| o |
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
for the fiscal year ended December 31, 2014
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
For the transition period from ________________ to ________________
|
|
o
|
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
Date of event requiring this shell company report
|
|
Title of each class
|
Name of each exchange on which registered
|
|
|
Common Shares, nominal value CHF 0.40 per share
|
The NASDAQ Stock Market LLC
|
|
Large accelerated filer
o
|
Accelerated filer
o
|
Non-accelerated filer
x
|
|
US GAAP
o
|
International Financial Reporting Standards as issued by the
International Accounting Standards Board x |
Other
o
|
|
|
·
|
our operation as a development stage company with limited operating history and a history of operating losses;
|
|
|
·
|
our need for substantial additional funding before we can expect to become profitable from sales of our products;
|
|
|
·
|
our dependence on the success of AM-101 and AM-111, which are still in clinical development and may eventually prove to be unsuccessful;
|
|
|
·
|
the chance that we may become exposed to costly and damaging liability claims resulting from the testing of our product candidates in the clinic or in the commercial stage;
|
|
|
·
|
uncertainty surrounding whether any of our product candidates will receive regulatory approval, which is necessary before they can be commercialized;
|
|
|
·
|
if our product candidates obtain regulatory approval, our being subject to expensive ongoing obligations and continued regulatory overview;
|
|
|
·
|
enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval and commercialization;
|
|
|
·
|
the chance that we do not obtain orphan drug exclusivity for AM-111, which would allow our competitors to sell products that treat the same conditions;
|
|
|
·
|
dependence on governmental authorities and health insurers establishing adequate reimbursement levels and pricing policies;
|
|
|
·
|
our products may not gain market acceptance, in which case we may not be able to generate product revenues;
|
|
|
·
|
our reliance on our current strategic relationships with INSERM or Xigen and the potential failure to enter into new strategic relationships;
|
|
|
·
|
our reliance on third parties to conduct our nonclinical and clinical trials and on third-party single-source suppliers to supply or produce our product candidates; and
|
|
|
·
|
other risk factors discussed under “Item 3. Key Information—D. Risk factors.”
|
|
B.
|
|
C.
|
|
For the years ended December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
(in thousands of CHF except for share and per share data)
|
||||||||||||
|
Profit or Loss and Other Comprehensive Loss:
|
||||||||||||
|
Research and development
|
(17,705 | ) | (13,254 | ) | (3,987 | ) | ||||||
|
General and administrative
|
(4,489 | ) | (1,362 | ) | (624 | ) | ||||||
|
Operating loss
|
(22,194 | ) | (14,616 | ) | (4,611 | ) | ||||||
|
Finance expense
|
(208 | ) | (159 | ) | (2 | ) | ||||||
|
Finance income
|
4,216 | 76 | 10 | |||||||||
|
Loss before tax
|
(18,185 | ) | (14,699 | ) | (4,602 | ) | ||||||
|
Income tax expense
|
— | (306 | ) | — | ||||||||
|
Net loss attributable to owners of the Company
|
(18,185 | ) | (15,005 | ) | (4,602 | ) | ||||||
|
Other comprehensive income:
|
||||||||||||
|
Items that will never be reclassified to profit or loss:
|
||||||||||||
|
Remeasurements of defined benefits liability
|
(1,102 | ) | (58 | ) | (55 | ) | ||||||
|
Items that are or may be reclassified to profit or loss:
|
||||||||||||
|
Foreign currency translation differences
|
(105 | ) | 32 | 22 | ||||||||
|
Other comprehensive loss
|
(1,207 | ) | (26 | ) | (32 | ) | ||||||
|
Total comprehensive loss attributable to owners of the Company
|
(19,392 | ) | (15,031 | ) | (4,635 | ) | ||||||
|
Net loss per share(1)
|
||||||||||||
|
Net loss per share, basic and diluted(2)
|
(0.66 | ) | (1.01 | ) | (0.40 | ) | ||||||
|
Weighted-average number of shares used to compute net loss per common share, basic and diluted
|
27,692,494 | 14,917,064 | 11,581,450 | |||||||||
|
(1)
|
For periods prior to the closing of our initial public offering, net loss per share includes preferred shares, which were converted on a one-for-one basis upon the closing of our initial public offering. See Note 12 to our audited financial statements included elsewhere in this Annual Report.
|
|
(2)
|
Basic net loss per common share and diluted net loss per common share are the same because outstanding options and convertible loans (to the extent outstanding during the applicable time period) would be anti-dilutive due to our net loss in these periods.
|
|
As of December 31,
|
||||||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Statement of Financial Position Data:
|
(in thousands of CHF)
|
|||||||||||
|
Cash and cash equivalents
|
56,934 | 23,866 | 64 | |||||||||
|
Total assets
|
59,493 | 26,252 | 866 | |||||||||
|
Total liabilities
|
6,210 | 17,219 | 1,110 | |||||||||
|
Share capital
|
11,604 | 6,487 | 4,633 | |||||||||
|
Total shareholders’ equity attributable to owners of the Company
|
53,283 | 9,034 | (244 | ) | ||||||||
|
Period-end
|
Average for
period
|
Low
|
High
|
|||||||||||||
|
(CHF per U.S. dollar)
|
||||||||||||||||
|
Year Ended December 31:
|
||||||||||||||||
|
2010
|
0.9369 | 1.0264 | 0.9369 | 1.1614 | ||||||||||||
|
2011
|
0.9374 | 0.8802 | 0.7296 | 0.9755 | ||||||||||||
|
2012
|
0.9155 | 0.9314 | 0.8949 | 0.9957 | ||||||||||||
|
2013
|
0.8904 | 0.9241 | 0.8856 | 0.9814 | ||||||||||||
|
2014
|
0.9934 | 0.9195 | 0.8712 | 0.9934 | ||||||||||||
|
Month Ended:
|
||||||||||||||||
|
September 30, 2014
|
0.9554 | 0.9370 | 0.9186 | 0.9554 | ||||||||||||
|
October 31, 2014
|
0.9623 | 0.9528 | 0.9425 | 0.9670 | ||||||||||||
|
November 28, 2014
|
0.9658 | 0.9642 | 0.9572 | 0.9702 | ||||||||||||
|
December 31, 2014
|
0.9934 | 0.9753 | 0.9604 | 0.9934 | ||||||||||||
|
January 31, 2015
|
0.9210 | 0.9443 | 0.8488 | 1.0195 | ||||||||||||
|
February 28, 2015
|
0.9513 | 0.9361 | 0.9228 | 0.9540 | ||||||||||||
|
March, 2015 (through March 20, 2015)
|
0.9766 | 0.9876 | 0.9553 | 1.0074 | ||||||||||||
|
D.
|
|
|
·
|
completing research and clinical development of our product candidates, including successfully completing Phase 3 clinical trials of AM-101 or AM-111;
|
|
|
·
|
obtaining marketing approvals for our product candidates, including AM-101 or AM-111, for which we complete clinical trials;
|
|
|
·
|
developing a sustainable and scalable manufacturing process for any approved product candidates and maintaining supply and manufacturing relationships with third parties that can conduct the process and provide adequate (in amount and quality) products to support clinical development and the market demand for our product candidates, if approved;
|
|
|
·
|
launching and commercializing product candidates for which we obtain marketing approval, either directly or with a collaborator or distributor;
|
|
|
·
|
obtaining market acceptance of our product candidates as viable treatment options;
|
|
|
·
|
addressing any competing technological and market developments;
|
|
|
·
|
identifying, assessing, acquiring and/or developing new product candidates;
|
|
|
·
|
negotiating favorable terms in any collaboration, licensing, or other arrangements into which we may enter;
|
|
|
·
|
maintaining, protecting, and expanding our portfolio of intellectual property rights, including patents, trade secrets, and know-how; and
|
|
|
·
|
attracting, hiring, and retaining qualified personnel.
|
|
|
·
|
the scope, rate of progress, results and cost of our clinical trials, nonclinical testing, and other related activities;
|
|
|
·
|
the cost of manufacturing clinical supplies, and establishing commercial supplies, of our product candidates and any products that we may develop;
|
|
|
·
|
the number and characteristics of product candidates that we pursue;
|
|
|
·
|
the cost, timing, and outcomes of regulatory approvals;
|
|
|
·
|
the cost and timing of establishing sales, marketing, and distribution capabilities; and
|
|
|
·
|
the terms and timing of any collaborative, licensing, and other arrangements that we may establish, including any required milestone and royalty payments thereunder.
|
|
|
·
|
completing clinical trials that demonstrate the efficacy and safety of our product candidates;
|
|
|
·
|
receiving marketing approvals from applicable regulatory authorities;
|
|
|
·
|
establishing commercial manufacturing capabilities;
|
|
|
·
|
launching commercial sales, marketing and distribution operations;
|
|
|
·
|
acceptance of our product candidates by patients, the medical community and third-party payors,
|
|
|
·
|
a continued acceptable safety profile following approval;
|
|
|
·
|
competing effectively with other therapies; and
|
|
|
·
|
qualifying for, maintaining, enforcing and defending our intellectual property rights and claims.
|
|
|
·
|
the delay or refusal of regulators or IRBs to authorize us to commence a clinical trial at a prospective trial site and changes in regulatory requirements, policies and guidelines;
|
|
|
·
|
delays or failure to reach agreement on acceptable terms with prospective CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
|
|
|
·
|
delays in patient enrollment and variability in the number and types of patients available for clinical trials;
|
|
|
·
|
the inability to enroll a sufficient number of patients in trials to ensure adequate statistical power to detect statistically significant treatment effects;
|
|
|
·
|
negative or inconclusive results, which may require us to conduct additional preclinical or clinical trials or to abandon projects that we expect to be promising;
|
|
|
·
|
safety or tolerability concerns could cause us to suspend or terminate a trial if we find that the participants are being exposed to unacceptable health risks;
|
|
|
·
|
regulators or IRBs requiring that we or our investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or safety concerns, among others;
|
|
|
·
|
lower than anticipated retention rates of patients and volunteers in clinical trials;
|
|
|
·
|
our CROs or clinical trial sites failing to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all, deviating from the protocol or dropping out of a trial;
|
|
|
·
|
delays relating to adding new clinical trial sites;
|
|
|
·
|
difficulty in maintaining contact with patients after treatment, resulting in incomplete data;
|
|
|
·
|
delays in establishing the appropriate dosage levels;
|
|
|
·
|
the quality or stability of the product candidate falling below acceptable standards;
|
|
|
·
|
the inability to produce or obtain sufficient quantities of the product candidate to complete clinical trials; and
|
|
|
·
|
exceeding budgeted costs due to difficulty in accurately predicting costs associated with clinical trials.
|
|
|
·
|
be delayed in obtaining marketing approval for our product candidates;
|
|
|
·
|
not obtain marketing approval at all;
|
|
|
·
|
obtain approval for indications or patient populations that are not as broad as intended or desired;
|
|
|
·
|
obtain approval with labeling that includes significant use or distribution restrictions or significant safety warnings, including boxed warnings;
|
|
|
·
|
be subject to additional post-marketing testing or other requirements; or
|
|
|
·
|
remove the product from the market after obtaining marketing approval.
|
|
|
·
|
regulatory authorities may withdraw approvals of such product;
|
|
|
·
|
regulatory authorities may require additional warnings on the label;
|
|
|
·
|
we may be required to create a medication guide outlining the risks of such side effects for distribution to patients;
|
|
|
·
|
we could be sued and held liable for harm caused to patients; and
|
|
|
·
|
our reputation and physician or patient acceptance of our products may suffer.
|
|
|
·
|
the FDA, EMA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials;
|
|
|
·
|
the population studied in the clinical program may not be sufficiently broad or representative to assure safety in the full population for which we seek approval;
|
|
|
·
|
the FDA, EMA or comparable foreign regulatory authorities may disagree with our interpretation of data from nonclinical trials or clinical trials;
|
|
|
·
|
the data collected from clinical trials of our product candidates may not be sufficient to support the submission of a new drug application, or NDA, or other submission or to obtain regulatory approval in the United States or elsewhere;
|
|
|
·
|
we may be unable to demonstrate to the FDA, EMA or comparable foreign regulatory authorities that a product candidate’s risk-benefit ratio for its proposed indication is acceptable;
|
|
|
·
|
the FDA, EMA or other regulatory authorities may fail to approve the manufacturing processes, test procedures and specifications, or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and
|
|
|
·
|
the approval policies or regulations of the FDA, EMA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval.
|
|
|
·
|
restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls;
|
|
|
·
|
fines, warning letters or holds on clinical trials;
|
|
|
·
|
refusal by the FDA to approve pending applications or supplements to approved applications filed by us, or suspension or revocation of product license approvals;
|
|
|
·
|
product seizure or detention, or refusal to permit the import or export of products; and
|
|
|
·
|
injunctions or the imposition of civil or criminal penalties.
|
|
|
·
|
the U.S. healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under U.S. government healthcare programs such as Medicare and Medicaid;
|
|
|
·
|
the U.S. False Claims Act imposes criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the U.S. government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
|
|
|
·
|
the U.S. Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
|
|
|
·
|
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information;
|
|
|
·
|
the transparency requirements under the Health Care Reform Law require manufacturers of drugs, devices, biologics and medical supplies to report to the U.S. Department of Health and Human Services information related to payments and other transfers of value made by such manufacturers to physicians and teaching hospitals, and ownership and investment interests held by physicians or their immediate family members; and
|
|
|
·
|
analogous laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures.
