|
INDIANA
|
|
82-5497352
|
|
(State or other jurisdiction of
|
|
(I.R.S. Employer
|
|
incorporation or organization)
|
|
Identification No.)
|
|
Title of each class
|
|
Common Stock, no par value
|
|
Name of each exchange on which registered
|
|
New York Stock Exchange
|
|
Large accelerated filer
o
|
Accelerated filer
o
|
|||||
|
Non-accelerated filer
ý
|
Smaller reporting company
o
|
|||||
|
|
Emerging growth company
o
|
|||||
|
Management's Discussion and Analysis of
Financial Condition and Results of Operations
|
||||
|
Part IV
|
||||
|
•
|
heightened competition, including from new innovation or generics;
|
|
•
|
the impact of disruptive innovations and advances in veterinary medical practices, animal health technologies and alternatives to animal-derived protein;
|
|
•
|
changes in regulatory restrictions on the use of antibiotics in food animals, as well as changing market demand regarding the use of antibiotics and productivity products;
|
|
•
|
impact of generic products;
|
|
•
|
our ability to implement our business strategies or achieve targeted cost efficiencies and gross margin improvements;
|
|
•
|
consolidation of our customers and distributors;
|
|
•
|
an outbreak of infectious disease carried by food animals;
|
|
•
|
the success of our R&D, acquisition and licensing efforts;
|
|
•
|
misuse or off-label use of our products;
|
|
•
|
unanticipated safety, quality or efficacy concerns associated with our products;
|
|
•
|
the impact of weather conditions and the availability of natural resources;
|
|
•
|
risks related to our presence in emerging markets;
|
|
•
|
changes in U.S. foreign trade policy, imposition of tariffs or trade disputes;
|
|
•
|
the impact of global macroeconomic conditions; and
|
|
•
|
the effect on our business of the transactions involving the separation of our business from that of Eli Lilly & Co. (Lilly) and distribution of Lilly's interest in us to its shareholders through an exchange offer or otherwise, if consummated.
|
|
(2)
|
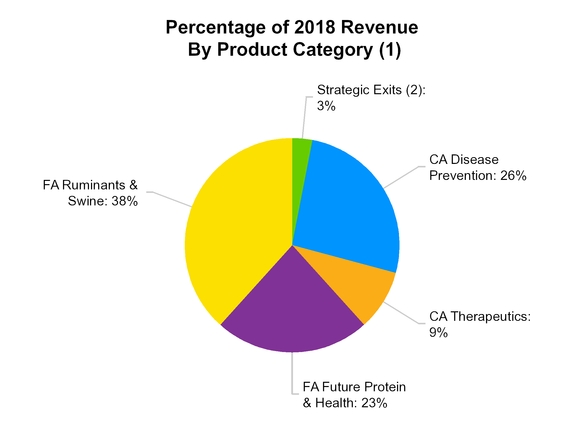
Strategic Exits include revenue from third-party manufacturing, distribution and other contractual arrangements, as well as an equine product not core to our business and transitional contract manufacturing activity associated with the supply of human growth hormone to Lilly, which we made the decision to exit
.
|
|
Primary
|
||
|
Product
|
Description
|
Species
|
|
Bronchi Shield III
and
Bronchi Shield Oral
(vaccines)
|
Bronchi Shield III - To protect against adenovirus, parainfluenza and Bordetella bronchiseptica (Bb) in dogs.
Bronchi Shield Oral - To protect against Bb in dogs.
|
Dogs
|
|
Comfortis
(spinosad)
|
To kill fleas and prevent and treat flea infestations (
Ctenocephalides felis
) in cats 14 weeks of age or older and weighing at least 4.1 lbs. and dogs 14 weeks of age or older and weighing at least 5.0 lbs.
|
Cats, Dogs
|
|
Credelio
(lotilaner)
|
To kill adult fleas and to treat flea infestations (
Ctenocephalides felis
) and treat and control tick infestations (
Amblyomma americanum
(lone star tick),
Dermacentor variabilis
(American dog tick),
Ixodes scapularis
(black‑legged tick) and
Rhipicephalus sanguineus
(brown dog tick)) for one month in dogs and puppies 8 weeks of age or older and weighing at least 4.4 lbs.
|
Dogs
|
|
Duramune
(vaccines)
|
Includes multiple products that collectively protect against distemper, adenovirus, parvovirus, corona, parainfluenza, leptospira canicola, and other diseases in dogs.
|
Dogs
|
|
Rabvac
(vaccines)
|
To protect against rabies, includes a 1‑year and 3‑year shot.
|
Cats, Dogs
|
|
Fel‑O‑Vax
(vaccines)
|
Includes multiple products that collectively protect against leukemia, rhinovirus, calicivirus, panleukopenia, and chlamydia in cats.
|
Cats
|
|
Fel‑O‑Guard
(vaccines)
|
Includes multiple products that collectively protect against leukemia, rhinovirus, calicivirus, panleukopenia, and chlamydia in cats.
|
Cats
|
|
Interceptor Plus
(milbemycin oxime/praziquantel)
|
To prevent heartworm disease caused by
Dirofilaria immitis
and for the treatment and control of adult roundworm (
Toxocara canis
and
Toxascaris leonina
), adult hookworm (
Ancylostoma caninum
), adult whipworm (
Trichuris vulpis
), and adult tapeworm (
Taenia pisiformis
,
Echinococcus multilocularis
, and
Echinococcus granulosus
) infections in dogs and puppies weighing at least 2 lbs. and 6 weeks of age or older.
Interceptor Plus
is a relaunch of a previously approved formula.
|
Dogs
|
|
Milbemax
(milbemycin
oxime +
praziquantel)
|
To treat and control parasitic infections due to adult hookworm, adult roundworm and adult tapeworm and to prevent heartworm disease caused by
Dirofilaria immitis
in cats and dogs.
|
Cats, Dogs
|
|
Trifexis
(spinosad +
milbemycin
oxime)
|
To prevent heartworm disease (
Dirofilaria immitis
) and to kill fleas.
Trifexis
is indicated for the prevention and treatment of flea infestations (
Ctenocephalides felis
), and the treatment and control of adult hookworm
(Ancylostoma caninum)
, adult roundworm (
Toxocara canis
and
Toxascaris leonina
) and adult whipworm (
Trichuris vulpis
) infections in dogs and puppies 8 weeks of age or older and weighing at least 5 lbs.
|
Dogs
|
|
Primary
|
||
|
Product
|
Description
|
Species
|
|
Atopica
(cyclosporine A)
|
To control atopic dermatitis in dogs weighing at least 4 lbs.
|
Dogs
|
|
Fortekor Plus
(benazepril +
pimobendan)
|
To treat congestive heart failure due to atrioventricular valve insufficiency or dilated cardiomyopathy in dogs.
|
Dogs
|
|
Galliprant
(grapiprant)
|
To control pain and inflammation associated with osteoarthritis in dogs.
|
Dogs
|
|
Onsior
(robenacoxib)
|
To control postoperative pain and inflammation associated with soft tissue surgery in dogs weighing at least 5.5 lbs. and 4 months of age or older and control postoperative pain and inflammation associated with orthopedic surgery, ovariohysterectomy and castration in cats weighing at least 5.5 lbs. and 6 months of age or older; for up to a maximum of 3 days.
|
Cats, Dogs
|
|
Osurnia
(terbinafine +
florfenicol +
betamethasone
acetate)
|
To treat otitis externa in dogs associated with susceptible strains of bacteria (
Staphylococcus pseudintermedius
) and yeast (
Malassezia pachydermatis
).
|
Dogs
|
|
Primary
|
||
|
Product
|
Description
|
Species
|
|
AviPro
(vaccines)
|
Includes multiple products that collectively protect against Newcastle disease, infectious bronchitis, fowl cholera, paramyxovirus Type 3, Bursal Disease, other diseases and foodborne pathogens like Salmonella in poultry.
|
Poultry
|
|
Clynav
(plasmid deoxyribonucleic acid vaccine)
|
To immunize Atlantic salmon to reduce impaired daily weight gain, and reduce mortality, and cardiac, pancreatic and skeletal muscle lesions caused by pancreas disease following infection with salmonid alphavirus subtype 3 (SAV3).
|
Fish (Salmon)
|
|
Coban
/
Elancoban
(monensin)
|
To aid in the prevention of coccidiosis in broiler and replacement chickens (caused by
Eimeria necatrix, E. tenella, E. acervulina, E. brunetti, E. mivati
, and
E. maxima
), in turkeys (caused by
Eimeria adenoeides, E. meleagrimitis
and
E. gallopavonis
) and in growing Bobwhite quail (caused by
Eimeria dispersa
and
E. lettyae
). Coban/Elancoban is an animal‑only antibiotic and an ionophore.
|
Poultry
|
|
Hemicell
(endo‑1, 4‑â‑mannanase)
|
Enzyme supplement for poultry and swine feeds that contain a source of â‑mannanase, which hydrolyses the â‑mannans present in soybean and corn meal.
|
Poultry, Swine
|
|
Imvixa
(lufenuron)
|
To prevent and control infestation caused by sea lice,
Caligus reogercresseyi,
in farmed salmon.
|
Fish (Salmon)
|
|
Maxiban
(narasin +
nicarbazin)
|
To prevent coccidiosis in broiler chickens caused by
Eimeria necatrix, E. tenella, E. acervulina, E. brunetti, E. mivati
and
E. maxima. Maxiban
is an animal‑only antibiotic and an ionophore.
|
Poultry
|
|
Monteban
(narasin)
|
To prevent coccidiosis in broiler chickens caused by
Eimeria necatrix, E. tenella, E. acervulina, E. brunetti, E. mivati
and
E. maxima. Monteban
is an animal‑only antibiotic and an ionophore.
|
Poultry
|
|
Surmax / Maxus / Inteprity
(avilamycin)
|
To prevent mortality caused by necrotic enteritis associated with
Clostridium perfringens
in broiler chickens.
Surmax, Maxis
and
Inteprity
are animal‑only antibiotics.
|
Poultry
|
|
Primary
|
||
|
Product
|
Description
|
Species
|
|
Denagard
(tiamulin)
|
To treat Swine Dysentery associated with
Serpulina
hyodysenteriae
susceptible to tiamulin and for treatment of swine bacterial enteritis caused by
Escherichia coli
and
Salmonella choleraesuis
sensitive to chlortetracycline and treatment of bacterial pneumonia caused by Pasteurella multocida sensitive to chlortetracycline.
Denagard
is a shared‑class antibiotic.
|
Swine
|
|
Optaflexx / Paylean
(ractopamine hydrochloride)
|
To increase rate of weight gain, improve feed efficiency and increase carcass leanness, and used as a top dress feed to increase rate of weight gain and improve feed efficiency in cattle fed in confinement for slaughter during the last 28 to 42 days on feed. Ractopamine, the active ingredient in
Paylean and Optaflexx,
is a beta adrenoreceptor agonist.
|
Cattle, Swine
|
|
Pulmotil
(tilmicosin)
|
For swine: To control swine respiratory disease associated with
Actinobacillus pleuropneumoniae
and
Pasteurella multocida.
For cattle: To control bovine respiratory disease (BRD) associated with
Mannheimia haemolytica, Pasteurella multocida
and
Histophilus somni
in groups of beef and non‑lactating dairy cattle, where active BRD has been diagnosed in at least 10% of the animals in the group.
Pulmotil
is a shared‑class antibiotic.
|
Cattle, Swine
|
|
Rumensin
(monensin)
|
For cattle fed in confinement for slaughter: To improve feed efficiency and prevent and control coccidiosis due to
Eimeria bovis
and
Eimeria zuernii
.
For dairy cows: To increase milk production efficiency (production of marketable solids‑corrected milk per unit of feed intake).
|
Cattle
|
|
For growing cattle on pasture or in dry lot (stocker and feeder and dairy and beef replacement heifers): To increase rate of weight gain and to prevent and control coccidiosis due to
Eimeria bovis
and
Eimeria zuernii
.
For mature reproducing beef cows: To improve feed efficiency when receiving supplemental feed and to prevent and control coccidiosis due to
Eimeria bovis
and
Eimeria zuernii
.
For goats: To prevent coccidiosis due to
Eimeria crandallis, Eimeria christenseni
and
Eimeria ninakohlyakimovae
in goats maintained in confinement.
For calves (excluding veal calves): To prevent and control coccidiosis due to
Eimeria bovis
and
Eimeria zuernii.
Rumensin
is an animal‑only antibiotic and an ionophore.
|
||
|
Tylan Premix
(tylosin phosphate)
|
To control porcine proliferative enteropathies associated with
Lawsonia intracellularis
and to control porcine proliferative enteropathies associated with
Lawsonia intracellularis
immediately after medicating with
Tylan Soluble
(tylosin tartrate) in drinking water.
