|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
|
For the quarterly period ended
|
|||||
| or | |||||
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
| For the transition period from to | |||||

|
|
|
|||||||
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |||||||
|
|
|
|||||||
| (Address of principal executive offices) | (Zip Code) | |||||||
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||||||||
|
|
|
|
||||||||||||
|
|
☒ | Accelerated filer | ☐ | |||||||||||
| Non-accelerated filer | ☐ | Smaller reporting company |
|
|||||||||||
| Emerging growth company |
|
|||||||||||||
| DexCom, Inc. | ||
| Table of Contents | ||
| Page | ||||||||
|
Consolidated Balance Sheets (unaudited) as of
March 31, 2024
and
December 31, 2023
|
||||||||
|
Consolidated Statements of Operations (unaudited) for the
three m
onths ended
March 31, 2024
and
2023
|
||||||||
|
Consolidated Statements of Comprehensive Income (unaudited) for the
three
months ended
March 31, 2024
and
2023
|
||||||||
|
Consolidated Statements of Stockholders’ Equity (unaudited) for the
three
months ended
March 31, 2024
and
2023
|
||||||||
|
Consolidated Statements of Cash Flows (unaudited) for the
three months ended
March 31, 2024
and
2023
|
||||||||
|
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
|
||
|
AVAILABLE INFORMATION
|
||
|
PART I. FINANCIAL INFORMATION
|
||
|
ITEM 1. FINANCIAL STATEMENTS
|
||
| DexCom, Inc. | ||
| Consolidated Balance Sheets | ||
| (Unaudited) | ||
|
(In millions, except share and par value data)
|
March 31, 2024 | December 31, 2023 | |||||||||
| Assets | |||||||||||
| Current assets: | |||||||||||
| Cash and cash equivalents | $ |
|
$ |
|
|||||||
| Short-term marketable securities |
|
|
|||||||||
| Accounts receivable, net |
|
|
|||||||||
| Inventory |
|
|
|||||||||
| Prepaid and other current assets |
|
|
|||||||||
| Total current assets |
|
|
|||||||||
| Property and equipment, net |
|
|
|||||||||
| Operating lease right-of-use assets |
|
|
|||||||||
| Goodwill |
|
|
|||||||||
| Intangibles, net |
|
|
|||||||||
| Deferred tax assets |
|
|
|||||||||
| Other assets |
|
|
|||||||||
| Total assets | $ |
|
$ |
|
|||||||
| Liabilities and Stockholders’ Equity | |||||||||||
| Current liabilities: | |||||||||||
| Accounts payable and accrued liabilities | $ |
|
$ |
|
|||||||
| Accrued payroll and related expenses |
|
|
|||||||||
| Short-term operating lease liabilities |
|
|
|||||||||
| Deferred revenue |
|
|
|||||||||
| Total current liabilities |
|
|
|||||||||
| Long-term senior convertible notes |
|
|
|||||||||
| Long-term operating lease liabilities |
|
|
|||||||||
| Other long-term liabilities |
|
|
|||||||||
| Total liabilities |
|
|
|||||||||
| Commitments and contingencies |
|
|
|||||||||
| Stockholders’ equity: | |||||||||||
|
Preferred stock, $
|
|
|
|||||||||
|
Common stock, $
|
|
|
|||||||||
| Additional paid-in capital |
|
|
|||||||||
|
Accumulated other comprehensive loss
|
(
|
(
|
|||||||||
| Retained earnings |
|
|
|||||||||
|
Treasury stock, at cost;
|
(
|
(
|
|||||||||
| Total stockholders’ equity |
|
|
|||||||||
| Total liabilities and stockholders’ equity | $ |
|
$ |
|
|||||||
| DexCom, Inc. | ||
| Consolidated Statements of Operations | ||
| (Unaudited) | ||
|
Three Months Ended
March 31, |
|||||||||||
| (In millions, except per share data) | 2024 | 2023 | |||||||||
| Revenue | $ |
|
$ |
|
|||||||
| Cost of sales |
|
|
|||||||||
| Gross profit |
|
|
|||||||||
| Operating expenses: | |||||||||||
| Research and development |
|
|
|||||||||
| Selling, general and administrative |
|
|
|||||||||
| Total operating expenses |
|
|
|||||||||
| Operating income |
|
|
|||||||||
|
Other income (expense), net
|
|
|
|||||||||
| Income before income taxes |
|
|
|||||||||
| Income tax expense (benefit) |
(
|
|
|||||||||
| Net income | $ |
|
$ |
|
|||||||
| Basic net income per share | $ |
|
$ |
|
|||||||
| Shares used to compute basic net income per share |
|
|
|||||||||
| Diluted net income per share | $ |
|
$ |
|
|||||||
| Shares used to compute diluted net income per share |
|
|
|||||||||
| DexCom, Inc. | ||
| Consolidated Statements of Comprehensive Income | ||
| (Unaudited) | ||
|
Three Months Ended
March 31, |
|||||||||||
| (In millions) | 2024 | 2023 | |||||||||
| Net income | $ |
|
$ |
|
|||||||
| Other comprehensive income (loss), net of tax: | |||||||||||
| Translation adjustments and other |
(
|
|
|||||||||
| Unrealized gain (loss) on marketable debt securities |
(
|
|
|||||||||
| Total other comprehensive income (loss), net of tax |
(
|
|
|||||||||
| Comprehensive income | $ |
|
$ |
|
|||||||
| DexCom, Inc. | ||
| Consolidated Statements of Stockholders’ Equity | ||
| (Unaudited) | ||
| Three Months Ended March 31, 2024 | ||||||||||||||||||||||||||||||||||||||||||||
| (In millions) | Common Stock |
Additional
Paid-In Capital |
Accumulated Other Comprehensive Loss | Retained Earnings | Treasury Stock |
Total
Stockholders’ Equity |
||||||||||||||||||||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2023 |
|
$ |
