|
þ
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
British Virgin Islands
|
Not applicable
|
|
|
State or Other Jurisdiction of Incorporation or Organization
|
I.R.S. Employer Identification No.
|
|
|
Building B15 and 25
Coyol Free Zone
Alajuela
Costa Rica
|
Not applicable
|
|
|
Address of Principal Executive Offices
|
Zip Code
|
|
|
+506 2434 2400
|
||||
|
Registrant’s Telephone Number, Including Area Code
|
||||
|
Not applicable
|
||||
|
Former Name, Former Address and Former Fiscal Year, if Changed Since Last Report
|
||||
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|
|
Common Shares, No Par Value
|
The Nasdaq Capital Market
|
|
|
Large accelerated filer
|
o
|
Accelerated filer
|
o
|
|
|
Non-accelerated filer
|
þ
|
Smaller reporting company
|
þ
|
|
|
Emerging growth company
|
þ
|
|||
|
|
|
Page
|
|
Total Breast Augmentation Procedures
|
|||
|
Rank *
|
Country
|
Procedures
|
Percentage of World-Wide Total
|
|
1
|
United States
|
345,236
|
20.6%
|
|
2
|
Brazil
|
235,950
|
14.1%
|
|
3
|
Mexico
|
67,478
|
4.0%
|
|
4
|
Italy
|
54,045
|
3.2%
|
|
5
|
Germany
|
46,165
|
2.8%
|
|
6
|
Colombia
|
45,570
|
2.7%
|
|
7
|
Thailand
|
14,614
|
0.9%
|
|
8
|
Japan
|
7,751
|
0.5%
|
|
* Rankings are based solely on those countries from which a sufficient survey response was received and data were considered to be representative.
|
|||
|
Sientra
5-Year |
Allergan
6-Year |
Mentor
6-Year |
||||
|
Number of Patients
|
N=1,788 Patients
|
N=455 Patients
(1)
|
N=1,008 Patients
|
|||
|
Ruptures
(2)
|
1.8%
|
5.5%
|
3.7%
|
|||
|
Capsular Contracture
|
9.0%
|
14.8%
|
13.4%
|
|||
|
Reoperations
|
23.8%
|
28.0%
|
26.1%
|
|||
|
Each of these prospective studies was conducted at multiple sites in the United States and submitted by each of these companies as their core study supporting approval, as that term is defined in the FDA Guidance on Breast Implants. Sientra, Inc., Mentor Worldwide LLC (a division of Johnson & Johnson), and Allergan plc studies commenced in 2002, 2000, and 1998, respectively, and the results described above were released in 2012, 2009, and 2007, respectively. Five-year and six-year data was chosen to increase comparability to our six-year data.
|
||||||
|
Kaplan-Meier risk rates were the primary method of analysis for the above data.
|
||||||
|
(1) Adverse events in the study were derived from the primary augmentation cohort. The overall patient population in the study was 715 patients.
|
||||||
|
(2) The total for Sientra is based on the total patient population in the study, and the totals for Allergan and Mentor are based on a substudy cohort of patients who underwent an MRI, which is lower than the overall number of patients participating in the study.
|
||||||
|
Sientra
10-Year |
Allergan
10-Year |
Mentor
10-Year |
||||
|
Number of Patients
|
N=1,116 Patients
|
N=455 Patients
|
N=552 Patients
|
|||
|
Ruptures
(1)
|
8.5%
|
9.3%
|
24.2%
|
|||
|
Capsular Contracture
|
12.9%
|
18.9%
|
12.1%
|
|||
|
Reoperations
|
24.0%
|
36.1%
|
25.5%
|
|||
|
Kaplan-Meier risk rates were the primary method of analysis for the above data. This table represents the final data from the primary cohort of the same study referenced in the above five- and six-year PMA studies conducted by our competitors. This 10-year data for Sientra, Allergan and Mentor were released in 2018, 2018, and 2015, respectively.
|
||||||
|
(1) The rupture rates represent the MRI cohort only for each respective study, which consisted of 571 patients for Sientra, 158 patients for Allergan and 202 patients for Mentor.
|
||||||
|
▪
|
Patient-centric innovative implant technologies.
We have developed our Motiva Implants by enhancing and creating novel product components for our implants, and then combining these components into products that deliver improved aesthetic outcomes, increased patient satisfaction and favorable safety profiles.
|
|
▪
|
Extensive suite of complementary products and services.
Our MotivaImagine product portfolio includes innovative products such as Divina 3D surgical simulation systems, Puregraft autologous fat grafting systems, and other surgical tools. We believe our branded surgical procedures, such as MotivaHybrid, Motiva MinimalScar and Motiva MINT, will address key unmet needs for both the physician and the patient.
|
|
▪
|
Proprietary internal manufacturing processes and capabilities.
We manufacture our silicone products in state-of-the-art manufacturing facilities in Costa Rica rather than relying on third-party manufacturers. In these facilities, we utilize our novel 3D imprinted molding method to create proprietary surface features that, in combination with other proprietary materials and methods, differentiate our products from those of our competitors. Our two manufacturing sites have gone through full site inspections and audits under the Medical Device Single Audit Program, or MDSAP, which were carried out by the British Standards Institute, or BSI, an agency which the FDA accepts as a substitute for routine agency inspections. We believe our modern facilities, focus on product quality and deep technological know-how have helped us establish and maintain a brand of consistency, quality and safety.
|
|
▪
|
Dynamic worldwide sales platform.
We sell our products both through exclusive arrangements with leading local distributors who have strong local surgeon relationships and our direct sales force in key markets such as Brazil and certain countries in Europe. Using this market specific approach, we have built an effective and efficient worldwide sales platform.
|
|
▪
|
Proven management team with expansive industry experience.
We have a highly experienced management team that is comprised of leaders from the medical aesthetic market.
|
|
▪
|
Expand revenues in existing markets.
We believe we can continue to grow market share in our existing markets due to the favorable safety profile and improved aesthetic outcomes of our Motiva Implants.
|
|
▪
|
Launch Motiva Implants in additional markets outside the United States.
We expect that continued geographic expansion will be a key driver of growth in the near term. In recent years, we started sales through distributors in Australia, Israel, Peru, Russia, Saudi Arabia and South Korea, as well as starting direct sales in Brazil, the second largest market for breast augmentations. Expansion into new countries in the Asia-Pacific region (China, India, Taiwan and Thailand) is expected in the next several years.
|
|
▪
|
Obtain FDA approval and enter the U.S. market.
We are conducting our IDE clinical trial in the United States, with the goal of obtaining approval from the FDA for a premarket application, or PMA, and commercializing our Motiva Implants in the United States. The first patient in the study was enrolled in April 2018, and we anticipate completing enrollment in Q2 2019.
|
|
▪
|
Optimize patient conversion through sales and marketing programs.
Our MotivaImagine Centers enable us to engage with and educate patients on the Motiva brand and the benefits of our products, as well as increase clinical efficiency for our physician collaborators. In the future, we expect our MotivaImagine Centers to have important strategic synergies with our branded surgeries, which are promoted globally. We employ a multi-faceted marketing strategy that includes social media engagement, conferences, advertisements and education.
|
|
▪
|
Seek out and pursue strategic acquisitions.
We intend to seek out other innovative products, services and branded procedures that meet unmet needs in the aesthetics space and complement our existing product portfolio, and we believe this can be additive to future revenue growth. We have purchased distributor networks in strategic markets and may acquire other third party sales organizations in the future. While we have no specific acquisitions or planned licensing agreements currently ongoing, we may engage in these, or other strategic transactions, with the goal of augmenting our existing product portfolio and global footprint.
|
|
▪
|
Continue a high level of engagement with key opinion leaders.
We promote Motiva Implants, in part, via an extensive and robust calendar of physician education events led by key opinion leaders in the field of aesthetic surgery. In 2018, we conducted
51
events through our MotivaEDGE educational platform. We also collaborate actively with respected and influential key opinion leader surgeons to identify and develop new clinical applications for our existing products, as well as new product and strategic opportunities.
