|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|
Common Stock, $.01 par value per share
|
New York Stock Exchange
|
|
Securities registered pursuant to Section 12(g) of the Act:
|
None
|
|
Documents Incorporated by Reference
|
Part of Form 10-K into
which incorporated
|
|
Document
|
|
|
Portions of the registrant's Proxy Statement to be filed by April 30, 2017
|
|
|
Item
|
Page
|
|
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
Item 15.
|
||
|
Index to Tables
|
|
|
Table 1 - Vision, Goals and Values
|
2
|
|
Table 2 - Two Point Business Strategy
|
2
|
|
Table 3 - Portfolio Growth
|
3
|
|
Table 4 - Strategies to Accelerate Growth
|
3
|
|
Table 5 - Key Professional Lab Services Offerings
|
4
|
|
Table 6 - Clinical Franchises
|
4
|
|
Table 7 - Consumer-Centric Initiatives to Accelerate Growth
|
5
|
|
Table 8 - 2016 Consumer-Centric Initiatives
|
5
|
|
Table 9 - Four Major Themes to Drive Operational Excellence
|
6
|
|
Table 10 - Invigorate Cost Excellence Program
|
6
|
|
Table 11 - Invigorate Cost Excellence Program - Savings
|
6
|
|
Table 12 - Positioned to Grow and Continue to Lead
|
7
|
|
Table 13 - Assets and Capabilities
|
8
|
|
Table 14 - New or Enhanced Disease Area Solutions
|
10
|
|
Table 15 - Sample Collaborations
|
12
|
|
Table 16 - 2016 Medical and Scientific
|
13
|
|
Table 17 - Quanum
TM
Health Information Technology Solutions
|
13
|
|
Table 18 - 2016 Net Revenues
|
14
|
|
Table 19 - U.S. Clinical Testing Industry
|
18
|
|
Table 20 - Key Trends
|
18
|
|
Table 21 - Customers
|
21
|
|
Table 22 - Sample Third Party Payers
|
23
|
|
Table 23 - Factors Traditionally Considered When Selecting a Diagnostics Information Services Provider
|
24
|
|
Table 24 - 2016 Medicare and Medicaid Revenues
|
27
|
|
Table 25 - Key Regulatory Schemes
|
28
|
|
Table 26 - Information Available at Our Corporate Governance Webpage
|
30
|
|
Table 27 - Executive Officers
|
31
|
|
Table 1 - Vision, Goals and Values
|
|
|
Vision
|
Empowering Better Health with Diagnostic Insights
|
|
Three Aspirational Goals
|
Promote a healthier world
Build value
Create an inspiring workplace
|
|
Values
|
Quality
Integrity
Accountability
Innovation
Collaboration
Leadership
|
|
Table 2 - Two Point Business Strategy
|
|
1. Accelerate growth
|
|
2. Drive operational excellence
|
|
Table 3 - Portfolio Growth
|
|||
|
Theme
|
Key Characteristics
|
At A Glance
|
Quest Value Proposition
|
|
General Diagnostics
|
Testing services generating strong cash flows and steady growth
|
Routine and non-routine testing services
Largest revenue stream
Essential portion of health care delivery
|
Scale
Operational excellence
Access and convenience
|
|
Advanced Diagnostics
|
Testing services providing faster growth through innovation testing model
|
Genetic and advanced molecular testing services
An important part of precision medicine
A growing set of unique, innovation-based competitors
|
Rich clinical, scientific and medical innovation expertise
Quality and reliability of new assays
Ability to manage potential new regulatory requirements
|
|
Diagnostic Services
|
Laboratory and data-related healthcare opportunities providing faster growth
|
Enables partners to deliver health care more efficiently (
e.g.
, risk assessment; Professional Lab Services; wellness)
Services to support population health (
e.g.
, data analytics; extended care services)
|
Extensive diagnostic capability
Large and growing database and analytics expertise
Partnerships with industry leaders across healthcare landscape
|
|
Table 4 - Strategies to Accelerate Growth
|
|
Organic growth through:
|
|
1. Partnerships with health plans, hospital systems and other risk bearing entities
|
|
2. Offering the broadest access to diagnostic innovation
|
|
3. Recognition as the consumer-friendly provider of diagnostic information services
|
|
4. Supporting population health with data analytics and extended care services
|
|
Additionally:
|
|
5. Grow 1-2% per year through accretive, strategic acquisitions
|
|
Table 5 - Key Professional Lab Services Offerings
|
|
|
Lab management outsourcing
|
Data diagnostics, consolidation and insights
|
|
Joint venture
|
Reference testing
|
|
Outreach acquisition
|
Supply chain management
|
|
Test menu management
|
Programs enabling effective patient care management
|
|
Table 6 - Clinical Franchises
|
|
|
Cardiovascular, Metabolic and Endocrinology
|
Oncology
|
|
General Health and Wellness
|
Prescription Drug Monitoring and Toxicology
|
|
Infectious Diseases and Immunology
|
Sports Science and Human Performance
|
|
Neurology
|
Women’s and Reproductive Health
|
|
Table 7 - Consumer-Centric Initiatives to Accelerate Growth
|
|
|
Consistent and superior consumer experience
|
Retail consumer partnerships
|
|
Information and connection
|
Consumer testing offerings
|
|
Table 8 - 2016 Consumer-Centric Initiatives
|
|
• Launched enhanced patient experience, including real-time payment determination for several major payers and electronic check-in.
|
|
• Announced partnership with Safeway to expand convenient access to testing services at select Safeway locations across the United States.
|
|
• Launched QuestDirect
TM
, our patient-initiated testing service, in Colorado and Missouri. Patient-initiated testing also is available in Arizona.
|
|
• >3.5 million registered users in our MyQuest
®
health portal and mobile connectivity solution. Implemented MyQuest Advanced Access
®
, which enables patients to access their historical laboratory test results and trends.
|
|
• Launched Blueprint for Athletes
®
, our service to empower athletes to track their progress and training, in the consumer market.
|
|
• Announced multi-year global collaboration with AncestryDNA to provide genotyping test services.
|
|
Table 9 - Four Major Themes to Drive Operational Excellence
|
|
|
Major Themes
|
Examples
|
|
Reduce denials by payers and bad debt
|
• Patient payment transparency
• Real-time payment determination
• Optum partnership
|
|
Standardize
|
• Lab information and billing systems
•
In vitro diagnostics
• Lab equipment asset management, maintenance and service
• Lab test menus
|
|
Enable digital services
|
• Pre-visit registration
• Enhanced appointment scheduling
• Patient check-in
• Test requisitions
• Same day sample pickup
• Ordering supplies
|
|
Optimize/Automate
|
• Optimize lab, patient services and administrative networks
• Automate microbiology and rest of laboratory
• Continuous improvement
|
|
Table 10 - Invigorate Cost Excellence Program
|
|
|
Flagship Programs
|
|
|
Revenue services
|
Organizational design
|
|
Information technology
|
Procurement
|
|
Laboratory
|
Field and customer service
|
|
Four Major Themes
|
|
|
Reduce denials by payers and bad debt
|
|
|
Standardize
|
|
|
Enable digital services
|
|
|
Optimize/Automate
|
|
|
Table 11 - Invigorate Cost Excellence Program - Savings
|
|
|
2012 Goal for run-rate savings exiting 2014
|
$600 Million
|
|
2013 Revised goal for run-rate savings exiting 2014
|
$700 Million
|
|
2014 Year-end run-rate savings
|
>$700 Million
|
|
2014 Goal for run-rate savings exiting 2017
|
$1.3 Billion
|
|
2015 Year-end run-rate savings
|
$990 Million
|
|
2016 Year-end run-rate savings
|
>$1.1 Billion
|
|
Table 12 - Positioned to Grow and Continue to Lead
|
|
|
•
A foundation of strong operating principles
|
•
Unmatched size, scale and capabilities
|
|
•
Leader in providing innovative solutions and diagnostic insights
|
•
Strong focus on quality and providing a superior customer experience
|
|
•
Strong collaborator, and strong relationships with healthcare stakeholders
|
•
Medical and scientific expertise
|
|
Table 13 - Assets and Capabilities
|
|
|
•
Provide healthcare connectivity solutions to >250,000 clinician and hospital accounts and interface with >675 EMRs
|
•
Own or control approximately 700 issued and 570 pending patents worldwide
|
|
•
Strong logistics capabilities
•
make nearly 80,000 stops daily
•
approximately 3,700 courier vehicles
•
23 aircraft serving the U.S.
|
•
One of the largest medical and scientific staffs in the industry to provide interpretive consultation
•
>650 M.D.s and Ph.D.s, many of whom are recognized leaders in their field
•
genetic counselors
|
|
•
>20,000 phlebotomists, paramedics, nurses and other health and wellness professionals
|
•
National access to patient testing, with most extensive network in the U.S., including phlebotomists in physician offices and >2,200 of our own patient service centers
|
|
•
Access to approximately 80% of U.S. insured lives
|
•
Processed over 160 million test requisitions in 2016
|
|
•
Industry-leading test menu
|
•
Access to >20 billion patient data points from test results delivered over past decade
|
|
Table 14 - New or Enhanced Disease Area Solutions
|
|
|
Cardiovascular, Metabolic and Endocrinology
|
We expanded our cardiovascular testing menu with the addition of the
CardioIQ
®
Advanced Lipid Panel and Inflammation Panel, which offers a more comprehensive assessment of risk for dyslipidemia and cardiovascular disease than a standard lipid panel.
We also introduced the Lp-PLA
2
Activity test. This test detects Lp-PLA
2
, an inflammation marker, to help assess, along with other traditional cardiovascular risk factor measures, risk of coronary artery disease and stroke.
|
|
Infectious Diseases and Immunology
|
We developed and launched a hepatitis C virus Genotype 3 NS5a resistance test. This test detects polymorphisms in the hepatitis C virus to insure that physicians prescribe the appropriate therapies.
We were the first commercial laboratory in the U.S. to offer a hepatitis B surface antigen quantitative test. This test is used for monitoring hepatitis B patients, enabling physicians to make better therapeutic decisions.
We received Emergency Use Authorization from the U.S. Food and Drug Administration for the Zika Virus RNA Qualitative Real-Time RT-PCR test; the test was the first from a commercial laboratory provider to be granted an Emergency Use Authorization for testing patients for Zika virus RNA. We now offer the complete suite of Zika tests (molecular/serology) for urine and serum samples.
We launched Borrelia miyamotoi DNA, Real Time PCR to aid in the diagnosis in humans of infection with Borrelia miyamotoi, a tick-borne infection.
|
|
Neurology
|
We introduced Cognisense
®
, a digital cognitive assessment tool that aids in a physician's assessment and diagnosis of individuals with cognitive dysfunction. It is designed to overcome several limitations of conventional paper-based cognitive assessment.
We began clinical implementation of an integrated dementia diagnostic solution based on our collaboration with UCSF. This offering integrates laboratory testing, cognitive exam, MRI and clinical evaluation to help primary doctors assess and diagnose dementia to identify treatable cause, shorten time to diagnosis and eliminate waste.
We introduced Myasthenia Gravis Panel 2 with Reflex to MuSK Antibody, enabling diagnosing myasthenia gravis by detecting hallmark diagnostic autoantibodies to neuromuscular transmitter Acetylcholine Receptor (AChR), and capturing additional cases by detecting autoantibodies to Muscle Specific Tyrosine Kinase (MuSK) in AChR antibody negative cases.
|
|
Oncology
|
We introduced IBM Watson
®
Genomics from Quest Diagnostics, a service that provides actionable insight tailored to the specific makeup of a patient's tumor by combining Quest's state of the art tumor analysis with an annotation service driven by IBM Watson's cognitive computing capability and the leading expertise of Memorial Sloan Kettering Cancer Center. The Broad Institute of MIT and Harvard is providing additional genome sequencing capabilities.
As part of our QuestVantage
TM
cancer test menu, we announced three new cancer test services regarding the risk of developing hereditary forms of cancer:
MYvantage
TM
34-gene Hereditary Comprehensive Cancer panel, which includes 34 high risk, moderate risk and emerging risk genes associated with a broad spectrum of hereditary cancers;
Glvantage
TM
Hereditary Colorectal Cancer Test, which includes 13 genes predominantly associated with colon and gastric cancers; and
Qvantage
TM
Hereditary Women's Health cancer test, which includes 14 genes predominantly associated with breast, colon, uterine and ovarian cancers.
We also introduced an expansion of our complementary test service for anti-PD-1 therapy to include melanoma, the third offering in our precision medicine menu for oncology immunotherapies.
|
|
Prescription Drug Monitoring and Toxicology
|
We launched Synthetic Cannabinoids Screen with Confirmation, Urine. This test enables physicians to detect use of a wide variety of psychoactive designer drugs, made with dried, shredded plant materials and chemical additives, that induce psychotic effects.
We introduced Pain Management, Naltrexone, Quantitative, Urine, providing physicians with an option for compliance monitoring for naltrexone use.
We offered Drug Toxicology Alcohol Metabolite, Quantitative, Oral Fluid, expanding our toxicology offerings.
|
|
Women’s and Reproductive Health
|
We offered QNatal Advanced
®
, our noninvasive prenatal screening service for detecting chromosomal abnormalities, to all pregnant women.
We introduced our expanded Prenatal Carrier Panel, which tests for whether the person carries a gene for genetic disorder. Our cystic fibrosis panel now tests for over 160 mutations.
We made it simpler for clinicians to order all guideline-supported cervical cancer screening tests based on a woman's age.
|
|
Table 15 - Sample Collaborations
|
|
|
Collaborator
|
Collaboration
|
|
IBM Watson
®
Health, Memorial Sloan Kettering Cancer Center and the Broad Institute of MIT and Harvard
|
IBM Watson
®
Genomics from Quest Diagnostics, a service that helps advance precision medicine by cognitive computing with genomic tumor sequencing. Memorial Sloan Kettering Cancer Center is supplementing Watson's corpus of scientific data with a precision oncology knowledge base to help inform precision treatment options for cancer patients, and the Broad Institute of MIT and Harvard is providing additional genome sequencing capabilities.
|
|
Optum
|
Our billing operations became part of Optum, helping us to reduce the complexity of our billing processes and fostering increased transparency of health care costs.
Advance new technology services to digitize our customer orders and workflows, with the goals of reducing bad debt and payer denials and increasing operational efficiency and productivity.
Increase the use of diagnostic information services, such as data analytics, population health insights and connectivity solutions, to help improve health care effectiveness and manage costs for health plans and care providers.
We became Optum's primary partner for member biometric screening services that Optum provides to employers and health plans.
|
|
AncestryDNA
|
We provide testing to help meet the rapidly growing consumer demand for genetic tests that provide insights into genetic ethnicity, origins and other factors.
Explore additional opportunities such as developing tools and applications to guide people on building and understanding their "family medical tree."
|
|
Safeway
|
We are providing diagnostic testing services in company-branded patient service centers in Safeway locations, enhancing convenient access to our services and diagnostic insights for patients
|
|
Inovalon
|
Data Diagnostics
®
, a tool that provides real-time patient-specific data analysis that clinicians can order at the point of care to identify and address gaps in quality, risk, utilization and medical history insights.
|
|
Inserm, the French National
Institute of Health and
Medical Research
|
BRCA Share
TM
, a novel data share initiative to provide scientists and laboratory organizations around the world with open access to BRCA1 and BRCA2 genetic data.
|
|
Perinatal Quality Foundation
|
The national initiative to advance clinically appropriate noninvasive prenatal screening.
|
|
University of California, San Francisco, the nation's leading university focused exclusively on health
|
To accelerate the translation of biomedical research into advanced diagnostics in the field of precision medicine. This collaboration has the overarching aim of enabling holistic and integrated diagnostic solutions that close gaps in care or enable new clinical value, with initial focus areas including autism, oncology, neurology and women's health.
|
|
U.S. Centers for Disease Control and Prevention ("CDC")
|
To improve public health analysis of hepatitis C screening, diagnosis and treatment, based on analysis of our database of national hepatitis C virus ("HCV") diagnostic information.
With CDC and the American Medical Association, to assess the prevalence of pre-diabetes.
|
|
National Institutes of Health
|
We participate in studies they sponsor (
e.g
., NIH National Children Study).
|
|
Quintiles IMS Holdings, Inc.
|
Joint venture, Q
2
Solutions
TM
, providing central lab testing services for clinical trials.
|
|
Table 16 - 2016 Medical and Scientific
|
|
|
Authored more than 130 publications, including approximately 80 articles in peer-reviewed journals
|
•
Insights into diagnostic testing; introduce novel diagnostic approaches; provide latest thinking in lab testing and disease diagnosis
- Addressed such topics as genetic testing in cancer, improving assessment of cardiovascular disease risk and noninvasive prenatal screening.
|
|
Authored textbooks or chapters
|
•
Used by academic institutions to train healthcare providers
|
|
Participated on scientific committees determining guidelines for diagnostic usage
|
•
Fields include HIV, HCV and testosterone testing
|
|
Published Quest Diagnostics Health Trends
TM
reports
|
•
Identify trends in disease and wellness.
- Recent reports focused on blood levels of lead in children and trends in rotavirus detection.
|
|
Table 17 - Quanum
TM
Health Information Technology Solutions
|
|
|
Analytics Solutions
|
|
|
Lab Utilization
|
On Demand Informatics
|
|
Data Diagnostics
®
|
Condition Management
|
|
Clinical and Financial Solutions
|
|
|
eLabs
|
Electronic Health Record System
|
|
ePrescribing
|
Practice Management
|
|
Interactive Insights
|
Revenue Cycle Management
|
|
Table 18 - 2016 Net Revenues
|
|
|
Activity
|
Approximate Percentage
of 2016 Net Revenues
|
|
Diagnostic Information Services
|
95
|
|
Routine clinical testing services
|
56
|
|
Gene-based and esoteric (including advanced diagnostics) testing services
|
31
|
|
Anatomic pathology testing services
|
8
|
|
Diagnostic Solutions: Healthcare information technology and risk assessment services
|
5
|
|
•
|
enhance the customer experience, including ease of use and patient and provider engagement;
|
|
•
|
deliver more precise, comprehensive solutions and actionable information;
|
|
•
|
provide increased and interactive insights and analytics to patients and providers;
|
|
•
|
foster greater adherence to clinical and reimbursement guidelines;
|
|
•
|
promote population health solutions;
|
|
•
|
tap the potential of large amounts of clinical information; and
|
|
•
|
advance the development of precision medicine.
|
|
Table 19 - U.S. Clinical Testing Industry
|
||
|
Testing
|
Approximate % of Total Industry
|
|
|
•
Hospital inpatient and outpatient testing
|
37%
|
|
|
•
Testing of persons who are not hospital patients, including testing done in commercial clinical laboratories, physician-office laboratories and other locations, as well as hospital outreach (non-hospital patients) testing
|
63%
Consists of approximately:
35% - hospital-affiliated laboratories
54% - commercial clinical laboratories
Balance - physician-office laboratories and other locations
|
|
|
Table 20 - Key Trends
|
|
|
Demographics
|
As the population continues to grow and age, the burden of chronic diseases and unmet diagnostic needs may increase the demand for diagnostic information services.
|
|
Prevention and wellness
|
We believe that the value of detection, prevention, wellness and personalized care now is well recognized. Consumers, employers, ACOs, IDNs, health plans and government agencies increasingly focus on helping the healthy stay healthy, detecting symptoms among those at risk and providing preventive insight and care that helps avoid disease.
Healthcare providers increasingly rely on diagnostic information services to help identify risk for a disease, to detect the symptoms of disease earlier, to aid in the choice of therapeutic regimen, to monitor patient compliance and to evaluate treatment results.
There is increased focus on a disease-oriented approach to diagnostics, treatment and management. Healthcare providers, consumers and payers increasingly recognize the value of diagnostic information services as a means to improve health and reduce the overall cost of healthcare through early detection, prevention and treatment.
|
|
Medical innovation
|
Medical advances allow for more accurate and earlier diagnosis and treatment of diseases.
Continuing advances in genomics and proteomics are expected to yield new, more sophisticated and specialized diagnostic tests. These advances also are spurring interest in and demand for precision medicine, which relies on diagnostic and prognostic testing and in which data information services and strategies are used to deliver the most effective healthcare to the right populations and individuals.
Pharmacogenomic testing increasingly is used as a parameter to help speed drug approval processes and to better focus therapy based on patient and tumor-specific genetic markers.
Demand also is growing toward comprehensive care management solutions that serve patients, payers and healthcare providers by improving clinical decision support and access to patient data, and by increasing patient participation in care management and population health management.
There is increasing focus on access to patient data and data-driven insights.
|
|
Customers and payers
|
Our customers and payers, including clinicians, health plans, IDNs, ACOs, employers and others, have been consolidating, converging and diversifying. For example, an increased number of hospital systems are considering establishing or have established health insurance plans, and health insurance plans are considering providing or are providing healthcare services.
