|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|
Common Stock, $.01 par value per share
|
New York Stock Exchange
|
|
Securities registered pursuant to Section 12(g) of the Act:
|
None
|
|
Large accelerated filer
x
|
Accelerated filer
o
|
|
Non-accelerated filer
o
|
Smaller reporting company
o
|
|
Emerging growth company
o
|
|
|
Documents Incorporated by Reference
|
Part of Form 10-K into
which incorporated
|
|
Document
|
|
|
Portions of the registrant's Proxy Statement to be filed by April 30, 2019
|
Part III
|
|
Item
|
Page
|
|
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
Item 15.
|
||
|
Item 16.
|
||
|
Guide to Tables
|
|
|
Table 1 - Portfolio Growth
|
3
|
|
Table 2 - Approaches to Accelerate Growth
|
3
|
|
Table 3 - Key Professional Laboratory Services Offerings
|
4
|
|
Table 4 - Clinical Franchises
|
4
|
|
Table 5 - Recent Consumer-Centric Initiatives
|
5
|
|
Table 6 - Major Themes to Drive Operational Excellence
|
6
|
|
Table 7 - Assets and Capabilities
|
8
|
|
Table 8 - 2018 Net Revenues
|
10
|
|
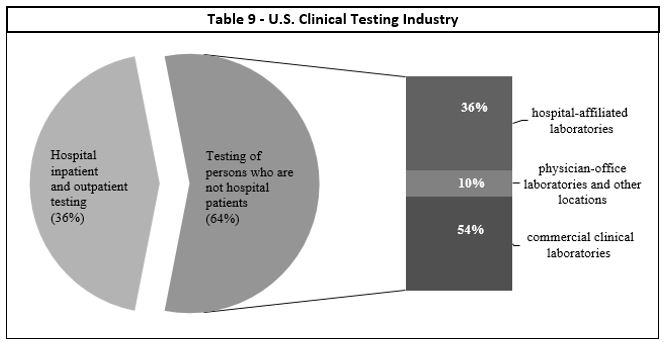
Table 9 - U.S. Clinical Testing Industry
|
13
|
|
Table 10 - Key Trends
|
13
|
|
Table 11 - Contributing to Reducing Healthcare Costs and Improving Care
|
17
|
|
Table 12 - Customers
|
18
|
|
Table 13 - Factors Considered When Selecting a Diagnostics Information Services Provider
|
21
|
|
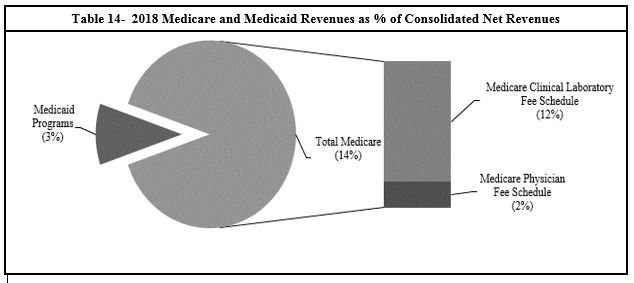
Table 14 - 2018 Medicare and Medicaid Revenues as % of Consolidated Net Revenues
|
24
|
|
Table 15 - Key Regulatory Schemes
|
24
|
|
Table 16 - Information Available at Our Corporate Governance Webpage
|
27
|
|
Table 17 - Executive Officers
|
28
|
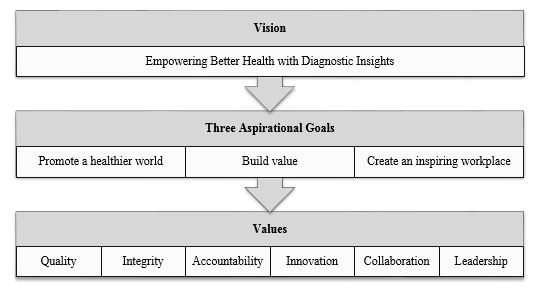
|
Table 1 - Portfolio Growth
|
|||
|
Theme
|
Key Characteristics
|
At A Glance
|
Quest Value Proposition
|
|
General Diagnostics
|
Testing services generating strong cash flows and steady growth
|
Routine and non-routine testing services
Largest revenue stream
Essential portion of health care delivery
|
Scale
Operational excellence
Access and convenience
|
|
Advanced Diagnostics
|
Testing services providing faster growth through innovation testing model
|
Genetic and advanced molecular testing services
An important part of precision medicine
A growing set of unique, innovation-based competitors
|
Rich clinical, scientific and medical innovation expertise
Quality and reliability of new assays
Ability to manage potential new regulatory requirements
|
|
Diagnostic Services
|
Laboratory and data-related healthcare opportunities providing faster growth
|
Enables partners to deliver health care more efficiently (
e.g.
, risk assessment; Professional Laboratory Services; wellness)
Services to support population health (
e.g.
, data analytics; extended care services)
|
Extensive diagnostic capability
Large and growing database and analytics expertise
Partnerships with industry leaders across healthcare landscape
|
|
Table 2 - Approaches to Accelerate Growth
|
|
1. Delivering a compound annual revenue growth rate of more than 2% through accretive, strategic acquisitions
|
|
Plus organic growth through:
|
|
2. Partnering with health plans, IDNs and other risk bearing entities
|
|
3. Offering the broadest access to diagnostic innovation
|
|
4. Being recognized as the consumer-friendly provider of diagnostic information services
|
|
5. Supporting population health with data analytics and extended care services
|
|
Table 3 - Key Professional Laboratory Services Offerings
|
|
|
Lab management outsourcing
|
Advanced data solutions
|
|
Joint venture
|
Reference testing, including advanced diagnostics
|
|
Outreach acquisition
|
Supply chain management and purchasing
|
|
Test menu optimization and spend consolidation
|
Blood utilization management
|
|
Table 4 - Clinical Franchises
|
|
|
Cardiovascular, Metabolic and Endocrinology
|
Oncology
|
|
General Health and Wellness
|
Prescription Drug Monitoring and Toxicology
|
|
Infectious Diseases and Immunology
|
Sports Science and Human Performance
|
|
Neurology
|
Women’s and Reproductive Health
|
|
Table 5 - Recent Consumer-Centric Initiatives
|
|
|
Enhance patient experience
|
• Electronic check-in at patient service centers.
• Improved on-line pre-registration and appointment scheduling.
• Real-time payment determination for additional payers.
|
|
Expand convenient access
|
• Partnerships with Walmart Stores and Safeway to expand convenient access to testing services at select Walmart and Safeway locations across the United States; the number of locations significantly increased in 2018 to over 200.
|
|
Consumer-initiated testing
|
• QuestDirect
TM
, our consumer-initiated testing service, is now available in 48 states.
• Consumers can choose from 35 test packages including general health, men's and women's health, digestive health, heart health, infectious disease and sexually transmitted disease testing.
|
|
Expand consumer connectivity and access to information
|
• >6.5 million registered users in our MyQuest
®
health portal and mobile connectivity solution. Implemented MyQuest Advanced Access
®
, which enables patients to access their historical laboratory test results and trends.
• Patients can manage healthcare for a circle of individuals and receive personal appointment reminders via text messaging.
• MyQuest
®
now supports Health Records using the Apple Health app.
|
|
Expand access to basic health care services
|
• Launched partnership with Walmart Stores to expand access to basic health care services.
|
|
Expand sports diagnostics offering
|
• Continued enhancement and expansion of our Blueprint for Athletes
®
offerings.
|
|
Self-collection technology
|
• Launched proprietary, consumer-friendly self-collection technology to engage consumers at home.
|
|
Expand consumer awareness
|
• Multi-year global collaboration with AncestryDNA to provide genotyping test services.
|
|
Table 6 - Major Themes to Drive Operational Excellence
|
|
|
Reduce denials and patient concessions
|
Standardize and automate
|
|
Digitize the customer experience
|
Optimize
|
|
•
|
Our organization is designed to align around future growth opportunities, coordinate units in our business for seamless execution and leverage our company-wide infrastructure to gain more capability, value and efficiency. The value creation side of our business includes product and commercial marketing and is organized by clinical franchise and focuses on customer solutions for the marketplace, including new test development and diagnostic insights. The value delivery side includes sales, laboratory operations, field operations, logistics and client services.
|
|
•
|
We use the Quest Management System to manage our Company. This system provides a foundation for day-to-day management, and includes best-in-class business performance tools to help us develop new capabilities to improve our Company. The system enables us to run the Company with a common language, approach and philosophy, and supports our efforts as we build a high-performance culture, with employees focused on behaviors to make us more agile, transparent, customer-focused, collaborative and performance oriented.
|
|
•
|
Our Everyday Excellence program, which includes guiding principles for our entire organization to support a superior customer experience and to inspire our employees to be their best every day, with every person and with every customer interaction. In 2018, we integrated these principles into our performance assessments and frontline employee behavioral standards.
|
|
•
|
Our Leading Quest Academy, which is designed to strengthen our more senior employee leaders through a highly experiential leadership development program focused on creating a high-performance culture and sharpening the capabilities needed to lead our organization, and leadership training programs for other employees.
|
|
•
|
Our Code of Ethics reinforces our commitment to integrity as one of our core values and aligns with our vision, goals and brand.
|
|
Table 7 - Assets and Capabilities
|
|
|
●
Provide healthcare connectivity solutions to >335,000 clinician and hospital accounts and interface with approximately 700 electronic health records systems
|
●
Own or control approximately 1000 issued and 475 pending patents worldwide in 2018
|
|
●
Strong logistics capabilities
•
make approximately 77,000 stops daily
•
approximately 3,750 courier vehicles
•
25 aircraft serving the U.S.
|
●
One of the largest medical and scientific staffs in the industry to provide interpretive consultation
•
>600 M.D.s and Ph.D.s, many of whom are recognized leaders in their field
•
Genetic counselors
|
|
●
Approximately 22,000 phlebotomists, paramedics, nurses and other health and wellness professionals
|
●
>6,600 patient access points, the most extensive network in the U.S., including phlebotomists in physician offices and >2,250 of our own patient service centers
|
|
●
Access to approximately 90% of U.S. insured lives
|
●
Processed approximately 168 million test requisitions in 2018
|
|
●
Industry-leading test menu
|
●
The largest private database of de-identified test results: >44 billion patient data points delivered over past decade
|

|
Table 10 - Key Trends
|
|
|
Prevention and wellness
|
We believe that the value of detection, prevention, wellness and personalized care is well recognized. Consumers, employers, ACOs, IDNs, health plans and government agencies increasingly focus on helping the healthy stay healthy, detecting symptoms among those at risk and providing preventive insight and care that helps avoid disease.
|
|
Medical innovation
|
Medical advances allow for more accurate and earlier diagnosis and treatment of diseases.
Continuing advances in genomics and proteomics are expected to yield new, more sophisticated and specialized diagnostic tests. These advances also are spurring interest in and demand for precision medicine, which relies on diagnostic and prognostic testing and in which data information services and strategies are used to deliver the most effective healthcare to the right populations and individuals.
Pharmacogenomic testing increasingly is used as a parameter to help speed drug approval processes and to better focus therapy based on patient and tumor-specific genetic markers.
Demand also is growing toward comprehensive care management solutions that serve patients, payers and healthcare providers by improving clinical decision support and access to patient data, and by increasing patient participation in care management and population health management.
There is increasing focus on access to patient data and data-driven insights.
|
|
Customers and payers; industry consolidation
|
Our customers and payers, including clinicians, health plans, IDNs, ACOs, employers and others, have been consolidating, converging and diversifying. For example, an increased number of hospital systems are considering establishing or have established health insurance plans, and health insurance plans are considering providing or are providing healthcare services. CVS Health, a leading provider of retail medical clinics and pharmacy benefits management services, has acquired Aetna, a leading health insurance provider. Cigna Corporation, a leading health insurance provider, has acquired Express Scripts, a leading pharmacy benefits manager. United Health Group, the parent of UnitedHealthcare, provides a wide array of health care services through its Optum subsidiaries. Health plans are entering agreements with other providers of healthcare services, including laboratory testing services providers, to partner on value-based approaches to delivering health care to populations.
Consolidation is increasing pricing transparency and bargaining power, and may encourage internalization of clinical testing.
Physicians frequently now are employed by hospital systems, IDNs, ACOs or large group practices integrated with healthcare systems, instead of organizing physician-owned practices, which is changing the dynamics for whether clinical testing is performed in or outside of a hospital. Physicians and other clinicians also increasingly are being employed by health plans or their affiliates.
Value-based reimbursement is contributing to changes in the healthcare system. ACOs and patient-centered medical homes have grown as a means to deliver patient care. Healthcare services increasingly are being provided by non-traditional providers (e.g., physician assistants), in non-traditional venues (e.g., retail medical clinics, urgent care centers) and using new technologies (e.g., telemedicine; digital pathology).
In addition, federal healthcare reform legislation adopted in 2010, the ACA, is resulting in changes in the way that some healthcare services are purchased and delivered in the United States. Hospitals and IDNs are under significant pressure, and are evolving.
Patients are also our customers. Increasingly, patients are engaged in their own healthcare, being empowered to manage and understand their healthcare and are bearing responsibility for payment for the services provided to them. See also the discussion under the heading Patients in table 12.
|
|
Pricing transparency
|
There has been a trend toward greater pricing transparency in the healthcare marketplace.
This transparency, combined with increased patient financial responsibility for medical care, is enhancing purchasing sophistication and changes in behavior in the healthcare marketplace. We believe that increased price transparency should benefit low cost, high value providers like our Company.
|
|
Competition
|
The diagnostic information services industry remains fragmented, is highly competitive and is subject to new competition.
Competition is emerging from new technologies (e.g., digital pathology) and growing from non-traditional competitors. Increased hospital acquisitions of physician practices may enhance clinician ties to hospital-affiliated laboratories and may strengthen their competitive position.
New industry entrants with extensive resources may make acquisitions or expand into our traditional areas of operations.
|
|
Healthcare utilization
|
In the past few years, healthcare utilization in the United States has fluctuated based on a number of factors. These factors include, without limitation, the economy, healthcare benefits design, patients delaying medical care and increased patient financial responsibility for medical care.
The ACA contained provisions eliminating patient cost-sharing for preventive services, and additional provisions that we believe have increased the number of patients that have health insurance, including Medicaid, and thus better access to diagnostic testing.
|
|
Reimbursement pressure; affordability
|
There is a strong focus in the United States on controlling the overall cost of healthcare.
Healthcare market participants, including governments, are focused on controlling costs. Examples of cost control approaches include reducing reimbursement for healthcare services, changing reimbursement for healthcare services (e.g., shift from fee for service to capitation), changing medical coverage policies (e.g., healthcare benefits design), denying coverage for services, requiring preauthorization of laboratory testing, requiring co-pays, introducing laboratory spend management utilities and payment and patient care innovations such as ACOs and patient-centered medical homes.
In light of continued pressure to reduce systemic healthcare costs, hospitals may change their approach to providing clinical testing services.
The Health Transformation Alliance, a group of over 40 major U.S. companies, was formed to improve and reform the healthcare system in the United States. The rising cost of healthcare in the United States was a key driver for the formation of this alliance.
In 2018, Amazon.com Inc., Berkshire Hathaway Inc. and JPMorgan Chase &Co., citing rising health care costs, announced plans to reduce their workers' health care costs by forming a non-profit venture that would provide simplified, high-quality healthcare for their workers.
Pursuant to PAMA, CMS has promulgated revised reimbursement rates schedules for clinical laboratory testing services provided under Medicare for 2018, 2019 and 2020. Under the revised Medicare Clinical Laboratory Fee Schedule, reimbursement rates for clinical laboratory testing was reduced in 2018 and is scheduled to be reduced again by approximately 10% in each of 2019 and 2020. PAMA calls for further revision of the Medicare Clinical Laboratory Fee Schedule for years after 2020, based on future surveys of market rates; further reduction in reimbursement rates may result from such revisions.
The American Clinical Laboratory Association, of which the Company is a member, initiated a lawsuit charging that in implementing PAMA, CMS failed to follow a Congressional directive to implement a market-based laboratory payment system. The lawsuit was dismissed; appeal of the dismissal is pending. The Company supports this lawsuit and also is pursuing a legislative solution from the revised Medicare Clinical Laboratory Fee Schedule implemented by CMS under PAMA, which the Company contends resulted from a flawed process and failed to protect access to laboratory services for Medicare beneficiaries.
In 2018, CMS finalized a national coverage determination for next-generation sequencing cancer panels. Under the determination, tests that gain FDA approval or clearance as an in vitro companion diagnostic will automatically receive full coverage, provided other coverage criteria are met. Coverage determinations for other diagnostic laboratory tests using next-generation sequencing will be made by Medicare Administrative Contractors. Clinical laboratory services providers are discussing this determination and others with CMS and Medicare Administrative Contractors to attempt to ensure that such providers can continue to provide these essential diagnostic services, but those discussions may not be successful.
While pressure to control healthcare costs poses a risk to our Company, it also creates opportunities, such as an opportunity for increased proper utilization of testing as an efficient means to manage the total cost of healthcare. We believe that it also creates greater opportunities for consolidation and gaining share for high value, low-cost providers, like our Company, as compared to other providers.
|
|
Legislative, regulatory and policy environment
|
Government oversight of and attention to the healthcare industry in the United States is significant and increasing; healthcare payment reform is a top issue.
In late 2018, legislation was introduced in Congress that would enable the FDA to regulate LDTs, in vitro diagnostics, software and other items used in the diagnosis of disease. If this legislation were to become law, the FDA could regulate diagnostic tests and components and platforms used as part of these tests. If such legislation were to become law, it could have a significant impact on the clinical laboratory testing industry, including regulating LDTs in new ways and creating avenues of opportunity and competition regarding clinical laboratory testing. New competitors may enter the industry, and competition may come in new forms.
The ACA has created significant uncertainty as healthcare markets react to changes. For example, more than half of the states have opted in to Medicaid expansion and employers may discontinue offering group health insurance to their employees, shifting more people to exchange products.
Certain aspects of the ACA have been repealed, delayed or modified. The scope and timing of any further legislation to repeal, amend, replace, or reform the rest of the ACA is uncertain, but if such legislation were to become law, it could have a significant impact on the U.S. healthcare system. In addition, uncertainty regarding the status of the ACA prior to any such repeal, amendment, replacement or reform could create uncertainty generally in the healthcare market.
A federal court has recently determined that the ACA is unconstitutional; that ruling has been appealed. Uncertainty about court rulings regarding the ACA could add to uncertainty in the healthcare market.
|
|
Informatics; technology; privacy concerns
|
The increased availability of healthcare data, including data made available as a result of next generation DNA sequencing, and the increased ability to effectively analyze that data at population and patient levels, is impacting healthcare practices. It is anticipated that the increased use of data in healthcare, coupled with mobile healthcare IT solutions for doctors and patients, will help to improve patient outcomes and reduce overall healthcare costs.
Informatics, including integrated diagnostic and decision support solutions, predictive analytics, use of population data and healthcare information technology, is spurring advances in precision medicine, including medical decision making and value, for populations and individuals. The increased focus on data and its use is increasing focus on maintaining the privacy of patient data.
