e20vf
UNITED STATES
SECURITIES AND EXCHANGE
COMMISSION
Washington, D.C. 20549
Form 20-F
|
|
|
|
|
(Mark One)
|
|
o
|
|
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b)
OR(g) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
or
|
|
þ
|
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
For the fiscal year ended December 31,
2009
|
|
or
|
|
o
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
For the transition period
from to
|
|
|
|
or
|
|
o
|
|
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF
THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
Date of event requiring this shell company report
|
Commission file number
001-32749
FRESENIUS MEDICAL CARE AG
& Co. KGaA
(Exact name of Registrant as
specified in its charter)
FRESENIUS MEDICAL CARE AG &
Co. KGaA
(Translation of Registrant’s
name into English)
Germany
(Jurisdiction of incorporation or
organization)
Else-Kröner Strasse 1,
61352 Bad Homburg, Germany
(Address of principal executive
offices)
Josef Dinger, +49 6172 608 2522,
Josef.Dinger@FMC-AG.com,
Else-Kröner Strasse 1,
61352 Bad Homburg, Germany
(Name, Telephone,
E-mail
and/or Facsimile number and Address of Company Contact Person)
Securities
registered or to be registered pursuant to Section 12(b) of
the Act:
|
|
|
|
|
Title of each class
|
|
Name of each exchange on which registered
|
|
|
|
American Depositary Shares representing Preference Shares
|
|
New York Stock Exchange
|
|
Preference Shares, no par value
|
|
New York Stock
Exchange
(1)
|
|
American Depositary Shares representing Ordinary Shares
|
|
New York Stock Exchange
|
|
Ordinary Shares, no par value
|
|
New York Stock
Exchange
(1)
|
|
|
|
|
(1)
|
Not for trading, but only in connection with the registration of
American Depositary Shares representing such shares.
|
Securities registered or to be registered pursuant to
Section 12(g) of the Act:
None
Securities for which there is a reporting obligation pursuant
to Section 15(d) of the Act:
7
7
/
8
%
USD Trust Preferred Securities due 2011
7
3
/
8
%
Euro Trust Preferred Securities due 2011
6
7
/
8
% Senior
Notes due 2017
Indicate the number of outstanding shares of each of the
issuer’s classes of capital or common stock as of the close
of the period covered by the annual report:
Preference Shares, no par value: 3,884,328
Ordinary Shares, no par value: 295,746,635
Indicate by check mark if the registrant is a well-known
seasoned issuer, as defined in Rule 405 of the Security Act.
Yes
þ
No
o
If this report is an annual or transition report, indicate by
check mark if the registrant is not required to file reports
pursuant to Section 13 or 15(d) of the Securities Exchange
Act of 1934.
Yes
o
No
þ
Note — Checking the box above will not relieve any
registrant required to file reports pursuant to Section 13
or 15(d) of the Securities Exchange Act of 1934 from their
obligations under those Sections.
Indicate by check mark whether the registrant (1) has filed
all reports required to be filed by Section 13 or 15(d) of
the Securities Exchange Act of 1934 during the preceding
12 months (or for such shorter period that the registrant
was required to file such reports) and (2) has been subject
to such filing requirements for the past 90 days.
Yes
þ
No
o
Indicate by check mark whether the registrant is a large
accelerated filer, an accelerated filer, or a non-accelerated
filer. See definition of “accelerated filer and large
accelerated filer” in
Rule 12b-2
of the Exchange Act. (Check one):
|
|
|
|
|
|
|
|
|
|
|
|
|
Large accelerated
filer
þ
|
|
Accelerated
filer
o
|
|
Non-accelerated
filer
o
|
Indicate by check mark which basis of accounting the registrant
has used to prepare the financial statements included in this
filing:
þ
U.S. GAAP
o
International
Financial Reporting Standards as issued by the International
Accounting Standards Board
o
Other
If “Other” has been checked in response to the
previous question, indicate by check mark which financial
statement item the registrant has elected to follow:
o
Item 17
o
Item 18
If this is an annual report, indicate by check mark whether the
registrant is a shell company (as defined in
Rule 12b-2
of the Exchange Act).
Yes
o
No
þ
Indicate by check mark whether the registrant has submitted
electronically and posted on its corporate Web site, if any,
every Interactive Date File required to be submitted and posted
pursuant to Rule 405 of
Regulation S-T
(§ 232,405 of this chapter) during the preceding
12 months (or for such shorter period that the registrant
was required to submit and post such files).
Yes
þ
No
o
Certain
Defined Terms
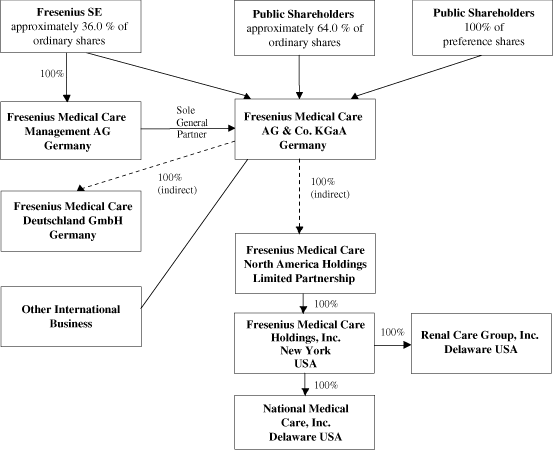
In this report, (1) the “Company” refers to both
Fresenius Medical Care AG prior to the transformation of legal
form discussed in Item 4.A, “Information on the
Company — History and Development of the
Company — History” below and to Fresenius Medical
Care AG & Co. KGaA after the transformation;
(2) “we”, “us” and “our”
refers either to the Company or the Company and its subsidiaries
on a consolidated basis both before and after the
transformation, as the context requires;
(3) “Fresenius Medical Care AG” and
“FMC-AG” refers to the Company as a German stock
corporation before the transformation of legal form and
“FMC-AG & Co. KGaA” refers to the Company as
a German partnership limited by shares after the transformation;
(4) “FMCH” and “D-GmbH” refer,
respectively, to Fresenius Medical Care Holdings, Inc., the
holding company for our North American operations and to
Fresenius Medical Care Deutschland GmbH, one of our German
subsidiaries; (5) “Fresenius SE” refers to
Fresenius SE, a European Company (Societas Europaea) previously
called Fresenius AG, a German stock corporation which owns 100%
of the share capital of our general partner and approximately
36.0% of our ordinary shares as of December 31, 2009 (and
which, prior to the transformation of our legal form, held
approximately 51.8% of our voting shares), and refers to that
company both before and after the conversion of Fresenius AG
from a stock corporation into a European Company on
July 13, 2007; and (6) “Management AG”
refers to Fresenius Medical Care Management AG,
FMC-AG & Co. KGaA’s general partner and a wholly
owned subsidiary of Fresenius SE.
Forward-looking
Statements
This report contains forward-looking statements within the
meaning of Section 27A of the Securities Act of 1933, as
amended, and Section 21E of the Securities Exchange Act of
1934, as amended. When used in this report, the words
“expects,” “anticipates,”
“intends,” “plans,” “believes,”
“seeks,” “estimates” and similar expressions
are generally intended to identify forward looking statements.
Although we believe that the expectations reflected in such
forward-looking statements are reasonable, forward-looking
statements are inherently subject to risks and uncertainties,
many of which cannot be predicted with accuracy and some of
which might not even be anticipated. We have based these
forward-looking statements on current estimates and assumptions
made to the best of our knowledge. By their nature, such
forward-looking statements involve risks, uncertainties,
assumptions and other factors which could cause actual results,
including our financial condition and profitability, to differ
materially and be more negative than the results expressly or
implicitly described in or suggested by these statements.
Moreover, forward-looking estimates or predictions derived from
third parties’ studies or information may prove to be
inaccurate. Consequently, we cannot give any assurance regarding
the future accuracy of the opinions set forth in this report or
the actual occurrence of the developments described herein. In
addition, even if our future results meet the expectations
expressed here, those results may not be indicative of our
performance in future periods.
These risks, uncertainties, assumptions, and other factors that
could cause actual results to differ from our projected results
include, among others, the following:
|
|
|
|
|
|
•
|
changes in governmental and commercial insurer reimbursement for
our products and services, including the mandated change
beginning in 2011 to an expanded “bundled” Medicare
reimbursement system for dialysis services;
|
|
|
|
|
•
|
reductions in erythropoietin, or EPO, utilization or EPO
reimbursement;
|
|
|
|
|
•
|
the outcome of ongoing government investigations;
|
|
|
|
|
•
|
the influence of private insurers and managed care organizations;
|
|
|
|
|
•
|
the impact of pending and future health care reforms;
|
|
|
|
|
•
|
product liability risks;
|
|
|
|
|
•
|
the outcome of ongoing potentially material litigation;
|
|
|
|
|
•
|
risks relating to the integration of acquisitions and our
dependence on additional acquisitions;
|
|
|
|
|
•
|
the impact of currency fluctuations;
|
|
|
|
|
•
|
changes in the cost of pharmaceuticals and utilization patterns;
|
|
|
|
|
•
|
introduction of generic or new pharmaceuticals that compete with
our pharmaceutical products;
|
|
|
|
|
•
|
changes in raw material and energy costs; and
|
|
|
|
|
•
|
other statements of our expectations, beliefs, future plans and
strategies, anticipated development and other matters that are
not historical facts.
|
1
Important factors that could contribute to such differences are
noted in this report under “Risk Factors”,
“Business Overview” in Item 4, “Information
on the Company”, Item 5, “Operating and Financial
Review and Prospects” and in Note 18 of the Notes to
Consolidated Financial Statements, “Legal Proceedings.”
Our business is also subject to other risks and uncertainties
that we describe from time to time in our public filings.
Developments in any of these areas could cause our results to
differ materially from the results that we or others have
projected or may project.
Our reported financial condition and results of operations are
sensitive to accounting methods, assumptions and estimates that
are the basis of our financial statements. The actual accounting
policies, the judgments made in the selection and application of
these policies, and the sensitivities of reported results to
changes in accounting policies, assumptions and estimates, are
factors to be considered along with our financial statements and
the discussion below under “Results of Operations”.
For a discussion of our critical accounting policies, see
Item 5, “Operating and Financial Review and
Prospects — Critical Accounting Policies”.
2
|
|
|
|
Item 1.
|
Identity
of Directors, Senior Management and Advisors
|
Not applicable
|
|
|
|
Item 2.
|
Other
Statistics and Expected Timetable
|
Not applicable
|
|
|
|
A.
|
Selected
Financial Data
|
The following table summarizes the consolidated financial
information for our business for each of the years 2009 through
2005. We derived the selected financial information from our
consolidated financial statements. We prepared our financial
statements in accordance with accounting principles generally
accepted in the United States of America and KPMG AG
Wirtschaftsprüfungsgesellschaft (“KPMG”), an
independent registered public accounting firm, audited these
financial statements. The statement of operations data for 2006
include the results of RCG and such financing costs from
April 1, 2006, the effective date of the RCG acquisition.
You should read this information together with our consolidated
financial statements and the notes to those statements appearing
elsewhere in this document and the information under
Item 5, “Operating and Financial Review and
Prospects.”
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
2005
|
|
|
|
|
(in millions except share and per share amounts)
|
|
|
|
|
Statement of Operations Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net revenues
|
|
$
|
11,247
|
|
|
$
|
10,612
|
|
|
$
|
9,720
|
|
|
$
|
8,499
|
|
|
$
|
6,772
|
|
|
Cost of revenues
|
|
|
7,415
|
|
|
|
6,983
|
|
|
|
6,364
|
|
|
|
5,621
|
|
|
|
4,564
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
3,832
|
|
|
|
3,629
|
|
|
|
3,356
|
|
|
|
2,878
|
|
|
|
2,208
|
|
|
Selling, general and administrative
|
|
|
1,982
|
|
|
|
1,877
|
|
|
|
1,709
|
|
|
|
1,549
|
|
|
|
1,218
|
|
|
Gain on sale of dialysis clinics
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(40
|
)
|
|
|
—
|
|
|
Research and development
|
|
|
94
|
|
|
|
80
|
|
|
|
67
|
|
|
|
51
|
|
|
|
51
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income
|
|
|
1,756
|
|
|
|
1,672
|
|
|
|
1,580
|
|
|
|
1,318
|
|
|
|
939
|
|
|
Interest expense, net
|
|
|
300
|
|
|
|
336
|
|
|
|
371
|
|
|
|
351
|
|
|
|
173
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income before income taxes
|
|
|
1,456
|
|
|
|
1,336
|
|
|
|
1,209
|
|
|
|
967
|
|
|
|
766
|
|
|
Net income attributable to FMC-AG & Co. KGaA
|
|
$
|
891
|
|
|
$
|
818
|
|
|
$
|
717
|
|
|
$
|
537
|
|
|
$
|
455
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average ordinary shares outstanding
|
|
|
294,418,795
|
|
|
|
293,233,477
|
|
|
|
291,929,141
|
|
|
|
290,621,904
|
|
|
|
210,000,000
|
|
|
Basic earnings per Ordinary share and Ordinary ADS
|
|
$
|
2.99
|
|
|
$
|
2.75
|
|
|
$
|
2.43
|
|
|
$
|
1.82
|
|
|
$
|
1.56
|
|
|
Fully diluted earnings per Ordinary share and Ordinary ADS
|
|
|
2.99
|
|
|
|
2.75
|
|
|
|
2.42
|
|
|
|
1.81
|
|
|
|
1.55
|
|
|
Basic earnings per Preference share and Preference ADS
|
|
|
3.02
|
|
|
|
2.78
|
|
|
|
2.45
|
|
|
|
1.85
|
|
|
|
1.58
|
|
|
Fully diluted earnings per Preference share and Preference ADS
|
|
|
3.02
|
|
|
|
2.78
|
|
|
|
2.44
|
|
|
|
1.84
|
|
|
|
1.57
|
|
|
Dividends declared and paid per Ordinary share
(€)
(a)
|
|
|
0.58
|
|
|
|
0.54
|
|
|
|
0.47
|
|
|
|
0.41
|
|
|
|
0.37
|
|
|
Dividends declared and paid per Preference share
(€)
(a)
|
|
|
0.60
|
|
|
|
0.56
|
|
|
|
0.49
|
|
|
|
0.43
|
|
|
|
0.39
|
|
|
Dividends declared and paid per Ordinary
share ($)
(a)
|
|
|
0.78
|
|
|
|
0.85
|
|
|
|
0.64
|
|
|
|
0.52
|
|
|
|
0.47
|
|
|
Dividends declared and paid per Preference
share ($)
(a)
|
|
|
0.81
|
|
|
|
0.88
|
|
|
|
0.67
|
|
|
|
0.55
|
|
|
|
0.49
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance Sheet Data at December 31:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Working capital
|
|
$
|
2,118
|
|
|
$
|
1,068
|
|
|
$
|
833
|
|
|
$
|
1,036
|
|
|
$
|
883
|
|
|
Total assets
|
|
|
15,821
|
|
|
|
14,920
|
|
|
|
14,170
|
|
|
|
13,045
|
|
|
|
7,983
|
|
|
Total long-term debt (excluding current portion)
|
|
|
5,084
|
|
|
|
4,598
|
|
|
|
4,668
|
|
|
|
5,083
|
|
|
|
1,895
|
|
|
Shareholders’ equity
|
|
|
7,030
|
|
|
|
6,123
|
|
|
|
5,681
|
|
|
|
4,945
|
|
|
|
3,988
|
|
|
Capital Stock — Preference shares — Nominal
Value
|
|
|
4
|
|
|
|
4
|
|
|
|
4
|
|
|
|
4
|
|
|
|
91
|
|
|
Capital Stock — Ordinary shares — Nominal
Value
|
|
|
366
|
|
|
|
363
|
|
|
|
361
|
|
|
|
360
|
|
|
|
271
|
|
|
|
|
|
(a)
|
Amounts shown for each year from 2009 to 2005 represent
dividends declared and paid in each such year with respect to
our operations in the year preceding payment. Our general
partner’s Management Board has proposed dividends with
respect to our operations in 2009 of €0.61 per Ordinary
share and €0.63 per Preference share. These dividends are
subject to approval by our shareholders at our Annual General
Meeting to be held on May 11, 2010.
|
We conduct our business on a global basis in various currencies,
although our operations are located principally in the United
States and Germany. We prepare our consolidated financial
statements, from which we derived the selected financial data
above, utilizing the U.S. dollar as our reporting currency.
We have consolidated the balance sheets of our
non-U.S. dollar
denominated operations into U.S. dollars at the exchange
rates prevailing at the balance sheet date. Revenues and
expenses are translated at the average exchange rates for the
period. For information regarding the exchange rates used in
preparing our consolidated financial statements, see
Item 11, “Quantitative and Qualitative Disclosures
About Market Risk — Management of Foreign Exchange and
Interest Rate Risks — Foreign Exchange Risks.”
3
D. Risk
Factors
Before you invest in our securities, you should be aware that
the occurrence of any of the events described in the following
risk factors, elsewhere in or incorporated by reference into
this report, and other events that we have not predicted or
assessed could have a material adverse effect on our results of
operations, financial condition and business. If the events
described below or other unpredicted events occur, then the
trading price of our securities could decline and you may lose
all or part of your investment.
Risks
Relating to Litigation and Regulatory Matters.
A
change in U.S. government reimbursement for dialysis care could
materially decrease our revenues and operating
profit.
For the twelve months ended December 31, 2009,
approximately 33% of our consolidated revenues resulted from
Medicare and Medicaid reimbursement. Legislative changes or
changes in government reimbursement practice may affect the
reimbursement rates for the services we provide, as well as the
scope of Medicare and Medicaid coverage. A decrease in Medicare
or Medicaid reimbursement rates or covered services could have a
material adverse effect on our business, financial condition and
results of operations. For a discussion of the rules proposed by
the Centers for Medicare and Medicaid Services to implement
recent Medicare reimbursement rate changes including provisions
for implementation of a “bundled rate” by
January 1, 2011, see Item 5, “Operating and
Financial Review and Prospects — Overview.”
A
reduction in reimbursement for or a change in the utilization of
EPO could materially reduce our revenue and operating profit. An
interruption of supply or our inability to obtain satisfactory
terms for EPO could reduce our revenues.
Revenue from the administration of erythropoietin, or EPO,
accounted for approximately 21% of total dialysis care revenue
in our North America segment for the year ended
December 31, 2009. Synthetic EPO is produced in the
U.S. by a single source manufacturer, Amgen Inc., under the
brand names
Epogen
®
(epoeitin alfa) and
Aranesp
®
(darbepoetin alfa). Our supply contract with Amgen USA, Inc., a
subsidiary of Amgen, Inc. covers the period from October 1,
2006 to December 31, 2011. Pricing is based on Amgen’s
list price and is subject to change within certain parameters.
Any of the following developments could materially adversely
affect our business, financial condition and results of
operations: (i) an increase in Amgen’s price for EPO
without a corresponding and timely increase in reimbursement for
EPO by the Centers for Medicare and Medicaid Services
(“CMS”), (ii) a reduction of the current overfill
amount in EPO vials which we currently use (liquid medications,
such as EPO, typically include a small overfill amount to ensure
that the fill volume can be extracted from the vial as
administered to the patient), (iii) an interruption of
supply of EPO, or (iv) decreased utilization of EPO. Under
the new expanded bundled reimbursement system to be implemented
by January 1, 2011, material increases in the utilization
of or acquisition costs for EPO or reduction in EPO overfill
could materially and adversely affect our business, financial
condition and results of operations.
If we
do not comply with the many governmental regulations applicable
to our business, we could be excluded from government health
care reimbursement programs or our authority to conduct business
could be terminated, either of which would result in a material
decrease in our revenue.
Our operations in both our provider business and our products
business are subject to extensive governmental regulation in
virtually every country in which we operate. We are also subject
to other laws of general applicability, including antitrust
laws. The applicable regulations, which differ from country to
country, cover areas that include:
|
|
|
|
|
|
•
|
the quality, safety and efficacy of medical and pharmaceutical
products and supplies;
|
|
|
|
|
•
|
the operation of manufacturing facilities, laboratories and
dialysis clinics;
|
|
|
|
|
•
|
accurate reporting and billing for government and third-party
reimbursement; and
|
|
|
|
|
•
|
compensation of medical directors and other financial
arrangements with physicians and other referral sources.
|
Failure to comply with one or more of these laws or regulations,
may give rise to a number of legal consequences. These include,
in particular, monetary and administrative penalties, increased
costs for compliance with government orders, complete or partial
exclusion from government reimbursement programs or complete or
partial curtailment of our authority to conduct business. Any of
these consequences could have a material adverse impact on our
business, financial condition and results of operations.
4
The Company’s pharmaceutical products are subject to
detailed, rigorous and continually changing regulation by the
U.S. Food and Drug Administration (“FDA”), and
numerous other national, supranational, federal and state
authorities. These include, among other things, regulations
regarding manufacturing practices, product labeling, quality
control, quality assurance, advertising and post-marketing
reporting, including adverse event reports and field alerts due
to manufacturing quality concerns. We cannot assure that all
necessary regulation approvals for new products or product
improvements will be granted on a timely basis or at all. In
addition, the Company’s facilities and procedures and those
of its suppliers are subject to periodic inspection by the FDA
and other regulatory authorities. The FDA and comparable
regulatory authorities outside the U.S. may suspend,
revoke, or adversely amend the authority necessary for
manufacture, marketing, or sale of our products and those of our
suppliers. The Company and its suppliers must incur expense and
spend time and effort to ensure compliance with these complex
regulations, and if such compliance is not maintained, could be
subject to significant adverse regulatory actions in the future.
These possible regulatory actions could include warning letters,
fines, damages, injunctions, civil penalties, recalls, seizures
of the Company’s products and criminal prosecution. These
actions could result in, among other things, substantial
modifications to the Company’s business practices and
operations; refunds; a total or partial shutdown of production
while the alleged violation is remedied; and withdrawals or
suspensions of current products from the market. Any of these
events, in combination or alone, could disrupt the
Company’s business and have a material adverse effect on
the Company’s business, financial condition and results of
operations.
We rely upon the Company’s management structure, regulatory
and legal resources and the effective operation of our
compliance programs to direct, manage and monitor our operations
to comply with government regulations. If employees were to
deliberately or inadvertently fail to adhere to these
regulations, then our authority to conduct business could be
terminated and our operations could be significantly curtailed.
Such actions could also lead to claims for repayment or other
sanctions. Any such terminations or reductions could materially
reduce our sales, with a resulting material adverse effect on
our business, financial condition and results of operations.
FMCH and its subsidiaries, including Renal Care Group
(“RCG”) (prior to the RCG Acquisition), received
subpoenas in 2005 from the U.S. Department of Justice for
the Eastern District of Missouri, in connection with a joint
civil and criminal investigation. The subpoenas require
production of a broad range of documents relating to FMCH’s
and RCG’s operations, with specific attention to documents
related to clinical quality programs, business development
activities, medical director compensation and physician
relationships, joint ventures, and anemia management programs,
RCG’s Method II home dialysis supply company,
pharmaceutical and other services that RCG provides to patients,
RCG’s relationships to pharmaceutical companies, and
RCG’s purchase of dialysis equipment from FMCH. The Office
of the Inspector General of the U.S. Department of Health
and Human Services and the U.S. Attorney’s office for
the District of Texas participated in the Eastern District of
Missouri’s investigation of FMCH’s and RCG’s
utilization of Epogen begun in 2005. On July 16, 2007, the
U.S. Attorney’s office filed a civil complaint against
RCG and FMCH in its capacity as RCG’s current corporate
parent in United States District Court, Eastern District of
Missouri. The complaint seeks monetary damages and penalties
with respect to issues arising out of the operation of
RCG’s Method II supply company through 2005, prior to
the date of FMCH’s acquisition of RCG. On August 11,
2009, the Court granted RCG’s motion to transfer venue to
the Middle District of Tennessee (Nashville), where the case is
proceeding toward trial. The Company believes that RCG’s
operation of its Method II supply company complied with
applicable law and will defend this litigation vigorously. We
will continue to cooperate in the ongoing investigation. An
adverse determination in this investigation or litigation or any
settlement arising out of this investigation or litigation could
result in significant financial penalties and possible exclusion
from participation in federal and state health care programs,
and any adverse determination in any litigation arising out of
the investigation could have a material adverse effect on the
Company’s business, financial condition and results of
operations.
If our
joint ventures violate the law, our business could be adversely
affected.
A number of the dialysis centers we operate are owned by joint
ventures in which we hold a controlling interest and one or more
hospitals, physicians or physician practice groups hold a
minority interest. The physician owners may also provide medical
director services to those centers or other centers we own and
operate. The majority of these joint ventures were acquired in
the RCG Acquisition. While we have structured our joint ventures
to comply with many of the criteria for safe harbor protection
under the Federal Anti-Kickback Statute, our investments in
these joint venture arrangements do not satisfy all elements of
such safe harbor. While we have established comprehensive
compliance policies, procedures and programs to ensure ethical
and compliant joint venture business operations, if one or more
of our joint ventures were found to be in violation of the
Anti-Kickback Statute or the Stark Law, we could be required to
restructure or terminate them. We also could be required to
repay to Medicare amounts received by the joint ventures
pursuant to any prohibited referrals, and we could be subject to
monetary penalties and exclusion from Medicare, Medicaid and
other federal and state health care programs.
5
Imposition of any of these penalties could have a material
adverse effect on our business, financial condition and results
of operations.
Proposals
for health care reform could decrease our revenues and operating
profit.
Many of the countries in which we operate have been considering
proposals to modify their current health care systems to improve
access to health care and control costs. We cannot predict
whether and when these reform proposals will be adopted in
countries in which we operate or what impact they might have on
us. In the U.S., Congress has debated bills that, if adopted,
would make significant changes to the health care system. It is
difficult to determine at this time whether a health care reform
bill will be passed and if passed, what the provisions of such a
bill would be. Any significant changes in health care reform
which radically change the financing and regulation of the
health care industry in countries in which we operate,
particularly significant changes in the U.S. Medicare and
Medicaid programs, could reduce our sales and profitability and
have a material adverse effect on our business, financial
condition and results of operations.
Risks
Relating to our Business
A
significant portion of our North American profits are dependent
on the services we provide to a minority of our patients who are
covered by private insurance.
In recent reviews of dialysis reimbursement, the Medicare
Payment Advisory Commission, also known as MedPAC, has noted
that Medicare payments for dialysis services are less than the
average costs that providers incur to provide the services.
Since Medicaid rates are comparable to those of Medicare and
because Medicare only pays us 80% of the Medicare allowable
amount (the patient, Medicaid or secondary insurance being
responsible for the remaining 20%), the amount we receive from
Medicare and Medicaid is less than our average cost per
treatment. As a result, the payments we receive from private
payors both subsidize the losses we incur on services for
Medicare and Medicaid patients and generate a substantial
portion of the profits we report. We estimate that Medicare and
Medicaid are the primary payors for approximately 80% of the
patients to whom we provide care in North America but that only
54% of our North America Dialysis Care net revenues in 2009 were
derived from Medicare and Medicaid. Therefore, if the private
payors who pay for the care of the other 20% of our patients
reduce their payments for our services, or if we experience a
shift in our revenue mix toward Medicare or Medicaid
reimbursement, then our revenue, cash flow and earnings would
decrease, and our cash flow and profits would be
disproportionately impacted.
Over the last few years, we have generally been able to
implement modest annual price increases for private insurers and
managed care organizations, but government reimbursement has
remained flat or has been increased at rates below typical
consumer price index (“CPI”) increases. There can be
no assurance of similar future price increases to private
insurers and managed care organizations. Any reductions in
reimbursement from private insurers and managed care
organizations could adversely impact our operating results. Any
reduction in our ability to attract private pay patients to
utilize our dialysis services relative to historical levels
could adversely impact our operating results. Any of the
following events could have a material adverse effect on our
operating results:
|
|
|
|
|
|
•
|
a portion of our business that is currently reimbursed by
private insurers or hospitals may become reimbursed by managed
care organizations, which generally have lower rates for our
services; or
|
|
|
|
|
•
|
a portion of our business that is currently reimbursed by
private insurers at rates based on our billed charges may become
reimbursed under a contract at lower rates.
|
We are
exposed to product liability, patent infringement and other
claims which could result in significant costs and liability
which we may not be able to insure on acceptable terms in the
future.
Health care companies are typically subject to claims alleging
negligence, product liability, breach of warranty, malpractice
and other legal theories that may involve large claims and
significant defense costs whether or not liability is ultimately
imposed. Health care products may also be subject to recalls and
patent infringement claims. We cannot assure you that such
claims will not be asserted against us, that significant adverse
verdicts will not be reached against us for patent infringements
or that large scale recalls of our products will not become
necessary. In addition, the laws of some of the countries in
which we operate provide legal rights to users of pharmaceutical
products that could increase the risk of product liability
claims. Product liability and patent infringement claims, other
actions for negligence or breach of contract and product recalls
or related sanctions could result in significant costs. These
costs could have a material adverse effect on our business,
financial condition and results of operations. See Note 18
of the Notes to Consolidated Financial Statements, “Legal
Proceedings.”
6
While we have been able to obtain liability insurance in the
past to partially cover our business risks, we cannot assure
that such insurance will be available in the future either on
acceptable terms or at all. In addition, FMCH, our largest
subsidiary, is partially self-insured for professional, product
and general liability, auto liability and worker’s
compensation claims, up to pre-determined levels above which our
third-party insurance applies. A successful claim in excess of
the limits of our insurance coverage could have a material
adverse effect on our business, results of operations and
financial condition. Liability claims, regardless of their merit
or eventual outcome, also may have a material adverse effect on
our business and reputation, which could in turn reduce our
sales and profitability.
The Company is vigorously defending certain patent infringement
lawsuits described in Note 18 of the Notes to Consolidated
Financial Statements, “Legal Proceedings —
Commercial Litigation”. While we believe we have valid
defenses to these claims, an adverse determination in any of
these matters could have a material adverse effect on the
Company’s business, financial condition and results of
operations.
Our
growth depends, in part, on our ability to continue to make
acquisitions.
The health care industry has experienced significant
consolidation in recent years, particularly in the dialysis
services sector. Our ability to make future acquisitions
depends, in part, on our available financial resources and could
be limited by restrictions imposed in the United States of
America or other countries competition laws or under our credit
agreements. If we make future acquisitions, we may need to incur
additional debt, assume significant liabilities or create
additional expenses relating to intangible assets, any of which
might reduce our reported earnings and our earnings per share
and cause our stock price to decline. In addition, any financing
that we might need for future acquisitions might be available to
us only on terms that restrict our business. We may also issue
ordinary shares for non-cash consideration without first
offering the shares to our existing shareholders, which could
dilute the holdings of these shareholders. Acquisitions that we
complete are also subject to risks relating to, among other
matters, integration of the acquired businesses (including
combining the acquired company’s infrastructure and
management information systems with ours, harmonization of its
marketing, patient service and logistical procedures with ours
and, potentially, reconciling divergent corporate and management
cultures), possible non-realization of anticipated synergies
from the combination, potential loss of key personnel or
customers of the acquired companies, and the risk of assuming
unknown liabilities not disclosed by the seller or not
identified during due diligence. If we are not able to effect
acquisitions on reasonable terms, there could be an adverse
effect on our business, financial condition and results of
operations.
We also compete with other dialysis products and services
companies in seeking suitable acquisition targets and the
continuing consolidation of dialysis providers and combinations
of dialysis providers with dialysis product manufacturers could
affect future growth of our product sales. If we are not able to
continue to effect acquisitions on reasonable terms, especially
in the international area, this could have an adverse effect on
our business, financial condition and results of operations.
We
face specific risks from international operations.
We operate dialysis clinics in more than 35 countries and sell a
range of equipment, products and services to customers in over
115 countries. Our international operations are subject to a
number of risks, including but not limited to the following:
|
|
|
|
|
|
•
|
the economic situation in developing countries could deteriorate;
|
|
|
|
|
•
|
fluctuations in exchange rates could adversely affect
profitability;
|
|
|
|
|
•
|
we could face difficulties in enforcing and collecting accounts
receivable under some countries’ legal systems;
|
|
|
|
|
•
|
local regulations could restrict our ability to obtain a direct
ownership interest in dialysis clinics or other operations;
|
|
|
|
|
•
|
political and economic instability, especially in developing and
newly industrializing countries, could disrupt our operations;
|
|
|
|
|
•
|
some customers and governments could have longer payment cycles,
with resulting adverse effects on our cash flow;
|
|
|
|
|
•
|
some countries could impose additional taxes or restrict the
import of our products; and
|
|
|
|
|
•
|
failure to receive or the loss of required licenses,
certifications or other regulatory approvals for operation of
dialysis clinics or sale of equipment, products, or services.
|
7
Any one or more of these or other factors could increase our
costs, reduce our revenues, or disrupt our operations, with
possible material adverse effects on our business, financial
condition and results of operations.
If
physicians and other referral sources cease referring patients
to our dialysis clinics or cease purchasing or prescribing our
dialysis products, our revenues would decrease.
Our dialysis services business is dependent upon patients
choosing our clinics as the location for their treatments.
Patients may select a clinic based, in whole or in part, on the
recommendation of their physician. We believe that physicians
and other clinicians typically consider a number of factors when
recommending a particular dialysis facility to an ESRD patient,
including, but not limited to, the quality of care at a clinic,
the competency of a clinic’s staff, convenient scheduling,
and a clinic’s location and physical condition. Physicians
may change their facility recommendations at any time, which may
result in the transfer of our existing patients to competing
clinics, including clinics established by the physicians
themselves. At most of our clinics, a relatively small number of
physicians often account for the referral of all or a
significant portion of the patient base. Our dialysis care
business also depends on recommendations by hospitals, managed
care plans and other health care institutions. If a significant
number of physicians, hospitals or other health care
institutions cease referring their patients to our clinics, this
would reduce our dialysis care revenue and would materially
adversely affect our overall operations.
The decision to purchase or prescribe our dialysis products and
other services or competing dialysis products and other services
will be made in some instances by medical directors and other
referring physicians at our dialysis clinics and by the managing
medical personnel and referring physicians at other dialysis
clinics, subject to applicable regulatory requirements. A
decline in physician recommendations or recommendations from
other sources for purchases of our products or ancillary
services would reduce our dialysis product and other services
revenue, and could materially adversely affect our business,
financial condition and results of operations.
Our
pharmaceutical product business could lose sales to generic drug
manufacturers or new branded drugs.
Our branded pharmaceutical product business is subject to
significant risk as a result of competition from manufacturers
of generic drugs and other new competing medicines or therapies.
For information regarding the impact of the launch of a generic
version of our phosphate binder
PhosLo
®
,
see Item 5, “Operating and Financial Review and
Prospects.” We are obligated to make certain minimum annual
royalty payments under certain of our pharmaceutical product
license agreements, irrespective of our annual sales of the
licensed products. Either the expiration or loss of patent
protection for one of our products, or the “at-risk”
launch by a generic manufacturer of a generic version of one of
our branded pharmaceutical products or the launch of new branded
drugs that compete with one or more of our products, could
result in the loss of a major portion of sales of that branded
pharmaceutical product in a very short time period with no
concomitant reduction in our license royalty payment
obligations, which could materially and adversely affect our
business, financial condition and results of operations.
Our
competitors could develop superior technology or otherwise
impact our sales.
We face numerous competitors in both our dialysis services
business and our dialysis products business, some of which may
possess substantial financial, marketing or research and
development resources. Competition and especially new
competitive developments could materially adversely affect the
future pricing and sale of our products and services. In
particular, technological innovation has historically been a
significant competitive factor in the dialysis products
business. The introduction of new products by competitors could
render one or more of our products or services less competitive
or even obsolete.
Global
economic conditions may have an adverse effect on our
businesses.
There was a material deterioration of the global economy and
tightening of the financial markets in 2008 and 2009, and the
outlook for the global economy in 2010 is uncertain. We depend
on the financial markets for access to capital, as do our renal
product customers and commercial healthcare insurers. Limited or
expensive access to capital could make it more difficult for
these customers to do business with us, or to do business
generally, which could adversely affect our businesses. The
continuation, or worsening, of domestic and global economic
conditions could continue to adversely affect our businesses and
results of operations.
If we
are unable to attract and retain skilled medical, technical and
engineering personnel, we may be unable to manage our growth or
continue our technological development.
Our continued growth in the provider business will depend upon
our ability to attract and retain skilled employees, such as
highly skilled nurses and other medical personnel. Competition
for those employees is intense and the current nursing shortage
has increased our personnel and recruiting costs. Moreover, we
believe that future
8
success in the provider business will be significantly dependent
on our ability to attract and retain qualified physicians to
serve as medical directors of our dialysis clinics. If we are
unable to achieve that goal or if doing so requires us to bear
increased costs this could adversely impact our growth and
results of operations.
Our dialysis products business depends on the development of new
products, technologies and treatment concepts to be competitive.
Competition is also intense for skilled engineers and other
technical research and development personnel. If we are unable
to obtain and retain the services of key personnel, the ability
of our officers and key employees to manage our growth would
suffer and our operations could suffer in other respects. These
factors could preclude us from integrating acquired companies
into our operations, which could increase our costs and prevent
us from realizing synergies from acquisitions. Lack of skilled
research and development personnel could impair our
technological development, which would increase our costs and
impair our reputation for production of technologically advanced
products.
Diverging
views of fiscal authorities could require us to make additional
tax payments.
We are in dispute with the German tax authorities and the
U.S. Internal Revenue Service (IRS) on certain tax
deductions disallowed in past tax audits. We are also subject to
ongoing tax audits in the U.S., Germany and other jurisdictions.
We have received notices of unfavorable adjustments and
disallowances in connection with certain of these audits and we
may be subject to additional unfavorable adjustments and
disallowances. We are contesting, and in some cases appealing or
litigating certain of the unfavorable determinations. If our
objections, audit appeals or court claims are unsuccessful, we
could be required to make additional tax payments, which could
have a material adverse impact on our results of operations and
operating cash flow in the relevant reporting period. (See
Item 5, “Operating and Financial Review and
Prospects — B. Liquidity and Capital
Resources — Liquidity” as well as Note 18 of
the Notes to Consolidated Financial Statements, “Legal
Proceedings.”)
Risks
Relating to our Securities
Our
indebtedness may limit our ability to pay dividends or implement
certain elements of our business strategy.
At December 31, 2009, we had consolidated debt of
$5,568 million, including $656 million of our trust
preferred securities, and consolidated total shareholders’
equity of $7,030 million. Our debt could have significant
consequences to our operations and our financial condition. For
example, it could require us to dedicate a substantial portion
of our cash flow from operations, as well as the proceeds of
certain financings and asset dispositions, to payments on our
indebtedness, thereby reducing the availability of our cash flow
and such proceeds to fund working capital, capital expenditures
and for other general corporate purposes.
Our 2006 Senior Credit Agreement, Senior Notes, European
Investment Bank Agreements, Euro Notes and the indentures
relating to our trust preferred securities include covenants
that require us to maintain certain financial ratios or meet
other financial tests. Under our senior credit agreement, we are
obligated to maintain a minimum consolidated fixed charge ratio
(ratio of EBITDAR — consolidated earnings before
interest, taxes, depreciation and amortization (EBITDA) plus
rent — to consolidated fixed charges) and subject to a
maximum consolidated leverage ratio (ratio of consolidated
funded debt to EBITDA).
Our 2006 Senior Credit Agreement and our indentures include
other covenants which, among other things, restrict or have the
effect of restricting our ability to dispose of assets, incur
debt, pay dividends and other restricted payments, create liens
or make investments or acquisitions. These covenants may
otherwise limit our activities. The breach of any of the
covenants could result in a default and acceleration of the
indebtedness under the credit agreement or the indentures, which
could, in turn, create additional defaults and acceleration of
the indebtedness under the agreements relating to our other
long-term indebtedness which would have an adverse effect on our
business, financial condition and results of operations
Fresenius
SE owns 100% of the shares in the general partner of our Company
and is able to exercise management control of FMC-AG &
Co. KGaA.
Fresenius SE owns approximately 36.0% of our voting ordinary
shares and 100% of the outstanding shares of the general partner
of the Company. As the sole shareholder of Fresenius Medical
Care Management AG, the general partner of the Company,
Fresenius SE has the sole right to elect the supervisory board
of the general partner which, in turn, appoints the management
board of the general partner. The management board of the
general partner is responsible for the management of the
Company. Through its ownership of the general partner, Fresenius
SE is able to exercise de facto management control of
FMC-AG & Co. KGaA even though it owns less than a
majority of our outstanding voting shares. Such de facto control
limits public shareholder influence on management of the
9
Company and precludes a takeover or change of control of the
Company without Fresenius SE’s consent, either or both of
which could adversely affect the prices of our shares.
Because
we are not organized under U.S. law, we are subject to certain
less detailed disclosure requirements under U.S. federal
securities laws.
Under the pooling agreement that we have entered into for the
benefit of minority holders of our ordinary shares and holders
of our preference shares (including, in each case, holders of
American Depositary Receipts representing beneficial ownership
of such shares), we have agreed to file quarterly reports with
the SEC, to prepare annual and quarterly financial statements in
accordance with United States generally accepted accounting
principles (“U.S. GAAP”), and to file information
with the SEC with respect to annual and general meetings of our
shareholders. These pooling agreements also require that the
supervisory board of Fresenius Medical Care Management AG, our
general partner, include at least two members who do not have
any substantial business or professional relationship with
Fresenius SE, Fresenius Medical Care Management AG or
FMC-AG & Co. KGaA and its affiliates and requires the
consent of those independent directors to certain transactions
between us and Fresenius SE and its affiliates.
We are a “foreign private issuer,” as defined in the
SEC’s regulations, and consequently we are not subject to
all of the same disclosure requirements applicable to domestic
companies. We are exempt from the SEC’s proxy rules, and
our annual reports contain less detailed disclosure than reports
of domestic issuers regarding such matters as management,
executive compensation and outstanding options, beneficial
ownership of our securities and certain related party
transactions. Also, our officers, directors and beneficial
owners of more than 10% of our equity securities are exempt from
the reporting requirements and short-swing profit recovery
provisions of Section 16 of the Securities Exchange Act of
1934. We are also generally exempt from most of the governance
rules applicable to companies listed on the New York Stock
Exchange, other than the obligation to maintain an audit
committee in accordance with Rule 10A-3 under the
Securities Exchange Act of 1934, as amended. These limits on
available information about our company and exemptions from many
governance rules applicable to U.S. domestic issuers may
adversely affect the market prices for our securities.
|
|
|
|
Item 4.
|
Information
on the Company
|
|
|
|
|
A.
|
History
and Development of the Company
|
General
Fresenius Medical Care AG & Co. KGaA
(“FMC-AG & Co. KGaA” or the
“Company”), is a German partnership limited by shares
(
Kommanditgesellschaft auf Aktien
), formerly known as
Fresenius Medical Care AG (“FMC-AG”), a German stock
corporation (
Aktiengesellschaft
) organized under the laws
of Germany.
The Company was originally incorporated on August 5, 1996
as a stock corporation and transformed into a partnership
limited by shares upon registration on February 10, 2006.
FMC-AG & Co. KGaA is registered with the commercial
register of the local court (Amtsgericht) of Hof an der Saale,
Germany, under the registration number HRB 4019. Our registered
office (
Sitz
) is Hof an der Saale, Germany. Our business
address is Else-Kröner-Strasse 1, 61352 Bad Homburg,
Germany, telephone +49-6172-609-0.
History
The Company was originally created by the transformation of
Sterilpharma GmbH (
Gesellschaft mit beschränkter
Haftung
), a limited liability company under German law
incorporated in 1975, into a stock corporation under German law
(
Aktiengesellschaft
). A shareholder’s meeting on
April 15, 1996 adopted the resolutions for this
transformation and the commercial register registered the
transformation on August 5, 1996.
On September 30, 1996, we completed a series of
transactions to consummate an Agreement and Plan of
Reorganization entered into on February 4, 1996 by
Fresenius SE (then Fresenius AG) and W.R. Grace which we refer
to as the “Merger” elsewhere in this report. Pursuant
to that agreement, Fresenius SE contributed Fresenius Worldwide
Dialysis, its global dialysis business, including its
controlling interest in Fresenius USA, Inc., in exchange for
105,630,000 FMC-AG Ordinary shares. Thereafter, we acquired:
|
|
|
|
|
|
•
|
all of the outstanding common stock of W.R. Grace &
Co., whose sole business at the time of the transaction
consisted of National Medical Care, Inc., its global dialysis
business, in exchange for 94,080,000 Ordinary shares; and
|
|
|
|
|
•
|
the publicly-held minority interest in Fresenius USA, Inc., in
exchange for 10,290,000 Ordinary shares.
|
10
Effective October 1, 1996, we contributed all our shares in
Fresenius USA, Inc., to Fresenius Medical Care Holdings, Inc.,
which conducts business under the trade name Fresenius Medical
Care North America, and which is the managing company for all of
our operations in the U.S., Canada and Mexico.
On February 10, 2006, the Company completed the
transformation of its legal form under German law as approved by
its shareholders during the Extraordinary General Meeting
(“EGM”) held on August 30, 2005. Upon
registration of the transformation of legal form in the
commercial register of the local court in Hof an der Saale, on
February 10, 2006, Fresenius Medical Care AG’s legal
form was changed from a stock corporation
(
Aktiengesellschaft
) to a partnership limited by shares
(
Kommanditgesellschaft auf Aktien
) with the name
Fresenius Medical Care AG & Co. KGaA. The Company as a
KGaA is the same legal entity under German law, rather than a
successor to the stock corporation. Fresenius Medical Care
Management AG (“Management AG”), a subsidiary of
Fresenius AG (now Fresenius SE), the majority voting shareholder
of FMC-AG prior to the transformation, is the general partner of
FMC-AG & Co. KGaA. Shareholders in FMC-AG &
Co. KGaA participate in all economic respects, including profits
and capital, to the same extent and (except as modified by the
share conversion described below) with the same number of
ordinary and preference shares in FMC-AG & Co. KGaA as
they held in FMC-AG prior to the transformation. Upon
effectiveness of the transformation of legal form, the share
capital of FMC-AG became the share capital of FMC-AG &
Co. KGaA, and persons who were shareholders of FMC-AG became
shareholders of the Company in its new legal form.
Prior to the effectiveness of the transformation, and as
approved by the EGM and by a separate vote of the Company’s
preference shareholders, the Company offered holders of its
non-voting preference shares (including preference shares
represented by American Depositary Shares (ADSs)) the
opportunity to convert their shares into ordinary shares, which
was accepted by the holders of approximately 96% of the
outstanding preference shares. Preference shares that were not
converted remained outstanding and became preference shares of
FMC-AG & Co. KGaA in the transformation.
On March 31, 2006, the Company completed the acquisition of
RCG (the “RCG Acquisition”), a Delaware corporation
with principal offices in Nashville, Tennessee, for an all cash
purchase price, net of cash acquired, of approximately
$4.2 billion including the concurrent repayment of
approximately $657.8 million of indebtedness of RCG.
All share and per share amounts in the consolidated financial
statements, the related notes and elsewhere in this report have
been restated to reflect our
three-for-one
share split completed June 15, 2007.
Capital
Expenditures
We invested, by business segment and geographical areas, the
amounts shown in the table below during the twelve month periods
ended December 31, 2009, 2008, and 2007
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Actual
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
(in millions)
|
|
|
|
|
Capital expenditures for property, plant and equipment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
North America
|
|
$
|
299
|
|
|
$
|
385
|
|
|
$
|
334
|
|
|
International
|
|
|
275
|
|
|
|
302
|
|
|
|
239
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Capital Expenditures
|
|
$
|
574
|
|
|
$
|
687
|
|
|
$
|
573
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquisitions and Investments
|
|
|
|
|
|
|
|
|
|
|
|
|
|
North America
|
|
$
|
124
|
|
|
$
|
135
|
|
|
$
|
63
|
|
|
International
|
|
|
68
|
|
|
|
82
|
|
|
|
92
|
|
|
Corporate
|
|
|
—
|
|
|
|
107
|
|
|
|
204
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Acquisitions and Investments
|
|
$
|
192
|
|
|
$
|
324
|
|
|
$
|
359
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Major areas of capital spending were for the maintenance of
existing clinics and equipment for new clinics. In addition,
expenditures were made for maintenance and expansion of
production facilities in North America, Germany, France, Japan
and China and for the capitalization of machines provided to
customers primarily in the International segment. We finance our
capital expenditures through cash flow from operations or under
existing or new credit facilities.
For additional information regarding our capital expenditures,
See Item 5.B, “Operational and Financial Review and
Prospects — Liquidity — Investing.” For
information regarding recent acquisitions, see Note 3 of
the Notes to Consolidated Financial Statements,
“Acquisitions.”
11
Our
Business
Based on publicly reported sales and number of patients treated,
we are the world’s largest kidney dialysis company,
operating in both the field of dialysis products and the field
of dialysis services. See “Renal Industry Overview”
below, for a description of our internal information data
gathering tool. Our dialysis business is vertically integrated,
providing dialysis treatment at our own dialysis clinics and
supplying these clinics with a broad range of products. In
addition, we sell dialysis products to other dialysis service
providers. At December 31, 2009, we provided dialysis
treatment to 195,651 patients in 2,553 clinics worldwide
located in more than 35 countries. In the U.S. we also
perform clinical laboratory testing and provide inpatient
dialysis services and other services under contract to
hospitals. In 2009, we provided approximately 29.4 million
dialysis treatments, an increase of approximately 6% compared to
2008. We also develop and manufacture a full range of equipment,
systems and disposable products, which we sell to customers in
over 115 countries. For the year ended December 31, 2009,
we had net revenues of $11.2 billion, a 6% increase (9% in
constant currency) over 2008 revenues. We derived 68% of our
revenues in 2009 from our North America operations and 32% from
our international operations, which include our operations in
Europe (22%), Latin America (5%) and Asia-Pacific (5%). Our
ordinary shares and our preference shares are listed in the
Frankfurt Stock Exchange and American Depositary Receipts
evidencing our ordinary shares and our preference shares on the
New York Stock Exchange, and on February 19, 2010 we had a
market capitalization of $15.2 billion.
We use the insight we gain when treating patients in developing
new and improved products. We believe that our size, our
activities in both dialysis care and dialysis products and our
concentration in specific geographic areas allow us to operate
more cost-effectively than many of our competitors.
We estimate that in 2009, the value of the global dialysis
market was approximately $65 billion and grew at 5%,
adjusted for foreign currency translation effects. Approximately
$55 billion represents dialysis services, including the
administration of dialysis drugs, and approximately
$10.5 billion represents sales of dialysis products. The
following table summarizes net revenues for our North America
segment and our International segment in our major categories of
activity -dialysis care and dialysis products- for the three
years ended December 31, 2009, 2008 and 2007.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
(in millions)
|
|
|
|
|
North America
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dialysis Care
|
|
$
|
6,794
|
|
|
$
|
6,247
|
|
|
$
|
6,002
|
|
|
Dialysis Products
|
|
|
818
|
|
|
|
758
|
|
|
|
661
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7,612
|
|
|
|
7,005
|
|
|
|
6,663
|
|
|
International
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dialysis Care
|
|
|
1,556
|
|
|
|
1,490
|
|
|
|
1,211
|
|
|
Dialysis Products
|
|
|
2,079
|
|
|
|
2,117
|
|
|
|
1,846
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3,635
|
|
|
|
3,607
|
|
|
|
3,057
|
|
Renal
Industry Overview
We offer life-maintaining and life-saving dialysis services and
products in a market which is characterized by favorable
demographic development. As a global market leader in dialysis
products and dialysis services, Fresenius Medical Care considers
it important to possess accurate and current information on the
status and development of the global, regional and national
markets.
To obtain and manage this information, Fresenius Medical Care
created an internal information tool called Market &
Competitor Survey (the “MCS”). The MCS is used within
the Company as a tool to collect, analyze and communicate
current, accurate and essential information on the dialysis
market, developing trends, the market position of Fresenius
Medical Care and those of its competitors. Country —
by — country surveys are performed at the end of each
calendar year which focus on the total number of patients
treated for ESRD, the treatment modality selected, products
used, treatment location and the structure of ESRD patient care
providers. The survey has been refined over the years to
facilitate access to more detailed information and to reflect
changes in the development of therapies and products as well as
changes to the structure of our competitive environment. The
questionnaires are distributed to professionals in the field of
dialysis who are in a position to provide ESRD-relevant country
specific information themselves or who can coordinate
appropriate input from contacts with the relevant know-how in
each country. The surveys are then centrally validated and
checked for consistency by cross-referencing them with the most
recent sources of national ESRD information (e.g. registry data
or publications if available) and with the
12
results of surveys performed in previous years. All information
received is consolidated at a global and regional level and
analyzed and reported together with publicly available
information published by our competitors.
Except as otherwise specified below, all patient and market data
in this Report has been derived using our MCS.
End-Stage
Renal Disease
ESRD is the stage of advanced chronic kidney disease that is
characterized by the irreversible loss of kidney function and
requires regular dialysis treatment or kidney transplantation to
sustain life. A normally functioning human kidney removes waste
products and excess water from the blood, which prevents toxin
buildup, water overload and the eventual poisoning of the body.
Most patients suffering from ESRD must rely on dialysis, which
is the removal of toxic waste products and excess fluids from
the body by artificial means. A number of conditions —
diabetes, hypertension, glomerulonephritis and inherited
diseases — can cause chronic kidney disease. The
majority of all people with ESRD acquire the disease as a
complication of one or more of these primary conditions.
There are currently only two methods for treating ESRD: dialysis
and kidney transplantation. Scarcity of compatible kidneys
limits transplants. Therefore, most patients suffering from ESRD
rely on dialysis.
We estimate that at the end of 2009, there were approximately
2.455 million ESRD patients worldwide, of which
approximately 560,000 kidney patients were living with a
transplanted kidney. For many years the number of donated organs
worldwide has continued to be significantly lower than the
number of patients on transplant waiting lists. Consequently,
less than one quarter of the global ESRD population lives with a
donor organ and the remainder receive renal replacement therapy
in the form of dialysis. Despite ongoing efforts by many
regional initiatives to increase awareness of and willingness
for kidney donation, the distribution of patients between the
various treatment modes has remained nearly unchanged over the
past ten years. In both the U.S. and Germany, approximately
30% of all ESRD patients live with a functioning kidney
transplant and approximately 70% require dialysis.
There are two major dialysis methods commonly used today,
hemodialysis (“HD”) and peritoneal dialysis
(“PD”). These are described below under “Dialysis
Treatment Options for ESRD.” Of the estimated
1.895 million dialysis patients treated in 2009,
approximately 1.69 million received HD and about 203,000
received PD. Generally, an ESRD patient’s physician, in
consultation with the patient, chooses the patient treatment
method, which is based on the patient’s medical conditions
and needs. The number of dialysis patients grew by approximately
6-7% in 2009.
The present annual patient growth rate in the U.S., the largest
dialysis market, is around 3-4% per year, while in many
developing countries we see annual growth rates of up to 10%. We
believe that worldwide growth will continue at around 6% per
year. At the end of 2009, there were approximately
465,000 patients in North America (including Mexico),
approximately 313,000 dialysis patients in the 27 countries of
the European Union (E.U.), approximately 237,000 patients
in Europe (excluding the E.U. countries), the Middle East and
Africa, approximately 200,000 patients in Latin America
(excluding Mexico), and approximately 680,000 patients in
Asia (including 294,000 patients in Japan).
Dialysis patient growth rates vary significantly from region to
region. A below average increase in the number of patients is
experienced in the U.S. and Japan, as well as Western and
Central Europe, where patients with terminal kidney failure have
had readily available access to treatment, usually dialysis, for
many years. In contrast, growth rates in the economically weaker
regions were above average, sometimes reaching double digit
figures. This indicates that accessibility to treatment is still
somewhat limited in these countries, but is gradually improving.
We estimate that about 20% of worldwide patients are treated in
the U.S., around 17% in E.U. and approximately 16% in Japan. The
remaining 47% of all dialysis patients are distributed
throughout more than 115 countries in different geographical
regions.
We believe that the continuing growth in the number of dialysis
patients is principally attributable to:
|
|
|
|
|
|
•
|
increased general life expectancy and the overall aging of the
general population;
|
|
|
|
|
•
|
shortage of donor organs for kidney transplants;
|
|
|
|
|
•
|
improved dialysis technology that makes life-prolonging dialysis
available to a larger patient population;
|
|
|
|
|
•
|
greater access to treatment in developing countries; and
|
|
|
|
|
•
|
better treatment and survival of patients with hypertension,
diabetes and other illnesses that lead to ESRD.
|
13
Dialysis
Treatment Options for ESRD
Hemodialysis.
Hemodialysis removes toxins and
excess fluids from the blood in a process in which the blood
flows outside the body through plastic tubes known as bloodlines
into a specially designed filter, called a dialyzer. The
dialyzer separates waste products and excess water from the
blood. Dialysis solution flowing through the dialyzer carries
away the waste products and excess water, and supplements the
blood with solutes which must be added due to renal failure. The
treated blood is returned to the patient. The hemodialysis
machine pumps blood, adds anti-coagulants, regulates the
purification process and controls the mixing of dialysis
solution and the rate of its flow through the system. This
machine can also monitor and record the patient’s vital
signs.
Hemodialysis patients generally receive treatment three times
per week, typically for three to five hours per treatment. The
majority of hemodialysis patients receive treatment at
outpatient dialysis clinics, such as ours, where hemodialysis
treatments are performed with the assistance of a nurse or
dialysis technician under the general supervision of a physician.
Patients can receive treatment at a clinic run by (1) a
public center (government or government subsidiary owned/run),
(2) a health care organization (non-profit organizations
for public benefit purposes), (3) a private center
(owned/run by individual doctors or a group of doctors) or
(4) a company-owned clinic, including multi-clinic
providers (owned/run by a company such as us). There were
approximately 5,400 Medicare-certified ESRD treatment clinics in
the U.S. in 2009 with only around 1% of patients receiving
care in public centers. In 2009, there were approximately 5,000
dialysis clinics in the E.U. treating dialysis patients. Around
47% of dialysis patients received care through public centers,
approximately 13% through centers owned by health care
organizations, around 23% through private centers and around 17%
through company-owned clinics, such as us. In Latin America,
private centers and company-owned clinics predominated, caring
for over 83% of all dialysis patients. In Japan, nephrologists
(doctors who specialize in the treatment of renal patients)
cared for around 80% of the population in their private centers.
Among company-owned clinics, the two largest providers are
Fresenius Medical Care, caring for 195,651 patients and
DaVita, caring for 118,000 patients at the end of 2009. All
other company-owned clinics care for less than
20,000 patients each.
Of the approximately 1.895 million patients who received
dialysis care in 2009, more than 89% were treated with
hemodialysis. Hemodialysis patients represented about 93% of all
dialysis patients in the U.S., approximately 96% of all dialysis
patients in Japan, and, 91% in the E.U. and 85% in the rest of
the world. Within the 15 largest dialysis countries (measured by
number of patients) that account for more than 75% of the world
dialysis population, hemodialysis is the predominate treatment
method in all countries, except Mexico. Based on these data, it
is clear that hemodialysis is the dominant therapy method
worldwide.
Peritoneal Dialysis.
Peritoneal dialysis
removes toxins from the blood using the peritoneum, the membrane
lining covering the internal organs located in the abdominal
area, as a filter. Most peritoneal dialysis patients administer
their own treatments in their own homes and workplaces, either
by a treatment known as continuous ambulatory peritoneal
dialysis or CAPD, or by a treatment known as continuous cycling
peritoneal dialysis or CCPD. In both of these treatments, a
surgically implanted catheter provides access to the peritoneal
cavity. Using this catheter, the patient introduces a sterile
dialysis solution from a solution bag through a tube into the
peritoneal cavity. The peritoneum operates as the filtering
membrane and, after a specified dwell time, the solution is
drained and disposed. A typical CAPD peritoneal dialysis program
involves the introduction and disposal of dialysis solution four
times a day. With CCPD, a machine pumps or “cycles”
solution to and from the patient’s peritoneal cavity while
the patient sleeps. During the day, one and a half to two liters
of dialysis solution remain in the abdominal cavity of the
patient. The human peritoneum can only be used as a dialyzer for
a limited period of time, ideally only if the kidneys are still
functioning to some extent.
14
Our
Strategy and Competitive Strengths
Growth
Objectives
GOAL 10 is our long-term strategy for sustained growth through
2010. The strategy was implemented in the spring of 2005. Our
GOAL 10 objectives, and our annual progress toward achieving
those objectives, are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Annual Progress
|
|
|
Goal
|
|
Outlook
|
|
|
|
|
2004
|
|
|
2005
|
|
|
2006
|
|
|
2007
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
2010
|
|
|
|
|
Revenue ($ million)
|
|
$
|
6,228
|
|
|
$
|
6,772
|
|
|
$
|
8,499
|
|
|
$
|
9,720
|
|
|
$
|
10,612
|
|
|
$
|
11,247
|
|
|
$>11,500
|
|
|
>12,000
|
|
|
Annual revenue growth
|
|
|
10%
|
|
|
|
8%
|
|
|
|
25%
|
|
|
|
12%
|
|
|
|
8%
|
|
|
|
9%
|
|
|
~6–9%
|
|
|
~7–9%
|
|
|
Share of dialysis market*
|
|
|
12%
|
|
|
|
13%
|
|
|
|
16%
|
|
|
|
16%
|
|
|
|
16%
|
|
|
|
17%
|
|
|
~18%
|
|
|
|
|
|
Market volume* ($ in billion)
|
|
$
|
~50
|
|
|
$
|
~52.5
|
|
|
$
|
~55
|
|
|
$
|
~61.5
|
|
|
$
|
~65
|
|
|
$
|
~65
|
|
|
$ ~67
|
|
|
|
|
|
Annual net income attributable to FMC-AG & Co. KGaA
growth percentage**
|
|
|
21%
|
|
|
|
17%
|
|
|
|
24%
|
|
|
|
25%
|
|
|
|
14%
|
|
|
|
9%
|
|
|
|
|
|
7-10%
|
|
|
Compound Annual Growth (Basis 2003)
|
|
|
21%
|
|
|
|
19%
|
|
|
|
21%
|
|
|
|
21%
|
|
|
|
20%
|
|
|
|
18%
|
|
|
Low to
mid teens
|
|
|
|
|
|
|
|
|
*
|
Company estimates
|
|
|
|
**
|
2005 excluding one-time effects, 2006 excluding one-time effects
and FAS 123(R) and 2007 excluding one-time effects
|
We increased our long-term revenue goals in 2006 mostly as a
result of the RCG acquisition in 2006. Our aim now is to
generate revenue greater than $11.5 billion by
2010 — $1.5 billion more than originally planned
in 2005, and we presently estimate that we will generate
revenues in excess of $12 billion in 2010. See Outlook in
table above and Item 5.B, “Operating and Financial
Review and Prospects — Liquidity and Capital
Resources — Outlook.” We expect to have an 18%
share of the worldwide dialysis market in 2010; we had
previously estimated it would be approximately 15%.
Growth
Paths
GOAL 10 defines four paths that the Company intends to take in
order to perform successfully in a broader spectrum of the
global dialysis market and to achieve our growth and
profitability objectives:
Path 1:
Organic Growth
In the coming years, we intend to achieve an annual organic
sales growth in dialysis care of 5% to 6%. To meet this goal, we
are further expanding our offer of integrated, innovative
treatment concepts such as
UltraCare
®
,
Nephro-Care and our recently introduced Protect, Preserve and
Prolong (“P3”) comprehensive PD therapy program as
well as Cardioprotective Hemodialysis, which uses our Body
Composition Monitor to measure patient water levels, a major
factor in the cardiovascular health of dialysis patients (see
Item 5.C, “Research and Development”) and
combining these treatments, for example, with our dialysis
drugs. With these measures, we want our portfolio of services to
stand out from those of our competitors. In addition, we plan to
increase our growth in revenue by opening new dialysis clinics
(including
70-80
new
clinics annually in the U.S. alone over the next three
years) and to further increase the number of patients whose
treatments are covered by private health insurance.
We also intend to continue to innovate with dialysis products.
New high-quality products such as the 5008 therapy system and
cost-effective manufacturing are intended to contribute
significantly to the further growth of our dialysis products
sector.
Path 2:
Acquisitions
We intend to make attractive, targeted acquisitions broadening
our network of dialysis clinics. In North America we want to
expand our clinic network in particularly attractive regions.
The acquisition of Renal Care Group is an excellent example of
this type of expansion although subsequent acquisitions have had
and future acquisitions in North America will have a smaller
financial scope.
Outside North America, we intend to participate in the
privatization process of healthcare systems and seek to achieve
above-average growth in Eastern Europe and Asia; acquisitions
will support these activities. In our clinic network outside
North America, we continue to focus on improving our strategic
position in selected markets.
Path 3:
Horizontal Expansion
We plan on opening up new growth opportunities in the dialysis
market by expanding our product portfolio beyond patient care
and dialysis products. To this end, beginning in 2006 we
increased our activities in some areas of dialysis medication
and will continue to do so in the future. Initially, we focused
on drugs regulating patients’
15
mineral and blood levels, including phosphate binders, iron and
Vitamin D supplements and calcimimetics. High phosphate levels
in the blood can lead to medium-term damage of patients’
bones and blood vessels. In 2006, we acquired the
PhosLo
®
phosphate binder business of Nabi Biopharmaceuticals, and in
2008 we entered into license and distribution agreements to
market and distribute intravenous iron products such as
Venofer
®
and
Ferinject
®
for dialysis treatment. See the discussion of “Renal
Pharmaceuticals” below.
Path 4:
Home Therapies
Around 11% of all dialysis patients perform dialysis at home,
principally PD, with the remaining 89% treated in clinics.
Still, we aim to achieve a long-term leading global position in
the relatively small field of home therapies, including
peritoneal dialysis and home hemodialysis. To achieve this goal,
we can combine our comprehensive and innovative product
portfolio with our expertise in patient care. In 2007 we
acquired Renal Solutions, Inc. which owns technology that can be
utilized to significantly reduce water volumes used in
hemodialysis, an important step in advancing home hemodialysis,
and on December 14, 2009, a subsidiary of FMCH entered into
an agreement to purchase substantially all the assets of
Xcorporeal, Inc. (“Xcorporeal”) and National Quality
Care, Inc. (“NQCI”). Xcorporeal, under license from
NQCI, has completed functional prototypes of a portable
artificial kidney for attended and home dialysis care and has
demonstrated a feasibility prototype of a wearable artificial
kidney.
We expect these strategic steps, expansion of our product
portfolio horizontally through an increase of our dialysis drug
activities (Path 3), further development of our home therapies
(Path 4) and organic growth in dialysis services (Path 1),
to produce average annual revenue growth of about 6% to 9%,
reaching more than $11.5 billion in 2010. Our annual net
income growth, in percent, is expected to range in the low to
mid teens by 2010, assuming constant currencies in 2009 and 2010.
Our
Competitive Strengths
We believe that we are well positioned to meet our strategic
objectives. Our competitive strengths include:
Our
Leading Market Position
Based on publicly reported sales and number of patients treated,
we are the world’s largest kidney dialysis company,
operating in both the field of dialysis products and the field
of dialysis services. We use the insight we gain when treating
patients in developing new and improved products. We believe
that our size, our activities in both dialysis care and dialysis
products and our concentration in specific geographic areas
allow us to operate more cost-effectively than many of our
competitors.
Our Full
Spectrum of Dialysis and Laboratory Services
We provide expanded and enhanced patient services, including
renal pharmaceutical products and laboratory services, to both
our own clinics and those of third parties. We have developed
disease state management methodologies, which involve the
coordination of holistic patient care for ESRD patients and
which we believe are attractive to managed care payors. We
provide ESRD and chronic kidney disease management programs to
about 4,000 patients. We also operate a surgical center for
the management and care of vascular access for ESRD patients,
which can decrease hospitalization.
Differentiated
Patient Care Programs from those of our Competitors
We believe that our
UltraCare
®
Patient Care program offered at our North American dialysis
facilities distinguishes and differentiates our patient care
from that of our competitors.
UltraCare
®
represents our commitment to deliver excellent care to patients
through innovative programs, the latest technology, continuous
quality improvement and a focus on superior customer service.
UltraCare
®
is delivered by highly trained staff, and demonstrated through
dedication, leadership and compassion by every team member,
every day.
Our
Reputation for High Standards of Patient Care and Quality
Products and our Extensive Clinic Network
We believe that our reputation for providing high standards of
patient care is a competitive advantage. With our large patient
population, we have developed proprietary patient statistical
databases which enable us to improve dialysis treatment
outcomes, and further improve the quality and effectiveness of
dialysis products. Our extensive network of dialysis clinics
enables physicians to refer their patients to conveniently
located clinics.
16
Our
Position as an Innovator in Product and Process
Technology
We are committed to technological leadership in both
hemodialysis and peritoneal dialysis products. Our research and
development teams focus on offering patients new products and
therapies in the area of dialysis and other extracorporeal
therapies to improve their quality of life and increase their
life expectancy. We believe that our extensive expertise in
patient treatment and clinical data will further enhance our
ability to develop more effective products and treatment
methodologies. Our ability to manufacture dialysis products on a
cost-effective and competitive basis results in large part from
our process technologies. Over the past several years, we have
reduced manufacturing costs per unit through development of
proprietary manufacturing technologies that have streamlined and
automated our production processes.
Our
Complete Dialysis Product Lines with Recurring Disposable
Products Revenue Streams
We offer broad and competitive hemodialysis and peritoneal
dialysis product lines. These product lines enjoy broad market
acceptance and enable us to serve as our customers’ single
source for all of their dialysis machines, systems and
disposable products.
Our
Worldwide Manufacturing Facilities
We operate
state-of-the-art
production facilities in all major regions — North
America, Europe, Latin America and Asia Pacific — to
meet the demand for our dialysis products, including dialysis
machines, dialyzers, and other equipment and disposables. We
have invested significantly in developing proprietary processes,
technologies and manufacturing equipment which we believe
provides a competitive advantage in manufacturing our products.
Our decentralized manufacturing structure adds to our economies
of scale by reducing transportation costs.
Dialysis
Care
Dialysis
Services
We provide dialysis treatment and related laboratory and
diagnostic services through our network of 2,553 outpatient
dialysis clinics, 1,784 of which are in North America (including
Mexico) and 769 of which are in more than 32 countries outside
of North America. Our operations within North America generated
81% of our 2009 dialysis care revenue and our operations outside
North America generated 19%. Our dialysis clinics are generally
concentrated in areas of high population density. In 2009, we
acquired a total of 73 existing clinics, opened 118 new clinics
and sold or consolidated 26 clinics. The number of patients we
treat at our clinics worldwide increased by about 6%, from
184,086 at December 31, 2008 to 195,651 at
December 31, 2009. For 2009, dialysis services accounted
for 74% of our total revenue.
With our large patient population, we have developed proprietary
patient statistical databases which enable us to improve
dialysis treatment outcomes, and further improve the quality and
effectiveness of dialysis products. We believe that local
physicians, hospitals and managed care plans refer their ESRD
patients to our clinics for treatment due to:
|
|
|
|
|
|
•
|
our reputation for quality patient care and treatment;
|
|
|
|
|
•
|
our extensive network of dialysis clinics, which enables
physicians to refer their patients to conveniently located
clinics; and
|
|
|
|
|
•
|
our reputation for technologically advanced products for
dialysis treatment.
|
At our clinics, we provide hemodialysis treatments at individual
stations through the use of dialysis machines and disposable
products. A nurse attaches the necessary tubing to the patient
and the dialysis machine and monitors the dialysis equipment and
the patient’s vital signs. The capacity of a clinic is a
function of the number of stations and such factors as type of
treatment, patient requirements, length of time per treatment,
and local operating practices and ordinances regulating hours of
operation.
In our North America and International segments, each of our
dialysis clinics is under the general supervision of a physician
medical director. (see “Patients, Physician and Other
Relationships.”) Each dialysis clinic also has an
administrator or clinical manager who supervises the
day-to-day
operations of the facility and the staff. The staff typically
consists of registered nurses and licensed practical nurses. Our
North America clinics also employ patient care technicians, a
social worker, a registered dietician, a unit clerk and
biomedical technicians, while in some countries within our
International segment, the staff also includes technicians,
social workers and dieticians.
As part of the dialysis therapy, we provide a variety of
services to ESRD patients at our dialysis clinics in the U.S.
These services include administering EPO, a synthetic engineered
hormone that stimulates the production of
17
red blood cells. EPO is used to treat anemia, a medical
complication that ESRD patients frequently experience, and we
administer EPO to most of our patients in the U.S. Revenues from
EPO accounted for approximately 21% of our total dialysis care
revenue in our North America segment for the year ended
December 31, 2009. We receive a substantial majority of
this revenue as reimbursements through the Medicare and Medicaid
programs. Amgen Inc. is the sole manufacturer of EPO in U.S. and
any interruption of supply could materially adversely affect our
business, financial condition and results of operations. Our
current contract with Amgen covers the period from October 2006
to December 2011. See “— Regulatory and Legal
Matters — Reimbursement — U.S. —
Erythropoietin (EPO).”
Our clinics also offer services for home dialysis patients, the
majority of whom receive peritoneal dialysis treatment. For
those patients, we provide materials, training and patient
support services, including clinical monitoring,
follow-up
assistance and arranging for delivery of the supplies to the
patient’s residence. (See “— Regulatory and Legal
Matters — Reimbursement — U.S.” for a
discussion of billing for these products and services.) We also
make dialysis services available to patients during vacations in
resorts and on cruise ships.
We also provide dialysis services under contract to hospitals in
the U.S. on an “as needed” basis for hospitalized ESRD
patients and for patients suffering from acute kidney failure.
Acute kidney failure can result from trauma or similar causes,
and requires dialysis until the patient’s kidneys recover
their normal function. We service these patients either at their
bedside, using portable dialysis equipment, or at the
hospital’s dialysis site. Contracts with hospitals provide
for payment at negotiated rates that are generally higher than
the Medicare reimbursement rates for chronic in-clinic
outpatient treatments.
We employ a centralized approach with respect to certain
administrative functions common to our operations. For example,
each dialysis clinic uses our proprietary manuals containing our
standardized operating and billing procedures. We believe that
centralizing and standardizing these functions enhance our
ability to perform services on a cost-effective basis.
The manner in which each clinic conducts its business depends,
in large part, upon applicable laws, rules and regulations of
the jurisdiction in which the clinic is located, as well as our
clinical policies. However, a patient’s attending
physician, who may be the clinic’s medical director or an
unaffiliated physician with staff privileges at the clinic, has
medical discretion to prescribe the particular treatment
modality and medications for that patient. Similarly, the
attending physician has discretion in prescribing particular
medical products, although the clinic typically purchases
equipment, regardless of brand, in consultation with its medical
director. As is generally true in the dialysis industry, at each
dialysis facility a small number of physicians often account for
all or a significant portion of the patient referral base. An
ESRD patient generally seeks treatment at a center that is
convenient to the patient and at which the patient’s
nephrologist has staff privileges.
In the more than 32 countries outside North America in which we
currently operate or manage dialysis clinics we face legal,
regulatory and economic environments varying significantly from
country to country. These individual environments can affect all
aspects of providing dialysis services including our legal
status, the extent to which we can provide dialysis services,
the way we have to organize these services and the system under
which we are reimbursed. (See “— Regulatory and Legal
Matters — Reimbursement — International
(Including Germany and Other
Non-U.S.)”
for further discussion of reimbursement.) Our approach to
managing this complexity utilizes local management to ensure the
strict adherence to the individual country rules and regulations
and international functional departments supporting country
management with processes and guidelines enabling the delivery
of the highest possible quality level of dialysis treatment. We
believe that with this bi-level organization we are able to
provide superior care to dialysis patients under the varying
local frameworks leading to improved patient well-being and to
lower social cost.
Fresenius
UltraCare
®
Program
The
UltraCare
®
program of our North America dialysis services group represents
our commitment to deliver excellent care to patients through
innovative programs,
state-of-the
art technology, continuous quality improvement and a focus on
superior patient service. It combines our latest product
technology with our highly trained and skilled staff to offer
our patients what we believe is a superior level of care. The
basis for this form of treatment is the
Optiflux
®
polysulfone single-use dialyzer.
Optiflux
®
single use dialyzers are combined with our
2008
tm
Hemodialysis Delivery System series, which has advanced online
patient monitoring and Ultra Pure Dialysate, all of which we
feel improve mortality rates and increase the quality of patient
care.
UltraCare
®
program also utilizes several systems to allow the tailoring of
treatment to meet individual patient needs. Among the other
capabilities of this system, staff will be alerted if toxin
clearance is less than the target prescribed for the patient,
and treatment can be adjusted accordingly. The
Ultracare
®
program also includes an annual training program for staff
18
recertification. In 2008 we launched UltraCare
at
Home
tm
which emphasized patient-centered care: offering the full range
of treatment modalities coupled with superior customer service
for patients desiring care in the home setting.
Laboratory
Services
We provide laboratory testing and marketing services in the U.S.
through Spectra Laboratories (“Spectra”). Spectra
provides blood, urine and other bodily fluid testing services to
determine the appropriate individual dialysis therapy for a
patient and to assist physicians in determining whether a
dialysis patient’s therapy regimen, diet and medicines
remain optimal. Spectra, the leading renal clinical laboratory
provider in North America, provides testing for dialysis related
treatments in its two operating laboratories located in New
Jersey and Northern California. During the year ended
December 31, 2009, Spectra performed nearly 57 million
tests for approximately 158,000 dialysis patients in over 2,300
clinics across the U.S., including clinics that we own or
operate.
Acquisitions
and Investments
A significant factor in the growth in our revenue and operating
earnings in prior years has been our ability to acquire health
care businesses, particularly dialysis clinics, on reasonable
terms. Worldwide, physicians own many dialysis clinics that are
potential acquisition candidates for us. In the U.S., doctors
might determine to sell their clinics to obtain relief from
day-to-day
administrative responsibilities and changing governmental
regulations, to focus on patient care and to realize a return on
their investment. Outside of the U.S., doctors might determine
to sell to us
and/or
enter
into joint ventures or other relationships with us to achieve
the same goals and to gain a partner with extensive expertise in
dialysis products and services. Privatization of health care in
Eastern Europe and Asia could present additional acquisition
opportunities.
During 2009 and 2008, we had total acquisitions and investments
of $192 million and $324 million, respectively. Of the
total 2009 acquisitions and investments, the cash consideration
amounted to approximately $188 million, primarily for
acquisitions of dialysis clinics and pharmaceutical licenses. In
2008, the cash consideration amounted to $277 million,
approximately $227 million primarily for acquisitions of
dialysis clinics and licenses and had an investment in a
$50 million loan to Fresenius SE which was repaid on
April 30, 2009. We continued to enhance our presence
outside the U.S. in 2009 by acquiring individual or small groups
of dialysis clinics in selected markets, expanding existing
clinics, and opening new clinics.
Quality
Assurance and Quality Management in Dialysis Care
In order to evaluate the quality of our dialysis treatments in
our worldwide operations, we make use of quality parameters that
are recognized by the dialysis industry, such as hemoglobin
values, phosphate levels, Kt/V values (the ratio of treatment
length to toxic molecule filtration), albumin levels for
assessment of nutritional condition and urea reduction ratio.
The number of days a patient spends hospitalized is also an
important indicator of treatment quality.
In our European region (includes our EU, European non-EU, Middle
East and Africa operations), our quality management activities
are primarily focused on comprehensive development and
implementation of an Integrated Management System
(“IMS”) for quality management. Our goals in this area
include not only meeting quality requirements for our dialysis
clinics and environmental concerns, but also managing the
quality of our dialysis care. This approach results in a IMS
structure that closely reflects existing corporate processes. We
are also able to use the IMS to fulfill many legal and normative
regulations covering service lines. In addition, the quality
management system standard offers a highly flexible structure
that allows us to adapt to future regulations. The most
important of these include, among others, ISO 9001:2000
requirements for quality management systems in combination with
the ISO 14001:2004 standard for environmental management
systems. Our IMS fulfils the ISO-Norm 9001:2000 requirements for
quality management systems and links it with the ISO-Norm
14001:2004 for environmental management systems. At the same
time, the IMS conforms to the medical devices requirements of
ISO-Norm 13485:2003.
Our dialysis clinics’ processes and documentation are
continuously inspected by internal auditors and external
parties. The underlying quality management system is certified
and found to be in compliance with relevant regulations,
requirements and company policies. Newly developed system
evaluation methods, allowing simpler performance comparisons,
are used to identify additional improvement possibilities. In
2009, we had 8 more employees qualified to carry out audits in
accordance with IMS requirements, especially in the nursing care
processes. We introduced our IMS system in 35 dialysis clinics
in 2009 and increased the portion of our European region clinics
that meet the quality management standard of ISO 9001:2000 from
71% to 76%.
19
Additionally, in 2009 in our European region, we developed the
Nephro-Care Excellence Program, which aims to optimize the
benefits of our dialysis care for all stakeholders beginning in
2010. Nephro-Care seeks to improve knowledge, performance and
satisfaction for our patients, our employees, our shareholders,
and the community through the employment of highly skilled and
motivated staff, innovative and efficient programs, the use of
cutting edge technology, and process of continuing improvement
through research and optimisation.
At each of our North America dialysis clinics, a quality
assurance committee is responsible for reviewing quality of care
data, setting goals for quality enhancement and monitoring the
progress of quality assurance initiatives. We believe that we
enjoy a reputation of providing high quality care to dialysis
patients. In 2009, the Company continued to develop and
implement programs to assist in achieving our quality goals. Our
Access Intervention Management Program detects and corrects
arteriovenous access failure in hemodialysis treatment, which is
the major cause of hospitalization and morbidity. In North
America, we have received five comprehensive FDA facility
inspections at our manufacturing locations over the last two
years without a single observation being made. Additionally, our
facilities have undergone annual ISO 13485:2003 inspections,
maintaining all certifications, with no major non-conformances
to the Quality Systems Standard being noted.
Our principal focus of our research and development activities
is the development of new products, technologies and treatment
concepts to optimize treatment quality for dialysis patients.
See Item 5.C, “Operating and Financial Review and
Prospects — Research and Development.”
Sources
of U.S. Dialysis Care Net Revenue
The following table provides information for the years ended
December 31, 2009, 2008 and 2007 regarding the percentage
of our U.S. dialysis treatment services net revenues from
(a) the Medicare ESRD program, (b) private/alternative
payors, such as commercial insurance and private funds,
(c) Medicaid and other government sources and
(d) hospitals.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Medicare ESRD program
|
|
|
50.0
|
%
|
|
|
53.2
|
%
|
|
|
53.2
|
%
|
|
Private / alternative payors
|
|
|
41.1
|
%
|
|
|
37.4
|
%
|
|
|
36.5
|
%
|
|
Medicaid and other government sources
|
|
|
3.6
|
%
|
|
|
3.8
|
%
|
|
|
4.2
|
%
|
|
Hospitals
|
|
|
5.3
|
%
|
|
|
5.6
|
%
|
|
|
6.1
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
100.0
|
%
|
|
|
100.0
|
%
|
|
|
100.0
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Under the Medicare ESRD program, Medicare reimburses dialysis
providers for the treatment of certain individuals who are
diagnosed as having ESRD, regardless of age or financial
circumstances. See “Regulatory and Legal
Matters — Reimbursement.”
Patient,
Physician and Other Relationships
We believe that our success in establishing and maintaining
dialysis clinics, both in the U.S. and in other countries,
depends significantly on our ability to obtain the acceptance of
and referrals from local physicians, hospitals and managed care
plans. A dialysis patient generally seeks treatment at a
conveniently located clinic at which the patient’s
nephrologist has staff privileges. In nearly all our dialysis
clinics, local nephrologists act as practitioners. Our ability
to provide high-quality dialysis care and to fulfill the
requirements of patients and doctors depends significantly on
our ability to enlist nephrologists for our dialysis clinics and
receive referrals from nephrologists, hospitals and general
practitioners.
Medicare ESRD program reimbursement regulations require that a
medical director generally supervise treatment at a dialysis
clinic. Generally, the medical director must be board certified
or board eligible in internal medicine or pediatrics, have
completed a board-approved training program in nephrology and
have at least twelve months of experience providing care to
patients undergoing dialysis. Our medical directors also
generally maintain their own private practices. We have entered
into written agreements with physicians who serve as medical
directors in our clinics. In North America these agreements
generally have an initial term between 5 to 10 years. The
compensation of our medical directors and other contracted
physicians is negotiated individually and depends in general on
local factors such as competition, the professional
qualification of the physician, their experience and their tasks
as well as the size and the offered services of the clinic. The
total annual compensation of the medical directors and the other
contracted physicians is stipulated at least one year in advance
and the medical directors agree to seek to continue to improve
efficiency and quality. We believe that the compensation of our
medical directors is in line with the market.
20
Almost all contracts we enter into with our medical directors in
the United States as well as the typical contracts which we
obtain when acquiring existing clinics, contain non-competition
clauses concerning certain activities in defined areas for a
defined period to time. These clauses do not enjoin the
physicians from performing patient services directly at other
locations/areas. As prescribed by law we do not require
physicians to send patients to us or to specific clinics or to
purchase or use specific medical products or ancillary services.
Competition
Dialysis Services.
Our largest competitors in
the North America segment are DaVita, Inc., Dialysis Clinic
Inc., Renal Advantage Inc. and Diversified Specialty Institutes,
Inc. and, in our International segment, our largest competitors
are Diaverum (formerly the
non-U.S.
dialysis services business of Gambro AB) and Kuratorium für
Heimdialyse in Europe, Show-Kai and Asia Renal Care in Asia
Pacific, and Diaverum and Baxter International Inc. in Latin
America. Ownership of dialysis clinics in the U.S. is
concentrated, consisting of a large number of company-owned
clinic providers, each owning 10 or fewer clinics and a small
number of larger company-owned, multi-clinic providers who own
the majority of U.S. clinics, of which we are the largest. Over
the last decade the dialysis industry has been characterized by
ongoing consolidations. In December 2008, Renal Advantage Inc.,
the fourth largest provider of dialysis services in the United
States, acquired National Renal Alliance, LLC, the tenth largest
provider of dialysis services in the United States.
Internationally, the dialysis services market is much more
fragmented, with a higher degree of public ownership in many
countries.
Many of our dialysis clinics are in urban areas, where there
frequently are many competing clinics in proximity to our
clinics. We experience direct competition from time to time from
former medical directors, former employees or referring
physicians who establish their own clinics. Furthermore, other
health care providers or product manufacturers, some of who have
significant operations, may decide to enter the dialysis
business in the future.
Because in the U.S., government programs are the primary source
of reimbursement for services to the majority of patients,
competition for patients in the U.S. is based primarily on
quality and accessibility of service and the ability to obtain
admissions from physicians with privileges at the facilities.
However, the extension of periods during which commercial
insurers are primarily responsible for reimbursement and the
growth of managed care have placed greater emphasis on service
costs for patients insured with private insurance.
In most countries other than the U.S., we compete primarily
against individual freestanding clinics and hospital-based
clinics. In many of these countries, especially the developed
countries, governments directly or indirectly regulate prices
and the opening of new clinics. Providers compete in all
countries primarily on the basis of quality and availability of
service and the development and maintenance of relationships
with referring physicians.
Laboratory Services.
Spectra competes in the
U.S. with large nationwide laboratories, dedicated dialysis
laboratories and numerous local and regional laboratories,
including hospital laboratories. In the laboratory services
market, companies compete on the basis of performance, including
quality of laboratory testing, timeliness of reporting test
results and cost-effectiveness. We believe that our services are
competitive in these areas.
Dialysis
Products
Based on internal estimates prepared using our MCS, publicly
available market data and our data of significant competitors,
we are the world’s largest manufacturer and distributor of
equipment and related products for hemodialysis and the second
largest manufacturer of peritoneal dialysis products, measured
by publicly reported revenues. We sell our dialysis products
directly and through distributors in over 115 countries. Most of
our customers are dialysis clinics. For the year 2009, dialysis
products accounted for 26% of our total revenue.
We produce a wide range of machines and disposables for
hemodialysis (“HD”), peritoneal (“PD”) and
acute dialysis:
|
|
|
|
|
|
•
|
HD machines and PD cyclers
|
|
|
|
|
•
|
Dialyzers, our largest product group
|
|
|
|
|
•
|
PD solutions in flexible bags
|
|
|
|
|
•
|
HD concentrates, solutions and granulates
|
|
|
|
|
•
|
Bloodlines
|
|
|
|
|
•
|
Systems for water treatment
|
Our product business also includes adsorbers, which are
specialized filters used in other extracorporeal therapies. In
addition we sell products from other producers, including
specific instruments for vascular access as
21
well as other supplies, such as bandages, clamps and injections.
We also include our
PhosLo
®
and
Venofer
®
iron products, and other renal pharmaceutical products business
as part of our dialysis product revenues.
The three largest manufacturers of dialysis products accounted
for almost 70% of the worldwide market in 2009. As the market
leader in this segment, we had approximately 32% of the market
share.
In 2009 in the U.S., our largest business region, our market
share for dialyzers and dialysis machines exceeded 75% of the
independent market, which is defined as all dialysis clinics not
belonging to a major
U.S.-wide
company-owned clinic provider. We manufactured approximately 80%
of all dialysis machines installed in dialysis clinics and
centers and we manufactured more than 90% of all new machines
purchased. Our second largest sales market for newly sold
hemodialysis machines in 2009 was China, where we supplied more
than 2,700 units. We produced over 45% of all hemodialysis
machines currently used in China. The annual growth rate for
product sales in China is estimated as high as 20% for some
product segments.
Overview
The following table shows the breakdown of our dialysis product
revenues into sales of hemodialysis products, peritoneal
dialysis products and other dialysis products.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Total
|
|
|
|
|
|
Total
|
|
|
|
|
|
Total
|
|
|
|
|
|
|
|
Product
|
|
|
% of
|
|
|
Product
|
|
|
% of
|
|
|
Product
|
|
|
% of
|
|
|
|
|
Revenues
|
|
|
Total
|
|
|
Revenues
|
|
|
Total
|
|
|
Revenues
|
|
|
Total
|
|
|
|
|
(in millions)
|
|
|
|
|
Hemodialysis Products
|
|
$
|
2,263.2
|
|
|
|
78
|
|
|
$
|
2,291.9
|
|
|
|
80
|
|
|
$
|
2,007.5
|
|
|
|
80
|
|
|
Peritoneal Dialysis Products
|
|
|
320.2
|
|
|
|
11
|
|
|
|
345.5
|
|
|
|
12
|
|
|
|
326.7
|
|
|
|
13
|
|
|
Other
|
|
|
313.8
|
|
|
|
11
|
|
|
|
237.4
|
|
|
|
8
|
|
|
|
173.1
|
|
|
|
7
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
2,897.2
|
|
|
|
100
|
|
|
$
|
2,874.8
|
|
|
|
100
|
|
|
$
|
2,507.3
|
|
|
|
100
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hemodialysis
Products
We offer a comprehensive hemodialysis product line, including HD
machines, modular components for dialysis machines, polysulfone
dialyzers, bloodlines, HD solutions and concentrates, needles,
connectors, machines for water treatment, data administration
systems and dialysis chairs. We also include our
PhosLo
®
and
Venofer
®
iron products, and other renal drug products as part of our
dialysis product revenues. We continually strive to expand and
improve the capabilities of our hemodialysis systems to offer an
advanced treatment mode at reasonable cost.
Dialysis Machines.
We sell our 4008 Series and
Series 5008 dialysis machines in our International segment.
