/22
|
|||||||||||
| 442,930 | 2.80 |
12/17/22
|
|||||||||||
| 30,000 | 2.10 |
06/30/23
|
|||||||||||
| 90,000 | 1.09 |
02/25/24
|
|||||||||||
| ---------- | |||||||||||||
| 977,930 | |||||||||||||
|
Patricia B. Prichep
|
5,000 | 3.30 |
04/26/15
|
||||||||||
| 3,000 | 4.80 |
09/21/15
|
|||||||||||
| 9,000 | 5.80 |
09/12/16
|
|||||||||||
| 10,000 | 6.30 |
09/13/17
|
|||||||||||
| 10,000 | 6.20 |
03/04/18
|
|||||||||||
| 71,710 | (1) | 2.50 |
04/23/19
|
||||||||||
| 15,000 | 3.80 |
07/20/19
|
|||||||||||
| 15,000 | 4.80 |
07/20/20
|
|||||||||||
| 15,000 | 6.90 |
04/14/21
|
|||||||||||
| 38,520 | 3.20 |
12/01/16
|
|||||||||||
| 20,000 | 3.90 |
05/17/22
|
|||||||||||
| 58,000 | 2.80 |
12/17/17
|
|||||||||||
| 23,390 | 2.80 |
12/17/22
|
|||||||||||
| 10,000 | 2.10 |
06/30/23
|
|||||||||||
| ---------- | |||||||||||||
| 303,620 | |||||||||||||
| 300,000 | (2) | 3.80 |
07/06/19
|
||||||||||
| 10,000 | 3.90 |
05/17/22
|
|||||||||||
| 126,610 | 2.80 |
12/17/22
|
|||||||||||
| 20,000 | 2.10 |
06/30/23
|
|||||||||||
| 60,000 | 1.09 |
02/25/24
|
|||||||||||
| ---------- | |||||||||||||
| 516,610 |
|
Eyal Talor, Ph.D
|
5,000 | 3.30 |
04/26/15
|
||||||||||
| 3,000 | 4.80 |
09/21/15
|
|||||||||||
| 8,000 | 5.80 |
09/12/16
|
|||||||||||
| 10,000 | 6.30 |
09/13/17
|
|||||||||||
| 10,000 | 6.20 |
03/04/18
|
|||||||||||
| 24,082 | (1) | 2.50 |
04/23/19
|
||||||||||
| 15,000 | 3.80 |
07/20/19
|
|||||||||||
| 15,000 | 4.80 |
07/20/20
|
|||||||||||
| 15,000 | 6.90 |
04/14/21
|
|||||||||||
| 27,773 | 3.20 |
12/01/16
|
|||||||||||
| 20,000 | 3.90 |
05/17/22
|
|||||||||||
| 37,417 | 2.80 |
12/17/17
|
|||||||||||
| 23,390 | 2.80 |
12/17/22
|
|||||||||||
| 10,000 | 2.10 |
06/30/23
|
|||||||||||
| ---------- | |||||||||||||
| 223,662 | |||||||||||||
| 300,000 | (2) | 3.80 |
07/06/19
|
||||||||||
| 10,000 | 3.90 |
05/17/22
|
|||||||||||
| 126,610 | 2.80 |
12/17/22
|
|||||||||||
| 20,000 | 2.10 |
06/30/23
|
|||||||||||
| 60,000 | 1.09 |
02/25/24
|
|||||||||||
| ---------- | |||||||||||||
| 516,610 | |||||||||||||
|
Daniel Zimmerman, Ph.D
|
5,000 | 3.30 |
04/16/15
|
||||||||||
| 3,000 | 4.80 |
09/21/15
|
|||||||||||
| 6,000 | 5.80 |
09/12/16
|
|||||||||||
| 7,500 | 6.30 |
09/13/17
|
|||||||||||
| 7,500 | 6.20 |
03/04/18
|
|||||||||||
| 15,000 | 4.80 |
07/20/20
|
|||||||||||
| 15,000 | 6.90 |
04/14/21
|
|||||||||||
| 25,200 | 3.20 |
12/01/16
|
|||||||||||
| 15,000 | 3.90 |
05/17/22
|
|||||||||||
| 39,200 | 2.80 |
12/17/17
|
|||||||||||
| 7,500 | 2.10 |
06/30/23
|
|||||||||||
| ---------- | |||||||||||||
| 145,900 | |||||||||||||
| 7,500 | 3.90 |
05/17/22
|
|||||||||||
| 15,000 | 2.10 |
06/30/23
|
|||||||||||
| 45,000 | 1.09 |
02/25/24
|
|||||||||||
| 200,000 | 1.10 |
08/05/24
|
|||||||||||
| ---------- | |||||||||||||
| 267,500 |
|
John Cipriano
|
3,000 | 4.80 |
09/21/15
|
||||||||||
| 6,000 | 5.80 |
09/12/16
|
|||||||||||
| 7,500 | 6.30 |
09/13/17
|
|||||||||||
| 7,500 | 6.20 |
03/04/18
|
|||||||||||
| 15,000 | 4.80 |
07/20/20
|
|||||||||||
| 15,000 | 6.90 |
04/14/21
|
|||||||||||
| 1,600 | 3.20 |
12/01/16
|
|||||||||||
| 10,000 | 2.50 |
09/30/19
|
|||||||||||
| 15,000 | 3.90 |
05/17/22
|
|||||||||||
| 7,500 | 2.10 |
06/30/23
|
|||||||||||
| ---------- | |||||||||||||
| 88,100 | |||||||||||||
| 7,500 | 3.90 |
05/17/22
|
|||||||||||
| 15,000 | 2.10 |
06/30/23
|
|||||||||||
| 45,000 | 1.09 |
02/25/24
|
|||||||||||
| ---------- | |||||||||||||
| 67,500 |
|
(1)
|
Options awarded to employees who did not collect a salary, or reduced or deferred their salary between September 15, 2008 and June 30, 2009. For example, Mr. de Clara, Mr. Kersten and Ms. Prichep did not collect any salary between September 30, 2008 and June 30, 2009.
|
|
(2)
|
Long-term performance options: The Board of Directors has identified the successful Phase III clinical trial for Multikine to be the most important corporate event to create shareholder value. Therefore, one third of the options can be exercised when the first 400 patients are enrolled in CEL-SCI's Phase III head and neck cancer clinical trial. One third of the options can be exercised when all of the patients have been enrolled in the Phase III clinical trial. One third of the options can be exercised when the Phase III trial is completed. The grant-date fair value of these options awarded to the senior management of the Company amounts to $3.3 million in total.