|
|
|
·
|
develop and commercialize products that are safer, more effective, less expensive, or more convenient or easier to administer;
|
|
|
·
|
obtain quicker regulatory approval;
|
|
|
·
|
establish superior proprietary positions;
|
|
|
·
|
have access to more manufacturing capacity;
|
|
|
·
|
implement more effective approaches to sales and marketing; or
|
|
|
·
|
form more advantageous strategic alliances.
|
|
|
·
|
how clinicians and potential patients perceive our novel products;
|
|
|
·
|
the timing of market introduction;
|
|
|
·
|
the number and clinical profile of competing products;
|
|
|
·
|
our ability to provide acceptable evidence of safety and efficacy;
|
|
|
·
|
the prevalence and severity of any side effects;
|
|
|
·
|
relative convenience and ease of administration, particularly as AM-101 and AM-111 require multiple outpatient procedures to administer the drug;
|
|
|
·
|
cost-effectiveness;
|
|
|
·
|
patient diagnostics and screening infrastructure in each market, particularly as AM-101 and AM-111 are being developed for the treatment of acute inner ear disorders and are thus dependent on a relatively rapid diagnosis and dosing process;
|
|
|
·
|
marketing and distribution support;
|
|
|
·
|
availability of coverage, reimbursement and adequate payment from health maintenance organizations and other third-party payors, both public and private; and
|
|
|
·
|
other potential advantages over alternative treatment methods.
|
|
|
·
|
we may not be able to control the amount and timing of resources that the collaboration partner devotes to the product development program;
|
|
|
·
|
the collaboration partner may experience financial difficulties;
|
|
|
·
|
we may be required to relinquish important rights such as marketing, distribution and intellectual property rights;
|
|
|
·
|
a collaboration partner could move forward with a competing product developed either independently or in collaboration with third parties, including our competitors; or
|
|
|
·
|
business combinations or significant changes in a collaboration partner’s business strategy may adversely affect our willingness to complete our obligations under any arrangement.
|
|
|
·
|
the scope of rights granted under the agreement and other interpretation-related issues;
|
|
|
·
|
the extent to which our technology and processes infringe on intellectual property of the licensor or partner that is not subject to the agreement;
|
|
|
·
|
the sublicensing of patent and other rights;
|
|
|
·
|
our diligence and commercialization obligations under the agreement and what activities satisfy those obligations;
|
|
|
·
|
the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors or partners and us and our collaborators; and
|
|
|
·
|
the priority of invention of patented technology.
|
|
|
·
|
positive or negative results of testing and clinical trials by us, strategic partners, or competitors;
|
|
|
·
|
delays in entering into strategic relationships with respect to development and/or commercialization of our product candidates or entry into strategic relationships on terms that are not deemed to be favorable to us;
|
|
|
·
|
technological innovations or commercial product introductions by us or competitors;
|
|
|
·
|
changes in government regulations;
|
|
|
·
|
developments concerning proprietary rights, including patents and litigation matters;
|
|
|
·
|
public concern relating to the commercial value or safety of any of our product candidates;
|
|
|
·
|
financing or other corporate transactions;
|
|
|
·
|
publication of research reports or comments by securities or industry analysts;
|
|
|
·
|
general market conditions in the pharmaceutical industry or in the economy as a whole; or
|
|
|
·
|
other events and factors beyond our control.
|
|
|
·
|
the non-Swiss court had jurisdiction pursuant to the Swiss Federal Act on Private International Law;
|
|
|
·
|
the judgment of such non-Swiss court has become final and non-appealable;
|
|
|
·
|
the judgment does not contravene Swiss public policy;
|
|
|
·
|
the court procedures and the service of documents leading to the judgment were in accordance with the due process of law; and
|
|
|
·
|
no proceeding involving the same position and the same subject matter was first brought in Switzerland, or adjudicated in Switzerland, or was earlier adjudicated in a third state and this decision is recognizable in Switzerland.
|
|
|
·
|
First mover advantage.
With two product candidates in late stage clinical development, we believe we are currently the clinically most advanced company working on inner ear therapeutics. We believe that AM-101 and AM-111 are the only drug candidates that have demonstrated positive efficacy in randomized placebo-controlled clinical trials in acute inner ear tinnitus and acute inner ear hearing loss. As a result, we believe we will be the first to market with FDA or EMA-approved products for these indications.
|
|
|
·
|
Barriers to entry.
Our products are protected not only through intellectual property rights but also by the orphan drug status granted to AM-111 as well as by the know-how across several disciplines that is required to formulate and reliably deliver drugs to the inner ear. Our proprietary gel formulation, its manufacturing and its application are part of our intellectual property, know-how and competitive advantage. In addition, we believe that our intellectual property broadly directed to polymer-based formulations for the treatment of middle or inner ear disorders will serve as barriers to entry beyond our current products.
|
|
|
·
|
Efficient commercialization.
Given that the market for our therapeutic product candidates can be efficiently accessed through a limited number of specialist ENT physicians and specialist neurotologists, we intend to build our own sales force in order to commercialize these products in the United States and key European markets.
|
|
|
·
|
Experienced management.
Having been focused on developing therapeutic products for inner ear indications for over a decade, we believe that our senior management provides us with significant capabilities. Our Chief Executive Officer and founder, Thomas Meyer, has played several pivotal roles in our development and evolution. Prior to Auris Medical, he was the CEO of Disetronic, a fast growing Swiss diabetes care company sold to Roche in 2003. Other key members of our management team bring significant experience in clinical, product and business development in biopharmaceutical companies.
|
|
|
·
|
Target inner ear disorders that have a defined pathophysiology and that are amenable to treatment.
We are focusing on inner ear disorders for which the pathophysiology is well characterized, can be effectively targeted and where affected patients seek medical attention proactively.
|
|
|
·
|
Use drug delivery techniques and proprietary drug formulations for effective, safe and rapid local administration to the inner ear.
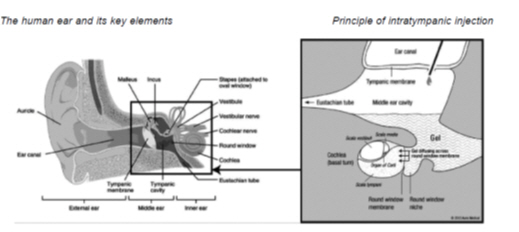
We are developing treatments for inner ear disorders based on i.t. injections into the middle ear. This short outpatient procedure allows us to deliver therapeutic concentrations of drug in a highly targeted fashion with only minimal systemic exposure. We are using proprietary, fully biocompatible and biodegradable gel formulations for optimum middle ear tolerance and effective diffusion of active substances into the inner ear.
|
|
|
·
|
Bring AM-101 and AM-111 to market.
We plan to focus most of our resources on the development and commercialization of our two lead product candidates. AM-101 is in two Phase 3 clinical trials, based on a SPA from the FDA and guidance from the EMA. The FDA held a pre-IND meeting with us in September 2014 and provided formal feedback and guidance on our pre-clinical and CMC development and specifically on HEALOS. In response to this feedback, we are in the process of finalizing the design of HEALOS. In addition, we obtained an open IND from the FDA for REACH. We plan to conduct REACH in the U.S., comparing a single 0.4 mg/mL dose of AM-111 to a placebo. If we obtain sufficient funding, we intend to start HEALOS in the second half of 2015 and REACH in 2016.
|
|
|
·
|
Build an efficient commercial infrastructure to maximize the value of our product candidates.
We intend to build commercial operations in the North American market and in select European markets. In those markets, we expect our commercial operations to include specialty sales forces targeting ENTs and specialists in neurotology both in hospitals and in private practice. In other markets, we expect to seek partnerships that would maximize our products’ commercial potential.
|
|
|
·
|
Expand our pipeline through internal development, academic collaborations, in-licensing and acquisitions.
Through our work with academic research partners on the pathophysiology of tinnitus and hearing loss and clinical development we have gained novel insights that will help us both to create new pipeline products that act by way of novel mechanisms as well as to expand the therapeutic focus for our existing product candidates beyond their current indications. We plan to further maximize our commercial potential through product life cycle management, and with licensing or acquisition of compounds that could augment our product offering in ENT disorders.
|
|
|
(1)
|
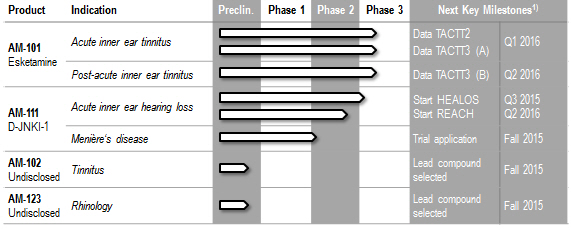
Dates of key milestones are indicative and subject to change.
|
|
AM-101
|
|||||
|
Placebo
|
Low Dose
|
High Dose
|
|||
|
Point improvement in tinnitus loudness (0-100 point scale)
|
|||||
|
LS means (n)
|
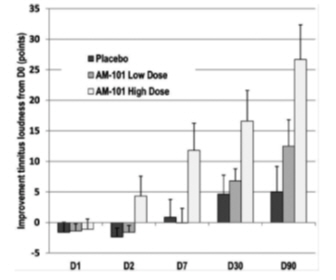
1.4 (23)
|
16.0 (25)
|
24.1 (29)
|
||
|
LS mean difference (95% confidence interval)
|
14.6 (1.4, 27.7)
|
22.7 (10.3, 35.1)
|
|||
|
P-value
|
0.0308*
|
0.0005***
|
|||
|
Point improvement in tinnitus annoyance (0-100 point scale)
|
|||||
|
LS means (n)
|
10.8 (23)
|
21.7 (25)
|
27.8 (29)
|
||
|
LS mean difference (95% confidence interval)
|
10.9 (1.4, 23.2)
|
17.0 (5.4, 28.6)
|
|||
|
P-value
|
0.0805
|
0.0047**
|
|||
|
Point improvement in difficulties falling asleep (0-100 point scale)
|
|||||
|
LS means (n)
|
11.8 (21)
|
29.8 (15)
|
38.7 (22)
|
||
|
LS mean difference (95% confidence interval)
|
18.1 (2.5, 33.6)
|
26.9 (13.0, 40.9)
|
|||
|
P-value
|
0.0234*
|
0.0003***
|
|||
|
Point improvement in tinnitus impact (0-24 point scale)
|
|||||
|
LS means (n)
|
2.5 (22)
|
5.5 (25)
|
5.9 (27)
|
||
|
LS mean difference (95% confidence interval)
|
3.0 (0.1, 5.8)
|
3.4 (0.8, 6.0)
|
|||
|
P-value
|
0.0400*
|
0.0124*
|
|||
|
AM-111
|
|||||
|
Placebo
|
Low Dose
|
High Dose
|
|||
|
Absolute hearing improvement, dB
|
17.9 (30)
|
29.9 (29)
|
22.7 (33)
|
||
|
LS means (n)
|
|||||
|
LS mean difference (95% confidence interval)
|
12.1 (2.2, 22.0)
|
4.9 (-4.8, 14.6)
|
|||
|
P-value
|
0.017*
|
0.319
|
|||
|
Relative hearing improvement, %
|
|||||
|
LS means (n)
|
30.9 (30)
|
50.4 (29)
|
37.6 (33)
|
||
|
LS mean difference (95% confidence interval)
|
19.5 (3.0, 35.9)
|
6.6 (-9.6, 22.8)
|
|||
|
P-value
|
0.021*
|
0.419
|
|||
|
Frequency complete hearing recovery, %
|
|
AM-111
|
|||||
|
Placebo
|
Low Dose
|
High Dose
|
|||
|
Mean (n)
|
13.3 (30)
|
31.0 (29)
|
24.2 (33)
|
||
|
Odds ratio (95% confidence interval)
|
5.5 (1.1, 29.0)
|
1.6 (0.4, 6.7)
|
|||
|
P-value
|
0.044*
|
0.530
|
|||
|
Speech discrimination score improvement, % points
|
|||||
|
LS means (n)
|
9.1 (29)
|
27.4 (29)
|
23.2 (33)
|
||
|
LS mean difference (95% confidence interval)
|
18.3 (3.1, 33.4)
|
14.1 (0.7, 28.9)
|
|||
|
P-value
|
0.019*
|
0.061*
|
|
|
·
|
Merz Pharmaceuticals GmbH has a product candidate that is a low affinity NMDA receptor antagonist and nicotinic acetylcholine receptor antagonist (neramexane) designed for oral treatment of tinnitus. In November 2011 Merz Pharma announced the suspension of its tinnitus development program with neramexane due to lack of efficacy in Phase 3 clinical trials in post-acute tinnitus; the product candidate is currently still being evaluated in a Phase 2 clinical trial by Merz’s Japanese collaboration partner Kyorin Pharmaceutical Co., Ltd.
|
|
|
·
|
Novartis Pharmaceuticals AG has a product candidate that is an AMPA receptor antagonist designed for oral administration (BGG492) and that has been tested in chronic tinnitus patients in a phase 2 clinical trial. The trial was completed in January 2012 with no outcomes being reported to date.
|
|
|
·
|
Autifony Therapeutics Ltd. has a Kv3 potassium channel agonist product candidate (AUT00063) that is designed for oral administration. In 2014 Autifony initiated a Phase 2 study with AUT00063 in patients with post-acute tinnitus. The trial is expected to complete enrollment in summer 2015.
