EVOGENE LTD.
FORM 20-F
ANNUAL REPORT FOR THE FISCAL YEAR ENDED DECEMBER 31, 2021
TABLE OF CONTENTS
CERTAIN TERMS AND CONVENTIONS
In this annual report, unless the context otherwise requires:
|
◾ |
references to “Evogene,” “we,” “us,” “our,” “our company” and “the
company” refer to Evogene Ltd. and its consolidated subsidiaries, consisting of AgPlenus Ltd., or AgPlenus, Biomica Ltd., or Biomica,
Canonic Ltd., or Canonic, Casterra Ag Ltd., or Casterra, Evogene Inc., Lavie Bio Ltd., or Lavie Bio, and their consolidated subsidiaries;
|
|
◾ |
references to “U.S. dollars,” “USD,” “$” or “dollars” are
to United States dollars; |
|
◾ |
references to “NIS” or “shekels” are to New Israeli Shekels; |
|
◾ |
references to the “U.S.” are to the United States; |
|
◾ |
references to “ordinary shares,” “our shares” and similar expressions refer to our Ordinary Shares, par value
NIS 0.02 per share; |
|
◾ |
references to the “articles of association” are to our Amended and Restated Articles of Association, which became effective
upon the closing of the U.S. initial public offering, as subsequently amended; |
|
◾ |
references to the “Companies Law” are to the Israeli Companies Law, 5759-1999, as amended; |
|
◾ |
references to the “Securities Act” are to the Securities Act of 1933, as amended; |
|
◾ |
references to the “Exchange Act” are to the Securities Exchange Act of 1934, as amended; |
|
◾ |
references to the “NYSE” are to the New York Stock Exchange; |
|
◾ |
references to the “Nasdaq” are to the Nasdaq Stock Market LLC; |
|
◾ |
references to the “TASE” are to the Tel Aviv Stock Exchange; and |
|
◾ |
references to the “SEC” are to the United States Securities and Exchange Commission. |
Unless derived from our financial statements or otherwise noted,
amounts presented in this annual report are translated at the rate of NIS 3.11 = USD 1.00, the exchange rate between the NIS and the U.S.
dollar reported by the Bank of Israel as of December 31, 2021.
This annual report includes other statistical, market and industry
data and forecasts which we obtained from publicly available information and independent industry publications and reports that we believe
to be reliable sources. Some data is also based on our good faith estimates, which are derived from management’s knowledge of the
industry and independent sources. These publicly available industry publications and reports generally state that they obtain their information
from sources that they believe to be reliable, but they do not guarantee the accuracy or completeness of the information. Although we
believe that these sources are reliable and are not aware of any misstatements regarding the industry data presented in this annual report,
we have not independently verified the information contained in such publications. Certain estimates and forecasts involve uncertainties
and risks and are subject to change based on various factors, including those discussed under the headings “—Special Note
Regarding Forward-Looking Statements” and “Item 3. Risk Factors—D. Risk Factors” in this annual report.
Throughout this annual report, we refer to various trademarks,
service marks and trade names that we use in our business. The “Evogene” design logo, “Evogene” and other trademarks
or service marks of Evogene Ltd. and its subsidiaries appearing in this annual report are the property of Evogene Ltd. or of its subsidiaries,
as applicable. We have several other registered trademarks, service marks and pending applications relating to our computational technologies.
Other trademarks and service marks appearing in this annual report are the property of their respective holders. Solely for convenience,
the trademarks and trade names in this annual report are referred to without the ® and ™ symbols, but such references should
not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights
thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement
or sponsorship of us by, any other companies.
SPECIAL
NOTE REGARDING FORWARD-LOOKING STATEMENTS
In addition to historical facts, this annual report contains forward-looking
statements within the meaning of Section 27A of the Securities Act, Section 21E of the Exchange Act, and the safe harbor provisions of
the U.S. Private Securities Litigation Reform Act of 1995. We have based these forward-looking statements on our current expectations
and projections about future events. Forward-looking statements include information concerning our possible or assumed future results
of our business, financial condition, results of operations, liquidity, anticipated growth strategies, anticipated trends in our industry,
market size, our potential growth opportunities, plans and objectives. Forward-looking statements include all statements that are not
historical facts and can be identified by terms such as “anticipates,” “believes,” “could,” “seeks,”
“estimates,” “expects,” “intends,” “may,” “plans,” “potential,”
“predicts,” “projects,” “should,” “will,” “would” or similar expressions that
convey uncertainty of future events or outcomes and the negatives of those terms.
Our actual future results, performance or achievements may differ
materially from what is expressed or implied by those forward-looking statements due to a variety of factors, some of which are beyond
our control, including the following factors:
|
◾ |
the extent to which we continue to maintain our holdings in our subsidiary companies; |
|
◾ |
the extent to which our discoveries and product candidates will have the desired effect so as to reach the stage of commercialization;
|
|
◾ |
whether we are able to achieve commercialization of our product candidates; |
|
◾ |
whether we and our collaborators are able to allocate the resources needed to develop commercial products from our discoveries and
product candidates; |
|
◾ |
the length and degree of complexity of the process of our developing commercial products based on our discoveries and product candidates
and the probability of our success, and the success of our collaborators, in developing such products; |
|
◾ |
whether we are able to efficiently produce and scale up the production of our products, whether ourselves or through third party
contractors, to achieve our commercialization targets; |
|
◾ |
the degree of success of third parties upon whom we rely to conduct certain activities, such as field-trials and pre-clinical studies;
|
|
◾ |
whether we are able to comply with regulatory requirements; |
|
◾ |
whether we and our subsidiaries are able to meet expected timelines in the performance of our activities (or are delayed, including
as a result of the effect of COVID-19); |
|
◾ |
the extent of the future growth of the agriculture, human health and industrial application industries in which we operate;
|
|
◾ |
whether we can maintain our current business models; |
|
◾ |
the actual commercial value of our key product candidates; |
|
◾ |
whether we or our collaborators receive regulatory approvals for the product candidates developed by us or our collaborators;
|
|
◾ |
whether products and product candidates containing or based on our discoveries are commercialized and earn us revenues or royalties;
|
|
◾ |
whether we are able to recruit, retain and develop knowledgeable or specialized personnel to perform our research and development
work; |
|
◾ |
the degree of our success at adapting to the continuous technological changes in our industries; |
|
◾ |
whether we can maintain our collaboration agreements with our current collaborators or enter into new collaboration agreements and
expand our research and development to new fields; |
|
◾ |
whether we can improve our existing, or develop and launch new, computational technologies and screening and validation systems;
|
|
◾ |
whether we can patent our discoveries and protect our trade secrets and proprietary know-how; and |
|
◾ |
the duration, degree of severity of, and strength of recovery from, the global COVID-19 pandemic, including government decisions
implemented to limit its spread. |
A number of additional important factors could cause our actual
results to differ materially from those indicated by our forward-looking statements, including, but not limited to, those factors described
in “Item 3. Key Information—D. Risk Factors,” “Item 4. Information on the Company” and “Item 5. Operating
and Financial Review and Prospects.”
You should not rely upon forward-looking statements as predictions
of future events. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee
that future results, levels of activity, performance and events and circumstances reflected in the forward-looking statements will be
achieved or will occur. All of the forward-looking statements that we have included in this annual report are based on information available
to us on the date of this annual report. Except as required by law, we undertake no obligation to publicly update or revise any forward-looking
statement, whether as a result of new information, future events, changes in our expectations or otherwise.
SUMMARY RISK FACTORS
The risk factors described below are a summary of
the principal risk factors associated with an investment in us. These are not the only risks we face. You should carefully
consider these risk factors, together with the risk factors set forth in Item 3D. of this annual report and the other reports
and documents filed by us with the SEC.
|
◾ |
If our equity holdings in our subsidiary companies are diluted, the benefits recognized by our shareholders from the value that may
be created in such subsidiary companies may be substantially reduced. |
|
◾ |
Our discoveries and product candidates may not achieve the desired effect required in order to create commercially viable products.
In addition, our product development cycle is lengthy and uncertain and various factors may delay or prevent commercialization of our
product candidates. We may never sell or earn royalties on the sale of commercial products based on our discoveries. |
|
◾ |
Due to mergers and consolidations, there is a reduced number of companies in the agriculture industry with which we might establish
strategic partnerships, and we rely on a limited number of collaborators to develop and commercialize product candidates containing our
seed trait, ag-chemical and ag-biological product candidates. In addition, a decrease in research expenditures by the major companies
in our target markets may jeopardize the continuation, or scope, of our collaborations with such companies and adversely impact our ability
to continue or extend existing collaborations or enter into new collaborations on favorable financial terms. |
|
◾ |
If we are unable to efficiently produce and scale up the production of our products, whether ourselves or through third party contractors,
we may be unable to achieve our commercialization targets. |
|
◾ |
We or our collaborators may fail to perform obligations under the collaboration agreements. |
|
◾ |
We are operating in multiple industries, each of which consists of multiple companies with much greater resources than us. If we
are unable to compete effectively, our financial resources will be diluted and our financial results will suffer. |
|
◾ |
We are working to develop and commercialize novel ag-biological products, ag-chemical products, seed-trait products, human microbiome-based
therapeutic product candidates, medical cannabis products, and our efforts with respect to any of these products may be unsuccessful.
|
|
◾ |
We are working to develop and commercialize castor seeds for industrial applications, and our efforts may be unsuccessful in achieving
a commercial presence in this market. |
|
◾ |
If Lavie Bio is unable to establish successful distribution and retail channels for the commercialization of its products, it will
not be able to meet its commercialization plans. |
|
◾ |
If Canonic is unable to establish successful marketing and distribution channels for the commercialization of its products, it will
not be able to meet its commercialization plans. |
|
◾ |
Biomica’s product candidates are based on microbiome therapeutics, which is an unproven approach to therapeutic intervention.
|
|
◾ |
Even if we are, or believe we are, entitled to royalties from our collaborators, we may not actually receive these royalties.
|
|
◾ |
Each of us and our subsidiaries depends on our key personnel and, if we are not able to attract and retain qualified scientific,
technological, business and managerial personnel, we may not be able to grow our business or develop and commercialize our product candidates.
|
|
◾ |
We develop certain discoveries independent om our collaborators, and we may need to finance the cost of the development of such technologies
product candidates ourselves. |
|
◾ |
Our business is subject to various government regulations and, if we or our collaborators are unable to obtain the necessary regulatory
approvals, we may not be able to continue our operations. |
|
◾ |
Our medical cannabis activity exposes us to legal and reputational risks associated with the cannabis industry. |
|
◾ |
If the cost we incur of Directors and Officers, or DO, liability insurance continues to increase, it will have an adverse effect
on our results of operations. |
|
◾ |
Disruption to our information technology, or IT, system could adversely affect our reputation and have a material adverse effect
on our business and results of operations. |
|
◾ |
The COVID-19 pandemic, or any other pandemic, epidemic or outbreak of an infectious disease has adversely impacted us and may continue
to adversely impact our operating results and financial condition. |
|
◾ |
Consumer and government resistance to genetically modified organisms, or GMOs, may negatively affect our public image and reduce
sales of plants containing our traits. |
|
◾ |
We have a history of operating losses and negative cash flow, and we may never achieve or maintain profitability. |
|
◾ |
The licenses we grant to our collaborators are in most cases exclusive with respect to a specified discovery, product type or market
area. This may limit our opportunities to enter into additional licensing or other arrangements with respect to such discoveries, product
types or market areas. |
|
◾ |
We may be required to pay substantial damages as a result of uninsured product liability claims. |
|
◾ |
Changes in laws and regulations to which we are subject, or to which we may become subject in the future, may materially increase
our costs of operation, decrease our operating revenues and disrupt our business. |
|
◾ |
Our success depends on our ability to protect our intellectual property and our proprietary technologies. If we are unable to protect
the confidentiality of our trade secrets, the value of our technology could be materially adversely affected and our business would be
harmed. In addition, we may not be able to protect our intellectual property rights throughout the world. |
|
◾ |
If we or one of our collaborators are sued for infringing the intellectual property rights of a third party, such litigation could
be costly and time consuming and could prevent us or our collaborators from developing or commercializing our product candidates.
|
|
◾ |
Our employment agreements with our employees and other agreements with our collaborators and third parties may not adequately prevent
disclosure of trade secrets, know-how and other proprietary information. In addition, we may not be able to fully enforce covenants not
to compete with our key employees. |
|
◾ |
Conditions in Israel could adversely affect our business. |
|
◾ |
Any appreciation of the NIS relative to the U.S. dollar would adversely impact our financial results. |
|
◾ |
Interest rate fluctuations may devalue our investments and could have an adverse impact on our financial condition. |
|
◾ |
The terms of our Israeli government grants for certain of our research and development activities may require us to satisfy specified
conditions in order to manufacture products and transfer technologies supported by such grants outside of Israel. In addition, in some
circumstances, we may be required to pay penalties in addition to repaying the grants. |
|
◾ |
The market price of our ordinary shares could be highly volatile and may fluctuate substantially as a result of many factors. In
addition, our ordinary shares are traded on more than one market and this may result in price variations. We believe we were a passive
foreign investment company, PFIC, for U.S. federal income tax purposes in 2021, and there is significant risk we will be a PFIC in 2022
as well. |
PART I
| ITEM 1. |
IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS |
Not applicable.
| ITEM 2. |
OFFER STATISTICS AND EXPECTED TIMETABLE |
Not applicable.
A. [Reserved]
B. Capitalization
and Indebtedness
Not applicable.
C. Reasons
for the Offer and Use of Proceeds
Not applicable.
D. Risk
Factors
Our
business faces significant risks. You should carefully consider all of the information set forth in this annual report and in our other
filings with the SEC, including the following risk factors which we face and which are faced by the industries in which we operate. Our
business, financial condition or results of operations could be materially adversely affected by any of these risks. This report also
contains forward-looking statements that involve risks and uncertainties. Our results could materially differ from those anticipated in
those forward-looking statements, as a result of certain factors, including the risks described below and elsewhere in this report and
our other SEC filings. See “Special Note Regarding Forward-Looking Statements” on page 5.
Risks Related to Our Business and Industry
If our equity holdings in our subsidiary companies
are diluted, the benefit recognized by our shareholders from value that may be created in such subsidiary companies may be substantially
reduced.
We initiated a new corporate strategy and structure at the beginning
of 2018, with the intent to make product development and go-to-market more efficient and to better reflect the individual value of each
of our market focused business units. Under the new corporate structure, we operate with Evogene acting as a technology hub and, below
it, a growing group of divisions and subsidiaries that benefit from the unique capabilities of Evogene’s Computational Predictive
Biology, or CPB platform and its technological engines. Each such subsidiary is responsible for advancing its product development and
pipeline, establishing its “go-to-market” strategy via direct sales or through existing and new collaborations, and securing
additional financial resources, if and when required. Due to our limited financial resources and other investment considerations, our
subsidiaries may obtain financing from external sources. External financing may result in a decrease of our percentage shareholdings in
our subsidiaries, which, in turn, may reduce the benefit we (and, indirectly, our shareholders) recognize from value established in such
subsidiaries, and potentially negatively affect our results of operations, financial condition, our long-term growth strategy and the
value of our shares.
Our discoveries and product candidates may
not achieve the desired effect required in order to create commercially-viable products.
Our success depends on our ability to develop products that have
the desired effects: in our agriculture activity, on plants, in our human health activity, on humans, and in our industrial applications
activity, on the relevant industrial inputs. Research and development in these industries entails considerable uncertainty. We may spend
many years developing product candidates that will never be commercialized. The science underlying the development of our product candidates
is highly complex and, although we use innovative approaches, there is no certainty that our discoveries will result in product candidates
that satisfy market requirements. Except for our first products in our medical cannabis and ag-biologicals activities and in our castor
oil activity, none of our discoveries and product candidates has completed the development process and become commercially available so
far and may never reach commercialization. If our discoveries and product candidates will not have the desired effects, we and our collaborators
may not develop commercial products that are based on them, which could materially and adversely affect our results of operations and
our long-term growth strategy.
Various factors may delay, hinder, or prevent commercialization
of our product candidates.
Our success depends in part on our ability to identify discoveries
that will improve crop performance, in our agriculture activity, obtain clinical benefits, in our human health activity, or improve industrial
inputs, in our industrial applications activity. To develop these discoveries and product candidates into commercial products, we either
license them to collaborators or develop them independently. Pursuant to our collaboration agreements in our agriculture activity, we
are usually entitled, subject to certain conditions, to receive royalties on products that are based on, or integrate, these discoveries.
In addition, certain of our agreements in our agriculture activity entitle us to upfront fees, research and development payments and milestone
payments in the event that specified milestones are met. Except for Casterra’s castor seed varieties and our first products in our
medical cannabis and ag-biologicals activities, none of our product candidates has completed the development process and become commercially
available, and there can be no guarantee that any of our current or future product candidates will ever reach commercialization. Therefore,
we currently do not earn royalties, nor do we have significant sales revenues from the sale of products based on our discoveries and product
candidates. Nevertheless, our long-term growth strategy is based in large part on the expectation that such royalties and revenues from
product sales will comprise a significant portion of our revenues in the future. If we or our collaborators are not successful in commercializing
products based on our discoveries, we will not realize revenues from sales or royalties and may not earn a profit on our discoveries,
which could materially and adversely affect our results of operations, financial condition and our long-term growth strategy and could
cause us to cease operations.
The manner in which we and our collaborators develop our product
candidates in our various fields of activity affects the period that will pass until such products are commercialized, if ever. Product
candidates based on our discoveries may never become commercialized for any of the following reasons:
|
◾ |
our discoveries and product candidates may not be successfully validated or may not have the desired effect required in order to
become, or to be incorporated into, commercial products; |
|
◾ |
the process of developing product candidates based on our discoveries is lengthy and expensive, and we or our collaborators may not
be able to allocate the resources needed to complete such development within the desired timeline; |
|
◾ |
we or our collaborators may decide to discontinue, pause, reduce, or alter the scope of the development efforts for our product candidates;
|
|
◾ |
we may fail to satisfy, in a timely manner or at all, relevant milestones under our agreements with our collaborators; |
|
◾ |
regulatory conditions related to our product candidates may change in different territories, thus negatively affecting the relevant
development processes and extending their length or limiting the commercialization of such product candidates; |
|
◾ |
we or our collaborators may be unable to obtain the requisite regulatory approvals for product candidates based on our discoveries;
|
|
◾ |
our competitors may launch competing or more effective products; |
|
◾ |
we or our collaborators may be unable to fully develop and commercialize product candidates containing our discoveries or may decide,
for whatever reason, not to commercialize, or to delay the commercialization of, such product candidates; |
|
◾ |
a market may not exist for products containing our discoveries or such products may not be commercially successful or relevant;
|
|
◾ |
we may be unable to protect the intellectual property underlying our discoveries in the necessary jurisdictions; and |
|
◾ |
we may encounter production and scale-up challenges with respect to our product candidates that hinder their commercialization.
|
Our product development cycle is lengthy and
uncertain, and we may never sell or earn royalties on the sale of commercial products based on our discoveries.
Research and development in our fields of activity is expensive
and prolonged and entails considerable uncertainty. We may spend many years and dedicate significant financial and other resources developing
product candidates that will never be commercialized. The process of discovering, developing and commercializing ag-chemicals, ag-biologicals,
seed traits, human microbiome-based therapeutics, medical cannabis products or castor varieties involves several phases and a long development
period. The timelines for development of product candidates by us or by our collaborators may extend beyond our expectations for many
reasons, such as:
|
◾ |
we or our collaborators may not be able to allocate the resources needed to develop product candidates based on our discoveries;
|
|
◾ |
we or our collaborators may revise the process of product development or make other decisions regarding the product development pipelines
that may extend the development period; |
|
◾ |
we or our collaborators may prioritize other development activities ahead of development activities with respect to the product candidates
on which we collaborate; |
|
◾ |
our discoveries may not be successfully validated or may not have the desired effect sought by us or by our collaborators; and
|
|
◾ |
we or our collaborators may be unable to obtain the requisite regulatory approvals for the product candidates based on our discoveries
within expected timelines or at all. |
Most of the product candidates we or our collaborators are developing
are in early development stages. We have little to no certainty as to which and when, if any, any of these product candidates will eventually
reach commercialization. Because of the long product development cycle and the complexities and uncertainties associated with research
in our fields of activity, there is significant uncertainty as to whether we will ever generate significant revenues or royalties, if
any, from the product candidates that we or our collaborators are developing. For more information on the product development cycle of
the product candidates we develop and a description of the phases of development, see the ‘Product Development Cycle’ paragraph
under the description of each of our activity divisions and subsidiaries in “Item 4. Information on the Company—B. Business
Overview”.
If we are unable to efficiently produce and
scale up the production of our products, whether ourselves or through third party contractors, we may be unable to achieve our commercialization
targets.
When we introduce a product to the market, and in certain cases
even in later stages of product development, we need to establish efficient production capabilities for our products. In most cases, our
products are, or are expected to be, produced by third party producers with whom we contract for such purpose. The production of our products,
and the scale up of such production, are complicated processes that require expertise. If we or our third party contractors are unable
to efficiently produce and scale up production as needed to meet the demand for our products, we may be unable to achieve our commercialization
targets, which may, in turn, materially and adversely affect our future results of operations.
Due to mergers and consolidations, there is
a reduced number of companies in the agriculture industry with which we might establish strategic partnerships, and we rely on a limited
number of collaborators to develop and commercialize product candidates containing our seed trait, ag-chemical and ag-biological product
candidates.
The agriculture markets are highly consolidated and dominated by
a relatively small number of large companies. In our agriculture operations, we are currently undertaking collaborations with several
of these companies to develop improved seed traits, ag-chemical and ag-biological product candidates. Due to the small number of major
companies in this industry, there are limited opportunities for us to grow our business with new collaborators. In addition, if we fail
to develop or maintain our relationships with any of our current collaborators, we could not only lose our opportunity to work with that
collaborator, but we could also suffer a reputational risk that could impact our relationships with other collaborators in what is a relatively
small industry community.
In our agriculture operations, we are currently working either
with collaborators or on independent projects to research and develop our different seed trait, ag-chemical and ag-biological product
candidates. While we seek to expand our portfolio of product candidates in the future, the research and development required to discover
and develop new product candidates is costly, time-intensive and requires significant infrastructure resources. If we are unable to enter
into new collaborations, or if we do not have the resources to develop the capabilities or resources necessary to discover and develop
such product candidates independently, we may not be able to expand our portfolio of these product candidates, which could have a material
adverse effect on our business prospects.
A decrease in research expenditures
by the major companies in our target markets may jeopardize the continuation, or scope, of our collaborations with such companies and
adversely impact our ability to continue or extend existing collaborations or enter into new collaborations on favorable financial terms.
The research and development expenditures of our existing and potential
collaborators in the agriculture, human health, and industrial applications markets we operate in may be reduced for reasons beyond our
control. For example, a global crisis or economic recession, a decrease in the prices of agricultural commodities, or the consolidation
trend in the seeds and ag-chemicals industries may result in decreased research and development expenditures in the markets relevant for
our seed trait, ag-biological and ag-chemical product candidates. Such developments may, in turn, adversely impact our ability to maintain
or extend our existing collaborations or enter into new collaborations on favorable financial terms. For example, we may not be able to
enter into new collaborations under which our collaborators cover our expenses through research and development payments.
We or our collaborators may fail to perform
obligations under the collaboration agreements.
We are obligated under our collaboration agreements to perform
research activities over a particular period of time. If we fail to perform our obligations under these agreements, in some cases our
collaborators may terminate our agreements with them and in other cases our collaborators’ obligations may be reduced and, as a
result, our anticipated revenues may decrease. In addition, any of our collaborators may fail to perform their obligations, which may
hinder development and commercialization of products containing the product candidates we develop and materially and adversely affect
our future results of operations. Furthermore, the various payments we receive from our collaborators are currently our primary source
of revenues. If our collaborators do not make these payments, either due to financial hardship, disagreement under the relevant collaboration
agreement or for any other reason, our results of operations and business could be materially and adversely affected. If disagreements
with a collaborator arise, any dispute with such collaborator may negatively affect our relationship with one or more of our other collaborators
and may hinder our ability to enter into future collaboration agreements, each of which could negatively impact our business and results
of operations.
We are operating in multiple industries, each
of which consists of multiple companies with much greater resources than us. Competition in our industries is intense and requires continuous
technological development. If we are unable to compete effectively, our financial resources will be diluted and our financial results
will suffer.
We currently face significant competition in the markets in which
we operate. The agriculture, human health and industrial applications markets in which we operate are intensely competitive and rapidly
changing. Many companies engage in research and development of products in such markets, and speed in getting a new product candidate
to market can be a significant competitive advantage. In most segments of our operations, the number of products available to the consumer
is steadily increasing as new products are introduced. We may be unable to compete successfully against our current and future competitors,
which may result in lower prices and margins and the inability to achieve market acceptance for our products. In addition, many of our
competitors have substantially greater financial, marketing, sales, distribution and technical resources than us and some of our collaborators
are significantly larger than us and have more experience in research and development, regulatory matters, manufacturing and marketing.
We anticipate increased competition in the future as new companies enter these markets and new technologies become available. Our technologies
may be rendered obsolete or uneconomical by technological advances or entirely different approaches developed by one or more of our competitors
or collaborators, which will prevent or limit our ability to receive any associated research and development payments or generate revenues
from the commercialization of our product candidates.
We are working to develop and commercialize
novel ag-biological products, and our efforts may be unsuccessful.
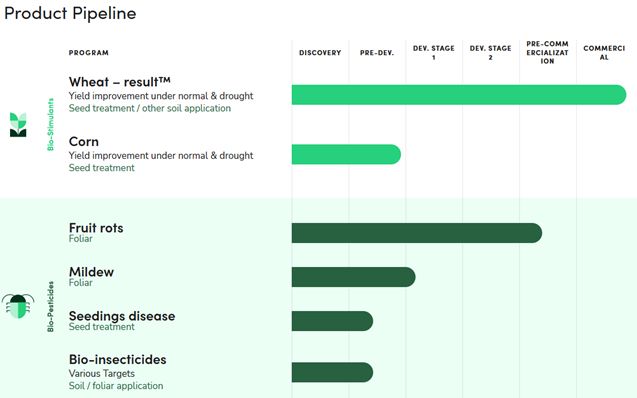
Our majority-owned subsidiary, Lavie Bio, is developing ag-biological
product candidates, currently focused mainly on microbial-based bio-stimulants and bio-pesticides, through a novel approach, focused on
plant-microbiome relationship. In certain of its ag-biological product programs, Lavie Bio funds its early stages of research and development
efforts, while in others it funds the entire development program towards launch of a commercial product. Lavie Bio’s efforts to
develop and commercialize novel ag-biological product candidates may fail for a variety of reasons, including:
|
◾ |
failure to establish the requisite infrastructure to enable the discovery and development of microbial bio-stimulants; |
|
◾ |
failure to identify and develop microbial candidates that enhance plant performance at the desired efficacy and stability;
|
|
◾ |
failure to successfully complete development of microorganisms to achieve cost-effective and commercially viable products;
|
|
◾ |
failure to obtain and maintain patent and trade secret protection for its product candidates; |
|
◾ |
failure to operate without infringing or violating the valid and enforceable patents or other intellectual property rights of third
parties; |
|
◾ |
inability to obtain sufficient funding to fully execute its business plan; |
|
◾ |
failure to meet regulatory requirements; |
|
◾ |
failure to establish efficient and reliable production and scale up capabilities of Lavie Bio’s products through third party
contractors; and |
|
◾ |
failure to establish cost-effective go-to-market models for selling its products. |
If Lavie Bio’s efforts to develop and commercialize ag-biological
product candidates are unsuccessful, our results of operations could be negatively impacted.
We
are working to develop and commercialize novel ag-chemical products, and our efforts may be unsuccessful.
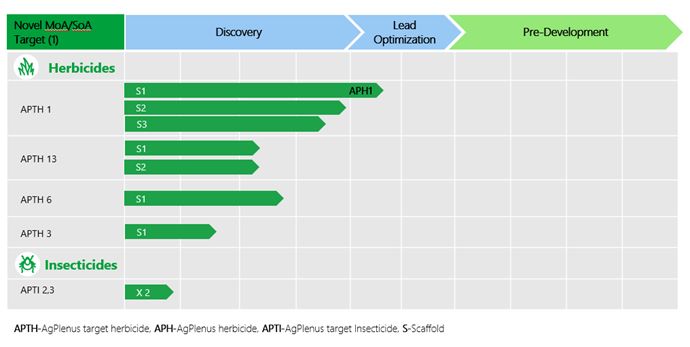
Our subsidiary, AgPlenus,
is currently developing solutions for crop protection through chemistry, or ag-chemistry. AgPlenus is developing these product candidates
through a novel approach, focused on biologically significant proteins called “targets”. AgPlenus’ efforts to develop
novel ag-chemical product candidates may fail for a variety of reasons, including:
|
◾ |
failure of its relatively novel target-based approach to lead to an effective product candidate or failure to identify chemical compounds
that will display required level of performance; |
|
◾ |
failure to establish cost-effective production of AgPlenus’ product candidates; |
|
◾ |
failure to obtain and maintain patent and trade secret protection for its product candidates; |
|
◾ |
failure to operate without infringing or violating the valid and enforceable patents or other intellectual property rights of third
parties; |
|
◾ |
inability to obtain sufficient funding to fully execute its ag-chemical business plan; and |
|
◾ |
failure to meet regulatory requirements. |
If AgPlenus’
efforts to develop ag-chemical product candidates are unsuccessful, our results of operations could be negatively impacted.
We are working to develop and commercialize
seed-trait products, and our efforts may be unsuccessful.
We are developing seed-trait and insect control product candidates
in our internal Ag-Seeds division. Our efforts to develop novel product candidates may fail for a variety of reasons, including:
|
◾ |
failure to identify and develop candidate genomic elements having the desired effect on the target trait in the plant of interest;
|
|
◾ |
failure to identify and develop toxin candidates having the desired effect on the target insects when inserted into the plants of
interest; |
|
◾ |
failure to obtain and maintain patent and trade secret protection for our product candidates; |
|
◾ |
failure to operate without infringing or violating the valid and enforceable patents or other intellectual property rights of third
parties; |
|
◾ |
inability to obtain sufficient funding to fully execute the business plan; |
|
◾ |
failure to successfully complete development of our seed trait product candidates; and |
|
◾ |
our failure to meet regulatory requirements for seed trait and insect control product candidates. |
Furthermore, even if we are able to discover and begin to develop
effective product candidates, we may not be successful if we are unable to find collaborators for further development and commercialization
of the product candidates. If our efforts to develop seed trait product candidates are unsuccessful, our results of operations could be
negatively impacted.
We are working to develop human microbiome-based
therapeutic product candidates, and our efforts may be unsuccessful.
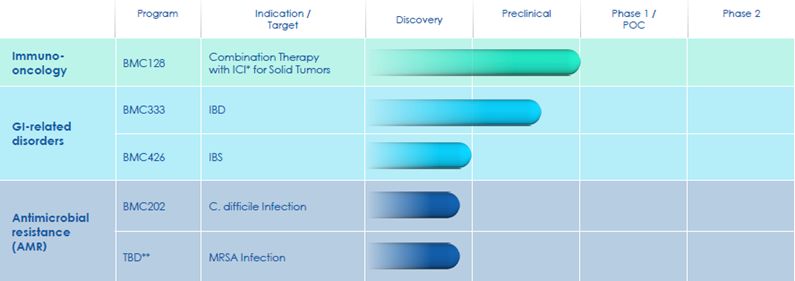
Our subsidiary, Biomica, is developing microbiome-based therapeutic
product candidates and is heavily dependent on the success of such product candidates, which are in pre-clinical and clinical development
stages. If Biomica is unable to advance its current or future product candidates through clinical trials, obtain marketing approval and
ultimately commercialize any product candidates it develops, or experiences significant delays in doing so, our business will be materially
harmed. Biomica’s product development efforts may be unsuccessful for a variety of reasons, including the following:
|
◾ |
failure to complete pre-clinical studies and clinical trials with positive results in which the FDA agrees with the design,
endpoints or implementation; |
|
◾ |
failure to receive regulatory approvals or authorizations for conducting our planned clinical trials or future clinical trials;
|
|
◾ |
failure to obtain sufficient financing for the development and commercialization of its product candidates; |
|
◾ |
failure to obtain and maintain patent and trade secret protection and regulatory exclusivity for its product candidates. |
|
◾ |
interruption of, or delays in receiving, supplies of our product candidates from our contract manufacturing organizations due to
staffing shortages, production slowdowns or stoppages and disruptions in delivery systems; |
|
◾ |
failure to launch commercial sales of its products, if and when approved, whether alone or in collaboration with others. |
|
◾ |
failure to enter into new collaborations throughout the development process as appropriate, from pre-clinical studies through to
commercialization; |
|
◾ |
failure to achieve acceptance of its products, if and when approved, by patients, the medical community and third-party payors;
|
|
◾ |
failure to effectively compete with companies developing and commercializing other therapies for the indications
that Biomica’s product candidates target; |
|
◾ |
failure to obtain and maintain coverage and adequate reimbursement by third-party payors, including government payors, for its products,
if approved; |
|
◾ |
failure to protect its rights in its intellectual property portfolio; |
|
◾ |
failure to operate without infringing or violating the valid and enforceable patents or other intellectual property rights of third
parties; |
|
◾ |
failure to maintain a continued acceptable safety profile of the products following approval; and |
|
◾ |
failure to maintain and develop an organization of scientists and business people who can develop and commercialize its products
and technology. |
If Biomica’s efforts to develop microbiome-based human
therapeutics are unsuccessful, our results of operations could be negatively impacted.
We are working to develop and commercialize
medical cannabis products, and our efforts may be unsuccessful.
Canonic, our subsidiary, is developing medical cannabis products.
Canonic’s efforts to develop and commercialize medical cannabis products may fail for a variety of reasons, including:
|
◾ |
failure to develop cannabis varieties having desired efficacy and stability; |
|
◾ |
failure to meet regulatory requirements; |
|
◾ |
failure to engage with, and successfully operate, contractors, in Israel and abroad, for performing cultivation and production services;
|
|
◾ |
failure to establish successful distribution channels, in Israel and abroad, for its medical cannabis products; |
|
◾ |
failure to satisfy the requirements for the export of seeds, seedlings of finished products; |
|
◾ |
failure to meet patients’ satisfaction; |
|
◾ |
inability to obtain sufficient funding to fully execute its business plan; |
|
◾ |
failure to secure cannabis cultivation facilities; |
|
◾ |
failure to establish efficient and reliable production capabilities for Canonic’s medical cannabis products through third party
contractors; and |
|
◾ |
the market for medical cannabis products is relatively new and suffers from high uncertainty in many aspects, including demand, supply,
pricing, regulation, customer preferences, etc. |
If Canonic’s efforts to develop and commercialize medical
cannabis products are unsuccessful, our results of operations could be negatively impacted.
We are working to develop and commercialize
castor seeds for industrial applications, and our efforts may be unsuccessful in achieving a commercial presence in this market.
Our subsidiary, Casterra, is developing improved, high-yield castor
bean seeds for use as a source of non-edible feedstock for industrial uses of castor oil. The supply chain in the market of castor oil
for industrial uses is not well established and is evolving. In order for Casterra’s castor bean seeds to be an attractive feedstock
for oil for industrial uses, it will need to demonstrate on a commercial scale that its castor beans can reliably be used as a cost-efficient
feedstock for castor oil production. Casterra’s efforts to develop castor been seeds for industrial uses may fail for a variety
of reasons, including:
|
◾ |
failure to reach desired yields of its castor seed varieties on a commercial scale to secure economic viability as bio-based oil
feedstock; |
|
◾ |
failure to establish an efficient mechanical harvest solution; |
|
◾ |
failure to establish a cost-effective production of castor bean grains, allowing grower profitability; |
|
◾ |
failure to reach large scale adoption of castor by growers, including the successful management of diseases and pests; |
|
◾ |
failure to address the health and environmental risks posed by castor bean seeds, which contain ricin, a naturally occurring poison;
|
|
◾ |
failure to comply with any regulatory requirement related to sales of castor beans, and in particular those related to the import
of such beans and the potential effects of ricin; and |
|
◾ |
failure to establish efficient and reliable production and scale up capabilities of castor seeds, independently or through third
party contractors. |
Casterra is operating in a new industry, with limited understanding
of the dynamics involved in producing and selling castor seeds. Casterra has made initial commercial sales of castor seeds; however, we
are unable to foresee as to when significant sales will commence. If Casterra is unable to adequately address any of these challenges,
we may not find a market for our castor bean seeds and our results of operations could be materially and adversely affected.
If Lavie Bio is unable to establish successful
distribution and retail channels for the commercialization of its products, it will not be able to meet its commercialization plans.
Our majority-owned subsidiary, Lavie Bio, intends to commercialize
part of its future ag-biological product portfolio through distribution and retail channels. Lavie Bio has little experience in establishing
such channels and may be unsuccessful in doing so. In addition, Lavie Bio will be dependent on its distributers in introducing its products
to the market. If Lavie Bio or its distributors are unsuccessful in their efforts to penetrate the market, our revenues and financial
results will be adversely affected.
If Canonic is unable to establish successful
marketing and distribution channels for the commercialization of its products, it will not be able to meet its commercialization plans.
Our subsidiary, Canonic, commercializes its medical cannabis products
through licensed distributors and pharmacies. Canonic has little experience in establishing such channels and may be unsuccessful in doing
so. In addition, Canonic will be dependent on its distribution and marketing channels in introducing its products to the market. If Canonic
is unsuccessful in its efforts to penetrate the market, our revenues and financial results will be adversely affected.
Biomica’s product candidates are based
on microbiome therapeutics, which is an unproven approach to therapeutic intervention.
Biomica’s product candidates are based on microbiome therapy, a therapeutic approach that is designed to treat disease by restoring
the function of a dysbiotic microbiome. To our knowledge, no company has received regulatory approval for a therapeutic based on this
approach. We cannot be certain that our approach will lead to the development of approvable or marketable products. In addition, the FDA
or other regulatory agencies may lack experience in evaluating the safety and efficacy of products based on microbiome therapeutics, which
could result in a longer than expected regulatory review process or evolving FDA standards and guidance, increase Biomica’s expected
development costs and delay or prevent commercialization of its product candidates. Regulatory requirements governing microbiome therapies
are still developing and may change in the future, which may lengthen the regulatory review process, require us to perform additional
preclinical studies or clinical trials, increase our development costs, delay or prevent approval and commercialization of our current
or future product candidates or lead to significant post-approval limitations or restrictions.
Even if we are entitled to royalties from our
collaborators, we may not actually receive these royalties, or we may experience difficulties in collecting the royalties that we believe
we are entitled to, potentially resulting in costly litigation and loss of reputation.
If and when our collaborators launch commercial products containing
our licensed discoveries, we will rely on our collaborators to report to us the sales they earn from these products and to accurately
calculate the royalties we are entitled to, a process that will involve complicated calculations. Although we seek to address these concerns
in our collaboration agreements, such provisions may not be effective. Additionally, we may not be able to achieve our long-term goal
of generating revenues from royalties, and in the coming years our revenues will be entirely dependent on fees we earn for our research
and development services and milestone payments from our collaborators.
In addition, our ability to generate royalty payments from our
collaboration agreements depends on our ability to clearly delineate our intellectual property rights under those agreements. We often
license patented discoveries and product candidates to our collaborators, who use them to develop and commercialize products. However,
a collaborator may use our intellectual property without our permission, dispute our ownership of certain intellectual property rights
or argue that our intellectual property does not cover their marketed product. If a dispute arises, it may result in costly litigation,
and our collaborator may refuse to pay us royalty payments while the dispute is ongoing. Furthermore, regardless of any resort to legal
action, a dispute with a collaborator over intellectual property rights may damage our relationship with that collaborator, and may also
harm our reputation in the industry.
Each of us and our subsidiaries depends on
our key personnel and, if we are not able to attract and retain qualified scientific, technological, business and managerial personnel,
we may not be able to grow our business or develop and commercialize our product candidates.
The vast majority of our workforce is involved in research and
development. Our business is therefore dependent on our ability to recruit and maintain a highly skilled and educated workforce with expertise
in a range of disciplines, including biology, chemistry, plant genetics, agronomics, entomology, mathematics, computer science and other
fields relevant to our operations. The number of qualified and highly educated personnel in the fields upon which our business focuses
in Israel, where most of our operations are located, is limited and competition for the services of such persons is intense. Although
we have employment agreements with all of our employees, most of these agreements may be terminated upon short notice. The failure to
hire and retain skilled and highly educated personnel could limit our growth and hinder our research and development efforts.
Competition for highly skilled scientific, technical and other personnel
is intense, and as a result we may fail to attract, recruit, retain and develop qualified employees, which could materially and adversely
impact our business, financial condition and results of operations.
We compete for personnel in a market characterized by rapidly changing
technologies and an evolving competitive landscape. In order for us to successfully compete and grow, we must attract, recruit, retain
and develop personnel with requisite qualifications to provide expertise across the entire spectrum of our intellectual capital and business
needs.
Our principal research and development activities are conducted
at our facilities in Israel, where we face significant competition. While there has been intense competition for qualified human resources
in the Israeli high-tech and bio-tech industries historically, these industries experienced record growth and activity in 2021. This flurry
of growth and activity has caused a sharp increase in job openings in Israeli high-tech and bio-tech companies, intensifying competition
between these employers to attract qualified employees in Israel. As a result, these industries in Israel have experienced significant
levels of employee attrition and are currently facing a severe shortage of skilled human capital, including research and development professionals.
Many of the companies with which we compete for qualified personnel
have significant resources, and we may not succeed in recruiting additional experienced or professional personnel, retaining personnel
or effectively replacing current personnel who may depart with qualified or effective successors. In addition, our employees may be increasingly
targeted for recruitment by competitors and other companies in the bio-tech industry, which may make it more difficult for us to retain
employees and may increase retention costs. Training of new employees with limited or no prior relevant experience could be time-consuming,
expensive and require significant resources.
In addition, as a result of the intense competition for qualified
human resources, the high-tech and bio-tech markets have also experienced and may continue to experience significant wage inflation. Accordingly,
our efforts to attract, retain and develop personnel may also result in significant additional expenses, which could adversely affect
our profitability. Furthermore, in making employment decisions, particularly in the high-tech and bio-tech industries, job candidates
often consider the value of the equity they are to receive in connection with their employment, which may force us to increase the amount
of equity awards we grant in order to recruit and retain talent.
In light of the foregoing, there can be no assurance that qualified
employees will remain in our employ or that we will be able to attract and retain qualified personnel in the future. Failure to retain
or attract qualified personnel could have a material adverse effect on our business, financial condition and results of operations.
We develop certain discoveries independent
of our collaborators, and we may need to finance the cost of the development of such product candidates ourselves.
