|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ý
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
|
36-4316614
|
|
(State or other jurisdiction of
incorporation or organization)
|
|
(I.R.S. Employer
Identification No.)
|
|
One Edwards Way, Irvine, California 92614
(Address of principal executive offices) (ZIP Code)
|
||
|
(949) 250-2500
Registrant's telephone number, including area code
|
||
|
Securities registered pursuant to Section 12(b) of the Act:
|
|
Name of each exchange on which registered:
|
|
Common Stock, par value $1.00 per share
|
|
New York Stock Exchange
|
|
Securities registered pursuant to Section 12(g) of the Act:
None
|
||
|
Large accelerated filer
ý
|
|
Accelerated filer
o
|
|
Non-accelerated filer
o
(Do not check if a smaller reporting company)
|
|
Smaller Reporting Company
o
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
•
|
federal, state, and foreign anti-kickback laws and regulations, which generally prohibit payments to physicians or other purchasers of medical products as an inducement to purchase a product;
|
|
•
|
the Stark law, which prohibits physicians from referring Medicare or Medicaid patients to a provider that bills these programs for the provision of certain designated health services if the physician (or a member of the physician's immediate family) has a financial relationship with that provider;
|
|
•
|
federal and state laws and regulations that protect the confidentiality of certain patient health information, including patient records, and restrict the use and disclosure of such information, in particular, the Health Insurance Portability and Accountability Act of 1996;
|
|
•
|
the Physician Payments Sunshine Act, which requires public disclosure of the financial relationships of United States physicians and teaching hospitals with applicable manufacturers, including medical device, pharmaceutical, and biologics companies;
|
|
•
|
the False Claims Act, which prohibits the submission of false or otherwise improper claims for payment to a federally funded health care program, and health care fraud statutes that prohibit false statements and improper claims to any third-party payor; and
|
|
•
|
the United States Foreign Corrupt Practices Act, which can be used to prosecute companies in the United States for arrangements with foreign government officials or other parties outside the United States.
|
|
•
|
product standards and specifications;
|
|
•
|
packaging requirements;
|
|
•
|
labeling requirements;
|
|
•
|
product collection and disposal requirements;
|
|
•
|
quality system requirements;
|
|
•
|
import restrictions;
|
|
•
|
tariffs;
|
|
•
|
duties; and
|
|
•
|
tax requirements.
|
|
•
|
changes in local medical reimbursement policies and programs;
|
|
•
|
changes in foreign regulatory requirements;
|
|
•
|
changes in a specific country's or region's political or economic conditions, including changing circumstances in emerging regions, that may reduce the number of procedures that use our products;
|
|
•
|
trade protection measures, quotas, embargoes, import or export licensing requirements, and duties, tariffs, or surcharges;
|
|
•
|
potentially negative impact of tax laws, including transfer pricing liabilities and tax costs associated with the repatriation of cash;
|
|
•
|
difficulty in staffing and managing global operations;
|
|
•
|
cultural, exchange rate, or other local factors affecting financial terms with customers;
|
|
•
|
local economic and financial conditions, including sovereign defaults and decline in sovereign credit ratings, affecting the collectability of receivables, including receivables from sovereign entities;
|
|
•
|
an outbreak of any life-threatening communicable disease;
|
|
•
|
economic and political instability and local economic and political conditions;
|
|
•
|
differing labor regulations; and
|
|
•
|
differing protection of intellectual property.
|
|
•
|
announcements of innovations, new products, strategic developments, or business combinations by us or our competitors;
|
|
•
|
demand for and clinical acceptance of products;
|
|
•
|
the timing and execution of customer contracts, particularly large contracts that would materially affect our operating results in a given quarter;
|
|
•
|
the timing of sales of products and of the introduction of new products;
|
|
•
|
the timing of marketing, training, and other expenses related to the introduction of new products;
|
|
•
|
the timing of regulatory approvals;
|
|
•
|
changes in foreign currency exchange rates;
|
|
•
|
delays or problems in introducing new products, such as slower than anticipated adoption of transcatheter heart valves;
|
|
•
|
changes in our pricing policies or the pricing policies of our competitors;
|
|
•
|
the timing of approvals of governmental reimbursement rates or changes in reimbursement rates for our products;
|
|
•
|
increased expenses, whether related to sales and marketing, raw materials or supplies, product development, or administration;
|
|
•
|
changes in the level of economic activity in the United States or other regions in which we do business;
|
|
•
|
costs related to acquisitions of technologies or businesses; and
|
|
•
|
our ability to expand our operations and the amount and timing of expansion-related expenditures.
|
|
North America
|
|
|
|
|
|
Irvine, California
|
(1
|
)
|
Corporate Headquarters, Research and Development, Regulatory and Clinical Affairs, Manufacturing, Administration
|
|
|
Draper, Utah
|
(1
|
)
|
Administration, Manufacturing
|
|
|
Haina, Dominican Republic
|
(2
|
)
|
Manufacturing
|
|
|
Añasco, Puerto Rico
|
(2
|
)
|
Manufacturing
|
|
|
Europe
|
|
|
|
|
|
Horw, Switzerland
|
(2
|
)
|
Manufacturing, Administration
|
|
|
Nyon, Switzerland
|
(1
|
)
|
Administration, Marketing
|
|
|
Prague, Czech Republic
|
(2
|
)
|
Administration
|
|
|
Asia
|
|
|
|
|
|
Tokyo, Japan
|
(2
|
)
|
Administration, Marketing, Distribution
|
|
|
Shanghai, China
|
(2
|
)
|
Administration
|
|
|
Singapore
|
(1),(2)
|
|
Manufacturing, Marketing, Distribution, Administration
|
|
|
(1)
|
Owned property.
|
|
(2)
|
Leased property.
|
|
|
2015
|
2014
|
|||||||||||||
|
|
High
|
Low
|
High
|
Low
|
|||||||||||
|
Calendar Quarter Ended:
|
|
|
|
|
|
|
|
|
|||||||
|
March 31
|
$
|
75.21
|
|
$
|
61.99
|
|
$
|
37.81
|
|
$
|
31.52
|
|
|||
|
June 30
|
73.65
|
|
61.38
|
|
44.10
|
|
36.40
|
|
|||||||
|
September 30
|
79.50
|
|
62.53
|
|
52.35
|
|
42.03
|
|
|||||||
|
December 31
|
83.43
|
|
70.32
|
|
67.14
|
|
48.54
|
|
|||||||
|
Period
|
Total Number
of Shares
(or Units)
Purchased (a)
|
Average
Price Paid
per Share
(or Unit) (b)
|
Total Number of
Shares (or Units)
Purchased as Part of Publicly Announced Plans or Programs
|
Maximum Number
(or Approximate
Dollar Value) of
Shares that
May Yet Be
Purchased
Under the Plans
or Programs
(in millions) (c)
|
|||||||||||||
|
October 1, 2015 through October 31, 2015
|
154
|
|
$
|
72.21
|
|
—
|
|
$
|
777.5
|
|
|||||||
|
November 1, 2015 through November 30, 2015
|
—
|
|
—
|
|
—
|
|
777.5
|
|
|||||||||
|
December 1, 2015 through December 31, 2015
|
1,240,262
|
|
80.61
|
|
1,240,262
|
|
677.5
|
|
|||||||||
|
Total
|
1,240,416
|
|
80.61
|
|
1,240,262
|
|
|||||||||||
|
(a)
|
The difference between the total number of shares (or units) purchased and the total number of shares (or units) purchased as part of publicly announced plans or programs is due to shares withheld by us to satisfy tax withholding obligations in connection with the vesting of restricted stock units issued to employees.
|
|
(b)
|
The two-for-one stock split paid on December 11, 2015 excluded treasury shares. However, because some purchases of treasury shares were at post-split prices, for consistency, we calculated the average price paid per share for all shares repurchased during the quarter based on post-split market prices.
|
|
(c)
|
On May 14, 2013, the Board of Directors approved a stock repurchase program authorizing us to purchase on the open market, including pursuant to a Rule 10b5-1 plan, and in privately negotiated transactions, up to $750.0 million of our common stock from time to time until December 31, 2016. During the fourth quarter of 2015, we exhausted the share repurchase authorization under this May 2013 program On July 10, 2014, the Board of Directors approved a new stock repurchase program without a specified end date providing for an additional $750.0 million of repurchases of our common
|

|
Total Cumulative Return
|
2011
|
2012
|
2013
|
2014
|
2015
|
||||||||||||||
|
Edwards Lifesciences
|
$
|
87.46
|
|
$
|
111.54
|
|
$
|
81.35
|
|
$
|
157.57
|
|
$
|
195.40
|
|
||||
|
S&P 500
|
102.11
|
|
118.45
|
|
156.82
|
|
178.29
|
|
180.75
|
|
|||||||||
|
S&P 500 Healthcare Equipment Index
|
106.02
|
|
124.33
|
|
157.80
|
|
193.86
|
|
204.79
|
|
|||||||||
|
|
|
As of or for the Years Ended December 31,
|
||||||||||||||||||
|
|
|
2015
|
2014
|
2013
|
2012
|
2011
|
||||||||||||||
|
|
|
(in millions, except per share data)
|
||||||||||||||||||
|
OPERATING RESULTS
|
Net sales
|
$
|
2,493.7
|
|
$
|
2,322.9
|
|
$
|
2,045.5
|
|
$
|
1,899.6
|
|
$
|
1,678.6
|
|
||||
|
Gross profit
|
1,876.5
|
|
1,697.3
|
|
1,528.9
|
|
1,408.6
|
|
1,189.2
|
|
||||||||||
|
Net income(a)
|
494.9
|
|
811.1
|
|
389.1
|
|
291.5
|
|
236.6
|
|
||||||||||
|
COMMON STOCK INFORMATION
|
Net income per common share(a)(c):
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Basic
|
$
|
2.30
|
|
$
|
3.81
|
|
$
|
1.74
|
|
$
|
1.27
|
|
$
|
1.03
|
|
|||||
|
Diluted
|
2.25
|
|
3.74
|
|
1.71
|
|
1.23
|
|
0.99
|
|
||||||||||
|
Cash dividends declared per common share
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||
|
BALANCE SHEET DATA
|
Total assets
|
$
|
4,059.3
|
|
$
|
3,523.0
|
|
$
|
2,709.9
|
|
$
|
2,209.3
|
|
$
|
1,970.0
|
|
||||
|
Long-term debt(b)
|
599.9
|
|
598.1
|
|
593.1
|
|
189.3
|
|
150.4
|
|
||||||||||
|
(a)
|
The above results include special charges of
$70.7 million
and
$16.3 million
during
2014
and
2013
, respectively. In addition, the above results include $750.0 million ($487.9 million, net of tax) in 2014 for an upfront payment received under a litigation settlement agreement, and $83.6 million ($52.3 million, net of tax) received in 2013 for a litigation award. See
"Management's Discussion and Analysis of Financial Condition and Results of Operations
" and Note 3 and Note 4 to the
"Consolidated Financial Statements"
for additional information.
|
|
(b)
|
In October 2013, we issued $600.0 million of 2.875% fixed-rate unsecured senior notes due October 15, 2018 ("the Notes").
|
|
(c)
|
The per share amounts for the prior periods presented have been retroactively adjusted to reflect the two-for-one stock split effected in the fourth quarter of 2015.
|
|
|
Years Ended December 31,
|
Change
|
|
|||||||||||||||
|
|
2015
|
2014
|
2013
|
2015
|
|
2014
|
|
|||||||||||
|
Net sales
|
$
|
2,493.7
|
|
$
|
2,322.9
|
|
$
|
2,045.5
|
|
7.4
|
%
|
13.6
|
%
|
|||||
|
Gross profit as a percentage of net sales
|
75.2
|
%
|
73.1
|
%
|
74.7
|
%
|
2.1
|
|
pts.
|
(1.6
|
)
|
pts.
|
||||||
|
Net income
|
$
|
494.9
|
|
$
|
811.1
|
|
$
|
389.1
|
|
(39.0
|
)%
|
108.5
|
%
|
|||||
|
Diluted earnings per share
|
$
|
2.25
|
|
$
|
3.74
|
|
$
|
1.71
|
|
(39.8
|
)%
|
118.7
|
%
|
|||||
|
•
|
final five-year clinical data for high-risk patients treated with the first-generation
SAPIEN
transcatheter aortic valve in The PARTNER Trial demonstrated equivalent outcomes to traditional open-heart surgery, and no structural valve deterioration requiring intervention;
|
|
•
|
30-day outcomes for intermediate-risk patients treated transfemorally with the
SAPIEN 3
transcatheter aortic valve at centers in Europe and Canada demonstrated very low mortality and stroke rates;
|
|
•
|
high-risk patients who received the advanced
Edwards SAPIEN 3
transcatheter aortic valve via transfemoral delivery had high one-year survival rates, as well as low rates of stroke and paravalvular leak;
|
|
•
|
we received FDA approval of the
Edwards SAPIEN 3
valve with the Commander Delivery System for the treatment of high-risk patients suffering from severe, symptomatic aortic stenosis;
|
|
•
|
we acquired CardiAQ, a privately-held company and developer of a transcatheter mitral valve replacement system; and
|
|
•
|
we received FDA approval for aortic valve-in-valve procedures using the
Edwards SAPIEN XT
transcatheter heart valve.
