|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CEL-SCI
CORPORATION
|
||
|
(Exact name of
registrant as specified in its charter)
|
||
|
|
||
|
COLORADO
|
|
84-0916344
|
|
(State or other
jurisdiction of incorporation or organization)
|
|
(I.R.S. Employer
Identification No.)
|
|
|
|
|
|
8229 Boone Blvd.,
Suite 802
|
|
|
|
Vienna,
Virginia
|
|
22182
|
|
(Address of
principal executive offices)
|
|
(Zip
Code)
|
|
Quarter Ending
|
High
|
Low
|
|
|
|
|
|
12/31/14
|
$
0.91
|
$
0.54
|
|
3/31/15
|
$
1.23
|
$
0.59
|
|
6/30/15
|
$
1.09
|
$
0.59
|
|
9/30/15
|
$
0.80
|
$
0.48
|
|
|
|
|
|
12/31/15
|
$
0.75
|
$
0.36
|
|
3/31/16
|
$
0.66
|
$
0.36
|
|
6/30/16
|
$
0.60
|
$
0.44
|
|
9/30/16
|
$
0.54
|
$
0.24
|
|
|
|
9/11
|
9/12
|
9/13
|
9/14
|
9/15
|
9/16
|
|
|
|
|
|
|
|
|
|
|
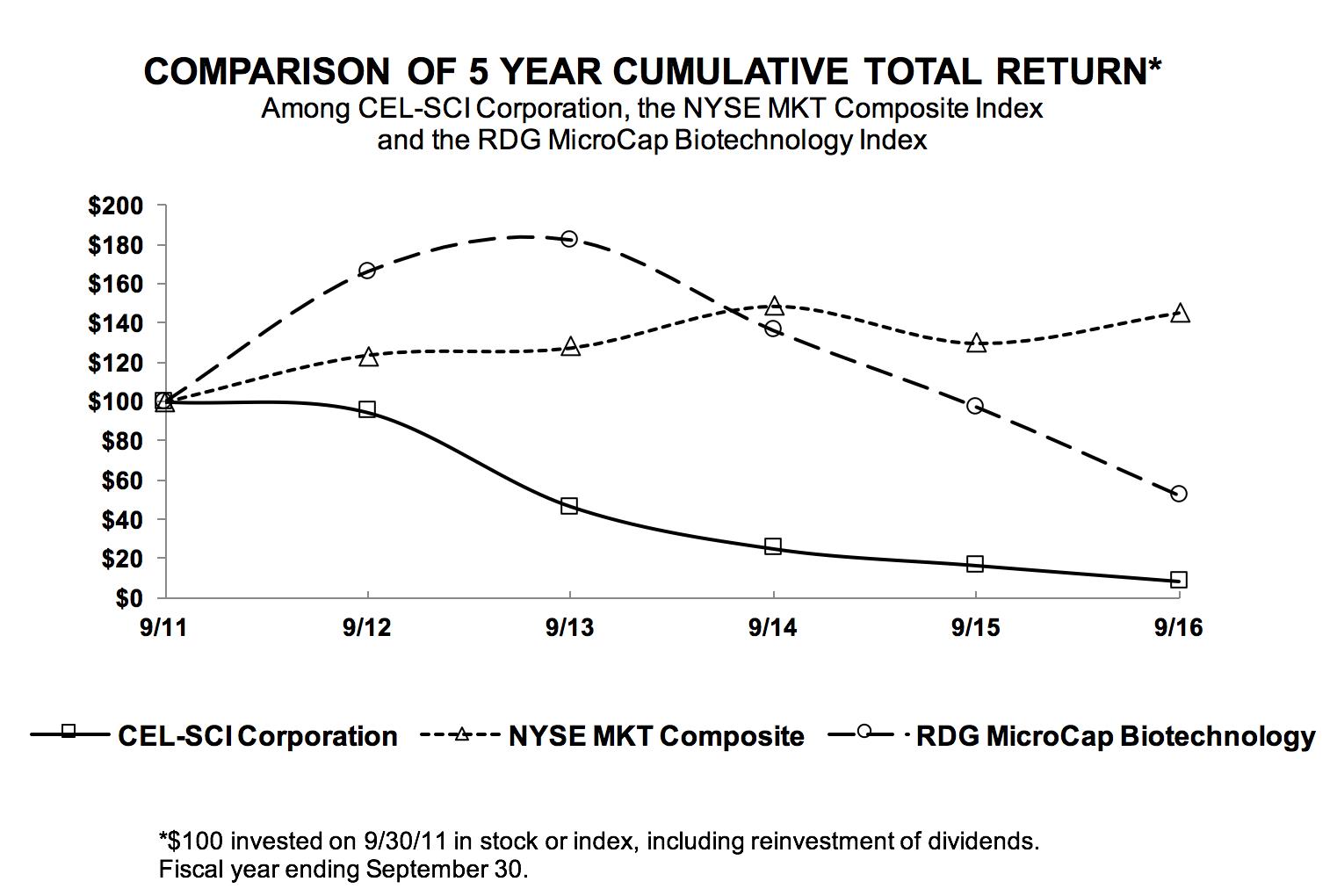
CEL-SCI Corporation
|
|
100.00
|
94.52
|
46.58
|
24.98
|
16.44
|
8.36
|
|
NYSE MKT Composite
|
|
100.00
|
123.99
|
127.59
|
148.86
|
129.94
|
145.53
|
|
RDG MicroCap Biotechnology
|
|
100.00
|
166.25
|
182.33
|
136.22
|
97.34
|
52.30
|
|
|
2016
|
2015
|
2014
|
2013
|
2012
|
|
Grant
revenue and other
|
$
285,055
|
$
657,377
|
$
264,033
|
$
159,583
|
$
254,610
|
|
Operating
expenses:
|
|
|
|
|
|
|
Research
and development
|
19,351,779
|
21,098,147
|
17,172,587
|
12,934,121
|
10,814,405
|
|
General
and administrative
|
6,486,501
|
13,855,775
|
10,665,558
|
7,093,738
|
6,683,045
|
|
Gain
on derivative instruments
|
14,013,726
|
282,616
|
248,767
|
10,750,666
|
1,911,683
|
|
Loss
on debt extinguishment
|
-
|
(620,457
)
|
-
|
-
|
-
|
|
Interest
income (expense), net
|
73,001
|
(40,260
)
|
(40,920
)
|
(53,337
)
|
(146,153
)
|
|
Net
loss
|
(11,466,498
)
|
(34,674,646
)
|
(27,366,265
)
|
(9,170,947
)
|
(15,477,310
)
|
|
Issuance of additional shares due to reset
provision
|
(1,117,447
)
|
-
|
(250,000
)
|
||
|
Modification
of warrants
|
|
|
|
(59,531
)
|
(325,620
)
|
|
Inducement
warrants
|
-
|
-
|
-
|
-
|
(1,593,000
)
|
|
Net
loss available to common shareholders
|
$
(11,466,498
)
|
$
(34,674,646
)
|
$
(28,483,712
)
|
$
(9,230,478
)
|
$
(17,645,930
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss per common share
|
|
|
|
|
|
|
Basic
|
$
(0.09
)
|
$
(0.42
)
|
$
(0.48
)
|
$
(0.30
)
|
$
(0.70
)
|
|
Diluted
|
$
(0.09
)
|
$
(0.42
)
|
$
(0.49
)
|
$
(0.66
)
|
$
(0.78
)
|
|
Weighted average common shares
outstanding
|
|
|
|
|
|
|
Basic
and diluted
|
121,655,108
|
82,519,027
|
58,804,622
|
30,279,442
|
25,183,654
|
|
|
2016
|
2015
|
2014
|
2013
|
2012
|
|
|
|
|
|
|
|
|
Working
capital (deficit)
|
$
1,875,874
|
$
2,127,718
|
$
8,496,076
|
$
(1,033,370
)
|
$
5,529,438
|
|
Total
assets
|
$
11,598,247
|
$
15,447,603
|
$
19,230,434
|
$
10,838,572
|
$
16,067,450
|
|
Derivative
instruments (a)
|
$
8,394,934
|
$
13,686,587
|
$
5,505,246
|
$
433,024
|
$
6,983,690
|
|
Total
liabilities
|
$
12,554,315
|
$
20,532,722
|
$
8,787,034
|
$
4,138,482
|
$
9,040,018
|
|
Stockholders'
(deficit) equity
|
$
(956,068
)
|
$
(5,085,119
)
|
$
10,443,400
|
$
6,700,090
|
$
7,027,432
|
|
|
|
Net income (loss) per share
|
|
|
Quarter
|
Net income (loss)
|
Basic
|
Diluted
|
|
|
|
|
|
|
12/31/15
|
$
2,341,813
|
$
0.02
|
$
0.02
|
|
3/31/16
|
$
(8,844,855
)
|
$
(0.07
)
|
$
(0.07
)
|
|
6/30/16
|
$
(3,849,324
)
|
$
(0.03
)
|
$
(0.03
)
|
|
9/30/16
|
$
(1,114,132
)
|
$
(0.01
)
|
$
(0.01
)
|
|
|
|
|
|
|
12/31/14
|
$
(7,845,318
)
|
$
(0.11
)
|
$
(0.14
)
|
|
3/31/2015
|
$
(12,556,236
)
|
$
(0.17
)
|
$
(0.17
)
|
|
6/30/2015
|
$
(4,429,137
)
|
$
(0.05
)
|
$
(0.06
)
|
|
9/30/2015
|
$
(9,843,955
)
|
$
(0.10
)
|
$
(0.10
)
|
|
|
2016
|
2015
|
2014
|
2013
|
2012
|
|
|
|
|
|
|
|
|
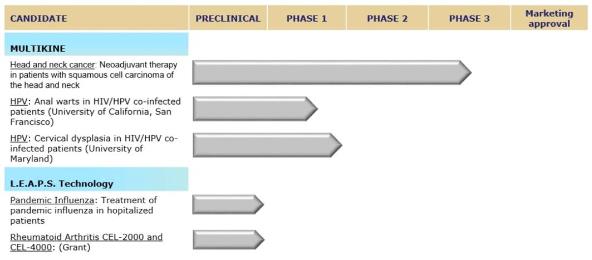
MULTIKINE
|
$
18,960,871
|
$
20,604,337
|
$
16,797,809
|
$
12,556,636
|
$
10,423,327
|
|
LEAPS
|
390,908
|
493,810
|
374,778
|
377,485
|
391,078
|
|
TOTAL
|
$
19,351,779
|
$
21,098,147
|
$
17,172,587
|
$
12,934,121
|
$
10,814,405
|
|
2017
|
$
1,930,244
|
|
2018
|
1,997,309
|
|
2019
|
2,066,329
|
|
2020
|
2,109,887
|
|
2021
|
2,099,785
|
|
Thereafter
|
15,830,794
|
|
|
$
26,034,348
|
|
Name
|
Age
|
Position
|
|
|
|
|
|
Geert R. Kersten, Esq.
