|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ý
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
94-3047598
|
|
(State or Other Jurisdiction of Incorporation or Organization)
|
(I.R.S. Employer Identification No.)
|
|
333 Lakeside Drive, Foster City, California
|
94404
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
Title of each class
|
Name of each exchange on which registered
|
|
Common Stock, $0.001 par value per share
|
The Nasdaq Global Select Market
|
|
Large accelerated filer
x
|
Accelerated filer
¨
|
Non-Accelerated filer
¨
|
Smaller reporting company
¨
|
|
(Do not check if a smaller reporting company)
|
|||
|
PART I
|
|
|
|
Item 1
|
||
|
Item 1A
|
||
|
Item 1B
|
||
|
Item 2
|
||
|
Item 3
|
||
|
Item 4
|
||
|
PART II
|
|
|
|
Item 5
|
||
|
Item 6
|
||
|
Item 7
|
||
|
Item 7A
|
||
|
Item 8
|
||
|
Item 9
|
||
|
Item 9A
|
||
|
Item 9B
|
||
|
PART III
|
|
|
|
Item 10
|
||
|
Item 11
|
||
|
Item 12
|
||
|
Item 13
|
||
|
Item 14
|
||
|
PART IV
|
|
|
|
Item 15
|
||
|
ITEM 1.
|
BUSINESS
|
|
•
|
Genvoya
is an oral formulation dosed once a day for the treatment of HIV-1 infection in adults. Genvoya is our fourth complete single tablet regimen for the treatment of HIV and is a fixed-dose combination of our antiretroviral medicines, Vitekta® (elvitegravir 85 mg and 150 mg), Tybost® (cobicistat), Emtriva
®
(emtricitabine) and TAF 10 mg. Genvoya was approved by FDA and the European Commission in November 2015.
|
|
•
|
Stribild
®
is an oral formulation dosed once a day for the treatment of HIV-1 infection in treatment-naive adults. Stribild is our third complete single tablet regimen for the treatment of HIV and is a fixed-dose combination of our antiretroviral medications, Vitekta, Tybost, Viread
®
and Emtriva.
|
|
•
|
Complera
®
/Eviplera
®
is an oral formulation dosed once a day for the treatment of HIV-1 infection in adults. The product, marketed in the United States as Complera and in Europe as Eviplera, is our second complete single tablet regimen for the treatment of HIV and is a fixed-dose combination of our antiretroviral medications, Viread and Emtriva, and Janssen's non-nucleoside reverse transcriptase inhibitor, Edurant (rilpivirine).
|
|
•
|
Atripla
®
is an oral formulation dosed once a day for the treatment of HIV infection in adults. Atripla is our first single tablet regimen for HIV intended as a stand-alone therapy or in combination with other antiretrovirals. It is a fixed-dose combination of our antiretroviral medications, Viread and Emtriva, and Bristol-Myers Squibb Company's (BMS's) non-nucleoside reverse transcriptase inhibitor, Sustiva (efavirenz).
|
|
•
|
Truvada
®
(emtricitabine and tenofovir disoproxil fumarate) is an oral formulation dosed once a day as part of combination therapy to treat HIV infection in adults. It is a fixed-dose combination of our antiretroviral medications, Viread and Emtriva. FDA also approved Truvada, in combination with safer sex practices, to reduce the risk of sexually acquired HIV-1 infection in adults at high risk; a strategy called pre-exposure prophylaxis (PrEP).
|
|
•
|
Viread
is an oral formulation of a nucleotide analog reverse transcriptase inhibitor, dosed once a day as part of combination therapy to treat HIV infection in patients two years of age and older. The European Commission also approved the use of Viread in combination with other antiretroviral agents for the treatment of HIV-1 infected adolescent patients aged two to less than 18 years with nucleoside reverse transcriptase inhibitor resistance or toxicities precluding the use of first-line pediatric agents. Viread is also approved for the treatment of chronic HBV.
|
|
•
|
Emtriva
is an oral formulation of a nucleoside analog reverse transcriptase inhibitor, dosed once a day as part of combination therapy to treat HIV infection in adults. In the United States and Europe, Emtriva is also available as an oral solution approved as part of combination therapy to treat HIV infection in children.
|
|
•
|
Tybost
is
a pharmacokinetic enhancer dosed once a day that boosts blood levels of certain HIV medicines. Tybost is indicated as a boosting agent for the HIV protease inhibitors atazanavir and darunavir as part of antiretroviral combination therapy in adults with HIV-1 infection.
|
|
•
|
Vitekta
is an oral formulation of an integrase inhibitor, dosed once a day as part of combination therapy to treat HIV infection in adults without known mutations associated with resistance to elvitegravir, the active ingredient of Vitekta. Vitekta is indicated for use as part of HIV treatment regimens that include a ritonavir-boosted protease inhibitor.
|
|
•
|
Harvoni
is an oral formulation of the NS5A inhibitor with a nucleotide analog polymerase inhibitor dosed once a day for the treatment of genotypes 1, 4, 5 and 6, HCV/HIV-1 co-infection, HCV genotype 1 and 4 liver transplant recipients, and genotype 1-infected patients with decompensated cirrhosis. In Europe, Harvoni is also indicated for certain patients with HCV genotype 4 infection, HCV genotype 3 infection with cirrhosis and/or prior treatment failure and those with HCV/HIV-1 co-infection.
|
|
•
|
Sovaldi
is an oral formulation of a nucleotide analog polymerase inhibitor dosed once a day for the treatment of HCV as a component of a combination antiviral treatment regimen. Sovaldi’s efficacy has been established in patients with HCV genotypes 1, 2, 3 or 4 infection (in United States and Europe) and genotypes 5 and 6 infection (in Europe), including those with hepatocellular carcinoma meeting Milan criteria (awaiting liver transplantation) and those with HCV/HIV-1 co-infection.
|
|
•
|
Viread
is an oral formulation of a nucleotide analog reverse transcriptase inhibitor, dosed once a day for the treatment of chronic HBV in adults with compensated and decompensated liver disease. We licensed to GlaxoSmithKline Inc. (GSK) the rights to commercialize Viread for the treatment of chronic HBV in China, Japan and Saudi Arabia. In 2012, the European Commission approved the use of Viread for the treatment of chronic HBV infection in adolescent patients aged 12 to less than 18 years with compensated liver disease and evidence of immune active disease. Viread is also approved for the treatment of HIV infection.
|
|
•
|
Hepsera
®
(adefovir dipivoxil) is an oral formulation of a nucleotide analog polymerase inhibitor, dosed once a day to treat chronic HBV in patients 12 years of age and older. We licensed to GSK the rights to commercialize Hepsera for the treatment of chronic HBV in Asia Pacific, Latin America and certain other territories.
|
|
•
|
Letairis
(ambrisentan) is an oral formulation of an endothelin receptor antagonist (ERA) indicated for the treatment of pulmonary arterial hypertension (PAH) (World Health Organization (WHO) Group 1) in patients with WHO Class II or III symptoms to improve exercise capacity and delay clinical worsening. We sublicensed to GSK the rights to ambrisentan, marketed by GSK as Volibris (ambrisentan), for PAH in territories outside of the United States.
|
|
•
|
Ranexa
®
(ranolazine) is an extended-release tablet for the treatment of chronic angina. We have licensed to Menarini International Operations Luxembourg SA the rights to Ranexa in territories outside of the United States.
|
|
•
|
Lexiscan
®
/Rapiscan
®
(regadenoson) injection is indicated for use as a pharmacologic stress agent in radionuclide myocardial perfusion imaging (MPI), a test that detects and characterizes coronary artery disease, in patients unable to undergo adequate exercise stress. Astellas US LLC (Astellas) has exclusive rights to manufacture and sell regadenoson under the name Lexiscan in the United States. Rapidscan Pharma Solutions, Inc. (RPS) holds the exclusive right to manufacture and sell regadenoson under the name Rapiscan in Europe and certain territories outside the United States. We receive royalties from Astellas and RPS for sales in these territories.
|
|
•
|
Zydelig
is a first-in-class PI3K delta inhibitor for the treatment of certain blood cancers. In the United States, Zydelig is approved in combination with rituximab for patients with relapsed chronic lymphocytic leukemia (CLL) for whom rituximab alone would be considered appropriate therapy and as monotherapy for patients with relapsed follicular B-cell non-Hodgkin lymphoma (FL) and small lymphocytic lymphoma (SLL) who have received at least two prior systemic therapies. In the European Union, Zydelig is approved for the treatment of CLL and FL.
|
|
•
|
Cayston
®
(aztreonam for inhalation solution) is an inhaled antibiotic for the treatment of respiratory systems in cystic fibrosis (CF) patients seven years of age and older with
Pseudomonas aeruginosa (P. aeruginosa)
.
|
|
•
|
Tamiflu
®
(oseltamivir phosphate) is an oral antiviral available in capsule form for the treatment and prevention of influenza A and B. Tamiflu is approved for the treatment of influenza in children and adults in more than 60 countries, including the United States, Japan and the European Union. Tamiflu is also approved for the prevention of influenza in children and adults in the United States, Japan and the European Union. We developed Tamiflu with F. Hoffmann-La Roche Ltd (together with Hoffmann-La Roche Inc., Roche). Roche has the
|
|
•
|
AmBisome
®
(amphotericin B liposome for injection) is a proprietary liposomal formulation of amphotericin B, an antifungal agent to treat serious invasive fungal infections caused by various fungal species in adults. Our corporate partner, Astellas Pharma US, Inc., promotes and sells AmBisome in the United States and Canada, and we promote and sell AmBisome in Europe, Australia and New Zealand.
|
|
•
|
Macugen
®
(pegaptanib sodium injection) is an intravitreal injection of an anti-angiogenic oligonucleotide for the treatment of neovascular age-related macular degeneration. Macugen was developed by Eyetech Inc. (Eyetech) using technology licensed from us and is now promoted in the United States by Valeant Pharmaceuticals, Inc. (Valeant), which acquired Eyetech in 2012. Valeant holds the exclusive rights to manufacture and sell Macugen in the United States, and Pfizer Inc. (Pfizer) holds the exclusive right to manufacture and sell Macugen in the rest of the world. We receive royalties from Valeant and Pfizer based on worldwide sales of Macugen.
|
|
•
|
Licenses with Generic Manufacturers.
We have entered into non-exclusive license agreements with Indian generic manufacturers, granting them rights to produce and distribute generic versions of TDF, emtricitabine, cobicistat, elvitegravir, including generic versions of combination product containing cobicistat, elvitegravir, TDF and emtricitabine for the treatment of HIV infection to low income countries around the world, which include India and many countries in our Gilead Access Program. We also included in these non-exclusive license agreements the ability to manufacture and distribute generic versions of TDF for the treatment of HBV in the same countries where they are authorized to sell generic versions of TDF for HIV. In 2014, we granted certain of our Indian partners direct licenses to produce and distribute generic TAF in the developing world, including single tablet regimens containing emtricitabine and fixed-dose combinations of TAF and emtricitabine co-formulated with our other HIV medicines. We also entered into collaborations with our Indian partners to produce and distribute generic versions in low-income countries and lower-middle income countries. In early 2015, we expanded our collaborations to allow our Indian partners to manufacture VEL and the single tablet regimen of SOF/VEL, once approved.
|
|
•
|
Medicines Patent Pool (the MPP).
In 2011, we entered into an agreement with the MPP, an organization that was established by the United Nations to increase global access to high-quality, low-cost antiretroviral therapy through the sharing of patents. We granted the MPP a non-exclusive license to identify generic pharmaceutical manufacturers in India who specialize in high-quality production of generic medicines and granted sublicenses to those Indian manufacturers to manufacture and distribute generic versions of our antiretrovirals in the developing world. Sublicensees through the MPP will be free to develop combination products and pediatric formulations of our HIV medicines. We also granted the MPP the right to grant sublicenses to generic versions of elvitegravir and cobicistat, the single tablet regimen consisting of elvitegravir, cobicistat, TDF and emtricitabine and TAF for HIV and HBV to developing countries, contingent on the medicine’s U.S. regulatory approval.
|
|
•
|
Special Partnerships.
We work with national governments and local organizations to increase access to our HIV and HCV medicines and strengthen healthcare systems. For example, we have established an agreement with the National AIDS Program of Myanmar to donate a generic version of our Atripla to 2,000 people living with HIV in the country, as well as provide HIV educational activities and financial support to strengthen the country’s health system. In Tanzania, we launched an HIV “test-and-treat” demonstration project with the Holy See’s Good Samaritan Foundation. The program's goal is to enable screening of 120,000 patients for HIV and provide HIV therapy to 20,000 HIV-positive individuals over five years. In Egypt, we have agreed to provide Sovaldi and Harvoni to the Egyptian Ministry of Health at a significantly reduced price. In addition, in partnership with the Ministry of Health, we invest in local HCV medical education and prevention efforts, as well as screening and patient awareness initiatives. In Georgia, we established an agreement with the Ministry of Labor, Health and Social Affairs of Georgia to help eliminate HCV in the country. The project aims to reduce the number of Georgians infected with HCV and lower the rate of new infections through universal screening, treatment, prevention and surveillance.
|
|
•
|
BMS.
In 2004, we entered into a collaboration arrangement with BMS to develop and commercialize a single tablet regimen containing our Truvada and BMS's Sustiva (efavirenz) in the United States. This combination was approved for use in the United States in 2006 and is sold under the brand name Atripla. We and BMS structured this collaboration as a joint venture that operates as a limited liability company named Bristol-Myers Squibb & Gilead Sciences, LLC, which we consolidate. We and BMS granted royalty-free sublicenses to the joint venture for the use of our respective company owned technologies and, in return, were granted a license by the joint venture to use any intellectual property that results from the collaboration. In 2006, we and BMS amended the joint venture's collaboration agreement to allow the joint venture to sell Atripla in Canada. The economic interests of the joint venture held by us and BMS (including share of revenues and out-of-pocket expenses) are based on the portion of the net selling price of Atripla attributable to efavirenz and Truvada. Since the net selling price for Truvada may change over time relative to the net selling price of efavirenz, both our and BMS's respective economic interests in the joint venture may vary annually. We and BMS shared marketing and sales efforts. Starting in 2011, except for a limited number of activities that are jointly managed, the parties no longer coordinate detailing and promotional activities in the United States, and the parties have reduced their joint promotional efforts since we launched Complera in August 2011 and Stribild in August 2012. Efavirenz purchased by the joint venture from BMS at BMS's estimated net selling price of efavirenz is included in inventories on our Consolidated Balance Sheets as of
December 31, 2015
and
2014
.The agreement will continue until terminated by the mutual agreement of the parties. In addition, either party may terminate the other party's participation in the collaboration within 30 days after the launch of at least one generic version of such other party's single agent products (or the double agent products). The terminating party then has the right to continue to sell Atripla and become the continuing party, but will be obligated to pay the terminated party certain royalties for a three-year period following the effective date of the termination.
|
|
•
|
Janssen.
In 2009, we entered into a collaboration agreement with Janssen to develop and commercialize a fixed-dose combination of our Truvada and Janssen's rilpivirine. The agreement was amended in 2011, 2013 and 2014. The combination was approved in the United States and European Union in 2011 and is sold under the brand name Complera in the United States and Eviplera in the European Union. The 2014 amendment expanded the collaboration to include another single tablet regimen containing Janssen’s rilpivirine and our emtricitabine and tenofovir alafenamide (R/F/TAF). Under the agreement, Janssen granted us an exclusive license to Complera/Eviplera and R/F/TAF worldwide but has the right to distribute both combination products in 18 countries including Mexico, Russia and Japan. Neither party is restricted from combining its drugs with any other drug products except those which are similar to the components of Complera/Eviplera and R/F/TAF.
|
|
•
|
Japan Tobacco.
