|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
45-4139254
|
|
|
(State or other jurisdiction of
incorporation or organization)
|
(I.R.S. Employer
Identification No.)
|
|
|
505 Penobscot Dr.
Redwood City, California
|
94063
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
|
Title of each class
|
Name of each exchange on which registered
|
|
|
Common Stock, par value $0.00001
|
The Nasdaq Stock Market LLC
|
|
|
Large accelerated filer
|
o
|
Accelerated filer
|
o
|
|
Non-accelerated filer
|
x
|
Smaller reporting company
|
o
|
|
Emerging growth company
|
x
|
||
|
Page
|
||
|
Business
|
||
|
Risk Factors
|
||
|
Unresolved Staff Comments
|
||
|
Properties
|
||
|
Legal Proceedings
|
||
|
Mine Safety Disclosures
|
||
|
PART
II
|
||
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
||
|
Selected Financial Data
|
||
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
||
|
Quantitative and Qualitative Disclosures About Market Risk
|
||
|
Financial Statements and Supplementary Data
|
||
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
|
||
|
Controls and Procedures
|
||
|
Other Information
|
||
|
PART II
I
|
||
|
Directors, Executive Officers and Corporate Governance
|
||
|
Executive Compensation
|
||
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
||
|
Certain Relationships and Related Transactions, and Director Independence
|
||
|
Principal Accounting Fees and Services
|
||
|
PART I
V
|
||
|
Exhibits, Financial Statement Schedules
|
||
|
Form 10-K Summary
|
||
|
Signatures
|
||
|
•
|
Increase awareness of our products by:
|
|
•
|
building awareness of liquid biopsy and pioneering a blood-first paradigm for genotyping cancer patients;
|
|
•
|
educating biopharmaceutical companies, KOLs and advocacy groups;
|
|
•
|
advocating for inclusion of our tests in treatment guidelines; and
|
|
•
|
expanding access to our products globally through direct investment and by leveraging our global network of partners.
|
|
•
|
Expand clinical utility and increase reimbursement for our products by:
|
|
•
|
working with private and public payers to establish coverage and reimbursement for our tests;
|
|
•
|
investing in clinical evidence directly and through relationships with academia and biopharmaceutical companies to establish expanded indications for use;
|
|
•
|
demonstrating improved clinical utility and health economics from use of our tests to patients, physicians and payers; and
|
|
•
|
pursuing FDA approval of our Guardant360 and future tests to facilitate reimbursement and global market access.
|
|
•
|
Strengthen our relationships with biopharmaceutical and academia customers by:
|
|
•
|
demonstrating the utility of our products in connection with standard of care biopharmaceutical treatments thereby encouraging clinical adoption;
|
|
•
|
developing and seeking approval of our products as companion diagnostics for targeted therapies and immuno-oncology therapies; and
|
|
•
|
providing earlier insights into emerging clinically relevant biomarkers.
|
|
•
|
Leverage our Guardant Health Oncology Platform to expand our product portfolio by:
|
|
•
|
using our commercial engine as a force multiplier of returns on research and development investment to generate data and analytical insights to enable development of new products;
|
|
•
|
taking a disciplined and systematic approach to product and market development, by starting with therapy selection and then expanding sequentially towards early cancer detection;
|
|
•
|
utilizing our data, sample biobank and insights into biology of circulating tumor-related biomarkers in blood to develop our LUNAR programs;
|
|
•
|
building on our regulatory and commercial infrastructure to accelerate new product launches and drive commercial efficiencies; and
|
|
•
|
using our strategic relationships, including our joint venture with SoftBank, to drive global commercialization of our products, with a near-term focus on Japan.
|
|
•
|
minimally invasive;
|
|
•
|
rapidly administered;
|
|
•
|
cost effective; and
|
|
•
|
readily available.
|
|
•
|
Vast data sets and deep insights:
We have targeted deep sequencing data in combination with low-coverage sequencing of whole genome from tens of thousands of cancer patients. This data has enabled discovery of novel epigenomic variations across multiple cancer types. We believe augmenting genomic with epigenomic signatures can enhance the clinical sensitivity and specificity of our tests significantly. Moreover, we developed a database of biological noise sources such as clonal hematopoiesis of indeterminate potential, which enables us to further enhance the sensitivity and specificity of our tests.
|
|
•
|
Extensive blood biobank:
We have a biobank of tens of thousands of cancer samples that we use for discovery and, more importantly, biomarker verification and validation. For example, we are analyzing these samples with whole genome sequencing to identify and confirm tumor associated signatures. Also, we have been collecting additional samples through multiple on-going research collaborations.
|
|
•
|
discovery of new targets and mechanisms of acquired resistance;
|
|
•
|
retrospective sample analysis to rapidly identify biomarkers associated with response and lack of response;
|
|
•
|
prospective screening and referral services to accelerate clinical trial enrollment; and
|
|
•
|
companion diagnostic development to support the approval and commercialization of therapeutics.
|
|
•
|
High-efficiency chemistry
: Overall efficiency of Guardant Health Digital Sequencing in recovery of ctDNA molecules from starting input amount of ctDNA to the post-sequencing analysis of reconstructed molecules indicates the vast majority of extracted ctDNA molecules are converted into a sequencing library, which exceeds most other next-generation sequencing preparations by more than 100%;
|
|
•
|
Error suppression via proprietary bioinformatics engine
: Error suppression through Guardant Health Digital Sequencing corresponds to a typical error rate of approximately one error per 3,000,000 reconstructed molecule nucleotides of high quality. This should be compared to the simplest single-end sequencing error rate of approximately one error per 1,000 sequenced nucleotides and approximately one error per 100,000 nucleotides that could be achieved by other assays relying on molecular barcoding alone.
|
|
•
|
applications and patents relating to our digital sequencing platform, including claims directed to methods for sequencing cell-free DNA, identifying CNVs, SNVs, indels and fusions in cell-free DNA and techniques for enriching nucleic acid samples;
|
|
•
|
applications and patents relating to detecting and monitoring cancer and other diseases by determining genetic variations in biological samples; and
|
|
•
|
applications and patents relating to early-stage cancer detection.
|
|
•
|
the FDA or other regulatory authorities do not approve a clinical trial protocol or a clinical trial, or place a clinical trial on hold;
|
|
•
|
patients do not enroll in clinical trials at the rate expected;
|
|
•
|
patients do not comply with trial protocols;
|
|
•
|
patient follow-up is not at the rate expected;
|
|
•
|
patients experience adverse events;
|
|
•
|
patients die during a clinical trial, even though their death may not be related to the products that are part of the trial;
|
|
•
|
device malfunctions occur with unexpected frequency or potential adverse consequences;
|
|
•
|
side effects or device malfunctions of similar products already in the market that change the FDA’s view toward approval of new or similar PMAs or result in the imposition of new requirements or testing;
|
|
•
|
institutional review boards and third-party clinical investigators may delay or reject the trial protocol;
|
|
•
|
third-party clinical investigators decline to participate in a trial or do not perform a trial on the anticipated schedule or consistent with the clinical trial protocol, investigator agreement, investigational plan, good clinical practices, the IDE regulations or other FDA or IRB requirements;
|
|
•
|
third-party investigators are disqualified by the FDA;
|
|
•
|
we or third-party organizations do not perform data collection, monitoring and analysis in a timely or accurate manner or consistent with the clinical trial protocol or investigational or statistical plans, or otherwise fail to comply with the IDE regulations governing responsibilities, records and reports of sponsors of clinical investigations;
|
|
•
|
third-party clinical investigators have significant financial interests related to us or our study such that the FDA deems the study results unreliable, or the company or investigators fail to disclose such interests;
|
|
•
|
regulatory inspections of our clinical trials or manufacturing facilities, which may, among other things, require us to undertake corrective action or suspend or terminate our clinical trials;
|
|
•
|
changes in government regulations or administrative actions;
|
|
•
|
the interim or final results of the clinical trial are inconclusive or unfavorable as to safety or efficacy; or
|
|
•
|
the FDA concludes that our trial designs are unreliable or inadequate to demonstrate safety and efficacy.
|
|
•
|
the device may not be shown safe or effective to the FDA’s satisfaction;
|
|
•
|
the data from pre-clinical studies and/or clinical trials may be found unreliable or insufficient to support approval;
|
|
•
|
the manufacturing process or facilities may not meet applicable requirements; and
|
|
•
|
changes in FDA approval policies or adoption of new regulations may require additional data.
|
|
•
|
the FDA’s QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, production, control, supplier/contractor selection, complaint handling, documentation and other quality assurance procedures during all aspects of the manufacturing process;
|
|
•
|
labeling regulations, unique device identification requirements and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label uses;
|
|
•
|
advertising and promotion requirements;
|
|
•
|
restrictions on sale, distribution or use of a device;
|
|
•
|
PMA annual reporting requirements;
|
|
•
|
PMA approval of product modifications, or the potential for new 510(k) clearances for certain modifications to 510(k)-cleared devices;
|
|
•
|
medical device reporting regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur;
|
|
•
|
medical device correction and removal reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health;
|
|
•
|
recall requirements, including a mandatory recall if there is a reasonable probability that the device would cause serious adverse health consequences or death;
|
|
•
|
an order of repair, replacement or refund;
|
|
•
|
device tracking requirements; and
|
|
•
|
post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device.
|
|
•
|
our ability to increase awareness of our tests and the benefits of liquid biopsy;
|
|
•
|
the rate of adoption and/or endorsement of our tests by clinicians, KOLs, advocacy groups and biopharmaceutical companies;
|
|
•
|
the timing and scope of any approval by the FDA for our tests;
|
|
•
|
our ability to obtain positive coverage decisions for our tests from additional commercial payers and to broaden the scope of indications included in such coverage decisions;
|
|
•
|
our ability to obtain reimbursement from government payers, including Medicare, which accounted for approximately 38% of our U.S. clinical test volume in
2018
;
|
|
•
|
the impact of our investments in product innovation and commercial growth;
|
|
•
|
negative publicity regarding ours or our competitors’ products resulting from defects or errors; and
|
|
•
|
our ability to further validate our technology through clinical research and accompanying publications.
|
|
•
|
the level of demand for any of our products, which may vary significantly;
|
|
•
|
the timing and cost of, and level of investment in, research, development, regulatory approval and commercialization activities relating to our products, which may change from time to time;
|
|
•
|
the volume and customer mix of our precision oncology testing;
|
|
•
|
the start and completion of projects in which our development services are utilized;
|
|
•
|
the introduction of new products or product enhancements by us or others in our industry;
|
|
•
|
coverage and reimbursement policies with respect to our products and products that compete with our products;
|
|
•
|
expenditures that we may incur to acquire, develop or commercialize additional products and technologies;
|
|
•
|
changes in governmental regulations or in the status of our regulatory approvals or applications;
|
|
•
|
future accounting pronouncements or changes in our accounting policies;
|
|
•
|
developments or disruptions in the business and operations of our clinical, commercial and other partners; and
|
|
•
|
general market conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors.
|
|
•
|
conduct substantial research and development, including validation studies and clinical trials;
|
|
•
|
further develop and scale our laboratory processes to accommodate different products; and
|
|
•
|
further develop and scale our infrastructure to be able to analyze increasingly large amounts of data.
|
|
•
|
failure of the product to perform as expected, including defects and errors;
|
|
•
|
lack of validation data; or
|
|
•
|
failure to demonstrate the clinical utility of the product.
|
|
•
|
multiple, conflicting and changing laws and regulations such as privacy regulations, tax laws, export and import restrictions, economic sanctions and embargoes, employment laws, regulatory requirements and other governmental approvals, permits and licenses;
|
|
•
|
failure by us, our distributors, our local partners or the Joint Venture with SoftBank to obtain regulatory approvals for the use of our products in various countries;
|
|
•
|
additional potentially blocking or relevant third-party patent or other intellectual property rights;
|
|
•
|
complexities and difficulties in obtaining intellectual property protection and enforcing our intellectual property;
|
|
•
|
difficulties in staffing and managing foreign operations;
|
|
•
|
complexities associated with managing multiple payer reimbursement regimes, government payers, or patient self-pay systems;
|
|
•
|
logistics and regulations associated with shipping blood samples, including infrastructure conditions and transportation delays;
|
|
•
|
limits in our ability to penetrate international markets if we are not able to perform our tests locally;
|
|
•
|
financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our products and exposure to foreign currency exchange rate fluctuations, currency controls and cash repatriation restrictions;
|
|
•
|
natural disasters, political and economic instability, including wars, terrorism, and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions; and
|
|
•
|
regulatory and compliance risks that relate to maintaining accurate information and control over sales and distributors’ activities that may fall within the purview of the U.S. Foreign Corrupt Practices Act, or FCPA, its books and records provisions, or its anti-bribery provisions.
|
|
•
|
increase our sales and marketing efforts to drive market adoption of Guardant360 and GuardantOMNI tests and address competitive developments;
|
|
•
|
fund development and marketing efforts of products from our LUNAR programs or any other future products we may develop;
|
|
•
|
expand our technologies into other types of cancer management and detection products;
|
|
•
|
acquire, license or invest in technologies;
|
|
•
|
acquire or invest in complementary businesses or assets; and
|
|
•
|
finance capital expenditures and general and administrative expenses.
