|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|
Common Stock, $.01 par value per share
|
New York Stock Exchange
|
|
Securities registered pursuant to Section 12(g) of the Act:
|
None
|
|
Documents Incorporated by Reference
|
Part of Form 10-K into
which incorporated
|
|
Document
|
|
|
Portions of the registrant's Proxy Statement to be filed by April 30, 2015
|
|
|
Item
|
Page
|
|
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
Item 15.
|
||
|
•
|
A healthier world;
|
|
•
|
Build a valuable company; and
|
|
•
|
Create an inspiring workplace.
|
|
•
|
Restore growth
|
|
•
|
Drive operational excellence
|
|
•
|
Simplify the organization
|
|
•
|
Refocus on diagnostic information services
|
|
•
|
Deliver disciplined capital deployment
|
|
•
|
we have unmatched size, scale and capabilities;
|
|
•
|
we are a leader in providing innovation and diagnostic insights;
|
|
•
|
we have a strong focus on quality and providing a superior customer experience; and
|
|
•
|
we are a high value, low cost provider.
|
|
•
|
enhance the customer experience, including ease of use and patient and provider engagement;
|
|
•
|
deliver more precise, comprehensive solutions and actionable information;
|
|
•
|
provide increased and interactive insights and analytics to patients and providers;
|
|
•
|
foster greater adherence to clinical and reimbursement guidelines;
|
|
•
|
promote population health solutions;
|
|
•
|
tap the potential of large amounts of clinical information; and
|
|
•
|
otherwise foster precision medicine.
|
|
Activity
|
Approximate Percentage
of 2014 Net Revenues
|
||
|
Diagnostic information services
|
92
|
||
|
Routine clinical testing services
|
55
|
||
|
Gene-based, esoteric and anatomic pathology testing services
|
34
|
||
|
Forensic drugs-of-abuse testing services
|
3
|
||
|
Diagnostic Solutions: Healthcare information technology, clinical trials testing, risk assessment services and diagnostic products
|
8
|
||
|
•
|
endocrinology and metabolism (the study of hormones and their effects on body growth and metabolism);
|
|
•
|
genetics (the study of chromosomes, genes and their expression);
|
|
•
|
hematology (the study of blood and bone marrow cells) and coagulation (the process of blood clotting);
|
|
•
|
neurology (the study of the nervous system, its structure and its diseases);
|
|
•
|
testing used in treatment selection and monitoring of patients with solid organ and bone marrow transplantation;
|
|
•
|
immunology (the study of the immune system, including antibodies, cytokines, immune system cells and their effect, receptor systems and autoimmune diseases);
|
|
•
|
microbiology and infectious diseases (the study of microscopic forms of life, including parasites, bacteria, viruses, fungi and other infectious agents);
|
|
•
|
oncology (the study of abnormal cell growth, including pre-cancerous conditions and cancer);
|
|
•
|
serology (a science dealing with body fluids and their analysis, including antibodies, proteins and other characteristics); and
|
|
•
|
toxicology (the study of chemicals and drugs and their intended and adverse effects on the body).
|
|
•
|
Cancer.
|
|
-
|
We introduced expanded pathology and blood test offerings to help identify and assess an individual's risk of Lynch syndrome, an inherited genetic disorder that increases the risk of colorectal and other cancers.
|
|
-
|
We introduced a new cancer test service based on the FDA-approved THxlD-BRAF
®
test from bioMerieux. This test is a companion diagnostic for two treatments for melanoma.
|
|
-
|
Based on our collaboration with Memorial Sloan Kettering Cancer Center, we introduced OncoVantage
TM
, a solution to enable molecular characterization of solid tumors, to improve physicians' ability to treat patients with breast, prostate, colon, lung and a variety of other solid tumors.
|
|
-
|
We introduced our BRCAvantagePlus
TM
solution, a suite of lab-developed test services for assessing genetic breast cancer risk based on clinically validated non-BRCA as well as BRCA genes.
|
|
-
|
We introduced BRCAvantage
TM
Ashkenazi Jewish Screen with Reflex BRCAvantage
TM
comprehensive testing that makes it more convenient for physicians to order testing for patients of Ashkenazi descent.
|
|
-
|
We released testing for tamoxifen metabolites by mass spectrometry to aid physicians in treating breast cancer patients.
|
|
•
|
Infectious Disease and Immunology.
|
|
-
|
We developed and introduced tests for Hepatitis C Viral NS5 a/b genotypes, to detect resistance for new Hepatitis C therapies.
|
|
-
|
We enhanced the Hepatitis C Viral NS3 genotype assay to include simeprevir; this assay may be used to detect boceprevir, telaprevir and simeprevir resistance-associated NS3 mutations in NS3 protease inhibitor treatment-experienced patients.
|
|
-
|
We developed and released molecular diagnostic tests for Influenza A/B & RSV; these tests detect and differentiate human influenza A/B and RSV.
|
|
-
|
We developed and introduced an updated C
difficile
test; this new test simultaneously detects C
difficile
glutamate dehydrogenase antigen and toxins A and B, conforming to testing guidelines.
|
|
-
|
We enhanced our chikungunya virus offering by developing and releasing a real-time polymerase chain reaction assay, in addition to our immune fluorescent antibody assay.
|
|
•
|
Cardiovascular and Metabolic Disease.
|
|
-
|
We released a simplified CardioIQ
®
interpretive report for our suite of CardioIQ
®
advanced cardiovascular tests.
|
|
-
|
We incorporated an ASCVD (atherosclerotic cardiovascular disease) Risk Panel as part of the CardioIQ
®
offering. This panel reports 10-year and lifetime ASCVD risk on the CardioIQ
®
report, conforming to the 2013 Cholesterol Guidelines of the American College of Cardiology and the American Heart Association.
|
|
-
|
We developed and released a diabetes risk assessment panel. This panel provides a comprehensive assessment of diabetes risk (incorporating a lipid panel, glucose, HbA1c and an 8-year diabetes risk score) that conforms to American Diabetes Association guidelines.
|
|
-
|
We developed and released a diabetes panel for non-alcoholic fatty liver disease that is supported by guidelines. This panel provides an easy and non-invasive way to assess liver fibrosis, which is prognostic for progression to more serious liver disease.
|
|
•
|
Neurology.
|
|
-
|
We launched a blood test panel to identify a severe, rapidly progressive but treatable form of autoimmune dementia and memory loss.
|
|
-
|
We launched a suite of new next-generation gene sequencing testing services to help more reliably and quickly diagnose the cause of several forms of epilepsy in adults and children.
|
|
-
|
We launched a new next-generation gene sequencing test service for initial assessment of Charcot-Marie Tooth disease.
|
|
•
|
Women's Health.
|
|
-
|
We introduced CFVantage
TM
, an evaluation of over 150 possible mutations for prenatal screening for cystic fibrosis.
|
|
-
|
We introduced access to the MaterniT21
TM
Plus prenatal test developed by Sequenom for pregnant women at increased risk for fetal chromosomal abnormalities.
|
|
-
|
We introduced a cervical cancer screening report that includes prior PAP and HPV testing results.
|
|
-
|
We introduced the APTIMA
TM
mRNA assay using Surepath
TM
vial samples types to enhance our cervical cancer screening portfolio.
|
|
•
|
Prescription Drug Monitoring and Toxicology.
|
|
-
|
We launched testing for prescription drugs on oral fluid sample types.
|
|
-
|
We expanded our testing for anticonvulsants, including for eslicarbazepine.
|
|
•
|
service capability and quality;
|
|
•
|
accuracy, timeliness and consistency in reporting test results;
|
|
•
|
patient insurance coverage;
|
|
•
|
number and type of tests performed;
|
|
•
|
pricing;
|
|
•
|
access to medical/scientific thought leaders for consultation;
|
|
•
|
number, convenience and geographic coverage of patient service centers;
|
|
•
|
reputation in the medical community;
|
|
•
|
healthcare information technology solutions;
|
|
•
|
qualifications of its staff; and
|
|
•
|
ability to develop new and useful tests and services.
|
|
•
|
“Client” fees charged to physicians, hospitals and institutions for which services are performed on a wholesale basis and which are billed on a monthly basis.
|
|
•
|
“Patient” fees charged to individual patients and certain third-party payers on a claim-by-claim basis.
|
|
Medicare Part B
Reimbursements
|
% of our
2014 Consolidated
Net Revenues
|
||
|
Clinical Laboratory Fee Schedule
|
12%
|
||
|
Physician Fee Schedule
|
2%
|
||
|
•
|
increase our administrative, billing or other operating costs;
|
|
•
|
decrease the amount of reimbursement related to diagnostic information services performed;
|
|
•
|
damage our reputation; or
|
|
•
|
adversely affect important business relationships with third parties.
|
|
•
|
Directors
|
|
•
|
Management
|
|
•
|
Code of Business Ethics
|
|
•
|
Integrity Commitment
|
|
•
|
Values
|
|
•
|
Corporate Governance Guidelines
|
|
•
|
Charters for the following committees of our Board of Directors: Audit and Finance; Compensation; Executive; Governance; and Quality, Safety and Compliance
|
|
•
|
Certificate of Incorporation
|
|
•
|
Bylaws
|
|
•
|
Corporate Political Contributions Policy
|
|
This Report also includes forward-looking statements that involve risks or uncertainties. Our results could differ materially from those anticipated in these forward-looking statements as
a result of certain factors, including the risks we face described below and elsewhere. See “Cautionary Factors that May Affect Future Results” on page
30
.
|
|
•
|
loss of key customers or employees;
|
|
•
|
difficulty in standardizing information and other systems;
|
|
•
|
difficulty in consolidating facilities and infrastructure;
|
|
•
|
failure to maintain the quality or timeliness of services that our Company has historically provided;
|
|
•
|
diversion of management's attention from the day-to-day business of our Company as a result of the need to deal with the foregoing disruptions and difficulties; and
|
|
•
|
the added costs of dealing with such disruptions.
|
|
•
|
billing and reimbursement of clinical testing;
|
|
•
|
certification or licensure of clinical laboratories;
|
|
•
|
the anti-self-referral and anti-kickback laws and regulations;
|
|
•
|
the laws and regulations administered by the FDA;
|
|
•
|
the corporate practice of medicine;
|
|
•
|
operational, personnel and quality requirements intended to ensure that clinical testing services are accurate, reliable and timely;
|
|
•
|
physician fee splitting;
|
|
•
|
relationships with physicians and hospitals;
|
|
•
|
safety and health of laboratory employees; and
|
|
•
|
handling, transportation and disposal of medical specimens, infectious and hazardous waste and radioactive materials.
|
|
•
|
diversion of management time and attention;
|
|
•
|
expenditure of large amounts of cash on legal fees, costs and payment of damages;
|
|
•
|
limitations on our ability to continue some of our operations;
|
|
•
|
enforcement actions, fines and penalties or the assertion of private litigation claims and damages;
|
|
•
|
decreased demand for our services ; and/or
|
|
•
|
injury to our reputation.
|
|
•
|
cease developing, performing or selling solutions or services that incorporate the challenged intellectual property;
|
|
•
|
obtain and pay for licenses from the holder of the infringed intellectual property right;
|
|
•
|
redesign or reengineer our tests;
|
|
•
|
change our business processes; or
|
|
•
|
pay substantial damages, court costs and attorneys' fees, including potentially increased damages for any infringement held to be willful.
|
|
•
|
changes in the local economic environment;
|
|
•
|
political instability;
|
|
•
|
social changes;
|
|
•
|
intellectual property legal protections and remedies;
|
|
•
|
trade regulations;
|
|
•
|
procedures and actions affecting approval, production, pricing, reimbursement and marketing of services;
|
|
•
|
exchange controls;
|
|
•
|
attracting and retaining qualified employees;
|
|
•
|
local market practices;
|
|
•
|
export and import controls;
|
|
•
|
weak legal systems which may affect our ability to enforce contractual rights;
|
|
•
|
changes in local laws or regulations; and
|
|
•
|
potentially longer payment and collection cycles.
|
|
(a)
|
Heightened competition from commercial clinical testing companies, hospitals, physicians and others.
|
|
(b)
|
Increased pricing pressure from customers and payers.
|
|
(c)
|
A decline in economic conditions.
|
|
(d)
|
Impact of changes in payer mix, including any shift from fee-for-service to discounted or capitated fee arrangements.
|
|
(e)
|
Adverse actions by government or other third-party payers, including healthcare reform that focuses on reducing healthcare costs but does not recognize the value and importance to healthcare of diagnostic testing, unilateral reduction of fee schedules payable to us, competitive bidding, and an increase in the practice of negotiating for exclusive arrangements that involve aggressively priced capitated or fee-for-service payments by health insurers or other payers.
|
|
(f)
|
The impact upon our testing volume and collected revenue or general or administrative expenses resulting from our compliance with Medicare and Medicaid administrative policies and requirements of third-party payers. These include:
|
|
(2)
|
inability to obtain from patients a valid advance beneficiary notice form for tests that cannot be billed without prior receipt of the form;
|
|
(3)
|
increased challenges in operating as a non-contracted provider with respect to health plans;
|
|
(4)
|
the impact of additional or expanded limited coverage policies and limits on the allowable number of test units; and
|
|
(5)
|
the impact of increased prior authorization programs for clinical testing.
|
|
(g)
|
Adverse results from pending or future government investigations, lawsuits or private actions. These include, in particular, monetary damages, loss or suspension of licenses, and/or suspension or exclusion from the Medicare and Medicaid programs and/or criminal penalties.
|
|
(h)
|
Failure to efficiently integrate acquired businesses and to manage the costs related to any such integration, or to retain key technical, professional or management personnel.
|
|
(i)
|
Denial, suspension or revocation of CLIA certification or other licenses for any of our clinical laboratories under the CLIA standards, revocation or suspension of the right to bill the Medicare and Medicaid programs or other adverse regulatory actions by federal, state and local agencies.
|
|
(j)
|
Changes in federal, state or local laws or regulations, including changes that result in new or increased federal or state regulation of commercial clinical laboratories, tests developed by commercial clinical laboratories or other products or services that we offer or activities in which we are engaged, including regulation by the FDA.
|
|
(k)
|
Inability to achieve expected benefits from our acquisitions of other businesses.
|
|
(l)
|
Inability to achieve additional benefits from our business performance tools and efficiency initiatives.
|
|
(m)
|
Adverse publicity and news coverage about the clinical testing industry or us.
|
|
(n)
|
Computer or other IT system failures that affect our ability to perform testing, report test results or properly bill customers, or result in the disclosure of confidential information, including potential failures resulting from implementing common IT systems and other system conversions, telecommunications failures, malicious human acts (such as electronic break-ins or computer viruses) or natural disasters.
