|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d)
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d)
|
|
DELAWARE
|
04-3744954
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
|
|
2560 General Armistead Avenue, Audubon, PA
|
19403
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
|
Registrant’s telephone number, including Area Code:
|
||
|
(610) 930-1800
|
||
|
Title of each class
|
Name of each exchange on which registered
|
|
Class A Common Stock, par value $.001 per share
|
New York Stock Exchange
|
|
Yes
o
|
No
x
|
|
Yes
o
|
No
x
|
|
Yes
x
|
No
o
|
|
Yes
x
|
No
o
|
|
Large accelerated filer
x
|
Accelerated filer
o
|
Non-accelerated filer
o
(Do not check if a smaller reporting company)
|
Smaller reporting company
o
|
|
Page
|
||
|
PART I
|
||
|
Item 1.
|
Business
|
|
|
Item 1A.
|
Risk Factors
|
|
|
Item 1B.
|
Unresolved Staff Comments
|
|
|
Item 2.
|
Properties
|
|
|
Item 3.
|
Legal Proceedings
|
|
|
Item 4.
|
Mine Safety Disclosures
|
|
|
PART II
|
||
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
|
|
Item 6.
|
Selected Financial Data
|
|
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
|
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
|
|
|
Item 8.
|
Financial Statements and Supplementary Data
|
|
|
Item 9.
|
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
|
|
|
Item 9A.
|
Controls and Procedures
|
|
|
Item 9B.
|
Other Information
|
|
|
PART III
|
||
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
|
|
Item 11.
|
Executive Compensation
|
|
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
|
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
|
|
Item 14.
|
Principal Accountant Fees and Services
|
|
|
PART IV
|
||
|
Item 15
|
Exhibits and Financial Statement Schedules
|
|
|
SIGNATURES
|
||
|
•
|
Leverage our integrated product development engine
. We plan to continue developing new spine products as well as additional robotic and trauma products using our product development engine. We believe our team-oriented approach, active surgeon input and demonstrated product development capabilities position us to maintain a rapid rate of new product launches. We launched
seventeen
new products in
2016
, have over
30
potential new products in various stages
|
|
•
|
Increase the size, scope and productivity of our exclusive U.S. sales force
. We believe there is significant opportunity for us to further penetrate existing markets and to enter new markets by increasing the size and geographic scope of our exclusive U.S. sales force. We expect to continue to increase the number of our direct and distributor sales representatives in the United States to expand into new geographic territories and to deepen our penetration in existing territories. We will also continue to provide our sales representatives with specialized development programs designed to improve their productivity. In addition, we have begun to build exclusive sales forces in the U.S. and internationally to support the anticipated launch of our robotics and trauma products.
|
|
•
|
Continue to expand into international markets
. In 2016, we significantly increased our international presence through the acquisition of the international operations and distribution channels of Alphatec Holdings, Inc. As of
December 31, 2016
, we had an existing direct or distributor sales presence in
49
countries outside the United States. We expect to continue to increase our international presence through the commercialization of additional products, including our robotics and trauma products, and through the expansion of our international sales force.
|
|
•
|
Pursue strategic acquisitions and alliances
. We intend to selectively pursue acquisitions and alliances in the future that will provide us with new or complementary technologies, personnel with significant relevant experience, or increased market penetration. We are currently evaluating a number of possible acquisitions or strategic relationships and believe that our resources and experience make us an attractive acquirer or partner.
|
|
•
|
Favorable patient demographics.
The number of people between 40 to 80 years old is large and growing. Improvements in healthcare have led to increasing life expectancies worldwide and the opportunity to lead more active lifestyles at advanced ages. These trends are expected to generate increased demand for spine surgeries.
|
|
•
|
Improving technologies leading to increased use in fusion procedures.
Due to the longevity of its practice and acceptable clinical outcomes, fusion has become a standard treatment option for patients presenting more advanced stages of spine disease. We expect that the development of
|
|
•
|
Disruptive Technologies driving earlier interventions and creating an expanded patient base.
Newer technology products and procedures are gaining increasing acceptance among patients and surgeons because they allow for novel surgical procedures, improvements to existing surgical procedures, the treatment of spine disorders by new physician specialties, and surgical intervention earlier in the continuum of care, all of which may result in better outcomes for patients. As a result, we expect Disruptive Technologies to drive accelerated growth and increase the size of the addressable patient population for spine surgery.
|
|
•
|
Continued growth of spine procedures worldwide.
While the United States comprises approximately
4%
of the worldwide population, we believe that approximately one-half of all spine surgeries occur in the United States. We believe that improvements to the standard of care outside of the United States will increase the international demand for spine products.
|
|
•
|
national and regional educational courses;
|
|
•
|
intensive hands-on cadaveric training on new products and new techniques;
|
|
•
|
research collaboration and support;
|
|
•
|
educational support; and
|
|
•
|
fellowship support.
|
|
•
|
product design and development;
|
|
•
|
product testing, manufacturing and safety;
|
|
•
|
post-market surveillance and reporting;
|
|
•
|
product labeling;
|
|
•
|
complaint handling;
|
|
•
|
post-market approval studies; and
|
|
•
|
product advertising, marketing and promotion.
|
|
•
|
untitled letters or warning letters;
|
|
•
|
fines, injunctions and civil penalties;
|
|
•
|
recall or seizure of our products;
|
|
•
|
operating restrictions, partial suspension or total shutdown of production;
|
|
•
|
refusing our request for 510(k) or
de novo
clearance or PMA of new products;
|
|
•
|
withdrawing 510(k) clearance or PMAs that are already granted;
|
|
•
|
refusal to grant export approval of our products; and
|
|
•
|
criminal prosecution.
|
|
•
|
lack of experience with MIS or our motion preservation or regenerative biologics technologies;
|
|
•
|
lack or perceived lack of evidence supporting additional patient benefits;
|
|
•
|
perceived liability risks generally associated with the use of new products and procedures;
|
|
•
|
limited or lack of availability of coverage and reimbursement within healthcare payment systems;
|
|
•
|
costs associated with the purchase of new products and equipment; and
|
|
•
|
the time commitment that may be required for training.
|
|
•
|
greater financial, human and other resources for product research and development, sales and marketing and litigation;
|
|
•
|
significantly greater name recognition;
|
|
•
|
established relationships with spine surgeons, hospitals and other healthcare providers;
|
|
•
|
large and established sales and marketing and distribution networks;
|
|
•
|
products supported by long-term clinical data;
|
|
•
|
greater experience in obtaining and maintaining regulatory clearances or approvals for products and product enhancements;
|
|
•
|
more expansive portfolios of intellectual property rights; and
|
|
•
|
greater ability to cross-sell their products or to incentivize hospitals or surgeons to use their products.
|
|
•
|
properly identify and anticipate surgeon and patient needs;
|
|
•
|
develop and introduce new products or product enhancements in a timely manner;
|
|
•
|
adequately protect our intellectual property and avoid infringing upon the intellectual property rights of third parties;
|
|
•
|
demonstrate the safety and efficacy of new products; and
|
|
•
|
obtain the necessary regulatory clearances or approvals for new products or product enhancements.
|
|
•
|
exposure to different legal and regulatory standards;
|
|
•
|
lack of stringent protection of intellectual property;
|
|
•
|
obstacles to obtaining domestic and foreign export, import and other governmental approvals, permits and licenses and compliance with foreign laws;
|
|
•
|
potentially adverse tax consequences and the complexities of foreign value-added tax systems;
|
|
•
|
adverse changes in tariffs and trade restrictions;
|
|
•
|
foreign exchange rate risk;
|
|
•
|
limitations on the repatriation of earnings;
|
|
•
|
difficulties in staffing and managing foreign operations;
|
|
•
|
transportation delays and difficulties of managing international distribution channels;
|
|
•
|
longer collection periods and difficulties in collecting receivables from foreign entities;
|
|
•
|
increased financing costs; and
|
|
•
|
political, social and economic instability and increased security concerns.
|
|
•
|
problems assimilating the purchased technologies, products or business operations;
|
|
•
|
issues maintaining uniform standards, procedures, controls and policies;
|
|
•
|
unanticipated costs associated with acquisitions;
|
|
•
|
diversion of management’s attention from our core business;
|
|
•
|
adverse effects on existing business relationships with suppliers and customers;
|
|
•
|
risks associated with entering new markets in which we have limited or no experience;
|
|
•
|
potential loss of key employees of acquired businesses; and
|
|
•
|
increased legal and accounting compliance costs.
|
|
•
|
sales and marketing, accounting and financial functions;
|
|
•
|
inventory management;
|
|
•
|
engineering and product development tasks; and
|
|
•
|
our research and development data.
|
|
•
|
earthquakes, fires, floods and other natural disasters;
|
|
•
|
terrorist attacks and attacks by computer viruses or hackers;
|
|
•
|
power losses; and
|
|
•
|
computer systems, or Internet, telecommunications or data network failures.
|
|
•
|
design, development and manufacturing;
|
|
•
|
testing, labeling, content and language of instructions for use and storage;
|
|
•
|
clinical trials;
|
|
•
|
product safety;
|
|
•
|
marketing, sales and distribution;
|
|
•
|
pre-market clearance and approval;
|
|
•
|
record keeping procedures;
|
|
•
|
advertising and promotion;
|
|
•
|
recalls and field safety corrective actions;
|
|
•
|
post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury;
|
|
•
|
post-market approval studies; and
|
|
•
|
product import and export.
|
|
•
|
we may not be able to demonstrate to the FDA’s satisfaction that our products are safe and effective for their intended uses;
|
|
•
|
the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval, where required; and
|
|
•
|
the manufacturing process or facilities we use may not meet applicable requirements.
|
|
•
|
warning letters;
|
|
•
|
fines;
|
|
•
|
injunctions;
|
|
•
|
civil penalties;
|
|
•
|
termination of distribution;
|
|
•
|
recalls or seizures of products;
|
|
•
|
delays in the introduction of products into the market;
|
|
•
|
total or partial suspension of production;
|
|
•
|
refusal of the FDA or other regulator to grant future clearances or approvals;
|
|
•
|
withdrawals or suspensions of current clearances or approvals, resulting in prohibitions on sales of our products;
|
|
•
|
refusal to grant export approvals; and/or
|
|
•
|
in the most serious cases, criminal penalties.
|
|
•
|
untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties;
|
|
•
|
customer notifications or repair, replacement, refunds, recall, detention or seizure of our products;
|
|
•
|
operating restrictions or partial suspension or total shutdown of production;
|
|
•
|
refusing or delaying our requests for 510(k) or
de novo
clearance or PMA of new products or modified products;
|
|
•
|
withdrawing 510(k) or
de novo
clearances or PMAs that have already been granted;
|
|
•
|
refusal to grant export approval for our products; or
|
|
•
|
criminal prosecution.
|
|
•
|
the Federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs;
|
|
•
|
federal false claims laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent;
|
|
•
|
the federal Health Insurance Portability and Accountability Act of 1996, which created federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
|
|
•
|
the Federal Trade Commission Act and similar laws regulating advertisement and consumer protections;
|
|
•
|
the FCPA, which prohibits corrupt payments, gifts or transfers of value to foreign officials;
|
|
•
|
foreign and U.S. state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers; and
|
|
•
|
the Physician Payment Sunshine Act, which requires medical device companies to report all compensation, gifts and benefits they have provided to certain healthcare professionals.
|
|
•
|
our ability to drive increased sales of our products;
|
|
•
|
our ability to establish and maintain an effective and dedicated sales force;
|
|
•
|
pricing pressure applicable to our products, including adverse third-party coverage and reimbursement outcomes;
|
|
•
|
results of clinical research and trials on our existing products and products in development;
|
|
•
|
the mix of our products sold because profit margins differ amongst our products;
|
|
•
|
timing of new product offerings, acquisitions, licenses or other significant events by us or our competitors;
|
|
•
|
the ability of our suppliers to timely provide us with an adequate supply of materials and components;
|
|
•
|
the evolving product offerings of our competitors;
|
|
•
|
regulatory approvals and legislative changes affecting the products we may offer or those of our competitors;
|
|
•
|
interruption in the manufacturing or distribution of our products;
|
|
•
|
the effect of competing technological, industry and market developments;
|
|
•
|
changes in our ability to obtain regulatory clearance or approval for our products; and
|
|
•
|
our ability to expand the geographic reach of our sales and marketing efforts.