|
$ |
|
$ |
(
|
$ |
|
$ |
(
|
$ |
|
|||||||||||||||||||||||||||||||
| Issuance of common stock under equity incentive plans |
|
— | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||
| Issuance of common stock for Employee Stock Purchase Plan |
|
— |
|
— | — | — |
|
|||||||||||||||||||||||||||||||||||||
| Issuance of common stock in connection with achievement of sales-based milestone, net of issuance costs |
|
— |
(
|
— | — |
|
|
|||||||||||||||||||||||||||||||||||||
| Exercise and settlement of warrants |
|
— |
(
|
— | — |
|
|
|||||||||||||||||||||||||||||||||||||
| Share-based compensation expense | — | — |
|
— | — | — |
|
|||||||||||||||||||||||||||||||||||||
| Net income | — | — | — | — |
|
— |
|
|||||||||||||||||||||||||||||||||||||
| Other comprehensive loss, net of tax | — | — | — |
(
|
— | — |
(
|
|||||||||||||||||||||||||||||||||||||
| Balance at March 31, 2024 |
|
$ |
|
$ |
|
$ |
(
|
$ |
|
$ |
(
|
$ |
|
|||||||||||||||||||||||||||||||
| Three Months Ended March 31, 2023 | ||||||||||||||||||||||||||||||||||||||||||||
| (In millions) | Common Stock |
Additional
Paid-In Capital |
Accumulated Other Comprehensive Loss | Retained Earnings | Treasury Stock |
Total
Stockholders’ Equity |
||||||||||||||||||||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2022 |
|
$ |
|
$ |
|
$ |
(
|
$ |
|
$ |
(
|
$ |
|
|||||||||||||||||||||||||||||||
| Issuance of common stock under equity incentive plans |
|
— | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||
| Issuance of common stock for Employee Stock Purchase Plan |
|
— |
|
— | — | — |
|
|||||||||||||||||||||||||||||||||||||
| Share-based compensation expense | — | — |
|
— | — | — |
|
|||||||||||||||||||||||||||||||||||||
| Net income | — | — | — | — |
|
— |
|
|||||||||||||||||||||||||||||||||||||
| Other comprehensive income, net of tax | — | — | — |
|
— | — |
|
|||||||||||||||||||||||||||||||||||||
| Balance at March 31, 2023 |
|
$ |
|
$ |
|
$ |
(
|
$ |
|
$ |
(
|
$ |
|
|||||||||||||||||||||||||||||||
| DexCom, Inc. | ||
| Consolidated Statements of Cash Flows | ||
| (Unaudited) | ||
|
Three Months Ended
March 31, |
|||||||||||
| (In millions) | 2024 | 2023 | |||||||||
| Operating activities | |||||||||||
| Net income | $ |
|
$ |
|
|||||||
|
Adjustments to reconcile net income to cash provided by operating activities:
|
|||||||||||
| Depreciation and amortization |
|
|
|||||||||
| Share-based compensation |
|
|
|||||||||
| Non-cash interest expense |
|
|
|||||||||
| Deferred income taxes |
(
|
(
|
|||||||||
| Other non-cash income and expenses |
(
|
(
|
|||||||||
| Changes in operating assets and liabilities: | |||||||||||
| Accounts receivable, net |
(
|
|
|||||||||
| Inventory |
(
|
(
|
|||||||||
| Prepaid and other assets |
|
(
|
|||||||||
| Operating lease right-of-use assets and liabilities, net |
(
|
(
|
|||||||||
| Accounts payable and accrued liabilities |
|
|
|||||||||
| Accrued payroll and related expenses |
(
|
(
|
|||||||||
| Deferred revenue and other liabilities |
|
|
|||||||||
| Net cash provided by operating activities |
|
|
|||||||||
| Investing activities | |||||||||||
| Purchases of marketable securities |
(
|
(
|
|||||||||
| Proceeds from sale and maturity of marketable securities |
|
|
|||||||||
| Purchases of property and equipment |
(
|
(
|
|||||||||
| Other investing activities |
|
|
|||||||||
| Net cash provided by (used in) investing activities |
|
(
|
|||||||||
| Financing activities | |||||||||||
| Net proceeds from issuance of common stock |
|
|
|||||||||
| Other financing activities |
(
|
(
|
|||||||||
| Net cash provided by financing activities |
|
|
|||||||||
| Effect of exchange rate changes on cash, cash equivalents and restricted cash |
(
|
|
|||||||||
| Increase (decrease) in cash, cash equivalents and restricted cash |
|
(
|
|||||||||
| Cash, cash equivalents and restricted cash, beginning of period |
|
|
|||||||||
| Cash, cash equivalents and restricted cash, end of period | $ |
|
$ |
|
|||||||
| Reconciliation of cash, cash equivalents and restricted cash, end of period: | |||||||||||
| Cash and cash equivalents | $ |
|
$ |
|
|||||||
| Restricted cash |
|
|
|||||||||
| Total cash, cash equivalents and restricted cash | $ |
|
$ |
|
|||||||
| Supplemental disclosure of non-cash investing and financing transactions: | |||||||||||
| Acquisition of property and equipment included in accounts payable and accrued liabilities | $ |
|
$ |
|
|||||||
| Right-of-use assets obtained in exchange for operating lease liabilities | $ |
|
$ |
|
|||||||
| Right-of-use assets obtained in exchange for finance lease liabilities | $ |
|
$ |
|
|||||||
| DexCom, Inc. | ||
| Notes to Consolidated Financial Statements | ||
| (Unaudited) | ||
| 1. Organization and Significant Accounting Policies | ||
| Organization and Business | ||
| Basis of Presentation and Principles of Consolidation | ||
| Significant Accounting Policies | ||
| Use of Estimates | ||
| Concentration of Credit Risk | ||
|
Contract Balances
|
||
| Net Income Per Share | ||
|
Three Months Ended