|
|
(o)
|
Development of technologies that substantially alter the practice of clinical testing, including technology changes that lead to the development of more convenient or cost-effective testing, or testing to be performed outside of a commercial clinical laboratory, such as (1) point-of-care testing that can be performed by physicians in their offices, (2) esoteric testing that can be performed by hospitals in their own laboratories or (3) home testing that can be carried out without requiring the services of clinical laboratories.
|
|
(p)
|
Negative developments regarding intellectual property and other property rights that could prevent, limit or interfere with our ability to develop, perform or sell our tests or operate our business. These include:
|
|
(1)
|
Issuance of patents or other property rights to our competitors or others; and
|
|
(2)
|
Inability to obtain or maintain adequate patent or other proprietary rights for our products and services or to successfully enforce our proprietary rights.
|
|
(q)
|
Development of tests by our competitors or others which we may not be able to license, or usage of our technology or similar technologies or our trade secrets or other intellectual property by competitors, any of which could negatively affect our competitive position.
|
|
(r)
|
Regulatory delay or inability to commercialize newly developed or licensed tests or technologies or to obtain appropriate reimbursements for such tests.
|
|
(s)
|
Inability to properly bill for our services or to obtain appropriate payments for services that we do bill.
|
|
(t)
|
Changes in interest rates and changes in our credit ratings from Standard & Poor's, Moody's Investor Services or Fitch Ratings causing an unfavorable impact on our cost of and access to capital.
|
|
(u)
|
Inability to hire and retain qualified personnel or the loss of the services of one or more of our key senior management personnel.
|
|
(v)
|
Terrorist and other criminal activities, hurricanes, earthquakes or other natural disasters, and health pandemics, which could affect our customers, transportation or systems, or our facilities, and for which insurance may not adequately reimburse us.
|
|
(w)
|
Difficulties and uncertainties in the discovery, development, regulatory environment and/or marketing of new services or solutions or new uses of existing tests.
|
|
(x)
|
Failure to adapt to changes in the healthcare system and healthcare delivery, including those stemming from the 2010 federal healthcare reform legislation.
|
|
(y)
|
Results and consequences of governmental inquiries.
|
|
(cc)
|
Inability to adapt to diverse and dynamic non-U.S. markets.
|
|
Location
|
Leased or Owned
|
|
|
Sacramento, California (laboratory)
|
Leased
|
|
|
West Hills, California (laboratory)
|
Leased
|
|
|
San Juan Capistrano, California (laboratory)
|
Owned
|
|
|
Tampa, Florida (laboratory)
|
Owned
|
|
|
Atlanta, Georgia (laboratory)
|
Owned
|
|
|
Chicago, Illinois (2) (laboratories)
|
One owned, one leased
|
|
|
Marlborough, Massachusetts (laboratories)
|
Leased
|
|
|
Baltimore, Maryland (laboratory)
|
Owned
|
|
|
Teterboro, New Jersey (laboratory)
|
Owned
|
|
|
Philadelphia, Pennsylvania (laboratory)
|
Leased
|
|
|
Norristown, Pennsylvania (offices)
|
Leased
|
|
|
Dallas, Texas (laboratory)
|
Leased
|
|
|
Chantilly, Virginia (laboratory)
|
Leased
|
|
|
Lenexa, Kansas (laboratory)
|
Owned
|
|
|
Greensboro, North Carolina (laboratory)
|
Leased
|
|
|
Common Stock
Market Price |
Dividends
Declared
|
||||||||||
|
High
|
Low
|
||||||||||
|
2013
|
|||||||||||
|
First Quarter
|
$
|
61.95
|
|
$
|
55.16
|
|
$
|
0.30
|
|
||
|
Second Quarter
|
63.40
|
|
55.26
|
|
0.30
|
|
|||||
|
Third Quarter
|
62.82
|
|
56.81
|
|
0.30
|
|
|||||
|
Fourth Quarter
|
64.10
|
|
52.50
|
|
0.30
|
|
|||||
|
2014
|
|||||||||||
|
First Quarter
|
$
|
60.50
|
|
$
|
50.46
|
|
$
|
0.33
|
|
||
|
Second Quarter
|
62.42
|
|
54.90
|
|
0.33
|
|
|||||
|
Third Quarter
|
64.38
|
|
58.56
|
|
0.33
|
|
|||||
|
Fourth Quarter
|
68.51
|
|
56.27
|
|
0.33
|
|
|||||
|
ISSUER PURCHASES OF EQUITY SECURITIES
|
||||||||||||||
|
Period
|
Total Number of
Shares
Purchased
|
Average Price
Paid per Share
|
Total Number of
Shares Purchased
as Part of Publicly
Announced Plans
or Programs
|
Approximate
Dollar Value of
Shares that May
Yet Be Purchased
Under the Plans
or Programs
(in thousands)
|
||||||||||
|
October 1, 2014 – October 31, 2014
|
|
|
|
|
|
|
|
|
||||||
|
Share Repurchase Program (A)
|
—
|
|
$
|
—
|
|
—
|
|
$
|
746,023
|
|
||||
|
Employee Transactions (B)
|
1,266
|
|
$
|
59.74
|
|
N/A
|
|
N/A
|
|
|||||
|
November 1, 2014 – November 30, 2014
|
||||||||||||||
|
Share Repurchase Program (A)
|
210,949
|
|
$
|
63.62
|
|
210,949
|
|
$
|
732,601
|
|
||||
|
Employee Transactions (B)
|
698
|
|
$
|
63.62
|
|
N/A
|
|
N/A
|
|
|||||
|
December 1, 2014 – December 31, 2014
|
||||||||||||||
|
Share Repurchase Program (A)
|
564,948
|
|
$
|
64.75
|
|
564,948
|
|
$
|
696,023
|
|
||||
|
Employee Transactions (B)
|
3,725
|
|
$
|
64.35
|
|
N/A
|
|
N/A
|
|
|||||
|
Total
|
||||||||||||||
|
Share Repurchase Program (A)
|
775,897
|
|
$
|
64.44
|
|
775,897
|
|
$
|
696,023
|
|
||||
|
Employee Transactions (B)
|
5,689
|
|
$
|
63.23
|
|
N/A
|
|
N/A
|
|
|||||
|
(A)
|
Since the share repurchase program's inception in May 2003, our Board of Directors has authorized $6.5 billion of share repurchases of our common stock through
December 31, 2014
. The share repurchase authority has no set expiration or termination date.
|
|
(B)
|
Includes: (1) shares delivered or attested to in satisfaction of the exercise price and/or tax withholding obligations by holders of stock options (granted under the Company's Amended and Restated Employee Long-Term Incentive Plan and its Amended and Restated Director Long-Term Incentive Plan, collectively the “Stock Compensation Plans”) who exercised options; and (2) shares withheld (under the terms of grants under the Stock Compensation Plans) to offset tax withholding obligations that occur upon the delivery of outstanding common shares underlying restricted share units and performance share units.
|
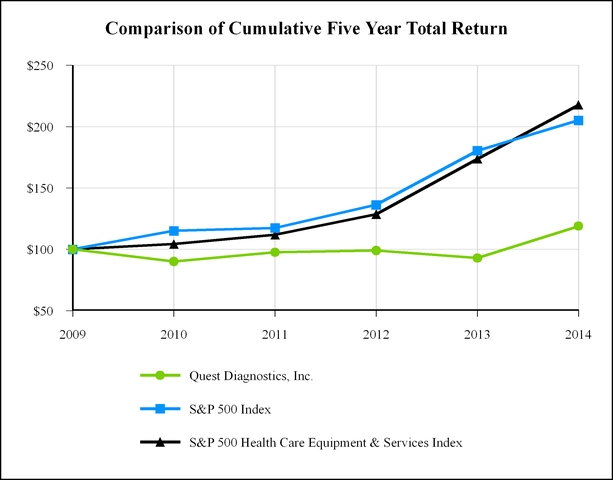
|
Closing DGX Price
|
Total Shareholder Return
|
Performance Graph Values
|
|||||||||||||||||||||
|
Date
|
DGX
|
S&P 500
|
S&P 500 H.C.
|
DGX
|
S&P 500
|
S&P 500 H.C.
|
|||||||||||||||||
|
12/31/2010
|
$53.97
|
(9.93
|
)%
|
15.06
|
%
|
4.31
|
%
|
$
|
90.07
|
|
$
|
115.06
|
|
$
|
104.31
|
|
|||||||
|
12/31/2011
|
$58.06
|
8.33
|
%
|
2.11
|
%
|
7.21
|
%
|
$
|
97.57
|
|
$
|
117.49
|
|
$
|
111.83
|
|
|||||||
|
12/30/2012
|
$58.27
|
1.49
|
%
|
16.00
|
%
|
15.02
|
%
|
$
|
99.03
|
|
$
|
136.30
|
|
$
|
128.63
|
|
|||||||
|
12/31/2013
|
$53.54
|
(6.24
|
)%
|
32.39
|
%
|
35.05
|
%
|
$
|
92.84
|
|
$
|
180.44
|
|
$
|
173.71
|
|
|||||||
|
12/31/2014
|
$67.06
|
28.06
|
%
|
13.69
|
%
|
25.34
|
%
|
$
|
118.89
|
|
$
|
205.14
|
|
$
|
217.72
|
|
|||||||
|
See page
42
.
|
|
See page
45
.
|
|
See page
64
.
|
|
(a)
|
Documents filed as part of this Report.
|
|
1.
|
Index to financial statements and supplementary data filed as part of this Report.
|
|
Item
|
Page
|
|
Financial Statements
|
|
|
2.
|
Financial Statement Schedule.
|
|
3.
|
Exhibits
|
|
(b)
|
Exhibits filed as part of this Report.
|
|
(c)
|
None.
|
|
QUEST DIAGNOSTICS INCORPORATED
|
||
|
(Registrant)
|
||
|
By:
|
/s/Stephen H. Rusckowski
|
|
|
Stephen H. Rusckowski
|
||
|
President and Chief Executive Officer
|
||
|
Signature
|
Capacity
|
|
|
/s/Stephen H. Rusckowski
Stephen H. Rusckowski
|
Director, President and Chief Executive Officer
(Principal Executive Officer)
|
|
|
/s/Mark J. Guinan
Mark J. Guinan
|
Senior Vice President and Chief Financial Officer
(Principal Financial Officer)
|
|
|
/s/Thomas F. Bongiorno
Thomas F. Bongiorno
|
Vice President, Corporate Controller and Chief Accounting Officer
(Principal Accounting Officer)
|
|
|
/s/John C. Baldwin, M.D.
John C. Baldwin, M.D.
|
Director
|
|
|
/s/Jenne K. Britell, Ph.D.
Jenne K. Britell, Ph.D.
|
Director
|
|
|
/s/Vicky B. Gregg
Vicky B. Gregg
|
Director
|
|
|
/s/Jeffry M. Leiden, M.D., Ph. D.
Jeffrey M. Leiden, M.D., Ph. D.
|
Director
|
|
|
/s/Timothy L. Main
Timothy L. Main
|
Director
|
|
|
/s/Gary M. Pfeiffer
Gary M. Pfeiffer
|
Director
|
|
|
/s/Timothy M. Ring
Timothy M. Ring
|
Director
|
|
|
/s/Daniel C. Stanzione, Ph.D.
Daniel C. Stanzione, Ph.D.
|
Chairman of the Board
|
|
|
/s/Gail R. Wilensky, Ph.D.
Gail R. Wilensky, Ph.D.
|
Director
|
|
|
/s/John B. Ziegler
John B. Ziegler
|
Director
|
|
|
Year Ended December 31,
|
|||||||||||||||||||
|
2014
|
2013
|
2012
|
2011
|
2010
|
|||||||||||||||
|
(dollars in millions, except per share data)
|
|||||||||||||||||||
|
Operations Data:
|
(a) (b)
|
(c) (d)
|
(e) (f)
|
(g) (h)
|
(i)
|
||||||||||||||
|
Net revenues
|
$
|
7,435
|
|
$
|
7,146
|
|
$
|
7,383
|
|
$
|
7,392
|
|
$
|
7,260
|
|
||||
|
Operating income
|
983
|
|
1,475
|
|
1,201
|
|
987
|
|
1,284
|
|
|||||||||
|
Income from continuing operations
|
587
|
|
848
|
|
666
|
|
494
|
|
745
|
|
|||||||||
|
Income (loss) from discontinued operations, net of taxes
|
5
|
|
35
|
|
(74
|
)
|
12
|
|
12
|
|
|||||||||
|
Net income
|
592
|
|
883
|
|
592
|
|
506
|
|
757
|
|
|||||||||
|
Less: Net income attributable to noncontrolling interests
|
36
|
|
34
|
|
36
|
|
35
|
|
36
|
|
|||||||||
|
Net income attributable to Quest Diagnostics
|
$
|
556
|
|
$
|
849
|
|
$
|
556
|
|
$
|
471
|
|
$
|
721
|
|
||||
|
Amounts attributable to Quest Diagnostics' stockholders:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
551
|
|
$
|
814
|
|
$
|
630
|
|
$
|
459
|
|
$
|
709
|
|
||||
|
Income (loss) from discontinued operations, net of taxes
|
5
|
|
35
|
|
(74
|
)
|
12
|
|
12
|
|
|||||||||
|
Net income
|
$
|
556
|
|
$
|
849
|
|
$
|
556
|
|
$
|
471
|
|
$
|
721
|
|
||||
|
Earnings per share attributable to Quest Diagnostics' common stockholders - basic:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
3.80
|
|
$
|
5.35
|
|
$
|
3.96
|
|
$
|
2.88
|
|
$
|
4.01
|
|
||||
|
Income (loss) from discontinued operations
|
0.03
|
|
0.23
|
|
(0.47
|
)
|
0.07
|
|
0.07
|
|
|||||||||
|
Net income
|
$
|
3.83
|
|
$
|
5.58
|
|
$
|
3.49
|
|
$
|
2.95
|
|
$
|
4.08
|
|
||||
|
Earnings per share attributable to Quest Diagnostics' common stockholders - diluted:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
3.78
|
|
$
|
5.31
|
|
$
|
3.92
|
|
$
|
2.85
|
|
$
|
3.98
|
|
||||
|
Income (loss) from discontinued operations
|
0.03
|
|
0.23
|
|
(0.46
|
)
|
0.07
|
|
0.07
|
|
|||||||||
|
Net income
|
$
|
3.81
|
|
$
|
5.54
|
|
$
|
3.46
|
|
$
|
2.92
|
|
$
|
4.05
|
|
||||
|
Dividends per common share
|
$
|
1.32
|
|
$
|
1.20
|
|
$
|
0.81
|
|
$
|
0.47
|
|
$
|
0.40
|
|
||||
|
Year Ended December 31,
|
|||||||||||||||||||
|
2014
|
2013
|
2012
|
2011
|
2010
|
|||||||||||||||
|
(dollars in millions)
|
|||||||||||||||||||
|
Balance Sheet Data (at end of year):
|
(a) (b)
|
(c) (d)
|
(e) (f)
|
(g) (h)
|
(i)
|
||||||||||||||
|
Cash and cash equivalents
|
$
|
192
|
|
$
|
187
|
|
$
|
296
|
|
$
|
165
|
|
$
|
449
|
|
||||
|
Total assets
|
9,877
|
|
8,948
|
|
9,284
|
|
9,313
|
|
8,527
|
|
|||||||||
|
Long-term debt
|
3,244
|
|
3,120
|
|
3,354
|
|
3,371
|
|
2,641
|
|
|||||||||
|
Total debt
|
3,762
|
|
3,332
|
|
3,364
|
|
4,025
|
|
2,990
|
|
|||||||||
|
Other Data:
|
|||||||||||||||||||
|
Net cash provided by operating activities
|
$
|
938
|
|
$
|
652
|
|
$
|
1,187
|
|
$
|
895
|
|
$
|
1,118
|
|
||||
|
Net cash (used in) provided by investing activities
|
(1,025
|
)
|
328
|
|
(217
|
)
|
(1,243
|
)
|
(217
|
)
|
|||||||||
|
Net cash provided by (used in) financing activities
|
92
|
|
(1,106
|
)
|
(822
|
)
|
64
|
|
(986
|
)
|
|||||||||
|
Capital expenditures
|
308
|
|
231
|
|
182
|
|
161
|
|
205
|
|
|||||||||
|
Purchases of treasury stock
|
132
|
|
1,037
|
|
200
|
|
935
|
|
750
|
|
|||||||||
|
(a)
|
On March 7, 2014, we completed the acquisition of Solstas Lab Partners Group ("Solstas). On April 18, 2014, we completed the acquisition of Summit Health, Inc. ("Summit Health"). On April 16, 2014, we completed the acquisition of the outreach laboratory service operations of Steward Healthcare, LLC ("Steward"). Consolidated operating results for 2014 include the results of operations of Solstas, Summit Health and Steward subsequent to the closing of the applicable acquisition. See Note 5 to the consolidated financial statements.