|
|
•
|
the revenues generated by sales of our products;
|
|
•
|
the costs associated with expanding our sales and marketing efforts;
|
|
•
|
the expenses we incur in manufacturing and selling our products;
|
|
•
|
the costs of developing and commercializing new products or technologies;
|
|
•
|
the cost of obtaining and maintaining regulatory approval or clearance of our products and products in development;
|
|
•
|
the number and timing of acquisitions and other strategic transactions;
|
|
•
|
the costs associated with our planned international expansion;
|
|
•
|
the costs associated with increased capital expenditures, including fixed asset purchases of instrument sets which we loan to hospitals to support surgeries; and
|
|
•
|
unanticipated general and administrative expenses.
|
|
•
|
stop selling products or using technology that contains the allegedly infringing intellectual property;
|
|
•
|
lose the opportunity to license our technology to others or to collect royalty payments based upon successful protection and assertion of our intellectual property rights against others;
|
|
•
|
incur significant legal expenses;
|
|
•
|
pay substantial damages to the party whose intellectual property rights we may be found to be infringing;
|
|
•
|
redesign those products that contain the allegedly infringing intellectual property, which could be costly and disruptive; or
|
|
•
|
attempt to obtain a license to the relevant intellectual property from third parties, which may not be available on reasonable terms or at all.
|
|
•
|
delaying, deferring or preventing a change in control of our company;
|
|
•
|
impeding a merger, consolidation, takeover or other business combination involving our company; or
|
|
•
|
causing us to enter into transactions or agreements that are not in the best interests of all stockholders.
|
|
Year Ended December 31, 2016:
|
High
|
Low
|
||||||
|
1st Quarter
|
$
|
27.64
|
|
$
|
21.56
|
|
||
|
2nd Quarter
|
25.99
|
|
21.90
|
|
||||
|
3rd Quarter
|
26.46
|
|
22.00
|
|
||||
|
4th Quarter
|
25.00
|
|
19.25
|
|
||||
|
Year Ended December 31, 2015:
|
High
|
Low
|
||||||
|
1st Quarter
|
$
|
26.00
|
|
$
|
23.04
|
|
||
|
2nd Quarter
|
26.30
|
|
23.15
|
|
||||
|
3rd Quarter
|
28.99
|
|
20.63
|
|
||||
|
4th Quarter
|
28.60
|
|
20.48
|
|
||||
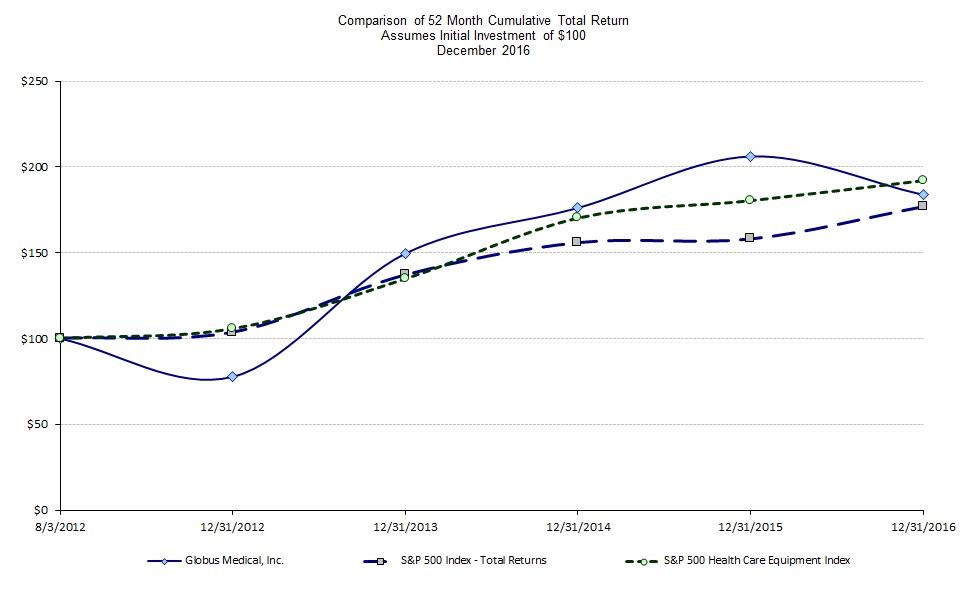
|
Company/Index
|
August 3,
2012 |
December 31,
2012 |
December 31,
2013 |
December 31,
2014 |
December 31,
2015 |
December 31,
2016 |
||||||
|
Globus Medical, Inc.
|
$100
|
$78
|
$149
|
$176
|
$206
|
$184
|
||||||
|
S&P 500 Index
|
$100
|
$104
|
$137
|
$156
|
$158
|
$177
|
||||||
|
S&P 500 Health Care Equipment
|
$100
|
$106
|
$135
|
$170
|
$181
|
$192
|
||||||
|
Statement of Income Data:
|
Year Ended December 31,
|
||||||||||||||||||
|
(In thousands, except per share amounts)
|
2016
|
2015
|
2014
|
2013
|
2012
|
||||||||||||||
|
Sales
|
$
|
563,994
|
|
$
|
544,753
|
|
$
|
474,371
|
|
$
|
434,459
|
|
$
|
385,994
|
|
||||
|
Cost of goods sold
|
134,705
|
|
132,333
|
|
110,769
|
|
100,343
|
|
75,199
|
|
|||||||||
|
Gross profit
|
429,289
|
|
412,420
|
|
363,602
|
|
334,116
|
|
310,795
|
|
|||||||||
|
Operating expenses:
|
|||||||||||||||||||
|
Research and development
|
44,532
|
|
36,312
|
|
31,166
|
|
26,389
|
|
27,802
|
|
|||||||||
|
Selling, general and administrative
|
222,156
|
|
210,241
|
|
188,632
|
|
182,348
|
|
168,420
|
|
|||||||||
|
Provision for litigation
|
3,156
|
|
(11,268
|
)
|
5,667
|
|
23,055
|
|
(786
|
)
|
|||||||||
|
Amortization of intangibles
|
3,478
|
|
1,561
|
|
712
|
|
531
|
|
447
|
|
|||||||||
|
Acquisition related costs
|
1,826
|
|
3,352
|
|
(937
|
)
|
120
|
|
119
|
|
|||||||||
|
Total operating expenses
|
275,148
|
|
240,198
|
|
225,240
|
|
232,443
|
|
196,002
|
|
|||||||||
|
Operating income
|
154,141
|
|
172,222
|
|
138,362
|
|
101,673
|
|
114,793
|
|
|||||||||
|
Other income/(expense), net
|
3,138
|
|
583
|
|
280
|
|
328
|
|
(140
|
)
|
|||||||||
|
Income before income taxes
|
157,279
|
|
172,805
|
|
138,642
|
|
102,001
|
|
114,653
|
|
|||||||||
|
Income tax provision
|
52,938
|
|
60,021
|
|
46,157
|
|
33,389
|
|
40,822
|
|
|||||||||
|
Net income
|
$
|
104,341
|
|
$
|
112,784
|
|
$
|
92,485
|
|
$
|
68,612
|
|
$
|
73,831
|
|
||||
|
Net income per common share:
|
|||||||||||||||||||
|
Basic
|
$
|
1.09
|
|
$
|
1.19
|
|
$
|
0.98
|
|
$
|
0.74
|
|
$
|
0.82
|
|
||||
|
Diluted
|
$
|
1.08
|
|
$
|
1.17
|
|
$
|
0.97
|
|
$
|
0.73
|
|
$
|
0.80
|
|
||||
|
Weighted average number of common shares:
|
|||||||||||||||||||
|
Basic
|
95,647
|
|
95,046
|
|
94,227
|
|
92,647
|
|
89,608
|
|
|||||||||
|
Diluted
|
96,432
|
|
96,073
|
|
95,457
|
|
94,192
|
|
92,208
|
|
|||||||||
|
Balance Sheet Data:
|
As of December 31,
|
||||||||||||||||||
|
(In thousands)
|
2016
|
2015
|
2014
|
2013
|
2012
|
||||||||||||||
|
Cash, cash equivalents and marketable securities
|
$
|
350,756
|
|
$
|
329,791
|
|
$
|
304,051
|
|
$
|
275,452
|
|
$
|
212,400
|
|
||||
|
Working capital
|
433,874
|
|
462,108
|
|
380,613
|
|
348,866
|
|
320,602
|
|
|||||||||
|
Total assets
|
927,637
|
|
834,100
|
|
703,547
|
|
566,304
|
|
447,133
|
|
|||||||||
|
Business acquisition liabilities, including current portion
(1)
|
20,080
|
|
33,314
|
|
26,276
|
|
17,258
|
|
11,344
|
|
|||||||||
|
Stockholders’ equity
|
$
|
832,078
|
|
$
|
715,324
|
|
$
|
585,454
|
|
$
|
472,360
|
|
$
|
386,502
|
|
||||
|
(1)
|
In connection with certain acquisitions completed in 2016 through 2011, we have certain contingent consideration obligations payable to the sellers in these transactions upon the achievement of certain regulatory and sales milestones. The maximum aggregate undiscounted amounts potentially payable were
$29.1 million
,
$35.9 million
,
$38.9 million
,
$23.9 million
and
$9.9 million
as of December 31, 2016, 2015, 2014, 2013, and 2012, respectively.