|
|
|
Years Ended December 31,
|
Change
|
Percent Change
|
||||||||||||||||||||||
|
|
2015
|
2014
|
2013
|
2015
|
2014
|
2015
|
|
2014
|
|||||||||||||||||
|
United States
|
$
|
1,262.9
|
|
$
|
1,047.3
|
|
$
|
939.6
|
|
$
|
215.6
|
|
$
|
107.7
|
|
20.6
|
%
|
11.5
|
%
|
||||||
|
Europe
|
717.3
|
|
744.5
|
|
616.5
|
|
(27.2
|
)
|
128.0
|
|
(3.6
|
)%
|
20.8
|
%
|
|||||||||||
|
Japan
|
246.2
|
|
257.9
|
|
243.6
|
|
(11.7
|
)
|
14.3
|
|
(4.6
|
)%
|
5.9
|
%
|
|||||||||||
|
Rest of World
|
267.3
|
|
273.2
|
|
245.8
|
|
(5.9
|
)
|
27.4
|
|
(2.1
|
)%
|
11.2
|
%
|
|||||||||||
|
International
|
1,230.8
|
|
1,275.6
|
|
1,105.9
|
|
(44.8
|
)
|
169.7
|
|
(3.5
|
)%
|
15.3
|
%
|
|||||||||||
|
Total net sales
|
$
|
2,493.7
|
|
$
|
2,322.9
|
|
$
|
2,045.5
|
|
$
|
170.8
|
|
$
|
277.4
|
|
7.4
|
%
|
13.6
|
%
|
||||||
|
|
Year Ended December 31,
|
Change
|
Percent Change
|
||||||||||||||||||||||
|
|
2015
|
|
2014
|
2013
|
2015
|
2014
|
2015
|
|
2014
|
||||||||||||||||
|
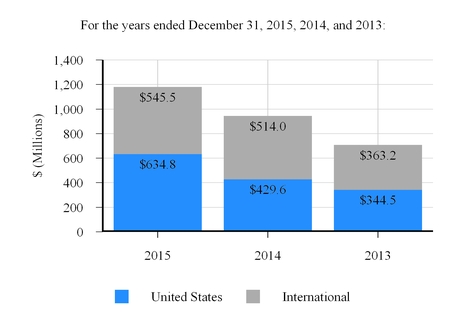
Transcatheter Heart Valve Therapy
|
$
|
1,180.3
|
|
|
$
|
943.6
|
|
|
$
|
707.7
|
|
$
|
236.7
|
|
$
|
235.9
|
|
25.1
|
%
|
33.3
|
%
|
||||
|
Surgical Heart Valve Therapy
|
785.0
|
|
|
826.1
|
|
|
801.2
|
|
(41.1
|
)
|
24.9
|
|
(5.0
|
)%
|
3.1
|
%
|
|||||||||
|
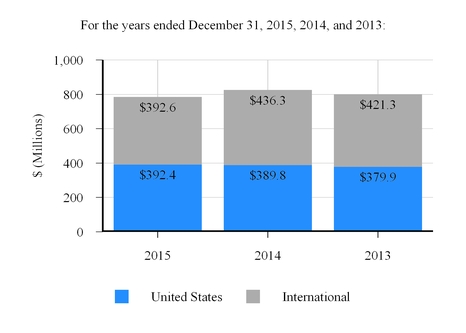
Critical Care
|
528.4
|
|
|
553.2
|
|
|
536.6
|
|
(24.8
|
)
|
16.6
|
|
(4.5
|
)%
|
3.1
|
%
|
|||||||||
|
Total net sales
|
$
|
2,493.7
|
|
|
$
|
2,322.9
|
|
|
$
|
2,045.5
|
|
$
|
170.8
|
|
$
|
277.4
|
|
7.4
|
%
|
13.6
|
%
|
||||
|
•
|
the
Edwards SAPIEN 3
valve, driven by its launch in July 2015; and
|
|
•
|
the
Edwards SAPIEN XT
valve, driven by its launch in June 2014;
|
|
•
|
lower sales of the
Edwards SAPIEN
valve as customers converted to
Edwards SAPIEN XT.
|
|
•
|
the
Edwards SAPIEN 3
valve, driven primarily by its launch in Europe in January 2014; and
|
|
•
|
the
Edwards SAPIEN XT
valve in Japan, driven by its launch in October 2013;
|
|
•
|
lower sales of the
Edwards SAPIEN XT
valve in Europe, as customers converted to
Edwards SAPIEN 3
; and
|
|
•
|
foreign currency exchange rate fluctuations, which decreased net sales by
$71.2 million
, due primarily to the weakening of the Euro against the United States dollar.
|
|
•
|
the
Edwards SAPIEN XT
valve, driven primarily by its launch in June 2014;
|
|
•
|
clinical sales of the
Edwards SAPIEN 3
valve; and
|
|
•
|
royalties received under a license agreement with Medtronic (see Note 3 to the "
Consolidated Financial Statements
").
|
|
•
|
the
Edwards SAPIEN 3
valve, driven primarily by its launch in Europe in January 2014; and
|
|
•
|
the
Edwards SAPIEN XT
valve in Japan, driven by its launch in October 2013;
|
|
•
|
lower sales of the
Edwards SAPIEN XT
valve in Europe, as customers converted to
Edwards SAPIEN 3
.
|
|
•
|
foreign currency exchange rate fluctuations, which decreased net sales by
$59.7 million
, due primarily to the weakening of the Euro and the Japanese yen against the United States dollar;
|
|
•
|
higher sales of (1) surgical heart valve products, driven by pericardial aortic tissue valves, primarily in Europe, Japan, and the United States, and (2)
EDWARDS INTUITY Elite
valves, primarily in Europe.
|
|
•
|
higher sales of (1) surgical heart valve products, driven by pericardial aortic tissue valves, primarily in the United States and Europe, and (2)
EDWARDS INTUITY Elite
valves, primarily in Europe;
|
|
•
|
foreign currency exchange rate fluctuations, which decreased net sales by $10.5 million, due to the weakening of various currencies against the United States dollar, mainly the Japanese yen, partially offset by the strengthening of the Euro against the United States dollar.
|

|
•
|
foreign currency exchange rate fluctuations, which decreased net sales by
$41.3 million
due primarily to the weakening of the Euro and the Japanese yen against the United States dollar;
|
|
•
|
higher sales of enhanced surgical recovery products in the United States, Europe, and Rest of World.
|
|
|
Years Ended December 31,
|
Change
|
|
|||||||||||
|
|
2015
|
2014
|
2013
|
2015
|
|
2014
|
|
|||||||
|
Gross profit as a percentage of net sales
|
75.2
|
%
|
73.1
|
%
|
74.7
|
%
|
2.1
|
pts.
|
(1.6
|
)
|
pts.
|
|||
|
•
|
a 1.9 percentage point increase due to the impact of foreign currency exchange rate fluctuations, including the settlement of foreign currency hedging contracts; and
|
|
•
|
a 0.9 percentage point increase in the United States and a 0.4 percentage point increase in international markets, due to an improved product mix, driven by THV products;
|
|
•
|
multiple investments in our operations, including an increase in costs to improve our manufacturing processes.
|
|
•
|
a 0.7 percentage point decrease due to the impact of foreign currency exchange rate fluctuations, including the settlement of foreign currency hedging contracts;
|
|
•
|
a 0.7 percentage point decrease due to higher performance-based incentive compensation; and
|
|
•
|
higher manufacturing costs, primarily for our operations in Utah;
|
|
•
|
a 0.8 percentage point increase due to an improved product mix in the United States, driven by THV products.
|
|
|
Years Ended December 31,
|
Change
|
|
|||||||||||||||||
|
|
2015
|
2014
|
2013
|
2015
|
|
2014
|
|
|||||||||||||
|
SG&A expenses
|
$
|
850.7
|
|
$
|
858.0
|
|
$
|
733.4
|
|
$
|
(7.3
|
)
|
$
|
124.6
|
|
|||||
|
SG&A expenses as a percentage of net sales
|
34.1
|
%
|
36.9
|
%
|
35.9
|
%
|
(2.8
|
)
|
pts.
|
1.0
|
|
pts.
|
||||||||
|
|
Years Ended December 31,
|
Change
|
|
|||||||||||||||||
|
|
2015
|
2014
|
2013
|
2015
|
|
2014
|
|
|||||||||||||
|
R&D expenses
|
$
|
383.1
|
|
$
|
346.5
|
|
$
|
323.0
|
|
$
|
36.6
|
|
$
|
23.5
|
|
|||||
|
R&D expenses as a percentage of net sales
|
15.4
|
%
|
14.9
|
%
|
15.8
|
%
|
0.5
|
|
pts.
|
(0.9
|
)
|
pts.
|
||||||||
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Foreign exchange losses, net
|
$
|
4.8
|
|
$
|
2.0
|
|
$
|
1.5
|
|
||
|
(Gain) loss on investments
|
(0.1
|
)
|
4.5
|
|
0.4
|
|
|||||
|
Promissory note impairment
|
—
|
|
4.0
|
|
—
|
|
|||||
|
Insurance settlement gain
|
—
|
|
(3.7
|
)
|
—
|
|
|||||
|
Lease contract termination costs
|
—
|
|
1.0
|
|
—
|
|
|||||
|
Other
|
(0.7
|
)
|
(0.1
|
)
|
(0.6
|
)
|
|||||
|
Total other expense, net
|
$
|
4.0
|
|
$
|
7.7
|
|
$
|
1.3
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Income tax expense at U.S. federal statutory rate
|
$
|
217.8
|
|
$
|
400.4
|
|
$
|
178.9
|
|
||
|
Foreign income taxed at different rates
|
(105.8
|
)
|
(67.1
|
)
|
(60.6
|
)
|
|||||
|
State and local taxes, net of federal tax benefit
|
3.1
|
|
19.3
|
|
5.8
|
|
|||||
|
Tax credits, federal and state
|
(15.7
|
)
|
(13.5
|
)
|
(19.8
|
)
|
|||||
|
Build (release) of reserve for uncertain tax positions for prior years
|
3.3
|
|
(4.8
|
)
|
(3.9
|
)
|
|||||
|
U.S. tax on foreign earnings, net of credits
|
20.5
|
|
(3.1
|
)
|
18.9
|
|
|||||
|
Nondeductible stock-based compensation
|
2.3
|
|
2.1
|
|
2.6
|
|
|||||
|
Other
|
2.0
|
|
(0.4
|
)
|
0.2
|
|
|||||
|
Income tax provision
|
$
|
127.5
|
|
$
|
332.9
|
|
$
|
122.1
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Unrecognized tax benefits, January 1
|
$
|
192.3
|
|
$
|
127.7
|
|
$
|
113.6
|
|
||
|
Current year tax positions
|
29.6
|
|
75.9
|
|
17.8
|
|
|||||
|
Increase prior year tax positions
|
2.2
|
|
0.6
|
|
5.7
|
|
|||||
|
Decrease prior year tax positions
|
(7.4
|
)
|
(10.5
|
)
|
(9.0
|
)
|
|||||
|
Settlements
|
(0.4
|
)
|
(1.0
|
)
|
(0.1
|
)
|
|||||
|
Lapse of statutes of limitations
|
(0.2
|
)
|
(0.4
|
)
|
(0.3
|
)
|
|||||
|
Unrecognized tax benefits, December 31
|
$
|
216.1
|
|
$
|
192.3
|
|
$
|
127.7
|
|
||
|
|
Payments Due by Period
|
||||||||||||||||||
|
Contractual Obligations
|
Total
|
Less Than
1 Year
|
1-3
Years
|
4-5
Years
|
After 5
Years
|
||||||||||||||
|
Debt
|
$
|
600.0
|
|
$
|
—
|
|
$
|
600.0
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Operating leases
|
72.8
|
|
20.1
|
|
24.3
|
|
9.6
|
|
18.8
|
|
|||||||||
|
Interest on debt
|
39.3
|
|
13.6
|
|
25.1
|
|
0.6
|
|
—
|
|
|||||||||
|
Pension obligations (a)
|
5.8
|
|
5.8
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Capital commitment obligations (b)
|
2.3
|
|
2.0
|
|
0.3
|
|
—
|
|
—
|
|
|||||||||
|
Purchase and other commitments
|
2.4
|
|
2.4
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Total contractual cash obligations (c) (d)
|
$
|
722.6
|
|
$
|
43.9
|
|
$
|
649.7
|
|
$
|
10.2
|
|
$
|
18.8
|
|
||||
|
(a)
|
The amount included in "Less Than 1 Year" reflects anticipated contributions to our various pension plans. Anticipated contributions beyond one year are not determinable. The total accrued benefit liability for our pension plans recognized as of
December 31, 2015
was
$43.0 million
. This amount is impacted by, among other items, pension expense funding levels, changes in plan demographics and assumptions, and investment return on plan assets. Therefore, we are unable to make a reasonably reliable estimate of the amount and period in which the liability might be paid, and did not include this amount in the contractual obligations table. See Note 12 to the "
Consolidated Financial Statements
" for further information.
|
|
(b)
|
Capital commitment obligations consist primarily of cash that we are obligated to pay to our limited partnership and limited liability corporation investees. These investees make equity investments in various development stage biopharmaceutical and medical device companies, and it is not certain if and/or when these payments will be made.
|
|
(c)
|
As of
December 31, 2015
, the liability for uncertain tax positions including interest was
$234.4 million
. We have entered into an APA process between the Switzerland and the United States governments for the years 2009 through 2015 covering transfer pricing matters. These transfer pricing matters are significant to our consolidated financial statements, and the final outcome of the negotiations between the two governments is uncertain. Management believes that adequate amounts of tax and related penalty and interest have been provided in income tax expense for any adjustments that may result for our uncertain tax positions. We are unable to make a reasonably reliable estimate of the amount and period in which the liability might be paid, and did not include this amount in the contractual obligations table.
|
|
(d)
|
We acquire assets still in development, enter into research and development arrangements, and sponsor certain clinical trials that often require milestone, royalty, or other future payments to third-parties, contingent upon the occurrence of certain future events. In situations where we have no ability to influence the achievement of the milestone or otherwise avoid the payment, we have included those payments in the table above. However, we have excluded from the table contingent milestone payments and other contingent liabilities for which we cannot reasonably predict future payments or for which we can avoid making payment by unilaterally deciding to stop development of a product or cease progress of a clinical trial. We estimate that these contingent payments could be up to approximately
$170.0 million
if all milestones or other contingent obligations were met.
|
|
•
|
timing and probability of success of clinical events or regulatory approvals;
|
|
•
|
timing and probability of success of meeting commercial milestones; and
|
|
•
|
discount rates.
