|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Mark One)
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
DELAWARE
|
01-0393723
|
|
(State or other jurisdiction of incorporation
|
(IRS Employer Identification No.)
|
|
or organization)
|
|
|
ONE IDEXX DRIVE, WESTBROOK, MAINE
|
04092
|
|
(Address of principal executive offices)
|
(ZIP Code)
|
|
Title of each class
|
Name of each exchange on which registered
|
|
Common Stock, $0.10 par value per share
|
NASDAQ Global Market
|
|
Large accelerated filer
|
x
|
Accelerated filer
|
¨
|
|||
|
Non-accelerated filer
|
¨
(Do not check if a smaller reporting company)
|
Smaller reporting company
|
¨
|
|
Item No.
|
Page No.
|
|
|
PART I
|
||
|
Item 1
|
Business
|
3
|
|
Item 1A
|
Risk Factors
|
12
|
|
Item 1B
|
Unresolved Staff Comments
|
18
|
|
Item 2
|
Properties
|
19
|
|
Item 3
|
Legal Proceedings
|
19
|
|
PART II
|
||
|
Item 5
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
22
|
|
Item 6
|
Selected Financial Data
|
25
|
|
Item 7
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
26
|
|
Item 7A
|
Quantitative and Qualitative Disclosure about Market Risk
|
51
|
|
Item 8
|
Financial Statements and Supplementary Data
|
52
|
|
Item 9
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
|
52
|
|
Item 9A
|
Controls and Procedures
|
53
|
|
Item 9B
|
Other Information
|
54
|
|
PART III
|
||
|
Item 10
|
Directors, Executive Officers and Corporate Governance
|
54
|
|
Item 11
|
Executive Compensation
|
54
|
|
Item 12
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
54
|
|
Item 13
|
Certain Relationships and Related Transactions and Director Independence
|
55
|
|
Item 14
|
Principal Accountant Fees and Services
|
55
|
|
PART IV
|
||
|
Item 15
|
Exhibits, Financial Statement Schedules
|
55
|
|
Signatures
|
56
|
|
|
Financial Statements and Supplementary Data – Index to Consolidated Financial Statements
|
F-1
|
|
|
Exhibit Index
|
||
|
ITEM 1.
|
BUSINESS
|
|
|
·
|
Point-of-care veterinary diagnostic products, comprising instruments and consumables and rapid assays;
|
|
|
·
|
Veterinary reference laboratory diagnostic and consulting services used by veterinarians;
|
|
|
·
|
Diagnostic and health-monitoring products for livestock and poultry;
|
|
|
·
|
Products that test water for certain microbiological contaminants;
|
|
|
·
|
Practice information systems and services, and digital radiography systems used by veterinarians;
|
|
|
·
|
Products that test milk for antibiotic residues and other contaminants; and
|
|
|
·
|
Point-of-care electrolytes and blood gas analyzers used in the human point-of-care medical diagnostics market.
|
|
|
·
|
SNAP
®
4Dx
®
, which tests for Lyme disease,
Ehrlichia canis
, canine heartworm, and
Anaplasma phagocytophilum
.
|
|
|
·
|
SNAP
®
3Dx
®
, which tests for Lyme disease,
Ehrlichia canis
and canine heartworm;
|
|
|
·
|
SNAP
®
Heartworm RT, which tests only for canine heartworm;
|
|
|
·
|
SNAP
®
Parvo, which tests for parvovirus;
|
|
|
·
|
SNAP
®
cPL
™
, which tests for canine pancreatitis; and
|
|
|
·
|
SNAP
®
Giardia
, which is a fecal test for soluble
Giardia
antigens.
|
|
|
·
|
SNAP
®
Feline Triple
®
, which tests for feline immunodeficiency virus (“FIV”) (which is similar to the human AIDS virus), feline leukemia virus (“FeLV”), and feline heartworm;
|
|
|
·
|
SNAP
®
FIV/FeLV Combo Test, which tests for FIV and FeLV;
|
|
|
·
|
SNAP
®
FeLV, which tests only for FeLV; and
|
|
|
·
|
SNAP
®
Giardia
, which is a fecal test for soluble
Giardia
antigens.
|
|
|
·
|
Exclusive licenses from Tulane University and the University of Texas to patents and patent applications that expire beginning in 2019 relating to the methods for detection of Lyme disease utilized in certain of our SNAP
®
products and a reference laboratory diagnostic test;
|
|
|
·
|
A patent concerning the Colilert
®
-18 product that expires in 2014;
|
|
|
·
|
A patent concerning the Quanti-Tray
®
product that expires in 2014;
|
|
|
·
|
A patent that relates to certain methods and kits for simultaneously detecting antigens and antibodies, which covers certain of our SNAP
®
products, including our canine and feline combination tests, that expires in 2014;
|
|
|
·
|
Patents covering various reagents, kits and/or immunoassays for detecting FIV antibodies utilized in certain of our SNAP
®
products that expire beginning in 2014;
|
|
|
·
|
An exclusive license from Boehringer Ingelheim to certain patents covering reagents and methods for detecting Porcine Reproductive and Respiratory Syndrome that expire beginning in 2012; and
|
|
|
·
|
An exclusive license from Cornell University to patents covering methods for detecting Bovine Viral Diarrhea Virus that expire beginning in 2017.
|
|
|
·
|
Veterinary diagnostic, water, livestock and poultry and dairy testing products
. We compete primarily on the basis of the ease of use, speed, accuracy, quality of the information provided, and other performance characteristics of our products and services (including unique tests), the breadth of our product line and services, the effectiveness of our sales and distribution channels, the quality of our technical and customer service, and our pricing relative to the value of our products in comparison with competitive products and services. We compete in most geographic locations in North America with Abaxis, Inc. in respect to our veterinary diagnostic products.
|
|
|
·
|
Veterinary reference laboratory diagnostic and consulting services
. We compete primarily on the basis of quality, consistency of service levels, technology, information management, medical consultation and our pricing relative to the value of our services in comparison with competitive products and services. We compete in most geographic locations in North America with Antech Diagnostics, a unit of VCA Antech, Inc.
|
|
|
·
|
Practice information management and digital radiography systems
. We compete primarily on the basis of functionality, connectivity to equipment and other systems, performance characteristics, effectiveness of our customer service, information handling capabilities, advances in technologies, and our pricing relative to the value of our products and services.
|
|
|
·
|
Electrolyte and blood gas analyzers for the human point-of-care medical diagnostics market
. We compete primarily with large human medical diagnostics companies such as Radiometer A/S, Siemens Medical Solutions Diagnostics, Instrumentation Laboratory, Abbott Diagnostics, and Roche Diagnostics. We compete primarily on the basis of the ease of use, menu, convenience, international distribution and service, instrument reliability, and our pricing relative to the value of our products.
|
|
ITEM 1A.
|
RISK FACTORS
|
|
|
·
|
Developing, manufacturing and marketing innovative new in-clinic laboratory analyzers that drive sales of IDEXX VetLab
®
instruments, grow our installed base of instruments, and create a recurring revenue stream from consumable products, services and accessories;
|
|
|
·
|
Developing and introducing new proprietary diagnostic tests and services that provide valuable medical information to our customers and effectively differentiate our products and services from those of our competitors;
|
|
|
·
|
Increasing the value to our customers of our companion animal products and services by enhancing the integration of these products and managing the diagnostic information derived from our products;
|
|
|
·
|
Achieving cost improvements in our worldwide network of laboratories by implementing global best practices including lean processing techniques, incorporating technological enhancements including laboratory automation and a global laboratory information management system, employing purchasing strategies to maximize leverage of our global scale, increasing the leverage of existing infrastructure and consolidating testing in high volume laboratory hubs;
|
|
|
·
|
Achieving cost improvements in the manufacture and service of our in-clinic laboratory analyzers by employing the benefits of economies of scale in both negotiating supply contracts and leveraging manufacturing overhead and improving reliability of our instruments;
|
|
|
·
|
Expanding our served market and growing our market share by strengthening our sales and marketing activities both within the U.S. and in geographies outside of the U.S.; and
|
|
|
·
|
Developing and implementing new technology and licensing strategies.
|
|
ITEM 1B.
|
UNRESOLVED STAFF COMMENTS
|
|
ITEM 2.
|
PROPERTIES
|
|
|
·
|
40,000 square feet of office and laboratory space located in the U.S., used for our Veterinary Reference Laboratory Diagnostic and Consulting Services line of business
|
|
|
·
|
23,000 square feet of office and laboratory space located in the U.K., used for our Veterinary Reference Laboratory Diagnostic and Consulting Services line of business
|
|
|
·
|
3,100 square feet of office and laboratory space located in Canada, used for our Veterinary Reference Laboratory Diagnostic and Consulting Services line of business
|
|
|
·
|
352,400 total square feet of laboratory, office and warehousing space located throughout the United States, Europe, Canada, Australia, Asia and Africa, primarily used for our Veterinary Reference Laboratory Diagnostic and Consulting Services line of business
|
|
|
·
|
108,600 square feet of distribution, warehousing and office space in the Netherlands, which serves as our European headquarters
|
|
|
·
|
113,500 square feet of industrial space in Tennessee for distribution and warehousing related to various lines of business
|
|
|
·
|
90,800 square feet of office space in Maine for Corporate, Customer Service and IT support services
|
|
|
·
|
70,100 square feet of office, manufacturing and warehousing space in Georgia related to our OPTI Medical Systems line of business
|
|
|
·
|
69,300 square feet of office and manufacturing space in Wisconsin related to our Practice Information Systems and Services line of business
|
|
|
·
|
64,400 total square feet of office and manufacturing space in France, Switzerland and Asia related to our Livestock and Poultry Diagnostics business
|
|
|
·
|
7,600 square feet of office and manufacturing space in the U.K. related to our Water business
|
|
ITEM 3.
|
LEGAL PROCEEDINGS
|
|
Name
|
Age
|
Title
|
||
|
Jonathan W. Ayers
|
54
|
Chairman of the Board of Directors, President and
Chief Executive Officer
|
||
|
William E. Brown III, PhD
|
56
|
Corporate Vice President and Chief Scientific Officer
|
||
|
Conan R. Deady
|
49
|
Corporate Vice President, General Counsel and Secretary
|
||
|
Thomas J. Dupree
(1)
|
42
|
Corporate Vice President
|
||
|
William B. Goodspeed
|
52
|
Corporate Vice President
|
||
|
Daniel V. Meyaard
|
53
|
Corporate Vice President
|
||
|
Ali Naqui, PhD
|
57
|
Corporate Vice President
|
||
|
James F. Polewaczyk
|
47
|
Corporate Vice President
|
||
|
Johnny D. Powers, PhD
|
49
|
Corporate Vice President
|
||
|
Merilee Raines
|
55
|
Corporate Vice President, Chief Financial Officer and Treasurer
|
||
|
Giovani Twigge
|
47
|
Corporate Vice President
|
||
|
Michael J. Williams, PhD
|
43
|
Corporate Vice President
|
|
(1)
|
Mr. Dupree has announced his intention to resign from the company effective June 30, 2011. See Item 9B.
|
|
ITEM 5.
|
MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
|
For the Quarter Ended
|
High
|
Low
|
||||||
|
March 31, 2009
|
$ | 36.89 | $ | 27.68 | ||||
|
June 30, 2009
|
46.90 | 33.07 | ||||||
|
September 30, 2009
|
55.12 | 43.47 | ||||||
|
December 31, 2009
|
55.69 | 47.52 | ||||||
|
March 31, 2010
|
59.95 | 49.03 | ||||||
|
June 30, 2010
|
68.57 | 57.31 | ||||||
|
September 30, 2010
|
64.00 | 54.80 | ||||||
|
December 31, 2010
|
72.40 | 59.65 | ||||||
|
Period
|
Total Number of
Shares Purchased
(a)
|
Average Price
Paid per Share
(b)
|
Total Number of
Shares Purchased as
Part of Publicly
Announced Plans or
Programs
(c)
|
Maximum Number of
Shares that May Yet Be
Purchased Under the
Plans or Programs
(d)
|
||||||||||||
|
October 1, 2010 to October 31, 2010
|
105,000 | $ | 62.11 | 105,000 | 4,108,372 | |||||||||||
|
November 1, 2010 to November 30, 2010
|
180,600 | 62.67 | 180,600 | 3,927,772 | ||||||||||||
|
December 1, 2010 to December 31, 2010
|
122,154 | 66.71 | 121,300 | 3,806,472 | ||||||||||||
|
Total
|
407,754 | $ | 63.74 | 406,900 | 3,806,472 | |||||||||||
|
December 31, 2010
|
||||||||||||
|
Plan Category
|
Number of Securities
to be Issued Upon Exercise
of Outstanding Options,
Warrants and Rights
(a)
|
Weighted Average
Exercise Price of
Outstanding Options,
Warrants and Rights
(3)
(b)
|
Number of Securities Remaining
Available for
Future Issuance Under Equity
Compensation Plans (Excluding
Securities Reflected in Column (a))
(c)
|
|||||||||
|
Equity compensation plans approved by security holders
|
4,383,061 | (1) | $ | 33.34 | 3,876,311 | (2) | ||||||
|
Equity compensation plans not approved by security holders
|
- | - | - | |||||||||
|
Total
|
4,383,061 | $ | 33.34 | 3,876,311 | ||||||||
|
(1)
|
Consists of shares of common stock subject to outstanding options, restricted stock units and deferred stock units under the following compensation plans: 1991 Stock Option Plan (356,554 shares), 1998 Stock Incentive Plan (574,832 shares), 2000 Director Option Plan (7,000 shares), 2003 Stock Incentive Plan (2,748,083 shares) and 2009 Stock Incentive Plan (696,592 shares). Excludes 260,657 shares issuable under the 1997 Employee Stock Purchase Plan in connection with the current and future offering periods.
|
|
(2)
|
Includes 3,615,654 shares available for issuance under our 2009 Plan. The 2009 Stock Incentive Plan provides for the issuance of incentive stock options, nonqualified stock options, stock appreciation rights, restricted stock unit awards and other stock unit awards. Also includes 260,657 shares issuable under our 1997 employee stock purchase plan in connection with the current and future offering periods. No new grants may be made under the other plans listed in footnote (1) except for the 2009 Stock Incentive Plan.
|
|
(3)
|
Only stock option awards were used in computing the weighted-average exercise price.