|
|
Name of Plan
|
Total Reserved Under Plans | Shares Outstandning Options | Shares Issued | Remaining Options/Shares Under Plans | ||||||||||||
|
Incentive Stock Option Plans
|
1,960,000 | 1,710,997 | N/A | 3,303 | ||||||||||||
|
Non-Qualified Stock Option Plans
|
5,680,000 | 5,120,152 | N/A | 2,929 | ||||||||||||
|
Stock Bonus Plans
|
1,594,000 | N/A | 1,058,896 | 534,348 | ||||||||||||
|
Stock Compensation Plan
|
1,350,000 | N/A | 1,098,621 | 251,379 | ||||||||||||
|
Incentive Stock Bonus Plan
|
16,000,000 | N/A | 15,700,000 | 300,000 | ||||||||||||
|
Plan category
|
Number of Securities to be Issued Upon Exercise of Outstandning Options (a) | Weighted-Average Exercise Price of Outstanding Options | Number of Securities Remaining Available For Future Issuance Under Equity Compensation Plans, Excluding Securities Reflected in Column (a) | |||||||||
|
Incentive Stock Option Plans
|
1,710,997 | $ | 3.05 | 3,303 | ||||||||
|
Non-Qualified Stock
Option Plans
|
5,120,152 | $ | 2.96 | 2,929 | ||||||||
|
Name and Address
|
Number of Shares
(1)
|
Percent of Class
(3)
|
||||||
|
Maximilian de Clara
|
698,448 | 0.80 | % | |||||
|
Bergstrasse 79
|
||||||||
|
6078 Lungern,
|
||||||||
|
Obwalden, Switzerland
|
||||||||
|
Geert R. Kersten
|
10,878,335 | (2) | 11.40 | % | ||||
|
8229 Boone Blvd., Suite 802
|
||||||||
|
Vienna, VA 22182
|
||||||||
|
Patricia B. Prichep
|
3,559,790 | 3.90 | % | |||||
|
8229 Boone Blvd., Suite 802
|
||||||||
|
Vienna, VA 22182
|
||||||||
|
Eyal Talor, Ph.D.
|
3,431,066 | 3.80 | % | |||||
|
8229 Boone Blvd., Suite 802
|
||||||||
|
Vienna, VA 22182
|
||||||||
|
Daniel H. Zimmerman, Ph.D.
|
220,168 | 0.20 | % | |||||
|
8229 Boone Blvd., Suite 802
|
||||||||
|
Vienna, VA 22182
|
||||||||
|
John Cipriano
|
1,703,100 | 1.90 | % | |||||
|
8229 Boone Blvd., Suite 802
|
||||||||
|
Vienna, VA 22182
|
||||||||
|
Alexander G. Esterhazy
|
279,549 | 0.30 | % | |||||
|
20 Chemin du Pre-Poiset
|
||||||||
|
CH- 1253 Vandoeuvres
|
||||||||
|
Geneve, Switzerland
|
||||||||
|
C. Richard Kinsolving, Ph.D.
|
297,125 | 0.30 | % | |||||
|
P.O. Box 20193
|
||||||||
|
Bradenton, FL 34204-0193
|
||||||||
|
Peter R. Young, Ph.D.
|
346,943 | 0.40 | % | |||||
|
208 Hewitt Drive
|
||||||||
|
Suite 103-143
|
||||||||
|
Waco, TX 76712
|
||||||||
|
All Officers and Directors as
a Group (9 persons)
|
21,414,524 | 21.90 | % | |||||
|
(1)
|
Includes shares issuable prior to February 28, 2015 upon the exercise of options or warrants granted to the following persons:
|
|
Name
|
Options or Warrants Exercisable
Prior to February 28, 2015
|
|||
|
Maximilian de Clara
|
673,325 | |||
|
Geert R. Kersten
|
4,336,822 | |||
|
Patricia B. Prichep
|
347,010 | |||
|
Eyal Talor, Ph.D.
|
267,052 | |||
|
Daniel Zimmerman
|
160,900 | |||
|
John Cipriano
|
103,100 | |||
|
Alexander G. Esterhazy
|
256,233 | |||
|
C. Richard Kinsolving, Ph.D.
|
266,900 | |||
|
Peter R. Young, Ph.D.
|
317,167 | |||
|
(2)
|
Amount includes shares held in trust for the benefit of Mr. Kersten's children and warrants held in the de Clara Trust, of which Mr. Kersten is the Trustee and a beneficiary. Geert R. Kersten is the stepson of Maximilian de Clara.
|
|
(3)
|
Amount includes shares referred to in (1) above but excludes shares which may be issued upon the exercise or conversion of other options, warrants and other convertible securities previously issued by CEL-SCI.
|
|
Year Ended September 30,
|
||||||||
|
2014
|
2013
|
|||||||
|
Audit Fees
|
$ | 397,000 | $ | 236,000 | ||||
|
Audit-Related Fees
|
- | - | ||||||
|
Tax Fees
|
- | - | ||||||
|
All Other Fees
|
- | - | ||||||
| Exhibits | ||||
|
3(a)
|
Articles of Incorporation
|
Incorporated by reference to Exhibit 3(a) of CEL-SCI's combined Registration Statement on Form S-1 and Post-Effective Amendment ("Registration Statement"), Registration Nos. 2-85547-D and 33-7531.
|
||
|
3(b)
|
Amended Articles
|
Incorporated by reference to Exhibit 3(a) of CEL-SCI's Registration Statement on Form S-1, Registration Nos. 2-85547-D and 33-7531.
|
||
|
3(c)
|
Amended Articles (Name change only)
|
Filed as Exhibit 3(c) to CEL-SCI's Registration Statement on Form S-1 Registration Statement (No. 33-34878).
|
||
|
3(d)
|
Bylaws
|
Incorporated by reference to Exhibit 3(b) of CEL-SCI's Registration Statement on Form S-1, Registration Nos. 2-85547-D and 33-7531.
|
||
| 4 |
Shareholders Rights Agreement
|
Incorporated by reference to Exhibit 4 of CEL-SCI’S report on Form 8-K dated November 7, 2007.
|
||
|
10(d)
|
Employment Agreement with
Maximilian de Clara
|
Incorporated by reference to Exhibit 10(d)
of CEL-SCI’s report on Form 8-K (dated April
21, 2005) and Exhibit 10(d) to CEL-SCI’s
report on Form 8-K dated September 8, 2006.
|
|
10(f)
|
Securities Purchase Agreement (together
with schedule required by Instruction 2 to
Item 601 of Regulation S-K) pertaining to
Series K notes and warrants, together with
The exhibits to the Securities Purchase
Agreement.
|
Incorporated by reference to Exhibit 10 to
CEL-SCI’s report on Form 8-K dated
August 4, 2006.
|
||
|
10(g)
|
Subscription Agreement (together with
Schedule required by Instruction 2 to
Item 601 of Regulation S-K) pertaining to
April 2007 sale of 20,000,000 shares of
CEL-SCI’s common stock, 10,000,000
Series L warrants and 10,000,000 Series M
Warrants
|
Incorporated by reference to Exhibit 10 of
CEL-SCI’s report on Form 8-K dated April
18, 2007
|
||
|
10(h)
|
Warrant Adjustment Agreement with
Laksya Ventures
|
Incorporated by reference to Exhibit 10(i) of
CEL-SCI’s report on Form 8-K dated August
3, 2010
|
||
|
10(i)
|
Employment Agreement with Patricia
Prichep (2013-2016)
|
Incorporated by reference to Exhibit 10(j) of
CEL-SCI’s report on Form 8-K dated August
30, 2013
|
||
|
10(j)
|
Employment Agreement with Eyal Taylor
(2013-2016)
|
Incorporated by reference to Exhibit 10(k) of
CEL-SCI’s report on Form 8-K dated August 30, 2013.
