|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FORM 10-K
|
|
ý
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
|
|
EXELIXIS, INC.
(Exact name of registrant as specified in its charter)
|
|
Delaware
|
04-3257395
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification Number)
|
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|
Common Stock $.001 Par Value per Share
|
The Nasdaq Stock Market LLC
|
|
|
|
Page
|
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
Item 15.
|
||
|
Item 16.
|
||
|
ITEM 1.
|
BUSINESS
|
|
•
|
CABOMETYX (cabozantinib)
was approved by the U.S. Food and Drug Administration, or FDA, on April 25, 2016, for the treatment of patients with advanced renal cell carcinoma, or RCC, who have received prior anti-angiogenic therapy. The European Commission, or EC, approved CABOMETYX on September 9, 2016 similarly for the treatment of advanced RCC in adults following prior vascular endothelial growth factor, or VEGF, targeted therapy. Outside the U.S. and Japan, CABOMETYX is being marketed by our collaboration partner Ipsen Pharma SAS, or Ipsen. Should CABOMETYX be approved in Japan, it will be marketed by our collaboration partner Takeda Pharmaceutical Company Limited, or Takeda. In 2016, we generated
$93.5 million
in net product revenue from sales of CABOMETYX in the United States.
|
|
•
|
COMETRIQ (cabozantinib)
,
our first marketed product, was approved by the FDA on November 29, 2012 for the treatment of patients with progressive, metastatic medullary thyroid carcinoma, or MTC. In March 2014, the EC granted COMETRIQ a similar, conditional marketing authorization for the treatment of adult patients with progressive, unresectable locally advanced or metastatic MTC. COMETRIQ is now commercialized in the European Union by Ipsen. In 2016, we generated
$39.4 million
in net product revenue from sales of COMETRIQ in the United States and received
$2.5 million
in product revenue from sales of COMETRIQ by our former distribution partner, Swedish Orphan Biovitrum, or Sobi. Cabozantinib has shown clinical anti-tumor activity in more than 20 forms of
|
|
•
|
Cotellic
(cobimetinib)
was approved by the FDA on November 10, 2015, in combination with vemurafenib for the treatment of patients with BRAF V600E or V600K mutation-positive advanced melanoma in the United States. It has also been approved in combination with vemurafenib in multiple other territories including the European Union, Switzerland, Canada, Australia and Brazil. In 2016, we recognized
$2.8 million
in collaboration revenue as a result of royalties on ex-U.S. sales of Cotellic and beginning in the fourth quarter of 2016, we also recognized a small net profit for our share of U.S. activities under the collaboration agreement. Genentech has an extensive clinical development program for this compound. For additional information on the cobimetinib development program, see “Cobimetinib Development Program
.
”
|
|
Indication
|
Combination Regimen
|
Status Update
|
|
Progressive, Metastatic Medullary Thyroid Cancer (MTC)
|
||
|
Approved in US and EU
|
||
|
Post-marketing study (EXAMINER)
|
||
|
Renal Cell Carcinoma (RCC)
|
||
|
Second-line
|
Approved in US and EU
|
|
|
First-line (intermediate- or poor-risk classification)
|
Preparing to file sNDA in 2017 based on results from CABOSUN† trial
|
|
|
First-line
|
+ nivolumab +/- ipilimumab
|
Phase 3 pivotal trial expected to begin in 2017, co-sponsored with Bristol-Myers Squibb
|
|
Hepatocellular Carcinoma
|
||
|
Second-line
|
Phase 3 (CELESTIAL), data anticipated in 2017
|
|
|
Non-Small Cell Lung Cancer
|
||
|
EGFR wild-type
|
Phase 2†
|
|
|
Molecular alterations in RET, ROS1, MET, AXL, or NTRK1
|
Phase 2*
|
|
|
Genitourinary Tumors, including Bladder and Urothelial Cancers
|
||
|
Genitourinary tumors
|
+ nivolumab +/- ipilimumab
|
Phase 1†
|
|
Advanced solid tumors
|
+ atezolizumab
|
Phase 1B* trial to begin in 2017, planned cohorts in RCC and urothelial carcinoma
|
|
Signal Search Opportunities to Inform Potential Development
|
||
|
Pancreatic neuroendocrine and carcinoid tumors
|
Phase 2*
|
|
|
Endometrial cancer
|
Phase 2†
|
|
|
Differentiated thyroid cancer
|
Phase 2*
|
|
|
Metastatic gastrointestinal stromal tumor
|
Phase 2 (CABOGIST)§
|
|
|
Breast cancer with brain metastases
|
+/- trastuzumab
|
Phase 2*
|
|
Metastatic, hormone-receptor-positive breast cancer
|
+ fulvestrant
|
Phase 2*
|
|
Metastatic, triple negative breast cancer
|
Phase 2*
|
|
|
Soft-tissue sarcomas
|
Phase 2†
|
|
|
High-grade uterine sarcomas
|
Phase 2§
|
|
|
Relapsed osteosarcoma or Ewing sarcoma
|
Phase 2†
|
|
|
Colorectal cancer
|
+/- panitumumab
|
Phase 1*
|
|
* Trial conducted through Exelixis' Investigator-Sponsored Trial program.
|
|
† Trial conducted through collaboration with NCI’s Cancer Therapy Evaluation Program.
|
|
§ Trial sponsored by the European Organization for Research and Treatment of Cancer (EORTC).
|
|
•
|
Phase 2 or phase 1/2 clinical trials to help prioritize future pivotal trials of cabozantinib in disease settings where there is substantial unmet medical need and in which cabozantinib has previously demonstrated clinical activity, consisting of randomized phase 2 clinical trials in ocular melanoma, prostate cancer and second/third line EGFR-wt NSCLC;
|
|
•
|
Additional phase 2 or phase 1/2 clinical trials to explore cabozantinib’s potential utility in other tumor types, including endometrial cancer, bladder cancer, sarcomas, NSCLC (EGFR-activating mutation positive), differentiated thyroid cancer, triple-negative breast cancer, hormone-receptor-positive breast cancer, cutaneous melanoma (molecularly selected patients), pancreatic neuroendocrine and carcinoid tumors. Positive results in these indications could lead to further study in randomized phase 2 or phase 3 clinical trials; and
|
|
•
|
Additional phase 1 clinical trials to further evaluate cabozantinib, consisting of a combination trial of cabozantinib and immuno-oncology agents (nivolumab with or without ipilumumab) in genitourinary tumors,
|
|
Indication
|
Combination Regimen
|
Status Update
|
|
Metastatic or Unresectable Locally Advanced Melanoma
|
||
|
BRAF mutation-positive
|
+ vemurafenib
|
Approved in US, EU and other territories
|
|
First-line BRAF mutation-positive
|
+ atezolizumab + vemurafenib
|
Phase 3 (IMspire150 TRILOGY)
|
|
First-line BRAF wild-type
|
+ atezolizumab
|
Phase 3 (IMspire170) planned for 2017
|
|
Colorectal Cancer
|
||
|
Third-line advanced or metastatic disease
|
+ atezolizumab
|
Phase 3 (IMblaze370)
|
|
Second/third-line metastatic disease
|
+ atezolizumab + bevacizumab
|
Phase 1
|
|
Breast Cancer
|
||
|
First-line metastatic triple negative disease
|
+ taxane +/- atezolizumab
|
Phase 1/2
(COLET)
|
|
•
|
The combination of cobimetinib and vemuarfenib in additional melanoma patient populations and settings;
|
|
•
|
A phase 2 trial of cobimetinib in combination with taxanes, with or without atezolizumab in first line triple negative breast cancer (COLET);
|
|
•
|
Phase 1 studies of cobimetinib in combination with atezolizumab in melanoma and NSCLC, in combination with vemurafenib and atezolizumab in melanoma, and in combination with venetoclax in relapsed or refractory acute myeloid leukemia; and
|
|
•
|
A phase 1b study evaluating the safety, tolerability and pharmacokinetics of cobimetinib in combination with atezolizumab and bevacizumab in patients with metastatic CRC.
|
|
•
|
preclinical laboratory and animal tests that must be conducted in accordance with Good Laboratory Practices;
|
|
•
|
submission of an IND, which must become effective before clinical trials may begin;
|
|
•
|
adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed drug candidate for its intended use;
|
|
•
|
submission of a New Drug Application, or NDA, to FDA for commercial marketing, or of a sNDA, for approval of a new indication if the product is already approved for another indication;
|
|
•
|
pre-approval inspection of manufacturing facilities and selected clinical investigators for their compliance with Good Manufacturing Practices, or GMP, and Good Clinical Practices, or GCP;
|
|
•
|
if FDA convenes an advisory committee, satisfactory completion of the advisory committee review; and
|
|
•
|
FDA approval of the NDA or sNDA.
|
|
•
|
Phase 1 - Studies, which involve the initial introduction of an IND into humans, are initially conducted in a limited number of subjects to test the product candidate for safety, dosage tolerance, absorption, metabolism, distribution and excretion in healthy humans or patients.
|
|
•
|
Phase 2 - Studies are conducted with groups of patients afflicted with a specified disease in order to provide enough data to evaluate the preliminary efficacy, optimal dosages and expanded evidence of safety. Multiple phase 2 clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more expensive phase 3 clinical trials. Phase 2 studies are typically well controlled, closely monitored, and conducted in a relatively small number of patients, usually involving no more than several hundred subjects. In some cases, a sponsor may decide to run what is referred to as a “phase 2b” evaluation, which is a second, confirmatory phase 2 trial that could, if positive, serve as a pivotal trial in the approval of a product candidate.
|
|
•
|
Phase 3 - When phase 2 evaluations demonstrate that a dosage range of the product is effective and has an acceptable safety profile, phase 3 trials are performed to gather the additional information about effectiveness and safety that is needed to evaluate the overall benefit-risk relationship of the drug and to provide an adequate basis for physician labeling. Phase 3 trials are undertaken in large patient populations to further evaluate dosage, to provide replicate statistically significant evidence of clinical efficacy and to further test for safety in an expanded patient population at multiple clinical trial sites.
|
|
•
|
require the FDA to establish a program to evaluate the potential use of real world evidence to help to support the approval of a new indication for an approved drug and to help to support or satisfy post-approval study requirements;
|
|
•
|
provide that the FDA may rely upon qualified data summaries to support the approval of a supplemental application with respect to a qualified indication for an already approved drug;
|
|
•
|
require FDA to issue guidance for purposes of assisting sponsors in incorporating complex adaptive and other novel trial designs into proposed clinical protocols and applications for new drugs; and
|
|
•
|
require FDA to establish a process for the qualification of drug development tools for use in supporting or obtaining FDA approval for or investigational use of a drug.
|
|
•
|
efficacy, safety and reliability of cabozantinib;
|
|
•
|
timing and scope of regulatory approval;
|
|
•
|
the speed at which we develop cabozantinib for the treatment of additional tumor types beyond its approved indications;
|
|
•
|
our ability to complete preclinical testing and clinical development and obtain regulatory approvals for cabozantinib;
|
|
•
|
our ability to manufacture and sell commercial quantities of cabozantinib product to the market;
|
|
•
|
our ability to successfully commercialize cabozantinib and secure coverage and adequate reimbursement in approved indications;
|
|
•
|
product acceptance by physicians and other health care providers;
|
|
•
|
the level of our collaboration partners’ investments in the resources necessary to successfully commercialize cabozantinib in territories where it is approved outside of the United States;
|
|
•
|
skills of our employees and our ability to recruit and retain skilled employees;
|
|
•
|
protection of our intellectual property; and
|
|
•
|
the availability of substantial capital resources to fund development and commercialization activities.
|
|
ITEM 1A.
|
RISK FACTORS
|
|
•
|
the effectiveness, or perceived effectiveness, of cabozantinib in comparison to competing products;
|
|
•
|
the safety of cabozantinib, including the existence of serious side effects of cabozantinib and their severity in comparison to those of any competing products;
|
|
•
|
cabozantinib’s relative convenience and ease of administration;
|
|
•
|
unexpected results connected with analysis of data from future or ongoing clinical trials;
|
|
•
|
the timing of cabozantinib label expansions for additional indications, if any, relative to competitive treatments;
|
|
•
|
the price of cabozantinib relative to competitive therapies and any new government initiatives affecting pharmaceutical pricing;
|
|
•
|
the strength of CABOMETYX sales efforts, marketing, medical affairs and distribution support;
|
|
•
|
the sufficiency of commercial and government insurance coverage and reimbursement; and
|
|
•
|
our ability to enforce our intellectual property rights with respect to cabozantinib.
|
|
•
|
the federal Anti-Kickback Statute, or AKS, which governs our business activities, including our marketing practices, educational programs, pricing policies, and relationships with healthcare providers or other entities. The AKS prohibits, among other things, persons and entities from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs. Remuneration is not defined in the AKS and has been broadly interpreted to include anything of value, including for example, gifts, discounts, coupons, the furnishing of supplies or equipment, credit arrangements, payments of cash, waivers of payments, ownership interests and providing anything at less than its fair market value. The AKS has been broadly interpreted to apply to manufacturer arrangements with prescribers, purchasers and formulary managers, among others;
|
|
•
|
the FDCA and its regulations which prohibit, among other things, the introduction or delivery for introduction into interstate commerce of any food, drug, device, or cosmetic that is adulterated or misbranded;
|
|
•
|
federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent, or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
|
|
•
|
federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
|
|
•
|
the Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, and their implementing regulations, which impose certain requirements relating to the privacy, security and transmission of individually identifiable health information;
|
|
•
|
state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payer, including commercial insurers, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts;
|
|
•
|
the Foreign Corrupt Practices Act, a U.S. law which regulates certain financial relationships with foreign government officials (which could include, for example, certain medical professionals);
|
|
•
|
federal and state consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers;
|
|
•
|
federal and state government price reporting laws that require us to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursement and/or discounts on our marketed drugs (participation in these programs and compliance with the applicable requirements may subject us to potentially significant discounts on our products, increased infrastructure costs, and could potentially affect our ability to offer certain marketplace discounts); and
|
|
•
|
federal and state financial transparency laws, which generally require certain types of expenditures in the United States to be tracked and reported (compliance with such requirements may require investment in infrastructure to ensure that tracking is performed properly, and some of these laws result in the public disclosure of various types of payments and relationships with healthcare providers and healthcare entities, which could potentially have a negative effect on our business and/or increase enforcement scrutiny of our activities).