|
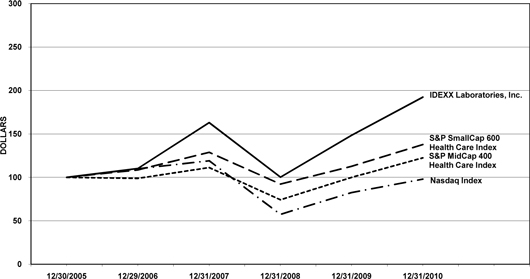
|
12/30/2005
|
12/29/2006
|
12/31/2007
|
12/31/2008
|
12/31/2009
|
12/31/2010
|
|||||||||||||||||||
|
IDEXX Laboratories, Inc.
|
$ | 100.00 | $ | 110.17 | $ | 162.91 | $ | 100.25 | $ | 148.51 | $ | 192.33 | ||||||||||||
|
S&P MidCap 400 Health Care Index
|
100.00 | 98.85 | 111.22 | 74.19 | 99.91 | 122.46 | ||||||||||||||||||
|
S&P SmallCap 600 Health Care Index
|
100.00 | 108.53 | 128.87 | 92.22 | 112.71 | 137.77 | ||||||||||||||||||
|
NASDAQ Index
|
100.00 | 109.84 | 119.14 | 57.41 | 82.53 | 97.95 | ||||||||||||||||||
|
ITEM 6.
|
SELECTED FINANCIAL DATA
|
|
For the Years Ended December 31,
(in thousands, except per share data)
|
||||||||||||||||||||
|
2010
|
2009
|
2008
|
2007
|
2006
|
||||||||||||||||
|
INCOME STATEMENT DATA:
|
||||||||||||||||||||
|
Revenue
|
$ | 1,103,392 | $ | 1,031,633 | $ | 1,024,030 | $ | 922,555 | $ | 739,117 | ||||||||||
|
Cost of revenue
|
524,769 | 505,352 | 494,264 | 459,033 | 359,588 | |||||||||||||||
|
Gross profit
|
578,623 | 526,281 | 529,766 | 463,522 | 379,529 | |||||||||||||||
|
Expenses:
|
||||||||||||||||||||
|
Sales and marketing
|
179,626 | 167,748 | 169,956 | 151,882 | 115,882 | |||||||||||||||
|
General and administrative
|
126,519 | 117,440 | 116,681 | 108,119 | 82,097 | |||||||||||||||
|
Research and development
|
68,597 | 65,124 | 70,673 | 67,338 | 53,617 | |||||||||||||||
|
Income from operations
|
203,881 | 175,969 | 172,456 | 136,183 | 127,933 | |||||||||||||||
|
Interest (expense) income, net
|
(1,752 | ) | (1,430 | ) | (2,269 | ) | (1,340 | ) | 2,817 | |||||||||||
|
Income before provision for income taxes
|
202,129 | 174,539 | 170,187 | 134,843 | 130,750 | |||||||||||||||
|
Provision for income taxes
|
60,809 | 52,304 | 54,018 | 40,829 | 37,224 | |||||||||||||||
|
Net income
|
141,320 | 122,235 | 116,169 | 94,014 | 93,526 | |||||||||||||||
|
Less: Net income attributable to noncontrolling interest
|
36 | 10 | - | - | (152 | ) | ||||||||||||||
|
Net income attributable to IDEXX Laboratories’ stockholders
|
$ | 141,284 | $ | 122,225 | $ | 116,169 | $ | 94,014 | $ | 93,678 | ||||||||||
|
Earnings per share
(1)
:
|
||||||||||||||||||||
|
Basic
|
$ | 2.45 | $ | 2.08 | $ | 1.94 | $ | 1.53 | $ | 1.49 | ||||||||||
|
Diluted
|
2.37 | 2.01 | 1.87 | 1.46 | 1.42 | |||||||||||||||
|
Weighted average shares outstanding
(1)
:
|
||||||||||||||||||||
|
Basic
|
57,713 | 58,809 | 59,953 | 61,560 | 62,866 | |||||||||||||||
|
Diluted
|
59,559 | 60,682 | 62,249 | 64,455 | 65,907 | |||||||||||||||
|
BALANCE SHEET DATA:
|
||||||||||||||||||||
|
Cash and investments
|
$ | 156,915 | $ | 106,728 | $ | 78,868 | $ | 60,360 | $ | 96,666 | ||||||||||
|
Working capital
|
175,479 | 120,033 | 60,598 | 82,271 | 177,520 | |||||||||||||||
|
Total assets
|
897,144 | 808,527 | 765,437 | 702,179 | 559,560 | |||||||||||||||
|
Total debt
|
133,280 | 123,884 | 156,479 | 76,683 | 7,125 | |||||||||||||||
|
Total stockholders’ equity
|
574,281 | 514,579 | 438,194 | 438,323 | 409,861 | |||||||||||||||
|
(1)
|
Share and per share amounts originally reported for 2006 have been adjusted as appropriate to reflect the effect of a two-for-one stock split, which was effected in the form of a common stock dividend distributed on November 26, 2007.
|
|
ITEM 7.
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
|
For the Years Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Revenue Reductions Recorded
|
||||||||||||
|
Practice Developer
®
program
(1)
|
$ | 5,025 | $ | 6,892 | $ | 7,521 | ||||||
|
SNAP
®
up the Savings
™
program
(1)
|
7,487 | 4,582 | 4,011 | |||||||||
|
Other programs
(1)
|
11,424 | 9,175 | 3,808 | |||||||||
|
Total revenue reductions
|
$ | 23,936 | $ | 20,649 | $ | 15,340 | ||||||
|
(1)
|
Practice Developer
®
, SNAP
®
up the Savings
™
and certain other customer program liabilities are settled through the issuance of IDEXX Points.
|
|
For the Years Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Accrued Customer Programs:
|
||||||||||||
|
Balance, beginning of the year
|
$ | 17,388 | $ | 15,183 | $ | 15,107 | ||||||
|
Current provision related to Practice Developer
®
program
(1)
|
5,025 | 6,892 | 7,521 | |||||||||
|
Current provision related to SNAP
®
up the Savings
™
program
(1)
|
7,487 | 4,582 | 4,011 | |||||||||
|
Current provision related to other programs
(1)
|
10,504 | 9,101 | 3,808 | |||||||||
|
Customer acquisition costs
|
6,037 | 100 | - | |||||||||
|
Breakage
|
(334 | ) | (367 | ) | (694 | ) | ||||||
|
Actual points redeemed and credits issued
|
(23,859 | ) | (18,256 | ) | (14,338 | ) | ||||||
|
Exchange impact on balances denominated in foreign currency
|
(34 | ) | 153 | (232 | ) | |||||||
|
Balance, end of year
|
$ | 22,214 | $ | 17,388 | $ | 15,183 | ||||||
|
(1)
|
Practice Developer
®
, SNAP
®
up the Savings
™
and certain other customer program liabilities are settled through the issuance of IDEXX Points.
|
|
For the Years Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Expected stock price volatility
|
31 | % | 31 | % | 25 | % | ||||||
|
Expected term, in years
(1)
|
4.9 | 4.8 | 4.9 | |||||||||
|
Risk-free interest rate
|
2.3 | % | 1.6 | % | 2.6 | % | ||||||
|
(1)
|
Options granted after January 1, 2006 have contractual terms of seven years. Options granted prior to January 1, 2006 have contractual terms of 10 years.
|
|
For the Years Ended December 31,
|
||||||||||||||||||||||||||||
|
Net Revenue
(dollars in thousands)
|
2010
|
2009
|
Dollar
Change
|
Percentage
Change
|
Percentage
Change from
Currency
(1)
|
Percentage
Change from
Acquisitions/
Divestitures
(2)
|
Organic
Revenue
Growth
(3)
|
|||||||||||||||||||||
|
CAG
|
$ | 905,655 | $ | 843,303 | $ | 62,352 | 7.4 | % | 0.5 | % | 0.5 | % | 6.4 | % | ||||||||||||||
|
Water
|
76,514 | 73,214 | 3,300 | 4.5 | % | 0.8 | % | - | 3.7 | % | ||||||||||||||||||
|
LPD
|
81,177 | 77,208 | 3,969 | 5.1 | % | (2.5 | %) | - | 7.6 | % | ||||||||||||||||||
|
Other
|
40,046 | 37,908 | 2,138 | 5.6 | % | - | - | 5.6 | % | |||||||||||||||||||
|
Total
|
$ | 1,103,392 | $ | 1,031,633 | $ | 71,759 | 7.0 | % | 0.3 | % | 0.4 | % | 6.3 | % | ||||||||||||||
|
(1)
|
The percentage change from currency is a non-U.S. GAAP measure. It represents the percentage change in revenue resulting from the difference between the average exchange rates during the twelve months ended December 31, 2010 and the same period of the prior year applied to foreign currency denominated revenues for the twelve months ended December 31, 2010.
|
|
(2)
|
Represents the percentage change in revenue during the year ended December 31, 2010 compared to the year ended December 31, 2009 attributed to incremental revenues from businesses acquired or revenues lost from businesses divested or discontinued subsequent to December 31, 2008.
|
|
(3)
|
Organic revenue is a non-U.S. GAAP measure and represents the percentage change in revenue during the year ended December 31, 2010 compared to the year ended December 31, 2009 net of acquisitions and divestitures and the effect from changes in foreign currency exchange rates.
|
|
For the Years Ended December 31,
|
||||||||||||||||||||||||||||
|
Net Revenue
(dollars in thousands)
|
2010
|
2009
|
Dollar
Change
|
Percentage
Change
|
Percentage
Change from
Currency
(1)
|
Percentage
Change from
Acquisitions/
Divestitures
(2)
|
Organic
Revenue
Growth
(3)
|
|||||||||||||||||||||
|
Instruments and consumables
|
$ | 354,239 | $ | 332,706 | $ | 21,533 | 6.5 | % | 0.1 | % | - | 6.4 | % | |||||||||||||||
|
Rapid assay products
|
146,538 | 147,078 | (540 | ) | (0.4 | %) | 0.3 | % | - | (0.7 | %) | |||||||||||||||||
|
Reference laboratory diagnostic and consulting services
|
329,666 | 298,410 | 31,256 | 10.5 | % | 1.0 | % | 1.2 | % | 8.3 | % | |||||||||||||||||
|
Practice information management systems and digital radiography
|
75,212 | 65,055 | 10,157 | 15.6 | % | 0.9 | % | 0.5 | % | 14.2 | % | |||||||||||||||||
|
Pharmaceutical products
|
- | 54 | (54 | ) | (100 | %) | - | - | (100 | %) | ||||||||||||||||||
|
Net CAG revenue
|
$ | 905,655 | $ | 843,303 | $ | 62,352 | 7.4 | % | 0.5 | % | 0.5 | % | 6.4 | % | ||||||||||||||
|
(1)
|
The percentage change from currency is a non-U.S. GAAP measure. It represents the percentage change in revenue resulting from the difference between the average exchange rates during the twelve months ended December 31, 2010 and the same period of the prior year applied to foreign currency denominated revenues for the twelve months ended December 31, 2010.
|
|
(2)
|
Represents the percentage change in revenue during the year ended December 31, 2010 compared to the year ended December 31, 2009 attributed to incremental revenues from businesses acquired or revenues lost from businesses divested or discontinued subsequent to December 31, 2008.
|
|
(3)
|
Organic revenue is a non-U.S. GAAP measure and represents the percentage change in revenue during the year ended December 31, 2010 compared to the year ended December 31, 2009 net of acquisitions and divestitures and the effect from changes in foreign currency exchange rates.