|
|
•
|
our ability to achieve revenue growth;
|
|
•
|
our rate of progress in establishing payer coverage and reimbursement arrangements with domestic and international commercial payers and government payers;
|
|
•
|
the cost of expanding our laboratory operations and product offerings, including our sales and marketing efforts;
|
|
•
|
our rate of progress in, and costs of our sales and marketing activities associated with, establishing adoption of and reimbursement for our Guardant360 and GuardantOMNI tests;
|
|
•
|
our rate of progress in, and costs of our research and development activities associated with, products in research and early development;
|
|
•
|
the effect of competing technological and market developments;
|
|
•
|
costs related to our international expansion; and
|
|
•
|
the potential costs of and delays in product development as a result of any existing or new regulatory oversight applicable to our products.
|
|
•
|
federal and state laws applicable to test ordering, documentation of tests ordered, billing practices and claims payment and/or regulatory agencies enforcing those laws and regulations;
|
|
•
|
federal and state health care fraud and abuse laws;
|
|
•
|
federal and state laboratory anti-mark-up laws;
|
|
•
|
coverage and reimbursement levels by Medicare, Medicaid, other governmental payers and private insurers;
|
|
•
|
restrictions on coverage of and reimbursement for tests;
|
|
•
|
federal and state laws governing laboratory testing, including CLIA, and state licensing laws;
|
|
•
|
federal and state laws and enforcement policies governing the development, use and distribution of diagnostic medical devices, including laboratory developed tests, or LDTs;
|
|
•
|
federal, state and local laws governing the handling and disposal of medical and hazardous waste;
|
|
•
|
federal and state Occupational Safety and Health Administration rules and regulations; and
|
|
•
|
the Health Insurance Portability and Accountability Act of 1996, or HIPAA, and similar state data privacy and security laws.
|
|
•
|
our inability to demonstrate to the satisfaction of the FDA that our products are safe or effective for their intended uses;
|
|
•
|
the disagreement of the FDA with the design, conduct or implementation of our clinical trials or the analysis or interpretation of data from our pre-clinical studies or clinical trials;
|
|
•
|
serious and unexpected adverse effects experienced by participants in our clinical trials;
|
|
•
|
the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval, where required;
|
|
•
|
our inability to demonstrate that the clinical and other benefits of any of our tests outweigh the risks;
|
|
•
|
an advisory committee, if convened by the FDA, may recommend against approval of our PMA or other application for any of our tests or may recommend that the FDA require, as a condition of approval, additional pre-clinical studies or clinical trials, limitations on approved labeling or distribution and use restrictions, or even if an advisory committee, if convened, makes a favorable recommendation, the FDA may still not approve the test;
|
|
•
|
the FDA may identify deficiencies in our marketing application, and in our manufacturing processes, facilities or analytical methods or those of our third-party contract manufacturers;
|
|
•
|
the potential for approval policies or regulations of the FDA to change significantly in a manner rendering our clinical data or regulatory filings insufficient for the clearance or approval; and
|
|
•
|
the FDA may audit our clinical trial data and conclude that the data is not sufficiently reliable to support a PMA application.
|
|
•
|
adverse publicity, warning letters, untitled letters, fines, injunctions, consent decrees and civil penalties;
|
|
•
|
repair, replacement, refunds, recalls, termination of distribution, administrative detention or seizures of our products;
|
|
•
|
operating restrictions, partial suspension or total shutdown of production;
|
|
•
|
customer notifications or repair, replacement or refunds;
|
|
•
|
refusing our requests for clearances or approvals of new products, new intended uses or modifications to existing products;
|
|
•
|
withdrawals of current clearances or approvals, resulting in prohibitions on sales of our products;
|
|
•
|
refusal to issue certificates needed to export products for sale in other countries; and
|
|
•
|
criminal prosecution.
|
|
•
|
the AKS, which prohibits knowingly and willfully offering, paying, soliciting or receiving remuneration, directly or indirectly, overtly or covertly, in cash or in kind (e.g. provision of free or discounted goods, services or items), in return for or to induce such person to refer an individual, or to purchase, lease, order, arrange for or recommend purchasing, leasing or ordering, any good, facility, item or service that is reimbursable, in whole or in part, under a federal healthcare program. The term ‘‘remuneration’’ has been broadly interpreted to include anything of value, such as phlebotomy kits. Although there are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, the exceptions and safe harbors are drawn narrowly, and practices that involve remuneration that are alleged to be intended to induce referrals, purchases or recommendations of covered items or services may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct
per se
illegal under the AKS. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all its facts and circumstances. Several courts have held that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the AKS has been violated. Moreover, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. Violations are subject to civil monetary penalties of up to $100,000 for each violation, plus up to three times the remuneration involved. Violations of the AKS may also result in criminal penalties, including fines of up to $100,000 and imprisonment of up to ten years, and exclusion from Medicare, Medicaid or other governmental healthcare programs;
|
|
•
|
the Stark Law, which prohibits a physician from making a referral for certain designated health services covered by the Medicare or Medicaid program, including laboratory and pathology services, if the physician or an immediate family member of the physician has a financial relationship with the entity providing the designated health services and prohibits that entity from billing, presenting or causing to be presented a claim for the designated health services furnished pursuant to the prohibited referral, unless an exception applies. Sanctions for violating the Stark Law include denial of payment, civil monetary penalties of up to $24,728 per claim submitted and exclusion from the federal health care programs. The statute also provides for a penalty of up to $164,992 for a circumvention scheme;
|
|
•
|
the federal Civil Monetary Penalties Law, which prohibits, among other things, the offering or transfer of remuneration to a Medicare or state healthcare program beneficiary if the person knows or should know it is likely to influence the beneficiary’s selection of a particular provider, practitioner or supplier of services reimbursable by Medicare or a state healthcare program, unless an exception applies. Violations can result in civil monetary penalties of up to $20,000 for each wrongful act;
|
|
•
|
federal and state “Anti-Markup” rules, which, among other things, typically prohibit a physician or supplier billing for clinical or diagnostic tests (with certain exceptions) from marking up the price of a purchased test performed by another physician or supplier that does not “share a practice” with the billing physician or supplier;
|
|
•
|
the federal Physician Payments Sunshine Act, which requires certain manufacturers of drugs, biologicals, and kits, medical devices or supplies that require premarket approval by or notification to the FDA, and for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program to report annually to CMS, information related to (i) payments and other transfers of value to physicians and teaching hospitals, and (ii) ownership and investment interests in such manufacturers held by physicians and their immediate family members. Failure to submit required information may result in civil monetary penalties of $11,278 per failure up to an aggregate of $169,170 per year (or up to an aggregate of $1.112 million per year for “knowing failures”), for all payments, transfers of value or ownership or investment interests that are not timely, accurately, and completely reported in an annual submission, and may result in liability under other federal laws or regulations;
|
|
•
|
the federal government may bring a lawsuit under the False Claims Act, or the FCA, against any party whom it believes has knowingly or recklessly presented, or caused to be presented, a false or fraudulent request for payment from the federal government, or who has made a false statement or used a false record to get a claim for payment approved. The federal government and a number of courts have taken the position that claims presented in violation of certain other statutes, including the AKS or the Stark Law, can also be considered a violation of the FCA based on the theory that a provider impliedly certifies compliance with all applicable laws, regulations, and other rules
|
|
•
|
the HIPAA fraud and abuse provisions, which created federal criminal statutes that prohibit, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private insurers, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation;
|
|
•
|
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their implementing regulations, also as amended, which imposes certain regulatory and contractual requirements regarding the privacy, security and transmission of protected health information, or PHI, and similar state health information privacy and data breach notification laws;
|
|
•
|
federal and state laws related to, among other things, unlawful schemes to defraud, excessive fees for services, unlawful trade practices, insurance fraud, kickbacks, patient inducement and statutory or common law fraud restrict the provision of products, services or items for free or at reduced charge to government or non-government healthcare program beneficiaries. These laws and regulations relating to the provision of items or services for free are complex and are subject to interpretation by the courts and by government agencies;
|
|
•
|
other federal and state fraud and abuse laws, such as state anti-kickback, self-referrals, false claims and anti-markup laws, any of which may extend to services reimbursable by any payer, including private insurers;
|
|
•
|
state laws that prohibit other specified practices, such as billing physicians for tests that they order; providing tests at no or discounted cost to induce adoption; waiving co-insurance, co-payments, deductibles or other amounts owed by patients; billing a state healthcare program at a price that is higher than what is charged to other payers; or employing, exercising control over or splitting fees with licensed medical professionals; and
|
|
•
|
similar foreign laws and regulations in the countries in which we operate or may operate in the future.
|
|
•
|
differences between the list prices for our tests and the reimbursement rates of payers;
|
|
•
|
compliance with complex federal and state regulations related to billing government healthcare programs, including Medicare and Medicaid, to the extent our tests are covered by such programs;
|
|
•
|
differences in coverage among payers and the effect of patient co-payments or co-insurance;
|
|
•
|
differences in information, pre-authorization and other billing requirements among payers;
|
|
•
|
changes to codes and coding instructions governing our tests;
|
|
•
|
incorrect or missing billing information; and
|
|
•
|
the resources required to manage the billing and claim appeals process.
|
|
•
|
the scope of rights granted under the license agreement and other interpretation-related issues;
|
|
•
|
the extent to which our products or product candidates, technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement;
|
|
•
|
the licensing of patent and other rights controlled by our licensors or developed under our collaborative development relationships to others;
|
|
•
|
our diligence obligations under the license agreement and what activities satisfy those diligence obligations;
|
|
•
|
the inventorship and ownership of inventions and know-how licensed to us or resulting from the joint creation or use of intellectual property by our licensors, us and/or our partners; and
|
|
•
|
the validity, enforceability or priority of licensed patent rights.
|
|
•
|
volume and customer mix for our precision oncology testing;
|
|
•
|
the introduction of new products or product enhancements by us or others in our industry;
|
|
•
|
disputes or other developments with respect to our or others’ intellectual property rights;
|
|
•
|
our ability to develop, obtain regulatory clearance or approval for, and market new and enhanced products on a timely basis;
|
|
•
|
product liability claims or other litigation;
|
|
•
|
quarterly or annual variations in our results of operations or those of others in our industry;
|
|
•
|
media exposure of our products or of those of others in our industry;
|
|
•
|
changes in governmental regulations or in the status of our regulatory approvals or applications;
|
|
•
|
changes in earnings estimates or recommendations by securities analysts; and
|
|
•
|
general market conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors.
|
|
•
|
faulty human judgment and simple errors, omissions or mistakes;
|
|
•
|
fraudulent action of an individual or collusion of two or more people;
|
|
•
|
inappropriate management override of procedures; and
|
|
•
|
the possibility that any enhancements to controls and procedures may still not be adequate to assure timely and accurate financial control.
|
|
•
|
our board of directors has the exclusive right to expand its size and to elect directors to fill a vacancy created by the expansion of the board or the resignation, death or removal of a director, which prevents stockholders from being able to fill vacancies on our board of directors;
|
|
•
|
our board of directors is divided into three classes, Class I, Class II and Class III, with each class serving staggered three-year terms, which may delay the ability of stockholders to change the membership of a majority of our board of directors;
|
|
•
|
our stockholders may not act by written consent, which forces stockholder action to be taken at an annual or special meeting of our stockholders;
|
|
•
|
a special meeting of stockholders may be called only by our board of directors, its chairman, our chief executive officer or our president, which may delay the ability of our stockholders to force consideration of a proposal or to take action, including the removal of directors;
|
|
•
|
our amended and restated certificate of incorporation prohibits cumulative voting in the election of directors, which limits the ability of minority stockholders to elect their director candidates;
|
|
•
|
our board of directors may alter our bylaws without obtaining stockholder approval;
|
|
•
|
approval of the holders of at least two-thirds of the shares entitled to vote at an election of directors is required to adopt, amend or repeal our bylaws or repeal the provisions of our amended and restated certificate of incorporation regarding the election and removal of directors;
|
|
•
|
stockholders must provide advance notice and additional disclosures in order to nominate candidates for election to the board of directors or to propose matters that can be acted upon at a stockholders’ meeting, which may discourage or deter a potential acquiror from conducting a solicitation of proxies to elect the acquiror’s own slate of directors or otherwise attempting to obtain control of our company; and
|
|
•
|
our board of directors is authorized to issue shares of preferred stock and to determine the terms of those shares, including preferences and voting rights, without stockholder approval, which could be used to significantly dilute the ownership of a hostile acquiror.
|
|
1.
|
We issued an aggregate of
320,289
shares of our common stock upon the exercise of common stock warrants, for aggregate cash consideration of approximately
$45,000
.
|
|
2.
|
We granted stock options to purchase an aggregate of
2,088,639
shares of our common stock at a weighted-average exercise price of
$7.19
per share, to certain of our employees, consultants and directors in connection with services provided to us by such persons.