We develop certain discoveries and product candidates independent
of our collaborators, with a goal of making such discoveries available to collaborators in later phases or developing and commercializing
end products. While we believe this will allow us to obtain more favorable license or commercialization terms with respect to such discoveries,
product candidates and products, the up-front cost to us of developing programs without a collaborator (and therefore without external
funding for the research and development expenditures we incur) in these early phases involves higher risks, since we need to fund the
research and development of such programs ourselves. If we are unsuccessful in discovering promising product candidates after having invested
significant funds, or if we are unable to find collaborators who are interested in such results and willing to fund subsequent phases
of development and commercialization, such failures could have a material and adverse effect on our business, financial condition and
results of operations. Regardless of the outcome of our research and development efforts, traditional financing sources such as bank financing
or public debt or equity financing, if available to us, could carry with them certain drawbacks, such as imposition of covenants restricting
our ability to operate, or substantial dilution to our existing shareholders.
Our business is subject to various government
regulations and, if we or our collaborators are unable to obtain the necessary regulatory approvals, we may not be able to continue our
operations.
Our business is generally subject to two types of regulations:
regulations that apply to how we operate and regulations that apply to product candidates and products. We may fail to comply with all
currently applicable regulations, and we may become subject to new or revised regulations or approvals in the future. Furthermore, any
violation of these regulations could expose us to civil and criminal penalties.
The relevant regulatory regimes may be particularly onerous; for
example, the U.S. federal government’s regulation of biotechnology is divided among the United States Environmental Protection Agency,
which regulates activity related to the invention of plant pesticides and herbicides, the United States Department of Agriculture, which
regulates the import, field testing and interstate movement of specific technologies that may be used in the creation of transgenic plants,
and the United States Food and Drug Administration, or the FDA, which regulates foods derived from new plant varieties. If we or our collaborators
are unable to obtain the requisite regulatory approvals or there is a delay in obtaining such approvals as a result of negative market
perception or heightened regulatory standards, such product candidates will not be commercialized, which would negatively impact our business
and results of operations.
Our medical cannabis activity exposes us to
legal and reputational risks associated with the cannabis industry.
Our involvement in cannabis-related
activity may expose us to legal and reputational risks. Such risks include:
|
◾ |
activities in the field of cannabis are subject to enhanced regulation in Israel and worldwide. For example, Israeli regulation requires
that we obtain a specific permit for each of the following activities: research, propagation, cultivation, production, marketing and distribution
and use; |
|
◾ |
changes in laws, regulations and guidelines related to cannabis may result in significant additional compliance costs for us or limit
our ability to operate in certain jurisdictions; |
|
◾ |
certain banks will not accept deposits from or provide other bank services to businesses involved with cannabis; |
|
◾ |
third parties with whom we do business may perceive that they are exposed to reputational risk as a result of our cannabis-related
business activities and may ultimately elect not to do business with us; |
|
◾ |
certain investors or investment banks are reluctant to work with companies affiliated with activity in the cannabis industry;
|
|
◾ |
future sales of medical cannabis products may expose us to consumer complaints or legal claims with respect to product quality or
activity; and |
|
◾ |
increased premiums under our DO liability insurance policies. |
Any of the foregoing factors could adversely affect our business
and results of operations
The cost we incur in procuring a DO liability
insurance has substantially increased during the last years. If this trend continues, it will have an adverse effect on our results of
operations.
DO liability insurance is intended to cover the liability
of the individuals serving as our directors and management, from losses incurred as a result of such service, our liability to indemnify
such individuals for such losses and to protect us from certain securities claims. During the last years, there has been a significant
increase in the cost of DO insurance for smaller, dual-listed public companies such as our Company. These increases have been tied
to perceived heightened levels of risk for DO insurers. Insurers have been increasing their level of compensation (in the form of
premiums), which they believe has not been commensurate with the risk being taken by them. In parallel, there has been an increase in
the amounts of the deductibles payable by public companies in situations in which an insurable event occurs. In addition, several insurers
are restricted from writing policies for companies active in the area of cannabis, which restricts the number of insurers that can provide
us with a DO liability insurance and limits our ability to negotiate the terms of such insurance. If these trends continue, it will
increase our operational expenses and have a negative effect on our financial results.
Disruption to our information technology, or
IT, system could adversely affect our reputation and have a material adverse effect on our business and results of operations.
Our computational technologies rely on our IT system to collect
and analyze the biological and chemical data we collect and discover. We store significant amounts of data, and to date, have compiled
several petabytes of data. There can be no assurance that our back-up storage arrangements will be effective if it becomes necessary to
rely on them. Furthermore, we can provide no assurance that our current IT system is fully protected against third-party intrusions, viruses,
hacker attacks, information or data theft or other similar threats. Disruption or failure of our IT system due to technical reasons, cyberattacks,
natural disasters or other unanticipated catastrophic events, including power interruptions, storms, fires, floods, earthquakes, terrorist
attacks and wars could significantly impair our internal development efforts and materially and adversely affect our collaborations, our
business and our results of operations.
As we continue to develop our computational technologies and expand
our datasets, we may need to update our IT system and storage capabilities. However, if our existing or future IT system does not function
properly, or if the IT system proves incompatible with our new technologies, we could experience interruptions in data transmissions and
slow response times, preventing us from completing routine research and business activities, which could adversely affect our business
and results of operations.
Development of our product candidates, particularly
during our validation and testing activities, may be adversely affected by circumstances caused by us or those beyond our control.
The industries we are engaged in are subject to various factors
that make our operations relatively unpredictable from period to period. For example, the testing of our product candidates may be adversely
affected by circumstances both caused by us and those that are beyond our control. Factors caused by us include any failure by us or our
collaborators to follow proper agronomic practice or suggested protocols for conducting our experiments, and failure to successfully complete
such experiments. Factors beyond our control include weather and climatic variations, such as droughts or heat stress, or other factors
we are unable to identify. For example, if there was prolonged or permanent disruption to the electricity, climate control or water supply
operating systems in our greenhouses or laboratories, the plants and pests on which we test our discoveries and product candidates and
the samples we store in freezers, both of which are essential to our research and development activities, would be severely damaged or
destroyed, adversely affecting our research and development activities and thereby our business and results of operations. We have experienced
these kind of failures in the past for unknown reasons, causing delays in our achievement of milestones and delivery of results, and necessitating
that we re-start the trials. Any test failure we may experience is not covered by our insurance policy, and therefore could result in
increased cost of the trials and development of our product candidates, which may negatively impact our business and results of operations.
The COVID-19 pandemic has caused and may continue
to cause disruption in certain of our activities.
The COVID-19 pandemic has caused states of emergency to be declared
in various countries, travel restrictions imposed globally, quarantines established in certain jurisdictions and various institutions
and companies being closed. Numerous government regulations and public advisories, as well as shifting social behaviors, temporarily and
from time to time limited or closed non-essential transportation, government functions, business activities and person-to-person interactions,
and the duration of such trends is difficult to predict.
The COVID-19 pandemic has lead to increased absences of our employees
due to illness and isolation requirements of our employees and their immediate family members. While we have facilitated remote work for
our employees, part of our activities, mainly laboratory and testing operations, cannot be conducted remotely, and are negatively affected
in times of increased employee absence, which may cause delays in our laboratory and experimental operations. Such delays may, in turn,
negatively affect our ability to meet our work plans, our contractual obligations and our targets.
While certain COVID-19 mitigation actions have been relaxed, no
assurance can be made that such actions, or other measures, will not be reimposed in the future. Although to date these restrictions have
not materially impacted our operations other than an increase in absences of our employees due to illness and isolation requirements,
the effect on our business, from the spread of COVID-19 and the COVID-19 mitigation actions implemented by the governments of the State
of Israel, the United States and other countries, may worsen over time. The extent to which COVID-19 impacts our business will depend
on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the
severity of COVID-19 and the actions to contain COVID-19 or treat its impact, among others.
Consumer and government resistance to GMOs
may negatively affect our public image and reduce potential sales of plants containing our traits.
A certain part of our seed traits activity includes research and
development of genetically modified, or GM, seeds. Foods made from such seeds are not accepted by many consumers and in certain countries
production of certain GM crops is effectively prohibited, including throughout the European Union, due to concerns over such products’
effects on food safety and the environment. The high public profile of biotechnology agriculture, especially in food production, and lack
of consumer acceptance of products to which we have devoted substantial resources could negatively affect our public image and results
of operations. For example, the prohibition on the production of certain GM crops in select countries and the current resistance from
consumer groups, particularly in Europe, to GM crops not only limits our access to such markets but also has the potential to spread to
and influence the acceptance of products developed through biotechnology in other regions of the world and may also influence regulators
in other countries to limit or ban production of GM crops, which could limit the commercial opportunities to exploit biotechnology.
GM crops are grown principally in the United States, Brazil and
Argentina where there are fewer restrictions on the production of GM crops. If these or other countries where GM crops are grown enact
laws or regulations that ban the production of such crops or make regulations more stringent, we could experience a longer product development
cycle for our product candidates and may even have to abandon projects related to certain crops or geographies, both of which would negatively
affect our business and results of operations and could cause us to have to cease operations. Furthermore, any changes in such laws and
regulations or consumer acceptance of GM crops could negatively impact our collaborators, who in turn might terminate or reduce the scope
of their collaborations with us or seek to alter the financial terms of our agreements with them.
We have a history of operating losses and negative
cash flow, and we may never achieve or maintain profitability.
We have a history of losses, and incurred operating losses of $31.0,
$24.8 million, and $21.2 million for the years ended December 31, 2021, 2020, and 2019, respectively. There is no assurance that
our efforts in developing our product candidates will result in commercially successful products. We expect to continue to incur losses
in future periods, until we begin earning significant revenues or royalties on our products, the product candidates we are currently developing
or any new product candidates we develop in the future, if at all. Because we will incur significant costs and expenses for these efforts
before we obtain any incremental revenues from them, our losses in future periods could be significant. In addition, we may find that
these efforts are more expensive than we anticipate or that they do not result in profitability in the time period we anticipate, which
would further increase our losses. For example, if governments across the globe continue to implement actions that limit movement and
activity, as a result of the COVID-19 pandemic or otherwise, we could face increased costs in order to meet our product development timeline.
If we are unable to adequately control the costs associated with operating our business, including our costs of development and sales,
we may deplete our cash resources and may be unable to continue to finance our business from our existing cash resources, and, our business,
financial condition, operating results and prospects will suffer. For more information concerning our cash resources, please see “Liquidity
and Capital Resources” in Item 5.B below.
The licenses we grant to our collaborators
to use our discoveries are in most cases exclusive with respect to a specified discovery, product type or market area. This may limit
our opportunities to enter into additional licensing or other arrangements with respect to such discoveries, product types or market areas.
Most of the licenses we grant our collaborators to our product
candidates or to use specific discoveries we have made are exclusive in the area of the license. That means that once these discoveries
are licensed to a collaborator, we are generally prohibited from licensing those discoveries to any third party for use in such area.
The limitations imposed by these exclusive licenses could prevent us from expanding our business and increasing our exposure to new licensees,
both of which could adversely affect our business and results of operations.
We may be required to pay substantial damages
as a result of uninsured product liability claims.
Once products integrating our discoveries and product candidates
reach commercialization, if ever, product liability claims will be a commercial risk for our business, particularly as some of the products
that we develop can be harmful to humans or the environment. Courts have levied substantial damages in the United States and elsewhere
against a number of companies in the agriculture and human health industries in past years based upon claims for injuries allegedly caused
by the use of their products. Product liability claims against us or our collaborators selling products that contain our product candidates
or allegations of product liability relating to products containing our discoveries could damage our reputation, harm our relationships
with our collaborators, and materially and adversely affect our business, results of operations, financial condition and prospects. We
currently do not have product liability insurance coverage. Any such insurance we may obtain in the future may be expensive and may not
cover our potential liability in full. Furthermore, while our collaboration agreements typically require that our collaborators indemnify
us for the cost of product liability claims brought against us, such indemnification provisions may not always be enforced, and we may
receive no indemnification if our own misconduct led to the claims.
Our facilities, in Israel and in the U.S.,
are located on leased properties. Termination of any of the leases, changes in lease terms, and long-term leases that may not be terminated
at will may jeopardize our activity and materially affect our financial condition or results of operations.
Our office spaces, labs, facilities, and farm are all situated
on properties that we lease pursuant to lease agreements, in Israel and in the U.S. Once a lease agreement ends, we may not be able to
renew it on favorable terms, or not at all, which may require us to increase our lease payments or take a new lease in another property,
adversely affecting our business and results of operations. In addition, a long-term lease may mean no or limited possibility to terminate
the lease at will before the completion of the lease period, which may lead to continued holding of an un-needed space or entry into a
sub-lease, which may adversely affect our results of operations. For more information regarding our facilities, please see “Item
4. Information on the Company—D. Property, Plants and Equipment.”
Lavie Bio’s research and development
facility in the U.S., our contracts with foreign businesses and any other current or future operations outside of Israel expose us to
additional market and operational risks, and failure to manage these risks may adversely affect our business and operating results.
Lavie Bio’s research and development facility in St. Louis,
Missouri may expose us to some of such operational risks, including:
|
◾ |
fluctuations in foreign currency exchange rates; |
|
◾ |
potentially adverse tax consequences; |
|
◾ |
difficulties in staffing and managing foreign operations; |
|
◾ |
hiring and retention of employees and/or consultants under foreign employment laws which are not familiar to us; |
|
◾ |
laws and business practices that sometimes favor local business; |
|
◾ |
compliance with foreign legislation, being subject to laws, regulations and the court systems of multiple jurisdictions; and
|
|
◾ |
tariffs, trade barriers and other regulatory or contractual limitations on our ability to develop (and, when applicable in the future,
sell) our solutions in certain foreign markets. |
Failure to manage the market and operational risks associated with
international operations effectively could limit the future growth of our business and adversely affect our operating results.
Our operations are subject to various health
and environmental risks associated with our use, handling and disposal of potentially toxic materials.
Our operations involve various health and environmental risks.
For example, as part of our seed traits operations, we assist in the development of GM crops by inserting new genes into the genomes of
certain plants. Though we introduce these genes in order to improve plant traits, we cannot always predict the effect that these genes
may have on the plant. In some cases, the genes may render the plant poisonous or toxic, or they may cause the plant to develop other
dangerous characteristics that could harm the plant’s surrounding environment. Furthermore, while we comply with relevant environmental
laws and regulations, there is a risk that, when testing genetically modified plants, the seeds of these plants may escape the greenhouse
or field in which they are being tested and contaminate nearby fields. Poisonous or toxic plants may therefore be inadvertently introduced
into the wild, or possibly enter the food production system, harming the people and animals who come in contact with them.
Moreover, as part of Lavie Bio’s operations, it develops
novel product candidates based on microbes in order to improve plants traits. Although microbes exist naturally in the environment, we
cannot always predict the effect that microbes have on the plant and its environment. There may be cases where the microbes render the
plant poisonous or toxic, or they may cause the plant to develop other dangerous characteristics that could harm the plant’s surrounding
environment.
Similar risks are relevant to our ag seeds operations, especially
with respect to GM seeds, AgPlenus’ ag-chemicals operations, Canonic’s cannabis operations and Casterra’s castor bean
operations.
Changes in laws and regulations to which we
are subject, or to which we may become subject in the future, may materially increase our costs of operation, decrease our operating revenues
and disrupt our business.
Laws and regulatory standards and procedures that impact our business
are continuously changing. Responding to these changes and meeting existing and new requirements may be costly and burdensome. Changes
in laws and regulations may occur that could:
|
◾ |
impair or eliminate our ability to research and develop our product candidates, including validating our product candidates through
lab, greenhouse, field or clinical trials; |
|
◾ |
increase our compliance and other costs of doing business through increases in the cost to patent or otherwise protect our intellectual
property or increases in the cost to our collaborators to obtain the necessary regulatory approvals to commercialize and market the product
candidates we develop with them; |
|
◾ |
require significant product redesign or systems redevelopment; |
|
◾ |
render our product candidates less profitable, obsolete or less attractive compared to competing products; |
|
◾ |
affect our collaborators’ willingness to do business with us; |
|
◾ |
jeopardize import or export of raw material or end products, such as with respect to medical cannabis seeds, seedlings and products;
|
|
◾ |
reduce the amount of revenues we receive from our collaborators through milestone payments or royalties; and |
|
◾ |
discourage our collaborators from offering, and consumers from purchasing, products that incorporate our discoveries. |
Any of these events could have a material adverse effect on our
business, results of operations and financial condition. For example, legislators and regulators have increased their focus on plant biotechnology
in recent years, with particular attention paid to GM crops as well as on ag-chemicals.
While none of our product candidates are currently available for
sale, other than Casterra’s castor seeds, our future growth relies on our ability and the ability of our collaborators to commercialize
and market our product candidates, and any restrictions on such activities could materially and adversely impact our business and results
of operations. Any changes in regulations in countries where our product candidates are used could result in our collaborators being unable
or unwilling to develop, commercialize or sell products that incorporate our discoveries. In addition, we rely on patents and other forms
of intellectual property protection. Legislation and jurisprudence on patent protection in the key target markets where we seek patent
protection, such as the United States and the European Union, is evolving and changes in laws could affect our ability to obtain or maintain
patent protection for our product candidates. Any changes to these existing laws and regulations may materially increase our costs of
operation, decrease our operating revenues and disrupt our business. For more information please see ‘Government Regulation of our
Operations’ and ‘Government Regulation of Product Candidates’ paragraphs under the description of each of our activity
divisions and subsidiaries under “Item 4. Information on the Company—B. Business Overview.”
Risks Related to Our Intellectual Property Rights
Our success depends on our ability to protect
our intellectual property and our proprietary technologies.
Our commercial success depends in part on our ability to obtain
and maintain patent protection and trade secret protection for our proprietary computational and experimental technologies, our discoveries
and their uses, as well as our ability to operate without infringing upon the proprietary rights of others. If we do not adequately protect
our intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage we may have,
which could harm our business and ability to achieve profitability.
While we expect our patent applications to receive approval, we
cannot be certain that we will obtain such results. Despite our efforts to protect our proprietary rights, unauthorized third parties
may attempt to use, copy or otherwise obtain and market or distribute our intellectual property rights or technology or otherwise develop
products or solutions with the same functionality as our solutions. In addition, the laws of some foreign countries provide less protection
for proprietary rights than U.S. law. We face the occasional risk, moreover, that third parties may assert copyright, trademark and other
intellectual property rights against us. Such claims may result in direct or indirect liability as we have contractually agreed to indemnify
certain parties for any damages suffered as a result of infringement by us of any third-party intellectual property rights.
If we are unable to protect the confidentiality
of our trade secrets, the value of our technology could be materially adversely affected and our business would be harmed.
We treat our proprietary computational and experimental technologies,
including unpatented know-how and other proprietary information, as trade secrets. We seek to protect these trade secrets, in part, by
entering into non-disclosure and confidentiality agreements with any third parties who have access to them, such as our consultants, independent
contractors, advisors, corporate collaborators and outside scientific collaborators. We also enter into confidentiality and invention
or patent assignment agreements with employees and certain consultants. Any party with whom we have executed such an agreement may breach
that agreement and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies
for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming,
and the outcome is unpredictable. In addition, if any of our trade secrets were to be lawfully obtained or independently developed by
a competitor, we would have no right to prevent such third party, or those to whom it communicates that technology or information, from
using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed
by a competitor, or if we otherwise lose protection for our trade secrets or proprietary know-how, the value of this information may be
greatly reduced and our business and competitive position could be harmed.
We may not be able to protect our intellectual
property rights throughout the world.
Filing, prosecuting, maintaining and defending patents on product
candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries
outside the United States are less extensive than those in the United States. In addition, the laws of some foreign countries do not protect
intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we are unable to prevent
third parties from using our inventions in all countries outside the United States, or from selling or importing products made using our
inventions in and into the jurisdictions in which we do not have patent protection. Competitors may use our technologies in jurisdictions
where we have not obtained patent protection to develop their own products, and we may be unable to prevent such competitors from importing
those infringing products into territories where we have patent protection but enforcement is not as strong as in the United States. These
products may compete with our product candidates and our patents and other intellectual property rights may not be effective or sufficient
to prevent them from competing in those jurisdictions. Moreover, farmers or others in the chain of commerce may raise legal challenges
against our intellectual property rights or may infringe upon our intellectual property rights, including through means that may be difficult
to prevent or detect. For example, the practice by some farmers of saving seeds from non-hybrid crops (such as soybeans, canola and cotton)
containing biotechnological traits may prevent us from realizing the full value of our intellectual property in countries outside of the
United States.
Many companies have encountered significant problems in protecting
and defending intellectual property rights in foreign jurisdictions, including China, where we have filed patent applications. The legal
systems of certain countries, including China, have not historically favored the enforcement of patents or other intellectual property
rights, which could hinder us from preventing the infringement of our patents or other intellectual property rights and result in substantial
risks to us. Proceedings to enforce our patent rights in the United States or foreign jurisdictions could result in substantial costs
and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted
narrowly and our patent applications at risk of not issuing and could provoke third parties to assert patent infringement or other claims
against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially
meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant
commercial advantage from the intellectual property that we develop or license from third parties.
If we or one of our collaborators are sued for infringing the intellectual
property rights of a third party, such litigation could be costly and time consuming and could prevent us or our collaborators from developing
or commercializing our product candidates.
Our ability to generate significant revenues from our product candidates
depends on our and our collaborators’ ability to develop, market and sell our product candidates and utilize our proprietary technology
without infringing the intellectual property and other rights of any third parties. In the United States and abroad there are numerous
third party patents and patent applications that may be applied toward our proprietary technology, business processes or product candidates,
some of which may be construed as containing claims that cover the subject matter of our product candidates or intellectual property.
Because of the rapid pace of technological change, the confidentiality of patent applications in some jurisdictions, and the fact that
patent applications can take many years to issue, there may be currently pending applications that are unknown to us that may later result
in issued patents upon which our product candidates or proprietary technologies infringe. Similarly, there may be issued patents relevant
to our product candidates of which we are not aware. These patents could reduce the value of the product candidates we develop or, to
the extent they cover key technologies on which we have unknowingly relied, require that we seek to obtain licenses or cease using the
technology, no matter how valuable to our business. We may not be able to obtain such a license on commercially reasonable terms. There
is a substantial amount of litigation involving patent and other intellectual property rights in the biotechnology industry generally.
If any third party patent or patent application covers our intellectual property or proprietary rights and we are not able to obtain a
license to it, we and our collaborators may be prevented from commercializing products containing our discoveries.
As the biotechnology industry continues to develop, we may become
party to, or threatened with, litigation or other adverse proceedings regarding intellectual property or proprietary rights in our technology,
processes or product candidates. Third parties may assert claims based on existing or future intellectual property rights and the outcome
of any proceedings is subject to uncertainties that cannot be adequately quantified in advance. Any litigation proceedings could be costly
and time consuming and negative outcomes could result in liability for monetary damages, including treble damages and attorneys’
fees if we are found to have willfully infringed a patent. There is also no guarantee that we would be able to obtain a license under
such infringed intellectual property on commercially reasonable terms or at all. A finding of infringement could prevent us or our collaborators
from developing, marketing or selling a product candidate or force us to cease some or all of our business operations. Even if we are
successful in these proceedings, we may incur substantial costs and the time and attention of our management and scientific personnel
may be diverted as a result of these proceedings, which could have a material adverse effect on us. Claims that we have misappropriated
the confidential information or trade secrets of third parties could similarly have a negative impact on our business.
We may be required to pay royalties to employees
who develop inventions that have been or will be commercialized by us, even if the rights to such inventions have been assigned to us
and the employees have waived their rights to royalties or other additional compensation.
A significant portion of our intellectual property has been developed
by our employees in the course of their employment for us. Under the Israeli Patent Law, 5727-1967, or the Patent Law, inventions conceived
by an employee in the course and as a result of or arising from his or her employment with a company are regarded as “service inventions,”
which belong to the employer, absent a specific agreement between the employee and employer giving the employee proprietary rights. The
Patent Law also provides under Section 134 that if there is no agreement between an employer and an employee as to whether the employee
is entitled to consideration for service inventions, and to what extent and under which conditions, the Israeli Compensation and Royalties
Committee, or the Committee, a body constituted under the Patent Law, shall determine these issues. Section 135 of the Patent Law provides
criteria for assisting the Committee in making its decisions. According to decisions of the Committee, an employee’s right to receive
consideration for service inventions is a personal right and is entirely separate from the proprietary rights in such invention. Therefore,
this right must be explicitly waived by the employee. A decision handed down in May 2014 by the Committee clarifies that the right to
receive consideration under Section 134 can be waived and that such waiver can be made orally, in writing or by behavior like any other
contract. The Committee will examine, on a case by case basis, the general contractual framework between the parties, using interpretation
rules of the general Israeli contract laws. Further, the Committee has not yet determined one specific formula for calculating this remuneration,
nor the criteria or circumstances under which an employee’s waiver of his right to remuneration will be disregarded. Similarly,
it remains unclear whether waivers by employees in their employment agreements of the alleged right to receive consideration for service
inventions should be declared as void being a depriving provision in a standard contract. All of our employees execute invention assignment
agreements upon commencement of employment, in which they assign their rights to potential inventions and acknowledge that they will not
be entitled to additional compensation or royalties from commercialization of inventions. Although our employees have agreed to assign
to us service invention rights and have specifically waived their right to receive any special remuneration for such service inventions
beyond their regular salary and benefits, we may face claims demanding remuneration in consideration for assigned inventions.
Changes in U.S. patent law could diminish the
value of patents in general, thereby impairing our ability to protect our product candidates.
As is the case with other biotechnology companies, our success
is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing biotechnology patents involves technological
and legal complexity, and is costly, time consuming, and inherently uncertain. In addition, the U.S. Supreme Court has ruled on several
patent cases, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners
in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination
of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the
federal courts, and the U.S. Patent and Trademark Office, the laws and regulations governing patents could change in unpredictable ways
that may weaken or undermine our ability to obtain new patents or to enforce our existing patents and patents we might obtain in the future.
Our employment agreements with our employees
and other agreements with our collaborators and third parties may not adequately prevent disclosure of trade secrets, know-how and other
proprietary information.
A substantial portion of our technologies and intellectual property
is protected by trade secret laws. We rely on a combination of patent and other intellectual property laws as well as our employment agreements
with our employees and other agreements with our collaborators and third parties to protect and otherwise seek to control access to, and
distribution of, our proprietary information. These measures may not prevent disclosure, infringement or misappropriation of our confidential
information. Our confidentiality, nondisclosure and assignment agreements or covenants may be breached, and we may not have adequate remedies
for such a breach that would effectively prevent the further dissemination of our confidential information. We have limited control over
the protection of trade secrets used by our collaborators and could lose future trade secret protection if any unauthorized disclosure
of such information occurs. In addition, others may independently discover our trade secrets and proprietary information, and in such
cases we could not assert any trade secret rights against such parties. Laws regarding trade secret rights in certain markets where we
operate may afford little or no protection of our trade secrets. Failure to obtain or maintain trade secret protection could adversely
affect our business, sales and competitive position.
We may not be able to fully enforce covenants
not to compete with our key employees, and therefore we may be unable to prevent our competitors from benefiting from the expertise of
such employees.
Our employment agreements with key employees, which include executive
officers, contain non-compete provisions. These provisions prohibit our key employees, if they cease working for us, from competing directly
with us or working for our competitors for one year. Under applicable U.S. and Israeli laws, we may be unable to enforce these provisions.
If we cannot enforce the non-compete provisions with our key employees, we may be unable to prevent our competitors from benefiting from
the expertise of such employees. Even if these provisions are enforceable, they may not adequately protect our interests. The defection
of one or more of our employees to a competitor could materially adversely affect our business, results of operations and ability to capitalize
on our proprietary information.
Risks Relating to Our Incorporation and Location in Israel
Conditions in Israel could adversely affect
our business.
We are incorporated under Israeli law and our principal offices
and research and development facilities are located in Israel. Accordingly, political, economic and military conditions in Israel directly
affect our business. Since the State of Israel was established in 1948, a number of armed conflicts have occurred between Israel and its
Arab neighbors. In recent years there has been an increase in unrest and terrorist activity, and several times since 2005 (when Israel
withdrew from the Gaza Strip) conflicts arose due to Hamas’ rocket attacks against Israeli civilian targets, during which Israel
responded to rocket attacks by engaging in an armed conflict with Hamas in the Gaza Strip. Our principal place of business is located
in Rehovot, Israel, which is approximately 30 miles from the nearest point of the border with the Gaza Strip. There can be no assurance
that attacks launched from the Gaza Strip will not reach our facilities, or that hostilities will not otherwise cause a significant disruption
to our operations, such as preventing our employees from reaching our facilities and limiting our ability to monitor and otherwise conduct
the crop and other experiments we conduct at the facilities.
Several countries still restrict doing business with Israel and
Israeli companies, and additional countries may impose restrictions on doing business with Israel and Israeli companies if hostilities
in Israel or political instability in the region continues or increases. These restrictions may limit materially our ability to sell our
product candidates to companies in these countries. Any hostilities involving Israel or the interruption or curtailment of trade between
Israel and its present trading partners, or significant downturn in the economic or financial condition of Israel, could adversely affect
our operations and research and development, cause our revenues to decrease and adversely affect the share price of publicly traded companies
having operations in Israel, such as ours. Further, the political and security situation in Israel may result in parties with whom we
have agreements involving performance in Israel claiming that they are not obligated to perform their commitments under those agreements
pursuant to force majeure provisions in such agreements. In addition, there have been increased efforts by activists to cause companies
and consumers to boycott Israeli goods for political reasons. Such actions, particularly if they become more widespread, may adversely
impact our ability to conduct business.
Furthermore, our business insurance does not cover losses that
may occur as a result of events associated with the security situation in the Middle East. Although the Israeli government currently covers
the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure that this government coverage
will be maintained. Any losses or damages incurred by us could have a material adverse effect on our business and financial condition.
Our operations may be disrupted by the obligations
of personnel to perform military service.
Many Israeli citizens are obligated to perform several days, and
in some cases more, of annual military reserve duty each year until they reach the age of 40 (or older, for reservists who are military
officers or who hold certain military positions) and, in the event of a military conflict, may be called to active duty. In response to
increases in terrorist activity, there have been periods of significant call-ups of military reservists. It is possible that there will
be military reserve duty call-ups in the future. Our operations could be disrupted by such call-ups, which may include the call-up of
our key employees and members of our management. Such disruption could materially adversely affect our business, financial condition and
results of operations.
Exchange rate fluctuations between the U.S.
dollar and the NIS may negatively affect our financial results.
The Company’s reporting currency is U.S. dollars. In view
that a substantial part of our expenses is in NIS, any appreciation of the NIS relative to the U.S. dollar would adversely impact our
financial results. The appreciation of the NIS in relation to the U.S. dollar amounted to 3.3%, 7.0% and 7.8% for the years ended December
31, 2021, 2020 and 2019, respectively. If we enter into hedging contracts in the future, we may be unsuccessful in protecting against
currency exchange rate fluctuations. See “Item 11. Quantitative and Qualitative Disclosure About Market Risk—Foreign Currency
Risk.”
Interest rate fluctuations may devalue our
investments and could have an adverse impact on our financial condition.
From time to time we hold corporate bonds and government treasury
notes denominated in New Israeli Shekels and in U.S. dollars. These investments expose us to the risk of interest rate fluctuations. An
increase in Israeli or in U.S. interest rates could cause the fair value of these investments to decrease.
We have received Israeli government grants
for certain of our research and development activities. The terms of these grants may require us to satisfy specified conditions in order
to manufacture products and transfer technologies supported by such grants outside of Israel. In addition, in some circumstances, we may
be required to pay penalties in addition to repaying the grants.
Our research and development operations have been partly financed
through certain governmental grants. Certain of these grants are royalty-bearing grants under the terms of which we are committed
to pay royalties at a rate of 3.0% on sales proceeds from our products that were developed under Israeli National Authority for Technological
Innovation, or the IIA, programs up to the total amount of grants received, linked to the U.S. dollar and bearing interest at an annual
rate of LIBOR applicable to U.S. dollar deposits. In September 2021, the Bank of Israel, which determines annual interest rates, published
a directive which stated that annual interest at a variable rate linked to the LIBOR rate for loans in U.S. dollars will be replaced by
the Secured Overnight Financing Rate, or the SOFR, in June 2023. While it is not currently possible to determine precisely whether, or
to what extent, the replacement of LIBOR with SOFR would affect us, the implementation of SOFR may increase our financial liabilities
to the IIA. Management continues to monitor the status and discussions regarding SOFR. We are not yet able to reasonably estimate
the expected impact.
In addition, these Israeli governmental grants impose certain restrictions
on the transfer outside of Israel of the underlying know-how and the manufacturing or manufacturing rights of the underlying products
and technologies. As of December 31, 2021, we had received from the IIA approximately $8.6 million (including accrued interest). We may
not receive the required approvals should we wish to transfer the know-how, technology or manufacturing rights related to such government
grants outside of Israel in the future or, if we receive such required approvals, they may be subject to certain conditions and payment
obligations. See “Item 5. Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Government
Grants.”
It may be difficult to enforce a U.S. judgment
against us, our officers and directors and the Israeli experts named in this annual report in Israel or the United States, or to assert
U.S. securities laws claims in Israel or serve process on our officers and directors and these experts.
We are incorporated in Israel. The majority of our directors and
executive officers reside outside the United States and the majority of our assets are located outside the United States. Therefore, it
may be difficult for an investor, or any other person or entity, to enforce a U.S. court judgment based upon the civil liability provisions
of the U.S. federal securities laws against us or any of these persons in a U.S. or Israeli court, or to effect service of process upon
these persons in the United States. Additionally, it may be difficult for an investor, or any other person or entity, to assert U.S. securities
law claims in original actions instituted in Israel. Israeli courts may refuse to hear a claim based on a violation of U.S. securities
laws on the grounds that Israel is not the most appropriate forum in which to bring such a claim. Even if an Israeli court agrees to hear
a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content
of applicable U.S. law must be proved as a fact, which can be a time-consuming and costly process. Certain matters of procedure will also
be governed by Israeli law. There is little binding case law in Israel addressing the matters described above.
Your rights and responsibilities as our shareholder
will be governed by Israeli law, which may differ in some respects from the rights and responsibilities of shareholders of U.S. corporations.
Since we are incorporated under Israeli law, the rights and responsibilities
of our shareholders are governed by Israeli law and by our articles of association. These rights and responsibilities differ in some respects
from the rights and responsibilities of shareholders of U.S.-based corporations. In particular, a shareholder of an Israeli company has
a duty to act in good faith and in a customary manner in exercising its rights and performing its obligations towards the company and
other shareholders and to refrain from abusing its power in the company, including, among other things, in voting at the general meeting
of shareholders on certain matters, such as an amendment to the company’s articles of association, an increase of the company’s
authorized share capital, a merger of the company and approval of related party transactions that require shareholder approval. A shareholder
also has a general duty to refrain from discriminating against other shareholders. In addition, a controlling shareholder or a shareholder
who knows that it possesses the power to determine the outcome of a shareholders’ vote or to appoint or prevent the appointment
of an office holder in the company has a duty to act in fairness towards the company. However, Israeli law does not define the substance
of this duty of fairness. See “Item 6. Directors, Senior Management and Employees—C. Board Practices—Shareholder Duties.”
Since Israeli corporate law underwent extensive revisions approximately 19 years ago, the parameters and implications of the provisions
that govern shareholder behavior have not been clearly determined. These provisions may be interpreted to impose additional obligations
and liabilities on our shareholders that are not typically imposed on shareholders of U.S. corporations.
Provisions of Israeli law may delay, prevent
or make undesirable an acquisition of all or a significant portion of our shares or assets.
Certain provisions of Israeli law and our articles of association
could have the effect of delaying or preventing a change in control and may make it more difficult for a third party to acquire us or
for our shareholders to elect different individuals to our board of directors, even if doing so would be beneficial to our shareholders,
and may limit the price that investors may be willing to pay in the future for our ordinary shares. For example, Israeli corporate law
regulates mergers and requires that a tender offer be effected when certain thresholds of percentage ownership of voting power in a company
are exceeded (subject to certain conditions). Further, Israeli tax considerations may make potential transactions undesirable to us or
to some of our shareholders whose country of residence does not have a tax treaty with Israel granting tax relief to such shareholders
from Israeli tax. With respect to mergers, Israeli tax law allows for tax deferral in certain circumstances but makes the deferral contingent
on the fulfillment of numerous conditions, including a holding period of two years from the date of the transaction during which certain
sales and dispositions of shares of the participating companies are restricted. Moreover, with respect to certain share swap transactions,
the tax deferral is limited in time, and when such time expires, the tax becomes payable even if no actual disposition of the shares has
occurred. See Exhibit 2.1 to this annual report.
Furthermore, under the Encouragement of Research, Development and
Technological Innovation in the Industry Law, 5744-1984 (formerly known as the Law for the Encouragement of Research and Development in
Industry 5744-1984), and the regulations, guidelines, rules, procedures and benefit tracks thereunder, collectively, the Innovation Law,
to which we are subject due to our receipt of grants from the IIA, a recipient of IIA grants such as our company must report to the IIA
regarding any change in the holding of any means of control of our company which transforms any non-Israeli citizen or resident into an
“interested party”, as defined in the Israeli Securities Law 5728-1968, and that such non-Israeli citizen or resident shall
execute an undertaking in favor of IIA, in a form prescribed by IIA.
Risks Related to Our Ordinary Shares and the Ownership and Trading
of Our Ordinary Shares
The price of our ordinary shares may fluctuate
significantly.
Our ordinary shares were first offered publicly in the United States
after our public offering in the United States in November 2013, at a price of $14.75 per share, and our ordinary shares have subsequently
traded on the NYSE (until December 2016) and on the Nasdaq (since December 2016) as high as $19.80 per share and as low as $0.75 and as
of March 30, 2022 were trading at $1.36 per share.
The market price of our ordinary shares could be highly volatile
and may fluctuate substantially as a result of many factors, including:
|
◾ |
our inability to obtain additional funding |
|
◾ |
any delay in filing a regulatory submission for any of our product or product candidates and any adverse development or perceived
adverse development with respect to the review of that regulatory submission by the applicable regulatory body |
|
◾ |
actual or anticipated fluctuations in our results of operations; |
|
◾ |
variance in our financial performance from the expectations of market analysts; |
|
◾ |
announcements by us or our competitors of significant business developments, changes in relationships with our collaborators, acquisitions
or expansion plans; |
|
◾ |
our involvement in litigation; |
|
◾ |
our sale, or the sale by our significant shareholders, of ordinary shares or other securities in the future; |
|
◾ |
failure to publish research or the publishing of inaccurate or unfavorable research; |
|
◾ |
market conditions in our industry and changes in estimates of the future size and growth rate of our markets; |
|
◾ |
changes in key personnel; |
|
◾ |
the trading volume of our ordinary shares; and |
|
◾ |
general economic and market conditions, including as a result of the scope and duration of the COVID-19 pandemic. |
Although our ordinary shares are listed on Nasdaq, an active trading
market on Nasdaq for our ordinary shares may not be sustained. If an active market for our ordinary shares is not sustained, it may be
difficult to sell ordinary shares in the U.S.
In addition, the stock markets have recently experienced extreme
price and volume fluctuations, including as a result of the COVID-19 pandemic. Broad market and industry factors may materially harm the
market price of our ordinary shares, regardless of our operating performance. In the past, following periods of volatility in the market
price of a company’s securities, securities class action litigation has often been instituted against that company. If we were involved
in any similar litigation, we could incur substantial costs and our management’s attention and resources could be diverted.
Our ordinary shares are traded on more than
one market and this may result in price variations.
Our ordinary shares have been traded on the TASE since 2007, and
are currently listed on Nasdaq. Trading in our ordinary shares on these markets will take place in different currencies (U.S. dollars
on Nasdaq and NIS on the TASE), and at different times (resulting from different time zones, trading days and public holidays in the United
States and Israel). The trading prices of our ordinary shares on these two markets may differ due to these and other factors. Any decrease
in the price of our ordinary shares on the TASE could cause a decrease in the trading price of our ordinary shares on Nasdaq or vice versa.
We could become subject to parallel reporting
obligations in Israel and the United States, which could increase compliance costs and divert management attention.
On July 28, 2013, our shareholders approved our plan to transition
solely to U.S. reporting standards under the rules and regulations of the SEC. However, should this change in the future, we may become
subject to parallel reporting obligations in Israel and the United States. While similar in many respects, certain differences between
Israeli and U.S. reporting schemes may impose on us disclosure obligations that are more stringent than those generally applied to foreign
private issuers whose securities are listed only in the United States. In addition, a requirement to comply with the separate reporting
obligations under U.S. and Israeli securities laws would require additional management attention and could burden us with additional costs.
The requirements of being a public company
in the United States and Israel may strain our resources and distract our management, which could make it difficult to manage our business.
Changing laws, regulations and standards, in the United States
or Israel, relating to corporate governance and public disclosure and other matters, may be implemented in the future, which may increase
our legal and financial compliance costs, make some activities more time consuming and divert management’s time and attention from
revenue-generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ
from the activities intended by regulatory or governing bodies due to ambiguities related to practice, regulatory authorities may initiate
legal proceedings against us and our business may be harmed. Being a publicly traded company in the United States and Israel and being
subject to U.S. and Israeli rules and regulations make it more expensive for us to obtain DO insurance, and we may be required to
accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us
to attract and retain qualified members of our board of directors, particularly to serve on our audit committee, and qualified executive
officers.
As a public company whose ordinary shares are listed in the United
States, we will continue to incur significant accounting, legal and other expenses, including costs associated with our reporting requirements
under the Exchange Act. We also incur additional costs associated with corporate governance requirements, including requirements under
Section 404 and other provisions of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, rules implemented by the SEC and the Nasdaq,
and provisions of Israeli corporate and securities laws applicable to public companies. The Exchange Act requires that we file annual
and certain other reports with respect to our business and financial condition. The Sarbanes-Oxley Act requires, among other things, that
we maintain effective disclosure controls and procedures and internal control over financial reporting. These rules and regulations could
continue to increase our legal and financial compliance costs, such as the cost of hiring consultants or testing compliance processes,
and make some activities more time-consuming and costly. These activities may divert management’s attention from other business
concerns, which could have a material adverse effect on our business, financial condition, results of operations and cash flows.
As a foreign private issuer we are not subject
to the provisions of Regulation FD or U.S. proxy rules and are exempt from filing certain Exchange Act reports.
As a foreign private issuer, we are exempt from compliance with
the rules and regulations under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors,
and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the
Exchange Act. In addition, we are not required under the Exchange Act to file annual and certain other reports and financial statements
with the SEC as frequently or as promptly as U.S. domestic companies whose securities are registered under the Exchange Act, we are permitted
to disclose limited compensation information for our executive officers on an individual basis and we are generally exempt from filing
quarterly reports with the SEC under the Exchange Act. Moreover, we are not required to comply with Regulation FD, which restricts the
selective disclosure of material nonpublic information to, among others, broker-dealers and holders of a company’s securities under
circumstances in which it is reasonably foreseeable that the holder will trade in the company’s securities on the basis of the information. These
exemptions and leniencies will reduce the frequency and scope of information and protections to which you may otherwise have been eligible
in relation to a U.S. domestic issuer.