|
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2016 |
December 31,
2015 |
$
|
%
|
||||||||||
|
Innovative Fusion
|
$
|
287,594
|
|
$
|
288,062
|
|
$
|
(468
|
)
|
(0.2
|
)%
|
|||
|
Disruptive Technology
|
276,400
|
|
256,691
|
|
19,709
|
|
7.7
|
%
|
||||||
|
Total sales
|
$
|
563,994
|
|
$
|
544,753
|
|
$
|
19,241
|
|
3.5
|
%
|
|||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2016 |
December 31,
2015 |
$
|
%
|
||||||||||
|
United States
|
$
|
500,226
|
|
$
|
498,191
|
|
$
|
2,035
|
|
0.4
|
%
|
|||
|
International
|
63,768
|
|
46,562
|
|
17,206
|
|
37.0
|
%
|
||||||
|
Total sales
|
$
|
563,994
|
|
$
|
544,753
|
|
$
|
19,241
|
|
3.5
|
%
|
|||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2016 |
December 31,
2015 |
$
|
%
|
||||||||||
|
Cost of goods sold
|
$
|
134,705
|
|
$
|
132,333
|
|
$
|
2,372
|
|
1.8
|
%
|
|||
|
Percentage of sales
|
23.9
|
%
|
24.3
|
%
|
||||||||||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2016 |
December 31,
2015 |
$
|
%
|
||||||||||
|
Research and development
|
$
|
44,532
|
|
$
|
36,312
|
|
$
|
8,220
|
|
22.6
|
%
|
|||
|
Percentage of sales
|
7.9
|
%
|
6.7
|
%
|
||||||||||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2016 |
December 31,
2015 |
$
|
%
|
||||||||||
|
Selling, general and administrative
|
$
|
222,156
|
|
$
|
210,241
|
|
$
|
11,915
|
|
5.7
|
%
|
|||
|
Percentage of sales
|
39.4
|
%
|
38.6
|
%
|
||||||||||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2016 |
December 31,
2015 |
$
|
%
|
||||||||||
|
Provision for litigation
|
$
|
3,156
|
|
$
|
(11,268
|
)
|
$
|
14,424
|
|
(128.0
|
)%
|
|||
|
Percentage of sales
|
0.6
|
%
|
(2.1
|
)%
|
||||||||||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2016 |
December 31,
2015 |
$
|
%
|
||||||||||
|
Amortization of intangibles
|
$
|
3,478
|
|
$
|
1,561
|
|
$
|
1,917
|
|
122.8
|
%
|
|||
|
Percentage of sales
|
0.6
|
%
|
0.3
|
%
|
||||||||||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2016 |
December 31,
2015 |
$
|
%
|
||||||||||
|
Acquisition related costs
|
$
|
1,826
|
|
$
|
3,352
|
|
$
|
(1,526
|
)
|
(45.5
|
)%
|
|||
|
Percentage of sales
|
0.3
|
%
|
0.6
|
%
|
||||||||||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2016 |
December 31,
2015 |
$
|
%
|
||||||||||
|
Other income, net
|
$
|
3,138
|
|
$
|
583
|
|
$
|
2,555
|
|
438.3
|
%
|
|||
|
Percentage of sales
|
0.6
|
%
|
0.1
|
%
|
||||||||||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2016 |
December 31,
2015 |
$
|
%
|
||||||||||
|
Income tax provision
|
$
|
52,938
|
|
$
|
60,021
|
|
$
|
(7,083
|
)
|
(11.8
|
)%
|
|||
|
Effective income tax rate
|
33.7
|
%
|
34.7
|
%
|
||||||||||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2015 |
December 31, 2014
|
$
|
%
|
||||||||||
|
Innovative Fusion
|
$
|
288,062
|
|
$
|
270,852
|
|
$
|
17,210
|
|
6.4
|
%
|
|||
|
Disruptive Technology
|
256,691
|
|
203,519
|
|
53,172
|
|
26.1
|
%
|
||||||
|
Total sales
|
$
|
544,753
|
|
$
|
474,371
|
|
$
|
70,382
|
|
14.8
|
%
|
|||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2015 |
December 31, 2014
|
$
|
%
|
||||||||||
|
United States
|
$
|
498,191
|
|
$
|
427,091
|
|
$
|
71,100
|
|
16.6
|
%
|
|||
|
International
|
46,562
|
|
47,280
|
|
(718
|
)
|
(1.5
|
)%
|
||||||
|
Total sales
|
$
|
544,753
|
|
$
|
474,371
|
|
$
|
70,382
|
|
14.8
|
%
|
|||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2015 |
December 31, 2014
|
$
|
%
|
||||||||||
|
Cost of goods sold
|
$
|
132,333
|
|
$
|
110,769
|
|
$
|
21,564
|
|
19.5
|
%
|
|||
|
Percentage of sales
|
24.3
|
%
|
23.4
|
%
|
||||||||||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2015 |
December 31,
2014 |
$
|
%
|
||||||||||
|
Research and development
|
$
|
36,312
|
|
$
|
31,166
|
|
$
|
5,146
|
|
16.5
|
%
|
|||
|
Percentage of sales
|
6.7
|
%
|
6.6
|
%
|
||||||||||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2015 |
December 31, 2014
|
$
|
%
|
||||||||||
|
Selling, general and administrative
|
$
|
210,241
|
|
$
|
188,632
|
|
$
|
21,609
|
|
11.5
|
%
|
|||
|
Percentage of sales
|
38.6
|
%
|
39.8
|
%
|
||||||||||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2015 |
December 31, 2014
|
$
|
%
|
||||||||||
|
Provision for litigation
|
$
|
(11,268
|
)
|
$
|
5,667
|
|
$
|
(16,935
|
)
|
(298.8
|
)%
|
|||
|
Percentage of sales
|
(2.1
|
)%
|
1.2
|
%
|
||||||||||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2015 |
December 31,
2014 |
$
|
%
|
||||||||||
|
Amortization of intangibles
|
$
|
1,561
|
|
$
|
712
|
|
$
|
849
|
|
119.2
|
%
|
|||
|
Percentage of sales
|
0.3
|
%
|
0.2
|
%
|
||||||||||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2015 |
December 31,
2014 |
$
|
%
|
||||||||||
|
Acquisition related costs
|
$
|
3,352
|
|
$
|
(937
|
)
|
$
|
4,289
|
|
(457.7
|
)%
|
|||
|
Percentage of sales
|
0.6
|
%
|
(0.2
|
)%
|
||||||||||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2015 |
December 31, 2014
|
$
|
%
|
||||||||||
|
Other income, net
|
$
|
583
|
|
$
|
280
|
|
$
|
303
|
|
108.2
|
%
|
|||
|
Percentage of sales
|
0.1
|
%
|
0.1
|
%
|
||||||||||
|
Year Ended
|
Change
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2015 |
December 31, 2014
|
$
|
%
|
||||||||||
|
Income tax provision
|
$
|
60,021
|
|
$
|
46,157
|
|
$
|
13,864
|
|
30.0
|
%
|
|||
|
Effective income tax rate
|
34.7
|
%
|
33.3
|
%
|
||||||||||
|
Year Ended
|
|||||||||||||
|
(In thousands, except percentages)
|
December 31,
2016 |
December 31,
2015 |
December 31,
2014 |
||||||||||
|
Net income
|
$
|
104,341
|
|
$
|
112,784
|
|
$
|
92,485
|
|
||||
|
Interest income, net
|
(3,057
|
)
|
(1,304
|
)
|
(805
|
)
|
|||||||
|
Provision for income taxes
|
52,938
|
|
60,021
|
|
46,157
|
|
|||||||
|
Depreciation and amortization
|
38,771
|
|
*
|
24,084
|
|
21,754
|
|
||||||
|
EBITDA
|
192,993
|
|
195,585
|
|
159,591
|
|
|||||||
|
Stock-based compensation expense
|
11,382
|
|
9,639
|
|
7,111
|
|
|||||||
|
Provision for litigation
|
3,156
|
|
(11,268
|
)
|
5,667
|
|
|||||||
|
Acquisition related costs/licensing
|
6,931
|
|
3,577
|
|
(937
|
)
|
|||||||
|
Prior period adjustment, excluding depreciation
|
(3,697
|
)
|
—
|
|
—
|
|
|||||||
|
Adjusted EBITDA
|
$
|
210,765
|
|
$
|
197,533
|
|
$
|
171,432
|
|
||||
|
Net income as a percentage of sales
|
18.5
|
%
|
20.7
|
%
|
19.5
|
%
|
|||||||
|
Adjusted EBITDA as a percentage of sales
|
37.4
|
%
|
36.3
|
%
|
36.1
|
%
|
|||||||
|
*
|
Included in this amount for the year ended
December 31, 2016
is
$5.5 million
related to depreciation amounts recognized in the current year related to the prior period adjustment.
|
|
Year Ended
|
||||||||||||
|
(In thousands)
|
December 31,
2016 |
December 31,
2015 |
December 31,
2014 |
|||||||||
|
Net income
|
$
|
104,341
|
|
$
|
112,784
|
|
$
|
92,485
|
|
|||
|
Provision for litigation
|
3,156
|
|
(11,268
|
)
|
5,667
|
|
||||||
|
Amortization of intangibles
|
3,478
|
|
1,561
|
|
712
|
|
||||||
|
Acquisition related costs/licensing
|
6,931
|
|
3,577
|
|
(937
|
)
|
||||||
|
Prior period adjustment
|
1,765
|
|
—
|
|
—
|
|
||||||
|
Tax effect of adjusting items
|
(5,166
|
)
|
2,127
|
|
(1,812
|
)
|
||||||
|
Non-GAAP net income
|
$
|
114,505
|
|
$
|
108,781
|
|
$
|
96,115
|
|
|||
|
Year Ended
|
||||||||||||
|
(Per share amounts)
|
December 31,
2016 |
December 31,
2015 |
December 31,
2014 |
|||||||||
|
Diluted earnings per share, as reported
|
$
|
1.08
|
|
$
|
1.17
|
|
$
|
0.97
|
|
|||
|
Provision for litigation
|
0.03
|
|
(0.12
|
)
|
0.06
|
|
||||||
|
Amortization of intangibles
|
0.04
|
|
0.02
|
|
0.01
|
|
||||||
|
Acquisition related costs/licensing
|
0.07
|
|
0.04
|
|
(0.01
|
)
|
||||||
|
Prior period adjustment
|
0.02
|
|
—
|
|
—
|
|
||||||
|
Tax effect of adjusting items
|
(0.05
|
)
|
0.02
|
|
(0.02
|
)
|
||||||
|
Non-GAAP diluted earnings per share
|
$
|
1.19
|
|
$
|
1.13
|
|
$
|
1.01
|
|
|||
|
Year Ended
|
||||||||||||
|
(Per share amounts)
|
December 31,
2016 |
December 31,
2015 |
December 31,
2014 |
|||||||||
|
Net cash provided by operating activities
|
$
|
171,893
|
|
$
|
121,957
|
|
$
|
79,172
|
|
|||
|
Adjustment for impact of restricted cash
|
(25,641
|
)
|
2,749
|
|
23,370
|
|
||||||
|
Purchases of property and equipment
|
(40,909
|
)
|
(50,760
|
)
|
(24,754
|
)
|
||||||
|
Free cash flow
|
$
|
105,343
|
|
$
|
73,946
|
|
$
|
77,788
|
|
|||
|
Year Ended
|
Reported Sales Growth
|
Currency Impact on 2016 Sales
|
Constant Currency Sales Growth
|
||||||||||||||
|
(In thousands, except percentages)
|
December 31,
2016 |
December 31,
2015 |
|||||||||||||||
|
United States
|
$
|
500,226
|
|
$
|
498,191
|
|
0.4
|
%
|
—
|
|
0.4
|
%
|
|||||
|
International
|
63,768
|
|
46,562
|
|
37.0
|
%
|
$
|
(1,594
|
)
|
40.4
|
%
|
||||||
|
Total sales
|
$
|
563,994
|
|
$
|
544,753
|
|
3.5
|
%
|
$
|
(1,594
|
)
|
3.8
|
%
|
||||
|
Year Ended
|
Reported Sales Growth
|
Currency Impact on 2015 Sales
|
Constant Currency Sales Growth
|
||||||||||||||
|
(In thousands, except percentages)
|
December 31,
2015 |
December 31,
2014 |
|||||||||||||||
|
United States
|
$
|
498,191
|
|
$
|
427,091
|
|
16.6
|
%
|
—
|
|
16.6
|
%
|
|||||
|
International
|
46,562
|
|
47,280
|
|
(1.5
|
%)
|
$
|
(5,544
|
)
|
10.2
|
%
|
||||||
|
Total sales
|
$
|
544,753
|
|
$
|
474,371
|
|
14.8
|
%
|
$
|
(5,544
|
)
|
16.0
|
%
|
||||
|
Year Ended
|
2016 - 2015 Change
|
2015 - 2014 Change
|
|||||||||||||||||
|
(In thousands)
|
December 31, 2016
|
December 31, 2015
|
December 31, 2014
|
$
|
$
|
||||||||||||||
|
Net cash provided by operating activities
|
$
|
171,893
|
|
$
|
121,957
|
|
$
|
79,172
|
|
$
|
49,936
|
|
$
|
42,785
|
|
||||
|
Net cash used in investing activities
|
(99,553
|
)
|
(150,550
|
)
|
(100,000
|
)
|
50,997
|
|
(50,550
|
)
|
|||||||||
|
Net cash provided by financing activities
|
2,041
|
|
6,327
|
|
12,946
|
|
(4,286
|
)
|
(6,619
|
)
|
|||||||||
|
Effect of foreign exchange rate changes on cash
|
(1,894
|
)
|
153
|
|
185
|
|
(2,047
|
)
|
(32
|
)
|
|||||||||
|
Increase/(decrease) in cash and cash equivalents
|
$
|
72,487
|
|
$
|
(22,113
|
)
|
$
|
(7,697
|
)
|
$
|
94,600
|
|
$
|
(14,416
|
)
|
||||
|
(In thousands)
|
December 31, 2016
|
December 31,
2015 |
|||||
|
Cash and cash equivalents
|
$
|
132,639
|
|
$
|
60,152
|
|
|
|
Short-term marketable securities
|
157,673
|
|
220,877
|
|
|||
|
Long-term marketable securities
|
60,444
|
|
48,762
|
|
|||
|
Total cash, cash equivalents and marketable securities
|
$
|
350,756
|
|
$
|
329,791
|
|
|
|
Available borrowing capacity under revolving credit facility
|
50,000
|
|
50,000
|
|
|||
|
Working capital
|
$
|
433,874
|
|
$
|
462,108
|
|
|
|
Payments Due by Period
|
||||||||||||||||||||
|
(In thousands)
|
Total
|
Less than 1 Year
|
1-3 Years
|
3-5 Years
|
More than 5 Years
|
|||||||||||||||
|
Operating leases
|
$
|
3,396
|
|
$
|
1,412
|
|
$
|
1,903
|
|
$
|
81
|
|
$
|
—
|
|
|||||
|
Purchase obligations
(1)
|
14,234
|
|
4,078
|
|
2,556
|
|
2,400
|
|
5,200
|
|
||||||||||
|
Total
(2)
|
$
|
17,630
|
|
$
|
5,490
|
|
$
|
4,459
|
|
$
|
2,481
|
|
$
|
5,200
|
|
|||||
|
(1)
|
Reflects minimum annual volume commitments to purchase inventory under certain of our supplier contracts as well as costs related to service agreements.