|
|
3.
|
We issued an aggregate of 880,086 shares of common stock to our employees, consultants and directors upon their exercise of stock options, for aggregate cash consideration of approximately $2.6 million.
|
|
Year Ended December 31,
|
||||||||||||
|
(in thousands, except per share data)
|
2018
|
2017
|
2016
|
|||||||||
|
Statements of Operations Data:
|
||||||||||||
|
Revenue:
|
||||||||||||
|
Precision oncology testing
|
$
|
78,407
|
|
$
|
42,088
|
|
$
|
24,496
|
|
|||
|
Development services
|
12,232
|
|
7,754
|
|
753
|
|
||||||
|
Total revenue
|
90,639
|
|
49,842
|
|
25,249
|
|
||||||
|
Costs and operating expenses:
|
||||||||||||
|
Cost of precision oncology testing
|
39,846
|
|
28,883
|
|
22,065
|
|
||||||
|
Cost of development services
|
3,364
|
|
2,735
|
|
59
|
|
||||||
|
Research and development expense
|
50,714
|
|
25,562
|
|
10,859
|
|
||||||
|
Sales and marketing expense
|
53,465
|
|
32,497
|
|
26,192
|
|
||||||
|
General and administrative expense
|
36,192
|
|
36,777
|
|
9,921
|
|
||||||
|
Total costs and operating expenses
|
183,581
|
|
126,454
|
|
69,096
|
|
||||||
|
Loss from operations
|
(92,942
|
)
|
(76,612
|
)
|
(43,847
|
)
|
||||||
|
Interest income
|
5,266
|
|
2,234
|
|
733
|
|
||||||
|
Interest expense
|
(1,251
|
)
|
(2,702
|
)
|
(3,018
|
)
|
||||||
|
Loss on debt extinguishment
|
—
|
|
(5,075
|
)
|
—
|
|
||||||
|
Other income (expense), net
|
4,702
|
|
(1,059
|
)
|
(1
|
)
|
||||||
|
Loss before provision for income taxes
|
(84,225
|
)
|
(83,214
|
)
|
(46,133
|
)
|
||||||
|
Provision for income taxes
|
38
|
|
7
|
|
6
|
|
||||||
|
Net loss
|
(84,263
|
)
|
(83,221
|
)
|
(46,139
|
)
|
||||||
|
Fair value adjustment of redeemable noncontrolling interest
|
(800
|
)
|
—
|
|
—
|
|
||||||
|
Net loss attributable to Guardant Health, Inc.
|
$
|
(85,063
|
)
|
$
|
(83,221
|
)
|
$
|
(46,139
|
)
|
|||
|
Deemed dividend related to repurchase of Series A convertible preferred stock
|
—
|
|
(4,716
|
)
|
—
|
|
||||||
|
Deemed dividend related to change in conversion rate of Series D convertible preferred stock
|
—
|
|
(1,058
|
)
|
—
|
|
||||||
|
Net loss attributable to Guardant Health, Inc. common stockholders
|
$
|
(85,063
|
)
|
$
|
(88,995
|
)
|
$
|
(46,139
|
)
|
|||
|
Net loss per share attributable to Guardant Health, Inc. common stockholders, basic and diluted
|
$
|
(2.80
|
)
|
$
|
(7.07
|
)
|
$
|
(3.53
|
)
|
|||
|
Weighted-average shares used in computing net loss per share attributable to Guardant Health, Inc. common stockholders, basic and diluted
|
30,403
|
|
12,582
|
|
13,053
|
|
||||||
|
As of December 31,
|
||||||||||||
|
(in thousands)
|
2018
|
2017
|
2016
|
|||||||||
|
Balance Sheet Data:
|
||||||||||||
|
Cash, cash equivalents and marketable securities
|
$
|
496,524
|
|
$
|
294,574
|
|
$
|
95,256
|
|
|||
|
Working capital(1)
|
422,047
|
|
223,308
|
|
88,813
|
|
||||||
|
Total assets
|
587,403
|
|
342,938
|
|
116,565
|
|
||||||
|
Total liabilities
|
62,451
|
|
34,332
|
|
36,869
|
|
||||||
|
Redeemable noncontrolling interest
|
41,800
|
|
—
|
|
—
|
|
||||||
|
Total stockholders’ equity
|
483,152
|
|
308,606
|
|
79,696
|
|
||||||
|
•
|
Testing volume, pricing and customer mix.
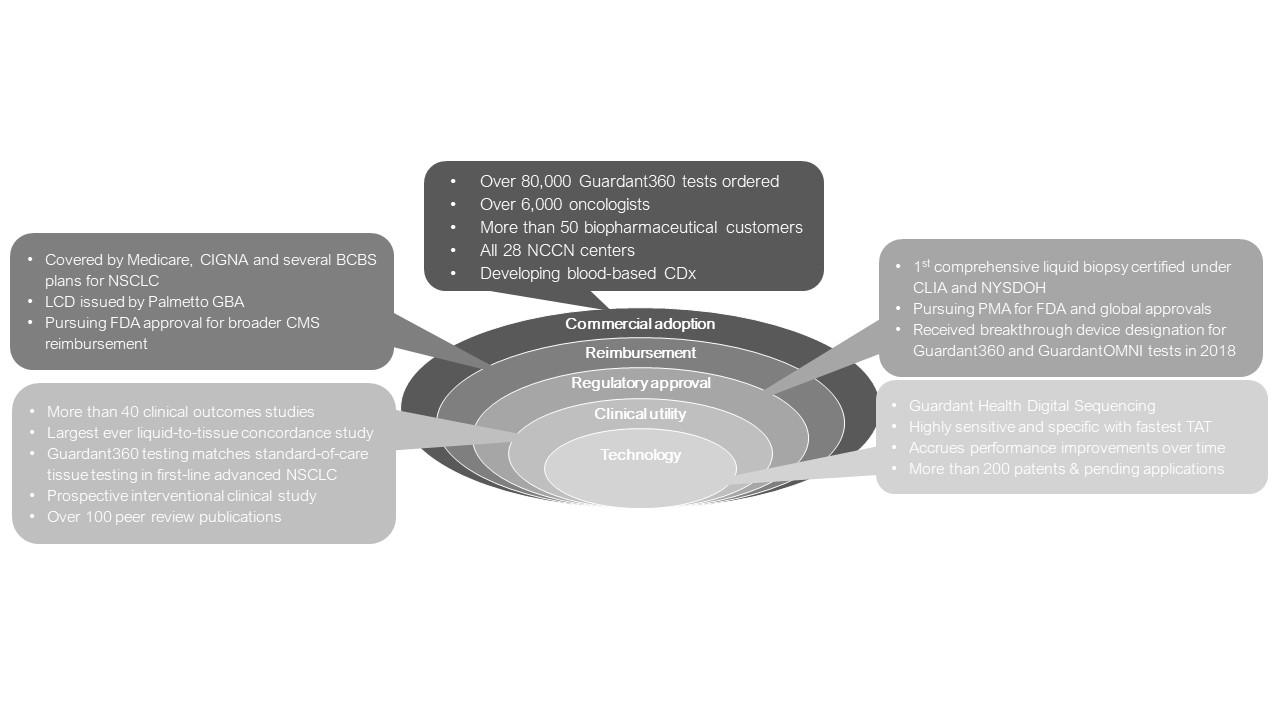
Our revenue and costs are affected by the volume of testing and mix of customers from period to period. We evaluate both the volume of tests that we perform for patients on behalf of clinicians and the number of tests we perform for biopharmaceutical companies. Our performance depends on our ability to retain and broaden adoption with existing customers, as well as attract new customers. We believe that the test volume we receive from clinicians and biopharmaceutical companies are indicators of growth in each of these customer verticals. Customer mix for our tests has the potential to significantly affect our results of operations, as the average selling price for biopharmaceutical sample testing is currently higher than our average selling price for clinical tests since we are not a contracted provider for, or our tests are not covered by clinical patients’ insurance for, the majority of the tests that we perform for patients on behalf of clinicians. Approximately 38% of our U.S. clinical tests in both
2018
and
2017
were for Medicare beneficiaries. Prior to the third quarter of 2018, Medicare did not cover our tests and we did not submit claims for reimbursement. In September 2018, we began to submit claims to Medicare for reimbursement for Guardant360 clinical tests for certain Medicare beneficiaries, and in October 2018, we began to receive payments from Medicare for these clinical tests.
|
|
•
|
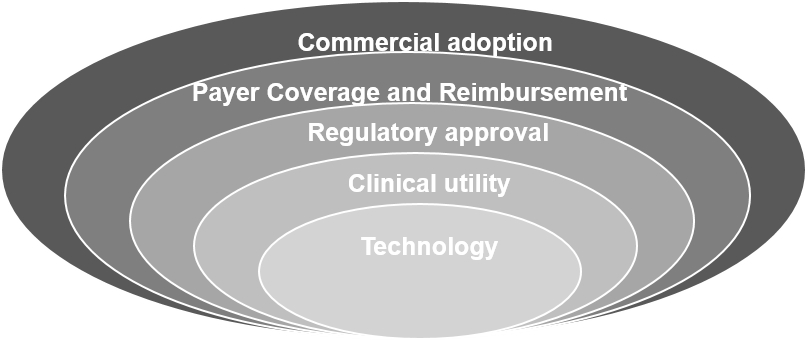
Regulatory approval .
Our Guardant360 test was the first comprehensive liquid biopsy test approved by NYSDOH. In addition, we believe our facility was the first comprehensive liquid biopsy laboratory to be CLIA-certified, CAP-accredited and NYSDOH-permitted. The FDA granted designation as a breakthrough device for our Guardant360 test in January 2018 and for our GuardantOMNI test in December 2018. An expedited access pathway and potentially faster review are provided for breakthrough devices that address unmet medical needs. While FDA approval is currently not required to market our tests in the United States, we intend to submit an application for a pre-market approval, or PMA, for each of our Guardant360 and GuardantOMNI tests. In March 2018, the Centers for Medicare and Medicaid Services, or CMS, published a Decision Memorandum for next-generation sequencing tests for patients with advanced cancer who meet certain clinical criteria, or the NGS Decision Memorandum. The NGS Decision Memorandum states that coverage would be available for next-generation sequencing FDA-approved tests offered within the FDA-approved labeling. FDA approval therefore provides a path to reimbursement
|
|
•
|
Payer coverage and reimbursement
. Our revenue depends on achieving broad coverage and reimbursement for our tests from third-party payers, including both commercial and government payers. Payment from commercial payers differs depending on whether we have entered into a contract with the payers as a “participating provider” or do not have a contract and are considered a "non-participating provider.” Payers often reimburse non-participating providers, if at all, at a lower amount than participating providers. We have received a substantial portion of our revenue from a limited number of commercial payers, most of which have not contracted with us to be a participating provider. We have received reimbursement for tests of patients with a variety of cancers, though for amounts that on average are significantly lower than for participating providers. Historically, we have experienced situations where commercial payers proactively reduced the amounts they were willing to reimburse for our tests, and in other situations, commercial payers have determined that the amounts they previously paid were too high and have sought to recover those perceived excess payments by deducting such amounts from payments otherwise being made. When we contract with a payer to serve as a participating provider, reimbursements by the payer are generally made pursuant to a negotiated fee schedule and are limited to only covered indications or where prior approval has been obtained. Becoming a participating provider generally results in higher reimbursement for covered indications and lack of reimbursement for non-covered indications. As a result, the impact of becoming a participating provider with a specific payer will vary based on historical reimbursement as a non-participating provider for that payer, and in some situations, the benefit of increased reimbursement for covered testing could be offset by the loss of reimbursement on tests for non-covered indications previously received when we served as a non-participating provider. Recently, Cigna, Priority Health and multiple regional Blue Cross Blue Shield plans adopted reimbursement policies that cover our Guardant360 test for the majority of NSCLC patients we test. If their reimbursement policies were to change in the future to cover additional cancer indications, we anticipate that our total reimbursement would increase. In September 2018, we began to submit claims for reimbursement at the rate of $3,500 per test with respect to Guardant360 clinical testing performed for NSCLC patients covered under Medicare’s Molecular Diagnostic Services Program who meet certain clinical criteria, and in October 2018, we began to receive payments from Medicare. If we fail to obtain or maintain coverage and adequate reimbursement from third-party payers, we may be unable to increase our testing volume and revenue as expected. Retrospective reimbursement adjustments can also negatively impact our revenue and cause our financial results to fluctuate.We have experienced situations where commercial payers proactively reduce the amounts they were willing to reimburse for our tests or determine that the amounts they previously paid were too high and have sought to recover those perceived excess payments by deducting such amounts from payments otherwise being made.
|
|
•
|
Biopharmaceutical customers.