There is a need for technology solutions to harness these opportunities. In addition, new technology, social media and mobile technology are changing the way that healthcare markets interact with each other, and the expectations that they have about how services are provided, what services are provided, and other capabilities of healthcare market participants. These developments are creating new opportunities and new challenges and disrupting the healthcare environment. For example, digital pathology is an emerging technology that may change the practice of pathology. Information technology that includes self-learning or "artificial intelligence" features is growing and may impact the healthcare industry.
Healthcare market participants, including pharmaceutical companies, health plans, clinicians, ACOs and IDNs, are striving to leverage interoperability, informatics and analytics to positively influence the health of patient populations while maintaining patient privacy.
|
|
Chronic diseases and conditions; gaps in care
|
We believe that the cost and challenges of identifying, treating and controlling chronic diseases and conditions such as diabetes and heart disease are now well recognized.
As a result of multiple factors, including increased focus on population health management and pressure to reduce the systemic costs associated with such diseases and conditions, there is increased focus on better identifying and attempting to reduce or eliminate the gaps in care historically associated with these diseases and conditions. Healthcare market participants are developing new approaches for this purpose.
|
|
Healthcare services delivery
|
Healthcare delivery is moving out of hospitals, doctor offices and other traditional locations in which it had been provided. Care is increasingly being provided in new settings, such as out-patient and home settings. For example, see the discussion of Emerging Retail Healthcare Providers in table 12. This dynamic offers new opportunities and challenges for healthcare providers and reflects not only efforts to take advantage of new technologies, but also the focus, discussed in this table above under the heading Reimbursement pressure; affordability, on controlling the overall cost of healthcare.
|
|
Table 11 - Contributing to Reducing Healthcare Costs and Improving Care
|
|
• Identifying patients at risk for disease before they require urgent care, hospital treatment or expensive therapies
|
|
• Helping clinicians to target the right medicines for the right patients (those who will benefit from the medicines)
|
|
• Identifying treatment-related side effects
|
|
• Early assessment of the efficacy of a therapy, enabling changes or discontinuation of ineffective therapies
|
|
• Enabling population health management by utilizing diagnostic information, identifying gaps in care and delivering targeted solutions to individuals who need care
|
|
• Identification and proactive management of individuals at risk for developing chronic diseases, to decrease progression and associated costs and morbidity
|
|
• Providing telemedicine services along with laboratory testing to help individuals interpret and obtain appropriate advice and referrals into needed care
|
|
Table 12 - Customers
|
|
|
Health plans including managed care organizations and other health insurance providers
|
These customers typically reimburse us as a contracted (or out-of-network) provider on behalf of their members. In certain locations, health plans may delegate to IPAs or other alternative delivery systems (
e.g
., physician hospital organizations, ACOs, patient-centered medical homes) the ability to negotiate for diagnostic information services on behalf of certain members.
Health plans and IPAs often require that diagnostic information services providers accept discounted fee structures or assume all or a portion of the financial risk associated with providing such services through capitated payment arrangements. Under capitated payment arrangements, we provide services at a predetermined monthly reimbursement rate for each covered member, generally regardless of the number or cost of services provided by us. Reimbursement under programs that do not provide for capitated payments is typically negotiated on a fee-for-service basis.
Health plans increasingly are adopting policies, practices and procedures based on requirements imposed by government payers such as Medicare and Medicaid. These policies, practices and procedures are subject to change, and may be changed without notice to us.
Reimbursement from our five largest health plans totaled approximately 20%, and no one health plan accounted for 10%, of our consolidated net revenues in 2018. Health plans typically negotiate directly or indirectly with a number of diagnostic information services providers, and represent approximately one-half of our total clinical testing volumes and approximately 35% of our net revenues from diagnostic information services. There has been a trend of consolidation among health plans. Some health plans also have narrowed their provider networks.
We are also sometimes a member of a “complementary network.” A complementary network generally is a set of contractual arrangements that a third party will maintain with various providers that provide discounted fees for the benefit of its customers. A member of a health plan may choose to access a non-contracted provider that is a member of a complementary network; if so, the provider will be reimbursed at a rate negotiated by the complementary network.
We offer to health plans services and programs that leverage our Company's expertise and resources, including our superior access, extensive test menu, medical staff, data, information technology solutions, and wellness and population health management capabilities.
Effective January 1, 2019, Quest Diagnostics had its best access to health plan members in over a decade, as a result of becoming a participating provider to UnitedHealthCare, Blue Cross Blue Shield of Georgia and Horizon Blue Cross and Blue Shield in New Jersey. With access to an additional approximately 43 million insured lives, the Company now has access to approximately 90% of the insured lives in the U.S., including very strong access in key high-population states. We believe that this improved access increases our attractiveness to other customer groups, including clinicians, patients and employers.
|
|
Clinicians
|
Clinicians, including primary care physicians, specialists and physician assistants, requiring diagnostic information services for patients are the primary referral source for our services when patients choose their diagnostic information services provider.
In recent years, there has been a marked increase in the number of physician practices owned by IDNs and hospital systems. There also has been a notable increase in some branches of medicine of the establishment of very large "rolled-up" specialty physician practice groups. Hospitals that own physician practices may require the practices to refer outreach testing to the hospital's affiliated laboratory. Large specialty physician groups may encourage their members to refer testing to other members of the group. In each case, referrals to independent diagnostic services providers may be reduced.
Clinicians determine which laboratory to recommend or use based on a variety of factors, including those set forth in table 13.
|
|
Hospitals
|
We believe that we are the industry's leader in servicing hospitals. We provide services to hospitals throughout the United States, including advanced testing services, in some cases helping manage their laboratories and serving as the medical directors of the hospital's histology or clinical laboratory, including through our Professional Laboratory Services offerings.
Hospitals generally maintain an on-site laboratory to perform the significant majority of clinical testing for their patients (inpatients and outpatients) and refer certain testing to outside service providers, which typically charge the hospitals on a negotiated fee-for-service basis. Fee schedules for hospital reference testing services often are negotiated on behalf of hospitals by group purchasing organizations.
Hospitals also provide outreach testing, and historically were able to negotiate higher reimbursement rates with health plans than commercial clinical laboratories for comparable services. They may seek to leverage their relationships with community clinicians by encouraging the clinicians to send their outreach testing to the hospital's laboratory. Increased hospital acquisitions of physician practices may enhance clinician ties to hospital-affiliated laboratories and may strengthen their competitive position.
We also have joint venture arrangements with leading hospitals or IDNs in several metropolitan areas. These joint venture arrangements, which provide diagnostic information services for affiliated hospitals as well as for unaffiliated clinicians and other local healthcare providers, serve as our principal facilities in their service areas. Typically, we have either a majority ownership interest in, or day-to-day management responsibilities for, our joint venture relationships.
In light of continued pressure to reduce systemic healthcare costs, hospitals may change their approach to providing clinical testing services, including by insourcing tests, seeking ways to improve profitability or to better utilize their laboratory capacity. We believe that our combination of services positions us to be an attractive partner for hospitals, offering a full range of strategic relationships.
|
|
ACOs and IDNs
|
An ACO is a network of providers and facilities that share financial risk in providing or arranging for the provision of healthcare. An IDN is a network of providers and facilities working together in providing or arranging for the provision of healthcare. ACOs and IDNs have increased in number; their impact on the provision of healthcare services to date has varied.
ACOs and IDNs may exercise operational and financial control over providers across the continuum of care, and may function as a payer. Thus, they may be able to manage the health of a population group within a defined geography, and also may be able to influence the cost and quality of healthcare delivery, for example through owned entities and through ancillary services. ACOs may be encouraged to consider exclusive arrangements with healthcare providers that become part of the ACO, or to limit service providers to the ACO, since members of the ACO share financial risk.
We are actively engaging with ACOs and IDNs to demonstrate the value of our services.
|
|
Employers
|
Employers use tests for drugs of abuse to determine an individual's employability and his or her “fitness for duty.” Companies with high employee turnover, safety conscious environments or regulatory testing requirements provide the highest volumes of testing. Factors such as the general economy and job market can impact the utilization of drugs-of-abuse testing.
Employers also are investing in health and wellness services. We meet their needs by providing nationwide access to our customizable wellness services (discussed above at page 11), directly and through health plan and health improvement providers. These services help employers, employees and others manage healthcare costs and capitalize on trends in personalized health.
We seek to grow our employer business through offering new and innovative programs to help them with their goals of (1) maintaining a safe and productive workplace, (2) improving healthcare for employees and (3) lowering healthcare costs for employees and employers.
|
|
Patients
|
Patients are taking increased interest in and responsibility for their healthcare. Some patients are interested in ordering their own diagnostics tests, rather than relying upon a healthcare professional to order the tests. In addition, patients often are bearing increased financial responsibility for their healthcare (
e.g
., high deductible health plans; rising deductibles). Patients are paying greater attention to their healthcare, are increasing their demands of healthcare providers, have increased expectations regarding their healthcare experiences and are becoming more sophisticated regarding healthcare. For example, in our experience, patients are more focused on transparency, ease of doing business and understanding diagnostics information services than they have been in the past. In addition, patients are seeking prompt, direct access to their test results.
The changing expectations of patients about their healthcare and their healthcare transactions are influencing the way that we think about our business and the services that we provide. We are well positioned to provide information and insights to patients to help them take actions to improve their healthcare, and increasingly we are providing patients with tools to do this. See the discussion of our consumer strategy at page 4.
|
|
Emerging Retail Healthcare Providers
|
In recent years, as the healthcare sector changes, retail providers of healthcare services have emerged and are growing. These providers include "big-box" retailers, pharmacy chains, supermarkets, urgent care centers and Internet-based service providers.
We are taking advantage of opportunities to work with these providers, not only to offer new access points for our services (
e.g
., our collaboration with Safeway), but also to grow our business by expanding our service offerings (
e.g
., our joint venture with Walmart). See the discussion of our consumer strategy at page 4.
|
|
Government Agencies
|
We provide services on a fee-for-service basis to federal, state and local governmental agencies.
Historically, most Medicare and Medicaid beneficiaries were covered under the traditional Medicare and Medicaid programs administered by the federal government. Over the last several years, the federal government has expanded its contracts with private health insurance plans for Medicare beneficiaries and has encouraged such beneficiaries to switch from the traditional programs to the private programs, called “Medicare Advantage” programs. There has been growth of health insurance providers offering Medicare Advantage programs and of beneficiary enrollment in these programs. States also have mandated that Medicaid beneficiaries enroll in private managed care arrangements.
|
|
Pharmaceutical companies
|
We offer an array of assets and services to support the development of companion diagnostics.
We also offer Quest Clinical Trials Connect
TM
, to help speed drugs to market through better patient recruitment and involvement and improved physician outreach.
|
|
Other Laboratories
|
We provide services on a fee-for-service basis to other commercial clinical laboratories.
|
|
Table 13 - Factors Considered When Selecting a Diagnostic Information Services Provider
|
|
|
• Service capability and quality
|
• Reputation in the medical community
|
|
• Accuracy, timeliness and consistency in reporting test results
|
• Healthcare information technology solutions, including connectivity options
|
|
• Access to medical/scientific thought leaders for consultation
|
• Patient access, including the number, convenience and geographic coverage of patient service centers
|
|
• Patient insurance coverage and experience
|
• Ability to develop new and useful tests and services
|
|
• Number and type of tests performed
|
• Qualifications of its staff
|
|
• Pricing and overall value
|
• Provider office workflow
|
|
• Real time payment determination
|
• Capabilities to support population health initiatives
|
|
•
|
Operational risk - risks arising from systems, processes, people and external events that affect the Company’s operational objectives or fundamental reason for its existence, including: product life-cycle and execution; service quality and performance; information management and data protection and security, including cybersecurity; supply chain and business disruption; and other risks, including human capital and reputation.
|
|
•
|
Financial risk - risks arising from the Company’s ability to meet its financial obligations pursuant to its strategic and operational objectives, including exposure to broad market and more specific industry risk that could impact liquidity, interest rate, credit, pricing and reimbursement, and also to internal and external financial reporting.
|
|
•
|
Legal and compliance risk - risks arising from government and regulatory environment and action, legal proceedings and compliance with integrity policies and procedures.
|
|
•
|
Strategic risk - risks that will impede the Company’s plan to achieve its mission and vision and apply its core values, including changes in the broad market and Company's industry, business development and restructuring activities, competitive threats and practices, technology and product innovation, and public policy.
|
|
•
|
“Client” fees charged to physicians, hospitals and institutions for which services are performed on a wholesale basis and which are billed on a monthly basis.
|
|
•
|
“Patient” fees charged to individual patients and certain third-party payers on a claim-by-claim basis.
|
|
Table 15 - Key Regulatory Schemes
|
|
|
CLIA and State Clinical Laboratory Licensing
|
CLIA regulates the operations of virtually all clinical laboratories, requiring that they be certified by the federal government and that they comply with various technical, operational, personnel and quality requirements intended to ensure that the services provided are accurate, reliable and timely.
State laws may require additional personnel qualifications or licenses, quality control, record maintenance, proficiency testing or detailed review of our scientific method validations and technical procedures for certain tests.
Violations of these laws and regulations may result in monetary fines, criminal and civil penalties and/or suspension or exclusion from participation in Medicare, Medicaid and other federal or state healthcare programs.
|
|
Medicare and Medicaid; Fraud and Abuse
|
Diagnostic testing services provided under Medicare and Medicaid programs are subject to complex, evolving, stringent and frequently ambiguous federal and state laws and regulations, including those relating to billing, coverage and reimbursement.
Anti-kickback laws and regulations prohibit making payments or furnishing other benefits to influence the referral of tests billed to Medicare, Medicaid or certain other federal or state healthcare programs.
In addition, federal and state anti-self-referral laws generally prohibit Medicare and Medicaid payments for clinical tests referred by physicians who have an ownership or investment interest in, or a compensation arrangement with, the testing laboratory, unless specific exceptions are met.
Federal substance abuse legislation enacted in 2018 contains anti-kickback provisions that are, by their terms, applicable to laboratory testing paid for by all payers. We are attempting to clarify the application of that legislation.
Some states have similar laws that are not limited in applicability to only Medicare and Medicaid referrals and could also affect tests that are paid for by health plans and other non-governmental payers.
Violations of these laws and regulations may result in monetary fines, criminal and civil penalties and/or suspension or exclusion from participation in Medicare, Medicaid and other federal or state healthcare programs.
|
|
FDA
|
The FDA has regulatory responsibility over, among other areas, instruments, software, test kits, reagents and other devices used by clinical laboratories to perform diagnostic testing in the United States. The FDA also regulates drugs-of-abuse testing for employers and insurers, testing for blood bank purposes and testing of donors of human cells for purposes such as in vitro fertilization.
A number of advanced tests we develop internally are offered as LDTs. The FDA has claimed regulatory authority over all LDTs, but has stated that it exercised enforcement discretion with regard to most LDTs performed by high complexity CLIA-certified laboratories.
Pursuant to the 21st Century Cures Act, the FDA issued guidance regarding its position on the regulation of clinical decision software, which may be used in, or in connection with, LDTs. The guidance attempts to address uncertainty regarding whether FDA approval of certain software is required. In January 2019 the FDA issued a draft guidance on a pre-certification pilot program to help software developers have a speedier and less restrictive path to clearance or approval of their software.
In late 2018, legislation was introduced in Congress that would enable the FDA to regulate LDTs, in vitro diagnostics, software and other items used in the diagnosis of disease. If this legislation were to become law, the FDA could regulate diagnostic tests and components and platforms used as part of these tests. If such legislation were to become law, it could have a significant impact on the clinical laboratory testing industry, including regulating LDTs in new ways and creating avenues of opportunity and competition regarding clinical laboratory testing. New competitors may enter the industry, and competition may come in new forms.
|
|
Environmental, Health and Safety
|
We are subject to laws and regulations related to the protection of the environment, the health and safety of employees and the handling, transportation and disposal of medical specimens, infectious and hazardous waste and radioactive materials.
For example, the U.S. Occupational Safety and Health Administration has established extensive requirements relating specifically to workplace safety for healthcare employers in the U.S. This includes requirements to develop and implement multi-faceted programs to protect workers from exposure to blood-borne pathogens, including preventing or minimizing any exposure through needle stick injuries.
For purposes of transportation, some biological materials and laboratory supplies are classified as hazardous materials and are subject to regulation by one or more of the following agencies: the U.S. Department of Transportation, the U.S. Public Health Service, the U.S. Postal Service and the International Air Transport Association.
|
|
Physicians
|
Our pathologists are required to hold a valid license to practice medicine in the jurisdiction in which they practice.
Many of our pathologists enter into an employment agreement. These agreements have varying terms, but generally can be terminated at any time, upon advance notice. Most of the agreements contain covenants generally limiting the activities of the pathologist within a defined geographic area for a limited period of time after termination of employment; the enforceability of these covenants may be limited under state law.
Several jurisdictions, including some in which our businesses are located, prohibit business corporations from engaging in the practice of medicine. In certain jurisdictions, business corporations are prohibited from employing licensed healthcare professionals to provide services on behalf of the corporation; these laws vary. In some jurisdictions, anatomic pathology services are delivered through physician-owned entities that employ the practicing pathologists. The manner in which licensed physicians can be organized to perform medical services may be governed by the laws of the jurisdictions in which medical services are provided and by the medical boards or other entities authorized by these jurisdictions to oversee the practice of medicine.
|
|
Privacy and Security of Health and Personal Information
|
We are subject to laws and regulations regarding protecting the security and privacy of certain healthcare and personal information, including: (a) the federal Health Insurance Portability and Accountability Act and the regulations thereunder, which establish (i) a complex regulatory framework including requirements for safeguarding protected health information and (ii) comprehensive federal standards regarding the uses and disclosures of protected health information; (b) state laws; and (c) the European Union's General Data Protection Regulation.
A healthcare provider may be subject to penalties for non-compliance and may be required to notify individuals or state, federal or county governments if the provider discovers certain breaches of personal information or protected health information.
|
|
Drug Testing; Controlled Substances
|
All U.S. laboratories that perform drug testing for certain public sector employees and employees of certain federally regulated businesses are required to be certified as meeting the detailed performance and quality standards of the Substance Abuse and Mental Health Services Administration.