Consolidation is increasing pricing transparency and bargaining power, and may encourage internalization of clinical testing.
Physicians increasingly are employed by hospital systems, IDNs, ACOs or large group practices integrated with healthcare systems, instead of organizing physician-owned practices, which is changing the dynamics for whether clinical testing is performed in or outside of a hospital. Physicians and other clinicians also increasingly are being employed by health plans or their affiliates.
Value-based reimbursement is contributing to changes in the healthcare system. ACOs and patient-centered medical homes are growing as a means to deliver patient care. Healthcare services increasingly are being provided by non-traditional providers (
e.g
., physician assistants), in non-traditional venues (
e.g
., retail medical clinics, urgent care centers) and using new technologies (
e.g
., telemedicine).
In addition, federal healthcare reform legislation adopted in 2010, the Affordable Care Act ("ACA"), is resulting in changes in the way that some healthcare services are purchased and delivered in the United States.
Patients are also our customers. Increasingly, patients are engaged in their own healthcare and are bearing increased responsibility for payment for the services that we provide to them.
|
|
Pricing transparency
|
There has been a trend toward greater pricing transparency in the healthcare marketplace.
This transparency, combined with increased patient financial responsibility for medical care, is enhancing purchasing sophistication and changes in behavior in the healthcare marketplace.
|
|
Competition
|
The clinical testing industry remains fragmented, is highly competitive and is subject to new competition.
Competition is growing from non-traditional competitors. Increased hospital acquisitions of physician practices enhance clinician ties to hospital-affiliated laboratories and may strengthen their competitive position.
New industry entrants with extensive resources may make acquisitions or expand into our traditional areas of operations.
|
|
Reimbursement pressure
|
There is a strong focus in the United States on controlling the overall cost of healthcare.
Healthcare market participants, including governments, are focused on controlling costs, including potentially by changing reimbursement for healthcare services (
e.g
., shift from fee for service to capitation), changing medical coverage policies (
e.g
., healthcare benefits design), pre-authorization of laboratory testing, requiring co-pays, introducing laboratory spend management utilities and payment and patient care innovations such as ACOs and patient-centered medical homes.
In light of continued pressure to reduce systemic healthcare costs, hospitals may change their approach to providing clinical testing services.
While pressure to control healthcare costs poses a risk to our Company, it also creates opportunities, such as an opportunity for increased proper utilization of testing as an efficient means to manage the total cost of healthcare. We believe that it also creates greater opportunities for high value, low-cost providers, like our Company, as compared to other providers.
|
|
Healthcare utilization
|
In the past few years, healthcare utilization in the United States has fluctuated based on a number of factors. These factors include, without limitation, the economy, healthcare benefits design, patients delaying medical care and increased patient financial responsibility for medical care.
The ACA contained provisions eliminating patient cost-sharing for preventive services, and additional provisions that we believe have increased the number of patients that have health insurance, including Medicaid, and thus better access to diagnostic testing.
|
|
Legislative, regulatory and policy environment
|
Government oversight of and attention to the healthcare industry in the United States is significant and increasing; healthcare payment reform is a top issue.
In 2015, the President of the United States launched the Precision Medicine Initiative to improve health and treat disease. The Initiative was intended to pioneer a new model of patient-powered research that promised to accelerate biomedical discoveries and provide clinicians with new tools, knowledge and therapies to select which treatments will work best for patients.
Pursuant to the federal Protecting Access to Medicare Act of 2014, which is targeted for implementation in 2018, it is expected that the Centers for Medicare and Medicaid Services (“CMS”) will revise reimbursement schedules for clinical laboratory testing services provided under Medicare. While we cannot determine the impact until we see the revised pricing schedules, we continue to believe that the impact will be manageable.
The FDA previously announced guidance initiatives that may impact the clinical laboratory testing business, including by increasing regulation of laboratory-developed tests ("LDTs"). More recently, it has offered suggestions for legislation to address this issue.
The ACA has created significant uncertainty as healthcare markets react to changes. For example, more than half of the states have opted in to Medicaid expansion and employers may discontinue offering group health insurance to their employees, shifting more people to exchange products.
The President of the United States has announced that he favors repealing the ACA in 2017, and leaders of the Republication-controlled federal legislature also have expressed a desire to repeal the ACA. The scope and timing of any legislation to repeal, amend, replace, or reform the ACA is uncertain, but if such legislation were to become law, it could have a significant impact on the U.S. healthcare system. In addition, uncertainty regarding the ACA prior to any such repeal, amendment, replacement or reform could create uncertainty generally in the healthcare market.
|
|
Globalization
|
There is a growing demand for healthcare services in emerging market countries.
Opportunities are arising to participate in the restructuring or growth of the healthcare systems outside the United States.
Demographic changes globally also may create opportunities.
|
|
Informatics; technology
|
The increased availability of healthcare data, including data made available as a result of next generation DNA sequencing, and the increased ability to effectively analyze that data at population and patient levels, is impacting healthcare practices. It is anticipated that the increased use of data in healthcare, coupled with mobile healthcare IT solutions for doctors and patients, will help to improve patient outcomes and reduce overall healthcare costs.
Informatics, including integrated diagnostic and decision support solutions, predictive analytics, use of population data and healthcare information technology, is spurring advances in precision medicine, including medical decision making and value, for populations and individuals.
There is a need for technology solutions to harness these opportunities. In addition, new technology, social media and mobile technology are changing the way that healthcare markets interact with each other, and the expectations that they have about how services are provided, what services are provided, and other capabilities of healthcare market participants. These developments are creating new opportunities and new challenges and disrupting the healthcare environment.
Healthcare market participants, including pharmaceutical companies, health plans, clinicians, ACOs and IDNs, are striving to leverage interoperability, informatics and analytics to positively influence the health of patient populations.
|
|
Table 21 - Customers
|
|
|
Health plans including managed care organizations and other health insurance providers
|
These customers typically reimburse us as a contracted (or out-of-network) provider on behalf of their members. In certain locations, health plans may delegate to independent physician associations (“IPAs”) or other alternative delivery systems (
e.g
., physician hospital organizations, ACOs, patient-centered medical homes) the ability to negotiate for diagnostic information services on behalf of certain members.
Health plans and IPAs often require that diagnostic information services providers accept discounted fee structures or assume all or a portion of the financial risk associated with providing such services through capitated payment arrangements. Under capitated payment arrangements, we provide services at a predetermined monthly reimbursement rate for each covered member, generally regardless of the number or cost of services provided by us. Reimbursement under programs that do not provide for capitated payments is typically negotiated on a fee-for-service basis.
Reimbursement from our five largest health plans totaled approximately 20%, and no one health plan accounted for 10%, of our consolidated net revenues in 2016. Health plans typically negotiate directly or indirectly with a number of diagnostic information services providers, and represent approximately one-half of our total clinical testing volumes and one-half of our net revenues from diagnostic information services. There has been a trend of consolidation among health plans. Some health plans also have narrowed their provider networks.
We are also sometimes a member of a “complementary network.” A complementary network generally is a set of contractual arrangements that a third party will maintain with various providers that provide discounted fees for the benefit of its customers. A member of a health plan may choose to access a non-contracted provider that is a member of a complementary network; if so, the provider will be reimbursed at a rate negotiated by the complementary network.
We attempt to strengthen our relationships with health plans and increase the volume of our services for their members by offering to health plans services and programs that leverage our Company's expertise and resources, including our superior access, extensive test menu, medical staff, data, IT solutions, and wellness and population health management capabilities.
|
|
Clinicians
|
Clinicians, including primary care physicians, specialists and physician assistants, requiring diagnostic information services for patients are the primary referral source of our services.
Clinicians determine which laboratory to recommend or use based on a variety of factors, including those set forth in Table 23 on page 24.
|
|
Hospitals
|
We believe that we are the industry's leader in servicing hospitals. We provide services to hospitals throughout the United States, including advanced testing services, in some cases helping manage their laboratories and serving as the medical directors of the hospital's histology or clinical laboratory, including through our Professional Laboratory Services offerings.
Hospitals generally maintain an on-site laboratory to perform the significant majority of clinical testing for their patients (inpatients or out patients) and refer certain testing to outside service providers, which typically charge the hospitals on a negotiated fee-for-service basis. Fee schedules for hospital reference testing services often are negotiated on behalf of hospitals by group purchasing organizations.
Hospitals also provide outreach testing, and historically were able to negotiate higher reimbursement rates with health plans than commercial clinical laboratories for comparable services. They may seek to leverage their relationships with community clinicians by encouraging the clinicians to send their outreach testing to the hospital's laboratory. In addition, hospitals that own physician practices may require the practices to refer outreach testing to the hospital's affiliated laboratory. In recent years, there has been a trend of hospitals acquiring physician practices, and as a result, an increased percentage of physician practices are owned by hospitals. Increased hospital acquisitions of physician practices enhance clinician ties to hospital-affiliated laboratories and may strengthen their competitive position.
We also have joint venture arrangements with leading hospitals or IDNs in several metropolitan areas. These joint venture arrangements, which provide diagnostic information services for affiliated hospitals as well as for unaffiliated clinicians and other local healthcare providers, serve as our principal facilities in their service areas. Typically, we have either a majority ownership interest in, or day-to-day management responsibilities for, our joint venture relationships.
In light of continued pressure to reduce systemic healthcare costs, hospitals may change their approach to providing clinical testing services, including by seeking ways to improve profitability or to better utilize their laboratory capacity. We believe that our combination of services positions us to be an attractive partner for hospitals, offering a full range of strategic relationships.
|
|
ACOs and IDNs
|
An ACO is a network of providers and facilities that share financial risk in providing or arranging for the provision of healthcare. An IDN is a network of providers and facilities working together in providing or arranging for the provision of healthcare. ACOs and IDNs are increasing in number and becoming more important constituents in delivering healthcare services; their impact on the provision of healthcare services to date has varied.
ACOs and IDNs may exercise operational and financial control over providers across the continuum of care, and may function as a payer. Thus, they may be able to manage the health of a population group within a defined geography, and also may be able to influence the cost and quality of healthcare delivery, for example through owned entities and through ancillary services. ACOs may be encouraged to consider exclusive arrangements with healthcare providers that become part of the ACO, or to limit service providers to the ACO, since members of the ACO share financial risk.
We are actively engaging with ACOs and IDNs to demonstrate the value of our services.
|
|
Employers
|
Employers use tests for drugs of abuse to determine an individual's employability and his or her “fitness for duty.” Companies with high employee turnover, safety conscious environments or regulatory testing requirements provide the highest volumes of testing. Factors such as the general economy and job market can impact the utilization of drugs-of-abuse testing.
Employers also are investing in health and wellness services. We meet their needs by providing nationwide access to our customizable biometric and laboratory wellness testing, reporting and analytics, incentive management and flu shot services, directly and through health plan and health improvement providers. These services help employers, employees and others manage healthcare costs and capitalize on trends in personalized health.
We seek to grow our employer business through offering new and innovative programs to help them with their goals of (1) maintaining a safe and productive workplace, (2) improving healthcare for employees and (3) lowering healthcare costs for employees and employers.
|
|
Patients
|
In the current environment, patients are taking increased interest in and responsibility for their healthcare. In addition, patients often are bearing increased financial responsibility for their healthcare (
e.g
., high deductible health plans). Patients are paying greater attention to their healthcare, are increasing their demands of healthcare providers, have increased expectations regarding their healthcare experiences and are becoming more sophisticated regarding healthcare. For example, in our experience, patients are more focused on transparency, ease of doing business and understanding diagnostics information services than they have been in the past.
The changing expectations of patients about their healthcare and their healthcare transactions are influencing the way that we think about our business and the services that we provide. We are well positioned to provide information and insights to patients to help them take actions to improve their healthcare, and increasingly we are providing patients with tools to do this.
|
|
Emerging Retail Healthcare Providers
|
In recent years, as the healthcare sector changes, retail providers of healthcare services have emerged and are growing. These providers include "big-box" retailers, pharmacy chains, supermarkets, urgent care centers and Internet-based service providers.
|
|
Other Laboratories and Other Customers
|
We also provide services on a fee-for-service basis to federal, state and local governmental agencies and to other commercial clinical laboratories
|
|
Table 22 - Sample Third Party Payers
|
|
|
•
Health plans
|
•
Patient-centered medical homes
|
|
•
Self-insured employer benefit funds
|
•
Traditional Medicare or Medicaid program
|
|
Table 23 - Factors Traditionally Considered When Selecting a Diagnostic Information Services Provider
|
|
|
•
Service capability and quality
|
•
Reputation in the medical community
|
|
•
Accuracy, timeliness and consistency in reporting test results
|
•
Healthcare information technology solutions, including connectivity options
|
|
•
Access to medical/scientific thought leaders for consultation
|
•
Patient access, including the number, convenience and geographic coverage of patient service centers
|
|
•
Patient insurance coverage and experience
|
•
Ability to develop new and useful tests and services
|
|
•
Number and type of tests performed
|
•
Qualifications of its staff
|
|
•
Pricing and overall value
|
•
Provider office workflow
|
|
•
|
Operational risk - risks arising from systems, processes, people and external events that affect the Company’s operational objectives or fundamental reason for its existence, including: product life-cycle and execution; service
|
|
•
|
Financial risk - risks arising from the Company’s ability to meet its financial obligations pursuant to its strategic and operational objectives, including exposure to broad market and more specific industry risk that could impact liquidity, interest rate, credit, pricing and reimbursement, and also to internal and external financial reporting.
|
|
•
|
Legal and compliance risk - risks arising from government and regulatory environment and action, legal proceedings and compliance with integrity policies and procedures.
|
|
•
|
Strategic risk - risks that will impede the Company’s plan to achieve its mission and vision and apply its core values, including changes in the broad market and Company's industry, business development and restructuring activities, competitive threats and practices, technology and product innovation, and public policy.
|
|
•
|
“Client” fees charged to physicians, hospitals and institutions for which services are performed on a wholesale basis and which are billed on a monthly basis.
|
|
•
|
“Patient” fees charged to individual patients and certain third-party payers on a claim-by-claim basis.
|
|
Table 24 - 2016 Medicare and Medicaid Revenues
|
|||
|
% of 2016
Consolidated Net Revenues
|
|||
|
Medicare Clinical Laboratory Fee Schedule
|
12
|
||
|
Medicare Physician Fee Schedule
|
2
|
||
|
Total Medicare
|
14
|
||
|
Medicaid Programs
|
3
|
||
|
Total Traditional Medicare and Medicaid
|
17
|
||
|
Table 25 - Key Regulatory Schemes
|
|
|
CLIA and State Clinical Laboratory Licensing
|
CLIA regulates the operations of virtually all clinical laboratories, requiring that they be certified by the federal government and that they comply with various technical, operational, personnel and quality requirements intended to ensure that the services provided are accurate, reliable and timely.
State laws may require additional personnel qualifications or licenses, quality control, record maintenance, proficiency testing or detailed review of our scientific method validations and technical procedures for certain tests.
Violations of these laws and regulations may result in monetary fines, criminal and civil penalties and/or suspension or exclusion from participation in Medicare, Medicaid and other federal or state healthcare programs.
|
|
Fraud and Abuse
|
Anti-kickback laws and regulations prohibit making payments or furnishing other benefits to influence the referral of tests billed to Medicare, Medicaid or certain other federal or state healthcare programs.
In addition, federal and state anti-self-referral laws generally prohibit Medicare and Medicaid payments for clinical tests referred by physicians who have an ownership or investment interest in, or a compensation arrangement with, the testing laboratory.
Some states have similar laws that are not limited to Medicare and Medicaid referrals and could also affect other tests referred by clinicians with investment or compensation arrangements with the testing laboratory.
Violations of these laws and regulations may result in monetary fines, criminal and civil penalties and/or suspension or exclusion from participation in Medicare, Medicaid and other federal or state healthcare programs.
|
|
FDA
|
The FDA has regulatory responsibility over, among other areas, instruments, software, test kits, reagents and other devices used by clinical laboratories to perform diagnostic testing in the United States. The FDA also regulates drugs-of-abuse testing for employers and insurers, testing for blood bank purposes and testing of donors of human cells for purposes such as in vitro fertilization.
A number of advanced tests we develop internally are offered as LDTs. The FDA has claimed regulatory authority over all LDTs, but has stated that it exercised enforcement discretion with regard to most LDTs performed by high complexity CLIA-certified laboratories.
The FDA recently announced its intention to refrain from issuing guidance on the oversight of LDTs, and published a "Discussion Document" that provides the FDA's views on legislation to govern LDTs. New legislation could significantly impact the clinical laboratory testing business, including by increasing or modifying the regulation of LDTs.
|
|
Environmental, Health and Safety
|
We are subject to laws and regulations related to the protection of the environment, the health and safety of employees and the handling, transportation and disposal of medical specimens, infectious and hazardous waste and radioactive materials.
For example, the U.S. Occupational Safety and Health Administration has established extensive requirements relating specifically to workplace safety for healthcare employers in the U.S. This includes requirements to develop and implement multi-faceted programs to protect workers from exposure to blood-borne pathogens, including preventing or minimizing any exposure through needle stick injuries.
For purposes of transportation, some biological materials and laboratory supplies are classified as hazardous materials and are subject to regulation by one or more of the following agencies: the U.S. Department of Transportation, the U.S. Public Health Service, the U.S. Postal Service and the International Air Transport Association.
|
|
Physicians
|
Our pathologists are required to hold a valid license to practice medicine in the jurisdiction in which they practice.
Many of our pathologists enter into an employment agreement. These agreements have varying terms, but generally can be terminated at any time, upon advance notice. Most of the agreements contain covenants generally limiting the activities of the pathologist within a defined geographic area for a limited period of time after termination of employment; the enforceability of these covenants may be limited under state law.
Several jurisdictions, including some in which our businesses are located, prohibit business corporations from engaging in the practice of medicine. In certain jurisdictions, business corporations are prohibited from employing licensed healthcare professionals to provide services on behalf of the corporation; these laws vary. In some jurisdictions, anatomic pathology services are delivered through physician-owned entities that employ the practicing pathologists. The manner in which licensed physicians can be organized to perform medical services may be governed by the laws of the jurisdictions in which medical services are provided and by the medical boards or other entities authorized by these jurisdictions to oversee the practice of medicine.
|
|
Privacy and Security of Health and Personal Information
|
We are subject to laws and regulations regarding protecting the security and privacy of certain healthcare and personal information, including the federal Health Insurance Portability and Accountability Act and the regulations thereunder, which establish (i) a complex regulatory framework including requirements for safeguarding protected health information and (ii) comprehensive federal standards regarding the uses and disclosures of protected health information.
A healthcare provider may be required to notify individuals or the government if the provider discovers certain breaches of personal information or protected health information.
|
|
Drug Testing; Controlled Substances
|
All U.S. laboratories that perform drug testing for certain public sector employees and employees of certain federally regulated businesses are required to be certified as meeting the detailed performance and quality standards of the Substance Abuse and Mental Health Services Administration.
To obtain access to controlled substances used to perform drugs-of-abuse testing in the United States, laboratories must be licensed by the Drug Enforcement Administration.
|
|
•
|
increase our administrative, billing or other operating costs;
|
|
•
|
decrease the amount of reimbursement related to diagnostic information services performed;
|
|
•
|
damage our reputation; or
|
|
•
|
adversely affect important business relationships with third parties.
|
|
Table 26 - Information Available at Our Corporate Governance Webpage
|
|
|
•
Directors
|
•
Corporate Governance Guidelines
|
|
•
Composition of the committees of our Board of Directors
|
•
Code of Ethics
|
|
•
Senior management
|
•
Certificate of Incorporation
|
|
•
Charters for the standing committees of our Board of Directors
|
•
Bylaws
|
|
•
Values
|
•
Corporate Political Contributions Policy
|
|
•
Statements of beneficial ownership of our equity securities filed by our directors, officers and others under Section 16 of the Exchange Act
|
|
|
Table 27 - Executive Officers
|
|
|
Name, Age, Title
|
Background
|
|
Stephen H. Rusckowski (59)
Chairman of the Board, President and Chief Executive Officer
|
Mr. Rusckowski joined the Company in May 2012 as President and Chief Executive Officer and became Chairman of the Board on January 1, 2017. From October 2006 until he joined the Company, he was Chief Executive Officer of Philips Healthcare, the largest unit of Royal Philips Electronics, and a member of the Board of Management of Royal Philips Electronics and its Executive Committee. Previously, he was CEO of the Imaging Systems business of Royal Phillips Electronics.
Before joining Philips in 2001, Mr. Rusckowski held numerous management positions with the healthcare division of Hewlett-Packard/Agilent Technologies.