Our revenue also depends on our ability to attract new, and to maintain and expand relationships with existing, biopharmaceutical customers, and we expect to increase our sales and marketing expense in furtherance of this goal. As we continue to develop these relationships, we expect to support a growing number of clinical trials both in the United States and internationally. If our relationships expand with biopharmaceutical customers, we believe we may continue to have opportunities to offer our platform to such customers for companion diagnostic development and for novel target discovery and validation, and to grow into other business opportunities. For example, we believe genomic data, in combination with clinical outcomes or claims data, has revenue-generating potential, including for novel target identification and clinical trial enrollment.
|
|
•
|
Research and development.
A significant aspect of our business is our investment in research and development, including the development of new products, such as those being developed as part of our LUNAR programs. In particular, we have invested heavily in clinical studies, including more than 40 clinical outcomes studies, the largest-ever liquid-to-tissue concordance study, and a prospective interventional clinical utility study demonstrating clinical overall response rates in line with tissue biopsy approaches. Our clinical research has resulted in over 100 peer-reviewed publications. In addition to clinical studies, we are collaborating with investigators from multiple academic cancer centers, including MD Anderson Cancer Center, the University of Colorado, Memorial Sloan Kettering Cancer Center, Massachusetts General Cancer Center, Wake Forest Cancer Center and the University of California San Francisco, as well as several international institutions. We believe these studies are critical to gaining physician adoption and driving favorable coverage decisions by payers, and expect our investments to increase. We expect to increase our research and development expense with the goal of fueling further innovation.
|
|
•
|
International expansion.
A component of our long-term growth strategy is to expand our commercial footprint internationally, and we expect to increase our sales and marketing expense to execute on this strategy. We currently offer our tests in countries outside the United States primarily through distributor relationships or direct contracts with hospitals. In May 2018, we formed and capitalized a joint venture, Guardant Health AMEA, Inc., which we refer to as the Joint Venture, with SoftBank, relating to the sale, marketing and distribution of our tests generally outside the Americas and Europe. We expect to rely on the Joint Venture to accelerate commercialization of our products in Asia, the Middle East and Africa, with our initial focus being on Japan.
|
|
Year Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Revenue:
|
|||||||||||
|
Precision oncology testing
|
$
|
78,407
|
|
$
|
42,088
|
|
$
|
24,496
|
|
||
|
Development services
|
12,232
|
|
7,754
|
|
753
|
|
|||||
|
Total revenue
|
90,639
|
|
49,842
|
|
25,249
|
|
|||||
|
Costs and operating expenses:
|
|||||||||||
|
Cost of precision oncology testing
(1)(2)
|
39,846
|
|
28,883
|
|
22,065
|
|
|||||
|
Cost of development services
|
3,364
|
|
2,735
|
|
59
|
|
|||||
|
Research and development expense
(1)(2)
|
50,714
|
|
25,562
|
|
10,859
|
|
|||||
|
Sales and marketing expense
(1)(2)
|
53,465
|
|
32,497
|
|
26,192
|
|
|||||
|
General and administrative expense
(1)(2)
|
36,192
|
|
36,777
|
|
9,921
|
|
|||||
|
Total costs and operating expenses
|
183,581
|
|
126,454
|
|
69,096
|
|
|||||
|
Loss from operations
|
(92,942
|
)
|
(76,612
|
)
|
(43,847
|
)
|
|||||
|
Interest income
|
5,266
|
|
2,234
|
|
733
|
|
|||||
|
Interest expense
|
(1,251
|
)
|
(2,702
|
)
|
(3,018
|
)
|
|||||
|
Loss on debt extinguishment
|
—
|
|
(5,075
|
)
|
—
|
|
|||||
|
Other income (expense), net
|
4,702
|
|
(1,059
|
)
|
(1
|
)
|
|||||
|
Loss before provision for income taxes
|
(84,225
|
)
|
(83,214
|
)
|
(46,133
|
)
|
|||||
|
Provision for income taxes
|
38
|
|
7
|
|
6
|
|
|||||
|
Net loss
|
$
|
(84,263
|
)
|
$
|
(83,221
|
)
|
$
|
(46,139
|
)
|
||
|
(1)
|
Amounts include stock-based compensation expense as follows:
|
|
Year Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Cost of precision oncology testing
|
$
|
512
|
|
$
|
162
|
|
$
|
225
|
|
||
|
Research and development expense
|
1,684
|
|
507
|
|
479
|
|
|||||
|
Sales and marketing expense
|
1,727
|
|
80
|
|
1,031
|
|
|||||
|
General and administrative expense
|
2,928
|
|
2,921
|
|
236
|
|
|||||
|
Total stock-based compensation expense
|
$
|
6,851
|
|
$
|
3,670
|
|
$
|
1,971
|
|
||
|
(2)
|
Amounts include compensation expenses associated with repurchase of common stock as follows:
|
|
Year Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Cost of precision oncology testing
|
$
|
—
|
|
$
|
72
|
|
$
|
2
|
|
||
|
Research and development expense
|
—
|
|
250
|
|
67
|
|
|||||
|
Sales and marketing expense
|
—
|
|
659
|
|
56
|
|
|||||
|
General and administrative expense
|
157
|
|
9,672
|
|
—
|
|
|||||
|
Total compensation expense associated with repurchase of common stock
|
$
|
157
|
|
$
|
10,653
|
|
$
|
125
|
|
||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2018
|
2017
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Precision oncology testing
|
$
|
78,407
|
|
$
|
42,088
|
|
$
|
36,319
|
|
86
|
%
|
|||
|
Development services
|
12,232
|
|
7,754
|
|
4,478
|
|
58
|
%
|
||||||
|
Total revenue
|
$
|
90,639
|
|
$
|
49,842
|
|
$
|
40,797
|
|
82
|
%
|
|||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2018
|
2017
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Cost of precision oncology testing
|
$
|
39,846
|
|
$
|
28,883
|
|
$
|
10,963
|
|
38
|
%
|
|||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2018
|
2017
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Cost of development services
|
$
|
3,364
|
|
$
|
2,735
|
|
$
|
629
|
|
23
|
%
|
|||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2018
|
2017
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Research and development
|
$
|
50,714
|
|
$
|
25,562
|
|
$
|
25,152
|
|
98
|
%
|
|||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2018
|
2017
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Sales and marketing
|
$
|
53,465
|
|
$
|
32,497
|
|
$
|
20,968
|
|
65
|
%
|
|||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2018
|
2017
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
General and administrative
|
$
|
36,192
|
|
$
|
36,777
|
|
$
|
(585
|
)
|
(2
|
)%
|
|||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2018
|
2017
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Interest income
|
$
|
5,266
|
|
$
|
2,234
|
|
$
|
3,032
|
|
136
|
%
|
|||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2018
|
2017
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Interest expense
|
$
|
1,251
|
|
$
|
2,702
|
|
$
|
(1,451
|
)
|
(54
|
)%
|
|||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2018
|
2017
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Loss on debt extinguishment
|
$
|
—
|
|
$
|
(5,075
|
)
|
$
|
5,075
|
|
(100
|
)%
|
|||
|
Year Ended December 31,
|
Change
|
||||||||||||
|
2018
|
2017
|
$
|
%
|
||||||||||
|
(in thousands)
|
|||||||||||||
|
Other income (expense), net
|
$
|
4,702
|
|
$
|
(1,059
|
)
|
$
|
5,761
|
|
*
|
|||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2018
|
2017
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Provision for income taxes
|
$
|
38
|
|
$
|
7
|
|
$
|
31
|
|
443
|
%
|
|||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2017
|
2016
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Precision oncology testing
|
$
|
42,088
|
|
$
|
24,496
|
|
$
|
17,592
|
|
72
|
%
|
|||
|
Development services
|
7,754
|
|
753
|
|
7,001
|
|
930
|
%
|
||||||
|
Total revenue
|
$
|
49,842
|
|
$
|
25,249
|
|
$
|
24,593
|
|
97
|
%
|
|||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2017
|
2016
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Cost of precision oncology testing
|
$
|
28,883
|
|
$
|
22,065
|
|
$
|
6,818
|
|
31
|
%
|
|||
|
Year Ended December 31,
|
Change
|
||||||||||||
|
2017
|
2016
|
$
|
%
|
||||||||||
|
(in thousands)
|
|||||||||||||
|
Cost of development services
|
$
|
2,735
|
|
$
|
59
|
|
$
|
2,676
|
|
*
|
|||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2017
|
2016
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Research and development
|
$
|
25,562
|
|
$
|
10,859
|
|
$
|
14,703
|
|
135
|
%
|
|||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2017
|
2016
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Sales and marketing
|
$
|
32,497
|
|
$
|
26,192
|
|
$
|
6,305
|
|
24
|
%
|
|||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2017
|
2016
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
General and administrative
|
$
|
36,777
|
|
$
|
9,921
|
|
$
|
26,856
|
|
271
|
%
|
|||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2017
|
2016
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Interest income
|
$
|
2,234
|
|
$
|
733
|
|
$
|
1,501
|
|
205
|
%
|
|||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2017
|
2016
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Interest expense
|
$
|
2,702
|
|
$
|
3,018
|
|
$
|
(316
|
)
|
(10
|
)%
|
|||
|
Year Ended December 31,
|
Change
|
||||||||||||
|
2017
|
2016
|
$
|
%
|
||||||||||
|
(in thousands)
|
|||||||||||||
|
Loss on debt extinguishment
|
$
|
5,075
|
|
$
|
—
|
|
$
|
5,075
|
|
*
|
|||
|
Year Ended December 31,
|
Change
|
||||||||||||
|
2017
|
2016
|
$
|
%
|
||||||||||
|
(in thousands)
|
|||||||||||||
|
Other income (expense), net
|
$
|
(1,059
|
)
|
$
|
(1
|
)
|
$
|
(1,058
|
)
|
*
|
|||
|
Year Ended December 31,
|
Change
|
|||||||||||||
|
2017
|
2016
|
$
|
%
|
|||||||||||
|
(in thousands)
|
||||||||||||||
|
Provision for income taxes
|
$
|
7
|
|
$
|
6
|
|
$
|
1
|
|
17
|
%
|
|||
|
Three Months Ended
|
||||||||||||||||||||||||||||||||
|
December 31, 2018
|
September 30, 2018
|
June 30,
2018
|
March 31, 2018
|
December 31, 2017
|
September 30, 2017
|
June 30,
2017
|
March 31, 2017
|
|||||||||||||||||||||||||
|
(unaudited)
|
||||||||||||||||||||||||||||||||
|
(in thousands)
|
||||||||||||||||||||||||||||||||
|
Revenue:
|
||||||||||||||||||||||||||||||||
|
Precision oncology testing
|
$
|
28,096
|
|
$
|
18,298
|
|
$
|
17,822
|
|
$
|
14,191
|
|
$
|
14,161
|
|
$
|
10,253
|
|
$
|
9,328
|
|
$
|
8,346
|
|
||||||||
|
Development services
|
4,777
|
|
3,394
|
|
1,560
|
|
2,501
|
|
5,841
|
|
879
|
|
868
|
|
166
|
|
||||||||||||||||
|
Total revenue
|
32,873
|
|
21,692
|
|
19,382
|
|
16,692
|
|
20,002
|
|
11,132
|
|
10,196
|
|
8,512
|
|
||||||||||||||||
|
Costs and operating expenses:
|
||||||||||||||||||||||||||||||||
|
Cost of precision oncology testing
|
12,624
|
|
9,671
|
|
9,506
|
|
8,045
|
|
7,955
|
|
7,603
|
|
6,954
|
|
6,371
|
|
||||||||||||||||
|
Cost of development services
|
1,323
|
|
380
|
|
453
|
|
1,208
|
|
1,193
|
|
1,058
|
|
481
|
|
3
|
|
||||||||||||||||
|
Research and development expense
|
16,652
|
|
14,253
|
|
11,554
|
|
8,255
|
|
8,120
|
|
7,246
|
|
5,494
|
|
4,702
|
|
||||||||||||||||
|
Sales and marketing expense
|
17,114
|
|
13,464
|
|
11,575
|
|
11,312
|
|
9,556
|
|
7,808
|
|
7,929
|
|
7,204
|
|
||||||||||||||||
|
General and administrative expense
|
12,547
|
|
8,129
|
|
8,997
|
|
6,519
|
|
8,795
|
|
16,095
|
|
6,675
|
|
5,212
|
|
||||||||||||||||
|
Total costs and operating expenses
|
60,260
|
|
45,897
|
|
42,085
|
|
35,339
|
|
35,619
|
|
39,810
|
|
27,533
|
|
23,492
|
|
||||||||||||||||
|
Loss from operations
|
(27,387
|
)
|
(24,205
|
)
|
(22,703
|
)
|
(18,647
|
)
|
(15,617
|
)
|
(28,678
|
)
|
(17,337
|
)
|
(14,980
|
)
|
||||||||||||||||
|
Interest income
|
2,334
|
|
958
|
|
989
|
|
985
|
|
1,012
|
|
657
|
|
360
|
|
205
|
|
||||||||||||||||
|
Interest expense
|
(299
|
)
|
(304
|
)
|
(317
|
)
|
(331
|
)
|
(304
|
)
|
(303
|
)
|
(1,056
|
)
|
(1,039
|
)
|
||||||||||||||||
|
Loss on debt extinguishment
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(5,075
|
)
|
—
|
|
||||||||||||||||
|
Other income (expense), net
|
115
|
|
43
|
|
395
|
|
4,149
|
|
(144
|
)
|
(266
|
)
|
(445
|
)
|
(204
|
)
|
||||||||||||||||
|
Loss before provision for income taxes
|
(25,237
|
)
|
(23,508
|
)
|
(21,636
|
)
|
(13,844
|
)
|
(15,053
|
)
|
(28,590
|
)
|
(23,553
|
)
|
(16,018
|
)
|
||||||||||||||||
|
Provision for income taxes
|
35
|
|
—
|
|
3
|
|
—
|
|
7
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||
|
Net loss
|
(25,272
|
)
|
(23,508
|
)
|
(21,639
|
)
|
(13,844
|
)
|
(15,060
|
)
|
(28,590
|
)
|
(23,553
|
)
|
(16,018
|
)
|
||||||||||||||||
|
Fair value adjustment of redeemable noncontrolling interest
|
150
|
|
(950
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||
|
Net loss attributable to Guardant Health, Inc.