In 2005, Japan Tobacco Inc. (Japan Tobacco) granted us exclusive rights to develop and commercialize elvitegravir, a novel HIV integrase inhibitor, in all countries of the world, excluding Japan, where Japan Tobacco retained such rights. Under the agreement, we are responsible for seeking regulatory approval in our territories and are required to use diligent efforts to commercialize elvitegravir for the treatment of HIV infection. We bear all costs and expenses associated with such commercialization efforts.
|
|
Product Candidates
|
Description
|
|
|
Marketing Applications Pending
|
||
|
Fixed-dose co-formulation of emtricitabine and TAF (F/TAF)
|
A fixed-dose co-formulation of emtricitabine and TAF is being evaluated for the treatment of HIV infection.
|
|
|
Single tablet regimen of emtricitabine, rilpivirine and TAF (R/F/TAF)
|
Under an agreement with Janssen, a single tablet regimen of emtricitabine, rilpivirine and TAF is being evaluated for the treatment of HIV infection.
|
|
|
Product in Phase 3
|
||
|
Single tablet regimen of GS-9883 (non-boosted integrase inhibitor) and F/TAF
|
A single tablet regimen of GS-9883 and F/TAF is being evaluated for the treatment of HIV infection.
|
|
|
Product in Phase 1
|
||
|
GS-9620
|
GS-9620, a TLR-7 agonist, is being evaluated for the treatment of HIV infection.
|
|
|
Product Candidates
|
Description
|
|
|
Market Applications Pending
|
||
|
Single tablet regimen of sofosbuvir (SOF) and velpatasvir (VEL)
|
A single tablet regimen of sofosbuvir and velpatasvir, a nucleotide NS5B inhibitor/pan-genotypic NS5A inhibitor, is being evaluated for the treatment of HCV.
|
|
|
TAF
|
TAF is a nucleotide reverse transcriptase inhibitor being evaluated for the treatment of HBV.
|
|
|
Product in Phase 3
|
||
|
Single tablet regimen of GS-9857 and SOF/VEL
|
A single tablet regimen of GS-9857, a pan-genotypic NS3 protease inhibitor, and SOF/VEL is being evaluated for the treatment of HCV.
|
|
|
Products in Phase 2
|
||
|
GS-4774
|
GS-4774, a Tarmogen T cell immunity stimulator, is being evaluated for the treatment of HBV.
|
|
|
GS-9620
|
GS-9620 is being evaluated for the treatment of HBV.
|
|
|
Simtuzumab
|
Simtuzumab, a monoclonal antibody, is being evaluated for the treatment of NASH and primary sclerosing cholangitis.
|
|
|
GS-4997
|
GS-4997, an ASK-1 inhibitor, is being evaluated for the treatment of diabetic nephropathy and NASH.
|
|
|
Product Candidates
|
Description
|
|
|
Product in Phase 3
|
|
|
|
Eleclazine
|
Eleclazine, a late sodium current inhibitor, is being evaluated for the treatment of Long QT-3 Syndrome.
|
|
|
Products in Phase 2
|
||
|
Eleclazine
|
Eleclazine is being evaluated for the treatment of hypertrophic cardiomyopathy and ventricular tachycardia/ventricular fibrillation.
|
|
|
GS-4997
|
GS-4997 is being evaluated for the treatment of pulmonary arterial hypertension.
|
|
|
Product Candidates
|
Description
|
|
|
Products in Phase 3
|
||
|
Idelalisib
|
Idelalisib, a PI3K delta inhibitor, is being evaluated for the treatment of frontline and relapsed refractory CLL and relapsed refractory iNHL.
|
|
|
Momelotinib
|
Momelotinib, a JAK inhibitor, is being evaluated for the treatment of myelofibrosis and pancreatic cancer.
|
|
|
GS-5745
|
GS-5745, a MMP9 maB inhibitor, is being evaluated for the treatment of gastric cancer.
|
|
|
Products in Phase 2
|
|
|
|
Entospletinib
|
Entospletinib, a spleen tyrosine kinase (Syk) inhibitor, is being evaluated for the treatment of hematological malignancies.
|
|
|
Idelalisib
|
Idelalisib is being evaluated for the treatment of frontline iNHL.
|
|
|
Products in Phase 1
|
||
|
GS-4059
|
GS-4059, a Bruton’s tyrosine kinase inhibitor, is being evaluated for the treatment of B-cell malignancies.
|
|
|
GS-5745
|
GS-5745 is being evaluated for the treatment of solid tumors.
|
|
|
GS-5829
|
GS-5829, a bromodomain and extra-terminal (BET) inhibitor, is being evaluated for the treatment of solid tumors.
|
|
|
Product Candidates
|
Description
|
|
|
Products in Phase 2
|
|
|
|
Filgotinib
|
Filgotinib, a JAK1-selective inhibitor, is being evaluated for the treatment of rheumatoid arthritis and Crohn's Disease.
|
|
|
GS-5745
|
GS-5745 is being evaluated for the treatment of ulcerative colitis and Crohn’s Disease.
|
|
|
Presatovir
|
Presatovir, a fusion inhibitor, is being evaluated for the treatment of respiratory syncytial virus.
|
|
|
Products in Phase 1
|
||
|
GS-5745
|
GS-5745 is being evaluated for the treatment of chronic obstructive pulmonary disease and rheumatoid arthritis.
|
|
|
GS-9876
|
GS-9876, a Syk inhibitor, is being evaluated for the treatment of rheumatoid arthritis.
|
|
|
Product Candidates
|
Description
|
|
|
Product in Phase 2
|
|
|
|
GS-4997
|
GS-4997 is being evaluated for the treatment of Diabetic Nephropathy.
|
|
|
Product in Phase 1
|
||
|
GS-5734
|
GS-5734, a nucleotide prodrug, is being evaluated for the treatment of Ebola.
|
|
|
Phase 3 Product Candidates
|
Patent Expiration
|
|||||
|
Product Candidates for the Treatment of HIV
|
U.S.
|
E.U.
|
||||
|
Single tablet regimen of emtricitabine and TAF
|
2022
|
2021
|
||||
|
Single tablet regimen of darunavir, cobicistat, emtricitabine and TAF
|
2029
|
2027
|
||||
|
Single tablet regimen of emtricitabine, rilpivirine and TAF
|
2022
|
2022
|
||||
|
Single tablet regimen of GS-9883 and F/TAF
|
2033
|
(2033)
|
||||
|
Product Candidates for the Treatment of Liver Diseases
|
|
|
||||
|
Single tablet regimen of sofosbuvir and velpatasvir for the treatment of HCV
|
2032
|
2032
|
||||
|
Single tablet regimen of sofosbuvir, velpatasvir and GS-9857 for the treatment of HCV
|
(2033)
|
(2033)
|
||||
|
Single agent TAF for the treatment of HBV
|
2022
|
2021
|
||||
|
Product Candidates for the Treatment of Oncology/Inflammation
|
||||||
|
Idelalisib for the treatment of frontline and relapsed refractory CLL and relapsed refractory iNHL.
|
2025
|
(2025)
|
||||
|
Momelotinib for the treatment of myelofibrosis and pancreatic cancer
|
2030
|
2028
|
||||
|
GS-5745 for the treatment of gastric cancer
|
2031
|
(2031)
|
||||
|
Product Candidates for the Treatment of Cardiovascular Diseases
|
|
|
||||
|
Eleclazine (formerly known as GS-6615) for the treatment of LQT-3 Syndrome
|
2032
|
(2032)
|
||||
|
Dates in parentheses reflect the estimated expiration date of patents which may issue from currently pending applications. The estimated expiration dates do not include any potential additional exclusivity (e.g., patent term extension, supplementary protection certificates or pediatric exclusivity) that has not yet been granted.
|
||||||
|
Products
|
Patent Expiration
|
|||||
|
U.S.
|
E.U.
|
|||||
|
Hepsera
|
2014
|
2016
|
||||
|
AmBisome
|
2016
|
2008
|
||||
|
Macugen
|
2017
|
2017
|
||||
|
Tamiflu
|
2017
|
2016
|
||||
|
Letairis
|
2018
|
2020
|
||||
|
Viread
|
2018*
|
2017
|
||||
|
Ranexa
|
2019**
|
2023
|
||||
|
Atripla
|
2021
|
2017
|
||||
|
Cayston
|
2021
|
2021
|
||||
|
Emtriva
|
2021
|
2016
|
||||
|
Truvada
|
2021
|
2017
|
||||
|
Lexiscan
|
2022
|
2025
|
||||
|
Complera/Eviplera
|
2022
|
2022
|
||||
|
Vitekta
|
2023
|
2028
|
||||
|
Zydelig
|
2025
|
(2025)
|
||||
|
Sovaldi
|
2029
|
2028
|
||||
|
Stribild
|
2029
|
2028
|
||||
|
Genvoya
|
2029
|
2028
|
||||
|
Tybost
|
2029
|
2027
|
||||
|
Harvoni
|
2030
|
2030
|
||||
|
Dates in parentheses reflect the estimated expiration date of patents which may issue from currently pending applications. The estimated expiration dates do not include any potential additional exclusivity (e.g., patent term extension, supplementary protection certificates or pediatric exclusivity) that has not yet been granted.
|
||||||
|
*
|
In 2013, Gilead and Teva Pharmaceuticals (Teva) reached an agreement in principle to settle the ongoing patent litigation concerning the four patents that protect tenofovir disoproxil fumarate in our Viread, Truvada and Atripla products. Under the agreement, Teva will be allowed to launch a generic version of Viread on December 15, 2017.
|
|
**
|
In 2013, Gilead and Lupin Limited (Lupin) reached an agreement to settle the patent litigation prior to issuance of the court’s decision. Under the agreement, Lupin will be allowed to launch a generic version of Ranexa on February 27, 2019.
|
|
•
|
Phase 1. The drug candidate is given to a small number of healthy human control subjects or patients suffering from the indicated disease, to test for safety, dose tolerance, pharmacokinetics, metabolism, distribution and excretion.
|
|
•
|
Phase 2. The drug candidate is given to a limited patient population to determine the effect of the drug candidate in treating the disease, the best dose of the drug candidate, and the possible side effects and safety risks of the drug candidate. It is not uncommon for a drug candidate that appears promising in Phase 1 clinical trials to fail in the more rigorous Phase 2 clinical trials.
|
|
•
|
Phase 3. If a drug candidate appears to be effective and safe in Phase 2 clinical trials, Phase 3 clinical trials are commenced to confirm those results. Phase 3 clinical trials are conducted over a longer term, involve a significantly larger population, are conducted at numerous sites in different geographic regions and are carefully designed to provide reliable and conclusive data regarding the safety and benefits of a drug candidate. It is not uncommon for a drug candidate that appears promising in Phase 2 clinical trials to fail in the more rigorous and extensive Phase 3 clinical trials.
|
|
ITEM 1A.
|
RISK FACTORS
|
|
•
|
As our HCV and HIV products are used over a longer period of time in many patients and in combination with other products, and additional studies are conducted, new issues with respect to safety, resistance and interactions with other drugs may arise, which could cause us to provide additional warnings or contraindications on our labels, narrow our approved indications or halt sales of a product, each of which could reduce our revenues.
|
|
•
|
As our products mature, private insurers and government payers often reduce the amount they will reimburse patients for these products, which increases pressure on us to reduce prices.
|
|
•
|
If physicians do not see the benefit of our HCV or HIV products, the sales of our HCV or HIV products will be limited.
|
|
•
|
As new HCV or new or generic HIV products are introduced into major markets, our ability to maintain pricing and market share may be affected.
|
|
•
|
we are unable to control the resources our corporate partners devote to our programs or products;
|
|
•
|
disputes may arise with respect to the ownership of rights to technology developed with our corporate partners;
|
|
•
|
disagreements with our corporate partners could cause delays in, or termination of, the research, development or commercialization of product candidates or result in litigation or arbitration;
|
|
•
|
contracts with our corporate partners may fail to provide significant protection or may fail to be effectively enforced if one of these partners fails to perform;
|
|
•
|
our corporate partners have considerable discretion in electing whether to pursue the development of any additional products and may pursue alternative technologies or products either on their own or in collaboration with our competitors;
|
|
•
|
our corporate partners with marketing rights may choose to pursue competing technologies or to devote fewer resources to the marketing of our products than they do to products of their own development; and
|
|
•
|
our distributors and our corporate partners may be unable to pay us, particularly in light of current economic conditions.
|
|
•
|
not provide us with accurate or timely information regarding their inventories, patient data or safety complaints;
|
|
•
|
not effectively sell or support Letairis or Cayston;
|
|
•
|
not devote the resources necessary to sell Letairis or Cayston in the volumes and within the time frames that we expect;
|
|
•
|
not be able to satisfy their financial obligations to us or others; or
|
|
•
|
cease operations.
|
|
•
|
obtain patents and licenses to patent rights;
|
|
•
|
preserve trade secrets;
|
|
•
|
defend against infringement and efforts to invalidate our patents; and
|
|
•
|
operate without infringing on the intellectual property of others.
|
|
ITEM 1B.
|
UNRESOLVED STAFF COMMENTS
|
|
ITEM 2.
|
PROPERTIES
|
|
ITEM 3.
|
LEGAL PROCEEDINGS
|
|
ITEM 4.
|
MINE SAFETY DISCLOSURES
|
|
ITEM 5.
|
MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
2015
|
2014
|
|||||||
|
|
High
|
Low
|
High
|
Low
|
||||
|
First Quarter
|
$107.77
|
$93.18
|
$84.88
|
$67.63
|
||||
|
Second Quarter
|
$123.37
|
$95.38
|
$84.45
|
$63.50
|
||||
|
Third Quarter
|
$120.37
|
$86.00
|
$110.64
|
$83.32
|
||||
|
Fourth Quarter
|
$111.11
|
$94.37
|
$116.83
|
$85.95
|
||||
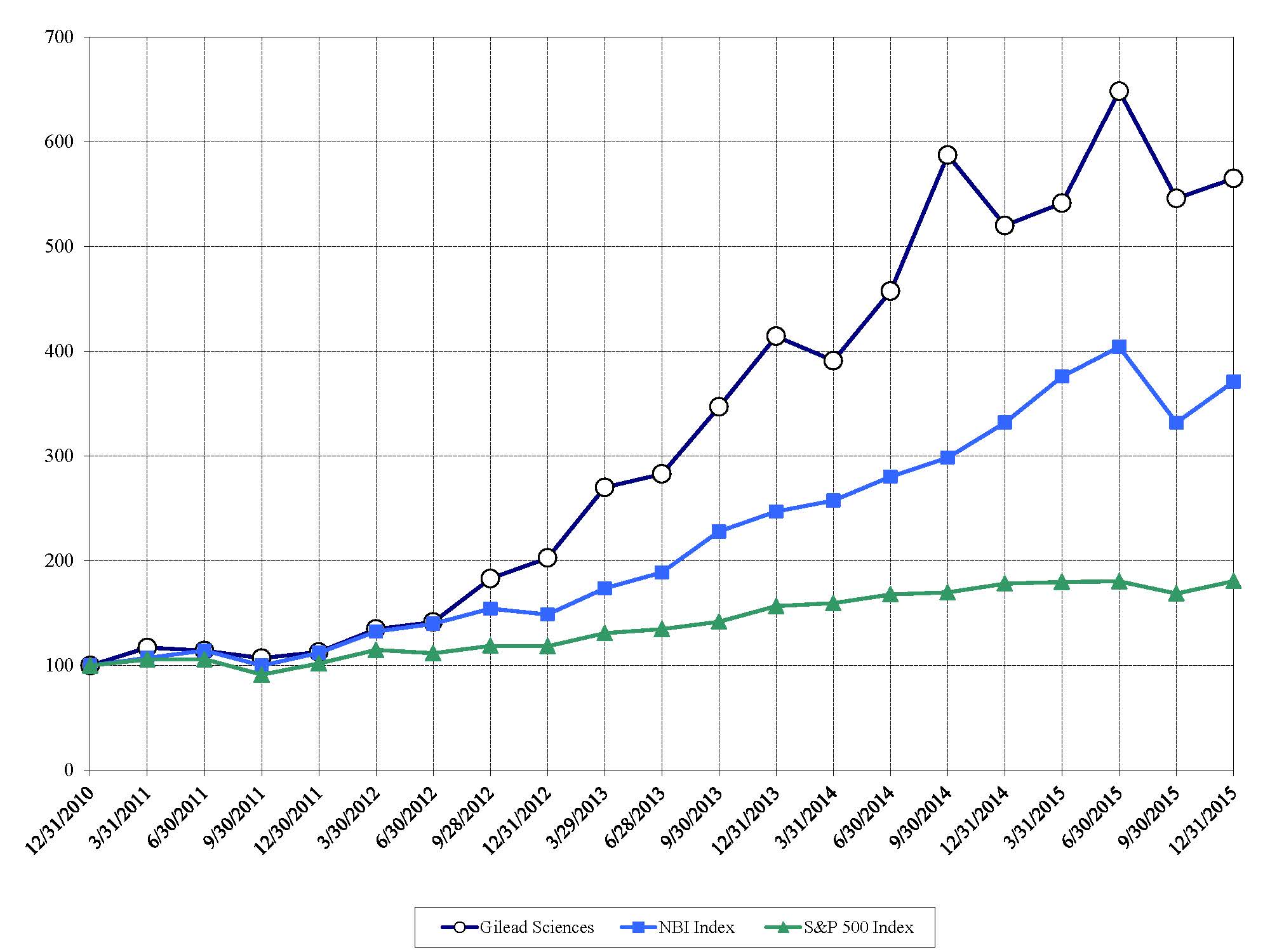
|
(1)
|
This section is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference in any of our filings under the Securities Act or the Exchange Act whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
|
|
(2)
|
Shows the cumulative return on investment assuming an investment of $100 in our common stock, the NBI Index and the S&P 500 Index on December 31, 2010, and that all dividends were reinvested.
|
|
Total Number of Shares Purchased
(in thousands)
|
Average Price Paid per Share
(in dollars)
|
Total Number of Shares Purchased as Part of Publicly Announced Program
(in thousands)
|
(1)
|
Maximum Fair Value of Shares that May Yet Be Purchased Under the Program
(in millions)
|
(1)
|
||||||||||
|
October 1 - October 31, 2015
|
8,367
|
|
$
|
102.25
|
|
8,313
|
|
$
|
10,200
|
|
|||||
|
November 1 - November 30, 2015
|
10,127
|
|
$
|
106.88
|
|
9,792
|
|
$
|
9,154
|
|
|||||
|
December 1 - December 31, 2015
|
11,259
|
|
$
|
102.69
|
|
11,239
|
|
$
|
8,000
|
|
|||||
|
Total
|
29,753
|
|
(2)
|
$
|
103.99
|
|
29,344
|
|
(2)
|
|
|
||||
|
(1)
|
Stock repurchases were made under the 2015 Program.
|
|
(2)
|
The difference between the total number of shares purchased and the total number of shares purchased as part of publicly announced programs is due to shares of common stock withheld by us from employee restricted stock awards in order to satisfy applicable tax withholding obligations.
|
|
ITEM 6.