As a foreign private issuer, we have elected
to follow home country corporate governance practices instead of certain Nasdaq corporate governance requirements, which may result in
less protection than is accorded to investors under rules applicable to domestic U.S. issuers.
As a foreign private issuer whose shares are listed on the Nasdaq
Global Market, we are permitted to follow certain home country corporate governance practices instead of those otherwise required under
the corporate governance standards for U.S. domestic issuers listed on Nasdaq. We currently follow Israeli home country practices, rather
than the requirements under the Nasdaq corporate governance rules, with regard to the (i) quorum requirement for shareholder meetings,
(ii) executive sessions for independent directors and non-management directors and (iii) the requirements to obtain shareholder approval
for certain dilutive events (such as for the establishment or amendment of certain equity-based compensation plans, issuances that will
result in a change of control of the company, certain transactions other than a public offering involving issuances of a 20% or more interest
in the company and certain acquisitions of the stock or assets of another company). See “Item 16G. Corporate Governance.”
Furthermore, we may in the future elect to follow Israeli home country practices with regard to other matters such as the requirement
to have a majority independent board of directors, have a compensation committee and have a nominating committee. Accordingly, our shareholders
may not be afforded the same protection as provided under Nasdaq corporate governance rules. Following our home country governance practices
as opposed to the requirements that would otherwise apply to a United States company listed on Nasdaq may provide less protection than
is accorded to investors of domestic issuers. For further discussion, see “Item 16G. Corporate Governance.”
We may lose our status as a foreign private
issuer, which would increase our compliance costs and could thereby negatively impact our results of operations.
We would lose our foreign private issuer status if (a) a majority
of our outstanding voting securities were either directly or indirectly owned of record by residents of the United States and (b)(i) a
majority of our executive officers or directors were United States citizens or residents, (ii) more than 50 percent of our assets were
located in the United States, or (iii) our business were administered principally outside the United States. Our loss of foreign private
issuer status would make U.S. regulatory provisions mandatory. The regulatory and compliance costs to us under U.S. securities laws as
a U.S. domestic issuer may be significantly higher. If we are not a foreign private issuer, we will be required to file periodic reports
and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available
to a foreign private issuer. We would also be required to follow U.S. proxy disclosure requirements, including the requirement to disclose,
under U.S. law, more detailed information about the compensation of our senior executive officers on an individual basis. We may also
be required to modify certain of our policies to comply with accepted governance practices associated with U.S. domestic issuers. Such
conversion and modifications will involve additional costs. In addition, we would lose our ability to rely upon exemptions from certain
corporate governance requirements on U.S. stock exchanges that are available to foreign private issuers, as described in the previous
risk factor above.
If a United States person is treated as owning
at least 10% of our ordinary shares, such holder may be subject to adverse U.S. federal income tax consequences.
If a United States person is treated as owning (directly, indirectly
or constructively) at least 10% of the value or voting power of our ordinary shares, such person may be treated as a “United States
shareholder” with respect to each “controlled foreign corporation” in our group (if any). If our group includes one
or more U.S. subsidiaries, certain of our non-U.S. subsidiaries could be treated as controlled foreign corporations (regardless of whether
we are or are not treated as a controlled foreign corporation). A United States shareholder of a controlled foreign corporation may be
required to annually report and include in its U.S. taxable income its pro rata share of “Subpart F income”, “global
intangible low-taxed income” and investments in U.S. property by controlled foreign corporations, whether or not we make any distributions.
An individual that is a United States shareholder with respect to a controlled foreign corporation generally would not be allowed certain
tax deductions or foreign tax credits that would be allowed to a United States shareholder that is a U.S. corporation. A failure to comply
with these reporting obligations may subject you to significant monetary penalties and may prevent the statute of limitations with respect
to your U.S. federal income tax return for the year for which reporting was due from starting. We cannot provide any assurances that we
will assist investors in determining whether any of our non-U.S. subsidiaries are treated as a controlled foreign corporation or whether
such investor is treated as a United States shareholder with respect to any of such controlled foreign corporations or furnish to any
United States shareholders information that may be necessary to comply with the aforementioned reporting and tax paying obligations. A
United States investor should consult their own advisors regarding the potential application of these rules to its investment in the ordinary
shares.
We believe we were a PFIC for U.S. federal
income tax purposes in 2021, and there is significant risk we will be a PFIC in 2022 as well. U.S. shareholders who held our ordinary
shares at any time during a taxable year in which we are a PFIC may suffer adverse tax consequences.
Generally, if for any taxable year 75% or more of our gross income
is passive income, or at least 50% of the average quarterly value of our assets (which may be determined in part by the market value of
our ordinary shares, which is subject to change) are held for the production of, or produce, passive income, we would be characterized
as a passive foreign investment company, or PFIC, for United States federal income tax purposes. According to these rules, a publicly
traded non-U.S. corporation may treat the aggregate fair market value of its assets as being equal to the sum of the aggregate value of
its outstanding shares, or Market Capitalization, and the total amount of its liabilities. We intend to take the position that the excess
of our Market Capitalization plus liabilities over the book value of all of our assets may generally be treated as attributable to non-passive
assets. Based on the book value of our assets and liabilities and our Market Capitalization in 2021, we believe that we met the PFIC asset
test described above for 2021 and, as a result, we were classified as a PFIC in 2021. Furthermore, because we currently hold, and expect
to continue to hold, a substantial amount of cash and cash equivalents and other passive assets used in our business, and because our
Market Capitalization is currently below the level necessary to avoid PFIC status for 2022, there is substantial risk we will be classified
as a PFIC for the 2022 taxable year as well. However, because PFIC status is determined after the close of each taxable year, we will
not be able to determine whether we will be a PFIC for the 2022 taxable year or for any future taxable year until after the close of such
year.
U.S. shareholders who held our ordinary shares at any time in 2021
or during any other taxable year in which we are a PFIC may suffer adverse tax consequences, including having gains realized on the sale
of our ordinary shares treated as ordinary income, rather than capital gain, the loss of the preferential rate applicable to dividends
received on our ordinary shares by individuals who are U.S. Holders (as defined in “Item 10. Additional Information—E. Taxation—United
States Federal Income Taxation”), and having interest charges apply to distributions by us and the proceeds of share sales. Certain
elections may be available that would alleviate some of the adverse consequences of PFIC status and result in an alternative treatment
(such as mark-to-market treatment) of our ordinary shares; however, we do not intend to provide the information necessary for U.S. holders
to make qualified electing fund elections. See “Item 10. Additional Information—E. Taxation—United States Federal Income
Taxation—Passive Foreign Investment Company Considerations.”
General Risk Factors
Any inability to meet the Nasdaq listing requirements
may have an adverse effect on our share price and lead to our delisting from Nasdaq.
We are required to meet the continued listing requirements of Nasdaq,
including those regarding minimum share price. In particular, we are required to maintain a minimum bid price for our listed ordinary
shares of $1.00 per share. If we do not meet Nasdaq’s continued listing requirements, Nasdaq could initiate delisting proceedings
and our ordinary shares could be delisted.
If Nasdaq initiates delisting proceedings or delists our ordinary
shares from trading on its exchange, we could face significant material adverse consequences including: reduced liquidity with respect
to our ordinary shares; limited amount of news and analyst coverage for our company; reputational damage; diminished investor, supplier
and employee confidence; and decreased ability to issue additional securities or obtain additional financing in the future.
If we fail to maintain effective internal control
over financial reporting, the price of our ordinary shares may be adversely affected.
Our internal control over financial reporting may have weaknesses and conditions that
could require correction or remediation, the disclosure of which may have an adverse impact on the price of our ordinary shares. We
are required to establish and maintain appropriate internal control over financial reporting. Failure to establish those controls,
or any failure of those controls once established, could adversely affect our public disclosures regarding our business, prospects, financial
condition or results of operations. In addition, management’s assessment of internal control over financial reporting may identify
weaknesses and conditions that need to be addressed in our internal control over financial reporting or other matters that may raise concerns
for investors. In addition, as a “non-accelerated filer,” we are exempt from the provisions of Section 404(b) of
the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness
of internal control over financial reporting. Decreased disclosures in our SEC filings due to our status as a “non-accelerated
filer” may make it harder for investors to analyze our results of operations and financial prospects and may make our ordinary shares
a less attractive investment. Any actual or perceived weaknesses and conditions that need to be addressed in our internal control over
financial reporting or disclosure of management’s assessment of our internal control over financial reporting may have an adverse
impact on the price of our ordinary shares.
| ITEM 4. |
INFORMATION ON THE COMPANY |
A. History
and Development of the Company
Our History
We are a leading computational biology company aiming to revolutionize
life-science product discovery and development across several market segments, including human health, agriculture, and other industrial
applications.
Our company was founded on October 10, 1999 as Agro Leads Ltd.,
a subsidiary of Compugen Ltd. In 2002, our company was spun-off as an independent corporation under the laws of the State of Israel, and
changed its name to Evogene Ltd.
In 2018 and 2019, we reorganized certain of our divisions into
wholly owned subsidiaries of the Company, as described in this annual report.
Our shares have been listed for trading on the TASE since 2007
and were listed for trading on the NYSE commencing with our U.S. initial public offering in November 2013, until December 2016, when we
transferred the listing to Nasdaq.
We are registered with the Israeli Registrar of Companies in Jerusalem.
Our registration number is 51-283872-3. Our purpose as set forth in our articles of association is to engage in any lawful business. Our
principal executive offices are located at 13 Gad Feinstein Street, Park Rehovot, Rehovot P.O. Box 4173 Ness Ziona, 7414002, Israel, and
our telephone number is +972-8-931-1900.
Our authorized representative in the United States and agent for
service of process in the United States, Puglisi Associates, is located at 850 Library Avenue, Suite 204, Newark, Delaware 19711.
Information contained on, or that can be accessed through, our website does not constitute a part of this annual report and is not incorporated
by reference herein.
The SEC maintains an internet site, http://www.sec.gov that contains
reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. Our internet
address is www.evogene.com. Neither such internet addresses are a part of this annual report.
Principal Capital Expenditures
Our capital expenditures for fiscal years 2021, 2020 and 2019 amounted
to $0.8 million, $0.7 million and $0.9 million, respectively. Our capital expenditures during those years consisted of investments in
property, plant and equipment. We anticipate our capital expenditures in fiscal year 2022 to include payments for maintenance and improvements
of our facilities in Israel in order to support our activities, which we anticipate we will finance with our currently available cash.
For a description of our principal capital expenditures and divestitures for the three years ended December 31, 2021 and for those currently
in progress, see Item 5. “Operating and Financial Review and Prospects—Liquidity and Capital Resources.”
B. Business
Overview
Overview
We are a leading computational biology company aiming to revolutionize
life-science product development across several market segments, including human health, agriculture, and other industries, by utilizing
cutting edge computational technologies.
The main challenge in product development in the life science industry
is finding the winning candidates out of a vast number of possible prospects that address a complex myriad of criteria to reach successful
products. We believe that by utilizing an advanced computational biology platform to identify the most promising candidates addressing
multiple development challenges towards successful life-science products, we can increase the probability of success while reducing time
and cost.
To achieve this mission, we established our unique Computational
Predictive Biology, or CPB, platform, leveraging big data and artificial intelligence and incorporating deep multidisciplinary understanding
in life sciences. The CPB platform is the basis for three technology engines, each focused on the direction and acceleration of the discovery
and development of products based on one of the following core components: Microbes – MicroBoost
AI, Small molecules – ChemPass AI, Genetic elements – GeneRator
AI.
We use our technological engines to support the development of
products for the life science industry through dedicated subsidiaries and with strategic partners. Currently, our main subsidiaries utilize
the technological engines to develop human microbiome-based therapeutics by Biomica, medical cannabis products by Canonic, ag-chemicals
by AgPlenus and ag-biologicals by Lavie Bio.
Business Model
Since 2015, the Company’s main business model is product
development through specific market-oriented divisions. At that time, Evogene began using its technology to develop its own product pipelines,
each focusing on a specific industrial segment. When such a division and product pipeline reach a certain level of maturity, the activity
is spun into a dedicated subsidiary. Each subsidiary focuses on continued development of its pipeline and adding new products to
its commercial offering, while using Evogene's technology as its core competitive advantage.
Currently, Evogene has four main subsidiaries, each focused on
a different type of product and target market. Each subsidiary has its own board of directors, management team, scientific advisory board,
research and development, or RD, and business development teams that focus on developing its own pipeline and go-to-market activities.
At the same time, each subsidiary benefits from using Evogene’s technology under an exclusive license from Evogene to use the CPB
platform’s discovery and development engines that are relevant to the subsidiary’s field of activity. The terms of these licenses
provide that the subsidiary owns the discoveries and product candidates that result from the utilization of the CPB platform, while Evogene
retains all rights to the CPB platform itself. According to the characteristics of the end-market, the subsidiaries can decide to commercialize
their products independently or in collaboration with partners.
Another business model, which was our main business model until
2014, is product development through collaborations. In this business model Evogene engages with partners for joint development of defined
products, requested by the partners. In this frame, Evogene typically conducts the initial RD activity, discovery and early-stage
development, while later stage development and commercialization are carried out by the partner. Under this model, Evogene’s potential
revenues include RD funding for activities that Evogene conducts in the collaboration, milestone payments for when the candidates
advance in our partners’ pipelines and revenue sharing from the end-product.
Until 2014, Evogene engaged in several collaborations of this type
with Bayer, Monsanto, DuPont and Syngenta, focused on improving seed traits using genetic modification, or GM, approach. Today, Evogene
has a number of smaller scale collaborations, and we aim to engage in additional collaborations in the future.
Fields of Activity
Given the broadly applicable capabilities of our technology, as
provided through our three engines, we can potentially enhance and improve product development in a variety of life science industries,
including human health and agriculture. Today, Evogene is applying its MicroBoost AI engine to direct and accelerate the discovery and
development of two types of products: human-microbiome based therapeutics in human health and ag-biological products in agriculture.
The ChemPass AI engine is used for the discovery and development of two types of products: drugs based on small molecules in human
health and ag-chemicals, such as herbicides and insecticides, in agriculture. The GeneRator AI engine is mainly applied for the discovery
and development of medical cannabis products in human health and improved seed traits in agriculture.
Evogene continuously evaluates new substantial industries with
well-recognized development roadblocks for which we can leverage our capabilities and assets for the development of next-generation products.
We will select the most suitable markets to focus on, based on a number of criteria, including: (i) market size; (ii) a well-recognized,
unmet need for next-generation products; (iii) an understanding of the scientific or technical road-blocks that challenge others from
developing next-generation products; and (iv) most importantly, the expectation that our technological engines and unique approach can
provide a significant competitive advantage in addressing these road-blocks.
Subsidiaries
As described above, since 2015 Evogene has used its three engines
to develop diverse product types through dedicated divisions and subsidiaries. In the area of human health, we established two subsidiaries:
Biomica, focusing on developing microbiome-based therapeutics, and Canonic, focusing on developing medical cannabis products. In Agriculture,
we established two subsidiaries: Lavie Bio focusing on developing ag-biologicals and AgPlenus focusing on ag-chemicals. In other industries,
we established one subsidiary, Casterra, focusing on developing ag-solutions for castor oil production.
Revenues
During 2021, except for initial sales of medical Cannabis products
by Canonic and seed sales by Casterra, our revenues consisted primarily of RD payments under various RD collaborations we and
our subsidiaries are engaged in, mainly in the fields of seed traits, ag-biologicals and ag-chemicals. A breakdown of our revenues by
business activity and geographic markets for each of the last three financial years is provided in “Item 5. Operating and Financial
Review and Prospects—Key Performance Indicators—Revenues.” In the future, we expect that we and our subsidiaries will
receive milestone payments and royalty revenues under such collaborations, as well as revenues from the sale of end-products or commercialization
of product candidates.
In 2022, through our subsidiaries or directly, we expect to continue
to develop our product pipelines and initiate new collaborations with an increased focus on strategic relationships for joint product
development. We also expect to continue to evolve our organization and to continue to examine new areas in which our technology engines
can serve as a competitive advantage and additional value can be created in a relatively short period of time.
Technology
Technology highlight
Our CPB platform aims to disrupt conventional life science product
discovery and development methodology, currently challenged by inefficiencies, such as long and expensive product development process
and low probability of success. By computational selection of the most relevant core components for life-science products, such as microbes,
small molecules and genes, and then computational optimization, we are aiming to reduce time, cost and most importantly increase the probability
of success to develop life-science based products. We provide these discovery and development capabilities through three dedicated engines:
MicroBoost AI for products based on microbes, ChemPass AI for products based on small molecules and GeneRator AI for products based on
changes in genetic elements.
The discovery phase, based on product definition, requires the
identification and selection of a reasonable number of candidates to initiate the development process. The challenge is that out of a
vast number of possible product candidates and numerous criteria that these candidates must address, finding the winning combination for
a successful product is extremely complex. Evogene believes that this complexity should be addressed using computational predictive biology.
Evogene’s technology, the CPB platform and its three engines, was designed to predict the most promising candidates that hold true
potential for a successful product. Through computationally screening databases, according to specific product criteria, candidates can
be narrowed down to focus on those most promising.
In addition to the selection of the candidates in the discovery
phase, the CPB platform is also used in the development phase. In the development phase, the chosen candidates undergo various validation
processes on the way to becoming a commercial product with certain desired attributes. In this process, the candidates’ ability
to pass the validation criteria is improved, as required, by using our technology. Our technology is able to identify the best optimization
proposal for a product candidate, improving a specific attribute of a product with minimal impairment of any of the other attributes.
CPB Platform
As described above, the mission of the CPB platform is to revolutionize
the product discovery and development approach in life science industries by decoding the biological world using computational biology.
This platform is the outcome of over a decade long multidisciplinary effort to integrate scientific concepts with big data and advanced
computational analytics in order to develop predictions of potential product candidates that later undergo experimental validation and
optimization toward commercialization. We believe that the uniqueness of our computational prediction approach stems from our ability
to successfully address multiple product attributes at the beginning of the discovery process, and during the optimization phase.
These efforts have been enabled by two parallel revolutions taking
place over the last decades: first, the data revolution – allowing the creation of enormous amounts of high-quality biological and
chemical data in a cost-effective manner; and second, the computational processing revolution – allowing the analysis of data with
advanced algorithms such as machine learning and other artificial intelligence methods.
The CPB platform represents a revolutionary approach for the design
and prediction of novel products, based on four pillars: first, computationally modeling the specific biological challenges in the discovery
and development of each product into pre-defined criteria, based on profound scientific understanding and know-how; second, designing
genomic, chemical and microbial databases holding diverse types of curated data specifically aimed at addressing the biological challenges
identified; third, developing state of the art computational tailored analytics, including artificial intelligence algorithms, designed
to provide more accurate predictions to those challenges; and fourth, utilizing screening and validation systems, comprised of multiple
tailored bioassays, to validate the product candidates and assist in their optimization.
Proprietary Databases
Our databases leverage multiple types of tailored big data from
various sources in order to support the different research and development activities powered by our technological engines. Specifically,
we focus on four different entities: microbial organisms, microbial genes, small molecules and plant genes. Our databases on different
entities are rich and highly interconnected, enabling our analysis platforms to maximize their predictive power. Our databases draw in
part from the public domain, and in part compile increasing amounts of proprietary data, generated either in-house or received from our
collaborators.
Discovery and Development
Engines
The CPB platform is the foundation for Evogene’s three technological
engines boosting the discovery and development of novel life science products. At the core of our engines are unique computational analysis
platforms, which are comprised of algorithms designed to address a vast number of parameters required for a product. These computational
analysis platforms, which increasingly utilize artificial intelligence, machine learning driven approaches and other sophisticated algorithms,
are designed to deliver innovative solutions to key bottlenecks in the product development process, such as efficacy and stability. As
our predictions undergo validation via dedicated validation systems, we continuously improve our predictions by feeding back these results
into our systems.
MicroBoost AI employs an innovative function-based approach based
on a proprietary microbial function catalog for the identification of novel microbial candidates. This engine not only aims to identify
candidates with high potential for a specific product, but also pinpoints the biological reasoning behind its selection, improving the
chances of the initial microbial candidate to pass the subsequent optimization and development phases.
ChemPass AI combines a large, well-organized, database of over
20 billion known molecules as well as a set of AI-based algorithms and innovative chemo-informatics tools which invent, prioritize and
analyze new small molecules prior to their expensive synthesis and testing phase. This platform is used to drive and accelerate the small
molecule product development process by using a set of high-end, validated tools and algorithms for virtual screening for the identification
of small molecule hits meeting multiple end-product attributes.
GeneRator AI aims to develop life science products via targeting
and modifying genetic elements. By using a set of computational and advanced AI tools and end-to-end discovery and development pipelines,
GeneRator AI identifies genomic elements of interest that can be then applied through genome editing, genetic engineering, as biomarkers,
or through additional applications.
We are constantly working to improve and expand our engines capabilities.
For example, we are working to improve the GeneRator AI engine through participating in the CRISPR-IL consortium. This consortium, funded
by the Israeli Innovation Authority, or IIA, aims to develop an artificial intelligence-based system, “Go-Genome”, providing
users improved genome-editing workflows. The system aims to provide end-to-end solutions from user interface to an accurate measurement
tool and is expected to include the computational design of on-target DNA modification with minimal accidental, off-target modifications,
improve modification efficiency and provide an accurate measuring tool to ensure the desired modification was made.
Validation and screening
systems
Our experimental technologies include bioassays as well as screening
and validation pipelines (i.e., sets of bioassays organized in a cascade of tests). They relate to diverse scientific fields, including
molecular biology and biochemistry, microbiology, organic chemistry, plant tissue culture and plant pathology, in laboratories, greenhouses
and field settings. All processes are accompanied by precise data gathering and are coordinated by pipeline management and quality assurance.
Our validation and screening systems support three key aspects
of our research and development approach: first, generating data sets to enable development and proof of concept of tailored computational
modules and their prediction performance evaluation; second, transforming computational-based recommendation to a physical entity output;
and third, validating and screening selected product candidates by the relevant scientific teams.
Major Occurrences and Developments
The following are major occurrences and developments in the Company
during 2021 and until the date of this annual report, reflecting advancement in all areas of activity:
Evogene
|
◾ |
CRISPR-IL consortium (January 2021) – we announced a 2020 year-end update for the CRISPR-IL consortium. |
|
◾ |
At-the-market, or ATM, offering (February 2021) – we completed a $28 million ATM offering and entered into a new ATM offering
sales agreement for the sale of up to $50 million of our ordinary shares. |
|
◾ |
Appointment of new Chairperson of the board (June 2021), Ms. Sarit Firon, replacing Mr. Martin Gerstel. |
|
◾ |
CRISPR-IL consortium (November 2021) – we announced participation in the second research period of the CRISPR-IL consortium.
|
Lavie Bio
|
◾ |
Go-to-Market (September 2021) – Lavie Bio and United Agronomy signed a distribution agreement for Lavie Bio’s inoculant
product. |
|
◾ |
Bio-stimulant program (November 2021) – announced commercial launch of its first microbiome-based product for yield improvement
– result™. |
|
◾ |
Bio-pesticide program (December 2021) – reported advancement in its bio-fungicide program for fruit rot. |
AgPlenus
|
◾ |
Herbicide program (July 2021) – announced positive results for an herbicide resistance trait for its leading candidate APH1.
|
Biomica
|
◾ |
Immuno-oncology program (April 2021) – positive pre-clinical results, demonstrating efficacy of BMC128 in Melanoma. |
|
◾ |
Immuno-oncology program (October 2021) – Biomica and Rambam Health Care Campus signed agreement for clinical trial of Biomica’s
microbiome-based immuno-oncology drug candidate. |
|
◾ |
Inflammatory Bowel Disorder (IBD) program (November 2021) – announced positive pre-clinical results. |
|
◾ |
Immuno-oncology program (January 2022) – announced clearance for first-in-human Phase I study of BMC-128 in combination with
Bristol Myers Squibb’s Anti-PD-1 Opdivo®. |
Canonic
|
◾ |
Precise product family (February 2021) – entered into a collaboration agreement with Tikun Olam-Cannbit Pharmaceuticals Ltd.
for the development of novel medical cannabis products. |
|
◾ |
MetaYield product family (March 2021) – announced identification of leading cannabis varieties to be further developed into
commercial varieties, towards expected commercial launch in Israel in 2022. |
|
◾ |
Go-to-market strategy (March 2021) – entered into agreements with Tikun Olam-Cannbit Pharmaceuticals Ltd. for production, packaging
and distribution of medical cannabis products under Canonic’s brand. |
|
◾ |
Commercialization (August 2021) – announced pre-launch of its first-generation medical cannabis products in Israel. |
|
◾ |
Commercialization (October 2021) – announced full commercial launch of its first medical cannabis products in Israel.
|
|
◾ |
Precise product family (January 2022) – announced positive results in pre-clinical studies identifying specific cannabis varieties
with anti-inflammatory and pain relief properties. |
Ag-Seeds Division
|
◾ |
Novel insect control traits (March 2021) – entered a collaboration agreement with Plastomics Inc. targeting novel insect control
traits for soybean. Evogene’s insect control genes demonstrating new modes of action (MoAs) will be introduced into soybeans
using Plastomics chloroplast technology. |
Market Segments
Agriculture
Lavie Bio Ltd.
Overview
In 2015, we initiated our activity for developing ag-biological
products as a division within Evogene and early in 2019 it was organized under Lavie-Bio Ltd., an independent company that upon establishment
was wholly owned by Evogene.
Lavie Bio aims to improve food quality, sustainability and agricultural
productivity through the introduction of microbiome-based ag-biologicals. Ag-biologicals are externally-applied products from biological
sources, such as microbial (micro-organisms) and naturally derived biochemistries, designed to improve crop productivity. A sub-segment
within the microbial biologicals is the “microbiome”, the microbial population living close or within the plant or other organisms,
such as pests.
Lavie Bio is focused on developing two main types of products:
(i) bio-pesticides, which are ag-biologicals for crop protection, addressing biotic stresses such as insects, diseases, and weeds and
(ii) bio-stimulants, which are ag-biologicals for crop enhancement, directly impacting crop yield or abiotic stress tolerance.
In August 2019, Corteva Agriscience invested in Lavie Bio in a
transaction that included the exchange of all shares of Corteva’s wholly owned subsidiary, Taxon Biosciences, along with a $10 million
equity investment by Corteva in Lavie Bio in consideration of approximately 28% of Lavie Bio’s equity. The assets of Taxon Biosciences
including, among others, a large microbial collection, were integrated into Lavie Bio’s microbial collection, technology platform
and pipeline. In addition, Corteva received certain commercial rights with respect to rights to Lavie Bio’s candidate products,
mainly in corn and soy.
Market
The market for ag-biological products was estimated at $10.6 billion
in 20211 and is a growing segment in the approximately
$250 billion agricultural input market, which includes the seed, crop protection and fertilizers segments. The sales of ag-biological
products significantly grew in past years, expanding from a market size of $3.2 billion in 2015 to its current size following a shift
in growers and consumer preferences to more sustainable and healthier practices, while driving agriculture productivity. According to
market estimates, this market is forecasted to reach sales of $18.5 billion in 20262,
anticipated to be driven by improvement of the product attributes of ag-biologicals, such as efficacy, stability and commercial viability.
Companies in this market can be generally divided into three groups:
(i) major seed and ag-chemical companies, such as BASF, Bayer, ChemChina and Corteva, with internal research and development units dedicated
to development of ag-biological products, (ii) small to mid-size biotech companies specializing in ag-biologicals with their own product
development programs, and (iii) academic and agricultural research institutions that pursue research activities in the field, typically
focusing on early stage activities.
Business Model
Lavie Bio has defined two main models for market access:
|
(i) |
Direct sales model – in fragmented markets Lavie Bio expects to complete product development
of its products independently, while establishing a tailored market access strategy per specific product and territory, such as commercialization
through distribution channels. Under this model, the production of Lavie Bio’s products is achieved through third party toll manufacturers.
Revenues may include sales to distributors. |
1 https://www.reportlinker.com/p04680744/Top-10-Trends-in-Agricultural-Biologicals-Market-Industry-Global-Forecast-to.html.
2 According to industry
publications.
Under the direct sales model, during the fourth quarter of 2021
Lavie Bio launched its inoculant Result™ towards the 2022 spring sowing season.
|
(ii) |
Collaboration model – Lavie Bio offers tailored solutions to potential partners. In
this model, Lavie Bio’s partner produces and commercializes the products being developed. Lavie Bio’s revenues in such engagements
may include research and development payments, payments upon achievement of development milestones and royalties. The scope of collaboration
may differ: |
|
- |
Broad collaboration model – in markets where Lavie Bio identifies strategic partners
that can drive the go-to-market for its products, the aim is to gain market access through collaborations with such partners. Such collaborations
would typically commence with candidate strains discovered and developed by Lavie Bio, which will then undergo co-development with the
partner towards commercialization. |
|
- |
Narrow collaboration model – where Lavie Bio utilizes its platform to optimize product
candidates in different stages of development. Such optimization may include addressing challenges of efficacy (such as impact against
pests), consistency (such as stability of field performance) and commercial viability (such as shelf life). Such collaborations may commence
with a partner’s strain candidate, which would undergo optimization to meet market commercial needs. |
Product Development Programs
Scientific Approach
Lavie Bio's approach is focused on 'Biology
Driven Design' for the discovery, optimization and development of effective, stable and cost-effective microbial-based ag-biologicals.
Lavie Bio’s approach is based on converging the plant, microbial and environmental factors to decode their complex interactions
in order to enable the amplification of the positive, elimination of the negative and retrieval of lost interactions within the biological
system.
Lavie Bio’s Biology Driven Design, or BDD, facilitates and
accelerates the design and development of microbiome-based products through the decoding of complex microbiome/host interactions and the
identification of the key genetic elements (functions) governing these interactions. This decoding, which enables amplification of positive,
elimination of negative, and the retrieval of lost interactions, is powered by big data and artificial intelligence, provides the basis
for products design. The enabling technologies for the establishment of the BDD platform are Evogene’s MicroBoost AI tech engine
and the Taxonia platform, which harnesses genomics and informatics to develop transformative applications to agriculture, acquired as
part of the Taxon Biosciences acquisition.
Product Development Cycle
Lavie Bio estimates that developing an ag-biological product based
on microbial sources takes, on average, between six to eight years. The length of the process may vary depending on several factors, such
as product type, target market and applicable regulatory or registration regime, type of application, type of natural source serving as
active ingredient, as well as number of active ingredients within the final products, which impacts the development activities required
to reach a commercially viable product.
The development process for microbial-based ag-biologicals is generally
divided into four steps, or phases, which include discovery, pre-development,
development, pre-commercialization, and ending with registration
approval and commercial launch. As this is a relatively young industry, the process is not yet well established and standardized and the
below outline is established based on our experience and estimations.
|
◾ |
Discovery: The identification of a candidate microbial strain, or microbial
strain teams, having the potential to improve the target trait. A collection of selected microbial candidates is typically tested on the
crop(s) of choice in greenhouse screens or limited field experiments for various efficacy, stability and commercial viability criteria.
Candidates that meet the testing criteria are referred to as “Hits”. Typically, the duration of the discovery phase is approximately
12-18 months. |
|
◾ |
Pre-development: Promising Hits are advanced to pre-development phase,
in order to further assess and optimize performance criteria such as shelf life, efficacy and stability. Successfully performing microbial
candidates are referred to as “Advanced Hits”. Typically, the duration of this phase is approximately 12-18 months.
|
|
◾ |
Development: This phase is usually divided into Development Stage 1, resulting
with a “Lead”, and Development Stage 2, resulting with a “Pre-Product”. In this phase, the fermentation and formulation
procedures are further optimized to allow for further testing and validation of efficacy and stability in the field as well as for commercial
scale production, addressing cost of good targets and compatibility with other agricultural inputs. Based on industry benchmarks and our
estimates, Lavie Bio estimates the duration of this stage to be approximately 24 months. |
|
◾ |
Pre-commercialization: In this phase, extensive field tests are undertaken
to demonstrate the effectiveness of product candidates in enhancing the target trait, including production of data to support product
positioning. Additional activities towards launch are performed, including packaging development, upscale manufacturing protocol, registration
and regulation. Based on industry benchmarks and our estimates, in the U.S. Lavie Bio expects the duration of this stage to be approximately
24 months for bio-stimulants and 36-48 months for bio-pesticides due to longer regulation processes. |
|
◾ |
Commercial: After initial commercialization of a product, different scale-up
activities are undertaken, such as production under toll-manufacturing agreements and deployment of end-product at point of sale. Toll
manufacturing involves development of production protocols for large fermentation vessels and down-stream-process protocol with the toll
manufacturer. |
Product Development Pipeline
The following table sets forth Lavie Bio’s main product development
programs:
With respect to its Bio-stimulants program for spring wheat, in
November 2021, Lavie Bio announced the commercial launch of its first product, LAV.211, an inoculant for yield improvement, under the
brand name result™. Initial market penetration for result™ is planned for the 2022 spring wheat season and will be limited
to target regions in North Dakota, under a distribution agreement with United Agronomy. Following the initial commercial introduction,
Lavie Bio intends to expand through additional distribution channels and to evaluate the opportunities for result™ in additional
territories for spring wheat, and for application to additional cereals.
With respect to its Bio-pesticides program against fruit rots,
in December 2021, Lavie Bio announced advancement to the pre-commercial stage and prioritization of LAV.311 for final development and
submission of a regulatory dossier expected to be filed with the federal U.S. Environmental Protection Agency, or EPA, and California
EPA during 2022.
Key Collaborations
Corteva (originally with DuPont-Pioneer)
In July 2017, Evogene entered a multiyear collaboration with DuPont-Pioneer
(now Corteva), for the research and development of novel microbial bio-stimulant seed treatments for the improvement of corn productivity
globally. Following the establishment of Lavie Bio, the collaboration agreement was assigned from Evogene to Lavie Bio. Under the agreement,
Lavie Bio is entitled to milestone payments for advancement of candidate strains, and royalties from product sales. Corteva and Lavie
Bio prioritized certain product programs to be executed by Lavie Bio, and Lavie Bio committed to allocate a certain part of its research
and development budget to these programs.
Intellectual Property
Lavie Bio files for patents to cover the use of microbial strains,
or strain teams, that are the core active ingredients of the products we develop, as well as enabling technologies. Other innovative and
proprietary technologies that we develop (such as computational predictive and design technologies), are typically protected as ‘trade
secrets’.
Raw Materials
Lavie Bio does not significantly rely upon any sources of raw materials
for its operations.
Seasonality
Lavie Bio's sale cycles are dependent on crop seasonality as
growing and harvest periods depend on crop seasonality. Also, RD activities are dependent on crop seasonality, as field trials are
highly dependent on crop seasonality and the time windows for conducting such trials are rigid.
Government Regulation of our Operations and of Product Candidates
In general, the regulatory landscape in the evolving field of ag-biological
products is still developing. As a result, it may face additional changes in the next few years. Complexity of regulatory processes varies
between bio-stimulants and bio-pesticides and between regulatory organizations.
In the U.S., the key focus market for the ag-biological products
Lavie Bio is currently developing, the Animal and Plant Health Inspection Service within the Department of Agriculture, or USDA APHIS,
is responsible for importation and field release permits for ag-biological products, and the EPA is in charge of the registration of plant
protection products. Most U.S. states also require certain registration processes for such products, which vary among states. Both U.S.
and European regulators are in the process of establishing a more defined regulation process for bio-stimulants. Under current EPA guidance,
bio-stimulants are regarded as plant inoculants, which currently does not require any regulatory action at the federal level but requires
registration at the state level. Bio-pesticides require registration at both federal and the state level.
In the European Union, bio-stimulants are currently regulated as
fertilizers, and bio-pesticides are regulated and registered as plant protection products.
AgPlenus Ltd.
Overview
In 2015, we initiated our activity for developing ag-chemical products
as a division within Evogene, and in 2018, we announced that it had been organized under AgPlenus Ltd., a separate company, wholly owned
by Evogene upon establishment. AgPlenus aims to design effective and sustainable crop protection products (crop protection refers to the
science and practice of managing risks of weed, plant diseases, and insects that damage agricultural crops and forestry) by leveraging
computational predictive biology and chemistry. AgPlenus’ activities focus on discovery and development of new MoA crop protection
products. During 2021, AgPlenus focused its efforts on its leading herbicide products under development.
Market
According to industry publications, the crop protection chemicals
market was estimated at approximately $63 billion in 2019 and is expected to grow to over $90 billion by 2026.3
Lack of available solutions for pest control and increasing resistance to existing crop protection solutions lead to a pressing need for
novel crop protection products. However, due to current technological limitations and increasing regulatory requirements, the development
of crop protection products is lengthy, complicated and expensive.
Competition
The ag-chemical RD market, as described above, can be classified
into four key groups of companies: (i) major seed and ag-chemical companies, such as BASF, Bayer, ChemChina and Corteva, with internal
research and development units dedicated to development of ag-chemical products, (ii) mid-size ag-chemical companies, mainly Japanese
companies focused on the Japanese market, that develop crop protection products, (iii) small to mid-size biotech companies that undertake
new approaches to research and development of novel crop protection products, and (iv) academic and agricultural research institutions,
typically focusing on early stage activities.
Business Model
AgPlenus’ business model is based on two commercialization
avenues:
Licensing of product candidates
– when product candidates advance towards what is referred to in the industry as a Lead,
at the end of the discovery stage, or further along the development pipeline, these product candidates gain increased value and can be
candidates for licensing to ag-chemical companies. A typical licensing agreement can include
upfront payments, payments upon achievement of pre-defined development milestones, and royalties from product sales.
RD collaborations
– early-stage collaborations, providing a tailored product offering per partner and product type, in order to build long-term research
and development relationships and to mitigate the risk associated with building an independent pipeline. A typical collaboration agreement
may include RD payments, payments upon achievement of pre-defined development milestones, and royalties from product sales, which
would typically be lower than the royalties under licensing agreements.
Currently, AgPlenus’ revenues are derived from research and
development payments under early-stage collaborations. In the longer term we expect that: (i) as AgPlenus’ product candidates advance
through development in our partners’ pipelines, and to the extent that they are commercialized by AgPlenus’ collaboration
partners, revenues are expected to include milestone payments and royalty payments; and (ii) as its internal pipeline product candidates
further advance, AgPlenus will license its product candidates.
Product Development Programs
Scientific Approach
AgPlenus’ approach is based on the disruption of the traditional
methods of ag-chemical discovery and optimization by implementing a target-based approach for identifying and developing new MoA crop
protection products to address the growing resistance of pests (weeds, insects, and fungi) to existing commercial products. AgPlenus utilizes
mainly Evogene’s ChemPass AI tech engine, as well as other advanced computational technologies and know-how, to drive its ag-chemical
discovery.
AgPlenus’ approach typically begins with the computational
and research-driven identification of protein ‘targets’, which are proteins that are essential to the function of performance
of the relevant weed, insect or fungi. Following the identification and validation of such targets, AgPlenus identifies candidate Hits,
which are chemical compounds (small molecules) that potentially inhibit these targets. AgPlenus screens candidate Hits to identify those
displaying effect on the pest of focus. Hits displaying confirmed activity in the initial validation screens, enter the Hit-to-Lead process,
which includes computational optimization and additional, more advanced, validation experiments.
3
Facts Factors - https://www.globenewswire.com/news-release/2021/02/02/2168067/0/en/Crop-Protection-Chemicals-Market-Size-Share-Will-Reach-to-USD-90-Billion-by-2026-Facts-Factors.html
In addition, these capabilities are also used independently of
each other to discover new Hits for known targets, to optimize an existing Hit-to-Lead and to optimize a commercial molecule.
Product Development Cycle
The product development cycle for ag-chemical products is generally
comprised of several stages, described as follows:
Discovery stage
|
◾ |
Identification of Targets – identification and validation of vital targets or proteins that when inhibited (for instance by
a chemical), lead to weed, insect or fungi death. |
|
◾ |
Identification of Hits – screening of chemical compounds for the identification of candidate Hits that potentially inhibit
identified vital targets and are capable of achieving the desired impact on the weeds, insects or fungi of interest. The discovery process
includes in-silico as well as biological screening and validation activities. |
|
◾ |
Hit-to-Lead process – Hits displaying confirmed activity in the initial validation screens will enter the Hit-to-Lead process,
including several optimization cycles, each constructed of compound design (in our case, focusing on computational optimization), synthesis
of compounds and validation experiments. This stage ends with a ‘Lead’ compound, which is a validated Hit that has confirmed
activity in advanced validation screens proving field translation in initial trials. |
Lead optimization
|
◾ |
In this stage, multiple field trials are conducted in diverse geographies, as well as greenhouse experiments on resistant weed biotypes
and on commercial crops, and the compound structure and formulation are finalized. Lead optimization also entails initial toxicology tests,
process engineering on the molecule and a significantly detailed cost of goods analysis. |
Pre-development stage
|
◾ |
In this stage, field trials to validate all commercial cases are conducted, including testing product mixtures, as well as additional
safety trials. This stage ends with a ‘Pre-Development’ compound. |
Development, Regulation Registration stage
|
◾ |
In the final development phases, new chemical products are registered with the proper regulatory authorities and then launched for
commercialization. We expect that these last stages of development will be conducted by our collaboration partners or licensor of our
product candidates. |
Product Development Pipeline
The following table sets forth AgPlenus’ main internal product
development programs:
Note: The table does not present product development programs undertaken
with collaborators that are subject to confidentiality restrictions.
In 2021, AgPlenus reached proof-of-concept for an herbicide tolerance
trait for a ‘Lead’ herbicide under its new MoA herbicide program.
Key Collaborations
Corteva – Herbicides
Overview
In March 2020, AgPlenus entered into a multi-year collaboration
with Corteva for the discovery and development of novel herbicides. Under the terms of the collaboration agreement, AgPlenus and Corteva
work together to optimize herbicide product candidates originating from AgPlenus’ pipeline. Successful candidates from this collaboration
are expected to be further developed by Corteva.
License Consideration
Pursuant to the collaboration agreement, Corteva obtained a worldwide,
royalty-bearing, exclusive license to use and modify chemical compounds identified under the collaboration to develop and commercialize
weed control products containing such compounds. Moreover, AgPlenus is entitled to research and development payments, milestone payments
upon achievement of certain development milestones as well as royalty payments from sales of products developed under the collaboration.
Intellectual Property
AgPlenus is seeking patent protection for intellectual property
rights covering its leading product candidates.
Government Regulation of our Operations
AgPlenus’ activities are performed at labs in Israel and
are regulated by the provisions of several Israeli governmental agencies. Violation of these regulations may expose us to criminal or
civil actions and may impose liability on us.
Government Regulation of Product Candidates
Regulatory approvals are required prior to the commercialization
and importation of ag-chemical products in most countries. Most of the key target markets where AgPlenus anticipates its collaborators
to sell products containing its compounds, including the U.S., the European Union, Brazil and Argentina, will require such regulatory
approvals prior to the commercialization of such products. Pursuant to AgPlenus’ collaboration agreements, its collaborators are
responsible for product regulation.
