|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _________________________ to _________________________
|
|
INTEGRITY APPLICATIONS, INC.
|
||
|
(Exact name of registrant as specified in its charter)
|
||
|
Delaware
|
98-0668934
|
|
|
(State or other jurisdiction of
incorporation or organization)
|
(I.R.S. Employer
Identification No.)
|
|
|
19 Ha'Yahalomim
Street
P.O. Box
12163
Ashdod
, Israel
|
L3
7760049
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
|
Registrant’s telephone number, including area code
972 (8) 675-7878
|
||
|
Securities registered pursuant to Section 12(b) of the Act:
None
|
||
|
Securities registered pursuant to Section 12(g) of the Act:
Common stock, par value $0.001 per share
|
||
|
Large accelerated filer
o
|
Accelerated filer
o
|
|
Non-accelerated filer
o
(Do not check if a smaller reporting company)
|
Smaller Reporting Company
x
|
|
4
|
|
|
4
|
|
|
5
|
|
|
5
|
|
|
30
|
|
|
48
|
|
|
48
|
|
|
48
|
|
| 48 | |
|
49
|
|
|
49
|
|
|
50
|
|
|
50
|
|
|
61
|
|
|
61
|
|
|
61
|
|
|
61
|
|
|
62
|
|
|
63
|
|
|
63
|
|
|
67
|
|
|
72
|
|
|
75
|
|
|
76
|
|
|
77
|
|
|
77
|
|
|
79
|
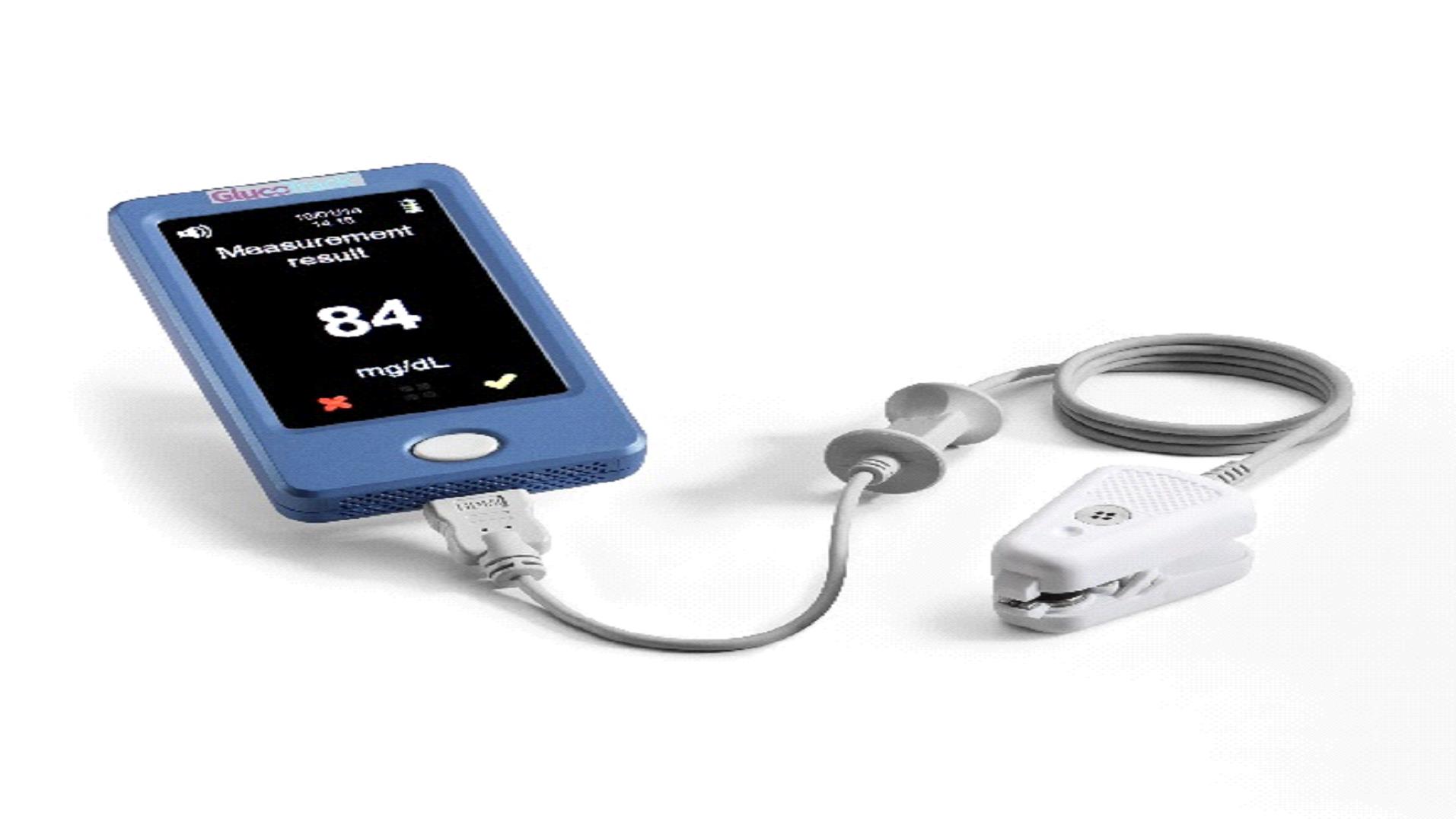
| · |
pain, as the GlucoTrack® model DF-F is a truly non-inveasive device; and
|
| · |
cost, as, despite the relatively high upfront cost of purchasing a GlucoTrack® model DF-F, we anticipate that the total cost of purchasing a device and purchasing replacement ear clips every six months (anticipated to be the only recurring cost, other than calibration costs, which are expected to be minimal) over the useful life of the device will be significantly lower than the cost of purchasing single use glucose sticks over that same period. See Figure B and the accompanying footnotes for a direct cost comparison of the GlucoTrack® model DF-F and conventional (invasive) spot finger stick devices.
|

| · |
Ultrasound
: The GlucoTrack® model DF-F uses ultrasound technology to measure the change of speed of sound through the earlobe, which is impacted by the glucose concentration in the capillary blood vessels.
|
| · |
Electromagnetic
: The GlucoTrack® model DF-F’s electromagnetic technology uses a measurement of conductivity to measure the change in tissue impedance, which is a function of glucose concentration. The GlucoTrack® model DF-F’s electromagnetic technology analyzes criteria similar to those analyzed by conventional invasive devices, such as spot finger stick devices, but does so in a non-invasive manner.
|
| · |
Thermal
: The GlucoTrack® model DF-F’s thermal technology uses a measurement of heat capacity characteristics of the tissue, which are influenced by glucose concentration.
|
| · |
The anti-kickback statute (Section 1128B(b) of the Social Security Act), which prohibits certain business practices and relationships that might affect the provision and cost of healthcare services reimbursable under Medicare, Medicaid and other federal healthcare programs, including the payment or receipt of remuneration for the referral of patients whose care will be paid by Medicare or other governmental programs;
|
| · |
The physician self-referral prohibition (Ethics in Patient Referral Act of 1989, as amended, commonly referred to as the Stark Law, Section 1877 of the Social Security Act), which prohibits referrals by physicians of Medicare or Medicaid patients to providers of a broad range of designated healthcare services in which the physicians (or their immediate family members) have ownership interests or with which they have certain other financial arrangements;
|
| · |
The anti-inducement provisions of the Civil Monetary Penalties Law (Section 1128A(a)(5) of the Social Security Act), which prohibit providers from offering anything to a Medicare or Medicaid beneficiary to induce that beneficiary to use items or services covered by either program;
|
| · |
The False Claims Act (31 U.S.C. § 3729 et seq.), which prohibits any person from knowingly presenting or causing to be presented false or fraudulent claims for payment to the federal government (including the Medicare and Medicaid programs); and
|
| · |
The Civil Monetary Penalties Law (Section 1128A of the Social Security Act), which authorizes the United States Department of Health and Human Services to impose civil penalties administratively for fraudulent or abusive acts.
|
| · |
significantly greater name recognition;
|
| · |
established relations with healthcare professionals, customers and third-party payors;
|
| · |
established distribution networks;
|
| · |
additional lines of products, and the ability to offer rebates or bundle products to offer higher discounts or incentives to gain a competitive advantage;
|
| · |
greater experience in conducting research and development, manufacturing, clinical trials, obtaining regulatory approval for products and marketing approved products; and
|
| · |
greater financial and human resources for product development, sales and marketing, and patent litigation.
|
|
Company
|
Product
|
Technology
|
Calibration
|
Type of Measurement
|
Technology Description
|
||||||
|
1.
|
Echo Therapeutics (MA, USA)
|
Symphony
|
UltraSound
|
Daily calibration
|
Continuous
|
Needle-free skin permeation and non-invasive, continuous transdermal glucose biosensor (device attached to skin).
|
|||||
|
2.
|
Freedom Meditech (MA, USA)
|
Optical Polarimetry (in front of the eye)
|
Not known
|
Spot; Screening
|
Non-invasive direct measurement of glucose levels in front of the eye via optical polarimetry.
|
||||||
|
3.
|
Cybiocare (Quebec, Canada)
|
OHD
|
Optical
|
Not known
|
Continuous
|
Through a device strapped to a patient’s arm, continuously measures glucose levels by using infrared light to detect hypoglycemia in the patient.
|
|||||
|
4.
|
CNOGA Medical (Israel)
|
TensorTip CGM Combo Glucometer
|
Optical Look-up table
|
At least 200 pricking within a few weeks
|
Spot
|
Four LED signals are beamed through the finger; color image sensor executes a special algorithm.
|
| · |
our ability to have partners manufacture and sell commercial quantities of any approved products to the market;
|
| · |
acceptance of product candidates by physicians and other health care providers;
|
| · |
the results of our clinical trials;
|
| · |
our ability to recruit and enroll patients for our clinical trials;
|
| · |
the efficacy, safety, performance and reliability of our product candidates;
|
| · |
the speed at which we develop product candidates;
|
| · |
our ability to obtain prompt and favorable IRB review and approval at each of our clinical sites;
|
| · |
our ability to commercialize and market any of our product candidates that may receive regulatory clearance or approval;
|
| · |
our ability to design and successfully execute appropriate clinical trials;
|
| · |
the timing and scope of regulatory clearances or approvals;
|
| · |
appropriate coverage and adequate levels of reimbursement under private and governmental health insurance plans, including Medicare; and
|
| · |
our ability to protect intellectual property rights related to our products.
|
| · |
the failure to obtain sufficient funding to pay for all necessary clinical trials;
|
| · |
limited number of, and competition for, suitable patients that meet the protocol’s inclusion criteria and do not meet any of the exclusion criteria;
|
| · |
limited number of, and competition for, suitable sites to conduct the clinical trials, and delay or failure to obtain FDA approval, if necessary, to commence a clinical trial;
|
| · |
delay or failure to obtain sufficient supplies of the product candidate for clinical trials;
|
| · |
requirements to provide the medical device required in clinical trials at cost, which may require significant expenditures that we are unable or unwilling to make;
|
| · |
delay or failure to reach agreement on acceptable clinical trial agreement terms or clinical trial protocols with prospective sites or investigators; and
|
| · |
delay or failure to obtain IRB approval or renewal of such approval to conduct a clinical trial at a prospective or accruing site, respectively.
|
| · |
slower than expected rates of patient recruitment and enrollment;
|
| · |
failure of patients to complete the clinical trial;
|
| · |
unforeseen safety issues;
|
| · |
lack of efficacy evidenced during clinical trials;
|
| · |
termination of clinical trials by one or more clinical trial sites;
|
| · |
inability or unwillingness of patients or medical investigators to follow clinical trial protocols; and
|
| · |
inability to monitor patients adequately during or after treatment.
|
| · |
restrictions on the products, manufacturers or manufacturing process;
|
| · |
adverse inspectional observations (Form 483), warning letters or non-warning letters incorporating inspectional observations, i.e., so-called “untitled letter”;
|
| · |
civil and criminal penalties;
|
| · |
injunctions;
|
| · |
suspension or withdrawal of regulatory clearances or approvals;
|
| · |
product seizures, detentions or import bans;
|
| · |
voluntary or mandatory product recalls and publicity requirements;
|
| · |
total or partial suspension of production;
|
| · |
imposition of restrictions on operations, including costly new manufacturing requirements; and
|
| · |
refusal to clear or approve pending applications or premarket notifications.