March 31, |
|||||||||||
| (In millions, except per share data) | 2024 | 2023 | |||||||||
| Net income | $ |
|
$ |
|
|||||||
| Add back interest expense, net of tax attributable to assumed conversion of senior convertible notes |
|
|
|||||||||
| Net income - diluted | $ |
|
$ |
|
|||||||
| Net income per common share | |||||||||||
| Basic | $ |
|
$ |
|
|||||||
| Diluted | $ |
|
$ |
|
|||||||
| Basic weighted average shares outstanding |
|
|
|||||||||
|
Dilutive potential securities:
|
|||||||||||
|
Collaborative sales-based milestones
|
|
|
|||||||||
|
RSUs and PSUs
|
|
|
|||||||||
| Senior convertible notes |
|
|
|||||||||
| Warrants |
|
|
|||||||||
| Diluted weighted average shares outstanding |
|
|
|||||||||
|
Three Months Ended
March 31, |
|||||||||||
| (In millions) | 2024 | 2023 | |||||||||
|
RSUs and PSUs
|
|
|
|||||||||
| Senior convertible notes |
|
|
|||||||||
| Total |
|
|
|||||||||
| Recent Accounting Guidance | ||
| 2. Development and Other Agreements | ||
| Collaboration with Verily Life Sciences | ||
| 3. Fair Value Measurements | ||
| Fair Value Measurements Using | |||||||||||||||||||||||
| (In millions) | Level 1 | Level 2 | Level 3 | Total | |||||||||||||||||||
| Cash equivalents | $ |
|
$ |
|
$ |
|
$ |
|
|||||||||||||||
| Debt securities, available-for-sale: | |||||||||||||||||||||||
|
U.S. government agencies
(1)
|
|
|
|
|
|||||||||||||||||||
| Commercial paper |
|
|
|
|
|||||||||||||||||||
| Corporate debt |
|
|
|
|
|||||||||||||||||||
| Total debt securities, available-for-sale |
|
|
|
|
|||||||||||||||||||
|
Other assets
(2)
|
|
|
|
|
|||||||||||||||||||
| Total assets measured at fair value on a recurring basis | $ |
|
$ |
|
$ |
|
$ |
|
|||||||||||||||
| Fair Value Measurements Using | |||||||||||||||||||||||
| (In millions) | Level 1 | Level 2 | Level 3 | Total | |||||||||||||||||||
| Cash equivalents | $ |
|
$ |
|
$ |
|
$ |
|
|||||||||||||||
| Debt securities, available-for-sale: | |||||||||||||||||||||||
|
U.S. government agencies
(1)
|
|
|
|
|
|||||||||||||||||||
| Commercial paper |
|
|
|
|
|||||||||||||||||||
| Corporate debt |
|
|
|
|
|||||||||||||||||||
| Total debt securities, available-for-sale |
|
|
|
|
|||||||||||||||||||
|
Other assets
(2)
|
|
|
|
|
|||||||||||||||||||
| Total assets measured at fair value on a recurring basis | $ |
|
$ |
|
$ |
|
$ |
|
|||||||||||||||
| Fair Value Measurements Using Level 1 | |||||||||||
| (In millions) | March 31, 2024 | December 31, 2023 | |||||||||
| Senior Convertible Notes due 2025 |
|
|
|||||||||
| Senior Convertible Notes due 2028 |
|
|
|||||||||
| Total fair value of outstanding senior convertible notes | $ |
|
$ |
|
|||||||
|
4. Balance Sheet Details and Other Financial Information
|
||
| Short-Term Marketable Securities | ||
| March 31, 2024 | |||||||||||||||||||||||
| (In millions) |
Amortized
Cost |
Gross
Unrealized Gains |
Gross
Unrealized Losses |
Estimated
Market Value |
|||||||||||||||||||
| Debt securities, available-for-sale: | |||||||||||||||||||||||
|
U.S. government agencies
(1)
|
$ |
|
$ |
|
$ |
(
|
$ |
|
|||||||||||||||
| Commercial paper |
|
|
(
|
|
|||||||||||||||||||
| Corporate debt |
|
|
(
|
|
|||||||||||||||||||
| Total debt securities, available-for-sale | $ |
|
$ |
|
$ |
(
|
$ |
|
|||||||||||||||
| December 31, 2023 | |||||||||||||||||||||||
| (In millions) |
Amortized
Cost |
Gross
Unrealized Gains |
Gross
Unrealized Losses |
Estimated
Market Value |
|||||||||||||||||||
| Debt securities, available-for-sale: | |||||||||||||||||||||||
|
U.S. government agencies
(1)
|
$ |
|
$ |
|
$ |
(
|
$ |
|
|||||||||||||||
| Commercial paper |
|
|
(
|
|
|||||||||||||||||||
| Corporate debt |
|
|
(
|
|
|||||||||||||||||||
| Total debt securities, available-for-sale | $ |
|
$ |
|
$ |
(
|
$ |
|
|||||||||||||||
|
Inventory
|
||
| (In millions) | March 31, 2024 | December 31, 2023 | |||||||||
| Raw materials | $ |
|
$ |
|
|||||||
| Work-in-process |
|
|
|||||||||
| Finished goods |
|
|
|||||||||
| Total inventory | $ |
|
$ |
|
|||||||
| Prepaid and Other Current Assets | ||
| (In millions) | March 31, 2024 | December 31, 2023 | |||||||||
| Prepaid expenses | $ |
|
$ |
|
|||||||
| Prepaid inventory |
|
|
|||||||||
| Deferred compensation plan assets |
|
|
|||||||||
| Income tax receivables |
|
|
|||||||||
| Other current assets |
|
|
|||||||||
| Total prepaid and other current assets | $ |
|
$ |
|
|||||||
|
Property and Equipment
|
||
| (In millions) | March 31, 2024 | December 31, 2023 | |||||||||
| Land and land improvements | $ |
|
$ |
|
|||||||
| Building |
|
|
|||||||||
| Furniture and fixtures |
|
|
|||||||||
| Computer software and hardware |
|
|
|||||||||
| Machinery and equipment |
|
|
|||||||||
| Leasehold improvements |
|
|
|||||||||
| Construction in progress |
|
|
|||||||||
| Total cost |
|
|
|||||||||
|
Less: accumulated depreciation and amortization
|
(
|
(
|
|||||||||
| Total property and equipment, net | $ |
|
$ |
|
|||||||
|
Other Assets
|
||
| (In millions) | March 31, 2024 | December 31, 2023 | |||||||||
| Long-term investments | $ |
|