|
|
(2)
|
In connection with certain acquisitions completed in 2011 through 2014, we have certain contingent consideration obligations payable to the sellers in these transactions upon the achievement of certain regulatory and sales milestones. The maximum aggregate undiscounted amounts potentially payable not included in the table above total
$29.1 million
.
|
|
Reports of Independent Registered Public Accounting Firms
|
|
|
Consolidated Balance Sheets
|
|
|
Consolidated Statements of Income
|
|
|
Consolidated Statements of Comprehensive Income
|
|
|
Consolidated Statements of Equity
|
|
|
Consolidated Statements of Cash Flows
|
|
|
Notes to Consolidated Financial Statements
|
|
|
(In thousands, except par value)
|
December 31,
2016 |
December 31,
2015 |
|||||
|
ASSETS
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
132,639
|
|
$
|
60,152
|
|
|
|
Restricted cash
|
477
|
|
26,119
|
|
|||
|
Short-term marketable securities
|
157,673
|
|
220,877
|
|
|||
|
Accounts receivable, net of allowances of $2,771 and $2,513, respectively
|
91,983
|
|
77,681
|
|
|||
|
Inventories
|
112,692
|
|
105,260
|
|
|||
|
Prepaid expenses and other current assets
|
14,502
|
|
7,351
|
|
|||
|
Income taxes receivable
|
3,800
|
|
8,672
|
|
|||
|
Deferred income taxes
|
—
|
|
38,687
|
|
|||
|
Total current assets
|
513,766
|
|
544,799
|
|
|||
|
Property and equipment, net of accumulated depreciation of $166,711 and $139,114, respectively
|
124,229
|
|
114,743
|
|
|||
|
Long-term marketable securities
|
60,444
|
|
48,762
|
|
|||
|
Note receivable
|
30,000
|
|
—
|
|
|||
|
Intangible assets, net
|
61,706
|
|
33,242
|
|
|||
|
Goodwill
|
105,926
|
|
91,964
|
|
|||
|
Other assets
|
928
|
|
590
|
|
|||
|
Deferred income taxes
|
30,638
|
|
—
|
|
|||
|
Total assets
|
$
|
927,637
|
|
$
|
834,100
|
|
|
|
LIABILITIES AND EQUITY
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
17,472
|
|
$
|
15,971
|
|
|
|
Accrued expenses
|
46,401
|
|
53,769
|
|
|||
|
Income taxes payable
|
1,911
|
|
763
|
|
|||
|
Business acquisition liabilities, current
|
14,108
|
|
12,188
|
|
|||
|
Total current liabilities
|
79,892
|
|
82,691
|
|
|||
|
Business acquisition liabilities, net of current portion
|
5,972
|
|
21,126
|
|
|||
|
Deferred income taxes
|
7,876
|
|
13,260
|
|
|||
|
Other liabilities
|
1,819
|
|
1,699
|
|
|||
|
Total liabilities
|
95,559
|
|
118,776
|
|
|||
|
Commitments and contingencies (Note 15)
|
|
|
|
|
|||
|
Equity:
|
|||||||
|
Class A common stock; $0.001 par value. Authorized 500,000 shares; issued and outstanding 72,052 and 71,442 shares at December 31, 2016 and 2015, respectively
|
72
|
|
71
|
|
|||
|
Class B common stock; $0.001 par value. Authorized 275,000 shares; issued and outstanding 23,878 shares at December 31, 2016 and 2015, respectively
|
24
|
|
24
|
|
|||
|
Additional paid-in capital
|
211,725
|
|
192,629
|
|
|||
|
Accumulated other comprehensive loss
|
(8,642
|
)
|
(1,958
|
)
|
|||
|
Retained earnings
|
628,899
|
|
524,558
|
|
|||
|
Total equity
|
832,078
|
|
715,324
|
|
|||
|
Total liabilities and equity
|
$
|
927,637
|
|
$
|
834,100
|
|
|
|
Year Ended
|
||||||||||||
|
(In thousands, except per share amounts)
|
December 31,
2016 |
December 31,
2015 |
December 31,
2014 |
|||||||||
|
Sales
|
$
|
563,994
|
|
$
|
544,753
|
|
$
|
474,371
|
|
|||
|
Cost of goods sold
|
134,705
|
|
132,333
|
|
110,769
|
|
||||||
|
Gross profit
|
429,289
|
|
412,420
|
|
363,602
|
|
||||||
|
Operating expenses:
|
||||||||||||
|
Research and development
|
44,532
|
|
36,312
|
|
31,166
|
|
||||||
|
Selling, general and administrative
|
222,156
|
|
210,241
|
|
188,632
|
|
||||||
|
Provision for litigation
|
3,156
|
|
(11,268
|
)
|
5,667
|
|
||||||
|
Amortization of intangibles
|
3,478
|
|
1,561
|
|
712
|
|
||||||
|
Acquisition related costs
|
1,826
|
|
3,352
|
|
(937
|
)
|
||||||
|
Total operating expenses
|
275,148
|
|
240,198
|
|
225,240
|
|
||||||
|
Operating income
|
154,141
|
|
172,222
|
|
138,362
|
|
||||||
|
Other income, net:
|
||||||||||||
|
Interest income, net
|
3,057
|
|
1,304
|
|
805
|
|
||||||
|
Foreign currency transaction loss
|
(482
|
)
|
(1,159
|
)
|
(899
|
)
|
||||||
|
Other income
|
563
|
|
438
|
|
374
|
|
||||||
|
Total other income, net
|
3,138
|
|
583
|
|
280
|
|
||||||
|
Income before income taxes
|
157,279
|
|
172,805
|
|
138,642
|
|
||||||
|
Income tax provision
|
52,938
|
|
60,021
|
|
46,157
|
|
||||||
|
Net income
|
$
|
104,341
|
|
$
|
112,784
|
|
$
|
92,485
|
|
|||
|
Earnings per share:
|
||||||||||||
|
Basic
|
$
|
1.09
|
|
$
|
1.19
|
|
$
|
0.98
|
|
|||
|
Diluted
|
$
|
1.08
|
|
$
|
1.17
|
|
$
|
0.97
|
|
|||
|
Weighted average shares outstanding:
|
||||||||||||
|
Basic
|
95,647
|
|
95,046
|
|
94,227
|
|
||||||
|
Dilutive stock options
|
785
|
|
1,027
|
|
1,230
|
|
||||||
|
Diluted
|
96,432
|
|
96,073
|
|
95,457
|
|
||||||
|
Anti-dilutive stock options excluded from weighted average calculation
|
5,481
|
|
3,348
|
|
1,666
|
|
||||||
|
Year Ended
|
||||||||||||
|
(In thousands)
|
December 31,
2016 |
December 31,
2015 |
December 31,
2014 |
|||||||||
|
Net income
|
$
|
104,341
|
|
$
|
112,784
|
|
$
|
92,485
|
|
|||
|
Other comprehensive loss:
|
||||||||||||
|
Unrealized loss on marketable securities, net of tax
|
(48
|
)
|
(55
|
)
|
(96
|
)
|
||||||
|
Foreign currency translation loss
|
(6,636
|
)
|
(246
|
)
|
(552
|
)
|
||||||
|
Total other comprehensive loss
|
(6,684
|
)
|
(301
|
)
|
(648
|
)
|
||||||
|
Comprehensive income
|
$
|
97,657
|
|
$
|
112,483
|
|
$
|
91,837
|
|
|||
|
Class A Common stock
|
Class B Common Stock
|
Additional paid-in capital
|
Accumulated other comprehensive income
|
Retained earnings
|
||||||||||||||||||||||||||
|
(In thousands)
|
Shares
|
$
|
Shares
|
$
|
Total
|
|||||||||||||||||||||||||
|
Balance at December 31, 2013
|
66,065
|
|
$
|
66
|
|
27,378
|
|
$
|
27
|
|
$
|
153,987
|
|
$
|
(1,009
|
)
|
$
|
319,289
|
|
$
|
472,360
|
|
||||||||
|
Conversion to Class A
|
3,500
|
|
3
|
|
(3,500
|
)
|
(3
|
)
|
—
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
7,111
|
|
—
|
|
—
|
|
7,111
|
|
||||||||||||||
|
Exercise of stock options
|
1,263
|
|
2
|
|
—
|
|
—
|
|
9,736
|
|
—
|
|
—
|
|
9,738
|
|
||||||||||||||
|
Tax benefit related to nonqualified stock options exercised
|
—
|
|
—
|
|
—
|
|
—
|
|
4,408
|
|
—
|
|
—
|
|
4,408
|
|
||||||||||||||
|
Comprehensive income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(648
|
)
|
92,485
|
|
91,837
|
|
||||||||||||||
|
Balance at December 31, 2014
|
70,828
|
|
71
|
|
23,878
|
|
24
|
|
175,242
|
|
(1,657
|
)
|
411,774
|
|
585,454
|
|
||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
9,860
|
|
—
|
|
—
|
|
9,860
|
|
||||||||||||||
|
Exercise of stock options
|
614
|
|
—
|
|
—
|
|
—
|
|
5,477
|
|
—
|
|
—
|
|
5,477
|
|
||||||||||||||
|
Tax benefit related to nonqualified stock options exercised
|
—
|
|
—
|
|
—
|
|
—
|
|
2,050
|
|
—
|
|
—
|
|
2,050
|
|
||||||||||||||
|
Comprehensive income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(301
|
)
|
112,784
|
|
112,483
|
|
||||||||||||||
|
Balance at December 31, 2015
|
71,442
|
|
71
|
|
23,878
|
|
24
|
|
192,629
|
|
(1,958
|
)
|
524,558
|
|
715,324
|
|
||||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
11,652
|
|
—
|
|
—
|
|
11,652
|
|
||||||||||||||
|
Exercise of stock options
|
610
|
|
1
|
|
—
|
|
—
|
|
5,873
|
|
—
|
|
—
|
|
5,874
|
|
||||||||||||||
|
Tax benefit related to nonqualified stock options exercised
|
—
|
|
—
|
|
—
|
|
—
|
|
1,571
|
|
—
|
|
—
|
|
1,571
|
|
||||||||||||||
|
Comprehensive income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(6,684
|
)
|
104,341
|
|
97,657
|
|
||||||||||||||
|
Balance at December 31, 2016
|
72,052
|
|
$
|
72
|
|
23,878
|
|
$
|
24
|
|
$
|
211,725
|
|
$
|
(8,642
|
)
|
$
|
628,899
|
|
$
|
832,078
|
|
||||||||
|
Year Ended
|
|||||||||||
|
(In thousands)
|
December 31,
2016 |
December 31,
2015 |
December 31,
2014 |
||||||||
|
Cash flows from operating activities:
|
|||||||||||
|
Net income
|
$
|
104,341
|
|
$
|
112,784
|
|
$
|
92,485
|
|
||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
|||||||||||
|
Depreciation and amortization
|
38,771
|
|
24,084
|
|
21,754
|
|
|||||
|
Amortization of premium on marketable securities
|
4,068
|
|
3,354
|
|
2,680
|
|
|||||