Among other regulatory requirements, our collaborators may need
to test new active ingredients for assessment of potential effects on mammals. These include tests on acute toxicity, carcinogenicity,
mutagenicity and reproduction. The results of these tests may impact the chemistry and formulation development stages.
In order to sell a crop protection ag-chemical product in most
countries, both the product and its active ingredient first need to be registered. This process may require the submission of over 100
toxicology and ecotoxicology studies, as well as detailed information on the chemistry of the active ingredient and the product. In the
United States, collaborators may need to seek regulatory approval from the EPA, which regulates the marketing and use of new plant pesticides
and herbicides. In addition, in Brazil, the commercialization of ag-chemical products is regulated by Anvisa, the federal agency in charge
of evaluating pesticide health risks. The approval process involves data collection and analysis, environmental impact assessments and
public hearings on certain products, and is similarly costly and time-intensive.
Raw Materials
AgPlenus does not significantly rely upon any sources of raw materials
for its operations.
Seasonality
The field testing of AgPlenus’ leading product candidates,
which have reached advanced stages of product development, are highly dependent on crop seasonality.
Currently, AgPlenus does not have any commercialized products
and therefore its revenues are not subject to variations based on seasonality. However, our expectation is that, in the future, sales
cycle of the products AgPlenus develops will be dependent on crop seasonality.
Ag-Seeds Division
Overview
The global population will expand to approximately 9.7 billion,
pushing food demand up 70%, by 20504,5.
Our seed traits activity is focused on the development of seed traits that have a direct impact on crop productivity through the use of
non-GM and GM approaches, aiming to fulfill such growing demand. We mainly target key commercial crops such as corn, soy, wheat, rice
and cotton.
The activities of this division are divided into four categories:
(i) yield abiotic stress tolerance, or YABST – increase crop performance and productivity by enhancing yield, tolerance
to abiotic stresses such as drought, heat and salinity and fertilizer use efficiency; (ii) disease resistance – increase crop resistance
to diseases such as fungi and nematodes; (iii) insect control – increase crop tolerance to pests; and (iv) quality traits –
increase production of natural plant products such as pigments and anti-aging agents.
In general, we utilize several biotechnology approaches with the
goal of improving plant traits, including: (i) genome editing technologies, enabling deletion or modification of specific genomic regions
in the crop's genome without inserting foreign DNA to the plant, (ii) genetic modification of plants, which involves the direct manipulation
of a plant’s genome by inserting a gene into the plant’s DNA, and (iii) advanced breeding methods, whereby plants with favorable
characteristics are selectively crossed through genomic-guided breeding schemes.
In recent years, the CRISPR genome editing technology has been
gaining popularity and is considered to have the potential to revolutionize the product development of new seed traits, while overcoming
environmental and other challenges, and is expected to expand the global crop industry by $110 billion between now and 2025 with an expected
growth of 25%6.
4The future of food and
agriculture, trends and challenges: http://www.fao.org/3/i6583e/i6583e.pdf.
5 The United Nations World
Water Development Report. Wastewater: The Untapped Resource, UNESCO. 2017: https://unesdoc.unesco.org/ark:/48223/pf0000247153.
6 CRISPR Genome-Editing-
Market Opportunity And Key Players: https://research.ark-invest.com/hubfs/1_Download_Files_ARK-Invest/White_Papers/ARK%20Invest_081318_White%20Paper_CRISPR%20Opportunity.pdf?hsCtaTracking=3e9ae410-326d-4658-9a4a-fd6c4cb7b263%7Cfa54a728-0144-4138-a518-b0051eae3b7f
Market
According to industry publications, in 2020 the GM seeds market
size was estimated at approximately $27.9 billion and is projected to reach to $45 billion by 2027. The GMO crops and seeds market in
the U.S. were estimated at $7.5 billion in the year 2020, while China is forecast to reach a projected market size of $10 billion by the
year 20277. According to industry publications, the market
potential for traits addressing plant insects and diseases was estimated to be between $7.5 billion to $8.5 billion, out of which the
commercial value of insect control products was approximately $4.5 billion.
Business Model
In the Ag Seeds activity, we collaborate with seed companies in
the development of improved seed traits. Our partners include world-leading seed companies, including Bayer and Corteva, as well as regional
seed companies such as Tropical Melhoramento Genética S/A, or TMG. Typically, under these collaborations we perform
the discovery phase, during which we discover and validate candidate trait-improving genetic elements. Subsequently, our collaborators,
under license from us, test and further develop these discoveries in their product development pipelines, starting Phase I, with the goal
of introducing them into commercial crop seeds. For more information on the product development pipeline, please see “—Product
Development Pipeline” below.
In most cases, we expect to generate revenue from our collaboration
agreements at two different points: first, we expect to receive milestone payments when certain specified results are achieved, such as
when a product candidate containing our traits is submitted for regulatory approval; second, we expect to receive royalty payments once
a commercial product containing our traits is launched into the market. Under several collaboration agreements, we also receive research
and development service payments to cover the costs of our research.
In the Ag-Seeds division, we currently generate revenues from research
and development payments for our activities. All of our product development programs under our Ag-Seeds activity are currently either
in the Discovery or in Phase I stages. For more information on our product development programs in this field, see “— Product
Development Programs.”
Product Development Programs
Scientific Approach
The division uses our expertise in plant and bacterial science
and genomics to improve commercial seed traits. Evogene’s proprietary CPB platform, specifically, the GeneRator AI, validation techniques
and other capabilities enable us to identify and optimize promising genetic elements that have the potential to improve our traits of
interest in target crops.
We have accumulated substantial scientific knowledge on plant,
diseases and insect mechanisms associated with yield, abiotic stress, fertilizer use efficiency, disease resistance traits and insect
control traits. We maintain a large proprietary genomic data from over 200 different plant species as well as large microbial data tailored
for insect and disease control. We have also established proprietary plant, disease and insect validation systems.
Product Development Cycle
The length of the process of developing and integrating seed
traits may vary depending on the technology being applied, the complexity of the trait and the type of crop involved. The development
process for seed traits is typically divided into discrete steps, or phases, as follows:
|
◾ |
Discovery: The identification of target genetic elements for enhancing
specified plant traits. We test these elements in different validation systems to determine their ability to enhance the specified trait.
In our experience, the Discovery phase takes approximately 6-18 months. The target genetic elements may be applicable to product development
through different technological approaches (i.e. genome editing, GM or advanced breeding). In our collaborations, we typically undertake
this phase. |
7 https://www.globenewswire.com/news-release/2020/09/14/2092784/0/en/Global-GMO-Crops-and-Seeds-Industry.html.
|
◾ |
Phase I, or “Proof of Concept”: Validated candidate genetic
elements are advanced to Phase I. In this phase, they are tested in target plants through greenhouse trials, field trials, or both, for
their efficacy in improving plant performance. Phase I may be conducted by us or by our collaborators, and in our experience, may last
between two and five years for a GM product or, three years for a genome editing or advanced breeding product. For products developed
through genome editing, deregulation process for classifying a product as non-GM is typically initiated during Phase I. |
|
◾ |
Phase II, or “Early Development”: In this phase, the field
tests are expanded, and our collaborators evaluate the genetic elements on multiple geographical locations and varieties, to reach commercially
viable success rates. We estimate the duration of Phase II is between two to four years. For a GM product, by the end of this phase, a
specific product candidate will be selected to advance to Phase III. For genome editing and advanced breeding products, the end of this
phase will lead straight to Phase IV (Pre-Launch). |
|
◾ |
Phase III, or “Advanced Development and Regulation”: This
phase is relevant only for the development of GM products. Extensive field trials are performed to test the effectiveness of the selected
product candidate across locations, and regulatory approvals are obtained, including potential environmental impact assessments, toxicity
and allergenicity. We estimate the duration of Phase III is between one to two years. |
|
◾ |
Phase IV, or “Pre-Launch”: This phase involves preparation
for commercial launch. The range of activities here includes preparing the seeds for commercial sales, formulation of a marketing strategy
and preparation of marketing materials. We estimate the duration of Phase IV is between one to two years. |
As indicated, the estimated timeframes of phase duration are based
on our experience and estimates according to available information. The total development time for a particular product may be longer
or shorter than the duration presented above depending on a range of factors.
Product Development Pipeline
The following table sets forth our key product development programs
in the AgSeeds division:
|
Program |
Crop |
Trait |
Technology |
Collaborator |
Development Phase |
|
YABST Programs: |
|
1 |
Corn |
YABST |
GM |
Bayer |
Phase I, at collaborator under license. |
|
2 |
(1) |
YABST |
Advanced breeding |
A consumer goods company (1) |
Undisclosed, at collaborator under license. |
|
Disease Resistance Programs: |
|
1 |
Corn |
Fusarium |
GM genome editing |
Bayer |
Undisclosed, at collaborator under license. |
|
2 |
Soybean |
Asian Soybean Rust |
GM |
Corteva |
Undisclosed, at collaborator under license. |
|
3 |
Soybean |
Nematodes |
Genome editing |
TMG |
Discovery |
|
4 |
Banana |
Black Sigatoka |
GM |
Rahan Meristem |
Phase I, at collaborator under license. |
|
Insect control Programs: |
|
1 |
Corn, Soybean, Cotton |
Lepidoptera |
GM |
Internal program |
Phase I |
|
2 |
Corn, Cotton |
Coleoptera |
GM |
Internal program |
Phase I |
|
3 |
Soybean |
Hemiptera |
GM |
Internal program |
Phase I |
__________
|
(1) |
Crop and collaborator name not disclosed. |
Key Collaborations
Bayer (originally with Monsanto)
In August 2008, we entered into a Collaboration and License Agreement
with Monsanto (now Bayer, following the completion of the acquisition of Monsanto by Bayer in June 2018 and a later assignment of the
agreement from Monsanto to Bayer CropScience LP), which was amended several times during collaboration and license phases.
Yield and Abiotic Stress
Tolerance Program
Pursuant to the agreement, Monsanto funded a research program under
which we identified and optimized genes with the potential to improve yield and abiotic stress tolerance in corn, soybean, cotton and
canola, and candidate genes have entered Phase I in Monsanto's product development pipeline. In July 2017, we announced completion of
candidate gene discovery stage in this collaboration.
Biotic
Stress Program - Fusarium
As part of the October 2013 amendment of the agreement, we identified
genes providing resistance to Fusarium, a type of fungi that is a main pathogen responsible for Stalk Rot disease in corn (a widespread,
yield-reducing disease). In July 2017, we announced that we had reached an important milestone in the collaboration with the demonstration
of positive Fusarium resistance results with Evogene-discovered genes. In July 2019, we announced that the collaboration was being refocused
on the identification of genome editing targets for evaluation against a broad range of corn diseases.
License
Consideration
We have granted Monsanto an exclusive, royalty-bearing, worldwide
license under our patents and know-how to commercially exploit and conduct research on the genes and other genetic elements we discovered
under the collaboration, in the specified crops.
Monsanto provided us with research and development payments, and
undertook to provide us with development milestone payments, if and when our product candidates reach significant milestones in its product
development pipeline, as well as royalty payments on any sales or other transfers of products it develops containing our licensed genes.
Corteva (Originally with DuPont-Pioneer)
In 2011, we entered a multi-year research and development collaboration
with DuPont-Pioneer (now Corteva, following the merger of Dow Chemicals and DuPont in September 2017), to improve resistance to Asian
Soybean Rust, or ASR, a devastating fungal disease in soybean. We amended and expanded the agreement in October 2013. Under this collaboration,
we identified relevant genes having the potential to improve in-plant resistance to ASR.
DuPont-Pioneer holds a worldwide, royalty-bearing, exclusive license
to develop and commercialize soybean products containing our licensed genes. Our compensation under the agreement is in the form of milestone
payments and royalty payments based on the sales of resulting products. Each party funded its expenses in performing its activities using
its own resources and a grant from the Israel-U.S. Binational Industrial Research and Development Foundation, or BIRD.
TMG
In December 2018, we entered into a multi-year collaboration and
license agreement with TMG, a major Brazilian developer and marketer of soybean varieties, for the development of nematode-resistant soybean
varieties using genome editing technologies. Under the agreement, we identified genomic elements for editing to attribute nematode resistance
in soybean and perform such edits on TMG’s commercial soybean germplasm. In turn, TMG validates the efficacy of the edited soybean
varieties in greenhouse assays and field trials in Brazil and for incorporation in its breeding pipeline.
Under the agreement, TMG obtained a worldwide, royalty-bearing
license to incorporate genome edits originating from the collaboration in its soybean varieties. Evogene, on the other hand, obtains a
non-exclusive, royalty-bearing license to commercialize such genome edits and soybean lines, subject to certain exclusivity restrictions.
According to the agreement, each party is entitled to receive royalty payments from the other party when the products of the collaboration
are commercialized. In addition, Evogene is entitled to success-based payments upon achievement of pre-defined development milestones.
Intellectual Property
In the AgSeeds division, we seek to obtain patent protection for
the use of the genes and genetic elements that we identify as linked to desired traits. In certain cases patent protection determines
our eligibility to receive royalties for seed traits under the licenses we grant our collaborators. To date, we have sought and obtained
patent protection for hundreds of plant genes in target territories.
Government Regulation of Product Candidates
In most of the key target markets where we anticipate our collaborators
will sell seeds containing our traits, including the United States, the European Union, Brazil and Argentina, regulatory approvals are
required prior to the commercialization and importation of biotechnologically enhanced seeds. Additional regulatory approvals are required
in countries importing grain produced from seeds containing our traits, such as China, India and certain countries in the European Union.
Pursuant to our collaboration agreements in the field of seed traits, our collaborators are typically responsible for applying for all
requisite regulatory approvals prior to commercialization of the product candidates we develop with them.
The regulatory status of products developed via genome editing
technologies is currently defined in most countries with the exception of the EU. In the United States, de-regulatory approvals are required
by the USDA prior to field testing of genomic edited seeds. Several ‘non-regulated organism’ approvals have been issued by
the USDA as well as the regulatory authorities of Japan and Argentina for products that are being commercialized or under development.
Government Regulation of our Operations
The business of the AgSeeds division is subject to regulation related
to agriculture, health and the environment. To operate, we must obtain various permits and licenses from government authorities and municipalities
in jurisdictions where we are active, and we must maintain our compliance with the terms of those permits, licenses and other government
standards as necessary. These laws and regulations, particularly in relation to biotechnology, are not fully settled, but continue to
evolve in order to keep pace with technological advances.
Our operations are carried out mainly in Israel and accordingly
are regulated by the Israeli Ministry of Agriculture and Rural Development, or ISARD, and more specifically by the ISARD’s Plants
Protection and Inspection Services. Our activities are subject to various laws, regulations, orders and procedures, which require us,
among other things, to obtain permits for conducting experiments on genetically enhanced plants and to satisfy special conditions determined
by the ISARD regarding the growing procedures of such seeds and plants. Violation of these regulations may expose the company to criminal
penalties. Pursuant to these regulations, we are also obligated to obtain separate permits to own and operate our greenhouses and testing
fields in Israel and we are routinely inspected by ISARD.
Raw Materials
Our AgSeeds division does not significantly rely upon any sources
of raw materials for our operations.
Seasonality
In general, seasonality has limited effect on up-stream research
activities, focused on computational discovery, laboratory work and greenhouse testing. Field trials, which are a central activity in
more advanced development stages, is dictated by crop seasonality.
Human Health
Biomica Ltd.
Overview
In 2017, we established Biomica, a subsidiary focused on the discovery
and development of innovative human microbiome-based therapeutics. The human microbiome is an array of more than 100 trillion microorganisms
that live on and in our bodies, creating a community of symbiotic, commensal and pathogenic bacteria, all of which call the human
body home. These microbes have numerous beneficial functions relevant to supporting life, such as digesting food, preventing disease-causing
pathogens from invading the body, and synthesizing essential nutrients and vitamins. Numerous studies have shown the connection between
the human microbiome and various medical disorders, and the search for microbiome therapies and treatments is a rapidly growing focus
for biotherapeutics research and development.
Biomica focuses on the development of human-microbiome based therapies
utilizing either rationally-designed microbial consortia or small molecule approaches for (i) immuno-oncology (ii) gastrointestinal inflammatory,
or GI related disorders, and (iii) antimicrobial resistance, or AMR, an antibiotic resistant bacteria.
Market
Biomica’s product development is currently focused in three
main markets:
Immune-Oncology
In oncology, checkpoint inhibitor antibodies, including those targeting
the programmed cell death protein/ligand 1, or PD-1/PD-L1 pathways, block the tumor’s ability to suppress the immune response. They
have significantly improved the treatment of many cancers. The cancer immunotherapy market size was estimated at $84 billion in 2018 and
is expected to reach a market size of $243 billion by 2026 according to a report published on July 17, 2019 by Reports and Data.8
Even in cancers, where checkpoint inhibition is considered the
frontline standard of care, a significant percentage of the patients do not respond to PD-1 + CTLA-4 inhibitor combination and a portion
of responders relapse within a few years. In all approved cancer indications, agents with differentiated immune mechanisms of action may
be complementary to checkpoint inhibitors by both augmenting existing effects and testing alternative pathways of immunotherapy in checkpoint
inhibitor non-responsive tumor types and patients.
Given a growing body of literature, it is becoming increasingly
clear that modulation of the gut microbiota may represent a novel and important adjunct to current anti-cancer therapeutic modalities.
GI related disorders
|
◾ |
Irritable Bowel Syndrome (IBS) is a common disorder that affects the large intestine.
Signs and symptoms include cramping, abdominal pain, bloating, gas, and diarrhea or constipation, or both. It is estimated that the total
market for IBS reached $1.5 billion in 2018, with 45 million patients in the U.S. alone and is expected to reach $3.3 billion in 20269,10.
Existing drugs for IBS mainly treat the symptoms of the condition, leaving patients exposed to cycles of remission and relapse that characterize
this chronic condition. |
|
◾ |
Inflammatory Bowel Disease (IBD) is a group of GI diseases, mainly comprised of Ulcerative
colitis and Crohn’s disease. IBDs cause long term chronic as well as severe inflammation in the gastrointestinal tract without any
known cause. According to the Centers for Disease Control and Prevention, or CDC, in 2015 an estimated 3.1 million people (1.3% of the
entire population) in the United States were diagnosed either with Crohn’s disease or with Ulcerative Colitis. The global IBD drug
market is estimated to grow from $15.9 billion in 2018 to $22.4 billion in 2026.11
|
8 https://www.globenewswire.com/news-release/2019/07/17/1884118/0/en/Cancer-Immunotherapy-Market-To-Reach-USD-242-86-Billion-By-2026-Reports-And-Data.html.
9 https://www.grandviewresearch.com/industry-analysis/irritable-bowel-syndrome-ibs-treatment-market.
10 https://www.bloomberg.com/press-releases/2019-07-23/ibs-treatment-market-size-worth-3-3-billion-by-2026-cagr-10-1-grand-view-research-inc.
11 https://www.benzinga.com/pressreleases/19/10/ab14683304/inflammatory-bowel-disease-treatment-market-is-projected-to-reach-22-4-billion-by-2026-grand-view.
AMR (antimicrobial resistance)
|
◾ |
Clostridium Difficile Infection (CDI) – The CDC has identified CDI as one of the top
three most urgent antibiotic-resistant bacterial threats in the United States. CDI is most often caused by the use of broad-spectrum antibiotics
which induce dysbiosis of the microbiome causing susceptibility to infection by C. difficile, a spore forming bacterium. It is the most
common cause of hospital acquired infection in the United States. |
CDI is responsible for the deaths of approximately 29,000 Americans
each year. Based on an epidemiological study conducted by the CDC, the incidence of CDI in the U.S. was estimated to be over 600,000.
CDI space across the seven major markets of the U.S., France, Germany, Italy, Spain, the UK and Japan is set to grow from just under $630
million in 2016 to almost $1.7 billion by 2026, representing a compound annual growth rate of 10.2%. The global CDI market is expected
to approach $1.7 billion by 2026.12
|
◾ |
Methicillin-Resistant Staphylococcus Aureus (MRSA) – One of the most common Staphylococcus
aureus infections is caused by MRSA, which is a multi-drug resistant bacterium, responsible for several difficult-to-treat infections
in humans, leading to tens of thousands of annual cases of mortality in the U.S. MRSA is the leading causative agent for hospital acquired
infections and has recently been documented as community-acquired as well as livestock-acquired. Current medical treatments include broad
spectrum antibiotics that are becoming increasingly ineffective. The current MRSA market was valued at approximately $922 million in 2018
and is projected to reach over $1.3 billion by 2026.13
|
Competition
The biotechnology and pharmaceutical industries are characterized
by rapid growth and a dynamic landscape of proprietary therapeutic candidates. The development and commercialization of new drug and biologic
products is highly competitive and is characterized by rapid and substantial technological development and product innovations. While
we believe that our computational platform and microbial drug candidates, coupled with our resources and industry expertise, give us a
competitive advantage in the field, we face competition from a variety of institutions, including larger pharmaceutical companies with
more resources. Specialty biotechnology companies, academic research institutions, governmental agencies, as well as public and private
institutions are also potential sources of competitive products and technologies.
In both inflammatory diseases and oncology, we anticipate intensifying
competition as new therapies are approved and advanced technologies become available. Many of our competitors, either alone or with strategic
partners, have considerably greater financial, technical, and human resources than we do.
Significant competition exists in the immuno-oncology and inflammatory
diseases field, where we are developing our first drug candidates in oncology and IBD. Although our rationally-designed microbial consortium
approach is unique relative to most other existing or investigational therapies in immuno-oncology, we will need to compete with all currently
or imminently available therapies within the indications where our development is focused. Although there is a wide range of potentially
competitive mechanisms, possible synergies between these and rationally-designed microbial consortia will also be evaluated.
Business Model
Biomica’s goal is to become a leading biopharmaceutical company
developing and commercializing microbiome therapeutics to address significant unmet medical needs, through strategic collaborations with
world-leading pharmaceutical companies.
12 https://www.globaldata.com/global-clostridium-difficile-infections-market-approach-1-7-billion-2026/.
13 https://www.bloomberg.com/press-releases/2019-09-24/global-methicillin-resistant-staphylococcus-aureus-mrsa-drugs-market-to-surpass-us-1-3-billion-by-2026.
Product Development Programs
Scientific Approach
Biomica aims to identify unique microbiome-based therapeutic entities
through multilayered analysis and integration of high resolution big-data originating from the human gut microbiome. Employing a holistic
approach, Biomica combines a profound understanding of the microbiome and its functions and their intricate relations with the human host.
Biomica’s approach relies on a multi-layered analysis of
omic and clinical / phenotypic data using an extensive nexus of modules in four key areas: (i) creation of microbial classifications –
enabling high-resolution taxonomy analysis of the microbial community down to the strain level, (ii) identification of microbial functions
– functional-level microbial community analysis profiling microbial genes, pathways and metabolites, (iii) identification of host
genomics – profiling of patients' genomic information (genetics and expression patterns), and (iv) clinical data – integrate
relevant phenotypic and physiological information manifested in patient.
Biomica’s discovery and development efforts are powered by
the predictive, high resolution, integrative selection of microbes, or PRISM, platform, which is powered by Evogene’s MicroBoost
AI engine. PRISM is a
proprietary metagenomics analysis platform for functional genomics profiling, utilizing internal comprehensive databases. These databases
have been specifically developed to allow the processing of large amounts of sequencing data, obtain high-resolution profiling of microbial
communities both at the taxonomic and the functional levels, and correlate them with specific clinically relevant host expression and
phenotypic profiles, enabling Biomica to achieve each of the below analyses:
|
◾ |
At the taxonomic level Biomica's analysis allows strain-level resolution and relies on an extensive proprietary strain database.
|
|
◾ |
At the functional level, Biomica's proprietary resources rely on a comprehensive catalog of microbial genes enabling mapping of an
average of 90% of the functions of the human gut microbiome obtained through metagenomics sequencing. |
In addition to its comprehensive computational solutions to profile
the microbiome, Biomica also utilizes Evogene's ChemPass AI engine, for virtual screening
of small molecular inhibitors to specifically target bacterial proteins of interest. This platform combines the physiochemical requirements
for binding a specific protein target and utilizes a comprehensive proprietary database of over 20 billion known molecules for the discovery
of potential therapeutics.
Product Development Pipeline
Immune-Oncology
BMC128 is an optimized consortium, which consists of four bacterial
strains derived from Biomica’s BMC121 and BMC127 (rationally-designed consortia that were identified using our computational analysis
and predictive capabilities designed to enhance an anti-tumor immune activity). BMC128 is a rationally-designed live biotherapeutic product,
comprised of unique bacterial strains, natural inhabitants of the human intestinal tract, that harbor specific functional capabilities
with the potential to enhance immunological therapeutic responses and facilitate anti-tumor immune activity though multiple biological
processes.
During 2021, Biomica continued the pre-clinical studies of BMC128,
obtaining positive results, and completed scale-up development and the first GMP batch production of its drug candidate, conducted by
Biose Industrie (Aurillac, France). The pre-clinical mice data showed that BMC128 administered prior to and in combination with an anti-PD1
demonstrated a 48% increase in anti-tumor objective response rate in a breast tumor model. Biomica reported additional positive pre-clinical
data in the use of BMC128 in treating melanoma demonstrating the potential efficacy of its microbiome therapeutics in the treatment of
different types of solid cancer tumors.
Biomica entered a clinical trial agreement with Rambam Health Care
Campus for initiating a first in-human proof-of-concept for BMC128 and in January 2022 Biomica received clearance to conduct such a trial
from the Israeli Ministry of Health. In 2022, Biomica expects to reach readout of this study.
GI Disorders
In the IBD program, BMC333 is an optimized consortium, which consists
of four bacterial strains derived from Biomica’s BMC321 and BMC322 (rationally-designed consortia that were identified using Biomica’s
computational analysis and predictive capabilities designed with specific emphasis on the anti-inflammatory activity of these strains
and their potential as novel therapeutic modality for IBD). During 2021, Biomica conducted pre-clinical trials pointing to reduction of
inflammation following treatment with BMC321 and BMC322. Following the insights provided by these pre-clinical studies, Biomica developed
BMC333, which also underwent pre-clinical trials and demonstrated BMC333’s ability to significantly reduce intestinal tissue damage
resulting from inflammation. During 2022, Biomica intends to initiate scale-up for GMP production of its drug candidate for IBD.
In the IBS program, we utilize proprietary data from several clinical
trials conducted in the U.S. to develop a novel microbiome-based drug. Biomica aims to push the barriers posed by existing therapies and
address the underlying cause of the disorder, rather than the symptoms, using bacteria or bacterial-associated factors affecting symptoms
and underlying pathophysiology.
AMR (antimicrobial resistance)
CDI
– Using Biomica's microbiome therapeutics platform, we are developing a small-molecule drug candidate (BMC201), designed to target
the main toxin secreted by the bacterium and hence repair dysbiosis in the colonic microbiome in the setting of primary or recurrent
CDI. BMC201 is being developed as an orally available drug.
MRSA
– Biomica is engaged in a collaboration with the Weizmann Institute of Science to develop a selective treatment against antibiotic
resistant strains of Staphylococcus aureus infection, in a microbiome focused approach. The company has in-licensed Prof. Ada Yonath’s,
Nobel Prize laureate, work and discoveries in high-resolution crystal structure of the large ribosomal subunit of the pathogenic Staphylococcus
aureus for the design and development of new types of selective, narrow spectrum antibiotics agents.
Intellectual Property
Biomica aims to protect the proprietary intellectual property that
it believes is important to Biomica’s business, including seeking international patent protection for its product candidates and
promptly file patent applications for new commercially valuable inventions of Biomica’s business. Biomica also relies on trade secrets
to protect aspects of its business that it does not consider appropriate for patent protection. Biomica’s success will depend on
its ability to obtain and maintain patent and other proprietary protection for commercially important technology, inventions and know-how
related to our business, as well as defend and enforce any patents that we may obtain.
Raw Materials
Biomica does not significantly rely upon any sources of raw materials
for its operations.
Seasonality
Biomica’s business in general is not subject to variations
based on seasonality.
Government Regulation of our Operations
The FDA and other regulatory authorities at federal, state and
local levels, as well as in other countries, extensively regulate, among other things, the research, development, testing, manufacture,
quality control, import, export, safety, effectiveness, labeling, packaging, storage, distribution, record keeping, approval, advertising,
promotion, marketing, post-approval monitoring and post-approval reporting of drugs and biologics such as those Biomica is developing.
Biomica, along with its contract manufacturers, will be required to navigate the various pre-clinical, clinical and commercial approval
requirements of the governing regulatory agencies of the countries in which it wishes to conduct studies or seek approval for its product
candidates. The process of obtaining regulatory approvals and ensuring subsequent compliance with appropriate federal, state, local and
foreign statutes and regulations requires the expenditure of substantial time and financial resources.
Government Regulation of Product Candidates
The development of therapeutic products targeting the underlying
biology of the human microbiome is an emerging field, and it is possible that the FDA and other regulatory authorities could issue regulations
or new policies in the future affecting our microbiome therapeutics that could adversely affect Biomica's product candidates. All of Biomica's
product candidates are based on microbiome therapy, a therapeutic approach that is designed to treat disease by restoring or providing
the targeted functions to a dysbiotic microbiome. Biomica has not, nor to its knowledge has any other company, received regulatory approval
for a therapeutic based on this approach.
Canonic Ltd.
Overview
In April 2019, we announced the establishment of Canonic, a wholly
owned subsidiary, focusing on the development and commercialization of precise and stable medical cannabis products for better therapeutic
effects using computational biology. In October 2021, Canonic began the commercialization of its first products in Israel.
Market
The global spending in the legal cannabis market is forecasted
to reach $42 billion in 202414. In 2019 the industry saw
an increase of approximately 45.7% in market size compared to 2018, reaching approximately $14.9 billion. In North America alone, the
size of this market increased to greater than $13 billion in 2019 and is estimated to reach $37 billion in 202415.
The global legal cannabis market is rapidly growing due to changes in regulatory acceptance and is divided into recreational and medical
products. According to industry publications, the market segment attributed to medical cannabis products is estimated to be 60% of the
overall market in 2024
In Israel alone, the Israeli Ministry of Health reported more than
100,000 medical cannabis patients at the end of 202116
and overall annual market of 40 tons that is estimated at an approximate market size of $260 million per year. According to industry estimations,
the number of patients is estimated to triple by 202517.
Canonic has identified three main challenges in the medical cannabis
market:
|
◾ |
Variety stability – Current cannabis varieties demonstrate high variability in active
compound concentration and other desired traits. Patients continuously seek more reliable consistent products. |
|
◾ |
Cannabinoid yield – Yield in cannabis refers to the active compounds or metabolites
found in the plant. Currently, low yield leads to higher production costs and subsequently higher costs for the patients. With the increasing
legalization of cannabis in more and more countries, the competition in the market is increasing, which leads to a price reduction per
gram of cannabis. The decreasing selling price of cannabis has made this product more sensitive to the cost of production, making yield
of active compounds per growing area a significant factor. |
14
State of legal cannabis markets, 2020, the Arcview group and BDS analytics.
15 State of legal cannabis
markets, 2020, the Arcview group and BDS analytics.
16 https://www.health.gov.il/Subjects/cannabis/Documents/licenses-status-september-2020.pdf
(the source is in Hebrew).
17 https://www.bizportal.co.il/capitalmarket/news/article/786443
(the source is in Hebrew).
|
◾ |
Cannabinoid specificity – Cannabis is known to contain hundreds of active compounds,
and a critical need is to connect specific active compounds to the relevant medical indication and to develop cannabis varieties and products
that include these specific active compounds in a stable and consistent manner. The lack of clinical data demonstrating correlation between
medical indications and the genomic and cannabinoid profile of cannabis plants creates difficulties to develop indication-specific products.
|
We believe that Canonic’s combination of assets and capabilities
in the use of computational technology, together with deep understanding of plant genomics and analysis of big data, can address these
challenges.
Competition
Canonic’s competitors include plant genomics companies aiming
to improve the properties of medical cannabis varieties, as well as companies commercializing
medical cannabis products.
Business Model
Canonic markets its medical cannabis products under its own label
to pharmacies through distributors in Israel. Canonic’s medical cannabis products are sold at over 50% of all licensed cannabis
pharmacies across Israel (approximately 90 out of a total of approximately 180 licensed pharmacies). After establishing its brand in the
Israeli market, Canonic intends to expand its activities to Europe and North America, where it intends to collaborate with local partners.
Canonic has
established a value chain from genomics to product, with certain parts of the chain, such as cultivation, production and distribution,
being outsourced to contractors. Canonic aims to focus on aspects in which it has a competitive advantage, such as the development of
improved cannabis varieties having commercially desirable traits. In executing its business model, Canonic has entered multiple production
and distribution agreements, to support production and commercialization of its products. In Israel, Canonic has executed this strategy
in 2020 and 2021, as demonstrated by the launch of its first two products.
Canonic expects to enter the European market with first products
in 2023. For this purpose, Canonic intends to establish a similar go-to-market strategy as in Israel and engage with cultivators, producers,
and distributers in Europe. In January 2022, Canonic announced that it has shipped a first batch of its cannabis varieties to Portugal,
following receiving approval to export from the Israeli Ministry of Health and Ministry of Agriculture. In the short term, Canonic intends
to grow its varieties in Portugal in a semi-commercial scale for lab testing and regulatory examinations, as required by European regulation,
before initiating commercial sales. Starting in 2023, Canonic intends that the site in Portugal will be its production site for its sales
to Europe.
Scientific Approach
Canonic is focused on the development of precise and stable medical
cannabis products based on proprietary cannabis varieties with unique genomic profiles. Leveraging Evogene's GeneRator AI tech engine
and high throughput screening capabilities, Canonic has established a unique cannabis database, which is based on its diverse genetic
collection. It has so far characterized more than 1,500 cannabis lines for their genetic and chemotype phenotype. This proprietary database,
coupled with genetic marker screening, has enabled the development of first cannabis varieties under the MetaYield program that exhibit
desired commercial attributes.
Similar methodology will enable Canonic in its Precise program
to identify genomic markers that correlate with desired active compounds in the cannabis plant, for the purpose of developing medical
grade cannabis products aimed at specific clinical indications. These genomic markers, which are used to screen our varieties for desired
medical traits, along with other advanced breeding techniques, reduce trial and error and guide Canonic while utilizing high throughput
systems to identify leading varieties for its pre-clinical testing stage.
Product Development
Canonic's product development efforts include the following main
stages:
|
◾ |
Development of varieties – pre-breeding and breeding activities of tailored cannabis
varieties (i.e., selective crossing of cannabis varieties) to achieve desired properties. During this stage Canonic also performs pre-clinical
trials to support and direct its medical product development pipeline. |
|
◾ |
Pre-production and pre-commercialization – testing of cannabis varieties, as well as
upscaling propagation and cultivation activities. |
|
◾ |
Production and commercialization – production of Canonic’s products through contractors
and distribution through regional distributors. |
|
◾ |
Post commercial data collection – this stage includes collecting patients’ feedback
on Canonic’s products in order to optimize traits and clinical effects for its future products. |
Product Development Pipeline
Canonic has two product families under development.
|
◾ |
MetaYield – focused on consumer traits and enhancement of total active compounds in
the plant. In this program, Canonic is developing various types of products, among which is the G-innovation series. In October 2021,
Canonic began the sale of its first MetaYield products, part of G-innovation series in Israel, following positive feedback received from
patients during Canonic’s pre-launch campaign: |
|
- |
G150 – marketed under the T15/C3 category (11%-19% THC18
0.5%-5.5% CBD), as defined by the Israeli Ministry of Health; and |
|
- |
G200 – marketed under the T20/C4 category (17%-24% THC 1%-7% CBD), as defined by the Israeli Ministry of Health.
|
Canonic is continuing the development of next generation product
candidates under this family of products.
|
◾ |
Precise – focused on the enhancement of
specific active compounds in the plant. The first-generation products Canonic is developing in this program target anti-inflammatory and
pain management properties. Canonic is planning commercial launch of a first Precise commercial variety in 2023, in Israel. |
In December 2020, Canonic announced positive results in pre-clinical
studies in inflammatory and pain model systems conducted by Hadassah Medical Center and by Migal - Galilee Research Institute. The results
support the identification of specific cannabis varieties with heightened anti-inflammatory and pain relief properties for Canonic’s
medical cannabis Precise product line and have led to a patent application filing.
Key Agreements
Cultivation Agreement
– Telcann
In December 2020 Canonic entered an agreement with Telcann Ltd,
a licensed Israeli medical cannabis cultivator, for the provision of plant growth services in Israel.
Collaboration Agreement
– Cannbit, a subsidiary of Tikun Olam-Cannbit
In February 2021 Canonic entered a collaboration agreement with
Cannbit Ltd, a subsidiary of Tikun Olam-Cannbit Ltd., a leading Israeli medical cannabis company, for joint development of novel medical
cannabis products. The development of the new products will be performed at Canonic’s RD facility and will be based on cannabis
strains that both companies will contribute to the collaboration. According to the agreement, each company will have full commercial rights
to the products arising from the collaboration, and there will be cross royalties by each to the other company.
18 Tetrahydrocannabinol
(THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified in the plant.
Production and Distribution
Agreements – Tikun Olam Production (Israel) Tikun Olam Supply and Distribution (Israel), subsidiaries of Tikun Olam-Cannbit
In March 2021, Canonic entered into agreements for the production
and distribution in Israel of Canonic’s medical cannabis products with Tikun Olam Production (Israel) and Tikun Olam Supply and
Distribution (Israel). The agreement is not exclusive for either party and consideration paid will be based on the scope of production
and related services provided.
Distribution Agreement
– Novolog
In August 2021, Canonic entered into an agreement for the distribution
in Israel of Canonic’s medical cannabis products with Novolog. According to the agreement, Novolog distributes Canonic’s medical
cannabis products in Israel through its distribution channels, on a consignment basis to licensed pharmacies, under the Canonic brand.
Consideration to be paid by Canonic will be based on a percentage of sales and for related services.
Production Agreement –
Cannasure
In November 2021, Canonic entered into an agreement for the production
of Canonic’s medical cannabis products with Cannasure. According to the agreement, Cannsure produces Canonic’s medical cannabis
products at its factory complying with good manufacturing practices, or GMP, standards of the Israeli Medical Cannabis Agency, or IMCA.
The agreement is not exclusive for either party and consideration paid will be based on the scope of production and related services provided.
Intellectual Property
Canonic expects its intellectual property to include three layers:
|
◾ |
Evogene's existing patent portfolio – patents regarding the use of plant genes for the improvement of plant traits and the
development of genetic markers, which is licensed exclusively to Canonic for cannabis; |
|
◾ |
Breeders’ rights – plant variety protection rights to be filed for cannabis varieties developed by Canonic. During 2021,
Canonic has filed breeders’ rights applications in Israel for several of its leading varieties; and |
|
◾ |
Patents – relating to the therapeutic attributes of active compounds within the cannabis plant, resulting from pre-clinical
or clinical trials conducted by Canonic. During 2021, Canonic has filed a provisional patent application for the pre-clinical activity
of the Precise products under development. |
Raw Materials
Canonic is producing its own reproductive materials, and therefore
it does not rely on external sources for that matter.
Seasonality
Canonic’s cultivation activities are performed under controlled
environments, which permit cultivation year-round, although in wintertime crop yield and active compound concentration may be negatively
affected.
Government Regulation of our Operations and Product Candidates
All cannabis related activities in Israel (including RD, cultivation,
manufacturing, distribution, import and export) are regulated by the IMCA. Every company with cannabis-related activity in Israel is subject
to the IMCA’s regulation and is required to obtain annually the relevant IMCA certifications for its activities. Relevant certifications
may include one or more of the following: (i) Good Security Practice, or GSP, (ii) Good Agriculture Practice, or GAP, (iii) Good Manufacturing
Practice, or GMP, (iv) Good Distribution Practice, or GDP, (v) Good Consumption Practice, or GCP, and (vi) Good Waste Disposal Practice,
or GWDP, depending on the specific activity undertaken by the company. In order to be eligible for a certain certification, a company
may be required to obtain certain preliminary approvals or licenses.
Canonic operates under the IMCA’s guidelines and has received
GSP certification, approval for its RD work plan, as well as IMC-GAP certification for its propagation farm. In addition, Canonic
has obtained relevant possession licenses, seed import permits and seedlings export permits.
Under the guidelines of the IMCA, medical cannabis can be manufactured
and marketed in Israel and exported to countries that permit the import of cannabis. Main potential target markets include Europe and
North America. In Europe, each country establishes its own regulations. In North America, Canada has legalized cannabis for both medical
and recreational use. In the U.S. regulation is established out on a state by state basis, while under federal law, the use and possession
of cannabis remains illegal.
Industrial Applications
Casterra Ag Ltd.
Overview
Our activities related to castor beans were initiated in 2007 and
in 2012 were organized under a wholly owned subsidiary, originally named Evofuel Ltd., and in 2019 re-named Casterra Ag Ltd., or Casterra.
Casterra focuses on the development of an integrated solution for castor cultivation, including advanced non-GMO high-yielding castor
bean varieties, growth protocols, and compatible agricultural machinery. Casterra’s main target market is Brazil, where large scale
castor agriculture and industry are well established, and it is also active in other selected markets.
Market
Castor beans are grown today for their high-quality oil, which
is used for the production of bio-polymers and lubricants for various industries such as the cosmetics, electronics, automotive and aerospace
industries. Currently treated as a “low-tech” crop in its key production areas around the world (for example, in India the
castor bean is grown using traditional techniques such as hand picking), according to industry estimations, the castor oil extracted from
the castor bean plant may hold great promise as an input for industrial markets. According to industry publications, the castor oil market
is expected to witness market growth at a rate of 6.7% between 2021 to 2028 and is expected to reach $3 billion by 2028.19
Competition
Casterra’s competition includes a few relatively small companies
that supply castor seeds to growers worldwide. During 2021, Casterra improved its competitive advantage by developing a proprietary dehulling
machine for castor grain. Farmers who use Casterra’s castor seed varieties gain access to Casterra’s dehulling machine
as well as to a mechanical harvester developed and marketed by Casterra’s partner.
Business Model
Casterra’s business model is to sell proprietary improved
castor seed varieties, together with targeted agro-technical growth protocols, to castor growers. These seed varieties and growth protocols
are adapted and targeted to localized characteristics. Casterra’s offering includes high yielding varieties with plant structure
suitable for mechanized harvest, best practices for large-scale castor growing, and advanced compatible mechanical harvest and dehulling
solutions.
Key agreements
Fantini s.r.l.
In October 2018, Casterra announced a breakthrough achieved in
the mechanical harvesting of castor beans with Fantini s.r.l., or Fantini, a leading manufacturer and distributor of agricultural equipment.
The lack of an available solution for mechanical harvesting has been a major challenge in the conversion of castor to a fully modernized
commercial crop, and the combination of the Fantini's harvester with Casterra’s proprietary varieties demonstrated significant improvement
in yield loss in field trials. The harvester is commercialized by Fantini to Casterra’s global partners.