|
|
Product
|
Motiva Implants
|
Divina
|
Puregraft
|

|

|
|
|
|
Description
|
Soft silicone-gel filled breast implants with improved appearance, feel and safety
|
3D simulation device and proprietary tissue modeling software
|
Autologous graft of healthy, viable adipose (fat) cells for filling and contouring
|
|
Product Catalog
|
Available in more than 1,000 product variations, including four projection heights
|
For use with breast surgeries
|
Available in three graft volumes: 50cc, 250cc, and 850cc
|
|
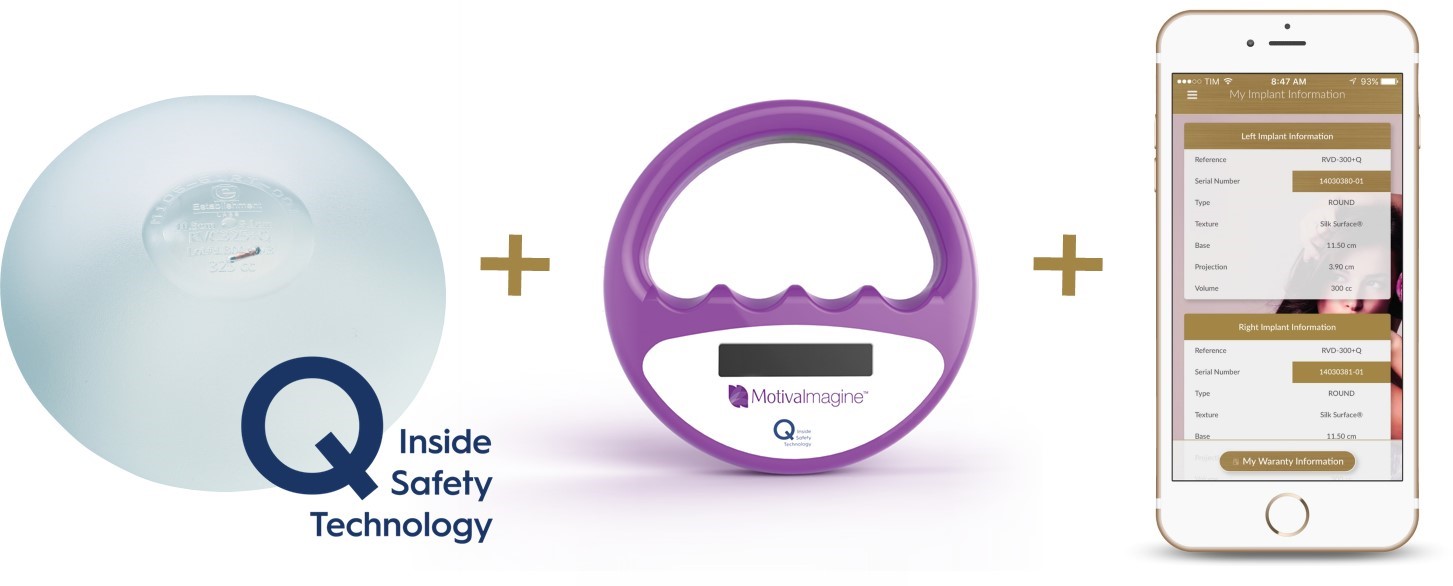
Key Features
|
▪
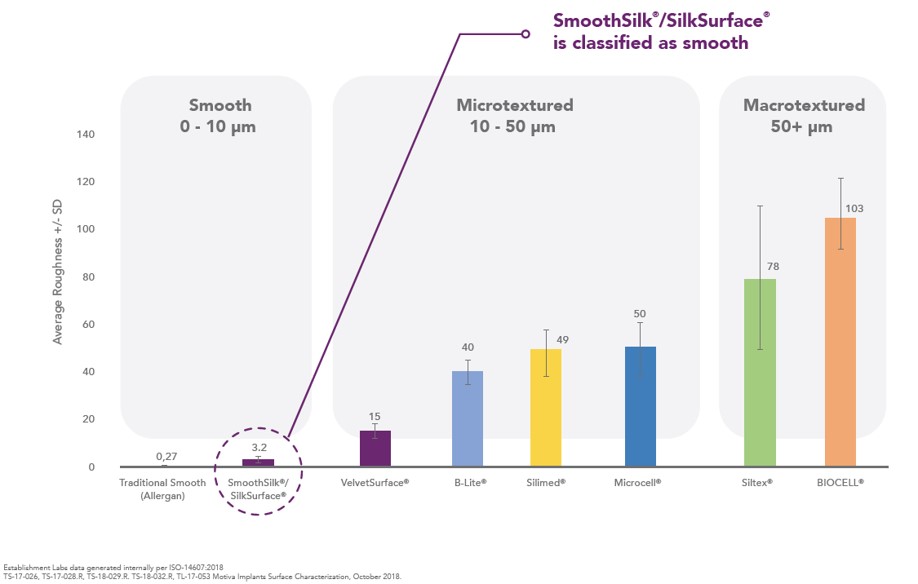
SilkSurface/SmoothSilk shell surface
▪
ProgressiveGel PLUS, ProgressiveGel Ultima, Silicone filling gels
▪
Ergonomix design
▪
TrueMonobloc construction
▪
QInside Safety Technology RFID microtransponder
▪
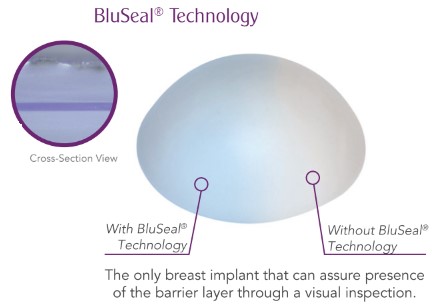
BluSeal shell barrier layer
|
▪
Pre-operative 3D planning that enables patients and physicians to visualize post-surgical result and measure pre-existing breast volume to optimize implant selection
▪
May increase clinical consultation efficiency
▪
MotivaHybrid: fat grafting can be used in conjunction with Motiva Implants by measuring pre-existing volume of the breast and calculating the appropriate ratio between silicone implant and fat graft
|
▪
Purifies adipose tissue through selective filtration technology
▪
Self-contained purification process preserves sterility
▪
MotivaHybrid: can be used in conjunction with Motiva Implants
▪
We are the exclusive distributor outside of the United States and Canada
|
|
Sales Territories
|
Over 60 countries outside the United States
|
||
|
|

|
▪
|
either our Divina or AX3 3D simulator, or a third party cloud-based visualization software that we sell in partnership with Crisalix systems;
|
|
▪
|
access to the full suite of MotivaImagine products that complement Motiva Implants;
|
|
▪
|
surgical staff trained by Establishment Labs in the optimal use of MotivaImagine products; and
|
|
▪
|
branding and design elements, according to company guidelines, that are intended to create a more luxurious and reassuring experience for patients.
|
|
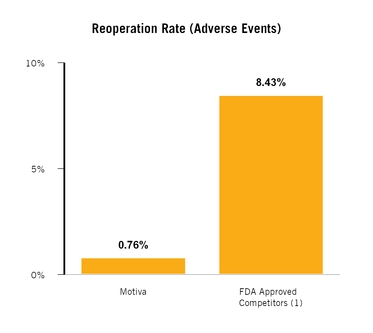
Motiva Implants
|
||
|
Number of Implants
|
N=650,078 Implants
(1)
|
|
|
Rupture
|
< 0.1%
|
|
|
Capsular Contracture
|
< 0.1%
|
|
|
Reoperation for Adverse Events
|
< 0.1%
|
|
|
Reoperation (All Causes)
|
N/A
(2)
|
|
|
(1) Data is internally tracked on an individual implant basis rather than by patient.
|
||
|
(2) Complaint database does not capture reoperations for reasons not related to safety.
|
||
|
▪
|
Massachusetts Institute of Technology
|
|
▪
|
Medical University of Innsbruck
|
|
▪
|
Plastic and Reconstructive Research Center at the University of Manchester
|
|
▪
|
Center for Biofilm Engineering of Montana State University
|
|
▪
|
The Chair of Plastic Surgery at the School of Medicine and Psychology of Sapienza University of Rome
|
|
▪
|
Microscopic Structure Research Center of the University of Costa Rica
|
|
▪
|
safety and outcomes data generated in clinical studies;
|
|
▪
|
regulatory approvals;
|
|
▪
|
technological characteristics of products;
|
|
▪
|
complementary platforms of non-implant products, such as facial fillers and fat grafting technologies;
|
|
▪
|
product price;
|
|
▪
|
customer service; and
|
|
▪
|
support by key opinion leaders.
|
|
▪
|
the submission of false claims or false information to government programs;
|
|
▪
|
deceptive or fraudulent conduct;
|
|
▪
|
excessive or unnecessary services or services at excessive prices; and
|
|
▪
|
prohibitions in defrauding private sector health insurers.
|
|
•
|
the outcomes of current and future clinical studies of Motiva Implants, including our ongoing PMA clinical trial, to demonstrate our products’ value in improving safety outcomes and/or patient satisfaction;
|
|
•
|
acceptance of Motiva Implants as safe and effective by patients, caregivers and the medical community;
|
|
•
|
an acceptable safety profile of Motiva Implants in the global market;
|
|
•
|
whether key thought leaders in the medical community accept that such clinical studies are sufficiently meaningful to influence their or their patients’ choices of product;
|
|
•
|
maintenance of our existing regulatory approvals and expansion of the geographies in which we have regulatory approvals;
|
|
•
|
commercially viable processes at a scale sufficient to meet anticipated demand at an adequate cost of manufacturing, and that are compliant with ISO 13485 Quality Management System requirements and/or good manufacturing practice, or GMP, requirements, as set forth in the FDA’s Quality System Regulation, Brazilian and other international regulations;
|
|
•
|
our success in educating physicians and patients about the benefits, administration and use of Motiva Implants, Motiva branded surgeries and value proposition of our MotivaImagine Centers;
|
|
•
|
the successful implementation of our MotivaImagine Centers with plastic surgery clinics;
|
|
•
|
the availability, perceived advantages, relative cost, relative safety and relative efficacy of alternative and competing treatments;
|
|
•
|
the willingness of patients to pay out-of-pocket for breast augmentation and reconstruction procedures in the absence of coverage and reimbursement for such procedures;
|
|
•
|
the success of our internal sales and marketing organization and the sales forces of our distributors; and
|
|
•
|
continued demand for breast augmentation and reconstruction procedures using silicone implants, which may be adversely affected by events involving either our products or those of our competitors, including FDA warnings to patients regarding Breast Implant-Associated Anaplasic Large Cell Lymphoma, or BIA-ALCL.
|
|
•
|
implement and execute our business strategy;
|
|
•
|
expand and improve the productivity of our direct sales force, distributors and marketing programs to grow sales of our existing and proposed products;
|
|
•
|
increase awareness of our brands and build loyalty among plastic surgeons and patients;
|
|
•
|
manage expanding operations;
|
|
•
|
respond effectively to competitive pressures and developments;
|
|
•
|
enhance our existing products and develop new products;
|
|
•
|
obtain regulatory clearance or approval to enhance our existing products and commercialize new products;
|
|
•
|
respond to changing regulations associated with medical devices across all geographies;
|
|
•
|
perform clinical trials with respect to our existing products and any new products;
|
|
•
|
attract, retain and motivate qualified personnel in various areas of our business; and
|
|
•
|
obtain and maintain coverage and adequate levels of reimbursement for our products.
|
|
•
|
clinical studies may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical studies or abandon product development programs;
|
|
•
|
the number of patients required for clinical studies may be larger than we anticipate, enrollment in these clinical studies may be insufficient or slower than we anticipate, or patients may drop out of these clinical studies at a higher rate than we anticipate;
|
|
•
|
the cost of clinical studies may be greater than we anticipate;
|
|
•
|
third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all;
|
|
•
|
we might suspend or terminate clinical studies of our planned products for various reasons, including a finding that our planned products have unanticipated serious side effects or other unexpected characteristics, or that the study subjects are being exposed to unacceptable health risks;
|
|
•
|
regulators may not approve our proposed clinical development plans;
|
|
•
|
regulators or independent institutional review boards, or IRBs, may not authorize us or our investigators to commence a clinical study or conduct a clinical study at a prospective study site;
|
|
•
|
regulators or IRBs may require that we, or our investigators, suspend or terminate clinical studies for various reasons, including noncompliance with regulatory requirements;
|
|
•
|
regulators in countries where Motiva Implants are currently marketed may require that we suspend commercial distribution if there is noncompliance with regulatory requirements or safety concerns;
|
|
•
|
regulators in countries where Motiva Implants are currently marketed may suspend commercial distribution of silicone breast implants due to safety or other concerns generally applicable to the product category;
|
|
•
|
the supply or quality of our planned products or other materials necessary to conduct clinical studies of our planned products may be insufficient or inadequate; and/or
|
|
•
|
the enactment of new regulatory requirements in Europe under the new Medical Device Regulation may make approval times longer and standards more difficult to pass.
|
|
•
|
be delayed in obtaining marketing approvals for Motiva Implants or our planned products;
|
|
•
|
not obtain marketing approval at all;
|
|
•
|
obtain approval for indications that are not as broad as intended;
|
|
•
|
have a product removed from the market after obtaining marketing approval;
|
|
•
|
be subject to additional post-marketing testing requirements; and/or
|
|
•
|
be subject to restrictions on how the product is distributed or used.