|
|
|
·
|
AudioCure Pharma GmbH has a ß-carboline product candidate (AC-002) in preclinical development that is designed for i.t. treatment of noise induced hearing loss in a gel-based formulation.
|
|
|
·
|
Autifony Therapeutics Ltd. has a Kv3 potassium channel agonist product candidate (AUT00063) that is designed for oral administration. Autifony is expected to initiate a Phase 2 trial with AUT00063 in the treatment of speech-in-noise deficits in elderly patients.
|
|
|
·
|
Sound Pharmaceuticals, Inc. has a product candidate (SPI-1005, ebselen), that mimics and prompts production of the enzyme glutathione peroxidase and is designed for oral administration, and that has been tested for the prevention and treatment of temporary inner ear hearing loss in a Phase 2 clinical trial.
|
|
|
·
|
Otologic Pharmaceutics, Inc. has a product candidate (HPN-07) designed for treatment of acute hearing loss by way of oral administration. A Phase 1 trial was initiated in 2014.
|
|
|
·
|
Southern Illinois University has an antioxidant product candidate (D-methionine) that is designed for oral administration in the prevention and treatment of noise induced hearing loss and currently being tested in a late stage study with the Department of Defense.
|
|
|
·
|
Otonomy Inc.’s product candidate OTO-104 is a micronized dexamethasone formulated in a poloxamer gel designed for single dose i.t. injection for which results of a Phase 2b clinical trial in the United States and Canada are expected to be reported in the second quarter of 2015. If successful, Otonomy is expected to initiate a second pivotal trial for OTO-104 in 2015.
|
|
|
·
|
Synphora AB is evaluating a latanoprost product candidate designed for i.t. injections in a Phase 2 clinical trial in Meniere’s Disease patients in Sweden.
|
|
|
·
|
the completion of preclinical laboratory tests and animal tests conducted under GLP regulations;
|
|
|
·
|
the submission to the FDA of an Investigational New Drug, or IND, application for human clinical testing, which must become effective before human clinical trials commence;
|
|
|
·
|
the performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the product candidate for each proposed indication and conducted in accordance with cGCP;
|
|
|
·
|
the submission to the FDA of a New Drug Application, or NDA;
|
|
|
·
|
the FDA’s acceptance of the NDA;
|
|
|
·
|
satisfactory completion of an FDA inspection of the manufacturing facilities at which the product is made to assess compliance with cGMPs; and
|
|
|
·
|
the FDA’s review and approval of an NDA prior to any commercial marketing or sale of the drug in the United States.
|
|
|
Phase 1.
|
Phase 1 clinical trials represent the initial introduction of a product candidate into human subjects, frequently healthy volunteers. In Phase 1, the product candidate is usually tested for safety, including adverse effects, dosage tolerance, absorption, distribution, metabolism, excretion and pharmacodynamics.
|
|
|
Phase 2.
|
Phase 2 clinical trials usually involve studies in a limited patient population to (1) evaluate the efficacy of the product candidate for specific indications, (2) determine dosage tolerance and optimal dosage and (3) identify possible adverse effects and safety risks.
|
|
|
Phase 3.
|
If a product candidate is found to be potentially effective and to have an acceptable safety profile in Phase 2 studies, the clinical trial program will be expanded to Phase 3 clinical trials to further demonstrate clinical efficacy, optimal dosage and safety within an expanded patient population at geographically dispersed clinical study sites.
|
|
|
·
|
restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls;
|
|
|
·
|
fines, warning letters or holds on post-approval clinical trials;
|
|
|
·
|
refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product license approvals;
|
|
|
·
|
product seizure or detention, or refusal to permit the import or export of products; or
|
|
|
·
|
injunctions or the imposition of civil or criminal penalties.
|
|
|
·
|
salaries for research and development staff and related expenses, including employee benefits;
|
|
|
·
|
costs for production of preclinical compounds and drug substances by contract manufacturers;
|
|
|
·
|
fees and other costs paid to contract research organizations in connection with additional preclinical testing and the performance of clinical trials;
|
|
|
·
|
costs of related facilities, materials and equipment;
|
|
|
·
|
costs associated with obtaining and maintaining patents;
|
|
|
·
|
costs related to the preparation of regulatory filings and fees; and
|
|
|
·
|
depreciation and amortization of tangible and intangible fixed assets used to develop our product candidates.
|
|
|
·
|
AM-101
. We have commenced a Phase 3 program of AM-101 comprising two Phase 3 studies and expect top-line data in early 2016. These two studies are extended into two open label extension studies. We anticipate that our research and development expenses will increase substantially in connection with these clinical trials.
|
|
|
·
|
AM-111
. We participated in a pre-IND meeting with the FDA in September 2014 in which the FDA provided formal feedback and guidance on our pre-clinical and CMC development and specifically on the planned HEALOS trial. In response to this feedback, we are in the process of finalizing the design of HEALOS. In addition, we obtained an open IND from the FDA for the REACH trial. We plan to conduct REACH in the U.S., comparing a single 0.4 mg/mL dose of AM-111 to a placebo. If we obtain sufficient funding, we intend to start HEALOS in the second half of 2015 and REACH in 2016. We anticipate that our research and development expenses will increase substantially in connection with commencement of additional clinical trials of AM-111.
|
|
|
·
|
Other development programs
. Other research and development expenses mainly relate to our preclinical studies of AM-102 and AM-123. The expenses mainly consist of salaries, costs for production of the preclinical compounds and costs paid to academic research institutions in conjunction with preclinical testing.
|
|
|
·
|
the scope, rate of progress, results and cost of our clinical trials, nonclinical testing, and other related activities;
|
|
|
·
|
the cost of manufacturing clinical supplies, and establishing commercial supplies, of our product candidates and any products that we may develop;
|
|
|
·
|
the number and characteristics of product candidates that we pursue;
|
|
|
·
|
the cost, timing, and outcomes of regulatory approvals;
|
|
|
·
|
the cost and timing of establishing sales, marketing, and distribution capabilities; and
|
|
|
·
|
the terms and timing of any collaborative, licensing, and other arrangements that we may establish, including any required milestone and royalty payments thereunder.
|
|
|
·
|
salaries for general and administrative staff and related expenses, including employee benefits;
|
|
|
·
|
business development expenses, including travel expenses;
|
|
|
·
|
administration costs including professional fees for auditors and other consulting expenses not related to research and development activities, professional fees for lawyers not related to the protection and maintenance of our intellectual property and IT expenses;
|
|
|
·
|
cost of facilities, communication and office expenses; and
|
|
|
·
|
depreciation and amortization of tangible and intangible fixed assets not related to research and development activities.
|
|
Year Ended December 31,
|
||||||||||||
|
2014
|
2013
|
Change
|
||||||||||
|
(in thousands of CHF)
|
%
|
|||||||||||
|
Research and development
|
(17,705 | ) | (13,254 | ) | 34 | % | ||||||
|
General and administrative
|
(4,489 | ) | (1,362 | ) | 230 | % | ||||||
|
Operating loss
|
(22,194 | ) | (14,616 | ) | 52 | % | ||||||
|
Finance expense
|
(208 | ) | (159 | ) | 31 | % | ||||||
|
Finance income
|
4,217 | 76 | 5,466 | % | ||||||||
|
Loss before tax
|
(18,185 | ) | (14,699 | ) | 24 | % | ||||||
|
Income tax expense
|
0 | (306 | ) | (100 | )% | |||||||
|
Net loss attributable to owners of the Company
|
(18,185 | ) | (15,005 | ) | 21 | % | ||||||
|
Other comprehensive loss:
|
||||||||||||
|
Items that will never be reclassified to profit or loss
|
||||||||||||
|
Remeasurements of defined benefits liability, net of taxes of CHF 0
|
(1,102 | ) | (58 | ) | 1,080 | % | ||||||
|
Items that are or may be reclassified to profit or loss
|
||||||||||||
|
Foreign currency translation differences, net of taxes of CHF 0
|
(105 | ) | 32 | (431 | )% | |||||||
|
Other comprehensive loss
|
(1,207 | ) | (26 | ) | 4,541 | % | ||||||
|
Total comprehensive loss attributable to owners of the Company
|
(19,392 | ) | (15,031 | ) | 29 | % | ||||||
|
Year Ended December 31,
|
||||||||||||
|
2014
|
2013
|
Change
|
||||||||||
|
(in thousands of CHF)
|
%
|
|||||||||||
|
Research and development expense
|
||||||||||||
|
Clinical projects
|
(12,142 | ) | (8,753 | ) | 39 | % | ||||||
|
Preclinical projects
|
(1,160 | ) | (2,078 | ) | (44 | )% | ||||||
|
Drug manufacture and substance
|
(1,384 | ) | (1,036 | ) | 34 | % | ||||||
|
Employee benefits
|
(1,718 | ) | (1,074 | ) | 60 | % | ||||||
|
Other research and development expenses
|
(1,301 | ) | (313 | ) | 316 | % | ||||||
|
Total
|
(17,705 | ) | (13,254 | ) | 34 | % | ||||||
|
|
·
|
Clinical projects
. Beginning in February and March 2014 we enrolled patients in two AM-101 Phase 3 trials, namely TACTT2 and TACTT3. In addition, roll-over of study participants into the open-label follow-on studies AMPACT1 and AMPACT2 started in May/June 2014. This resulted in the incurrence of substantial service and pass through costs from our CROs and other service providers. In 2013, AM-101 clinical costs were lower as Phase 2 completed in February and we mainly incurred costs related to the preparation for the Phase 3 trials (notably for feasibility assessments, investigator and site selections, investigator meetings, validation and translation work on questionnaires and other study documents, procurement of electronic patient diaries, set-up of electronic data capture systems, databases and procedures as well as submissions to regulatory agencies and institutional review boards) . For AM-111, in 2014, we incurred significantly higher clinical costs for the preparation of the Phase 3 program, most notably, feasibility assessments, as well as investigator and site selections. In contrast, in 2013 expense levels related to the AM-111 project were lower because a Phase 2 clinical trial had been completed.
|
|
|
·
|
Preclinical projects
. In 2014 we finished toxicology studies with repeated AM-101 dosing and therefore incurred lower expenses as compared to 2013. In contrast, in 2014 we incurred higher expenses for AM-111 as we started a repeated dose toxicology study and finalized the analytical assay development. Costs incurred in 2013 related to project AM-102, including screening of compounds for a new pharmacological target in tinnitus. In addition, we initiated additional toxicology studies with repeated AM-101 dosing in rodents, and conducted reproduction toxicology studies with AM-111.
|
|
|
·
|
Drug manufacture and substance
. In 2014 we incurred substantial costs related to the manufacture of clinical supplies for AM-111 in preparation for the Phase 3 trial program as well as for analytical development and validation. In contrast, in 2013 we mainly incurred costs related to the manufacture, labelling and packaging of supplies for the AM-101 Phase 3 trials.
|
|
|
·
|
Employee benefits
. Headcount continued to increase in 2014 in line with the expansion of our research and development activities as well as our administrative functions.
|
|
Year Ended December 31,
|
||||||||||||
|
2014
|
2013
|
Change
|
||||||||||
|
General and administrative expense
|
(in thousands of CHF)
|
%
|
||||||||||
|
Employee benefits
|
(1,137 | ) | (196 | ) | 481 | % | ||||||
|
Administration costs
|
(2,014 | ) | (556 | ) | 262 | % | ||||||
|
Initial public offering costs, expensed
|
(822 | ) | 0 | 100 | % | |||||||
|
Other
|
(516 | ) | (610 | ) | (15 | )% | ||||||
|
Total
|
(4,489 | ) | (1,362 | ) | 230 | % | ||||||
|
|
·
|
Employee benefits
. Headcount continued to increase in 2014 in line with the expansion of our administrative staff after becoming a publicly listed company.
|
|
|
·
|
Administration costs
. The increase reflects higher legal and auditing expenses in connection with the preparation for our initial public offering and expenses associated with operating as a public company after August 2014.
|
|
|
·
|
Initial public offering costs, expensed
. These initial public offering costs expensed in the three months ended March, 31, 2014, as management determined that successful completion was not deemed more likely than not. No such costs were incurred in 2013.
|
|
|
·
|
Other
. These costs were on aggregate broadly in line with 2013 and comprise facility, business development and travel costs.