|
$
|
(25,122
|
)
|
$
|
(24,458
|
)
|
$
|
(21,639
|
)
|
$
|
(13,844
|
)
|
$
|
(15,060
|
)
|
$
|
(28,590
|
)
|
$
|
(23,553
|
)
|
$
|
(16,018
|
)
|
||||||||
|
Deemed dividend related to repurchase of Series A convertible preferred stock
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(4,716
|
)
|
—
|
|
—
|
|
||||||||||||||||
|
Deemed dividend related to change in conversion rate of Series D convertible preferred stock
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(1,058
|
)
|
—
|
|
||||||||||||||||
|
Net loss attributable to Guardant Health, Inc. common stockholders
|
$
|
(25,122
|
)
|
$
|
(24,458
|
)
|
$
|
(21,639
|
)
|
$
|
(13,844
|
)
|
$
|
(15,060
|
)
|
$
|
(33,306
|
)
|
$
|
(24,611
|
)
|
$
|
(16,018
|
)
|
||||||||
|
Net loss per share attributable to Guardant Health, Inc. common stockholders, basic and diluted
|
$
|
(0.30
|
)
|
$
|
(1.94
|
)
|
$
|
(1.75
|
)
|
$
|
(1.16
|
)
|
$
|
(1.27
|
)
|
$
|
(2.76
|
)
|
$
|
(1.85
|
)
|
$
|
(1.22
|
)
|
||||||||
|
Weighted-average shares used in computing net loss per share attributable to Guardant Health, Inc. common stockholders, basic and diluted
|
84,123
|
|
12,582
|
|
12,388
|
|
11,920
|
|
11,851
|
|
12,073
|
|
13,276
|
|
13,182
|
|
||||||||||||||||
|
Year Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Cash used in operating activities
|
$
|
(72,185
|
)
|
$
|
(72,235
|
)
|
$
|
(36,710
|
)
|
||
|
Cash provided by (used in) investing activities
|
(153,028
|
)
|
(170,416
|
)
|
26,202
|
|
|||||
|
Cash provided by financing activities
|
293,161
|
|
281,656
|
|
39,840
|
|
|||||
|
Payments due by period
|
|||||||||||||||||||
|
Total
|
|
Less than
1 year
|
|
1-3 years
|
|
3-5 years
|
|
More than 5 years
|
|
||||||||||
|
(in thousands)
|
|||||||||||||||||||
|
Operating lease obligations(1)
|
$
|
42,178
|
|
$
|
4,099
|
|
$
|
10,631
|
|
$
|
11,662
|
|
$
|
15,786
|
|
||||
|
Capital lease obligation(2)
|
285
|
|
141
|
|
144
|
|
—
|
|
—
|
|
|||||||||
|
Royalty obligation(3)
|
13,449
|
|
1,431
|
|
2,862
|
|
3,434
|
|
5,722
|
|
|||||||||
|
Total
|
$
|
55,912
|
|
$
|
5,671
|
|
$
|
13,637
|
|
$
|
15,096
|
|
$
|
21,508
|
|
||||
|
(1)
|
We lease our office and laboratory space in Redwood City, California under an operating lease that expires in December 2025. We also have operating leases for manufacturing and office equipment through February 2023.
|
|
(2)
|
As of
December 31, 2018
, we had two capital leases for our manufacturing equipment which expire at various dates through April 2021.
|
|
(3)
|
We have patent license agreements with four parties. Under these agreements, we have made one-time and milestone license fee payments that we have capitalized and are amortizing to expense ratably over the useful life of the applicable underlying patent rights. Under some of these agreements, we are obligated to pay low single-digit percentage running royalties on net sales where the patent right(s) are used in the product or service sold, subject to minimum annual royalties or fees in certain agreements.
|
|
Year Ended December 31,
|
||||||
|
2018
|
2017
|
2016
|
||||
|
Expected term (in years)
|
5.01 – 6.51
|
6.02 – 6.08
|
6.02 – 6.43
|
|||
|
Expected volatility
|
68.7% – 78.8%
|
74.1% – 75.1%
|
65.2% – 67.7%
|
|||
|
Risk-free interest rate
|
2.5% – 3.0%
|
1.9% – 2.2%
|
1.2% – 2.3%
|
|||
|
Expected dividend yield
|
—%
|
—%
|
—%
|
|||
|
Page
|
|
|
As of December 31,
|
|||||||
|
2018
|
2017
|
||||||
|
ASSETS
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
140,544
|
|
$
|
72,280
|
|
|
|
Short-term marketable securities
|
278,417
|
|
149,040
|
|
|||
|
Accounts receivable
|
35,690
|
|
12,787
|
|
|||
|
Inventory
|
9,136
|
|
7,287
|
|
|||
|
Prepaid expenses and other current assets
|
5,204
|
|
1,541
|
|
|||
|
Total current assets
|
468,991
|
|
242,935
|
|
|||
|
Long-term marketable securities
|
77,563
|
|
73,254
|
|
|||
|
Property and equipment, net
|
31,003
|
|
16,036
|
|
|||
|
Capitalized license fees
|
7,800
|
|
8,739
|
|
|||
|
Other assets
|
2,046
|
|
1,974
|
|
|||
|
Total Assets
(1)
|
$
|
587,403
|
|
$
|
342,938
|
|
|
|
LIABILITIES, REDEEMABLE NONCONTROLLING INTEREST AND STOCKHOLDERS’ EQUITY
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
10,642
|
|
$
|
4,998
|
|
|
|
Accrued compensation
|
12,986
|
|
4,911
|
|
|||
|
Accrued expenses
|
7,081
|
|
6,406
|
|
|||
|
Capital lease, current
|
97
|
|
199
|
|
|||
|
Deferred revenue
|
16,138
|
|
3,113
|
|
|||
|
Total current liabilities
|
46,944
|
|
19,627
|
|
|||
|
Capital lease, net of current portion
|
119
|
|
460
|
|
|||
|
Deferred rent, net of current portion
|
7,844
|
|
6,537
|
|
|||
|
Obligation related to royalty
|
7,338
|
|
7,708
|
|
|||
|
Other long-term liabilities
|
206
|
|
—
|
|
|||
|
Total Liabilities
(1)
|
62,451
|
|
34,332
|
|
|||
|
Commitments and contingencies (Note 8)
|
|
|
|
|
|||
|
Redeemable noncontrolling interest
|
41,800
|
|
—
|
|
|||
|
Stockholders’ equity:
|
|||||||
|
Convertible preferred stock, par value of $0.00001 per share; no shares authorized, issued or outstanding as of December 31, 2018; 80,104,464 shares authorized, 78,627,369 shares issued and outstanding as of December 31, 2017
|
—
|
|
499,974
|
|
|||
|
Preferred stock, par value of $0.00001 per share; 10,000,000 shares authorized, no shares issued or outstanding as of December 31, 2018; no shares authorized, issued or outstanding as of December 31, 2017
|
—
|
|
—
|
|
|||
|
Common stock, par value of $0.00001 per share; 350,000,000 and 111,853,396 shares authorized as of December 31, 2018 and 2017, respectively; 85,832,454 and 11,896,882 shares issued and outstanding as of December 31, 2018 and 2017, respectively
|
1
|
|
—
|
|
|||
|
Additional paid-in capital
|
764,033
|
|
4,900
|
|
|||
|
Accumulated other comprehensive loss
|
(83
|
)
|
(532
|
)
|
|||
|
Accumulated deficit
|
(280,799
|
)
|
(195,736
|
)
|
|||
|
Total Stockholders’ Equity
|
483,152
|
|
308,606
|
|
|||
|
Total Liabilities, Redeemable Noncontrolling Interest and Stockholders’ Equity
|
$
|
587,403
|
|
$
|
342,938
|
|
|
|
Year Ended December 31,
|
||||||||||||
|
2018
|
2017
|
2016
|
||||||||||
|
Revenue:
|
||||||||||||
|
Precision oncology testing
|
$
|
78,407
|
|
$
|
42,088
|
|
$
|
24,496
|
|
|||
|
Development services
|
12,232
|
|
7,754
|
|
753
|
|
||||||
|
Total revenue
|
90,639
|
|
49,842
|
|
25,249
|
|
||||||
|
Costs and operating expenses:
|
||||||||||||
|
Cost of precision oncology testing
|
39,846
|
|
28,883
|
|
22,065
|
|
||||||
|
Cost of development services
|
3,364
|
|
2,735
|
|
59
|
|
||||||
|
Research and development expense
|
50,714
|
|
25,562
|
|
10,859
|
|
||||||
|
Sales and marketing expense
|
53,465
|
|
32,497
|
|
26,192
|
|
||||||
|
General and administrative expense
|
36,192
|
|
36,777
|
|
9,921
|
|
||||||
|
Total costs and operating expenses
|
183,581
|
|
126,454
|
|
69,096
|
|
||||||
|
Loss from operations
|
(92,942
|
)
|
(76,612
|
)
|
(43,847
|
)
|
||||||
|
Interest income
|
5,266
|
|
2,234
|
|
733
|
|
||||||
|
Interest expense
|
(1,251
|
)
|
(2,702
|
)
|
(3,018
|
)
|
||||||
|
Loss on debt extinguishment
|
—
|
|
(5,075
|
)
|
—
|
|
||||||
|
Other income (expense), net
|
4,702
|
|
(1,059
|
)
|
(1
|
)
|
||||||
|
Loss before provision for income taxes
|
(84,225
|
)
|
(83,214
|
)
|
(46,133
|
)
|
||||||
|
Provision for income taxes
|
38
|
|
7
|
|
6
|
|
||||||
|
Net loss
|
(84,263
|
)
|
(83,221
|
)
|
(46,139
|
)
|
||||||
|
Fair value adjustment of redeemable noncontrolling interest
|
(800
|
)
|
—
|
|
—
|
|
||||||
|
Net loss attributable to Guardant Health, Inc.
|
$
|
(85,063
|
)
|
$
|
(83,221
|
)
|
$
|
(46,139
|
)
|
|||
|
Deemed dividend related to repurchase of Series A convertible preferred stock
|
—
|
|
(4,716
|
)
|
—
|
|
||||||
|
Deemed dividend related to change in conversion rate of Series D convertible preferred stock
|
—
|
|
(1,058
|
)
|
—
|
|
||||||
|
Net loss attributable to Guardant Health, Inc. common stockholders
|
$
|
(85,063
|
)
|
$
|
(88,995
|
)
|
$
|
(46,139
|
)
|
|||
|
Net loss per share attributable to Guardant Health, Inc. common stockholders, basic and diluted
|
$
|
(2.80
|
)
|
$
|
(7.07
|
)
|
$
|
(3.53
|
)
|
|||
|
Weighted-average shares used in computing net loss per share attributable to Guardant Health, Inc. common stockholders, basic and diluted
|
30,403
|
|
12,582
|
|
13,053
|
|
||||||
|
Year Ended December 31,
|
||||||||||||
|
2018
|
2017
|
2016
|
||||||||||
|
Net loss
|
$
|
(84,263
|
)
|
$
|
(83,221
|
)
|
$
|
(46,139
|
)
|
|||
|
Other comprehensive income (loss), net of tax impact:
|
||||||||||||
|
Unrealized gain (loss) on available-for-sale securities
|
449
|
(446
|
)
|
(76
|
)
|
|||||||
|
Other comprehensive income (loss)
|
449
|
(446)
|
(76)
|
|||||||||
|
Comprehensive loss
|
$
|
(83,814
|
)
|
$
|
(83,667
|
)
|
$
|
(46,215
|
)
|
|||
|
Comprehensive loss attributable to redeemable noncontrolling interest
|
(800
|
)
|
—
|
|
—
|
|
||||||
|
Comprehensive loss attributable to Guardant Health, Inc.