19
https://www.databridgemarketresearch.com/reports/global-castor-oil-market.
Intellectual Property
Casterra’s policy is to register relevant castor varieties
in destination territories. To date Casterra has registered several of its varieties in several Latin America countries, including Brazil.
In addition, Casterra filed a patent application with respect to the dehulling machine it developed.
Government Regulation of our Operations
Casterra’s activities in Israel in the field of seeds are
regulated by the Israeli Ministry of Environmental Protection. Pursuant to these regulations, Casterra is required, among other things,
to obtain toxins permits, which allow it to conduct experiments using “hazardous materials,” as such term is defined in the
applicable regulations, and to follow specific rules regarding waste disposal. Violation of these regulations may expose Casterra to criminal
penalties, administrative sanctions and responsibility to compensate those injured for any environmental damages.
Government Regulation of Product Candidates
All seed production designated for export to Casterra's partners
is subject to field and warehouse inspection by the regulator in the country of destination for compliance with the local regulations,
including sampling and inspection for pests and diseases.
Raw Materials
Casterra does not significantly rely upon any sources of raw materials
for its operations.
Seasonality
Casterra’s castor seed business in general, and revenues
in particular, generated from sales of castor seeds and related agro-technical services to local castor growers, are subject to variations
based on crop seasonality. The timing of Casterra’s seed production, as well as the delivery of castor seeds to its partners and
revenue recognition with respect to such seed sales, derive substantially from the seasonality of castor growing in the locations where
it produces seeds and in its target markets.
C. Organizational
Structure
The legal name of our company is Evogene Ltd. and we are organized
under the laws of the State of Israel. As of the date of this report, we held directly and indirectly the percentage indicated of the
outstanding capital stock of the following significant subsidiaries:
|
Name
of Subsidiary |
|
Jurisdiction
|
|
Ownership
Interest |
|
AgPlenus Ltd. |
|
Israel |
|
98.3% (1)
|
|
Biomica Ltd. |
|
Israel |
|
93.2% (2)
|
|
Canonic Ltd. |
|
Israel |
|
100% |
|
Casterra Ag Ltd. (formerly
known as Evofuel Ltd.). |
|
Israel |
|
100% |
|
Lavie Bio Ltd. |
|
Israel |
|
70.7%
(3) |
|
(1) |
The remaining 1.7% of AgPlenus Ltd.’s outstanding share capital is held by AgPlenus’ former Chief Executive Officer and
current director as a result of exercise of options. |
|
(2) |
The remaining 6.8% of Biomica Ltd.’s outstanding share capital is held by Biomica's Chief Technology Officer. |
|
(3) |
The remaining 29.3% of Lavie Bio Ltd.’s outstanding share capital is held by (i) Pioneer Hi-Bred International, Inc. (also
known by the name Corteva), who holds 27.3%, and (ii) Lavie Bio’s former Chief Executive Officer, who holds 2.0% as a result of
exercise of options. |
D. Property,
Plants and Equipment
Our principal facility is located in Rehovot, Israel and consists
of 3,209 square meters (approximately 34,500 square feet) of leased office space accommodating our corporate offices and our molecular,
microbial and crop protection labs. The lease for this facility will expire December 31, 2024. A portion (500 square meters, or approximately
5,382 square feet) of the leased space is subleased to two unaffiliated companies.
We perform most of our testing in plants, or in-planta
testing, at our “Evogene Farm”, located on two adjacent lots that we lease outside Rehovot, which also hosts Canonic’s
cannabis RD facility. The first lease covers approximately 13,500 square meters (approximately 145,000 square feet) of land, and
expires on July 21, 2025, and we hold an option to renew such lease for an additional 36-month period. The second lease covers approximately
10,000 square meters (approximately 108,000 square feet) of land and expires on May 14, 2026, and we hold an option to renew such lease
for an additional 24-month period.
The Evogene Farm contains greenhouses, which are used for various
in-planta experiments of the company and its subsidiaries. During 2019, we converted part of the
Evogene Farm to a designated area for cannabis greenhouse as part of the activities of Canonic, our subsidiary which is focused on the
area of medical cannabis. In addition, the Evogene Farm contains warehouses, office facilities and seed banks.
Until October 2021, we leased approximately 5,750 square feet lab
facility in BRDG Park, at the Donald Danforth Plant Science Center in St. Louis, Missouri, of which approximately 1,200 square feet were
subleased since December 2017. Starting March 2020, the facility accommodated the activities of Lavie Bio Inc., a wholly owned subsidiary
of our subsidiary Lavie Bio. As of October 2021, Lavie Bio Inc. moved its operations to a subleased research and development facility
located in the City Foundry STL Project in St. Louis, Missouri, consisting of approximately 4,050 square feet, under a three-year sublease
agreement, expiring on September 30, 2024.
Unless otherwise stated, all of our facilities are fully utilized.
We have no material tangible fixed assets apart from the leased properties described above. We believe that our currently leased facilities
meet our needs for the short and mid-terms.
| ITEM 4A. |
UNRESOLVED STAFF COMMENTS |
There are no unresolved staff comments.
| ITEM 5. |
OPERATING AND FINANCIAL REVIEW AND PROSPECTS |
The information contained in this section should
be read in conjunction with our consolidated financial statements as of, and for the year ended, December 31, 2021 and related notes and
the information contained elsewhere in this annual report. Our financial statements have been prepared in accordance with IFRS as issued
by the IASB. This discussion contains forward-looking statements that are subject to known and unknown risks and uncertainties. As a result
of many factors, such as those set forth under “Item 3. Key Information—D. Risk Factors” and “Special Note Regarding
Forward-Looking Statements,” our actual results may differ materially from those anticipated in these forward-looking statements.
Summary
We are a leading computational biology company aiming to revolutionize
life-science product development across several market segments, including human health, agriculture, and other industries, by utilizing
cutting edge computational technologies.
The main challenge in product development in the life science industry
is finding the winning candidates out of a vast number of possible prospects that address a complex myriad of criteria to reach successful
products. We believe that by utilizing an advanced computational biology platform to identify the most promising candidates addressing
multiple development challenges towards successful life-science products, we can increase the probability of success while reducing time
and cost.
To achieve this mission, we established our unique Computational
Predictive Biology, or CPB, platform, leveraging big data and artificial intelligence and incorporating deep multidisciplinary understanding
in life sciences. The CPB platform is the basis for three technology engines, each focused on the direction and acceleration of the discovery
and development of products based on one of the following core components: Microbes – MicroBoost
AI, Small molecules – ChemPass AI, Genetic elements – GeneRator
AI.
We use our technological engines to support the development of
products for the life science industry through dedicated subsidiaries and with strategic partners. Currently, our main subsidiaries utilize
the technological engines to develop human microbiome-based therapeutics by Biomica, medical cannabis products by Canonic, ag-chemicals
by AgPlenus and ag-biologicals by Lavie Bio.
Since 2015, the Company’s main business model is product
development through specific market-oriented divisions. At that time, Evogene began using its technology to develop its own product pipelines,
each focusing on a specific industrial segment. When such a division and product pipeline reach a certain level of maturity, the activity
is spun into a dedicated subsidiary. Each subsidiary focuses on continued development of its pipeline and adding new products to
its commercial offering, while using Evogene's technology as its core competitive advantage.
Currently, Evogene has four main subsidiaries, each focused on
a different type of product and target market. Each subsidiary has its own board of directors, management team, scientific advisory board,
research and development, or RD, and business development teams that focus on developing its own pipeline and go-to-market activities.
At the same time, each subsidiary benefits from using Evogene’s technology under an exclusive license from Evogene to use the CPB
platform’s discovery and development engines that are relevant to the subsidiary’s field of activity. The terms of these licenses
provide that the subsidiary owns the discoveries and product candidates that result from the utilization of the CPB platform, while Evogene
retains all rights to the CPB platform itself. According to the characteristics of the end-market, the subsidiaries can decide to commercialize
their products independently or in collaboration with partners.
Another business model, which was our main business model until
2014, is product development through collaborations. In this business model Evogene engages with partners for joint development of defined
products, requested by the partners. In this frame, Evogene typically conducts the initial RD activity, discovery and early-stage
development, while later stage development and commercialization are carried out by the partner. Under this model, Evogene’s potential
revenues include RD funding for activities that Evogene conducts in the collaboration, milestone payments for when the candidates
advance in our partners’ pipelines and revenue sharing from the end-product.
Until 2014, Evogene engaged in several collaborations of this type
with Bayer, Monsanto, DuPont and Syngenta, focused on improving seed traits using genetic modification, or GM, approach. Today, Evogene
has a number of smaller scale collaborations, and we aim to engage in additional collaborations in the future.
Given the broadly applicable capabilities of our technology, as
provided through our three engines, we can potentially enhance and improve product development in a variety of life science industries,
including human health and agriculture. Today, Evogene is applying its MicroBoost AI engine to direct and accelerate the discovery and
development of two types of products: human-microbiome based therapeutics in human health and ag-biological products in agriculture.
The ChemPass AI engine is used for the discovery and development of two types of products: drugs based on small molecules in human
health and ag-chemicals, such as herbicides and insecticides, in agriculture. The GeneRator AI engine is mainly applied for the discovery
and development of medical cannabis products in human health and improved seed traits in agriculture.
As described above, since 2015 Evogene has used its three engines
to develop diverse product types through dedicated divisions and subsidiaries. In the area of human health, we established two subsidiaries:
Biomica, focusing on developing microbiome-based therapeutics, and Canonic, focusing on developing medical cannabis products. In Agriculture,
we established two subsidiaries: Lavie Bio focusing on developing ag-biologicals and AgPlenus focusing on ag-chemicals. In other industries,
we established one subsidiary, Casterra, focusing on developing ag-solutions for castor oil production.
Key Performance Indicators
Revenues
Our revenues are principally derived from research and development
payments under our collaboration agreements and related arrangements with our collaborators. Some of our agreements with collaborators
also provide for success-based payments, such as milestone payments paid by our collaborators upon the occurrence of certain specified
events and royalty revenues based on the sales or transfer of products our collaborators develop that contain, or are based on, our discoveries,
which we license to them. We have not yet generated revenues from royalty payments. In October 2021, Canonic, our subsidiary in the field
of medical cannabis, began the commercialization of its first products to pharmacies in Israel.
Breakdown of Revenues by Operating Segment:
The following table presents a breakdown of net revenues by operating
segment for the periods indicated.
| |
|
Year ended December 31, |
|
|
Operating Segment: |
|
2021 |
|
|
2020 |
|
|
2019 |
|
| |
|
(U.S. dollars, in thousands) |
|
|
Agriculture |
|
$ |
628 |
|
|
$ |
862 |
|
|
$ |
651 |
|
|
Industry |
|
|
40 |
|
|
|
33 |
|
|
|
26 |
|
|
Human |
|
|
183 |
|
|
|
75 |
|
|
|
- |
|
|
Unallocated |
|
|
79 |
|
|
|
70 |
|
|
|
76 |
|
|
Total |
|
$ |
930 |
|
|
$ |
1,040 |
|
|
$ |
753 |
|
Geographical Breakdown of Net Revenues
The following table presents net revenues by geographic breakdown
of customers as a percentage of our total net revenues for the periods indicated. This data refers to the location of the customer and
does not take into consideration the location of the end-user (to the extent it is different).
| |
|
Year ended December 31,
|
|
|
Geographical Region: |
|
2021 |
|
|
2020 |
|
|
2019
|
|
|
United States |
|
|
56 |
% |
|
|
65 |
% |
|
|
33 |
% |
|
Israel |
|
|
38 |
% |
|
|
22 |
% |
|
|
35 |
% |
|
Brazil |
|
|
2 |
% |
|
|
11 |
% |
|
|
28 |
% |
|
Other |
|
|
4 |
% |
|
|
2 |
% |
|
|
4 |
% |
|
Total |
|
|
100 |
% |
|
|
100 |
%
|
|
|
100 |
% |
Cost of Revenues
Cost of revenues primarily consists of development costs incurred
in conjunction with our collaborations, which include: salaries and related personnel costs for our research and development employees
working on the collaborations; payments to third party suppliers that assist us in producing genomic data; and the cost of disposable
materials (such as seeds, laboratory supplies, fertilizer, water and soil), and expenses related to retaining advisors, who primarily
consist of biological experts.
Operating Expenses
Research and Development Expenses, net: Research
and development expenses primarily consist of costs related to our internal or independent research and development activities, as opposed
to development costs incurred in connection with our collaborations (which are included in cost of revenues). These independent activities
of ours include the further development of our product pipeline, enhancement and expansion of our CPB platform and improvement of our
computational, scientific and validation technologies, know-how and capabilities used by our subsidiaries and product divisions. Research
and development costs include: salaries and related personnel costs (including share-based compensation); payments to third party suppliers
and subcontractors, including scale-up development of GMP batch production of drug candidate, field-trials and pre-clinical studies carried
out by third parties; cost of disposable materials; expenses associated with participation in professional conferences; operational overhead
costs, which include costs related to leasing and operating our office, laboratory facilities and greenhouses; depreciation of property,
plant and equipment; and amortization of intangible assets. Expenses related to our intellectual property, such as legal and other costs
associated with patent applications, are also included as research and development expenses. We expect that our research and developments
expenses will increase during 2022 due to the expected advancement in the pipeline of our subsidiaries and expansion in our product development
activities.
Business Development Expenses:
Business development expenses consist of costs primarily related to commercialization activities of our subsidiaries for product launch
and maintaining our relationships with our collaborators and establishing new collaborations. These costs include salaries and related
personnel costs (including share-based compensation) and expenses related to legal and professional services. We expect our business development
expenses will increase during 2022 due to expansion of our commercialization efforts. Travel expenses and other related expenses may increase
if COVID-19 ceases to be a global pandemic.
General and Administrative Expenses:
General and administrative expenses mainly consist of salaries and related personnel costs (including share-based compensation) for our
general and administrative employees; legal, DO liability insurance, and professional services; expenses related to HR activities
and employee benefits and welfare; expenses for consulting; and other expenses associated with being a U.S. publicly listed company. We
expect that our general and administrative expenses will remain at the current level during 2022.
In view of the COVID-19 pandemic, which has disrupted our operations
since March 2020, we adjusted our work plans and budget, reducing and delaying certain activities. The impact of these changes has been
minimal and by the end of 2020 and during 2021 we had resumed our full activities. With respect to 2022, we expect that the impact of
the COVID-19 pandemic on our operations (assuming the pandemic to be at the level as of the date of this annual report) will be minimal.
Financing Income and Expenses
Financing income primarily consists of interest income on our cash
bank deposits and securities; income related to a revaluation of the marketable securities we hold, which consist of money market funds,
corporate bonds and government treasury notes; and foreign currency exchange income.
Financing expenses primarily consist of issuance expenses and revaluation
of pre-funded warrants issued as part of our November 2020 $12 million fundraising; expenses related to bank charges and commissions;
expenses related to a revaluation of the marketable securities we hold; interest expense for our operating lease liability; and foreign
currency exchange expense. The interest due on government grants is also considered a financial expense and is recognized beginning on
the date on which we receive the grant until the date on which the grant is expected to be repaid.
Taxes on Income
We do not generate taxable income in Israel, as we have historically
incurred operating losses resulting in carryforward tax losses totaling approximately $164 million as of December 31, 2021, to be
carried forward indefinitely to future tax years. Accordingly, we do not expect to pay taxes in Israel for the foreseeable future, until
we have taxable income after the full utilization of our carryforward tax losses.
Our U.S. subsidiaries, Evogene Inc., Lavie Bio Inc., Lavie Bio
Tec Inc., Taxon Biosciences Inc. and AgPlenus Inc. are subject to U.S. income taxes. In 2021, the tax rates applicable to those
companies were approximately 21% and 6.5% (federal tax and state tax, respectively, where those companies operate).
Segment Data
We divide our operations into three operating segments –
agriculture, human health and industrial applications, as follows:
|
◾ |
Agriculture: our agriculture segment includes our division and subsidiaries engaged in agricultural
activities, including seed traits activity, ag-chemicals activity (through our subsidiary AgPlenus) and ag-biologicals activity (through
our subsidiary Lavie Bio). |
|
◾ |
Human Health: our human health segment focuses mainly on discovery and development of human
microbiome-based therapeutics (through our subsidiary Biomica) and cannabis activity (through our subsidiary Canonic). |
|
◾ |
Industrial Applications: our industrial applications segment focuses on the development and
commercialization of improved castor bean seeds for industrial uses (through our subsidiary Casterra). |
The following table presents our revenues and operating loss by
segment for the periods presented:
| |
|
Agriculture |
|
|
Industrial Applications |
|
|
Human Health |
|
|
Unallocated |
|
|
Total |
|
| |
|
|
(in thousands) |
|
|
|
|
|
Year ended December 31, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues |
|
$
|
628 |
|
|
$
|
40
|
|
|
$ |
183 |
|
|
$
|
79 |
|
|
$
|
930
|
|
|
Operating loss |
|
|
(12,248 |
) |
|
|
(169 |
) |
|
|
(10,087 |
) |
|
|
(8,449 |
) |
|
|
(30,953 |
) |
|
Year ended December 31, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues |
|
$
|
862 |
|
|
$
|
33 |
|
|
$
|
75 |
|
|
$
|
70 |
|
|
$
|
1,040
|
|
|
Operating loss |
|
|
(8,687 |
) |
|
|
(333 |
) |
|
|
(4,669 |
) |
|
|
(11,125 |
) |
|
|
(24,814 |
) |
|
Year ended December 31, 2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues |
|
$
|
651 |
|
|
$
|
26 |
|
|
$
|
- |
|
|
$ |
76 |
|
|
$ |
753 |
|
|
Operating loss |
|
|
(10,062 |
) |
|
|
(419 |
) |
|
|
(3,219 |
) |
|
|
(7,466 |
) |
|
|
(21,166 |
) |
A.
Operating Results
The following table sets forth our overall results of operations
(on an unsegmented basis) for the years ended December 31, 2019, 2020 and 2021. The below discussion of our results of operations omits
a comparison of our results for the years ended December 31, 2019 and 2020. In order to view that discussion, please see “Item 5.
Operating and Financial Review and Prospects—A. Operating Results—Comparison of Period-to-Period Results of Operations”
in our Annual Report on
Form 20-F for the year
ended December 31, 2020, which we filed with the SEC on April 2, 2021.
| |
|
2021 |
|
|
2020 |
|
|
2019 |
|
| |
|
|
|
|
|
|
|
|
|
|
Consolidated Statements of Comprehensive loss: |
|
|
|
|
|
|
|
|
|
|
(U.S. dollars, in thousands) |
|
|
|
|
|
|
|
|
|
|
Total Revenues |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of revenues |
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development, net
|
|
|
21,125 |
|
|
|
17,287 |
|
|
|
15,791 |
|
|
Business development
|
|
|
2,738 |
|
|
|
2,672 |
|
|
|
2,029 |
|
|
General and administrative
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating loss |
|
|
(30,953 |
) |
|
|
(24,814 |
) |
|
|
(21,166 |
) |
|
Financing income |
|
|
1,935 |
|
|
|
1,591 |
|
|
|
2,630 |
|
|
Financing expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss before taxes on income |
|
|
|
|
|
|
|
|
|
|
|
|
|
Taxes on income |
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss |
|
$ |
(30,445 |
) |
|
$ |
(26,206 |
) |
|
$ |
(19,115 |
) |
Year Ended December 31, 2021 Compared
to Year Ended December 31, 2020
Revenues
Our total revenues decreased by $0.1 million, or 10.6%, to $0.9
million for the year ended December 31, 2021 from $1.0 million for the year ended December 31, 2020. The decrease was primarily
related to a decrease in the revenues in the agriculture segment, partially offset by revenues from initial product sales in the human
health segment.
Cost of Revenues
Cost of revenues increased by $0.2 million, or 33.6%, to $0.8 million
for the year ended December 31, 2021 from $0.6 million for the year ended December 31, 2020. The increase was primarily related
to an increase in the revenues in the human health segment as well as changes in the composition of our revenues under our collaboration
agreements in the agriculture segment.
Gross Profit
Gross profit decreased by $0.3 million, or 65.0%, to $0.2 million
for the year ended December 31, 2021 from $0.5 million for the year ended December 31, 2020, due to the combined impact of changes
in our revenues and cost of revenues, as described above.
Operating Expenses
Research and Development Expenses,
Net. Research and development expenses increased by $3.8 million, or 22.2%, to $21.1 million for the year ended December 31,
2021 from $17.3 million for the year ended December 31, 2020. This increase was attributable to payments made to third parties for
(i) pre-clinical studies conducted for Biomica, (ii) Biomica’s GMP microbe drug production for its first-in-human proof-of-concept
study in the immuno-oncology program; (iii) field trials conducted in target locations for Lavie Bio and (iv) a decrease in participation
in respect of government grants to cover certain research and development expenses.
Business Development Expenses.
Business development expenses increased by $0.1 million, or 2.4%, to $2.73 million for the year ended December 31, 2021 from $2.67
million for the year ended December 31, 2020. This increase was attributable to our subsidiaries’ efforts to commercialize
their products.
General and Administrative Expenses.
General and administrative expenses increased by $2 million, or 36.3%, to $7.3 million for the year ended December 31, 2021 from
$5.3 million for the year ended December 31, 2020. This increase was attributable to (a) the impact of an industry-wide increase
in the cost of DO liability insurance and (b) an increase in salary-based expenses due to an increase in market demand for highly
skilled workers.
Financing Income and Expenses
Financing Income. Financing
income increased by $0.3 million, or 21.6%, to $1.9 million for the year ended December 31, 2021 from $1.6 million for the year ended
December 31, 2020. This increase was attributable to the change in the fair value of marketable securities and exchange rate differences
between the USD and NIS.
Financing Expenses. Financing
expenses decreased by $1.6 million, or 52.1%, to $1.4 million for the year ended December 31, 2021 from $3.0 million for the year
ended December 31, 2020. This decrease was primarily attributable to the revaluation of pre-funded warrants in 2021.
Taxes on Income
For the years ended December 31, 2021 and 2020, we recorded
insignificant amounts for taxes on income in Israel and an insignificant amount of taxes with respect to U.S. subsidiaries.
Loss
The amount of our overall loss increased by 16.2% to $30.4 million
for the year ended December 31, 2021, from $26.2 million for the year ended December 31, 2020. This increase reflected the cumulative
effect of all of the above-described line items from our consolidated statements of comprehensive loss.
B.
Liquidity and Capital Resources
Our working capital requirements generally reflect the growth in
our business and have historically been provided by cash raised from our investors, payments from our collaborators and government grants.
As of December 31, 2021, we had cash, short term bank deposits and marketable securities of $53.9 million, of which $29.6 million were
attributable to equity financing, net, and working capital of $50.0 million, which is calculated by subtracting our current liabilities
from our current assets. As of December 31, 2021, we had $4.3 million of outstanding long-term indebtedness related to government grants.
In 2021, our primary sources of liquidity were cash on hand, cash proceeds raised from
public offering of our ordinary shares and the exercise of options, proceeds from collaboration agreements and revenues from the selling
of medical cannabis products. We used these funds primarily to finance our business operations.
Public Offerings of Ordinary Shares
Cantor Controlled Equity OfferingSM Sales
Agreement
On January 14, 2021 and February 19, 2021, we entered into Controlled Equity OfferingSM
Sales Agreements, or the January Sales Agreement and February Sales Agreement, respectively, with Cantor Fitzgerald Co., or the
Agent, pursuant to which the Company could offer and sell, from time to time, its ordinary shares, through the Agent in an ATM offering,
as defined in Rule 415(a)(4) promulgated under the Securities Act, for aggregate offering price of up to $28.0 million and $50.0 million,
respectively. In February 2021, we completed the sales of ordinary shares under the January Sales Agreement and issued 3,803,594 ordinary
shares, with a weighted average selling price of $7.36 per share, resulting in gross proceeds of approximately $28 million. Subsequently
the company entered into the February Sales Agreement. As of December 31, 2021, we sold 726,832 ordinary shares with a weighted average
selling price of $3.64 per share, resulting in gross proceeds of approximately $2.6 million. We are not obligated to make any sales of
ordinary shares under the February Sales Agreement and no assurance can be given that we will sell any ordinary shares under such agreement,
or, if we do, as to the price or number of such shares that we will sell or the dates on which any such sales will take place.
Registered Direct Offerings
On September 2, 2020, we issued 5,882,353 ordinary shares in a registered direct offering.
Each ordinary share was sold at $1.70 per share resulting in gross proceeds of $10 million. On November 2, 2020, we completed a second
registered direct offering with certain institutional investors for the purchase of 3,920,000 ordinary shares at a share price of $2.50
per ordinary share and 883,534 pre-funded warrants with an exercise price of $0.01 per share at price of $2.49 per warrant (which were
exercised in the beginning of January 2021), resulting in gross proceeds of $12 million.
Shelf Registration Statement
On February 19, 2021, we filed a shelf registration statement on Form F-3 with the SEC
under which we may offer and sell from time to time in one or more offerings, our ordinary shares, debt securities, rights, warrants and
units having an aggregate offering price of up to $200 million. We registered up to $50 million under this Form F-3 in connection with
for the February Sales Agreement. We may seek additional capital or strategic considerations, even if we believe we have sufficient funds
for our current or future operating plans.
Under our RD collaboration agreements, our revenues typically
include RD funding for activities that we conduct in the collaboration, as well as milestone payments for when the candidates advance
in our partners’ pipelines and revenue sharing from the end-product.
We expect that our sources of liquidity for 2022 will include cash on hand, proceeds
raised from the public offering of our ordinary shares and the exercise of options, proceeds from collaboration agreements and revenues
from the selling of medical cannabis products, cash held in our bank accounts, including bank deposits and marketable securities,
proceeds generated from agreements with collaborators, proceeds from grants and financing transactions.
In the future, cash may serve us in effecting MA transactions
for achieving inorganic growth in our different segments of operation. We believe that our existing cash as of December 31, 2021 will
be sufficient to meet our projected cash requirements for at least 12 months. Nevertheless, in order to accelerate our subsidiaries growth
and to strengthen their position as independent companies, we are in different levels of discussions with potential strategic and financial
investors towards potential fundraisings.
Although we have sufficient cash, cash equivalents, short-term bank deposits, and marketable
securities that we believe will enable us to fund our operations during the next 12-month period at current annual expenditures, our ability
to fund our capital needs depends on our ongoing ability to generate cash from existing and future collaborations, our revenues, and from
our ability to raise additional funds. To the extent that existing cash, and cash equivalents, short-term bank deposits, and marketable
securities are insufficient to fund our future activities, we may need to raise additional funding through debt and equity financing.
Additional funds may not be available when we need them on terms that are acceptable to us, or at all.
If adequate funds are not available to us on a timely basis, we
may be required to delay, limit, scale back or cease our research and development activities, establishment and maintenance of sales and
marketing capabilities or other activities that may be necessary to commercialize our product candidates.
Cash Flows
The following table presents the major components of net cash flows
used in or provided by (as applicable) operating, investing and financing activities for the periods presented. For a discussion of our
net cash flows for the year ended December 31, 2019, please see “Item 5. Operating and Financial Review and Prospects— B.
Liquidity and Capital Resources— Cash Flows” in our
Annual
Report on Form 20-F for the year ended December 31, 2020, which we filed with the SEC on April 2, 2021:
| |
|
2021 |
|
|
2020 |
|
|
2019 |
|
|
(U.S. dollars, in thousands) |
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities |
|
$ |
(24,716 |
) |
|
$ |
(19,514 |
) |
|
$ |
(17,666 |
) |
|
Net cash provided by (used in) investing activities |
|
|
(20,566 |
) |
|
|
9,415 |
|
|
|
37,139 |
|
|
Net cash provided by financing activities |
|
|
30,276 |
|
|
|
20,374 |
|
|
|
9,306 |
|
|
Exchange rate differences - cash and cash equivalents balances |
|
|
1,102 |
|
|
|
1,206 |
|
|
|
159 |
|
|
Increase (decrease) in cash and cash equivalents |
|
$ |
(13,904 |
) |
|
$ |
11,481 |
|
|
$ |
28,938 |
|
Cash Used in Operating Activities
Cash used in operating activities for the year ended December 31,
2021 was $24.7 million and primarily reflects our overall loss of $30.4 million, as adjusted downwards to eliminate certain non-cash items
that were taken into account in calculating, and that increased, our overall loss, including $2.6 million of share-based compensation
expenses, $1.3 million of depreciation expenses and $0.9 million amortization of intangible assets and the movement in balance sheet items
of $1.7 million. These downwards adjustments to cash used were partially offset by $0.9 million of non-cash net financing income.
Cash used in operating activities for the year ended December 31,
2020 was $19.5 million and primarily reflected our overall loss of $26.2 million, as adjusted downwards to eliminate certain non-cash
items that were taken into account in calculating, and that increased, our overall loss, including $4.1 million of share-based compensation
expenses, $1.8 million of depreciation expenses, $1.0 million of net financing income, and $0.9 million amortization of intangible assets.
These downwards adjustments to cash used were partially offset by the movement in balance sheet items, including an increase in other
receivables of $1.3 million.
Cash Provided by (Used In) Investing Activities
Cash used in investing activities was $20.6 million for the year
ended December 31, 2021. That primarily reflects $23.1 million of net cash invested in the purchase of marketable securities, $1.0 million
of cash withdrawal from bank deposits and $0.8 million of cash used for the purchase of property, plant and equipment, offset by $4.4
million of net cash proceeds from the sale of marketable securities.
Cash provided by investing activities was $9.4 million for the
year ended December 31, 2020. That primarily reflects $2.1 million of net cash proceeds from the sale of marketable securities and $8.0
million of cash withdrawal from bank deposits, partially offset by $0.7 million of cash used for the purchase of property, plant and equipment.
Cash Provided by Financing Activities
Cash provided by financing activities was $30.3 million for the
year ended December 31, 2021. That was primarily attributable to $29.6 million of cash provided by the sale of newly-issued ordinary
shares in connection with the Controlled Equity OfferingSM
Sales Agreements, the net proceeds of government grants of $0.8 million and $0.5 million proceeds from exercise of options, partially
offset by $0.6 million for the repayment of an operating lease liability.
Cash provided by financing activities was $20.4 million for the
year ended December 31, 2020. That was primarily attributable to $18.7 million of net cash provided by the sale of newly issued ordinary
shares, $2.0 million for the sale of pre-funded warrants and the net proceeds of government grants of $0.3 million, partially offset by
the use of $0.6 million for the repayment of an operating lease liability.
Government Grants
Our research and development efforts have been financed, in part,
through grants from IIA, BIRD, CIIRDF and the EU. From our inception through 2021, we received grants totaling $8.6 million (including
accrued interest) from the IIA, and repaid $3.5 million, in respect of refundable projects. We also received an additional $4.3 million
from the IIA in respect of several non-refundable projects. We have received grants totaling approximately $1 million (linked to the U.S.
Consumer Price Index) from BIRD and have repaid $0.5 million, whereas the amount of $0.4 million of grants from BIRD have been cancelled,
as we decided to withdraw from the relevant project, as detailed in Note 12 to the financial statements included in this annual report
under Item 18. We have received grants totaling $1.1 million from the EU, which are not required to be repaid. As of December 31,
2021, we had two active research grants under which we have received funding from the IIA.
See “Item 3. Key Information—D. Risk Factors—Risks
Relating to Our Incorporation and Location in Israel—We have received Israeli government grants for certain of our research and
development activities. The terms of these grants may require us to satisfy specified conditions in order to manufacture products and
transfer technologies supported by such grants outside of Israel. We may be required to pay penalties in addition to repayment of the
grants.”
IIA Grants
Under the Innovation Law, research and development programs that
meet specified criteria and are approved by a committee of the IIA are eligible for grants. The grants awarded are typically up to 50%
of a project’s expenditures, as determined by the IIA committee and subject to the benefit track under which the grant was awarded.
A company that receives a grant from the IIA is typically required to pay 3% royalties to the IIA on income generated from products incorporating
know-how developed using that grant (including income derived from services associated with such products), until 100% of the U.S. dollar-linked
grant, plus interest at the annual London Interbank Offered Rate, or LIBOR, is repaid. Certain benefit tracks do not require payment of
royalties.
The obligation to pay royalties is contingent on actual income
generated from such products and services. In the absence of such income, no payment of royalties is required. It should be noted that
the restrictions under the Innovation Law, including restrictions on the sale, transfer or assignment outside of Israel of know-how developed
as part of the programs under which the grants were given will continue to apply even after the repayment of such royalties in full.
The terms of the grants under the Innovation Law also require that
the products developed as part of the programs under which the grants were given be manufactured in Israel and that the know-how developed
thereunder may not be transferred outside of Israel, unless prior written approval is received from the IIA (such approval is not required
for the transfer of a portion of the manufacturing capacity which does not exceed, in the aggregate, 10% of the manufacturing (in which
case only notification is required)), and additional payments are required to be made to the IIA, as described below. It should be noted
that this does not restrict the export of products that incorporate the funded know-how.
Ordinarily, as a condition to obtaining approval to manufacture
outside Israel, we may be required to pay royalties at an increased rate, and up to an increased cap amount of up to three or six times
the total amount of the relevant IIA grant, plus interest accrued thereon, depending on the manufacturing volume to be performed outside
of Israel.
The Innovation Law restricts the ability to transfer know-how funded
by the IIA. Transfer of IIA-funded know-how outside of Israel requires prior approval and is subject to payment of a redemption fee to
the IIA calculated according to a formula provided under the Innovation Law. A transfer for the purpose of the Innovation Law is generally
interpreted very broadly and includes, inter alia, any actual sale of the IIA-funded know-how, any license to develop the IIA-funded know-how
or the products resulting from such IIA-funded know-how or any other transaction, which, in essence, constitutes a transfer of the IIA-funded
know-how.
The IIA approval to transfer know-how created, in whole or
in part, in connection with an IIA-funded project to a third party outside Israel is subject to payment of a redemption fee to the IIA
calculated according to a formula provided under the Innovation Law that is based, in general, on the value of the transferred know-how,
multiplied by the amount of grants received from the IIA (including the accrued interest), divided by the total amounts expended by the
grant recipient on RD. To the extent any royalties were paid on account of the grants, such royalties will be deducted from the calculation.
The redemption fee is subject to a cap of six times the total amount of the IIA grants, plus interest accrued thereon, namely the total
liability to the IIA, including the accrued interest, multiplied by six. If the grant recipient undertakes that for a period of not less
than three years, at least 75% of its relevant RD positions will remain in Israel, then the cap will be reduced to three times (rather
than six times) the total liability to the IIA, calculated as set out above.
Subject to prior approval of the IIA, we may transfer the IIA-funded
know-how to another Israeli company. If the IIA-funded know-how is transferred to another Israeli entity, the transfer would still require
IIA approval but will not be subject to the payment of the redemption fee (although there will be an obligation to pay royalties to the
IIA from the income of such sale transaction as part of the royalty payment obligation). In such case, the acquiring company would have
to assume all of the selling company’s restrictions and obligations towards the IIA (including the restrictions on the transfer
of know-how and manufacturing capacity outside of Israel) as a condition to IIA approval.
We are required to pay up to 100% of the amount of grants received
by us from the IIA, plus interest at the LIBOR. In addition to paying any royalty due, we must abide by other restrictions associated
with receiving such grants under the Innovation Law. Those restrictions may impair our ability to outsource development of products containing
our traits, engage in change of control transactions or otherwise transfer our know-how outside of Israel and may require us to obtain
the approval from the IIA for certain actions and transactions and pay additional royalties and other amounts to the IIA. We cannot be
certain that any approval of the IIA will be obtained on terms that are acceptable to us, or at all. We may not receive the required approvals
should we wish to transfer IIA-funded know-how, manufacturing and/or development outside of Israel in the future. Furthermore, in the
event that we undertake a transaction involving the transfer to a non-Israeli entity of know-how developed with IIA-funding pursuant to
a merger or similar transaction, the consideration available to our shareholders may be reduced by the amounts we are required to pay
to IIA. Any approval, if given, will generally be subject to additional financial obligations. Failure to comply with the requirements
under the Innovation Law may subject us to mandatory repayment of grants received by us (together with interest and penalties), as well
as expose us to criminal proceedings. In addition, the IIA may from time to time conduct royalties audits and such audits may lead to
additional royalties being payable on additional products. Such grants may be terminated or reduced in the future, which would increase
our costs. IIA approval is not required for the marketing of products resulting from the IIA-funded research or development in the ordinary
course of business.
In January 2018, we announced participation in a three-year IIA-sponsored
Phenomics Consortium to develop tools and systems for precision agriculture and innovative development of agriculture products. In addition
to Evogene, the Phenomics Consortium consists of several Israeli industrial companies and academic institutions. The goal of the consortium
is to develop plant phenotyping technologies, including the generation of comprehensive agricultural ‘Big-Data’ and the development
of artificial intelligence algorithms for real time analysis of phenotypic data. The grant for the consortium was originally approved
for calendar year 2018 in an amount of approximately $5 million, of which approximately $1.4 million was granted to Evogene. By the end
of 2018, the grant was extended by an additional six months to a total period of 18 months until mid-2019, and the grant amount was updated
to approximately $7.6 million total, of which approximately $2.5 million was granted to Evogene. In June 2019, the IIA approved the continuation
of the consortium following such 18-month period, until the end of 2020, which would complete a three-year workplan, and granted an additional
amount of approximately $7.5 million, of which approximately $1.9 million was granted to Evogene.
In June 2020, we announced participation in a three-year workplan,
IIA-sponsored CRISPR-IL Consortium to develop an artificial intelligence based, end-to-end system for genome-editing to be used in multi-species
including human, plant, and certain animal DNA, applicable to market segments in pharma, agriculture and aquaculture. In addition to Evogene,
the CRISPR-IL Consortium consists of several Israeli industrial companies and academic institutions. The goal of the consortium is to
develop an artificial intelligence-based system, “Go-Genome”, providing users improved genome-editing workflows. The system
aims to provide end-to-end solutions, from user interface to an accurate measurement tool. The total budget for the consortium was approved
for the first 18 months in an amount of approximately $10.2 million, of which approximately $1.3 million was allocated to us. After the
first 18 months, the consortium was extended to an additional 18 months with an approved budget allocated to the Company of $1.8 million.
Participation in the IIA-sponsored consortium programs as described above does not obligate us to pay royalties to the IIA; however, the
know-how developed in such consortium programs is subject to the provisions and restrictions under the Innovation Law.
In March 2020 and March 2021, Lavie Bio obtained an IIA
approval to receive a grant for its third and fourth year programs, respectively, for bio fungicides for mildew in fruit and vegetables.
The total approved budgets for each of the third and fourth year programs were NIS 3.9 million (approximately $1.1 million for the
third year and approximately $1.2 for the fourth year).
In 2020, AgPlenus obtained IIA approval to receive a grant
for its first-year program for development of novel herbicides. The total budget was approved for NIS 3.1 million (approximately
$1.0 million).
We entered into agreements with certain of our subsidiaries in
the framework of which they were granted permission to use our technology and related know how, which was funded by the IIA. Evogene remains
responsible to the IIA for the obligations regarding such IIA funding.
BIRD Grants
We have received two BIRD grants, covering the following programs:
(i) a joint development program with DuPont-Pioneer (now Corteva) of research and development improvements to soybean rust resistance,
which the Company has repaid in full; and (ii) a joint research and development program with Marrone Bio Innovations, or MBI, for discovery
of novel modes of biological action for insect control, which the Company has decided to withdraw from.
Under the MBI BIRD program, the grant for the joint development
will be repaid: (a) from revenues received for the licensing of products developed under the project; (b) from revenues generated from
sales of products developed under the project; (c) from proceeds received from the outright sale of the technology developed under the
project; (d) if we and our partner have concluded the development of a product within the period of development defined under each of
the programs; or (e) if within 60 months from the original grant date we and MBI did not conclude the development of a product but nevertheless
decide to continue the project. In each such case, the repayment will be in an amount of up to 150% of the total grant received, depending
on the timing of the repayment.
CIIRDF Grant
The CIIRDF grant that we have received was also provided to us
as part of a previous joint project of ours with Saskatchewan Wheat Pool Inc., operating under the name of Viterra, to develop canola
with improved yield and abiotic stress tolerance. This grant will be repaid from income resulting from the commercialization of a product
developed pursuant to the grant project, at a rate of 2.5% of royalties on sales of such product, in an amount up to 100% of the total
grant received. Alternatively, we may repay the grant as royalties of 2.5% of the income we receive from licensing the product developed
pursuant to the grant. Payment of such royalties is not required if commercial revenues are not generated as a result of the project.
EU Grant
In early 2016, a grant application for a consortium for research
in photosynthesis in which we participate within the EU Horizon 2020 Program for Research and Innovation was confirmed. The consortium’s
research program is focused on an innovative approach to modulate photosynthesis related pathways aiming to improve photosynthetic efficiency.
Beyond us, the consortium includes academic institutions such as the Max Planck Institute of Molecular Plant Physiology and the Institute
of Terrestrial Microbiology, the Weizmann Institute of Science, and the Imperial College of Science, Technology and Medicine. Overall,
we received a total amount of €0.9 million for our participation in the consortium during the five-year project.
C. Research
and Development, Patents and Licenses, etc.
We continuously invest, and have for at least the last three years
historically invested, in maintaining the technological capabilities of our CPB platform, which includes tailored ‘big-data’
databases, interconnected data hubs and proprietary analysis and prediction algorithms. We also maintain laboratories, greenhouses and
fields for conducting biological validation activities for our computational predictions.
Our ongoing research and development activities are funded mainly
by internal resources, collaboration research and development payments and governmental grants. As of December 31, 2021, 96 of our employees,
representing approximately 72% of our entire work force, were engaged in research and development on a full-time basis. For more information
regarding our research and development activities, intellectual property and licenses, please see Item 4.B. “Information on the
Company—Business Overview.”
D.
Trend Information
In recent years we experienced an increase in the cost we incur
for procuring DO liability insurance, resulting from a general increase in the cost of DO liability insurance for smaller, dual-listed
public companies such as us. This general increase has been tied to perceived heightened levels of risk for DO insurers. Insurers
have been increasing their level of compensation, in the form of premiums, which they believe have not been commensurate with the risk
being taken by them. In parallel, there has been an increase in the amounts of the deductibles payable by public companies in situations
in which an insurable event occurs. If this trend continues, it will increase our operational expenses and have a negative effect on our
financial results.
A significant portion of our expenses is denominated in currencies
other than the U.S. dollar. The Company is therefore subject to non-U.S. currency risks and non-U.S. exchange exposure, especially the
NIS. Exchange rates can be volatile and a substantial change of foreign currencies against the U.S. dollar could increase or reduce the
Company’s expenses and net loss and impact the comparability of results from period to period. The devaluation of the U.S. dollar
against the NIS was 3.3%, 7.0% and 7.8% in 2021, 2020 and 2019, respectively. For example, for the year ended December 31, 2021, assuming
a 10% devaluation of the U.S. dollar against the NIS, we would have experienced a decrease in our net loss of approximately $1.3 million,
while assuming a 10% appreciation of the U.S. dollar against the NIS, we would experience an increase in our net loss of approximately
$1.3 million.
