|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Mark One)
|
|
|
x
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
For the quarterly period ended March 31, 2019
|
|
OR
|
|
|
☐
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
For the transition period from to
|

|
Delaware
|
14-1902018
|
|
(State or Other Jurisdiction of
Incorporation or Organization)
|
(I.R.S. Employer
Identification No.)
|
|
|
|
|
400 Professional Drive, Suite 400
|
|
|
Gaithersburg, Maryland
|
20879
|
|
(Address of Principal Executive Offices)
|
(Zip Code)
|
|
Securities registered pursuant to Section 12(b) of the Act
|
||
|
Title of each class
|
Trading Symbol(s)
|
Name of each exchange on which registered
|
|
Common Stock, Par Value $0.001 per share
|
EBS
|
New York Stock Exchange
|
|
Large accelerated filer
x
|
Accelerated filer ☐
|
|
|
Non-accelerated filer ☐
|
Smaller reporting company ☐
|
|
|
Emerging growth company ☐
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
•
|
appropriations for the procurement of BioThrax® (Anthrax Vaccine Adsorbed) and our other products addressing public health threats (PHTs);
|
|
•
|
our ability to perform under our contracts with the U.S. government (USG) related to BioThrax, our AV7909
product candidate, and our other public health threat products, including the timing of and specifications relating to deliveries;
|
|
•
|
our ability to commence deliveries based on BARDA’s procurement of AV7909 (anthrax vaccine adsorbed with CPG 7909 adjuvant) for the Strategic National Stockpile (SNS), to receive Emergency Use Authorization (EUA) and eventual licensure of AV7909 from the U.S. Food and Drug Administration (FDA);
|
|
•
|
the availa
bility of funding for our U.S. government grants and contracts;
|
|
•
|
our ability to secure follow-on procurement contracts for our PHTs that are under procurement contracts that have expired or will be expiring;
|
|
•
|
our ability and the ability of our collaborators to
defend underlying patents from infringement by generic naloxone entrants
;
|
|
•
|
our ability to identify and acquire companies, businesses, products or product candidates that satisfy our selection criteria;
|
|
•
|
our ability to successfully integrate and realize the benefits of our acquisitions of PaxVax Holding Company Ltd. (PaxVax) and Adapt Pharma Limited (Adapt), both of which were acquired in October 2018;
|
|
•
|
our ability to successfully identify and respond to new development contracts with the USG, as well as successfully maintain, through achievement of development milestones, current development contracts with the USG;
|
|
•
|
our ability and the ability of our contractors and suppliers to maintain compliance with current good manufacturing practices and other regulatory obligations;
|
|
•
|
the results of regulatory inspections;
|
|
•
|
the operating and financial restrictions placed on us and our subsidiaries under our senior secured credit facilities;
|
|
•
|
our ability to obtain and maintain regulatory approvals for our product candidates and the timing of any such approvals
;
|
|
•
|
the procurement of products by USG entities under regulatory exemptions prior to approval by the FDA and corresponding procurement by government entities outside of the United States under regulatory exemptions prior to approval by the corresponding regulatory authorities in the applicable country;
|
|
•
|
the success of our commercialization, marketing and manufacturing capabilities and strategy; and
|
|
•
|
the accuracy of our estimates regarding future revenues, expenses, capital requirements and needs for additional financing.
|
|
|
March 31, 2019
|
December 31, 2018
|
|||||
|
ASSETS
|
|
||||||
|
Current assets:
|
|
|
|||||
|
Cash and cash equivalents
|
$
|
137.2
|
|
$
|
112.2
|
|
|
|
Restricted cash
|
0.2
|
|
0.2
|
|
|||
|
Accounts receivable, net
|
121.5
|
|
262.5
|
|
|||
|
Inventories
|
211.0
|
|
205.8
|
|
|||
|
Prepaid expenses and other current assets
|
58.6
|
|
40.1
|
|
|||
|
Total current assets
|
528.5
|
|
620.8
|
|
|||
|
Property, plant and equipment, net
|
513.4
|
|
510.2
|
|
|||
|
Intangible assets, net
|
757.1
|
|
761.6
|
|
|||
|
In-process research and development
|
41.0
|
|
50.0
|
|
|||
|
Goodwill
|
267.7
|
|
259.7
|
|
|||
|
Other assets
|
46.0
|
|
27.1
|
|
|||
|
Total assets
|
$
|
2,153.7
|
|
$
|
2,229.4
|
|
|
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
78.6