|
SELECTED FINANCIAL DATA
|
|
|
Year Ended December 31,
|
||||||||||||||||||
|
|
2015
|
2014
|
2013
|
2012
|
2011
|
||||||||||||||
|
CONSOLIDATED STATEMENT OF INCOME DATA:
|
|
|
|
|
|
||||||||||||||
|
Total revenues
(1)
|
$
|
32,639
|
|
$
|
24,890
|
|
$
|
11,202
|
|
$
|
9,702
|
|
$
|
8,385
|
|
||||
|
Total costs and expenses
(1)
|
$
|
10,446
|
|
$
|
9,625
|
|
$
|
6,678
|
|
$
|
5,692
|
|
$
|
4,596
|
|
||||
|
Income from operations
|
$
|
22,193
|
|
$
|
15,265
|
|
$
|
4,524
|
|
$
|
4,010
|
|
$
|
3,790
|
|
||||
|
Provision for income taxes
|
$
|
3,553
|
|
$
|
2,797
|
|
$
|
1,151
|
|
$
|
1,038
|
|
$
|
862
|
|
||||
|
Net income attributable to Gilead
|
$
|
18,108
|
|
$
|
12,101
|
|
$
|
3,075
|
|
$
|
2,592
|
|
$
|
2,804
|
|
||||
|
Net income per share attributable to Gilead
common stockholders - basic
|
$
|
12.37
|
|
$
|
7.95
|
|
$
|
2.01
|
|
$
|
1.71
|
|
$
|
1.81
|
|
||||
|
Shares used in per share calculation-basic
|
1,464
|
|
1,522
|
|
1,529
|
|
1,515
|
|
1,550
|
|
|||||||||
|
Net income per share attributable to Gilead
common stockholders - diluted
|
$
|
11.91
|
|
$
|
7.35
|
|
$
|
1.81
|
|
$
|
1.64
|
|
$
|
1.77
|
|
||||
|
Shares used in per share calculation-diluted
|
1,521
|
|
1,647
|
|
1,695
|
|
1,583
|
|
1,580
|
|
|||||||||
|
Cash dividends declared per share
|
$
|
1.29
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
|
December 31,
|
||||||||||||||||||
|
|
2015
|
2014
|
2013
|
2012
|
2011
|
||||||||||||||
|
CONSOLIDATED BALANCE SHEET DATA:
|
|
|
|
|
|
||||||||||||||
|
Cash, cash equivalents and marketable securities
(2)
|
$
|
26,208
|
|
$
|
11,726
|
|
$
|
2,571
|
|
$
|
2,582
|
|
$
|
9,964
|
|
||||
|
Working capital
(2)
|
$
|
14,872
|
|
$
|
11,953
|
|
$
|
590
|
|
$
|
1,918
|
|
$
|
11,432
|
|
||||
|
Total assets
(2)
|
$
|
51,839
|
|
$
|
34,664
|
|
$
|
22,579
|
|
$
|
21,240
|
|
$
|
17,303
|
|
||||
|
Other long-term obligations
(3)
|
$
|
395
|
|
$
|
586
|
|
$
|
262
|
|
$
|
281
|
|
$
|
180
|
|
||||
|
Senior unsecured notes, convertible senior notes
and credit facility
(2)
|
$
|
22,178
|
|
$
|
12,404
|
|
$
|
6,636
|
|
$
|
8,224
|
|
$
|
7,606
|
|
||||
|
Retained earnings
|
$
|
18,001
|
|
$
|
12,732
|
|
$
|
6,106
|
|
$
|
3,705
|
|
$
|
1,777
|
|
||||
|
Total stockholders' equity
|
$
|
19,113
|
|
$
|
15,819
|
|
$
|
11,745
|
|
$
|
9,544
|
|
$
|
6,867
|
|
||||
|
(1)
|
See Item 7, Management's Discussion and Analysis for a description of our results of operations for 2015.
|
|
|
(2)
|
During 2015, we issued $10.0 billion principal amount of senior unsecured notes in a registered offering. We also repaid $213 million of principal balance of convertible senior notes due in 2016 and $784 million in cash related to the conversion spread of the notes.
|
|
|
During 2014, we issued $8.0 billion principal amount of senior unsecured notes in registered offerings. We also repaid $912 million of principal balance of convertible senior notes due in 2014, $2.5 billion in cash related to the conversion spread of the notes, $750 million for senior unsecured notes and $600 million under the five-year revolving credit facility agreement (the Five-Year Revolving Credit Agreement).
|
||
|
During 2013, we repaid $1.5 billion of principal balance of convertible senior notes and repaid $150 million under our Five-Year Revolving Credit Agreement.
|
||
|
During 2012, we completed the acquisition of Pharmasset, Inc. and we recognized consideration transferred of $11.1 billion which was primarily recorded in intangible assets. We financed the transaction with approximately $5.2 billion in cash on hand, $2.2 billion in bank debt issued in January 2012 and $3.7 billion in senior unsecured notes issued in December 2011.
|
||
|
(3)
|
Prior year amounts have been reclassified to conform to current presentation.
|
|
|
ITEM 7.
|
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
|
•
|
U.S. Food and Drug Administration (FDA) and European Commission approved Genvoya for the treatment of HIV-1 infection. Genvoya is our first
t
enofovir alafenamide (TAF)-based regimen.
|
|
•
|
We submitted marketing applications to FDA and European Medicines Agency (EMA) for an investigational, once-daily single tablet regimen that combines our emtricitabine 200 mg and TAF 25 mg with rilpivirine 25 mg (R/F/TAF) from Janssen Sciences Ireland UC, one of the Janssen Pharmaceutical Companies of Johnson & Johnson, for the treatment of HIV-1 infection in adult and pediatric patients 12 years of age and older.
|
|
•
|
We submitted marketing applications to FDA and EMA for two doses of F/TAF (200/10 mg and 200/25 mg) for the treatment of HIV-1 infection in adults and pediatric patients age 12 years and older, in combination with other HIV antiretroviral agents.
|
|
•
|
FDA approved Harvoni for expanded use in patients with genotype 4, 5 and 6 HCV infection and in patients co-infected with HIV. In addition, Harvoni plus ribavirin for 12 weeks was approved as an alternate therapy to 24 weeks of Harvoni for treatment-experienced, genotype 1 patients with cirrhosis.
|
|
•
|
Japanese Ministry of Health, Labour and Welfare approved Sovaldi for the suppression of viremia in patients with genotype 2 chronic HCV infection with or without compensated cirrhosis and Harvoni, the first once-daily single-tablet regimen for the treatment of chronic HCV genotype 1 infection in adults with or without compensated cirrhosis, with a treatment duration of 12 weeks.
|
|
•
|
We submitted marketing applications to FDA and EMA for an investigational, once-daily fixed-dose combination of the nucleotide analog polymerase inhibitor sofosbuvir (SOF) 400 mg and velpatasvir (VEL) 100 mg, an investigational pan-genotypic NS5A inhibitor, for the treatment of genotype 1-6 chronic HCV infection.
|
|
•
|
We received reimbursement approval for Sovaldi and Harvoni in various countries in the European Union.
|
|
•
|
FDA approved the use of Letairis (ambrisentan) in combination with tadalafil for the treatment of pulmonary arterial hypertension (PAH) to reduce the risks of disease progression and hospitalization for worsening PAH, and to improve exercise ability.
|
|
•
|
We filed a supplemental new drug application for the use of Zydelig (idelalisib) in combination with ofatumumab in previously-treated patients with chronic lymphocytic leukemia.
|
|
•
|
We entered into a collaboration and license agreement with Galapagos NV, which became effective January 2016, for the development and commercialization of the JAK1-selective inhibitor filgotinib for inflammatory disease indications. This collaboration represents an opportunity to add complementary clinical programs to our growing inflammation research and development efforts.
|
|
(In millions, except percentages)
|
2015
|
Change
|
2014
|
Change
|
2013
|
|||||||||||||
|
Revenues:
|
|
|
|
|
|
|||||||||||||
|
Product sales
|
$
|
32,151
|
|
31
|
%
|
$
|
24,474
|
|
127
|
%
|
$
|
10,804
|
|
|||||
|
Royalty, contract and other revenues
|
488
|
|
17
|
%
|
416
|
|
5
|
%
|
398
|
|
||||||||
|
Total revenues
|
32,639
|
|
31
|
%
|
24,890
|
|
122
|
%
|
11,202
|
|
||||||||
|
(In millions, except percentages)
|
2015
|
Change
|
2014
|
Change
|
2013
|
|||||||||||||
|
Antiviral products:
|
|
|
|
|
|
|||||||||||||
|
Harvoni
|
$
|
13,864
|
|
*
|
|
$
|
2,127
|
|
*
|
|
—
|
|
||||||
|
Sovaldi
|
5,276
|
|
(49
|
)%
|
10,283
|
|
*
|
|
$
|
139
|
|
|||||||
|
Truvada
|
3,459
|
|
4
|
%
|
3,340
|
|
7
|
%
|
3,136
|
|
||||||||
|
Atripla
|
3,134
|
|
(10
|
)%
|
3,470
|
|
(5
|
)%
|
3,648
|
|
||||||||
|
Stribild
|
1,825
|
|
52
|
%
|
1,197
|
|
122
|
%
|
539
|
|
||||||||
|
Complera/Eviplera
|
1,427
|
|
16
|
%
|
1,228
|
|
52
|
%
|
810
|
|
||||||||
|
Viread
|
1,108
|
|
5
|
%
|
1,058
|
|
10
|
%
|
959
|
|
||||||||
|
Genvoya
|
45
|
|
*
|
|
—
|
|
*
|
|
—
|
|
||||||||
|
Other antiviral
|
69
|
|
(22
|
)%
|
88
|
|
(21
|
)%
|
111
|
|
||||||||
|
Total antiviral products
|
30,207
|
|
33
|
%
|
22,791
|
|
144
|
%
|
9,342
|
|
||||||||
|
Other products:
|
||||||||||||||||||
|
Letairis
|
700
|
|
18
|
%
|
595
|
|
14
|
%
|
520
|
|
||||||||
|
Ranexa
|
588
|
|
15
|
%
|
510
|
|
14
|
%
|
449
|
|
||||||||
|
AmBisome
|
350
|
|
(10
|
)%
|
388
|
|
10
|
%
|
352
|
|
||||||||
|
Zydelig
|
132
|
|
*
|
|
23
|
|
*
|
|
—
|
|
||||||||
|
Other
|
174
|
|
4
|
%
|
167
|
|
18
|
%
|
141
|
|
||||||||
|
Total product sales
|
$
|
32,151
|
|
31
|
%
|
$
|
24,474
|
|
127
|
%
|
$
|
10,804
|
|
|||||
|
•
|
Harvoni
|
|
•
|
Sovaldi
|
|
•
|
Truvada
|
|
•
|
Atripla
|
|
•
|
Stribild
|
|
•
|
Complera/Eviplera
|
|
•
|
Viread
|
|
(In millions, except percentages)
|
2015
|
Change
|
2014
|
Change
|
2013
|
|||||||||||||
|
Royalty, contract and other revenues
|
$
|
488
|
|
17
|
%
|
$
|
416
|
|
5
|
%
|
$
|
398
|
|
|||||
|
(In millions, except percentages)
|
2015
|
Change
|
2014
|
Change
|
2013
|
|||||||||||||
|
Total product sales
|
$
|
32,151
|
|
31
|
%
|
$
|
24,474
|
|
127
|
%
|
$
|
10,804
|
|
|||||
|
Cost of goods sold
|
$
|
4,006
|
|
6
|
%
|
$
|
3,788
|
|
32
|
%
|
$
|
2,859
|
|
|||||
|
Product gross margin
|
88
|
%
|
|
|
85
|
%
|
|
|
74
|
%
|
||||||||
|
(In millions, except percentages)
|
2015
|
Change
|
2014
|
Change
|
2013
|
|||||||||||||
|
Research and development
|
$
|
3,014
|
|
6
|
%
|
$
|
2,854
|
|
35
|
%
|
$
|
2,120
|
|
|||||
|
(In millions, except percentages)
|
2015
|
2014
|
2013
|
|||||||||
|
Clinical studies and outside services
|
$
|
1,634
|
|
$
|
1,688
|
|
$
|
1,147
|
|
|||
|
Personnel and infrastructure expenses
|
1,041
|
|
900
|
|
714
|
|
||||||
|
Facilities, IT and other costs
|
339
|
|
266
|
|
259
|
|
||||||
|
Total
|
$
|
3,014
|
|
$
|
2,854
|
|
$
|
2,120
|
|
|||
|
(In millions, except percentages)
|
2015
|
Change
|
2014
|
Change
|
2013
|
|||||||||||||
|
Selling, general and administrative
|
$
|
3,426
|
|
15
|
%
|
$
|
2,983
|
|
76
|
%
|
$
|
1,699
|
|
|||||
|
(in millions)
|
2015
|
2014
|
2013
|
||||||||||
|
As of December 31:
|
|
|
|
||||||||||
|
Cash, cash equivalents and marketable securities
|
$
|
26,208
|
|
$
|
11,726
|
|
$
|
2,571
|
|
||||
|
Working capital
|
$
|
14,872
|
|
$
|
11,953
|
|
$
|
590
|
|
||||
|
(in millions)
|
2015
|
2014
|
2013
|
||||||||||
|
Cash provided by (used in):
|
|
|
|
|
|
|
|||||||
|
Operating activities
|
$
|
20,329
|
|
$
|
12,818
|
|
$
|
3,105
|
|
||||
|
Investing activities
|
$
|
(12,475
|
)
|
$
|
(1,823
|
)
|
$
|
(254
|
)
|
||||
|
Financing activities
|
$
|
(4,963
|
)
|
$
|
(3,025
|
)
|
$
|
(2,544
|
)
|
||||
|
(in millions)
|
2015
|
2014
|
2013
|
||||||||||
|
Shares repurchased and retired
|
95
|
|
59
|
|
10
|
|
|||||||
|
Amount
|
$
|
10,002
|
|
$
|
5,349
|
|
$
|
582
|
|
||||
|
•
|
the commercial performance of our current and future products;
|
|
•
|
the progress and scope of our R&D efforts, including preclinical studies and clinical trials;
|
|
•
|
the cost, timing and outcome of regulatory reviews;
|
|
•
|
the expansion of our sales and marketing capabilities;
|
|
•
|
administrative expenses;
|
|
•
|
the possibility of acquiring additional manufacturing capabilities or office facilities;
|
|
•
|
the possibility of acquiring other companies or new products;
|
|
•
|
costs associated with the settlement and conversion of our convertible senior notes and related warrants;
|
|
•
|
the establishment of additional collaborative relationships with other companies; and
|
|
•
|
costs associated with the defense, settlement and adverse results of litigation and government investigations.
|
|
Accrued government and other rebates and chargebacks:
|
Balance at Beginning of Year
|
Decrease/(Increase) to Product Sales
|
Payments
|
Balance at End of Year
|
||||||||||||
|
Year ended December 31, 2015:
|
|
|
|
|
||||||||||||
|
Activity related to 2015 sales
|
$
|
—
|
|
$
|
16,400
|
|
$
|
(11,597
|
)
|
$
|
4,803
|
|
||||
|
Activity related to sales prior to 2015
|
2,536
|
|
7
|
|
(2,321
|
)
|
222
|
|
||||||||
|
Total
|
$
|
2,536
|
|
$
|
16,407
|
|
$
|
(13,918
|
)
|
$
|
5,025
|
|
||||
|
Year ended December 31, 2014:
|
|
|
|
|
|
|
|
|
||||||||
|
Activity related to 2014 sales
|
$
|
—
|
|
$
|
6,113
|
|
$
|
(3,650
|
)
|
$
|
2,463
|
|
||||
|
Activity related to sales prior to 2014
|
1,167
|
|
(109
|
)
|
(985
|
)
|
73
|
|
||||||||
|
Total
|
$
|
1,167
|
|
$
|
6,004
|
|
$
|
(4,635
|
)
|
$
|
2,536
|
|
||||
|
•
|
estimates of revenues and operating profits related to the products or product candidates;
|
|
•
|
the probability of success for unapproved product candidates considering their stages of development;
|
|
•
|
the time and resources needed to complete the development and approval of product candidates;
|
|
•
|
the life of the potential commercialized products and associated risks, including the inherent difficulties and uncertainties in developing a product candidate such as obtaining FDA and other regulatory approvals; and
|
|
•
|
risks related to the viability of and potential alternative treatments in any future target markets.
|
|
|
Payments due by Period
|
|||||||||||||||||||
|
Contractual Obligations
|
Total
|
Less than one
year
|
1-3 years
|
3-5 years
|
More than 5
years
|
|||||||||||||||
|
Debt
(1)
|
$
|
36,003
|
|
$
|
1,819
|
|
$
|
2,645
|
|
$
|
4,587
|
|
$
|
26,952
|
|
|||||
|
Operating lease obligations
|
317
|
|
66
|
|
114
|
|
74
|
|
63
|
|
||||||||||
|
Capital commitments
(2)
|
847
|
|
387
|
|
438
|
|
22
|
|
—
|
|
||||||||||
|
Purchase obligations
(3)(4)
|
3,084
|
|
1,759
|
|
1,034
|
|
208
|
|
83
|
|
||||||||||
|
Clinical trials
(5)
|
1,336
|
|
683
|
|
481
|
|
126
|
|
46
|
|
||||||||||
|
Total
|
$
|
41,587
|
|
$
|
4,714
|
|
$
|
4,712
|
|
$
|
5,017
|
|
$
|
27,144
|
|
|||||
|
(1)
|
Our debt obligations include senior unsecured notes and convertible senior notes. Interest payments are incurred and calculated based on terms of the related notes. For further information, see Item 8, Note
10
Debt and Credit Facility in our Consolidated Financial Statements included in this Annual Report on Form 10-K.
|
|
(2)
|
At
December 31, 2015
, we had firm capital project commitments of approximately
$847 million
primarily relating to construction of new buildings.
|
|
(3)
|
At
December 31, 2015
, we had firm purchase commitments related to active pharmaceutical ingredients and certain inventory-related items. These amounts include minimum purchase requirements.
|
|
(4)
|
In addition to the above, we have committed to make potential future milestone payments to third parties as part of licensing, collaboration and development arrangements. Payments under these agreements generally become due and payable only upon achievement of certain developmental, regulatory and/or commercial milestones. Because the achievement of these milestones is neither probable nor reasonably estimable, such contingencies have not been recorded on our Consolidated Balance Sheets and have not been included in the table above.
|
|
(5)
|
At
December 31, 2015
, we had several clinical studies in various clinical trial phases. Our most significant clinical trial expenditures are to contract research organizations (CROs). Although all of our material contracts with CROs are cancelable, we historically have not canceled such contracts. These amounts reflect commitments based on existing contracts and do not reflect any future modifications to, or terminations of, existing contracts or anticipated or potential new contracts.
|
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
|
•
|
safety and preservation of principal and diversification of risk;
|
|
•
|
liquidity of investments sufficient to meet cash flow requirements; and
|
|
•
|
competitive after-tax rate of return.
|
|
|
Expected Maturity
|
|
Total Fair Value
|
|||||||||||||||||||||||||||||
|
|
2016
|
2017
|
2018
|
2019
|
2020
|
Thereafter
|
Total
|
|||||||||||||||||||||||||
|
Assets
|
|
|
|
|
|
|
|
|
||||||||||||||||||||||||
|
Available-for-sale debt securities
|
$
|
1,759
|
|
$
|
6,020
|
|
$
|
4,466
|
|
$
|
525
|
|
$
|
384
|
|
$
|
206
|
|
$
|
13,360
|
|
$
|
13,360
|
|
||||||||
|
Average interest rate
|
0.75
|
%
|
1.21
|
%
|
1.58
|
%
|
1.36
|
%
|
1.48
|
%
|
1.71
|
%
|
|
|
|
|
||||||||||||||||
|
Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
Debt
(1)
|
$
|
985
|
|
$
|
—
|
|
$
|
1,000
|
|
$
|
500
|
|
$
|
2,500
|
|
$
|
17,250
|
|
$
|
22,235
|
|
$
|
23,738
|
|
||||||||
|
Average interest rate
|
2.64
|
%
|
—
|
%
|
1.85
|
%
|
2.05
|
%
|
2.51
|
%
|
4.24
|
%
|
|
|
|
|
||||||||||||||||
|
(1)
|
As of December 31, 2015 our debt consisted of senior unsecured notes and convertible senior notes with an aggregate carrying value of
$22.2 billion
.