|
|
•
|
lack of efficacy or harmful side effects;
|
|
•
|
negative or inconclusive clinical trial results may require us to conduct further testing or to abandon projects that we had expected to be promising;
|
|
•
|
our competitors may discover or commercialize other compounds or therapies that show significantly improved safety or efficacy compared to our product candidates;
|
|
•
|
our inability to identify and maintain a sufficient number of trial sites, many of which may already be engaged in other clinical trial programs;
|
|
•
|
patient registration or enrollment in our clinical testing may be lower than we anticipate, resulting in the delay or cancellation of clinical testing;
|
|
•
|
failure of our third-party contract research organization or investigators to satisfy their contractual obligations, including deviating from trial protocol; and
|
|
•
|
regulators or institutional review boards may withhold authorization to commence or conduct clinical trials of a product candidate, or delay, suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or their determination that participating patients are being exposed to unacceptable health risks.
|
|
•
|
the number of patients who ultimately participate in the clinical trial;
|
|
•
|
the duration of patient follow-up that is appropriate in view of the results or required by regulatory authorities;
|
|
•
|
the number of clinical sites included in the trials; and
|
|
•
|
the length of time required to enroll suitable patient subjects.
|
|
•
|
the commercial success of both CABOMETYX and COMETRIQ and the revenues we generate from those approved products;
|
|
•
|
costs associated with maintaining our expanded sales, marketing, medical affairs and distribution capabilities for CABOMETYX in advanced RCC and COMETRIQ in the approved MTC indications;
|
|
•
|
the achievement of stated regulatory and commercial milestones under our collaboration with Ipsen;
|
|
•
|
the commercial success of Cotellic and the calculation of our share of related profits and losses for the commercialization of Cotellic in the U.S. and royalties from Cotellic sales outside the U.S. under our collaboration with Genentech;
|
|
•
|
the outcome of our arbitration against Genentech in which we have asserted claims related to Genentech’s clinical development, pricing and commercialization of Cotellic, and cost and revenue allocations arising from Cotellic’s commercialization in the United States;
|
|
•
|
the potential regulatory approval of cabozantinib as a treatment for previously untreated advanced RCC and in other indications, both in the United States and abroad;
|
|
•
|
future clinical trial results, notably the results from CELESTIAL, our phase 3 pivotal trial in patients with advanced HCC;
|
|
•
|
our future investments in the expansion of our pipeline through drug discovery and corporate development activities;
|
|
•
|
our repayment and any potential mandatory prepayment of the Secured Convertible Notes due 2018, or the Deerfield Notes, (see “Note 7. Debt” to our “Notes to Consolidated Financial Statements” contained in Part II, Item 8 of this Annual Report on Form 10-K for a description of these notes), which mature on July 1, 2018, and which we intend to repay on or about July 1, 2017;
|
|
•
|
our ability to control costs;
|
|
•
|
our ability to remain in compliance with, or amend or cause to be waived, financial covenants contained in agreements with third parties;
|
|
•
|
the cost of clinical drug supply for our clinical trials;
|
|
•
|
trends and developments in the pricing of oncologic therapeutics in the United States and abroad, especially in the European Union;
|
|
•
|
scientific developments in the market for oncologic therapeutics and the timing of regulatory approvals for competing oncologic therapies; and
|
|
•
|
the filing, maintenance, prosecution, defense and enforcement of patent claims and other intellectual property rights.
|
|
•
|
increasing our vulnerability to adverse economic and industry conditions;
|
|
•
|
limiting our ability to obtain additional financing;
|
|
•
|
requiring the dedication of a substantial portion of our cash flow from operations to service our indebtedness, thereby reducing the amount of our cash flow available for other purposes, including clinical trials, research and development, capital expenditures, working capital and other general corporate purposes;
|
|
•
|
limiting our flexibility in planning for, or reacting to, changes in our business;
|
|
•
|
dilution experienced by our existing stockholders as a result of a conversion of the Deerfield Notes, at our discretion, into shares of common stock; and
|
|
•
|
placing us at a possible competitive disadvantage with less leveraged competitors and competitors that may have better access to capital resources.
|
|
•
|
we are not able to control the amount and timing of resources that our collaborators or potential future collaborators will devote to the development or commercialization of drug candidates or to their marketing and distribution;
|
|
•
|
we are not able to control the U.S. commercial resourcing decisions made and resulting costs incurred by Genentech for cobimetinib, which reasonable costs we are obligated to share, in part, under our collaboration agreement with Genentech;
|
|
•
|
collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a drug candidate, repeat or conduct new clinical trials or require a new formulation of a drug candidate for clinical testing;
|
|
•
|
disputes may arise between us and our collaborators that result in the delay or termination of the research, development or commercialization of our drug candidates, or that diminish or delay receipt of the economic benefits we are entitled to receive under the collaboration, or that result in costly litigation or arbitration that diverts management’s attention and resources;
|
|
•
|
collaborators may experience financial difficulties;
|
|
•
|
collaborators may not be successful in their efforts to obtain regulatory approvals in a timely manner, or at all;
|
|
•
|
collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our proprietary information or expose us to potential litigation;
|
|
•
|
collaborators may not comply with applicable healthcare regulatory laws;
|
|
•
|
business combinations or significant changes in a collaborator’s business strategy may adversely affect a collaborator’s willingness or ability to complete its obligations under any arrangement;
|
|
•
|
a collaborator could independently move forward with a competing drug candidate developed either independently or in collaboration with others, including our competitors;
|
|
•
|
we may be precluded from entering into additional collaboration arrangements with other parties in an area or field of exclusivity;
|
|
•
|
future collaborators may require us to relinquish some important rights, such as marketing and distribution rights; and
|
|
•
|
collaborations may be terminated or allowed to expire, which would delay, and may increase the cost of development of our drug candidates.
|
|
•
|
the commercial success of both CABOMETYX and COMETRIQ and the revenues we generate from those approved products;
|
|
•
|
customer ordering patterns for CABOMETYX and COMETRIQ, which may vary significantly from period to period;
|
|
•
|
the overall level of demand for CABOMETYX and COMETRIQ, including the impact of any competitive products and the duration of therapy for patients receiving CABOMETYX or COMETRIQ;
|
|
•
|
costs associated with maintaining our expanded sales, marketing, medical affairs and distribution capabilities for CABOMETYX, COMETRIQ and Cotellic;
|
|
•
|
our ability to obtain regulatory approval for cabozantinib as a treatment of first-line advanced RCC;
|
|
•
|
the achievement of stated regulatory and commercial milestones, under our collaboration with Ipsen;
|
|
•
|
the outcome of our arbitration against Genentech in which we have asserted claims related to Genentech’s clinical development, pricing and commercialization of Cotellic, and cost and revenue allocations arising from Cotellic’s commercialization in the United States;
|
|
•
|
the progress and scope of other development and commercialization activities for cabozantinib and our other compounds;
|
|
•
|
future clinical trial results, notably the results from CELESTIAL, our phase 3 pivotal trial in patients with advanced HCC;
|
|
•
|
our future investments in the expansion of our pipeline through drug discovery and corporate development activities;
|
|
•
|
the inability to obtain adequate product supply for any approved drug product or inability to do so at acceptable prices;
|
|
•
|
recognition of upfront licensing or other fees or revenues;
|
|
•
|
payments of non-refundable upfront or licensing fees, or payment for cost-sharing expenses, to third parties;
|
|
•
|
the introduction of new technologies or products by our competitors;
|
|
•
|
the timing and willingness of collaborators to further develop or, if approved, commercialize our product candidates out-licensed to them;
|
|
•
|
the termination or non-renewal of existing collaborations or third party vendor relationships;
|
|
•
|
regulatory actions with respect to our product candidates and any approved products or our competitors’ products;
|
|
•
|
disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies;
|
|
•
|
the timing and amount of expenses incurred for clinical development and manufacturing of cabozantinib;
|
|
•
|
adjustments to expenses accrued in prior periods based on management’s estimates after the actual level of activity relating to such expenses becomes more certain;
|
|
•
|
the impairment of acquired goodwill and other assets;
|
|
•
|
additions and departures of key personnel;
|
|
•
|
general and industry-specific economic conditions that may affect our or our collaborators’ research and development expenditures; and
|
|
•
|
other factors described in this “Risk Factors” section.
|
|
•
|
adverse results or delays in our or our collaborators’ clinical trials;
|
|
•
|
the announcement of FDA approval or non-approval, or delays in the FDA review process, of cabozantinib or our collaborators’ product candidates or those of our competitors or actions taken by regulatory agencies with respect to our, our collaborators’ or our competitors’ clinical trials;
|
|
•
|
the commercial success of both CABOMETYX and COMETRIQ and the revenues we generate from those approved products;
|
|
•
|
the timing of achievement of our clinical, regulatory, partnering and other milestones, such as the commencement of clinical development, the completion of a clinical trial, the filing for regulatory approval or the establishment of collaborative arrangements for cabozantinib or any of our other programs or compounds;
|
|
•
|
actions taken by regulatory agencies with respect to cabozantinib or our clinical trials for cabozantinib;
|
|
•
|
the announcement of new products by our competitors;
|
|
•
|
quarterly variations in our or our competitors’ results of operations;
|
|
•
|
developments in our relationships with our collaborators, including the termination or modification of our agreements;
|
|
•
|
the announcement of an in-licensed product candidate or strategic acquisition;
|
|
•
|
conflicts or litigation with our collaborators, including the outcome of our arbitration with Genentech regarding Cotellic;
|
|
•
|
litigation, including intellectual property infringement and product liability lawsuits, involving us;
|
|
•
|
failure to achieve operating results projected by securities analysts;
|
|
•
|
changes in earnings estimates or recommendations by securities analysts;
|
|
•
|
the satisfaction of outstanding debt obligations or entry into new financing arrangements;
|
|
•
|
developments in the biotechnology, biopharmaceutical or pharmaceutical industry;
|
|
•
|
sales of large blocks of our common stock or sales of our common stock by our executive officers, directors and significant stockholders;
|
|
•
|
departures of key personnel or board members;
|
|
•
|
FDA or international regulatory actions;
|
|
•
|
third-party coverage and reimbursement policies;
|
|
•
|
disposition of any of our technologies or compounds; and
|
|
•
|
general market, economic and political conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors.
|
|
•
|
a classified Board of Directors;
|
|
•
|
a prohibition on actions by our stockholders by written consent;
|
|
•
|
the inability of our stockholders to call special meetings of stockholders;
|
|
•
|
the ability of our Board of Directors to issue preferred stock without stockholder approval, which could be used to institute a “poison pill” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our Board of Directors;
|
|
•
|
limitations on the removal of directors; and
|
|
•
|
advance notice requirements for director nominations and stockholder proposals.
|
|
ITEM 1B.
|
UNRESOLVED STAFF COMMENTS
|
|
ITEM 2.
|
PROPERTIES
|
|
•
|
The first two leases cover two buildings for a total of 130,964 square feet and expire in May 2017. We have subleased a total of 93,243 square feet of portions of these buildings to five different subtenants. The terms of the subleases expire at the end of our lease terms.
|
|
•
|
The third lease covers two buildings for a total of 116,063 square feet and expires in June 2018. We have one five-year options to extend the term of the lease prior to expiration.
|
|
ITEM 3.
|
LEGAL PROCEEDINGS
|
|
ITEM 4.
|
MINE SAFETY DISCLOSURES
|
|
ITEM 5.
|
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
|
Common Stock Price
|
||||||
|
|
High
|
Low
|
|||||
|
Year ended December 30, 2016:
|
|||||||
|
Quarter ended April 1, 2016
|
$
|
5.85
|
|
$
|
3.55
|
|
|
|
Quarter ended July 1, 2016
|
$
|
8.19
|
|
$
|
4.11
|
|
|
|
Quarter ended September 30, 2016
|
$
|
15.58
|
|
$
|
7.93
|
|
|
|
Quarter ended December 30, 2016
|
$
|
18.29
|
|
$
|
10.04
|
|
|
|
Year ended January 1, 2016:
|
|||||||
|
Quarter ended April 3, 2015
|
$
|
3.16
|
|
$
|
1.54
|
|
|
|
Quarter ended July 3, 2015
|
$
|
4.18
|
|
$
|
2.51
|
|
|
|
Quarter ended October 2, 2015
|
$
|
6.81
|
|
$
|
3.31
|
|
|
|
Quarter ended January 1, 2016
|
$
|
6.42
|
|
$
|
4.70
|
|
|
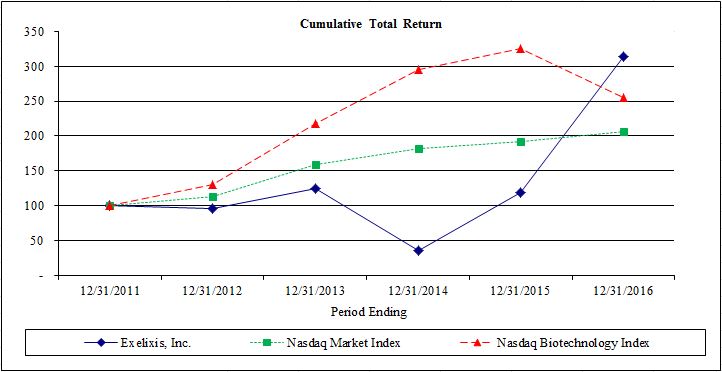
|
December 31,
|
|||||||||||||||||
|
2011
|
2012
|
2013
|
2014
|
2015
|
2016
|
||||||||||||
|
Exelixis, Inc.
|
100
|
|
95
|
|
125
|
|
35
|
|
119
|
|
315
|
|
|||||
|
NASDAQ Market Index
|
100
|
|
114
|
|
160
|
|
181
|
|
192
|
|
207
|
|
|||||
|
NASDAQ Biotechnology Index
|
100
|
|
130
|
|
218
|
|
295
|
|
326
|
|
256
|
|
|||||
|
ITEM 6.