|
|
$
|
80.7
|
|
|
|
Accrued expenses
|
49.6
|
|
30.7
|
|
|||
|
Contingent consideration, current portion
|
62.7
|
|
5.6
|
|
|||
|
Accrued compensation
|
36.9
|
|
58.2
|
|
|||
|
Long-term indebtedness, current portion
|
10.1
|
|
10.1
|
|
|||
|
Other current liabilities
|
10.5
|
|
15.1
|
|
|||
|
Total current liabilities
|
248.4
|
|
200.4
|
|
|||
|
Contingent consideration
|
10.0
|
|
54.4
|
|
|||
|
Long-term indebtedness
|
732.4
|
|
784.5
|
|
|||
|
Deferred tax liability
|
66.4
|
|
67.5
|
|
|||
|
Deferred revenue, net of current portion
|
64.7
|
|
62.5
|
|
|||
|
Other liabilities
|
44.3
|
|
49.2
|
|
|||
|
Total liabilities
|
$
|
1,166.2
|
|
$
|
1,218.5
|
|
|
|
Commitments and contingencies (Notes 8 & 14)
|
|
|
|
|
|||
|
Stockholders' equity:
|
|||||||
|
Preferred stock, $0.001 par value; 15.0 shares authorized, no shares issued or outstanding at both 2019 and 2018
|
—
|
|
—
|
|
|||
|
Common stock, $0.001 par value; 200.0 shares authorized, 52.6 shares issued and 51.4 shares outstanding at 2019; 52.4 shares issued and 51.2 shares outstanding at 2018
|
0.1
|
|
0.1
|
|
|||
|
Treasury stock, at cost, 1.2 common shares at both 2019 and 2018
|
(39.6
|
)
|
(39.6
|
)
|
|||
|
Additional paid-in capital
|
690.2
|
|
688.6
|
|
|||
|
Accumulated other comprehensive loss
|
(4.5
|
)
|
(5.5
|
)
|
|||
|
Retained earnings
|
341.3
|
|
367.3
|
|
|||
|
Total stockholders' equity
|
987.5
|
|
1,010.9
|
|
|||
|
Total liabilities and stockholders' equity
|
$
|
2,153.7
|
|
$
|
2,229.4
|
|
|
|
|
Three Months Ended March 31,
|
||||||
|
|
2019
|
2018
|
|||||
|
Revenues:
|
|
|
|
||||
|
Product sales, net
|
$
|
153.0
|
|
$
|
75.8
|
|
|
|
Contract manufacturing
|
15.9
|
|
26.1
|
|
|||
|
Contracts and grants
|
21.7
|
|
15.9
|
|
|||
|
Total revenues
|
190.6
|
|
117.8
|
|
|||
|
Operating expenses:
|
|||||||
|
Cost of product sales and contract manufacturing
|
91.8
|
|
54.3
|
|
|||
|
Research and development
|
46.1
|
|
29.1
|
|
|||
|
Selling, general and administrative
|
65.4
|
|
40.0
|
|
|||
|
Amortization of intangible assets
|
14.5
|
|
3.9
|
|
|||
|
Total operating expenses
|
217.8
|
|
127.3
|
|
|||
|
Loss from operations
|
(27.2
|
)
|
(9.5
|
)
|
|||
|
Other income (expense):
|
|||||||
|
Interest expense
|
(9.6
|
)
|
(0.2
|
)
|
|||
|
Other income (expense), net
|
(1.0
|
)
|
0.3
|
|
|||
|
Total other income (expense), net
|
(10.6
|
)
|
0.1
|
|
|||
|
Loss before benefit from income taxes
|
(37.8
|
)
|
(9.4
|
)
|
|||
|
Income tax benefit
|
(11.8
|
)
|
(4.5
|
)
|
|||
|
Net loss
|
$
|
(26.0
|
)
|
$
|
(4.9
|
)
|
|
|
Net loss per common share
|
|||||||
|
Basic
|
$
|
(0.51
|
)
|
$
|
(0.10
|
)
|
|
|
Diluted
|
$
|
(0.51
|
)
|
$
|
(0.10
|
)
|
|
|
Shares used in computing loss per share
|
|||||||
|
Basic
|
51.2
|
|
49.6
|
|
|||
|
Diluted
|
51.2
|
|
49.6
|
|
|||
|
|
Three Months Ended March 31,
|
||||||
|
|
2019
|
2018
|
|||||
|
Net loss
|
$
|
(26.0
|
)
|
$
|
(4.9
|
)
|
|
|
Other comprehensive income (loss), net of tax:
|
|
|
|
|
|||
|
Foreign currency translations, net of tax
|
1.2
|
|
0.4
|
|
|||
|
Unrealized losses on pension benefit obligation
|
(0.2
|
)
|
—
|
|
|||
|
Total other comprehensive income, net of tax
|
1.0
|
|
0.4
|
|
|||
|
Comprehensive loss
|
$
|
(25.0
|
)
|
$
|
(4.5
|
)
|
|
|
|
Three Months Ended March 31,
|
||||||
|
|
2019
|
2018
|
|||||
|
Cash flows from operating activities:
|
|||||||
|
Net Loss
|
$
|
(26.0
|
)
|
$
|
(4.9
|
)
|
|
|
Adjustments to reconcile to net cash provided by (used in) operating activities:
|
|||||||
|
Share-based compensation expense
|
6.8
|
|
7.3
|
|
|||
|
Depreciation and amortization
|
26.6
|
|
12.3
|
|
|||
|
Amortization of deferred financing costs
|
0.7
|
|
0.1
|
|
|||
|
Deferred income taxes
|
(11.4
|
)
|
(4.5
|
)
|
|||
|
Change in fair value of contingent consideration
|
1.7
|
|
1.0
|
|
|||
|
Other
|
(0.1
|
)
|
0.1
|
|
|||
|
Changes in operating assets and liabilities:
|
|||||||
|
Accounts receivable
|
141.6
|
|
21.8
|
|
|||
|
Inventories
|
(5.2
|
)
|
(12.4
|
)
|
|||
|
Prepaid expenses and other assets
|
(16.6
|
)
|
(7.7
|
)
|
|||
|
Accounts payable
|
4.2
|
|
3.6
|
|
|||
|
Accrued expenses
|
1.7
|
|
2.2
|
|
|||
|
Accrued compensation
|
(21.3
|
)
|
(13.4
|
)
|
|||
|
Deferred revenue
|
2.1
|
|
(6.5
|
)
|
|||
|
Net cash provided by (used in) operating activities:
|
104.8
|
|
(1.0
|
)
|
|||
|
Cash flows from investing activities:
|
|||||||
|
Purchases of property, plant and equipment and other
|
(21.4
|
)
|
(11.6
|