|
||
|
10(k)
|
Amendment to Employment Agreement
with Maximilian de Clara
|
Incorporated by reference to Exhibit 10(l) of
CEL-SCI’s report on Form 8-K dated August 30, 2010 and Exhibit 10(l) of CEL-SCI’s report on Form 8-K dated August 30, 2013.
|
||
|
10(l)
|
Amendment to Development Supply
and Distribution Agreement with Orient
Europharma. (part of Exhibit 10(m) has
been omitted pursuant to a request for
confidential treatment).
|
Incorporated by reference to Exhibit 10(m)
filed with CEL-SCI’s 10-K report for the
year ended September 30, 2010.
|
||
|
10(m)
|
Licensing Agreement with Teva
Pharmaceutical Industries Ltd. (parts
of Exhibit 10(n) have been omitted
pursuantto a request for confidential
treatment.)
|
Incorporated by reference to Exhibit 10(n)
filed with CEL-SCI’s 10-K report for the
year ended September 30, 2010.
|
||
|
10(n)
|
Lease Agreement (parts of Exhibit 10(o)
have been omitted pursuant to a request
for confidential treatment).
|
Incorporated by reference to Exhibit 10(o)
filed with CEL-SCI’s 10-K report for the
year ended September 30, 2010.
|
||
|
10(o)
|
Loan Agreements with Maximilian de
Clara
|
Incorporated by reference to Exhibit 10(p)
filed with CEL-SCI’s 10-K report for the
year ended September 30, 2010.
|
||
|
10(p)
|
Licensing Agreement with Byron
Biopharma
|
Incorporated by reference to Exhibit 10(i) of
CEL-SCI’s report on Form 8-K dated March
27, 2009
|
||
|
10(q)
|
At Market Issuance Sales Agreement
with McNicoll, Lewis & Vlak LLC
|
Incorporated by reference to Exhibit 10(r)
filed with CEL-SCI’s 10-K report for the
year ended September 30, 2010
|
||
|
10(z)
|
Development, Supply and Distribution
Agreement with Orient Europharma
|
Incorporated by reference to Exhibit 10(z)
filed with CEL-SCI’s report on Form
10-K for the year ended September 30, 2003.
|
||
|
10(za)
|
Employment Agreement with
Geert Kersten. Amendment to
Employment Agreement
|
Incorporated by reference to Exhibit 10(za)
to CEL-SCI’s report on Form 8-K dated
September 1, 2011 and Exhibit 10(za) of
CEL-SCI’s report on Form 8-K dated August
30, 2013.
|
||
|
10(aa)
|
Securities Purchase Agreement and
form of the Series F warrants, which is
and exhibit to the Securities Purchase
Agreement.
|
Incorporated by reference to Exhibit 10(aa)
of CEL-SCI’s report on Form 8-K dated
October 3, 2011.
|
|
10(bb)
|
Placement Agent Agreement
|
Incorporated by reference to Exhibit 10(bb)
of CEL-SCI’s report on Form 8-K dated
October 3, 2011.
|
|
10(cc)
|
Securities Purchase Agreement, together with the form of the Series H warrant, which is an exhibit to the securities Purchase Agreement.
|
Incorporated by reference to Exhibit 10(cc) of CEL-SCI’s report on Form 8-K dated January 25, 2012.
|
||
|
10(dd)
|
Placement Agent Agreement
|
Incorporated by reference to Exhibit 10(dd) of CEL-SCI’s report on Form 8-K dated January 25, 2012.
|
||
|
10(ee)
|
Warrant Amendment Agreement, together with the form of the Series P warrant, which is an exhibit to the Warrant Amendment Agreement.
|
Incorporated by reference to Exhibit 10(ee) of CEL-SCI’s report on Form 8-K dated February 10, 2012.
|
||
|
10(ff)
|
Placement Agent Agreement
|
Incorporated by reference to Exhibit 10(ff) of CEL-SCI’s report on Form 8-K dated February 10, 2012.
|
||
|
10(gg)
|
Securities Purchase Agreement and the form of the Series Q warrant, which is an exhibit to the Securities Purchase Agreement.
|
Incorporated by reference to Exhibit 10(gg) of CEL-SCI’s report on Form 8-K dated June 18, 2012.
|
||
|
10(hh)
|
Placement Agent Agreement
|
Incorporated by reference to Exhibit 10(hh) of CEL-SCI’s report on Form 8-K dated June 18, 2012.
|
||
|
10 (ii)
|
Securities Purchase Agreement and the form of the Series R warrant, which is an exhibit to the Securities Purchase Agreement.
|
Incorporated by reference to Exhibit 10(ii) of CEL-SCI’s report on Form 8-K dated December 5, 2012.
|
||
|
10 (jj)
|
Placement Agent Agreement
|
Incorporated by reference to Exhibit 10(jj) of CEL-SCI’s report on Form 8-K dated December 5, 2012.
|
||
|
10 (nn)
|
Underwriting Agreement, together with the form of Series S warrant which is an exhibit to the underwriting agreement.
|
Incorporated by reference to Exhibit 1.1 of CEL-SCI’s report on Form 8-K dated October 8, 2013.
|
||
|
10 (oo)
|
Underwriting Agreement, together with the form of Series S warrant which is an exhibit to the underwriting agreement.
|
Incorporated by reference to Exhibit 1.1 of CEL-SCI’s report on Form 8-K dated December 19, 2013.
|
||
|
10 (pp)
|
Underwriting Agreement, together with the form of Series T warrant which is an exhibit to the warrant agent agreement.
|
Incorporated by reference to Exhibit 1.1 of CEL-SCI’s report on Form 8-K dated April 15, 2014.
|
||
|
10 (qq)
|
Underwriting Agreement, together with the form of Series S warrant which is an exhibit to the warrant agent agreement.
|
Incorporated by reference to Exhibit 1.1 of CEL-SCI’s report on Form 8-K dated October 23, 2014.