|
|
Financial Statements:
|
|
|
For the Years Ended December 31, 2015, 2014, and 2013:
|
|
|
Other schedules are not applicable and have not been submitted
|
|
|
December 31,
|
|||||||
|
|
2015
|
2014
|
|||||
|
ASSETS
|
|||||||
|
Current assets
|
|
|
|
|
|||
|
Cash and cash equivalents
|
$
|
718.4
|
|
$
|
653.8
|
|
|
|
Short-term investments (Note 6)
|
506.3
|
|
785.0
|
|
|||
|
Accounts receivable, net (Note 5)
|
315.4
|
|
288.0
|
|
|||
|
Other receivables
|
28.7
|
|
37.0
|
|
|||
|
Inventories (Note 5)
|
339.9
|
|
296.8
|
|
|||
|
Prepaid expenses
|
45.1
|
|
48.8
|
|
|||
|
Other current assets
|
94.1
|
|
121.7
|
|
|||
|
Total current assets
|
2,047.9
|
|
2,231.1
|
|
|||
|
Long-term accounts receivable, net (Note 5)
|
3.6
|
|
5.8
|
|
|||
|
Long-term investments (Note 6)
|
379.9
|
|
240.9
|
|
|||
|
Property, plant, and equipment, net (Note 5)
|
482.5
|
|
442.9
|
|
|||
|
Goodwill (Note 8)
|
628.3
|
|
376.0
|
|
|||
|
Other intangible assets, net (Note 8)
|
205.4
|
|
23.4
|
|
|||
|
Deferred income taxes
|
180.5
|
|
153.7
|
|
|||
|
Other assets
|
131.2
|
|
49.2
|
|
|||
|
Total assets
|
$
|
4,059.3
|
|
$
|
3,523.0
|
|
|
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
|||||||
|
Current liabilities
|
|
|
|
|
|||
|
Accounts payable
|
$
|
63.9
|
|
$
|
58.2
|
|
|
|
Accrued and other liabilities (Note 5)
|
412.3
|
|
367.9
|
|
|||
|
Total current liabilities
|
476.2
|
|
426.1
|
|
|||
|
Long-term debt (Note 9)
|
599.9
|
|
598.1
|
|
|||
|
Uncertain tax positions
|
194.7
|
|
194.8
|
|
|||
|
Other long-term liabilities
|
285.4
|
|
112.6
|
|
|||
|
Commitments and contingencies (Notes 9 and 17)
|
|
|
|
|
|||
|
Stockholders' equity
(Note 13)
|
|
|
|
|
|||
|
Preferred stock, $.01 par value, authorized 50.0 shares, no shares outstanding
|
—
|
|
—
|
|
|||
|
Common stock, $1.00 par value, 350.0 shares authorized, 239.1 and 128.9 shares issued, and 215.4 and 107.8 shares outstanding, respectively
|
239.1
|
|
128.9
|
|
|||
|
Additional paid-in capital
|
946.8
|
|
878.4
|
|
|||
|
Retained earnings
|
3,336.8
|
|
2,841.9
|
|
|||
|
Accumulated other comprehensive loss
|
(182.6
|
)
|
(100.9
|
)
|
|||
|
Treasury stock, at cost, 23.7 and 21.1 shares, respectively
|
(1,837.0
|
)
|
(1,556.9
|
)
|
|||
|
Total stockholders' equity
|
2,503.1
|
|
2,191.4
|
|
|||
|
Total liabilities and stockholders' equity
|
$
|
4,059.3
|
|
$
|
3,523.0
|
|
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Net sales
|
$
|
2,493.7
|
|
$
|
2,322.9
|
|
$
|
2,045.5
|
|
||
|
Cost of sales
|
617.2
|
|
625.6
|
|
516.6
|
|
|||||
|
Gross profit
|
1,876.5
|
|
1,697.3
|
|
1,528.9
|
|
|||||
|
Selling, general, and administrative expenses
|
850.7
|
|
858.0
|
|
733.4
|
|
|||||
|
Research and development expenses
|
383.1
|
|
346.5
|
|
323.0
|
|
|||||
|
Intellectual property litigation expenses (income), net (Note 3)
|
7.0
|
|
(740.4
|
)
|
(61.5
|
)
|
|||||
|
Special charges (Note 4)
|
—
|
|
70.7
|
|
16.3
|
|
|||||
|
Interest expense
|
17.2
|
|
17.2
|
|
9.8
|
|
|||||
|
Interest income
|
(7.9
|
)
|
(6.4
|
)
|
(4.6
|
)
|
|||||
|
Other expense, net (Note 15)
|
4.0
|
|
7.7
|
|
1.3
|
|
|||||
|
Income before provision for income taxes
|
622.4
|
|
1,144.0
|
|
511.2
|
|
|||||
|
Provision for income taxes (Note 16)
|
127.5
|
|
332.9
|
|
122.1
|
|
|||||
|
Net income
|
$
|
494.9
|
|
$
|
811.1
|
|
$
|
389.1
|
|
||
|
Share information
(Note 2):
|
|
|
|
|
|
|
|||||
|
Earnings per share:
|
|
|
|
|
|
|
|||||
|
Basic
|
$
|
2.30
|
|
$
|
3.81
|
|
$
|
1.74
|
|
||
|
Diluted
|
$
|
2.25
|
|
$
|
3.74
|
|
$
|
1.71
|
|
||
|
Weighted-average number of common shares outstanding:
|
|
|
|
|
|
|
|||||
|
Basic
|
215.5
|
|
213.0
|
|
223.4
|
|
|||||
|
Diluted
|
220.3
|
|
217.0
|
|
227.6
|
|
|||||
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Net income
|
$
|
494.9
|
|
$
|
811.1
|
|
$
|
389.1
|
|
||
|
Other comprehensive (loss) income, net of tax (Note 14):
|
|
|
|
|
|
|
|||||
|
Foreign currency translation adjustments
|
(65.1
|
)
|
(96.2
|
)
|
5.6
|
|
|||||
|
Unrealized (loss) gain on cash flow hedges
|
(20.5
|
)
|
28.8
|
|
(3.5
|
)
|
|||||
|
Defined benefit pension plans—net actuarial gain (loss) and other
|
5.4
|
|
(5.6
|
)
|
9.3
|
|
|||||
|
Unrealized loss on available-for-sale investments for the period
|
(2.6
|
)
|
(0.3
|
)
|
(1.1
|
)
|
|||||
|
Reclassification of net realized investment loss to earnings
|
1.1
|
|
—
|
|
—
|
|
|||||
|
Other comprehensive (loss) income, net of tax
|
(81.7
|
)
|
(73.3
|
)
|
10.3
|
|
|||||
|
Comprehensive income
|
$
|
413.2
|
|
$
|
737.8
|
|
$
|
399.4
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Cash flows from operating activities
|
|
|
|
|
|
|
|||||
|
Net income
|
$
|
494.9
|
|
$
|
811.1
|
|
$
|
389.1
|
|
||
|
Adjustments to reconcile net income to cash provided by operating activities:
|
|
|
|
|
|||||||
|
Depreciation and amortization
|
65.8
|
|
68.6
|
|
62.9
|
|
|||||
|
Stock-based compensation (Notes 2 and 13)
|
49.9
|
|
48.3
|
|
47.4
|
|
|||||
|
Excess tax benefit from stock plans (Notes 2 and 13)
|
(41.3
|
)
|
(49.4
|
)
|
(73.5
|
)
|
|||||
|
Deferred income taxes
|
(95.0
|
)
|
(71.1
|
)
|
(12.4
|
)
|
|||||
|
Purchased in-process research and development (Note 4)
|
—
|
|
10.6
|
|
—
|
|
|||||
|
Other
|
11.0
|
|
13.9
|
|
6.7
|
|
|||||
|
Changes in operating assets and liabilities:
|
|
|
|
|
|||||||
|
Accounts and other receivables, net
|
(38.3
|
)
|
(26.8
|
)
|
8.5
|
|
|||||
|
Inventories
|
(67.7
|
)
|
(30.5
|
)
|
(44.4
|
)
|
|||||
|
Accounts payable and accrued liabilities
|
29.4
|
|
112.9
|
|
0.2
|
|
|||||
|
Income taxes
|
134.5
|
|
128.1
|
|
80.6
|
|
|||||
|
Prepaid expenses and other current assets
|
(0.2
|
)
|
(0.9
|
)
|
(6.3
|
)
|
|||||
|
Other
|
6.7
|
|
7.5
|
|
13.9
|
|
|||||
|
Net cash provided by operating activities
|
549.7
|
|
1,022.3
|
|
472.7
|
|
|||||
|
Cash flows from investing activities
|
|
|
|
|
|||||||
|
Capital expenditures
|
(102.7
|
)
|
(82.9
|
)
|
(109.0
|
)
|
|||||
|
Purchases of held-to-maturity investments (Note 6)
|
(928.5
|
)
|
(1,956.4
|
)
|
(823.2
|
)
|
|||||
|
Proceeds from held-to-maturity investments (Note 6)
|
1,260.1
|
|
1,611.2
|
|
526.4
|
|
|||||
|
Purchases of available-for-sale investments (Note 6)
|
(380.3
|
)
|
(160.4
|
)
|
—
|
|
|||||
|
Proceeds from available-for-sale investments (Note 6)
|
179.6
|
|
1.7
|
|
—
|
|
|||||
|
Investments in unconsolidated affiliates (Note 6)
|
(5.1
|
)
|
(11.2
|
)
|
(3.0
|
)
|
|||||
|
Proceeds from unconsolidated affiliates (Note 6)
|
3.0
|
|
2.1
|
|
0.3
|
|
|||||
|
Investments in trading securities, net
|
(9.2
|
)
|
(14.4
|
)
|
(1.4
|
)
|
|||||
|
Acquisitions (Notes 7 and 8)
|
(331.6
|
)
|
(15.0
|
)
|
—
|
|
|||||
|
Investments in intangible assets and in-process research and development
|
(3.8
|
)
|
(10.8
|
)
|
(1.1
|
)
|
|||||
|
Proceeds from sale of assets
|
2.4
|
|
3.1
|
|
2.3
|
|
|||||
|
Other
|
—
|
|
—
|
|
(4.0
|
)
|
|||||
|
Net cash used in investing activities
|
(316.1
|
)
|
(633.0
|
)
|
(412.7
|
)
|
|||||
|
Cash flows from financing activities
|
|
|
|
|
|
|
|||||
|
Proceeds from issuance of debt
|
31.4
|
|
226.3
|
|
1,305.0
|
|
|||||
|
Payments on debt and capital lease obligations
|
(29.5
|
)
|
(239.0
|
)
|
(895.4
|
)
|
|||||
|
Purchases of treasury stock
|
(280.1
|
)
|
(300.9
|
)
|
(496.9
|
)
|
|||||
|
Proceeds from stock plans
|
87.2
|
|
113.3
|
|
45.5
|
|
|||||
|
Excess tax benefit from stock plans (Notes 2 and 13)
|
41.3
|
|
49.4
|
|
73.5
|
|
|||||
|
Other
|
(8.9
|
)
|
(2.1
|
)
|
3.2
|
|
|||||
|
Net cash (used in) provided by financing activities
|
(158.6
|
)
|
(153.0
|
)
|
34.9
|
|
|||||
|
Effect of currency exchange rate changes on cash and cash equivalents
|
(10.4
|
)
|
(2.9
|
)
|
14.6
|
|
|||||
|
Net increase in cash and cash equivalents
|
64.6
|
|
233.4
|
|
109.5
|
|
|||||
|
Cash and cash equivalents at beginning of year
|
653.8
|
|
420.4
|
|
310.9
|
|
|||||
|
Cash and cash equivalents at end of year
|
$
|
718.4
|
|
$
|
653.8
|
|
$
|
420.4
|
|
||
|
Supplemental disclosures:
|
|
|
|
|
|
|
|||||
|
Cash paid during the year for:
|
|
|
|
|
|
|
|||||
|
Interest
|
$
|
17.2
|
|
$
|
15.5
|
|
$
|
4.3
|
|
||
|
Income taxes
|
$
|
86.9
|
|
$
|
274.7
|
|
$
|
54.0
|
|
||
|
Non-cash investing and financing transactions:
|
|
|
|
|
|
|
|||||
|
Capital expenditures accruals
|
$
|
15.1
|
|
$
|
8.3
|
|
$
|
8.4
|
|
||
|
Capital additions transferred from inventory
|
$
|
3.0
|
|
$
|
4.0
|
|
$
|
7.8
|
|
||
|
Capital lease obligations incurred
|
$
|
—
|
|
$
|
13.3
|
|
$
|
—
|
|
||
|
|
Common Stock
|
Treasury Stock
|
|||||||||||||||||||||||||||
|
|
Shares
|
Par Value
|
Shares
|
Amount
|
Additional Paid-in Capital
|
Retained Earnings
|
Accumulated Other Comprehensive (Loss) Income
|
Total Stockholders' Equity
|
|||||||||||||||||||||
|
BALANCE AT DECEMBER 31, 2012
|
124.2
|
|
$
|
124.2
|
|
9.9
|
|
$
|
(749.9
|
)
|
$
|
489.0
|
|
$
|
1,641.7
|
|
$
|
(37.9
|
)
|
$
|
1,467.1
|
|
|||||||
|
Net income
|
|
|
|
|
|
|
|
|
|
|
389.1
|
|
|
|
389.1
|
|
|||||||||||||
|
Other comprehensive income, net of tax
|
|
|
|
|
|
|
|
|
|
|
|
|
10.3
|
|
10.3
|
|
|||||||||||||
|
Common stock issued under equity plans, including tax benefits
|
1.8
|
|
1.8
|
|
|
|
|
|
125.7
|
|
|
|
|
|
127.5
|
|
|||||||||||||
|
Stock-based compensation expense
|
|
|
|
|
|
|
|
|
47.4
|
|
|
|
|
|
47.4
|
|
|||||||||||||
|
Purchases of treasury stock
|
|
|
|
|
6.8
|
|
(506.1
|
)
|
9.1
|
|
|
|
|
|
(497.0
|
)
|
|||||||||||||
|
BALANCE AT DECEMBER 31, 2013
|
126.0
|
|
126.0
|
|
16.7
|
|
(1,256.0
|
)
|
671.2
|
|
2,030.8
|
|
(27.6
|
)
|
1,544.4
|
|
|||||||||||||
|
Net income
|
|
|
|
|
|
|
|
|
|
|
811.1
|
|
|
|
811.1
|
|
|||||||||||||
|
Other comprehensive loss, net of tax
|
|
|
|
|
|
|
|
|
|
|
|
|
(73.3
|
)
|
(73.3
|
)
|
|||||||||||||
|
Common stock issued under equity plans, including tax benefits
|
2.9
|
|
2.9
|
|
|
|
|
|
158.9
|
|
|
|
|
|
161.8
|
|
|||||||||||||
|
Stock-based compensation expense
|
|
|
|
|
|
|
|
|
48.3
|
|
|
|
|
|
48.3
|
|
|||||||||||||
|
Purchases of treasury stock
|
|
|
|
|
4.4
|
|
(300.9
|
)
|
|
|
|
|
|
|
(300.9
|
)
|
|||||||||||||
|
BALANCE AT DECEMBER 31, 2014
|
128.9
|
|
128.9
|
|
21.1
|
|
(1,556.9
|
)
|
878.4
|
|
2,841.9
|
|
(100.9
|
)
|
2,191.4
|
|
|||||||||||||
|
Net income
|
|
|
|
|
|
|
|
|
|
|
494.9
|
|
|
|
494.9
|
|
|||||||||||||
|
Other comprehensive loss, net of tax
|
|
|
|
|
|
|
|
|
|
|
|
|
(81.7
|
)
|
(81.7
|
)
|
|||||||||||||
|
Common stock issued under equity plans, including tax benefits
|
2.0
|
|
2.0
|
|
|
|
|
|
126.7
|
|
|
|
|
|
128.7
|
|
|||||||||||||
|
Stock-based compensation expense
|
|
|
|
|
|
|
|
|
49.9
|
|
|
|
|
|
49.9
|
|
|||||||||||||
|
Purchases of treasury stock
|
|
|
|
|
2.6
|
|
(280.1
|
)
|
|
|
|
|
|
|
(280.1
|
)
|
|||||||||||||
|
Stock issued to effect stock split
|
108.2
|
|
108.2
|
|
(108.2
|
)
|
—
|
|
|||||||||||||||||||||
|
BALANCE AT DECEMBER 31, 2015
|
239.1
|
|
$
|
239.1
|
|
23.7
|
|
$
|
(1,837.0
|
)
|
$
|
946.8
|
|
$
|
3,336.8
|
|
$
|
(182.6
|
)
|
$
|
2,503.1
|
|
|||||||
|
•
|
the duration and extent to which the market value has been less than cost;
|
|
•
|
the financial condition and near term prospects of the investee/issuer;
|
|
•
|
the reasons for the decline in market value;
|
|
•
|
the Company's ability and intent to hold the investment for a period of time sufficient to allow for any anticipated recovery in market value; and
|
|
•
|
the investee's performance against product development milestones.