)
|
|||
|
Net cash used in investing activities:
|
(21.4
|
)
|
(11.6
|
)
|
|||
|
Cash flows from financing activities:
|
|||||||
|
Proceeds from revolving credit facility
|
30.0
|
|
—
|
|
|||
|
Principal payments on revolving credit facility
|
(80.0
|
)
|
—
|
|
|||
|
Principal payments on term loan facility
|
(2.8
|
)
|
—
|
|
|||
|
Issuances of stock under share-based benefit plans
|
0.9
|
|
4.7
|
|
|||
|
Taxes paid on behalf of employees for equity activity
|
(6.0
|
)
|
(5.9
|
)
|
|||
|
Contingent consideration payments
|
(0.5
|
)
|
(0.8
|
)
|
|||
|
Purchase of treasury stock
|
—
|
|
(0.1
|
)
|
|||
|
Net cash used in financing activities:
|
(58.4
|
)
|
(2.1
|
)
|
|||
|
Net increase (decrease) in cash, cash equivalents and restricted cash
|
25.0
|
|
(14.7
|
)
|
|||
|
Cash, cash equivalents and restricted cash at beginning of period
|
112.4
|
|
179.3
|
|
|||
|
Cash, cash equivalents and restricted cash at end of period
|
$
|
137.4
|
|
$
|
164.6
|
|
|
|
|
$0.001 Par Value Common Stock
|
Additional Paid-In Capital
|
Treasury Stock
|
Accumulated Other Comprehensive Loss
|
Retained Earnings
|
Total Stockholders' Equity
|
||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||||
|
Three Months Ended March 31, 2019
|
||||||||||||||||||||||||||||||
|
Balance at December 31, 2018
|
52.4
|
|
$
|
0.1
|
|
$
|
688.6
|
|
(1.2
|
)
|
$
|
(39.6
|
)
|
$
|
(5.5
|
)
|
$
|
367.3
|
|
$
|
1,010.9
|
|
||||||||
|
Employee equity plans activity
|
0.2
|
|
—
|
|
1.6
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1.6
|
|
||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(26.0
|
)
|
(26.0
|
)
|
||||||||||||||
|
Other comprehensive income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1.0
|
|
—
|
|
1.0
|
|
||||||||||||||
|
Balance at March 31, 2019
|
52.6
|
|
$
|
0.1
|
|
$
|
690.2
|
|
(1.2
|
)
|
$
|
(39.6
|
)
|
$
|
(4.5
|
)
|
$
|
341.3
|
|
$
|
987.5
|
|
||||||||
|
Three Months Ended March 31, 2018
|
||||||||||||||||||||||||||||||
|
Balance at December 31, 2017
|
50.6
|
|
$
|
0.1
|
|
$
|
618.3
|
|
(1.2
|
)
|
$
|
(39.5
|
)
|
$
|
(3.7
|
)
|
$
|
337.1
|
|
$
|
912.3
|
|
||||||||
|
Adoption of new revenue accounting standard (ASC 606), net of tax
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(32.5
|
)
|
(32.5
|
)
|
||||||||||||||
|
Balance at January 1, 2018
|
50.6
|
|
0.1
|
|
618.3
|
|
(1.2
|
)
|
(39.5
|
)
|
(3.7
|
)
|
304.6
|
|
879.8
|
|
||||||||||||||
|
Employee equity plans activity
|
0.4
|
|
—
|
|
6.1
|
|
—
|
|
—
|
|
—
|
|
—
|
|
6.1
|
|
||||||||||||||
|
Treasury stock
|
—
|
|
—
|
|
—
|
|
—
|
|
(0.1
|
)
|
—
|
|
—
|
|
(0.1
|
)
|
||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(4.9
|
)
|
(4.9
|
)
|
||||||||||||||
|
Other comprehensive income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
0.4
|
|
—
|
|
0.4
|
|
||||||||||||||
|
Balance at March 31, 2018
|
51.0
|
|
$
|
0.1
|
|
$
|
624.4
|
|
(1.2
|
)
|
$
|
(39.6
|
)
|
$
|
(3.3
|
)
|
$
|
299.7
|
|
$
|
881.3
|
|
||||||||
|
•
|
Vaccines and Anti-Infectives
-
BioThrax® (Anthrax Vaccine Adsorbed), ACAM2000® (Smallpox (Vaccinia) Vaccine, Live), Vaxchora® (Cholera Vaccine, Live, Oral), and Vivotif® (Typhoid Vaccine, Live, Oral Ty21a).
|
|
•
|
Devices
-
NARCAN® (naloxone HCl) Nasal Spray for opioid overdose, RSDL® (Reactive Skin Decontamination Lotion Kit), and the Trobigard® (atropine sulfate, obidoxime chloride a nerve agent countermeasure) auto-injector.
|
|
•
|
Antibody Therapeutics -
raxibacumab (Anthrax Monoclonal antibody therapeutic for anthrax),
Anthrasil®( Anthrax Immune Globulin Intravenous (Human)), BAT®(Botulism Antitoxin Heptavalent), and VIGIV (Vaccinia Immune Globulin Intravenous (Human) therapeutic) for complications from smallpox vaccinations.
|
|
October 15, 2018
|
||||
|
Cash
|
$
|
581.5
|
|
|
|
Equity
|
37.7
|
|
||
|
Fair value of contingent purchase consideration
|
48.0
|
|
||
|
Preliminary purchase consideration
|
667.2
|
|
||
|
Adjustments
|
1.5
|
|
||
|
Updated purchase consideration
|
$
|
668.7
|
|
|
|
October 15, 2018
|
Measurement Period Adjustments
|
Updated October 15, 2018
|
|||||||||
|
Estimated fair value of tangible assets acquired and liabilities assumed:
|
|||||||||||
|
Cash
|
$
|
17.7
|
|
$
|
—
|
|
$
|
17.7
|
|
||
|
Accounts receivable
|
21.3
|
|
—
|
|
21.3
|
|
|||||
|
Inventory
|
41.4
|
|
—
|
|
41.4
|
|
|||||
|
Prepaid expenses and other assets
|
7.8
|
|
3.0
|
|
10.8
|
|
|||||
|
Accounts payable
|
(32.2
|
)
|
—
|
|
(32.2
|
)
|
|||||
|