|
|
•
|
a product candidate may not be deemed to be safe and effective;
|
|
•
|
FDA officials may not find the data from clinical and preclinical studies sufficient;
|
|
•
|
the FDA may not approve our or our suppliers’ processes or facilities; or
|
|
•
|
the FDA may change its approval policies or adopt new regulations.
|
|
•
|
decreased demand for any planned products we may develop;
|
|
•
|
injury to our reputation and significant negative media attention;
|
|
•
|
withdrawal of patients from clinical studies or cancellation of studies;
|
|
•
|
significant costs to defend the related litigation and distraction to our management team;
|
|
•
|
substantial monetary awards to plaintiffs;
|
|
•
|
loss of revenue; and
|
|
•
|
the inability to commercialize any products that we may develop.
|
|
•
|
failure to complete sterilization on time or in compliance with the required regulatory standards;
|
|
•
|
transportation and import and export risk, particularly given the global nature of our supply and distribution chains;
|
|
•
|
delays in analytical results or failure of analytical techniques that we depend on for quality control and release of products;
|
|
•
|
natural or other disasters, labor disputes, financial distress, lack of raw material supply, issues with facilities and equipment or other forms of disruption to business operations affecting our manufacturer or its suppliers;
|
|
•
|
latent defects that may become apparent after products have been released and that may result in a recall of such products;
|
|
•
|
contamination of our raw materials or manufactured products; and
|
|
•
|
inclusion of vendors of raw materials not in compliance with ISO-13485 requirements.
|
|
•
|
compliance with the free zone regime regulations under which the manufacturing sites operate;
|
|
•
|
different regulatory requirements for device approvals in international markets;
|
|
•
|
multiple, conflicting and changing laws and regulations such as tariffs and tax laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits and licenses;
|
|
•
|
potential failure by us or our distributors to obtain and/or maintain regulatory approvals for the sale or use of our products in various countries;
|
|
•
|
difficulties in managing global operations;
|
|
•
|
logistics and regulations associated with shipping products, including infrastructure conditions and transportation delays;
|
|
•
|
limits on our ability to penetrate international markets if our distributors do not execute successfully;
|
|
•
|
governmental price controls, differing reimbursement regimes and other market regulations;
|
|
•
|
financial risks, such as longer payment cycles, difficulty enforcing contracts and collecting accounts receivable, and exposure to currency exchange rate fluctuations;
|
|
•
|
reduced protection for intellectual property rights, or lack of them in certain jurisdictions, forcing more reliance on our trade secrets, if available;
|
|
•
|
economic weakness, political and economic instability, including wars, terrorism and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions;
|
|
•
|
the March 2017 Article 50 notice of withdrawal that formally began the process of a British exit from the EU, including with respect to its effect on the value of the British pound relative to other currencies;
|
|
•
|
failure to comply with the Foreign Corrupt Practices Act, including its books and records provisions and its anti-bribery provisions, by maintaining accurate information and control over sales activities and distributors’ activities;
|
|
•
|
unexpected changes in tariffs, trade barriers and regulatory requirements;
|
|
•
|
compliance with tax, employment, immigration and labor laws;
|
|
•
|
taxes, including withholding of payroll taxes;
|
|
•
|
currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country;
|
|
•
|
workforce uncertainty in countries where labor unrest is more common than in the United States;
|
|
•
|
production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and
|
|
•
|
business and shipping interruptions resulting from natural or other disasters including earthquakes, volcanic activity, hurricanes, floods and fires.
|
|
•
|
managing our clinical trials effectively, which we anticipate being conducted at numerous clinical sites;
|
|
•
|
identifying, recruiting, maintaining, motivating and integrating additional employees with the expertise and experience we will require, in multiple countries;
|
|
•
|
managing our internal development efforts effectively while complying with our contractual obligations to licensors, licensees, contractors and other third parties;
|
|
•
|
managing additional relationships with various distributors, suppliers, and other third parties;
|
|
•
|
improving our managerial, development, operational and finance reporting systems and procedures; and
|
|
•
|
expanding our facilities.
|
|
•
|
failure to complete sterilization on time or in compliance with the required regulatory standards;
|
|
•
|
transportation and import and export risk, particularly given the global nature of our supply and distribution chains;
|
|
•
|
delays in analytical results or failure of analytical techniques that we depend on for quality control and release of products;
|
|
•
|
natural or other disasters, labor disputes, financial distress, lack of raw material supply, issues with facilities and equipment or other forms of disruption to business operations affecting our manufacturer or its suppliers;
|
|
•
|
latent defects that may become apparent after products have been released and that may result in a recall of such products;
|
|
•
|
contamination of our raw materials or manufactured products; and
|
|
•
|
inclusion of vendors of raw materials not in compliance with ISO-13485 requirements.
|
|
•
|
it may not be able, or willing, to manufacture silicone raw materials with our agreed-upon specifications;
|
|
•
|
it may not be able, or willing, to manufacture our needed raw materials in compliance with regulatory requirements, or our its manufacturing facilities may not be able to maintain compliance with regulatory requirements;
|
|
•
|
it may not be able to supply sufficient quantities of each raw material quickly enough for us to respond to rapid increases in demand;
|
|
•
|
it may unintentionally convey information to our competitors that is helpful in understanding our proprietary compositions and other trade secrets of our manufacturing processes;
|
|
•
|
we may be subject to price fluctuations if we fail to meet certain minimum order requirements, or if our existing contract expires or is renegotiated;
|
|
•
|
it may lose access to critical services and components, resulting in interruption in manufacture or shipment of medical-grade silicone;
|
|
•
|
its facilities may be affected by earthquakes, wild fires, mud slides or other natural disasters, which could delay or impede production of our raw materials;
|
|
•
|
we may be required to obtain regulatory approvals related to any change in our supply chain;
|
|
•
|
NuSil may wish to discontinue supply of products to us due to its existing relationships with our competitors;
|
|
•
|
NuSil may claim ownership of the intellectual property associated with our ProgressiveGel family of silicone gel rheologies; and
|
|
•
|
NuSil or its parent entity may encounter financial or other hardships unrelated to our demand for products, which could negatively impact their ability to fulfill our orders and support our regulatory approvals.
|
|
•
|
warning letters;
|
|
•
|
civil or criminal penalties and fines;
|
|
•
|
injunctions;
|
|
•
|
suspension or withdrawal of regulatory approval;
|
|
•
|
suspension of any ongoing clinical studies;
|
|
•
|
voluntary or mandatory product recalls and publicity requirements;
|
|
•
|
refusal to accept or approve applications for marketing approval of new devices or supplements to approved applications filed by us;
|
|
•
|
restrictions on operations, including costly new manufacturing requirements; or
|
|
•
|
seizure or detention of our products or import bans.
|
|
•
|
the federal Anti-Kickback Statute, which prohibits, among other things, any person from knowingly and willfully offering, soliciting, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal health care programs, such as the Medicare and Medicaid programs;
|
|
•
|
the federal physician self-referral law, commonly known as the Stark Law, which prohibits, among other things, physicians who have a financial relationship, including an investment, ownership or compensation relationship with an entity, from referring Medicare and Medicaid patients to that entity for designated health services, unless an exception applies. Similarly, entities may not bill Medicare, Medicaid or any other party for services furnished pursuant to a prohibited referral. Unlike the federal Anti-Kickback Statute, the Stark Law is a strict liability statute, meaning that all of the requirements of a Stark Law exception must be met in order to be compliant with the law;
|
|
•
|
the federal civil and criminal false claims and civil monetary penalties laws, including the federal False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, false claims, or knowingly using false statements, to obtain payment from the federal government;
|
|
•
|
HIPAA, which prohibits, executing a scheme to defraud any health care benefit program or making false statements relating to health care matters;
|
|
•
|
the federal transparency requirements under the PPACA which requires certain manufacturers of drugs, devices, biologics and medical supplies to annual report to the HHS information related to physician payments and other transfers of value made to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members;
|
|
•
|
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, which governs the conduct of certain electronic health care transactions and protects the security and privacy of protected health information;
|
|
•
|
state law equivalents of each of the above federal laws, such as anti-kickback, transparency and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers, as well as state post-marketing compliance laws; and
|
|
•
|
state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
|
|
•
|
our ability to successfully commercialize, and realize revenues from sales of, Motiva Implants, MotivaImagine Centers and Motiva branded surgeries;
|
|
▪
|
the success of competitive products or technologies;
|
|
▪
|
results of clinical studies of Motiva Implants or planned products or those of our competitors;
|
|
•
|
regulatory or legal developments in the United States and other countries, especially changes in laws or regulations applicable to our products;
|
|
•
|
introductions and announcements of new products by us, our commercialization partners, or our competitors, and the timing of these introductions or announcements;
|
|
•
|
actions taken by regulatory agencies with respect to our products, clinical studies, manufacturing processes or sales and marketing terms;
|
|
•
|
variations in our financial results or those of companies that are perceived to be similar to us;
|
|
•
|
the success of our efforts to acquire or in-license additional products or planned products;
|
|
•
|
developments concerning our collaborations, including but not limited to those with our sources of manufacturing supply and our commercialization partners;
|
|
•
|
developments concerning our ability to bring our manufacturing processes to scale in a cost-effective manner;
|
|
•
|
announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments;
|
|
•
|
developments or disputes concerning patents or other proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our products;
|
|
•
|
our ability or inability to raise additional capital and the terms on which we raise it;
|
|
•
|
the recruitment or departure of key personnel;
|
|
•
|
changes in the structure of health care payment systems;
|
|
•
|
negative shifts in the economy effecting the number of aesthetic breast procedures;
|
|
•
|
market conditions in the pharmaceutical and biotechnology sectors;
|
|
•
|
actual or anticipated changes in earnings estimates or changes in securities analyst recommendations regarding our common shares, other comparable companies or our industry generally;
|
|
•
|
trading volume of our common shares;
|
|
•
|
sales of our common shares by us or our shareholders;
|
|
•
|
general economic, industry and market conditions; and
|
|
•
|
the other risks described in this “Risk Factors” section.
|
|
•
|
our Board of Directors is divided into three classes with staggered three-year terms which may delay or prevent a change of our management or a change in control;
|
|
•
|
our Board of Directors has the right to elect directors to fill a vacancy created by the expansion of our Board of Directors or the resignation, death or removal of a director, which will prevent shareholders from being able to fill vacancies on our Board of Directors;
|
|
•
|
our shareholders are not be able to act by written consent, as a result, a holder, or holders, controlling a majority of our shares are not be able to take certain actions other than at annual shareholders’ meetings or special shareholders’ meetings;
|
|
•
|
our amended and restated memorandum and articles of association do not allow cumulative voting in the election of directors, which limits the ability of minority shareholders to elect director candidates;
|
|
•
|
amendments of our amended and restated memorandum and articles of association will require the approval of shareholders holding 66 2/3% of our outstanding voting shares (unless amended by the Board of Directors);
|
|
•
|
our shareholders are required to provide advance notice and additional disclosures in order to nominate individuals for election to our Board of Directors or to propose matters that can be acted upon at a shareholders’ meeting, which may discourage or deter a potential acquiror from conducting a solicitation of proxies to elect the acquiror’s own slate of directors or otherwise attempting to obtain control of our company; and
|
|
•
|
our Board of Directors is able to issue, without shareholder approval, preferred shares with voting or other rights or preferences that could impede the success of any attempt to acquire us.