|
|
For the Years Ended December 31,
|
||||||||||||||||||||||||
|
Gross Profit
(dollars in thousands)
|
2010
|
Percent of
Revenue
|
2009
|
Percent of
Revenue
|
Dollar
Change
|
Percentage
Change
|
||||||||||||||||||
|
CAG
|
$ | 457,519 | 50.5 | % | $ | 410,356 | 48.7 | % | $ | 47,163 | 11.5 | % | ||||||||||||
|
Water
|
47,676 | 62.3 | % | 47,233 | 64.5 | % | 443 | 0.9 | % | |||||||||||||||
|
LPD
|
54,898 | 67.6 | % | 51,256 | 66.4 | % | 3,642 | 7.1 | % | |||||||||||||||
|
Other
|
18,297 | 45.7 | % | 17,067 | 45.0 | % | 1,230 | 7.2 | % | |||||||||||||||
|
Unallocated amounts
|
233 | N/A | 369 | N/A | (136 | ) | (36.9 | %) | ||||||||||||||||
|
Total Company
|
$ | 578,623 | 52.4 | % | $ | 526,281 | 51.0 | % | $ | 52,342 | 9.9 | % | ||||||||||||
|
For the Years Ended December 31,
|
||||||||||||||||||||||||
|
Operating Expenses
(dollars in thousands)
|
2010
|
Percent of
Revenue
|
2009
|
Percent of
Revenue
|
Dollar
Change
|
Percentage
Change
|
||||||||||||||||||
|
CAG
|
$ | 297,793 | 32.9 | % | $ | 274,235 | 32.5 | % | $ | 23,558 | 8.6 | % | ||||||||||||
|
Water
|
16,600 | 21.7 | % | 15,618 | 21.3 | % | 982 | 6.3 | % | |||||||||||||||
|
LPD
|
35,810 | 44.1 | % | 33,985 | 44.0 | % | 1,825 | 5.4 | % | |||||||||||||||
|
Other
|
13,714 | 34.2 | % | 13,642 | 36.0 | % | 72 | 0.5 | % | |||||||||||||||
|
Unallocated amounts
|
10,825 | N/A | 12,832 | N/A | (2,007 | ) | (15.6 | %) | ||||||||||||||||
|
Total Company
|
$ | 374,742 | 34.0 | % | $ | 350,312 | 34.0 | % | $ | 24,430 | 7.0 | % | ||||||||||||
|
Operating Income
(dollars in thousands)
|
2010
|
Percent of Revenue
|
2009
|
Percent of Revenue
|
Dollar Change
|
Percentage Change
|
||||||||||||||||||
|
CAG
|
$ | 159,726 | 17.6 | % | $ | 136,121 | 16.1 | % | $ | 23,605 | 17.3 | % | ||||||||||||
|
Water
|
31,076 | 40.6 | % | 31,615 | 43.2 | % | (539 | ) | (1.7 | %) | ||||||||||||||
|
LPD
|
19,088 | 23.5 | % | 17,271 | 22.4 | % | 1,817 | 10.5 | % | |||||||||||||||
|
Other
|
4,583 | 11.4 | % | 3,425 | 9.0 | % | 1,158 | 33.8 | % | |||||||||||||||
|
Unallocated amounts
|
(10,592 | ) | N/A | (12,463 | ) | N/A | 1,871 | (15.0 | %) | |||||||||||||||
|
Total Company
|
$ | 203,881 | 18.5 | % | $ | 175,969 | 17.1 | % | $ | 27,912 | 15.9 | % | ||||||||||||
|
For the Years Ended December 31,
|
||||||||||||||||||||||||
|
Operating Expenses
(dollars in thousands)
|
2010
|
Percent of
Revenue
|
2009
|
Percent of
Revenue
|
Dollar
Change
|
Percentage
Change
|
||||||||||||||||||
|
Sales and marketing
|
$ | 151,068 | 16.7 | % | $ | 141,681 | 16.8 | % | $ | 9,387 | 6.6 | % | ||||||||||||
|
General and administrative
|
102,775 | 11.3 | % | 92,122 | 10.9 | % | 10,653 | 11.6 | % | |||||||||||||||
|
Research and development
|
43,950 | 4.9 | % | 40,432 | 4.8 | % | 3,518 | 8.7 | % | |||||||||||||||
|
Total operating expenses
|
$ | 297,793 | 32.9 | % | $ | 274,235 | 32.5 | % | $ | 23,558 | 8.6 | % | ||||||||||||
|
For the Years Ended December 31,
|
||||||||||||||||||||||||
|
Operating Expenses
(dollars in thousands)
|
2010
|
Percent of
Revenue
|
2009
|
Percent of
Revenue
|
Dollar
Change
|
Percentage
Change
|
||||||||||||||||||
|
Sales and marketing
|
$ | 7,877 | 10.3 | % | $ | 7,115 | 9.7 | % | $ | 762 | 10.7 | % | ||||||||||||
|
General and administrative
|
6,320 | 8.3 | % | 5,851 | 8.0 | % | 469 | 8.0 | % | |||||||||||||||
|
Research and development
|
2,403 | 3.1 | % | 2,652 | 3.6 | % | (249 | ) | (9.4 | %) | ||||||||||||||
|
Total operating expenses
|
$ | 16,600 | 21.7 | % | $ | 15,618 | 21.3 | % | $ | 982 | 6.3 | % | ||||||||||||
|
For the Years Ended December 31,
|
||||||||||||||||||||||||
|
Operating Expenses
(dollars in thousands)
|
2010
|
Percent of
Revenue
|
2009
|
Percent of
Revenue
|
Dollar
Change
|
Percentage
Change
|
||||||||||||||||||
|
Sales and marketing
|
$ | 13,793 | 17.0 | % | $ | 12,650 | 16.4 | % | $ | 1,143 | 9.0 | % | ||||||||||||
|
General and administrative
|
12,246 | 15.1 | % | 12,845 | 16.6 | % | (599 | ) | (4.7 | %) | ||||||||||||||
|
Research and development
|
9,771 | 12.0 | % | 8,490 | 11.0 | % | 1,281 | 15.1 | % | |||||||||||||||
|
Total operating expenses
|
$ | 35,810 | 44.1 | % | $ | 33,985 | 44.0 | % | $ | 1,825 | 5.4 | % | ||||||||||||
|
For the Years Ended December 31,
|
||||||||||||||||||||||||||||
|
Net Revenue
(dollars in thousands)
|
2009
|
2008
|
Dollar
Change
|
Percentage
Change
|
Percentage
Change from
Currency
(1)
|
Percentage
Change from
Acquisitions/
Divestitures
(2)
|
Organic
Revenue
Growth
(3)
|
|||||||||||||||||||||
|
CAG
|
$ | 843,303 | $ | 834,056 | $ | 9,247 | 1.1 | % | (2.3 | %) | (2.0 | %) | 5.4 | % | ||||||||||||||
|
Water
|
73,214 | 74,469 | (1,255 | ) | (1.7 | %) | (3.4 | %) | - | 1.7 | % | |||||||||||||||||
|
LPD
|
77,208 | 80,762 | (3,554 | ) | (4.4 | %) | (3.4 | %) | - | (1.0 | %) | |||||||||||||||||
|
Other
|
37,908 | 34,743 | 3,165 | 9.1 | % | (0.4 | %) | - | 9.5 | % | ||||||||||||||||||
|
Total
|
$ | 1,031,633 | $ | 1,024,030 | $ | 7,603 | 0.7 | % | (2.4 | %) | (1.6 | %) | 4.7 | % | ||||||||||||||
|
(1)
|
The percentage change from currency is a non-U.S. GAAP measure. It represents the percentage change in revenue resulting from the difference between the average exchange rates during the twelve months ended December 31, 2009 and the same period of the prior year applied to foreign currency denominated revenues for the twelve months ended December 31, 2009.
|
|
(2)
|
Represents the percentage change in revenue during the year ended December 31, 2009 compared to the year ended December 31, 2008 attributed to incremental revenues from businesses acquired or revenues lost from businesses divested or discontinued subsequent to December 31, 2007.
|
|
(3)
|
Organic revenue is a non-U.S. GAAP measure and represents the percentage change in revenue during the year ended December 31, 2009 compared to the year ended December 31, 2008 net of acquisitions and divestitures and the effect from changes in foreign currency exchange rates.
|
|
For the Years Ended December 31,
|
||||||||||||||||||||||||||||
|
Net Revenue
(dollars in thousands)
|
2009
|
2008
|
Dollar
Change
|
Percentage
Change
|
Percentage
Change from
Currency
(1)
|
Percentage
Change from
Acquisitions/
Divestitures
(2)
|
Organic
Revenue
Growth
(3)
|
|||||||||||||||||||||
|
Instruments and consumables
|
$ | 332,706 | $ | 318,533 | $ | 14,173 | 4.4 | % | (2.7 | %) | - | 7.1 | % | |||||||||||||||
|
Rapid assay products
|
147,078 | 146,867 | 211 | 0.1 | % | (0.7 | %) | - | 0.8 | % | ||||||||||||||||||
|
Reference laboratory diagnostic and consulting services
|
298,410 | 288,244 | 10,166 | 3.5 | % | (3.0 | %) | 0.7 | % | 5.8 | % | |||||||||||||||||
|
Practice information management systems and digital radiography
|
65,055 | 61,291 | 3,764 | 6.1 | % | (0.8 | %) | 0.2 | % | 6.7 | % | |||||||||||||||||
|
Pharmaceutical products
|
54 | 19,121 | (19,067 | ) | (99.7 | %) | - | (99.6 | %) | (0.1 | %) | |||||||||||||||||
|
Net CAG revenue
|
$ | 843,303 | $ | 834,056 | $ | 9,247 | 1.1 | % | (2.3 | %) | (2.0 | %) | 5.4 | % | ||||||||||||||
|
(1)
|
The percentage change from currency is a non-U.S. GAAP measure. It represents the percentage change in revenue resulting from the difference between the average exchange rates during the twelve months ended December 31, 2009 and the same period of the prior year applied to foreign currency denominated revenues for the twelve months ended December 31, 2009.
|
|
(2)
|
Represents the percentage change in revenue during the year ended December 31, 2009 compared to the year ended December 31, 2008 attributed to incremental revenues from businesses acquired or revenues lost from businesses divested or discontinued subsequent to December 31, 2007.
|
|
(3)
|
Organic revenue is a non-U.S. GAAP measure and represents the percentage change in revenue during the year ended December 31, 2009 compared to the year ended December 31, 2008 net of acquisitions and divestitures and the effect from changes in foreign currency exchange rates.
|
|
For the Years Ended December 31,
|
||||||||||||||||||||||||
|
Gross Profit
(dollars in thousands)
|
2009
|
Percent of
Revenue
|
2008
|
Percent of
Revenue
|
Dollar
Change
|
Percentage
Change
|
||||||||||||||||||
|
CAG
|
$ | 410,356 | 48.7 | % | $ | 412,199 | 49.4 | % | $ | (1,843 | ) | (0.4 | %) | |||||||||||
|
Water
|
47,233 | 64.5 | % | 47,052 | 63.2 | % | 181 | 0.4 | % | |||||||||||||||
|
LPD
|
51,256 | 66.4 | % | 55,005 | 68.1 | % | (3,749 | ) | (6.8 | %) | ||||||||||||||
|
Other
|
17,067 | 45.0 | % | 15,131 | 43.6 | % | 1,936 | 12.8 | % | |||||||||||||||
|
Unallocated amounts
|
369 | N/A | 379 | N/A | (10 | ) | (2.6 | %) | ||||||||||||||||
|
Total Company
|
$ | 526,281 | 51.0 | % | $ | 529,766 | 51.7 | % | $ | (3,485 | ) | (0.7 | %) | |||||||||||
|
For the Years Ended December 31,
|
||||||||||||||||||||||||
|
Operating Expenses
(dollars in thousands)
|
2009
|
Percent of
Revenue
|
2008
|
Percent of
Revenue
|
Dollar
Change
|
Percentage
Change
|
||||||||||||||||||
|
CAG
|
$ | 274,235 | 32.5 | % | $ | 282,579 | 33.9 | % | $ | (8,344 | ) | (3.0 | %) | |||||||||||
|
Water
|
15,618 | 21.3 | % | 15,722 | 21.1 | % | (104 | ) | (0.7 | %) | ||||||||||||||
|
LPD
|
33,985 | 44.0 | % | 33,245 | 41.2 | % | 740 | 2.2 | % | |||||||||||||||
|
Other
|
13,642 | 36.0 | % | 13,576 | 39.1 | % | 66 | 0.5 | % | |||||||||||||||
|
Unallocated amounts
|
12,832 | N/A | 12,188 | N/A | 644 | 5.3 | % | |||||||||||||||||
|
Total Company
|
$ | 350,312 | 34.0 | % | $ | 357,310 | 34.9 | % | $ | (6,998 | ) | (2.0 | %) | |||||||||||
|
Operating Income
(dollars in thousands)
|
2009
|
Percent of
Revenue
|
2008
|
Percent of
Revenue
|
Dollar
Change
|
Percentage
Change
|
||||||||||||||||||
|
CAG
|
$ | 136,121 | 16.1 | % | $ | 129,620 | 15.5 | % | $ | 6,501 | 5.0 | % | ||||||||||||
|
Water
|
31,615 | 43.2 | % | 31,330 | 42.1 | % | 285 | 0.9 | % | |||||||||||||||
|
LPD
|
17,271 | 22.4 | % | 21,760 | 26.9 | % | (4,489 | ) | (20.6 | %) | ||||||||||||||
|
Other
|
3,425 | 9.0 | % | 1,555 | 4.5 | % | 1,870 | 120.3 | % | |||||||||||||||
|
Unallocated amounts
|
(12,463 | ) | N/A | (11,809 | ) | N/A | (654 | ) | (5.5 | %) | ||||||||||||||
|
Total Company
|
$ | 175,969 | 17.1 | % | $ | 172,456 | 16.8 | % | $ | 3,513 | 2.0 | % | ||||||||||||
|
For the Years Ended December 31,
|
||||||||||||||||||||||||
|
Operating Expenses
(dollars in thousands)
|
2009
|
Percent of
Revenue
|
2008
|
Percent of
Revenue
|
Dollar
Change
|
Percentage
Change
|
||||||||||||||||||
|
Sales and marketing
|
$ | 141,681 | 16.8 | % | $ | 143,644 | 17.2 | % | $ | (1,963 | ) | (1.4 | %) | |||||||||||
|
General and administrative
|
92,122 | 10.9 | % | 93,008 | 11.2 | % | (886 | ) | (1.0 | %) | ||||||||||||||
|
Research and development
|
40,432 | 4.8 | % | 45,927 | 5.5 | % | (5,495 | ) | (12.0 | %) | ||||||||||||||
|
Total operating expenses
|
$ | 274,235 | 32.5 | % | $ | 282,579 | 33.9 | % | $ | (8,344 | ) | (3.0 | %) | |||||||||||
|
For the Years Ended December 31,
|
||||||||||||||||||||||||
|
Operating Expenses
(dollars in thousands)
|
2009
|
Percent of
Revenue
|
2008
|
Percent of
Revenue
|
Dollar
Change
|
Percentage
Change
|
||||||||||||||||||
|
Sales and marketing
|
$ | 7,115 | 9.7 | % | $ | 7,504 | 10.1 | % | $ | (389 | ) | (5.2 | %) | |||||||||||
|
General and administrative
|
5,851 | 8.0 | % | 5,674 | 7.6 | % | 177 | 3.1 | % | |||||||||||||||
|
Research and development
|
2,652 | 3.6 | % | 2,544 | 3.4 | % | 108 | 4.2 | % | |||||||||||||||
|
Total operating expenses
|
$ | 15,618 | 21.3 | % | $ | 15,722 | 21.1 | % | $ | (104 | ) | (0.7 | %) | |||||||||||
|
For the Years Ended December 31,
|
||||||||||||||||||||||||
|
Operating Expenses
(dollars in thousands)
|
2009
|
Percent of
Revenue
|
2008
|
Percent of
Revenue
|
Dollar
Change
|
Percentage
Change
|
||||||||||||||||||
|
Sales and marketing
|
$ | 12,650 | 16.4 | % | $ | 12,982 | 16.1 | % | $ | (332 | ) | (2.6 | %) | |||||||||||
|
General and administrative
|
12,845 | 16.6 | % | 12,416 | 15.4 | % | 429 | 3.5 | % | |||||||||||||||
|
Research and development
|
8,490 | 11.0 | % | 7,847 | 9.7 | % | 643 | 8.2 | % | |||||||||||||||
|
Total operating expenses
|
$ | 33,985 | 44.0 | % | $ | 33,245 | 41.2 | % | $ | 740 | 2.2 | % | ||||||||||||
|
For the Three Months Ended
|
||||||||||||||||||||
|
December 31,
2010
|
September
30,
2010
|
June 30,
2010
|
March 31,
2010
|
December 31,
2009
|
||||||||||||||||
|
Days sales outstanding
(1)
|
38.7 | 41.9 | 41.8 | 41.7 | 38.9 | |||||||||||||||
|
Inventory turns
(2)
|
1.8 | 1.7 | 1.9 | 2.0 | 2.2 | |||||||||||||||
|
For the Years Ended December 31,
|
||||||||||||
|
(dollars in thousands)
|
2010
|
2009
|
Dollar Change
|
|||||||||
|
Net cash provided by operating activities
|
$ | 178,833 | $ | 174,952 | $ | 3,881 | ||||||
|
Net cash (used) provided by investing activities
|
(43,190 | ) | (53,621 | ) | 10,431 | |||||||
|
Net cash (used) provided by financing activities
|
(86,769 | ) | (95,295 | ) | 8,526 | |||||||
|
Net effect of changes in exchange rates on cash
|
1,313 | 1,824 | (511 | ) | ||||||||
|
Net increase in cash and cash equivalents
|
$ | 50,187 | $ | 27,860 | $ | 22,327 | ||||||
|
For the Years Ended December 31,
|
||||||||||||
|
(dollars in thousands)
|
2010
|
2009
|
Dollar Change
|
|||||||||
|
Accounts receivable
|
$ | (6,914 | ) | $ | (1,155 | ) | $ | (5,759 | ) | |||
|
Inventories
|
(19,469 | ) | 6,223 | (25,692 | ) | |||||||
|
Other assets
|
(13,208 | ) | (7,842 | ) | (5,366 | ) | ||||||
|
Accounts payable
|
3,482 | (9,156 | ) | 12,638 | ||||||||
|
Accrued liabilities
|
30,604 | 705 | 29,899 | |||||||||
|
Deferred revenue
|
2,370 | 925 | 1,445 | |||||||||
|
Tax benefit from exercises of stock options and vesting of restricted stock units
|
(18,126 | ) | (5,194 | ) | (12,932 | ) | ||||||
|
Total change in cash due to changes in operating assets and liabilities and the tax benefit from exercises of stock options and vesting of restricted stock units
|
$ | (21,261 | ) | $ | (15,494 | ) | $ | (5,767 | ) | |||
|
|
·
|
We have management and non-management employee incentive programs that provide for the payment of annual bonuses in the first quarter following the year for which the bonuses were earned.