|
Write-down for excess and obsolete inventories
|
12,836
|
|
9,924
|
|
6,962
|
|
|||||
|
Stock-based compensation expense
|
11,382
|
|
9,639
|
|
7,111
|
|
|||||
|
Excess tax benefit related to nonqualified stock options
|
(1,571
|
)
|
(2,050
|
)
|
(4,408
|
)
|
|||||
|
Allowance for doubtful accounts
|
685
|
|
1,465
|
|
318
|
|
|||||
|
Change in fair value of contingent consideration
|
2,866
|
|
3,118
|
|
(1,131
|
)
|
|||||
|
Non-cash settlement of accrued expenses
|
(4,632
|
)
|
(8,405
|
)
|
—
|
|
|||||
|
Impairment of intangible assets
|
3,472
|
|
—
|
|
—
|
|
|||||
|
Change in deferred income taxes
|
(3,810
|
)
|
6,235
|
|
(4,379
|
)
|
|||||
|
(Increase)/decrease in:
|
|||||||||||
|
Restricted cash
|
25,641
|
|
(2,749
|
)
|
(23,370
|
)
|
|||||
|
Accounts receivable
|
(4,668
|
)
|
(4,193
|
)
|
(12,667
|
)
|
|||||
|
Inventories
|
(10,503
|
)
|
(19,327
|
)
|
(18,001
|
)
|
|||||
|
Prepaid expenses and other assets
|
4,568
|
|
(1,203
|
)
|
(249
|
)
|
|||||
|
Increase/(decrease) in:
|
|||||||||||
|
Accounts payable
|
(23
|
)
|
(3,825
|
)
|
4,628
|
|
|||||
|
Accounts payable to related party
|
—
|
|
(5,359
|
)
|
2,703
|
|
|||||
|
Accrued expenses and other liabilities
|
(18,164
|
)
|
(878
|
)
|
5,149
|
|
|||||
|
Income taxes payable/receivable
|
6,634
|
|
(657
|
)
|
(413
|
)
|
|||||
|
Net cash provided by operating activities
|
171,893
|
|
121,957
|
|
79,172
|
|
|||||
|
Cash flows from investing activities:
|
|||||||||||
|
Purchases of marketable securities
|
(287,263
|
)
|
(297,707
|
)
|
(251,422
|
)
|
|||||
|
Maturities of marketable securities
|
281,885
|
|
188,702
|
|
184,567
|
|
|||||
|
Sales of marketable securities
|
52,802
|
|
57,728
|
|
27,737
|
|
|||||
|
Purchases of property and equipment
|
(40,909
|
)
|
(50,760
|
)
|
(24,754
|
)
|
|||||
|
Issuance of note receivable
|
(30,000
|
)
|
—
|
|
—
|
|
|||||
|
Acquisition of businesses, net of cash acquired
|
(76,068
|
)
|
(48,513
|
)
|
(36,128
|
)
|
|||||
|
Net cash used in investing activities
|
(99,553
|
)
|
(150,550
|
)
|
(100,000
|
)
|
|||||
|
Cash flows from financing activities:
|
|||||||||||
|
Payment of business acquisition liabilities
|
(5,404
|
)
|
(1,200
|
)
|
(1,200
|
)
|
|||||
|
Proceeds from exercise of stock options
|
5,874
|
|
5,477
|
|
9,738
|
|
|||||
|
Excess tax benefit related to nonqualified stock options
|
1,571
|
|
2,050
|
|
4,408
|
|
|||||
|
Net cash provided by financing activities
|
2,041
|
|
6,327
|
|
12,946
|
|
|||||
|
Effect of foreign exchange rate on cash
|
(1,894
|
)
|
153
|
|
185
|
|
|||||
|
Net increase/(decrease) in cash and cash equivalents
|
72,487
|
|
(22,113
|
)
|
(7,697
|
)
|
|||||
|
Cash and cash equivalents, beginning of period
|
60,152
|
|
82,265
|
|
89,962
|
|
|||||
|
Cash and cash equivalents, end of period
|
$
|
132,639
|
|
$
|
60,152
|
|
$
|
82,265
|
|
||
|
Supplemental disclosures of cash flow information:
|
|||||||||||
|
Interest paid
|
35
|
|
9
|
|
32
|
|
|||||
|
Income taxes paid
|
$
|
50,087
|
|
$
|
57,100
|
|
$
|
51,096
|
|
||
|
(In thousands)
|
|||
|
Consideration:
|
|||
|
Cash paid at closing
|
$
|
80,000
|
|
|
Net working capital adjustment due
|
(2,217
|
)
|
|
|
Fair value of consideration
|
$
|
77,783
|
|
|
Identifiable assets acquired and liabilities assumed:
|
|||
|
Cash acquired
|
$
|
4,010
|
|
|
Accounts receivable
|
12,402
|
|
|
|
Inventory
|
10,579
|
|
|
|
Customer relationships
|
38,800
|
|
|
|
Property and equipment
|
4,800
|
|
|
|
Deferred tax assets
|
1,436
|
|
|
|
Other assets
|
8,092
|
|
|
|
Accounts payable and accrued expenses
|
(8,119
|
)
|
|
|
Deferred tax liabilities
|
(9,002
|
)
|
|
|
Total identifiable net assets
|
62,998
|
|
|
|
Goodwill
|
14,785
|
|
|
|
Total allocated purchase price
|
$
|
77,783
|
|
|
Year Ended
|
|||||||
|
(pro forma, unaudited, in thousands, except per share amounts)
|
December 31,
2016 |
December 31,
2015 |
|||||
|
Net sales
|
$
|
595,698
|
|
$
|
598,386
|
|
|
|
Net income
|
110,611
|
|
115,181
|
|
|||
|
Earnings per share:
|
|||||||
|
Basic
|
$
|
1.16
|
|
$
|
1.21
|
|
|
|
Diluted
|
$
|
1.15
|
|
$
|
1.20
|
|
|
|
(In thousands)
|
|||
|
Consideration:
|
|||
|
Cash paid at closing
|
$
|
57,042
|
|
|
Deferred consideration
|
5,290
|
|
|
|
Closing adjustments payable
|
944
|
|
|
|
Fair value of consideration
|
$
|
63,276
|
|
|
Identifiable assets acquired and liabilities assumed:
|
|||
|
Cash acquired
|
$
|
9,026
|
|
|
Accounts receivable
|
88
|
|
|
|
Inventory
|
4,753
|
|
|
|
Other assets
|
444
|
|
|
|
Property and equipment
|
14,862
|
|
|
|
Accounts payable and accrued expenses
|
(1,585
|
)
|
|
|
Deferred tax liability, net
|
(3,280
|
)
|
|
|
Total identifiable net assets
|
24,308
|
|
|
|
Goodwill
|
38,968
|
|
|
|
Total allocated purchase price
|
$
|
63,276
|
|
|
Year Ended
|
|||||||
|
(pro forma, unaudited, in thousands, except per share amounts)
|
December 31,
2015 |
December 31,
2014 |
|||||
|
Net sales
|
$
|
544,578
|
|
$
|
474,544
|
|
|
|
Net income
|
115,915
|
|
92,945
|
|
|||
|
Earnings per share:
|
|||||||
|
Basic
|
$
|
1.22
|
|
$
|
0.99
|
|
|
|
Diluted
|
$
|
1.21
|
|
$
|
0.97
|
|
|
|
(In thousands)
|
||||
|
Consideration:
|
||||
|
Cash paid at closing
|
$
|
36,128
|
|
|
|
Contingent consideration
|
11,300
|
|
(1)
|
|
|
Fair value of consideration
|
$
|
47,428
|
|
|
|
Identifiable assets acquired and liabilities assumed:
|
||||
|
Inventory
|
$
|
9,599
|
|
|
|
Supplier network
|
4,000
|
|
||
|
Customer relationships
|
1,600
|
|
||
|
Accounts receivable
|
1,529
|
|
||
|
Equipment
|
518
|
|
||
|
Trade names
|
300
|
|
||
|
Other assets
|
292
|
|
||
|
Accounts payable and accrued expenses
|
(5,034
|
)
|
||
|
Total identifiable net assets
|
12,804
|
|
||
|
|
||||
|
Goodwill
|
34,624
|
|
||
|
Total allocated purchase price
|
$
|
47,428
|
|
|
|
(1)
|
The contingent consideration relates to the achievement of certain sales milestones. As of December 31, 2014, the aggregate, undiscounted amount of contingent consideration that we could pay related to the acquisitions ranges from zero to
$15.0 million
(see
“Note 6. Fair Value Measurements”
below).
|
|
December 31, 2016
|
|||||||||||||
|
(In thousands)
|
Weighted-
Average Amortization Period (in years) |
Gross
Carrying Amount |
Accumulated Amortization
|
Intangible
Assets, net |
|||||||||
|
In-process research & development
|
—
|
$
|
20,460
|
|
$
|
—
|
|
$
|
20,460
|
|
|||
|
Supplier network
|
10.0
|
4,000
|
|
(867
|
)
|
3,133
|
|
||||||
|
Customer relationships & other intangibles
|
6.8
|
40,936
|
|
(5,201
|
)
|
35,735
|
|
||||||
|
Patents
|
16.1
|
3,035
|
|
(657
|
)
|
2,378
|
|
||||||
|
Total intangible assets
|
$
|
68,431
|
|
$
|
(6,725
|
)
|
$
|
61,706
|
|
||||
|
December 31, 2015
|
|||||||||||||
|
(In thousands)
|
Weighted-
Average Amortization Period (in years) |
Gross
Carrying Amount |
Accumulated Amortization
|
Intangible
Assets, net |
|||||||||
|
In-process research & development
|
—
|
$
|
24,560
|
|
$
|
—
|
|
$
|
24,560
|
|
|||
|
Supplier network
|
10.0
|
4,000
|
|
(467
|
)
|
3,533
|
|
||||||
|
Customer relationships & other intangibles
|
7.3
|
5,525
|
|
(2,384
|
)
|
3,141
|
|
||||||
|
Patents
|
17.0
|
2,495
|
|
(487
|
)
|
2,008
|
|
||||||
|
Total intangible assets
|
$
|
36,580
|
|
$
|
(3,338
|
)
|
$
|
33,242
|
|
||||
|
(In thousands)
|
Annual Amortization
|
|||
|
Year ending December 31:
|
||||
|
2017
|
$
|
6,889
|
|
|
|
2018
|
6,338
|
|
||
|
2019
|
6,200
|
|
||
|
2020
|
5,934
|
|
||
|
2021
|
5,698
|
|
||
|
Thereafter
|
10,187
|
|
||
|
Total
|
$
|
41,246
|
|
|
|
December 31, 2016
|
|||||||||||||||||
|
(In thousands)
|
Contractual Maturity
(in years)
|
Amortized Cost
|
Gross Unrealized Gains
|
Gross Unrealized Losses
|
Fair Value
|
||||||||||||
|
Short-term:
|
|||||||||||||||||
|
Municipal bonds
|
Less than 1
|
$
|
114,826
|
|
$
|
2
|
|
$
|
(88
|
)
|
$
|
114,740
|
|
||||
|
Corporate debt securities
|
Less than 1
|
36,020
|
|
21
|
|
(4
|
)
|
36,037
|
|
||||||||
|
Commercial paper
|
Less than 1
|
6,898
|
|
—
|
|
(2
|
)
|
6,896
|
|
||||||||
|
Total short-term marketable securities
|
$
|
157,744
|
|
$
|
23
|
|
$
|
(94
|
)
|
$
|
157,673
|
|
|||||
|
Long-term:
|
|||||||||||||||||
|
Municipal bonds
|
1-2
|
$
|
30,207
|
|
$
|
—
|
|
$
|
(137
|
)
|
$
|
30,070
|
|
||||
|
Corporate debt securities
|
1-2
|
15,278
|
|
9
|