$ |
|
|||||||
| Long-term deposits |
|
|
|||||||||
| Other assets |
|
|
|||||||||
| Total other assets | $ |
|
$ |
|
|||||||
|
Accounts Payable and Accrued Liabilities
|
||
|
(In millions)
|
March 31, 2024 | December 31, 2023 | |||||||||
| Accounts payable trade | $ |
|
$ |
|
|||||||
| Accrued tax, audit, and legal fees |
|
|
|||||||||
| Accrued rebates |
|
|
|||||||||
| Accrued warranty |
|
|
|||||||||
|
Income tax payable
|
|
|
|||||||||
| Deferred compensation plan liabilities |
|
|
|||||||||
| Other accrued liabilities |
|
|
|||||||||
| Total accounts payable and accrued liabilities | $ |
|
$ |
|
|||||||
|
Accrued Payroll and Related Expenses
|
||
| (In millions) | March 31, 2024 | December 31, 2023 | |||||||||
| Accrued wages, bonus and taxes | $ |
|
$ |
|
|||||||
| Other accrued employee benefits |
|
|
|||||||||
| Total accrued payroll and related expenses | $ |
|
$ |
|
|||||||
|
Other Long-Term Liabilities
|
||
| (In millions) | March 31, 2024 | December 31, 2023 | |||||||||
|
Finance lease obligations
|
$ |
|
$ |
|
|||||||
| Deferred revenue, long-term |
|
|
|||||||||
| Asset retirement obligation |
|
|
|||||||||
| Other tax liabilities |
|
|
|||||||||
| Other liabilities |
|
|
|||||||||
| Total other long-term liabilities | $ |
|
$ |
|
|||||||
|
Other Income (Expense), Net
|
||
|
Three Months Ended
March 31, |
|||||||||||
| (In millions) | 2024 | 2023 | |||||||||
|
Interest and dividend income
|
$ |
|
$ |
|
|||||||
|
Interest expense
|
(
|
(
|
|||||||||
|
Other expense, net
|
(
|
(
|
|||||||||
|
Total other income (expense), net
|
$ |
|
$ |
|
|||||||
| 5. Debt | ||
| Senior Convertible Notes | ||
| (In millions) | March 31, 2024 | December 31, 2023 | |||||||||
| Principal amount: | |||||||||||
| Senior Convertible Notes due 2025 |
|
|
|||||||||
| Senior Convertible Notes due 2028 |
|
|
|||||||||
| Total principal amount |
|
|
|||||||||
| Unamortized debt issuance costs |
(
|
(
|
|||||||||
| Carrying amount of senior convertible notes | $ |
|
$ |
|
|||||||
| (In millions) | March 31, 2024 | December 31, 2023 | |||||||||
| Senior Convertible Notes due 2025 |
|
|
|||||||||
| Senior Convertible Notes due 2028 |
|
|
|||||||||
|
Total by which the notes
’
if-converted value exceeds their principal amount
|
$ |
|
$ |
|
|||||||
|
Three Months Ended
March 31, |
|||||||||||
| (In millions) | 2024 | 2023 | |||||||||
| Cash interest expense: | |||||||||||
|
Contractual coupon interest
(1)
|
$ |
|
$ |
|
|||||||
| Non-cash interest expense: | |||||||||||
| Amortization of debt issuance costs |
|
|
|||||||||
| Total interest expense recognized on senior notes | $ |
|
$ |
|
|||||||
| Effective interest rate: | |||||||||||
|
Senior Convertible Notes due 2023
(2)
|
* |
|
% | ||||||||
| Senior Convertible Notes due 2025 |
|
% |
|
% | |||||||
| Senior Convertible Notes due 2028 |
|
% | * | ||||||||
|
(1)
Interest on our unsecured senior convertible notes due 2023, or the 2023 Notes, began accruing upon issuance and was payable semi-annually on June 1 and December 1 of each year until the 2023 Notes matured on December 1, 2023. Interest on our unsecured senior convertible notes due 2025, or the 2025 Notes, began accruing upon issuance and is payable semi-annually on May 15 and November 15 of each year. Interest on our unsecured senior convertible notes due 2028, or the 2028 Notes, began accruing upon issuance and is payable semi-annually on May 15 and November 15 of each year.
|
|||||||||||
|
(2)
The effective interest rate presented represents the rate applicable for the period outstanding. The 2023 Notes matured on December 1, 2023 and are no longer outstanding.
|
|||||||||||
|
* Not applicable as no notes were outstanding at this date.
|
|||||||||||
|
Senior Convertible Notes
|
Offering Completion Date
|
Maturity Date
|
Stated Interest Rate
|
Aggregate Principal Amount Issued
|
Net Proceeds
(1)
|
Initial Conversion Rate
(2)
(per $1,000 principal amount)
|
Conversion Price
(per share)
|
Settlement Methods
(3)
|
||||||||||||||||||
|
2023 Notes
(4)
|
November 2018
|
December 1, 2023
|
|
$
|
$
|
24.3476 shares
|
$
|
Cash and/or shares
|
||||||||||||||||||
|
2025 Notes
|
May 2020
|
November 15, 2025
|
|
$
|
$
|
6.6620 shares
|
$
|
Cash and/or shares
|
||||||||||||||||||
|
2028 Notes
|
May 2023
|
May 15, 2028
|
|
$
|
$
|
6.1571 shares
|
$
|
Cash and/or shares
|
||||||||||||||||||
|
(1)
Net proceeds is calculated by deducting the initial purchasers’ discounts and estimated costs directly related to the offering from the aggregate principal amount of the applicable series of notes.
|
||||||||||||||||||||||||||
|
(2)
Subject to adjustments as defined in the applicable indentures.
|
||||||||||||||||||||||||||
|
(3)
The 2025 Notes and 2028 Notes may be settled in cash, stock, or a combination thereof, solely at our discretion. The 2023 Notes, while outstanding, could be settled in cash, stock, or a combination thereof, solely at our discretion.
|
||||||||||||||||||||||||||
|
(4)
The 2023 Notes matured on December 1, 2023 and are no longer outstanding.