Tylan Premix
is a shared‑class antibiotic.
|
Swine, Cattle, Poultry
|
|
Vira Shield
(vaccines)
|
Includes multiple products that protect against infection, bovine rhinotracheitis, bovine viral diarrhea, bovine respiratory syncytial virus, bovine respiratory disease, leptospira canicola and other diseases in cattle.
|
Cattle
|
|
Site
|
Location
|
Site
|
Location
|
|||
|
Clinton
|
Indiana, U.S.
|
Prince Edward Island
|
Canada
|
|||
|
Speke
|
Liverpool, U.K.
|
Winslow
|
Maine, U.S.
|
|||
|
Kansas City
|
Kansas, U.S.
|
Fort Dodge
|
Iowa, U.S.
|
|||
|
Huningue
|
France
|
Cuxhaven
|
Germany
|
|||
|
Wusi
|
China
|
Chungli
|
Taiwan
|
|||
|
Terre Haute
|
Indiana, U.S.
|
Barueri
|
Brazil
|
|||
|
•
|
the failure of us or any of our vendors or suppliers, including logistical service providers, to comply with applicable regulations and quality assurance guidelines;
|
|
•
|
mislabeling;
|
|
•
|
construction delays;
|
|
•
|
equipment malfunctions;
|
|
•
|
shortages of materials;
|
|
•
|
labor problems;
|
|
•
|
natural disasters;
|
|
•
|
power outages;
|
|
•
|
criminal and terrorist activities;
|
|
•
|
changes in manufacturing production sites and limits to manufacturing capacity due to regulatory requirements, changes in types of products produced, shipping distributions or physical limitations; and
|
|
•
|
the outbreak of any highly contagious diseases near our production sites.
|
|
•
|
pay monetary damages;
|
|
•
|
obtain a license in order to continue manufacturing or marketing the affected products, which may not be available on commercially reasonable terms, or at all; or
|
|
•
|
stop activities, including any commercial activities, relating to the affected products, which could include a recall of the affected products and/or a cessation of sales in the future.
|
|
•
|
volatility in the international financial markets;
|
|
•
|
compliance with governmental controls;
|
|
•
|
difficulties enforcing contractual and intellectual property rights;
|
|
•
|
parallel trade in our products (importation of our products from EU countries where our products are sold at lower prices into EU countries where the products are sold at higher prices);
|
|
•
|
compliance with a wide variety of laws and regulations, such as the U.S. Foreign Corrupt Practices Act (the FCPA) and similar non
‑
U.S. laws and regulations;
|
|
•
|
compliance with foreign labor laws;
|
|
•
|
burdens to comply with multiple and potentially conflicting foreign laws and regulations, including those relating to environmental, health and safety requirements;
|
|
•
|
changes in laws, regulations, government controls or enforcement practices with respect to our business and the businesses of our customers, including the imposition of limits on our profitability;
|
|
•
|
political and social instability, including crime, civil disturbance, terrorist activities and armed conflicts;
|
|
•
|
trade restrictions and restrictions on direct investments by foreign entities, including restrictions administered by the Office of Foreign Assets Control of the U.S. Department of the Treasury and the EU, in relation to our products or the products of farmers and other customers;
|
|
•
|
government limitations on foreign ownership;
|
|
•
|
government takeover or nationalization of business;
|
|
•
|
changes in tax laws and tariffs;
|
|
•
|
imposition of anti
‑
dumping and countervailing duties or other trade
‑
related sanctions;
|
|
•
|
costs and difficulties and compliance risks in staffing, managing and monitoring international operations, including in the use of overseas third
‑
party goods and service providers;
|
|
•
|
corruption risk inherent in business arrangements and regulatory contacts with foreign government entities;
|
|
•
|
longer payment cycles and increased exposure to counterparty risk; and
|
|
•
|
additional limitations on transferring personal information between countries or other restrictions on the processing of personal information.
|
|
•
|
improving strategic and operational flexibility and streamlining decision
‑
making by providing the flexibility to implement our strategic plan and to respond more effectively to different customer needs and the changing economic and industry environment;
|
|
•
|
allowing us to adopt the investment policy and dividend policy best suited to our financial profile and business needs, and allowing us to raise capital as an independent business;
|
|
•
|
creating an independent equity structure that makes possible future acquisitions utilizing our common stock as well as compensation arrangements; and
|
|
•
|
facilitating incentive compensation arrangements for employees more directly tied to the performance of our business, and enhancing employee hiring and retention by, among other things, improving the alignment of management and employee incentives with performance and growth objectives of our business.
|
|
•
|
making it more difficult for us to satisfy our obligations with respect to our debt;
|
|
•
|
limiting our ability to obtain additional financing to fund future working capital, capital expenditures, business development or other general corporate requirements, including dividends;
|
|
•
|
increasing our vulnerability to general adverse economic and industry conditions;
|
|
•
|
exposing us to the risk of increased interest rates as certain of our borrowings are and may in the future be at variable rates of interest;
|
|
•
|
limiting our flexibility in planning for and reacting to changes in the animal health industry;
|
|
•
|
impacting our effective tax rate; and
|
|
•
|
increasing our cost of borrowing.
|
|
•
|
our historical consolidated and combined financial data does not reflect the separation;
|
|
•
|
our historical consolidated and combined financial data reflects expense allocations for certain support functions that are provided on a centralized basis within Lilly, such as expenses for executive oversight, treasury, legal, finance, human resources, tax, internal audit, financial reporting, information technology and investor relations that may be higher or lower than the comparable expenses we would have actually incurred, or will incur in the future, as a standalone company;
|
|
•
|
our cost of debt and our capital structure is different from that reflected in our historical consolidated and combined financial statements;
|
|
•
|
significant increases may occur in our costs as a result of us being a standalone public company, including costs related to public company reporting, investor relations and compliance with the Sarbanes
‑
Oxley Act; and
|
|
•
|
loss of economies of scale as a result of no longer being a part of Lilly.
|
|
•
|
our announcements or our competitors’ announcements regarding new products, enhancements, significant contracts, acquisitions or strategic investments;
|
|
•
|
changes in earnings estimates or recommendations by securities analysts, if any, who cover our common stock;
|
|
•
|
failures to meet external expectations or management guidance;
|
|
•
|
fluctuations in our quarterly financial results or the quarterly financial results of companies perceived to be similar to us;
|
|
•
|
changes in our capital structure or dividend policy, including as a result of the exchange offer, future issuances of securities, sales of large blocks of common stock by our shareholders, including Lilly, or our incurrence of additional debt;
|
|
•
|
reputational issues arising from, among other things, negative publicity about us, our industry or personnel, including as a result of changing public attitudes regarding our products;
|
|
•
|
changes in general economic and market conditions in any of the regions in which we conduct our business;
|
|
•
|
changes in industry conditions or perceptions;
|
|
•
|
changes in applicable laws, rules or regulations and other dynamics; and
|
|
•
|
announcements or actions taken by Lilly, if Lilly were to retain a significant portion of our common stock following the exchange offer.
|
|
•
|
a board of directors divided into three classes with staggered terms;
|
|
•
|
advance notice requirements regarding how our shareholders may present proposals or nominate directors for election at shareholder meetings (except for, depending on the number of shares validly tendered and whether Lilly retains a significant portion of our common stock, Lilly’s designation of persons for nomination by the board of directors);
|
|
•
|
the right of our board of directors to issue one or more series of preferred stock with such powers, rights and preferences as the board of directors shall determine;
|
|
•
|
only the board of directors being able to fill newly
‑
created directorships or vacancies on Our board of directors;
|
|
•
|
limitations on the ability of shareholders to call special meetings of shareholders and the requirement that all shareholder action be taken at a meeting rather than by written consent;
|
|
•
|
a two
‑
thirds shareholder vote requirement to amend our amended and restated articles of incorporation;
|
|
•
|
the exclusive right of our board of directors to amend our amended and restated bylaws; and
|
|
•
|
the requirement that a 66
2
/
3
% vote is necessary to remove directors. These limitations may adversely affect the prevailing market price and market for our common stock if they are viewed as limiting the liquidity of our stock or discouraging takeover attempts in the future.
|
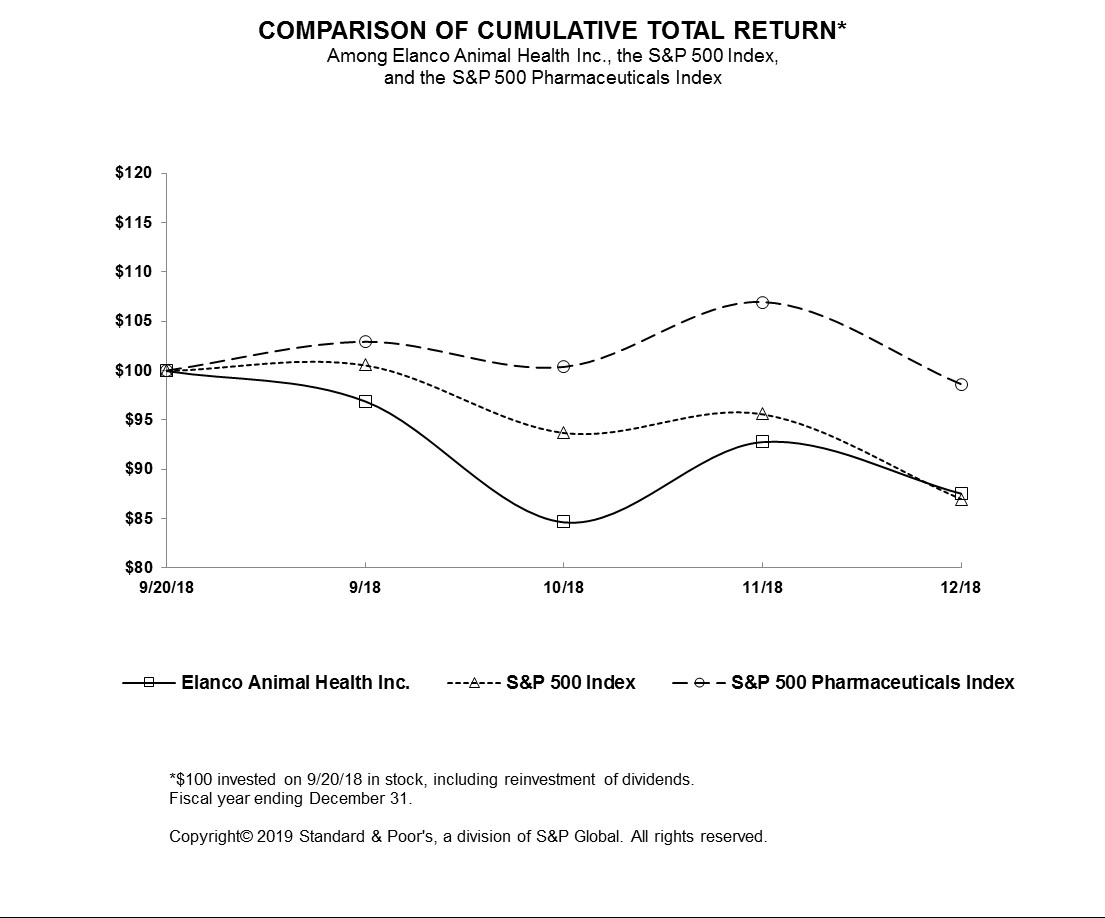
|
9/20/18
|
9/30/18
|
10/31/18
|
11/30/18
|
12/31/18
|
||
|
Elanco Animal Health Inc.