|
57
|
Director, Chief Executive Officer and Treasurer
|
|
Patricia B. Prichep
|
65
|
Senior Vice President of Operations and Corporate
Secretary
|
|
Dr. Eyal Talor
|
60
|
Chief Scientific Officer
|
|
Dr. Daniel H. Zimmerman
|
75
|
Senior Vice President of Research, Cellular Immunology
|
|
John Cipriano
|
74
|
Senior Vice President of Regulatory Affairs
|
|
Alexander G. Esterhazy
|
75
|
Director
|
|
Dr. Peter R. Young
|
71
|
Director
|
|
Bruno Baillavoine
|
64
|
Director
|
|
Name
and
Principal
Position
|
|
Fiscal
Year
|
Salary (1)
|
Bonus (2)
|
Restricted Stock Awards (3)
|
Option Awards (4)
|
All Other Compen-sation(5)
|
Total
|
|
|
|
|
$
|
$
|
$
|
$
|
$
|
$
|
|
|
|
|
|
|
|
|
|
|
|
Maximilian de
Clara,
|
|
2016
|
345,618
|
--
|
--
|
46,352
|
40,000
|
431,970
|
|
Former President
(6)
|
|
2015
|
332,750
|
--
|
--
|
69,190
|
40,000
|
441,940
|
|
|
|
2014
|
393,250
|
--
|
--
|
298,648
|
73,183
|
765,081
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Geert R.
Kersten,
|
|
2016
|
558,432
|
--
|
15,900
|
--
|
54,981
|
629,314
|
|
Chief
Executive
|
|
2015
|
514,083
|
--
|
16,050
|
--
|
54,981
|
585,114
|
|
Officer and
Treasurer
|
|
2014
|
584,621
|
--
|
3,236,526
|
82,917
|
57,581
|
3,961,645
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Patricia B.
Prichep,
|
|
2016
|
245,804
|
--
|
14,725
|
--
|
9,031
|
269,559
|
|
Senior Vice
President
|
|
2015
|
235,702
|
--
|
14,128
|
--
|
6,906
|
256,736
|
|
of Operations
and
|
|
2014
|
247,852
|
--
|
1,735,938
|
55,278
|
6,531
|
2,045,599
|
|
Secretary
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Eyal Talor,
Ph.D.,
|
|
2016
|
303,597
|
--
|
9,600
|
--
|
6,031
|
319,227
|
|
Chief Scientific
Officer
|
|
2015
|
290,983
|
--
|
9,600
|
--
|
6,031
|
306,613
|
|
|
|
2014
|
283,283
|
--
|
1,731,290
|
55,278
|
6,031
|
2,075,882
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Daniel Zimmerman,
Ph.D.,
|
|
2016
|
228,413
|
--
|
13,708
|
37,081
|
6,031
|
285,233
|
|
Senior Vice
President of
|
|
2015
|
219,026
|
--
|
13,148
|
52,003
|
6,031
|
290,209
|
|
Research,
Cellular
|
|
2014
|
213,231
|
--
|
13,274
|
227,319
|
6,031
|
459,855
|
|
Immunology
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
John
Cipriano,
|
|
2016
|
211,405
|
--
|
--
|
--
|
31
|
211,437
|
|
Senior Vice
President of
|
|
2015
|
202,718
|
--
|
--
|
--
|
31
|
202,749
|
|
Regulatory
Affairs
|
|
2014
|
197,354
|
--
|
888,614
|
41,549
|
31
|
1,127,458
|
|
Name
|
Paid in Cash
|
Stock Awards (1)
|
Option Awards (2)
|
Total
|
|
Maximilian de Clara
(3)
|
$
40,000
|
-
|
46,352
|
$
86,352
|
|
Geert
Kersten
|
$
40,000
|
-
|
-
|
$
40,000
|
|
Alexander
Esterhazy
|
$
45,000
|
-
|
46,352
|
$
91,352
|
|
Peter R.
Young
|
$
50,000
|
-
|
46,352
|
$
96,352
|
|
Bruno
Baillavoine
|
$
45,000
|
-
|
46,352
|
$
91,352
|
|
Name
|
|
Grant
Date
|
Options Granted
|
Price Per Share
|
Expiration
Date
|
|
Daniel
Zimmerman
|
|
7/22/2016
|
100,000
|
$
0.47
|
7/21/2026
|
|
Alexander
Esterhazy
|
|
7/22/2016
|
125,000
|
$
0.47
|
7/21/2026
|
|
Peter
Young
|
|
7/22/2016
|
125,000
|
$
0.47
|
7/21/2026
|
|
Bruno
Baillavoine
|
|
7/22/2016
|
125,000
|
$
0.47
|
7/21/2026
|
|
Maximilian de
Clara
|
|
7/22/2016
|
125,000
|
$
0.47
|
7/21/2026
|
|
|
|
|
|
|
|
|
|
|
Weighted
Average
|
Weighted Avergage
Remaining
Contractual
|
|
Employee
|
Total
Options
|
Exercise
Price
|
Term
(Years)
|
|
|
|
|
|
|
|
Date
of
|
Shares
Acquired
|
Value
|
|
Name
|
Exercise
|
On
Exercise
|
Realized
|
|
|
|
|
|
|
|
Shares underlying unexercised
|
|
|
|
|
|
Option which are:
|
Exercise
|
Expiration
|
|
|
Name
|
Exercisable
|
Unexercisable
|
Price
|
Date
|
|
|
|
|
|
|
|
Maximilian
de Clara
|
20,000
|
|
6.30
|
09/13/17
|
|
|
20,000
|
|
6.20
|
03/04/18
|
|
|
143,625
|
|
2.50
|
04/23/19
|
|
|
50,000
(2)
|
|
3.80
|
07/06/19
|
|
|
25,000
|
|
3.80
|
07/20/19
|
|
|
25,000
|
|
4.80
|
07/20/20
|
|
|
25,000
|
|
6.90
|
04/14/21
|
|
|
47,200
|
|
3.20
|
12/01/16
|
|
|
37,500
|
|
3.90
|
05/17/22
|
|
|
100,000
|
|
2.80
|
12/17/17
|
|
|
37,500
|
|
2.10
|
06/30/23
|
|
|
75,000
|
|
1.09
|
02/25/24
|
|
|
100,000
|
|
1.10
|
08/05/24
|
|
|
150,000
|
|
1.08
|
08/25/24
|
|
|
125,000
|
|
0.66
|
06/21/25
|
|
|
125,000
|
|
0.47
|
07/21/26
|
|
|
1,105,825
|
|
|
|
|
|
Shares underlying unexercised
|
|
|
|
|
|
Option which are:
|
Exercise
|
Expiration
|
|
|
Name
|
Exercisable
|
Unexercisable
|
Price
|
Date
|
|
|
|
|
|
|
|
Geert
R. Kersten
|
20,000
|
|
6.30
|
09/13/17
|
|
|
20,000
|
|
6.20
|
03/04/18
|
|
|
183,861
(1)
|
|
2.50
|
04/23/19
|
|
|
133,334
(2)
|
|
3.80
|
07/06/19
|
|
|
30,000
|
|
3.80
|
07/20/19
|
|
|
30,000
|
|
4.80
|
07/20/20
|
|
|
30,000
|
|
6.90
|
04/14/21
|
|
|
125,440
|
|
3.20
|
12/01/16
|
|
|
45,000
|
|
3.90
|
05/17/22
|
|
|
189,000
|
|
2.80
|
12/17/17
|
|
|
195,549
|
|
2.80
|
12/17/22
|
|
|
45,000
|
|
2.10
|
06/30/23
|
|
|
60,000
|
|
1.09
|
02/25/24
|
|
|
----------
|
|
|
|
|
|
1,107,184
|
|
|
|
|
|
|
266,666
(2)
|
3.80
|
07/06/19
|
|
|
|
304,451
|
2.80
|
12/17/22
|
|
|
|
30,000
|
1.09
|
02/25/24
|
|
|
|
----------
|
|
|
|
|
|
601,117
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Patricia
B. Prichep
|
10,000
|
|
6.30
|
09/13/17
|
|
|
10,000
|
|
6.20
|
03/04/18
|
|
|
71,710
(1)
|
|
2.50
|
04/23/19
|
|
|
100,000
(2)
|
|
3.80
|
07/06/19
|
|
|
15,000
|
|
3.80
|
07/20/19
|
|
|
15,000
|
|
4.80
|
07/20/20
|
|
|
15,000
|
|
6.90
|
04/14/21
|
|
|
38,520
|
|
3.20
|
12/01/16
|
|
|
30,000
|
|
3.90
|
05/17/22
|
|
|
58,000
|
|
2.80
|
12/17/17
|
|
|
81,187
|
|
2.80
|
12/17/22
|
|
|
30,000
|
|
2.10
|
06/30/23
|
|
|
40,000
|
|
1.09
|
02/25/24
|
|
|
----------
|
|
|
|
|
|
514,417
|
|
|
|
|
|
|
200,000
(2)
|
3.80
|
07/06/19
|
|
|
|
68,813
|
2.80
|
12/17/22
|
|
|
|
20,000
|
1.09
|
02/25/24
|
|
|
|
----------
|
|
|
|
|
|
288,813
|
|
|
|
|
|
|
|
|
|
|
Shares underlying unexercised
|
|
|
|
|
|
Option which are:
|
Exercise
|
Expiration
|
|
|
Name
|
Exercisable
|
Unexercisable
|
Price
|
Date
|
|
Eyal
Talor, Ph.D.