To obtain access to controlled substances used to perform drugs-of-abuse testing in the United States, laboratories must be licensed by the Drug Enforcement Administration.
|
|
•
|
increase our administrative, billing or other operating costs;
|
|
•
|
decrease the amount of reimbursement related to diagnostic information services performed;
|
|
•
|
damage our reputation; or
|
|
•
|
adversely affect important business relationships with third parties.
|
|
Table 16 - Information Available at Our Corporate Governance Webpage
|
|
|
•
Directors
|
•
Corporate Governance Guidelines
|
|
•
Composition of the committees of our Board of Directors
|
•
Code of Ethics
|
|
•
Senior management
|
•
Certificate of Incorporation
|
|
•
Charters for the standing committees of our Board of Directors
|
•
Bylaws
|
|
•
Information about our corporate political contributions
|
•
Values
|
|
•
Statements of beneficial ownership of our equity securities filed by our directors, officers and others under Section 16 of the Exchange Act
|
|
|
Table 17 - Executive Officers
|
|
|
Name, Age, Title
|
Background
|
|
Stephen H. Rusckowski (61)
Chairman of the Board, President and Chief Executive Officer
|
Mr. Rusckowski joined the Company in May 2012 as President and Chief Executive Officer and became Chairman of the Board on January 1, 2017. From October 2006 until he joined the Company, he was Chief Executive Officer of Philips Healthcare, the largest unit of Royal Philips Electronics, and a member of the Board of Management of Royal Philips Electronics and its Executive Committee. Previously, he was CEO of the Imaging Systems business of Royal Phillips Electronics.
Before joining Philips in 2001, Mr. Rusckowski held numerous management positions with the healthcare division of Hewlett-Packard/Agilent Technologies.
Mr. Rusckowski has been a director of the Company since May 2012. He was a director of Xerox Corporation from February 2015 to 2018, and a director of Covidien plc from December 2013 to January 2015. Mr. Rusckowski served as Chairman of the American Clinical Laboratory Association from 2013 to 2016.
|
|
Everett V. Cunningham (52)
Senior Vice President, Commercial
|
Mr. Cunningham is responsible for the commercial organization for the Company's Diagnostic Information Services business.
Prior to joining the Company in October 2012, he spent 21 years with Pfizer, Inc., where he served from June 2011 to October 2012 as Regional President, Established Products, Asia. From 2009 to 2011, Mr. Cunningham served as Regional President, West Business Unit, Primary Care. From 2007 to 2009, he served as Vice President, Human Resources, Corporate Groups. Before that Mr. Cunningham served Pfizer in a series of sales and leadership and general management roles.
|
|
James E. Davis (56)
Executive Vice President, General Diagnostics
|
In January 2017, Mr. Davis became Executive Vice President, General Diagnostics; previously he was Senior Vice President and Group Executive - Regional Businesses. In January 2015, he assumed responsibility for the general management of the Company's regional Diagnostic Information Services business. Mr. Davis was responsible for our products business from February 2014 until 2016. From February 2014 to January 2015, he was responsible for operations for the Company's Diagnostic Information Services business. He joined Quest Diagnostics in April 2013 as Senior Vice President, Diagnostics Solutions, with responsibility for the healthcare information technology, risk assessment, clinical trials, diagnostic products and employer solutions businesses.
Prior to joining Quest Diagnostics, from March 2012 to April 2013, Mr. Davis served as Lead Director, and then as Chief Executive Officer, of InSightec, Inc., a medical device company that designs and develops ultrasound ablation devices that are guided by magnetic resonance imaging systems.
Previously, Mr. Davis held a number of senior positions in General Electric’s healthcare business, including from 2007 to 2012 as Vice President and General Manager of GE Healthcare’s magnetic resonance imaging business. Prior to joining GE Healthcare, Mr. Davis held leadership positions in GE’s aviation business and led the development of strategic and operational improvement initiatives for clients of McKinsey & Company, Inc.
|
|
Catherine T. Doherty (56)
Senior Vice President and Group Executive - Clinical Franchise Solutions and Marketing
|
Since January 2013, Ms. Doherty has been responsible for overseeing the development of clinical franchise solutions in the areas of general health and wellness, cardiovascular, metabolic and endocrinology, infectious disease and immunology, and prescription drug monitoring and toxicology, as well as enterprise-wide marketing. Ms. Doherty is also responsible for the employer solutions and risk assessment businesses. Additionally, in October 2018, QuestDirect, our consumer initiated testing platform was launched under her leadership. She also was responsible for clinical franchise solutions in the areas of neurology and women's health from January 2013 to January 2017 and for the healthcare information technology business from February 2014 to January 2017.
From May 2011 to December 2012, she served as Senior Vice President, Physician Services. Prior to May 2011, Ms. Doherty held a variety of positions of increasing responsibility since joining the Company in 1990, including Vice President, Hospital Services; Vice President, Office of the Chairman; Vice President, Finance and Administration for the Hospital business; Vice President, Communications and Investor Relations; and Chief Accounting Officer.
|
|
Carrie Eglinton Manner (44)
Senior Vice President, Advanced Diagnostics
|
Ms. Eglinton Manner joined the Company in January 2017. She is responsible for the Company's advanced testing activities, including overseeing the development of clinical franchise solutions in the areas of neurology, oncology and women's health.
Previously, Ms. Eglinton Manner spent over 20 years in various leadership roles in healthcare businesses at General Electric. From 2015 to 2016, she served as President and CEO of the Detection and Guidance Solutions business, delivering advanced x-ray technologies spanning the continuum of healthcare. From 2013 to 2015, Ms. Eglinton Manner served as President and CEO of OEC Surgical Mobile C-arm systems. She was President and CEO of General Electric's diagnostic pathology laboratory services business from 2012 to 2013, and President of the Maternal Infant Care Business from 2009 to 2012.
|
|
Mark J. Guinan (57)
Executive Vice President and Chief Financial Officer
|
Mr. Guinan joined the Company in July 2013. From 2010 until joining Quest Diagnostics in 2013, he served as Chief Financial Officer for Hill-Rom Holdings Inc., a manufacturer and provider of medical technologies and related services for the health care industry. Mr. Guinan has served as a director of Myovant Sciences, Ltd. since July 2018.
Previously, he had served in a number of finance and operations roles in a long career at Johnson & Johnson including 2009 to 2010 as Vice President, Chief Procurement Officer, and 2005 to 2009 as Vice President, Group Finance Pharmaceuticals. Before joining Johnson & Johnson in 1997, he held a number of financial roles at Procter & Gamble.
|
|
Michael E. Prevoznik (57)
Senior Vice President and General Counsel
|
Mr. Prevoznik joined the Company as Vice President and General Counsel in August 1999. In 2003, he assumed responsibility for governmental affairs. From 1999 until April 2009, Mr. Prevoznik also had responsibility for the Company's Compliance Department.
In addition, from April 2011 to January 2017, he had management responsibility for the Company's diagnostic information services activities outside the U.S., and from April 2011 to January 2013, he had management responsibility for the Company's clinical trials business.
Prior to joining the Company, Mr. Prevoznik served in positions of increasing responsibility within the compliance organization at SmithKline Beecham, most recently as Vice President, Compliance, with responsibility for coordinating all SmithKline Beecham compliance activities worldwide.
|
|
This Report also includes forward-looking statements that involve risks or uncertainties. Our results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks we face described below and elsewhere. See “Cautionary Factors that May Affect Future Results” on page
38
.
|
|
•
|
loss of key customers or employees;
|
|
•
|
difficulty in standardizing information and other systems;
|
|
•
|
difficulty in consolidating facilities and infrastructure;
|
|
•
|
failure to maintain the quality or timeliness of services that our Company has historically provided;
|
|
•
|
diversion of management's attention from the day-to-day business of our Company as a result of the need to deal with the foregoing disruptions and difficulties; and
|
|
•
|
the added costs of dealing with such disruptions.
|
|
•
|
billing and reimbursement of clinical testing;
|
|
•
|
certification or licensure of clinical laboratories;
|
|
•
|
the anti-self-referral and anti-kickback laws and regulations;
|
|
•
|
the laws and regulations administered by the FDA;
|
|
•
|
the corporate practice of medicine;
|
|
•
|
operational, personnel and quality requirements intended to ensure that clinical testing services are accurate, reliable and timely;
|
|
•
|
physician fee splitting;
|
|
•
|
relationships with physicians and hospitals;
|
|
•
|
safety and health of laboratory employees; and
|
|
•
|
handling, transportation and disposal of medical specimens, infectious and hazardous waste and radioactive materials.
|
|
•
|
diversion of management time and attention;
|
|
•
|
expenditure of large amounts of cash on legal fees, costs and payment of damages;
|
|
•
|
limitations on our ability to continue some of our operations;
|
|
•
|
enforcement actions, fines and penalties or the assertion of private litigation claims and damages;
|
|
•
|
decreased demand for our services; and/or
|
|
•
|
injury to our reputation.
|
|
•
|
cease developing, performing or selling solutions or services that incorporate the challenged intellectual property;
|
|
•
|
obtain and pay for licenses from the holder of the infringed intellectual property right;
|
|
•
|
redesign or re-engineer our tests;
|
|
•
|
change our business processes; or
|
|
•
|
pay substantial damages, court costs and attorneys' fees, including potentially increased damages for any infringement held to be willful.
|
|
(a)
|
Heightened competition from commercial clinical testing companies, hospitals, physicians and others.
|
|
(b)
|
Increased pricing pressure from customers, including payers and patients.
|
|
(c)
|
A decline in economic conditions.
|
|
(d)
|
Impact of changes in payment mix, including increased patient financial responsibility and any shift from fee-for-service to discounted, capitated or bundled fee arrangements.
|
|
(e)
|
Adverse actions by government or other third-party payers, including healthcare reform that focuses on reducing healthcare costs but does not recognize the value and importance to healthcare of clinical testing or innovative solutions, unilateral reduction of fee schedules payable to us, unilateral recoupment of amounts allegedly owed and competitive bidding.
|
|
(f)
|
The impact upon our testing volume and collected revenue or general or administrative expenses resulting from compliance with policies and requirements imposed by Medicare, Medicaid and other third-party payers. These include:
|
|
(1)
|
the requirements of government and other payers to provide diagnosis codes and other information for many tests;
|
|
(2)
|
inability to obtain from patients a valid advance consent form for tests that cannot be billed without prior receipt of the form;
|
|
(3)
|
the impact of additional or expanded limited coverage policies and limits on the allowable number of test units or ordering frequency of same; and
|
|
(4)
|
the impact of increased prior authorization programs.
|
|
(g)
|
Adverse results from pending or future government investigations, lawsuits or private actions. These include, in particular, monetary damages, loss or suspension of licenses, and/or suspension or exclusion from the Medicare and Medicaid programs and/or criminal penalties.
|
|
(h)
|
Failure to efficiently integrate acquired businesses and to manage the costs related to any such integration, or to retain key technical, professional or management personnel.
|
|
(i)
|
Denial, suspension or revocation of CLIA certification or other licenses for any of our clinical laboratories under the CLIA standards, revocation or suspension of the right to bill the Medicare and Medicaid programs or other adverse regulatory actions by federal, state and local agencies.
|
|
(j)
|
Changes in and complexity of federal, state or local laws or regulations, including changes that result in new or increased federal or state regulation of commercial clinical laboratories, tests developed by commercial clinical laboratories or other products or services that we offer or activities in which we are engaged, including regulation by the FDA.
|
|
(k)
|
Inability to achieve expected benefits from our acquisitions of other businesses.
|
|
(l)
|
Inability to achieve additional benefits from our business performance tools and efficiency initiatives.
|
|
(m)
|
Adverse publicity and news coverage about the diagnostic information services industry or us.
|
|
(n)
|
Failure of the Company to maintain, defend and secure its financial, accounting, technology, customer data and other operational systems from cyberattacks, IT system outages, telecommunications failures, malicious human acts and failure of the systems of third parties upon which the Company relies.
|
|
(o)
|
Development of technologies that substantially alter the practice of clinical testing, including technology changes that lead to the development of more convenient or cost-effective testing, or testing to be performed outside of a
|
|
(p)
|
Negative developments regarding intellectual property and other property rights that could prevent, limit or interfere with our ability to develop, perform or sell our tests or operate our business. These include:
|
|
(1)
|
Issuance of patents or other property rights to our competitors or others; and
|
|
(2)
|
Inability to obtain or maintain adequate patent or other proprietary rights for our products and services or to successfully enforce our proprietary rights.
|
|
(q)
|
Development of tests by our competitors or others which we may not be able to license, or usage of our technology or similar technologies or our trade secrets or other intellectual property by competitors, any of which could negatively affect our competitive position.
|
|
(r)
|
Regulatory delay or inability to commercialize newly developed or licensed tests or technologies or to obtain appropriate reimbursements for such tests.
|
|
(s)
|
The complexity of billing and revenue recognition for clinical laboratory testing.
|
|
(t)
|
Changes in interest rates and changes in our credit ratings from Standard & Poor's, Moody's Investor Services or Fitch Ratings causing an unfavorable impact on our cost of and access to capital.
|
|
(u)
|
Inability to hire or retain qualified or key senior management personnel.
|
|
(v)
|
Terrorist and other criminal activities, hurricanes, earthquakes or other natural disasters, and health pandemics, which could affect our customers, transportation or systems, or our facilities, and for which insurance may not adequately reimburse us.
|
|
(w)
|
Difficulties and uncertainties in the discovery, development, regulatory environment and/or marketing of new services or solutions or new uses of existing tests.
|
|
(x)
|
Failure to adapt to changes in the healthcare system (including the medical laboratory testing market) and healthcare delivery, including those stemming from the ACA (or its repeal, amendment or replacement), PAMA, trends in utilization of the healthcare system and increased patient financial responsibility for services.
|
|
(y)
|
Results and consequences of governmental inquiries.
|
|
Location
|
Leased or Owned
|
|
|
Sacramento, California (laboratory)
|
Leased
|
|
|
West Hills, California (laboratory)
|
Leased
|
|
|
San Juan Capistrano, California (laboratory)
|
Owned
|
|
|
Tampa, Florida (laboratory)
|
Owned
|
|
|
Atlanta, Georgia (laboratory)
|
Owned
|
|
|
Chicago, Illinois (2) (laboratories)
|
One owned, one leased
|
|
|
Marlborough, Massachusetts (laboratories)
|
Leased
|
|
|
Baltimore, Maryland (laboratory)
|
Owned
|
|
|
Teterboro, New Jersey (laboratory)
|
Owned
|
|
|
Philadelphia, Pennsylvania (laboratory)
|
Leased
|
|
|
Dallas, Texas (laboratory)
|
Leased
|
|
|
Chantilly, Virginia (laboratory)
|
Leased
|
|
|
Lenexa, Kansas (laboratory)
|
Owned
|
|
|
Greensboro, North Carolina (laboratory)
|
Leased
|
|
|
Lewisville, Texas (laboratory)
|
Leased
|
|
|
Cleveland, Ohio (laboratory)
|
Leased
|
|
|
ISSUER PURCHASES OF EQUITY SECURITIES
|
||||||||||||||
|
Period
|
Total Number of
Shares
Purchased
|
Average Price
Paid per Share
|
Total Number of
Shares Purchased
as Part of Publicly
Announced Plans
or Programs
|
Approximate
Dollar Value of
Shares that May
Yet Be Purchased
Under the Plans
or Programs
(in thousands)
|
||||||||||
|
October 1, 2018 – October 31, 2018
|
|
|
|
|
|
|
|
|
||||||
|
Share Repurchase Program (A)
|
130,414
|
|
$
|
92.01
|
|
130,414
|
|
$
|
755,124
|
|
||||
|
Employee Transactions (B)
|
797
|
|
$
|
103.14
|
|
N/A
|
|
N/A
|
|
|||||
|
November 1, 2018 – November 30, 2018
|
||||||||||||||
|
Share Repurchase Program (A)
|
482,952
|
|
$
|
95.24
|
|
482,952
|
|
$
|
709,126
|
|
||||
|
Employee Transactions (B)
|
714
|
|
$
|
96.06
|
|
N/A
|
|
N/A
|
|
|||||
|
December 1, 2018 – December 31, 2018
|
||||||||||||||
|
Share Repurchase Program (A)
|
1,365,222
|
|
$
|
85.70
|
|
1,365,222
|
|
$
|
592,126
|
|
||||
|
Employee Transactions (B)
|
1,902
|
|
$
|
82.74
|
|
N/A
|
|
N/A
|
|
|||||
|
Total
|
||||||||||||||
|
Share Repurchase Program (A)
|
1,978,588
|
|
$
|
88.45
|
|
1,978,588
|
|
$
|
592,126
|
|
||||
|
Employee Transactions (B)
|
3,413
|
|
$
|
90.29
|
|
N/A
|
|
N/A
|
|
|||||
|
(A)
|
Since the share repurchase program's inception in May 2003, our Board of Directors has authorized $8.0 billion of share repurchases of our common stock through
December 31, 2018
. The share repurchase authority has no set expiration or termination date.
|
|
(B)
|
Includes: (1) shares delivered or attested to in satisfaction of the exercise price and/or tax withholding obligations by holders of stock options (granted under the Company's Amended and Restated Employee Long-Term Incentive Plan) who exercised options; and (2) shares withheld (under the terms of grants under the Long-Term Incentive Plan) to offset tax withholding obligations that occur upon the delivery of outstanding common shares underlying restricted share units and performance share units.
|
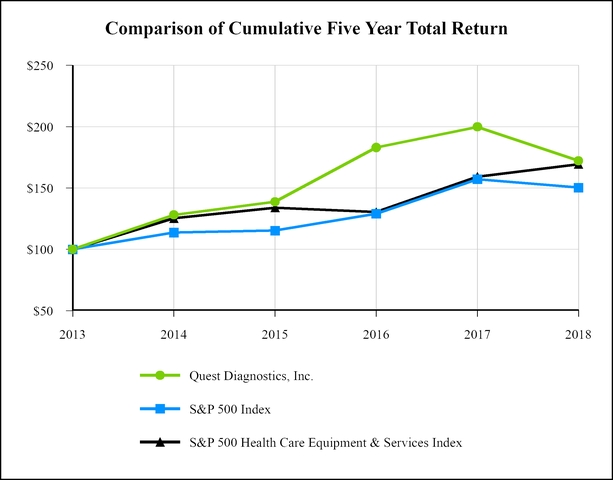
|
Closing DGX Price
|
Total Shareholder Return
|
Performance Graph Values
|
|||||||||||||||||||||||
|
Date
|
DGX
|
S&P 500
|
S&P 500 H.C.
|
DGX
|
S&P 500
|
S&P 500 H.C.
|
|||||||||||||||||||
|
12/31/2014
|
$
|
67.06
|
|
28.06
|
%
|
13.69
|
%
|
25.34
|
%
|
$
|
128.06
|
|
$
|
113.69
|
|
$
|
125.34
|
|
|||||||
|
12/31/2015
|
$
|
71.14
|
|
8.35
|
%
|
1.38
|
%
|
6.89
|
%
|
$
|
138.75
|
|
$
|
115.26
|
|
$
|
133.97
|
|
|||||||
|
12/30/2016
|
$
|
91.90
|
|
31.89
|
%
|
11.96
|
%
|
(2.69
|
)%
|
$
|
183.01
|
|
$
|
129.05
|
|
$
|
130.37
|
|
|||||||
|
12/29/2017
|
$
|
98.49
|
|
9.16
|
%
|
21.83
|
%
|
22.08
|
%
|
$
|
199.77
|
|
$
|
157.22
|
|
$
|
159.15
|
|
|||||||
|
12/31/2018
|
$
|
83.27
|
|
(13.84
|
)%
|
(4.38
|
)%
|
6.47
|
%
|
$
|
172.12
|
|
$
|
150.33
|
|
$
|
169.44
|
|
|||||||
|
See page
49
.
|
|
See page
53
.
|
|
See page
72
.
|
|
(a)
|
Documents filed as part of this Report.
|
|
1.
|
Index to financial statements and supplementary data filed as part of this Report.
|
|
Item
|
Page
|
|
Financial Statements
|
|
|
2.
|
Financial Statement Schedule.
|
|
3.
|
Exhibits
|
|
(b)
|
Exhibits filed as part of this Report.
|
|
(c)
|
None.
|
|
QUEST DIAGNOSTICS INCORPORATED
|
||
|
(Registrant)
|
||
|
By:
|
/s/Stephen H. Rusckowski
|
|
|
Stephen H. Rusckowski
|
||
|
Chairman of the Board, President and Chief Executive Officer
|
||
|
Signature
|
Capacity
|
|
|
/s/Stephen H. Rusckowski
Stephen H. Rusckowski
|
Chairman of the Board, President and Chief Executive Officer
(Principal Executive Officer)
|
|
|
/s/Mark J. Guinan
Mark J. Guinan
|
Executive Vice President and Chief Financial Officer
(Principal Financial Officer)
|
|
|
/s/Robert A. Klug
Robert A. Klug
|
Vice President, Corporate Controller and Chief Accounting Officer
(Principal Accounting Officer)
|
|
|
/s/Jenne K. Britell, Ph.D.