Mr. Rusckowski has been a director of the Company since May 2012. He has been a director of Xerox Corporation since February 2015, and was a director of Covidien plc from December 2013 to January 2015. Mr. Rusckowski is the Chairman of the American Clinical Laboratory Association.
|
|
Jon R. Cohen, M.D. (62)
Senior Vice President and Group Executive - Diagnostic Solutions
|
Dr. Cohen joined the company in March 2009 as Chief Medical Officer. From May 2011 to January 2013, he also had responsibility for Hospital Services. In January 2013, Dr. Cohen assumed responsibility for anatomic pathology services, sports science and human performance and professional laboratory services, and he was responsible for the oncology clinical franchise from January 2013 until January 2017. From February 2014 to July 2015, he had responsibility for our clinical trials business.
Dr. Cohen served as the Senior Adviser to New York Governor David Patterson from 2008 to 2009, where he was responsible for all policy and strategic planning.
Previously, Dr. Cohen was a managing director, health industries advisory services, at PricewaterhouseCoopers LLP, and spent 21 years with North Shore-Long Island Jewish Health System, one of the nation's largest not-for-profit health systems, including serving as its Chief Medical Officer from 2000 to 2006.
|
|
Everett V. Cunningham (50)
Senior Vice President, Commercial
|
Mr. Cunningham is responsible for the commercial organization for the Company's Diagnostic Information Services business.
Prior to joining the Company in October 2012, he spent 21 years with Pfizer, Inc., where he served from June 2011 to October 2012 as Regional President, Established Products, Asia. From 2009 to 2011, Mr. Cunningham served as Regional President, West Business Unit, Primary Care. From 2007 to 2009, he served as Vice President, Human Resources, Corporate Groups. Before that Mr. Cunningham served Pfizer in a series of sales and leadership and general management roles.
|
|
James E. Davis (54)
Executive Vice President, General Diagnostics
|
In January 2017, Mr. Davis became Executive Vice President, General Diagnostics; previously he was Senior Vice President and Group Executive - Regional Businesses. In January 2015, he assumed responsibility for the general management of the Company's regional Diagnostic Information Services businesses. Mr. Davis was responsible for our products business from February 2014 until 2016. From February 2014 to January 2015, he was responsible for operations for the Company's Diagnostic Information Services business. He joined Quest Diagnostics in April 2013 as Senior Vice President, Diagnostics Solutions, with responsibility for the healthcare information technology, risk assessment, clinical trials, diagnostic products and employer solutions businesses.
Prior to joining Quest Diagnostics, from March 2012 to April 2013, Mr. Davis served as Lead Director, and then as Chief Executive Officer, of InSightec, Inc., a medical device company that designs and develops ultrasound ablation devices that are guided by magnetic resonance imaging systems.
Previously, Mr. Davis held a number of senior positions in General Electric’s healthcare business, including from 2007 to 2012 as Vice President and General Manager of GE Healthcare’s magnetic resonance imaging business. Prior to joining GE Healthcare, Mr. Davis held leadership positions in GE’s aviation business and led the development of strategic and operational improvement initiatives for clients of McKinsey & Company, Inc.
|
|
Catherine T. Doherty (54)
Senior Vice President and Group Executive - Clinical Franchise Solutions and Marketing
|
Since January 2013, Ms. Doherty has been responsible for overseeing the development of clinical franchise solutions in the areas of cardiovascular, infectious disease and immunology, and prescription drug monitoring and toxicology, as well as enterprise-wide strategic marketing. From January 2013 to January 2017, she also was responsible for clinical franchise solutions in the areas of neurology, women's health and general wellness. In February 2014, Ms. Doherty assumed responsibility for the employer solutions and risk assessment businesses. From February 2014 to January 2017, she also was responsible for the healthcare information technology business.
From May 2011 to December 2012, she served as Senior Vice President, Physician Services. Prior to May 2011, Ms. Doherty held a variety of positions of increasing responsibility since joining the Company in 1990, including Vice President, Hospital Services; Vice President, Office of the Chairman; Vice President, Finance and Administration for the Hospital business; Vice President, Communications and Investor Relations; and Chief Accounting Officer.
|
|
Mark J. Guinan (55)
Executive Vice President and Chief Financial Officer
|
Mr. Guinan joined the Company in July 2013. From 2010 until joining Quest Diagnostics in 2013, he served as Chief Financial Officer for Hill-Rom Holdings Inc., a manufacturer and provider of medical technologies and related services for the health care industry.
Previously, he had served in a number of finance and operations roles in a long career at Johnson & Johnson including 2009 to 2010 as Vice President, Chief Procurement Officer, and 2005 to 2009 as Vice President, Group Finance Pharmaceuticals. Before joining Johnson & Johnson in 1997, he held a number of financial roles at Procter & Gamble.
|
|
Carrie Eglinton Manner (42)
Senior Vice President, Advanced Diagnostics
|
Ms. Eglinton Manner joined the Company in January 2017. She is responsible for the Company's advanced testing activities, including overseeing the development of clinical franchise solutions in the areas of neurology, oncology and women's health.
Previously, Ms. Eglinton Manner spent over 20 years in various leadership roles in healthcare businesses at General Electric. From 2015-16, she served as President and CEO of the Detection and Guidance Solutions business, delivering advanced x-ray technologies spanning the continuum of healthcare. From 2013-15, Ms. Eglinton Manner served as President and CEO of OEC Surgical Mobile C-arm systems. She was President and CEO of General Electric's diagnostic pathology laboratory services business from 2012-13, and President of the Maternal Infant Care Business from 2009-2012.
|
|
Michael E. Prevoznik (55)
Senior Vice President and General Counsel
|
Mr. Prevoznik joined the Company as Vice President and General Counsel in August 1999. In 2003, he assumed responsibility for governmental affairs. From 1999 until April 2009, Mr. Prevoznik also had responsibility for the Company's Compliance Department.
In addition, from April 2011 to January 2017, he had management responsibility for the Company's diagnostic information services activities outside the U.S., and from April 2011 to January 2013, he had management responsibility for the Company's clinical trials business.
Prior to joining the Company, Mr. Prevoznik served in positions of increasing responsibility within the compliance organization at SmithKline Beecham, most recently as Vice President, Compliance, with responsibility for coordinating all SmithKline Beecham compliance activities worldwide.
|
|
This Report also includes forward-looking statements that involve risks or uncertainties. Our results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks we face described below and elsewhere. See “Cautionary Factors that May Affect Future Results” on page
41
.
|
|
•
|
loss of key customers or employees;
|
|
•
|
difficulty in standardizing information and other systems;
|
|
•
|
difficulty in consolidating facilities and infrastructure;
|
|
•
|
failure to maintain the quality or timeliness of services that our Company has historically provided;
|
|
•
|
diversion of management's attention from the day-to-day business of our Company as a result of the need to deal with the foregoing disruptions and difficulties; and
|
|
•
|
the added costs of dealing with such disruptions.
|
|
•
|
billing and reimbursement of clinical testing;
|
|
•
|
certification or licensure of clinical laboratories;
|
|
•
|
the anti-self-referral and anti-kickback laws and regulations;
|
|
•
|
the laws and regulations administered by the FDA;
|
|
•
|
the corporate practice of medicine;
|
|
•
|
operational, personnel and quality requirements intended to ensure that clinical testing services are accurate, reliable and timely;
|
|
•
|
physician fee splitting;
|
|
•
|
relationships with physicians and hospitals;
|
|
•
|
safety and health of laboratory employees; and
|
|
•
|
handling, transportation and disposal of medical specimens, infectious and hazardous waste and radioactive materials.
|
|
•
|
diversion of management time and attention;
|
|
•
|
expenditure of large amounts of cash on legal fees, costs and payment of damages;
|
|
•
|
limitations on our ability to continue some of our operations;
|
|
•
|
enforcement actions, fines and penalties or the assertion of private litigation claims and damages;
|
|
•
|
decreased demand for our services; and/or
|
|
•
|
injury to our reputation.
|
|
•
|
cease developing, performing or selling solutions or services that incorporate the challenged intellectual property;
|
|
•
|
obtain and pay for licenses from the holder of the infringed intellectual property right;
|
|
•
|
redesign or re-engineer our tests;
|
|
•
|
change our business processes; or
|
|
•
|
pay substantial damages, court costs and attorneys' fees, including potentially increased damages for any infringement held to be willful.
|
|
•
|
changes in the local economic environment;
|
|
•
|
political instability;
|
|
•
|
social changes;
|
|
•
|
intellectual property legal protections and remedies;
|
|
•
|
trade regulations;
|
|
•
|
procedures and actions affecting approval, production, pricing, reimbursement and marketing of services;
|
|
•
|
exchange controls;
|
|
•
|
attracting and retaining qualified employees;
|
|
•
|
local market practices;
|
|
•
|
export and import controls;
|
|
•
|
weak legal systems which may affect our ability to enforce contractual rights;
|
|
•
|
changes in local laws or regulations; and
|
|
•
|
potentially longer payment and collection cycles.
|
|
(a)
|
Heightened competition from commercial clinical testing companies, hospitals, physicians and others.
|
|
(b)
|
Increased pricing pressure from customers and payers.
|
|
(c)
|
A decline in economic conditions.
|
|
(d)
|
Impact of changes in payment mix, including any shift from fee-for-service to discounted, capitated or bundled fee arrangements.
|
|
(e)
|
Adverse actions by government or other third-party payers, including healthcare reform that focuses on reducing healthcare costs but does not recognize the value and importance to healthcare of clinical testing or innovative solutions, unilateral reduction of fee schedules payable to us, competitive bidding, and an increase in the practice of negotiating for exclusive arrangements that involve aggressively priced capitated or fee-for-service payments by health insurers or other payers.
|
|
(f)
|
The impact upon our testing volume and collected revenue or general or administrative expenses resulting from our compliance with Medicare and Medicaid administrative policies and requirements of third-party payers. These include:
|
|
(2)
|
inability to obtain from patients a valid advance beneficiary notice form for tests that cannot be billed without prior receipt of the form;
|
|
(3)
|
increased challenges in operating as a non-contracted provider with respect to health plans;
|
|
(4)
|
the impact of additional or expanded limited coverage policies and limits on the allowable number of test units; and
|
|
(5)
|
the impact of increased prior authorization programs for clinical testing.
|
|
(g)
|
Adverse results from pending or future government investigations, lawsuits or private actions. These include, in particular, monetary damages, loss or suspension of licenses, and/or suspension or exclusion from the Medicare and Medicaid programs and/or criminal penalties.
|
|
(h)
|
Failure to efficiently integrate acquired businesses and to manage the costs related to any such integration, or to retain key technical, professional or management personnel.
|
|
(i)
|
Denial, suspension or revocation of CLIA certification or other licenses for any of our clinical laboratories under the CLIA standards, revocation or suspension of the right to bill the Medicare and Medicaid programs or other adverse regulatory actions by federal, state and local agencies.
|
|
(j)
|
Changes in and complexity of federal, state or local laws or regulations, including changes that result in new or increased federal or state regulation of commercial clinical laboratories, tests developed by commercial clinical laboratories or other products or services that we offer or activities in which we are engaged, including regulation by the FDA.
|
|
(k)
|
Inability to achieve expected benefits from our acquisitions of other businesses.
|
|
(l)
|
Inability to achieve additional benefits from our business performance tools and efficiency initiatives.
|
|
(m)
|
Adverse publicity and news coverage about the clinical testing industry or us.
|
|
(n)
|
Computer or other IT system or IT security failures that affect our ability to perform testing, report test results or properly bill customers, or result in the disclosure of confidential information, including potential failures resulting from implementing common IT systems and other system conversions, telecommunications failures, malicious human acts (such as electronic break-ins or computer viruses) or natural disasters.
|
|
(o)
|
Development of technologies that substantially alter the practice of clinical testing, including technology changes that lead to the development of more convenient or cost-effective testing, or testing to be performed outside of a commercial clinical laboratory, such as (1) point-of-care testing that can be performed by physicians in their offices, (2) advanced testing that can be performed by hospitals in their own laboratories or (3) home testing that can be carried out without requiring the services of clinical laboratories.
|
|
(p)
|
Negative developments regarding intellectual property and other property rights that could prevent, limit or interfere with our ability to develop, perform or sell our tests or operate our business. These include:
|
|
(1)
|
Issuance of patents or other property rights to our competitors or others; and
|
|
(2)
|
Inability to obtain or maintain adequate patent or other proprietary rights for our products and services or to successfully enforce our proprietary rights.
|
|
(q)
|
Development of tests by our competitors or others which we may not be able to license, or usage of our technology or similar technologies or our trade secrets or other intellectual property by competitors, any of which could negatively affect our competitive position.
|
|
(r)
|
Regulatory delay or inability to commercialize newly developed or licensed tests or technologies or to obtain appropriate reimbursements for such tests.
|
|
(s)
|
Failure to properly bill for our services or to obtain appropriate payments for services that we do bill.
|
|
(t)
|
Changes in interest rates and changes in our credit ratings from Standard & Poor's, Moody's Investor Services or Fitch Ratings causing an unfavorable impact on our cost of and access to capital.
|
|
(u)
|
Inability to hire or retain qualified or key senior management personnel.
|
|
(v)
|
Terrorist and other criminal activities, hurricanes, earthquakes or other natural disasters, and health pandemics, which could affect our customers, transportation or systems, or our facilities, and for which insurance may not adequately reimburse us.
|
|
(w)
|
Difficulties and uncertainties in the discovery, development, regulatory environment and/or marketing of new services or solutions or new uses of existing tests.
|
|
(x)
|
Failure to adapt to changes in the healthcare system and healthcare delivery, including those stemming from the ACA (or its repeal, amendment or replacement), trends in utilization of the healthcare system and increased patient financial responsibility for services.
|
|
(y)
|
Results and consequences of governmental inquiries.
|
|
(bb)
|
Political, legal, operational and other changes and challenges in international markets.
|
|
Location
|
Leased or Owned
|
|
|
Sacramento, California (laboratory)
|
Leased
|
|
|
West Hills, California (laboratory)
|
Leased
|
|
|
San Juan Capistrano, California (laboratory)
|
Owned
|
|
|
Tampa, Florida (laboratory)
|
Owned
|
|
|
Atlanta, Georgia (laboratory)
|
Owned
|
|
|
Chicago, Illinois (2) (laboratories)
|
One owned, one leased
|
|
|
Marlborough, Massachusetts (laboratories)
|
Leased
|
|
|
Baltimore, Maryland (laboratory)
|
Owned
|
|
|
Teterboro, New Jersey (laboratory)
|
Owned
|
|
|
Philadelphia, Pennsylvania (laboratory)
|
Leased
|
|
|
Dallas, Texas (laboratory)
|
Leased
|
|
|
Chantilly, Virginia (laboratory)
|
Leased
|
|
|
Lenexa, Kansas (laboratory)
|
Owned
|
|
|
Greensboro, North Carolina (laboratory)
|
Leased
|
|
|
Common Stock
Market Price |
Dividends
Declared
|
||||||||||
|
High
|
Low
|
||||||||||
|
2015
|
|||||||||||
|
First Quarter
|
$
|
78.33
|
|
$
|
66.09
|
|
$
|
0.38
|
|
||
|
Second Quarter
|
89.00
|
|
69.47
|
|
0.38
|
|
|||||
|
Third Quarter
|
75.25
|
|
60.07
|
|
0.38
|
|
|||||
|
Fourth Quarter
|
72.43
|
|
60.15
|
|
0.38
|
|
|||||
|
2016
|
|||||||||||
|
First Quarter
|
$
|
72.64
|
|
$
|
59.66
|
|
$
|
0.40
|
|
||
|
Second Quarter
|
81.41
|
|
70.92
|
|
0.40
|
|
|||||
|
Third Quarter
|
86.85
|
|
80.27
|
|
0.40
|
|
|||||
|
Fourth Quarter
|
93.57
|
|
79.12
|
|
0.45
|
|
|||||
|
ISSUER PURCHASES OF EQUITY SECURITIES
|
||||||||||||||
|
Period
|
Total Number of
Shares
Purchased
|
Average Price
Paid per Share
|
Total Number of
Shares Purchased
as Part of Publicly
Announced Plans
or Programs
|
Approximate
Dollar Value of
Shares that May
Yet Be Purchased
Under the Plans
or Programs
(in thousands)
|
||||||||||
|
October 1, 2016 – October 31, 2016
|
|
|
|
|
|
|
|
|
||||||
|
Share Repurchase Program (A)
|
—
|
|
$
|
—
|
|
—
|
|
$
|
532,116
|
|
||||
|
Employee Transactions (B)
|
727
|
|
$
|
83.97
|
|
N/A
|
|
N/A
|
|
|||||
|
November 1, 2016 – November 30, 2016
|
||||||||||||||
|
Share Repurchase Program (A)
|
488,007
|
|
$
|
86.06
|
|
488,007
|
|
$
|
490,119
|
|
||||
|
Employee Transactions (B)
|
2,370
|
|
$
|
85.02
|
|
N/A
|
|
N/A
|
|
|||||
|
December 1, 2016 – December 31, 2016
|
||||||||||||||
|
Share Repurchase Program (A)
|
1,196,549
|
|
$
|
90.26
|
|
1,196,549
|
|
$
|
1,382,116
|
|
||||
|
Employee Transactions (B)
|
1,047
|
|
$
|
88.04
|
|
N/A
|
|
N/A
|
|
|||||
|
Total
|
||||||||||||||
|
Share Repurchase Program (A)
|
1,684,556
|
|
$
|
89.04
|
|
1,684,556
|
|
$
|
1,382,116
|
|
||||
|
Employee Transactions (B)
|
4,144
|
|
$
|
85.60
|
|
N/A
|
|
N/A
|
|
|||||
|
(A)
|
Since the share repurchase program's inception in May 2003, our Board of Directors has authorized $8.0 billion of share repurchases of our common stock through
December 31, 2016
. The share repurchase authority has no set expiration or termination date.
|
|
(B)
|
Includes: (1) shares delivered or attested to in satisfaction of the exercise price and/or tax withholding obligations by holders of stock options (granted under the Company's Amended and Restated Employee Long-Term Incentive Plan) who exercised options; and (2) shares withheld (under the terms of grants under the Long-Term Incentive Plan) to offset tax withholding obligations that occur upon the delivery of outstanding common shares underlying restricted share units and performance share units.
|
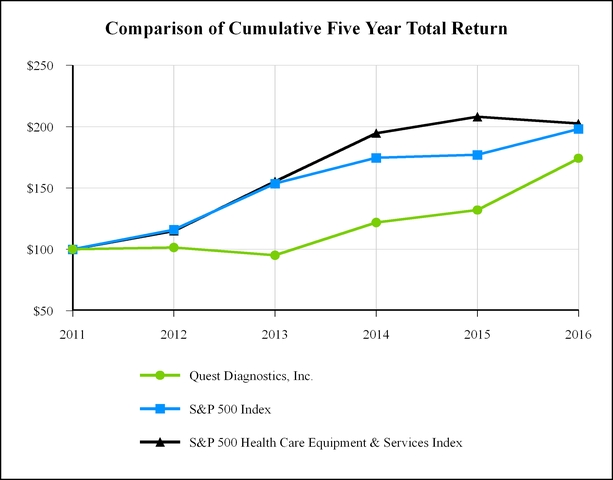
|
Closing DGX Price
|
Total Shareholder Return
|
Performance Graph Values
|
|||||||||||||||||||||
|
Date
|
DGX
|
S&P 500
|
S&P 500 H.C.
|
DGX
|
S&P 500
|
S&P 500 H.C.
|
|||||||||||||||||
|
12/31/2012
|
$58.27
|
1.49
|
%
|
16.00
|
%
|
15.02
|
%
|
$
|
101.49
|
|
$
|
116.00
|
|
$
|
115.02
|
|
|||||||
|
12/31/2013
|
$53.54
|
(6.24
|
)%
|
32.39
|
%
|
35.05
|
%
|
$
|
95.15
|
|
$
|
153.57
|
|
$
|
155.33
|
|
|||||||
|
12/31/2014
|
$67.06
|
28.06
|
%
|
13.69
|
%
|
25.34
|
%
|
$
|
121.85
|
|
$
|
174.60
|
|
$
|
194.69
|
|
|||||||
|
12/31/2015
|
$71.14
|
8.35
|
%
|
1.38
|
%
|
6.89
|
%
|
$
|
132.03
|
|
$
|
177.01
|
|
$
|
208.11
|
|
|||||||
|
12/30/2016
|
$91.90
|
31.89
|
%
|
11.96
|
%
|
(2.69
|
)%
|
$
|
174.14
|
|
$
|
198.18
|
|
$
|
202.51
|
|
|||||||
|
See page
52
.
|
|
See page
56
.
|
|
See page
76
.
|
|
(a)
|
Documents filed as part of this Report.
|
|
1.
|
Index to financial statements and supplementary data filed as part of this Report.
|
|
Item
|
Page
|
|
Financial Statements
|
|
|
2.
|
Financial Statement Schedule.
|
|
3.
|
Exhibits
|
|
(b)
|
Exhibits filed as part of this Report.
|
|
(c)
|
None.
|
|
QUEST DIAGNOSTICS INCORPORATED
|
||
|
(Registrant)
|
||
|
By:
|
/s/Stephen H. Rusckowski
|
|
|
Stephen H. Rusckowski
|
||
|
Chairman of the Board, President and Chief Executive Officer
|
||
|
Signature
|
Capacity
|
|
|
/s/Stephen H. Rusckowski
Stephen H. Rusckowski
|
Chairman of the Board, President and Chief Executive Officer
(Principal Executive Officer)
|
|
|
/s/Mark J. Guinan
Mark J. Guinan
|
Executive Vice President and Chief Financial Officer
(Principal Financial Officer)
|
|
|
/s/Robert A. Klug
Robert A. Klug
|
Vice President, Corporate Controller and Chief Accounting Officer
(Principal Accounting Officer)
|
|
|
/s/Jenne K. Britell, Ph.D.