|
10,000
|
|
6.30
|
09/13/17
|
|
|
10,000
|
|
6.20
|
03/04/18
|
|
|
24,082
(1)
|
|
2.50
|
04/23/19
|
|
|
100,000
(2)
|
|
3.80
|
07/06/19
|
|
|
15,000
|
|
3.80
|
07/20/19
|
|
|
15,000
|
|
4.80
|
07/20/20
|
|
|
15,000
|
|
6.90
|
04/14/21
|
|
|
27,773
|
|
3.20
|
12/01/16
|
|
|
30,000
|
|
3.90
|
05/17/22
|
|
|
37,417
|
|
2.80
|
12/17/17
|
|
|
81,187
|
|
2.80
|
12/17/22
|
|
|
30,000
|
|
2.10
|
06/30/23
|
|
|
40,000
|
|
1.09
|
02/25/24
|
|
|
----------
|
|
|
|
|
|
435,459
|
|
|
|
|
|
|
200,000
(2)
|
3.80
|
07/06/19
|
|
|
|
68,813
|
2.80
|
12/17/22
|
|
|
|
20,000
|
1.09
|
02/25/24
|
|
|
|
----------
|
|
|
|
|
|
288,813
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Daniel
Zimmerman, Ph.D.
|
7,500
|
|
6.30
|
09/13/17
|
|
|
7,500
|
|
6.20
|
03/04/18
|
|
|
15,000
|
|
4.80
|
07/20/20
|
|
|
15,000
|
|
6.90
|
04/14/21
|
|
|
25,200
|
|
3.20
|
12/01/16
|
|
|
22,500
|
|
3.90
|
05/17/22
|
|
|
39,200
|
|
2.80
|
12/17/17
|
|
|
22,500
|
|
2.10
|
06/30/23
|
|
|
30,000
|
|
1.09
|
02/25/24
|
|
|
133,334
|
|
1.10
|
08/05/24
|
|
|
33,334
|
|
0.62
|
06/25/25
|
|
|
----------
|
|
|
|
|
|
351,068
|
|
|
|
|
|
|
15,000
|
1.09
|
02/25/24
|
|
|
|
66,666
|
1.10
|
08/05/24
|
|
|
|
66,666
|
0.62
|
06/25/25
|
|
|
|
100,000
|
0.47
|
07/21/26
|
|
|
|
----------
|
|
|
|
|
|
248,332
|
|
|
|
|
Shares underlying unexercised
|
|
|
|
|
|
Option which are:
|
Exercise
|
Expiration
|
|
|
Name
|
Exercisable
|
Unexercisable
|
Price
|
Date
|
|
John
Cipriano
|
7,500
|
|
6.30
|
09/13/17
|
|
|
7,500
|
|
6.20
|
03/04/18
|
|
|
15,000
|
|
4.80
|
07/20/20
|
|
|
15,000
|
|
6.90
|
04/14/21
|
|
|
1,600
|
|
3.20
|
12/01/16
|
|
|
10,000
|
|
2.50
|
09/30/19
|
|
|
22,500
|
|
3.90
|
05/17/22
|
|
|
22,500
|
|
2.10
|
06/30/23
|
|
|
30,000
|
|
1.09
|
02/25/24
|
|
|
----------
|
|
|
|
|
|
131,600
|
|
|
|
|
|
|
15,000
|
1.09
|
02/25/24
|
|
|
|
----------
|
|
|
|
|
|
15,000
|
|
|
|
Name of
Plan
|
Total Shares Reserved Under Plans
|
Shares Reserved for Outstanding
Options
|
Shares Issued
|
Remaining Options/Shares Under
Plans
|
|
|
|
|
|
|
|
Incentive Stock
Option Plans
|
3,460,000
|
1,648,966
|
N/A
|
1,511,334
|
|
Non-Qualified Stock
Option
Plans
|
9,680,000
|
6,940,321
|
N/A
|
2,059,261
|
|
Bonus
Plans
|
5,594,000
|
N/A
|
3,161,211
|
2,431,962
|
|
Stock Compensation
Plan
|
3,350,000
|
N/A
|
1,985,037
|
1,331,912
|
|
Incentive Stock
Bonus Plan
|
16,000,000
|
N/A
|
15,600,000
|
400,000
|
|
Plan
category
|
Number of Securities to be Issued Upon Exercise of
Outstanding Options (a)
|
Weighted-Average Exercise Price of Outstanding
Options
|
Number of Securities Remaining Available For Future
Issuance Under Equity Compensation Plans, Excluding
SecuritiesReflected in Column (a)
|
|
|
|
|
|
|
Incentive Stock
Option Plans
|
1,648,966
|
$
2.97
|
1,511,334
|
|
Non-Qualified Stock
Option Plans
|
6,940,321
|
$
2.23
|
2,059,261
|
|
Name and Address
|
Number of Shares (1)
|
Percent of Class (2)
|
|
|
|
|
|
Geert R.
Kersten
8229 Boone Blvd.,
Suite 802
Vienna, VA
22182
|
19,352,961
(3)
|
11.8
%
|
|
|
|
|
|
Patricia B.
Prichep
8229 Boone Blvd.,
Suite 802
Vienna, VA
22182
|
3,871,696
|
2.5
%
|
|
|
|
|
|
Eyal Talor,
Ph.D.
8229 Boone Blvd.,
Suite 802
Vienna, VA
22182
|
3,732,377
|
2.4
%
|
|
|
|
|
|
Daniel H.
Zimmerman, Ph.D.
8229 Boone Blvd.,
Suite 802
Vienna, VA
22182
|
509,154
|
0.3
%
|
|
John
Cipriano
8229 Boone Blvd.,
Suite 802
Vienna, VA
22182
|
1,774,318
|
1.1
%
|
|
|
|
|
|
Alexander G.
Esterhazy
20 Chemin du
Pre-Poiset
CH- 1253
Vandoeuvres
Geneve,
Switzerland
|
384,716
|
0.2
%
|
|
|
|
|
|
Peter R. Young,
Ph.D.
208 Hewitt Drive,
Suite 103-143
Waco, TX
76712
|
400,110
|
0.3
%
|
|
|
|
|
|
Bruno
Baillavoine
8229 Boone Blvd.,
Suite 802
Vienna, VA
22182
|
41,667
|
0.0
%
|
|
|
|
|
|
All Officers and
Directors
as a Group (8
persons)
|
30,066,999
|
18.1
%
|
|
Name
|
Options or Warrants ExercisablePrior to February 28,
2017
|
|
|
|
|
Geert R. Kersten,
Esq.
|
7,432,220
(3)
|
|
Patricia B.
Prichep
|
568,824
|
|
Eyal Talor,
Ph.D.
|
489,866
|
|
Daniel Zimmerman,
Ph.D.
|
366,068
|
|
John
Cipriano
|
146,600
|
|
Alexander G.
Esterhazy
|
361,400
|
|
Peter R. Young,
Ph.D.
|
370,334
|
|
Bruno
Baillavoine
|
41,667
|
|
|
Year Ended September 30,
|
|
|
|
2016
|
2015
|
|
|
|
|
|
Audit
Fees
|
$
311,000
|
$
362,000
|
|
Audit Related
Fees
|
-
|
-
|
|
Tax
Fees
|
-
|
-
|
|
All Other
Fees
|
-
|
-
|
|
Exhibits
|
|
|
|
|
|
|
|
3(a)
|
Articles
of Incorporation
|
Incorporated
by reference to Exhibit 3(a) of CEL-SCI's combined Registration
Statement on Form S-1 and Post-Effective Amendment ("Registration
Statement"), Registration Nos. 2-85547-D and 33-7531.
|
|
|
|
|
|
3(b)
|
Amended
Articles
|
Incorporated
by reference to Exhibit 3(a) of CEL-SCI's Registration Statement on
Form S-1, Registration Nos. 2-85547-D and 33-7531.
|
|
|
|
|
|
3(c)
|
Amended
Articles (Name change only)
|
Filed
as Exhibit 3(c) to CEL-SCI's Registration Statement on Form S-1
Registration Statement (No. 33-34878).
|
|
|
|
|
|
3(d)
|
Bylaws
|
Incorporated
by reference to Exhibit 3(b) of CEL-SCI's Registration Statement on
Form S-1, Registration Nos. 2-85547-D and 33-7531.
|
|
|
|
|
|
3(e)
|
Amended
Bylaws
|
Incorporated
by reference to Exhibit 3(ii) of CEL-SCI’s report on Form 8-K
dated March 16, 2015.
|
|
|
|
|
|
4
|
Shareholders
Rights Agreement, as Amended
|
Incorporated
by reference to Exhibit 4 filed with CEL-SCI’s 10-K
report for the year ended September 30, 2015.
|
|
|
|
|
|
4(b)
|
Incentive
Stock Option Plan
|
Incorporated
by reference to Exhibit 4 (b) filed on September 25, 2012 with the
Company’s registration statement on Form S¬8 (File
number 333-184092.