Jenne K. Britell, Ph.D.
|
Director
|
|
|
/s/Vicky B. Gregg
Vicky B. Gregg
|
Director
|
|
|
/s/Jeffrey M. Leiden, M.D., Ph. D.
Jeffrey M. Leiden, M.D., Ph. D.
|
Director
|
|
|
/s/Timothy L. Main
Timothy L. Main
|
Director
|
|
|
/s/Denise M. Morrison
Denise M. Morrison
|
Director
|
|
|
/s/Gary M. Pfeiffer
Gary M. Pfeiffer
|
Director
|
|
|
/s/Timothy M. Ring
Timothy M. Ring
|
Director
|
|
|
/s/Daniel C. Stanzione, Ph.D.
Daniel C. Stanzione, Ph.D.
|
Director
|
|
|
/s/Helen I. Torley, M.B. Ch. B., M.R.C.P.
Helen I. Torley, M.B. Ch. B., M.R.C.P.
|
Director
|
|
|
/s/Gail R. Wilensky, Ph.D.
Gail R. Wilensky, Ph.D.
|
Director
|
|
|
Year Ended December 31,
|
|||||||||||||||||||
|
2018
|
2017
|
2016
|
2015
|
2014
|
|||||||||||||||
|
(dollars in millions, except per share data)
|
|||||||||||||||||||
|
Operations Data:
|
(a) (b) (c)
|
(a) (d) (e)
|
(a) (f) (g)
|
(a) (h) (i)
|
(a) (j) (k)
|
||||||||||||||
|
Net revenues
|
$
|
7,531
|
|
$
|
7,402
|
|
$
|
7,214
|
|
$
|
7,493
|
|
$
|
7,435
|
|
||||
|
Operating income
|
1,101
|
|
1,165
|
|
1,277
|
|
1,399
|
|
983
|
|
|||||||||
|
Income from continuing operations
|
788
|
|
824
|
|
696
|
|
753
|
|
587
|
|
|||||||||
|
Income from discontinued operations, net of taxes
|
—
|
|
—
|
|
—
|
|
—
|
|
5
|
|
|||||||||
|
Net income
|
788
|
|
824
|
|
696
|
|
753
|
|
592
|
|
|||||||||
|
Less: Net income attributable to noncontrolling interests
|
52
|
|
52
|
|
51
|
|
44
|
|
36
|
|
|||||||||
|
Net income attributable to Quest Diagnostics
|
$
|
736
|
|
$
|
772
|
|
$
|
645
|
|
$
|
709
|
|
$
|
556
|
|
||||
|
Amounts attributable to Quest Diagnostics' stockholders:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
736
|
|
$
|
772
|
|
$
|
645
|
|
$
|
709
|
|
$
|
551
|
|
||||
|
Income from discontinued operations, net of taxes
|
—
|
|
—
|
|
—
|
|
—
|
|
5
|
|
|||||||||
|
Net income
|
$
|
736
|
|
$
|
772
|
|
$
|
645
|
|
$
|
709
|
|
$
|
556
|
|
||||
|
Earnings per share attributable to Quest Diagnostics' common stockholders - basic:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
5.39
|
|
$
|
5.63
|
|
$
|
4.58
|
|
$
|
4.92
|
|
$
|
3.80
|
|
||||
|
Income from discontinued operations
|
—
|
|
—
|
|
—
|
|
—
|
|
0.03
|
|
|||||||||
|
Net income
|
$
|
5.39
|
|
$
|
5.63
|
|
$
|
4.58
|
|
$
|
4.92
|
|
$
|
3.83
|
|
||||
|
Earnings per share attributable to Quest Diagnostics' common stockholders - diluted:
|
|||||||||||||||||||
|
Income from continuing operations
|
$
|
5.29
|
|
$
|
5.50
|
|
$
|
4.51
|
|
$
|
4.87
|
|
$
|
3.78
|
|
||||
|
Income from discontinued operations
|
—
|
|
—
|
|
—
|
|
—
|
|
0.03
|
|
|||||||||
|
Net income
|
$
|
5.29
|
|
$
|
5.50
|
|
$
|
4.51
|
|
$
|
4.87
|
|
$
|
3.81
|
|
||||
|
Dividends per common share
|
$
|
2.03
|
|
$
|
1.80
|
|
$
|
1.65
|
|
$
|
1.52
|
|
$
|
1.32
|
|
||||
|
Year Ended December 31,
|
|||||||||||||||||||
|
2018
|
2017
|
2016
|
2015
|
2014
|
|||||||||||||||
|
(dollars in millions)
|
|||||||||||||||||||
|
Balance Sheet Data (at end of year):
|
(a) (b) (c)
|
(a) (d) (e)
|
(a) (f) (g)
|
(a) (h) (i)
|
(a) (j) (k)
|
||||||||||||||
|
Cash and cash equivalents
|
$
|
135
|
|
$
|
137
|
|
$
|
359
|
|
$
|
133
|
|
$
|
192
|
|
||||
|
Total assets
|
11,003
|
|
10,503
|
|
10,100
|
|
9,962
|
|
9,857
|
|
|||||||||
|
Long-term debt
|
3,429
|
|
3,748
|
|
3,728
|
|
3,492
|
|
3,224
|
|
|||||||||
|
Total debt
|
3,893
|
|
3,784
|
|
3,734
|
|
3,651
|
|
3,742
|
|
|||||||||
|
Redeemable noncontrolling interest
|
77
|
|
80
|
|
77
|
|
70
|
|
—
|
|
|||||||||
|
Other Data:
|
|||||||||||||||||||
|
Net cash provided by operating activities
|
$
|
1,200
|
|
$
|
1,175
|
|
$
|
1,116
|
|
$
|
967
|
|
$
|
944
|
|
||||
|
Net cash used in investing activities
|
(801
|
)
|
(830
|
)
|
(127
|
)
|
(362
|
)
|
(1,025
|
)
|
|||||||||
|
Net cash (used in) provided by financing activities
|
(401
|
)
|
(592
|
)
|
(738
|
)
|
(664
|
)
|
86
|
|
|||||||||
|
Capital expenditures
|
383
|
|
252
|
|
293
|
|
263
|
|
308
|
|
|||||||||
|
Purchases of treasury stock
|
322
|
|
465
|
|
590
|
|
224
|
|
132
|
|
|||||||||
|
Dividends paid
|
266
|
|
247
|
|
223
|
|
212
|
|
187
|
|
|||||||||
|
(a)
|
Net revenues for the years ended December 31, 2017 and 2016 have been restated to reflect the impact of new revenue recognition rules that became effective January 1, 2018 and were adopted on a retrospective basis; Net revenues for the years ended December 31, 2015 and 2014 have not been restated. Cash flow data for the years ended December 31, 2017, 2016, 2015 and 2014 have been restated to reflect the impact of the adoption of two new accounting standards that clarify presentation and classification in the statement of cash flows on a retrospective basis. See Note 2 to the consolidated financial statements for further details on the adoption of new accounting standards. During the third quarter of 2006, we completed the wind down of NID, a test kit manufacturing subsidiary. As a result, the NID operations have been classified as discontinued operations for all periods presented. We will continue to report NID as a discontinued operation until uncertain tax benefits associated with NID are resolved.
|
|
(b)
|
On February 1, 2018, we completed the acquisition of Mobile Medical Examination Services, LLC. ("MedXM"). On June 18, 2018, we completed the acquisition of the outreach laboratory service business of Cape Cod Healthcare, Inc. On September 19, 2018, we completed the acquisition of ReproSource, Inc. ("ReproSource"). On November 6, 2018, we completed the acquisition of the U.S. laboratory service business of Oxford Immunotec, Inc. ("Oxford"). Consolidated operating results for 2018 include the results of operations of MedXM, the outreach laboratory service business of Cape Cod Healthcare, Inc., ReproSource and Oxford subsequent to the closing of the applicable acquisition. For further details regarding our acquisitions, see Note 6 to the consolidated financial statements.
|
|
(c)
|
Operating income included (for 2018):
|
|
•
|
pre-tax charges of $122 million, primarily associated with workforce reductions, systems conversions and integration incurred in connection with further restructuring and integrating our business; and
|
|
•
|
pre-tax charges of $2 million, primarily associated with costs incurred related to certain legal matters and a loss on the sale of a foreign subsidiary partially offset by a gain associated with the decrease in the fair value of the contingent consideration accrual associated with our MedXM acquisition and an insurance claim for hurricane related losses.
|
|
•
|
excess tax benefits associated with stock-based compensation arrangements of
$18 million
; and
|
|
•
|
income tax benefit of $14 million primarily associated with a change in a tax return accounting method that enabled our Company to accelerate the deduction of certain expenses on its 2017 tax return at the federal corporate statutory tax rate in effect during 2017 partially offset by an income tax expense associated with finalizing the impact of the enactment of the Tax Cuts and Jobs Act ("TCJA").
|
|
(d)
|
On May 1, 2017, we completed the acquisition of the outreach laboratory service business of PeaceHealth Laboratories ("PHL"). On July 14, 2017, we completed the acquisition of Med Fusion, LLC and Clearpoint Diagnostic Laboratories, LLC ("Med Fusion"). On September 28, 2017, we completed the acquisition of the outreach laboratory service businesses of two hospitals of Hartford HealthCare Corporation ("HHC"), The William W. Backus Hospital and The Hospital of Central Connecticut. On December 1, 2017, we completed the acquisition of Cleveland HeartLab, Inc. ("CHL"). On December 7, 2017, we completed the acquisition of certain assets of the clinical and anatomic pathology laboratory business of Shiel Holdings, LLC ("Shiel"). Consolidated operating results for 2017 include the results of operations of PHL, Med Fusion, HHC, CHL and Shiel subsequent to the closing of the applicable acquisition. For further details regarding our acquisitions, see Note 6 to the consolidated financial statements.
|
|
(e)
|
Operating income included (for 2017):
|
|
•
|
pre-tax charges of $105 million, primarily associated with systems conversions, integration and workforce reductions incurred in connection with further restructuring and integrating our business; and
|
|
•
|
pre-tax charges of $12 million, primarily a result of non-cash asset impairment charges and incremental costs incurred as a result of hurricanes and costs incurred related to certain legal matters.
|
|
•
|
a net pre-tax gain of $2 million, primarily a result of a gain on the sale of an interest in an equity method investment partially offset by non-cash asset impairment charges associated with an investment;
|
|
•
|
$1 million of pre-tax restructuring and integration charges associated with our Q
2
Solutions joint venture;
|
|
•
|
a provisional estimated income tax benefit of $106 million associated with the TCJA, including a deferred income tax benefit of $115 million primarily due to the remeasurement of our net deferred tax liabilities and reserves at the new combined federal and state tax rate, partially offset by $9 million of current tax expense primarily due to the mandatory repatriation toll charge on undistributed foreign earnings and profits;
|
|
•
|
excess tax benefits associated with stock-based compensation arrangements of $37 million; and
|
|
•
|
income tax expense of $3 million primarily a result of recording a valuation allowance against certain net operating loss carryforwards in a geography impacted by hurricanes.
|
|
(f)
|
On February 29, 2016, we completed the acquisition of the outreach laboratory service business of Clinical Laboratory Partners, LLC ("CLP"), a wholly-owned subsidiary of HHC. Consolidated operating results for 2016 include the results of operations of CLP subsequent to the closing of the acquisition. On May 13, 2016, we completed the sale of our Focus Diagnostics products business ("Focus Sale"). Our Focus Diagnostics products business has not been classified as a discontinued operation. For further details regarding dispositions, see Note 7 to the consolidated financial statements.
|
|
(g)
|
Operating income included (for 2016):
|
|
•
|
a pre-tax gain of $118 million associated with the Focus Sale;
|
|
•
|
pre-tax charges of $78 million, primarily associated with systems conversions and integration incurred in connection with further restructuring and integrating our business; and
|
|
•
|
a net pre-tax gain of $7 million, primarily a result of a non-taxable gain on an escrow recovery associated with an acquisition, partially offset by costs associated with winding down subsidiaries, non-cash asset impairment charges and costs incurred related to certain legal matters.
|
|
•
|
income tax expense of $84 million associated with the Focus Sale, consisting of $91 million of current income tax expense and a deferred income tax benefit of $7 million;
|
|
•
|
$48 million of pre-tax charges on the retirement of debt associated with the March 2016 cash tender offer and the related income tax benefit of $18 million;
|
|
•
|
non-cash asset impairment charges of $7 million associated with certain investments;
|
|
•
|
$4 million of pre-tax restructuring and integration charges associated with our Q
2
Solutions joint venture; and
|
|
•
|
excess tax benefits associated with stock-based compensation arrangements of $9 million.
|
|
•
|
a $17 million cash tax benefit on the retirement of debt associated with the March 2016 cash tender offer;
|
|
•
|
$54 million of proceeds received from the termination of interest rate swap agreements; and
|
|
•
|
$91 million of income taxes paid in connection with the Focus Sale.
|
|
(h)
|
On August 3, 2015, we completed the acquisition of MemorialCare Health System's laboratory outreach business ("MemorialCare"). On November 16, 2015, we completed the acquisition of the business assets of Superior Mobile Medics, Inc. ("Superior Mobile Medics"). Consolidated operating results for 2015 include the results of operations of MemorialCare and Superior Mobile Medics subsequent to the closing of the applicable acquisition. In July 2015, we contributed our clinical trials testing business to a newly formed global clinical trials central laboratory services joint venture with IQVIA Holdings Inc., Q
2
Solutions ("Clinical Trials Contribution"). Our clinical trials testing business was not classified as a discontinued operation.
|
|
(i)
|
Operating income included (for 2015):
|
|
•
|
pre-tax gain of $334 million associated with the Clinical Trials Contribution;
|
|
•
|
pre-tax charges of $105 million, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating our business; and
|
|
•
|
net pre-tax charges of $33 million primarily associated with non-cash asset impairment charges and other costs associated with winding down our Celera products business and another subsidiary, costs incurred related to certain legal matters and a pre-tax gain of $13 million associated with a decrease in the fair value of the contingent consideration accrual associated with our Summit Health, Inc. ("Summit Health") acquisition.
|
|
•
|
$144 million of pre-tax charges on retirement of debt associated with the March 2015 cash tender offer and the April 2015 redemption and the related income tax benefit of $57 million;
|
|
•
|
deferred income tax expense of $145 million associated with the gain on the Clinical Trials Contribution;
|
|
•
|
$58 million deferred income tax benefit associated with winding down a subsidiary; and
|
|
•
|
$5 million of pre-tax restructuring and integration charges associated with our Q
2
Solutions joint venture.
|
|
•
|
a $57 million income tax benefit on the retirement of debt associated with the March 2015 cash tender offer and April 2015 redemption;
|
|
•
|
payments associated with an additional payroll cycle in 2015; and
|
|
•
|
an income tax payment in the third quarter of 2015 associated with certain tax contingencies.
|
|
•
|
$139 million of pre-tax cash charges on retirement of debt associated with the March 2015 cash tender offer and the April 2015 redemption, principally consisting of premiums paid;
|
|
•
|
$51 million of deferred acquisition consideration payments, primarily to UMass Memorial Medical Center ("UMass"), related to the business acquisition in 2013; and
|
|
•
|
$63 million of proceeds from the sale of a noncontrolling interest in a subsidiary to UMass.
|
|
(j)
|
On March 7, 2014, we completed the acquisition of Solstas Lab Partners Group ("Solstas"). On April 18, 2014, we completed the acquisition of Summit Health. On April 16, 2014, we completed the acquisition of the outreach laboratory service operations of Steward Healthcare, LLC ("Steward"). Consolidated operating results for 2014 include the results of operations of Solstas, Summit Health and Steward subsequent to the closing of the applicable acquisition.
|
|
(k)
|
Operating income included (for 2014):
|
|
•
|
pre-tax charges of $121 million, primarily associated with workforce reductions and professional fees incurred in connection with further restructuring and integrating our business;
|
|
•
|
pre-tax charges of $24 million principally associated with costs related to certain legal matters; and
|
|
•
|
pre-tax gain of $9 million associated with a decrease in the fair value of the contingent consideration accrual associated with our Summit Health acquisition.
|
|
•
|
Our total net revenues of
$7.5 billion
were
1.7%
above the prior year.
|
|
•
|
In DIS:
|
|
◦
|
Revenues of
$7.2 billion
increased by
1.9%
compared to the prior year, which reflects the impact of recent acquisitions, partially offset by a decrease in organic revenue (revenue growth excluding the impact of acquisitions).
|
|
◦
|
Volume, measured by the number of requisitions, increased 2.5% compared to the prior year.
|
|
◦
|
Revenue per requisition decreased by 1.2% compared to the prior year primarily due to pricing pressure including the impact of the Protecting Access to Medicare Act ("PAMA"), increased denials and higher patient concessions.
|
|
•
|
DS revenues of
$327 million
were
2.3%
below the prior year primarily due to certain royalty revenues received in 2017 related to a royalty agreement, retained from the sale of our products business, that has since expired.
|
|
•
|
Net income attributable to Quest Diagnostics' stockholders was
$736 million
, or
$5.29
per diluted share, in
2018
, compared to
$772 million
, or
$5.50
per diluted share, in 2017.
|
|
•
|
Net cash provided by operating activities was
$1.2 billion
in both
2018
and 2017.
|
|
•
|
revenues and accounts receivable associated with DIS;
|
|
•
|
reserves for general and professional liability claims;
|
|
•
|
reserves for other legal proceedings;
|
|
•
|
accounting for and recoverability of goodwill; and
|
|
•
|
accounting for stock-based compensation expense.