|
100.00
|
96.92
|
84.67
|
92.81
|
87.58
|
|
|
S&P 500 Index
|
100.00
|
100.57
|
93.70
|
95.60
|
86.97
|
|
|
S&P 500 Pharmaceuticals Index
|
100.00
|
102.91
|
100.36
|
106.93
|
98.62
|
|
|
ELANCO ANIMAL HEALTH INCORPORATED
(Dollars in millions, except per-share data)
|
2018
|
2017
|
2016
|
2015
|
2014
|
|||||||||||||||
|
Operations
|
||||||||||||||||||||
|
Revenue
|
$
|
3,066.8
|
|
$
|
2,889.0
|
|
$
|
2,913.5
|
|
$
|
2,909.1
|
|
$
|
2,066.0
|
|
|||||
|
Cost of sales
|
1,573.8
|
|
1,493.9
|
|
1,409.0
|
|
1,533.7
|
|
932.6
|
|
||||||||||
|
Research and development
|
246.6
|
|
251.7
|
|
265.8
|
|
291.0
|
|
208.5
|
|
||||||||||
|
Marketing, selling and administrative
|
735.2
|
|
779.8
|
|
784.8
|
|
916.0
|
|
561.2
|
|
||||||||||
|
Amortization of intangible assets
|
197.4
|
|
221.2
|
|
170.7
|
|
163.0
|
|
57.6
|
|
||||||||||
|
Asset impairment, restructuring and other special charges
|
128.8
|
|
375.1
|
|
308.4
|
|
263.3
|
|
38.8
|
|
||||||||||
|
Interest expense, net of capitalized interest
|
29.6
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
Other (income) expense, net
|
41.3
|
|
(0.1
|
)
|
(2.8
|
)
|
1.6
|
|
1.4
|
|
||||||||||
|
Income (loss) before income tax expense
|
114.1
|
|
(232.6
|
)
|
(22.4
|
)
|
(259.5
|
)
|
265.9
|
|
||||||||||
|
Income tax expense (benefit)
|
27.6
|
|
78.1
|
|
25.5
|
|
(48.7
|
)
|
101.0
|
|
||||||||||
|
Net income (loss)
|
$
|
86.5
|
|
$
|
(310.7
|
)
|
$
|
(47.9
|
)
|
$
|
(210.8
|
)
|
$
|
164.9
|
|
|||||
|
Net income (loss) as a percent of revenue
|
3
|
%
|
(11
|
)%
|
(2
|
)%
|
(7
|
)%
|
8
|
%
|
||||||||||
|
Net income (loss) per share - basic and diluted
|
$
|
0.28
|
|
$
|
(1.06
|
)
|
$
|
(0.16
|
)
|
$
|
(0.72
|
)
|
$
|
0.56
|
|
|||||
|
Weighted-average number of shares outstanding-diluted
|
313.7
|
|
293.3
|
|
293.3
|
|
293.3
|
|
293.3
|
|
||||||||||
|
Financial Position
|
||||||||||||||||||||
|
Total assets
|
$
|
8,956.7
|
|
$
|
8,940.3
|
|
$
|
8,099.7
|
|
$
|
8,433.6
|
|
$
|
2,980.6
|
|
|||||
|
Long term debt
|
$
|
2,443.3
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||||
|
Total liabilities
|
$
|
3,759.2
|
|
$
|
1,160.0
|
|
$
|
1,082.3
|
|
$
|
1,004.1
|
|
$
|
551.5
|
|
|||||
|
Total equity
|
$
|
5,197.5
|
|
$
|
7,780.3
|
|
$
|
7,017.4
|
|
$
|
7,429.5
|
|
$
|
2,429.1
|
|
|||||
|
•
|
CA Disease Prevention includes parasiticides and vaccine products for dogs and cats;
|
|
•
|
CA Therapeutics includes products for the treatment of pain, osteoarthritis, otitis, cardiovascular and dermatology indications in dogs and cats;
|
|
•
|
FA Future Protein & Health includes vaccines, antibiotics, parasiticides and other products used in poultry and aquaculture production, as well as functional nutritional health products, including enzymes, probiotics and prebiotics;
|
|
•
|
FA Ruminants & Swine includes vaccines, antibiotics, implants, parasiticides, and other products used in ruminants and swine production, as well as certain other food animal products; and
|
|
•
|
Strategic Exits includes business activities that we have either exited or made the strategic decision to exit, including the transitional contract manufacturing activity that we acquired in connection with our acquisition of the BI Vetmedica U.S. vaccines portfolio, two terminated legacy U.S. distribution agreements, a terminated distribution agreement outside the U.S.; an equine product not core to our business and a transitional contract manufacturing activity associated with the supply to Lilly of human growth hormone.
|
|
|
Year Ended December 31,
|
% Change
|
|||||||||||||
|
|
2018
|
2017
|
2016
|
18/17
|
17/16
|
||||||||||
|
Revenue
|
$
|
3,066.8
|
|
$
|
2,889.0
|
|
$
|
2,913.5
|
|
6%
|
(1)%
|
||||
|
Costs, expenses and other:
|
|||||||||||||||
|
Cost of sales
|
1,573.8
|
|
1,493.9
|
|
1,409.0
|
|
5%
|
6%
|
|||||||
|
% of revenue
|
51
|
%
|
52
|
%
|
48
|
%
|
|||||||||
|
Research and development
|
246.6
|
|
251.7
|
|
265.8
|
|
(2)%
|
(5)%
|
|||||||
|
% of revenue
|
8
|
%
|
9
|
%
|
9
|
%
|
|||||||||
|
Marketing, selling and administrative
|
735.2
|
|
779.8
|
|
784.8
|
|
(6)%
|
(1)%
|
|||||||
|
% of revenue
|
24
|
%
|
27
|
%
|
27
|
%
|
|||||||||
|
Amortization of intangible assets
|
197.4
|
|
221.2
|
|
170.7
|
|
(11)%
|
30%
|
|||||||
|
% of revenue
|
6
|
%
|
8
|
%
|
6
|
%
|
|||||||||
|
Asset impairment, restructuring and other special charges
|
128.8
|
|
375.1
|
|
308.4
|
|
(66)%
|
22%
|
|||||||
|
Interest expense, net of capitalized interest
|
29.6
|
|
—
|
|
—
|
|
NM
|
NM
|
|||||||
|
Other (income) expense, net
|
41.3
|
|
(0.1
|
)
|
(2.8
|
)
|
NM
|
NM
|
|||||||
|
Income (loss) before taxes
|
114.1
|
|
(232.6
|
)
|
(22.4
|
)
|
NM
|
NM
|
|||||||
|
% of revenue
|
4
|
%
|
(8
|
)%
|
(1
|
)%
|
NM
|
NM
|
|||||||
|
Income tax expense
|
27.6
|
|
78.1
|
|
25.5
|
|
NM
|
NM
|
|||||||
|
Net income (loss)
|
$
|
86.5
|
|
$
|
(310.7
|
)
|
$
|
(47.9
|
)
|
NM
|
NM
|
||||
|
Year Ended December 31,
|
% Change
|
||||||||||||||
|
2018
|
2017
|
2016
|
18/17
|
17/16
|
|||||||||||
|
CA Disease Prevention
|
$
|
804.6
|
|
$
|
660.2
|
|
$
|
628.4
|
|
22%
|
5%
|
||||
|
CA Therapeutics
(1)
|
283.1
|
|
260.8
|
|
255.6
|
|
9%
|
2%
|
|||||||
|
FA Future Protein & Health
|
711.2
|
|
649.2
|
|
630.8
|
|
10%
|
3%
|
|||||||
|
FA Ruminants & Swine
|
1,174.0
|
|
1,175.0
|
|
1,309.2
|
|
(0)%
|
(10)%
|
|||||||
|
Subtotal
|
2,972.9
|
|
2,745.2
|
|
2,824.0
|
|
8%
|
(3)%
|
|||||||
|
Strategic Exits
(1)
|
93.9
|
|
143.8
|
|
89.5
|
|
(35)%
|
61%
|
|||||||
|
Total
|
$
|
3,066.8
|
|
$
|
2,889.0
|
|
$
|
2,913.5
|
|
6%
|
(1)%
|
||||
|
(1)
|
Represents revenue from business activities we have either exited or made a strategic decision to exit. On June 30, 2018, Elanco made the decision to exit an equine product not core to its business. Revenue from this product is reflected in Strategic Exits for the year ended December 31, 2018 and in CA Therapeutics for the years ended December 31, 2017 and 2016. Revenue from this product was $1.6 million, $3.4 million and $3.7 million, for the years ended December 31, 2018, 2017 and 2016, respectively.
|
|
Full year 2018
|
Revenue
|
Price
|
FX Rate
|
Volume
|
Total
|
CER*
|
||||||
|
CA Disease Prevention
|
$804.6
|
8%
|
0%
|
14%
|
22%
|
22%
|
||||||
|
CA Therapeutics
|
283.1
|
7%
|
1%
|
0%
|
9%
|
7%
|
||||||
|
FA Future Protein & Health
|
711.2
|
4%
|
(0)%
|
6%
|
10%
|
10%
|
||||||
|
FA Ruminants & Swine
|
1,174.0
|
(1)%
|
(0)%
|
1%
|
(0)%
|
(0)%
|
||||||
|
Core Revenue
|
$2,972.9
|
3%
|
0%
|
5%
|
8%
|
8%
|
||||||
|
Strategic Exits
|
93.9
|
(0)%
|
0%
|
(34)%
|
(35)%
|
(35)%
|
||||||
|
Total Elanco
|
$3,066.8
|
3%
|
0%
|
3%
|
6%
|
6%
|
||||||
|
•
|
an increase in revenue of $142.1 million or 22% from CA Disease Prevention products, excluding the impact of foreign exchange rates;
|
|
•
|
an increase in revenue of $18.4 million or 7% from CA Therapeutics products, excluding the impact of foreign exchange rates;
|
|
•
|
an increase in revenue of $63.8 million or 10% from FA Future Protein & Health products, excluding the impact of foreign exchange rates and
|
|
•
|
a decrease in revenue of $0.8 million or 0% from FA Ruminants & Swine, excluding the impact of foreign exchange rates and
|
|
•
|
a decrease in revenue of $49.9 million or 35% from Strategic Exits, excluding the impact of foreign exchange rates.
|
|
•
|
CA Disease Prevention revenue increased by $144.4 million or 22% due primarily to a reduction in channel inventory in 2017 providing a favorable year-on-year comparison, continued uptake of Credelio and Interceptor Plus, as well as realized price increases primarily impacting Trifexis, Capstar (a flea treatment) and Comfortis, partially offset by volume declines in certain parasiticides, primarily Trifexis and Comfortis volumes.
|
|
•
|
CA Therapeutics revenue increased by $22.3 million or 9% due primarily to the continued uptake of Galliprant and Osurnia, as well as increased demand for Onsior, partially offset by a temporary supply shortage of Percorten V used for the treatment of canine Addison’s Disease.
|
|
•
|
FA Future Protein & Health revenue increased by $62.0 million or 10% due primarily to the launch of Imvixa and the growth in poultry animal-only antibiotics and poultry vaccines.
|
|
•
|
FA Ruminants & Swine revenue decreased by $1.0 million due primarily to competitive headwinds for ractopamine based products, offset by growth in animal-only antibiotics, primarily in cattle.
|
|
•
|
Strategic Exits revenue decreased by $49.9 million or 35% due primarily to the termination of a legacy U.S. distribution agreement in the third quarter of 2017, partially offset by revenue from the contract manufacturing agreement to supply human growth hormone to Lilly.
|
|
(Dollars in millions)
|
Year Ended December 31,
|
% Change
|
|||||||||||||||
|
Net cash provided by (used in):
|
2018
|
2017
|
2016
|
18/17
|
17/16
|
||||||||||||
|
Operating activities
|
$
|
487.3
|
|
$
|
173.8
|
|
$
|
155.9
|
|
180
|
%
|
11
|
%
|
||||
|
Investing activities
|
(127.0
|
)
|
(964.6
|
)
|
(182.1
|
)
|
(87
|
)%
|
430
|
%
|
|||||||
|
Financing activities
|
(35.2
|
)
|
847.5
|
|
(149.6
|
)
|
(104
|
)%
|
(667
|
)%
|
|||||||
|
Effect of exchange-rate changes on cash and cash equivalents
|
29.0
|
|
7.9
|
|
(26.0
|
)
|
267
|
%
|
(130
|
)%
|
|||||||
|
Net increase in cash, cash equivalents and restricted cash
|
$
|
354.1
|
|
$
|
64.6
|
|
$
|
(201.8
|
)
|
448
|
%
|
(132
|
)%
|
||||
|
•
|
a decrease in receivables in 2017 as compared to an increase in 2016 due to a one-time impact of standardizing payment terms across our acquired businesses as well as payment receipt timing due to integration of acquired assets;
|
|
•
|
a decrease in other assets in 2017 as compared to an increase in 2016 primarily due to the timing of tax payments; and
|
|
•
|
increased net losses.