|
|
|
|
|
|
4(c)
|
Non-Qualified
Stock Option Plan
|
Incorporated
by reference to Exhibit 4 (b) filed on August 19, 2014 with the
Company’s registration statement on Form S¬8 (File
number 333-198244).
|
|
|
|
|
|
4(d)
|
Stock
Bonus Plan
|
Incorporated
by reference to Exhibit 4 (d) filed on September 25, 2012 with the
Company’s registration statement on Form S¬8 (File
number 333-184092.
|
|
|
|
|
|
4(e)
|
Stock
Compensation Plan
|
Incorporated
by reference to Exhibit 4 (e) filed on September 25, 2012 with the
Company’s registration statement on Form S¬8 (File
number 333-184092.
|
|
|
|
|
|
4(f)
|
2014
Incentive Stock Bonus Plan
|
Filed
with this Amendment No. 2 to the Company’s annual report on
Form 10-K for the year ended September 30, 2014.
|
|
10(f)
|
Securities
Purchase Agreement (together with schedule required by
Instruction 2 to Item 601 of Regulation S-K) pertaining
to Series K notes and warrants, together with the exhibits to
the Securities Purchase Agreement
|
Incorporated
by reference to Exhibit 10 to CEL-SCI’s report on Form
8-K dated August 4, 2006.
|
|
|
|
|
|
10(g)
|
Subscription
Agreement (together with Schedule required by Instruction 2
toItem 601 of Regulation S-K) pertaining to April 2007 sale of
20,000,000 shares of CEL-SCI’s common stock,
10,000,000 Series L warrants and 10,000,000 Series
M Warrants
|
Incorporated
by reference to Exhibit 10 of CEL-SCI’s report on Form
8-K dated April 18, 2007
|
|
|
|
|
|
10(h)
|
Warrant
Adjustment Agreement with Laksya Ventures
|
Incorporated
by reference to Exhibit 10(i) of CEL-SCI’s report on
Form 8-K dated August 3, 2010
|
|
|
|
|
|
10(l)
|
First
Amendment to Development Supply and Distribution Agreement
with Orient Europharma.
|
Incorporated
by reference to Exhibit 10(m) filed with CEL-SCI’s 10-K
report for the year ended September 30, 2010.
|
|
|
|
|
|
10(m)
|
Exclusive
License and Distribution Agreement with
Teva Pharmaceutical Industries Ltd.
|
Incorporated
by reference to Exhibit 10(n) filed with CEL-SCI’s 10-K
report for the year ended September 30, 2010.
|
|
|
|
|
|
10(n)
|
Lease
Agreement
|
Incorporated
by reference to Exhibit 10(o) filed with CEL-SCI’s 10-K
report for the year ended September 30, 2010.
|
|
|
|
|
|
10(o)
|
Promissory
Note with Maximilian de Clara, together with Amendments 1 and
2
|
Incorporated
by reference to Exhibit 10(p) filed with CEL-SCI’s 10-K
report for the year ended September 30, 2010.
|
|
|
|
|
|
10(p)
|
Licensing
Agreement with Byron Biopharma
|
Incorporated
by reference to Exhibit 10(i) of CEL-SCI’s report on
Form 8-K dated March 27, 2009
|
|
|
|
|
|
10(z)
|
Development,
Supply and Distribution Agreement with Orient
Europharma
|
Incorporated
by reference to Exhibit 10(z) filed with CEL-SCI’s
report on Form 10-K for the year ended September 30,
2003.
|
|
|
|
|
|
10(aa)
|
Securities
Purchase Agreement and form of the Series F warrants, which
is and exhibit to the Securities
Purchase Agreement
|
Incorporated
by reference to Exhibit 10(aa) of CEL-SCI’s report on
Form 8-K dated October 3, 2011.
|
|
|
|
|
|
10(bb)
|
Placement
Agent Agreement
|
Incorporated
by reference to Exhibit 10(bb) of CEL-SCI’s report on
Form 8-K dated October 3, 2011.
|
|
|
|
|
|
10(cc)
|
Securities Purchase
Agreement, together with the form of the
Series H warrant, which is an exhibit to the securities Purchase
Agreement
|
Incorporated
by reference to Exhibit 10(cc) of CEL-SCI’s report on Form
8-K dated January 25, 2012.
|
|
|
|
|
|
10(dd)
|
Placement
Agent Agreement
|
Incorporated
by reference to Exhibit 10(dd) of CEL-SCI’s report on
Form 8-K dated January 25, 2012.
|
|
|
|
|
|
10(ee)
|
Warrant
Amendment Agreement, together with the form of the Series P
warrant, which is an exhibit to the Warrant Amendment
Agreement
|
Incorporated
by reference to Exhibit 10(ee) of CEL-SCI’s report on
Form 8-K dated February 10, 2012.
|
|
|
|
|
|
10(ff)
|
Placement
Agent Agreement
|
Incorporated
by reference to Exhibit 10(ff) of CEL-SCI’s report on
Form 8-K dated February 10, 2012.
|
|
|
|
|
|
10(gg)
|
Securities Purchase Agreement and
the form of the Series
Q warrant, which is
an exhibit to the Securities Purchase
Agreement
|
Incorporated
by reference to Exhibit 10(gg) of CEL-SCI’s report on
Form 8-K dated June 18, 2012.
|
|
|
|
|
|
10(hh)
|
Placement
Agent Agreement
|
Incorporated
by reference to Exhibit 10(hh) of CEL-SCI’s report on
Form 8-K dated June 18, 2012.
|
|
|
|
|
|
10
(ii)
|
Securities Purchase Agreement and
the form of the Series
R warrant, which is
an exhibit to the Securities Purchase
Agreement
|
Incorporated
by reference to Exhibit 10(ii) of CEL-SCI’s report on
Form 8-K dated December 5, 2012.
|
|
|
|
|
|
10
(jj)
|
Placement
Agent Agreement
|
Incorporated
by reference to Exhibit 10(jj) of CEL-SCI’s report
on Form 8-K dated December 5, 2012.
|
|
|
|
|
|
10
(nn)
|
Underwriting
Agreement, together with the form of Series S warrant which is an
exhibit to the underwriting agreement
|
Incorporated
by reference to Exhibit 1.1 of CEL-SCI’s report on Form 8-K
dated October 8, 2013.
|
|
|
|
|
|
10
(oo)
|
Underwriting
Agreement, together with the form of Series S warrant which is an
exhibit to the underwriting agreement
|
Incorporated
by reference to Exhibit 1.1 of CEL-SCI’s report on Form 8-K
dated December 19, 2013.
|
|
|
|
|
|
10
(pp)
|
Underwriting
Agreement, together with the form of Series T warrant which is an
exhibit to the warrant agent agreement
|
Incorporated
by reference to Exhibit 1.1 of CEL-SCI’s report on Form 8-K
dated April 15, 2014.
|
|
|
|
|
|
10
(qq)
|
Underwriting
Agreement, together with the form of Series S warrant which is an
exhibit to the warrant agent agreement
|
Incorporated
by reference to Exhibit 1.1 of CEL-SCI’s report on Form 8-K
dated October 23, 2014.
|
|
|
|
|
|
10
(rr)
|
Assignment
and Assumption Agreement with Teva Pharmaceutical Industries, Ltd.
and GCP Clinical Studies, Ltd.
|
Incorporated
by reference to Exhibit 10(rr) of CEL-SCI’s report on Form
10-K/A report for the year ended September 30, 2014 dated
April 17, 2015.
|
|
|
|
|
|
10
(ss)
|
Service
Agreement with GCP Clinical Studies, Ltd., together with Amendment
1 thereto*
|
Incorporated
by reference to Exhibit 10(ss) of CEL-SCI’s first amendment
to its Form 10-K report for the year ended September 30, 2014
dated April 17, 2015.
|
|
|
|
|
|
10
(tt)
|
Joinder
Agreement with PLIVA Hrvatska d.o.o.
|
Incorporated
by reference to Exhibit 10(tt) of CEL-SCI’s first amendment
to its Form 10-K report for the year ended September 30, 2014
dated April 17, 2015.
|
|
|
|
|
|
10
(uu)
|
Master
Service Agreement with Ergomed Clinical Research,
Ltd., and Clinical Trial Orders thereunder
|
Incorporated
by reference to Exhibit 10(uu) of CEL-SCI’s first amendment
to its Form 10-K report for the year ended September 30, 2014
dated April 17, 2015.
|
|
|
|
|
|
10
(vv)
|
Co-Development
and Revenue Sharing Agreement with Ergomed Clinical Research Ltd.,
dated April 19, 2013, as amended
|
Incorporated
by reference to Exhibit 10(vv) of CEL-SCI’s first amendment
to its Form 10-K report for the year ended September 30, 2014
dated April 17, 2015.
|
|
|
|
|
|
10
(ww)
|
Co-Development
and Revenue Sharing Agreement II: Cervical
Intraepithelial Neoplasia in HIV/HPV co-infected women, with
Ergomed Clinical Research Ltd., dated October 10, 2013, as
amended
|
Incorporated
by reference to Exhibit 10(ww) of CEL- first amendment to its Form
10-K report for the year ended September 30, 2014 dated April
17, 2015.
|
|
|
|
|
|
10
(xx)
|
Co-Development
and Revenue Sharing Agreement III: Anal warts and anal
intraepithelial neoplasia in HIV/HPV co-infected patients, with
Ergomed Clinical Research Ltd., dated October 24, 2013
|
Incorporated
by reference to Exhibit 10(xx) of CEL-SCI’s first amendment
to its Form 10-K report for the year ended September 30, 2014
dated April 17, 2015.
|
|
|
|
|
|
10
(yy)
|
Master
Services Agreement with Aptiv Solutions, Inc.
|
Incorporated
by reference to Exhibit 10(yy) of CEL-SCI’s first amendment
to its Form 10-K report for the year ended September 30, 2014
dated April 17, 2015.