|
|
•
|
Healthcare Insurers
|
|
•
|
Government Payers
|
|
•
|
Client Payers
|
|
•
|
Patients
|
|
% of
|
% of
|
||
|
Total
|
Consolidated
|
||
|
Volume
|
Net Revenues
|
||
|
Healthcare Insurers
|
46
|
35
|
|
|
Government Payers
|
13
|
16
|
|
|
Client Payers
|
37
|
32
|
|
|
Patients *
|
1
|
13
|
|
|
Total DIS
|
97
|
96
|
|
|
% of
|
|
|
Consolidated
|
|
|
Net Accounts
|
|
|
Receivable
|
|
|
Healthcare Insurers
|
22
|
|
Government Payers
|
13
|
|
Client Payers
|
41
|
|
Patients (including coinsurance and deductible responsibilities)
|
20
|
|
Total DIS
|
96
|
|
•
|
DIS business;
|
|
•
|
Risk assessment services business which is part of our DS businesses
|
|
$ Increase (Decrease)
|
% Increase (Decrease)
|
||||||||||||||||||||||||
|
2018
|
2017
|
2016
|
2018 vs. 2017
|
2017 vs. 2016
|
2018 vs. 2017
|
2017 vs. 2016
|
|||||||||||||||||||
|
(dollars in millions, except per share data)
|
|||||||||||||||||||||||||
|
Net revenues:
|
|||||||||||||||||||||||||
|
DIS business
|
$
|
7,204
|
|
$
|
7,068
|
|
$
|
6,837
|
|
$
|
136
|
|
$
|
231
|
|
1.9
|
%
|
3.4
|
%
|
||||||
|
DS businesses
|
327
|
|
334
|
|
377
|
|
(7
|
)
|
(43
|
)
|
(2.3
|
)
|
(11.3
|
)
|
|||||||||||
|
Total net revenues
|
$
|
7,531
|
|
$
|
7,402
|
|
$
|
7,214
|
|
$
|
129
|
|
$
|
188
|
|
1.7
|
%
|
2.6
|
%
|
||||||
|
Operating costs and expenses and other operating income:
|
|||||||||||||||||||||||||
|
Cost of services
|
$
|
4,926
|
|
$
|
4,719
|
|
$
|
4,616
|
|
$
|
207
|
|
$
|
103
|
|
4.4
|
%
|
2.2
|
%
|
||||||
|
Selling, general and administrative
|
1,424
|
|
1,443
|
|
1,380
|
|
(19
|
)
|
63
|
|
(1.3
|
)
|
4.6
|
|
|||||||||||
|
Amortization of intangible assets
|
90
|
|
74
|
|
72
|
|
16
|
|
2
|
|
21.2
|
|
2.5
|
|
|||||||||||
|
Loss (gain) on disposition of business
|
4
|
|
—
|
|
(118
|
)
|
4
|
|
118
|
|
NM
|
|
NM
|
|
|||||||||||
|
Other operating (income) expense, net
|
(14
|
)
|
1
|
|
(13
|
)
|
(15
|
)
|
14
|
|
NM
|
|
NM
|
|
|||||||||||
|
Total operating costs and expenses, net
|
$
|
6,430
|
|
$
|
6,237
|
|
$
|
5,937
|
|
$
|
193
|
|
$
|
300
|
|
3.1
|
%
|
5.1
|
%
|
||||||
|
Operating income
|
$
|
1,101
|
|
$
|
1,165
|
|
$
|
1,277
|
|
$
|
(64
|
)
|
$
|
(112
|
)
|
(5.5
|
)%
|
(8.8
|
)%
|
||||||
|
Other (expense) income:
|
|||||||||||||||||||||||||
|
Interest expense, net
|
$
|
(167
|
)
|
$
|
(151
|
)
|
$
|
(143
|
)
|
$
|
(16
|
)
|
$
|
(8
|
)
|
10.8
|
%
|
5.3
|
%
|
||||||
|
Other (expense) income, net
|
(8
|
)
|
16
|
|
(48
|
)
|
(24
|
)
|
64
|
|
NM
|
|
NM
|
|
|||||||||||
|
Total non-operating expenses, net
|
$
|
(175
|
)
|
$
|
(135
|
)
|
$
|
(191
|
)
|
$
|
(40
|
)
|
$
|
56
|
|
28.9
|
%
|
(29.1
|
)%
|
||||||
|
Income tax expense
|
$
|
(182
|
)
|
$
|
(241
|
)
|
$
|
(429
|
)
|
$
|
59
|
|
$
|
188
|
|
(24.2
|
)%
|
(43.9
|
)%
|
||||||
|
Effective income tax rate
|
19.7
|
%
|
23.4
|
%
|
39.5
|
%
|
-370 bps
|
|
-1610 bps
|
|
NM
|
|
NM
|
|
|||||||||||
|
Equity in earnings of equity method investees, net of taxes
|
$
|
44
|
|
$
|
35
|
|
$
|
39
|
|
$
|
9
|
|
$
|
(4
|
)
|
24.4
|
%
|
(9.6
|
)%
|
||||||
|
Net income attributable to Quest Diagnostics' stockholders
|
$
|
736
|
|
$
|
772
|
|
$
|
645
|
|
$
|
(36
|
)
|
$
|
127
|
|
(4.7
|
)%
|
19.8
|
%
|
||||||
|
Diluted earnings per common share attributable to Quest Diagnostics’ common stockholders
|
$
|
5.29
|
|
$
|
5.50
|
|
$
|
4.51
|
|
$
|
(0.21
|
)
|
$
|
0.99
|
|
(3.8
|
)%
|
22.0
|
%
|
||||||
|
2018
|
2017
|
2016
|
||||||
|
Net revenues:
|
||||||||
|
DIS business
|
95.7
|
%
|
95.5
|
%
|
94.8
|
%
|
||
|
DS businesses
|
4.3
|
|
4.5
|
|
5.2
|
|
||
|
Total net revenues
|
100.0
|
%
|
100.0
|
%
|
100.0
|
%
|
||
|
Operating costs and expenses and other operating income:
|
|
|
|
|
|
|
||
|
Cost of services
|
65.4
|
%
|
63.8
|
%
|
64.0
|
%
|
||
|
Selling, general and administrative
|
18.9
|
|
19.5
|
|
19.1
|
|
||
|
Amortization of intangible assets
|
1.2
|
|
1.0
|
|
1.0
|
|
||
|
Loss (gain) on disposition of business
|
0.1
|
|
—
|
|
(1.5
|
)
|
||
|
Other operating (income) expense, net
|
(0.2
|
)
|
—
|
|
(0.3
|
)
|
||
|
Total operating costs and expenses, net
|
85.4
|
%
|
84.3
|
%
|
82.3
|
%
|
||
|
Operating income
|
14.6
|
%
|
15.7
|
%
|
17.7
|
%
|
||
|
•
|
pre-tax charges of $122 million ($56 million in cost of services, $65 million in selling, general and administrative expenses, and $1 million in
other operating (income) expense, net
), or $0.66 per diluted share, primarily associated with workforce reductions, systems conversions and integration incurred in connection with further restructuring and integrating our business;
|
|
•
|
excess tax benefits associated with stock-based compensation arrangements of
$18 million
, or
$0.13
per diluted share, recorded in income tax expense;
|
|
•
|
an income tax benefit of $14 million, or $0.09 per diluted share, associated with a change in a tax return accounting method that enabled our Company to accelerate the deduction of certain expenses on its 2017 tax return at the federal corporate statutory tax rate in effect during 2017 partially offset by an income tax expense associated with finalizing the impact of the enactment of the Tax Cuts and Jobs Act ("TCJA");
|
|
•
|
net pre-tax charges of $2 million ($12 million in cost of services and $4 million in loss (gain) on disposition of business partially offset by $14 million gain in
other operating (income) expense, net
), or $0.01 per diluted share, primarily attributable to costs incurred related to certain legal matters and a loss on the sale of a foreign subsidiary which were partially offset by a gain associated with the decrease in the fair value of the contingent consideration accrual associated with our MedXM acquisition and an insurance claim for hurricane related losses.
|
|
•
|
excess tax benefits associated with stock-based compensation arrangements of
$37 million
, or
$0.27
per diluted share, recorded in income tax expense;
|
|
•
|
a provisional estimated income tax benefit of $106 million, or $0.77 per diluted share, associated with the TCJA, including a deferred income tax benefit of $115 million primarily due to the remeasurement of our net deferred tax liabilities and reserves at the new combined federal and state tax rate, partially offset by $9 million of current tax expense primarily due to the mandatory repatriation toll charge on undistributed foreign earnings and profits;
|
|
•
|
pre-tax charges of $106 million ($45 million in cost of services, $60 million in selling, general and administrative expenses and $1 million in equity in earnings of equity method investees, net of taxes), or $0.47 per diluted share, primarily associated with systems conversions, integration and workforce reductions incurred in connection with further restructuring and integrating our business; and
|
|
•
|
net pre-tax charges of $10 million ($5 million in cost of services, $7 million in selling, general and administrative expenses and $2 million benefit in
other (expense) income, net
), or $0.07 per diluted share primarily associated with non-cash asset impairment charges associated with an investment, non-cash asset impairment charges and incremental costs incurred as a result of hurricanes, and costs incurred related to certain legal matters, partially offset by gain on the sale of an interest in an equity method investment.
|
|
•
|
excess tax benefits associated with stock-based compensation arrangements of $9 million, or $0.06 per diluted share, recorded in income tax expense;
|
|
•
|
pre-tax gain of $118 million, or $0.24 per diluted share, related to the Focus Sale recorded in loss (gain) on disposition of business;
|
|
•
|
pre-tax charges of $82 million ($40 million in cost of services, $37 million in selling, general and administrative expenses, $1 million in
other operating (income) expense, net
and $4 million in equity in earnings of equity method investees, net of taxes), or $0.35 per diluted share, primarily associated with systems conversions and integration costs incurred in connection with further restructuring and integrating our business;
|
|
•
|
pre-tax charges of $48 million, or $0.21 per diluted share, related to the 2016 loss on retirement of debt associated with the March 2016 cash tender offer ("2016 Tender Offer"), in which we purchased $73 million of our Senior Notes due 2037 and $127 million of our Senior Notes due 2040, recorded in other (expense) income, net; and
|
|
•
|
pre-tax costs of $6 million in selling, general and administrative expenses, a net pre-tax gain of $13 million in
other operating (income) expense, net
and pre-tax costs of $7 million in
other (expense) income, net
that on a combined basis benefited diluted earnings per share by $0.06, primarily a result of a non-taxable gain on an escrow recovery associated with an acquisition, partially offset by costs associated with winding down subsidiaries, non-cash asset impairment charges and costs incurred related to certain legal matters.
|
|
2018
|
2017
|
2016
|
|||||||||
|
(dollars in millions)
|
|||||||||||
|
Net cash provided by operating activities
|
$
|
1,200
|
|
$
|
1,175
|
|
$
|
1,116
|
|
||
|
Net cash used in investing activities
|
(801
|
)
|
(830
|
)
|
(127
|
)
|
|||||
|
Net cash used in financing activities
|
(401
|
)
|
(592
|
)
|
(738
|
)
|
|||||
|
Net change in cash and cash equivalents and restricted cash
|
$
|
(2
|
)
|
$
|
(247
|
)
|
$
|
251
|
|
||
|
•
|
a decrease in 2018 tax payments of $159 million primarily due to the impact of TCJA; partially offset by;
|
|
•
|
lower operating income in 2018 as compared to 2017; and
|
|
•
|
timing of movements in our working capital accounts.
|
|
•
|
a decrease in 2017 tax payments associated with the realization of a $62 million deferred tax benefit in 2017 and a $91 million tax payment in 2016 related to the Focus Sale; and
|
|
•
|
improved operating performance in 2017; partially offset by;
|
|
•
|
$54 million of proceeds received in the third quarter of 2016 from the termination of interest swap agreements.
|
|
•
|
$160 million
decrease in cash paid for business acquisitions; partially offset by;
|
|
•
|
$131 million
increase in capital expenditures.
|
|
•
|
$442 million increase in cash paid for business acquisitions; and
|
|
•
|
$294 million decrease in proceeds from the disposition of businesses, primarily a result of the Focus Sale in 2016; partially offset by;
|
|
•
|
$41 million decrease in capital expenditures
|
|
•
|
$143 million
decrease in cash paid for repurchases of our common stock (see "Share Repurchases" for further details) in 2018; and
|
|
•
|
$124 million in net borrowings (proceeds from borrowings less repayments of debt) in 2018, compared to $23 million in net borrowings in 2017; partially offset by;
|
|
•
|
$31 million
decrease in proceeds from the exercise of stock options, which was a result of a decrease in the volume of stock options exercised over the past year.
|
|
•
|
$125 million decrease in repurchases of our common stock (see "Share Repurchases" for further details) in 2017;
|
|
•
|
$80 million increase in bank overdrafts, which are generally settled in cash the following business day;
|
|
•
|
$57 million increase in proceeds from the exercise of stock options, which was a result of an increase in the volume of stock options exercised compared to the prior year; and
|
|
•
|
$43 million of payments related to the retirement of debt in 2016; partially offset by;
|
|
•
|
$23 million in net borrowings (proceeds from borrowings less repayments of debt) in 2017, compared to $141 million in net borrowings in 2016; and
|
|
•
|
$24 million increase in dividends paid.
|
|
Payments due by period
|
||||||||||||||||||||
|
Contractual Obligations
|
Total
|
Less than
1 year
|
1-3 years
|
4-5 years
|
After 5 years
|
|||||||||||||||
|
Outstanding debt
|
$
|
3,936
|
|
$
|
460
|
|
$
|
1,350
|
|
$
|
—
|
|
$
|
2,126
|
|
|||||
|
Capital lease obligations
|
36
|
|
4
|
|
6
|
|
4
|
|
22
|
|
||||||||||
|
Interest payments on outstanding debt
|
1,476
|
|
169
|
|
269
|
|
214
|
|
824
|
|
||||||||||
|
Operating leases
|
691
|
|
181
|
|
249
|
|
139
|
|
122
|
|
||||||||||
|
Purchase obligations
|
1,831
|
|
300
|
|
546
|
|
440
|
|
545
|
|
||||||||||
|
Merger consideration obligation
|
14
|
|
9
|
|
5
|
|
—
|
|
—
|
|
||||||||||
|
Total contractual obligations
|
$
|
7,984
|
|
$
|
1,123
|
|
$
|
2,425
|
|
$
|
797
|
|
$
|
3,639
|
|
|||||
|
/s/
|
PricewaterhouseCoopers LLP
|
|
Florham Park, New Jersey
|
|
|
February 21, 2019
|
|
|
2018
|
2017
|
||||||
|
Assets
|
|
|
|
|
|||
|
Current assets:
|
|
|
|
|
|||
|
Cash and cash equivalents
|
$
|
135
|
|
$
|
137
|
|
|
|
Accounts receivable, net of allowance for doubtful accounts of $15 and $13 as of December 31, 2018 and 2017, respectively
|
1,012
|
|
924
|
|
|||
|
Inventories
|
99
|
|
95
|
|
|||
|
Prepaid expenses and other current assets
|
144
|
|
150
|
|
|||
|
Total current assets
|
1,390
|
|
1,306
|
|
|||
|
Property, plant and equipment, net
|
1,288
|
|
1,145
|
|
|||
|
Goodwill
|
6,563
|
|
6,335
|
|
|||
|
Intangible assets, net
|
1,207
|
|
1,119
|
|
|||
|
Investments in equity method investees
|
436
|
|
462
|
|
|||
|
Other assets
|
119
|
|
136
|
|
|||
|
Total assets
|
$
|
11,003
|
|
$
|
10,503
|
|
|
|
Liabilities and Stockholders’ Equity
|
|
|
|
|
|||
|
Current liabilities:
|
|
|
|
|
|||
|
Accounts payable and accrued expenses
|
$
|
1,021
|
|
$
|
1,021
|
|
|
|
Current portion of long-term debt
|
464
|
|
36
|
|
|||
|
Total current liabilities
|
1,485
|
|
1,057
|
|
|||
|
Long-term debt
|
3,429
|
|
3,748
|
|
|||
|
Other liabilities
|
745
|
|
663
|
|
|||
|
Commitments and contingencies
|
|
|
|
|
|||
|
Redeemable noncontrolling interest
|
77
|
|
80
|
|
|||
|
Stockholders’ equity:
|
|
|
|
|
|||
|
Quest Diagnostics stockholders’ equity:
|
|
|
|
|
|||
|
Common stock, par value $0.01 per share; 600 shares authorized as of both December 31, 2018 and 2017; 217 and 216 shares issued as of December 31, 2018 and 2017, respectively
|
2
|
|
2
|
|
|||
|
Additional paid-in capital
|
2,667
|
|
2,612
|
|
|||
|
Retained earnings
|
7,602
|
|
7,138
|
|
|||
|
Accumulated other comprehensive loss
|
(59
|
)
|
(48
|
)
|
|||
|
Treasury stock, at cost; 82 shares and 81 shares as of December 31, 2018 and 2017, respectively
|
(4,996
|
)
|
(4,783
|
)
|
|||
|
Total Quest Diagnostics stockholders’ equity
|
5,216
|
|
4,921
|
|
|||
|
Noncontrolling interests
|
51
|
|
34
|
|
|||
|
Total stockholders’ equity
|
5,267
|
|
4,955
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
11,003
|
|
$
|
10,503
|
|
|
|
2018
|