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Basic:
|
|
|
|
|
|
|
|||||
|
Net income
|
$
|
494.9
|
|
$
|
811.1
|
|
$
|
389.1
|
|
||
|
Weighted-average shares outstanding
|
215.5
|
|
213.0
|
|
223.4
|
|
|||||
|
Basic earnings per share
|
$
|
2.30
|
|
$
|
3.81
|
|
$
|
1.74
|
|
||
|
Diluted:
|
|
|
|
|
|
|
|||||
|
Net income
|
$
|
494.9
|
|
$
|
811.1
|
|
$
|
389.1
|
|
||
|
Weighted-average shares outstanding
|
215.5
|
|
213.0
|
|
223.4
|
|
|||||
|
Dilutive effect of stock plans
|
4.8
|
|
4.0
|
|
4.2
|
|
|||||
|
Dilutive weighted-average shares outstanding
|
220.3
|
|
217.0
|
|
227.6
|
|
|||||
|
Diluted earnings per share
|
$
|
2.25
|
|
$
|
3.74
|
|
$
|
1.71
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Cost of sales
|
$
|
6.8
|
|
$
|
6.1
|
|
$
|
5.9
|
|
||
|
Selling, general, and administrative expenses
|
34.3
|
|
34.9
|
|
34.7
|
|
|||||
|
Research and development expenses
|
8.8
|
|
7.3
|
|
6.8
|
|
|||||
|
Total stock-based compensation expense
|
$
|
49.9
|
|
$
|
48.3
|
|
$
|
47.4
|
|
||
|
Past damages
|
$
|
754.3
|
|
|
License agreement
|
238.0
|
|
|
|
Covenant not to sue
|
77.7
|
|
|
|
Total
|
$
|
1,070.0
|
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
|
(in millions)
|
||||||||||
|
Charitable foundation contribution
|
$
|
—
|
|
$
|
50.0
|
|
$
|
—
|
|
||
|
Acquisition of IPR&D
|
—
|
|
10.2
|
|
—
|
|
|||||
|
Settlement
|
—
|
|
7.5
|
|
—
|
|
|||||
|
Asset write-down
|
—
|
|
3.0
|
|
—
|
|
|||||
|
Realignment expenses
|
—
|
|
—
|
|
10.4
|
|
|||||
|
IPR&D impairment
|
—
|
|
—
|
|
5.9
|
|
|||||
|
Total special charges
|
$
|
—
|
|
$
|
70.7
|
|
$
|
16.3
|
|
||
|
|
As of December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
|
(in millions)
|
||||||
|
Accounts receivable, net (a)
|
|
|
|
|
|||
|
Trade accounts receivable
|
$
|
322.2
|
|
$
|
293.1
|
|
|
|
Allowance for doubtful accounts
|
(6.8
|
)
|
(5.1
|
)
|
|||
|
$
|
315.4
|
|
$
|
288.0
|
|
||
|
Inventories
|
|
|
|
|
|||
|
Raw materials
|
$
|
63.8
|
|
$
|
67.4
|
|
|
|
Work in process
|
64.1
|
|
59.3
|
|
|||
|
Finished products
|
212.0
|
|
170.1
|
|
|||
|
$
|
339.9
|
|
$
|
296.8
|
|
||
|
Property, plant, and equipment, net
|
|
|
|
|
|||
|
Land
|
$
|
25.1
|
|
$
|
25.4
|
|
|
|
Buildings and leasehold improvements
|
293.4
|
|
270.6
|
|
|||
|
Machinery and equipment
|
328.6
|
|
320.8
|
|
|||
|
Equipment with customers
|
34.6
|
|
41.0
|
|
|||
|
Software
|
97.4
|
|
99.6
|
|
|||
|
Construction in progress
|
75.2
|
|
50.9
|
|
|||
|
854.3
|
|
808.3
|
|
||||
|
Accumulated depreciation
|
(371.8
|
)
|
(365.4
|
)
|
|||
|
$
|
482.5
|
|
$
|
442.9
|
|
||
|
Long-term accounts receivable, net (a)
|
|
|
|
|
|||
|
Long-term trade accounts receivable
|
$
|
9.9
|
|
$
|
12.0
|
|
|
|
Allowance for doubtful accounts
|
(6.3
|
)
|
(6.2
|
)
|
|||
|
$
|
3.6
|
|
$
|
5.8
|
|
||
|
Accrued and other liabilities
|
|
|
|
|
|||
|
Employee compensation and withholdings
|
$
|
209.4
|
|
$
|
190.5
|
|
|
|
Research and development accruals
|
38.6
|
|
39.9
|
|
|||
|
Property, payroll, and other taxes
|
34.5
|
|
32.7
|
|
|||
|
Accrued rebates
|
23.9
|
|
11.7
|
|
|||
|
Taxes payable
|
14.5
|
|
9.1
|
|
|||
|
Severance and realignment reserves
|
19.1
|
|
11.8
|
|
|||
|
Litigation reserves (Note 17)
|
5.6
|
|
4.4
|
|
|||
|
Fair value of derivatives
|
4.2
|
|
2.6
|
|
|||
|
Other accrued liabilities
|
62.5
|
|
65.2
|
|
|||
|
$
|
412.3
|
|
$
|
367.9
|
|
||
|
(a)
|
As of December 31,
2015
and
2014
, the Company's accounts receivables, net of the allowance for doubtful accounts, from customers in certain European countries were
$58.4 million
and
$69.7 million
, respectively. Balances from customers located in these countries that are expected to be collected beyond one year have been discounted to present value based on the estimated collection date.
|
|
|
December 31, 2015
|
December 31, 2014
|
|||||||||||||||||||||||||||||
|
Held-to-maturity
|
Cost
|
Gross Unrealized Gains
|
Gross Unrealized Losses
|
Fair Value
|
Cost
|
Gross Unrealized Gains
|
Gross Unrealized Losses
|
Fair Value
|
|||||||||||||||||||||||
|
Bank time deposits
|
$
|
440.1
|
|
$
|
—
|
|
$
|
—
|
|
$
|
440.1
|
|
$
|
661.5
|
|
$
|
—
|
|
$
|
—
|
|
$
|
661.5
|
|
|||||||
|
Commercial paper
|
—
|
|
—
|
|
—
|
|
—
|
|
80.0
|
|
—
|
|
—
|
|
80.0
|
|
|||||||||||||||
|
U.S. government and agency securities
|
32.5
|
|
—
|
|
(0.2
|
)
|
32.3
|
|
58.9
|
|
0.1
|
|
(0.1
|
)
|
58.9
|
|
|||||||||||||||
|
Asset-backed securities
|
1.2
|
|
—
|
|
—
|
|
1.2
|
|
8.2
|
|
—
|
|
—
|
|
8.2
|
|
|||||||||||||||
|
Corporate debt securities
|
16.4
|
|
—
|
|
—
|
|
16.4
|
|
24.7
|
|
—
|
|
—
|
|
24.7
|
|
|||||||||||||||
|
Municipal securities
|
5.2
|
|
—
|
|
—
|
|
5.2
|
|
6.1
|
|
—
|
|
—
|
|
6.1
|
|
|||||||||||||||
|
$
|
495.4
|
|
$
|
—
|
|
$
|
(0.2
|
)
|
$
|
495.2
|
|
$
|
839.4
|
|
$
|
0.1
|
|
$
|
(0.1
|
)
|
$
|
839.4
|
|
||||||||
|
Available-for-sale
|
|||||||||||||||||||||||||||||||
|
Commercial paper
|
$
|
28.1
|
|
$
|
—
|
|
$
|
—
|
|
$
|
28.1
|
|
$
|
13.0
|
|
$
|
—
|
|
$
|
—
|
|
$
|
13.0
|
|
|||||||
|
U.S. government and agency securities
|
38.7
|
|
—
|
|
(0.2
|
)
|
38.5
|
|
1.0
|
|
—
|
|
—
|
|
1.0
|
|
|||||||||||||||
|
Asset-backed securities
|
62.8
|
|
—
|
|
(0.2
|
)
|
62.6
|
|
42.9
|
|
—
|
|
—
|
|
42.9
|
|
|||||||||||||||
|
Corporate debt securities
|
230.0
|
|
—
|
|
(1.3
|
)
|
228.7
|
|
103.6
|
|
—
|
|
(0.4
|
)
|
103.2
|
|
|||||||||||||||
|
Municipal securities
|
4.7
|
|
—
|
|
—
|
|
4.7
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||
|
$
|
364.3
|
|
$
|
—
|
|
$
|
(1.7
|
)
|
$
|
362.6
|
|
$
|
160.5
|
|
$
|
—
|
|
$
|
(0.4
|
)
|
$
|
160.1
|
|
||||||||
|
|
Held-to-Maturity
|
Available-for-Sale
|
|||||||||||||
|
|
Cost
|
Fair Value
|
Cost
|
Fair Value
|
|||||||||||
|
|
(in millions)
|
||||||||||||||
|
Due in 1 year or less
|
$
|
466.8
|
|
$
|
466.8
|
|
$
|
39.5
|
|
$
|
39.5
|
|
|||
|
Due after 1 year through 5 years
|
16.0
|
|
15.9
|
|
265.1
|
|
263.6
|
|
|||||||
|
Instruments not due at a single maturity date
|
12.6
|
|
12.5
|
|
59.7
|
|
59.5
|
|
|||||||
|
$
|
495.4
|
|
$
|
495.2
|
|
$
|
364.3
|
|
$
|
362.6
|
|
||||
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
|
(in millions)
|
||||||
|
Available-for-sale investments
|
|
|
|
|
|||
|
Cost
|
$
|
—
|
|
$
|
—
|
|
|
|
Unrealized gains
|
0.2
|
|
0.4
|
|
|||
|
Fair value of available-for-sale investments
|
0.2
|
|
0.4
|
|
|||
|
Equity method investments
|
|
|
|
|
|||
|
Cost
|
10.9
|
|
12.8
|
|
|||
|
Equity in losses
|
(4.2
|
)
|
(3.5
|
)
|
|||
|
Carrying value of equity method investments
|
6.7
|
|
9.3
|
|
|||
|
Cost method investments
|
|
|
|
|
|||
|
Carrying value of cost method investments
|
21.3
|
|
16.7
|
|
|||
|
Total investments in unconsolidated affiliates
|
$
|
28.2
|
|
$
|
26.4
|
|
|
|
Current assets
|
$
|
28.1
|
|
||
|
Property and equipment, net
|
0.2
|
|
|||
|
Goodwill
|
258.9
|
|
|||
|
IPR&D
|
190.0
|
|
|||
|
Current liabilities assumed
|
(32.9
|
)
|
|||
|
Deferred income taxes
|
(66.0
|
)
|
|||
|
Contingent consideration
|
(30.3
|
)
|
|||
|
Total cash purchase price
|
348.0
|
|
|||
|
Less: cash acquired
|
(27.9
|
)
|
|||
|
Total cash purchase price, net of cash acquired
|
$
|
320.1
|
|
||
|
|
United
States |
Europe
|
Total
|
||||||||
|
|
(in millions)
|
||||||||||
|
Goodwill at December 31, 2013
|
$
|
308.3
|
|
$
|
77.1
|
|
$
|
385.4
|
|
||
|
Currency translation adjustment
|
—
|
|
(9.4
|
)
|
(9.4
|
)
|
|||||
|
Goodwill at December 31, 2014
|
308.3
|
|
67.7
|
|
376.0
|
|
|||||
|
Goodwill acquired during the year
|
258.9
|
|
—
|
|
258.9
|
|
|||||
|
Currency translation adjustment
|
—
|
|
(6.6
|
)
|
(6.6
|
)
|
|||||
|
Goodwill at December 31, 2015
|
$
|
567.2
|
|
$
|
61.1
|
|
$
|
628.3
|
|
||
|
|
December 31,
|
||||||||||||||||||||||
|
|
2015
|
2014
|
|||||||||||||||||||||
|
|
Cost
|
Accumulated
Amortization |
Net
Carrying Value |
Cost
|
Accumulated
Amortization |
Net
Carrying Value |
|||||||||||||||||
|
Amortizable intangible assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Patents
|
$
|
180.6
|
|
$
|
(172.3
|
)
|
$
|
8.3
|
|
$
|
181.1
|
|
$
|
(168.6
|
)
|
$
|
12.5
|
|
|||||
|
Developed technology
|
43.6
|
|
(37.9
|
)
|
5.7
|
|
45.6
|
|
(36.7
|
)
|
8.9
|
|
|||||||||||
|
Other
|
10.0
|
|
(8.6
|
)
|
1.4
|
|
10.4
|
|
(8.4
|
)
|
2.0
|
|
|||||||||||
|
234.2
|
|
(218.8
|
)
|
15.4
|
|
237.1
|
|
(213.7
|
)
|
23.4
|
|
||||||||||||
|
Unamortizable intangible assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
IPR&D
|
190.0
|
|
—
|
|
190.0
|
|
—
|
|
—
|
|
—
|
|
|||||||||||
|
$
|
424.2
|
|
$
|
(218.8
|
)
|
$
|
205.4
|
|
$
|
237.1
|
|
$
|
(213.7
|
)
|
$
|
23.4
|
|
||||||
|
2016
|
$
|
6.9
|
|
|
2017
|
6.3
|
|
|
|
2018
|
1.2
|
|
|
|
2019
|
0.7
|
|
|
|
2020
|
0.2
|
|
|
|
|
December 31,
|
||||||||||||
|
|
2015
|
|
2014
|
||||||||||
|
|
Amount
|
Effective
Interest Rate |
|
Amount
|
Effective
Interest Rate |
||||||||
|
(in millions)
|
(in millions)
|
||||||||||||
|
Fixed-rate 2.875% notes due October 15, 2018
|
$
|
600.0
|
|
2.983
|
%
|
$
|
600.0
|
|
2.983
|
%
|
|||
|
Unamortized discount
|
(1.7
|
)
|
|
|
|
(2.3
|
)
|
|
|
||||
|
Hedge accounting fair value adjustments (see Note 11)
|
1.6
|
|
|
|
|
0.4
|
|
|
|
||||
|
Total carrying amount
|
$
|
599.9
|
|
|
|
|
$
|
598.1
|
|
|
|
||
|
|
Operating
Leases |
Aggregate
Debt Maturities |
|||||
|
2016
|
$
|
20.1
|
|
$
|
—
|
|
|
|
2017
|
14.9
|
|
—
|
|
|||
|
2018
|
9.4
|
|
600.0
|
|
|||
|
2019
|
5.1
|
|
—
|
|
|||
|
2020
|
4.5
|
|
—
|
|
|||
|
Thereafter
|
18.8
|
|
—
|
|
|||
|
Total obligations and commitments
|
$
|
72.8
|
|
$
|
600.0
|
|
|
|
December 31, 2015
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||||
|
Assets
|
|
|
|
|
|
|
|
|
|||||||
|
Cash equivalents
|
$
|
3.5
|
|
$
|
8.5
|
|
$
|
—
|
|
$
|
12.0
|
|
|||
|
Available-for-sale investments:
|
|
|
|||||||||||||
|
Corporate debt securities
|
—
|
|
228.7
|
|
—
|
|
228.7
|
|
|||||||
|
Asset-backed securities
|
—
|
|
62.6
|
|
—
|
|
62.6
|
|
|||||||
|
U.S. government and agency securities
|
9.6
|
|
28.9
|
|
—
|
|
38.5
|
|
|||||||
|
Commercial paper
|
—
|
|
28.1
|
|
—
|
|
28.1
|
|
|||||||
|
Municipal securities
|
—
|
|
4.7
|
|
—
|
|
4.7
|
|
|||||||
|
Equity investments in unconsolidated affiliates
|
0.1
|
|
—
|
|
—
|
|
0.1
|
|
|||||||
|
Investments held for deferred compensation plans
|
35.3
|
|
—
|
|
—
|
|
35.3
|
|
|||||||
|
Derivatives
|
—
|
|
23.3
|
|
—
|
|
23.3
|
|
|||||||
|
$
|
48.5
|
|
$
|
384.8
|
|
$
|
—
|
|
$
|
433.3
|
|
||||
|
Liabilities
|
|
|
|
|
|
|
|
|
|||||||
|
Derivatives
|
$
|
—
|
|
$
|
4.2
|
|
$
|
—
|
|
$
|
4.2
|
|
|||
|
Deferred compensation plans
|
35.5
|
|
—
|
|
—
|
|
35.5
|
|
|||||||
|
Contingent consideration obligation
|
—
|
|
—
|
|
30.5
|
|
30.5
|
|
|||||||
|
$
|
35.5
|
|
$
|
4.2
|
|
$
|
30.5
|
|
$
|
70.2
|
|
||||
|
December 31, 2014
|