Since these instruments bear interest at fixed rates, changes in interest rates do not affect interest expense or cash flows. However, the fair value of these instruments fluctuates when interest rates change. See Note
10
, Debt and Credit Facility in our Consolidated Financial Statements included in this Annual Report on Form 10-K for additional information.
|
|
ITEM 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
|
Report of Independent Registered Public Accounting Firm
|
||
|
Audited Consolidated Financial Statements:
|
||
|
Selected Quarterly Financial Information
(Unaudited)
|
||
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Assets
|
|
|
|||||
|
Current assets:
|
|
|
|||||
|
Cash and cash equivalents
|
$
|
12,851
|
|
$
|
10,027
|
|
|
|
Short-term marketable securities
|
1,756
|
|
101
|
|
|||
|
Accounts receivable, net of allowances of $1,032 at December 31, 2015 and $356 at December 31, 2014
|
5,854
|
|
4,635
|
|
|||
|
Inventories
|
1,955
|
|
1,386
|
|
|||
|
Deferred tax assets
|
828
|
|
508
|
|
|||
|
Prepaid and other current assets
|
1,519
|
|
1,057
|
|
|||
|
Total current assets
|
24,763
|
|
17,714
|
|
|||
|
Property, plant and equipment, net
|
2,276
|
|
1,674
|
|
|||
|
Long-term portion of prepaid royalties
|
400
|
|
466
|
|
|||
|
Long-term deferred tax assets
|
324
|
|
236
|
|
|||
|
Long-term marketable securities
|
11,601
|
|
1,598
|
|
|||
|
Intangible assets, net
|
10,247
|
|
11,073
|
|
|||
|
Goodwill
|
1,172
|
|
1,172
|
|
|||
|
Other long-term assets
|
1,056
|
|
731
|
|
|||
|
Total assets
|
$
|
51,839
|
|
$
|
34,664
|
|
|
|
|
|
|
|
||||
|
Liabilities and Stockholders’ Equity
|
|
|
|
|
|||
|
Current liabilities:
|
|
|
|
|
|||
|
Accounts payable
|
$
|
1,178
|
|
$
|
955
|
|
|
|
Accrued government and other rebates
|
4,118
|
|
2,316
|
|
|||
|
Other accrued liabilities
|
3,172
|
|
1,873
|
|
|||
|
Deferred revenues
|
440
|
|
134
|
|
|||
|
Current portion of long-term debt and other obligations, net
|
983
|
|
483
|
|
|||
|
Total current liabilities
|
9,891
|
|
5,761
|
|
|||
|
Long-term debt, net
|
21,195
|
|
11,921
|
|
|||
|
Long-term income taxes payable
|
1,243
|
|
562
|
|
|||
|
Other long-term obligations
|
395
|
|
586
|
|
|||
|
Commitments and contingencies (Note 11)
|
|
|
|
|
|||
|
Equity component of currently redeemable convertible notes
|
2
|
|
15
|
|
|||
|
Stockholders’ equity:
|
|
|
|
|
|||
|
Preferred stock, par value $0.001 per share; 5 shares authorized; none outstanding
|
—
|
|
—
|
|
|||
|
Common stock, par value $0.001 per share; shares authorized of 5,600 at December 31, 2015 and December 31, 2014; shares issued and outstanding of 1,422 at December 31, 2015 and 1,499 at December 31, 2014
|
1
|
|
2
|
|
|||
|
Additional paid-in capital
|
444
|
|
2,391
|
|
|||
|
Accumulated other comprehensive income
|
88
|
|
301
|
|
|||
|
Retained earnings
|
18,001
|
|
12,732
|
|
|||
|
Total Gilead stockholders’ equity
|
18,534
|
|
15,426
|
|
|||
|
Noncontrolling interest
|
579
|
|
393
|
|
|||
|
Total stockholders’ equity
|
19,113
|
|
15,819
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
51,839
|
|
$
|
34,664
|
|
|
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Revenues:
|
|
|
|
|||||||||
|
Product sales
|
$
|
32,151
|
|
$
|
24,474
|
|
$
|
10,804
|
|
|||
|
Royalty, contract and other revenues
|
488
|
|
416
|
|
398
|
|
||||||
|
Total revenues
|
32,639
|
|
24,890
|
|
11,202
|
|
||||||
|
Costs and expenses:
|
|
|||||||||||
|
Cost of goods sold
|
4,006
|
|
3,788
|
|
2,859
|
|
||||||
|
Research and development expenses
|
3,014
|
|
2,854
|
|
2,120
|
|
||||||
|
Selling, general and administrative expenses
|
3,426
|
|
2,983
|
|
1,699
|
|
||||||
|
Total costs and expenses
|
10,446
|
|
9,625
|
|
6,678
|
|
||||||
|
Income from operations
|
22,193
|
|
15,265
|
|
4,524
|
|
||||||
|
Interest expense
|
(688
|
)
|
(412
|
)
|
(307
|
)
|
||||||
|
Other income (expense), net
|
154
|
|
3
|
|
(9
|
)
|
||||||
|
Income before provision for income taxes
|
21,659
|
|
14,856
|
|
4,208
|
|
||||||
|
Provision for income taxes
|
3,553
|
|
2,797
|
|
1,151
|
|
||||||
|
Net income
|
18,106
|
|
12,059
|
|
3,057
|
|
||||||
|
Net loss attributable to noncontrolling interest
|
(2
|
)
|
(42
|
)
|
(18
|
)
|
||||||
|
Net income attributable to Gilead
|
$
|
18,108
|
|
$
|
12,101
|
|
$
|
3,075
|
|
|||
|
Net income per share attributable to Gilead common stockholders - basic
|
$
|
12.37
|
|
$
|
7.95
|
|
$
|
2.01
|
|
|||
|
Shares used in per share calculation - basic
|
1,464
|
|
1,522
|
|
1,529
|
|
||||||
|
Net income per share attributable to Gilead common stockholders - diluted
|
$
|
11.91
|
|
$
|
7.35
|
|
$
|
1.81
|
|
|||
|
Shares used in per share calculation - diluted
|
1,521
|
|
1,647
|
|
1,695
|
|
||||||
|
Cash dividends declared per share
|
$
|
1.29
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
Year Ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
Net income
|
$
|
18,106
|
|
$
|
12,059
|
|
$
|
3,057
|
|
|||
|
Other comprehensive income (loss):
|
||||||||||||
|
Net foreign currency translation gain (loss), net of tax
|
9
|
|
(9
|
)
|
(44
|
)
|
||||||
|
Available-for-sale securities:
|
||||||||||||
|
Net unrealized gain (loss), net of tax impact of $(17), $0 and $4
|
(29
|
)
|
1
|
|
5
|
|
||||||
|
Reclassifications to net income, net of tax impact of $1, $0 and $0
|
1
|
|
(1
|
)
|
—
|
|
||||||
|
Net change
|
(28
|
)
|
—
|
|
5
|
|
||||||
|
Cash flow hedges:
|
||||||||||||
|
Net unrealized gain (loss), net of tax impact of $21, $16 and $4
|
389
|
|
430
|
|
(60
|
)
|
||||||
|
Reclassification to net income, net of tax impact of $(19), $(4) and $(1)
|
(583
|
)
|
4
|
|
21
|
|
||||||
|
Net change
|
(194
|
)
|
434
|
|
(39
|
)
|
||||||
|
Other comprehensive income (loss)
|
(213
|
)
|
425
|
|
(78
|
)
|
||||||
|
Comprehensive income
|
17,893
|
|
12,484
|
|
2,979
|
|
||||||
|
Comprehensive loss attributable to noncontrolling interest
|
(2
|
)
|
(42
|
)
|
(18
|
)
|
||||||
|
Comprehensive income attributable to Gilead
|
$
|
17,895
|
|
$
|
12,526
|
|
$
|
2,997
|
|
|||
|
|
Gilead Stockholders' Equity
|
Noncontrolling
Interest
|
Total
Stockholders'
Equity
|
||||||||||||||||||||||||
|
Common Stock
|
Additional
Paid-In
Capital
|
Accumulated
Other
Comprehensive
Income (Loss)
|
Retained
Earnings
|
||||||||||||||||||||||||
|
Shares
|
Amount
|
||||||||||||||||||||||||||
|
Balance at December 31, 2012
|
1,519
|
|
$
|
2
|
|
$
|
5,642
|
|
$
|
(46
|
)
|
$
|
3,705
|
|
$
|
241
|
|
$
|
9,544
|
|
|||||||
|
Contributions from noncontrolling interest
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
152
|
|
152
|
|
|||||||||||||
|
Net income (loss)
|
—
|
|
—
|
|
—
|
|
—
|
|
3,075
|
|
(18
|
)
|
3,057
|
|
|||||||||||||
|
Other comprehensive loss, net of tax
|
—
|
|
—
|
|
—
|
|
(78
|
)
|
—
|
|
—
|
|
(78
|
)
|
|||||||||||||
|
Issuances under employee stock purchase plan
|
3
|
|
—
|
|
55
|
|
—
|
|
—
|
|
—
|
|
55
|
|
|||||||||||||
|
Issuances under equity incentive plans
|
24
|
|
—
|
|
258
|
|
—
|
|
—
|
|
—
|
|
258
|
|
|||||||||||||
|
Tax benefits from employee stock plans
|
—
|
|
—
|
|
285
|
|
—
|
|
—
|
|
—
|
|
285
|
|
|||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
254
|
|
—
|
|
—
|
|
—
|
|
254
|
|
|||||||||||||
|
Repurchases of common stock
|
(12
|
)
|
—
|
|
(14
|
)
|
—
|
|
(674
|
)
|
—
|
|
(688
|
)
|
|||||||||||||
|
Warrants settlement
|
—
|
|
—
|
|
(1,040
|
)
|
—
|
|
—
|
|
—
|
|
(1,040
|
)
|
|||||||||||||
|
Convertible notes settlement
|
—
|
|
—
|
|
(2,771
|
)
|
—
|
|
—
|
|
—
|
|
(2,771
|
)
|
|||||||||||||
|
Convertible notes hedge settlement
|
—
|
|
—
|
|
2,774
|
|
—
|
|
—
|
|
—
|
|
2,774
|
|
|||||||||||||
|
Reclassification to equity component of currently redeemable convertible notes
|
—
|
|
—
|
|
(57
|
)
|
—
|
|
—
|
|
—
|
|
(57
|
)
|
|||||||||||||
|
Balance at December 31, 2013
|
1,534
|
|
2
|
|
5,386
|
|
(124
|
)
|
6,106
|
|
375
|
|
11,745
|
|
|||||||||||||
|
Change in noncontrolling interest
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
60
|
|
60
|
|
|||||||||||||
|
Net income (loss)
|
—
|
|
—
|
|
—
|
|
—
|
|
12,101
|
|
(42
|
)
|
12,059
|
|
|||||||||||||
|
Other comprehensive income, net of tax
|
—
|
|
—
|
|
—
|
|
425
|
|
—
|
|
—
|
|
425
|
|
|||||||||||||
|
Issuances under employee stock purchase plan
|
3
|
|
—
|
|
72
|
|
—
|
|
—
|
|
—
|
|
72
|
|
|||||||||||||
|
Issuances under equity incentive plans
|
24
|
|
—
|
|
260
|
|
—
|
|
—
|
|
—
|
|
260
|
|
|||||||||||||
|
Tax benefits from employee stock plans
|
—
|
|
—
|
|
484
|
|
—
|
|
—
|
|
—
|
|
484
|
|
|||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
362
|
|
—
|
|
—
|
|
—
|
|
362
|
|
|||||||||||||
|
Repurchases of common stock
|
(62
|
)
|
—
|
|
(133
|
)
|
—
|
|
(5,475
|
)
|
—
|
|
(5,608
|
)
|
|||||||||||||
|
Warrants settlement
|
—
|
|
—
|
|
(4,093
|
)
|
—
|
|
—
|
|
—
|
|
(4,093
|
)
|
|||||||||||||
|
Convertible notes settlement
|
—
|
|
—
|
|
(2,513
|
)
|
—
|
|
—
|
|
—
|
|
(2,513
|
)
|
|||||||||||||
|
Convertible notes hedge settlement
|
—
|
|
—
|
|
2,543
|
|
—
|
|
—
|
|
—
|
|
2,543
|
|
|||||||||||||
|
Purchases of convertible note hedges
|
—
|
|
—
|
|
(26
|
)
|
—
|
|
—
|
|
—
|
|
(26
|
)
|
|||||||||||||
|
Reclassification to equity component of currently redeemable convertible notes
|
—
|
|
—
|
|
49
|
|
—
|
|
—
|
|
—
|
|
49
|
|
|||||||||||||
|
Balance at December 31, 2014
|
1,499
|
|
2
|
|
2,391
|
|
301
|
|
12,732
|
|
393
|
|
15,819
|
|
|||||||||||||
|
Change in noncontrolling interest
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
188
|
|
188
|
|
|||||||||||||
|
Net income (loss)
|
—
|
|
—
|
|
—
|
|
—
|
|
18,108
|
|
(2
|
)
|
18,106
|
|
|||||||||||||
|
Other comprehensive loss, net of tax
|
—
|
|
—
|
|
—
|
|
(213
|
)
|
—
|
|
—
|
|
(213
|
)
|
|||||||||||||
|
Issuances under employee stock purchase plan
|
1
|
|
—
|
|
86
|
|
—
|
|
—
|
|
—
|
|
86
|
|
|||||||||||||
|
Issuances under equity incentive plans
|
21
|
|
—
|
|
235
|
|
—
|
|
—
|
|
—
|
|
235
|
|
|||||||||||||
|
Tax benefits from employee stock plans
|
—
|
|
—
|
|
586
|
|
—
|
|
—
|
|
—
|
|
586
|
|
|||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
384
|
|
—
|
|
—
|
|
—
|
|
384
|
|
|||||||||||||
|
Repurchases of common stock
|
(99
|
)
|
(1
|
)
|
(222
|
)
|
—
|
|
(10,115
|
)
|
—
|
|
(10,338
|
)
|
|||||||||||||
|
Warrants settlement
|
—
|
|
—
|
|
(3,031
|
)
|
—
|
|
(834
|
)
|
—
|
|
(3,865
|
)
|
|||||||||||||
|
Convertible notes settlement
|
—
|
|
—
|
|
(782
|
)
|
—
|
|
—
|
|
—
|
|
(782
|
)
|
|||||||||||||
|
Convertible notes hedge settlement
|
—
|
|
—
|
|
784
|
|
—
|
|
—
|
|
—
|
|
784
|
|
|||||||||||||
|
Dividends declared
|
—
|
|
—
|
|
—
|
|
—
|
|
(1,890
|
)
|
—
|
|
(1,890
|
)
|
|||||||||||||
|
Reclassification to equity component of currently redeemable convertible notes
|
—
|
|
—
|
|
13
|
|
—
|
|
—
|
|
—
|
|
13
|
|
|||||||||||||
|
Balance at December 31, 2015
|
1,422
|
|
$
|
1
|
|
$
|
444
|
|
$
|
88
|
|
$
|
18,001
|
|
$
|
579
|
|
$
|
19,113
|
|
|||||||
|
Year Ended December 31,
|
||||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Operating Activities:
|
||||||||||||
|
Net income
|
$
|
18,106
|
|
$
|
12,059
|
|
$
|
3,057
|
|
|||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
||||||||||||
|
Depreciation expense
|
161
|
|
125
|
|
103
|
|
||||||
|
Amortization expense
|
937
|
|
925
|
|
242
|
|
||||||
|
Stock-based compensation expense
|
382
|
|
360
|
|
252
|
|
||||||
|
Excess tax benefits from stock-based compensation
|
(585
|
)
|
(482
|
)
|
(279
|
)
|
||||||
|
Tax benefits from exercise and vesting of stock-based awards
|
586
|
|
484
|
|
285
|
|
||||||
|
Deferred income taxes
|
(393
|
)
|
(236
|
)
|
(98
|
)
|
||||||
|
Other
|
(24
|
)
|
101
|
|
105
|
|
||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Accounts receivable, net
|
(1,397
|
)
|
(2,578
|
)
|
(315
|
)
|
||||||
|
Inventories
|
(855
|
)
|
143
|
|
(343
|
)
|
||||||
|
Prepaid expenses and other assets
|
(90
|
)
|
(371
|
)
|
(170
|
)
|
||||||
|
Accounts payable
|
226
|
|
(289
|
)
|
(98
|
)
|
||||||
|
Income taxes payable
|
269
|
|
533
|
|
30
|
|
||||||
|
Accrued liabilities
|
2,632
|
|
2,013
|
|
312
|
|
||||||
|
Deferred revenues
|
374
|
|
31
|
|
22
|
|
||||||
|
Net cash provided by operating activities
|
20,329
|
|
12,818
|
|
3,105
|
|
||||||
|
Investing Activities:
|
||||||||||||
|
Purchases of marketable securities
|
(17,239
|
)
|
(2,107
|
)
|
(257
|
)
|
||||||
|
Proceeds from sales of marketable securities
|
4,792
|
|
807
|
|
494
|
|
||||||
|
Proceeds from maturities of marketable securities
|
719
|
|
52
|
|
78
|
|
||||||
|
Other investments
|
—
|
|
(18
|
)
|
—
|
|
||||||
|
Acquisitions, net of cash acquired
|
—
|
|
—
|
|
(379
|
)
|
||||||
|
Capital expenditures
|
(747
|
)
|
(557
|
)
|
(190
|
)
|
||||||
|
Net cash used in investing activities
|
(12,475
|
)
|
(1,823
|
)
|
(254
|
)
|
||||||
|
Financing Activities:
|
||||||||||||
|
Proceeds from debt financing, net of issuance costs
|
9,902
|
|
7,932
|
|
—
|
|
||||||
|
Proceeds from convertible note hedges
|
784
|
|
2,543
|
|
2,774
|
|
||||||
|
Purchases of convertible note hedges
|
—
|
|
(26
|
)
|
—
|
|
||||||
|
Proceeds from issuances of common stock
|
319
|
|
331
|
|
313
|
|
||||||
|
Repurchases of common stock
|
(10,002
|
)
|
(5,349
|
)
|
(582
|
)
|
||||||
|
Repayments of debt and other obligations
|
(997
|
)
|
(4,779
|
)
|
(4,440
|
)
|
||||||
|
Payments to settle warrants
|
(3,865
|
)
|
(4,093
|
)
|
(1,040
|
)
|
||||||
|
Excess tax benefits from stock-based compensation
|
585
|
|
482
|
|
279
|
|
||||||
|
Payment of contingent consideration
|
(3
|
)
|
(101
|
)
|
—
|
|
||||||
|
Payment of dividends
|
(1,874
|
)
|
—
|
|
—
|
|
||||||
|
Contributions from noncontrolling interest
|
188
|
|
35
|
|
152
|
|
||||||
|
Net cash used in financing activities
|
(4,963
|
)
|
(3,025
|
)
|
(2,544
|
)
|
||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
(67
|
)
|
(56
|
)
|
2
|
|
||||||
|
Net change in cash and cash equivalents
|
2,824
|
|
7,914
|
|
309
|
|
||||||
|
Cash and cash equivalents at beginning of period
|
10,027
|
|
2,113
|
|
1,804
|
|
||||||
|
Cash and cash equivalents at end of period
|
$
|
12,851
|
|
$
|
10,027
|
|
$
|
2,113
|
|
|||
|
Supplemental disclosure of cash flow information:
|
||||||||||||
|
Interest paid, net of amounts capitalized
|
$
|
529
|
|
$
|
330
|
|
$
|
238
|
|
|||
|
Income taxes paid
|
$
|
3,137
|
|
$
|
2,060
|
|
$
|
1,051
|
|
|||
|
1
.