In North America, we sell our
2008
®
Series machines, modeled on the 4008 Series. The 4008/2008
series is the most widely sold machine for hemodialysis
treatment. In our International segment in 2009, we introduced
our 4008S classic machine which is a basic dialysis machine for
performing conventional HD treatments with limited therapy
options for budget-focused customers. Following the successful
launch of the 5008 series in 2005, we concentrated on the
continued improvement of the reliable operation of our model
5008 dialysis machine in clinical use and under increasingly
varied conditions in international applications during 2009.
These efforts for improvement have taken into account
considerable feedback from our own dialysis clinics as well as
from other customers while focusing on therapeutic, technical,
and economic aspects of the machine. The 5008 series is intended
to gradually replace most of the 4008 series in the coming
years. The successor 5008 contains a number of newly developed
technical components for revised and improved dialysis processes
and is offering the most efficient therapy modality,
Online-Hemodiafilitration, as a standard feature. Significant
advances in the field of electronics enables highly complex
treatment procedures to be controlled and monitored safely and
clearly through dedicated interfaces.
Our dialysis machines offer the following features and
advantages:
|
|
|
|
|
|
•
|
Volumetric dialysate balancing and ultrafiltration control
system. This system, which we introduced in 1977, provides for
safe and more efficient use of highly permeable dialyzers,
permitting efficient dialysis with controlled rates of fluid
removal;
|
|
|
|
|
•
|
Proven hydraulic systems, providing reliable operation and
servicing flexibility;
|
|
|
|
|
•
|
Compatibility with all manufacturers’ dialyzers and a
variety of bloodlines and dialysis solutions, permitting maximum
flexibility in both treatment and disposable products usage;
|
22
|
|
|
|
|
|
•
|
Modular design, which permits us to offer dialysis clinics a
broad range of options to meet specific patient or regional
treatment requirements and specialized modules that provide
monitoring and response capability for selected biophysical
patient parameters, such as body temperature and relative blood
volume. Modular design also allows upgrading through module
substitution without replacing the entire machine;
|
|
|
|
|
•
|
Sophisticated microprocessor controls, touchscreen interfaces,
displays
and/or
readout panels that are adaptable to local language requirements;
|
|
|
|
|
•
|
Battery backup, which continues operation of the blood circuit
and all protective systems up to 20 minutes following a power
failure;
|
|
|
|
|
•
|
Online clearance, measurement of dialyzer clearance for quality
assurance with On-Line Clearance Monitoring, providing immediate
effective clearance information, real time treatment outcome
monitoring, and therapy adjustment during dialysis without
requiring invasive procedures or blood samples;
|
|
|
|
|
•
|
In the series 5008 and 4008H, the most efficient therapy
mode Online-Hemodiafilitration as standard;
|
|
|
|
|
•
|
Online data collection capabilities and computer interfacing
with our TDMS
and/or
FDS08
systems. Our systems enable us to:
|
|
|
|
|
|
|
—
|
monitor and assess prescribed therapy;
|
|
|
|
|
—
|
connect a large number of hemodialysis machines and peripheral
devices, such as patient scales, blood chemistry analyzers and
blood pressure monitors, to a computer network;
|
|
|
|
|
—
|
enter nursing records automatically at bedside;
|
|
|
|
|
—
|
adapt to new data processing devices and trends;
|
|
|
|
|
—
|
perform home hemodialysis with remote monitoring by a staff
caregiver; and
|
|
|
|
|
—
|
record and analyze trends in medical outcome factors in
hemodialysis patients.
|
Dialyzers.
We manufacture our F-Series and FX
premium series of dialyzers using hollow fiber Fresenius
Polysulfone
®
and Helixone membranes from synthetic materials, including our
Optiflux
®
polysulfone single-use dialyzer. We estimate that we are the
leading worldwide producer of polysulfone dialyzers. We believe
that polysulfone offers the following superior performance
characteristics compared to other materials used in dialyzers:
|
|
|
|
|
|
•
|
higher biological compatibility, resulting in reduced incidence
of adverse reactions to the fibers;
|
|
|
|
|
•
|
greater capacity to clear uremic toxins from patient blood
during dialysis, permitting more thorough, more rapid dialysis,
resulting in shorter treatment time; and
|
|
|
|
|
•
|
a complete range of permeability or membrane pore size, which
permits dialysis at prescribed rates — high flux and
low flux, as well as ultra flux for acute dialysis and allows
tailoring of dialysis therapy to individual patients.
|
Other
Hemodialysis Products
We manufacture and distribute arterial, venous, single needle
and pediatric bloodlines. We produce both liquid and dry
dialysate concentrates. Liquid dialysate concentrate is mixed
with purified water by the hemodialysis machine to produce
dialysis solution, which removes the toxins and excess water
from the patient’s blood during dialysis. Dry concentrate,
developed more recently, is less labor-intensive to use,
requires less storage space and may be less prone to bacterial
growth than liquid solutions. We also produce dialysis solutions
in bags, including solutions for priming and rinsing
hemodialysis bloodlines, as well as connection systems for
central concentrate supplies and devices for mixing dialysis
solutions and supplying them to hemodialysis machines. Other
products include solutions for disinfecting and decalcifying
hemodialysis machines, fistula needles, hemodialysis catheters,
and products for acute renal treatment.
Peritoneal
Dialysis Products
We offer a full line of peritoneal dialysis systems and
solutions which include both continuous ambulatory peritoneal
dialysis (CAPD) and continuous cycling peritoneal dialysis
(CCPD) also called automated peritoneal dialysis (APD). In 2008,
we introduced our Body Composition Monitor for home dialysis,
which determines a
23
patient’s body composition (water, body mass and fat) which
assesses a patient’s hydration state to assist in
determining the patient’s therapy. See Item 5.C,
“Operating and Financial Review and Prospects —
Research and Development.”
CAPD Therapy:
We manufacture both systems and
solutions for CAPD therapy. Our product range offers the
following advantages for patients including:
|
|
|
|
|
|
•
|
Fewer possibilities for touch
contamination.
Our unique PIN and DISC technology
was designed to reduce the number of steps in the fluid exchange
process and by doing so has lessened the risk of infection,
particularly in the disconnection step in which the patient
connector is closed automatically without the need for manual
intervention.
|
|
|
|
|
•
|
Improved biocompatibility.
Our new balance and
bicaVera
®
solutions are pH neutral and have very low glucose degradation
products providing greater protection for the peritoneal
membrane.
|
|
|
|
|
•
|
Environmentally friendly material:
Our
stay•safe
®
system is made of
Biofine
®
,
a material, developed by Fresenius, which upon combustion is
reduced to carbon dioxide and water and does not contain any
plasticizers.
|
APD Therapy:
We have been at the forefront of
the development of automated peritoneal dialysis machines since
1980. APD therapy differs from that of CAPD in that fluid is
infused into the patient’s peritoneal cavity while they
sleep. The effectiveness of the therapy is dependant on the
dwell time, the composition of the solution used, the volume of
solution and the time of the treatment, usually 8-10 hours. APD
offers a number of benefits to patients:
|
|
|
|
|
|
•
|
Improved quality of life.
The patient is
treated at night and can lead a more normal life during the day
without fluid exchange every few hours.
|
|
|
|
|
•
|
Improved adequacy of dialysis.
By adjusting
the parameters of treatment it is possible to provide more
dialysis to the patient compared to conventional CAPD therapy.
This therapy offers important options to physicians such as
improving the delivered dose of dialysis for certain patients.
|
Our automated peritoneal dialysis equipment incorporates
microprocessor technology. This offers physicians the
opportunity to program specific prescriptions for individual
patients. Our APD equipment product line includes:
|
|
|
|
|
|
•
|
sleep•safe:
The sleep•safe machine
has been used since 1999. It has automated connection technology
thus further reducing the risk on touch contamination. Another
key safety feature is the barcode recognition system for the
types of solution bags used. This improves compliance and
ensures that the prescribed dosage is administered to the
patient. There is also a pediatric option for the treatment of
infants.
|
|
|
|
|
•
|
North American cycler portfolio:
This
includes: (a) the new
Liberty
®
cycler introduced in 2008 incorporating many new operational and
safety features with an innovative piston driven pumping
cassette design, (b) the
Freedom
®
and
90/2
®
cyclers for pediatric and acute markets, (c) the
Freedom
®
Cycler PD+ with IQ
card
tm
and (d) the Newton
IQ
®
Cycler. The credit card-sized
IQcard
tm
can provide actual treatment details and results for compliance
monitoring to the physician and, when used with the Newton
IQ
tm
Cycler, can upload the patient’s prescription into the
machine. The Newton
IQ
tm
Cycler also pumps waste dialysate directly into a receptacle.
|
|
|
|
|
•
|
Patient Management Software:
We have developed
specific patient management software tools to support both CAPD
and APD therapies in the different regions of the world. These
include: PatientOnLine,
Pack-PD
®
and
Finesse
®
.
These tools can be used by physicians and nurses to design and
monitor treatment protocols thus ensuring that therapy is
optimized and that patient care is maximized.
|
Renal
Pharmaceuticals
We acquired the rights to
PhosLo
®
from Nabi Pharmaceuticals in November 2006. During 2007, we
submitted an application to the U.S. FDA to extend the
PhosLo
®
label indication to pre-dialysis patients with chronic kidney
disease. We also applied for approval of
PhosLo
®
in selected European countries and of OsvaRen, another phosphate
binder that supports bone health, in most EU member states. In
October 2008, a competitor’s generic phosphate binder that
competes with
PhosLo
®
was introduced in the U.S. market. See Item 5,
“Operating and Financial Review and Prospects —
Results of Operations — Year Ended December 31,
2009 Compared to Year Ended December 31, 2008.” In
October 2009, we launched a competing authorized generic version
of the
PhosLo
®
existing gelcap formulation in the U.S. In July 2009, a new drug
application for the liquid formulation of
PhosLo
®
was submitted to the FDA.
24
In July 2008, we entered into two separate and independent
license and distribution agreements, one for the U.S. (with
Galenica Ltd. and Luitpold Pharmaceuticals Inc.) and one for
certain countries in Europe and the Middle East (with Galenica
AG and Vifor (International) AG), to market and distribute
intravenous iron products, such as
Venofer
®
and
Ferinject
®
.
Both drugs are used to treat iron deficiency anemia experienced
by dialysis patients.
Venofer
®
is the leading intravenous iron product worldwide. The agreement
concerns all commercialization activities for these intravenous
iron products in the field of dialysis and became effective on
January 1st, 2009. In North America, a separate license
agreement effective November 1, 2009 provides our
subsidiary FUSA Manufacturing Inc. (“FMI”) with
exclusive rights to manufacture and distribute
Venofer
®
to freestanding (non-hospital based) U.S. dialysis facilities
and, in addition, grants FMI similar rights for
Ferinject
®
(ferric carboxymaltose), a proposed new intravenous iron
medication currently under clinical study in the U.S. The U.S.
license agreement has a term of ten years and includes FMI
extension options. The international agreement has a term of
20 years. For additional information regarding the terms of
the license, see Note 7 of the Notes to Consolidated
Financial Statements, “Intangible Assets and
Goodwill — Intangible Assets: License and Distribution
Agreements.”
Our dialysis services include administering EPO, a synthetic
engineered hormone used to treat anemia in ESRD patients. We
purchase EPO under a multi year contract with Amgen, Inc., the
sole U.S. manufacturer (see “Dialysis Care —
Dialysis Services” above and “— Regulatory
and Legal Matters — Reimbursement —
U.S. — Erythropoietin (EPO)” below).
We estimate that the worldwide market for dialysis drugs
(excluding erythropoietin stimulating agents, such as EPO) in
2009 was more than $2.5 billion. As part of our horizontal
expansion growth path, we intend to continue to integrate the
use of dialysis drugs with our existing product technology,
dialysis treatment and laboratory services.
Customers,
Marketing, Distribution and Service
We sell most of our products to clinics, hospitals and
specialized treatment clinics. With our comprehensive product
line and years of experience in dialysis, we believe that we
have been able to establish and maintain very close
relationships with our clinic customer base on a global basis.
Close interaction between our Sales & Marketing and
R&D personnel enables us to integrate concepts and ideas
that originate in the field into product development. We
maintain a direct sales force of trained salespersons engaged in
the sale of both hemodialysis and peritoneal dialysis products.
Sales & Marketing engages in direct promotional
efforts, including visits to physicians, clinical specialists,
hospitals, clinics and dialysis clinics, and represents us at
industry trade shows. We also sponsor medical conferences and
scientific symposia as a means for disseminating scientific or
technical information. Our clinical nurses provide clinical
support, training and assistance to customers and assist our
sales force. We also use outside distributors to provide sales
coverage in countries that our internal sales force does not
service.
In our basic distribution system, we ship products from
factories to central warehouses which are frequently located
near the factories. From these central warehouses, we distribute
our dialysis products to regional warehouses. We distribute
peritoneal dialysis products to the patient at home, and ship
hemodialysis products directly to dialysis clinics and other
customers. Local sales forces, independent distributors, dealers
and sales agents sell all our products. In the U.S., products
are sold at the customer’s request.
In January 2009, we consolidated our German warehouses in
Gernsheim and Darmstadt into the new central distribution center
in Biebesheim. All warehouse activities and business have been
transferred, resulting in one distribution center servicing
customers in more than 140 countries worldwide. Through this
consolidation, we have been able to increase service level,
quality and responsiveness on customer demands, as well as
decreased stock levels and lower costs.
We offer customer service, training and education in the
applicable local language, and technical support such as field
service, repair shops, maintenance, and warranty regulation for
each country in which we sell dialysis products. We provide
training sessions on our equipment at our facilities in
Schweinfurt, Germany, Waukegan, Illinois, Coppell, Texas and
Manila, Philippines and we also maintain regional service
centers that are responsible for
day-to-day
international service support.
Manufacturing
Operations
We operate
state-of-the-art
production facilities worldwide to meet the demand for machines,
cyclers, dialyzers, solutions, concentrates, mixes, bloodlines,
and disposable tubing assemblies and equipment for water
treatment in dialysis clinics. We have invested significantly in
developing proprietary processes, technologies and manufacturing
equipment which we believe provide a competitive advantage in
manufacturing our products. Our strategically located production
and distribution centers help to reduce transport costs. We are
using our facilities in
25
St. Wendel, Germany and Ogden, Utah as centers of competence for
development and manufacturing. For example, in St. Wendel we
developed in-house an automatic bundling machine for processing
polysulfone fibers. The machine automatically carries out all
steps required to convert hollow fibers for dialyzer production
and to create bundles with a fixed number of fibers —
the core of the dialyzer. We integrated the first automatic
bundling machine into production in 2008 and three additional
machines are scheduled to enter into service in 2010.
We produce and assemble hemodialysis machines and CCPD cyclers
in our Schweinfurt, Germany and our Walnut Creek, California
facilities. We also maintain facilities at our service and local
distribution centers in Argentina, Egypt, France, Italy, The
Netherlands, China, Brazil and Russia for testing and
calibrating dialysis machines manufactured or assembled
elsewhere, to meet local end user market needs. We manufacture
and assemble dialyzers and polysulfone membranes in our St.
Wendel, Germany, L’Arbresle, France, Vrsac, Serbia and
Inukai and Buzen, Japan facilities and at production facilities
of our joint ventures in Belarus, Saudi Arabia and Japan. At our
Ogden, Utah facilities, we manufacture and assemble dialyzers
and polysulfone membranes and manufacture PD solutions. We
manufacture hemodialysis concentrate at various facilities
worldwide, including Italy, Great Britain, Spain, Turkey,
Serbia, Morocco, Argentina, Brazil, Columbia, Australia,
Germany, Canada, Mexico and the U.S. Our PD products are
manufactured in North America, Europe, Latin America, and Asia,
with two of our largest plants for production of PD products in
Germany and the U.S. Our plant in Reynosa, Mexico is the
world’s largest (by volume) bloodline manufacturing
facility. In 2009, our facility in Jiangsu, China, which
produces bloodlines, received approval from health authorities
to produce peritoneal dialysis solutions, and we are in a
position to start the second and final phase of the process for
obtaining pharmaceutical and medical product approval. We are
also pursing the approval process for manufacture of
hemodialysis concentrate and dialyzers in Jiangsu. Our
facilities are inspected on a regular basis by national
and/or
international authorities.
We estimate that in 2009, we supplied approximately 45% of
global dialyzer production. To meet the ever-growing demand for
dialyzers from Fresenius Medical Care, we have just completed a
€38 million expansion of our production capacity for
FX-class premium dialyzers in Germany. With this investment, our
installed dialyzer capacity has increased by 40% from
25 million to 35 million F- and FX-class dialyzers. We
have also expanded our dialyzer production capacities in the
U.S. (Ogden, Utah), from 32 million to 37 million, and
a new assembly line scheduled to commence production in 2010
will further increase capacity to approximately 45 million
dialyzers. In the coming years, our Ogden site will implement
two additional production lines for polysulfone fiber bundles.
We estimate that we manufactured approximately 55% of all
hemodialysis machines sold worldwide in 2009. Due to strong
demand for our dialysis machines, we kept our production for
these machines for the U.S. market on the increased level of
2008. In 2009, production of series 5008 machines for our
International segment rose by 10.6% compared to 2008, due to new
sales of the series 5008 machines as well as replacement
sales for the series 4008 machines although total machine
sales were lower than the previous year.
We operate a comprehensive quality management system in our
production facilities. Raw materials delivered for the
production of solutions are subjected to infra-red and
ultra-violet testing as well as physical and chemical analysis
to ensure their quality and consistency. During the production
cycle, sampling and testing take place in accordance with
applicable quality control measures to assure sterility, safety
and effectiveness of the finished products. The pressure,
temperature and time required for the various processes are
monitored to ensure consistency of unfinished products during
the production process. Through monitoring of environmental
conditions, particle and bacterial content are kept below
permitted limits. We provide regular ongoing training for our
employees in the areas of quality control and proper production
practice. In North America, we are gearing our manufacturing
processes to the “Lean Six Sigma” management system
which is also utilized in our Schweinfurt facility. The focus of
Lean Six Sigma is to achieve a very low error rate which would
result in better quality production results while shortening the
time it takes to manufacture our products. IMS fulfills
ISO 9001:2000 requirements for quality control systems in
combination with the ISO norm 14001:2004 for environmental
control systems. At the same time, IMS conforms to the
requirements for medical devices of ISO norm 13485:2003. We have
implemented our IMS in all our European production sites. (see
also Item 4. Regulatory and Legal Matters —
Facilities and Operational Regulations.)
Environmental
Management
We have integrated environmental protection targets into our
operations. To reach these goals, our IMS has been in use at our
production facilities as well as at a number of dialysis
clinics. IMS fulfills the requirements of quality management
systems as well as environmental management. Environmental goals
are set, adhered to and monitored during all stages of the lives
of our products, from their development to their disposal.
We continually seek to improve our production processes for
environmental compatibility, which frequently generates cost
savings. In 2009, we rolled out the efficiency initiative
“Energy squeeze” in our main European
26
production plants. The target is to save annually 5% of energy
consumption. We have rolled out the integrated software solution
e-con
5 for
management of eco-controlling data, the aim being to reduce the
working time effort while increasing the data quality and
possibilities for data analysis at the place of origin.
In our dialysis facilities, we establish, depending on the
facility and situation concerned, a priority environmental
protection target on which our dialysis clinics concentrate for
at least one year. Environmental performance in other dialysis
facilities is used as the basis for comparisons and targets.
Improvements are implemented on a
site-by-site
basis after evaluation of the site’s performance. More than
200 of our European region dialysis clinics are now certified
according to the environmental management standard ISO
14001-2004.
Our European region dialysis clinics participate also in the
Corporate Environmental Program, the purpose of which is to
improve environmental awareness and ecological efficiency,
comply with new environmental regulations, and expand the number
of ISO
14001-2004
certified clinics. In our North America dialysis clinics, we
have been able to reduce fresh water consumption by one third by
means of a new system of production of purified water and to
reduce electricity consumption, and have implemented recycling
programs for corrugated materials and hemodialysis machines. Use
of heat exchangers enables us to obtain residual heat from water
used for industrial purposes, which we use to heat fresh water
used for dialysis treatment. Our clinics in North America
commenced a reusable sharp containers program in 2009. Targeted
environmental performance criteria in other locations include
fresh water consumption and improved separation of waste.
Sources
of Supply
Our purchasing policy combines worldwide sourcing of
high-quality materials with the establishment of long-term
relationships with our suppliers. Additionally, we carefully
assess the reliability of all materials purchased to ensure that
they comply with the rigorous quality and safety standards
required for our dialysis products and we outsource only if we
believe that a supplier can exceed our own quality standards. An
interactive information system links all our global projects to
ensure that they are standardized and constantly monitored.
We focus on further optimizing procurement logistics and
reducing purchasing costs. Supplemental raw material contracts
for all manufacturers of semi-finished goods will enable us to
improve purchasing terms for our complete network. We also plan
to intensify, where appropriate, our use of internet-based
procurement tools by purchasing raw materials through special
on-line auctions. Our sophisticated routing software enables us
to distribute our supplies to best accommodate customer requests
while maintaining operational efficiency.
New
Product Introductions
The field of dialysis products is mainly characterized by
constant development and refinement of existing product groups
and less by break-through innovations. In 2009, we continued to
expand our activities in the field of ONLINE HDF with the
introduction of MIXED HDF, which tailors dialysis treatment even
more precisely to individual patient needs. We also introduced
the 4008S classic hemodialysis machine in the International
market and obtained FDA approval for the 2008T hemodialysis
machine for the U.S. market, which we expect to launch in 2010.
In addition, we continued research to further improve treatment
quality both in the clinical and home environment. We have
continued to launch the Body Composition Monitor
(“BCM”) in additional markets and are continuing to
research ways to reduce water consumption per treatment. Actual
expenditures on research and development in 2009 were
$94 million (see Item 5.C, “Operating and
Financial Review and Prospects — Research and
Development”).
Patents
and Licenses
As the owner of patents or licensee under patents throughout the
world, we currently hold rights in about 2,700 patents and
patent applications in major markets. Patented technologies that
relate to dialyzers include our in line sterilization method for
in line sterilized medical devices. The generation of
DiaSafe
plus
®
filters and
FX
®
dialyzers are also the subject of patents and pending patent
applications.
The connector-container system for our biBag bicarbonate
concentrate powder container for the 4008 dialysis equipment
series has been patented in the United States, Norway, Finland,
Japan and Europe. The German part of the European patent has
been the subject of invalidity proceedings. A final court
decision in 2009 confirmed the validity of the patent. For
additional information regarding patent infringement claims made
against us, see Note 18 of the Notes to Consolidated
Financial Statements, “Legal Proceedings —
Commercial Litigation.”
A number of patents and pending patent applications relate to
components of the more recent 5008 dialysis equipment series,
including, for example, the pump technology, extracorporeal
blood pressure measurement and connector system for a modified
biBag bicarbonate concentrate container. New applications are
also pending
27
relating to our new
Liberty
®
peritoneal dialysis cycler which has a number of innovative
attributes such as its multi-channel disposable cassette, dual
piston pump and pneumatically locking door.
In 2007 Fresenius Medical Care acquired Renal Solutions Inc. and
its substantial portfolio of patents and applications for renal
sorbent technology. Many of the patents and applications
represent new technology that the Company hopes to utilize in
future products. We recently filed several new patent
applications for improved sorbent designs/formulations developed
since the acquisition as well as for future dialysis devices
that utilize the acquired technology.
One of Fresenius Medical Care AG & Co. KGaA’s
more significant patents, the in-line sterilization method
patent, will expire in 2010 in Germany, the United States and
other countries. The patent for the 4008 biBag connector expires
in 2013 in Germany, the United States, and other countries. The
dates given represent the maximum patent life of the
corresponding patents. Our patent for the polysulfone hollow
fiber has expired. We believe that even after expiration of
patents, our proprietary know how for the manufacture of our
products and our continuous efforts in obtaining targeted patent
protection for newly developed upgrade products will continue to
constitute a competitive advantage. In addition, multiple new
applications have been filed for our next generation
2008
®
hemodialysis machine to be introduced in North America in the
near future.
For peritoneal dialysis, Fresenius Medical Care AG &
Co. KGaA holds protective rights for our polyolefine film,
Biofine
®
,
which is suitable for packaging intravenous and peritoneal
dialysis fluids. This film is currently used only in
non-U.S.
markets. Patents have been granted in Australia, Brazil, Canada,
Germany, Europe, South Korea, Belarus and the United States. A
Japanese patent was revoked as a result of opposition
proceedings. A further patent family describes a special film
for a peelable, non-PVC, multi chamber bag for peritoneal
dialysis solutions. These patents have been granted in Brazil,
Europe, Germany, Japan, South Korea and the United States.
However, proceedings against the registration of this patent are
currently pending in Europe. A series of patents covering tubing
sets for peritoneal dialysis expire in 2010 and 2012 in the
United States.
We believe that our success will continue to depend
significantly on our technology. As a standard practice, we
obtain the legal protections we believe are appropriate for our
intellectual property. Nevertheless, we are in a position to
successfully market a material number of products for which
patent protection has lapsed or where only particular features
have been patented. From time to time our patents may be
infringed by third parties and in such case we will assert our
rights. Initially registered patents may also be subject to
invalidation claims made by competitors in formal proceedings
(oppositions, trials, re-examinations, etc.) either in part or
in whole. In addition, technological developments could suddenly
and unexpectedly reduce the value of some of our existing
intellectual property.
Trademarks
Our principal trademarks are the name “Fresenius” and
the “F” logo, for which we hold a perpetual,
royalty-free license from Fresenius SE the sole stockholder of
our general partner (see Item 7.B, “Related Party
Transactions — Trademarks”).
Competition
The markets in which we sell our dialysis products are highly
competitive. Our competitors in the sale of hemodialysis and
peritoneal dialysis products include Gambro AB, Baxter
International Inc., Asahi Kasei Kuraray Medical Co. Ltd., Bellco
S.r.l., B. Braun Melsungen AG, Nipro Corporation Ltd., Nikkiso
Co., Ltd., NxStage Medical, Inc., Terumo Corporation, Kawasumi
Laboratories Inc., Fuso Pharmaceuticals Industries Ltd., and
Toray Industries, Inc.
Risk
Management
We see risk management as the ongoing task of assessing,
analyzing and evaluating the spectrum of possible and actual
developments and, if necessary, taking corrective measures. Our
far-reaching risk management system enables management to
identify and reduce risks that could threaten our going concern
or growth at an early stage and minimize their impact as much as
possible.
Risk management is part of our integrated management information
system and is based upon group-wide controlling as well as an
internal monitoring system. Regional monitoring systems form the
backbone of our risk management system and watch over all
inherent industry- and market-specific risks. Our management
board receives status reports from the responsible risk managers
twice yearly and immediate information regarding anticipated
risks as the information is developed. These reports include
qualitative and quantitative appraisals of the likelihood of
risks that have been identified as potentially harmful to us as
well as the potential extent of damage.
28
Efficient reporting is essential for controlling and monitoring
risks as well as for taking preventative measures. Management
receives information on a monthly and quarterly basis about the
state of the health care industry and our operative and
non-operative business, as well as analyses of our asset,
financial and earnings position.
Our risk management system is strengthened by the internal audit
department. The department operates in compliance with the
Institute of Internal Auditors (IIA) standards and is
independent of the regions. Annual worldwide audit assignments
are selected based on a risk assessment model. The audit plan is
reviewed by the Management Board and approved by the Audit and
Corporate Governance Committee of the Supervisory Board. This
plan includes financial audits of individual units as well as
full-scope audits of all business processes of a subsidiary or
business unit. The audit reports are sent to the Management
Board and our external auditor. The internal audit department
also monitors the implementation of measures documented in the
audit reports. The Management Board is regularly informed about
the implementation status. In addition, the Audit and Corporate
Governance Committee of the Supervisory Board is informed about
the audit results.
As of May 2009, we are subject to the “German Act on the
Modernisation of Accounting Law (“BilMoG”). The act
contains a number of provisions intended to enhance and improve
the corporate governance of companies participating in the
capital markets. Management has taken BilMoG as an opportunity
for additional review and, if necessary, improvement of the
existing internal reporting and control processes.
As a company required to file reports under the Securities
Exchange Act of 1934, we are subject to the provisions of the
Sarbanes-Oxley Act of 2002. For further information on this
requirement, see Items 15.A. and 15.B, “Disclosure
Controls and Procedures” and “Management’s annual
report on internal control over financial reporting.”
Regulatory
and Legal Matters
Regulatory
Overview
Our operations are subject to extensive governmental regulation
by virtually every country in which we operate including, most
notably, in the U.S., at the federal, state and local levels.
Although these regulations differ from country to country, in
general,
non-U.S.
regulations are designed to accomplish the same objectives as
U.S. regulations regarding the operation of dialysis clinics,
laboratories and manufacturing facilities, the provision of
quality health care for patients, compliance with labor laws,
the maintenance of occupational, health, safety and
environmental standards and the provision of accurate reporting
and billing for governmental payments
and/or
reimbursement. In the U.S., some states establish regulatory
processes that must be satisfied prior to the establishment of
new dialysis clinics. Outside the U.S., each country has its own
payment and reimbursement rules and procedures, and some
countries prohibit ownership of health care providers or
establish other regulatory barriers to direct ownership by
foreign companies. In all jurisdictions, we work within the
framework of applicable laws to establish alternative
contractual arrangements to provide services to those facilities.
Any of the following matters could have a material adverse
effect on our business, financial condition and results of
operations:
|
|
|
|
|
|
•
|
failure to receive required licenses, certifications or other
approvals for new facilities or products or significant delays
in such receipt;
|
|
|
|
|
•
|
complete or partial loss of various federal certifications,
licenses, or other permits required under the laws of any state
or other governmental authority by withdrawal, revocation,
suspension, or termination or restrictions of such certificates
and licenses by the imposition of additional requirements or
conditions, or the initiation of proceedings possibly leading to
such restrictions or the partial or complete loss of the
required certificates, licenses or permits;
|
|
|
|
|
•
|
a non-appealable finding of material violations of U.S. fraud
and abuse laws; and
|
|
|
|
|
•
|
changes resulting from health care reform or other government
actions that reduce reimbursement or reduce or eliminate
coverage for particular services we provide.
|
We must comply with all U.S., German and other legal and
regulatory requirements under which we operate, including the
U.S. federal Medicare and Medicaid Fraud and Abuse Amendments of
1977, as amended, generally referred to as the
“Anti-Kickback Statute”, the federal False Claims Act,
the federal restrictions on certain physician referrals,
commonly known as the “Stark Law”, U.S. federal rules
under the Health Insurance Portability and Accountability Act of
1996 that protect the privacy and security of patient medical
records and prohibit inducements to patients to select a
particular health care provider, commonly known as
“HIPAA”, and other fraud and abuse laws and similar
state statutes, as well as similar laws in other countries.
Moreover, there can be no
29
assurance that applicable laws, or the regulations thereunder,
will not be amended, or that enforcement agencies or the courts
will not make interpretations inconsistent with our own, any one
of which could have a material adverse effect on our business,
reputation, financial condition and operating results. Sanctions
for violations of these statutes may include criminal or civil
penalties, such as imprisonment, fines or forfeitures, denial of
payments, and suspension or exclusion from the Medicare and
Medicaid programs. In the U.S., some of these laws have been
broadly interpreted by a number of courts, and significant
government funds and personnel have been devoted to their
enforcement because such enforcement has become a high priority
for the federal government and some states. Our company, and the
health care industry in general, will continue to be subject to
extensive federal, state and foreign regulation, the full scope
of which cannot be predicted. In addition, the U.S. Congress and
federal and state regulatory agencies continue to consider
modifications to health care laws that may create further
restrictions.
We maintain a comprehensive worldwide compliance program under
the overall supervision of our general partner’s Member of
the Management Board responsible for Legal, who is also our
general counsel and chief compliance officer. The program
includes a compliance staff, a written code of conduct
applicable worldwide, training programs, regulatory compliance
policies and procedures, provisions for anonymous reporting of
violations of applicable laws or Company policies and periodic
internal audits of our compliance procedures. Nevertheless, we
operate many facilities throughout the United States and other
countries in which we do business. In such a decentralized
system, it is often difficult to maintain the desired level of
oversight and control over the thousands of individuals employed
by many affiliated companies. We rely on our management
structure, regulatory and legal resources, and the effective
operation of our compliance program to direct, manage and
monitor the activities of these employees. If our employees,
deliberately or inadvertently, were to submit inadequate or
false billings to any federally-funded health care program, or
engage in impermissible conduct with physicians or other
referral sources or vendors with which we do business, the
actions of such persons could subject us and our subsidiaries to
liability under the Anti-Kickback Statute, the Stark Statute or
the False Claims Act, among other laws. See Note 18 of the
Notes to Consolidated Financial Statements, “Legal
Proceedings — Other Litigation and Potential
Exposures.”
Product
Regulation
U.S.
In the U.S. numerous regulatory bodies, including the Food and
Drug Administration (“FDA”) and comparable state
regulatory agencies impose requirements on certain of our
subsidiaries as a manufacturer and a seller of medical products
and supplies under their jurisdiction. We are required to
register with the FDA as a device manufacturer. As a result, we
are subject to periodic inspection by the FDA for compliance
with the FDA’s Quality System Regulation (21 C.F.R.
Part 820) requirements and other regulations. These
regulations require us to manufacture products in accordance
with current Good Manufacturing Practices (“GMP”) and
that we comply with FDA requirements regarding the design,
safety, labeling, record keeping and distribution of our
products. In 2006, our production site for dialyzers in St.
Wendel successfully passed an FDA GMC audit conducted by
TÜV Süd Product Service Munich under the mutual
recognition agreement between the European Union and the U.S.
Further, we are required to comply with various FDA and other
agency requirements for labeling and promotion. The Medical
Device Reporting regulations require that we provide information
to the FDA whenever there is evidence to reasonably suggest that
a device may have caused or contributed to a death or serious
injury. In addition, the FDA prohibits us from promoting a
product for unapproved indications.
If the FDA believes that a company is not in compliance with
applicable regulations, it can issue a warning letter, issue a
recall order, institute proceedings to detain or seize products,
impose operating restrictions, enjoin future violations and
assess civil penalties against a company, its officers or its
employees and can recommend criminal prosecution to the
Department of Justice.
In addition, in order to clinically test, produce and market
certain medical products and other disposables (including
hemodialysis and peritoneal dialysis equipment, dialyzers,
bloodlines and other disposables) for human use, we must satisfy
mandatory procedures and safety and efficacy requirements
established by the FDA or comparable foreign governmental
agencies. After approval or clearance to market is given, the
FDA, upon the occurrence of certain events, has the power to
withdraw the approval or clearance or require changes to a
device, its manufacturing process, or its labeling or may
require additional proof that regulatory requirements have been
met. Such rules generally require that products be approved or
cleared by the FDA as safe and effective for their intended use
prior to being marketed.
We cannot assure that all necessary regulatory approvals,
including approvals for new products or product improvements,
will be granted on a timely basis, if at all. Delays in or
failure to receive approval, product recalls or warnings and
other regulatory actions and penalties can materially affect
operating results.
30
Some of our products — including our peritoneal
dialysis solutions,
PhosLo
®
,
and
Venofer
®
—
are designated as drugs by the FDA and, as such, are subject to
additional regulation under the Food, Drug, and Cosmetic Act of
1938, as amended. Many of these requirements are similar to
those for devices. Thus, we are required to register with the
FDA and are required to comply with regulatory requirements
regarding drug manufacturing, labeling, distribution, and
recordkeeping. Our drug products must be manufactured in
accordance with cGMP (21 C.F.R. Part 211), and we are
required to provide information to the FDA whenever we become
aware of a report of an adverse drug experience associated with
the use of one of our drug products that is both serious and
unexpected, as defined in FDA regulations. In addition, as with
our medical devices, our drug products must satisfy mandatory
procedures and safety and efficacy requirements in order to
market the products and the FDA prohibits us from promoting a
pharmaceutical product for unapproved indications. Finally, if
the FDA believes that a company is not in compliance with
applicable drug regulations, it has similar enforcement
authorities as those discussed above with respect to medical
devices.
International
(Including Germany and Other Non-U.S).
Most countries maintain different regulatory regimes for
medicinal products and for medical devices. In almost every
country, there are rules regarding the quality, effectiveness,
and safety of products and regulating their testing, production,
and distribution. Treaties or other international law and
standards and guidelines under treaties or laws may supplement
or supersede individual country regulations.
Drugs.
Some of our products, such as
peritoneal dialysis solutions and
PhosLo
®
,
are considered medicinal products and are, therefore subject to
the specific drug law provisions in the various countries. The
European Union has issued a directive on medicinal products,
No. 65/65/EWG (January 26, 1965), as amended. Each
member of the European Union is responsible for conforming its
law to comply with this directive. In Germany the German Drug
Law
(Arzneimittelgesetz)
(“AMG”), which
implements European Union requirements, is the primary
regulation applicable to medicinal products.
The provisions of the German Drug Law are comparable with the
legal standards in other European countries. As in many other
countries, the AMG provides that in principle a medicinal
product may only be placed on the market if it has been granted
a corresponding marketing authorization. Such marketing
authorization is granted by the competent licensing authorities
only if the quality, efficacy and safety of the medicinal
product has been scientifically proven. Medicinal products
marketed on the basis of a corresponding marketing authorization
are subject to ongoing control by the competent authorities. The
marketing authorization may also be subsequently restricted or
made subject to specific requirements. It may be withdrawn or
revoked if there was a reason for the refusal of the marketing
authorization upon its grant or such a reason arises
subsequently, or if the medicinal product is not an effective
therapy or its therapeutic effect has been insufficiently proven
according to the relevant state of scientific knowledge. Such a
reason for refusal is, inter alia, found to exist if there is a
well-founded suspicion that the medicinal product has not been
sufficiently examined in accordance with the current state of
scientific knowledge, that the medicinal product does not show
the appropriate quality, or that the medicinal product, when
properly used as intended, produces detrimental effects going
beyond the extent justifiable according to the current state of
knowledge of medicinal science. The marketing authorization can
also be withdrawn or revoked in the case of incorrect or
incomplete information supplied in the authorization documents,
if the quality checks prescribed for the medicinal product were
insufficient or have not been sufficiently carried out, or if
the withdrawal or revocation is required to comply with a
decision made by the European Commission or the Council of the
European Union. Instead of a withdrawal or revocation, the
suspension of the marketing authorization may be ordered for a
limited period.
The provisions of the AMG and a statutory order,
Arzneimittel- und Wirkstoffherstellungsverordnung
(“AMWHV”), also contain special requirements for the
manufacture of medicinal products. The production of medicinal
products requires a corresponding manufacturing license which is
granted by the competent authorities of the relevant Member
State for a specific manufacturing facility and for specific
medicinal products and forms of medicinal products. The
manufacturing license is granted only if the manufacturing
facility, production techniques and production processes comply
with the national drug law requirements, with the principles and
guidelines of EU-good manufacturing practice
(“EU-GMP”) as well as the terms of the particular
marketing authorization. A manufacturer of medicinal products
must, inter alia, employ pharmacists, chemists, biologists, or
physicians responsible for the quality, safety and efficacy of
the medicinal products. The manufacturer must name several
responsible persons: a Qualified Person (QP) for the release of
the medicinal product into the market possessing the expert
knowledge specified by the AMG, a head of production, a head of
quality control, and, if the manufacturer markets the medicinal
products itself, a commissioner for the so-called graduated plan
(
Stufenplanbeauftragter
for Germany, a Qualified Person
for Pharmacovigilance (QPP) for the European Union) and an
information officer. It is the responsibility of the QP to
ensure that each batch of the medicinal products is produced and
examined in
31
compliance with the statutory provisions of the AMG. The QPP
must, among other things, collect and assess any reported risks
associated with the medicinal products and coordinate any
necessary measures according to German Drug Law. The QPP,
residing within the European Economic Area, is responsible for
pharmacovigilance and the establishment of a system for handling
of all suspected adverse reactions that need to be reported. The
information officer is in charge of the scientific information
relating to the medicinal products. All these persons may be
held personally liable under German criminal law for any breach
of the AMG.
International guidelines also govern the manufacture of
medicinal products and, in many cases, overlap with national
requirements. Material regulations concerning manufacture and
registration related to medicinal products have been issued by
the European Commission and the International Conference on
Harmonization of Technical Requirements for Human Use
(“ICH”). In particular, the Pharmaceutical Inspection
Co-operation Scheme
(“PIC/S”)
an international treaty, contains rules binding many countries
in which medicinal products are manufactured. Among other
things, the European Commission,
PIC/S,
ICH,
a.s.o. establish requirements for GMP which are then adopted at
the national level. Another international standard, which is
non-binding for medicinal products, is the ISO9001:2000 system
for assuring quality management system requirements. This system
has a broader platform than EU-GMP, which is more detailed and
is primarily acknowledged outside the field of medicinal
products, e.g., with respect to medical devices.
Medical Devices.
In the past, medical devices
were subject to less stringent regulation than medicinal
products in some countries. In the last decade, however,
statutory requirements have been increased. In the EU, the
requirements to be satisfied by medical devices are laid down in
three European directives to be observed by all Member States
and all Member States of the European Economic Area
(“EEA”), as well as all future accession states:
(1) Directive 90/385/EEC of 20 June 1990 relating to
active implantable medical devices (“AIMDs”), as last
amended (“AIMD Directive”), (2) Directive
93/42/EEC of June 14, 1993 relating to medical devices, as
last amended (“MD Directive”), and (3) Directive
98/79/EC of October 27, 1998 relating to in vitro
diagnostic medical devices as last amended (“IVD
Directive”). In addition, Directive
2001/95/EC
of December 3, 2001, as last amended, concerning product
safety should be noted. With regard to the MD Directive, the
Commission submitted an amendment,
2007/47/EC,
intended to achieve improvements, for instance in the following
areas: clinical assessment by specification of the requirements
in more detail; monitoring of the devices after their placing on
the market; and decision making by enabling the Commission to
make binding decisions in case of contradictory opinions of
states regarding the classification of a product as a medical
device. Member States had to incorporate the new Directive into
national law by December 31, 2008 and all manufacturers
must come into compliance by March 21, 2010. We will be in
compliance as of March 21, 2010.
According to the directives relating to medical devices, the CE
mark (the abbreviation of Conformité Européenne
signifying that the device complies with all applicable
requirements) shall serve as a general product passport for all
Member States of the EU and the EEA. Upon receipt of a CE
certificate for a product according to the applicable conformity
assessment procedure, e.g. a certified full quality management
system for medical devices according to ISO13485:2003 and
AC2007, and the documented declaration and proof of conformity
of our products to the harmonized European norms (Declaration of
Conformity), we as the legal manufacturer are able to mark
products as being in compliance with the EC requirements. If
able to do so, the manufacturer has to put a “CE” mark
on the products. Medical devices that do not bear the
“CE” mark cannot be imported, sold or distributed
within the European Community.
The right to affix the CE mark is granted to any manufacturer
who has observed the conformity assessment procedure prescribed
for the relevant medical device and submitted the EC declaration
of conformity before placing the medical device on the market.
The conformity assessment procedures were standardized by
Council Decision 93/465/EEC of July 22, 1993, which
established modules for the various phases of the conformity
assessment procedures intended to be used in the technical
harmonization norms and the rules for the affixing and use of
the CE conformity mark. The conformity assessment modules to be
used differ depending on the risk class of the medical device to
be placed on the market. The classification rules for medical
devices are, as a general rule, based upon the potential risk of
causing harm to the human body. Annex IX to the MD
Directive (making a distinction between four product
classes I, IIa, IIb, and III) and Annex II to the
IVD Directive (including a list of the products from lists A and
B) contain classification criteria for products and product
lists that are, in turn, assigned to specific conformity
assessment modules. AIMDs represent a product class of their own
and are subject to the separate AIMD Directive. Special rules
apply, for example, to custom-made medical devices, medical
devices manufactured in-house, medical devices intended for
clinical investigation or in vitro diagnostic medical devices
intended for performance evaluation, as well as for diagnostic
medical devices for in-house use (“lay use”),
combination devices and accessories to medical devices.
32
The conformity assessment procedures for Class I devices
with a low degree of invasiveness in the human body (e.g.
devices without a measuring function that are not subject to any
sterilization requirements), can be made under the sole
responsibility of the manufacturer by submitting an EC
declaration of conformity (a self-certification or
self-declaration). For Class IIa devices, the participation
of a so-called “Notified Body” is binding for the
production phase. Devices of classes IIb and III involving
a high risk potential are subject to inspection by the Notified
Body not only in relation to their manufacture (as for
class IIa devices), but also in relation to their
specifications and design. Class III is reserved for the
most critical devices the marketing of which is subject to an
explicit prior authorization with regard to their conformity. In
risk categories IIa, IIb and III, the manufacturer can make use
of several different conformity assessment modules.
To maintain the high quality standards and performance of our
operations, we have subjected our entire European business to
the most comprehensive procedural module, which is also the
fastest way to launch a new product in the European Union. This
module requires the certification of a full quality management
system by a Notified Body charged with supervising the quality
management system from design, manufacture, and distribution, to
after sales service.
Our Series 4008 dialysis machines and their therapy
modifications, our 5008 dialysis machine and its accessories and
devices, our PD-NIGHT cycler, our Sleep-safe cycler for
automated PD treatment, the multiFiltrate system, and our other
active medical devices distributed in the European market, as
well as our dialysis filters and dialysis tubing systems and
accessories, all bear the “CE” mark. We expect to
continue to obtain additional certificates for newly developed
products or product groups.
Environmental
Regulation
The Company uses substances regulated under U.S. environmental
laws, primarily in manufacturing and sterilization processes.
While it is difficult to quantify, we believe the ongoing impact
of compliance with environmental protection laws and regulations
will not have a material impact on the Company’s financial
position or results of operations.
The Environmental Management System (“EMS”) based on
ISO 14001:2004 has been established in the main production
plants and in a high number of dialysis clinics in the European
region. Compliance with environmental regulations is an
essential corporate requirement of the EMS. Internal and
external audits are organized and performed to ensure that these
corporate requirements are annually evaluated.
Facilities
and Operational Regulation
U.S.
The Clinical Laboratory Improvement Amendments of 1988
(“CLIA”) subjects virtually all clinical laboratory
testing facilities, including ours, to the jurisdiction of the
Department of Health and Human Services (“HHS”). CLIA
establishes national standards for assuring the quality of
laboratories based upon the complexity of testing performed by a
laboratory. Certain of our operations are also subject to
federal laws governing the repackaging and dispensing of drugs
and the maintenance and tracking of certain life sustaining and
life-supporting equipment.
Our operations are subject to various U.S. Department of
Transportation, Nuclear Regulatory Commission, Environmental
Protection Agency, and Occupational Safety and Health
Administration (“OSHA”) requirements and other
federal, state and local hazardous and medical waste disposal
laws. As currently in effect, laws governing the disposal of
hazardous waste do not classify most of the waste produced in
connection with the provision of dialysis, or laboratory
services as hazardous, although disposal of nonhazardous medical
waste is subject to specific state regulation. Our operations
are also subject to various air emission and wastewater
discharge regulations.
Federal, state and local regulations require us to meet various
standards relating to, among other things, the management of
facilities, personnel qualifications and licensing, maintenance
of proper records, equipment, quality assurance programs, the
operation of pharmacies, and dispensing of controlled
substances. All of our operations in the U.S. are subject to
periodic inspection by federal and state agencies and other
governmental authorities to determine if the operations,
premises, equipment, personnel and patient care meet applicable
standards. To receive Medicare reimbursement, our dialysis
centers, renal diagnostic support business and laboratories must
be certified by CMS, an agency within HHS. All of our dialysis
centers, and laboratories that furnish Medicare or Medicaid
services have the required certification.
Certain of our facilities and certain of their employees are
also subject to state licensing statutes and regulations. These
statutes and regulations are in addition to federal and state
rules and standards that must be met to qualify for payments
under Medicare, Medicaid and other government reimbursement
programs. Licenses and
33
approvals to operate these centers and conduct certain
professional activities are customarily subject to periodic
renewal and to revocation upon failure to comply with the
conditions under which they were granted.
OSHA regulations require employers to provide employees who work
with blood or other potentially infectious materials with
prescribed protections against blood-borne and air-borne
pathogens. The regulatory requirements apply to all health care
facilities, including dialysis centers and laboratories, and
require employers to make a determination as to which employees
may be exposed to blood or other potentially infectious
materials and to have in effect a written exposure control plan.
In addition, employers are required to provide hepatitis B
vaccinations, personal protective equipment, blood-borne
pathogens training, post-exposure evaluation and
follow-up,
waste disposal techniques and procedures, engineering and work
practice controls and other OSHA-mandated programs for
blood-borne and air-borne pathogens.
Some states in which we operate have certificate of need
(“CON”) laws that require any person or entity seeking
to establish a new health care service or to expand an existing
service to apply for and receive an administrative determination
that the service is needed. We currently operate in several
states, as well as the District of Columbia and Puerto Rico that
have CON laws applicable to dialysis centers. These requirements
could, as a result of a state’s internal determination of
its dialysis services needs, prevent entry to new companies
seeking to provide services in these states, and could constrain
our ability to expand our operations in these states.
International
(Including Germany and Other
Non-U.S.)
Most countries outside of the U.S. regulate operating conditions
of dialysis clinics and hospitals and the manufacturing of
dialysis products, medicinal products and medical devices.
We are subject to a broad spectrum of regulation in almost all
countries. Our operations must comply with various environmental
and transportation regulations in the various countries in which
we operate. Our manufacturing facilities and dialysis clinics
are also subject to various standards relating to, among other
things, facilities, management, personnel qualifications and
licensing, maintenance of proper records, equipment, quality
assurance programs, the operation of pharmacies, the protection
of workers from blood-borne diseases and the dispensing of
controlled substances. All of our operations are subject to
periodic inspection by various governmental authorities to
determine if the operations, premises, equipment, personnel and
patient care meet applicable standards. Our dialysis clinic
operations and our related activities generally require
licenses, which are subject to periodic renewal and may be
revoked for violation of applicable regulatory requirements.
In addition, many countries impose various investment
restrictions on foreign companies. For instance, government
approval may be required to enter into a joint venture with a
local partner. Some countries do not permit foreign investors to
own a majority interest in local companies or require that
companies organized under their laws have at least one local
shareholder. Investment restrictions therefore affect the
corporate structure, operating procedures and other
characteristics of our subsidiaries and joint ventures in these
and other countries.
We believe our facilities are currently in compliance in all
material respects with the applicable national and local
requirements in the jurisdictions in which they operate.
Reimbursement
As a global dialysis care provider and supplier of dialysis
services and products, we are represented in more than 115
countries throughout the world facing the challenge of meeting
the needs of patients in very different economic environments
and health care systems.
The health care systems and rules for the reimbursement of the
treatment of patients suffering from ESRD vary in the individual
countries. In general, the government, frequently in
coordination with private insurers, is responsible for financing
the health care system through tax payments and other sources of
income, social security contributions or a combination of such
sources.
However, in a large number of developing countries, the
government or charitable institutions grant only minor aid so
that dialysis patients must bear all or a large part of their
treatment expenses themselves. In some countries, dialysis
patients do not receive treatment on a regular basis, but only
if and to the extent available funds so allow.
U.S.
Dialysis Services.
Our dialysis centers
provide outpatient hemodialysis treatment and related services
for ESRD patients. In addition, some of the Company’s
centers offer services for the provision of peritoneal dialysis
and hemodialysis treatment at home, and dialysis for
hospitalized patients.
34
The Medicare program is the largest single source of dialysis
services revenues from dialysis treatment. For example, in 2009,
approximately 54% of North America dialysis services revenues
resulted from Medicare’s ESRD program. As a preliminary
matter, in order to be eligible for reimbursement by Medicare,
ESRD facilities must meet conditions for coverage established by
CMS. New conditions for coverage became effective in October of
2008, with the exception of two provisions relating to physical
environment and infection control which became effective in
February of 2009. We believe we have made the necessary
modifications to meet these new requirements.
Certain products and services delivered by our dialysis centers
are reimbursed by the Medicare program in accordance with a
“basic case-mix adjusted prospective payment system,”
which provides a fixed payment for each dialysis treatment,
comprised of a composite rate component, a drug add-on
adjustment component, case-mix adjustments and a regional wage
index adjustment. The payment rates under this system are now
subject to adjustment from time to time through changes in the
Medicare statute (in the case of basic services included in the
“composite rate”) or through annual adjustments (in
the case of a portion of the payment referred to as the drug
add-on, case-mix and wage index adjustments).
For calendar year 2009, CMS set the drug add-on adjustment at
$20.33 per treatment, or 15.2% of the total per-treatment
composite payment. For 2010, CMS kept the drug add-on amount
constant at $20.33 per treatment, while it increased the base
portion of the composite rate by 1% pursuant to the requirement
in the Medicare Improvements for Patients and Providers Act of
2008 (“MIPPA”). As a result, the drug add-on amount,
constant in dollar terms, declined to 15% of the total
per-treatment payment in 2010. The base portion of the composite
rate, unlike many other payment rates in Medicare, has not been
automatically updated each year. As a result, this portion of
the composite payment rate has not received an annual update in
the absence of a statutory change. In MIPPA, Congress provided
for a 1.0% increase in the base portion of the composite rate in
each of 2009 and 2010. Further, Congress eliminated a provision
that previously paid hospital-based facilities slightly more
than independent (or “free-standing”) facilities.
Thus, in 2009, all facilities were paid at the 2008 independent
facility rate increased by 1.0%.
For 2010, the base composite rate is $135.15 for both
independent and hospital-based facilities, an increase of 1.0%
from the 2009 rate. CMS updated the wage index adjustment
applicable to ESRD facilities from the
25/75 blend
between adjustments based on old metropolitan statistical areas
(“MSA”) and those based on new core-based statistical
areas (“CBSA”) used in 2008. For 2009, CMS completed
the transition from the MSA definition to the CBSA definition,
and facilities will henceforth be paid according to the CBSA
rate. For 2010, CMS reduced the wage index floor from 0.70 to
0.65.
Certain other items and services that we furnish at our dialysis
centers are not now included in the composite rate and are
eligible for separate Medicare reimbursement. The most
significant of these items are drugs or biologicals, such as
erythropoietin-stimulating agents (“ESAs”), vitamin D
analogs, and iron, which are reimbursed at 106% of the average
sales price as reported to CMS by the manufacturer. Products and
support services furnished to ESRD patients receiving dialysis
treatment at home are also reimbursed separately under a
reimbursement structure comparable to the in-center composite
rate. Although these reimbursement methodologies limit the
allowable charge per treatment, they provide us with predictable
per treatment revenues.
Any significant decreases in Medicare reimbursement rates could
have material adverse effects on our provider business and,
because the demand for products is affected by Medicare
reimbursement, on our products business. To the extent that
increases in operating costs that are affected by inflation,
such as labor and supply costs, are not fully reflected in a
compensating increase in reimbursement rates, our business and
results of operations may be adversely affected. For a
discussion of the rules proposed by CMS to implement recent
Medicare reimbursement rate changes including provisions for
implementation of a “bundled rate” by January 1,
2011, see “— Erythropoietin stimulating
agents,” below.
For Medicare-primary patients, Medicare is responsible for
payment of 80% of the prospective payment amount and separately
reimbursable drugs or biologicals amounts set by Congress for
dialysis treatments and the patient or third-party insurance
payors, including employer-sponsored health insurance plans,
commercial insurance carriers and the Medicaid program, are
responsible for paying any co-payment amounts for approved
services not paid by Medicare (typically the annual deductible
and 20% co-insurance), subject to the specific coverage policies
of such payors. Each third-party payor, including Medicaid,
makes payment under contractual or regulatory reimbursement
provisions that may or may not cover the full 20% co-payment or
annual deductible. Where the patient has no third-party
insurance or the third-party insurance does not cover the
co-payment or deductible, the patient is responsible for paying
the co-payments or the deductible, which we frequently do not
fully collect despite reasonable collection efforts. Under an
advisory opinion from the Office of the Inspector General of the
Department of Health and Human Services, subject to specified
conditions, we and other similarly situated providers may make
contributions to a non-profit organization that has agreed to
make premium payments for
35
supplemental medical insurance
and/or
“Medigap” insurance on behalf of indigent ESRD
patients, including some of our patients.
Medicaid Rebate Program and Other Government Drug Pricing
Program Requirements.
Manufacturers of drugs that are
covered by the Medicaid program or that are reimbursed by
Part B of the Medicare program are subject to various price
determination and reporting requirements under federal statutes,
including the Medicaid and Medicare statutes as well as the
Public Health Service Act (“PHSA”) and the Veterans
Health Care Act (“VHCA”). Compliance with the Medicaid
rebate statute, the VHCA, the Medicare statute, and
Section 340B of the PHSA requires us to calculate
and/or
report a number of different pricing metrics (
e.g.
,
Average Manufacturer Price (“AMP”), Best Price
(“BP”), Average Sales Price (“ASP”), Federal
Ceiling Price (“FCP”), non-federal average
manufacturer price (“Non-FAMP”), and 340B ceiling
price) to federal authorities responsible for monitoring and
enforcing drug manufacturer compliance with federal law and
policy.
We participate in the Federal Medicaid rebate program
established by the Omnibus Budget Reconciliation Act of 1990, as
well as several state supplemental rebate programs, and we make
our pharmaceutical products available to authorized users of the
Federal Supply Schedule (“FSS”) of the General
Services Administration under an FSS contract negotiated by the
Department of Veterans Affairs (“DVA”). With the
recent acquisition of a license to market and distribute the IV
Iron product
Venofer
®
to freestanding dialysis clinics, we also are considered, for
statutory price reporting purposes, to be the
“manufacturer” of
Venofer
®
which is reimbursed under Part B of the Medicare program.
Our products also are subject to a federal requirement that any
company participating in the Medicaid rebate or Medicare
Part B program extend discounts comparable to the rebates
paid to State Medicaid agencies to qualified purchasers under
the Public Health Services (“PHS”) pharmaceutical
pricing program managed by the Health Resources and Services
Administration of HHS (also known as the “340B
program” by virtue of the section of the PHSA that created
the program). The PHS pricing program extends these deep
discounts on drugs to a variety of community health clinics and
other entities that receive health services grants from the PHS,
as well as hospitals that serve a disproportionate share of poor
Medicare and Medicaid beneficiaries.
Under the Medicaid rebate program, we pay a rebate to each state
Medicaid program based upon sales of our pharmaceutical products
that are reimbursed by those programs. Rebate calculations are
complex and, in certain respects, subject to interpretations of
law, regulation, or policy guidance by us, government or
regulatory agencies and the courts. The Medicaid rebate amount
is computed each quarter based on our submission to CMS of our
current average manufacturer price and best price for our
pharmaceutical products. The Veterans Health Care Act of 1992
imposes a requirement that the prices we charge to certain
federal entities under the FSS must be no greater than a Federal
Ceiling Price determined by applying a statutory discount to the
AMP charged to non-federal customers (a pricing metric referred
to as “Non-FAMP”). Because the amount the government
pays to reimburse the cost of a drug under Part B of the
Medicare program is ordinarily based on the ASP charged by the
manufacturer to purchasers of the drug, additional price
calculation and reporting obligations are imposed on the
manufacturers of Part B drugs under that program. Since
Venofer
®
is a Part B drug (
i.e
., one ordinarily administered
incident to a physician service), we are responsible for
compiling and utilizing a wide range of sales data elements to
determine the ASP of
Venofer
®
marketed under our labeler code, and reporting it to the CMS. We
are subject to specific ASP reporting obligations with respect
to our
Venofer
®
sales under a consent order issued by the Federal Trade
Commission in October 2008 (FTC File
No. 081-0146).
We anticipate that the new expanded bundled ESRD reimbursement
system will replace ASP-based drug reimbursement for dialysis
products as the new system is implemented starting in 2011.
Government agencies may make changes in program interpretations,
requirements or conditions of participation, and retain the
right to audit the accuracy of our computations of rebates and
pricing, some of which may have or result in implications (such
as recoupment) for amounts previously estimated or paid and may
have a material adverse effect on the Company’s revenues,
profitability and financial condition.
Laboratory Tests.
Spectra obtains a
substantial portion of its net revenue from Medicare, which pays
for clinical laboratory services provided to dialysis patients
in two ways.
First, payment for certain routine tests is included in the
composite rate paid to our dialysis centers. The centers obtain
the laboratory services from laboratories and pay the
laboratories for the services. In accordance with industry
practice, Spectra usually provides such testing services under
capitation agreements with its customers pursuant to which it
bills a fixed amount per patient per month to cover the
laboratory tests included in the composite rate at the
designated frequencies.
Second, laboratory tests performed by Spectra for Medicare
beneficiaries that are not included in the composite rate are
billed separately directly to Medicare. Such tests are paid at
100% of the Medicare fee schedule amounts, which vary to some
extent across different geographic areas but which cannot exceed
national ceilings on
36
payment rates, called national limitation amounts
(“NLAs”). The Medicare statute calls for updating the
payment rates to reflect inflation by the change in consumer
price index. Congress has frequently reduced or eliminated this
update, most recently by imposing a five-year freeze for
calendar years 2004 through 2008. In MIPPA, Congress specified
updates for 2009 to 2013 as the CPI change minus one-half
percentage point.
Erythropoietin stimulating agents.
ESAs,
including
Epogen
®
and
Aranesp
®
are used for anemia management of patients with renal disease.
The administration of ESAs is separately billable under the
Medicare program, and accounted for 21% of our 2009 North
America segment dialysis care revenues.
Anemia severity is commonly monitored by measuring a
patient’s hematocrit, an indicator of the proportion of red
blood cells in a patient’s whole blood, or by evaluating a
patient’s hemoglobin level. The amount of ESA that is
appropriate for a patient varies by several factors, including
the severity of the patient’s anemia.
We believe our policies on billing for ESAs comply with CMS
policies. We have recommended to our treating physicians that
they review and understand the package label insert and the
K/DOQI guidelines as they make their anemia management decisions.
Any of the following changes relating to ESAs could adversely
affect our business, and results of operations, possibly
materially:
|
|
|
|
|
|
•
|
future changes in the ESA reimbursement methodology
and/or
rate;
|
|
|
|
|
•
|
a material reduction in the typical dosage per administration; or
|
|
|
|
|
•
|
increases in the cost of ESAs without offsetting increases in
the ESA reimbursement rate.
|
|
|
|
|
•
|
reduction by the manufacturer of ESAs of the amount of overfill
in the ESA vials
|
With the enactment of MIPPA, in 2008 Congress mandated the
development of an expanded ESRD bundled payment system for
services furnished on or after January 1, 2011. MIPPA
requires CMS to implement by January 1, 2011 a bundled ESRD
payment system under which CMS will reimburse dialysis
facilities with a single payment for (i) all items and
services included in the composite rate, (ii) all ESAs and
other pharmaceuticals (other drugs and biologicals, other than
vaccines) furnished to the patients that were previously
reimbursed separately, (iii) diagnostic laboratory tests
and (iv) other services furnished to individuals for the
treatment of ESRD. The initial bundled reimbursement rate will
be set based on 98% of estimated 2011 Medicare program costs of
dialysis care as calculated under the current reimbursement
system using the lowest per patient utilization data from 2007,
2008 or 2009 for all Medicare beneficiaries. The bundled payment
will be subject to case mix adjustments that may take into
account individual patient characteristics (e.g., age, weight,
body mass) and co-morbidities. Payments will also be adjusted
for (i) certain high cost patient outliers due to unusual
variations in medically necessary care, (ii) disparately
high costs incurred by low volume facilities relative to other
facilities and (iii) such other adjustments as the
Secretary of HHS deems appropriate. Beginning in 2012, the
bundled payment amount will be subject to annual increases based
on increases in the costs of a “market basket” of
dialysis items and services to be determined by HHS minus 1%.
MIPPA requires CMS to implement
pay-for-performance
standards, effective in 2012. Dialysis facilities that fail to
achieve the established quality standards will have payments
reduced by up to 2%. Facility quality standards are expected to
be limited at the outset to measures of anemia management and
hemodialysis adequacy and facility performance scores will be
made available to the public. The bundled system will be phased
in over four years with full implementation for all dialysis
facilities on January 1, 2014. However, providers may elect
in November 2010 to become fully subject to the new system
starting in January 2011. MIPPA extends the authority of
specialized Medicare Advantage (“MA”) plans to target
enrollment to certain populations through December 31, 2010
and revises definitions, care management requirements and
quality reporting standards for all specialized plans. On
September 29, 2009, CMS published a proposed rule
implementing the case-mix adjusted bundled prospective payment
system (“PPS”) for ESRD dialysis facilities in
accordance with MIPPA. If implemented in its current form, the
provisions of the proposed rule relating to case mix and
transition adjustments would result in reimbursement reductions.
The proposed rule, if adopted without further changes, would
fail to provide adequate funding for ESRD facilities’
delivery of oral ESRD medications currently covered under
Medicare Part D and would not adequately address the
coordination of secondary insurance coverage. While it is clear
that the expanded ESRD bundled payment system will materially
affect how the Company is paid for pharmaceuticals and other
items and services, the Company cannot estimate the overall
effect of the new system on its business until adoption of the
final CMS regulations.
Coordination of Benefits.
Medicare entitlement
begins for most patients in the fourth month after the
initiation of chronic dialysis treatment at a dialysis center.
During the first three months, considered to be a waiting
period, the patient or patient’s insurance, Medicaid or a
state renal program are responsible for payment.
37
Patients who are covered by Medicare and are also covered by an
employer group health plan (“EGHP”) are subject to a
30-month
coordination period during which the EGHP is the primary payor
and Medicare the secondary payor. During this coordination
period the EGHP pays a negotiated rate or in the absence of such
a rate, our standard rate or a rate defined by its plan
documents. The EGHP payments are generally higher than the
Medicare payment. EGHP insurance, when available, will therefore
generally cover as the primary payor a total of 33 months,
the
3-month
waiting period plus the
30-month
coordination period.
Possible Changes in Statutes or
Regulations.
Further legislation or regulations
may be enacted in the future that could substantially modify or
reduce the amounts paid for services and products offered by us
and our subsidiaries. It is also possible that statutes may be
adopted or regulations may be promulgated in the future that
impose additional eligibility requirements for participation in
the federal and state health care programs. Such new legislation
or regulations could, depending upon the detail of the
provisions, have positive or adverse effects, possibly material,
on our businesses and results of operations. See Item 3,
“Risk Factors — Risks Relating to Litigation and
Regulatory Matters — Proposals for health care reform
could decrease our revenues and operating profit,” and
“— Health Care Reform” below.
International
(Including Germany and Other
Non-U.S.)
As a global company delivering dialysis care and dialysis
products in more than 115 countries worldwide, we face the
challenge of addressing the needs of dialysis patients in widely
varying economic and health care environments.
Health care systems and reimbursement structures for ESRD
treatment vary by country. In general, the government pays for
health care and finances its payments through taxes and other
sources of government income, from social contributions, or a
combination of those sources. However, not all health care
systems provide for dialysis treatment. In many developing
countries, only limited subsidies from government or charitable
institutions are available, and dialysis patients must finance
all or substantially all of the cost of their treatment. In some
countries patients in need of dialysis do not receive treatment
on a regular basis but rather when the financial resources allow
it.
In the major European and British Commonwealth countries, health
care systems are generally based on one of two models. The
German model, the “Bismarck system”, is based on
mandatory employer and employee contributions dedicated to
health care financing. The British model, the “Beveridge
system”, provides a national health care system funded by
taxes. Within these systems, provision for the treatment of
dialysis has been made either through allocation of a national
budget or a billing system reimbursing on a
fee-for-service
basis. The health care systems of countries such as Japan,
France, Belgium, Austria, Czech Republic, Poland, Hungary,
Turkey and the Netherlands are based on the Bismarck-type
system. Countries like Canada, Denmark, Finland, Portugal,
Sweden, Taiwan and Italy established their national health
services using the Beveridge-type system. For information on the
distribution of clinic ownership in various countries in which
we operate, see “Renal Industry Overview —
Dialysis Treatment Options for ESRD,” above.
Financing policies for ESRD treatment also differ from
country-to-country.
There are three main types of reimbursement modalities: budget
transfer, fee for service and flat rate. In some cases, the
reimbursement modality varies within the same country depending
on the type of provider (public or private). Budget transfer is
a reimbursement modality used mainly for public providers in
most of the European countries where the funding is based on
taxation and in some of the countries where it is based on
social security (e.g. Czech Republic). Fee for service is the
most common reimbursement modality for private providers in all
European countries (with exceptions, such as Hungary, where
reimbursement to private providers is based on budget) and for
public providers in countries where the funding system is based
on social security payments.
In 2008, the Portuguese Ministry of Health and Anadial, the
national association of privately run dialysis centers, agreed
on a new reimbursement model for ambulatory care to hemodialysis
patients in Portugal. The new model “Comprehensive Price
Payment” is an integrated and quality-driven approach that
bundles a variety of dialysis related services and products. It
requires the implementation and functioning of an integrated
disease management model in order to achieve, simultaneously,
health benefits, quality improvement and system rationalization.
The “Comprehensive Price Payment” model includes all
necessary dialysis services, the deployment of dialysis-related
products, laboratory services and other complementary medical
tests and the administration of renal drugs for anemia
management, bone management, blood pressure and cardiovascular
control as well as vitamins. The new reimbursement structure
provides for a quality oriented flat-rate payment of a national
reimbursement rate per week per patient. The main characteristic
is that the amount of this reimbursement will directly depend on
the fulfillment of certain treatment results and quality control
parameters with the dialysis services provided. The therapeutic
goals include, among others, the adequacy of dialysis, targets
for hemoglobin
38
levels, bone metabolism status, water quality as well as outcome
measures such as mortality rate and hospitalization days. These
goals mirror the good practices guidelines, both national and
international, for dialysis care to patients, which will serve
as support for contractual monitoring. The establishment of
auditing, information, monitoring, attendance and evaluation
mechanisms is a pre-requisite for a participating dialysis
provider. We treat approximately 4,200 patients in 34 dialysis
clinics in Portugal. Under the new model, our reimbursement rate
(including reimbursement for additional services that we provide
as part of the new flat rate) has increased by approximately 50%.
Treatment components included in the cost of dialysis may vary
from
country-to-country
or even within countries, depending on the structure and cost
allocation principles. Where treatment is reimbursed on a
fee-for-service
basis, reimbursement rates are sometimes allocated in accordance
with the type of treatment performed. We believe that it is not
appropriate to calculate a global reimbursement amount because
the services and costs for which reimbursement is provided in
any such global amount would likely bear little relation to the
actual reimbursement system in any one country. Generally, in
countries with established dialysis programs, reimbursements
range from $100 to more than $300 per treatment. However, a
comparison from country to country would not be meaningful if
made in the absence of a detailed analysis of the cost
components reimbursed, services rendered and the structure of
the dialysis clinic in each country being compared.
Health care expenditures are consuming an ever-increasing
portion of gross domestic product worldwide. In the developed
economies of Europe, Asia and Latin America, health care
spending is in the range of 5%-15% of gross domestic product. In
many countries, dialysis costs consume a disproportionately high
amount of health care spending and these costs may be considered
a target for implementation of cost containment measures. Today,
there is increasing awareness of the correlation between the
quality of care delivered in the dialysis unit and the total
health care expenses incurred by the dialysis patient.
Accordingly, developments in reimbursement policies might
include higher reimbursement rates for practices which are
believed to improve the overall state of health of the ESRD
patient and reduce the need for additional medical treatment.
Anti-Kickback
Statutes, False Claims Act, Health Insurance Portability and
Accountability Act of 1996, Civil Monetary Penalties Law, Stark
Law and Other Fraud and Abuse Laws in the United
States
Some of our operations are subject to federal and state statutes
and regulations governing financial relationships between health
care providers and potential referral sources and reimbursement
for services and items provided to Medicare and Medicaid
patients. Such laws include the Anti-Kickback Statute, the False
Claims Act, the Stark Law, and other federal health care fraud
and abuse laws and similar state laws.
The U.S. Government, many individual states and private
third-party risk insurers have devoted increasing resources to
combat fraud, waste, and abuse in the health care sector. The
Office of the Inspector General of HHS (“OIG”), state
Medicaid fraud control units, and other enforcement agencies
have dedicated substantial resources to their efforts to detect
agreements between physicians and service providers that may
violate fraud and abuse laws. In its most recent Work Plan for
Fiscal Year 2010, the OIG has scheduled several ESRD-related
audits in the coming year on: (i) the extent to which
ambulance services are used to transport ESRD beneficiaries to
and from dialysis facilities; (ii) Medicare payments for
selected ESRD drugs; and (iii) whether a dialysis
facility’s dosing guidelines (to the extent a facility
adopts dosing guidelines) for erythopoiesis-stimulating agents
adhere to FDA labelling recommendations.
Anti-Kickback
Statutes
The federal Anti-Kickback Statute establishes criminal
prohibitions against and civil penalties for the knowing and
willful solicitation, receipt, offer or payment of any
remuneration, whether direct or indirect, in return for or to
induce the referral of patients or the ordering or purchasing of
items or services payable in whole or in part under Medicare,
Medicaid or other federal health care programs. Sanctions for
violations of the Anti-Kickback Statute include criminal and
civil penalties, such as imprisonment
and/or
criminal fines of up to $25,000 per violation, and civil
penalties of up to $50,000 per violation, and exclusion from the
Medicare or Medicaid programs and other federal programs.
The OIG has the authority to promulgate regulations referred to
as “safe harbors” that define certain business
relationships and arrangements that would not be subject to
civil sanction or criminal enforcement under the Anti-Kickback
Statute. Failure to comply with a safe harbor provision does not
make the activity illegal. Rather, the safe harbors set forth
specific criteria that, if fully met, will assure the entities
involved of not being prosecuted criminally or civilly for the
arrangement under the Anti-Kickback Statute.
39
Many states also have enacted statutes similar to the
Anti-Kickback Statute, which may include criminal penalties,
applicable to referrals of patients regardless of payor source,
and may contain exceptions different from state to state and
from those contained in the federal Anti-Kickback Statute.
False
Claims Act and Related Criminal Provisions
The federal False Claims Act (the “False Claims Act”)
imposes civil penalties for knowingly making or causing to be
made false claims with respect to governmental programs, such as
Medicare and Medicaid, for services billed but not rendered, or
for misrepresenting actual services rendered, in order to obtain
higher reimbursement. Under the interpretation of certain
courts, claims submitted for services furnished in violation of
the Anti-Kickback Statute or Stark Law could also violate the
False Claims Act. Moreover, private individuals may bring qui
tam or “whistle blower” suits against providers under
the False Claims Act, which authorizes the payment of
15-30%
of
any recovery to the individual bringing suit. Such actions are
initially required to be filed under seal pending their review
by the Department of Justice. The False Claims Act generally
provides for the imposition of civil penalties of $5,000 to
$10,000 per claim and for treble damages, resulting in the
possibility of substantial financial penalties for small billing
errors that are replicated in a large number of claims, as each
individual claim could be deemed to be a separate violation of
the False Claims Act. Some states also have enacted statutes
similar to the False Claims Act which may include criminal
penalties, substantial fines, and treble damages. Effective,
January 1, 2007, section 1909 of the Social Security
Act (enacted by section 6031 of the Deficit Reduction Act
of 2005) provides a financial incentive for states to enact
false claims acts that establish liability to the state for the
submission of false or fraudulent claims to the state’s
Medicaid program. If a state false claims act is determined to
meet certain enumerated requirements, the state is entitled to
an increase in the amounts recovered under a state action
brought under such law. The OIG, in consultation with the
Attorney General of the United States and the Department of
Justice, determines whether a state false claims act meets these
enumerated requirements to qualify for the added financial
incentive. As of December 2009, the OIG reviewed and approved
state false claims acts promulgated by California, Georgia,
Hawaii, Illinois, Indiana, Massachusetts, Nevada, New York,
Rhode Island, Tennessee, Texas, Virginia, and Wisconsin.
The
Health Insurance Portability and Accountability Act of 1996
(“HIPAA”)
HIPAA was enacted in August 1996 and expanded federal fraud and
abuse laws by increasing their reach to all federal health care
programs, establishing new bases for exclusions and mandating
minimum exclusion terms, creating an additional statutory
exception to the Anti-Kickback Statute for risk-sharing
arrangements, requiring the Secretary of Health and Human
Services to issue advisory opinions, increasing civil money
penalties to $10,000 (formerly $2,000) per item or service and
assessments to three times (formerly twice) the amount claimed,
creating a specific health care fraud offense and related health
fraud crimes, and expanding investigative authority and
sanctions applicable to health care fraud. It also prohibits a
provider from offering anything of value which the provider
knows or should know would be likely to induce a federal health
care program beneficiary to select or continue with the provider.
HIPAA included a health care fraud provision which prohibits
knowingly and willfully executing a scheme or artifice to
defraud any “health care benefit program,” which
includes any public or private plan or contract affecting
commerce under which any medical benefit, item, or service is
provided to any individual, and includes any individual or
entity who is providing a medical benefit, item, or service for
which payment may be made under the plan or contract. Penalties
for violating this statute include criminal penalties, exclusion
from the Medicare and Medicaid programs, freezing of assets and
forfeiture of property traceable to commission of a health care
fraud.
HIPAA regulations establish national standards for certain
electronic health care transactions, the use and disclosure of
certain individually identifiable patient health information,
and the security of the electronic systems maintaining this
information. These are commonly known as the HIPAA Privacy and
Security Rules. Health insurance payers and healthcare providers
like us must comply with the HIPAA regulations. Violations of
these HIPAA regulations may include civil money penalties and
potential criminal sanctions.
Many U.S. states also have enacted state health care privacy and
data security breach laws governing patient information, medical
records and personal information, including sensitive
information such as financial and identity data. The HIPAA
Privacy Rule establishes a minimum U.S. federal standard for
protecting privacy and preempts all contrary U.S. state privacy
laws. The Privacy Rule does not, however, preempt U.S. state
privacy laws that are more stringent or more protective. In such
instances, we would need to comply with the U.S. state privacy
law. In addition, almost all U.S. states now regulate data
breaches (unless the data is effectively encrypted) by requiring
burdensome reporting and notification requirements, with
significant financial penalties for noncompliance.
40
The Health Information Technology for Economic and Clinical
Health Act (“HITECH Act”), pursuant to the American
Recovery and Reinvestment Act of 2009 (“ARRA”), makes
sweeping changes to the health information privacy and security
regulations of HIPAA by expanding the scope and application of
the statute. These changes include, among other things,
(i) establishing an affirmative obligation to provide
patient data breach notification in the event of the
unauthorized acquisition, access, use or disclosure of unsecured
protected health information (“PHI”);
(ii) defining the “minimum necessary” information
that a covered entity may use, disclose or request in the event
the disclosure of a limited data set (partially de-identified
data) is insufficient to accomplish the appropriate objectives;
(iii) restricting the use of PHI for marketing purposes
(expanding definition of marketing activities requiring
authorization); (iv) prohibiting the sale of PHI;
(v) establishing an affirmative obligation to provide an
accounting of disclosures made for payment, treatment and health
care operations (up to 3 years); (vi) permitting
individual requests to restrict disclosure in certain
circumstances; (vii) applying the privacy and security
rules to business associates; and (viii) limiting
application of the amendments to personal health records (PHR)
vendors. The U.S. government has promulgated interim final
regulations, effective September 23, 2009, that address the
obligation to provide patient data breach notifications, which
subjects the Company to additional administrative requirements
in the U.S. The U.S. government has stated, however, that
sanctions would not be imposed for the failure to provide the
required notifications for breaches that are discovered before
February 22, 2010. The Company cannot estimate the overall
effect of the remaining regulatory changes until adoption of
final regulations implementing those statutory provisions.
Civil
Monetary Penalties Law
Individuals or entities who have either (1) directly
submitted, or caused to be submitted, claims which are improper
or false; (2) arranged or contracted with an individual or
entity that the person knows or should know is excluded from
participation in federal health care programs; or
(3) offered or received kickbacks may also be subject to
monetary penalties or exclusion under the Civil Monetary
Penalties Law (“CMPL”) at the discretion of the OIG.
Penalties are generally not more than $10,000 for each item or
service. However, under the CMPL, violators of the federal
Anti-Kickback Statute provisions may also be subject to
additional civil money penalties of $50,000 per violation.
Violators are also subject to an assessment of up to three times
the amount claimed for each item or service in lieu of damages
sustained by the United States or a state agency because of such
claim, or damages of up to three times the total amount of
renumeration offered, paid, solicited, or received. In addition,
any person or entity who violates this section may be excluded
from participation in the federal or state health care programs.
Stark
Law
The original Ethics in Patient Referrals Act of 1989 (commonly
referred to as the “Stark Law”) was enacted as part of
the Omnibus Budget Reconciliation Act (“OBRA”) of
1989, and prohibited a physician from referring Medicare
patients for clinical laboratory services to entities with which
the physician (or an immediate family member) has a financial
relationship, unless an exception applies. Sanctions for
violations of the Stark Law may include denial of payment,
refund obligations, civil monetary penalties and exclusion of
the provider from the Medicare and Medicaid programs. In
addition, the Stark Law prohibits the entity receiving the
referral from filing a claim or billing for services arising out
of the prohibited referral.
Provisions of OBRA 1993, known as “Stark II,” amended
the Stark Law to revise and expand upon various statutory
exceptions, expanded the services regulated by the statute to a
list of “Designated Health Services,” and expanded the
reach of the statute to the Medicaid program. The provisions of
Stark II generally became effective on January 1, 1995. The
additional Designated Health Services, in addition to clinical
laboratory services, include: physical therapy, occupational
therapy and speech language pathology services; radiology and
certain other imaging services; radiation therapy services and
supplies; durable medical equipment and supplies; parenteral and
enteral nutrients, equipment and supplies; prosthetics,
orthotics, and prosthetic devices and supplies; home health
services; outpatient prescription drugs; and inpatient and
outpatient hospital services. The first phase of Stark
regulations was finalized on January 4, 2001. Most portions
of the first phase regulations became effective in 2002. The
first phase of the final regulations implementing the Stark Law
(the “Phase I regulations”) contains an exception for
Epogen
®
and certain other dialysis-related outpatient prescription drugs
furnished in or by an ESRD facility under many circumstances. In
addition, the regulations made clear that services reimbursed by
Medicare to a dialysis facility under the ESRD composite rate do
not implicate the Stark Law. Further, the final Phase I
regulations also adopted a definition of durable medical
equipment which effectively excludes ESRD equipment and supplies
from the category of Designated Health Services. Phase II of the
Stark regulations was published on March 26, 2004, and
became effective on July 26, 2004. This phase of the
regulations finalized all of the compensation exceptions to the
Stark Law, including those for “personal services
arrangements” and “indirect
41
compensation arrangements.” In addition, Phase II revised
the exception for
Epogen
®
and certain other dialysis-related outpatient prescription drugs
furnished in or by an ESRD facility to include certain
additional drugs.
On September 5, 2007, CMS published Phase III of the Stark
regulations. While this rulemaking was intended to be the final
phase of the Stark rulemaking process, CMS has continued to
address the Stark Law as part of its annual rulemaking process
for reimbursement under the Medicare Part B Physician Fee
Schedule.
Finally, it should be noted that many states in which we operate
have enacted self-referral statutes similar to the Stark Law.
Such state self-referral laws may apply to referrals of patients
regardless of payor source and may contain exceptions different
from each other and from those contained in the Stark Law.
Other
Fraud and Abuse Laws
Our operations are also subject to a variety of other federal
and state fraud and abuse laws, principally designed to ensure
that claims for payment to be made with public funds are
complete, accurate and fully comply with all applicable program
rules.
Health
Care Reform
Health care reform is considered by many countries to be a
national priority. The United States Congress has debated bills
that, if adopted, would make significant changes to the health
care system. It is difficult to determine at this time whether a
health care reform bill will be passed and if passed, what the
provisions of such a bill would be. In addition, several U.S.
states and many other countries in which we operate are also
currently considering health care proposals. Any health care
reform which radically changes the financing and regulation of
the health care industry could have a material adverse effect on
our business and the results of our operations.
|
|
|
|
C.
|
Organizational
Structure
|
The following chart shows our organizational structure and our
significant subsidiaries. Fresenius Medical Care Holdings, Inc.
conducts its business as “Fresenius Medical Care North
America.”
42
|
|
|
|
D.
|
Property,
plant and equipment
|
Property
The table below describes our principal facilities. We do not
own the land and buildings comprising our principal facilities
in Germany. Rather, we lease those facilities on a long-term
basis from Fresenius SE or one of its affiliates. These leases
are described under “Item 7.B. Related Party
Transactions — Real Property Lease.”
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Currently
|
|
|
|
|
|
|
|
|
|
|
Owned or
|
|
|
|
|
|
|
|
Floor Area
|
|
|
Leased by
|
|
|
|
|
|
|
|
(Approximate
|
|
|
Fresenius
|
|
Lease
|
|
|
|
Location
|
|
Square Meters)
|
|
|
Medical Care
|
|
Expiration
|
|
Use
|
|
|
|
Bad Homburg, Germany
|
|
|
17,600
|
|
|
leased
|
|
December 2016
|
|
Corporate headquarters and administration
|
|
Bad Homburg, Germany
|
|
|
4,556
|
|
|
leased
|
|
December 2011
|
|
Administration Building FMC GmbH Central Europe
|
|
St. Wendel, Germany
|
|
|
58,767
|
|
|
leased
|
|
December 2016
|
|
Manufacture of polysulfone membranes, dialyzers and peritoneal
dialysis solutions; research and development
|
|
Biebesheim, Germany
|
|
|
30,000
|
|
|
leased
|
|
December 2023
|
|
Central distribution Europe, Asia Pacific and Latin America
|
|
Gernsheim, Germany
|
|
|
17,291
|
|
|
leased
|
|
December 2010
|
|
Central distribution Europe, Asia Pacific and Latin America
|
|
Schweinfurt, Germany
|
|
|
24,900
|
|
|
leased
|
|
December 2016
|
|
Manufacture of hemodialysis machines and peritoneal dialysis
cyclers; research and development
|
|
Bad Homburg (OE), Germany
|
|
|
10,304
|
|
|
leased
|
|
December 2016
|
|
Manufacture of hemodialysis concentrate solutions / Technical
Services / Logistics services
|
|
Darmstadt, Germany
|
|
|
21,597
|
|
|
leased
|
|
November 2010
|
|
Central Europe Regional Distribution Center
|
|
Palazzo Pignano, Italy
|
|
|
19,990
|
|
|
owned
|
|
|
|
Manufacture of bloodlines and tubing
|
|
L’Arbresle, France
|
|
|
13,524
|
|
|
owned
|
|
|
|
Manufacture of polysulfone dialyzers, special filters and dry
hemodialysis concentrates
|
|
Nottinghamshire, UK
|
|
|
5,110
|
|
|
owned
|
|
|
|
Manufacture of hemodialysis concentrate solutions
|
|
Vrsac, Serbia
|
|
|
2,642
|
|
|
owned
|
|
|
|
Production area, laboratory, maintenance, administration,
logistics
|
|
Barcelona, Spain
|
|
|
2,000
|
|
|
owned
|
|
|
|
Manufacture of hemodialysis concentrate solutions
|
|
Antalya, Turkey
|
|
|
8,676
|
|
|
leased
|
|
December 2022
|
|
Manufacture of bloodlines
|
|
Casablanca, Morocco
|
|
|
2,823
|
|
|
owned
|
|
|
|
Manufacture of hemodialysis concentrate solutions
|
|
Guadalajara, México
|
|
|
26,984
|
|
|
owned
|
|
|
|
Manufacture of peritoneal dialysis bags
|
|
Buenos Aires, Argentina
|
|
|
10,500
|
|
|
owned
|
|
|
|
Manufacture of hemodialysis concentrate solutions, dry
hemodialysis concentrates, bloodlines and desinfectants
|
|
São Paulo, Brazil
|
|
|
8,615
|
|
|
owned
|
|
|
|
Manufacture of hemodialysis concentrate solutions, dry
hemodialysis concentrates, peritoneal dialysis bags, intravenous
solutions bags, peritoneal dialysis and blood lines sets
|
|
São Paulo, Brazil
|
|
|
5,000
|
|
|
leased
|
|
October 2010
|
|
Warehouse
|
|
Rio de Janeiro, Brazil
|
|
|
2,185
|
|
|
leased
|
|
July 2011
|
|
Head Office
|
43
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Currently
|
|
|
|
|
|
|
|
|
|
|
Owned or
|
|
|
|
|
|
|
|
Floor Area
|
|
|
Leased by
|
|
|
|
|
|
|
|
(Approximate
|
|
|
Fresenius
|
|
Lease
|
|
|
|
Location
|
|
Square Meters)
|
|
|
Medical Care
|
|
Expiration
|
|
Use
|
|
|
|
Bogotá, Colombia
|
|
|
18,947
|
|
|
owned
|
|
|
|
Manufacture of hemodialysis concentrate solutions, peritoneal
dialysis bags, intravenous solutions, administration
|
|
Valencia, Venezuela
|
|
|
3,562
|
|
|
leased
|
|
May 2010
|
|
Head Office and Warehouse
|
|
Hong Kong
|
|
|
1,770
|
|
|
leased
|
|
February 2012
|
|
Various leases of Warehouse facility
|
|
Suzhou, China (Changshu Plant)
|
|
|
25,168
|
|
|
owned
|
|
|
|
Manufacture of hemodialysis bloodline sets / AV Fistula set
|
|
Smithfield NSW, Australia
|
|
|
5,350
|
|
|
owned
|
|
|
|
Manufacture of hemodialysis concentrate & Warehouse
|
|
Auckland, New Sealand
|
|
|
2,170
|
|
|
leased
|
|
May 2030
|
|
Warehouse
|
|
Selangor, Malaysia
|
|
|
3,149
|
|
|
leased
|
|
May 2012
|
|
Administration / Warehouse
|
|
Yongin, South Korea
|
|
|
2,645
|
|
|
leased
|
|
May 2012
|
|
Warehouse
|
|
Oita, Japan (Inukai Plant)
|
|
|
3,065
|
|
|
owned
|
|
|
|
Manufacture of polysulfone filters
|
|
Fukuoka, Japan (Buzen Plant)
|
|
|
37,092
|
|
|
owned
|
|
|
|
Manufacture of peritoneal dialysis bags
|
|
Saga, Japan
|
|
|
6,668
|
|
|
leased
|
|
January 2010
with 1 year
renewal option
|
|
Warehouse
|
|
Waltham, Massachusetts
|
|
|
25,588
|
|
|
leased
|
|
April 2017 - July 2017 with a 10 year renewal and a second 5
year renewal option
|
|
North American corporate headquarters
|
|
Walnut Creek, California
|
|
|
7,897
|
|
|
leased
|
|
July 2012 with 5 year renewal option
|
|
Manufacture of Hemodialysis machines and peritoneal
|
|
Pittsburg, California
|
|
|
5,574
|
|
|
leased
|
|
July 2012 with 5 year renewal option
|
|
Warehouse
|
|
Ogden, Utah
|
|
|
74,322
|
|
|
owned
|
|
|
|
Manufacture polysulfone membranes and dialyzers and peritoneal
dialysis solutions; research and development
|
|
Ogden, Utah
|
|
|
9,755
|
|
|
leased
|
|
July 2033
|
|
Plant expansion, manufacturing operations
|
|
Ogden, Utah
|
|
|
24,452
|
|
|
leased
|
|
December 2011
|
|
Warehouse
|
|
Ogden, Utah
|
|
|
8,933
|
|
|
leased
|
|
December 2011
|
|
Warehouse
|
|
Ogden, Utah
|
|
|
2,072
|
|
|
leased
|
|
year-to-year lease
|
|
Warehouse
|
|
Oregon, Ohio
|
|
|
13,934
|
|
|
leased
|
|
April 2019
|
|
Manufacture of liquid hemodialysis concentrate solutions
|
|
Livingston, California
|
|
|
7,885
|
|
|
leased
|
|
December 2011
|
|
Manufacture of liquid hemodialysis concentrates and resupply
|
|
Milpitas, California
|
|
|
8,670
|
|
|
leased
|
|
December 2015 with 5-year renewal option
|
|
Clinical laboratory testing
|
|
Rockleigh, New Jersey
|
|
|
9,812
|
|
|
leased
|
|
May 2012
|
|
Clinical laboratory testing
|
|
Irving, Texas
|
|
|
6,506
|
|
|
leased
|
|
December 2010
|
|
Manufacture of liquid hemodialysis solution
|
|
Reynosa, Mexico
|
|
|
21,015
|
|
|
leased
|
|
June 2013
|
|
Manufacture of bloodlines
|
|
Reynosa, Mexico
|
|
|
4,645
|
|
|
owned
|
|
|
|
Warehouse
|
|
Lachine, Canada
|
|
|
3,601
|
|
|
leased
|
|
March 2011
|
|
Warehouse
|
|
Montreal, Canada
|
|
|
4,036
|
|
|
leased
|
|
September 2010
|
|
Warehouse
|
44
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Currently
|
|
|
|
|
|
|
|
|
|
|
Owned or
|
|
|
|
|
|
|
|
Floor Area
|
|
|
Leased by
|
|
|
|
|
|
|
|
(Approximate
|
|
|
Fresenius
|
|
Lease
|
|
|
|
Location
|
|
Square Meters)
|
|
|
Medical Care
|
|
Expiration
|
|
Use
|
|
|
|
Richmond , Canada
|
|
|
2,286
|
|
|
leased
|
|
January 2016
|
|
Warehouse
|
|
Richmond Hill, Canada
|
|
|
5,948
|
|
|
leased
|
|
November 2016
|
|
Warehouse and administrative offices
|
|
Oklahoma City, OK
|
|
|
3,427
|
|
|
leased
|
|
October 2010
|
|
Manufacture of sorbent cartridges
|
We lease most of our dialysis clinics, manufacturing,
laboratory, warehousing and distribution and administrative and
sales facilities in the U.S. and other countries on terms which
we believe are customary in the industry. We own those dialysis
clinics and manufacturing facilities that we do not lease.