|
||
|
Consent of BDO USA, LLP
|
||||
|
Rule 13a-14(a) Certifications
|
||||
|
Section 1350 Certifications
|
|
CEL-SCI CORPORATION
|
|||
|
|
By:
|
/s/ Maximilian de Clara | |
|
Maximilian de Clara, President
|
|||
|
Signature
|
Title
|
Date
|
||
|
/s/
Maximilian de Clara
|
Director
|
December __, 2014
|
||
|
Maximilian de Clara
|
||||
|
/s/
Geert R. Kersten
|
Chief Executive, Principal
Accounting, Principal Financial
Officer and a Director
|
December __, 2014
|
||
|
Geert R. Kersten
|
||||
|
/s/
Alexander G. Esterhazy
|
Director
|
December __, 2014
|
||
|
Alexander G. Esterhazy
|
||||
|
/s/ Dr. C. Richard Kinsolving
|
Director
|
December __, 2014
|
||
|
Dr. C. Richard Kinsolving
|
||||
|
/s/ Dr. Peter R. Young
|
Director
|
December __, 2014
|
||
|
Dr. Peter R. Young
|
| Page | ||
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
|
F-2 | |
|
FINANCIAL STATEMENTS FOR THE YEARS
ENDED SEPTEMBER 30, 2014, 2013 and 2012:
|
||
|
Balance Sheets
|
F-3 | |
|
Statements of Operations
|
F-4 | |
|
Statements of Stockholders’ Equity
|
F-5 | |
|
Statements of Cash Flows
|
F-6 | |
|
Notes to Financial Statements
|
F-8 |
|
ASSETS
|
2014
|
2013
|
||||||
|
CURRENT ASSETS:
|
||||||||
|
Cash and cash equivalents
|
$ | 8,513,620 | $ | 41,612 | ||||
|
Receivables
|
81,820 | 74,263 | ||||||
|
Prepaid expenses
|
907,526 | 780,523 | ||||||
|
Deposits - current portion
|
150,000 | - | ||||||
|
Inventory used for R&D and manufacturing
|
1,452,020 | 1,016,628 | ||||||
|
Deferred rent - current portion
|
544,074 | 598,717 | ||||||
|
Total current assets
|
11,649,060 | 2,511,743 | ||||||
|
RESEARCH AND OFFICE EQUIPMENT, net
|
403,004 | 489,336 | ||||||
|
PATENT COSTS, net
|
323,588 | 318,195 | ||||||
|
DEFERRED RENT - net of current portion
|
4,733,865 | 5,448,381 | ||||||
|
DEPOSITS
|
2,120,917 | 2,070,917 | ||||||
|
TOTAL ASSETS
|
$ | 19,230,434 | $ | 10,838,572 | ||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
CURRENT LIABILITIES:
|
||||||||
|
Accounts payable
|
$ | 1,160,783 | $ | 1,924,482 | ||||
|
Accrued expenses
|
547,208 | 113,496 | ||||||
|
Due to employees
|
307,961 | 386,337 | ||||||
|
Related party loan
|
1,104,057 | 1,104,057 | ||||||
|
Deferred rent - current portion
|
6,375 | 8,529 | ||||||
|
Lease obligation - current portion
|
8,495 | 8,212 | ||||||
|
Derivative instruments - current portion
|
18,105 | - | ||||||
|
Total current liabilities
|
3,152,984 | 3,545,113 | ||||||
|
Derivative instruments - net of current portion
|
5,487,141 | 433,024 | ||||||
|
Deferred revenue
|
126,591 | 126,545 | ||||||
|
Deferred rent - net of current portion
|
6,290 | 7,875 | ||||||
|
Lease obligation - net of current portion
|
9,028 | 20,925 | ||||||
|
Deposits held
|
5,000 | 5,000 | ||||||
|
Total liabilities
|
8,787,034 | 4,138,482 | ||||||
|
COMMITMENTS AND CONTINGENCIES
|
||||||||
|
STOCKHOLDERS' EQUITY
|
||||||||
|
Preferred stock, $.01 par value-200,000 shares authorized;
|
||||||||
|
-0- shares issued and outstanding
|
- | - | ||||||
|
Common stock, $.01 par value - 600,000,000 shares authorized;
|
||||||||
|
81,902,471 and 31,025,019 shares issued and outstanding
|
||||||||
|
at September 30, 2014 and 2013, respectively
|
819,025 | 310,250 | ||||||
|
Additional paid-in capital
|
249,151,208 | 218,550,408 | ||||||
|
Accumulated deficit
|
(239,526,833 | ) | (212,160,568 | ) | ||||
|
Total stockholders' equity
|
10,443,400 | 6,700,090 | ||||||
|
TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY
|
$ | 19,230,434 | $ | 10,838,572 | ||||
|
2014
|
2013
|
2012
|
||||||||||
|
GRANT INCOME AND OTHER
|
$ | 264,033 | $ | 159,583 | $ | 254,610 | ||||||
|
OPERATING EXPENSES:
|
||||||||||||
|
Research and development (excluding
|
||||||||||||
|
R&D depreciation of $172,442, $253,072
|
||||||||||||
|
and $445,710 respectively, included below)
|
17,000,145 | 12,681,049 | 10,368,695 | |||||||||
|
Depreciation and amortization
|
231,752 | 364,124 | 533,468 | |||||||||
|
General & administrative
|
10,606,248 | 6,982,686 | 6,595,287 | |||||||||
|
Total operating expenses
|
27,838,145 | 20,027,859 | 17,497,450 | |||||||||
|
OPERATING LOSS
|
(27,574,112 | ) | (19,868,276 | ) | (17,242,840 | ) | ||||||
|
GAIN ON DERIVATIVE INSTRUMENTS
|
248,767 | 10,750,666 | 1,911,683 | |||||||||
|
INTEREST INCOME
|
122,854 | 117,086 | 116,061 | |||||||||
|
INTEREST EXPENSE
|
(163,774 | ) | (170,423 | ) | (262,214 | ) | ||||||
|
NET LOSS
|
(27,366,265 | ) | (9,170,947 | ) | (15,477,310 | ) | ||||||
|
ISSUANCE OF ADDITIONAL SHARES DUE TO RESET PROVISIONS
|
(1,117,447 | ) | - | (250,000 | ) | |||||||
|
MODIFICATIONS OF WARRANTS
|
- | (59,531 | ) | (325,620 | ) | |||||||
|
INDUCEMENT WARRANTS
|
- | - | (1,593,000 | ) | ||||||||
|
NET LOSS AVAILABLE TO COMMON SHAREHOLDERS
|
$ | (28,483,712 | ) | $ | (9,230,478 | ) | $ | (17,645,930 | ) | |||
|
NET LOSS PER COMMON SHARE
|
||||||||||||
|
BASIC
|
$ | (0.48 | ) | $ | (0.30 | ) | $ | (0.70 | ) | |||
|
DILUTED
|
$ | (0.49 | ) | $ | (0.66 | ) | $ | (0.78 | ) | |||
|
WEIGHTED AVERAGE COMMON SHARES
|
||||||||||||
|
OUTSTANDING
|
||||||||||||
|
BASIC and DILUTED
|
58,804,622 | 30,279,442 | 25,183,654 | |||||||||
|
Additional
|
||||||||||||||||||||
|
Common
|
Stock
|
Paid-In
|
Accumulated
|
|||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Deficit
|
Total
|
||||||||||||||||
|
BALANCE, October 1, 2011
|
21,473,461 | $ | 214,735 | $ | 196,376,400 | $ | (187,512,311 | ) | $ | 9,078,824 | ||||||||||
|
Sale of stock
|
4,616,667 | 46,167 | 14,243,351 | - | 14,289,518 | |||||||||||||||