|
SELECTED FINANCIAL DATA
|
|
|
Year Ended December 31,
|
||||||||||||||||||
|
|
2016
|
2015
|
2014
|
2013
|
2012
|
||||||||||||||
|
|
(In thousands, except per share data)
|
||||||||||||||||||
|
Consolidated Statements of Operations Data:
|
|||||||||||||||||||
|
Revenues
|
$
|
191,454
|
|
$
|
37,172
|
|
$
|
25,111
|
|
$
|
31,338
|
|
$
|
47,450
|
|
||||
|
Operating expenses:
|
|||||||||||||||||||
|
Cost of goods sold
|
6,552
|
|
3,895
|
|
2,043
|
|
1,118
|
|
—
|
|
|||||||||
|
Research and development
|
95,967
|
|
96,351
|
|
189,101
|
|
178,763
|
|
128,878
|
|
|||||||||
|
Selling, general and administrative
|
116,145
|
|
57,305
|
|
50,829
|
|
50,958
|
|
31,837
|
|
|||||||||
|
Restructuring charge
|
914
|
|
1,042
|
|
7,596
|
|
1,231
|
|
9,171
|
|
|||||||||
|
Total operating expenses
|
219,578
|
|
158,593
|
|
249,569
|
|
232,070
|
|
169,886
|
|
|||||||||
|
Loss from operations
|
(28,124
|
)
|
(121,421
|
)
|
(224,458
|
)
|
(200,732
|
)
|
(122,436
|
)
|
|||||||||
|
Other expense, net
(1)
|
(42,098
|
)
|
(40,268
|
)
|
(37,021
|
)
|
(37,556
|
)
|
(22,792
|
)
|
|||||||||
|
Loss before taxes
|
(70,222
|
)
|
(161,689
|
)
|
(261,479
|
)
|
(238,288
|
)
|
(145,228
|
)
|
|||||||||
|
Income tax provision (benefit)
|
—
|
|
55
|
|
(182
|
)
|
(96
|
)
|
107
|
|
|||||||||
|
Net loss
(1)
|
$
|
(70,222
|
)
|
$
|
(161,744
|
)
|
$
|
(261,297
|
)
|
$
|
(238,192
|
)
|
$
|
(145,335
|
)
|
||||
|
Net loss per share, basic and diluted
(1)
|
$
|
(0.28
|
)
|
$
|
(0.77
|
)
|
$
|
(1.34
|
)
|
$
|
(1.29
|
)
|
$
|
(0.91
|
)
|
||||
|
Shares used in computing basic and diluted loss per share amounts
|
250,531
|
|
209,227
|
|
194,299
|
|
184,062
|
|
160,138
|
|
|||||||||
|
|
December 31,
|
||||||||||||||||||
|
|
2016
|
2015
|
2014
|
2013
|
2012
|
||||||||||||||
|
|
(In thousands)
|
||||||||||||||||||
|
Consolidated Balance Sheet Data:
|
|||||||||||||||||||
|
Cash and investments
|
$
|
479,554
|
|
$
|
253,310
|
|
$
|
242,760
|
|
$
|
415,862
|
|
$
|
633,961
|
|
||||
|
Working capital (deficit)
|
$
|
200,215
|
|
$
|
126,414
|
|
$
|
(3,188
|
)
|
$
|
178,756
|
|
$
|
350,837
|
|
||||
|
Total assets
|
$
|
597,541
|
|
$
|
332,342
|
|
$
|
323,269
|
|
$
|
497,951
|
|
$
|
714,142
|
|
||||
|
Long-term obligations
(1)
|
$
|
237,635
|
|
$
|
420,897
|
|
$
|
312,163
|
|
$
|
395,599
|
|
$
|
394,311
|
|
||||
|
Accumulated deficit
(1)
|
$
|
(1,983,147
|
)
|
$
|
(1,912,925
|
)
|
$
|
(1,751,181
|
)
|
$
|
(1,489,884
|
)
|
$
|
(1,251,692
|
)
|
||||
|
Total stockholders’ equity (deficit)
(1)
|
$
|
89,318
|
|
$
|
(140,806
|
)
|
$
|
(159,324
|
)
|
$
|
14,498
|
|
$
|
238,127
|
|
||||
|
(1)
|
Prior periods have been revised to reflect the correction of the accounting for non-cash interest expense associated with the 2019 Notes. See “Note 1. Organization and Summary of Significant Accounting Policies - Correction of an Immaterial Error” in the “Notes to the Consolidated Financial Statements” for additional information on the correction.
|
|
ITEM 7.
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
|
•
|
In February 2016, we entered into a collaboration and license agreement with Ipsen for the commercialization and further development of cabozantinib. Pursuant to the terms of the collaboration agreement, Ipsen received exclusive commercialization rights for current and potential future cabozantinib indications outside of the United States, Canada and Japan. The collaboration agreement was subsequently amended in December 2016 to include commercialization rights in Canada.
|
|
•
|
In April 2016, based on results of our phase 3 pivotal trial METEOR, which met its primary endpoint of improving PFS, as well as its secondary endpoints of improving OS and ORR, the FDA approved CABOMETYX for the treatment of patients with advanced RCC who have received prior anti-angiogenic therapy.
|
|
•
|
In May 2016, we announced that CABOSUN met its primary endpoint, demonstrating a statistically significant and clinically meaningful improvement in PFS compared with sunitinib in patients with advanced intermediate- or poor-risk RCC. Based on these results, we are working towards the submission of a sNDA in the third quarter of 2017 for cabozantinib as a treatment for first-line advanced RCC.
|
|
•
|
In June 2016, we presented results from our phase 3 pivotal trial METEOR at the ASCO 2016 Annual Meeting, showing that CABOMETYX demonstrated a statistically significant and clinically meaningful increase in OS. Compared with everolimus, CABOMETYX was associated with a 34% reduction in the rate of death and median OS was 21.4 months for patients receiving CABOMETYX versus 16.5 months for those receiving everolimus (HR=0.66, 95% CI 0.53-0.83, P=0.0003).
|
|
•
|
In June 2016, our collaboration partner Genentech announced preliminary results from a phase 1b trial evaluating the safety and clinical activity of the combination of cobimetinib with ateolizumab in patients with metastatic CRC, which included 23 patients with advanced CRC (22 with mutant KRAS and one with wild-type KRAS). The ORR for the combination was 17%, including four confirmed PRs; additionally five patients achieved SD. Responses were seen in tumors with the microsatellite stable, or MSS, phenotype, which comprises 95% of CRC. MSS CRC has historically been refractory to immuno-oncology agents. The median duration of response was not yet reached, with a range of 5.4 months to more than 11.1 months. No dose-limiting toxicities were observed.
|
|
•
|
In September 2016, the EC approved CABOMETYX for the treatment of adult patients with advanced RCC following prior VEGF-targeted therapy and in December 2016, Ipsen recorded its first commercial sales in Europe.
|
|
•
|
In October 2016, we announced positive results from the NCI-CTEP-sponsored phase 1 trial of cabozantinib in combination with nivolumab in patients with previously treated genitourinary tumors.
|
|
•
|
In November 2016, we announced Genentech’s efforts to advance the development program for cobimetinib, through the initiation and announcement of multiple phase 3 pivotal trials exploring the combination of cobimetinib with other targeted and immuno-oncology agents for the treatment of melanoma and CRC.
|
|
•
|
In January 2017, we entered into a collaboration and license agreement with Takeda for the commercialization and further clinical development of cabozantinib in Japan.
|
|
•
|
Our total net product revenue increased by
$101.2 million
, or
296%
, in 2016 compared to 2015, primarily due to the commercial launch of CABOMETYX as a treatment for patients with advanced RCC in April 2016 and, to a lesser extent, an increase in COMETRIQ product sales.
|
|
•
|
Our collaboration revenue increased by
$53.1 million
in 2016 compared to 2015, primarily due to upfront payments and milestones received as a result of entering into our collaboration and license agreement with Ipsen.
|
|
•
|
Cash and investments increased to
$479.6 million
at
December 31, 2016
as compared to
$253.3 million
at
December 31, 2015
.
|
|
•
|
Between August and November 2016, we retired all $287.5 million of the 2019 Notes through privately negotiated exchange transactions and redemption procedures provided for by the 2019 Notes. For additional information on the retirement of the 2019 Notes, see “Note 7. Debt,” to our “Notes to Consolidated Financial Statements contained in Part II, Item 8 of this Annual Report on Form 10-K.
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Gross product revenues
|
$
|
151,499
|
|
$
|
36,650
|
|
$
|
28,963
|
|
||
|
Discounts and allowances
|
(16,124
|
)
|
(2,492
|
)
|
(3,852
|
)
|
|||||
|
Net product revenues
|
135,375
|
|
34,158
|
|
25,111
|
|
|||||
|
Royalty and product supply revenues, net
|
2,795
|
|
14
|
|
—
|
|
|||||
|
License revenues
(1)
|
13,284
|
|
—
|
|
—
|
|
|||||
|
Contract revenues
(2)
|
40,000
|
|
3,000
|
|
—
|
|
|||||
|
Collaboration revenues
|
56,079
|
|
3,014
|
|
—
|
|
|||||
|
Total revenues
|
$
|
191,454
|
|
$
|
37,172
|
|
$
|
25,111
|
|
||
|
Dollar change
|
$
|
154,282
|
|
$
|
12,061
|
|
|||||
|
Percentage change
|
415
|
%
|
48
|
%
|
|||||||
|
(1)
|
Includes amortization of upfront payments.
|
|
(2)
|
Includes milestone payments.
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
CABOMETYX
|
$
|
93,481
|
|
$
|
—
|
|
$
|
—
|
|
||
|
COMETRIQ
|
41,894
|
|
34,158
|
|
25,111
|
|
|||||
|
Net product revenues
|
$
|
135,375
|
|
$
|
34,158
|
|
$
|
25,111
|
|
||
|
Dollar change
|
$
|
101,217
|
|
$
|
9,047
|
|
|||||
|
Percentage change
|
296
|
%
|
36
|
%
|
|||||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Diplomat Specialty Pharmacy
|
$
|
63,826
|
|
$
|
30,856
|
|
$
|
24,832
|
|
||
|
Ipsen
|
33,252
|
|
—
|
|
—
|
|
|||||
|
Others, individually less than 10% of total revenues for all periods presented
|
94,376
|
|
6,316
|
|
279
|
|
|||||
|
Total revenues
|
$
|
191,454
|
|
$
|
37,172
|
|
$
|
25,111
|
|
||
|
Customer credits and co-pay assistance
|
Rebates
|
Chargebacks
|
Returns
|
Total
|
|||||||||||||||
|
Balance at December 31, 2014
|
$
|
2,320
|
|
$
|
484
|
|
$
|
(10
|
)
|
$
|
—
|
|
$
|
2,794
|
|
||||
|
Provision related to sales made in:
|
—
|
|
|||||||||||||||||
|
Current period
|
1,014
|
|
1,539
|
|
69
|
|
38
|
|
2,660
|
|
|||||||||
|
Prior periods
|
—
|
|
(197
|
)
|
10
|
|
—
|
|
(187
|
)
|
|||||||||
|
Payments
|
(3,003
|
)
|
(935
|
)
|
(30
|
)
|
—
|
|
(3,968
|
)
|
|||||||||
|
Balance at December 31, 2015
|
331
|
|
891
|
|
39
|
|
38
|
|
1,299
|
|
|||||||||
|
Provision related to sales made in:
|
—
|
|
|||||||||||||||||
|
Current period
|
5,721
|
|
5,105
|
|
5,297
|
|
359
|
|
16,482
|
|
|||||||||
|
Prior periods
|
2
|
|
(313
|
)
|
(39
|
)
|
(8
|
)
|
(358
|
)
|
|||||||||
|
Payments
|
(4,779
|
)
|
(3,056
|
)
|
(3,976
|
)
|
(38
|
)
|
(11,849
|
)
|
|||||||||
|
Balance at December 31, 2016
|
$
|
1,275
|
|
$
|
2,627
|
|
$
|
1,321
|
|
$
|
351
|
|
$
|
5,574
|
|
||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Cost of goods sold
|
$
|
6,552
|
|
$
|
3,895
|
|
$
|
2,043
|
|
||
|
Gross margin
|
95
|
%
|
89
|
%
|
92
|
%
|
|||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Research and development expenses
|
$
|
95,967
|
|
$
|
96,351
|
|
$
|
189,101
|
|
||
|
Dollar change
|
$
|
(384
|
)
|
$
|
(92,750
|
)
|
|||||
|
Percentage change
|
less than 1%
|
|
(49
|
)%
|
|||||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Selling, general and administrative expenses
|
$
|
116,145
|
|
$
|
57,305
|
|
$
|
50,829
|
|
||
|
Dollar change
|
$
|
58,840
|
|
$
|
6,476
|
|
|||||
|
Percentage change
|
103
|
%
|
13
|
%
|
|||||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Interest income and other, net
|
$
|
4,863
|
|
$
|
412
|
|
$
|
4,341
|
|
||
|
Interest expense
|
(33,060
|
)
|
(40,680
|
)
|
(41,362
|
)
|
|||||
|
Loss on extinguishment of debt
|
(13,901
|
)
|
—
|
|
—
|
|
|||||
|
Total other expense, net
|
$
|
(42,098
|
)
|
$
|
(40,268
|
)
|
$
|
(37,021
|
)
|
||
|
Dollar change
|
$
|
(1,830
|
)
|
$
|
(3,247
|
)
|
|||||
|
Percentage change
|
5
|
%
|
9
|
%
|
|||||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Net loss
|
$
|
(70,222
|
)
|
$
|
(161,744
|
)
|
$
|
(261,297
|
)
|
||
|
Adjustments to reconcile net loss to net cash used in operating activities
|
49,251
|
|
46,004
|
|
36,169
|
|
|||||
|
Changes in operating assets and liabilities
|
227,267
|
|
(25,845
|
)
|
(10,277
|
)
|
|||||
|
Net cash provided by (used in) operating activities
|
206,296
|
|
(141,585
|
)
|
(235,405
|
)
|
|||||
|
Net cash (used in) provided by investing activities
|
(216,048
|
)
|
50,077
|
|
146,330
|
|
|||||
|
Net cash provided by financing activities
|
19,804
|
|
152,747
|
|
65,492
|
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
10,052
|
|
61,239
|
|
(23,583
|
)
|
|||||
|
Cash and cash equivalents at beginning of year
|
141,634
|
|
80,395
|
|
103,978
|
|
|||||
|
Cash and cash equivalents at end of year
|
$
|
151,686
|
|
$
|
141,634
|
|
$
|
80,395
|
|
||
|
|
Payments Due by Period
|
|||||||||||||||
|
Contractual Obligations
|
Total
|
Less than
1 year
|
1-3
Years
|
More than 3
years
|
||||||||||||
|
Deerfield notes
(1)
|
$
|
124,972
|
|
$
|
—
|
|
$
|
124,972
|
|
$
|
—
|
|
||||
|
Loans payable
(2)
|
80,000
|
|
80,000
|
|
—
|
|
—
|
|
||||||||
|
Operating leases
(3)
|
11,481
|
|
8,474
|
|
3,007
|
|
—
|
|
||||||||
|
Purchase obligations
(4)
|
1,112
|
|
1,112
|
|
—
|
|
—
|
|
||||||||
|
Total contractual cash obligations
|
$
|
217,565
|
|
$
|
89,586
|
|
$
|
127,979
|
|
$
|
—
|
|
||||
|
(1)
|
Due date is based on our contractual obligations under the Deerfield Notes. We intend to repay the Deerfield Notes on or about July 1, 2017 and as a result, we have classified the Deerfield Notes as a current liability as of December 31, 2016. See “Note 7. Debt” of the Notes to Consolidated Financial Statements regarding the terms of the Deerfield Notes.
