Washington, D.C. 20549
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes
☐
No
☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes
☐
No
☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes
☒
No
☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes
☒
No
☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K
☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act): Yes
☐
No
☒
The Registrant's common stock did not have a market price as of the last day of the Registrant's second fiscal quarter, therefore the aggregate market value of the outstanding shares of common stock as of such date cannot be calculated.
As of March 23, 2016, there were outstanding 25,411,800 shares of common stock, no par value.
Portions of the registrant's Proxy Statement for 2016 Annual Meeting of Shareholders are incorporated by reference in Part III
Certain statements contained herein are forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements pertaining to future financial and/or operating results, future growth in research, technology, clinical development, and potential opportunities for OncoCyte, along with other statements about the future expectations, beliefs, goals, plans, or prospects expressed by management constitute forward-looking statements. Any statements that are not historical fact (including, but not limited to statements that contain words such as “will,” “believes,” “plans,” “anticipates,” “expects,” “estimates”) should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. Actual results may differ materially from the results anticipated in these forward-looking statements and as such should be evaluated together with the many uncertainties that affect the businesses of OncoCyte, particularly those mentioned in the cautionary statements found in OncoCyte’s filings with the Securities and Exchange Commission. OncoCyte disclaims any intent or obligation to update these forward-looking statements.
References to “OncoCyte,” “our” or “us” mean OncoCyte Corporation.
The description or discussion, in this Form 10-K, of any contract or agreement is a summary only and is qualified in all respects by reference to the full text of the applicable contract or agreement.
This Annual Report on Form 10-K contains market data and industry forecasts that were obtained from industry publications, third party market research and publicly available information. These publications generally state that the information contained therein has been obtained from sources believed to be reliable. While we believe that the information from these publications is reliable, we have not independently verified such information.
This Annual Report on Form 10-K also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. We obtained the industry and market data in this Report from our own research as well as from industry and general publications, surveys and studies conducted by third parties, some of which may not be publicly available. Such data involves a number of assumptions and limitations and contains projections and estimates of the future performance of the industries in which we operate that are subject to a high degree of uncertainty. We caution you not to give undue weight to such projections, assumptions and estimates.
As of March 23, 2016, we had 289 shareholders of record and there were 25,411,800 shares of our common stock outstanding, of which 14,674,244 shares were held by our parent BioTime, Inc. ("BioTime"). These shares held by BioTime account for 57.7% of our common stock outstanding as a whole. Accordingly, we are a consolidated subsidiary of BioTime.
On November 18, 2015, OncoCyte effected a 1-for-2 reverse stock split of its common stock. All references to common stock, warrants, and options to purchase common stock, and all per share data and related information, including the price at which shares of common stock have been sold or may be issued, have been retroactively adjusted, where applicable, to reflect the reverse stock split of OncoCyte common stock as if it had occurred at the beginning of the earliest period presented.
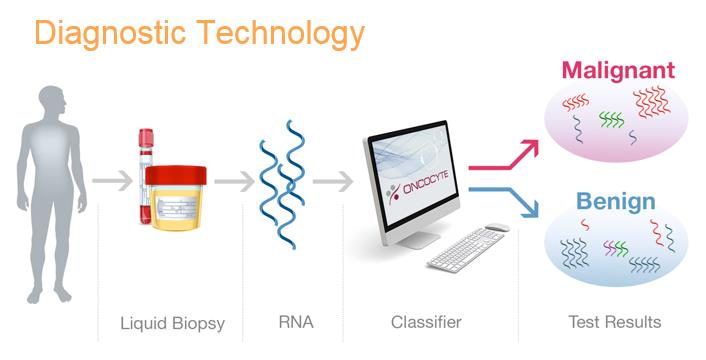
Our mission is to develop highly accurate, easy to administer, non-invasive molecular diagnostic tests to reduce unnecessary and risky medical procedures and, thereby reduce cost to the healthcare system, while improving patient outcomes. Our initial focus will be confirmatory diagnostics, utilizing novel liquid biopsy technology, for use in conjunction with imaging to confirm initial diagnoses within certain oncology indications. In addition, we will be developing screening diagnostics as potential replacements for screening imaging procedures that do not meet the needs of patients, health care providers or payers. For some indications, we will also be pursuing the probability of recurrence of a specific cancer through the development of prognostics; or companion diagnostics that help a physician determine which therapy is the optimal treatment for the patient.