|
||||||||||||||||||||||||||
|
Fiscal Period
|
Converted Notes
|
Aggregate Principal Amount Converted
|
Shares Issued for Settlement
|
Shares Received from Exercise of 2023 Note Hedge
|
||||||||||
|
1/1/2023 - 12/31/2023
|
2023 Notes
|
$
|
|
|
||||||||||
|
Summary of Conversions Rights at the Option of the Holders for the 2025 Notes and 2028 Notes, which we refer to collectively as the Notes
|
||||||||
|
Conversion Rights at the Option of the Holders
|
Holders of the Notes have the ability to convert all or a portion of their notes in multiples of $1,000 principal amount, at their option prior to 5:00 p.m., New York City time, on the business day immediately preceding August 15, 2025 and February 15, 2028 for the 2025 Notes and 2028 Notes, respectively, only under the following circumstances:
|
|||||||
|
Circumstance 1
(1)
|
During any calendar quarter commencing after the applicable period (and only during such calendar quarter), if the last reported sale price of Dexcom’s common stock for at least
|
|||||||
|
Circumstance 2
|
During the
|
|||||||
|
Circumstance 3
|
If we call any or all of the Notes for redemption, at any time prior to the close of business on the scheduled trading day immediately preceding the redemption date (only with respect to the notes called or deemed called for redemption)
|
|||||||
|
Circumstance 4
|
Upon the occurrence of specified corporate events
|
|||||||
|
Circumstance 5
(2)
|
Holders of the Notes may convert all or a portion of their Notes regardless of the foregoing circumstances prior to the close of business on the business day immediately preceding the maturity date for the 2025 Notes and prior to the close of business on the second scheduled trading day immediately preceding the maturity date for the 2028 Notes
|
|||||||
|
(1)
Circumstance 1 is available after the calendar quarter ended September 30, 2020 and September 30, 2023 for the 2025 Notes and 2028 Notes, respectively.
|
||||||||
|
(2)
Circumstance 5 is available on or after August 15, 2025 and February 15, 2028 for the 2025 Notes and 2028 Notes, respectively.
|
||||||||
|
Summary of Conversion Right at the Option of the Company for the 2025 Notes and 2028 Notes
|
||||||||
|
Conversion Right at Our Option
(1)
|
Dexcom may redeem for cash all or part of the Notes, at its option, if the last reported sale price of our common stock has been at least
|
|||||||
|
(1)
Dexcom does not have the right to redeem the Notes prior to May 20, 2023 and May 20, 2026 for the 2025 Notes and 2028 Notes, respectively. Dexcom has the right to redeem the notes on or after May 20, 2023 and prior to August 15, 2025 for the 2025 Notes, and on or after May 20, 2026 and prior to February 15, 2028 for the 2028 Notes.
|
||||||||
| Revolving Credit Agreement | ||
| (In millions) | March 31, 2024 | ||||
| Available principal amount |
|
||||
| Letters of credit sub-facility |
|
||||
| Outstanding borrowings |
|
||||
| Outstanding letters of credit |
|
||||
| Total available balance | $ |
|
|||
|
Range
|
ABR Spread
|
Term Benchmark/CDOR Spread and RFR Spread
|
Unused Commitment Fee Rate
|
||||||||
|
Minimum
|
|
|
|
||||||||
|
Maximum
|
|
|
|
||||||||
| 6. Contingencies | ||
| Litigation | ||
| 7. Income Taxes | ||
| 8. Stockholders’ Equity | ||
|
Three Months Ended
March 31, |
|||||||||||
| (In millions) | 2024 | 2023 | |||||||||
| Cost of sales | $ |
|
$ |
|
|||||||
| Research and development |
|
|
|||||||||
| Selling, general and administrative |
|
|
|||||||||
| Total share-based compensation expense | $ |
|
$ |
|
|||||||
| 9. Business Segment and Geographic Information | ||
|
Three Months Ended
March 31, 2024 |
Three Months Ended
March 31, 2023 |
||||||||||||||||||||||
| (In millions) | Amount | % of Total | Amount | % of Total | |||||||||||||||||||
| United States | $ |
|
|
% | $ |
|
|
% | |||||||||||||||
| International |
|
|
% |
|
|
% | |||||||||||||||||
| Total revenue | $ |
|
|
% | $ |
|
|
% | |||||||||||||||
|
Three Months Ended
March 31, 2024 |
Three Months Ended
March 31, 2023 |
||||||||||||||||||||||
| (In millions) | Amount | % of Total | Amount | % of Total | |||||||||||||||||||
| Distributor | $ |
|
|
% | $ |
|
|
% | |||||||||||||||
| Direct |
|
|
% |
|
|
% | |||||||||||||||||
| Total revenue | $ |
|
|
% | $ |
|
|
% | |||||||||||||||
|
ITEM 2 - MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
||
| Overview | ||
| Who We Are | |||||
|
We are a medical device company primarily focused on the design, development and commercialization of continuous glucose monitoring, or CGM, systems for the management of diabetes by patients, caregivers, and clinicians around the world.
We received approval from the Food and Drug Administration, or FDA, and commercialized our first product in 2006. We launched our latest generation system, the Dexcom G6
®
integrated Continuous Glucose Monitoring System, or G6, in 2018, and we launched the Dexcom G7
®
, or G7,
in 2023. In March 2024, we received marketing clearance from the FDA on Stelo, our new 15-day sensor designed for people with Type 2 diabetes who do not use insulin, as the first glucose biosensor that does not require a prescription.