2017
|
2016
|
|||||||||
|
Net revenues
|
$
|
7,531
|
|
$
|
7,402
|
|
$
|
7,214
|
|
||
|
Operating costs and expenses and other operating income:
|
|
|
|
|
|
|
|||||
|
Cost of services
|
4,926
|
|
4,719
|
|
4,616
|
|
|||||
|
Selling, general and administrative
|
1,424
|
|
1,443
|
|
1,380
|
|
|||||
|
Amortization of intangible assets
|
90
|
|
74
|
|
72
|
|
|||||
|
Loss (gain) on disposition of business
|
4
|
|
—
|
|
(118
|
)
|
|||||
|
Other operating (income) expense, net
|
(14
|
)
|
1
|
|
(13
|
)
|
|||||
|
Total operating costs and expenses, net
|
6,430
|
|
6,237
|
|
5,937
|
|
|||||
|
Operating income
|
1,101
|
|
1,165
|
|
1,277
|
|
|||||
|
Other (expense) income:
|
|
|
|
|
|
|
|||||
|
Interest expense, net
|
(167
|
)
|
(151
|
)
|
(143
|
)
|
|||||
|
Other (expense) income, net
|
(8
|
)
|
16
|
|
(48
|
)
|
|||||
|
Total non-operating expenses, net
|
(175
|
)
|
(135
|
)
|
(191
|
)
|
|||||
|
Income before income taxes and equity in earnings of equity method investees
|
926
|
|
1,030
|
|
1,086
|
|
|||||
|
Income tax expense
|
(182
|
)
|
(241
|
)
|
(429
|
)
|
|||||
|
Equity in earnings of equity method investees, net of taxes
|
44
|
|
35
|
|
39
|
|
|||||
|
Net income
|
788
|
|
824
|
|
696
|
|
|||||
|
Less: Net income attributable to noncontrolling interests
|
52
|
|
52
|
|
51
|
|
|||||
|
Net income attributable to Quest Diagnostics
|
$
|
736
|
|
$
|
772
|
|
$
|
645
|
|
||
|
Earnings per share attributable to Quest Diagnostics’ common stockholders:
|
|
|
|
|
|||||||
|
Basic
|
$
|
5.39
|
|
$
|
5.63
|
|
$
|
4.58
|
|
||
|
Diluted
|
$
|
5.29
|
|
$
|
5.50
|
|
$
|
4.51
|
|
||
|
2018
|
2017
|
2016
|
|||||||||
|
Net income
|
$
|
788
|
|
$
|
824
|
|
$
|
696
|
|
||
|
Other comprehensive (loss) income:
|
|||||||||||
|
Currency translation
|
(11
|
)
|
20
|
|
(34
|
)
|
|||||
|
Investment adjustments, net of taxes
|
—
|
|
3
|
|
(2
|
)
|
|||||
|
Net deferred loss on cash flow hedges, net of tax
|
2
|
|
1
|
|
2
|
|
|||||
|
Other comprehensive (loss) income
|
(9
|
)
|
24
|
|
(34
|
)
|
|||||
|
Comprehensive income
|
779
|
|
848
|
|
662
|
|
|||||
|
Less: Comprehensive income attributable to noncontrolling interests
|
52
|
|
52
|
|
51
|
|
|||||
|
Comprehensive income attributable to Quest Diagnostics
|
$
|
727
|
|
$
|
796
|
|
$
|
611
|
|
||
|
2018
|
2017
|
2016
|
|||||||||
|
Cash flows from operating activities:
|
|
|
|
|
|||||||
|
Net income
|
$
|
788
|
|
$
|
824
|
|
$
|
696
|
|
||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
|
|
|
|
|
|
|||||
|
Depreciation and amortization
|
309
|
|
270
|
|
249
|
|
|||||
|
Provision for doubtful accounts
|
6
|
|
8
|
|
7
|
|
|||||
|
Deferred income tax provision
|
73
|
|
9
|
|
37
|
|
|||||
|
Stock-based compensation expense
|
61
|
|
79
|
|
69
|
|
|||||
|
Loss (gain) on disposition of business
|
4
|
|
—
|
|
(118
|
)
|
|||||
|
Payment of debt extinguishment costs
|
—
|
|
—
|
|
43
|
|
|||||
|
Other, net
|
8
|
|
(6
|
)
|
(2
|
)
|
|||||
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|||||
|
Accounts receivable
|
(65
|
)
|
9
|
|
(42
|
)
|
|||||
|
Accounts payable and accrued expenses
|
(19
|
)
|
(8
|
)
|
56
|
|
|||||
|
Income taxes payable
|
4
|
|
16
|
|
42
|
|
|||||
|
Termination of interest rate swap agreements
|
—
|
|
—
|
|
54
|
|
|||||
|
Other assets and liabilities, net
|
31
|
|
(26
|
)
|
25
|
|
|||||
|
Net cash provided by operating activities
|
1,200
|
|
1,175
|
|
1,116
|
|
|||||
|
Cash flows from investing activities:
|
|
|
|
|
|||||||
|
Business acquisitions, net of cash acquired
|
(421
|
)
|
(581
|
)
|
(139
|
)
|
|||||
|
Proceeds from disposition of business
|
2
|
|
1
|
|
295
|
|
|||||
|
Capital expenditures
|
(383
|
)
|
(252
|
)
|
(293
|
)
|
|||||
|
Decrease in investments and other assets
|
1
|
|
2
|
|
10
|
|
|||||
|
Net cash used in investing activities
|
(801
|
)
|
(830
|
)
|
(127
|
)
|
|||||
|
Cash flows from financing activities:
|
|
|
|
|
|||||||
|
Proceeds from borrowings
|
2,090
|
|
205
|
|
1,869
|
|
|||||
|
Repayments of debt
|
(1,966
|
)
|
(182
|
)
|
(1,728
|
)
|
|||||
|
Purchases of treasury stock
|
(322
|
)
|
(465
|
)
|
(590
|
)
|
|||||
|
Exercise of stock options
|
99
|
|
130
|
|
73
|
|
|||||
|
Employee payroll tax withholdings on stock issued under stock-based compensation plans
|
(21
|
)
|
(23
|
)
|
(10
|
)
|
|||||
|
Dividends paid
|
(266
|
)
|
(247
|
)
|
(223
|
)
|
|||||
|
Distributions to noncontrolling interest partners
|
(54
|
)
|
(51
|
)
|
(41
|
)
|
|||||
|
Payment of debt extinguishment costs
|
—
|
|
—
|
|
(43
|
)
|
|||||
|
Contributions from noncontrolling interest partners
|
16
|
|
4
|
|
—
|
|
|||||
|
Other financing activities, net
|
23
|
|
37
|
|
(45
|
)
|
|||||
|
Net cash used in financing activities
|
(401
|
)
|
(592
|
)
|
(738
|
)
|
|||||
|
Net change in cash and cash equivalents and restricted cash
|
(2
|
)
|
(247
|
)
|
251
|
|
|||||
|
Cash and cash equivalents and restricted cash, beginning of year
|
137
|
|
384
|
|
133
|
|
|||||
|
Cash and cash equivalents and restricted cash, end of year
|
$
|
135
|
|
$
|
137
|
|
$
|
384
|
|
||
|
Cash and cash equivalents
|
$
|
135
|
|
$
|
137
|
|
$
|
359
|
|
||
|
Restricted cash
|
—
|
|
—
|
|
25
|
|
|||||
|
Cash and cash equivalents and restricted cash, end of year
|
$
|
135
|
|
$
|
137
|
|
$
|
384
|
|
||
|
Quest Diagnostics Stockholders’ Equity
|
||||||||||||||||||||||||||||
|
Shares of
Common Stock Out-
standing
|
Common
Stock
|
Additional
Paid-In
Capital
|
Retained
Earnings
|
Accumulated
Other
Comprehensive
Loss
|
Treasury
Stock, at
Cost
|
Non-
controlling
Interests
|
Total Stock-holders’
Equity
|
Redeemable Non-controlling Interest
|
||||||||||||||||||||
|
Balance, December 31, 2015
|
143
|
|
$
|
2
|
|
$
|
2,481
|
|
$
|
6,199
|
|
$
|
(38
|
)
|
$
|
(3,960
|
)
|
$
|
29
|
|
$
|
4,713
|
|
$
|
70
|
|
||
|
Net income
|
|
|
|
|
|
|
645
|
|
|
|
|
|
44
|
|
689
|
|
7
|
|
||||||||||
|
Other comprehensive loss, net of tax
|
|
|
|
|
|
|
|
|
(34
|
)
|
|
|
|
|
(34
|
)
|
||||||||||||
|
Dividends declared
|
|
|
|
|
|
|
(231
|
)
|
|
|
|
|
|
|
(231
|
)
|
||||||||||||
|
Distributions to noncontrolling interest partners
|
|
|
|
|
|
|
|
|
|
|
|
|
(41
|
)
|
(41
|
)
|
|
|
||||||||||
|
Issuance of common stock under benefit plans
|
|
|
|
|
7
|
|
|
|
|
|
15
|
|
|
|
22
|
|
||||||||||||
|
Stock-based compensation expense
|
|
|
|
|
65
|
|
|
|
|
|
4
|
|
|
|
69
|
|
||||||||||||
|
Exercise of stock options
|
1
|
|
|
|
2
|
|
|
|
|
|
71
|
|
|
|
73
|
|
||||||||||||
|
Shares to cover employee payroll tax withholdings on stock issued under stock-based compensation plans
|
|
|
|
|
(10
|
)
|
|
|
|
|
|
|
|
|
(10
|
)
|
||||||||||||
|
Purchases of treasury stock
|
(7
|
)
|
|
|
|
|
|
|
|
|
(590
|
)
|
|
|
(590
|
)
|
||||||||||||
|
Balance, December 31, 2016
|
137
|
|
$
|
2
|
|
$
|
2,545
|
|
$
|
6,613
|
|
$
|
(72
|
)
|
$
|
(4,460
|
)
|
$
|
32
|
|
$
|
4,660
|
|
$
|
77
|
|
||
|
Net income
|
|
|
|
|
|
|
772
|
|
|
|
|
|
45
|
|
817
|
|
7
|
|
||||||||||
|
Other comprehensive income, net of tax
|
|
|
|
|
|
|
|
|
24
|
|
|
|
|
|
24
|
|
||||||||||||
|
Dividends declared
|
|
|
|
|
|
|
(247
|
)
|
|
|
|
|
|
|
(247
|
)
|
||||||||||||
|
Distributions to noncontrolling interest partners
|
|
|
|
|
|
|
|
|
|
|
|
|
(47
|
)
|
(47
|
)
|
(4
|
)
|
||||||||||
|
Issuance of common stock under benefit plans
|
|
|
|
|
11
|
|
|
|
|
|
12
|
|
|
|
23
|
|
||||||||||||
|
Stock-based compensation expense
|
|
|
|
|
75
|
|
|
|
|
|
4
|
|
|
|
79
|
|
||||||||||||
|
Exercise of stock options
|
3
|
|
|
|
4
|
|
|
|
|
|
126
|
|
|
|
130
|
|
||||||||||||
|
Shares to cover employee payroll tax withholdings on stock issued under stock-based compensation plans
|
|
|
|
|
(23
|
)
|
|
|
|
|
|
|
|
|
(23
|
)
|
||||||||||||
|
Purchases of treasury stock
|
(5
|
)
|
|
|
|
|
|
|
|
|
(465
|
)
|
|
|
(465
|
)
|
||||||||||||
|
Contributions from noncontrolling interest partners
|
|
|
4
|
|
4
|
|
|
|
||||||||||||||||||||
|
Balance, December 31, 2017
|
135
|
|
$
|
2
|
|
$
|
2,612
|
|
$
|
7,138
|
|
$
|
(48
|
)
|
$
|
(4,783
|
)
|
$
|
34
|
|
$
|
4,955
|
|
$
|
80
|
|
||
|
Net income
|
|
|
|
|
|
|
736
|
|
|
|
|
|
45
|
|
781
|
|
7
|
|
||||||||||
|
Other comprehensive loss, net of tax
|
|
|
|
|
|
|
|
|
(9
|
)
|
|
|
|
|
(9
|
)
|
||||||||||||
|
Dividends declared
|
|
|
|
|
|
|
(274
|
)
|
|
|
|
|
|
|
(274
|
)
|
||||||||||||
|
Distributions to noncontrolling interest partners
|
|
|
|
|
|
|
|
|
|
|
|
|
(44
|
)
|
(44
|
)
|
(10
|
)
|
||||||||||
|
Issuance of common stock under benefit plans
|
|
|
|
|
14
|
|
|
|
|
|
14
|
|
|
|
28
|
|
||||||||||||
|
Stock-based compensation expense
|
|
|
|
|
56
|
|
|
|
|
|
5
|
|
|
|
61
|
|
||||||||||||
|
Exercise of stock options
|
3
|
|
|
|
6
|
|
|
|
|
|
93
|
|
|
|
99
|
|
||||||||||||
|
Shares to cover employee payroll tax withholdings on stock issued under stock-based compensation plans
|
|
|
|
|
(21
|
)
|
|
|
|
|
|
|
|
|
(21
|
)
|
||||||||||||
|
Purchases of treasury stock
|
(3
|
)
|
|
|
|
|
|
|
|
|
(325
|
)
|
|
|
(325
|
)
|
||||||||||||
|
Contributions from noncontrolling interest partners
|
|
|
|
|
|
|
16
|
|
16
|
|
|
|||||||||||||||||
|
Reclassification of stranded tax effects resulting from enactment of the Tax Cuts and Jobs Act
|
|
|
|
2
|
|
(2
|
)
|
|
|
|
—
|
|
|
|||||||||||||||
|
Balance, December 31, 2018
|
135
|
|
$
|
2
|
|
$
|
2,667
|
|
$
|
7,602
|
|
$
|
(59
|
)
|
$
|
(4,996
|
)
|
$
|
51
|
|
$
|
5,267
|
|
$
|
77
|
|
||
|
•
|
buildings and improvements, ranging up to
thirty-one and a half
years;
|
|
•
|
laboratory equipment and furniture and fixtures, ranging from
five
to
twelve
years;
|
|
•
|
leasehold improvements, the lesser of the useful life of the improvement or the remaining life of the building or lease, as applicable; and
|
|
•
|
computer software developed or obtained for internal use,
five
to
ten
years.
|
|
•
|
Equity investments with readily determinable fair values which are comprised of participant-directed investments of deferred employee compensation and related Company matching contributions held in trusts pursuant to the Company's supplemental deferred compensation plans (see Note 17). These investments are measured at fair value with both realized and unrealized gains and losses recorded in current earnings as a component of non-operating expense within other (expense) income, net in the consolidated statement of operations. For the years ended
December 31, 2018
,
2017
and
2016
, gains and (losses) from these equity securities totaled
$(2) million
,
$8 million
, and
$3 million
, respectively. The carrying value of these investments, which are included in other assets on the consolidated balance sheet, were
$53 million
and
$58 million
at
December 31, 2018
and
2017
, respectively.
|
|
•
|
Equity investments that do not have readily determinable fair values which consist of investments in preferred and common shares of privately held companies. These investments are measured at cost minus impairment, if any, plus or minus changes resulting from observable price changes. The Company regularly evaluates these equity investments to determine if there are any indicators that the investment is impaired; no impairment charges were recognized related to these investments for the years ended
December 31, 2018
,
2017
, and
2016
. The carrying value of these investments, which are included in other assets on the consolidated balance sheet, were
$10 million
and
$9 million
at
December 31, 2018
and
2017
, respectively.
|
|
•
|
Foreign currency translation adjustments;
|
|
•
|
Net deferred loss on cash flow hedges, which represents deferred losses, net of tax on interest rate related derivative financial instruments designated as cash flow hedges, net of amounts reclassified to interest expense (see Note 16).
|
|
As Previously Reported
|
Adjustment for New Accounting Standard on Revenue Recognition
|
As Restated
|
|||||||||
|
Year Ended December 31, 2017
|
|||||||||||
|
Consolidated Statements of Operations:
|
|||||||||||
|
Net revenues
|
$
|
7,709
|
|
$
|
(307
|
)
|
$
|
7,402
|
|
||
|
Selling, general and administrative expenses
|
$
|
1,750
|
|
$
|
(307
|
)
|
$
|
1,443
|
|
||
|
Net income attributable to Quest Diagnostics
|
$
|
772
|
|
$
|
—
|
|
$
|
772
|
|
||
|
Consolidated Statements of Cash Flows:
|
|||||||||||
|
Provision for doubtful accounts
|
$
|
315
|
|
$
|
(307
|
)
|
$
|
8
|
|
||
|
Changes in operating assets and liabilities:
|
|||||||||||
|
Accounts receivable
|
$
|
(298
|
)
|
$
|
307
|
|
$
|
9
|
|
||
|
As Previously Reported
|
Adjustment for New Accounting Standard on Revenue Recognition
|
As Restated
|
|||||||||
|
Year Ended December 31, 2016
|
|||||||||||
|
Consolidated Statements of Operations:
|
|||||||||||
|
Net revenues
|
$
|
7,515
|
|
$
|
(301
|
)
|
$
|
7,214
|
|
||
|
Selling, general and administrative expenses
|
$
|
1,681
|
|
$
|
(301
|
)
|
$
|
1,380
|
|
||
|
Net income attributable to Quest Diagnostics
|
$
|
645
|
|
$
|
—
|
|
$
|
645
|
|
||
|
Consolidated Statements of Cash Flows:
|
|||||||||||
|
Provision for doubtful accounts
|
$
|
308
|
|
$
|
(301
|
)
|
$
|
7
|
|
||
|
Changes in operating assets and liabilities:
|
|||||||||||
|
Accounts receivable
|
$
|
(343
|
)
|
$
|
301
|
|
$
|
(42
|
)
|
||
|
Balance, December 31, 2017
|
|||||||||||
|
Consolidated Balance Sheets:
|
|||||||||||
|
Accounts receivable
|
$
|
1,193
|
|
$
|
(256
|
)
|
$
|
937
|
|
||
|
Allowance for doubtful accounts
|
$
|
269
|
|
$
|
(256
|
)
|
$
|
13
|
|
||
|
Accounts receivable, net of allowance for doubtful accounts
|
$
|
924
|
|
$
|
—
|
|
$
|
924
|
|
||
|
•
|
Amounts generally described as restricted cash and restricted cash equivalents are now presented with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. As a result of adoption, there was no impact to cash flows from operating, investing or financing activities for the year ended
December 31, 2018
. For the year ended December 31, 2016, proceeds from the disposition of business within cash flows from investing activities now includes
$25 million
of proceeds associated with the sale of the Focus Diagnostics products business which were initially held in escrow and included in restricted cash. The receipt of the escrow proceeds, which was previously reported as a cash inflow from investing activities for the year ended
December 31, 2017
, is no longer presented within the net change in cash and cash equivalents and restricted cash for 2017 as it is included in the beginning-of-period balance of restricted cash. Refer to Note 7 to the consolidated financial statements for more information regarding the disposition of the Focus Diagnostics products business.