|
$
|
(84,614
|
)
|
$
|
(83,667
|
)
|
$
|
(46,215
|
)
|
|||
|
Redeemable Noncontrolling Interest
|
|
Convertible
Preferred Stock |
Common Stock
|
Additional
Paid-in Capital |
|
Accumulated
Other
Comprehensive Loss
|
|
Accumulated
Deficit |
|
Total Stockholders’ Equity
|
|
||||||||||||||||||||||
|
Shares
|
|
Amount
|
|
Shares
|
|
Amount
|
|
||||||||||||||||||||||||||
|
Balance as of December 31, 2015
|
$
|
—
|
|
34,821,414
|
|
$
|
139,948
|
|
13,109,829
|
|
$
|
—
|
|
$
|
4,990
|
|
$
|
(10
|
)
|
$
|
(61,486
|
)
|
$
|
83,442
|
|
||||||||
|
Issuance of Series D convertible preferred stock, net of issuance cost of $30
|
—
|
|
5,360,509
|
|
40,049
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
40,049
|
|
|||||||||||||||
|
Issuance of common stock upon exercise of stock options
|
—
|
|
—
|
|
—
|
|
132,681
|
|
—
|
|
266
|
|
—
|
|
—
|
|
266
|
|
|||||||||||||||
|
Issuance of common stock upon early exercise of stock options
|
—
|
|
—
|
|
—
|
|
1,844
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||
|
Issuance of common stock upon exercise of warrants
|
—
|
|
—
|
|
—
|
|
1,589
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||
|
Vesting of common stock exercised early
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
283
|
|
—
|
|
—
|
|
283
|
|
|||||||||||||||
|
Repurchase of common stock
|
—
|
|
—
|
|
—
|
|
(61,729
|
)
|
—
|
|
(100
|
)
|
—
|
|
—
|
|
(100
|
)
|
|||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
|
—
|
|
1,971
|
|
—
|
|
—
|
|
1,971
|
|
||||||||||||||||
|
Other comprehensive loss, net of tax impact
|
—
|
|
—
|
|
—
|
|
|
—
|
|
—
|
|
(76
|
)
|
—
|
|
(76
|
)
|
||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
|
—
|
|
—
|
|
—
|
|
(46,139
|
)
|
(46,139
|
)
|
||||||||||||||||
|
Balance as of December 31, 2016
|
—
|
|
40,181,923
|
|
179,997
|
|
13,184,214
|
|
—
|
|
7,410
|
|
(86
|
)
|
(107,625
|
)
|
79,696
|
|
|||||||||||||||
|
Cumulative effect adjustment for ASU 2016-09 adoption
|
—
|
|
—
|
|
—
|
|
|
—
|
|
174
|
|
—
|
|
(174
|
)
|
—
|
|
||||||||||||||||
|
Issuance of Series D convertible preferred stock in exchange for a technology license agreement
|
—
|
|
141,774
|
|
1,060
|
|
|
—
|
|
—
|
|
—
|
|
—
|
|
1,060
|
|
||||||||||||||||
|
Issuance of Series E convertible preferred stock, net of issuance cost of $883
|
—
|
|
38,970,592
|
|
319,536
|
|
|
—
|
|
—
|
|
—
|
|
—
|
|
319,536
|
|
||||||||||||||||
|
Repurchase of Series A convertible preferred stock
|
—
|
|
(666,920
|
)
|
(619
|
)
|
|
—
|
|
—
|
|
—
|
|
(4,716
|
)
|
(5,335
|
)
|
||||||||||||||||
|
Issuance of common stock upon exercise of stock options
|
—
|
|
—
|
|
—
|
|
342,946
|
|
—
|
|
753
|
|
—
|
|
—
|
|
753
|
|
|||||||||||||||
|
Issuance of common stock upon exercise of warrants
|
—
|
|
—
|
|
—
|
|
89,030
|
|
—
|
|
12
|
|
—
|
|
—
|
|
12
|
|
|||||||||||||||
|
Vesting of common stock exercised early
|
—
|
|
—
|
|
—
|
|
|
—
|
|
103
|
|
—
|
|
—
|
|
103
|
|
||||||||||||||||
|
Repurchase of common stock
|
—
|
|
—
|
|
—
|
|
(1,719,308
|
)
|
—
|
|
(7,222
|
)
|
—
|
|
—
|
|
(7,222
|
)
|
|||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
|
—
|
|
3,670
|
|
—
|
|
—
|
|
3,670
|
|
||||||||||||||||
|
Other comprehensive loss, net of tax impact
|
—
|
|
—
|
|
—
|
|
|
—
|
|
—
|
|
(446
|
)
|
—
|
|
(446
|
)
|
||||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
|
—
|
|
—
|
|
—
|
|
(83,221
|
)
|
(83,221
|
)
|
||||||||||||||||
|
Balance as of December 31, 2017
|
—
|
|
78,627,369
|
|
499,974
|
|
11,896,882
|
|
—
|
|
4,900
|
|
(532
|
)
|
(195,736
|
)
|
308,606
|
|
|||||||||||||||
|
Conversion of convertible preferred stock to common stock upon initial public offering
|
—
|
|
(78,627,369
|
)
|
(499,974
|
)
|
58,264,577
|
|
1
|
|
499,973
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||
|
Issuance of common stock upon initial public offering, net of offering costs of $4,475
|
—
|
|
—
|
|
—
|
|
14,375,000
|
|
—
|
|
249,531
|
|
—
|
|
—
|
|
249,531
|
|
|||||||||||||||
|
Issuance of common stock upon exercise of stock options
|
—
|
|
—
|
|
—
|
|
963,119
|
|
—
|
|
2,905
|
|
—
|
|
—
|
|
2,905
|
|
|||||||||||||||
|
Issuance of common stock upon early exercise of stock options
|
—
|
|
—
|
|
—
|
|
44,268
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||
|
Issuance of common stock upon exercise of warrants
|
—
|
|
—
|
|
—
|
|
320,289
|
|
—
|
|
45
|
|
—
|
|
—
|
|
45
|
|
|||||||||||||||
|
Repurchase of common stock
|
—
|
|
—
|
|
—
|
|
(31,681
|
)
|
—
|
|
(172
|
)
|
—
|
|
—
|
|
(172
|
)
|
|||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
6,851
|
|
—
|
|
—
|
|
6,851
|
|
|||||||||||||||
|
Issuance of equity interests in redeemable noncontrolling interest
|
41,000
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||
|
Fair value adjustment of redeemable noncontrolling interest
|
800
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(800
|
)
|
(800
|
)
|
|||||||||||||||
|
Other comprehensive loss, net of tax impact
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
449
|
|
—
|
|
449
|
|
|||||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(84,263
|
)
|
(84,263
|
)
|
|||||||||||||||
|
Balance as of December 31, 2018
|
$
|
41,800
|
|
—
|
|
$
|
—
|
|
85,832,454
|
|
$
|
1
|
|
$
|
764,033
|
|
$
|
(83
|
)
|
$
|
(280,799
|
)
|
$
|
483,152
|
|
||||||||
|
Year Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
OPERATING ACTIVITIES:
|
|||||||||||
|
Net loss
|
$
|
(84,263
|
)
|
$
|
(83,221
|
)
|
$
|
(46,139
|
)
|
||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|||||||||||
|
Depreciation and amortization
|
7,136
|
|
5,206
|
|
3,693
|
|
|||||
|
Unrealized translation (gains) losses on obligation related to royalty
|
(357
|
)
|
980
|
|
—
|
|
|||||
|
Non-cash stock-based compensation
|
6,851
|
|
3,670
|
|
1,971
|
|
|||||
|
Non-cash interest expense
|
(13
|
)
|
685
|
|
1,024
|
|
|||||
|
Loss on debt extinguishment
|
—
|
|
5,075
|
|
—
|
|
|||||
|
Amortization of premium (discount) on marketable securities
|
(412
|
)
|
359
|
|
287
|
|
|||||
|
Changes in operating assets and liabilities:
|
|||||||||||
|
Accounts receivable
|
(22,903
|
)
|
(9,292
|
)
|
(2,786
|
)
|
|||||
|
Inventory
|
(1,849
|
)
|
(4,518
|
)
|
(351
|
)
|
|||||
|
Prepaid expenses and other current assets
|
(3,663
|
)
|
30
|
|
(610
|
)
|
|||||
|
Other assets
|
(451
|
)
|
(883
|
)
|
12
|
|
|||||
|
Accounts payable
|
5,046
|
|
1,250
|
|
2,145
|
|
|||||
|
Accrued compensation
|
8,075
|
|
2,348
|
|
1,374
|
|
|||||
|
Accrued expenses and other current liabilities
|
286
|
|
4,657
|
|
508
|
|
|||||
|
Deferred rent
|
1,307
|
|
204
|
|
467
|
|
|||||
|
Deferred revenue
|
13,025
|
|
1,215
|
|
1,695
|
|
|||||
|
Net cash used in operating activities
|
(72,185
|
)
|
(72,235
|
)
|
(36,710
|
)
|
|||||
|
INVESTING ACTIVITIES:
|
|||||||||||
|
Purchase of marketable securities
|
(287,450
|
)
|
(236,835
|
)
|
(341,415
|
)
|
|||||
|
Maturity of marketable securities
|
154,625
|
|
75,402
|
|
369,383
|
|
|||||
|
Purchase of property and equipment
|
(20,203
|
)
|
(6,681
|
)
|
(1,766
|
)
|
|||||
|
Payment in connection with a license agreement
|
—
|
|
(2,302
|
)
|
—
|
|
|||||
|
Net cash provided by (used in) investing activities
|
(153,028
|
)
|
(170,416
|
)
|
26,202
|
|
|||||
|
FINANCING ACTIVITIES:
|
|||||||||||
|
Payment related to settlement of debt and buyout of royalty obligations
|
—
|
|
(25,844
|
)
|
(379
|
)
|
|||||
|
Payments made on capital lease obligations
|
(443
|
)
|
(244
|
)
|
4
|
|
|||||
|
Proceeds from issuance of convertible preferred stock, net of issuance costs
|
—
|
|
319,536
|
|
40,049
|
|
|||||
|
Proceeds from issuance of common stock upon exercise of stock options
|
3,111
|
|
753
|
|
266
|
|
|||||
|
Proceeds from issuance of common stock upon the exercise of warrants
|
45
|
|
12
|
|
—
|
|
|||||
|
Repurchase of convertible preferred stock
|
—
|
|
(5,335
|
)
|
—
|
|
|||||
|
Repurchase of common stock
|
(172
|
)
|
(7,222
|
)
|
(100
|
)
|
|||||
|
Proceeds from initial public offering, net of underwriting discounts and commissions
|
254,006
|
|
—
|
|
—
|
|
|||||
|
Payment of offering costs related to initial public offering
|
(4,386
|
)
|
—
|
|
—
|
|
|||||
|
Net proceeds from issuance of equity interests in redeemable noncontrolling interest
|
41,000
|
|
—
|
|
—
|
|
|||||
|
Net cash provided by financing activities
|
293,161
|
|
281,656
|
|
39,840
|
|
|||||
|
Net increase in cash, cash equivalents and restricted cash
|
67,948
|
|
39,005
|
|
29,332
|
|
|||||
|
Cash, cash equivalents and restricted cash - Beginning of period
|
72,596
|
|
33,591
|
|
4,259
|
|
|||||
|
Cash, cash equivalents and restricted cash - End of period
|
$
|
140,544
|
|
$
|
72,596
|
|
$
|
33,591
|
|
||
|
Supplemental Disclosures of Cash Flow Information:
|
|||||||||||
|
Cash paid for interest
|
$
|
1,251
|
|
$
|
1,339
|
|
$
|
2,243
|
|
||
|
Cash paid for income taxes
|
$
|
102
|
|
$
|
26
|
|
$
|
14
|
|
||
|
Supplemental Disclosures of Noncash Investing and Financing Activities:
|
|||||||||||
|
Capitalized license fees financed through future royalty payment
|
$
|
—
|
|
$
|
6,302
|
|
$
|
—
|
|
||
|
Issuance of Series D convertible preferred stock in exchange for a technology license agreement
|
$
|
—
|
|
$
|
1,060
|
|
$
|
—
|
|
||
|
Increase in purchases of property and equipment included in accounts payable and accrued expenses
|
$
|
897
|
|
$
|
591
|
|
$
|
34
|
|
||
|
Vesting of common stock exercised early
|
$
|
—
|
|
$
|
103
|
|
$
|
283
|
|
||
|
Property and equipment acquired under capital leases
|
$
|
—
|
|
$
|
346
|
|
$
|
468
|
|
||
|
Conversion of convertible preferred stock to common stock upon initial public offering
|
$
|
499,974
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Deferred offering costs included in accounts payable and accrued expenses
|
$
|
89
|
|
$
|
—
|
|
$
|
—
|
|
||
|
1.
|
Description of Business
|
|
2.