|
|
Years
|
||||||||||||||||||||
|
(Dollars in millions)
|
Total
(2)
|
Less Than 1 Year
|
1 - 3 Years
|
4 - 5 Years
|
More Than 5 Years
|
|||||||||||||||
|
Long-term debt obligations
|
$
|
2,958.4
|
|
$
|
79.9
|
|
$
|
1,137.9
|
|
$
|
829.7
|
|
$
|
910.9
|
|
|||||
|
Operating leases
|
95.6
|
|
25.2
|
|
33.6
|
|
18.3
|
|
18.5
|
|
||||||||||
|
Purchase obligations
(1)
|
1,207.9
|
|
1,108.9
|
|
42.8
|
|
39.8
|
|
16.4
|
|
||||||||||
|
Other long-term liabilities
|
12.3
|
|
0.5
|
|
10.8
|
|
0.1
|
|
0.9
|
|
||||||||||
|
Total
|
$
|
4,274.2
|
|
$
|
1,214.5
|
|
$
|
1,225.1
|
|
$
|
887.9
|
|
$
|
946.7
|
|
|||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2018
|
2017
|
2016
|
||||||||
|
Revenue
|
$
|
3,066.8
|
|
$
|
2,889.0
|
|
$
|
2,913.5
|
|
||
|
Costs, expenses and other:
|
|||||||||||
|
Cost of sales
|
1,573.8
|
|
1,493.9
|
|
1,409.0
|
|
|||||
|
Research and development
|
246.6
|
|
251.7
|
|
265.8
|
|
|||||
|
Marketing, selling and administrative
|
735.2
|
|
779.8
|
|
784.8
|
|
|||||
|
Amortization of intangible assets
|
197.4
|
|
221.2
|
|
170.7
|
|
|||||
|
Asset impairments, restructuring and other special charges (Note 7)
|
128.8
|
|
375.1
|
|
308.4
|
|
|||||
|
Interest expense, net of capitalized interest
|
29.6
|
|
—
|
|
—
|
|
|||||
|
Other (income) expense, net
|
41.3
|
|
(0.1
|
)
|
(2.8
|
)
|
|||||
|
2,952.7
|
|
3,121.6
|
|
2,935.9
|
|
||||||
|
Income (loss) before income taxes
|
114.1
|
|
(232.6
|
)
|
(22.4
|
)
|
|||||
|
Income tax expense
|
27.6
|
|
78.1
|
|
25.5
|
|
|||||
|
Net income (loss)
|
$
|
86.5
|
|
$
|
(310.7
|
)
|
$
|
(47.9
|
)
|
||
|
Earnings (loss) per share:
|
|||||||||||
|
Basic and diluted
|
$
|
0.28
|
|
$
|
(1.06
|
)
|
$
|
(0.16
|
)
|
||
|
Weighted average shares outstanding:
|
|||||||||||
|
Basic and diluted
|
313.7
|
|
293.3
|
|
293.3
|
|
|||||
|
Year Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
Net income (loss)
|
$
|
86.5
|
|
$
|
(310.7
|
)
|
$
|
(47.9
|
)
|
||
|
Other comprehensive income (loss):
|
|||||||||||
|
Change in foreign currency translation gains (losses)
|
(47.1
|
)
|
210.1
|
|
(230.7
|
)
|
|||||
|
Change in defined benefit pension and retiree health benefit plans, net of taxes
|
25.4
|
|
(9.8
|
)
|
(4.3
|
)
|
|||||
|
Other comprehensive income (loss), net of taxes
|
(21.7
|
)
|
200.3
|
|
(235.0
|
)
|
|||||
|
Comprehensive income (loss)
|
$
|
64.8
|
|
$
|
(110.4
|
)
|
$
|
(282.9
|
)
|
||
|
December 31, 2018
|
December 31, 2017
|
||||||
|
Assets
|
|
||||||
|
Current Assets
|
|||||||
|
Cash and cash equivalents
|
$
|
474.8
|
|
$
|
323.4
|
|
|
|
Accounts receivable, net of allowances of $8.4 (2018) and $9.8 (2017)
|
651.8
|
|
567.4
|
|
|||
|
Other receivables
|
57.6
|
|
34.5
|
|
|||
|
Inventories (Note 8)
|
1,004.1
|
|
1,062.3
|
|
|||
|
Prepaid expenses and other
|
113.9
|
|
136.1
|
|
|||
|
Restricted cash (Note 19)
|
202.7
|
|
—
|
|
|||
|
Total current assets
|
2,504.9
|
|
2,123.7
|
|
|||
|
Noncurrent Assets
|
|||||||
|
Investments (Note 10)
|
15.3
|
|
12.3
|
|
|||
|
Goodwill (Note 11)
|
2,958.0
|
|
2,969.2
|
|
|||
|
Other intangibles, net (Note 11)
|
2,453.0
|
|
2,672.8
|
|
|||
|
Other noncurrent assets
|
103.1
|
|
242.0
|
|
|||
|
Property and equipment, net (Note 12)
|
922.4
|
|
920.3
|
|
|||
|
Total assets
|
$
|
8,956.7
|
|
$
|
8,940.3
|
|
|
|
Liabilities and Equity
|
|||||||
|
Current Liabilities
|
|||||||
|
Accounts payable
|
$
|
205.2
|
|
$
|
203.8
|
|
|
|
Employee compensation
|
98.9
|
|
89.3
|
|
|||
|
Sales rebates and discounts
|
169.9
|
|
165.5
|
|
|||
|
Current portion of long term debt
|
29.0
|
|
—
|
|
|||
|
Other current liabilities
|
199.0
|
|
184.5
|
|
|||
|
Payable to Lilly (Note 19)
|
268.7
|
|
—
|
|
|||
|
Total current liabilities
|
970.7
|
|
643.1
|
|
|||
|
Noncurrent Liabilities
|
|||||||
|
Long-term debt (Note 9)
|
2,443.3
|
|
—
|
|
|||
|
Accrued retirement benefits (Note 17)
|
109.1
|
|
139.0
|
|
|||
|
Deferred taxes (Note 14)
|
114.6
|
|
251.9
|
|
|||
|
Other noncurrent liabilities
|
121.5
|
|
126.0
|
|
|||
|
Total liabilities
|
3,759.2
|
|
1,160.0
|
|
|||
|
Commitments and Contingencies (Note 15)
|
|
|
|||||
|
Equity
|
|||||||
|
Net parent company investment
|
—
|
|
8,036.9
|
|
|||
|
Common stock, no par value, 5,000,000,000 shares authorized 365,643,911 shares issued and outstanding as of December 31, 2018
|
—
|
|
—
|
|
|||
|
Additional paid-in capital
|
5,403.3
|
|
—
|
|
|||
|
Retained earnings
|
16.4
|
|
—
|
|
|||
|
Accumulated other comprehensive loss
|
(222.2
|
)
|
(256.6
|
)
|
|||
|
Total equity
|
5,197.5
|
|
7,780.3
|
|
|||
|
Total liabilities and equity
|
$
|
8,956.7
|
|
$
|
8,940.3
|
|
|
|
Common Stock
|
Accumulated Other Comprehensive Income (Loss)
|
|||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Additional Paid-in Capital
|
Net Parent Company Investment
|
Retained Earnings
|
Foreign Currency Translation
|
|
Defined Benefit Pension and Retiree Health Benefit Plans
|
Total
|
Total Equity
|
|||||||||||||||||||||||||
|
January 1, 2016
|
293.3
|
|
$
|
—
|
|
$
|
—
|
|
$
|
7,651.4
|
|
$
|
—
|
|
$
|
(206.6
|
)
|
$
|
(15.3
|
)
|
$
|
(221.9
|
)
|
$
|
7,429.5
|
|
||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
(47.9
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(47.9
|
)
|
||||||||||||||||
|
Other comprehensive income, net of tax
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(230.7
|
)
|
(4.3
|
)
|
(235.0
|
)
|
(235.0
|
)
|
||||||||||||||||
|
Transfers (to)/from Lilly, net
|
—
|
|
—
|
|
—
|
|
(129.2
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(129.2
|
)
|
||||||||||||||||
|
December 31, 2016
|
293.3
|
|
—
|
|
—
|
|
7,474.3
|
|
—
|
|
(437.3
|
)
|
(19.6
|
)
|
(456.9
|
)
|
7,017.4
|
|
||||||||||||||||
|
Net (loss)
|
—
|
|
—
|
|
—
|
|
(310.7
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(310.7
|
)
|
||||||||||||||||
|
Other comprehensive income (loss), net of tax
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
210.1
|
|
(9.8
|
)
|
200.3
|
|
200.3
|
|
||||||||||||||||
|
Transfers (to)/from Lilly, net
|
—
|
|
—
|
|
—
|
|
873.3
|
|
—
|
|
—
|
|
—
|
|
—
|
|
873.3
|
|
||||||||||||||||
|
December 31, 2017
|
293.3
|
|
—
|
|
—
|
|
8,036.9
|
|
—
|
|
(227.2
|
)
|
(29.4
|
)
|
(256.6
|
)
|
7,780.3
|
|
||||||||||||||||
|
Adoption of Accounting Standards Update 2016-16
|
—
|
|
—
|
|
—
|
|
(0.3
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(0.3
|
)
|
||||||||||||||||
|
Net income
|
—
|
|
—
|
|
—
|
|
70.1
|
|
16.4
|
|
|
—
|
|
—
|
|
86.5
|
|
|||||||||||||||||
|
Other comprehensive income (loss), net of tax
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(47.1
|
)
|
25.4
|
|
(21.7
|
)
|
(21.7
|
)
|
||||||||||||||||
|
Transfers (to)/from Lilly, net
|
—
|
|
—
|
|
—
|
|
(226.3
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
(226.3
|
)
|
||||||||||||||||
|
Separation adjustments
|
—
|
|
—
|
|
—
|
|
43.5
|
|
—
|
|
56.1
|
|
—
|
|
56.1
|
|
99.6
|
|
||||||||||||||||
|
Issuance of common stock
|
72.3
|
|
—
|
|
1,659.7
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,659.7
|
|
||||||||||||||||
|
Consideration to Lilly in connection with the Separation
|
—
|
|
—
|
|
(4,194.9
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(4,194.9
|
)
|
||||||||||||||||
|
Reclassification of net parent company investment
|
—
|
|
—
|
|
7,923.9
|
|
(7,923.9
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||
|
Shared base compensation
|
—
|
|
—
|
|
1.8
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|
1.8
|
|
|||||||||||||||
|
Capital contribution from Lilly
|
—
|
|
—
|
|
12.8
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|
12.8
|
|
|||||||||||||||
|
December 31, 2018
|
365.6
|
|
$
|
—
|
|
$
|
5,403.3
|
|
$
|
—
|
|
$
|
16.4
|
|
$
|
(218.2
|
)
|
$
|
(4.0
|
)
|
$
|
(222.2
|
)
|
$
|
5,197.5
|
|
||||||||
|
Year Ended December 31,
|
||||||||||
|
2018
|
2017
|
2016
|
||||||||
|
Cash Flows from Operating Activities
|
||||||||||
|
Net income (loss)
|
$
|
86.5
|
|
|
$
|
(310.7
|
)
|
$
|
(47.9
|
)
|
|
Adjustments to reconcile net income (loss) to cash flows from operating activities:
|
|
|
|
|||||||
|
Depreciation and amortization
|
296.0
|
|
|
318.4
|
|
254.4
|
|
|||
|
Change in deferred income taxes
|
(60.7
|
)
|
|
(13.4
|
)
|
(5.9
|
)
|
|||
|
Stock-based compensation expense
|
26.0
|
|
|
25.0
|
|
20.4
|
|
|||
|
Asset impairment charges
|
120.5
|
|
|
110.6
|
|
98.3
|
|
|||
|
Gain on sale of assets
|
(0.8
|
)
|
|
(19.6
|
)
|
—
|
|
|||
|
Other non-cash operating activities, net
|
49.0
|
|
10.0
|
|
6.0
|
|
||||
|
Other changes in operating assets and liabilities, net of acquisitions and divestitures:
|
|
|||||||||
|
Receivables
|
(122.0
|
)
|
48.4
|
|
(80.7
|
)
|
||||
|
Inventories
|
(20.1
|
)
|
(39.0
|
)
|
(89.1
|
)
|
||||
|
Other assets
|
(3.2
|
)
|
52.5
|
|
(36.7
|
)
|
||||
|
Accounts payable and other liabilities
|
116.1
|
|
(8.4
|
)
|
37.1
|
|
||||
|
Net Cash Provided by Operating Activities
|
487.3
|
|
173.8
|
|
155.9
|
|
||||
|
Cash Flows from Investing Activities
|
||||||||||
|
Purchases of property and equipment
|
(134.5
|
)
|
(98.6
|
)
|
(110.3
|
)
|
||||
|
Disposals of property and equipment
|
9.4
|
|
37.6
|
|
7.4
|
|
||||
|
Cash paid for acquisitions, net of cash acquired
|
—
|
|
|
(882.1
|
)
|
(45.0
|
)
|
|||
|
Other investing activities, net
|
(1.9
|
)
|
|
(21.5
|
)
|
(34.2
|
)
|
|||
|
Net Cash Used for Investing Activities
|
(127.0
|
)
|
(964.6
|
)
|
(182.1
|
)
|
||||
|
Cash Flows from Financing Activities
|
||||||||||
|
Proceeds from issuance of long-term debt (Note 9)
|
2,500.0
|
|
—
|
|
—
|
|
||||
|
Repayments of borrowings
|
(7.5
|
)
|
—
|
|
—
|
|
||||
|
Proceeds from issuance of common stock (Note 1)
|
1,659.7
|
|
—
|
|
—
|
|
||||
|
Debt issuance costs
|
(24.5
|
)
|
—
|
|
—
|
|
||||
|
Consideration paid to Lilly in connection with the Separation (Note 1)
|
(3,991.3
|
)
|
|
—
|
|
—
|
|
|||
|
Other financing activities, net
|
(17.2
|
)
|
|
(0.8
|
)
|
—
|
|
|||
|
Other net transactions with Lilly
|
(154.4
|
)
|
|
848.3
|
|
(149.6
|
)
|
|||
|
Net Cash Provided by (Used for) Financing Activities
|
(35.2
|
)
|
847.5
|
|
(149.6
|
)
|
||||
|
Effect of exchange rate changes on cash and cash equivalents
|
29.0
|
|
|
7.9
|
|
(26.0
|
)
|
|||
|
Net increase in cash, cash equivalents and restricted cash
|
354.1
|
|
64.6
|
|
(201.8
|
)
|
||||
|
Cash, cash equivalents and restricted cash at January 1
|
323.4
|
|
258.8
|
|
460.6
|
|
||||
|
Cash, cash equivalents and restricted cash at December 31
|
$
|
677.5
|
|
$
|
323.4
|
|
$
|
258.8
|
|
|
|
December 31,
|
|||||||
|
2018
|
2017
|
||||||
|
Cash and cash equivalents
|
$
|
474.8
|
|
|
$
|
323.4
|
|
|
Restricted cash (Note 19)
|
202.7
|
|
|
—
|
|
||
|
Cash, cash equivalents and restricted cash at December 31
|
$
|
677.5
|
|
|
$
|
323.4
|
|
|
•
|
Milestone payment obligations incurred prior to regulatory approval of the product, which are accrued when the event requiring payment of the milestone occurs.
