|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ý
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
|
88-0488686
|
|
(State or other jurisdiction of incorporation or organization)
|
|
(I.R.S. Employer Identification No.)
|
|
11388 Sorrento Valley Road, San Diego, CA
|
|
92121
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
|
Large accelerated filer
ý
|
Accelerated filer
¨
|
|
Non-accelerated filer
¨
|
Smaller reporting company
¨
|
Emerging growth company
¨
|
|
(Do not check if a smaller reporting company)
|
|||||
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
¨
|
|||||
|
|
|
Page
|
||
|
Item 1.
|
||||
|
Three Months Ended March 30, 2017 and 2016
|
||||
|
March 31, 2017 and 2016
|
||||
|
Item 2.
|
||||
|
Item 3.
|
||||
|
Item 4.
|
||||
|
Item 1.
|
||||
|
Item 1A.
|
||||
|
Item 2.
|
||||
|
Item 3.
|
||||
|
Item 4.
|
||||
|
Item 5.
|
||||
|
Item 6.
|
||||
|
Item 1.
|
Financial Statements
|
|
March 31,
2017 |
December 31,
2016 |
|||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
45,188
|
|
$
|
66,764
|
|
||
|
Marketable securities, available-for-sale
|
133,797
|
|
138,217
|
|
||||
|
Accounts receivable, net
|
12,452
|
|
15,680
|
|
||||
|
Inventories
|
14,290
|
|
14,623
|
|
||||
|
Prepaid expenses and other assets
|
16,627
|
|
21,248
|
|
||||
|
Total current assets
|
222,354
|
|
256,532
|
|
||||
|
Property and equipment, net
|
3,741
|
|
4,264
|
|
||||
|
Prepaid expenses and other assets
|
172
|
|
219
|
|
||||
|
Restricted cash
|
500
|
|
500
|
|
||||
|
Total assets
|
$
|
226,767
|
|
$
|
261,515
|
|
||
|
LIABILITIES AND STOCKHOLDERS’ DEFICIT
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$
|
3,079
|
|
$
|
3,578
|
|
||
|
Accrued expenses
|
24,935
|
|
28,821
|
|
||||
|
Deferred revenue, current portion
|
4,093
|
|
4,793
|
|
||||
|
Current portion of long-term debt, net
|
29,601
|
|
17,393
|
|
||||
|
Total current liabilities
|
61,708
|
|
54,585
|
|
||||
|
Deferred revenue, net of current portion
|
38,802
|
|
39,825
|
|
||||
|
Long-term debt, net
|
184,430
|
|
199,228
|
|
||||
|
Other long-term liabilities
|
326
|
|
358
|
|
||||
|
Commitments and contingencies (Note 9)
|
|
|
||||||
|
Stockholders’ deficit:
|
||||||||
|
Preferred stock - $0.001 par value; 20,000 shares authorized; no shares
issued and outstanding
|
—
|
|
—
|
|
||||
|
Common stock - $0.001 par value; 200,000 shares authorized; 129,822 and
129,502 shares issued and outstanding at March 31, 2017 and
December 31, 2016, respectively
|
130
|
|
130
|
|
||||
|
Additional paid-in capital
|
559,659
|
|
552,737
|
|
||||
|
Accumulated other comprehensive loss
|
(49
|
)
|
(6
|
)
|
||||
|
Accumulated deficit
|
(618,239
|
)
|
(585,342
|
)
|
||||
|
Total stockholders’ deficit
|
(58,499
|
)
|
(32,481
|
)
|
||||
|
Total liabilities and stockholders’ deficit
|
$
|
226,767
|
|
$
|
261,515
|
|
||
|
Three Months Ended
March 31, |
||||||||
|
|
2017
|
2016
|
||||||
|
Revenues:
|
||||||||
|
Product sales, net
|
$
|
11,434
|
|
$
|
12,940
|
|
||
|
Royalties
|
13,982
|
|
11,387
|
|
||||
|
Revenues under collaborative agreements
|
4,152
|
|
18,172
|
|
||||
|
Total revenues
|
29,568
|
|
42,499
|
|
||||
|
Operating expenses:
|
||||||||
|
Cost of product sales
|
7,544
|
|
7,762
|
|
||||
|
Research and development
|
36,935
|
|
40,100
|
|
||||
|
Selling, general and administrative
|
12,615
|
|
10,806
|
|
||||
|
Total operating expenses
|
57,094
|
|
58,668
|
|
||||
|
Operating loss
|
(27,526
|
)
|
(16,169
|
)
|
||||
|
Other income (expense):
|
||||||||
|
Investment and other income, net
|
287
|
|
229
|
|
||||
|
Interest expense
|
(5,448
|
)
|
(3,876
|
)
|
||||
|
Net loss before income taxes
|
(32,687
|
)
|
(19,816
|
)
|
||||
|
Income tax expense
|
210
|
|
—
|
|
||||
|
Net loss
|
$
|
(32,897
|
)
|
$
|
(19,816
|
)
|
||
|
Net loss per share:
|
||||||||
|
Basic and diluted
|
$
|
(0.26
|
)
|
$
|
(0.16
|
)
|
||
|
Shares used in computing net loss per share:
|
||||||||
|
Basic and diluted
|
128,615
|
|
127,615
|
|
||||
|
Three Months Ended
March 31, |
||||||||
|
2017
|
2016
|
|||||||
|
Net loss
|
$
|
(32,897
|
)
|
$
|
(19,816
|
)
|
||
|
Other comprehensive (loss) income:
|
||||||||
|
Unrealized (loss) gain on marketable securities
|
(40
|
)
|
187
|
|
||||
|
Foreign currency translation adjustment
|
(4
|
)
|
—
|
|
||||
|
Unrealized gain on foreign currency
|
1
|
|
—
|
|
||||
|
Total comprehensive loss
|
$
|
(32,940
|
)
|
$
|
(19,629
|
)
|
||
|
Three Months Ended
March 31, |
||||||||
|
|
2017
|
2016
|
||||||
|
Operating activities:
|
||||||||
|
Net loss
|
$
|
(32,897
|
)
|
$
|
(19,816
|
)
|
||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||
|
Share-based compensation
|
7,315
|
|
5,817
|
|
||||
|
Depreciation and amortization
|
602
|
|
489
|
|
||||
|
Non-cash interest expense
|
487
|
|
1,054
|
|
||||
|
Payment-in-kind interest expense on long-term debt
|
—
|
|
1,890
|
|
||||
|
Amortization of premiums on marketable securities, net
|
16
|
|
213
|
|
||||
|
Loss on disposal of equipment
|
44
|
|
—
|
|
||||
|
Recognition of deferred revenue
|
(1,723
|
)
|
(1,524
|
)
|
||||
|
Recognition of deferred rent
|
(114
|
)
|
(70
|
)
|
||||
|
Other
|
(5
|
)
|
—
|
|
||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Accounts receivable, net
|
3,228
|
|
6,867
|
|
||||
|
Inventories
|
333
|
|
(856
|
)
|
||||
|
Prepaid expenses and other assets
|
4,668
|
|
(2,522
|
)
|
||||
|
Accounts payable and accrued expenses
|
(4,413
|
)
|
(4,565
|
)
|
||||
|
Net cash used in operating activities
|
(22,459
|
)
|
(13,023
|
)
|
||||
|
Investing activities:
|
||||||||
|
Purchases of marketable securities
|
(54,830
|
)
|
(126,431
|
)
|
||||
|
Proceeds from maturities of marketable securities
|
59,194
|
|
21,908
|
|
||||
|
Purchases of property and equipment
|
(99
|
)
|
(1,099
|
)
|
||||
|
Net cash provided by (used in) investing activities
|
4,265
|
|
(105,622
|
)
|
||||
|
Financing activities:
|
||||||||
|
Proceeds from issuance of long-term debt, net
|
—
|
|
148,046
|
|
||||
|
Repayment of long-term debt
|
(2,989
|
)
|
(3,885
|
)
|
||||
|