|
| · |
a medical device candidate may not be deemed safe or effective, in the case of a PMA;
|
| · |
a medical device candidate may not be deemed to be substantially equivalent to a lawfully marketed non-premarket approval device in the case of a 510(k)-premarket notification;
|
| · |
FDA officials may not find the data from the clinical trials sufficient;
|
| · |
the FDA might not approve our third-party manufacturer’s processes or facilities; or
|
| · |
the FDA may change its clearance or approval policies or adopt new regulations.
|
| · |
restrictions on the products, manufacturers or manufacturing process;
|
| · |
adverse inspectional observations (Form 483), warning letters, or non-warning letters incorporating inspectional observations;
|
| · |
civil or criminal penalties or fines;
|
| · |
injunctions;
|
| · |
product seizures, detentions or import bans;
|
| · |
voluntary or mandatory product recalls and publicity requirements;
|
| · |
suspension or withdrawal of regulatory clearances or approvals;
|
| · |
total or partial suspension of production;
|
| · |
imposition of restrictions on operations, including costly new manufacturing requirements; and
|
| · |
refusal to clear or approve pending applications or premarket notifications.
|
| · |
timing of market introduction of competitive products;
|
| · |
safety and efficacy of our product;
|
| · |
prevalence and severity of any side effects;
|
| · |
potential advantages or disadvantages over alternative treatments;
|
| · |
strength of marketing and distribution support;
|
| · |
price of our product candidates, both in absolute terms and relative to alternative treatments; and
|
| · |
availability of coverage and reimbursement from government and other third-party payors.
|
| · |
difficulties in compliance with non-U.S. laws and regulations;
|
| · |
changes in non-U.S. regulations and customs;
|
| · |
changes in non-U.S. currency exchange rates and currency controls;
|
| · |
changes in a specific country’s or region’s political or economic environment;
|
| · |
trade protection measures, import or export licensing requirements or other restrictive actions by U.S. or non-U.S. governments;
|
| · |
negative consequences from changes in tax laws; and
|
| · |
difficulties associated with staffing and managing foreign operations, including differing labor relations.
|
| · |
any major hostilities involving Israel;
|
| · |
a full or partial mobilization of the reserve forces of the Israeli army;
|
| · |
the interruption or curtailment of trade between Israel and its present trading partners; and
|
| · |
a significant downturn in the economic or financial conditions in Israel.
|
| · |
the announcement of new products or product enhancements by us or our competitors;
|
| · |
developments concerning intellectual property rights and regulatory approvals;
|
| · |
variations in our and our competitors’ results of operations;
|
| · |
changes in earnings estimates or recommendations by securities analysts, if the common stock is covered by analysts;
|
| · |
developments in the medical device industry;
|
| · |
the results of product liability or intellectual property lawsuits;
|
| · |
future issuances of common stock or other securities;
|
| · |
the addition or departure of key personnel;
|
| · |
announcements by us or our competitors of acquisitions, investments or strategic alliances; and
|
| · |
general market conditions and other factors, including factors unrelated to our operating performance.
|
|
Quarter Ended
|
High Bid
|
Low Bid
|
||||||
|
December 31, 2016
|
$
|
3.09
|
$
|
2.30
|
||||
|
September 30, 2016
|
$
|
3.75
|
$
|
1.70
|
||||
|
June 30, 2016
|
$
|
4.50
|
$
|
3.25
|
||||
|
March 31, 2016
|
$
|
4.50
|
$
|
2.00
|
||||
|
December 31, 2015
|
$
|
5.83
|
$
|
3.25
|
||||
|
September 30, 2015
|
$
|
6.75
|
$
|
4.00
|
||||
|
June 30, 2015
|
$
|
7.50
|
$
|
4.25
|
||||
|
March 31, 2015
|
$
|
6.25
|
$
|
4.90
|
||||
| 1. |
Accounting Standard Update 2014-09, “Revenue from Contracts with Customers”
|
| 2. |
Accounting Standards Update 2014-15, “Presentation of Financial Statements—Going Concern”
|
| 3. |
Accounting Standard Update 2014-16, “Determining Whether the Host Contract in a Hybrid Financial Instrument Issued in the Form of a Share Is More Akin to Debt or to Equity”.
|
| 4. |
Accounting Standard Update 2015-11, “Simplifying the Measurement of Inventory”
|
|
Name
|
Age
|
Position
|
||
|
Dr. Robert Fischell
|
87
|
Director, member of the Compensation and Nominating and Corporate Governance committees
|
||
|
John Graham
|
56
|
Chairman and Chief Executive Officer
|
||
|
Leslie Seff
|
66
|
Director, member of the Compensation and Nominating and Corporate Governance committees
|
||
|
Angela Strand
|
48
|
Vice Chairman, member of the Compensation and Nominating and Corporate Governance committees
|
||
|
Revan Schwartz
|
71
|
Director
|
||
|
Avner Gal
|
62 | Director |
|
Name
|
Age
|
Position
|
||
|
John Graham
|
56 |
Chairman and Chief Executive Officer
|
||
|
David Malka
|
51
|
Vice President of Operations
|
||
|
Sami Sassoun
|
49
|
Chief Financial Officer
|
||
|
Avner Gal
|
62 |
Chief Executive Officer, Integrity Israel
|
|
Name and Principal Position
|
Year
|
Salary
|
Bonus
|
Option Awards
|
All Other Compensation
|
Total Compensation
|
||||||||||||||||
|
Avner Gal
|
||||||||||||||||||||||
|
Former Chief Executive Officer
|
2016 (1)
|
$
|
125,722
|
-
|
-
|
$
|
61,664
|
(2)
|
$
|
187,386
|
||||||||||||
|
2015 (6)
|
$
|
124,564
|
-
|
-
|
$
|
81,227
|
(7)
|
$
|
205,791
|
|||||||||||||
|
Eran Hertz
|
||||||||||||||||||||||
|
Former Chief Financial Officer
|
2016 (1)
|
$
|
87,963
|
-
|
$
|
2,234
|
$
|
51,135
|
(3)
|
$
|
141,332
|
|||||||||||
|
2015 (6)
|
$
|
87,435
|
-
|
$
|
7,964
|
$
|
48,433
|
(8)
|
$
|
143,832
|
||||||||||||
|
David Malka
|
2016 (1)
|
$
|
63,114
|
-
|
-
|
$
|
48,045
|
(4)
|
$
|
111,160
|
||||||||||||
|
Vice President of Operations
|
2015 (6)
|
$
|
62,716
|
-
|
-
|
$
|
43,687
|
(9)
|
$
|
106,403
|
||||||||||||
|
Eran Cohen
|
||||||||||||||||||||||
|
Former Chief Operating Officer
|
2016 (1)
|
$
|
162,938
|
-
|
$
|
11,057
|
$
|
66,966
|
(5)
|
$
|
240,960
|
|||||||||||
| (1) |
Calculated based on the average exchange rate for the year of New Israeli Shekels to U.S. Dollars of NIS 3.832 = U.S. $1.00.
|
| (2) |
Includes $18,727 in automobile expenses paid by Integrity, including leasing costs, insurance premiums, gasoline and/or repairs incurred in connection with the executive’s automobile, $313 in cellular communications expenses paid by Integrity, representing the estimated costs of our cellular communications expenses attributable to the executive, $12,539 in tax gross-up payments, and contributions to the (a) Severance Pay-Fund, (b) retirement plan feature of Managers’ Insurance (Kupat Gemel), (c) disability insurance (Ovdan Kosher Avoda) and (d) and statutory national insurance (Bituach Leumi) in the aggregate total amount of $30,085.
|
| (3) |
Includes $18,163 in automobile expenses paid by Integrity, including leasing costs, insurance premiums, gasoline and/or repairs incurred in connection with the executive’s automobile, $313 in cellular communications expenses paid by Integrity, representing the estimated costs of our cellular communications expenses attributable to the executive, $11,474 in tax gross-up payments, and contributions to the (a) Severance Pay-Fund, (b) retirement plan feature of Managers’ Insurance (Kupat Gemel), (c) disability insurance (Ovdan Kosher Avoda) and (d) statutory national insurance (Bituach Leumi) in the aggregate total amount of $21,185.
|
| (4) |
Includes $20,292 in automobile expenses paid by Integrity, including leasing costs, insurance premiums, gasoline and/or repairs incurred in connection with the executive’s automobile, $313 in cellular communications expenses paid by Integrity, representing the estimated costs of our cellular communications expenses attributable to the executive, $11,574 in tax gross-up payments, and contributions to the (a) Severance Pay-Fund, (b) retirement plan feature of Managers’ Insurance (Kupat Gemel), (c) disability insurance (Ovdan Kosher Avoda) and (d) statutory national insurance (Bituach Leumi) in the aggregate total amount of $15,866.
|
| (5) |
Includes $19,854 in automobile expenses paid by Integrity, including leasing costs, insurance premiums, gasoline and/or repairs incurred in connection with the executive’s automobile, $313 in cellular communications expenses paid by Integrity, representing the estimated costs of our cellular communications expenses attributable to the executive, $12,113 in tax gross-up payments, and contributions to the (a) Severance Pay-Fund, (b) retirement plan feature of Managers’ Insurance (Kupat Gemel), (c) disability insurance (Ovdan Kosher Avoda) (d) statutory national insurance (Bituach Leumi) and (e) vacation pay-out in the aggregate total amount of $34,686.
|
| (6) |
Calculated based on the average exchange rate for the year of New Israeli Shekels to U.S. Dollars of NIS 3.888 = U.S. $1.00.
|
| (7) |
Includes $18,456 in automobile expenses paid by Integrity, including leasing costs, insurance premiums, gasoline and/or repairs incurred in connection with the executive’s automobile, $360 in cellular communications expenses paid by Integrity, representing the estimated costs of our cellular communications expenses attributable to the executive, $12,649 in tax gross-up payments, and contributions to the (a) Severance Pay-Fund, (b) retirement plan feature of Managers’ Insurance (Kupat Gemel), (c) disability insurance (Ovdan Kosher Avoda) and (d) statutory national insurance (Bituach Leumi) in the aggregate total amount of $49,762
|
| (8) |
Includes $17,900 in automobile expenses paid by Integrity, including leasing costs, insurance premiums, gasoline and/or repairs incurred in connection with the executive’s automobile, $10,780 in tax gross-up payments, and contributions to the (a) Severance Pay-Fund, (b) retirement plan feature of Managers’ Insurance (Kupat Gemel), (c) disability insurance (Ovdan Kosher Avoda) and (d) statutory national insurance (Bituach Leumi) in the aggregate total amount of $19,753.
|
| (9) |
Includes $19,999 in automobile expenses paid by Integrity, including leasing costs, insurance premiums, gasoline and/or repairs incurred in connection with the executive’s automobile, $527 in cellular communications expenses paid by Integrity, representing the estimated costs of our cellular communications expenses attributable to the executive, $10,648 in tax gross-up payments, and contributions to the (a) Severance Pay-Fund, (b) retirement plan feature of Managers’ Insurance (Kupat Gemel), (c) disability insurance (Ovdan Kosher Avoda) (d) statutory national insurance (Bituach Leumi) and (e) vacation pay-out in the aggregate total amount of $12,513.