|
(40
|
)
|
15,247
|
|
||||||||
|
Asset backed securities
|
1-2
|
10,146
|
|
6
|
|
(1
|
)
|
10,151
|
|
||||||||
|
Securities of U.S. government-sponsored agencies
|
1-2
|
5,002
|
|
—
|
|
(26
|
)
|
4,976
|
|
||||||||
|
Total long-term marketable securities
|
$
|
60,633
|
|
$
|
15
|
|
$
|
(204
|
)
|
$
|
60,444
|
|
|||||
|
December 31, 2015
|
|||||||||||||||||
|
(In thousands)
|
Contractual Maturity
(in years)
|
Amortized Cost
|
Gross Unrealized Gains
|
Gross Unrealized Losses
|
Fair Value
|
||||||||||||
|
Short-term:
|
|||||||||||||||||
|
Municipal bonds
|
Less than 1
|
$
|
108,402
|
|
$
|
15
|
|
$
|
(81
|
)
|
$
|
108,336
|
|
||||
|
Corporate debt securities
|
Less than 1
|
53,759
|
|
2
|
|
(57
|
)
|
53,704
|
|
||||||||
|
Commercial paper
|
Less than 1
|
42,149
|
|
3
|
|
(1
|
)
|
42,151
|
|
||||||||
|
Securities of U.S. government-sponsored agencies
|
Less than 1
|
14,511
|
|
4
|
|
(4
|
)
|
14,511
|
|
||||||||
|
Asset backed securities
|
Less than 1
|
2,175
|
|
—
|
|
—
|
|
2,175
|
|
||||||||
|
Total short-term marketable securities
|
$
|
220,996
|
|
$
|
24
|
|
$
|
(143
|
)
|
$
|
220,877
|
|
|||||
|
Long-term:
|
|||||||||||||||||
|
Municipal bonds
|
1-2
|
$
|
18,508
|
|
$
|
—
|
|
$
|
(25
|
)
|
$
|
18,483
|
|
||||
|
Corporate debt securities
|
1-2
|
12,033
|
|
—
|
|
(25
|
)
|
12,008
|
|
||||||||
|
Asset backed securities
|
1-2
|
18,294
|
|
—
|
|
(23
|
)
|
18,271
|
|
||||||||
|
Total long-term marketable securities
|
$
|
48,835
|
|
$
|
—
|
|
$
|
(73
|
)
|
$
|
48,762
|
|
|||||
|
Balance at
|
|||||||||||||||
|
(In thousands)
|
December 31,
2016 |
Level 1
|
Level 2
|
Level 3
|
|||||||||||
|
Assets
|
|||||||||||||||
|
Cash equivalents
|
$
|
76,157
|
|
$
|
957
|
|
$
|
75,200
|
|
$
|
—
|
|
|||
|
Municipal bonds
|
144,810
|
|
—
|
|
144,810
|
|
—
|
|
|||||||
|
Corporate debt securities
|
51,284
|
|
—
|
|
51,284
|
|
—
|
|
|||||||
|
Commercial paper
|
6,896
|
|
—
|
|
6,896
|
|
—
|
|
|||||||
|
Asset-backed securities
|
10,151
|
|
—
|
|
10,151
|
|
—
|
|
|||||||
|
Securities of U.S. government-sponsored agencies
|
4,976
|
|
—
|
|
4,976
|
|
—
|
|
|||||||
|
Liabilities
|
|||||||||||||||
|
Contingent consideration
|
19,849
|
|
—
|
|
—
|
|
19,849
|
|
|||||||
|
Balance at
|
|||||||||||||||
|
(In thousands)
|
December 31,
2015 |
Level 1
|
Level 2
|
Level 3
|
|||||||||||
|
Assets
|
|||||||||||||||
|
Cash equivalents
|
$
|
12,700
|
|
$
|
1,701
|
|
$
|
10,999
|
|
$
|
—
|
|
|||
|
Municipal bonds
|
126,819
|
|
—
|
|
126,819
|
|
—
|
|
|||||||
|
Corporate debt securities
|
65,712
|
|
—
|
|
65,712
|
|
—
|
|
|||||||
|
Commercial paper
|
42,151
|
|
—
|
|
42,151
|
|
—
|
|
|||||||
|
Asset-backed securities
|
20,446
|
|
—
|
|
20,446
|
|
—
|
|
|||||||
|
Securities of U.S. government-sponsored agencies
|
14,511
|
|
—
|
|
14,511
|
|
—
|
|
|||||||
|
Liabilities
|
|||||||||||||||
|
Contingent consideration
|
26,617
|
|
—
|
|
—
|
|
26,617
|
|
|||||||
|
Fair Value at
|
||||||||||||||
|
(In thousands)
|
December 31,
2016 |
Valuation technique
|
Unobservable input
|
Range
|
||||||||||
|
Discount rate
|
3.1
|
%
|
-
|
8.5
|
%
|
|||||||||
|
Revenue-based payments
|
$
|
15,218
|
|
Discounted cash flow
|
Probability of payment
|
87.0
|
%
|
-
|
97.5
|
%
|
||||
|
Projected year of payment
|
2017
|
|
-
|
2029
|
|
|||||||||
|
Discount rate
|
5.3
|
%
|
-
|
13.5
|
%
|
|||||||||
|
Milestone-based payments
|
$
|
4,631
|
|
Discounted cash flow
|
Probability of payment
|
80.0
|
%
|
-
|
100.0
|
%
|
||||
|
Projected year of payment
|
2016
|
|
-
|
2020
|
|
|||||||||
|
Year Ended
|
||||||||
|
(In thousands)
|
December 31,
2016 |
December 31,
2015 |
||||||
|
Beginning balance
|
$
|
26,617
|
|
$
|
24,335
|
|
||
|
Purchase price contingent consideration
|
—
|
|
—
|
|
||||
|
Contingent payments
|
(5,002
|
)
|
(836
|
)
|
||||
|
Non-cash settlement of certain contingent consideration
|
(4,632
|
)
|
—
|
|
||||
|
Changes in fair value of contingent consideration
|
2,866
|
|
3,118
|
|
||||
|
Ending balance
|
$
|
19,849
|
|
$
|
26,617
|
|
||
|
(In thousands)
|
December 31, 2016
|
December 31, 2015
|
|||||
|
Raw materials
|
$
|
13,257
|
|
$
|
12,308
|
|
|
|
Work in process
|
10,747
|
|
7,091
|
|
|||
|
Finished goods
|
88,688
|
|
85,861
|
|
|||
|
Total
|
$
|
112,692
|
|
$
|
105,260
|
|
|
|
(In thousands)
|
Useful Life
|
December 31, 2016
|
December 31, 2015
|
|||||||
|
Land
|
—
|
$
|
8,271
|
|
$
|
8,254
|
|
|||
|
Buildings and improvements
|
30
|
22,225
|
|
19,809
|
|
|||||
|
Equipment
|
5-7
|
49,919
|
|
40,998
|
|
|||||
|
Instruments
|
3
|
173,668
|
|
147,961
|
|
|||||
|
Modules and cases
|
3
|
21,692
|
|
28,519
|
|
|||||
|
Other property and equipment
|
3-5
|
15,165
|
|
8,316
|
|
|||||
|
290,940
|
|
253,857
|
|
|||||||
|
Less: accumulated depreciation
|
(166,711
|
)
|
(139,114
|
)
|
||||||
|
Total
|
$
|
124,229
|
|
$
|
114,743
|
|
||||
|
Year Ended
|
||||||||||||
|
(In thousands)
|
December 31, 2016
|
December 31, 2015
|
December 31, 2014
|
|||||||||
|
Depreciation
|
$
|
35,293
|
|
$
|
22,522
|
|
$
|
21,044
|
|
|||
|
(In thousands)
|
December 31,
2016 |
December 31,
2015 |
|||||
|
Compensation and other employee-related costs
|
$
|
23,214
|
|
$
|
21,151
|
|
|
|
Legal and other settlements and expenses
|
734
|
|
13,617
|
|
|||
|
Accrued non-income taxes
|
6,946
|
|
6,808
|
|
|||
|
Royalties
|
4,671
|
|
6,787
|
|
|||
|
Other
|
10,836
|
|
5,406
|
|
|||
|
Total accrued expenses
|
$
|
46,401
|
|
$
|
53,769
|
|
|
|
(Shares)
|
Class A Common
|
Class B
Common
|
Total
|
|||||
|
December 31, 2016
|
72,052,360
|
|
23,877,556
|
|
95,929,916
|
|
||
|
December 31, 2015
|
71,442,166
|
|
23,877,556
|
|
95,319,722
|
|
||
|
(In thousands)
|
Unrealized loss on marketable securities, net of tax
|
Foreign currency translation adjustments
|
Accumulated other comprehensive loss
|
||||||||||
|
Accumulated other comprehensive loss, net of tax, at December 31, 2014
|
$
|
(64
|
)
|
$
|
(1,593
|
)
|
$
|
(1,657
|
)
|
||||
|
Other comprehensive loss before reclassifications
|
(67
|
)
|
(246
|
)
|
(313
|
)
|
|||||||
|
Amounts reclassified from accumulated other comprehensive loss, net of tax
|
12
|
|
—
|
|
12
|
|
|||||||
|
Other comprehensive loss, net of tax
|
(55
|
)
|
(246
|
)
|
(301
|
)
|
|||||||
|
Accumulated other comprehensive loss, net of tax, at December 31, 2015
|
(119
|
)
|
(1,839
|
)
|
(1,958
|
)
|
|||||||
|
Other comprehensive loss before reclassifications
|
(74
|
)
|
(6,636
|
)
|
(6,710
|
)
|
|||||||
|
Amounts reclassified from accumulated other comprehensive loss, net of tax
|
26
|
|
—
|
|
26
|
|
|||||||
|
Other comprehensive loss, net of tax
|
(48
|
)
|
(6,636
|
)
|
(6,684
|
)
|
|||||||
|
Accumulated other comprehensive loss, net of tax, at December 31, 2016
|
$
|
(167
|
)
|
$
|
(8,475
|
)
|
$
|
(8,642
|
)
|
||||
|
Year Ended
|
||||||||||||
|
December 31,
2016 |
December 31,
2015 |
December 31,
2014 |
||||||||||
|
Weighted average grant date per share fair value
|
$
|
7.62
|
|
$
|
8.63
|
|
$
|
9.63
|
|
|||
|
Year Ended
|
|||||||||||||||||
|
December 31,
2016 |
December 31,
2015 |
December 31,
2014 |
|||||||||||||||
|
Risk-free interest rate
|
1.03
|
%
|
-
|
2.01
|
%
|
1.39
|
%
|
-
|
2.11
|
%
|
1.51
|
%
|
-
|
1.95
|
%
|
||
|
Expected term (years)
|
5.8
|
-
|
6.5
|
5.1
|
-
|
9.9
|
5.3
|
-
|
6.4
|
||||||||
|
Expected volatility
|
28.0
|
%
|
-
|
29.0
|
%
|
29.0
|
%
|
-
|
38.0
|
%
|
35.0
|
%
|
-
|
41.0
|
%
|
||
|
Expected dividend yield
|
—%
|
—%
|
—%
|
||||||||||||||
|
Option Shares (thousands)
|
Weighted average exercise price
|
Weighted average remaining contractual life (years)
|
Aggregate intrinsic value (thousands)
|
|||||||||
|
Outstanding at December 31, 2013
|
4,886
|
|
$
|
10.04
|
|
|||||||
|
Granted
|
1,495
|
|
23.45
|
|
||||||||
|
Exercised
|
(1,263
|
)
|
7.71
|
|
||||||||
|
Forfeited
|
(264
|
)
|
15.33
|
|
||||||||
|
Outstanding at December 31, 2014
|
4,854
|
|
14.50
|
|
||||||||
|
Granted
|
2,828
|
|
24.69
|
|
||||||||
|
Exercised
|
(614
|
)
|
8.92
|
|
||||||||
|
Forfeited
|
(391
|
)
|
17.84
|
|
||||||||
|
Outstanding at December 31, 2015
|
6,677
|
|
19.14
|
|
||||||||
|
Granted
|
2,309
|
|
24.22
|
|
||||||||
|
Exercised
|
(610
|
)
|
9.63
|
|
||||||||
|
Forfeited
|
(635
|
)
|
23.10
|
|
||||||||
|
Outstanding at December 31, 2016
|
7,741
|
|
21.08
|
|
7.6
|
$
|
30,608
|
|
||||
|