|
Issuance of warrants in connection with
|
||||||||||||||||||||
|
sale of common stock
|
- | - | (6,706,667 | ) | - | (6,706,667 | ) | |||||||||||||
|
401(k) contributions paid
|
||||||||||||||||||||
|
in common stock
|
42,627 | 426 | 154,090 | - | 154,516 | |||||||||||||||
|
Exercise of warrants and stock options
|
1,019,119 | 10,191 | 2,654,348 | - | 2,664,539 | |||||||||||||||
|
Stock issued to nonemployees for service
|
160,618 | 1,606 | 556,686 | - | 558,292 | |||||||||||||||
|
Exercise of derivative liabilities
|
- | - | 122,367 | - | 122,367 | |||||||||||||||
|
Modification of options issued to consultants
|
- | - | 54,789 | - | 54,789 | |||||||||||||||
|
Modification of options issued to employees
|
- | - | 36,990 | - | 36,990 | |||||||||||||||
|
Equity based compensation - employees
|
- | - | 2,229,326 | - | 2,229,326 | |||||||||||||||
|
Equity based compensation - non-employees
|
- | - | 22,248 | - | 22,248 | |||||||||||||||
|
Net loss
|
- | - | - | (15,477,310 | ) | (15,477,310 | ) | |||||||||||||
|
BALANCE, SEPTEMBER 30, 2012
|
27,312,492 | 273,125 | 209,743,928 | (202,989,621 | ) | 7,027,432 | ||||||||||||||
|
Sale of stock
|
3,500,000 | 35,000 | 9,753,769 | - | 9,788,769 | |||||||||||||||
|
Issuance of warrants in connection with
|
||||||||||||||||||||
|
sale of common stock
|
- | - | (4,200,000 | ) | - | (4,200,000 | ) | |||||||||||||
|
401(k) contributions paid
|
||||||||||||||||||||
|
in common stock
|
74,230 | 742 | 158,114 | - | 158,856 | |||||||||||||||
|
Stock issued to nonemployees for service
|
138,297 | 1,383 | 359,542 | - | 360,925 | |||||||||||||||
|
Equity based compensation - employees
|
- | - | 2,636,905 | - | 2,636,905 | |||||||||||||||
|
Equity based compensation - non-employees
|
- | - | 98,150 | - | 98,150 | |||||||||||||||
|
Net loss
|
- | - | - | (9,170,947 | ) | (9,170,947 | ) | |||||||||||||
|
BALANCE, SEPTEMBER 30, 2013
|
31,025,019 | 310,250 | 218,550,408 | (212,160,568 | ) | 6,700,090 | ||||||||||||||
|
Sale of stock
|
31,755,494 | 317,555 | 28,129,691 | - | 28,447,246 | |||||||||||||||
|
Issuance of warrants in connection with
|
||||||||||||||||||||
|
sale of common stock
|
- | - | (7,791,448 | ) | - | (7,791,448 | ) | |||||||||||||
|
401(k) contributions paid
|
||||||||||||||||||||
|
in common stock
|
164,787 | 1,647 | 153,787 | - | 155,434 | |||||||||||||||
|
Exercise of warrants
|
2,668,508 | 26,686 | 4,253,632 | - | 4,280,318 | |||||||||||||||
| Conversion of warrant liability to equity | - | - | 1,308,528 | - | 1,308,528 | |||||||||||||||
|
Stock issued to nonemployees for service
|
579,968 | 5,800 | 621,318 | - | 627,118 | |||||||||||||||
|
Stock issued for patents
|
8,695 | 87 | 9,912 | - | 9,999 | |||||||||||||||
|
Modification of options issued to consultants
|
- | - | 76,991 | - | 76,991 | |||||||||||||||
|
Issuance of restricted stock
|
15,700,000 | 157,000 | (157,000 | ) | - | - | ||||||||||||||
|
Equity based compensation - employees
|
- | - | 3,958,637 | - | 3,958,637 | |||||||||||||||
|
Equity based compensation - non-employees
|
- | - | 36,752 | - | 36,752 | |||||||||||||||
|
Net loss
|
- | - | - | (27,366,265 | ) | (27,366,265 | ) | |||||||||||||
|
BALANCE, SEPTEMBER 30, 2014
|
81,902,471 | $ | 819,025 | $ | 249,151,208 | $ | (239,526,833 | ) | $ | 10,443,400 | ||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||||||||||
|
Net loss
|
$ | (27,366,265 | ) | $ | (9,170,947 | ) | $ | (15,477,310 | ) | |||
|
Adjustments to reconcile net loss to
|
||||||||||||
|
net cash used in operating activities:
|
||||||||||||
|
Depreciation and amortization
|
231,752 | 364,124 | 533,468 | |||||||||
|
Issuance of common stock, warrants and options for services
|
694,955 | 454,855 | 527,207 | |||||||||
|
Modification of warrants issued to consultants
|
76,991 | - | 54,789 | |||||||||
|
Modification of stock options issued to employees
|
- | - | 36,990 | |||||||||
|
Equity based compensation
|
3,958,637 | 2,636,905 | 2,229,326 | |||||||||
|
Common stock contributed to 401(k) plan
|
155,434 | 158,856 | 154,516 | |||||||||
|
Impairment loss on abandonment of patents
|
1,182 | 22,628 | 44,921 | |||||||||
|
Loss on retired equipment
|
268 | 4,350 | 9,399 | |||||||||
|
Gain on derivative instruments
|
(248,767 | ) | (10,750,666 | ) | (1,911,683 | ) | ||||||
|
(Increase)/decrease in assets:
|
||||||||||||
|
Receivables
|
(7,557 | ) | 84,351 | 298,723 | ||||||||
|
Deferred rent
|
769,159 | 544,028 | 598,714 | |||||||||
|
Prepaid expenses
|
(158,088 | ) | 529,738 | 775,823 | ||||||||
|
Inventory used for R&D and manufacturing
|
(435,392 | ) | 367,856 | 186,698 | ||||||||
|
Deposits
|
(200,000 | ) | (400,000 | ) | - | |||||||
|
Increase/(decrease) in liabilities:
|
||||||||||||
|
Accounts payable
|
(751,971 | ) | 1,316,964 | (168,463 | ) | |||||||
|
Accrued expenses
|
433,712 | 101,995 | (99,006 | ) | ||||||||
|
Deferred revenue
|
46 | 45 | 1,500 | |||||||||
|
Due to employees
|
(78,376 | ) | 186,446 | (2,611 | ) | |||||||
|
Deferred rent liability
|
(3,739 | ) | (108 | ) | 11,986 | |||||||
|
Deposits held
|
- | - | 5,000 | |||||||||
|
Net cash used in operating activities
|
(22,928,019 | ) | (13,548,580 | ) | (12,190,013 | ) | ||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||||||
|
Purchases of equipment
|
(103,977 | ) | (102,033 | ) | (54,637 | ) | ||||||
|
Expenditures for patent costs
|
(34,887 | ) | (30,728 | ) | (78,959 | ) | ||||||
|
Net cash used in investing activities
|