|
ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
|
|
Description
|
Estimated Useful Life
|
|
Buildings and improvements
|
20-35
|
|
Laboratory and manufacturing equipment
|
4-10
|
|
Office and computer equipment
|
3-7
|
|
Leasehold improvements
|
Shorter of useful life or lease term
|
|
2
.
|
FAIR VALUE MEASUREMENTS
|
|
•
|
Level 1 inputs which include quoted prices in active markets for identical assets or liabilities;
|
|
•
|
Level 2 inputs which include observable inputs other than Level 1 inputs, such as quoted prices for similar assets or liabilities; quoted prices for identical or similar assets or liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the asset or liability. For our marketable securities, we review trading activity and pricing as of the measurement date. When sufficient quoted pricing for identical securities is not available, we use market pricing and other observable market inputs for similar securities obtained from various third-party data providers. These inputs either represent quoted prices for similar assets in active markets or have been derived from observable market data; and
|
|
•
|
Level 3 inputs which include unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the underlying asset or liability. Our Level 3 assets and liabilities include those whose
|
|
|
December 31, 2015
|
December 31, 2014
|
|||||||||||||||||||||||||||||
|
|
Level 1
|
Level 2
|
Level 3
|
Total
|
Level 1
|
Level 2
|
Level 3
|
Total
|
|||||||||||||||||||||||
|
Assets:
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
|
Money market funds
|
$
|
10,161
|
|
$
|
—
|
|
$
|
—
|
|
$
|
10,161
|
|
$
|
7,926
|
|
$
|
—
|
|
$
|
—
|
|
$
|
7,926
|
|
|||||||
|
Corporate debt securities
|
—
|
|
5,773
|
|
—
|
|
5,773
|
|
—
|
|
938
|
|
—
|
|
938
|
|
|||||||||||||||
|
U.S. treasury securities
|
4,389
|
|
—
|
|
—
|
|
4,389
|
|
363
|
|
—
|
|
—
|
|
363
|
|
|||||||||||||||
|
Residential mortgage and asset-backed securities
|
—
|
|
1,695
|
|
—
|
|
1,695
|
|
—
|
|
269
|
|
—
|
|
269
|
|
|||||||||||||||
|
U.S. government agencies securities
|
—
|
|
707
|
|
—
|
|
707
|
|
—
|
|
113
|
|
—
|
|
113
|
|
|||||||||||||||
|
Certificates of deposit
|
—
|
|
448
|
|
—
|
|
448
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||
|
Non-U.S. government securities
|
—
|
|
313
|
|
—
|
|
313
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||
|
Municipal debt securities
|
—
|
|
34
|
|
—
|
|
34
|
|
—
|
|
16
|
|
—
|
|
16
|
|
|||||||||||||||
|
Foreign currency derivative contracts
|
—
|
|
210
|
|
—
|
|
210
|
|
—
|
|
349
|
|
—
|
|
349
|
|
|||||||||||||||
|
Deferred compensation plan
|
66
|
|
—
|
|
—
|
|
66
|
|
54
|
|
—
|
|
—
|
|
54
|
|
|||||||||||||||
|
|
$
|
14,616
|
|
$
|
9,180
|
|
$
|
—
|
|
$
|
23,796
|
|
$
|
8,343
|
|
$
|
1,685
|
|
$
|
—
|
|
$
|
10,028
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
Liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
Contingent consideration
|
$
|
—
|
|
$
|
—
|
|
$
|
59
|
|
$
|
59
|
|
$
|
—
|
|
$
|
—
|
|
$
|
133
|
|
$
|
133
|
|
|||||||
|
Deferred compensation plan
|
66
|
|
—
|
|
—
|
|
66
|
|
54
|
|
—
|
|
—
|
|
54
|
|
|||||||||||||||
|
Foreign currency derivative contracts
|
—
|
|
41
|
|
—
|
|
41
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||||
|
|
$
|
66
|
|
$
|
41
|
|
$
|
59
|
|
$
|
166
|
|
$
|
54
|
|
$
|
—
|
|
$
|
133
|
|
$
|
187
|
|
|||||||
|
December 31, 2015
|
December 31, 2014
|
|||||||||||||||||||||||||||||||
|
|
Amortized
Cost |
Gross
Unrealized Gains |
Gross
Unrealized Losses |
Estimated
Fair Value |
Amortized
Cost |
Gross
Unrealized Gains |
Gross
Unrealized Losses |
Estimated
Fair Value |
||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
|
Money market funds
|
$
|
10,161
|
|
$
|
—
|
|
$
|
—
|
|
$
|
10,161
|
|
$
|
7,926
|
|
$
|
—
|
|
$
|
—
|
|
$
|
7,926
|
|
||||||||
|
Corporate debt securities
|
5,795
|
|
1
|
|
(23
|
)
|
5,773
|
|
941
|
|
—
|
|
(3
|
)
|
938
|
|
||||||||||||||||
|
U.S. treasury securities
|
4,407
|
|
—
|
|
(18
|
)
|
4,389
|
|
363
|
|
—
|
|
—
|
|
363
|
|
||||||||||||||||
|
Residential mortgage and asset-backed securities
|
1,701
|
|
—
|
|
(6
|
)
|
1,695
|
|
269
|
|
—
|
|
—
|
|
269
|
|
||||||||||||||||
|
U.S. government agencies securities
|
709
|
|
—
|
|
(2
|
)
|
707
|
|
113
|
|
—
|
|
—
|
|
113
|
|
||||||||||||||||
|
Certificates of deposit
|
448
|
|
—
|
|
—
|
|
448
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||
|
Non-U.S. government securities
|
315
|
|
—
|
|
(2
|
)
|
313
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||||
|
Municipal debt securities
|
34
|
|
—
|
|
—
|
|
34
|
|
16
|
|
—
|
|
—
|
|
16
|
|
||||||||||||||||
|
Total
|
$
|
23,570
|
|
$
|
1
|
|
$
|
(51
|
)
|
$
|
23,520
|
|
$
|
9,628
|
|
$
|
—
|
|
$
|
(3
|
)
|
$
|
9,625
|
|
||||||||
|
|
December 31, 2015
|
December 31, 2014
|
|||||
|
Cash and cash equivalents
|
$
|
10,163
|
|
$
|
7,926
|
|
|
|
Short-term marketable securities
|
1,756
|
|
101
|
|
|||
|
Long-term marketable securities
|
11,601
|
|
1,598
|
|
|||
|
Total
|
$
|
23,520
|
|
$
|
9,625
|
|
|
|
|
December 31, 2015
|
||||||
|
|
Amortized Cost
|
Fair Value
|
|||||
|
Less than one year
|
$
|
11,921
|
|
$
|
11,919
|
|
|
|
Greater than one year but less than five years
|
11,442
|
|
11,395
|
|
|||
|
Greater than five years but less than ten years
|
186
|
|
184
|
|
|||
|
Greater than ten years
|
21
|
|
22
|
|
|||
|
Total
|
$
|
23,570
|
|
$
|
23,520
|
|
|
|
|
Less Than 12 Months
|
12 Months or Greater
|
Total
|
|||||||||||||||||||||
|
|
Gross
Unrealized Losses |
Estimated
Fair Value |
Gross
Unrealized Losses |
Estimated
Fair Value |
Gross
Unrealized Losses |
Estimated
Fair Value |
||||||||||||||||||
|
December 31, 2015
|
|
|
|
|
|
|
||||||||||||||||||
|
Debt securities:
|
|
|
|
|
|
|
||||||||||||||||||
|
Corporate debt securities
|
$
|
(23
|
)
|
$
|
4,891
|
|
$
|
—
|
|
$
|
43
|
|
$
|
(23
|
)
|
$
|
4,934
|
|
||||||
|
U.S. treasury securities
|
(18
|
)
|
4,342
|
|
—
|
|
—
|
|
(18
|
)
|
4,342
|
|
||||||||||||
|
Residential mortgage and asset-backed securities
|
(6
|
)
|
1,626
|
|
—
|
|
20
|
|
(6
|
)
|
1,646
|
|
||||||||||||
|
U.S. government agencies securities
|
(2
|
)
|
707
|
|
—
|
|
—
|
|
(2
|
)
|
707
|
|
||||||||||||
|
Non-U.S. government securities
|
(2
|
)
|
313
|
|
—
|
|
—
|
|
(2
|
)
|
313
|
|
||||||||||||
|
Municipal debt securities
|
—
|
|
21
|
|
—
|
|
—
|
|
—
|
|
21
|
|
||||||||||||
|
Total
|
$
|
(51
|
)
|
$
|
11,900
|
|
$
|
—
|
|
$
|
63
|
|
$
|
(51
|
)
|
$
|
11,963
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
December 31, 2014
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
Debt securities:
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
Corporate debt securities
|
$
|
(3
|
)
|
$
|
802
|
|
$
|
—
|
|
$
|
—
|
|
$
|
(3
|
)
|
$
|
802
|
|
||||||
|
Residential mortgage and asset-backed securities
|
—
|
|
227
|
|
—
|
|
1
|
|
—
|
|
228
|
|
||||||||||||
|
U.S. treasury securities
|
—
|
|
206
|
|
—
|
|
—
|
|
—
|
|
206
|
|
||||||||||||
|
U.S. government agencies securities
|
—
|
|
22
|
|
—
|
|
—
|
|
—
|
|
22
|
|
||||||||||||
|
Municipal debt securities
|
—
|
|
2
|
|
—
|
|
—
|
|
—
|
|
2
|
|
||||||||||||
|
Total
|
$
|
(3
|
)
|
$
|
1,259
|
|
$
|
—
|
|
$
|
1
|
|
$
|
(3
|
)
|
$
|
1,260
|
|
||||||
|
4
.
|
DERIVATIVE FINANCIAL INSTRUMENTS
|
|
|
December 31, 2015
|
|||||||||||
|
|
Asset Derivatives
|
Liability Derivatives
|
||||||||||
|
|
Classification
|
Fair Value
|
Classification
|
Fair
Value
|
||||||||
|
Derivatives designated as hedges:
|
|
|
|
|
||||||||
|
Foreign currency exchange contracts
|
Other current assets
|
$
|
200
|
|
Other accrued liabilities
|
$
|
(32
|
)
|
||||
|
Foreign currency exchange contracts
|
Other long-term assets
|
9
|
|
Other long-term obligations
|
(8
|
)
|
||||||
|
Total derivatives designated as hedges
|
|
209
|
|
|
(40
|
)
|
||||||
|
Derivatives not designated as hedges:
|
|
|
|
|
|
|
||||||
|
Foreign currency exchange contracts
|
Other current assets
|
1
|
|
Other accrued liabilities
|
(1
|
)
|
||||||
|
Total derivatives not designated as hedges
|
|
1
|
|
|
(1
|
)
|
||||||
|
Total derivatives
|
|
$
|
210
|
|
|
$
|
(41
|
)
|
||||
|
|
December 31, 2014
|
|||||||||||
|
|
Asset Derivatives
|
Liability Derivatives
|
||||||||||
|
|
Classification
|
Fair Value
|
Classification
|
Fair
Value
|
||||||||
|
Derivatives designated as hedges:
|
|
|
|
|
||||||||
|
Foreign currency exchange contracts
|
Other current assets
|
$
|
314
|
|
Other accrued liabilities
|
$
|
—
|
|
||||
|
Foreign currency exchange contracts
|
Other long-term assets
|
35
|
|
Other long-term obligations
|
—
|
|
||||||
|
Total derivatives
|
|
$
|
349
|
|
|
$
|
—
|
|
||||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Derivatives designated as hedges:
|
|
|
||||||||||
|
Gains (losses) recognized in accumulated OCI (effective portion)
|
$
|
410
|
|
$
|
446
|
|
$
|
(55
|
)
|
|||
|
Gains (losses) reclassified from accumulated OCI into product sales (effective portion)
|
$
|
602
|
|
$
|
—
|
|
$
|
(20
|
)
|
|||
|
Gains (losses) recognized in other income (expense), net (ineffective portion and amounts excluded from effectiveness testing)
|
$
|
13
|
|
$
|
(7
|
)
|
$
|
2
|
|
|||
|
Derivatives not designated as hedges:
|
|
|
|
|
|
|
||||||
|
Gains (losses) recognized in other income (expense), net
|
$
|
117
|
|
$
|
135
|
|
$
|
(17
|
)
|
|||
|
As of December 31, 2015
|
||||||||||||||||||||||||
|
Offsetting of Derivative Assets/Liabilities
|
||||||||||||||||||||||||
|
Gross Amounts Not Offset in the Consolidated Balance Sheet
|
||||||||||||||||||||||||
|
Description
|
Gross Amounts of Recognized Assets/Liabilities
|
Gross Amounts Offset in the Consolidated Balance Sheet
|
Amounts of Assets/Liabilities Presented
in the Consolidated
Balance Sheet
|
Derivative Financial Instruments
|
Cash Collateral Received/Pledged
|
Net Amount (Legal Offset)
|
||||||||||||||||||
|
Derivative assets
|
$
|
210
|
|
$
|
—
|
|
$
|
210
|
|
$
|
(38
|
)
|
$
|
—
|
|
$
|
172
|
|
||||||
|
Derivative liabilities
|
(41
|
)
|
—
|
|
(41
|
)
|
38
|
|
—
|
|
(3
|
)
|
||||||||||||
|
As of December 31, 2014
|
||||||||||||||||||||||||
|
Offsetting of Derivative Assets/Liabilities
|
||||||||||||||||||||||||
|
Gross Amounts Not Offset in the Consolidated Balance Sheet
|
||||||||||||||||||||||||
|
Description
|
Gross Amounts of Recognized Assets/Liabilities
|
Gross Amounts Offset in the Consolidated Balance Sheet
|
Amounts of Assets/Liabilities Presented in the Consolidated Balance Sheet
|
Derivative Financial Instruments
|
Cash Collateral Received/Pledged
|
Net Amount (Legal Offset)
|
||||||||||||||||||
|
Derivative assets
|
$
|
349
|
|
$
|
—
|
|
$
|
349
|
|
$
|
—
|
|
$
|
—
|
|
$
|
349
|
|
||||||
|
Derivative liabilities
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||
|
5
.
|
INVENTORIES
|
|
|
December 31,
|
|||||||
|
|
2015
|
2014
|
||||||
|
Raw materials
|
$
|
1,332
|
|
$
|
909
|
|
||
|
Work in process
|
542
|
|
500
|
|
||||
|
Finished goods
|
852
|
|
466
|
|
||||
|
Total
|
$
|
2,726
|
|
$
|
1,875
|
|
||
|
Reported as:
|
||||||||
|
Inventories
|
$
|
1,955
|
|
$
|
1,386
|
|
||
|
Other long-term assets
|
771
|
|
489
|
|
||||
|
Total
|
$
|
2,726
|
|
$
|
1,875
|
|
||
|
6
.
|
PROPERTY, PLANT AND EQUIPMENT
|
|
|
December 31,
|
|||||||
|
|
2015
|
2014
|
||||||
|
Buildings and improvements (including leasehold improvements)
|
$
|
1,320
|
|
$
|
997
|
|
||
|
Laboratory and manufacturing equipment
|
377
|
|
327
|
|
||||
|
Office and computer equipment
|
395
|
|
305
|
|
||||
|
Construction in progress
|
554
|
|
411
|
|
||||
|
Subtotal
|
2,646
|
|
2,040
|
|
||||
|
Less accumulated depreciation and amortization (including $0 for 2015 and $2 for 2014 related to capitalized leased equipment)
|
(763
|
)
|
(620
|
)
|
||||
|
Subtotal
|
1,883
|
|
1,420
|
|
||||
|
Land
|
393
|
|
254
|
|
||||
|
Total
|
$
|
2,276
|
|
$
|
1,674
|
|
||
|
7
.