Exercisable at December 31, 2016
|
3,512
|
|
17.38
|
|
6.2
|
26,533
|
|
|||||
|
Expected to vest at December 31, 2016
|
4,229
|
|
$
|
24.15
|
|
8.7
|
$
|
4,074
|
|
|||
|
Year Ended
|
||||||||||||
|
(In thousands)
|
December 31,
2016 |
December 31,
2015 |
December 31,
2014 |
|||||||||
|
Intrinsic value of stock options exercised
|
$
|
8,824
|
|
$
|
9,984
|
|
$
|
20,216
|
|
|||
|
Stock-based compensation expense
|
$
|
11,382
|
|
$
|
9,639
|
|
$
|
7,111
|
|
|||
|
Net stock-based compensation capitalized into inventory
|
270
|
|
221
|
|
—
|
|
||||||
|
Total stock-based compensation cost
|
$
|
11,652
|
|
$
|
9,860
|
|
$
|
7,111
|
|
|||
|
Year ended
|
||||||||||||
|
(In thousands)
|
December 31, 2016
|
December 31, 2015
|
December 31, 2014
|
|||||||||
|
Domestic
|
$
|
154,377
|
|
$
|
171,278
|
|
$
|
137,643
|
|
|||
|
Foreign
|
2,902
|
|
1,527
|
|
999
|
|
||||||
|
Total
|
$
|
157,279
|
|
$
|
172,805
|
|
$
|
138,642
|
|
|||
|
Year ended
|
||||||||||||
|
(In thousands)
|
December 31, 2016
|
December 31, 2015
|
December 31, 2014
|
|||||||||
|
Current:
|
||||||||||||
|
Federal
|
$
|
51,785
|
|
$
|
45,813
|
|
$
|
43,561
|
|
|||
|
State
|
4,533
|
|
7,193
|
|
6,097
|
|
||||||
|
Foreign
|
748
|
|
673
|
|
519
|
|
||||||
|
57,066
|
|
53,679
|
|
50,177
|
|
|||||||
|
Deferred:
|
||||||||||||
|
Federal
|
(4,527
|
)
|
5,926
|
|
(3,630
|
)
|
||||||
|
State
|
204
|
|
480
|
|
(357
|
)
|
||||||
|
Foreign
|
195
|
|
(64
|
)
|
(33
|
)
|
||||||
|
(4,128
|
)
|
6,342
|
|
(4,020
|
)
|
|||||||
|
Total
|
$
|
52,938
|
|
$
|
60,021
|
|
$
|
46,157
|
|
|||
|
Year ended
|
|||||||||
|
December 31, 2016
|
December 31, 2015
|
December 31, 2014
|
|||||||
|
Statutory U.S. federal tax rate
|
35.0
|
%
|
35.0
|
%
|
35.0
|
%
|
|||
|
State income taxes, net of federal benefit
|
2.2
|
|
3.0
|
|
2.6
|
|
|||
|
Domestic production activities deduction
|
(2.7
|
)
|
(2.6
|
)
|
(2.7
|
)
|
|||
|
Tax credits
|
(1.3
|
)
|
(0.9
|
)
|
(1.2
|
)
|
|||
|
Nondeductible expenses
|
0.5
|
|
0.3
|
|
0.7
|
|
|||
|
Other
|
—
|
|
(0.1
|
)
|
(1.1
|
)
|
|||
|
Effective tax rate
|
33.7
|
%
|
34.7
|
%
|
33.3
|
%
|
|||
|
(In thousands)
|
December 31, 2016
|
December 31, 2015
|
||||||
|
Deferred tax assets:
|
||||||||
|
Inventory reserve
|
$
|
31,202
|
|
$
|
26,741
|
|
||
|
Accruals, reserves, and other currently not deductible
|
13,269
|
|
13,333
|
|
||||
|
Stock-based compensation
|
10,595
|
|
8,014
|
|
||||
|
Foreign net operating loss carryforwards
|
227
|
|
329
|
|
||||
|
Total deferred tax assets
|
55,293
|
|
48,417
|
|
||||
|
Valuation allowance
|
(83
|
)
|
(43
|
)
|
||||
|
Total deferred tax assets, net of valuation allowance
|
55,210
|
|
48,374
|
|
||||
|
Deferred tax liabilities:
|
||||||||
|
Depreciation and amortization
|
(32,448
|
)
|
(22,666
|
)
|
||||
|
Total deferred tax liabilities
|
(32,448
|
)
|
(22,666
|
)
|
||||
|
Net deferred tax assets
|
$
|
22,762
|
|
$
|
25,708
|
|
||
|
Year ended
|
||||||||||||
|
(In thousands)
|
December 31, 2016
|
December 31, 2015
|
December 31, 2014
|
|||||||||
|
Unrecognized tax benefits at the beginning of the year
|
$
|
1,575
|
|
$
|
3,228
|
|
$
|
3,978
|
|
|||
|
Additions related to current year tax positions
|
—
|
|
316
|
|
—
|
|
||||||
|
Additions related to prior year tax positions
|
287
|
|
261
|
|
614
|
|
||||||
|
Reductions related to prior year tax positions
|
—
|
|
(2,230
|
)
|
(209
|
)
|
||||||
|
Lapse of statute of limitations
|
—
|
|
—
|
|
(202
|
)
|
||||||
|
Settlements
|
—
|
|
—
|
|
(953
|
)
|
||||||
|
Unrecognized tax benefits at the end of the year
|
$
|
1,862
|
|
$
|
1,575
|
|
$
|
3,228
|
|
|||
|
(In thousands)
|
December 31, 2016
|
December 31, 2015
|
December 31, 2014
|
|||||||||
|
Portion of total unrecognized tax benefits that, if recognized, would affect the effective income tax rate
|
$
|
1,542
|
|
$
|
1,335
|
|
$
|
838
|
|
|||
|
(In thousands)
|
||||
|
Year ending December 31:
|
||||
|
2017
|
$
|
1,412
|
|
|
|
2018
|
795
|
|
||
|
2019
|
1,108
|
|
||
|
2020
|
74
|
|
||
|
2021
|
7
|
|
||
|
Thereafter
|
—
|
|
||
|
Total
|
$
|
3,396
|
|
|
|
Year ended
|
||||||||||||
|
(In thousands)
|
December 31, 2016
|
December 31, 2015
|
December 31, 2014
|
|||||||||
|
Rent expense
|
$
|
1,500
|
|
$
|
1,113
|
|
$
|
676
|
|
|||
|
Year ended
|
||||||||||||
|
(In thousands)
|
December 31, 2016
|
December 31, 2015
|
December 31, 2014
|
|||||||||
|
401(k) and other retirement plan contributions
|
$
|
2,772
|
|
$
|
2,303
|
|
$
|
2,185
|
|
|||
|
Year Ended
|
||||||||||||
|
(In thousands)
|
December 31,
2016 |
December 31,
2015 |
December 31,
2014 |
|||||||||
|
United States
|
$
|
500,226
|
|
$
|
498,191
|
|
$
|
427,091
|
|
|||
|
International
|
63,768
|
|
46,562
|
|
47,280
|
|
||||||
|
Total sales
|
$
|
563,994
|
|
$
|
544,753
|
|
$
|
474,371
|
|
|||
|
Year Ended
|
||||||||||||
|
(In thousands)
|
December 31,
2016 |
December 31,
2015 |
December 31,
2014 |
|||||||||
|
Innovative Fusion
|
$
|
287,594
|
|
$
|
288,062
|
|
$
|
270,852
|
|
|||
|
Disruptive Technology
|
276,400
|
|
256,691
|
|
203,519
|
|
||||||
|
Total sales
|
$
|
563,994
|
|
$
|
544,753
|
|
$
|
474,371
|
|
|||
|
(unaudited)
|
||||||||||||||||
|
(In thousands, except per share amounts)
|
March 31,
2016 |
June 30,
2016 |
September 30,
2016 |
December 31,
2016 |
||||||||||||
|
Sales
|
$
|
139,264
|
|
$
|
137,489
|
|
$
|
135,651
|
|
$
|
151,590
|
|
||||
|
Gross profit
|
107,745
|
|
104,758
|
|
104,198
|
|
112,588
|
|
||||||||
|
Net income
|
28,010
|
|
25,806
|
|
26,227
|
|
24,298
|
|
||||||||
|
Net earnings per common share - basic
|
0.29
|
|
0.27
|
|
0.27
|
|
0.25
|
|
||||||||
|
Net earnings per common share - diluted
|
0.29
|
|
0.27
|
|
0.27
|
|
0.25
|
|
||||||||
|
(unaudited)
|
||||||||||||||||
|
(In thousands, except per share amounts)
|
March 31,
2015 |
June 30,
2015 |
September 30,
2015 |
December 31,
2015 |
||||||||||||
|
Sales
|
$
|
131,604
|
|
$
|
133,570
|
|
$
|
136,992
|
|
$
|
142,587
|
|
||||
|
Gross profit
|
99,592
|
|
101,116
|
|
104,065
|
|
107,647
|
|
||||||||
|
Net income
|
24,648
|
|
24,054
|
|
26,481
|
|
37,601
|
|
||||||||
|
Net earnings per common share - basic
|
0.26
|
|
0.25
|
|
0.28
|
|
0.39
|
|
||||||||
|
Net earnings per common share - diluted
|
0.26
|
|
0.25
|
|
0.28
|
|
0.39
|
|
||||||||
|
•
|
recorded an updated depreciation and scrap methodology for instruments and cases included in December 31, 2016 financial statements
|
|
•
|
reviewed results using the updated methodology to validate the prior period adjustment
|
|
•
|
implementation of the new methodology within the fixed asset sub-ledger
|
|
•
|
additional account detail within the general ledger to provide added visibility to monitor amounts scrapped.
|
|
Page
|
|
|
Report of Independent Registered Public Accounting Firm
|
|
|
Consolidated Balance Sheets
|
|
|
Consolidated Statements of Income
|
|
|
Consolidated Statements of Comprehensive Income
|
|
|
Consolidated Statements of Equity
|
|
|
Consolidated Statements of Cash Flows
|
|
|
Notes to Consolidated Financial Statements
|
|
|
(In thousands)
|
Beginning of period
|
Additions
|
Write-offs
|
End of period
|
|||||||||||
|
Year ended December 31, 2014
|
$
|
1,581
|
|
$
|
318
|
|
$
|
(252
|
)
|
$
|
1,647
|
|
|||
|
Year ended December 31, 2015
|
1,647
|
|
1,465
|
|
(599
|
)
|
2,513
|
|
|||||||
|
Year ended December 31, 2016
|
$
|
2,513
|
|
$
|
865
|
|
$
|
(607
|
)
|
$
|
2,771
|
|
|||
|
(In thousands)
|
Beginning of period
|
Additions
|
Write-offs
|
End of period
|
|||||||||||
|
Year ended December 31, 2014
|
$
|
327
|
|
$
|
—
|
|
$
|
(282
|
)
|
$
|
45
|
|
|||
|
Year ended December 31, 2015
|
45
|
|
—
|
|
(2
|
)
|
43
|
|
|||||||
|
Year ended December 31, 2016
|
$
|
43
|
|
$
|
40
|
|
$
|
—
|
|
$
|
83
|
|
|||
|
Exhibit No.
|
Item
|
|
|
2.1
|
Purchase and Sale Agreement, dated as of July 25, 2016, by and among Globus Medical Ireland, Ltd., and Alphatec Holdings, Inc. (incorporated by reference to Exhibit 2.1 of Globus Medical Inc.’s Current Report on Form 8-K filed on July 27, 2016).
|
|
|
2.2
|
First Amendment to Purchase and Sale Agreement, dated as of September 1, 2016, by and among Globus Medical Ireland, Ltd. and Alphatec Holdings, Inc. (incorporated by reference to Exhibit 2.1 of Globus Medical Inc.’s Current Report on Form 8-K filed on September 2, 2016).