|
|
(b)
|
Operating income includes pre-tax charges $121 million, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating our business. In addition, operating income includes pre-tax charges of $24 million principally associated with costs related to legal matters, partially offset by a pre-tax gain of $9 million associated with a decrease in the fair value of the contingent consideration accrual associated with our Summit Health acquisition.
|
|
(c)
|
On January 2, 2013, we completed the acquisition of the clinical outreach and anatomic pathology businesses of UMass Memorial Medical Center ("UMass"). On May 15, 2013, we completed the acquisition of the toxicology and clinical laboratory business of Advanced Toxicology Network ("ATN") from Concentra, a subsidiary of Humana Inc. On June 22, 2013, we completed the acquisition of certain lab-related clinical outreach service operations of Dignity Health ("Dignity"), a hospital system in California. On October 7, 2013, we completed the acquisition of ConVerge Diagnostic Services, LLC ("ConVerge"), a leading full-service laboratory providing clinical, cytology and anatomic pathology testing services to patients, physicians and hospitals in New England. Consolidated operating results for 2013 include the results of operations of UMass, ATN, Dignity and ConVerge subsequent to the closing of the applicable acquisition. See Note 5 to the consolidated financial statements.
|
|
(d)
|
Operating income includes pre-tax charges of $115 million, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating our business. In addition, operating income includes a pre-tax gain on sale of royalty rights of $474 million and a pre-tax loss of $40 million associated with the sale of the Enterix. For further details regarding the sale of royalty rights and Enterix, see Note 6 to the consolidated financial statements.
|
|
(e)
|
On January 6, 2012, we completed the acquisition of S.E.D. Medical Laboratories ("S.E.D.") from Lovelace Health System. Consolidated operating results for 2012 include the results of operations of S.E.D. subsequent to the closing of the acquisition. See Note 5 to the consolidated financial statements.
|
|
(f)
|
Operating income includes $106 million of pre-tax charges incurred in conjunction with further restructuring and integrating our business. Results for 2012 also include pre-tax charges of $10 million, principally representing severance and other separation benefits as well as accelerated vesting of certain equity awards in connection with the succession of our prior CEO. In addition, we estimate that the impact of severe weather during the fourth quarter of 2012 adversely affected operating income for 2012 by approximately $16 million.
|
|
(g)
|
On April 4, 2011, we completed the acquisition of Athena Diagnostics (“Athena”). On May 17, 2011, we completed the acquisition of Celera Corporation (“Celera”). Consolidated operating results for 2011 include the results of operations of Athena and Celera subsequent to the closing of the applicable acquisition.
|
|
(h)
|
Operating income includes a pre-tax charge to earnings in the first quarter of 2011 of $236 million which represented the cost to resolve a previously disclosed civil lawsuit brought by a California competitor in which the State of California intervened (the “California Lawsuit”). Also includes $52 million of pre-tax charges incurred in conjunction with further restructuring and integrating our business, consisting of $42 million of pre-tax charges principally associated with workforce reductions, with the remainder principally professional fees. Results for 2011 also include $17 million of pre-tax transaction costs, primarily related to professional fees, associated with the acquisitions of Athena and Celera. In addition, operating income includes pre-tax charges of $6 million, principally representing severance and other separation benefits as well as accelerated vesting of certain equity awards in connection with the succession of our prior CEO. In addition, we estimate that the impact of severe weather during the first quarter of 2011 adversely affected operating income for 2011 by $19 million.
|
|
(i)
|
Operating income includes $27 million of costs principally associated with workforce reductions and $10 million of costs associated with the settlement of employment litigation. In addition, we estimate that the impact of severe weather during the first quarter of 2010 adversely affected operating income for 2010 by $14 million.
|
|
•
|
We grew revenues in 2014 as compared to 2013 primarily due to the acquisitions of Solstas, Summit Health and Steward.
|
|
•
|
Our cost excellence program, Invigorate, delivered run-rate savings of more than $700 million; and in November 2014, we announced our goal to deliver an additional $600 million in run-rate savings as we exit 2017. We now expect run-rate savings of $1.3 billion as we exit 2017, compared to 2011.
|
|
•
|
We opened our clinical testing laboratory in Marlborough, Massachusetts, which will use advanced automation technology to improve the quality and efficiency of clinical testing for the New England market.
|
|
•
|
On January 29, 2015, we announced that our Board of Directors authorized a 15% increase in our quarterly dividend from $0.33 per share to $0.38 per share, or $1.52 annually, commencing with the dividend payable in April 2015.
|
|
•
|
We repurchased approximately
$132 million
of our common stock as part of our stock repurchase program.
|
|
•
|
by major cost type, of the pre-tax charges expected to be incurred in connection with the course of action; or
|
|
•
|
of the pre-tax charges that will result in future cash expenditures.
|
|
•
|
revenues and accounts receivable associated with DIS;
|
|
•
|
reserves for general and professional liability claims;
|
|
•
|
reserves for other legal proceedings;
|
|
•
|
accounting for and recoverability of goodwill; and
|
|
•
|
accounting for stock-based compensation expense.
|
|
% of
|
% of
|
||
|
DIS
|
DIS
|
||
|
Volume
|
Revenues
|
||
|
Healthcare Insurers
|
44% - 48%
|
48% - 52%
|
|
|
Government Payers
|
14% - 18%
|
17% - 21%
|
|
|
Client Payers
|
34% - 38%
|
26% - 30%
|
|
|
Patients
|
1% - 5%
|
1% - 5%
|
|
|
•
|
DIS business;
|
|
•
|
Diagnostic products business;
|
|
•
|
Risk assessment services business; and
|
|
•
|
Clinical trials testing business.
|
|
2014
|
2013
|
2012
|
2014 vs. 2013 Increase
(Decrease) |
2013 vs. 2012 Increase
(Decrease) |
2014 vs. 2013 % Increase
(Decrease) |
2013 vs. 2012 % Increase
(Decrease) |
|||||||||||||||||||
|
(dollars in millions, except per share amounts)
|
|||||||||||||||||||||||||
|
Net revenues:
|
|||||||||||||||||||||||||
|
DIS business
|
$
|
6,873
|
|
$
|
6,587
|
|
$
|
6,820
|
|
$
|
286
|
|
$
|
(233
|
)
|
4.3
|
%
|
(3.4
|
)%
|
||||||
|
DS businesses
|
562
|
|
559
|
|
563
|
|
3
|
|
(4
|
)
|
0.5
|
|
(0.7
|
)
|
|||||||||||
|
Total net revenues
|
$
|
7,435
|
|
$
|
7,146
|
|
$
|
7,383
|
|
$
|
289
|
|
$
|
(237
|
)
|
4.0
|
%
|
(3.2
|
)%
|
||||||
|
Operating costs and expenses:
|
|
|
|
|
|
|
|||||||||||||||||||
|
Cost of services
|
$
|
4,637
|
|
$
|
4,326
|
|
$
|
4,365
|
|
$
|
311
|
|
$
|
(39
|
)
|
7.2
|
%
|
(0.9
|
)%
|
||||||
|
Selling, general and administrative
|
1,728
|
|
1,704
|
|
1,745
|
|
24
|
|
(41
|
)
|
1.4
|
|
(2.3
|
)
|
|||||||||||
|
Amortization of intangible assets
|
94
|
|
79
|
|
75
|
|
15
|
|
4
|
|
18.5
|
|
5.3
|
|
|||||||||||
|
Gain on sale of royalty rights
|
—
|
|
(474
|
)
|
—
|
|
474
|
|
(474
|
)
|
NM
|
|
NM
|
|
|||||||||||
|
Other operating (income) expense, net
|
(7
|
)
|
36
|
|
(3
|
)
|
(43
|
)
|
39
|
|
NM
|
|
NM
|
|
|||||||||||
|
Total operating costs and expenses
|
$
|
6,452
|
|
$
|
5,671
|
|
$
|
6,182
|
|
$
|
781
|
|
$
|
(511
|
)
|
13.8
|
%
|
(8.3
|
)%
|
||||||
|
Operating income
|
$
|
983
|
|
$
|
1,475
|
|
$
|
1,201
|
|
$
|
(492
|
)
|
$
|
274
|
|
(33.3
|
)%
|
22.8
|
%
|
||||||
|
Other income (expense):
|
|||||||||||||||||||||||||
|
Interest expense, net
|
$
|
(164
|
)
|
$
|
(159
|
)
|
$
|
(165
|
)
|
$
|
5
|
|
$
|
(6
|
)
|
2.8
|
%
|
(3.6
|
)%
|
||||||
|
Equity in earnings of equity method investees
|
26
|
|
24
|
|
26
|
|
2
|
|
(2
|
)
|
7.7
|
|
(7.7
|
)
|
|||||||||||
|
Other income, net
|
4
|
|
8
|
|
6
|
|
(4
|
)
|
2
|
|
NM
|
|
NM
|
|
|||||||||||
|
Total non-operating expenses, net
|
$
|
(134
|
)
|
$
|
(127
|
)
|
$
|
(133
|
)
|
$
|
7
|
|
$
|
(6
|
)
|
5.1
|
%
|
(4.5
|
)%
|
||||||
|
Income tax expense
|
$
|
262
|
|
$
|
500
|
|
$
|
402
|
|
$
|
(238
|
)
|
$
|
98
|
|
(47.5
|
)%
|
24.4
|
%
|
||||||
|
Effective income tax rate
|
30.9
|
%
|
37.1
|
%
|
37.6
|
%
|
(6.2
|
)%
|
(0.5
|
)%
|
NM
|
|
NM
|
|
|||||||||||
|
Income (loss) from discontinued operations, net of taxes
|
$
|
5
|
|
$
|
35
|
|
$
|
(74
|
)
|
$
|
(30
|
)
|
$
|
109
|
|
NM
|
|
NM
|
|
||||||
|
Income from continuing operations attributable to Quest Diagnostics' stockholders
|
$
|
551
|
|
$
|
814
|
|
$
|
630
|
|
$
|
(263
|
)
|
$
|
184
|
|
(32.3
|
)%
|
29.2
|
%
|
||||||
|
Diluted earnings per common share from continuing operations attributable to Quest Diagnostics’ common stockholders
|
$
|
3.78
|
|
$
|
5.31
|
|
$
|
3.92
|
|
$
|
(1.53
|
)
|
$
|
1.39
|
|
(28.8
|
)%
|
35.5
|
%
|
||||||
|
2014
|
2013
|
2012
|
||||||
|
Net revenues:
|
||||||||
|
DIS business
|
92.4
|
%
|
92.2
|
%
|
92.4
|
%
|
||
|
DS businesses
|
7.6
|
%
|
7.8
|
%
|
7.6
|
%
|
||
|
Total net revenues
|
100.0
|
%
|
100.0
|
%
|
100.0
|
%
|
||
|
Operating costs and expenses:
|
|
|
|
|
|
|
||
|
Cost of services
|
62.4
|
%
|
60.5
|
%
|
59.1
|
%
|
||
|
Selling, general and administrative
|
23.2
|
|
23.8
|
|
23.6
|
|
||
|
Amortization of intangible assets
|
1.3
|
|
1.1
|
|
1.0
|
|
||
|
Gain on sale of royalty rights
|
—
|
|
(6.6
|
)
|
—
|
|
||
|
Other operating (income) expense, net
|
(0.1
|
)
|
0.6
|
|
—
|
|
||
|
Total operating costs and expenses
|
86.8
|
%
|
79.4
|
%
|
83.7
|
%
|
||
|
Operating income
|
13.2
|
%
|
20.6
|
%
|
16.3
|
%
|
||
|
Bad debt as a percentage of net revenues
|
4.0
|
%
|
3.8
|
%
|
3.6
|
%
|
||
|
2014 vs. 2013 Increase
(Decrease) |
2013 vs. 2012 Increase
(Decrease) |
||||||||||||||||||
|
|
2014
|
2013
|
2012
|
||||||||||||||||
|
|