|
|
(2)
|
Consists of our obligations under our loan from Silicon Valley Bank. See “Note 7. Debt” of the Notes to Consolidated Financial Statements regarding the terms of our loan from Silicon Valley Bank.
|
|
(3)
|
The operating lease payments do not include
$1.2 million
to be received in 2017 in connection with the subleases of our South San Francisco buildings.
|
|
(4)
|
At December 31, 2016, we had firm purchase commitments related to manufacturing and maintenance of inventory. These commitments include a portion of our 2017 contractual minimum purchase obligation. Our actual purchases are expected to significantly exceed these amounts.
|
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
|
ITEM 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
|
|
Page
|
|
|
December 31,
|
||||||
|
|
2016
|
2015
|
|||||
|
ASSETS
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
151,686
|
|
$
|
141,634
|
|
|
|
Short-term investments
|
268,117
|
|
25,426
|
|
|||
|
Trade and other receivables
|
42,246
|
|
5,183
|
|
|||
|
Inventory
|
3,338
|
|
2,616
|
|
|||
|
Prepaid expenses and other current assets
|
5,416
|
|
3,806
|
|
|||
|
Total current assets
|
470,803
|
|
178,665
|
|
|||
|
Long-term investments
|
55,601
|
|
83,600
|
|
|||
|
Long-term restricted cash and investments
|
4,150
|
|
2,650
|
|
|||
|
Property and equipment, net
|
2,071
|
|
1,434
|
|
|||
|
Goodwill
|
63,684
|
|
63,684
|
|
|||
|
Other long-term assets
|
1,232
|
|
2,309
|
|
|||
|
Total assets
|
$
|
597,541
|
|
$
|
332,342
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT)
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
6,565
|
|
$
|
6,401
|
|
|
|
Accrued compensation and benefits
|
20,334
|
|
3,629
|
|
|||
|
Accrued clinical trial liabilities
|
14,131
|
|
18,071
|
|
|||
|
Accrued collaboration liability
|
—
|
|
10,938
|
|
|||
|
Current portion of convertible notes
|
109,122
|
|
—
|
|
|||
|
Current portion of term loan payable
|
80,000
|
|
—
|
|
|||
|
Current portion of deferred revenue
|
19,665
|
|
—
|
|
|||
|
Other current liabilities
|
20,771
|
|
13,212
|
|
|||
|
Total current liabilities
|
270,588
|
|
52,251
|
|
|||
|
Long-term portion of convertible notes
|
—
|
|
337,937
|
|
|||
|
Long-term portion of term loan payable
|
—
|
|
80,000
|
|
|||
|
Long-term portion of deferred revenue
|
237,094
|
|
—
|
|
|||
|
Other long-term liabilities
|
541
|
|
2,960
|
|
|||
|
Total liabilities
|
508,223
|
|
473,148
|
|
|||
|
Commitments (Note 13)
|
|
|
|||||
|
Stockholders’ equity (deficit):
|
|||||||
|
Preferred stock, $0.001 par value, 10,000,000 shares authorized and no shares issued
|
—
|
|
—
|
|
|||
|
Common stock, $0.001 par value; 400,000,000 shares authorized; issued and
outstanding: 289,923,798 and 227,960,943 shares at December 31, 2016 and 2015,
respectively
|
290
|
|
228
|
|
|||
|
Additional paid-in capital
|
2,072,591
|
|
1,772,123
|
|
|||
|
Accumulated other comprehensive loss
|
(416
|
)
|
(232
|
)
|
|||
|
Accumulated deficit
|
(1,983,147
|
)
|
(1,912,925
|
)
|
|||
|
Total stockholders’ equity (deficit)
|
89,318
|
|
(140,806
|
)
|
|||
|
Total liabilities and stockholders’ equity (deficit)
|
$
|
597,541
|
|
$
|
332,342
|
|
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Revenues:
|
|||||||||||
|
Net product revenues
|
$
|
135,375
|
|
$
|
34,158
|
|
$
|
25,111
|
|
||
|
Collaboration revenues
|
56,079
|
|
3,014
|
|
—
|
|
|||||
|
Total revenues
|
191,454
|
|
37,172
|
|
25,111
|
|
|||||
|
Operating expenses:
|
|||||||||||
|
Cost of goods sold
|
6,552
|
|
3,895
|
|
2,043
|
|
|||||
|
Research and development
|
95,967
|
|
96,351
|
|
189,101
|
|
|||||
|
Selling, general and administrative
|
116,145
|
|
57,305
|
|
50,829
|
|
|||||
|
Restructuring charges
|
914
|
|
1,042
|
|
7,596
|
|
|||||
|
Total operating expenses
|
219,578
|
|
158,593
|
|
249,569
|
|
|||||
|
Loss from operations
|
(28,124
|
)
|
(121,421
|
)
|
(224,458
|
)
|
|||||
|
Other expense, net:
|
|||||||||||
|
Interest income and other, net
|
4,863
|
|
412
|
|
4,341
|
|
|||||
|
Interest expense
|
(33,060
|
)
|
(40,680
|
)
|
(41,362
|
)
|
|||||
|
Loss on extinguishment of debt
|
(13,901
|
)
|
—
|
|
—
|
|
|||||
|
Total other expense, net
|
(42,098
|
)
|
(40,268
|
)
|
(37,021
|
)
|
|||||
|
Loss before income taxes
|
(70,222
|
)
|
(161,689
|
)
|
(261,479
|
)
|
|||||
|
Income tax provision (benefit)
|
—
|
|
55
|
|
(182
|
)
|
|||||
|
Net loss
|
$
|
(70,222
|
)
|
$
|
(161,744
|
)
|
$
|
(261,297
|
)
|
||
|
Net loss per share, basic and diluted
|
$
|
(0.28
|
)
|
$
|
(0.77
|
)
|
$
|
(1.34
|
)
|
||
|
Shares used in computing basic and diluted net loss per share
|
250,531
|
|
209,227
|
|
194,299
|
|
|||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Net loss
|
$
|
(70,222
|
)
|
$
|
(161,744
|
)
|
$
|
(261,297
|
)
|
||
|
Other comprehensive loss
(1)
|
(184
|
)
|
(111
|
)
|
(267
|
)
|
|||||
|
Comprehensive loss
|
$
|
(70,406
|
)
|
$
|
(161,855
|
)
|
$
|
(261,564
|
)
|
||
|
(1)
|
Other comprehensive loss consisted solely of unrealized losses, net on available-for-sale securities arising during the periods presented. There were
no
reclassification adjustments to net loss resulting from realized gains or losses on the sale of securities and there was
no
income tax expense related to other comprehensive loss during those years.
|
|
Common
Stock
Shares
|
Common
Stock
Amount
|
Additional
Paid-in
Capital
|
Accumulated
Other
Comprehensive
(Loss) Income
|
Accumulated
Deficit
|
Total
Stockholders’
Equity (Deficit)
|
|||||||||||||||||
|
Balance at December 31, 2013
|
184,533,651
|
|
$
|
184
|
|
$
|
1,504,052
|
|
$
|
146
|
|
$
|
(1,489,884
|
)
|
$
|
14,498
|
|
|||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(261,297
|
)
|
(261,297
|
)
|
||||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
(267
|
)
|
—
|
|
(267
|
)
|
||||||||||
|
Sale of shares of common stock, net
|
10,000,000
|
|
10
|
|
75,633
|
|
—
|
|
—
|
|
75,643
|
|
||||||||||
|
Issuance of common stock under stock plans
|
1,362,118
|
|
2
|
|
2,091
|
|
—
|
|
—
|
|
2,093
|
|
||||||||||
|
Stock-based compensation expense
|
—
|
|
—
|
|
10,006
|
|
—
|
|
—
|
|
10,006
|
|
||||||||||
|
Balance at December 31, 2014
|
195,895,769
|
|
196
|
|
1,591,782
|
|
(121
|
)
|
(1,751,181
|
)
|
(159,324
|
)
|
||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(161,744
|
)
|
(161,744
|
)
|
||||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
(111
|
)
|
—
|
|
(111
|
)
|
||||||||||
|
Sale of shares of common stock, net
|
28,750,000
|
|
29
|
|
145,620
|
|
—
|
|
—
|
|
145,649
|
|
||||||||||
|
Warrants transferred from other long-term liabilities
|
—
|
|
—
|
|
1,470
|
|
—
|
|
—
|
|
1,470
|
|
||||||||||
|
Issuance of common stock under stock plans
|
3,315,174
|
|
3
|
|
11,274
|
|
—
|
|
—
|
|
11,277
|
|
||||||||||
|
Stock-based compensation expense
|
—
|
|
—
|
|
21,977
|
|
—
|
|
—
|
|
21,977
|
|
||||||||||
|
Balance at December 31, 2015
|
227,960,943
|
|
228
|
|
1,772,123
|
|
(232
|
)
|
(1,912,925
|
)
|
(140,806
|
)
|
||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
(70,222
|
)
|
(70,222
|
)
|
||||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
(184
|
)
|
—
|
|
(184
|
)
|
||||||||||
|
Issuance of common stock in settlement of convertible notes
|
54,009,279
|
|
54
|
|
253,026
|
|
—
|
|
—
|
|
253,080
|
|
||||||||||
|
Issuance of common stock under stock plans
|
7,953,576
|
|
8
|
|
24,530
|
|
—
|
|
—
|
|
24,538
|
|
||||||||||
|
Stock-based compensation expense
|
—
|
|
—
|
|
22,912
|
|
—
|
|
—
|
|
22,912
|
|
||||||||||
|
Balance at December 31, 2016
|
289,923,798
|
|
$
|
290
|
|
$
|
2,072,591
|
|
$
|
(416
|
)
|
$
|
(1,983,147
|
)
|
$
|
89,318
|
|
|||||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Cash flows from operating activities:
|
|||||||||||
|
Net loss
|
$
|
(70,222
|
)
|
$
|
(161,744
|
)
|
$
|
(261,297
|
)
|
||
|
Adjustments to reconcile net loss to net cash provided by (used in) operating activities:
|
|||||||||||
|
Depreciation and amortization
|
1,002
|
|
1,406
|
|
2,391
|
|
|||||
|
Stock-based compensation expense
|
22,912
|
|
21,977
|
|
10,006
|
|
|||||
|
Loss on extinguishment of debt
|
13,901
|
|
—
|
|
—
|
|
|||||
|
Amortization of debt discounts and debt issuance costs
|
8,432
|
|
17,041
|
|
22,289
|
|
|||||
|
Accrual of interest paid in kind
|
8,008
|
|
3,817
|
|
—
|
|
|||||
|
Gain on sale of business and other equity investment
|
(2,494
|
)
|
(112
|
)
|
(838
|
)
|
|||||
|
Changes in the fair value of warrants
|
—
|
|
548
|
|
(1,840
|
)
|
|||||
|
Other
|
(2,510
|
)
|
1,327
|
|
4,161
|
|
|||||
|
Changes in assets and liabilities:
|
|||||||||||
|
Trade and other receivables
|
(37,002
|
)
|
(646
|
)
|
(941
|
)
|
|||||
|
Inventory
|
(722
|
)
|
(235
|
)
|
509
|
|
|||||
|
Prepaid expenses and other current assets
|
(1,610
|
)
|
(325
|
)
|
1,526
|
|
|||||
|
Other long-term assets
|
1,077
|
|
1,340
|
|
(2,149
|
)
|
|||||
|
Accounts payable
|
164
|
|
(12
|
)
|
(2,932
|
)
|
|||||
|
Accrued compensation and benefits
|
16,705
|
|
279
|
|
(9,447
|
)
|
|||||
|
Accrued clinical trial liabilities
|
(3,940
|
)
|
(23,474
|
)
|
6,587
|
|
|||||
|
Accrued collaboration liability
|
(10,938
|
)
|
|
10,206
|
|
732
|
|
||||
|
Deferred revenue
|
256,759
|
|
(2,582
|
)
|
1,133
|
|
|||||
|
Other current and long-term liabilities
|
6,774
|
|
(10,396
|
)
|
(5,295
|
)
|
|||||
|
Net cash provided by (used in) operating activities
|
206,296
|
|
(141,585
|
)
|
(235,405
|
)
|
|||||
|
Cash flows from investing activities:
|
|||||||||||
|
Purchases of property and equipment
|
(1,703
|
)
|
(447
|
)
|
(474
|
)
|
|||||
|
Proceeds from sale of property and equipment
|
97
|
|
1,346
|
|
392
|
|
|||||
|
Proceeds from sale of business and other equity investments
|
2,494
|
|
95
|
|
838
|
|
|||||
|
Proceeds from maturities of restricted cash and investments
|
7,150
|
|
19,789
|
|
20,354
|
|
|||||
|
Purchase of restricted cash and investments
|
(8,650
|
)
|
(5,650
|
)
|
(8,143
|
)
|
|||||
|
Proceeds from sale of investments
|
2,266
|
|
—
|
|
—
|
|
|||||
|
Proceeds from maturities of investments
|
151,485
|
|
178,936
|
|
252,891
|
|
|||||
|
Purchases of investments
|
(369,187
|
)
|
(143,992
|
)
|
(119,528
|
)
|
|||||
|
Net cash (used in) provided by investing activities
|
(216,048
|
)
|
50,077
|
|
146,330
|
|
|||||
|
Cash flows from financing activities:
|
|||||||||||
|
Proceeds from issuance of common stock, net
|
—
|
|
145,649
|
|
75,643
|
|
|||||
|
Proceeds from exercise of stock options
|
25,327
|
|
10,911
|
|
120
|
|
|||||
|
Proceeds from employee stock purchase plan
|
2,187
|
|
568
|
|
1,438
|
|
|||||
|
Principal payments on debt
|
—
|
|
(4,381
|
)
|
(11,709
|
)
|
|||||
|
Redemption of convertible notes
|
(575
|
)
|
—
|
|
—
|
|
|||||
|
Payments on conversion of convertible notes
|
(7,135
|
)
|
—
|
|
—
|
|
|||||
|
Net cash provided by financing activities
|
19,804
|
|
152,747
|
|
65,492
|
|
|||||
|
Net increase (decrease) in cash and cash equivalents
|
10,052
|
|
61,239
|
|
(23,583
|
)
|
|||||
|
Cash and cash equivalents at beginning of year
|
141,634
|
|
80,395
|
|
103,978
|
|
|||||
|
Cash and cash equivalents at end of year
|
$
|
151,686
|
|
$
|
141,634
|
|
$
|
80,395
|
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Supplemental cash flow disclosure:
|
|||||||||||
|
Cash paid for interest
|
$
|
21,044
|
|
$
|
19,822
|
|
$
|
19,109
|
|
||
|
Cash paid for taxes
|
$
|
190
|
|
$
|
192
|
|
$
|
60
|
|
||
|
Non-cash financing activity:
|
|||||||||||
|
Issuance of common stock in settlement of convertible notes
|
$
|
286,925
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Issuance of warrants in connection with amendment to convertible notes
|
$
|
—
|
|
$
|
—
|
|
$
|
2,762
|
|
||
|
Year ended December 31,
|
|||||||||||||||
|
2015
|
2014
|
2013
|
2012
|
||||||||||||
|
Statements of Operations:
|
|||||||||||||||
|
Interest expense, overstated by $7,993, $7,245, $6,568, $2,310 for the years ended December 31, 2015, 2014, 2013 and 2012, respectively
|
$
|
(40,680
|
)
|
$
|
(41,362
|
)
|
$
|
(38,779
|
)
|
$
|
(24,778
|
)
|
|||
|
Total other expense, net, overstated by $7,993, $7,245, $6,568, $2,310 for the years ended December 31, 2015, 2014, 2013 and 2012, respectively
|
$
|
(40,268
|
)
|
$
|
(37,021
|
)
|
$
|
(37,556
|
)
|
$
|
(22,792
|
)
|
|||
|
Net loss, overstated by $7,993, $7,245, $6,568, $2,310 for the years ended December 31, 2015, 2014, 2013 and 2012, respectively
|
$
|
(161,744
|
)
|
$
|
(261,297
|
)
|
$
|
(238,192
|
)
|
$
|
(145,335
|
)
|
|||
|
Net loss per share, basic and diluted, overstated by $0.04, $0.04, $0.04, $0.01 for the years ended December 31, 2015, 2014, 2013 and 2012, respectively
|
$
|
(0.77
|
)
|
$
|
(1.34
|
)
|
$
|
(1.29
|
)
|
$
|
(0.91
|
)
|
|||
|
Statements of Comprehensive Loss:
|
|||||||||||||||
|
Comprehensive loss, overstated by $7,993, $7,245, $6,568, $2,310 for the years ended December 31, 2015, 2014, 2013 and 2012, respectively
|
$
|
(161,855
|
)
|
$
|
(261,564
|
)
|
$
|
(237,954
|
)
|
$
|
(145,289
|
)
|
|||
|
Statements of Cash Flows
(1)
:
|
|||||||||||||||
|
Net loss, overstated by $7,993, $7,245, $6,568, $2,310 for the years ended December 31, 2015, 2014, 2013 and 2012, respectively
|
$
|
(161,744
|
)
|
$
|
(261,297
|
)
|
$
|
(238,192
|
)
|
$
|
(145,335
|
)
|
|||
|
Accretion of debt discount and debt issuance costs, overstated by $7,993, $7,245, $6,568, $2,310 for the years ended December 31, 2015, 2014, 2013 and 2012, respectively
|
$
|
17,041
|
|
$
|
22,289
|
|
$
|
19,722
|
|
$
|
12,442
|
|
|||
|
(1)
|
The error did not impact our net cash provided by or used in operating activities, financing activities or investing activities for any of the periods presented.