|
|
•
|
Between February 2018 and June 2018, we issued an aggregate of $6.2 million of Class G ordinary shares at a purchase price of $16.00 per share to several investors;
|
|
•
|
In May 2018, we issued an aggregate $10.0 million Class G-1 ordinary shares at a purchase price of $16.00 per share to entities affiliated with RTW Investments;
|
|
•
|
In October 2018, we issued 5,000 common shares to Belle Health LTD as partial consideration in an asset acquisition; and
|
|
•
|
In December 2018, we issued 33,333 common shares to Femiline AB as contingent consideration for a milestone achieved related to our acquisition of certain assets from Femiline AB.
|
|
2018
|
2017
|
||||||
|
(in thousands)
|
|||||||
|
Revenue
|
$
|
61,208
|
|
$
|
34,681
|
|
|
|
Cost of revenue
|
25,090
|
|
16,979
|
|
|||
|
Gross profit
|
36,118
|
|
17,702
|
|
|||
|
Operating expenses:
|
|||||||
|
Sales, general and administrative
|
47,295
|
|
30,821
|
|
|||
|
Research and development
|
12,687
|
|
6,864
|
|
|||
|
Total operating expenses
|
59,982
|
|
37,685
|
|
|||
|
Loss from operations
|
(23,864
|
)
|
(19,983
|
)
|
|||
|
Interest expense
|
(8,814
|
)
|
(10,420
|
)
|
|||
|
Change in fair value of derivative instruments
|
15,894
|
|
(2,428
|
)
|
|||
|
Other income (expense), net
|
(4,099
|
)
|
(1,961
|
)
|
|||
|
Loss before income taxes
|
(20,883
|
)
|
(34,792
|
)
|
|||
|
Provision for income taxes
|
(215
|
)
|
(105
|
)
|
|||
|
Net loss
|
$
|
(21,098
|
)
|
$
|
(34,897
|
)
|
|
|
2018
|
2017
|
||||||
|
(in thousands)
|
|||||||
|
Revenue
|
$
|
61,208
|
|
$
|
34,681
|
|
|
|
Cost of revenue
|
25,090
|
|
16,979
|
|
|||
|
Gross profit
|
$
|
36,118
|
|
$
|
17,702
|
|
|
|
Gross margin
|
59.0
|
%
|
51.0
|
%
|
|||
|
2018
|
2017
|
||||||
|
(in thousands)
|
|||||||
|
Operating expenses:
|
|||||||
|
Sales, general and administrative
|
$
|
47,295
|
|
$
|
30,821
|
|
|
|
Research and development
|
12,687
|
|
6,864
|
|
|||
|
Total operating expenses
|
$
|
59,982
|
|
$
|
37,685
|
|
|
|
▪
|
the degree and rate of market adoption of our products;
|
|
▪
|
the cost and timing of our regulatory activities, especially the PMA clinical trial to obtain regulatory approval for our Motiva Implants in the United States;
|
|
▪
|
the emergence of new competing technologies and products;
|
|
▪
|
the costs of R&D activities we undertake to develop and expand our products;
|
|
▪
|
the costs of commercialization activities, including sales, marketing and manufacturing;
|
|
▪
|
the level of working capital required to support our growth; and
|
|
▪
|
our need for additional personnel, information technology or other operating infrastructure to support our growth and operations as a public company.
|
|
2018
|
2017
|
||||||
|
Net cash provided by (used in):
|
|||||||
|
Operating activities
|
$
|
(33,885
|
)
|
$
|
(31,970
|
)
|
|
|
Investing activities
|
(5,731
|
)
|
(845
|
)
|
|||
|
Financing activities
|
81,527
|
|
42,993
|
|
|||
|
Effect of exchange rate changes on cash
|
(136
|
)
|
207
|
|
|||
|
Net increase in cash
|
$
|
41,775
|
|
$
|
10,385
|
|
|
|
▪
|
Fair Value of Common Shares
. As the ordinary shares are not publicly traded, we must estimate its fair value, as discussed under Common Share Valuations below. The fair value of ordinary shares was determined on a periodic basis by our board of directors, with the assistance of an independent third-party valuation firm. The board of directors intended all options granted to be exercisable at a price per share not less than the estimated per share fair value of the shares underlying those options on the date of grant.
|
|
▪
|
Risk-Free Interest Rate
. We base the risk-free interest rate used in the Black-Scholes valuation model on the implied yield available on U.S. Treasury zero-coupon issues with a term equivalent to that of the expected term of the options for each option group.
|
|
▪
|
Expected Term
. The expected term represents the period that our share-based awards are expected to be outstanding. Because of the limitations on the sale or transfer of our shares as a privately held company, we do not believe our historical exercise pattern is indicative of the pattern it will experience as a publicly traded company. We have consequently used the Staff Accounting Bulletin 110, or SAB 110, simplified method to calculate the expected term, which is the average of the contractual term and vesting period. We plan to continue to use the SAB 110 simplified method until it has sufficient trading history as a publicly traded company.
|
|
▪
|
Volatility
. We determine the price volatility based on the historical volatilities of industry peers as it has no trading history for its stock. Industry peers consist of several public companies in the medical device industry with comparable characteristics, including revenue growth, operating model and working capital requirements. We intend to continue to consistently apply this process using the same or a similar peer group of public companies until a sufficient amount of historical information regarding the volatility of our shares becomes available, or unless circumstances change such that the identified peer companies are
|
|
▪
|
Dividend Yield
. The expected dividend assumption is based on our current expectations about its anticipated dividend policy. We have no expectation t
hat we will declare dividends on its ordinary shares, and therefore have used an expected dividend yield of zero.
|
|
Exhibit Number
|
Description of Exhibit
|
Incorporation by Reference
|
||
|
1.1
|
Incorporated by reference from Registrant’s Form S-1/A filed July 13, 2018.
|
|||
|
3.1
|
Incorporated by reference from Registrant’s Form S-1 filed June 21, 2018.
|
|||
|
3.2
|
Incorporated by reference from Registrant’s Form S-1/A filed July 9, 2018.
|
|||
|
3.3
|
Incorporated by reference from Registrant’s Form S-1 filed June 21, 2018.
|
|||
|
4.1
|
Incorporated by reference from Registrant’s Form S-1/A filed July 13, 2018.
|
|||
|
4.2
|
Incorporated by reference from Registrant’s Form S-1 filed June 21, 2018.
|
|||
|
4.3
|
Incorporated by reference from Registrant’s Form S-1 filed June 21, 2018.
|
|||
|
4.4
|
Incorporated by reference from Registrant’s Form S-1 filed June 21, 2018.
|
|||
|
4.5
|
Incorporated by reference from Registrant’s Form S-1 filed June 21, 2018.
|
|||
|
4.6
|
Incorporated by reference from Registrant’s Form S-1 filed June 21, 2018.
|
|||
|
4.7
|
Incorporated by reference from Registrant’s Form S-1 filed June 21, 2018.
|
|||
|
10.1
|
Incorporated by reference from Registrant’s Form S-1 filed June 21, 2018.
|
|||
|
Exhibit Number
|
Description of Exhibit
|
Incorporation by Reference
|
||
|
10.2+
|
Incorporated by reference from Registrant’s Form S-1 filed June 21, 2018.
|
|||
|
10.3+
|
Incorporated by reference from Registrant’s Form S-1/A filed July 9, 2018.
|
|||
|
10.4+
|
Incorporated by reference from Registrant’s Form S-1/A filed July 9, 2018.
|
|||
|
10.5+
|
Incorporated by reference from Registrant’s Form S-1 filed June 21, 2018.
|
|||
|
10.6+
|
Incorporated by reference from Registrant’s Form S-1/A filed July 13, 2018.
|
|||
|
10.7+
|
Incorporated by reference from Registrant’s Form S-1/A filed July 13, 2018.
|
|||
|
10.8+
|
Incorporated by reference from Registrant’s Form S-1 filed June 21, 2018.
|
|||
|
10.9
|
Incorporated by reference from Registrant’s Form S-1 filed June 21, 2018.
|
|||
|
10.10
‡
|
Incorporated by reference from Registrant’s Form S-1/A filed July 13, 2018.
|
|||
|
10.11
|
Incorporated by reference from Registrant’s Form S-1 filed June 21, 2018.
|
|||
|
10.12‡
|
Incorporated by reference from Registrant’s Form S-1/A filed July 13, 2018.
|
|||
|
10.13
‡
|
Incorporated by reference from Registrant’s Form S-1/A filed July 13, 2018.
|
|||
|
10.14
|
Incorporated by reference from Registrant’s Form S-1/A filed July 13, 2018.
|
|||
|
10.15
|
Incorporated by reference from Registrant’s Form S-1/A filed July 13, 2018.
|
|||
|
10.16
|
Incorporated by reference from Registrant’s Form S-1/A filed July 13, 2018.
|
|||
|
10.17
|
Incorporated by reference from Registrant’s Form S-1 filed June 21, 2018.
|
|||
|
10.18
|
Incorporated by reference from Registrant’s Form S-1 filed June 21, 2018.
|
|||
|
10.19
‡
|
Incorporated by reference from Registrant’s Form S-1/A filed July 13, 2018.
|
|||
|
10.20
‡
|
Incorporated by reference from Registrant’s Form S-1 filed July 13, 2018.
|
|||
|
10.21+
|
Incorporated by reference from Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2018.
|
|||
|
10.22
|
Incorporated by reference from Registrant’s Current Report on Form 8-K filed October 10, 2018.
|
|||
|
Exhibit Number
|
Description of Exhibit
|
Incorporation by Reference
|
||
|
10.23
|
Incorporated by reference from Registrant’s Current Report on Form 8-K filed October 10, 2018.
|
|||
|
10.24
|
Incorporated by reference from Registrant’s Current Report on Form 8-K filed October 10, 2018.
|
|||
|
10.25+
|
Incorporated by reference from Registrant’s Current Report on Form 8-K filed December 28, 2018.
|
|||
|
10.26+
|
Incorporated by reference from Registrant’s Current Report on Form 8-K filed December 28, 2018.
|
|||
|
10.27+
|
Incorporated by reference from Registrant’s Current Report on Form 8-K filed December 28, 2018.