|
|
|
·
|
We have agreements with certain suppliers that require us to make minimum annual inventory purchases, in some cases in order to retain exclusive distribution rights, and we have other agreements with suppliers that provide for lower pricing based on annual purchase volumes. We may place a higher volume of purchase orders for inventory during the fourth quarter in order to meet our minimum commitments or realize volume pricing discounts and we receive that inventory in the fourth or first quarters and pay in the first quarter. The specific facts and circumstances that we consider in determining the timing and level of inventory purchases throughout the year related to these agreements may yield inconsistent cash flows from operations, most typically in the first and fourth quarters. The timing
of inventory receipts also impacts our inventory turnover metrics. To the extent we receive large inventory shipments at the end of a quarter our inventory turnover will be negatively affected.
|
|
(in thousands)
|
Total
|
2011
|
2012–2013
|
2014–2015
|
After 2015
|
|||||||||||||||
|
Long-term debt obligations
(1)
|
$
|
4,726
|
$
|
1,091
|
$
|
2,181
|
$
|
1,454
|
$
|
-
|
||||||||||
|
Operating leases
|
76,951
|
13,777
|
22,736
|
13,619
|
26,819
|
|||||||||||||||
|
Purchase obligations
(2)
|
84,999
|
82,999
|
2,000
|
-
|
-
|
|||||||||||||||
|
Minimum royalty payments
|
6,714
|
813
|
1,841
|
1,490
|
2,570
|
|||||||||||||||
|
Total contractual cash obligations
|
$
|
173,390
|
$
|
98,680
|
$
|
28,758
|
$
|
16,563
|
$
|
29,389
|
||||||||||
|
(1)
|
Long-term debt amounts include interest payments associated with long-term debt.
|
|
(2)
|
Purchase obligations include agreements to purchase goods or services that are enforceable and legally binding and that specify all significant terms, including fixed or minimum quantities, pricing, and approximate timing of purchase transactions.
|
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK
|
|
ITEM 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
|
ITEM 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
|
ITEM 9A.
|
CONTROLS AND PROCEDURES
|
|
|
·
|
Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company;
|
|
|
·
|
Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the Company; and
|
|
|
·
|
Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements.
|
|
ITEM 9B.
|
OTHER INFORMATION
|
|
ITEM 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
|
ITEM 11.
|
EXECUTIVE COMPENSATION
|
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
|
|
ITEM 14.
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
|
ITEM 15.
|
EXHIBITS, FINANCIAL STATEMENT SCHEDULES
|
|
(a) (1) and (a) (2)
|
The financial statements set forth in the Index to Consolidated Financial Statements and the Consolidated Financial Statement Schedule are filed as a part of this Annual Report on Form 10-K commencing on page F-1.
|
|
(a)(3) and (c)
|
The exhibits listed in the accompanying Exhibit Index are filed as part of this Annual Report on Form 10-K and either filed herewith or incorporated by reference herein, as applicable.
|
|
IDEXX LABORATORIES, INC.
|
|
|
By: /s/ Jonathan W. Ayers
|
|
|
Date: February 22, 2011
|
Jonathan W. Ayers
|
|
President and Chief Executive Officer
|
|
|
SIGNATURE
|
TITLE
|
DATE
|
||
|
/s/ Jonathan W. Ayers
|
President, Chief Executive Officer and
|
February 22, 2011
|
||
|
Jonathan W. Ayers
|
Chairman of the Board of Directors
|
|||
|
/s/ Merilee Raines
|
Corporate Vice President, Chief Financial
|
February 22, 2011
|
||
|
Merilee Raines
|
Officer and Treasurer
(Principal Financial and Accounting Officer)
|
|||
|
/s/ Thomas Craig
|
Director
|
February 22, 2011
|
||
|
Thomas Craig
|
||||
|
/s/ William T. End
|
Director
|
February 22, 2011
|
||
|
William T. End
|
||||
|
/s/ Rebecca M. Henderson, PhD
|
Director
|
February 22, 2011
|
||
|
Rebecca M. Henderson, PhD
|
||||
|
/s/ Barry C. Johnson, PhD
|
Director
|
February 22, 2011
|
||
|
Barry C. Johnson, PhD
|
||||
|
/s/ Brian P. McKeon
|
Director
|
February 22, 2011
|
||
|
Brian P. McKeon
|
||||
|
/s/ Joseph V. Vumbacco
|
Director
|
February 22, 2011
|
||
|
Joseph V. Vumbacco
|
||||
|
/s/ Robert J. Murray
|
Director
|
February 22, 2011
|
||
|
Robert J. Murray
|
|
Page No.
|
|
|
Report of Independent Registered Public Accounting Firm
|
F-2
|
|
Consolidated Balance Sheets as of December 31, 2010 and 2009
|
F-3
|
|
Consolidated Statements of Income for the Years Ended December 31, 2010, 2009 and 2008
|
F-4
|
|
Consolidated Statements of Stockholders’ Equity for the Years Ended December 31, 2010, 2009 and 2008
|
F-5
|
|
Consolidated Statements of Cash Flows for the Years Ended December 31, 2010, 2009 and 2008
|
F-7
|
|
Notes to Consolidated Financial Statements
|
F-8
|
|
Schedule II
|
|
|
Valuation and Qualifying Accounts
|
F-39
|
|
December 31,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
ASSETS
|
||||||||
|
Current Assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
156,915
|
$
|
106,728
|
||||
|
Accounts receivable, net of reserves of $2,828 in 2010 and $2,331 in 2009
|
120,080
|
115,107
|
||||||
|
Inventories, net
|
127,885
|
110,425
|
||||||
|
Deferred income tax assets
|
26,203
|
25,188
|
||||||
|
Other current assets
|
29,508
|
18,890
|
||||||
|
Total current assets
|
460,591
|
376,338
|
||||||
|
Long-Term Assets:
|
||||||||
|
Property and equipment, net
|
201,725
|
199,946
|
||||||
|
Goodwill
|
149,112
|
148,705
|
||||||
|
Intangible assets, net
|
55,752
|
63,907
|
||||||
|
Other long-term assets, net
|
29,964
|
19,631
|
||||||
|
Total long-term assets
|
436,553
|
432,189
|
||||||
|
TOTAL ASSETS
|
$
|
897,144
|
$
|
808,527
|
||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
||||||||
|
Current Liabilities:
|
||||||||
|
Accounts payable
|
$
|
22,669
|
$
|
19,133
|
||||
|
Accrued liabilities
|
118,598
|
104,959
|
||||||
|
Line of credit
|
128,999
|
118,790
|
||||||
|
Current portion of long-term debt
|
863
|
813
|
||||||
|
Current portion of deferred revenue
|
13,983
|
12,610
|
||||||
|
Total current liabilities
|
285,112
|
256,305
|
||||||
|
Long-Term Liabilities:
|
||||||||
|
Deferred income tax liabilities
|
18,661
|
18,283
|
||||||
|
Long-term debt, net of current portion
|
3,418
|
4,281
|
||||||
|
Long-term deferred revenue, net of current portion
|
4,627
|
3,813
|
||||||
|
Other long-term liabilities
|
11,045
|
11,266
|
||||||
|
Total long-term liabilities
|
37,751
|
37,643
|
||||||
|
Total liabilities
|
322,863
|
293,948
|
||||||
|
Commitments and Contingencies (Note 14)
|
||||||||
|
Stockholders’ Equity:
|
||||||||
|
Common stock, $0.10 par value: Authorized: 120,000 shares;
Issued: 97,968 and 96,334 shares in 2010 and 2009, respectively
|
9,797
|
9,633
|
||||||
|
Additional paid-in capital
|
641,645
|
580,797
|
||||||
|
Deferred stock units: Outstanding: 118 and 117 units in 2010 and 2009, respectively
|
4,433
|
4,301
|
||||||
|
Retained earnings
|
965,540
|
824,256
|
||||||
|
Accumulated other comprehensive income
|
13,467
|
10,341
|
||||||
|
Treasury stock, at cost: 40,657 and 38,118 shares in 2010 and 2009, respectively
|
(1,060,647
|
)
|
(914,759
|
)
|
||||
|
Total IDEXX Laboratories’ stockholders’ equity
|
574,235
|
514,569
|
||||||
|
Noncontrolling interest
|
46
|
10
|
||||||
|
Total stockholders’ equity
|
574,281
|
514,579
|
||||||
|
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY
|
$
|
897,144
|
$
|
808,527
|
||||
|
For the Years Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Revenue:
|
||||||||||||
|
Product revenue
|
$ | 718,107 | $ | 687,010 | $ | 693,320 | ||||||
|
Service revenue
|
385,285 | 344,623 | 330,710 | |||||||||
|
Total revenue
|
1,103,392 | 1,031,633 | 1,024,030 | |||||||||
|
Cost of revenue:
|
||||||||||||
|
Cost of product revenue
|
285,936 | 281,043 | 270,163 | |||||||||
|
Cost of service revenue
|
238,833 | 224,309 | 224,101 | |||||||||
|
Total cost of revenue
|
524,769 | 505,352 | 494,264 | |||||||||
|
Gross profit
|
578,623 | 526,281 | 529,766 | |||||||||
|
Expenses:
|
||||||||||||
|
Sales and marketing
|
179,626 | 167,748 | 169,956 | |||||||||
|
General and administrative
|
126,519 | 117,440 | 116,681 | |||||||||
|
Research and development
|
68,597 | 65,124 | 70,673 | |||||||||
|
Income from operations
|
203,881 | 175,969 | 172,456 | |||||||||
|
Interest expense
|
(2,415 | ) | (1,916 | ) | (4,589 | ) | ||||||
|
Interest income
|
663 | 486 | 2,320 | |||||||||
|
Income before provisions for income taxes
|
202,129 | 174,539 | 170,187 | |||||||||
|
Provision for income taxes
|
60,809 | 52,304 | 54,018 | |||||||||
|
Net income
|
141,320 | 122,235 | 116,169 | |||||||||
|
Less: Net income attributable to noncontrolling interest
|
36 | 10 | - | |||||||||
|
Net income attributable to IDEXX Laboratories, Inc. stockholders
|
141,284 | 122,225 | 116,169 | |||||||||
|
Earnings per share:
|
||||||||||||
|
Basic
|
$ | 2.45 | $ | 2.08 | $ | 1.94 | ||||||
|
Diluted
|
$ | 2.37 | $ | 2.01 | $ | 1.87 | ||||||
|
Weighted average shares outstanding:
|
||||||||||||
|
Basic
|
57,713 | 58,809 | 59,953 | |||||||||
|
Diluted
|
59,559 | 60,682 | 62,249 | |||||||||
|
The accompanying notes are an integral part of these consolidated financial statements.