Other than as described immediately above or disclosed elsewhere
in this annual report, we are not aware of any trends, uncertainties, demands, commitments or events during our current fiscal year that
are reasonably likely to have a material effect on our net revenue, income, profitability, liquidity or capital resources, or that would
cause the financial information included in this annual report to be not necessarily indicative of our future operating results or financial
condition.
E. Critical
Accounting Estimates
Application of Critical Accounting Policies and Estimates
Our consolidated financial statements are prepared in conformity
with IFRS. Our accounting policies affecting our financial condition and results of operations are more fully described in our consolidated
financial statements included elsewhere in this annual report. The preparation of our financial statements requires management to make
judgments, estimates and assumptions that affect the amounts reflected in the consolidated financial statements and accompanying notes,
and related disclosure of contingent assets and liabilities. We base our estimates upon various factors, including past experience, where
applicable, external sources and on other assumptions that we believe are reasonable under the circumstances, the results of which form
the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual
results may differ from these estimates under different assumptions or conditions and could have a material adverse effect on our reported
results.
In many cases, the accounting treatment of a particular transaction,
event or activity is specifically dictated by accounting principles and does not require management’s judgment in its application,
while in other cases, management’s judgment is required in the selection of the most appropriate alternative among the available
accounting principles, that allow different accounting treatment for similar transactions.
We believe that the accounting policies discussed below are
critical to our financial results and to the understanding of our past and future performance as these policies relate to the more significant
areas involving management’s estimates and assumptions. We consider an accounting estimate to be critical if: (1) it requires
us to make assumptions because information was not available at the time or it included matters that were highly uncertain at the time
we were making our estimate; and (2) changes in the estimate or different estimates that we could have selected may have had a material
impact on our financial condition or results of operations.
Revenue Recognition
We recognize revenues when the control over the goods or services
is transferred to the customer. The transaction price is the amount of the consideration that is expected to be received based on the
contract terms, excluding amounts collected on behalf of third parties (such as taxes).
We have entered into research collaboration and license agreements
under which we grant to our collaborators an exclusive license to intellectual property rights for the development and commercialization
of our proprietary product candidates. The agreements contain multiple performance obligations, including funding from periodic payments
for research and development services, payments based on achievement of specified milestones and royalties on sales of products sold by
our collaborators that include the licensed traits.
Revenues from research and development services as part of our research collaboration
and license agreements are recognized over time, during the period the customer simultaneously receives and consumes the benefits provided
by our performance. Recognition of the service is throughout the services period and is determined based on the proportion of actual costs
incurred for each reporting period to the estimated total costs, subject to the enforceable rights. We charge our customers based on payment
terms agreed upon in specific agreements. When payments are made before or after the service is performed, we recognize the resulting
contract asset or liability.
If the contract contains a single performance obligation, the entire
transaction price is allocated to the single performance obligation. Contracts that contain multiple performance obligations such as licenses,
services, royalties and milestone events require an allocation of the transaction price to each performance obligation based on a relative
standalone selling price, or SSP, basis. The Company establishes SSP based on management judgment, considering internal factors such as
margin objectives, pricing practices and historical sales.
Revenues from milestone events stipulated in the agreements are
recognized upon the occurrence of event or achievement of the milestone specified in the agreement.
Share-Based Compensation
We account for share-based compensation in accordance with the
fair value recognition provision of IFRS guidance on share-based compensation. Under these provisions, share-based compensation is measured
at the grant date based on the fair value of the award and is recognized as an expense, net of estimated forfeitures, over the requisite
service period, which is generally the vesting period of the respective award. Share-based compensation expense was $2.6 million, $4.1
million and $1.6 million in 2021, 2020 and 2019, respectively. We selected the binomial option-pricing model as the most appropriate method
for determining the estimated fair value of our share-based compensation. The determination of the grant date fair value of options using
an option-pricing model is affected by estimates and assumptions regarding a number of complex and subjective variables. These variables
include the estimated period of time that we expect employees to hold their options, the expected volatility of our share price over the
expected term of the options (estimated using historical data from prior years, including historical forfeiture rates), share option exercise
and cancellation behaviors, risk-free interest rates, expected dividend yields (assumed to be zero as we have historically not paid and
do not intend to pay dividends on our ordinary shares) and the price of our ordinary shares. In addition, our compensation expense is
affected by our estimate of the number of awards that will ultimately vest. In the future, if the number of equity awards that are forfeited
by employees is lower than expected, the expense recognized in future periods will be higher.
Government Grants
Government grants received from the IIA are recognized as a liability
if future economic benefits are expected from the projects that will result in royalty-bearing sales.
A liability for a grant is first measured at fair value using a
discount rate that reflects a market rate of interest. The difference between the amount of the grant received and the fair value of the
liability is accounted for as a government grant and recognized as a reduction of research and development expenses. After initial recognition,
the liability is measured at amortized cost using the effective interest method. Royalty payments we make to repay the grant are treated
as a reduction of the liability. If no economic benefits are expected from the research activity, the grant receipts are recognized as
a reduction of research and development expenses, in which case, the royalty obligation is treated as a contingent liability.
There is uncertainty regarding the estimates of future cash flows
and the estimate of the capitalization rate that is used for determining the amount of the liability recognized. At the end of each reporting
period, we evaluate whether there is reasonable assurance that the liability recognized, in whole or in part, will not be repaid (since
we will not be required to pay royalties) based on the best estimate of future sales, and if so, the appropriate amount of the liability
is recognized as a reduction of research and development expenses.
Leases
We cannot readily determine the interest rate implicit in our operating
lease for our principal facility in Rehovot, Israel. We therefore use our incremental borrowing rate, IBR, to measure lease liabilities.
The IBR is the rate of interest that we would have to pay to borrow over a similar term, and with a similar security, the funds necessary
to obtain an asset of a similar value to the right-of-use asset in a similar economic environment. The IBR therefore reflects what we
‘would have to pay’, which requires estimation when no observable rates are available or when they need to be adjusted to
reflect the terms and conditions of the lease.
We estimate the IBR using observable inputs (such as market interest
rates) when available and we are required to make certain entity-specific estimates (such as the Company's stand-alone credit rating).
Intangible
assets
On August 6, 2019, Corteva invested in the Company's agriculture
biologicals subsidiary, Lavie Bio, by way of a contribution of all Corteva’s holdings in its wholly owned subsidiary Taxon Biosciences,
which included several intangible assets, and payment of an amount of $10 million in cash.
The fair value of intangible assets received through the Corteva
investment is determined upon initial recognition by either one of three traditional methods in valuating an asset. These methods include
the market approach, the income approach and the cost approach. The pipeline products and potential products were valued by applying the
income approach and the Microorganisms collection was valued using the cost approach.
The Company’s significant estimates in this analysis included,
but were not limited to, future cash flow projections, the weighted average cost of capital, the terminal growth rate, and the tax rate.
The Company believes the current assumptions and estimates utilized were both reasonable and appropriate. Future cash flow estimates
are, by their nature, subjective and actual results may differ materially from the Company’s estimates. If the Company’s
ongoing estimates of future cash flows are not met, the Company may have to record impairment charges in future periods. The Company’s
estimates of future cash flows are based on current regulatory and economic climates, recent operating results, and planned business strategy.
These estimates could be negatively affected by changes in federal, state, or local regulations or economic downturns.
The useful economic life of the intangible assets acquired by
us in this transaction was determined through years of development until final year of projected sales. When applying the income approach,
the cash flows expected to be generated by intangible assets are discounted to their present value equivalent using a rate of return that
reflects the relative risk of the investment, as well as the time value of money. For each intangible asset, a specific discount rate
was valuated using “Modified CAPM Build-Up Method”.
The Company evaluates the need to record an impairment of non-financial
assets whenever events or changes in circumstances indicate that the carrying amount is not recoverable. If the carrying amount of non-financial
assets exceeds their recoverable amount, the assets are reduced to their recoverable amount. The recoverable amount is the higher of fair
value less costs of sale and value in use. In measuring value in use, the expected future cash flows are discounted using a pre-tax discount
rate that reflects the risks specific to the asset. The recoverable amount of an asset that does not generate independent cash flows is
determined for the cash-generating unit to which the asset belongs. Impairment losses are recognized in profit or loss.
Impact of Israeli Tax Policies and Government Programs on Our Operating
Results
Tax regulations have a material impact on our business, particularly
in Israel where we have our headquarters. The following summary describes the current tax structure applicable to companies in Israel,
with special reference to its effect on us.
General Corporate Tax Structure in Israel
Israeli companies are generally subject to corporate tax on their
taxable income. In 2021, the corporate tax rate was 23%. Capital gains derived by an Israeli company are generally subject to tax at the
prevailing regular corporate tax rate.
Law for the Encouragement of Industry (Taxes),
5729-1969
The Law for the Encouragement of Industry (Taxes), 5729-1969, generally
referred to as the Industry Encouragement Law, provides several tax benefits for an “Industrial Company”.
The Industry Encouragement Law defines an “Industrial Company”
as an Israeli resident company which was incorporated in Israel, of which 90% or more of its income in any tax year, other than income
from certain government loans, is derived from an “Industrial Enterprise” owned by it and located in Israel. An “Industrial
Enterprise” is defined as an enterprise that is held by an Industrial Company whose principal activity in a given tax year is industrial
production.
The following tax benefits, among others, are available to Industrial
Companies:
|
◾ |
amortization over an eight-year period of the cost of purchased know-how and patents and rights to use a patent and know-how which
are used for the development or advancement of the Industrial Enterprise, commencing in the year in which such rights were first exercised;
|
|
◾ |
under limited conditions, an election to file consolidated tax returns together with Israeli Industrial Companies controlled by it;
and |
|
◾ |
expenses related to a public offering are deductible in equal amounts over a three-year period, commencing in the year of the offering.
|
Eligibility for benefits under the Industry Encouragement Law is
not contingent upon the approval of any governmental authority. We believe that we currently qualify as an Industrial Company within the
meaning of the Industry Encouragement Law. There can be no assurance that we will continue to qualify as an Industrial Company or that
the benefits described above will be available in the future.
Law for the Encouragement of Capital Investments,
5719-1959
The Law for the Encouragement of Capital Investments, 5719-1959,
generally referred to as the Investment Law, provides certain incentives for capital investments in production facilities (or other eligible
assets) by “Industrial Enterprises” (as defined under the Investment Law).
The Investment Law was significantly amended effective April 1,
2005 (which we refer to as the 2005 Amendment), further amended as of January 1, 2011 (which we refer to as the 2011 Amendment) and
further amended as of January 1, 2017 (which we refer to as the 2017 Amendment). Pursuant to the 2005 Amendment, tax benefits granted
in accordance with the provisions of the Investment Law prior to its revision by the 2005 Amendment remain in force but any benefits granted
subsequently are subject to the provisions of the 2005 Amendment. Similarly, the 2011 Amendment introduced new benefits to replace those
granted in accordance with the provisions of the Investment Law in effect prior to the 2011 Amendment. However, companies entitled to
benefits under the Investment Law as in effect prior to January 1, 2011 were entitled to choose to continue to enjoy such benefits,
provided that certain conditions are met, or elect instead irrevocably to forego such benefits and have the benefits of the 2011 Amendment
apply. The 2017 Amendment introduced new benefits for Technological Enterprises, alongside the existing tax benefits.
On October 24, 2010, we received a tax ruling from the Israel
Tax Authority, according to which, among other things, our activity has been qualified as an “industrial activity”, as defined
in the Investment Law and is also eligible to tax benefits as a Beneficiary Enterprise, which will apply to the turnover attributed to
such enterprise. The benefit period under this tax ruling ended in 2018, and since we did not generate any taxable income in tax year
2018, we were not entitled to any tax benefits under this tax regime.
In addition, we have reviewed and evaluated the implications and
effect of the benefits under the 2011 and 2017 Amendments, and, while potentially eligible for such benefits, we have not yet chosen to
be subject to the tax benefits introduced by the 2011 or the 2017 Amendments.
| ITEM 6. |
DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES |
A. Directors
and Senior Management
The following table sets forth the name, age and position of each of our executive officers
and directors as of the date of this annual report.
|
|
|
|
|
Position |
|
Executive officers |
|
|
|
|
|
Mr. Ofer Haviv |
|
55 |
|
President and Chief Executive Officer |
|
Dr. Nir Arbel |
|
42 |
|
Chief Product Officer |
|
Dr. Brian Ember |
|
46 |
|
Chief Executive Officer of AgPlenus Ltd. |
|
Dr. Elran Haber |
|
41 |
|
Chief Executive Officer of Biomica Ltd. |
|
Dr. Arnon Heyman |
|
45 |
|
Chief Executive Officer of Canonic Ltd. |
|
Mr. Mark Kapel |
|
45 |
|
|
|
Ms. Dorit Kreiner |
|
50 |
|
Chief Financial Officer |
|
Mr. Sassi Masliah |
|
43 |
|
Executive Vice President Corporate Development |
| |
|
|
|
|
|
Directors |
|
|
|
|
|
Ms. Sarit Firon(3)(4)
|
|
55 |
|
Chairperson of the Board |
|
Mr. Dan Falk(1)(2)(4)
|
|
77 |
|
Director |
|
Mr. Ziv Kop(1)(2)(3)(4)
|
|
50 |
|
Director |
|
Dr. Adrian Percy(4)
|
|
56 |
|
Director |
|
Mr. Leon Y. Recanati(3)(4)
|
|
73 |
|
Director |
|
Dr. Oded Shoseyov(1)(2)(4)
|
|
65 |
|
Director |
____________________________
|
(1) |
Member of our Audit Committee. |
|
(2) |
Member of our Compensation and Nominating Committee. |
|
(3) |
Member of our Corporate Development Committee. |
|
(4) |
Independent director under the Nasdaq Listing Rules. |
Executive Officers
Mr. Ofer Haviv has
served as Evogene's President and Chief Executive Officer since December 2004 after having joined the company in January 2002 as Chief
Financial Officer. Mr. Haviv serves as Chairperson of the Board of Directors of our subsidiaries. From 2006 to 2007, Mr. Haviv
served as a director of the company. Mr. Haviv is a Certified Public Accountant and holds a BA in Accounting and Economics from Tel-Aviv
University, Israel.
Dr. Nir Arbel has
served as Chief Product Officer of Evogene since August 2021. Dr. Arbel has over ten years of experience in biotechnology and medical
technology companies in product development and commercialization. Prior to joining the Company, Dr. Arbel served as Chief Executive Officer
and Co-Founder of Carmentix Pte Ltd, Singapore, a company focused on high-risk pregnancy prognosis, from 2015 to 2020, and as the Operating
Partner in Esco Ventures, a medical technology fund based in Singapore, from 2016 to 2020. Dr. Arbel holds a Ph.D. in Biochemistry from
Ben-Gurion University, Israel.
Dr. Brian Ember
has served as Chief Executive Officer of AgPlenus Ltd., a subsidiary of Evogene, since December 2021. Dr. Ember has previously held various
senior leadership roles, including, during 2021, Head of Global Portfolio Management and Head of Marketing and Business Development, Americas
for Biotalys, an agricultural technology company focused on reinventing food protection with protein-based biocontrol solutions; Senior
Director, Business Development for AgriMetis, an innovative crop protection company, between 2018 and 2021; and various management roles
at BASF from 2012 to 2018 and Syngenta from 2008 to 2012. Dr. Ember holds a B.Sc. in Chemistry from the University of Florida, Gainesville,
Florida; a Ph.D. in Organic Chemistry from the University of Georgia, Athens, Georgia; and an MBA from Kenan-Flagler Business School at
the University of North Carolina, Chapel Hill, North Carolina.
Dr. Elran Haber
has served as Chief Executive Officer of Biomica Ltd., a subsidiary of Evogene, since January 2018. Dr. Haber previously served as Chief
Executive Officer of Therapix Biosciences Ltd. (now known as SciSparc Ltd.) (NASDAQ: SPRC) beginning in November 2015. Prior to that,
from March 2014, Dr. Haber served as our Vice President of Business Strategy and Innovation. Dr. Haber served for more than 10 years as
Chairperson and board member of several publicly traded and privately held companies, including Issta Lines Ltd. (TASE: ISTA) from 2007
to 2012, and American Express Global Business Travel – Israel (Histour-Eltive Ltd.) from 2010 to 2012, and has been a member of
various board committees and has served in senior executive roles in various life science companies. Dr. Haber holds a Ph.D. in Pharmaceutical
Science and an MBA in Finance Financial Engineering, both from The Hebrew University of Jerusalem, Israel.
Dr. Arnon Heyman
has served as Chief Executive Officer of Canonic Ltd., a subsidiary of Evogene, since April 2019. He previously served as Vice President
General Manager of Ag-Seeds and as director of project management crop protection from 2018. Prior to Evogene, Dr. Heyman served
as Chief Technology Officer of BondX Technologies Ltd. from 2009-2014. Dr. Heyman holds a Ph.D. in Biotechnology from The Hebrew University
of Jerusalem, Israel, and an MBA from the College of Management, Israel.
Mr. Mark Kapel
was appointed as Executive Vice President Technology in February 2018, previously serving as Director of Information Technologies
Data Management from 2013. Mr. Kapel joined Evogene in 2005 and has held various positions in the company over the years. Mr. Kapel
holds a B.Sc. in Physics Computers from the Ben Gurion University of Negev, Israel, an MBA specializing in Management of Technology
from Tel-Aviv University’s Faculty of Management – Recanati Graduate School of Business Administration, Israel.
Ms. Dorit Kreiner
has served as Chief Financial Officer of Evogene since February 2019. Ms. Kreiner has previously served as CFO of a number of companies,
including NRGene (TASE: NRGN) between 2014 and 2018 and Therapix Biosciences Ltd. (now known as SciSparc Ltd.) (NASDAQ: SPRC) between
2011 and 2014. Ms. Kreiner also previously filled the position of Director of Finance of Evogene between 2004 and 2011. Ms. Kreiner holds
a B.A. in accounting and economics and an MBA in Finance and Marketing from the Tel-Aviv University, Israel, and an LL.B. from the College
of Management, Israel.
Mr. Sassi Masliah
has served as Executive Vice President Corporate Development since February 2022. Mr. Masliah has held various positions within Evogene
over the last 11 years, most recently as Evogene’s Vice President for Legal Affairs and Corporate Secretary. Mr. Masliah holds an
LL.B. and B.A. in economics from the Tel-Aviv University, Israel.
Directors
Mr. Dan Falk
has served as a director of our Company since he was appointed by the Board in November 2021. Mr. Falk has extensive experience
of more than 20 years in serving as a financial expert on public and private company boards, most recently on the boards of Nice Ltd.
(NASDAQ: NICE), Ormat Technologies Inc. (NYSE: ORA) and Innoviz Technologies Ltd. (NASDAQ: INVZ). Additionally, in the past Mr. Falk held
various executive positions in Orbotech Ltd. between 1985 and 1999, and Sapiens International Corporation (NASDAQ: SPNS) between 1999-2001.
Mr. Falk holds a B.A. in Economics and Political Sciences, and an M.A. in Business Administration both from the Hebrew University of Jerusalem,
Israel.
Ms. Sarit Firon has
served as a director of our Company since she was appointed by the Board in August 2016, and as chairperson since August 2021. Ms. Firon
is managing partner of Team8 Group and co-founder and managing partner of Team8 Capital, the investment arm of Team8 Group, which invests
in early-stage technology startups. Previously, she was a managing partner of Cerca Partners, an Israeli venture capital fund. She has
served at Extreme Reality Ltd., as its Chief Executive Officer from December 2012 to November 2014 and as a director since December 2014.
From November 2011 to November 2012, Ms. Firon was the Chief Financial Officer of Kenshoo Ltd. From November 2007 to October 2011, Ms.
Firon was the Chief Financial Officer of MediaMind Technologies Inc., a Nasdaq listed company which was acquired by DG, Inc. in August
2011. From May 2005 to June 2007, Ms. Firon was the Chief Financial Officer of OliveSoftware and from January 2000 to October 2004, she
was the CFO of P-Cube, a private company which was acquired in October 2004 by Cisco Systems, Inc. (Nasdaq: CSCO). From October 2004 to
January 2005, Ms. Firon was employed by Cisco to be responsible for the post-merger integration of P-Cube. From January 1995 to December
1999, Ms. Firon served in various positions at Radcom Ltd. (Nasdaq: RDCM), including as its Chief Financial Officer from September 1997
to December 1999. Since July 2015, she has served as chairperson of the board of directors of myThings Israel Ltd. Since June 2014, Ms.
Firon has served as a director of Mediwound Ltd. (Nasdaq: MDWD), and since June 2012, Ms. Firon has served as a director of Datorama Ltd.
From October 2000 to December 2006, Ms. Firon served as a director of MetaLink Ltd. (OTCMKTS: MTLK). Ms. Firon holds a B.A. in Accounting
and Economics from Tel-Aviv University, Israel.
Mr. Ziv Kop has
served as a director of our Company since January 2014. Mr. Kop serves as a director of Outbrain Inc. and Outbrain Ltd. Mr. Kop currently
serves as Managing Partner at OG Tech Partners. From 2017 to 2019, Mr. Kop served as Partner at Marker/Innovation Endeavors venture capital
fund. From February 2014 to June 2016, Mr. Kop served as Chief Operating Officer and board member of Outbrain Inc. a web-based content discovery
platform. Previously, and since its inception in 2003 until June 2013, Mr. Kop was a Managing Partner at GlenRock Israel., a private equity
investment firm, where he managed a portfolio of growth companies in the fields of advanced technologies and healthcare, and served on
the board of more than ten private and public companies. Prior to his role at GlenRock, Mr. Kop served as Chief Executive Officer of POC
Management Consulting, a leading Israeli consultancy in the field of strategic planning. Mr. Kop holds an LL.B. from the Tel-Aviv University
Law School and Business School, Israel, and is a graduate of INSEAD’s Young Managers Program, France.
Dr. Adrian
Percy has served as a director of our Company since February 2019. Dr. Percy serves on the board of directors of BioLumic,
HiFidelity Genetics and FA Bio. He is a member of the science and technology boards of Terramera, Oerth Bio, Harpe Bio, Biotalys
and Rothamsted Research. Dr. Percy is currently a venture partner with Finistere Ventures and frequently acts as an advisor to companies
through his own consultancy company, Nomad Technology Consulting. From 2019-2021, Dr. Percy served as Chief Technology Officer at UPL
Ltd. From 2014-2018, he served as the head of research and development for the Crop Science division of Bayer as part of its executive
committee. During his 16-year tenure at Bayer, he also led regulatory affairs activities across the entire division of Crop Science between
2013 and 2014 and crop protection development activities for Bayer in North America between 2011 and 2013. Dr. Percy has held positions
in the research and development departments of Aventis CropScience between 2000 and 2002, Rhone Poulenc between 1996 and 2000, and Bayer
in France, Germany and the United States. Dr. Percy earned a bachelor’s degree in pharmacology at the University of Liverpool, England,
as well as a master’s degree in toxicology and a doctorate in biochemistry at the University of Birmingham, England.
Mr. Leon Y.
Recanati has served as a director of our Company since May 2005. Mr. Recanati has served as chairperson and chief executive
officer of GlenRock Israel Ltd. since 2003. Previously, Mr. Recanati was chief executive officer or chairperson positions at IDB
Holding Corporation, Clal Industries, Azorim Investment Development and Construction Co., Delek Israel Fuel Corporation, and Super-Sol.
He also founded Clal Biotechnologies Industries, a biotechnology investment company operating in Israel. Mr. Recanati holds an MBA
from The Hebrew University of Jerusalem, Israel, and Honorary Doctorates from the Technion Institute of Technology, Israel, and Tel-Aviv
University, Israel.
Dr. Oded Shoseyov has
served as a director of our Company since November 2018. Dr. Shoseyov is the scientific founder of 15 companies, including: Futuragene
Ltd., Collplant Ltd., Biobetter Ltd., GemmaCert Ltd., SP-Nano materials Ltd., Melodea Ltd., Valentis Nanotech. Ltd., Paulee CleanTec Ltd.,
Smart Resilin Ltd., Sensogenic Ltd., SavorEat Ltd. and Karme Yosef Winery. Dr. Shoseyov is a faculty member of The Hebrew University of
Jerusalem, Israel, where he conducts research in plant molecular biology protein engineering and nano-biotechnology. His group’s
focus is on Bio-Inspired Nanocomposite materials. He has authored or co-authored more than 300 scientific publications and is the inventor
or co-inventor of 94 patents. Dr. Shoseyov is a TED speaker and a co-owner and winemaker of Bravdo winery. Dr. Shoseyov received the Outstanding
Scientist Polak Award for 2002, the 1999 and 2010 Kay Award for Innovative and Applied Research, the 2012 Israel Prime Minister Citation
for Entrepreneurship and Innovation, and the 2018 Presidential Award for his contribution to the Economy and Society of Israel. Dr. Shoseyov
holds a B.Sc., an M.Sc. and a Ph.D. from The Hebrew University of Jerusalem, Israel.
Arrangements for Election of Directors and Members of Management;
Family Relationships
There are no arrangements or understandings with major shareholders,
customers, suppliers or others related to the election of our board of directors or the appointment of members of our senior management.
There are furthermore no family relationships among any directors or members of our senior management.
B. Compensation
Aggregate and Individual Compensation of Officers and Directors
The aggregate compensation, including non-cash share-based compensation
(consisting of expenses related to option grants), accrued by us in respect of the year ended December 31, 2021 to all persons who served
as directors and/or executive officers during that year, was approximately $3.5 million. That amount includes approximately $0.4 million
of gross compensation set aside or accrued for executive officers for purposes of pension, severance, retirement, car, phone or similar
benefits or expenses, but does not include business travel, relocation, professional and business association dues and expenses reimbursed
to executive officers, and other expenses commonly reimbursed by companies in Israel. These amounts include the partial-year compensation
paid to five executive officers and two directors, who served in their positions over the course of 2021 and whose employment as directors
or executive officers either was terminated or has commenced during 2021.
During 2021, we granted to our executive officers and directors
an aggregate amount of 440,500 options, of which 175,000 were granted with an exercise price of NIS 8.45 ($2.72), 240,000 were granted
with an exercise price of NIS 9.17 ($2.95), 2,500 were granted with an exercise price of NIS 12.75 ($4.10), 2,500 were granted with an
exercise price of NIS 15.49 ($4.98), 2,500 were granted with an exercise price of NIS 20.61 ($6.63), and 18,000 were granted with an exercise
price of $2.98. The options detailed above expire within ten years from the date of grant. The option grants detailed above include grants
to one executive officer, who served in his position over the course of 2021 and whose employment terminated in 2022 before the date of
this report. In addition, during 2021, one executive officer, who serves as chief executive officer in one of our subsidiaries, was granted
options to purchase equity of such subsidiary, which are not detailed above.
The following table presents information regarding compensation
accrued in our financial statements for the year ended December 31, 2021 for our five most highly compensated executive officers, as required
under Israeli Securities Law 5728-1968 and the regulations promulgated thereunder.
| |
|
(in
thousands, US$)(1)
|
|
|
|
|
Salary and related benefits
|
|
|
|
|
|
Value of Options Granted(3) |
|
|
|
|
|
Ofer Haviv
President and Chief Executive Officer |
|
|
420 |
|
|
|
52 |
|
|
|
58 |
|
|
|
530 |
|
|
Ido Dor
CEO Lavie Bio |
|
|
249 |
|
|
|
- |
|
|
|
153 |
|
|
|
402 |
|
|
Dr. Elran Haber
CEO of Biomica |
|
|
256 |
|
|
|
31 |
|
|
|
197 |
|
|
|
484 |
|
|
Mark Kapel
EVP Technology |
|
|
266 |
|
|
|
29 |
|
|
|
14 |
|
|
|
309 |
|
|
Douglas A. Eisner
|
|
|
179 |
|
|
|
- |
|
|
|
185 |
|
|
|
364 |
|
|
(1) |
All amounts reported in the table are in terms of cost to the Company, as recorded in our financial statements. |
|
(2) |
Bonus amounts shown in this table reflect bonuses that were paid in 2022 relating to the office holders’ service in our Company
in 2021, as approved by our compensation and nominating committee and board of directors, and, to the extent required, also by our shareholders.
|
|
(3) |
Consists of amounts recognized as non-cash expenses in our statement of profit or loss for the year ended December 31, 2021 in respect
of option grants. Some of our office holders were granted options to purchase equity of our subsidiaries for which they serve as officers,
for which the related expenses were recorded in our statement of profit or loss. |
Compensation Policy
As required by the Companies Law, we have adopted a policy regarding
the terms of engagement of office holders, or a compensation policy. Under the Companies Law, the term “office holders” includes
directors and certain officers, including the general manager (i.e., chief executive officer, or CEO), chief business manager, deputy
CEO, vice CEO, any other person assuming the responsibilities of any of the foregoing positions without regard to such person’s
title, and any director or manager who reports directly to the CEO. The compensation policy serves as the basis for determining the financial
terms of employment or engagement of office holders, including exculpation, insurance, indemnification or any monetary payment or obligation
of payment in respect of employment or engagement. The compensation policy must relate to certain factors specified in the Companies Law,
including advancement of the company’s objectives, the company’s business and its long-term strategy, and creation of appropriate
incentives for executives. It must also consider, among other things, the company’s risk management, size and the nature of its
operations. The Companies Law describes what factors have to be considered by, and what principles must be included in, the compensation
policy.
Our current compensation policy was adopted in August 2021, at
an annual general meeting of our shareholders, following the recommendation of our compensation committee and our board of directors.
Approvals Required for Compensation of Directors and Officers
Under the Companies Law, the compensation of each of our directors
and our CEO requires the approval of our compensation committee, the subsequent approval of the board of directors and, unless exempted
under the regulations promulgated under the Companies Law, the approval of our shareholders at a general meeting (in the case of our CEO,
the shareholder approval must include the special majority described under “Item 6. Directors, Senior Management and Employees—
C. Board Practices— Approval of Related Party Transactions under Israeli Law— Disclosure of Personal Interests of an
Office Holder and Approval of Certain Transactions”). The compensation of any other office holder (who is neither a director nor
our Chief Executive Officer), if consistent with our compensation policy, requires the approval of our compensation committee, followed
by our board of directors. Compensation of any such office holder that deviates from our compensation policy also requires shareholders
approval, including by the special majority described under “Item 6. Directors, Senor Management and Employees— C. Board Practices—
Approval of Related Party Transactions under Israeli Law— Disclosure of Personal Interests of an Office Holder and Approval of Certain
Transactions.”
Compensation of Executive Officers
Our compensation for our executive officers is paid pursuant to
written employment agreements that we have entered with each of our executive officers and is based, in part, on each executive officer’s
personal contribution to our management, operations and success. Such compensation is determined consistent with our compensation policy.
For more information on our compensation policy, please see “—Compensation Policy” above.
Each executive officer’s entitlement to an annual bonus is
determined according to a formula that links financial and qualitative target-based goals and metrics related to the specific objectives
within the responsibility of the relevant executive officer. In the case of executive officers who are also office holders, their annual
bonus is also required to be consistent with our compensation policy. The goals and objectives of Evogene Ltd.’s office holders
are determined by the compensation and nominating committee and our board of directors. For each fiscal year, our compensation and nominating
committee and board of directors determine the maximum target bonus for each of our office holders, including our CEO. Further, for our
CEO, all terms of employment, including bonus terms, require, in general, approval by a majority of our shareholders present and voting
(in person or by proxy) at a meeting of shareholders, subject to fulfillment of one of the two additional conditions described in “Item
6. Directors, Senior Management and Employees—C. Board Practices—Approval of Related Party Transactions under Israeli Law—Disclosure
of Personal Interests of an Office Holder and Approval of Certain Transactions.”
Each of the employment agreements with our executive officers contains
provisions regarding non-competition, confidentiality of information and assignment of inventions. The non-competition provision applies
for a period that is generally 12 months following termination of employment. The enforceability of covenants not to compete in Israel
and in the United States is subject to limitations. The employment agreement of each executive officer is terminable at will upon 60 days
written notice by either party to the agreement, except for the employment agreement with Mr. Ofer Haviv, our President and CEO, which
is terminable at will upon 90 days written notice, by either party to the agreement.
Director Compensation
Our directors are entitled to cash compensation and equity compensation
as follows:
Cash Compensation of
Directors
All of our directors receive annual fees and per-meeting fees
for their service on our board and its committees, in the following amounts:
|
◾ |
Annual fees in an amount of approximately $19,200 for directors not classified as experts and approximately $25,600 for directors
classified as experts; and |
|
◾ |
Per-meeting fees in an amount of approximately $1,000 for directors not classified as experts and approximately $1,300 for directors
classified as experts; 60% of such amounts for participation in meetings via telecommunication and 50% of such amounts for resolutions
adopted in writing. |
Such amounts are within the range for cash compensation for
external and unaffiliated directors of a company of our size (based on level of shareholders’ equity) under the Companies Law.
Cash Compensation of
Chairperson of the Board
In accordance with shareholders’ approval from August
2021, a chairperson of the board who is determined by the Board to be an “active chairperson” in light of increased involvement
in our activities and increased time investment in the performance of the duties accompanying the chairperson’s position compared
to the other directors, shall be entitled to increased compensation relative to our other directors of approximately $7,700 per month
(equal to NIS 25,000). Our Board has determined that Ms. Sarit Firon, our chairperson of the board, is an active chairperson, and accordingly
her fees as active chairperson are as aforesaid.
Equity Compensation of
Directors
In accordance with our shareholders’ approval from August
2021, and in compliance with our compensation policy, each non-employee director is granted options to purchase 18,000 ordinary shares
of the Company on the date of our annual general shareholders meeting at which such director is elected or re-elected to the board. The
chairperson of our board is granted options to purchase 36,000 ordinary shares. These options vest over a period of one year, with 25%
of the options vesting at the end of each successive three-month period following the director’s appointment or re-appointment (as
applicable) by the general meeting of shareholders, subject to continued service through each vesting date.
All option grants to directors following the approval of our 2021
Share Incentive Plan by our shareholders (i.e., as of August 10, 2021), are subject to the terms of our 2021 Share Incentive Plan and
are granted at an exercise price equal to the average closing sales price per ordinary share on the TASE over the thirty day calendar
period preceding the subject date (utilizing all trading days during such 30 calendar day period) (but not less than “fair market
value” with respect to grantees subject to U.S. tax). All option grants to directors prior to August 10, 2021, are subject to the
terms of our 2013 Share Option Plan and were granted at an exercise price equal to the higher of (i) the average closing price of our
ordinary shares on the TASE during the 30 trading days prior to the date of option allocation, plus 5% and (ii) the closing price of our
ordinary shares on the TASE on the date of option allocation. All such options expire 10 years following the date of grant thereof.
Information regarding the options to purchase our ordinary shares
(including number of options, exercise price and expiration date of all such options) held by each of our directors and executive officers
who beneficially owns our ordinary shares, after including ordinary shares underlying options held by them, which, as of March 20, 2022,
were exercisable or exercisable within 60 days, appears in the beneficial ownership table in Item 7.A below and in the footnotes thereto.
Share Option and Incentive Plans
Company Option and Incentive
Plans
We maintain four share option and incentive plans: Evogene Ltd.
2002 Share Option Plan, Evogene Ltd. 2003 Key Employee Share Incentive Plan, Evogene Ltd. 2013 Share Option Plan, and Evogene Ltd. 2021
Share Incentive Plan, or the 2021 Plan. No new grants will be made under the first three plans, although outstanding awards under those
plans remain subject to the terms of those plans. All such option and incentive plans provide for the grant of options to purchase our
ordinary shares, and the 2021 Plan also provides for the issuance of restricted shares, the grant of restricted shares units, or RSUs,
and the issuance or grant of other equity-based awards.
As of March 20, 2022, options to purchase 4,029,644 ordinary
shares, having a weighted average exercise price of NIS 17.12 per share, and 217,912 RSUs, having no exercise price, were outstanding
under our option and incentive plans, of which, options to purchase 2,665,732 ordinary shares were exercisable and 16,613 RSUs were vested.
An additional 1,291,659 ordinary shares remained available for future grant under our 2021 Plan as of that date.
Among other types of equity-based awards, our share option and
incentive plans provide for granting awards in compliance with Section 102 of the Israeli Income Tax Ordinance [New Version], 5721-1961
(the “Tax Ordinance”), which provides to employees, directors and officers, who are not controlling shareholders (i.e.,
who hold less than 10% of our share capital) and are Israeli residents, favorable tax treatment for compensation in the form of shares,
options, RSUs or other types of equity awards issued or granted, as applicable, to a trustee under the “capital gains track”
for the benefit of the relevant employee, director or officer and are held by the trustee for at least two years after the date of grant
or issuance. Under the “capital gains track”, we are not allowed to deduct an expense with respect to the grant or issuance
of the relevant equity-based awards.
The 2021 Plan also permits us to grant equity-based awards to
U.S. residents, in accordance with the applicable provisions of the U.S. Internal Revenue Code of 1986, as amended, or the Code.
Awards granted under our plans may be subject to vesting schedules.
Options to purchase our ordinary shares granted under our plans expire 10 years from the grant date. The plans address the treatment of
vested and unvested awards upon the termination of employment of the award holder as well as upon consummation of a merger or consolidation
of our company, or sale of all or substantially all of our shares or assets.
Subsidiary Equity Incentive
Plans
In addition to the share option and incentive plans at our parent
company level, each of our subsidiaries has adopted its own equity incentive plan. The following table presents information regarding
our subsidiaries’ equity incentive plans, including the percentage of the equity of those companies that may be issued or granted
as equity incentives to employees, directors or service providers of those companies and the percentage of that equity that has been issued
or granted as of March 20, 2022 (in both cases, after including shares underlying options).
|
|
|
Percentage of Subsidiary's Equity Issuable as Equity Incentives
|
|
|
Percentage of Equity Granted to Date as Equity
Incentives |
|
|
AgPlenus |
|
|
9.1 |
% |
|
|
4.2 |
% |
|
Biomica |
|
|
22.1 |
% |
|
|
18.7 |
% |
|
Casterra |
|
|
8.0 |
% |
|
|
3.6 |
% |
|
Canonic |
|
|
9.1 |
% |
|
|
7.1 |
% |
|
Lavie Bio |
|
|
10.7 |
% |
|
|
10.2 |
% |
Grants under our subsidiaries’ equity incentive plans may
qualify for favorable treatment under the tax law provisions of the United States or Israel.
The share-based payments under our subsidiary equity incentive
plans are presented as non-controlling interests in the financial statements and were valued at $1.7 million in 2021, as detailed in Note
17.f. to the financial statements included in this annual report under Item 18.
C.
Board Practices
Board of Directors
Under the Companies Law and our articles of association, the supervision
of the management of our business is vested in our board of directors. Our board of directors may exercise all powers and may take all
actions that are not specifically granted to our shareholders or to executive management. Our Chief Executive Officer (referred to as
a “general manager” under the Companies Law) is responsible for our day-to-day management. Our Chief Executive Officer is
appointed by, and serves at the discretion of, our board of directors, subject to the employment agreement that we have entered into with
him. All other executive officers are appointed by the Chief Executive Officer and are subject to the terms of any applicable employment
agreements that we may enter into with them.
Under our articles of association and the Companies Law, our board
of directors must consist of not less than three and no more than seven directors. Currently our board of directors consists of six directors.
Our directors are elected annually, by a simple majority vote of
holders of our voting shares participating and voting at the annual meeting of our shareholders, for a one-year term, from the annual
general meeting of our shareholders at which they are elected until the next annual general meeting and until their respective successors
are elected and qualified or until their earlier removal by our shareholders at a general meeting, or upon the occurrence of certain events,
in accordance with the Companies Law and our articles of association. The duration of service of each of our current directors can be
found in their respective biographies in Item 6.A. above.
In addition, under our articles of association, our board of directors
may appoint directors to fill vacancies or as new directors for a term of office that lasts until the next annual meeting of our shareholders.
In the event of a vacancy resulting in the board consisting of less than the minimum number of directors required by our articles of association,
our board of directors may only act in order to convene a general meeting of our shareholders for the purpose of electing such additional
number of directors.
Pursuant to the terms of a put option agreement we entered into
with Monsanto (now Bayer) in October 2013, Monsanto has the right to nominate a non-voting observer to our board of directors so long
as Monsanto holds at least 5% of our voting rights. In addition, pursuant to a share purchase agreement we entered into with Bayer in
December 2010 and as amended in June 2013, Bayer also has the right to appoint one observer to our board of directors so long as Bayer
holds at least 3% of our issued and outstanding shares. In each case, the observer is entitled to be advised reasonably in advance of
board meetings and is to receive copies of all material distributed in connection with such meetings. The observer would not have any
voting rights. To date, neither Monsanto nor Bayer has appointed an observer.
Chairperson of the Board
Our articles of association provide that the chairperson of the
board is appointed by the members of the board of directors and serves as chairperson of the board throughout his term as a director,
unless resolved otherwise by the board of directors. Under the Companies Law, the general manager (i.e., the Chief Executive Officer)
or a relative of the general manager may not serve as the chairperson of the board of directors, and the chairperson or a relative of
the chairperson may not be vested with authorities of the general manager, in each case without shareholder approval consisting of a majority
vote of the shares present and voting at a shareholders meeting, provided that either:
|
◾ |
such majority includes at least 2/3 of the shares held by all shareholders who are not controlling shareholders and do not have a
personal interest in such appointment, present and voting at such meeting; or |
|
◾ |
the total number of shares of non-controlling shareholders who do not have a personal interest in such appointment voting against
such appointment does not exceed two percent of the aggregate voting rights in the company. |
In addition, a person subordinated, directly or indirectly, to
the general manager may not serve as the chairperson of the board of directors; the chairperson of the board may not be vested with authorities
that are granted to those subordinated to the general manager; and the chairperson of the board may not serve in any other position in
the company or a controlled company, except that he may serve as a director or chairperson of a subsidiary.
External Directors
In general, under the Companies Law, the board of directors of
an Israeli public company (such as ours) is required to include at least two external directors. According to regulations promulgated
under the Companies Law, a person may be appointed as an external director if such person has either professional qualifications or accounting
and financial expertise. In addition, at least one of the external directors must be determined by our board of directors to have accounting
and financial expertise.
However, pursuant to regulations enacted under the Companies Law
in 2016, the board of directors of a company whose shares are listed on certain non-Israeli stock exchanges (including the Nasdaq Global
Market), which company does not have a controlling shareholder (as such term is defined in the Companies Law), may elect not to comply
with the requirements of the Companies Law relating to the election of external directors and to the composition of the audit committee
and compensation committee. Such an election may be made by the board of directors, and is contingent upon the company’s satisfaction,
in an ongoing manner, of the applicable foreign country stock exchange rules that apply to companies organized in that country relating
to the appointment of independent directors and the composition of the audit and compensation committees.