|
|
•
|
Acquired in-process research and development (IPR&D) expense, which includes the initial costs of IPR&D projects, acquired directly in a transaction other than a business combination that do not have an alternative future use.
|
|
Standard
|
Description
|
Effect on the financial statements or other significant matters
|
||
|
Accounting Standards Update 2014-09 and various other related updates,
Revenue from Contracts with Customers
|
This standard replaced existing revenue recognition standards and requires entities to recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. An entity can apply the new revenue standard retrospectively to each prior reporting period presented or with the cumulative effect of initially applying the standard recognized at the date of initial application in retained earnings. We applied the latter approach.
|
Application of the new standard to applicable contracts had no impact to net parent company investment as of January 1, 2018. Disclosures required by the new standard are included in Note 5.
|
||
|
Accounting Standards Update 2016-16,
Income Taxes: Intra-Entity Transfers of Assets Other Than Inventory
|
This standard requires entities to recognize the income tax consequences of intra-entity transfers of assets other than inventory at the time of transfer. This standard requires a modified retrospective approach to adoption.
|
Upon adoption, the cumulative effect of applying the standard resulted in a decrease to net parent company investment of approximately $0.3 million. Adoption of this standard did not result in a material change in net income for the twelve months ended December 31, 2018.
|
||
|
Accounting Standards Update 2017-07,
Compensation-Retirement Benefits: Improving the Presentation of Net Periodic Pension Cost and Net Periodic Postretirement Benefit Cost
|
This standard was issued to improve the transparency and comparability among organizations by requiring entities to separate their net periodic pension cost and net periodic postretirement benefit cost into a service cost component and other components. Previously, the costs of the other components along with the service cost component were classified based upon the function of the employee. This standard requires entities to classify the service cost component in the same financial statement line item or items as other compensation costs arising from services rendered by pertinent employees. The other components of net benefit cost are now presented separately from the line items that include the service cost component. When applicable, the service cost component is now the only component eligible for capitalization. An entity should apply the new standard retrospectively for the classification of the service cost and other components and prospectively for the capitalization of the service cost component.
|
Upon adoption of this standard, pension and postretirement benefit cost components other than service costs are presented in other (income) expense, net. Retrospective application was not material to the combined statement of operations for the twelve months ended December 31, 2017. We do not expect application of the new standard to have a material impact on an ongoing basis.
|
||
|
Accounting Standards Update 2017-12,
Derivatives and Hedging
|
This standard amends the hedge accounting recognition and presentation requirements and is intended to better align hedge accounting with companies' risk management strategies. This standard eliminates the requirements to separately measure and report hedge ineffectiveness and generally requires that the entire change in fair value of a hedging instrument be presented in the same income statement line item as the respective hedged item. The standard also modifies certain disclosure requirements.
|
We elected to early adopt this guidance as of January 1, 2018. There were no hedging contracts in effect as of the date of adoption. We do not expect application of the new standard to have a material impact on an ongoing basis.
|
||
|
Standard
|
Description
|
Effective Date
|
Effect on the financial statements or other significant matters
|
|||
|
Accounting Standards Update 2016-02,
Leases
|
This standard was issued to increase transparency and comparability among organizations by recognizing lease assets and lease liabilities, including leases classified as operating leases under current GAAP, on the balance sheet and requiring additional disclosures about leasing arrangements. An entity can apply the new leases standard retrospectively to each prior reporting period presented or with the cumulative effect of initially applying the standard recognized at the date of initial application in retained earnings. We plan to use the latter approach.
|
This standard is effective January 1, 2019, with early adoption permitted. We intend to adopt this standard on that date.
|
We expect to record a right-of-use asset and lease liability for operating leases of approximately $75-95 million on our consolidated balance sheet on January 1, 2019. Our accounting for capital leases will remain substantially unchanged. This standard will not have a material impact on our consolidated statement of operations.
|
|||
|
•
|
Most of our products are sold to wholesale distributors. We initially invoice our customers contractual list prices. Contracts with direct and indirect customers may provide for various rebates and discounts that may differ in each contract. As a consequence, to determine the appropriate transaction price for our product sales at the time we recognize a sale to a direct customer, we must estimate any rebates or discounts that ultimately will be due to the direct customer and other customers in the distribution chain under the terms of our contracts. Significant judgments are required in making these estimates.
|
|
•
|
The rebate and discount amounts are recorded as a deduction to arrive at our net product sales. We estimate these accruals using an expected value approach.
|
|
•
|
In determining the appropriate accrual amount, we consider our historical experience with similar incentives programs and current sales data to estimate the impact of such programs on revenue and continually monitor the impact of this experience and adjust as necessary. Although we accrue a liability for rebates related to these programs at the time the sale is recorded, the rebate related to that sale is typically paid up to
six months
after rebate or incentive period expires. Because of this time lag, in any particular period rebate adjustments may incorporate revisions of accruals for several periods.
|
|
Year Ended December 31,
|
||||||||
|
|
2018
|
2017
|
||||||
|
Beginning balance
|
$
|
114.8
|
|
$
|
116.1
|
|
||
|
Reduction of revenue
|
221.0
|
|
236.1
|
|
||||
|
Payments
|
(217.3
|
)
|
(237.4
|
)
|
||||
|
Ending balance
|
$
|
118.5
|
|
$
|
114.8
|
|
||
|
•
|
We estimate a reserve for future product returns related to product sales using an expected value approach. This estimate is based on several factors, including: local returns policies and practices; returns as a percentage of revenue; an understanding of the reasons for past returns; estimated shelf life by product; and estimate of the amount of time between shipment and return. Adjustments to the returns reserve have been and may in the future be required based on revised estimates to our assumptions, which would have an impact on our consolidated results of operations. We record the return amounts as a deduction to arrive at our net product sales.
|
|
•
|
Actual product returns have been approximately
1%
of net revenue for the year ended December 31, 2018 and 2017 and have not fluctuated significantly as a percentage of revenue.
|
|
2018
|
2017
|
2016
|
||||||||||
|
Companion Animal Disease Prevention
|
$
|
804.6
|
|
$
|
660.2
|
|
$
|
628.4
|
|
|||
|
Companion Animal Therapeutics
|
283.1
|
|
260.8
|
|
255.6
|
|
||||||
|
Food Animal Future Protein & Health
|
711.2
|
|
649.2
|
|
630.8
|
|
||||||
|
Food Animal Ruminants Swine
|
1,174.0
|
|
1,175.0
|
|
1,309.2
|
|
||||||
|
Other
|
93.9
|
|
143.8
|
|
89.5
|
|
||||||
|
Total Revenue
|
$
|
3,066.8
|
|
$
|
2,889.0
|
|
$
|
2,913.5
|
|
|||
|
Estimated Fair Value at January 3, 2017
|
||||
|
Inventories
(1)
|
$
|
108.6
|
|
|
|
Marketed products
(2)
|
297.0
|
|
||
|
Property and equipment
|
148.2
|
|
||
|
Other assets and liabilities — net
|
8.2
|
|
||
|
Total identifiable net assets
|
562.0
|
|
||
|
Goodwill
(3)
|
320.1
|
|
||
|
Total consideration transferred — net of cash acquired
|
$
|
882.1
|
|
|
|
Estimated Fair Value at April 22, 2016
|
||||
|
Deferred tax assets
|
$
|
15.3
|
|
|
|
Acquired in-process research and development
|
31.6
|
|
||
|
Marketed products(1)
|
57.0
|
|
||
|
Deferred tax liabilities
|
(15.3
|
)
|
||
|
Total consideration
|
88.6
|
|
||
|
Less: Contingent consideration
|
(43.6
|
)
|
||
|
Total cash paid
|
$
|
45.0
|
|
|
|
2018
|
2017
|
2016
|
|||||||||
|
Cash expense:
|
|||||||||||
|
Severance and other
|
$
|
15.5
|
|
$
|
162.0
|
|
$
|
42.1
|
|
||
|
Integration
|
26.5
|
|
90.3
|
|
154.8
|
|
|||||
|
Facility exit costs
|
5.7
|
|
31.8
|
|
13.2
|
|
|||||
|
Total cash expense
|
47.7
|
|
284.1
|
|
210.1
|
|
|||||
|
Non-cash expense:
|
|||||||||||
|
Asset impairment
|
81.9
|
|
110.6
|
|
98.3
|
|
|||||
|
Total non-cash expense
|
81.9
|
|
110.6
|
|
98.3
|
|
|||||
|
Gain on sale of fixed assets
|
(0.8
|
)
|
(19.6
|
)
|
—
|
|
|||||
|
Total expense
|
$
|
128.8
|
|
$
|
375.1
|
|
$
|
308.4
|
|
||
|
Exit costs
|
Severance
|
Total
|
|||||||||
|
Balance at December 31, 2016
|
$
|
11.5
|
|
$
|
26.6
|
|
$
|
38.1
|
|
||
|
Charges
|
31.8
|
|
162.0
|
|
193.8
|
|
|||||
|
Reserve adjustment
|
1.4
|
|
(3.9
|
)
|
(2.5
|
)
|
|||||
|
Cash paid
|
(9.8
|
)
|
(141.6
|
)
|
(151.4
|
)
|
|||||
|