(dollars in millions)
|
||||||||||||||||||
|
Net revenues
|
$
|
—
|
|
$
|
28
|
|
$
|
117
|
|
$
|
(28
|
)
|
$
|
(89
|
)
|
||||
|
Income (loss) from discontinued operations before taxes
|
1
|
|
25
|
|
(74
|
)
|
(24
|
)
|
99
|
|
|||||||||
|
Income tax benefit
|
(4
|
)
|
(10
|
)
|
—
|
|
(6
|
)
|
10
|
|
|||||||||
|
Income (loss) from discontinued operations, net of taxes
|
$
|
5
|
|
$
|
35
|
|
$
|
(74
|
)
|
$
|
(30
|
)
|
$
|
109
|
|
||||
|
2014
|
2013
|
2012
|
|||||||||
|
(dollars in millions)
|
|||||||||||
|
Net cash provided by operating activities
|
$
|
938
|
|
$
|
652
|
|
$
|
1,187
|
|
||
|
Net cash (used in) provided by investing activities
|
(1,025
|
)
|
328
|
|
(217
|
)
|
|||||
|
Net cash provided by (used in) financing activities
|
92
|
|
(1,106
|
)
|
(822
|
)
|
|||||
|
Net change in cash and cash equivalents
|
$
|
5
|
|
$
|
(126
|
)
|
$
|
148
|
|
||
|
Payments due by period
|
||||||||||||||||||||
|
(in millions)
|
||||||||||||||||||||
|
Contractual Obligations
|
Total
|
Less than
1 year
|
1-3 years
|
3-5 years
|
After 5 years
|
|||||||||||||||
|
Outstanding debt
|
$
|
3,706
|
|
$
|
506
|
|
$
|
675
|
|
$
|
300
|
|
$
|
2,225
|
|
|||||
|
Capital lease obligations
|
33
|
|
12
|
|
15
|
|
5
|
|
1
|
|
||||||||||
|
Interest payments on outstanding debt
|
1,787
|
|
170
|
|
272
|
|
215
|
|
1,130
|
|
||||||||||
|
Operating leases
|
721
|
|
193
|
|
252
|
|
113
|
|
163
|
|
||||||||||
|
Purchase obligations
|
235
|
|
80
|
|
111
|
|
21
|
|
23
|
|
||||||||||
|
Merger consideration obligation
|
85
|
|
56
|
|
28
|
|
1
|
|
—
|
|
||||||||||
|
Total contractual obligations
|
$
|
6,567
|
|
$
|
1,017
|
|
$
|
1,353
|
|
$
|
655
|
|
$
|
3,542
|
|
|||||
|
/s/
|
PricewaterhouseCoopers LLP
|
|
Florham Park, New Jersey
|
|
|
February 24, 2015
|
|
|
2014
|
2013
|
||||||
|
Assets
|
|
|
|
|
|||
|
Current assets:
|
|
|
|
|
|||
|
Cash and cash equivalents
|
$
|
192
|
|
$
|
187
|
|
|
|
Accounts receivable, net of allowance for doubtful accounts of $250 and $236 at December 31, 2014 and 2013, respectively
|
932
|
|
852
|
|
|||
|
Inventories
|
110
|
|
91
|
|
|||
|
Deferred income taxes
|
169
|
|
148
|
|
|||
|
Prepaid expenses and other current assets
|
200
|
|
105
|
|
|||
|
Total current assets
|
1,603
|
|
1,383
|
|
|||
|
Property, plant and equipment, net
|
933
|
|
805
|
|
|||
|
Goodwill
|
6,032
|
|
5,649
|
|
|||
|
Intangible assets, net
|
1,071
|
|
896
|
|
|||
|
Other assets
|
238
|
|
215
|
|
|||
|
Total assets
|
$
|
9,877
|
|
$
|
8,948
|
|
|
|
Liabilities and Stockholders’ Equity
|
|
|
|
|
|||
|
Current liabilities:
|
|
|
|
|
|||
|
Accounts payable and accrued expenses
|
$
|
1,191
|
|
$
|
920
|
|
|
|
Current portion of long-term debt
|
518
|
|
212
|
|
|||
|
Total current liabilities
|
1,709
|
|
1,132
|
|
|||
|
Long-term debt
|
3,244
|
|
3,120
|
|
|||
|
Other liabilities
|
594
|
|
723
|
|
|||
|
Commitments and contingencies
|
|||||||
|
Stockholders’ equity:
|
|
|
|
|
|||
|
Quest Diagnostics stockholders’ equity:
|
|
|
|
|
|||
|
Common stock, par value $0.01 per share; 600 shares authorized at both December 31, 2014 and 2013; 215 shares issued at both December 31, 2014 and 2013
|
2
|
|
2
|
|
|||
|
Additional paid-in capital
|
2,418
|
|
2,379
|
|
|||
|
Retained earnings
|
5,723
|
|
5,358
|
|
|||
|
Accumulated other comprehensive loss
|
(27
|
)
|
(8
|
)
|
|||
|
Treasury stock, at cost; 71 shares at both December 31, 2014 and 2013
|
(3,815
|
)
|
(3,783
|
)
|
|||
|
Total Quest Diagnostics stockholders’ equity
|
4,301
|
|
3,948
|
|
|||
|
Noncontrolling interests
|
29
|
|
25
|
|
|||
|
Total stockholders’ equity
|
4,330
|
|
3,973
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
9,877
|
|
$
|
8,948
|
|
|
|
2014
|
2013
|
2012
|
|||||||||
|
Net revenues
|
$
|
7,435
|
|
$
|
7,146
|
|
$
|
7,383
|
|
||
|
Operating costs and expenses:
|
|
|
|
|
|
|
|||||
|
Cost of services
|
4,637
|
|
4,326
|
|
4,365
|
|
|||||
|
Selling, general and administrative
|
1,728
|
|
1,704
|
|
1,745
|
|
|||||
|
Amortization of intangible assets
|
94
|
|
79
|
|
75
|
|
|||||
|
Gain on sale of royalty rights
|
—
|
|
(474
|
)
|
—
|
|
|||||
|
Other operating (income) expense, net
|
(7
|
)
|
36
|
|
(3
|
)
|
|||||
|
Total operating costs and expenses
|
6,452
|
|
5,671
|
|
6,182
|
|
|||||
|
Operating income
|
983
|
|
1,475
|
|
1,201
|
|
|||||
|
Other income (expense):
|
|
|
|
|
|
|
|||||
|
Interest expense, net
|
(164
|
)
|
(159
|
)
|
(165
|
)
|
|||||
|
Equity in earnings of equity method investees
|
26
|
|
24
|
|
26
|
|
|||||
|
Other income, net
|
4
|
|
8
|
|
6
|
|
|||||
|
Total non-operating expenses, net
|
(134
|
)
|
(127
|
)
|
(133
|
)
|
|||||
|
Income from continuing operations before taxes
|
849
|
|
1,348
|
|
1,068
|
|
|||||
|
Income tax expense
|
262
|
|
500
|
|
402
|
|
|||||
|
Income from continuing operations
|
587
|
|
848
|
|
666
|
|
|||||
|
Income (loss) from discontinued operations, net of taxes
|
5
|
|
35
|
|
(74
|
)
|
|||||
|
Net income
|
592
|
|
883
|
|
592
|
|
|||||
|
Less: Net income attributable to noncontrolling interests
|
36
|
|
34
|
|
36
|
|
|||||
|
Net income attributable to Quest Diagnostics
|
$
|
556
|
|
$
|
849
|
|
$
|
556
|
|
||
|
Amounts attributable to Quest Diagnostics’ stockholders:
|
|
|
|
|
|||||||
|
Income from continuing operations
|
$
|
551
|
|
$
|
814
|
|
$
|
630
|
|
||
|
Income (loss) from discontinued operations, net of taxes
|
5
|
|
35
|
|
(74
|
)
|
|||||
|
Net income
|
$
|
556
|
|
$
|
849
|
|
$
|
556
|
|
||
|
Earnings per share attributable to Quest Diagnostics’ common stockholders - basic:
|
|
|
|
|
|||||||
|
Income from continuing operations
|
$
|
3.80
|
|
$
|
5.35
|
|
$
|
3.96
|
|
||
|
Income (loss) from discontinued operations
|
0.03
|
|
0.23
|
|
(0.47
|
)
|
|||||
|
Net income
|
$
|
3.83
|
|
$
|
5.58
|
|
$
|
3.49
|
|
||
|
Earnings per share attributable to Quest Diagnostics’ common stockholders - diluted:
|
|
|
|
|
|||||||
|
Income from continuing operations
|
$
|
3.78
|
|
$
|
5.31
|
|
$
|
3.92
|
|
||
|
Income (loss) from discontinued operations
|
0.03
|
|
0.23
|
|
(0.46
|
)
|
|||||
|
Net income
|
$
|
3.81
|
|
$
|
5.54
|
|
$
|
3.46
|
|
||
|
Dividends per common share
|
$
|
1.32
|
|
$
|
1.20
|
|
$
|
0.81
|
|
||
|
2014
|
2013
|
2012
|
|||||||||
|
Net income
|
$
|
592
|
|
$
|
883
|
|
$
|
592
|
|
||
|
Other comprehensive (loss) income:
|
|||||||||||
|
Currency translation
|
(7
|
)
|
(27
|
)
|
24
|
|
|||||
|
Market valuation, net of tax
|
(1
|
)
|
(1
|
)
|
—
|
|
|||||
|
Net deferred loss on cash flow hedges, net of tax
|
(10
|
)
|
2
|
|
1
|
|
|||||
|
Other
|
(1
|
)
|
4
|
|
(3
|
)
|
|||||
|
Other comprehensive (loss) income
|
(19
|
)
|
(22
|
)
|
22
|
|
|||||
|
Comprehensive income
|
573
|
|
861
|
|
614
|
|
|||||
|
Less: Comprehensive income attributable to noncontrolling interests
|
36
|
|
34
|
|
36
|
|
|||||
|
Comprehensive income attributable to Quest Diagnostics
|
$
|
537
|
|
$
|
827
|
|
$
|
578
|
|
||
|
2014
|
2013
|
2012
|
|||||||||
|
Cash flows from operating activities:
|
|
|
|
|
|||||||
|
Net income
|
$
|
592
|
|
$
|
883
|
|
$
|
592
|
|
||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
|
|
|
|
|
|
|||||
|
Depreciation and amortization
|
314
|
|
283
|
|
287
|
|
|||||
|
Provision for doubtful accounts
|
296
|
|
270
|
|
269
|
|
|||||
|
Deferred income tax provision
|
23
|
|
19
|
|
7
|
|
|||||
|
Stock-based compensation expense
|
51
|
|
28
|
|
50
|
|
|||||
|
Excess tax benefits from stock-based compensation arrangements
|
—
|
|
(4
|
)
|
(4
|
)
|
|||||
|
Gain on sale of royalty rights
|
—
|
|
(474
|
)
|
—
|
|
|||||
|
Asset impairment and loss on sale of businesses, net
|
—
|
|
17
|
|
86
|
|
|||||
|
Other, net
|
(12
|
)
|
2
|
|
(8
|
)
|
|||||
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|||||
|
Accounts receivable
|
(312
|
)
|
(247
|
)
|
(243
|
)
|
|||||
|
Accounts payable and accrued expenses
|
68
|
|
(21
|
)
|
(13
|
)
|
|||||
|
Income taxes payable
|
(84
|
)
|
(93
|
)
|
100
|
|
|||||
|
Termination of interest rate swap agreements
|
—
|
|
—
|
|
72
|
|
|||||
|
Other assets and liabilities, net
|
2
|
|
(11
|
)
|
(8
|
)
|
|||||
|
Net cash provided by operating activities
|
938
|
|
652
|
|
1,187
|
|
|||||
|
Cash flows from investing activities:
|
|
|
|
|
|||||||
|
Business acquisitions, net of cash acquired
|
(728
|
)
|
(213
|
)
|
(51
|
)
|
|||||
|
Proceeds from sale of businesses
|
—
|
|
296
|
|
—
|
|
|||||
|
Proceeds from sale of royalty rights
|
—
|
|
474
|
|
—
|
|
|||||
|
Capital expenditures
|
(308
|
)
|
(231
|
)
|
(182
|
)
|
|||||
|
Decrease in investments and other assets
|
11
|
|
2
|
|
16
|
|
|||||
|
Net cash (used in) provided by investing activities
|
(1,025
|
)
|
328
|
|
(217
|
)
|
|||||
|
Cash flows from financing activities:
|
|
|
|
|
|||||||
|
Proceeds from borrowings
|
2,018
|
|
896
|
|
715
|
|
|||||
|
Repayments of debt
|
(1,647
|
)
|
(900
|
)
|
(1,369
|
)
|
|||||
|
Purchases of treasury stock
|
(132
|
)
|
(1,037
|
)
|
(200
|
)
|
|||||
|
Exercise of stock options
|
78
|
|
138
|
|
162
|
|
|||||
|
Excess tax benefits from stock-based compensation arrangements
|
—
|
|
4
|
|
4
|
|
|||||
|
Dividends paid
|
(187
|
)
|
(185
|
)
|
(108
|
)
|
|||||
|
Distributions to noncontrolling interests
|
(31
|
)
|
(32
|
)
|
(38
|
)
|
|||||
|
Other financing activities, net
|
(7
|
)
|
10
|
|
12
|
|
|||||
|
Net cash provided by (used in) financing activities
|
92
|
|
(1,106
|
)
|
(822
|
)
|
|||||
|
Net change in cash and cash equivalents
|
5
|
|
(126
|
)
|
148
|
|
|||||
|
Change in cash and cash equivalents included in current assets held for sale
|
—
|
|
17
|
|
(17
|
)
|
|||||
|
Cash and cash equivalents, beginning of year
|
187
|
|
296
|
|
165
|
|
|||||
|
Cash and cash equivalents, end of year
|
$
|
192
|
|
$
|
187
|
|
$
|
296
|
|
||
|
Quest Diagnostics Stockholders’ Equity
|
|||||||||||||||||||||||
|
Shares of
Common Stock Outstanding |
Common
Stock
|
Additional
Paid-In
Capital
|
Retained
Earnings
|
Accumulated
Other
Compre-
hensive (Loss) Income
|
Treasury
Stock, at
Cost
|
Non-
controlling
Interests
|
Total Stock-
holders’
Equity
|
||||||||||||||||
|
Balance, December 31, 2011
|
157
|
|
$
|
2
|
|
$
|
2,347
|
|
$
|
4,264
|
|
$
|
(8
|
)
|
$
|
(2,912
|
)
|
$
|
22
|
|
$
|
3,715
|
|
|
Net income
|
|
|
|
|
|
|
556
|
|
|
|
|
|
36
|
|
592
|
|
|||||||
|
Other comprehensive income, net of tax