|||||||||||||||
|
Assets
|
|||||||||||||||
|
Cash equivalents
|
$
|
32.6
|
|
$
|
12.0
|
|
$
|
—
|
|
$
|
44.6
|
|
|||
|
Available-for-sale investments:
|
|
|
|
|
|
|
|
|
|||||||
|
Corporate debt securities
|
—
|
|
103.2
|
|
—
|
|
103.2
|
|
|||||||
|
Asset-backed securities
|
—
|
|
42.9
|
|
—
|
|
42.9
|
|
|||||||
|
U.S. government and agency securities
|
—
|
|
1.0
|
|
—
|
|
1.0
|
|
|||||||
|
Commercial paper
|
—
|
|
13.0
|
|
—
|
|
13.0
|
|
|||||||
|
Equity investments in unconsolidated affiliates
|
0.4
|
|
—
|
|
—
|
|
0.4
|
|
|||||||
|
Investments held for deferred compensation plans
|
28.2
|
|
—
|
|
—
|
|
28.2
|
|
|||||||
|
Derivatives
|
—
|
|
50.7
|
|
—
|
|
50.7
|
|
|||||||
|
$
|
61.2
|
|
$
|
222.8
|
|
$
|
—
|
|
$
|
284.0
|
|
||||
|
Liabilities
|
|
|
|
|
|
|
|
|
|||||||
|
Derivatives
|
$
|
—
|
|
$
|
2.6
|
|
$
|
—
|
|
$
|
2.6
|
|
|||
|
Deferred compensation plans
|
28.7
|
|
—
|
|
—
|
|
28.7
|
|
|||||||
|
$
|
28.7
|
|
$
|
2.6
|
|
$
|
—
|
|
$
|
31.3
|
|
||||
|
Balance at December 31, 2014
|
$
|
—
|
|
|
Additions
|
30.3
|
|
|
|
Changes in fair value
|
0.2
|
|
|
|
Balance at December 31, 2015
|
$
|
30.5
|
|
|
|
Notional Amount
|
||||||
|
|
December 31, 2015
|
December 31, 2014
|
|||||
|
|
(in millions)
|
||||||
|
Foreign currency forward exchange contracts
|
$
|
1,061.6
|
|
$
|
761.2
|
|
|
|
Interest rate swap agreements
|
300.0
|
|
300.0
|
|
|||
|
Foreign currency option contracts
|
—
|
|
9.2
|
|
|||
|
|
|
Fair Value
|
|||||||
|
|
Balance Sheet Location
|
December 31, 2015
|
December 31, 2014
|
||||||
|
Derivatives designated as hedging instruments
|
|
|
|
|
|
||||
|
Assets
|
|
|
|
|
|
||||
|
Foreign currency contracts
|
Other current assets
|
$
|
15.0
|
|
$
|
45.2
|
|
||
|
Interest rate swap agreements
|
Other assets
|
$
|
1.6
|
|
$
|
0.4
|
|
||
|
Liabilities
|
|
|
|
|
|
||||
|
Foreign currency contracts
|
Accrued and other liabilities
|
$
|
4.2
|
|
$
|
2.6
|
|
||
|
Derivatives not designated as hedging instruments
|
|
|
|
|
|
||||
|
Assets
|
|
|
|
|
|
||||
|
Foreign currency contracts
|
Other assets
|
$
|
6.7
|
|
$
|
5.1
|
|
||
|
|
|
|
|
Gross Amounts Not Offset in the Consolidated Balance Sheet
|
|
||||||||||||||||||
|
|
|
Gross Amounts
Offset in the
Consolidated
Balance Sheet
|
Net Amounts
Presented in the
Consolidated
Balance Sheet
|
||||||||||||||||||||
|
December 31, 2015
|
Gross
Amounts
|
Financial
Instruments
|
Cash
Collateral
Received
|
Net
Amount
|
|||||||||||||||||||
|
Derivative Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Foreign currency contracts
|
$
|
21.7
|
|
$
|
—
|
|
$
|
21.7
|
|
$
|
(4.0
|
)
|
$
|
—
|
|
$
|
17.7
|
|
|||||
|
Interest rate swap agreements
|
$
|
1.6
|
|
$
|
—
|
|
$
|
1.6
|
|
$
|
—
|
|
$
|
—
|
|
$
|
1.6
|
|
|||||
|
Derivative Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Foreign currency contracts
|
$
|
4.2
|
|
$
|
—
|
|
$
|
4.2
|
|
$
|
(4.0
|
)
|
$
|
—
|
|
$
|
0.2
|
|
|||||
|
December 31, 2014
|
|
|
|
|
|
|
|||||||||||||||||
|
Derivative Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Foreign currency contracts
|
$
|
50.3
|
|
$
|
—
|
|
$
|
50.3
|
|
$
|
(2.6
|
)
|
$
|
—
|
|
$
|
47.7
|
|
|||||
|
Interest rate swap agreements
|
$
|
0.4
|
|
$
|
—
|
|
$
|
0.4
|
|
$
|
—
|
|
$
|
—
|
|
$
|
0.4
|
|
|||||
|
Derivative Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
Foreign currency contracts
|
$
|
2.6
|
|
$
|
—
|
|
$
|
2.6
|
|
$
|
(2.6
|
)
|
$
|
—
|
|
$
|
—
|
|
|||||
|
|
Amount of Gain or (Loss) Recognized in OCI on Derivative (Effective Portion)
|
Location of Gain or (Loss) Reclassified from Accumulated OCI into Income
|
Amount of Gain or (Loss) Reclassified from Accumulated OCI into Income
|
||||||||||||||
|
|
|||||||||||||||||
|
|
2015
|
2014
|
2015
|
2014
|
|||||||||||||
|
(in millions)
|
|
(in millions)
|
|||||||||||||||
|
Cash flow hedges
|
|||||||||||||||||
|
Foreign currency contracts
|
$
|
35.3
|
|
$
|
54.3
|
|
Cost of sales
|
$
|
67.1
|
|
$
|
7.6
|
|
||||
|
Selling, general and administrative expenses
|
$
|
0.9
|
|
$
|
—
|
|
|||||||||||
|
Net investment hedges
|
|||||||||||||||||
|
Foreign currency contracts
|
$
|
2.9
|
|
$
|
—
|
|
Other expense, net
|
$
|
—
|
|
$
|
—
|
|
||||
|
|
|
Amount of Gain or
(Loss) Recognized in
Income on Derivative (a)
|
|||||||||||
|
|
Location of Gain or
(Loss) Recognized in
Income on Derivative
|
||||||||||||
|
|
2015
|
2014
|
2013
|
||||||||||
|
|
(in millions)
|
||||||||||||
|
Fair value hedges
|
|||||||||||||
|
Interest rate swap agreements
|
Interest expense
|
$
|
1.2
|
|
$
|
4.4
|
|
$
|
(4.0
|
)
|
|||
|
(a)
|
The gains and losses on the interest rate swap agreements are fully offset by the changes in the fair value of the fixed-rate debt being hedged.
|
|
|
|
Amount of Gain or
(Loss) Recognized in
Income on Derivative
|
|||||||||||
|
|
Location of Gain or
(Loss) Recognized in
Income on Derivative
|
||||||||||||
|
|
2015
|
2014
|
2013
|
||||||||||
|
|
(in millions)
|
||||||||||||
|
Derivatives not designated as hedging instruments
|
|||||||||||||
|
Foreign currency contracts
|
Other expense, net
|
$
|
6.6
|
|
$
|
13.7
|
|
$
|
18.4
|
|
|||
|
|
Years Ended
December 31, |
||||||
|
|
2015
|
2014
|
|||||
|
(in millions)
|
|||||||
|
Change in projected benefit obligation:
|
|
|
|
|
|||
|
Beginning of year
|
$
|
123.1
|
|
$
|
111.2
|
|
|
|
Service cost
|
7.0
|
|
6.3
|
|
|||
|
Interest cost
|
1.5
|
|
2.2
|
|
|||
|
Participant contributions
|
1.8
|
|
1.9
|
|
|||
|
Actuarial loss (gain)
|
(1.4
|
)
|
12.0
|
|
|||
|
Benefits paid
|
(1.3
|
)
|
0.3
|
|
|||
|
Plan amendment
|
(2.9
|
)
|
(1.0
|
)
|
|||
|
Settlements
|
(4.1
|
)
|
—
|
|
|||
|
Currency exchange rate changes and other
|
(5.6
|
)
|
(9.8
|
)
|
|||
|
End of year
|
$
|
118.1
|
|
$
|
123.1
|
|
|
|
Change in fair value of plan assets:
|
|
|
|
|
|||
|
Beginning of year
|
$
|
73.8
|
|
$
|
68.5
|
|
|
|
Actual return on plan assets
|
1.3
|
|
4.2
|
|
|||
|
Employer contributions
|
6.1
|
|
5.0
|
|
|||
|
Participant contributions
|
1.8
|
|
1.9
|
|
|||
|
Settlements
|
(4.1
|
)
|
—
|
|
|||
|
Benefits paid
|
(1.3
|
)
|
0.3
|
|
|||
|
Currency exchange rate changes and other
|
(2.5
|
)
|
(6.1
|
)
|
|||
|
End of year
|
$
|
75.1
|
|
$
|
73.8
|
|
|
|
Years Ended
December 31, |
|||||||
|
2015
|
2014
|
||||||
|
(in millions)
|
|||||||
|
Funded Status
|
|
|
|
|
|||
|
Projected benefit obligation
|
$
|
(118.1
|
)
|
$
|
(123.1
|
)
|
|
|
Plan assets at fair value
|
75.1
|
|
73.8
|
|
|||
|
Underfunded status
|
$
|
(43.0
|
)
|
$
|
(49.3
|
)
|
|
|
Net amounts recognized on the consolidated balance sheet:
|
|
|
|
|
|||
|
Other long-term liabilities
|
$
|
43.0
|
|
$
|
49.3
|
|
|
|
Accumulated other comprehensive loss, net of tax:
|
|
|
|
|
|||
|
Net actuarial loss
|
$
|
(20.0
|
)
|
$
|
(24.1
|
)
|
|
|
Net prior service credit
|
5.1
|
|
2.6
|
|
|||
|
Deferred income tax benefit
|
3.5
|
|
4.7
|
|
|||
|
Total
|
$
|
(11.4
|
)
|
$
|
(16.8
|
)
|
|
|
|
Years Ended
December 31, |
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Service cost, net
|
$
|
7.0
|
|
$
|
6.3
|
|
$
|
7.6
|
|
||
|
Interest cost
|
1.5
|
|
2.2
|
|
2.0
|
|
|||||
|
Expected return on plan assets
|
(1.5
|
)
|
(1.6
|
)
|
(1.2
|
)
|
|||||
|
Settlement
|
0.6
|
|
—
|
|
—
|
|
|||||
|
Amortization of actuarial loss
|
1.0
|
|
0.5
|
|
1.3
|
|
|||||
|
Amortization of prior service credit
|
(0.4
|
)
|
(0.3
|
)
|
(0.3
|
)
|
|||||
|
Net periodic pension benefit cost
|
$
|
8.2
|
|
$
|
7.1
|
|
$
|
9.4
|
|
||
|
|
December 31,
|
||||
|
|
2015
|
2014
|
|||
|
Discount rate
|
1.0
|
%
|
1.4
|
%
|
|
|
Rate of compensation increase
|
2.7
|
%
|
3.0
|
%
|
|
|
Social securities increase
|
1.6
|
%
|
1.6
|
%
|
|
|
Pension increase
|
2.0
|
%
|
2.0
|
%
|
|
|
|
Years ended
December 31,
|
|||||||
|
|
2015
|
2014
|
2013
|
|||||
|
Discount rate
|
1.4
|
%
|
2.2
|
%
|
1.9
|
%
|
||
|
Expected return on plan assets
|
1.9
|
%
|
2.6
|
%
|
2.1
|
%
|
||
|
Rate of compensation increase
|
3.0
|
%
|
3.1
|
%
|
3.1
|
%
|
||
|
Social securities increase
|
1.6
|
%
|
1.8
|
%
|
1.8
|
%
|
||
|
Pension increase
|
2.0
|
%
|
2.0
|
%
|
2.0
|
%
|
||
|
Insurance contracts
|
80.5
|
%
|
|
Equity securities
|
11.9
|
%
|
|
Debt securities
|
7.6
|
%
|
|
Total
|
100.0
|
%
|
|
December 31, 2015
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||||
|
Asset Category
|
|
|
|
|
|
|
|
|
|||||||
|
Cash
|
$
|
2.7
|
|
$
|
—
|
|
$
|
—
|
|
$
|
2.7
|
|
|||
|
Equity securities:
|
|
|
|
|
|
|
|
|
|||||||
|
United States equities
|
3.5
|
|
—
|
|
—
|
|
3.5
|
|
|||||||
|
International equities
|
7.3
|
|
—
|
|
—
|
|
7.3
|
|
|||||||
|
Debt securities:
|
|
|
|
|
|
|
|
|
|||||||
|
United States government bonds
|
0.7
|
|
—
|
|
—
|
|
0.7
|
|
|||||||
|
International government bonds
|
4.1
|
|
—
|
|
—
|
|
4.1
|
|
|||||||
|
Insurance contracts
|
—
|
|
—
|
|
56.8
|
|
56.8
|
|
|||||||
|
$
|
18.3
|
|
$
|
—
|
|
$
|
56.8
|
|
$
|
75.1
|
|
||||
|
December 31, 2014
|
|
|
|
|
|
|
|
|
|||||||
|
Asset Category
|
|
|
|
|
|
|
|
|
|||||||
|
Cash
|
$
|
0.5
|
|
$
|
—
|
|
$
|
—
|
|
$
|
0.5
|
|
|||
|
Equity securities:
|
|
|
|
|
|
|
|
|
|||||||
|
United States equities
|
2.8
|
|
—
|
|
—
|
|
2.8
|
|
|||||||
|
International equities
|
6.8
|
|
—
|
|
—
|
|
6.8
|
|
|||||||
|
Debt securities:
|
|
|
|
|
|
|
|
|
|||||||
|
United States government bonds
|
0.6
|
|
—
|
|
—
|
|
0.6
|
|
|||||||
|
International government bonds
|
4.7
|
|
—
|
|
—
|
|
4.7
|
|
|||||||
|
Insurance contracts
|
—
|
|
—
|
|
58.4
|
|
58.4
|
|
|||||||
|
$
|
15.4
|
|
$
|
—
|
|
$
|
58.4
|
|
$
|
73.8
|
|
||||
|
|
Insurance
Contracts |
||
|
Balance at December 31, 2013
|
$
|
54.6
|
|
|
Actual return on plan assets:
|
|
|
|
|
Relating to assets still held at December 31, 2014
|
3.0
|
|
|
|
Purchases, sales and settlements
|
4.9
|
|
|
|
Currency exchange rate impact
|
(4.1
|
)
|
|
|
Balance at December 31, 2014
|
58.4
|
|
|
|
Actual return on plan assets:
|
|
|
|
|
Relating to assets still held at December 31, 2015
|
(0.3
|
)
|
|
|
Purchases, sales and settlements
|
0.7
|
|
|
|
Currency exchange rate impact
|
(2.0
|
)
|
|
|
Balance at December 31, 2015
|
$
|
56.8
|
|
|
2016
|
$
|
3.9
|
|
|
2017
|
3.9
|
|
|
|
2018
|
3.7
|
|
|
|
2019
|
3.7
|
|
|
|
2020
|
4.4
|
|
|
|
2021-2025
|
33.6
|
|
|
|
|
|
Initial Delivery
|
Final Settlement
|
||||||||||||||||||||
|
Agreement Date
|
Amount
Paid
|
Shares
Received
|
Price per
Share (a)
|
Value of
Shares as %
of Contract
Value
|
Settlement
Date
|
Total Shares
Received
|
Average Price
per Share (a)
|
||||||||||||||||
|
August 2013
|
$
|
250.0
|
|
3.1
|
|
$
|
72.39
|
|
90
|
%
|
October 2013
|
3.5
|
|
$
|
71.24
|
|
|||||||
|
(a)
|
The
two
-for-one stock split paid on December 11, 2015 excluded treasury shares. The shares and per share prices in the table above are reflected at the pre-split amounts and prices at the time of the transaction.