|
|
December 31,
|
|||||||||||||||
|
2015
|
2014
|
2013
|
2012
|
||||||||||||
|
Balance Sheets:
|
|||||||||||||||
|
Long-term portion of convertible notes, understated by $36,502, $44,494, $51,739, $58,307 as of December 31, 2015, 2014, 2013 and 2012, respectively
|
$
|
337,937
|
|
$
|
223,629
|
|
$
|
301,550
|
|
$
|
291,828
|
|
|||
|
Liabilities, understated by $36,502, $44,494, $51,739, $58,307 as of December 31, 2015, 2014, 2013 and 2012, respectively
|
$
|
473,148
|
|
$
|
482,592
|
|
$
|
483,452
|
|
$
|
476,015
|
|
|||
|
Additional paid-in capital, overstated by $60,618 as of all dates presented
|
$
|
1,772,123
|
|
$
|
1,591,782
|
|
$
|
1,504,052
|
|
$
|
1,489,727
|
|
|||
|
Accumulated deficit, overstated by $24,116, $16,124, $8,879, $2,310 as of December 31, 2015, 2014, 2013 and 2012, respectively
|
$
|
(1,912,925
|
)
|
$
|
(1,751,181
|
)
|
$
|
(1,489,884
|
)
|
$
|
(1,251,692
|
)
|
|||
|
Stockholders’ equity (deficit), misstated by $36,502, $44,494, $51,739, $58,307 as of December 31, 2015, 2014, 2013 and 2012, respectively
|
$
|
(140,806
|
)
|
$
|
(159,324
|
)
|
$
|
14,498
|
|
$
|
238,127
|
|
|||
|
Statements of Stockholders’ Equity (Deficit):
|
|||||||||||||||
|
Net loss, overstated by $7,993, $7,245, $6,568, $2,310 for the years ended December 31, 2015, 2014, 2013 and 2012, respectively
|
$
|
(161,744
|
)
|
$
|
(261,297
|
)
|
$
|
(238,192
|
)
|
$
|
(145,335
|
)
|
|||
|
Additional paid-in capital, overstated by $60,618 as of all dates presented
|
$
|
1,772,123
|
|
$
|
1,591,782
|
|
$
|
1,504,052
|
|
$
|
1,489,727
|
|
|||
|
Accumulated deficit, overstated by $24,116, $16,124, $8,879, $2,310 as of December 31, 2015, 2014, 2013 and 2012, respectively
|
$
|
(1,912,925
|
)
|
$
|
(1,751,181
|
)
|
$
|
(1,489,884
|
)
|
$
|
(1,251,692
|
)
|
|||
|
Stockholders’ equity (deficit), misstated by $36,502, $44,494, $51,739, $58,307 as of December 31, 2015, 2014, 2013 and 2012, respectively
|
$
|
(140,806
|
)
|
$
|
(159,324
|
)
|
$
|
14,498
|
|
$
|
238,127
|
|
|||
|
Equipment and furniture
|
5 years
|
|
Computer equipment and software
|
3 years
|
|
Leasehold improvements
|
Shorter of lease life or 7 years
|
|
Year Ended December 31, 2016
|
|||
|
Milestones achieved
|
$
|
20,000
|
|
|
Amortization of upfront payments and deferred milestone
|
13,284
|
|
|
|
Royalty revenue
|
175
|
|
|
|
Product supply agreement revenue
|
1,612
|
|
|
|
Cost of supplied product
|
(1,555
|
)
|
|
|
Royalty payable to GlaxoSmithKline on net sales by Ipsen
|
(264
|
)
|
|
|
Collaboration revenues under the collaboration agreement
|
$
|
33,252
|
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Collaboration revenues:
|
|||||||||||
|
Royalty revenues on ex-U.S. sales of COTELLIC
|
$
|
2,827
|
|
$
|
14
|
|
$
|
—
|
|
||
|
(Loss) net cost recovery under the collaboration agreement included in Selling, general and administrative expenses
|
$
|
8,771
|
|
$
|
(16,600
|
)
|
|
$
|
(2,916
|
)
|
|
|
2010 Restructurings
|
2014 Restructuring
|
||||||||||||||||||||||
|
Facility
Charges |
Other
|
Employee
Severance
and Other Benefits
|
Facility
Charges |
Other
|
Total
|
||||||||||||||||||
|
Restructuring liability as of December 31, 2013
|
$
|
13,460
|
|
$
|
12
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
13,472
|
|
|||||
|
Restructuring charge (recovery)
|
1,626
|
|
(117
|
)
|
5,775
|
|
65
|
|
247
|
|
7,596
|
|
|||||||||||
|
Proceeds from sale of assets
|
—
|
|
199
|
|
—
|
|
—
|
|
100
|
|
299
|
|
|||||||||||
|
Other cash payments, net
|
(5,644
|
)
|
(8
|
)
|
(4,507
|
)
|
(65
|
)
|
(12
|
)
|
(10,236
|
)
|
|||||||||||
|
Other items
|
12
|
|
(86
|
)
|
22
|
|
—
|
|
(288
|
)
|
(340
|
)
|
|||||||||||
|
Restructuring liability as of December 31, 2014
|
9,454
|
|
—
|
|
1,290
|
|
—
|
|
47
|
|
10,791
|
|
|||||||||||
|
Restructuring charge (recovery)
|
757
|
|
—
|
|
(269
|
)
|
1,582
|
|
(1,028
|
)
|
1,042
|
|
|||||||||||
|
Proceeds from sale of assets
|
—
|
|
—
|
|
—
|
|
—
|
|
1,325
|
|
1,325
|
|
|||||||||||
|
Other cash payments, net
|
(6,449
|
)
|
—
|
|
(1,021
|
)
|
(1,357
|
)
|
—
|
|
(8,827
|
)
|
|||||||||||
|
Other items
|
325
|
|
—
|
|
—
|
|
278
|
|
(344
|
)
|
259
|
|
|||||||||||
|
Restructuring liability as of December 31, 2015
|
4,087
|
|
—
|
|
—
|
|
503
|
|
—
|
|
4,590
|
|
|||||||||||
|
Restructuring charge
|
902
|
|
—
|
|
—
|
|
12
|
|
—
|
|
914
|
|
|||||||||||
|
Other cash payments, net
|
(4,039
|
)
|
—
|
|
—
|
|
(500
|
)
|
(34
|
)
|
(4,573
|
)
|
|||||||||||
|
Other items
|
975
|
|
—
|
|
—
|
|
—
|
|
34
|
|
1,009
|
|
|||||||||||
|
Restructuring liability as of December 31, 2016
|
$
|
1,925
|
|
$
|
—
|
|
$
|
—
|
|
$
|
15
|
|
$
|
—
|
|
$
|
1,940
|
|
|||||
|
Restructuring charge (recovery) from implementation to date
|
$
|
32,517
|
|
$
|
23,933
|
|
(1)
|
$
|
5,506
|
|
$
|
1,659
|
|
$
|
(781
|
)
|
(1)
|
$
|
62,834
|
|
|||
|
(1)
|
Other restructuring charge from implementation to date for the 2010 Restructurings includes
$21.7 million
in charges related to employee severance and other benefits for periods ended prior to December 31, 2013. The remainder of Other activity for both restructurings relates primarily to the impairment and sale of property and equipment.