For information regarding plans to expand our facilities and
related capital expenditures, see “Item 4.A. History
and Development of the Company — Capital
Expenditures.”
|
|
|
|
Item 4A.
|
Unresolved
Staff Comments
|
Not applicable.
|
|
|
|
Item 5.
|
Operating
and Financial Review and Prospects
|
You should read the following discussion and analysis of the
results of operations of Fresenius Medical Care AG &
Co. KGaA and its subsidiaries in conjunction with our historical
consolidated financial statements and related notes contained
elsewhere in this report. Some of the statements contained
below, including those concerning future revenue, costs and
capital expenditures and possible changes in our industry and
competitive and financial conditions include forward-looking
statements. We made these forward-looking statements based on
the expectations and beliefs of the management of the
Company’s General Partner concerning future events which
may affect us, but we cannot assure that such events will occur
or that the results will be as anticipated. Because such
statements involve risks and uncertainties, actual results may
differ materially from the results which the forward-looking
statements express or imply. Such statements include the matters
and are subject to the uncertainties that we described in the
discussion in this report entitled
“Introduction — Forward-Looking Statements.”
(See also Item 3.D., “Key Information — Risk
Factors.”)
Our business is also subject to other risks and uncertainties
that we describe from time to time in our public filings.
Developments in any of these areas could cause our results to
differ materially from the results that we or others have
projected or may project.
Critical
Accounting Policies
The Company’s reported financial condition and results of
operations are sensitive to accounting methods, assumptions and
estimates that are the basis for our financial statements. The
critical accounting policies, the judgments made in the creation
and application of these policies, and the sensitivities of
reported results to changes in accounting policies, assumptions
and estimates are factors to be considered along with the
Company’s financial statements, and the discussion below in
“Results of Operations.”
Recoverability
of Goodwill and Intangible Assets
The growth of our business through acquisitions has created a
significant amount of intangible assets, including goodwill,
trade names and management contracts. At December 31, 2009,
the carrying amount of goodwill amounted to $7,511 million
and
non-amortizable
intangible assets amounted to $430 million representing in
total approximately 50% of our total assets.
In accordance with current accounting standards, we perform an
impairment test of goodwill and
non-amortizable
intangible assets at least once a year for each reporting unit,
or if we become aware of events that occur or if circumstances
change that would indicate the carrying value might be impaired
(See also Note 1f) in the Notes to Consolidated Financial
Statements).
To comply with the provisions of the current accounting
standards for the impairment testing, the fair value of the
reporting unit is compared to the reporting unit’s carrying
amount. We estimate the fair value of each reporting unit using
estimated future cash flows for the unit discounted by a
weighted average cost of capital (“WACC”) specific to
that reporting unit. Estimating the discounted future cash flows
involves significant assumptions, especially regarding future
reimbursement rates and sales prices, treatments and sales
volumes and costs. In
45
determining discounted cash flows, the Company utilizes for
every reporting unit, its three-year budget, projections for
years 4 to 10 and a corresponding growth rate for all remaining
years. Projections for up to ten years are possible due to the
stability of the Company’s business which, due to the
non-discretionary nature of the healthcare services we provide,
the need for products utilized to provide such services and the
availability of government reimbursement for a substantial
portion of our services, has been largely independent from the
economic cycle. The Company’s weighted average cost of
capital consists of a basic rate of 6.45% for 2009. This basic
rate is then adjusted by a country specific risk rate within
each reporting.
If the fair value of the reporting unit is less than its
carrying value, a second step is performed which compares the
fair value of the reporting unit’s goodwill to the carrying
value of its goodwill. If the fair value of the goodwill is less
than its carrying value, the difference is recorded as an
impairment.
A prolonged downturn in the healthcare industry with lower than
expected increases in reimbursement rates
and/or
higher than expected costs for providing healthcare services and
for procuring and selling products could adversely affect our
estimated future cash flows. Future adverse changes in a
reporting unit’s economic environment could affect the
discount rate. A decrease in our estimated future cash flows
and/or
a
decline in a reporting unit’s economic environment could
result in impairment charges to goodwill and other intangible
assets which could materially and adversely affect our future
financial position and operating results.
Legal
Contingencies
We are party to litigation and subject to investigations
relating to a number of matters as described in Note 18 of
the Notes to Consolidated Financial Statements, “Legal
Proceedings.” The outcome of these matters may have a
material effect on our financial position, results of operations
or cash flows.
We regularly analyze current information including, as
applicable, our defenses and we provide accruals for probable
contingent losses including the estimated legal expenses to
resolve the matters. We use the resources of our internal legal
department as well as external lawyers for the assessment. In
making the decision regarding the need for loss accrual, we
consider the degree of probability of an unfavorable outcome and
our ability to make a reasonable estimate of the amount of loss.
The filing of a suit or formal assertion of a claim or
assessment, or the disclosure of any such suit or assertion,
does not automatically indicate that accrual of a loss may be
appropriate.
Accounts
Receivable and Allowance for Doubtful Accounts
Trade accounts receivable are a significant asset of ours and
the allowance for doubtful accounts is a significant estimate
made by management. Trade accounts receivable were
$2,286 million and $2,176 million at December 31,
2009 and 2008, respectively, net of allowances for doubtful
accounts of $266 million and $263 million at
December 31, 2009 and 2008, respectively. Approximately
half of our receivables relates to business in our North America
segment.
Dialysis care revenues are recognized and billed at amounts
estimated to be receivable under reimbursement arrangements with
third party payors. Medicare and Medicaid programs are billed at
pre-determined net realizable rates per treatment that are
established by statute or regulation. Revenues for
non-governmental payors where we have contracts or letters of
agreement in place are recognized at the prevailing contract
rates. The remaining non-governmental payors are billed at our
standard rates for services and, in our North America segment, a
contractual adjustment is recorded to recognize revenues based
on historic reimbursement experience with those payors for which
contracted rates are not predetermined. The contractual
adjustment and the allowance for doubtful accounts are reviewed
quarterly for their adequacy. No material changes in estimates
were recorded for the contractual allowance in the periods
presented.
The allowance for doubtful accounts is based on local payment
and collection experience. We sell dialysis products directly or
through distributors in over 115 countries and dialysis services
in more than 35 countries through owned or managed clinics. Most
payors are government institutions or government-sponsored
programs with significant variations between the countries and
even between payors within one country in local payment and
collection practices. Specifically, public health institutions
in a number of countries outside the U.S. require a significant
amount of time until payment is made. Payment differences are
mainly due to the timing of the funding by the local, state or
federal government to the agency that is sponsoring the program
that purchases our services or products. The collection of
accounts receivable from product sales to dialysis clinics is
affected by the same underlying causes, since these buyers of
our products are reimbursed as well by government institutions
or government sponsored programs.
46
In our U.S. operations, the collection process is usually
initiated 30 days after service is provided or upon the
expiration of the time provided by contract. For Medicare and
Medicaid, once the services are approved for payment, the
collection process begins upon the expiration of a period of
time based upon experience with Medicare and Medicaid. In all
cases where co-payment is required the collection process
usually begins within 30 days after service has been
provided. In those cases where claims are approved for amounts
less than anticipated or if claims are denied, the collection
process usually begins upon notice of approval of the lesser
amounts or upon denial of the claim. The collection process can
be confined to internal efforts, including the accounting and
sales staffs and, where appropriate, local management staff. If
appropriate, external collection agencies may be engaged.
For our international operations, a significant number of payors
are government entities whose payments are often determined by
local laws and regulations. Depending on local facts and
circumstances, the period of time to collect can be quite
lengthy. In those instances where there are commercial payors,
the same type of collection process is initiated as in the U.S.
Due to the number of our subsidiaries and different countries
that we operate in, our policy of determining when a valuation
allowance is required considers the appropriate local facts and
circumstances that apply to an account. While payment and
collection practices vary significantly between countries and
even agencies within one country, government payors usually
represent low credit risks. Accordingly, the length of time to
collect does not, in and of itself, indicate an increased credit
risk and it is our policy to determine when receivables should
be classified as bad debt on a local basis taking into account
local practices. In all instances, local review of accounts
receivable is performed on a regular basis, generally monthly.
When all efforts to collect a receivable, including the use of
outside sources where required and allowed, have been exhausted,
and after appropriate management review, a receivable deemed to
be uncollectible is considered a bad debt and written off.
Estimates for the allowances for doubtful accounts receivable
from the dialysis service business are mainly based on local
payment and past collection history. Specifically, the
allowances for the North American operations are based on an
analysis of collection experience, recognizing the differences
between payors and aging of accounts receivable. From time to
time, accounts receivable are reviewed for changes from the
historic collection experience to ensure the appropriateness of
the allowances. The allowances in the International segment and
the products business are also based on estimates and consider
various factors, including aging, creditor and past collection
history. Write offs are taken on a claim by claim basis when the
collection efforts are exhausted. Due to the fact that a large
portion of our reimbursement is provided by public health care
organizations and private insurers, we expect that most of our
accounts receivables will be collectable, albeit potentially
slightly more slowly in the International segment in the
immediate future, particularly in countries most severely
affected by the current global financial crisis. A significant
change in our collection experience, a deterioration in the
aging of receivables and collection difficulties could require
that we increase our estimate of the allowance for doubtful
accounts. Any such additional bad debt charges could materially
and adversely affect our future operating results.
If, in addition to our existing allowances, 1% of the gross
amount of our trade accounts receivable as of December 31,
2009 were uncollectible through either a change in our estimated
contractual adjustment or as bad debt, our operating income for
2009 would have been reduced by approximately 1.5%.
The following tables show the portion and aging of trade
accounts receivable of major debtors or debtor groups at
December 31, 2009 and 2008. No single debtor other than
U.S. Medicaid and Medicare accounted for more than 5% of total
trade accounts receivable in either year. Trade accounts
receivable in the International segment are for a large part due
from government or government-sponsored organizations that are
established in the various countries
47
within which we operate. Amounts pending approval from third
party payors represent less than 1% at December 31, 2009.
Aging of Net Trade Accounts Receivable by Major Payor Groups:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, 2009
|
|
|
|
|
|
|
|
|
|
|
overdue
|
|
|
overdue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
overdue
|
|
|
more than
|
|
|
more than
|
|
|
overdue
|
|
|
|
|
|
% of
|
|
|
|
|
|
|
|
by
|
|
|
3 months
|
|
|
6 months
|
|
|
by
|
|
|
|
|
|
net
|
|
|
|
|
|
|
|
up to
|
|
|
up to
|
|
|
up to
|
|
|
more than
|
|
|
|
|
|
trade
|
|
|
|
|
current
|
|
|
3 months
|
|
|
6 months
|
|
|
1 year
|
|
|
1 year
|
|
|
Total
|
|
|
A/R
|
|
|
|
|
(in millions)
|
|
|
|
|
U.S. Medicare and Medicaid Programs
|
|
$
|
287
|
|
|
$
|
74
|
|
|
$
|
32
|
|
|
$
|
22
|
|
|
$
|
22
|
|
|
$
|
437
|
|
|
|
19
|
|
|
U.S. Commercial Payors
|
|
|
256
|
|
|
|
140
|
|
|
|
52
|
|
|
|
40
|
|
|
|
30
|
|
|
|
518
|
|
|
|
23
|
|
|
U.S. Hospitals
|
|
|
88
|
|
|
|
19
|
|
|
|
3
|
|
|
|
2
|
|
|
|
2
|
|
|
|
114
|
|
|
|
5
|
|
|
Self-Pay of U.S. patients
|
|
|
2
|
|
|
|
6
|
|
|
|
6
|
|
|
|
3
|
|
|
|
1
|
|
|
|
18
|
|
|
|
1
|
|
|
Other North America
|
|
|
2
|
|
|
|
1
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
3
|
|
|
|
—
|
|
|
International product customers and dialysis payors
|
|
|
699
|
|
|
|
232
|
|
|
|
106
|
|
|
|
86
|
|
|
|
73
|
|
|
|
1,196
|
|
|
|
52
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
1,334
|
|
|
$
|
472
|
|
|
$
|
199
|
|
|
$
|
153
|
|
|
$
|
128
|
|
|
$
|
2,286
|
|
|
|
100
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, 2008
|
|
|
|
|
|
|
|
|
|
|
overdue
|
|
|
overdue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
overdue
|
|
|
more than
|
|
|
more than
|
|
|
overdue
|
|
|
|
|
|
% of
|
|
|
|
|
|
|
|
by
|
|
|
3 months
|
|
|
6 months
|
|
|
by
|
|
|
|
|
|
net
|
|
|
|
|
|
|
|
up to
|
|
|
up to
|
|
|
up to
|
|
|
more than
|
|
|
|
|
|
trade
|
|
|
|
|
current
|
|
|
3 months
|
|
|
6 months
|
|
|
1 year
|
|
|
1 year
|
|
|
Total
|
|
|
A/R
|
|
|
|
|
(in millions)
|
|
|
|
|
U.S. Medicare and Medicaid Programs
|
|
$
|
311
|
|
|
$
|
56
|
|
|
$
|
47
|
|
|
$
|
34
|
|
|
$
|
34
|
|
|
$
|
482
|
|
|
|
22
|
|
|
U.S. Commercial Payors
|
|
|
215
|
|
|
|
176
|
|
|
|
62
|
|
|
|
47
|
|
|
|
41
|
|
|
|
541
|
|
|
|
25
|
|
|
U.S. Hospitals
|
|
|
83
|
|
|
|
25
|
|
|
|
3
|
|
|
|
1
|
|
|
|
1
|
|
|
|
113
|
|
|
|
5
|
|
|
Self-Pay of U.S. patients
|
|
|
1
|
|
|
|
5
|
|
|
|
3
|
|
|
|
2
|
|
|
|
—
|
|
|
|
11
|
|
|
|
1
|
|
|
Other North America
|
|
|
7
|
|
|
|
1
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
8
|
|
|
|
—
|
|
|
International product customers and dialysis payors
|
|
|
620
|
|
|
|
185
|
|
|
|
84
|
|
|
|
66
|
|
|
|
66
|
|
|
|
1,021
|
|
|
|
47
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
1,237
|
|
|
$
|
448
|
|
|
$
|
199
|
|
|
$
|
150
|
|
|
$
|
142
|
|
|
$
|
2,176
|
|
|
|
100
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Self-Insurance
Programs
Under the insurance programs for professional, product and
general liability, auto liability and worker’s compensation
claims, FMCH, our largest subsidiary, is partially self-insured
for professional liability claims. For all other coverages we
assume responsibility for incurred claims up to predetermined
amounts above which third party insurance applies. Reported
liabilities for the year represent estimated future payments of
the anticipated expense for claims incurred (both reported and
incurred but not reported) based on historical experience and
existing claim activity. This experience includes both the rate
of claims incidence (number) and claim severity (cost) and is
combined with individual claim expectations to estimate the
reported amounts.
Financial
Condition and Results of Operations
Overview
We are engaged primarily in providing dialysis services and
manufacturing and distributing products and equipment for the
treatment of end-stage renal disease. In the U.S., we also
perform clinical laboratory testing. We estimate that providing
dialysis services and distributing dialysis products and
equipment represents an over $65 billion worldwide market
with expected annual worldwide patient growth of around 6%.
Patient growth results from factors such as the aging population
and increased life expectancies; shortage of donor organs for
kidney transplants, increasing incidence and better treatment of
and survivial of patients with diabetes and hypertension, which
frequently precede the onset of ESRD; improvements in treatment
quality, which prolong patient life; and improving standards of
living in developing countries, which make life-saving dialysis
treatment available. Key to continued growth in revenue is our
ability to attract new patients in order to increase the number
of treatments performed each year. For that reason, we believe
the number of treatments performed each year is a strong
indicator of continued revenue growth and success. In addition,
the reimbursement and ancillary services utilization environment
significantly influences our business. In the past we
experienced and also expect in the future generally stable
reimbursements for dialysis services. This includes the
balancing of unfavorable reimbursement changes in certain
countries with favorable changes in other countries. The
majority of treatments are paid for by
48
governmental institutions such as Medicare in the United States.
As a consequence of the pressure to decrease health care costs,
reimbursement rate increases have been limited. Our ability to
influence the pricing of our services is limited. Profitability
depends on our ability to manage rising labor, drug and supply
costs.
A majority of our U.S. dialysis services are paid for by the
Medicare program. Medicare payments for dialysis services are
based on a composite rate which includes a drug add-on
adjustment, case-mix adjustments, and a regional wage index
adjustment. The drug add-on adjustment was established under the
Medicare Prescription Drug, Improvement and Modernization Act of
2003 (“MMA”) to account for differences in Medicare
reimbursement for separately billable pharmaceuticals
pre-MMA
and
the new average sales price reimbursement system established by
the MMA.
For calendar year 2009, the Centers for Medicare and Medicaid
Services (“CMS”) set the drug add-on adjustment at
$20.33 per treatment, or 15.2% of the total per-treatment
composite rate payment. For 2010, CMS kept the drug add-on
amount constant at $20.33 per treatment, while it increased the
base portion of the composite rate by 1% pursuant to the
requirement in the Medicare Improvements for Patients and
Providers Act of 2008 (“MIPPA”). As a result, the drug
add-on amount, constant in dollar terms, declined to 15% of the
total per-treatment payment in 2010. The base portion of the
composite rate, unlike many other payment rates in Medicare, has
not been automatically updated each year. As a result, this
portion of the composite payment rate has not received an annual
update in the absence of a statutory change. In MIPPA, Congress
provided for a 1.0% increase in the base portion of the
composite rate in each of 2009 and 2010. Further, Congress
eliminated a provision that previously paid hospital-based
facilities slightly more than independent (or
“free-standing”) facilities. For 2010, the base
composite rate is $135.15 for both independent and
hospital-based facilities, an increase of 1.0% from the 2009
rate. CMS updated the wage index adjustment applicable to ESRD
facilities from the 25/75 blend between adjustments based on old
metropolitan statistical areas (“MSAs”) and those
based on new core-based statistical areas (“CBSAs”)
used in 2008. For 2009, CMS completed the transition from the
MSA definition to the CBSA definition, and facilities are now
paid according to the CBSA rate. For 2010, CMS reduced the wage
index floor from 0.70 to 0.65. For a discussion of the composite
rate for reimbursement of dialysis treatments, see
Item 4.B, “Business Overview — Regulatory
and Legal Matters — Reimbursement”.
Certain other items and services that we furnish at our dialysis
centers are not now included in the composite rate and are
eligible for separate Medicare reimbursement. The most
significant of these items are drugs or biologicals, such as
erythropoietin-stimulating agents (“ESAs”), vitamin D
analogs, and iron, which are reimbursed at 106% of the average
sales price as reported to CMS by the manufacturer. Products and
support services furnished to ESRD patients receiving dialysis
treatment at home are also reimbursed separately under a
reimbursement structure comparable to the in-center composite
rate. Although these reimbursement methodologies limit the
allowable charge per treatment, they provide us with predictable
per treatment revenues.
With the enactment of MIPPA in 2008, Congress mandated the
development of an expanded ESRD bundled payment system for
services furnished on or after January 1, 2011. MIPPA
requires CMS to implement by January 1, 2011 a bundled ESRD
payment system under which CMS will reimburse dialysis
facilities with a single payment for (i) all items and
services included in the composite rate, (ii) all ESAs and
other pharmaceuticals (other drugs and biologicals, other than
vaccines) furnished to the patients that were previously
reimbursed separately, (iii) diagnostic laboratory tests
and (iv) other services furnished to individuals for the
treatment of ESRD. The initial bundled reimbursement rate will
be set based on 98% of estimated 2011 Medicare program costs of
dialysis care as calculated under the current reimbursement
system using the lowest per patient utilization data from 2007,
2008 or 2009 for all Medicare beneficiaries. The bundled payment
will be subject to case mix adjustments that may take into
account individual patient characteristics (e.g., age, weight,
body mass) and co-morbidities. Payments will also be adjusted
for (i) certain high cost patient outliers due to unusual
variations in medically necessary care, (ii) disparately
high costs incurred by low volume facilities relative to other
facilities and (iii) such other adjustments as the
Secretary of Health and Human Services (“HHS”) deems
appropriate. Beginning in 2012, the bundled payment amount will
be subject to annual increases based on increases in the costs
of a “market basket” of dialysis items and services to
be determined by HHS minus 1%. MIPPA requires, CMS to implement
pay-for-performance
standards, effective in 2012. Dialysis facilities that fail to
achieve the established quality standards will have payments
reduced by up to 2%. Facility quality standards are expected to
be limited at the outset to anemia management and hemodialysis
adequacy and facility performance scores will be made available
to the public. The bundled system will be phased in over four
years with full implementation for all dialysis facilities on
January 1, 2014. However, providers may elect in November
2010 to become fully subject to the new system starting in
January 2011. MIPPA extends the authority of specialized
Medicare Advantage (“MA”) plans to target enrollment
to certain populations through December 31, 2010 and
revises definitions, care management requirements and quality
reporting standards for all specialized plans. On
September 29, 2009, CMS published a proposed rule
implementing the case-mix adjusted bundled prospective payment
system (“PPS”) for ESRD
49
dialysis facilities in accordance with MIPPA. If implemented in
its current form, the provisions of the proposed rule relating
to case mix and transition adjustments would result in
reimbursement reductions. The proposed rule, if adopted without
further changes, would fail to provide adequate funding for ESRD
facilities’ delivery of oral ESRD medications currently
covered under Medicare Part D and would not adequately
address the coordination of secondary insurance coverage. While
it is clear that the expanded ESRD bundled payment system will
materially affect how the Company is paid for pharmaceuticals
and other items and services, the Company cannot estimate the
overall effect of the new system on its business until adoption
of the final CMS regulations.
We have identified three operating segments, North America,
International, and Asia Pacific. For reporting purposes, we have
aggregated the International and Asia Pacific segments as
“International.” We aggregated these segments due to
their similar economic characteristics. These characteristics
include same services provided and same products sold, same type
patient population, similar methods of distribution of products
and services and similar economic environments. The general
partner’s Management Board member responsible for the
profitability and cash flow of each segment’s various
businesses supervises the management of each operating segment.
The accounting policies of the operating segments are the same
as those we apply in preparing our consolidated financial
statements under accounting principles generally accepted in the
United States (“U.S. GAAP”). Our management evaluates
each segment using a measure that reflects all of the
segment’s controllable revenues and expenses.
With respect to the performance of our business operations, our
management believes the most appropriate measure in this regard
is operating income which measures our source of earnings.
Financing is a corporate function which segments do not control.
Therefore, we do not include interest expense relating to
financing as a segment measurement. We also regard income taxes
to be outside the segments’ control. Similarly, we do not
allocate “corporate costs,” which relate primarily to
certain headquarters overhead charges, including accounting and
finance, professional services, etc. because we believe that
these costs are also not within the control of the individual
segments. In addition, certain acquisitions and intangible
assets are not allocated to a segment but are accounted for as
“corporate.” Accordingly, all of these items are
excluded from our analysis of segment results and are discussed
below in the discussion of our consolidated results of
operations.
The following tables summarize our financial performance and
certain operating results by principal business segment for the
periods indicated. Inter-segment sales primarily reflect sales
of medical equipment and supplies from the International segment
to the North America segment. We prepared the information using
a management
50
approach, consistent with the basis and manner in which our
management internally disaggregates financial information to
assist in making internal operating decisions and evaluating
management performance.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
(in millions)
|
|
|
|
|
Total revenue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
North America
|
|
$
|
7,615
|
|
|
$
|
7,007
|
|
|
$
|
6,664
|
|
|
International
|
|
|
3,713
|
|
|
|
3,688
|
|
|
|
3,134
|
|
|
Corporate
|
|
|
—
|
|
|
|
1
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Totals
|
|
|
11,328
|
|
|
|
10,696
|
|
|
|
9,798
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Inter-segment revenue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
North America
|
|
|
3
|
|
|
|
2
|
|
|
|
1
|
|
|
International
|
|
|
78
|
|
|
|
82
|
|
|
|
77
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Totals
|
|
|
81
|
|
|
|
84
|
|
|
|
78
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total net revenue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
North America
|
|
|
7,612
|
|
|
|
7,005
|
|
|
|
6,663
|
|
|
International
|
|
|
3,635
|
|
|
|
3,606
|
|
|
|
3,057
|
|
|
Corporate
|
|
|
—
|
|
|
|
1
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Totals
|
|
|
11,247
|
|
|
|
10,612
|
|
|
|
9,720
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortization and depreciation
|
|
|
|
|
|
|
|
|
|
|
|
|
|
North America
|
|
|
265
|
|
|
|
238
|
|
|
|
220
|
|
|
International
|
|
|
183
|
|
|
|
171
|
|
|
|
141
|
|
|
Corporate
|
|
|
9
|
|
|
|
7
|
|
|
|
2
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Totals
|
|
|
457
|
|
|
|
416
|
|
|
|
363
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
North America
|
|
|
1,250
|
|
|
|
1,168
|
|
|
|
1,130
|
|
|
International
|
|
|
637
|
|
|
|
616
|
|
|
|
544
|
|
|
Corporate
|
|
|
(131
|
)
|
|
|
(112
|
)
|
|
|
(94
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Totals
|
|
|
1,756
|
|
|
|
1,672
|
|
|
|
1,580
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
21
|
|
|
|
25
|
|
|
|
29
|
|
|
Interest expense
|
|
|
(321
|
)
|
|
|
(361
|
)
|
|
|
(400
|
)
|
|
Income tax expense
|
|
|
(491
|
)
|
|
|
(476
|
)
|
|
|
(454
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
|
965
|
|
|
|
860
|
|
|
|
755
|
|
|
Less: Net income attributable to Noncontrolling interests
|
|
|
74
|
|
|
|
42
|
|
|
|
38
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Income attributable to FMC-AG & Co. KGaA
|
|
$
|
891
|
|
|
$
|
818
|
|
|
$
|
717
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year
ended December 31, 2009 compared to year ended
December 31, 2008
Highlights
Revenues increased by 6% to $11,247 million (9% at constant
rates) mainly due to organic growth at 8% and acquisitions at 1%.
Operating income (EBIT) increased 5%.
Net Income increased by 9%.
51
Consolidated
Financials
Key
Indicators for Consolidated Financials
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in %
|
|
|
|
|
|
|
|
|
|
|
|
|
|
at constant
|
|
|
|
|
2009
|
|
|
2008
|
|
|
as reported
|
|
|
exchange rates
|
|
|
|
|
Number of treatments
|
|
|
29,425,758
|
|
|
|
27,866,573
|
|
|
|
6
|
%
|
|
|
|
|
|
Same market treatment growth in %
|
|
|
4.1
|
%
|
|
|
4.5
|
%
|
|
|
|
|
|
|
|
|
|
Revenue in $ million
|
|
|
11,247
|
|
|
|
10,612
|
|
|
|
6
|
%
|
|
|
9
|
%
|
|
Gross profit in % of revenue
|
|
|
34.1
|
%
|
|
|
34.2
|
%
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative costs in % of revenue
|
|
|
17.6
|
%
|
|
|
17.7
|
%
|
|
|
|
|
|
|
|
|
|
Net income attributable to FMC-AG & Co. KGaA in
$ million
|
|
|
891
|
|
|
|
818
|
|
|
|
9
|
%
|
|
|
|
|
We provided 29,425,758 treatments during the year ended
December 31, 2009, an increase of 6% over the same period
in 2008. Same market treatment growth contributed 4% and growth
from acquisitions contributed 2%.
At December 31, 2009, we owned, operated or managed
(excluding those managed but not consolidated in the U.S.) 2,553
clinics compared to 2,388 clinics at December 31, 2008.
During 2009, we acquired 73 clinics, opened 118 clinics and
combined or closed 26 clinics. The number of patients treated in
clinics that we own, operate or manage (excluding patients of
clinics managed but not consolidated in the U.S.) increased by
6% to 195,651 at December 31, 2009 from 184,086 at
December 31, 2008. Including 30 clinics managed but not
consolidated in the U.S., the total number of patients was
197,358.
Net revenue increased by 6% (9% at constant exchange rates) for
the year ended December 31, 2009 over the comparable period
in 2008 due to growth in both dialysis care and dialysis
products revenues.
Dialysis care revenue grew by 8% to $8,350 million (10% at
constant exchange rates) in 2009 mainly due to growth in same
market treatments (4%), revenue per treatment (5%) and
acquisitions (1%), partially offset by exchange rate
fluctuations (2%).
Dialysis product revenue increased by 1% to $2,897 million
(increased by 6% at constant exchange rates) in the same period
driven by pharmaceutical sales, especially of the newly licensed
intravenous iron products and increased sales of dialyzers,
bloodlines, solutions and concentrates, as well as sales of
products for acute care treatments. These increases were
partially offset by decreased sales of our phosphate binding
drug
PhosLo
®
following a competitor’s launch of a generic version of the
drug in the U.S. in October 2008 and lower sales of hemodialysis
machines.
The slight decrease in gross profit margin reflects a decrease
in gross profit margin in North America, partially offset by an
increase in the International segment. North America was
impacted by cost increases for pharmaceuticals as well as lower
margin contribution from our pharmaceutical business due to a
competitor’s launch of a generic version of
PhosLo
®
in the U.S. in October 2008, increased depreciation related to
recently acquired computer equipment and recently installed
leasehold improvements and higher personnel costs, partially
offset by increased revenue rates. The increase in International
was due to the positive effect of an inventory adjustment in the
first quarter of 2009, lower production costs caused by lower
prices for certain raw material and energy as well as economies
of scale, partially offset by unfavorable foreign exchange
transaction effects related to the purchase of products produced
in Europe and Japan due to the appreciation of the Euro and the
Yen against local currencies.
Selling, general and administrative (“SG&A”)
expenses increased to $1,982 million in 2009 from
$1,876 million in the same period of 2008. SG&A costs
as a percentage of sales decreased slightly to 17.6% in 2009
from 17.7% in the same period of 2008. The slight decrease was
due to a decrease in North America driven by economies of scale
and lower bad debt expenses partially offset by higher personnel
expenses. Bad debt expense for the year ended December 31,
2009 was $210 million as compared to $214 million in
2008, representing 1.9% of sales for the year ended
December 31, 2009, as compared to 2.0% for the same period
in 2008.
Research and development (“R&D”) expenses
increased to $94 million in 2009 from $80 million for
the same period in 2008 due to additional programs in the field
of hemodialysis equipment and extracorporeal critical care
therapies.
52
Operating income increased to $1,756 million in 2009 from
$1,672 million for the same period in 2008. Operating
income margin decreased to 15.6% in 2009 as compared to 15.8%
for the same period in 2008 due to the decreased gross profit
margin as noted above partially offset by decreased SG&A
expenses as a percentage of sales as described above.
Interest expense decreased by 11% to $321 million in 2009
from $361 million for the same period in 2008 as a result
of decreased short-term interest rates.
Income tax expense increased to $491 million for the year
ended December 31, 2009 from $476 million for the same
period in 2008 as a result of higher earnings in 2009. The
effective tax rate for 2009 decreased to 33.7% from 35.6% in
2008 mainly as a result of an increase in non-taxable
noncontrolling interests in North America in 2009.
Net income attributable to FMC-AG & Co. KGaA for 2009
increased to $891 million from $818 million for the
same period in 2008 as a result of the combined effects of the
items discussed above.
We employed 67,988 people (full-time equivalents) as of
December 31, 2009 compared to 64,666 as of
December 31, 2008, an increase of 5.1% primarily due to
overall growth in our business.
The following discussions pertain to our business segments and
the measures we use to manage these segments.
North
America Segment
Key
Indicators for North America Segment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
Change in %
|
|
|
|
|
Number of treatments
|
|
|
19,867,465
|
|
|
|
19,146,084
|
|
|
|
4
|
%
|
|
Same market treatment growth in %
|
|
|
3.5
|
%
|
|
|
2.9
|
%
|
|
|
|
|
|
Revenue in $ million
|
|
|
7,612
|
|
|
|
7,005
|
|
|
|
9
|
%
|
|
Depreciation and amortization in $ million
|
|
|
265
|
|
|
|
238
|
|
|
|
11
|
%
|
|
Operating income in $ million
|
|
|
1,250
|
|
|
|
1,168
|
|
|
|
7
|
%
|
|
Operating income margin in %
|
|
|
16.4
|
%
|
|
|
16.7
|
%
|
|
|
|
|
Revenue
Treatments increased by 4% for the year ended December 31,
2009 as compared to the same period in 2008 mostly due to same
market growth (4%) and acquisitions (1%), partially offset by
the effect of one less dialysis day of 1%. At December 31,
2009, 132,262 patients (a 5% increase over the same period in
the prior year) were being treated in the 1,784 clinics that we
own or operate in the North America segment, compared to 125,857
patients treated in 1,686 clinics at December 31, 2008.
Average North America revenue per treatment was $341 for the
year ended December 31, 2009 and $326 in the same period in
2008. In the U.S., average revenue per treatment was $347 for
the year ended December 31, 2009 and $330 for the same
period in 2008. The increase was mainly attributable to a
revenue per treatment increase, including increased commercial
payor revenue, increased utilization of pharmaceuticals,
including EPO, Medicare reimbursement increases for
pharmaceuticals (ASP+6%) and the 1% 2009 Medicare composite rate
increase.
Net revenue for the North America segment for 2009 increased as
a result of increases in dialysis care revenue by 9% to
$6,794 million from $6,247 million in the same period
of 2008 and in dialysis product revenue by 8% to
$818 million from $758 million in 2008.
The dialysis care revenue increase was driven by same market
treatment growth (4%), increased revenue per treatment (4%) and
acquisitions (1%). The administration of EPO represented
approximately 21% of total North America dialysis care revenue
for the year ended December 31, 2009 and 20% for the year
ended December 31, 2008.
The dialysis product revenue increase was driven mostly by a
higher pharmaceutical sales, especially of the newly licensed
intravenous iron products, partially offset by lower
PhosLo
®
revenues as a result of a competitor’s launch of a generic
version of
PhosLo
®
in the United States in October 2008.
53
Operating
Income
Operating income increased to $ 1,250 million for the year
ended December 31, 2009 from $ 1,168 million for the
same period in 2008. Operating income margin decreased to 16.4%
in 2009 from 16.7% in 2008 due to increased costs for
pharmaceuticals, lower margin contribution from our
pharmaceutical business due to a competitor’s launch of a
generic version of
PhosLo
®
in October 2008, higher personnel costs and increased
depreciation related to recently acquired computer equipment and
recently installed leasehold improvements, partially offset by
increased revenue per treatment as described above and decreased
bad debt expense. Cost per treatment increased to $283 in 2009
from $273 in 2008.
International
Segment
Key
Indicators for International Segment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in %
|
|
|
|
|
|
|
|
|
|
|
|
|
|
at constant
|
|
|
|
|
2009
|
|
|
2008
|
|
|
as reported
|
|
|
exchange rates
|
|
|
|
|
Number of treatments
|
|
|
9,558,293
|
|
|
|
8,720,489
|
|
|
|
10
|
%
|
|
|
|
|
|
Same market treatment growth in %
|
|
|
5.3
|
%
|
|
|
8.6
|
%
|
|
|
|
|
|
|
|
|
|
Revenue in $ million
|
|
|
3,635
|
|
|
|
3,606
|
|
|
|
1
|
%
|
|
|
9
|
%
|
|
Depreciation and amortization in $ million
|
|
|
183
|
|
|
|
171
|
|
|
|
8
|
%
|
|
|
|
|
|
Operating income in $ million
|
|
|
637
|
|
|
|
616
|
|
|
|
3
|
%
|
|
|
|
|
|
Operating income margin in %
|
|
|
17.5
|
%
|
|
|
17.1
|
%
|
|
|
|
|
|
|
|
|
Revenue
Treatments increased by 10% in 2009 over 2008 mainly due to same
market growth (5%) and acquisitions (5%). As of
December 31, 2009, 63,389 patients (a 9% increase over the
same period of the prior year) were being treated at 769 clinics
that we own, operate or manage in the International segment
compared to 58,229 patients treated at 702 clinics at
December 31, 2008. Average revenue per treatment decreased
to $163 from $171 due to the weakening of local currencies
against the U.S. dollar ($15) partially offset by increased
reimbursement rates and changes in country mix ($7).
Net revenues for the International segment for the year ended
December 31, 2009 increased by 1% as compared to the same
period in 2008 as a result of an increase in dialysis care
revenue partially offset by a decrease in dialysis product
revenue. Organic growth during the period of 8% and a
contribution from acquisitions of approximately 2% were
partially offset by a negative impact of exchange rate
fluctuations of 8% as well as the effect of closed or sold
clinics of 1%.
Including the effects of acquisitions, European region revenue
decreased 1% (8% increase at constant exchange rates), Latin
America region revenue increased 5% (16% increase at constant
exchange rates), and Asia Pacific region revenue increased 6%
(8% increase at constant exchange rates).
Total dialysis care revenue for the International segment
increased during 2009 by 4% (14% increase at constant exchange
rates) to $1,556 million from $1,490 million in the
same period of 2008. This increase is a result of increases in
revenue per treatment of 6%, same market growth of 5% and a 4%
increase in contributions from acquisitions, partially offset by
of the negative impact of exchange rate fluctuations of
approximately 10% as well as the effect of one less dialysis day
of 1%.
Total dialysis product revenue for 2009 decreased by 2% (6%
increase at constant exchange rates) to $2,079 million.
Increased pharmaceutical sales especially related to newly
licensed intraveneous iron products, and increased sales of
dialyzers, bloodlines, hemodialysis solutions and concentrates
as well as sales of products for acute care treatment were more
than offset by the negative impact of exchange rate fluctuations
(8%) and lower sales of hemodialysis machines.
Operating
Income
Operating income increased by 3% to $637 million. Operating
income margin increased to 17.5% for the year ended
December 31, 2009 from 17.1% for the same period in 2008
due to lower production costs as a result of lower prices for
certain raw material and energy, economies of scale and the
positive effect of an inventory adjustment in
54
the first quarter of 2009, partially offset by unfavorable
foreign currency transaction effects related to the purchase of
products produced in Europe and Japan due to the appreciation of
the Euro and Yen against local currencies.
Year
ended December 31, 2008 compared to year ended
December 31, 2007
Consolidated
Financials
Key
Indicators for Consolidated Financials
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in %
|
|
|
|
|
|
|
|
|
|
|
|
|
|
at constant
|
|
|
|
|
2008
|
|
|
2007
|
|
|
as reported
|
|
|
exchange rates
|
|
|
|
|
Number of treatments
|
|
|
27,866,573
|
|
|
|
26,442,421
|
|
|
|
5
|
%
|
|
|
|
|
|
Same market treatment growth in %
|
|
|
4.5
|
%
|
|
|
3.9
|
%
|
|
|
|
|
|
|
|
|
|
Revenue in $ million
|
|
|
10,612
|
|
|
|
9,720
|
|
|
|
9
|
%
|
|
|
8
|
%
|
|
Gross profit in % of revenue
|
|
|
34.2
|
%
|
|
|
34.5
|
%
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative costs in % of revenue
|
|
|
17.7
|
%
|
|
|
17.6
|
%
|
|
|
|
|
|
|
|
|
|
Net income attributable to FMC-AG & Co. KGaA in $
million
|
|
|
818
|
|
|
|
717
|
|
|
|
14
|
%
|
|
|
|
|
We provided 27,866,573 treatments during the year ended
December 31, 2008, an increase of 5% over the same period
in 2007. Same market treatment growth contributed 4% and growth
from acquisitions contributed 1%.
At December 31, 2008, we owned, operated or managed
(excluding those managed but not consolidated in the U.S.) 2,388
clinics compared to 2,238 clinics at December 31, 2007.
During 2008, we acquired 48 clinics, opened 127 clinics and
combined or closed 25 clinics. The number of patients treated in
clinics that we own, operate or manage (excluding patients of
clinics managed but not consolidated in the U.S.) increased by
6% to 184,086 at December 31, 2008 from 173,863 at
December 31, 2007. Including 32 clinics managed but not
consolidated in the U.S., the total number of patients was
185,768.
Net revenue increased by 9% (8% at constant exchange rates) for
the year ended December 31, 2008 over 2007 due to growth in
revenue in both dialysis care and dialysis products.
Dialysis care revenue grew by 7% to $7,737 million (6% at
constant exchange rates) in 2008 mainly due to growth in same
market treatments (4%), revenue per treatment (2%), acquisitions
(1%), and exchange rate fluctuations (1%), partially offset by
sold or closed clinics (1%).
Dialysis product revenue increased by 15% to $2,875 million
(11% at constant exchange rates) mainly as a result of increased
sales of hemodialysis machines, dialyzers, bloodlines,
concentrates, and peritoneal dialysis products and higher
revenues attributable to the phosphate binding drug,
PhosLo
®
and to the sales of the newly licensed intravenous iron products.
The decrease in gross margin reflects reductions in gross margin
in the International segment. North America was impacted by
higher personnel and other operating costs, decreased
utilization of and reduced reimbursement rates for EPO, higher
material costs, and increased costs for the anticoagulant drug
heparin, fully offset by increased commercial payor revenue.
International was affected by strong growth in dialysis care
business which has lower than average margins and unfavorable
foreign currency transaction effects related to purchases from
Europe due to the appreciation of the Euro against local
currencies. Both segments experienced higher depreciation
expense in 2008 as compared to 2007 as a result of expansion of
production capacities. The availability of these new capacities
allowed a more normalized summer maintenance program in our
International facilities, in contrast to the prior year’s
shortened program.
Selling, general and administrative (“SG&A”)
costs increased to $1,876 million in 2008 from
$1,709 million in 2007. SG&A costs as a percentage of
sales increased to 17.7% in 2008 from 17.6% in 2007. The
percentage increased in the North America segment and decreased
in the International segment. North America was impacted by
higher personnel costs and higher bad debt expense, partially
offset by economies of scale and gains on the sale of minority
interests in subsidiaries. International benefited from lower
foreign currency losses in Europe, lower bad debt expense and
with respect to SG&A as a percentage of sales, from revenue
growth in excess of the increase of SG&A. These were
partially offset by higher corporate expenses relating to the
operating expenses of Renal Solutions Inc., reported under
corporate, and compensation expense for stock options. Bad debt
expense for the year
55
ended December 31, 2008 was $214 million as compared
to $202 million in 2007, representing 2.0% of sales for the
year ended December 31, 2008 and 2.1% for 2007.
Research and development (“R&D”) expenses
increased to $80 million in 2008 from $67 million for
the same period in 2007 mainly as a result of the additional
R&D programs related to continued development of
hemodialysis machines, field testing of new products and
extracorporeal and home therapy programs.
Operating income increased to $1,672 million in 2008 from
$1,580 million for 2007. Operating income margin decreased
to 15.8% for the year ended December 31, 2008 from 16.3%
for 2007 due to the decreased gross margins, increased SG&A
as a percentage of sales, and increased R&D costs as
discussed above.
Interest expense decreased 10% to $361 million in 2008 from
$400 million for 2007 mainly as a result of decreased
interest rates and the more favorable financing structure
following the repayment of a portion of our trust preferred
securities. This was partially offset by the slightly increased
debt level resulting from the acquisition of Renal Solutions,
Inc. in the fourth quarter of 2007 as well as higher capital
expenditures in 2008.
Income tax expense increased to $476 million for the year
ended December 31, 2008 from $454 million in 2007 due
to increased earnings. The effective tax rate for 2008 decreased
to 35.6% from 37.5% for 2007 mainly due to a German corporate
tax rate reduction which became effective January 1, 2008.
Net income attributable to FMC-AG & Co. KGaA for 2008
increased to $818 million from $717 million for 2007
mainly as a result of the combined effects of the items
discussed above.
We employed 64,666 people (full-time equivalents) as of
December 31, 2008 compared to 61,406 as of
December 31, 2007, an increase of 5% primarily due to our
overall growth in business.
The following discussions pertain to our business segments and
the measures we use to manage these segments.
North
America Segment
Key
Indicators for North America Segment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2007
|
|
|
Change in %
|
|
|
|
|
Number of treatments
|
|
|
19,146,084
|
|
|
|
18,451,381
|
|
|
|
4
|
%
|
|
Same market treatment growth in %
|
|
|
2.9
|
%
|
|
|
2.9
|
%
|
|
|
|
|
|
Revenue in $ million
|
|
|
7,005
|
|
|
|
6,663
|
|
|
|
5
|
%
|
|
Depreciation and amortization in $ million
|
|
|
238
|
|
|
|
220
|
|
|
|
8
|
%
|
|
Operating income in $ million
|
|
|
1,168
|
|
|
|
1,130
|
|
|
|
3
|
%
|
|
Operating income margin in %
|
|
|
16.7
|
%
|
|
|
17.0
|
%
|
|
|
|
|
Revenue
Treatments increased by 4% for the year ended December 31,
2008 as compared to 2007 due to same market growth (3%) and
acquisitions (1%). At December 31, 2008,
125,857 patients (a 4% increase over the same period in the
prior year) were being treated in the 1,686 clinics that we own
or operate in the North America segment, compared to
121,431 patients treated in 1,602 clinics at
December 31, 2007. Average North America revenue per
treatment was $326 for the year ended December 31, 2008 and
$323 for 2007. In the U.S., average revenue per treatment was
$330 for the year ended December 31, 2008 and $327 in 2007,
mainly due to increased commercial payor revenue.
Net revenue for the North America segment for 2008 increased as
a result of increases in dialysis care revenue by 4% to
$6,247 million from $6,002 million in 2007 and in
dialysis product revenue by 15% to $758 million from
$661 million in 2007.
The dialysis care revenue increase was driven by same market
treatment growth of 3%, increased revenue per treatment (1%),
and 1% resulting from acquisitions partially offset by the
effects of sold or closed clinics (1%). The administration of
EPO represented approximately 20% and 21% of total North America
dialysis care revenue for 2008 and 2007, respectively.
The product revenue increase was driven mostly by a higher sales
volume of dialysis machines, concentrate, bloodlines, dialyzers,
and peritoneal products, as well as increased pricing and sales
of the newly licensed intravenous iron products and higher
revenue attributable to the phosphate binding drug,
PhosLo
®
,
which we
56
acquired in late 2006. However, we experienced substantial
reductions in our
PhosLo
®
sales following a competitor’s launch of a generic version
of
PhosLo
®
in the U.S. in October 2008.
Operating
Income
Operating income increased by 3% to $1,168 million for 2008
from $1,130 million for 2007. Operating income margin
decreased to 16.7% for 2008 as compared to 17.0% for 2007
primarily due to increased personnel and other operating costs,
higher raw material costs, decreased utilization of and reduced
reimbursement rates for EPO, heparin cost increases, and higher
depreciation expense due to expansion of production capacities,
partially offset by increased commercial payor revenue. Cost per
treatment increased to $273 in 2008 from $267 in 2007.
International
Segment
Key
Indicators for International Segment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in %
|
|
|
|
|
|
|
|
|
|
|
|
|
|
at constant
|
|
|
|
|
2008
|
|
|
2007
|
|
|
as reported
|
|
|
exchange rates
|
|
|
|
|
Number of treatments
|
|
|
8,720,489
|
|
|
|
7,991,040
|
|
|
|
9
|
%
|
|
|
|
|
|
Same market treatment growth in %
|
|
|
8.6
|
%
|
|
|
6.2
|
%
|
|
|
|
|
|
|
|
|
|
Revenue in $ million
|
|
|
3,606
|
|
|
|
3,057
|
|
|
|
18
|
%
|
|
|
13
|
%
|
|
Depreciation and amortization in $ million
|
|
|
171
|
|
|
|
141
|
|
|
|
21
|
%
|
|
|
|
|
|
Operating income in $ million
|
|
|
616
|
|
|
|
544
|
|
|
|
13
|
%
|
|
|
|
|
|
Operating income margin in %
|
|
|
17.1
|
%
|
|
|
17.8
|
%
|
|
|
|
|
|
|
|
|
Revenue
Treatments increased by 9% in 2008 over 2007 mainly due to same
market growth (9%), and acquisitions (1%), partially offset by
sold or closed clinics (1%). As of December 31, 2008,
58,229 patients (a 11% increase over the prior year) were
being treated at 702 clinics that we own, operate or manage in
the International segment compared to 52,432 patients
treated at 636 clinics at December 31, 2007. Average
revenue per treatment increased to $171 from $152 due to
increased reimbursement rates and changes in country mix ($11)
and the strengthening of local currencies against the
U.S. dollar ($8).
The increase in net revenues for the International segment for
2008 over 2007 resulted from increases in both dialysis care and
dialysis product revenues. Organic growth during the period was
12% and acquisitions contributed approximately 1%. Exchange rate
fluctuations contributed 5%.
Including the effects of acquisitions, European region revenue
increased 19% (12% at constant exchange rates), Latin America
region revenue increased 23% (19% at constant exchange rates),
and Asia Pacific region revenue increased 12% (11% at constant
exchange rates).
Total dialysis care revenue for the International segment
increased during 2008 by 23% (18% at constant exchange rates) to
$1,490 million from $1,211 million for 2007. This
increase is a result of same market treatment growth of 9% and a
1% increase in contributions from acquisitions and one
additional dialysis day (1%), partially offset by sold or closed
clinics (1%). Increases in revenue per treatment contributed 8%
and exchange rate fluctuations contributed approximately 5%.
Total dialysis product revenue for 2008 increased by 15% (10% at
constant exchange rates) to $2,117 million mostly due to
higher dialyzer and machine sales.
Operating
Income
Operating income increased by 13% to $616 million primarily
as a result of increases in the number of treatments, revenue
per treatment and increases in units of products sold. Operating
income margin decreased to 17.1% for the year ended
December 31, 2008 from 17.8% in 2007. The margin decrease
resulted from the effects of stronger growth in dialysis care
business which has lower than average margins and higher
depreciation expense due to expansion of production capacities.
The availability of these new capacities allowed a more
normalized summer maintenance program in our facilities in 2008,
in contrast to the prior year’s shortened program. In
addition, the International margin was impacted by unfavorable
foreign currency transaction effects in Asia Pacific related to
purchase of products from Europe due to the appreciation of the
Euro against local currencies.
57
B. Liquidity
and Capital Resources
Our primary sources of liquidity have historically been cash
from operations, cash from borrowings from third parties and
related parties, as well as cash from issuance of equity and
debt securities. We require this capital primarily to finance
working capital needs, to fund acquisitions and develop
free-standing renal dialysis centers, to purchase equipment for
existing or new renal dialysis centers and production sites, to
repay debt and to pay dividends.
At December 31, 2009, we had cash and cash equivalents of
$301 million and unused sources of credit of
$1,051 million available to us which are discussed in more
detail below.
Operations
In the past three years, we have generated cash flows from
operations of $1,339 million, $1,016 million and
$1,200 million, respectively. Cash from operations is
impacted by the profitability of our business, the development
of our working capital, principally receivables, and cash
outflows that occur due to a number of singular specific items
(especially payments in relation to disallowed tax deductions
and legal proceedings). The increase in 2009 versus 2008 was
mainly a result of favorable days sales outstanding
(“DSO”) development in North America and increased
earnings partially offset by higher income tax payments in 2009
while 2008 had been favorably impacted by a $37 million tax
refund in the U.S. as a result of the settlement agreement
with the IRS to resolve our appeal of the IRS’ disallowance
of deductions for the civil settlement payments made to qui tam
relators in connection with the resolution of the 2000 OIG
investigation.
The profitability of our business depends significantly on
reimbursement rates. Approximately 74% of our revenues are
generated by providing dialysis treatment, a major portion of
which is reimbursed by either public health care organizations
or private insurers. For 2009, approximately 33% of our
consolidated revenues were attributable to U.S. federal
health care benefit programs, such as Medicare and Medicaid
reimbursement. Legislative changes could affect Medicare
reimbursement rates for all the services we provide, as well as
the scope of Medicare coverage. A decrease in reimbursement
rates or the scope of coverage could have a material adverse
effect on our business, financial condition and results of
operations and thus on our capacity to generate cash flow. In
the past we experienced and also expect in the future generally
stable reimbursements for our dialysis services. This includes
the balancing of unfavorable reimbursement changes in certain
countries with favorable changes in other countries. See
“Overview” above for a discussion of recent Medicare
reimbursement rate changes including provisions for
implementation of a “bundled rate” commencing
January 1, 2011.
Our working capital was $2,118 million at December 31,
2009 which increased from $1,068 million at
December 31, 2008, mainly as a result of decreases in
short-term debt mostly as a result of the repayment of
short-term Euro Notes in the third quarter of 2009 with the
proceeds from the issuance of new long-term debt in the second
quarter of 2009; increases in prepaid expenses, inventories,
accounts receivables from related parties and cash, and a
decrease in the accounts receivable facility. Our ratio of
current assets to current liabilities was 1.8.
Cash from operations depends on the collection of accounts
receivable. Customers and governments generally have different
payment cycles. A lengthening of their payment cycles could have
a material adverse effect on our capacity to generate cash flow.
In addition, we could face difficulties in enforcing and
collecting accounts receivable under some countries’ legal
systems. Accounts receivable balances at December 31, 2009
and December 31, 2008, net of valuation allowances,
represented approximately 72 and 77 of DSO, respectively.
The development of DSO by operating segment is shown in the
table below:
Development
of Days Sales Outstanding
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
North America days sales outstanding
|
|
|
52
|
|
|
|
60
|
|
|
|
|
|
|
|
|
|
|
|
|
International days sales outstanding
|
|
|
110
|
|
|
|
107
|
|
|
|
|
|
|
|
|
|
|
|
|
FMC AG & Co. KGaA average days sales outstanding
|
|
|
72
|
|
|
|
77
|
|
|
|
|
|
|
|
|
|
|
|
The decrease in DSO in the North America segment is mainly a
result of prior changes made to our management and structure of
the billing groups as well as the continued work flow and
process improvements to drive cash collections. The increase in
DSO for the International segment mainly reflects slight average
payment delays by government and private entities most recently
impacted by the worldwide financial crises. Due to the fact
58
that a large portion of our reimbursement is provided by public
health care organizations and private insurers, we expect that
most of our accounts receivables will be collectable, albeit
potentially slightly more slowly in the International segment in
the immediate future, particularly in countries most severely
affected by the current global financial crisis. Interest and
income tax payments also have a significant impact on our cash
from operations.
There are a number of tax and other items we have identified
that will or could impact our cash flows from operations in the
immediate future as follows:
We have filed claims for refunds contesting the Internal Revenue
Service’s (“IRS”) disallowance of FMCH’s
civil settlement payment deductions taken by Fresenius Medical
Care Holdings, Inc. (“FMCH”) in prior year tax
returns. As a result of a settlement agreement with the IRS to
resolve our appeal of the IRS’s disallowance of deductions
for the civil settlement payments made to qui tam relators in
connection with the resolution of the 2000 U.S. government
investigation, we received a refund in September 2008 of
$37 million, inclusive of interest. The settlement
preserves our right to continue to pursue claims in the
U.S. Federal courts for refunds of all other disallowed
deductions.
For the tax year 1997, we recognized an impairment of one of our
subsidiaries which the German tax authorities disallowed in 2003
at the conclusion of its audit for the years 1996 and 1997. We
have filed a complaint with the appropriate German court to
challenge the tax authority’s decision. As a result of a
change in judgment based on new information which became
available in the second quarter of 2009 we have increased our
recognition of the tax benefit related to this claim by
$14.6 million (€10.4 million).
The IRS tax audits of FMCH for the years 2002 through 2006 have
been completed. The IRS has disallowed all deductions taken
during these audit periods related to intercompany mandatorily
redeemable preferred shares. We have protested the disallowed
deductions and will avail ourselves of all remedies. An adverse
determination with respect to the disallowed deductions related
to intercompany mandatorily redeemable preferred shares could
have a material adverse effect on our results of operations and
liquidity. In addition, the IRS proposed other adjustments which
have been recognized in the financial statements.
We are subject to ongoing tax audits in the U.S., Germany and
other jurisdictions. We have received notices of unfavorable
adjustments and disallowances in connection with certain of the
audits, including those described above. We are contesting,
including appealing, certain of these unfavorable
determinations. If our objections and any final audit appeals
are unsuccessful, we could be required to make additional tax
payments, including payments to state tax authorities reflecting
the adjustments made in our federal tax returns in the
U.S. With respect to other potential adjustments and
disallowances of tax matters currently under review or where
tentative agreement has been reached, we do not anticipate that
an unfavorable ruling could have a material impact on our
results of operations. We are not currently able to determine
the timing of these potential additional tax payments.
W.R. Grace & Co. and certain of its subsidiaries filed
for reorganization under Chapter 11 of the
U.S. Bankruptcy Code (the “Grace Chapter 11
Proceedings”) on April 2, 2001. The settlement
agreement with the asbestos creditors committees on behalf of
the W.R. Grace & Co. bankruptcy estate (see
Note 18 of the Notes to Consolidated Financial Statements,
“Legal Proceedings”) provides for payment by the
Company of $115 million upon approval of the settlement
agreement by the U.S. District Court, which has occurred,
and confirmation of a W.R. Grace & Co. bankruptcy
reorganization plan that includes the settlement. The
$115 million obligation was included in the special charge
we recorded in 2001 to address 1996 merger-related legal
matters. The payment obligation is not interest-bearing.
If all potential additional tax payments and the Grace
Chapter 11 Proceedings settlement payment were to occur
contemporaneously, there could be a material adverse impact on
our operating cash flow in the relevant reporting period.
Nonetheless, we anticipate that cash from operations and, if
required, our senior credit agreement and other sources of
liquidity will be sufficient to satisfy all such obligations if
and when they come due.
Investing
We used net cash of $698 million, $891 million and
$777 million in investing activities in 2009, 2008 and
2007, respectively.
Capital expenditures for property, plant and equipment, net of
disposals were $562 million in 2009, $673 in 2008 and $543
in 2007. In 2009, capital expenditures were $295 million in
the North America segment and $267 million for the
International segment. Capital expenditures in 2008 were
$384 million in the North America segment and
$289 million for the International segment. In 2007,
capital expenditures were $314 in the North America Segment and
$229 for the International Segment. The majority of our capital
expenditures was used for maintaining existing clinics,
equipping new clinics, and maintenance and expansion of
production facilities
59
primarily in North America, Germany, France, Japan and China and
capitalization of machines provided to our customers, primarily
in the International segment. Capital expenditures were
approximately 5%, 6% and 6% of total revenue for 2009, 2008 and
2007, respectively.
We invested approximately $188 million cash in 2009,
primarily for acquisitions of dialysis clinics and recently
acquired pharmaceutical licenses, ($124 million in the
North America segment, $64 million in the International
segment) as compared to $227 million cash in 2008
($113 million in the North America segment,
$57 million in the International segment and
$57 million at Corporate) and $143 million in 2007
($63 million in the North America segment and
$80 million in the International segment). In addition, in
2007 we paid approximately $120 million in conjunction with
the Renal Solutions acquisition. We also received
$2 million, $59 million and $29 million in
conjunction with divestitures in 2009, 2008 and 2007,
respectively.
In 2008, we granted a loan of $50 million to Fresenius SE,
our parent, which was repaid on April 30, 2009. See
Item 7, “Major Shareholders and Related Party
Transactions-Related party transactions-Financing.”
We anticipate capital expenditures of approximately $550 to
$650 million and expect to make acquisitions of up to
$400 million in 2010. See “Outlook” below.
Financing
Net cash used in financing was $558 million in 2009
compared to $156 million in 2008 and $341 million in
2007.
In 2009, cash was mainly used for the repayment of the current
portion of long-term debt including the Euro Notes in the amount
of $279 million (€200 million) that were due and
repaid on July 27, 2009, reducing the amount outstanding
under our accounts receivable securitization program, and the
payment of dividends partially offset by the issuance of
long-term debt and borrowings under other existing long-term
debt facilities. In 2008, cash was mainly used for redemption of
trust preferred securities ($678 million), the payment of
dividends ($252 million) and the payment in November 2008
of the remaining financial liability relating to the 2007 RSI
Acquisition ($56 million); we raised cash from our accounts
receivable securitization facility (“A/R Facility”)
and other existing long-term credit facilities. In 2007, cash
was mainly used to pay down our A/R Facility and other debt and
for payment of dividends; we raised net proceeds of
$484 million from the issuance of our
6
7
/
8
% Senior
Notes due 2017
(“6
7
/
8
% Senior
Notes”).
The following table summarizes the Company’s available
sources of liquidity at December 31, 2009:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Available Sources of Liquidity
|
|
|
|
|
Expiration per period of
|
|
|
in millions
|
|
Total
|
|
|
1 Year
|
|
|
2-5 Years
|
|
|
|
|
Accounts receivable
facility
(a)
|
|
$
|
436
|
|
|
$
|
436
|
|
|
$
|
—
|
|
|
2006 Senior Credit Agreement
|
|
|
308
|
|
|
|
—
|
|
|
|
308
|
|
|
Other Unused long-term Lines of Credit
|
|
|
98
|
|
|
|
—
|
|
|
|
98
|
|
|
Other Unused short-term Lines of Credit
|
|
|
209
|
|
|
|
209
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
1,051
|
|
|
$
|
645
|
|
|
$
|
406
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a)
|
Subject to availability of sufficient accounts receivable
meeting funding criteria.
|
The amount of guarantees and other commercial commitments at
December 31, 2009 is not significant.
At December 31, 2009, we have short-term borrowings,
excluding the current portion of long-term debt, of
$310 million.
60
The following table summarizes, as of December 31, 2009,
our obligations and commitments to make future payments under
our long-term debt, trust preferred securities and other
long-term obligations, and our commitments and obligations under
lines of credit and letters of credit.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contractual Obligations and Commitments
|
|
|
|
|
Payments due by period of
|
|
|
in millions
|
|
Total
|
|
|
1 Year
|
|
|
2-5 Years
|
|
|
Over 5 Years
|
|
|
|
|
Trust Preferred
Securities
(a)
|
|
$
|
732
|
|
|
$
|
50
|
|
|
$
|
682
|
|
|
$
|
—
|
|
|
Long Term
Debt
(b)(c)
|
|
|
5,218
|
|
|
|
344
|
|
|
|
4,261
|
|
|
|
613
|
|
|
Capital Lease Obligations
|
|
|
20
|
|
|
|
5
|
|
|
|
10
|
|
|
|
5
|
|
|
Operating Leases
|
|
|
2,551
|
|
|
|
455
|
|
|
|
1,294
|
|
|
|
802
|
|
|
Unconditional Purchase Obligations
|
|
|
2,414
|
|
|
|
408
|
|
|
|
1,044
|
|
|
|
962
|
|
|
Other Long-term Obligations
|
|
|
48
|
|
|
|
35
|
|
|
|
12
|
|
|
|
1
|
|
|
Letters of Credit
|
|
|
97
|
|
|
|
97
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
11,080
|
|
|
$
|
1,394
|
|
|
$
|
7,303
|
|
|
$
|
2,383
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a)
|
Interest payments are determined on these debt instruments until
their respective maturity dates and based on their applicable
balances and fixed interest rates for each period presented.
|
|
|
|
(b)
|
Includes expected interest payments which are based upon the
principal repayment schedules and fixed interest rates or
estimated variable interest rates considering the applicable
interest rates (e.g. Libor, Prime), the applicable margins, and
the effects of related interest rate swaps.
|
|
|
|
(c)
|
Excludes our 5.50% Senior Notes due 2016 issued on
January 20, 2010 (“5.50% Senior Notes”).
|
Our obligations under the 2006 Senior Credit Agreement are
secured by pledges of capital stock of certain material
subsidiaries, including FMCH and D-GmbH, in favor of the
lenders. Our 2006 Senior Credit Agreement, EIB agreements, Euro
Notes,
6
7
/
8
% Senior
Notes, 5.50% Senior Notes and the indentures relating to
our trust preferred securities include covenants that require us
to maintain certain financial ratios or meet other financial
tests. Under our 2006 Senior Credit Agreement, we are obligated
to maintain a minimum consolidated fixed charge ratio (ratio of
consolidated EBITDAR (sum of EBITDA plus Rent expense under
operation leases) to Consolidated Fixed Charges as these terms
are defined in the 2006 Senior Credit Agreement) and a maximum
consolidated leverage ratio (ratio of consolidated funded debt
to consolidated EBITDA as these terms are defined in the 2006
Senior Credit Agreement). Other covenants in one or more of each
of these agreements restrict or have the effect of restricting
our ability to dispose of assets, incur debt, pay dividends and
make other restricted payments, create liens or engage in
sale-lease backs.
The breach of any of the covenants in any of the instruments or
agreements governing our long-term debt — the 2006
Senior Credit Agreement, the EIB agreements, the Euro Notes, the
6
7
/
8
% Senior
Notes, the 5.50% Senior Notes or the notes underlying our
trust preferred securities — could, in turn, create
additional defaults under one or more of the other instruments
or agreements. In default, the outstanding balance under the
Senior Credit Agreement becomes due at the option of the lenders
under that agreement, and the “cross default”
provisions in our other long-term debt permit the lenders to
accelerate the maturity of the debt upon such a default as well.
As of December 31, 2009, we are in compliance with all
covenants under the 2006 Senior Credit Agreement and our other
financing agreements. For information regarding our 2006 Senior
Credit Agreement, EIB agreements, Euro Notes,
6
7
/
8
% Senior
Notes, and the indentures relating to our trust preferred
securities, see Note 10 and Note 12 of the Notes to
Consolidated Financial Statements, “Long-Term Debt and
Capital Lease Obligations” and “Mandatorily Redeemable
Trust Preferred Securities.” For information regarding
our 5.50% Senior Notes issued in January 2010, see Note 2
of the Notes to Consolidated Financial Statements,
“Subsequent Event.”
Although we are not immune from the current worldwide financial
crises, we believe that we are well positioned to continue to
grow our business while meeting our financial obligations as
they come due. Our business is generally not cyclical. A
substantial portion of our accounts receivable are generated by
governmental payers. While payment and collection practices vary
significantly between countries and even between agencies within
one country, government payors usually represent low credit
risks. Our syndicated credit facility is comprised of
approximately 60 lenders for the revolving credit facility, none
of which contribute more than 5.5% of our revolving facility
under the 2006 Senior Credit Agreement. Although one of the 60
participating banks in this syndicated facility defaulted on its
obligation to provide funds under the terms of the revolving
facility during the fourth quarter 2008, we do not anticipate
any major issues in having funds available for us when we
utilize this credit facility. As we deemed the amount in default
immaterial, we took no action to amend our 2006 Senior Credit
Agreement to replace the defaulting bank. However, limited or
expensive access to capital could make it more difficult for our
customers to do business with us, or to do business generally,
which could adversely affect our business by causing
61
our customers to reduce or delay their purchases of our dialysis
products. See “Results of Operations” above. If the
current conditions in the credit and equity markets continue, or
worsen, they could also increase our financing costs and limit
our financial flexibility.
Following our earnings-driven dividend policy, our General
Partner’s Management Board will propose to the shareholders
at the Annual General meeting on May 11, 2010, a dividend
with respect to 2009 and payable in 2010, of €0.61 per
ordinary share (for 2008 paid in 2009: €0.58) and
€0.63 per preference share (for 2008 paid in 2009:
€0.60). The total expected dividend payment is
approximately €183 million (approximately
$263 million based upon the December 31, 2009 spot
rate) compared to dividends of €173 million
($232 million) paid in 2009 with respect to 2008. Our 2006
Senior Credit Agreement limits disbursements for dividends and
other payments for the acquisition of our equity securities (and
rights to acquire them, such as options or warrants) during 2010
to $300 million in total.
We will focus our financing activities in the coming years on
reducing subordinated debt. In this respect we did not refinance
the subordinated trust-preferred securities issued by Fresenius
Medical Care Capital Trust II and III which matured in
February 2008 by issuing new subordinated debt, but used our
existing senior credit facilities instead. Our target for
maturing long-term debt is to refinance through more senior and
unsecured debt instruments. We have sufficient financial
resources — consisting of only partly drawn credit
facilities and our accounts receivable facility —
which we intend to preserve in the next years. We aim to keep
committed and unutilized credit facilities to a minimum of $300
to $500 million.
On April 27, 2009, the Company issued euro denominated
notes (“Euro Notes”) totaling €200 million
which are senior, unsecured obligations and guaranteed by FMCH
and D-GmbH, consisting of 4 tranches having terms of 3.5 and
5.5 years with floating and fixed interest rate tranches.
The initial average interest rate was 6.95%. Proceeds of
€69.5 million of the newly issued Euro Notes were used
in April 2009 to voluntarily retire a portion of the Euro Notes
that were due in July 2009 with the remaining proceeds used to
repay the balance of the notes on their scheduled maturity date
of July 27, 2009. Our immediate refinancing need for 2010
is limited to the annual renewal of our accounts receivable
facility which, in November 2009, was increased from
$550 million to $650 million and extended to
October 15, 2010. On January 20, 2010, our wholly
owned subsidiary, FMC Finance VI S.A., issued
€250 million ($353.3 million at date of issuance)
of 5.50% Senior Notes at an issue price of 98.6636%. The
5.50% Senior Notes have a yield to maturity of 5.75% and
are due July 15, 2016. Proceeds were used to repay
short-term indebtedness and for general corporate purposes. The
5.50% Senior Notes are guaranteed on a senior basis jointly
and severally by us, Fresenius Medical Care Holdings, Inc. and
Fresenius Medical Care Deutschland GmbH. For additional
information regarding our 5.50% Senior Notes, see
Note 2 of the Notes to Consolidated Financial Statements,
“Subsequent Event.”
In addition to the annual renewal of our accounts receivable
facility described above, our 2010 financing needs are limited
to the dividend payment of approximately $263 million in
May 2010 which is expected to be covered by cash flow from
operations and from existing credit facilities. The Company
plans to refinance the amounts due in 2011 for Term Loan A and
the Revolving Credit facility under the 2006 Senior Credit
Agreement (See Note 10 of the Notes to Consolidated
Financial Statements, “Long-term Debt and Capital Lease
Obligations — 2006 Senior Credit Agreement”) and
the amounts due under the Trust Preferred Securities (See
Note 12 of the Notes to Consolidated Financial Statements,
“Mandatorily Redeemable Trust Preferred
Securities”), in all totaling approximately
$2,624 million at December 31, 2009, by renewing the
credit agreement or by entering into diverse capital market
transactions. We also expect to pay the anticipated dividend
payment in 2011 from our cash flows and by using credit
facilities existing at the time the dividends are paid. We
currently have sufficient flexibility under our debt covenants
to meet our financing needs in the near future. Generally, we
believe that we will have sufficient financing to achieve our
goals in the future and to continue to promote our growth.
The rating agencies identified in the table below assign credit
ratings to us based on their assessments of our financing
strategy, resources and financial performance. Our cost of
borrowing is indirectly influenced by these ratings. The table
below shows the ratings as of December 31, 2009:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Standard &
|
|
|
|
|
|
|
|
Poor’s
|
|
Moody’s
|
|
Fitch
|
|
|
|
Corporate Credit Rating
|
|
|
BB
|
|
|
|
Ba1
|
|
|
|
BB
|
|
|
Outlook
|
|
|
stable
|
|
|
|
stable
|
|
|
|
stable
|
|
62
Outlook
Below is a table showing our growth outlook for 2010 and 2011:
|
|
|
|
|
|
|
|
|
2010
|
|
2011
|
|
|
|
($ in millions)
|
|
($ in millions)
|
|
|
|
Net Revenues
|
|
> $12,000
|
|
|
|
Revenue growth
|
|
7-9%
|
|
5-8%
|
|
Net Income attributable to FMC-AG & Co. KGaA
|
|
$950-$980
|
|
|
|
Increase in Net Income attributable to FMC-AG & Co.
KGaA
|
|
7-10%
|
|
> revenue growth
|
|
Dividend
|
|
based on
|
|
based on
|
|
|
|
development of
|
|
development of
|
|
|
|
earnings
|
|
earnings
|
|
Capital Expenditures
|
|
$550-$650
|
|
|
|
Acquisitions
|
|
up to $400
|
|
|
|
Total Capital Expenditures and Acquisitions in % of Revenue
|
|
8-9%
|
|
7-9%
|
|
Tax Rate
|
|
34.5-35.5%
|
|
34.5-35.5%
|
|
Debt/EBITDA
|
|
< 2.5x
|
|
< 2.5x
|
Debt
covenant disclosure — EBITDA
EBITDA (earnings before interest, tax, depreciation and
amortization expenses) was approximately $2,213 million,
19.7% of revenues for 2009, $2,088 million, 19.7% of
revenues for 2008 and $1,944 million, 20.0% of revenues for
2007. EBITDA is the basis for determining compliance with
certain covenants contained in our 2006 Senior Credit Agreement,
Euro Notes, EIB, and the indentures relating to our
6
7
/
8
% Senior
Notes, our 5.50% Senior Notes and our outstanding trust
preferred securities (See “Financing” above). You
should not consider EBITDA to be an alternative to net earnings
determined in accordance with U.S. GAAP or to cash flow
from operations, investing activities or financing activities.
In addition, not all funds depicted by EBITDA are available for
management’s discretionary use. For example, a substantial
portion of such funds are subject to contractual restrictions
and functional requirements for debt service, to fund necessary
capital expenditures and to meet other commitments from time to
time as described in more detail elsewhere in this report.
EBITDA, as calculated, may not be comparable to similarly titled
measures reported by other companies. A reconciliation of EBITDA
to cash flow provided by operating activities, which we believe
to be the most directly comparable U.S. GAAP financial
measure, is calculated as follows:
Reconciliation
of measures for consolidated totals.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the years ended December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
(in thousands)
|
|
|
|
|
Total EBITDA
|
|
$
|
2,212,681
|
|
|
$
|
2,088,103
|
|
|
$
|
1,943,451
|
|
|
Interest expense (net of interest income)
|
|
|
(299,963
|
)
|
|
|
(336,742
|
)
|
|
|
(371,047
|
)
|
|
Income tax expense, net
|
|
|
(490,413
|
)
|
|
|
(475,702
|
)
|
|
|
(453,765
|
)
|
|
Change in deferred taxes, net
|
|
|
22,002
|
|
|
|
133,047
|
|
|
|
1,177
|
|
|
Changes in operating assets and liabilities
|
|
|
(139,494
|
)
|
|
|
(403,123
|
)
|
|
|
51,933
|
|
|
Compensation expense
|
|
|
33,746
|
|
|
|
31,879
|
|
|
|
24,208
|
|
|
Other items, net
|
|
|
58
|
|
|
|
(21,064
|
)
|
|
|
3,617
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by operating activities
|
|
$
|
1,338,617
|
|
|
$
|
1,016,398
|
|
|
$
|
1,199,574
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Recently
Issued Accounting Standards
For a discussion of recently issued accounting standards, see
Note 1 of the Notes to Consolidated Financial Statements
“The Company, Basis of Presentation and Summary of
Significant Accounting Policies — Summary of
Significant Accounting Policies — u) Recent
Pronouncements”.
C. Research
and Development
As a leading global dialysis company, we focus our research and
development (“R&D”) strategy on three essential
objectives: first, to continuously enhance the quality of life
of patients with chronic kidney disease using
63
innovative products and treatment concepts; second, to offer our
customers high-quality services while keeping our prices as low
as possible; and third, based on these, to continue to expand
our position as the dialysis market leader. Due to our vertical
integration, our research and development department can apply
our experience as the world’s largest provider of dialysis
treatments to product development, and our technical department
benefits from our daily practical experience as a provider of
dialysis treatment and being directly in-touch with doctors,
nurses and patients to keep track of and meet customer and
patient needs. In addition, our research and development units
are usually located at production sites, enabling direct
exchange of ideas with our production staff. We conduct annual
internal R&D conferences which our employees attend every
year. In addition, our employees visit research events worldwide
and participate actively in scientific discourse. This not only
enables them to inject new concepts into their work, but also
strengthens our reputation in the international professional
community. We also maintain close contacts with universities and
research institutions. We are cooperating closely with the
University of Michigan (on a longitudinal study of chronic
kidney patients), Danube University Krems in Krems, Austria (on
extracorporeal methods), and the Renal Research Institute (RRI)
in the United States. RRI was founded in 1997 as a joint venture
between Fresenius Medical Care North America and the Beth Israel
Medical Center, a hospital in New York. The goal of RRI is to
improve the treatment and care of kidney patients by exploring
new technologies. We are currently constructing a new research
laboratory in Krems specializing in sorbent technology that is
set to open in 2010.
The task of our research and development group, which employs
approximately 477 full time equivalents, is to continually
develop and improve our products and treatments. Our largest
research and development department is R&D International
with 303 employees, most of whom work at our Schweinfurt
and Bad Homburg locations. Smaller teams also work in St. Wendel
and in Romania, where an R&D competency center specializing
in software development has been established. Apart from
R&D International, we have research and development
departments in North America and in the Asia Pacific regions.
All of these units are closely connected and cooperate on many
projects.
In 2009, research and development expenditures amounted to
$94 million (equivalent to 3.2% of our total dialysis
product sales), compared to $80 million in 2008 and
$67 million in 2007. Our 2009 expenditures focused on
continuously enhancing and improving our products and treatment
concepts for our patients and users and less on inventing new
products entailing high investments, resulting in expenditures
that are well within the range typically observed in the
dialysis industry. In 2007, we acquired Renal Solutions, Inc.
(RSI), which is continuing to do intensive research in the field
of sorbent-based technology, helping to create a potential
platform for the eventual development of a wearable artificial
kidney. In addition, we expanded our activities in key areas of
strategic development of hemodialysis machines, field testing of
new products and extracorporeal and home therapy programs. A
discussion of each of these activities follows below.
ONLINE
HDF and MIXED HDF
In 2009, we expanded our activities in the key areas of
strategic development, the field of ONLINE hemodiafiltration
(“HDF”) and the 5008 therapy system based upon it. In
May 2009, we presented another innovation built upon this
development platform at the industry conference ERA-EDTA in
Milan: MIXED HDF. In May 2009, we presented another
innovation built upon this development platform at the industry
conference ERA-EDTA in Milan: MIXED HDF. Due to an innovative
new control technology, MIXED HDF is a flexible way to infuse
volume replacement fluid, called infusate or substitution fluid,
simultaneously pre and post dialyzer in a manner which allows
for the most efficient solute removal process from the blood of
an individual patient, allowing the treatment to be tailored
even more precisely to the medical needs of each patient. This
means that even more patients can benefit from the advantages of
the 5008 therapy system, with excellent treatment results and a
better life expectancy. We are the first company to have MIXED
HDF ready for market launch. The technology is the result of
many years of work by a team comprised of dialysis, software and
membrane specialists as well as experts in the field of
nephrology. We believe this innovation will further contribute
to establishing HDF as a standard dialysis treatment. The ONLINE
HDF mode of our 5008 therapy system also saves on resources
since the device uses up to 30% less energy, water and
concentrate than traditional hemodialysis methods during
treatment.
HDF technology combines two difference methods for treating
chronic kidney disease- hemodialysis and hemofiltration.
Therefore, it can be used to effectively clean patients’
blood and boost their life expectancy, as have become
increasingly apparent in scientific studies. In HDF, the
dialysis machine automatically produces the infusion solution
for treatment. This solution must have the right medical
composition and be free of all chemical and microbiological
contamination (e.g. microorganisms and their metabolic waste
products, or fragments of dead microorganisms). The ONLINE
method is a safe, user-friendly, resource-saving and
cost-efficient alternative to ready-made infusion solutions in
bags. Due to its considerable potential for the medical world,
we see HDF as a long-term innovation platform.
64
Hemodialysis
Machines
In 2009, we improved and continued to develop our traditional HD
products. At the ERA-EDTA congress in 2009, we introduced our
4008S classic HD machine for our International segment. This
device offers exceptional treatment quality along with high
reliability and safety at an affordable price due to its
high-quality basic configuration. The cost effectiveness and
simple operability should result in access to high-quality
dialysis treatment for even more dialysis patients, particularly
in areas with poor infrastructures.
In the U.S. in 2009, our 2008T HD machine, a new product
generation of the 2008 series that we sell in the U.S., gained
FDA approval. The 2008T is the first HD machine on the American
market to use an integrated computer system to automatically
compile clinical treatment data. Data compilation will be
necessary to establish satisfaction of the treatment quality
critical under the “bundled” reimbursement system to
be implemented in 2011 (See Item 5, “Operating and
Financial Review and Prospects — Overview”), and
the 2008T offers a distinct advantage in meeting these
requirements. The 2008T is flexible, as it can connect to
various data management systems used in dialysis clinics across
the U.S. The 2008T can also be used with citrate based
dialysate, which utilizes the natural substance citric acid as a
partial replacement for heparin to inhibit blood coagulation in
many dialysis patients more effectively, gently and at a lower
cost. We presented the 2008T at the American Society of
Nephrology Conference in 2009 and intend to launch the device in
2010.
Home
Dialysis and Sorbent Systems
We continued to develop our portfolio in the area of home
dialysis in 2009, another of our strategic development
platforms. In 2008, we introduced the Liberty Cycler, our
therapy system for peritoneal dialysis, in the U.S. It was
a resounding success, with other 2,500 patients now being
treated with the device. Our strategy is to make this system a
core product in North America. To achieve this, we have
continued to improve the Liberty Cycler since it was introduced,
including adding an expanded alarm system to help users avoid
application errors. The ongoing development of the device’s
software means that patients’ individual treatment settings
and results can be processed even more comprehensively and
transmitted to the attending clinic. There, the data is
regularly checked to adapt the treatment to individual patients
in the best possible way.
As a home dialysis treatment method, PD requires a high level of
individual responsibility from patients as they usually carry it
out themselves. Therefore, it is crucial that these patients
receive intensive training on hygiene and safety matters. We
believe that, with its intuitive user interface and
easy-to-understand
instructions, which guide patients through the device settings
step-by-step
via a screen, the Liberty Cycler is one of the simplest and
safest devices in this respect. To further increase the
user-friendliness of the cycler, we are currently working on a
new help software which uses short instructional videos and text
information to demonstrate how the device should be used. It
will also allow patients to receive prompt answers to their
questions via a help menu, even during treatment. We intend to
make this new feature standard for the device in 2011.
In HD, a dialyzer outside of the body filters the blood.
Traditionally, 120 liters of water are needed for each
treatment. For this reason, among others, PD is presently the
home therapy of choice. However, we are researching ways of
reducing water consumption per treatment, which would enable
widespread use of HD as a therapy outside of dialysis clinics.
In 2009, RRI continued research in the field of sorbent systems.
This field focuses on sorbents, substances that bind toxins in
liquids so that they can be removed. These sorbents can be used
to recycle dialysis solution, which absorbs toxins during HD or
PD treatment that have been filtered out of the patients’
blood. By cleansing and then recycling the dialysate with the
help of sorbents, the amount of water typically needed during
dialysis treatment can be reduced from 120 to 200 liters to
approximately six to ten liters. This innovative sorbent
technology is particularly important for our “wearable
kidney” project, as a device of this kind must be able to
function with a substantially smaller amount of liquid to be
light and small enough to be worn on the body.
Body
Composition Monitor (BCM)
Another development focus is the Body Composition Monitor, which
we originally introduced in select markets in 2008 and
successfully launched in additional markets in 2009. The BCM can
determine the exact
make-up
of
the human body and its fluids (body water, fat and
fat-free
body mass). This provides doctors with information on the
patients’ general health, such as the constitution of their
blood vessels, and helps them to determine to what extent a
patient may be suffering from overhydration. Such information
can substantially improve the treatment quality of dialysis, as
both heart and vascular diseases and overhydration are common
side effects of chronic kidney disease and have been shown to
reduce the life expectancy of HD patients considerably. We are
currently working on making the advantages of BCM technology,
which up to the point have only been documented in the treatment
of HD patients, available to other patient groups. This will
enable us to tap into new markets for the BCM. Initial studies
have shown that PD patients can also benefit immensely from
professional
65
“fluid management,” a regular check of their fluid
status with the treatment adjusted accordingly. Another group of
patients whose treatment could be improved with the use of BCM
technology are people who suffer from acute kidney failure.
Outlook
We intend to continue investing in developing and improving
life-sustaining products and treatment concepts in the years to
come, thus improving the quality of life for as many patients as
possible with financially viable, environmentally-friendly
innovations based on strategic technology platforms. We plan to
spend approximately $95 million and $105 million on
research and development in 2010 and 2011, respectively. Two of
the topics we intend to focus on in 2010 are IT solutions for
managing clinic and therapy data and products that increase the
safety of dialysis treatment. In the U.S., we intend to
concentrate on further improving our Liberty Cycler system and
enhancing our hemodialysis portfolio. Platform technologies like
our 5008 therapy system and ONLINE and MIXED HDF will continue
to play an important role in the future in developing and
improving our products.
In the coming years, we will place a greater overall focus on
designing products and processes that are more environmentally
compatible and cost effective. Cost efficiency combined with
high-quality products is becoming an increasingly important
factor in the healthcare industry, particularly in view of
health care reform efforts in countries like the U.S. In
addition, we will focus on the biochemical effects of uremia, a
toxic condition of ESRD patients resulting from the presence of
excessive amounts of urea in the blood. We will then use our
findings to develop particularly high-performance membranes
which, together with developments in sorbent technology, could
be used in a “wearable kidney”. In connection with the
wearable kidney, we are also working on further reducing the
size of dialysis machines and accessories to allow patients to
carry the device on their body in the long term. Over the long
term, we intend to conduct research in the transferability of
the blood-cleansing dialysis process to other illnesses, such as
liver disease or certain autoimmune and metabolic disorders.
For information regarding significant trends in our business see
Item 5.A, “Operating Financial Review and
Prospects.”
|
|
|
|
Item 6.
|
Directors,
Senior Management and Employees
|
|
|
|
|
A.
|
Directors
and senior management
|
General
As a partnership limited by shares, under the German Stock
Corporation Act (
Aktiengesetz
), our corporate bodies are
our general partner, our supervisory board and our general
meeting of shareholders. Our sole general partner is Fresenius
Medical Care Management AG (“Management AG”), a
wholly-owned
subsidiary of Fresenius SE. Management AG is required to devote
itself exclusively to the management of Fresenius Medical Care
AG & Co. KGaA.
For a detailed discussion of the legal and management structure
of Fresenius Medical Care AG & Co. KGaA, including the
more limited powers and functions of the supervisory board
compared to those of the general partner, see Item 16.G,
below, “Governance — The Legal Structure of
Fresenius Medical Care AG & Co. KGaA.”
The general partner has a Supervisory Board and a Management
Board. These two boards are separate and no individual may
simultaneously be a member of both boards. A person may,
however, serve on both the supervisory board of our general
partner and on our supervisory board.
The
General Partner’s Supervisory Board
The Supervisory Board of Management AG consists of six members
who are elected by Fresenius SE as the sole shareholder of
Management AG. Pursuant to pooling agreements for the benefit of
the public holders of our ordinary shares and the holders of our
preference shares, at least one-third (but no fewer than two) of
the members of the general partner’s Supervisory Board are
required to be independent directors as defined in the pooling
agreements, i.e., persons with no substantial business or
professional relationship with us, Fresenius SE, the general
partner, or any affiliate of any of them.
Unless resolved otherwise by the general meeting of
shareholders, the terms of each of the members of the
Supervisory Board of Management AG will expire at the end of the
general meeting of shareholders in which the shareholders
discharge the Supervisory Board for the fourth fiscal year
following the year in which the Management AG supervisory board
member was elected by Fresenius SE, but not counting the fiscal
year in which such
66
member’s term begins. Members of the general partner’s
Supervisory Board may be removed only by a resolution of
Fresenius SE in its capacity as sole shareholder of the general
partner. Neither our shareholders nor the separate supervisory
board of FMC AG & Co. KGaA has any influence on the
appointment of the Supervisory Board of the general partner.
The general partner’s Supervisory Board ordinarily acts by
simple majority vote and the Chairman has a tie-breaking vote in
case of any deadlock. The principal function of the general
partner’s Supervisory Board is to appoint and to supervise
the general partner’s Management Board in its management of
the Company, and to approve mid-term planning, dividend payments
and matters which are not in the ordinary course of business and
are of fundamental importance to us.
The table below provides the names of the members of the
Supervisory Board of Management AG and their ages as of
December 31, 2009.
|
|
|
|
|
|
|
|
|
Age as of
|
|
|
|
|
December 31,
|
|
|
Name
|
|
2009
|
|
|
|
|
Dr. Ulf M. Schneider,
Chairman
(1)
|
|
|
44
|
|
|
Dr. Dieter Schenk, Vice
Chairman
(5)
|
|
|
57
|
|
|
Dr. Gerd
Krick
(1)(2)
|
|
|
71
|
|
|
Dr. Walter L.
Weisman
(1)(2)(4)
|
|
|
74
|
|
|
Mr. John G.
Kringel
(3)(4)(5)
|
|
|
70
|
|
|
Mr. William P.