|
|
|
|
|
|
10
(zz)
|
Project
Agreement Number 1 with Aptiv Solutions, Inc. together with
Amendments 1 and 2 thereto*
|
Incorporated
by reference to Exhibit 10(zz) of CEL-SCI’s first amendment
to its Form 10-K report for the year ended September 30, 2014
dated April 17, 2015.
|
|
|
|
|
|
10
(aaa)
|
Second
Amendment to Development Supply and Distribution Agreement with
Orient Europharma
|
Incorporated
by reference to Exhibit 10(aaa) of CEL-SCI’s first amendment
to its Form 10-K report for the year ended September 30, 2014
dated April 17, 2015.
|
|
|
|
|
|
10
(bbb)
|
Amended
and Restated Promissory Note with Maximilian de Clara
|
Incorporated
by reference to Exhibit 10(bbb) of CEL-SCI’s report on Form
10-K/A report for the year ended September 30, 2014 dated
April 17, 2015.
|
|
|
|
|
|
10
(ccc)
|
Placement
Agent Agreement dated May 22,
2015 by
and among CEL-SCI Corporation and Dawson James Securities,
Inc.
|
Incorporated
by reference to Exhibit 1.1 of CEL-SCI’s report on Form 8-K
filed on May 26, 2015.
|
|
|
|
|
|
10
(ddd)
|
Warrant
Agent Agreement (as amended),
Series
V warrants
|
Incorporated
by reference to Exhibit 10 (ccc) of CEL-SCI’s report on Form
8-K filed on May 29, 2015.
|
|
|
|
|
|
10
(eee)
|
Assignment
of Proceeds and Investment Agreement between CEL-SCI Corporation
and Lake Whillans Vehicle 1.
|
Incorporated
by reference to Exhibit 10 (ddd) of CEL-SCI’s report on Form
8-K filed on October 16, 2015.
|
|
|
|
|
|
10
(fff)
|
Placement
Agent Agreement dated October 22, 2015 by and among CEL-SCI
Corporation and Dawson James Securities, Inc.
|
Incorporated
by reference to Exhibit 1.1 of CEL-SCI’s report on Form 8-K
filed on October 23, 2015.
|
|
|
|
|
|
10
(ggg)
|
Warrant
Agent Agreement, Series W warrants
|
Incorporated
by reference to Exhibit 10 (eee) of CEL-SCI’s report on Form
8-K filed on October 23, 2015.
|
|
|
|
|
|
10
(iii)
|
Amendment
to Co-Development and Revenue
Sharing
Agreement with Ergomed Clinical
Research,
Ltd., dated September 15, 2015
|
Incorporated
by reference to Exhibit 10 (iii) filed with CEL-SCI’s
10-K report for the year ended September 30,
2015.
|
|
|
|
|
|
10
(jjj)
|
Securities
Purchase Agreement
|
Incorporated
by reference to Exhibit 10(jjj) of CEL-SCI’s report on Form
8-K dated May 19, 2016.
|
|
|
|
|
|
10
(kkk)
|
Securities
Purchase Agreement
|
Incorporated
by reference to Exhibit 10(kkk) of CEL-SCI’s report on Form
8-K dated August 24, 2016.
|
|
|
|
|
|
10
(lll)
|
Termination
Agreement with Maximilian de Clara
|
Incorporated
by reference to Exhibit 10(lll) of CEL-SCI’s report on Form
8-K dated September 2, 2016.
|
|
|
|
|
|
10
(mmm)
|
Employment
Agreement with Geert Kersten (2016-2019)
|
Incorporated
by reference to Exhibit 10(mmm) of CEL-SCI’s report on Form
8-K dated September 2, 2016.
|
|
|
|
|
|
10
(nnn)
|
Employment
Agreement with Patricia Prichep (2016-2019)
|
Incorporated
by reference to Exhibit 10(nnn) of CEL-SCI’s report on Form
8-K dated September 2, 2016.
|
|
|
|
|
|
10
(000)
|
Employment
Agreement with Eyal Taylor (2016-2019)
|
Incorporated
by reference to Exhibit 10(ooo) of CEL-SCI’s report on Form
8-K dated September 2, 2016.
|
|
|
|
|
|
23.1
|
Consent
of BDO USA, LLP
|
|
|
|
|
|
|
31
|
Rule 13a-14(a) Certifications |
|
|
|
|
|
|
32
|
Section 1350 Certifications |
|
|
|
Page
|
|
|
|
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING
FIRM
|
F- 2
|
|
|
|
|
FINANCIAL STATEMENTS FOR THE YEARS
ENDED
SEPTEMBER 30, 2016, 2015 and 2014:
|
|
|
|
|
|
Balance Sheets
|
F- 3
|
|
|
|
|
Statements of Operations
|
F- 4
|
|
|
|
|
Statements of Stockholders’ (Deficit)
Equity
|
F- 5
|
|
|
|
|
Statements of Cash Flows
|
F- 6
|
|
|
|
|
Notes to Financial Statements
|
F- 8
|
|
ASSETS
|
2016
|
2015
|
|
|
|
|
|
CURRENT
ASSETS:
|
|
|
|
Cash
and cash equivalents
|
$
2,917,996
|
$
5,726,682
|
|
Receivables
|
394,515
|
87,214
|
|
Prepaid
expenses
|
981,677
|
979,655
|
|
Deposits
- current portion
|
154,995
|
150,000
|
|
Inventory
used for R&D and manufacturing
|
1,008,642
|
1,401,839
|
|
Deferred
rent - current portion
|
429,821
|
487,793
|
|
|
|
|
|
Total
current assets
|
5,887,646
|
8,833,183
|
|
|
|
|
|
RESEARCH
AND OFFICE EQUIPMENT, net
|
226,216
|
307,466
|
|
|
|
|
|
PATENT
COSTS, net
|
256,547
|
291,564
|
|
DEFERRED
RENT - net of current portion
|
3,406,921
|
4,044,473
|
|
|
|
|
|
DEPOSITS
- net of current portion
|
1,820,917
|
1,970,917
|
|
|
|
|
|
TOTAL
ASSETS
|
$
11,598,247
|
$
15,447,603
|
|
|
|
|
|
LIABILITIES
AND STOCKHOLDERS' DEFICIT
|
|
|
|
|
|
|
|
CURRENT
LIABILITIES:
|
|
|
|
Accounts
payable
|
$
3,091,512
|
$
5,128,682
|
|
Accrued
expenses
|
378,672
|
88,575
|
|
Due
to employees
|
538,278
|
365,131
|
|
Related
party loan
|
-
|
1,104,057
|
|
Deferred
rent - current portion
|
3,310
|
9,997
|
|
Lease
obligation - current portion
|
-
|
9,028
|
|
|
|
|
|
Total
current liabilities
|
4,011,772
|
6,705,470
|
|
|
|
|
|
Derivative
instruments
|
8,394,934
|
13,686,587
|
|
Deferred
revenue
|
125,000
|
126,639
|
|
Deferred
rent - net of current portion
|
17,609
|
9,026
|
|
Deposits
held
|
5,000
|
5,000
|
|
|
|
|
|
Total
liabilities
|
12,554,315
|
20,532,722
|
|
|
|
|
|
COMMITMENTS
AND CONTINGENCIES
|
|
|
|
|
|
|
|
STOCKHOLDERS'
DEFICIT
|
|
|
|
Preferred
stock, $.01 par value-200,000 shares authorized;
|
|
|
|
-0-
shares issued and outstanding
|
-
|
-
|
|
Common
stock, $.01 par value - 600,000,000 shares authorized;
|
|
|
|
155,962,079
and 112,360,568 shares issued and outstanding
|
|
|
|
at
September 30, 2016 and 2015, respectively
|
1,559,621
|
1,123,606
|
|
Additional
paid-in capital
|
283,152,288
|
267,992,754
|
|
Accumulated
deficit
|
(285,667,977
)
|
(274,201,479
)
|
|
|
|
|
|
Total
stockholders' deficit
|
(956,068
)
|
(5,085,119
)
|
|
|
|
|
|
TOTAL
LIABILITIES AND STOCKHOLDERS' DEFICIT
|
$
11,598,247
|
$
15,447,603
|
|
|
2016
|
2015
|
2014
|
|
|
|
|
|
|
GRANT
INCOME AND OTHER
|
$
285,055
|
$
657,377
|
$
264,033
|
|
|
|
|
|
|
OPERATING
EXPENSES:
|
|
|
|
|
Research
and development
|
19,351,779
|
21,098,147
|
17,172,587
|
|
General
& administrative
|
6,486,501
|
13,855,775
|
10,665,558
|
|
|
|
|
|
|
Total
operating expenses
|
25,838,280
|
34,953,922
|
27,838,145
|
|
|
|
|
|
|
OPERATING
LOSS
|
(25,553,225
)
|
(34,296,545
)
|
(27,574,112
)
|
|
|
|
|
|
|
GAIN
ON DERIVATIVE INSTRUMENTS
|
14,013,726
|
282,616
|
248,767
|
|
|
|
|
|
|
LOSS
ON DEBT EXTINGUISHMENT
|
-
|
(620,457
)
|
-
|
|
|
|
|
|
|
INTEREST
INCOME (EXPENSE), net
|
73,001
|
(40,260
)
|
(40,920
)
|
|
|
|
|
|
|
NET
LOSS
|
(11,466,498
)
|
(34,674,646
)
|
(27,366,265
)
|
|
|
|
|
|
|
ISSUANCE