Jenne K. Britell, Ph.D.
|
Director
|
|
|
/s/Vicky B. Gregg
Vicky B. Gregg
|
Director
|
|
|
/s/Jeffrey M. Leiden, M.D., Ph. D.
Jeffrey M. Leiden, M.D., Ph. D.
|
Director
|
|
|
/s/Timothy L. Main
Timothy L. Main
|
Director
|
|
|
/s/Gary M. Pfeiffer
Gary M. Pfeiffer
|
Director
|
|
|
/s/Timothy M. Ring
Timothy M. Ring
|
Director
|
|
|
/s/Daniel C. Stanzione, Ph.D.
Daniel C. Stanzione, Ph.D.
|
Director
|
|
|
/s/Gail R. Wilensky, Ph.D.
Gail R. Wilensky, Ph.D.
|
Director
|
|
|
/s/John B. Ziegler
John B. Ziegler
|
Director
|
|
|
Year Ended December 31,
|
|||||||||||||||||||
|
2016
|
2015
|
2014
|
2013
|
2012
|
|||||||||||||||
|
(dollars in millions, except per share data)
|
|||||||||||||||||||
|
Operations Data:
|
(a) (b) (c)
|
(a) (d) (e)
|
(a) (f) (g)
|
(a) (h) (i)
|
(a) (j) (k)
|
||||||||||||||
|
Net revenues
|
$
|
7,515
|
|
$
|
7,493
|
|
$
|
7,435
|
|
$
|
7,146
|
|
$
|
7,383
|
|
||||
|
Operating income
|
1,277
|
|
1,399
|
|
983
|
|
1,475
|
|
1,201
|
|
|||||||||
|
Income from continuing operations
|
696
|
|
753
|
|
587
|
|
848
|
|
666
|
|
|||||||||
|
Income (loss) from discontinued operations, net of taxes
|
—
|
|
—
|
|
5
|
|
35
|
|
(74
|
)
|
|||||||||
|
Net income
|
696
|
|
753
|
|
592
|
|
883
|
|
592
|
|
|||||||||
|
Less: Net income attributable to noncontrolling interests
|
51
|
|
44
|
|
36
|
|
34
|
|
36
|
|
|||||||||
|
Net income attributable to Quest Diagnostics
|
$
|
645
|
|
$
|
709
|
|
$
|
556
|
|
$
|
849
|
|
$
|
556
|
|
||||
|
Amounts attributable to Quest Diagnostics' stockholders:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
645
|
|
$
|
709
|
|
$
|
551
|
|
$
|
814
|
|
$
|
630
|
|
||||
|
Income (loss) from discontinued operations, net of taxes
|
—
|
|
—
|
|
5
|
|
35
|
|
(74
|
)
|
|||||||||
|
Net income
|
$
|
645
|
|
$
|
709
|
|
$
|
556
|
|
$
|
849
|
|
$
|
556
|
|
||||
|
Earnings per share attributable to Quest Diagnostics' common stockholders - basic:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
4.58
|
|
$
|
4.92
|
|
$
|
3.80
|
|
$
|
5.35
|
|
$
|
3.96
|
|
||||
|
Income (loss) from discontinued operations
|
—
|
|
—
|
|
0.03
|
|
0.23
|
|
(0.47
|
)
|
|||||||||
|
Net income
|
$
|
4.58
|
|
$
|
4.92
|
|
$
|
3.83
|
|
$
|
5.58
|
|
$
|
3.49
|
|
||||
|
Earnings per share attributable to Quest Diagnostics' common stockholders - diluted:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
4.51
|
|
$
|
4.87
|
|
$
|
3.78
|
|
$
|
5.31
|
|
$
|
3.92
|
|
||||
|
Income (loss) from discontinued operations
|
—
|
|
—
|
|
0.03
|
|
0.23
|
|
(0.46
|
)
|
|||||||||
|
Net income
|
$
|
4.51
|
|
$
|
4.87
|
|
$
|
3.81
|
|
$
|
5.54
|
|
$
|
3.46
|
|
||||
|
Dividends per common share
|
$
|
1.65
|
|
$
|
1.52
|
|
$
|
1.32
|
|
$
|
1.20
|
|
$
|
0.81
|
|
||||
|
Year Ended December 31,
|
|||||||||||||||||||
|
2016
|
2015
|
2014
|
2013
|
2012
|
|||||||||||||||
|
(dollars in millions)
|
|||||||||||||||||||
|
Balance Sheet Data (at end of year):
|
(a) (b) (c)
|
(a) (d) (e)
|
(a) (f) (g)
|
(a) (h) (i)
|
(a) (j) (k)
|
||||||||||||||
|
Cash and cash equivalents
|
$
|
359
|
|
$
|
133
|
|
$
|
192
|
|
$
|
187
|
|
$
|
296
|
|
||||
|
Total assets
|
10,100
|
|
9,962
|
|
9,857
|
|
8,930
|
|
9,263
|
|
|||||||||
|
Long-term debt
|
3,728
|
|
3,492
|
|
3,224
|
|
3,102
|
|
3,333
|
|
|||||||||
|
Total debt
|
3,734
|
|
3,651
|
|
3,742
|
|
3,314
|
|
3,343
|
|
|||||||||
|
Redeemable noncontrolling interest
|
77
|
|
70
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Other Data:
|
|||||||||||||||||||
|
Net cash provided by operating activities
|
$
|
1,069
|
|
$
|
821
|
|
$
|
944
|
|
$
|
667
|
|
$
|
1,211
|
|
||||
|
Net cash (used in) provided by investing activities
|
(152
|
)
|
(362
|
)
|
(1,025
|
)
|
328
|
|
(217
|
)
|
|||||||||
|
Net cash (used in) provided by financing activities
|
(691
|
)
|
(518
|
)
|
86
|
|
(1,121
|
)
|
(846
|
)
|
|||||||||
|
Capital expenditures
|
293
|
|
263
|
|
308
|
|
231
|
|
182
|
|
|||||||||
|
Purchases of treasury stock
|
590
|
|
224
|
|
132
|
|
1,037
|
|
200
|
|
|||||||||
|
Dividends paid
|
223
|
|
212
|
|
187
|
|
185
|
|
108
|
|
|||||||||
|
(a)
|
During the third quarter of 2006, we completed the wind down of NID, a test kit manufacturing subsidiary. As a result, the operations NID have been classified as discontinued operations for all periods presented. We will continue to report NID as a discontinued operation until uncertain tax benefits associated with NID are resolved. For further details regarding our discontinued operations, see Note 18 to the consolidated financial statements.
|
|
(b)
|
On February 29, 2016, we completed the acquisition of the outreach laboratory service business of Clinical Laboratory Partners, LLC ("CLP"), a wholly-owned subsidiary of Hartford HealthCare Corporation. Consolidated operating results for 2016 include the results of operations of CLP subsequent to the closing of the acquisition. On May 13, 2016, we completed the sale of our Focus Diagnostics products business to DiaSorin S.p.A ("Focus Sale"), which has not been classified as a discontinued operation. For further details regarding our acquisitions and dispositions, see Note 5 and Note 6, respectively, to the consolidated financial statements.
|
|
(c)
|
Operating income included:
|
|
•
|
a pre-tax gain of $118 million associated with the Focus Sale;
|
|
•
|
pre-tax charges of $78 million, primarily associated with systems conversions and integration costs incurred in connection with further restructuring and integrating our business; and
|
|
•
|
a net pre-tax gain of $7 million, primarily a result of a non-taxable gain on an escrow recovery associated with an acquisition, partially offset by costs associated with winding down subsidiaries, non-cash asset impairment charges and costs incurred related to certain legal matters.
|
|
•
|
income tax expense of $84 million associated with the Focus Sale, consisting of $91 million of current income tax expense and a deferred income tax benefit of $7 million;
|
|
•
|
$48 million of pre-tax charges on retirement of debt associated with the March 2016 cash tender offer and the related income tax benefit of $18 million;
|
|
•
|
non-cash asset impairment charges associated with certain investments of $7 million; and
|
|
•
|
$4 million of pre-tax restructuring and integration charges associated with our Q
2
Solutions joint venture.
|
|
•
|
$47 million of pre-tax cash charges, or $30 million after the related cash tax benefit, on the retirement of debt associated with the March 2016 cash tender offer;
|
|
•
|
$54 million of proceeds received in 2016 from the termination of interest rate swap agreements; and
|
|
•
|
$91 million of income taxes paid in connection with the Focus Sale.
|
|
(d)
|
On August 3, 2015, we completed the acquisition of MemorialCare Health System's laboratory outreach business ("MemorialCare"). On November 16, 2015, we completed the acquisition of the business assets of Superior Mobile Medics, Inc. ("Superior Mobile Medics"). Consolidated operating results for 2015 include the results of operations of MemorialCare and Superior Mobile Medics subsequent to the closing of the applicable acquisition. In July 2015, we contributed our clinical trials testing business to a newly formed global clinical trials central laboratory services joint venture with Quintiles IMS Holdings, Inc., Q
2
Solutions ("Clinical Trials Contribution"). The disposition of our clinical trials testing business was not classified as a discontinued operation.
|
|
(e)
|
Operating income included:
|
|
•
|
pre-tax gain of $334 million associated with the Clinical Trials Contribution;
|
|
•
|
pre-tax charges of $105 million, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating our business; and
|
|
•
|
net pre-tax charges of $33 million primarily associated with non-cash asset impairment charges and other costs associated with winding down our Celera products business and another subsidiary, costs incurred related to certain legal matters and a pre-tax gain of $13 million associated with a decrease in the fair value of the contingent consideration accrual associated with our Summit Health, Inc. acquisition.
|
|
•
|
$144 million of pre-tax charges on retirement of debt associated with the March 2015 cash tender offer and the April 2015 redemption and the related income tax benefit of $57 million;
|
|
•
|
deferred income tax expense of $145 million associated with the gain on the Clinical Trials Contribution;
|
|
•
|
$58 million deferred income tax benefit associated with winding down a subsidiary; and
|
|
•
|
$5 million of pre-tax restructuring and integration charges associated with our Q
2
Solutions joint venture.
|
|
•
|
$146 million of pre-tax cash charges, or $89 million after the related cash tax benefit, on the retirement of debt associated with the March 2015 cash tender offer and April 2015 redemption;
|
|
•
|
payments associated with an additional payroll cycle in 2015; and
|
|
•
|
an income tax payment in the third quarter of 2015 associated with certain tax contingencies.
|
|
•
|
$51 million of deferred acquisition consideration payments, primarily to UMass Memorial Medical Center ("UMass"), related to the business acquisition in 2013; and
|
|
•
|
$63 million of proceeds from the sale of a noncontrolling interest in a subsidiary to UMass.
|
|
(f)
|
On March 7, 2014, we completed the acquisition of Solstas Lab Partners Group ("Solstas"). On April 18, 2014, we completed the acquisition of Summit Health, Inc. ("Summit Health"). On April 16, 2014, we completed the acquisition of the outreach laboratory service operations of Steward Healthcare, LLC ("Steward"). Consolidated operating results for 2014 include the results of operations of Solstas, Summit Health and Steward subsequent to the closing of the applicable acquisition.
|
|
(g)
|
Operating income included:
|
|
•
|
pre-tax charges of $121 million, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating our business;
|
|
•
|
pre-tax charges of $24 million principally associated with costs related to certain legal matters; and
|
|
•
|
pre-tax gain of $9 million associated with a decrease in the fair value of the contingent consideration accrual associated with our Summit Health acquisition.
|
|
(h)
|
On January 2, 2013, we completed the acquisition of the clinical outreach and anatomic pathology businesses of UMass. On May 15, 2013, we completed the acquisition of the toxicology and clinical laboratory business of Advanced Toxicology Network ("ATN") from Concentra, a subsidiary of Humana Inc. On June 22, 2013, we completed the acquisition of certain lab-related clinical outreach service operations of Dignity Health ("Dignity"), a hospital system in California. On October 7, 2013, we completed the acquisition of ConVerge Diagnostic Services, LLC ("ConVerge"), a leading full-service laboratory providing clinical, cytology and anatomic pathology testing services to patients, physicians and hospitals in New England. Consolidated operating results for 2013 include the results of operations of UMass, ATN, Dignity and ConVerge subsequent to the closing of the applicable acquisition. In September 2013, we completed the sale of our Enterix products business, which was not classified as a discontinued operation.
|
|
(i)
|
Operating income included:
|
|
•
|
pre-tax charges of $115 million, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating our business;
|
|
•
|
pre-tax gain on sale of the ibrutinib royalty rights of $474 million; and
|
|
•
|
pre-tax loss of $40 million associated with the sale of the Enterix products business.
|
|
•
|
gain of $14 million (including foreign currency translation adjustments, partially offset by income tax expense and transaction costs) associated with the sale of our HemoCue products business; and
|
|
•
|
discrete tax benefits of $20 million associated with favorable resolution of certain tax contingencies related to our NID business.
|
|
•
|
income tax payments of $175 million associated with the sale of the ibrutinib royalty rights; and
|
|
•
|
$70 million of income tax payments which were deferred from the fourth quarter of 2012 under a program offered to companies whose principal place of business was in states most affected by Hurricane Sandy.
|
|
•
|
proceeds from the sale of the ibrutinib royalty rights of $474 million, net of transaction costs; and
|
|
•
|
proceeds from the sales of HemoCue and Enterix of $296 million.
|
|
(j)
|
On January 6, 2012, we completed the acquisition of S.E.D. Medical Laboratories ("S.E.D.") from Lovelace Health System. Consolidated operating results for 2012 include the results of operations of S.E.D. subsequent to the closing of the acquisition. During the fourth quarter of 2012, we sold our OralDNA salivary diagnostics business and committed to a plan to sell our HemoCue diagnostic products business. The sale of HemoCue was completed in April 2013. As a result, the operations for HemoCue and OralDNA have been classified as discontinued operations for all periods presented. As of December 31, 2012, the assets and liabilities of HemoCue were reported as held for sale.
|
|
(k)
|
Operating income included:
|
|
•
|
pre-tax charges of $106 million incurred in conjunction with further restructuring and integrating our business; and
|
|
•
|
pre-tax charges of $10 million, principally representing severance and other separation benefits as well as accelerated vesting of certain equity awards in connection with the succession of our prior CEO.
|
|
•
|
$86 million of charges for the asset impairment associated with HemoCue and the loss on sale associated with OralDNA;
|
|
•
|
$8 million of income tax expense related to the re-valuation of deferred tax assets associated with HemoCue and a $4 million income tax benefit related to the remeasurement of deferred taxes associated with HemoCue as a result of an enacted income tax rate change in Sweden.
|
|
•
|
receipts of $72 million from the termination of certain interest rate swap agreements; and
|
|
•
|
the deferral of approximately $70 million of income tax payments into the first quarter of 2013, which was offered to companies whose principal place of business was in states most affected by Hurricane Sandy.
|
|
•
|
Our total net revenues of
$7.52 billion
were
0.3%
above the prior year. The Clinical Trials Contribution, Focus Sale and winding down of our Celera products business ("Celera Products") negatively impacted total net revenues by 2.3% compared to the prior year.
|
|
•
|
DIS revenues of
$7.1 billion
were
2.5%
above the prior year. DIS volume increased 2.0% compared to the prior year. DIS revenue per requisition increased 0.4% compared to the prior year.
|
|
•
|
DS revenues of
$377 million
were 28.5% below the prior year due to the Clinical Trials Contribution, Focus Sale and winding down of Celera Products.
|
|
•
|
Net income attributable to Quest Diagnostics' stockholders was
$645 million
, or
$4.51
per diluted share, in
2016
, compared to
$709 million
, or
$4.87
per diluted share, in 2015. The decreases in net income attributable to Quest Diagnostics' stockholders and diluted earnings per share in 2016, compared to the prior year, were primarily a result of the gain on the Clinical Trials Contribution and a deferred income tax benefit associated with winding down a subsidiary in 2015, partially offset by the gain on the Focus Sale in 2016.
|
|
•
|
revenues and accounts receivable associated with DIS;
|
|
•
|
reserves for general and professional liability claims;
|
|
•
|
reserves for other legal proceedings;
|
|
•
|
accounting for and recoverability of goodwill; and
|
|
•
|
accounting for stock-based compensation expense.
|
|
% of
|
% of
|
||
|
DIS
|
DIS
|
||
|
Volume
|
Revenues
|
||
|
Healthcare Insurers (including coinsurance and deductible responsibilities)
|
47
|
51
|
|
|
Government Payers
|
15
|
18
|
|
|
Client Payers
|
37
|
29
|
|
|
Patients
|
1
|
2
|
|
|
% of
|
|
|
Consolidated
|
|
|
Net Accounts
|
|
|
Receivable
|
|
|
Healthcare Insurers
|
17
|
|
Government Payers
|
15
|
|
Client Payers
|
45
|
|
Patients (including coinsurance and deductible responsibilities)
|
17
|
|
Total DIS
|
94
|
|
•
|
DIS business;
|
|
•
|
Risk assessment services business;
|
|
•
|
Diagnostic products business (disposed of on May 13, 2016 as a result of the Focus Sale); and
|
|
•
|
Clinical trials testing business (disposed of on July 1, 2015 as a result of the Clinical Trials Contribution).