Accrued expenses and other liabilities
|
(50.4
|
)
|
—
|
|
(50.4
|
)
|
|||||
|
Deferred tax liability, net
|
(62.4
|
)
|
(0.5
|
)
|
(62.9
|
)
|
|||||
|
Total estimated fair value of tangible assets acquired and liabilities assumed
|
(56.8
|
)
|
2.5
|
|
(54.3
|
)
|
|||||
|
Acquired in-process research and development
|
41.0
|
|
—
|
|
41.0
|
|
|||||
|
Acquired intangible assets
|
534.0
|
|
—
|
|
534.0
|
|
|||||
|
Goodwill
|
149.0
|
|
(1.0
|
)
|
148.0
|
|
|||||
|
Total purchase price
|
$
|
667.2
|
|
$
|
1.5
|
|
$
|
668.7
|
|
||
|
October 4, 2018
|
Measurement Period Adjustments
|
Updated October 4, 2018
|
|||||||||
|
Estimated fair value of tangible assets acquired and liabilities assumed:
|
|||||||||||
|
Cash
|
$
|
9.0
|
|
$
|
—
|
|
$
|
9.0
|
|
||
|
Accounts receivable
|
4.1
|
|
—
|
|
4.1
|
|
|||||
|
Inventory
|
19.7
|
|
—
|
|
19.7
|
|
|||||
|
Prepaid expenses and other assets
|
12.2
|
|
—
|
|
12.2
|
|
|||||
|
Property, plant and equipment
|
57.8
|
|
—
|
|
57.8
|
|
|||||
|
Deferred tax assets
|
3.8
|
|
—
|
|
3.8
|
|
|||||
|
Accounts payable
|
(3.5
|
)
|
—
|
|
(3.5
|
)
|
|||||
|
Accrued expenses and other liabilities
|
(33.6
|
)
|
—
|
|
(33.6
|
)
|
|||||
|
Total estimated fair value of tangible assets acquired and liabilities assumed
|
69.5
|
|
—
|
|
69.5
|
|
|||||
|
Acquired in-process research and development
|
9.0
|
|
(9.0
|
)
|
—
|
|
|||||
|
Acquired intangible assets
|
133.0
|
|
—
|
|
133.0
|
|
|||||
|
Goodwill
|
61.6
|
|
9.0
|
|
70.6
|
|
|||||
|
Total purchase price
|
$
|
273.1
|
|
$
|
—
|
|
$
|
273.1
|
|
||
|
March 31, 2019
|
|||
|
Revenue
|
$
|
74.9
|
|
|
Operating loss
|
(3.8
|
)
|
|
|
March 31, 2019
|
December 31, 2018
|
|||||||
|
Raw materials and supplies
|
$
|
57.3
|
|
$
|
51.8
|
|
||
|
Work-in-process
|
112.7
|
|
103.2
|
|
||||
|
Finished goods
|
41.0
|
|
50.8
|
|
||||
|
Total inventories
|
$
|
211.0
|
|
$
|
205.8
|
|
||
|
March 31, 2019
|
December 31, 2018
|
|||||||
|
Land and improvements
|
$
|
44.3
|
|
$
|
44.6
|
|
||
|
Buildings, building improvements and leasehold improvements
|
223.4
|
|
216.2
|
|
||||
|
Furniture and equipment
|
316.8
|
|
293.9
|
|
||||
|
Software
|
55.5
|
|
55.2
|
|
||||
|
Construction-in-progress
|
57.2
|
|
71.8
|
|
||||
|
Property, plant and equipment, gross
|
697.2
|
|
681.7
|
|
||||
|
Less: Accumulated depreciation and amortization
|
(183.8
|
)
|
(171.5
|
)
|
||||
|
Total property, plant and equipment, net
|
$
|
513.4
|
|
$
|
510.2
|
|
||
|
March 31, 2019
|
||||
|
Operating lease cost:
|
||||
|
Amortization of right-of-use assets
|
$
|
0.6
|
|
|
|
Interest on lease liabilities
|
0.1
|
|
||
|
Total operating lease cost
|
$
|
0.7
|
|
|
|
(In millions, except lease term and discount rate)
|
March 31, 2019
|
|||
|
Operating lease right-of-use assets
|
$
|
12.9
|
|
|
|
Other current liabilities
|
2.0
|
|
||
|
Operating lease liabilities
|
11.6
|
|
||
|
Total operating lease liabilities
|
$
|
13.6
|
|
|
|
Operating leases:
|
|
|
||
|
Weighted Average Remaining Lease Term
|
9.3
|
|
||
|
Weighted Average Discount Rate
|
4.27
|
%
|
||
|
March 31, 2019
|
|||||||||||||||||||||||
|
Estimated Life (years)
|
Cost
|
Measurement Period Adjustment
|
Additions
|
Gross Total
|
Accumulated
Amortization
|
Net
|
|||||||||||||||||
|
Intangible assets, net
|
5-22
|
$
|
818.4
|
|
—
|
|
$
|
10.0
|
|
$
|
828.4
|
|
$
|
(71.3
|
)
|
$
|
757.1
|
|
|||||
|
IPR&D
|
indefinite
|
50.0
|
|
(9.0
|
)
|
—
|
|
41.0
|
|
—
|
|
41.0
|
|
||||||||||
|
Goodwill
|
indefinite
|
259.7
|
|
8.0
|
|
—
|
|
267.7
|
|
—
|
|
267.7
|
|
||||||||||
|
December 31, 2018
|
||||||||||||||||||||||
|
Estimated Life (years)
|
Cost
|
Measurement Period Adjustment
|
Additions
|
Gross Total
|
Accumulated
Amortization
|
Net
|
||||||||||||||||
|
Intangible assets, net
|
5-22
|
$
|
151.4
|
|
—
|
|
667.0
|
|
$
|
818.4
|
|
$
|
(56.8
|
)
|
$
|
761.6
|
|
|||||
|
IPR&D
|
indefinite
|
50.0
|
|
—
|
|
—
|
|
50.0
|
|
—
|
|
50.0
|
|
|||||||||
|
Goodwill
|
indefinite
|
49.1
|
|
—
|
|
210.6
|
|
259.7
|
|
—
|
|
259.7
|
|
|||||||||
|
|
|||
|
Balance at December 31, 2018
|
$
|
60.0
|
|
|
Milestone achievement - asset acquisition
|
10.0
|
|
|
|
Measurement period adjustment
|
1.5
|
|
|
|
Change in fair value
|
1.7
|
|
|
|
Settlements
|
(0.5
|
)
|
|
|
Balance at March 31, 2019
|
$
|
72.7
|
|
|
Three Months Ended March 31, 2019
|
Three Months Ended March 31, 2018
|
|||||||||||||||||||||||
|
|
U.S
Government
|
Non-U.S.
Government
|
Total
|
U.S
Government
|
Non-U.S.