|
||||||||||||
|
Accumulated
|
Total IDEXX
|
|||||||||||||||||||||||||||||||||||||||
|
Common Stock
|
Additional
|
Other
|
Laboratories’
|
Total
|
||||||||||||||||||||||||||||||||||||
|
Number of
|
$
0.10
|
Paid-in
|
Deferred
|
Retained
|
Comprehensive
|
Treasury
|
Stockholders’
|
Noncontrolling
|
Stockholders’
|
|||||||||||||||||||||||||||||||
|
Shares
|
Par Value
|
Capital
|
Stock Units
|
Earnings
|
Income
|
Stock
|
Equity
|
Interest
|
Equity
|
|||||||||||||||||||||||||||||||
|
Balance January 1, 2008
|
94,504 | $ | 9,450 | $ | 514,254 | $ | 2,720 | $ | 585,862 | $ | 22,705 | $ | (696,668 | ) | $ | 438,323 | $ | - | $ | 438,323 | ||||||||||||||||||||
|
Comprehensive income (loss):
|
||||||||||||||||||||||||||||||||||||||||
|
Net income
|
- | - | - | - | 116,169 | - | - | 116,169 | - | 116,169 | ||||||||||||||||||||||||||||||
|
Unrealized loss on investments, net of tax of $275
|
- | - | - | - | - | (469 | ) | - | (469 | ) | - | (469 | ) | |||||||||||||||||||||||||||
|
Unrealized net gain on derivative instruments, net of tax of $3,647
|
- | - | - | - | - | 8,118 | - | 8,118 | - | 8,118 | ||||||||||||||||||||||||||||||
|
Translation adjustment
|
- | - | - | - | - | (24,679 | ) | - | (24,679 | ) | - | (24,679 | ) | |||||||||||||||||||||||||||
|
Total comprehensive income
|
- | - | - | - | - | - | - | 99,139 | - | 99,139 | ||||||||||||||||||||||||||||||
|
Purchase of treasury stock
|
- | - | - | - | - | - | (133,722 | ) | (133,722 | ) | - | (133,722 | ) | |||||||||||||||||||||||||||
|
Common stock issued under employee stock options, including excess tax benefit
|
728 | 73 | 20,076 | - | - | - | - | 20,149 | - | 20,149 | ||||||||||||||||||||||||||||||
|
Common stock issued under employee purchase plan, including excess tax benefit
|
80 | 8 | 3,153 | - | - | - | - | 3,161 | 3,161 | |||||||||||||||||||||||||||||||
|
Common stock issued under employee restricted and deferred stock plans
|
75 | 8 | 428 | (38 | ) | - | - | - | 398 | - | 398 | |||||||||||||||||||||||||||||
|
Issuance of deferred stock units
|
- | - | - | 515 | - | - | - | 515 | - | 515 | ||||||||||||||||||||||||||||||
|
Vesting of deferred stock units
|
- | - | (450 | ) | 450 | - | - | - | - | - | - | |||||||||||||||||||||||||||||
|
Share-based compensation cost recognized
|
- | - | 10,231 | - | - | - | - | 10,231 | - | 10,231 | ||||||||||||||||||||||||||||||
|
Balance December 31, 2008
|
95,387 | 9,539 | 547,692 | 3,647 | 702,031 | 5,675 | (830,390 | ) | 438,194 | - | 438,194 | |||||||||||||||||||||||||||||
|
Comprehensive income (loss):
|
||||||||||||||||||||||||||||||||||||||||
|
Net income, attributable to stockholders
|
- | - | - | - | 122,225 | - | - | 122,225 | 10 | 122,235 | ||||||||||||||||||||||||||||||
|
Unrealized gain on investments, net of tax of $224
|
- | - | - | - | - | 401 | - | 401 | - | 401 | ||||||||||||||||||||||||||||||
|
Unrealized net loss on derivative instruments, net of tax of $4,607
|
- | - | - | - | - | (10,105 | ) | - | (10,105 | ) | - | (10,105 | ) | |||||||||||||||||||||||||||
|
Translation adjustment
|
- | - | - | - | - | 14,370 | - | 14,370 | - | 14,370 | ||||||||||||||||||||||||||||||
|
Total comprehensive income
|
126,891 | 10 | 126,901 | |||||||||||||||||||||||||||||||||||||
|
Purchase of treasury stock
|
- | - | - | - | - | - | (84,369 | ) | (84,369 | ) | - | (84,369 | ) | |||||||||||||||||||||||||||
|
Common stock issued under employee stock options, including excess tax benefit
|
755 | 75 | 19,058 | - | - | - | - | 19,133 | - | 19,133 | ||||||||||||||||||||||||||||||
|
Common stock issued under employee purchase plan, including excess tax benefit
|
88 | 9 | 3,277 | - | - | - | - | 3,286 | - | 3,286 | ||||||||||||||||||||||||||||||
|
Common stock issued under employee restricted and deferred stock plans
|
104 | 10 | (335 | ) | (34 | ) | - | - | - | (359 | ) | - | (359 | ) | ||||||||||||||||||||||||||
|
Issuance of deferred stock units
|
- | - | - | 418 | - | - | - | 418 | - | 418 | ||||||||||||||||||||||||||||||
|
Vesting of deferred stock units
|
- | - | (270 | ) | 270 | - | - | - | - | - | - | |||||||||||||||||||||||||||||
|
Share-based compensation cost recognized
|
- | - | 11,375 | - | - | - | - | 11,375 | - | 11,375 | ||||||||||||||||||||||||||||||
|
Balance December 31, 2009
|
96,334 | $ | 9,633 | $ | 580,797 | $ | 4,301 | $ | 824,256 | $ | 10,341 | $ | (914,759 | ) | $ | 514,569 | $ | 10 | $ | 514,579 | ||||||||||||||||||||
|
Accumulated
|
Total IDEXX
|
|||||||||||||||||||||||||||||||||||||||
|
Common Stock
|
Additional
|
Other
|
Laboratories’
|
Total
|
||||||||||||||||||||||||||||||||||||
|
Number of
|
$0.10
|
Paid-in
|
Deferred
|
Retained
|
Comprehensive
|
Treasury
|
Stockholders’
|
Noncontrolling
|
Stockholders’
|
|||||||||||||||||||||||||||||||
|
Shares
|
Par Value
|
Capital
|
Stock Units
|
Earnings
|
Income
|
Stock
|
Equity
|
Interest
|
Equity
|
|||||||||||||||||||||||||||||||
|
Balance December 31, 2009
|
96,334 | $ | 9,633 | $ | 580,797 | $ | 4,301 | $ | 824,256 | $ | 10,341 | $ | (914,759 | ) | $ | 514,569 | $ | 10 | $ | 514,579 | ||||||||||||||||||||
|
Comprehensive income (loss):
|
||||||||||||||||||||||||||||||||||||||||
|
Net income, attributable to stockholders
|
- | - | - | - | 141,284 | - | - | 141,284 | 36 | 141,320 | ||||||||||||||||||||||||||||||
|
Unrealized gain on investments, net of tax of $108
|
- | - | - | - | - | 176 | - | 176 | - | 176 | ||||||||||||||||||||||||||||||
|
Unrealized net gain on derivative instruments, net of tax of $240
|
- | - | - | - | - | 730 | - | 730 | - | 730 | ||||||||||||||||||||||||||||||
|
Translation adjustment
|
- | - | - | - | - | 2,220 | - | 2,220 | - | 2,220 | ||||||||||||||||||||||||||||||
|
Total comprehensive income
|
144,410 | 36 | 144,446 | |||||||||||||||||||||||||||||||||||||
|
Purchase of treasury stock
|
- | - | - | - | - | - | (145,888 | ) | (145,888 | ) | - | (145,888 | ) | |||||||||||||||||||||||||||
|
Common stock issued under employee stock options, including excess tax benefit
|
1,411 | 141 | 43,501 | - | - | - | - | 43,642 | - | 43,642 | ||||||||||||||||||||||||||||||
|
Common stock issued under employee purchase plan, including excess tax benefit
|
64 | 7 | 3,384 | - | - | - | - | 3,391 | - | 3,391 | ||||||||||||||||||||||||||||||
|
Common stock issued under employee restricted and deferred stock plans
|
159 | 16 | 1,119 | (455 | ) | - | - | - | 680 | - | 680 | |||||||||||||||||||||||||||||
|
Issuance of deferred stock units
|
- | - | - | 362 | - | - | - | 362 | - | 362 | ||||||||||||||||||||||||||||||
|
Vesting of deferred stock units
|
- | - | (225 | ) | 225 | - | - | - | - | - | - | |||||||||||||||||||||||||||||
|
Share-based compensation cost recognized
|
- | - | 13,069 | - | - | - | - | 13,069 | - | 13,069 | ||||||||||||||||||||||||||||||
|
Balance December 31, 2010
|
97,968 | $ | 9,797 | $ | 641,645 | $ | 4,433 | $ | 965,540 | $ | 13,467 | $ | (1,060,647 | ) | $ | 574,235 | $ | 46 | $ | 574,281 | ||||||||||||||||||||
|
For the Years Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Cash Flows from Operating Activities:
|
||||||||||||
|
Net income
|
$
|
141,320
|
$
|
122,235
|
$
|
116,169
|
||||||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
||||||||||||
|
Depreciation and amortization
|
45,956
|
49,773
|
47,984
|
|||||||||
|
Loss on disposal of property and equipment
|
1,599
|
2,474
|
835
|
|||||||||
|
Increase (decrease) in deferred compensation liability
|
290
|
484
|
(726
|
)
|
||||||||
|
(Gain) loss on disposition of pharmaceutical product lines and related restructuring
|
(3,000
|
)
|
(2,000
|
)
|
1,479
|
|||||||
|
Write-down of equine digital radiography intangible assets
|
-
|
1,511
|
-
|
|||||||||
|
Write-down of marketable securities
|
-
|
150
|
-
|
|||||||||
|
Provision for uncollectible accounts
|
1,575
|
926
|
1,180
|
|||||||||
|
(Benefit of) provision for deferred income taxes
|
(908
|
)
|
3,270
|
5,634
|
||||||||
|
Share-based compensation expense
|
13,262
|
11,623
|
10,501
|
|||||||||
|
Tax benefit from exercises of stock options and vesting of restricted stock units
|
(18,126
|
)
|
(5,194
|
)
|
(6,237
|
)
|
||||||
|
Changes in assets and liabilities, net of acquisitions:
|
||||||||||||
|
Accounts receivable
|
(6,914
|
)
|
(1,155
|
)
|
(10,266
|
)
|
||||||
|
Inventories
|
(19,469
|
)
|
6,223
|
(18,468
|
)
|
|||||||
|
Other assets
|
(13,208
|
)
|
(7,842
|
)
|
(3,902
|
)
|
||||||
|
Accounts payable
|
3,482
|
(9,156
|
)
|
(4,327
|
)
|
|||||||
|
Accrued liabilities
|
30,604
|
705
|
4,257
|
|||||||||
|
Deferred revenue
|
2,370
|
925
|
(805
|
)
|
||||||||
|
Net cash provided by operating activities
|
178,833
|
174,952
|
143,308
|
|||||||||
|
Cash Flows from Investing Activities:
|
||||||||||||
|
Purchases of property and equipment
|
(38,908
|
)
|
(50,663
|
)
|
(89,971
|
)
|
||||||
|
Proceeds from disposition of pharmaceutical product lines
|
-
|
3,377
|
7,025
|
|||||||||
|
Proceeds from sale of property and equipment
|
112
|
2,079
|
-
|
|||||||||
|
Acquisition of intangible assets
|
(394
|
)
|
(500
|
)
|
(4,758
|
)
|
||||||
|
Investment in notes receivable and related business
|
(4,000
|
)
|
-
|
-
|
||||||||
|
Acquisitions of businesses, net of cash acquired
|
-
|
(7,914
|
)
|
(3,891
|
)
|
|||||||
|
Net cash used by investing activities
|
(43,190
|
)
|
(53,621
|
)
|
(91,595
|
)
|
||||||
|
Cash Flows from Financing Activities:
|
||||||||||||
|
Borrowings (payments) on revolving credit facilities, net
|
10,143
|
(32,830
|
)
|
79,550
|
||||||||
|
Payment of other notes payable
|
(813
|
)
|
(926
|
)
|
(595
|
)
|
||||||
|
Purchases of treasury stock
|
(143,090
|
)
|
(83,099
|
)
|
(132,342
|
)
|
||||||
|
Proceeds from exercises of stock options and employee stock purchase plans
|
28,865
|
16,366
|
16,360
|
|||||||||
|
Tax benefit from exercises of stock options and vesting of restricted stock units
|
18,126
|
5,194
|
6,237
|
|||||||||
|
Net cash used by financing activities
|
(86,769
|
)
|
(95,295
|
)
|
(30,790
|
)
|
||||||
|
Net effect of changes in exchange rates on cash
|
1,313
|
1,824
|
(2,415
|
)
|
||||||||
|
Net increase in cash and cash equivalents
|
50,187
|
27,860
|
18,508
|
|||||||||
|
Cash and cash equivalents at beginning of period
|
106,728
|
78,868
|
60,360
|
|||||||||
|
Cash and cash equivalents at end of period
|
$
|
156,915
|
$
|
106,728
|
$
|
78,868
|
||||||
|
Supplemental Disclosures of Cash Flow Information:
|
||||||||||||
|
Interest paid
|
$
|
2,598
|
$
|
2,773
|
$
|
5,076
|
||||||
|
Income taxes paid
|
$
|
48,113
|
$
|
45,731
|
$
|
49,547
|
||||||
|
Supplemental Disclosure of Non-Cash Information:
|
||||||||||||
|
Market value of common shares received from employees in connection with
share-based compensation – see Note 18
|
$
|
2,797
|
$
|
1,270
|
$
|
1,380
|
||||||
|
Receivable on disposition of pharmaceutical product lines
|
$
|
3,000
|
$
|
-
|
$
|
1,377
|
||||||
|
(a)
|
Estimates
|
|
(
b
)
|
Cash and cash equivalents
|
|
(c)
|
Inventories
|
|
(d)
|
Property and Equipment
|
|
Asset Classification
|
Estimated Useful Life
|
|
|
Land improvements
|
15 years
|
|
|
Buildings and improvements
|
15–40 years
|
|
|
Leasehold improvements
|
Shorter of life of lease or useful life
|
|
|
Machinery and equipment
|
3–7 years
|
|
|
Office furniture and equipment
|
3–7 years
|
|
|
Computer hardware and software
|
3–7 years
|
|
(e)
|
Goodwill and Other Intangible Assets
|
|
Asset Classification
|
Estimated
Useful Life
|
|
|
Patents
|
7–15 years
|
|
|
Product rights
(1)
|
5–15 years
|
|
|
Customer-related intangible assets
(2)
|
7–15 years
|
|
|
Noncompete agreements
|
3–9 years
|
|
(1)
|
Product rights comprise certain technologies, licenses and trade names acquired from third parties.
|
|
|
(2)
|
Customer-related intangible assets comprise customer lists and customer relationships acquired from third parties.
|
|
For the Years Ended December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Balance, beginning of year
|
$
|
3,104
|
$
|
2,837
|
||||
|
Provision for warranty expense
|
3,113
|
4,424
|
||||||
|
Change in estimate, balance beginning of year
|
(1,298
|
)
|
(819
|
)
|
||||
|
Settlement of warranty liability
|
(2,723
|
)
|
(3,338
|
)
|
||||
|
Balance, end of year
|
$
|
2,196
|
$
|
3,104
|
||||
|
(g)
|
Income Taxes
|
|
|
·
|
We recognize revenue at the time of shipment to U.S. distributors for substantially all products sold through distributors because title and risk of loss pass to the distributors on delivery to the common carrier. Our distributors do not have the right to return products. We recognize revenue for the remainder of our customers, including distributors outside of the U.S., when the product is delivered to the customer, except as noted below.
|
|
|
·
|
We recognize revenue from the sales of instruments, non-cancelable software licenses and hardware systems upon installation (and completion of training if applicable) and the customer’s acceptance of the instrument or system as we have no significant further obligations after this point in time.
|
|
|
·
|
We recognize service revenue at the time the service is performed.
|
|
|
·
|
We recognize revenue associated with extended maintenance agreements (“EMAs”) over the life of the contracts using the straight-line method, which approximates the expected timing in which applicable services are performed. Amounts collected in advance of revenue recognition are recorded as a current or long-term liability based on the time from the balance sheet date to the future date of revenue recognition.
|
|
|
·
|
We recognize revenue on certain instrument systems under rental programs over the life of the rental agreement using the straight-line method. Amounts collected in advance of revenue recognition are recorded as a current or long-term liability based on the time from the balance sheet date to the future date of revenue recognition.