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation of Globus Medical, Inc. (incorporated by reference to Exhibit 3.1 of the Registrant’s Amendment No. 5 to the Registration Statement on Form S-1 filed on August 2, 2012).
|
|
|
3.2
|
Certificate of Amendment of the Amended and Restated Certificate of Incorporation, dated July 31, 2012 (incorporated by reference to Exhibit 3.2 of the Registrant’s Amendment No. 5 to the Registration Statement on Form S-1 filed on August 2, 2012).
|
|
|
3.3
|
Certificate of Amendment of the Amended and Restated Certificate of Incorporation, dated August 20, 2012 (incorporated by reference to Exhibit 3.1 of the Registrant’s Form 10-Q/A filed on September 19, 2012).
|
|
|
3.4
|
Amended and Restated Bylaws of Globus Medical, Inc. (incorporated by reference to Exhibit 3.4 of the Registrant’s Form 10-K filed on February 29, 2016).
|
|
|
4.1
|
Specimen Certificate for Class A Common Stock (incorporated by reference to Exhibit 4.1 of the Registrant’s Amendment No. 3 to the Registration Statement on Form S-1 filed on July 16, 2012).
|
|
|
10.1
|
Globus Medical, Inc. Amended and Restated 2003 Stock Plan (incorporated by reference to Exhibit 10.4 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.2
|
First Amendment to the Globus Medical, Inc. Amended and Restated 2003 Stock Plan (incorporated by reference to Exhibit 10.5 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.3
|
Globus Medical, Inc. 2008 Stock Plan (incorporated by reference to Exhibit 10.6 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.4
|
Globus Medical, Inc. 2012 Equity Incentive Plan (incorporated by reference to Exhibit 10.7 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.5
|
Form of Grant Notice and Stock Option Agreement under 2003 Stock Plan (incorporated by reference to Exhibit 10.8 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.6
|
Form of Grant Notice and Stock Option Agreement under 2008 Stock Plan (incorporated by reference to Exhibit 10.9 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.7
|
Form of Incentive Stock Option Grant Notice and Incentive Stock Option Agreement under 2012 Equity Incentive Plan (incorporated by reference to Exhibit 10.10 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.8
|
Form of Nonqualified Stock Option Grant Notice and Nonqualified Stock Option Agreement under 2012 Equity Incentive Plan (incorporated by reference to Exhibit 10.11 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.9
|
Vice President Employment Agreement, dated June 1, 2005, by and between Globus Medical, Inc. and Brett Murphy (incorporated by reference to Exhibit 10.13 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.10
|
First Amendment to Vice President Employment Agreement, dated November 1, 2006, by and between Globus Medical, Inc. and Brett Murphy (incorporated by reference to Exhibit 10.14 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.11
|
Second Amendment to Vice President Employment Agreement, dated February 8, 2011, by and between Globus Medical, Inc. and Brett Murphy (incorporated by reference to Exhibit 10.15 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.12
|
Form of Indemnification Agreement (incorporated by reference to Exhibit 10.18 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.13
|
Form of No Competition and Non-Disclosure Agreement (incorporated by reference to Exhibit 10.19 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.14
|
Agreement and Plan of Merger, dated as of February 24, 2015, by and among Branch Medical Group, Inc., Globus Medical, Inc., BM Acquisition, Inc. and Spine Therapy Technologies, Inc. (incorporated by reference to Exhibit 2.1 to our Current Report on Form 8-K filed on March 2, 2015).
|
|
|
10.15
|
Executive Employment Agreement, dated June 26, 2014, by and between Globus Medical, Inc. and Anthony L. Williams (incorporated by reference to Exhibit 10.1 to our Form 10-Q filed on May 5, 2015).
|
|
|
10.16
|
Employment Agreement, dated September 14, 2015, by and between Globus Medical, Inc. and David M. Demski (incorporated by reference to Exhibit 10.1 to our Current Report on Form 8-K filed on September 17, 2015).
|
|
|
10.17
|
Executive Employment Agreement, dated May 3, 2016 by and between Globus Medical, Inc. and Daniel T. Scavilla (incorporated by reference to Exhibit 10.1 to our Form 10-Q filed on May 4, 2016).
|
|
|
10.18
|
Credit Agreement, dated May 3, 2016, by and between Globus Medical, Inc. and Globus Medical North America, Inc., and Wells Fargo Bank, National Association (incorporated by reference to Exhibit 10.1 to our Form 10-Q filed on July 27, 2016).
|
|
|
21.1*
|
Subsidiaries of Globus Medical, Inc.
|
|
|
23.1*
|
Consent of independent registered public accounting firm - Grant Thornton LLP.
|
|
|
23.2*
|
Consent of independent registered public accounting firm - KPMG LLP.
|
|
|
31.1*
|
Certification by Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
31.2*
|
Certification by Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
32**
|
Certifications pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
101.INS*
|
XBRL Instance Document
|
|
|
101.SCH*
|
XBRL Taxonomy Extension Schema Document
|
|
|
101.CAL*
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
|
101.LAB*
|
XBRL Taxonomy Extension Label Linkbase Document
|
|
|
101.PRE*
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
|
101.DEF*
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
|
*
|
Filed herewith.
|
|
|
**
|
Furnished herewith.
|
|
|
GLOBUS MEDICAL, INC.
|
||
|
Dated:
|
March 16, 2017
|
/s/ DAVID C. PAUL
|
|
David C. Paul
|
||
|
Chairman
|
||
|
Chief Executive Officer
|
||
|
(Principal Executive Officer)
|
||
|
Dated:
|
March 16, 2017
|
/s/ DANIEL T. SCAVILLA
|
|
Daniel T. Scavilla
|
||
|
Senior Vice President
|
||
|
Chief Financial Officer
|
||
|
(Principal Financial Officer)
|
||
|
Dated:
|
March 16, 2017
|
/s/ STEVEN M. PAYNE
|
|
Steven M. Payne
|
||
|
Chief Accounting Officer
|
||
|
(Principal Accounting Officer)
|
||
|
SIGNATURE
|
TITLE
|
DATE
|
|
|
/s/ David C. Paul
|
Chief Executive Officer and Director
|
March 16, 2017
|
|
|
David C. Paul
|
(Principal Executive Officer)
|
||
|
/s/ Daniel T. Scavilla
|
Senior Vice President
|
March 16, 2017
|
|
|
Daniel T. Scavilla
|
and Chief Financial Officer
|
||
|
(Principal Financial Officer)
|
|||
|
/s/ Steven M. Payne
|
Chief Accounting Officer
|
March 16, 2017
|
|
|
Steven M. Payne
|
(Principal Accounting Officer)
|
||
|
/s/ David M. Demski
|
President, Emerging Technologies
|
March 16, 2017
|
|
|
David M. Demski
|
and Director
|
||
|
/s/ David D. Davidar
|
Director
|
March 16, 2017
|
|
|
David D. Davidar
|
|||
|
/s/ Kurt C. Wheeler
|
Director
|
March 16, 2017
|
|
|
Kurt C. Wheeler
|
|||
|
/s/ Robert W. Liptak
|
Director
|
March 16, 2017
|
|
|
Robert W. Liptak
|
|||
|
/s/ Daniel T. Lemaitre
|
Director
|
March 16, 2017
|
|
|
Daniel T. Lemaitre
|
|||
|
/s/ Ann D. Rhoads
|
Director
|
March 16, 2017
|
|
|
Ann D. Rhoads
|
|||
|
/s/ James R. Tobin
|
Director
|
March 16, 2017
|
|
|
James R. Tobin
|
|||
|
Exhibit No.
|
Item
|
|
|
2.1
|
Purchase and Sale Agreement, dated as of July 25, 2016, by and among Globus Medical Ireland, Ltd., and Alphatec Holdings, Inc. (incorporated by reference to Exhibit 2.1 of Globus Medical Inc.’s Current Report on Form 8-K filed on July 27, 2016).
|
|
|
2.2
|
First Amendment to Purchase and Sale Agreement, dated as of September 1, 2016, by and among Globus Medical Ireland, Ltd. and Alphatec Holdings, Inc. (incorporated by reference to Exhibit 2.1 of Globus Medical Inc.’s Current Report on Form 8-K filed on September 2, 2016).
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation of Globus Medical, Inc. (incorporated by reference to Exhibit 3.1 of the Registrant’s Amendment No. 5 to the Registration Statement on Form S-1 filed on August 2, 2012).
|
|
|
3.2
|
Certificate of Amendment of the Amended and Restated Certificate of Incorporation, dated July 31, 2012 (incorporated by reference to Exhibit 3.2 of the Registrant’s Amendment No. 5 to the Registration Statement on Form S-1 filed on August 2, 2012).
|
|
|
3.3
|
Certificate of Amendment of the Amended and Restated Certificate of Incorporation, dated August 20, 2012 (incorporated by reference to Exhibit 3.1 of the Registrant’s Form 10-Q/A filed on September 19, 2012).
|
|
|
3.4
|
Amended and Restated Bylaws of Globus Medical, Inc. (incorporated by reference to Exhibit 3.4 of the Registrant’s Form 10-K filed on February 29, 2016).
|
|
|
4.1
|
Specimen Certificate for Class A Common Stock (incorporated by reference to Exhibit 4.1 of the Registrant’s Amendment No. 3 to the Registration Statement on Form S-1 filed on July 16, 2012).
|
|
|
10.1
|
Globus Medical, Inc. Amended and Restated 2003 Stock Plan (incorporated by reference to Exhibit 10.4 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.2
|
First Amendment to the Globus Medical, Inc. Amended and Restated 2003 Stock Plan (incorporated by reference to Exhibit 10.5 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.3
|
Globus Medical, Inc. 2008 Stock Plan (incorporated by reference to Exhibit 10.6 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.4
|
Globus Medical, Inc. 2012 Equity Incentive Plan (incorporated by reference to Exhibit 10.7 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.5
|
Form of Grant Notice and Stock Option Agreement under 2003 Stock Plan (incorporated by reference to Exhibit 10.8 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.6
|
Form of Grant Notice and Stock Option Agreement under 2008 Stock Plan (incorporated by reference to Exhibit 10.9 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.7
|
Form of Incentive Stock Option Grant Notice and Incentive Stock Option Agreement under 2012 Equity Incentive Plan (incorporated by reference to Exhibit 10.10 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.8
|
Form of Nonqualified Stock Option Grant Notice and Nonqualified Stock Option Agreement under 2012 Equity Incentive Plan (incorporated by reference to Exhibit 10.11 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.9
|
Vice President Employment Agreement, dated June 1, 2005, by and between Globus Medical, Inc. and Brett Murphy (incorporated by reference to Exhibit 10.13 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.10
|
First Amendment to Vice President Employment Agreement, dated November 1, 2006, by and between Globus Medical, Inc. and Brett Murphy (incorporated by reference to Exhibit 10.14 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.11
|
Second Amendment to Vice President Employment Agreement, dated February 8, 2011, by and between Globus Medical, Inc. and Brett Murphy (incorporated by reference to Exhibit 10.15 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.12
|
Form of Indemnification Agreement (incorporated by reference to Exhibit 10.18 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.13
|
Form of No Competition and Non-Disclosure Agreement (incorporated by reference to Exhibit 10.19 of the Registrant’s Amendment No. 1 to the Registration Statement on Form S-1 filed on May 8, 2012).
|
|
|
10.14
|
Agreement and Plan of Merger, dated as of February 24, 2015, by and among Branch Medical Group, Inc., Globus Medical, Inc., BM Acquisition, Inc. and Spine Therapy Technologies, Inc. (incorporated by reference to Exhibit 2.1 to our Current Report on Form 8-K filed on March 2, 2015).
|
|
|
10.15
|
Executive Employment Agreement, dated June 26, 2014, by and between Globus Medical, Inc. and Anthony L. Williams (incorporated by reference to Exhibit 10.1 to our Form 10-Q filed on May 5, 2015).
|
|
|
10.16
|
Employment Agreement, dated September 14, 2015, by and between Globus Medical, Inc. and David M. Demski (incorporated by reference to Exhibit 10.1 to our Current Report on Form 8-K filed on September 17, 2015).
|
|
|
10.17
|
Executive Employment Agreement, dated May 3, 2016 by and between Globus Medical, Inc. and Daniel T. Scavilla (incorporated by reference to Exhibit 10.1 to our Form 10-Q filed on May 4, 2016).
|
|
|
10.18
|
Credit Agreement, dated May 3, 2016, by and between Globus Medical, Inc. and Globus Medical North America, Inc., and Wells Fargo Bank, National Association (incorporated by reference to Exhibit 10.1 to our Form 10-Q filed on July 27, 2016).
|
|
|
21.1*
|
Subsidiaries of Globus Medical, Inc.
|
|
|
23.1*
|
Consent of independent registered public accounting firm - Grant Thornton LLP.
|
|
|
23.2*
|
Consent of independent registered public accounting firm - KPMG LLP.
|
|
|
31.1*
|
Certification by Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
31.2*
|
Certification by Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|
|
32**
|
Certifications pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
101.INS*
|
XBRL Instance Document
|
|
|
101.SCH*
|
XBRL Taxonomy Extension Schema Document
|
|
|
101.CAL*
|
XBRL Taxonomy Extension Calculation Linkbase Document
|
|
|
101.LAB*
|
XBRL Taxonomy Extension Label Linkbase Document
|
|
|
101.PRE*
|
XBRL Taxonomy Extension Presentation Linkbase Document
|
|
|
101.DEF*
|
XBRL Taxonomy Extension Definition Linkbase Document
|
|
|
*
|
Filed herewith.
|
|
|
**
|
Furnished herewith.
|
|