|
|||
|
10.28+
|
Incorporated by reference from Registrant’s Current Report on Form 8-K filed December 28, 2018.
|
|||
|
21.1
|
Filed herewith.
|
|||
|
23.1
|
Filed herewith.
|
|||
|
31.1*
|
Filed herewith.
|
|||
|
31.2*
|
Filed herewith.
|
|||
|
32.1*
|
Filed herewith.
|
|||
|
101.INS
|
XBRL Instance Document
|
|||
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
|||
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|||
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|||
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document
|
|||
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|||
|
+
|
Indicates management contract or compensatory plan or arrangement.
|
|
‡
|
Portions omitted, or to be omitted, pursuant to a request for confidential treatment.
|
|
PAGE
|
|
|
Consolidated Financial Statements
|
|
|
Consolidated Statements of Shareholders’ Equity (Deficit)
|
|
|
December 31,
|
|||||||
|
2018
|
2017
|
||||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash
|
$
|
52,639
|
|
$
|
10,864
|
|
|
|
Accounts receivable, net of allowance for doubtful accounts of $926 and $1,512
|
17,648
|
|
13,108
|
|
|||
|
Inventory, net
|
24,845
|
|
13,173
|
|
|||
|
Prepaid expenses and other current assets
|
4,303
|
|
2,237
|
|
|||
|
Total current assets
|
99,435
|
|
39,382
|
|
|||
|
Long-term assets:
|
|||||||
|
Property and equipment, net of accumulated depreciation of $5,230 and $3,179
|
12,913
|
|
13,500
|
|
|||
|
Goodwill
|
465
|
|
465
|
|
|||
|
Intangible assets, net of accumulated amortization of $1,213 and $573
|
3,445
|
|
3,401
|
|
|||
|
Restricted cash
|
—
|
|
75
|
|
|||
|
Other non-current assets
|
315
|
|
272
|
|
|||
|
Total assets
|
$
|
116,573
|
|
$
|
57,095
|
|
|
|
Liabilities and shareholders’ equity (deficit)
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
6,239
|
|
$
|
9,131
|
|
|
|
Accrued liabilities
|
6,125
|
|
2,326
|
|
|||
|
Notes payable related party, including accrued interest
|
—
|
|
4,921
|
|
|||
|
Note payable, Madryn, net of debt discount and issuance costs
|
—
|
|
19,167
|
|
|||
|
Madryn put option
|
—
|
|
20,302
|
|
|||
|
Madryn call option
|
—
|
|
360
|
|
|||
|
Other liabilities, short term
|
4,083
|
|
1,228
|
|
|||
|
Total current liabilities
|
16,447
|
|
57,435
|
|
|||
|
Long-term liabilities:
|
|||||||
|
Note payable, Madryn, net of debt discount and issuance costs
|
22,322
|
|
—
|
|
|||
|
Madryn put option
|
4,768
|
|
—
|
|
|||
|
Other liabilities, long term
|
3,551
|
|
4,673
|
|
|||
|
Total liabilities
|
47,088
|
|
62,108
|
|
|||
|
Commitments and contingencies (Note 7)
|
|
|
|||||
|
Shareholders’ equity (deficit):
|
|||||||
|
Ordinary shares - $1.00 par value (class A and B), zero and 21,206,630 shares authorized at December 31, 2018 and 2017, respectively; zero and 13,427,536 shares issued at December 31, 2018 and 2017, respectively; zero and 12,206,326 shares outstanding at December 31, 2018 and 2017, respectively
|
—
|
|
13,427
|
|
|||
|
Ordinary shares - no par value (class C, D, E, F, G and G-1), zero and 2,316,169 shares authorized at December 31, 2018 and 2017, respectively; zero and 2,316,169 shares issued and outstanding at December 31, 2018 and 2017, respectively
|
—
|
|
27,840
|
|
|||
|
Common shares - zero and $1.00 par value as of December 31, 2018 and 2017, respectively, unlimited amount and 84,050,000 shares authorized at December 31, 2018 and 2017, respectively; 20,672,025 and zero shares issued at December 31, 2018 and 2017, respectively; 20,263,955 and zero shares outstanding at December 31, 2018 and 2017, respectively
|
145,709
|
|
—
|
|
|||
|
Additional paid-in-capital
|
15,156
|
|
27,986
|
|
|||
|
Treasury shares, at cost, 408,070 and 1,221,210 shares held at December 31, 2018 and 2017, respectively
|
(2,854
|
)
|
(6,465
|
)
|
|||
|
Accumulated deficit
|
(88,975
|
)
|
(67,877
|
)
|
|||
|
Accumulated other comprehensive income
|
449
|
|
76
|
|
|||
|
Total shareholders’ equity (deficit)
|
69,485
|
|
(5,013
|
)
|
|||
|
Total liabilities and shareholders’ equity (deficit)
|
$
|
116,573
|
|
$
|
57,095
|
|
|
|
Year Ended December 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
Revenue
|
$
|
61,208
|
|
$
|
34,681
|
|
||
|
Cost of revenue
|
25,090
|
|
16,979
|
|
||||
|
Gross profit
|
36,118
|
|
17,702
|
|
||||
|
Operating expenses:
|
||||||||
|
Sales, general and administrative
|
47,295
|
|
30,821
|
|
||||
|
Research and development
|
12,687
|
|
6,864
|
|
||||
|
Total operating expenses
|
59,982
|
|
37,685
|
|
||||
|
Loss from operations
|
(23,864
|
)
|
(19,983
|
)
|
||||
|
Interest income
|
16
|
|
19
|
|
||||
|
Interest expense
|
(8,814
|
)
|
(10,420
|
)
|
||||
|
Change in fair value of derivative instruments
|
15,894
|
|
(2,428
|
)
|
||||
|
Change in fair value of contingent consideration
|
(1,727
|
)
|
—
|
|
||||
|
Initial public offering expenses
|
—
|
|
(1,585
|
)
|
||||
|
Other income (expense), net
|
(2,388
|
)
|
(395
|
)
|
||||
|
Loss before income taxes
|
(20,883
|
)
|
(34,792
|
)
|
||||
|
Benefit (provision) for income taxes
|
(215
|
)
|
(105
|
)
|
||||
|
Net loss
|
$
|
(21,098
|
)
|
$
|
(34,897
|
)
|
||
|
Basic and diluted loss per share
|
$
|
(1.22
|
)
|
$
|
(3.41
|
)
|
||
|
Weighted average outstanding shares used for basic and diluted net loss per share
|
17,350,705
|
|
10,230,586
|
|
||||
|
Year Ended December 31,
|
|||||||
|
2018
|
2017
|
||||||
|
Net loss
|
$
|
(21,098
|
)
|
$
|
(34,897
|
)
|
|
|
Other comprehensive income:
|
|||||||
|
Foreign currency translation gain
|
373
|
|
76
|
|
|||
|
Other comprehensive gain
|
373
|
|
76
|
|
|||
|
Comprehensive loss
|
$
|
(20,725
|
)
|
$
|
(34,821
|
)
|
|
|
Common Shares
|
Ordinary Shares
|
Treasury Shares
|
Additional
Paid-In Capital |
Accumulated
Deficit |
Accumulated Other Comprehensive Income
|
Total
|
|||||||||||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
||||||||||||||||||||||||||||||||||||||||||
|
Balance at January 1, 2017
|
—
|
|
$
|
—
|
|
7,118,753
|
|
$
|
7,118
|
|
(813,140
|
)
|
$
|
(3,611
|
)
|
$
|
3,038
|
|
$
|
(32,980
|
)
|
$
|
—
|
|
$
|
(26,435
|
)
|
||||||||||||||||||||
|
Issuance of ordinary shares
|
—
|
|
—
|
|
2,316,169
|
|
27,840
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
$
|
27,840
|
|
||||||||||||||||||||||||||
|
Cumulative change in accounting principle (adoption of ASU 2017-11)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
958
|
|
—
|
|
—
|
|
$
|
958
|
|
||||||||||||||||||||||||||
|
Extinguishment of warrant with related party
|
—
|
|
—
|
|
207,716
|
|
208
|
|
—
|
|
—
|
|
2,192
|
|
—
|
|
—
|
|
$
|
2,400
|
|
||||||||||||||||||||||||||
|
Conversion of related party convertible notes payable
|
—
|
|
—
|
|
5,869,417
|
|
5,869
|
|
—
|
|
—
|
|
18,383
|
|
—
|
|
—
|
|
$
|
24,252
|
|
||||||||||||||||||||||||||
|
Issuance of shares in a business combination
|
—
|
|
—
|
|
35,714
|
|
36
|
|
—
|
|
—
|
|
308
|
|
—
|
|
—
|
|
$
|
344
|
|
||||||||||||||||||||||||||
|
Repurchase of ordinary shares
|
—
|
|
—
|
|
—
|
|
—
|
|
(408,070
|
)
|
(2,854
|
)
|
—
|
|
—
|
|
—
|
|
$
|
(2,854
|
)
|
||||||||||||||||||||||||||
|
Share-based compensation
|
—
|
|
—
|
|
195,936
|
|
196
|
|
—
|
|
—
|
|
3,107
|
|
—
|
|
—
|
|
$
|
3,303
|
|
||||||||||||||||||||||||||
|
Foreign currency translation gain
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
76
|
|
$
|
76
|
|
||||||||||||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(34,897
|
)
|
—
|
|
$
|
(34,897
|
)
|