|
|
$ Increase (Decrease)
|
% Increase (Decrease)
|
||||||||||||||||||||||||
|
2016
|
2015
|
2014
|
2016 vs. 2015
|
2015 vs. 2014
|
2016 vs. 2015
|
2015 vs. 2014
|
|||||||||||||||||||
|
(dollars in millions, except per share data)
|
|||||||||||||||||||||||||
|
Net revenues:
|
|||||||||||||||||||||||||
|
DIS business
|
$
|
7,138
|
|
$
|
6,965
|
|
$
|
6,873
|
|
$
|
173
|
|
$
|
92
|
|
2.5
|
%
|
1.3
|
%
|
||||||
|
DS businesses
|
377
|
|
528
|
|
562
|
|
(151
|
)
|
(34
|
)
|
(28.5
|
)
|
(6.1
|
)
|
|||||||||||
|
Total net revenues
|
$
|
7,515
|
|
$
|
7,493
|
|
$
|
7,435
|
|
$
|
22
|
|
$
|
58
|
|
0.3
|
%
|
0.8
|
%
|
||||||
|
Operating costs, expenses and other income:
|
|
|
|||||||||||||||||||||||
|
Cost of services
|
$
|
4,616
|
|
$
|
4,657
|
|
$
|
4,637
|
|
$
|
(41
|
)
|
$
|
20
|
|
(0.9
|
)%
|
0.4
|
%
|
||||||
|
Selling, general and administrative
|
1,681
|
|
1,679
|
|
1,728
|
|
2
|
|
(49
|
)
|
0.1
|
|
(2.8
|
)
|
|||||||||||
|
Amortization of intangible assets
|
72
|
|
81
|
|
94
|
|
(9
|
)
|
(13
|
)
|
(10.7
|
)
|
(14.1
|
)
|
|||||||||||
|
Gain on disposition of business
|
(118
|
)
|
(334
|
)
|
—
|
|
216
|
|
(334
|
)
|
NM
|
|
NM
|
|
|||||||||||
|
Other operating (income) expense, net
|
(13
|
)
|
11
|
|
(7
|
)
|
(24
|
)
|
18
|
|
NM
|
|
NM
|
|
|||||||||||
|
Total operating costs and expenses, net
|
$
|
6,238
|
|
$
|
6,094
|
|
$
|
6,452
|
|
$
|
144
|
|
$
|
(358
|
)
|
2.4
|
%
|
(5.5
|
)%
|
||||||
|
Operating income
|
$
|
1,277
|
|
$
|
1,399
|
|
$
|
983
|
|
$
|
(122
|
)
|
$
|
416
|
|
(8.7
|
)%
|
42.3
|
%
|
||||||
|
Other income (expense):
|
|||||||||||||||||||||||||
|
Interest expense, net
|
$
|
(143
|
)
|
$
|
(153
|
)
|
$
|
(164
|
)
|
$
|
(10
|
)
|
$
|
(11
|
)
|
(6.3
|
)%
|
(6.7
|
)%
|
||||||
|
Other (expense) income, net
|
(48
|
)
|
(143
|
)
|
4
|
|
(95
|
)
|
147
|
|
NM
|
|
NM
|
|
|||||||||||
|
Total non-operating expenses, net
|
$
|
(191
|
)
|
$
|
(296
|
)
|
$
|
(160
|
)
|
$
|
(105
|
)
|
$
|
136
|
|
(35.1
|
)%
|
85.8
|
%
|
||||||
|
Income tax expense
|
$
|
(429
|
)
|
$
|
(373
|
)
|
$
|
(262
|
)
|
$
|
56
|
|
$
|
111
|
|
15.3
|
%
|
41.9
|
%
|
||||||
|
Effective income tax rate
|
39.5
|
%
|
33.8
|
%
|
31.8
|
%
|
570 bps
|
|
200 bps
|
|
NM
|
|
NM
|
|
|||||||||||
|
Equity in earnings of equity method investees, net of taxes
|
$
|
39
|
|
$
|
23
|
|
$
|
26
|
|
$
|
16
|
|
$
|
(3
|
)
|
73.3
|
%
|
(10.8
|
)%
|
||||||
|
Income from discontinued operations, net of taxes
|
$
|
—
|
|
$
|
—
|
|
$
|
5
|
|
$
|
—
|
|
$
|
(5
|
)
|
NM
|
|
NM
|
|
||||||
|
Income from continuing operations attributable to Quest Diagnostics' stockholders
|
$
|
645
|
|
$
|
709
|
|
$
|
551
|
|
$
|
(64
|
)
|
$
|
158
|
|
(9.1
|
)%
|
28.7
|
%
|
||||||
|
Diluted earnings per common share from continuing operations attributable to Quest Diagnostics’ common stockholders
|
$
|
4.51
|
|
$
|
4.87
|
|
$
|
3.78
|
|
$
|
(0.36
|
)
|
$
|
1.09
|
|
(7.4
|
)%
|
28.8
|
%
|
||||||
|
2016
|
2015
|
2014
|
||||||
|
Net revenues:
|
||||||||
|
DIS business
|
95.0
|
%
|
93.0
|
%
|
92.4
|
%
|
||
|
DS businesses
|
5.0
|
|
7.0
|
|
7.6
|
|
||
|
Total net revenues
|
100.0
|
%
|
100.0
|
%
|
100.0
|
%
|
||
|
Operating costs, expenses and other income:
|
|
|
|
|
|
|
||
|
Cost of services
|
61.4
|
%
|
62.1
|
%
|
62.4
|
%
|
||
|
Selling, general and administrative
|
22.4
|
|
22.4
|
|
23.2
|
|
||
|
Amortization of intangible assets
|
1.0
|
|
1.1
|
|
1.3
|
|
||
|
Gain on disposition of business
|
(1.5
|
)
|
(4.4
|
)
|
—
|
|
||
|
Other operating (income) expense, net
|
(0.3
|
)
|
0.1
|
|
(0.1
|
)
|
||
|
Total operating costs and expenses, net
|
83.0
|
%
|
81.3
|
%
|
86.8
|
%
|
||
|
Operating income
|
17.0
|
%
|
18.7
|
%
|
13.2
|
%
|
||
|
Bad debt
|
4.1
|
%
|
4.0
|
%
|
4.0
|
%
|
||
|
•
|
pre-tax gain of $118 million, or $0.24 per diluted share, related to the Focus Sale recorded in gain on disposition of business;
|
|
•
|
pre-tax charges of $82 million ($40 million in cost of services, $37 million in selling, general and administrative expenses, $1 million in other operating (income) expense, net and $4 million in equity in earnings of equity method investees, net of taxes), or $0.35 per diluted share, primarily associated with systems conversions and integration costs in connection with further restructuring and integrating our business;
|
|
•
|
pre-tax charges of $48 million, or $0.21 per diluted share, related to the 2016 Tender Offer recorded in other (expense) income, net; and
|
|
•
|
pre-tax costs of $6 million in selling, general and administrative expenses, a net pre-tax gain of $13 million in other operating (income) expense, net and pre-tax costs of $7 million in other (expense) income, net that on a combined basis benefited diluted earnings per share by $0.06, primarily a result of a non-taxable gain on an escrow recovery associated with an acquisition, partially offset by costs associated with winding down subsidiaries, non-cash asset impairment charges and costs incurred related to certain legal matters.
|
|
•
|
pre-tax gain of $334 million, or $1.30 per diluted share, related to the Clinical Trials Contribution recorded in gain on disposition of business;
|
|
•
|
pre-tax charges of $150 million ($6 million in interest expense, net and $144 million in other (expense) income, net), or $0.62 per diluted share, related to the loss on retirement of debt and related refinancing charges in connection with the: March 2015 cash tender offer ("2015 Tender Offer"), in which we purchased $250 million aggregate principal amount of our Senior Notes due 2037 and Senior Notes due 2040; and the April 2015 redemption ("2015 Redemption"), in which we redeemed all of our $500 million Senior Notes due November 2015, $150 million, or 50%, of our Senior Notes due April 2016 and all of our $375 million Senior Notes due July 2017;
|
|
•
|
pre-tax charges of $110 million, or $0.46 per diluted share, related to restructuring costs primarily associated with workforce reductions, integration costs associated with acquisitions and professional fees associated with the further restructuring and integrating our business ($63 million in cost of services, $42 million in selling, general and administrative expenses and $5 million in equity in earnings of equity method investees, net of taxes);
|
|
•
|
a deferred income tax benefit of $58 million, or $0.40 per diluted share, associated with winding down a subsidiary; and
|
|
•
|
net pre-tax costs of $31 million ($2 million in cost of services, $21 million in selling, general and administrative expenses, $10 million in other operating (income) expense, net and $(2) million on other (expense) income, net), or $0.14 per diluted share, primarily associated with non-cash asset impairment charges and other costs associated with Celera Products and winding down of another subsidiary as well as costs incurred related to certain legal matters, partially offset by a pre-tax gain of $13 million associated with a decrease in the fair value of the contingent consideration accrual associated with our Summit Health, Inc. acquisition.
|
|
•
|
pre-tax charges of $121 million ($50 million in cost of services, $69 million in selling, general and administrative expenses and $2 million in other operating (income) expense, net), or $0.53 per diluted share, related to restructuring costs primarily associated with workforce reductions, integration costs associated with acquisitions and professional fees associated with the further restructuring and integrating our business;
|
|
•
|
a discrete tax benefit of $44 million, or $0.30 per diluted share, associated with the favorable resolution of certain tax contingencies; and
|
|
•
|
net pre-tax costs of $15 million ($24 million in selling, general and administrative expenses and $(9) million in other operating (income) expense, net), or $0.09 per diluted share, primarily associated with costs related to certain legal matters, partially offset by a pre-tax gain of $9 million associated with a decrease in the fair value of the contingent consideration accrual associated with our Summit Health, Inc. acquisition.
|
|
2016
|
2015
|
2014
|
|||||||||
|
(dollars in millions)
|
|||||||||||
|
Net cash provided by operating activities
|
$
|
1,069
|
|
$
|
821
|
|
$
|
944
|
|
||
|
Net cash used in investing activities
|
(152
|
)
|
(362
|
)
|
(1,025
|
)
|
|||||
|
Net cash (used in) provided by financing activities
|
(691
|
)
|
(518
|
)
|
86
|
|
|||||
|
Net change in cash and cash equivalents
|
$
|
226
|
|
$
|
(59
|
)
|
$
|
5
|
|
||
|
•
|
$99 million decrease in payments related to the retirement of debt, principally comprised of premiums paid, associated with the 2016 Tender Offer as compared to the 2015 Tender Offer and 2015 Redemption;
|
|
•
|
an additional payroll cycle in 2015;
|
|
•
|
$54 million of proceeds received in 2016 from the termination of interest rate swap agreements;
|
|
•
|
$25 million decrease in restructuring payments;
|
|
•
|
$24 million decrease in interest paid; and
|
|
•
|
improved operating performance.
|
|
•
|
$146 million of payments related to the retirement of debt, principally comprised of premiums paid, associated with the 2015 Tender Offer and 2015 Redemption;
|
|
•
|
an additional payroll cycle in 2015;
|
|
•
|
an income tax payment in the third quarter of 2015 associated with certain tax contingencies; and
|
|
•
|
higher performance-based compensation payments.
|
|
•
|
$270 million
increase in proceeds from the disposition of business, principally related to the Focus Sale in 2016; and
|
|
•
|
$33 million
decrease in investment in equity method investee, related to cash included in our Clinical Trials Contribution in 2015.
|
|
•
|
$72 million
increase in cash paid for business acquisitions in 2016, principally a result of the CLP acquisition in 2016; and
|
|
•
|
$30 million
increase in capital expenditures.
|
|
•
|
a $661 million decrease in business acquisitions in 2015, primarily as a result of the Solstas Lab Partners Group and Summit Health, Inc. acquisitions in 2014; and
|
|
•
|
a $45 million decrease in capital expenditures.
|
|
•
|
$366 million
increase in repurchases of our common stock (discussed in "
Share Repurchases
" below); and
|
|
•
|
$63 million
decrease in proceeds from the sale of noncontrolling interest in a subsidiary, as a result of the sale of noncontrolling interest in a subsidiary to UMass Memorial Medical Center ("UMass") in 2015.
|
|
•
|
$145 million
in net borrowings (proceeds from borrowings less repayments of debt) in 2016, compared to
$84 million
in net repayments in 2015; and
|
|
•
|
$51 million
decrease in payment of deferred acquisition consideration principally a result of a payment to UMass in 2015 related to the business acquisition in 2013.
|
|
•
|
$84 million in net repayments (proceeds from borrowings less repayments of debt) in 2015, compared to $371 million in net borrowings in 2014;
|
|
•
|
$92 million increase in repurchases of our common stock (discussed in "
Share Repurchases
" below);
|
|
•
|
$25 million increase in dividends paid as a result of higher dividend rates in 2015 (discussed in "
Dividend Program
" below);
|
|
•
|
$18 million decrease in proceeds from the exercise of stock options; and
|
|
•
|
$51 million of deferred acquisition consideration payments, primarily to UMass related to the business acquisition in 2013.
|
|
Payments due by period
|
||||||||||||||||||||
|
Contractual Obligations
|
Total
|
Less than
1 year
|
1-3 years
|
3-5 years
|
After 5 years
|
|||||||||||||||
|
Outstanding debt
|
$
|
3,775
|
|
$
|
—
|
|
$
|
300
|
|
$
|
1,350
|
|
$
|
2,125
|
|
|||||
|
Capital lease obligations
|
13
|
|
6
|
|
6
|
|
1
|
|
—
|
|
||||||||||
|
Interest payments on outstanding debt
|
1,619
|
|
150
|
|
296
|
|
223
|
|
950
|
|
||||||||||
|
Operating leases
|
661
|
|
179
|
|
221
|
|
108
|
|
153
|
|
||||||||||
|
Purchase obligations
|
2,139
|
|
302
|
|
490
|
|
415
|
|
932
|
|
||||||||||
|
Merger consideration obligation
|
4
|
|
3
|
|
1
|
|
—
|
|
—
|
|
||||||||||
|
Total contractual obligations
|
$
|
8,211
|
|
$
|
640
|
|
$
|
1,314
|
|
$
|
2,097
|
|
$
|
4,160
|
|
|||||
|
/s/
|
PricewaterhouseCoopers LLP
|
|
Florham Park, New Jersey
|
|
|
February 22, 2017
|
|
|
2016
|
2015
|
||||||
|
Assets
|
|
|
|
|
|||
|
Current assets:
|
|
|
|
|
|||
|
Cash and cash equivalents
|
$
|
359
|
|
$
|
133
|
|
|
|
Accounts receivable, net of allowance for doubtful accounts of $265 and $254 as of December 31, 2016 and 2015, respectively
|
926
|
|
901
|
|
|||
|
Inventories
|
82
|
|
84
|
|
|||
|
Prepaid expenses and other current assets
|
155
|
|
207
|
|
|||
|
Assets held for sale
|
9
|
|
176
|
|
|||
|
Total current assets
|
1,531
|
|
1,501
|
|
|||
|
Property, plant and equipment, net
|
1,029
|
|
925
|
|
|||
|
Goodwill
|
6,000
|
|
5,905
|
|
|||
|
Intangible assets, net
|
949
|
|
984
|
|
|||
|
Investments in equity method investees
|
443
|
|
473
|
|
|||
|
Other assets
|
148
|
|
174
|
|
|||
|
Total assets
|
$
|
10,100
|
|
$
|
9,962
|
|
|
|
Liabilities and Stockholders’ Equity
|
|
|
|
|
|||
|
Current liabilities:
|
|
|
|
|
|||
|
Accounts payable and accrued expenses
|
$
|
975
|
|
$
|
1,014
|
|
|
|
Current portion of long-term debt
|
6
|
|
159
|
|
|||
|
Total current liabilities
|
981
|
|
1,173
|
|
|||
|
Long-term debt
|
3,728
|
|
3,492
|
|
|||
|
Other liabilities
|
654
|
|
514
|
|
|||
|
Commitments and contingencies
|
|
|
|
|
|||
|
Redeemable noncontrolling interest
|
77
|
|
70
|
|
|||
|
Stockholders’ equity:
|
|
|
|
|
|||
|
Quest Diagnostics stockholders’ equity:
|
|
|
|
|
|||
|
Common stock, par value $0.01 per share; 600 shares authorized as of both December 31, 2016 and 2015; 216 shares issued as of both December 31, 2016 and 2015
|
2
|
|
2
|
|
|||
|
Additional paid-in capital
|
2,545
|
|
2,481
|
|
|||
|
Retained earnings
|
6,613
|
|
6,199
|
|
|||
|
Accumulated other comprehensive loss
|
(72
|
)
|
(38
|
)
|
|||
|
Treasury stock, at cost; 79 shares and 73 shares as of December 31, 2016 and 2015, respectively
|
(4,460
|
)
|
(3,960
|
)
|
|||
|
Total Quest Diagnostics stockholders’ equity
|
4,628
|
|
4,684
|
|
|||
|
Noncontrolling interests
|
32
|
|
29
|
|
|||
|
Total stockholders’ equity
|
4,660
|
|
4,713
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
10,100
|
|
$
|
9,962
|
|
|
|
2016
|
2015
|
2014
|
|||||||||
|
Net revenues
|
$
|
7,515
|
|
$
|
7,493
|
|
$
|
7,435
|
|
||
|
Operating costs, expenses and other income:
|
|
|
|
|
|
|
|||||
|
Cost of services
|
4,616
|
|
4,657
|
|
4,637
|
|
|||||
|
Selling, general and administrative
|
1,681
|
|
1,679
|
|
1,728
|
|
|||||
|
Amortization of intangible assets
|
72
|
|
81
|
|
94
|
|
|||||
|
Gain on disposition of business
|
(118
|
)
|
(334
|
)
|
—
|
|
|||||
|
Other operating (income) expense, net
|
(13
|
)
|
11
|
|
(7
|
)
|
|||||
|
Total operating costs, expenses, net
|
6,238
|
|
6,094
|
|
6,452
|
|
|||||
|
Operating income
|
1,277
|
|
1,399
|
|
983
|
|
|||||
|
Other income (expense):
|
|
|
|
|
|
|
|||||
|
Interest expense, net
|
(143
|
)
|
(153
|
)
|
(164
|
)
|
|||||
|
Other (expense) income, net
|
(48
|
)
|
(143
|
)
|
4
|
|
|||||
|
Total non-operating expenses, net
|
(191
|
)
|
(296
|
)
|
(160
|
)
|
|||||
|
Income from continuing operations before income taxes and equity in earnings of equity method investees
|
1,086
|
|
1,103
|
|
823
|
|
|||||
|
Income tax expense
|
(429
|
)
|
(373
|
)
|
(262
|
)
|
|||||
|
Equity in earnings of equity method investees, net of taxes
|
39
|
|
23
|
|
26
|
|
|||||
|
Income from continuing operations
|
696
|
|
753
|
|
587
|
|
|||||
|
Income from discontinued operations, net of taxes
|
—
|
|
—
|
|
5
|
|
|||||
|
Net income
|
696
|
|
753
|
|
592
|
|
|||||
|
Less: Net income attributable to noncontrolling interests
|
51
|
|
44
|
|
36
|
|
|||||
|
Net income attributable to Quest Diagnostics
|
$
|
645
|
|
$
|
709
|
|
$
|
556
|
|
||
|
Amounts attributable to Quest Diagnostics’ stockholders:
|
|
|
|
|
|||||||
|
Income from continuing operations
|
$
|
645
|
|
$
|
709
|
|
$
|
551
|
|
||
|
Income from discontinued operations, net of taxes
|
—
|
|
—
|
|
5
|
|
|||||
|
Net income
|
$
|
645
|
|
$
|
709
|
|
$
|
556
|
|
||
|
Earnings per share attributable to Quest Diagnostics’ common stockholders - basic:
|
|
|
|
|
|||||||
|
Income from continuing operations
|
$
|
4.58
|
|
$
|
4.92
|
|
$
|
3.80
|
|
||
|
Income from discontinued operations
|
—
|
|
—
|
|
0.03
|
|
|||||
|
Net income
|
$
|
4.58
|
|
$
|
4.92
|
|
$
|
3.83
|
|
||
|
Earnings per share attributable to Quest Diagnostics’ common stockholders - diluted:
|
|
|
|
|
|||||||
|
Income from continuing operations
|
$
|
4.51
|
|
$
|
4.87
|
|
$
|
3.78
|
|
||
|
Income from discontinued operations
|
—
|
|
—
|
|
0.03
|
|
|||||
|
Net income
|
$
|
4.51
|
|
$
|
4.87
|
|
$
|
3.81
|
|
||
|
Dividends per common share
|
$
|
1.65
|
|
$
|
1.52
|
|
$
|
1.32
|
|
||
|
2016
|
2015
|
2014
|
|||||||||
|
Net income
|
$
|
696
|
|
$
|
753
|
|
$
|
592
|
|
||
|
Other comprehensive (loss) income:
|
|||||||||||
|
Currency translation
|
(34
|
)
|
(15
|
)
|
(7
|
)
|
|||||
|
Market valuation, net of tax
|
(2
|
)
|
—
|
|
(1
|
)
|
|||||
|
Net deferred loss on cash flow hedges, net of tax
|
2
|
|
3
|
|
(10
|
)
|
|||||
|
Other
|
—
|
|
1
|
|
(1
|
)
|
|||||
|
Other comprehensive loss
|
(34
|
)
|
(11
|
)
|
(19
|
)
|
|||||
|
Comprehensive income
|
662
|
|
742
|
|
573
|
|
|||||
|
Less: Comprehensive income attributable to noncontrolling interests
|
51
|
|
44
|
|
36
|
|
|||||
|
Comprehensive income attributable to Quest Diagnostics
|
$
|
611
|
|
$
|
698
|
|
$
|
537
|
|
||
|
2016
|
2015
|
2014
|
|||||||||
|