|
|
Year Ended December 31,
|
||||||||||||
|
2013
|
2012
|
Change
|
||||||||||
|
(in thousands of CHF)
|
%
|
|||||||||||
|
Research and development
|
(13,254 | ) | (3,987 | ) | 232 | % | ||||||
|
General and administrative
|
(1,362 | ) | (624 | ) | 118 | % | ||||||
|
Operating loss
|
(14,616 | ) | (4,611 | ) | 217 | % | ||||||
|
Finance expense
|
(159 | ) | (2 | ) | 8,713 | % | ||||||
|
Finance income
|
76 | 10 | 666 | % | ||||||||
|
Loss before tax
|
(14,699 | ) | (4,602 | ) | 219 | % | ||||||
|
Income tax expense
|
(306 | ) | — | |||||||||
|
Net loss attributable to owners of the Company
|
(15,005 | ) | (4,602 | ) | 226 | % | ||||||
|
Other comprehensive loss:
|
||||||||||||
|
Items that will never be reclassified to profit or loss
|
||||||||||||
|
Remeasurements of defined benefit liability, net of taxes of CHF 0
|
(58 | ) | (55 | ) | 6 | % | ||||||
|
Items that are or may be reclassified to profit or loss
|
||||||||||||
|
Foreign currency translation differences, net of taxes of CHF 0
|
32 | 22 | 42 | % | ||||||||
|
Other comprehensive loss, net of taxes of CHF 0
|
(26 | ) | (32 | ) | (20 | )% | ||||||
|
Total comprehensive loss attributable to owners of the Company
|
(15,031 | ) | (4,635 | ) | 224 | % | ||||||
|
Year Ended December 31,
|
||||||||||||
|
2013
|
2012
|
Change
|
||||||||||
|
(in thousands of CHF)
|
%
|
|||||||||||
|
Research and development expense
|
||||||||||||
|
Clinical projects
|
(8,753 | ) | (1,687 | ) | 419 | % | ||||||
|
Preclinical projects
|
(2,078 | ) | (298 | ) | 598 | % | ||||||
|
Drug manufacture and substance
|
(1,036 | ) | (915 | ) | 13 | % | ||||||
|
Employee benefits
|
(1,074 | ) | (770 | ) | 40 | % | ||||||
|
Other research and development expenses
|
(313 | ) | (317 | ) | (1 | )% | ||||||
|
Total
|
(13,254 | ) | (3,987 | ) | 232 | % | ||||||
|
|
·
|
Clinical Projects
. In 2013 we completed the second Phase 2 study with AM-101. At the same time we incurred substantial costs related to the preparation of the AM-101 Phase 3 program (TACTT2 and TACTT3), notably for feasibility assessments, investigator and site selections, investigator meetings, validation and translation work on questionnaires and other study documents, procurement of electronic patient diaries, set-up of electronic data capture systems, databases and procedures as well as submissions to regulatory agencies and institutional review boards. In contrast, expense levels related to the AM-111 project decreased from 2012 levels because Phase 2 clinical trial work was completed.
|
|
|
·
|
Preclinical projects
. In 2013, we stepped up our activities related to project AM-102, including screening of compounds for a new pharmacological target in tinnitus. In addition, we initiated additional toxicology studies with repeated AM-101 dosing in rodents, and conducted reproduction toxicology studies with AM-111.
|
|
|
·
|
Drug manufacture and substance
. In 2013 we incurred substantial costs related to the manufacture, labeling and packaging of supplies for the AM-101 Phase 3 trials.
|
|
|
·
|
Employee benefits
. Headcount continued to increase in 2013 in line with the expansion of our research and development activities.
|
|
Year Ended December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
(in thousands of CHF)
|
||||||||
|
Cash used in operating activities
|
(19,316 | ) | (14,044 | ) | ||||
|
Net cash used in investing activities
|
(1,186 | ) | (35 | ) | ||||
|
Net cash from financing activities
|
49,609 | 37,881 | ||||||
|
Net effect of currency translation on cash
|
3,961 | 0 | ||||||
|
Cash and cash equivalents at the beginning of the period
|
23,866 | 64 | ||||||
|
Cash and cash equivalents at the end of the period
|
56,934 | 23,866 | ||||||
|
Year Ended December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
(in thousands of CHF)
|
||||||||
|
Cash used in operating activities
|
(14,044 | ) | (4,499 | ) | ||||
|
Net cash used in investing activities
|
(35 | ) | (53 | ) | ||||
|
Net cash from financing activities
|
37,881 | 3,862 | ||||||
|
Net effect of currency translation on cash
|
0 | 1 | ||||||
|
Cash and cash equivalents at the beginning of the period
|
64 | 753 | ||||||
|
Cash and cash equivalents at the end of the period
|
23,866 | 64 | ||||||
|
Equity Capital and Preferred Shares
|
Loans
|
Total
|
||||||||||
|
(in thousands of CHF)
|
||||||||||||
|
2014
|
50,038 | — | 50,038 | |||||||||
|
2013
|
24,111 | 13,770 | 37,881 | |||||||||
|
2012
|
3,862 | — | 3,862 | |||||||||
|
Total
|
78,011 | 13,770 | 91,781 | |||||||||
|
|
·
|
the scope, rate of progress, results and cost of our clinical trials, nonclinical testing, and other related activities;
|
|
|
·
|
the cost of manufacturing clinical supplies, and establishing commercial supplies, of our product candidates and any products that we may develop;
|
|
|
·
|
the number and characteristics of product candidates that we pursue;
|
|
|
·
|
the cost, timing, and outcomes of regulatory approvals;
|
|
|
·
|
the cost and timing of establishing sales, marketing, and distribution capabilities; and
|
|
|
·
|
the terms and timing of any collaborative, licensing, and other arrangements that we may establish, including any required milestone and royalty payments thereunder.
|
|
|
·
|
temporary differences on the initial recognition of assets or liabilities in a transaction that is not a business combination and that affects neither accounting nor taxable profit or loss;
|
|
|
·
|
temporary differences related to investments in subsidiaries to the extent that the Company is able to control the timing of the reversal of the temporary differences and it is probable that they will not reverse in the foreseeable future; and
|
|
|
·
|
taxable temporary differences arising on the initial recognition of goodwill.
|
|
|
·
|
not providing an auditor attestation report on our system of internal controls over financial reporting;
|
|
|
·
|
not providing all of the compensation disclosure that may be required of non-emerging growth public companies under the U.S. Dodd-Frank Wall Street Reform and Consumer Protection Act;
|
|
|
·
|
not disclosing certain executive compensation-related items such as the correlation between executive compensation and performance and comparisons of the Chief Executive Officer’s compensation to median employee compensation; and
|
|
|
·
|
not complying with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis).
|
|
Payments Due by Period
|
||||||||||||
|
Less Than
1 Year
|
Between 1 and 5 Years
|
Total
|
||||||||||
|
(in thousands of CHF)
|
||||||||||||
|
Operating lease obligations(1)
|
104 | 59 | 163 | |||||||||
|
Total
|
104 | 59 | 163 | |||||||||
|
(1)
|
Operating lease obligations consist of payments pursuant to non-cancellable operating lease agreements relating to our lease of office space and are not accounted for on the balance sheet. The lease term is 5 years with an early termination option as of March 2016.
|
|
G.
|
|
Name
|
Position
|
Age
|
Initial Year of Appointment
|
|||||||
|
Executive Officers
|
||||||||||
|
Thomas Meyer
|
Chairman and Chief Executive Officer
|
47 | 2003 | |||||||
|
Bettina Stubinski
|
Chief Medical Officer
|
48 | 2013 | |||||||
|
Sven Zimmermann
|
Chief Financial Officer
|
44 | 2014 | |||||||
|
Non-Executive Directors
|
||||||||||
|
Wolfgang Arnold
|
Director
|
73 | 2007 | |||||||
|
James I. Healy
|
Director
|
50 | 2013 | |||||||
|
Oliver Kubli
|
Director
|
42 | 2010 | |||||||
|
Alain Munoz
|
Director
|
65 | 2007 | |||||||
|
Antoine Papiernik
|
Director
|
48 | 2013 | |||||||
|
B.
|
|
|
·
|
the appointment, compensation, retention and oversight of any auditor or accounting firm engaged for the purpose of preparing or issuing an audit report or performing other audit, review or attest services;
|
|
|
·
|
pre-approving the audit services and non-audit services to be provided by our independent auditor before the auditor is engaged to render such services;
|
|
|
·
|
reviewing and discussing with the independent auditor its responsibilities under generally accepted auditing standards, the planned scope and timing of the independent auditor’s annual audit plan(s) and significant findings from the audit;
|
|
|
·
|
obtaining and reviewing a report from the independent auditor describing all relationships between the independent auditor and the Company consistent with the applicable PCAOB requirements regarding the independent auditor’s communications with the audit committee concerning independence;
|
|
|
·
|
confirming and evaluating the rotation of the audit partners on the audit engagement team as required by law;
|
|
|
·
|
reviewing with management and the independent auditor, in separate meetings whenever the Audit Committee deems appropriate, any analyses or other written communications prepared by the Management and/or the independent auditor setting forth significant financial reporting issues and judgments made in connection with the preparation of the financial statements, including analyses of the effects of alternative IFRS methods on the financial statements; and other critical accounting policies and practices of the Company;
|
|
|
·
|
reviewing, in conjunction with the Chief Executive Officer and Chief Financial Officer of the Company, the Company’s disclosure controls and procedures and internal control over financial reporting;
|
|
|
·
|
establishing procedures for the receipt, retention and treatment of complaints received by the Company regarding accounting, internal accounting controls or auditing matters, and the confidential, anonymous submission by employees of the Company of concerns regarding questionable accounting or auditing matters;
|
|
|
·
|
approving or ratifying any related person transaction (as defined in our related person transaction policy) in accordance with our related person transaction policy.
|
|
D.
|
|
|
·
|
each person, or group of affiliated persons, known by us to own beneficially 5% or more of our outstanding common shares;
|
|
|
·
|
each of our executive officers and directors; and
|
|
|
·
|
all executive officers and directors as a group.
|
|
Shares Beneficially Owned
|
||||||||
|
Shareholder
|
Number
|
Percent
|
||||||
|
5% Shareholders
|
||||||||
|
Sofinnova Venture
Partners VIII, L.P.(1)
|
5,818,175 | 20.1 | % | |||||
|
Sofinnova Capital VII FCPR(2)
|
5,384,450 | 18.6 | % | |||||
|
Entities affiliated with ZKB(3)
|
2,169,625 | 7.5 | % | |||||
|
Entities affiliated with Idinvest Partners(4)
|
2,065,233 | 7.1 | % | |||||
|
Clifton Park Capital Management, LLC(5)
|
1,666,667 | 5.7 | % | |||||
|
FMR LLC (6)
|
1,666,667 | 5.7 | % | |||||
|
Executive Officers and Directors
|
||||||||
|
Thomas Meyer, Ph.D.(11)
|
6,742,500 | 23.2 | % | |||||
|
Wolfgang Arnold, M.D.(11)
|
12,500 | * | ||||||
|
Alain Munoz, M.D.(7)(11)
|
12,500 | * | ||||||
|
James I. Healy, M.D., Ph.D.(8)
|
5,818,175 | 20.1 | % | |||||
|
Oliver Kubli(9)(11)
|
2,188,375 | 7.5 | % | |||||
|
Antoine Papiernik(10)
|
5,384,450 | 18.6 | % | |||||
|
Bettina Stubinski, M.D.
|
25,862 | * | ||||||
|
Sven Zimmermann, Ph.D.
|
29,019 | * | ||||||
|
*
|
Indicates beneficial ownership of less than 1% of the total outstanding common shares.
|
|
(1)
|
James I. Healy and the other managing members of Sofinnova Management VIII, L.L.C., which is the general partner of Sofinnova Venture Partners VIII, L.P., share the power to vote or dispose of these shares and therefore may be deemed to have voting and investment power with respect to such shares. Each of the managing members disclaims beneficial ownership of such shares except to the extent of his pecuniary interest
|
|
|
therein, if any. The address for Sofinnova Venture Partners VIII, L.P. and Sofinnova Management VIII, L.L.C. is 2800 Sand Hill Road, Suite 150, Menlo Park, California 94025, USA.
|
|
(2)
|
Consists of 5,384,450 common shares held by Sofinnova Capital VII FCPR. Sofinnova Partners SAS, a French corporation and the management company of Sofinnova Capital VII FCPR, may be deemed to have sole voting and investment power, and Denis Lucquin, Antoine Papiernik, Rafaele Tordjman and Monique Saulnier, the managing partners of Sofinnova Partners SAS, may be deemed to have shared voting and investment power with respect to such shares. All of the managing partners of Sofinnova Partners SAS disclaim beneficial ownership of such shares except to the extent of their pecuniary interest therein. The address for Sofinnova Capital VII FCPR is 16-18 Rue du Quatre Septembre, 75002 Paris, France.
|
|
(3)
|
Consists of 575,000 common shares held by Adamant Global Generika Funds, 418,750 common shares held by Adamant Global Biotech Funds and 238,375 shares held by Adamant Global Medtech Funds (collectively, the “AG Funds”) and 937,500 common shares held by ZKB Pharma Vision Funds. Voting and investment power over the shares is exercised by Balfidor Fondsleitung AG, Peter Merian-Strasse 47, 4002 Basel, Switzerland. The address for the AG Funds is c/o Adamant Biomedical Investments AG, Freischützgasse 3, 8004 Zürich, Switzerland. The address for ZKB Pharma Vision Funds is c/o Zurcher Kantonalbank, Bahnhofstrasse 9, 8001 Zurich, Switzerland.
|
|
(4)
|
Consists of 805,481 common shares held by Allianz Innovation 8 FCPI; 578,257 common shares held by Idinvest Croissance 2005 FCPI; 454,360 shares held by Allianz Innovation 7 FCPI and 227,135 shares held by La Banque Postale Innovation 3 FCPI. These entities are collectively referred to as the “Idinvest Funds.” Idinvest Partners is the investment management company to the Idinvest Funds. Christophe Baviere and Benoist Grossmann are respectively CEO and Managing Partner of Idinvest Partners and as such represent the interests of the Idinvest Funds over the common shares held by them. Each of Christophe Baviere and Benoist Grossmann disclaim beneficial ownership of all applicable shares except to the extent of any pecuniary interest therein. The address for each of the Idinvest Funds is c/o Idinvest Partners, 117, avenue des Champs Elysées, 75008 Paris, France.