|
|
December 31, 2016
|
|||||||||||||||
|
|
Amortized
Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Fair Value
|
|||||||||||
|
Cash and cash equivalents
|
$
|
151,686
|
|
$
|
—
|
|
$
|
—
|
|
$
|
151,686
|
|
|||
|
Short-term investments
|
268,234
|
|
13
|
|
(130
|
)
|
268,117
|
|
|||||||
|
Long-term investments
|
55,792
|
|
1
|
|
(192
|
)
|
55,601
|
|
|||||||
|
Long-term restricted investments
|
4,150
|
|
—
|
|
—
|
|
4,150
|
|
|||||||
|
Total cash and investments
|
$
|
479,862
|
|
$
|
14
|
|
$
|
(322
|
)
|
$
|
479,554
|
|
|||
|
December 31, 2015
|
|||||||||||||||
|
|
Amortized
Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Fair Value
|
|||||||||||
|
Cash and cash equivalents
|
$
|
141,634
|
|
$
|
—
|
|
$
|
—
|
|
$
|
141,634
|
|
|||
|
Short-term investments
|
25,484
|
|
5
|
|
(63
|
)
|
25,426
|
|
|||||||
|
Long-term investments
|
83,665
|
|
2
|
|
(67
|
)
|
83,600
|
|
|||||||
|
Long-term restricted investments
|
2,650
|
|
—
|
|
—
|
|
2,650
|
|
|||||||
|
Total cash and investments
|
$
|
253,433
|
|
$
|
7
|
|
$
|
(130
|
)
|
$
|
253,310
|
|
|||
|
December 31, 2016
|
|||||||||||||||
|
Amortized
Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Fair Value
|
||||||||||||
|
Money market funds
|
$
|
71,457
|
|
$
|
—
|
|
$
|
—
|
|
$
|
71,457
|
|
|||
|
Commercial paper
|
165,375
|
|
—
|
|
—
|
|
165,375
|
|
|||||||
|
Corporate bonds
|
152,712
|
|
3
|
|
(308
|
)
|
152,407
|
|
|||||||
|
U.S. Treasury and government sponsored enterprises
|
70,730
|
|
11
|
|
(14
|
)
|
70,727
|
|
|||||||
|
Total investments
|
$
|
460,274
|
|
$
|
14
|
|
$
|
(322
|
)
|
$
|
459,966
|
|
|||
|
December 31, 2015
|
|||||||||||||||
|
Amortized
Cost
|
Gross
Unrealized
Gains
|
Gross
Unrealized
Losses
|
Fair Value
|
||||||||||||
|
Money market funds
|
$
|
72,000
|
|
$
|
—
|
|
$
|
—
|
|
$
|
72,000
|
|
|||
|
Commercial paper
|
78,155
|
|
—
|
|
—
|
|
78,155
|
|
|||||||
|
Corporate bonds
|
72,205
|
|
4
|
|
(118
|
)
|
72,091
|
|
|||||||
|
U.S. Treasury and government sponsored enterprises
|
28,434
|
|
1
|
|
(12
|
)
|
28,423
|
|
|||||||
|
Marketable equity securities
|
16
|
|
2
|
|
—
|
|
18
|
|
|||||||
|
Total investments
|
$
|
250,810
|
|
$
|
7
|
|
$
|
(130
|
)
|
$
|
250,687
|
|
|||
|
Mature within One Year
|
After One Year through Two Years
|
Fair Value
|
|||||||||
|
Money market funds
|
$
|
71,457
|
|
$
|
—
|
|
$
|
71,457
|
|
||
|
Commercial paper
|
165,375
|
|
—
|
|
165,375
|
|
|||||
|
Corporate bonds
|
99,455
|
|
52,952
|
|
152,407
|
|
|||||
|
U.S. Treasury and government sponsored enterprises
|
68,078
|
|
2,649
|
|
70,727
|
|
|||||
|
Total
|
$
|
404,365
|
|
$
|
55,601
|
|
$
|
459,966
|
|
||
|
|
December 31,
|
||||||
|
|
2016
|
2015
|
|||||
|
Raw materials
|
$
|
863
|
|
$
|
1,037
|
|
|
|
Work in process
|
2,343
|
|
2,251
|
|
|||
|
Finished goods
|
738
|
|
583
|
|
|||
|
Total
|
3,944
|
|
3,871
|
|
|||
|
Less: non-current portion included in Other long-term assets
|
(606
|
)
|
(1,255
|
)
|
|||
|
Inventory
|
$
|
3,338
|
|
$
|
2,616
|
|
|
|
|
December 31,
|
||||||
|
|
2016
|
2015
|
|||||
|
Laboratory equipment
|
$
|
4,310
|
|
$
|
4,749
|
|
|
|
Computer equipment and software
|
13,738
|
|
11,890
|
|
|||
|
Furniture and fixtures
|
2,240
|
|
2,253
|
|
|||
|
Leasehold improvements
|
6,646
|
|
6,395
|
|
|||
|
Construction-in-progress
|
19
|
|
456
|
|
|||
|
26,953
|
|
25,743
|
|
||||
|
Less: accumulated depreciation and amortization
|
(24,882
|
)
|
(24,309
|
)
|
|||
|
Property and equipment, net
|
$
|
2,071
|
|
$
|
1,434
|
|
|
|
|
December 31,
|
||||||
|
|
2016
|
2015
|
|||||
|
Secured Convertible Notes due 2018 (“Deerfield Notes”)
|
$
|
109,122
|
|
$
|
102,727
|
|
|
|
Term loan payable
|
80,000
|
|
80,000
|
|
|||
|
2019 Notes
|
—
|
|
235,210
|
|
|||
|
Total debt
|
189,122
|
|
417,937
|
|
|||
|
Less: current portion
|
(189,122
|
)
|
—
|
|
|||
|
Long-term debt
|
$
|
—
|
|
$
|
417,937
|
|
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Stated coupon interest
|
$
|
8,008
|
|
$
|
6,792
|
|
$
|
6,000
|
|
||
|
Interest paid in kind
|
8,008
|
|
3,817
|
|
—
|
|
|||||
|
Amortization of debt discount and debt issuance costs
|
457
|
|
5,461
|
|
11,731
|
|
|||||
|
Total interest expense
|
$
|
16,473
|
|
$
|
16,070
|
|
$
|
17,731
|
|
||
|
Inducements included in August 9, 2016 and August 19, 2016 agreements:
|
|||
|
Cash inducements
|
$
|
2,394
|
|
|
Repayments of interest required upon a conversion under the terms of the indenture that were not repaid under the terms of the exchange agreements
|
3,572
|
|
|
|
Difference between the total settlement consideration attributed to the liability component of the 2019 Notes and the net carrying value of the liability, described above
|
7,338
|
|
|
|
Unamortized discount on redeemed notes
|
83
|
|
|
|
Third party costs
|
514
|
|
|
|
Loss on extinguishment of debt
|
$
|
13,901
|
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Stated coupon interest
|
$
|
7,799
|
|
$
|
12,218
|
|
$
|
12,253
|
|
||
|
Amortization of debt discount and debt issuance costs
|
7,975
|
|
11,581
|
|
10,525
|
|
|||||
|
Total interest expense
|
$
|
15,774
|
|
$
|
23,799
|
|
$
|
22,778
|
|
||
|
Year Ending December 31,
(1)
|
|
||
|
2017
|
$
|
80,000
|
|
|
2018
|
124,972
|
|
|
|
Thereafter
|
—
|
|
|
|
(1)
|
As described above, we intend to repay the Deerfield Notes, which have a contractual maturity of July 1, 2018, on or about July 1, 2017. The actual timing of payments may differ materially.
|
|
December 31, 2016
|
|||||||||||
|
Level 1
|
Level 2
|
Total
|
|||||||||
|
Money market funds
|
$
|
71,457
|
|
$
|
—
|
|
$
|
71,457
|
|
||
|
Commercial paper
|
—
|
|
165,375
|
|
165,375
|
|
|||||
|
Corporate bonds
|
—
|
|
152,407
|
|
152,407
|
|
|||||
|
U.S. Treasury and government sponsored enterprises
|
—
|
|
70,727
|
|
70,727
|
|
|||||
|
Total financial assets
|
$
|
71,457
|
|
$
|
388,509
|
|
$
|
459,966
|
|
||
|
December 31, 2015
|
|||||||||||
|
Level 1
|
Level 2
|
Total
|
|||||||||
|
Money market funds
|
$
|
72,000
|
|
$
|
—
|
|
$
|
72,000
|
|
||
|
Commercial paper
|
—
|
|
78,155
|
|
78,155
|
|
|||||
|
Corporate bonds
|
—
|
|
72,091
|
|
72,091
|
|
|||||
|
U.S. Treasury and government sponsored enterprises
|
—
|
|
28,423
|
|
28,423
|
|
|||||
|
Marketable equity securities
|
18
|
|
—
|
|
18
|
|
|||||
|
Total financial assets
|
$
|
72,018
|
|
$
|
178,669
|
|
$
|
250,687
|
|
||
|
Balance at December 31, 2014
|
$
|
921
|
|
|
Unrealized loss at final re-measurement of warrants on March 18, 2015,
included in Interest income and other, net
|
549
|
|
|
|
Transfer of warrants from Other long-term liabilities to Additional paid-in capital at their estimated fair value upon warrant repricing on March 18, 2015
|
(1,470
|
)
|
|
|
Balance at December 31, 2015
|
$
|
—
|
|
|
December 31, 2016
|
December 31, 2015
|
||||||||||||||
|
Carrying
Amount
|
Fair Value
|
Carrying
Amount
|
Fair Value
|
||||||||||||
|
Deerfield Notes
|
$
|
109,122
|
|
$
|
121,220
|
|
$
|
102,727
|
|
$
|
101,096
|
|
|||
|
Term loan payable
|
$
|
80,000
|
|
$
|
79,784
|
|
$
|
80,000
|
|
$
|
79,815
|
|
|||
|
2019 Notes
|
$
|
—
|
|
$
|
—
|
|
$
|
235,210
|
|
$
|
336,260
|
|
|||
|
•
|
When available, we value investments based on quoted prices for those financial instruments, which is a Level 1 input. Our remaining investments are valued using third-party pricing sources, which use observable market prices, interest rates and yield curves observable at commonly quoted intervals of similar assets as observable inputs for pricing, which is a Level 2 input.
|
|
•
|
The 2019 Notes were valued using a third-party pricing model that is based in part on average trading prices, which is a Level 2 input. The 2019 Notes were not marked-to-market and are shown at their initial fair value less the unamortized discount; the portion of the value allocated to the conversion option is included in Stockholders’ equity (deficit) on the accompanying Consolidated Balance Sheets.
|
|
•
|
We estimate the fair value of our other debt instruments, where possible, using the net present value of the payments. For the Silicon Valley Bank term loan and line of credit, we use an interest rate that is consistent with money-market rates that would have been earned on our non-interest-bearing compensating balances as our discount rate, which is a Level 2 input. For the Deerfield Notes, we used a discount rate of
9.5%
, which we estimate as our current borrowing rate for similar debt as of
December 31, 2016
, which is a Level 3 input.
|
|
•
|
The settlement consideration comprises, in part, shares of our Common Stock. The fair value of our Common Stock was determined based on the closing market price of our Common Stock on the various settlement dates of the conversions, which are level 1 inputs;
|
|
•
|
The carrying value of the remaining settlement consideration, which includes cash and the forgiveness of the repayment of certain prior interest payments, approximates fair value;
|
|
•
|
We estimated the fair value of the liability component of the 2019 Notes using the net present value of estimated future cash flows through maturity. We used a discount rate of
9.5%
, which we estimated as our current borrowing rate for straight debt as of September 30, 2016, which is a Level 3 input.
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Research and development expense
|
$
|
9,366
|
|
$
|
11,691
|
|
$
|
3,245
|
|
||
|
Selling, general and administrative expense
|
13,546
|
|
10,286
|
|
6,783
|
|
|||||
|
Restructuring related recovery
|
—
|
|
—
|
|
(22
|
)
|
|||||
|
Total employee stock-based compensation expense
|
$
|
22,912
|
|
$
|
21,977
|
|
$
|
10,006
|
|
||
|
|
2016
|
2015
|
2014
|
||||||||
|
Stock options
|
$
|
4.77
|
|
$
|
2.55
|
|
$
|
1.46
|
|
||
|
ESPP
|
$
|
2.17
|
|
$
|
1.20
|
|
$
|
1.28
|
|
||
|
|
Stock Options
|
|||||||
|
|
2016
|
2015
|
2014
|
|||||
|
Risk-free interest rate
|
1.15
|
%
|
1.22
|
%
|
1.80
|
%
|
||
|
Dividend yield
|
—
|
%
|
—
|
%
|
—
|
%
|
||
|
Volatility
|
76
|
%
|
93
|
%
|
85
|
%
|
||
|
Expected life
|
4.4 years
|
|
4.5 years
|
|
5.5 years
|
|
||
|
|
ESPP
|
|||||||
|
|
2016
|
2015
|
2014
|
|||||
|
Risk-free interest rate
|
0.55
|
%
|
0.15
|
%
|
0.06
|
%
|
||
|
Dividend yield
|
—
|
%
|
—
|
%
|
—
|
%
|
||
|
Volatility
|
65
|
%
|
98
|
%
|
69
|
%
|
||
|
Expected life
|
6 months
|
|
6 months
|
|
6 months
|
|
||
|
Shares
|
Weighted
Average
Exercise Price
|
Weighted
Average
Remaining
Contractual
Term
|
Aggregate
Intrinsic
Value
|
|||||||||
|
Options outstanding at December 31, 2015
|
27,425,854
|
|
$
|
4.22
|
|
|||||||
|
Granted
|
4,200,950
|
|
$
|
8.29
|
|
|||||||
|
Exercised
|
(6,239,022
|
)
|
$
|
4.07
|
|
|||||||
|
Forfeited
|
(307,601
|
)
|
$
|
4.67
|
|
|||||||
|
Expired
|
(80,516
|
)
|
$
|
10.49
|
|
|||||||
|
Options outstanding at December 31, 2016
|
24,999,665
|
|
$
|
4.91
|
|
4.54 years
|
$
|
250,996
|
|
|||
|
Exercisable at December 31, 2016
|
17,731,361
|
|
$
|
4.01
|
|
3.98 years
|
$
|
193,288
|
|
|||
|
|
Options Outstanding
|
Options Outstanding and
Exercisable
|
|||||||||||||||
|
Exercise Price Range
|
Number
|
Weighted
Average
Remaining
Contractual Life
|
Weighted
Average
Exercise
Price
|
Number
Exercisable
|
Weighted
Average
Exercise
Price
|
||||||||||||
|
$1.46 - $1.90
|
8,231,617
|
|
4.64 years
|
$
|
1.77
|
|
8,088,721
|
|
$
|
1.77
|
|
||||||
|
$2.57 - $4.05
|
2,707,474
|
|
5.57 years
|
$
|
3.56
|
|
1,130,251
|
|
$
|
3.27
|
|
||||||
|
$4.16 - $5.55
|
5,957,725
|
|
3.58 years
|
3,186,063
|
|
$
|
5.18
|
|
4,520,554
|
|
$
|
5.31
|
|
||||
|
$5.61 - $6.21
|
4,076,881
|
|
6,881
|
|
5.17 years
|
$
|
6.08
|
|
1,861,457
|
|
$
|
6.00
|
|
||||
|
$6.25 - $18.25
|
4,025,968
|
|
4.42 years
|
$
|
10.67
|
|
2,130,378
|
|
$
|
8.42
|
|
||||||
|
24,999,665
|
|
4.54 years
|
$
|
4.91
|
|
17,731,361
|
|
$
|
4.01
|
|
|||||||
|
Shares
|
Weighted
Average
Grant Date
Fair Value
|
Weighted
Average
Remaining
Contractual
Term
|
Aggregate
Intrinsic
Value
|
|||||||||
|
Awards outstanding at December 31, 2015
|
1,002,188
|
|
$
|
5.16
|
|
|||||||
|
Awarded
|
3,138,236
|
|
$
|
7.58
|
|
|||||||
|
Vested and released
|
(1,640,324
|
)
|
$
|
4.49
|
|
|||||||
|
Forfeited
|
(30,309
|
)
|
$
|
4.77
|
|
|||||||
|
Awards outstanding at December 31, 2016
|
2,469,791
|
|
$
|
8.69
|
|
1.93 years
|
$
|
36,825
|
|
|||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Domestic
|
$
|
(70,222
|
)
|
$
|
(150,846
|
)
|
$
|
(230,535
|
)
|
||
|
Foreign
|
—
|
|
(10,843
|
)
|
(30,944
|
)
|
|||||
|
Total
|
$
|
(70,222
|
)
|
$
|
(161,689
|
)
|
$
|
(261,479
|
)
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Current:
|
|||||||||||
|
Federal
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||
|
State
|
—
|
|
55
|
|
(182
|
)
|
|||||
|
Total current tax expense
|
—
|
|
55
|
|
(182
|
)
|
|||||
|
Deferred:
|
|||||||||||
|
Federal
|
—
|
|
—
|
|
—
|
|
|||||
|
State
|
—
|
|
—
|
|
—
|
|
|||||
|
Total deferred tax expense
|
—
|
|
—
|
|
—
|
|
|||||
|
Income tax provision (benefit)
|
$
|
—
|
|
$
|
55
|
|
$
|
(182
|
)
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
U.S. federal income tax benefit at statutory rate
|
$
|
(23,876
|
)
|
$
|
(54,974
|
)
|
$
|
(88,903
|
)
|
||
|
Unutilized net operating losses
|
6,377
|
|
51,421
|
|
84,985
|
|
|||||
|
State tax expense
|
6,520
|
|
55
|
|
(182
|
)
|
|||||
|
Debt extinguishment
|
4,726
|
|
—
|
|
—
|
|
|||||
|
Non-deductible interest
|
2,680
|
|
3,308
|
|
3,598
|
|
|||||
|
Stock-based compensation
|
3,155
|
|
195
|
|
255
|
|
|||||
|
Other
|
418
|
|
50
|
|
65
|
|
|||||
|
Income tax (benefit) provision
|
$
|
—
|
|
$
|
55
|
|
$
|
(182
|
)
|
||
|
|
December 31,
|
||||||
|
|
2016
|
2015
|
|||||
|
Deferred tax assets:
|
|||||||
|
Net operating loss carry-forwards
|
$
|
471,327
|
|
$
|
464,504
|
|
|
|
Book over tax depreciation and amortization
|
70,617
|
|
1,752
|
|
|||
|
Tax credit and charitable contribution carry-forwards
|
64,367
|
|
64,350
|
|
|||
|
Amortization of deferred stock compensation – non-qualified
|
14,780
|
|
14,615
|
|
|||
|
Accruals and reserves not currently deductible
|
8,117
|
|
7,775
|
|
|||
|
Other
|
106
|
|
—
|
|
|||
|
Total deferred tax assets
|
629,314
|
|
552,996
|
|
|||
|
Valuation allowance
|
(629,062
|
)
|
(536,327
|
)
|
|||
|
Net deferred tax assets
|
252
|
|
16,669
|
|
|||
|
Deferred tax liabilities:
|
|||||||
|
Unrealized gain on derivatives
|
(252
|
)
|
(497
|
)
|
|||
|
Convertible debt
|
—
|
|
(16,172
|
)
|
|||
|
Total deferred tax liabilities
|
(252
|
)
|
(16,669
|
)
|
|||
|
Net deferred taxes
|
$
|
—
|
|
$
|
—
|
|
|
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Beginning balance
|
$
|
88,638
|
|
$
|
58,215
|
|
$
|
55,077
|
|
||
|
Decrease (increase) relating to prior year provision
|
(29,110
|
)
|
21,696
|
|
719
|
|
|||||
|
Increase relating to current year provision
|
2,304
|
|
8,727
|
|
2,706
|
|
|||||
|
Reductions based on the lapse of the applicable statutes of limitations
|
(23
|
)
|
—
|
|
(287
|
)
|
|||||
|
Ending balance
|
$
|
61,809
|
|
$
|
88,638
|
|
$
|
58,215
|
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2016
|
2015
|
2014
|
||||||||
|
Numerator:
|
|||||||||||
|
Net loss
|
$
|
(70,222
|
)
|
$
|
(161,744
|
)
|
$
|
(261,297
|
)
|
||
|
Denominator:
|
|||||||||||
|
Shares used in computing basic and diluted net loss per share
|
250,531
|
|
209,227
|
|
194,299
|
|
|||||
|
Net loss per share, basic and diluted
|
$
|
(0.28
|
)
|
$
|
(0.77
|
)
|
$
|
(1.34
|
)
|
||
|
|
December 31,
|
|||||||
|
|
2016
|
2015
|
2014
|
|||||
|
Convertible debt
|
33,890
|
|
88,008
|
|
75,734
|
|
||
|
Outstanding stock options, unvested RSUs and ESPP contributions
|
27,568
|
|
28,470
|
|
28,930
|
|
||
|
Warrants
|
1,000
|
|
1,000
|
|
1,000
|
|
||
|
Total potentially dilutive shares
|
62,458
|
|
117,478
|
|
105,664
|
|
||
|
Year Ending December 31,
|
Operating
Leases (1)
|
||
|
2017
|
8,474
|
|
|
|
2018
|
3,007
|
|
|
|
$
|
11,481
|
|
|
|
(1)
|
Minimum payments have not been reduced by minimum sublease rentals of
$1.2 million
due in the future under noncancelable subleases.