Proceeds from issuance of common stock under equity incentive plans, net of taxes paid related to net share settlement
|
(393
|
)
|
285
|
|
||||
|
Net cash (used in) provided by financing activities
|
(3,382
|
)
|
144,446
|
|
||||
|
Net (decrease) increase in cash, cash equivalents and restricted cash
|
(21,576
|
)
|
25,801
|
|
||||
|
Cash, cash equivalents and restricted cash at beginning of period
|
67,264
|
|
43,792
|
|
||||
|
Cash, cash equivalents and restricted cash at end of period
|
$
|
45,688
|
|
$
|
69,593
|
|
||
|
1.
|
The consideration is commensurate with either the entity’s performance to achieve the milestone or the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the entity’s performance to achieve the milestone;
|
|
2.
|
The consideration relates solely to past performance; and
|
|
3.
|
The consideration is reasonable relative to all of the deliverables and payment terms within the arrangement.
|
|
Standard
|
Description
|
Effective Date
|
Effect on the Financial
Statements or Other Significant Matters
|
|||
|
In July 2015, the FASB issued ASU 2015-11, Inventory: Simplifying the Measurement of Inventory.
|
The new guidance requires that for entities that measure inventory using the first-in, first-out method, inventory should be measured at the lower of cost or net realizable value. Topic 330, Inventory, currently requires an entity to measure inventory at the lower of cost or market. Market could be replacement cost, net realizable value, or net realizable value less an approximate normal profit margin. Net realizable value is the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation.
|
January 1, 2017.
|
The adoption did not have a material impact on our condensed consolidated financial position or results of operations.
|
|||
|
In August 2016, the FASB issued ASU 2016-15, Statement of Cash Flows: Classification of Certain Cash Receipts and Cash Payments.
In November 2016, the FASB issued ASU 2016-18, Statement of Cash Flows: Restricted Cash. |
Current U.S. GAAP either is unclear or does not include specific guidance on the eight cash flow classification issues included in ASU 2016-15. The new guidance is an improvement to U.S. GAAP and is intended to reduce the current and potential future diversity in practice. ASU 2016-18 provides additional classification guidance for restricted cash, which requires that restricted cash be included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows.
|
January 1, 2018. We have elected to early adopt as of January1, 2017.
|
Cash and cash equivalents at the beginning-of-period and end-of-period total amounts in the Condensed Consolidated Statements of Cash Flows have been adjusted to include $0.5 million of restricted cash for each of the periods presented.
|
|||
|
Standard
|
Description
|
Effective Date
|
Effect on the Financial
Statements or Other Significant Matters
|
|||
|
In January 2016, the FASB issued ASU 2016-01, Financial Instruments - Overall; Recognition and Measurement of Financial Assets and Financial Liabilities.
|
The new guidance supersedes the guidance to classify equity securities with readily determinable fair values into different categories (that is, trading or available-for-sale) and requires equity securities to be measured at fair value with changes in the fair value recognized through net income. The new guidance requires public business entities that are required to disclose fair value of financial instruments measured at amortized cost on the balance sheet to measure that fair value using the exit price notion consistent with Topic 820, Fair Value Measurement.
|
January 1, 2018.
|
We currently do not hold equity securities, and we are evaluating the effect that the updated standard will have on our consolidated financial statements and related disclosures.
|
|||
|
Standard
|
Description
|
Effective Date
|
Effect on the Financial
Statements or Other Significant Matters
|
|||
|
In May 2014, the FASB issued ASU 2014-09, Revenue from Contracts with Customers (Topic 606). In March, April, May and December 2016, the FASB issued additional guidance related to Topic 606.
|
The new standard will supersede nearly all existing revenue recognition guidance. Under Topic 606, an entity is required to recognize revenue upon transfer of promised goods or services to customers in an amount that reflects the expected consideration to be received in exchange for those goods or services. Topic 606 defines a five-step process in order to achieve this core principle, which may require the use of judgment and estimates, and also requires expanded qualitative and quantitative disclosures relating to the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers, including significant judgments and estimates used. The new standard also defines accounting for certain costs related to origination and fulfillment of contracts with customers, including whether such costs should be capitalized. The new standard permits adoption either by using (i) a full retrospective approach for all periods presented in the period of adoption or (ii) a modified retrospective approach where the new standard is applied in the financial statements starting with the year of adoption. Under both approaches, cumulative impact of the adoption is reflected as an adjustment to retained earnings (accumulated deficit) as of the earliest date presented in accordance with the new standard.
|
January 1, 2018. Early adoption is permitted.
|
We plan to implement the new guidance on January 1, 2018. We currently plan to adopt using the modified retrospective approach; however, a final decision regarding the adoption method has not been finalized at this time. We are currently evaluating the effect that the updated standard will have on our consolidated financial statements and related disclosures. However, we anticipate an impact to the timing of recognition of payments related to certain of our license and collaboration agreements
(1)
and the timing of recognition of our sales-based royalties.