Johnston
(1)(2)(4)(5)
|
|
|
65
|
|
|
|
|
|
(1)
|
Members of the Human Resources Committee of the Supervisory
Board of Management AG
|
|
|
|
(2)
|
Members of the Audit and Corporate Governance Committee of
FMC-AG & Co. KGaA
|
|
|
|
(3)
|
Member of the Audit and Corporate Governance Committee of
FMC-AG & Co. KGaA until March 2009
|
|
|
|
(4)
|
Independent director for purposes of our pooling agreement
|
|
|
|
(5)
|
Member of the Regulatory and Reimbursement Assessment Committee
of the Supervisory Board of Management AG
|
DR. ULF M. SCHNEIDER has been Chairman of the Supervisory Board
of Management AG from April 15, 2005. He was a member of
the Fresenius Medical Care AG Supervisory Board from May 2004
and Chairman of its Supervisory Board until the effective date
of the transformation when he resigned upon the Company’s
transformation to a KGaA. He was Chief Financial Officer of
FMC-AG from November 2001 until May 2003. On March 7, 2003,
Dr. Schneider announced his resignation from the FMC-AG
Management Board to become Chairman of the Management Board of
Fresenius AG (now Fresenius SE), effective May 28, 2003.
Previously he was Group Finance Director for Gehe UK plc., a
pharmaceutical wholesale and retail distributor, in Coventry,
United Kingdom. He has held several senior executive and
financial positions since 1989 with Gehe’s majority
shareholder, Franz Haniel & Cie. GmbH, Duisburg, a
diversified German multinational company. Dr. Schneider is
Chairman of the Supervisory Board of Fresenius Kabi AG, HELIOS
Kliniken GmbH and Fresenius Medical Care Groupe France S.A.S.,
France. He was Chairman of the Supervisory Board of Eufets AG
until May 31, 2009. Dr. Schneider is member of the
Supervisory Boards of Fresenius Kabi Austria GmbH, Austria,
Fresenius Kabi Espana S.A., Spain and Fresenius HemoCare
Nederlands B.V., Netherlands. Dr. Schneider is Chairman of
the Board of Directors of APP Pharmaceuticals, Inc., USA and was
Chairman of the Board of Directors of Fresenius Kabi
Pharmaceuticals Holding, Inc., USA until November 1, 2009.
He remains a member of the Board of Directors of Fresenius Kabi
Pharmaceuticals Holding, Inc., USA and is a member of the Board
of Directors of FHC (Holdings), Ltd., Great Britain.
DR. DIETER SCHENK has been a member of the Supervisory Board of
Management AG since April 8, 2005 and Vice Chairman of the
Supervisory Board of Management AG since April 15, 2005 and
was Vice Chairman of the Supervisory Board of FMC-AG from 1996
until the transformation of legal form to a KGaA. He is also
Vice Chairman of the Supervisory Board of FMC-AG & Co.
KGaA. He is an attorney and tax advisor and has been a partner
in the law firm of Noerr LLP (formerly Nörr Stiefenhofer
Lutz) since 1986. Dr. Schenk is also Vice Chairman of the
Supervisory Board of Fresenius SE and Chairman of the Advisory
Board of Else-Kröner-Fresenius-Stiftung, which owns
approximately 58% of the ordinary shares of Fresenius SE. He
also serves as the Chairman of the Supervisory Board of Gabor
Shoes AG and TOPTICA Photonics AG and as a Vice-Chairman of the
Supervisory Board of Greiffenberger AG.In September 2008,
Dr. Schenk resigned from his position as Chairman of the
Supervisory Board of NSL Consulting AG.
DR. GERD KRICK has been a member of the Supervisory Board of
Management AG since December 28, 2005 and was Chairman of
the Supervisory Board of FMC-AG from January 1, 1998 until
the transformation of
67
legal form to a KGaA. He is also Chairman of the Supervisory
Boards of FMC-AG & Co. KGaA and Fresenius SE. He was
Chairman of the Fresenius AG Management Board from 1992 to May
2003 at which time he became chairman of its Supervisory Board.
Prior to 1992, he was a Director of the Medical Systems Division
of Fresenius AG and Vice-Chairman of the Fresenius AG Management
Board. From September 1996 until December 1997, Dr. Krick
was Chairman of the Management Board of FMC-AG. Dr. Krick
was a member of the Advisory Board of HDI Haftpflichtverband der
deutschen Industrie V.a.G until December 31, 2008. He is
also the Chairman of the Supervisory Board of VAMED AG, Austria
and was a member of the Supervisory Board of Allianz Private
Krankenversicherungs-AG until April 16, 2008.
MR. JOHN G. KRINGEL has been a member of the Supervisory Board
of Management AG since December 28, 2005 and was a member
of the Supervisory Board of FMC-AG from October 20, 2004,
when his appointment to fill a vacancy was approved by the local
court, until the transformation of legal form to a KGaA. His
election to the Supervisory Board was subsequently approved by
the shareholders of FMC-AG at the Annual General Meeting held
May 24, 2005. He is also a member of the Supervisory Board
of FMC-AG & Co. KGaA. He has the following other
mandates: Natures View, LLC, Alpenglow Development, LLC,
Justice, LLC, River Walk, LLC. Formerly he was also an Advisory
Board member of Visionary Medical Device Fund. Mr. Kringel
spent 18 years with Abbott Laboratories prior to his
retirement as Senior Vice President, Hospital Products, in 1998.
Prior to Abbott Laboratories, he spent three years as Executive
Vice President of American Optical Corporation, a subsidiary of
Warner Lambert Co. and ten years in the U.S. Medical Division of
Corning Glassworks.
DR. WALTER L. WEISMAN has been a member of the Supervisory Board
of Management AG since December 28, 2005 and was a member
of the Supervisory Board of FMC-AG from 1996 until the
transformation of legal form to a KGaA. He is also a member of
the Supervisory Board of FMC-AG & Co. KGaA. He is a
private investor and a former Chairman, President and Chief
Executive Officer of American Medical International, Inc., and
is a member of the Board of Directors of Occidental Petroleum
Corporation. He is Senior Trustee of the Board of Trustees for
the California Institute of Technology, life trustee of the
Board of Trustees of the Los Angeles County Museum of Art, and
Chairman of the Board of Trustees of the Sundance Institute.
Dr. Weisman was Vice-Chairman and Lead Director of Maguire
Properties, Inc. until September 1, 2008 and was
Vice-Chairman of the Board of Trustees of the Samuel H. Kress
Foundation until November 1, 2008.
MR. WILLIAM P. JOHNSTON was elected to the Supervisory Board of
Management AG on August 30, 2006. He has been a member of
the Supervisory Board of FMC-AG & Co. KGaA since May
2006. In February 2008, Mr. Johnston was appointed as a
member of the Board of Directors of HCR-Manor Care, Inc. He was
the former Chairman of the Board of Directors of Renal Care
Group, Inc. Mr. Johnston has been a Senior Advisor of The
Carlyle Group since June 2006. He is also a member of the Board
of Directors of The Hartford Mutual Funds, Inc., HCR-Manor Care,
Inc., LifeCare Holdings, Inc. and Multiplan, Inc.
Mr. Johnston is a member of the Board of Directors of
Georgia O’Keeffe Museum.
The
General Partner’s Management Board
Each member of the Management Board of Management AG is
appointed by the Supervisory Board of Management AG for a
maximum term of five years and is eligible for reappointment
thereafter. Their terms of office expire in the years listed
below.
68
The table below provides names, positions and terms of office of
the members of the Management Board of Management AG and their
ages as of January 1, 2010.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Age as of
|
|
|
|
|
|
|
|
|
|
Jan. 1,
|
|
|
|
|
Year term
|
|
|
Name
|
|
2010
|
|
|
Position
|
|
expires
|
|
|
|
|
Dr. Ben J. Lipps
|
|
|
69
|
|
|
Chairman of the Management Board, Chief Executive Officer of
FMC-AG & Co. KGaA
|
|
|
2012
|
|
|
Rice Powell
|
|
|
54
|
|
|
Deputy Chairman of the Management Board and Chief Executive
Officer, Fresenius Medical Care North America
|
|
|
2014
|
|
|
Michael Brosnan
|
|
|
54
|
|
|
Chief Financial Offier of FMC-AG & Co. KGaA
|
|
|
2012
|
|
|
Roberto Fusté
|
|
|
58
|
|
|
Chief Executive Officer for Asia Pacific
|
|
|
2011
|
|
|
Dr. Emanuele Gatti
|
|
|
54
|
|
|
Chief Executive Officer for Europe, Middle East, Africa and
Latin America and Chief Strategist for FMC-AG & Co. KGaA
|
|
|
2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dr. Rainer Runte
|
|
|
50
|
|
|
Chief Administrative Officer, General Counsel, Chief Compliance
Officer and Labor Relations Director for Germany
|
|
|
2015
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Kent Wanzek
|
|
|
50
|
|
|
Head of Global Manufacturing Operations
|
|
|
2012
|
|
DR. BEN J. LIPPS became Chairman and Chief Executive Officer of
the Management Board of Management AG on December 21, 2005.
He served as acting Chief Financial Officer from
September 1, 2009 until December 31, 2009. He was
Chairman and Chief Executive Officer of the Management Board of
FMC-AG from May 1, 1999 until the transformation of legal
form to a KGaA and was Vice Chairman of the Management Board
until May 1999. He was Chief Executive Officer of Fresenius
Medical Care North America until February 2004. He was
President, Chief Executive Officer, Chief Operating Officer and
a director of Fresenius USA from October 1989 through February
2004, and served in various capacities with Fresenius USA’s
predecessor from 1985 through 1989. He is a member of the
management board of Fresenius SE. He has been active in the
field of dialysis for more than 40 years. After earning his
master’s and doctoral degrees at the Massachusetts
Institute of Technology in chemical engineering, Dr. Lipps
led the research team that developed the first commercial hollow
fiber artificial kidney at the end of the 1960s. Before joining
the Fresenius Group in 1985, Dr. Lipps held several
research management positions, among them with DOW Chemical.
RICE POWELL became Deputy Chairman of the Management Board and
Chief Executive Officer of Fresenius Medical Care North America
effective January 1, 2010. He was a member of the
Management Board of FMC-AG from February 2004 until the
transformation of legal form and was Co-Chief Executive Officer
of Fresenius Medical Care North America and CEO of Renal Therapy
Group (RTG) of Fresenius Medical Care North America. He has more
than 30 years of experience in the healthcare industry.
From 1978 to 1996 he held various positions within Baxter
International Inc. (USA), Biogen Inc. (USA) and Ergo Sciences
Inc. (USA).
MICHAEL BROSNAN became a member of the Management Board of
Management AG and Chief Financial Officer on January 1,
2010. For the past seven years, he has served as Chief Financial
Officer and member of the Board of Directors of Fresenius
Medical Care North America. Mr. Brosnan joined the Company
in 1998 as Vice President of Finance and Administration for
Spectra Renal Management, the Company’s laboratory services
organization. Since then, he has held several executive
positions in North America. Prior to joining Fresenius Medical
Care, Mr. Brosnan held senior financial positions at
Polaroid Corporation and was an audit partner at KPMG.
DR. EMANUELE GATTI became a member of the Management Board of
Management AG and Chief Executive Officer for Europe, Latin
America, Middle East and Africa on December 21, 2005. He
became Chief Strategist effective January 1, 2010. He held
such positions in FMC-AG from May 1997 until the transformation
of legal form. After completing his studies in bioengineering,
Dr. Gatti lectured at several biomedical institutions. He
continues to be involved in comprehensive research and
development activities focusing on dialysis and blood
purification, biomedical signal analysis, medical device safety
and health care economics. Dr. Gatti has been with the
company since 1989. Before being appointed to the Management
Board in 1997, he was responsible for the Company’s
dialysis business in Southern Europe.
ROBERTO FUSTÉ became a member of the Management Board of
Management AG and Chief Executive Officer for Asia Pacific on
December 21, 2005. He held such positions in FMC-AG from
January 1, 1999 until the
69
transformation of legal form. After finishing his studies in
economic sciences at the University of Valencia, he founded the
company Nephrocontrol S.A. in 1983. In 1991, Nephrocontrol was
acquired by the Fresenius Group, where Mr. Fusté has
since worked. Before being appointed to the Management Board of
FMC-AG in 1999, Mr. Fusté held several senior
positions within the Company in Europe and the Asia Pacific
region.
DR. RAINER RUNTE is the Company’s Chief Administrative
Officer (General Counsel, Chief Compliance Officer and Labor
Relations Director for Germany). He has been a member of the
Management Board of Management AG since December 2005. He became
Chief Administrative Officer including, among other
responsibilities, Labor Relations Director for Germany,
effective January 1, 2010. He was a member of the
Management Board responsible for Law, Compliance &
Corporate Governance and Intellectual Property of FMC-AG from
January 1, 2004 until the transformation of legal form to a
KGaA. He has worked for the Fresenius group for 19 years.
Previously he served as scientific assistant to the law
department of the Johann Wolfgang Goethe University in Frankfurt
and as an attorney in a law firm specialized in economic law.
Dr. Runte took the position as Senior Vice President for
Law of Fresenius Medical Care in 1997 and was appointed as
deputy member of the Management Board in 2002.
KENT WANZEK became a member of the Management Board of
Management AG effective January 1, 2010, with
responsibility for Global Manufacturing Operations. Previously,
Mr. Wanzek was in charge of North American Operations for
the Renal Therapies Group at Fresenius Medical Care North
America since 2004. Prior to joining the Company in 2003,
Mr. Wanzek held several senior executive positions with
companies in the health care industry, including Philips Medical
Systems, Perkin-Elmer, Inc. and Baxter Healthcare Corporation.
The business address of all members of our Management Board and
Supervisory Board is Else-Kröner-Strasse 1, 61352 Bad
Homburg, Germany.
The
Supervisory Board of FMC-AG & Co. KGaA
The Supervisory Board of FMC-AG & Co. KGaA consists of
six members who are elected by the shareholders of
FMC-AG & Co. KGaA in a general meeting. Fresenius SE,
as the sole shareholder of Management AG, the general partner,
is barred from voting for election of the Supervisory Board of
FMC-AG & Co. KGaA but, nevertheless has and will
retain significant influence over the membership of the
FMC-AG & Co. KGaA Supervisory Board in the foreseeable
future. See Item 16.G, below, “Governance —
The Legal Structure of FMC-AG & Co. KGaA.”
The current Supervisory Board of FMC-AG & Co. KGaA
consists of six persons, five of whom —
Messrs. Schenk, Krick, Kringel, Weisman and
Johnston — are also members of the Supervisory Board
of our General Partner. For information regarding the names,
ages, terms of office and business experience of those members
of the Supervisory Board of FMC-AG & Co. KGaA, see
“The General Partner’s Supervisory Board,” above.
The sixth member of the Supervisory Board of FMC-AG &
Co. KGaA is Prof. Dr. Bernd Fahrholz. Information regarding
his age, term of office and business experience is as follows:
PROF. DR. BERND FAHRHOLZ, age 62, was a member of the
Supervisory Board of Management AG from April 8, 2005 until
August 30, 2006 and was a member of the Supervisory Board
of FMC-AG from 1998 until the transformation of legal form to a
KGaA and a member of the Supervisory Board of FMC-AG &
Co. KGaA following the transformation. He is a member of our
Audit and Corporate Governance Committee. He is partner in the
law firm of Dewey & LeBoeuf, LLP, and from 2004 until
September 30, 2005 was a partner in the law firm of
Nörr Stiefenhofer Lutz (now Noerr LLP). He was a member of
the Management Board of Dresdner Bank AG since 1998 and was
Chairman from April 2000 until he resigned in March of 2003. He
also served as the vice-chairman of the Management Board of
Allianz AG and chairman of the Supervisory Board of Advance
Holding AG until March 25, 2003. He served on the
Supervisory Boards of BMW AG until May 13, 2004 and
Heidelberg Cement AG until May 6, 2004. Prof.
Dr. Fahrholz is Chairman of the Supervisory Board of
SMARTRAC N.V.
The terms of office of the aforesaid members of the Supervisory
Board of FMC-AG & Co. KGaA will expire at the end of
the general meeting of shareholders of FMC-AG & Co.
KGaA, in which the shareholders discharge the Supervisory Board
for the fourth fiscal year following the year in which they were
elected, but not counting the fiscal year in which such
member’s term begins. Members of the FMC-AG & Co.
KGaA Supervisory Board may be removed only by a resolution of
the shareholders of FMC-AG & Co. KGaA with a majority
of three quarters of the votes cast at such general meeting.
Fresenius SE is barred from voting on such resolutions. The
Supervisory Board of FMC-AG & Co. KGaA ordinarily acts
by simple majority vote and the Chairman has a tie-breaking vote
in case of any deadlock.
The principal function of the Supervisory Board of
FMC-AG & Co. KGaA is to oversee the management of the
Company but, in this function, the supervisory board of a
partnership limited by shares has less power and scope for
70
influence than the supervisory board of a stock corporation. The
Supervisory Board of FMC-AG & Co. KGaA is not entitled
to appoint the general partner or its executive bodies, nor may
it subject the general partner’s management measures to its
consent or issue rules of procedure for the general partner.
Only the Supervisory Board of Management AG, elected solely by
Fresenius SE, has the authority to appoint or remove members of
the general partner’s Management Board. See Item 16.G,
below, “Governance — The Legal Structure of
FMC-AG & Co. KGaA.” Among other matters, the
Supervisory Board of FMC-AG & Co. KGaA will, together
with the general partner, fix the agenda for the annual general
meeting and make recommendations with respect to approval of the
company’s annual financial statements and dividend
proposals. The Supervisory Board of FMC-AG & Co. KGaA
will also propose nominees for election as members of its
Supervisory Board and propose the Company’s auditors for
approval by shareholders.
Report
of the Management Board of Management AG, our General
Partner
The compensation report of Fresenius Medical Care AG &
Co. KGaA summarizes the main elements of the compensation system
for the members of the Management Board of Fresenius Medical
Care Management AG as general partner of Fresenius Medical
AG & Co. KGaA and in this connection notably explains
the amounts and structure of the compensation paid to the
Management Board. The compensation report is prepared on the
basis of the recommendations made by the German Corporate
Governance Code and also includes the disclosures as required
pursuant to the applicable statutory regulations, notably in
accordance with the German Commercial Code (HGB).
Compensation
of the Management Board
The Supervisory Board of Fresenius Medical Care Management AG is
responsible for determining the compensation of the Management
Board. The Supervisory Board is assisted in this task by a
personnel committee, the Human Resources Committee. In 2009, the
Human Resources Committee was composed of Dr. Ulf
M. Schneider, Dr. Gerd Krick, William P. Johnston and
Dr. Walter Weisman.
The objective of the compensation system is to enable the
members of the Management Board to participate reasonably in the
sustainable development of the Company’s business with the
compensation paid and to reward them based on their duties and
performance as well as their success in managing the
Company’s economic and financial position while giving due
regard to the peer environment.
The compensation of the Management Board is, as a whole,
performance-oriented and was composed of three elements in the
fiscal year 2009:
|
|
|
|
|
|
•
|
non-performance-related compensation (basic salary)
|
|
|
|
|
•
|
performance-related compensation (variable bonus)
|
|
|
|
|
•
|
components with long-term incentive effects (stock options and
share-based compensation with cash settlement)
|
In addition, four members of the Management Board had pension
commitments in the reporting period.
The individual components are designed on the basis of the
following criteria:
In fiscal year 2009, each member of the general partner’s
Management Board received a non-performance-related basic salary
paid in twelve monthly installments. Moreover, the members of
the Management Board received additional benefits consisting
mainly of insurance premiums, the private use of company cars,
special payments such as foreign supplements, rent supplements
and reimbursement of certain other charges and additional
contributions to pension and health insurance.
Performance-related compensation will also be granted for fiscal
year 2009 as a variable bonus. The amount of the bonus in each
case depends on the achievement of individual and common targets:
The targets for the members of the Management Board are measured
by reference to operating earnings (EBIT), net consolidated
earnings (EAT) and its growth, as well as the development of
cash flow, and achievement of the targets are in part subject to
a comparison with the previous year’s figures and can be
derived in another part from the comparison of budgeted and
actually achieved figures. Furthermore, targets are divided into
Group level targets and those to be achieved in individual
regions. Lastly, the various target parameters are weighted
differently by their relative share in the aggregate amount of
variable compensation depending on the respective (regional)
areas of responsibility assumed by the members of the Management
Board.
71
For 2009, all members of the Management Board were assessed on
the basis of growth rates for Group-wide after-tax earnings (EAT
growth). The floor relevant for variable compensation for
Group-wide growth in EAT to be achieved was at least 6%, whereas
the top relevant growth rate for this was set at 15% (cap).
Besides, the members of the Management Board assuming Group
functions and the members of the Management Board with regional
responsibilities were evaluated in terms of the development of
the respective cash flow within the Group or in the relevant
regions during the period under review, with the targets subject
to compensation being within a corridor of growth rates between
3% and 6% with reference to the respective cash flow. The growth
rates achieved during the period under review in terms of
regional operating earnings (regional EBIT) were moreover
compensated for the respective Board members with regional
responsibilities in each case within a target corridor between
13% and 19%.
As a rule, growth rates for after-tax earnings (EAT growth) for
members of the Management Board with Group functions are
compensated at a share of 80% in variable compensation and are
thus weighted higher than for Board members having
responsibility for regional earnings where the share is 60%. The
achievement of the target for free cash flow is assessed at the
uniform rate of 20% of variable compensation for all members of
the Management Board; likewise, the valuation of operating
earnings (EBIT margin) in the regions is weighted at 20% of the
variable compensation component.
In 2009, the bonus component in principle consisted
proportionately of cash payments (short-term) and a further
share-based compensation component (long-term) to be paid by way
of cash settlement based on the performance of the stock price
of the ordinary shares of Fresenius Medical Care AG &
Co. KGaA. Once the annual targets were or are achieved, the cash
was or will be paid after the end of the respective fiscal year
in which the target is achieved. The share-based compensation
also to be granted yearly in these cases is subject to a several
year vesting period, although a shorter period may apply in
special cases (e.g. professional incapacity, entry into
retirement). The amount of cash payment of this share-based
compensation correspond to the share price of Fresenius Medical
Care AG & Co. KGaA ordinary shares upon exercise after
the several year vesting period, and is for that reason,
attributed to the long-term incentive compensation components.
The amount of the maximum achievable bonus for each of the
members of the Management Board is capped.
In addition, a special bonus component applied in some cases for
fiscal years 2006, 2007 and 2008 which was linked to the
achievement of targets as measured only over this three-year
period but whose payment to a certain extent is also subject to
a vesting period of several years and consequently will take
place up to 2012. This bonus component also included special
components linked to the achievement of extraordinary financial
targets related to special integration measures (e.g. in
connection with the acquisition of Renal Care Group in the U.S.)
and thus required the achievement of an extraordinary increase
in earnings. The present report also reflects those payments
based on this earlier bonus component but exercised and paid
only in the year under review.
For fiscal years 2009 and 2008 the amount of cash payments of
the management board of Fresenius Medical Care Management AG
without long-term incentive components consisted of the
following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Compensation
|
|
|
|
|
Non-Performance Related
|
|
|
Performance
|
|
|
(without long-term
|
|
|
|
|
Compensation
|
|
|
Related Compensation
|
|
|
Incentive Components)
|
|
|
|
|
Salary
|
|
|
Other
1)
|
|
|
Bonus
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2009
|
|
|
2008
|
|
|
2009
|
|
|
2008
|
|
|
2009
|
|
|
2008
|
|
|
|
|
in thousands
|
|
|
in thousands
|
|
|
in thousands
|
|
|
in thousands
|
|
|
|
|
Dr. Ben Lipps
|
|
$
|
1,200
|
|
|
$
|
1,200
|
|
|
$
|
350
|
|
|
$
|
297
|
|
|
$
|
1,674
|
|
|
$
|
1,417
|
|
|
$
|
3,224
|
|
|
$
|
2,914
|
|
|
Roberto Fusté
|
|
|
558
|
|
|
|
515
|
|
|
|
258
|
|
|
|
270
|
|
|
|
724
|
|
|
|
290
|
|
|
|
1,540
|
|
|
|
1,075
|
|
|
Dr. Emanuele Gatti
|
|
|
767
|
|
|
|
809
|
|
|
|
155
|
|
|
|
95
|
|
|
|
1,021
|
|
|
|
968
|
|
|
|
1,943
|
|
|
|
1,872
|
|
|
Rice Powell
|
|
|
750
|
|
|
|
750
|
|
|
|
39
|
|
|
|
44
|
|
|
|
1,210
|
|
|
|
1,053
|
|
|
|
1,999
|
|
|
|
1,847
|
|
|
Lawrence A. Rosen
|
|
|
372
|
|
|
|
589
|
|
|
|
110
|
|
|
|
126
|
|
|
|
247
|
|
|
|
750
|
|
|
|
729
|
|
|
|
1,465
|
|
|
Dr. Rainer Runte
|
|
|
530
|
|
|
|
486
|
|
|
|
42
|
|
|
|
42
|
|
|
|
629
|
|
|
|
644
|
|
|
|
1,201
|
|
|
|
1,172
|
|
|
Mats Wahlstrom
|
|
|
850
|
|
|
|
850
|
|
|
|
39
|
|
|
|
46
|
|
|
|
1,627
|
|
|
|
1,244
|
|
|
|
2,516
|
|
|
|
2,140
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
5,027
|
|
|
$
|
5,199
|
|
|
$
|
993
|
|
|
$
|
920
|
|
|
$
|
7,132
|
|
|
$
|
6,366
|
|
|
$
|
13,152
|
|
|
$
|
12,485
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1)
|
Includes, insurance premiums, private use of company cars,
contributions to pension and health insurance and other benefits.
|
72
In addition to the aforementioned share-based compensation
components with cash settlement, stock options under Stock
Option Plan 2006 were granted as (further) components with
long-term incentive effects in fiscal year 2009. The principles
of Stock Option Plan 2006 are described in more detail in the
Notes to Consolidated Financial Statements (see Note 15,
“Stock Options — Fresenius Medical Care
AG & Co. KGaA Stock Option Plan 2006”).
As of January 1, 2009, the Company still had three
additional Employee Participation Programs secured by
conditional capital which entitled their participants to
convertible bonds or stock options, and from which, however, in
fiscal year 2009 no further options could be issued. In
continuation with these successful employee participation
programs of the past fiscal years, Fresenius Medical Care
AG & Co. KGaA implemented Stock Option Plan 2006
approved by resolution of the general meeting on May 9,
2006 and amended by resolution of the general meeting of
May 15, 2007 (reflecting the share split 1: 3).
During 2009, a total of 2,585,196 stock options were granted
under this Stock Option Plan, of which 348,600 were granted to
the members of the Management Board.
For fiscal years 2009 and 2008 the number and value of stock
options issued and the value of other share-based compensation
with cash settlement is shown individually in the following
table.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Components with Long-term Incentive Effect
|
|
|
|
|
|
|
|
Share-based
|
|
|
|
|
|
|
|
|
|
|
Compensation with Cash
|
|
|
|
|
|
|
|
Stock Options
|
|
|
Settlement
|
|
|
Total
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2009
|
|
|
2008
|
|
|
2009
|
|
|
2008
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Number
|
|
|
in thousands
|
|
|
in thousands
|
|
|
in thousands
|
|
|
|
|
Dr. Ben Lipps
|
|
|
99,600
|
|
|
|
99,600
|
|
|
$
|
1,086
|
|
|
$
|
1,537
|
|
|
$
|
475
|
|
|
$
|
626
|
|
|
$
|
1,561
|
|
|
$
|
2,163
|
|
|
Roberto Fusté
|
|
|
49,800
|
|
|
|
49,800
|
|
|
|
543
|
|
|
|
768
|
|
|
|
176
|
|
|
|
—
|
|
|
|
719
|
|
|
|
768
|
|
|
Dr. Emanuele Gatti
|
|
|
49,800
|
|
|
|
49,800
|
|
|
|
543
|
|
|
|
768
|
|
|
|
340
|
|
|
|
260
|
|
|
|
883
|
|
|
|
1,028
|
|
|
Rice Powell
|
|
|
49,800
|
|
|
|
49,800
|
|
|
|
543
|
|
|
|
768
|
|
|
|
337
|
|
|
|
348
|
|
|
|
880
|
|
|
|
1,116
|
|
|
Lawrence A. Rosen
|
|
|
—
|
|
|
|
49,800
|
|
|
|
—
|
|
|
|
768
|
|
|
|
—
|
|
|
|
307
|
|
|
|
—
|
|
|
|
1,075
|
|
|
Dr. Rainer Runte
|
|
|
49,800
|
|
|
|
49,800
|
|
|
|
543
|
|
|
|
768
|
|
|
|
209
|
|
|
|
253
|
|
|
|
752
|
|
|
|
1,021
|
|
|
Mats Wahlstrom
|
|
|
49,800
|
|
|
|
49,800
|
|
|
|
543
|
|
|
|
768
|
|
|
|
—
|
|
|
|
395
|
|
|
|
543
|
|
|
|
1,163
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
348,600
|
|
|
|
398,400
|
|
|
$
|
3,801
|
|
|
$
|
6,145
|
|
|
$
|
1,537
|
|
|
$
|
2,189
|
|
|
$
|
5,338
|
|
|
$
|
8,334
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The stated values of the stock options granted to the members of
the Management Board in fiscal year 2009 correspond to their
fair value at the time of being granted, namely a value of $
10.90 (€ 7.64) (2008 — $ 15.43/€ 9.80)
per stock option. The exercise price for the stock options
granted is $ 45.62 (€ 31.97) (2008 — $
55.88/€ 35.49).
At the end of the fiscal year 2009, the members of the
Management Board held a total of 2,041,121 stock options (2008:
2,159,720 stock options).
73
The development and status of stock options of the members of
the Management Board in fiscal year 2009 are shown in more
detail in the following table:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dr. Ben
|
|
|
Roberto
|
|
|
Dr. Emanuele
|
|
|
Rice
|
|
|
Lawrence
|
|
|
Dr. Rainer
|
|
|
Mats
|
|
|
|
|
|
|
|
Lipps
|
|
|
Fusté
|
|
|
Gatti
|
|
|
Powell
|
|
|
A. Rosen
|
|
|
Runte
|
|
|
Wahlstrom
|
|
|
Total
|
|
|
|
|
Options outstanding at January 1, 2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number
|
|
|
818,411
|
|
|
|
291,276
|
|
|
|
276,276
|
|
|
|
177,177
|
|
|
|
227,604
|
|
|
|
207,753
|
|
|
|
161,223
|
|
|
|
2,159,720
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average exercise price in $
|
|
|
35.40
|
|
|
|
35.47
|
|
|
|
36.16
|
|
|
|
43.58
|
|
|
|
40.68
|
|
|
|
42.56
|
|
|
|
46.59
|
|
|
|
38.26
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options granted during the fiscal year
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number
|
|
|
99,600
|
|
|
|
49,800
|
|
|
|
49,800
|
|
|
|
49,800
|
|
|
|
—
|
|
|
|
49,800
|
|
|
|
49,800
|
|
|
|
348,600
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average exercise price in $
|
|
|
45.62
|
|
|
|
45.62
|
|
|
|
45.62
|
|
|
|
45.62
|
|
|
|
—
|
|
|
|
45.62
|
|
|
|
45.62
|
|
|
|
45.62
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options exercised during the fiscal year
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number
|
|
|
214,595
|
|
|
|
25,000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
128,004
|
|
|
|
—
|
|
|
|
—
|
|
|
|
367,599
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average exercise price in $
|
|
|
22.07
|
|
|
|
22.78
|
|
|
|
—
|
|
|
|
—
|
|
|
|
33.44
|
|
|
|
—
|
|
|
|
—
|
|
|
|
26.07
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average share price in $
|
|
|
41.06
|
|
|
|
51.80
|
|
|
|
—
|
|
|
|
—
|
|
|
|
49.40
|
|
|
|
—
|
|
|
|
—
|
|
|
|
44.69
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options forfeited during the fiscal year
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
99,600
|
|
|
|
—
|
|
|
|
—
|
|
|
|
99,600
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average exercise price in $
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
49.99
|
|
|
|
—
|
|
|
|
—
|
|
|
|
49.99
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options outstanding at December 31, 2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number
|
|
|
703,416
|
|
|
|
316,076
|
|
|
|
326,076
|
|
|
|
226,977
|
|
|
|
—
|
|
|
|
257,553
|
|
|
|
211,023
|
|
|
|
2,041,121
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average exercise price in $
|
|
|
40.97
|
|
|
|
38.15
|
|
|
|
37.67
|
|
|
|
44.13
|
|
|
|
—
|
|
|
|
43.23
|
|
|
|
46.46
|
|
|
|
41.20
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average remaining contractual life in years
|
|
|
4.2
|
|
|
|
4.4
|
|
|
|
4.5
|
|
|
|
4.9
|
|
|
|
—
|
|
|
|
4.7
|
|
|
|
5.1
|
|
|
|
4.5
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Range of exercise price in $
|
|
|
20.85 - 51.13
|
|
|
|
16.45 - 51.13
|
|
|
|
16.45 - 51.13
|
|
|
|
16.45 -51.13
|
|
|
|
—
|
|
|
|
20.69 - 51.13
|
|
|
|
29.19 - 51.13
|
|
|
|
16.45 - 51.13
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options exercisable at December 31, 2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number
|
|
|
404,616
|
|
|
|
166,676
|
|
|
|
176,676
|
|
|
|
77,577
|
|
|
|
—
|
|
|
|
108,153
|
|
|
|
61,623
|
|
|
|
995,321
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average exercise price in $
|
|
|
35.27
|
|
|
|
28.70
|
|
|
|
28.37
|
|
|
|
35.35
|
|
|
|
—
|
|
|
|
35.71
|
|
|
|
41.10
|
|
|
|
33.36
|
|
Based on the targets achieved in fiscal year 2009, additional
rights for share-based compensation with cash settlement
totaling $1,537,296 (2008: $ 2,189,419) were earned, on the
basis of which the number of share-based compensation rights is
distributed. Since the actual distribution will not take place
until March 2010, the specific number of shares of such
share-based compensation rights will be determined by the
Supervisory Board at that time by reference to the then current
price of the ordinary shares of Fresenius Medical Care
AG & Co. KGaA. Such number of shares will then serve
as a basis and multiplier for calculating of the payment after
the several year vesting period.
The amount of the total compensation of the Management Board of
Fresenius Medical Care Management AG for fiscal years 2009 and
2008 in shown in the following table:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Compensation
|
|
|
Components with
|
|
|
Total Compensation
|
|
|
|
|
(without long-term
|
|
|
long-term
|
|
|
(including long-term
|
|
|
|
|
Incentive components)
|
|
|
Incentive Effect
|
|
|
Incentive Components)
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2009
|
|
|
2008
|
|
|
2009
|
|
|
2008
|
|
|
|
|
in thousands
|
|
|
in thousands
|
|
|
in thousands
|
|
|
|
|
Dr. Ben Lipps
|
|
$
|
3,224
|
|
|
$
|
2,914
|
|
|
$
|
1,561
|
|
|
$
|
2,163
|
|
|
$
|
4,785
|
|
|
$
|
5,077
|
|
|
Roberto Fusté
|
|
|
1,540
|
|
|
|
1,075
|
|
|
|
719
|
|
|
|
768
|
|
|
|
2,259
|
|
|
|
1,843
|
|
|
Dr. Emanuele Gatti
|
|
|
1,943
|
|
|
|
1,872
|
|
|
|
883
|
|
|
|
1,028
|
|
|
|
2,826
|
|
|
|
2,900
|
|
|
Rice Powell
|
|
|
1,999
|
|
|
|
1,847
|
|
|
|
880
|
|
|
|
1,116
|
|
|
|
2,879
|
|
|
|
2,963
|
|
|
Lawrence A. Rosen
|
|
|
729
|
|
|
|
1,465
|
|
|
|
—
|
|
|
|
1,075
|
|
|
|
729
|
|
|
|
2,540
|
|
|
Dr. Rainer Runte
|
|
|
1,201
|
|
|
|
1,172
|
|
|
|
752
|
|
|
|
1,021
|
|
|
|
1,953
|
|
|
|
2,193
|
|
|
Mats Wahlstrom
|
|
|
2,516
|
|
|
|
2,140
|
|
|
|
543
|
|
|
|
1,163
|
|
|
|
3,059
|
|
|
|
3,303
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
13,152
|
|
|
$
|
12,485
|
|
|
$
|
5,338
|
|
|
$
|
8,334
|
|
|
$
|
18,490
|
|
|
$
|
20,819
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Compensation components with long-term incentive effects, i.e.
stock options as well as share-based compensation with cash
settlement, can be exercised only after the expiry of the
specified vesting period. Their
74
value is recognized over the vesting period as expense in the
respective fiscal year of the vesting period. Share-based
compensation expenses attributable to fiscal years 2009 and 2008
are shown in the following table:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expense
|
|
|
|
|
|
|
|
Expense
|
|
|
for Long-term
|
|
|
|
|
|
|
|
for Long-term
|
|
|
Incentive
|
|
|
|
|
|
|
|
Incentive
|
|
|
Components
|
|
|
Total Expense
|
|
|
|
|
Components
|
|
|
by Share-based
|
|
|
for
|
|
|
|
|
with Equity
|
|
|
Compensation with
|
|
|
Share-based
|
|
|
|
|
Instruments
|
|
|
Cash Settlement
|
|
|
Compensation
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2009
|
|
|
2008
|
|
|
2009
|
|
|
2008
|
|
|
|
|
in thousands
|
|
|
in thousands
|
|
|
in thousands
|
|
|
|
|
Dr. Ben Lipps
|
|
$
|
1,318
|
|
|
$
|
1,188
|
|
|
$
|
1,272
|
|
|
$
|
796
|
|
|
$
|
2,590
|
|
|
$
|
1,984
|
|
|
Roberto Fusté
|
|
|
659
|
|
|
|
594
|
|
|
|
—
|
|
|
|
—
|
|
|
|
659
|
|
|
|
594
|
|
|
Dr. Emanuele Gatti
|
|
|
659
|
|
|
|
594
|
|
|
|
424
|
|
|
|
265
|
|
|
|
1,083
|
|
|
|
859
|
|
|
Rice Powell
|
|
|
659
|
|
|
|
594
|
|
|
|
805
|
|
|
|
488
|
|
|
|
1,464
|
|
|
|
1,082
|
|
|
Lawrence A. Rosen
|
|
|
(279
|
)
|
|
|
594
|
|
|
|
(501
|
)
|
|
|
385
|
|
|
|
(780
|
)
|
|
|
979
|
|
|
Dr. Rainer Runte
|
|
|
659
|
|
|
|
594
|
|
|
|
508
|
|
|
|
340
|
|
|
|
1,167
|
|
|
|
934
|
|
|
Mats Wahlstrom
|
|
|
1,606
|
|
|
|
594
|
|
|
|
1,633
|
|
|
|
558
|
|
|
|
3,239
|
|
|
|
1,152
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
5,281
|
|
|
$
|
4,752
|
|
|
$
|
4,141
|
|
|
$
|
2,832
|
|
|
$
|
9,422
|
|
|
$
|
7,584
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-performance-related compensation components and the basic
structures of performance-related compensation components have
been agreed with the individual members of the Management Board
in their employment contracts/service agreements. Stock options
are granted to members of the Management Board on an annual
basis by the Supervisory Board.
Commitments
to Members of the Management Board for the Event of the
Termination of their Appointment
There are individual contractual pension commitments for the
Management Board members Roberto Fusté, Dr. Emanuele
Gatti, Dr. Rainer Runte and Lawrence A. Rosen (the latter
having left the Company with effect from August 31, 2009).
Under these commitments, Fresenius Medical Care as of
December 31, 2009 has aggregate pension obligations of $
4,776,471 (at December 31, 2008: $ 3,354,178). Additions to
pension obligations in fiscal year 2009 amounted to $ 983,407
(2008: $ 422,394). Each of the pension commitments provides for
a pension and survivor benefit, depending on the amount of the
recipient’s most recent basic salary, from age 65, or,
in the case of termination because of professional or
occupational incapacity, from the time of ending active work.
The starting percentage of 30% increases with every year of
service by 1.5 percentage points up to a maximum of 45%.
30% of the gross amount of any later income from an activity of
the Management Board member is set off against the pension
obligation. The acquired pro rata pension commitment of
Mr. Lawrence A. Rosen became vested, after leaving the
Company on August 31, 2009.
With the Chairman of the Management Board, Dr. Ben Lipps,
there is an individual agreement instead of a pension provision,
to the effect that, taking account of a non-compete covenant
upon termination of his employment contract/service agreement
with Fresenius Medical Care Management AG, he will be retained
to render consulting services to the Company for a period of
10 years. The annual consideration for such services would
amount to approximately 33% of the non-performance-linked
compensation components paid to him in fiscal year 2009.
Under individual agreements, the Management Board members
Dr. Emanuele Gatti and Rice Powell are entitled to benefits
(severance payments, calculated on the basis of guaranteed
simple annual income, based on the relevant basic salary) in the
event that their employment with Fresenius Medical Care
Management AG should end. Such severance payments will be
reduced by one half of any additional compensation which the
said Board members would be entitled to in connection with
existing post-contractual non-compete covenants. The employment
contracts of Board members contain no express provisions for the
case of a change of control.
With Mr. Mats Wahlstrom, who resigned from the Management
Board on December 31, 2009 it was agreed that all
outstanding cash-settled share-based compensation related to the
special bonus component from the years 2006 to 2008 became
vested at the time of his resignation from the Board on
December 31, 2009. It was agreed that he can exercise these
rights at any date of his choice in February 2010. For the
fiscal year Mr. Mats Wahlstrom is also entitled to receive
payment for the agreed annual bonus component in accordance with
the determinations of the Supervisory Board. It was further
agreed with Mr. Mats Wahlstrom that he will render future
services under a service contract with a U.S. subsidiary of the
Fresenius Medical Care Group in the position of Senior Vice
President of Fresenius Medical Care and will act as a senior
advisor to the Chairman of the Management Board of Fresenius
Medical Care Management AG for a period of five years. For this
activity Mr. Mats Wahlstorm receives a separate
75
contractual remuneration. It was also agreed that
Mr. Wahlstrom shall remain entitled to all stock options
granted while serving as a Board Member. The latter also applies
in the case that Mr. Wahlstrom should resign from the
Fresenius Medical Care Group.
Miscellaneous
In fiscal year 2009, no loans or advance payments of future
compensation components were made to members of the Management
Board of Fresenius Medical Care Management AG.
To the extent permitted by law, Fresenius Medical Care
Management AG undertook to indemnify the members of the
Management Board from claims against them arising out of their
work for the Company and its affiliates, if such claims exceed
their liability under German law. To secure such obligations,
the Company has concluded Directors&Officers liability
insurance with an appropriate excess. To the extent that
adjustments are to be made with respect to the excess on the
basis of the German Act on the Appropriateness of Executive
Board Compensation (VorstAG), such adjustments shall be made no
later than the expiry of the transitional period on
June 30, 2010. The aforementioned indemnity applies for the
time in which each member of the Management Board is in office
and for claims in this connection after termination of
membership on the Management Board in each case.
Former members of the Management Board did not receive any
compensation in fiscal year 2009.
Adjustments
to System of Compensation of Members of the Management
Board
In view of the revised requirements for the system of
compensation of the Management Board arising from the German Act
on the Appropriateness of Executive Board Compensation (VorstAG)
which took effect on August 5, 2009, which requirements are
applicable to all directors’ employment contracts/service
agreements having been newly concluded or amended/adjusted in
their provisions, the Supervisory Board of Fresenius Medical
Care Management AG, in the context of the reorganization of
responsibilities within the Management Board as of
January 1, 2010, by resolution of December 8, 2009,
resolved to apply an adjusted system of compensation of the
members of the Management Board.
First of all, each member of the Management Board, as before,
receives an annual fixed basic compensation to be paid out in
twelve equal monthly installments, the amount of which is
assessed differently depending on the member of the Management
Board so as to reflect the particular individual tasks and
responsibilities assumed by the various members of the
Management Board.
In terms of the performance-related assessment of its variable
compensation components, the new compensation model observes the
principles described above under “Compensation of the
Management Board”. This assessment includes the respective
regional operating earnings (EBIT margin), growth in the net
consolidated income (EAT growth) and the development of the
Company-wide as well as regional cash flow before acquisitions
(FCF). All figures are calculated from a comparison of budgeted
figures with actually achieved figures.
Performance-related variable compensation is split into
(i) a component which is paid out in cash after
determination of the annual targets achieved (annual bonus),
(ii) a share-based compensation component with cash
settlement subject to a several years vesting period, and
(iii) a further component with long-term incentive effects
in the form of stock options. In determining the variable
compensation, due care is exercised to ensure that the share of
long-term incentive components accounts for at least 50% of the
entire variable compensation. The achievement of the targets in
terms of the aforementioned key ratios is assessed at 120%
maximum and is subject to a fixed multiplier so as to provide
for the possibility of limiting the variable compensation. As
already in the past, the new compensation system contains a
provision on capping the share-based compensation components in
the event of extraordinary developments.
The amount of the basic compensation and the total compensation
of the members of the Management Board was assessed giving
particular regard to the relevant comparison values of other DAX
companies and similar companies of comparable size and
performance from the relevant industrial sector. In this regard,
a conservative position below the respective mean value of
comparative companies was chosen on average. In addition to this
horizontal comparative view, due significance was also attached
to the vertical (company-internal) comparative view. In this
regard also the Company’s position is conservative relative
to similar companies.
Overall, the selection of compensation-relevant ratios and their
weighting within the compensation system was defined in such a
way that, on the whole, a compensation structure is provided
which is geared towards sustainable company development.
76
Compensation
of the Supervisory Board of Fresenius Medical Care &
Co KGaA and Supervisory Board of Management AG
Our supervisory board consists of six members, five of whom are
also members of the supervisory board of Management AG, our
general partner. Management AG has one additional supervisory
board member who is not a member of our supervisory board. Each
member of our supervisory board is paid an annual retainer fee
of $80,000. The Chairman is paid twice that amount and the
Vice-Chairman 150% of that amount. Supervisory Board members are
reimbursed for their reasonable travel and accommodation
expenses, including value added tax, incurred with respect to
their duties as Supervisory Board members. Supervisory board
members who serve on committees receive an additional retainer
of $30,000 per year ($50,000 per year in the case of committee
chairs). In accordance with our by-laws, we pay 50% of the fees
directly to the board member for the five supervisory board
members who are also members of the Management AG board and 100%
of the sixth (unaffiliated) member’s compensation directly
to him. In addition, under the management agreement with our
general partner, the general partner pays the remaining 50% of
the retainer fees of five members of our supervisory board and
100% of the fees payable to the general partner’s sixth
board member (who has no position with FMC-AG & Co.
KGaA or its Supervisory Board). By agreement, we reimburse
Management AG for 100% of all fees it incurs (including
compensation paid to the general partner’s supervisory
board). The aggregate compensation reported does not include
amounts paid as fees for services rendered by certain business
or professional entities with which some of the Supervisory
Board members are associated.
For the years 2009 and 2008 the
compensation
1)
for
the members of the supervisory boards were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fixed
|
|
|
Fixed
|
|
|
|
|
|
|
|
|
|
|
|
|
|
compensation for
|
|
|
compensation for
|
|
|
Compensation for
|
|
|
Compensation for
|
|
|
|
|
|
|
|
Supervisory Board
|
|
|
Supervisory Board
|
|
|
committee services
|
|
|
committee services
|
|
|
|
|
|
|
|
at FMC
|
|
|
at FMC-AG
|
|
|
at FMC
|
|
|
at FMC-AG
|
|
|
Total
|
|
|
|
|
Management AG
|
|
|
& Co. KGaA
|
|
|
Management AG
|
|
|
& Co. KGaA
|
|
|
compensation
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2009
|
|
|
2008
|
|
|
2009
|
|
|
2008
4)
|
|
|
2009
|
|
|
2008
|
|
|
2009
|
|
|
2008
|
|
|
|
|
in thousand
|
|
|
in thousand
|
|
|
in thousand
|
|
|
in thousand
|
|
|
in thousand
|
|
|
Dr. Gerd Krick
|
|
$
|
40
|
|
|
$
|
40
|
|
|
$
|
120
|
|
|
$
|
120
|
|
|
$
|
40
|
|
|
$
|
20
|
|
|
$
|
30
|
|
|
$
|
30
|
|
|
$
|
230
|
|
|
$
|
210
|
|
|
Dr. Dieter Schenk
|
|
|
60
|
|
|
|
60
|
|
|
|
60
|
|
|
|
60
|
|
|
|
30
|
|
|
|
15
|
|
|
|
—
|
|
|
|
—
|
|
|
|
150
|
|
|
|
135
|
|
|
Dr. Ulf
M. Schneider
2)
|
|
|
160
|
|
|
|
160
|
|
|
|
—
|
|
|
|
—
|
|
|
|
50
|
|
|
|
25
|
|
|
|
—
|
|
|
|
—
|
|
|
|
210
|
|
|
|
185
|
|
|
Dr. Walter L. Weisman
|
|
|
40
|
|
|
|
40
|
|
|
|
40
|
|
|
|
40
|
|
|
|
30
|
|
|
|
15
|
|
|
|
50
|
|
|
|
50
|
|
|
|
160
|
|
|
|
145
|
|
|
John Gerhard
Kringel
5)
|
|
|
40
|
|
|
|
40
|
|
|
|
40
|
|
|
|
40
|
|
|
|
40
|
|
|
|
20
|
|
|
|
10
|
|
|
|
30
|
|
|
|
130
|
|
|
|
130
|
|
|
Dr. William P. Johnston
|
|
|
40
|
|
|
|
40
|
|
|
|
40
|
|
|
|
40
|
|
|
|
80
|
|
|
|
40
|
|
|
|
30
|
|
|
|
30
|
|
|
|
190
|
|
|
|
150
|
|
|
Prof. Dr. Bernd
Fahrholz
3)
|
|
|
—
|
|
|
|
—
|
|
|
|
80
|
|
|
|
80
|
|
|
|
—
|
|
|
|
—
|
|
|
|
30
|
|
|
|
30
|
|
|
|
110
|
|
|
|
110
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
380
|
|
|
$
|
380
|
|
|
$
|
380
|
|
|
$
|
380
|
|
|
$
|
270
|
|
|
$
|
135
|
|
|
$
|
150
|
|
|
$
|
170
|
|
|
$
|
1,180
|
|
|
$
|
1,065
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1)
|
|
Shown without VAT and withholding
tax
|
|
|
|
2)
|
|
Chairman of the supervisory board
of FMC Management AG, but not member of the supervisory board of
FMC-AG & Co. KGaA; fixed compensation paid by FMC
Management AG
|
|
|
|
3)
|
|
Member of the supervisory board of
FMC-AG & Co. KGaA, but not member of the supervisory
board of FMC Management AG; fixed compensation paid by
FMC-AG & Co. KGaA
|
|
|
|
4)
|
|
at FMC Management AG level
committees have been established in Q3 2008 only; hence, the
respective compensation for 2008 was paid on a pro rata basis
|
|
|
|
5)
|
|
Member of Audit and Corporate
Governance Committee of FMC-AG & Co. KGaA until Q2 2009
|
For information relating to the terms of office of the
Management Board and the Supervisory Board of the general
partner, Fresenius Medical Care Management AG, and of the
Supervisory Board of FMC-AG & Co. KGaA, and the
periods in which the members of those bodies have served in
office, see Item 6.A, “Directors, Senior Management
and Employees — Directors and Senior Management,”
above. For information regarding certain compensation payable to
certain members of the general partner’s management board
after termination of employment, see Item 6.B,
“Directors, Senior Management and Employees —
Compensation — Commitments to Members of Management
for the Event of the Termination of their
Employment.“above. The functions usually performed by a
remuneration committee, particularly evaluation and assessment
of the compensation of the members of the general partner’s
Management Board, are performed by the Human Resources Committee
of the general partner’s Supervisory Board, the members of
which are Dr. Ulf M. Schneider (Chairman),
Dr. Gerd Krick, Mr. William P. Johnston and
Dr. Walter L. Weisman. Determination of the compensation
system and of the compensation to be granted is to be made by
the full Supervisory Board of Management AG. In 2009, the Audit
and Corporate Governance Committee of FMC-AG & Co.
KGaA consisted of Dr. Gerd Krick, Prof. Dr. Bernd
Fahrholz,
77
Dr. Walter L. Weisman (Chairman), Mr. William P.
Johnston and Mr. John Gerhard Kringel (until his
resignation from the committee in March 2009), all of whom are
independent directors for purposes of SEC
Rule 10A-3.
The primary function of the Audit and Corporate Governance
Committee is to assist FMC-AG & Co. KGaA’s
supervisory board in fulfilling its oversight responsibilities,
primarily through:
|
|
|
|
|
|
•
|
overseeing management’s conduct of our financial reporting
process and the internal accounting and financial control
systems and auditing of our financial statements;
|
|
|
|
|
•
|
monitoring our internal controls risk program;
|
|
|
|
|
•
|
monitoring our corporate governance performance according to the
German corporate governance codex;
|
|
|
|
|
•
|
monitoring the independence and performance of our outside
auditors;
|
|
|
|
|
•
|
providing an avenue of communication among the outside auditors,
management and the Supervisory Board;
|
|
|
|
|
•
|
reviewing the report of our general partner on relations with
related parties and for reporting to the overall supervisory
board thereon;
|
|
|
|
|
•
|
recommending the appointment of our independent auditors to
audit our German statutory financial statements (subject to the
approval by our shareholders at our Annual General Meeting) and
approval of their fees;
|
|
|
|
|
•
|
retaining the services of our independent auditors to audit our
U.S. GAAP financial statements and approval of their fees; and
|
|
|
|
|
•
|
pre-approval of all audit and non-audit services performed by
KPMG, our independent auditors.
|
In connection with the settlement of the shareholder proceedings
contesting the resolutions of the Extraordinary General Meeting
(“EGM”) held August 30, 2005 that approved the
transformation, the conversion of our preference shares into
ordinary shares and related matters, we established a joint
committee (the “Joint Committee”) (
gemeinsamer
Ausschuss
) of the supervisory boards of Management AG and
FMC-AG & Co. KGaA consisting of two members designated
by each supervisory board to advise and decide on certain
extraordinary management measures, including:
|
|
|
|
|
|
•
|
transactions between us and Fresenius SE with a value in excess
of 0.25% of our consolidated revenue, and
|
|
|
|
|
•
|
acquisitions and sales of significant participations and parts
of our business, the spin-off of significant parts of our
business, initial public offerings of significant subsidiaries
and similar matters. A matter is “significant” for
purposes of this approval requirement if 40% of our consolidated
revenues, our consolidated balance sheet total assets or
consolidated profits, determined by reference to the arithmetic
average of the said amounts shown in our audited consolidated
accounts for the previous three fiscal years, are affected by
the matter.
|
The supervisory board of our general partner, Management AG, is
supported by a Regulatory and Reimbursement Assessment Committee
(the “RRAC”) whose members are Mr. William P.
Johnston (Chairman), Mr. John Gerhard Kringel and
Dr. Dieter Schenk. The primary function of the RRAC is to
assist and to represent the board in fulfilling its
responsibilities, primarily through assessing the Company’s
affairs in the area of its regulatory obligations and
reimbursement structures for dialysis services. In the United
States, these reimbursement regulations are mandated by the HHS
and CMS for dialysis services. Similar regulatory agencies exist
country by country in the International regions to address the
conditions for payment of dialysis treatments.
At December 31, 2009, we had 67,988 employees (full-time
equivalents) as compared to 64,666 at December 31, 2008,
and 61,406 at December 31, 2007. The 5% increase in 2009
was mainly due to the overall
78
growth in our business. The following table shows the number of
employees by our major category of activities for the last three
fiscal years.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
North America
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dialysis Care
|
|
|
35,188
|
|
|
|
33,694
|
|
|
|
32,087
|
|
|
Dialysis Products
|
|
|
6,916
|
|
|
|
6,752
|
|
|
|
7,007
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
42,104
|
|
|
|
40,446
|
|
|
|
39,094
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
International
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dialysis Care
|
|
|
16,413
|
|
|
|
15,180
|
|
|
|
13,728
|
|
|
Dialysis Products
|
|
|
9,312
|
|
|
|
8,903
|
|
|
|
8,454
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
25,725
|
|
|
|
24,083
|
|
|
|
22,182
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Corporate
|
|
|
159
|
|
|
|
137
|
|
|
|
130
|
|
|
Total Company
|
|
|
67,988
|
|
|
|
64,666
|
|
|
|
61,406
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
We are members of the Chemical Industry Employers Association
for most sites in Germany and we are bound by union agreements
negotiated with the respective union representatives in those
sites. We generally apply the principles of the Association and
the related union agreements for those sites where we are not
members. We are also party to additional shop agreements
negotiated with works councils at individual facilities that
relate to those facilities. In addition, approximately 5% of our
U.S. employees are covered by collective bargaining agreements.
During the last three fiscal years, we have not suffered any
labor-related work disruptions.
As of December 31, 2009, no member of the Supervisory Board
or the Management Board beneficially owned 1% or more of our
outstanding Ordinary shares or our outstanding Preference
shares. At December 31, 2009, Management Board members of
the General Partner held options to acquire 2,041,121 ordinary
shares of which options to purchase 995,321 ordinary shares were
exercisable at a weighted average exercise price of €23.16
($33.36) (see Item 6.B, “Directors, Senior Management
and Employees — Compensation”). Those options
expire at various dates between 2011and 2016.
Options
to Purchase Our Securities
Stock
Option and Other Share Based Plans
Incentive
plan
In 2009, Management Board members were eligible for
performance-related compensation that depended upon achievement
of individual and common targets. The targets are based upon
operating earnings (EBIT), net consolidated earnings (EAT) and
its growth, as well as the development of cash flow, and are in
part developed by a comparison with the previous year’s
figures, budgeted figures and actually achieved figures. Targets
are divided into Group level targets and those to be achieved in
individual regions. For detailed information relating to targets
and calculation of bonus payments, see Item 6.B above.
The bonus for fiscal year 2009 will, in principle, consist
proportionately of a cash component and a share-based component
which will be paid in cash. Upon meeting the annual targets, the
cash component was or will be paid after the end of 2009. The
share-based component is subject to a several year vesting
period, although a shorter period may apply in special cases.
The amount of cash payment relating to the share-based component
will correspond to the share price of Fresenius Medical Care
AG & Co. KGaA ordinary shares upon exercise after the
several year vesting period. The amount of the maximum
achievable bonus for each of the members of the Management Board
is capped.
In 2006, Fresenius Medical Care Management AG adopted a
performance related compensation plan for the members of its
management board in the form of a variable bonus. A special
bonus component (award) for some of the management board members
consists in equal parts of cash payments and share-based
compensation based on the development of the stock price of
Fresenius Medical Care AG & Co. KGaA’s ordinary
shares. The amount of the award in each case depends on the
achievement of certain performance targets. The targets are
measured by reference to revenue growth, operating income,
consolidated net income and cash flow development. These
performance targets relate to a three-year period comprising the
fiscal years 2007, 2008 and 2009 only. Annual targets have been
achieved and the cash portion of the award has been paid after
the end of the respective fiscal year. The share-based
compensation portion has been granted but subject to a
three-year vesting period. The amount of
79
cash payment of the share-based compensation portion corresponds
to the share price of Fresenius Medical Care AG & Co.
KGaA’s ordinary shares on exercise, i.e. at the end of the
vesting period, and is also made in cash.
The share-based compensation incurred under these plans for
years 2009, 2008 and 2007 was $1,537, $2,189 and $4,595,
respectively. Such share-based compensation paid to the members
of the general partner’s management board is included in
the amounts shown in the table entitled “Components with
Long-Term Incentive Effect” in Item 6.B, Directors,
Senior Management and Employees —
Compensation — Compensation of the Management
Board.”
Fresenius
Medical Care AG & Co. KGaA Stock Option Plan
2006
On May 9, 2006, as amended on May 15, 2007 for a
three-for-one
share split (the “Share Split”), the Fresenius Medical
Care AG & Co. KGaA Stock Option Plan 2006 (the
“Amended 2006 Plan”) was established by resolution of
our annual general meeting with a conditional capital increase
up to €15,000,000 subject to the issue of up to fifteen
million no par value bearer ordinary shares with a nominal value
of €1.00 each. Under the Amended 2006 Plan, up to fifteen
million options can be issued, each of which can be exercised to
obtain one ordinary share, with up to three million options
designated for members of the Management Board of the General
Partner, up to three million options designated for members of
management boards of direct or indirect subsidiaries of the
Company and up to nine million options designated for managerial
staff members of the Company and such subsidiaries. With respect
to participants who are members of the General Partner’s
Management Board, the general partner’s Supervisory Board
has sole authority to grant stock options and exercise other
decision making powers under the Amended 2006 Plan (including
decisions regarding certain adjustments and forfeitures). The
General Partner’s Management Board has such authority with
respect to all other participants in the Amended 2006 Plan.
Options under the Amended 2006 Plan can be granted the last
Monday in July
and/or
the
first Monday in December. The exercise price of options granted
under the Amended 2006 Plan shall be the average closing price
on the Frankfurt Stock Exchange of our ordinary shares during
the 30 calendar days immediately prior to each grant date.
Options granted under the Amended 2006 Plan have a seven-year
term but can be exercised only after a three-year vesting
period. The vesting of options granted is subject to achievement
of performance targets, measured over a three-year period from
the grant date. For each such year, the performance target is
achieved if our adjusted basic income per ordinary share
(“EPS”), as calculated in accordance with the Amended
2006 Plan, increases by at least 8% year over year during the
vesting period, beginning with EPS for the year of grant as
compared to EPS for the year preceding such grant. Calculation
of EPS under the Amended 2006 Plan excludes, among other items,
the costs of the transformation of our legal form to a KGaA and
the conversion of preference shares into ordinary shares. For
each grant, one-third of the options granted are forfeited for
each year in which EPS does not meet or exceed the 8% target.
The performance targets for 2009, 2008, and 2007 were met but
the options that vested will not be exercisable until expiration
of the full
3-year
vesting period of each year’s grants. Vesting of the
portion or portions of a grant for a year or years in which the
performance target is met does not occur until completion of the
entire three-year vesting period. Upon exercise of vested
options, we have the right to reissue treasury shares or issue
new shares.
During 2009, we awarded 2,585,196 options, including 348,600 to
members of the Management Board of the General Partner, at a
weighted average exercise price of $46.22 (€32.08), a
weighted average fair value of $10.95 each and a total fair
value of approximately $28.3 million which will be
amortized on a straight line basis over the three-year vesting
period. For information regarding options granted to each member
of the general partner’s management board, see
Item 6.B, “- Compensation of the Management
Board” above.
Options granted under the Amended 2006 Plan to U.S. participants
are non-qualified stock options under the United States Internal
Revenue Code of 1986, as amended. Options under the Amended 2006
Plan are not transferable by a participant or a
participant’s heirs, and may not be pledged, assigned, or
otherwise disposed of.
At December 31, 2009, we had awards outstanding under the
terms of various prior stock-based compensation plans, including
the 2001 plan. Under the 2001 plan, convertible bonds with a
principal of up to €10,240,000 were issued to the members
of the Management Board and other employees of the Company
representing grants for up to 4 million non-voting
Preference shares. Following the Share Split, the convertible
bonds have a par value of €0.85 and bear interest at a rate
of 5.5%. Except for the members of the Management Board,
eligible employees were able to purchase the bonds by issuing a
non-recourse note with terms corresponding to the terms of and
secured by the bond. We have the right to offset our obligation
on a bond against the employee’s obligation on the related
note; therefore, the convertible bond obligations and employee
note receivables represent stock options we issued and are not
reflected in the consolidated financial statements. The options
expire in ten years and one third of each grant can be exercised
beginning after two, three or four years from the date of the
grant. Bonds issued to Board members who did not issue a note to
us are recognized as a liability on our balance sheet.
80
Upon issuance of the option, the employees had the right to
choose options with or without a stock price target. The
conversion price of options subject to a stock price target
becomes the stock exchange quoted price of the shares upon the
first time the stock exchange quoted price exceeds the initial
value by at least 25%. The initial value (“Initial
Value”) is the average price of the shares during the last
30 trading days prior to the date of grant. In the case of
options not subject to a stock price target, the number of
convertible bonds awarded to the eligible employee would be 15%
less than if the employee elected options subject to the stock
price target. The conversion price of the options without a
stock price target is the Initial Value, as adjusted in
accordance to the Share Split. Each option entitles the holder
thereof, upon payment the respective conversion price, to
acquire one share. Up to 20% of the total amount available for
the issuance of awards under the 2001 plan could be issued each
year through May 22, 2006. Effective May 2006, no further
grants could be issued under the 2001 plan.
During 1998, we adopted two stock incentive plans (“FMC98
Plan 1” and “FMC98 Plan 2”) for our key
management and executive employees. These stock incentive plans
were replaced by the 2001 plan and no options have been granted
since 2001. Under these plans eligible employees had the right
to acquire our shares. Options granted under these plans have a
ten-year term, and one third of them vest on each of the second,
third and fourth anniversaries of the award date. Each option
can be exercised for one share.
At December 31, 2009, the Management Board members of the
General Partner held 2,041,121 stock options for ordinary shares
and employees of the Company held 9,852,942 stock options for
ordinary shares with an average remaining contractual life of
5.01 years and 146,601 stock options for preference shares
with an average remaining contractual life of 3.91 years
with 146,601 exercisable preference options at a weighted
average exercise price of $26.43 and 4,589,390 exercisable
ordinary options at a weighted average exercise price of $36.40.
Item 7.
Major
Shareholders and Related Party Transactions
Security
Ownership of Certain Beneficial Owners of Fresenius Medical
Care
Our outstanding share capital consists of Ordinary shares and
non-voting Preference shares that are issued only in bearer
form. Accordingly, unless we receive information regarding
acquisitions of our shares through a filing with the Securities
and Exchange Commission or through the German statutory
requirements referred to below, or except as described below
with respect to our shares held in American Depository Receipt
(“ADR”) form, we face difficulties precisely
determining who our shareholders are at any specified time or
how many shares any particular shareholder owns. Because we are
a foreign private issuer under the rules of the Securities and
Exchange Commission, our directors and officers are not required
to report their ownership of our equity securities or their
transactions in our equity securities pursuant to
Section 16 of the Exchange Act. However, persons who become
“beneficial owners” of more than 5% of our ordinary
shares are required to report their beneficial ownership
pursuant to Section 13(d) of the Exchange act. In addition,
under the German Securities Trading Act
(
Wertpapierhandelsgesetz
), persons who discharge
managerial responsibilities within an issuer of shares are
obliged to notify the issuer and the German Federal Financial
Supervisory Authority of their own transactions in shares of the
issuer in excess of €5,000 in any year. This obligation
also applies to persons who are closely associated with the
persons discharging managerial responsibility. Additionally,
holders of voting securities of a German company listed on the
Regulated Market (Regulierter Markt) of a German stock exchange
or a corresponding trading segment of a stock exchange within
the European Union are obligated to notify the company of the
level of their holding whenever such holding reaches, exceeds or
falls below certain thresholds, which have been set at 3%, 5%,
10%, 15%, 20%, 25%, 30%, 50% and 75% of a company’s
outstanding voting rights. Such notification obligations will
also apply to option agreements (excluding the 3% threshold).
We have been informed that as of December 31, 2009,
Fresenius SE owned approximately 36.0% of our Ordinary shares.
In August 2008, an indirect wholly-owned subsidiary of Fresenius
SE issued €554.4 million aggregate principal amount of
Mandatory Exchangeable Bonds due 2011 with each bond having a
nominal value of €50,000 (the “FSE Bonds”).
Fresenius SE may redeem the FSE Bonds at or prior to maturity
solely by delivery of our Ordinary shares. The actual number of
ordinary shares deliverable by Fresenius SE upon redemption of
the FSE Bonds will depend upon the exchange ratio for the FSE
Bonds at the time of exchange, subject to an adjusted minimum
exchange price of €32.34 and an adjusted maximum exchange
price of €38.16. Upon maturity of the FSE Bonds, Fresenius
SE’s holding of our Ordinary shares could decrease to
between approximately 31% at the maximum exchange price and 30%
at the minimum exchange price.
All of our ordinary shares have the same voting rights. However,
as the sole shareholder of our general partner, Fresenius SE is
barred from voting its ordinary shares on certain matters. See
Item 16.G, “Corporate Governance —
Supervisory Board.”
81
Bank of New York Mellon, our ADR depositary, informed us, that
as of December 31, 2009, 18,538,422 Ordinary ADSs, each
representing one Ordinary share, were held of record by 4,864
U.S. holders and there were 84,341 Preference ADSs, each
representing one Preference share, held of record by 1 U.S.
holder. For more information regarding ADRs and ADSs see
Item 10.B, “Memorandum and Articles of
Association — Description of American Depositary
Receipts.”
Security
Ownership of Certain Beneficial Owners of Fresenius
SE
Fresenius SE’s share capital consists of ordinary shares
and non-voting preference shares. Both classes of shares are
issued only in bearer form. Accordingly, Fresenius SE has
difficulties precisely determining who its shareholders are at
any specified time or how many shares any particular shareholder
owns. However, under the German Securities Trading Act, holders
of voting securities of a German company listed on the Regulated
Market (Regulierter Markt) of a German stock exchange or a
corresponding trading segment of a stock exchange within the
European Union are obligated to notify the company of certain
levels of holdings, as described above.
Based on the most recent information available,
Else-Kröner-Fresenius Stiftung owns approximately 58% of
the Fresenius SE Ordinary shares. See Item 7.B,
“Related party transactions — Other
interests,” below. According to Allianz
Lebensversicherungs-AG, they hold between 5%-10% of the
Fresenius SE Ordinary shares.
|
|
|
|
B.
|
Related
party transactions
|
In connection with the formation of FMC-AG, and the combination
of the dialysis businesses of Fresenius SE and W.R.
Grace & Co. in the second half 1996, Fresenius SE and
its affiliates and Fresenius Medical Care and its affiliates
entered into several agreements for the purpose of giving effect
to the merger and defining our ongoing relationship. Fresenius
SE and W.R. Grace & Co. negotiated these agreements.
The information below summarizes the material aspects of certain
agreements, arrangements and transactions between Fresenius
Medical Care and Fresenius SE and their affiliates. The
following descriptions are not complete and are qualified in
their entirety by reference to those agreements, which have been
filed with the Securities and Exchange Commission and the New
York Stock Exchange. We believe that the leases, the supply
agreements and the service agreements are no less favorable to
us and no more favorable to Fresenius SE than would have been
obtained in arm’s-length bargaining between independent
parties. The trademark and other intellectual property
agreements summarized below were negotiated by Fresenius SE and
W.R. Grace & Co., and, taken independently, are not
necessarily indicative of market terms.
Dr. Gerd Krick, Chairman of our Supervisory Board, is also a
member of the Supervisory Board of our general partner and
Chairman of the Supervisory Board of Fresenius SE.
Dr. Dieter Schenk, Vice Chairman of the Supervisory Board
of our general partner and of the Supervisory Board of
FMC-AG & Co. KGaA, is also a member of the Supervisory
Board of Fresenius SE, and Dr. Ulf M. Schneider,
Chairman of the Supervisory Board of our general partner and a
former member of the Supervisory Board of FMC-AG, is Chairman of
the Management Board and CEO of Fresenius SE. Each of
Mr. John G. Kringel, Dr. Walter L. Weisman and
Dr. William P. Johnston is a member of both our Supervisory
Board and our general Partner’s Supervisory Board.
In the discussion below regarding our contractual and other
relationships with Fresenius SE:
|
|
|
|
|
|
•
|
the term “we (or us) and our affiliates” refers only
to Fresenius Medical Care AG & Co. KGaA and its
subsidiaries; and
|
|
|
|
|
•
|
the term “Fresenius SE and its affiliates” refers only
to Fresenius SE and affiliates of Fresenius SE other than
Fresenius Medical Care AG & Co. KGaA and its
subsidiaries.
|
Real
Property Lease
We did not acquire the land and buildings in Germany that
Fresenius Worldwide Dialysis used when we were formed in the
second half of 1996. Fresenius SE or its affiliates have leased
part of the real property to us, directly, and transferred the
remainder of that real property to two limited partnerships.
Fresenius SE is the sole limited partner of each partnership,
and the sole shareholder of the general partner of each
partnership. These limited partnerships, as landlords, have
leased the properties to us and to our affiliates, as
applicable, for use in our respective businesses. The aggregate
annual rent payable by us under these leases is approximately
€16.6 million, which was approximately
$23.1 million as of December 31, 2009, exclusive of
maintenance and other costs, and is subject to escalation, based
upon development of the German consumer-price-index determined
by the Federal Statistical Office. The leases for manufacturing
facilities have a ten-year term, followed by two successive
optional renewal terms of ten years each at our election. In
December 2006, the Company exercised its option to renew the
lease for manufacturing facilities and the other leases were
amended to extend their terms and add renewal options.
82
The leases for the other facilities have a term of ten years. In
December 2007, we amended the lease for the Schweinfurt, Germany
facility, to add additional manufacturing capacity. Based upon
an appraisal, we believe that the rents under the leases
represent fair market value for such properties. For information
with respect to our principal properties in Germany, see
“Item 4.D. Property, plants and equipment.”
Trademarks
Fresenius SE continues to own the name and mark
“Fresenius” and its “F” logo. Fresenius SE
and Fresenius Medical Care Deutschland GmbH, one of our German
subsidiaries, have entered into agreements containing the
following provisions. Fresenius SE has granted to our German
subsidiary, for our benefit and that of our affiliates, an
exclusive, worldwide, royalty-free, perpetual license to use
“Fresenius Medical Care” in our company names, and to
use the Fresenius marks, including some combination marks
containing the Fresenius name that were used by Fresenius
Worldwide Dialysis, and the Fresenius Medical Care name as a
trade name, in all aspects of the renal business. Our German
subsidiary, for our benefit and that of our affiliates, has also
been granted a worldwide, royalty-free, perpetual license:
|
|
|
|
|
|
•
|
to use the “Fresenius Medical Care” mark in the then
current National Medical Care non-renal business if it is used
as part of “Fresenius Medical Care” together with one
or more descriptive words, such as “Fresenius Medical Care
Home Care” or “Fresenius Medical Care
Diagnostics”;
|
|
|
|
|
•
|
to use the “F” logo mark in the National Medical Care
non-renal business, with the consent of Fresenius SE. That
consent will not be unreasonably withheld if the mark using the
logo includes one or more additional descriptive words or
symbols; and
|
|
|
|
|
•
|
to use “Fresenius Medical Care” as a trade name in the
renal business
|
We and our affiliates have the right to use “Fresenius
Medical Care” as a trade name in other medical businesses
only with the consent of Fresenius SE. Fresenius SE may not
unreasonably withhold its consent. In the U.S. and Canada,
Fresenius SE will not use “Fresenius” or the
“F” logo as a trademark or service mark, except that
it is permitted to use “Fresenius” in combination with
one or more additional words such as “Pharma Home
Care” as a service mark in connection with its home care
business and may use the “F” logo as a service mark
with the consent of our principal German subsidiary. Our
subsidiary will not unreasonably withhold its consent if the
service mark includes one or more additional descriptive words
or symbols. Similarly, in the U.S. and Canada, Fresenius SE has
the right to use “Fresenius” as a trade name, but not
as a mark, only in connection with its home care and other
medical businesses other than the renal business and only in
combination with one or more other descriptive words, provided
that the name used by Fresenius SE is not confusingly similar to
our marks and trade names. Fresenius SE’s ten-year covenant
not to compete with us, granted in 1996, has expired, and
Fresenius SE may use “Fresenius” in its corporate
names if it is used in combination with one or more additional
distinctive word or words, provided that the name used by
Fresenius SE is not confusingly similar to the Fresenius Medical
Care marks or corporate or trade names.
Other
Intellectual Property
Some of the patents, patent applications, inventions, know-how
and trade secrets that Fresenius Worldwide Dialysis used prior
to our formation were also used by other divisions of Fresenius
SE. For Biofine, the polyvinyl chloride-free packaging material,
Fresenius SE has granted to our principal German subsidiary, for
our benefit and for the benefit of our affiliates, an exclusive
license for the renal business and a non-exclusive license for
all other fields except other non-renal medical businesses. Our
German subsidiary and Fresenius SE share equally any royalties
from licenses of the Biofine intellectual property by either our
German subsidiary or by Fresenius SE to third parties outside
the renal business and the other non-renal medical businesses.
In addition, Fresenius SE transferred to our German subsidiary
the other patents, patent applications, inventions, know-how and
trade secrets that were used predominantly in Fresenius
SE’s dialysis business. In certain cases Fresenius
Worldwide Dialysis and the other Fresenius SE divisions as a
whole each paid a significant part of the development costs for
patents, patent applications, inventions, know-how and trade
secrets that were used by both prior to the merger. Where our
German subsidiary acquired those jointly funded patents, patent
applications, inventions, know-how and trade secrets, our
subsidiary licensed them back to Fresenius SE exclusively in the
other non-renal medical businesses and non-exclusively in all
other fields. Where Fresenius SE retained the jointly funded
patents, patent applications, inventions, know-how and trade
secrets, Fresenius SE licensed them to our German subsidiary
exclusively in the renal business and non-exclusively in all
other fields.
83
Supply
Agreements and Arrangements
We produce most of our products in our own facilities. However,
Fresenius Kabi AG, a subsidiary of Fresenius SE, manufactures
some of our products for us, principally dialysis concentrates
and other solutions. These facilities are located in Germany,
Brazil, France and South Africa. Conversely, our facilities in
Germany and Italy produce products for Fresenius Kabi AG.
Our local subsidiaries and those of Fresenius SE have entered
into supply agreements for the purchase and sale of products
from the above facilities. Prices under the supply agreements
are determined by good-faith negotiation between the parties.
During 2009, we sold products to Fresenius SE in the amount of
$13.6 million. In 2009, we made purchases from Fresenius SE
in the amount of $43.3 million.
The parties may modify existing or enter into additional supply
agreements, arrangements and transactions. Any future
modifications, agreements, arrangements and transactions will be
negotiated between the parties and will be subject to the
approval provisions of the pooling agreements and the regulatory
provisions of German law regarding dominating enterprises.
In January and February 2008, Baxter Healthcare Corporation
and/or
its
parent corporation, Baxter International, Inc., issued recalls
and suspended production of its sodium heparin injection
products in response to reports of adverse patient reactions.
Heparin is a blood thinning drug that is widely and routinely
used in the treatment of dialysis patients to prevent
life-threatening blood clots. Prior to the recalls, FMCH
purchased a majority of its heparin requirements from Baxter. As
a result of the recalls, APP Pharmaceuticals, Inc. (“APP
Inc.”), is the only remaining U.S. supplier of FDA-approved
heparin used in dialysis. APP Inc. has substantially increased
FMCH’s acquisition costs for this product. On
September 10, 2008, Fresenius Kabi AG, a wholly-owned
subsidiary of Fresenius SE, acquired APP Inc. The acquisition
has had no impact on the purchase price of heparin. FMCH
currently purchases heparin supplied by APP Inc. through
MedAssets, Inc. MedAssets Inc. is a publicly-traded U.S.
corporation that provides inventory purchasing services to
healthcare providers through a group purchasing organization
(GPO) structure. A GPO is an organization that endeavors to
manage supply and service costs for hospitals and health care
providers by negotiating discounted prices with manufacturers,
distributors and other vendors. Vendors discount their prices
and pay administrative fees to GPOs because GPOs provide access
to a large customer base, thus reducing vendors’ sales and
marketing costs and overhead. FMCH is one of many U.S.
healthcare providers that participate in the MedAssets GPO. FMCH
purchases pharmaceuticals and supplies used in its dialysis
services business through the MedAssets GPO contract. During
2009, we acquired $31.3 million of heparin from APP Inc.
through the GPO.
During the third quarter of 2009, we acquired production lines
from Fresenius SE for a purchase price of $3.4 million, net
of value added tax (VAT).
Services
Agreement
We obtain administrative and other services from Fresenius SE
headquarters and from other divisions and subsidiaries of
Fresenius SE. These services relate to, among other things,
administrative services, management information services,
employee benefit administration, insurance, IT services, tax
services and treasury services. For 2009, Fresenius SE and its
affiliates charged us approximately $68.2 million for these
services. Conversely, we have provided certain services to other
divisions and subsidiaries of Fresenius SE relating to research
and development, central purchasing, patent administration and
warehousing. For 2009 we charged approximately
$13.5 million to Fresenius SE and its subsidiaries for
services we rendered to them.
We and Fresenius SE may modify existing or enter into additional
services agreements, arrangements and transactions. Any such
future modifications, agreements, arrangements and transactions
will be negotiated between the parties and will be subject to
the approval provisions of the pooling agreements and the
regulations of German law regarding dominating enterprises.
Financing
We are party to an Amended and Restated Subordinated Loan Note
with Fresenius SE under which we or our subsidiaries may request
and receive one or more advances up to an aggregate amount of
$400 million during the period ending March 31, 2011.
See Note 9 of the Notes to Consolidated Financial
Statements, “Short-Term Borrowings, Other Financial
Liabilities and Short-Term Borrowings from Related
Parties — Short-Term Borrowings from Related
Parties.” During 2009, we received advances between
€1.3 million and €72.0 million which carried
interest at rates between 1.05% and 2.05% per annum. On
December 31, 2009, the Company had no advances outstanding
due to Fresenius SE. On August 19, 2009, the Company
borrowed $2.2 million from the general partner at 1.335%,
due August 19, 2010.
84
We were party to a German consolidated trade tax return with
Fresenius SE and certain of its German subsidiaries for the
fiscal years
1998-2001.
During the second quarter of 2009, we reclassified an accounts
payable relating to taxes payable by the Company in the amount
of €77.7 million ($110 million at June 30,
2009) to Fresenius SE to short-term borrowings from related
parties. Of this amount, €5.7 million
($8.3 million at December 31, 2009) was
outstanding at December 31, 2009 and will be repaid in 2010
with an interest rate of 6%.
On November 7, 2008, we entered into a loan agreement with
Fresenius SE under which we advanced Fresenius SE
$50 million at 6.45% interest. The loan was fully repaid on
April 30, 2009.
Other
Interests
Dr. Gerd Krick, chairman of the Supervisory Board of
FMC-AG & Co. KGaA and member of the supervisory board
of Management AG, was a member of the administration board of
Dresdner Bank, Luxembourg, S.A., a subsidiary of Dresdner Bank
AG. See “— Security Ownership of Certain
Beneficial Owners of Fresenius SE.” Dresdner Bank AG,
through its New York and Cayman branches, was a documentation
agent and was one of the joint lead arrangers and book managers
under our senior credit agreement in effect prior to our current
2006 Senior Credit Agreement. Dr. Dieter Schenk, Vice
Chairman of the Supervisory Boards of Management AG and of
FMC-AG Co. KGaA and a member of the Supervisory Board of
Fresenius SE, is a partner in the law firm of Noerr LLP
(formerly Nörr Stiefenhofer Lutz Partnerschaft), which has
provided legal services to Fresenius SE and Fresenius Medical
Care. The portion of said legal services to Fresenius Medical
Care for the period January 1, 2009 through
September 30, 2009, has been approved by our supervisory
board, with Dr. Schenk abstaining from the vote. Services
for the fourth quarter 2009 will be reviewed in the first
quarter 2010. During 2009, Noerr LLP was paid approximately
$1.4 million for these services. Dr. Schenk is one of
the executors of the estate of the late Mrs. Else
Kröner. Else Kröner-Fresenius-Stiftung, a charitable
foundation established under the will of the late
Mrs. Kröner, owns the majority of the voting shares of
Fresenius SE. Dr. Schenk is also the chairman of the
advisory board of Else-Kröner-Fresenius-Stiftung. See
“— Security Ownership of Certain Beneficial
Owners of Fresenius SE.”
Under the articles of association of FMC AG & Co.
KGaA, we are obligated to pay Fresenius SE a guaranteed return
on its capital investment in our general partner. See
Item 1.6G, “Corporate Governance — The Legal
Structure of FMC AG & Co. KGaA,” below.
General
Partner Reimbursement
Management AG, the Company’s general partner, is a 100%
wholly-owned subsidiary of Fresenius SE. The Company’s
Articles of Association provide that the General Partner shall
be reimbursed for any and all expenses in connection with
management of the Company’s business, including
compensation of the members of the General Partner’s
supervisory board and the General Partner’s management
board. The aggregate amount reimbursed to Management AG for 2009
was approximately $7.8 million for its management services
during 2009 including $0.08 million as compensation for
their exposure to risk as General Partner. The Company’s
Articles of Association fix this compensation as a guaranteed
return of 4% of the amount of the General Partner’s
invested capital (€1.5 million). See Item 16G
“Governance — The Legal Structure of
FMC-AG & Co. KGaA” below.
Item 8.
Financial
information
The information called for by parts 8.A.1 through 8.A.6 of this
item is in the section beginning on
Page F-1.
8.A.7. Legal
Proceedings
The information in Note 18 of the Notes to Consolidated
Financial Statements, “Legal Proceedings,” in
Part III, Item 18 of this report is incorporated by
this reference in response to this item. For information
regarding certain tax audits and related claims, see
Note 16 of the Notes to Consolidated Financial Statements,
“Income Taxes.”
8.A.8. Dividend
Policy
We generally pay annual dividends on both our preference shares
and our ordinary shares in amounts that we determine on the
basis of Fresenius Medical Care AG & Co. KGaA’s
prior year unconsolidated earnings as shown in the statutory
financial statements that we prepare under German law on the
basis of the accounting principles of the German Commercial Code
(
Handelsgesetzbuch
or
HGB
), subject to
authorization by a resolution to be passed at our general
meeting of shareholders. Under our articles of association, the
minimum dividend payable on the preference shares is €0.04
per share and, if we declare dividends, holders of our
preference shares must receive €0.02 per share
85
more than the dividend on an ordinary share. Under German law,
we must, in all cases, pay the annual dividend declared on our
preference shares before we pay dividends declared on our
ordinary shares.
The general partner and our Supervisory Board propose dividends
and the shareholders approve dividends for payment in respect of
a fiscal year at the Annual General Meeting in the following
year. Since all of our shares are in bearer form, we remit
dividends to the depositary bank (
Depotbank
) on behalf of
the shareholders.
Our 2006 Senior Credit Agreement and outstanding euro notes, as
well as the senior subordinated indentures relating to our trust
preferred securities, restrict our ability to pay dividends.
Item 5.B, “Operating and Financial Review and
Prospects — Liquidity and Capital Resources” and
the notes to our consolidated financial statements appearing
elsewhere in this report discuss this restriction.
The table below provides information regarding the annual
dividend per share that we paid on our Preference shares and
Ordinary shares. These payments were paid in the years shown for
the results of operations in the year preceding the payment.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Per Share Amount
|
|
2009
|
|
2008
|
|
2007
|
|
|
|
Preference share
|
|
€
|
0.60
|
|
|
€
|
0.56
|
|
|
€
|
0.49
|
|
|
Ordinary share
|
|
€
|
0.58
|
|
|
€
|
0.54
|
|
|
€
|
0.47
|
|
We have announced that the general partner’s Management
Board and our Supervisory Board have proposed dividends for 2009
payable in 2010 of €0.63 per preference share and
€0.61 per ordinary share. These dividends are subject to
approval by our shareholders at our Annual General Meeting to be
held on May 11, 2010.
Except as described herein, holders of ADSs will be entitled to
receive dividends on the ordinary shares and the preference
shares represented by the respective ADSs. We will pay any cash
dividends payable to such holders to the depositary in euros
and, subject to certain exceptions, the depositary will convert
the dividends into U.S. dollars. See Item 10,
“Additional Information —Description of American
Depositary Receipts — Share Dividends and Other
Distributions.” Fluctuations in the exchange rate between
the U.S. dollar and the euro will affect the amount of dividends
that ADS holders receive. Dividends paid on the preference
shares and dividends paid to holders and beneficial holders of
the ADSs will be subject to deduction of German withholding tax.
You can find a discussion of German withholding tax below in
“Item 10.E. Taxation”.
Item 9.
The
Offer and Listing Details
A.4. and
C. Information regarding the trading markets for price history
of our stock
Trading
Markets
The principal trading market for our ordinary shares and the
preference shares is the Frankfurt Stock Exchange
(FWB
®
Frankfurter Wertpapierbörse). All ordinary shares and
preference shares have been issued in bearer form. Accordingly,
we face difficulties determining precisely who our holders of
ordinary and preference shares are or how many shares any
particular shareholder owns, with the exception of the number of
shares held in ADR form in the United States. For more
information regarding ADRs see “Item 10.B. Memorandum
and articles of association — Description of American
Depositary Receipts.” However, under the German Securities
Trading Act, holders of voting securities of a German company
listed on a stock exchange within the EU are obligated to notify
the company of certain levels of holdings as described in
“Item 7.A. Major Shareholders”. Additionally,
persons discharging managerial responsibilities and affiliated
persons are obliged to notify the supervising authority and the
Company of trades in their shares in excess of €5,000 in
any year. The ordinary shares of Fresenius Medical Care AG had
been listed on the Frankfurt Stock Exchange since
October 2, 1996, the preference shares since
November 25, 1996. Trading in the ordinary shares and
preference shares of FMC-AG & Co. KGaA on the
Frankfurt Stock Exchange commenced on February 13, 2006.
Our shares have been listed on the Official Market (Amtlicher
Markt) of the Frankfurt Stock Exchange, which has been combined
with another market and renamed as the Regulated Market
(Regulierter Markt) as of November 1, 2007, and on the
sub-segment
Prime Standard of the Regulated Market. The Prime Standard is a
sub-segment
of the Regulated Market with additional post-admission
obligations. Admission to the Prime Standard requires the
fulfillment of the following transparency criteria: publication
of quarterly reports; preparation of financial statements in
accordance with international accounting standards (IFRS or U.S.
GAAP); publication of a company calendar; convening of at least
one analyst conference per year; and publication of ad-hoc
messages (i.e., certain announcements of material developments
and events) in English. Companies aiming to be listed in this
segment have to apply for admission. Listing in the Prime
Standard is a prerequisite for inclusion of shares in the
selection indices of the Frankfurt Stock Exchange, such as the
DAX
®
,
the index of 30 major German stocks.
86
Since October 1, 1996, ADSs each originally representing
one-third of an Ordinary share and, commencing June 15,
2007, each representing one Ordinary share (the “Ordinary
ADSs”), have been listed and traded on the New York Stock
Exchange (“NYSE”) under the symbol FMS. Since
November 25, 1996, ADSs, each originally representing
one-third of a Preference share and, commencing June 15,
2007, each representing one Preference share (the
“Preference ADSs”), have been listed and traded on the
NYSE under the symbol FMS/P. At December 31, 2009, there
were 84,341 preference ADSs outstanding. Accordingly, while the
preference ADSs remain listed on the New York Stock Exchange,
the trading market for the preference ADSs is highly illiquid.
In addition, in connection with the New Your Stock Exchange
listing of our ADSs upon consummation of our transformation and
the related conversion offer, the New York Stock Exchange
advised us that if the number of publicly held preference ADSs
falls below 100,000, which has occurred, the preference ADSs
could be delisted. The Depositary for both the Ordinary ADSs and
the Preference ADSs is Bank of New York Mellon (the
“Depositary”).
Trading
on the Frankfurt Stock Exchange
Deutsche Börse AG operates the Frankfurt Stock Exchange,
which is the most significant of the six German stock exchanges.
As of December 2009, the most recent figures available, the
shares of more than 10,000 companies traded on the Regulated
Market and the Regulated Unofficial Market of the Frankfurt
Stock Exchange.
Trading on the floor of the Frankfurt Stock Exchange begins
every business day at 9:00 a.m. and ends at 8:00 p.m.,
Central European Time (“CET”). In floor trading, lead
brokers are responsible for price determination and quotation
for the shares supported by them. The order book in which all
buy and sell orders are compiled serves as their basis. Thereby,
only one lead broker is in charge of each security. A
performance measurement for price determination on the floor
includes minimum requirements and therefore ensures
|
|
|
|
|
|
•
|
permanent quotation during trading hours
|
|
|
|
|
•
|
best price execution (in terms of spread and speed)
|
|
|
|
|
•
|
full execution.
|
Our shares are traded on
Xetra
®
,
the electronic trading system of the Deutsche Börse, in
addition to being traded on the Frankfurt floor. The trading
hours for Xetra are between 9:00 a.m. and 5:30 p.m.