|
|
|
Original
Term
(Expiration)
|
Renewal Options
|
Future
Minimum
Lease
Payments
|
||
|
Building Lease #1 and 2
|
May 2017
|
none
|
$
|
3,425
|
|
|
Building Lease #3
|
July 2018
|
1 additional period of 5 years
|
8,056
|
|
|
|
Total
|
$
|
11,481
|
|
||
|
|
Year Ended December 31,
|
|||||||
|
|
2016
|
2015
|
2014
|
|||||
|
Diplomat Specialty Pharmacy
|
33
|
%
|
83
|
%
|
99
|
%
|
||
|
Ipsen
|
17
|
%
|
—
|
%
|
—
|
%
|
||
|
Year Ended December 31,
|
|||||||||||
|
2016
|
2015
|
2014
|
|||||||||
|
U.S.
|
$
|
140,709
|
|
$
|
33,869
|
|
$
|
24,832
|
|
||
|
Europe
|
35,745
|
|
3,303
|
|
279
|
|
|||||
|
Rest of world
|
15,000
|
|
—
|
|
—
|
|
|||||
|
|
Quarter Ended
|
||||||||||||||
|
December 31,
|
September 30,
|
June 30,
|
March 31,
|
||||||||||||
|
2016:
|
|||||||||||||||
|
Revenues
|
$
|
77,581
|
|
$
|
62,194
|
|
$
|
36,252
|
|
$
|
15,427
|
|
|||
|
Gross profit
|
$
|
50,064
|
|
$
|
40,287
|
|
$
|
30,058
|
|
$
|
8,414
|
|
|||
|
Income (loss) from operations
|
$
|
38,883
|
|
$
|
7,264
|
|
$
|
(25,136
|
)
|
$
|
(49,135
|
)
|
|||
|
Net income (loss)
|
$
|
35,123
|
|
$
|
(11,284
|
)
|
$
|
(34,838
|
)
|
$
|
(59,223
|
)
|
|||
|
Net income (loss) per share, basic and diluted
|
$
|
0.12
|
|
$
|
(0.04
|
)
|
$
|
(0.15
|
)
|
$
|
(0.26
|
)
|
|||
|
2015:
|
|||||||||||||||
|
Revenues
|
$
|
9,938
|
|
$
|
9,854
|
|
$
|
7,992
|
|
$
|
9,388
|
|
|||
|
Gross profit
|
$
|
8,915
|
|
$
|
8,434
|
|
$
|
7,306
|
|
$
|
8,622
|
|
|||
|
Loss from operations
|
$
|
(31,600
|
)
|
$
|
(35,781
|
)
|
$
|
(31,280
|
)
|
$
|
(22,760
|
)
|
|||
|
Net loss
(1)
|
$
|
(41,568
|
)
|
$
|
(45,542
|
)
|
$
|
(41,389
|
)
|
$
|
(33,245
|
)
|
|||
|
Net loss per share, basic and diluted
(1)
|
$
|
(0.18
|
)
|
$
|
(0.21
|
)
|
$
|
(0.21
|
)
|
$
|
(0.17
|
)
|
|||
|
(1)
|
Prior period balances reflect revisions due to a correction of an immaterial error with regards to the 2019 Notes. The immaterial error resulted in an overstatement of the discount on the 2019 Notes and therefore overstated the related interest expense. Therefore, net loss was overstated by
$2.2 million
,
$2.1 million
,
$2.1 million
,
$2.0 million
,
$2.0 million
,
$1.9 million
for the quarters ended June 30 2016 and March 31, 2016, December 31, 2015, September 30, 2015, June 30 2015 and March 31, 2015, respectively, and net loss per share, basic and diluted was overstated by
$0.01
, for each of those quarters. See “Note 1. Organization and Summary of Significant Accounting Policies - Correction of an Immaterial Error” for additional information on the correction of the immaterial error.
|
|
ITEM 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
|
ITEM 9B.
|
OTHER INFORMATION
|
|
ITEM 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
|
ITEM 11.
|
EXECUTIVE COMPENSATION
|
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
|
Plan Category
|
Number of
securities to be issued upon exercise of outstanding
options, warrants and rights
|
Weighted-average exercise price of outstanding
options, warrants and
rights (1)
|
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a))
|
|||||||
|
|
(a)
|
(b)
|
(c)
|
|||||||
|
Equity compensation plans approved by stockholders (2)
|
28,433,956
|
|
$
|
4.84
|
|
5,670,544
|
|
|||
|
Equity compensation plans not approved by stockholders (3)
|
35,500
|
|
$
|
14.91
|
|
1,749,937
|
|
|||
|
Total
|
28,469,456
|
|
$
|
4.86
|
|
7,420,481
|
|
|||
|
(1)
|
The weighted average exercise price does not take into account the shares subject to outstanding restricted stock units, or RSUs, which have no exercise price.
|
|
(2)
|
Represents shares of our common stock issuable pursuant to the 2000 Plan, the 2011 Plan, the 2014 Plan, the Director Plan and the ESPP.
|
|
(3)
|
Represents shares of our common stock issuable pursuant to the 2016 Plan and 401(k) Plan. We sponsor a 401(k) Plan whereby eligible employees may elect to contribute up to the lesser of 50% of their annual compensation or the statutorily prescribed annual limit allowable under Internal Revenue Service regulations. The 401(k) Plan permits us to make matching contributions on behalf of all participants. We match 100% of the first 3% of participant contributions into the 401(k) Plan in the form of our common stock.
|
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
|
ITEM 14.
|
PRINCIPAL ACCOUNTING FEES AND SERVICES
|
|
ITEM 15.
|
EXHIBITS, FINANCIAL STATEMENT SCHEDULES
|
|
(a)
|
The following documents are being filed as part of this report:
|
|
|
Page
|
|
ITEM 16.
|
FORM 10-K SUMMARY
|
|
E
XELIXIS
, I
NC
.
|
||
|
By:
|
|
/s/ M
ICHAEL
M. M
ORRISSEY
|
|
|
Michael M. Morrissey, Ph.D.
|
|
|
|
President and Chief Executive Officer
|
|
|
Signatures
|
Title
|
|
Date
|
|
|
/s/ M
ICHAEL
M. M
ORRISSEY
|
Director, President and
|
|
February 27, 2017
|
|
|
Michael M. Morrissey, Ph.D.
|
Chief Executive Officer (Principal Executive Officer)
|
|||
|
/s/ C
HRISTOPHER
S
ENNER
|
Executive Vice President and Chief Financial Officer
|
|
February 27, 2017
|
|
|
Christopher Senner
|
(Principal Financial and Accounting Officer)
|
|||
|
/s/ S
TELIOS
P
APADOPOULOS
|
Chairman of the Board
|
|
February 27, 2017
|
|
|
Stelios Papadopoulos, Ph.D.
|
||||
|
/s/ C
HARLES
C
OHEN
|
Director
|
|
February 27, 2017
|
|
|
Charles Cohen, Ph.D.
|
||||
|
/s/ C
ARL
B. F
ELDBAUM
|
Director
|
|
February 27, 2017
|
|
|
Carl B. Feldbaum, Esq.
|
||||
|
/s/ A
LAN
M. G
ARBER
|
|
Director
|
|
February 27, 2017
|
|
Alan M. Garber, M.D., Ph.D.
|
||||
|
Signatures
|
|
Title
|
|
Date
|
|
/s/ V
INCENT
T. M
ARCHESI
|
|
Director
|
|
February 27, 2017
|
|
Vincent T. Marchesi, M.D., Ph.D.
|
||||
|
/s/ G
EORGE
P
OSTE
|
|
Director
|
|
February 27, 2017
|
|
George Poste, D.V.M., Ph.D.
|
||||
|
/s/ G
EORGE
A. S
CANGOS
|
|
Director
|
|
February 27, 2017
|
|
George A. Scangos, Ph.D.
|
||||
|
/s/ J
ULIE
A. S
MITH
|
|
Director
|
|
February 27, 2017
|
|
Julie A. Smith
|
||||
|
/s/ L
ANCE
W
ILLSEY
|
|
Director
|
|
February 27, 2017
|
|
Lance Willsey, M.D.
|
||||
|
/s/ J
ACK
L. W
YSZOMIERSKI
|
|
Director
|
|
February 27, 2017
|
|
Jack L. Wyszomierski
|
||||
|
Exhibit
Number
|
Exhibit Description
|
Incorporation by Reference
|
Filed
Herewith
|
|||||||||
|
Form
|
File Number
|
Exhibit/
Appendix
Reference
|
Filing Date
|
|||||||||
|
3.1
|
Amended and Restated Certificate of Incorporation of Exelixis, Inc.
|
10-K
|
000-30235
|
3.1
|
3/10/2010
|
|||||||
|
3.2
|
Certificate of Amendment of Amended and Restated Certificate of Incorporation of Exelixis, Inc.
|
10-K
|
000-30235
|
3.2
|
3/10/2010
|
|||||||
|
3.3
|
Certificate of Amendment of Amended and Restated Certificate of Incorporation of Exelixis, Inc.
|
8-K
|
000-30235
|
3.1
|
5/25/2012
|
|||||||
|
3.4
|
Certificate of Ownership and Merger Merging X-Ceptor Therapeutics, Inc. with and into Exelixis, Inc.
|
8-K
|
000-30235
|
3.2
|
10/15/2014
|
|||||||
|
3.5
|
Certificate of Change of Registered Agent and/or Registered Office of Exelixis, Inc.
|
8-K
|
000-30235
|
3.1
|
10/15/2014
|
|||||||
|
3.6
|
Amended and Restated Bylaws of Exelixis, Inc.
|
8-K
|
000-30235
|
3.1
|
12/5/2011
|
|||||||
|
4.1
|
Specimen Common Stock Certificate.
|
S-1,
as amended
|
333-96335
|
4.1
|
4/7/2000
|
|||||||
|
4.2
|
Amended and Restated Secured Convertible Note dated July 1, 2015 in favor of Deerfield Partners, L.P.
|
10-Q
|
000-30235
|
4.2
|
8/11/2015
|
|||||||
|
4.3
|
Amended and Restated Secured Convertible Note dated July 1, 2015 in favor of Deerfield International Master Fund, L.P.
|
10-Q
|
000-30235
|
4.3
|
8/11/2015
|
|||||||
|
4.4
|
Registration Rights Agreement dated January 22, 2014 by and among Exelixis, Inc., Deerfield Partners, L.P. and Deerfield International Master Fund, L.P.
|
8-K
|
000-30235
|
4.2
|
1/22/2014
|
|||||||
|
4.5
|
Form of Warrant to Purchase Common Stock of Exelixis, Inc. issued to OTA LLC
|
10-Q
|
000-30235
|
4.5
|
11/10/2015
|
|||||||
|
10.1†
|
Form of Indemnity Agreement.