(2)
We anticipate that this standard will have a material impact on our consolidated financial statements. Additional areas of impact may be identified as we continue our evaluation. We cannot reasonably estimate additional quantitative information related to the impact of the new standard on our consolidated financial statements at this time.
|
|||
|
In February 2016, the FASB issued ASU 2016-02, Leases.
|
The new guidance requires lessees to recognize assets and liabilities for most leases and provides enhanced disclosures.
|
January 1, 2019. Early adoption is permitted.
|
We are currently evaluating the effect that the updated standard will have on our consolidated financial statements and related disclosures. However, we anticipate recognition of additional assets and corresponding liabilities related to our leases on our consolidated balance sheet.
|
|||
|
(1)
|
Under the new standard, we are required to assess whether licenses granted under our collaboration and license agreements are distinct from other performance obligations and functional when granted. We expect that license-related amounts, including upfront payments, exclusive designation fees, annual license maintenance fees and sales-based milestones will be recognized, generally, when earned. Currently, these amounts related to certain of our license and collaboration agreements are being amortized over the term of the collaboration agreement. For example, during the year ended December 31, 2016, we recognized revenue from amortization of license payments of
$4.1 million
, and total deferred revenue related to license payments under collaboration agreements as of December 31, 2016 was
$43.9 million
. While we have not completed our evaluation at this time, we anticipate a potential reduction or elimination of our associated deferred revenue balances upon adoption of Topic 606.
|
|
(2)
|
Under the new standard, we expect sales-based royalties will be recognized in the quarter they are earned based on estimates, with true-up to actual results following in the subsequent quarter. Sales-based royalty revenue earned under our collaboration and license agreements is presently recognized when the royalty reports are made available. Upon adoption of Topic 606, we will evaluate and reduce our accumulated deficit and increase our accounts receivable, net, by the amount earned but not yet reported in our consolidated balance sheet at the time of adoption. We are establishing a process to estimate sales-based royalty revenues in the quarter in which the sales occur.
|
|
March 31, 2017
|
||||||||||||||||
|
Amortized Cost
|
Gross Unrealized Gains
|
Gross Unrealized Losses
|
Estimated Fair Value
|
|||||||||||||
|
Corporate debt securities
|
$
|
33,158
|
|
$
|
—
|
|
$
|
(18
|
)
|
$
|
33,140
|
|
||||
|
U.S. Treasury securities
|
70,002
|
|
1
|
|
(29
|
)
|
69,974
|
|
||||||||
|
Commercial paper
|
30,683
|
|
—
|
|
—
|
|
30,683
|
|
||||||||
|
$
|
133,843
|
|
$
|
1
|
|
$
|
(47
|
)
|
$
|
133,797
|
|
|||||
|
December 31, 2016
|
||||||||||||||||
|
Amortized Cost
|
Gross Unrealized Gains
|
Gross Unrealized Losses
|
Estimated Fair Value
|
|||||||||||||
|
Corporate debt securities
|
$
|
40,221
|
|
$
|
1
|
|
$
|
(15
|
)
|
$
|
40,207
|
|
||||
|
U.S. Treasury securities
|
94,002
|
|
24
|
|
(16
|
)
|
94,010
|
|
||||||||
|
Commercial paper
|
$
|
4,000
|
|
$
|
—
|
|
$
|
—
|
|
$
|
4,000
|
|
||||
|
$
|
138,223
|
|
$
|
25
|
|
$
|
(31
|
)
|
$
|
138,217
|
|
|||||
|
March 31, 2017
|
December 31, 2016
|
|||||||||||||||||||||||
|
Level 1
|
Level 2
|