|
INTANGIBLE ASSETS
|
|
|
December 31,
|
|||||||
|
|
2015
|
2014
|
||||||
|
Finite-lived intangible assets
|
$
|
9,815
|
|
$
|
10,641
|
|
||
|
Indefinite-lived intangible assets
|
432
|
|
432
|
|
||||
|
Total intangible assets
|
$
|
10,247
|
|
$
|
11,073
|
|
||
|
|
December 31, 2015
|
December 31, 2014
|
||||||||||||||
|
|
Gross Carrying
Amount
|
Accumulated
Amortization
|
Gross Carrying
Amount
|
Accumulated
Amortization
|
||||||||||||
|
Intangible asset - sofosbuvir
|
$
|
10,720
|
|
$
|
1,456
|
|
$
|
10,720
|
|
$
|
757
|
|
||||
|
Intangible asset - Ranexa
|
688
|
|
363
|
|
688
|
|
277
|
|
||||||||
|
Other
|
455
|
|
229
|
|
455
|
|
188
|
|
||||||||
|
Total
|
$
|
11,863
|
|
$
|
2,048
|
|
$
|
11,863
|
|
$
|
1,222
|
|
||||
|
Fiscal Year
|
Amount
|
||
|
2016
|
$
|
839
|
|
|
2017
|
844
|
|
|
|
2018
|
849
|
|
|
|
2019
|
741
|
|
|
|
2020
|
713
|
|
|
|
Thereafter
|
5,829
|
|
|
|
Total
|
$
|
9,815
|
|
|
|
Amount
|
|||
|
Indefinite-lived intangible asset - momelotinib
|
$
|
315
|
|
|
|
Indefinite-lived intangible assets - Other
|
117
|
|
||
|
Total
|
$
|
432
|
|
|
|
8
.
|
OTHER FINANCIAL INFORMATION
|
|
December 31,
|
||||||||
|
|
2015
|
2014
|
||||||
|
Prepaid taxes
|
$
|
773
|
|
$
|
391
|
|
||
|
Prepaid expenses
|
240
|
|
194
|
|
||||
|
Other current assets
|
506
|
|
472
|
|
||||
|
Total prepaid and other current assets
|
$
|
1,519
|
|
$
|
1,057
|
|
||
|
December 31,
|
||||||||
|
|
2015
|
2014
|
||||||
|
Income taxes payable
|
$
|
65
|
|
$
|
105
|
|
||
|
Compensation and employee benefits
|
380
|
|
316
|
|
||||
|
Branded Prescription Drug Fee
|
649
|
|
186
|
|
||||
|
Accrued royalties
|
237
|
|
355
|
|
||||
|
Other accrued expenses
|
1,841
|
|
911
|
|
||||
|
Total other accrued liabilities
|
$
|
3,172
|
|
$
|
1,873
|
|
||
|
9
.
|
COLLABORATIVE ARRANGEMENTS
|
|
December 31,
|
||||||||
|
|
2015
|
2014
|
||||||
|
Total assets
|
$
|
2,464
|
|
$
|
2,138
|
|
||
|
Cash and cash equivalents
|
166
|
|
250
|
|
||||
|
Accounts receivable, net
|
269
|
|
297
|
|
||||
|
Inventories
|
2,027
|
|
1,590
|
|
||||
|
Total liabilities
|
1,055
|
|
1,157
|
|
||||
|
Accounts payable
|
606
|
|
749
|
|
||||
|
Other accrued liabilities
|
449
|
|
408
|
|
||||
|
10
.
|
|
|
Stated
|
December 31,
|
|||||||||||||
|
Type of Borrowing
|
Issue Date
|
Due Date
|
Interest Rate
|
2015
|
2014
|
|||||||||
|
Convertible Senior
|
July 2010
|
May 2016
|
1.625%
|
$
|
283
|
|
$
|
483
|
|
|||||
|
Senior Unsecured
|
December 2011
|
December 2016
|
3.05%
|
700
|
|
700
|
|
|||||||
|
Senior Unsecured
|
September 2015
|
September 2018
|
1.85%
|
1,000
|
|
—
|
|
|||||||
|
Senior Unsecured
|
March 2014
|
April 2019
|
2.05%
|
499
|
|
499
|
|
|||||||
|
Senior Unsecured
|
November 2014
|
February 2020
|
2.35%
|
499
|
|
499
|
|
|||||||
|
Senior Unsecured
|
September 2015
|
September 2020
|
2.55%
|
1,997
|
|
—
|
|
|||||||
|
Senior Unsecured
|
March 2011
|
April 2021
|
4.50%
|
995
|
|
995
|
|
|||||||
|
Senior Unsecured
|
December 2011
|
December 2021
|
4.40%
|
1,248
|
|
1,248
|
|
|||||||
|
Senior Unsecured
|
September 2015
|
September 2022
|
3.25%
|
999
|
|
—
|
|
|||||||
|
Senior Unsecured
|
March 2014
|
April 2024
|
3.70%
|
1,748
|
|
1,747
|
|
|||||||
|
Senior Unsecured
|
November 2014
|
February 2025
|
3.50%
|
1,748
|
|
1,748
|
|
|||||||
|
Senior Unsecured
|
September 2015
|
March 2026
|
3.65%
|
2,739
|
|
—
|
|
|||||||
|
Senior Unsecured
|
September 2015
|
September 2035
|
4.60%
|
997
|
|
—
|
|
|||||||
|
Senior Unsecured
|
December 2011
|
December 2041
|
5.65%
|
998
|
|
998
|
|
|||||||
|
Senior Unsecured
|
March 2014
|
April 2044
|
4.80%
|
1,747
|
|
1,747
|
|
|||||||
|
Senior Unsecured
|
November 2014
|
February 2045
|
4.50%
|
1,740
|
|
1,740
|
|
|||||||
|
Senior Unsecured
|
September 2015
|
March 2046
|
4.75%
|
2,241
|
|
—
|
|
|||||||
|
Total debt, net
|
$
|
22,178
|
|
$
|
12,404
|
|
||||||||
|
Less current portion
|
983
|
|
483
|
|
||||||||||
|
Total long-term debt, net
|
$
|
21,195
|
|
$
|
11,921
|
|
||||||||
|
|
Principal repayments
|
Conversion value paid in excess of principal
|
Net proceeds from convertible note hedges
|
|||||||||||||||||||||
|
|
Year Ended December 31,
|
Year Ended December 31,
|
Year Ended December 31,
|
|||||||||||||||||||||
|
|
2015
|
2014
|
2015
|
2014
|
2015
|
2014
|
||||||||||||||||||
|
May Notes
|
$
|
213
|
|
$
|
912
|
|
$
|
784
|
|
$
|
2,517
|
|
$
|
784
|
|
$
|
2,517
|
|
||||||
|
|
Carrying Value of
Equity Component
|
Net Carrying Amount of
Liability Component
|
Unamortized Discount of
Liability Component
|
|||||||||||||||||||||
|
|
December 31,
|
December 31,
|
December 31,
|
|||||||||||||||||||||
|
|
2015
|
2014
|
2015
|
2014
|
2015
|
2014
|
||||||||||||||||||
|
May 2016 Notes
|
$
|
35
|
|
$
|
61
|
|
$
|
283
|
|
$
|
483
|
|
$
|
(2
|
)
|
$
|
(15
|
)
|
||||||
|
Year
|
2016
|
2017
|
2018
|
2019
|
2020
|
|||||||||||||||
|
Contractual Maturities
|
$
|
985
|
|
$
|
—
|
|
$
|
1,000
|
|
$
|
500
|
|
$
|
2,500
|
|
|||||
|
11
.
|
COMMITMENTS AND CONTINGENCIES
|
|
2016
|
$
|
66
|
|
|
2017
|
63
|
|
|
|
2018
|
51
|
|
|
|
2019
|
43
|
|
|
|
2020
|
31
|
|
|
|
Thereafter
|
63
|
|
|
|
Total
|
$
|
317
|
|
|
12
.
|
STOCKHOLDERS' EQUITY
|
|
Year ended December 31,
|
|||||||||||||
|
2015
(1)
|
2014
(2)
|
2013
(3)
|
|||||||||||
|
Shares repurchased and retired
|
95
|
|
59
|
|
10
|
|
|||||||
|
Amount
|
$
|
10,002
|
|
$
|
5,349
|
|
$
|
582
|
|
||||
|
Average price per share
|
$
|
104.91
|
|
$
|
90.29
|
|
$
|
60.78
|
|
||||
|
(1)
|
Includes 65 million shares repurchased for $7.0 billion under the 2015 Program and 30 million shares repurchased for $3.0 billion under the 2014 Program.
|
|
(2)
|
Includes 19 million shares repurchased for $2.0 billion under the 2014 Program and 40 million shares repurchased for $3.3 billion under the 2011 Program.
|
|
(3)
|
All shares repurchased under the 2011 Program.
|
|
Year ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
Reduction of common stock and APIC
|
$
|
223
|
|
$
|
133
|
|
$
|
14
|
|
|||
|
Charge to retained earnings
|
$
|
10,115
|
|
$
|
5,475
|
|
$
|
674
|
|
|||
|
|
Dividend Per Share
|
Amount
|
||||||
|
2015:
|
||||||||
|
Second quarter
|
$
|
0.43
|
|
$
|
639
|
|
||
|
Third quarter
|
0.43
|
|
631
|
|
||||
|
Fourth quarter
|
0.43
|
|
620
|
|
||||
|
Total
|
$
|
1.29
|
|
$
|
1,890
|
|
||
|
Foreign Currency Items
|
Unrealized Gains and Losses on Available-for-Sale Securities
|
Unrealized Gains and Losses on Cash Flow Hedges
|
Total
|
|||||||||||||
|
Balance at December 31, 2013
|
$
|
(45
|
)
|
$
|
12
|
|
$
|
(91
|
)
|
$
|
(124
|
)
|
||||
|
Other comprehensive income (loss) before reclassifications
|
(9
|
)
|
—
|
|
430
|
|
421
|
|
||||||||
|
Amounts reclassified from accumulated other comprehensive income
|
—
|
|
—
|
|
4
|
|
4
|
|
||||||||
|
Net current period other comprehensive income (loss)
|
(9
|
)
|
—
|
|
434
|
|
425
|
|
||||||||
|
Balance at December 31, 2014
|
(54
|
)
|
12
|
|
343
|
|
301
|
|
||||||||
|
Other comprehensive income (loss) before reclassifications
|
9
|
|
(29
|
)
|
389
|
|
369
|
|
||||||||
|
Amounts reclassified from accumulated other comprehensive income
|
—
|
|
1
|
|
(583
|
)
|
(582
|
)
|
||||||||
|
Net current period other comprehensive income (loss)
|
9
|
|
(28
|
)
|
(194
|
)
|
(213
|
)
|
||||||||
|
Balance at December 31, 2015
|
$
|
(45
|
)
|
$
|
(16
|
)
|
$
|
149
|
|
$
|
88
|
|
||||
|
13
.
|
EMPLOYEE BENEFITS
|
|
Shares
(in thousands)
|
Weighted-
Average
Exercise Price
(in dollars)
|
Weighted-Average Remaining Contractual Term (Years)
|
Aggregate Intrinsic Value (in millions)
|
||||||||||
|
Outstanding at December 31, 2014
|
39,144
|
|
$
|
22.63
|
|
||||||||
|
Granted
|
1,356
|
|
$
|
102.97
|
|
||||||||
|
Forfeited
|
(110
|
)
|
$
|
69.11
|
|
||||||||
|
Expired
|
(3
|
)
|
$
|
21.06
|
|
||||||||
|
Exercised
|
(12,974
|
)
|
$
|
18.11
|
|
||||||||
|
Outstanding at December 31, 2015
|
27,413
|
|
$
|
28.56
|
|
3.6
|
$
|
1,995
|
|
||||
|
Exercisable at December 31, 2015
|
24,731
|
|
$
|
23.11
|
|
3.1
|
$
|
1,931
|
|
||||
|
Expected to vest, net of estimated forfeitures at December 31, 2015
|
2,576
|
|
$
|
78.10
|
|
8.2
|
$
|
63
|
|
||||
|
Shares
(1)
(in thousands)
|
Weighted-
Average Grant-Date Fair Value Per Share (1)
(in dollars)
|
||||||
|
Outstanding at December 31, 2014
|
827
|
|
$
|
51.52
|
|
||
|
Granted
|
1,219
|
|
$
|
61.71
|
|
||
|
Vested
|
(1,554
|
)
|
$
|
48.60
|
|
||
|
Forfeited
|
(5
|
)
|
$
|
98.32
|
|
||
|
Outstanding at December 31, 2015
|
487
|
|
$
|
85.83
|
|
||
|
|
Shares
(in thousands) |
Weighted-
Average Grant-Date Fair Value Per Share (in dollars) |
|||||
|
Outstanding at December 31, 2014
|
14,483
|
|
$
|
49.37
|
|
||
|
Granted
|
4,065
|
|
$
|
103.19
|
|
||
|
Vested
|
(6,397
|
)
|
$
|
38.86
|
|
||
|
Forfeited
|
(1,123
|
)
|
$
|
62.96
|
|
||
|
Outstanding at December 31, 2015
|
11,028
|
|
$
|
73.93
|
|
||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Cost of goods sold
|
$
|
11
|
|
$
|
10
|
|
$
|
7
|
|
|||
|
Research and development expenses
|
173
|
|
152
|
|
109
|
|
||||||
|
Selling, general and administrative expenses
|
198
|
|
198
|
|
136
|
|
||||||
|
Stock-based compensation expense included in total costs and expenses
|
382
|
|
360
|
|
252
|
|
||||||
|
Income tax effect
|
(131
|
)
|
(64
|
)
|
(67
|
)
|
||||||
|
Stock-based compensation expense, net of tax
|
$
|
251
|
|
$
|
296
|
|
$
|
185
|
|
|||
|
|
Year Ended December 31,
|
||||||||
|
|
2015
|
2014
|
2013
|
||||||
|
Expected volatility:
|
|
|
|
||||||
|
Stock options
|
35
|
%
|
34
|
%
|
29
|
%
|
|||
|
ESPP
|
32
|
%
|
32
|
%
|
31
|
%
|
|||
|
Expected term in years:
|
|
|
|
|
|
|
|||
|
Stock options
|
5.7
|
|
5.5
|
|
5.7
|
|
|||
|
ESPP
|
1.2
|
|
1.2
|
|
1.2
|
|
|||
|
Risk-free interest rate:
|
|
|
|
|
|
|
|||
|
Stock options
|
1.4
|
%
|
1.8
|
%
|
1.1
|
%
|
|||
|
ESPP
|
1.4
|
%
|
1.5
|
%
|
1.1
|
%
|
|||
|
Expected dividend yield
|
1.7
|
%
|
—
|
%
|
—
|
%
|
|||
|
14
.
|
NET INCOME PER SHARE ATTRIBUTABLE TO GILEAD COMMON STOCKHOLDERS
|
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Net income attributable to Gilead
|
$
|
18,108
|
|
$
|
12,101
|
|
$
|
3,075
|
|
|||
|
Shares used in per share calculation — basic
|
1,464
|
|
1,522
|
|
1,529
|
|
||||||
|
Effect of dilutive securities:
|
|
|
|
|
|
|
||||||
|
Stock options and equivalents
|
23
|
|
33
|
|
40
|
|
||||||
|
Conversion spread related to the Convertible Notes
|
14
|
|
30
|
|
63
|
|
||||||
|
Warrants related to the Convertible Notes
|
20
|
|
62
|
|
63
|
|
||||||
|
Shares used in per share calculation — diluted
|
1,521
|
|
1,647
|
|
1,695
|
|
||||||
|
Net income per share attributable to Gilead common stockholders — basic
|
$
|
12.37
|
|
$
|
7.95
|
|
$
|
2.01
|
|
|||
|
Net income per share attributable to Gilead common stockholders — diluted
|
$
|
11.91
|
|
$
|
7.35
|
|
$
|
1.81
|
|
|||
|
15
.
|
SEGMENT INFORMATION
|
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Antiviral products:
|
|
|
|
|||||||||
|
Harvoni
|
$
|
13,864
|
|
$
|
2,127
|
|
$
|
—
|
|
|||
|
Sovaldi
|
5,276
|
|
10,283
|
|
139
|
|
||||||
|
Truvada
|
3,459
|
|
3,340
|
|
3,136
|
|
||||||
|
Atripla
|
3,134
|
|
3,470
|
|
3,648
|
|
||||||
|
Stribild
|
1,825
|
|
1,197
|
|
539
|
|
||||||
|
Complera/Eviplera
|
1,427
|
|
1,228
|
|
810
|
|
||||||
|
Viread
|
1,108
|
|
1,058
|
|
959
|
|
||||||
|
Genvoya
|
45
|
|
—
|
|
—
|
|
||||||
|
Other antiviral
|
69
|
|
88
|
|
111
|
|
||||||
|
Total antiviral products
|
30,207
|
|
22,791
|
|
9,342
|
|
||||||
|
Other products:
|
||||||||||||
|
Letairis
|
700
|
|
595
|
|
520
|
|
||||||
|
Ranexa
|
588
|
|
510
|
|
449
|
|
||||||
|
AmBisome
|
350
|
|
388
|
|
352
|
|
||||||
|
Zydelig
|
132
|
|
23
|
|
—
|
|
||||||
|
Other
|
174
|
|
167
|
|
141
|
|
||||||
|
Total product sales
|
$
|
32,151
|
|
$
|
24,474
|
|
$
|
10,804
|
|
|||
|
Year Ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
Revenues:
|
||||||||||||
|
United States
|
$
|
21,234
|
|
$
|
18,182
|
|
$
|
6,695
|
|
|||
|
Europe
|
7,528
|
|
5,442
|
|
3,614
|
|
||||||
|
Other countries
|
3,877
|
|
1,266
|
|
893
|
|
||||||
|
Total revenues
|
$
|
32,639
|
|
$
|
24,890
|
|
$
|
11,202
|
|
|||
|
|
Year Ended December 31,
|
||||||||
|
|
2015
|
2014
|
2013
|
||||||
|
McKesson Corp.
|
24
|
%
|
24
|
%
|
16
|
%
|
|||
|
AmerisourceBergen Corp.
|
19
|
%
|
25
|
%
|
13
|
%
|
|||
|
Cardinal Health, Inc.