|
|
|
|
|
|
|
|
|
22
|
|
|
|
|
|
22
|
|
|||||||
|
Dividends declared
|
|
|
|
|
|
|
(130
|
)
|
|
|
|
|
|
|
(130
|
)
|
|||||||
|
Distributions to noncontrolling interests
|
|
|
|
|
|
|
|
|
|
|
|
|
(38
|
)
|
(38
|
)
|
|||||||
|
Issuance of common stock under benefit plans
|
1
|
|
|
|
4
|
|
|
|
|
|
17
|
|
|
|
21
|
|
|||||||
|
Stock-based compensation expense
|
|
|
|
|
46
|
|
|
|
|
|
4
|
|
|
|
50
|
|
|||||||
|
Exercise of stock options
|
3
|
|
|
|
(15
|
)
|
|
|
|
|
177
|
|
|
|
162
|
|
|||||||
|
Shares to cover employee payroll tax withholdings on stock issued under benefit plans
|
|
|
|
|
(20
|
)
|
|
|
|
|
|
|
|
|
(20
|
)
|
|||||||
|
Tax benefits associated with stock-based compensation plans
|
|
|
|
|
9
|
|
|
|
|
|
|
|
|
|
9
|
|
|||||||
|
Purchases of treasury stock
|
(3
|
)
|
|
|
|
|
|
|
|
|
(200
|
)
|
|
|
(200
|
)
|
|||||||
|
Other
|
|
|
|
|
|
|
|
|
|
|
|
|
3
|
|
3
|
|
|||||||
|
Balance, December 31, 2012
|
158
|
|
$
|
2
|
|
$
|
2,371
|
|
$
|
4,690
|
|
$
|
14
|
|
$
|
(2,914
|
)
|
$
|
23
|
|
$
|
4,186
|
|
|
Net income
|
|
|
|
|
|
|
849
|
|
|
|
|
|
34
|
|
883
|
|
|||||||
|
Other comprehensive loss, net of tax
|
|
|
|
|
|
|
|
|
(22
|
)
|
|
|
|
|
(22
|
)
|
|||||||
|
Dividends declared
|
|
|
|
|
|
|
(181
|
)
|
|
|
|
|
|
|
(181
|
)
|
|||||||
|
Distributions to noncontrolling interests
|
|
|
|
|
|
|
|
|
|
|
|
|
(32
|
)
|
(32
|
)
|
|||||||
|
Issuance of common stock under benefit plans
|
1
|
|
|
|
3
|
|
|
|
|
|
17
|
|
|
|
20
|
|
|||||||
|
Stock-based compensation expense
|
|
|
|
|
24
|
|
|
|
|
|
4
|
|
|
|
28
|
|
|||||||
|
Exercise of stock options
|
3
|
|
|
|
(9
|
)
|
|
|
|
|
147
|
|
|
|
138
|
|
|||||||
|
Shares to cover employee payroll tax withholdings on stock issued under benefit plans
|
(1
|
)
|
|
|
(11
|
)
|
|
|
|
|
|
|
|
|
(11
|
)
|
|||||||
|
Tax benefits associated with stock-based compensation plans
|
|
|
|
|
1
|
|
|
|
|
|
|
|
|
|
1
|
|
|||||||
|
Purchases of treasury stock
|
(17
|
)
|
|
|
|
|
|
|
|
|
(1,037
|
)
|
|
|
(1,037
|
)
|
|||||||
|
Balance, December 31, 2013
|
144
|
|
$
|
2
|
|
$
|
2,379
|
|
$
|
5,358
|
|
$
|
(8
|
)
|
$
|
(3,783
|
)
|
$
|
25
|
|
$
|
3,973
|
|
|
Net income
|
|
|
|
|
|
|
556
|
|
|
|
|
|
36
|
|
592
|
|
|||||||
|
Other comprehensive loss, net of tax
|
|
|
|
|
|
|
|
|
(19
|
)
|
|
|
|
|
(19
|
)
|
|||||||
|
Dividends declared
|
|
|
|
|
|
|
(191
|
)
|
|
|
|
|
|
|
(191
|
)
|
|||||||
|
Distributions to noncontrolling interests
|
|
|
|
|
|
|
|
|
|
|
|
|
(31
|
)
|
(31
|
)
|
|||||||
|
Issuance of common stock under benefit plans
|
1
|
|
|
|
2
|
|
|
|
|
|
17
|
|
|
|
19
|
|
|||||||
|
Stock-based compensation expense
|
|
|
|
|
48
|
|
|
|
|
|
3
|
|
|
|
51
|
|
|||||||
|
Exercise of stock options
|
1
|
|
|
|
(2
|
)
|
|
|
|
|
80
|
|
|
|
78
|
|
|||||||
|
Shares to cover employee payroll tax withholdings on stock issued under benefit plans
|
|
|
|
|
(6
|
)
|
|
|
|
|
|
|
|
|
(6
|
)
|
|||||||
|
Tax benefits associated with stock-based compensation plans
|
|
|
|
|
(3
|
)
|
|
|
|
|
|
|
|
|
(3
|
)
|
|||||||
|
Purchases of treasury stock
|
(2
|
)
|
|
|
|
|
|
|
|
|
(132
|
)
|
|
|
(132
|
)
|
|||||||
|
Other
|
|
|
|
|
|
|
|
|
|
|
|
|
(1
|
)
|
(1
|
)
|
|||||||
|
Balance, December 31, 2014
|
144
|
|
$
|
2
|
|
$
|
2,418
|
|
$
|
5,723
|
|
$
|
(27
|
)
|
$
|
(3,815
|
)
|
$
|
29
|
|
$
|
4,330
|
|
|
2014
|
2013
|
||||||
|
Available-for-sale equity securities
|
$
|
9
|
|
$
|
—
|
|
|
|
Trading equity securities
|
49
|
|
50
|
|
|||
|
Cash surrender value of life insurance policies
|
30
|
|
29
|
|
|||
|
Other investments
|
8
|
|
13
|
|
|||
|
Total
|
$
|
96
|
|
$
|
92
|
|
|
|
|
2014
|
2013
|
2012
|
||||||||
|
Amounts attributable to Quest Diagnostics’ stockholders:
|
|
|
|
|
|||||||
|
Income from continuing operations
|
$
|
551
|
|
$
|
814
|
|
$
|
630
|
|
||
|
Income (loss) from discontinued operations, net of taxes
|
5
|
|
35
|
|
(74
|
)
|
|||||
|
Net income attributable to Quest Diagnostics’ common stockholders
|
$
|
556
|
|
$
|
849
|
|
$
|
556
|
|
||
|
Income from continuing operations
|
$
|
551
|
|
$
|
814
|
|
$
|
630
|
|
||
|
Less: Earnings allocated to participating securities
|
2
|
|
3
|
|
2
|
|
|||||
|
Earnings available to Quest Diagnostics’ common stockholders – basic and diluted
|
$
|
549
|
|
$
|
811
|
|
$
|
628
|
|
||
|
Weighted average common shares outstanding – basic
|
145
|
|
152
|
|
159
|
|
|||||
|
Effect of dilutive securities:
|
|
|
|
|
|||||||
|
Stock options and performance share units
|
—
|
|
1
|
|
1
|
|
|||||
|
Weighted average common shares outstanding – diluted
|
145
|
|
153
|
|
160
|
|
|||||
|
Earnings per share attributable to Quest Diagnostics’ common stockholders – basic:
|
|
|
|
|
|||||||
|
Income from continuing operations
|
$
|
3.80
|
|
$
|
5.35
|
|
$
|
3.96
|
|
||
|
Income (loss) from discontinued operations
|
0.03
|
|
0.23
|
|
(0.47
|
)
|
|||||
|
Net income
|
$
|
3.83
|
|
$
|
5.58
|
|
$
|
3.49
|
|
||
|
Earnings per share attributable to Quest Diagnostics’ common stockholders – diluted:
|
|
|
|
|
|||||||
|
Income from continuing operations
|
$
|
3.78
|
|
$
|
5.31
|
|
$
|
3.92
|
|
||
|
Income (loss) from discontinued operations
|
0.03
|
|
0.23
|
|
(0.46
|
)
|
|||||
|
Net income
|
$
|
3.81
|
|
$
|
5.54
|
|
$
|
3.46
|
|
||
|
2014
|
2013
|
2012
|
||||||
|
Stock options and performance share units
|
2
|
|
1
|
|
2
|
|
||
|
2014
|
2013
|
2012
|
|||||||||
|
Employee separation costs
|
$
|
31
|
|
$
|
69
|
|
$
|
57
|
|
||
|
Facility-related costs
|
12
|
|
6
|
|
1
|
|
|||||
|
Asset impairment charges
|
1
|
|
—
|
|
1
|
|
|||||
|
Accelerated vesting of stock-based compensation
|
—
|
|
1
|
|
2
|
|
|||||
|
Total restructuring charges
|
$
|
44
|
|
$
|
76
|
|
$
|
61
|
|
||
|
Employee Separation Costs
|
Facility-Related Costs
|
Total
|
|||||||||
|
Balance, December 31, 2012
|
$
|
40
|
|
$
|
—
|
|
$
|
40
|
|
||
|
Income statement expense
|
69
|
|
6
|
|
75
|
|
|||||
|
Cash payments
|
(81
|
)
|
(1
|
)
|
(82
|
)
|
|||||
|
Other / adjustments
|
3
|
|
—
|
|
3
|
|
|||||
|
Balance, December 31, 2013
|
31
|
|
5
|
|
36
|
|
|||||
|
Income statement expense
|
31
|
|
12
|
|
43
|
|
|||||
|
Cash payments
|
(44
|
)
|
(6
|
)
|
(50
|
)
|
|||||
|
Balance, December 31, 2014
|
$
|
18
|
|
$
|
11
|
|
$
|
29
|
|
||
|
Solstas
|
Summit Health
|
||||||||||
|
Cash
|
$
|
572
|
|
$
|
125
|
|
|||||
|
Estimated fair value of contingent consideration
|
—
|
|
22
|
|
|||||||
|
Transaction related costs due to sellers
|
—
|
|
5
|
|
|||||||
|
Total consideration
|
$
|
572
|
|
$
|
152
|
|
|||||
|
Solstas
|
Summit Health
|
||||||||||
|
Allocation of purchase price:
|
Fair Value
|
Weighted Average Useful Life (in years)
|
Fair Value
|
Weighted Average Useful Life (in years)
|
|||||||
|
Cash and cash equivalents
|
$
|
9
|
|
$
|
1
|
|
|||||
|
Accounts receivable, net
|
48
|
|
9
|
|
|||||||
|
Current deferred income taxes
|
7
|
|
—
|
|
|||||||
|
Other current assets
|
12
|
|
16
|
|
|||||||
|
Property, plant and equipment, net
|
49
|
|
6
|
|
|||||||
|
Goodwill
|
270
|
|
92
|
|
|||||||
|
Intangible assets:
|
|||||||||||
|
Customer relationships
|
203
|
|
20
|
33
|
|
15
|
|||||
|
Tradename
|
7
|
|
2
|
2
|
|
1
|
|||||
|
Software
|
—
|
|
3
|
|
4
|
||||||
|
Total intangible assets
|
210
|
|
38
|
|
|||||||
|
Non-current deferred income taxes
|
42
|
|
—
|
|
|||||||
|
Total assets acquired
|
647
|
|
162
|
|
|||||||
|
Current liabilities
|
63
|
|
10
|
|
|||||||
|
Non-current deferred income taxes
|
4
|
|
—
|
|
|||||||
|
Other non-current liabilities
|
8
|
|
—
|
|
|||||||
|
Total liabilities assumed
|
75
|
|
10
|
|
|||||||
|
Net assets acquired
|
$
|
572
|
|
$
|
152
|
|
|||||
|
2014
|
2013
|
||||||
|
(unaudited)
|
|||||||
|
Pro forma net revenues
|
$
|
7,520
|
|
$
|
7,622
|
|
|
|
Pro forma income from continuing operations
|
$
|
585
|
|
$
|
855
|
|
|
|
Earnings per share attributable to Quest Diagnostics’ common stockholders - basic:
|
|||||||
|
Pro forma income from continuing operations
|
$
|
3.79
|
|
$
|
5.40
|
|
|
|
Earnings per share attributable to Quest Diagnostics’ common stockholders - diluted:
|
|||||||
|
Pro forma income from continuing operations
|
$
|
3.77
|
|
$
|
5.36
|
|
|
|
Basis of Fair Value Measurements
|
|||||||||||||||
|
Quoted
Prices in
Active
Markets for
Identical
Assets /
Liabilities
|
Significant
Other
Observable
Inputs
|
Significant
Unobservable
Inputs
|
|||||||||||||
|
December 31, 2014
|
Total
|
Level 1
|
Level 2
|
Level 3
|
|||||||||||
|
Assets:
|
|
|
|
|
|
|
|
|
|||||||
|
Trading securities
|
$
|
49
|
|
$
|
49
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Cash surrender value of life insurance policies
|
30
|
|
—
|
|
30
|
|
—
|
|
|||||||
|
Interest rate swaps
|
17
|
|
—
|
|
17
|
|
—
|
|
|||||||
|
Available-for-sale equity securities
|
9
|
|
9
|
|
—
|
|
—
|
|
|||||||
|
Put Option
|
1
|
|
—
|
|
—
|
|
1
|
|
|||||||
|
Total
|
$
|
106
|
|
$
|
58
|
|
$
|
47
|
|
$
|
1
|
|
|||
|
Liabilities:
|
|
|
|
|
|
|
|
|
|||||||
|
Deferred compensation liabilities
|
$
|
85
|
|
$
|
—
|
|
$
|
85
|
|
$
|
—
|
|
|||
|
Contingent consideration
|
17
|
|
—
|
|
—
|
|
17
|
|
|||||||
|
Forward starting interest rate swaps
|
15
|
|
—
|
|
15
|
|
—
|
|
|||||||
|
Interest rate swaps
|
13
|
|
—
|
|
13
|
|
—
|
|
|||||||
|
Call option
|
5
|
|
—
|
|
—
|
|
5
|
|
|||||||
|
Total
|
$
|
135
|
|
$
|
—
|
|
$
|
113
|
|
$
|
22
|
|
|||
|
December 31, 2013
|
|||||||||||||||
|
Assets:
|
|
|
|
|
|
||||||||||
|
Trading securities
|
$
|
50
|
|
$
|
50
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Cash surrender value of life insurance policies
|
29
|
|
—
|
|
29
|
|
—
|
|
|||||||
|
Put option
|
4
|
|
—
|
|
—
|
|
4
|
|
|||||||
|
Forward starting interest rate swaps
|
2
|
|
—
|
|
2
|
|
—
|
|
|||||||
|
Total
|
$
|
85
|
|
$
|
50
|
|
$
|
31
|
|
$
|
4
|
|
|||
|
Liabilities:
|
|
|
|
|
|
|
|
|
|||||||
|
Deferred compensation liabilities
|
$
|
84
|
|
$
|
—
|
|
$
|
84
|
|
$
|
—
|
|
|||
|
Interest rate swaps
|
34
|
|
—
|
|
34
|
|
—
|
|
|||||||
|
Call option
|
8
|
|
—
|
|
—
|
|
8
|
|
|||||||
|
Total
|
$
|
126
|
|
$
|
—
|
|
$
|
118
|
|
$
|
8
|
|
|||
|
Available-for-Sale Equity Securities
|
Put Option Derivative Asset
|
Total
|
|||||||||
|
Balance, December 31, 2012
|
$
|
1
|
|
$
|
—
|
|
$
|
1
|
|
||
|
Purchases, additions and issuances
|
—
|
|
8
|
|
8
|
|
|||||
|
Total gains (losses) - realized/ unrealized:
|
|||||||||||