|
|
2015
|
2014
|
2013
|
|||||||||
|
Average risk-free interest rate
|
1.4
|
%
|
1.5
|
%
|
0.8
|
%
|
|||||
|
Expected dividend yield
|
None
|
|
None
|
|
None
|
|
|||||
|
Expected volatility
|
30
|
%
|
31
|
%
|
31
|
%
|
|||||
|
Expected life (years)
|
4.6
|
|
4.6
|
|
4.6
|
|
|||||
|
Fair value, per share
|
$
|
18.13
|
|
$
|
11.75
|
|
$
|
9.73
|
|
||
|
2015
|
2014
|
2013
|
|||||||||
|
Average risk-free interest rate
|
0.2
|
%
|
0.1
|
%
|
0.1
|
%
|
|||||
|
Expected dividend yield
|
None
|
|
None
|
|
None
|
|
|||||
|
Expected volatility
|
28
|
%
|
30
|
%
|
33
|
%
|
|||||
|
Expected life (years)
|
0.6
|
|
0.6
|
|
0.6
|
|
|||||
|
Fair value, per share
|
$
|
15.59
|
|
$
|
8.59
|
|
$
|
9.93
|
|
||
|
|
Shares
|
Weighted-
Average
Exercise
Price
|
Weighted-
Average
Remaining
Contractual
Term
|
Aggregate
Intrinsic Value
|
||||||||
|
Outstanding as of December 31, 2014
|
12.8
|
|
$
|
33.93
|
|
|
|
|
||||
|
Options granted
|
1.8
|
|
66.41
|
|
|
|
|
|||||
|
Options exercised
|
(2.7
|
)
|
23.31
|
|
|
|
|
|||||
|
Options forfeited
|
(0.3
|
)
|
43.39
|
|
|
|
|
|||||
|
Outstanding as of December 31, 2015
|
11.6
|
|
41.14
|
|
3.8 years
|
$
|
438.0
|
|
||||
|
Exercisable as of December 31, 2015
|
7.1
|
|
35.70
|
|
2.9 years
|
308.8
|
|
|||||
|
Vested and expected to vest as of December 31, 2015
|
11.0
|
|
40.70
|
|
3.8 years
|
422.0
|
|
|||||
|
|
Shares
|
Weighted-
Average
Grant-Date
Fair Value
|
||||
|
Nonvested as of December 31, 2014
|
1.8
|
|
$
|
40.27
|
|
|
|
Granted (a)
|
0.5
|
|
67.40
|
|
||
|
Vested
|
(0.6
|
)
|
42.56
|
|
||
|
Forfeited
|
(0.1
|
)
|
42.76
|
|
||
|
Nonvested as of December 31, 2015
|
1.6
|
|
47.99
|
|
||
|
(a)
|
Includes
68,000
shares of market-based restricted stock units granted during
2015
, which represents the targeted number of shares to be issued. As described above, the actual number of shares ultimately issued will be determined based on the Company's total shareholder return relative to a selected industry peer group. In addition, includes
43,000
shares of performance-based restricted stock units granted during 2015 which vest upon achievement of specified milestones.
|
|
|
Foreign
Currency
Translation
Adjustments
|
Unrealized Gain
on Cash
Flow Hedges
|
Unrealized Gain
(Loss) on
Available-for-sale
Investments
|
Unrealized
Pension
Costs (a)
|
Total
Accumulated
Other
Comprehensive
Loss
|
||||||||||||||
|
|
(in millions)
|
||||||||||||||||||
|
December 31, 2012
|
$
|
(25.8
|
)
|
$
|
7.0
|
|
$
|
1.4
|
|
$
|
(20.5
|
)
|
$
|
(37.9
|
)
|
||||
|
Other comprehensive income (loss) before reclassifications
|
5.6
|
|
16.3
|
|
(1.2
|
)
|
9.9
|
|
30.6
|
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive loss
|
—
|
|
(21.5
|
)
|
—
|
|
1.0
|
|
(20.5
|
)
|
|||||||||
|
Deferred income tax benefit (expense)
|
—
|
|
1.7
|
|
0.1
|
|
(1.6
|
)
|
0.2
|
|
|||||||||
|
December 31, 2013
|
(20.2
|
)
|
3.5
|
|
0.3
|
|
(11.2
|
)
|
(27.6
|
)
|
|||||||||
|
Other comprehensive (loss) income before reclassifications
|
(96.2
|
)
|
54.3
|
|
(0.8
|
)
|
(7.1
|
)
|
(49.8
|
)
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive loss
|
—
|
|
(7.6
|
)
|
0.4
|
|
0.2
|
|
(7.0
|
)
|
|||||||||
|
Deferred income tax (expense) benefit
|
—
|
|
(17.9
|
)
|
0.1
|
|
1.3
|
|
(16.5
|
)
|
|||||||||
|
December 31, 2014
|
(116.4
|
)
|
32.3
|
|
—
|
|
(16.8
|
)
|
(100.9
|
)
|
|||||||||
|
Other comprehensive (loss) income before reclassifications
|
(64.0
|
)
|
35.3
|
|
(2.6
|
)
|
5.4
|
|
(25.9
|
)
|
|||||||||
|
Amounts reclassified from accumulated other comprehensive loss
|
—
|
|
(68.0
|
)
|
1.1
|
|
1.2
|
|
(65.7
|
)
|
|||||||||
|
Deferred income tax (expense) benefit
|
(1.1
|
)
|
12.2
|
|
—
|
|
(1.2
|
)
|
9.9
|
|
|||||||||
|
December 31, 2015
|
$
|
(181.5
|
)
|
$
|
11.8
|
|
$
|
(1.5
|
)
|
$
|
(11.4
|
)
|
$
|
(182.6
|
)
|
||||
|
(a)
|
For the years ended
December 31, 2015
,
2014
, and
2013
, the change in unrealized pension costs consisted of the following (in millions):
|
|
|
Pre-Tax
Amount |
Tax (Expense) Benefit
|
Net of Tax
Amount |
||||||||
|
2015
|
|
|
|
|
|
|
|||||
|
Prior service credit arising during period
|
$
|
2.9
|
|
$
|
(0.3
|
)
|
$
|
2.6
|
|
||
|
Amortization of prior service credit
|
(0.4
|
)
|
0.1
|
|
(0.3
|
)
|
|||||
|
Net prior service credit arising during period
|
2.5
|
|
(0.2
|
)
|
2.3
|
|
|||||
|
Net actuarial gain arising during period
|
4.1
|
|
(1.0
|
)
|
3.1
|
|
|||||
|
Unrealized pension credits, net
|
$
|
6.6
|
|
$
|
(1.2
|
)
|
$
|
5.4
|
|
||
|
2014
|
|
|
|
|
|
|
|||||
|
Prior service credit arising during period
|
$
|
0.8
|
|
$
|
—
|
|
$
|
0.8
|
|
||
|
Amortization of prior service credit
|
(0.3
|
)
|
—
|
|
(0.3
|
)
|
|||||
|
Net prior service credit arising during period
|
0.5
|
|
—
|
|
0.5
|
|
|||||
|
Net actuarial loss arising during period
|
(7.4
|
)
|
1.3
|
|
(6.1
|
)
|
|||||
|
Unrealized pension costs, net
|
$
|
(6.9
|
)
|
$
|
1.3
|
|
$
|
(5.6
|
)
|
||
|
2013
|
|
|
|
|
|
|
|||||
|
Prior service cost arising during period
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Amortization of prior service credit
|
(0.3
|
)
|
—
|
|
(0.3
|
)
|
|||||
|
Net prior service cost arising during period
|
(0.3
|
)
|
—
|
|
(0.3
|
)
|
|||||
|
Net actuarial gain arising during period
|
11.2
|
|
(1.6
|
)
|
9.6
|
|
|||||
|
Unrealized pension credits, net
|
$
|
10.9
|
|
$
|
(1.6
|
)
|
$
|
9.3
|
|
||
|
|
Years Ended December 31,
|
|
|||||||
|
Details about Accumulated Other Comprehensive Loss
Components
|
2015
|
2014
|
Affected Line on Consolidated
Statements of Operations
|
||||||
|
Gain on cash flow hedges
|
$
|
68.0
|
|
$
|
7.6
|
|
Cost of sales
|
||
|
(25.0
|
)
|
(2.9
|
)
|
Provision for income taxes
|
|||||
|
$
|
43.0
|
|
$
|
4.7
|
|
Net of tax
|
|||
|
Loss on available-for-sale investments
|
$
|
(1.1
|
)
|
$
|
(0.4
|
)
|
Other expense, net
|
||
|
—
|
|
—
|
|
Provision for income taxes
|
|||||
|
$
|
(1.1
|
)
|
$
|
(0.4
|
)
|
Net of tax
|
|||
|
Amortization of pension adjustments
|
$
|
(1.2
|
)
|
$
|
(0.2
|
)
|
(a)
|
||
|
0.2
|
|
0.1
|
|
Provision for income taxes
|
|||||
|
$
|
(1.0
|
)
|
$
|
(0.1
|
)
|
Net of tax
|
|||
|
(a)
|
This item is included in the components of net periodic benefit costs. See Note 12 for additional information.