CET. Only brokers and banks that have been admitted to Xetra by
the Frankfurt Stock Exchange have direct access to the system
and may trade on it. Private investors can trade on Xetra
through their banks and brokers.
Deutsche Börse AG publishes information for all traded
securities on the Internet,
http://www.deutsche-boerse.com.
Transactions on Xetra and the Frankfurt Stock Exchange settle on
the second business day following the trade except for trades
executed on Xetra International Markets, the European Blue Chip
segment of Deutsche Börse AG, which settle on the third
business day following a trade. The Frankfurt Stock Exchange can
suspend a quotation if orderly trading is temporarily endangered
or if a suspension is deemed to be necessary to protect the
public.
The Hessian Stock Exchange Supervisory Authority (Hessische
Börsenaufsicht) and the Trading Monitoring Unit of the
Frankfurt Stock Exchange (HÜST
Handelssüberwachungsstelle) both monitor trading on the
Frankfurt Stock Exchange.
The Federal Financial Supervisory Authority (
Bundesanstalt
für Finanzdienstleistungsaufsicht
), an independent
federal authority, is responsible for the general supervision of
securities trading pursuant to provisions of the German
Securities Trading Act (
Wertpapierhandelsgesetz
) and
other laws.
The table below sets forth for the periods indicated, the high
and low closing sales prices in euro for the Ordinary shares and
the Preference shares on the Frankfurt Stock Exchange, as
reported by the Frankfurt Stock Exchange Xetra system. Since
January 4, 1999, all shares on German stock exchanges trade
in euro. All share prices have been adjusted to reflect our
one-for-three
share splits in June 2007.
87
As of February 19, 2010, the share prices for the Ordinary
and Preference shares traded on the Frankfurt Stock Exchange
were €37.28 and €29.70, respectively.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Price per
|
|
|
Price per
|
|
|
|
|
|
|
|
ordinary share (€)
|
|
|
preference share (€)
|
|
|
|
|
|
|
|
High
|
|
|
Low
|
|
|
High
|
|
|
Low
|
|
|
|
|
|
2010
|
|
|
January
|
|
|
37.85
|
|
|
|
36.10
|
|
|
|
31.70
|
|
|
|
29.70
|
|
|
|
2009
|
|
|
December
|
|
|
37.70
|
|
|
|
35.90
|
|
|
|
31.90
|
|
|
|
30.50
|
|
|
|
|
|
|
November
|
|
|
36.00
|
|
|
|
32.80
|
|
|
|
33.50
|
|
|
|
31.30
|
|
|
|
|
|
|
October
|
|
|
33.90
|
|
|
|
31.90
|
|
|
|
33.00
|
|
|
|
30.90
|
|
|
|
|
|
|
September
|
|
|
34.20
|
|
|
|
30.30
|
|
|
|
33.50
|
|
|
|
28.70
|
|
|
|
|
|
|
August
|
|
|
32.80
|
|
|
|
30.00
|
|
|
|
33.00
|
|
|
|
29.90
|
|
|
|
2009
|
|
|
Fourth Quarter
|
|
|
37.71
|
|
|
|
31.86
|
|
|
|
33.46
|
|
|
|
30.50
|
|
|
|
|
|
|
Third Quarter
|
|
|
34.20
|
|
|
|
30.03
|
|
|
|
34.00
|
|
|
|
28.72
|
|
|
|
|
|
|
Second Quarter
|
|
|
32.19
|
|
|
|
27.64
|
|
|
|
30.00
|
|
|
|
25.24
|
|
|
|
|
|
|
First Quarter
|
|
|
35.48
|
|
|
|
27.07
|
|
|
|
35.30
|
|
|
|
25.60
|
|
|
|
2008
|
|
|
Fourth Quarter
|
|
|
37.75
|
|
|
|
31.42
|
|
|
|
34.50
|
|
|
|
28.51
|
|
|
|
|
|
|
Third Quarter
|
|
|
38.27
|
|
|
|
33.54
|
|
|
|
36.38
|
|
|
|
32.30
|
|
|
|
|
|
|
Second Quarter
|
|
|
36.10
|
|
|
|
31.18
|
|
|
|
34.60
|
|
|
|
29.90
|
|
|
|
|
|
|
First Quarter
|
|
|
39.10
|
|
|
|
29.73
|
|
|
|
37.60
|
|
|
|
28.31
|
|
|
|
2009
|
|
|
Annual
|
|
|
37.71
|
|
|
|
26.07
|
|
|
|
35.30
|
|
|
|
25.24
|
|
|
|
2008
|
|
|
Annual
|
|
|
39.10
|
|
|
|
29.73
|
|
|
|
37.60
|
|
|
|
28.31
|
|
|
|
2007
|
|
|
Annual
|
|
|
38.67
|
|
|
|
33.05
|
|
|
|
36.78
|
|
|
|
31.32
|
|
|
|
2006
|
|
|
Annual
|
|
|
36.30
|
|
|
|
27.50
|
|
|
|
33.83
|
|
|
|
25.03
|
|
|
|
2005
|
|
|
Annual
|
|
|
29.82
|
|
|
|
19.12
|
|
|
|
26.44
|
|
|
|
13.87
|
|
The average daily trading volume of the Ordinary shares and the
Preference shares traded on the Frankfurt Stock Exchange during
2009 was 1,040,200 shares and 1,144 shares,
respectively. The foregoing numbers are based on total yearly
turnover statistics supplied by the Frankfurt Stock Exchange.
Trading
on the New York Stock Exchange
As of February 19, 2010, the share prices for the Ordinary
and Preference shares traded on the NYSE were $50.79 and $42.79,
respectively.
The table below sets forth, for the periods indicated, the high
and low closing sales prices for the Ordinary ADSs and the
Preference ADSs on the NYSE:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Price per
|
|
|
|
|
|
|
|
|
|
|
ordinary ADS ($)
|
|
|
Price per preference ADS ($)
|
|
|
|
|
|
|
|
High
|
|
|
Low
|
|
|
High
|
|
|
Low
|
|
|
|
|
|
2010
|
|
|
January
|
|
|
54.60
|
|
|
|
50.40
|
|
|
|
45.60
|
|
|
|
42.70
|
|
|
|
2009
|
|
|
December
|
|
|
55.00
|
|
|
|
52.80
|
|
|
|
48.30
|
|
|
|
44.80
|
|
|
|
|
|
|
November
|
|
|
54.30
|
|
|
|
48.50
|
|
|
|
49.60
|
|
|
|
45.70
|
|
|
|
|
|
|
October
|
|
|
50.30
|
|
|
|
47.60
|
|
|
|
47.40
|
|
|
|
45.70
|
|
|
|
|
|
|
September
|
|
|
50.30
|
|
|
|
43.40
|
|
|
|
49.20
|
|
|
|
45.00
|
|
|
|
|
|
|
August
|
|
|
47.00
|
|
|
|
42.30
|
|
|
|
45.00
|
|
|
|
45.00
|
|
|
|
2009
|
|
|
Fourth Quarter
|
|
|
54.96
|
|
|
|
47.57
|
|
|
|
49.60
|
|
|
|
44.75
|
|
|
|
|
|
|
Third Quarter
|
|
|
50.29
|
|
|
|
42.31
|
|
|
|
49.18
|
|
|
|
40.00
|
|
|
|
|
|
|
Second Quarter
|
|
|
45.04
|
|
|
|
36.94
|
|
|
|
50.00
|
|
|
|
32.00
|
|
|
|
|
|
|
First Quarter
|
|
|
47.67
|
|
|
|
35.66
|
|
|
|
43.00
|
|
|
|
35.00
|
|
|
|
2008
|
|
|
Fourth Quarter
|
|
|
52.22
|
|
|
|
39.84
|
|
|
|
50.00
|
|
|
|
28.87
|
|
|
|
|
|
|
Third Quarter
|
|
|
59.01
|
|
|
|
50.49
|
|
|
|
54.00
|
|
|
|
40.00
|
|
|
|
|
|
|
Second Quarter
|
|
|
56.25
|
|
|
|
49.53
|
|
|
|
48.00
|
|
|
|
45.00
|
|
|
|
|
|
|
First Quarter
|
|
|
57.59
|
|
|
|
45.98
|
|
|
|
55.00
|
|
|
|
45.00
|
|
|
|
2009
|
|
|
Annual
|
|
|
54.96
|
|
|
|
35.66
|
|
|
|
50.00
|
|
|
|
32.00
|
|
|
|
2008
|
|
|
Annual
|
|
|
59.01
|
|
|
|
39.84
|
|
|
|
55.00
|
|
|
|
28.87
|
|
|
|
2007
|
|
|
Annual
|
|
|
56.70
|
|
|
|
43.69
|
|
|
|
53.50
|
|
|
|
40.00
|
|
|
|
2006
|
|
|
Annual
|
|
|
47.60
|
|
|
|
34.50
|
|
|
|
40.00
|
|
|
|
31.00
|
|
|
|
2005
|
|
|
Annual
|
|
|
35.22
|
|
|
|
25.09
|
|
|
|
31.20
|
|
|
|
18.16
|
|
88
Item 10.
Additional
information
|
|
|
|
B.
|
Articles
of Association
|
FMC-AG & Co. KGaA is a partnership limited by shares
(
Kommanditgesellschaft auf Aktien
) organized under the
laws of Germany. FMC-AG & Co. KGaA is registered with
the commercial register of the local court
(Amtsgericht)
of Hof an der Saale, Germany under HRB 4019. Our registered
office
(Sitz)
is Hof an der Saale, Germany. Our business
address is Else-Kröner-Strasse 1, 61352 Bad Homburg,
Germany,
telephone +49-6172-609-0.
The following summary of the material provisions of our articles
of association is qualified in its entirety by reference to the
complete text of our articles of association. An English
convenience translation of our articles of association has been
filed with the Securities and Exchange Commission and can also
be found on our website under
www.fmc-ag.com
. For a
summary of certain other provisions of our Articles of
Association relating to management by our general partner and
required ownership of our share capital by the shareholder of
our general partner, See Item 16.G,
“Governance — the Articles of Association of
FMC-AG & Co. KGaA” above.
Corporate
Purposes
Under our articles of association, our business purposes are:
|
|
|
|
|
|
•
|
the development, production and distribution of as well as the
trading in health care products, systems and procedures,
including dialysis;
|
|
|
|
|
•
|
the projecting, planning, establishment, acquisition and
operation of health care businesses, including dialysis centers,
also in separate enterprises or through third parties as well as
the participation in such dialysis centers;
|
|
|
|
|
•
|
the development, production and distribution of other
pharmaceutical products and the provision of services in this
field;
|
|
|
|
|
•
|
the provision of advice in the medical and pharmaceutical areas
as well as scientific information and documentation;
|
|
|
|
|
•
|
the provision of laboratory services for dialysis and
non-dialysis patients and homecare medical services.
|
We conduct our business directly and through subsidiaries within
and outside Germany.
General
Information Regarding Our Share Capital
As of February 19, 2010, our share capital consists of
€299,636,822, divided into 295,746,635 bearer ordinary
shares without par value (
Stückaktien
) and 3,890,187
bearer non-voting preference shares without par value
(
Stückaktien
). Our share capital has been fully paid
in.
All shares of FMC-AG & Co. KGaA are in bearer form.
Our shares are deposited as share certificates in global form
(
Sammelurkunden
) with Clearstream Banking AG, Frankfurt
am Main. Shareholders are not entitled to have their
shareholdings issued in certificated form. All shares of
FMC-AG & Co. KGaA are freely transferable, subject to
any restrictions imposed by applicable securities laws.
General
provisions on Increasing the Capital of Stock Corporations and
Partnerships Limited by Shares
Under the German Stock Corporation Act (
Aktiengesetz
),
the capital of a stock corporation or of a partnership limited
by shares may be increased by a resolution of the general
meeting, passed with a majority of three quarters of the capital
represented at the vote, unless the articles of association of
the stock corporation or the partnership limited by shares
provide for a different majority.
In addition, the general meeting of a stock corporation or a
partnership limited by shares may create authorized capital
(also called approved capital) (
genehmigtes Kapital
). The
resolution creating authorized capital requires the affirmative
vote of a majority of three quarters of the capital represented
at the vote and may authorize the management board to issue
shares up to a stated amount for a period of up to five years.
The nominal value of the authorized capital may not exceed half
of the share capital at the time of the authorization.
In addition, the general meeting of a stock corporation or of a
partnership limited by shares may create conditional capital
(
bedingtes Kapital
) for the purpose of issuing
(i) shares to holders of convertible bonds or other
securities which grant a right to shares, (ii) shares as
consideration to prepare a merger with another company, or
(iii) shares offered to members of the management board or
employees of the company or of an affiliated company. In each
case, the authorizing resolution requires the affirmative vote
of a majority of three quarters of the capital
89
represented at the vote. The nominal value of the conditional
capital may not exceed half or, in the case of conditional
capital created for the purpose of issuing shares to members of
the management board and employees, 10% of the company’s
share capital at the time of the resolution.
In a partnership limited by shares all resolutions increasing
the capital of the partnership limited by shares also require
the consent of the general partner for their effectiveness.
Voting
Rights
Each ordinary share entitles the holder thereof to one vote at
general meetings of shareholders of FMC-AG & Co. KGaA.
Resolutions are passed at an ordinary general or an
extraordinary general meeting of our shareholders by a majority
of the votes cast, unless a higher vote is required by law or
our articles of association. By statute, Fresenius SE as
shareholder of the general partner is not entitled to vote its
ordinary shares in the election or removal of members of the
supervisory board of FMC-AG & Co. KGaA, the
ratification of the acts of the general partners and members of
the supervisory board, the appointment of special auditors, the
assertion of compensation claims against members of the
executive bodies arising out of the management of the Company,
the waiver of compensation claims and the appointment of
auditors. In the case of resolutions regarding such matters
Fresenius SE’s voting rights may not be exercised by any
other person.
Our preference shares do not have any voting rights, except as
otherwise regulated by law. If we do not pay the minimum annual
dividend payable on the preference shares for any year in the
following year, and we do not pay both the dividend arrearage
and the dividend payable on the preference shares for such
following year in full in the next following year, then the
preference shares shall have the same voting rights as the
ordinary shares (one vote for each share held or for each ADS
held) until all preference share dividend arrearages are fully
paid up. In addition, holders of preference shares are entitled
to vote on most matters affecting their preferential rights,
such as changes in the rate of the preferential dividend. Any
such vote requires the affirmative vote of 75% of the votes cast
in a meeting of holders of preference shares.
Dividend
Rights
The general partner and our supervisory board will propose any
dividends for approval at the annual general meeting of
shareholders. Usually, shareholders vote on a recommendation
made by management (i.e., the general partner) and the
supervisory board as to the amount of dividends to be paid. Any
dividends are paid once a year, generally, immediately following
our annual general meeting.
Under German law, dividends may only be paid from our balance
sheet profits (
Bilanzgewinn
) as determined by our
unconsolidated annual financial statements as approved by our
annual general meeting of shareholders and the general partner.
Unlike our consolidated annual financial statements, which are
prepared on the basis of accounting principles generally
accepted in the United States of America (U.S. GAAP), the
unconsolidated annual financial statements referred to above are
prepared on the basis of the accounting principles of the German
Commercial Code (
Handelsgesetzbuch
or
HGB
). Since
our ordinary shares and our preference shares that are entitled
to dividend payments are held in a clearing system, the
dividends will be paid in accordance with the rules of the
individual clearing system. We will publish notice of the
dividends paid and the appointment of the paying agent or agents
for this purpose in the electronic version of the German Federal
Gazette (
elektronischer Bundesanzeiger
). If dividends are
declared, preference shareholders will receive €0.02 per
share more than the dividend payable on our ordinary shares, but
not less than €0.04 per share, according to our articles of
association. Under German law, we must pay the annual dividend
for our preference shares prior to paying any dividends on the
ordinary shares. If the profit shown on the balance sheet in one
or more fiscal years is not adequate to permit distribution of a
dividend of €0.04 per preference share, the shortfall
without interest must be made good out of the profit on the
balance sheet in the following fiscal year or years after
distribution of the minimum dividend on the preference shares
for that year or years and prior to the distribution of a
dividend on the ordinary shares. The right to this payment is an
integral part of the profit share of the fiscal year from which
the shortfall in the preference share dividend is made good.
In the case of holders of ADRs, the depositary will receive all
cash dividends and distributions on all deposited securities and
will, as promptly as practicable, distribute the dividends and
distributions to the holders of ADRs entitled to the dividend.
See “Description of American Depositary
Receipts — Share Dividends and Other
Distributions.”
90
Liquidation
Rights
Our company may be dissolved by a resolution of our general
shareholders’ meeting passed with a majority of three
quarters of our share capital represented at such general
meeting and the approval of the general partner. In accordance
with the German Stock Corporation Act (
Aktiengesetz
), in
such a case, any liquidation proceeds remaining after paying all
of our liabilities will be distributed among our shareholders in
proportion to the total number of shares held by each
shareholder. Our preference shares are not entitled to a
preference in liquidation.
Pre-emption
Rights
Under the German Stock Corporation Act, each shareholder in a
stock corporation or partnership limited by shares has a
preferential right to subscribe for any issue by that company of
shares, debt instruments convertible into shares, e.g.
convertible bonds or option bonds, and participating debt
instruments, e.g. profit participation rights or participating
certificates, in proportion to the number of shares held by that
shareholder in the existing share capital of the company. Such
pre-emption rights are freely assignable. These rights may also
be traded on German stock exchanges within a specified period of
time prior to the expiration of the subscription period. Our
general shareholders’ meeting may exclude pre-emption
rights by passing a resolution with a majority of at least three
quarters of our share capital represented at the general meeting
at which the resolution to exclude the pre-emption rights is
passed. In addition, an exclusion of pre-emption rights requires
a report by the general partner justifying the exclusion by
explaining why the interest of FMC-AG & Co. KGaA in
excluding the pre-emption rights outweighs our
shareholders’ interests in receiving such rights. However,
such justification is not required for any issue of new shares
if:
|
|
|
|
|
|
•
|
we increase our share capital against contributions in cash;
|
|
|
|
|
•
|
the amount of the capital increase does not exceed 10% of our
existing share capital; and
|
|
|
|
|
•
|
the issue price of the new shares is not significantly lower
than the price for the shares quoted on a stock exchange.
|
Exclusion
of Minority Shareholders
Under the provisions of Sections 327a et seq. of the German
Stock Corporation Act concerning squeeze-outs, a shareholder who
owns 95% of the issued share capital (a “principal
shareholder”) may request that the annual
shareholders’ meeting of a stock corporation or a
partnership limited by shares resolve to transfer the shares of
the other minority shareholders to the principal shareholder in
return for adequate cash compensation. In a partnership limited
by shares, the consent of the general partner(s) is not
necessary for the effectiveness of the resolution. The amount of
cash compensation to be paid to the minority shareholders must
take account of the issuer’s financial condition at the
time the resolution is passed. The full value of the issuer,
which is normally calculated using the capitalization of
earnings method (
Ertragswertmethode
), is decisive for
determining the compensation amount.
In addition to the provisions for squeeze-outs of minority
shareholders, Sections 319 et seq. of the German Stock
Corporation Act provides for the integration of stock
corporations. In contrast to the squeeze-out of minority
shareholders, integration is only possible when the future
principal company is a stock corporation with a stated domicile
in Germany. A partnership limited by shares can not be
integrated into another company.
General
Meeting
Our annual general meeting must be held within the first eight
months of each fiscal year at the location of FMC-AG &
Co. KGaA’s registered office, or in a German city where a
stock exchange is situated or at the location of a registered
office of a domestic affiliated company. To attend the general
meeting and exercise voting rights, shareholders must register
for the general meeting and prove ownership of shares. The
relevant reporting date is the beginning of the 21st day prior
to the general meeting.
Amendments
to the Articles of Association
An amendment to our articles of association requires both a
voting majority of 75% of the shares entitled to vote
represented at the general meeting and the approval of the
general partner.
91
Description
of American Depositary Receipts
General
The Bank of New York Mellon, a New York banking corporation, is
the depositary for our ordinary shares and preference shares.
Each American Depositary Share (ADS) represents an ownership
interest in one ordinary share or one preference share. The
deposited shares are deposited with a custodian, as agent of the
depositary, under the deposit agreements among ourselves, the
depositary and all of the ADS holders of the applicable class
from time to time. Each ADS also represents any securities, cash
or other property deposited with the depositary but not
distributed by it directly to ADS holders. The ADSs may be
evidenced by certificates called American depositary receipts or
ADRs. ADSs may also be uncertificated. If ADSs are issued in
uncertificated form, owners will receive periodic statements
from the depositary showing their ownership of ADSs.
The depositary’s office is located at 101 Barclay Street,
New York, NY 10286, U.S.A.
An investor may hold ADSs either directly or indirectly through
a broker or other financial institution. Investors who hold ADSs
directly, by having ADSs registered in their names on the books
of the depositary, are ADS holders. This description assumes an
investor holds ADSs directly. Investors who hold ADSs through
their brokers or financial institution nominees must rely on the
procedures of their brokers or financial institutions to assert
the rights of an ADS holder described in this section. Investors
should consult with their brokers or financial institutions to
find out what those procedures are.
As an ADS holder, we will not treat you as one of our
shareholders and you will not have shareholder rights. German
law governs shareholder rights. The depositary will be the
holder of the shares underlying your ADSs. As a registered
holder of ADSs, you will have ADS holder rights. The applicable
deposit agreement sets out ADS holder rights as well as the
rights and obligations of the depositary. New York law governs
the deposit agreements and the ADSs.
As of December 31, 2009, we had 84,341 preference share
ADSs outstanding. Accordingly, while the preference share ADSs
remain listed on the New York Stock Exchange, the trading market
for the preference share ADSs is highly illiquid. In addition,
the New York Stock Exchange has advised us that if the number of
publicly held preference share ADSs falls below 100,000
preference share ADSs could be delisted.
The following is a summary of the material terms of the deposit
agreements. Because it is a summary, it does not contain all the
information that may be important to investors. Except as
specifically noted, the description covers both ordinary share
ADSs and preference share ADSs. For more complete information,
investors should read the entire applicable deposit agreement
and the form of ADR of the relevant class which contains the
terms of the ADSs. Investors may obtain a copy of the deposit
agreements at the SEC’s Public Reference Room, located at
100 F Street N.E., Washington, D.C. 20549. Electronic
copies of the deposit agreements are also available on the
website maintained by the SEC,
www.sec.gov
.
Share
Dividends and Other Distributions
We may make different types of distributions with respect to our
ordinary shares and our preference shares. The depositary has
agreed to pay to investors the cash dividends or other
distributions it or the custodian receives on the shares or
other deposited securities, after deducting its fees and
expenses. Investors will receive these distributions in
proportion to the number of underlying shares of the applicable
class their ADSs represent.
Except as stated below, to the extent the depositary is legally
permitted it will deliver distributions to ADS holders in
proportion to their interests in the following manner:
|
|
|
|
|
|
•
|
Cash.
The depositary shall convert cash
distributions from foreign currency to U.S. dollars if this is
permissible and can be done on a reasonable basis. The
depositary will endeavor to distribute cash in a practicable
manner, and may deduct any taxes or other governmental charges
required to be withheld, any expenses of converting foreign
currency and transferring funds to the United States, and
certain other fees and expenses. In addition, before making a
distribution the depositary will deduct any taxes withheld. If
exchange rates fluctuate during a time when the depositary
cannot convert a foreign currency, investors may lose some or
all of the value of the distribution.
|
|
|
|
|
•
|
Shares.
If we make a distribution in shares,
the depositary may deliver additional ADSs to represent the
distributed shares, unless the number of ordinary shares or
preference shares represented by our ADSs is adjusted in
connection with the distribution. Only whole ADSs will be
issued. Any shares which would result in fractional ADSs will be
sold and the net proceeds will be distributed to the ADS holders
otherwise entitled to receive fractional ADSs.
|
92
|
|
|
|
|
|
•
|
Rights to receive additional shares.
In the
case of a distribution of pre-emption rights to subscribe for
ordinary shares or preference shares, or other subscription
rights, if we provide satisfactory evidence that the depositary
may lawfully distribute the rights, the depositary may arrange
for ADS holders to instruct the depositary as to the exercise of
the rights. However, if we do not furnish the required evidence
or if the depositary determines it is not practical to
distribute the rights, the depositary may:
|
|
|
|
|
|
|
•
|
allow the rights to lapse, in which case ADS holders will
receive nothing, or
|
|
|
|
|
•
|
sell the rights if practicable and distribute the net proceeds
as cash.
|
We have no obligation to file a registration statement under the
U.S. Securities Act of 1933, as amended (the “Securities
Act”) in order to make any rights available to ADS holders.
|
|
|
|
|
|
•
|
Other Distributions.
If we make a distribution
of securities or property other than those described above, the
depositary may either:
|
|
|
|
|
|
|
•
|
distribute the securities or property in any manner it deems
fair and equitable;
|
|
|
|
|
•
|
sell the securities or property and distribute any net proceeds
in the same way it distributes cash; or
|
|
|
|
|
•
|
hold the distributed property in which case the ADSs will also
represent the distributed property.
|
Any U.S. dollars will be distributed by checks drawn on a bank
in the United States for whole dollars and cents (fractional
cents will be rounded to the nearest whole cent).
The depositary may choose any practical method of distribution
for any specific ADS holder, including the distribution of
foreign currency, securities or property, or it may retain the
items, without paying interest on or investing them, on behalf
of the ADS holder as deposited securities.
The depositary is not responsible if it decides that it is
unlawful or impractical to make a distribution available to any
ADS holders.
There can be no assurance that the depositary will be able to
convert any currency at a specified exchange rate or sell any
property, rights, shares or other securities at a specified
price, or that any of these transactions can be completed within
a specified time period.
Deposit,
Withdrawal and Cancellation
The depositary will deliver ADSs if an investor or his broker
deposits ordinary shares or preference shares or evidence of
rights to receive ordinary shares or preference shares with the
custodian. Shares deposited with the custodian must be
accompanied by certain documents, including instruments showing
that such shares have been properly transferred or endorsed to
the person on whose behalf the deposit is being made.
The custodian will hold all deposited shares for the account of
the depositary. ADS holders thus have no direct ownership
interest in the shares and only have the rights that are
contained in the deposit agreements. The custodian will also
hold any additional securities, property and cash received on or
in substitution for the deposited shares. The deposited shares
and any additional items are referred to as “deposited
securities.”
Upon each deposit of shares, receipt of related delivery
documentation and compliance with the other provisions of the
deposit agreement, including the payment of the fees and charges
of the depositary and any taxes or other fees or charges owing,
the depositary will deliver ADSs of the applicable class in the
name of the person entitled to them.
All ADSs issued will, unless specifically requested to the
contrary, be delivered through the book-entry settlement system
of The Depository Trust Company, also referred to as DTC,
or be uncertificated and held through the depositary’s
book-entry direct registration system (“DRS”), and a
registered holder will receive periodic statements from the
depositary which will show the number of ADSs registered in the
holder’s name. An ADS holder can request that the ADSs not
be held through the depositary’s DRS and that an ADR be
issued to evidence those ADSs. ADRs will be delivered at the
depositary’s principal New York office or any other
location that it may designate as its transfer office.
Profile is a required feature of DRS which allows a participant
in DTC, claiming to act on behalf of a registered holder of
ADSs, to direct the depositary to register a transfer of those
ADSs to DTC or its nominee and to deliver those ADSs to the DTC
account of that DTC participant without receipt by the
depositary of prior authorization from the ADS registered holder
to register that transfer.
93
In connection with and in accordance with the arrangements and
procedures relating to DRS/Profile, the parties to the deposit
agreements understand that the depositary will not verify,
determine or otherwise ascertain that the DTC participant which
is claiming to be acting on behalf of an ADS registered holder
in requesting registration of transfer and delivery described in
the paragraph above has the actual authority to act on behalf of
the ADS registered holder (notwithstanding any requirements
under the Uniform Commercial Code). In the deposit agreements,
the parties agree that the depositary’s reliance on and
compliance with instructions received by the depositary through
the DRS/Profile System and in accordance with the deposit
agreement, shall not constitute negligence or bad faith on the
part of the depositary.
When an investor surrenders ADSs at the depositary’s
office, the depositary will, upon payment of certain applicable
fees, charges and taxes, and upon receipt of proper
instructions, deliver the whole number of ordinary shares or
preference shares represented by the ADSs turned in to the
account the investor directs within Clearstream Banking AG, the
central German clearing firm.
The depositary may restrict the withdrawal of deposited
securities only in connection with:
|
|
|
|
|
|
•
|
temporary delays caused by closing our transfer books or those
of the depositary, or the deposit of shares in connection with
voting at a shareholders’ meeting, or the payment of
dividends,
|
|
|
|
|
•
|
the payment of fees, taxes and similar charges, or
|
|
|
|
|
•
|
compliance with any U.S. or foreign laws or governmental
regulations relating to the ADRs.
|
This right of withdrawal may not be limited by any other
provision of the applicable deposit agreement.
Voting
Rights
You may instruct the depositary to vote the number of shares
your ADSs represent. The depositary will notify you of
shareholders’ meetings and arrange to deliver our voting
materials to you if we ask it to. Those materials will describe
the matters to be voted on and explain how you may instruct the
depositary how to vote. For instructions to be valid, they must
reach the depositary by a date set by the depositary.
The depositary will try, as far as practical, subject to German
law and the provisions of our constitutive documents, to vote
the number of shares or other deposited securities represented
by your ADSs as you instruct. The depositary will only vote or
attempt to vote as you instruct or as described below.
We cannot ensure that you will receive voting materials or
otherwise learn of an upcoming shareholders’ meeting in
time to ensure that you can instruct the depositary to vote your
shares. In addition, the depositary and its agents are not
responsible for failing to carry out voting instructions or for
the manner of carrying out voting instructions. This means that
you may not be able to vote and there may be nothing you can do
if your shares are not voted as you requested.
If (i) we timely asked the depositary to solicit your
voting instructions, (ii) the depositary receives a
recommendation as to how to vote from the custodian pursuant to
the German Stock Corporation Act before it mails voting
materials to ADS holders and (iii) the depositary does not
receive voting instructions from you by the specified date, it
will consider you to have authorized and directed it to give a
discretionary proxy to the custodian to vote the number of
deposited securities represented by your ADSs in accordance with
the custodian’s recommendation. The depositary will give a
discretionary proxy in those circumstances with respect to each
question covered by the recommendation unless we notify the
depositary that:
|
|
|
|
|
|
•
|
we do not wish a discretionary proxy to be given;
|
|
|
|
|
•
|
we think there is substantial shareholder opposition to the
particular question; or
|
|
|
|
|
•
|
we think the particular question would have an adverse impact on
our shareholders.
|
Fees and
Expenses
For information regarding fees and expenses payable by holders
of ADSs and amounts payable by the Depository to the Company,
see Item 12.D, “American Depositary Shares.”
Payment
of Taxes
ADS holders must pay any tax or other governmental charge
payable by the custodian or the depositary on any ADS or ADR,
deposited security or distribution. If an ADS holder owes any
tax or other governmental charge, the depositary may
(i) deduct the amount thereof from any cash distributions,
or (ii) sell deposited securities and deduct
94
the amount owing from the net proceeds of such sale. In either
case the ADS holder remains liable for any shortfall.
Additionally, if any tax or governmental charge is unpaid, the
depositary may also refuse to effect any registration,
registration of transfer,
split-up
or
combination of deposited securities or withdrawal of deposited
securities (except under limited circumstances mandated by
securities regulations). If any tax or governmental charge is
required to be withheld on any non-cash distribution, the
depositary may sell the distributed property or securities to
pay such taxes and distribute any remaining net proceeds to the
ADS holders entitled thereto.
Limitations on Obligations and Liability
Limits on
our Obligations and the Obligations of the Depositary; Limits on
Liability to Holders of ADSs
The deposit agreements expressly limit our obligations and the
obligations of the depositary. They also limit our liability and
the liability of the depositary. We and the depositary:
|
|
|
|
|
|
•
|
are only obligated to take the actions specifically set forth in
the applicable deposit agreement without negligence or bad faith;
|
|
|
|
|
•
|
are not liable if we are or it is prevented or delayed by law or
circumstances beyond our control from performing our or its
obligations under the applicable deposit agreement;
|
|
|
|
|
•
|
are not liable if we or it exercises discretion permitted under
the applicable deposit agreement;
|
|
|
|
|
•
|
have no obligation to become involved in a lawsuit or other
proceeding related to the ADSs or the applicable deposit
agreement on your behalf or on behalf of any other person; and
|
|
|
|
|
•
|
may rely upon any documents we believe or it believes in good
faith to be genuine and to have been signed or presented by the
proper person.
|
In the deposit agreements, we and the depositary agree to
indemnify each other under certain circumstances.
Requirements
for Depositary Actions
Before the depositary will deliver or register a transfer of an
ADS, make a distribution on an ADS, or permit withdrawal of
shares, the depositary may require:
|
|
|
|
|
|
•
|
payment of stock transfer or other taxes or other governmental
charges and transfer or registration fees charged by third
parties for the transfer of any shares or other deposited
securities;
|
|
|
|
|
•
|
satisfactory proof of the identity and genuineness of any
signature or other information it deems necessary; and
|
|
|
|
|
•
|
compliance with regulations it may establish, from time to time,
consistent with the deposit agreement, including presentation of
transfer documents.
|
The depositary may refuse to deliver ADSs or register transfers
of ADSs generally when the transfer books of the depositary or
our transfer books are closed or at any time if the depositary
or we think it advisable to do so.
Shareholder
communications; inspection of register of holders of
ADSs
The depositary will make available for your inspection at its
office all communications that it receives from us as a holder
of deposited securities that we make generally available to
holders of deposited securities. The depositary will send you
copies of those communications if we ask it to. You have a right
to inspect the register of holders of ADSs, but not for the
purpose of contacting those holders about a matter unrelated to
our business or the ADSs.
Description
of the Pooling Arrangements
Prior to the transformation of legal form of FMC-AG to
FMC-AG & Co. KGaA, FMC-AG, Fresenius SE and the
independent directors (as defined in the pooling agreements
referred to below) of FMC-AG were parties to two pooling
agreements for the benefit of the holders of our ordinary shares
and the holders of our preference shares (other than Fresenius
SE and its affiliates). Upon consummation of the conversion and
the transformation, we entered into pooling arrangements that we
believe provide similar benefits for the holders of the ordinary
shares and preference shares of FMC-AG & Co. KGaA. The
following is a summary of the material provisions of the pooling
arrangements which we have entered into with Fresenius SE and
our independent directors.
95
General
The pooling arrangements have been entered into for the benefit
of all persons who, from time to time, beneficially own our
ordinary shares, including owners of ADSs evidencing our
ordinary shares, other than Fresenius SE and its affiliates or
their agents and representatives, and persons from time to time
beneficially owning our preference shares, including (if the
preference ADSs are eligible for listing on the New York Stock
Exchange), ADSs evidencing our preference shares, other than
Fresenius SE and its affiliates or their agents and
representatives. Beneficial ownership is determined in
accordance with the beneficial ownership rules of the SEC.
Independent
Directors
Under the pooling arrangements, no less than one-third of the
supervisory board of Management AG, the general partner of
FMC-AG & Co. KGaA, must be independent directors, and
there must be at least two independent directors. Independent
directors are persons without a substantial business or
professional relationship with us, Fresenius SE, or any
affiliate of either, other than as a member of the supervisory
board of FMC-AG & Co. KGaA or as a member of the
supervisory board of Management AG. If an independent director
resigns, is removed, or is otherwise unable or unwilling to
serve in that capacity, a new person shall be appointed to serve
as an independent director in accordance with the provisions of
the articles of association of the general partner, and the
pooling arrangements, if as a result of the resignation or
removal the number of independent directors falls below the
required minimum. The provisions of the pooling agreement
relating to independent directors are in addition to the
functions of the joint committee established in connection with
the transformation of our legal form and conversion of our
preference shares, and are also in addition to the requirement
of
Rule 10A-3
under the Securities Exchange Act of 1934 that our audit
committee be composed solely of independent directors as defined
in that rule.
Extraordinary
Transactions
Under the pooling arrangements, we and our affiliates on the one
hand, and Management AG and Fresenius SE and their affiliates on
the other hand, must comply with all provisions of German law
regarding: any merger, consolidation, sale of all or
substantially all assets, recapitalization, other business
combination, liquidation or other similar action not in the
ordinary course of our business, any issuance of shares of our
voting capital stock representing more than 10% of our total
voting capital stock outstanding, and any amendment to our
articles of association which adversely affects any holder of
ordinary shares or preference shares, as applicable.
Interested
Transactions
We and Management AG and Fresenius SE have agreed that while the
pooling arrangements are in effect, a majority of the
independent directors must approve any transaction or contract,
or any series of related transactions or contracts, between
Fresenius SE, Management AG or any of their affiliates (other
than us or our controlled affiliates), on the one hand, and us
or our controlled affiliates, on the other hand, which involves
aggregate payments in any calendar year in excess of
€5 million for each individual transaction or
contract, or a related series of transactions or contracts.
However, approval is not required if the transaction or
contract, or series of related transactions or contracts, has
been described in a business plan or budget that a majority of
independent directors has previously approved. In any year in
which the aggregate amount of transactions that require approval
(or that would have required approval in that calendar year but
for the fact that such payment or other consideration did not
exceed €5 million) has exceeded €25 million,
a majority of the independent directors must approve all further
interested transactions involving more than
€2.5 million. However, approval is not required if the
transaction or contract, or series of related transactions or
contracts, has been described in a business plan or budget that
a majority of independent directors has previously approved.
Listing
of American Depositary Shares; SEC Filings
During the term of the pooling agreement, Fresenius SE has
agreed to use its best efforts to exercise its rights as the
direct or indirect holder of the general partner interest in
Fresenius Medical Care AG & Co. KGaA to cause us to,
and we have agreed to:
|
|
|
|
|
|
•
|
maintain the effectiveness of (i) the deposit agreement for
the ordinary shares, or a similar agreement, and to assure that
the ADSs evidencing the ordinary shares are listed on either the
New York Stock Exchange or the Nasdaq Stock Market and (ii),
while the preference ADSs are eligible for listing on the New
York Exchange or the Nasdaq Stock Market, the deposit agreement
for the preference shares, or a similar agreement, and to assure
that, if eligible for such listing, the ADSs evidencing the
preference shares are listed on either the New York Stock
Exchange or the Nasdaq Stock Market;
|
96
|
|
|
|
|
|
•
|
file all reports, required by the New York Stock Exchange or the
Nasdaq Stock Market, as applicable, the Securities Act, the
Securities Exchange Act of 1934, as amended, and all other
applicable laws;
|
|
|
|
|
•
|
prepare all financial statements required for any filing in
accordance with generally accepted accounting principles of the
U.S. (“U.S. GAAP”);
|
|
|
|
|
•
|
on an annual basis, prepare audited consolidated financial
statements in accordance with U.S. GAAP, and, on a quarterly
basis, prepare and furnish to the SEC consolidated financial
statements prepared in accordance with U.S. GAAP under cover of
form 6-K
or a comparable successor form;
|
|
|
|
|
•
|
furnish materials with the SEC with respect to annual and
special shareholder meetings under cover of
Form 6-K
and make the materials available to the depositary for
distribution to holders of ordinary share ADSs and, if we
maintain a preference share ADS facility, to holders of
preference share ADSs at any time that holders of preference
shares are entitled to voting rights; and
|
|
|
|
|
•
|
make available to the depositary for distribution to holders of
ADSs representing our ordinary shares and, if we maintain a
preference share ADS facility, ADSs representing our preference
shares on an annual basis, a copy of any report prepared by the
supervisory board or the supervisory board of the general
partner and provided to our shareholders generally pursuant to
Section 314(2) of the German Stock Corporation Act, or any
successor provision. These reports concern the results of the
supervisory board’s examination of the managing
board’s report on our relation with affiliated enterprises.
|
Term
The pooling arrangements will terminate if:
|
|
|
|
|
|
•
|
Fresenius SE or its affiliates acquire all our voting shares;
|
|
|
|
|
•
|
Fresenius SE’s beneficial ownership of our outstanding
share capital is reduced to less than 25%;
|
|
|
|
|
•
|
Fresenius SE or an affiliate of Fresenius SE ceases to own the
general partner interest in FMC-AG & Co. KGaA; or
|
|
|
|
|
•
|
we no longer meet the minimum threshold for obligatory
registration of the ordinary shares or ADSs representing our
ordinary shares and the preference shares or ADSs representing
our preference shares, as applicable, under
Section 12(g)(1) of the Securities Exchange Act of 1934, as
amended, and
Rule 12g-1
thereunder.
|
Amendment
Fresenius SE and a majority of the independent directors may
amend the pooling arrangements, provided, that beneficial owners
of 75% of the ordinary shares held by shareholders other than
Fresenius SE and its affiliates at a general meeting of
shareholders and 75% of the preference shares at a general
meeting of preference shareholders, as applicable, approve such
amendment.
Enforcement;
Governing Law
The pooling arrangements are governed by New York law and may be
enforced in the state and federal courts of New York. The
Company and Fresenius SE have confirmed their intention to abide
by the terms of the pooling arrangements as described above.
Directors
and Officers Insurance
Subject to any mandatory restrictions imposed by German law,
FMC-AG has obtained and FMC-AG & Co. KGaA will
continue to maintain directors and officers insurance in respect
of all liabilities arising from or relating to the service of
the members of the supervisory board and our officers, subject
to legally mandated deductibles. We believe that our acquisition
of that insurance is in accordance with customary and usual
policies followed by public corporations in the U.S.
For information regarding certain of our material contracts, see
“Item 7.B. Major Shareholders and Related Party
Transactions — Related Party Transactions.” For a
description of our stock option plans, see “Item 6.E.
Directors, Senior Management and Employees — Share
Ownership — Options to Purchase our Securities.”
For a
97
description of our 2006 Senior Credit Agreement and our
agreements relating to our long-term and short-term
indebtedness, see Notes 9 and 10 of the Notes to
Consolidated Financial Statements.
Our material agreements include the settlement agreement that
we, FMCH and NMC entered into with the Official Committee of
Asbestos Injury Claimants, and the Official Committee of
Asbestos Property Damage Claimants of W.R. Grace &
Co., a description of which appears in Note 18 of the Notes
to Consolidated Financial Statements, “Legal
Proceedings,” and the merger agreement among us, FMCH and
RCG. For a description of our license and distribution
agreements for injectable iron products, see Note 7 of the
Notes to Consolidated Financial Statements, “Intangible
Assets and Goodwill.”
Exchange
Controls and Other Limitations Affecting Security
Holders.
At the present time, Germany does not restrict the export or
import of capital, except for certain restrictions on
transactions based on international embargo or terror prevention
resolutions concerning for example Iraq, Iran, the Democratic
Republic of Korea, Myanmar, or Sudan. However, the Federal
Ministry of Economics and Technology (
Bundesministerium
für Wirtschaft und Technologie
) may — in
exceptional cases — review and prohibit the direct or
indirect acquisition of 25% or more of the shares or voting
rights in a German company by a person or company resident
outside of the European Union or the European Free Trade Area if
such acquisition constitutes a material threat to the public
security or order. Further, for statistical purposes only, every
resident individual or corporation residing in Germany must
report to the German Federal Bank (Deutsche Bundesbank), subject
only to certain immaterial exceptions, any payment received from
or made to an individual or a corporation resident outside of
Germany if such payment exceeds €12,500. In addition,
residents must report any claims against, or any liabilities
payable to, non-residents individuals or corporations, if such
claims or liabilities, in the aggregate exceed
€5 million at the end of any month.
There are no limitations imposed by German law or our articles
of association (
Satzung
) on the right of a non-resident
to hold the Preference shares or Ordinary shares or the ADSs
evidencing Preference shares or Ordinary shares.
U.S. and
German Tax Consequences of Holding ADSs
The discussion below is not a complete analysis of all of the
potential U.S. federal and German tax consequences of holding
ADSs of FMC-AG & Co. KGaA. In addition, the U.S.
federal and German tax consequences to particular U.S. holders,
such as insurance companies, tax-exempt entities, investors
holding ADSs through partnerships or other fiscally transparent
entities, investors liable for the alternative minimum tax,
investors that hold ADSs as part of a straddle or a hedge,
investors whose functional currency is not the U.S. dollar,
financial institutions and dealers in securities, and to
non-U.S.
holders may be different from that discussed herein.
Germany and the United States of America have agreed on a
Protocol amending the existing Income Tax Treaty. On
December 28, 2007, the Protocol entered into force. The
Protocol is effective in respect of withholding taxes for
amounts paid on or after January 1, 2007. Changes related
to other taxes on income became effective on January 1,
2008.
Investors should consult their tax advisors with respect to the
particular United States federal and German tax consequences
applicable to holding ADSs of FMC-AG & Co.KGaA.
Tax
Treatment of Dividends
German corporations are required to withhold tax on dividends
paid to resident and non-resident shareholders. The German
Business Tax Reform 2008 increased the withholding tax rate on
dividends to 25% (plus solidarity surcharges) starting
January 1, 2009. Also effective January 1, 2009 for
corporate non-German holders, forty percent (40%) of the
withheld and remitted withholding tax may be refunded upon
application at the German Federal Tax Office (at the address
noted below), which would generally result in a net withholding
of 15% (plus solidarity surcharge). The entitlement of corporate
non-German holders to further reductions of the withholding tax
under an applicable income tax treaty remains unaffected. A
partial refund of this withholding tax can be obtained by U.S.
holders under the
U.S.-German
Tax Treaty (“Treaty”). For U.S. federal income tax
purposes, U.S. holders are taxable on dividends paid by German
corporations subject to a foreign tax credit for certain German
income taxes paid. The amount of the refund of German
withholding tax and the determination of the foreign tax credit
98
allowable against U.S. federal income tax depend on whether the
U.S. holder is a corporation owning at least 10% of the voting
stock of the German corporation (“Holder 1”).
In the case of any U.S. holder (“Holder 2”) other than
a Holder 1, the German withholding tax is partially refunded
under the Treaty to reduce the withholding tax to 15% of the
gross amount of the dividend. In this case, for each $100 of
gross dividend that we pay to a Holder 2, the dividend is
subject to withholding tax of $26.38, $11.38 which is refunded,
resulting in a net tax of $15. For U.S. foreign tax credit
purposes, the U.S. holder would report dividend income of $100
(to the extent paid out of current and accumulated earnings and
profits) and foreign taxes paid of $15, for purposes of
calculating the foreign tax credit or the deduction for taxes
paid.
Subject to certain exceptions, dividends received by a
non-corporate U.S. holder will be subject to a maximum U.S.
federal income tax rate of 15%. The lower rate applies to
dividends only if the ADSs in respect of which such dividend is
paid have been held for at least 61 days during the
121 day period beginning 60 days before the
ex-dividend date. Periods during which you hedge a position in
our ADSs or related property may not count for purposes of the
holding period test. The dividends would also not be eligible
for the lower rate if you elect to take dividends into account
as investment income for purposes of limitations on deductions
for investment income. U.S. holders should consult their
own tax advisors regarding the availability of the reduced
dividend rate in light of their own particular circumstances.
In the case of a Holder 1, the 26.375% German withholding tax is
reduced under the Treaty to 5% of the gross amount of the
dividend. Such a holder may, therefore, apply for a refund of
German withholding tax in the amount of 21.375% of the gross
amount of the dividends. A corporate U.S. holder will generally
not be eligible for the dividends-received deduction generally
allowed to U.S. corporations in respect of dividends received
from other U.S. corporations.
Subject to certain complex limitations, a U.S. holder is
generally entitled to a foreign tax credit equal to the portion
of the withholding tax that cannot be refunded under the Treaty.
Dividends paid in Euros to a U.S. holder of ADSs will be
included in income in a dollar amount calculated by reference to
the exchange rate in effect on the date the dividends, including
the deemed refund of German withholding tax, are included in
income by such a U.S. holder. If dividends paid in Euros are
converted into dollars on the date included in income, U.S.
holders generally should not be required to recognize foreign
currency gain or loss in respect of the dividend income.
Under the Treaty the refund of German tax, including the
withholding tax, Treaty payment and solidarity surcharge, will
not be granted when the ADSs are part of the business property
of a U.S. holder’s permanent establishment located in
Germany or are part of the assets of an individual U.S.
holder’s fixed base located in Germany and used for the
performance of independent personal services. In this case,
however, withholding tax and solidarity surcharge may be
credited against German income tax liability.
Refund
Procedures
To claim a refund under the Treaty, the U.S. holder must submit
a claim for refund to the German tax authorities, with the
original bank voucher, or certified copy thereof issued by the
paying entity documenting the tax withheld within four years
from the end of the calendar year in which the dividend is
received. Claims for refund are made on a special German claim
for refund form, which must be filed with the German Federal Tax
Office: Bundeszentralamt für Steuern, An der Küppe 1,
D-53225 Bonn, Germany. The claim refund forms may be obtained
from the German Federal Tax Office at the same address where the
applications are filed, or from the Embassy of the Federal
Republic of Germany, 4645 Reservoir Road, N.W., Washington, D.C.
20007-1998,
or from the Office of International Operations, Internal Revenue
Service, 1325 K Street, N.W., Washington, D.C. 20225, Attention:
Taxpayer Service Division, Room 900 or can be downloaded
from the homepage of the Bundeszentralamt für Steuern
(www.bzst.bund.de).
U.S. holders must also submit to the German tax authorities
certification of their last filed U.S. federal income tax
return. Certification is obtained from the office of the
Director of the Internal Revenue Service Center by filing a
request for certification with the Internal Revenue Service
Center, Foreign Certificate Request, P.O. Box 16347,
Philadelphia, PA
19114-0447.
Requests for certification are to be made in writing and must
include the U.S. holder’s name, address, phone number,
social security number or employer identification number, tax
return form number and tax period for which certification is
requested. The Internal Revenue Service will send the
certification back to the U.S. holder for filing with the German
tax authorities.
U.S. holders of ADSs who receive a refund attributable to
reduced withholding taxes under the Treaty may be required to
recognize foreign currency gain or loss, which will be treated
as ordinary income or loss, to the extent
99
that the dollar value of the refund received by the U.S. holders
differs from the dollar equivalent of the refund on the date the
dividend on which such withholding taxes were imposed was
received by the depositary or the U.S. holder, as the case may
be.
Taxation
of Capital Gains
Under the Treaty, a U.S. holder who is not a resident of Germany
for German tax purposes will not be liable for German tax on
capital gains realized or accrued on the sale or other
disposition of ADSs unless the ADSs are part of the business
property of a permanent establishment located in Germany or are
part of the assets of a fixed base of an individual located in
Germany and used for the performance of independent personal
services.
Upon a sale or other disposition of the ADSs, a U.S. holder will
recognize gain or loss for U.S. federal income tax purposes in
an amount equal to the difference between the amount realized
and the U.S. holder’s tax basis in the ADSs. Such gain or
loss will generally be capital gain or loss if the ADSs are held
by the U.S. holder as a capital asset, and will be long-term
capital gain or loss if the U.S. holder’s holding period
for the ADSs exceeds one year. Individual U.S. holders are
generally taxed at a maximum 15% rate on net long-term capital
gains.
Gift and
Inheritance Taxes
The
U.S.-Germany
estate, inheritance and gift tax treaty provides that an
individual whose domicile is determined to be in the U.S. for
purposes of such treaty will not be subject to German
inheritance and gift tax, the equivalent of the U.S. federal
estate and gift tax, on the individual’s death or making of
a gift unless the ADSs are part of the business property of a
permanent establishment located in Germany or are part of the
assets of a fixed base of an individual located in Germany and
used for the performance of independent personal services. An
individual’s domicile in the U.S., however, does not
prevent imposition of German inheritance and gift tax with
respect to an heir, donee, or other beneficiary who is domiciled
in Germany at the time the individual died or the gift was made.
Such treaty also provides a credit against U.S. federal estate
and gift tax liability for the amount of inheritance and gift
tax paid in Germany, subject to certain limitations, in a case
where ADSs are subject to German inheritance or gift tax and
U.S. federal estate or gift tax.
Other
German Taxes
There are no German transfer, stamp or other similar taxes that
would apply to U.S. holders who purchase or sell ADSs.
United
States Information Reporting and Backup Withholding
Dividends and payments of the proceeds on a sale of ADSs, paid
within the United States or through
U.S.-related
financial intermediaries are subject to information reporting
and may be subject to backup withholding unless you (1) are
a corporation or other exempt recipient or (2) provide a
taxpayer identification number and certify (on Internal Revenue
Service
Form W-9)
that no loss of exemption from backup withholding has occurred.
Non-U.S.
shareholders are not U.S. persons generally subject to
information reporting or backup withholding. However, a
non-U.S.
holder may be required to provide a certification (generally on
Internal Revenue Service
Form W-8BEN)
of its
non-U.S.
status in connection with payments received in the United States
or through a
U.S.-related
financial intermediary.
We file periodic reports and information with the Securities and
Exchange Commission and the New York Stock Exchange. You may
inspect a copy of these reports without charge at the Public
Reference Room of the Securities and Exchange Commission at 100
F Street N.E., Washington, D.C. 20549 or at the Securities and
Exchange Commission’s regional offices 233 Broadway, New
York, New York 10279 and 500 West Madison Street,
Suite 1400, Chicago, Illinois 60661. The public may obtain
information on the operation of the Public Reference Room by
calling the Securities and Exchange Commission at
1-800-SEC-0330.
The Securities and Exchange Commission also maintains an
Internet site that contains reports, proxy and information
statements and other information regarding registrants that file
electronically with the Securities and Exchange Commission. The
Securities and Exchange Commission’s World Wide Web address
is
http://www.sec.gov.
The New York Stock Exchange currently lists American Depositary
Shares representing our Preference shares and American
Depositary Shares representing our Ordinary shares. As a result,
we are subject to the periodic
100
reporting requirements of the Securities Exchange Act of 1934,
as amended, and we file reports and other information with the
Securities and Exchange Commission. These reports, proxy
statements and other information and the registration statement
and exhibits and schedules thereto may be inspected without
charge at, and copies thereof may be obtained at prescribed
rates from, the public reference facilities of the Securities
and Exchange Commission and the electronic sources listed in the
preceding paragraph. In addition, these materials are available
for inspection and copying at the offices of the New York Stock
Exchange, 20 Broad Street, New York, New York 10005, USA.
We prepare annual and quarterly reports. Our annual reports
contain financial statements examined and reported upon, with
opinions expressed by our independent auditors. Our consolidated
financial statements included in these annual reports are
prepared in conformity with U.S. GAAP. Our annual and quarterly
reports to our shareholders are posted under
“Publications” on the “Investor Relations”
page of our website at
http://www.fmc-ag.com.
In furnishing our web site address in this report, however, we
do not intend to incorporate any information on our web site
into this report, and any information on our web site should not
be considered to be part of this report.
We will also furnish the depositary with all notices of
shareholder meetings and other reports and communications that
are made generally available to our shareholders. The
depositary, to the extent permitted by law, shall arrange for
the transmittal to the registered holders of American Depositary
Receipts of all notices, reports and communications, together
with the governing instruments affecting our shares and any
amendments thereto. Such documents are also available for
inspection by registered holders of American Depositary Receipts
at the principal office of the depositary.
Documents referred to in this report which relate to us as well
as future annual and interim reports prepared by us may also be
inspected at our offices, Else-Kröner-Strasse 1, 61352 Bad
Homburg.
Item 11.
Quantitative
and Qualitative Disclosures About Market Risk
Market
Risk
Our businesses operate in highly competitive markets and are
subject to changes in business, economic and competitive
conditions. Our business is subject to:
|
|
|
|
|
|
•
|
changes in reimbursement rates;
|
|
|
|
|
•
|
intense competition;
|
|
|
|
|
•
|
foreign exchange rate fluctuations;
|
|
|
|
|
•
|
varying degrees of acceptance of new product introductions;
|
|
|
|
|
•
|
technological developments in our industry;
|
|
|
|
|
•
|
uncertainties in litigation or investigative proceedings and
regulatory developments in the health care sector;and
|
|
|
|
|
•
|
the availability of financing.
|
Our business is also subject to other risks and uncertainties
that we describe from time to time in our public filings. See
Item 3.D, “Key Information — Risk
Factors.” Developments in any of these areas could cause
our results to differ materially from the results that we or
others have projected or may project.
Reimbursement
Rates
We obtained approximately 33% of our worldwide revenue for 2009
from sources subject to regulations under U.S. government health
care programs. In the past, U.S. budget deficit reduction and
health care reform measures have changed the reimbursement rates
under these programs, including the Medicare composite rate, the
reimbursement rate for EPO, and the reimbursement rates for
other dialysis and non-dialysis related services and products,
as well as other material aspects of these programs, and they
may change in the future. See Item 4.B, “Information
on the Company — Business Overview —
Regulatory and Legal Matters — Reimbursement” and
“— Health Care Reform.”
We also obtain a significant portion of our net revenues from
reimbursement by non-government payors. Historically, these
payors’ reimbursement rates generally have been higher than
government program rates in their respective countries. However,
non-governmental payors are imposing cost containment measures
that are creating significant downward pressure on reimbursement
levels that we receive for our services and products.
101
Inflation
The effects of inflation during the periods covered by the
consolidated financial statements have not been significant to
our results of operations. However, a major portion of our net
revenues from dialysis care are subject to reimbursement rates
regulated by governmental authorities, and a significant portion
of other revenues, especially revenues from the U.S., is
received from customers whose revenues are subject to these
regulated reimbursement rates. Non-governmental payors are also
exerting downward pressure on reimbursement rates. Increased
operation costs that are subject to inflation, such as labor and
supply costs, may not be recoverable through price increases in
the absence of a compensating increase in reimbursement rates
payable to us and our customers, and could materially adversely
affect our business, financial condition and results of
operations.
Management
of Foreign Exchange and Interest Rate Risks
We are primarily exposed to market risk from changes in foreign
exchange rates and changes in interest rates. In order to manage
the risks from these foreign exchange rate and interest rate
fluctuations, we enter into various hedging transactions, as
authorized by the Management Board of the general partner, with
banks which generally have ratings in the “A” Category
or better. We do not use financial instruments for trading or
other speculative purposes.
Fresenius SE, as provided for under a service agreement,
conducts financial instrument activity for us and its other
subsidiaries under the control of a single centralized
department. Fresenius SE has established guidelines, that we
have agreed to, for risk assessment procedures and controls for
the use of financial instruments. They include a clear
segregation of duties with regard to execution on one side and
administration, accounting and controlling on the other.
Foreign
Exchange Risk
We conduct our business on a global basis in various currencies,
although our operations are located principally in the United
States and Germany. For financial reporting purposes, we have
chosen the U.S. dollar as our reporting currency. Therefore,
changes in the rate of exchange between the U.S. dollar and the
local currencies in which the financial statements of our
international operations are maintained, affect our results of
operations and financial position as reported in our
consolidated financial statements. We have consolidated the
balance sheets of our
non-U.S.
dollar denominated operations into U.S. dollars at the exchange
rates prevailing at the balance sheet date. Revenues and
expenses are translated at the average exchange rates for the
period.
Our exposure to market risk for changes in foreign exchange
rates relates to transactions such as sales and purchases. We
have significant amounts of sales of products invoiced in euro
from our European manufacturing facilities to our other
international operations. This exposes our subsidiaries to
fluctuations in the rate of exchange between the euro and the
currency in which their local operations are conducted. For the
purpose of hedging existing and foreseeable foreign exchange
transaction exposures we enter into foreign exchange forward
contracts and, on a small scale, foreign exchange options. Our
policy, which has been consistently followed, is that financial
derivatives be used only for purposes of hedging foreign
currency exposures. We have not used such instruments for
purposes other than hedging.
In connection with intercompany loans in foreign currency, we
normally use foreign exchange swaps thus assuring that no
foreign exchange risks arise from those loans.
The Company is exposed to potential losses in the event of
non-performance by counterparties to financial instruments. We
do not expect any counterparty to fail to meet its obligations.
The current credit exposure of foreign exchange derivatives is
represented by the fair value of those contracts with a positive
fair value at the reporting date. The table below provides
information about our foreign exchange forward contracts at
December 31, 2009. The information is provided in U.S.
dollar equivalent amounts. The table presents the notional
amounts by year of maturity, the fair values of the contracts,
which show the unrealized net gain (loss) on existing contracts
as of December 31, 2009, and the credit risk inherent to
those contracts with positive market values as of
December 31, 2009. All contracts expire within
35 months after the reporting date.
102
Foreign
Currency Risk Management
December 31, 2009
(USD in millions)
Nominal amount
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Credit
|
|
|
|
|
2010
|
|
|
2011
|
|
|
2012
|
|
|
Total
|
|
|
Fair value
|
|
|
risk
|
|
|
|
|
Purchase of EUR against US$
|
|
$
|
309
|
|
|
|
697
|
|
|
|
—
|
|
|
$
|
1,006
|
|
|
$
|
7
|
|
|
$
|
9
|
|
|
Sale of EUR against US$
|
|
|
85
|
|
|
|
—
|
|
|
|
—
|
|
|
|
85
|
|
|
|
(1
|
)
|
|
|
—
|
|
|
Purchase of EUR against others
|
|
|
595
|
|
|
|
39
|
|
|
|
—
|
|
|
|
634
|
|
|
|
(2
|
)
|
|
|
9
|
|
|
Sale of EUR against others
|
|
|
40
|
|
|
|
—
|
|
|
|
—
|
|
|
|
40
|
|
|
|
—
|
|
|
|
—
|
|
|
Others
|
|
|
55
|
|
|
|
7
|
|
|
|
—
|
|
|
|
62
|
|
|
|
1
|
|
|
|
3
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
1,084
|
|
|
|
743
|
|
|
|
—
|
|
|
$
|
1,827
|
|
|
$
|
5
|
|
|
$
|
21
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A summary of the high and low exchange rates for the euro to
U.S. dollars and the average exchange rates for the last five
years is set forth below. The European Central Bank
(“ECB”) determines such rates (“Reference
Rates”) based on the regular daily averaging of rates
between central banks within and outside the European banking
system. The ECB normally publishes the Reference Rates daily at
2:15 p.m. (CET). In preparing our consolidated financial
statements and in converting certain U.S. dollar amounts in this
report, we have used the following Year’s Average Reference
Rate of $1.3948 or Year’s Close Reference Rate of $1.4406
per €1.00.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year’s
|
|
|
Year’s
|
|
|
Year’s
|
|
|
Year’s
|
|
|
Year ending December 31,
|
|
High
|
|
|
Low
|
|
|
Average
|
|
|
Close
|
|
|
|
|
2005 US$ per EUR
|
|
|
1.3507
|
|
|
|
1.1667
|
|
|
|
1.2442
|
|
|
|
1.1797
|
|
|
2006 US$ per EUR
|
|
|
1.3331
|
|
|
|
1.1826
|
|
|
|
1.2558
|
|
|
|
1.3170
|
|
|
2007 US$ per EUR
|
|
|
1.4874
|
|
|
|
1.2893
|
|
|
|
1.3705
|
|
|
|
1.4721
|
|
|
2008 US$ per EUR
|
|
|
1.5990
|
|
|
|
1.2460
|
|
|
|
1.4713
|
|
|
|
1.3917
|
|
|
2009 US$ per EUR
|
|
|
1.5120
|
|
|
|
1.2555
|
|
|
|
1.3948
|
|
|
|
1.4406
|
|
|
|
|
The Reference Rate on February 19, 2010 was $1.3626 per
€1.00.
|
Foreign
Exchange Sensitivity Analysis
In order to estimate and quantify the transaction risks from
foreign currencies, the Company considers the cash flows
reasonably expected for the three months following the reporting
date as the relevant assessment basis for a sensitivity
analysis. For this analysis, the Company assumes that all
foreign exchange rates in which the Company had unhedged
positions as of the reporting date would be negatively impacted
by 10%. By multiplying the calculated unhedged risk positions
with this factor, the maximum possible negative impact of the
foreign exchange transaction risks on the Company’s results
of operations would be $7 million.
Interest
Rate Risk
We are exposed to changes in interest rates that affect our
variable-rate borrowings. We enter into debt obligations and
into accounts receivable securitizations to support our general
corporate purposes including capital expenditures and working
capital needs. Consequently, we enter into derivatives,
particularly interest rate swaps to protect interest rate
exposures arising from borrowings at floating rates by
effectively swapping them into fixed rates.
We enter into interest rate swap agreements that are designated
as cash flow hedges effectively converting the major part of
variable interest rate payments due on our 2006 Senior Credit
Agreement denominated in U.S. dollars into fixed interest rate
payments. Those swap agreements, all of which expire at various
dates between 2010 and 2012, in the notional amount of
$2.40 billion, effectively fix the Company’s variable
interest rate on the majority of its U.S. dollar-denominated
loans at an average interest rate of 4.29% plus an applicable
margin. Interest payable and interest receivable under the swap
agreements are accrued and recorded as an adjustment to interest
expense at each reporting date. At December 31, 2009, the
negative fair value of these agreements is $106 million.
103
The table below presents principal amounts and related weighted
average interest rates by year of maturity for interest rate
swaps and for our significant debt obligations.
Interest
Rate Exposure
December 31,
2009
(in
millions)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dec. 31,
|
|
|
|
|
2010
|
|
|
2011
|
|
|
2012
|
|
|
2013
|
|
|
2014
|
|
|
There-after
|
|
|
Totals
|
|
|
2009
|
|
|
|
|
FLOATING RATE US$ DEBT
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Principal payments on Senior Credit Agreement
|
|
$
|
134
|
|
|
|
1,550
|
|
|
|
1,142
|
|
|
|
379
|
|
|
|
|
|
|
|
|
|
|
$
|
3,205
|
|
|
$
|
3,113
|
|
|
Variable interest rate = 1.37%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable securitization programs
|
|
$
|
214
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
214
|
|
|
$
|
214
|
|
|
Variable interest rate = 0.41%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EIB loans
|
|
$
|
|
|
|
|
|
|
|
|
|
|
|
|
84
|
|
|
|
|
|
|
|
|
|
|
$
|
84
|
|
|
$
|
84
|
|
|
Variable interest rate = 0.38%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FLOATING RATE € DEBT
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Principal payments on Senior Credit Agreement
|
|
$
|
|
|
|
|
317
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
317
|
|
|
$
|
317
|
|
|
Variable interest rate = 1.19%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Euro Notes 2009/2012
|
|
$
|
|
|
|
|
|
|
|
|
172
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
172
|
|
|
$
|
174
|
|
|
Variable interest rate = 6.019%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Euro Notes 2009/2014
|
|
$
|
|
|
|
|
|
|
|
|
5
|
|
|
|
5
|
|
|
|
34
|
|
|
|
|
|
|
$
|
44
|
|
|
$
|
45
|
|
|
Variable interest rate = 6.519%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EIB loan
|
|
$
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
130
|
|
|
|
|
|
|
$
|
130
|
|
|
$
|
130
|
|
|
Variable interest rate = 0.695%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FIXED RATE US$ DEBT
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Company obligated mandatorily redeemable preferred securities of
subsidiaries
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fresenius Medical Care Capital Trusts
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fixed interest rate = 7.875% / issued in 2001
|
|
$
|
|
|
|
|
224
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
224
|
|
|
$
|
233
|
|
|
Senior Notes 2007/2017; fixed interest rate = 6.875%
|
|
$
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
493
|
|
|
$
|
493
|
|
|
$
|
499
|
|
|
FIXED RATE € DEBT
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Company obligated mandatorily redeemable preferred securities of
subsidiaries
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fresenius Medical Care Capital Trusts
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fixed interest rate = 7.375% / issued in 2001
|
|
$
|
|
|
|
|
432
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
432
|
|
|
$
|
455
|
|
|
Euro Notes 2009/2012
|
|
$
|
|
|
|
|
|
|
|
|
51
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
51
|
|
|
$
|
57
|
|
|
Fixed interest rate = 7.4065%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Euro Notes 2009/2014
|
|
$
|
|
|
|
|
|
|
|
|
3
|
|
|
|
3
|
|
|
|
15
|
|
|
|
|
|
|
$
|
21
|
|
|
$
|
24
|
|
|
Fixed interest rate = 8.3835%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INTEREST RATE DERIVATIVES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
US$ Payer Swaps Notional amount
|
|
$
|
250
|
|
|
|
1,000
|
|
|
|
1,150
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
2,400
|
|
|
$
|
(106
|
)
|
|
Average fixed pay rate = 4.29%
|
|
|
4.28
|
%
|
|
|
4.10
|
%
|
|
|
4.45
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.29
|
%
|
|
|
|
|
|
Receive rate =
3-month
$LIBOR
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All variable interest rates dipicted above are as of
December 31, 2009
|
Interest
Rate Sensitivity Analysis
For purposes of analyzing the impact of changes in the relevant
reference interest rates on the Company’s results of
operations, the Company calculates the portion of financial debt
which bears variable interest and which has not been hedged by
means of interest rate swaps or options against rising interest
rates. For this particular part of its liabilities, the Company
assumes an increase in the reference rates of 0.5% compared to
the actual rates as of reporting date. The corresponding
additional annual interest expense is then compared to the
Company’s net income. This analysis shows that an increase
of 0.5% in the relevant reference rates would have an effect of
less than 1% on the consolidated net income of the Company.
104
Item 12.
Description
of Securities other than Equity Securities
|
|
|
|
D.
|
American
Depositary Shares
|
For a description of our American Depositary Shares, see
Item 10B, “Additional Information — Articles
of Association — Description of American Depositary
Receipts.”
D.3. Fees
and expenses
ADS holders will be charged a fee for each issuance of ADSs,
including issuances resulting from distributions of shares,
rights and other property, and for each surrender of ADSs in
exchange for deposited securities. The fee in each case is up to
$5.00 for each 100 ADSs (or any portion thereof) issued or
surrendered.
The following additional charges shall be incurred by the ADS
holders, by any party depositing or withdrawing shares or by any
party surrendering ADSs or to whom ADSs are issued (including,
without limitation, issuance pursuant to a stock dividend or
stock split declared by the Company or an exchange of stock
regarding the ADSs or the deposited securities or a distribution
of ADRs), whichever is applicable:
|
|
|
|
|
|
•
|
a fee of $0.02 or less per ADS (or portion thereof) for any cash
distribution made pursuant to the deposit agreement;
|
|
|
|
|
•
|
a fee of $0.02 per ADS (or portion thereof) per year for
services performed by the depositary in administering our ADS
program (which fee shall be assessed against holders of ADSs as
of the record date set by the depositary not more than once each
calendar year and shall be payable in the manner described in
the next succeeding provision);
|
|
|
|
|
•
|
any other charge payable by any of the depositary, any of the
depositary’s agents, including, without limitation, the
custodian, or the agents of the depositary’s agents in
connection with the servicing of our shares or other deposited
securities (which charge shall be assessed against registered
holders of our ADSs as of the record date or dates set by the
depositary and shall be payable at the sole discretion of the
depositary by billing such registered holders or by deducting
such charge from one or more cash dividends or other cash
distributions);
|
|
|
|
|
•
|
a fee for the distribution of securities (or the sale of
securities in connection with a distribution), such fee being in
an amount equal to the fee for the execution and delivery of
ADSs which would have been charged as a result of the deposit of
such securities (treating all such securities as if they were
shares) but which securities or the net cash proceeds from the
sale thereof are instead distributed by the depositary to those
holders entitled thereto;
|
|
|
|
|
•
|
stock transfer or other taxes and other governmental charges;
|
|
|
|
|
•
|
cable, telex and facsimile transmission and delivery charges
incurred at the request of holders of our shares;
|
|
|
|
|
•
|
transfer or registration fees for the registration of transfer
of deposited securities on any applicable register in connection
with the deposit or withdrawal of deposited securities; and
|
|
|
|
|
•
|
expenses of the depositary in connection with the conversion of
foreign currency into U.S. dollars.
|
We will pay all other charges and expenses of the depositary and
any agent of the depositary (except the custodian) pursuant to
agreements from time to time between us and the depositary. The
fees described above may be amended from time to time.
|
|
|
|
D.4.
|
Amounts
payable by the depositary to the Company
|
Fees
Incurred in Past Annual Period
Under the fee agreement between us and the depositary, the
depositary agrees to pay certain fees relating to the
maintenance of the ADRs. Certain fees we encounter related to
our ADRs are reimbursed to us by the depositary. From
January 1, 2009 to February 19, 2010, we received from
the depositary $21,075, $48,205 and $69,420 for continuing
annual stock exchange listing fees, standard
out-of-pocket
maintenance costs for the ADRs (consisting of the expenses of
postage and envelopes for mailing annual and interim financial
reports, printing and distributing dividend checks, electronic
filing of U.S. Federal tax information, mailing required tax
forms, stationary, postage, facsimile, and telephone calls), any
applicable performance indicators relating to the ADR facility
and legal fees.
Fees
to be Paid in the Future
The Bank of New York Mellon, as depositary, has agreed to
reimburse us for expenses we incur that are related to
establishment and maintenance expenses of the ADS program. The
depositary has agreed to reimburse us for its continuing annual
stock exchange listing fees. The depositary has also agreed to
pay the standard
out-of-pocket
maintenance costs for the ADRs, which consist of the expenses of
postage and envelopes for mailing annual and
105
interim financial statements, printing and distributing dividend
checks, electronic filing of U.S. Federal tax information,
mailing required tax forms, stationary, postage, facsimile, and
telephone calls. It has also agreed to reimburse us annually for
certain investor relations programs or special investor
relations promotion activities. In certain instances, the
depositary has agreed to provide additional payments to us based
on any applicable performance indicators relating to the ADR
facility. There are limits on the amount of expenses for which
the depositary will reimburse the Company, but the amount of
reimbursement available to us is not necessarily tied to the
amount of fees the depositary collects from investors.
The depositary collects its fees for delivery and surrender of
ADSs directly from investors depositing shares or surrendering
ADSs for the purpose of withdrawal or from intermediaries acting
for them. The depositary collects fees from the amounts
distributed or by selling a portion of distributable property to
pay the fees. The depositary may collect its annual fee for
depositary services by deduction from cash distributions or by
directly billing investors or by charging the book-entry system
accounts of participants acting for them. The depositary may
generally refuse to provide fee-attracting services until its
fees for those services are paid.
|
|
|
|
Item 13.
|
Defaults,
Dividend Arrearages and Delinquencies
|
None
|
|
|
|
Item 14.
|
Material
Modifications to the Rights of Security Holders and Use of
Proceeds
|
Not applicable
|
|
|
|
Item 15A.
|
Disclosure
Controls and Procedures
|
The Company’s management, including the members of the
Management Board of our general partner performing the functions
Chief Executive Officer and Chief Financial Officer, has
conducted an evaluation of the effectiveness of the design and
operation of the Company’s disclosure controls and
procedures as of the end of the period covered by this report,
as contemplated by Securities Exchange Act
Rule 13a-15.
Based on that evaluation, the Chief Executive Officer of our
general partner and Chief Financial Officer of our general
partner concluded that the disclosure controls and procedures
were effective in ensuring that all material information
required to be filed in this annual report has been made known
to them in a timely fashion. There have been no significant
changes in internal controls, or in factors that could
significantly affect internal controls, subsequent to the date
the Chief Executive Officer and Chief Financial Officer
completed their evaluation.
|
|
|
|
Item 15B.
|
Management’s
annual report on internal control over financial
reporting
|
Management of the Company is responsible for establishing and
maintaining adequate internal control over financial reporting,
as such term is defined in Exchange Act
Rules 13a-15(f).
The Company’s internal control over financial reporting is
a process designed by or under the supervision of the Chief
Executive Officer of our general partner and Chief Financial
Officer of our general partner, to provide reasonable assurance
regarding the reliability of financial reporting and the
preparation of the Company’s financial statements for
external reporting purposes in accordance with U.S. generally
accepted accounting principles.
As of December 31, 2009, management conducted an assessment
of the effectiveness of the Company’s internal control over
financial reporting based on the criteria established in
Internal Control — Integrated Framework issued by the
Committee of Sponsoring Organizations of the Treadway Commission
(COSO). Based on this assessment, management has determined that
the Company’s internal control over financial reporting as
of December 31, 2009 is effective.
The Company’s internal control over financial reporting
includes policies and procedures that (1) pertain to the
maintenance of records that, in reasonable detail, accurately
and fairly reflect transactions and dispositions of our assets;
(2) provide reasonable assurances that the Company’s
transactions are recorded as necessary to permit preparation of
financial statements in accordance with U.S. generally accepted
accounting principles, and that the Company’s receipts and
expenditures are being made only in accordance with
authorizations of management; and (3) provide reasonable
assurance regarding prevention or timely detection of
unauthorized acquisition, use or disposition of the
Company’s assets that could have a material effect on the
Company’s financial statements.
Because of its inherent limitation, internal control over
financial reporting, no matter how well designed, cannot provide
absolute assurance of achieving financial reporting objectives
and may not prevent or detect
106
misstatements. Therefore, even if the internal control over
financial reporting is determined to be effective it can provide
only reasonable assurance with respect to financial statement
preparation and presentation. Also, projections of any
evaluation of effectiveness to future periods are subject to the
risk that controls may become inadequate because of changes in
conditions, or that the degree of compliance with the policies
or procedures may deteriorate.
The effectiveness of our internal control over financial
reporting as of December 31, 2009, has been audited by
KPMG, an independent registered public accounting firm, as
stated in their report included on
page F-4.
|
|
|
|
Item 15C.
|
Attestation
report of the registered public accounting firm
|
The attestation report of KPMG with respect to Management’s
Report on Internal Control Over Financial Reporting appears at
page F-4.
|
|
|
|
Item 15D.
|
Changes
in Internal Control over Financial Reporting
|
There have been no changes in the Company’s internal
control over financial reporting that occurred during fiscal
year 2009, which have materially affected or are reasonably
likely to materially affect the Company’s internal control
over financial reporting.
|
|
|
|
Item 16A.
|
Audit
Committee Financial Expert
|
Our Supervisory Board has determined that each of Prof.
Dr. Bernd Fahrholz, Dr. Walter L. Weisman and
Mr. William P. Johnston qualifies as an independent audit
committee financial expert and is “independent” as
defined in
Rule 10A-3
under the Exchange Act, in accordance with the provisions of
Item 16A of
Form 20-F.
In 2003, our Management Board adopted through our worldwide
compliance program a code of ethics, titled the
Code of
Business Conduct,
which as adopted applied to members of the
Management Board, including its chairman and the responsible
member for Finance & Controlling, other senior
officers and all Company employees. After the transformation of
legal form, our Code of Business Conduct applies to the members
of the Management Board of our general partner and all Company
employees, including senior officers. A copy of the
Company’s Code of Business Conduct is available on our
website under “Our Company — Compliance” at:
http://www.fmc-ag.com/Code_of_Conduct.htm
|
|
|
|
Item 16C.
|
Principal
Accountant Fees and Services.
|
In the annual general meeting held on May 7, 2009, our
shareholders approved the appointment of KPMG to serve as our
independent auditors for the 2009 fiscal year. KPMG billed the
following fees to us for professional services in each of the
last two years:
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(in thousands)
|
|
|
|
|
Audit fees
|
|
$
|
9,838
|
|
|
$
|
10,405
|
|
|
Audit related fees
|
|
|
187
|
|
|
|
103
|
|
|
Tax fees
|
|
|
909
|
|
|
|
1,046
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
10,934
|
|
|
$
|
11,554
|
|
|
|
|
|
|
|
|
|
|
|
“Audit Fees” are the aggregate fees billed by KPMG for
the audit of our German statutory and U.S. GAAP consolidated and
annual financial statements, reviews of interim financial
statements and attestation services that are provided in
connection with statutory and regulatory filings or engagements.
Fees related to the audit of internal control are included in
Audit Fees. “Audit-Related Fees” are fees charged by
KPMG for assurance and related services that are reasonably
related to the performance of the audit or review of our
financial statements and are not reported under “Audit
Fees.” This category comprises fees billed for comfort
letters, consultation on accounting issues, the audit of
employee benefit plans and pension schemes,
agreed-upon
procedure engagements and other attestation services subject to
regulatory requirements. “Tax Fees” are fees for
professional services rendered by KPMG for tax compliance, tax
advice on implications for actual or contemplated transactions,
tax consulting associated with international transfer prices,
and expatriate employee tax services.
107
Audit
Committee’s pre-approval policies and procedures
As a German company, we prepare statutory financial statements
under German law on the basis of the accounting principles of
the German Commercial Code (
Handelsgesetzbuch
or
HGB
). Our supervisory board engages our independent
auditors to audit these financial statements, in consultation
with our Audit and Governance Committee and subject to
approval by our shareholders at our AGM in accordance with
German law. We also prepare financial statements in accordance
with U.S. GAAP, which are included in registration statements
and reports that we file with the Securities and Exchange
Commission. Our Audit and Corporate Governance Committee engages
our independent auditors to audit these financial statements in
accordance with rule 10A-3 under the Exchange Act and Rule
303A.06 of the NYSE Governance Rules. See also the description
in “Item 6C. Directors, Senior Management and
Employees - Board Practices.” In 2003, Fresenius Medical
Care AG’s audit committee also adopted a policy requiring
management to obtain the committee’s approval before
engaging our independent auditors to provide any audit or
permitted non-audit services to us or our subsidiaries. Pursuant
to this policy, which is designed to assure that such
engagements do not impair the independence of our auditors, the
Audit and Corporate Governance Committee pre-approves annually a
catalog of specific audit and non-audit services in the
categories Audit Services, Audit-Related Services, Tax
Consulting Services, and Other Services that may be performed by
our auditors as well as additional approval requirements based
on fee amount.
The general partner’s Chief Financial Officer reviews all
individual management requests to engage our auditors as a
service provider in accordance with this catalog and, if the
requested services are permitted pursuant to the catalog and fee
level, approves the request accordingly. We inform the Audit and
Corporate Governance Committee about these approvals on an
annual basis. Services that are not included in the catalog or
exceed applicable fee level require pre-approval by the Audit
and Corporate Governance Committee’s chairman or full
committee on a
case-by-case
basis. Neither the chairman of our Audit and Corporate
Governance Committee nor the full committee is permitted to
approve any engagement of our auditors if the services to be
performed either fall into a category of services that are not
permitted by applicable law or the services would be
inconsistent with maintaining the auditors’ independence.
During 2009, the total fees paid to the Audit and Corporate
Governance Committee members for service on the committee were
$0.148 million.
|
|
|
|
Item 16D.
|
Exemptions
from the Listing Standards for Audit Committees
|
Not applicable
|
|
|
|
Item 16E.
|
Purchase
of Equity Securities by the Issuer and Affiliated
Purchasers
|
We did not purchase any of our equity securities during the
fiscal year covered by this report.
|
|
|
|
Item 16F.
|
Change
in Registrant’s Certifying Accountant
|
Not applicable
|
|
|
|
Item 16G.
|
Corporate
Governance
|
Introduction
American Depositary Shares representing our Ordinary shares and
our Preference shares are listed on the New York Stock Exchange
(“NYSE”). However, because we are a “foreign
private issuer,” as defined in the rules of the Securities
and Exchange Commission, we are exempt from substantially all of
the governance rules set forth in Section 303A of the
NYSE’s Listed Companies Manual, other than the obligation
to maintain an audit committee in accordance with
Rule 10A-3
under the Securities Exchange Act of 1934, as amended, the
obligation to notify the NYSE if any of our executive officers
becomes aware of any material non-compliance with any applicable
provisions of Section 303A, and the obligation to file
annual and interim written affirmations, on forms mandated by
the NYSE, relating to our compliance with applicable NYSE
governance rules. Instead, the rules of both the SEC and the
NYSE require that we disclose the significant ways in which our
corporate practices differ from those applicable to U.S.
domestic companies under NYSE listing standards.
As a German company FMC-AG & Co. KGaA follows German
Corporate Governance practices. German corporate governance
practices generally derive from the provisions of the German
Stock Corporation Act (
“AktG”
) including
capital market related laws, the German Codetermination Act
(
“MitBestG”
) and the German Corporate
Governance Code which was adopted in 2002 and revised
periodically thereafter by the German government commission,
most recently in June 2009. Our Articles of Association also
include provisions affecting
108
our corporate governance. German standards differ from the
corporate governance listing standards applicable to U.S.
domestic companies which have been adopted by the NYSE. The
discussion below provides certain information regarding our
organizational structure, management arrangements and
governance, including information regarding the legal structure
of a partnership limited by shares, or KGaA, management by our
general partner, certain provisions of our Articles of
Association and the role of our supervisory board in monitoring
the management of our company by the general partner. It
includes a brief, general summary of the principal differences
between German and U.S. corporate governance practices, together
with, as appropriate, a comparison to U.S. principles or
practices.
The
Legal Structure of FMC-AG & Co. KGaA
A KGaA (
“Kommanditgesellschaft auf Aktien”
) is
a mixed form of entity under German corporate law, which has
elements of both a partnership and a corporation. Like a stock
corporation, the share capital of a KGaA is held by its
shareholders. KGaA and stock corporation
(
“Aktiengesellschaft”
or
“AG”
)
are the only legal forms provided by German law for entities
whose shares trade on the stock exchange. A KGaA is similar to a
limited partnership because there are two groups of owners, the
general partner on the one hand, and the KGaA shareholders on
the other hand. Our general partner, Management AG, is a
wholly-owned subsidiary of Fresenius SE.
A KGaA’s corporate bodies are its general partner, its
supervisory board and the general meeting of shareholders. A
KGaA may have one or more general partners who conduct the
business of the KGaA. However, unlike a stock corporation, in
which the supervisory board appoints the management board, the
supervisory board of a KGaA has no influence on appointment of
the managing body — the general partner. Likewise, the
removal of the general partner from office is subject to very
strict conditions. The general partner(s) may, but are not
required to, purchase shares of the KGaA. The general partner(s)
are personally liable for the liabilities of the KGaA in
relations with third parties subject, in the case of corporate
general partners, to applicable limits on liability of
corporations generally.
Management
and Oversight
The management structure of FMC-AG & Co. KGaA is
illustrated as follows (percentage ownership amounts refer to
ownership of the Company’s total share capital of all
classes):
General
Partner
Management AG, a stock corporation and a wholly owned subsidiary
of Fresenius SE, is the sole general partner of
FMC-AG & Co. KGaA and will conduct its business and
represent it in external relations. Use of a stock corporation
as the legal form of the general partner enables the Company to
maintain a management structure substantially similar to
FMC-AG’s management structure prior to the transformation
into a KGaA. The internal corporate governance structure of the
general partner is substantially similar to the prior structure
at FMC-AG. In particular, the general partner has substantially
the same provisions in its articles of association concerning
the relationship between the general partner’s management
board and the general partner’s supervisory board and,
109
subject to applicable statutory law, substantially the same
rules of procedure for its executive bodies. Management AG was
incorporated on April 8, 2005 and registered with the
commercial register in Hof an der Saale on May 10, 2005.
The registered share capital of Management AG is
€1.5 million.
The general partner has not made a capital contribution to the
Company and, therefore, will not participate in its assets or
its profits and losses. However, the general partner will be
compensated for all outlays in connection with conducting the
business of the Company, including the remuneration of members
of the general partner’s management board and supervisory
board. See “The Articles of Association of
FMC-AG & Co. KGaA — Organization of the
Company” below. FMC-AG & Co. KGaA itself will
bear all expenses of its administration. Management AG will
devote itself exclusively to the management of
FMC-AG & Co. KGaA. The general partner will receive
annual compensation amounting to 4% of its capital for assuming
the liability and the management of FMC-AG & Co.
AG & Co. KGaA. This payment of €60,000 per annum
constitutes a guaranteed return on Fresenius SE’s
investment in the share capital of Management AG. This payment
is required for tax reasons, to avoid a constructive dividend by
the general partner to Fresenius SE in the amount of reasonable
compensation for undertaking liability for the obligations of
Fresenius Medical Care AG & Co. KGaA. FMC
AG & Co. KGaA will also reimburse the general partner
for the remuneration paid to the members of its management board
and its supervisory board. See Item 7.B, “Major
Shareholders and Related Party Transactions.”
The status of the general partner or partners in a KGaA is
stronger than that of the shareholders based on: (i) the
management powers of the general partners, (ii) the
existing de facto veto rights regarding material resolutions
adopted by the general meeting and (iii) the independence
of the general partner from the influence of the KGaA
shareholders as a collective body (See “General
Meeting”, below). Because Fresenius SE is the sole
shareholder of Management AG, the general partner, Fresenius SE
has the sole power to elect the supervisory board of Management
AG which appoints the members of the management board of
Management AG, who act on behalf of the general partner in the
conduct of the company’s business and in relations with
third parties.
The statutory provisions governing a partnership, including a
KGaA, provide in principle that the consent of the KGaA
shareholders at a general meeting is required for transactions
that are not in the ordinary course of business. However, as
permitted by statute, the articles of association of
FMC-AG & Co. KGaA permit such decisions to be made by
Management AG as general partner without the consent of the
FMC-AG & Co. KGaA shareholders. This negation of the
statutory restrictions on the authority of Management AG as
general partner is intended to replicate governance arrangements
in FMC-AG, our corporate form prior to transformation of legal
form, by retaining for the management board of the general
partner the level of operating flexibility that existed prior to
the transformation. Prior to the transformation of legal form,
the shareholders of FMC-AG did not have any such veto right
regarding determinations of its management board. This does not
affect the general meeting’s right of approval with regard
to measures of unusual significance, such as a spin-off of a
substantial part of a company’s assets, as developed in
German Federal Supreme Court decisions.
The general partner’s supervisory board appoints the
members of the general partner’s management board and
supervises and advises them in managing the Company. The general
partner’s management board conducts the business activities
of our Company in accordance with the rules of procedure adopted
by the general partner’s supervisory board pursuant to the
German Corporate Governance Code. The relationship between the
Management AG supervisory board and the Management AG management
board is substantially similar to the governance provisions at
FMC-AG prior to the transformation. In particular, under the
articles of association of Management AG, the same transactions
are subject to the consent of the supervisory board of
Management AG as previously required the consent of the
supervisory board of FMC-AG. These transactions include, among
others:
|
|
|
|
|
|
•
|
The acquisition, disposal and encumbrance of real property if
the value or the amount to be secured exceeds a specified
threshold (€5 million);
|
|
|
|
|
•
|
The acquisition, formation, disposal or encumbrance of an equity
participation in other enterprises if the value of the
transaction exceeds a specified threshold (€5 million);
|
|
|
|
|
•
|
The adoption of new or the abandonment of existing lines of
business or establishments;
|
|
|
|
|
•
|
Conclusion, amendment and termination of affiliation agreements;
and
|
|
|
|
|
•
|
Certain inter-company legal transactions.
|
Five of the six members of the supervisory board of
FMC-AG & Co. KGaA are also members of the supervisory
board of Management AG. The Company and Fresenius SE have
entered into a pooling agreement requiring that at least
one-third (and not less than two) members of the general
partner’s supervisory board be “independent
directors” — i.e., persons without a substantial
business or professional relationship with the Company,
Fresenius SE, or any affiliate of either, other than as a member
of the supervisory board of the Company
110
or the general partner. (See Item 10.B, “Additional
Information — Articles of Association —
Description of the Pooling Arrangements.”)