Balance at December 31, 2017
|
34.9
|
|
43.1
|
|
78.0
|
|
|||||
|
Charges
|
11.7
|
|
15.5
|
|
27.2
|
|
|||||
|
Separation adjustment
|
(5.9
|
)
|
—
|
|
(5.9
|
)
|
|||||
|
Reserve adjustment
|
(6.0
|
)
|
|
—
|
|
|
(6.0
|
)
|
|||
|
Cash paid
|
(25.4
|
)
|
(23.5
|
)
|
(48.9
|
)
|
|||||
|
Balance at December 31, 2018
|
$
|
9.3
|
|
$
|
35.1
|
|
$
|
44.4
|
|
||
|
2018
|
2017
|
||||||
|
Finished products
|
$
|
400.7
|
|
$
|
452.0
|
|
|
|
Work in process
|
570.4
|
|
580.0
|
|
|||
|
Raw materials and supplies
|
80.4
|
|
70.4
|
|
|||
|
Total (approximates replacement cost)
|
1,051.5
|
|
1,102.4
|
|
|||
|
Decrease to LIFO cost
|
(47.4
|
)
|
(40.1
|
)
|
|||
|
Inventories
|
$
|
1,004.1
|
|
$
|
1,062.3
|
|
|
|
December 31, 2018
|
|||
|
Term credit facility
|
$
|
492.5
|
|
|
3.912% Senior Notes due 2021
|
500.0
|
|
|
|
4.272% Senior Notes due 2023
|
750.0
|
|
|
|
4.900% Senior Notes due 2028
|
750.0
|
|
|
|
Other obligations
|
0.5
|
|
|
|
Unamortized debt issuance costs
|
(20.7
|
)
|
|
|
2,472.3
|
|
||
|
Less current portion of long-term debt
|
(29.0
|
)
|
|
|
Total long-term debt
|
$
|
2,443.3
|
|
|
|
|
Fair Value Measurements Using
|
|
||||||||||||||||
|
Financial statement line item
|
Carrying Amount
|
Quoted Prices in Active Markets for Identical Assets (Level 1)
|
Significant Other Observable Inputs (Level 2)
|
Significant Unobservable Inputs (Level 3)
|
Fair Value
|
||||||||||||||
|
December 31, 2018
|
|||||||||||||||||||
|
Other current liabilities- contingent consideration
|
$
|
5.1
|
|
$
|
—
|
|
$
|
—
|
|
$
|
5.1
|
|
$
|
5.1
|
|
||||
|
Other noncurrent liabilities- contingent consideration
|
69.0
|
|
—
|
|
—
|
|
69.0
|
|
69.0
|
|
|||||||||
|
Other noncurrent liabilities- cross currency interest rate contracts designated as net investment hedges
|
7.4
|
|
|
—
|
|
|
7.4
|
|
|
—
|
|
|
7.4
|
|
|||||
|
December 31, 2017
|
|||||||||||||||||||
|
Other current liabilities- contingent consideration
|
1.3
|
|
—
|
|
—
|
|
1.3
|
|
1.3
|
|
|||||||||
|
Other noncurrent liabilities- contingent consideration
|
45.2
|
|
—
|
|
—
|
|
45.2
|
|
45.2
|
|
|||||||||
|
2018
|
2017
|
|||||||||||||||||||||||
|
Description
|
Carrying Amount, Gross
|
Accumulated Amortization
|
Carrying Amount, Net
|
Carrying Amount, Gross
|
Accumulated Amortization
|
Carrying Amount, Net
|
||||||||||||||||||
|
Finite-lived intangible assets:
|
||||||||||||||||||||||||
|
Marketed products
|
$
|
3,193.5
|
|
$
|
(779.2
|
)
|
$
|
2,414.3
|
|
$
|
3,151.2
|
|
$
|
(599.8
|
)
|
$
|
2,551.4
|
|
||||||
|
Other
|
53.1
|
|
(34.0
|
)
|
19.1
|
|
54.1
|
|
(29.9
|
)
|
24.2
|
|
||||||||||||
|
Total finite-lived intangible assets
|
3,246.6
|
|
(813.2
|
)
|
2,433.4
|
|
3,205.3
|
|
(629.7
|
)
|
2,575.6
|
|
||||||||||||
|
Indefinite-lived intangible assets:
|
||||||||||||||||||||||||
|
Acquired in-process research and development
|
19.6
|
|
—
|
|
19.6
|
|
97.2
|
|
—
|
|
97.2
|
|
||||||||||||
|
Other intangibles
|
$
|
3,266.2
|
|
$
|
(813.2
|
)
|
$
|
2,453.0
|
|
$
|
3,302.5
|
|
$
|
(629.7
|
)
|
$
|
2,672.8
|
|
||||||
|
2019
|
2020
|
2021
|
2022
|
2023
|
||||||||||||||||
|
Estimated amortization expense
|
$
|
197.9
|
|
$
|
198.3
|
|
$
|
198.0
|
|
$
|
196.0
|
|
$
|
195.8
|
|
|||||
|
2018
|
2017
|
|||||||
|
Land
|
$
|
27.6
|
|
$
|
25.1
|
|
||
|
Buildings
|
567.2
|
|
557.7
|
|
||||
|
Equipment
|
1,025.1
|
|
994.5
|
|
||||
|
Construction in progress
|
181.1
|
|
177.1
|
|
||||
|
1,801
|
|
1,754.4
|
|
|||||
|
Less accumulated depreciation
|
(878.6
|
)
|
(834.1
|
)
|
||||
|
Property and equipment, net
|
$
|
922.4
|
|
$
|
920.3
|
|
||
|
2018
|
2017
|
2016
|
||||||||||
|
Depreciation expense
|
$
|
81.3
|
|
$
|
79.8
|
|
$
|
75.7
|
|
|||
|
Rental expense
|
47.5
|
|
47.1
|
|
41.8
|
|
||||||
|
2019
|
2020
|
2021
|
2022
|
2023
|
After 2023
|
|||||||||||||||||||
|
Lease commitments
|
$
|
25.2
|
|
$
|
20.1
|
|
$
|
13.5
|
|
$
|
10.0
|
|
$
|
8.3
|
|
$
|
18.5
|
|
||||||
|
(Percents)
|
2018
|
2017
|
2016
|
||||||
|
Expected dividend yield
|
2.50
|
%
|
2.50
|
%
|
2.00
|
%
|
|||
|
Risk-free interest rate
|
2.31
|
|
1.38
|
|
0.92
|
|
|||
|
Volatility
|
22.26
|
|
22.91
|
|
21.68
|
|
|||
|
2018
|
|||
|
Expected dividend yield
(1)
|
0.70
|
%
|
|
|
Risk-free interest rate
(2)
|
3.07
|
%
|
|
|
Expected stock price volatility
(3)
|
28.25
|
%
|
|
|
Expected term
(4)
(years)
|
6.5
|
|
|
|
Shares of Common Stock Attributable to Options
|
Weighted-Average Exercise Price of Options
|
||||||
|
Outstanding at January 1, 2018
|
—
|
|
$
|
—
|
|
||
|
Granted
|
421,297
|
|
31.61
|
|
|||
|
Exercised
|
—
|
|
—
|
|
|||
|
Forfeited or expired
|
—
|
|
—
|
|
|||
|
Outstanding at December 31, 2018
|
421,297
|
|
31.61
|
|
|||
|
Exercisable at December 31, 2018
|
58,766
|
|
31.61
|
|
|||
|
2018
|
2017
|
2016
|
|||||||||
|
Federal
|
$
|
12.2
|
|
$
|
(133.2
|
)
|
$
|
(12.5
|
)
|
||
|
Foreign
|
101.9
|
|
(99.4
|
)
|
(9.9
|
)
|
|||||
|
Income (loss) before income taxes
|
$
|
114.1
|
|
$
|
(232.6
|
)
|
$
|
(22.4
|
)
|
||
|
2018
|
2017
|
2016
|
|||||||||
|
Current:
|
|||||||||||
|
Federal
|
$
|
45.1
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
Foreign
|
45.5
|
|
91.6
|
|
31.1
|
|
|||||
|
State
|
(2.3
|
)
|
(0.1
|
)
|
0.3
|
|
|||||
|
Total current tax expense
|
88.3
|
|
91.5
|
|
31.4
|
|
|||||
|
Deferred:
|
|||||||||||
|
Federal
|
(56.8
|
)
|
42.6
|
|
18.4
|
|
|||||
|
Foreign
|
(5.6
|
)
|
(16.6
|
)
|
(26.8
|
)
|
|||||
|
State
|
1.7
|
|
(6.3
|
)
|
2.5
|
|
|||||
|
2017 Tax Act
|
—
|
|
(33.1
|
)
|
—
|
|
|||||
|
Total deferred tax benefit
|
(60.7
|
)
|
(13.4
|
)
|
(5.9
|
)
|
|||||
|
Income taxes
|
$
|
27.6
|
|
$
|
78.1
|
|
$
|
25.5
|
|
||
|
2018
|
2017
|
||||||
|
Deferred tax assets:
|
|||||||
|
Compensation and benefits
|
$
|
32.2
|
|
$
|
34.8
|
|
|
|
Accruals and reserves
|
47.8
|
|
12.0
|
|
|||
|
Tax credit carryovers
|
1.9
|
|
19.2
|
|
|||
|
Tax loss carryovers
|
21.7
|
|
144.9
|
|
|||
|
Other
|
23.5
|
|
26.6
|
|
|||
|
Total gross deferred tax assets
|
127.1
|
|
237.5
|
|
|||
|
Valuation allowances
|
(21.4
|
)
|
(127.7
|
)
|
|||
|
Total deferred tax assets
|
105.7
|
|
109.8
|
|
|||
|
Deferred tax liabilities:
|
|||||||
|
Intangibles
|
(130.8
|
)
|
(165.2
|
)
|
|||
|
Property and equipment
|
(50.8
|
)
|
(43.1
|
)
|
|||
|
Other
|
(2.7
|
)
|
(7.4
|
)
|
|||
|
Total deferred tax liabilities
|
(184.3
|
)
|
(215.7
|
)
|
|||
|
Deferred tax liabilities - net
|
$
|
(78.6
|
)
|
$
|
(105.9
|
)
|
|
|
2018
|
2017
|
||||||
|
January 1
|
$
|
(127.7
|
)
|
$
|
(39.1
|
)
|
|
|
Adjustment related to Separation
|
110.4
|
|
|
—
|
|
||
|
January 1
|
(17.3
|
)
|
|
(39.1
|
)
|
||
|
Increase
|
(5.8
|
)
|
(97.4
|
)
|
|||
|
Release
|
1.7
|
|
8.8
|
|
|||
|
December 31
|
$
|
(21.4
|
)
|
|
$
|
(127.7
|
)
|
|
2018
|
2017
|
2016
|
|||||||||
|
Cash payments of income taxes
|
$
|
26.9
|
|
$
|
35.7
|
|
$
|
53.6
|
|
||
|
2018
|
2017
|
2016
|
|||||||||
|
Income tax at the U.S. federal statutory tax rate
|
$
|
24.0
|
|
$
|
(81.4
|
)
|
$
|
(7.8
|
)
|
||
|
Add (deduct):
|
|||||||||||
|
International operations and change in foreign tax rates
|
11.5
|
|
55.6
|
|
8.4
|
|
|||||
|
State taxes
|
4.4
|
|
5.4
|
|
2.8
|
|
|||||
|
Income tax credits
|
(17.3
|
)
|
(1.8
|
)
|
(1.7
|
)
|
|||||
|
Foreign inclusion items
|
9.0
|
|
4.2
|
|
2.4
|
|
|||||
|
IPO and separation costs
|
2.3
|
|
|
—
|
|
|
—
|
|
|||
|
Other permanent adjustments
|
0.9
|
|
|
1.6
|
|
|
0.2
|
|
|||
|
Change in uncertain tax positions
|
(1.7
|
)
|
6.2
|
|
5.2
|
|
|||||
|
Change in valuation allowance
|
(1.7
|
)
|
122.2
|
|
18.1
|
|
|||||
|
2017 Tax Act
|
—
|
|
(33.1
|
)
|
—
|
|
|||||
|
Other
|
(3.8
|
)
|
(0.8
|
)
|
(2.1
|
)
|
|||||
|
Income taxes
|
$
|
27.6
|
|
$
|
78.1
|
|
$
|
25.5
|
|
||
|
2018
|
2017
|
2016
|
|||||||||
|
Beginning balance at January 1
|
$
|
29.6
|
|
$
|
25.7
|
|
$
|
25.5
|
|
||
|
Adjustments related to Separation
|
(17.6
|
)
|
|
—
|
|
|
—
|
|
|||
|
Beginning balance at January 1
|
12.0
|
|
|
25.7
|
|
|
25.5
|
|
|||
|
Additions based on tax positions related to the current year
|
2.2
|
|
7.9
|
|
7.4
|
|
|||||
|
Additions for tax positions of prior years
|
4.0
|
|
—
|
|
—
|
|
|||||
|
Settlements
|
(3.0
|
)
|
(4.0
|
)
|
(7.1
|
)
|
|||||
|
Changes related to the impact of foreign currency translation
|
(0.5
|
)
|
—
|
|
(0.1
|
)
|
|||||
|
Ending balance at December 31
|
$
|
14.7
|
|
$
|
29.6
|
|
$
|
25.7
|
|
||
|
2018
|
2017
|
2016
|
|||||||||
|
Income tax expense (benefit)
|
$
|
(2.5
|
)
|
$
|
2.5
|
|
$
|
5.5
|
|
||
|
2018
|
2017
|
2016
|
||||||||||
|
Geographic Information
|
||||||||||||
|
Revenue — to unaffiliated customers
(1)
:
|
||||||||||||
|
United States
|
$
|
1,483.2
|
|
$
|
1,373.0
|
|
$
|
1,361.6
|
|
|||
|
International
|
1,583.6
|
|
1,516.0
|
|
1,551.9
|
|
||||||
|
Revenue
|
$
|
3,066.8
|
|
$
|
2,889.0
|
|
$
|
2,913.5
|
|
|||
|
Long-lived assets
(2)
:
|
||||||||||||
|
United States
|
$
|
602.6
|
|
$
|
604.7
|
|
$
|
463.8
|
|
|||
|
United Kingdom
|
187.5
|
|
204.4
|
|
190.6
|
|
||||||
|
Other foreign countries
|
195.8
|
|
190.2
|
|
173.0
|
|
||||||
|
Long-lived assets
|
$
|
985.9
|
|
$
|
999.3
|
|
$
|
827.4
|
|
|||
|
|
2018
|
2017
|
|||||
|
Change in benefit obligation:
|
|||||||
|
Benefit obligation at beginning of year
|
$
|
258.6
|
|
$
|
225.0
|
|
|
|
Service cost
|
11.3
|
|
10.5
|
|
|||
|
Interest cost
|
2.5
|
|
1.8
|
|
|||
|
Actuarial (gain) loss
|
(44.7
|
)
|
24.4
|
|
|||
|
Benefits paid
|
(2.7
|
)
|
(18.5
|
)
|
|||
|
Foreign currency exchange rate changes and other adjustments
|
9.8
|
|
15.4
|
|
|||
|
Benefit obligation at end of year
|
234.8
|
|
258.6
|
|
|||
|
Change in plan assets:
|
|||||
|
Fair value of plan assets at beginning of year
|
131.5
|
|
123.7
|
|
|
|
Actual return on plan assets
|
(10.2
|
)
|
13.3
|
|
|
|
Employer contribution
|
5.7
|
|
3.9
|
|
|
|
Benefits paid
|
(2.7
|
)
|
(18.5
|
)
|
|
|
Foreign currency exchange rate changes and other adjustments
|
7.3
|
|
9.1
|
|
|
|
Fair value of plan assets at end of year
|
131.6
|
|
131.5
|
|
|
|
Funded status
|
(103.2
|
)
|
(127.1