Our initial liquid biopsy diagnostic tests will be confirmatory diagnostics and are being developed to reduce false positive results associated with current diagnostic techniques. These new diagnostic tests are intended to:
We are currently developing diagnostic tests for three types of cancer: lung cancer, breast cancer, and bladder cancer.
OncoCyte Corporation is a majority-owned subsidiary of BioTime, Inc. We were incorporated in 2009 in the state of California. Our principal executive offices are located at 1010 Atlantic Avenue, Suite 102, Alameda, California 94501. Our telephone number is (510) 775-0515.
Our strategy is to identify medical indications where current screening technology is insufficient, leading to poor early detection or excessive false positives, requiring the patient to endure unnecessary, costly and risky additional confirmatory procedures. By focusing on what we believe to be the biggest unmet needs with the lowest technological hurdles and potential shortest time to market, we believe our strategy is an efficient and risk-balanced use of capital and human resources.
We are developing liquid biopsy molecular cancer diagnostics utilizing a discovery platform that focuses on identifying genetic markers broadly expressed in numerous types of cancer. The diagnostic markers we have discovered thus far may address unmet needs in cancer diagnostic indications that have a strong potential to generate short- to mid-term revenues.
Our current development strategy for cancer diagnostic tests is to evaluate and validate specific diagnostics using methods of detecting proteins, messenger RNA (“mRNA”) or micro RNA (“miRNA”) approach based on unmet medical need, market size and ease of use. We believe that this approach allows us to have a broader look into the genetic markers that differentially express in cancer. Our development strategy will be matched to our market planning strategy to determine which:
For the near term, we plan to devote most of our financial resources to the development and commercialization of our initial laboratory diagnostic tests for certain types of cancer. While diagnostics are presently our primary focus, we may devote a portion of our resources to cancer therapeutic development based on technology, pertaining to homing peptides and the derivation of vascular cells engineered to deliver a toxic payload to the developing blood vessels of a malignant tumor to destroy the tumor. The extent of our work in the cancer therapeutics field will depend in part on the financial resources available to us, whether from revenues from the development and commercialization of cancer diagnostics, or from funds obtained through capital transactions. Because the development of cancer therapeutics will be a longer term and more capital intensive project than diagnostic test development, in lieu of completing the development of therapeutics products ourselves, we may seek to license out the development of potential cancer therapeutics to biopharmaceutical companies focused on therapeutic development and commercialization.
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012. We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.0 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act of 1933, as amended (the “Securities Act”); (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission, or the SEC. We refer to the Jumpstart Our Business Startups Act of 2012 herein as the “JOBS Act,” and references herein to “emerging growth company” shall have the meaning associated with it in the JOBS Act.
As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable, in general, to public companies that are not emerging growth companies. These provisions include:
We may take advantage of these exemptions for up to five years or such earlier time that we are no longer an emerging growth company.
Based on substantial unmet needs, large markets, and data generated thus far from patient serum or urine screening, we are focusing our efforts on biomarkers associated with lung, breast and bladder cancers. Our approach is based on utilizing detectable amounts of cancer-associated biomarkers in patients with early-stage disease. Our identification of certain combinations of biomarkers in lung, breast and bladder cancer has led us to identify these three diseases as promising initial targets.
The relative ease of administering a liquid biopsy diagnostic and cost savings due to the elimination of costlier and invasive biopsy procedures, we believe, will make liquid biopsy diagnostic tests useful as routine tests that could be performed in men and women of any age and at any desired frequency to detect lung, breast or bladder cancer. If successful, our tests will initially reduce diagnosis uncertainty and eliminate unnecessary down-stream procedures resulting from indeterminate low dose computed tomography (“LDCT”), cytology or mammogram tests.