Cash flows from operating activities:
|
|
|
|
|
|||||||
|
Net income
|
$
|
696
|
|
$
|
753
|
|
$
|
592
|
|
||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
|
|
|
|
|
|
|||||
|
Depreciation and amortization
|
249
|
|
304
|
|
314
|
|
|||||
|
Provision for doubtful accounts
|
308
|
|
297
|
|
296
|
|
|||||
|
Deferred income tax provision
|
37
|
|
112
|
|
23
|
|
|||||
|
Stock-based compensation expense
|
69
|
|
52
|
|
51
|
|
|||||
|
Gain on disposition of business
|
(118
|
)
|
(334
|
)
|
—
|
|
|||||
|
Other, net
|
(6
|
)
|
6
|
|
(12
|
)
|
|||||
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|||||
|
Accounts receivable
|
(343
|
)
|
(262
|
)
|
(312
|
)
|
|||||
|
Accounts payable and accrued expenses
|
56
|
|
(24
|
)
|
74
|
|
|||||
|
Income taxes payable
|
42
|
|
(41
|
)
|
(84
|
)
|
|||||
|
Termination of interest rate swap agreements
|
54
|
|
—
|
|
—
|
|
|||||
|
Other assets and liabilities, net
|
25
|
|
(42
|
)
|
2
|
|
|||||
|
Net cash provided by operating activities
|
1,069
|
|
821
|
|
944
|
|
|||||
|
Cash flows from investing activities:
|
|
|
|
|
|||||||
|
Business acquisitions, net of cash acquired
|
(139
|
)
|
(67
|
)
|
(728
|
)
|
|||||
|
Proceeds from sale of businesses
|
270
|
|
—
|
|
—
|
|
|||||
|
Capital expenditures
|
(293
|
)
|
(263
|
)
|
(308
|
)
|
|||||
|
Investment in equity method investee
|
—
|
|
(33
|
)
|
—
|
|
|||||
|
Decrease in investments and other assets
|
10
|
|
1
|
|
11
|
|
|||||
|
Net cash used in investing activities
|
(152
|
)
|
(362
|
)
|
(1,025
|
)
|
|||||
|
Cash flows from financing activities:
|
|
|
|
|
|||||||
|
Proceeds from borrowings
|
1,869
|
|
2,453
|
|
2,018
|
|
|||||
|
Repayments of debt
|
(1,724
|
)
|
(2,537
|
)
|
(1,647
|
)
|
|||||
|
Purchases of treasury stock
|
(590
|
)
|
(224
|
)
|
(132
|
)
|
|||||
|
Exercise of stock options
|
73
|
|
60
|
|
78
|
|
|||||
|
Employee payroll tax withholdings on stock issued under stock-based compensation plans
|
(10
|
)
|
(7
|
)
|
(6
|
)
|
|||||
|
Dividends paid
|
(223
|
)
|
(212
|
)
|
(187
|
)
|
|||||
|
Distributions to noncontrolling interests
|
(41
|
)
|
(42
|
)
|
(31
|
)
|
|||||
|
Sale of noncontrolling interest in subsidiary
|
—
|
|
63
|
|
—
|
|
|||||
|
Payment of deferred business acquisition consideration
|
—
|
|
(51
|
)
|
—
|
|
|||||
|
Other financing activities, net
|
(45
|
)
|
(21
|
)
|
(7
|
)
|
|||||
|
Net cash (used in) provided by financing activities
|
(691
|
)
|
(518
|
)
|
86
|
|
|||||
|
Net change in cash and cash equivalents
|
226
|
|
(59
|
)
|
5
|
|
|||||
|
Cash and cash equivalents, beginning of year
|
133
|
|
192
|
|
187
|
|
|||||
|
Cash and cash equivalents, end of year
|
$
|
359
|
|
$
|
133
|
|
$
|
192
|
|
||
|
Quest Diagnostics Stockholders’ Equity
|
||||||||||||||||||||||||||||
|
Shares of
Common Stock Out-
standing
|
Common
Stock
|
Additional
Paid-In
Capital
|
Retained
Earnings
|
Accumulated
Other
Comprehensive
Loss
|
Treasury
Stock, at
Cost
|
Non-
controlling
Interests
|
Total Stock-holders’
Equity
|
Redeemable Non-controlling Interest
|
||||||||||||||||||||
|
Balance, December 31, 2013
|
144
|
|
$
|
2
|
|
$
|
2,379
|
|
$
|
5,358
|
|
$
|
(8
|
)
|
$
|
(3,783
|
)
|
$
|
25
|
|
$
|
3,973
|
|
$
|
—
|
|
||
|
Net income
|
|
|
|
|
|
|
556
|
|
|
|
|
|
36
|
|
592
|
|
|
|
||||||||||
|
Other comprehensive loss, net of tax
|
|
|
|
|
|
|
|
|
(19
|
)
|
|
|
|
|
(19
|
)
|
||||||||||||
|
Dividends declared
|
|
|
|
|
|
|
(191
|
)
|
|
|
|
|
|
|
(191
|
)
|
||||||||||||
|
Distributions to noncontrolling interests
|
|
|
|
|
|
|
|
|
|
|
|
|
(31
|
)
|
(31
|
)
|
|
|
||||||||||
|
Issuance of common stock under benefit plans
|
1
|
|
|
|
2
|
|
|
|
|
|
17
|
|
|
|
19
|
|
||||||||||||
|
Stock-based compensation expense
|
|
|
|
|
48
|
|
|
|
|
|
3
|
|
|
|
51
|
|
||||||||||||
|
Exercise of stock options
|
1
|
|
|
|
(2
|
)
|
|
|
|
|
80
|
|
|
|
78
|
|
||||||||||||
|
Shares to cover employee payroll tax withholdings on stock issued under stock-based compensation plans
|
|
|
|
|
(6
|
)
|
|
|
|
|
|
|
|
|
(6
|
)
|
||||||||||||
|
Tax benefits associated with stock-based compensation plans
|
|
|
|
|
(3
|
)
|
|
|
|
|
|
|
|
|
(3
|
)
|
||||||||||||
|
Purchases of treasury stock
|
(2
|
)
|
|
|
|
|
|
|
|
|
(132
|
)
|
|
|
(132
|
)
|
||||||||||||
|
Other
|
|
|
|
|
|
|
|
|
|
|
|
|
(1
|
)
|
(1
|
)
|
||||||||||||
|
Balance, December 31, 2014
|
144
|
|
$
|
2
|
|
$
|
2,418
|
|
$
|
5,723
|
|
$
|
(27
|
)
|
$
|
(3,815
|
)
|
$
|
29
|
|
$
|
4,330
|
|
$
|
—
|
|
||
|
Net income
|
|
|
|
|
|
|
709
|
|
|
|
|
|
42
|
|
751
|
|
2
|
|
||||||||||
|
Other comprehensive loss, net of tax
|
|
|
|
|
|
|
|
|
(11
|
)
|
|
|
|
|
(11
|
)
|
||||||||||||
|
Dividends declared
|
|
|
|
|
|
|
(219
|
)
|
|
|
|
|
|
|
(219
|
)
|
||||||||||||
|
Distributions to noncontrolling interests
|
|
|
|
|
|
|
|
|
|
|
|
|
(42
|
)
|
(42
|
)
|
|
|
||||||||||
|
Issuance of common stock under benefit plans
|
1
|
|
|
|
6
|
|
|
|
|
|
15
|
|
|
|
21
|
|
||||||||||||
|
Stock-based compensation expense
|
|
|
|
|
48
|
|
|
|
|
|
4
|
|
|
|
52
|
|
||||||||||||
|
Exercise of stock options
|
1
|
|
|
|
|
|
|
|
|
|
60
|
|
|
|
60
|
|
||||||||||||
|
Shares to cover employee payroll tax withholdings on stock issued under stock-based compensation plans
|
|
|
|
|
(7
|
)
|
|
|
|
|
|
|
|
|
(7
|
)
|
||||||||||||
|
Tax benefits associated with stock-based compensation plans
|
|
|
|
|
5
|
|
|
|
|
|
|
|
|
|
5
|
|
||||||||||||
|
Purchases of treasury stock
|
(3
|
)
|
|
|
|
|
|
|
|
|
(224
|
)
|
|
|
(224
|
)
|
||||||||||||
|
Sale of redeemable noncontrolling interest
|
11
|
|
11
|
|
54
|
|
||||||||||||||||||||||
|
Adjustment to fair value
|
(14
|
)
|
(14
|
)
|
14
|
|
||||||||||||||||||||||
|
Balance, December 31, 2015
|
143
|
|
$
|
2
|
|
$
|
2,481
|
|
$
|
6,199
|
|
$
|
(38
|
)
|
$
|
(3,960
|
)
|
$
|
29
|
|
$
|
4,713
|
|
$
|
70
|
|
||
|
Net income
|
|
|
|
|
|
|
645
|
|
|
|
|
|
44
|
|
689
|
|
7
|
|
||||||||||
|
Other comprehensive loss, net of tax
|
|
|
|
|
|
|
|
|
(34
|
)
|
|
|
|
|
(34
|
)
|
||||||||||||
|
Dividends declared
|
|
|
|
|
|
|
(231
|
)
|
|
|
|
|
|
|
(231
|
)
|
||||||||||||
|
Distributions to noncontrolling interests
|
|
|
|
|
|
|
|
|
|
|
|
|
(41
|
)
|
(41
|
)
|
|
|
||||||||||
|
Issuance of common stock under benefit plans
|
|
|
|
|
7
|
|
|
|
|
|
15
|
|
|
|
22
|
|
||||||||||||
|
Stock-based compensation expense
|
|
|
|
|
65
|
|
|
|
|
|
4
|
|
|
|
69
|
|
||||||||||||
|
Exercise of stock options
|
1
|
|
|
|
2
|
|
|
|
|
|
71
|
|
|
|
73
|
|
||||||||||||
|
Shares to cover employee payroll tax withholdings on stock issued under stock-based compensation plans
|
|
|
|
|
(10
|
)
|
|
|
|
|
|
|
|
|
(10
|
)
|
||||||||||||
|
Purchases of treasury stock
|
(7
|
)
|
|
|
|
|
|
|
|
|
(590
|
)
|
|
|
(590
|
)
|
||||||||||||
|
Balance, December 31, 2016
|
137
|
|
$
|
2
|
|
$
|
2,545
|
|
$
|
6,613
|
|
$
|
(72
|
)
|
$
|
(4,460
|
)
|
$
|
32
|
|
$
|
4,660
|
|
$
|
77
|
|
||
|
•
|
buildings and improvements, ranging up to
thirty-one and a half
years;
|
|
•
|
laboratory equipment and furniture and fixtures, ranging from
five
to
twelve
years;
|
|
•
|
leasehold improvements, the lesser of the useful life of the improvement or the remaining life of the building or lease, as applicable; and
|
|
•
|
computer software developed or obtained for internal use,
five
years.
|
|
•
|
Trading securities represent participant-directed investments of deferred employee compensation and related Company matching contributions held in trusts pursuant to the Company's supplemental deferred compensation plans (see Note 16). Trading securities are carried at fair value with both realized and unrealized gains and losses recorded currently in earnings as a component of non-operating expenses within other (expense) income, net in the consolidated statements of operations. For the years ended
December 31, 2016
,
2015
and
2014
, gains from trading equity securities totaled
$3 million
,
$0 million
, and
$3 million
, respectively.
|
|
•
|
Available-for-sale equity securities consists of an investment in registered shares of a public corporation. Available-for-sale equity securities are carried at fair value with unrealized gains and losses, net of tax, recorded as a component of accumulated other comprehensive loss within stockholders' equity and realized gains and losses recorded in other (expense) income, net in the consolidated statements of operations. As of
December 31, 2016
, the Company had gross unrealized losses from available-for-sale equity securities of
$5 million
.
|
|
•
|
Other investments do not have readily determinable fair values and consist of investments in preferred and common shares of privately held companies and are accounted for under the cost method.
|
|
2016
|
2015
|
||||||
|
Available-for-sale equity securities
|
$
|
3
|
|
$
|
6
|
|
|
|
Trading equity securities
|
51
|
|
49
|
|
|||
|
Other investments
|
6
|
|
8
|
|
|||
|
Total
|
$
|
60
|
|
$
|
63
|
|
|
|
•
|
Excess income tax benefits and deficiencies from stock-based compensation arrangements are recognized as a discrete item within income tax expense, rather than additional paid-in capital. The adoption of this provision, which was done on a prospective basis, resulted in the classification of
$9 million
of tax benefits in income tax expense for the year ended December 31, 2016. In addition, excess income tax benefits and deficiencies are no longer considered when applying the treasury stock method for computing the effect of dilutive securities, which resulted in an increase in the effect of the dilutive securities for the year ended December 31, 2016.
|
|
•
|
Excess income tax benefits from stock-based compensation arrangements are classified as an operating activity and cash paid for employee payroll tax withholdings by directly withholding shares are classified as a financing activity in
|
|
◦
|
$5 million
and
$0 million
of excess tax benefits related to the settlement of stock-based compensation awards from financing to operating activities for the year ended December 31, 2015 and 2014, respectively; and
|
|
◦
|
$7 million
and
$6 million
of taxes paid related to employee payroll tax withholdings on stock issued under stock-based compensation plans from operating to financing activities for the years ended December 31, 2015 and 2014, respectively.
|
|
|
2016
|
2015
|
2014
|
||||||||
|
Amounts attributable to Quest Diagnostics’ stockholders:
|
|
|
|
|
|||||||
|
Income from continuing operations
|
$
|
645
|
|
$
|
709
|
|
$
|
551
|
|
||
|
Income from discontinued operations, net of taxes
|
—
|
|
—
|
|
5
|
|
|||||
|
Net income attributable to Quest Diagnostics’ common stockholders
|
$
|
645
|
|
$
|
709
|
|
$
|
556
|
|
||
|
Income from continuing operations
|
$
|
645
|
|
$
|
709
|
|
$
|
551
|
|
||
|
Less: Earnings allocated to participating securities
|
3
|
|
3
|
|
2
|
|
|||||
|
Earnings available to Quest Diagnostics’ common stockholders – basic and diluted
|
$
|
642
|
|
$
|
706
|
|
$
|
549
|
|
||
|
Weighted average common shares outstanding – basic
|
140
|
|
144
|
|
145
|
|
|||||
|
Effect of dilutive securities:
|
|
|
|
|
|||||||
|
Stock options and performance share units
|
2
|
|
1
|
|
—
|
|
|||||
|
Weighted average common shares outstanding – diluted
|
142
|
|
145
|
|
145
|
|
|||||
|
Earnings per share attributable to Quest Diagnostics’ common stockholders – basic:
|
|
|
|
|
|||||||
|
Income from continuing operations
|
$
|
4.58
|
|
$
|
4.92
|
|
$
|
3.80
|
|
||
|
Income from discontinued operations
|
—
|
|
—
|
|
0.03
|
|
|||||
|
Net income
|
$
|
4.58
|
|
$
|
4.92
|
|
$
|
3.83
|
|
||
|
Earnings per share attributable to Quest Diagnostics’ common stockholders – diluted:
|
|
|
|
|
|||||||
|
Income from continuing operations
|
$
|
4.51
|
|
$
|
4.87
|
|
$
|
3.78
|
|
||
|
Income from discontinued operations
|
—
|
|
—
|
|
0.03
|
|
|||||
|
Net income
|
$
|
4.51
|
|
$
|
4.87
|
|
$
|
3.81
|
|
||
|
2016
|
2015
|
2014
|
||||||
|
Stock options and performance share units
|
1
|
|
2
|
|
2
|
|
||
|
2016
|
2015
|
2014
|
|||||||||
|
Employee separation costs
|
$
|
9
|
|
$
|
38
|
|
$
|
31
|
|
||
|
Facility-related costs
|
2
|
|
1
|
|
12
|
|
|||||
|
Asset impairment charges
|
—
|
|
—
|
|
1
|
|
|||||
|
Total restructuring charges
|
$
|
11
|
|
$
|
39
|
|
$
|
44
|
|
||
|
Employee Separation Costs
|
Facility-Related Costs
|
Total
|
|||||||||
|
Balance, December 31, 2014
|
$
|
18
|
|
$
|
11
|
|
$
|
29
|
|
||
|
Income statement expense
|
38
|
|
1
|
|
39
|
|
|||||
|
Cash payments
|
(40
|
)
|
(6
|
)
|
(46
|
)
|
|||||
|
Other / adjustments
|
—
|
|
(3
|
)
|
(3
|
)
|
|||||
|
Balance, December 31, 2015
|
16
|
|
3
|
|
19
|
|
|||||
|
Income statement expense
|
9
|
|
2
|
|
11
|
|
|||||
|
Cash payments
|
(19
|
)
|
(2
|
)
|
(21
|
)
|
|||||
|
Balance, December 31, 2016
|
$
|
6
|
|
$
|
3
|
|
$
|
9
|
|
||
|
Solstas
|
Summit Health
|
||||||||||
|
Cash
|
$
|
572
|
|
$
|
125
|
|
|||||
|
Estimated fair value of contingent consideration
|
—
|
|
22
|
|
|||||||
|
Transaction related costs due to sellers
|
—
|
|
5
|
|
|||||||
|
Total consideration
|
$
|
572
|
|
$
|
152
|
|
|||||
|
Solstas
|
Summit Health
|
||||||||||
|
Fair Value
|
Weighted Average Useful Life (in years)
|
Fair Value
|
Weighted Average Useful Life (in years)
|
||||||||
|
Allocation of purchase price:
|
|||||||||||
|
Cash and cash equivalents
|
$
|
9
|
|
$
|
1
|
|
|||||
|
Accounts receivable, net
|
48
|
|
9
|
|
|||||||
|
Other current assets
|
12
|
|
16
|
|
|||||||
|
Property, plant and equipment, net
|
49
|
|
6
|
|
|||||||
|
Goodwill
|
271
|
|
92
|
|
|||||||
|
Intangible assets:
|
|||||||||||
|
Customer relationships
|
203
|
|
20
|
33
|
|
15
|
|||||
|
Tradename
|
7
|
|
2
|
2
|
|
1
|
|||||
|
Software
|
—
|
|
3
|
|
4
|
||||||
|
Total intangible assets
|
210
|
|
38
|
|
|||||||
|
Non-current deferred income taxes
|
48
|
|
—
|
|
|||||||
|
Total assets acquired
|
647
|
|
162
|
|
|||||||
|
Current liabilities
|
64
|
|
10
|
|
|||||||
|
Non-current deferred income taxes
|
3
|
|
—
|
|
|||||||
|
Other non-current liabilities
|
8
|
|
—
|
|
|||||||
|
Total liabilities assumed
|
75
|
|
10
|
|
|||||||
|
Net assets acquired
|
$
|
572
|
|
$
|
152
|
|
|||||
|
2014
|
|||
|
(unaudited)
|
|||
|
Pro forma net revenues
|
$
|
7,520
|
|
|
Pro forma income from continuing operations
|
$
|
585
|
|
|
Earnings per share attributable to Quest Diagnostics’ common stockholders - basic:
|
|||
|
Pro forma income from continuing operations
|
$
|
3.79
|
|
|
Earnings per share attributable to Quest Diagnostics’ common stockholders - diluted:
|
|||
|
Pro forma income from continuing operations
|
$
|
3.77
|
|
|
Basis of Fair Value Measurements
|
|||||||||||||||
|
Total
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
December 31, 2016
|
|||||||||||||||
|
Assets:
|
|
|
|
|
|
|
|
|
|||||||
|
Trading securities
|
$
|
51
|
|
$
|
51
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Cash surrender value of life insurance policies
|
32
|
|
—
|
|
32
|
|
—
|
|
|||||||
|
Available-for-sale equity securities
|
3
|
|
3
|
|
—
|
|
—
|
|
|||||||
|
Total
|
$
|
86
|
|
$
|
54
|
|
$
|
32
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|
|
|
|
|
|
|
|
|||||||
|
Deferred compensation liabilities
|
$
|
91
|
|
$
|
—
|
|
$
|
91
|
|
$
|
—
|
|
|||
|
Interest rate swaps
|
88
|
|
—
|
|
88
|
|
—
|
|
|||||||
|
Contingent consideration
|
3
|
|
—
|
|
—
|
|
3
|
|
|||||||
|
Total
|
$
|
182
|
|
$
|
—
|
|
$
|
179
|
|
$
|
3
|
|
|||
|
December 31, 2015
|
|||||||||||||||
|
Assets:
|
|
|
|
|
|
||||||||||
|
Trading securities
|
$
|
49
|
|
$
|
49
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Cash surrender value of life insurance policies
|
29
|
|
—
|
|
29
|
|
—
|
|
|||||||
|
Interest rate swaps
|
23
|
|
—
|
|
23
|
|
—
|
|
|||||||
|
Available-for-sale equity securities
|
6
|
|
6
|
|
—
|
|
—
|
|
|||||||
|
Total
|
$
|
107
|
|
$
|
55
|
|
$
|
52
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|
|
|
|
|
|
|
|
|||||||
|
Deferred compensation liabilities
|
$
|
85
|
|
$
|
—
|
|
$
|
85
|
|
$
|
—
|
|
|||
|
Interest rate swaps
|
6
|
|
—
|
|
6
|
|
—
|
|
|||||||
|
Contingent consideration
|
3
|
|
—
|
|
—
|
|
3
|
|
|||||||
|
Total
|
$
|
94
|
|
$
|
—
|
|
$
|
91
|
|
$
|
3
|
|
|||
|
Contingent Consideration
|
|||
|
Balance, December 31, 2014
|
$
|
17
|
|
|
Settlements
|
(1
|
)
|
|
|
Total (gains) losses - realized/unrealized:
|
|||
|
Included in earnings
|
(13
|
)
|
|
|
Balance, December 31, 2015
|
3
|
|
|
|
Balance, December 31, 2016
|
$
|
3
|
|
|
2016
|
2015
|
2014
|
|||||||||
|
Current:
|
|||||||||||
|
Federal
|
$
|
346
|
|
$
|
231
|
|
$
|
204
|
|
||
|
State and local
|
45
|
|
27
|
|
34
|
|
|||||
|
Foreign
|
1
|
|
3
|
|
3
|
|
|||||
|
Deferred:
|
|||||||||||
|
Federal
|
33
|
|
104
|
|
28
|
|
|||||
|
State and local
|
4
|
|
7
|
|
(6
|
)
|
|||||
|
Foreign
|
—
|
|
1
|
|
(1
|
)
|
|||||
|
Total
|
$
|
429
|
|
$
|
373
|
|
$
|
262
|
|
||
|
2016
|
2015
|
2014
|
||||||
|
Tax provision at statutory rate
|
35.0
|
%
|
35.0
|
%
|
35.0
|
%
|
||
|
State and local income taxes, net of federal benefit
|
3.3
|
|
2.6
|
|
3.2
|
|
||
|
Gains and losses on book and tax basis difference
|
3.3
|
|
(2.7
|
)
|
—
|
|
||
|
Adjustments to unrecognized tax positions
|
0.5
|
|
(0.4
|
)
|
(5.1
|
)
|
||
|
Impact of noncontrolling interests
|
(1.8
|
)
|
(1.6
|
)
|
(1.7
|
)
|
||
|
Impact of equity earnings
|
1.0
|
|
0.7
|
|
1.1
|
|
||
|
Other, net
|
(1.8
|
)
|
0.2
|
|
(0.7
|
)
|
||
|
Effective tax rate
|
39.5
|
%
|
33.8
|
%
|
31.8
|
%
|
||
|
2016
|
2015
|
||||||
|
Non-current deferred tax assets (liabilities):
|
|||||||
|
Accounts receivable reserves
|
$
|
94
|
|
$
|
95
|
|
|
|
Liabilities not currently deductible
|
189
|
|
202
|
|
|||
|
Stock-based compensation