(138,864 | ) | (132,761 | ) | (133,596 | ) | ||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
||||||||||||
|
Proceeds from issuance of common stock and warrants
|
28,428,641 | 9,788,769 | 14,289,518 | |||||||||
|
Proceeds from exercise of warrants and stock options
|
3,118,387 | - | 2,664,539 | |||||||||
|
Payments on convertible debt
|
- | - | (4,950,000 | ) | ||||||||
|
Payments on obligations under capital lease
|
(8,137 | ) | (6,858 | ) | - | |||||||
|
Net cash provided by financing activities
|
31,538,891 | 9,781,911 | 12,004,057 | |||||||||
|
NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS
|
8,472,008 | (3,899,430 | ) | (319,552 | ) | |||||||
|
CASH AND CASH EQUIVALENTS, BEGINNING OF YEAR
|
41,612 | 3,941,042 | 4,260,594 | |||||||||
|
CASH AND CASH EQUIVALENTS, END OF YEAR
|
$ | 8,513,620 | $ | 41,612 | $ | 3,941,042 | ||||||
|
2014
|
2013
|
2012
|
||||||||||
|
ISSUANCE OF WARRANTS:
|
||||||||||||
|
Increase in derivative liabilities
|
$ | (7,791,448 | ) | $ | (4,200,000 | ) | $ | (6,706,667 | ) | |||
|
Decrease in additional paid-in capital
|
7,791,448 | 4,200,000 | 6,706,667 | |||||||||
| $ | - | $ | - | $ | - | |||||||
|
ISSUANCE OF ADDITIONAL SHARES:
|
||||||||||||
|
Increase in common stock
|
$ | (15,631 | ) | $ | - | $ | (8,333 | ) | ||||
|
Increase in additional paid-in capital
|
(1,101,816 | ) | - | (241,667 | ) | |||||||
|
Decrease in additional paid-in capital
|
1,117,447 | - | 250,000 | |||||||||
| $ | - | $ | - | $ | - | |||||||
|
EXERCISE OF WARRANTS
|
||||||||||||
|
Increase in common stock
|
$ | (657 | ) | $ | - | $ | - | |||||
|
Increase in additional paid-in capital
|
(1,161,274 | ) | - | (122,367 | ) | |||||||
|
Decrease in derivative liabilities
|
1,161,931 | - | 122,367 | |||||||||
| $ | - | $ | - | $ | - | |||||||
|
INDUCEMENT WARRANTS
|
||||||||||||
|
Increase in additional paid-in capital
|
$ | - | $ | - | $ | (1,593,000 | ) | |||||
|
Decrease in additional paid-in capital
|
- | - | 1,593,000 | |||||||||
| $ | - | $ | - | $ | - | |||||||
|
RECLASSIFICATION/MODIFICATION OF WARRANTS:
|
||||||||||||
|
Increase in additional paid-in capital
|
$ | (1,308,528 | ) | $ | - | $ | (325,620 | ) | ||||
|
Decrease in additional paid-in capital
|
325,620 | |||||||||||
|
Decrease in derivative liabilities
|
1,308,528 | - | - | |||||||||
| $ | - | $ | - | $ | - | |||||||
|
ISSUANCE OF COMMON STOCK FOR PREPAID SERVICES
|
||||||||||||
|
Increase in additional paid-in capital
|
$ | (31,085 | ) | $ | (57,553 | ) | $ | (53,333 | ) | |||
|
Increase in prepaid expenses
|
31,085 | 57,553 | 53,333 | |||||||||
| $ | - | $ | - | $ | - | |||||||
|
ISSUANCE OF COMMON STOCK FOR PATENT COSTS
|
||||||||||||
|
Increase in common stock
|
$ | (87 | ) | $ | - | $ | - | |||||
|
Increase in additional paid in capital
|
(9,912 | ) | - | - | ||||||||
|
Increase in patent costs
|
9,999 | - | - | |||||||||
| $ | - | $ | - | $ | - | |||||||
|
PATENT COSTS INCLUDED IN ACCOUNTS PAYABLE
|
||||||||||||
|
Increase in patent costs
|
$ | 4,474 | $ | 14,024 | $ | 22,379 | ||||||
|
Increase in accounts payable
|
(4,474 | ) | (14,024 | ) | (22,379 | ) | ||||||
| $ | - | $ | - | $ | - | |||||||
|
NON-CASH EQUIPMENT CHANGES
|
||||||||||||
|
Increase (decrease) in research and office equipment
|
$ | (1,074 | ) | $ | 36,622 | $ | - | |||||
|
Increase in accounts payable
|
(2,345 | ) | - | - | ||||||||
|
Decrease (increase) in capital lease obligation
|
3,419 | (36,622 | ) | - | ||||||||
| $ | - | $ | - | $ | - | |||||||
|
CAPITAL LEASE PAYMENTS INCLUDED IN
|
||||||||||||
|
ACCOUNTS PAYABLE:
|
||||||||||||
|
Decrease in capital lease obligation
|
$ | 58 | $ | 627 | $ | - | ||||||
|
Increase in accounts payable
|
(58 | ) | (627 | ) | - | |||||||
| $ | - | $ | - | $ | - | |||||||
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOWS
|
||||||||||||
|
INFORMATION:
|
||||||||||||
|
Cash expenditure for interest expense
|
$ | 180,654 | $ | 156,225 | $ | 377,715 | ||||||
|
Warrants
|
Issue Date
|
Shares Issuable upon Exercise of
Warrants
|
Exercise
Price
|
Expiration Date
|
Refer-ence
|
||||||||||
|
Series N
|
8/18/08
|
2,844,627 | 0.53 |
8/18/15
|
1 | ||||||||||
|
Series A
|
6/24/09
|
130,347 | 5.00 |
12/24/14
|
1 | ||||||||||
|
Schleuning (Series A)
|
7/8/09
|
16,750 | 5.00 |
1/8/15
|
1 | ||||||||||
|
Series B
|
9/4/09
|
- | 6.80 |
9/4/14
|
1 | ||||||||||
|
Series C
|
8/20/09 – 8/26/09
|
463,487 | 5.50 |
2/20/15
|
1 | ||||||||||
|
Series E
|
9/21/09
|
- | 17.50 |
8/12/14
|
1 | ||||||||||
|
Series F
|
10/6/11
|
1,200,000 | 4.00 |
10/6/14
|
1 | ||||||||||
|
Series G
|
10/6/11
|
- | 4.00 |
8/12/14
|
1 | ||||||||||
|
Series H
|
1/26/12
|
1,200,000 | 5.00 |
8/1/15
|
1 | ||||||||||
|
Series Q
|
6/21/12
|
1,200,000 | 5.00 |
12/22/15
|
1 | ||||||||||
|
Series R
|
12/6/12
|
2,625,000 | 4.00 |
12/6/16
|
1 | ||||||||||
|
Series S
|
10/11/13-12/24/13
|
23,624,326 | 1.25 |
10/11/18
|
1 | ||||||||||
|
Series T
|
4/17/14
|
1,782,057 | 1.58 |
10/17/14
|
1 | ||||||||||
|
Series U
|
4/17/14
|
445,514 | 1.75 |
10/17/17
|
1 | ||||||||||
|
Series L
|
4/18/07
|
- | 7.50 |
4/17/14
|
2 | ||||||||||
|
Series L (repriced)
|
4/18/07
|
70,000 | 2.50 |
4/2/15
|
2 | ||||||||||
|
Series P
|
2/10/12
|
590,001 | 4.50 |
3/6/17
|
3 | ||||||||||
|
Private Investor
|
7/18/05
|
- | 6.50 |
7/18/14
|
4 | ||||||||||
|
Warrants held by Officer and Director
|
6/24/09 – 7/6/09
|
349,754 | 4.00 – 5.00 |
12/24/14 – 1/6/15
|
5 | ||||||||||
|
Consultants
|
2/15/05 – 4/25/14
|
149,500 | 0.85 – 20.00 |
2/15/15 - 12/27/17
|
6 | ||||||||||
|
1.