We intend to initially develop and market a lung cancer diagnostic test in the United States before seeking regulatory approvals required to market the diagnostic test in other countries. The test developed will be a blood screening test for cancer markers, which will be regulated under the Clinical Laboratory Improvements Amendment (“CLIA”) as a laboratory diagnostic test (“LDT”). We may also pursue approvals from the United States Food and Drug Administration (the “FDA”) or through the European Directive on
in vitro
diagnostics (“IVDs”) for any
IVDs that we may develop.
We plan to start the process to establish a laboratory in mid-2016, including ordering the equipment and hiring the personnel, for which we will apply for CLIA registration and certification.
Once we have completed development of a liquid biopsy diagnostic test, we may commence marketing that diagnostic test for one or more specific kinds of use which relate to the kind of diagnostic evaluation that a physician is performing for a patient. Our diagnostics may have one or more of four different types of use depending on the type of cancer and the performance of the diagnostic. These intended uses include:
Currently we are focused on diagnostics to initially detect cancer due to the market opportunity associated with these types of diagnostics. Piper Jaffrey estimated that the domestic revenue opportunity for initial diagnosis diagnostics was $15 billion. This is over twice the size of companion/treatment monitoring diagnostics or recurrence diagnostics.
Source: The 2015 Liquid Biopsy Report Piper Jaffrey September 2015
We first announced the development of our confirmatory and screening diagnostics in December 2011 and have achieved several key advances since then, including:
Our liquid biopsy diagnostic tests for cancer will each go through four stages of development prior to commercialization: the research stage; assay development; validation studies; and CLIA validation. Clinical utility studies will also be conducted after commencement of the marketing of a diagnostic test.
The first stage of the development of a CLIA LDT is the research stage. In the research stage of a molecular diagnostic, biological markers are analyzed to determine if specific markers are differentially expressed in certain diseases. We are developing blood and urine tests that differentiate malignant patient samples from benign patient samples by looking at differences in the amount of specific mRNA and miRNA expressed in whole blood or urine. The objective of this phase of the development process is to delineate promising biomarkers.
In the second stage, assay development, the best performing mRNA and miRNA biomarkers are combined into an assay. The optimal combination of biomarkers that are to be utilized in the final diagnostic are determined through bioinformatics and machine learning software strategies, and assay/marker reliability and usability. The end result of this stage is a “locked down” assay.
The locked down assay is first engineered and tested for reliability, reproducibility, accuracy, precision and stability in series of research and development studies, which we referred to as
R&D validation studies
that result in the validation of the assay. In these R&D validation studies, blinded samples are run through the assay to confirm that the results reported by the assay are consistent across an appropriate range of real world, day to day variables including operating temperature variances and sample differences.
After the completion of the R&D validation study, studies and analysis are run in the CLIA laboratory – the laboratory where the diagnostic will be performed after commercial launch – in order to confirm the reliability of the diagnostic test and the full test system in the clinical environment. The
CLIA validation
phase confirms that the diagnostic test being used routinely in the clinical oncology market meets the appropriate regulatory and clinical standards. Successful completion of this stage results in the finalization and lockdown of the commercial diagnostic test system. At this point, the laboratory undergoes the inspection and certification process, which allows the marketing of the diagnostic in specific states or countries.
The final phase of the diagnostic pathway occurs after the final diagnostic test has been launched and consists of carrying out one or more
clinical utility studies.
These studies are important for obtaining coverage and reimbursement by payers such as Medicare, Medicaid, third party commercial insurers, health maintenance organizations (“HMOs”), and large corporations that self-insure. Clinical utility studies analyze the healthcare economics associated with a diagnostic test, and in particular whether it results in overall patient benefits and decreased expenditures for the healthcare system. These studies track the progress of patients who have had the diagnostic test administered; where the diagnostic test has ruled out the possibility of a disease, downstream procedures are tracked to see if physician behavior has changed. The results of this phase may be published in peer review journals and are generally compiled in dossiers to share with managed care groups, including both public and commercial payers. Obtaining positive results in clinical utility studies is very important in obtaining positive coverage and reimbursement decisions by payers.