|
Summary of Significant Accounting Policies
|
|
As of December 31,
|
|||||||
|
2018
|
2017
|
||||||
|
(in thousands)
|
|||||||
|
Cash and cash equivalents
|
$
|
140,544
|
|
$
|
72,280
|
|
|
|
Restricted cash
|
—
|
|
316
|
|
|||
|
Total cash and cash equivalents and restricted cash
|
$
|
140,544
|
|
$
|
72,596
|
|
|
|
Revenue
|
Accounts Receivable
|
|||||||||||||
|
Year Ended December 31,
|
As of December 31,
|
|||||||||||||
|
2018
|
2017
|
2016
|
2018
|
2017
|
||||||||||
|
Customer A
|
*
|
|
13
|
%
|
19
|
%
|
*
|
|
*
|
|
||||
|
Customer B
|
18
|
%
|
13
|
%
|
*
|
|
65
|
%
|
24
|
%
|
||||
|
Customer C
|
*
|
|
*
|
|
*
|
|
*
|
|
23
|
%
|
||||
|
Customer D
|
*
|
|
*
|
|
*
|
|
*
|
|
13
|
%
|
||||
|
*
|
less than 10%
|
|
Property and Equipment
|
Estimated Useful Life
|
|
|
Machinery and equipment
|
3 – 5 years
|
|
|
Furniture and fixtures
|
7 years
|
|
|
Computer hardware and computer software
|
3 years
|
|
|
Leasehold improvements
|
Lesser of estimated useful life or remaining lease term
|
|
|
3.
|
Investment in Joint Venture
|
|
4.
|
Consolidated Balance Sheet Components
|
|
As of December 31,
|
|||||||
|
2018
|
2017
|
||||||
|
(in thousands)
|
|||||||
|
Machinery and equipment
|
$
|
23,440
|
|
$
|
15,676
|
|
|
|
Computer hardware
|
4,949
|
|
1,939
|
|
|||
|
Leasehold improvements
|
13,965
|
|
6,766
|
|
|||
|
Furniture and fixtures
|
1,522
|
|
1,347
|
|
|||
|
Computer software
|
643
|
|
655
|
|
|||
|
Construction in progress
|
3,118
|
|
349
|
|
|||
|
Property and equipment, gross
|
47,637
|
|
26,732
|
|
|||
|
Less: accumulated depreciation and amortization
|
(16,634
|
)
|
(10,696
|
)
|
|||
|
Property and equipment, net
|
$
|
31,003
|
|
$
|
16,036
|
|
|
|
As of December 31,
|
|||||||
|
2018
|
2017
|
||||||
|
(in thousands)
|
|||||||
|
Accrued royalty obligations
|
$
|
707
|
|
$
|
766
|
|
|
|
Accrued litigation settlement expense
|
—
|
|
3,000
|
|
|||
|
Accrued legal expenses
|
814
|
|
561
|
|
|||
|
Accrued tax liabilities
|
1,470
|
|
905
|
|
|||
|
Accrued professional services
|
1,791
|
|
336
|
|
|||
|
Purchases of property and equipment included in accrued expenses
|
343
|
|
—
|
|
|||
|
Other
|
1,956
|
|
838
|
|
|||
|
Total accrued expenses
|
$
|
7,081
|
|
$
|
6,406
|
|
|
|
5.
|
Fair Value Measurements, Cash Equivalents and Marketable Securities
|
|
December 31, 2018
|
|||||||||||||||
|
Fair Value
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Financial Assets:
|
|||||||||||||||
|
Money market funds
|
$
|
25,796
|
|
$
|
25,796
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Total cash equivalents
|
25,796
|
|
25,796
|
|
—
|
|
—
|
|
|||||||
|
Corporate bonds
|
38,397
|
|
—
|
|
38,397
|
|
—
|
|
|||||||
|
U.S. government debt securities
|
235,016
|
|
—
|
|
235,016
|
|
—
|
|
|||||||
|
U.S. government agency bonds
|
5,004
|
|
—
|
|
5,004
|
|
—
|
|
|||||||
|
Total short-term marketable securities
|
278,417
|
|
—
|
|
278,417
|
|
—
|
|
|||||||
|
Corporate bonds
|
3,805
|
|
—
|
|
3,805
|
|
—
|
|
|||||||
|
U.S. government debt securities
|
73,758
|
|
—
|
|
73,758
|
|
—
|
|
|||||||
|
Total long-term marketable securities
|
77,563
|
|
—
|
|
77,563
|
|
—
|
|
|||||||
|
Total
|
$
|
381,776
|
|
$
|
25,796
|
|
$
|
355,980
|
|
$
|
—
|
|
|||
|
December 31, 2017
|
|||||||||||||||
|
Fair Value
|
Level 1
|
Level 2
|
Level 3
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Financial Assets:
|
|||||||||||||||
|
Money market funds
|
$
|
33,485
|
|
$
|
33,485
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Total cash equivalents
|
33,485
|
|
33,485
|
|
—
|
|
—
|
|
|||||||
|
Corporate bonds
|
48,075
|
|
—
|
|
48,075
|
|
—
|
|
|||||||
|
U.S. government debt securities
|
100,965
|
|
—
|
|
100,965
|
|
—
|
|
|||||||
|
Total short-term marketable securities
|
149,040
|
|
—
|
|
149,040
|
|
—
|
|
|||||||
|
Corporate bonds
|
6,698
|
|
—
|
|
6,698
|
|
—
|
|
|||||||
|
U.S. government debt securities
|
66,556
|
|
—
|
|
66,556
|
|
—
|
|
|||||||
|
Total long-term marketable securities
|
73,254
|
|
—
|
|
73,254
|
|
—
|
|
|||||||
|
Total
|
$
|
255,779
|
|
$
|
33,485
|
|
$
|
222,294
|
|
$
|
—
|
|
|||
|
December 31, 2018
|
|||||||||||||||
|
Amortized Cost
|
Gross Unrealized Gain
|
Gross Unrealized Loss
|
Estimated Fair Value
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Money market fund
|
$
|
25,796
|
|
$
|
—
|
|
$
|
—
|
|
$
|
25,796
|
|
|||
|
Corporate bond
|
42,273
|
|
—
|
|
(71
|
)
|
42,202
|
|
|||||||
|
U.S. government debt securities
|
308,775
|
|
235
|
|
(236
|
)
|
308,774
|
|
|||||||
|
U.S. government agency bonds
|
$
|
5,014
|
|
$
|
—
|
|
$
|
(10
|
)
|
$
|
5,004
|
|
|||
|
Total
|
$
|
381,858
|
|
$
|
235
|
|
$
|
(317
|
)
|
$
|
381,776
|
|
|||
|
December 31, 2017
|
|||||||||||||||
|
Amortized Cost
|
Gross Unrealized Gain
|
Gross Unrealized Loss
|
Estimated Fair Value
|
||||||||||||
|
(in thousands)
|
|||||||||||||||
|
Money market fund
|
$
|
33,485
|
|
$
|
—
|
|
$
|
—
|
|
$
|
33,485
|
|
|||
|
Corporate bond
|
54,878
|
|
—
|
|
(105
|
)
|
54,773
|
|
|||||||
|
U.S. government debt securities
|
167,947
|
|
—
|
|
(426
|
)
|
167,521
|
|
|||||||
|
Total
|
$
|
256,310
|
|
$
|
—
|
|
$
|
(531
|
)
|
$
|
255,779
|
|
|||
|
6.
|
Patent License Agreement
|
|
7.
|
Senior Term Loan and Royalty Purchase Agreement
|
|
8
.
|
Commitments and Contingencies
|
|
Year Ending December 31,
|
|||
|
(in thousands)
|
|||
|
2019
|
$
|
4,099
|
|
|
2020
|
5,273
|
|
|
|
2021
|
5,358
|
|
|
|
2022
|
5,557
|
|
|
|
2023
|
6,105
|
|
|
|
2024 and thereafter
|
15,786
|
|
|
|
Total
|
$
|
42,178
|
|
|
Year Ending December 31,
|
|||
|
(in thousands)
|
|||
|
2019
|
$
|
141
|
|
|
2020
|
108
|
|
|
|
2021
|
36
|
|
|
|
Total minimum capital lease payments
|
285
|
|
|
|
Less: amount representing interest
|
(69
|
)
|
|
|
Present value of net minimum capital lease payments
|
216
|
|
|
|
Less: current installments of obligations under capital lease
|
(97
|
)
|
|
|
Obligations under capital lease, excluding current installments
|
$
|
119
|
|
|
Year Ending December 31,
|
|||
|
(in thousands)
|
|||
|
2019
|
$
|
1,431
|
|
|
2020
|
1,431
|
|
|
|
2021
|
1,431
|
|
|
|
2022
|
1,717
|
|
|
|
2023
|
1,717
|
|
|
|
2024 and thereafter
|
5,722
|
|
|
|
Total future minimum royalty payments
|
13,449
|
|
|
|
Less: amount representing interest
|
(6,111
|
)
|
|
|
Present value of future minimum royalty payments
|
$
|
7,338
|
|
|
9.
|
Common Stock
|
|
As of December 31,
|
||||
|
2018
|
2017
|
|||
|
Conversion of outstanding convertible preferred stock
|
—
|
|
58,264,577
|
|
|
Shares underlying outstanding stock options
|
7,588,405
|
7,391,052
|
||
|
Shares available for future stock option grants
|
3,556,507
|
1,698,790
|
||
|
Exercise and conversion of preferred stock warrants
|
—
|
|
7,636
|
|
|
Exercise of common stock warrants
|
—
|
|
313,741
|
|
|
Total
|
11,144,912
|
67,675,796
|
||
|
10.
|
Warrants
|
|
11.
|
Convertible Preferred Stock
|
|
December 31, 2017
|
|||||||||||
|
Shares Authorized
|
Shares Issued and Outstanding
|
Aggregate Liquidation Preference
|
Net Carrying Value
|
||||||||
|
(in thousands)
|
|||||||||||
|
Series A
|
9,935,864
|
9,263,558
|
$
|
8,598
|
|
$
|
8,531
|
|
|||
|
Series B
|
10,320,952
|
10,297,182
|
32,490
|
|
32,428
|
|
|||||
|
Series C
|
8,873,996
|
8,873,996
|
55,999
|
|
55,921
|
|
|||||
|
Series D
|
11,222,041
|
11,222,041
|
83,904
|
|
83,559
|
|
|||||
|
Series E
|
39,751,611
|
38,970,592
|
320,419
|
|
319,535
|
|
|||||
|
Total convertible preferred stock
|
80,104,464
|
78,627,369
|
$
|
501,410
|
|
$
|
499,974
|
|
|||
|
12.
|
Stock-Based Compensation
|
|
Options Outstanding
|
|||||||||||||||
|
Shares
Available for Grant
|
Shares Subject to Options Outstanding
|
Weighted-Average Exercise Price
|
Weighted-Average Remaining Contractual Life (Years)
|
Aggregate Intrinsic Value
|
|||||||||||
|
(in thousands)
|
|||||||||||||||
|
Balance as of December 31, 2016
|
699,462
|
|
3,592,185
|
|
$
|
2.77
|
|
8.6
|
$
|
3,325
|
|
||||
|
Shares authorized
|
5,141,141
|
|
—
|
|
|||||||||||
|
Granted
|
(4,685,577
|
)
|
4,685,577
|
|
4.17
|
|
|||||||||
|
Exercised
|
—
|
|
(342,946
|
)
|
2.25
|
|
|||||||||
|
Canceled
|
543,764
|
|
(543,764
|
)
|
3.48
|
|
|||||||||
|
Balance as of December 31, 2017
|
1,698,790
|
|
7,391,052
|
|
3.63
|
|
8.8
|
7,595
|
|
||||||
|
Shares authorized
|
3,658,602
|
|
—
|
|
|||||||||||
|
Shares forfeited
|
(508,847
|
)
|
—
|
|
|||||||||||
|
Granted
|
(2,088,639
|
)
|
2,088,639
|
|
7.19
|
|
|||||||||
|
Exercised
|
—
|
|
(1,007,387
|
)
|
3.09
|
|
|||||||||
|
Canceled
|
795,371
|
|
(883,899
|
)
|
4.57
|
|
|||||||||
|
Repurchase of early exercised shares
|
1,230
|
|
—
|
|
|||||||||||
|
Balance as of December 31, 2018
|
3,556,507
|
|
7,588,405
|
|
$
|
4.58
|
|
8.3
|
$
|
250,495
|
|
||||
|
Vested and Exercisable as of December 31, 2018
|
3,066,648
|
|
$
|
3.49
|
|
7.5
|
$
|
104,560
|
|
||||||
|
Year Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Cost of precision oncology testing
|
$
|
512
|
|
$
|
162
|
|
$
|
225
|
|
||
|
Research and development expense
|
1,684
|
|
507
|
|
479
|
|
|||||
|
Sales and marketing expense
|
1,727
|
|
80
|
|
1,031
|
|
|||||
|
General and administrative expense
|
2,928
|
|
2,921
|
|
236
|
|
|||||
|
Total stock-based compensation expense
|
$
|
6,851
|
|
$
|
3,670
|
|
$
|
1,971
|
|
||
|
Year Ended December 31,
|
||||||
|
2018
|
2017
|
2016
|
||||
|
Expected term (in years)
|
5.01 – 6.51
|
6.02 – 6.08
|
6.02 – 6.43
|
|||
|
Expected volatility
|
68.7% – 78.8%
|
74.1% – 75.1%
|
65.2% – 67.7%
|
|||
|
Risk-free interest rate
|
2.5% – 3.0%
|
1.9% – 2.2%
|
1.2% – 2.3%
|
|||
|
Expected dividend yield
|
—%
|
—%
|
—%
|
|||
|
Year Ended December 31,
|
||||||
|
2018
|
2017
|
2016
|
||||
|
Expected term (in years)
|
5.1 – 10.0
|
6.0 – 10.0
|
6.0 – 10.0
|
|||
|
Expected volatility
|
64.9% – 79.4%
|
66.1% – 73.4%
|
66.2% – 69.5%
|
|||
|
Risk-free interest rate
|
2.3% – 3.2%
|
1.2% – 2.3%
|
1.2% – 2.3%
|
|||
|
Expected dividend yield
|
—%
|
—%
|
—%
|
|||
|
13.