|
|
•
|
The classification of how certain cash receipts and payments are presented within the statement of cash flows has been clarified. As a result, the payment of debt extinguishment costs and the repayment of original issue debt discount of
$43 million
and
$4 million
, respectively, for the year ended December 31, 2016 are now presented as a financing cash outflow in the consolidated statement of cash flows for the year ended
|
|
Twelve Months Ended December 31,
|
|||||||||
|
2018
|
2017
|
2016
|
|||||||
|
Healthcare insurers:
|
|||||||||
|
Fee-for-service
|
32
|
%
|
34
|
%
|
34
|
%
|
|||
|
Capitated
|
3
|
|
3
|
|
4
|
|
|||
|
Total healthcare insurers
|
35
|
|
37
|
|
38
|
|
|||
|
Government payers
|
16
|
|
17
|
|
17
|
|
|||
|
Client payers
|
32
|
|
30
|
|
29
|
|
|||
|
Patient
|
13
|
|
12
|
|
11
|
|
|||
|
Total DIS
|
96
|
|
96
|
|
95
|
|
|||
|
DS
|
4
|
|
4
|
|
5
|
|
|||
|
Net revenues
|
100
|
%
|
100
|
%
|
100
|
%
|
|||
|
|
2018
|
2017
|
2016
|
||||||||
|
Amounts attributable to Quest Diagnostics’ common stockholders:
|
|
|
|
|
|||||||
|
Net income attributable to Quest Diagnostics
|
$
|
736
|
|
$
|
772
|
|
$
|
645
|
|
||
|
Less: Earnings allocated to participating securities
|
3
|
|
3
|
|
3
|
|
|||||
|
Earnings available to Quest Diagnostics’ common stockholders – basic and diluted
|
$
|
733
|
|
$
|
769
|
|
$
|
642
|
|
||
|
Weighted average common shares outstanding – basic
|
136
|
|
137
|
|
140
|
|
|||||
|
Effect of dilutive securities:
|
|
|
|
|
|||||||
|
Stock options and performance share units
|
3
|
|
3
|
|
2
|
|
|||||
|
Weighted average common shares outstanding – diluted
|
139
|
|
140
|
|
142
|
|
|||||
|
Earnings per share attributable to Quest Diagnostics’ common stockholders:
|
|
|
|
|
|||||||
|
Basic
|
$
|
5.39
|
|
$
|
5.63
|
|
$
|
4.58
|
|
||
|
Diluted
|
$
|
5.29
|
|
$
|
5.50
|
|
$
|
4.51
|
|
||
|
2018
|
2017
|
2016
|
||||||
|
Stock options
|
2
|
|
2
|
|
1
|
|
||
|
2018
|
2017
|
2016
|
|||||||||
|
Employee separation costs
|
$
|
45
|
|
$
|
29
|
|
$
|
9
|
|
||
|
Facility-related costs
|
4
|
|
1
|
|
2
|
|
|||||
|
Asset impairment charges
|
2
|
|
3
|
|
—
|
|
|||||
|
Total restructuring charges
|
$
|
51
|
|
$
|
33
|
|
$
|
11
|
|
||
|
Employee Separation Costs
|
Facility-Related Costs
|
Total
|
|||||||||
|
Balance, December 31, 2016
|
$
|
6
|
|
$
|
3
|
|
$
|
9
|
|
||
|
Income statement expense
|
29
|
|
1
|
|
30
|
|
|||||
|
Cash payments
|
(14
|
)
|
(3
|
)
|
(17
|
)
|
|||||
|
Balance, December 31, 2017
|
21
|
|
1
|
|
22
|
|
|||||
|
Income statement expense
|
45
|
|
4
|
|
49
|
|
|||||
|
Cash payments
|
(29
|
)
|
(4
|
)
|
(33
|
)
|
|||||
|
Balance, December 31, 2018
|
$
|
37
|
|
$
|
1
|
|
$
|
38
|
|
||
|
Basis of Fair Value Measurements
|
|||||||||||||||
|
Total
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
December 31, 2018
|
|||||||||||||||
|
Assets:
|
|
|
|
|
|
|
|
|
|||||||
|
Trading securities
|
$
|
53
|
|
$
|
53
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Cash surrender value of life insurance policies
|
34
|
|
—
|
|
34
|
|
—
|
|
|||||||
|
Total
|
$
|
87
|
|
$
|
53
|
|
$
|
34
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|
|
|
|
|
|
|
|
|||||||
|
Deferred compensation liabilities
|
$
|
96
|
|
$
|
—
|
|
$
|
96
|
|
$
|
—
|
|
|||
|
Interest rate swaps
|
93
|
|
—
|
|
93
|
|
—
|
|
|||||||
|
Contingent consideration
|
14
|
|
—
|
|
—
|
|
14
|
|
|||||||
|
Total
|
$
|
203
|
|
$
|
—
|
|
$
|
189
|
|
$
|
14
|
|
|||
|
December 31, 2017
|
|||||||||||||||
|
Assets:
|
|
|
|
|
|
||||||||||
|
Trading securities
|
$
|
58
|
|
$
|
58
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Cash surrender value of life insurance policies
|
37
|
|
—
|
|
37
|
|
—
|
|
|||||||
|
Equity securities
|
2
|
|
2
|
|
—
|
|
—
|
|
|||||||
|
Total
|
$
|
97
|
|
$
|
60
|
|
$
|
37
|
|
$
|
—
|
|
|||
|
Liabilities:
|
|
|
|
|
|
|
|
|
|||||||
|
Deferred compensation liabilities
|
$
|
103
|
|
$
|
—
|
|
$
|
103
|
|
$
|
—
|
|
|||
|
Interest rate swaps
|
89
|
|
—
|
|
89
|
|
—
|
|
|||||||
|
Contingent consideration
|
7
|
|
—
|
|
—
|
|
7
|
|
|||||||
|
Total
|
$
|
199
|
|
$
|
—
|
|
$
|
192
|
|
$
|
7
|
|
|||
|
Contingent Consideration
|
|||
|
Balance, December 31, 2016
|
$
|
3
|
|
|
Purchases, additions and issuances
|
6
|
|
|
|
Settlements
|
(2
|
)
|
|
|
Balance, December 31, 2017
|
7
|
|
|
|
Purchases, additions and issuances
|
19
|
|
|
|
Settlements
|
(1
|
)
|
|
|
Total (gains)/losses included in earnings - realized/unrealized
|
(11
|
)
|
|
|
Balance, December 31, 2018
|
$
|
14
|
|
|
2018
|
2017
|
2016
|
|||||||||
|
Current:
|
|||||||||||
|
Federal
|
$
|
82
|
|
$
|
226
|
|
$
|
346
|
|
||
|
State and local
|
26
|
|
5
|
|
45
|
|
|||||
|
Foreign
|
1
|
|
1
|
|
1
|
|
|||||
|
Deferred:
|
|||||||||||
|
Federal
|
66
|
|
(20
|
)
|
33
|
|
|||||
|
State and local
|
10
|
|
27
|
|
4
|
|
|||||
|
Foreign
|
(3
|
)
|
2
|
|
—
|
|
|||||
|
Total
|
$
|
182
|
|
$
|
241
|
|
$
|
429
|
|
||
|
2018
|
2017
|
2016
|
||||||
|
Tax provision at statutory rate
|
21.0
|
%
|
35.0
|
%
|
35.0
|
%
|
||
|
State and local income taxes, net of federal benefit
|
4.7
|
|
3.8
|
|
3.3
|
|
||
|
Gains and losses on book and tax basis difference
|
—
|
|
(0.1
|
)
|
3.3
|
|
||
|
Impact of noncontrolling interests
|
(1.4
|
)
|
(1.9
|
)
|
(1.8
|
)
|
||
|
Excess tax benefits on stock-based compensation arrangements
|
(1.9
|
)
|
(3.6
|
)
|
(0.8
|
)
|
||
|
Return to provision true-ups
|
(1.4
|
)
|
(2.0
|
)
|
(0.8
|
)
|
||
|
Impact of TCJA enactment
|
0.1
|
|
(10.4
|
)
|
—
|
|
||
|
Change in accounting method
|
(1.6
|
)
|
—
|
|
—
|
|
||
|
Other, net
|
0.2
|
|
2.6
|
|
1.3
|
|
||
|
Effective tax rate
|
19.7
|
%
|
23.4
|
%
|
39.5
|
%
|
||
|
2018
|
2017
|
||||||
|
Non-current deferred tax assets (liabilities):
|
|||||||
|
Accounts receivable reserves
|
$
|
66
|
|
$
|
63
|
|
|
|
Liabilities not currently deductible
|
137
|
|
129
|
|
|||
|
Stock-based compensation
|
38
|
|
41
|
|
|||
|
Basis differences in investments, joint ventures and subsidiaries
|
(80
|
)
|
(79
|
)
|
|||
|
Net operating loss carryforwards, net of valuation allowance
|
80
|
|
83
|
|
|||
|
Depreciation and amortization
|
(484
|
)
|
(403
|
)
|
|||
|
Total non-current deferred tax liabilities, net
|
$
|
(243
|
)
|
$
|
(166
|
)
|
|
|
2018
|
2017
|
2016
|
|||||||||
|
Balance, beginning of year
|
$
|
115
|
|
$
|
98
|
|
$
|
91
|
|
||
|
Additions:
|
|||||||||||
|
For tax positions of current year
|
2
|
|
5
|
|
3
|
|
|||||
|
For tax positions of prior years
|
11
|
|
23
|
|
12
|
|
|||||
|
Reductions:
|
|||||||||||
|
Changes in judgment
|
(6
|
)
|
(2
|
)
|
(1
|
)
|
|||||
|
Expirations of statutes of limitations
|
(15
|
)
|
(6
|
)
|
(7
|
)
|
|||||
|
Settlements
|
—
|
|
(3
|
)
|
—
|
|
|||||
|
Balance, end of year
|
$
|
107
|
|
$
|
115
|
|
$
|
98
|
|
||
|
2018
|
2017
|
2016
|
|||||||||
|
Depreciation expense
|
$
|
219
|
|
$
|
196
|
|
$
|
177
|
|
||
|
Amortization expense
|
90
|
|
74
|
|
72
|
|
|||||
|
Depreciation and amortization expense
|
$
|
309
|
|
$
|
270
|
|
$
|
249
|
|
||
|
Interest expense
|
$
|
(169
|
)
|
$
|
(153
|
)
|
$
|
(144
|
)
|
||
|
Interest income
|
2
|
|
2
|
|
1
|
|
|||||
|
Interest expense, net
|
$
|
(167
|
)
|
$
|
(151
|
)
|
$
|
(143
|
)
|
||
|
Interest paid
|
$
|
174
|
|
$
|
159
|
|
$
|
148
|
|
||
|
Income taxes paid
|
$
|
84
|
|
$
|
243
|
|
$
|
361
|
|
||
|
Assets acquired under capital leases
|
$
|
1
|
|
$
|
7
|
|
$
|
—
|
|
||
|
Accounts payable associated with capital expenditures
|
$
|
11
|
|
$
|
26
|
|
$
|
9
|
|
||
|
Accounts payable associated with purchases of treasury stock
|
$
|
3
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Dividends payable
|
$
|
71
|
|
$
|
61
|
|
$
|
62
|
|
||
|
Businesses acquired:
|
|
|
|
|
|||||||
|
Fair value of assets acquired
|
$
|
453
|
|
$
|
657
|
|
$
|
139
|
|
||
|
Fair value of liabilities assumed
|
7
|
|
58
|
|
—
|
|
|||||
|
Fair value of net assets acquired
|
446
|
|
599
|
|
139
|
|
|||||
|
Merger consideration paid (payable), net
|
(19
|
)
|
(6
|
)
|
—
|
|
|||||
|
Cash paid for business acquisitions
|
427
|
|
593
|
|
139
|
|
|||||
|
Less: Cash acquired
|
6
|
|
12
|
|
—
|
|
|||||
|
Business acquisitions, net of cash acquired
|
$
|
421
|
|
$
|
581
|
|
$
|
139
|
|
||
|
2018
|
2017
|
||||||
|
Land
|
$
|
29
|
|
$
|
29
|
|
|
|
Buildings and improvements
|
429
|
|
430
|
|
|||
|
Laboratory equipment and furniture and fixtures
|
1,691
|
|
1,594
|
|
|||
|
Leasehold improvements
|
606
|
|
544
|
|
|||
|
Computer software developed or obtained for internal use
|
1,013
|
|
934
|
|
|||
|
Construction-in-progress
|
202
|
|
140
|
|
|||
|
3,970
|
|
3,671
|
|
||||
|
Less: Accumulated depreciation and amortization
|
(2,682
|
)
|
(2,526
|
)
|
|||
|
Total
|
$
|
1,288
|
|
$
|
1,145
|
|
|
|
2018
|
2017
|
||||||
|
Balance, beginning of year
|
$
|
6,335
|
|
$
|
6,000
|
|
|
|
Goodwill acquired during the year
|
228
|
|
335
|
|
|||
|
Balance, end of year
|
$
|
6,563
|
|
$
|
6,335
|
|
|
|
Weighted
Average
Amortization
Period (in years)
|
December 31, 2018
|
December 31, 2017
|
|||||||||||||||||||||||
|
Cost
|
Accumulated
Amortization
|
Net
|
Cost
|
Accumulated
Amortization
|
Net
|
||||||||||||||||||||
|
Amortizing intangible assets:
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
Customer-related
|
18
|
$
|
1,355
|
|
$
|
(478
|
)
|
$
|
877
|
|
$
|
1,210
|
|
$
|
(404
|
)
|
$
|
806
|
|
||||||
|
Non-compete agreements
|
8
|
3
|
|
(2
|
)
|
1
|
|
7
|
|
(5
|
)
|
2
|
|
||||||||||||
|
Technology
|
17
|
104
|
|
(50
|
)
|
54
|
|
95
|
|
(45
|
)
|
50
|
|
||||||||||||
|
Other
|
9
|
114
|
|
(75
|
)
|
39
|
|
105
|
|
(80
|
)
|
25
|
|
||||||||||||
|
Total
|
17
|
1,576
|
|
(605
|
)
|
971
|
|
1,417
|
|
(534
|
)
|
883
|
|
||||||||||||
|
Intangible assets not subject to amortization:
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
Trade names
|
|
235
|
|
—
|
|
235
|
|
235
|
|
—
|
|
235
|
|
||||||||||||
|
Other
|
|
1
|
|
—
|
|
1
|
|
1
|
|
—
|
|
1
|
|
||||||||||||
|
Total intangible assets
|
$
|
1,812
|
|
$
|
(605
|
)
|
$
|
1,207
|
|
$
|
1,653
|
|
$
|
(534
|
)
|
$
|
1,119
|
|
|||||||
|
Year Ending December 31,
|
|
|
|
|
2019
|
$
|
97
|
|
|
2020
|
96
|
|
|
|
2021
|
90
|
|
|
|
2022
|
86
|
|
|
|
2023
|
85
|
|
|
|
Thereafter
|
517
|
|
|
|
Total
|
$
|
971
|
|
|
2018
|
2017
|
||||||
|
Accrued wages and benefits (including incentive compensation)
|
$
|
249
|
|
$
|
325
|
|
|
|
Accrued expenses
|
274
|
|
246
|
|
|||
|
Trade accounts payable
|
222
|
|
224
|
|
|||
|
Overdrafts
|
98
|
|
71
|
|
|||
|
Dividend payable
|
71
|
|
61
|
|
|||
|
Accrued interest
|
47
|
|
46
|
|
|||
|
Accrued insurance
|
29
|
|
32
|
|
|||
|
Income taxes payable
|
17
|
|
9
|
|
|||
|
Merger consideration payable
|
14
|
|
7
|
|
|||
|
Total
|
$
|
1,021
|
|
$
|
1,021
|
|
|
|
2018
|
2017
|
||||||
|
Secured Receivables Credit Facility (3.39% and 2.27% at December 31, 2018 and 2017, respectively)
|
$
|
160
|
|
$
|
30
|
|
|
|
2.70% Senior Notes due April 2019
|
300
|
|
300
|
|
|||
|
4.75% Senior Notes due January 2020
|
507
|
|
514
|
|
|||
|
2.50% Senior Notes due March 2020
|
300
|
|
300
|
|
|||
|
4.70% Senior Notes due April 2021
|
557
|
|
559
|
|
|||
|
4.25% Senior Notes due April 2024
|
299
|
|
303
|
|
|||
|
3.50% Senior Notes due March 2025
|
562
|
|
566
|
|
|||
|
3.45% Senior Notes due June 2026
|
469
|
|
470
|
|
|||
|
6.95% Senior Notes due July 2037
|
175
|
|
174
|
|
|||
|
5.75% Senior Notes due January 2040
|
244
|
|
244
|
|
|||
|
4.70% Senior Notes due March 2045
|
300
|
|
300
|
|
|||
|
Other
|
37
|
|
44
|
|
|||
|
Debt issuance costs
|
(17
|
)
|
(20
|
)
|
|||
|
Total long-term debt
|
3,893
|
|
3,784
|
|
|||
|
Less: Current portion of long-term debt
|
464
|
|
36
|
|
|||
|
Total long-term debt, net of current portion
|
$
|
3,429
|
|
$
|
3,748
|
|
|
|
Year Ending December 31,
|
|||
|
2019
|
$
|
464
|
|
|
2020
|
803
|
|
|
|
2021
|
553
|
|
|
|
2022
|
3
|
|
|
|
2023
|
1
|
|
|
|
Thereafter
|
2,148
|
|
|
|
Total maturities of long-term debt
|
3,972
|
|
|
|
Unamortized discount
|
(9
|
)
|
|
|
Debt issuance costs
|
(17
|
)
|
|
|
Fair value basis adjustments attributable to hedged debt
|
(53
|
)
|
|
|
Total long-term debt
|
3,893
|
|
|
|
Less: Current portion of long-term debt
|
464
|
|
|
|
Total long-term debt, net of current portion
|
$
|
3,429
|
|
|
Notional Amount
|
||||||||
|
Debt Instrument
|
2018
|
2017
|
||||||
|
4.25% Senior Notes due April 2024
|
250
|
|
250
|
|
||||
|
3.50% Senior Notes due March 2025
|
600
|
|
600
|
|
||||
|
3.45% Senior Notes due June 2026
|
350
|
|
350
|
|
||||
|
$
|
1,200
|
|
$
|
1,200
|
|
|||
|
Carrying Amount of Hedged Long-Term Debt
|
Hedge Accounting Basis Adjustment (a)
|
Carrying Amount of Hedged Long-Term Debt
|
Hedge Accounting Basis Adjustment (a)
|
||||||||||||||
|
Balance Sheet Classification
|
December 31, 2018
|
December 31, 2018
|
December 31, 2017
|
December 31, 2017
|
|||||||||||||
|
Long-term debt
|
$
|
1,125
|
|
$
|
(53
|
)
|
$
|
1,132
|
|
$
|
(33
|
)
|
|||||
|
Year Ended December 31,
|
|||||||
|
2018
|
2017
|
||||||
|
Other (expense) income, net
|
Other (expense) income, net
|
||||||
|
Total for line item in which the effects of fair value hedges are recorded
|
(8
|
)
|
16
|
|
|||
|
Gain (loss) on fair value hedging relationships:
|
|||||||
|
Hedged items (Long-term debt)
|
4
|
|
1
|
|
|||
|
Derivatives designated as hedging instruments
|
(4
|
)
|
(1
|
)
|
|||
|
December 31, 2018
|
December 31, 2017
|
||||||||||
|
Balance Sheet
Classification
|
Fair Value
|
Balance Sheet
Classification
|
Fair Value
|
||||||||
|
Derivatives Designated as Hedging Instruments
|
|
|
|
|
|
||||||
|
Interest rate swaps
|
Other liabilities
|
$
|
93
|
|
Other liabilities
|
$
|
89
|
|
|||
|
•
|
Foreign currency translation adjustments;
|
|
•
|
Net deferred loss on cash flow hedges, which represents deferred losses, net of tax on interest rate related derivative financial instruments designated as cash flow hedges, net of amounts reclassified to interest expense (see Note 15).