|
|
(5)
|
Based on a Schedule 13G filed with the SEC on February 13, 2015 by FMR LLC. Members of the family of Edward C. Johnson 3d, Director and Chairman of FMR LLC, including Abigail P. Johnson, Vice Chairman, Chief Executive Officer and President of FMR LLC, are the predominant owners, directly or through trusts, of 49% of the voting power of FMR LLC. FMR LLC reports that members of the Johnson family may be deemed, under the Investment Company Act of 1940, to form a controlling group with respect to FMR LLC. FMR LLC reports that neither FMR LLC nor Edward C. Johnson 3d nor Abigail P. Johnson has the sole power to vote or direct the voting of the shares owned directly by the various investment companies registered under the Investment Company Act (“Fidelity Funds”) advised by Fidelity Management & Research Company (“Fidelity”), a wholly owned subsidiary of FMR LLC, which power resides with the Fidelity Funds’ Boards of Trustees. Fidelity carries out the voting of the shares under written guidelines established by the Fidelity Funds’ Boards of Trustees. FMR LLC reports that the shares it beneficially owns do not reflect securities, if any, beneficially owned by certain other companies whose beneficial ownership of securities is disaggregated from that of its subsidiaries, affiliates and other companies in accordance with Securities and Exchange Commission Release No. 34-39538 (January 12, 1998). The address of FMR LLC and Fidelity is 245 Summer Street, Boston, Massachusetts 02210.
|
|
(6)
|
Based on a Schedule 13G filed with the SEC on February 13, 2015 by Clifton Park Capital Management, LLC. The address of Clifton Park Capital Management, LLC is 2711 Centerville Road, Suite 400, Wilmington, Delaware 19808-1645.
|
|
(7)
|
Consists of 12,500 common shares held by Dr. Munoz.
|
|
(8)
|
Consists of 5,818,175 common shares held by Sofinnova Venture Partners VIII, L.P. Dr. Healy is a managing member of Sofinnova Management VIII, L.L.C., the general partner of Sofinnova Venture Partners VIII, L.P., and may be considered to have beneficial ownership of Sofinnova Venture Partners VIII, L.P.’s interest in us. Dr. Healy disclaims beneficial ownership of all shares held by Sofinnova Venture Partners VIII, L.P., except to the extent of his pecuniary interest therein.
|
|
(9)
|
Consists of 1,232,125 common shares held by the AG Funds and 937,500 common shares held by the ZKB Pharma Vision Funds. Mr. Kubli is a Senior Portfolio manager for the AG Funds and the ZKB Fonds Aktien Gesundheit. He disclaims beneficial ownership of such shares except to the extent of his pecuniary interest therein. Also consists of 18,750 common shares acquired by Mr. Kubli pursuant to the exercise of Plan A options.
|
|
(10)
|
Consists of 5,384,450 common shares held by Sofinnova Capital VII FCPR. Mr. Papiernik disclaims any beneficial ownership of the shares held by Sofinnova Capital VII FCPR except to the extent of his pecuniary interest therein.
|
|
(11)
|
Upon the closing of our initial public offering, all 181,000 options outstanding under our Stock Option Plan A vested and became immediately exercisable. Mr. Meyer, Dr. Arnold, Dr. Munoz and Mr. Kubli own 50,000, 18,750, 18,750 and 6,250 Stock Option Plan A options, respectively.
|
|
Name and Address of Beneficial Owner
|
C Shares
|
Purchase Price (CHF)
|
||||||
|
Sofinnova Venture Partners VIII, L.P (1)
|
3,693,175 | 5.28 | ||||||
|
Sofinnova Capital VII FCPR (2)
|
3,551,150 | 5.28 | ||||||
|
(1)
|
James I. Healy and the other managing members of Sofinnova Management VIII, L.L.C., which is the general partner of Sofinnova Venture Partners VIII, L.P., share the power to vote or dispose of these shares and therefore may be deemed to have voting and investment power with respect to such shares. Each of the
|
|
|
managing members disclaims beneficial ownership of such shares except to the extent of his pecuniary interest therein, if any.
|
|
(2)
|
Consists of 3,551,150 shares held by Sofinnova Capital VII FCPR. Sofinnova Partners SAS, a French corporation and the management company of Sofinnova Capital VII FCPR, may be deemed to have sole voting and investment power, and Denis Lucquin, Antoine Papiernik, Rafaele Tordjman and Monique Saulnier, the managing partners of Sofinnova Partners SAS, may be deemed to have shared voting and investment power with respect to such shares. All of the managing partners of Sofinnova Partners SAS disclaim beneficial ownership of such shares except to the extent of their pecuniary interest therein.
|
|
C.
|
|
High
|
Low
|
|||||||
|
Year Ended December 31, 2014:
|
||||||||
|
Third Quarter
|
7.23 | 5.37 | ||||||
|
Fourth Quarter
|
5.75 | 3.505 | ||||||
|
Month Ended:
|
||||||||
|
September 30, 2014
|
7.23 | 5.37 | ||||||
|
October 31, 2014
|
5.75 | 4.17 | ||||||
|
November 28, 2014
|
4.67 | 4.19 | ||||||
|
December 31, 2014
|
4.66 | 3.505 | ||||||
|
January 31, 2015
|
4.00 | 3.81 | ||||||
|
February 28, 2015
|
5.75 | 5.44 | ||||||
|
March, 2015 (through March 20, 2015)
|
6.70 | 5.67 | ||||||
|
E.
|
|
E.
|
|
|
·
|
certain financial institutions;
|
|
|
·
|
dealers or traders in securities who use a mark-to-market method of tax accounting;
|
|
|
·
|
persons holding common shares as part of a hedging transaction, straddle, wash sale, conversion transaction or integrated transaction or persons entering into a constructive sale with respect to the common shares;
|
|
|
·
|
persons whose functional currency for U.S. federal income tax purposes is not the U.S. dollar;
|
|
|
·
|
entities classified as partnerships for U.S. federal income tax purposes;
|
|
|
·
|
tax-exempt entities, including an “individual retirement account” or “Roth IRA”;
|
|
|
·
|
persons that own or are deemed to own ten percent or more of our voting stock;
|
|
|
·
|
persons who acquired our common shares pursuant to the exercise of an employee stock option or otherwise as compensation; or
|
|
|
·
|
persons holding shares in connection with a trade or business conducted outside of the United States.
|
|
|
·
|
an individual who is a citizen or resident of the United States;
|
|
|
·
|
a corporation, or other entity taxable as a corporation, created or organized in or under the laws of the United States, any state therein or the District of Columbia; or
|
|
|
·
|
an estate or trust the income of which is subject to U.S. federal income taxation regardless of its source.
|
|
A.
|
|
2014
|
2013
|
|||||||
|
Audit Fees
|
870 | 15 | ||||||
|
Total fees
|
870 | 15 | ||||||
|
|
1.1*
|
Articles of Association of Auris Medical Holding AG
|
|
|
2.1
|
Form of Registration Rights Agreement between Auris Medical Holding AG and the shareholders listed therein (incorporated by reference to exhibit 4.1 of the Auris Medical Holding AG registration statement on Form F-1 (Registration no. 333-197105) filed with the Commission on July 21, 2014)
|
|
|
4.1†
|
Collaboration and License Agreement, dated October 21, 2003, between Auris Medical AG and Xigen SA (incorporated by reference to exhibit 10.1 of the Auris Medical Holding AG registration statement on Form F-1 (Registration no. 333-197105) filed with the Commission on June 27, 2014)
|
|
|
4.2†
|
Co-Ownership and Exploitation Agreement, dated September 29, 2003, between Auris Medical AG and INSERM (incorporated by reference to exhibit 10.2 of the Auris Medical Holding AG registration statement on Form F-1 (Registration no. 333-197105) filed with the Commission on June 27, 2014)
|
|
|
4.3
|
Series C Investment Agreement, dated April 5, 2013 (incorporated by reference to exhibit 10.3 of the Auris Medical Holding AG registration statement on Form F-1 (Registration no. 333-197105) filed with the Commission on June 27, 2014)
|
|
|
4.4
|
Series C Shareholders’ Agreement, dated April 5, 2013 (incorporated by reference to exhibit 10.4 of the Auris Medical Holding AG registration statement on Form F-1 (Registration no. 333-197105) filed with the Commission on June 27, 2014)
|
|
|
4.5
|
Convertible Loan Agreement, dated December 2013, between Auris Medical AG and Sofinnova Venture Partners VIII, L.P. and Sofinnova Capital VII FCPR (incorporated by reference to exhibit 10.5 of the Auris Medical Holding AG registration statement on Form F-1 (Registration no. 333-197105) filed with the Commission on June 27, 2014)
|
|
|
4.6
|
Service Agreement, dated January 2011 between Auris Medical AG and Altamira Pharma GmbH (incorporated by reference to exhibit 10.6 of the Auris Medical Holding AG registration statement on Form F-1 (Registration no. 333-197105) filed with the Commission on June 27, 2014)
|
|
|
4.7
|
Termination of Service Agreement, dated February 2014 between Auris Medical AG and Altamira Pharma GmbH (incorporated by reference to exhibit 10.7 of the Auris Medical Holding AG registration statement on Form F-1 (Registration no. 333-197105) filed with the Commission on June 27, 2014)
|
|
|
4.8
|
Loan Agreement, dated January 2013 between Auris Medical AG and Altamira Pharma GmbH (incorporated by reference to exhibit 10.8 of the Auris Medical Holding AG registration statement on Form F-1 (Registration no. 333-197105) filed with the Commission on June 27, 2014)
|
|
|
4.9
|
Form of Indemnification Agreement (incorporated by reference to exhibit 10.9 of the Auris Medical Holding AG registration statement on Form F-1 (Registration no. 333-197105) filed with the Commission on July 21, 2014)
|
|
|
4.10
|
English language translation of Lease Agreement between Auris Medical AG and Privera AG (incorporated by reference to exhibit 10.10 of the Auris Medical Holding AG registration statement on Form F-1 (Registration no. 333-197105) filed with the Commission on June 27, 2014)
|
|
|
4.11
|
Stock Option Plan A (incorporated by reference to exhibit 10.11 of the Auris Medical Holding AG registration statement on Form F-1 (Registration no. 333-197105) filed with the Commission on June 27, 2014)
|
|
|
4.12
|
Stock Option Plan C (incorporated by reference to exhibit 10.12 of the Auris Medical Holding AG registration statement on Form F-1 (Registration no. 333-197105) filed with the Commission on June 27, 2014)
|
|
|
8.1
|
List of subsidiaries (incorporated by reference to exhibit 21.1 of the Auris Medical Holding AG registration statement on Form F-1 (Registration no. 333-197105) filed with the Commission on June 27, 2014)
|
|
|
12.1*
|
Certification of Thomas Meyer pursuant to 17 CFR 240.13a-14(a)
|
|
|
12.2*
|
Certification of Sven Zimmermann pursuant to 17 CFR 240.13a-14(a).
|
|
|
13.1*
|
Certification of Thomas Meyer pursuant to 17 CFR 240.13a-14(b) and 18 U.S.C.1350
|
|
|
13.2*
|
Certification of Sven Zimmermann pursuant to 17 CFR 240.13a-14(b) and 18 U.S.C.1350
|
|
|
15.1*
|
Consent of
Deloitte AG
|
|
|
15.2*
|
Consent of KPMG AG
|
|
*
|
Filed herewith
|
|
†
|
Confidential treatment requested as to portions of the exhibit. Confidential materials omitted and filed separately with the Securities and Exchange Commission.