Government
|
Total
|
||||||||||||||||||
|
Product sales
|
$
|
73.3
|
|
$
|
79.7
|
|
$
|
153.0
|
|
$
|
66.0
|
|
$
|
9.8
|
|
$
|
75.8
|
|
||||||
|
Contract manufacturing
|
—
|
|
15.9
|
|
15.9
|
|
—
|
|
26.1
|
|
26.1
|
|
||||||||||||
|
Contracts and grants
|
20.4
|
|
1.3
|
|
21.7
|
|
14.8
|
|
1.1
|
|
15.9
|
|
||||||||||||
|
Total revenues
|
$
|
93.7
|
|
$
|
96.9
|
|
$
|
190.6
|
|
$
|
80.8
|
|
$
|
37.0
|
|
$
|
117.8
|
|
||||||
|
December 31, 2018
|
$
|
73.1
|
|
|
|
Deferral of revenue
|
4.9
|
|
||
|
Revenue recognized
|
(2.8
|
)
|
||
|
March 31, 2019
|
$
|
75.2
|
|
|
|
March 31, 2019
|
December 31, 2018
|
|||||||
|
Billed, net
|
$
|
89.6
|
|
|
$
|
234.0
|
|
|
|
Unbilled
|
31.9
|
|
|
28.5
|
|
|||
|
Total, net
|
$
|
121.5
|
|
|
$
|
262.5
|
|
|
|
(in millions, except share and per share data)
|
Three Months Ended March 31,
|
|||||||
|
2019
|
2018
|
|||||||
|
Numerator:
|
|
|
||||||
|
Net loss
|
$
|
(26.0
|
)
|
$
|
(4.9
|
)
|
||
|
Denominator:
|
||||||||
|
Weighted-average number of shares—basic
|
51.2
|
|
49.6
|
|
||||
|
Dilutive securities—equity awards
|
—
|
|
—
|
|
||||
|
Weighted-average number of shares—diluted
|
51.2
|
|
49.6
|
|
||||
|
Net loss per share - basic
|
$
|
(0.51
|
)
|
$
|
(0.10
|
)
|
||
|
Net loss per share - diluted
|
$
|
(0.51
|
)
|
$
|
(0.10
|
)
|
||
|
Three Months Ended March 31, 2019
|
||||
|
Net service cost
|
$
|
0.3
|
|
|
|
Expected return on plan assets, net of expenses
|
(0.1
|
)
|
||
|
Total
|
$
|
0.2
|
|
|
|
March 31, 2019
|
December 31, 2018
|
|||||||
|
Cash and cash equivalents
|
$
|
137.2
|
|
$
|
112.2
|
|
||
|
Restricted cash
|
0.2
|
|
0.2
|
|
||||
|
Total cash, cash equivalents and restricted cash
|
$
|
137.4
|
|
$
|
112.4
|
|
||
|
•
|
BioThrax® (Anthrax Vaccine Adsorbed), the only vaccine licensed by the FDA for the general use prophylaxis and post-exposure prophylaxis of anthrax disease;
|
|
•
|
ACAM2000® (Smallpox (Vaccinia) Vaccine, Live), the only smallpox vaccine licensed by the FDA for active immunization against smallpox disease for persons determined to be at high risk for smallpox infection;
|
|
•
|
Vivotif® (Typhoid Vaccine Live Oral Ty21a), the only oral vaccine licensed by the FDA for the prevention of typhoid fever; and
|
|
•
|
Vaxchora® (Cholera Vaccine, Live, Oral), the only FDA-licensed vaccine for the prevention of cholera.
|
|
•
|
NARCAN® (naloxone HCl) Nasal Spray, the first needle-free formulation of naloxone approved by the FDA and Health Canada, for the emergency treatment of known or suspected opioid overdose as manifested by respiratory and/or central nervous system depression;
|
|
•
|
RSDL® (Reactive Skin Decontamination Lotion Kit), the only medical device cleared by the FDA to remove or neutralize the following chemical warfare agents from the skin: tabun, sarin, soman, cyclohexyl sarin, VR, VX, mustard gas and T-2 toxin; and
|
|
•
|
Trobigard
®
(atropine sulfate, obidoxime chloride), an auto-injector device designed for intramuscular self-injection of atropine sulfate and obidoxime chloride, as a nerve agent countermeasure. This product is not currently approved or cleared by the FDA or any similar regulatory body, and is only distributed to authorized government buyers for use outside the United States. This product is not dist
ributed in the United States.
|
|
•
|
raxibacumab (Anthrax Monoclonal), the first fully human monoclonal antibody therapeutic licensed by the FDA for the treatment and prophylaxis of inhalational anthrax;
|
|
•
|
Anthrasil® (Anthrax Immune Globulin Intravenous (Human)), the only polyclonal antibody therapeutic licensed by the FDA and Health Canada for the treatment of inhalational anthrax;
|
|
•
|
BAT® (Botulism Antitoxin Heptavalent (A,B,C,D,E,F,G)-(Equine)), the only heptavalent antibody therapeutic licensed by the FDA and Health Canada for the treatment of botulism; and
|
|
•
|
VIGIV (Vaccinia Immune Globulin Intravenous (Human)), the only polyclonal antibody therapeutic licensed by the FDA and Health Canada to address certain complications from smallpox vaccination.
|
|
•
|
On April 16, 2019, we announced results from an interim analysis of our Phase 2 clinical study evaluating the safety and immunogenicity of the Company’s chikungunya virus virus-like particle vaccine candidate across a series of dosing regimens. The interim analysis has shown that with a single dose administered, up to 98% of study participants produced a neutralizing antibody response against the chikungunya virus by day 7. Further, the immune response was shown to be persistent through the six-month visit, including in the one-dose regimen.
|
|
•
|
On March 19, 2019, we announced the initiation of a Phase 3 trial to evaluate the lot consistency, immunogenicity, and safety of AV7909 (anthrax vaccine adsorbed with CPG 7909 adjuvant) following a two-dose schedule administered intramuscularly in healthy adults. AV7909 is being developed for post-exposure prophylaxis of disease resulting from suspected or confirmed Bacillus anthracis exposure.