|
Included in earnings
|
—
|
|
(4
|
)
|
(4
|
)
|
|||||
|
Included in other comprehensive income (loss)
|
(1
|
)
|
—
|
|
(1
|
)
|
|||||
|
Balance, December 31, 2013
|
—
|
|
4
|
|
4
|
|
|||||
|
Total gains (losses) - realized/ unrealized:
|
|||||||||||
|
Included in earnings
|
—
|
|
(3
|
)
|
(3
|
)
|
|||||
|
Balance, December 31, 2014
|
$
|
—
|
|
$
|
1
|
|
$
|
1
|
|
||
|
Contingent Consideration
|
Call Option Derivative Liability
|
Total
|
|||||||||
|
Balance, December 31, 2012
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Purchases, additions and issuances
|
—
|
|
11
|
|
11
|
|
|||||
|
Total (gains) losses - realized/ unrealized:
|
|||||||||||
|
Included in earnings
|
—
|
|
(3
|
)
|
(3
|
)
|
|||||
|
Balance, December 31, 2013
|
—
|
|
8
|
|
8
|
|
|||||
|
Purchases, additions and issuances
|
26
|
|
—
|
|
26
|
|
|||||
|
Total (gains) losses - realized/ unrealized:
|
|||||||||||
|
Included in earnings
|
(9
|
)
|
(3
|
)
|
(12
|
)
|
|||||
|
Balance, December 31, 2014
|
$
|
17
|
|
$
|
5
|
|
$
|
22
|
|
||
|
2014
|
2013
|
2012
|
|||||||||
|
Current:
|
|||||||||||
|
Federal
|
$
|
204
|
|
$
|
417
|
|
$
|
332
|
|
||
|
State and local
|
34
|
|
59
|
|
61
|
|
|||||
|
Foreign
|
3
|
|
4
|
|
3
|
|
|||||
|
Deferred:
|
|||||||||||
|
Federal
|
28
|
|
27
|
|
13
|
|
|||||
|
State and local
|
(6
|
)
|
(6
|
)
|
(6
|
)
|
|||||
|
Foreign
|
(1
|
)
|
(1
|
)
|
(1
|
)
|
|||||
|
Total
|
$
|
262
|
|
$
|
500
|
|
$
|
402
|
|
||
|
2014
|
2013
|
2012
|
||||||
|
Tax provision at statutory rate
|
35.0
|
%
|
35.0
|
%
|
35.0
|
%
|
||
|
State and local income taxes, net of federal benefit
|
3.1
|
|
2.8
|
|
3.4
|
|
||
|
Impact of foreign operations
|
(0.2
|
)
|
(0.3
|
)
|
(0.3
|
)
|
||
|
Tax credits
|
(0.8
|
)
|
(0.4
|
)
|
(0.2
|
)
|
||
|
Adjustments to unrecognized tax positions (the net benefit mainly results from favorable resolution of certain tax contingencies)
|
(4.9
|
)
|
1.4
|
|
1.2
|
|
||
|
Non-deductible expenses, primarily meals and entertainment expenses
|
0.4
|
|
0.3
|
|
0.3
|
|
||
|
Impact of noncontrolling interests
|
(1.6
|
)
|
(1.0
|
)
|
(1.3
|
)
|
||
|
Other, net
|
(0.1
|
)
|
(0.7
|
)
|
(0.5
|
)
|
||
|
Effective tax rate
|
30.9
|
%
|
37.1
|
%
|
37.6
|
%
|
||
|
2014
|
2013
|
||||||
|
Current deferred tax assets:
|
|||||||
|
Accounts receivable reserves
|
$
|
91
|
|
$
|
85
|
|
|
|
Liabilities not currently deductible
|
78
|
|
63
|
|
|||
|
Total current deferred tax assets
|
$
|
169
|
|
$
|
148
|
|
|
|
Non-current deferred tax assets (liabilities):
|
|||||||
|
Liabilities not currently deductible
|
$
|
138
|
|
$
|
144
|
|
|
|
Stock-based compensation
|
42
|
|
43
|
|
|||
|
Capitalized R&D expense
|
3
|
|
6
|
|
|||
|
Net operating loss carryforwards, net of valuation allowance
|
165
|
|
114
|
|
|||
|
Depreciation and amortization
|
(519
|
)
|
(475
|
)
|
|||
|
Total non-current deferred tax liabilities, net
|
$
|
(171
|
)
|
$
|
(168
|
)
|
|
|
2014
|
2013
|
2012
|
|||||||||
|
Balance, beginning of year
|
$
|
168
|
|
$
|
199
|
|
$
|
195
|
|
||
|
Additions:
|
|||||||||||
|
For tax positions of current year
|
17
|
|
11
|
|
12
|
|
|||||
|
For tax positions of prior years
|
1
|
|
12
|
|
10
|
|
|||||
|
Reductions:
|
|||||||||||
|
Changes in judgment
|
(56
|
)
|
(23
|
)
|
(2
|
)
|
|||||
|
Expirations of statutes of limitations
|
(6
|
)
|
(2
|
)
|
(6
|
)
|
|||||
|
Settlements
|
(2
|
)
|
(29
|
)
|
(10
|
)
|
|||||
|
Balance, end of year
|
$
|
122
|
|
$
|
168
|
|
$
|
199
|
|
||
|
2014
|
2013
|
2012
|
|||||||||
|
Depreciation expense
|
$
|
220
|
|
$
|
204
|
|
$
|
207
|
|
||
|
Amortization expense
|
94
|
|
79
|
|
80
|
|
|||||
|
Interest paid
|
170
|
|
167
|
|
163
|
|
|||||
|
Income taxes paid
|
327
|
|
568
|
|
305
|
|
|||||
|
Assets acquired under capital leases
|
12
|
|
13
|
|
6
|
|
|||||
|
Account payable associated with capital expenditures
|
26
|
|
28
|
|
—
|
|
|||||
|
Dividend payable
|
48
|
|
43
|
|
48
|
|
|||||
|
Businesses acquired:
|
|
|
|
|
|||||||
|
Fair value of assets acquired
|
853
|
|
280
|
|
51
|
|
|||||
|
Fair value of liabilities assumed
|
85
|
|
16
|
|
—
|
|
|||||
|
Fair value of net assets acquired
|
768
|
|
264
|
|
51
|
|
|||||
|
Merger consideration paid (payable)
|
(30
|
)
|
(50
|
)
|
—
|
|
|||||
|
Cash paid for business acquisitions
|
738
|
|
214
|
|
51
|
|
|||||
|
Less: Cash acquired
|
10
|
|
1
|
|
—
|
|
|||||
|
Business acquisitions, net of cash acquired
|
$
|
728
|
|
$
|
213
|
|
$
|
51
|
|
||
|
2014
|
2013
|
2012
|
|||||||||
|
Depreciation expense
|
$
|
220
|
|
$
|
203
|
|
$
|
204
|
|
||
|
Interest expense
|
(167
|
)
|
(162
|
)
|
(168
|
)
|
|||||
|
Interest income
|
3
|
|
3
|
|
3
|
|
|||||
|
Interest expense, net
|
$
|
(164
|
)
|
$
|
(159
|
)
|
$
|
(165
|
)
|
||
|
2014
|
2013
|
||||||
|
Land
|
$
|
28
|
|
$
|
30
|
|
|
|
Buildings and improvements
|
367
|
|
365
|
|
|||
|
Laboratory equipment, furniture and fixtures
|
1,386
|
|
1,248
|
|
|||
|
Leasehold improvements
|
538
|
|
452
|
|
|||
|
Computer software developed or obtained for internal use
|
675
|
|
581
|
|
|||
|
Construction-in-progress
|
132
|
|
130
|
|
|||
|
3,126
|
|
2,806
|
|
||||
|
Less: Accumulated depreciation and amortization
|
(2,193
|
)
|
(2,001
|
)
|
|||
|
Total
|
$
|
933
|
|
$
|
805
|
|
|
|
2014
|
2013
|
||||||
|
Balance, beginning of year
|
$
|
5,649
|
|
$
|
5,536
|
|
|
|
Goodwill acquired during the year
|
383
|
|
150
|
|
|||
|
Write-off associated with sale of business during the year
|
—
|
|
(37
|
)
|
|||
|
Balance, end of year
|
$
|
6,032
|
|
$
|
5,649
|
|
|
|
Weighted
Average
Amort-ization
Period (Years)
|
December 31, 2014
|
December 31, 2013
|
|||||||||||||||||||||||
|
Cost
|
Accumulated
Amortization
|
Net
|
Cost
|
Accumulated
Amortization
|
Net
|
||||||||||||||||||||
|
Amortizing intangible assets:
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
Customer-related intangibles
|
18
|
$
|
929
|
|
$
|
(259
|
)
|
$
|
670
|
|
$
|
670
|
|
$
|
(210
|
)
|
$
|
460
|
|
||||||
|
Non-compete agreements
|
4
|
43
|
|
(37
|
)
|
6
|
|
43
|
|
(27
|
)
|
16
|
|
||||||||||||
|
Technology
|
14
|
118
|
|
(38
|
)
|
80
|
|
119
|
|
(28
|
)
|
91
|
|
||||||||||||
|
Other
|
8
|
152
|
|
(82
|
)
|
70
|
|
141
|
|
(57
|
)
|
84
|
|
||||||||||||
|
Total
|
16
|
1,242
|
|
(416
|
)
|
826
|
|
973
|
|
(322
|
)
|
651
|
|
||||||||||||
|
Intangible assets not subject to amortization:
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
Tradenames
|
|
244
|
|
—
|
|
244
|
|
244
|
|
—
|
|
244
|
|
||||||||||||
|
Other
|
|
1
|
|
—
|
|
1
|
|
1
|
|
—
|
|
1
|
|
||||||||||||
|
Total intangible assets
|
$
|
1,487
|
|
$
|
(416
|
)
|
$
|
1,071
|
|
$
|
1,218
|
|
$
|
(322
|
)
|
$
|
896
|
|
|||||||
|
Year Ending December 31,
|
|
|
|
|
2015
|
$
|
85
|
|
|
2016
|
74
|
|
|
|
2017
|
71
|
|
|
|
2018
|
64
|
|
|
|
2019
|
62
|
|
|
|
Thereafter
|
470
|
|
|
|
Total
|
$
|
826
|
|
|
2014
|
2013
|
||||||
|
Trade accounts payable
|
$
|
257
|
|
$
|
258
|
|
|
|
Accrued wages and benefits, including incentive compensation
|
364
|
|
283
|
|
|||
|
Income taxes payable
|
63
|
|
7
|
|
|||
|
Accrued interest
|
67
|
|
61
|
|
|||
|
Accrued insurance
|
59
|
|
30
|
|
|||
|
Merger consideration payable
|
56
|
|
1
|
|
|||
|
Dividend payable
|
48
|
|
43
|
|
|||
|
Accrued expenses
|
277
|
|
237
|
|
|||
|
Total
|
$
|
1,191
|
|
$
|
920
|
|
|
|
2014
|
2013
|
||||||
|
Floating Rate Senior Notes due March 2014 (bearing interest at three-month LIBOR plus 0.85%)
|
$
|
—
|
|
$
|
200
|
|
|
|
5.45% Senior Notes due November 2015
|
500
|
|
500
|
|
|||
|
3.20% Senior Notes due April 2016
|
304
|
|
307
|
|
|||
|
6.40% Senior Notes due July 2017
|
375
|
|
375
|
|
|||
|
2.70% Senior Notes due April 2019
|
300
|
|
—
|
|
|||
|
4.75% Senior Notes due January 2020
|
524
|
|
520
|
|
|||
|
4.70% Senior Notes due April 2021
|
549
|
|
533
|
|
|||
|
4.25% Senior Notes due April 2024
|
311
|
|
—
|
|
|||
|
6.95% Senior Notes due July 2037
|
421
|
|
421
|
|
|||
|
5.75% Senior Notes due January 2040
|
439
|
|
439
|
|
|||
|
Other
|
39
|
|
37
|
|
|||
|
Total long-term debt
|
3,762
|
|
3,332
|
|
|||
|
Less: current portion of long-term debt
|
518
|
|
212
|
|
|||
|
Total long-term debt, net of current portion
|
$
|
3,244
|
|
$
|
3,120
|
|
|
|
Year Ending December 31,
|
|||
|
2016
|
$
|
309
|
|
|
2017
|
381
|
|
|
|
2018
|
4
|
|
|
|
2019
|
301
|
|
|
|
2020
|
500
|
|
|
|
Thereafter
|
1,726
|
|
|
|
Total maturities of long-term debt
|
3,221
|
|
|
|
Unamortized discount
|
(20
|
)
|
|
|
Fair value basis adjustments attributable to hedged debt
|
43
|
|
|
|
Total long-term debt, net of current portion
|
$
|
3,244
|
|
|
Notional Amount
|
|||||||
|
2014
|
2013
|
||||||
|
Forward Starting Interest Rate Swaps
|
$
|
150
|
|
$
|
100
|
|
|
|
Floating Rate
|
Notional Amount
|
|||||||||
|
Debt Instrument
|
Paid by the Company
|
2014
|
2013
|
|||||||
|
3.20% Senior Notes due April 2016
|
Six-month LIBOR plus a 2.3% spread
|
$
|
200
|
|
$
|
200
|
|
|||
|
4.75% Senior Notes due January 2020
|
One-month LIBOR plus a 3.6% spread
|
350
|
|
350
|
|
|||||
|
4.70% Senior Notes due April 2021
|
One-month LIBOR plus a 2.45% to 3.39% spread
|
400
|
|
400
|
|
|||||
|
4.25% Senior Notes due April 2024
|
One-month LIBOR plus a 1.54% to 1.59% spread
|
250
|
|
—
|
|
|||||
|
$
|
1,200
|
|
$
|
950
|
|
|||||
|
December 31, 2014
|
December 31, 2013
|
||||||||||
|
Balance Sheet
Classification
|
Fair Value
|
Balance Sheet
Classification
|
Fair Value
|
||||||||
|
Derivatives Designated as Hedging Instruments
|
|
|
|
|
|
||||||
|
Asset Derivatives:
|
|
|
|
|
|
|
|||||
|
Interest rate swaps
|
Other assets
|
$
|
17
|
|
$
|
—
|
|
||||
|
Forward starting interest rate swaps
|
—
|
|
Other assets
|
2
|
|
||||||
|
Total Asset Derivatives
|
17
|
|
2
|
|
|||||||
|
Liability Derivatives:
|
|||||||||||
|
Interest rate swaps
|
Other liabilities
|
13
|
|
Other liabilities
|
34
|
|
|||||
|
Forward starting interest rate swaps
|
Other liabilities
|
15
|
|
—
|
|
||||||
|
Total Liability Derivatives
|
28
|
|
34
|
|
|||||||
|
Derivatives Not Designated as Hedging Instruments
|
|
|
|
|
|
||||||
|
Asset Derivatives:
|
|
|
|
|
|
|
|||||
|
Put option
|
Prepaid expenses and other current assets
|
1
|
|
Other assets
|
4
|
|
|||||
|
Liability Derivatives:
|
|
|
|
|
|
|
|||||
|
Call option
|
Accounts payable and accrued expenses
|
5
|
|
Other liabilities