Unless the context requires otherwise, the terms “we,” “us,” “our,” the “company,” or “Dexcom” refer to DexCom, Inc. and its subsidiaries.
|
|||||
| Global Presence | |||||
|
We have built a direct sales organization in North America and certain international markets to call on health care professionals, such as endocrinologists, physicians and diabetes educators, who can educate and influence patient adoption of continuous glucose monitoring. To complement our direct sales efforts, we have entered into distribution arrangements in North America and several international markets that allow distributors to sell our products.
|
|||||
| Future Developments | |||||
|
Product Development:
We plan to develop future generations of technologies that are focused on improved performance and convenience and that will enable intelligent insulin administration. Over the longer term, we plan to continue to develop and improve networked platforms with open architecture, connectivity and transmitters capable of communicating with other devices. We also intend to expand our efforts to accumulate CGM patient data and metrics and apply predictive modeling and machine learning to generate interactive CGM insights that can inform patient behavior.
|
|||||
|
Partnerships:
We also continue to pursue and support development partnerships with insulin pump companies and companies or institutions developing insulin delivery systems, including automated insulin delivery systems.
|
|||||
|
New Opportunities:
We are also exploring how to extend our offerings to other opportunities, including for people with Type 2 diabetes that are non-insulin using, people with pre-diabetes, people who are obese, people who are pregnant, and people in the hospital setting. Eventually, we may apply our technological expertise to products beyond glucose monitoring.
|
|||||
| Critical Accounting Estimates | ||
| Overview of Financial Results | ||||||||||||||||||||||||||||||||||||||
|
The most important financial indicators that we use to assess our business are revenue, gross profit, operating income, net income, and operating cash flow.
|
||||||||||||||||||||||||||||||||||||||
|
Key Highlights for the Three Months Ended March 31, 2024 include the following:
|
||||||||||||||||||||||||||||||||||||||
| Revenue | Gross Profit | Operating Income | Net Income |
Operating
Cash Flow |
||||||||||||||||||||||||||||||||||
| $921.0 million | $561.9 million | $101.1 million | $146.4 million | $209.2 million | ||||||||||||||||||||||||||||||||||
|
up 24% from the same period 2023
|
up 21% from the same period 2023
|
up 114% from the same period 2023
|
up 201% from the same period 2023
|
up 35% from the same period 2023
|
||||||||||||||||||||||||||||||||||
| Results of Operations | ||
| Financial Overview | ||
|
Three Months Ended March 31, 2024 Compared to Three Months Ended March 31, 2023
|
|||||||||||||||||||||||||||||||||||
| Three Months Ended March 31, | 2024 - 2023 | ||||||||||||||||||||||||||||||||||
| (In millions, except per share amounts) | 2024 |
% of Revenue
(1)
|
2023 |
% of Revenue
(1)
|
$ Change | % Change | |||||||||||||||||||||||||||||
| Revenue | $ | 921.0 | 100 | % | $ | 741.5 | 100 | % | $ | 179.5 | 24 | % | |||||||||||||||||||||||
| Cost of sales | 359.1 | 39 | % | 278.9 | 38 | % | 80.2 | 29 | % | ||||||||||||||||||||||||||
| Gross profit | 561.9 | 61.0 | % | 462.6 | 62.4 | % | 99.3 | 21 | % | ||||||||||||||||||||||||||
| Operating expenses: | |||||||||||||||||||||||||||||||||||
| Research and development | 141.5 | 15 | % | 119.0 | 16 | % | 22.5 | 19 | % | ||||||||||||||||||||||||||
| Selling, general and administrative | 319.3 | 35 | % | 296.4 | 40 | % | 22.9 | 8 | % | ||||||||||||||||||||||||||
| Total operating expenses | 460.8 | 50 | % | 415.4 | 56 | % | 45.4 | 11 | % | ||||||||||||||||||||||||||
| Operating income | 101.1 | 11 | % | 47.2 | 6 | % | 53.9 | 114 | % | ||||||||||||||||||||||||||
| Other income (expense), net | 31.4 | 3 | % | 17.3 | 2 | % | 14.1 | 82 | % | ||||||||||||||||||||||||||
| Income before income taxes | 132.5 | 14 | % | 64.5 | 9 | % | 68.0 | ** | |||||||||||||||||||||||||||
| Income tax expense (benefit) | (13.9) | (2) | % | 15.9 | 2 | % | (29.8) | ** | |||||||||||||||||||||||||||
| Net income | $ | 146.4 | 16 | % | $ | 48.6 | 7 | % | $ | 97.8 | 201 | % | |||||||||||||||||||||||
| Basic net income per share | $ | 0.38 | ** | $ | 0.13 | ** | $ | 0.25 | ** | ||||||||||||||||||||||||||
| Diluted net income per share | $ | 0.36 | ** | $ | 0.12 | ** | $ | 0.24 | ** | ||||||||||||||||||||||||||
|
(1)
The sum of the individual percentages may not equal the total due to rounding.
|
|||||||||||||||||||||||||||||||||||
|
** Not meaningful
|
|||||||||||||||||||||||||||||||||||
|
Three Months Ended March 31, 2024 Compared to Three Months Ended March 31, 2023
|
||||||||||||||

| Three Months Ended March 31, | 2024 - 2023 | |||||||||||||||||||||||||||||||||||||
| 2024 | 2023 | Change in Revenues | ||||||||||||||||||||||||||||||||||||
| (In millions) | Revenue | % of Total | Revenue | % of Total | $ |
%
|
||||||||||||||||||||||||||||||||
|
United States
|
$ | 653.2 | 71% | $ | 526.0 | 71% | $ | 127.2 | 24% | |||||||||||||||||||||||||||||
| International | 267.8 | 29% | 215.5 | 29% | 52.3 | 24% | ||||||||||||||||||||||||||||||||
| Total Revenue | $ | 921.0 | 100% | $ | 741.5 | 100% | $ | 179.5 | 24% | |||||||||||||||||||||||||||||
|
Three Months Ended March 31, 2024 Compared to
Three Months Ended March 31, 2023
|
||||||||||||||||||||||||||||||||||||||
| Revenue |
The revenue increase was primarily driven by increased sales volume of our disposable sensors due to the continued growth of our worldwide customer base. We added approximately 600,000 users to our worldwide customer base in 2023.
Disposable sensor and other revenue comprised approximately 93% of total revenue and Reusable Hardware revenue comprised approximately 7% of total revenue for the three months ended March 31, 2024. Disposable sensor and other revenue comprised approximately 88% of total revenue and Reusable Hardware revenue comprised approximately 12% of total revenue for the three months ended March 31, 2023.
|
|||||||||||||||||||||||||||||||||||||
| Cost of sales & Gross profit |
Cost of sales and gross profit increased primarily due to an increase in sales volume driven by the addition of approximately 600,000 users to our worldwide customer base in 2023.