|
|
•
|
On February 28, 2019, we announced that we have signed an indefinite-delivery, indefinite-quantity contract with the U.S. Department of State to establish a long-term, reliable, and stable supply chain for medical countermeasures that address the treatment prosed by chemical warfare agents. The contract is comprised of a five-year base period of performance along with five one-year option periods with a total contract value of a minimum of approximately $7 million to a maximum of
|
|
Three Months Ended March 31,
|
|||||||||||||||
|
2019
|
2018
|
Change
|
% Change
|
||||||||||||
|
Product sales net:
|
|
|
|
|
|||||||||||
|
NARCAN Nasal Spray
|
$
|
65.5
|
|
|
$
|
—
|
|
|
$
|
65.5
|
|
NM
|
|
||
|
ACAM2000
|
45.6
|
|
21.8
|
|
23.8
|
|
109
|
%
|
|||||||
|
BioThrax
|
11.7
|
|
20.2
|
|
(8.5
|
)
|
(42
|
%)
|
|||||||
|
Other
|
30.2
|
|
33.8
|
|
(3.6
|
)
|
(11
|
)%
|
|||||||
|
Total product sales, net
|
153.0
|
|
75.8
|
|
77.2
|
|
102
|
%
|
|||||||
|
Contract manufacturing
|
15.9
|
|
26.1
|
|
(10.2
|
)
|
(39
|
)%
|
|||||||
|
Contracts and grants
|
21.7
|
|
15.9
|
|
5.8
|
|
36
|
%
|
|||||||
|
Total revenues
|
190.6
|
|
117.8
|
|
72.8
|
|
62
|
%
|
|||||||
|
Operating expenses:
|
|||||||||||||||
|
Cost of product sales and contract manufacturing
|
91.8
|
|
54.3
|
|
37.5
|
|
69
|
%
|
|||||||
|
Research and development
|
46.1
|
|
29.1
|
|
17.0
|
|
58
|
%
|
|||||||
|
Selling, general and administrative
|
65.4
|
|
40.0
|
|
25.4
|
|
64
|
%
|
|||||||
|
Amortization of intangible assets
|
14.5
|
|
3.9
|
|
10.6
|
|
NM
|
|
|||||||
|
Total operating expenses
|
217.8
|
|
127.3
|
|
90.5
|
|
71
|
%
|
|||||||
|
Loss from operations
|
(27.2
|
)
|
(9.5
|
)
|
(17.7
|
)
|
NM
|
|
|||||||
|
Other income (expense):
|
|||||||||||||||
|
Interest expense
|
(9.6
|
)
|
(0.2
|
)
|
(9.4
|
)
|
NM
|
|
|||||||
|
Other income (expense), net
|
(1.0
|
)
|
0.3
|
|
(1.3
|
)
|
NM
|
|
|||||||
|
Total other income (expense), net
|
(10.6
|
)
|
0.1
|
|
(10.7
|
)
|
NM
|
|
|||||||
|
Loss before benefit from income taxes
|
(37.8
|
)
|
(9.4
|
)
|
(28.4
|
)
|
NM
|
|
|||||||
|
Income tax benefit
|
(11.8
|
)
|
(4.5
|
)
|
(7.3
|
)
|
NM
|
|
|||||||
|
Net loss
|
$
|
(26.0
|
)
|
$
|
(4.9
|
)
|
$
|
(21.1
|
)
|
NM
|
|
||||

|
NARCAN Nasal Spray
|
Other Product Sales
|
||
|
ACAM2000
|
Contract Manufacturing
|
||
|
BioThrax
|
Contracts and Grants
|
||
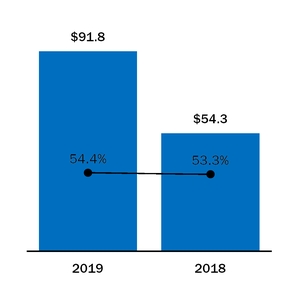
|
Cost of Product Sales and Contract Manufacturing
|
|
|
l
|
Cost of Sales as a Percentage of Product Sales and Contract Manufacturing Revenue
|

|
Research and Development expense
|
|
|
l
|
Research and Development expense, net of contracts and grants revenue
|

|
Selling, General and Administrative
|
|
|
l
|
SG&A as a percentage of total revenue
|
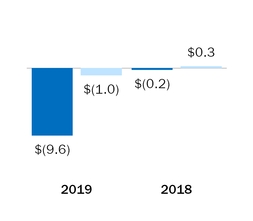
|
Interest expense
|
|
|
Other income (expense)
|
|
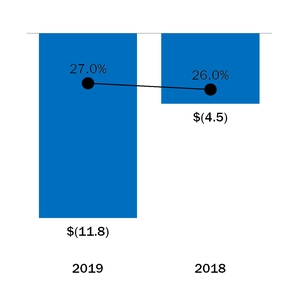
|
Benefit from income taxes
|
|
|
l
|
Effective tax rate
|
|
Three months ended March 31, 2019
|
||||||||
|
2019
|
2018
|
|||||||
|
Net cash provided by (used in):
|
|
|
||||||
|
Operating activities(i)
|
$
|
104.8
|
|
$
|
(1.0
|
)
|
||
|
Investing activities
|
(21.4
|
)
|
(11.6
|
)
|
||||
|
Financing activities
|
(58.4
|
)
|
(2.1
|
)
|
||||
|
Net increase (decrease) in cash and cash equivalents
|
$
|
25.0
|
|
$
|
(14.7
|
)
|
||
|
•
|
existing cash and cash equivalents;
|
|
•
|
net proceeds from the sale of our products and contract manufacturing services;
|
|
•
|
development contracts and grants funding; and
|
|
•
|
our senior secured credit facilities and any other lines of credit we may establish from time to time.
|
|
•
|
the level, timing and cost of product sales and contract manufacturing services;
|
|
•
|
the extent to which we acquire or invest in and integrate companies, businesses, products or technologies;
|
|
•
|
the acquisition of new facilities and capital improvements to new or existing facilities;
|
|
•
|
the payment obligations under our indebtedness;
|
|
•
|
the scope, progress, results and costs of our development activities;
|
|
•
|
our ability to obtain funding from collaborative partners, government entities and non-governmental organizations for our development programs;
|
|
•
|
the extent to which we repurchase additional common stock under our authorized share repurchase program; and
|
|
•
|
the costs of commercialization activities, including product marketing, sales and distribution.
|
|
•
|
the possibility that we may be ineligible to respond to a request for proposal issued by the government;
|
|
•
|
the commitment of substantial time and attention of management and key employees to the preparation of bids and proposals for contracts that may not be awarded to us;
|
|
•
|
the need to accurately estimate the resources and cost structure that will be required to perform any contract that we might be awarded;
|
|
•
|
the submission by third parties of protests to our responses to requests for proposal that could result in delays or withdrawals of those requests for proposal; and
|
|
•
|
in the event our competitors protest or challenge contract or grant awards made to us pursuant to competitive bidding, the potential that we may incur expenses or delays, and that any such protest or challenge could result in the resubmission of bids based on modified specifications, or in the termination, reduction or modification of the awarded contract.