|
8
|
|
|||||
|
Total Net Derivatives Liabilities
|
|
$
|
(15
|
)
|
|
$
|
(36
|
)
|
|||
|
Foreign
Currency
Translation
Adjustment
|
Market Value
Adjustment
|
Net Deferred Loss on Cash Flow Hedges
|
Other
|
Accumulated Other Comprehensive (Loss) Income
|
|||||||||||||||
|
Balance, December 31, 2011
|
$
|
1
|
|
$
|
1
|
|
$
|
(8
|
)
|
$
|
(2
|
)
|
$
|
(8
|
)
|
||||
|
Other comprehensive income (loss) before reclassifications
|
24
|
|
—
|
|
—
|
|
(3
|
)
|
21
|
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive income (loss)
|
—
|
|
—
|
|
1
|
|
—
|
|
1
|
|
|||||||||
|
Net current period other comprehensive income (loss)
|
24
|
|
—
|
|
1
|
|
(3
|
)
|
22
|
|
|||||||||
|
Balance, December 31, 2012
|
25
|
|
1
|
|
(7
|
)
|
(5
|
)
|
14
|
|
|||||||||
|
Other comprehensive income (loss) before reclassifications
|
2
|
|
(1
|
)
|
1
|
|
1
|
|
3
|
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive income (loss)
|
(29
|
)
|
—
|
|
1
|
|
3
|
|
(25
|
)
|
|||||||||
|
Net current period other comprehensive (loss) income
|
(27
|
)
|
(1
|
)
|
2
|
|
4
|
|
(22
|
)
|
|||||||||
|
Balance, December 31, 2013
|
(2
|
)
|
—
|
|
(5
|
)
|
(1
|
)
|
(8
|
)
|
|||||||||
|
Other comprehensive loss before reclassifications
|
(7
|
)
|
(1
|
)
|
(11
|
)
|
(1
|
)
|
(20
|
)
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive loss
|
—
|
|
—
|
|
1
|
|
—
|
|
1
|
|
|||||||||
|
Net current period other comprehensive loss
|
(7
|
)
|
(1
|
)
|
(10
|
)
|
(1
|
)
|
(19
|
)
|
|||||||||
|
Balance, December 31, 2014
|
$
|
(9
|
)
|
$
|
(1
|
)
|
$
|
(15
|
)
|
$
|
(2
|
)
|
$
|
(27
|
)
|
||||
|
2014
|
2013
|
2012
|
|||
|
Weighted average fair value of options at grant date
|
$10.99
|
$12.64
|
$15.87
|
||
|
Expected volatility
|
25.1%
|
25.8%
|
27%
|
||
|
Dividend yield
|
2.1%
|
1.4%
|
0.9%
|
||
|
Risk-free interest rate
|
1.6% - 2.0%
|
1.1% - 1.3%
|
1.3% - 1.5%
|
||
|
Expected holding period, in years
|
5.5 - 6.6
|
5.5 - 6.7
|
6.7 - 7.5
|
||
|
Shares
(in millions)
|
Weighted
Average Exercise Price
|
Weighted Average Remaining Contractual Term
(in years)
|
Aggregate Intrinsic Value
|
|||||||||
|
Options outstanding, beginning of year
|
6.3
|
|
$
|
54.20
|
|
|||||||
|
Options granted
|
2.5
|
|
52.39
|
|
||||||||
|
Options exercised
|
(1.5
|
)
|
51.65
|
|
||||||||
|
Options forfeited and canceled
|
(0.7
|
)
|
50.47
|
|
||||||||
|
Options outstanding, end of year
|
6.6
|
|
$
|
54.46
|
|
7.3
|
$
|
83
|
|
|||
|
Exercisable, end of year
|
3.1
|
|
$
|
55.00
|
|
5.6
|
$
|
37
|
|
|||
|
Vested and expected to vest, end of year
|
6.4
|
|
$
|
54.50
|
|
7.2
|
$
|
81
|
|
|||
|
2014
|
2013
|
2012
|
||||||||||||||||||
|
Shares
(in millions)
|
Weighted
Average
Grant Date
Fair Value
|
Shares
(in millions) |
Weighted
Average
Grant Date
Fair Value
|
Shares
(in millions)
|
Weighted
Average
Grant Date
Fair Value
|
|||||||||||||||
|
Shares outstanding, beginning of year
|
0.7
|
|
$
|
57.20
|
|
1.2
|
|
$
|
56.84
|
|
2.0
|
|
$
|
54.61
|
|
|||||
|
Shares granted
|
0.7
|
|
52.72
|
|
0.8
|
|
56.79
|
|
0.8
|
|
57.78
|
|
||||||||
|
Shares vested
|
(0.3
|
)
|
57.14
|
|
(0.5
|
)
|
56.25
|
|
(0.9
|
)
|
52.62
|
|
||||||||
|
Shares forfeited and canceled
|
—
|
|
—
|
|
(0.1
|
)
|
56.92
|
|
(0.1
|
)
|
57.09
|
|
||||||||
|
Adjustment to estimate of performance share units to be earned
|
0.1
|
|
56.10
|
|
(0.7
|
)
|
56.84
|
|
(0.6
|
)
|
57.06
|
|
||||||||
|
Shares outstanding, end of year
|
1.2
|
|
$
|
54.37
|
|
0.7
|
|
$
|
57.20
|
|
1.2
|
|
$
|
56.84
|
|
|||||
|
Year Ending December 31,
|
|||
|
2015
|
$
|
193
|
|
|
2016
|
148
|
|
|
|
2017
|
104
|
|
|
|
2018
|
66
|
|
|
|
2019
|
47
|
|
|
|
2020 and thereafter
|
163
|
|
|
|
Minimum lease payments
|
721
|
|
|
|
Noncancelable sub-lease income
|
—
|
|
|
|
Net minimum lease payments
|
$
|
721
|
|
|
2014
|
2013
|
2012
|
|||||||||
|
Net revenues
|
$
|
—
|
|
$
|
28
|
|
$
|
117
|
|
||
|
Income (loss) from discontinued operations before taxes
|
1
|
|
25
|
|
(74
|
)
|
|||||
|
Income tax expense benefit
|
(4
|
)
|
(10
|
)
|
—
|
|
|||||
|
Income (loss) from discontinued operations, net of taxes
|
$
|
5
|
|
$
|
35
|
|
$
|
(74
|
)
|
||
|
2014
|
2013
|
2012
|
|||||||||
|
Net revenues:
|
|
|
|
|
|||||||
|
DIS business
|
$
|
6,873
|
|
$
|
6,587
|
|
$
|
6,820
|
|
||
|
All other operating segments
|
562
|
|
559
|
|
563
|
|
|||||
|
Total net revenues
|
$
|
7,435
|
|
$
|
7,146
|
|
$
|
7,383
|
|
||
|
Operating earnings (loss):
|
|
|
|
|
|||||||
|
DIS business
|
$
|
1,068
|
|
$
|
1,201
|
|
$
|
1,370
|
|
||
|
All other operating segments
|
94
|
|
76
|
|
67
|
|
|||||
|
General corporate activities
|
(179
|
)
|
198
|
|
(236
|
)
|
|||||
|
Total operating income
|
983
|
|
1,475
|
|
1,201
|
|
|||||
|
Non-operating expenses, net
|
(134
|
)
|
(127
|
)
|
(133
|
)
|
|||||
|
Income from continuing operations before taxes
|
849
|
|
1,348
|
|
1,068
|
|
|||||
|
Income tax expense
|
262
|
|
500
|
|
402
|
|
|||||
|
Income from continuing operations
|
587
|
|
848
|
|
666
|
|
|||||
|
Income (loss) from discontinued operations, net of taxes
|
5
|
|
35
|
|
(74
|
)
|
|||||
|
Net income
|
592
|
|
883
|
|
592
|
|
|||||
|
Less: Net income attributable to noncontrolling interests
|
36
|
|
34
|
|
36
|
|
|||||
|
Net income attributable to Quest Diagnostics
|
$
|
556
|
|
$
|
849
|
|
$
|
556
|
|
||
|
2014
|
2013
|
2012
|
|||||||||
|
Depreciation and amortization:
|
|||||||||||
|
DIS business
|
$
|
206
|
|
$
|
189
|
|
$
|
188
|
|
||
|
All other operating segments
|
13
|
|
12
|
|
13
|
|
|||||
|
General corporate
|
95
|
|
82
|
|
77
|
|
|||||
|
314
|
|
283
|
|
278
|
|
||||||
|
Adjustments: Discontinued operations
|
—
|
|
—
|
|
9
|
|
|||||
|
Total depreciation and amortization
|
$
|
314
|
|
$
|
283
|
|
$
|
287
|
|
||
|
Capital expenditures:
|
|||||||||||
|
DIS business
|
$
|
283
|
|
$
|
196
|
|
$
|
145
|
|
||
|
All other operating segments
|
17
|
|
26
|
|
24
|
|
|||||
|
General corporate
|
8
|
|
9
|
|
11
|
|
|||||
|
308
|
|
231
|
|
180
|
|
||||||
|
Adjustments: Discontinued operations
|
—
|
|
—
|
|
2
|
|
|||||
|
Total capital expenditures
|
$
|
308
|
|
$
|
231
|
|
$
|
182
|
|
||
|
2014 (a)
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
Total
Year
|
||||||||||||||
|
(b)
|
(c)
|
(d)
|
(e)
|
||||||||||||||||
|
Net revenues
|
$
|
1,746
|
|
$
|
1,902
|
|
$
|
1,904
|
|
$
|
1,883
|
|
$
|
7,435
|
|
||||
|
Gross profit
|
645
|
|
728
|
|
726
|
|
699
|
|
2,798
|
|
|||||||||
|
Income from continuing operations
|
111
|
|
142
|
|
139
|
|
195
|
|
587
|
|
|||||||||
|
Income from discontinued operations, net of taxes
|
—
|
|
—
|
|
—
|
|
5
|
|
5
|
|
|||||||||
|
Net income
|
111
|
|
142
|
|
139
|
|
200
|
|
592
|
|
|||||||||
|
Less: Net income attributable to noncontrolling interests
|
7
|
|
9
|
|
10
|
|
10
|
|
36
|
|
|||||||||
|
Net income attributable to Quest Diagnostics
|
$
|
104
|
|
$
|
133
|
|
$
|
129
|
|
$
|
190
|
|
$
|
556
|
|
||||
|
Amounts attributable to Quest Diagnostics' stockholders:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
104
|
|
$
|
133
|
|
$
|
129
|
|
$
|
185
|
|
$
|
551
|
|
||||
|
Income from discontinued operations, net of taxes
|
—
|
|
—
|
|
—
|
|
5
|
|
5
|
|
|||||||||
|
Net income
|
$
|
104
|
|
$
|
133
|
|
$
|
129
|
|
$
|
190
|
|
$
|
556
|
|
||||
|
Earnings per share attributable to Quest Diagnostics' stockholders - basic:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
0.72
|
|
$
|
0.92
|
|
$
|
0.89
|
|
$
|
1.27
|
|
$
|
3.80
|
|
||||
|
Income from discontinued operations
|
—
|
|
—
|
|
—
|
|
0.03
|
|
0.03
|
|
|||||||||
|
Net income
|
$
|
0.72
|
|
$
|
0.92
|
|
$
|
0.89
|
|
$
|
1.30
|
|
$
|
3.83
|
|
||||
|
Earnings per share attributable to Quest Diagnostics' stockholders - diluted:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
0.71
|
|
$
|
0.92
|
|
$
|
0.88
|
|
$
|
1.26
|
|
$
|
3.78
|
|
||||
|
Income from discontinued operations
|
—
|
|
—
|
|
—
|
|
0.03
|
|
0.03
|
|
|||||||||
|
Net income
|
$
|
0.71
|
|
$
|
0.92
|
|
$
|
0.88
|
|
$
|
1.29
|
|
$
|
3.81
|
|
||||
|
2013 (a)
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
Total
Year
|
||||||||||||||
|
(f)
|
(g)
|
(h)
|
(i)
|
||||||||||||||||
|
Net revenues
|
$
|
1,787
|
|
$
|
1,815
|
|
$
|
1,788
|
|
$
|
1,756
|
|
$
|
7,146
|
|
||||
|
Gross profit
|
695
|
|
721
|
|
699
|
|
705
|
|
2,820
|
|
|||||||||
|
Income from continuing operations
|
124
|
|
161
|
|
412
|
|
151
|
|
848
|
|
|||||||||
|
Income from discontinued operations, net of taxes
|
20
|
|
13
|
|
2
|
|
—
|
|
35
|
|
|||||||||
|
Net income
|
144
|
|
174
|
|
414
|
|
151
|
|
883
|
|
|||||||||
|
Less: Net income attributable to noncontrolling interests
|
8
|
|
9
|
|
9
|
|
8
|
|
34
|
|
|||||||||
|
Net income attributable to Quest Diagnostics
|
$
|
136
|
|
$
|
165
|
|
$
|
405
|
|
$
|
143
|
|
$
|
849
|
|
||||
|
Amounts attributable to Quest Diagnostics' stockholders:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
116
|
|
$
|
152
|
|
$
|
403
|
|
$
|
143
|
|
$
|
814
|
|
||||
|
Income from discontinued operations, net of taxes
|
20
|
|
13
|
|
2
|
|
—
|
|
35
|
|
|||||||||
|
Net income
|
$
|
136
|
|
$
|
165
|
|
$
|
405
|
|
$
|
143
|
|
$
|
849
|
|
||||
|
Earnings per share attributable to Quest Diagnostics' stockholders - basic:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
0.73
|
|
$
|
0.99
|
|
$
|
2.68
|
|
$
|
0.98
|
|
$
|
5.35
|
|
||||
|
Income (loss) from discontinued operations
|
0.13
|
|
0.08
|
|
0.02
|
|
(0.01
|
)
|
0.23
|
|
|||||||||
|
Net income
|
$
|
0.86
|
|
$
|
1.07
|
|
$
|
2.70
|
|
$
|
0.97
|
|
$
|
5.58
|
|
||||
|
Earnings per share attributable to Quest Diagnostics' stockholders - diluted:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
0.72
|
|
$
|
0.99
|
|
$
|
2.66
|
|
$
|
0.97
|
|
$
|
5.31
|
|
||||
|
Income from discontinued operations
|
0.13
|
|
0.08
|
|
0.02
|
|
—
|
|
0.23
|
|
|||||||||
|
Net income
|
$
|
0.85
|
|
$
|
1.07
|
|
$
|
2.68
|
|
$
|
0.97
|
|
$
|
5.54
|
|
||||
|
(a)
|
In December 2012, the Company committed to a plan to sell HemoCue and completed the sale of OralDNA. During the third quarter of 2006, the Company completed its wind down of NID and classified the operations of NID as discontinued operations. Results of operations have been prepared to report the results of HemoCue, OralDNA and NID as discontinued operations for all periods presented (see Note 18).