Because our company did not have, in May 2016, and still does not
have, a controlling shareholder, and as we comply with the Nasdaq Listing Rules applicable to domestic U.S. companies with respect to
a majority of our directors being independent and with respect to the composition of our audit committee and compensation committee, our
board of directors determined, in May 2016, to opt-out of the requirement to elect external directors. If in the future we were to have
a controlling shareholder, we would likely again be required to comply with the Companies Law requirements relating to external directors
and composition of the audit committee and compensation committee.
The term controlling shareholder, as used in the Companies Law
for purposes of all matters related to external directors and for certain other purposes, means a shareholder that has the ability to
direct the activities of the company, other than by virtue of being an office holder. For purposes of all matters related to external
directors, a shareholder is presumed to be a controlling shareholder if the shareholder holds 50% or more of the voting rights in the
company or has the right to appoint the majority of the directors of the company or its general manager (chief executive officer).
Directors’ Service Contracts
There are no arrangements or understandings between us, on the
one hand, and any of our directors, on the other hand, providing for benefits upon termination of their service as directors of our company.
Financial Statements Review and Audit Committee
Our financial statements review and audit committee, or audit committee,
consists of Mr. Dan Falk, Mr. Ziv Kop and Dr. Oded Shoseyov. Mr. Falk serves as the Chairperson of the audit committee.
Requirements as to Composition
Following the promulgation of the regulations described above,
we may comply with the requirements of the Companies Law by appointing an audit committee whose composition complies with the Nasdaq Listing
Rules. Under the Nasdaq Listing Rules, we are required to maintain an audit committee consisting of at least three independent directors,
each of whom is financially literate and at least one of whom has accounting or related financial management expertise.
All members of our audit committee meet the requirements for independence
and financial literacy under the Nasdaq Listing Rules. Our board of directors has determined that each of Mr. Dan Falk and Mr. Ziv Kop
is furthermore an audit committee financial expert, as defined by the SEC rules, and has the requisite financial experience required under
the Nasdaq Listing Rules.
Each of the members of the audit committee is also “independent”
as required by, and as such term is defined in, Rule 10A-3(b)(1) under the Exchange Act, which is different from the general test for
independence of board and committee members under the Nasdaq Listing Rules.
Audit Committee Role
Our board of directors (following the approval by our audit committee)
has adopted an audit committee charter setting forth the required composition, meeting procedures and other matters related to the terms
of operation of the committee. The charter also describes the responsibilities of the audit committee consistent with the rules of the
SEC and the Nasdaq Listing Rules, which include, among others:
|
◾ |
retaining and terminating the services of our independent auditors, subject to the approval of the board of directors and shareholders;
|
|
◾ |
pre-approval of audit and non-audit services to be provided by the independent auditors; |
|
◾ |
reviewing with management and our independent directors our financial reports prior to their submission to the SEC; and |
|
◾ |
approval of certain transactions with office holders and other related-party transactions. |
The charter of the audit committee is available on our website.
The contents of that website do not constitute a part of this annual report.
Our audit committee provides assistance to our board of directors
in fulfilling its legal and fiduciary obligations in matters involving our accounting, auditing, financial reporting, internal control
and legal compliance functions by pre-approving the services performed by our independent accountants and reviewing their reports regarding
our accounting practices and systems of internal control over financial reporting. Our audit committee also oversees the audit efforts
of our independent accountants and takes those actions that it deems necessary to satisfy itself that the accountants are independent
of management.
Additionally, under the Companies Law, an audit committee is required,
among other things, to (i) identify deficiencies in the administration of the company (including by consulting with the internal auditor),
and recommend remedial actions with respect to such deficiencies, (ii) review and approve certain related party transactions, including
determining whether or not such transactions are extraordinary transactions or insignificant transactions, and (iii) adopt procedures
with respect to processing employee complaints in connection with deficiencies in the administration of the company, and the appropriate
means of protection afforded to such employees. In addition, the audit committee is responsible for overseeing the internal control procedures
of the company. Under the Companies Law, the approval of the audit committee is required for specified actions and transactions with office
holders and controlling shareholders. See “—Approval of Related Party Transactions under Israeli Law.”
Compensation and Nominating Committee
Our compensation and nominating committee, or compensation committee,
consists of Mr. Dan Falk, Mr. Ziv Kop and Dr. Oded Shoseyov. Mr. Kop serves as the Chairperson of the committee.
Requirements as to Composition
Following the promulgation of the regulations described above,
we may comply with the requirements of the Companies Law by appointing a compensation committee whose composition complies with the Nasdaq
Listing Rules. Under the Nasdaq Listing Rules, we are required to maintain a compensation committee consisting of at least two members,
each of whom qualifies as an independent director (as defined under the Nasdaq Listing Rules). Each compensation committee member must
furthermore be deemed by our board of directors to meet the enhanced independence requirements for members of the compensation committee
under the Nasdaq Listing Rules, which require that our board of directors consider (among other things) the source of each such committee
member’s compensation in determining whether he or she is independent.
Our board of directors has determined that each of the members
of our compensation committee is considered “independent” under the Nasdaq Listing Rules and meets the enhanced independence
requirements for members of the compensation committee under the Nasdaq Listing Rules and Rule 10C-1 under the Exchange Act.
Compensation and Nominating
Committee Role
Our board of directors (following approval by our compensation
committee) has adopted a compensation and nominating committee charter setting forth the required composition, meeting procedures and
other matters related to the terms of operation of the committee. The charter also describes the responsibilities of the compensation
committee consistent with the Nasdaq Listing Rules and the Companies Law, which include, among others:
|
◾ |
reviewing and recommending an overall compensation policy with respect to our Chief Executive Officer and other executive officers,
as described above under “Item 6. Directors, Senior Management and Employees—B. Compensation—Compensation Policy”;
|
|
◾ |
reviewing and approving corporate goals and objectives relevant to the compensation of our Chief Executive Officer and other executive
officers, including evaluating their performance in light of such goals and objectives; |
|
◾ |
reviewing and recommending to our board of directors to approve the granting of options and other incentive awards; |
|
◾ |
reviewing, evaluating and making recommendations regarding the compensation and benefits for our non-employee directors; and
|
|
◾ |
advising our board of directors in selecting individuals who are best able to fulfill the responsibilities of a director or executive
officer of our company. |
The charter of the compensation and nominating committee is available
on our website. The contents of that website do not constitute a part of this annual report.
Corporate Development Committee
Our board of directors has formed a corporate development committee,
of which Ms. Sarit Firon, Mr. Ziv Kop and Mr. Leon Recanati serve as members. Ms. Firon serves as the Chairperson of the committee. The
corporate development committee assists our board of directors in fulfilling its oversight
responsibilities across the principal areas of corporate development for our company and its subsidiaries. This committee may also assist
the board of directors by reviewing such matters as corporate and division strategy and MA opportunities and making recommendations
for consideration by our board of directors.
Internal Auditor
Under the Companies Law, the board of directors of an Israeli public
company must appoint an internal auditor recommended by the audit committee. Under the Companies Law, the internal auditor may be an employee
of the company but not an office holder, an affiliate, or a relative of an office holder or affiliate, and may not be the company’s
independent accountant or its representative.
The role of the internal auditor is to examine, among other things,
our compliance with applicable law and orderly business procedures. The audit committee is required to oversee the activities and to assess
the performance of the internal auditor as well as to review the internal auditor’s work plan. Mr. Yisrael Gewirtz, CPA, has been
appointed as our internal auditor. Mr. Gewirtz is a certified internal auditor and a partner of Fahn Kanne Control Management Ltd., an
affiliate of Grant Thornton LLP.
Our internal auditor also provides management and the audit committee
ongoing assessments of our risk management processes and our internal controls.
Approval of Related Party Transactions under Israeli Law
Fiduciary Duties of Directors and Executive
Officers
The Companies Law codifies the fiduciary duties that office holders
owe to a company. Many of the executive officers listed in the table under “Item 6. Directors, Senior Management and Employees—
A. Directors and Senior Management” are also office holders under the Companies Law. An office holder’s fiduciary duties consist
of a duty of care and a duty of loyalty. The duty of care requires an office holder to act with the level of care with which a reasonable
office holder in the same position would have acted under the same circumstances. The duty of loyalty requires that an office holder act
in good faith and in the best interests of the company. The duty of care includes a duty to use reasonable means to obtain (i) information
on the appropriateness of a given action submitted for his or her approval or performed by virtue of his or her position; and (ii) all
other important information pertaining to these actions. The duty of loyalty includes a duty to (i) refrain from any conflict of interest
between the performance of his or her duties in the company and his or her personal affairs; (ii) refrain from any activity that is competitive
with the business of the company; (iii) refrain from exploiting any business opportunity of the company in order to receive a personal
gain for himself or herself or others; and (iv) disclose to the company any information or documents relating to the company’s affairs
which the office holder received as a result of his or her position as an office holder.
Disclosure of Personal Interests of an Office
Holder and Approval of Certain Transactions
The Companies Law requires that an office holder promptly disclose
to the board of directors any conflict of interest (referred to under the Companies Law as a “personal interest”) that he
or she may have and all related material information known to him or her concerning any existing or proposed transaction with the company.
If it is determined that an office holder has a personal interest in a transaction, approval by the board of directors is required for
the transaction, unless the company’s articles of association provide for a different method of approval. Our articles of association
provide that for non-extraordinary interested party transactions, the board of directors may delegate its approval, or may provide a general
approval to certain types of non-extraordinary interested party transactions. Every interested party transaction requires that our board
of directors determine affirmatively that the transaction is favorable to the company. Approval first by the company’s audit committee
and subsequently by the board of directors is required for an extraordinary transaction, meaning any transaction that is not in the ordinary
course of business, not on market terms or that is likely to have a material impact on the company’s profitability, assets or liabilities.
A director and any other office holder who has a personal interest in a transaction which is considered at a meeting of the board of directors
or the audit committee may generally (unless it is with respect to a transaction which is not an extraordinary transaction) not be present
at such a meeting or vote on that matter unless a majority of the directors or members of the audit committee, as applicable, have a personal
interest in the matter. If a majority of the members of the audit committee or the board of directors has a personal interest in the approval
of such a transaction then all of the directors may participate in deliberations of the audit committee or board of directors, as applicable,
with respect to such transaction and vote on the approval thereof and, in such case, shareholder approval is also required.
Pursuant to the Companies Law, extraordinary transactions with
our office holders who are not directors require audit committee approval and subsequent approval by our board of directors. Compensation,
insurance, indemnification or exculpation arrangements for office holders who are not directors require approval by our compensation committee,
followed by our board of directors and, if deviating from our compensation policy, our shareholders as well, via a special majority. Compensation
arrangements with directors, including in their capacities as executive officers, or with our Chief Executive Officer, as well as insurance
(unless exempted under the applicable regulations), indemnification or exculpation of directors or our Chief Executive Officer, require
the approval of the compensation committee, the board of directors and our shareholders, in that order. In the case of our Chief Executive
Officer, the shareholder approval must fulfill, in addition to an ordinary majority, one of the following two special majority requirements:
|
◾ |
at least a majority of the voting rights in the company held by non-controlling shareholders who have no conflict of interest (referred
to under the Companies Law as a “personal interest”) in the transaction or arrangement and who are present and voting (in
person or by proxy) at the general meeting, must be voted in favor of approving the transaction or arrangement (for this purpose, abstentions
are disregarded); or |
|
◾ |
the voting rights held by non-controlling, non-conflicted shareholders (as described in the previous bullet point) who are present
and voting (in person or by proxy) at the general meeting, and who vote against the transaction, do not exceed two percent of the voting
rights in the company. |
As described above (concerning votes related to external directors),
a shareholder is presumed to be a controlling shareholder if the shareholder holds 50% or more of the voting rights in the company or
has the right to appoint the majority of the directors of the company or its general manager (chief executive officer). In addition, as
it relates to the approval of related party transactions, a controlling shareholder is furthermore deemed to include any shareholder possessing
25% or more of the voting rights if no other shareholder possesses more than 50% of the voting rights.
If the transaction or compensation arrangement of the office holder
brought for approval amends an existing arrangement, then only the approval of the audit committee or compensation committee (as appropriate)
is required if that committee determines that the amendment is not material in relation to the existing arrangement.
Disclosure of Personal Interests of Controlling
Shareholders and Approval of Certain Transactions
Pursuant to the Companies Law, the disclosure requirements regarding
personal interests that apply to directors and executive officers also apply to a controlling shareholder of a public company. In the
case of an extraordinary transaction between a public company and a controlling shareholder, or in which a controlling shareholder has
a personal interest, the shareholder approval requirement—by a special majority—that applies to a compensation arrangement
for the chief executive officer (as described above) also applies to the extraordinary transaction (except that a controlling shareholder’s
vote is not excluded from the special majority determination, unless the controlling shareholder possesses a conflict of interest/ personal
interest). We currently do not have a controlling shareholder.
Shareholder Duties
Pursuant to the Companies Law, a shareholder has a duty to act
in good faith and in a customary manner toward the company and other shareholders and to refrain from abusing his, her or its power with
respect to the company, including, among other things, in voting at a general meeting and at shareholder class meetings with respect to
the following matters: (i) an amendment to the company’s articles of association; (ii) an increase of the company’s authorized
share capital; (iii) a merger; or (iv) an interested party transaction that requires shareholder approval.
In addition, a shareholder has a general duty to refrain from discriminating
against other shareholders. Certain shareholders also have a duty of fairness toward the company. These shareholders include any controlling
shareholder, any shareholder who knows that it has the power to determine the outcome of a shareholder vote and any shareholder who has
the power to appoint or to prevent the appointment of an office holder of the company or exercise any other rights available to it under
the company’s articles of association with respect to the company. The Companies Law does not define the substance of this duty
of fairness, except to state that the remedies generally available upon a breach of contract will also apply in the event of a breach
of the duty of fairness. Israeli courts have not yet interpreted the scope or nature of any of these duties.
Approval of Private Placements
Under the Companies Law, a significant private placement of securities
requires approval by the board of directors and the shareholders by a simple majority. A private placement is considered a significant
private placement if it results in a person becoming a controlling shareholder, or if all of the following conditions are met: (i) the
securities issued amount to 20% or more of the company’s outstanding voting rights before the issuance; (ii) some or all of the
consideration is other than cash or listed securities or the transaction is not on market terms; and (iii) the transaction will increase
the relative holdings of a shareholder who holds 5% or more of the company’s outstanding share capital or voting rights, or will
cause any person to become, as a result of the issuance, a holder of more than 5% of the company’s outstanding share capital or
voting rights.
Exculpation, Insurance and Indemnification
of Office Holders
Under the Companies Law, a company may not exculpate an office
holder from liability for a breach of the duty of loyalty. An Israeli company may exculpate an office holder in advance from liability
to the company, in whole or in part, for damages caused to the company as a result of a breach of duty of care but only if a provision
authorizing such exculpation is included in its articles of association. Our articles of association include such a provision. An Israeli
company may not exculpate a director from liability arising out of a prohibited dividend or distribution to shareholders.
An Israeli company may indemnify an office holder in respect of
the following liabilities and expenses incurred for acts performed as an office holder, either in advance of an event or following an
event, provided a provision authorizing such indemnification is contained in its articles of association:
|
◾ |
financial liability imposed on him or her in favor of another person pursuant to a judgment, settlement or arbitrator’s award
approved by a court. However, if an undertaking to indemnify an office holder with respect to such liability is provided in advance, then
such an undertaking must be limited to events which, in the opinion of the board of directors, can be foreseen based on the company’s
activities when the undertaking to indemnify is given, and to an amount or according to criteria determined by the board of directors
as reasonable under the circumstances, and such undertaking shall detail the abovementioned events and amount or criteria; |
|
◾ |
reasonable litigation expenses, including attorneys’ fees, incurred by the office holder as a result of an investigation or
proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (i) no
indictment was filed against such office holder as a result of such investigation or proceeding; and (ii) no financial liability,
such as a criminal penalty, was imposed upon him or her as a substitute for the criminal proceeding as a result of such investigation
or proceeding or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal
intent; and |
|
◾ |
reasonable litigation expenses, including attorneys’ fees, incurred by the office holder or imposed by a court in proceedings
instituted against him or her by the company, on its behalf or by a third party or in connection with criminal proceedings in which the
office holder was acquitted or as a result of a conviction for an offense that does not require proof of criminal intent. |
An Israeli company may insure an office holder against the following
liabilities incurred for acts performed as an office holder if and to the extent provided in the company’s articles of association:
(i) a breach of the duty of loyalty to the company, to the extent that the office holder acted in good faith and had a reasonable basis
to believe that the act would not prejudice the company; (ii) a breach of the duty of care to the company or to a third party, including
a breach arising out of the negligent conduct of the office holder; (iii) a financial liability imposed on the office holder in favor
of a third party; (iv) a financial liability imposed on the office holder in favor of a third party harmed by a breach in an administrative
proceeding; and (v) reasonable litigation expenses, including attorneys’ fees, incurred by the office holder as a result of an administrative
proceeding instituted against him or her.
An Israeli company may not indemnify or insure an office holder
against any of the following: (i) a breach of the duty of loyalty, except to the extent that the office holder acted in good faith and
had a reasonable basis to believe that the act would not prejudice the company; (ii) a breach of the duty of care committed intentionally
or recklessly, excluding a breach arising out of the negligent conduct of the office holder; (iii) an act or omission committed with intent
to derive illegal personal benefit; or (iv) a fine or forfeit levied against the office holder.
Under the Companies Law, exculpation, indemnification and insurance
of office holders must be approved by the compensation and nominating committee and the board of directors and, with respect to directors
and our Chief Executive Officer, also by our shareholders (in the case of our Chief Executive Officer, by a special majority, as described
above under “—Approval of Related Party Transactions under Israeli Law—Disclosure of Personal Interests of an Officer
Holder and Approval of Certain Transactions”, unless an applicable exemption applies).
Our articles of association allow us to indemnify and insure our
office holders for any liability imposed on them as a consequence of an act which was performed by virtue of being an office holder. Our
shareholders have approved an amendment to our articles of association that extends such indemnification and insurance to cover omissions
by our office holders (in their role as such) as well. Our office holders are currently covered by a directors’ and officers’
insurance policy.
We have entered into agreements with each of our directors and
executive officers. Each such agreement exculpates our director or officer, to the fullest extent permitted by law, from liability to
us for damages caused to us as a result of a breach of duty of care and undertaking to indemnify them to the fullest extent permitted
by law. This indemnification is limited to events determined as foreseeable by the board of directors based on our activities, and to
an amount or according to criteria determined by the board of directors as reasonable under the circumstances.
The maximum indemnification amount set forth in such agreements
is limited to an amount equal to 25% of our shareholders’ equity as reflected in our most recent consolidated financial statements
prior to the date on which the indemnity payment is made. If the amount equal to 25% of our shareholders’ equity is insufficient
to cover all indemnity amounts payable with respect to all indemnifiable directors and executive officers, such amount will be allocated
among our directors and executive officers pro rata, in accordance with their relative culpabilities, as finally determined by a court
with respect to a particular claim. The maximum amount set forth in such agreements is in addition to any amount paid (if paid) under
insurance and/or by a third party pursuant to an indemnification arrangement. In the opinion of the SEC, indemnification of directors
and office holders for liabilities arising under the Securities Act is against public policy and therefore unenforceable.
D. Employees
The total number of employees in Evogene and its subsidiaries as
of December 31, 2019, 2020 and 2021 was 143, 130 and 141, respectively. As of December 31, 2021, our research and development activities
involved 96 employees amounting to approximately 72% of our total full-time workforce, of which 51 were employed by Evogene and 45 were
employed by our subsidiaries. Our staff possesses multidisciplinary and wide-ranging expertise, with employees specializing in biology,
chemistry, plant genetics, agronomics, mathematics, computer and data science and other related fields and 44 of our employees hold a
Ph.D. As of March 20, 2022, the total number of employees in Evogene and its subsidiaries was 149.
As of the date hereof, all of our employees are based in Israel,
except for eight U.S.-based employees. Of our eight U.S.-based employees, seven are employed by Lavie Bio Inc., a U.S. subsidiary of Lavie
Bio Ltd., the majority of whom are based at Lavie Bio’s U.S. research and development site in St. Louis, Missouri. In addition,
AgPlenus Inc.’s CEO is located in North Carolina. In addition, during 2021, we had, on average, approximately 27 hourly employees
who are based in Israel. The following table shows the breakdown of our employees by division/category of activity and by location as
of December 31, 2019, 2020 and 2021, excluding hourly employees:
|
|
As of December 31, 2019 |
|
|
As of December 31, 2020 |
|
|
As of December 31, 2021 |
|
| |
|
Israel |
|
|
U.S. |
|
|
Total |
|
|
Israel |
|
|
U.S. |
|
|
Total |
|
|
Israel |
|
|
U.S. |
|
|
Total |
|
|
Executive management |
|
|
4 |
|
|
|
- |
|
|
|
4 |
|
|
|
4 |
|
|
|
- |
|
|
|
4 |
|
|
|
5 |
|
|
|
- |
|
|
|
5 |
|
|
Lavie Bio Ltd. |
|
|
15 |
|
|
|
- |
|
|
|
15 |
|
|
|
17 |
|
|
|
5 |
|
|
|
22 |
|
|
|
17 |
|
|
|
7 |
|
|
|
24 |
|
|
AgPlenus Ltd. |
|
|
16 |
|
|
|
- |
|
|
|
16 |
|
|
|
11 |
|
|
|
1 |
|
|
|
12 |
|
|
|
11 |
|
|
|
1 |
|
|
|
12 |
|
|
Ag-Seeds division |
|
|
5 |
|
|
|
- |
|
|
|
5 |
|
|
|
2 |
|
|
|
- |
|
|
|
2 |
|
|
|
2 |
|
|
|
- |
|
|
|
2 |
|
|
Casterra Ag Ltd. |
|
|
2 |
|
|
|
- |
|
|
|
2 |
|
|
|
1 |
|
|
|
- |
|
|
|
1 |
|
|
|
1 |
|
|
|
- |
|
|
|
1 |
|
|
Biomica Ltd. |
|
|
7 |
|
|
|
- |
|
|
|
7 |
|
|
|
7 |
|
|
|
- |
|
|
|
7 |
|
|
|
13 |
|
|
|
- |
|
|
|
13 |
|
|
Canonic Ltd. |
|
|
4 |
|
|
|
- |
|
|
|
4 |
|
|
|
6 |
|
|
|
- |
|
|
|
6 |
|
|
|
10 |
|
|
|
- |
|
|
|
10 |
|
|
Technology platform |
|
|
57 |
|
|
|
4 |
|
|
|
61 |
|
|
|
48 |
|
|
|
- |
|
|
|
48 |
|
|
|
41 |
|
|
|
- |
|
|
|
41 |
|
General and administrative |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
33 |
|
|
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Israeli labor laws govern the length of the workday, minimum wages
for employees, procedures for hiring and dismissing employees, determination of severance pay, annual leave, sick days, advance notice
of termination of employment, equal opportunity and anti-discrimination laws and other conditions of employment. Subject to certain exceptions,
Israeli law generally requires severance pay upon the retirement, death or dismissal of an employee, and requires us and our employees
to make payments to the National Insurance Institute, which is similar to the U.S. Social Security Administration. Our employees have
pension plans that comply with the applicable Israeli legal requirements.
While none of our employees is party to any collective bargaining
agreements, certain provisions of the collective bargaining agreements between the “Histadrut”
(the General Union of Workers in Israel) and the Coordination Bureau of Economic Organizations (including the Industrialists’ Associations)
are applicable to our employees in Israel by order of the Israeli Ministry of the Economy and Industry. These provisions primarily concern
pension fund benefits for all employees, insurance for work-related accidents, recuperation pay and travel expenses.
None of our employees is represented by a labor union or covered
under a collective bargaining agreement. We have never experienced any employment-related work stoppages and believe our relationships
with our employees are good.
The employees of our U.S. subsidiaries are subject to the U.S.
labor laws and have insurance coverage, health benefits and are covered by certain plans, such as (i) medical and dental care; (ii) long
term disability protection plans; (iii) life insurance; and (iv) a 401(k) savings plan.
E.
Share Ownership
For information regarding the share ownership of our directors
and executive officers, please refer to the table in “Item 7. Major Shareholders and Related Party Transactions—A. Major
Shareholders.” For information regarding our equity incentive plans, see Item 6.B. “Director,
Senior Management and Employees—Compensation—Equity Incentive Plans.”
| ITEM 7. |
MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS |
A. Major
Shareholders
The following table sets forth information with respect to the
beneficial ownership of our shares as of March 20, 2022 (unless otherwise indicated) by: (i) each person or entity known by us to own
beneficially more than 5% of our outstanding shares; (ii) each of our directors and executive officers individually; and (iii) all of
our executive officers and directors as a group.
The beneficial ownership of ordinary shares is determined in accordance
with the rules of the SEC, and generally includes any ordinary shares over which a person exercises sole or shared voting or investment
power, or the right to receive the economic benefit of ownership. For purposes of the table below, we deem shares subject to options that
are currently exercisable or exercisable within 60 days of March 20, 2022, to be outstanding and to be beneficially owned by the
person holding the options for the purposes of computing the percentage ownership of that person, but we do not treat them as outstanding
for the purpose of computing the percentage ownership of any other person.
The percentage of shares beneficially owned by any shareholder
has been calculated based on 41,188,280 ordinary shares outstanding as of March 20, 2022. Unless otherwise indicated below, to our
knowledge, all persons named in the table have sole voting and investment power with respect to their shares, except to the extent that
authority is shared by spouses under community property laws.
Unless otherwise noted below, each shareholder’s address
is c/o Evogene Ltd., 13 Gad Feinstein Street, Park Rehovot, Rehovot P.O. Box 4173, Ness Ziona, 7414002, Israel. The shareholders listed
below (including our directors and executive officers) do not have any different voting rights than any of our other shareholders. We
know of no arrangements that would, at a subsequent date, result in a change of control of our company. A description of any material
relationship that our principal shareholders have had with us or any of our predecessors or affiliates within the past year is included
under “Item 7. Major Shareholders and Related Party Transactions—B. Related Party Transactions.”
|
|
Shares Beneficially Held
|
|
|
Name of Beneficial Owner |
|
Number
|
|
|
Percentage of Class
|
|
|
Principal Shareholders |
|
|
|
|
|
|
|
ARK Investment Management LLC (1)
|
|
|
2,515,657 |
|
|
|
6.11 |
% |
|
Executive Officers and Directors |
|
|
|
|
|
|
|
|
|
Ofer Haviv |
|
|
860,000 |
(2) |
|
|
2 |
% |
|
Dr. Nir Arbel |
|
|
0 |
|
|
|
* |
|
|
Lazer Bezdin |
|
|
0( |
**) |
|
|
* |
|
|
Ido Dor |
|
|
255,500 |
(3)(**) |
|
|
|
|
|
Douglas A. Eisner |
|
|
0 |
(**) |
|
|
* |
|
|
|
|
|
0 |
|
|
|
* |
|
|
Dr. Eyal Emmanuel |
|
|
71,204 |
(4)(**) |
|
|
|
|
|
Dr. Elran Haber |
|
|
15,624 |
(5) |
|
|
* |
|
|
Dr. Arnon Heyman |
|
|
103,000 |
(6) |
|
|
* |
|
|
Mark Kapel |
|
|
163,382 |
(7) |
|
|
* |
|
|
Dorit Kreiner |
|
|
117,739 |
(8) |
|
|
* |
|
|
Sassi Masliah |
|
|
97,125 |
(9) |
|
|
* |
|
|
Martin S. Gerstel |
|
|
636,506 |
(10)(**) |
|
|
1.5 |
% |
|
Dan Falk |
|
|
0 |
|
|
|
* |
|
|
Sarit Firon |
|
|
41,375 |
(11) |
|
|
* |
|
|
Ziv Kop |
|
|
34,125 |
(12) |
|
|
* |
|
|
Dr. Adrian Percy |
|
|
21,625 |
(13) |
|
|
* |
|
|
Leon Y. Recanati |
|
|
869,234 |
(14) |
|
|
2.1 |
% |
|
Dr. Oded Shoseyov |
|
|
22,875 |
(15) |
|
|
* |
|
|
All directors and executive officers as a group (19 persons**) |
|
|
3,309,314 |
|
|
|
7.4 |
% |
_______________________________
|
** |
The engagement of each of Mr. Bezdin, Mr. Dor, Mr. Eisner and Dr. Emmanuel as executive officers and of Mr. Gerstel as a director
ended during 2021. Beneficial ownership information for such persons is based on our own internal records as of the date on which their
engagement ended. |
|
(1) |
This information is based upon a Schedule 13G/A filed by ARK Investment Management LLC with the SEC on February 9, 2022. ARK Investment
Management LLC is a Delaware limited liability company and possesses sole voting and dispositive power over these ordinary shares. The
principal address for ARK Investment Management LLC is 3 East 28th Street, 7th Floor, New York, NY 10016. |
|
(2) |
Consists of 860,000 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days
of March 20, 2021, of which options to purchase the following number of shares expire on the following dates, respectively: 215,000 on
July 17, 2023, 170,000 on March 22, 2025, 225,000 on August 8, 2027 and 250,000 on April 21, 2030. The weighted average exercise price
of these options is NIS 25.86. |
|
(3) |
Ido Dor served as the CEO of our subsidiary company Lavie Bio, and as such, he holds options to purchase shares of Lavie Bio. In
addition, Mr. Dor also holds options to purchase 255,500 ordinary shares of Evogene that are currently exercisable or exercisable within
60 days of March 20, 2021, which expire on November 20, 2022. The exercise price of these options is NIS 35.58 per ordinary share.
|
|
(4) |
Consists of 71,204 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days
of March 20, 2021, of which options to purchase the following number of shares expire on the following dates, respectively: 35,602 on
November 13, 2028, and 35,602on December 23, 2028. The weighted average exercise price of these options is NIS 10.16. |
|
(5) |
Consists of 15,624 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days
of March 20, 2021, which expire on September 1, 2031. The exercise price of these options is NIS 9.17 per ordinary share. Elran Haber
serves as the CEO of our subsidiary company Biomica, and as such, also holds options to purchase shares of Biomica. For a description
of our subsidiaries’ equity incentive plans, please see Item 6 “Directors, Senior Management and Employees—B. Compensation—Share
Option and Incentive Plans—Subsidiary Equity Incentive Plans”. |
|
(6) |
Consists of 103,000 ordinary shares of Evogene that are currently exercisable or exercisable within 60 days of March 20, 2021, of
which options to purchase the following number of shares expire on the following dates, respectively: 10,000 on November 9, 2024, 18,000
on May 18, 2026, 50,000 on August 8, 2027, and 25,000 on February 26, 2028. The weighted average exercise price of these options is NIS
21.27. Arnon Heyman serves as the CEO of our subsidiary company Canonic Ltd., and as such, also holds options to purchase shares of Biomica.
For a description of our subsidiaries’ equity incentive plans, please see Item 6 “Directors, Senior Management and Employees—B.
Compensation—Share Option and Incentive Plans—Subsidiary Equity Incentive Plans”. |
|
(7) |
Consists of 163,382 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days
of March 20, 2021, of which options to purchase the following number of shares expire on the following dates, respectively: 13,500 on
July 15, 2023, 12,000 on March 22, 2025, 23,000 on August 8, 2027, 60,000 on February 26, 2028, 24,375 on February 4, 2029, 24,057 on
July 30, 2029 and 6,250 on November 16, 2031. The weighted average exercise price of these options is NIS 16.63. |
|
(8) |
Includes 116,239 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days of
March 20, 2021, of which options to purchase the following number of shares expire on the following dates, respectively: 28,433 on February
4, 2029, 86,244 on July 30, 2029 and 1,562 on November 16, 2031. The weighted average exercise price of these options is NIS 6.45. Also
includes 1,500 ordinary shares held by a trustee in favor of Ms. Kreiner. As previously reported, Ms. Kreiner’s engagement
with the Company is expected to terminate on March 31, 2022. In the context of such termination, the exercise period of all of Ms. Kreiner’s
options that are vested on the date of such termination shall be extended, such that, unless exercised earlier, all such options shall
expire 12 months following such termination. |
|
(9) |
Includes 97,125 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days of
March 20, 2021, of which options to purchase the following number of shares expire on the following dates, respectively: 5,000 on July
15, 2023, 17,000 on March 22, 2025, 18,000 on August 8, 2027, 3,125 on May 28, 2028, 6,250 on February 4, 2029, 16,500 on July 30, 2029,
18,750 on September 22, 2030 and 12,500 on November 16, 2031. The weighted average exercise price of these options is NIS 16.33.
|
|
(10) |
Includes 636,506 ordinary shares, consisting of: (a) 37,500 ordinary shares held by a trustee in favor of Mr. Gerstel; (b) 383,815
ordinary shares held by Martin Gerstel; and (c) 215,191 ordinary shares held by Shomar Corporation with respect to which Martin Gerstel
and his wife Mrs. Shoshana Gerstel possess voting and investment power. |
|
(11) |
Consists of 41,375 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days
of March 20, 2021, of which options to purchase the following number of shares expire on the following dates, respectively: 10,000 on
August 10, 2026, 2,500 on August 8, 2027, 1,875 on August 6, 2028 and 27,000 on September 1, 2031. The weighted average exercise price
of these options is NIS 14.11. |
|
(12) |
Consists of 34,125 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days
of March 20, 2021, of which options to purchase the following number of shares expire on the following dates, respectively: 10,000 on
March 20, 2024, 2,500 on March 22, 2025, 2,500 on February 28, 2026, 2,500 on January 12, 2027, 2,500 on January 11, 2028, 625 on February
4, 2029 and 13,500 on September 1, 2031. The weighted average exercise price of these options is NIS 32.40. |
|
(13) |
Consists of 21,625 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days
of March 20, 2021, of which options to purchase the following number of shares expire on the following dates, respectively: 8,125 on December
23, 2028 and 13,500 on August 10, 2031. The weighted average exercise price of these options is USD $2.82. |
|
(14) |
Includes 838,859 ordinary shares held by Mr. Recanati. Also includes 30,375 ordinary shares issuable upon exercise of options that
are currently exercisable or exercisable within 60 days of March 20, 2021, of which options to purchase the following number of shares
expire on the following dates, respectively: 2,500 on June 11, 2022, 2,500 on September 15, 2023, 2,500 on August 17, 2024, 2,500 on July
2, 2025, 2,500 on May 18, 2026, 2,500 on May 16, 2027, 1,875 on June 25, 2028 and 13,500 on September 1, 2031. The weighted average exercise
price of these options is NIS 23.23. |
|
(15) |
Consists of 22,875 ordinary shares issuable upon exercise of options that are currently exercisable or exercisable within 60 days
of March 20, 2021, of which options to purchase the following number of shares expire on the following dates, respectively: 8,750 on November
13, 2028, 625 on December 19, 2029 and 13,500 on September 1, 2031. The exercise price of these options is NIS 9.65. |
Changes in Percentage Ownership by Major Shareholders
Over the course of 2021, there was a decrease in the percentage
ownership of a significant shareholder (which we define as a holder of at least 5% of our issued and outstanding share capital), ARK Investment
Management LLC (from 11.4% to 6.01%). In addition, over the course of 2021, based on publicly available information, we believe that entities
affiliated with Waddell Reed Financial, Inc. were acquired by Macquarie Group Ltd. or its affiliates. Macquarie Group Ltd.
filed with the SEC a Schedule 13G/A dated February 11, 2022 to report that it had decreased its ownership of our ordinary shares and has
ceased to be the beneficial owner of more than 5% of our ordinary shares. Waddell Reed Financial, Inc. and its affiliates had previously
reported on a Schedule 13G/A ownership of 6.8% of our issued and outstanding share capital.
Over the course of 2020, there were increases in the percentage
ownership of ARK Investment Management LLC (from below 5% to 11.4%). On the other hand, there were decreases in the percentage ownership
of some of our former significant shareholders, each of which fell below 5% beneficial ownership of our issued and outstanding share capital
from the following ownership percentage levels, including: (i) entities affiliated with Phoenix Holdings Ltd. (formerly 7.6%), (ii) entities
affiliated with Senvest Management, LLC, (formerly 8.5%), (iii) Monsanto Company (formerly 6.4%), (iii) entities affiliated with UBS Group
AG (formerly 5.3%), and (iv) Alpha Capital Anstalt (formerly 8.8%).
Over the course of 2019, there were increases in the percentage
ownership of some of our major shareholders at the time, including entities affiliated with (i) The Phoenix Holdings Ltd. (from 6.8% to
7.6%) and (ii) Senvest Management, LLC, which first reported in April 2019 that they held 6.8%, and as of December 31, 2019 held 8.5%.
On the other hand, there were decreases in the percentage ownership of entities affiliated with (x) our former significant shareholder,
Migdal Insurance Financial Holdings Ltd. (from 7.6% to 0.2%) and (y) Waddell Reed Financial, Inc. (from 10.9% to 10.7%).
The information above regarding changes in percentage ownership
by major shareholders during the years ended December 31, 2019 through 2021 is based solely on information contained in Schedule 13Gs
(as may be amended) filed by such persons with the SEC.
Record Holders
As of March 20, 2022, all of our issued and outstanding ordinary
shares were held of record in the United States, in the name of a single record shareholder — Cede Co., as nominee for the
Depository Trust Company. The number of record holders is not representative of the number of beneficial holders of our ordinary shares,
nor is it representative of where such beneficial holders reside, since the shares held in the name of Cede Co. are listed for trading
on Nasdaq and the TASE and are beneficially owned by a wide range of underlying beneficial shareholders who hold their shares in “street
name,” including Israeli and other non-U.S. shareholders.
B.
Related Party Transactions
Except as described below or elsewhere in this annual report, since
January 1, 2021, we have had no transaction, nor do we have any presently proposed transaction, and neither we nor our subsidiaries have
had any loan, nor do we or our subsidiaries have any presently proposed loan, involving any related party described in Item 7.B. of this
annual report.
Agreements with Directors and Officers
Employment Agreements
We have entered into written employment agreements with all of
our executive officers. Each of these agreements contains provisions regarding non-competition, confidentiality of information and assignment
of inventions. The non-competition provision applies for a period that is generally 12 months following termination of employment. The
enforceability of covenants not to compete in Israel and in the United States is subject to limitations.
Equity Awards
See “Item 6. Directors, Senior Management and Employee—B. Compensation—Share
Option and Incentive Plans.”
Indemnification Agreements
Our articles of association allow us to indemnify and insure our
office holders for any liability imposed on them as a consequence of an act which was performed by virtue of being an office holder. They
also allow us to exculpate an office holder in advance from liability to the company, in whole or in part, for damages caused to the company
as a result of a breach of duty of care. In furtherance of such allowance, we have entered into agreements with each of our directors
and executive officers exculpating them, to the fullest extent permitted by law, from liability to us for damages caused to us as a result
of a breach of duty of care, and undertaking to indemnify them to the fullest extent permitted by law. See “Item 6. Directors, Senior
Management and Employees—C. Board Practices—Exculpation, Insurance and Indemnification of Office Holders.”
C. Interests
of Experts and Counsel
Not applicable.
| ITEM 8. |
FINANCIAL INFORMATION |
A. Consolidated
Statements and Other Financial Information
Consolidated financial statements
We have appended our consolidated financial statements at the end
of this annual report, together with the report of our independent auditor on those financial statements, beginning on page F-2, as part
of this annual report.
Legal Proceedings
From time to time, we may be subject to legal proceedings and claims
in the ordinary course of business. We are currently not involved in any pending or contemplated legal proceedings that could reasonably
be expected to have a significant effect on our financial position, profitability or cash flows. We may become involved in material legal
proceedings in the future. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs,
diversion of management resources and other factors.
Dividend Policy
Since our inception, we have not declared or paid any cash or other
form of dividends on our ordinary shares. We currently intend to retain any earnings for use in our business and do not currently intend
to pay cash dividends on our ordinary shares. Dividends, if any, on our outstanding ordinary shares will be declared by and subject to
the discretion of our board of directors. Even if our board of directors decides to distribute dividends, the form, frequency and amount
of such dividends will depend upon our future operations and earnings, capital requirements and surplus, general financial condition,
contractual restrictions and other factors our board of directors may deem relevant.
In addition, the distribution of dividends may be limited by Israeli
law, which permits the distribution of dividends only out of distributable profits. See “Dividend and Liquidation Rights”
in Exhibit 2.1 to this annual report.
B.
Significant Changes
No significant changes have occurred since December 31, 2020, except
as otherwise disclosed in this annual report.
| ITEM 9. |
THE OFFER AND LISTING |
A. Offer
and Listing Details
Our ordinary shares have been listed for trading on the TASE since
2007 and were listed for trading on the NYSE commencing with our U.S. initial public offering in November 2013 until December 2016, at
which point we transferred the listing to Nasdaq, where they have been listed from December 2016 to the present time. “EVGN”
has served as the trading symbol for each such listing.
B.
Plan of Distribution
Not applicable.
C. Markets
See “—A. Offer and Listing Details” above.
D. Selling
Shareholders
Not applicable.
E. Dilution
Not applicable.
F.
Expenses of the Issue
Not applicable.
| ITEM 10. |
ADDITIONAL INFORMATION |
A. Share
Capital
Not applicable.
B. Memorandum
and Articles of Association
For a discussion of the provisions of the company’s articles
of association with respect to the powers of directors, see “Item 6. Directors, Senior Management and Employees—C. Board Practices.”
A copy of our articles of association is attached as Exhibit 1.1 to this annual report. The information called for by this Item 10.B is
set forth in Exhibit 2.1 to this annual report and is incorporated by reference into this annual report.
C. Material
Contracts
The following is a summary of each material contract, other than
material contracts entered into in the ordinary course of business, to which we are or have been a party, for the two years immediately
preceding the date of this annual report:
Controlled Equity Offering Sales Agreements
On January 14, 2021 and February 19, 2021, we entered into Controlled
Equity OfferingSM Sales Agreements, or the January
Sales Agreement and February Sales Agreement, respectively, with Cantor Fitzgerald Co., or the Agent, pursuant to which the Company
could offer and sell, from time to time, its ordinary shares, through the Agent in an at-the-market offering, as defined in Rule 415(a)(4)
promulgated under the Securities Act, for aggregate offering price of up to $28.0 million and $50.0 million, respectively. In February
2021, we completed the sales of ordinary shares under the January Sales Agreement and subsequently entered into the February Sales Agreement.
We are not obligated to make any sales of ordinary shares under the February Sales Agreement and no assurance can be given that we will
sell any ordinary shares under such agreement, or, if we do, as to the price or number of such shares that we will sell, or the dates
on which any such sales will take place.
Lavie Bio Share Purchase Agreement with Corteva
In August 2019, we entered into a share purchase agreement with
Corteva, whereby Corteva invested in our subsidiary Lavie Bio. That investment consisted of Corteva’s (i) contribution of its shares
in Taxon Biosciences to Lavie Bio and (ii) payment of $10 million for equity of Lavie Bio. In exchange, Lavie Bio issued to Corteva approximately
28% of Lavie Bio’s shares. Information on that transaction is set forth in this annual report under “Item 4. Information on
the Company— B. Business Overview— Market Segments— Ag-Business Market— Lavie Bio Ltd.— Investment by Corteva”
and is incorporated by reference herein.