For example, in our first product candidate - the lung confirmatory diagnostic - patients who have received a suspicious finding in LDCT screening will be tested with our diagnostic. During our post marketing clinical utility studies, we will be tracking patients with a benign result to see if any unnecessary downstream procedures (bronchoscopy or surgical biopsy) are still performed. In other words, we will track whether our diagnostic test prevents unnecessary procedures and reduce the overall cost of diagnosing lung cancer, or whether it is used in addition to downstream procedures, and thereby increases overall costs.
Our three most advanced diagnostic tests (lung, breast and bladder cancer tests) are in the assay development stage. We anticipate that our lung cancer diagnostic test will move into the R&D validation stage in 2016 and finish the CLIA validation stage by early 2017 but there can be no assurance that the development of that diagnostic test will advance in that time frame.
The current standard of care for diagnosing lung cancer in high risk patients is LDCT scanning. The United States Preventive Services Task force (“USPSTF”) guidelines recommend annual LDCTs for patients at high risk for lung cancer. The USPSTF was created in 1984 as an independent, volunteer panel of national experts in prevention and evidence-based medicine. The USPSTF works to improve the health of all Americans by making evidence-based recommendations about clinical preventive services such as screenings, counseling services, and preventive medications.
The guidelines, released in December of 2013, recommend annual LDCT scans for all Americans aged 55 to 80 years old who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years. A 30 pack-year smoking history is defined as the number of cigarette packs smoked per day times the number of years smoked. A 30 pack-year patient would include the following types of patients:
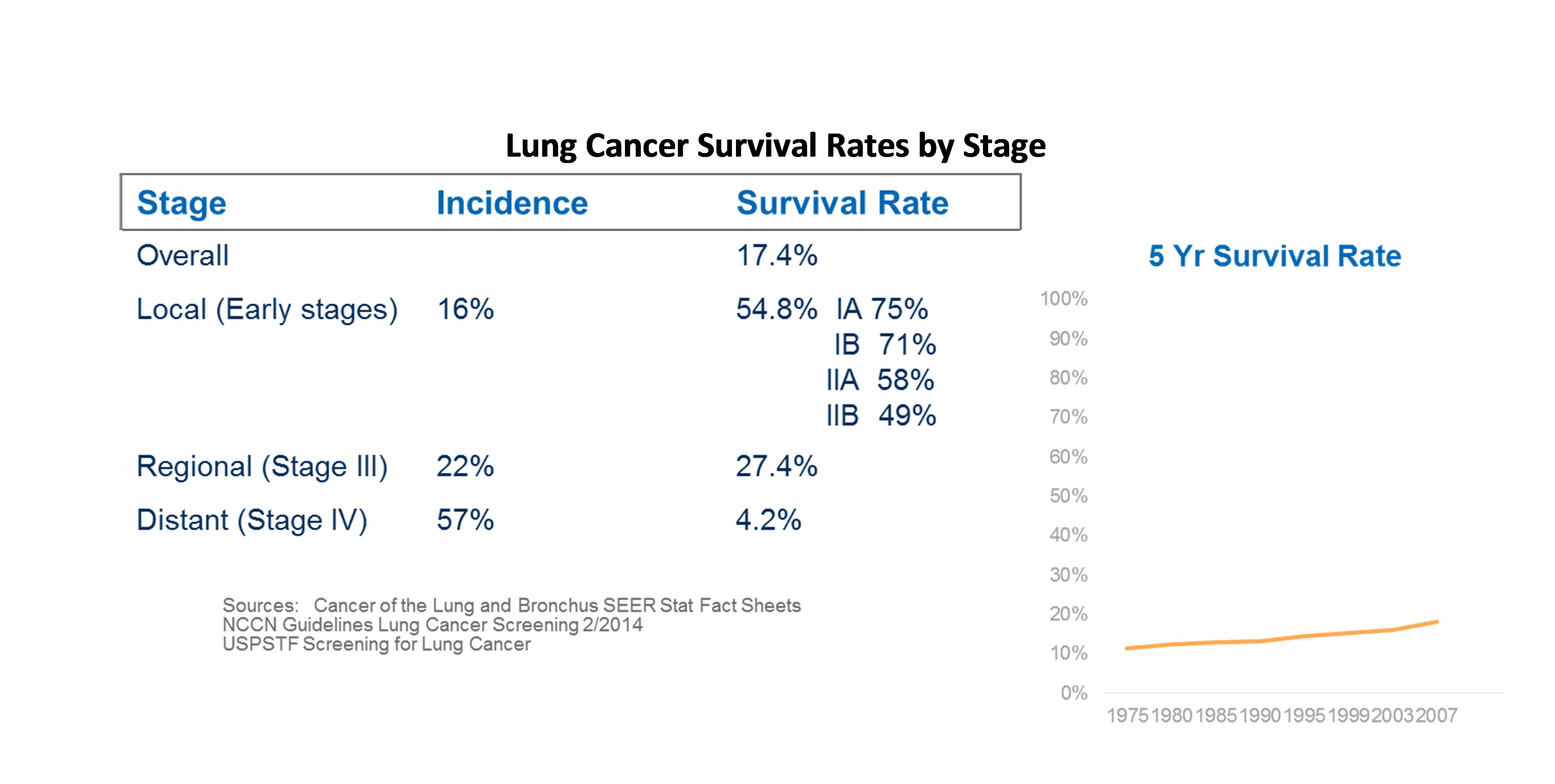
These guidelines were driven by a need to improve the standard of care for diagnosing lung cancer. Currently, the survival rate for lung cancer is very low – only 17% of people are still alive five years after a lung cancer diagnosis. These low survival rates result in one of the highest mortality rates for lung cancer, which is projected to kill 158,000 Americans in 2015.