Fresenius SE’s de facto control of the Company through
ownership of the general partner is conditioned upon its
ownership of a substantial amount of the Company’s share
capital (See “The Articles of Association of
FMC-AG & Co. KGaA — Organization of the
Company”, below).
Supervisory
Board
The supervisory board of a KGaA is similar in certain respects
to the supervisory board of a stock corporation. Like the
supervisory board of a stock corporation, the supervisory board
of a KGaA is under an obligation to oversee the management of
the business of the Company. The supervisory board is elected by
the KGaA shareholders at the general meeting. Shares in the KGaA
held by the general partner or its affiliated companies are not
entitled to vote for the election of the supervisory board
members of the KGaA. Accordingly, Fresenius SE is not entitled
to vote its shares for the election of FMC-AG & Co.
KGaA’s supervisory board members.
Although Fresenius SE will not be able to vote in the election
of FMC-AG & Co. KGaA’s supervisory board,
Fresenius SE will nevertheless retain influence on the
composition of the supervisory board of FMC-AG & Co.
KGaA. Because (i) four of the six former members of the
FMC-AG supervisory board continue to hold office as four of the
six current members of the supervisory board of
FMC-AG & Co. KGaA (except for Dr. Ulf
M. Schneider and Mr. William P. Johnston) and
(ii) in the future, the FMC-AG & Co. KGaA
supervisory board will propose future nominees for election to
its supervisory board (subject to the right of shareholders to
make nominations), Fresenius SE is likely to retain de facto
influence over the selection of the supervisory board of
FMC-AG & Co. KGaA. However, under our articles of
association, a resolution for the election of members of the
supervisory board requires the affirmative vote of 75% of the
votes cast at the general meeting. Such a high vote requirement
could be difficult to achieve, which could result in the need to
apply for court appointment of members to the supervisory board
after the end of the terms of the members in office.
The supervisory board of FMC-AG & Co. KGaA has less
power and scope for influence than the supervisory board of the
Company as a stock corporation. The supervisory board of
FMC-AG & Co. KGaA is not entitled to appoint the
general partner or its executive bodies. Nor may the supervisory
board subject the management measures of the general partner to
its consent, or issue rules of procedure for the general
partner. Management of the Company will be conducted by the
management board of the general partner and only the supervisory
board of the general partner (all of whose members will be
elected solely by Fresenius SE) has the authority to appoint or
remove them. FMC-AG & Co. KGaA’s supervisory
board will represent FMC-AG & Co. KGaA in transactions
with the general partner.
FMC-AG & Co. KGaA’s annual financial statements
are submitted to the Company’s shareholders for approval at
the Company’s general meeting. Except for making a
recommendation to the general meeting regarding such approval,
this matter is not within the competence of the supervisory
board.
Under certain conditions supervisory boards of large German
stock corporations will include both shareholder representatives
and a certain percentage of labor representatives, referred to
as “co-determination.” Depending on the company’s
total number of employees, up to one half of the supervisory
board members are being elected by the company’s employees.
In these cases traditionally the chairman is a representative of
the shareholders. In case of a tie vote, the supervisory board
chairman may cast the decisive tie-breaking vote. We are not
currently subject to German law co-determination requirements.
In recent history, there has been a trend towards selecting
shareholder representatives for supervisory boards from a wider
spectrum of candidates, including representatives from
non-German companies, in an effort to introduce a broader range
of experiences and expertise and a larger degree of
independence. German law also has several rules applicable to
supervisory board members which are designed to ensure a certain
degree of independence of the board’s members. In addition
to prohibiting members of the management board from serving on
the supervisory board, German law requires members of the
supervisory board to act in the best interest of the company.
They do not have to follow direction or instruction from third
parties. Any service, consulting or similar agreements between
the company and any of its supervisory board members must be
approved by the supervisory board.
General
Meeting
The annual general meeting is the resolution body of the KGaA
shareholders. Shareholders can exercise their voting rights at
the general meeting themselves, by proxy via a representative of
their choice, or by a Company-nominated proxy acting on their
instructions. Among other matters, the general meeting of a KGaA
approves its
111
annual financial statements. The internal procedure of the
general meeting corresponds to that of the general meeting of a
stock corporation. The agenda for the general meeting is fixed
by the general partner and the KGaA supervisory board except
that the general partner cannot propose nominees for election as
members of the KGaA supervisory board or proposals for the
Company auditors.
KGaA shareholders exercise influence in the general meeting
through their voting rights but, in contrast to a stock
corporation, the general partner of a KGaA has a de facto veto
right with regard to material resolutions. The members of the
supervisory board of a KGaA are elected by the general meeting
as in a stock corporation. Although Fresenius SE, as sole
shareholder of the general partner of the Company is not
entitled to vote its shares in the election of the supervisory
board of FMC-AG & Co. KGaA, Fresenius SE will retain a
degree of influence on the composition of the supervisory board
of FMC-AG & Co. KGaA (see “The Supervisory
Board”, above).
Under German law, resolutions may be adopted by the vote of a
majority of the shares present at the meeting. Therefore, based
on Fresenius SE’s ownership of approximately 36.0% of the
Company’s voting ordinary shares, as long as less than
approximately 72.0% of the Company’s ordinary shares are
present at a meeting, Fresenius SE will continue to possess a
controlling vote on most matters presented to the shareholders,
other than election of the supervisory board and the matters
subject to a ban on voting as set forth below, at least until
the Company would issue additional ordinary shares in a capital
increase in which Fresenius SE would not participate.
Fresenius SE is subject to various bans on voting at general
meetings due to its ownership of the shares of the general
partner. Fresenius SE is banned from voting on resolutions
concerning the election and removal from office of the
FMC-AG & Co. KGaA supervisory board, ratification or
discharge of the actions of the general partner and members of
the supervisory board, the appointment of special auditors, the
assertion of compensation claims against members of the
executive bodies, the waiver of compensation claims, and the
selection of auditors of the annual financial statements.
Certain matters requiring a resolution at the general meeting
will also require the consent of the general partner, such as
amendments to the articles of association, dissolution of the
Company, mergers, a change in the legal form of the partnership
limited by shares and other fundamental changes. The general
partner therefore has a de facto veto right on these matters.
Annual financial statements are subject to approval by both the
KGaA shareholders and the general partner.
The
Articles of Association of FMC-AG & Co.
KGaA
The articles of association of FMC-AG & Co. KGaA are
based on the articles of association of FMC-AG formerly in
effect, particularly with respect to capital structure, the
supervisory board and the general meeting. Other provisions of
the articles of association, such as those dealing with
management of FMC-AG & Co. KGaA, have been adjusted to
the KGaA legal form. Certain material provisions of the articles
of association are explained below, especially variations from
the articles of association of FMC-AG. The following summary is
qualified in its entirety by reference to the complete form of
articles of association of FMC-AG & Co. KGaA, an
English translation of which is on file with the SEC. In
addition, it can be found on the Company’s website under
www.fmc-ag.com
.
Organization
of the Company
The articles of association of FMC-AG & Co. KGaA
contain several provisions relating to the general partner of
FMC-AG & Co. KGaA. The general partner is Management
AG with its registered office in Hof an der Saale, Germany.
Under the articles of association, possession of the power to
control management of the Company through ownership of the
general partner is conditioned upon ownership of a specific
minimum portion of the Company’s share capital. Under
German law, Fresenius SE could significantly reduce its holdings
in the Company’s share capital while at the same time
retaining its de facto control over the Company’s
management through its ownership of the shares of the general
partner. Under the Company’s prior legal form as a stock
corporation, a shareholder had to hold more than 50% of the
Company’s voting ordinary shares to exercise a controlling
influence. If half the Company’s total share capital had
been issued as preference shares (the maximum permissible by
law), such controlling interest would represent more than 25% of
the Company’s total share capital. This minimum threshold
for control of more than 25% of the total share capital of a
stock corporation is the basis for a provision in the articles
of association of FMC-AG & Co. KGaA requiring that a
parent company within the group shall hold an interest of more
than 25% of the share capital of FMC-AG & Co. KGaA. As
a result, the general partner will be required to withdraw from
FMC-AG & Co. KGaA if its shareholder no longer holds,
directly or indirectly, more than 25% of the Company’s
share capital. The effect of this provision is that the parent
company within the group may not reduce
112
its capital participation in FMC-AG & Co. KGaA below
such amount without causing the withdrawal of the general
partner. The articles of association also permit a transfer of
all shares in the general partner to the Company, which would
have the same effect as withdrawal of the general partner.
The articles of association also provide that the general
partner must withdraw if the shares of the general partner are
acquired by a person who does not make an offer under the German
Securities Acquisition and Takeover Act to acquire the shares of
the Company’s other shareholders within three months of the
acquisition of the general partner. The consideration to be
offered to shareholders must include any portion of the
consideration paid for the general partner’s shares in
excess of the general partner’s equity capital, even if the
parties to the sale allocate the premium solely to the general
partner’s shares. The Company’s articles of
association provide that the general partner can be acquired
only by a purchaser who at the same time acquires more than 25%
of FMC-AG & Co. KGaA’s share capital. These
provisions would therefore trigger a takeover offer at a lower
threshold than the German Securities Acquisition and Takeover
Act, which requires that a person who acquires at least 30% of a
company’s shares make an offer to all shareholders. The
provisions will enable shareholders to participate in any
potential control premium payable for the shares of the general
partner, although the obligations to make the purchase offer and
extend the control premium to outside shareholders could also
discourage an acquisition of the general partner, thereby
discouraging a change of control.
In the event that the general partner withdraws from
FMC-AG & Co. KGaA as described above or for other
reasons, the articles of association provide for continuation of
the Company as a so-called “unified KGaA”
(
Einheits-KGaA
), i.e., a KGaA in which the general
partner is a wholly-owned subsidiary of the KGaA. Upon the
coming into existence of a “unified KGaA”, the
shareholders of FMC-AG & Co. KGaA would ultimately be
restored to the status as shareholders in a stock corporation,
since the shareholding rights in the general partner would be
exercised by FMC-AG & Co. KGaA’s supervisory
board pursuant to the articles of association. If the KGaA is
continued as a “unified KGaA,” an extraordinary or the
next ordinary general meeting would vote on a change in the
legal form of the partnership limited by shares into a stock
corporation. In such a case, the change of legal form back to
the stock corporation would be facilitated by provisions of the
articles of association requiring only a simple majority vote
and that the general partner consent to the transformation of
legal form.
The articles of association provide that to the extent legally
required, the general partner must declare or refuse its consent
to resolutions adopted by the meeting directly at the general
meeting.
After formation, the articles of association of a KGaA may be
amended only through a resolution of the general meeting adopted
by a qualified 75% majority and with the consent of the general
partner. Therefore, neither group (i.e., the KGaA shareholders
and the general partner(s)) can unilaterally amend the articles
of association without the consent of the other group. Fresenius
SE will, however, continue to be able to exert significant
influence over amendments to the articles of association of
FMC-AG & Co. KGaA through its ownership of a
significant percentage of the Company’s ordinary shares
after the transformation, since such amendments require a 75%
vote of the shares present at the meeting rather than three
quarters of the outstanding shares.
Annual
Financial Statement and Allocation of Profits
The articles of association of FMC AG & Co. KGaA on
rendering of accounts require that the annual financial
statement and allocation of profits of FMC-AG & Co.
KGaA be submitted for approval to the annual general meeting of
the Company.
Corresponding to the articles of FMC-AG, the articles of
association of FMC-AG & Co. KGaA provide that
Management AG is authorized to transfer up to a maximum of half
of the annual surplus of FMC-AG & Co. KGaA to other
retained earnings when setting up the annual financial
statements.
Articles
of Association of Management AG
As a separate corporation, FMC AG & Co. KGaA’s
general partner, Management AG, has its own articles of
association.
The articles of association of Management AG are based
essentially on FMC-AG’s articles of association formerly in
effect. In particular, the provisions of its articles of
association on relations between the management board and the
supervisory board have been incorporated into the articles of
association of Management AG. The amount of Management AG’s
share capital is €1,500,000, issued as 1,500,000 registered
shares without par value. By law, notice of any transfer of
Management AG’s shares must be provided to the management
board of Management AG in order for the transferee to be
recognized as a new shareholder by Management AG.
113
Directors’
Share Dealings
According to article 15a of the German Securities Trading
Act (
Wertpapierhandelsgesetz,
), members of the Management
and Supervisory Boards or other employees in management
positions are required to inform the Company when buying or
selling our shares and related financial instruments if the
volume exceeds €5,000 within a single year. We publish the
information received in these reports on our web site in
accordance with the regulations as well as in our Annual Report
to Shareholders.
Comparison
with U.S. and NYSE Governance Standards and
Practices
The listing standards of the NYSE require that independent
directors of a U.S. domestic listed company meet in regularly
scheduled sessions without management. U.S. listed companies
also must adopt corporate governance guidelines that address
director qualification standards, director responsibilities,
director access to management and independent advisors, director
compensation, director orientation and continuing education,
management succession, and an annual performance evaluation of
the board. Several of these concepts are addressed (but not
mandated) by the German Corporate Governance Code (the
“Code”) issued by the German Federal Ministry of
Justice. The most recent version of the Code is dated
June 18, 2009. While the Code’s governance rules
applicable to German corporations are not legally binding,
companies failing to comply with the Code’s recommendations
must disclose publicly how and for what reason their practices
differ from those recommended by the Code. A convenience
translation of our most recent annual “Declaration of
Compliance” under the Code, will be posted on our web site,
www.fmc-ag.com
on the Investor Relations page under
“Corporate Governance/Declaration of Compliance”
together with our declarations for prior years. Some of the
Code’s recommendations address the independence and
qualifications of supervisory board members. Specifically, the
Corporate Governance Code recommends that the supervisory board
should take into account potential conflicts of interest when
nominating candidates for election to the supervisory board.
Similarly, if a material conflict of interest arises during the
term of a member of the supervisory board, the Corporate
Governance Code recommends that the term of that member be
terminated. The Corporate Governance Code further recommends
that at any given time not more than two former members of the
management board should serve on the supervisory board. For
nominations for the election of members of the Supervisory
Board, care shall be taken that the Supervisory Board, at all
times, is composed of members who have the required knowledge,
abilities and expert experience to properly complete their
tasks. The only recommendations with which we do not currently
comply are the imposition of age limits for service on the
Supervisory Board and the Management Board and payment of
performance related compensation (including components based on
the enterprise’s long-term performance) to members of the
Supervisory Board. We believe that any such age limit would
limit our selection of qualified candidates for service on the
Supervisory Board. Furthermore, we currently pay only fixed
compensation to the members of the Supervisory Board; however,
the possible introduction of performance-related compensation to
the members of the Supervisory Board, linked to the success of
the Company, is still under review. The Corporate Governance
Code furthermore includes the suggestion that supervisory board
members meet without any representatives of the management board
attending, whenever necessary. Deviations from this
recommendation are, however, not required to be disclosed
publicly.
As noted in the Introduction, as a company listed on the NYSE,
we are required to maintain an audit committee in accordance
with
Rule 10A-3
under the Securities Exchange Act of 1934. The NYSE’s
listing standards applicable to U.S. domestic listed companies
require that such companies also maintain a nominating committee
to select nominees to the board of directors and a compensation
committee, each consisting solely of directors who are
“independent” as defined in the NYSE’s governance
rules.
In contrast to U.S. practice, with one exception, German
corporate law does not mandate the creation of specific
supervisory board committees. In certain cases, German
corporations are required to establish what is called a
mediation committee with a charter to resolve any disputes among
the members of the supervisory board that may arise in
connection with the appointment or dismissal of members of the
management board. The German Stock Corporation Act provides that
the supervisory board may establish, and the German Corporate
Governance Code recommends that a supervisory board establish an
audit committee to handle the formal engagement of the
company’s independent auditors once they have been approved
by the general meeting of shareholders. Under the Corporate
Governance Code, the audit committee would also address issues
of accounting, risk management and auditor independence and,
under the Stock Corporation Act, an audit committee should
supervise the effectiveness of the internal control system, the
risk management system and the internal audit function. Our
Audit and Corporate Governance Committee within the supervisory
board of FMC-AG & Co. KGaA functions in each of these areas
and also serves as our audit committee as required by Rule
10A-3
under
the Exchange Act and the NYSE rules. As sole shareholder of our
general partner, Fresenius SE elects the supervisory board of
our general partner (subject to the requirements of our pooling
agreement discussed above). In practice, many supervisory boards
have also constituted other committees to facilitate the work of
the supervisory board. For example, a presidential committee
114
is frequently constituted to deal with executive compensation
and nomination issues as well as service agreements with members
of the supervisory board. At the present time, these functions
are carried out by our general partner’s supervisory board,
as a whole assisted, with respect to compensation matters, by
its Human Resources Committee. We have also established a joint
committee (the “Joint Committee”) (
gemeinsamer
Ausschuss
) together with Fresenius SE and our general
partner, Management AG, of the supervisory boards of Management
AG and FMC-AG & Co. KGaA consisting of two members
designated by each supervisory board to advise and decide on
certain extraordinary management measures.
For information regarding the members of our Audit and Corporate
Governance Committee as well as the functions of the Audit and
Corporate Governance Committee, the Joint Committee, our general
partner’s Regulatory and Reimbursement Assessment Committee
and our general partner’s Human Resources Committee, see
Item 6.C, “Directors, Senior Management and
Employees — Board Practices.”
|
|
|
|
Item 17.
|
Financial
Statements
|
Not applicable. See “Item 18. Financial
Statements.”
|
|
|
|
Item 18.
|
Financial
Statements
|
The information called for by this item commences on
Page F-1.
Pursuant to the provisions of the Instructions for the filings
of Exhibits to Annual Reports on
Form 20-F,
Fresenius Medical Care AG & Co. KGaA (the
“Registrant”) is filing the following exhibits
1.1 Articles of Association (Satzung) of the Registrant
(Incorporated by reference to Exhibit 10.1 to the
Registrant’s Report on
Form 6-K
for the month of April 2009, filed April 9, 2009).
2.1 Amended and Restated Deposit Agreement between The Bank
of New York (now The Bank of New York Mellon) and Fresenius
Medical Care AG & Co. KGaA dated as of
February 26, 2007 relating to Ordinary Share ADSs
(Incorporated by reference to Exhibit 3.a. to the
Registration Statement on
Form F-6,
Registration
No. 333-140664,
filed February 13, 2007).
2.2 Amended and Restated Deposit Agreement between The Bank
of New York (now The Bank of New York Mellon) and Fresenius
Medical Care AG & Co. KGaA dated as of
February 26, 2007 relating to Preference Share ADSs
(Incorporated by reference to Exhibit 3.a. to the
Registration Statement on
Form F-6,
Registration
No. 333-140730,
filed February 15, 2007).
2.3 Pooling Agreement dated February 13, 2006 by and
between Fresenius AG, Fresenius Medical Care Management AG and
the individuals acting from time to time as Independent
Directors. (Incorporated by reference to Exhibit 2.3 to the
Registrant’s Annual Report on
Form 20-F
for the year ended December 31, 2005, filed March 2,
2006).
2.4 (Intentionally Omitted.)
2.5 (Intentionally Omitted.)
2.6 Indenture dated as of July 2, 2007 by and among
FMC Finance III S.A., the Registrant and the other Guarantors
party thereto and U.S. Bank National Association, as Trustee
relating to the 6
7
/
8
%
Senior Notes due 2017 of FMC Finance III S.A (Incorporated by
reference to Exhibit 4.3 to the Report on
Form 6-K
furnished to the SEC by Registrant on August 2, 2007).
2.7 Form of Note Guarantee for
6
7
/
8
%
Senior Note due 2017 (included in Exhibit 2.4).
(Incorporated by reference to Exhibit 4.3 to the Report on
Form 6-K
furnished to the SEC by Registrant on August 2, 2007).
2.8 Declaration of Trust of Fresenius Medical Care Capital
Trust IV, dated February 12, 1998 (Incorporated by
reference to Exhibit no. 4.41 to the Registration Statement
on
Form F-4
of Fresenius Medical Care AG
(“FMC-AG”)
et al filed August 2, 2001, Registration
No. 333-66558).
115
2.9 First Amendment to Declaration of Trust of Fresenius
Medical Care Capital Trust IV, dated June 5, 2001
(Incorporated by reference to Exhibit No. 4.42 to the
Registration Statement on
Form F-4
of FMC — AG et al filed August 2, 2001,
Registration
No. 333-66558).
2.10 Declaration of Trust of Fresenius Medical Care Capital
Trust V, dated June 1, 2001 (Incorporated by reference
to Exhibit No. 4.43 to the Registration Statement on
Form F-4
of FMC — AG et al filed August 2, 2001,
Registration
No. 333-66558).
2.11 Amended and Restated Declaration of Trust of Fresenius
Medical Care Capital Trust IV, dated as of June 6,
2001 (Incorporated by reference to Exhibit No. 4.44 to
the Registration Statement on
Form F-4
of FMC — AG et al filed August 2, 2001,
Registration
No. 333-66558).
2.12 Amended and Restated Declaration of Trust of Fresenius
Medical Care Capital Trust V, dated as of June 15,
2000 (Incorporated by reference to Exhibit No. 4.45 to
the Registration Statement on
Form F-4
of FMC — AG et al filed August 2, 2001,
Registration
No. 333-66558).
2.13 Senior Subordinated Indenture (U.S. Dollar
denominated) dated as of June 6, 2001, among FMC-AG, FMC
Trust Finance S.à.r.l. Luxembourg-III, State Street
Bank and Trust Company, as Trustee, and the Subsidiary
Guarantors named therein (Incorporated by reference to
Exhibit No. 4.46 to the Registration Statement on
Form F-4
of FMC — AG et al filed August 2, 2001,
Registration
No. 333-66558).
2.14 Senior Subordinated Indenture (Euro denominated) dated
as of June 15, 2001, among FMC-AG, FMC Trust Finance
S.à.r.l. Luxembourg-III, State Street Bank and
Trust Company, as Trustee, and the Subsidiary Guarantors
named therein (Incorporated by reference to
Exhibit No. 4.47 to the Registration Statement on
Form F-4
of FMC — AG et al filed August 2, 2001,
Registration
No. 333-66558).
2.15 Guarantee Agreement dated as of June 6, 2001
between FMC — AG and State Street Bank and
Trust Company as Trustee, with respect to Fresenius Medical
Care Capital Trust IV (Incorporated by reference to
Exhibit No. 4.48 to the Registration Statement on
Form F-4
of FMC — AG et al filed August 2, 2001,
Registration
No. 333-66558).
2.16 Guarantee Agreement dated as of June 15, 2001
between FMC — AG and State Street Bank and
Trust Company as Trustee, with respect to Fresenius Medical
Care Capital Trust V (Incorporated by reference to
Exhibit No. 4.49 to the Registration Statement on
Form F-4
of FMC — AG et al filed August 2, 2001,
Registration
No. 333-66558).
2.17 Agreement as to Expenses and Liabilities between
FMC — AG and Fresenius Medical Care Capital
Trust IV dated as of June 6, 2001 (Incorporated by
reference to Exhibit No. 4.50 to the Registration
Statement on
Form F-4
of FMC — AG et al filed August 2, 2001,
Registration
No. 333-66558).
2.18 Agreement as to Expenses and Liabilities between
FMC — AG and Fresenius Medical Care Capital
Trust V dated as of June 15, 2001 (Incorporated by
reference to Exhibit No. 4.51 to the Registration
Statement on
Form F-4
of FMC — AG et al filed August 2, 2001,
Registration
No. 333-66558).
2.19 First Supplemental Indenture dated as of
December 23, 2004 among FMC-AG, FMC Trust Finance
S.à.r.l. Luxembourg-III, US Bank, National Association,
successor to State Street Bank and Trust Company, as
Trustee, and the Subsidiary Guarantors named therein
(incorporated by reference to Exhibit No. 2.28 to the
Annual Report of FMC — AG for the year ended
December 31, 2004 filed March 1, 2005).
2.20 Fifth Amended and Restated Transfer and Administration
Agreement dated as of November 17, 2009 by and among NMC
Funding Corporation, as transferor, National Medical Care, Inc.,
as initial collection agent, Paradigm Funding LLC, and other
conduit investors party thereto, the financial institutions
party thereto, The Bank of Nova Scotia, Barclays Bank PLC,
Bayeriche Landesbank, New York Branch, Calyon New York Branch
and Royal Bank of Canada, as administrative agents, and WestLB
AG, New York Branch, as administrative agent and as agent (filed
herewith).
2.21 Amended and Restated Receivables Purchase Agreement
dated October 16, 2008 between National Medical Care, Inc.
and NMC Funding Corporation (incorporated by reference to
Exhibit 10.1 to the
Form 6-K
of the Registrant for the six months ended June 30, 2009,
furnished August 5,
2009).
(1)
2.22 Amendment No. 1 dated November 17, 2009 to
Amended and Restated Receivables Purchase Agreement (filed
herewith).
2.23 Bank Credit Agreement dated as of March 31, 2006
among the Registrant, Fresenius Medical Care Holdings, Inc., and
certain subsidiaries of the Registrant as Borrowers and
Guarantors, Bank of America N.A., as Administrative Agent,
Deutsche Bank AG New York Branch, as Sole Syndication Agent, The
Bank of Nova Scotia,
116
Credit Suisse, Cayman Islands Branch, and JPMorgan Chase Bank,
National Association, as Co-Documentation Agents and the Lenders
named therein (incorporated by reference to
Exhibit No. 4.1 to the
Form 6-K
of the Registrant for the three months ended March 31, 2006
furnished May 17,
2006).
(2)
2.24 Term Loan Credit Agreement dated as of March 31,
2006 among and the Registrant, Fresenius Medical Care Holdings,
Inc., and certain subsidiaries of the Registrant as Borrowers
and Guarantors, Bank of America N.A., as Administrative Agent,
Deutsche Bank AG New York Branch, as Sole Syndication Agent, The
Bank of Nova Scotia, Credit Suisse, Cayman Islands Branch, and
JPMorgan Chase Bank, National Association, as Co-Documentation
Agents and the Lenders named therein (incorporated by reference
to Exhibit 4.2 to the
Form 6-K
of the Registrant for the three month period ended
March 31, 2006 furnished May 17,
2006).
(2)
2.25 Amendment No. 1 dated as of June 26, 2007 to
Bank Credit Agreement dated as of March 31, 2006 among the
Company, Fresenius Medical Care Holdings, Inc., and certain
subsidiaries of the Company as Borrowers and Guarantors, Bank of
America N.A., as Administrative Agent, Deutsche Bank AG New York
Branch, as Sole Syndication Agent, The Bank of Nova Scotia,
Credit Suisse, Cayman Islands Branch, and JPMorgan Chase Bank,
National Association, as Co-Documentation Agents and the Lenders
named therein (incorporated by reference to Exhibit 4.1 to
the Report on
Form 6-K
furnished to the SEC by FMC-KGaA on August 2, 2007).
2.26 Amendment No. 1 dated as of June 26, 2007 to
Term Loan Credit Agreement dated as of March 31, 2006 among
and the Company, Fresenius Medical Care Holdings, Inc., and
certain subsidiaries of the Company as Borrowers and Guarantors,
Bank of America N.A., as Administrative Agent, Deutsche Bank AG
New York Branch, as Sole Syndication Agent, The Bank of Nova
Scotia, Credit Suisse, Cayman Islands Branch, and JPMorgan Chase
Bank, National Association, as Co-Documentation Agents and the
Lenders named therein (Incorporated by reference to
Exhibit 4.2 to the Report on
Form 6-K
furnished to the SEC by FMC-KGaA on August 2, 2007).
2.27 Amendment No. 2 dated as of January 31, 2008
to Bank Credit Agreement dated as of March 31, 2006 among
the Company, Fresenius Medical Care Holdings, Inc., and certain
subsidiaries of the Company as Borrowers and Guarantors, Bank of
America N.A., as Administrative Agent, Deutsche Bank AG New York
Branch, as Sole Syndication Agent, The Bank of Nova Scotia,
Credit Suisse, Cayman Islands Branch, and JPMorgan Chase Bank,
National Association, as Co-Documentation Agents and the Lenders
named therein (Incorporated by reference to Exhibit 2.24 to
the Registrant’s Annual Report on
Form 20-F
for the year ended December 31, 2008 filed on
February 20, 2009).
2.28 Amendment No. 2 dated as of January 31, 2008
to Term Loan Credit Agreement dated as of March 31, 2006
among and the Company, Fresenius Medical Care Holdings, Inc.,
and certain subsidiaries of the Company as Borrowers and
Guarantors, Bank of America N.A., as Administrative Agent,
Deutsche Bank AG New York Branch, as Sole Syndication Agent, The
Bank of Nova Scotia, Credit Suisse, Cayman Islands Branch, and
JPMorgan Chase Bank, National Association, as Co-Documentation
Agents and the Lenders named therein (Incorporated by reference
to Exhibit 2.24 to the Registrant’s Annual Report on
Form 20-F
for the year ended December 31, 2008 filed on
February 20, 2009).
4.1 Agreement and Plan of Reorganization dated as of
February 4, 1996 between W.R. Grace & Co. and
Fresenius AG. (Incorporated by reference to Appendix A to
the Joint Proxy Statement-Prospectus of FMC-AG, W.R.
Grace & Co. and Fresenius USA, Inc., dated
August 2, 1996).
4.2 Distribution Agreement by and among W.R.
Grace & Co., W.R., Grace & Co. —
Conn. and Fresenius AG dated as of February 4, 1996.
(Incorporated by reference to Appendix A to the Joint Proxy
Statement-Prospectus of FMC-AG, W.R. Grace & Co. and
Fresenius USA, Inc., dated August 2, 1996).
4.3 Contribution Agreement by and among Fresenius AG,
Sterilpharma GmbH and W.R. Grace & Co. —
Conn. dated February 4, 1996. (Incorporated by reference to
Appendix E to the Joint Proxy Statement-Prospectus of
FMC-AG, W.R. Grace & Co. and Fresenius USA, Inc.,
dated August 2, 1996).
4.4 Renewed Post-Closing Covenants Agreement effective
January 1, 2007 between Fresenius AG and Registrant
(Incorporated by reference to Exhibit 4.4 to the
Registrant’s Amended Annual Report on
Form 20-F/A
for the year ended December 31, 2006 filed on
February 26, 2007).
4.5 Lease Agreement for Office Buildings dated
September 30, 1996 by and between Fresenius AG and
Fresenius Medical Care Deutschland GmbH. (Incorporated by
reference to Exhibit 10.3 to FMC-AG’s Registration
Statement on
Form F-1,
Registration
No. 333-05922,
filed November 18, 1996).
4.6 Amendment for Lease Agreement for Office Buildings
dated December 19, 2006 by and between Fresenius AG and
Fresenius Medical Care Deutschland GmbH (Incorporated by
reference to Exhibit 4.5 to the
117
Registrant’s Amended Annual Report on
Form 20-F/A
for the year ended December 31, 2006 filed on
February 26, 2007).
4.7 Lease Agreement for Manufacturing Facilities dated
September 30, 1996 by and between Fresenius
Immobilien-Verwaltungs-GmbH & Co. Objekt Schweinfurt
KG and Fresenius Medical Care Deutschland GmbH. (Incorporated by
reference to Exhibit 10.4.1 to FMC-AG’s Registration
Statement on
Form F-1,
Registration
No. 333-05922,
filed November 16, 1996).
4.8 Amendment for Lease Agreement for Manufacturing
Facilities dated December 19, 2006 by and between Fresenius
Immobilien-Verwaltungs-GmbH & Co. Objekt Schweinfurt
KG and Fresenius Medical Care Deutschland GmbH. (Incorporated by
reference to Exhibit 4.6 to the Registrant’s Amended
Annual Report on
Form 20-F/A
for the year ended December 31, 2006 filed on
February 26, 2007).
4.9 Schweinfurt facility rental agreement between Fresenius
Immobilien-Verwaltungs-GmbH & Co, Objekt Schweinfurt
KG, as Lessor, and Fresenius Medical Care Deutschland GmbH, as
Lessee, dated February 6, 2008 and effective
October 1, 2007, supplementing the Principal Lease dated
December 18, 2006 (Incorporated by reference to
Exhibit 10.1 to the Report of
Form 6-K
furnished to the SEC by FMC-KGaA on April 30, 2008).
4.10 Lease Agreement for Manufacturing Facilities dated
September, 1996 by and between Fresenius
Immobilien-Verwaltungs-GmbH & Co. Objekt St. Wendel KG
and Fresenius Medical Care Deutschland GmbH. (Incorporated by
reference to Exhibit 10.4.2 to FMC-AG’s Registration
Statement on
Form F-1,
Registration
No. 333-05922,
filed November 16, 1996).
4.11 Amendment for Lease Agreement for Manufacturing
Facilities dated December 19, 2006 by and between Fresenius
Immobilien-Verwaltungs-GmbH & Co. Objekt St. Wendel KG
and Fresenius Medical Care Deutschland GmbH. (Incorporated by
reference to Exhibit 4.7 to the Registrant’s Amended
Annual Report on
Form 20-F/A
for the year ended December 31, 2006 filed on
February 26, 2007).
4.12 Lease Agreement for Manufacturing Facilities dated
September 30, 1996 by and between Fresenius AG and
Fresenius Medical Care Deutschland GmbH (Ober-Erlenbach)
(Incorporated by reference to Exhibit 10.5 to FMC-AG’s
Registration Statement on
Form F-1,
Registration
No. 333-05922,
filed November 18, 1996).
4.13 Amendment for Lease Agreement for Manufacturing
Facilities dated December 19, 2006 by and between Fresenius
AG and Fresenius Medical Care Deutschland GmbH (Ober-Erlenbach).
(Incorporated by reference to Exhibit 4.8 to the
Registrant’s Amended Annual Report on
Form 20-F/A
for the year ended December 31, 2006 filed on
February 26, 2007).
4.14 Trademark License Agreement dated September 27,
1996 by and between Fresenius AG and FMC-AG. (Incorporated by
reference to Exhibit 10.8 to FMC-AG’s Registration
Statement on
Form F-1,
Registration
No. 333-05922,
filed November 16, 1996).
4.15 Technology License Agreement (Biofine) dated
September 27, 1996 by and between Fresenius AG and FMC-AG.
(Incorporated by reference to Exhibit 10.9 to FMC-AG’s
Registration Statement on
Form F-1,
Registration
No. 333-05922,
filed November 16, 1996).
4.16 Cross-License Agreement dated September 27, 1996
by and between Fresenius AG and FMC-AG. (Incorporated by
reference to Exhibit 10.10 to FMC-AG’s Registration
Statement on
Form F-1,
Registration
No. 333-05922,
filed November 16, 1996).
4.17 Lease Agreement for Office Buildings dated
September 30, 1996 by and between Fresenius AG and
Fresenius Medical Care Deutschland GmbH (Daimler Str.)
(Incorporated by reference to Exhibit 2.8 to FMC-AG’s
Annual Report on
Form 20-F
for the year ended December 31, 1996, filed April 7,
1997).
4.18 Amendment for Lease Agreement for Office Buildings
dated December 19, 2006 by and between Fresenius AG and
Fresenius Medical Care Deutschland GmbH (Daimler Str.).
(Incorporated by reference to Exhibit 4.12 to the
Registrant’s Amended Annual Report on
Form 20-F/A
for the year ended December 31, 2006 filed on
February 26, 2007).
4.19 FMC — AG 1998 Stock Incentive Plan adopted
effective as of April 6, 1998. (Incorporated by reference
to Exhibit 4.8 to FMC-AG’s Report on
Form 6-K
for the three months ended March 31, 1998, furnished
May 14, 1998).
4.20 FMC — AG Stock Option Plan of June 10,
1998 (for non-North American employees). (Incorporated by
reference to Exhibit 1.2 to FMC-AG’s Annual Report on
Form 20-F,
for the year ended December 31, 1998, filed March 24,
1999).
118
4.21 Fresenius Medical Care Aktiengesellschaft 2001
International Stock Incentive Plan (Incorporated by reference to
Exhibit No. 10.17 to the Registration Statement on
Form F-4
of FMC — AG et al filed August 2, 2001,
Registration
No. 333-66558).
4.22 Stock Option Plan 2006 of Fresenius Medical Care
AG & Co. KGaA (Incorporated by reference to
Exhibit 10.2 to the Registrant’s
Form 6-K/A
for the six-month period ended June 30, 2006 furnished
August 11, 2006).
4.23 Sourcing and Supply Agreement dated October 13,
2006, by and among Amgen, Inc., Amgen USA, Inc., and Fresenius
Medical Care Holdings, Inc. (Incorporated by reference to
Exhibit 4.18 to the Registrant’s Amended Annual Report
on
Form 20-F/A
for the year ended December 31, 2006 filed on
February 26,
2007).
(1)
4.24 Amendment No. 1 to Sourcing and Supply agreement
dated effective October 1, 2006, among Fresenius Medical
Care Holdings, Inc. Amgen Inc. and Amgen USA Inc. (Incorporated
by reference to Exhibit 10.2 to the Report on
Form 6-K
furnished to the SEC by FMC-KGaA on August 2,
2007).
(2)
4.25 Amendment No. 2 to Sourcing and Supply agreement
dated effective October 1, 2006, among Fresenius Medical
Care Holdings, Inc. Amgen Inc. and Amgen USA Inc. (Incorporated
by reference to Exhibit 10.3 to the Report on
Form 6-K
furnished to the SEC by FMC-KGaA on August 2,
2007).
(2)
4.26 Amendment No. 3 to Sourcing and Supply agreement
dated effective October 1, 2006, among Fresenius Medical
Care Holdings, Inc. Amgen Inc. and Amgen USA Inc. (Incorporated
by reference to Exhibit 10.4 the Report on
Form 6-K
furnished to the SEC by FMC-KGaA on August 2,
2007).
(2)
4.27 Amendment No. 4 to Sourcing and Supply agreement
dated effective October 1, 2006, among Fresenius Medical
Care Holdings, Inc. Amgen Inc. and Amgen USA Inc. (Incorporated
by reference to Exhibit 10.5 to the Report on
Form 6-K
furnished to the SEC by FMC-KGaA on August 2,
2007).
(2)
4.28 Settlement Agreement dated as of February 6, 2003
by and among FMC-AG, Fresenius Medical Care Holdings, National
Medical Care, Inc., the Official Committee of Asbestos Personal
Injury Claimants, and the Official Committee of Asbestos
Property Damage Claimants of W.R. Grace & Co.
(incorporated by reference to Exhibit No. 10.18 on
Form 10-K
of Fresenius Medical Care Holdings, Inc. for the year ended
December 31, 2002 filed March 17, 2002).
4.29 Amended and Restated Subordinated Loan Note dated as
of March 31, 2006, among National Medical Care, Inc. and
certain of its subsidiaries as borrowers and Fresenius AG as
lender (incorporated herein by reference to Exhibit 4.3 to
the Registrant’s
Form 6-K
for the three month period ended March 31, 2006 furnished
May 17,
2006).
(1)
4.30 Agreement Containing Consent Orders, United States of
America before Federal Trade Commission, In the Matter of
Fresenius AG, File
No. 051-0154.
(Incorporated by reference to Exhibit 10.1 to the
Registrant’s
Form 6-K
for the three-month period ended March 31, 2006 furnished
May 17, 2006).
4.31 Decision and Order, United States of America before
Federal Trade Commission, In the Matter of Fresenius AG.
(Incorporated by reference to Exhibit 10.3 to the
Registrant’s
Form 6-K
for the three-month period ended March 31, 2006 furnished
May 17, 2006).
4.32 License, Distribution, Manufacturing and Supply
Agreement by and between Luitpold Pharmaceuticals, Inc.,
American Regent, Inc., and Fresenius USA Manufacturing, Inc.,
dated June, 2008 (Incorporated by reference to Exhibit 10.1
to the Report of
Form 6-K
furnished to the SEC FMC-KGaA on November 5,
2008).
(2)
4.33 First Amendment dated September 13, 2008 to the
License, Distribution, Manufacturing and Supply Agreement by and
between Luipold Pharmaceuticals, Inc., American Regent, Inc.,
and Fresenius USA Manufacturing, Inc., dated September, 2008
(Incorporated by reference to Exhibit 10.2 to the Report of
Form 6-K
furnished to the SEC by FMC-KGaA on November 5,
2008).
(2)
8.1 List of Significant Subsidiaries. Our significant
subsidiaries are identified in “Item 4.C. Information
on the Company — Organizational Structure.”
11.1 Code of Business Conduct. A copy of the
Registrant’s Code of Business Conduct is available on the
Registrant’s web site at:
http://www.fmc-ag.com/Code_of_Conduct.htm
.
12.1 Certification of Chief Executive Officer of the
general partner of the Registrant Pursuant to Section 302
of the Sarbanes-Oxley Act of 2002 (filed herewith).
12.2 Certification of Chief Financial Officer of the
general partner of the Registrant Pursuant to Section 302
of the Sarbanes-Oxley Act of 2002 (filed herewith).
119
13.1 Certification of Chief Executive Officer and Chief
Financial Officer of the general partner of the Registrant
Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to
Section 906 of the Sarbanes-Oxley Act of 2002 (furnished
herewith). (This Exhibit is furnished herewith, but not deemed
“filed” for purposes of Section 18 of the
Securities Exchange Act of 1934, as amended, or otherwise
subject to liability under that section. Such certification will
not be deemed to be incorporated by reference into any filing
under the Securities Act or the Exchange Act, except to the
extent that we explicitly incorporate it by reference.)
14.1 Consent of KPMG, independent registered public
accounting firm (filed herewith).
101 The following financial statements as of and for the
year ended December 31, 2009 from FMC-AG & Co.
KGaA’s Report on
Form 20-F
for the month of February 2010, formatted in XBRL (eXtensible
Business Language Reporting Language): (i) Consolidated
Statements of Income, (ii) Consolidated Statements of
Comprehensive Income, (iii) Consolidated Balance Sheets,
(iv) Consolidated Statements of Cash Flows,
(v) Consolidated Statements of Shareholders’ Equity
and (vi) Notes to Consolidated Financial Statements, tagged
as blocks of text.
|
|
|
|
(1)
|
Portions of this exhibit have been omitted pursuant to a request
for confidential treatment and file separately with the
Securities and Exchange Commission.
|
|
|
|
(2)
|
Confidential treatment has been granted as to certain portions
of this document in accordance with the applicable rules of the
Securities and Exchange Commission.
|
120
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of
1934, the Registrant has duly caused this report to be signed on
its behalf by the undersigned thereunto duly authorized.
DATE: February 24, 2010
Fresenius Medical Care
AG & Co. KG
aA
a partnership limited by shares, represented by:
fresenius medical care
management ag,
its general partner
Name: Dr. Ben J. Lipps
|
|
|
|
|
|
Title:
|
Chief Executive Officer and
|
Chairman of the Management Board
of the General Partner
Name: Michael Brosnan
|
|
|
|
|
|
Title:
|
Chief Financial Officer and Member
|
of the Management Board of the
General Partner
121
INDEX OF
FINANCIAL STATEMENTS
|
|
|
|
|
|
|
Audited Consolidated Financial Statements
|
|
|
|
|
|
|
|
|
F-2
|
|
|
|
|
|
F-3
|
|
|
|
|
|
F-4
|
|
|
|
|
|
F-5
|
|
|
|
|
|
F-6
|
|
|
|
|
|
F-7
|
|
|
|
|
|
F-8
|
|
|
|
|
|
F-9
|
|
|
|
|
|
F-10
|
|
|
|
|
|
S-II
|
|
F-1
MANAGEMENT’S
ANNUAL REPORT ON INTERNAL CONTROL
OVER FINANCIAL REPORTING
Management of the Company is responsible for establishing and
maintaining adequate internal control over financial reporting,
as such term is defined in Exchange Act
Rule 13a-15(f).
The Company’s internal control over financial reporting is
a process designed by or under the supervision of the
Company’s chief executive officer and chief financial
officer to provide reasonable assurance regarding the
reliability of financial reporting and the preparation of the
Company’s financial statements for external reporting
purposes in accordance with U.S. generally accepted accounting
principles.
As of December 31, 2009, management conducted an assessment
of the effectiveness of the Company’s internal control over
financial reporting based on the criteria established in
Internal Control — Integrated Framework issued by the
Committee of Sponsoring Organizations of the Treadway Commission
(COSO). Management’s assessment follows the guidance for
management of the evaluation of internal controls over financial
reporting released by the Securities and Exchange Commission on
May 23, 2007. Based on this assessment, management has
determined that the Company’s internal control over
financial reporting is effective as of December 31, 2009.
The Company’s internal control over financial reporting
includes policies and procedures that (1) pertain to the
maintenance of records that in reasonable detail accurately and
fairly reflect transactions and dispositions of assets;
(2) provide reasonable assurance that the Company’s
transactions are recorded as necessary to permit preparation of
financial statements in accordance with U.S. generally accepted
accounting principles, and that the Company’s receipts and
expenditures are being made only in accordance with
authorizations of the Company’s management and directors;
and (3) provide reasonable assurance regarding prevention
or timely detection of unauthorized acquisition, use or
disposition of the Company’s assets that could have a
material effect on the Company’s financial statements.
Because of its inherent limitation, internal control over
financial reporting, no matter how well designed, cannot provide
absolute assurance of achieving financial reporting objectives
and may not prevent or detect misstatements. Therefore, even if
the internal control over financial reporting is determined to
be effective it can provide only reasonable assurance with
respect to financial statement preparation and presentation.
Also, projections of any evaluation of effectiveness to future
periods are subject to the risk that controls may become
inadequate because of changes in conditions, or that the degree
of compliance with the policies or procedures may deteriorate.
The effectiveness of the Company’s internal control over
financial reporting as of December 31, 2009 has been
audited by KPMG AG Wirtschaftsprüfungsgesellschaft, an
independent registered public accounting firm, as stated in
their report included on
page F-4.
Date: February 24, 2010
Fresenius Medical Care
AG & Co.
KGaA,
a partnership limited by shares, represented by:
fresenius medical care
management ag
, its
General Partner
By: /s/ DR. BEN LIPPS
Name: Dr. Ben Lipps
|
|
|
|
|
|
Title:
|
Chief Executive Officer and Chairman
|
of the Management Board of the
General Partner
By: /s/ MICHAEL BROSNAN
Name: Michael Brosnan
|
|
|
|
|
|
Title:
|
Chief Financial Officer and member
|
of the Management Board of the
General Partner
F-2
REPORT OF
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Supervisory Board
Fresenius Medical Care AG & Co. KGaA:
We have audited the accompanying consolidated balance sheets of
Fresenius Medical Care AG & Co. KGaA and subsidiaries
(“Fresenius Medical Care” or the “Company”)
as of December 31, 2009 and 2008 and the related
consolidated statements of income, shareholders’ equity,
and cash flows for each of the years in the three-year period
ended December 31, 2009. In connection with our audits of
the consolidated financial statements, we have also audited the
financial statement schedule as listed in the accompanying
index. These consolidated financial statements and the financial
statement schedule are the responsibility of the Company’s
management. Our responsibility is to express an opinion on these
consolidated financial statements and the financial statement
schedule based on our audits.
We conducted our audits in accordance with the standards of the
Public Company Accounting Oversight Board (United States). Those
standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are
free of material misstatement. An audit includes examining, on a
test basis, evidence supporting the amounts and disclosures in
the financial statements. An audit also includes assessing the
accounting principles used and significant estimates made by
management, as well as evaluating the overall financial
statement presentation. We believe that our audits provide a
reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred
to above present fairly, in all material respects, the financial
position of Fresenius Medical Care as of December 31, 2009
and 2008, and the results of their operations and their cash
flows for each of the years in the three-year period ended
December 31, 2009, in conformity with U.S. generally
accepted accounting principles. Also in our opinion, the related
financial statement schedule, when considered in relation to the
consolidated financial statements taken as a whole, presents
fairly, in all material respects, the information set forth
therein.
We also have audited, in accordance with the standards of the
Public Company Accounting Oversight Board (United States), the
effectiveness of Fresenius Medical Care’s internal control
over financial reporting as of December 31, 2009, based on
criteria established in Internal Control — Integrated
Framework issued by the Committee of Sponsoring Organizations of
the Treadway Commission (COSO), and our report dated
February 24, 2010 expressed an unqualified opinion on the
effective operation of internal control over financial reporting.
Frankfurt am Main, Germany
February 24, 2010
/s/ KPMG AG
Wirtschaftsprüfungsgesellschaft
F-3
REPORT OF
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
ON INTERNAL CONTROL OVER FINANCIAL REPORTING
The Supervisory Board
Fresenius Medical Care AG & Co. KGaA:
We have audited the internal control over financial reporting of
Fresenius Medical Care AG & Co. KGaA and subsidiaries
(“Fresenius Medical Care” or the “Company”)
as of December 31, 2009, based on criteria established in
Internal Control-Integrated Framework issued by the Committee of
Sponsoring Organizations of the Treadway Commission (COSO).
Fresenius Medical Care’s management is responsible for
maintaining effective internal control over financial reporting
and its assessment of the effectiveness of internal control over
financial reporting included in the accompanying
Management’s Annual Report on Internal Control over
Financial Reporting. Our responsibility is to express an opinion
on the effectiveness of the Company’s internal control over
financial reporting based on our audit.
We conducted our audit in accordance with the standards of the
Public Company Accounting Oversight Board (United States). Those
standards require that we plan and perform the audit to obtain
reasonable assurance about whether effective internal control
over financial reporting was maintained in all material
respects. Our audit included obtaining an understanding of
internal control over financial reporting, assessing the risk
that a material weakness exists, and testing and evaluating the
design and operating effectiveness of internal control based on
the assessed risk and performing such other procedures as we
considered necessary in the circumstances. We believe that our
audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a
process designed to provide reasonable assurance regarding the
reliability of financial reporting and the preparation of
financial statements for external purposes in accordance with
generally accepted accounting principles. A company’s
internal control over financial reporting includes those
policies and procedures that (1) pertain to the maintenance
of records that, in reasonable detail, accurately and fairly
reflect the transactions and dispositions of the assets of the
company; (2) provide reasonable assurance that transactions
are recorded as necessary to permit preparation of financial
statements in accordance with generally accepted accounting
principles, and that receipts and expenditures of the company
are being made only in accordance with authorizations of
management and directors of the company; and (3) provide
reasonable assurance regarding prevention or timely detection of
unauthorized acquisition, use, or disposition of the
company’s assets that could have a material effect on the
financial statements.
Because of its inherent limitations, internal control over
financial reporting may not prevent or detect misstatements.
Also, projections of any evaluation of effectiveness to future
periods are subject to the risk that controls may become
inadequate because of changes in conditions, or that the degree
of compliance with the policies or procedures may deteriorate.
In our opinion, Fresenius Medical Care maintained, in all
material respects, effective internal control over financial
reporting as of December 31, 2009, based on criteria
established in Internal Control-Integrated Framework issued by
the Committee of Sponsoring Organizations of the Treadway
Commission (COSO).
We also have audited, in accordance with the standards of the
Public Company Accounting Oversight Board (United States), the
consolidated balance sheets of Fresenius Medical Care as of
December 31, 2009 and 2008, and the related consolidated
statements of income, shareholders’ equity, and cash flows
for each of the years in the three-year period ended
December 31, 2009, and our report dated February 24,
2010 expressed an unqualified opinion on those consolidated
financial statements.
Frankfurt am Main, Germany
February 24, 2010
/s/ KPMG AG
Wirtschaftsprüfungsgesellschaft
F-4
FRESENIUS
MEDICAL CARE AG & Co. KGaA
Consolidated Statements of Income
For the years ended December 31,
(in thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Net revenue:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dialysis Care
|
|
$
|
8,350,233
|
|
|
$
|
7,737,498
|
|
|
$
|
7,213,000
|
|
|
Dialysis Products
|
|
|
2,897,244
|
|
|
|
2,874,825
|
|
|
|
2,507,314
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
11,247,477
|
|
|
|
10,612,323
|
|
|
|
9,720,314
|
|
|
Costs of revenue:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dialysis Care
|
|
|
5,945,724
|
|
|
|
5,547,615
|
|
|
|
5,130,287
|
|
|
Dialysis Products
|
|
|
1,470,241
|
|
|
|
1,435,860
|
|
|
|
1,234,232
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7,415,965
|
|
|
|
6,983,475
|
|
|
|
6,364,519
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
3,831,512
|
|
|
|
3,628,848
|
|
|
|
3,355,795
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative
|
|
|
1,982,106
|
|
|
|
1,876,177
|
|
|
|
1,709,150
|
|
|
Research and development
|
|
|
93,810
|
|
|
|
80,239
|
|
|
|
66,523
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income
|
|
|
1,755,596
|
|
|
|
1,672,432
|
|
|
|
1,580,122
|
|
|
Other (income) expense:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income
|
|
|
(21,397
|
)
|
|
|
(24,811
|
)
|
|
|
(28,588
|
)
|
|
Interest expense
|
|
|
321,360
|
|
|
|
361,553
|
|
|
|
399,635
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income before income taxes
|
|
|
1,455,633
|
|
|
|
1,335,690
|
|
|
|
1,209,075
|
|
|
Income tax expense
|
|
|
490,413
|
|
|
|
475,702
|
|
|
|
453,765
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
|
965,220
|
|
|
|
859,988
|
|
|
|
755,310
|
|
|
Less: Net income attributable to Noncontrolling interests
|
|
|
74,082
|
|
|
|
42,381
|
|
|
|
38,180
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income attributable to FMC-AG & Co. KGaA
|
|
$
|
891,138
|
|
|
$
|
817,607
|
|
|
$
|
717,130
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic income per ordinary share
|
|
$
|
2.99
|
|
|
$
|
2.75
|
|
|
$
|
2.43
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fully diluted income per ordinary share
|
|
$
|
2.99
|
|
|
$
|
2.74
|
|
|
$
|
2.42
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes to consolidated financial statements.
F-5
FRESENIUS
MEDICAL CARE AG & Co. KGaA
Consolidated Statements of Comprehensive Income
For the years ended December 31,
(in thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Net Income
|
|
$
|
965,220
|
|
|
$
|
859,988
|
|
|
$
|
755,310
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gain (loss) related to cash flow hedges
|
|
|
30,082
|
|
|
|
(108,240
|
)
|
|
|
(88,374
|
)
|
|
Actuarial gains (losses) on defined benefit pension plans
|
|
|
9,708
|
|
|
|
(28,551
|
)
|
|
|
35,729
|
|
|
Foreign currency translation
|
|
|
82,545
|
|
|
|
(168,336
|
)
|
|
|
146,308
|
|
|
Income tax (expense) benefit related to components of other
comprehensive income
|
|
|
(18,971
|
)
|
|
|
55,692
|
|
|
|
21,891
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income (loss), net of tax
|
|
|
103,364
|
|
|
|
(249,435
|
)
|
|
|
115,554
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income
|
|
$
|
1,068,584
|
|
|
$
|
610,553
|
|
|
$
|
870,864
|
|
|
Comprehensive income attributable to Noncontrolling interests
|
|
|
75,886
|
|
|
|
45,108
|
|
|
|
47,440
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income attributable to FMC-AG & Co. KGaA
|
|
$
|
992,698
|
|
|
$
|
565,445
|
|
|
$
|
823,424
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes to consolidated financial statements.
F-6
FRESENIUS
MEDICAL CARE AG & Co. KGaA
(in thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
301,225
|
|
|
$
|
221,584
|
|
|
Trade accounts receivable less allowance for doubtful accounts
of $266,449 in 2009 and $262,836 in 2008
|
|
|
2,285,909
|
|
|
|
2,176,316
|
|
|
Accounts receivable from related parties
|
|
|
272,886
|
|
|
|
175,525
|
|
|
Inventories
|
|
|
821,654
|
|
|
|
707,050
|
|
|
Prepaid expenses and other current assets
|
|
|
729,306
|
|
|
|
607,399
|
|
|
Deferred taxes
|
|
|
316,820
|
|
|
|
324,123
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
4,727,800
|
|
|
|
4,211,997
|
|
|
|
|
|
|
|
|
|
|
|
|
Property, plant and equipment, net
|
|
|
2,419,570
|
|
|
|
2,236,078
|
|
|
Intangible assets
|
|
|
859,195
|
|
|
|
846,496
|
|
|
Goodwill
|
|
|
7,511,434
|
|
|
|
7,309,910
|
|
|
Deferred taxes
|
|
|
64,749
|
|
|
|
92,805
|
|
|
Other assets
|
|
|
238,567
|
|
|
|
222,390
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
15,821,315
|
|
|
$
|
14,919,676
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and shareholders’ equity
|
|
|
|
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
362,407
|
|
|
$
|
366,017
|
|
|
Accounts payable to related parties
|
|
|
277,429
|
|
|
|
239,243
|
|
|
Accrued expenses and other current liabilities
|
|
|
1,335,553
|
|
|
|
1,288,433
|
|
|
Short-term borrowings and other financial liabilities
|
|
|
316,344
|
|
|
|
683,155
|
|
|
Short-term borrowings from related parties
|
|
|
10,440
|
|
|
|
1,330
|
|
|
Current portion of long-term debt and capital lease obligations
|
|
|
157,634
|
|
|
|
455,114
|
|
|
Income tax payable
|
|
|
116,978
|
|
|
|
82,468
|
|
|
Deferred taxes
|
|
|
32,930
|
|
|
|
28,652
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
2,609,715
|
|
|
|
3,144,412
|
|
|
|
|
|
|
|
|
|
|
|
|
Long-term debt and capital lease obligations, less current
portion
|
|
|
4,427,921
|
|
|
|
3,957,379
|
|
|
Other liabilities
|
|
|
307,112
|
|
|
|
319,602
|
|
|
Pension liabilities
|
|
|
147,327
|
|
|
|
136,755
|
|
|
Income tax payable
|
|
|
215,921
|
|
|
|
171,747
|
|
|
Deferred taxes
|
|
|
427,530
|
|
|
|
426,299
|
|
|
Company-obligated mandatorily redeemable preferred securities of
subsidiary Fresenius Medical Care Capital Trusts holding solely
|
|
|
|
|
|
|
|
|
|
Company-guaranteed debentures of subsidiaries
|
|
|
656,096
|
|
|
|
640,696
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
8,791,622
|
|
|
|
8,796,890
|
|
|
Shareholders’ equity:
|
|
|
|
|
|
|
|
|
|
Preference shares, no par value, €1.00 nominal value,
12,356,880 shares authorized, 3,884,328 issued and
outstanding
|
|
|
4,343
|
|
|
|
4,240
|
|
|
Ordinary shares, no par value, €1.00 nominal value,
373,436,220 shares authorized, 295,746,635 issued and
outstanding
|
|
|
365,672
|
|
|
|
363,076
|
|
|
Additional paid-in capital
|
|
|
3,389,111
|
|
|
|
3,293,918
|
|
|
Retained earnings
|
|
|
3,111,530
|
|
|
|
2,452,332
|
|
|
Accumulated other comprehensive (loss) income
|
|
|
(49,724
|
)
|
|
|
(151,284
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total FMC-AG & Co. KGaA shareholders’ equity
|
|
|
6,820,932
|
|
|
|
5,962,282
|
|
|
|
|
|
|
|
|
|
|
|
|
Noncontrolling interests
|
|
|
208,761
|
|
|
|
160,504
|
|
|
Total equity
|
|
|
7,029,693
|
|
|
|
6,122,786
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and equity
|
|
$
|
15,821,315
|
|
|
$
|
14,919,676
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes to consolidated financial statements.
F-7
FRESENIUS
MEDICAL CARE AG & Co. KGaA
Consolidated Statements of Cash Flows
For the years ended December 31,
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Operating Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
965,220
|
|
|
$
|
859,988
|
|
|
$
|
755,310
|
|
|
Adjustments to reconcile net income to net cash
|
|
|
|
|
|
|
|
|
|
|
|
|
|
provided by operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
457,085
|
|
|
|
415,671
|
|
|
|
363,330
|
|
|
Change in deferred taxes, net
|
|
|
22,002
|
|
|
|
133,047
|
|
|
|
1,177
|
|
|
(Gain) loss on sale of investments
|
|
|
(1,250
|
)
|
|
|
(24,049
|
)
|
|
|
913
|
|
|
Loss on sale of fixed assets
|
|
|
1,308
|
|
|
|
2,985
|
|
|
|
2,703
|
|
|
Compensation expense related to stock options
|
|
|
33,746
|
|
|
|
31,879
|
|
|
|
24,208
|
|
|
Changes in assets and liabilities, net of amounts from
businesses acquired:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade accounts receivable, net
|
|
|
(41,994
|
)
|
|
|
(241,967
|
)
|
|
|
(62,735
|
)
|
|
Inventories
|
|
|
(88,933
|
)
|
|
|
(94,112
|
)
|
|
|
(72,825
|
)
|
|
Prepaid expenses, other current and non-current assets
|
|
|
(147,105
|
)
|
|
|
(84,089
|
)
|
|
|
(6,623
|
)
|
|
Accounts receivable from related parties
|
|
|
(144,224
|
)
|
|
|
(32,747
|
)
|
|
|
56,538
|
|
|
Accounts payable to related parties
|
|
|
138,506
|
|
|
|
64,999
|
|
|
|
(78,803
|
)
|
|
Accounts payable, accrued expenses and other current and
non-current liabilities
|
|
|
71,092
|
|
|
|
(17,040
|
)
|
|
|
113,960
|
|
|
Income tax payable
|
|
|
73,164
|
|
|
|
1,833
|
|
|
|
102,421
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by operating activities
|
|
|
1,338,617
|
|
|
|
1,016,398
|
|
|
|
1,199,574
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Investing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant and equipment
|
|
|
(573,606
|
)
|
|
|
(687,356
|
)
|
|
|
(572,721
|
)
|
|
Proceeds from sale of property, plant and equipment
|
|
|
11,730
|
|
|
|
13,846
|
|
|
|
29,668
|
|
|
Acquisitions and investments, net of cash acquired,
|
|
|
|
|
|
|
|
|
|
|
|
|
|
and net purchases of intangible assets
|
|
|
(188,113
|
)
|
|
|
(276,473
|
)
|
|
|
(263,395
|
)
|
|
Proceeds from divestitures
|
|
|
51,965
|
|
|
|
58,582
|
|
|
|
29,495
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash (used in) investing activities
|
|
|
(698,024
|
)
|
|
|
(891,401
|
)
|
|
|
(776,953
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from short-term borrowings and other financial
liabilities
|
|
|
107,192
|
|
|
|
176,104
|
|
|
|
96,995
|
|
|
Repayments of short-term borrowings and other financial
liabilities
|
|
|
(169,175
|
)
|
|
|
(183,210
|
)
|
|
|
(107,793
|
)
|
|
Proceeds from short-term borrowings from related parties
|
|
|
18,830
|
|
|
|
168,641
|
|
|
|
43,554
|
|
|
Repayments of short-term borrowings from related parties
|
|
|
(118,422
|
)
|
|
|
(169,573
|
)
|
|
|
(46,071
|
)
|
|
Proceeds from long-term debt and capital lease obligations (net
of
|
|
|
709,540
|
|
|
|
458,951
|
|
|
|
516,762
|
|
|
debt issuance costs of $16,703 in 2007)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Repayments of long-term debt and capital lease obligations
|
|
|
(566,241
|
)
|
|
|
(135,492
|
)
|
|
|
(486,513
|
)
|
|
Redemption of trust preferred securities
|
|
|
—
|
|
|
|
(678,379
|
)
|
|
|
—
|
|
|
(Decrease) increase of accounts receivable securitization program
|
|
|
(325,000
|
)
|
|
|
454,000
|
|
|
|
(181,000
|
)
|
|
Proceeds from exercise of stock options
|
|
|
72,394
|
|
|
|
43,887
|
|
|
|
46,934
|
|
|
Repurchase of preferred stock
|
|
|
—
|
|
|
|
—
|
|
|
|
(7,660
|
)
|
|
Dividends paid
|
|
|
(231,940
|
)
|
|
|
(252,395
|
)
|
|
|
(188,407
|
)
|
|
Distributions to Noncontrolling interests
|
|
|
(68,004
|
)
|
|
|
(38,592
|
)
|
|
|
(27,469
|
)
|
|
Contributions from Noncontrolling interests
|
|
|
12,699
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash (used in) financing activities
|
|
|
(558,127
|
)
|
|
|
(156,058
|
)
|
|
|
(340,668
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate changes on cash and cash equivalents
|
|
|
(2,825
|
)
|
|
|
7,955
|
|
|
|
3,727
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and Cash Equivalents:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash and cash equivalents
|
|
|
79,641
|
|
|
|
(23,106
|
)
|
|
|
85,680
|
|
|
Cash and cash equivalents at beginning of period
|
|
|
221,584
|
|
|
|
244,690
|
|
|
|
159,010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at end of period
|
|
$
|
301,225
|
|
|
$
|
221,584
|
|
|
$
|
244,690
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes to consolidated financial statements.
F-8
FRESENIUS
MEDICAL CARE AG & Co. KGaA
Consolidated Statement of Shareholders’ Equity
For the years ended December 31, 2009, 2008 and 2007
(in thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
FMC-AG &
|
|
|
|
|
|
|
|
|
|
|
Preference Shares
|
|
|
Ordinary Shares
|
|
|
Additional
|
|
|
|
|
|
Other
|
|
|
Co. KGaA
|
|
|
|
|
|
|
|
|
|
|
Number of
|
|
|
No par
|
|
|
Number of
|
|
|
No par
|
|
|
paid in
|
|
|
Retained
|
|
|
comprehensive
|
|
|
shareholders’
|
|
|
Noncontrolling
|
|
|
|
|
|
|
|
shares
|
|
|
value
|
|
|
shares
|
|
|
value
|
|
|
capital
|
|
|
earnings
|
|
|
income (loss)
|
|
|
equity
|
|
|
interests
|
|
|
Total
|
|
|
|
|
Balance at December 31, 2006
|
|
|
3,711,435
|
|
|
$
|
4,098
|
|
|
|
291,449,673
|
|
|
$
|
359,527
|
|
|
$
|
3,153,556
|
|
|
$
|
1,358,397
|
|
|
$
|
(5,416
|
)
|
|
$
|
4,870,162
|
|
|
$
|
75,158
|
|
|
$
|
4,945,320
|
|
|
Proceeds from exercise of options and related tax effects
|
|
|
66,652
|
|
|
|
93
|
|
|
|
1,336,910
|
|
|
|
1,857
|
|
|
|
43,880
|
|
|
|
—
|
|
|
|
|
|
|
|
45,830
|
|
|
|
|
|
|
|
45,830
|
|
|
Compensation expense related to stock options
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
24,208
|
|
|
|
—
|
|
|
|
|
|
|
|
24,208
|
|
|
|
|
|
|
|
24,208
|
|
|
Dividends paid
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(188,407
|
)
|
|
|
|
|
|
|
(188,407
|
)
|
|
|
(27,469
|
)
|
|
|
(215,876
|
)
|
|
Purchase/ sale of Noncontrolling interests
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
|
5,628
|
|
|
|
5,628
|
|
|
Contributions from Noncontrolling interests
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
|
5,057
|
|
|
|
5,057
|
|
|
Net income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
717,130
|
|
|
|
|
|
|
|
717,130
|
|
|
|
38,180
|
|
|
|
755,310
|
|
|
Other comprehensive income (loss)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
106,294
|
|
|
|
106,294
|
|
|
|
9,260
|
|
|
|
115,554
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
106,294
|
|
|
|
823,424
|
|
|
|
47,440
|
|
|
|
870,864
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2007
|
|
|
3,778,087
|
|
|
$
|
4,191
|
|
|
|
292,786,583
|
|
|
$
|
361,384
|
|
|
$
|
3,221,644
|
|
|
$
|
1,887,120
|
|
|
$
|
100,878
|
|
|
$
|
5,575,217
|
|
|
$
|
105,814
|
|
|
$
|
5,681,031
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from exercise of options and related tax effects
|
|
|
32,453
|
|
|
|
49
|
|
|
|
1,145,453
|
|
|
|
1,692
|
|
|
|
40,395
|
|
|
|
—
|
|
|
|
|
|
|
|
42,136
|
|
|
|
—
|
|
|
|
42,136
|
|
|
Compensation expense related to stock options
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
31,879
|
|
|
|
—
|
|
|
|
|
|
|
|
31,879
|
|
|
|
—
|
|
|
|
31,879
|
|
|
Dividends paid
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(252,395
|
)
|
|
|
|
|
|
|
(252,395
|
)
|
|
|
(38,592
|
)
|
|
|
(290,987
|
)
|
|
Purchase/ sale of Noncontrolling interests
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
|
31,000
|
|
|
|
31,000
|
|
|
Contributions from Noncontrolling interests
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
|
17,174
|
|
|
|
17,174
|
|
|
Net income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
817,607
|
|
|
|
|
|
|
|
817,607
|
|
|
|
42,381
|
|
|
|
859,988
|
|
|
Other comprehensive income (loss)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(252,162
|
)
|
|
|
(252,162
|
)
|
|
|
2,727
|
|
|
|
(249,435
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(252,162
|
)
|
|
|
565,445
|
|
|
|
45,108
|
|
|
|
610,553
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2008
|
|
|
3,810,540
|
|
|
|
4,240
|
|
|
|
293,932,036
|
|
|
|
363,076
|
|
|
|
3,293,918
|
|
|
|
2,452,332
|
|
|
|
(151,284
|
)
|
|
|
5,962,282
|
|
|
|
160,504
|
|
|
|
6,122,786
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from exercise of options and related tax effects
|
|
|
73,788
|
|
|
|
103
|
|
|
|
1,814,599
|
|
|
|
2,596
|
|
|
|
64,585
|
|
|
|
—
|
|
|
|
—
|
|
|
|
67,284
|
|
|
|
—
|
|
|
|
67,284
|
|
|
Compensation expense related to stock options
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
33,746
|
|
|
|
—
|
|
|
|
|
|
|
|
33,746
|
|
|
|
—
|
|
|
|
33,746
|
|
|
Dividends paid
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(231,940
|
)
|
|
|
|
|
|
|
(231,940
|
)
|
|
|
(61,499
|
)
|
|
|
(293,439
|
)
|
|
Purchase/ sale of Noncontrolling interests
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(3,138
|
)
|
|
|
|
|
|
|
|
|
|
|
(3,138
|
)
|
|
|
25,477
|
|
|
|
22,339
|
|
|
Contributions from Noncontrolling interests
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
|
8,393
|
|
|
|
8,393
|
|
|
Net income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
891,138
|
|
|
|
|
|
|
|
891,138
|
|
|
|
74,082
|
|
|
|
965,220
|
|
|
Other comprehensive income (loss)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101,560
|
|
|
|
101,560
|
|
|
|
1,804
|
|
|
|
103,364
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
101,560
|
|
|
|
992,698
|
|
|
|
75,886
|
|
|
|
1,068,584
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009
|
|
|
3,884,328
|
|
|
$
|
4,343
|
|
|
|
295,746,635
|
|
|
$
|
365,672
|
|
|
$
|
3,389,111
|
|
|
$
|
3,111,530
|
|
|
$
|
(49,724
|
)
|
|
$
|
6,820,932
|
|
|
$
|
208,761
|
|
|
$
|
7,029,693
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes to consolidated financial statements.
F-9
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share data)
|
|
|
|
1.
|
The
Company, Basis of Presentation and Summary of Significant
Accounting Policies
|
The
Company
Fresenius Medical Care AG & Co. KGaA
(“FMC-AG & Co. KGaA” or the
“Company,” “we,” “us” or
“our” and together with its subsidiaries on a
consolidated basis, as the context requires), a German
partnership limited by shares (
Kommanditgesellschaft auf
Aktien
), is the world’s largest kidney dialysis
company, operating in both the field of dialysis services and
the field of dialysis products for the treatment of end-stage
renal disease (“ESRD”). The Company’s dialysis
business is vertically integrated, providing dialysis treatment
at dialysis clinics it owns or operates and supplying these
clinics with a broad range of products. In addition, the Company
sells dialysis products to other dialysis service providers. In
the United States, the Company also performs clinical laboratory
testing and provides inpatient dialysis services and other
services under contract to hospitals.
Basis of
Presentation
On July 1, 2009, the Financial Accounting Standards Board
(“FASB”) issued
FASB Accounting Standards
Codification
tm
(“ASC”) 105,
Generally Accepted Accounting
Principles
(originally issued
as FASB Statement
No. 168-FASB
accounting Standards Codification and the Hierarchy of Generally
Accepted Accounting Principles
). ASC 105 establishes the
FASB ASC as the exclusive authoritative reference for
nongovernmental United States generally accepted accounting
principles (“U.S. GAAP”) for use in financial
statements issued for interim and annual periods ending after
September 15, 2009, except for SEC rules and interpretive
releases, which are also authoritative GAAP for SEC registrants.
This divides nongovernmental U.S. GAAP into the authoritative
ASC and guidance that is nonauthoritative. The contents of the
ASC carry the same level of authority, eliminating the
four-level GAAP hierarchy previously set forth in FASB
Statement No. 162, which has been superseded by the ASC.
The ASC supersedes or makes nonauthoritative all other existing
non-grandfathered, non-SEC accounting literature and reporting
standards not included in the ASC. The accompanying consolidated
financial statements have been prepared in accordance with U.S.
GAAP.
The Company evaluated the financial statements for subsequent
events through the date of the submission of this 20-F to the
Securities and Exchange Commission. See Note 2.
Income tax expense in the amount of $13,440 and $11,887 for the
years ended December 31, 2008 and 2007, in the prior
year’s comparative consolidated financial statements has
been reclassified to income attributable to noncontrolling
interests to conform with the current year’s presentation.
Summary
of Significant Accounting Policies
a) Principles
of Consolidation
The consolidated financial statements include all companies in
which the Company has legal or effective control. In addition,
the Company consolidates variable interest entities
(“VIEs”) for which it is deemed the primary
beneficiary. In accordance with current accounting principles,
the Company also consolidates certain clinics that it manages.
The equity method of accounting is used for investments in
associated companies (20% to 50% owned). Noncontrolling
interests represent the proportionate equity interests of owners
in the Company’s consolidated entities that are not wholly
owned. All significant intercompany transactions and balances
have been eliminated.
The Company entered into various arrangements with certain
dialysis clinics to provide management services, financing and
product supply. A group of these clinics has negative equity and
are unable to provide their own funding, therefore the Company
has agreed to fund their operations for at least a six year
period. The funding carries no interest but the Company is
entitled to a pro rata share of profits, if any, and has a right
of first refusal in the event the owners sell the business or
assets. These clinics are VIEs in which the Company has been
determined to be the primary beneficiary and which therefore
have been fully consolidated. They generated approximately
$87,999,
F-10
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
$88,508, and $79,164 in revenue in 2009, 2008, and 2007,
respectively. The table below shows the carrying amounts of the
assets and liabilities of these VIEs:
|
|
|
|
|
|
|
Trade accounts receivable, net
|
|
$
|
31,060
|
|
|
Other current assets
|
|
|
11,576
|
|
|
Property, plant and equipment, intangible assets &
other non-current assets
|
|
|
8,921
|
|
|
Goodwill
|
|
|
18,941
|
|
|
Accounts payable, accrued expenses and other liabilities
|
|
|
(25,108
|
)
|
|
Non-current loans to related parties
|
|
|
(4,016
|
)
|
|
Equity
|
|
|
(41,375
|
)
|
b) Cash
and Cash Equivalents
Cash and cash equivalents comprise cash funds and all
short-term, liquid investments with original maturities of up to
three months.
c) Allowance
for Doubtful Accounts
Estimates for the allowances for accounts receivable from the
dialysis care business are based mainly on past collection
history. Specifically, the allowances for the North America
services division are based on an analysis of collection
experience, recognizing the differences between payors and aging
of accounts receivable. From time to time, accounts receivable
are reviewed for changes from the historic collection experience
to ensure the appropriateness of the allowances. The allowances
in the International Segment and the products business are based
on estimates and consider various factors, including aging,
debtor and past collection history.
d) Inventories
Inventories are stated at the lower of cost (determined by using
the average or
first-in,
first-out method) or market value (see Note 5). Costs
included in inventories are based on invoiced costs
and/or
production costs as applicable. Included in production costs are
material, direct labor and production overhead, including
depreciation charges.
e) Property,
Plant and Equipment
Property, plant, and equipment are stated at cost less
accumulated depreciation (see Note 6). Significant
improvements are capitalized; repairs and maintenance costs that
do not extend the useful lives of the assets are charged to
expense as incurred. Property and equipment under capital leases
are stated at the present value of future minimum lease payments
at the inception of the lease, less accumulated depreciation.
Depreciation on property, plant and equipment is calculated
using the straight-line method over the estimated useful lives
of the assets ranging from 5 to 50 years for buildings and
improvements with a weighted average life of 12 years and 3
to 15 years for machinery and equipment with a weighted
average life of 10 years. Equipment held under capital
leases and leasehold improvements are amortized using the
straight-line method over the shorter of the lease term or the
estimated useful life of the asset. Internal use platform
software that is integral to the computer equipment it supports
is included in property, plant and equipment. The Company
capitalizes interest on borrowed funds during construction
periods. Interest capitalized during 2009, 2008, and 2007 was
$10,395, $8,723, and $5,323, respectively.
f) Intangible
Assets and Goodwill
Intangible assets such as non-compete agreements, technology,
distribution rights, patents, licenses to treat, licenses to
manufacture, distribute and sell pharmaceutical drugs, trade
names, management contracts, application software, acute care
agreements, lease agreements, and licenses acquired in a
purchase method business combination are recognized and reported
apart from goodwill (see Note 7).
Goodwill and identifiable intangibles with indefinite useful
lives are not amortized but tested for impairment annually or
when an event becomes known that could trigger an impairment.
The Company identified trade names and certain qualified
management contracts as intangible assets with indefinite useful
lives because, based on an
F-11
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
analysis of all of the relevant factors, there is no foreseeable
limit to the period over which those assets are expected to
generate net cash inflows for the Company. Intangible assets
with finite useful lives are amortized over their respective
useful lives to their residual values. The Company amortizes
non-compete agreements over their average useful life of
8 years. Technology is amortized over its useful life of
15 years. Licenses to manufacture, distribute and sell
pharmaceutical drugs are amortized over their average useful
life of 10 years. The U.S. intravenous iron products
distribution and manufacturing agreement is amortized over its
10 year contractual license period based upon the annual
estimated units of sale of the licensed product. All other
intangible assets are amortized over their weighted average
useful lives of 6 years. The average useful life of all
amortizable intangible assets is 9 years. Intangible assets
with finite useful lives are evaluated for impairment when
events have occurred that may give rise to an impairment.
To perform the annual impairment test of goodwill, the Company
identified its reporting units and determined their carrying
value by assigning the assets and liabilities, including the
existing goodwill and intangible assets, to those reporting
units. A reporting unit is usually defined one level below the
segment level based on regions or legal entities. Two reporting
units were identified in the segment North America (Renal
Therapy Group and Fresenius Medical Services). The segment
International is divided into two reporting units (Europe and
Latin America), while only one reporting unit exists in the
segment Asia Pacific.
In a first step, the Company compares the fair value of a
reporting unit to its carrying amount. Fair value is determined
using estimated future cash flows for the unit discounted by a
weighted average cost of capital (“WACC”) specific to
that reporting unit. Estimating the discounted future cash flows
involves significant assumptions, especially regarding future
reimbursement rates and sales prices, number of treatments,
sales volumes and costs. In determining discounted cash flows,
the Company utilizes for every reporting unit, its three-year
budget, projections for years 4 to 10 and a corresponding growth
rate for all remaining years. Projections for up to ten years
are possible due to the stability of the Company’s business
which, due to the non-discretionary nature of the healthcare
services we provide, the need for products utilized to provide
such services and the availability of government reimbursement
for a substantial portion of our services, has been largely
independent from the economic cycle. The reporting units’
respective expected growth rates for the period beyond ten years
are: Renal Therapy Group 1%, Fresenius Medical Services 1%,
Europe 0%, Latin America 4%, and Asia Pacific 4%. The discount
factor is determined by the WACC of the respective reporting
unit. The Company’s WACC consists of a basic rate of 6.45%
for 2009. The basic rate is then adjusted by a country-specific
risk rate within each reporting unit. In 2009, WACCs for the
reporting units ranged from 6.45% to 12.05%.
In the case that the fair value of the reporting unit is less
than its book value, a second step is performed which compares
the fair value of the reporting unit’s goodwill to the
carrying value of its goodwill. If the fair value of the
goodwill is less than the book value, the difference is recorded
as an impairment.
To evaluate the recoverability of intangible assets with
indefinite useful lives, the Company compares the fair values of
intangible assets with their carrying values. An intangible
asset’s fair value is determined using a discounted cash
flow approach or other methods, if appropriate.
g) Derivative
Financial Instruments
Derivative financial instruments which primarily include foreign
currency forward contracts and interest rate swaps are
recognized as assets or liabilities at fair value in the balance
sheet (see Note 19). Changes in the fair value of
derivative financial instruments classified as fair value hedges
and in the corresponding underlyings are recognized periodically
in earnings. The effective portion of changes in fair value of
cash flow hedges is recognized in accumulated other
comprehensive income (loss) in shareholders’ equity. The
ineffective portion of cash flow hedges is recognized in
earnings immediately. The change in fair value of derivatives
that do not qualify for hedge accounting are recorded in the
income statement and usually offset the changes in value
recorded in the income statement for the underlying asset or
liability.
h) Foreign
Currency Translation
For purposes of these consolidated financial statements, the
U.S. dollar is the reporting currency. Substantially all assets
and liabilities of the parent company and all
non-U.S.
subsidiaries are translated at year-end exchange rates, while
revenues and expenses are translated at average exchange rates.
Adjustments for foreign currency translation fluctuations are
excluded from net earnings and are reported in accumulated other
comprehensive
F-12
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
income (loss). In addition, the translation adjustments of
certain intercompany borrowings, which are considered foreign
equity investments, are reported in accumulated other
comprehensive income (loss).
i) Revenue
Recognition Policy
Dialysis care revenues are recognized on the date services and
related products are provided and the payor is obligated to pay
at amounts estimated to be received under reimbursement
arrangements with third party payors. Medicare and Medicaid in
North America and programs involving other government payors in
the International Segment are billed at pre-determined rates per
treatment that are established by statute or regulation. Most
non-governmental payors are billed at our standard rates for
services net of contractual allowances to reflect the estimated
amounts to be received under reimbursement arrangements with
these payors.
Dialysis product revenues are recognized when title to the
product passes to the customers either at the time of shipment,
upon receipt by the customer or upon any other terms that
clearly define passage of title. As product returns are not
typical, no return allowances are established. In the event a
return is required, the appropriate reductions to sales,
accounts receivables and cost of sales are made. Sales are
stated net of discounts and rebates.
A minor portion of International Segment product revenues is
generated from arrangements which give the customer, typically a
health care provider, the right to use dialysis machines. In the
same contract the customer agrees to purchase the related
treatment disposables at a price marked up from the standard
price list. In this type of contract, FMC-AG & Co.
KGaA does not recognize revenue upon delivery of the dialysis
machine but recognizes revenue, including the
mark-up,
on
the sale of disposables. In certain other contracts of this
type, the contract is structured as a sales type lease whereby
ownership of the dialysis machine is transferred to the user
upon installation of the dialysis machine at the customer site.
In this type of contract, revenue is recognized in accordance
with the accounting principles for sales type leases.
Any tax assessed by a governmental authority that is incurred as
a result of a revenue transaction (e.g. sales tax) is excluded
from revenues and reported on a net basis.
j) Research
and Development expenses
Research and development expenses are expensed as incurred.
k) Income
Taxes
The Company recognizes deferred tax assets and liabilities for
future consequences attributable to temporary differences
between the financial statement carrying amounts of existing
assets and liabilities and their respective tax basis as well as
on consolidation procedures affecting net income and tax loss
carryforwards which are more likely than not to be utilized.
Deferred tax assets and liabilities are measured using enacted
tax rates expected to apply to taxable income in the years in
which those temporary differences are expected to be recovered
or settled. A valuation allowance is recorded to reduce the
carrying amount of the deferred tax assets unless it is more
likely than not that such assets will be realized (see
Note 16).
It is the Company’s policy to recognize interest and
penalties related to its tax positions as income tax expense.
l) Impairment
The Company reviews the carrying value of its long-lived assets
or asset groups with definite useful lives to be held and used
for impairment whenever events or changes in circumstances
indicate that the carrying value of these assets may not be
recoverable. Recoverability of these assets is measured by a
comparison of the carrying value of an asset to the future net
cash flows directly associated with the asset. If assets are
considered to be impaired, the impairment recognized is the
amount by which the carrying value exceeds the fair value of the
asset. The Company uses a discounted cash flow approach or other
methods, if appropriate, to assess fair value.
Long-lived assets to be disposed of by sale are reported at the
lower of carrying value or fair value less cost to sell and
depreciation is ceased. Long-lived assets to be disposed of
other than by sale are considered to be held and used until
disposal.
F-13
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
m) Debt
Issuance Costs
Costs related to the issuance of debt are amortized over the
term of the related obligation (see Note 10).
n) Self-Insurance
Programs
Under the insurance programs for professional, product and
general liability, auto liability and worker’s compensation
claims, the Company’s largest subsidiary is partially
self-insured for professional liability claims. For all other
coverages, the Company assumes responsibility for incurred
claims up to predetermined amounts above which third party
insurance applies. Reported liabilities for the year represent
estimated future payments of the anticipated expense for claims
incurred (both reported and incurred but not reported) based on
historical experience and existing claim activity. This
experience includes both the rate of claims incidence (number)
and claim severity (cost) and is combined with individual claim
expectations to estimate the reported amounts.
o) Use
of Estimates
The preparation of consolidated financial statements in
conformity with U.S. GAAP requires management to make estimates
and assumptions that affect the reported amounts of assets and
liabilities and disclosure of contingent assets and liabilities
at the date of the consolidated financial statements and the
reported amounts of revenues and expenses during the reporting
period. Actual results could differ from those estimates.
p) Concentration
of Risk
The Company is engaged in the manufacture and sale of products
for all forms of kidney dialysis, principally to health care
providers throughout the world, and in providing kidney dialysis
treatment, clinical laboratory testing, and other medical
ancillary services. The Company performs ongoing evaluations of
its customers’ financial condition and, generally, requires
no collateral.
Approximately 33%, 35%, and 36% of the Company’s worldwide
revenues were earned and subject to regulations under
governmental health care programs, Medicare and Medicaid,
administered by the United States government in 2009, 2008, and
2007, respectively.
See Note 5 for concentration of supplier risks.
q) Legal
Contingencies
From time to time, during the ordinary course of the
Company’s operations, the Company is party to litigation
and arbitration and is subject to investigations relating to
various aspects of its business (see Note 18). The Company
regularly analyzes current information about such claims for
probable losses and provides accruals for such matters,
including the estimated legal expenses and consulting services
in connection with these matters, as appropriate. The Company
utilizes its internal legal department as well as external
resources for these assessments. In making the decision
regarding the need for loss accrual, the Company considers the
degree of probability of an unfavorable outcome and its ability
to make a reasonable estimate of the amount of loss.
The filing of a suit or formal assertion of a claim or
assessment, or the disclosure of any such suit or assertion,
does not necessarily indicate that accrual of a loss is
appropriate.
r) Earnings
per Ordinary share and Preference share
Basic earnings per ordinary share and basic earnings per
preference share for all years presented have been calculated
using the two-class method required under U.S. GAAP based upon
the weighted average number of ordinary and preference shares
outstanding. Basic earnings per share is computed by dividing
net income less preference amounts by the weighted average
number of ordinary shares and preference shares outstanding
during the year. Basic earnings per preference share is derived
by adding the preference per preference share to the basic
earnings per share. Diluted earnings per share include the
effect of all potentially dilutive instruments on ordinary
shares and preference shares that would have been outstanding
during the year.
The awards granted under the Company’s stock incentive
plans (see Note 15), are potentially dilutive equity
instruments.
F-14
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
s) Employee
Benefit Plans
The Company recognizes the underfunded status of its defined
benefit plans, measured as the difference between plan assets at
fair value and the benefit obligation, as a liability. Changes
in the funded status of a plan, net of tax, resulting from
actuarial gains or losses and prior service costs or credits
that are not recognized as components of the net periodic
benefit cost will be recognized through accumulated other
comprehensive income in the year in which they occur. Actuarial
gains or losses and prior service costs are subsequently
recognized as components of net periodic benefit cost when
realized. The Company uses December 31 as the measurement date
when measuring the funded status of all plans.
t) Stock
Option Plans
Effective January 1, 2006, the Company adopted the
provisions of the accounting standards for share-based payments
using the modified prospective transition method (see
Note 15). Under this transition method, compensation cost
recognized in 2006 and subsequent years includes applicable
amounts of: (a) compensation cost of all stock-based
payments granted prior to, but not yet vested as of,
January 1, 2006, and (b) compensation cost for all
stock-based payments subsequent to January 1, 2006 based on
the grant-date fair value estimated in accordance with the
provisions of these standards.
u) Recent
Pronouncements
Recently
Implemented Accounting Statements
In January 2010, the Financial Accounting Standards Board
(“FASB”) issued
Accounting Standards Update
2010-06
(“ASU
2010-06”),
an update for ASC (see “Basis of Presentation”, above)
820-10,
Fair-Value Measurements and Disclosures,
resulting in new
disclosure requirements with regard to the following areas:
|
|
|
|
|
|
•
|
Fair-value measurements are to be disaggregated by class, as
opposed to the current disclosure requirement of by major
category
|
|
|
|
|
•
|
Disclosure of significant transfers of assets and liabilities in
and/or
out
of Level 1 and Level 2, in addition to transfers in
and/or
out
of the Level 3 category
|
|
|
|
|
•
|
Purchases, sales, issuances, and settlements of Level 3
assets and liabilities are to be disclosed separately
|
|
|
|
|
•
|
Disclosure of the valuation techniques and inputs used to
determine fair value for Level 2 and Level 3 fair-value
measurements, as well as changes in valuation techniques used
and the reasons for the changes
|
The disclosures required under ASU
2010-06
are
effective for reporting periods beginning after
December 15, 2009, with the exception of the disclosures
about purchases, sales, issuances, and settlements in the roll
forward of Level 3 activity, which are effective for fiscal
years beginning after December 31, 2010, and for interim
periods within those fiscal years. Early adoption is permitted
for the additional disclosures. The Company adopted all
disclosures required under this update as of December 31,
2009.
Recently
Issued Accounting Statements
In June 2009, the FASB issued
Accounting Standards Update
2009-17
(“ASU
2009-17”)
(originally issued as FASB Statement No. 167), ASC 810,
Consolidations — Improvements to Financial
Reporting by Enterprises Involved with Variable Interest
Entities
. ASU
2009-17
requires reporting entities to evaluate former Qualifying
Special Purpose Entities (“QSPE”) for consolidation
and changes the approach to determining a VIE’s primary
beneficiary from a quantitative assessment to a qualitative
assessment designed to identify a controlling financial
interest. In addition, ASU
2009-17
increases the frequency of required reassessments to determine
whether a company is the primary beneficiary of a VIE. It also
clarifies, but does not significantly change, the
characteristics that identify a VIE. ASU
2009-17
also
requires additional year-end and interim disclosures about risks
related to continuing involvement in transferred financial
assets.
The amendments contained in ASU
2009-17
are
effective as of the beginning of a company’s first fiscal
year that begins after November 15, 2009 and for subsequent
interim and annual reporting periods. All former QSPEs and other
variable interest entities will need to be reevaluated under the
amended consolidation requirements as of
F-15
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
the beginning of the first annual reporting period that begins
after November 15, 2009. Early adoption is prohibited. The
Company will implement the amendments prescribed by ASU
2009-17
as
of January 1, 2010.