OF ADDITIONAL SHARES DUE TO RESET PROVISIONS
|
-
|
-
|
(1,117,447
)
|
|
|
|
|
|
|
NET
LOSS AVAILABLE TO COMMON SHAREHOLDERS
|
$
(11,466,498
)
|
$
(34,674,646
)
|
$
(28,483,712
)
|
|
|
|
|
|
|
NET
LOSS PER COMMON SHARE
|
|
|
|
|
BASIC
|
$
(0.09
)
|
$
(0.42
)
|
$
(0.48
)
|
|
DILUTED
|
$
(0.09
)
|
$
(0.42
)
|
$
(0.49
)
|
|
|
|
|
|
|
WEIGHTED
AVERAGE COMMON SHARES
|
|
|
|
|
OUTSTANDING
|
|
|
|
|
BASIC
and DILUTED
|
121,655,108
|
82,519,027
|
58,804,622
|
|
|
|
|
Additional
|
|
|
|
|
Common
|
Stock
|
Paid-In
|
Accumulated
|
|
|
|
Shares
|
Amount
|
Capital
|
Deficit
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BALANCE,
OCTOBER 1, 2013
|
31,025,019
|
310,250
|
218,550,408
|
(212,160,568
)
|
6,700,090
|
|
|
|
|
|
|
|
|
Sale
of common stock
|
31,755,494
|
317,555
|
28,129,691
|
-
|
28,447,246
|
|
Issuance
of warrants in connection with
|
|
|
|
|
|
|
sale
of common stock
|
-
|
-
|
(7,791,448
)
|
-
|
(7,791,448
)
|
|
401(k)
contributions paid
|
|
|
|
|
|
|
in
common stock
|
164,787
|
1,647
|
153,787
|
-
|
155,434
|
|
Exercise
of warrants
|
2,668,508
|
26,686
|
4,253,632
|
-
|
4,280,318
|
|
Conversion
of warrant liability to equity
|
-
|
-
|
1,308,528
|
-
|
1,308,528
|
|
Stock
issued to nonemployees for service
|
579,968
|
5,800
|
621,318
|
-
|
627,118
|
|
Stock
issued for patents
|
8,695
|
87
|
9,912
|
-
|
9,999
|
|
Modification
of options issued to consultants
|
-
|
-
|
76,991
|
-
|
76,991
|
|
Issuance
of restricted stock
|
15,700,000
|
157,000
|
(157,000
)
|
-
|
-
|
|
Equity
based compensation - employees
|
-
|
-
|
3,958,637
|
-
|
3,958,637
|
|
Equity
based compensation - non-employees
|
-
|
-
|
36,752
|
-
|
36,752
|
|
Net
loss
|
-
|
-
|
-
|
(27,366,265
)
|
(27,366,265
)
|
|
|
|
|
|
|
|
|
BALANCE,
SEPTEMBER 30, 2014
|
81,902,471
|
819,025
|
249,151,208
|
(239,526,833
)
|
10,443,400
|
|
|
|
|
|
|
|
|
Sale
of common stock
|
29,467,901
|
294,679
|
20,853,699
|
-
|
21,148,378
|
|
Issuance
of warrants in connection with
|
|
|
|
|
|
|
sale
of common stock
|
-
|
-
|
(8,463,957
)
|
-
|
(8,463,957
)
|
|
401(k)
contributions paid
|
|
|
|
|
|
|
in
common stock
|
243,178
|
2,432
|
163,214
|
-
|
165,646
|
|
Stock
issued to nonemployees for service
|
739,968
|
7,400
|
526,576
|
-
|
533,976
|
|
Modification
of warrants and extingishment loss
|
-
|
-
|
620,457
|
-
|
620,457
|
|
Forfeiture
of unvested restricted stock
|
(100,000
)
|
(1,000
)
|
1,000
|
-
|
-
|
|
Equity
based compensation - employees
|
107,050
|
1,070
|
5,104,757
|
-
|
5,105,827
|
|
Equity
based compensation - non-employees
|
-
|
-
|
35,800
|
-
|
35,800
|
|
Net
loss
|
-
|
-
|
-
|
(34,674,646
)
|
(34,674,646
)
|
|
|
|
|
|
|
|
|
BALANCE,
SEPTEMBER 30, 2015
|
112,360,568
|
1,123,606
|
267,992,754
|
(274,201,479
)
|
(5,085,119
)
|
|
|
|
|
|
|
|
|
Sale
of common stock
|
41,523,248
|
415,232
|
20,958,464
|
-
|
21,373,696
|
|
Issuance
of warrants in connection with
|
|
|
|
|
|
|
sale
of common stock
|
-
|
-
|
(8,722,073
)
|
-
|
(8,722,073
)
|
|
401(k)
contributions paid
|
|
|
|
|
|
|
in
common stock
|
408,497
|
4,085
|
157,486
|
-
|
161,571
|
|
Stock
issued to nonemployees for service
|
1,248,831
|
12,489
|
677,824
|
-
|
690,313
|
|
Equity
based compensation - employees
|
420,935
|
4,209
|
2,008,474
|
-
|
2,012,683
|
|
Equity
based compensation - non-employees
|
-
|
-
|
79,359
|
-
|
79,359
|
|
Net
loss
|
-
|
-
|
-
|
(11,466,498
)
|
(11,466,498
)
|
|
|
|
|
|
|
|
|
BALANCE,
SEPTEMBER 30, 2016
|
155,962,079
|
$
1,559,621
|
$
283,152,288
|
$
(285,667,977
)
|
$
(956,068
)
|
|
|
2016
|
2015
|
2014
|
|
CASH
FLOWS FROM OPERATING ACTIVITIES:
|
|
|
|
|
Net
loss
|
$
(11,466,498
)
|
$
(34,674,646
)
|
$
(27,366,265
)
|
|
Adjustments
to reconcile net loss to
|
|
|
|
|
net
cash used in operating activities:
|
|
|
|
|
Depreciation
and amortization
|
150,243
|
206,750
|
231,752
|
|
Issuance
of common stock and options for services -
non-employees
|
751,651
|
565,915
|
694,955
|
|
Modification
of warrants issued to consultants
|
-
|
-
|
76,991
|
|
Equity
based compensation
|
2,113,433
|
5,105,827
|
3,958,637
|
|
Common
stock contributed to 401(k) plan
|
161,571
|
165,646
|
155,434
|
|
Impairment
loss on abandonment of patents
|
-
|
-
|
1,182
|
|
Loss
on retired equipment
|
248
|
313
|
268
|
|
Gain
on derivative instruments
|
(14,013,726
)
|
(282,616
)
|
(248,767
)
|
|
Loss
on debt extinguishment
|
-
|
620,457
|
-
|
|
(Increase)/decrease
in assets:
|
|
|
|
|
Receivables
|
(1,960
)
|
(5,394
)
|
(7,557
)
|
|
Deferred
rent
|
695,524
|
745,673
|
769,159
|
|
Prepaid
expenses
|
15,999
|
(68,268
)
|
(158,088
)
|
|
Inventory
used for R&D and manufacturing
|
393,197
|
50,181
|
(435,392
)
|
|
Deposits
|
145,005
|
150,000
|
(200,000
)
|
|
Increase/(decrease)
in liabilities:
|
|
|
|
|
Accounts
payable
|
(2,389,931
)
|
3,981,886
|
(751,971
)
|
|
Accrued
expenses
|
290,097
|
(458,633
)
|
433,712
|
|
Deferred
revenue
|
(1,639
)
|
48
|
46
|
|
Due
to employees
|
72,397
|
57,170
|
(78,376
)
|
|
Deferred
rent liability
|
1,896
|
6,358
|
(3,739
)
|
|
|
|
|
|
|
Net
cash used in operating activities
|
(23,082,493
)
|
(23,833,333
)
|
(22,928,019
)
|
|
|
|
|
|
|
CASH
FLOWS FROM INVESTING ACTIVITIES:
|
|
|
|
|
Purchases
of equipment
|
(31,405
)
|
(73,399
)
|
(103,977
)
|
|
Expenditures
for patent costs
|
(2,819
)
|
(20,132
)
|
(34,887
)
|
|
|
|
|
|
|
Net
cash used in investing activities
|
(34,224
)
|
(93,531
)
|
(138,864
)
|
|
|
|
|
|
|
CASH
FLOWS FROM FINANCING ACTIVITIES:
|
|
|
|
|
Proceeds
from issuance of common stock and warrants
|
21,420,301
|
21,148,378
|
28,428,641
|
|
Payment
on related party loan
|
(1,104,057
)
|
|
|
|
Proceeds
from exercise of warrants
|
-
|
-
|
3,118,387
|
|
Payments
on obligations under capital lease
|
(8,213
)
|
(8,452
)
|
(8,137
)
|
|
|
|
|
|
|
Net
cash provided by financing activities
|
20,308,031
|
21,139,926
|
31,538,891
|
|
|
|
|
|
|
NET
(DECREASE) INCREASE IN CASH AND CASH EQUIVALENTS
|
(2,808,686
)
|
(2,786,938
)
|
8,472,008
|
|
|
|
|
|
|
CASH
AND CASH EQUIVALENTS, BEGINNING OF YEAR
|
5,726,682
|
8,513,620
|
41,612
|
|
|
|
|
|
|
CASH
AND CASH EQUIVALENTS, END OF YEAR
|
$
2,917,996
|
$
5,726,682
|
$
8,513,620
|
|
SUPPLEMENTAL SCHEDULE OF NON-CASH INVESTING AND FINANCING
ACTIVITIES:
|
|
||
|
|
|
|
|
|
|
2016
|
2015
|
2014
|
|
Receivable
due under the litigation funding arrangement offset by the same
amount
|
|
|
|
|
payable
to the legal firm providing the services
|
$
305,341
|
$
-
|
$
-
|
|
Research
and office equipment included in accounts payable at year
end
|
$
-
|
$
(2,345
)
|
$
(1,074
)
|
|
Capitalizable
patent costs included in accounts payable at year end.