|
Derivative Liabilities
|
|
2014
|
2013
|
|||||||
|
Series N warrants
|
$ | - | $ | 41,501 | ||||
|
Series A through E warrants
|
6,105 | 6,106 | ||||||
|
Series F and G warrants
|
- | 12,667 | ||||||
|
Series H warrants
|
12,000 | 36,000 | ||||||
|
Series Q warrants
|
12,000 | 48,000 | ||||||
|
Series R warrants
|
157,500 | 288,750 | ||||||
|
Series S warrants
|
5,197,352 | - | ||||||
|
Series T warrants
|
- | - | ||||||
|
Series U warrants
|
120,289 | - | ||||||
|
|
||||||||
|
Total derivative liabilities
|
$ | 5,505,246 | $ | 433,024 | ||||
|
2.
|
Series L and M Warrants
|
|
4.
|
Private Investor Warrants
|
|
5.
|
Warrants held by Officer and Director
|
|
6.
|
Options and Shares Issued to Consultants
|
| 2014 | 2013 | |||||||
|
Research equipment
|
$ | 3,230,882 | $ | 3,184,779 | ||||
|
Furniture and equipment
|
141,269 | 139,992 | ||||||
|
Leasehold improvements
|
131,910 | 131,910 | ||||||
| 3,504,061 | 3,456,681 | |||||||
|
Less: Accumulated depreciation and amortization
|
(3,101,057 | ) | (2,967,345 | ) | ||||
|
Net research and office equipment
|
$ | 403,004 | $ | 489,336 | ||||
| 2014 | 2013 | |||||||
|
Patents
|
$ | 1,517,344 | $ | 1,470,047 | ||||
|
Accumulated amortization
|
(1,193,756 | ) | (1,151,852 | ) | ||||
|
Net Patents
|
$ | 323,588 | $ | 318,195 | ||||
|
Year ending September 30,
|
||||
| 2015 | $ | 36,051 | ||
| 2016 | 36,051 | |||
| 2017 | 36,051 | |||
| 2018 | 35,716 | |||
| 2019 | 34,014 | |||
| Thereafter | 145,705 | |||
| $ | 323,588 | |||
| 2014 | 2013 | |||||||
|
Net operating loss carryforwards
|
$ | 55,229,799 | $ | 50,485,248 | ||||
|
R&D credit
|
1,221,487 | 1,221,487 | ||||||
|
Stock-based compensation
|
4,054,450 | 3,323,353 | ||||||
|
Fixed assets and intangibles
|
26,329 | - | ||||||
|
Capitalized R&D
|
9,897,041 | 5,542,816 | ||||||
|
Vacation and other
|
108,891 | 270,121 | ||||||
|
Total deferred tax assets
|
70,537,997 | 60,843,025 | ||||||
|
|
||||||||
|
Fixed assets and intangibles
|
- | (1,968 | ) | |||||
|
Total deferred tax liabilities
|
- | (1,968 | ) | |||||
|
Valuation allowance
|
(70,537,997 | ) | (60,841,057 | ) | ||||
|
Net deferred tax asset
|
$ | - | $ | - | ||||
| 2014 | 2013 | 2012 | ||||||||||
| Federal Rate | 34.00 | % | 34.00 | % | 34.00 | % | ||||||
| State tax rate, net of federal benefit | 5.15 | 4.97 | 5.21 | |||||||||
| State tax rate change | 0.93 | (3.77 | ) | 18.07 | ||||||||
| Other adjustments | 0.00 | 0.00 | (0.53 | ) | ||||||||
| Expired tax attributes | 0.00 | (87.87 | ) | (33.54 | ) | |||||||
| Adjustment to deferreds | 19.13 | 14.30 | 0.00 | |||||||||
| Permanent differences | (0.43 | ) | (1.59 | ) | (0.68 | ) | ||||||
| Change in valuation allowance | (58.78 | ) | 39.96 | (23.53 | ) | |||||||
| Effective tax rate | 0.00 | % | 0.00 | % | 0.00 | % | ||||||
|
Fiscal Year Ended September 30,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Employees
|
$ | 3,958,637 | $ | 2,636,905 | $ | 2,266,316 | ||||||
|
Non-employees
|
$ | 771,946 | $ | 454,855 | $ | 581,996 | ||||||
| 2014 | 2013 | 2012 | ||||
|
Expected stock price volatility
|
72.81 – 86.87%
|
84.41-92.28%
|
87.72-94.93%
|
|||
|
Risk-free interest rate
|
0.59 – 2.65%
|
0.75-2.73%
|
0.83-1.92%
|
|||
|
Expected life of options
|
3.0 – 9.76 Years
|
4.85-9.77 Years
|
4.82-9.66 Years
|
|||
|
Expected dividend yield
|
- | - | - |
|
Outstanding
|
Exercisable
|
|||||||||||||||||||||||||||||||
|
Number of
Shares
|
Weighted
Average
|
Weighted
Ave
|
Aggregate
Intrinsic
|
Number of
Shares
|
Weighted
Average
|
Weighted
Ave
|
Aggregate
Intrinsic
|
|||||||||||||||||||||||||
|
Outstanding at October 1, 2013
|
5,188,141 | $ | 3.62 | 6.53 | $ | 133 | 2,422,997 | $ | 4.00 | 4.95 | $ | 133 | ||||||||||||||||||||
|
Vested
|
1,094,803 | $ | 2.14 | |||||||||||||||||||||||||||||
|
Granted (a)
|
1,723,240 | $ | 1.09 | |||||||||||||||||||||||||||||
|
Exercised
|
||||||||||||||||||||||||||||||||
|
Forfeited
|
6,316 | $ | 1.60 | |||||||||||||||||||||||||||||
|
Expired
|
73,916 | $ | 4.29 | 73,916 | $ | 4.29 | ||||||||||||||||||||||||||
|
Cancelled
|
||||||||||||||||||||||||||||||||
|
Outstanding at September 30, 2014
|
6,831,149 | $ | 2.98 | 6.55 | $ | 3,600 | 3,443,884 | $ | 3.40 | 5.49 | $ | 3,600 | ||||||||||||||||||||
|
(a)
|
During the year ending September 30, 2014, 80,000 stock options were granted to consultants.