|
|
•
|
the Federal Acquisition Regulation (FAR), and agency-specific regulations supplemental to FAR, which comprehensively regulate the award, formation, administration and performance of government contracts;
|
|
•
|
the Defense Federal Acquisition Regulations (DFARs), and agency-specific regulations supplemental to DFARs, which comprehensively regulate the award, formation, administration and performance of U.S. Department of Defense (DoD) government contracts;
|
|
•
|
the Department of State Acquisition Regulation (DOSAR), which regulates the relationship between a Department of State organization and a contractor or potential contractor;
|
|
•
|
business ethics and public integrity obligations, which govern conflicts of interest and the hiring of former government employees, restrict the granting of gratuities and funding of lobbying activities and incorporate other requirements such as the Anti-Kickback Act, the Procurement Integrity Act, the False Claims Act and the Foreign Corrupt Practices Act;
|
|
•
|
export and import control laws and regulations, including but not limited to International Traffic in Arms Regulations; and
|
|
•
|
laws, regulations and executive orders restricting the use and dissemination of information classified for national security purposes and the exportation of certain products and technical data.
|
|
•
|
terminate existing contracts, in whole or in part, for any reason or no reason;
|
|
•
|
unilaterally reduce or modify contracts or subcontracts, including by imposing equitable price adjustments;
|
|
•
|
cancel multi-year contracts and related orders, if funds for contract performance for any subsequent year become unavailable;
|
|
•
|
decline, in whole or in part, to exercise an option to purchase product under a procurement contract or to fund additional development under a development contract;
|
|
•
|
decline to renew a procurement contract;
|
|
•
|
claim rights to facilities or to products, including intellectual property, developed under the contract;
|
|
•
|
require repayment of contract funds spent on construction of facilities in the event of contract default;
|
|
•
|
take actions that result in a longer development timeline than expected;
|
|
•
|
direct the course of a development program in a manner not chosen by the government contractor;
|
|
•
|
suspend or debar the contractor from doing business with the government or a specific government agency;
|
|
•
|
pursue civil or criminal remedies under acts such as the False Claims Act and False Statements Act; and
|
|
•
|
control or prohibit the export of products.
|
|
•
|
the federal Anti-Kickback Statute makes it illegal for any person or entity, including a prescription drug manufacturer (or a party acting on its behalf) to knowingly and willfully solicit, receive, offer or pay remuneration, directly or indirectly, overtly or covertly, to induce, or in return for, either the referral of an individual, or the purchase, lease, prescribing or recommendation of an item, good, facility or service reimbursable by a federally funded healthcare program, such as the Medicare or Medicaid program. The term “remuneration” has been interpreted broadly and may constrain our marketing practices, educational programs, pricing policies and relationships with healthcare providers or other entities, among other activities;
|
|
•
|
federal civil and criminal false claims, including the federal False Claims Act, and false statement laws and civil monetary penalty laws, which impose criminal and civil penalties, including through civil whistleblower or qui tam actions, on individuals or entities for, among other things,
|
|
•
|
the U.S. federal Health Insurance Portability and Accountability Act of 1996 (HIPAA), which imposes criminal and civil liability for, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false statement, in connection with the delivery of, or payment for, healthcare benefits, items or services. Similar to the U.S. federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation;
|
|
•
|
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health (HITECH), and their respective implementing regulations mandates, among other things, the adoption of uniform standards for the electronic exchange of information in common healthcare transactions, as well as standards relating to the privacy, security and transmission of individually identifiable health information, which require the adoption of administrative, physical and technical safeguards to protect such information. Among other things, HITECH makes HIPAA's security standards directly applicable to “business associates,” or independent contractors or agents of covered entities that create, receive or obtain protected health information in connection with providing a service for or on behalf of a covered entity;
|
|
•
|
the Physician Payments Sunshine Act and its implementing regulations, which requires certain manufacturers of drugs, biologics, medical devices and medical supplies for which payment is available under Medicare, Medicaid or the Centers for Medicare & Medicaid Services (CMS), certain payments and transfers of value made to physicians and teaching hospitals, and
|
|
•
|
state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payor, including commercial insurers; state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts; state, local and foreign laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, obtain pharmaceutical agent licensure, and/or otherwise restrict payments that may be made to healthcare providers and entities; and state, local and foreign laws that require drug manufacturers to report information related to payments and other transfers of value to healthcare providers or entities, or marketing expenditures.
|
|
•
|
Diversion of management time and attention;
|
|
•
|
Expenditure of large amounts of cash on legal fees, costs and payment of damages or penalties;
|
|
•
|
Limitations on our ability to continue some of our operations;
|
|
•
|
Decreased demand for our products; and
|
|
•
|
Injury to our reputation.
|
|
•
|
warning letters and other communications;
|
|
•
|
product seizure or withdrawal of the product from the market;
|
|
•
|
restrictions on the marketing or manufacturing of a product;
|
|
•
|
suspension or withdrawal of regulatory approvals or refusal to approve pending applications or supplements to approved applications;
|
|
•
|
fines or disgorgement of profits or revenue; and
|
|
•
|
injunctions or the imposition of civil or criminal penalties.
|
|
•
|
equipment malfunctions or failures;
|
|
•
|
technology malfunctions;
|
|
•
|
cyber-attacks;
|
|
•
|
work stoppages or slow-downs;
|
|
•
|
protests, including by animal rights activists;
|
|
•
|
injunctions;
|
|
•
|
damage to or destruction of the facility; and
|
|
•
|
product contamination or tampering.
|
|
•
|
retaining existing customers and attracting new customers;
|
|
•
|
retaining key employees;
|
|
•
|
diversion of management attention and resources;
|
|
•
|
conforming internal controls, policies and procedures, business cultures and compensation programs;
|
|
•
|
consolidating corporate and administrative infrastructures;
|
|
•
|
successfully executing technology transfers and obtaining required regulatory approvals;
|
|
•
|
consolidating sales and marketing operations;
|
|
•
|
identifying and eliminating redundant and underperforming operations and assets;
|
|
•
|
assumption of known and unknown liabilities;
|
|
•
|
coordinating geographically dispersed organizations; and
|
|
•
|
managing tax costs or inefficiencies associated with integrating operations.