The decrease in gross profit margin percentage in the first quarter of 2024 compared to the first quarter of 2023 was primarily driven by product and channel mix changes.
|
|||||||||||||||||||||||||||||||||||||
|
|
Three Months Ended March 31, 2024 Compared to
Three Months Ended March 31, 2023
|
|||||||
| Research and development expense |
Research and development expense increased primarily due to $6.8 million in compensation-related costs most notably due to higher headcount, $5.0 million in supplies and other support costs, and $3.2 million in clinical trials costs.
We continue to believe that focused investments in research and development are critical to our future growth and competitive position in the marketplace, and to the development of new and updated products and services that are central to our core business strategy.
|
|||||||
| Selling, general and administrative expense |
Selling, general and administrative expense increased primarily due to $16.0 million in compensation-related costs most notably due to higher headcount, $5.9 million of travel related expenses, and $2.5 million related to the divestiture of certain non-CGM assets, partially offset by $9.6 million in lower advertising and marketing costs.
|
|||||||
| Other income (expense), net |
Other income (expense), net, increased primarily due to $14.0 million in interest and dividend income on our cash, cash equivalents, and marketable securities portfolio. The increase in interest income was related to a change in market interest rates, as well as an increase in the average invested balances compared to the same period in 2023.
|
|||||||
| Income tax expense (benefit) |
The income tax benefit recorded for the three months ended March 31, 2024 was primarily attributable to income tax expense from normal, recurring operations
at an estimated annual effective tax rate of 23.8%, offset by discrete excess tax benefits recognized for share-based compensation for employees, net of disallowed executive compensation and the Verily milestone payment.
The income tax expense recorded for the three months ended March 31, 2023 was primarily attributable to income tax expense from normal, recurring operations, partially offset by discrete excess tax benefits recognized for share-based compensation for employees, net of disallowed executive compensation. The decrease in our effective tax rate for the three months ended March 31, 2024 compared to the same period in 2023 is primarily attributable to impacts of the prior year tax restructuring and the Verily milestone payment.
|
|||||||
| Liquidity and Capital Resources | ||
| Overview, Capital Resources, and Capital Requirements | ||
|
The evolution of the international expansion of our business and the revenue generated by sales of our approved products and any future products;
|
Our ability to efficiently scale our operations to meet demand for our current and any future products; | The success of our research and development efforts; | |||||||||||||||||||||
| The expenses we incur in manufacturing, developing, selling and marketing our products; | The costs, timing and risks of delays of additional regulatory approvals; | The costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; | |||||||||||||||||||||
| The quality levels of our products and services; | The emergence of competing or complementary technological developments; | The terms and timing of any collaborative, licensing and other arrangements that we may establish; and | |||||||||||||||||||||
| The third-party reimbursement of our products for our customers; | The rate of progress and cost of our clinical trials and other development activities; | The acquisition of businesses, products and technologies and our ability to integrate and manage any acquired businesses, products and technologies. | |||||||||||||||||||||
|
Cash Flows
|
||
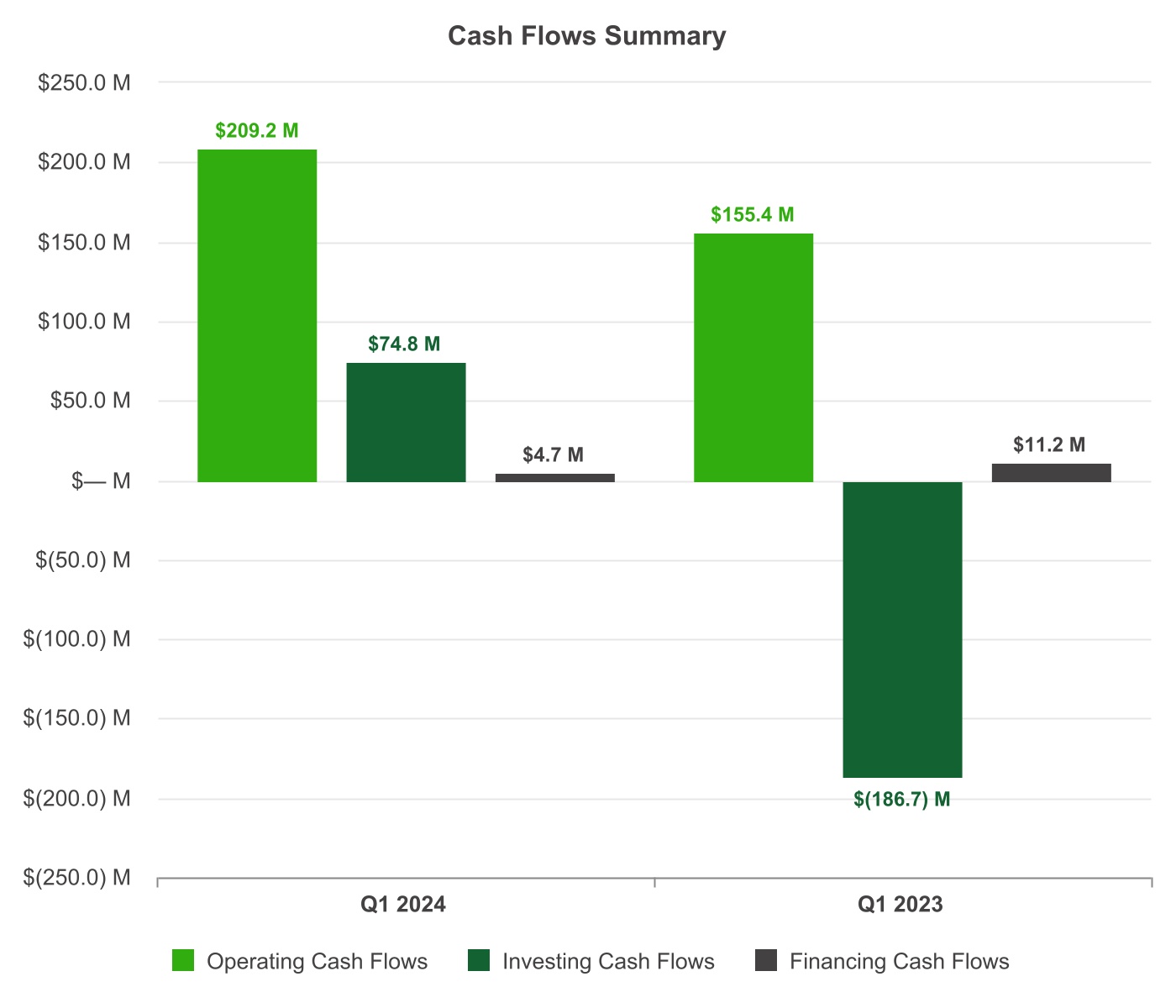
| Three Months Ended | ||||||||||||||||||||
| March 31, 2024 | March 31, 2023 | |||||||||||||||||||
| Operating Cash Flows | + |
$146.4 million of net income, $43.8 million of net non-cash adjustments, and a net increase of $19.0 million in changes of working capital balances
|
+ |
$48.6 million of net income, $55.3 million of net non-cash adjustments, and a net increase of $51.5 million in changes of working capital balances
|
||||||||||||||||
|
Net non-cash adjustments were primarily related to depreciation and amortization, share-based compensation, and deferred income taxes.