In June 2009, the FASB issued
Accounting Standards Update
2009-16
(“ASU
2009-16”)
(originally issued as FASB Statement No. 166), ASC 860,
Transfers and Servicing — Accounting for Transfers
of Financial Assets
. ASU
2009-16
eliminates the QSPE concept, creates more stringent conditions
for reporting a transfer of a portion of a financial asset as a
sale, clarifies the derecognition criteria, revises how retained
interests are initially measured, and removes the guaranteed
mortgage securitization recharacterization provisions. ASU
2009-16
also
requires additional year-end and interim disclosures about risks
related to variable interest entities.
ASU
2009-16
is effective as of the beginning of a company’s first
fiscal year that begins after November 15, 2009, and for
subsequent interim and annual reporting periods. ASU
2009-16’s
disclosure requirements must be applied to transfers that
occurred before and after its effective date. Early adoption is
prohibited. The Company will adopt provisions of ASU
2009-16
as
of January 1, 2010.
On January 20, 2010, the Company’s wholly owned
subsidiary, FMC Finance VI S.A (“Finance VI”), issued
€250,000 ($353,300 at date of issuance) of senior unsecured
notes (the “5.50% Senior Notes”) with a coupon of
5.50% at an issue price of 98.6636%. The 5.50% Senior Notes have
a yield to maturity of 5.75% and are due July 15, 2016.
Finance VI may redeem the 5.50% Senior Notes at any time at 100%
of principal plus accrued interest and a premium calculated
pursuant to the terms of the indenture. The holders have a right
to request that Finance IV repurchase the 5.50% Senior Notes at
101% of principal plus accrued interest upon the occurrence of a
change of control followed by a decline in the rating of the
5.50% Senior Notes. Proceeds were used to repay short-term
indebtedness and for general corporate purposes. The 5.50%
Senior Notes are guaranteed on a senior basis jointly and
severally by the Company, Fresenius Medical Care Holdings, Inc.
and Fresenius Medical Care Deutschland GmbH.
RSI
Acquisition
On November 26, 2007, the Company completed the acquisition
of all the common stock of Renal Solutions, Inc.
(“RSI”), an Indiana corporation with principal offices
in Warrendale, PA. The RSI acquisition agreement provided for
total consideration of up to $203,666, consisting of $20,000
previously advanced to RSI in the form of a loan, $99,854 paid
at closing, $60,000 paid in November, 2008, $3,572 receivable
related to a working capital adjustment which was received in
2008, and up to $30,000 in milestone payments over a three year
period contingent upon the achievement of certain performance
criteria, of which $20,000 was paid in 2009. In 2007, the
Company recorded a liability of $27,384 representing the net
present value of the $30,000 milestone payments. At
December 31, 2009, the net book value of the remaining
liability was $9,488. The purchase price was allocated to
goodwill ($159,386), intangible assets ($34,480) and other net
assets ($9,800). RSI holds key patents and other intellectual
property worldwide related to sorbent-based technology
(“SORB”). SORB technology purifies potable water to
dialysate quality and allows dialysis for up to 8 hours with
only 6 liters of potable water through a process of dialysate
regeneration and toxin adsorption. This regeneration capability
significantly reduces the water volume requirement for a typical
hemodialysis treatment and is an important step in advancing
home hemodialysis and helping to create a potential platform for
eventual development of a wearable kidney.
The assets and liabilities of all acquisitions were recorded at
their estimated fair values at the dates of the acquisitions and
are included in the Company’s Consolidated Financial
Statements and operating results from the effective date of
acquisition.
|
|
|
|
4.
|
Related
Party Transactions
|
a) Service
Agreements and Leases
The Company is party to service agreements with Fresenius SE,
the sole stockholder of its General Partner and its largest
shareholder with approximately 36.0% ownership of the
Company’s voting shares, and certain affiliates of
Fresenius SE that are not also subsidiaries of the Company to
receive services, including, but not limited to: administrative
services, management information services, employee benefit
administration, insurance, IT services,
F-16
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
tax services and treasury management services. Fees for these
services are negotiated by the involved parties based on
requested service volume and full absorption cost plus a 5%
mark-up.
For
the years 2009, 2008, and 2007, amounts charged by Fresenius SE
to the Company under the terms of these agreements are $68,234,
$59,038, and $44,143, respectively. The Company also provides
certain services to Fresenius SE and certain affiliates of
Fresenius SE, including research and development, central
purchasing, patent administration and warehousing. The fees for
these services are negotiated on the same basis as the fees for
services provided to the Company by Fresenius SE. The Company
charged $13,540, $9,798, and $9,784 for services rendered to
Fresenius SE in 2009, 2008, and 2007, respectively.
Under operating lease agreements for real estate entered into
with Fresenius SE, the Company paid Fresenius SE $23,109,
$23,485, and $19,211 during 2009, 2008, and 2007, respectively.
The majority of the leases expires in 2016 and contain renewal
options.
The Company’s Articles of Association provide that the
General Partner shall be reimbursed for any and all expenses in
connection with management of the Company’s business,
including remuneration of the members of the General
Partner’s supervisory board and the General Partner’s
management board. The aggregate amount reimbursed to Management
AG for 2009, 2008, and 2007 was $7,783, $9,230, and $10,348,
respectively, for its management services during those years and
included $84, $88, and $82 as compensation for their exposure to
risk as General Partner for 2009, 2008, and 2007, respectively.
The Company’s Articles of Association set the annual
compensation for assuming unlimited liability at 4% of the
amount of the General Partner’s invested capital
(€1,500).
b) Products
During the years ended December 31, 2009, 2008, and 2007,
the Company sold products to Fresenius SE for $13,601, $36,704
and $34,133 respectively. During 2009, 2008, and 2007, the
Company made purchases from Fresenius SE in the amount of
$43,320, $45,084 and $52,280 respectively.
In addition to the purchases noted above, the Company currently
purchases heparin supplied by APP Inc., through a group
purchasing organization (“GPO”). In September 2008,
Fresenius Kabi AG, a wholly-owned subsidiary of Fresenius SE,
acquired 100% of APP Inc. The Company has no direct supply
agreement with APP Inc. and does not submit purchase orders
directly to APP Inc. In the twelve-month periods ended
December 31, 2009 and 2008, Fresenius Medical Care
Holdings, Inc. (“FMCH”) acquired approximately $31,300
and $19,564, respectively, of heparin from APP Inc. through the
GPO contract, which was negotiated by the GPO at arm’s
length on behalf of all members of the GPO.
c) Financing
Provided by and to Fresenius SE
The Company receives short-term financing from and provides
short-term financing to Fresenius SE.
On August 19, 2009, the Company borrowed $2,161
(€1,500) from its General Partner at 1.335%, due on
August 19, 2010.
During the second quarter 2009, the Company reclassified an
account payable to Fresenius SE in the amount of €77,745
($109,885 at June 30, 2009) from account payable to
related parties to short-term borrowings from related parties.
The amount represents taxes payable by the Company arising from
the period
1997-2001
during which German trade taxes were paid by Fresenius SE on
behalf of the Company. Of this amount, €5,747 ($8,279 at
December 31, 2009) was outstanding at
December 31, 2009 and will be repaid in 2010 with an
interest rate of 6%. Interest expense incurred on the
€71,998 of the loan repaid in 2009 was $4,313 (€3,092).
See Note 9 for further information on the short-term
borrowings from related parties balance at December 31,
2009.
In addition to the above, there was $1,330 owed to Fresenius SE
at December 31, 2008 which was repaid in 2009.
On November 7, 2008, the Company entered into a loan
agreement with Fresenius SE whereby it advanced Fresenius SE
$50,000 at 6.45% interest which was due and repaid on
April 30, 2009.
F-17
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
d) Other
During the third quarter of 2009 the Company acquired production
lines from Fresenius SE for a purchase price of $3,416, net of
value added tax (VAT).
The Chairman of the Company’s Supervisory Board is also the
Chairman of the Supervisory Board of Fresenius SE. He is also a
member of the Supervisory Board of the Company’s General
Partner.
The Vice Chairman of the Company’s Supervisory Board is a
member of the Supervisory Board of Fresenius SE and Vice
Chairman of the Supervisory Board of the Company’s General
Partner. He is also a partner in a law firm which provided
services to the Company. The Company paid the law firm
approximately $1,445, $1,098, and $969 in 2009, 2008, and 2007,
respectively. Five of the six members of the Company’s
Supervisory Board, including the Chairman and Vice Chairman, are
also members of the Supervisory Board of the Company’s
General Partner.
As of December 31, 2009 and 2008, inventories consisted of
the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Raw materials and purchased components
|
|
$
|
154,599
|
|
|
$
|
145,756
|
|
|
Work in process
|
|
|
63,683
|
|
|
|
60,960
|
|
|
Finished goods
|
|
|
481,047
|
|
|
|
385,607
|
|
|
Health care supplies
|
|
|
122,325
|
|
|
|
114,727
|
|
|
|
|
|
|
|
|
|
|
|
|
Inventories
|
|
$
|
821,654
|
|
|
$
|
707,050
|
|
|
|
|
|
|
|
|
|
|
|
During the first quarter, 2009, inventory adjustments led to an
increase in value of inventory at January 1, 2009, of
$23,327 and a corresponding reduction in costs of revenues sold
during the three month period ending March 31, 2009.
Under the terms of certain unconditional purchase agreements,
the Company is obligated to purchase approximately $2,414,214 of
materials, of which $407,889 is committed at December 31,
2009 for 2010. The terms of these agreements run 1 to
9 years.
Inventories as of December 31, 2009 and 2008 include
$34,788 and $35,143, respectively, of Erythropoietin
(“EPO”), which is supplied by a single source supplier
in the United States. In October 2006, the Company entered into
a five-year exclusive sourcing and supply agreement with its EPO
supplier. Revenues from EPO accounted for approximately 21%,
20%, and 21% of total dialysis care revenue in the North America
segment for 2009, 2008, and 2007, respectively. Delays,
stoppages, or interruptions in the supply of EPO could adversely
affect the operating results of the Company.
|
|
|
|
6.
|
Property,
Plant and Equipment
|
As of December 31, 2009 and 2008, property, plant and
equipment consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Land and improvements
|
|
$
|
44,837
|
|
|
$
|
40,156
|
|
|
Buildings and improvements
|
|
|
1,727,681
|
|
|
|
1,535,017
|
|
|
Machinery and equipment
|
|
|
2,630,925
|
|
|
|
2,352,344
|
|
|
Machinery, equipment and rental equipment under capitalized
leases
|
|
|
29,557
|
|
|
|
22,718
|
|
|
Construction in progress
|
|
|
259,711
|
|
|
|
238,583
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4,692,711
|
|
|
|
4,188,818
|
|
|
Accumulated depreciation
|
|
|
(2,273,141
|
)
|
|
|
(1,952,740
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Property, plant and equipment, net
|
|
$
|
2,419,570
|
|
|
$
|
2,236,078
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation expense for property, plant and equipment amounted
to $396,860, $368,300, and $328,595 for the years ended
December 31, 2009, 2008, and 2007, respectively.
F-18
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
Included in property, plant and equipment as of
December 31, 2009 and 2008 were $364,118 and $299,778,
respectively, of peritoneal dialysis cycler machines which the
Company leases to customers with end-stage renal disease on a
month-to-month
basis and hemodialysis machines which the Company leases to
physicians under operating leases.
Accumulated depreciation related to machinery, equipment and
rental equipment under capital leases was $14,010 and $10,984 at
December 31, 2009 and 2008, respectively.
|
|
|
|
7.
|
Intangible
Assets and Goodwill
|
As of December 31, 2009 and 2008, the carrying value and
accumulated amortization of intangible assets other than
goodwill consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Gross
|
|
|
|
|
|
Gross
|
|
|
|
|
|
|
|
Carrying
|
|
|
Accumulated
|
|
|
Carrying
|
|
|
Accumulated
|
|
|
|
|
Amount
|
|
|
Amortization
|
|
|
Amount
|
|
|
Amortization
|
|
|
|
|
Amortizable Intangible Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-compete Agreements
|
|
$
|
224,579
|
|
|
$
|
(157,717
|
)
|
|
$
|
218,245
|
|
|
$
|
(142,974
|
)
|
|
Technology
|
|
|
100,016
|
|
|
|
(18,109
|
)
|
|
|
100,016
|
|
|
|
(11,490
|
)
|
|
License and distribution agreements
|
|
|
184,219
|
|
|
|
(59,677
|
)
|
|
|
173,244
|
|
|
|
(41,336
|
)
|
|
Self-developed Software
|
|
|
31,230
|
|
|
|
(9,405
|
)
|
|
|
8,656
|
|
|
|
(1,815
|
)
|
|
Other
|
|
|
277,468
|
|
|
|
(210,484
|
)
|
|
|
261,816
|
|
|
|
(197,374
|
)
|
|
Construction in progress
|
|
|
67,113
|
|
|
|
—
|
|
|
|
49,886
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
884,625
|
|
|
$
|
(455,392
|
)
|
|
$
|
811,863
|
|
|
$
|
(394,989
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of December 31, 2009 and 2008 the carrying value of
non-amortizable
intangible assets other than goodwill consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Carrying Amount
|
|
|
Carrying Amount
|
|
|
|
|
Non-amortizable
Intangible Assets
|
|
|
|
|
|
|
|
|
|
Tradename
|
|
$
|
210,348
|
|
|
$
|
210,156
|
|
|
Management contracts
|
|
|
219,614
|
|
|
|
219,466
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
429,962
|
|
|
$
|
429,622
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Intangible Assets
|
|
$
|
859,195
|
|
|
$
|
846,496
|
|
|
|
|
|
|
|
|
|
|
|
The tables below show the amortization expense related to the
amortizable intangible assets for the years presented and the
estimated amortization expense of these assets for the following
five years.
|
|
|
|
|
|
|
Amortization Expense
|
|
|
|
|
|
|
2007
|
|
$
|
34,003
|
|
|
2008
|
|
$
|
47,384
|
|
|
2009
|
|
$
|
60,225
|
|
|
|
|
|
|
|
|
Estimated Amortization
Expense
|
|
|
|
|
|
|
2010
|
|
$
|
61,448
|
|
|
2011
|
|
$
|
57,647
|
|
|
2012
|
|
$
|
54,743
|
|
|
2013
|
|
$
|
53,345
|
|
|
2014
|
|
$
|
52,976
|
|
F-19
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
Intangible
Assets: License and Distribution Agreements
In July 2008, Fresenius Medical Care entered into two separate
license and distribution agreements, one for the U.S. (with
Galenica Ltd. and Luitpold Pharmaceuticals Inc.), the “U.S.
Agreement,” and one for certain countries in Europe and the
Middle East (with Galenica AG and Vifor (International) AG), the
“International Agreement,” to market and distribute
Galenica Ltd’s and Luitpold Pharmaceuticals Inc.’s
intravenous iron products, such as
Venofer
®
and
Ferinject
®
for dialysis treatment. In North America, the license agreement
among our subsidiary, FUSA Manufacturing Inc. (“FMI”),
Luitpold Pharmaceuticals Inc, American Regent, Inc. and Vifor
(International), Inc. provides FMI with exclusive rights to
manufacture and distribute
Venofer
®
to freestanding (non-hospital based) U.S. dialysis facilities.
In addition, it grants FMI similar rights for
Injectafer
®
(ferric carboxymaltose), a proposed new intravenous iron
medication currently under clinical study in the U.S. The U.S.
license agreement has a term of ten years, includes FMI
extension options, and requires payment by FMI over the ten year
term of approximately $2,000,000, which the Company will expense
as incurred (based upon the annual estimated units of sale of
the licensed product), subject to certain early termination
provisions. In addition to these payments, the Company will pay
a total of approximately $47,000 over a four year period for the
U.S Agreement of which $6,111 and $22,000 was paid in 2009 and
2008, respectively. The Company recorded a liability for the
balance. The cost of the U.S. Agreement and related transaction
costs of $5,957 will be amortized over their
10-year
expected useful life (based upon the annual estimated units of
sale of the licensed product). The Company paid $14,566 upon
signing of the International Agreement in 2008 and could pay up
to €40,000 more upon certain milestones being met. The
International Agreement costs will be amortized over their
expected
20-year
useful life. Milestone payments will be capitalized and
amortized over their useful lives at the time the milestone
payments are made, of which $20,922 (€15,000) was paid in
2009.
Goodwill
Changes in the carrying amount of goodwill are mainly a result
of acquisitions and the impact of foreign currency translations.
During 2009 and 2008, the Company’s acquisitions consisted
primarily of clinics in the normal course of operations. The
segment detail is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
North
|
|
|
|
|
|
|
|
|
|
|
|
|
|
America
|
|
|
International
|
|
|
Corporate
|
|
|
Total
|
|
|
|
|
Balance as of January 1, 2008
|
|
$
|
6,508,475
|
|
|
$
|
577,729
|
|
|
$
|
159,385
|
|
|
$
|
7,245,589
|
|
|
Goodwill acquired
|
|
|
64,809
|
|
|
|
30,577
|
|
|
|
432
|
|
|
|
95,818
|
|
|
Reclassifications
|
|
|
(1,231
|
)
|
|
|
12,773
|
|
|
|
—
|
|
|
|
11,542
|
|
|
Foreign Currency Translation Adjustment
|
|
|
(642
|
)
|
|
|
(42,397
|
)
|
|
|
—
|
|
|
|
(43,039
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2008
|
|
$
|
6,571,411
|
|
|
$
|
578,682
|
|
|
$
|
159,817
|
|
|
$
|
7,309,910
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill acquired
|
|
|
123,303
|
|
|
|
52,011
|
|
|
|
—
|
|
|
|
175,314
|
|
|
Foreign Currency Translation Adjustment
|
|
|
(3
|
)
|
|
|
26,213
|
|
|
|
—
|
|
|
|
26,210
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2009
|
|
$
|
6,694,711
|
|
|
$
|
656,906
|
|
|
$
|
159,817
|
|
|
$
|
7,511,434
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8.
|
Accrued
Expenses and Other Current Liabilities
|
As at December 31, 2009 and 2008 accrued expenses and other
current liabilities consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Accrued salaries and wages
|
|
$
|
320,295
|
|
|
$
|
301,923
|
|
|
Unapplied cash and receivable credits
|
|
|
192,626
|
|
|
|
205,187
|
|
|
Accrued insurance
|
|
|
169,866
|
|
|
|
125,713
|
|
|
Special charge for legal matters
|
|
|
115,000
|
|
|
|
115,000
|
|
|
Other
|
|
|
537,766
|
|
|
|
540,610
|
|
|
|
|
|
|
|
|
|
|
|
|
Total accrued expenses and other current liabilities
|
|
$
|
1,335,553
|
|
|
$
|
1,288,433
|
|
|
|
|
|
|
|
|
|
|
|
F-20
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
In 2001, the Company recorded a $258,159 special charge to
address legal matters relating to transactions pursuant to the
Agreement and Plan of Reorganization dated as of
February 4, 1996 by and between W.R. Grace & Co.
and Fresenius SE (the “Merger”), estimated liabilities
and legal expenses arising in connection with the W.R.
Grace & Co. Chapter 11 proceedings (the
“Grace Chapter 11 Proceedings”) and the cost of
resolving pending litigation and other disputes with certain
commercial insurers. During the second quarter of 2003, the
court supervising the Grace Chapter 11 Proceedings approved
a definitive settlement agreement entered into among the
Company, the committees representing the asbestos creditors and
W.R. Grace & Co. Under the settlement agreement, the
Company will pay $115,000, without interest, upon plan
confirmation (see Note 18). With the exception of the
proposed $115,000 payment under the Settlement Agreement, all
other matters included in the special charge have been resolved.
The other item in the table above includes accruals for
operating expenses, interest, withholding tax, value added tax,
legal and compliance costs, physician compensation, commissions,
short-term portion of pension liabilities, bonuses and rebates,
derivatives and accrued rents.
|
|
|
|
9.
|
Short-Term
Borrowings and Short-Term Borrowings from Related
Parties
|
As of December 31, 2009 and 2008, short-term borrowings and
short-term borrowings from related parties consisted of the
following:
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Borrowings under lines of credit
|
|
$
|
95,720
|
|
|
$
|
121,476
|
|
|
Accounts receivable facility
|
|
|
214,000
|
|
|
|
539,000
|
|
|
Other financial liabilities
|
|
|
6,624
|
|
|
|
22,679
|
|
|
|
|
|
|
|
|
|
|
|
|
Short-term borrowings and other financial liabilities
|
|
|
316,344
|
|
|
|
683,155
|
|
|
Short-term borrowings from related parties
|
|
|
10,440
|
|
|
|
1,330
|
|
|
|
|
|
|
|
|
|
|
|
|
Short-term borrowings, Other financial liabilities and
Short-term borrowings from related parties
|
|
$
|
326,784
|
|
|
$
|
684,485
|
|
|
|
|
|
|
|
|
|
|
|
Short-term
Borrowings and Other Financial Liabilities
Lines of
Credit
Short-term borrowings of $95,720 and $121,476 at
December 31, 2009 and 2008, respectively, represent amounts
borrowed by the Company and certain of its subsidiaries under
lines of credit with commercial banks. The average interest
rates on these borrowings at December 31, 2009 and 2008
were 2.94% and 5.30%, respectively.
Excluding amounts available under the 2006 Senior Credit
Agreement (see Note 10 below), at December 31, 2009
and 2008, the Company had $208,952 and $226,221 available under
other commercial bank agreements. In some instances, lines of
credit are secured by assets of the Company’s subsidiary
that is party to the agreement or may require the Company’s
guarantee. In certain circumstances, the subsidiary may be
required to meet certain covenants.
Accounts
Receivable Facility
The Company has an asset securitization facility (the “A/R
Facility”) which is typically renewed in October of each
year and was most recently renewed and increased from $550,000
to $650,000 on November 17, 2009. Under the AR Facility,
certain receivables are sold to NMC Funding Corporation
(“NMC Funding”), a wholly-owned subsidiary. NMC
Funding then assigns percentage ownership interests in the
accounts receivable to certain bank investors. Under the terms
of the AR Facility, NMC Funding retains the right, at any time,
to recall all the then outstanding transferred interests in the
accounts receivable. Consequently, the receivables remain on the
Company’s Consolidated Balance Sheet and the proceeds from
the transfer of percentage ownership interests are recorded as
short-term borrowings.
At December 31, 2009 there are outstanding short-term
borrowings under the AR Facility of $214,000. NMC Funding pays
interest to the bank investors, calculated based on the
commercial paper rates for the particular
F-21
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
tranches selected. The average interest rate during 2009 was
2.90%. Annual refinancing fees, which include legal costs and
bank fees (if any), are amortized over the term of the facility.
Other
Financial Liabilities
At December 31, 2009 and 2008, the Company had $6,624 and
$22,679 of other financial liabilities which were mainly related
to the 2008
Venofer
®
transaction (see Note 7).
Short-term
Borrowings from related parties
From time to time during each of the years presented, the
Company received advances under the existing loan agreements
with Fresenius SE for those years. During the year ended
December 31, 2009, the Company received advances ranging
from €1,300 to €72,000 with interest rates ranging
from 1.05% to 2.05%. During the year ended December 31,
2008, the Company received advances ranging from €13,200 to
€153,400 with interest rates ranging from 4.02% to 5.11%.
On December 31, 2009, the Company had advances outstanding
with Fresenius SE in the amount of $8,279 (€5,747) with an
interest rate of 6%. Furthermore the Company had advances
outstanding with the Company’s general partner in the
amount of $2,161 (€1,500) with an interest rate of 1.335%.
On December 31, 2008, the Company had advances outstanding
with Fresenius SE in the amount of $1,330 with an interest rate
of 7.25%. Annual interest expense on the borrowings during the
years presented was $188, $3,388, and $652 for the years 2009,
2008, and 2007, respectively.
|
|
|
|
10.
|
Long-term
Debt and Capital Lease Obligations
|
As of December 31, 2009 and 2008, long-term debt and
capital lease obligations consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
2006 Senior Credit Agreement
|
|
$
|
3,522,040
|
|
|
$
|
3,366,079
|
|
|
6
7
/
8
%
Senior Notes
|
|
|
493,344
|
|
|
|
492,456
|
|
|
Euro Notes
|
|
|
288,120
|
|
|
|
278,340
|
|
|
EIB Agreements
|
|
|
213,460
|
|
|
|
174,059
|
|
|
Capital lease obligations
|
|
|
17,600
|
|
|
|
13,394
|
|
|
Other
|
|
|
50,991
|
|
|
|
88,165
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4,585,555
|
|
|
|
4,412,493
|
|
|
Less current maturities
|
|
|
(157,634
|
)
|
|
|
(455,114
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
4,427,921
|
|
|
$
|
3,957,379
|
|
|
|
|
|
|
|
|
|
|
|
Senior
Debt
The Company’s senior debt consists mainly of borrowings
related to its 2006 Senior Credit Agreement, its
6
7
/
8
%
Senior Notes, its Euro Notes and borrowings under its European
Investment Bank Agreements as follows:
2006
Senior Credit Agreement
The Company, Fresenius Medical Care Holdings, and certain other
subsidiaries of the Company that are borrowers
and/or
guarantors thereunder, including Fresenius Medical Care
Deutschland GmbH, entered into a $4,600,000 syndicated credit
facility (the “2006 Senior Credit Agreement”) with
Bank of America, N.A. (“BofA”); Deutsche Bank AG New
York Branch; The Bank of Nova Scotia, Credit Suisse, Cayman
Islands Branch; JPMorgan Chase Bank, National Association; and
certain other lenders (collectively, the “Lenders”) on
March 31, 2006 which replaced its prior credit agreement.
The 2006 Senior Credit Agreement consists of:
|
|
|
|
|
|
•
|
a
5-year
$1,000,000 revolving credit facility (of which up to $250,000 is
available for letters of credit, up to $300,000 is available for
borrowings in certain
non-U.S.
currencies, up to $150,000 is available as swing line loans in
U.S. dollars, up to $250,000 is available as a competitive loan
facility and up to $50,000 is
|
F-22
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
|
|
|
|
|
|
|
available as swing line loans in certain
non-U.S.
currencies, the total of which cannot exceed $1,000,000) which
will be due and payable on March 31, 2011.
|
|
|
|
|
|
|
•
|
a
5-year
term loan facility (“Term Loan A”) of $1,850,000, also
scheduled to mature on March 31, 2011. The 2006 Senior
Credit Agreement requires 19 quarterly payments on Term Loan A
of $30,000 each that permanently reduce the term loan facility
which began June 30, 2006 and continue through
December 31, 2010. The remaining amount outstanding is due
on March 31, 2011. As a result of the voluntary repayment
made in July 2007 from the proceeds of the issuance of the
6
7
/
8
%
Senior Notes (see
“6
7
/
8
%
Senior Notes,” below) which reduced the principal balance
outstanding; the quarterly payments were reduced to $29,430
beginning with the payment for September 30, 2008.
|
|
|
|
|
•
|
a
7-year
term loan facility (“Term Loan B”) of $1,750,000
scheduled to mature on March 31, 2013. The terms of the
2006 Senior Credit Agreement require 28 quarterly payments on
Term Loan B that permanently reduce the term loan facility. The
repayment began June 30, 2006. The first 24 quarterly
payments are $4,375 and payments 25 through 28 are $411,250 with
the final payment of the remaining balance due on March 31,
2013, subject to an early repayment requirement on March 1,
2011 if the Trust Preferred Securities due June 15,
2011 are not repaid or refinanced or their maturity is not
extended prior to that date. As a result of the voluntary
repayment made in July 2007 from the proceeds of the issuance of
the
6
7
/
8
%
Senior Notes (see
“6
7
/
8
%
Senior Notes,” below) the balance of the remaining payments
of $4,375 were reduced to $4,036 beginning with the
September 30, 2008 payment, and payments 25 through 28 were
reduced to $379,396.
|
Interest on these facilities will be, at the Company’s
option, depending on the interest periods chosen, at a rate
equal to either (i) LIBOR plus an applicable margin or
(ii) the higher of (a) BofA’s prime rate or
(b) the Federal Funds rate plus 0.5%, plus an applicable
margin.
The applicable margin is variable and depends on the
Company’s Consolidated Leverage Ratio which is a ratio of
its Consolidated Funded Debt less up to $30,000 cash and cash
equivalents to Consolidated EBITDA (as these terms are defined
in the 2006 Senior Credit Agreement).
In addition to scheduled principal payments, indebtedness
outstanding under the 2006 Senior Credit Agreement will be
reduced by mandatory prepayments utilizing portions of the net
cash proceeds from certain sales of assets, securitization
transactions other than the Company’s existing
A/R
Facility, the issuance of subordinated debt other than certain
intercompany transactions, certain issuances of equity and
excess cash flow.
Obligations under the 2006 Senior Credit Agreement are secured
by pledges of capital stock of certain material subsidiaries in
favor of the lenders. The 2006 Senior Credit Agreement contains
affirmative and negative covenants with respect to the Company
and its subsidiaries and other payment restrictions. Certain of
the covenants limit indebtedness of the Company and investments
by the Company, and require the Company to maintain certain
financial ratios defined in the agreement. Additionally, the
2006 Senior Credit Agreement provides for a limitation on
dividends and other restricted payments which is $300,000 for
dividends in 2010, and increases in subsequent years. The
Company paid dividends of $231,940 in May of 2009 which was in
compliance with the restrictions set forth in the 2006 Senior
Credit Agreement. In default, the outstanding balance under the
2006 Senior Credit Agreement becomes immediately due and payable
at the option of the Lenders. As of December 31, 2009, the
Company is in compliance with all covenants under the 2006
Senior Credit Agreement.
The Company incurred fees of approximately $85,828 in
conjunction with the 2006 Senior Credit Agreement which are
being amortized over the life of this agreement and wrote off
approximately $14,735 in unamortized fees related to its prior
senior credit agreement in 2006.
F-23
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
The following table shows the available and outstanding amounts
under the 2006 Senior Credit Agreement at December 31, 2009
and 2008, respectively:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Maximum Amount Available
|
|
|
Balance Outstanding
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Revolving Credit
|
|
$
|
1,000,000
|
|
|
$
|
1,000,000
|
|
|
$
|
594,714
|
|
|
$
|
304,887
|
|
|
Term Loan A
|
|
|
1,373,418
|
|
|
|
1,491,139
|
|
|
|
1,373,418
|
|
|
|
1,491,139
|
|
|
Term Loan B
|
|
|
1,553,908
|
|
|
|
1,570,053
|
|
|
|
1,553,908
|
|
|
|
1,570,053
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
3,927,326
|
|
|
$
|
4,061,192
|
|
|
$
|
3,522,040
|
|
|
$
|
3,366,079
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In addition, at December 31, 2009 and 2008, respectively,
$97,287 and $111,994 were utilized as letters of credit which
are not included as part of the balances outstanding at those
dates.
In January 2008, the 2006 Senior Credit Agreement was amended in
order to increase certain types of permitted borrowings and to
remove all limitations on capital expenditures.
6
7
/
8
%
Senior Notes
In July 2007, FMC Finance III S.A. (“Finance III”), a
wholly-owned subsidiary of the Company, issued $500,000
aggregate principal amount of
6
7
/
8
%
senior notes due 2017 (the
“6
7
/
8
%
Senior Notes”) at a discount resulting in an effective
interest rate of
7
1
/
8
%.
The
6
7
/
8
%
Senior Notes are guaranteed on a senior basis jointly and
severally by the Company and by its subsidiaries Fresenius
Medical Care Holdings, Inc. (“FMCH”) and Fresenius
Medical Care Deutschland GmbH (“D-GmbH”). Finance III
may redeem the
6
7
/
8
%
Senior Notes at any time at 100% of principal plus accrued
interest and a premium calculated pursuant to the terms of the
indenture. The holders have a right to request that Finance III
repurchase the
6
7
/
8
%
Senior Notes at 101% of principal plus accrued interest upon the
occurrence of a change of control followed by a decline in the
rating of the
6
7
/
8
%
Senior Notes. The proceeds, net of discounts, investment bank
fees and other offering related expenses, were applied to reduce
Term Loan A and Term Loan B under the Company’s 2006 Senior
Credit Agreement (See 2006 Senior Credit Agreement above) and
were used to pay down the then outstanding balance under its
short-term
A/R
Facility
(See above). The discount is being amortized over the life of
the
6
7
/
8
%
Senior Notes.
Euro
Notes
On April 27, 2009, the Company issued euro denominated
notes (“Euro Notes”) totaling €200,000 ($288,120
at December 31, 2009), which are senior, unsecured and
guaranteed by FMCH and D-GmbH, consisting of 4 tranches
having terms of 3.5 and 5.5 years with floating and fixed
interest rate tranches. The initial average interest rate was
6.95%. Proceeds of €69,500 of the newly issued Euro Notes
were used in April 2009 to voluntarily retire a portion of the
2005 Euro Notes (see below) that were due in July 2009 with the
remaining proceeds used to repay the balance of those notes on
their scheduled maturity date of July 27, 2009.
In July 2005, FMC Finance IV Luxembourg issued euro denominated
notes (“2005 Euro Notes”)
(
Schuldscheindarlehen
) totaling $278,340 (€200,000)
with a €126,000 tranche at a fixed interest rate and a
€74,000 tranche with a floating rate at EURIBOR plus
applicable margin. The 2005 Euro Notes, guaranteed by the
Company, matured and were fully repaid on July 27, 2009 and
were included in the short term portion of long-term debt in our
balance sheet at December 31, 2008.
European
Investment Bank Agreements
The Company entered into various credit agreements with the
European Investment Bank (“EIB”) in 2005 and 2006
totaling €221,000. In addition, in December 2009, the
Company entered into an additional EIB loan agreement providing
for a term loan of €50,000. The loan has a four-year term
and bears interest at the Euribor Rate plus an applicable
margin. The EIB is a
not-for-profit
long-term lending institution of the European Union and lends
funds at favorable rates for the purpose of capital investment
and R&D projects, normally for up to half of the funds
required for such projects.
F-24
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
The Company will use the proceeds of the 2009 EIB loan to
refinance certain research and development projects carried out
in 2007 through 2009 and uses the funds under the other
agreements to refinance certain R&D projects, to make
investments in expansion and optimization of existing production
facilities in Germany, and for financing and refinancing of
certain clinic refurbishing and improvement projects. Currently
all agreements with the EIB have variable interest rates that
change quarterly, with FMC-AG & Co. KGaA having
options to convert the variable rates into fixed rates. Advances
under some agreements can be denominated in certain foreign
currencies including U.S. dollars.
The Company has four credit facilities available at
December 31, 2009 under these agreements with the maximum
amounts available and outstanding balances as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Maximum amount available
|
|
|
Balance outstanding
|
|
|
|
|
December 31,
|
|
|
December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Revolving Credit
|
|
€
|
90,000
|
|
|
€
|
90,000
|
|
|
$
|
35,000
|
|
|
$
|
—
|
|
|
Loan 2005
|
|
|
41,000
|
|
|
|
41,000
|
|
|
|
48,806
|
|
|
|
48,806
|
|
|
Loan 2006
|
|
|
90,000
|
|
|
|
90,000
|
|
|
|
129,654
|
|
|
|
125,253
|
|
|
Loan 2009
|
|
|
50,000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
€
|
271,000
|
|
|
€
|
221,000
|
|
|
$
|
213,460
|
|
|
$
|
174,059
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Company’s U.S. dollar borrowings under the Loan 2005
agreement had interest rates of 0.384% and 2.03%, and the euro
borrowings under the Loan 2006 agreement had interest rates of
0.695% and 4.77% at December 31, 2009 and 2008,
respectively.
Borrowings under the 2005 and 2006 agreements are secured by
bank guarantees while the 2009 agreement is guaranteed by FMCH
and D-GmbH. All EIB agreements have customary covenants.
Annual
Payments
Aggregate annual payments applicable to the 2006 Senior Credit
Agreement,
6
7
/
8
%
Senior Notes, Euro Notes, EIB agreements, capital leases and
other borrowings (excluding the Company’s trust preferred
securities, see Note 12) for the five years subsequent
to December 31, 2009 are:
|
|
|
|
|
|
|
2010
|
|
$
|
157,634
|
|
|
2011
|
|
|
1,882,699
|
|
|
2012
|
|
|
1,385,638
|
|
|
2013
|
|
|
476,980
|
|
|
2014
|
|
|
181,675
|
|
|
Thereafter
|
|
|
507,585
|
|
|
|
|
|
|
|
|
|
|
$
|
4,592,211
|
|
|
|
|
|
|
|
|
|
|
|
11.
|
Employee
Benefit Plans
|
General
FMC-AG & Co. KGaA recognizes pension costs and related
pension liabilities for current and future benefits to qualified
current and former employees of the Company. The Company’s
pension plans are structured differently according to the legal,
economic and fiscal circumstances in each country. The Company
currently has two types of plans, defined benefit and defined
contribution plans. In general plan benefits in defined benefit
plans are based on all or a portion of the employees’ years
of services and final salary. Plan benefits in defined
contribution plans are determined by the amount of contribution
by the employee and the employer, both of which may be limited
by legislation, and the returns earned on the investment of
those contributions.
F-25
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
Upon retirement under defined benefit plans, the Company is
required to pay defined benefits to former employees when the
defined benefits become due. Defined benefit plans may be funded
or unfunded. The Company has two major defined benefit plans,
one funded plan in North America and an unfunded plan in Germany.
Actuarial assumptions generally determine benefit obligations
under defined benefit plans. The actuarial calculations require
the use of estimates. The main factors used in the actuarial
calculations affecting the level of the benefit obligations are:
assumptions on life expectancy, the discount rate and future
salary and benefit levels. Under the Company’s funded
plans, assets are set aside to meet future payment obligations.
An estimated return on the plan assets is recognized as income
in the respective period. Actuarial gains and losses are
generated when there are variations in the actuarial assumptions
and differences between the actual and the estimated return on
plan assets for that year. The company’s pension liability
is impacted by these actuarial gains or losses.
In the case of the Company’s funded plan, the defined
benefit obligation is offset against the fair value of plan
assets. A pension liability is recognized in the balance sheet
if the defined benefit obligation exceeds the fair value of plan
assets. A pension asset is recognized (and reported under other
assets in the balance sheet) if the fair value of plan assets
exceeds the defined benefit obligation and if the Company has a
right of reimbursement against the fund or a right to reduce
future payments to the fund.
Under defined contribution plans, the Company pays defined
contributions during the employee’s service life which
satisfies all obligations of the Company to the employee. The
Company has a defined contribution plan in North America.
Defined
Benefit Pension Plans
During the first quarter of 2002, FMCH, the Company’s North
America subsidiary, curtailed its defined benefit and
supplemental executive retirement plans. Under the curtailment
amendment for substantially all employees eligible to
participate in the plan, benefits have been frozen as of the
curtailment date and no additional defined benefits for future
services will be earned. The Company has retained all employee
benefit obligations as of the curtailment date. Each year FMCH
contributes at least the minimum amount required by the Employee
Retirement Income Security Act of 1974, as amended. There was no
minimum funding requirement for FMCH for the defined benefit
plan in 2009. FMCH voluntarily contributed $759 during 2009.
Expected funding for 2010 is $607.
The benefit obligation for all defined benefit plans at
December 31, 2009, is $386,852 (2008: $353,961) which
consists of the benefit obligation of $261,282 (2008: $245,070)
for the North America plan, which is funded by plan assets, and
the benefit obligation of $125,570 (2008: $108,891) for the
German unfunded plan.
The following table shows the changes in benefit obligations,
the changes in plan assets, and the funded status of the pension
plans. Benefits paid as shown in the changes in benefit
obligations represent payments made from
F-26
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
both the funded and unfunded plans while the benefits paid as
shown in the changes in plan assets include only benefit
payments from the Company’s funded benefit plan.
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Change in benefit obligation:
|
|
|
|
|
|
|
|
|
|
Benefit obligation at beginning of year
|
|
$
|
353,961
|
|
|
$
|
331,649
|
|
|
Foreign currency translation
|
|
|
4,235
|
|
|
|
(6,288
|
)
|
|
Service cost
|
|
|
7,500
|
|
|
|
8,357
|
|
|
Interest cost
|
|
|
21,397
|
|
|
|
20,393
|
|
|
Transfer of plan participants
|
|
|
96
|
|
|
|
2,228
|
|
|
Actuarial (gain) loss
|
|
|
13,216
|
|
|
|
4,472
|
|
|
Benefits paid
|
|
|
(7,560
|
)
|
|
|
(6,850
|
)
|
|
Curtailments and Settlements
|
|
|
(5,993
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
Benefit obligation at end of year
|
|
$
|
386,852
|
|
|
$
|
353,961
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in plan assets:
|
|
|
|
|
|
|
|
|
|
Fair value of plan assets at beginning of year
|
|
$
|
214,616
|
|
|
$
|
228,581
|
|
|
Actual return on plan assets
|
|
|
29,382
|
|
|
|
(9,092
|
)
|
|
Employer contributions
|
|
|
759
|
|
|
|
684
|
|
|
Benefits paid
|
|
|
(6,063
|
)
|
|
|
(5,557
|
)
|
|
Settlements
|
|
|
(2,061
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair value of plan assets at end of year
|
|
$
|
236,633
|
|
|
$
|
214,616
|
|
|
|
|
|
|
|
|
|
|
|
|
Funded status at end of year
|
|
$
|
150,219
|
|
|
$
|
139,345
|
|
|
|
|
|
|
|
|
|
|
|
The Company had a pension liability of $150,219 and $139,345 at
December 31, 2009 and 2008, respectively. The pension
liability consists of a current portion of $2,892 (2008: $2,590)
which is recognized as a current liability in the line item
“accrued expenses and other current liabilities” in
the balance sheet. The non-current portion of $147,327 (2008:
$136,755) is recorded as non-current pension liability in the
balance sheet. Approximately 85% of the beneficiaries are
located in North America with the majority of the remaining 15%
located in Germany.
The accumulated benefit obligation for all defined benefit
pension plans was $367,182 and $334,951 at December 31,
2009 and 2008, respectively. The accumulated benefit obligation
for all defined benefit pension plans with an obligation in
excess of plan assets was $367,182 and $334,951 at
December 31, 2009 and 2008, respectively; the related plan
assets had a fair value of $236,633 and $214,616 at
December 31, 2009 and 2008, respectively.
The pre-tax changes in the table below reflect actuarial losses
(gains) in other comprehensive income relating to pension
liabilities. As of December 31, 2009, there are no
cumulative effects of prior service costs included in other
comprehensive income.
F-27
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
|
|
|
|
|
|
|
|
|
Actuarial
|
|
|
|
|
losses (gains)
|
|
|
|
|
Adjustments related to pensions at January 1, 2008
|
|
$
|
48,375
|
|
|
Additions
|
|
|
30,494
|
|
|
Releases
|
|
|
(1,944
|
)
|
|
Foreign Currency Translation Adjustment
|
|
|
1
|
|
|
|
|
|
|
|
|
Adjustments related to pensions at December 31, 2008
|
|
$
|
76,926
|
|
|
|
|
|
|
|
|
Additions
|
|
|
(4,331
|
)
|
|
Releases
|
|
|
(5,404
|
)
|
|
Foreign Currency Translation Adjustment
|
|
|
27
|
|
|
|
|
|
|
|
|
Adjustments related to pensions at December 31, 2009
|
|
$
|
67,218
|
|
|
|
|
|
|
|
The actuarial loss expected to be amortized from other
comprehensive income into net periodic pension cost over the
next year is $4,788.
The discount rates for all plans are based upon yields of
portfolios of equity and highly rated debt instruments with
maturities that mirror the plan’s benefit obligation. The
Company’s discount rate is the weighted average of these
plans based upon their benefit obligations at December 31,
2009. The following weighted-average assumptions were utilized
in determining benefit obligations as of December 31:
|
|
|
|
|
|
|
|
|
|
|
in %
|
|
2009
|
|
2008
|
|
|
|
Discount rate
|
|
|
6.00
|
|
|
|
6.15
|
|
|
Rate of compensation increase
|
|
|
4.01
|
|
|
|
4.19
|
|
The defined benefit pension plans’ net periodic benefit
costs are comprised of the following components for each of the
years ended December 31:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Components of net periodic benefit cost:
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Service cost
|
|
$
|
7,500
|
|
|
$
|
8,357
|
|
|
$
|
8,835
|
|
|
Interest cost
|
|
|
21,397
|
|
|
|
20,393
|
|
|
|
18,506
|
|
|
Expected return on plan assets
|
|
|
(15,767
|
)
|
|
|
(16,931
|
)
|
|
|
(16,362
|
)
|
|
Amortization of unrealized losses
|
|
|
4,592
|
|
|
|
1,944
|
|
|
|
5,163
|
|
|
Settlement loss
|
|
|
812
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net periodic benefit costs
|
|
$
|
18,534
|
|
|
$
|
13,763
|
|
|
$
|
16,142
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following weighted-average assumptions were used in
determining net periodic benefit cost for the year ended
December 31:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
in %
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Discount rate
|
|
|
6.15
|
|
|
|
6.16
|
|
|
|
5.52
|
|
|
Expected return of plan assets
|
|
|
7.50
|
|
|
|
7.50
|
|
|
|
7.50
|
|
|
Rate of compensation increase
|
|
|
4.19
|
|
|
|
4.16
|
|
|
|
4.18
|
|
F-28
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
Expected benefit payments for the next five years and in the
aggregate for the five years thereafter are as follows:
|
|
|
|
|
|
|
2010
|
|
$
|
10,441
|
|
|
2011
|
|
|
11,070
|
|
|
2012
|
|
|
12,131
|
|
|
2013
|
|
|
13,356
|
|
|
2014
|
|
|
14,663
|
|
|
2015-2019
|
|
|
99,204
|
|
Plan
Assets
The following table presents the fair values of the
Company’s pension plan assets at December 31, 2009.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value Measurements at December 31, 2009
|
|
|
|
|
|
|
|
Quoted Prices in
|
|
|
Significant
|
|
|
|
|
|
|
|
Active Markets
|
|
|
Observable
|
|
|
|
|
|
|
|
for Identical Assets
|
|
|
Inputs
|
|
|
Asset Category
|
|
Total
|
|
|
(Level 1)
|
|
|
(Level 2)
|
|
|
|
|
Equity Investments
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stocks
|
|
$
|
5,904
|
|
|
$
|
5,904
|
|
|
$
|
—
|
|
|
Index
Funds
1
|
|
|
71,406
|
|
|
|
71,406
|
|
|
|
—
|
|
|
Fixed Income Investments
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Government
Bonds
2
|
|
|
3,655
|
|
|
|
394
|
|
|
|
3,261
|
|
|
Corporate
Bonds
3
|
|
|
149,367
|
|
|
|
—
|
|
|
|
149,367
|
|
|
Other
Bonds
4
|
|
|
163
|
|
|
|
—
|
|
|
|
163
|
|
|
U.S. Treasury Money Market
Funds
5
|
|
|
5,776
|
|
|
|
5,776
|
|
|
|
—
|
|
|
Other types of investments
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash, Money Market and Mutual
Funds
6
|
|
|
362
|
|
|
|
362
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
236,633
|
|
|
$
|
83,842
|
|
|
$
|
152,791
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1
|
|
This Category comprises low-cost
equity index funds not actively managed that track the S&P
500, S&P 400, Russell 2000, MSCI EAFE Index, MSCI Emerging
Markets Index and the Barclays Capital Long Corporate Index
|
|
2
|
|
This Category comprises government
fixed income investments with the majority coming from U.S.,
Finland, Canada and Norway
|
|
3
|
|
This Category represents investment
grade bonds of U.S. issuers from diverse industries
|
|
4
|
|
This Category comprises private
placement bonds
|
|
5
|
|
This Category represents funds that
invest in treasury bills and treasury backed instruments
|
|
6
|
|
This Category represents cash,
money market funds as well as mutual funds comprised of high
grade corporate bonds
|
The methods and inputs used to measure the fair value of plan
assets are as follows:
Common stocks and index funds are valued at their market prices
as of the balance sheet date.
The majority of the fair values of the government bonds are
measured based on market quotes. The remaining government bonds
are valued at their market prices.
Corporate bonds and other bonds are valued based on market
quotes as of the balance sheet date.
Cash is stated at nominal value which equals the fair value.
US Treasury money market funds as well as other money market and
mutual funds are valued at their market price.
F-29
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
Plan
Investment Policy and Strategy
For the North America funded plan, the Company periodically
reviews the assumption for long-term expected return on pension
plan assets. As part of the assumptions review, a range of
reasonable expected investment returns for the pension plan as a
whole was determined based on an analysis of expected future
returns for each asset class weighted by the allocation of the
assets. The range of returns developed relies both on forecasts,
which include the actuarial firm’s expected long-term rates
of return for each significant asset class or economic
indicator, and on broad-market historical benchmarks for
expected return, correlation, and volatility for each asset
class. As a result, the Company’s expected rate of return
on pension plan assets was 7.5% for 2009.
The Company’s overall investment strategy is to achieve a
mix of approximately 98% of investments for long-term growth and
2% for near-term benefit payments with a wide diversification of
asset types, fund strategies and fund managers.
The investment policy, utilizing a revised target investment
allocation of 35% equity and 65% long-term U.S. bonds, considers
that there will be a time horizon for invested funds of more
than 5 years. The total portfolio will be measured against
a policy index that reflects the asset class benchmarks and the
target asset allocation. The Plan policy does not allow
investments in securities of the Company or other related party
securities. The performance benchmarks for the separate asset
classes include: S&P 500 Index, S&P 400 Index, Russell
2000 Growth Index, MSCI EAFE Index, MSCI Emerging Markets Index,
Barclays Capital Long Term Government Index and Barclays Capital
20 Year US Treasury Strip Index.
Defined
Contribution Plans
Most FMCH employees are eligible to join a 401(k) savings plan.
Employees can deposit up to 75% of their pay up to a maximum of
$16.5 if under 50 years old ($22.0 if 50 or over) under
this savings plan. The Company will match 50% of the employee
deposit up to a maximum Company contribution of 3% of the
employee’s pay. The Company’s total expense under this
defined contribution plan for the years ended December 31,
2009, 2008, and 2007, was $28,567, $26,096, and $23,534,
respectively.
|
|
|
|
12.
|
Mandatorily
Redeemable Trust Preferred Securities
|
The Company issued Trust Preferred Securities through
Fresenius Medical Care Capital Trusts, statutory trusts
organized under the laws of the State of Delaware.
FMC-AG & Co. KGaA owns all of the common securities of
these trusts. The sole asset of each trust is a senior
subordinated note of FMC-AG & Co. KGaA or a
wholly-owned subsidiary of FMC-AG & Co. KGaA.
FMC-AG & Co. KGaA, D-GmbH and FMCH have guaranteed
payment and performance of the senior subordinated notes to the
respective Fresenius Medical Care Capital Trusts. The
Trust Preferred Securities are guaranteed by
FMC-AG & Co. KGaA through a series of undertakings by
the Company, FMCH and D-GmbH.
The Trust Preferred Securities entitle the holders to
distributions at a fixed annual rate of the stated amount and
are mandatorily redeemable after 10 years. Earlier
redemption at the option of the holders may also occur upon a
change of control followed by a rating decline or defined events
of default including a failure to pay interest. Upon liquidation
of the trusts, the holders of Trust Preferred Securities
are entitled to a distribution equal to the stated amount. The
Trust Preferred Securities do not hold voting rights in the
trust except under limited circumstances.
The indentures governing the notes held by the Fresenius Medical
Care Capital Trusts contain affirmative and negative covenants
with respect to the Company and its subsidiaries and other
payment restrictions. Some of the covenants limit the
Company’s indebtedness and its investments, and require the
Company to maintain certain ratios defined in the indentures. As
of December 31, 2009, the Company is in compliance with all
financial covenants under all Trust Preferred Securities
agreements.
F-30
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
The Trust Preferred Securities outstanding as of
December 31, 2009 and 2008 are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mandatory
|
|
|
|
|
|
|
|
|
|
|
Year
|
|
|
Stated
|
|
|
Interest
|
|
|
Redemption
|
|
|
|
|
|
|
|
|
|
|
Issued
|
|
|
Amount
|
|
|
Rate
|
|
|
Date
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Fresenius Medical Care Capital Trust IV
|
|
|
2001
|
|
|
|
$225,000
|
|
|
|
7
7
/
8
|
%
|
|
|
June 15, 2011
|
|
|
$
|
224,451
|
|
|
$
|
224,068
|
|
|
Fresenius Medical Care Capital Trust V
|
|
|
2001
|
|
|
|
€300,000
|
|
|
|
7
3
/
8
|
%
|
|
|
June 15, 2011
|
|
|
|
431,645
|
|
|
|
416,628
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
656,096
|
|
|
$
|
640,696
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Company redeemed the securities issued by Trust II and
Trust III which were due and paid on February 1, 2008,
primarily with funds obtained under its existing credit
facilities.
Capital
Stock
The General Partner has no equity interest in the Company and,
therefore, does not participate in either the assets or the
profits and losses of the Company. However, the General Partner
is compensated for all outlays in connection with conducting the
Company’s business, including the remuneration of members
of the management board and the supervisory board (see
Note 4).
The general meeting of a partnership limited by shares may
approve Authorized Capital (
genehmigtes Kapital
). The
resolution creating Authorized Capital requires the affirmative
vote of a majority of three quarters of the capital represented
at the vote and may authorize the management board to issue
shares up to a stated amount for a period of up to five years.
The nominal value of the Authorized Capital may not exceed half
of the capital stock at the time of the authorization.
In addition, the general meeting of a partnership limited by
shares may create Conditional Capital (
bedingtes Kapital
)
for the purpose of issuing (i) shares to holders of
convertible bonds or other securities which grant a right to
shares, (ii) shares as the consideration in a merger with
another company, or (iii) shares offered to management or
employees. In each case, the authorizing resolution requires the
affirmative vote of a majority of three quarters of the capital
represented at the vote. The nominal value of the Conditional
Capital may not exceed half or, in the case of Conditional
Capital created for the purpose of issuing shares to management
and employees, 10% of the company’s capital at the time of
the resolution.
All resolutions increasing the capital of a partnership limited
by shares also require the consent of the General Partner for
their effectiveness.
Authorized
Capital
By resolution of the Extraordinary General Meeting
(“EGM”) of shareholders on August 30, 2005,
Management AG was authorized, with the approval of the
supervisory board, to increase, on one or more occasions, the
Company’s share capital until August 29, 2010 by a
maximum amount of €35,000 through issue of new ordinary
shares against cash contributions, Authorized Capital I. The
General Partner is entitled, subject to the approval of the
supervisory board, to decide on the exclusion of statutory
pre-emption rights of the shareholders. However, such an
exclusion of pre-emption rights will be permissible for
fractional amounts. Additionally, the newly issued shares may be
taken up by certain credit institutions determined by the
General Partner if such credit institutions are obliged to offer
the shares to the shareholders (indirect pre-emption rights).
In addition, by resolution of the EGM of shareholders on
August 30, 2005, the General Partner was authorized, with
the approval of the supervisory board, to increase, on one or
more occasions, the share capital of the Company until
August 29, 2010 by a maximum amount of €25,000 through
the issue of new ordinary shares against cash contributions or
contributions in kind, Authorized Capital II. The General
Partner is entitled, subject to the approval of the supervisory
board, to decide on an exclusion of statutory pre-emption rights
of the shareholders. However, such exclusion of pre-emption
rights will be permissible only if (i) in case of a capital
increase against cash contributions, the nominal value of the
issued shares does not exceed 10% of the nominal share value of
the Company’s share capital and the issue price for the new
shares is at the time of the determination by the General
Partner not significantly lower than the stock price in Germany
of the existing listed shares of the same type and
F-31
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
with the same rights or, (ii) in case of a capital increase
against contributions in kind, the purpose of such increase is
to acquire an enterprise, parts of an enterprise or an interest
in an enterprise.
The Company’s Authorized Capital I and Authorized Capital
II became effective upon registration with the commercial
register of the local court in Hof an der Saale on
February 10, 2006.
Conditional
Capital
By resolution of the Company’s Annual General Meeting of
shareholders (“AGM”) on May 9, 2006, as amended
by the AGM on May 15, 2007, resolving a
three-for-one
share split, the Company’s share capital was conditionally
increased by up to €15,000 corresponding to 15 million
ordinary shares with no par value and a nominal value of
€1.00. This Conditional Capital increase can only be
effected by the exercise of stock options under the
Company’s Stock Option Plan 2006 with each stock option
awarded exercisable for one ordinary share (see Note 14).
The Company has the right to deliver ordinary shares that it
owns or purchases in the market in place of increasing capital
by issuing new shares.
Through the Company’s other employee participation
programs, the Company has issued convertible bonds and stock
option/subscription rights (
Bezugsrechte
) to employees
and the members of the Management Board of the General Partner
and employees and members of management of affiliated companies
that entitle these persons to receive preference shares or,
following the conversion offer in 2005, ordinary shares. At
December 31, 2009, 146,601 convertible bonds or options for
preference shares remained outstanding with a remaining average
term of 3.91 years and 11,894,063 convertible bonds or
options for ordinary shares remained outstanding with a
remaining average term of 5.01 years under these programs.
For the year ending December 31, 2009, 73,788 options for
preference shares and 1,814,599 options for ordinary shares had
been exercised under these employee participation plans and
$64,384 (€44,686) remitted to the Company.
As the result of the Company’s
three-for-one
stock split for both preference and ordinary shares on
June 15, 2007, and with the approval of the shareholders as
the AGM on May 15, 2007, the Company’s Conditional
Capital was increased by €4,454 ($6,557). Conditional
Capital available for all programs at December 31, 2009 is
€26,162 ($37,689) which includes €14,444 ($20,808) for
the 2006 Plan and €11,718 ($16,881) for all other plans.
Dividends
Under German law, the amount of dividends available for
distribution to shareholders is based upon the unconsolidated
retained earnings of Fresenius Medical Care AG & Co.
KGaA as reported in its balance sheet determined in accordance
with the German Commercial Code (
Handelsgesetzbuch
).
If no dividends on the Company’s preference shares are
declared for two consecutive years after the year for which the
preference shares are entitled to dividends, then the holders of
such preference shares would be entitled to the same voting
rights as holders of ordinary shares until all arrearages are
paid. In addition, the payment of dividends by
FMC-AG & Co. KGaA is subject to limitations under the
2006 Senior Credit Agreement (see Note 9).
Cash dividends of $231,940 for 2008 in the amount of €0.60
per preference share and €0.58 per ordinary share were paid
on May 8, 2009.
Cash dividends of $252,395 for 2007 in the amount of €0.56
per preference share and €0.54 per ordinary share were paid
on May 21, 2008
Cash dividends of $188,407 for 2006 in the amount of €0.49
per preference share and €0.47 per ordinary share were paid
on May 16, 2007.
Noncontrolling
Interests
The Company has purchase obligations under options held by the
holders of noncontrolling interests in certain of its
subsidiaries. These obligations result from put options and are
exercisable by the owners of the noncontrolling interests. If
these put options were exercised, the Company would be required
to purchase the noncontrolling owners’ interests for cash
equal to the then fair value. As of December 31, 2009 and
2008 the Company’s potential obligations under these put
options are $211,000 and $112,000 of which, at December 31,
2009, $78,000 were exercisable and another $4,000 is exercisable
within one year. In the last three fiscal years ending
December 31, 2009, three puts have been exercised for a
total consideration of $13,000.
F-32
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
During 2008 and 2007, the Company received cash contributions
from holders of noncontrolling interests in the amounts of
$17,174 and $5,057, respectively. These amounts were recorded in
net cash provided by operating activities in the respective cash
flow statements.
The following table contains reconciliations of the numerators
and denominators of the basic and diluted earnings per share
computations for the years ending December 31:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Numerators:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income attributable to FMC-AG & Co. KGaA
|
|
$
|
891,138
|
|
|
$
|
817,607
|
|
|
$
|
717,130
|
|
|
less:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dividend Preference on Preference shares
|
|
|
107
|
|
|
|
112
|
|
|
|
103
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income available to all class of shares
|
|
$
|
891,031
|
|
|
$
|
817,495
|
|
|
$
|
717,027
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Denominators:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ordinary shares outstanding
|
|
|
294,418,795
|
|
|
|
293,233,477
|
|
|
|
291,929,141
|
|
|
Preference shares outstanding
|
|
|
3,842,586
|
|
|
|
3,795,248
|
|
|
|
3,739,470
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total weighted average shares outstanding
|
|
|
298,261,381
|
|
|
|
297,028,725
|
|
|
|
295,668,611
|
|
|
Potentially dilutive Ordinary shares
|
|
|
—
|
|
|
|
777,848
|
|
|
|
1,079,683
|
|
|
Potentially dilutive Preference shares
|
|
|
66,314
|
|
|
|
98,060
|
|
|
|
127,324
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total weighted average Ordinary shares outstanding assuming
dilution
|
|
|
294,418,795
|
|
|
|
294,011,325
|
|
|
|
293,008,824
|
|
|
Total weighted average Preference shares outstanding assuming
dilution
|
|
|
3,908,900
|
|
|
|
3,893,308
|
|
|
|
3,866,794
|
|
|
Basic income per Ordinary share
|
|
$
|
2.99
|
|
|
$
|
2.75
|
|
|
$
|
2.43
|
|
|
Plus preference per Preference share
|
|
|
0.03
|
|
|
|
0.03
|
|
|
|
0.02
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic income per Preference Share
|
|
$
|
3.02
|
|
|
$
|
2.78
|
|
|
$
|
2.45
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fully diluted income per Ordinary share
|
|
$
|
2.99
|
|
|
$
|
2.74
|
|
|
$
|
2.42
|
|
|
Plus preference per Preference share
|
|
|
0.03
|
|
|
|
0.03
|
|
|
|
0.02
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fully diluted income per Preference share
|
|
$
|
3.02
|
|
|
$
|
2.77
|
|
|
$
|
2.44
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In connection with its stock option program, the Company
incurred compensation expense of $33,746, $31,879, and $24,208
for the years ending December 31, 2009, 2008, and 2007,
respectively. There were no capitalized compensation costs in
any of the three years presented. The Company also recorded a
related deferred income tax of $9,740, $9,158, and $6,880 for
the years ending December 31, 2009, 2008, and 2007,
respectively.
Stock
Options and other Share-Based Plans
At December 31, 2009, the Company has awards outstanding
under various stock-based compensation plans.
Incentive
plan
In 2009, Management Board members were eligible for
performance-related compensation that depended upon achievement
of individual and common targets. The targets are based upon
operating earnings (EBIT), net consolidated earnings (EAT) and
its growth, as well as the development of cash flow, and are in
part developed by a comparison with the previous year’s
figures, budgeted figures and actually achieved figures. Targets
are divided into Group level targets and those to be achieved in
individual regions.
The bonus for fiscal year 2009 will, in principle, consist
proportionately of a cash component and a share-based component
which will be paid in cash. Upon meeting the annual targets, the
cash component was or will be paid after the end of 2009. The
share-based component is subject to a several year vesting
period, although a shorter
F-33
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
period may apply in special cases. The amount of cash payment
relating to the share-based component will correspond to the
share price of Fresenius Medical Care AG & Co. KGaA
ordinary shares upon exercise after the several year vesting
period. The amount of the maximum achievable bonus for each of
the members of the Management Board is capped.
In 2006, Fresenius Medical Care Management AG adopted a
three-year performance related compensation plan for fiscal
years 2008, 2007 and 2006, for the members of its management
board in the form of a variable bonus. A special bonus component
(award) for some of the management board members consists in
equal parts of cash payments and a share-based compensation
based on development of the share price of Fresenius Medical
Care AG & Co. KGaA’s ordinary shares. The amount
of the award in each case depends on the achievement of certain
performance targets. The targets are measured by reference to
revenue growth, operating income, consolidated net income, and
cash flow development. Annual targets have been achieved, the
cash portion of the award has been paid after the end of the
respective fiscal year. The share-based compensation portion of
the award has been granted but subject to a three-year vesting
period beginning after the respective fiscal year in which the
target has been met and is amortized over the same three-year
vesting period. The payment of the share-based compensation
portion corresponds to the share price of Fresenius Medical Care
AG & Co. KGaA’s ordinary shares on exercise, i.e.
at the end of the vesting period, and is also made in cash. The
share-based compensation is revalued each reporting period
during the vesting period to reflect the market value of the
stock as of the reporting date with any changes in value
recorded in the reporting period.
The share-based compensation incurred under these plans for
years 2009, 2008 and 2007 was $1,537, $2,189 and $4,595,
respectively.
Fresenius
Medical Care AG & Co. KGaA Stock Option Plan
2006
On May 9, 2006, as amended on May 15, 2007, the
Fresenius Medical Care AG & Co. KGaA Stock Option Plan
2006 (the “Amended 2006 Plan”) was established by
resolution of the Company’s AGM with a conditional capital
increase up to €15,000 subject to the issue of up to
fifteen million no par value bearer ordinary shares with a
nominal value of €1.00 each. Under the Amended 2006 Plan,
up to fifteen million options can be issued, each of which can
be exercised to obtain one ordinary share, with up to three
million options designated for members of the Management Board
of the General Partner, up to three million options designated
for members of management boards of direct or indirect
subsidiaries of the Company and up to nine million options
designated for managerial staff members of the Company and such
subsidiaries. With respect to participants who are members of
the General Partner’s Management Board, the general
partner’s Supervisory Board has sole authority to grant
stock options and exercise other decision making powers under
the Amended 2006 Plan (including decisions regarding certain
adjustments and forfeitures). The General Partner has such
authority with respect to all other participants in the Amended
2006 Plan.
Options under the Amended 2006 Plan can be granted the last
Monday in July
and/or
the
first Monday in December. The exercise price of options granted
under the Amended 2006 Plan shall be the average closing price
on the Frankfurt Stock Exchange of the Company’s ordinary
shares during the 30 calendar days immediately prior to each
grant date. Options granted under the Amended 2006 Plan have a
seven-year term but can be exercised only after a three-year
vesting period. The vesting of options granted is subject to
achievement of performance targets measured over a three-year
period from the grant date. For each such year, the performance
target is achieved if the Company’s adjusted basic income
per ordinary share (“EPS”), as calculated in
accordance with the Amended 2006 Plan, increases by at least 8%
year over year during the vesting period, beginning with EPS for
the year of grant as compared to EPS for the year preceding such
grant. Calculation of EPS under the Amended 2006 Plan excluded,
among other items, the costs of the transformation of the
Company’s legal form and the conversion of preference
shares into ordinary shares. For each grant, one-third of the
options granted are forfeited for each year in which EPS does
not meet or exceed the 8% target. The performance targets for
2009, 2008 and 2007 were met. Vesting of the portion or portions
of a grant for a year or years in which the performance target
is met does not occur until completion of the entire three-year
vesting period. Upon exercise of vested options, the Company has
the right to reissue treasury shares or issue new shares.
During 2009, the Company awarded 2,585,196 options under the
Amended 2006 Plan, including 348,600 options granted to members
of the Management Board of Fresenius Medical Care Management AG,
the Company’s
F-34
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
general partner, at a weighted average exercise price of $46.22
(€32.08), a weighted average fair value of $10.95 each and
a total fair value of $28,318 which will be amortized over the
three year vesting period.
During 2008, the Company awarded 2,523,729 options under the
Amended 2006 Plan, including 398,400 options granted to members
of the Management Board of the General Partner, at a weighted
average exercise price of $49.38 (€35.48), a weighted
average fair value of $15.37 each and a total fair value of
$38,788, which will be amortized on a straight line basis over
the three-year vesting period.
During 2007, the Company awarded 2,395,962 options under the
Amended 2006 Plan, including 398,400 options granted to members
of the Management Board of the General Partner, at a weighted
average exercise price of $46.22 (€33.91), a weighted
average fair value of $13.23 (€9.71) each and a total fair
value of $31,709, which will be amortized on a straight line
basis over the three-year vesting period.
Options granted under the Amended 2006 Plan to US participants
are non-qualified stock options under the United States Internal
Revenue Code of 1986, as amended. Options under the Amended 2006
Plan are not transferable by a participant or a
participant’s heirs, and may not be pledged, assigned, or
otherwise disposed of.
Fresenius
Medical Care 2001 International Stock Option Plan
Under the Fresenius Medical Care 2001 International Stock
Incentive Plan (the “2001 Plan”), options in the form
of convertible bonds with a principal of up to €10,240 were
issued to the members of the Management Board and other
employees of the Company representing grants for up to
4 million non-voting preference shares. The convertible
bonds originally had a par value of €2.56 and bear interest
at a rate of 5.5%. In connection with the share split effected
in 2007, the principal amount was adjusted in the same
proportion as the share capital out of the capital increase and
the par value of the convertible bonds was adjusted to
€0.85 without affecting the interest rate. Except for the
members of the Management Board, eligible employees may purchase
the bonds by issuing a non-recourse note with terms
corresponding to the terms of and secured by the bond. The
Company has the right to offset its obligation on a bond against
the employee’s obligation on the related note; therefore,
the convertible bond obligations and employee note receivables
represent stock options issued by the Company and are not
reflected in the Consolidated Financial Statements. The options
expire ten years from issuance and can be exercised beginning
two, three or four years after issuance. Compensation costs
related to awards granted under this plan are amortized on a
straight-line basis over the vesting period for each separately
vesting portion of the awards. Bonds issued to Management Board
members who did not issue a note to the Company are recognized
as a liability on the Company’s balance sheet.
Upon issuance of the option, the employees had the right to
choose options with or without a stock price target. The
conversion price of options subject to a stock price target
corresponds to the stock exchange quoted price of the preference
shares upon the first time the stock exchange quoted price
exceeds the initial value by at least 25%. The initial value
(“Initial Value”) is the average price of the
preference shares during the last 30 trading days prior to the
date of grant. In the case of options not subject to a stock
price target, the number of convertible bonds awarded to the
eligible employee would be 15% less than if the employee elected
options subject to the stock price target. The conversion price
of the options without a stock price target is the Initial
Value. Each option entitles the holder thereof, upon payment of
the respective conversion price, to acquire one preference
share. Effective May 2006, no further grants can be issued under
the 2001 Plan and no options were granted under the 2001 Plan
after 2005.
At December 31, 2009, the Management Board members of the
General Partner held 2,041,121 stock options for ordinary shares
and employees of the Company held 9,852,942 stock options for
ordinary shares and 146,601 stock options for preference shares,
under the various stock-based compensation plans of the Company.
The Table below provides reconciliations for options outstanding
at December 31, 2009, as compared to December 31, 2008.
F-35
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
Weighted
|
|
|
|
|
|
|
|
average
|
|
|
average
|
|
|
|
|
Options
|
|
|
exercise
|
|
|
exercise
|
|
|
|
|
(in thousands)
|
|
|
price
|
|
|
price
|
|
|
|
|
|
|
|
€
|
|
|
$
|
|
|
|
|
Ordinary shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2008
|
|
|
11,280
|
|
|
|
29.15
|
|
|
|
41.99
|
|
|
Granted
|
|
|
2,585
|
|
|
|
32.08
|
|
|
|
46.22
|
|
|
Exercised
|
|
|
1,815
|
|
|
|
24.08
|
|
|
|
34.69
|
|
|
Forfeited
|
|
|
156
|
|
|
|
33.18
|
|
|
|
47.80
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009
|
|
|
11,894
|
|
|
|
30.50
|
|
|
|
43.94
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preference shares
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2008
|
|
|
242
|
|
|
|
16.18
|
|
|
|
23.31
|
|
|
Exercised
|
|
|
74
|
|
|
|
13.38
|
|
|
|
19.28
|
|
|
Forfeited
|
|
|
21
|
|
|
|
11.04
|
|
|
|
15.90
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009
|
|
|
147
|
|
|
|
18.35
|
|
|
|
26.44
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following table provides a summary of fully vested options
outstanding and exercisable for both preference and ordinary
shares at December 31, 2009:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fully Vested Outstanding and Exercisable Options
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
|
average
|
|
Weighted
|
|
Weighted
|
|
|
|
|
|
|
|
Number
|
|
remaining
|
|
average
|
|
average
|
|
Aggregate
|
|
Aggregate
|
|
|
|
of
|
|
contractual
|
|
exercise
|
|
exercise
|
|
intrinsic
|
|
intrinsic
|
|
|
|
Options
|
|
life in years
|
|
price
|
|
price
|
|
value
|
|
value
|
|
|
|
(in thousands)
|
|
|
|
€
|
|
US$
|
|
€
|
|
US$
|
|
|
|
Options for preference shares
|
|
|
147
|
|
|
|
3.91
|
|
|
|
18.35
|
|
|
|
26.43
|
|
|
|
1,861
|
|
|
|
2,681
|
|
|
Options for ordinary shares
|
|
|
4,589
|
|
|
|
4.02
|
|
|
|
25.27
|
|
|
|
36.40
|
|
|
|
53,560
|
|
|
|
77,158
|
|
At December 31, 2009, there were $45,441 of total
unrecognized compensation costs related to non-vested options
granted under all plans. These costs are expected to be
recognized over a weighted-average period of 1.6 years.
During the years ended December 31, 2009, 2008, and 2007,
the company received cash of $64,271, $36,755, and $38,757,
respectively, from the exercise of stock options. The intrinsic
value of options exercised for the twelve-month periods ending
December 31, 2009, 2008, and 2007, were $28,170, $27,135,
and $27,591, respectively. The Company recorded a related tax
benefit of $8,123, $7,132, and $8,177 for the years ending
December 31, 2009, 2008, and 2007, respectively.
Fair
Value Information
The Company used a binomial option-pricing model in determining
the fair value of the awards under the 2006 Plan. Option
valuation models require the input of highly subjective
assumptions including expected stock price volatility. The
Company’s assumptions are based upon its past experiences,
market trends and the experiences of other entities of the same
size and in similar industries. Expected volatility is based on
historical volatility of the Company’s shares. To
incorporate the effects of expected early exercise in the model,
an early exercise of vested options was assumed as soon as the
share price exceeds 155% of the exercise price. The
Company’s stock options have characteristics that vary
significantly from traded options and changes in subjective
assumptions can
F-36
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
materially affect the fair value of the option. The assumptions
used to determine the fair value of the 2009 and 2008 grants are
as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Expected dividend yield
|
|
|
2.39%
|
|
|
|
1.85%
|
|
|
Risk-free interest rate
|
|
|
3.11%
|
|
|
|
4.38%
|
|
|
Expected volatility
|
|
|
25.85%
|
|
|
|
25.58%
|
|
|
Expected life of options
|
|
|
7 years
|
|
|
|
7 years
|
|
|
Weighted average exercise price
|
|
€
|
32.08
|
|
|
€
|
35.48
|
|
|
(Weighted average exercise price in US$)
|
|
($
|
46.22
|
)
|
|
($
|
49.38
|
)
|
Income before income taxes is attributable to the following
geographic locations:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Germany
|
|
$
|
296,326
|
|
|
$
|
372,174
|
|
|
$
|
281,633
|
|
|
United States
|
|
|
904,083
|
|
|
|
773,089
|
|
|
|
724,839
|
|
|
Other
|
|
|
255,224
|
|
|
|
190,427
|
|
|
|
202,603
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
1,455,633
|
|
|
$
|
1,335,690
|
|
|
$
|
1,209,075
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense (benefit) for the years ended
December 31, 2009, 2008, and 2007, consisted of the
following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Current:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Germany
|
|
$
|
68,442
|
|
|
$
|
62,609
|
|
|
$
|
124,598
|
|
|
United States
|
|
|
318,589
|
|
|
|
198,763
|
|
|
|
272,032
|
|
|
Other
|
|
|
81,236
|
|
|
|
77,134
|
|
|
|
75,534
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
468,267
|
|
|
|
338,506
|
|
|
|
472,164
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Germany
|
|
|
5,041
|
|
|
|
43,593
|
|
|
|
(11,377
|
)
|
|
United States
|
|
|
22,498
|
|
|
|
105,152
|
|
|
|
3,483
|
|
|
Other
|
|
|
(5,393
|
)
|
|
|
(11,549
|
)
|
|
|
(10,505
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
22,146
|
|
|
|
137,196
|
|
|
|
(18,399
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
490,413
|
|
|
$
|
475,702
|
|
|
$
|
453,765
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In 2009 and 2008, the Company is subject to German federal
corporation income tax at a base rate of 15% plus a solidarity
surcharge of 5.5% on federal corporation taxes payable.
In 2007, the Company was subject to German federal corporation
income tax at a base rate of 25% plus a solidarity surcharge of
5.5% on federal corporation taxes payable.
A reconciliation between the expected and actual income tax
expense is shown below. The expected corporate income tax
expense is computed by applying the German corporation tax rate
(including the solidarity surcharge) and the effective trade tax
rate on income before income taxes. The respective combined tax
rates are 29.13%, 29.58% and 38.47% for the fiscal years ended
December 31, 2009, 2008, and 2007.
F-37
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Expected corporate income tax expense
|
|
$
|
423,953
|
|
|
$
|
395,097
|
|
|
$
|
465,131
|
|
|
Tax free income
|
|
|
(33,284
|
)
|
|
|
(49,309
|
)
|
|
|
(50,131
|
)
|
|
Foreign tax rate differential
|
|
|
96,237
|
|
|
|
93,877
|
|
|
|
(5,434
|
)
|
|
Non-deductible expenses
|
|
|
3,947
|
|
|
|
5,494
|
|
|
|
5,081
|
|
|
Taxes for prior years
|
|
|
6,663
|
|
|
|
21,371
|
|
|
|
41,868
|
|
|
Change in valuation allowance
|
|
|
8,950
|
|
|
|
4,168
|
|
|
|
3,627
|
|
|
Book income of consolidated partnership attributable to
Noncontrolling interests
|
|
|
(26,876
|
)
|
|
|
(13,440
|
)
|
|
|
(11,887
|
)
|
|
Change of German tax rate
|
|
|
—
|
|
|
|
—
|
|
|
|
(4,257
|
)
|
|
Other
|
|
|
10,823
|
|
|
|
18,444
|
|
|
|
9,767
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Actual income tax expense
|
|
$
|
490,413
|
|
|
$
|
475,702
|
|
|
$
|
453,765
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effective tax rate
|
|
|
33.7
|
%
|
|
|
35.6
|
%
|
|
|
37.5
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The tax effects of the temporary differences that give rise to
deferred tax assets and liabilities at December 31, 2009
and 2008, are presented below:
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Deferred tax assets:
|
|
|
|
|
|
|
|
|
|
Accounts receivable, primarily due to allowance for doubtful
accounts
|
|
$
|
37,571
|
|
|
$
|
37,431
|
|
|
Inventory, primarily due to additional costs capitalized for tax
purposes, and inventory reserve accounts
|
|
|
33,798
|
|
|
|
35,029
|
|
|
Plant, equipment, intangible assets and other non current
assets, principally due to differences in depreciation and
amortization
|
|
|
50,925
|
|
|
|
41,103
|
|
|
Accrued expenses and other liabilities for financial accounting
purposes, not currently tax deductible
|
|
|
291,767
|
|
|
|
305,898
|
|
|
Net operating loss carryforwards, tax credit carryforwards and
interest carryforwards
|
|
|
78,730
|
|
|
|
79,389
|
|
|
Derivatives
|
|
|
52,283
|
|
|
|
67,800
|
|
|
Stock-based compensation expense
|
|
|
22,981
|
|
|
|
17,405
|
|
|
Other
|
|
|
21,530
|
|
|
|
10,679
|
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred tax assets
|
|
$
|
589,585
|
|
|
$
|
594,734
|
|
|
Less: valuation allowance
|
|
|
(63,497
|
)
|
|
|
(56,169
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net deferred tax assets
|
|
$
|
526,088
|
|
|
$
|
538,565
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred tax liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
$
|
10,670
|
|
|
$
|
11,015
|
|
|
Inventory, primarily due to inventory reserve accounts for tax
purposes
|
|
|
9,643
|
|
|
|
4,615
|
|
|
Accrued expenses and other liabilities deductible for tax prior
to financial accounting recognition
|
|
|
14,941
|
|
|
|
50,229
|
|
|
Plant, equipment and intangible assets, principally due to
differences in depreciation and amortization
|
|
|
513,254
|
|
|
|
432,367
|
|
|
Derivatives
|
|
|
3,128
|
|
|
|
11,830
|
|
|
Other
|
|
|
53,343
|
|
|
|
66,532
|
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred tax liabilities
|
|
|
604,979
|
|
|
|
576,588
|
|
|
|
|
|
|
|
|
|
|
|
|
Net deferred tax assets (liabilities)
|
|
$
|
(78,891
|
)
|
|
$
|
(38,023
|
)
|
|
|
|
|
|
|
|
|
|
|
The valuation allowance increased by $7,328 in 2009 and by
$4,843 in 2008.
F-38
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
The expiration of net operating losses is as follows:
|
|
|
|
|
|
|
2009
|
|
$
|
45,648
|
|
|
2010
|
|
|
6,012
|
|
|
2011
|
|
|
8,444
|
|
|
2012
|
|
|
11,950
|
|
|
2013
|
|
|
17,337
|
|
|
2014
|
|
|
8,941
|
|
|
2015
|
|
|
9,517
|
|
|
2016
|
|
|
22,492
|
|
|
2017
|
|
|
10,450
|
|
|
2018 and thereafter
|
|
|
4,071
|
|
|
Without expiration date
|
|
|
84,558
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
229,420
|
|
|
|
|
|
|
|
In assessing the realizability of deferred tax assets,
management considers whether it is more-likely-than-not that
some portion or all of the deferred tax assets will be realized.
The ultimate realization of deferred tax assets is dependent
upon the generation of future taxable income during the periods
in which those temporary differences become deductible.
Management considers the scheduled reversal of deferred tax
liabilities and projected future taxable income in making this
assessment. Based upon the level of historical taxable income
and projections for future taxable income over the periods in
which the deferred tax assets are deductible, management
believes it is more-likely-than-not the Company will realize the
benefits of these deductible differences, net of the existing
valuation allowances at December 31, 2009.
The Company provides for income taxes on the cumulative earnings
of foreign subsidiaries that will not be reinvested. At
December 31, 2009, the Company provided for $13,289 of
deferred tax liabilities associated with earnings that are
likely to be distributed in 2010 and the following years.
Provision has not been made for additional taxes on $2,733,920
undistributed earnings of foreign subsidiaries as these earnings
are considered permanently reinvested. The earnings could become
subject to additional tax if remitted or deemed remitted as
dividends; however calculation of such additional tax is not
practical. These taxes would predominantly comprise foreign
withholding tax on dividends of foreign subsidiaries, and German
income tax of approx 1.5 percent on all dividends and
capital gains.
FMC-AG & Co. KGaA companies are subject to tax audits
in Germany and the U.S. on a regular basis and on-going tax
audits in other jurisdictions. In Germany, the tax audit for the
years 1998 until 2001 has been finalized. The Company recognized
and recorded the results of the audit in 2006 and thereafter
paid all amounts due to the tax authorities. Fiscal years 2002
through 2005 are currently under audit and fiscal years 2006,
2007, 2008 and 2009 are open to audit.
For the tax year 1997, the Company recognized an impairment of
one of its subsidiaries which the German tax authorities
disallowed in 2003 at the conclusion of its audit for the years
1996 and 1997. The Company has filed a complaint with the
appropriate German court to challenge the tax authority’s
decision. The Company has included the related unrecognized tax
benefit in the total unrecognized tax benefit noted below.
The Company filed claims for refunds contesting the Internal
Revenue Service’s (“IRS”) disallowance of
FMCH’s civil settlement payment deductions taken by
Fresenius Medical Care Holdings, Inc. (“FMCH”) in
prior year tax returns. As a result of a settlement agreement
with the IRS to resolve the Company’s appeal of the
IRS’s disallowance of deductions for the civil settlement
payments made to qui tam relators in connection with the
resolution of the 2000 U.S. government investigation, the
Company received a refund in September 2008 of $37,000,
inclusive of interest. The settlement agreement preserves the
right to continue to pursue claims in the U.S. Federal courts
for refunds of all other disallowed deductions. The unrecognized
tax benefit relating to these deductions is included in the
total unrecognized tax benefit noted below.
The IRS tax audits of FMCH for the years 2002 through 2006 have
been completed. The IRS has disallowed all deductions taken
during these audit periods related to intercompany mandatorily
redeemable preferred shares. The Company has protested the
disallowed deductions and will avail itself of all remedies. An
adverse determination with respect to the disallowed deductions
related to the intercompany mandatorily redeemable preferred
shares
F-39
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
could have a material adverse effect on the results of
operations and liquidity. In addition, the IRS proposed other
adjustments which have been recognized in the financial
statements.
Fiscal years 2007, 2008 and 2009 are open to audit. There are a
number of state audits in progress and various years are open to
audit in various states. All expected results have been
recognized in the financial statements.
Subsidiaries of FMC-AG & Co. KGaA in a number of
countries outside of Germany and the U.S. are also subject to
tax audits. The Company estimates that the effects of such tax
audits are not material to these consolidated financial
statements.
The following table shows the reconciliation of the beginning
and ending amounts of unrecognized tax benefits:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Unrecognized tax benefits (net of interest)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at January 1, 2009
|
|
$
|
379,327
|
|
|
$
|
354,050
|
|
|
$
|
302,552
|
|
|
Increases in unrecognized tax benefits prior periods
|
|
|
59,833
|
|
|
|
24,074
|
|
|
|
29,236
|
|
|
Decreases in unrecognized tax benefits prior periods
|
|
|
(13,911
|
)
|
|
|
(36,334
|
)
|
|
|
9,965
|
|
|
Increases in unrecognized tax benefits current period
|
|
|
7,587
|
|
|
|
20,180
|
|
|
|
14,893
|
|
|
Changes related to settlements with tax authorities
|
|
|
(8,599
|
)
|
|
|
(2,042
|
)
|
|
|
2,960
|
|
|
Foreign currency translation
|
|
|
(14,221
|
)
|
|
|
19,399
|
|
|
|
20,294
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009
|
|
$
|
410,016
|
|
|
$
|
379,327
|
|
|
$
|
354,050
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Included in the balance at December 31, 2009 are $379,674
of unrecognized tax benefits which would affect the effective
tax rate if recognized. The Company is currently not in a
position to forecast the timing and magnitude of changes in the
unrecognized tax benefits.
During the year ended December 31, 2009 the Company
recognized $16,609 in interest and penalties. The Company had a
total accrual of $47,383 of tax related interest and penalties
at December 31, 2009.
The Company leases buildings and machinery and equipment under
various lease agreements expiring on dates through 2034. Rental
expense recorded for operating leases for the years ended
December 31, 2009, 2008 and 2007 was $532,465, $497,875 and
$461,490, respectively.
Future minimum rental payments under noncancelable operating
leases for the five years succeeding December 31, 2009 and
thereafter are:
|
|
|
|
|
|
|
2010
|
|
$
|
454,833
|
|
|
2011
|
|
|
403,366
|
|
|
2012
|
|
|
348,214
|
|
|
2013
|
|
|
298,414
|
|
|
2014
|
|
|
244,528
|
|
|
Thereafter
|
|
|
802,093
|
|
|
|
|
|
|
|
|
|
|
$
|
2,551,448
|
|
|
|
|
|
|
|
Legal
Proceedings
The Company is routinely involved in numerous claims, lawsuits,
regulatory and tax audits, investigations and other legal
matters arising, for the most part, in the ordinary course of
its business of providing healthcare services and products. The
outcome of litigation and other legal matters is always
difficult to accurately predict and outcomes that are not
consistent with the Company’s view of the merits can occur.
The Company believes that it has valid defenses to the legal
matters pending against it and is defending itself vigorously.
Nevertheless, it is possible that the resolution of one or more
of the legal matters currently pending or threatened could have
a material adverse effect on its business, results of operations
and financial condition.
F-40
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
Commercial
Litigation
The Company was originally formed as a result of a series of
transactions it completed pursuant to the Agreement and Plan of
Reorganization dated as of February 4, 1996, by and between
W.R. Grace & Co. and Fresenius SE (the
“Merger”). At the time of the Merger, a W.R.
Grace & Co. subsidiary known as W.R. Grace &
Co.-Conn. had, and continues to have, significant liabilities
arising out of product-liability related litigation (including
asbestos-related actions), pre-Merger tax claims and other
claims unrelated to National Medical Care, Inc.
(“NMC”), which was W.R. Grace & Co.’s
dialysis business prior to the Merger. In connection with the
Merger, W.R. Grace & Co.-Conn. agreed to indemnify the
Company, FMCH, and NMC against all liabilities of W.R.
Grace & Co., whether relating to events occurring
before or after the Merger, other than liabilities arising from
or relating to NMC’s operations. W.R. Grace & Co.
and certain of its subsidiaries filed for reorganization under
Chapter 11 of the U.S. Bankruptcy Code (the “Grace
Chapter 11 Proceedings”) on April 2, 2001.
Prior to and after the commencement of the Grace Chapter 11
Proceedings, class action complaints were filed against W.R.
Grace & Co. and FMCH by plaintiffs claiming to be
creditors of W.R. Grace & Co.-Conn., and by the
asbestos creditors’ committees on behalf of the W.R.
Grace & Co. bankruptcy estate in the Grace
Chapter 11 Proceedings, alleging among other things that
the Merger was a fraudulent conveyance, violated the uniform
fraudulent transfer act and constituted a conspiracy. All such
cases have been stayed and transferred to or are pending before
the U.S. District Court as part of the Grace Chapter 11
Proceedings.
In 2003, the Company reached agreement with the asbestos
creditors’ committees on behalf of the W.R.
Grace & Co. bankruptcy estate and W.R.
Grace & Co. in the matters pending in the Grace
Chapter 11 Proceedings for the settlement of all fraudulent
conveyance and tax claims against it and other claims related to
the Company that arise out of the bankruptcy of W.R.
Grace & Co. Under the terms of the settlement
agreement as amended (the “Settlement Agreement”),
fraudulent conveyance and other claims raised on behalf of
asbestos claimants will be dismissed with prejudice and the
Company will receive protection against existing and potential
future W.R. Grace & Co. related claims, including
fraudulent conveyance and asbestos claims, and indemnification
against income tax claims related to the non-NMC members of the
W.R. Grace & Co. consolidated tax group upon
confirmation of a W.R. Grace & Co. bankruptcy
reorganization plan that contains such provisions. Under the
Settlement Agreement, the Company will pay a total of $115,000
without interest to the W.R. Grace & Co. bankruptcy
estate, or as otherwise directed by the Court, upon plan
confirmation. No admission of liability has been or will be
made. The Settlement Agreement has been approved by the U.S.
District Court. Subsequent to the Merger, W.R. Grace &
Co. was involved in a multi-step transaction involving Sealed
Air Corporation (“Sealed Air,” formerly known as Grace
Holding, Inc.). The Company is engaged in litigation with Sealed
Air to confirm its entitlement to indemnification from Sealed
Air for all losses and expenses incurred by the Company relating
to pre-Merger tax liabilities and Merger-related claims. Under
the Settlement Agreement, upon confirmation of a plan that
satisfies the conditions of the Company’s payment
obligation, this litigation will be dismissed with prejudice.
On April 4, 2003, FMCH filed a suit in the U. S. District
Court for the Northern District of California, styled Fresenius
USA, Inc., et al., v. Baxter International Inc., et al., Case
No. C
03-1431,
seeking a declaratory judgment that FMCH does not infringe
patents held by Baxter International Inc. and its subsidiaries
and affiliates (“Baxter”), that the patents are
invalid, and that Baxter is without right or authority to
threaten or maintain suit against FMCH for alleged infringement
of Baxter’s patents. In general, the alleged patents
concern the use of touch screen interfaces for hemodialysis
machines. Baxter filed counterclaims against FMCH seeking more
than $140,000 in monetary damages and injunctive relief, and
alleging that FMCH willfully infringed on Baxter’s patents.