|
|
•
|
successful development, formulation and cGMP scale-up of manufacturing that meets FDA or other foreign regulatory requirements;
|
|
•
|
successful program partnering;
|
|
•
|
successful completion of clinical or non-clinical development, including toxicology studies and studies in approved animal models;
|
|
•
|
receipt of marketing approvals from the FDA and equivalent foreign regulatory authorities;
|
|
•
|
establishment of commercial manufacturing processes and product supply arrangements;
|
|
•
|
training of a commercial sales force for the product, whether alone or in collaboration with others;
|
|
•
|
successful registration and maintenance of relevant patent and/or other proprietary protection; and
|
|
•
|
acceptance of the product by potential government and other customers.
|
|
•
|
our inability to manufacture sufficient quantities of materials for use in trials;
|
|
•
|
the unavailability or variability in the number and types of subjects for each study;
|
|
•
|
safety issues or inconclusive or incomplete testing, trial or study results;
|
|
•
|
drug immunogenicity;
|
|
•
|
lack of efficacy of product candidates during the trials;
|
|
•
|
government or regulatory restrictions or delays; and
|
|
•
|
greater than anticipated costs of trials.
|
|
•
|
Pfizer, Inc. an oligonucleotide adjuvant, CPG 7909, for use in our AV7909 (anthrax vaccine adsorbed with CPG 7909 adjuvant) anthrax vaccine product candidate.
|
|
•
|
Opiant Pharmaceuticals, Inc. formulations of naloxone, for use in our NARCAN® Nasal Spray.
|
|
•
|
Pharma Consult GmbH autoinjectors, including the autoinjector used for our Trobigard® (atropine sulfate, obidoxime chloride) autoinjector.*
|
|
•
|
requiring us to dedicate a substantial portion of any cash flow from operations to payment on our debt, which would reduce the amounts available to fund other corporate initiatives;
|
|
•
|
increasing the amount of interest that we have to pay on debt with variable interest rates, if market rates of interest increase;
|
|
•
|
subjecting us, as under our senior secured credit facilities, to restrictive covenants that may reduce our ability to take certain corporate actions, acquire companies, products or technology, or obtain further debt financing;
|
|
•
|
requiring us to pledge our assets as collateral, which could limit our ability to obtain additional debt financing;
|
|
•
|
limiting our flexibility in planning for, or reacting to, general adverse economic and industry conditions; and
|
|
•
|
placing us at a competitive disadvantage compared to our competitors that have less debt, better debt servicing options or stronger debt servicing capacity.
|
|
•
|
the level, timing and cost of product sales and contract manufacturing services;
|
|
•
|
the extent to which we acquire or invest in and integrate companies, businesses, products or technologies;
|
|
•
|
the acquisition of new facilities and capital improvements to new or existing facilities;
|
|
•
|
the payment obligations under our indebtedness;
|
|
•
|
the scope, progress, results and costs of our development activities;
|
|
•
|
our ability to obtain funding from collaborative partners, government entities and non-governmental organizations for our development programs;
|
|
•
|
the extent to which we repurchase additional common stock under our authorized share repurchase program; and
|
|
•
|
the costs of commercialization activities, including product marketing, sales and distribution.
|
|
•
|
decreased demand or withdrawal of a product;
|
|
•
|
injury to our reputation;
|
|
•
|
withdrawal of clinical trial participants;
|
|
•
|
costs to defend the related litigation;
|
|
•
|
substantial monetary awards to trial participants or patients;
|
|
•
|
loss of revenue; and
|
|
•
|
an inability to commercialize products that we may develop.
|
|
•
|
the classification of our directors;
|
|
•
|
limitations on changing the number of directors then in office;
|
|
•
|
limitations on the removal of directors;
|
|
•
|
limitations on filling vacancies on the board;
|
|
•
|
advance notice requirements for stockholder nominations of candidates for election to the Board of Directors and other proposals;
|
|
•
|
the inability of stockholders to act by written consent;
|
|
•
|
the inability of stockholders to call special meetings; and
|
|
•
|
the ability of our Board of Directors to designate the terms of and issue a new series of preferred stock without stockholder approval.
|
|
•
|
contracts, decisions and procurement policies by the USG affecting BioThrax and our other products and product candidates;
|
|
•
|
the success of competitive products or technologies;
|
|
•
|
results of clinical and non-clinical trials of our product candidates;
|
|
•
|
announcements of acquisitions, financings or other transactions by us;
|
|
•
|
litigation or legal proceedings;
|
|
•
|
public concern as to the safety of our products;
|
|
•
|
termination or delay of a development program;
|
|
•
|
the recruitment or departure of key personnel;
|
|
•
|
variations in our product revenue and profitability; and
|
|
•
|
the other factors described in this “Risk Factors” section.
|
|
Exhibit
Number
|
Description
|
|
10.1
#
|
|
|
10.2
|
|
|
31.1
#
|
|
|
31.2
#
|
|
|
32.1
#
|
|
|
32.2
#
|
|
|
101. INS
|
XBRL Instance Document.
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document.
|
|
101.CAL
|
XBRL Taxonomy Calculation Linksbase Document.
|
|
101.DEF
|
XBRL Taxonomy Definition Linksbase Document.
|
|
101.LAB
|
XBRL Taxonomy Label Linksbase Document.
|
|
101.PRE
|
XBRL Taxonomy Presentation Linksbase Document.
|
|
(i)
|
Condensed Consolidated Statements of Operations for the three months ended
March 31, 2019
and
2018
;
|
|
(ii)
|
Condensed Consolidated Statements of Comprehensive Loss for the three months ended
March 31, 2019
and 2018;
|
|
(iii)
|
Condensed Consolidated Balance Sheets at
March 31, 2019
and
December 31, 2018
;
|
|
(iv)
|
Condensed Consolidated Statements of Cash Flows for the three months ended
March 31, 2019
and
2018
; and
|
|
(v)
|
Condensed Consolidated Statement of Changes in Stockholders' Equity for the three months ended
March 31, 2019
and
2018
; and
|
|
(vi)
|
Notes to Condensed Consolidated Financial Statements.
|
|
#
|
Filed herewith.
|
|
EMERGENT BIOSOLUTIONS INC.
|
|
By:
/s/ROBERT G. KRAMER, SR.
Robert G. Kramer, Sr.
President and Chief Executive Officer
(Principal Executive Officer)
|
|
Date: May 8, 2019
|
|
By:
/s/RICHARD S. LINDAHL
Richard S. Lindahl
Executive Vice President, Chief Financial Officer and Treasurer
(Principal Financial and Accounting Officer)
|
|
Date: May 8, 2019
|