|
Net non-cash adjustments were primarily related to depreciation and amortization and share-based compensation.
|
|||||||||||||||||||
| Investing Cash Flows | + |
$129.4 million in net proceeds from marketable securities
|
- |
$112.0 million in net purchases of marketable securities
|
||||||||||||||||
| - |
$56.9 million in capital expenditures
|
- |
$74.7 million in capital expenditures
|
|||||||||||||||||
| Financing Cash Flows | + |
$13.4 million in proceeds from issuance of common stock under our employee stock plans
|
+ |
$12.3 million in proceeds from issuance of common stock under our employee stock plans
|
||||||||||||||||
| Contractual Obligations | ||
| Recent Accounting Guidance | ||
| ITEM 3 - QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK | ||
| ITEM 4 - CONTROLS AND PROCEDURES | ||
| PART II. OTHER INFORMATION | ||
| ITEM 1 - LEGAL PROCEEDINGS | ||
| ITEM 1A - RISK FACTORS | ||
| ITEM 2 - UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS | ||
| ITEM 3 - DEFAULTS UPON SENIOR SECURITIES | ||
| ITEM 4 - MINE SAFETY DISCLOSURES | ||
| ITEM 5 - OTHER INFORMATION | ||
| Name | Title | Action |
Action Date
|
Aggregate Number of Shares to be Sold
(1)
|
Expiration Date
(2)
|
||||||||||||
|
|
|
|
|
|
3/15/2025 | ||||||||||||
|
|
|
|
|
|
3/21/2025 | ||||||||||||
|
|
|
|
|
|
3/15/2025 | ||||||||||||
|
(1)
The actual number of shares sold may depend on the net shares vested as a result of tax withholding obligations, the vesting of certain PSUs, the number of shares purchased under employee stock purchase plans, one or more limit orders, as applicable, and therefore, may not be determinable at this time.
|
|||||||||||||||||
|
(2)
Each trading arrangement permitted or permits transactions through and including the date listed in the table.
|
|||||||||||||||||
|
(3)
Adopted on behalf of a trust for which Mr. Foletta serves as a trustee.
|
|||||||||||||||||
| ITEM 6 - EXHIBITS | ||
| Incorporated by Reference | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Exhibit
Number |
Exhibit Description | Form | File No. |
Date of
First Filing |
Exhibit
Number |
Provided
Herewith |
||||||||||||||||||||||||||||||||||||||||||||
| 8-K | 000-51222 | February 15, 2024 |
10.1
|
|||||||||||||||||||||||||||||||||||||||||||||||
| — | — | — | — | X | ||||||||||||||||||||||||||||||||||||||||||||||
| — | — | — | — | X | ||||||||||||||||||||||||||||||||||||||||||||||
| — | — | — | — | X | ||||||||||||||||||||||||||||||||||||||||||||||
| — | — | — | — | X | ||||||||||||||||||||||||||||||||||||||||||||||
| 101.INS |
Inline XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document
|
X | ||||||||||||||||||||||||||||||||||||||||||||||||
| 101.SCH | Inline XBRL Taxonomy Extension Schema Document | X | ||||||||||||||||||||||||||||||||||||||||||||||||
| 101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase Document | X | ||||||||||||||||||||||||||||||||||||||||||||||||
| 101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document | X | ||||||||||||||||||||||||||||||||||||||||||||||||
| 101.LAB | Inline XBRL Taxonomy Extension Label Linkbase Document | X | ||||||||||||||||||||||||||||||||||||||||||||||||
| 101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase Document | X | ||||||||||||||||||||||||||||||||||||||||||||||||
| Incorporated by Reference | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Exhibit
Number |
Exhibit Description | Form | File No. |
Date of
First Filing |
Exhibit
Number |
Provided
Herewith |
||||||||||||||||||||||||||||||||||||||||||||
| 104 |
Cover Page Interactive Data File - the cover page from the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2024 is formatted in Inline XBRL
|
X | ||||||||||||||||||||||||||||||||||||||||||||||||
| * | Portions of this exhibit have been omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K. | |||||||
| ** |
This certification is not deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liability of that section. Such certification will not be deemed to be incorporated by reference into any filing under the Securities Act or the Exchange Act, except to the extent that Dexcom specifically incorporates it by reference.
|
|||||||
| SIGNATURES | ||
|
DEXCOM, INC.
(Registrant)
|
|||||||||||||||||
| Dated: | April 25, 2024 | By: | /s/ KEVIN R. SAYER | ||||||||||||||
|
Kevin R. Sayer,
Chairman of the Board of Directors,
President and Chief Executive Officer
(Principal Executive Officer)
|
|||||||||||||||||
| Dated: | April 25, 2024 | By: | /s/ JEREME M. SYLVAIN | ||||||||||||||
|
Jereme M. Sylvain,
Executive Vice President and Chief Financial Officer
(Principal Financial and Accounting Officer)
|
|||||||||||||||||