Total estimated fair value
|
Level 1
|
Level 2
|
Total estimated fair value
|
|||||||||||||||||||
|
Cash equivalents:
|
||||||||||||||||||||||||
|
Money market funds
|
$
|
34,814
|
|
$
|
—
|
|
$
|
34,814
|
|
$
|
60,916
|
|
$
|
—
|
|
$
|
60,916
|
|
||||||
|
Commercial paper
|
4,000
|
|
4,000
|
|
—
|
|
—
|
|
—
|
|
||||||||||||||
|
Available-for-sale marketable
securities:
|
||||||||||||||||||||||||
|
Corporate debt securities
|
—
|
|
33,140
|
|
33,140
|
|
—
|
|
40,207
|
|
40,207
|
|
||||||||||||
|
U.S. Treasury securities
|
69,974
|
|
—
|
|
69,974
|
|
94,010
|
|
—
|
|
94,010
|
|
||||||||||||
|
Commercial paper
|
—
|
|
30,683
|
|
30,683
|
|
—
|
|
4,000
|
|
4,000
|
|
||||||||||||
|
$
|
104,788
|
|
$
|
67,823
|
|
$
|
172,611
|
|
$
|
154,926
|
|
$
|
44,207
|
|
$
|
199,133
|
|
|||||||
|
As of
March 31, 2017 |
|||
|
Upfront license fee payment for the application of rHuPH20 to the initial exclusive targets
|
$
|
20,000
|
|
|
Election of additional exclusive targets and annual license maintenance fees for the right to
designate the remaining targets as exclusive targets
|
23,000
|
|
|
|
Clinical development milestone payments
|
13,000
|
|
|
|
Regulatory milestone payments
|
8,000
|
|
|
|
Sales-based milestone payments
|
15,000
|
|
|
|
Total payments received
|
$
|
79,000
|
|
|
As of
March 31, 2017 |
|||
|
Upfront license fee payment for the application of rHuPH20 to the initial exclusive target
|
$
|
10,000
|
|
|
Regulatory milestone payments
|
3,000
|
|
|
|
Sales-based milestone payments
|
4,000
|
|
|
|
Total payments received
|
$
|
17,000
|
|
|
As of
March 31, 2017 |
|||
|
Lilly
|
$
|
33,000
|
|
|
AbbVie
|
29,000
|
|
|
|
Janssen
|
15,250
|
|
|
|
Pfizer
|
16,500
|
|
|
|
Total payments received
|
$
|
93,750
|
|
|
March 31,
2017 |
December 31,
2016 |
|||||||
|
Accounts receivable from product sales to collaborators
|
$
|
8,023
|
|
$
|
7,854
|
|
||
|
Accounts receivable from other product sales
|
2,090
|
|
2,234
|
|
||||
|
Accounts receivable from revenues under collaborative agreements
|
2,921
|
|
6,151
|
|
||||
|
Subtotal
|
13,034
|
|
16,239
|
|
||||
|
Allowance for distribution fees and discounts
|
(582
|
)
|
(559
|
)
|
||||
|
Total accounts receivable, net
|
$
|
12,452
|
|
$
|
15,680
|
|
||
|
March 31,
2017 |
December 31,
2016 |
|||||||
|
Raw materials
|
$
|
868
|
|
$
|
761
|
|
||
|
Work-in-process
|
12,885
|
|
12,850
|
|
||||
|
Finished goods
|
537
|
|
1,012
|
|
||||
|
Total inventories
|
$
|
14,290
|
|
$
|
14,623
|
|
||
|
March 31,
2017 |
December 31,
2016 |
|||||||
|
Prepaid manufacturing expenses
|
$
|
6,327
|
|
$
|
9,663
|
|
||
|
Prepaid research and development expenses
|
8,345
|
|
8,613
|
|
||||
|
Other prepaid expenses
|
1,525
|
|
1,661
|
|
||||
|
Other assets
|
602
|
|
1,530
|
|
||||
|
Total prepaid expenses and other assets
|
16,799
|
|
21,467
|
|
||||
|
Less long-term portion
|
172
|
|
219
|
|
||||
|
Total prepaid expenses and other assets, current
|
$
|
16,627
|
|
$
|
21,248
|
|
||
|
March 31,
2017 |
December 31,
2016 |
|||||||
|
Research equipment
|
$
|
10,483
|
|
$
|
10,479
|
|
||
|
Computer and office equipment
|
3,395
|
|
3,373
|
|
||||
|
Leasehold improvements
|
2,345
|
|
2,331
|
|
||||
|
Subtotal
|
16,223
|
|
16,183
|
|
||||
|
Accumulated depreciation and amortization
|
(12,482
|
)
|
(11,919
|
)
|
||||
|
Property and equipment, net
|
$
|
3,741
|
|
$
|
4,264
|
|
||
|
March 31,
2017 |
December 31,
2016 |
|||||||
|
Accrued outsourced research and development expenses
|
$
|
11,970
|
|
$
|
9,522
|
|
||
|
Accrued compensation and payroll taxes
|
5,045
|
|
11,539
|
|
||||
|