|
58
|
|
49
|
|
|||
|
Capitalized R&D expense
|
—
|
|
1
|
|
|||
|
Basis differences in investments, joint ventures and subsidiaries
|
(87
|
)
|
(90
|
)
|
|||
|
Net operating loss carryforwards, net of valuation allowance
|
120
|
|
140
|
|
|||
|
Depreciation and amortization
|
(533
|
)
|
(517
|
)
|
|||
|
Total non-current deferred tax liabilities, net
|
$
|
(159
|
)
|
$
|
(120
|
)
|
|
|
2016
|
2015
|
2014
|
|||||||||
|
Balance, beginning of year
|
$
|
91
|
|
$
|
122
|
|
$
|
168
|
|
||
|
Additions:
|
|||||||||||
|
For tax positions of current year
|
3
|
|
5
|
|
17
|
|
|||||
|
For tax positions of prior years
|
12
|
|
5
|
|
1
|
|
|||||
|
Reductions:
|
|||||||||||
|
Changes in judgment
|
(1
|
)
|
(11
|
)
|
(56
|
)
|
|||||
|
Expirations of statutes of limitations
|
(7
|
)
|
(3
|
)
|
(6
|
)
|
|||||
|
Settlements
|
—
|
|
(27
|
)
|
(2
|
)
|
|||||
|
Balance, end of year
|
$
|
98
|
|
$
|
91
|
|
$
|
122
|
|
||
|
2016
|
2015
|
2014
|
|||||||||
|
Depreciation expense
|
$
|
177
|
|
$
|
223
|
|
$
|
220
|
|
||
|
Amortization expense
|
72
|
|
81
|
|
94
|
|
|||||
|
Depreciation and amortization expense
|
$
|
249
|
|
$
|
304
|
|
$
|
314
|
|
||
|
Interest expense
|
$
|
(144
|
)
|
$
|
(154
|
)
|
$
|
(167
|
)
|
||
|
Interest income
|
1
|
|
1
|
|
3
|
|
|||||
|
Interest expense, net
|
$
|
(143
|
)
|
$
|
(153
|
)
|
$
|
(164
|
)
|
||
|
Interest paid
|
$
|
148
|
|
$
|
172
|
|
$
|
170
|
|
||
|
Income taxes paid
|
$
|
361
|
|
$
|
319
|
|
$
|
327
|
|
||
|
Assets acquired under capital leases
|
$
|
—
|
|
$
|
3
|
|
$
|
12
|
|
||
|
Accounts payable associated with capital expenditures
|
$
|
9
|
|
$
|
15
|
|
$
|
26
|
|
||
|
Dividend payable
|
$
|
62
|
|
$
|
55
|
|
$
|
48
|
|
||
|
Businesses acquired:
|
|
|
|
|
|||||||
|
Fair value of assets acquired
|
$
|
139
|
|
$
|
63
|
|
$
|
853
|
|
||
|
Fair value of liabilities assumed
|
—
|
|
—
|
|
85
|
|
|||||
|
Fair value of net assets acquired
|
139
|
|
63
|
|
768
|
|
|||||
|
Merger consideration paid (payable), net
|
—
|
|
4
|
|
(30
|
)
|
|||||
|
Cash paid for business acquisitions
|
139
|
|
67
|
|
738
|
|
|||||
|
Less: Cash acquired
|
—
|
|
—
|
|
10
|
|
|||||
|
Business acquisitions, net of cash acquired
|
$
|
139
|
|
$
|
67
|
|
$
|
728
|
|
||
|
2016
|
2015
|
||||||
|
Land
|
$
|
28
|
|
$
|
28
|
|
|
|
Buildings and improvements
|
379
|
|
370
|
|
|||
|
Laboratory equipment and furniture and fixtures
|
1,462
|
|
1,407
|
|
|||
|
Leasehold improvements
|
533
|
|
535
|
|
|||
|
Computer software developed or obtained for internal use
|
834
|
|
756
|
|
|||
|
Construction-in-progress
|
193
|
|
136
|
|
|||
|
3,429
|
|
3,232
|
|
||||
|
Less: Accumulated depreciation and amortization
|
(2,400
|
)
|
(2,307
|
)
|
|||
|
Total
|
$
|
1,029
|
|
$
|
925
|
|
|
|
2016
|
2015
|
||||||
|
Balance, beginning of year
|
$
|
5,905
|
|
$
|
6,032
|
|
|
|
Goodwill acquired during the year
|
95
|
|
33
|
|
|||
|
Reclassification to assets held for sale
|
—
|
|
(160
|
)
|
|||
|
Balance, end of year
|
$
|
6,000
|
|
$
|
5,905
|
|
|
|
Weighted
Average
Amort-ization
Period (Years)
|
December 31, 2016
|
December 31, 2015
|
|||||||||||||||||||||||
|
Cost
|
Accumulated
Amortization
|
Net
|
Cost
|
Accumulated
Amortization
|
Net
|
||||||||||||||||||||
|
Amortizing intangible assets:
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
Customer-related intangibles
|
18
|
$
|
971
|
|
$
|
(346
|
)
|
$
|
625
|
|
$
|
936
|
|
$
|
(296
|
)
|
$
|
640
|
|
||||||
|
Non-compete agreements
|
6
|
6
|
|
(4
|
)
|
2
|
|
6
|
|
(3
|
)
|
3
|
|
||||||||||||
|
Technology
|
18
|
93
|
|
(40
|
)
|
53
|
|
93
|
|
(35
|
)
|
58
|
|
||||||||||||
|
Other
|
9
|
103
|
|
(70
|
)
|
33
|
|
106
|
|
(59
|
)
|
47
|
|
||||||||||||
|
Total
|
18
|
1,173
|
|
(460
|
)
|
713
|
|
1,141
|
|
(393
|
)
|
748
|
|
||||||||||||
|
Intangible assets not subject to amortization:
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
Tradenames
|
|
235
|
|
—
|
|
235
|
|
235
|
|
—
|
|
235
|
|
||||||||||||
|
Other
|
|
1
|
|
—
|
|
1
|
|
1
|
|
—
|
|
1
|
|
||||||||||||
|
Total intangible assets
|
$
|
1,409
|
|
$
|
(460
|
)
|
$
|
949
|
|
$
|
1,377
|
|
$
|
(393
|
)
|
$
|
984
|
|
|||||||
|
Year Ending December 31,
|
|
|
|
|
2017
|
$
|
69
|
|
|
2018
|
65
|
|
|
|
2019
|
64
|
|
|
|
2020
|
64
|
|
|
|
2021
|
57
|
|
|
|
Thereafter
|
394
|
|
|
|
Total
|
$
|
713
|
|
|
2016
|
2015
|
||||||
|
Trade accounts payable
|
$
|
261
|
|
$
|
279
|
|
|
|
Accrued wages and benefits (including incentive compensation)
|
316
|
|
305
|
|
|||
|
Income taxes payable
|
3
|
|
4
|
|
|||
|
Accrued interest
|
46
|
|
52
|
|
|||
|
Accrued insurance
|
31
|
|
60
|
|
|||
|
Merger consideration payable
|
2
|
|
2
|
|
|||
|
Dividend payable
|
62
|
|
55
|
|
|||
|
Accrued expenses
|
254
|
|
257
|
|
|||
|
Total
|
$
|
975
|
|
$
|
1,014
|
|
|
|
2016
|
2015
|
||||||
|
3.20% Senior Notes due April 2016
|
—
|
|
150
|
|
|||
|
2.70% Senior Notes due April 2019
|
300
|
|
300
|
|
|||
|
4.75% Senior Notes due January 2020
|
521
|
|
522
|
|
|||
|
2.50% Senior Notes due March 2020
|
299
|
|
299
|
|
|||
|
4.70% Senior Notes due April 2021
|
563
|
|
554
|
|
|||
|
4.25% Senior Notes due April 2024
|
307
|
|
313
|
|
|||
|
3.50% Senior Notes due March 2025
|
568
|
|
601
|
|
|||
|
3.45% Senior Notes due June 2026
|
469
|
|
—
|
|
|||
|
6.95% Senior Notes due July 2037
|
174
|
|
247
|
|
|||
|
5.75% Senior Notes due January 2040
|
244
|
|
368
|
|
|||
|
4.70% Senior Notes due March 2045
|
300
|
|
300
|
|
|||
|
Other
|
13
|
|
22
|
|
|||
|
Debt issuance costs
|
(24
|
)
|
(25
|
)
|
|||
|
Total long-term debt
|
3,734
|
|
3,651
|
|
|||
|
Less: Current portion of long-term debt
|
6
|
|
159
|
|
|||
|
Total long-term debt, net of current portion
|
$
|
3,728
|
|
$
|
3,492
|
|
|
|
Year Ending December 31,
|
|||
|
2017
|
$
|
6
|
|
|
2018
|
4
|
|
|
|
2019
|
302
|
|
|
|
2020
|
801
|
|
|
|
2021
|
550
|
|
|
|
Thereafter
|
2,125
|
|
|
|
Total maturities of long-term debt
|
3,788
|
|
|
|
Unamortized discount
|
(13
|
)
|
|
|
Debt issuance costs
|
(24
|
)
|
|
|
Fair value basis adjustments attributable to hedged debt
|
(17
|
)
|
|
|
Total long-term debt
|
3,734
|
|
|
|
Less: Current portion of long-term debt
|
6
|
|
|
|
Total long-term debt, net of current portion
|
$
|
3,728
|
|
|
Notional Amount
|
||||||||
|
Debt Instrument
|
2016
|
2015
|
||||||
|
4.75% Senior Notes due January 2020
|
$
|
—
|
|
$
|
350
|
|
||
|
4.70% Senior Notes due April 2021
|
—
|
|
400
|
|
||||
|
4.25% Senior Notes due April 2024
|
250
|
|
250
|
|
||||
|
3.50% Senior Notes due March 2025
|
600
|
|
200
|
|
||||
|
3.45% Senior Notes due June 2026
|
350
|
|
—
|
|
||||
|
$
|
1,200
|
|
$
|
1,200
|
|
|||
|
December 31, 2016
|
December 31, 2015
|
||||||||||
|
Balance Sheet
Classification
|
Fair Value
|
Balance Sheet
Classification
|
Fair Value
|
||||||||
|
Derivatives Designated as Hedging Instruments
|
|
|
|
|
|
||||||
|
Asset Derivatives:
|
|
|
|
|
|
|
|||||
|
Interest rate swaps
|
|
$
|
—
|
|
Other assets
|
$
|
23
|
|
|||
|
Liability Derivatives:
|
|||||||||||
|
Interest rate swaps
|
Other liabilities
|
88
|
|
Other liabilities
|
6
|
|
|||||
|
Total Net Derivatives (Liabilities) Assets
|
|
$
|
(88
|
)
|
|
$
|
17
|
|
|||
|
Foreign
Currency
Translation
Adjustment
|
Market Value
Adjustment
|
Net Deferred Loss on Cash Flow Hedges
|
Other
|
Accumulated Other Comprehensive Income (Loss)
|
|||||||||||||||
|
Balance, December 31, 2013
|
$
|
(2
|
)
|
$
|
—
|
|
$
|
(5
|
)
|
$
|
(1
|
)
|
$
|
(8
|
)
|
||||
|
Other comprehensive income (loss) before reclassifications
|
(7
|
)
|
(1
|
)
|
(11
|
)
|
(1
|
)
|
(20
|
)
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive income (loss)
|
—
|
|
—
|
|
1
|
|
—
|
|
1
|
|
|||||||||
|
Net current period other comprehensive income (loss)
|
(7
|
)
|
(1
|
)
|
(10
|
)
|
(1
|
)
|
(19
|
)
|
|||||||||
|
Balance, December 31, 2014
|
(9
|
)
|
(1
|
)
|
(15
|
)
|
(2
|
)
|
(27
|
)
|
|||||||||
|
Other comprehensive income (loss) before reclassifications
|
(15
|
)
|
—
|
|
—
|
|
1
|
|
(14
|
)
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive income (loss)
|
—
|
|
—
|
|
3
|
|
—
|
|
3
|
|
|||||||||
|
Net current period other comprehensive income (loss)
|
(15
|
)
|
—
|
|
3
|
|
1
|
|
(11
|
)
|
|||||||||
|
Balance, December 31, 2015
|
(24
|
)
|
(1
|
)
|
(12
|
)
|
(1
|
)
|
(38
|
)
|
|||||||||
|
Other comprehensive income (loss) before reclassifications
|
(34
|
)
|
(2
|
)
|
—
|
|
—
|
|
(36
|
)
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive income (loss)
|
—
|
|
—
|
|
2
|
|
—
|
|
2
|
|
|||||||||
|
Net current period other comprehensive income (loss)
|
(34
|
)
|
(2
|
)
|
2
|
|
—
|
|
(34
|
)
|
|||||||||
|
Balance, December 31, 2016
|
$
|
(58
|
)
|
$
|
(3
|
)
|
$
|
(10
|
)
|
$
|
(1
|
)
|
$
|
(72
|
)
|
||||
|
2016
|
2015
|
2014
|
|||
|
Fair value at grant date
|
$10.35
|
$11.57
|
$10.99
|
||
|
Expected volatility
|
21.6%
|
21.0%
|
25.1%
|
||
|
Dividend yield
|
2.4%
|
2.1%
|
2.1%
|
||
|
Risk-free interest rate
|
1.4%
|
1.7%
|
1.6% - 2.0%
|
||
|
Expected holding period, in years
|
5.3
|
5.3
|
5.5 - 6.6
|
||
|
Shares
|
Weighted
Average Exercise Price
|
Weighted Average Remaining Contractual Term
(in years)
|
Aggregate Intrinsic Value
|
|||||||||
|
Options outstanding, beginning of year
|
7.7
|
|
$
|
59.65
|
|
|||||||
|
Options granted
|
2.8
|
|
66.87
|
|
||||||||
|
Options exercised
|
(1.3
|
)
|
56.17
|
|
||||||||
|
Options forfeited and canceled
|
(0.1
|
)
|
66.53
|
|
||||||||
|
Options outstanding, end of year
|
9.1
|
|
$
|
62.27
|
|
7.2
|
$
|
269
|
|
|||
|
Exercisable, end of year
|
4.2
|
|
$
|
57.84
|
|
5.7
|
$
|
144
|
|
|||
|
Vested and expected to vest, end of year
|
8.8
|
|
$
|
62.10
|
|
7.2
|
$
|
262
|
|
|||
|
2016
|
2015
|
2014
|
||||||||||||||||||
|
Shares
|
Weighted
Average
Grant Date
Fair Value
|
Shares
|
Weighted
Average
Grant Date
Fair Value
|
Shares
|
Weighted
Average
Grant Date
Fair Value
|
|||||||||||||||
|
Shares outstanding, beginning of year
|
1.7
|
|
$
|
59.92
|
|
1.9
|
|
$
|
55.50
|
|
1.9
|
|
$
|
57.08
|
|
|||||
|
Shares granted
|
0.6
|
|
67.26
|
|
0.6
|
|
71.17
|
|
0.7
|
|
52.72
|
|
||||||||
|
Shares vested
|
(0.4
|
)
|
58.98
|
|
(0.3
|
)
|
55.74
|
|
(0.3
|
)
|
57.14
|
|
||||||||
|
Shares forfeited and canceled
|
(0.4
|
)
|
57.31
|
|
(0.5
|
)
|
58.18
|
|
(0.4
|
)
|
56.44
|
|
||||||||
|
Shares outstanding, end of year
|
1.5
|
|
$
|
63.88
|
|
1.7
|
|
$
|
59.92
|
|
1.9
|
|
$
|
55.50
|
|
|||||
|
Year Ending December 31,
|
|||
|
2017
|
$
|
179
|
|
|
2018
|
129
|
|
|
|
2019
|
92
|
|
|
|
2020
|
64
|
|
|
|
2021
|
44
|
|
|
|
Thereafter
|
153
|
|
|
|
Minimum lease payments
|
$
|
661
|
|
|
2016
|
2015
|
2014
|
|||||||||
|
Net revenues
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Income from discontinued operations before taxes
|
—
|
|
—
|
|
1
|
|
|||||
|
Income tax benefit
|
—
|
|
—
|
|
(4
|
)
|
|||||
|
Income from discontinued operations, net of taxes
|
$
|
—
|
|
$
|
—
|
|
$
|
5
|
|
||
|
2016
|
2015
|
2014
|
|||||||||
|
Net revenues:
|
|
|
|
|
|||||||
|
DIS business
|
$
|
7,138
|
|
$
|
6,965
|
|
$
|
6,873
|
|
||
|
All other operating segments
|
377
|
|
528
|
|
562
|
|
|||||
|
Total net revenues
|
$
|
7,515
|
|
$
|
7,493
|
|
$
|
7,435
|
|
||
|
Operating earnings (loss):
|
|
|
|
|
|||||||
|
DIS business
|
$
|
1,244
|
|
$
|
1,118
|
|
$
|
1,068
|
|
||
|
All other operating segments
|
64
|
|
110
|
|
94
|
|
|||||
|
General corporate activities
|
(31
|
)
|
171
|
|
(179
|
)
|
|||||
|
Total operating income
|
1,277
|
|
1,399
|
|
983
|
|
|||||
|
Non-operating expenses, net
|
(191
|
)
|
(296
|
)
|
(160
|
)
|
|||||
|
Income from continuing operations before income taxes and equity in earnings of equity method investees
|
1,086
|
|
1,103
|
|
823
|
|
|||||
|
Income tax expense
|
(429
|
)
|
(373
|
)
|
(262
|
)
|
|||||
|
Equity in earnings of equity method investees, net of taxes
|
39
|
|
23
|
|
26
|
|
|||||
|
Income from continuing operations
|
696
|
|
753
|
|
587
|
|
|||||
|
Income from discontinued operations, net of taxes
|
—
|
|
—
|
|
5
|
|
|||||
|
Net income
|
696
|
|
753
|
|
592
|
|
|||||
|
Less: Net income attributable to noncontrolling interests
|
51
|
|
44
|
|
36
|
|
|||||
|
Net income attributable to Quest Diagnostics
|
$
|
645
|
|
$
|
709
|
|
$
|
556
|
|
||
|
2016
|
2015
|
2014
|
|||||||||
|
DIS business
|
$
|
170
|
|
$
|
212
|
|
$
|
206
|
|
||
|
All other operating segments
|
6
|
|
10
|
|
13
|
|
|||||
|
General corporate
|
73
|
|
82
|
|
95
|
|
|||||
|
Total depreciation and amortization
|
$
|
249
|
|
$
|
304
|
|
$
|
314
|
|
||
|
2016
|
2015
|
2014
|
|||||||||
|
DIS business
|
$
|
264
|
|
$
|
243
|
|
$
|
283
|
|
||
|
All other operating segments
|
21
|
|
16
|
|
17
|
|
|||||
|
General corporate
|
8
|
|
4
|
|
8
|
|
|||||
|
Total capital expenditures
|
$
|
293
|
|
$
|
263
|
|
$
|
308
|
|
||
|
2016
|
2015
|
2014
|
|||||||||
|
Routine clinical testing services
|
$
|
4,179
|
|
$
|
4,078
|
|
$
|
4,066
|
|
||
|
Gene-based and esoteric (including advanced diagnostics) testing services
|
2,335
|
|
2,256
|
|
2,158
|
|
|||||
|
Anatomic pathology testing services
|
624
|
|
631
|
|
649
|
|
|||||
|
All other
|
377
|
|
528
|
|
562
|
|
|||||
|
Total net revenues
|
$
|
7,515
|
|
$
|
7,493
|
|
$
|
7,435
|
|
||
|
2016 (a)
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
Total
Year
|
||||||||||||||
|
(b)
|
(c)
|
(d)
|
(e)
|
||||||||||||||||
|
Net revenues
|
$
|
1,863
|
|
$
|
1,906
|
|
$
|
1,885
|
|
$
|
1,861
|
|
$
|
7,515
|
|
||||
|
Gross profit
|
719
|
|
751
|
|
728
|
|
701
|
|
2,899
|
|
|||||||||
|
Income from continuing operations
|
115
|
|
209
|
|
205
|
|
167
|
|
696
|
|
|||||||||
|
Income from discontinued operations, net of taxes
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Net income
|
115
|
|
209
|
|
205
|
|
167
|
|
696
|
|
|||||||||
|
Less: Net income attributable to noncontrolling interests
|
12
|
|
14
|
|
13
|
|
12
|
|
51
|
|
|||||||||
|
Net income attributable to Quest Diagnostics
|
$
|
103
|
|
$
|
195
|
|
$
|
192
|
|
$
|
155
|
|
$
|
645
|
|
||||
|
Amounts attributable to Quest Diagnostics' stockholders:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
103
|
|
$
|
195
|
|
$
|
192
|
|
$
|
155
|
|
$
|
645
|
|
||||
|
Income from discontinued operations, net of taxes
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Net income
|
$
|
103
|
|
$
|
195
|
|
$
|
192
|
|
$
|
155
|
|
$
|
645
|
|
||||
|
Earnings per share attributable to Quest Diagnostics' stockholders - basic:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
0.72
|
|
$
|
1.38
|
|
$
|
1.37
|
|
$
|
1.11
|
|
$
|
4.58
|
|
||||
|
Income from discontinued operations
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Net income
|
$
|
0.72
|
|
$
|
1.38
|
|
$
|
1.37
|
|
$
|
1.11
|
|
$
|
4.58
|
|
||||
|
Earnings per share attributable to Quest Diagnostics' stockholders - diluted:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
0.71
|
|
$
|
1.37
|
|
$
|
1.34
|
|
$
|
1.09
|
|
$
|
4.51
|
|
||||
|
Income from discontinued operations
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Net income
|
$
|
0.71
|
|
$
|
1.37
|
|
$
|
1.34
|
|
$
|
1.09
|
|
$
|
4.51
|
|
||||
|
2015 (a)
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
Total
Year
|
||||||||||||||
|
(f)
|
(g)
|
(h)
|
(i)
|
||||||||||||||||
|
Net revenues
|
$
|
1,839
|
|
$
|
1,925
|
|
$
|
1,880
|
|
$
|
1,849
|
|
$
|
7,493
|
|
||||
|
Gross profit
|
676
|
|
743
|
|
718
|
|
699
|
|
2,836
|
|
|||||||||
|
Income from continuing operations
|
70
|
|
129
|
|
354
|
|
200
|
|
753
|
|
|||||||||
|
Income from discontinued operations, net of taxes
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Net income
|
70
|
|
129
|
|
354
|
|
200
|
|
753
|
|
|||||||||
|
Less: Net income attributable to noncontrolling interests
|
9
|
|
11
|
|
12
|
|
12
|
|
44
|
|
|||||||||
|
Net income attributable to Quest Diagnostics
|
$
|
61
|
|
$
|
118
|
|
$
|
342
|
|
$
|
188
|
|
$
|
709
|
|
||||
|
Amounts attributable to Quest Diagnostics' stockholders:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
61
|
|
$
|
118
|
|
$
|
342
|
|
$
|
188
|
|
$
|
709
|
|
||||
|
Income from discontinued operations, net of taxes
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Net income
|
$
|
61
|
|
$
|
118
|
|
$
|
342
|
|
$
|
188
|
|
$
|
709
|
|
||||
|
Earnings per share attributable to Quest Diagnostics' stockholders - basic:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
0.42
|
|
$
|
0.82
|
|
$
|
2.37
|
|
$
|
1.31
|
|
$
|
4.92
|
|
||||
|
Income from discontinued operations
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Net income
|
$
|
0.42
|
|
$
|
0.82
|
|
$
|
2.37
|
|
$
|
1.31
|
|
$
|
4.92
|
|
||||
|
Earnings per share attributable to Quest Diagnostics' stockholders - diluted:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
0.42
|
|
$
|
0.81
|
|
$
|
2.35
|
|
$
|
1.29
|
|
$
|
4.87
|
|
||||
|
Income from discontinued operations
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Net income
|
$
|
0.42
|
|
$
|
0.81
|
|
$
|
2.35
|
|
$
|
1.29
|
|
$
|
4.87
|
|
||||
|
(a)
|
During the third quarter of 2006, the Company completed its wind down of NID and classified the operations of NID as discontinued operations. Results of operations have been prepared to report the results of NID as discontinued operations for all periods presented (see Note 18). In May 2016, the Company completed the sale of Focus Diagnostics (see Note 6). In July 2015, the Company contributed its clinical trials testing business to a newly formed global clinical trials central laboratory services joint venture, Q
2
Solutions (see Note 6). Subsequent to the contribution, the Company's ownership interest in the joint venture is being accounted for under the equity method of accounting.