|
$
-
|
$
(11,685
)
|
$
4,474
|
|
Patent
costs purchased with common stock
|
$
-
|
$
-
|
$
9,999
|
|
Lease
payments included in accounts payable at year end
|
$
815
|
$
43
|
$
3,477
|
|
Fair
value of warrant liabilities on the date of issuance reclassed to
liabilities
|
$
(8,722,073
)
|
$
(8,463,957
)
|
$
(5,320,989
)
|
|
Financing
costs included in accounts payable at year end
|
$
46,605
|
$
-
|
$
-
|
|
Forfeiture
of unvested restricted stock
|
$
-
|
$
1,000
|
$
-
|
|
Stock
issued under an anti-dilution provision and cashless exercise of
warrants
|
$
-
|
$
-
|
$
(16,375
)
|
|
Prepaid
amount under consulting services paid with issuance of common
stock
|
$
18,021
|
$
3,861
|
$
31,085
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
paid for interest expense
|
$
43,673
|
$
147,166
|
$
180,654
|
|
Warrant
|
|
Issue
Date
|
Shares Issuable upon Exercise of
Warrant
|
Exercise Price
|
Expiration
Date
|
Refer-ence
|
|
|
|
|
|
|
|
|
|
Series
R
|
|
12/6/12
|
2,625,000
|
$
4.00
|
12/6/16
|
1
|
|
Series
S
|
|
10/11/13
-10/24/14
|
25,928,010
|
$
1.25
|
10/11/18
|
1
|
|
Series
U
|
|
4/17/14
|
445,514
|
$
1.75
|
10/17/17
|
1
|
|
Series
V
|
|
5/28/15
|
20,253,164
|
$
0.79
|
5/28/20
|
1
|
|
Series
W
|
|
10/28/15
|
17,223,248
|
$
0.67
|
10/28/20
|
1
|
|
Series
X
|
|
1/13/16
|
3,000,000
|
$
0.37
|
1/13/21
|
2
|
|
Series
Y
|
|
2/15/16
|
650,000
|
$
0.48
|
2/15/21
|
2
|
|
Series
Z
|
|
5/23/16
|
6,600,000
|
$
0.55
|
11/23/21
|
1
|
|
Series
ZZ
|
|
5/23/16
|
500,000
|
$
0.55
|
5/18/21
|
1
|
|
Series
AA
|
|
8/26/16
|
5,000,000
|
$
0.55
|
2/22/22
|
1
|
|
Series
BB
|
|
8/26/16
|
400,000
|
$
0.55
|
8/22/21
|
1
|
|
Series
N
|
|
8/18/08
|
2,844,627
|
$
0.53
|
8/18/17
|
2
|
|
Series
P
|
|
2/10/12
|
590,001
|
$
4.50
|
3/6/17
|
2
|
|
Consultants
|
|
12/2/11-
7/1/16
|
640,000
|
$
0.37- $3.50
|
10/27/16-
6/30/19
|
3
|
|
Warrants
|
|
Issue
Date
|
Shares Issuable upon Exercise of
Warrants
|
Exercise Price
|
Expiration
Date
|
Refer-ence
|
|
|
|
|
|
|
|
|
|
Series
N
|
|
8/18/08
|
2,844,627
|
0.53
|
8/18/17
|
1
|
|
Series
Q
|
|
6/21/12
|
1,200,000
|
5.00
|
12/22/15
|
1
|
|
Series
R
|
|
12/6/12
|
2,625,000
|
4.00
|
12/6/16
|
1
|
|
Series
S
|
|
10/11/13-
10/24/14
|
25,928,010
|
1.25
|
10/11/18
|
1
|
|
Series
U
|
|
4/17/14
|
445,514
|
1.75
|
10/17/17
|
1
|
|
Series
V
|
|
5/28/15
|
20,253,164
|
0.79
|
5/28/20
|
1
|
|
Series
P
|
|
2/10/12
|
590,001
|
4.50
|
3/6/17
|
2
|
|
Consultants
|
|
10/14/05 –
7/1/15
|
238,000
|
0.66 – 20.00
|
10/14/15 -
6/30/18
|
3
|
|
|
2016
|
2015
|
|
Series S
warrants
|
$
3,111,361
|
$
7,363,555
|
|
Series U
warrants
|
-
|
44,551
|
|
Series V
warrants
|
1,620,253
|
6,278,481
|
|
Series W
warrants
|
1,799,858
|
-
|
|
Series Z
warrants
|
970,604
|
-
|
|
Series ZZ
warrants
|
70,609
|
-
|
|
Series AA
warrants
|
763,661
|
-
|
|
Series BB
warrants
|
58,588
|
-
|
|
|
|
|
|
Total derivative
liabilities
|
$
8,394,934
|
$
13,686,587
|
|
Warrant
Series
|
2016
|
2015
|
2014
|
|
Series A -
E
|
$
-
|
$
6,105
|
$
1
|
|
Series F and
G
|
-
|
-
|
12,667
|
|
Series
H
|
-
|
12,000
|
24,000
|
|
Series
N
|
-
|
-
|
(1,404,027
)
|
|
Series
Q
|
-
|
12,000
|
36,000
|
|
Series
R
|
-
|
157,500
|
131,250
|
|
Series
S
|
4,252,193
|
(1,705,466
)
|
1,098,787
|
|
Series
T
|
-
|
-
|
276,122
|
|
Series
U
|
44,552
|
75,738
|
73,967
|
|
Series
V
|
4,658,228
|
1,724,739
|
-
|
|
Series
W
|
3,260,913
|
-
|
-
|
|
Series
Z
|
997,226
|
-
|
-
|
|
Series
ZZ
|
75,229
|
-
|
-
|
|
Series
AA
|
672,246
|
-
|
-
|
|
Series
BB
|
53,139
|
-
|
-
|
|
|
|
|
|
|
Net
gain
|
$
14,013,726
|
$
282,616
|
$
248,767
|
|
|
2016
|
2015
|
|
|
|
|
|
Research
equipment
|
$
3,158,633
|
$
3,268,757
|
|
Furniture
and equipment
|
133,499
|
141,347
|
|
Leasehold
improvements
|
131,910
|
131,910
|
|
|
|
|
|
|
3,424,042
|
3,542,014
|
|
|
|
|
|
Accumulated
depreciation and amortization
|
(3,197,826
)
|
(3,234,548
)
|
|
|
|
|
|
Net
research and office equipment
|
$
226,216
|
$
307,466
|
|
|
2016
|
2015
|
|
|
|
|
|
Patents
|
$
1,528,610
|
$
1,525,791
|
|
Accumulated
amortization
|
(1,272,063.00
)
|
(1,234,227.00
)
|
|
|
|
|
|
Net
Patents
|
$
256,547
|
$
291,564
|
|
Years ending September
30,
|
|
|
2017
|
$
37,000
|
|
2018
|
36,000
|
|
2019
|
35,000
|
|
2020
|
31,000
|
|
2021
|
28,000
|
|
Thereafter
|
90,000
|
|
|
$
257,000
|
|
|
2016
|
2015
|
|
|
|
|
|
Net
operating loss carryforwards
|
$
64,366,000
|
$
61,363,000
|
|
|
|
|
|
R&D
credit
|
1,221,000
|
1,221,000
|
|
Stock-based
compensation
|
6,379,000
|
5,855,000
|
|
Fixed
assets and intangibles
|
57,000
|
41,000
|
|
Capitalized
R&D
|
18,508,000
|
15,082,000
|
|
Vacation
and other
|
179,000
|
114,000
|
|
Loan
modification
|
-
|
57,000
|
|
|
|
|
|
Total
deferred tax assets
|
90,710,000
|
83,733,000
|
|
|
|
|
|
Valuation
allowance
|
(90,710,000
)
|
(83,733,000
)
|
|
Net
deferred tax asset
|
$
-
|
$
-
|
|
|
2016
|
2015
|
2014
|
|
|
|
|
|
|
Federal
Rate
|
34.00
%
|
34.00
%
|
34.00
%
|
|
State
tax rate, net of federal benefit
|
3.92
|
5.12
|
5.15
|
|
State
tax rate change
|
(22.13
)
|
(0.15
)
|
(0.93
)
|
|
Other
adjustments
|
(0.03
)
|
(0.21
)
|
0.00
|
|
Adjustment
to deferreds
|
0.00
|
0.00
|
19.13
|
|
Permanent
differences (1)
|
45.08
|
(0.71
)
|
(0.43
)
|
|
Change
in valuation allowance
|
(60.84
)
|
(38.05
)
|
(58.78
)
|
|
Effective
tax rate
|
0.00
%
|
0.00
%
|
0.00
%
|
|
|
Year Ended September
30,
|
||
|
|
2016
|
2015
|
2014
|
|
Employees
|
$
2,113,433
|
$
5,105,827
|
$
3,958,637
|
|
Non-employees
|
$
751,651
|
$
565,915
|
$
771,946
|
|
|
2016
|
2015
|
2014
|
|
Expected stock
price volatility
|
75.58
– 80.9
%
|
73.38
– 86.19
%
|
72.81
– 86.87
%
|
|
Risk-free interest
rate
|
0.71
– 1.56
%
|
0.93
– 2.35
%
|
0.59
– 2.65
%
|
|
Expected life of
options
|
3.0 – 9.69 Years
|
3.0 – 9.76 Years
|
3.0 –
9.76 Years
|
|
Expected dividend
yield
|
-
|
-
|
-
|
|
|
Outstanding
|
Exercisable
|
||||||
|
|
|
|
Weighted
|
|
|
|
Weighted
|
|
|
|
|
|
Ave
|
|
|
|
Ave
|
|
|
|
Number of
|
Weighted
Average |
Remaining Contractual
|
Aggregate
Intrinsic |
Number of
|
Weighted
Average |
Remaining Contractual
|
Aggregate
Intrinsic |
|
|
Shares
|
Exercise Price
|
Term (Years)
|
Value
|
Shares
|
Exercise Price
|
Term (Years)
|
Value
|
|
Outstanding
at October 1, 2014
|
6,831,149
|
$
2.98
|
6.55
|
$
3,600
|
3,443,884
|
$
3.40
|
5.49
|
$
3,600
|
|
|
|
|
|
|
|