Accrued outsourced manufacturing expenses
|
3,670
|
|
3,225
|
|
||||
|
Other accrued expenses
|
4,276
|
|
4,552
|
|
||||
|
Total accrued expenses
|
24,961
|
|
28,838
|
|
||||
|
Less long-term portion
|
26
|
|
17
|
|
||||
|
Total accrued expenses, current
|
$
|
24,935
|
|
$
|
28,821
|
|
||
|
March 31,
2017 |
December 31,
2016 |
|||||||
|
Collaborative agreements
|
||||||||
|
License fees and event-based payments:
|
||||||||
|
Roche
|
$
|
34,877
|
|
$
|
35,709
|
|
||
|
Other
|
8,018
|
|
8,209
|
|
||||
|
42,895
|
|
43,918
|
|
|||||
|
Reimbursement for research and development services
|
—
|
|
700
|
|
||||
|
Total deferred revenue
|
42,895
|
|
44,618
|
|
||||
|
Less current portion
|
4,093
|
|
4,793
|
|
||||
|
Deferred revenue, net of current portion
|
$
|
38,802
|
|
$
|
39,825
|
|
||
|
Three Months Ended
March 31, |
||||||||
|
|
2017
|
2016
|
||||||
|
Research and development
|
$
|
3,274
|
|
$
|
2,584
|
|
||
|
Selling, general and administrative
|
4,041
|
|
3,233
|
|
||||
|
Share-based compensation expense
|
$
|
7,315
|
|
$
|
5,817
|
|
||
|
Three Months Ended
March 31, |
||||||||
|
|
2017
|
2016
|
||||||
|
Stock options
|
$
|
4,749
|
|
$
|
3,708
|
|
||
|
RSAs, RSUs and PRSUs
|
2,566
|
|
2,109
|
|
||||
|
$
|
7,315
|
|
$
|
5,817
|
|
|||
|
Three Months Ended
March 31, |
||||
|
|
2017
|
2016
|
||
|
Expected volatility
|
71.0-71.7%
|
67.5-69.1%
|
||
|
Average expected term (in years)
|
5.6
|
5.4
|
||
|
Risk-free interest rate
|
1.92-1.94%
|
1.27-1.73%
|
||
|
Expected dividend yield
|
—
|
—
|
||
|
March 31, 2017
|
||||||
|
|
Unrecognized
Expense
|
Remaining
Weighted-Average
Recognition Period
(years)
|
||||
|
Stock options
|
$
|
53,470
|
|
2.9
|
||
|
RSAs
|
$
|
7,281
|
|
2.1
|
||
|
RSUs
|
$
|
22,742
|
|
3.3
|
||
|
Item 2.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
|
•
|
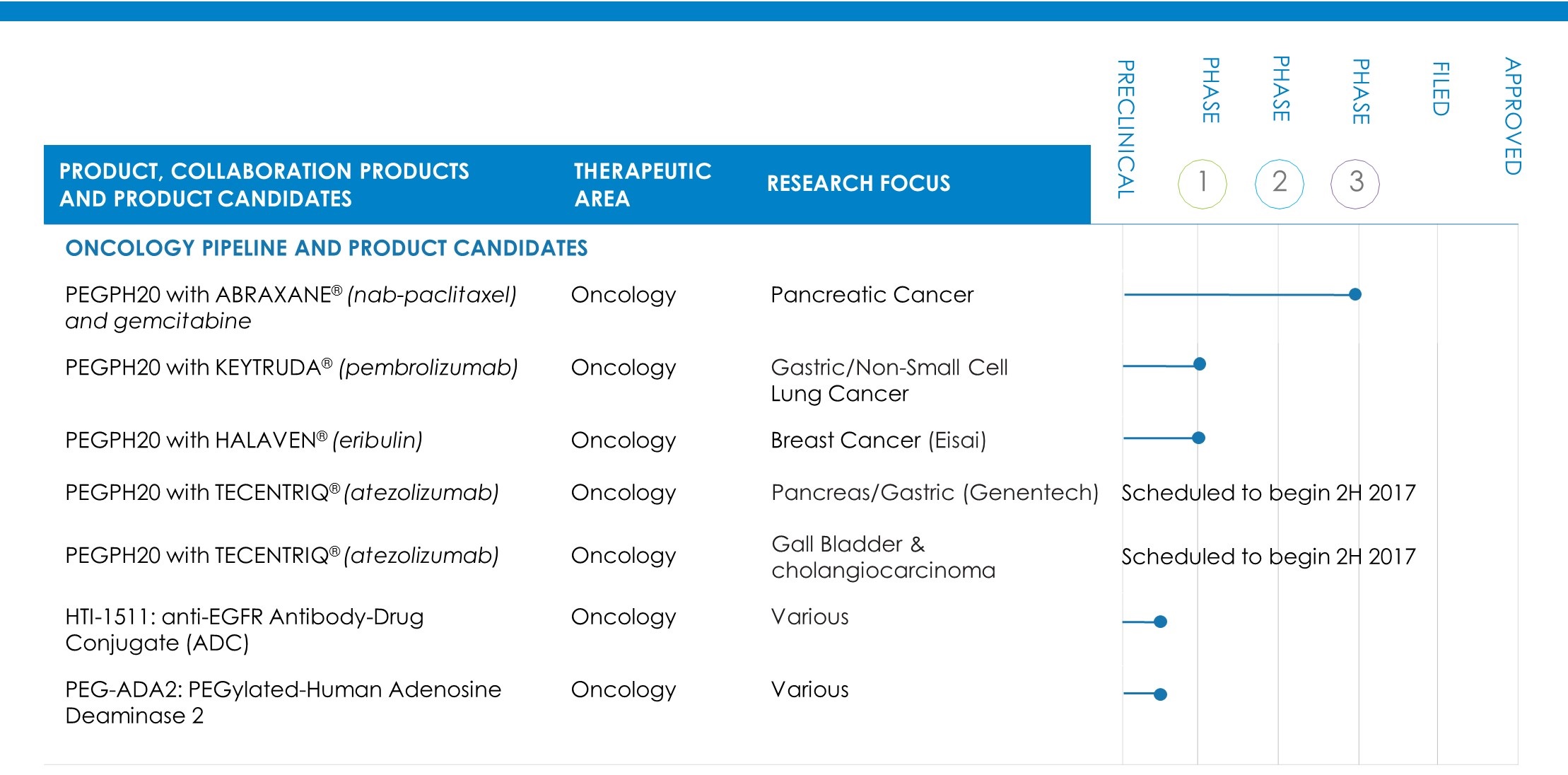
As announced in March 2017, SWOG, an independent network of researchers that design and conduct cancer clinical trials, stopped enrollment in a Phase 1b/2 trial evaluating PEGPH20 plus modified FOLFIRINOX chemotherapy versus modified FOLFIRINOX alone in patients with previously untreated metastatic pancreas cancer. PEGPH20 is a targeted investigational therapy for patients with high levels of HA. The SWOG study, which was initiated in October 2013, was enrolling patients irrespective of HA levels, referred to as an all-comer population. During a planned early futility analysis, SWOG’s independent Data Monitoring Committee found, based on preliminary data, that the addition of PEGPH20 given every two weeks to modified FOLFIRINOX in this all-comer population would be unlikely to demonstrate a statistically significant improvement in the primary endpoint of overall survival. SWOG further reported that a higher rate of death was observed in the PEGPH20 arm versus modified FOLFIRINOX alone. SWOG has stopped the study and continues its ongoing effort to collect and clean outstanding data. Upon completion, we will work with SWOG to analyze and evaluate the dataset. Our PEGPH20 studies and clinical collaborations in combination with agents other than modified FOLFIRINOX continue unchanged.
|
|
•
|
In April 2017, we presented at the annual meeting of the American Association of Cancer Research (AACR) that, in preclinical models, PEGPH20 increases the number of cancer-fighting white blood cells accumulating in the tumor and the effectiveness of immunotherapies, which builds upon prior preclinical findings and continues to support the potential benefits of remodeling the tumor microenvironment.