|
| Number of Shares | Weighted Average Grant Date Fair Value | |||||||
|
Unvested at October 1, 2012
|
1,649,063 | $ | 3.60 | |||||
|
Vested
|
(729,087 | ) | ||||||
|
Granted
|
1,859,387 | |||||||
|
Forfeited
|
(14,219 | ) | ||||||
|
Unvested at October 1, 2013
|
2,765,144 | $ | 2.79 | |||||
|
Vested
|
(1,094,803 | ) | ||||||
|
Granted
|
1,723,240 | |||||||
|
Forfeited
|
(6,316 | ) | ||||||
|
Unvested at September 30, 2014
|
3,387,265 | $ | 2.15 | |||||
| 2015 | $ | 1,785,873 | ||
| 2016 | 1,769,497 | |||
| 2017 | 1,746,328 | |||
| 2018 | 1,746,802 | |||
| 2019 | 1,808,302 | |||
|
2020 and thereafter
|
19,570,627 | |||
|
Total minimum lease payments:
|
$ | 28,427,429 | ||
|
o
|
Level 1 – Observable inputs such as quoted prices in active markets for identical assets or liabilities
|
|
o
|
Level 2 – Inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active and amounts derived from valuation models where all significant inputs are observable in active markets
|
|
o
|
Level 3 – Unobservable inputs that reflect management’s assumptions
|
| Quoted Prices in Active Markets for Identical Assets or Liabilities (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | Total | |||||||||||||
|
Derivative Instruments
|
$ | 5,197,352 | $ | - | $ | 307,894 | $ | 5,505,246 | ||||||||
| Quoted Prices in Active Markets for Identical Assets or Liabilities (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | Total | |||||||||||||
|
Derivative Instruments
|
$ | - | $ | - | $ | 433,024 | $ | 433,024 | ||||||||
|
2014
|
2013
|
|||||||
|
Beginning balance
|
$ | 433,024 | $ | 6,983,690 | ||||
|
Issuances
|
7,791,448 | 4,200,000 | ||||||
|
Settlements
|
(1,445,528 | ) | - | |||||
|
Transfers to Level 1
|
(7,321,071 | ) | - | |||||
|
Realized and unrealized losses/(gains) recorded in earnings
|
850,021 | (10,750,066 | ) | |||||
|
Ending balance
|
$ | 307,894 | $ | 433,024 | ||||
| 2014 | 2013 | 2012 | ||||||||||
|
Net loss – available to
common shareholders
|
$ | (28,483,712 | ) | $ | (9,230,478 | ) | $ | (17,645,930 | ) | |||
|
Less: Gain on derivative
Instruments
|
(248,767 | ) | (10,750,666 | ) | (1,911,683 | ) | ||||||
|
Net loss - diluted
|
$ | (28,732,479 | ) | $ | (19,981,144 | ) | $ | (19,557,613 | ) | |||
|
Weighted average number of
shares - basic and diluted
|
58,804,622 | 30,279,442 | 25,183,654 | |||||||||
|
Loss per share - basic
|
$ | (0.48 | ) | $ | (0.30 | ) | $ | (0.70 | ) | |||
|
Loss per share - diluted
|
$ | (0.49 | ) | $ | (0.66 | ) | $ | (0.78 | ) | |||
|
Fiscal 2014
|
||||||||||||||||||||
|
Three months
|
Three months
|
Three months
|
Three months
|
|||||||||||||||||
|
ended
|
ended
|
ended
|
Ended
|
Year ended
|
||||||||||||||||
|
December 31
2013
|
March 31,
2014
|
June 30,
2014
|
September 30,
2014
|
September 30,
2014
|
||||||||||||||||
|
Revenue
|
$ | 113,144 | $ | 67,157 | $ | 15,914 | $ | 67,818 | $ | 264,033 | ||||||||||
|
Operating expenses
|
6,047,454 | 6,293,592 | 6,917,243 | 8,579,856 | 27,838,145 | |||||||||||||||
|
Non-operating expenses, net
|
(10,925 | ) | (6,797 | ) | (10,927 | ) | (12,271 | ) | (40,920 | ) | ||||||||||
|
Gain (loss) on derivative instruments
|
1,610,817 | (7,132,348 | ) | 4,467,776 | 1,302,522 | 248,767 | ||||||||||||||
|
Net loss
|
(4,334,418 | ) | (13,365,580 | ) | (2,444,480 | ) | (7,221,787 | ) | (27,366,265 | ) | ||||||||||
|
Issuance of shares due to reset provisions
|
(1,117,447 | ) | - | - | - | (1,117,447 | ) | |||||||||||||
|
Net loss available to
|
||||||||||||||||||||
|
common shareholders
|
$ | (5,451,865 | ) | $ | (13,365,580 | ) | $ | (2,444,480 | ) | $ | (7,221,787 | ) | $ | (28,483,712 | ) | |||||
|
Net loss per share-basic
|
$ | (0.11 | ) | $ | (0.24 | ) | $ | (0.04 | ) | $ | (0.11 | ) | $ | (0.48 | ) | |||||
|
Net loss per share-diluted
|
$ | (0.15 | ) | $ | (0.24 | ) | $ | (0.11 | ) | $ | (0.13 | ) | $ | (0.49 | ) | |||||
|
Weighted average shares-basic and diluted
|
48,215,919 | 56,239,562 | 64,664,274 | 66,091,826 | 58,804,622 | |||||||||||||||
|
Fiscal 2013
|
||||||||||||||||||||
|
Three months
|
Three months
|
Three months
|
Three months
|
|||||||||||||||||
|
ended
|
ended
|
ended
|
ended
|
Year ended
|
||||||||||||||||
|
December 31
2012
|
March 31,
2013
|
June 30,
2013
|
September 30,
2013
|
September 30,
2013
|
||||||||||||||||
|
Revenue
|
$ | 15,000 | $ | 15,405 | $ | 113,728 | $ | 15,450 | $ | 159,583 | ||||||||||
|
Operating expenses
|
5,059,457 | 4,255,229 | 5,626,927 | 5,087,191 | 20,027,859 | |||||||||||||||
|
Non operating expenses, net
|
(11,987 | ) | (11,811 | ) | (13,666 | ) | (14,928 | ) | (53,337 | ) | ||||||||||
|
Gain on derivative instruments
|
2,746,198 | 3,538,264 | 1,079,392 | 3,386,812 | 10,750,666 | |||||||||||||||
|
Net loss
|
(2,310,246 | ) | (713,371 | ) | (4,447,473 | ) | (1,699,857 | ) | (9,170,947 | ) | ||||||||||
|
Modification of warrants
|
- | - | (59,531 | ) | - | (59,531 | ) | |||||||||||||
|
Net loss available to
|
||||||||||||||||||||
|
common shareholders
|
$ | (2,310,246 | ) | $ | (713,371 | ) | $ | (4,507,004 | ) | $ | (1,699,857 | ) | $ | (9,230,478 | ) | |||||
|
Net loss per share-basic
|
$ | (0.08 | ) | $ | (0.02 | ) | $ | (0.15 | ) | $ | (0.05 | ) | $ | (0.30 | ) | |||||
|
Net loss per share-diluted
|
$ | (0.18 | ) | $ | (0.14 | ) | $ | (0.18 | ) | $ | (0.16 | ) | $ | (0.66 | ) | |||||
|
Weighted average shares-basic and diluted
|
28,311,602 | 30,901,177 | 30,930,650 | 30,994,932 | 30,279,442 | |||||||||||||||
|
CEL-SCI CORPORATION
|
|||
|
|
By:
|
/s/ Maximilian de Clara | |
|
Maximilian de Clara, President
|
|||
|
Signature
|
Title
|
Date
|
||
|
/s/
Maximilian de Clara
|
Director
|
December 23, 2014
|
||
|
Maximilian de Clara
|
||||
|
/s/
Geert R. Kersten
|
Chief Executive, Principal
Accounting, Principal Financial
Officer and a Director
|
December 23, 2014
|
||
|
Geert R. Kersten
|
||||
|
/s/
Alexander G. Esterhazy
|
Director
|
December 23, 2014
|
||
|
Alexander G. Esterhazy
|
||||
|
/s/ Dr. C. Richard Kinsolving
|
Director
|
December 23, 2014
|
||
|
Dr. C. Richard Kinsolving
|
||||
|
/s/ Dr. Peter R. Young
|
Director
|
December 23, 2014
|
||
|
Dr. Peter R. Young
|