|
|
Name
|
Number of Securities Underlying Unexercised Options (#) Exercisable
|
Number of Securities Underlying Unexercised Options (#) Unexercisable
|
Option Exercise Price($)
|
Option Expiration Date
|
|||||||||
|
Avner Gal
|
176,519
|
88,259
|
$
|
6.25
|
(1)
|
March 11, 2022
|
|||||||
|
Former Chief Executive Officer
|
|||||||||||||
|
Eran Hertz
|
10,000
|
-
|
$
|
7.00
|
(2)
|
June 30, 2024
|
|||||||
|
Former Chief Financial Officer
|
|||||||||||||
|
David Malka
|
52,956
|
26,478
|
$
|
6.25
|
(3)
|
March 11, 2022
|
|||||||
|
Vice President of Operations
|
|||||||||||||
|
Eran Cohen
|
6,000
|
10,000
|
$
|
4.75
|
(4)
|
January 1, 2026
|
|||||||
|
Former Chief Operating Officer
|
|||||||||||||
|
Name
|
Fees earned or
paid in cash
|
Option Awards
Vested
(1) |
All other
compensation
|
Total
|
||||||||||||
|
Robert Fischell
|
$
|
20,000
|
$
|
18,975
|
(2)
|
-
|
$
|
38,975
|
||||||||
|
Angela Strand
|
$
|
22,500
|
$
|
18,975
|
(3)
|
$
|
20,000
|
(5)
|
$
|
61,475
|
||||||
|
Leslie Seff
|
$
|
20,000
|
$
|
18,975
|
(4)
|
-
|
$
|
38,975
|
||||||||
|
Philip Darivoff
|
-
|
-
|
-
|
-
|
||||||||||||
|
Revan Schwartz
|
-
|
-
|
-
|
-
|
||||||||||||
|
|
$
|
62,500
|
$
|
56,925
|
$
|
20,000
|
$
|
139,425
|
||||||||
| · |
an annual cash payment in the amount of $15,000, payable in four equal quarterly installments of $3,750 each on the last day of each calendar quarter commencing with the second quarter of 2016, subject to the director’s continued service as of each such date; and
|
| · |
an annual cash payment to the chairperson of the Nominating and Corporate Governance Committee in the amount of $10,000, payable in four equal quarterly installments of $2,500 each, on the last day of each calendar quarter commencing with the second quarter of 2016, subject to the chairperson’s continued service as of each such date.
|
|
Number of securities to be issued upon exercise of
outstanding options, warrants and rights |
Weighted-average exercise price of outstanding options, warrants and rights
|
Number of securities remaining available for future issuance under equity compensation
plans (excluding securities reflected in column (a))
|
||||||||||
|
|
(a)
|
(b)
|
(c)
|
|||||||||
|
Equity compensation plans approved by security holders
|
562,803
|
$
|
5.64
|
437,197
|
(1)
|
|||||||
|
Equity compensation plans not approved by security holders
|
1,529,969
|
(2)
|
$
|
5.99
|
—
|
|||||||
|
Total
|
2,092,772
|
$
|
5.90
|
437,197
|
||||||||
| (1) |
On March 17, 2016, the Board approved an amendment to the Plan to increase the number of shares of the Company’s Common Stock reserved for issuance under the Plan from 529,555 shares to 1,000,000 shares. The number of securities remaining available for future issuance under equity compensation as of December 31, 2016, as reflected in column (c), includes the additional securities authorized by such amendment.
|
| (2) |
Consists of: (i) warrants to purchase 129,556 shares of Common Stock issuable to Andrew Garrett, Inc., as partial consideration for its services as the placement agent for Integrity’s private placement of 1,295,545 shares of Common Stock completed in July 2011, (ii) warrants to purchase 256,769 shares of Common Stock issued or issuable to Andrew Garrett, Inc., as partial consideration for its services as placement agent for Integrity’s private placement of the Series A Units; (iii) warrants to purchase 439,674 shares of Common Stock issued or issuable to Andrew Garrett, Inc., as partial consideration for its services as placement agent for Integrity’s private placement of the Series B Units; (iv) warrants to purchase 388,600 shares of Common Stock issued or issuable to Andrew Garrett, Inc., as partial consideration for its services as placement agent for Integrity’s private placement of the Series C Units; (v) warrants to purchase 244,572 shares of Common Stock issued pursuant to the anti-dilution provisions of outstanding warrants held by Andrew Garrett, Inc.; (vi) options to purchase 17,656 shares of Common Stock issued to the Company’s former investor relations provider, as partial consideration for their services; (vii) options to purchase 21,640 shares of Common Stock issued in consideration of regulatory services and (viii) options to purchase 31,502 shares of Common Stock issued in consideration of finder’s fee.
|
|
Name of Beneficial Owner
|
Class of Security
|
Number of Shares Beneficially Owned
|
Percent of Class (1)
|
|||||||
|
Executive Officers and Directors
:
|
||||||||||
|
John Graham
|
Common Stock
|
170,975
|
(2)
|
2.8
|
%
|
|||||
|
Dr. Robert Fischell
|
Common Stock
|
53,103
|
(3)
|
0.9
|
%
|
|||||
|
Avner Gal
|
Common Stock
|
450,507
|
(4)
|
7.5
|
%
|
|||||
|
David Malka
|
Common Stock
|
176,651
|
(
5
)
|
2.9
|
%
|
|||||
|
Angela Strand
|
Common Stock
|
27,779
|
(
6
)
|
0.5
|
%
|
|||||
|
Leslie Seff
|
Common Stock
|
13,334
|
(
6
)
|
0.2
|
%
|
|||||
|
Sami Sassoun
|
Common Stock
|
0
|
*
|
|||||||
|
Revan Schwartz
|
Common Stock
|
0
|
*
|
|||||||
|
All Executive Officers and Directors as a Group (8 Persons)
|
Common Stock
|
892,349
|
14.8
|
%
|
||||||
|
Principal Stockholders (Common Stock)
:
|
||||||||||
|
Amos and Daughters Investments and Properties Ltd. (
7
)
|
Common Stock
|
393,714
|
6.5
|
%
|
||||||
|
Joel L. Gold (
8
)
|
Common Stock
|
318,432
|
(
9
)
|
5.3
|
%
|
|||||
|
Vayikra Capital, LLC (
10
)
|
Common Stock
|
607,591
|
(
1
1
)
|
9.9
|
%
|
|||||
|
Y.H. Dimri Holdings (
1
2
)
|
Common Stock
|
1,160,650
|
19.3
|
%
|
||||||
|
Principal Stockholders (Series A Preferred Stock)
:
|
||||||||||
|
H. Applebaum Family Trust U/A Dtd 07/17/92 (
1
3
)
|
Series A Preferred Stock
|
50
|
13.3
|
%
|
||||||
|
Chunlin Chiang (
1
4
)
|
Series A Preferred Stock
|
100
|
26.6
|
%
|
||||||
|
James Waring (
1
5
)
|
Series A Preferred Stock
|
100
|
26.6
|
%
|
||||||
|
James H. Smith (
1
6
)
|
Series A Preferred Stock
|
100
|
26.6
|
%
|
||||||
|
Principal Stockholders (Series B Preferred Stock)
:
|
||||||||||
|
RBC Capital Markets (
1
7
)
|
Series B Preferred Stock
|
1,310
|
8.7
|
%
|
||||||
|
Principal Stockholders (Series C Preferred Stock)
:
|
||||||||||
|
John Ballantyne (
18
)
|
Series C Preferred Stock
|
1,500
|
17.1
|
%
|
||||||
|
Vayikra Capital, LLC (
9
)
|
Series C Preferred Stock
|
1,300
|
14.8
|
%
|
||||||
|
(1)
|
Common stock percentages are based on 6,047,640 shares of Common Stock outstanding as of March 27, 2017. Series A Preferred Stock percentages are based on 376 shares of Series A Preferred Stock outstanding as of March 27 , 2017. Series B Preferred Stock percentages are based on 15,031 outstanding as of March 27, 2017. Series C Preferred Stock percentages are based on 8,793 shares of Series C Preferred Stock outstanding as of March 27, 2017.
|
|
(2)
|
Includes 170,975 shares of Common Stock issuable upon the exercise of stock options exercisable within 60 days
|
|
(3)
|
Includes 10,000 shares of Common Stock issuable upon the exercise of stock options exercisable within 60 days.
|
| (4) |
Includes 176,519 shares of Common Stock issuable upon the exercise of stock options exercisable within 60 days.
|
| (5) |
Includes 52,956 shares of Common Stock issuable upon the exercise of stock options exercisable within 60 days.
|
| ( 6 ) |
Includes 10,000 shares of Common Stock issuable upon the exercise of stock options exercisable within 60 days.
|
| ( 7 ) |
The address of Amos and Daughters Investments and Properties Ltd. is Shekel House, 111 Arlozorov St. Tel-Aviv 61216 Israel. Eri Steimatzky has voting and investment control over the shares held by Amos and Daughters Investments and Properties Ltd.
|
| ( 8 ) |
The address of Joel L. Gold is 874 East 9th Street, Brooklyn, NY 11230.
|
| ( 9 ) |
Includes 218,280 shares held by relatives and affiliates of Mr. Gold.
|
| ( 10 ) |
The address of Vayikra Capital, LLC is 1 Farmstead Road, Short Hills NJ, 07078. Philip M. Darivoff has voting and investment control over the shares held by Vayikra Capital, LLC.
|
| ( 11 ) |
Includes 138,890 shares of Common Stock issuable upon the conversion of shares of Series B Preferred Stock which are convertible within 60 days and 54,329 shares of Common Stock issuable upon the exercise of Series B-1 Warrants which are exercisable within 60 days. Excludes 361,190 shares of Common Stock issuable upon the exercise of Series B-1 and Series B-2 Warrants not convertible within 60 days. The conversion of such Series B-1 and Series B-2 Warrants is limited by the beneficial ownership limitation included in Vayikra Capital, LLC’s Series B-1 and Series B-2 Warrants which provides that Vayikra Capital, LLC will not be permitted to exercise such warrants if such conversion would cause such holder to beneficially own more than 9.99% of the outstanding number of shares of our Common Stock outstanding after giving effect to such conversion.
|
| ( 1 2 ) |
The address of Y.H. Dimri Holdings is 1 Jerusalem St. Netivot, 87710 Israel. Y.H. Dimri is entitled to these subject to the fulfillment of certain requirements. Yigal Dimri has voting and investment control over the shares held by Y.H. Dimri Holdings.
|
| ( 1 3 ) |
Howard Applebaum has voting and investment control over the shares held by the H Applebaum Family Trust U/A Dtd 07/17/92.
|
| ( 1 4 ) |
The address of Chunlin Chiang is 140-14 Cherry Avenue, Flushing, NY
11355.
|
| ( 1 5 ) |
The address of James Waring is 21221 349
th
Avenue, Ree Heights SD
57371.
|
| ( 1 6 ) |
The address of James H. Smith is 1525 NW 25
th
Avenue, Chiefland, FL
32626.
|
| ( 1 7 ) |
The address of RBC Capital Markets is 60 South 6
th
Street, Minneapolis MN
55402.
|
| ( 1 8 ) |
The address of John Ballantyne is
1101 28th Ave South, Fargo, ND 58103.