||||||||||||||||||||||||||
|
Balance at December 31, 2017
|
—
|
|
$
|
—
|
|
15,743,705
|
|
$
|
41,267
|
|
(1,221,210
|
)
|
$
|
(6,465
|
)
|
$
|
27,986
|
|
$
|
(67,877
|
)
|
$
|
76
|
|
$
|
(5,013
|
)
|
||||||||||||||||||||
|
Issuance of ordinary shares
|
—
|
|
—
|
|
—
|
|
—
|
|
1,011,174
|
|
—
|
|
16,180
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(78
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
16,102
|
|
|||||||||
|
Issuance of common shares, IPO
|
4,272,568
|
|
70,055
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
70,055
|
|
|||||||||||||||||||||||||||
|
Issuance of common shares in a business combination
|
33,333
|
|
862
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
862
|
|
|||||||||||||||||||||||||||
|
Conversion of ordinary shares to common shares
|
16,215,710
|
|
74,256
|
|
(16,215,710
|
)
|
(56,908
|
)
|
—
|
|
—
|
|
(17,348
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||||||||||||
|
Issuance of shares in an asset acquisition
|
5,000
|
|
120
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
120
|
|
|||||||||||||||||||||||||||
|
Warrant exercises
|
38,785
|
|
128
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(7
|
)
|
—
|
|
—
|
|
121
|
|
|||||||||||||||||||||||||||
|
Stock option exercises
|
25,843
|
|
207
|
|
106,248
|
|
106
|
|
—
|
|
—
|
|
330
|
|
—
|
|
—
|
|
643
|
|
|||||||||||||||||||||||||||
|
Share-based compensation
|
80,786
|
|
81
|
|
167,723
|
|
168
|
|
—
|
|
—
|
|
7,071
|
|
—
|
|
—
|
|
7,320
|
|
|||||||||||||||||||||||||||
|
Retirement of treasury shares
|
—
|
|
—
|
|
(813,140
|
)
|
(813
|
)
|
813,140
|
|
3,611
|
|
(2,798
|
)
|
—
|
|
—
|
|
—
|
|
|||||||||||||||||||||||||||
|
Foreign currency translation gain (loss)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
373
|
|
373
|
|
|||||||||||||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(21,098
|
)
|
—
|
|
(21,098
|
)
|
|||||||||||||||||||||||||||
|
Balance at December 31, 2018
|
20,672,025
|
|
$
|
145,709
|
|
—
|
|
$
|
—
|
|
$
|
(408,070
|
)
|
$
|
(2,854
|
)
|
$
|
15,156
|
|
$
|
(88,975
|
)
|
$
|
449
|
|
$
|
69,485
|
|
|||||||||||||||||||
|
Year Ended December 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
Cash flows from operating activities:
|
||||||||
|
Net loss
|
$
|
(21,098
|
)
|
$
|
(34,897
|
)
|
||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||
|
Depreciation and amortization
|
2,810
|
|
1,939
|
|
||||
|
(Bad debt recovery) provision for doubtful accounts
|
(547
|
)
|
943
|
|
||||
|
Provision for inventory obsolescence
|
204
|
|
—
|
|
||||
|
Share-based compensation
|
7,320
|
|
3,303
|
|
||||
|
Loss from disposal of property and equipment
|
79
|
|
—
|
|
||||
|
Write off of deferred offering costs
|
—
|
|
1,585
|
|
||||
|
Unrealized foreign currency (gain) loss, net
|
2,217
|
|
—
|
|
||||
|
Change in fair value of derivative instruments
|
(15,894
|
)
|
2,428
|
|
||||
|
Change in fair value of contingent consideration
|
1,727
|
|
—
|
|
||||
|
Conversion of accrued interest into principal and amortization of debt discount
|
3,317
|
|
7,054
|
|
||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Accounts receivable
|
(4,667
|
)
|
(6,911
|
)
|
||||
|
Inventory
|
(6,984
|
)
|
(6,437
|
)
|
||||
|
Prepaid expenses and other current assets
|
(2,192
|
)
|
(1,681
|
)
|
||||
|
Other assets
|
(45
|
)
|
(570
|
)
|
||||
|
Accounts payable
|
(3,905
|
)
|
(878
|
)
|
||||
|
Accrued liabilities
|
3,801
|
|
1,103
|
|
||||
|
Other liabilities
|
(28
|
)
|
1,049
|
|
||||
|
Net cash used in operating activities
|
(33,885
|
)
|
(31,970
|
)
|
||||
|
Cash flows from investing activities:
|
||||||||
|
Purchases of property and equipment
|
(1,731
|
)
|
(901
|
)
|
||||
|
Cash used in asset acquisitions
|
(3,959
|
)
|
—
|
|
||||
|
Cost incurred for intangible assets
|
(41
|
)
|
(40
|
)
|
||||
|
Increase (decrease) in restricted cash
|
—
|
|
96
|
|
||||
|
Net cash used in investing activities
|
(5,731
|
)
|
(845
|
)
|
||||
|
Cash flows from financing activities:
|
||||||||
|
Borrowings on short-term notes payable
|
—
|
|
1,000
|
|
||||
|
Borrowings under Madryn credit agreement, net of issuance costs
|
—
|
|
38,465
|
|
||||
|
Repayments on short term notes payable
|
—
|
|
(1,200
|
)
|
||||
|
Repayments on related-party notes payable
|
(5,083
|
)
|
—
|
|
||||
|
Repayments under Perceptive credit agreement
|
—
|
|
(15,000
|
)
|
||||
|
Payments of deferred offering costs
|
—
|
|
(914
|
)
|
||||
|
Repayments on capital leases
|
(311
|
)
|
(291
|
)
|
||||
|
Proceeds from issuance of ordinary shares, net of issuance costs
|
16,102
|
|
27,840
|
|
||||
|
Proceeds from issuance of common shares, net of underwriters’ discount
|
71,523
|
|
—
|
|
||||
|
Deferred equity issuance costs, IPO
|
(1,468
|
)
|
—
|
|
||||
|
Cash used to repurchase warrants
|
—
|
|
(2,400
|
)
|
||||
|
Proceeds from stock option exercises
|
643
|
|
—
|
|
||||
|
Proceeds from warrant exercises
|
121
|
|
—
|
|
||||
|
Shares repurchased
|
—
|
|
(4,507
|
)
|
||||
|
Net cash provided by financing activities
|
81,527
|
|
42,993
|
|
||||
|
Effect of exchange rate changes on cash
|
(136
|
)
|
207
|
|
||||
|
Net increase in cash
|
41,775
|
|
10,385
|
|
||||
|
Cash at beginning of period
|
10,864
|
|
479
|
|
||||
|
Cash at end of period
|
$
|
52,639
|
|
$
|
10,864
|
|
||
|
Year Ended December 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
Supplemental disclosures:
|
||||||||
|
Cash paid for interest
|
$
|
5,379
|
|
$
|
2,862
|
|
||
|
Cash paid for income taxes
|
$
|
136
|
|
$
|
147
|
|
||
|
Supplemental disclosures of non-cash investing and financing activities:
|
||||||||
|
Unpaid balance for property and equipment
|
$
|
717
|
|
$
|
783
|
|
||
|
Assets acquired under capital leases
|
$
|
94
|
|
$
|
209
|
|
||
|
Extinguishment of warrants with related party
|
$
|
—
|
|
$
|
958
|
|
||
|
Equity consideration in an asset acquisition
|
$
|
120
|
|
$
|
—
|
|
||
|
Consideration payable related to asset acquisitions
|
$
|
1,276
|
|
$
|
—
|
|
||
|
Conversion of related party convertible notes payable into ordinary shares
|
$
|
—
|
|
$
|
24,252
|
|
||
|
Liability to issues shares in business acquisition
|
$
|
—
|
|
$
|
964
|
|
||
|
Consideration payable related to business acquisition
|
$
|
—
|
|
$
|
1,704
|
|
||
|
Goodwill recorded in business combination
|
$
|
—
|
|
$
|
210
|
|
||
|
Property, equipment and prepaids acquired in business acquisition
|
$
|
—
|
|
$
|
1,498
|
|
||
|
Intangible assets acquired in business acquisition
|
$
|
—
|
|
$
|
1,304
|
|
||
|
Issuance of common shares in partial settlement of contingent consideration
|
$
|
863
|
|
$
|
—
|
|
||
|
Transfer from restricted cash to prepaid expenses and other current assets
|
$
|
75
|
|
$
|
—
|
|
||
|
•
|
the Company amended and restated its Memorandum of Association and Articles of Association, or the Articles, to automatically convert all outstanding classes of the Company’s stock into common shares of a single class of no par value. An unlimited number of common shares was authorized.
|
|
•
|
The Board of Directors determined no further awards would be issued from the 2015 Equity Plan and approved the 2018 Equity Incentive Plan, or the 2018 Plan, with an initial reserve of
1,500,000
of the Company’s common shares for issuance;
|
|
•
|
The Board of Directors adopted the 2018 Employee Share Purchase Plan, or the ESPP, with an initial reserve of
100,000
of the Company’s common shares for issuance.