|
|
|
·
|
We recognize revenue on practice information management systems sales, where the system includes software that is considered more than incidental to the utility and value of the system, either by allocating the revenue to each element of the sale based on relative fair values of the elements, including post-contract support when fair value for all elements is available, or by use of the residual method when only the fair value of the post-contract support is available. We recognize revenue for the system upon installation and customer acceptance and recognize revenue equal to the fair value of the post-contract support over the support period.
|
|
|
·
|
Shipping costs reimbursed by the customer are included in revenue.
|
|
(m)
|
Foreign Currency Translation
|
|
December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Unrealized loss on investments, net of tax
|
$
|
(179
|
)
|
$
|
(355
|
)
|
||
|
Derivative Instruments:
|
||||||||
|
Unrealized loss on forward currency exchange contracts, net of tax
|
(1,544
|
)
|
(2,913
|
)
|
||||
|
Unrealized loss on interest rate swap agreements, net of tax
|
(1,013
|
)
|
(375
|
)
|
||||
|
Cumulative translation adjustment
|
16,203
|
13,984
|
||||||
|
$
|
13,467
|
$
|
10,341
|
|||||
|
For the Years Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Share-based compensation expense included in cost of revenue
|
$ | 1,290 | $ | 1,280 | $ | 1,120 | ||||||
|
Share-based compensation expense included in operating expenses
|
11,779 | 10,095 | 9,111 | |||||||||
|
Total share-based compensation expense
|
13,069 | 11,375 | 10,231 | |||||||||
|
Income tax benefit in net income for share-based compensation expense
|
(3,902 | ) | (3,367 | ) | (2,835 | ) | ||||||
|
Income tax benefit in net income for employees’ disqualifying dispositions of shares
acquired through the exercise of stock options and employee stock purchase rights
|
(695 | ) | (460 | ) | (415 | ) | ||||||
|
Total income tax benefit
|
(4,597 | ) | (3,827 | ) | (3,250 | ) | ||||||
|
Net impact of share-based compensation on net income
|
$ | 8,472 | $ | 7,548 | $ | 6,981 | ||||||
|
For the Years Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Cash proceeds from employee stock purchases and options exercised under all share-based payment arrangements
|
$ | 28,865 | $ | 16,366 | $ | 16,360 | ||||||
|
Reduction of income taxes payable due to employee’s share-based compensation tax
|
$ | 22,784 | $ | 7,971 | $ | 9,037 | ||||||
|
For the Years Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Expected stock price volatility
|
31
|
%
|
31
|
%
|
25
|
%
|
||||||
|
Expected term, in years
|
4.9
|
4.8
|
4.9
|
|||||||||
|
Risk-free interest rate
|
2.3
|
%
|
1.6
|
%
|
2.6
|
%
|
||||||
|
Weighted average fair value of options granted
|
$
|
16.56
|
$
|
9.97
|
$
|
14.63
|
||||||
|
Number of
Options (000)
|
Weighted
Average
Exercise Price
|
Weighted
Average
Remaining
Contractual
Term
|
Aggregate
Intrinsic Value
($000)
|
|||||||||||||
|
Outstanding at December 31, 2009
|
4,789 | $ | 27.12 | |||||||||||||
|
Granted
|
436 | 53.51 | ||||||||||||||
|
Exercised
|
(1,411 | ) | 18.05 | |||||||||||||
|
Forfeited
|
(56 | ) | 45.06 | |||||||||||||
|
Expired
|
(4 | ) | 25.46 | |||||||||||||
|
Outstanding at December 31, 2010
|
3,754 | $ | 33.34 | 3.5 | $ | 134,713 | ||||||||||
|
Fully vested at December 31, 2010
|
2,509 | $ | 27.00 | 2.8 | $ | 105,934 | ||||||||||
|
Fully vested and expected to vest, at December 31, 2010
|
3,667 | $ | 33.02 | 3.5 | $ | 132,739 | ||||||||||
|
For the Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Number of purchase rights issued
|
64 | 89 | 80 | |||||||||
|
Weighted average fair value per purchase right issued
|
$ | 9.29 | $ | 6.54 | $ | 7.02 | ||||||
|
Number
of Units
(000)
|
Weighted
Average Grant-
Date Fair Value
|
|||||||
|
Unvested at December 31, 2009
|
555 | $ | 41.70 | |||||
|
Granted
|
152 | $ | 53.48 | |||||
|
Vested
|
(153 | ) | $ | 41.93 | ||||
|
Forfeited
|
(43 | ) | $ | 43.95 | ||||
|
Unvested at December 31, 2010
|
511 | $ | 44.94 | |||||
|
Fully vested at December 31, 2010
|
31 | $ | 41.09 | |||||
|
Fully vested and expected to vest, at December 31, 2010
|
477 | $ | 44.60 | |||||
|
December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Raw materials
|
$
|
26,758
|
$
|
28,426
|
||||
|
Work-in-process
|
13,790
|
17,761
|
||||||
|
Finished goods
|
87,337
|
64,238
|
||||||
|
$
|
127,885
|
$
|
110,425
|
|||||
|
December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Land and improvements
|
$
|
7,446
|
$
|
7,389
|
||||
|
Buildings and improvements
|
109,090
|
101,725
|
||||||
|
Leasehold improvements
|
20,080
|
18,515
|
||||||
|
Machinery and equipment
|
110,406
|
103,072
|
||||||
|
Office furniture and equipment
|
26,806
|
24,866
|
||||||
|
Computer hardware and software
|
81,971
|
66,979
|
||||||
|
Construction in progress
|
20,826
|
24,046
|
||||||
|
376,625
|
346,592
|
|||||||
|
Less accumulated depreciation and amortization
|
174,900
|
146,646
|
||||||
|
Total property and equipment, net
|
$
|
201,725
|
$
|
199,946
|
||||
|
December 31,
|
||||||||
|
Description
|
2010
|
2009
|
||||||
|
Deferred tax assets, net
|
$
|
630
|
$
|
1,017
|
||||
|
Cost of rental instruments sold under recourse, net
|
2,644
|
1,665
|
||||||
|
Investment in long-term product supply arrangements
|
12,120
|
11,320
|
||||||
|
Notes receivable
|
3,742
|
-
|
||||||
|
Customer acquisition costs, net
|
5,470
|
1,343
|
||||||
|
Other assets
|
5,358
|
4,286
|
||||||
|
$
|
29,964
|
$
|
19,631
|
|||||
|
December 31, 2010
|
December 31, 2009
|
|||||||||||||||
|
Cost
|
Accumulated
Amortization
|
Cost
|
Accumulated
Amortization
|
|||||||||||||
|
Patents
|
$ | 9,377 | $ | 5,825 | $ | 9,446 | $ | 4,885 | ||||||||
|
Product rights
(1)
|
31,641 | 17,974 | 30,334 | 14,505 | ||||||||||||
|
Customer-related intangible assets
(2)
|
58,941 | 21,655 | 58,544 | 17,147 | ||||||||||||
|
Noncompete agreements
|
5,949 | 4,702 | 6,127 | 4,007 | ||||||||||||
| $ | 105,908 | $ | 50,156 | $ | 104,451 | $ | 40,544 | |||||||||
|
|
(1)
|
Product rights comprise certain technologies, licenses and trade names acquired from third parties.
|
|
|
(2)
|
Customer-related intangible assets comprise customer lists and customer relationships acquired from third parties.
|
|
Amortization
Expense
|
||||
|
2011
|
$ | 8,485 | ||
|
2012
|
8,177 | |||
|
2013
|
7,292 | |||
|
2014
|
6,483 | |||
|
2015
|
6,109 | |||
|
Thereafter
|
19,206 | |||
| $ | 55,752 | |||
|
December 31,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Companion animal group segment
|
$
|
118,131
|
$
|
117,955
|
||||
|
Water segment
|
13,648
|
14,002
|
||||||
|
Livestock and poultry diagnostics segment
|
10,802
|
10,217
|
||||||
|
Other segment
|
6,531
|
6,531
|
||||||
|
$
|
149,112
|
$
|
148,705
|
|||||
|
December 31,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Accrued expenses
|
$
|
36,150
|
$
|
33,094
|
||||
|
Accrued employee compensation and related expenses
|
47,914
|
44,497
|
||||||
|
Accrued taxes
|
12,320
|
9,980
|
||||||
|
Accrued customer programs
|
22,214
|
17,388
|
||||||
|
$
|
118,598
|
$
|
104,959
|
|||||
|
Years Ending December 31,
|
Amount
|
|||
|
2011
|
$ | 863 | ||
|
2012
|
917 | |||
|
2013
|
1,107 | |||
|
2014
|
1,035 | |||
|
2015
|
359 | |||
| $ | 4,281 | |||
|
For the Years Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Domestic
|
$ | 151,660 | $ | 124,974 | $ | 123,632 | ||||||
|
International
|
50,469 | 49,565 | 46,555 | |||||||||
| $ | 202,129 | $ | 174,539 | $ | 170,187 | |||||||
|
For the Years Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Current
|
||||||||||||
|
Federal
|
$ | 44,833 | $ | 34,043 | $ | 33,276 | ||||||
|
State
|
5,079 | 3,984 | 3,839 | |||||||||
|
International
|
11,805 | 11,007 | 11,269 | |||||||||
| 61,717 | 49,034 | 48,384 | ||||||||||
|
Deferred
|
||||||||||||
|
Federal
|
958 | 4,876 | 9,365 | |||||||||
|
State
|
(230 | ) | (107 | ) | 540 | |||||||
|
International
|
(1,636 | ) | (1,499 | ) | (4,271 | ) | ||||||
| (908 | ) | 3,270 | 5,634 | |||||||||
| $ | 60,809 | $ | 52,304 | $ | 54,018 | |||||||
|
For the Years Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
U.S. federal statutory rate
|
35.0 | % | 35.0 | % | 35.0 | % | ||||||
|
State income tax, net of federal tax benefit
|
1.4 | 1.4 | 1.8 | |||||||||
|
International income taxes
|
(3.6 | ) | (4.5 | ) | (5.5 | ) | ||||||
|
Domestic manufacturing exclusions
|
(1.6 | ) | (0.9 | ) | (0.7 | ) | ||||||
|
Research and experiment credit
|
(1.2 | ) | (1.1 | ) | (1.2 | ) | ||||||
|
Pharmaceutical non-deductible goodwill write-off
|
- | - | 1.5 | |||||||||
|
Other, net
|
0.1 | 0.1 | 0.8 | |||||||||
|
Effective tax rate
|
30.1 | % | 30.0 | % | 31.7 | % | ||||||
|
December 31, 2010
|
December 31, 2009
|
|||||||||||||||
|
Current
|
Long-Term
|
Current
|
Long-Term
|
|||||||||||||
|
Assets:
|
||||||||||||||||
|
Accrued expenses
|
$ | 18,820 | $ | - | $ | 15,600 | $ | - | ||||||||
|
Accounts receivable reserves
|
702 | - | 647 | - | ||||||||||||
|
Deferred revenue
|
804 | 1,059 | 1,865 | 168 | ||||||||||||
|
Inventory basis differences
|
3,415 | - | 4,465 | - | ||||||||||||
|
Property-based differences
|
- | 1,228 | - | 1,258 | ||||||||||||
|
Share-based compensation
|
2,041 | 6,298 | 1,789 | 5,461 | ||||||||||||
|
Other
|
296 | 13 | 535 | 126 | ||||||||||||
|
Net operating loss carryforwards
|
731 | 4,720 | 20 | 5,352 | ||||||||||||
|
Unrealized losses on foreign exchange
contracts and investments
|
609 | - | 299 | - | ||||||||||||
|
Total assets
|
27,418 | 13,318 | 25,220 | 12,365 | ||||||||||||
|
Valuation allowance
|
(281 | ) | (4,323 | ) | (514 | ) | (4,617 | ) | ||||||||
|
Total assets, net of valuation allowance
|
27,137 | 8,995 | 24,706 | 7,748 | ||||||||||||
|
Liabilities:
|
||||||||||||||||
|
Cost of rental instruments sold under recourse
|
- | (526 | ) | - | (287 | ) | ||||||||||
|
Property-based differences
|
- | (13,776 | ) | - | (10,723 | ) | ||||||||||
|
Intangible asset basis differences
|
- | (13,035 | ) | - | (12,891 | ) | ||||||||||
|
Other
|
(168 | ) | (21 | ) | (163 | ) | (371 | ) | ||||||||
|
Total liabilities
|
(168 | ) | (27,358 | ) | (163 | ) | (24,272 | ) | ||||||||
|
Net deferred tax assets (liabilities)
|
$ | 26,969 | (18,363 | ) | $ | 24,543 | $ | (16,524 | ) | |||||||
|
For the Years Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Total amounts of unrecognized tax benefits, beginning of period
|
$ | 5,429 | $ | 5,850 | $ | 5,086 | ||||||
|
Gross decreases in unrecognized tax benefits as a result of tax positions taken during a prior period
|
- | - | - | |||||||||
|
Gross increases in unrecognized tax benefits as a result of tax positions taken in the current period
|
1,143 | 1,120 | 1,447 | |||||||||
|
Decreases in unrecognized tax benefits relating to settlements with taxing authorities
|
- | (513 | ) | - | ||||||||
|
Decreases in unrecognized tax benefits as a result of a lapse of the applicable statutes of limitations
|
(1,425 | ) | (1,141 | ) | (345 | ) | ||||||
|
Net effect of foreign currency translation
|
(171 | ) | 113 | (338 | ) | |||||||
|
Total amounts of unrecognized tax benefits, end of period
|
$ | 4,976 | $ | 5,429 | $ | 5,850 | ||||||
|
For the Years Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Shares outstanding for basic earnings per share:
|
||||||||||||
|
Weighted average shares outstanding
|
57,591 | 58,695 | 59,855 | |||||||||
|
Weighted average vested deferred stock units outstanding
|
122 | 114 | 98 | |||||||||
| 57,713 | 58,809 | 59,953 | ||||||||||
|
Shares outstanding for diluted earnings per share:
|
||||||||||||
|
Shares outstanding for basic earnings per share
|
57,713 | 58,809 | 59,953 | |||||||||
|
Dilutive effect of options issued
|
1,645 | 1,724 | 2,198 | |||||||||
|
Dilutive effect of restricted stock units issued
|
199 | 142 | 93 | |||||||||
|
Dilutive effect of unvested deferred stock units issued
|
2 | 7 | 5 | |||||||||
| 59,559 | 60,682 | 62,249 | ||||||||||
|
For the Years Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Weighted average number of shares underlying anti-dilutive options
|
501
|
878