|
|
Exhibit Number
|
Description
|
|
|
2.1
|
Merger Agreement and Plan of Reorganization, dated as of May 25, 2010, by and among Integrity Applications, Inc., Integrity Acquisition Ltd. and A.D. Integrity Applications Ltd. (1)
|
|
|
3.1
|
Certificate of Incorporation of Integrity Applications, Inc. (1)
|
|
|
3.2
|
Certificate of Amendment to Certificate of Incorporation of Integrity Applications, Inc. (1)
|
|
|
3.3
|
Certificate of Designation of Preferences and Rights of Series A 5% Convertible Preferred Stock (2)
|
|
|
3.4
|
Bylaws of Integrity Applications, Inc. (1)
|
|
|
3.5
|
Certificate of Designation of Preferences and Rights of Series B 5.5% Convertible Preferred Stock (3)
|
|
|
3.6
|
Certificate of Designation of Preferences and Rights of Series C 5.5% Convertible Preferred Stock (4)
|
|
|
4.1
|
Specimen Certificate Evidencing Shares of Common Stock (1)
|
|
|
4.2
|
Form of Common Stock Purchase Warrant (1)
|
|
|
4.3
|
Form of Series A Securities Purchase Agreement (2)
|
|
|
4.4
|
Form of Series A Common Stock Purchase Warrant (2)
|
|
|
4.5
|
Form of Series A Registration Rights Agreement (2)
|
|
|
4.6
|
Form of Series B Securities Purchase Agreement (3)
|
|
|
4.7
|
Form of Series B-1 Common Stock Purchase Warrant (3)
|
|
|
4.8
|
Form of Series B-2 Common Stock Purchase Warrant (3)
|
|
|
4.9
|
Form of Series B Registration Rights Agreement (3)
|
|
|
4.10
|
Form of Series C Securities Purchase Agreement (4)
|
|
|
4.11
|
Form of Series C Registration Rights Agreement (4)
|
|
|
4.12
|
Form of Series C-1 Common Stock Purchase Warrant (4)
|
|
|
4.13
|
Form of Series C-2 Common Stock Purchase Warrant (4)
|
|
|
10.1*
|
Integrity Applications, Inc. 2010 Incentive Compensation Plan (1)
|
|
|
10.2*
|
Form of Director and Officer Indemnification Agreement (1)
|
|
Exhibit Number
|
Description
|
|
10.3*
|
Personal Employment Agreement, dated as of July 22, 2009, between A.D. Integrity Applications Ltd. and Avner Gal (1)
|
|
|
10.4*
|
Personal Employment Agreement, dated as of July 22, 2010, between A.D. Integrity Applications Ltd. and David Malka (1)
|
|
|
10.5
|
Irrevocable Undertaking of Indemnification, dated as of July 26, 2010, by and among Integrity Applications, Inc., Avner Gal, Zvi Cohen, Ilana Freger, David Malka and Alexander Raykhman (1)
|
|
|
10.6
|
Agreement, dated as of November 1, 2005 by and between A.D. Integrity Applications Ltd. and Diabeasy Diabeasy cc. (5)
|
|
|
10.7
|
Agreement, dated as of October 2, 2005, by and between Technology Transfer Group and Integrity Applications Ltd. (1)
|
|
|
10.8*
|
Form of Stock Option Agreement (1)
|
|
|
10.9*
|
Form of Stock Option Agreement (ESOP) (1)
|
|
|
10.10
|
Letter of Approval, addressed to Integrity Applications Ltd. from the Ministry of Industry, Trade and Employment of the State of Israel (6)
|
|
|
10.11
|
Letter of Undertaking, addressed to the Ministry of Industry, Trade and Employment of the State of Israel - Office of the Chief Scientist from Integrity Applications Ltd. (7)
|
|
|
10.12
|
Investment Agreement, dated March 16, 2004, by and among A.D. Integrity Applications Ltd., Yitzhak Fisher, Asher Kugler and Nir Tarlovsky (5)
|
|
|
10.13*
|
Personal Employment Agreement, dated as of October 22, 2013, between A.D. Integrity Applications Ltd. and Eran Hertz (8)
|
|
|
10.14*
|
Personal Employment Agreement, dated as of February 1, 2017, between A.D. Integrity Applications Ltd. and Sami Sassoun
|
|
|
10.15*
|
Amended and Restated Consulting Agreement, dated as of February 6, 2017, between Integrity Applications, Inc. and Strand Strategy
|
|
|
10.16*
|
Personal Employment Agreement, dated as of March 20, 2017, between Integrity Applications, Inc. and John Graham
|
|
|
14.1
|
Code of Ethics
|
|
|
21.1
|
Subsidiaries of Integrity Applications, Inc. (1)
|
|
|
31.1
|
Certification of Chief Executive Officer Pursuant to Exchange Act Rule 13a-14(a) or 15(d)-14(a), as Adopted Pursuant to Section 302 of the Sarbanes Oxley Act of 2002
|
|
|
31.2
|
Certification of Chief Financial Officer Pursuant to Exchange Act Rule 13a-14(a) or 15(d)-14(a), as Adopted Pursuant to Section 302 of the Sarbanes Oxley Act of 2002
|
|
|
32.1
|
Certification of Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes Oxley Act of 2002
|
|
|
32.2
|
Certification of Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes Oxley Act of 2002
|
|
|
101.INS
|
XBRL Instance Document (9)
|
|
|
101.SCH
|
XBRL Schema Document (9)
|
|
|
101.CAL
|
XBRL Calculation Linkbase Document (9)
|
|
|
101.LAB
|
XBRL Label Linkbase Document (9)
|
|
|
101.PRE
|
XBRL Presentation Linkbase Document (9)
|
|
|
101.DEF
|
XBRL Definition Linkbase Document (9)
|
| (1) |
Previously filed as an exhibit to the Company’s Registration Statement on Form S-1, as filed with the SEC on August 22, 2011.
|
| (2) |
Previously filed as an exhibit to the Company’s Current Report on Form 8-K, as filed with the SEC on March 18, 2013.
|
| (3) |
Previously filed as an exhibit to the Company’s Current Report on Form 8-K, as filed with the SEC on September 5, 2014.
|
| (4) |
Previously filed as an exhibit to the Company’s Current Report on Form 8-K, as filed with the SEC on April 14, 2016.
|
| (5) |
Previously filed as an exhibit to Amendment No. 2 to the Company’s Registration Statement on Form S-1, as filed with the SEC on October 27, 2011.
|
| (6) |
Previously filed as an exhibit to Amendment No. 3 to the Company’s Registration Statement on Form S-1, as filed with the SEC on November 10, 2011.
|
| (7) |
Previously filed as an exhibit to Amendment No. 1 to the Company’s Registration Statement on Form S-1, as filed with the SEC on October 7, 2011.
|
| (8) |
Previously filed as an exhibit to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2013, as filed with the SEC on March 28, 2014.
|
| (9) |
Pursuant to Rule 406T of Regulation S-T, the interactive files are deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, are deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and otherwise are not subject to liability under those sections.
|
|
INTEGRITY APPLICATIONS, INC.
|
|||
|
|
By:
|
/s/ John Graham | |
| Name: John Graham | |||
| Title: Chief Executive Officer | |||
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/ John Graham
|
|
Chairman and Chief Executive Officer
|
|
March 31 2017
|
|
John Graham
|
|
(Principal Executive Officer)
|
|
|
|
|
|
|
|
|
|
/s/ Sami Sassoun
|
|
Chief Financial Officer
|
|
March 31 2017
|
|
Sami Sassoun
|
|
(Principal Financial Officer and Principal Accounting Officer)
|
|
|
|
|
|
|
|
|
|
|
|
Director
|
|
March 31 2017
|
|
Dr. Robert Fischell
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Angela Strand
|
|
Vice Chairman
|
|
March 31 2017
|
|
Angela Strand
|
|
|
|
|
|
/s/ Leslie Seff
|
|
Director
|
|
March 31 2017
|
|
Leslie Seff
|
|
|
|
|
|
/s/ Revan Schwartz
|
|
Director
|
|
March 31 2017
|
|
Revan Schwartz
|
|
|
|
|
|
/s/ Avner Gal
|
Director
|
|||
|
Avner Gal
|
|
Page
|
|
|
F-2
|
|
|
Consolidated Financial Statements
|
|
|
F-3
|
|
|
F-4
|
|
|
F-5 – F-7
|
|
|
F-8 – F-9
|
|
|
F-10 – F-44
|

|
Fahn Kanne & Co.
Head Office
32 Hamasger Street
Tel-Aviv 6721118, ISRAEL
PO Box 36172, 6136101
T +972 3 7106666
F +972 3 7106660
www.gtfk.co.il
|
|
US dollars (except share data)
|
||||||||
|
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
A S S E T S
|
||||||||
|
Current Assets
|
||||||||
|
Cash and cash equivalents
|
148,836
|
608,701
|
||||||
|
Accounts receivable, net
|
92,061
|
18,446
|
||||||
|
Inventories (Note 3)
|
1,419,604
|
816,223
|
||||||
|
Other current assets (Note 4)
|
356,994
|
268,792
|
||||||
|
Total current assets
|
2,017,495
|
1,712,162
|
||||||
|
Property and Equipment, Net (Note 5)
|
240,452
|
220,463
|
||||||
|
Long-Term Restricted Cash
|
35,673
|
35,152
|
||||||
|
Funds in Respect of Employee Rights Upon Retirement
|
167,326
|
164,883
|
||||||
|
Total assets
|
2,460,946
|
2,132,660
|
||||||
|
LIABILITIES, TEMPORARY EQUITY AND STOCKHOLDERS’ DEFICIT
|
||||||||
|
Current Liabilities
|
||||||||
|
Accounts payable
|
1,634,642
|
1,082,546
|
||||||
|
Other current liabilities (Note 6)
|
713,549
|
427,886
|
||||||
|
Total current liabilities
|
2,348,191
|
1,510,432
|
||||||
|
Long-Term Liabilities
|
||||||||
|
Long-Term Loans from Stockholders (Note 8)
|
162,034
|
160,314
|
||||||
|
Liability for Employee Rights Upon Retirement (Note 2J)
|
176,719
|
174,137
|
||||||
|
Warrants with Down-Round Protection (Note 10E)
|
681,970
|
321,695
|
||||||
|
Total long-term liabilities
|
1,020,723
|
656,146
|
||||||
|
Total liabilities
|
3,368,914
|
2,166,578
|
||||||
|
Commitments and Contingent Liabilities (Note 9)
|
||||||||
|
Temporary Equity (Note 10.A)
|
||||||||
|
Convertible Preferred Stock of $ 0.001 par value ("Preferred Stock"):
|
||||||||
|
10,000,000 shares of Preferred Stock authorized as of December 31, 2016, and as of December 31, 2015
|
||||||||
|
Preferred Stock Series A issued and outstanding 376 shares as of December 31, 2016, and 376 shares as of December 31, 2015
|
221,152
|
221,152
|
||||||
|
Preferred Stock Series B issued and outstanding 15,031 shares as of December 31, 2016, and 15,031 shares as of December 31, 2015
|
6,715,844
|
6,715,844
|
||||||
|
Preferred Stock Series C issued and outstanding 5,829 shares as of December 31, 2016, and 0 shares as of December 31, 2015
|
3,104,466
|
-
|
||||||
|
Total Temporary Equity
|
10,041,462
|
6,936,996
|
||||||
|
Stockholders' Deficit
|
||||||||
|
Common Stock of $ 0.001 par value ("Common Stock"):
|
||||||||
|
40,000,000 shares authorized as of December 31, 2016, and December 31, 2015; issued and outstanding 6,026,527 shares and 5,690,097 shares as of December 31, 2016, and December 31, 2015, respectively
|
6,028
|
5,691
|
||||||
|
Additional paid in capital
|
24,586,142
|
22,309,742
|
||||||
|
Accumulated other comprehensive income
|
62,576
|
90,168
|
||||||
|
Accumulated deficit
|
(35,604,176
|
)
|
(29,376,515
|
)
|
||||
|
Total stockholders' deficit
|
(10,949,430
|
)
|
(6,970,914
|
)
|
||||
|
Total liabilities, temporary equity and stockholders’ deficit
|
2,460,946
|
2,132,660
|
||||||
|
US dollars (except share data)
|
||||||||||||
|
Year ended December 31,
|
||||||||||||
|
2016
|
2015
|
2014
|
||||||||||
|
Revenues
|
611,689
|
143,167
|
59,775
|
|||||||||
|
Research and development expenses (Note 11)
|
2,881,817
|
2,268,345
|
1,849,624
|
|||||||||
|
Selling and Marketing expenses (Note 12)
|
1,127,915
|
1,127,434
|
230,759
|
|||||||||
|
General and administrative expenses (Note 13)
|
2,257,799
|
1,402,741
|
1,586,751
|
|||||||||
|
Total operating expenses
|
6,267,531
|
4,798,520
|
3,667,134
|
|||||||||
|
Operating loss
|
5,655,842
|
4,655,353
|
3,607,359
|
|||||||||
|
Financing (income) expenses, net (Note 14)
|
(246,105
|
)
|
1,186,819
|
(6,587,785
|
)
|
|||||||
|
Income (loss) for the period
|
(5,409,737
|
)
|
(5,842,172
|
)
|
2,980,426
|
|||||||
|
Other comprehensive income (loss):
|
||||||||||||
|
Foreign currency translation adjustment
|
(27,592
|
)
|
23,498
|
13,968
|
||||||||
|
Comprehensive income (loss) for the period
|
(5,437,329
|
)
|
(5,818,674
|
)
|
2,994,394
|
|||||||
|
Income (loss) per share (Basic) (Note 16)
|
(1.08
|
)
|
(1.15
|
)
|
0.37
|
|||||||
|
Income (loss) per share (Diluted) (Note 16)
|
(1.08
|
)
|
(1.15
|
)
|
0.37
|
|||||||
|
Common shares used in computing Basic income (loss) per share (Note 16)
|
5,788,842
|
5,476,870
|
5,304,500
|
|||||||||
|
Common shares used in computing Diluted income (loss) per share (Note 16)
|
5,788,842
|
5,476,870
|
5,349,242
|
|||||||||
|
US Dollars (except share data)
|
||||||||||||||||||||||||
|
Common Stock
|
Accumulated
other
|
|
Total
|
|||||||||||||||||||||
|
Number
of shares |
Amount
|
Additional paid
in capital
|
comprehensive
income
|
Accumulated
deficit
|
stockholders’
deficit
|
|||||||||||||||||||
|
Balance as of January 1, 2014
|
5,301,693
|
5,302
|
14,532,068
|
52,702
|
(25,653,190
|
)
|
(11,063,118
|
)
|
||||||||||||||||
|
Income for the year
|
-
|
-
|
-
|
-
|
2,980,426
|
2,980,426
|
||||||||||||||||||
|
Other comprehensive income
|
-
|
-
|
-
|
13,968
|
-
|
13,968
|
||||||||||||||||||
|
Amounts allocated to Series B-1 and Series B-2 Warrants, net
|
-
|
-
|
3,320,429
|
-
|
-
|
3,320,429
|
||||||||||||||||||
|
Amount classified out of stockholders deficit and presented as Warrants with Down-Round Protection within long-term liabilities
|
-
|
-
|
(400,671
|
)
|
-
|
-
|
(400,671
|
)
|
||||||||||||||||
|
Conversion of Series A Preferred Stock into Common Stock
|
1,725
|
2
|
5,886
|
-
|
-
|
5,888
|
||||||||||||||||||
|
Warrants issued as consideration for placement services
|
-
|
-
|
630,936
|
-
|
-
|
630,936
|
||||||||||||||||||
|
Stock dividend to certain Common Stock holders
|
654
|
1
|
(1
|
)
|
-
|
-
|
-
|
|||||||||||||||||
|
Stock dividend on Series B Preferred Stock
|
18,986
|
19
|
43,839
|
-
|
(43,858
|
)
|
-
|
|||||||||||||||||
|
Cash dividend on Series A Preferred Stock
|
-
|
-
|
-
|
-
|
(370,441
|
)
|
(370,441
|
)
|
||||||||||||||||
|
Stock-based compensation
|
-
|
-
|
50,380
|
-
|
-
|
50,380
|
||||||||||||||||||
|
Balance as of December 31, 2014
|
5,323,058
|
5,324
|
18,182,866
|
66,670
|
(23,087,063
|
)
|
(4,832,203
|
)
|
||||||||||||||||
|
The accompanying notes are an integral part of the consolidated financial statements.