|
|
•
|
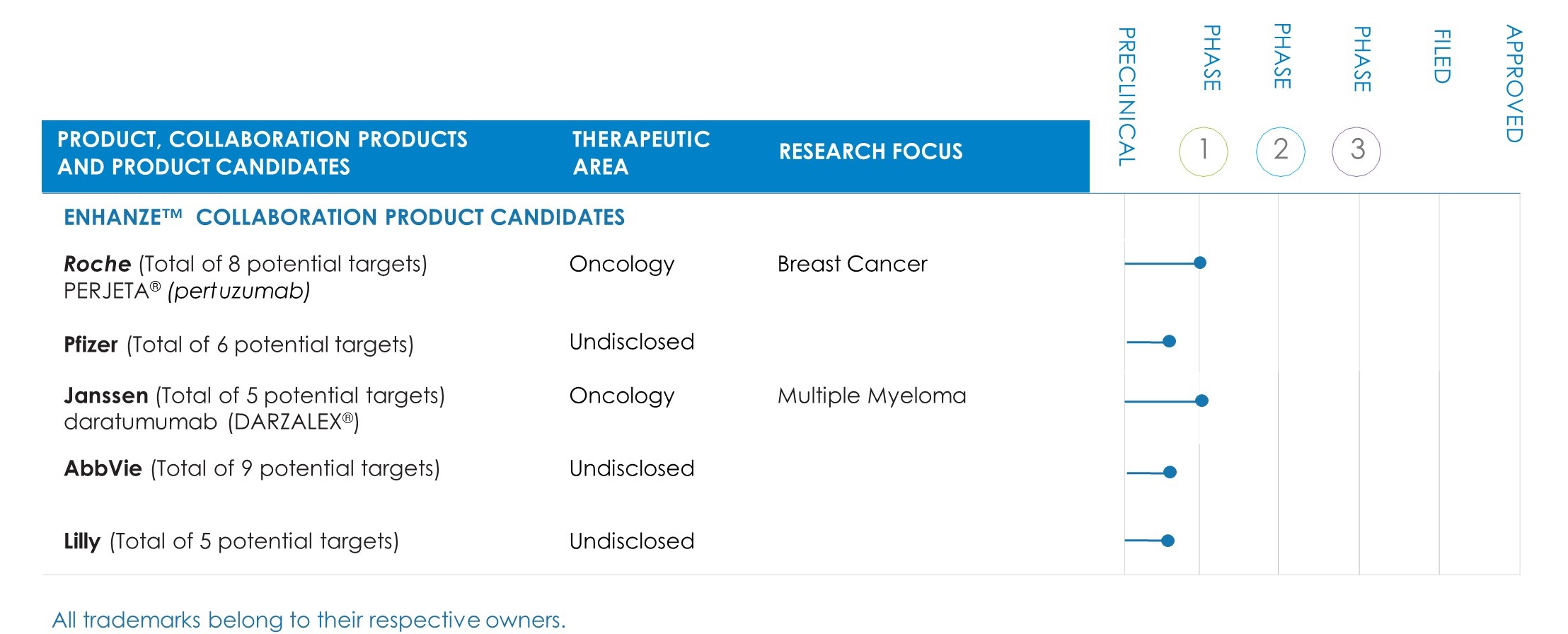
In March 2017, an Oncologic Drug Advisory Committee of the FDA voted 11 to 0 that the benefit/risk of rituximab SC was favorable for patients in the proposed indications in follicular lymphoma, diffuse large B-cell lymphoma and chronic lymphocytic leukemia. The FDA action date is June 26, 2017. Efficacy, safety, pharmacokinetic and patient-reported outcome data from five clinical studies presented by Genentech, a member of the Roche Group, at the advisory committee meeting supported the rituximab SC application. This is a co-formulation with rHuPH20, which is approved and marketed under the MabThera SC brand in countries outside the U.S.
|
|
•
|
In January 2017, we announced topline results from the combined analysis of Stage 1 and Stage 2, and Stage 2 alone, of the Study 109-202, based on a December 2016 data cutoff. Among the findings, the overall study population showed a statistically significant increase in progression-free survival (PFS) in the 84 total HA-High patients treated with PEGPH20 plus ABRAXANE and gemcitabine when compared to HA-High patients receiving ABRAXANE and gemcitabine alone. Stage 2 of the study, which completed enrollment in February 2016, showed a 91 percent improvement in median PFS for HA-High patients in the PEGPH20 arm, 8.6 months compared to 4.5 months in the control arm, and achieved its primary endpoint to evaluate and demonstrate a reduction in the rate of TE events in the PEGPH20 arm.
|

|
•
|
The primary endpoint of PFS in the efficacy evaluable population (total of 231 patients) was met with statistical significance with a median PFS of 6.0 months in the PAG arm compared to 5.3 months in the AG arm, hazard ratio (HR) with a 95% confidence interval (CI): 0.73 (0.53, 1.00); p=0.048;
|
|
•
|
The secondary endpoint of PFS in the HA-High intent to treat population (total of 84 HA-High patients) was met with statistical significance with a median PFS of 9.2 months in the PAG arm compared to 5.2 months in the AG arm, HR 0.51 (95% CI: 0.26, 1.00); p=0.048;
|
|
•
|
The exploratory analysis of median OS was 11.5 months vs. 8.5 months in the PAG vs. AG arms, respectively. Factors potentially having an impact on these results include less aggressive disease among patients in the AG arm within the Stage 1 patient population, and 9 of the 24 patients in the PAG arm (approximately 40 percent) discontinued PEGPH20 treatment at the time of the clinical hold, resulting in many patients receiving AG alone in both arms.
|
|
•
|
Median PFS was 8.6 months in the PAG arm compared to 4.5 months in the AG arm, hazard ratio of 0.63 (95% CI: 0.21, 1.93);
|
|
•
|
Median overall survival (OS) was 11.7 months in the PAG arm compared to 7.8 months in the AG arm, hazard ratio of 0.52 (95% CI: 0.22, 1.23);
|
|
•
|
The primary safety endpoint of decreasing the rate of TE events in Stage 2 was also met with the rate of TE events reducing from 43 percent to 10 percent in the PAG arm and from 25 percent to 6 percent in the AG arm, following a protocol amendment that excluded patients at high risk of TE events and with the introduction of prophylaxis with low molecular weight heparin (enoxaparin) in Stage 2 of the study with the current 1mg/kg/day dose of enoxaparin prophylaxis given in both treatment arms of the study.
|
|
•
|
Magnitude of the PFS treatment effect observed;
|
|
•
|
Toxicity profile; and
|
|
•
|
Interim OS data.