|
833
|
|||||||||
|
Weighted average exercise price per underlying share of anti-dilutive options
|
$
|
54.53
|
$
|
49.40
|
$
|
50.10
|
||||||
|
Weighted average number of shares underlying anti-dilutive restricted stock units
|
-
|
2
|
134
|
|||||||||
|
December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Closing price per share of our common stock
|
$
|
69.22
|
$
|
53.45
|
||||
|
Number of shares underlying outstanding options with exercise prices below the closing price
|
3,754
|
4,427
|
||||||
|
Number of shares underlying outstanding options with exercise prices equal to or above the closing price
|
-
|
362
|
||||||
|
Total number of shares underlying outstanding options
|
3,754
|
4,789
|
||||||
|
Years Ending December 31,
|
Amount
|
|||
|
2011
|
$ | 13,777 | ||
|
2012
|
12,424 | |||
|
2013
|
10,312 | |||
|
2014
|
7,449 | |||
|
2015
|
6,170 | |||
|
Thereafter
|
26,819 | |||
| $ | 76,951 | |||
|
For the Years Ended December 31,
|
||||||||||||||||||||||||
|
CAG
|
Water
|
LPD
|
Other
|
Unallocated Amounts
|
Consolidated Total
|
|||||||||||||||||||
|
2010
|
||||||||||||||||||||||||
|
Revenue
|
$ | 905,655 | $ | 76,514 | $ | 81,177 | $ | 40,046 | $ | - | $ | 1,103,392 | ||||||||||||
|
Income (loss) from operations
|
$ | 159,726 | $ | 31,076 | $ | 19,088 | $ | 4,583 | $ | (10,592 | ) | $ | 203,881 | |||||||||||
|
Interest expense, net
|
1,752 | |||||||||||||||||||||||
|
Income before provision for income taxes
|
202,129 | |||||||||||||||||||||||
|
Provision for income taxes
|
60,809 | |||||||||||||||||||||||
|
Net income
|
141,320 | |||||||||||||||||||||||
|
Net income attributable to noncontrolling interest
|
36 | |||||||||||||||||||||||
|
Net income attributable to IDEXX Laboratories’ stockholders
|
$ | 141,284 | ||||||||||||||||||||||
|
Depreciation and amortization
|
$ | 38,211 | $ | 1,532 | $ | 3,809 | $ | 2,404 | $ | - | $ | 45,956 | ||||||||||||
|
Segment assets
|
551,492 | 41,884 | 57,390 | 38,028 | 208,350 | 897,144 | ||||||||||||||||||
|
Expenditures for long-lived assets
(1)
|
31,499 | 1,642 | 2,815 | 2,952 | - | 38,908 | ||||||||||||||||||
|
2009
|
||||||||||||||||||||||||
|
Revenue
|
$ | 843,303 | $ | 73,214 | $ | 77,208 | $ | 37,908 | $ | - | $ | 1,031,633 | ||||||||||||
|
Income (loss) from operations
|
$ | 136,121 | $ | 31,615 | $ | 17,271 | $ | 3,425 | $ | (12,463 | ) | $ | 175,969 | |||||||||||
|
Interest expense, net
|
1,430 | |||||||||||||||||||||||
|
Income before provision for income taxes
|
174,539 | |||||||||||||||||||||||
|
Provision for income taxes
|
52,304 | |||||||||||||||||||||||
|
Net income
|
122,235 | |||||||||||||||||||||||
|
Net income attributable to noncontrolling interest
|
10 | |||||||||||||||||||||||
|
Net income attributable to IDEXX Laboratories’ stockholders
|
$ | 122,225 | ||||||||||||||||||||||
|
Depreciation and amortization
|
$ | 41,865 | $ | 1,457 | $ | 4,544 | $ | 1,907 | $ | - | $ | 49,773 | ||||||||||||
|
Segment assets
|
519,098 | 43,893 | 57,897 | 35,779 | 151,860 | 808,527 | ||||||||||||||||||
|
Expenditures for long-lived assets
(1)
|
41,111 | 3,110 | 3,337 | 4,774 | - | 52,332 | ||||||||||||||||||
|
2008
|
||||||||||||||||||||||||
|
Revenue
|
$ | 834,056 | $ | 74,469 | $ | 80,762 | $ | 34,743 | $ | - | $ | 1,024,030 | ||||||||||||
|
Income (loss) from operations
|
$ | 129,620 | $ | 31,330 | $ | 21,760 | $ | 1,555 | $ | (11,809 | ) | $ | 172,456 | |||||||||||
|
Interest expense, net
|
2,269 | |||||||||||||||||||||||
|
Income before provision for income taxes
|
170,187 | |||||||||||||||||||||||
|
Provision for income taxes
|
54,018 | |||||||||||||||||||||||
|
Net income
|
116,169 | |||||||||||||||||||||||
|
Net income attributable to noncontrolling interest
|
- | |||||||||||||||||||||||
|
Net income attributable to IDEXX Laboratories’ stockholders
|
$ | 116,169 | ||||||||||||||||||||||
|
Depreciation and amortization
|
$ | 39,913 | $ | 600 | $ | 5,075 | $ | 2,396 | $ | - | $ | 47,984 | ||||||||||||
|
Segment assets
|
500,824 | 41,429 | 58,019 | 33,009 | 132,156 | 765,437 | ||||||||||||||||||
|
Expenditures for long-lived assets
(1)
|
74,145 | 4,761 | 6,794 | 3,573 | - | 89,273 | ||||||||||||||||||
|
(1)
|
Expenditures for long-lived assets exclude expenditures for intangible assets. See Note 4 and Note 9 for information regarding acquisitions of intangible assets during the years ended December 31, 2010, 2009 and 2008.
|
|
For the Years Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
CAG segment revenue:
|
||||||||||||
|
Instruments and consumables
|
$ | 354,239 | $ | 332,706 | $ | 318,533 | ||||||
|
Rapid assay products
|
146,538 | 147,078 | 146,867 | |||||||||
|
Reference laboratory diagnostic and consulting services
|
329,666 | 298,410 | 288,244 | |||||||||
|
Practice information systems and digital radiography
|
75,212 | 65,055 | 61,291 | |||||||||
|
Pharmaceutical products
|
- | 54 | 19,121 | |||||||||
|
CAG segment revenue
|
905,655 | 843,303 | 834,056 | |||||||||
|
Water segment revenue
|
76,514 | 73,214 | 74,469 | |||||||||
|
Livestock and poultry diagnostics segment revenue
|
81,177 | 77,208 | 80,762 | |||||||||
|
Other segment revenue
|
40,046 | 37,908 | 34,743 | |||||||||
|
Total revenue
|
$ | 1,103,392 | $ | 1,031,633 | $ | 1,024,030 | ||||||
|
For the Years Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Americas
|
||||||||||||
|
United States
|
$ | 652,026 | $ | 614,517 | $ | 610,056 | ||||||
|
Canada
|
59,806 | 55,105 | 61,456 | |||||||||
|
Other Americas
|
16,343 | 12,416 | 10,794 | |||||||||
| 728,175 | 682,038 | 682,306 | ||||||||||
|
Europe
|
||||||||||||
|
United Kingdom
|
56,493 | 55,835 | 62,274 | |||||||||
|
Germany
|
68,318 | 62,480 | 62,611 | |||||||||
|
France
|
42,895 | 41,756 | 42,801 | |||||||||
|
Other Europe
|
106,186 | 104,364 | 103,818 | |||||||||
| 273,892 | 264,435 | 271,504 | ||||||||||
|
Asia Pacific Region
|
||||||||||||
|
Japan
|
36,260 | 31,794 | 27,424 | |||||||||
|
Australia
|
36,296 | 29,177 | 25,360 | |||||||||
|
Other Asia Pacific
|
28,769 | 24,189 | 17,436 | |||||||||
| 101,325 | 85,160 | 70,220 | ||||||||||
|
Total
|
$ | 1,103,392 | $ | 1,031,633 | $ | 1,024,030 | ||||||
|
December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Americas
|
||||||||||||
|
United States
|
$ | 173,070 | $ | 169,933 | $ | 163,107 | ||||||
|
Canada
|
3,628 | 4,373 | 5,403 | |||||||||
| 176,698 | 174,306 | 168,510 | ||||||||||
|
Europe
|
||||||||||||
|
United Kingdom
|
10,341 | 9,520 | 6,209 | |||||||||
|
Germany
|
2,670 | 3,210 | 3,271 | |||||||||
|
Switzerland
|
2,164 | 2,870 | 3,800 | |||||||||
|
France
|
2,270 | 2,813 | 2,665 | |||||||||
|
Netherlands
|
3,525 | 3,532 | 2,538 | |||||||||
|
Other Europe
|
802 | 965 | 912 | |||||||||
| 21,772 | 22,910 | 19,395 | ||||||||||
|
Asia Pacific Region
|
||||||||||||
|
Japan
|
737 | 709 | 439 | |||||||||
|
Australia
|
1,704 | 1,650 | 1,049 | |||||||||
|
Other Asia Pacific
|
814 | 371 | 253 | |||||||||
| 3,255 | 2,730 | 1,741 | ||||||||||
|
Total
|
$ | 201,725 | $ | 199,946 | $ | 189,646 | ||||||
|
Level 1
|
Quoted prices in active markets for identical assets or liabilities that the entity has the ability to access at the measurement date.
|
|
Level 2
|
Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Foreign currency exchange contracts classified as derivative instruments are valued based on the present value of the forward rate less the contract rate multiplied by the notional amount. The product of this calculation is then adjusted for counterparty risk. Interest rate swaps classified as derivative instruments
are valued utilizing a discounted cash flow analysis based on the terms of the contract and the interest rate curve, and adjusted for counterparty risk
.
|
|
Level 3
|
Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. At December 31, 2010 and 2009, we had no Level 3 assets or liabilities.
|
|
As of December 31, 2010
|
Quoted Prices
in Active
Markets for
Identical Assets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
Balance at
December 31,
2010
|
||||||||||||
|
Assets
|
||||||||||||||||
|
Money market funds
(1)
|
$ | 67,025 | $ | - | $ | - | $ | 67,025 | ||||||||
|
Equity mutual funds
(2)
|
2,222 | - | - | 2,222 | ||||||||||||
|
Liabilities
|
||||||||||||||||
|
Foreign currency exchange contracts
(3)
|
- | 2,234 | - | 2,234 | ||||||||||||
|
Deferred compensation
(4)
|
2,222 | - | - | 2,222 | ||||||||||||
|
Interest rate swaps
(5)
|
- | 1,611 | - | 1,611 | ||||||||||||
|
As of December 31, 2009
|
Quoted Prices
in Active
Markets for
Identical Assets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
Balance at
December 31,
2009
|
||||||||||||
|
Assets
|
||||||||||||||||
|
Money market funds
(1)
|
$ | 47,021 | $ | - | $ | - | $ | 47,021 | ||||||||
|
Equity mutual funds
(2)
|
1,891 | - | - | 1,891 | ||||||||||||
|
Liabilities
|
||||||||||||||||
|
Foreign currency exchange contracts
(3)
|
- | 4,221 | - | 4,221 | ||||||||||||
|
Deferred compensation
(4)
|
1,891 | - | - | 1,891 | ||||||||||||
|
Interest rate swaps
(5)
|
- | 595 | - | 595 | ||||||||||||
|
(1)
|
Money market funds are included within cash and cash equivalents.
|
|
(2)
|
Equity mutual funds relate to a deferred compensation plan that was assumed as part of a previous business combination. This amount is included within other long-term assets. See number (4) below for a discussion of the related deferred compensation liability.
|
|
(3)
|
Foreign currency exchange contracts are included within accrued liabilities.
|
|
(4)
|
Deferred compensation plans are included within other long-term liabilities. The fair value of our deferred compensation plan is indexed to the performance of the underlying equity mutual funds discussed in number (2) above.
|
|
(5)
|
Interest rate swaps are included within accrued liabilities.
|
|
Currency Sold
|
U.S. Dollar Equivalent
|
|||||||||||
|
December 31,
|
December 31,
|
December 31,
|
||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Euro
|
$ | 59,360 | $ | 53,091 | $ | 44,907 | ||||||
|
British Pound
|
21,144 | 19,238 | 20,540 | |||||||||
|
Canadian Dollar
|
21,776 | 18,849 | 16,960 | |||||||||
|
Australian Dollar
|
7,930 | 7,086 | 3,641 | |||||||||
|
Japanese Yen
|
10,427 | 9,795 | 6,318 | |||||||||
| $ | 120,637 | $ | 108,059 | $ | 92,366 | |||||||
|
Currency Purchased
|
U.S. Dollar Equivalent
|
|||||||||||
|
December 31,
|
December 31,
|
December 31,
|
||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Swiss Franc
|
$ | 12,542 | $ | 8,808 | $ | 5,383 | ||||||
|
U.S. Dollar Equivalent
|
||||||||||||
|
December 31,
|
December 31,
|
December 31,
|
||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Interest rate swaps
|
$ | 80,000 | $ | 80,000 | $ | - | ||||||
|
Liability Derivatives
|
|||||||||||
|
December 31, 2010
|
December 31, 2009
|
||||||||||
|
Balance Sheet Classification
|
Fair Value
|
Balance Sheet Classification
|
Fair Value
|
||||||||
|
Derivatives designated as hedging instruments
|
|||||||||||
|
Foreign currency exchange contracts
|
Accrued expenses
|
$
|
2,234
|
Accrued expenses
|
$
|
4,221
|
|||||
|
Interest rate swaps
|
Accrued expenses
|
1,611
|
Accrued expenses
|
595
|
|||||||
|
Total derivative instruments
|
$
|
3,845
|
$
|
4,816
|
|||||||
|
Gain (Loss) Recognized in OCI on Derivative Instruments (Effective Portion)
|
||||||||||||
|
For the Years Ended December 31,
|
||||||||||||
|
Derivative instruments
|
2010
|
2009
|
2008
|
|||||||||
|
Foreign currency exchange contracts, net of tax
|
$
|
1,368
|
$
|
(9,730
|
)
|
$
|
8,118
|
|||||
|
Interest rate swaps, net of tax
|
(638
|
)
|
(375
|
)
|
-
|
|||||||
|
Total gain (loss), net of tax
(1)
|
$
|
730
|
$
|
(10,105
|
)
|
$
|
8,118
|
|||||
|
(1)
|
Total loss at December 31, 2010 is shown net of $0.2 million in taxes from foreign exchange contracts with 2011 expiration dates and interest rate swap contracts. Total loss at December 31, 2009 is shown net of $4.6 million in taxes from foreign exchange contracts with 2010 expiration dates and interest rate swap contracts. Total gain at December 31, 2008 is shown net of $3.6 million in taxes from foreign exchange contracts with 2009 expiration dates.