|
|
|
|
Vested
|
|
|
|
|
1,153,357
|
$
2.48
|
|
|
|
Granted
(a)
|
893,700
|
$
0.66
|
|
|
|
|
|
|
|
Forfeited
|
116,665
|
$
1.87
|
|
|
|
|
|
|
|
Expired
|
70,499
|
$
4.15
|
|
|
70,499
|
$
4.15
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding
at September 30, 2015
|
7,537,685
|
$
2.71
|
5.98
|
$
50
|
4,526,742
|
$
3.15
|
5.01
|
$
0
|
|
Vested
|
|
|
|
|
1,401,716
|
$
1.27
|
|
|
|
Granted
(b)
|
1,213,600
|
$
0.48
|
|
|
|
|
|
|
|
Forfeited
|
55,998
|
$
0.86
|
|
|
|
|
|
|
|
Expired
|
106,000
|
$
5.80
|
|
|
106,000
|
$
5.80
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding
at September 30, 2016
|
8,589,287
|
$
2.37
|
5.35
|
$
0
|
5,822,458
|
$
2.65
|
4.76
|
$
0
|
|
|
|
Weighted Average
|
|
|
Number of
|
Grant Date
|
|
|
Options
|
Fair Value
|
|
|
|
|
|
Unvested
at October 1, 2014
|
3,387,265
|
$
2.15
|
|
Vested
|
(1,153,357
)
|
|
|
Granted
|
893,700
|
|
|
Forfeited
|
(116,665
)
|
|
|
Unvested
at September 30, 2015
|
3,010,943
|
$
1.72
|
|
Vested
|
(1,401,716
)
|
|
|
Granted
|
1,213,600
|
|
|
Forfeited
|
(55,998
)
|
|
|
Unvested
at September 30, 2016
|
2,766,829
|
$
1.48
|
|
|
Number of Shares
|
Weighted Average Grant Date Fair
Value
|
|
Unvested at
October 1, 2014
|
15,700,000
|
$
0.55
|
|
Vested
|
(500,000
)
|
|
|
Granted
|
-
|
|
|
Forfeited
|
(100,000
)
|
|
|
Unvested at
September 30, 2015
|
15,100,000
|
$
0.55
|
|
Vested
|
-
|
|
|
Unvested at
September 30, 2016
|
15,100,000
|
$
0.55
|
|
Years
Ending September 30,
|
|
|
2017
|
$
1,930,000
|
|
2018
|
1,997,000
|
|
2019
|
2,066,000
|
|
2020
|
2,110,000
|
|
2021
|
2,100,000
|
|
Thereafter
|
15,831,000
|
|
Total
minimum lease payments:
|
$
26,034,000
|
|
|
Quoted Prices in
|
Significant
|
|
|
|
|
Active Markets for
|
Other
|
Significant
|
|
|
|
Identical
|
Observable
|
Unobservable
|
|
|
|
Liabilities (Level 1)
|
Inputs (Level 2)
|
Inputs (Level 3)
|
Total
|
|
|
|
|
|
|
|
Derivative
Instruments
|
$
3,111,361
|
|
$
5,283,573
|
$
8,394,934
|
|
|
Quoted
Prices in
|
Significant
|
|
|
|
|
Active
Markets for
|
Other
|
Significant
|
|
|
|
Identical
|
Observable
|
Unobservable
|
|
|
|
Liabilities
(Level 1)
|
Inputs
(Level 2)
|
Inputs
(Level 3)
|
Total
|
|
|
|
|
|
|
|
Derivative
Instuments
|
$
7,365,555
|
|
$
6,323,032
|
$
13,686,587
|
|
|
2016
|
2015
|
|
|
|
|
|
Beginning
balance
|
$
6,323,032
|
$
307,894
|
|
Issuances
|
8,722,073
|
8,003,220
|
|
Net realized
and unrealized derivative gain
|
(9,761,532
)
|
(1,988,082
)
|
|
Ending
balance
|
$
5,283,573
|
$
6,323,032
|
|
|
2016
|
2015
|
2014
|
|
|
|
|
|
|
Net
loss available to
|
|
|
|
|
common
shareholders
|
$
(11,466,498
)
|
$
(34,674,646
)
|
$
(28,483,712
)
|
|
Less:
Gain on derivative
|
|
|
|
|
Instruments
|
-
|
-
|
(248,767
)
|
|
Net
loss - diluted
|
$
(11,466,498
)
|
$
(34,674,646
)
|
$
(28,732,479
)
|
|
|
|
|
|
|
Weighted
average number of
|
|
|
|
|
shares
- basic and diluted
|
121,655,108
|
82,519,027
|
58,804,622
|
|
|
|
|
|
|
Loss
per share - basic
|
$
(0.09
)
|
$
(0.42
)
|
$
(0. 48
)
|
|
Loss
per share - diluted
|
$
(0.09
)
|
$
(0.42
)
|
$
(0. 49
)
|
|
|
2016
|
2015
|
2014
|
|
|
|
|
|
|
Options and
Warrants
|
91,882,022
|
58,421,058
|
39,994,707
|
|
Convertible
Debt
|
-
|
1,871,283
|
276,014
|
|
Unvested Restricted
Stock
|
15,100,000
|
15,100,000
|
15,700,000
|
|
Total
|
106,982,022
|
75,392,341
|
55,970,721
|
|
Fiscal
2016
|
|
|
|
|
|
|
|
Three months
|
Three months
|
Three months
|
Three months
|
|
|
|
ended
|
ended
|
ended
|
Ended
|
Year ended
|
|
|
December 31
2015 |
March 31,
2016 |
June 30,
2016 |
September 30, 2016 |
September 30,
2016 |
|
Grant
income and other
|
$
20,976
|
$
32,775
|
$
129,975
|
$
101,329
|
$
285,055
|
|
Operating
expenses
|
5,804,108
|
6,306,378
|
6,512,722
|
7,215,072
|
25,838,280
|
|
Non-operating
(expense) income, net
|
1,985
|
22,478
|
24,679
|
23,859
|
73,001
|
|
Gain
(loss) on derivative instruments
|
8,122,960
|
(2,593,730
)
|
2,508,744
|
5,975,752
|
14,013,726
|
|
Net
income (loss) available to
|
|
|
|
|
|
|
common
shareholders
|
$
2,341,813
|
$
(8,844,855
)
|
$
(3,849,324
)
|
$
(1,114,132
)
|
$
(11,466,498
)
|
|
EPS
(LPS) -basic and diluted
|
$
0.02
|
$
(0.07
)
|
$
(0.03
)
|
$
(0.01
)
|
$
(0.09
)
|
|
Weighted
average shares:
|
|
|
|
|
|
|
Basic
|
109,768,502
|
118,420,327
|
124,132,500
|
134,290,870
|
121,655,108
|
|
Diluted
|
111,639,785
|
118,420,327
|
124,132,500
|
134,290,870
|
121,655,108
|
|
Fiscal
2015
|
|
|
|
|
|
|
|
Three months
|
Three months
|
Three months
|
Three months
|
|
|
|
ended
|
ended
|
ended
|
Ended
|
Year ended
|
|
|
December 31
2014 |
March 31,
2015 |
June 30,
2015 |
September 30,
2015 |
September 30,
2015 |
|
Grant
income and other
|
$
136,838
|
$
197,620
|
$
389,223
|
$
(66,304
)
|
$
657,377
|
|
Operating
expenses
|
10,132,579
|
7,956,963
|
8,590,698
|
8,273,682
|
34,953,922
|
|
Non-operating
(expense) income, net
|
(12,547
)
|
(14,097
)
|
(35,985
)
|
22,369
|
(40,260
)
|
|
Gain
(loss) on derivative instruments
|
2,162,970
|
(4,782,796
)
|
4,428,780
|
(1,526,338
)
|
282,616
|
|
Loss
on debt extinguishment
|
-
|
-
|
(620,457
)
|
-
|
(620,457
)
|
|
Net
loss available to
|
|
|
|
|
|
|
common
shareholders
|
$
(7,845,318
)
|
$
(12,556,236
)
|
$
(4,429,137
)
|
$
(9,843,955
)
|
$
(34,674,646
)
|
|
Net
loss per share-basic
|
$
(0.11
)
|
$
(0.17
)
|
$
(0.05
)
|
$
(0.10
)
|
$
(0.42
)
|
|
Net
loss per share-diluted
|
$
(0.14
)
|
$
(0.17
)
|
$
(0.06
)
|
$
(0.10
)
|
$
(0.42
)
|
|
Weighted
average shares:
|
|
|
|
|
|
|
Basic
|
73,260,783
|
75,847,869
|
83,796,311
|
97,040,004
|
82,519,027
|
|
Diluted
|
73,260,783
|
75,847,869
|
85,134,107
|
97,040,004
|
82,519,027
|
|
|
CEL-SCI CORPORATION
|
|
|
|
|
|
|
|
|
|
By:
|
/s/
Geert
R. Kersten
|
|
|
|
|
Geert R.
Kersten
, Chief Executive Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
Signature
|
Title
|
Date
|
|
|
|
|
|
|
|
|
|
/s/ Geert R.
Kersten
|
Chief Executive,
Principal
|
|
|
Geert R.
Kersten
|
Accounting,
Principal Financial
|
|
|
|
Officer and a
Director
|
December 14,
2016
|
|
|
|
|
|
|
|
|
|
/s/ Alexander G.
Esterhazy
|
Director
|
December 14,
2016
|
|
Alexander G.
Esterhazy
|
|
|
|
|
|
|
|
|
|
|
|
/s/Peter R.
Young
|
Director
|
December 14,
2016
|
|
Dr. Peter R.
Young
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Bruno
Baillavoine
|
Director
|
December 14,
2016
|