|
|
US Dollars (except share data)
|
||||||||||||||||||||||||
|
Common Stock
|
Accumulated
other
|
|
Total
|
|||||||||||||||||||||
|
Number
of shares |
Amount
|
Additional paid
in capital
|
comprehensive
income
|
Accumulated
deficit
|
stockholders’
deficit
|
|||||||||||||||||||
|
Balance as of January 1, 2015
|
5,323,058
|
5,324
|
18,182,866
|
66,670
|
(23,087,063
|
)
|
(4,832,203
|
)
|
||||||||||||||||
|
Loss for the year
|
-
|
-
|
-
|
-
|
(5,842,172
|
)
|
(5,842,172
|
)
|
||||||||||||||||
|
Other comprehensive income
|
-
|
-
|
-
|
23,498
|
-
|
23,498
|
||||||||||||||||||
|
Issuance of Series B-1 and Series B-2 Warrants
|
-
|
-
|
3,445,337
|
-
|
-
|
3,445,337
|
||||||||||||||||||
|
Conversion of Series A and Series B Preferred Stock into Common Stock
|
86,208
|
86
|
237,451
|
-
|
-
|
237,537
|
||||||||||||||||||
|
Stock dividend to certain Common Stock holders
|
92,136
|
92
|
(92
|
)
|
-
|
-
|
-
|
|||||||||||||||||
|
Stock dividend on Series B Preferred Stock
|
168,926
|
169
|
390,050
|
-
|
(390,219
|
)
|
-
|
|||||||||||||||||
|
Cash dividend on Series A Preferred Stock
|
-
|
-
|
-
|
-
|
(57,061
|
)
|
(57,061
|
)
|
||||||||||||||||
|
Exercise of employees’ stock options
|
19,769
|
20
|
36,117
|
-
|
-
|
36,137
|
||||||||||||||||||
|
Stock-based compensation
|
-
|
-
|
18,013
|
-
|
-
|
18,013
|
||||||||||||||||||
|
Balance as of December 31, 2015
|
5,690,097
|
5,691
|
22,309,742
|
90,168
|
(29,376,515
|
)
|
(6,970,914
|
)
|
||||||||||||||||
|
The accompanying notes are an integral part of the consolidated financial statements.
|
|
US Dollars (except share data)
|
||||||||||||||||||||||||
|
Common Stock
|
Accumulated
other
|
|
Total
|
|||||||||||||||||||||
|
Number
of shares |
Amount
|
Additional paid
in capital
|
comprehensive
income
|
Accumulated
deficit
|
stockholders’
deficit
|
|||||||||||||||||||
|
Balance as of January 1, 2016
|
5,690,097
|
5,691
|
22,309,742
|
90,168
|
(29,376,515
|
)
|
(6,970,914
|
)
|
||||||||||||||||
|
Loss for the year
|
-
|
-
|
-
|
-
|
(5,409,737
|
)
|
(5,409,737
|
)
|
||||||||||||||||
|
Other comprehensive loss
|
-
|
-
|
-
|
(27,592
|
)
|
-
|
(27,592
|
)
|
||||||||||||||||
|
Amounts allocated to Series C-1 and Series C-2 Warrants, net
|
-
|
-
|
1,537,380
|
-
|
-
|
1,537,380
|
||||||||||||||||||
|
Amount classified out of stockholders’ deficit and presented as Warrants with Down-Round Protection within long-term liabilities
|
-
|
-
|
(341,662
|
)
|
-
|
-
|
(341,662
|
)
|
||||||||||||||||
|
Incremental fair market value adjustments of modified warrants issued to placement agent
|
-
|
-
|
211,077
|
-
|
-
|
211,077
|
||||||||||||||||||
|
Stock dividend on Series C Preferred Stock
|
64,148
|
65
|
152,415
|
-
|
(152,480
|
)
|
-
|
|||||||||||||||||
|
Stock dividend on Series B Preferred Stock
|
272,282
|
272
|
646,943
|
-
|
(647,215
|
)
|
-
|
|||||||||||||||||
|
Cash dividend on Series A Preferred Stock
|
-
|
-
|
-
|
-
|
(18,229
|
)
|
(18,229
|
)
|
||||||||||||||||
|
Stock-based compensation
|
-
|
-
|
70,247
|
-
|
70,247
|
|||||||||||||||||||
|
Balance as of December 31, 2016,
|
6,026,527
|
6, 028
|
24,586,142
|
62,576
|
(35,604,176
|
)
|
(10,949,430
|
)
|
||||||||||||||||
|
The accompanying notes are an integral part of the consolidated financial statements.
|
|
US dollars
|
||||||||||||
|
Year ended December 31,
|
||||||||||||
|
2016
|
2015
|
2014
|
||||||||||
|
Cash flows from operating activities:
|
||||||||||||
|
Income (loss) for the year
|
(5,409,737
|
)
|
(5,842,172
|
)
|
2,980,426
|
|||||||
|
Adjustments to reconcile income (loss) for the year to net cash used in operating activities:
|
||||||||||||
|
Depreciation
|
59,584
|
44,891
|
34,683
|
|||||||||
|
Stock-based compensation
|
70,247
|
18,013
|
50,380
|
|||||||||
|
Incremental fair market value adjustments of modified warrants issued to placement agent
|
211,077
|
-
|
-
|
|||||||||
|
Change in the fair value of warrants issued with down-round protection
|
(289,626
|
)
|
(149,092
|
)
|
(6,559,758
|
)
|
||||||
|
Linkage difference on principal of loans from stockholders
|
(629
|
)
|
(2,521
|
)
|
(556
|
)
|
||||||
|
Loss on partial extinguishment of Series A Preferred Stock and Series A Warrants
|
-
|
1,284,354
|
-
|
|||||||||
|
Changes in assets and liabilities:
|
||||||||||||
|
Decrease (increase) in accounts receivable
|
(73,440
|
)
|
4,131
|
(24,539
|
)
|
|||||||
|
Increase in inventory
|
(590,616
|
)
|
(737,554
|
)
|
(94,895
|
)
|
||||||
|
Increase in other current assets
|
(85,415
|
)
|
(156,302
|
)
|
(32,416
|
)
|
||||||
|
Increase in accounts payable
|
526,560
|
982,215
|
68,838
|
|||||||||
|
Increase (decrease) in other current liabilities
|
270,165
|
(11,187
|
)
|
216,192
|
||||||||
|
Net cash used in operating activities
|
(5,311,830
|
)
|
(4,565,224
|
)
|
(3,361,645
|
)
|
||||||
|
Cash flows from investment activities:
|
||||||||||||
|
Increase in funds in respect of employee rights upon retirement
|
-
|
(24,279
|
)
|
(28,058
|
)
|
|||||||
|
Purchase of property and equipment
|
(76,455
|
)
|
(143,736
|
)
|
(63,455
|
)
|
||||||
|
Increase in long-term restricted cash
|
-
|
(35,152
|
)
|
-
|
||||||||
|
Net cash used in investment activities
|
(76,455
|
)
|
(203,167
|
)
|
(91,513
|
)
|
||||||
|
Cash flows from financing activities
|
||||||||||||
|
Cash dividend on Series A Preferred Stock
|
`(13,529
|
) |
(57,061
|
)
|
(370,441
|
)
|
||||||
|
Proceeds allocated to Series B Preferred Stock, net of cash issuance expenses
|
-
|
-
|
3,710,860
|
|||||||||
|
Proceeds allocated to Series B Warrants, net of cash issuance expenses
|
-
|
-
|
3,632,531
|
|||||||||
|
Proceeds allocated to Series C Preferred Stock, net of cash issuance expenses
|
3,310,617
|
-
|
-
|
|||||||||
|
Proceeds allocated to Series C Warrants, net of cash issuance expenses
|
1,639,468
|
-
|
-
|
|||||||||
|
Proceeds from exercise of employees’ stock options
|
-
|
36,137
|
-
|
|||||||||
|
Repayment of loan from stockholders
|
-
|
(439,939
|
)
|
-
|
||||||||
|
Net cash provided by (used in) financing activities
|
4,936,556
|
(460,863
|
)
|
6,972,950
|
||||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
(8,136
|
)
|
10,395
|
(78,143
|
)
|
|||||||
|
Increase (decrease) in cash and cash equivalents
|
(459,865
|
)
|
(5,218,859
|
)
|
3,441,649
|
|||||||
|
Cash and cash equivalents at beginning of the year
|
608,701
|
5,827,560
|
2,385,911
|
|||||||||
|
Cash and cash equivalents at end of the year
|
148,836
|
608,701
|
5,827,560
|
|||||||||
|
NOTE 1 -
|
GENERAL
|
| A. |
Integrity Applications, Inc. (the "Company") was incorporated on May 18, 2010 under the laws of the State of Delaware. On July 15, 2010, Integrity Acquisition Corp. Ltd. (hereinafter: "Integrity Acquisition"), a wholly owned Israeli subsidiary of the Company, which was established on May 23, 2010, completed a merger with A.D. Integrity Applications Ltd. (hereinafter: "Integrity Israel"), an Israeli corporation that was previously held by the stockholders of the Company. Pursuant to the merger, all equity holders of Integrity Israel received the same proportional ownership in the Company as they had in Integrity Israel prior to the merger. Following the merger, Integrity Israel remained a wholly-owned subsidiary of the Company. As the merger transaction constituted a structural reorganization, the merger has been accounted for at historical cost in a manner similar to a pooling of interests. Integrity Israel was incorporated in 2001 and commenced its operations in 2002. Integrity Israel, a medical device company, focuses on the design, development and commercialization of non-invasive glucose monitoring devices for use by people with diabetes.