|
S-1,
as amended
|
333-96335
|
10.1
|
3/17/2000
|
|||||||
|
10.2
†
|
2000 Equity Incentive Plan.
|
10-Q
|
000-30235
|
10.1
|
5/3/2007
|
|||||||
|
10.3
†
|
Form of Stock Option Agreement under the 2000 Equity Incentive Plan (early exercise permissible).
|
10-Q
|
000-30235
|
10.2
|
11/8/2004
|
|||||||
|
10.4
†
|
Form of Stock Option Agreement under the 2000 Equity Incentive Plan (early exercise may be restricted).
|
8-K
|
000-30235
|
10.1
|
12/15/2004
|
|||||||
|
10.5
†
|
2000 Non-Employee Directors’ Stock Option Plan.
|
10-K
|
000-30235
|
10.6
|
2/20/2014
|
|||||||
|
10.6
†
|
Form of Stock Option Agreement under the 2000 Non-Employee Directors’ Stock Option Plan.
|
10-K
|
000-30235
|
10.7
|
2/22/2011
|
|||||||
|
10.7
†
|
2000 Employee Stock Purchase Plan.
|
Schedule 14A
|
000-30235
|
A
|
4/13/2016
|
|||||||
|
10.8
†
|
2011 Equity Incentive Plan.
|
8-K
|
000-30235
|
10.1
|
5/24/2011
|
|||||||
|
Exhibit
Number
|
Exhibit Description
|
Incorporation by Reference
|
Filed
Herewith
|
|||||||||
|
Form
|
File Number
|
Exhibit/
Appendix
Reference
|
Filing Date
|
|||||||||
|
10.9
†
|
Form of Stock Option Agreement under the 2011 Equity Incentive Plan
|
10-Q
|
000-30235
|
10.3
|
8/4/2011
|
|||||||
|
10.10
†
|
Form of Restricted Stock Unit Agreement under the 2011 Equity Incentive Plan
|
10-Q
|
000-30235
|
10.4
|
8/4/2011
|
|||||||
|
10.11
†
|
Exelixis, Inc. 2014 Equity Incentive Plan
|
8-K
|
000-30235
|
10.1
|
5/29/2014
|
|||||||
|
10.12
†
|
Form of Stock Option Agreement under the Exelixis, Inc. 2014 Equity Incentive Plan
|
10-Q
|
000-30235
|
10.2
|
7/31/2014
|
|||||||
|
10.13
†
|
Form of Stock Option Agreement (International) under the Exelixis, Inc. 2014 Equity Incentive Plan
|
10-Q
|
000-30235
|
10.3
|
7/31/2014
|
|||||||
|
10.14
†
|
Form of Stock Option Agreement (Non-Employee Director) under the Exelixis, Inc. 2014 Equity Incentive Plan
|
10-Q
|
000-30235
|
10.4
|
7/31/2014
|
|||||||
|
10.15
†
|
Form of Restricted Stock Unit Agreement under the Exelixis, Inc. 2014 Equity Incentive Plan
|
10-Q
|
000-30235
|
10.5
|
7/31/2014
|
|||||||
|
10.16
†
|
Form of Restricted Stock Unit Agreement (Non-Employee Director) under the Exelixis, Inc. 2014 Equity Incentive Plan
|
8-K
|
000-30235
|
10.1
|
10/16/2014
|
|||||||
|
10.17
†
|
Non-Employee Director Equity Compensation Policy under the Exelixis, Inc. 2014 Equity Incentive Plan
|
X
|
||||||||||
|
10.18
†
|
Exelixis, Inc. 2016 Inducement Award Plan
|
8-K
|
000-30235
|
10.1
|
11/22/2016
|
|||||||
|
10.19
†
|
Form of Stock Option Agreement under the 2016 Inducement Award Plan
|
8-K
|
000-30235
|
10.2
|
11/22/2016
|
|||||||
|
10.20
†
|
Form of Restricted Stock Unit Agreement under the 2016 Inducement Award Plan
|
8-K
|
000-30235
|
10.2
|
11/22/2016
|
|||||||
|
10.21
†
|
Offer Letter Agreement, dated February 3, 2000, between Michael Morrissey, Ph.D., and Exelixis, Inc.
|
10-Q
|
000-30235
|
10.43
|
8/5/2004
|
|||||||
|
10.22
†
|
Offer Letter Agreement, dated June 30, 2015, between Christopher Senner, and Exelixis, Inc.
|
10-Q
|
000-30235
|
10.5
|
11/10/2015
|
|||||||
|
10.23
†
|
Offer Letter Agreement, dated June 20, 2006, between Exelixis, Inc. and Gisela M. Schwab, M.D.
|
8-K
|
000-30235
|
10.1
|
6/26/2006
|
|||||||
|
10.24
†
|
Offer Letter Agreement, dated February 10, 2014, between Exelixis, Inc. and Jeffrey J. Hessekiel.
|
10-Q
|
000-30235
|
10.4
|
5/1/2014
|
|||||||
|
10.25
†
|
Offer Letter Agreement, dated August 11, 2000, between Exelixis, Inc. and Peter Lamb.
|
10-K
|
000-30235
|
10.24
|
2/29/2016
|
|||||||
|
10.26
†
|
Offer Letter Agreement, dated August 19, 2010, between Exelixis, Inc. and Patrick J. Haley
|
X
|
||||||||||
|
Exhibit
Number
|
Exhibit Description
|
Incorporation by Reference
|
Filed
Herewith
|
|||||||||
|
Form
|
File Number
|
Exhibit/
Appendix
Reference
|
Filing Date
|
|||||||||
|
10.27
†
|
Resignation Agreement dated July 22, 2010, by and between Exelixis, Inc. and George A. Scangos
|
10-Q
|
000-30235
|
10.1
|
11/4/2010
|
|||||||
|
10.28
†
|
Compensation Information for Named Executive Officers (2016 cash bonus and 2017 compensation)
|
8-K
|
000-30235
|
|
2/27/2017
|
|||||||
|
10.29
†
|
Compensation Information for Non-Employee Directors.
|
X
|
||||||||||
|
10.30
†
|
Exelixis, Inc. Change in Control and Severance Benefit Plan, as amended and restated.
|
10-Q
|
000-30235
|
10.2
|
10/27/2011
|
|||||||
|
10.31
|
Lease Agreement, dated May 27, 2005, between Exelixis, Inc. and Britannia Pointe Grand Limited Partnership.
|
8-K
|
000-30235
|
10.1
|
5/27/2005
|
|||||||
|
10.32
|
Loan and Security Agreement, dated May 22, 2002, by and between Silicon Valley Bank and Exelixis, Inc.
|
10-Q
|
000-30235
|
10.34
|
8/6/2002
|
|||||||
|
10.33
|
Loan Modification Agreement, dated December 21, 2004, between Silicon Valley Bank and Exelixis, Inc.
|
8-K
|
000-30235
|
10.1
|
12/23/2004
|
|||||||
|
10.34
|
Amendment No. 7, dated December 21, 2006, to the Loan and Security Agreement, dated May 22, 2002, between Silicon Valley Bank and Exelixis, Inc.
|
8-K
|
000-30235
|
10.1
|
12/27/2006
|
|||||||
|
10.35
|
Amendment No. 8, dated December 21, 2007, to the Loan and Security Agreement, dated May 22, 2002, between Silicon Valley Bank and Exelixis, Inc.
|
8-K
|
000-30235
|
10.1
|
12/26/2007
|
|||||||
|
10.36
|
Amendment No. 9, dated December 22, 2009, to the Loan and Security Agreement, dated May 22, 2002, between Silicon Valley Bank and Exelixis, Inc.
|
8-K
|
000-30235
|
10.1
|
12/23/2009
|
|||||||
|
10.37*
|
Amendment No. 10, dated June 2, 2010, to the Loan and Security Agreement, dated May 22, 2002, by and between Silicon Valley Bank and Exelixis, Inc.
|
10-Q
|
000-30235
|
10.3
|
8/5/2010
|
|||||||
|
10.38*
|
Amendment No. 11, dated August 18, 2011, to the Loan and Security Agreement, dated May 22, 2002, by and between Silicon Valley Bank and Exelixis, Inc.
|
10-Q
|
000-30235
|
10.7
|
10/27/2011
|
|||||||
|
10.39
|
Note Purchase Agreement, dated June 2, 2010, by and between Deerfield Private Design Fund, L.P., Deerfield Private Design International, L.P. and Exelixis, Inc.
|
10-Q
|
000-30235
|
10.1
|
8/5/2010
|
|||||||
|
10.40
|
Consent and Amendment dated as of August 6, 2012 to Note Purchase Agreement, dated as of June 2, 2010, between Exelixis, Inc., Deerfield Private Design Fund, L.P. and Deerfield Private Design International, L.P.
|
8-K
|
000-30235
|
10.1
|
8/6/2012
|
|||||||
|
Exhibit
Number
|
Exhibit Description
|
Incorporation by Reference
|
Filed
Herewith
|
|||||||||
|
Form
|
File Number
|
Exhibit/
Appendix
Reference
|
Filing Date
|
|||||||||
|
10.41
|
Amendment No. 2 dated as of August 1, 2013 to Note Purchase Agreement, dated as of June 2, 2010, between Exelixis, Inc., Deerfield Private Design Fund, L.P. and Deerfield Private Design International, L.P.
|
10-Q
|
000-30235
|
10.1
|
10/30/2013
|
|||||||
|
10.42
|
Amendment No. 3 dated as of January 22, 2013 to Note Purchase Agreement, dated as of June 2, 2010, by and among Exelixis, Inc., Deerfield Private Design Fund, L.P., Deerfield Private Design International, L.P., Deerfield Partners L.P. and Deerfield International Master Fund, L.P.
|
8-K
|
000-30235
|
10.1
|
1/22/2014
|
|||||||
|
10.43
|
Amendment No. 4 dated as of July 10, 2014 to Note Purchase Agreement, dated as of June 2, 2010, by and among Exelixis, Inc., Deerfield Private Design Fund, L.P., Deerfield Private Design International, L.P., Deerfield Partners L.P. and Deerfield International Master Fund, L.P.
|
10-Q
|
000-30235
|
10.1
|
11/4/2014
|
|||||||
|
10.44
|
Security Agreement, dated July 1, 2010, by and between Deerfield Private Design Fund, L.P., Deerfield Private Design International, L.P. and Exelixis, Inc.
|
10-Q
|
000-30235
|
10.2
|
8/5/2010
|
|||||||
|
10.45**
|
Cooperative Research and Development Agreement for Extramural-PHS Clinical Research by and between The U.S. Department of Health and Human Services, as represented by National Cancer Institute, an Institute, Center, or Division of the National Institutes of Health and Exelixis, Inc. dated October 5, 2011
|
X
|
||||||||||
|
10.46
|
Amendment #1 dated April 16, 2013, to Cooperative Research and Development Agreement for Extramural-PHS Clinical Research by and between The U.S. Department of Health and Human Services, as represented by National Cancer Institute, an Institute, Center, or Division of the National Institutes of Health and Exelixis, Inc. dated October 5, 2011
|
X
|
||||||||||
|
10.47
|
Amendment #2 dated July 18, 2016, to Cooperative Research and Development Agreement for Extramural-PHS Clinical Research by and between The U.S. Department of Health and Human Services, as represented by National Cancer Institute, an Institute, Center, or Division of the National Institutes of Health and Exelixis, Inc. dated October 5, 2011
|
X
|
||||||||||
|
Exhibit
Number
|
Exhibit Description
|
Incorporation by Reference
|
Filed
Herewith
|
|||||||||
|
Form
|
File Number
|
Exhibit/
Appendix
Reference
|
Filing Date
|
|||||||||
|
10.48*
|
Collaboration and License Agreement dated February 29, 2016 by and between Exelixis, Inc. and Ipsen Pharma SAS
|
10-Q/A
|
000-30235
|
10.3
|
9/30/2016
|
|||||||
|
10.49**
|
First Amendment dated December 20, 2016, to the Collaboration and License Agreement dated February 29, 2016, by and between Exelixis, Inc. and Ipsen Pharma SAS
|
X
|
||||||||||
|
10.50*
|
Supply Agreement dated February 29, 2016, by and between Exelixis, Inc. and Ipsen Pharma SAS
|
10-Q/A
|
000-30235
|
10.4
|
9/30/2016
|
|||||||
|
10.51**
|
Collaboration Agreement, dated December 22, 2006, between Exelixis, Inc. and Genentech, Inc.
|
X
|
||||||||||
|
10.52**
|
First Amendment, dated March 13, 2008, to the Collaboration Agreement, dated December 22, 2006, between Exelixis, Inc. and Genentech, Inc.
|
X
|
||||||||||
|
10.53
|
Second Amendment, dated April 30, 2010, to the Collaboration Agreement, dated December 22, 2006, between Exelixis, Inc. and Genentech, Inc.
|
10-Q
|
000-30235
|
10.5
|
8/5/2010
|
|||||||
|
12.1
|
Statement Re Computation of Earnings to Fixed Charges
|
X
|
||||||||||
|
21.1
|
Subsidiaries of Exelixis, Inc.
|
X
|
||||||||||
|
23.1
|
Consent of Independent Registered Public Accounting Firm.
|
X
|
||||||||||
|
24.1
|
Power of Attorney (contained on signature page).
|
X
|
||||||||||
|
31.1
|
Certification required by Rule 13a-14(a) or Rule 15d-14(a).
|
X
|
||||||||||
|
31.2
|
Certification required by Rule 13a-14(a) or Rule 15d-14(a).
|
X
|
||||||||||
|
32.1‡
|
Certification by the Chief Executive Officer and the Chief Financial Officer of Exelixis, Inc., as required by Rule 13a-14(b) or 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. 1350).
|
X
|
||||||||||
|
101.INS
|
XBRL Instance Document
|
X
|
||||||||||
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
X
|
||||||||||
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
X
|
||||||||||
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase
|
X
|
||||||||||
|
101.LAB
|
XBRL Taxonomy Extension Labels Linkbase Document
|
X
|
||||||||||
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
X
|
||||||||||
|
†
|
Management contract or compensatory plan.
|
|
*
|
Confidential treatment granted for certain portions of this exhibit.
|
|
**
|
Confidential treatment requested for certain portions of this exhibit.
|
|
‡
|
This certification accompanies this Annual Report on Form 10-K, is not deemed filed with the SEC and is not to be incorporated by reference into any filing of the Company under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of this Annual Report on Form 10-K), irrespective of any general incorporation language contained in such filing.
|