|
|
(b)
|
Included pre-tax charges of
$21 million
, primarily associated with systems conversions and integration costs incurred in connection with further restructuring and integrating the Company (
$7 million
in cost of services,
$12 million
in selling, general and administrative expenses and
$2 million
in equity in earnings of equity method investees, net of taxes); pre-tax charges of
$1 million
in other operating (income) expense, net, representing non-cash asset impairment charges; pre-tax charges in selling, general and administrative expenses of
$2 million
, primarily representing costs incurred related to certain legal matters; pre-tax charges of
$48 million
on retirement of debt associated with the March 2016 cash tender offer in other (expense) income, net (see Note 13); and pre-tax charges of
$1 million
representing non-cash asset impairment charges associated with an investment.
|
|
(c)
|
Included a pre-tax gain of
$118 million
associated with the sales of the Focus Diagnostics; pre-tax charges of
$19 million
, primarily associated with systems conversions and integration costs incurred in connection with further restructuring and integrating the Company (
$10 million
in cost of services,
$8 million
in selling, general and administrative expenses and
$1 million
in equity in earnings of equity method investees, net of taxes); pre-tax charges in selling, general and administrative expenses of
$1 million
, primarily representing costs incurred related to certain legal matters; and pre-tax charges of
$6 million
representing non-cash asset impairment charges associated with certain investments in other (expense) income, net.
|
|
(d)
|
Included pre-tax charges of
$18 million
, primarily associated with systems conversions and integration costs incurred in connection with further restructuring and integrating the Company (
$8 million
in cost of services and
$10 million
in selling, general and administrative expenses); and pre-tax gain of
$21 million
, principally a result of a gain on escrow recovery associated with an acquisition in other operating (income) expense, net.
|
|
(e)
|
Included pre-tax charges of
$24 million
, primarily associated with systems conversions and integration costs incurred in connection with further restructuring and integrating the Company (
$15 million
in cost of services,
$7 million
in selling, general and administrative expenses,
$1 million
in other operating (income) expense, net and
$1 million
in equity in earnings of equity method investees, net of taxes); and pre-tax charges of
$6 million
in other operating (income) expense, net, representing non-cash asset impairment charges.
|
|
(f)
|
Included pre-tax charges of
$31 million
, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating the Company (
$20 million
in cost of services and
$11 million
in selling, general and administrative expenses); pre-tax charges of
$8 million
in other operating (income) expense, net, representing non-cash asset impairment charges associated with our Celera products business; pre-tax charges in selling, general and administrative expenses of
$2 million
, principally representing costs incurred related to certain legal matters; and pre-tax charges of
$79 million
on retirement of debt associated with the March 2015 cash tender offer in other (expense) income, net (see Note 13).
|
|
(g)
|
Included pre-tax charges of
$23 million
, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating the Company (
$11 million
in cost of services and
$12 million
in selling, general and administrative expenses); a pre-tax gain included in other operating (income) expense, net of
$13 million
associated with a decrease in the fair value of the contingent consideration accrual associated with our Summit Health acquisition (see Note 5 and Note 7); pre-tax charges in selling, general and administrative expenses of
$5 million
, principally representing costs incurred related to certain legal matters; pre-tax charges of
$5 million
in other operating (income) expense, net, representing non-cash asset impairment charges; and pre-tax charges of
$65 million
on retirement of debt associated with the April 2015 redemption in other (expense) income, net (see Note 13).
|
|
(h)
|
Included a pre-tax gain of
$334 million
associated with the contribution of the Company's clinical trials testing business to Q
2
Solutions (see Note 6); and pre-tax charges of
$34 million
, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating the Company (
$20 million
in cost of services,
$9 million
in selling, general and administrative expenses and
$5 million
equity in earnings of equity method investees, net of taxes).
|
|
(i)
|
Included pre-tax charges of
$22 million
, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating the Company (
$12 million
in cost of services and
$10 million
in selling, general and administrative expenses); pre-tax charges of
$11 million
in other operating (income) expense, net, representing non-cash asset impairment charges associated with winding down a subsidiary; and pre-tax charges in selling, general and administrative expenses of
$10 million
, principally representing costs incurred related to certain legal matters. Income from continuing operations includes a deferred income tax benefit of
$58 million
associated with winding down a subsidiary.
|
|
Balance at
Beginning of Year
|
Provision for Doubtful Accounts
|
Net Deductions
and Other
|
Balance at
End of Year
|
||||||||||||
|
Year Ended December 31, 2016
|
|||||||||||||||
|
Doubtful accounts and allowances
|
$
|
254
|
|
$
|
308
|
|
$
|
297
|
|
(a)
|
$
|
265
|
|
||
|
Year Ended December 31, 2015
|
|||||||||||||||
|
Doubtful accounts and allowances
|
$
|
250
|
|
$
|
297
|
|
$
|
293
|
|
(a)
|
$
|
254
|
|
||
|
Year Ended December 31, 2014
|
|||||||||||||||
|
Doubtful accounts and allowances
|
$
|
236
|
|
$
|
296
|
|
$
|
282
|
|
(a)
|
$
|
250
|
|
||
|
(a)
|
Primarily represents the write-off of accounts receivable, net of recoveries.
|
|
Exhibit
Number
|
Description
|
|
3.1
|
Restated Certificate of Incorporation (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: May 20, 2014) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
3.2
|
Amended and Restated By-Laws of the Company (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: February 25, 2016) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.1
|
Form of 6.95% Senior Note due 2037 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: June 19, 2007) and incorporated herein by reference) (Commission file Number 001-12215)
|
|
4.2
|
Form of 4.750% Senior Note due 2020 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: November 17, 2009) and incorporated herein by reference) (Commission file Number 001-12215)
|
|
4.3
|
Form of 5.750% Senior Note due 2040 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: November 17, 2009) and incorporated herein by reference) (Commission file Number 001-12215)
|
|
4.4
|
Form of 4.700% Senior Note due 2021 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: March 21, 2011) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.5
|
Form of 2.700% Senior Note due 2019 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: March 12, 2014) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.6
|
Form of 4.250% Senior Note due 2024 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: March 12, 2014) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.7
|
Form of 2.500% Senior Note due 2020 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: March 10, 2015) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.8
|
Form of 3.500% Senior Note due 2025 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: March 10, 2015) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.9
|
Form of 4.700% Senior Note due 2045 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: March 10, 2015) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.10
|
Form of 3.450% Senior Note due 2026 (filed as an Exhibit to the Company’s current report on Form 8-K (Date of Report: May 23, 2016) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.11
|
Indenture dated as of June 27, 2001, among the Company, the Subsidiary Guarantors, and the Trustee (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: June 27, 2001) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.12
|
First Supplemental Indenture, dated as of June 27, 2001, among the Company, the Subsidiary Guarantors, and The Bank of New York (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: June 27, 2001) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.13
|
Second Supplemental Indenture, dated as of November 26, 2001, among the Company, the Subsidiary Guarantors, and The Bank of New York (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: November 26, 2001) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.14
|
Third Supplemental Indenture, dated as of April 4, 2002, among the Company, the Additional Subsidiary Guarantors, and The Bank of New York (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: April 1, 2002) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.15
|
Fourth Supplemental Indenture dated as of March 19, 2003, among Unilab Corporation (f/k/a Quest Diagnostics Newco Incorporated), the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's quarterly report on Form 10-Q for the quarter ended March 31, 2003 and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.16
|
Fifth Supplemental Indenture dated as of April 16, 2004, among Unilab Acquisition Corporation (d/b/a FNA Clinics of America), the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's quarterly report on Form 10-Q for the quarter ended March 31, 2004 and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.17
|
Sixth Supplemental Indenture dated as of October 31, 2005, among the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: October 31, 2005) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.18
|
Seventh Supplemental Indenture dated as of November 21, 2005, among the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: November 21, 2005) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.19
|
Eighth Supplemental Indenture dated as of July 31, 2006, among the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: July 31, 2006) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.20
|
Ninth Supplemental Indenture dated as of September 30, 2006, among the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: September 30, 2006) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.21
|
Tenth Supplemental Indenture dated as of June 22, 2007, among the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: June 19, 2007) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.22
|
Eleventh Supplemental Indenture dated as of June 22, 2007, among the Company, The Bank of New York, and the Additional Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: June 19, 2007) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.23
|
Twelfth Supplemental Indenture dated as of June 25, 2007, among the Company, The Bank of New York, and the Additional Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: June 19, 2007) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.24
|
Thirteenth Supplemental Indenture dated as of November 17, 2009, among the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: November 17, 2009) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.25
|
Fourteenth Supplemental Indenture dated as of March 24, 2011, among the Company, The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: March 21, 2011) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.26
|
Fifteenth Supplemental Indenture dated as of November 30, 2011, among the Company, The Bank of New York Mellon Trust Company, N.A., as successor trustee to The Bank of New York, and the Subsidiary Guarantors (filed as an Exhibit to the Company's 2011 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.27
|
Sixteenth Supplemental Indenture dated as of March 12, 2014, among the Company, The Bank of New York Mellon Trust Company, N.A., (filed as an Exhibit to the Company's current report on Form 8-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.28
|
Seventeenth Supplemental Indenture dated as of March 10, 2015, among the Company and The Bank of New York Mellon (filed as an Exhibit to the Company’s current report on Form 8-K (Date of Report: March 5, 2015) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
4.29
|
Eighteenth Supplemental Indenture dated as of May 26, 2016, among the Company and The Bank of New York Mellon (filed as an Exhibit to the Company’s current report on Form 8-K (Date of Report: May 23, 2016) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.1‡
|
Amended and Restated Employee Stock Purchase Plan (filed as an Exhibit to the Company's quarterly report on form 10-Q for the quarter ended June 30, 2016 and incorporated herein by reference) (Commission file number 001-12215)
|
|
10.2‡
|
Amended and Restated Quest Diagnostics Incorporated Employee Long-Term Incentive Plan as amended May 15, 2015 (filed as an exhibit to the Company's 2015 quarterly report on Form 10-Q for the quarter ended June 30, 2015 and incorporated herein by reference) (Commission file number 001-12215)
|
|
10.3‡
|
Form of Equity Award Agreement dated as of February 23, 2015 (filed as an exhibit to the Company’s 2015 annual report on Form 10-K and incorporated herein by reference) (Commission file number 001-12215)
|
|
10.4‡
|
Quest Diagnostics Supplemental Deferred Compensation Plan (Post 2004) amended December 22, 2008 (filed as an Exhibit to the Company's 2008 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.5‡
|
Amendment No. 1 dated November 27, 2012 to Quest Diagnostics Supplemental Deferred Compensation Plan (Post 2004) amended December 22, 2008 (filed as an Exhibit to the Company's 2012 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.6‡
|
Quest Diagnostics Supplemental Deferred Compensation Plan (Pre-2005) amended and restated November 27, 2012 (filed as an Exhibit to the Company's 2012 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.7‡
|
Quest Diagnostics Incorporated Senior Management Incentive Plan (filed as Appendix A to the Company's Definitive Proxy Statement dated March 28, 2003 and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.8‡*
|
Amended and Restated Quest Diagnostics Incorporated Executive Officer Severance Plan as amended February 20, 2017
|
|
10.9‡
|
AmeriPath Group Holdings, Inc. 2006 Stock Option and Restricted Stock Purchase Plan (filed as an Exhibit to the Company's registration statement on Form S-8 (Registration No. 333-143889 filed with the Commission on June 19, 2007 and incorporated herein by reference) (Commission File Number 333-143889)
|
|
10.10‡
|
Amendment dated as of August 17, 2007 to the AmeriPath Group Holdings, Inc. 2006 Stock Option and Restricted Stock Purchase Plan (filed as an Exhibit to the Company's 2007 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.11‡
|
The Profit Sharing Plan of Quest Diagnostics Incorporated (Amendment and Restatement, effective as of January 1, 2016) (filed as an exhibit to the Company’s 2015 annual report on Form 10-K and incorporated herein by reference) (Commission file number 001-12215)
|
|
10.12‡
|
Quest Diagnostics Incorporated Amended and Restated Deferred Compensation Plan For Directors as amended effective January 1, 2016 (filed as an exhibit to the Company’s 2015 annual report on Form 10-K and incorporated herein by reference) (Commission file number 001-12215)
|
|
10.13‡
|
Amended and Restated Quest Diagnostics Incorporated Long-Term Incentive Plan for Non-Employee Directors as amended December 3, 2015 (filed as an exhibit to the Company’s 2015 annual report on Form 10-K and incorporated herein by reference) (Commission file number 001-12215)
|
|
10.14‡
|
Form of Non-Employee Director Equity Award Agreement (filed as an Exhibit to the Company's 2011 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.15‡
|
Form of Non-Employee Director Equity Award Agreement dated May 15, 2015 (filed as an exhibit to the Company’s 2015 annual report on Form 10-K and incorporated herein by reference) (Commission file number 001-12215)
|
|
10.16‡
|
Form of Non-Employee Director Elective Option Award Agreement (filed as an Exhibit to the Company's 2011 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.17‡
|
Employment Agreement between Stephen H. Rusckowski and Quest Diagnostics Incorporated, dated April 3, 2012 (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: April 9, 2012) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.18‡
|
Amendment to Employment Agreement between Stephen H. Rusckowski and Quest Diagnostics Incorporated, dated June 11, 2015 (filed as an Exhibit to the Company’s current report on Form 8-K (Date of Report: June 11, 2015) and incorporated herein by reference) (Commission File Number 001-12215)
|
|
10.19‡
|
Aircraft Timesharing Agreement dated as of December 17, 2013 between Quest Diagnostics Incorporated and Stephen H. Rusckowski (filed as an Exhibit to the Company’s 2013 annual report on Form 10-K and incorporated herein by reference) (Commission File Number 001-12215)
|
|
11.1
|
Statement re: Computation of Earnings Per Common Share (the calculation of per share earnings is in Part II, Item 8, Note 3 to the consolidated financial statements (Earnings Per Share) and is omitted in accordance with Item 601(b)(11) of Regulation S-K)
|
|
21.1*
|
Subsidiaries of Quest Diagnostics Incorporated
|
|
23.1*
|
Consent of PricewaterhouseCoopers LLP
|
|
24.1*
|
Power of Attorney (included on signature page)
|
|
31.1*
|
Rule 13a-14(a) Certification of Chief Executive Officer
|
|
31.2*
|
Rule 13a-14(a) Certification of Chief Financial Officer
|
|
32.1**
|
Section 1350 Certification of Chief Executive Officer
|
|
32.2**
|
Section 1350 Certification of Chief Financial Officer
|
|
101.INS*
|
dgx-20161231.xml
|
|
101.SCH*
|
dgx-20161231.xsd
|
|
101.CAL*
|
dgx-20161231_cal.xml
|
|
101.DEF*
|
dgx-20161231_def.xml
|
|
101.LAB*
|
dgx-20161231_lab.xml
|
|
101.PRE*
|
dgx-20161231_pre.xml
|
|
*
|
Filed herewith.
|
|
**
|
Furnished herewith.
|
|
‡
|
Management contract or compensatory plan or arrangement required to be filed as an exhibit to this Form 10-K pursuant to Item 15(b) of Form 10-K.
|