|
|
Three Months Ended
|
||||||||||||
|
March 31,
|
||||||||||||
|
2017
|
2016
|
Change
|
||||||||||
|
Sales of bulk rHuPH20:
|
||||||||||||
|
Roche
|
$
|
5,268
|
|
$
|
6,357
|
|
$
|
(1,089
|
)
|
|||
|
Baxalta
|
2,716
|
|
2,327
|
|
389
|
|
||||||
|
Other
|
245
|
|
386
|
|
(141
|
)
|
||||||
|
Sales of
Hylenex
|
3,205
|
|
3,870
|
|
(665
|
)
|
||||||
|
Total product sales, net
|
$
|
11,434
|
|
$
|
12,940
|
|
$
|
(1,506
|
)
|
|||
|
Three Months Ended
|
||||||||||||
|
March 31,
|
||||||||||||
|
2017
|
2016
|
Change
|
||||||||||
|
Upfront license fees, license fees for the election of additional targets, license maintenance fees and amortization of deferred upfront and other license fees and event-based payments:
|
||||||||||||
|
Roche
|
$
|
832
|
|
$
|
832
|
|
$
|
—
|
|
|||
|
Baxalta
|
191
|
|
191
|
|
—
|
|
||||||
|
Other
|
—
|
|
15,500
|
|
(15,500
|
)
|
||||||
|
1,023
|
|
16,523
|
|
(15,500
|
)
|
|||||||
|
Reimbursements for research and development services
|
3,129
|
|
1,649
|
|
1,480
|
|
||||||
|
Total revenues under collaborative agreements
|
$
|
4,152
|
|
$
|
18,172
|
|
$
|
(14,020
|
)
|
|||
|
Three Months Ended
|
||||||||||||
|
|
March 31,
|
|||||||||||
|
Programs
|
2017
|
2016
|
Change
|
|||||||||
|
PEGPH20
|
$
|
27,737
|
|
$
|
32,109
|
|
$
|
(4,372
|
)
|
|||
|
Enhanze collaborations and rHuPH20 platform
|
4,748
|
|
5,242
|
|
(494
|
)
|
||||||
|
Other
|
4,450
|
|
2,749
|
|
1,701
|
|
||||||
|
Total research and development expenses
|
$
|
36,935
|
|
$
|
40,100
|
|
$
|
(3,165
|
)
|
|||
|
•
|
clinical results may not meet prescribed endpoints for the studies or otherwise provide sufficient data to support the efficacy of our product candidates;
|
|
•
|
clinical and nonclinical test results may reveal side effects, adverse events or unexpected safety issues associated with the use of our product candidates; for example, in April 2014, a clinical hold was placed on patient enrollment and dosing of PEGPH20 in Study 202 as a result of a possible difference in the TE event rate that had been observed at that time in the trial between the group of patients treated with PEGPH20 versus the group of patients treated without PEGPH20. The clinical hold was lifted by the FDA in June 2014, and we have completed enrollment and continue to monitor ongoing patients who remain either on treatment or in follow-up on Study 202 under a revised clinical protocol;
|
|
•
|
completion of clinical trials may be delayed for a variety of reasons including the amount of time it may take to identify and enroll patients with high levels of HA in our target population, and the ability to procure drug supply required in clinical trial protocols;
|
|
•
|
third parties, such as contract research organizations, upon whom we rely to help conduct and manage our clinical trials may not perform satisfactorily, fulfill their contractual obligations to us, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols;
|
|
•
|
regulatory review may not find a product candidate safe or effective enough to merit either continued testing or final approval;
|
|
•
|
regulatory review may not find that the data from preclinical testing and clinical trials justifies approval;
|
|
•
|
regulatory authorities may require that we change our studies or conduct additional studies which may significantly delay or make continued pursuit of approval commercially unattractive;
|
|
•
|
a regulatory agency may reject our trial data or disagree with our interpretations of either clinical trial data or applicable regulations;
|
|
•
|
a regulatory agency may approve only a narrow use of our product or may require additional safety monitoring and reporting through Risk Evaluation and Mitigation Strategies or conditions to assure safe use programs;
|
|
•
|
the cost of clinical trials required for product approval may be greater than what we originally anticipate, and we may decide to not pursue regulatory approval for such a product;
|
|
•
|
a regulatory agency may not approve our manufacturing processes or facilities, or the processes or facilities of our collaborators, our contract manufacturers or our raw material suppliers;
|
|
•
|
a regulatory agency may identify problems or other deficiencies in our existing manufacturing processes or facilities, or the existing processes or facilities of our collaborators, our contract manufacturers or our raw material suppliers;
|
|
•
|
a regulatory agency may change its formal or informal approval requirements and policies, act contrary to previous guidance, adopt new regulations or raise new issues or concerns late in the approval process; or
|
|
•
|
a product candidate may be approved only for indications that are narrow or under conditions that place the product at a competitive disadvantage, which may limit the sales and marketing activities for such product candidate or otherwise adversely impact the commercial potential of a product.
|
|
•
|
restrictions on our products or manufacturing processes;
|
|
•
|
warning letters;
|
|
•
|
withdrawal of the products from the market;
|
|
•
|
voluntary or mandatory recall;
|
|
•
|
fines;
|
|
•
|
suspension or withdrawal of regulatory approvals;
|
|
•
|
suspension or termination of any of our ongoing clinical trials;
|
|
•
|
refusal to permit the import or export of our products;
|
|
•
|
refusal to approve pending applications or supplements to approved applications that we submit;
|
|
•
|
product seizure;
|
|
•
|
injunctions; or
|
|
•
|
imposition of civil or criminal penalties.
|
|
•
|
if any payment of principal is not made within three days of when such payment is due and payable or otherwise made in accordance with the terms of the Credit Agreement;
|
|
•
|
if any representations or warranties made in the Credit Agreement or any other transaction document proves to be incorrect or misleading in any material respect when made;
|
|
•
|
if there occurs a default in the performance of affirmative and negative covenants set forth in the Credit Agreement or any other transaction document;
|
|
•
|
the failure by either Baxalta or Roche to pay material amounts owed under our collaboration agreements because of an actual breach or default by us under the collaboration agreements;
|
|
•
|
the voluntary or involuntary commencement of bankruptcy proceedings by either Halozyme or Halozyme Royalty and other insolvency related defaults;
|
|
•
|
any materially adverse effect on the binding nature of any of the transaction documents or the collaboration agreements with Baxalta and Roche; or
|
|
•
|
Halozyme ceases to own, of record and beneficially, 100% of the equity interests in Halozyme Royalty.
|
|
•
|
the price of products relative to other therapies for the same or similar treatments;
|
|
•
|
the perception by patients, physicians and other members of the health care community of the effectiveness and safety of these products for their prescribed treatments relative to other therapies for the same or similar treatments;
|
|
•
|
our ability to fund our sales and marketing efforts and the ability and willingness of our collaborators to fund sales and marketing efforts;
|
|
•
|
the degree to which the use of these products is restricted by the approved product label;
|
|
•
|
the effectiveness of our sales and marketing efforts and the effectiveness of the sales and marketing efforts of our collaborators;
|
|
•
|
the introduction of generic competitors; and
|
|
•
|
the extent to which reimbursement for our products and related treatments will be available from third party payors including government insurance programs (Medicare and Medicaid) and private insurers.