|
|
(b)
|
Includes pre-tax charges of
$24 million
, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating the Company. Of these costs,
$12 million
and
$12 million
were included in cost of services and selling, general and administrative expenses, respectively. Also includes pre-tax charges in selling, general and administrative expenses of
$4 million
, principally representing costs incurred related to legal matters.
|
|
(c)
|
Includes pre-tax charges of
$27 million
, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating the Company. Of these costs,
$11 million
and
$16 million
were included in cost of services and selling, general and administrative expenses, respectively. Also includes pre-tax charges in selling, general and administrative expenses of
$7 million
, principally representing costs incurred related to legal matters.
|
|
(d)
|
Includes pre-tax charges of
$40 million
, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating the Company. Of these costs,
$14 million
,
$25 million
and
$1 million
were included in cost of services, selling, general and administrative expenses and other operating (income) expense, net, respectively. Also includes pre-tax charges in selling, general and administrative expenses of
$8 million
, principally representing costs incurred related to legal matters.
|
|
(e)
|
Includes pre-tax charges of
$30 million
, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating the Company. Of these costs,
$13 million
,
$16 million
and
$1 million
were included in cost of services, selling, general and administrative expenses and other operating (income) expense, net, respectively. Also includes pre-tax charges in selling, general and administrative expenses of
$5 million
, principally representing costs incurred related to legal matters, and a pre-tax gain included in other operating (income) expense, net of
|
|
(f)
|
Includes pre-tax charges of
$45 million
, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating the Company. Of these costs,
$18 million
and
$27 million
were included in cost of services and selling, general and administrative expenses, respectively.
|
|
(g)
|
Includes pre-tax charges of
$19 million
, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating the Company. Of these costs,
$7 million
and
$12 million
were included in cost of services and selling, general and administrative expenses, respectively. Income from discontinued operations, net of taxes includes a gain on the sale of HemoCue of
$14 million
(see Note 18).
|
|
(h)
|
Includes pre-tax charges of
$39 million
, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating the Company. Of these costs,
$11 million
and
$28 million
were included in cost of services and selling, general and administrative expenses, respectively. Also includes pre-tax gain on sale of royalty rights of
$474 million
and the pre-tax loss of
$40 million
associated with the sale of the Enterix (see Note 6).
|
|
(i)
|
Includes pre-tax charges of
$12 million
, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating the Company. Of these costs,
$7 million
and
$5 million
were included in cost of services and selling, general and administrative expenses, respectively.
|
|
Balance at
1-1-14
|
Provision for Doubtful Accounts
|
Net Deductions
and Other
|
Balance at
12-31-14
|
||||||||||||
|
Year Ended December 31, 2014
|
|||||||||||||||
|
Doubtful accounts and allowances
|
$
|
236
|
|
$
|
296
|
|
$
|
282
|
|
(a)
|
$
|
250
|
|
||
|
Balance at
1-1-13
|
Provision for Doubtful Accounts
|
Net Deductions
and Other
|
Balance at
12-31-13
|
||||||||||||
|
Year Ended December 31, 2013
|
|||||||||||||||
|
Doubtful accounts and allowances
|
$
|
236
|
|
$
|
270
|
|
$
|
270
|
|
(a)
|
$
|
236
|
|
||
|
Balance at
1-1-12
|
Provision for Doubtful Accounts
|
Net Deductions
and Other
|
Balance at
12-31-12
|
||||||||||||
|
Year Ended December 31, 2012
|
|||||||||||||||
|
Doubtful accounts and allowances
|
$
|
237
|
|
$
|
269
|
|
$
|
270
|
|
(a)
|
$
|
236
|
|
||
|
(a)
|
Primarily represents the write-off of accounts receivable, net of recoveries.
|
|
Exhibit
Number
|
Description
|
|
3.1
|
Restated Certificate of Incorporation (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: May 20, 2014) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
3.2
|
Amended and Restated By-Laws of the Company (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: May 20, 2014) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.1
|
Form of 5.45% Exchange Senior Note due 2015 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: November 1, 2005) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.2
|
Form of 6.40% Senior Note due 2017 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: June 19, 2007) and incorporated herein by reference) (Commission file Number 001-12215)
|
|
4.3
|
Form of 6.95% Senior Note due 2037 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: June 19, 2007) and incorporated herein by reference) (Commission file Number 001-12215)
|
|
4.4
|
Form of 4.750% Senior Note due 2020 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: November 17, 2009) and incorporated herein by reference) (Commission file Number 001-12215)
|
|
4.5
|
Form of 5.750% Senior Note due 2040 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: November 17, 2009) and incorporated herein by reference) (Commission file Number 001-12215)
|
|
4.6
|
Form of 3.200% Senior Note due 2016 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: March 21, 2011) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.7
|
Form of 4.700% Senior Note due 2021 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: March 21, 2011) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.8
|
Form of 2.700% Senior Note due 2019 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: March 12, 2014) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.9
|
Form of 4.250% Senior Note due 2024 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: March 12, 2014) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.10
|
Indenture dated as of June 27, 2001, among the Company, the Subsidiary Guarantors, and the Trustee (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: June 27, 2001) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.11
|
First Supplemental Indenture, dated as of June 27, 2001, among the Company, the Subsidiary Guarantors, and The Bank of New York (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: June 27, 2001) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.12
|
Second Supplemental Indenture, dated as of November 26, 2001, among the Company, the Subsidiary Guarantors, and The Bank of New York (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: November 26, 2001) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.13
|
Third Supplemental Indenture, dated as of April 4, 2002, among the Company, the Additional Subsidiary Guarantors, and The Bank of New York (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: April 1, 2002) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.14
|
Fourth Supplemental Indenture dated as of March 19, 2003, among Unilab Corporation (f/k/a Quest Diagnostics Newco Incorporated), the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's quarterly report on Form 10-Q for the quarter ended March 31, 2003 and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.15
|
Fifth Supplemental Indenture dated as of April 16, 2004, among Unilab Acquisition Corporation (d/b/a FNA Clinics of America), the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's quarterly report on Form 10-Q for the quarter ended March 31, 2004 and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.16
|
Sixth Supplemental Indenture dated as of October 31, 2005, among the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: October 31, 2005) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.17
|
Seventh Supplemental Indenture dated as of November 21, 2005, among the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: November 21, 2005) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.18
|
Eighth Supplemental Indenture dated as of July 31, 2006, among the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: July 31, 2006) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.19
|
Ninth Supplemental Indenture dated as of September 30, 2006, among the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: September 30, 2006) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.20
|
Tenth Supplemental Indenture dated as of June 22, 2007, among the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: June 19, 2007) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.21
|
Eleventh Supplemental Indenture dated as of June 22, 2007, among the Company, The Bank of New York, and the Additional Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: June 19, 2007) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.22
|
Twelfth Supplemental Indenture dated as of June 25, 2007, among the Company, The Bank of New York, and the Additional Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: June 19, 2007) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.23
|
Thirteenth Supplemental Indenture dated as of November 17, 2009, among the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: November 17, 2009) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.24
|
Fourteenth Supplemental Indenture dated as of March 24, 2011, among the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: March 21, 2011) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.25
|
Fifteenth Supplemental Indenture dated as of November 30, 2011, among the Company, The Bank of New York Mellon Trust Company, N.A., as successor trustee to The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's 2011 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.26
|
Sixteenth Supplemental Indenture dated as of March 12, 2014, among the Company, The Bank of New York Mellon Trust Company, N.A., (filed as an Exhibit to the Company's current report on Form 8-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.1‡
|
Amended and Restated Employee Stock Purchase Plan (filed as an Exhibit to the Company's quarterly report on form 10-Q for the quarter ended June 30, 2014 and incorporated herein by reference) (Commission file number 001-12215)
|
|
10.2‡
|
Amended and Restated Quest Diagnostics Incorporated Employee Long-Term Incentive Plan as amended February 14, 2014 (filed as an exhibit to the Company's 2013 annual report on Form 10-K and incorporated herein by reference) (Commission file number 001-12215)
|
|
10.3‡
|
Amended and Restated Deferred Compensation Plan For Directors as amended October 31, 2008 (filed as an Exhibit to the Company's 2008 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.4‡
|
Form of Equity Award Agreement dated as of February 25, 2013 (filed as an Exhibit to the Company's quarterly Report on Form 10-Q for the quarter ended March 31, 2013 and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.5‡
|
Form of Equity Award Agreement dated as of August 19, 2013 (filed as an Exhibit to the Company's quarterly Report on Form 10-Q for the quarter ended September 30, 2013 and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.6‡
|
Quest Diagnostics Supplemental Deferred Compensation Plan (Post 2004) amended December 22, 2008 (filed as an Exhibit to the Company's 2008 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.7‡
|
Amendment No. 1 dated November 27, 2012 to Quest Diagnostics Supplemental Deferred Compensation Plan (Post 2004) amended December 22, 2008 (filed as an Exhibit to the Company's 2012 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.8‡
|
Quest Diagnostics Supplemental Deferred Compensation Plan (Pre-2005) amended and restated November 27, 2012 (filed as an Exhibit to the Company's 2012 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.9‡
|
Senior Management Incentive Plan (filed as Appendix A to the Company's Definitive Proxy Statement dated March 28, 2003 and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.10‡
|
Amended and Restated Quest Diagnostics Incorporated Executive Officer Severance Plan (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: May 20, 2014) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.11‡
|
AmeriPath Group Holdings, Inc. 2006 Stock Option and Restricted Stock Purchase Plan (filed as an Exhibit to the Company's registration statement on Form S-8 (Registration No. 333-143889 filed with the Commission on June 19, 2007) and incorporated herein by reference) (Commission File Number 333-143889)
|
|
10.12‡
|
Amendment dated as of August 17, 2007 to the AmeriPath Group Holdings, Inc. 2006 Stock Option and Restricted Stock Purchase Plan (filed as an Exhibit to the Company's 2007 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.13
|
The Profit Sharing Plan of Quest Diagnostics Incorporated, Amended and Restated effective as of January 1, 2012 (filed as an Exhibit to the Company's 2012 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.14
|
401(k) Savings Plan of Quest Diagnostics Incorporated, Amended and Restated effective as of January 1, 2012 (filed as an Exhibit to the Company's 2012 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.15‡
|
Form of Non-Employee Director Equity Award Agreement (filed as an Exhibit to the Company's 2011 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.16‡
|
Form of Non-Employee Director Elective Option Award Agreement (filed as an Exhibit to the Company's 2011 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.17‡
|
Amended and Restated Quest Diagnostics Incorporated Long-Term Incentive Plan for Non-Employee Directors as amended February 14, 2014 (filed as an Exhibit to the Company's 2013 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.18‡
|
Employment Agreement between Stephen H. Rusckowski and the Company, dated April 3, 2012 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: April 9, 2012) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.19‡
|
Aircraft Timesharing Agreement dated as of December 17, 2013 between the Company and Stephen H. Rusckowski
|
|
11.1
|
Statement re: Computation of Earnings Per Common Share (the calculation of per share earnings is in Part II, Item 8, Note 3 to the consolidated financial statements (Earnings Per Share) and is omitted in accordance with Item 601(b)(11) of Regulation S-K)
|
|
21.1*
|
Subsidiaries of Quest Diagnostics Incorporated
|
|
23.1*
|
Consent of PricewaterhouseCoopers LLP
|
|
24.1*
|
Power of Attorney (included on signature page)
|
|
31.1*
|
Rule 13a-14(a) Certification of Chief Executive Officer
|
|
31.2*
|
Rule 13a-14(a) Certification of Chief Financial Officer
|
|
32.1**
|
Section 1350 Certification of Chief Executive Officer
|
|
32.2**
|
Section 1350 Certification of Chief Financial Officer
|
|
101.INS*
|
dgx-20141231.xml
|
|
101.SCH*
|
dgx-20141231.xsd
|
|
101.CAL*
|
dgx-20141231_cal.xml
|
|
101.DEF*
|
dgx-20141231_def.xml
|
|
101.LAB*
|
dgx-20141231_lab.xml
|
|
101.PRE*
|
dgx-20141231_pre.xml
|
|
*
|
Filed herewith.
|
|
**
|
Furnished herewith.
|
|
‡
|
Management contract or compensatory plan or arrangement required to be filed as an exhibit to this Form 10-K pursuant to Item 15(b) of Form 10-K.
|