|
|
Foreign
Currency
Translation
Adjustment
|
Investment Adjustments
|
Net Deferred Loss on Cash Flow Hedges
|
Other
|
Accumulated Other Comprehensive Income (Loss)
|
|||||||||||||||
|
Balance, December 31, 2015
|
$
|
(24
|
)
|
$
|
(1
|
)
|
$
|
(12
|
)
|
$
|
(1
|
)
|
$
|
(38
|
)
|
||||
|
Other comprehensive loss before reclassifications
|
(34
|
)
|
(2
|
)
|
—
|
|
—
|
|
(36
|
)
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive loss
|
—
|
|
—
|
|
2
|
|
—
|
|
2
|
|
|||||||||
|
Net current period other comprehensive (loss) income
|
(34
|
)
|
(2
|
)
|
2
|
|
—
|
|
(34
|
)
|
|||||||||
|
Balance, December 31, 2016
|
(58
|
)
|
(3
|
)
|
(10
|
)
|
(1
|
)
|
(72
|
)
|
|||||||||
|
Other comprehensive income before reclassifications
|
20
|
|
—
|
|
—
|
|
—
|
|
20
|
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive loss
|
—
|
|
3
|
|
1
|
|
—
|
|
4
|
|
|||||||||
|
Net current period other comprehensive income
|
20
|
|
3
|
|
1
|
|
—
|
|
24
|
|
|||||||||
|
Balance, December 31, 2017
|
(38
|
)
|
—
|
|
(9
|
)
|
(1
|
)
|
(48
|
)
|
|||||||||
|
Other comprehensive loss before reclassifications
|
(15
|
)
|
—
|
|
—
|
|
—
|
|
(15
|
)
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive loss
|
4
|
|
—
|
|
2
|
|
|
|
6
|
|
|||||||||
|
Net current period other comprehensive loss
|
(11
|
)
|
—
|
|
2
|
|
—
|
|
(9
|
)
|
|||||||||
|
Reclassification of stranded tax effects resulting from enactment of the Tax Cuts and Jobs Act
|
—
|
|
—
|
|
(2
|
)
|
—
|
|
(2
|
)
|
|||||||||
|
Balance, December 31, 2018
|
$
|
(49
|
)
|
$
|
—
|
|
$
|
(9
|
)
|
$
|
(1
|
)
|
$
|
(59
|
)
|
||||
|
2018
|
2017
|
2016
|
|||
|
Fair value at grant date
|
$18.14
|
$15.98
|
$10.35
|
||
|
Expected volatility
|
19.1%
|
19.8%
|
21.6%
|
||
|
Dividend yield
|
1.9%
|
1.9%
|
2.4%
|
||
|
Risk-free interest rate
|
2.8%
|
2.1%
|
1.4%
|
||
|
Expected holding period, in years
|
5.3
|
5.2
|
5.3
|
||
|
Shares
|
Weighted
Average Exercise Price
|
Weighted Average Remaining Contractual Term
(in years)
|
Aggregate Intrinsic Value
|
|||||||||
|
Options outstanding, beginning of year
|
8.5
|
|
$
|
70.11
|
|
|||||||
|
Options granted
|
1.6
|
|
103.56
|
|
||||||||
|
Options exercised
|
(1.6
|
)
|
63.08
|
|
||||||||
|
Options forfeited and canceled
|
(0.1
|
)
|
93.51
|
|
||||||||
|
Options outstanding, end of year
|
8.4
|
|
$
|
77.35
|
|
6.7
|
$
|
102
|
|
|||
|
Exercisable, end of year
|
5.1
|
|
$
|
67.17
|
|
5.6
|
$
|
90
|
|
|||
|
Vested and expected to vest, end of year
|
8.3
|
|
$
|
76.98
|
|
6.6
|
$
|
102
|
|
|||
|
2018
|
2017
|
2016
|
||||||||||||||||||
|
Shares
|
Weighted
Average
Grant Date
Fair Value
|
Shares
|
Weighted
Average
Grant Date
Fair Value
|
Shares
|
Weighted
Average
Grant Date
Fair Value
|
|||||||||||||||
|
Shares outstanding, beginning of year
|
1.3
|
|
$
|
77.90
|
|
1.5
|
|
$
|
63.88
|
|
1.7
|
|
$
|
59.92
|
|
|||||
|
Shares granted
|
0.4
|
|
103.51
|
|
0.4
|
|
96.27
|
|
0.6
|
|
67.26
|
|
||||||||
|
Shares vested
|
(0.5
|
)
|
74.00
|
|
(0.6
|
)
|
57.59
|
|
(0.4
|
)
|
58.98
|
|
||||||||
|
Shares forfeited and canceled
|
(0.1
|
)
|
90.16
|
|
—
|
|
—
|
|
(0.4
|
)
|
57.31
|
|
||||||||
|
Shares outstanding, end of year
|
1.1
|
|
$
|
88.13
|
|
1.3
|
|
$
|
77.90
|
|
1.5
|
|
$
|
63.88
|
|
|||||
|
Year Ending December 31,
|
|||
|
2019
|
$
|
181
|
|
|
2020
|
143
|
|
|
|
2021
|
106
|
|
|
|
2022
|
79
|
|
|
|
2023
|
60
|
|
|
|
Thereafter
|
122
|
|
|
|
Minimum lease payments
|
$
|
691
|
|
|
2018
|
2017
|
2016
|
|||||||||
|
Net revenues:
|
|
|
|
|
|||||||
|
DIS business
|
$
|
7,204
|
|
$
|
7,068
|
|
$
|
6,837
|
|
||
|
All other operating segments
|
327
|
|
334
|
|
377
|
|
|||||
|
Total net revenues
|
$
|
7,531
|
|
$
|
7,402
|
|
$
|
7,214
|
|
||
|
Operating earnings (loss):
|
|
|
|
|
|||||||
|
DIS business
|
$
|
1,235
|
|
$
|
1,313
|
|
$
|
1,244
|
|
||
|
All other operating segments
|
47
|
|
52
|
|
64
|
|
|||||
|
General corporate activities
|
(181
|
)
|
(200
|
)
|
(31
|
)
|
|||||
|
Total operating income
|
1,101
|
|
1,165
|
|
1,277
|
|
|||||
|
Non-operating expenses, net
|
(175
|
)
|
(135
|
)
|
(191
|
)
|
|||||
|
Income before income taxes and equity in earnings of equity method investees
|
926
|
|
1,030
|
|
1,086
|
|
|||||
|
Income tax expense
|
(182
|
)
|
(241
|
)
|
(429
|
)
|
|||||
|
Equity in earnings of equity method investees, net of taxes
|
44
|
|
35
|
|
39
|
|
|||||
|
Net income
|
788
|
|
824
|
|
696
|
|
|||||
|
Less: Net income attributable to noncontrolling interests
|
52
|
|
52
|
|
51
|
|
|||||
|
Net income attributable to Quest Diagnostics
|
$
|
736
|
|
$
|
772
|
|
$
|
645
|
|
||
|
2018
|
2017
|
2016
|
|||||||||
|
DIS business
|
$
|
213
|
|
$
|
189
|
|
$
|
170
|
|
||
|
All other operating segments
|
6
|
|
6
|
|
6
|
|
|||||
|
General corporate
|
90
|
|
75
|
|
73
|
|
|||||
|
Total depreciation and amortization
|
$
|
309
|
|
$
|
270
|
|
$
|
249
|
|
||
|
2018
|
2017
|
2016
|
|||||||||
|
DIS business
|
$
|
330
|
|
$
|
219
|
|
$
|
264
|
|
||
|
All other operating segments
|
16
|
|
15
|
|
21
|
|
|||||
|
General corporate
|
37
|
|
18
|
|
8
|
|
|||||
|
Total capital expenditures
|
$
|
383
|
|
$
|
252
|
|
$
|
293
|
|
||
|
2018
|
2017
|
2016
|
|||||||||
|
Routine clinical testing services
|
$
|
4,217
|
|
$
|
4,006
|
|
$
|
3,878
|
|
||
|
Gene-based and esoteric (including advanced diagnostics) testing services
|
2,409
|
|
2,449
|
|
2,335
|
|
|||||
|
Anatomic pathology testing services
|
578
|
|
612
|
|
624
|
|
|||||
|
All other
|
327
|
|
335
|
|
377
|
|
|||||
|
Total net revenues
|
$
|
7,531
|
|
$
|
7,402
|
|
$
|
7,214
|
|
||
|
2018 (a)
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
Total
Year
|
||||||||||||||
|
(b)
|
(c)
|
(d)
|
(e)
|
||||||||||||||||
|
Net revenues
|
$
|
1,884
|
|
$
|
1,919
|
|
$
|
1,889
|
|
$
|
1,839
|
|
$
|
7,531
|
|
||||
|
Gross profit
|
658
|
|
676
|
|
667
|
|
604
|
|
2,605
|
|
|||||||||
|
Net income
|
189
|
|
233
|
|
227
|
|
139
|
|
788
|
|
|||||||||
|
Less: Net income attributable to noncontrolling interests
|
12
|
|
14
|
|
14
|
|
12
|
|
52
|
|
|||||||||
|
Net income attributable to Quest Diagnostics
|
$
|
177
|
|
$
|
219
|
|
$
|
213
|
|
$
|
127
|
|
$
|
736
|
|
||||
|
Earnings per share attributable to Quest Diagnostics' stockholders:
|
|||||||||||||||||||
|
Basic
|
$
|
1.30
|
|
$
|
1.60
|
|
$
|
1.56
|
|
$
|
0.93
|
|
$
|
5.39
|
|
||||
|
Diluted
|
$
|
1.27
|
|
$
|
1.57
|
|
$
|
1.53
|
|
$
|
0.92
|
|
$
|
5.29
|
|
||||
|
2017 (a)
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
Total
Year
|
||||||||||||||
|
(f)
|
(g)
|
(h)
|
(i)
|
||||||||||||||||
|
Net revenues
|
$
|
1,817
|
|
$
|
1,864
|
|
$
|
1,856
|
|
$
|
1,865
|
|
$
|
7,402
|
|
||||
|
Gross profit
|
652
|
|
694
|
|
666
|
|
671
|
|
2,683
|
|
|||||||||
|
Net income
|
175
|
|
207
|
|
175
|
|
267
|
|
824
|
|
|||||||||
|
Less: Net income attributable to noncontrolling interests
|
11
|
|
14
|
|
14
|
|
13
|
|
52
|
|
|||||||||
|
Net income attributable to Quest Diagnostics
|
$
|
164
|
|
$
|
193
|
|
$
|
161
|
|
$
|
254
|
|
$
|
772
|
|
||||
|
Earnings per share attributable to Quest Diagnostics' stockholders:
|
|||||||||||||||||||
|
Basic
|
$
|
1.19
|
|
$
|
1.40
|
|
$
|
1.18
|
|
$
|
1.86
|
|
$
|
5.63
|
|
||||
|
Diluted
|
$
|
1.16
|
|
$
|
1.37
|
|
$
|
1.15
|
|
$
|
1.82
|
|
$
|
5.50
|
|
||||
|
(a)
|
The process for estimating revenues and the ultimate collection of accounts receivable involves significant judgment and estimation. The Company follows a standard process, which considers historical denial and collection experience and other factors, to estimate contractual allowances and implicit price concessions, recording adjustments in the current period as changes in estimates. Based on this process, during the fourth quarter of 2018, the Company increased its reserves for revenues and accounts receivable by approximately
$35 million
(see Note 3 to the consolidated financial statements). Net revenues for 2017 have been restated to reflect the impact of new revenue recognition rules that became effective January 1, 2018 and were adopted on a retrospective basis (see Note 2 to the consolidated financial statements).
|
|
(b)
|
Included pre-tax charges of
$31 million
, primarily associated with workforce reduction, systems conversions and integration incurred in connection with further restructuring and integrating the Company (
$12 million
in cost of services,
$18 million
in selling, general and administrative expenses and
$1 million
in other operating (income) expense, net); and excess tax benefits associated with stock-based compensation arrangements of
$8 million
recorded in income tax expense.
|
|
(c)
|
Included pre-tax charges of
$25 million
, primarily associated with workforce reduction, systems conversions and integration incurred in connection with further restructuring and integrating the Company (
$14 million
in cost of services and
$11 million
in selling, general and administrative expenses); net pre-tax charges of
$10 million
, primarily associated with certain legal matters partially offset by a gain associated with an insurance claim for hurricane related losses (
$11 million
in cost of services offset by a
$1 million
gain in other operating (income) expense, net); excess tax benefits associated with stock-based compensation arrangements of
$5 million
recorded in income tax expense; and an income tax benefit of
$15 million
associated with a change in a tax return accounting method that enabled the company to accelerate the deduction of certain expenses on its 2017 tax return at the federal corporate statutory tax rate in effect during 2017.
|
|
(d)
|
Included pre-tax charges of
$19 million
, primarily associated with workforce reduction, systems conversions and integration incurred in connection with further restructuring and integrating the Company (
$10 million
in cost of services and
$9 million
in selling, general and administrative expenses); a pre-tax benefit of
$12 million
primarily associated with the decrease in the fair value of the contingent consideration accrual associated with the MedXM acquisition partially offset by non-cash asset impairment charges (
$13 million
gain in other operating (income) expense, net offset by
$1 million
in cost of services); and excess tax benefits associated with stock-based compensation arrangements of
$4 million
recorded in income tax expense.
|
|
(e)
|
Included pre-tax charges of
$47 million
, primarily associated with workforce reductions, systems conversions and integration incurred in connection with further restructuring and integrating the Company (
$20 million
in cost of services and
$27 million
in selling, general and administrative expenses); pre-tax charges of
$4 million
, primarily associated with the loss on the sale of a foreign subsidiary recorded in loss (gain) on disposition of business;
$1 million
of income tax expense associated with finalizing the impact of the enactment of TCJA; and excess tax benefits associated with stock-based compensation arrangements of
$1 million
recorded in income tax expense.
|
|
(f)
|
Included pre-tax charges of
$18 million
, primarily associated with systems conversions and integration incurred in connection with further restructuring and integrating the Company (
$10 million
in cost of services and
$8 million
in selling, general and administrative expenses); and excess tax benefits associated with stock-based compensation arrangements of
$16 million
recorded in income tax expense.
|
|
(g)
|
Included pre-tax charges of
$23 million
, primarily associated with systems conversions and integration incurred in connection with further restructuring and integrating the Company (
$9 million
in cost of services,
$13 million
in selling, general and administrative expenses, and
$1 million
in equity in earnings of equity method investees, net of taxes); pre-tax gain of
$7 million
related to the sale of an interest in an equity method investment recorded in other (expense) income, net;
$2 million
in costs incurred related to certain legal matters recorded in selling, general and administrative expenses; and excess tax benefits associated with stock-based compensation arrangements of
$13 million
recorded in income tax expense.
|
|
(h)
|
Included pre-tax charges of
$23 million
, primarily associated with systems conversions and integration incurred in connection with further restructuring and integrating the Company (
$12 million
in cost of services and
$11 million
in selling, general and administrative expenses); pre-tax charges of
$9 million
primarily associated with non-cash asset impairment charges and incremental costs incurred as a result of hurricanes (
$3 million
in cost of services,
$1 million
in selling, general and administrative expenses, and
$5 million
in other (expense) income, net); and excess tax benefits associated with stock-based compensation arrangements of
$7 million
recorded in income tax expense.
|
|
(i)
|
Included pre-tax charges of
$42 million
, primarily associated with systems conversions, integration and workforce reductions incurred in connection with further restructuring and integrating the Company (
$14 million
in cost of services and
$28 million
in selling, general and administrative expenses); pre-tax charges of
$6 million
, primarily related to non-cash asset impairment charges and incremental costs incurred as a result of the hurricanes (
$2 million
in cost of services and
$4 million
in selling, general and administrative expenses); a provisional estimated income tax benefit of
$106 million
associated with the TCJA, including a deferred income tax benefit of
$115 million
primarily due to the remeasurement of net deferred tax liabilities and reserves at the new combined federal and state tax rate, partially offset by
$9 million
of current tax expense primarily due to the mandatory repatriation toll charge on undistributed foreign earnings and profits; and excess tax benefits associated with stock-based compensation arrangements of
$1 million
recorded in income tax expense.
|
|
Balance at
Beginning of Year
|
Provision for Doubtful Accounts
|
Net Deductions
and Other
|
Balance at
End of Year
|
||||||||||||
|
Year Ended December 31, 2018
|
|||||||||||||||
|
Doubtful accounts and allowances
|
$
|
13
|
|
$
|
6
|
|
$
|
4
|
|
(a)
|
$
|
15
|
|
||
|
Year Ended December 31, 2017
|
|||||||||||||||
|
Doubtful accounts and allowances
|
$
|
6
|
|
$
|
8
|
|
$
|
1
|
|
(a)
|
$
|
13
|
|
||
|
Year Ended December 31, 2016
|
|||||||||||||||
|
Doubtful accounts and allowances
|
9
|
|
$
|
7
|
|
$
|
10
|
|
(a)
|
$
|
6
|
|
|||
|
(a)
|
Primarily represents the write-off of accounts receivable, net of recoveries.
|
|
Exhibit
Number
|
Description
|
|
3.1
|
|
|
3.2
|
|
|
4.1
|
|
|
4.2
|
|
|
4.3
|
|
|
4.4
|
|
|
4.5
|
|
|
4.6
|
|
|
4.7
|
|
|
4.8
|
|
|
4.9
|
|
|
4.10
|
|
|
4.11
|
|
|
4.12
|
|
|
4.13
|
|
|
4.14
|
|
|
4.15
|
|
|
4.16
|
|
|
4.17
|
|
|
4.18
|
|
|
4.19
|
|
|
4.20
|
|
|
4.21
|
|
|
4.22
|
|
|
4.23
|
|
|
4.24
|
|
|
4.25
|
|
|
4.26
|
|
|
4.27
|
|
|
4.28
|
|
|
4.29
|
|
|
10.1‡
|
|
|
10.2‡
|
|
|
10.3‡
|
|
|
10.4‡
|
|
|
10.5‡
|
|
|
10.6‡
|
|
|
10.7‡
|
|
|
10.8‡
|
|
|
10.9‡
|
|
|
10.10‡
|
|
|
10.11‡*
|
|
|
10.12‡
|
|
|
10.13‡
|
|
|
10.14‡
|
|
|
10.15‡
|
|
|
10.16‡
|
|
|
10.17‡
|
|
|
10.18‡
|
|
|
10.19‡
|
|
|
11.1
|
Statement re: Computation of Earnings Per Common Share (the calculation of per share earnings is in Part II, Item 8, Note 4 to the consolidated financial statements (Earnings Per Share) and is omitted in accordance with Item 601(b)(11) of Regulation S-K)
|
|
21.1*
|
|
|
23.1*
|
|
|
24.1*
|
|
|
31.1*
|
|
|
31.2*
|
|
|
32.1**
|
|
|
32.2**
|
|
|
101.INS*
|
dgx-20181231.xml
|
|
101.SCH*
|
dgx-20181231.xsd
|
|
101.CAL*
|
dgx-20181231_cal.xml
|
|
101.DEF*
|
dgx-20181231_def.xml
|
|
101.LAB*
|
dgx-20181231_lab.xml
|
|
101.PRE*
|
dgx-20181231_pre.xml
|
|
*
|
Filed herewith.
|
|
**
|
Furnished herewith.
|
|
‡
|
Management contract or compensatory plan or arrangement required to be filed as an exhibit to this Form 10-K pursuant to Item 15(b) of Form 10-K.
|