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
(in millions)
|
|||||||||||
|
Foreign exchange losses, net
|
$
|
4.8
|
|
$
|
2.0
|
|
$
|
1.5
|
|
||
|
Loss on investments
|
(0.1
|
)
|
4.5
|
|
0.4
|
|
|||||
|
Promissory note impairment
|
—
|
|
4.0
|
|
—
|
|
|||||
|
Insurance settlement gain
|
—
|
|
(3.7
|
)
|
—
|
|
|||||
|
Lease contract termination costs
|
—
|
|
1.0
|
|
—
|
|
|||||
|
Other
|
(0.7
|
)
|
(0.1
|
)
|
(0.6
|
)
|
|||||
|
Total other expense, net
|
$
|
4.0
|
|
$
|
7.7
|
|
$
|
1.3
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
United States
|
$
|
182.8
|
|
$
|
791.1
|
|
$
|
215.5
|
|
||
|
International, including Puerto Rico
|
439.6
|
|
352.9
|
|
295.7
|
|
|||||
|
$
|
622.4
|
|
$
|
1,144.0
|
|
$
|
511.2
|
|
|||
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Current
|
|
|
|
|
|
|
|||||
|
United States:
|
|
|
|
|
|
|
|||||
|
Federal
|
$
|
102.4
|
|
$
|
341.5
|
|
$
|
42.5
|
|
||
|
State and local
|
7.4
|
|
23.3
|
|
5.7
|
|
|||||
|
International, including Puerto Rico
|
33.5
|
|
34.8
|
|
27.9
|
|
|||||
|
Current income tax expense
|
$
|
143.3
|
|
$
|
399.6
|
|
$
|
76.1
|
|
||
|
Deferred
|
|
|
|
|
|
|
|||||
|
United States:
|
|
|
|
|
|
|
|||||
|
Federal
|
$
|
(12.5
|
)
|
$
|
(46.4
|
)
|
$
|
39.8
|
|
||
|
State and local
|
(2.6
|
)
|
(8.1
|
)
|
0.8
|
|
|||||
|
International, including Puerto Rico
|
(0.7
|
)
|
(12.2
|
)
|
5.4
|
|
|||||
|
Deferred income tax (benefit) expense
|
(15.8
|
)
|
(66.7
|
)
|
46.0
|
|
|||||
|
Total income tax provision
|
$
|
127.5
|
|
$
|
332.9
|
|
$
|
122.1
|
|
||
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Deferred tax assets
|
|
|
|
|
|||
|
Compensation and benefits
|
$
|
94.0
|
|
$
|
82.3
|
|
|
|
Benefits from uncertain tax positions
|
50.0
|
|
41.4
|
|
|||
|
Net tax credit carryforwards
|
39.3
|
|
32.1
|
|
|||
|
Net operating loss carryforwards
|
27.0
|
|
31.8
|
|
|||
|
Accrued liabilities
|
24.2
|
|
25.3
|
|
|||
|
Inventories
|
11.9
|
|
11.7
|
|
|||
|
State income taxes
|
0.5
|
|
6.8
|
|
|||
|
Investments
|
2.6
|
|
2.6
|
|
|||
|
Other
|
4.5
|
|
4.8
|
|
|||
|
Total deferred tax assets
|
254.0
|
|
238.8
|
|
|||
|
Deferred tax liabilities
|
|
|
|
|
|||
|
Property, plant, and equipment
|
(25.1
|
)
|
(25.8
|
)
|
|||
|
Cash flow hedges
|
(0.4
|
)
|
(11.9
|
)
|
|||
|
Deferred tax on foreign earnings
|
(10.9
|
)
|
(4.9
|
)
|
|||
|
Inventories
|
(0.9
|
)
|
(3.1
|
)
|
|||
|
Other intangible assets
|
(0.3
|
)
|
(0.5
|
)
|
|||
|
Other
|
(0.5
|
)
|
(1.7
|
)
|
|||
|
Total deferred tax liabilities
|
(38.1
|
)
|
(47.9
|
)
|
|||
|
Valuation allowance
|
(45.2
|
)
|
(47.7
|
)
|
|||
|
Net deferred tax assets
|
$
|
170.7
|
|
$
|
143.2
|
|
|
|
|
Carryforward
Amount |
Tax Benefit
Amount |
Valuation
Allowance |
Net Tax
Benefit |
Carryforward
Period Ends |
||||||||||||
|
United States state net operating losses
|
$
|
9.7
|
|
$
|
0.6
|
|
$
|
(0.4
|
)
|
$
|
0.2
|
|
2016-2033
|
||||
|
Non-United States net operating losses
|
53.4
|
|
13.3
|
|
(12.7
|
)
|
0.6
|
|
2016-2024
|
||||||||
|
Non-United States net operating losses
|
39.6
|
|
13.3
|
|
(13.2
|
)
|
0.1
|
|
Indefinite
|
||||||||
|
Total
|
$
|
102.7
|
|
$
|
27.2
|
|
$
|
(26.3
|
)
|
$
|
0.9
|
|
|
||||
|
|
Carryforward
Amount |
Valuation
Allowance |
Net Tax
Benefit |
Carryforward
Period Ends |
|||||||||
|
California research expenditure tax credits
|
$
|
63.6
|
|
$
|
—
|
|
$
|
63.6
|
|
Indefinite
|
|||
|
Puerto Rico purchases credit
|
14.4
|
|
(14.4
|
)
|
—
|
|
Indefinite
|
||||||
|
Total
|
$
|
78.0
|
|
$
|
(14.4
|
)
|
$
|
63.6
|
|
|
|||
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Income tax expense at U.S. federal statutory rate
|
$
|
217.8
|
|
$
|
400.4
|
|
$
|
178.9
|
|
||
|
Foreign income taxed at different rates
|
(105.8
|
)
|
(67.1
|
)
|
(60.6
|
)
|
|||||
|
State and local taxes, net of federal tax benefit
|
3.1
|
|
19.3
|
|
5.8
|
|
|||||
|
Tax credits, federal and state
|
(15.7
|
)
|
(13.5
|
)
|
(19.8
|
)
|
|||||
|
Build (release) of reserve for uncertain tax positions for prior years
|
3.3
|
|
(4.8
|
)
|
(3.9
|
)
|
|||||
|
U.S. tax on foreign earnings, net of credits
|
20.5
|
|
(3.1
|
)
|
18.9
|
|
|||||
|
Nondeductible stock-based compensation
|
2.3
|
|
2.1
|
|
2.6
|
|
|||||
|
Other
|
2.0
|
|
(0.4
|
)
|
0.2
|
|
|||||
|
Income tax provision
|
$
|
127.5
|
|
$
|
332.9
|
|
$
|
122.1
|
|
||
|
|
December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Unrecognized tax benefits, January 1
|
$
|
192.3
|
|
$
|
127.7
|
|
$
|
113.6
|
|
||
|
Current year tax positions
|
29.6
|
|
75.9
|
|
17.8
|
|
|||||
|
Increase prior year tax positions
|
2.2
|
|
0.6
|
|
5.7
|
|
|||||
|
Decrease prior year tax positions
|
(7.4
|
)
|
(10.5
|
)
|
(9.0
|
)
|
|||||
|
Settlements
|
(0.4
|
)
|
(1.0
|
)
|
(0.1
|
)
|
|||||
|
Lapse of statutes of limitations
|
(0.2
|
)
|
(0.4
|
)
|
(0.3
|
)
|
|||||
|
Unrecognized tax benefits, December 31
|
$
|
216.1
|
|
$
|
192.3
|
|
$
|
127.7
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Segment Net Sales
|
|
|
|
|
|
|
|||||
|
United States
|
$
|
1,262.8
|
|
$
|
1,047.3
|
|
$
|
939.6
|
|
||
|
Europe
|
842.9
|
|
741.4
|
|
622.2
|
|
|||||
|
Japan
|
297.2
|
|
270.8
|
|
293.7
|
|
|||||
|
Rest of World
|
315.1
|
|
285.1
|
|
252.8
|
|
|||||
|
Total segment net sales
|
$
|
2,718.0
|
|
$
|
2,344.6
|
|
$
|
2,108.3
|
|
||
|
Segment Pre-tax Income
|
|
|
|
|
|
|
|||||
|
United States
|
$
|
747.8
|
|
$
|
605.6
|
|
$
|
550.5
|
|
||
|
Europe
|
409.1
|
|
328.1
|
|
287.7
|
|
|||||
|
Japan
|
139.4
|
|
125.2
|
|
145.6
|
|
|||||
|
Rest of World
|
82.2
|
|
78.6
|
|
68.3
|
|
|||||
|
Total segment pre-tax income
|
$
|
1,378.5
|
|
$
|
1,137.5
|
|
$
|
1,052.1
|
|
||
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Net Sales Reconciliation
|
|
|
|
|
|
|
|||||
|
Segment net sales
|
$
|
2,718.0
|
|
$
|
2,344.6
|
|
$
|
2,108.3
|
|
||
|
Foreign currency
|
(224.3
|
)
|
(21.7
|
)
|
(62.8
|
)
|
|||||
|
Consolidated net sales
|
$
|
2,493.7
|
|
$
|
2,322.9
|
|
$
|
2,045.5
|
|
||
|
Pre-tax Income Reconciliation
|
|
|
|
|
|
|
|||||
|
Segment pre-tax income
|
$
|
1,378.5
|
|
$
|
1,137.5
|
|
$
|
1,052.1
|
|
||
|
Unallocated amounts:
|
|
|
|||||||||
|
Corporate items
|
(711.3
|
)
|
(659.2
|
)
|
(568.5
|
)
|
|||||
|
Special charges
|
—
|
|
(70.7
|
)
|
(16.3
|
)
|
|||||
|
Intellectual property (expenses) income, net
|
(7.0
|
)
|
740.4
|
|
61.5
|
|
|||||
|
Interest expense, net
|
(9.3
|
)
|
(10.8
|
)
|
(5.2
|
)
|
|||||
|
Foreign currency
|
(28.5
|
)
|
6.8
|
|
(12.4
|
)
|
|||||
|
Consolidated pre-tax income
|
$
|
622.4
|
|
$
|
1,144.0
|
|
$
|
511.2
|
|
||
|
|
As of or for the Years Ended
December 31, |
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
(in millions)
|
|||||||||||
|
Net Sales by Geographic Area
|
|
|
|
|
|
|
|||||
|
United States
|
$
|
1,262.9
|
|
$
|
1,047.3
|
|
$
|
939.6
|
|
||
|
Europe
|
717.3
|
|
744.5
|
|
616.5
|
|
|||||
|
Japan
|
246.2
|
|
257.9
|
|
243.6
|
|
|||||
|
Rest of World
|
267.3
|
|
273.2
|
|
245.8
|
|
|||||
|
$
|
2,493.7
|
|
$
|
2,322.9
|
|
$
|
2,045.5
|
|
|||
|
Net Sales by Major Product Area
|
|
|
|
|
|
|
|||||
|
Transcatheter Heart Valve Therapy
|
$
|
1,180.3
|
|
$
|
943.6
|
|
$
|
707.7
|
|
||
|
Surgical Heart Valve Therapy
|
785.0
|
|
826.1
|
|
801.2
|
|
|||||
|
Critical Care
|
528.4
|
|
553.2
|
|
536.6
|
|
|||||
|
$
|
2,493.7
|
|
$
|
2,322.9
|
|
$
|
2,045.5
|
|
|||
|
Long-lived Tangible Assets by Geographic Area
|
|
|
|
|
|
|
|||||
|
United States
|
$
|
473.6
|
|
$
|
347.6
|
|
$
|
308.2
|
|
||
|
Europe
|
36.0
|
|
42.1
|
|
40.9
|
|
|||||
|
Japan
|
8.1
|
|
8.5
|
|
10.8
|
|
|||||
|
Rest of World
|
96.0
|
|
93.9
|
|
97.1
|
|
|||||
|
$
|
613.7
|
|
$
|
492.1
|
|
$
|
457.0
|
|
|||
|
Years Ended December 31,
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
Total
Year
|
||||||||||||||
|
|
(in millions, except per share data)
|
||||||||||||||||||
|
2015
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Net sales
|
$
|
590.3
|
|
$
|
616.8
|
|
$
|
615.5
|
|
$
|
671.1
|
|
$
|
2,493.7
|
|
||||
|
Gross profit
|
454.3
|
|
458.2
|
|
468.8
|
|
495.2
|
|
1,876.5
|
|
|||||||||
|
Net income
|
123.4
|
|
112.7
|
|
118.1
|
|
140.7
|
|
494.9
|
|
|||||||||
|
Earnings per common share:
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Basic
|
0.57
|
|
0.52
|
|
0.55
|
|
0.65
|
|
2.30
|
|
|||||||||
|
Diluted
|
0.56
|
|
0.51
|
|
0.54
|
|
0.64
|
|
2.25
|
|
|||||||||
|
Market price:
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
High
|
$
|
75.21
|
|
$
|
73.65
|
|
$
|
79.50
|
|
$
|
83.43
|
|
$
|
83.43
|
|
||||
|
Low
|
61.99
|
|
61.38
|
|
62.53
|
|
70.32
|
|
61.38
|
|
|||||||||
|
2014
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Net sales
|
$
|
522.4
|
|
$
|
575.1
|
|
$
|
607.4
|
|
$
|
618.0
|
|
$
|
2,322.9
|
|
||||
|
Gross profit
|
376.5
|
|
423.9
|
|
439.3
|
|
457.6
|
|
1,697.3
|
|
|||||||||
|
Net income (a)
|
60.3
|
|
547.0
|
|
94.6
|
|
109.2
|
|
811.1
|
|
|||||||||
|
Earnings per common share (a):
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Basic
|
0.28
|
|
2.59
|
|
0.44
|
|
0.51
|
|
3.81
|
|
|||||||||
|
Diluted
|
0.28
|
|
2.55
|
|
0.44
|
|
0.50
|
|
3.74
|
|
|||||||||
|
Market price:
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
High
|
$
|
37.81
|
|
$
|
44.10
|
|
$
|
52.35
|
|
$
|
67.14
|
|
$
|
67.14
|
|
||||
|
Low
|
31.52
|
|
36.40
|
|
42.03
|
|
48.54
|
|
31.52
|
|
|||||||||
|
(a)
|
The second quarter of 2014 includes a
$750.0 million
gain for an upfront payment received under a litigation settlement agreement and a
$50.0 million
charge for the contribution to the Edwards Lifesciences Foundation.
|
|
|
|
Additions
|
|
|
|||||||||||||||
|
|
Balance at
Beginning
of Period
|
Charged to
Costs and
Expenses
|
Charged to
Other
Accounts
|
Deductions
From
Reserves
|
Balance at
End of
Period
|
||||||||||||||
|
|
(in millions)
|
||||||||||||||||||
|
Year ended December 31, 2015
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Allowance for doubtful accounts (a)
|
$
|
11.3
|
|
$
|
3.8
|
|
$
|
—
|
|
$
|
(2.0
|
)
|
$
|
13.1
|
|
||||
|
Tax valuation allowance (b)
|
47.7
|
|
4.8
|
|
—
|
|
(7.3
|
)
|
45.2
|
|
|||||||||
|
Year ended December 31, 2014
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Allowance for doubtful accounts (a)
|
$
|
12.2
|
|
$
|
0.8
|
|
$
|
—
|
|
$
|
(1.7
|
)
|
$
|
11.3
|
|
||||
|
Tax valuation allowance (b)
|
46.4
|
|
2.0
|
|
—
|
|
(0.7
|
)
|
47.7
|
|
|||||||||
|
Year ended December 31, 2013
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
Allowance for doubtful accounts (a)
|
$
|
12.0
|
|
$
|
2.4
|
|
$
|
—
|
|
$
|
(2.2
|
)
|
$
|
12.2
|
|
||||
|
Tax valuation allowance (b)
|
38.6
|
|
8.2
|
|
—
|
|
(0.4
|
)
|
46.4
|
|
|||||||||
|
(a)
|
The deductions related to allowances for doubtful accounts represent accounts receivable which are written off and product which is returned from customers.