|
|
AURIS MEDICAL HOLDING AG
|
|||
|
By:
|
/s/ Thomas Meyer
|
||
|
Name:
|
Thomas Meyer
|
||
|
Title:
|
Chief Executive Officer
|
||
|
/s/ James D. Horiguchi
|
/s/ Matthias Gschwend
|
|
Partner
|
Director
|
|
Auditor in Charge
|
|
/s/ Martin Rohrbach
|
/s/ Charles Errico
|
|
|
Martin Rohrbach
|
Charles Errico
|
|
Note
|
2014
|
2013
|
2012
|
|||||||||||||
|
Research and development
|
17 | -17,704,461 | -13,253,638 | -3,986,691 | ||||||||||||
|
General and administrative
|
18 | -4,489,051 | -1,362,211 | -623,812 | ||||||||||||
|
Operating loss
|
-22,193,512 | -14,615,849 | -4,610,503 | |||||||||||||
|
Finance expense
|
20 | -207,825 | -158,641 | -1,800 | ||||||||||||
|
Finance income
|
20 | 4,216,322 | 75,747 | 9,894 | ||||||||||||
|
Loss before tax
|
-18,185,015 | -14,698,743 | -4,602,409 | |||||||||||||
|
Income tax expense
|
21 | – | -305,750 | – | ||||||||||||
|
Net loss attributable to owners of the Company
|
-18,185,015 | -15,004,493 | -4,602,409 | |||||||||||||
|
Other comprehensive loss:
|
||||||||||||||||
|
Items that will never be reclassified to profit or loss
*
|
||||||||||||||||
|
Remeasurements of defined benefit liability, net of taxes of CHF 0
|
19 | -1,101,468 | -57,716 | -54,577 | ||||||||||||
|
Items that are or may be reclassified to profit or loss
|
||||||||||||||||
|
Foreign currency translation differences, net of taxes of CHF 0
|
-105,104 | 31,720 | 22,275 | |||||||||||||
|
Other comprehensive loss, net of taxes of CHF 0
*
|
-1,206,572 | -25,996 | -32,302 | |||||||||||||
|
Total comprehensive loss attributable to owners of the Company
|
-19,391,587 | -15,030,489 | -4,634,711 | |||||||||||||
|
Basic and diluted loss per share
|
22 | -0.66 | -1.01 | -0.40 | ||||||||||||
|
*
|
the net effect of taxes was CHF 0 for 2013 and 2012
|
|
Note
|
December 31,
2014
|
December 31,
2013
|
||||||||
|
ASSETS
|
||||||||||
|
Non-current assets
|
||||||||||
|
Property and equipment
|
7 | 235,427 | 195,915 | |||||||
|
Intangible assets
|
8 | 1,482,520 | 1,482,520 | |||||||
|
Deferred Tax Asset
|
21 | 32,761 | – | |||||||
|
Total non-current assets
|
1,750,708 | 1,678,435 | ||||||||
|
Current assets
|
||||||||||
|
Other receivables
|
9 | 542,538 | 524,786 | |||||||
|
Prepayments
|
10 | 265,170 | 183,137 | |||||||
|
Cash and cash equivalents
|
11 | 56,934,325 | 23,865,842 | |||||||
|
Total current assets
|
57,742,033 | 24,573,765 | ||||||||
|
Total assets
|
59,492,741 | 26,252,200 | ||||||||
|
EQUITY AND LIABILITIES
|
||||||||||
|
Equity
|
||||||||||
|
Share capital
|
12 | 11,604,156 | 6,487,130 | |||||||
|
Share premium
|
93,861,171 | 35,608,210 | ||||||||
|
Foreign currency translation reserve
|
-51,108 | 53,995 | ||||||||
|
Accumulated deficit
|
-52,131,426
|
-3,115,689 | ||||||||
|
Total shareholders’ equity attributable to owners of the Company
|
53,282,793 | 9,033,646 | ||||||||
|
Non-current liabilities
|
||||||||||
|
Employee benefits
|
19 | 1,410,598 | 328,342 | |||||||
|
Deferred tax liabilities
|
21 | 360,398 | 327,637 | |||||||
|
Total non-current liabilities
|
1,770,996 | 655,979 | ||||||||
|
Current liabilities
|
||||||||||
|
Convertible loans
|
14 | – | 13,711,200 | |||||||
|
Trade and other payables
|
15 | 3,234,384 | 954,257 | |||||||
|
Accrued expenses
|
16 | 1,204,568 | 1,897,118 | |||||||
|
Total current liabilities
|
4,438,952 | 16,562,575 | ||||||||
|
Total liabilities
|
6,209,948 | 17,218,554 | ||||||||
|
Total equity and liabilities
|
59,492,741 | 26,252,200 | ||||||||
|
Attributable to Owners of the Company
|
||||||||||||||||||||||||||
|
Note
|
Share Capital
|
Share Premium
|
Treasury Shares
|
Foreign Currency Translation Reserve
|
Accumulated Deficit
|
Total Equity
|
||||||||||||||||||||
|
As of January 1, 2012
|
4,632,580 | 10,006,179 | -526,320 | – | -13,639,857 | 472,582 | ||||||||||||||||||||
|
Total comprehensive loss
|
||||||||||||||||||||||||||
|
Net loss
|
– | – | – | – | -4,602,409 | -4,602,409 | ||||||||||||||||||||
|
Other comprehensive income (loss)
|
– | – | – | 22,275 | -54,577 | -32,302 | ||||||||||||||||||||
|
Total comprehensive loss
|
– | – | – | 22,275 | -4,656,986 | -4,634,711 | ||||||||||||||||||||
|
Transactions with owners of the Company
|
||||||||||||||||||||||||||
|
Additional paid in capital
|
– | 3,335,763 | – | – | – | 3,335,763 | ||||||||||||||||||||
|
Share based payments
|
– | – | – | – | 56,013 | 56,013 | ||||||||||||||||||||
|
Treasury shares sold
|
– | – | 526,320 | – | – | 526,320 | ||||||||||||||||||||
|
Balance at December 31, 2012
|
4,632,580 | 13,341,942 | – | 22,275 | -18,240,831 | -244,034 | ||||||||||||||||||||
|
As of January 1, 2013
|
4,632,580 | 13,341,942 | – | 22,275 | -18,240,831 | -244,034 | ||||||||||||||||||||
|
Total comprehensive loss
|
– | |||||||||||||||||||||||||
|
Net loss
|
– | – | – | – | -15,004,493 | -15,004,493 | ||||||||||||||||||||
|
Other comprehensive income (loss)
|
– | – | – | 31,720 | -57,716 | -25,996 | ||||||||||||||||||||
|
Total comprehensive loss
|
– | – | – | 31,720 | -15,062,209 | -15,030,489 | ||||||||||||||||||||
|
Transactions with owners of the Company
|
||||||||||||||||||||||||||
|
Issue of ordinary shares
|
1,854,550 | 22,625,510 | – | – | – | 24,480,060 | ||||||||||||||||||||
|
Share issuance costs
|
– | -359,242 | – | – | – | -359,242 | ||||||||||||||||||||
|
Convertible loans - equity component
|
14 | – | – | – | – | 99,038 | 99,038 | |||||||||||||||||||
|
Convertible loans - deferred tax
|
14 | – | – | – | – | -21,886 | -21,886 | |||||||||||||||||||
|
Share based payments
|
13 | 110,198 | 110,198 | |||||||||||||||||||||||
|
Balance at December 31, 2013
|
6,487,130 | 35,608,210 | – | 53,995 | -33,115,689 | 9,033,646 | ||||||||||||||||||||
|
As of January 1, 2014
|
6,487,130 | 35,608,210 | – | 53,995 | -33,115,689 | 9,033,646 | ||||||||||||||||||||
|
Total comprehensive loss
|
||||||||||||||||||||||||||
|
Net loss
|
– | – | – | – | -18,185,015 | -18,185,015 | ||||||||||||||||||||
|
Other comprehensive loss
|
– | – | – | -105,104 | -1,101,468 | -1,206,572 | ||||||||||||||||||||
|
Total comprehensive loss
|
– | – | – | -105,104 | -19,286,483 | -19,391,587 | ||||||||||||||||||||
|
Transactions with owners of the Company
|
||||||||||||||||||||||||||
|
Issue of ordinary shares associated with Initial Public Offering (“IPO”)
|
12 | 4,045,294 | 47,261,446 | – | – | – | 51,306,740 | |||||||||||||||||||
|
Issuance costs associated with IPO
|
12 | – | -1,815,056 | – | – | – | -1,815,056 | |||||||||||||||||||
|
Conversion of convertible loan
|
14 | 1,043,180 | 12,717,655 | – | – | – | 13,760,835 | |||||||||||||||||||
|
Share issuance costs
|
– |
-136,697
|
– | – | – | -136,697 | ||||||||||||||||||||
|
Share based payments
|
13 | – | – | – | – | 270,747 | 270,747 | |||||||||||||||||||
|
Share options exercised
|
13 | 28,552 | 225,613 | – | – | – | 254,165 | |||||||||||||||||||
|
Balance at December 31, 2014
|
11,604,156 | 93,861,171 | – | -51,109 | -52,131,426 | 53,282,793 | ||||||||||||||||||||
|
Note
|
2014
|
2013
|
2012
|
|||||||||||
|
Cash flows from operating activities
|
||||||||||||||
|
Net loss
|
-18,185,015 | -15,004,493 | -4,602,409 | |||||||||||
|
Adjustments for:
|
||||||||||||||
|
Depreciation
|
17,18 | 73,984 | 37,517 | 9,242 | ||||||||||
|
Unrealized Net foreign currency exchange (gains) loss
|
-4,066,452 | 32,076 | 20,920 | |||||||||||
|
Net interest income
|
20 | -2,498 | -23,859 | -5,825 | ||||||||||
|
Share option costs
|
13 | 270,747 | 110,198 | 56,013 | ||||||||||
|
Employee benefits
|
-19,211 | 27,980 | 9,545 | |||||||||||
|
Income tax expense
|
21 | – | 305,750 | 0 | ||||||||||
| -21,928,445 | -14,514,831 | -4,512,514 | ||||||||||||
|
Changes in:
|
||||||||||||||
|
Other receivables
|
-17,634 | -288,765 | -125,782 | |||||||||||
|
Prepayments
|
-82,033 | -98,812 | -15,659 | |||||||||||
|
Trade and other payables
|
2,279,626 | 530,080 | 254,063 | |||||||||||
|
Accrued expenses
|
432,449 | 328,719 | -98,575 | |||||||||||
|
Cash used in operating activities
|
-19,316,037 | -14,043,609 | -4,498,467 | |||||||||||
|
Cash flows from investing activities
|
||||||||||||||
|
Purchase of property and equipment
|
7 | -113,496 | -108,936 | -131,751 | ||||||||||
|
Purchase of intangibles
|
-1,125,000 | – | – | |||||||||||
|
Sale of financial asset
|
– | – | 72,850 | |||||||||||
|
Interest received
|
20 | 52,133 | 74,036 | 5,825 | ||||||||||
|
Net cash used in investing activities
|
-1,186,363 | -34,900 | -53,076 | |||||||||||
|
Cash flows from financing activities
|
||||||||||||||
|
Proceeds from share capital increase
|
13 | – | 24,120,818 | 3,335,762 | ||||||||||
|
Proceeds from exercise of options
|
13 | 254,165 | – | – | ||||||||||
|
Share issuance costs
|
-136,697 | – | – | |||||||||||
|
Proceeds from issue of convertible loans
|
14 | – | 13,769,976 | – | ||||||||||
|
Proceeds from IPO, net (net of underwriting fees and IPO costs)
|
12 | 50,037,847 | – | – | ||||||||||
|
Share issuance costs IPO
|
12 | -546,163 | – | – | ||||||||||
|
Sale of treasury shares
|
– | – | 526,320 | |||||||||||
|
Interest paid
|
20 | – | -9,915 | – | ||||||||||
|
Net cash from financing activities
|
49,609,152 | 37,880,879 | 3,862,082 | |||||||||||
|
Net increase in cash and cash equivalents
|
29,106,752 | 23,802,370 | -689,461 | |||||||||||
|
Cash and cash equivalents at beginning of the period
|
23,865,842 | 63,967 | 752,874 | |||||||||||
|
Net effect of currency translation on cash
|
3,961,731 | -495 | 554 | |||||||||||
|
Cash and cash equivalents at end of the period
|
56,934,325 | 23,865,842 | 63,967 | |||||||||||
|
·
|
Auris Medical AG, Basel, Switzerland (100%)
|
|
·
|
Otolanum AG, Zug, Switzerland (100%)
|
|
·
|
Auris Medical Inc., Chicago, United States (100%)
|
|
·
|
Auris Medical Ltd., Dublin, Ireland (100%)
|
|
Currency
|
Geographical area
|
Reporting
entities
|
December 31,
2014
|
December 31,
2013
|
December 31,
2012
|
|||||||||||||||
|
CHF
|
Swiss Franc
|
Switzerland
|
3/1 | * | 1.0000 | 1.0000 | 1.0000 | |||||||||||||
|
USD
|
Dollar
|
United States
|
1 | 0.9895 | 0.8894 | 0.9154 | ||||||||||||||
|
EUR
|
Euro
|
Europe
|
1 | 1.2027 | 1.2255 | 1.2068 | ||||||||||||||
|
*
There were three operating entities in 2014 and one operating entity in each of 2013 and 2012.
|
|
Currency
|
Geographical area
|
Reporting
entities
|
2014
|
2013
|
2012
|
|||||||||||||||
|
CHF
|
Swiss Franc
|
Switzerland
|
3/1 | * | 1.0000 | 1.0000 | 1.0000 | |||||||||||||
|
USD
|
Dollar
|
United States
|
1 | * | 0.9150 | 0.9391 | 0.9481 | |||||||||||||
|
EUR
|
Euro
|
Europe
|
1 | * | 1.2144 | 1.2414 | 1.2196 | |||||||||||||
|
*
There were three operating entities in 2014 and one operating entity in each of 2013 and 2012.
|
|
Production equipment
|
5 years
|
|
Office furniture and electronic data processing equipment (“EDP”)
|
3 years
|
|
Leasehold improvements
|
5 years
|
|
·
|
default or delinquency by a debtor;
|
|
·
|
indications that a debtor or issuer will enter bankruptcy;
|
|
·
|
adverse changes in the payment status of borrowers or issuers;
|
|
·
|
the disappearance of an active market for a security; or
|
|
·
|
observable data indicating that there is measurable decrease in expected cash flows from a group of financial assets.
|
|
·
|
temporary differences on the initial recognition of assets or liabilities in a transaction that is not a business combination and that affects neither accounting nor taxable profit or loss;
|
|
·
|
temporary differences related to investments in subsidiaries to the extent that the Group is able to control the timing of the reversal of the temporary differences and it is probable that they will not reverse in the foreseeable future; and
|
|
·
|
taxable temporary differences arising on the initial recognition of goodwill.