On July 17, 2006, the court entered judgment on a jury
verdict in favor of FMCH finding that all the asserted claims of
the Baxter patents are invalid as obvious
and/or
anticipated in light of prior art.
On February 13, 2007, the court granted Baxter’s
motion to set aside the jury’s verdict in favor of FMCH and
reinstated the patents and entered judgment of infringement.
Following a trial on damages, the court entered judgment on
November 6, 2007 in favor of Baxter on a jury award of
$14,300. On April 4, 2008, the court denied Baxter’s
motion for a new trial, established a royalty payable to Baxter
of 10% of the sales price for continuing sales of FMCH’s
2008K hemodialysis machines and 7% of the sales price of related
disposables, parts and service beginning November 7, 2007,
and enjoined sales of the touchscreen-equipped 2008K machine
effective January 1, 2009. We appealed the court’s
rulings to the Court of Appeals for the Federal Circuit. On
September 10, 2009, the Court of Appeals reversed the
district court’s decision and determined that the asserted
claims in two of the three patents at issue are invalid. As to
the third patent, the Court of Appeals affirmed the district
court’s decision;
F-41
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
however, the Court of Appeals vacated the injunction and award
of damages. These issues have been remanded to the lower court
for reconsideration in light of the invalidity ruling on most of
the claims. As a result, FMCH is no longer required to fund the
court-approved escrow account set up to hold the royalty
payments ordered by the district court, although funds already
contributed will remain in escrow until the case is concluded.
The remaining patent has been found invalid in re-examination by
the U.S. Patent and Trademark Office (USPTO) and Baxter has
appealed this finding. If we prevail with respect to the
invalidity of the final remaining patent, the escrowed funds
will be returned to us with interest. In October 2008, we
completed design modifications to the 2008K machine that
eliminate any incremental hemodialysis machine royalty payment
exposure under the original district court order, irrespective
of the outcome of the remanded issues.
On April 28, 2008, Baxter filed suit in the U.S. District
Court for the Northern District of Illinois, Eastern Division
(Chicago), styled Baxter International, Inc. and Baxter
Healthcare Corporation v. Fresenius Medical Care Holdings, Inc.
and Fresenius USA, Inc., Case No. CV 2389, asserting that
FMCH’s hemodialysis machines infringe four recently issued
patents (late
2007-2008),
all of which are based on one of the patents at issue in the
April 2003 Baxter case described above. The new patents expire
in April 2011 and relate to trend charts shown on touch screen
interfaces and the entry of ultrafiltration profiles
(ultrafiltration is the removing of liquid from a patient’s
body using osmotic pressure). This case is currently stayed
pursuant to court order. The Company believes that its
hemodialysis machines do not infringe any valid claims of the
Baxter patents at issue, all of which are now subject to
re-examination at, and to preliminary findings of invalidity by,
the USPTO.
On October 17, 2006, Baxter and DEKA Products Limited
Partnership (DEKA) filed suit in the U.S. District Court for the
Eastern District of Texas which was subsequently transferred to
the Northern District of California, styled Baxter Healthcare
Corporation and DEKA Products Limited Partnership v. Fresenius
Medical Care Holdings, Inc. d/b/a Fresenius Medical Care North
America and Fresenius USA, Inc., Case No. CV 438 TJW. The
complaint alleges that FMCH’s Liberty peritoneal cyclers
infringe certain patents owned by or licensed to Baxter. Sales
of the Liberty cyclers commenced in July 2008. The Company
believes that the Liberty peritoneal cycler does not infringe
any valid claims of the Baxter/DEKA patents.
A patent infringement action has been pending in Germany between
Gambro Industries (“Gambro”) on the one side and
Fresenius Medical Care Deutschland GmbH (“D-GmbH”) and
FMC-AG & Co. KGaA on the other side (hereinafter
collectively “Fresenius Medical Care”). Gambro herein
alleged patent infringements by Fresenius Medical Care
concerning a patent on a device for the preparation of medical
solutions. The District Court of Mannheim rendered a judgment on
June 27, 2008 deciding in favor of Gambro and declaring
that Fresenius Medical Care has infringed a patent. Accordingly,
the court ordered Fresenius Medical Care to pay compensation (to
be determined in a separate court proceeding which was recently
initiated by Gambro; a hearing has been scheduled in February
2010) for alleged infringement and to stop offering the
alleged patent infringing technology in its original form in
Germany. D-GmbH brought an invalidity action in the Federal
German Patent Court (“BPatG”) against Gambro’s
patent. This case is currently pending with the Federal Court of
Justice as the court of appeal. Fresenius Medical Care has also
filed an appeal against the District Court’s verdict. On
January 5, 2009, Gambro enforced such verdict provisionally
by way of security. However, preceding such enforcement
Fresenius Medical Care had already developed design
modifications, being an alternative technical solution, and
replaced the alleged patent infringing technology in all of the
affected devices. In view of the pending appeal against
BPatG’s verdict and Fresenius Medical Care’s appeal
against the District Court’s verdict, Fresenius Medical
Care continues to believe that the alleged patent infringing
technology does not infringe any valid patent claims of Gambro.
Therefore, the Company has made no provision in the financial
statements for any potential liability in this matter.
Other
Litigation and Potential Exposures
Renal Care Group, Inc. (“RCG”) was named as a nominal
defendant in a second amended complaint filed September 13,
2006 in the Chancery Court for the State of Tennessee Twentieth
Judicial District at Nashville against former officers and
directors of RCG which purports to constitute a class action and
derivative action relating to alleged unlawful actions and
breaches of fiduciary duty in connection with the Company’s
acquisition of RCG (the “RCG Acquisition”) and in
connection with alleged improper backdating
and/or
timing of stock option grants by RCG. The amended complaint was
styled Indiana State District Council of Laborers and Hod
Carriers Pension Fund v. Gary Brukardt et al. The complaint
sought damages against defendant and its former officers and
directors but did not state a claim for money damages directly
against RCG. As of August 24, 2009, appellate
F-42
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
proceedings that reversed the trial court’s dismissal of
the complaint had concluded. The litigation is accordingly
proceeding toward trial in the Chancery Court.
FMCH and its subsidiaries, including RCG (prior to the RCG
Acquisition), received subpoenas from the U.S. Department of
Justice, U.S. Attorney for the Eastern District of Missouri, in
connection with a joint civil and criminal investigation. FMCH
received its subpoena in April 2005. RCG received its subpoena
in August 2005. The subpoenas require production of a broad
range of documents relating to FMCH’s and RCG’s
operations, with specific attention to documents related to
clinical quality programs, business development activities,
medical director compensation and physician relationships, joint
ventures, and anemia management programs, RCG’s supply
company, pharmaceutical and other services that RCG provides to
patients, RCG’s relationships to pharmaceutical companies,
and RCG’s purchase of dialysis equipment from FMCH. The
Office of Inspector General of the United States Department of
Health and Human Services and the United States Attorney for the
Eastern District of Texas participated in the Eastern District
of Missouri’s investigation of FMCH’s and RCG’s
utilization of Epogen begun in 2005. Subsequently, the review of
Epogen utilization was transferred to the Eastern District of
Texas, where a qui tam relator’s complaint has been pending
under seal since 2005 (qui tam is a legal provision under the
United States False Claims Act, which allows private individuals
to bring suit on behalf of the U.S. federal government, as far
as such individuals believe to have knowledge of presumable
fraud committed by third parties). The qui tam relator’s
complaint was made public by the United States District Court
for the Eastern District of Texas during the 4th quarter 2009
and was dismissed by the Court on January 11, 2010 with
respect to FMCH and its subsidiaries following the
relator’s motion to dismiss FMCH and its subsidiaries and
with the United States’ consent.
On July 17, 2007, the U.S. Attorney’s office filed a
civil complaint against RCG and FMCH in its capacity as
RCG’s current corporate parent in United States District
Court, Eastern District of Missouri. The complaint seeks
monetary damages and penalties with respect to issues arising
out of the operation of RCG’s Method II supply company
through 2005, prior to the date of FMCH’s acquisition of
RCG. The complaint is styled United States of America ex rel.
Julie Williams et al. vs. Renal Care Group, Renal Care Group
Supply Company and FMCH. On August 11, 2009, the Court
granted RCG’s motion to transfer venue to the Middle
District of Tennessee (Nashville), where the case is proceeding
toward trial. The Company believes that RCG’s operation of
its Method II supply company was in compliance with applicable
law and will defend this litigation vigorously.
On November 27, 2007, the United States District Court for
the Western District of Texas (El Paso) unsealed and permitted
service of two complaints previously filed under seal by a qui
tam relator, a former FMCH local clinic employee. The first
complaint alleges that a nephrologist unlawfully employed in his
practice an assistant to perform patient care tasks that the
assistant was not licensed to perform and that Medicare billings
by the nephrologist and FMCH therefore violated the False Claims
Act. The second complaint alleges that FMCH unlawfully
retaliated against the relator by discharging her from
employment constructively. The United States Attorney for the
Western District of Texas declined to intervene and to prosecute
on behalf of the United States. Litigation on the relator’s
complaint is proceeding to trial.
On June 25, 2009, FMCH received a subpoena from the U.S.
Department of Justice, U.S. Attorney for the District of
Massachusetts. The subpoena seeks information relating to the
results of certain laboratory tests ordered for patients treated
in FMCH’s dialysis facilities during the years 2004 through
2009. The Company intends to cooperate fully in the
government’s investigation.
We have filed claims for refunds contesting the Internal Revenue
Service’s (“IRS”) disallowance of FMCH’s
civil settlement payment deductions taken by Fresenius Medical
Care Holdings, Inc. (“FMCH”) in prior year tax
returns. As a result of a settlement agreement with the IRS to
resolve our appeal of the IRS’s disallowance of deductions
for the civil settlement payments made to qui tam relators in
connection with the resolution of the 2000 U.S. government
investigation, we received a refund in September 2008 of
$37,000, inclusive of interest. The settlement preserves our
right to continue to pursue claims in the U.S. Federal courts
for refunds of all other disallowed deductions.
For the tax year 1997, the Company recognized an impairment of
one of our subsidiaries which the German tax authorities
disallowed in 2003 at the conclusion of its audit for the years
1996 and 1997. The Company has filed a complaint with the
appropriate German court to challenge the tax authority’s
decision.
F-43
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
The IRS tax audits of FMCH for the years 2002 through 2006 have
been completed. The IRS has disallowed all deductions taken
during these audit periods related to intercompany mandatorily
redeemable preferred shares. The Company has protested the
disallowed deductions and will avail itself of all remedies. An
adverse determination with respect to the disallowed deductions
related to intercompany mandatorily redeemable preferred shares
could have a material adverse effect on our results of
operations and liquidity. In addition, the IRS proposed other
adjustments which have been recognized in the financial
statements.
Following Fresenius Medical Care & Co KGaA’s
Annual General Meeting of Shareholders (“AGM”) on
May 7, 2009, two shareholders challenged, on the basis of
alleged insufficient disclosure during the AGM, resolutions
taken by the shareholders on (i) the approval of the
actions of the General Partner and (ii) the approval of the
actions of the members of the Supervisory Board. Upon conclusion
of the proceedings, the court will either uphold the respective
resolutions or order their annulment. The Company is of the
opinion that the challenges are without merit and will defend
this litigation vigorously. A hearing has been scheduled in
March 2010.
From time to time, the Company is a party to or may be
threatened with other litigation or arbitration, claims or
assessments arising in the ordinary course of its business.
Management regularly analyzes current information including, as
applicable, the Company’s defenses and insurance coverage
and, as necessary, provides accruals for probable liabilities
for the eventual disposition of these matters.
The Company, like other health care providers, conducts its
operations under intense government regulation and scrutiny. It
must comply with regulations which relate to or govern the
safety and efficacy of medical products and supplies, the
operation of manufacturing facilities, laboratories and dialysis
clinics, and environmental and occupational health and safety.
The Company must also comply with the Anti-Kickback Statute, the
False Claims Act, the Stark Law, and other federal and state
fraud and abuse laws. Applicable laws or regulations may be
amended, or enforcement agencies or courts may make
interpretations that differ from the Company’s
interpretations or the manner in which it conducts its business.
Enforcement has become a high priority for the federal
government and some states.
In addition, the provisions of the False Claims Act authorizing
payment of a portion of any recovery to the party bringing the
suit encourage private plaintiffs to commence “whistle
blower” actions. In May 2009, the scope of the False Claims
Act was expanded and additional protections for whistle blowers
and procedural provisions to aid whistle blowers’ ability
to proceed in a False Claims Act case were added. By virtue of
this regulatory environment, the Company’s business
activities and practices are subject to extensive review by
regulatory authorities and private parties, and continuing
audits, investigative demands, subpoenas, other inquiries,
claims and litigation relating to the Company’s compliance
with applicable laws and regulations. The Company may not always
be aware that an inquiry or action has begun, particularly in
the case of “whistle blower” actions, which are
initially filed under court seal.
The Company operates many facilities throughout the United
States. In such a decentralized system, it is often difficult to
maintain the desired level of oversight and control over the
thousands of individuals employed by many affiliated companies.
The Company relies upon its management structure, regulatory and
legal resources, and the effective operation of its compliance
program to direct, manage and monitor the activities of these
employees. On occasion, the Company may identify instances where
employees, deliberately or inadvertently, have submitted
inadequate or false billings. The actions of such persons may
subject the Company and its subsidiaries to liability under the
Anti-Kickback Statute, the Stark Law and the False Claims Act,
among other laws.
Physicians, hospitals and other participants in the health care
industry are also subject to a large number of lawsuits alleging
professional negligence, malpractice, product liability,
worker’s compensation or related claims, many of which
involve large claims and significant defense costs. The Company
has been and is currently subject to these suits due to the
nature of its business and expects that those types of lawsuits
may continue. Although the Company maintains insurance at a
level which it believes to be prudent, it cannot assure that the
coverage limits will be adequate or that insurance will cover
all asserted claims. A successful claim against the Company or
any of its subsidiaries in excess of insurance coverage could
have a material adverse effect upon it and the results of its
operations. Any claims, regardless of their merit or eventual
outcome, could have a material adverse effect on the
Company’s reputation and business.
The Company has also had claims asserted against it and has had
lawsuits filed against it relating to alleged patent
infringements or businesses that it has acquired or divested.
These claims and suits relate both to operation of
F-44
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
the businesses and to the acquisition and divestiture
transactions. The Company has, when appropriate, asserted its
own claims, and claims for indemnification. A successful claim
against the Company or any of its subsidiaries could have a
material adverse effect upon its business, financial condition,
and the results of its operations. Any claims, regardless of
their merit or eventual outcome, could have a material adverse
effect on the Company’s reputation and business.
Accrued
Special Charge for Legal Matters
At December 31, 2001, the Company recorded a pre-tax
special charge of $258,159 to reflect anticipated expenses
associated with the defense and resolution of pre-Merger tax
claims, Merger-related claims, and commercial insurer claims.
The costs associated with the Settlement Agreement and
settlements with insurers have been charged against this
accrual. With the exception of the proposed $115,000 payment
under the Settlement Agreement in the Grace Chapter 11
Proceedings, all other matters included in the special charge
have been resolved. While the Company believes that its
remaining accrual reasonably estimates its currently anticipated
costs related to the continued defense and resolution of this
matter, no assurances can be given that its actual costs
incurred will not exceed the amount of this accrual.
|
|
|
|
19.
|
Financial
Instruments
|
As a global supplier of dialysis services and products in more
than 115 countries throughout the world, the Company is faced
with a concentration of credit risks due to the nature of the
reimbursement systems which are often provided by the
governments of the countries in which the Company operates.
Changes in reimbursement rates or the scope of coverage could
have a material adverse effect on the Company’s business,
financial condition and results of operations and thus on its
capacity to generate cash flow. In the past the Company
experienced and also expects in the future generally stable
reimbursements for its dialysis services. This includes the
balancing of unfavorable reimbursement changes in certain
countries with favorable changes in other countries. Due to the
fact that a large portion of the Company’s reimbursement is
provided by public health care organizations and private
insurers, the Company expects that most of its accounts
receivables will be collectable, albeit potentially slightly
more slowly in the International segment in the immediate
future, particularly in countries most severely affected by the
current global financial crisis.
Non-derivative
Financial Instruments
The following table presents the carrying amounts and fair
values of the Company’s non-derivative financial
instruments at December 31, 2009, and December 31,
2008.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Carrying
|
|
|
Fair
|
|
|
Carrying
|
|
|
Fair
|
|
|
|
|
Amount
|
|
|
Value
|
|
|
Amount
|
|
|
Value
|
|
|
|
|
Non-derivatives
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
301,225
|
|
|
$
|
301,225
|
|
|
$
|
221,584
|
|
|
$
|
221,584
|
|
|
Receivables
|
|
|
2,558,795
|
|
|
|
2,558,795
|
|
|
|
2,351,841
|
|
|
|
2,351,841
|
|
|
Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
|
639,836
|
|
|
|
639,836
|
|
|
|
605,260
|
|
|
|
605,260
|
|
|
Short-term borrowings
|
|
|
316,344
|
|
|
|
316,344
|
|
|
|
683,155
|
|
|
|
683,155
|
|
|
Short-term borrowings from related parties
|
|
|
10,440
|
|
|
|
10,440
|
|
|
|
1,330
|
|
|
|
1,330
|
|
|
Long term debt, excluding 2006 Senior Credit Agreement, Euro
Notes and
6
7
/
8
%
Senior Notes
|
|
|
282,051
|
|
|
|
282,051
|
|
|
|
275,618
|
|
|
|
275,618
|
|
|
2006 Senior Credit Agreement
|
|
|
3,522,040
|
|
|
|
3,429,470
|
|
|
|
3,366,079
|
|
|
|
3,366,079
|
|
|
Trust Preferred Securities
|
|
|
656,096
|
|
|
|
688,026
|
|
|
|
640,696
|
|
|
|
626,241
|
|
|
Euro Notes
|
|
|
288,120
|
|
|
|
299,621
|
|
|
|
278,340
|
|
|
|
276,154
|
|
|
6
7
/
8
%
Senior Notes
|
|
|
493,344
|
|
|
|
498,750
|
|
|
|
492,456
|
|
|
|
465,625
|
|
The carrying amounts in the table are included in the
consolidated balance sheet under the indicated captions.
F-45
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
The significant methods and assumptions used in estimating the
fair values of non-derivative financial instruments are as
follows:
Cash and cash equivalents are stated at nominal value which
equals the fair value.
Short-term financial instruments such as accounts receivable and
accounts payable and short-term borrowings are valued at their
carrying amounts, which are reasonable estimates of the fair
value due to the relatively short period to maturity of these
instruments.
The fair values of the major long-term financial liabilities are
calculated on the basis of market information. Instruments for
which market quotes are available are measured using these
quotes. The fair values of the other long-term financial
liabilities are calculated at the present value of the
respective future cash flows. To determine these present values,
the prevailing interest rates and credit spreads for the Company
as of the balance sheet date are used.
Derivative
Financial Instruments
The Company is exposed to market risk from changes in interest
rates and foreign exchange rates. In order to manage the risk of
interest rate and currency exchange rate fluctuations, the
Company enters into various hedging transactions by means of
derivative instruments with highly rated financial institutions
as authorized by the Company’s General Partner. On a
quarterly basis an assessment of the Company’s counterparty
credit risk is performed, which we consider currently to be low.
The Company does not use financial instruments for trading
purposes.
The Company established guidelines for risk assessment
procedures and controls for the use of financial instruments.
They include a clear segregation of duties with regard to
execution on one side and administration, accounting and
controlling on the other.
Foreign
Exchange Risk Management
The Company conducts business on a global basis in various
currencies, though its operations are mainly in Germany and the
United States. For financial reporting purposes, the Company has
chosen the U.S. dollar as its reporting currency. Therefore,
changes in the rate of exchange between the U.S. dollar and the
local currencies in which the financial statements of the
Company’s international operations are maintained affect
its results of operations and financial position as reported in
its consolidated financial statements.
The Company’s exposure to market risk for changes in
foreign exchange rates relates to transactions such as sales and
purchases. The Company has significant amounts of sales of
products invoiced in euro from its European manufacturing
facilities to its other international operations and, to a
lesser extent, sales of products invoiced in other
non-functional currencies. This exposes the subsidiaries to
fluctuations in the rate of exchange between the euro and the
currency in which their local operations are conducted. For the
purpose of hedging existing and foreseeable foreign exchange
transaction exposures the Company enters into foreign exchange
forward contracts and, on a small scale, foreign exchange
options. The Company’s policy, which has been consistently
followed, is that financial derivatives be used only for the
purpose of hedging foreign currency exposure. As of
December 31, 2009 the Company had no foreign exchange
options.
Changes in the fair value of foreign exchange forward contracts
designated and qualifying as cash flow hedges of forecasted
product purchases and sales are reported in accumulated other
comprehensive income (loss). These amounts are subsequently
reclassified into earnings as a component of cost of revenues,
in the same period in which the hedged transaction affects
earnings. The notional amounts of foreign exchange forward
contracts in place that are designated and qualify as cash flow
hedges that hedge exposures from operations totaled $405,675.
In connection with intercompany loans in foreign currency the
Company uses foreign exchange swaps thus assuring that no
foreign exchange risks arise from those loans. At
December 31, 2009 the notional amounts of contracts for
which hedge accounting is applied totaled $670,542.
In certain instances, the Company enters into derivative
contracts of forecasted product purchases and sales and for
intercompany loans in foreign currency that do not qualify for
hedge accounting but are utilized for economic purposes
(“economic hedges”). In these cases, the change in
value of the economic hedge is recorded in the income statement
and usually offsets the change in value recorded in the income
statement for the underlying
F-46
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
asset or liability. The notional amounts of economic hedges for
forecasted product purchases and sales totaled $343,725 and for
intercompany loans totaled $407,087
Interest
Rate Risk Management
The Company enters into derivatives, particularly interest rate
swaps and to a certain extent, interest rate options, to hedge
interest rate exposures arising from long-term debt at floating
rates by effectively swapping them into fixed rates.
Cash
Flow Hedges of Variable Rate Debt
The Company enters into interest rate swap agreements that are
designated as cash flow hedges effectively converting the major
part of payments based on variable interest rates applicable to
the Company’s 2006 Senior Credit Agreement denominated in
U.S. dollars into payments at a fixed interest rate. Those swap
agreements, all of which expire at various dates between 2010
and 2012, in the notional amount of $2,400,000, effectively fix
the Company’s variable interest rate at an average interest
rate of 4.29% plus an applicable margin. Gains and losses were
deferred in accumulated other comprehensive income
(“OCI”); an amount of $33 net gains are reclassified
from accumulated OCI to interest income.
Interest payable and receivable under the swap agreements is
accrued and recorded as an adjustment to interest expense.
Derivative
Financial Instruments Valuation
The following table shows the Company’s derivatives at
December 31, 2009 and 2008.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2009
|
|
|
December 31, 2008
|
|
|
|
|
Assets
(2)
|
|
|
Liabilities
(2)
|
|
|
Assets
(2)
|
|
|
Liabilities
(2)
|
|
|
|
|
Derivatives in cash flow hedging
relationships
(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign exchange contracts
|
|
$
|
8,899
|
|
|
$
|
(9,251
|
)
|
|
$
|
27,904
|
|
|
$
|
(12,216
|
)
|
|
Interest rate contracts (Dollar)
|
|
|
—
|
|
|
|
(305
|
)
|
|
|
—
|
|
|
|
(8,526
|
)
|
|
Non—current
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign exchange contracts
|
|
|
5,284
|
|
|
|
(830
|
)
|
|
|
2,624
|
|
|
|
(2,547
|
)
|
|
Interest rate contracts (Dollar)
|
|
|
—
|
|
|
|
(105,810
|
)
|
|
|
—
|
|
|
|
(140,420
|
)
|
|
Interest rate contracts (Yen)
|
|
|
—
|
|
|
|
(3
|
)
|
|
|
—
|
|
|
|
(9
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
14,183
|
|
|
$
|
(116,199
|
)
|
|
$
|
30,528
|
|
|
$
|
(163,718
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Derivatives not designated as hedging
instruments
(1)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign exchange contracts
|
|
$
|
7,696
|
|
|
$
|
(6,217
|
)
|
|
$
|
22,182
|
|
|
$
|
(24,832
|
)
|
|
Non-current
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign exchange contracts
|
|
|
9
|
|
|
|
—
|
|
|
|
921
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
7,705
|
|
|
$
|
(6,217
|
)
|
|
$
|
23,103
|
|
|
$
|
(24,832
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1)
|
|
As of December 31, 2009, the
valuation of the Company’s derivatives was determined using
Significant Other Observable Inputs (Level 2) in
accordance with the fair value hierarchy levels established in
the Codification.
|
|
|
|
(2)
|
|
Derivative instruments are marked
to market each reporting period resulting in carrying amounts
being equal to fair values at reporting date.
|
The carrying amounts for the current portion of derivatives
indicated as assets in the table above are included in Prepaid
expenses and other current assets in the Consolidated Balance
Sheets while the current portion of those indicated as
liabilities are included in Accrued expenses and other current
liabilities. The non-current portions indicated as assets or
liabilities are included in the Consolidated Balance Sheets in
Other assets or Other liabilities, respectively.
F-47
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
The significant methods and assumptions used in estimating the
fair values of derivative financial instruments are as follows:
The fair value of interest rate swaps is calculated by
discounting the future cash flows on the basis of the market
interest rates applicable for the remaining term of the contract
as of the balance sheet date. To determine the fair value of
foreign exchange forward contracts, the contracted forward rate
is compared to the current forward rate for the remaining term
of the contract as of the balance sheet date. The result is then
discounted on the basis of the market interest rates prevailing
at the balance sheet date for the applicable currency.
The Company includes its own credit risk for financial
instruments deemed liabilities and counterparty-credit risks for
financial instruments deemed assets when measuring the fair
value of derivative financial instruments.
The
Effect of Derivatives on the Consolidated Financial
Statements
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amount of (Gain)
|
|
|
|
|
Amount of Gain or
|
|
|
Location of (Gain)
|
|
reclassified from
|
|
|
|
|
(Loss) Recognized in
|
|
|
reclassified from
|
|
Accumulated OCI in
|
|
|
|
|
OCI on Derivative
|
|
|
Accumulated OCI in
|
|
Income
|
|
Derivatives in Cash Flow
|
|
(Effective Portion)
|
|
|
Income
|
|
(Effective Portion)
|
|
|
Hedging Relationships
|
|
2009
|
|
|
(Effective Portion)
|
|
2009
|
|
|
|
|
Interest rate contracts (Dollar)
|
|
$
|
42,832
|
|
|
Interest income/expense
|
|
$
|
(33
|
)
|
|
Interest rate contracts (Yen)
|
|
|
6
|
|
|
Interest income/expense
|
|
|
—
|
|
|
Foreign exchange contracts
|
|
|
(6,785
|
)
|
|
Costs of Revenue
|
|
|
(5,938
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
36,053
|
|
|
|
|
$
|
(5,971
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amount of (Gain) or
|
|
|
|
|
|
|
Location of (Gain) or
|
|
Loss Recognized in
|
|
|
|
Derivatives not Designated as
|
|
Loss Recognized in
|
|
Income on Derivative
|
|
|
|
|
Hedging Instruments
|
|
Income on Derivative
|
|
2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign exchange contracts
|
|
Selling, general and
administrative expense
|
|
$
|
(3,309
|
)
|
|
|
|
|
|
Interest income/expense
|
|
|
3,883
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
574
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Company expects to recognize $ 4,277 of losses deferred in
accumulated other comprehensive income at December 31,
2009, in earnings during the next twelve months.
As of December 31, 2009, the Company had foreign exchange
derivatives with maturities of up to 35 months and interest
rate swaps with maturities of up to 27 months.
|
|
|
|
20.
|
Other
Comprehensive Income (Loss)
|
The changes in the components of other comprehensive income
(loss) for the years ended December 31, 2009, 2008, and
2007 are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, 2009
|
|
|
Year ended December 31, 2008
|
|
|
Year ended December 31, 2007
|
|
|
|
|
|
|
|
Tax
|
|
|
|
|
|
|
|
|
Tax
|
|
|
|
|
|
|
|
|
Tax
|
|
|
|
|
|
|
|
Pretax
|
|
|
Effect
|
|
|
Net
|
|
|
Pretax
|
|
|
Effect
|
|
|
Net
|
|
|
Pretax
|
|
|
Effect
|
|
|
Net
|
|
|
|
|
Other comprehensive income (loss) relating to cash flow hedges:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Changes in fair value of cash flow hedges during the period
|
|
$
|
36,053
|
|
|
$
|
(16,419
|
)
|
|
$
|
19,634
|
|
|
$
|
(107,316
|
)
|
|
$
|
42,764
|
|
|
$
|
(64,552
|
)
|
|
$
|
(83,919
|
)
|
|
$
|
32,961
|
|
|
$
|
(50,958
|
)
|
|
Reclassification adjustments
|
|
|
(5,971
|
)
|
|
|
1,375
|
|
|
|
(4,596
|
)
|
|
|
(924
|
)
|
|
|
296
|
|
|
|
(628
|
)
|
|
|
(4,455
|
)
|
|
|
1,360
|
|
|
|
(3,095
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total other comprehensive income (loss) relating to cash flow
hedges:
|
|
|
30,082
|
|
|
|
(15,044
|
)
|
|
|
15,038
|
|
|
|
(108,240
|
)
|
|
|
43,060
|
|
|
|
(65,180
|
)
|
|
|
(88,374
|
)
|
|
|
34,321
|
|
|
|
(54,053
|
)
|
|
Foreign-currency translation adjustment
|
|
|
82,545
|
|
|
|
—
|
|
|
|
82,545
|
|
|
|
(168,336
|
)
|
|
|
—
|
|
|
|
(168,336
|
)
|
|
|
146,308
|
|
|
|
—
|
|
|
|
146,308
|
|
|
Adjustments related to pension obligations
|
|
|
9,708
|
|
|
|
(3,927
|
)
|
|
|
5,781
|
|
|
|
(28,551
|
)
|
|
|
12,632
|
|
|
|
(15,919
|
)
|
|
|
35,729
|
|
|
|
(12,430
|
)
|
|
|
23,299
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income (loss)
|
|
$
|
122,335
|
|
|
$
|
(18,971
|
)
|
|
$
|
103,364
|
|
|
$
|
(305,127
|
)
|
|
$
|
55,692
|
|
|
$
|
(249,435
|
)
|
|
$
|
93,663
|
|
|
$
|
21,891
|
|
|
$
|
115,554
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-48
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
|
|
|
|
21.
|
Business
Segment Information
|
The Company has identified three business segments, North
America, International, and Asia Pacific, which were determined
based upon how the Company manages its businesses. All segments
are primarily engaged in providing dialysis services and
manufacturing and distributing products and equipment for the
treatment of end-stage renal disease. In the U.S., the Company
also engages in performing clinical laboratory testing and
providing inpatient dialysis services, and other services under
contract to hospitals. The Company has aggregated the
International and Asia Pacific operating segments as
“International.” The segments are aggregated due to
their similar economic characteristics. These characteristics
include the same services provided and the same products sold,
the same type patient population, similar methods of
distribution of products and services and similar economic
environments.
Management evaluates each segment using a measure that reflects
all of the segment’s controllable revenues and expenses.
Management believes that the most appropriate measure in this
regard is operating income which measures the Company’s
source of earnings. Financing is a corporate function, which the
Company’s segments do not control. Therefore, the Company
does not include interest expense relating to financing as a
segment measure. Similarly, the Company does not allocate
“corporate costs” which relate primarily to certain
headquarters overhead charges, including accounting and finance,
professional services, etc., because the Company believes that
these costs are also not within the control of the individual
segments. The Company also regards income taxes to be outside
the segment’s control. In addition, certain acquisitions
and intangible assets are not allocated to a segment but are
accounted for as “corporate.”
F-49
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
North
|
|
|
|
|
|
Segment
|
|
|
|
|
|
|
|
|
|
|
America
|
|
|
International
|
|
|
Total
|
|
|
Corporate
|
|
|
Total
|
|
|
|
|
2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net revenue
|
|
$
|
7,611,500
|
|
|
$
|
3,635,373
|
|
|
$
|
11,246,873
|
|
|
$
|
604
|
|
|
$
|
11,247,477
|
|
|
Inter-segment revenue
|
|
|
2,752
|
|
|
|
77,856
|
|
|
|
80,608
|
|
|
|
(80,608
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
|
7,614,252
|
|
|
|
3,713,229
|
|
|
|
11,327,481
|
|
|
|
(80,004
|
)
|
|
|
11,247,477
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
(264,785
|
)
|
|
|
(183,405
|
)
|
|
|
(448,190
|
)
|
|
|
(8,895
|
)
|
|
|
(457,085
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating Income
|
|
|
1,249,769
|
|
|
|
636,665
|
|
|
|
1,886,434
|
|
|
|
(130,838
|
)
|
|
|
1,755,596
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Segment assets
|
|
|
11,202,999
|
|
|
|
4,253,058
|
|
|
|
15,456,057
|
|
|
|
365,258
|
|
|
|
15,821,315
|
|
|
Capital expenditures, acquisitions and
investments
(1)
|
|
|
422,537
|
|
|
|
338,000
|
|
|
|
760,537
|
|
|
|
1,182
|
|
|
|
761,719
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net revenue
|
|
$
|
7,005,401
|
|
|
$
|
3,606,270
|
|
|
$
|
10,611,671
|
|
|
$
|
652
|
|
|
$
|
10,612,323
|
|
|
Inter-segment revenue
|
|
|
2,100
|
|
|
|
82,283
|
|
|
|
84,383
|
|
|
|
(84,383
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
|
7,007,501
|
|
|
|
3,688,553
|
|
|
|
10,696,054
|
|
|
|
(83,731
|
)
|
|
|
10,612,323
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
(238,300
|
)
|
|
|
(169,999
|
)
|
|
|
(408,299
|
)
|
|
|
(7,372
|
)
|
|
|
(415,671
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating Income
|
|
|
1,168,173
|
|
|
|
616,034
|
|
|
|
1,784,207
|
|
|
|
(111,775
|
)
|
|
|
1,672,432
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Segment assets
|
|
|
10,960,264
|
|
|
|
3,557,247
|
|
|
|
14,517,511
|
|
|
|
402,165
|
|
|
|
14,919,676
|
|
|
Capital expenditures, acquisitions and
investments
(2)
|
|
|
497,612
|
|
|
|
358,930
|
|
|
|
856,542
|
|
|
|
107,287
|
|
|
|
963,829
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2007
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net revenue
|
|
$
|
6,663,221
|
|
|
$
|
3,057,030
|
|
|
$
|
9,720,251
|
|
|
$
|
63
|
|
|
$
|
9,720,314
|
|
|
Inter-segment revenue
|
|
|
516
|
|
|
|
77,492
|
|
|
|
78,008
|
|
|
|
(78,008
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
|
|
6,663,737
|
|
|
|
3,134,522
|
|
|
|
9,798,259
|
|
|
|
(77,945
|
)
|
|
|
9,720,314
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
(220,210
|
)
|
|
|
(140,968
|
)
|
|
|
(361,178
|
)
|
|
|
(2,151
|
)
|
|
|
(363,329
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating Income
|
|
|
1,129,801
|
|
|
|
544,214
|
|
|
|
1,674,015
|
|
|
|
(93,893
|
)
|
|
|
1,580,122
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Segment assets
|
|
|
10,586,316
|
|
|
|
3,330,955
|
|
|
|
13,917,271
|
|
|
|
252,994
|
|
|
|
14,170,265
|
|
|
Capital expenditures, acquisitions and
investments
(3)
|
|
|
396,705
|
|
|
|
319,105
|
|
|
|
715,810
|
|
|
|
120,306
|
|
|
|
836,116
|
|
|
|
|
|
|
(1)
|
|
International acquisitions exclude
$4,151 of non-cash acquisitions for 2009.
|
|
|
|
(2)
|
|
North America and International
acquisitions exclude $22,542 and $24,710, respectively, of
non-cash acquisitions for 2008.
|
|
|
|
(3)
|
|
International and Corporate
acquisitions exclude $9,964 and $83,812, respectively, of
non-cash acquisitions for 2007.
|
F-50
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
For the geographic presentation, revenues are attributed to
specific countries based on the end user’s location for
products and the country in which the service is provided.
Information with respect to the Company’s geographic
operations is set forth in the table below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
North
|
|
|
Rest of
|
|
|
|
|
|
|
|
Germany
|
|
|
America
|
|
|
the World
|
|
|
Total
|
|
|
|
|
2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net revenue
|
|
$
|
358,060
|
|
|
$
|
7,611,500
|
|
|
$
|
3,277,917
|
|
|
$
|
11,247,477
|
|
|
Long-lived assets
|
|
|
350,194
|
|
|
|
8,864,165
|
|
|
|
1,809,114
|
|
|
|
11,023,473
|
|
|
2008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net revenue
|
|
$
|
350,995
|
|
|
$
|
7,005,401
|
|
|
$
|
3,255,927
|
|
|
$
|
10,612,323
|
|
|
Long-lived assets
|
|
|
306,963
|
|
|
|
8,706,790
|
|
|
|
1,597,576
|
|
|
|
10,611,329
|
|
|
2007
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net revenue
|
|
$
|
308,603
|
|
|
$
|
6,663,221
|
|
|
$
|
2,748,490
|
|
|
$
|
9,720,314
|
|
|
Long-lived assets
|
|
|
195,846
|
|
|
|
8,471,870
|
|
|
|
1,558,364
|
|
|
|
10,226,080
|
|
|
|
|
|
22.
|
Supplementary
Cash Flow Information
|
The following additional information is provided with respect to
the consolidated statements of cash flows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Supplementary cash flow information :
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid for interest
|
|
$
|
332,731
|
|
|
$
|
357,295
|
|
|
$
|
407,882
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid for income
taxes
(1)
|
|
$
|
425,945
|
|
|
$
|
343,224
|
|
|
$
|
349,058
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash inflow for income taxes from stock option exercises
|
|
$
|
8,123
|
|
|
$
|
7,132
|
|
|
$
|
8,177
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosures of cash flow information :
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Details for acquisitions:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets acquired
|
|
$
|
(241,745
|
)
|
|
$
|
(129,711
|
)
|
|
$
|
(431,289
|
)
|
|
Liabilities assumed
|
|
|
20,574
|
|
|
|
9,858
|
|
|
|
47,779
|
|
|
Noncontrolling interests
|
|
|
35,448
|
|
|
|
(3,706
|
)
|
|
|
13,040
|
|
|
Notes assumed in connection with acquisition
|
|
|
4,151
|
|
|
|
2,490
|
|
|
|
93,775
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid
|
|
|
(181,572
|
)
|
|
|
(121,069
|
)
|
|
|
(276,695
|
)
|
|
Less cash acquired
|
|
|
7,059
|
|
|
|
714
|
|
|
|
18,818
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash paid for acquisitions
|
|
$
|
(174,513
|
)
|
|
$
|
(120,355
|
)
|
|
$
|
(257,877
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
22.
|
Supplemental
Condensed Combining Information
|
In February 1998 FMC Trust Finance S.à.r.l.
Luxembourg, and in June 2001 FMC Trust Finance
S.à.r.l. Luxembourg III, each of which is a wholly-owned
subsidiary of FMC-AG & Co. KGaA, issued senior
subordinated debt securities, fully and unconditionally
guaranteed, jointly and severally, on a senior subordinated
basis, by FMC-AG & Co. KGaA, D-GmbH and FMCH (D-GmbH
and FMCH being the “Guarantor Subsidiaries”). The
senior subordinated debt securities were issued to statutory
trusts organized under the laws of the State of Delaware, which
issued trust preferred securities that were guaranteed by the
Company through a series of undertakings by the Company and the
Guarantor Subsidiaries, and the Company acquired all of the
common securities of these trusts. (See Note 12). In
December 2004, the Company assumed the obligations of its wholly
owned subsidiaries as the issuer of senior subordinated notes
denominated in Deutschmark and euro held by Fresenius Medical
Care Capital Trust III and Fresenius Medical Care Capital
Trust V, respectively. FMC Trust Finance S.à.r.l.
Luxembourg repaid $450 and DM300 aggregate principal amount of
senior subordinated debt securities on February 1, 2008 in
connection with the mandatory redemption on the same date of the
related trust preferred securities issued by Fresenius Medical
Care Capital Trust II and Fresenius Medical Care Capital
Trust III.
F-51
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
In addition, FMC Finance III S.A., a wholly-owned subsidiary of
the Company, is the obligor on senior debt securities which are
fully and unconditionally guaranteed, jointly and severally on a
senior basis, by the Company and by the Guarantors (see
Note 9). The following combining financial information for
the Company is as of December 31, 2009 and 2008 and for the
years ended December 31, 2009, 2008 and 2007, segregated
between FMC Finance III S.A., the Company, D-GmbH, FMCH, and
each of the Company’s other businesses (the
“Non-Guarantor Subsidiaries”). For purposes of the
condensed combining information, the Company and the Guarantors
carry their investments under the equity method. Other (income)
expense includes income (loss) related to investments in
consolidated subsidiaries recorded under the equity method for
purposes of the condensed combining information. In addition,
other (income) expense includes income and losses from profit
and loss transfer agreements as well as dividends received.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the year ended December 31, 2009
|
|
|
|
|
Issuer
|
|
|
Guarantors
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FMC
|
|
|
FMC - AG &
|
|
|
|
|
|
|
|
|
Non-Guarantor
|
|
|
Combining
|
|
|
Combined
|
|
|
|
|
Finance III
|
|
|
Co. KGaA
|
|
|
D-GmbH
|
|
|
FMCH
|
|
|
Subsidiaries
|
|
|
Adjustment
|
|
|
Total
|
|
|
|
|
Net revenue
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
2,818,124
|
|
|
$
|
—
|
|
|
$
|
10,744,709
|
|
|
$
|
(2,315,356
|
)
|
|
$
|
11,247,477
|
|
|
Cost of revenue
|
|
|
—
|
|
|
|
—
|
|
|
|
2,293,550
|
|
|
|
—
|
|
|
|
7,437,867
|
|
|
|
(2,315,452
|
)
|
|
|
7,415,965
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
—
|
|
|
|
—
|
|
|
|
524,574
|
|
|
|
—
|
|
|
|
3,306,842
|
|
|
|
96
|
|
|
|
3,831,512
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses (income):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative
|
|
|
28
|
|
|
|
87,774
|
|
|
|
173,215
|
|
|
|
(19,877
|
)
|
|
|
1,753,586
|
|
|
|
(12,620
|
)
|
|
|
1,982,106
|
|
|
Research and development
|
|
|
—
|
|
|
|
—
|
|
|
|
64,911
|
|
|
|
—
|
|
|
|
28,899
|
|
|
|
—
|
|
|
|
93,810
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating (loss) income
|
|
|
(28
|
)
|
|
|
(87,774
|
)
|
|
|
286,448
|
|
|
|
19,877
|
|
|
|
1,524,357
|
|
|
|
12,716
|
|
|
|
1,755,596
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other (income) expense:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest, net
|
|
|
(720
|
)
|
|
|
35,184
|
|
|
|
6,070
|
|
|
|
56,269
|
|
|
|
231,559
|
|
|
|
(28,399
|
)
|
|
|
299,963
|
|
|
Other, net
|
|
|
—
|
|
|
|
(1,032,515
|
)
|
|
|
190,345
|
|
|
|
(560,286
|
)
|
|
|
—
|
|
|
|
1,402,456
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before income taxes
|
|
|
692
|
|
|
|
909,557
|
|
|
|
90,033
|
|
|
|
523,894
|
|
|
|
1,292,798
|
|
|
|
(1,361,341
|
)
|
|
|
1,455,633
|
|
|
Income tax expense (benefit)
|
|
|
197
|
|
|
|
18,419
|
|
|
|
86,728
|
|
|
|
(14,338
|
)
|
|
|
518,329
|
|
|
|
(118,922
|
)
|
|
|
490,413
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
|
495
|
|
|
|
891,138
|
|
|
|
3,305
|
|
|
|
538,232
|
|
|
|
774,469
|
|
|
|
(1,242,419
|
)
|
|
|
965,220
|
|
|
Net income attributable to Noncontrolling interests
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
74,082
|
|
|
|
74,082
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) attributable to the group
|
|
$
|
495
|
|
|
$
|
891,138
|
|
|
$
|
3,305
|
|
|
$
|
538,232
|
|
|
$
|
774,469
|
|
|
$
|
(1,316,501
|
)
|
|
$
|
891,138
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-52
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the year ended December 31, 2008
|
|
|
|
|
Issuer
|
|
|
Guarantors
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FMC
|
|
|
FMC - AG &
|
|
|
|
|
|
|
|
|
Non-Guarantor
|
|
|
Combining
|
|
|
Combined
|
|
|
|
|
Finance III
|
|
|
Co. KGaA
|
|
|
D-GmbH
|
|
|
FMCH
|
|
|
Subsidiaries
|
|
|
Adjustment
|
|
|
Total
|
|
|
|
|
Net revenue
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
2,875,322
|
|
|
$
|
—
|
|
|
$
|
10,088,483
|
|
|
$
|
(2,351,482
|
)
|
|
$
|
10,612,323
|
|
|
Cost of revenue
|
|
|
—
|
|
|
|
—
|
|
|
|
2,212,088
|
|
|
|
—
|
|
|
|
7,085,966
|
|
|
|
(2,314,579
|
)
|
|
|
6,983,475
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
—
|
|
|
|
—
|
|
|
|
663,234
|
|
|
|
—
|
|
|
|
3,002,517
|
|
|
|
(36,903
|
)
|
|
|
3,628,848
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses (income):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative
|
|
|
93
|
|
|
|
49,245
|
|
|
|
208,299
|
|
|
|
(5,503
|
)
|
|
|
1,600,385
|
|
|
|
23,658
|
|
|
|
1,876,177
|
|
|
Research and development
|
|
|
—
|
|
|
|
—
|
|
|
|
55,448
|
|
|
|
—
|
|
|
|
24,791
|
|
|
|
—
|
|
|
|
80,239
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating (loss) income
|
|
|
(93
|
)
|
|
|
(49,245
|
)
|
|
|
399,487
|
|
|
|
5,503
|
|
|
|
1,377,341
|
|
|
|
(60,561
|
)
|
|
|
1,672,432
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other (income) expense:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest, net
|
|
|
(721
|
)
|
|
|
13,597
|
|
|
|
14,565
|
|
|
|
79,688
|
|
|
|
260,600
|
|
|
|
(30,987
|
)
|
|
|
336,742
|
|
|
Other, net
|
|
|
—
|
|
|
|
(945,938
|
)
|
|
|
255,501
|
|
|
|
(568,804
|
)
|
|
|
—
|
|
|
|
1,259,241
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before income taxes
|
|
|
628
|
|
|
|
883,096
|
|
|
|
129,421
|
|
|
|
494,619
|
|
|
|
1,116,741
|
|
|
|
(1,288,815
|
)
|
|
|
1,335,690
|
|
|
Income tax expense (benefit)
|
|
|
185
|
|
|
|
65,489
|
|
|
|
114,279
|
|
|
|
(29,118
|
)
|
|
|
376,169
|
|
|
|
(51,302
|
)
|
|
|
475,702
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
|
443
|
|
|
|
817,607
|
|
|
|
15,142
|
|
|
|
523,737
|
|
|
|
740,572
|
|
|
|
(1,237,513
|
)
|
|
|
859,988
|
|
|
Net income attributable to Noncontrolling interests
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
42,381
|
|
|
|
42,381
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) attributable to the group
|
|
$
|
443
|
|
|
$
|
817,607
|
|
|
$
|
15,142
|
|
|
$
|
523,737
|
|
|
$
|
740,572
|
|
|
$
|
(1,279,894
|
)
|
|
$
|
817,607
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the year ended December 31, 2007
|
|
|
|
|
Issuer
|
|
|
Guarantors
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FMC
|
|
|
FMC - AG &
|
|
|
|
|
|
|
|
|
Non-Guarantor
|
|
|
Combining
|
|
|
Combined
|
|
|
|
|
Finance III
|
|
|
Co. KGaA
|
|
|
D-GmbH
|
|
|
FMCH
|
|
|
Subsidiaries
|
|
|
Adjustment
|
|
|
Total
|
|
|
|
|
Net revenue
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
2,423,597
|
|
|
$
|
—
|
|
|
$
|
9,239,917
|
|
|
$
|
(1,943,200
|
)
|
|
$
|
9,720,314
|
|
|
Cost of revenue
|
|
|
—
|
|
|
|
—
|
|
|
|
1,871,403
|
|
|
|
—
|
|
|
|
6,400,239
|
|
|
|
(1,907,123
|
)
|
|
|
6,364,519
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
—
|
|
|
|
—
|
|
|
|
552,194
|
|
|
|
—
|
|
|
|
2,839,678
|
|
|
|
(36,077
|
)
|
|
|
3,355,795
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses (income):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative
|
|
|
37
|
|
|
|
104,449
|
|
|
|
181,283
|
|
|
|
(1,900
|
)
|
|
|
1,477,793
|
|
|
|
(52,512
|
)
|
|
|
1,709,150
|
|
|
Research and development
|
|
|
—
|
|
|
|
—
|
|
|
|
45,047
|
|
|
|
—
|
|
|
|
21,476
|
|
|
|
—
|
|
|
|
66,523
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating (loss) income
|
|
|
(37
|
)
|
|
|
(104,449
|
)
|
|
|
325,864
|
|
|
|
1,900
|
|
|
|
1,340,409
|
|
|
|
16,435
|
|
|
|
1,580,122
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other (income) expense:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest, net
|
|
|
(358
|
)
|
|
|
18,536
|
|
|
|
15,257
|
|
|
|
188,644
|
|
|
|
192,335
|
|
|
|
(43,367
|
)
|
|
|
371,047
|
|
|
Other, net
|
|
|
—
|
|
|
|
(893,558
|
)
|
|
|
196,415
|
|
|
|
(591,969
|
)
|
|
|
—
|
|
|
|
1,289,112
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before income taxes
|
|
|
321
|
|
|
|
770,573
|
|
|
|
114,192
|
|
|
|
405,225
|
|
|
|
1,148,074
|
|
|
|
(1,229,310
|
)
|
|
|
1,209,075
|
|
|
Income tax expense (benefit)
|
|
|
94
|
|
|
|
53,443
|
|
|
|
123,247
|
|
|
|
(74,698
|
)
|
|
|
413,981
|
|
|
|
(62,302
|
)
|
|
|
453,765
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
|
227
|
|
|
|
717,130
|
|
|
|
(9,055
|
)
|
|
|
479,923
|
|
|
|
734,093
|
|
|
|
(1,167,008
|
)
|
|
|
755,310
|
|
|
Net income attributable to Noncontrolling interests
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
38,180
|
|
|
|
38,180
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) attributable to the group
|
|
$
|
227
|
|
|
$
|
717,130
|
|
|
$
|
(9,055
|
)
|
|
$
|
479,923
|
|
|
$
|
734,093
|
|
|
$
|
(1,205,188
|
)
|
|
$
|
717,130
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-53
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, 2009
|
|
|
|
|
Issuer
|
|
|
Guarantors
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FMC
|
|
|
FMC - AG &
|
|
|
|
|
|
|
|
|
Non-Guarantor
|
|
|
Combining
|
|
|
Combined
|
|
|
|
|
Finance III
|
|
|
Co. KGaA
|
|
|
D-GmbH
|
|
|
FMCH
|
|
|
Subsidiaries
|
|
|
Adjustment
|
|
|
Total
|
|
|
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
108
|
|
|
$
|
24
|
|
|
$
|
194
|
|
|
$
|
—
|
|
|
$
|
286,205
|
|
|
$
|
14,694
|
|
|
$
|
301,225
|
|
|
Trade accounts receivable, less allowance for doubtful accounts
|
|
|
—
|
|
|
|
—
|
|
|
|
158,089
|
|
|
|
—
|
|
|
|
2,128,308
|
|
|
|
(488
|
)
|
|
|
2,285,909
|
|
|
Accounts receivable from related parties
|
|
|
16,543
|
|
|
|
1,837,748
|
|
|
|
628,819
|
|
|
|
539,867
|
|
|
|
2,600,656
|
|
|
|
(5,350,747
|
)
|
|
|
272,886
|
|
|
Inventories
|
|
|
—
|
|
|
|
—
|
|
|
|
202,837
|
|
|
|
—
|
|
|
|
701,429
|
|
|
|
(82,612
|
)
|
|
|
821,654
|
|
|
Prepaid expenses and other current assets
|
|
|
1
|
|
|
|
110,117
|
|
|
|
16,072
|
|
|
|
50
|
|
|
|
608,990
|
|
|
|
(5,924
|
)
|
|
|
729,306
|
|
|
Deferred taxes
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
294,214
|
|
|
|
22,606
|
|
|
|
316,820
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
16,652
|
|
|
|
1,947,889
|
|
|
|
1,006,011
|
|
|
|
539,917
|
|
|
|
6,619,802
|
|
|
|
(5,402,471
|
)
|
|
|
4,727,800
|
|
|
Property, plant and equipment, net
|
|
|
—
|
|
|
|
266
|
|
|
|
191,445
|
|
|
|
—
|
|
|
|
2,322,145
|
|
|
|
(94,286
|
)
|
|
|
2,419,570
|
|
|
Intangible assets
|
|
|
—
|
|
|
|
622
|
|
|
|
50,263
|
|
|
|
—
|
|
|
|
808,310
|
|
|
|
—
|
|
|
|
859,195
|
|
|
Goodwill
|
|
|
—
|
|
|
|
—
|
|
|
|
3,508
|
|
|
|
—
|
|
|
|
7,507,926
|
|
|
|
—
|
|
|
|
7,511,434
|
|
|
Deferred taxes
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
91,346
|
|
|
|
(26,597
|
)
|
|
|
64,749
|
|
|
Other assets
|
|
|
493,344
|
|
|
|
7,001,455
|
|
|
|
1,193,451
|
|
|
|
9,142,162
|
|
|
|
(6,254,725
|
)
|
|
|
(11,337,120
|
)
|
|
|
238,567
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
509,996
|
|
|
$
|
8,950,232
|
|
|
$
|
2,444,678
|
|
|
$
|
9,682,079
|
|
|
$
|
11,094,804
|
|
|
$
|
(16,860,474
|
)
|
|
$
|
15,821,315
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
4
|
|
|
$
|
217
|
|
|
$
|
19,131
|
|
|
$
|
—
|
|
|
$
|
343,055
|
|
|
$
|
—
|
|
|
$
|
362,407
|
|
|
Accounts payable to related parties
|
|
|
200
|
|
|
|
867,147
|
|
|
|
600,951
|
|
|
|
1,500,829
|
|
|
|
2,672,902
|
|
|
|
(5,364,600
|
)
|
|
|
277,429
|
|
|
Accrued expenses and other current liabilities
|
|
|
15,868
|
|
|
|
42,304
|
|
|
|
98,966
|
|
|
|
791
|
|
|
|
1,178,644
|
|
|
|
(1,020
|
)
|
|
|
1,335,553
|
|
|
Short-term borrowings
|
|
|
—
|
|
|
|
130
|
|
|
|
—
|
|
|
|
—
|
|
|
|
316,214
|
|
|
|
—
|
|
|
|
316,344
|
|
|
Short-term borrowings from related parties
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,161
|
|
|
|
8,279
|
|
|
|
10,440
|
|
|
Current portion of long-term debt and capital lease obligations
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
133,866
|
|
|
|
23,768
|
|
|
|
—
|
|
|
|
157,634
|
|
|
Income tax payable
|
|
|
30
|
|
|
|
32,342
|
|
|
|
—
|
|
|
|
—
|
|
|
|
83,958
|
|
|
|
648
|
|
|
|
116,978
|
|
|
Deferred taxes
|
|
|
—
|
|
|
|
2,569
|
|
|
|
8,692
|
|
|
|
—
|
|
|
|
24,288
|
|
|
|
(2,619
|
)
|
|
|
32,930
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
16,102
|
|
|
|
944,709
|
|
|
|
727,740
|
|
|
|
1,635,486
|
|
|
|
4,644,990
|
|
|
|
(5,359,312
|
)
|
|
|
2,609,715
|
|
|
Long term debt and capital lease obligations, less current
portion
|
|
|
493,344
|
|
|
|
1,063,346
|
|
|
|
—
|
|
|
|
1,576,242
|
|
|
|
4,096,766
|
|
|
|
(2,801,777
|
)
|
|
|
4,427,921
|
|
|
Long term borrowings from related parties
|
|
|
—
|
|
|
|
4,543
|
|
|
|
226,936
|
|
|
|
493,344
|
|
|
|
430,743
|
|
|
|
(1,155,566
|
)
|
|
|
—
|
|
|
Other liabilities
|
|
|
—
|
|
|
|
105,810
|
|
|
|
7,693
|
|
|
|
—
|
|
|
|
170,121
|
|
|
|
23,488
|
|
|
|
307,112
|
|
|
Pension liabilities
|
|
|
—
|
|
|
|
3,702
|
|
|
|
114,666
|
|
|
|
—
|
|
|
|
28,959
|
|
|
|
—
|
|
|
|
147,327
|
|
|
Income tax payable
|
|
|
—
|
|
|
|
1,139
|
|
|
|
—
|
|
|
|
—
|
|
|
|
100,917
|
|
|
|
113,865
|
|
|
|
215,921
|
|
|
Deferred taxes
|
|
|
—
|
|
|
|
6,051
|
|
|
|
3,110
|
|
|
|
—
|
|
|
|
428,448
|
|
|
|
(10,079
|
)
|
|
|
427,530
|
|
|
Company obligated mandatorily redeemable preferred securities of
subsidiary Fresenius Medical Care Capital Trusts holding solely
Company-guaranteed debentures of subsidiary
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
656,096
|
|
|
|
—
|
|
|
|
656,096
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
509,446
|
|
|
|
2,129,300
|
|
|
|
1,080,145
|
|
|
|
3,705,072
|
|
|
|
10,557,040
|
|
|
|
(9,189,381
|
)
|
|
|
8,791,622
|
|
|
Total FMC-AG & Co. KGaA shareholders’ equity
|
|
|
550
|
|
|
|
6,820,932
|
|
|
|
1,364,533
|
|
|
|
5,977,007
|
|
|
|
329,003
|
|
|
|
(7,671,093
|
)
|
|
|
6,820,932
|
|
|
Noncontrolling interests
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
208,761
|
|
|
|
—
|
|
|
|
208,761
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
550
|
|
|
|
6,820,932
|
|
|
|
1,364,533
|
|
|
|
5,977,007
|
|
|
|
537,764
|
|
|
|
(7,671,093
|
)
|
|
|
7,029,693
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and equity
|
|
$
|
509,996
|
|
|
$
|
8,950,232
|
|
|
$
|
2,444,678
|
|
|
$
|
9,682,079
|
|
|
$
|
11,094,804
|
|
|
$
|
(16,860,474
|
)
|
|
$
|
15,821,315
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-54
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At December 31, 2008
|
|
|
|
|
Issuer
|
|
|
Guarantors
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FMC
|
|
|
FMC - AG &
|
|
|
|
|
|
|
|
|
Non-Guarantor
|
|
|
Combining
|
|
|
Combined
|
|
|
|
|
Finance III
|
|
|
Co. KGaA
|
|
|
D-GmbH
|
|
|
FMCH
|
|
|
Subsidiaries
|
|
|
Adjustment
|
|
|
Total
|
|
|
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
23
|
|
|
$
|
—
|
|
|
$
|
44
|
|
|
$
|
—
|
|
|
$
|
221,003
|
|
|
$
|
514
|
|
|
$
|
221,584
|
|
|
Trade accounts receivable, less allowance for doubtful accounts
|
|
|
—
|
|
|
|
—
|
|
|
|
182,421
|
|
|
|
—
|
|
|
|
1,993,779
|
|
|
|
116
|
|
|
|
2,176,316
|
|
|
Accounts receivable from related parties
|
|
|
16,552
|
|
|
|
1,520,238
|
|
|
|
692,195
|
|
|
|
468,871
|
|
|
|
2,040,953
|
|
|
|
(4,563,284
|
)
|
|
|
175,525
|
|
|
Inventories
|
|
|
—
|
|
|
|
—
|
|
|
|
182,223
|
|
|
|
—
|
|
|
|
614,879
|
|
|
|
(90,052
|
)
|
|
|
707,050
|
|
|
Prepaid expenses and other current assets
|
|
|
1
|
|
|
|
82,188
|
|
|
|
28,794
|
|
|
|
50
|
|
|
|
496,393
|
|
|
|
(27
|
)
|
|
|
607,399
|
|
|
Deferred taxes
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
300,068
|
|
|
|
24,055
|
|
|
|
324,123
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
16,576
|
|
|
|
1,602,426
|
|
|
|
1,085,677
|
|
|
|
468,921
|
|
|
|
5,667,075
|
|
|
|
(4,628,678
|
)
|
|
|
4,211,997
|
|
|
Property, plant and equipment, net
|
|
|
—
|
|
|
|
272
|
|
|
|
176,148
|
|
|
|
—
|
|
|
|
2,141,714
|
|
|
|
(82,056
|
)
|
|
|
2,236,078
|
|
|
Intangible assets
|
|
|
—
|
|
|
|
470
|
|
|
|
44,546
|
|
|
|
—
|
|
|
|
801,480
|
|
|
|
—
|
|
|
|
846,496
|
|
|
Goodwill
|
|
|
—
|
|
|
|
—
|
|
|
|
3,389
|
|
|
|
—
|
|
|
|
7,306,521
|
|
|
|
—
|
|
|
|
7,309,910
|
|
|
Deferred taxes
|
|
|
—
|
|
|
|
13,408
|
|
|
|
243
|
|
|
|
—
|
|
|
|
89,744
|
|
|
|
(10,590
|
)
|
|
|
92,805
|
|
|
Other assets
|
|
|
492,456
|
|
|
|
6,511,354
|
|
|
|
1,207,785
|
|
|
|
8,305,121
|
|
|
|
(4,007,726
|
)
|
|
|
(12,286,600
|
)
|
|
|
222,390
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
509,032
|
|
|
$
|
8,127,930
|
|
|
$
|
2,517,788
|
|
|
$
|
8,774,042
|
|
|
$
|
11,998,808
|
|
|
$
|
(17,007,924
|
)
|
|
$
|
14,919,676
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
—
|
|
|
$
|
752
|
|
|
$
|
28,714
|
|
|
$
|
—
|
|
|
$
|
336,551
|
|
|
$
|
—
|
|
|
$
|
366,017
|
|
|
Accounts payable to related parties
|
|
|
1
|
|
|
|
1,229,275
|
|
|
|
621,598
|
|
|
|
1,460,218
|
|
|
|
1,466,838
|
|
|
|
(4,538,687
|
)
|
|
|
239,243
|
|
|
Accrued expenses and other current liabilities
|
|
|
15,887
|
|
|
|
37,994
|
|
|
|
104,128
|
|
|
|
1,939
|
|
|
|
1,121,326
|
|
|
|
7,159
|
|
|
|
1,288,433
|
|
|
Short-term borrowings
|
|
|
—
|
|
|
|
55,668
|
|
|
|
—
|
|
|
|
—
|
|
|
|
627,487
|
|
|
|
—
|
|
|
|
683,155
|
|
|
Short-term borrowings from related parties
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
111,232
|
|
|
|
(109,902
|
)
|
|
|
1,330
|
|
|
Current portion of long-term debt and capital lease obligations
|
|
|
—
|
|
|
|
786
|
|
|
|
—
|
|
|
|
133,866
|
|
|
|
320,462
|
|
|
|
—
|
|
|
|
455,114
|
|
|
Income tax payable
|
|
|
190
|
|
|
|
13,958
|
|
|
|
—
|
|
|
|
—
|
|
|
|
71,649
|
|
|
|
(3,329
|
)
|
|
|
82,468
|
|
|
Deferred taxes
|
|
|
—
|
|
|
|
1,177
|
|
|
|
7,250
|
|
|
|
—
|
|
|
|
23,339
|
|
|
|
(3,114
|
)
|
|
|
28,652
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
16,078
|
|
|
|
1,339,610
|
|
|
|
761,690
|
|
|
|
1,596,023
|
|
|
|
4,078,884
|
|
|
|
(4,647,873
|
)
|
|
|
3,144,412
|
|
|
Long term debt and capital lease
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
obligations, less current portion
|
|
|
492,456
|
|
|
|
635,904
|
|
|
|
—
|
|
|
|
1,519,843
|
|
|
|
4,661,820
|
|
|
|
(3,355,137
|
)
|
|
|
3,954,886
|
|
|
Long term borrowings from related parties
|
|
|
—
|
|
|
|
4,388
|
|
|
|
223,332
|
|
|
|
492,456
|
|
|
|
697,047
|
|
|
|
(1,414,730
|
)
|
|
|
2,493
|
|
|
Other liabilities
|
|
|
—
|
|
|
|
140,420
|
|
|
|
11,497
|
|
|
|
—
|
|
|
|
148,172
|
|
|
|
19,513
|
|
|
|
319,602
|
|
|
Pension liabilities
|
|
|
—
|
|
|
|
3,030
|
|
|
|
107,152
|
|
|
|
—
|
|
|
|
26,573
|
|
|
|
—
|
|
|
|
136,755
|
|
|
Income tax payable
|
|
|
—
|
|
|
|
42,296
|
|
|
|
—
|
|
|
|
—
|
|
|
|
86,420
|
|
|
|
43,031
|
|
|
|
171,747
|
|
|
Deferred taxes
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
395,375
|
|
|
|
30,924
|
|
|
|
426,299
|
|
|
Company obligated mandatorily redeemable preferred securities of
subsidiary Fresenius Medical Care Capital Trusts holding solely
Company-guaranteed debentures of subsidiary
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
640,696
|
|
|
|
—
|
|
|
|
640,696
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
508,534
|
|
|
|
2,165,648
|
|
|
|
1,103,671
|
|
|
|
3,608,322
|
|
|
|
10,734,987
|
|
|
|
(9,324,272
|
)
|
|
|
8,796,890
|
|
|
Total FMC-AG & Co. KGaA shareholders’ equity
|
|
|
498
|
|
|
|
5,962,282
|
|
|
|
1,414,117
|
|
|
|
5,165,720
|
|
|
|
1,103,317
|
|
|
|
(7,683,652
|
)
|
|
|
5,962,282
|
|
|
Noncontrolling interests
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
160,504
|
|
|
|
—
|
|
|
|
160,504
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
498
|
|
|
|
5,962,282
|
|
|
|
1,414,117
|
|
|
|
5,165,720
|
|
|
|
1,263,821
|
|
|
|
(7,683,652
|
)
|
|
|
6,122,786
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and equity
|
|
$
|
509,032
|
|
|
$
|
8,127,930
|
|
|
$
|
2,517,788
|
|
|
$
|
8,774,042
|
|
|
$
|
11,998,808
|
|
|
$
|
(17,007,924
|
)
|
|
$
|
14,919,676
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-55
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the year ended December 31, 2009
|
|
|
|
|
Issuer
|
|
|
Guarantors
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FMC
|
|
|
FMC - AG &
|
|
|
|
|
|
|
|
|
Non-Guarantor
|
|
|
Combining
|
|
|
Combined
|
|
|
|
|
Finance III
|
|
|
Co. KGaA
|
|
|
D-GmbH
|
|
|
FMCH
|
|
|
Subsidiaries
|
|
|
Adjustment
|
|
|
Total
|
|
|
|
|
Operating Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
$
|
495
|
|
|
$
|
891,138
|
|
|
$
|
3,305
|
|
|
$
|
538,232
|
|
|
$
|
774,469
|
|
|
$
|
(1,242,419
|
)
|
|
$
|
965,220
|
|
|
Adjustments to reconcile net income to net cash provided by
(used in) operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity affiliate income
|
|
|
—
|
|
|
|
(635,395
|
)
|
|
|
—
|
|
|
|
(560,286
|
)
|
|
|
—
|
|
|
|
1,195,681
|
|
|
|
—
|
|
|
Depreciation and amortization
|
|
|
—
|
|
|
|
1,470
|
|
|
|
38,029
|
|
|
|
888
|
|
|
|
439,196
|
|
|
|
(22,498
|
)
|
|
|
457,085
|
|
|
Change in deferred taxes, net
|
|
|
—
|
|
|
|
23,191
|
|
|
|
4,707
|
|
|
|
—
|
|
|
|
(15,491
|
)
|
|
|
9,595
|
|
|
|
22,002
|
|
|
(Gain) Loss on sale of fixed assets and investments
|
|
|
—
|
|
|
|
—
|
|
|
|
411
|
|
|
|
—
|
|
|
|
(353
|
)
|
|
|
—
|
|
|
|
58
|
|
|
Loss (gain) on investments
|
|
|
—
|
|
|
|
7,063
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(7,063
|
)
|
|
|
—
|
|
|
Write-off of loans from related parties
|
|
|
—
|
|
|
|
50
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(50
|
)
|
|
|
—
|
|
|
Compensation expense related to stock options
|
|
|
—
|
|
|
|
33,746
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
33,746
|
|
|
Changes in assets and liabilities, net of amounts from
businesses acquired:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade accounts receivable, net
|
|
|
—
|
|
|
|
—
|
|
|
|
(13,874
|
)
|
|
|
—
|
|
|
|
(28,120
|
)
|
|
|
—
|
|
|
|
(41,994
|
)
|
|
Inventories
|
|
|
—
|
|
|
|
—
|
|
|
|
(27,435
|
)
|
|
|
—
|
|
|
|
(49,213
|
)
|
|
|
(12,285
|
)
|
|
|
(88,933
|
)
|
|
Prepaid expenses and other current and non-current assets
|
|
|
—
|
|
|
|
(37,138
|
)
|
|
|
9,921
|
|
|
|
(18,344
|
)
|
|
|
(93,954
|
)
|
|
|
(7,590
|
)
|
|
|
(147,105
|
)
|
|
Accounts receivable from / payable to related parties
|
|
|
208
|
|
|
|
(388,546
|
)
|
|
|
7,308
|
|
|
|
39,091
|
|
|
|
256,906
|
|
|
|
79,315
|
|
|
|
(5,718
|
)
|
|
Accounts payable, accrued expenses and other current and
non-current liabilities
|
|
|
(15
|
)
|
|
|
16,210
|
|
|
|
12,731
|
|
|
|
(1,149
|
)
|
|
|
38,065
|
|
|
|
5,250
|
|
|
|
71,092
|
|
|
Income tax payable
|
|
|
(160
|
)
|
|
|
(23,961
|
)
|
|
|
—
|
|
|
|
(14,338
|
)
|
|
|
71,931
|
|
|
|
39,692
|
|
|
|
73,164
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) operating activities
|
|
|
528
|
|
|
|
(112,172
|
)
|
|
|
35,103
|
|
|
|
(15,906
|
)
|
|
|
1,393,436
|
|
|
|
37,628
|
|
|
|
1,338,617
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Investing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant and equipment
|
|
|
—
|
|
|
|
(152
|
)
|
|
|
(65,684
|
)
|
|
|
—
|
|
|
|
(537,167
|
)
|
|
|
29,397
|
|
|
|
(573,606
|
)
|
|
Proceeds from sale of property, plant and equipment
|
|
|
—
|
|
|
|
—
|
|
|
|
731
|
|
|
|
—
|
|
|
|
10,999
|
|
|
|
—
|
|
|
|
11,730
|
|
|
Disbursement of loans to related parties
|
|
|
—
|
|
|
|
(7,270
|
)
|
|
|
178
|
|
|
|
17,240
|
|
|
|
—
|
|
|
|
(10,148
|
)
|
|
|
—
|
|
|
Acquisitions and investments, net of cash acquired, and net
purchases of intangible assets
|
|
|
—
|
|
|
|
(11,841
|
)
|
|
|
(1,900
|
)
|
|
|
—
|
|
|
|
(185,878
|
)
|
|
|
11,506
|
|
|
|
(188,113
|
)
|
|
Proceeds from divestitures
|
|
|
—
|
|
|
|
13,380
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,965
|
|
|
|
36,620
|
|
|
|
51,965
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash (used in) provided by investing activities
|
|
|
—
|
|
|
|
(5,883
|
)
|
|
|
(66,675
|
)
|
|
|
17,240
|
|
|
|
(710,081
|
)
|
|
|
67,375
|
|
|
|
(698,024
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short-term borrowings, net
|
|
|
—
|
|
|
|
(95,795
|
)
|
|
|
31,716
|
|
|
|
—
|
|
|
|
10,943
|
|
|
|
(108,439
|
)
|
|
|
(161,575
|
)
|
|
Long-term debt and capital lease obligations, net
|
|
|
—
|
|
|
|
396,013
|
|
|
|
—
|
|
|
|
(1,334
|
)
|
|
|
(261,528
|
)
|
|
|
10,148
|
|
|
|
143,299
|
|
|
(Decrease) increase of accounts receivable securitization program
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(325,000
|
)
|
|
|
—
|
|
|
|
(325,000
|
)
|
|
Proceeds from exercise of stock options
|
|
|
—
|
|
|
|
64,271
|
|
|
|
—
|
|
|
|
—
|
|
|
|
8,123
|
|
|
|
—
|
|
|
|
72,394
|
|
|
Dividends paid
|
|
|
(443
|
)
|
|
|
(231,940
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(5,321
|
)
|
|
|
5,764
|
|
|
|
(231,940
|
)
|
|
Capital increase (decrease)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,874
|
)
|
|
|
1,874
|
|
|
|
—
|
|
|
Distributions to Noncontrolling interests
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(68,004
|
)
|
|
|
—
|
|
|
|
(68,004
|
)
|
|
Contributions from Noncontrolling interests
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
12,699
|
|
|
|
—
|
|
|
|
12,699
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash (used in) provided by financing activities
|
|
|
(443
|
)
|
|
|
132,549
|
|
|
|
31,716
|
|
|
|
(1,334
|
)
|
|
|
(629,962
|
)
|
|
|
(90,653
|
)
|
|
|
(558,127
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate changes on cash and cash equivalents
|
|
|
—
|
|
|
|
(14,470
|
)
|
|
|
6
|
|
|
|
—
|
|
|
|
11,590
|
|
|
|
49
|
|
|
|
(2,825
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and Cash Equivalents:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash and cash equivalents
|
|
|
85
|
|
|
|
24
|
|
|
|
150
|
|
|
|
—
|
|
|
|
64,983
|
|
|
|
14,399
|
|
|
|
79,641
|
|
|
Cash and cash equivalents at beginning of period
|
|
|
23
|
|
|
|
—
|
|
|
|
44
|
|
|
|
—
|
|
|
|
221,517
|
|
|
|
—
|
|
|
|
221,584
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at end of period
|
|
$
|
108
|
|
|
$
|
24
|
|
|
$
|
194
|
|
|
$
|
—
|
|
|
$
|
286,500
|
|
|
$
|
14,399
|
|
|
$
|
301,225
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-56
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the year ended December 31, 2008
|
|
|
|
|
Issuer
|
|
|
Guarantors
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FMC
|
|
|
FMC - AG &
|
|
|
|
|
|
|
|
|
Non-Guarantor
|
|
|
Combining
|
|
|
Combined
|
|
|
|
|
Finance III
|
|
|
Co. KGaA
|
|
|
D-GmbH
|
|
|
FMCH
|
|
|
Subsidiaries
|
|
|
Adjustment
|
|
|
Total
|
|
|
|
|
Operating Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
$
|
443
|
|
|
$
|
817,607
|
|
|
$
|
15,142
|
|
|
$
|
523,737
|
|
|
$
|
740,572
|
|
|
$
|
(1,237,513
|
)
|
|
$
|
859,988
|
|
|
Adjustments to reconcile net income to net cash provided by
(used in) operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity affiliate income
|
|
|
—
|
|
|
|
(462,412
|
)
|
|
|
—
|
|
|
|
(568,804
|
)
|
|
|
—
|
|
|
|
1,031,216
|
|
|
|
—
|
|
|
Depreciation and amortization
|
|
|
—
|
|
|
|
1,472
|
|
|
|
40,895
|
|
|
|
888
|
|
|
|
393,558
|
|
|
|
(21,142
|
)
|
|
|
415,671
|
|
|
Change in deferred taxes, net
|
|
|
—
|
|
|
|
(7,951
|
)
|
|
|
3,169
|
|
|
|
—
|
|
|
|
97,726
|
|
|
|
40,103
|
|
|
|
133,047
|
|
|
(Gain) Loss on sale of fixed assets and investments
|
|
|
—
|
|
|
|
(422
|
)
|
|
|
(55
|
)
|
|
|
—
|
|
|
|
(21,009
|
)
|
|
|
422
|
|
|
|
(21,064
|
)
|
|
Write-up
of
loans from related parties
|
|
|
—
|
|
|
|
(17,727
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
17,727
|
|
|
|
—
|
|
|
Compensation expense related to stock options
|
|
|
—
|
|
|
|
31,879
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
31,879
|
|
|
Changes in assets and liabilities, net of amounts from
businesses acquired:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade accounts receivable, net
|
|
|
—
|
|
|
|
—
|
|
|
|
(34,889
|
)
|
|
|
—
|
|
|
|
(207,078
|
)
|
|
|
—
|
|
|
|
(241,967
|
)
|
|
Inventories
|
|
|
—
|
|
|
|
—
|
|
|
|
(27,549
|
)
|
|
|
—
|
|
|
|
(82,328
|
)
|
|
|
15,765
|
|
|
|
(94,112
|
)
|
|
Prepaid expenses and other current and non-current assets
|
|
|
—
|
|
|
|
(32,757
|
)
|
|
|
(25,384
|
)
|
|
|
(3,964
|
)
|
|
|
(30,156
|
)
|
|
|
8,172
|
|
|
|
(84,089
|
)
|
|
Accounts receivable from / payable to related parties
|
|
|
899
|
|
|
|
(318,373
|
)
|
|
|
43,853
|
|
|
|
34,620
|
|
|
|
104,895
|
|
|
|
166,358
|
|
|
|
32,252
|
|
|
Accounts payable, accrued expenses and other current and
non-current liabilities
|
|
|
(1,237
|
)
|
|
|
1,140
|
|
|
|
22,438
|
|
|
|
(4,538
|
)
|
|
|
(38,463
|
)
|
|
|
3,620
|
|
|
|
(17,040
|
)
|
|
Income tax payable
|
|
|
96
|
|
|
|
(49,994
|
)
|
|
|
—
|
|
|
|
(29,118
|
)
|
|
|
91,779
|
|
|
|
(10,930
|
)
|
|
|
1,833
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) operating activities
|
|
|
201
|
|
|
|
(37,538
|
)
|
|
|
37,620
|
|
|
|
(47,179
|
)
|
|
|
1,049,496
|
|
|
|
13,798
|
|
|
|
1,016,398
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Investing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant and equipment
|
|
|
—
|
|
|
|
(186
|
)
|
|
|
(77,381
|
)
|
|
|
—
|
|
|
|
(646,396
|
)
|
|
|
36,607
|
|
|
|
(687,356
|
)
|
|
Proceeds from sale of property, plant and equipment
|
|
|
—
|
|
|
|
16
|
|
|
|
1,348
|
|
|
|
—
|
|
|
|
12,482
|
|
|
|
—
|
|
|
|
13,846
|
|
|
Disbursement of loans to related parties
|
|
|
—
|
|
|
|
(123,908
|
)
|
|
|
177
|
|
|
|
164,746
|
|
|
|
—
|
|
|
|
(41,015
|
)
|
|
|
—
|
|
|
Acquisitions and investments, net of cash acquired, and net
purchases of intangible assets
|
|
|
—
|
|
|
|
(36,148
|
)
|
|
|
(39,721
|
)
|
|
|
—
|
|
|
|
(186,477
|
)
|
|
|
(14,127
|
)
|
|
|
(276,473
|
)
|
|
Proceeds from divestitures
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
58,582
|
|
|
|
—
|
|
|
|
58,582
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash (used in) provided by investing activities
|
|
|
—
|
|
|
|
(160,226
|
)
|
|
|
(115,577
|
)
|
|
|
164,746
|
|
|
|
(761,809
|
)
|
|
|
(18,535
|
)
|
|
|
(891,401
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short-term borrowings, net
|
|
|
—
|
|
|
|
36,847
|
|
|
|
78,179
|
|
|
|
—
|
|
|
|
(123,064
|
)
|
|
|
—
|
|
|
|
(8,038
|
)
|
|
Long-term debt and capital lease obligations, net
|
|
|
—
|
|
|
|
366,231
|
|
|
|
(221
|
)
|
|
|
(117,567
|
)
|
|
|
(644,378
|
)
|
|
|
41,015
|
|
|
|
(354,920
|
)
|
|
Increase (decrease) of accounts receivable securitization program
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
454,000
|
|
|
|
—
|
|
|
|
454,000
|
|
|
Proceeds from exercise of stock options
|
|
|
—
|
|
|
|
36,755
|
|
|
|
—
|
|
|
|
—
|
|
|
|
7,132
|
|
|
|
—
|
|
|
|
43,887
|
|
|
Dividends paid
|
|
|
(222
|
)
|
|
|
(252,395
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
163
|
|
|
|
59
|
|
|
|
(252,395
|
)
|
|
Capital increase (decrease)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
35,873
|
|
|
|
(35,873
|
)
|
|
|
—
|
|
|
Distributions to Noncontrolling interests
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(38,592
|
)
|
|
|
—
|
|
|
|
(38,592
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash (used in) provided by financing activities
|
|
|
(222
|
)
|
|
|
187,438
|
|
|
|
77,958
|
|
|
|
(117,567
|
)
|
|
|
(308,866
|
)
|
|
|
5,201
|
|
|
|
(156,058
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate changes on cash and cash equivalents
|
|
|
—
|
|
|
|
10,326
|
|
|
|
(2
|
)
|
|
|
—
|
|
|
|
(2,419
|
)
|
|
|
50
|
|
|
|
7,955
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and Cash Equivalents:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (decrease) increase in cash and cash equivalents
|
|
|
(21
|
)
|
|
|
—
|
|
|
|
(1
|
)
|
|
|
—
|
|
|
|
(23,598
|
)
|
|
|
514
|
|
|
|
(23,106
|
)
|
|
Cash and cash equivalents at beginning of period
|
|
|
44
|
|
|
|
—
|
|
|
|
45
|
|
|
|
—
|
|
|
|
244,601
|
|
|
|
—
|
|
|
|
244,690
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at end of period
|
|
$
|
23
|
|
|
$
|
—
|
|
|
$
|
44
|
|
|
$
|
—
|
|
|
$
|
221,003
|
|
|
$
|
514
|
|
|
$
|
221,584
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-57
FRESENIUS
MEDICAL CARE AG & Co. KGaA
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS —
(Continued)
(in thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the year ended December 31, 2007
|
|
|
|
|
Issuer
|
|
|
Guarantors
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FMC
|
|
|
FMC - AG &
|
|
|
|
|
|
|
|
|
Non-Guarantor
|
|
|
Combining
|
|
|
Combined
|
|
|
|
|
Finance III
|
|
|
Co. KGaA
|
|
|
D-GmbH
|
|
|
FMCH
|
|
|
Subsidiaries
|
|
|
Adjustment
|
|
|
Total
|
|
|
|
|
Operating Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
$
|
227
|
|
|
$
|
717,130
|
|
|
$
|
(9,055
|
)
|
|
$
|
479,923
|
|
|
$
|
734,093
|
|
|
$
|
(1,167,008
|
)
|
|
$
|
755,310
|
|
|
Adjustments to reconcile net income to net cash provided by
(used in) operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity affiliate income
|
|
|
—
|
|
|
|
(559,674
|
)
|
|
|
—
|
|
|
|
(591,969
|
)
|
|
|
—
|
|
|
|
1,151,643
|
|
|
|
—
|
|
|
Depreciation and amortization
|
|
|
—
|
|
|
|
2,025
|
|
|
|
33,620
|
|
|
|
—
|
|
|
|
344,844
|
|
|
|
(17,159
|
)
|
|
|
363,330
|
|
|
Change in deferred taxes, net
|
|
|
—
|
|
|
|
(14,012
|
)
|
|
|
396
|
|
|
|
—
|
|
|
|
21,821
|
|
|
|
(7,028
|
)
|
|
|
1,177
|
|
|
(Gain) Loss on sale of fixed assets and investments
|
|
|
—
|
|
|
|
(303
|
)
|
|
|
776
|
|
|
|
—
|
|
|
|
3,527
|
|
|
|
(384
|
)
|
|
|
3,616
|
|
|
Write-up
of
loans from related parties
|
|
|
—
|
|
|
|
17,390
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(17,390
|
)
|
|
|
—
|
|
|
Compensation expense related to stock options
|
|
|
—
|
|
|
|
24,208
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
24,208
|
|
|
Changes in assets and liabilities, net of amounts from
businesses acquired:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade accounts receivable, net
|
|
|
—
|
|
|
|
—
|
|
|
|
(18,536
|
)
|
|
|
—
|
|
|
|
(44,199
|
)
|
|
|
—
|
|
|
|
(62,735
|
)
|
|
Inventories
|
|
|
—
|
|
|
|
—
|
|
|
|
(13,322
|
)
|
|
|
—
|
|
|
|
(72,294
|
)
|
|
|
12,791
|
|
|
|
(72,825
|
)
|
|
Prepaid expenses and other current and non-current assets
|
|
|
49
|
|
|
|
(7,907
|
)
|
|
|
13,690
|
|
|
|
8,588
|
|
|
|
(406
|
)
|
|
|
(20,637
|
)
|
|
|
(6,623
|
)
|
|
Accounts receivable from / payable to related parties
|
|
|
(17,450
|
)
|
|
|
(77,549
|
)
|
|
|
(53,019
|
)
|
|
|
55,923
|
|
|
|
82,296
|
|
|
|
(12,466
|
)
|
|
|
(22,265
|
)
|
|
Accounts payable, accrued expenses and other current and
non-current liabilities
|
|
|
17,124
|
|
|
|
15,247
|
|
|
|
17,312
|
|
|
|
(1,973
|
)
|
|
|
74,258
|
|
|
|
(8,008
|
)
|
|
|
113,960
|
|
|
Income tax payable
|
|
|
94
|
|
|
|
38,393
|
|
|
|
—
|
|
|
|
(74,698
|
)
|
|
|
99,923
|
|
|
|
38,709
|
|
|
|
102,421
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) operating activities
|
|
|
44
|
|
|
|
154,948
|
|
|
|
(28,138
|
)
|
|
|
(124,206
|
)
|
|
|
1,243,863
|
|
|
|
(46,937
|
)
|
|
|
1,199,574
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Investing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant and equipment
|
|
|
—
|
|
|
|
(217
|
)
|
|
|
(62,954
|
)
|
|
|
—
|
|
|
|
(541,701
|
)
|
|
|
32,151
|
|
|
|
(572,721
|
)
|
|
Proceeds from sale of property, plant and equipment
|
|
|
—
|
|
|
|
4
|
|
|
|
1,153
|
|
|
|
—
|
|
|
|
28,511
|
|
|
|
—
|
|
|
|
29,668
|
|
|
Disbursement of loans to related parties
|
|
|
—
|
|
|
|
3,435
|
|
|
|
155
|
|
|
|
120,437
|
|
|
|
(9,527
|
)
|
|
|
(114,500
|
)
|
|
|
—
|
|
|
Acquisitions and investments, net of cash acquired, and net
purchases of intangible assets
|
|
|
—
|
|
|
|
35,377
|
|
|
|
(1,015
|
)
|
|
|
—
|
|
|
|
(261,738
|
)
|
|
|
(36,019
|
)
|
|
|
(263,395
|
)
|
|
Proceeds from divestitures
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
29,495
|
|
|
|
—
|
|
|
|
29,495
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) investing activities
|
|
|
—
|
|
|
|
38,599
|
|
|
|
(62,661
|
)
|
|
|
120,437
|
|
|
|
(754,960
|
)
|
|
|
(118,368
|
)
|
|
|
(776,953
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short-term borrowings, net
|
|
|
—
|
|
|
|
(3,015
|
)
|
|
|
91,080
|
|
|
|
—
|
|
|
|
(101,380
|
)
|
|
|
—
|
|
|
|
(13,315
|
)
|
|
Long-term debt and capital lease obligations, net
|
|
|
—
|
|
|
|
(38,916
|
)
|
|
|
(274
|
)
|
|
|
11,897
|
|
|
|
(56,958
|
)
|
|
|
114,500
|
|
|
|
30,249
|
|
|
(Decrease) increase of accounts receivable securitization program
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(181,000
|
)
|
|
|
—
|
|
|
|
(181,000
|
)
|
|
Proceeds from exercise of stock options
|
|
|
—
|
|
|
|
38,757
|
|
|
|
—
|
|
|
|
—
|
|
|
|
8,177
|
|
|
|
—
|
|
|
|
46,934
|
|
|
Cash paid for repurchase preferred stock
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(7,660
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(7,660
|
)
|
|
Dividends paid
|
|
|
—
|
|
|
|
(188,407
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(12,671
|
)
|
|
|
12,671
|
|
|
|
(188,407
|
)
|
|
Capital (decrease) increase
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(36,018
|
)
|
|
|
36,018
|
|
|
|
—
|
|
|
Distributions to Noncontrolling interests
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(468
|
)
|
|
|
(27,001
|
)
|
|
|
—
|
|
|
|
(27,469
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash (used in) provided by financing activities
|
|
|
—
|
|
|
|
(191,581
|
)
|
|
|
90,806
|
|
|
|
3,769
|
|
|
|
(406,851
|
)
|
|
|
163,189
|
|
|
|
(340,668
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate changes on cash and cash equivalents
|
|
|
—
|
|
|
|
(1,988
|
)
|
|
|
4
|
|
|
|
—
|
|
|
|
3,595
|
|
|
|
2,116
|
|
|
|
3,727
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and Cash Equivalents:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash and cash equivalents
|
|
|
44
|
|
|
|
(22
|
)
|
|
|
11
|
|
|
|
—
|
|
|
|
85,647
|
|
|
|
—
|
|
|
|
85,680
|
|
|
Cash and cash equivalents at beginning of period
|
|
|
—
|
|
|
|
22
|
|
|
|
34
|
|
|
|
—
|
|
|
|
158,954
|
|
|
|
—
|
|
|
|
159,010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at end of period
|
|
$
|
44
|
|
|
$
|
—
|
|
|
$
|
45
|
|
|
$
|
—
|
|
|
$
|
244,601
|
|
|
$
|
—
|
|
|
$
|
244,690
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-58
FRESENIUS
MEDICAL CARE AG & Co. KGaA
(in
thousands, except share data)
Development
of allowance for doubtful accounts
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
|
|
Allowance for doubtful accounts as of January 1
|
|
$
|
262,836
|
|
|
$
|
247,800
|
|
|
$
|
207,293
|
|
|
Change in valuation allowances as recorded in the consolidated
statements of income
|
|
|
210,124
|
|
|
|
213,586
|
|
|
|
201,998
|
|
|
Write-offs and recoveries of amounts previously written-off
|
|
|
(210,166
|
)
|
|
|
(192,626
|
)
|
|
|
(167,519
|
)
|
|
Foreign currency translation
|
|
|
3,656
|
|
|
|
(5,924
|
)
|
|
|
6,028
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Allowance for doubtful accounts as of December 31
|
|
$
|
266,449
|
|
|
$
|
262,836
|
|
|
$
|
247,800
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S-II