|
)
|
|||
|
Unrecognized net actuarial loss
|
0.5
|
|
29.1
|
|
|||
|
Unrecognized prior service cost
|
0.8
|
|
0.7
|
|
|||
|
Net amount recognized
|
$
|
(101.9
|
)
|
$
|
(97.3
|
)
|
|
|
Amounts recognized in the combined balance sheet consisted of:
|
|||||||
|
Noncurrent assets
|
$
|
2.3
|
|
$
|
2.4
|
|
|
|
Other current liabilities
|
(0.3
|
)
|
(0.3
|
)
|
|||
|
Accrued retirement benefits
|
(105.2
|
)
|
(129.2
|
)
|
|||
|
Accumulated other comprehensive loss before income taxes
|
1.3
|
|
29.8
|
|
|||
|
Net amount recognized
|
$
|
(101.9
|
)
|
$
|
(97.3
|
)
|
|
|
Unrecognized net actuarial loss
|
$
|
0.5
|
|
|
Unrecognized prior service cost
|
0.8
|
|
|
|
Total
|
$
|
1.3
|
|
|
(Percents)
|
2018
|
2017
|
2016
|
|||
|
Discount rate for benefit obligation
|
1.5%
|
1.1%
|
1.0%
|
|||
|
Discount rate for net benefit costs
|
1.1
|
1.0
|
1.0
|
|||
|
Rate of compensation increase for benefit obligation
|
2.2
|
2.1
|
3.1
|
|||
|
Rate of compensation increase for net benefit costs
|
2.1
|
3.1
|
3.0
|
|||
|
Expected return on plan assets for net benefit costs
|
4.0
|
4.4
|
4.9
|
|||
|
2019
|
2020
|
2021
|
2022
|
2023
|
2024-2028
|
||||||||||||||||||
|
Benefit payments
|
$
|
5.8
|
|
$
|
6.4
|
|
$
|
7.1
|
|
$
|
6.1
|
|
$
|
6.3
|
|
$
|
35.9
|
|
|||||
|
|
2018
|
2017
|
|||||
|
Projected benefit obligation
|
$
|
229.2
|
|
$
|
251.6
|
|
|
|
Fair value of plan assets
|
124.1
|
|
121.8
|
|
|||
|
|
2018
|
2017
|
|||||
|
Accumulated benefit obligation
|
$
|
194.3
|
|
$
|
223.1
|
|
|
|
Fair value of plan assets
|
124.1
|
|
121.8
|
|
|||
|
|
2018
|
2017
|
2016
|
||||||||
|
Service cost
|
$
|
11.3
|
|
$
|
10.5
|
|
$
|
9.3
|
|
||
|
Interest cost
|
2.5
|
|
1.8
|
|
1.8
|
|
|||||
|
Expected return on plan assets
|
(6.2
|
)
|
(2.4
|
)
|
(3.4
|
)
|
|||||
|
Amortization of prior service cost
|
0.2
|
|
0.1
|
|
0.1
|
|
|||||
|
Amortization of net actuarial loss
|
1.9
|
|
1.4
|
|
1.0
|
|
|||||
|
Other
|
0.5
|
|
—
|
|
—
|
|
|||||
|
Net pension expense
|
$
|
10.2
|
|
$
|
11.4
|
|
$
|
8.8
|
|
||
|
2018
|
2017
|
2016
|
|||||||||
|
Actuarial gain (loss) arising during period
|
$
|
28.3
|
|
$
|
(17.0
|
)
|
$
|
(6.1
|
)
|
||
|
Amortization of prior service cost included in net loss
|
0.2
|
|
0.1
|
|
0.1
|
|
|||||
|
Amortization of net actuarial loss included in net loss
|
1.9
|
|
1.4
|
|
1.0
|
|
|||||
|
Foreign currency exchange rate changes and other
|
(1.9
|
)
|
3.5
|
|
3.0
|
|
|||||
|
Total other comprehensive income (loss) during period
|
$
|
28.5
|
|
$
|
(12.0
|
)
|
$
|
(2.0
|
)
|
||
|
•
|
Fixed-income securities - Swiss Bonds, Global Aggregates, Global Aggregate Corporates and Emerging Markets Local Currencies.
|
|
•
|
Equity investments - Swiss Equities, World Equities MSCI, Low Volatility Equities (to reduce risk), Emerging Markets Equities and real estate investment trusts.
|
|
•
|
Real Estate in Switzerland - investment foundations and funds
|
|
•
|
Other investments - represents primarily private equity like investments, hedge funds, insurance-linked securities, cash and mark-to-market derivatives.
|
|
|
|
|
Fair Value Measurements Using
|
|||||||||||||||||
|
Asset Class
|
Total
|
Quoted Prices in Active Markets for Identical Assets (Level 1)
|
Significant Observable Inputs (Level 2)
|
Significant Unobservable Inputs (Level 3)
|
Investments Valued at Net Asset Value
(1)
|
|||||||||||||||
|
Public equity securities
|
$
|
2.2
|
|
$
|
1.0
|
|
$
|
—
|
|
$
|
—
|
|
$
|
1.2
|
|
|||||
|
Fixed income:
|
||||||||||||||||||||
|
Developed markets
|
29.9
|
|
7.8
|
|
0.1
|
|
—
|
|
22.0
|
|
||||||||||
|
Emerging markets
|
6.4
|
|
0.7
|
|
0.4
|
|
—
|
|
5.3
|
|
||||||||||
|
Private alternative investments:
|
—
|
|
||||||||||||||||||
|
Hedge funds
|
6.6
|
|
—
|
|
—
|
|
—
|
|
6.6
|
|
||||||||||
|
Equity-like funds
|
49.0
|
|
—
|
|
—
|
|
—
|
|
49.0
|
|
||||||||||
|
Real estate
|
20.1
|
|
0.1
|
|
—
|
|
—
|
|
20.0
|
|
||||||||||
|
Other
|
17.4
|
|
0.3
|
|
2.3
|
|
—
|
|
14.8
|
|
||||||||||
|
Total
|
$
|
131.6
|
|
$
|
9.9
|
|
$
|
2.8
|
|
$
|
—
|
|
$
|
118.9
|
|
|||||
|
(1)
|
Certain investments that are measured at fair value using the NAV per share (or its equivalent) as a practical expedient have not been classified in the fair value hierarchy.
|
|
|
|
Fair Value Measurements Using
|
||||||||||||||||||
|
Asset Class
|
Total
|
Quoted Prices in Active Markets for Identical Assets (Level 1)
|
Significant Observable Inputs (Level 2)
|
Significant Unobservable Inputs (Level 3)
|
Investments Valued at Net Asset Value
(1)
|
|||||||||||||||
|
Public equity securities
|
$
|
0.8
|
|
$
|
0.6
|
|
$
|
—
|
|
$
|
—
|
|
$
|
0.2
|
|
|||||
|
Fixed income:
|
||||||||||||||||||||
|
Developed markets
|
29.9
|
|
8.2
|
|
0.1
|
|
—
|
|
21.6
|
|
||||||||||
|
Emerging markets
|
7.2
|
|
0.6
|
|
0.3
|
|
—
|
|
6.3
|
|
||||||||||
|
Private alternative investments:
|
|
|||||||||||||||||||
|
Hedge funds
|
6.8
|
|
—
|
|
—
|
|
—
|
|
6.8
|
|
||||||||||
|
Equity-like funds
|
52.7
|
|
—
|
|
—
|
|
—
|
|
52.7
|
|
||||||||||
|
Real estate
|
20.2
|
|
—
|
|
—
|
|
—
|
|
20.2
|
|
||||||||||
|
Other
|
13.9
|
|
0.1
|
|
0.1
|
|
13.7
|
|
||||||||||||
|
Total
|
$
|
131.5
|
|
$
|
9.5
|
|
$
|
0.5
|
|
$
|
—
|
|
$
|
121.5
|
|
|||||
|
(1)
|
Certain investments that are measured at fair value using the NAV per share (or its equivalent) as a practical expedient have not been classified in the fair value hierarchy.
|
|
•
|
Transitional Services Agreement. Historically, Lilly has provided us significant shared services and resources related to corporate functions such as executive oversight, treasury, legal, finance, human resources, tax, internal audit, financial reporting, information technology and investor relations, which we refer to collectively as the "Lilly Services." Under the terms of the TSA, we will be able to use Lilly Services for a fixed term established on a service-by-service basis. We will pay Lilly mutually agreed-upon fees for the Lilly Services provided under the TSA, which will be based on Lilly's cost (including third-party costs) of providing the Lilly Services through
March 31, 2021
, and subject to a mark-up of
7%
thereafter, with additional inflation-based escalation beginning
January 1, 2020
. The fees under the TSA become payable for all periods beginning after
October 1, 2018
.
|
|
•
|
Intellectual Property and Technology License Agreement. We entered into an intellectual property and technology license agreement with Lilly immediately prior to the completion of the IPO. Under the intellectual property and technology license agreement, Lilly granted Elanco an exclusive, perpetual license to exploit products in the animal health field that utilize or use certain of Lilly's intellectual property (excluding trademarks). In addition, Lilly granted Elanco non-exclusive, non-sublicensable license to screen certain compounds in Lilly's compound libraries to exploit products in the animal use certain of Lilly's intellectual property. This screening license has an initial term of
two years
, subject to
three
one
-year extensions, each of which requires Lilly's consent.
|
|
2018
(1)
|
2017
|
2016
|
|||||||||
|
Cost of sales
|
$
|
21.8
|
|
$
|
31.8
|
|
$
|
32.5
|
|
||
|
Research and development
|
2.2
|
|
2.8
|
|
2.3
|
|
|||||
|
Marketing, selling and administrative
|
81.2
|
|
117.1
|
|
110.5
|
|
|||||
|
Total
|
$
|
105.2
|
|
$
|
151.7
|
|
$
|
145.3
|
|
||
|
2018
|
Fourth
|
Third
|
Second
|
First
|
||||||||||||
|
Revenue
|
$
|
799.3
|
|
$
|
761.1
|
|
$
|
770.2
|
|
$
|
736.2
|
|
||||
|
Cost of sales
|
412.5
|
|
369.8
|
|
431.5
|
|
360.0
|
|
||||||||
|
Operating expenses
(1)
|
246.2
|
|
237.9
|
|
252.5
|
|
245.2
|
|
||||||||
|
Asset Impairment, restructuring, and other special charges
|
46.0
|
|
12.4
|
|
68.0
|
|
2.4
|
|
||||||||
|
Interest expense, net of capitalized interest
|
21.0
|
|
|
8.6
|
|
|
—
|
|
|
—
|
|
|||||
|
Income (loss) before income taxes
|
(2.2
|
)
|
78.8
|
|
(40.0
|
)
|
77.5
|
|
||||||||
|
Income taxes
|
(18.6
|
)
|
18.6
|
|
22.8
|
|
4.8
|
|
||||||||
|
Net income (loss)
|
16.4
|
|
60.2
|
|
(62.8
|
)
|
72.7
|
|
||||||||
|
Earnings (loss) per share—basic and diluted
|
0.04
|
|
0.20
|
|
(0.21
|
)
|
0.25
|
|
||||||||
|
|
|
|
||||||||||||||
|
2017
|
Fourth
|
Third
|
Second
|
First
|
||||||||||||
|
Revenue
|
$
|
754.3
|
|
$
|
697.1
|
|
$
|
732.8
|
|
$
|
704.8
|
|
||||
|
Cost of sales
|
405.0
|
|
376.2
|
|
374.0
|
|
338.6
|
|
||||||||
|
Operating expenses
(1)
|
258.8
|
|
256.6
|
|
257.8
|
|
258.3
|
|
||||||||
|
Asset Impairment, restructuring, and other special charges
|
185.8
|
|
23.7
|
|
58.8
|
|
106.8
|
|
||||||||
|
Interest expense, net of capitalized interest
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|||||
|
Income (loss) before income taxes
|
(155.4
|
)
|
(9.1
|
)
|
(15.2
|
)
|
(52.9
|
)
|
||||||||
|
Income taxes
|
6.1
|
|
11.6
|
|
15.0
|
|
45.4
|
|
||||||||
|
Net income (loss)
|
(161.5
|
)
|
(20.7
|
)
|
(30.2
|
)
|
(98.3
|
)
|
||||||||
|
Earnings (loss) per share—basic and diluted
|
(0.55
|
)
|
(0.07
|
)
|
(0.10
|
)
|
(0.34
|
)
|
||||||||
|
•
|
Consolidated and Combined Statements of Operations—Years Ended December 31, 2018, 2017, and 2016
|
|
•
|
Consolidated and Combined Statements of Comprehensive Income—Years Ended December 31, 2018, 2017, and 2016
|
|
•
|
Consolidated and Combined Balance Sheets—December 31, 2018 and 2017
|
|
•
|
Consolidated and Combined Statements of Shareholders' Equity—Years Ended December 31, 2018, 2017, and 2016
|
|
•
|
Consolidated and Combined Statements of Cash Flows—Years Ended December 31, 2018, 2017, and 2016
|
|
•
|
Notes to Consolidated and Combined Financial Statements
|
|
Exhibit Number
|
|
Description
|
|
|
|
|
Amended and Restated Articles of Incorporation of Elanco Animal Health Incorporated, effective September 18, 2018 (incorporated by reference to Exhibit 3.1 of the Current Report on Form 8-K filed with the SEC on September 26, 2018).