|
| B. |
Going concern uncertainty and management plans
|
|
NOTE 1 –
|
GENERAL (cont.)
|
| C. |
Risk factors
|
|
NOTE 2 –
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
|
| A. |
Use of estimates in the preparation of financial statements
|
| B. |
Functional currency
|
|
Year ended December 31,
|
||||||||||||
|
2016
|
2015
|
2014
|
||||||||||
|
Official exchange rate of NIS 1 to US dollar
|
0.260
|
0.256
|
0.257
|
|||||||||
|
NOTE 2 –
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
|
| C. |
Principles of consolidation
|
| D. |
Cash and cash equivalents
|
| E. |
Inventories
|
| F. |
Property and equipment, net
|
| 1. |
Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets. When an asset is retired or otherwise disposed of, the related carrying value and accumulated depreciation are removed from the respective accounts and the net difference less any amount realized from disposition is reflected in the statements of operations.
|
| 2. |
Rates of depreciation:
|
|
%
|
|
|
Computers
|
33
|
|
Furniture and office equipment
|
7-15
|
|
Leasehold improvements
|
Shorter of lease term
and 10 years |
| G. |
Impairment of long-lived assets
|
|
NOTE 2 –
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
|
| H. |
Long-term restricted cash
|
| I. |
Income tax
|
| J. |
Liability for employee rights upon retirement
|
| K. |
Revenue recognition
|
|
NOTE 2 –
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
|
| L. |
Research and development expenses
|
| M. |
Royalty-bearing grants
|
| O. |
Basic and diluted income (loss) per share
|
| P. |
Stock-based compensation
|
|
NOTE 2 –
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
|
| Q. |
Fair value of financial instruments
|
| R. |
Concentrations of credit risk
|
| S. |
Contingencies
|
| 1. |
Temporary Equity Classification
|
|
NOTE 2 –
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
|
| 2. |
Initial Measurement
|
| 3. |
Subsequent Measurement
|
| 4. |
Conversion Feature Analysis
|
| 5. |
Modifications or Exchanges
|
|
NOTE 2 –
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
|
| V. |
Recently Issued Accounting Pronouncements
|
| 1. |
Accounting Standard Update 2014-09, “Revenue from Contracts with Customers”
|
|
NOTE 2 –
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
|
| V. |
Recently Issued Accounting Pronouncements (cont.)
|
| 2. |
Accounting Standards Update 2014-15, “Presentation of Financial Statements—Going Concern”
|
| 3. |
Accounting Standard Update 2014-16, “Determining Whether the Host Contract in a Hybrid Financial Instrument Issued in the Form of a Share Is More Akin to Debt or to Equity”
|
|
NOTE 2 –
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
|
| V. |
Recently Issued Accounting Pronouncements (cont.)
|
| 3. |
Accounting Standard Update 2014-16, “Determining Whether the Host Contract in a Hybrid Financial Instrument Issued in the Form of a Share Is More Akin to Debt or to Equity” (cont.)
|
| 4. |
Accounting Standard Update 2015-11, “Simplifying the Measurement of Inventory”
|
| W. |
Certain prior year amounts have been reclassified for consistency with the current year presentation. These reclassifications did not have material effect on the reported results of operations, shareholder’s deficit or cash flows
.
|
|
NOTE 3 –
|
INVENTORIES
|
|
US dollars
|
||||||||
|
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Raw materials
|
735,201
|
205,645
|
||||||
|
Work in process
|
633,132
|
551,111
|
||||||
|
Finished products
|
51,271
|
59,467
|
||||||
|
1,419,604
|
816,223
|
|||||||
| NOTE 4 – |
OTHER CURRENT ASSETS
|
|
US dollars
|
||||||||
|
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Prepaid expenses
|
90,563
|
57,206
|
||||||
|
Government Institution (*)
|
266,431
|
211,586
|
||||||
|
356,994
|
268,792
|
|||||||
| (*) |
Represents amounts advanced by Integrity Israel to the Israeli tax authorities or amounts owed to Integrity Israel by the Israeli Value Added Tax authorities.
|
|
US dollars
|
||||||||
|
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Computers
|
274,693
|
240,066
|
||||||
|
Furniture and office equipment
|
237,240
|
200,548
|
||||||
|
Leasehold improvements
|
44,686
|
32,898
|
||||||
|
556,619
|
473,512
|
|||||||
|
Less – accumulated depreciation
|
(316,167
|
)
|
(253,049
|
)
|
||||
|
240,452
|
220,463
|
|||||||
|
US dollars
|
||||||||
|
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Employees and related institutions
|
363,738
|
240,358
|
||||||
|
Accrued expenses and other
|
349,811
|
187,528
|
||||||
|
713,549
|
427,886
|
|||||||
| A. |
On March 4, 2004, the OCS provided Integrity Israel with a grant of approximately $93,300 (NIS 420,000), for its plan to develop a non-invasive blood glucose monitor (the “Development Plan”). Integrity Israel is required to pay royalties to the OCS at a rate ranging between 3-5% of the proceeds from the sale of the Group's products arising from the Development Plan up to an amount equal to $93,300, plus interest at LIBOR from the date of grant. As of December 31, 2016, the remaining contingent liability with respect to royalty payment on future sales equals approximately $69,330, excluding interest. Such contingent obligation has no expiration date.
|
| B. |
Until mid of December 2015, Integrity Israel leased approximately 3,100 sq. ft. of office space in the city of Ashkelon, Israel as its principal offices and prototype laboratory. Pursuant to a verbal agreement with the landlord, Integrity Israel leased this facility on a monthly basis at a cost of approximately $2,934 (NIS 11,500). Currently, Integrity Israel leases approximately 5,500 sq. ft. of office space in the city of Ashdod, Israel for its principal offices. The lease term began on December 1, 2015 for a period of 5 years which can be extended for an additional 5 years at the option of the Company. Monthly lease payments including maintenance approximate $10,000. The Company estimates that its minimal rent and maintenance payments will approximate $120,000 per year over each of the next 5 years. In connection with the lease agreement, Integrity Israel provided the landlord a bank guarantee in the amount of approximately $35,000 (NIS 137,162) that can be exercised by the landlord in the case Integrity Israel fails to pay the monthly rent payments. The guarantee is renewed on an annual basis for a period of 5 years and is secured by funds on deposit with the bank, which generally must be sufficient to cover the principal amount guarantee.
|
| C. |
During 2014, the Company engaged the Placement Agent in connection with an offering of up $25,000,000 of its securities, which engagement ultimately culminated in the issuance and sale of $8,500,000 Series B Units (the “2014 Offering”). Pursuant to a placement agent agreement with the Placement Agent entered into with respect to such private placement, the Placement Agent (or its sub-agents) was paid, as a commission, an amount equal to 6% of the funds raised in each, such offering, plus 4% of the funds as a management fee plus a 3% non-accountable expense allowance (13% in the aggregate), all in cash. In addition, pursuant to such placement agent agreement, the Company was required to and did issue to the Placement Agent (or its sub-agents) warrants to purchase up to 10% of the shares of Common Stock issued to investors (or underlying convertible securities issued to investors) in connection with such offerings at a price per share equal to the offering price subject to certain price adjustments. Based on the agreement signed in 2014, the Placement Agent will have a right of first refusal on any private equity, debt or rights offering for a period of 24 months from the date on which marketing efforts with respect to the 2014 offering began. In addition, pursuant to such agreement, the Company was required to use its reasonable efforts to have the Placement Agent named as a co-underwriter in any secondary public offering by the Company in the 12 months following completion of the 2014 offering. In addition, starting in September 2013, the Company retained the Placement Agent to provide certain advisory services for a monthly fee of $12,500 for the period starting September 2013 until December 2014 and $20,000 per month starting from January 2015.
|
| A. | 1 . | Description of the rights attached to the Common Stock |
| 2 . |
Description of the rights attached to the Series A Preferred Stock
|
| A. | 2 . | Description of the rights attached to the Series A Preferred Stock (cont.) |
| A. | 3 . | Description of the rights attached to the Series B Preferred Stock |
| A. | 3 . | Description of the rights attached to the Series B Preferred Stock (cont.) |
| A. | 4 . | Description of the rights attached to the Series C Preferred Stock |
| A. | 4 . | Description of the rights attached to the Series C Preferred Stock (cont.) |
| B. |
The 2012 Offering
|
| C. |
The 2014 Offering
|
| C. |
The 2014 Offering (cont.)
|
| D. |
The 2016 Offering
|
| D. |
The 2016 Offering (cont.)
|
| E. |
Warrants with down round protection
|
| E. |
Warrants with down round protection (cont.)
|
|
Warrants with
Down-Round Protection
|
||||||||
|
December 31,
|
||||||||
|
2016
|
2015
|
|||||||
|
Balance, Beginning of the year
|
321,695
|
2,057,618
|
||||||
|
Warrants issued as consideration for placement services
|
308,239
|
-
|
||||||
|
Amount classified out of stockholders’ deficit and presented as Warrants with Down-Round Protection
|
341,662
|
-
|
||||||
|
Exchange of Series A Warrants pursuant to the “Most favored nation” provision
|
(1,586,831
|
)
|
||||||
|
Change in fair value of Warrants with Down-Round Protection
|
(289,626
|
)
|
(149,092
|
)
|
||||
|
Balance, End of year
|
681,970
|
321,695
|
||||||
|
December 31,
2016 |
December 31,
2015 |
|||||||
|
Dividend yield (%)
|
-
|
-
|
||||||
|
Expected volatility (%) (*)
|
56.59
|
105.14
|
||||||
|
Risk free interest rate (%)
|
0.92
|
0.99
|
||||||
|
Expected term of options (years) (**)
|
1.20
|
2.20
|
||||||
|
Exercise price (US dollars)
|
4.50
|
5.80
|
||||||
|
Share price (US dollars) (***)
|
2.38
|
2.31
|
||||||
|
Fair value (US dollars)
|
0.16
|
0.84
|
||||||
| (*) |
Due to the low trading volume of the Company’s Common Stock, the expected volatility was based on the historical volatility of the share price of other public companies that operate in the same industry sector as the Company.
|
| (**) |
Due to the fact that the Company does not have sufficient historical exercise data, the expected term was determined based on the "simplified method" in accordance with Staff Accounting Bulletin No. 110.
|
| (***) |
The Common Stock price, per share reflects the Company’s management’s estimation of the fair value per share of Common Stock as of December 31, 2016, and 2015. In reaching its estimation for such periods, management considered, among other things, a valuation prepared by a third-party valuation firm following the issuance of the Series C Units at December 31, 2016, as applicable to each reporting period.
|
| F. |
Stock-based compensation
|
| 1. |
Grants to non-employees
|
| a. |
In August 2005, Integrity Israel granted 21,640 options with an exercise price of $0.001 per share in consideration of regulatory services. In October 2006, Integrity Israel granted 45,531 options with an exercise price of $4.305 per share in consideration of investor finders, of which 17,657 were forfeited. In November 2008, Integrity Israel granted 8,989 options with an exercise price of $5.517 per share in consideration of investor finders, of which 5,365 were forfeited.
|
| b. |
In connection with the 2010 Offering, the Company issued to the Placement Agent warrants to purchase 45,097 and 84,459 shares of the Company's Common Stock, with an exercise price of $6.25 per share, in 2011 and 2010, respectively. The warrants expire on the fifth anniversary of the date on which the shares of Common Stock underlying such warrants are fully registered with the SEC (See Note 9C regarding the extension of the warrants). The warrants include customary adjustment provisions for stock splits, reorganizations and other similar transactions and in addition a down-round protection provision. As a result of the issuance of the Series B Units, pursuant to the terms of the warrants, on August 29, 2014, the exercise price per share of the applicable warrants decreased from $6.25 per share to $5.80 per share and the number of shares of Common Stock issuable upon exercise of each such warrant, in the aggregate, increased to 139,608. As a result of the initial issuance and sale of the Series C Units, on April 8, 2016, the exercise price per share of the Warrants decreased again from $5.80 per share to $4.50 per share and the number of shares of Common Stock issuable upon exercise of each warrants, in the aggregate, increased to 179,939.