|
|
Subsidiary
|
Incorporation/Acquisition Date
|
|
Establishment Labs, S.A. (Costa Rica)
|
January 18, 2004
|
|
Motiva USA, LLC (USA)
|
February 20, 2014
|
|
JAMM Technologies, Inc. (USA)
|
October 27, 2015
|
|
Establishment Labs Produtos par Saude Ltda (Brazil)
|
January 4, 2016
|
|
European Distribution Center Motiva BVBA (Belgium)
|
March 4, 2016
|
|
Motiva Implants France SAS (France)
|
September 12, 2016
|
|
JEN-Vault AG (Switzerland)
|
November 22, 2016
|
|
Motiva Nordica AB (Sweden)
|
November 2, 2017
|
|
Motiva Implants UK Limited
|
July 31, 2018
|
|
Motiva Italy S.R.L
|
July 31, 2018
|
|
December 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
(in thousands)
|
||||||||
|
Raw materials
|
$
|
3,349
|
|
$
|
1,978
|
|
||
|
Work in process
|
1,244
|
|
1,132
|
|
||||
|
Finished goods
|
20,252
|
|
10,063
|
|
||||
|
$
|
24,845
|
|
$
|
13,173
|
|
|||
|
December 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
(in thousands)
|
||||||||
|
Machinery and equipment
|
$
|
7,145
|
|
$
|
5,473
|
|
||
|
Vehicles
|
400
|
|
353
|
|
||||
|
Furniture and fixtures
|
2,536
|
|
2,110
|
|
||||
|
Leasehold improvements
|
8,062
|
|
8,743
|
|
||||
|
Total
|
18,143
|
|
16,679
|
|
||||
|
Less: Accumulated depreciation and amortization
|
(5,230
|
)
|
(3,179
|
)
|
||||
|
$
|
12,913
|
|
$
|
13,500
|
|
|||
|
December 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
(in thousands)
|
||||||||
|
Bonus
|
$
|
3,058
|
|
$
|
748
|
|
||
|
Payroll and related expenses
|
1,300
|
|
798
|
|
||||
|
Commissions
|
487
|
|
210
|
|
||||
|
Other
|
1,280
|
|
570
|
|
||||
|
$
|
6,125
|
|
$
|
2,326
|
|
|||
|
December 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
(in thousands)
|
||||||||
|
Repurchase of warrants
|
$
|
2,261
|
|
$
|
—
|
|
||
|
Contingent equity consideration (see Note 11)
|
914
|
|
321
|
|
||||
|
Deferred revenue
|
908
|
|
907
|
|
||||
|
$
|
4,083
|
|
$
|
1,228
|
|
|||
|
December 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
(in thousands)
|
||||||||
|
Repurchase of warrants
|
$
|
—
|
|
$
|
2,261
|
|
||
|
Contingent equity consideration (see Note 11)
|
914
|
|
643
|
|
||||
|
Deferred revenue
|
1,186
|
|
927
|
|
||||
|
Cash payable for asset acquisitions (see Note 11)
|
883
|
|
—
|
|
||||
|
Other
|
568
|
|
842
|
|
||||
|
$
|
3,551
|
|
$
|
4,673
|
|
|||
|
Balance as of January 1, 2018
|
Additions
|
Accumulated Impairment Losses
|
Balance as of December 31, 2018
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Goodwill
|
$
|
465
|
|
$
|
—
|
|
$
|
—
|
|
$
|
465
|
|
|||
|
Gross Carrying Amount
|
Accumulated Amortization
|
Net Carrying Amount
|
Estimated Useful Lives
|
||||||||||
|
(in thousands)
|
(in years)
|
||||||||||||
|
Patents and license rights
|
$
|
1,669
|
|
$
|
(564
|
)
|
$
|
1,105
|
|
7-12
|
|||
|
Customer relationships
|
1,896
|
|
(381
|
)
|
1,515
|
|
4-10
|
||||||
|
510(k) authorization
|
567
|
|
(118
|
)
|
449
|
|
15
|
||||||
|
Developed technology
|
62
|
|
(34
|
)
|
28
|
|
10
|
||||||
|
Capitalized software development costs
|
98
|
|
(98
|
)
|
—
|
|
2
|
||||||
|
Other
|
75
|
|
(18
|
)
|
57
|
|
2-5
|
||||||
|
Capitalized patents and license rights not yet amortized
|
291
|
|
—
|
|
291
|
|
|||||||
|
$
|
4,658
|
|
$
|
(1,213
|
)
|
$
|
3,445
|
|
|||||
|
Gross Carrying Amount
|
Accumulated Amortization
|
Net Carrying Amount
|
Estimated Useful Lives
|
||||||||||
|
(in thousands)
|
(in years)
|
||||||||||||
|
Patents and license rights
|
$
|
1,635
|
|
$
|
(361
|
)
|
$
|
1,274
|
|
7-12
|
|||
|
Customer relationships
|
1,304
|
|
(40
|
)
|
1,264
|
|
10
|
||||||
|
510(k) authorization
|
567
|
|
(80
|
)
|
487
|
|
15
|
||||||
|
Developed technology
|
62
|
|
(28
|
)
|
34
|
|
10
|
||||||
|
Capitalized software development costs
|
98
|
|
(49
|
)
|
49
|
|
2
|
||||||
|
Other
|
17
|
|
(15
|
)
|
2
|
|
2-3
|
||||||
|
Capitalized patents and license rights not yet amortized
|
291
|
|
—
|
|
291
|
|
|||||||
|
$
|
3,974
|
|
$
|
(573
|
)
|
$
|
3,401
|
|
|||||
|
Year Ending December 31,
|
(in thousands)
|
|||
|
2019
|
$
|
729
|
|
|
|
2020
|
714
|
|
||
|
2021
|
674
|
|
||
|
2022
|
345
|
|
||
|
2023
|
109
|
|
||
|
Thereafter
|
583
|
|
||
|
Total
|
$
|
3,154
|
|
|
|
▪
|
Level I Unadjusted quoted prices in active markets for identical assets or liabilities;
|
|
▪
|
Level II Inputs other than quoted prices included within Level I that are observable, unadjusted quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the related assets or liabilities; and
|
|
▪
|
Level III Uno
bservable inputs that are supported by little or no market activity for the related assets or liabilities.
|
|
|
Fair Value Measurements at December 31, 2018
|
||||||||||||||
|
|
Total
|
Level 1
|
Level 2
|
Level 3
|
|||||||||||
|
(in thousands)
|
|||||||||||||||
|
Liabilities
|
|||||||||||||||
|
Madryn put option liability
|
$
|
4,768
|
|
$
|
—
|
|
$
|
—
|
|
$
|
4,768
|
|
|||
|
Acquisition-related contingent consideration
|
1,828
|
|
—
|
|
—
|
|
1,828
|
|
|||||||
|
$
|
6,596
|
|
$
|
—
|
|
$
|
—
|
|
$
|
6,596
|
|
||||
|
|
Fair Value Measurements at December 31, 2017
|
||||||||||||||
|
|
Total
|
Level 1
|
Level 2
|
Level 3
|
|||||||||||
|
(in thousands)
|
|||||||||||||||
|
Liabilities
|
|||||||||||||||
|
Madryn put option liability
|
$
|
20,302
|
|
$
|
—
|
|
$
|
—
|
|
$
|
20,302
|
|
|||
|
Madryn call option liability
|
360
|
|
—
|
|
—
|
|
360
|
|
|||||||
|
Acquisition-related contingent consideration
|
964
|
|
—
|
|
—
|
|
964
|
|
|||||||
|
$
|
21,626
|
|
$
|
—
|
|
$
|
—
|
|
$
|
21,626
|
|
||||
|
December 31, 2018
|
December 31,
2017
|
||
|
Interest rate volatility
|
17.4%
|
21.0%
|
|
|
Market yield rate
|
13.0%
|
12.5%
|
|
|
Term (in years)
|
4.5
|
5.5
|
|
|
Dividend yield
|
—%
|
—%
|
|
|
December 31, 2018
|
December 31,
2017
|
||
|
Interest rate volatility
|
--
|
21.0%
|
|
|
Market yield rate
|
--
|
12.5%
|
|
|
Term (in years)
|
--
|
5.5
|
|
|
Dividend yield
|
--
|
—%
|
|
|
Warrant
Liability |
Acquisition-related Contingent Consideration
|
Put Option Liability (Madryn)
|
Call Option Liability (Madryn)
|
||||||||||||
|
Balance at December 31, 2016
|
$
|
3,983
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Issuance of financial instruments
|
—
|
|
964
|
|
20,044
|
|
83
|
|
|||||||
|
Change in fair value
|
4,035
|
|
—
|
|
258
|
|
277
|
|
|||||||
|
Repurchase of warrants
|
(7,060
|
)
|
—
|
|
—
|
|
—
|
|
|||||||
|
De-recognition during period
|
(958
|
)
|
—
|
|
—
|
|
—
|
|
|||||||
|
Balance at December 31, 2017
|
$
|
—
|
|
$
|
964
|
|
$
|
20,302
|
|
$
|
360
|
|
|||
|
Change in fair value
|
—
|
|
1,727
|
|
(15,534
|
)
|
(360
|
)
|
|||||||
|
De-recognition during period
|
—
|
|
(863
|
)
|
—
|
|
—
|
|
|||||||
|
Balance at December 31, 2018
|
$
|
—
|
|
$
|
1,828
|
|
$
|
4,768
|
|
$
|
—
|
|
|||
|
December 31,
2018 |
December 31,
2017 |
|||||||
|
(in thousands)
|
||||||||
|
Principal
|
$
|
—
|
|
$
|
4,218
|
|
||
|
Accrued interest
|
—
|
|
703
|
|
||||
|
Total principal and accrued interest at end of period
|
$
|
—
|
|
$
|
4,921
|
|
||
|
2018
|
2017
|
||||||
|
(in thousands)
|
|||||||
|
Principal
|
$
|
40,000
|
|
$
|
40,000
|
|
|
|
Net unamortized debt discount and issuance costs
|
(17,678
|
)
|
(20,833
|
)
|
|||
|
Net carrying value of Madryn debt
|
$
|
22,322
|
|
$
|
19,167
|
|
|
|
Years Ending December 31,
|
Operating
Leases
|
|||
|
(in thousands)
|
||||
|
2019
|
$
|
888
|
|
|
|
2020
|
824
|
|
||
|
2021
|
765
|
|
||
|
2022
|
764
|
|
||
|
2023
|
792
|
|
||
|
Thereafter
|
3,137
|
|
||
|
$
|
7,170
|
|
||
|
Years Ending December 31,
|
Capital
Leases
|
|||
|
(in thousands)
|
||||
|
2019
|
$
|
355
|
|
|
|
2020
|
263
|
|
||
|
2021
|
154
|
|
||
|
2022
|
25
|
|
||
|
797
|
|
|||
|
Interest included in the above payments
|
(81
|
)
|
||
|
Amount payable without interest
|
716
|
|
||
|
Short-term minimum capital lease payments (included in accrued liabilities)
|
336
|
|
||
|
Long-term minimum capital lease payments
|
$
|
380
|
|
|
|
2018
|
2017
|
|||||
|
Warrants to purchase common shares
|
104,826
|
|
145,000
|
|
||
|
Options to purchase shares
|
1,486,363
|
|
863,932
|
|
||
|
Remaining shares available under the 2015 Equity Incentive Plan
|
—
|
|
91,181
|
|
||
|
Remaining shares available under the 2018 Equity Incentive Plan
|
1,015,148
|
|
—
|
|
||
|
Shares issuable on vesting of restricted stock awards
|
314,123
|
|
585,056
|
|
||
|
Remaining shares available under the 2018 ESPP
|
100,000
|
|
—
|
|
||
|
Total
|
3,020,460
|
|
1,685,169
|
|
||
|
Warrant Holder
|
Issue Date
|
In Connection With
|
Warrant to
Purchase |
Shares
|
Exercise
Price |
Expiration Date
|
|||||||||
|
Rockport
|
3/3/2017
|
Loan agreement
|
Common
|
104,826
|
|
$
|
3.80
|
|
8/28/2022
|
||||||
|
2018
|
2017
|
|||||||
|
(in thousands)
|
||||||||
|
Sales, general and administrative
|
$
|
3,400
|
|
$
|
1,221
|
|
||
|
Research and development
|
3,920
|
|
2,082
|
|
||||
|
Total
|
$
|
7,320
|
|
$
|
3,303
|
|
||
|
|
Number of Options Outstanding
|
|
Weighted-Average Exercise Price
|
|
Weighted-Average Remaining Contractual Term (in years)
|
|
Aggregate Intrinsic Value (in thousands)
|
||||||
|
Balances at December 31, 2017
|
863,932
|
|
$
|
5.36
|
|
7.6
|
$
|
4,199
|
|
||||
|
Granted (weighted-average fair value of $10.43 per share)
|
810,628
|
|
16.52
|
|
|||||||||
|
Exercised
|
(132,091
|
)
|
4.87
|
|
|||||||||
|
Forfeited/canceled
|
(56,106
|
)
|
7.54
|
|
|||||||||
|
Balances at December 31, 2018
|
1,486,363
|
|
$
|
11.43
|
|
8.74
|
$
|
23,778
|
|
||||
|
▪
|
Fair Value of Common Shares.
Following the IPO, the closing price of the Company’s publicly-traded common shares on the date of grant and at quarter-end is used as the fair value of the shares. Prior to
|
|
▪
|
Risk-Free Interest Rate.
The Company bases the risk-free interest rate used in the Black-Scholes valuation model on the implied yield available on U.S. Treasury zero-coupon issues with a term equivalent to that of the term of the options for each option group on the measurement date.
|
|
▪
|
Term.