|
15
|
%
|
14
|
%
|
17
|
%
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Domestic
|
$
|
7,953
|
|
$
|
6,678
|
|
$
|
3,470
|
|
|||
|
Foreign
|
13,706
|
|
8,178
|
|
738
|
|
||||||
|
Total income before provision for income taxes
|
$
|
21,659
|
|
$
|
14,856
|
|
$
|
4,208
|
|
|||
|
|
Year Ended December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Federal:
|
|
|
|
|||||||||
|
Current
|
$
|
3,568
|
|
$
|
2,810
|
|
$
|
1,156
|
|
|||
|
Deferred
|
(313
|
)
|
(190
|
)
|
(71
|
)
|
||||||
|
|
3,255
|
|
2,620
|
|
1,085
|
|
||||||
|
State:
|
|
|
|
|
|
|
||||||
|
Current
|
158
|
|
152
|
|
62
|
|
||||||
|
Deferred
|
(21
|
)
|
(30
|
)
|
(22
|
)
|
||||||
|
|
137
|
|
122
|
|
40
|
|
||||||
|
Foreign:
|
|
|
|
|
|
|
||||||
|
Current
|
212
|
|
85
|
|
46
|
|
||||||
|
Deferred
|
(51
|
)
|
(30
|
)
|
(20
|
)
|
||||||
|
|
161
|
|
55
|
|
26
|
|
||||||
|
Provision for income taxes
|
$
|
3,553
|
|
$
|
2,797
|
|
$
|
1,151
|
|
|||
|
|
Year Ended December 31,
|
||||||||
|
|
2015
|
2014
|
2013
|
||||||
|
Federal statutory rate
|
35.0
|
%
|
35.0
|
%
|
35.0
|
%
|
|||
|
State taxes, net of federal benefit
|
0.5
|
%
|
0.6
|
%
|
0.5
|
%
|
|||
|
Foreign earnings at different rates
|
(18.5
|
)%
|
(16.9
|
)%
|
(6.6
|
)%
|
|||
|
Research and other credits
|
(0.7
|
)%
|
(0.9
|
)%
|
(3.0
|
)%
|
|||
|
Net unbenefitted stock compensation
|
0.1
|
%
|
0.2
|
%
|
0.6
|
%
|
|||
|
Other
|
—
|
%
|
0.8
|
%
|
0.8
|
%
|
|||
|
Effective tax rate
|
16.4
|
%
|
18.8
|
%
|
27.3
|
%
|
|||
|
|
December 31,
|
|||||||
|
|
2015
|
2014
|
||||||
|
Deferred tax assets:
|
|
|
||||||
|
Net operating loss carryforwards
|
$
|
199
|
|
$
|
215
|
|
||
|
Stock-based compensation
|
222
|
|
157
|
|
||||
|
Reserves and accruals not currently deductible
|
676
|
|
383
|
|
||||
|
Deferred revenue
|
55
|
|
46
|
|
||||
|
Depreciation related
|
63
|
|
55
|
|
||||
|
Research and other credit carryforwards
|
135
|
|
91
|
|
||||
|
Other, net
|
118
|
|
125
|
|
||||
|
Total deferred tax assets before valuation allowance
|
1,468
|
|
1,072
|
|
||||
|
Valuation allowance
|
(6
|
)
|
(9
|
)
|
||||
|
Total deferred tax assets
|
1,462
|
|
1,063
|
|
||||
|
Deferred tax liabilities:
|
|
|
|
|
||||
|
Intangibles
|
(280
|
)
|
(328
|
)
|
||||
|
Unremitted foreign earnings
|
—
|
|
(16
|
)
|
||||
|
Other
|
(50
|
)
|
(34
|
)
|
||||
|
Total deferred tax liabilities
|
(330
|
)
|
(378
|
)
|
||||
|
Net deferred tax assets
|
$
|
1,132
|
|
$
|
685
|
|
||
|
|
December 31,
|
|||||||||||
|
|
2015
|
2014
|
2013
|
|||||||||
|
Balance, beginning of period
|
$
|
661
|
|
$
|
237
|
|
$
|
157
|
|
|||
|
Tax positions related to current year:
|
|
|
|
|
|
|
||||||
|
Additions
|
675
|
|
430
|
|
112
|
|
||||||
|
Reductions
|
—
|
|
—
|
|
—
|
|
||||||
|
Tax positions related to prior years:
|
|
|
|
|
||||||||
|
Additions
|
45
|
|
21
|
|
13
|
|
||||||
|
Reductions
|
—
|
|
(20
|
)
|
—
|
|
||||||
|
Settlements
|
(24
|
)
|
(5
|
)
|
(39
|
)
|
||||||
|
Lapse of statute of limitations
|
(7
|
)
|
(2
|
)
|
(6
|
)
|
||||||
|
Balance, end of period
|
$
|
1,350
|
|
$
|
661
|
|
$
|
237
|
|
|||
|
17
.
|
SUBSEQUENT EVENT
|
|
|
1st Quarter
|
2nd Quarter
|
3rd Quarter
|
4th Quarter
|
||||||||||||
|
2015
|
|
|
|
|||||||||||||
|
Total revenues
|
$
|
7,594
|
|
$
|
8,244
|
|
$
|
8,295
|
|
$
|
8,506
|
|
||||
|
Gross profit on product sales
|
$
|
6,523
|
|
$
|
7,128
|
|
$
|
7,147
|
|
$
|
7,347
|
|
||||
|
Net income
|
$
|
4,332
|
|
$
|
4,497
|
|
$
|
4,592
|
|
$
|
4,685
|
|
||||
|
Net income attributable to Gilead
|
$
|
4,333
|
|
$
|
4,492
|
|
$
|
4,600
|
|
$
|
4,683
|
|
||||
|
Net income per share attributable to Gilead common stockholders-basic
|
$
|
2.91
|
|
$
|
3.05
|
|
$
|
3.14
|
|
$
|
3.26
|
|
||||
|
Net income per share attributable to Gilead common stockholders-diluted
|
$
|
2.76
|
|
$
|
2.92
|
|
$
|
3.06
|
|
$
|
3.18
|
|
||||
|
2014
|
|
|
|
|
|
|
||||||||||
|
Total revenues
|
$
|
4,999
|
|
$
|
6,535
|
|
$
|
6,042
|
|
$
|
7,314
|
|
||||
|
Gross profit on product sales
|
$
|
4,058
|
|
$
|
5,488
|
|
$
|
4,981
|
|
$
|
6,159
|
|
||||
|
Net income
|
$
|
2,223
|
|
$
|
3,650
|
|
$
|
2,724
|
|
$
|
3,462
|
|
||||
|
Net income attributable to Gilead
|
$
|
2,227
|
|
$
|
3,656
|
|
$
|
2,731
|
|
$
|
3,487
|
|
||||
|
Net income per share attributable to Gilead common stockholders-basic
|
$
|
1.45
|
|
$
|
2.39
|
|
$
|
1.80
|
|
$
|
2.32
|
|
||||
|
Net income per share attributable to Gilead common stockholders-diluted
|
$
|
1.33
|
|
$
|
2.20
|
|
$
|
1.67
|
|
$
|
2.18
|
|
||||
|
Balance at Beginning of Period
|
Additions/Charged to Expense
|
Deductions
|
Balance at End of Period
|
|||||||||||||
|
Year ended December 31, 2015:
|
||||||||||||||||
|
Accounts receivable allowances
(1)
|
$
|
356
|
|
$
|
6,934
|
|
$
|
6,258
|
|
$
|
1,032
|
|
||||
|
Sales return allowance
|
$
|
171
|
|
$
|
219
|
|
$
|
19
|
|
$
|
371
|
|
||||
|
Valuation allowances for deferred tax assets
(2)
|
$
|
9
|
|
$
|
—
|
|
$
|
3
|
|
$
|
6
|
|
||||
|
Year ended December 31, 2014:
|
||||||||||||||||
|
Accounts receivable allowances
(1)
|
$
|
252
|
|
$
|
2,867
|
|
$
|
2,763
|
|
$
|
356
|
|
||||
|
Sales return allowance
|
$
|
82
|
|
$
|
104
|
|
$
|
15
|
|
$
|
171
|
|
||||
|
Valuation allowances for deferred tax assets
(2)
|
$
|
9
|
|
$
|
—
|
|
$
|
—
|
|
$
|
9
|
|
||||
|
Year ended December 31, 2013:
|
||||||||||||||||
|
Accounts receivable allowances
(1)
|
$
|
188
|
|
$
|
1,870
|
|
$
|
1,806
|
|
$
|
252
|
|
||||
|
Sales return allowance
|
$
|
73
|
|
$
|
21
|
|
$
|
12
|
|
$
|
82
|
|
||||
|
Valuation allowances for deferred tax assets
(2)
|
$
|
9
|
|
$
|
—
|
|
$
|
—
|
|
$
|
9
|
|
||||
|
(1)
|
Allowances are for doubtful accounts, cash discounts and chargebacks.
|
|
(2)
|
Valuation allowance for deferred tax assets includes $4
million and $6 million as of December 31, 2015 and 2014, respectively, related to our acquisitions.
|
|
ITEM 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
|
ITEM 9A.
|
CONTROLS AND PROCEDURES
|
|
ITEM 9B.
|
OTHER INFORMATION
|
|
ITEM 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
|
ITEM 11.
|
EXECUTIVE COMPENSATION
|
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
|
ITEM 14.
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
|
Audited Consolidated Financial Statements
|
|
|
ITEM 15.
|
EXHIBITS
|
|
Exhibit
Footnote
|
Exhibit Number
|
Description of Document
|
||
|
(1)
|
1.1
|
Underwriting Agreement, dated September 9, 2015, among Registrant and Merrill Lynch, Pierce, Fenner & Smith Incorporated and J.P. Morgan Securities LLC, as representatives of the several underwriters listed in Schedule 1 thereto
|
||
|
†(2)
|
2.1
|
Agreement and Plan of Merger among Registrant, Merger Sub and Pharmasset, Inc., dated as of November 21, 2011
|
||
|
(3)
|
3.1
|
Restated Certificate of Incorporation of Registrant
|
||
|
(4)
|
3.2
|
Amended and Restated Bylaws of Registrant
|
||
|
4.1
|
Reference is made to Exhibit 3.1 and Exhibit 3.2
|
|||
|
(5)
|
4.2
|
Indenture related to the Convertible Senior Notes due 2016 (2016 Notes), between Registrant and Wells Fargo Bank, National Association, as trustee (including form of 1.625% Convertible Senior Note due 2016), dated July 30, 2010
|
||
|
(6)
|
4.3
|
Indenture related to Senior Notes, dated as of March 30, 2011, between Registrant and Wells Fargo, National Association, as Trustee
|
||
|
(6)
|
4.4
|
First Supplemental Indenture related to Senior Notes, dated as of March 30, 2011, between Registrant and Wells Fargo, National Association, as Trustee (including form of Senior Notes)
|
||
|
(7)
|
4.5
|
Second Supplemental Indenture related to Senior Notes, dated as of December 13, 2011, between Registrant and Wells Fargo, National Association, as Trustee (including Form of 2014 Note, Form of 2016 Note, Form of 2021 Note, Form of 2041 Note)
|
||
|
(8)
|
4.6
|
Third Supplemental Indenture related to Senior Notes, dated as of March 7, 2014, between Registrant and Wells Fargo, National Association, as Trustee (including Form of 2019 Note, Form of 2024 Note, Form of 2044 Note)
|
||
|
(9)
|
4.7
|
Fourth Supplemental Indenture related to Senior Notes, dated as of November 17, 2014, between Registrant and Wells Fargo, National Association, as Trustee (including Form of 2020 Note, Form of 2025 Note, Form of 2045 Note)
|
||
|
(1)
|
4.8
|
Fifth Supplemental Indenture, dated as of September 14, 2015, between Registrant and Wells Fargo Bank, National Association, as Trustee (including Form of 2018 Note, Form of 2020 Note, Form of 2022 Note, Form of 2026 Note, Form of 2035 Note and Form of 2046 Note)
|
||
|
(10)
|
10.1
|
Confirmation of OTC Convertible Note Hedge related to 2016 Notes, dated July 26, 2010, between Registrant and Goldman, Sachs & Co.
|
||
|
(10)
|
10.2
|
Confirmation of OTC Convertible Note Hedge related to 2016 Notes, dated July 26, 2010, between Registrant and JPMorgan Chase Bank, National Association
|
||
|
(10)
|
10.3
|
Confirmation of OTC Warrant Transaction, dated July 26, 2010, between Registrant and Goldman, Sachs & Co. for warrants expiring in 2016
|
||
|
(10)
|
10.4
|
Confirmation of OTC Warrant Transaction, dated July 26, 2010, between Registrant and JPMorgan Chase Bank, National Association for warrants expiring in 2016
|
||
|
(11)
|
10.5
|
Confirmation of OTC Additional Convertible Note Hedge related to 2016 Notes, dated August 5, 2010, between Registrant and Goldman, Sachs & Co.
|
||
|
(11)
|
10.6
|
Confirmation of OTC Additional Convertible Note Hedge related to 2016 Notes, dated August 5, 2010, between Registrant and JPMorgan Chase Bank, National Association
|
||
|
(11)
|
10.7
|
Confirmation of OTC Additional Warrant Transaction, dated August 5, 2010, between Registrant and Goldman, Sachs & Co. for warrants expiring in 2016
|
||
|
(11)
|
10.8
|
Confirmation of OTC Additional Warrant Transaction, dated August 5, 2010, between Registrant and JPMorgan Chase Bank, National Association for warrants expiring in 2016
|
||
|
(11)
|
10.9
|
Amendment to Confirmation of OTC Convertible Note Hedge related to 2016 Notes, dated August 30, 2010, between Registrant and Goldman, Sachs & Co.
|
||
|
(11)
|
10.10
|
Amendment to Confirmation of OTC Convertible Note Hedge related to 2016 Notes, dated August 30, 2010, between Registrant and JPMorgan Chase Bank, National Association
|
||
|
(11)
|
10.11
|
Amendment to Confirmation of OTC Additional Convertible Note Hedge related to 2016 Notes, dated August 30, 2010, between Registrant and Goldman, Sachs & Co.
|
||
|
(11)
|
10.12
|
Amendment to Confirmation of OTC Additional Convertible Note Hedge related to 2016 Notes, dated August 30, 2010, between Registrant and JPMorgan Chase Bank, National Association
|
||
|
(12)
|
10.13
|
Amendment to Base Warrants (2016), dated May 8, 2015, between Registrant and Goldman, Sachs & Co.