|
Net Loss Per Share Attributable to Guardant Health, Inc. Common Stockholders
|
|
Year Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
(in thousands, except per share data)
|
|||||||||||
|
Net loss
|
$
|
(84,263
|
)
|
$
|
(83,221
|
)
|
$
|
(46,139
|
)
|
||
|
Fair value adjustment of redeemable noncontrolling interest
|
(800
|
)
|
—
|
|
—
|
|
|||||
|
Net loss attributable to Guardant Health, Inc.
|
(85,063
|
)
|
(83,221
|
)
|
(46,139
|
)
|
|||||
|
Deemed dividend related to repurchase of Series A convertible preferred stock
|
—
|
|
(4,716
|
)
|
—
|
|
|||||
|
Deemed dividend related to change in conversion rate of Series D convertible preferred stock
|
—
|
|
(1,058
|
)
|
—
|
|
|||||
|
Net loss attributable to Guardant Health, Inc. common stockholders, basic and diluted
|
$
|
(85,063
|
)
|
$
|
(88,995
|
)
|
$
|
(46,139
|
)
|
||
|
Net loss per share attributable to Guardant Health, Inc. common stockholders, basic and diluted
|
$
|
(2.80
|
)
|
$
|
(7.07
|
)
|
$
|
(3.53
|
)
|
||
|
Weighted-average shares used in computing net loss per share attributable to Guardant Health, Inc. common stockholders, basic and diluted
|
30,403
|
|
12,582
|
|
13,053
|
|
|||||
|
Year Ended December 31,
|
||||||||
|
2018
|
2017
|
2016
|
||||||
|
(in thousands)
|
||||||||
|
Convertible preferred stock (on an as if converted basis)
|
43,898
|
|
44,818
|
|
29,009
|
|
||
|
Stock options issued and outstanding
|
7,527
|
|
5,179
|
|
3,043
|
|
||
|
ESPP obligation
|
22
|
|
—
|
|
—
|
|
||
|
Preferred stock warrants (on an as if converted basis)
|
6
|
|
8
|
|
8
|
|
||
|
Common stock warrants
|
208
|
|
382
|
|
404
|
|
||
|
Common stock subject to repurchase
|
46
|
|
28
|
|
101
|
|
||
|
Total
|
51,707
|
|
50,415
|
|
32,565
|
|
||
|
14.
|
Income Taxes
|
|
Year Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
(in thousands)
|
|||||||||||
|
United States
|
$
|
(84,313
|
)
|
$
|
(83,214
|
)
|
$
|
(46,133
|
)
|
||
|
Foreign
|
88
|
|
—
|
|
—
|
|
|||||
|
Total
|
$
|
(84,225
|
)
|
$
|
(83,214
|
)
|
$
|
(46,133
|
)
|
||
|
Year Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Current:
|
|||||||||||
|
State
|
$
|
4
|
|
$
|
—
|
|
$
|
—
|
|
||
|
Foreign
|
34
|
|
7
|
|
6
|
|
|||||
|
Total current tax expense
|
38
|
|
7
|
|
6
|
|
|||||
|
Total provision for income taxes
|
$
|
38
|
|
$
|
7
|
|
$
|
6
|
|
||
|
Year Ended December 31,
|
|||||||
|
2018
|
2017
|
||||||
|
(in thousands)
|
|||||||
|
Deferred tax assets:
|
|||||||
|
Net operating losses carryforwards
|
$
|
36,783
|
|
$
|
25,657
|
|
|
|
Intangible assets
|
17,107
|
|
10,436
|
|
|||
|
Accruals and reserves
|
5,127
|
|
3,553
|
|
|||
|
Research and development credits
|
5,753
|
|
2,823
|
|
|||
|
Stock-based compensation
|
1,289
|
|
727
|
|
|||
|
Other
|
210
|
|
484
|
|
|||
|
Total deferred tax asset
|
$
|
66,269
|
|
$
|
43,680
|
|
|
|
Deferred tax liabilities:
|
|||||||
|
Property and equipment
|
$
|
(73
|
)
|
$
|
—
|
|
|
|
Less: valuation allowance
|
(66,196
|
)
|
(43,680
|
)
|
|||
|
Net deferred tax assets
|
$
|
—
|
|
$
|
—
|
|
|
|
Year Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Tax at the statutory federal rate
|
$
|
(17,690
|
)
|
$
|
(28,293
|
)
|
$
|
(15,687
|
)
|
||
|
Other nondeductible items
|
329
|
|
371
|
|
360
|
|
|||||
|
Stock-based compensation
|
497
|
|
3,819
|
|
413
|
|
|||||
|
Research and development credits
|
(1,726
|
)
|
(714
|
)
|
(304
|
)
|
|||||
|
Change in valuation allowance
|
22,516
|
|
5,415
|
|
16,640
|
|
|||||
|
State taxes, net of federal benefits
|
(4,231
|
)
|
(1,868
|
)
|
(1,224
|
)
|
|||||
|
Change in tax rate due to Tax Act
|
—
|
|
21,346
|
|
—
|
|
|||||
|
Other
|
343
|
|
(69
|
)
|
(192
|
)
|
|||||
|
Total provision for income taxes
|
$
|
38
|
|
$
|
7
|
|
$
|
6
|
|
||
|
Year Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
(in thousands)
|
|||||||||||
|
Unrecognized tax benefits - Beginning of period
|
$
|
1,712
|
|
$
|
884
|
|
$
|
1,691
|
|
||
|
Increases related to current year’s tax positions
|
1,635
|
|
828
|
|
353
|
|
|||||
|
Increases related to prior years' tax positions
|
80
|
|
—
|
|
—
|
|
|||||
|
Decreases related to prior years’ tax positions
|
—
|
|
—
|
|
(1,160
|
)
|
|||||
|
Unrecognized tax benefits - End of period
|
$
|
3,427
|
|
$
|
1,712
|
|
$
|
884
|
|
||
|
15.
|
Employee Benefit Plan
|
|
16.
|
Segment and Geographic Information
|
|
Year Ended December 31,
|
|||||||||||
|
2018
|
2017
|
2016
|
|||||||||
|
(in thousands)
|
|||||||||||
|
United States
|
$
|
77,916
|
|
$
|
43,715
|
|
$
|
21,641
|
|
||
|
International(1)
|
12,723
|
|
6,127
|
|
3,608
|
|
|||||
|
Total revenue
|
$
|
90,639
|
|
$
|
49,842
|
|
$
|
25,249
|
|
||
|
(1)
|
No single country outside of the United States accounted for more than 10% of total revenue during the year ended
December 31, 2018
,
2017
and
2016
.
|
|
17.
|
Related Party Transactions
|
|
Incorporated by Reference
|
||||||||||||
|
Exhibit Number
|
Exhibit Description
|
Form
|
File No.
|
Exhibit
|
Filing Date
|
Filed/Furnished Herewith
|
||||||
|
3.1
|
8-K
|
001-38683
|
3.1
|
10/9/2018
|
||||||||
|
3.2
|
8-K
|
001-38683
|
3.2
|
10/9/2018
|
||||||||
|
10.1
|
S-1
|
333-227206
|
10.1
|
9/6/2018
|
||||||||
|
10.2#
|
S-1
|
333-227206
|
10.3
|
9/6/2018
|
||||||||
|
10.2(a)#
|
S-1
|
333-227206
|
10.4
|
9/6/2018
|
||||||||
|
10.3#
|
S-8
|
333-227762
|
99.2(a)
|
10/10/2018
|
||||||||
|
10.3(a)#
|
S-1/A
|
333-227206
|
10.9(a)
|
9/21/2018
|
||||||||
|
10.3(b)#
|
S-1/A
|
333-227206
|
10.9(b)
|
9/21/2018
|
||||||||
|
10.3(c)#
|
S-1/A
|
333-227206
|
10.9(c)
|
9/21/2018
|
||||||||
|
10.4#
|
S-8
|
333-227762
|
99.3
|
10/10/2018
|
||||||||
|
10.4(a)#
|
*
|
|||||||||||
|
10.5#
|
Executive Severance Plan
|
S-1/A
|
333-227206
|
10.13
|
9/21/2018
|
|||||||
|
10.5(a)#
|
*
|
|||||||||||
|
10.6#
|
S-1/A
|
333-227206
|
10.14
|
9/21/2018
|
||||||||
|
10.7#
|
10-Q
|
001-38683
|
10.9
|
11/19/2018
|
||||||||
|
10.8#
|
10-Q
|
001-38683
|
10.10
|
11/19/2018
|
||||||||
|
10.9
|
S-1/A
|
333-227206
|
10.8
|
9/18/2018
|
||||||||
|
10.10
|
S-1
|
333-227206
|
10.2
|
9/6/2018
|
||||||||
|
10.11
|
S-1
|
333-227206
|
10.2(a)
|
9/6/2018
|
||||||||
|
10.12§
|
S-1
|
333-227206
|
10.5
|
9/6/2018
|
||||||||
|
10.13§
|
S-1
|
333-227206
|
10.6
|
9/6/2018
|
||||||||
|
10.14§
|
S-1
|
333-227206
|
10.7
|
9/6/2018
|
||||||||
|
10.15§
|
S-1
|
333-227206
|
10.7(a)
|
9/6/2018
|
||||||||
|
10.16§
|
S-1
|
333-227206
|
10.7(b)
|
9/6/2018
|
||||||||
|
10.17§
|
S-1
|
333-227206
|
10.7(c)
|
9/6/2018
|
||||||||
|
10.18§
|
S-1
|
333-227206
|
10.7(d)
|
9/6/2018
|
||||||||
|
10.19#
|
*
|
|||||||||||
|
21.1
|
*
|
|||||||||||
|
23.1
|
*
|
|||||||||||
|
31.1
|
*
|
|||||||||||
|
31.2
|
*
|
|||||||||||
|
32.1
|
**
|
|||||||||||
|
32.2
|
**
|
|||||||||||
|
101.INS
|
XBRL Instance Document
|
*
|
||||||||||
|
101.SCH
|
XBRL Taxonomy Extension Schema Document
|
*
|
||||||||||
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
*
|
||||||||||
|
101.DEF
|
XBRL Taxonomy Extension Definition Linkbase Document
|
*
|
||||||||||
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document
|
*
|
||||||||||
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
*
|
||||||||||
|
*
|
Filed herewith.
|
|
**
|
Furnished herewith.
|
|
#
|
Indicates management contract or compensatory plan.
|
|
§
|
Portions of this exhibit (indicated by asterisks) have been omitted pursuant to a request for confidential treatment pursuant to Rule 24b-2 under the Securities Exchange Act of 1934, as amended.
|
|
GUARDANT HEALTH, INC.
|
|||
|
Dated:
|
March 19, 2019
|
By:
|
/s/ Helmy Eltoukhy
|
|
Name:
|
Helmy Eltoukhy
|
||
|
Title:
|
Chief Executive Officer
|
||
|
Signature
|
Title
|
Date
|
||
|
/s/ Helmy Eltoukhy
|
Chief Executive Officer and Director
(Principal Executive Officer)
|
March 19, 2019
|
||
|
Helmy Eltoukhy
|
||||
|
/s/ Derek Bertocci
|
Chief Financial Officer
(Principal Accounting Officer and Principal Financial Officer)
|
March 19, 2019
|
||
|
Derek Bertocci
|
||||
|
/s/ AmirAli Talasaz
|
President, Chief Operating Officer and Chairman of the Board of Directors
|
March 19, 2019
|
||
|
AmirAli Talasaz
|
||||
|
/s/ Ian Clark
|
Director
|
March 19, 2019
|
||
|
Ian Clark
|
||||
|
/s/ Aaref Hilaly
|
Director
|
March 19, 2019
|
||
|
Aaref Hilaly
|
||||
|
/s/ Samir Kaul
|
Director
|
March 19, 2019
|
||
|
Samir Kaul
|
||||
|
/s/ Stanley Meresman
|
Director
|
March 19, 2019
|
||
|
Stanley Meresman
|
||||
|
/s/ Dipchand Nishar
|
Director
|
March 19, 2019
|
||
|
Dipchand Nishar
|
||||