|
|
(b)
|
The tax valuation allowances are provided for other-than-temporary impairments and unrealized losses related to certain investments that may not be recognized due to the uncertainty of the ready marketability of certain impaired investments, and net operating loss and credit carryforwards that may not be recognized due to insufficient taxable income.
|
|
|
EDWARDS LIFESCIENCES CORPORATION
|
||
|
February 19, 2016
|
By:
|
|
/s/ MICHAEL A. MUSSALLEM
|
|
Michael A. Mussallem
Chairman of the Board and
Chief Executive Officer
|
|||
|
Signature
|
Title
|
|
Date
|
|
|
|
|
|
|
/s/ MICHAEL A. MUSSALLEM
|
Chairman of the Board and Chief Executive Officer
|
|
February 19, 2016
|
|
Michael A. Mussallem
|
(Principal Executive Officer)
|
||
|
/s/ SCOTT B. ULLEM
|
Corporate Vice President, Chief Financial Officer
|
|
February 19, 2016
|
|
Scott B. Ullem
|
(Principal Financial Officer)
|
||
|
/s/ ROBERT W.A. SELLERS
|
Vice President, Corporate Controller
|
|
February 19, 2016
|
|
Robert W.A. Sellers
|
(Principal Accounting Officer)
|
||
|
/s/ JOHN T. CARDIS
|
Director
|
|
February 18, 2016
|
|
John T. Cardis
|
|||
|
/s/ KIERAN T. GALLAHUE
|
Director
|
|
February 18, 2016
|
|
Kieran T. Gallahue
|
|||
|
/s/ WILLIAM J. LINK, PH.D.
|
Director
|
|
February 19, 2016
|
|
William J. Link, Ph.D.
|
|||
|
/s/ MARTHA H. MARSH
|
Director
|
|
February 18, 2016
|
|
Martha H. Marsh
|
|||
|
/s/ BARBARA J. MCNEIL, M.D., PH.D.
|
Director
|
|
February 18, 2016
|
|
Barbara J. McNeil, M.D., Ph.D.
|
|||
|
/s/ WESLEY W. VON SCHACK
|
Director
|
|
February 18, 2016
|
|
Wesley W. von Schack
|
|||
|
/s/ NICHOLAS J. VALERIANI
|
Director
|
|
February 18, 2016
|
|
Nicholas J. Valeriani
|
|||
|
Exhibit No.
|
Exhibit No.
|
|
|
3.1
|
|
Amended and Restated Certificate of Incorporation of Edwards Lifesciences Corporation dated May 16, 2013 (incorporated by reference to Exhibit 3.1 in Edwards Lifesciences' report on Form 8-K dated May 17, 2013)
|
|
3.2
|
|
Bylaws of Edwards Lifesciences Corporation amended and restated as of February 20, 2014 (incorporated by reference to Exhibit 3.1 in Edwards Lifesciences' report on Form 8-K dated February 26, 2014)
|
|
4.1
|
|
Specimen form of certificate representing Edwards Lifesciences Corporation common stock (incorporated by reference to Exhibit 4.1 in Edwards Lifesciences' Registration Statement on Form 10 (File No. 001-15525) filed on March 15, 2000)
|
|
4.2
|
|
Indenture, dated as of September 6, 2013, between Edwards Lifesciences Corporation and Wells Fargo Bank, National Association, as trustee (incorporated by reference to Exhibit 4.5 in Edwards Lifesciences' Registration Statement on Form S-3 (File No. 333-191022) filed on September 6, 2013) (the "Indenture")
|
|
4.3
|
|
First Supplemental Indenture, dated as of October 3, 2013, to the Indenture (incorporated by reference to Exhibit 4.1 in Edwards Lifesciences' report on Form 8-K, filed on October 3, 2013) ("First Supplemental Indenture")
|
|
4.4
|
|
Form of Global Note for the 2.875% Senior Notes due 2018 (incorporated by reference to Exhibit A in the First Supplemental Indenture filed as Exhibit 4.1 in Edwards Lifesciences' report on Form 8-K, filed on October 3, 2013)
|
|
10.1
|
|
Five-Year Credit Agreement, dated as of July 18, 2014, among Edwards Lifesciences Corporation and certain of its subsidiaries, as Borrowers; the lenders signatory thereto, Bank of America, N.A., as Administrative Agent, Swing Line Lender and Issuing Bank; JPMorgan Chase Bank, N.A. and Wells Fargo Bank, National Association, as Co-Syndication Agents; and Deutsche Bank Securities Inc., HSBC Bank USA, National Association, PNC Bank, National Association, The Bank of Tokyo-Mitsubishi UFJ, Ltd., and U.S. Bank National Association, as Co-Documentation Agents (incorporated by reference to Exhibit 10.1 in Edwards Lifesciences' report on Form 8-K, filed on July 24, 2014)
|
|
#10.2
|
|
Settlement Agreement, dated May 19, 2014, between Edwards Lifesciences Corporation and Medtronic, Inc. (incorporated by reference to Exhibit 10.2 in Edwards Lifesciences' report on Form 10-Q for the quarterly period ended June 30, 2014)
|
|
*10.3
|
|
Edwards Lifesciences Corporation Form of Employment Agreement (incorporated by reference to Exhibit 10.8 in Edwards Lifesciences' report on Form 10-Q for the quarterly period ended March 31, 2003)
|
|
*10.4
|
|
Edwards Lifesciences Corporation Amended and Restated Employment Agreement for Michael A. Mussallem dated March 30, 2009 (incorporated by reference to Exhibit 10.2 in Edwards Lifesciences' report on Form 10-Q for the quarterly period ended March 31, 2009)
|
|
*10.5
|
|
Edwards Lifesciences Corporation Amended and Restated Chief Executive Officer Change-in-Control Severance Agreement, dated October 9, 2012 (incorporated by reference to Exhibit 10.1 in Edwards Lifesciences' report on Form 10-Q for the quarterly period ended September 30, 2012)
|
|
*10.6
|
|
Edwards Lifesciences Corporation Form of Change-in-Control Severance Agreement (incorporated by reference to Exhibit 10.2 in Edward Lifesciences' report on Form 10-Q for the quarterly period ended September 30, 2012)
|
|
*10.7
|
|
Edwards Lifesciences Corporation 2015 Edwards Incentive Plan (incorporated by reference to Appendix A in Edwards Lifesciences’ Definitive Proxy Statement filed on March 30, 2015)
|
|
*10.8
|
|
Edwards Lifesciences Corporation Long-Term Stock Incentive Compensation Program, as amended and restated as of February 19, 2015 (the "Long-Term Stock Program") (incorporated by reference to Appendix B in Edwards Lifesciences' Definitive Proxy Statement filed on March 30, 2015)
|
|
*10.9
|
|
Edwards Lifesciences Corporation Form of Participant Stock Option Statement and related Long-Term Stock Program Global Nonqualified Stock Option Award Agreement for awards granted prior to May 2015 (incorporated by reference to Exhibit 10.1 in Edwards Lifesciences' report on Form 10-Q for the quarterly period ended March 31, 2011)
|
|
*10.10
|
|
Edwards Lifesciences Corporation Form of Participant Restricted Stock Unit Statement and related Long-Term Stock Program Global Restricted Stock Unit Award Agreement for awards granted prior to May 2015 (incorporated by reference to Exhibit 10.4 in Edwards Lifesciences' report on Form 10-Q for the quarterly period ended March 31, 2011)
|
|
*10.11
|
|
Edwards Lifesciences Corporation Form of Performance-Based Restricted Stock Unit Statement and related Long-Term Stock Program Global Performance-Based Restricted Stock Unit Award Agreement for awards granted prior to May 2015 (incorporated by reference to Exhibit 10.4 in Edwards Lifesciences' report on Form 10-Q for the quarterly period ended June 30, 2012)
|
|
*10.12
|
|
Edwards Lifesciences Corporation Long-Term Stock Program Global Nonqualified Stock Option Award Agreement for awards granted beginning May 2015 (incorporated by reference to Exhibit 10.3 in Edwards Lifesciences' report on Form 10-Q for the quarterly period ended June 30, 2015)
|
|
Exhibit No.
|
Exhibit No.
|
|
|
*10.13
|
|
Edwards Lifesciences Corporation Long-Term Stock Program Global Restricted Stock Unit Award Agreement for awards granted beginning May 2015 (incorporated by reference to Exhibit 10.4 in Edwards Lifesciences' report on Form 10-Q for the quarterly period ended June 30, 2015)
|
|
*10.14
|
|
Edwards Lifesciences Corporation Form of Performance-Based Restricted Stock Unit Statement and related Long-Term Stock Program Global Performance-Based Restricted Stock Unit Award Agreement for awards granted beginning May 2015 (incorporated by reference to Exhibit 10.4 in Edwards Lifesciences' report on Form 10-Q for the quarterly period ended June 30, 2015)
|
|
*10.15
|
|
Edwards Lifesciences Corporation Nonemployee Directors Stock Incentive Program, as amended and restated as of May 14, 2013 (incorporated by reference to Exhibit 10.1 in Edwards Lifesciences' report on Form 10-Q for the quarterly period ended June 30, 2013)
|
|
*10.16
|
|
Edwards Lifesciences Corporation Form of Participant Stock Option Statement and related Nonemployee Directors Stock Incentive Program Nonqualified Stock Option Award Agreement (incorporated by reference to Exhibit 10.2 in Edwards Lifesciences' report on Form 10-Q for the quarterly period ended June 30, 2013)
|
|
*10.17
|
|
Edwards Lifesciences Corporation Form of Nonemployee Directors Stock Incentive Program Restricted Stock Units Agreement (incorporated by reference to Exhibit 10.4 in Edwards Lifesciences' report on Form 10-Q for the quarterly period ended March 31, 2011)
|
|
*10.18
|
|
Edwards Lifesciences Corporation Form of Nonemployee Directors Stock Incentive Program Restricted Stock Agreement (incorporated by reference to Exhibit 10.5 in Edwards Lifesciences' report on Form 10-Q for the quarterly period ended March 31, 2011)
|
|
*10.19
|
|
Edwards Lifesciences Corporation Severance Pay Plan, restated effective January 1, 2013 (incorporated by reference to Exhibit 10.1 in Edwards Lifesciences' report on Form 10-Q for the quarterly period ended March 31, 2013)
|
|
*10.20
|
|
Edwards Lifesciences Corporation Executive Deferred Compensation Plan, as amended and restated effective November 9, 2011 (incorporated by reference to Exhibit 10.7 in Edwards Lifesciences' report on Form 10-K for the fiscal year ended December 31, 2011)
|
|
*10.21
|
|
Edwards Lifesciences Corporation of Puerto Rico Savings and Investment Plan, as amended and restated January 1, 2011 (incorporated by reference to Exhibit 10.17 in Edwards Lifesciences' report on Form 10-K for the fiscal year ended December 31, 2012)
|
|
*10.22
|
|
Edwards Lifesciences Corporation 401(k) Savings and Investment Plan, as amended and restated January 1, 2009 (incorporated by reference to Exhibit 10.18 in Edwards Lifesciences' report on Form 10-K for the fiscal year ended December 31, 2012)
|
|
*10.23
|
|
Amendment #1 to the Edwards Lifesciences Corporation 401(k) Savings and Investment Plan, dated April 1, 2011 (incorporated by reference to Exhibit 10.19 in Edwards Lifesciences' report on Form 10-K for the fiscal year ended December 31, 2012)
|
|
*10.24
|
|
Amendment #2 to the Edwards Lifesciences Corporation 401(k) Savings and Investment Plan, dated September 13, 2011 (incorporated by reference to Exhibit 10.20 in Edwards Lifesciences' report on Form 10-K for the fiscal year ended December 31, 2012)
|
|
*10.25
|
|
Amendment #3 to the Edwards Lifesciences Corporation 401(k) Savings and Investment Plan, dated October 21, 2011 (incorporated by reference to Exhibit 10.21 in Edwards Lifesciences' report on Form 10-K for the fiscal year ended December 31, 2012)
|
|
*10.26
|
|
Amendment #4 to the Edwards Lifesciences Corporation 401(k) Savings and Investment Plan, dated November 12, 2014 (incorporated by reference to Exhibit 10.23 in Edwards Lifesciences’ report on Form 10-K for the fiscal year ended December 31, 2015)
|
|
*10.27
|
|
Amendment #5 to the Edwards Lifesciences Corporation 401(k) Savings and Investment Plan, dated September 1, 2015 (incorporated by reference to Exhibit 10.1 in Edwards Lifesciences’ report on Form 10-Q for the quarterly period ended September 30, 2015)
|
|
*10.28
|
|
Edwards Lifesciences Corporation 2001 Employee Stock Purchase Plan for United States Employees, as amended and restated November 10, 2009 (incorporated by reference to Appendix B in Edwards Lifesciences' Definitive Proxy Statement filed on March 29, 2013)
|
|
*10.29
|
|
Edwards Lifesciences Corporation 2001 Employee Stock Purchase Plan for International Employees, as amended and restated February 20, 2014 (incorporated by reference to Appendix B in Edwards Lifesciences' Definitive Proxy Statement filed on March 28, 2014)
|
|
*10.30
|
|
Edwards Lifesciences Corporation 2010 Edwards Incentive Plan (incorporated by reference to Appendix C in Edwards Lifesciences' Definitive Proxy Statement filed on March 31, 2010)
|
|
*10.31
|
|
Edwards Lifesciences Corporation Officer Perquisite Program Guidelines, as of February 20, 2013 (incorporated by reference to Exhibit 10.25 in Edwards Lifesciences' report on Form 10-K for the fiscal year ended December 31, 2012)
|
|
*10.32
|
|
Edwards Lifesciences Corporation Form of Indemnification Agreement (incorporated by reference to Exhibit 10.20 in Edwards Lifesciences' report on Form 10-K for the fiscal year ended December 31, 2011)
|
|
Exhibit No.
|
Exhibit No.
|
|
|
12.1
|
|
Ratio of Earnings to Fixed Charges
|
|
21.1
|
|
Subsidiaries of Edwards Lifesciences Corporation
|
|
23
|
|
Consent of Independent Registered Public Accounting Firm
|
|
31.1
|
|
Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
31.2
|
|
Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
32
|
|
Certification Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
|
101
|
|
The following financial statements from Edwards Lifesciences' Annual Report on Form 10-K for the year ended December 31, 2015, formatted in XBRL (eXtensible Business Reporting Language): (i) the Consolidated Balance Sheets, (ii) the Consolidated Statements of Operations, (iii) the Consolidated Statements of Comprehensive Income, (iv) the Consolidated Statements of Cash Flows, (v) the Consolidated Statements of Stockholders' Equity and (vi) Notes to Consolidated Financial Statements.
|