Indemnification Agreements
We have entered into indemnification agreements with our office
holders. Information on the indemnification agreements may be found in this annual report under “Item 7. Major Shareholders and
Related Party Transactions—B. Related Party Transactions—Agreements with Directors and Officers—Indemnification Agreements,”
and is incorporated herein by reference.
Other Compensation Agreements
|
◾ |
Evogene Ltd. Officers Compensation Policy. See Item 6. “Directors, Senior Management and Employees” for more information
about this document. |
|
◾ |
Evogene Share Option Plan (2002). See Item 6. “Directors, Senior Management and Employees” for more information about
this document. |
|
◾ |
Evogene Ltd. Key Employee Share Incentive Plan, 2003. See Item 6. “Directors, Senior Management and Employees” for more
information about this document. |
|
◾ |
Evogene Ltd. 2013 Share Option Plan. See Item 6. “Directors, Senior Management and Employees” for more information about
this document. |
|
◾ |
Evogene 2021 Share Incentive Plan. See Item 6. “Directors, Senior Management and Employees” for more information about
this document. |
D. Exchange
Controls
Other than general anti-money laundering regulations, there are
currently no Israeli currency control regulations in effect that restrict our import or export of capital to or from the State of Israel,
or the availability of cash and cash equivalents for use by our affiliated companies. Under the Bank of Israel Law, 5770-2010, the Governor
of the Bank of Israel, with the approval of the monetary policy committee of the Bank of Israel, is authorized to issue an administrative
order restricting the transfer of funds to or from Israel. However, such an order is only likely to be issued under emergency circumstances
and only for a temporary period, if necessary for the achievement of the goals of the Bank of Israel or the carrying out of its responsibilities
under Israeli law. Furthermore, Israel has agreed, pursuant to international agreements to which it is a party (including incident to
Israel’s having joined the International Monetary Fund) to allow for the free flow of capital to and from within its borders. Certain
transactions nevertheless require the filing of reports with the Bank of Israel.
Similarly, there are no currently effective Israeli governmental
laws, decrees, regulations or other legislation that restrict the payment of dividends or other distributions with respect to our ordinary
shares or the proceeds from the sale of the shares, except for the obligation of Israeli residents to file reports with the Bank of Israel
regarding some transactions. However, legislation remains in effect under which currency controls can be imposed by administrative action
at any time.
E. Taxation
Israel Income Tax Consequences
This section discusses the material Israeli income tax consequences
concerning the ownership and disposition of our ordinary shares by our non-Israeli shareholders. This summary does not discuss all the
aspects of Israeli tax law that may be relevant to a particular investor in light of his or her personal investment circumstances or to
some types of investors subject to special treatment under Israeli law. Examples of such investors include traders in securities who are
subject to special tax regimes not covered in this discussion. Because parts of this discussion are based on new tax legislation that
has not yet been subject to judicial or administrative interpretation, we cannot assure you that the appropriate tax authorities or the
courts will accept the views expressed in this discussion. The discussion below is subject to change, including due to amendments under
Israeli law or changes to the applicable judicial or administrative interpretations of Israeli law, which change could affect the tax
consequences described below.
Taxation of Our Non-Israeli Shareholders
Capital Gains Taxes Applicable
to Non-Israeli Resident Shareholders. A non-Israeli resident (whether individual or corporation) who derives capital gains from
the sale of shares in an Israeli resident company that were purchased after the company was listed for trading on a stock exchange outside
of Israel should generally be exempt from Israeli tax so long as the shares were not held through a permanent establishment that the non-resident
maintains in Israel and that such shareholder is not subject to the Israeli Income Tax Law (Inflationary Adjustments) 5745-1985. However,
non-Israeli corporations will not be entitled to the foregoing exemption if Israeli residents: (i) have a controlling interest of
more than 25% in such non-Israeli corporation or (ii) are the beneficiaries of, or are entitled to, 25% or more of the revenues or
profits of such non-Israeli corporation, whether directly or indirectly. Additionally, such exemption is not applicable to a person whose
gains from selling or otherwise disposing of the shares are deemed to be business income.
If not exempt, a non-Israeli resident shareholder would generally
be subject to tax on capital gain at the ordinary corporate tax rate (23% in 2021), if generated by a company, or at the rate of 25%,
if generated by an individual, or 30%, if generated by an individual who is a “substantial shareholder” (as defined under
the Tax Ordinance), at the time of sale or at any time during the preceding 12-month period (or if the shareholder claims a deduction
for interest and linkage differences expenses in connection with the purchase and holding of such shares). A “substantial shareholder”
is generally a person who alone or together with such person’s relative or another person who collaborates with such person on a
permanent basis, holds, directly or indirectly, at least 10% of any of the “means of control” of the corporation. “Means
of control” generally include, among others, the right to vote, receive profits, nominate a director or an executive officer, receive
assets upon liquidation, or order someone who holds any of the aforesaid rights how to act, regardless of the source of such right. Individual
and corporate shareholders dealing in securities in Israel are taxed at the tax rates applicable to business income (a corporate tax rate
for a corporation (23% in 2021) and a marginal tax rate of up to 47% for an individual in 2021 (excluding excess tax as discussed below))
unless contrary provisions in a relevant tax treaty apply.
Additionally, a sale of shares by a non-Israeli resident may be
exempt from Israeli capital gains tax under the provisions of an applicable tax treaty (subject to the receipt in advance of a valid certificate
from the Israel Tax Authority allowing for an exemption). For example, under the United States-Israel Tax Treaty, the disposition of shares
by a shareholder who is a United States resident (for purposes of the United States-Israel Tax Treaty) holding the shares as a capital
asset and is entitled to claim the benefits afforded to such person by the treaty, is generally exempt from Israeli capital gains
tax unless, among other things, (i) the capital gain arising from the disposition can be attributed to a permanent establishment of the
shareholder which is maintained in Israel; (ii) the shareholder holds, directly or indirectly, shares representing 10% or more of
the voting capital during any part of the 12-month period preceding such sale, exchange or disposition, subject to certain conditions;
or (iii) such U.S. resident is an individual and was present in Israel for a period or periods aggregating to 183 days or more during
the relevant taxable year. In each case, the sale, exchange or disposition of such shares would be subject to Israeli tax, to the extent
applicable; however, under the United States-Israel Tax Treaty, the United States resident would be permitted to claim a credit for the
Israeli tax against the United States federal income tax imposed with respect to the sale, exchange or disposition, subject to the limitations
in United States laws applicable to foreign tax credits. The United States-Israel Tax Treaty does not relate to U.S. state or local taxes.
In some instances, where our shareholders may be liable for Israeli
tax on the sale of their ordinary shares, the payment of the consideration may be subject to the withholding of Israeli tax at source.
Shareholders may be required to demonstrate that they are exempt from tax on their capital gains in order to avoid withholding at source
at the time of sale. Specifically, in transactions involving a sale of all of the shares of an Israeli resident company, in the form of
a merger or otherwise, the Israel Tax Authority may require from shareholders who are not liable for Israeli tax to sign declarations
in forms specified by this authority or obtain a specific exemption from the Israel Tax Authority to confirm their status as non-Israeli
resident, and, in the absence of such declarations or exemptions, may require the purchaser of the shares to withhold taxes at source.
Taxation of Non-Israeli Shareholders
on Receipt of Dividends. Non-Israeli residents (whether individuals or corporations) are generally subject to Israeli income tax
on the receipt of dividends paid on our ordinary shares at the rate of 25%. With respect to a person who is a “substantial shareholder”
(as defined above) at the time of receiving the dividend or on any time during the preceding twelve months, the applicable tax rate is
30%. “Means of control” generally include the right to vote, receive profits, nominate a director or an executive officer,
receive assets upon liquidation, or order someone who holds any of the aforesaid rights how to act, regardless of the source of such right.
Dividends paid on publicly traded shares, which are registered with and held by a nominee company, to non-Israeli residents are generally
subject to Israeli withholding tax at a rate of 25% (whether the recipient is a “substantial shareholder” or not), unless
a lower rate is provided under an applicable tax treaty between Israel and the shareholder’s country of residence and provided that
a certificate from the Israel Tax Authority allowing for a reduced withholding tax rate is obtained in advance.
In this regard, under the United States-Israel Tax Treaty, the
maximum rate of tax withheld at source in Israel on dividends paid to a holder of our ordinary shares who is a United States resident
(for purposes of the United States-Israel Tax Treaty) is 25%. However, generally, the maximum rate of withholding tax on dividends, not
generated by an Approved Enterprise or a Beneficiary Enterprise, that are paid to a United States corporation holding at least 10% or
more of our outstanding voting capital throughout the tax year in which the dividend is distributed as well as during the previous tax
year, is 12.5%, provided that no more than 25% of our gross income for such preceding year consists of certain types of dividends and
interest. Notwithstanding the foregoing, dividends distributed from income attributed to an Approved Enterprise, Benefited Enterprise
or Preferred Enterprise are not entitled to such reduction under such tax treaty but are subject to withholding tax at the rate of 15%
or 20% for such a United States corporate shareholder, provided that the conditions related to the holding of 10% of our voting capital
and to our gross income for the previous year (as set forth in the previous sentence) are met. The aforementioned rates under the
United States-Israel Tax Treaty would not apply if the dividend income is derived through a permanent establishment of the U.S. resident
in Israel. We cannot assure you that we will designate the profits that we may distribute in a way that will reduce shareholders’
tax liability. United States residents who are subject to Israeli withholding tax on a dividend may be entitled to a credit or deduction
for Untied States federal income tax purposes in the amount of the taxes withheld, subject to detailed rules contained in United States
tax legislation.
A non-Israeli resident who receives dividends from which tax was
withheld is generally exempt from the obligation to file tax returns in Israel with respect to such income, provided that (i) such
income was not derived from a business conducted in Israel by the taxpayer, (ii) the taxpayer has no other taxable sources of income
in Israel with respect to which a tax return is required to be filed, and (iii) the taxpayer is not obliged to pay excess tax (as further
explained below).
Excess Tax
Individuals who are subject to tax in Israel (whether any such
individual is an Israeli resident or non-Israeli resident) are also subject to an additional tax at a rate of 3% on annual taxable income
(including, but not limited to, dividends, interest and capital gain) exceeding a certain threshold (NIS 647,640 for 2021), which amount
is linked to the annual change in the Israeli consumer price index, including, but not limited to, dividends, interest and capital
gain.
United States Federal Income Taxation
The following is a description of the material United States
federal income tax consequences to U.S. Holders (as defined below) of the acquisition, ownership and disposition of our ordinary shares.
This description addresses only the United States federal income tax consequences to holders of our ordinary shares that hold such ordinary
shares as capital assets. This description does not address tax considerations applicable to holders that may be subject to special tax
rules, including, without limitation:
|
◾ |
banks, financial institutions or insurance companies; |
|
◾ |
real estate investment trusts, regulated investment companies or grantor trusts; |
|
◾ |
dealers or traders in securities, commodities or currencies; |
|
◾ |
certain former citizens or long-term residents of the United States; |
|
◾ |
persons that received our shares as compensation for the performance of services; |
|
◾ |
persons that will hold our shares as part of a “hedging,” “integrated” or “conversion” transaction
or as a position in a “straddle” for United States federal income tax purposes; |
|
◾ |
partnerships (including entities classified as partnerships for United States federal income tax purposes) or other pass-through
entities, or holders that will hold our shares through such an entity; |
|
◾ |
persons subject to special tax accounting rules as a result of any item of gross income with respect to the ordinary shares being
taken into account in an “applicable financial statement” pursuant to Section 451(b) of the Code; |
|
◾ |
U.S. Holders (as defined below) whose “functional currency” is not the U.S. dollar; or |
|
◾ |
holders that own directly, indirectly or through attribution 10.0% or more of the voting power or value of our shares. |
Moreover, this description does not address the United States federal
estate, gift or alternative minimum tax consequences, or any state, local or foreign tax consequences, of the acquisition, ownership and
disposition of our ordinary shares.
This description is based on the Code, existing, proposed and temporary
United States Treasury Regulations and judicial and administrative interpretations thereof, in each case as in effect and available on
the date hereof. Each of the foregoing is subject to change, which change could apply retroactively and could affect the tax consequences
described below. There can be no assurances that the U.S. Internal Revenue Service will not take a different position concerning the tax
consequences of the acquisition, ownership and disposition of our ordinary shares or that such a position would not be sustained.
For purposes of this description, a “U.S. Holder” is
a beneficial owner of our ordinary shares that, for United States federal income tax purposes, is:
|
◾ |
a citizen or resident of the United States; |
|
◾ |
a corporation (or other entity treated as a corporation for United States federal income tax purposes) created or organized in or
under the laws of the United States or any state thereof, including the District of Columbia; |
|
◾ |
an estate the income of which is subject to United States federal income taxation regardless of its source; or |
|
◾ |
a trust if such trust has validly elected to be treated as a United States person for United States federal income tax purposes or
if (1) a court within the United States is able to exercise primary supervision over its administration and (2) one or more
United States persons have the authority to control all of the substantial decisions of such trust. |
A “Non-U.S. Holder” is a beneficial owner of our ordinary
shares that is neither a U.S. Holder nor a partnership (or other entity treated as a partnership for United States federal income tax
purposes).
If a partnership (or any other entity treated as a partnership
for United States federal income tax purposes) holds our ordinary shares, the tax treatment of a partner in such partnership will generally
depend on the status of the partner and the activities of the partnership. Such a partner or partnership is encouraged to consult its
tax advisor as to its tax consequences.
You are encouraged to consult your advisor with
respect to the United States federal, state, local and foreign tax consequences of acquiring, owning and disposing of our ordinary shares.
Distributions
Subject to the discussion below under “Passive Foreign Investment
Company Considerations,” if you are a U.S. Holder, the gross amount of any distribution that we pay you with respect to our ordinary
shares before reduction for any Israeli taxes withheld therefrom generally will be includible in your income as dividend income to the
extent such distribution is paid out of our current or accumulated earnings and profits as determined under United States federal income
tax principles. To the extent that the amount of any cash distribution exceeds our current and accumulated earnings and profits as determined
under United States federal income tax principles, it will be treated first as a tax-free return of your adjusted tax basis in our ordinary
shares and thereafter as capital gain. We do not expect to maintain calculations of our earnings and profits under United States federal
income tax principles. Therefore, if you are a U.S. Holder you should expect that the entire amount of any cash distribution generally
will be reported as dividend income to you; provided, however, that distributions of ordinary shares to U.S. Holders that are part of
a pro rata distribution to all of our shareholders generally will not be subject to United States federal income tax. Subject to the PFIC
rules discussed below, non-corporate U.S. Holders may qualify for the lower rates of taxation with respect to dividends on ordinary shares
applicable to long-term capital gains (i.e., gains from the sale of capital assets held for more
than one year), provided that certain conditions are met, including certain holding period requirements and the absence of certain risk
reduction transactions. Moreover, such reduced rate shall not apply if we are a PFIC for the taxable year in which we pay a dividend,
or were a PFIC for the preceding taxable year. As discussed below, we believe we were classified as a PFIC for the year ending December
31, 2021. Dividends will not be eligible for the dividends received deduction generally allowed to corporate U.S. Holders.
If you are a U.S. Holder, dividends that we pay you with respect
to our ordinary shares will be treated as foreign source income, which may be relevant in calculating your foreign tax credit limitation.
Subject to certain conditions and limitations, Israeli tax withheld on dividends may be deducted from your taxable income or credited
against your United States federal income tax liability. The limitation on foreign taxes eligible for credit is calculated separately
with respect to specific classes of income. For this purpose, dividends that we distribute generally should constitute “passive
category income.” A foreign tax credit for foreign taxes imposed on distributions may be denied if you do not satisfy certain minimum
holding period requirements. The rules relating to the determination of the foreign tax credit are complex, and you are encouraged to
consult your tax advisor to determine whether and to what extent you will be entitled to this credit.
Sale, Exchange or Other Disposition of Ordinary
Shares
Subject to the discussion below under “Passive Foreign Investment
Company Considerations,” if you are a U.S. Holder, you generally will recognize an amount of gain or loss on the sale, exchange
or other disposition of our ordinary shares equal to the difference between the amount realized on such sale, exchange or other disposition
and your tax basis in our ordinary shares, and such gain or loss will be capital gain or loss. The tax basis in an ordinary share generally
will equal the cost of such ordinary share. If you are a non-corporate U.S. Holder, capital gain from the sale, exchange or other disposition
of ordinary shares generally will be eligible for a preferential rate of taxation applicable to capital gains, if your holding period
for such ordinary shares exceeds one year. The deductibility of capital losses for United States federal income tax purposes is subject
to limitations under the Code. However, as discussed below, we believe we were classified as a PFIC for the year ending December 31, 2021,
in which case special rules may apply as explained below. Any such gain or loss that a U.S. Holder recognizes generally will be treated
as U.S. source income or loss for foreign tax credit limitation purposes.
Passive Foreign Investment Company Considerations
Based on certain estimates of our gross income and gross assets
and the nature of our business, we believe that we were classified as a PFIC for the taxable year ending December 31, 2021. As a
result, a U.S. Holder who held our ordinary shares at any time during 2020 would be subject to special rules generally intended to reduce
or eliminate any benefits from the deferral of U.S. federal income tax that a U.S. Holder could derive from investing in a non-U.S. company
that does not distribute all of its earnings on a current basis.
A non-U.S. corporation will be classified as a PFIC for federal
income tax purposes in any taxable year in which, after applying certain look-through rules, either:
|
◾ |
at least 75% of its gross income is “passive income”; or |
|
◾ |
at least 50% of the average quarterly value of its gross assets (which may be determined in part by the market value of our ordinary
shares, which is subject to change) is attributable to assets that produce “passive income” or are held for the production
of passive income. |
Passive income for this purpose generally includes dividends, interest,
royalties, rents, gains from commodities and securities transactions, the excess of gains over losses from the disposition of assets which
produce passive income, and includes amounts derived by reason of the temporary investment of funds raised in offerings of our ordinary
shares. If a non-U.S. corporation owns at least 25% by value of the stock of another corporation, the non-U.S. corporation is treated
for purposes of the PFIC tests as owning its proportionate share of the assets of the other corporation and as receiving directly its
proportionate share of the other corporation’s income. For publicly traded corporations, the PFIC asset test described above is
applied using the fair market value of the non-U.S. corporation’s assets. For purposes of a the PFIC asset test, a publicly traded
non-U.S. corporation may treat the aggregate fair market value of its assets as being equal to the sum of its Market Capitalization and
the total amount of its liabilities. We intend to take the position that the excess of our Market Capitalization plus liabilities over
the book value of all of our assets may generally be treated as attributable to non-passive asset. If we are classified as a PFIC in any
year with respect to which a U.S. Holder owns our ordinary shares, we will continue to be treated as a PFIC with respect to such U.S.
Holder in all succeeding years during which the U.S. Holder owns our ordinary shares, regardless of whether we continue to meet the tests
described above.
Based on the book value of our assets and liabilities and our Market
Capitalization in 2021, we believe that we met the PFIC asset test described above for 2021 and, as a result, we were classified as a
PFIC in 2021. Furthermore, because we currently hold, and expect to continue to hold, a substantial amount of cash and cash equivalents
and other passive assets used in our business, and because our Market Capitalization is currently below the level necessary to avoid PFIC
status for 2022, there is substantial risk we will be classified as a PFIC for the 2022 taxable year as well. However, because PFIC status
is based on our income, assets and activities for the entire taxable year, and our Market Capitalization, it is not possible to determine
whether we will be characterized as a PFIC for the 2022 taxable year until after the close of the year. Moreover, we must determine our
PFIC status annually after the close of each taxable year based on tests which are factual in nature, and our status in future years will
depend on our income, assets, activities and Market Capitalization in those years. Thus, there can be no assurance that we will not be
considered a PFIC for the current taxable year or any future taxable year.
If we are a PFIC for any taxable year during which a U.S. Holder
owns ordinary shares, we will generally continue to be treated as a PFIC with respect to such U.S. Holder for all succeeding years during
which such U.S. Holder owns such ordinary shares, unless we cease to be a PFIC and the U.S. Holder makes a “deemed sale” election
with respect to such ordinary shares. If such election is made, the U.S. Holder will be deemed to have sold the ordinary shares it holds
at their fair market value and any gain from the deemed sale would be subject to the rules described in the following paragraph. After
the deemed sale election, so long as we do not become a PFIC in a subsequent taxable year, the ordinary shares with respect to which such
election was made will not be treated as shares in a PFIC and will not be subject to the rules described below with respect to any “excess
distribution” the U.S. Holder receives from us or any gain from an actual sale or other disposition of such ordinary shares. U.S.
Holders are strongly urged to consult their tax advisors as to the possibility and consequences of making a deemed sale election if we
were to become and then cease to be a PFIC, and such election becomes available.
If you are a U.S. Holder that owns our ordinary shares during 2020
or any other taxable year for which we are a PFIC, then unless you make one of the elections described below, a special tax regime will
apply to both (a) any “excess distribution” by us to you (generally, your ratable portion of distributions in any year
which are greater than 125% of the average annual distribution received by you in the shorter of the three preceding years or your holding
period for our ordinary shares) and (b) any gain realized on the sale or other disposition of the ordinary shares. Under this regime,
any excess distribution and realized gain will be treated as ordinary income (even if you hold the ordinary shares as capital assets)
and will be subject to tax as if (a) the excess distribution or gain had been realized ratably over your holding period, (b) the
amount deemed realized in each year had been subject to tax in each year of that holding period at the highest marginal rate for such
year (other than income allocated to the current period or any taxable period before we became a PFIC, which would be subject to tax at
the U.S. Holder’s regular ordinary income rate for the current year and would not be subject to the interest change discussed below),
and (c) the interest charge generally applicable to underpayments of tax had been imposed on the taxes deemed to have been payable
in those years. The tax liability for amounts allocated to years prior to the year of disposition or excess distribution cannot be offset
by any net operating losses for such years.
If we are a PFIC for any taxable year during which a U.S. Holder
holds our ordinary shares, then in lieu of being subject to the tax and interest charge rules discussed above, a U.S. Holder may make
an election to include gain on the stock of a PFIC as ordinary income under a mark-to-market method, provided that such ordinary shares
are “regularly traded” on a “qualified exchange.” In general, our ordinary shares will be treated as “regularly
traded” for a given calendar year if more than a de minimis quantity of our ordinary shares
are traded on a qualified exchange on at least 15 days during each calendar quarter of such calendar year. Our ordinary shares are listed,
and we expect them to continue to be listed for the foreseeable future, on the New York Stock Exchange, which is a qualifying exchange
for this purpose. However, no assurance can be given that our ordinary shares will continue to be regularly traded on a “qualified
exchange” for purposes of the mark-to-market election. In addition, because a mark-to-market election cannot be made for any lower-tier
PFICs that we may own, a U.S. Holder may continue to be subject to the PFIC rules discussed above with respect to such holder’s
indirect interest in any investments we hold that are treated as an equity interest in a PFIC for United States federal income tax purposes.
If a U.S. Holder makes an effective mark-to-market election, in
each year that we are a PFIC, such U.S. Holder will include in each year that we are a PFIC as ordinary income the excess of the fair
market value of such U.S. Holder’s ordinary shares at the end of the year over such U.S. Holder’s adjusted tax basis in the
shares. Such U.S. Holder will be entitled to deduct as an ordinary loss in each such year the excess of such U.S. Holder’s adjusted
tax basis in the ordinary shares over their fair market value at the end of the year, but only to the extent of the net amount previously
included in income as a result of the mark-to-market election. If a U.S. Holder makes an effective mark-to-market election, in each year
that we are a PFIC, any gain such U.S. Holder recognizes upon the sale or other disposition of such U.S. Holder’s ordinary shares
will be treated as ordinary income and any loss will be treated as ordinary loss, but only to the extent of the net amount of previously
included income as a result of the mark-to-market election.
A U.S. Holder’s adjusted tax basis in the ordinary shares
will be increased by the amount of any income inclusion and decreased by the amount of any deductions under the mark-to-market rules discussed
above. If a U.S. Holder makes an effective mark-to-market election, it will be effective for the taxable year for which the election is
made and all subsequent taxable years unless the ordinary shares are no longer regularly traded on a qualified exchange or the IRS consents
to the revocation of the election. U.S. Holders are encouraged to consult their tax advisers about the availability of the mark-to-market
election, and whether making the election would be advisable in their particular circumstances.
In certain circumstances, a U.S. equity holder in a PFIC may avoid
the adverse tax and interest-charge regime described above by making a “qualified electing fund” election to include in income
its share of the corporation’s income on a current basis. However, a U.S. Holder may make a qualified electing fund election with
respect to the ordinary shares only if we agree to furnish you annually with a PFIC annual information statement as specified in the applicable
Treasury regulations.
We do not intend to provide the information necessary for U.S.
Holders to make qualified electing fund elections if we are classified as a PFIC. U.S. Holders are encouraged to consult their tax advisors
to determine whether any of these elections would be available and if so, what the consequences of the alternative treatments would be
in their particular circumstances.
If we are determined to be a PFIC for any year in which a U.S.
Holder holds our ordinary shares, the general tax treatment for the U.S. Holder described in this paragraph would apply to indirect distributions
and gains deemed to be realized by the U.S. Holders in respect of any of our subsidiaries that also may be determined to be PFICs.
If a U.S. Holder owns ordinary shares during any year in which
we are a PFIC and the U.S. Holder recognizes gain on a disposition of our ordinary shares or receives distributions with respect to our
ordinary shares, the U.S. Holder generally will be required to file an IRS Form 8621 with respect to the company, generally with the U.S.
Holder’s federal income tax return for that year. If our company were a PFIC for a given taxable year, then you are encouraged to
consult your tax advisor concerning your annual filing requirements.
U.S. Holders are strongly encouraged to consult
their tax advisors regarding the consequences of our classification as a PFIC for our 2021 taxable year, our potential classification
as a PFIC in 2022 and future taxable years, and the application of the PFIC rules on their investment.
Backup Withholding Tax and Information Reporting
Requirements
United States backup withholding tax and information reporting
requirements may apply to certain payments to certain holders of stock. Information reporting generally will apply to payments of dividends
on, and to proceeds from the sale or redemption of, our ordinary shares made within the United States, or by a United States payor or
United States middleman, to a holder of our ordinary shares, other than an exempt recipient (including a payee that is not a United States
person that provides an appropriate certification and certain other persons). A payor will be required to withhold backup withholding
tax from any payments of dividends on, or the proceeds from the sale or redemption of, ordinary shares within the United States, or by
a United States payor or United States middleman, to a holder, other than an exempt recipient, if such holder fails to furnish its correct
taxpayer identification number or otherwise fails to comply with, or establish an exemption from, such backup withholding tax requirements.
Any amounts withheld under the backup withholding rules will be allowed as a credit against the beneficial owner’s United States
federal income tax liability, if any, and any excess amounts withheld under the backup withholding rules may be refunded, provided that
the required information is timely furnished to the Internal Revenue Service.
Foreign Asset Reporting
Certain U.S. Holders who are individuals are required to report
information relating to an interest in our ordinary shares, subject to certain exceptions (including an exception for shares held in accounts
maintained by financial institutions). U.S. Holders are encouraged to consult their tax advisors regarding their information reporting
obligations, if any, with respect to their ownership and disposition of our ordinary shares.
Medicare Tax
Certain U.S. Holders that are individuals, estates or trusts are
subject to a 3.8% tax on all or a portion of their “net investment income,” which may include all or a portion of their dividend
income and net gains from the disposition of ordinary shares. Each U.S. Holder that is an individual, estate or trust is encouraged to
consult its tax advisors regarding the applicability of the Medicare tax to its income and gains in respect of its investment in the ordinary
shares.
The above description is not intended to constitute
a complete analysis of all tax consequences relating to acquisition, ownership and disposition of our ordinary shares. You are encouraged
to consult your tax advisor concerning the tax consequences of your particular situation.
F.
Dividends and Paying Agents
Not applicable.
G. Statement
by Experts
Not applicable.
H. Documents
on Display
We are currently subject to the informational requirements of the
Exchange Act applicable to foreign private issuers and fulfill the obligations of these requirements by filing reports with the SEC. As
a foreign private issuer, we are exempt from the rules under the Exchange Act relating to the furnishing and content of proxy statements,
and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained
in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements
with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. However, we intend
to file with the SEC, within 120 days after the end of each subsequent fiscal year, an annual report on Form 20-F containing financial
statements which will be examined and reported on, with an opinion expressed, by an independent public accounting firm. We also intend
to furnish to the SEC reports of foreign private issuer on Form 6-K containing unaudited quarterly financial information.
The SEC maintains an Internet website at http://www.sec.gov that
contains reports, including this annual report and the documents referred to herein, proxy statements, information statements and other
material that are filed through the SEC’s Electronic Data Gathering, Analysis and Retrieval, or “EDGAR” system.
We also file annual and special reports and other information with
the Israeli Securities Authority through its fair disclosure electronic system called MAGNA. You may review these filings on the website
of the MAGNA system operated by the Israeli Securities Authority at www.magna.isa.gov.il or on the website of the TASE at www.tase.co.il.
Our ordinary shares are quoted on the TASE and, since December
2016, on Nasdaq (after being listed on the NYSE from November 2013 until December 2016). Information about us is also available on our
website at http://www.evogene.com. Our website and the information contained therein or connected thereto will not be deemed to be incorporated
into this annual report and you should not rely on any such information in making your decision whether to purchase our ordinary shares.
I. Subsidiary
Information
Not applicable.
| ITEM 11. |
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
We are exposed to a variety of risks, including foreign currency
exchange fluctuations, commodity price risk and other risks. We regularly assess the risks to minimize any adverse effects on our business.
For sensitivity analysis of our exposure to foreign currency exchange fluctuations and changes in market prices of listed securities,
see Note 13d to our consolidated financial statements as of, and for the year ended, December 31, 2021 included elsewhere in this annual
report.
Foreign Currency Risk
Most of our revenues are denominated in U.S. dollars. By contrast,
we incur expenses primarily denominated in NIS. As a result, any appreciation of the NIS relative to the U.S. dollar adversely impacts
our profitability due to the portion of our expenses that are incurred in NIS. As of December 31, 2021, we did not have any open forward
currency contracts. In the future we may enter into hedging transactions in order to decrease our foreign currency risk; however, these
transactions may not fully protect us from such risk.
The following table presents information about the changes in the
exchange rate of the NIS against the U.S. dollar:
|
Period |
|
Depreciation (Appreciation)
of the U.S. dollar against the NIS (%) Based on Average of Daily Exchange Rates Throughout Year Compared to Previous Year |
|
|
2021 |
|
|
(6.0 |
) |
|
2020 |
|
|
(7.0 |
) |
|
2019 |
|
|
(7.7 |
) |
|
2018 |
|
|
(0.1 |
) |
|
2017 |
|
|
(6.3 |
) |
| |
|
|
|
|
Our exposure related to exchange rate changes on our net asset
position denominated in currencies other than U.S. dollars varies with changes in our net asset position. Net asset position refers to
financial assets, such as trade receivables and cash and cash deposits, less financial liabilities, such as trade payable and other payables.
The impact of any such transaction gains or losses is reflected in financing expenses or income. Our most significant exposure relates
to a potential change in the U.S. dollar-NIS exchange rates. Assuming a 10% increase in the U.S. dollar relative to the NIS, and assuming
no other change, our financing expenses would have increased by $1.3 million in 2021, increased by $1.0 million in 2020, and increased
by $0.8 million in 2019 due to our positive current net asset position denominated in NIS as of December 31, 2021 2020 and 2019. As of
December 31, 2021, we did not have any hedge arrangements in place to protect our exposure to foreign currency risk.
Commodity Price Risk
Changes in commodity prices in the agriculture markets may affect
our reported operating results and cash flows in view of our activity in the agriculture segment. For example, a decrease in the prices
of corn and soy grains may adversely impact the budget for, and size of, research and development expenditures of our existing and potential
collaborators and, in turn, our ability to continue or extend existing collaborations or enter into new ones. Further, the royalties we
may receive from our collaborators on the sales and transfers of seeds containing the traits
we develop could be affected by fluctuations in seed commodity prices. As of December 31, 2021, we did not have any hedge arrangements
in place to protect our exposure to commodity price fluctuations.
| ITEM 12. |
DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES |
Not applicable.
PART II
| ITEM 13. |
DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES |
None.
| ITEM 14. |
MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS |
The effective date of the registration statement on Form F-1, File
No. 333-191315, for our U.S. initial public offering of ordinary shares was November 20, 2013. The offering commenced on November 21,
2013 and was closed on November 26, 2013. Credit Suisse Securities and Deutsche Bank Securities acted as joint book-running managers for
the offering, and Oppenheimer Co. and Piper Jaffray Co. acted as co-managers. We registered and sold 5,750,000 of our ordinary
shares in our U.S. initial public offering. The aggregate offering price of the shares registered was approximately $84.8 million, as
was the aggregate price of the shares sold. The total expenses of the offering, including underwriting discounts and commissions, were
approximately $8 million. The net proceeds that we received from the offering were approximately $76.8 million.
None of the net proceeds of our U.S. initial public offering was
paid directly or indirectly to any director, officer, general partner of ours or to their associates, persons owning ten percent or more
of any class of our equity securities, or to any of our affiliates.
The Company has used all the offering proceeds from its U.S. initial public offering that closed on November 26, 2013.
| ITEM 15. |
CONTROLS AND PROCEDURES |
|
(a) |
Disclosure Controls and Procedures |
Our management, including our Chief Executive Officer and Chief
Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e)
and 15d-15(e) under the Exchange Act) as of December 31, 2021. Our management recognizes that any controls and procedures, no matter how
well designed and operated, can provide only reasonable assurance of achieving their objectives and our management necessarily applies
its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on this evaluation, our Chief Executive
Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of December 31, 2021, to provide
reasonable assurance that the information required to be disclosed in filings and submissions under the Exchange Act, is recorded, processed,
summarized and reported within the time periods specified by the SEC’s rules and forms, and that such information related to us
and our consolidated subsidiaries is accumulated and communicated to management, including the Chief Executive Officer and Chief Financial
Officer, as appropriate to allow timely decisions about required disclosure.
|
(b) |
Management’s Annual Report on Internal Control Over Financial Reporting |
Our management is responsible for establishing and maintaining
adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Our internal control
over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the
preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Our management recognizes that there are inherent limitations in
the effectiveness of any system of internal control over financial reporting, including the possibility of human error and the circumvention
or override of internal control. Accordingly, even effective internal control over financial reporting can provide only reasonable assurance
with respect to financial statement preparation and may not prevent or detect all misstatements. Further, because of changes in conditions,
the effectiveness of internal control over financial reporting may vary over time.
Our management assessed the effectiveness of our internal control
over financial reporting as of December 31, 2021. In conducting its assessment of internal control over financial reporting, management
used the framework and criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring
Organizations of the Treadway Commission (COSO) as of the end of the period covered by this report. Based on that evaluation, our management
has concluded that our internal control over financial reporting was effective as of December 31, 2021.
|
(c) |
Attestation Report of Registered Public Accounting Firm |
Not applicable (we are exempt from this requirement due to our
status under the Exchange Act as a non-accelerated filer as of the current time).
|
(d) |
Changes in internal control over financial reporting |
During the period covered by this annual report, no changes in
our internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange
Act), have occurred that have materially affected, or are reasonably likely to materially affect, our internal control over financial
reporting.
| ITEM 16A. |
AUDIT COMMITTEE FINANCIAL EXPERT |
Our board of directors has determined that each of Mr. Dan Falk
and Mr. Ziv Kop qualifies as an audit committee financial expert, as defined by the rules of the SEC, and has the requisite financial
experience required by the Nasdaq Listing Rules. In addition, each of Mr. Falk and Mr. Kop is independent, as such term is defined in
Rule 10A-3(b)(1) under the Exchange Act and under the Nasdaq Listing Rules.
We have adopted a Code of Ethics and Proper Business Conduct applicable
to our executive officers, directors and all other employees, which is a “code of ethics” as defined in this Item 16B of Form
20-F promulgated by the SEC. We have also implemented a training program for new and existing employees concerning our Code of Ethics
and Proper Business Conduct. A copy of the code is delivered to every employee of Evogene Ltd. and all of its subsidiaries, and is available
to investors and others, without charge, on our website at http://www.evogene.com/investor-relations/corporate-governance/ or by contacting
our investor relations department. Information contained on, or that can be accessed through, our website does not constitute a part of
this Form 20-F and is not incorporated by reference herein. Under Item 16B of Form 20-F, if a waiver or amendment of the Code
of Business Conduct and Ethics applies to our principal executive officer, principal financial officer, principal accounting officer,
controller or other persons performing similar functions and relates to standards promoting any of the values described in Item 16B(b)
of Form 20-F, we will disclose such waiver or amendment on our website within four business days following the date of amendment
or waiver in accordance with the requirements of the Nasdaq listing rules and Instruction 4 to such Item 16B. We granted no
waivers under our Code of Ethics and Proper Business Conduct in 2021. We also intend to disclose any amendments to, or waivers of, the
Code of Ethics and Proper Business Conduct applicable to our directors or executive officers on our website.
| ITEM 16C. |
PRINCIPAL ACCOUNTANT FEES AND SERVICES |
Principal Accountant Fees and Services.
We paid or accrued the following fees for professional services
rendered by Kost, Forer, Gabbay Kasierer, a member of Ernst Young Global and an independent registered public accounting firm,
for the years ended December 31, 2020 and 2021:
| |
|
|
|
|
|
|
|
Audit Fees |
|
$ |
215,000 |
|
|
|
260,000 |
|
|
Audit-Related Fees |
|
|
- |
|
|
|
- |
|
|
Tax Fees |
|
|
16,905 |
|
|
|
25,000 |
|
|
All Other Fees |
|
|
- |
|
|
|
- |
|
|
Total |
|
|
|
|
|
|
|
|
“Audit fees” are the aggregate fees billed for the
audit of our annual financial statements. This category also includes services that generally the independent accountant provides, such
as consents and assistance with and review of documents filed with the SEC.
“Tax fees” include fees for professional services rendered
by our auditors for tax compliance and tax consulting in connection with international transfer pricing.
Our audit committee has adopted a pre-approval policy for the engagement
of our independent accountant to perform certain audit and non-audit services. Pursuant to this policy, which is designed to assure that
such engagements do not impair the independence of our auditors, the audit committee pre-approves annually any specific audit and non-audit
services, audit-related services and tax services that may be performed by our independent accountants. Pursuant to that policy, our audit
committee pre-approved all fees paid to our auditors for the year ended December 31, 2021.
| ITEM 16D. |
EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES |
Not applicable.
| ITEM 16E. |
PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS |
Not applicable.
| ITEM 16F. |
CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT |
Not applicable.
| ITEM 16G. |
CORPORATE GOVERNANCE |
Except as otherwise indicated, we are in compliance with corporate
governance standards as currently applicable to us under Israeli, U.S., SEC and Nasdaq laws, rules and/or regulations, as applicable.
Under the Nasdaq Listing Rules, as a foreign private issuer (as such term is defined in Rule 3b-4 under the Securities Exchange Act of
1934, as amended), we may elect to follow certain corporate governance practices permitted under the Companies Law in lieu of compliance
with corresponding corporate governance requirements otherwise imposed by the Nasdaq Listing Rules for U.S. domestic issuers. We currently
follow the provisions of the Companies Law, rather than the Nasdaq Listing Rules, solely with respect to the following requirements:
|
◾ |
Quorum. As permitted under the Companies Law, pursuant to our articles of association, the
quorum required for an ordinary meeting of shareholders consists of at least two shareholders present in person, by proxy or by other
voting instrument in accordance with the Companies Law, who hold at least 25% of the voting power of our shares (and in an adjourned meeting,
with some exceptions, at least two shareholders), instead of 33 1/3% of the issued share capital, as required under the Nasdaq Listing
Rules. |
|
◾ |
Executive sessions of independent directors. Israeli law does not require executive sessions
of independent directors. Although all of our current directors are “independent directors” under the applicable Nasdaq criteria,
we do not intend to comply with this requirement if we have directors who are not independent. |
|
◾ |
Shareholder approval. We seek shareholder approval for all corporate actions requiring such
approval under the Companies Law, which include (i) transactions with directors concerning the terms of their service or indemnification,
exemption and insurance for their service (or for any other position that they may hold at our company), (ii) transactions concerning
the compensation, indemnification, exculpation and insurance of the chief executive officer; (iii) the compensation policy recommended
by the compensation committee of our board of directors and approved by our board of directors (and any amendments thereto); (iv) extraordinary
transactions with, and the terms of employment or other engagement of, a controlling shareholder (if and when this becomes relevant to
our company), (v) amendments to our articles of association, and (vi) certain non-public issuances of securities. In addition,
under the Companies Law, a merger requires approval of the shareholders of each of the merging companies. We are not required, however,
to seek shareholder approval for any of the following events described in the Nasdaq Listing Rules: |
|
◾ |
certain issuances of shares in excess of 20% of the outstanding shares of the Company; |
|
◾ |
an issuance that will result in a change of control of our company; and |
|
◾ |
adoption of, or material changes to, our equity compensation plans. |
| ITEM 16H. |
MINE SAFETY DISCLOSURE |
Not applicable.
ITEM 16I. DISCLOSURE REGARDING
FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable
PART III
| ITEM 17. |
FINANCIAL STATEMENTS |
We have provided financial statements pursuant to Item 18.
| ITEM 18. |
FINANCIAL STATEMENTS |
The audited consolidated financial statements as required under
Item 18 are attached hereto starting on page F-2 of this Annual Report. The audit report of Kost Forer Gabbay Kasierer, a member
of Ernst Young Global, an independent registered public accounting firm, is included herein preceding the audited consolidated financial
statements.
ANNUAL REPORT ON FORM 20-F
INDEX OF EXHIBITS
|
Exhibit No. |
|
Description |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101 |
|
The following financial information from Evogene Ltd.’s Annual Report on Form 20-F
for the year ended December 31, 2021 formatted in inline XBRL (eXtensible Business Reporting Language): (i) Consolidated Statements of
Financial Position at December 31, 2021 and 2020; (ii) Consolidated Statements of Profit or Loss for the years ended December 31, 2021,
2020 and 2019; (iii) Consolidated Statements of Changes in Equity for the years ended December 31, 2021, 2020 and 2019; (iv) Consolidated
Statements of Cash Flows for the years ended December 31, 2021, 2020 and 2019; and (v) Notes to Consolidated Financial Statements, tagged
as blocks of text. † |
|
104 |
|
Cover Page Interactive Data File 101 |
|
* |
Portions of this exhibit have been omitted in accordance with the rules of the SEC.
|
SIGNATURES
The registrant hereby certifies that it meets all of the requirements
for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this Annual Report on its behalf.
| |
|
Evogene Ltd.
|
|
|
|
By: /s/ Ofer Haviv
Name: Ofer
Haviv
Title: President and Chief Executive Officer |