|
| c. |
In connection with the 2012 Offering, the Company issued to the Placement Agent (a) 5 year warrants to purchase up to 128,277 shares of Common Stock at an exercise price of $5.80 per share and (b) 5 year warrants to purchase up to 128,277 shares of Common Stock at an exercise price of $6.96 per share and (c) 5 year warrants to purchase up to 215 shares of Common Stock at an exercise price of $7.00 per share. Such warrants have substantially the same terms as those issued to the Series A Unit Purchasers except that the Placement Agent warrants may also be exercisable on a cashless basis at all times. As a result of the issuance of the Series B Units, pursuant to the terms of the warrants, on August 29, 2014, the exercise price per share of the applicable warrants decreased from $6.96 and $7.00 per share to $5.80 per share and the number of shares of Common Stock issuable upon exercise of each such warrant, in the aggregate, increased such that the aggregate exercise price payable thereunder, after taking into account the decrease in the exercise price, will be equal to the aggregate exercise price prior to such adjustment. As a result of the initial issuance and sale of the Series C Units, on April 8, 2016, the exercise price per share of the Warrants decreased again from $5.80 per share to $4.50 per share and the number of shares of Common Stock issuable upon exercise of each Warrants, in the aggregate, increased such that the aggregate exercise price payable thereunder, after taking into account the decrease in the exercise price, will be equal to the aggregate exercise price prior to such adjustment. As of December 31, 2016, and 2015 the Placement Agent was entitled to an aggregate of 364,071 and 282,469 shares of Common Stock, respectively, at an exercise price of $4.50 in connection with the 2012 offering. In addition, as of December 31, 2016, the placement agent was also entitled to 42,549 shares of Common Stock, at an exercise price of $7.75 in connection with the 2012 offering.
|
| F. |
Stock-based compensation (cont.)
|
| 1. |
Grants to non-employees (cont.)
|
| d. |
In connection with the 2014 Offering, the Company issued to the Placement Agent (a) 5 year warrants to purchase up to 293,115 shares of Common Stock at an exercise price of $5.80 per share and (b) 5 year warrants to purchase up to 146,559 shares of Common Stock at an exercise price of $10.00 per share. The terms of the Placement Agent warrants are substantially similar to the terms of the Series B Warrants except that the Placement Agent warrants may also be exercisable on a cashless basis at all times. The average fair value per warrant of approximately $1.40 was estimated using the following assumptions: dividend yield of 0%, expected volatility of 105.14%, risk free interest rate of 1.66%, stock price of $2.31 and exercise price of $5.80 and 10.00, as applicable.
|
| e. |
In connection with the 2016 Offering, the Company issued to the Placement Agent (a) 5 year warrants to purchase up to 259,068 shares of Common Stock at an exercise price of $4.50 per share and (b) 5 year warrants to purchase up to 129,533 shares of Common Stock at an exercise price of $7.75 per share. The terms of the Placement Agent warrants are substantially similar to the terms of the Series C Warrants except that the Placement Agent warrants may also be exercisable on a cashless basis at all times. The average fair value per warrant of approximately $0.79 was estimated using the following assumptions: dividend yield of 0%, expected volatility of 62.16%, risk free interest rate of 1.21%, stock price of $2.38 and exercise price of $4.50 and $7.75, as applicable.
|
| 2. |
Grants to employees
|
| F. |
Stock-based compensation (cont.)
|
| 2. |
Grants to employees (cont.)
|
|
Number
|
Weighted
average
exercise
price (US$)
|
|||||||
|
Year ended December 31, 2016
|
||||||||
|
Balance outstanding at beginning of year
|
413,473
|
6.04
|
||||||
|
Granted
|
149,330
|
4.53
|
||||||
|
Balance outstanding at end of the year
|
562,803
|
5.64
|
||||||
|
Balance exercisable at the end of the year
|
334,735
|
5.81
|
||||||
| F. |
Stock-based compensation (cont.)
|
| 2. |
Grants to employees (cont.)
|
|
Number
|
Weighted
average
exercise
price (US$)
|
|||||||
|
Year ended December 31, 2015
|
||||||||
|
Balance outstanding at beginning of year
|
450,847
|
5.85
|
||||||
|
Exercised
|
(19,769
|
)
|
1.82
|
|||||
|
Forfeited
|
(17,605
|
)
|
6.01
|
|||||
|
Balance outstanding at end of the year
|
413,473
|
6.04
|
||||||
|
Balance exercisable at the end of the year
|
293,736
|
5.95
|
||||||
|
Number
|
Weighted
average
exercise
price (US$)
|
|||||||
|
Year ended December 31, 2014
|
||||||||
|
Balance outstanding at beginning of year
|
414,847
|
5.74
|
||||||
|
Granted
|
44,000
|
7.00
|
||||||
|
Forfeited
|
(8,000
|
)
|
6.25
|
|||||
|
Balance outstanding at end of the year
|
450,847
|
5.85
|
||||||
|
Balance exercisable at the end of the year
|
302,360
|
5.58
|
||||||
|
Exercise
price (US$) |
Outstanding at
December 31, 2016
|
Weighted
average
remaining
contractual
life (years)
|
Exercise
price (US$) |
Exercisable at
December 31, 2016
|
Weighted
average
remaining
contractual
life (years)
|
|||||||||||||||||
|
1.72
|
21,758
|
0.60
|
1.72
|
21,758
|
0.60
|
|||||||||||||||||
|
3.63
|
3,730
|
0.60
|
3.63
|
3,730
|
0.60
|
|||||||||||||||||
|
6.01
|
4,273
|
1.03
|
6.01
|
4,273
|
1.03
|
|||||||||||||||||
|
6.25
|
351,712
|
5.19
|
6.25
|
236,975
|
5.19
|
|||||||||||||||||
|
7.00
|
32,000
|
7.50
|
7.00
|
32,000
|
7.50
|
|||||||||||||||||
|
4.75
|
16,000
|
9.01
|
4.75
|
6,000
|
9.01
|
|||||||||||||||||
|
4.50
|
79,998
|
9.21
|
4.50
|
30,000
|
9.21
|
|||||||||||||||||
|
4.50
|
53,332
|
9.88
|
4.50
|
-
|
9.88
|
|||||||||||||||||
|
562,803
|
334,736
|
|||||||||||||||||||||
| F. |
Stock-based compensation (cont.)
|
| 2. |
Grants to employees (cont.)
|
|
2016
|
2015
|
2014
|
||||||||||
|
Dividend yield (%)
|
0
|
-
|
0
|
|||||||||
|
Expected volatility (%) (*)
|
62.16
|
-
|
105.14
|
|||||||||
|
Risk free interest rate (%)
|
1.08
|
-
|
1.61
|
|||||||||
|
Expected term of options (years) (**)
|
6
|
-
|
5-6
|
|||||||||
|
Exercise price (US dollars)
|
4.50, 4.75
|
-
|
7.00
|
|||||||||
|
Stock price (US dollars) (***)
|
2.38
|
-
|
2.31
|
|||||||||
|
Fair value (US dollars)
|
1.01, 098
|
-
|
1.59
|
|||||||||
| (*) |
Due to the low trading volume of the Company’s Common Stock, the expected volatility was based on the historical volatility of the share price of other public companies that operate in the same industry sector as the Company.
|
|
(**)
|
Due to the fact that the Company does not have sufficient historical exercise data, the expected term was determined based on the "simplified method" in accordance with Staff Accounting Bulletin No. 110.
|
| (***) |
The Common Stock price, per share for the year ended December 31, 2014 and 2016 reflects the Company’s management’s estimation of the fair value per share of Common Stock. In reaching its estimation for December 31, 2014, management considered, among other things, a valuation prepared by a third-party valuation firm following the issuance of the 2014 Offering.
In reaching its estimation for December 31, 2016, management considered, among other things, a valuation prepared by a third-party valuation firm following the issuance of the Series C Units.
|
|
US dollars
|
||||||||||||
|
Year ended December 31,
|
||||||||||||
|
2016
|
2015
|
2014
|
||||||||||
|
Salaries and related expenses
|
1,510,491
|
1,279,216
|
992,991
|
|||||||||
|
Professional fees
|
58,954
|
71,930
|
83,597
|
|||||||||
|
Regulations related expenses
|
620,535
|
488,536
|
413,985
|
|||||||||
|
Patents
|
132,344
|
159,624
|
188,825
|
|||||||||
|
Materials
|
346,238
|
153,669
|
98,129
|
|||||||||
|
Depreciation
|
32,508
|
19,133
|
16,156
|
|||||||||
|
Travel expenses
|
66,211
|
39,324
|
27,867
|
|||||||||
|
Vehicle maintenance
|
91,935
|
54,936
|
25,888
|
|||||||||
|
Other
|
22,601
|
1,977
|
2,186
|
|||||||||
|
2,881,817
|
2,268,345
|
1,849,624
|
||||||||||
|
US dollars
|
||||||||||||
|
Year ended December 31,
|
||||||||||||
|
2016
|
2015
|
2014
|
||||||||||
|
Salaries and related expenses
|
707,111
|
318,716
|
-
|
|||||||||
|
Professional fees
|
271,984
|
416,575
|
132,504
|
|||||||||
|
Travel & expenses
|
76,022
|
178,167
|
26,943
|
|||||||||
|
Exhibitions and Shows
|
72,798
|
175,176
|
49,084
|
|||||||||
|
Other
|
-
|
38,800
|
22,228
|
|||||||||
|
1,127,915
|
1,127,434
|
230,759
|
||||||||||
|
US dollars
|
||||||||||||
|
Year ended December 31,
|
||||||||||||
|
2016
|
2015
|
2014
|
||||||||||
|
Salaries and related expenses
|
938,203
|
584,058
|
548,323
|
|||||||||
|
Professional fees
|
1,046,987
|
573,815
|
749,167
|
|||||||||
|
Travel & expenses
|
130,533
|
114,783
|
173,405
|
|||||||||
|
Depreciation
|
27,076
|
25,759
|
18,527
|
|||||||||
|
Insurance
|
63,182
|
63,146
|
56,401
|
|||||||||
|
Vehicle maintenance
|
51,818
|
41,180
|
40,087
|
|||||||||
|
Other
|
-
|
-
|
841
|
|||||||||
|
2,257,799
|
1,402,741
|
1,586,751
|
||||||||||
|
US dollars
|
||||||||||||
|
Year ended December 31,
|
||||||||||||
|
2016
|
2015
|
2014
|
||||||||||
|
Israeli CPI linkage difference on principal of loans from stockholders
|
(629
|
)
|
(2,521
|
)
|
(556
|
)
|
||||||
|
Exchange rate differences
|
27,934
|
38,873
|
(25,282
|
)
|
||||||||
|
Change in fair value of Warrants with down-round protection
|
(289,626
|
)
|
(149,092
|
)
|
(6,559,758
|
)
|
||||||
|
Interest expenses on credit from banks and other
|
16,216
|
15,205
|
(2,189
|
)
|
||||||||
|
Loss on partial extinguishment of Series A Preferred Stock and Series A Warrants
|
-
|
1,284,354
|
-
|
|||||||||
|
(246,105
|
)
|
1,186,819
|
(6,587,785
|
)
|
||||||||
| A. |
Measurement of results for tax purposes under the Israeli Income Tax (Inflationary Adjustments) Law, 1985 (the “Inflationary Adjustment Law”)
|
| B. |
Changes in the Israeli corporate tax rates
|
| C. |
Tax assessments
|
| D. |
Carryforward tax losses
|
| E. |
The following is a reconciliation between the theoretical tax on pre-tax income, at the tax rate applicable to the Company (federal tax rate) and the tax expense reported in the financial statements:
|
|
US dollars
|
||||||||||||
|
Year ended December 31,
|
||||||||||||
|
2016
|
2015
|
2014
|
||||||||||
|
Pretax income (loss)
|
(5,409,737
|
)
|
(5,842,172
|
)
|
2,980,426
|
|||||||
|
Federal tax rate
|
34
|
%
|
34
|
%
|
35
|
%
|
||||||
|
Income tax expenses (benefit) computed at the ordinary tax rate
|
(1,839,311
|
)
|
(1,986, 338
|
)
|
1,043,149
|
|||||||
|
Non-deductible expenses
|
34,500
|
31,050
|
27,250
|
|||||||||
|
Stock-based compensation
|
17,562
|
4,503
|
14,415
|
|||||||||
|
Warrants with down round protection
|
(98,473
|
)
|
(50,691
|
)
|
(2,295,915
|
)
|
||||||
|
Loss on partial extinguishment of Series A Preferred Stock and Series A Warrants
|
-
|
436,680
|
-
|
|||||||||
|
Tax in respect of differences in corporate tax rates
|
396,956
|
340,263
|
278,466
|
|||||||||
|
Losses and timing differences in respect of which no deferred taxes assets were recognized
|
1,488,766
|
1,224,533
|
932,635
|
|||||||||
|
-
|
-
|
-
|
||||||||||
| F. |
Deferred taxes result principally from temporary differences in the recognition of certain revenue and expense items for financial and income tax reporting purposes. Significant components of the Group's future tax assets are as follows:
|
|
US dollars
|
||||||||||||
|
December 31,
|
||||||||||||
|
2016
|
2015
|
2014
|
||||||||||
|
Composition of deferred tax assets:
|
||||||||||||
|
Provision for employee-related obligation
|
28,526
|
23,494
|
20,220
|
|||||||||
|
Non-capital loss carry forwards
|
7,823,828
|
6,233,314
|
4,810,780
|
|||||||||
|
Valuation allowance
|
(7,852,354
|
)
|
(6,256,808
|
)
|
(4,831,000
|
)
|
||||||
|
-
|
-
|
-
|
||||||||||
|
US dollars
|
||||||||||||
|
Year ended December 31,
|
||||||||||||
|
2016
|
2015
|
2014
|
||||||||||
|
Income (loss) for the year
|
(5,409,737
|
)
|
(5,842,172
|
)
|
2,980,426
|
|||||||
|
Cash dividend on Series A Preferred Stock
|
(18,229
|
)
|
(57,061
|
)
|
(370,441
|
)
|
||||||
|
Stock dividend on Series B Preferred Stock
|
(647,215
|
)
|
(390,219
|
)
|
(43,858
|
)
|
||||||
|
Stock dividend on Series C Preferred Stock
|
(152,480
|
)
|
-
|
-
|
||||||||
|
Income attributable to participating securities (Preferred Stock)
|
-
|
-
|
(596,472
|
)
|
||||||||
|
Income (loss) for the period attributable to common stockholders
|
(6,227,661
|
)
|
(6,289,452
|
)
|
1,969,655
|
|||||||
|
Number of shares
|
||||||||||||
|
Year ended December 31,
|
||||||||||||
|
2016
|
2015
|
2014
|
||||||||||
|
Common shares used in computing Basic income (loss) per share
|
5,788,842
|
5,476,870
|
5,304,500
|
|||||||||
|
Common shares used in computing Diluted income (loss) per share
(*)
|
5,788,842
|
5,476, 870
|
5,349,242
|
|||||||||
|
Total weighted average number of Common shares related to outstanding convertible Preferred Stock, options and warrants excluded from the calculations of diluted income (loss) per share (**)
|
12,745,874
|
9,431,728
|
4,557,612
|
|||||||||
| (*) |
In applying the treasury method, the average market price of Common Stock was based on management estimate. For
December 31, 2016, management considered, among other things, a valuation prepared by a third-party valuation firm following the issuance of the Series C Units.