|
|
Gain (Loss) Reclassified from Accumulated OCI into Income (Effective Portion)
|
||||||||||||||
|
For the Years Ended December 31,
|
||||||||||||||
|
Derivative instruments
|
Classification
|
2010
|
2009
|
2008
|
||||||||||
|
Foreign currency exchange contracts
|
Cost of revenue
|
$
|
(743
|
)
|
$
|
4,813
|
$
|
913
|
||||||
|
Gain (Loss) Recognized in Income Related to De-designated Cash Flow Hedges
|
||||||||||||||
|
For the Years Ended December 31,
|
||||||||||||||
|
De-designated derivative instruments
|
Classification
|
2010
|
2009
|
2008
|
||||||||||
|
Foreign currency exchange contracts
|
General and
administrative expense
|
$
|
-
|
$
|
(80
|
)
|
$
|
-
|
||||||
|
For the Years Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Treasury shares acquired
|
2,539 | 1,954 | 2,664 | |||||||||
|
Total cost of treasury shares
|
$ | 145,888 | $ | 84,369 | $ | 133,722 | ||||||
|
Average cost per share
|
$ | 57.46 | $ | 43.17 | $ | 50.19 | ||||||
|
December 31,
|
||||
|
2008
|
||||
|
Expenses:
|
||||
|
Sales and marketing
|
$ | 263 | ||
|
General and administrative
|
1,095 | |||
|
Research and development
|
121 | |||
| $ | 1,479 | |||
|
For the Three Months Ended
|
||||||||||||||||
|
March 31,
|
June 30,
|
September 30,
|
December 31,
|
|||||||||||||
|
2010
|
||||||||||||||||
|
Revenue
|
$ | 268,525 | $ | 281,482 | $ | 269,628 | $ | 283,757 | ||||||||
|
Gross profit
|
142,361 | 149,284 | 142,207 | 144,771 | ||||||||||||
|
Operating income
|
48,428 | 54,835 | 49,814 | 50,804 | ||||||||||||
|
Net income attributable to stockholders
|
33,026 | 37,193 | 34,694 | 36,371 | ||||||||||||
|
Earnings per share:
|
||||||||||||||||
|
Basic
|
$ | 0.57 | $ | 0.64 | $ | 0.60 | $ | 0.63 | ||||||||
|
Diluted
|
$ | 0.55 | $ | 0.62 | $ | 0.59 | $ | 0.62 | ||||||||
|
2009
|
||||||||||||||||
|
Revenue
|
$ | 236,455 | $ | 265,723 | $ | 259,120 | $ | 270,335 | ||||||||
|
Gross profit
|
124,433 | 138,440 | 130,477 | 132,931 | ||||||||||||
|
Operating income
|
38,441 | 49,176 | 44,205 | 44,147 | ||||||||||||
|
Net income attributable to stockholders
|
26,071 | 33,667 | 31,536 | 30,951 | ||||||||||||
|
Earnings per share:
|
||||||||||||||||
|
Basic
|
$ | 0.44 | $ | 0.57 | $ | 0.54 | $ | 0.53 | ||||||||
|
Diluted
|
$ | 0.43 | $ | 0.55 | $ | 0.52 | $ | 0.51 | ||||||||
|
Balance at
Beginning
of Year
|
Charges to
Costs and
Expenses
|
Write-Offs/
Cash
Payments
|
Foreign
Currency
Translation
|
Balance at End
of Year
|
||||||||||||||||
|
Reserves for doubtful accounts receivable:
|
||||||||||||||||||||
|
December 31, 2008
|
$ | 1,742 | 1,180 | (746 | ) | (83 | ) | $ | 2,093 | |||||||||||
|
December 31, 2009
|
2,093 | 926 | (783 | ) | 95 | 2,331 | ||||||||||||||
|
December 31, 2010
|
2,331 | 1,575 | (1,024 | ) | (54 | ) | 2,828 | |||||||||||||
|
Valuation allowance for deferred tax assets:
|
||||||||||||||||||||
|
December 31, 2008
|
$ | 4,241 | 1,013 | (585 | ) | (78 | ) | $ | 4,591 | |||||||||||
|
December 31, 2009
|
4,591 | 904 | (400 | ) | 36 | 5,131 | ||||||||||||||
|
December 31, 2010
|
5,131 | 278 | (847 | ) | 42 | 4,604 | ||||||||||||||
|
Exhibit No.
|
Description
|
|
|
3.1
|
Restated Certificate of Incorporation of the Company, as amended (filed as Exhibit No. 3(i) to Quarterly Report on Form 10-Q for the quarter ended June 30, 2006, File No. 0-19271, and incorporated herein by reference).
|
|
|
3.2
|
Amended and Restated By-Laws of the Company (filed as Exhibit No. 3.1 to Form 8-K filed July 21, 2009, File No. 0-19271, and incorporated herein by reference).
|
|
|
4.3
|
Instruments with respect to other long-term debt of the Company and its consolidated subsidiaries are omitted pursuant to Item 601(b)(4)(iii) of Regulation S-K since the total amount authorized under each such omitted instrument does not exceed 10 percent of the total assets of the Company and its subsidiaries on a consolidated basis. The Company hereby agrees to furnish a copy of any such instrument to the Securities and Exchange Commission upon request.
|
|
|
10.1*
|
U.S. Supply Agreement, effective as of October 16, 2003, between the Company and Ortho-Clinical Diagnostics, Inc. (“Ortho”) (filed as Exhibit No. 10.7 to Annual Report on Form 10-K for the year ended December 31, 2003, File No. 0-19271 (“2003 Form 10-K”), and incorporated herein by reference).
|
|
|
10.2*
|
Amendment No. 1 to U.S. Supply Agreement effective as of January 1, 2005, between the Company and Ortho (filed as Exhibit No. 10.1 to Quarterly Report on Form 10-Q for the quarter ended June 30, 2005, File No. 0-19271 (“June 2005 Form 10-Q”), and incorporated herein by reference).
|
|
|
10.3*
|
Amendment No. 2 to U.S. Supply Agreement effective as of October 15, 2006, between the Company and Ortho (filed as Exhibit No. 10.4 to Annual Report on Form 10-K for the year ended December 31, 2007, File No. 0-19271 (“2007 Form 10-K”), and incorporated herein by reference).
|
|
|
10.4*
|
Amendment No. 3 to U.S. Supply Agreement effective as of January 18, 2008, between the Company and Ortho (filed as Exhibit No. 10.5 to 2007 Form 10-K, and incorporated herein by reference).
|
|
|
10.5*
|
European Supply Agreement, effective as of October 17, 2003, between the Company and Ortho (filed as Exhibit No. 10.8 to 2003 Form 10-K, and incorporated herein by reference).
|
|
|
10.6*
|
Amendment No. 1 to European Supply Agreement effective as of January 1, 2005, between the Company and Ortho (filed as Exhibit No. 10.2 to June 2005 10-Q, and incorporated herein by reference).
|
|
|
10.7*
|
Amendment No. 2 to European Supply Agreement effective as of January 18, 2008, between the Company and Ortho (filed as Exhibit No. 10.8 to 2007 Form 10-K, and incorporated herein by reference).
|
|
|
10.8
|
Amendment, Release and Settlement Agreement dated as of September 12, 2002, among the Company, IDEXX Europe B.V., and Ortho (filed as Exhibit No. 10.1 to Quarterly Report on Form 10-Q for the quarter ended September 30, 2002, File No. 0-19271, and incorporated herein by reference).
|
|
|
10.9*
|
Supply Agreement, effective as of May 7, 2007 between the Company and Moss, Inc. (filed as Exhibit No. 10.1 to Quarterly Report on Form 10-Q for the quarter ended June 30, 2010, File No. 0-19271 (“June 2010 Form 10-Q”), and incorporated herein by reference).
|
|
|
10.10**
|
Employment Agreement dated January 22, 2002, between the Company and Jonathan W. Ayers (filed as Exhibit No. 10.13 to Annual Report on Form 10-K for the year ended December 31, 2001, File No. 0-19271, and incorporated herein by reference).
|
|
|
10.11**
|
Executive Employment Agreement dated October 1, 2010, between the Company and Jonathan W. Ayers (filed as Exhibit No. 10.1 to Current Report on Form 8-K filed October 7, 2010, File No. 0-19271 (“October 7, 2010 Form 8-K”), and incorporated herein by reference).
|
|
|
10.12**
|
Executive Employment Agreement dated October 1, 2010, between the Company and Merilee Raines (filed as Exhibit No. 10.2 to October 7, 2010 Form 8-K, and incorporated herein by reference).
|
|
|
10.13**
|
Form of Executive Employment Agreement dated October 1, 2010, between the Company and each of Thomas J. Dupree, Johnny D. Powers, PhD, and Michael J. Williams, PhD (filed as Exhibit No. 10.3 to October 7, 2010 Form 8-K, and incorporated herein by reference).
|
|
10.14**
|
Restated Director Deferred Compensation Plan, as amended (filed as Exhibit No. 10.1 to Quarterly Report on Form 10-Q for the quarter ended September 30, 2010, File No. 0-19271, and incorporated herein by reference).
|
|
10.15**
|
Restated Executive Deferred Compensation Plan, as amended (filed as Exhibit No. 10.3 to June 2010 Form 10-Q, and incorporated herein by reference).
|
|
10.16**
|
Form of Director Stock Option Agreement, as amended pursuant to the 2009 Stock Incentive Plan (filed as Exhibit No. 10.1 to Quarterly Report on Form 10-Q for the quarter ended March 31, 2010, File No. 0-19271 (“March 2010 Form 10-Q”), and incorporated herein by reference).
|
|
10.17**
|
Form of Employee Stock Option Agreement, as amended pursuant to the 2009 Stock Incentive Plan (filed as Exhibit No. 10.2 to March 2010 Form 10-Q, and incorporated herein by reference).
|
|
10.18**
|
1997 Employee Stock Purchase Plan, as amended (filed as Exhibit No. 99.1 to Registration Statement on Form S-8 filed June 19, 2009, File No. 333-160085 and incorporated herein by reference).
|
|
10.19**
|
Form of Restricted Stock Unit Agreement, as amended pursuant to the 2009 Stock Incentive Plan (filed as Exhibit 10.24 to Annual Report on Form 10-K for the year ended December 31, 2009, File No. 0-19271, and incorporated herein by reference).
|
|
10.20**
|
2008 Incentive Compensation Plan (filed as Exhibit 10.2 to Current Report on Form 8-K filed May 13, 2008, File No. 0-19271, and incorporated herein by reference).
|
|
10.21**
|
2009 Stock Incentive Plan (filed as Exhibit No. 99.1 to Registration Statement on Form S-8 filed June 19, 2009, File No. 333-160083, and incorporated herein by reference).
|
|
10.22
|
Credit Agreement among the Company, as borrower, certain material subsidiaries of the Company, as guarantors, JPMorgan Chase Bank, National Association, as administrative agent, and JPMorgan Chase Bank, National Association, Toronto Branch, as Toronto agent (filed as Exhibit No. 10.1 to Current Report on Form 8-K filed January 31, 2007, File No. 0-19271, and incorporated herein by reference).
|
|
10.23
|
Amended and Restated Credit Agreement among the Company, IDEXX Distribution, Inc., IDEXX Operations, Inc., IDEXX Reference Laboratories, Inc., OPTI Medical Systems, Inc. and IDEXX Laboratories Canada Corporation, as borrowers, the lenders party thereto, JPMorgan Chase Bank, National Association, as administrative agent, JPMorgan Chase Bank, National Association, Toronto Branch, as Toronto agent, Bank of America, N.A., as syndication agent, Wachovia Bank, N.A., as documentation agent, LaSalle Bank National Association, as co-agent and J.P. Morgan Securities Inc., as sole bookrunner and lead arranger (filed as Exhibit 10.1 to Current Report on Form 8-K filed April 5, 2007, File No. 0-19271, and incorporated herein by
reference).
|
|
10.24
|
Modification to Credit Agreement, dated as of February 22, 2008, among the Company, IDEXX Distribution, Inc., IDEXX Operations, Inc., IDEXX Reference Laboratories, Inc., OPTI Medical Systems, Inc. and IDEXX Laboratories Canada Corporation, the lenders party thereto, JPMorgan Chase Bank, National Association, as administrative agent, and JPMorgan Chase Bank, National Association, Toronto Branch, as Toronto agent (filed as Exhibit No. 10.1 to Current Report on Form 8-K filed February 25, 2008, File No. 0-19271 (“February 25, 2008 Form 8-K”), and incorporated herein by reference).
|
|
10.25
|
Amendment No. 1 to Credit Agreement, dated as of February 22, 2008, among the Company, IDEXX Distribution, Inc. IDEXX Operations, Inc., IDEXX Reference Laboratories, Inc., OPTI Medical Systems, Inc. and IDEXX Laboratories Canada Corporation, the lenders party thereto, JPMorgan Chase Bank, National Association, as administrative agent, and JPMorgan Chase Bank, National Association, Toronto Branch, as Toronto agent, (filed as Exhibit 10.2 to February 25, 2008 Form 8-K, and incorporated herein by reference).
|
|
21
|
Subsidiaries of the Company (filed herewith).
|
|
23
|
Consent of PricewaterhouseCoopers LLP (filed herewith).
|
|
31.1
|
Certification by Chief Executive Officer (filed herewith).
|
|
31.2
|
Certification by Corporate Vice President, Chief Financial Officer and Treasurer (filed herewith).
|
|
32.1
|
Certification by Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (filed herewith).
|
|
32.2
|
Certification by Corporate Vice President, Chief Financial Officer and Treasurer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (filed herewith).
|
|
101.INS†
|
XBRL Instance Document.
|
|
101.SCH†
|
XBRL Taxonomy Extension Schema Document.
|
|
101.CAL†
|
XBRL Taxonomy Extension Calculation Linkbase Document.
|
|
101.DEF†
|
XBRL Taxonomy Extension Definition Linkbase Document.
|
|
101.LAB†
|
XBRL Taxonomy Extension Label Linkbase Document.
|
|
101.PRE†
|
XBRL Taxonomy Extension Presentation Linkbase Document.
|
|
*
|
Confidential treatment requested as to certain portions.
|
|
**
|
Management contract or compensatory arrangement required to be filed as an exhibit pursuant to Item 15(a)(3) of Form 10-K.
|
|
†
|
In accordance with Rule 406T of Regulation S-T, these interactive data files are deemed “not filed” for purposes of Section 18 of the Exchange Act, and otherwise are not subject to liability under that section.
|