|
|
•
|
we may have to issue convertible debt or equity securities to complete an acquisition, which would dilute our stockholders and could adversely affect the market price of our common stock;
|
|
•
|
an acquisition may negatively impact our results of operations because it may require us to amortize or write down amounts related to goodwill and other intangible assets, or incur or assume substantial debt or liabilities, or it may cause adverse tax consequences, substantial depreciation or deferred compensation charges;
|
|
•
|
we may encounter difficulties in assimilating and integrating the business, products, technologies, personnel or operations of companies that we acquire;
|
|
•
|
certain acquisitions may impact our relationship with existing or potential collaborators who are competitive with the acquired business, products or technologies;
|
|
•
|
acquisitions may require significant capital infusions and the acquired businesses, products or technologies may not generate sufficient value to justify acquisition costs;
|
|
•
|
we may take on liabilities from the acquired company such as debt, legal liabilities or business risk which could be significant;
|
|
•
|
an acquisition may disrupt our ongoing business, divert resources, increase our expenses and distract our management;
|
|
•
|
acquisitions may involve the entry into a geographic or business market in which we have little or no prior experience; and
|
|
•
|
key personnel of an acquired company may decide not to work for us.
|
|
•
|
the presence of competitive products to those being developed by us;
|
|
•
|
failure (actual or perceived) of our collaborators to devote attention or resources to the development or commercialization of product candidates licensed to such collaborator;
|
|
•
|
a dispute regarding our failure, or the failure of one of our third party collaborators, to comply with the terms of a collaboration agreement;
|
|
•
|
the termination, for any reason, of any of our collaboration agreements;
|
|
•
|
the sale of common stock by any significant stockholder, including, but not limited to, direct or indirect sales by members of management or our Board of Directors;
|
|
•
|
the resignation, or other departure, of members of management or our Board of Directors;
|
|
•
|
general negative conditions in the healthcare industry;
|
|
•
|
general negative conditions in the financial markets;
|
|
•
|
the cost associated with obtaining regulatory approval for any of our proprietary or collaboration product candidates;
|
|
•
|
the failure, for any reason, to secure or defend our intellectual property position;
|
|
•
|
for those products that are not yet approved for commercial sale, the failure or delay of applicable regulatory bodies to approve such products;
|
|
•
|
identification of safety or tolerability issues;
|
|
•
|
failure of clinical trials to meet efficacy endpoints;
|
|
•
|
suspensions or delays in the conduct of clinical trials or securing of regulatory approvals;
|
|
•
|
adverse regulatory action with respect to our and our collaborators’ products and product candidates such as clinical holds, imposition of onerous requirements for approval or product recalls;
|
|
•
|
our failure, or the failure of our third party collaborators, to successfully commercialize products approved by applicable regulatory bodies such as the FDA;
|
|
•
|
our failure, or the failure of our third party collaborators, to generate product revenues anticipated by investors;
|
|
•
|
disruptions in our clinical or commercial supply chains, including disruptions caused by problems with a bulk rHuPH20 contract manufacturer or a fill and finish manufacturer for any product or product candidate;
|
|
•
|
the sale of additional debt and/or equity securities by us;
|
|
•
|
our failure to obtain financing on acceptable terms or at all; or
|
|
•
|
a restructuring of our operations.
|
|
•
|
we will be able to obtain patent protection for our products and technologies;
|
|
•
|
the scope of any of our issued patents will be sufficient to provide commercially significant exclusivity for our products and technologies;
|
|
•
|
others will not independently develop similar or alternative technologies or duplicate our technologies and obtain patent protection before we do; and
|
|
•
|
any of our issued patents, or patent pending applications that result in issued patents, will be held valid, enforceable and infringed in the event the patents are asserted against others.
|
|
Item 3.
|
Quantitative and Qualitative Disclosures About Market Risk
|
|
Item 4.
|
Controls and Procedures
|
|
Item 1.
|
Legal Proceedings
|
|
Item 1A.
|
Risk Factors
|
|
Item 2.
|
Unregistered Sales of Equity Securities and Use of Proceeds
|
|
Item 3.
|
Defaults Upon Senior Securities
|
|
Item 4.
|
Mine Safety Disclosures
|
|
Item 5.
|
Other Information
|
|
Item 6.
|
Exhibits
|
|
3.1
|
|
Composite Certificate of Incorporation (1)
|
|
3.2
|
|
Bylaws, as amended (2)
|
|
31.1
|
|
Certification of Chief Executive Officer pursuant to Rule 13a-14(a) and 15d-14(a) of the Securities Exchange Act of 1934, as amended
|
|
31.2
|
|
Certification of Chief Financial Officer pursuant to Rule 13a-14(a) and 15d-14(a) of the Securities Exchange Act of 1934, as amended
|
|
32
|
|
Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
|
101.INS
|
|
Instance Document
|
|
101.SCH
|
Taxonomy Extension Schema Document
|
|
|
101.CAL
|
Taxonomy Extension Calculation Linkbase Document
|
|
|
101.DEF
|
Taxonomy Extension Definition Linkbase Document
|
|
|
101.LAB
|
Taxonomy Extension Label Linkbase Document
|
|
|
101.PRE
|
Taxonomy Extension Presentation Linkbase Document
|
|
|
(1)
|
Incorporated by reference to the Registrant’s Quarterly Report on Form 10-Q, filed August 7, 2013 (File No. 001-32335).
|
|
(2)
|
Incorporated by reference to the Registrant’s Current Report on Form 8-K, filed December 19, 2016 (File No. 001-32335).
|
|
|
Halozyme Therapeutics, Inc.,
a Delaware corporation
|
||
|
|
|||
|
Dated:
|
May 9, 2017
|
/s/ Helen I. Torley, M.B. Ch.B., M.R.C.P.
|
|
|
|
Helen I. Torley, M.B. Ch.B., M.R.C.P.
President and Chief Executive Officer
(Principal Executive Officer)
|
||
|
|
|||
|
|
|||
|
Dated:
|
May 9, 2017
|
/s/ Laurie D. Stelzer
|
|
|
|
Laurie D. Stelzer
Senior Vice President and Chief Financial Officer
(Principal Financial and Accounting Officer)
|
||
|
|
|||
|
|
|||