For December 31, 2015 and 2014, management estimation considered, among other things, a valuation prepared by a third-party valuation firm following the issuance of the Series B Units (See Note 10C).
|
| (**) |
The Company excludes from the calculation of diluted income (loss) per share, shares that will be issued upon the exercise of options and warrants with exercise prices, that are greater than the estimated average market value of the Company’s Common Stock
and shares issuable upon conversion of Preferred Stock because their effect would be anti-dilutive. Outstanding shares that will be issued upon conversion or exercise, as applicable, of all convertible Preferred Stock, stock options and warrants, have been excluded from the calculation of the diluted net loss per share for all the reported periods for which net loss was reported because the effect of the common shares issuable as a result of the exercise or conversion of these instruments was anti-dilutive.
|
|
Year ended December 31,
|
||||||||||||
|
Revenues based on the customer’s location:
|
2016
|
2015
|
2014
|
|||||||||
|
Europe
|
431,772
|
13,420
|
32,000
|
|||||||||
|
Asia and Pacific
|
179,917
|
129,747
|
27,775
|
|||||||||
|
Total
|
611,689
|
143,167
|
59,775
|
|||||||||
| A. |
Avner Gal, the beneficial owner of approximately 4.12% of the Company's outstanding Common Stock as of December 31, 2016, entered into an employment agreement with Integrity Israel in July 2010 pursuant to which Mr. Gal agreed to continue to serve as the chief executive officer and managing director of Integrity Israel. The agreement was approved by the board of directors and stockholders of Integrity Israel. Mr. Gal’s employment agreement provides for an annual salary of approximately $125,722, $123,457 and $134,769 (NIS 480,000) for the year ended December 31, 2016, 2015 and 2014 respectively. In addition, Mr. Gal is entitled to an annual bonus to be determined by the board of directors and an additional sum provided that Mr. Gal reaches certain milestones approved by the board, as well as the payment of certain social and insurance benefits and the use of a car. During the year ended December 31, 2016, 2015 and 2014 the Company did not pay Mr. Gal any bonuses. The agreement also provides for a renegotiation of Mr. Gal’s annual salary on the one-year anniversary thereof and the renegotiation of Mr. Gal’s bonus formula once Integrity Israel has begun commercialization of its products. The agreement is terminable by either party on 180 days’ notice, immediately by Integrity Israel with the payment of an amount equal to 180 days of annual salary, or immediately by Integrity Israel for cause (as defined in the agreement) without the payment of severance. Mr. Gal is subject to a non-compete and a confidentiality agreement during the term of the agreement and for one year thereafter. As of December 31, 2016, the Company did not make any amendments to Mr. Gal’s employment agreement.
|
| B. |
David Malka, the beneficial owner of 1.64% of the Company's outstanding Common Stock as of December 31, 2016, entered into an employment agreement with Integrity Israel in July 2010 pursuant to which Mr. Malka agreed to continue to serve as the vice president of operations of Integrity Israel. The agreement was approved by the board of directors and stockholders of Integrity Israel. Mr. Malka’s employment agreement provides for an annual salary of approximately $63,114, 61,728 and $67,302 (NIS 240,000) for the year ended December 31, 2016, 2015 and 2014 respectively. In addition, Mr. Malka is entitled to an annual bonus to be determined by the Board of Directors in its sole discretion and an additional sum provided that Mr. Malka reaches certain milestones approved by the Board, as well as the payment of certain social and insurance benefits and the use of a group three car. During the year ended December 31, 2016, 2015 and 2014 the Company did not pay Mr. Malka any bonuses. The agreement also provided for a renegotiation of Mr. Malka’s annual salary on the one-year anniversary thereof and the renegotiation of Mr. Malka’s bonus formula once Integrity Israel has begun commercialization of its products. The agreement is terminable by either party on 90 days’ notice, immediately by Integrity Israel with the payment of an amount equal to 90 days of annual salary, or immediately by Integrity Israel for cause (as defined in the agreement) without the payment of severance. Mr. Malka is subject to a non-compete and confidentiality agreement during the term of the agreement and for one year thereafter. As of December 31, 2016, the Company did not make any amendments to Mr. Malka’s employment agreement.
|
| A. |
On January 5, 2017, and March 8, 2017 the Company, entered into a Securities Purchase Agreement with certain accredited investors (the “Purchasers”) pursuant to which, the Company issued to the Purchasers an aggregate of 403.9 and 2,560, respectively, units of the Company’s Series C 5.5% Convertible Preferred Stock, par
value
$0.001 per share, as described in note 10D above. The shares of Preferred Stock comprising the Units are convertible into an aggregate of 658,649 shares of Common Stock, and the Warrants comprising the Units are exercisable for an aggregate of 1,317,298 shares of Common Stock, in each case subject to certain adjustments. The Company received aggregate gross proceeds of $2,963,900 from the sale of the Units pursuant to the Purchase Agreement.
|
| B. |
On January 31, 2017, the Board of Directors of the Company unanimously voted to appoint Sami Sassoun as the Chief Financial Officer of the Company, commencing on February 1, 2017. On February 1, 2017, the Subsidiary entered into an employment agreement (the “
Employment Agreement
”) with Mr. Sassoun to serve as Chief Financial Officer of the Company and the Subsidiary. The Employment Agreement provides for a monthly
base
gross salary of NIS 30,000 (approximately $8,000 based on the exchange rate of NIS 3.7461 / $1.00 USD in effect on February 1, 2017), as well as the payment of certain social benefits and the use of a company car. In addition, pursuant to the Employment Agreement, the Company has agreed to grant to Mr. Sassoun, on the one year anniversary of the commencement of his employment with the Company, options to purchase such number of shares of Common Stock of the Company, at an exercise price of $4.50 per share, with the number of options to be issued and the vesting provisions applicable thereto to be determined by the Board of Directors of the Company. The Employment Agreement is terminable by either party on 90 days’ notice or immediately by the Subsidiary for Cause (as defined in the Employment Agreement) without the payment of severance. The Employment Agreement contains non-compete obligations applicable during the term of the agreement and for one year thereafter and confidentiality obligations that survive the termination of the agreement indefinitely
|
| C. |
On February 6, 2017, the Company, entered into an amended and restated consulting agreement (the “
A&R Consulting Agreement
”) with Strand Strategy, a healthcare consulting firm (“
Strand Strategy
”), relating to the retention of Strand Strategy’s services as an independent contractor on a temporary basis, effective as of December 1,
2016
, (the “
Effective Date
”). The founder and managing director of Strand Strategy, Angela Strand, is an independent member of the board of directors of the Company (the “
Board
”), is a member of the Compensation Committee and Chairperson of the Nominating and Corporate Governance Committee of the Board. Pursuant to the terms of the A&R Consulting Agreement, Strand Strategy agreed to assist the Company with its corporate strategy, business development and communication management for a 90-day period, beginning on the Effective Date (the “
Services
”). As consideration for the Services, the Company agreed to pay Strand Strategy a fee of $60,000, 25% of which shall be paid in cash and 75% of which shall be paid by the grant to Strand Strategy of 10,000 shares of Common Stock, par value $0.001 per share, of the Company. The A&R Consulting Agreement may be terminated immediately by either party, upon written notice to the other party, if the other party materially breached the A&R Consulting Agreement, and such breach is incapable of cure. With respect to a breach capable of cure, the non-breaching party may terminate the A&R Consulting Agreement if the breaching party fails to cure within five (5) days after receipt of written notice of breach.
The A&R Consulting Agreement contains confidentiality obligations that survive the termination of the A&R Consulting Agreement indefinitely.
|
| D. |
On March 20, 2017, the Company entered into an employment agreement (the “
Graham Employment Agreement
”) with Mr. Graham to serve as Chief Executive Officer of the Company. Pursuant to the terms of the Graham Employment Agreement, Mr. Graham will (1) receive a base salary of $500,000 per year; (2) be eligible to earn an annual performance bonus between 35-72% of his current base salary, subject to certain performance criteria and, provided that Mr. Graham continues to be an employee through and on March 15, 2018, Mr. Graham’s performance bonus for his first year is guaranteed up to $225,000; (3) be eligible to earn a one-time milestone bonus equal to $500,000 based upon satisfaction of the Company attaining cash on hand in the Company equal to or greater than $20,000,000 on or before December 31, 2018, provided that Mr. Graham continues to be an employee through and on the date of payment of such one-time bonus; and (4) receive certain equity awards (pursuant to the Company’s 2010 Incentive Compensation Plan, as amended) under the terms and conditions as set forth in the Graham Employment Agreement. The Graham Employment Agreement is terminable by the Company on 90 days’ prior written notice to Mr. Graham, without Cause, or immediately by the Company for Cause (as defined in the Graham Employment Agreement). The Graham Employment Agreement contains non-compete obligations applicable during the term of the agreement and for one year thereafter and confidentiality obligations that survive the termination of the agreement indefinitely.
|