|
||
|
(12)
|
10.14
|
Amendment to Base Warrants (2016), dated May 8, 2015, between Registrant and JPMorgan Chase Bank, National Association
|
||
|
(13)
|
10.15
|
5-Year Revolving Credit Facility Credit Agreement among Registrant and Gilead Biopharmaceutics Ireland UC (formerly Gilead Biopharmaceutics Ireland Corporation), as Borrowers, Bank of America, N.A., as Administrative Agent, Swing Line Lender and L/C Issuer, certain other lenders parties thereto, Barclays Capital, as Syndication Agent, and Goldman Sachs Bank USA, JPMorgan Chase Bank, N.A., Royal Bank of Canada and Wells Fargo Bank, N.A., as Co-Documentation Agents, dated as of January 12, 2012
|
||
|
(13)
|
10.16
|
Parent Guaranty Agreement (5-Year Revolving Credit Facility), dated as of January 12, 2012, by Registrant
|
||
|
*(3)
|
10.17
|
Gilead Sciences, Inc. 2004 Equity Incentive Plan, as amended through May 8, 2013
|
||
|
*(14)
|
10.18
|
Form of employee stock option agreement used under 2004 Equity Incentive Plan (for grants prior to February 2008)
|
||
|
*(15)
|
10.19
|
Form of employee stock option agreement used under 2004 Equity Incentive Plan (for grants made February 2008 through April 2009)
|
||
|
*(16)
|
10.20
|
Form of employee stock option agreement used under 2004 Equity Incentive Plan (for grants commencing in May 2009)
|
||
|
*(17)
|
10.21
|
Form of employee stock option agreement used under 2004 Equity Incentive Plan (for grants commencing in February 2010)
|
||
|
*(18)
|
10.22
|
Form of employee stock option agreement used under 2004 Equity Incentive Plan (for 2011 and subsequent year grants)
|
||
|
*(15)
|
10.23
|
Form of non-employee director stock option agreement used under 2004 Equity Incentive Plan (for grants prior to 2008)
|
||
|
*(15)
|
10.24
|
Form of non-employee director option agreement used under 2004 Equity Incentive Plan (for initial grants made in 2008)
|
||
|
*(15)
|
10.25
|
Form of non-employee director option agreement used under 2004 Equity Incentive Plan (for annual grants made in May 2008 and through May 2012)
|
||
|
*(16)
|
10.26
|
Form of non-employee director option agreement used under 2004 Equity Incentive Plan (for annual grants commencing in May 2009 and through May 2012)
|
||
|
*(19)
|
10.27
|
Form of non-employee director option agreement used under 2004 Equity Incentive Plan (for annual grants made in May 2013)
|
||
|
*(19)
|
10.28
|
Form of non-employee director option agreement (non-U.S.) used under 2004 Equity Incentive Plan (for annual grants made in May 2013)
|
||
|
*(20)
|
10.29
|
Form of non-employee director option agreement used under 2004 Equity Incentive Plan (for annual grants made in and after May 2014)
|
||
|
*(21)
|
10.30
|
Form of restricted stock unit issuance agreement used under 2004 Equity Incentive Plan (for annual grants to non-employee directors in May 2012)
|
||
|
*(16)
|
10.31
|
Form of restricted stock award agreement used under 2004 Equity Incentive Plan (for annual grants to certain non-employee directors prior to May 2012)
|
||
|
*(19)
|
10.32
|
Form of restricted stock unit issuance agreement used under 2004 Equity Incentive Plan (for annual grants to non-employee directors commencing in May 2013)
|
||
|
*(20)
|
10.33
|
Form of restricted stock unit issuance agreement used under 2004 Equity Incentive Plan (for annual grants to non-employee directors commencing in and after May 2014)
|
||
|
*(19)
|
10.34
|
Form of restricted stock unit issuance agreement (non-U.S.) used under 2004 Equity Incentive Plan (for annual grants to non-employee directors commencing in May 2013)
|
||
|
*(16)
|
10.35
|
Form of performance share award agreement used under the 2004 Equity Incentive Plan (for grants to certain executive officers made in 2009)
|
||
|
*(17)
|
10.36
|
Form of performance share award agreement used under the 2004 Equity Incentive Plan (for grants to certain executive officers made in 2010)
|
||
|
*(18)
|
10.37
|
Form of performance share award agreement used under the 2004 Equity Incentive Plan (for grants to certain executive officers made in 2011)
|
||
|
*(19)
|
10.38
|
Form of performance share award agreement used under the 2004 Equity Incentive Plan (for grants to certain executive officers made in 2012)
|
||
|
*(22)
|
10.39
|
Form of performance share award agreement used under the 2004 Equity Incentive Plan (for TSR Goals in 2013 and 2014)
|
||
|
*(23)
|
10.40
|
Form of performance share award agreement used under the 2004 Equity Incentive Plan (for Revenue Goals in 2013 and 2014)
|
||
|
*(24)
|
10.41
|
Form of performance share award agreement used under the 2004 Equity Incentive Plan (for TSR Goals - Non-US in 2015)
|
||
|
*(24)
|
10.42
|
Form of performance share award agreement used under the 2004 Equity Incentive Plan (for Revenue Goals - Non-US in 2015)
|
||
|
*(25)
|
10.43
|
Form of restricted stock unit issuance agreement used under the 2004 Equity Incentive Plan (for grants to certain executive officers made prior to May 2009)
|
||
|
*(16)
|
10.44
|
Form of restricted stock unit issuance agreement used under the 2004 Equity Incentive Plan (for grants to certain executive officers commencing in May 2009)
|
||
|
*(26)
|
10.45
|
Form of restricted stock unit issuance agreement used under the 2004 Equity Incentive Plan (service-based vesting for certain executive officers commencing in November 2009)
|
||
|
*(18)
|
10.46
|
Form of restricted stock unit issuance agreement used under the 2004 Equity Incentive Plan (service-based vesting for certain executive officers commencing in 2011)
|
||
|
*(27)
|
10.47
|
Gilead Sciences, Inc. Employee Stock Purchase Plan, restated on January 22, 2015
|
||
|
*(28)
|
10.48
|
Gilead Sciences, Inc. Deferred Compensation Plan-Basic Plan Document
|
||
|
*(26)
|
10.49
|
Gilead Sciences, Inc. Deferred Compensation Plan-Adoption Agreement
|
||
|
*(28)
|
10.50
|
Addendum to the Gilead Sciences, Inc. Deferred Compensation Plan
|
||
|
*(29)
|
10.51
|
Gilead Sciences, Inc. 2005 Deferred Compensation Plan, as amended and restated on October 23, 2008
|
||
|
*(22)
|
10.52
|
Gilead Sciences, Inc. Severance Plan, as amended on January 26, 2012
|
||
|
*
|
10.53
|
Gilead Sciences, Inc. Corporate Bonus Plan, amended on November 4, 2015
|
||
|
*(30)
|
10.54
|
Amended and Restated Gilead Sciences, Inc. Code Section 162(m) Bonus Plan
|
||
|
*(31)
|
10.55
|
2016 Base Salaries for the Named Executive Officers
|
||
|
*(32)
|
10.56
|
Offer Letter dated April 16, 2008 between Registrant and Robin Washington
|
||
|
*(33)
|
10.57
|
Form of Indemnity Agreement entered into between Registrant and its directors and executive officers
|
||
|
*(34)
|
10.58
|
Form of Employee Proprietary Information and Invention Agreement entered into between Registrant and certain of its officers and key employees
|
||
|
*(17)
|
10.59
|
Form of Employee Proprietary Information and Invention Agreement entered into between Registrant and certain of its officers and key employees (revised in September 2006)
|
||
|
+ (35)
|
10.60
|
Amended and Restated Collaboration Agreement by and among Registrant, Gilead Holdings, LLC, Bristol-Myers Squibb Company, E.R. Squibb & Sons, L.L.C., and Bristol-Myers Squibb & Gilead Sciences, LLC, dated September 28, 2006
|
||
|
+ (15)
|
10.61
|
Commercialization Agreement by and between Gilead Sciences Ireland UC (formerly Gilead Sciences Limited) and Bristol-Myers Squibb Company, dated December 10, 2007
|
||
|
+ (36)
|
10.62
|
Amendment Agreement, dated October 25, 1993, between Registrant, the Institute of Organic Chemistry and Biochemistry (IOCB) and Rega Stichting v.z.w. (REGA), together with the following exhibits: the License Agreement, dated December 15, 1991, between Registrant, IOCB and REGA (the 1991 License Agreement), the License Agreement, dated October 15, 1992, between Registrant, IOCB and REGA (the October 1992 License Agreement) and the License Agreement, dated December 1, 1992, between Registrant, IOCB and REGA (the December 1992 License Agreement)
|
||
|
+ (37)
|
10.63
|
Amendment Agreement between Registrant and IOCB/REGA, dated December 27, 2000 amending the 1991 License Agreement and the December 1992 License Agreement
|
||
|
+ (35)
|
10.64
|
Sixth Amendment Agreement to the License Agreement, between IOCB/REGA and Registrant, dated August 18, 2006 amending the October 1992 License Agreement and the December 1992 License Agreement
|
||
|
+ (38)
|
10.65
|
Seventh Amendment Agreement to the License Agreement, between IOCB/REGA and Registrant dated July 1, 2013 amending the October 1992 License Agreement and the December 1992 License Agreement
|
||
|
+ (39)
|
10.66
|
Exclusive License Agreement between Registrant (as successor to Triangle Pharmaceuticals, Inc.), Glaxo Group Limited, The Wellcome Foundation Limited, Glaxo Wellcome Inc. and Emory University, dated May 6, 1999
|
||
|
+ (40)
|
10.67
|
Royalty Sale Agreement by and among Registrant, Emory University and Investors Trust & Custodial Services (Ireland) Limited, solely in its capacity as Trustee of Royalty Pharma, dated July 18, 2005
|
||
|
+ (40)
|
10.68
|
Amended and Restated License Agreement between Registrant, Emory University and Investors Trust & Custodial Services (Ireland) Limited, solely in its capacity as Trustee of Royalty Pharma, dated July 21, 2005
|
||
|
+ (41)
|
10.69
|
License Agreement between Japan Tobacco Inc. and Registrant, dated March 22, 2005
|
||
|
+ (42)
|
10.70
|
First Amendment to License Agreement between Japan Tobacco Inc. and Registrant, dated May 19, 2005
|
||
|
+ (42)
|
10.71
|
Second Amendment to License Agreement between Japan Tobacco Inc. and Registrant, dated May 17, 2010
|
||
|
+(12)
|
10.72
|
Third Amendment (Revised) to License Agreement between Japan Tobacco Inc. and Registrant, dated June 10, 2015
|
||
|
+ (42)
|
10.73
|
Fourth Amendment to License Agreement between Japan Tobacco Inc. and Registrant, dated July 5, 2011
|
||
|
+(43)
|
10.74
|
Amendment to License Agreement between Japan Tobacco Inc. and Registrant, dated October 10, 2013
|
||
|
+(44)
|
10.75
|
Fifth Amendment to License Agreement between Japan Tobacco Inc. and Registrant, dated September 29, 2014
|
||
|
+(45)
|
10.76
|
Amended and Restated Collaboration Agreement by and among Registrant, Gilead Sciences Ireland UC (formerly Gilead Sciences Limited) and Janssen R&D Ireland, dated December 23, 2014
|
||
|
+(46)
|
10.77
|
Master Clinical and Commercial Supply Agreement between Gilead World Markets, Limited, Registrant and Patheon Inc., dated January 1, 2003
|
||
|
+(47)
|
10.78
|
Restated and Amended Toll Manufacturing Agreement between Gilead Sciences Ireland UC (formerly Gilead Sciences Limited), Registrant and Takeda GmbH (formerly Nycomed GmbH and Altana Pharma Oranienburg GmbH), dated November 7, 2005
|
||
|
21.1
|
Subsidiaries of Registrant
|
|||
|
23.1
|
Consent of Independent Registered Public Accounting Firm
|
|||
|
31.1
|
Certification of Chief Executive Officer, as required by Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as amended
|
|||
|
31.2
|
Certification of Chief Financial Officer, as required by Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as amended
|
|||
|
32.1**
|
Certifications of Chief Executive Officer and Chief Financial Officer, as required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. §1350)
|
|||
|
101***
|
The following materials from Registrant's Annual Report on Form 10-K for the year ended December 31, 2015, formatted in Extensible Business Reporting Language (XBRL) includes: (i) Consolidated Balance Sheets at December 31, 2015 and 2014, (ii) Consolidated Statements of Income for the years ended December 31, 2015, 2014 and 2013, (iii) Consolidated Statements of Comprehensive Income for the years ended December 31, 2015, 2014 and 2013, (iv) Consolidated Statements of Stockholders' Equity for the years ended December 31, 2015, 2014 and 2013 (v) Consolidated Statements of Cash Flows for years ended December 31, 2015, 2014 and 2013, and (vi) Notes to Consolidated Financial Statements.
|
|||
|
(1)
|
Filed as an exhibit to Registrant's Current Report on Form 8-K filed on September 14, 2015, and incorporated herein by reference.
|
|
(2)
|
Filed as an exhibit to Registrant's Current Report on Form 8-K filed on November 25, 2011, and incorporated herein by reference.
|
|
(3)
|
Filed as an exhibit to Registrant's Current Report on Form 8-K filed on May 8, 2014, and incorporated herein by reference.
|
|
(4)
|
Filed as an exhibit to Registrant's Current Report on Form 8-K filed on December 23, 2015, and incorporated herein by reference.
|
|
(5)
|
Filed as an exhibit to Registrant's Current Report on Form 8-K filed on August 2, 2010, and incorporated herein by reference.
|
|
(6)
|
Filed as an exhibit to Registrant's Current Report on Form 8-K filed on April 1, 2011, and incorporated herein by reference.
|
|
(7)
|
Filed as an exhibit to Registrant's Current Report on Form 8-K filed on December 13, 2011, and incorporated herein by reference.
|
|
(8)
|
Filed as an exhibit to Registrant's Current Report on Form 8-K filed on March 7, 2014, and incorporated herein by reference.
|
|
(9)
|
Filed as an exhibit to Registrant's Current Report on Form 8-K filed on November 17, 2014, and incorporated herein by reference.
|
|
(10)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended June 30, 2010, and incorporated herein by reference.
|
|
(11)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended September 30, 2010, and incorporated herein by reference.
|
|
(12)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended June 30, 2015, and incorporated herein by reference.
|
|
(13)
|
Filed as an exhibit to Registrant's Current Report on Form 8-K filed on January 17, 2012, and incorporated herein by reference.
|
|
(14)
|
Filed as an exhibit to Registrant's Current Report on Form 8-K/A filed on February 22, 2006, and incorporated herein by reference.
|
|
(15)
|
Filed as an exhibit to Registrant's Annual Report on Form 10-K for the fiscal year ended December 31, 2007, and incorporated herein by reference.
|
|
(16)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended June 30, 2009, and incorporated herein by reference.
|
|
(17)
|
Filed as an exhibit to Registrant's Annual Report on Form 10-K for the fiscal year ended December 31, 2009, and incorporated herein by reference.
|
|
(18)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended March 31, 2011, and incorporated herein by reference.
|
|
(19)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended June 30, 2013, and incorporated herein by reference
|
|
(20)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended June 30, 2014, and incorporated herein by reference.
|
|
(21)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended June 30, 2012, and incorporated herein by reference.
|
|
(22)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended March 31, 2012, and incorporated herein by reference.
|
|
(23)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended March 31, 2013, and incorporated herein by reference.
|
|
(24)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended March 31, 2015, and incorporated herein by reference.
|
|
(25)
|
Filed as an exhibit to Registrant's Current Report on Form 8-K first filed on December 19, 2007, and incorporated herein by reference.
|
|
(26)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended March 31, 2010, and incorporated herein by reference.
|
|
(27)
|
Filed as an exhibit to Registrant's Current Report on Form 8-K filed on May 8, 2015, and incorporated herein by reference.
|
|
(28)
|
Filed as an exhibit to Registrant's Annual Report on Form 10-K for the fiscal year ended December 31, 2001, and incorporated herein by reference.
|
|
(29)
|
Filed as an exhibit to Registrant's Annual Report on Form 10-K for the fiscal year ended December 31, 2008, and incorporated herein by reference.
|
|
(30)
|
Filed as an exhibit to Registrant's Current Report on Form 8-K filed on May 13, 2013, and incorporated herein by reference.
|
|
(31)
|
Filed as an exhibit to Registrant's Current Report on Form 8-K filed on February 3, 2016, and incorporated herein by reference.
|
|
(32)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended June 30, 2008, and incorporated herein by reference.
|
|
(33)
|
Filed as an exhibit to Registrant's Registration Statement on Form S-1 (No. 33-55680), as amended, and incorporated herein by reference.
|
|
(34)
|
Filed as an exhibit to Registrant's Registration Statement on Form S-8 (No. 333-102912) filed on January 31, 2003, and incorporated herein by reference.
|
|
(35)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended September 30, 2006, and incorporated herein by reference.
|
|
(36)
|
Filed as an exhibit to Registrant's Annual Report on Form 10-K for the fiscal year ended March 31, 1994, and incorporated herein by reference.
|
|
(37)
|
Filed as an exhibit to Registrant's Annual Report on Form 10-K for the fiscal year ended December 31, 2000, and incorporated herein by reference.
|
|
(38)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended September 30, 2013, and incorporated herein by reference.
|
|
(39)
|
Filed as an exhibit to Triangle Pharmaceuticals, Inc.'s Quarterly Report on Form 10-Q/A filed on November 3, 1999, and incorporated herein by reference.
|
|
(40)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended September 30, 2005, and incorporated herein by reference.
|
|
(41)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended March 31, 2005, and incorporated herein by reference.
|
|
(42)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended September 30, 2011, and incorporated herein by reference.
|
|
(43)
|
Filed as an exhibit to Registrant's Annual Report on Form 10-K for the fiscal year ended December 31, 2013, and incorporated herein by reference.
|
|
(44)
|
Filed as an exhibit to Registrant's Quarterly Report on Form 10-Q for the quarter ended September 30, 2014, and incorporated herein by reference.
|
|
(45)
|
Filed as an exhibit to Registrant's Annual Report on Form 10-K for the fiscal year ended December 31, 2014, and incorporated herein by reference.
|
|
(46)
|
Filed as an exhibit to Registrant's Annual Report on Form 10-K for the fiscal year ended December 31, 2003, and incorporated herein by reference.
|
|
(47)
|
Filed as an exhibit to Registrant's Annual Report on Form 10-K for the fiscal year ended December 31, 2005, and incorporated herein by reference.
|
|
†
|
The Agreement and Plan of Merger (the Pharmasset Merger Agreement) contains representations and warranties of Registrant, Merger Sub and Pharmasset, Inc. made solely to each other as of specific dates. Those representations and warranties were made solely for purposes of the Pharmasset Merger Agreement and may be subject to important qualifications and limitations agreed to by Registrant, Merger Sub and Pharmasset, Inc. Moreover, some of those representations and warranties may not be accurate or complete as of any specified date, may be subject to a standard of materiality provided for in the Pharmasset Merger Agreement and have been used for the purpose of allocating risk among Registrant, Merger Sub and Pharmasset, Inc. rather than establishing matters as facts.
|
|
*
|
Management contract or compensatory plan or arrangement.
|
|
**
|
This certification accompanies the Form 10-K to which it relates, is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference into any filing of Registrant under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of the Form 10-K), irrespective of any general incorporation language contained in such filing.
|
|
***
|
XBRL information is filed herewith.
|
|
+
|
Certain confidential portions of this Exhibit were omitted by means of marking such portions with an asterisk (the Mark). This Exhibit has been filed separately with the Secretary of the Securities and Exchange Commission without the Mark pursuant to Registrant's Application Requesting Confidential Treatment under Rule 24b-2 under the Securities Exchange Act of 1934, as amended.
|
|
G
ILEAD
S
CIENCES
, I
NC
.
|
|
|
By:
|
/
S
/ J
OHN
C. M
ARTIN
|
|
|
John C. Martin, Ph.D.
Chairman and Chief Executive Officer
|
|
Signature
|
Title
|
Date
|
||
|
/
S
/ J
OHN
C. M
ARTIN
|
Chairman and Chief Executive Officer
|
February 24, 2016
|
||
|
John C. Martin, Ph.D.
|
(Principal Executive Officer)
|
|||
|
/
S
/ R
OBIN
L. W
ASHINGTON
|
Executive Vice President and Chief Financial Officer
|
February 24, 2016
|
||
|
Robin L. Washington
|
(Principal Financial and Accounting Officer)
|
|||
|
/
S
/ J
OHN
F. C
OGAN
|
Director
|
February 24, 2016
|
||
|
John F. Cogan
|
||||
|
/
S
/ E
TIENNE
F. D
AVIGNON
|
Director
|
February 24, 2016
|
||
|
Etienne F. Davignon
|
||||
|
/
S
/ C
ARLA
A. H
ILLS
|
Director
|
February 24, 2016
|
||
|
Carla A. Hills
|
||||
|
/
S
/ K
EVIN
E. L
OFTON
|
Director
|
February 24, 2016
|
||
|
Kevin E. Lofton
|
||||
|
/
S
/ J
OHN
W. M
ADIGAN
|
Director
|
February 24, 2016
|
||
|
John W. Madigan
|
||||
|
/
S
/ J
OHN
F. M
ILLIGAN
|
Director
|
February 24, 2016
|
||
|
John F. Milligan
|
||||
|
/
S
/ N
ICHOLAS
G. M
OORE
|
Director
|
February 24, 2016
|
||
|
Nicholas G. Moore
|
||||
|
/
S
/ R
ICHARD
J. W
HITLEY
|
Director
|
February 24, 2016
|
||
|
Richard J. Whitley
|
||||
|
/
S
/ G
AYLE
E. W
ILSON
|
Director
|
February 24, 2016
|
||
|
Gayle E. Wilson
|
||||
|
/
S
/ P
ER
W
OLD
-O
LSEN
|
Director
|
February 24, 2016
|
||
|
Per Wold-Olsen
|
||||