|
|
Standard/Interpretation
|
Impact
|
Effective date
|
Planned
application by the Group
|
|||||
|
New standards, interpretations or amendments
|
||||||||
|
IAS 19
|
Employee Contributions (Amendments)
|
1)
|
July 1, 2014
|
Reporting year 2015
|
||||
|
Various
|
Annual Improvements to IFRSs 2010-2012 Cycle
|
1)
|
July 1, 2014
|
Reporting year 2015
|
||||
|
Various
|
Annual Improvements to IFRSs 2011-2013 Cycle
|
1)
|
July 1, 2014
|
Reporting year 2015
|
||||
|
IFRS 9
|
Financial Instruments
|
2)
|
January 1, 2016
|
To be determined
|
||||
|
IFRS 14
|
Regulatory Deferral Accounts
|
2)
|
January 1, 2016
|
To be determined
|
||||
|
IFRS 15
|
Revenue from contract with customers
|
2)
|
January 1, 2017
|
To be determined
|
||||
|
IFRS 11 amendment
|
Joint Arrangement
|
2)
|
January 1, 2016
|
To be determined
|
||||
|
IAS 16 & 38
|
Property Plant and Equipment, Intangible Assets
|
2)
|
January 1, 2016
|
To be determined
|
||||
|
IAS 16 & 41 amendments
|
Property Plant and Equipment, Agriculture
|
2)
|
January 1, 2016
|
To be determined
|
||||
|
IAS 27 amendments
|
Consolidated and Separate Financial Statements
|
2)
|
January 1, 2016
|
To be determined
|
||||
|
Various
|
Annual Improvements to IFRSs:2012-2014 Cycle
|
2)
|
January 1, 2016
|
To be determined
|
||||
|
IFRS 10,12, & 28 amendments
|
Consolidated Financial Statements, Disclosure of Interests in Other Entities
|
2)
|
January 1, 2016
|
To be determined
|
||||
|
IAS 1 amendments
|
Presentation of Financial Statements
|
2)
|
January 1, 2016
|
To be determined
|
||||
|
1)
|
No or no significant impacts are expected on the consolidated financial statements of the Group.
|
|
2)
|
The impact on the consolidated financial statements of Group cannot yet be determined with sufficient reliability.
|
|
December 31,
2014
|
December 31,
2013
|
|||||||
|
Financial assets
|
||||||||
|
Available for sale
|
||||||||
|
Current financial assets
|
— | — | ||||||
|
Loans and receivables
|
||||||||
|
Cash and cash equivalents
|
56,934,325 | 23,865,842 | ||||||
|
Other receivables
|
451,355 | 451,206 | ||||||
|
Total financial assets
|
57,385,680 | 24,317,048 | ||||||
|
Financial liabilities
|
||||||||
|
At amortized cost
|
||||||||
|
Convertible loans from shareholders
|
— | 13,711,200 | ||||||
|
Trade and other accounts payable
|
3,234,383 | 954,257 | ||||||
|
Accrued expenses
|
1,162,988 | 1,871,598 | ||||||
|
Total financial liabilities
|
4,397,371 | 16,537,055 | ||||||
|
Carrying
amount
|
Less than 3
months
|
Between 3
months and
2 years
|
2 years
and later
|
Total
|
||||||||||||||||
|
December 31, 2014
|
||||||||||||||||||||
|
Convertible loans from shareholders
|
— | — | — | — | — | |||||||||||||||
|
Trade and other accounts payable
|
3,234,383 | 3,234,383 | — | — | 3,234,383 | |||||||||||||||
|
Accrued expenses
|
1,162,988 | 1,162,988 | — | — | 1,162,988 | |||||||||||||||
|
Total
|
4,397,371 | 4,397,371 | — | — | 4,397,371 | |||||||||||||||
|
Carrying
amount
|
Less than 3
months
|
Between 3
months and
2 years
|
2 years
and later
|
Total
|
||||||||||||||||
|
December 31, 2013
|
||||||||||||||||||||
|
Convertible loans from shareholders
|
13,711,200 | 13,769,976 | — | — | 13,769,976 | |||||||||||||||
|
Trade and other accounts payable
|
954,257 | 954,257 | — | — | 954,257 | |||||||||||||||
|
Accrued expenses
|
1,871,598 | 1,871,598 | — | — | 1,871,598 | |||||||||||||||
|
Total
|
16,537,055 | 16,595,831 | — | — | 16,595,831 | |||||||||||||||
|
December 31,
2014
|
December 31,
2013
|
|||||||
|
Financial assets
|
||||||||
|
Cash and cash equivalents
|
56,934,325 | 23,865,842 | ||||||
|
Other receivables
|
451,355 | 451,206 | ||||||
|
Total
|
57,385,680 | 24,317,048 | ||||||
|
2014
|
2013
|
|||||||||||||||
|
in CHF
|
USD
|
EUR
|
USD
|
EUR
|
||||||||||||
|
Prepayments
|
183,779 | 11,489 | 11,321 | 112,133 | ||||||||||||
|
Other receivables
|
145,636 | 267,533 | 145,636 | 273,438 | ||||||||||||
|
Cash and cash equivalents
|
46,433,371 | 1,949,684 | 4,267,768 | 2,309,318 | ||||||||||||
|
Trade and other accounts payable
|
-1,686,733 | -1,238,171 | -334,896 | -502,645 | ||||||||||||
|
Accrued expenses
|
-354,397 | -590,001 | -219,304 | -435,046 | ||||||||||||
|
Net
statement of financial position exposure - asset/(liability)
|
44,721,656
|
400,534 | 3,870,525 | 1,757,198 | ||||||||||||
|
December 31,
2014
|
December 31,
2013
|
|||||||
|
Switzerland
|
1,750,708
|
1,678,435 | ||||||
|
Total
|
1,750,708
|
1,678,435 |
|
Production
equipment
|
Office
furniture
and EDP
|
Leasehold
improvements
|
Total
|
|||||||||||||
|
At cost
|
||||||||||||||||
|
As at January 1, 2013
|
123,330 | 83,661 | — | 206,991 | ||||||||||||
|
Additions
|
43,420 | 48,384 | 17,132 | 108,936 | ||||||||||||
|
As at December 31, 2013
|
166,750 | 132,045 | 17,132 | 315,927 | ||||||||||||
|
Additions
|
63,499 | 49,997 | — | 113,496 | ||||||||||||
|
As at December 31, 2014
|
230,249 | 182,042 | 17,132 | 429,423 | ||||||||||||
|
Accumulated depreciation
|
||||||||||||||||
|
As at January 1, 2013
|
-5,787 | -76,708 | — | -82,495 | ||||||||||||
|
Charge for the year
|
- 25,575 | - 9,580 | - 2,362 | - 37,517 | ||||||||||||
|
As at December 31, 2013
|
-31,362 | -86,288 | -2,362 | -120,012 | ||||||||||||
|
Charge for the year
|
- 42,230 | - 28,251 | - 3,503 |
-73,984
|
||||||||||||
|
As at December 31, 2014
|
-73,592 | -114,539 | -5,865 |
-193,996
|
||||||||||||
|
Net book value
|
||||||||||||||||
|
As at December 31, 2013
|
135,388 | 45,757 | 14,770 | 195,915 | ||||||||||||
|
As at December 31, 2014
|
156,657 | 67,502 | 11,267 |
235,427
|
||||||||||||
|
Licenses
|
||||
|
At cost
|
||||
|
As at January 1, 2013
|
357,520 | |||
|
Additions
|
1,125,000 | |||
|
As at December 31, 2013
|
1,482,520 | |||
|
As at December 31, 2014
|
1,482,520 | |||
|
Accumulated amortization and impairment losses
|
||||
|
As at December 31, 2013
|
— | |||
|
As at December 31, 2014
|
— | |||
|
Net book value
|
||||
|
As at December 31, 2013
|
1,482,520 | |||
|
As at December 31, 2014
|
1,482,520 | |||
|
December 31,
2014
|
December 31,
2013
|
|||||||
|
Advance payments to suppliers
|
413,169 | 413,169 | ||||||
|
Value added tax receivable
|
74,065 | 47,714 | ||||||
|
Withholding tax receivable
|
17,118 | 25,866 | ||||||
|
Deposit for rent
|
38,066 | 38,037 | ||||||
|
Other
|
120 | — | ||||||
|
Total other receivables
|
542,538 | 524,786 | ||||||
|
December 31,
2014
|
December 31,
2013
|
|||||||
|
Clinical projects and related activities
|
85,299 | 114,076 | ||||||
|
Insurance
|
179,871 | 4,323 | ||||||
|
Social charges
|
— | 22,450 | ||||||
|
Capital taxes
|
— | 32,910 | ||||||
|
Other
|
— | 9,378 | ||||||
|
Total prepayments
|
265,170 | 183,137 | ||||||
|
December 31,
2014
|
December 31,
2013
|
|||||||
|
Cash in bank accounts
|
56,932,993 | 23,865,165 | ||||||
|
Petty cash
|
1,332 | 677 | ||||||
|
Total cash and cash equivalents
|
56,934,325 | 23,865,842 | ||||||
|
December 31, 2014
|
December 31, 2013
|
|||||||||||||||
|
Number
|
CHF
|
Number
|
CHF
|
|||||||||||||
|
Common shares with a nominal value of CHF 0.40 each
|
29,010,391 | 11,604,156 | 72,600 | 29,040 | ||||||||||||
|
Preferred shares Series A with a nominal value of CHF 0.40 each
|
— | — | 5,999,750 | 2,399,900 | ||||||||||||
|
Preferred shares Series B with a nominal value of CHF 0.40 each
|
— | — | 5,509,100 | 2,203,640 | ||||||||||||
|
Preferred shares Series C with a nominal value of CHF 0.40 each
|
— | — | 4,636,375 | 1,854,550 | ||||||||||||
|
Total
|
29,010,391 | 11,604,156 | 16,217,825 | 6,487,130 | ||||||||||||
|
Common Shares (Number)
|
Preferred Shares (Number)
|
|||||||||||||||
|
2014
|
2013
|
2014
|
2013
|
|||||||||||||
|
As of January 1
|
72,600 | 72,600 | 16,145,225 | 11,508,850 | ||||||||||||
|
Common share stock options with a nominal value of CHF 0.40 each
|
71,381 | — | — | — | ||||||||||||
|
Shares issued for cash
|
— | — | — | 4,636,375 | ||||||||||||
|
Preferred shares C issued for conversion of convertible loans with a nominal value of CHF 0.40 each
|
— | — | 2,607,950 | — | ||||||||||||
|
Common share issued for the IPO with a nominal value of CHF 0.40 each cash
|
10,113,235 | — | — | — | ||||||||||||
|
Common share issued with a nominal value of CHF 0.40 each resulting from conversion of preference shares at the IPO
|
18,753,175 | — | -18,753,175 | — | ||||||||||||
|
Total, as at December 31
|
29,010,391 | 72,600 | — | 16,145,225 | ||||||||||||
| Plan |
Number of
instruments awarded (1)
|
Vesting conditions
|
Contractual
life of
options
|
||||
|
Stock option plan A
|
236,500 |
3 years’ service from grant date
|
5 years
|
||||
|
Stock
option plan B
|
93,231 |
Immediately
|
3 months
|
||||
|
Stock option plan C
|
173,750 |
4 years’ service from grant date
|
6 years
|
||||
|
Equity Incentive Plan
|
99,260 |
2 years’ service from grant date (50%),
3 years’ service from grant date (50%)
|
8 years
|
||||
|
(1)
|
Number of instruments adjusted for the increase in the number of issued shares resulting from the 25:1 stock split in December 2013.