|
|
|
|
|
Amended and Restated Bylaws of Elanco Animal Health Incorporated, effective September 19, 2018 (incorporated by reference to Exhibit 3.2 of the Current Report on Form 8-K filed with the SEC on September 26, 2018).
|
|
|
|
|
Form of Certificate of Common Stock (incorporated by reference to Exhibit 4.1 of Amendment No. 1 to Registration Statement on Form S-1 (Registration No. 333-226536) filed with the SEC on August 28, 2018).
|
|
|
|
|
Indenture, dated August 28, 2018, between Elanco Animal Health Incorporated and Deutsche Bank Trust Company Americas, as trustee (incorporated by reference to Exhibit 4.2 of Amendment No. 1 to Registration Statement on Form S-1 (Registration No. 333-226536) filed with the SEC on August 28, 2018).
|
|
|
|
|
First Supplemental Indenture, dated August 28, 2018, between Elanco Animal Health Incorporated and Deutsche Bank Trust Company Americas, as trustee (incorporated by reference to Exhibit 4.3 of Amendment No. 1 to Registration Statement on Form S-1 (Registration No. 333-226536) filed with the SEC on August 28, 2018).
|
|
|
|
|
Exchange and Registration Rights Agreement, dated August 28, 2018, between Elanco Animal Health Incorporated and Goldman Sachs & Co. LLC, J.P. Morgan Securities LLC and Morgan Stanley & Co. LLC, as representatives of the several initial purchasers (incorporated by reference to Exhibit 4.4 of Amendment No. 1 to Registration Statement on Form S-1 (Registration No. 333-226536) filed with the SEC on August 28, 2018).
|
|
|
|
|
Master Separation Agreement, dated September 24, 2018, between Eli Lilly and Company and Elanco Animal Health Incorporated (incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the SEC on September 26, 2018).
|
|
|
|
|
Transitional Services Agreement, dated September 24, 2018, between Eli Lilly and Company and Elanco Animal Health Incorporated (incorporated by reference to Exhibit 10.2 of the Current Report on Form 8-K filed with the SEC on September 26, 2018).
|
|
|
|
|
Tax Matters Agreement, dated September 24, 2018, between Eli Lilly and Company and Elanco Animal Health Incorporated (incorporated by reference to Exhibit 10.3 of the Current Report on Form 8-K filed with the SEC on September 26, 2018).
|
|
|
|
|
Employee Matters Agreement, dated September 24, 2018, between Eli Lilly and Company and Elanco Animal Health Incorporated (incorporated by reference to Exhibit 10.4 of the Current Report on Form 8-K filed with the SEC on September 26, 2018).
|
|
|
|
|
Toll Manufacturing and Supply Agreement, dated September 24, 2018, between Eli Lilly Export S.A. and Elanco UK AH Limited (incorporated by reference to Exhibit 10.5 of the Current Report on Form 8-K filed with the SEC on September 26, 2018).
|
|
|
|
|
Registration Rights Agreement, dated September 24, 2018, between Eli Lilly and Company and Elanco Animal Health Incorporated (incorporated by reference to Exhibit 10.6 of the Current Report on Form 8-K filed with the SEC on September 26, 2018).
|
|
|
|
Transitional Trademark License Agreement, dated September 24, 2018, among Eli Lilly and Company, Elanco Animal Health Incorporated and Elanco US Inc. (incorporated by reference to Exhibit 10.7 of the Current Report on Form 8-K filed with the SEC on September 26, 2018).
|
||
|
|
Intellectual Property and Technology License Agreement, dated September 24, 2018, among Eli Lilly and Company, Elanco Animal Health Incorporated and Elanco US Inc. (incorporated by reference to Exhibit 10.8 of the Current Report on Form 8-K filed with the SEC on September 26, 2018).
|
||
|
|
Revolving Loan Credit Agreement, dated as of September 5, 2018, among Elanco Animal Health Incorporated, as borrower, JPMorgan Chase Bank, N.A., as administrative agent and the other Lenders party thereto (incorporated by reference to Exhibit 10.24 of Amendment No. 2 to Registration Statement on Form S-1 (Registration No. 333-226536) filed with the SEC on September 6, 2018).
|
||
|
|
Term Loan Credit Agreement, dated as of September 5, 2018, among Elanco Animal Health Incorporated, as borrower, JPMorgan Chase Bank, N.A., as administrative agent and the other Lenders party thereto (incorporated by reference to Exhibit 10.25 of Amendment No. 2 to Registration Statement on Form S-1 (Registration No. 333-226536) filed with the SEC on September 6, 2018).
|
||
|
|
2018 Elanco Stock Plan (incorporated by reference to Exhibit 4.3 of Registration Statement on Form S-8 (Registration No. 333-227447) filed with the SEC on September 20, 2018).*
|
||
|
|
Elanco Animal Health Incorporated Directors’ Deferral Plan (incorporated by reference to Exhibit 4.4 of Registration Statement on Form S-8 (Registration No. 333-227447) filed with the SEC on September 20, 2018)*
|
||
|
|
Director Letter Agreement between Emu Holdings Company and R. David Hoover, dated as of May 25, 2018 (incorporated by reference to Exhibit 10.19 of Elanco Animal Health Incorporated's registration statement on Form S-1 (File No. 333-226536) filed with the SEC on August 2, 2018)*
|
||
|
|
Form of 2018 Change in Control Severance Pay Plan for Select Employees (incorporated by reference to Exhibit 10.20 of Amendment No. 1 to Elanco Animal Health Incorporated's registration statement on Form S-1 (File No. 333-226536) filed with the SEC on August 28, 2018)
.
*
|
||
|
|
Form of Elanco Animal Health Incorporated Restricted Stock Unit Awards Agreement (incorporated by reference to Exhibit 10.21 of Amendment No. 1 to Elanco Animal Health Incorporated's registration statement on Form S-1 (File No. 333-226536) filed with the SEC on August 28, 2018).*
|
||
|
|
Form of Elanco Animal Health Incorporated Nonqualified Stock Option Award Agreement (incorporated by reference to Exhibit 10.22 of Amendment No. 1 to Elanco Animal Health Incorporated's registration statement on Form S-1 (File No. 333-226536) filed with the SEC on August 28, 2018).*
|
||
|
|
Retention Bonus Agreement, dated October 18, 2018, by and between Elanco US Inc. and Todd S. Young (incorporated by reference to Exhibit 10.2 to Elanco Animal Health Incorporated's Report on Form 8-K filed with the SEC on October 30, 2018).*
|
||
|
|
Employment Offer Letter with Mr. Todd S. Young, dated October 15, 2018, by and between Elanco US Inc. and Todd S. Young (incorporated by reference to Exhibit 10.1 to Elanco Animal Health Incorporated's Report on Form 8-K filed with the SEC on October 30, 2018).*
|
||
|
|
Form of Performance Award Agreement (Incorporated by reference to Exhibit 10.1 to Form 8-K filed with the SEC on February 19, 2019)*
|
||
|
|
Form of Restricted Stock Unit Award Agreement (Incorporated by reference to Exhibit 10.2 to Form 8-K filed with the SEC on February 19, 2019)*
|
||
|
|
Form of Restricted Stock Unit Award Agreement (filed herewith)*
|
||
|
|
Form of Replacement Performance Award Agreement for Certain Named Executive Officers (filed herewith)*
|
||
|
|
Form of Replacement Performance Award Agreement for Jeffery N. Simmons (filed herewith)*
|
||
|
|
Form of Replacement Restricted Stock Unit Award Agreement for Certain Named Executive Officers (filed herewith)*
|
||
|
|
2002 Lilly Stock Plan, as amended (incorporated by reference to Appendix C to Eli Lilly and Company's proxy statement on Schedule 14A filed on March 19, 2018)* |
||
|
|
The Eli Lilly and Company Bonus Plan, as amended (incorporated by reference to Exhibit 10.7 to Eli Lilly and Company's Report on Form 10-K for the year ended December 31, 2013)*
|
||
|
|
Form of Performance Award under the 2002 Lilly Stock Plan (incorporated by reference to Exhibit 10.2 to Eli Lilly and Company's Report on Form 10-K for the year ended December 31, 2017)*
|
||
|
|
Form of Shareholder Value Award under the 2002 Lilly Stock Plan (incorporated by reference to Exhibit 10.3 to Eli Lilly and Company's Report on Form 10-K for the year ended December 31, 2017)*
|
||
|
|
The Lilly Deferred Compensation Plan, as amended (incorporated by reference to Exhibit 10.5 to Eli Lilly and Company's Report on Form 10-K for the year ended December 31, 2013)*
|
||
|
|
The Eli Lilly and Company Executive Offer Incentive Plan (incorporated by reference to Appendix B to Eli Lily and Company's proxy statement on Schedule 14A filed on March 7, 2011 (SEC File No. 001-06351, Film No. 11666753))*
|
||
|
|
2007 Change in Control Severance Pay Plan (incorporated by reference to Exhibit 10 to Eli Lilly and Company's Report on Form 10-Q for the quarter ended September 30, 2010 (SEC File No. 001-06351, Film No. 101149876))*
|
||
|
|
The Elanco Corporate Bonus Plan (incorporated by reference to Exhibit 10.16 of Elanco Animal Health Incorporated's registration statement on Form S-1 (File No. 333-226536))*
|
||
|
|
The Lilly Severance Pay Plan (incorporated by reference to Exhibit 10.23 of Elanco Animal Health Incorporated's registration statement on Form S-1 (File No. 333-226536))*
|
||
|
|
Subsidiaries of Elanco Animal Health Incorporated (filed herewith)
|
||
|
|
Consent of Ernst & Young LLP (filed herewith)
|
||
|
|
Section 302 Certification of the Chief Executive Officer pursuant to Rule 13a-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith).
|
||
|
|
Section 302 Certification of the Chief Financial Officer pursuant to Rule 13a-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith).
|
||
|
|
Certification of the Chief Executive Officer and the Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (filed herewith).
|
||
|
101
|
|
Interactive Data Files.
|
|
|
ELANCO ANIMAL HEALTH INCORPORATED
|
||
|
(Registrant)
|
||
|
Date:
|
February 20, 2019
|
/s/ Jeffrey N. Simmons
|
|
Jeffrey N. Simmons
|
||
|
President and Chief Executive Officer
|
||
|
/s/ Jeffrey N. Simmons
|
Date:
|
February 20, 2019
|
|
Jeffrey N. Simmons
|
||
|
President and Chief Executive Officer (principal executive officer) and Director
|
||
|
/s/ Todd S. Young
|
Date:
|
February 20, 2019
|
|
Todd S. Young
|
||
|
Executive Vice President, Chief Financial Officer (principal financial officer)
|
||
|
/s/ James M. Meer
|
Date:
|
February 20, 2019
|
|
James M. Meer
|
||
|
Chief Accounting Officer (principal accounting officer)
|
||
|
/s/ R. David Hoover
|
Date:
|
February 20, 2019
|
|
R. David Hoover
|
||
|
Chairman of the Board
|
||
|
/s/ Kapila Kapur Anand
|
Date:
|
February 20, 2019
|
|
Kapila Kapur Anand
|
||
|
Director
|
||
|
/s/ Michael J. Harrington
|
Date:
|
February 20, 2019
|
|
Michael J. Harrington
|
||
|
Director
|
||
|
/s/ Lawrence E. Kurzius
|
Date:
|
February 20, 2019
|
|
Lawrence E. Kurzius
|
||
|
Director
|
||
|
/s/ Carl L. McMillian Ph.D.
|
Date:
|
February 20, 2019
|
|
Carl L. McMillian
|
||
|
Director
|
||
|
/s/ David A. Ricks
|
Date:
|
February 20, 2019
|
|
David A. Ricks
|
||
|
Director
|
||
|
/s/ Aarti S. Shah Ph.D.
|
Date:
|
February 20, 2019
|
|
Aarti S. Shah
|
||
|
Director
|
||
|
/s/ Joshua L. Smiley
|
Date:
|
February 20, 2019
|
|
Joshua L. Smiley
|
||
|
Director
|
||