For employee stock options, the expected term represents the period that the Company’s share-based awards are expected to be outstanding. Because of the limitations on the sale or transfer of the Company’s shares as a privately held company, the Company does not believe its historical exercise pattern is indicative of the pattern it will experience as a publicly traded company. The Company has consequently used the Staff Accounting Bulletin 110, or SAB 110, simplified method to calculate the expected term of employee stock options, which is the average of the contractual term and vesting period. The Company plans to continue to use the SAB 110 simplified method until it has sufficient trading history as a publicly traded company. For consultant stock options, the term used is equal to the remaining contractual term on the measurement date.
|
|
▪
|
Volatility.
The Company determines the price volatility based on the historical volatilities of industry peers as it does not have sufficient trading history for its shares. Industry peers consist of several public companies in the medical device industry with comparable characteristics, including revenue growth, operating model and working capital requirements. The Company intends to continue to consistently apply this process using the same or a similar peer group of public companies until a sufficient amount of historical information regarding the volatility of its own shares becomes available, or unless circumstances change such that the identified peer companies are no longer similar, in which case other suitable peer companies whose common share prices are publicly available would be utilized in the calculation. The volatility is calculated based on the term on the measurement date.
|
|
▪
|
Dividend Yield.
The expected dividend assumption is based on the Company’s current expectations about its anticipated dividend policy. The Company has no expectation that it will declare dividends on its common shares, and therefore has used an expected dividend yield of zero.
|
|
2018
|
||
|
Volatility
|
56.0% - 57.0%
|
|
|
Risk-free interest rate
|
2.7% - 3.1%
|
|
|
Term (in years)
|
6.25
|
|
|
Dividend yield
|
0%
|
|
|
2018
|
2017
|
|||
|
Volatility
|
58.0% - 59.0%
|
59.0%
|
||
|
Risk-free interest rate
|
2.8% - 3.1%
|
2.4%
|
||
|
Term (in years)
|
10.0
|
10.0
|
||
|
Dividend yield
|
0.0%
|
0.0%
|
||
|
|
Restricted Stock Awards
|
|
Weighted-
Average
Grant Date
Fair Value
|
||||
|
Outstanding unvested at December 31, 2017
|
585,056
|
|
|
$
|
7.57
|
|
|
|
Granted
|
90,301
|
|
13.27
|
|
|||
|
Vested
|
(245,066
|
)
|
7.42
|
|
|||
|
Forfeited/canceled
|
(116,168
|
)
|
9.60
|
|
|||
|
Outstanding unvested at December 31, 2018
|
314,123
|
|
$
|
8.57
|
|
||
|
|
Restricted Stock Units
|
|
Weighted-
Average
Grant Date
Fair Value
|
||||
|
Outstanding unvested at December 31, 2017
|
—
|
|
|
$
|
—
|
|
|
|
Granted
|
3,852
|
|
25.35
|
|
|||
|
Vested
|
(3,852
|
)
|
25.35
|
|
|||
|
Forfeited/canceled
|
—
|
|
—
|
|
|||
|
Outstanding unvested at December 31, 2018
|
—
|
|
$
|
—
|
|
||
|
Purchase Price:
|
(in thousands)
|
|||
|
Cash consideration
|
$
|
1,000
|
|
|
|
Fair market value of Class A ordinary shares issued
|
344
|
|
||
|
Contingent consideration
|
964
|
|
||
|
Cash paid for inventory
|
704
|
|
||
|
Total purchase price
|
$
|
3,012
|
|
|
|
Allocation of Purchase Price:
|
(in thousands)
|
|||
|
Inventory
|
$
|
1,498
|
|
|
|
Customer relationships
|
1,280
|
|
||
|
Covenant not to compete
|
24
|
|
||
|
Goodwill
|
210
|
|
||
|
Total purchase price allocated
|
$
|
3,012
|
|
|
|
Purchase Price:
|
(in thousands)
|
|||
|
Cash consideration
|
$
|
544
|
|
|
|
Minimum guaranteed milestone payments
|
1,161
|
|
||
|
Cash paid for inventory
|
917
|
|
||
|
Total purchase price
|
$
|
2,622
|
|
|
|
Allocation of Purchase Price:
|
(in thousands)
|
|||
|
Inventory
|
$
|
2,137
|
|
|
|
Customer relationships
|
428
|
|
||
|
Covenant not to compete
|
57
|
|
||
|
Total purchase price allocated
|
$
|
2,622
|
|
|
|
Purchase Price:
|
(in thousands)
|
|||
|
Cash consideration
|
$
|
1,838
|
|
|
|
Cash paid for inventory
|
1,774
|
|
||
|
Total purchase price
|
$
|
3,612
|
|
|
|
Allocation of Purchase Price:
|
(in thousands)
|
|||
|
Inventory
|
$
|
3,560
|
|
|
|
Customer relationships
|
52
|
|
||
|
Total purchase price allocated
|
$
|
3,612
|
|
|
|
Purchase Price:
|
(in thousands)
|
|||
|
Cash consideration
|
$
|
100
|
|
|
|
Fair market value of common shares issued on effective date
|
120
|
|
||
|
Cash paid for inventory
|
123
|
|
||
|
Total purchase price
|
$
|
343
|
|
|
|
Allocation of Purchase Price:
|
(in thousands)
|
|||
|
Inventory
|
$
|
235
|
|
|
|
Customer relationships
|
108
|
|
||
|
Total purchase price allocated
|
$
|
343
|
|
|
|
2018
|
2017
|
||||||
|
(in thousands)
|
|||||||
|
Costa Rica Operations
|
$
|
3,422
|
|
$
|
(6,887
|
)
|
|
|
Non-Costa Rica Operations
|
(24,305
|
)
|
(27,905
|
)
|
|||
|
$
|
(20,883
|
)
|
$
|
(34,792
|
)
|
||
|
2018
|
2017
|
||||||
|
(in thousands)
|
|||||||
|
Current
|
|||||||
|
Costa Rica
|
$
|
105
|
|
$
|
—
|
|
|
|
Non-Costa Rica
|
110
|
|
105
|
|
|||
|
Total Current
|
215
|
|
105
|
|
|||
|
Deferred
|
|||||||
|
Costa Rica
|
—
|
|
—
|
|
|||
|
Non-Costa Rica
|
—
|
|
—
|
|
|||
|
Total deferred
|
—
|
|
—
|
|
|||
|
Total provision
|
$
|
215
|
|
$
|
105
|
|
|
|
2018
|
2017
|
||||||||||||
|
(in thousands)
|
|||||||||||||
|
Tax benefit at Costa Rica statutory rate
|
$
|
(6,265
|
)
|
30
|
%
|
$
|
(10,438
|
)
|
30
|
%
|
|||
|
Foreign tax rate differential
|
4,335
|
|
(21
|
)
|
5,665
|
|
(15
|
)
|
|||||
|
Tax rate changes
|
42
|
|
—
|
|
562
|
|
(2
|
)
|
|||||
|
Return to provision adjustment
|
553
|
|
(3
|
)
|
679
|
|
(1
|
)
|
|||||
|
Tax credits
|
(75
|
)
|
—
|
|
(26
|
)
|
—
|
|
|||||
|
Change in valuation allowance
|
2,439
|
|
(12
|
)
|
1,768
|
|
(4
|
)
|
|||||
|
Net operating loss expiration
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||
|
Tax holiday benefit
|
(912
|
)
|
4
|
|
1,653
|
|
(8
|
)
|
|||||
|
Other
|
98
|
|
1
|
|
242
|
|
—
|
|
|||||
|
$
|
215
|
|
(1
|
)%
|
$
|
105
|
|
—
|
%
|
||||
|
2018
|
2017
|
||||||
|
(in thousands)
|
|||||||
|
Accruals and reserves
|
$
|
62
|
|
$
|
91
|
|
|
|
Deferred revenue
|
—
|
|
33
|
|
|||
|
Intangibles
|
73
|
|
69
|
|
|||
|
Stock compensation
|
131
|
|
5
|
|
|||
|
Net operating loss
|
5,738
|
|
3,384
|
|
|||
|
R&D credits
|
97
|
|
28
|
|
|||
|
Other
|
(46
|
)
|
6
|
|
|||
|
Valuation allowance
|
(6,055
|
)
|
(3,616
|
)
|
|||
|
Total net deferred tax liabilities
|
$
|
—
|
|
$
|
—
|
|
|
|
Year Ended December 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
(in thousands, except share and per share data)
|
||||||||
|
Numerator:
|
||||||||
|
Net loss
|
$
|
(21,098
|
)
|
$
|
(34,897
|
)
|
||
|
Denominator:
|
||||||||
|
Weighted average common shares used for basic and diluted earnings per share
|
17,350,705
|
|
10,230,586
|
|
||||
|
Loss per share:
|
||||||||
|
Basic and diluted
|
$
|
(1.22
|
)
|
$
|
(3.41
|
)
|
||
|
2018
|
2017
|
|||||
|
Options to purchase shares
|
1,486,363
|
|
863,932
|
|
||
|
Shares issuable on vesting of restricted stock awards
|
314,123
|
|
585,056
|
|
||
|
Warrants to purchase common shares
|
104,826
|
|
145,000
|
|
||
|
Total
|
1,905,312
|
|
1,593,988
|
|
||
|
ESTABLISHMENT LABS HOLDINGS INC.
|
|||
|
Dated:
|
March 20, 2019
|
By:
|
/s/ Juan Jose Chacon Quiros
|
|
Name:
|
Juan Jose Chacon Quiros
|
||
|
Title:
|
Chief Executive Officer and Director
|
||
|
Signature
|
Title
|
Date
|
||
|
/s/ Juan Jose Chacon Quiros
|
President, Chief Executive Officer and Director
(Principal Executive Officer)
|
March 20, 2019
|
||
|
Juan Jose Chacon Quiros
|
||||
|
/s/ Renee M. Gaeta
|
Chief Financial Officer
(Principal Financial and Accounting Officer)
(Authorized Representative in the United States)
|
March 20, 2019
|
||
|
Renee M. Gaeta
|
||||
|
/s/ Nicholas Lewin
|
Chairman of the Board of Directors
|
March 20, 2019
|
||
|
Nicholas Lewin
|
||||
|
/s/ Dennis Condon
|
Director
|
March 20, 2019
|
||
|
Dennis Condon
|
||||
|
/s/ Lisa N. Colleran
|
Director
|
March 20, 2019
|
||
|
Lisa N. Colleran
|
||||
|
/s/ Edward Schutter
|
Director
|
March 20, 2019
|
||
|
Edward Schutter
|
||||
|
/s/ David Hung, M.D.
|
Director
|
March 20, 2019
|
||
|
David Hung, M.D.
|
||||
|
Director
|
||||
|
Allan Weinstein
|
||||