|
|
Stock Option Plan
|
|||||||
|
Equity Incentive Plan2014
|
Plan C 2014
(CHF)
|
Plan C 2013
(CHF)
|
Plan A 2013
(CHF)
|
||||
|
Fair value at grant date
|
USD 1.572 (2 years vesting); USD 1.902 (3 year vesting)
|
3.03
|
3.03
|
2.43
|
|||
|
Share price at grant date
|
USD 3.92
|
5.28
|
5.28
|
4.80
|
|||
|
Exercise price
|
USD 4.05
|
5.28
|
5.28
|
4.80
|
|||
|
Expected volatility
|
74%
|
74%
|
78%
|
78%
|
|||
|
Expected life
|
2 and 3 years
|
4 years
|
4 years
|
3 years
|
|||
|
Expected dividends
|
—
|
—
|
—
|
—
|
|||
|
Risk-free interest rate
|
2.22%
|
0.96%
|
1.0%
|
1.0%
|
|||
|
2014
|
2013
|
2012
|
||||||||||||||||||||||||||||||||||
|
Number of
options
|
Weighted
average
exercise
price
|
Weighted
average
remaining term
|
Number of
options
|
Weighted
average
exercise
price
|
Weighted
average
remaining term
|
Number of
options
|
Weighted
average
exercise
price
|
Weighted
average
remaining term
|
||||||||||||||||||||||||||||
|
Outstanding at January 1
|
272,750 | 4.32 |
3.64
|
146,500 | 3.66 | 3.29 | 109,000 | 3.20 | 3.70 | |||||||||||||||||||||||||||
|
Forfeited during the year
|
— | — | — | — | — | — | -10,000 |
4.00
|
— | |||||||||||||||||||||||||||
|
Exercised during the year
|
50,500 | 3.20 | — | — | — | — | — | — | ||||||||||||||||||||||||||||
|
Granted during the year
|
196,760 | 4.64 | 6.71 | 126,250 | 5.09 | 5.24 | 47,500 | 4.80 | 4.77 | |||||||||||||||||||||||||||
|
Outstanding at December 31
|
419,010 | 4.61 | 4.86 | 272,750 | 4.32 | 3.64 | 146,500 | 3.66 | 3.29 | |||||||||||||||||||||||||||
|
Exercisable at December 31
|
146,000 | 4.21 | 2.33 | 69,000 | 3.20 | 1.12 | 38,000 | 3.20 | 1.60 | |||||||||||||||||||||||||||
|
December 31,
2013
|
||||
|
Nominal value of the convertible loans
|
13,769,976 | |||
|
Difference to fair value, recognized in equity
|
-99,038 | |||
|
Fair value on initial recognition
|
13,670,938 | |||
|
Imputed interest expense
|
40,262 | |||
|
Convertible loans at December 31
|
13,711,200 | |||
|
December 31,
2014
|
||||
|
Convertible loans balance at January 1, 2014
|
13,711,200 | |||
|
Loss on de-recognition
|
9,141 | |||
|
Imputed interest expense
|
49,635 | |||
|
De-recognition of liability at conversion into equity
|
13,769,976 | |||
|
Convertible loans at December 31
|
— |
|
December 31,
2014
|
December 31,
2013
|
|||||||
|
Trade accounts payable - third parties
|
3,141,194 | 946,215 | ||||||
|
Other
|
93,190 | 8,042 | ||||||
|
Total trade and other payables
|
3,234,384 | 954,257 | ||||||
|
December 31,
2014
|
December 31,
2013
|
|||||||
|
Accrued research and development costs including milestone payments
|
949,561 | 1,791,638 | ||||||
|
Professional fees
|
178,000 | 57,950 | ||||||
|
Accrued vacation & overtime
|
41,580 | 25,520 | ||||||
|
Board of Directors fees
|
32,056 | 9,836 | ||||||
|
Social security
|
— | 10,700 | ||||||
|
Other
|
3,371 | 1,474 | ||||||
|
Total accrued expenses
|
1,204,568 | 1,897,118 | ||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Pre-clinical projects
|
1,160,058 | 2,078,407 | 297,960 | |||||||||
|
Clinical projects
|
12,141,571 | 8,753,398 | 1,687,368 | |||||||||
|
Drug manufacturing and substance
|
1,383,581
|
1,036,152 | 915,368 | |||||||||
|
Employee benefits
|
1,718,212 | 1,074,398 | 770,124 | |||||||||
|
Lease expenses
|
68,082 | 74,065 | 35,556 | |||||||||
|
Patents and trademarks
|
665,023 | 100,702 | 53,473 | |||||||||
|
Regulatory projects
|
519,104 | 106,325 | 219,106 | |||||||||
|
Depreciation tangible assets
|
48,830
|
30,191 | 7,736 | |||||||||
|
Total research and development expense
|
17,704,461 | 13,253,638 | 3,986,691 | |||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Employee benefits
|
1,136,677 | 195,739 | 116,251 | |||||||||
|
Business development
|
237,720 | 479,027 | 86,231 | |||||||||
|
Travel expenses
|
169,602 | 77,616 | 9,551 | |||||||||
|
Administration costs
|
2,014,178
|
556,445 | 375,155 | |||||||||
|
IPO costs, expensed
|
822,367 | — | — | |||||||||
|
Lease expenses
|
35,072 | 3,968 | 2,340 | |||||||||
|
Depreciation tangible assets
|
25,153 | 7,326 | 1,506 | |||||||||
|
Capital tax expenses
|
48,281 | 42,090 | 32,778 | |||||||||
|
Total general and administrative expenses
|
4,489,051 | 1,362,211 | 623,812 | |||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Salaries
|
2,259,112 | 836,686 | 711,883 | |||||||||
|
Pension costs
|
118,755 | 78,917 | 50,046 | |||||||||
|
Other social benefits
|
131,939 | 71,878 | 39,921 | |||||||||
|
Share option costs
|
270,748 | 110,198 | 56,013 | |||||||||
|
Other
|
74,334 | 172,458 | 28,512 | |||||||||
|
Total employee benefits
|
2,854,888 | 1,270,137 | 886,375 | |||||||||
|
2014
|
2013
|
|||||||
|
Defined benefit obligation at January 1
|
1,626,241 | 1,030,145 | ||||||
|
Service cost
|
111,513 | 72,803 | ||||||
|
Plan participants’ contribution
|
137,966 | 50,937 | ||||||
|
Interest cost
|
52,097 | 23,133 | ||||||
|
Actuarial losses
|
1,484,222 | 136,933 | ||||||
|
Benefits paid
|
-539,920 | -107,303 | ||||||
|
Transfer-in amounts of new employees
|
2,023,548 | 419,593 | ||||||
|
Defined benefit obligation at December 31
|
4,895,667 | 1,626,241 | ||||||
|
2014
|
2013
|
|||||||
|
Fair value of plan assets at January 1
|
1,297,899 | 787,499 | ||||||
|
Interest income
|
47,909 | 19,394 | ||||||
|
Return on plan assets excluding interest income
|
382,755 | 79,217 | ||||||
|
Employer contributions
|
137,966 | 50,937 | ||||||
|
Plan participants’ contributions
|
137,966 | 50,937 | ||||||
|
Benefits paid
|
-539,920 | -107,303 | ||||||
|
Transfer-in amounts of new employees
|
2,023,548 | 419,593 | ||||||
|
Administration expense
|
-3,054 | -2,375 | ||||||
|
Fair value of plan assets at December 31
|
3,485,069 | 1,297,899 | ||||||
|
December 31,
2014
|
December 31,
2013
|
|||||||
|
Present value of funded defined benefit obligation
|
4,895,667 | 1,626,241 | ||||||
|
Fair value of plan assets
|
-3,485,069 | -1,297,899 | ||||||
|
Net defined benefit liability
|
1,410,598 | 328,342 | ||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Service cost
|
111,513 | 72,803 | 45,036 | |||||||||
|
Net interest expense
|
4,188 | 3,739 | 3,313 | |||||||||
|
Administration expense
|
3,054 | 2,375 | 1,697 | |||||||||
|
Total defined benefit cost for the year recognized in profit or loss
|
118,755 | 78,917 | 50,046 | |||||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Actuarial loss (gain) arising from changes in financial assumptions
|
699,456 | -44,737 | 49,754 | |||||||||
|
Actuarial loss arising from experience adjustments
|
784,766 | 181,670 | 57,684 | |||||||||
|
Return on plan assets excluding interest income
|
-382,755 | -79,217 | -52,861 | |||||||||
|
Total defined benefit cost for the year recognized in other comprehensive loss
|
1,101,467 | 57,716 | 54,577 | |||||||||
|
At December 31
|
2014
|
2013
|
2012
|
|||||||||
|
Discount rate
|
1.20 | % | 2.20 | % | 1.95 | % | ||||||
|
Future salary increase
|
1.50 | % | 1.50 | % | 1.50 | % | ||||||
|
Pension indexation
|
0.00 | % | 0.00 | % | 0.00 | % | ||||||
|
Mortality and disability rates
|
BVG 2010G
|
BVG 2010G
|
BVG 2010G
|
|||||||||
|
December 31, 2014
|
||||||||
|
Change in assumption
|
0.25% increase
|
0.25% decrease
|
||||||
|
Discount rate
|
-228,394 | 249,899 | ||||||
|
Salary increase
|
29,192 | -33,212 | ||||||
|
Pension indexation
|
135,368 | N/A | ||||||
|
Change in assumption
|
+1 year
|
-1
year
|
||||||
|
Life expectancy
|
99,859 | -101,889 | ||||||
|
2014
|
2013
|
2012
|
||||||||||
|
Interest income
|
52,133 | 74,036 | 5,825 | |||||||||
|
Investment income
|
— | — | 1,216 | |||||||||
|
Net foreign exchange gain
|
4,164,189 | 1,711 | 2,853 | |||||||||
|
Total finance income
|
4,216,322 | 75,747 | 9,894 | |||||||||
|
Interest expense related parties
|
49,635 | 50,177 | — | |||||||||
|
Bank charges
|
6,175 | 2,454 | 1,800 | |||||||||
|
Net foreign exchange loss
|
152,015 | 106,010 | — | |||||||||
|
Total finance expense
|
207,825 | 158,641 | 1,800 | |||||||||
|
Finance income/(expense), net
|
4,008,497 | -82,894 | 8,094 | |||||||||
|
2014
|
2013
|
|||||||
|
Deferred income tax expense
|
-32,761 | -305,750 | ||||||
|
Deferred income tax gain
|
32,761 | — | ||||||
|
Total income tax expense
|
— | -305,750 | ||||||
|
Reconciliation
|
2014
|
2013
|
2012
|
|||||||||
|
Loss before income tax
|
-18,185,015 | -14,698,743 | -4,602,409 | |||||||||
|
Income tax at statutory tax rates applicable to results in the respective countries
|
4,177,780 | 3,488,916 | 1,071,976 | |||||||||
|
Effect of unrecognized temporary differences
|
-273,073 | 1,343,556 | 823,403 | |||||||||
|
Effect of unrecognized taxable losses
|
-4,160,118 | -5,160,108 | -1,895,379 | |||||||||
|
Effect of unrecognized taxable losses in equity
|
-99,406 | — | ||||||||||
|
Effect on unrecognized deferred tax due to change in income tax rate
|
156,005 | — | — | |||||||||
|
Deferred tax recognized directly in equity
|
— | 21,886 | — | |||||||||
|
Income tax expense
|
— | -305,750 | — | |||||||||
|
Deferred Tax Liabilities
|
December 31,
2014
|
December 31,
2013
|
||||||
|
Intangible assets
|
-327,637 | -327,637 | ||||||
|
Provisions
|
-32,761 | — | ||||||
|
Total
|
-360,398 | -327,637 | ||||||
|
Deferred Tax Asset
|
||||||||
|
Net operating loss (NOL)
|
32,761 | — | ||||||
|
Total
|
32,761 | — | ||||||
|
Deferred Tax, net
|
-327,637 | -327,637 | ||||||
|
Deferred Tax 2014
|
Opening Balance
|
Recognized in Profit or Loss
|
Recognized in Equity
|
Closing Balance
|
||||||||||||
|
Intangible assets
|
-327,637 | — | — | -327,637 | ||||||||||||
|
Provisions
|
— | -32,761 | — | -32,761 | ||||||||||||
|
Net operating loss (NOL)
|
— | 32,761 | 32,761 | |||||||||||||
|
Total
|
-327,637 | — | — | -327,637 | ||||||||||||
|
Deferred Tax 2013
|
Opening Balance
|
Recognized in Profit or Loss
|
Recognized in Equity
|
Closing Balance
|
||||||||||||
|
Intangible assets/
Convertible loan
|
— | -305,751 | -21,886 | -327,637 | ||||||||||||
|
Total
|
— |
-305,751
|
-21,886 | -327,637 | ||||||||||||
|
December 31,
2014
|
December 31,
2013
|
December 31,
2012
|
||||||||||
|
Within 1 year
|
2,068,441 | 18,568 | 32,654 | |||||||||
|
Between 1 and 3 years
|
3,456,587 | 3,755,427 | 2,087,009 | |||||||||
|
Between 3 and 7 years
|
51,963,606 | 34,194,937 | 13,474,978 | |||||||||
|
More than 7 years
|
1,056,556 | 938,560 | 785,439 | |||||||||
|
Total
|
58,635,190 | 38,907,492 | 16,380,080 | |||||||||
|
December 31,
2014
|
December 31,
2013
|
|||||||
|
Deductible temporary differences
|
||||||||
|
Employee benefit plan
|
311,742 | 75,174 | ||||||
|
Stock option plans
|
114,145 | 56,262 | ||||||
|
Accrued expenses
|
— | 26,097 | ||||||
|
Total potential tax assets
|
425,887 | 157,533 | ||||||
|
Taxable unrecognized temporary differences
|
||||||||
|
Property and equipment
|
— | 4,719 | ||||||
|
Total unrecognized potential tax liabilities
|
— | 4,719 | ||||||
|
Offsetting potential tax liabilities with potential tax assets
|
— | 4,719 | ||||||
|
Net potential tax assets from temporary differences not recognized
|
425,887 | 152,814 | ||||||
|
Potential tax assets from loss carry-forwards not recognized
|
13,067,988 | 8,907,870 | ||||||
|
Total potential tax assets from loss carry-forwards and temporary differences not recognized
|
13,493,874 | 9,060,684 | ||||||
|
December 31,
2014
|
December 31,
2013
|
|||||||
|
Loss attributable to owners of the Company
|
-18,185,015 | -15,004,493 | ||||||
|
Weighted average number of shares outstanding
|
27,692,494 | 14,917,064 | ||||||
|
Basic and diluted loss per share
|
-0.66 | -1.01 | ||||||
|
December 31,
2014
|
December 31,
2013
|
|||||||
|
Within one year
|
103,572 | 91,572 | ||||||
|
Between one and five years
|
58,893 | 122,096 | ||||||
|
Total
|
162,465 | 213,668 | ||||||
|
December 31,
2014
|
December 31,
2013
|
|||||||
|
Convertible loans from shareholders
|
— | 13,711,200 | ||||||
|
Trade and other payables
|
— | 22 | ||||||
|
Total liabilities to related parties
|
— | 13,711,222 | ||||||
|
2014
|
2013
|
|||||||
|
Interest expense related parties
|
-49,635 | -50,177 | ||||||
|
Net interest expense—related parties
|
-49,635 | -50,177 | ||||||