Moreover, the survival rate, unlike many other types of cancer, has not increased significantly in the last 30 years. The low probability of surviving lung cancer is significantly affected by the late diagnosis – with more than half of all patients diagnosed after the point that the cancer has spread. USPSTF guidelines were developed to increase the probability of detecting lung cancer in earlier stages, which can significantly improve the survival rates.
However, the earlier detection of lung cancer will not come without risks. LDCTs are highly sensitive imaging procedures and they result in many false positives. Clinical studies have shown that 26% of LDCTs are indeterminate of which 96% are shown to be false positives. This results in patients being referred for risky downstream procedures including bronchoscopies, needle biopsies and surgery. These invasive procedures have been shown to result in morbidity and mortality including:
Source: Evaluation of Individuals with Pulmonary Nodules: When is it Lung Cancer? Chest 2013 May: 143 (Suppl):e83-e120.
Lung cancer is a primary cause of cancer-related death, in part because there is no effective diagnostic test to screen patients for lung cancer at an early stage.
USPSTF guidelines, which recommend LDCT scans for patients at high risk for lung cancer, may impact up to 10 million Americans who fit the criteria of 30 pack-year smokers. Research has shown that 26% of patients will have suspicious LDCT results, and around 96% of those indeterminate results will be false positives which could result in as many as 2 million unnecessary lung biopsy procedures.
We will initially focus on patients with indeterminate diagnoses of larger nodules over 8 millimeters, which is shown as Intended Use 1 in the graph below. These nodules are most likely to be sent for downstream biopsies. This potential market is estimated to include between 180,000 and 250,000 patients annually. We intend to expand the use of our lung cancer diagnostic into Intended Use 2 which targets patients with smaller nodules, who currently are put into a wait and hold pattern and can be scheduled for repeated LDCTs, risking the increase radiation exposure and incurring incremental costs to determine whether the nodule is growing. This will increase the potential patient population to 1.8 to 2.5 million patients. Finally, we will work on a diagnostic that could be used as a screening diagnostic and potentially replace LDCTs for the 7-10 million patients who meet the USPSTF guidelines for high risk, which is represented as Intended Use 3 in the following graph.
We are collaborating with The Wistar Institute of Anatomy and Biology (“Wistar”) to develop the confirmatory lung cancer diagnostic test in a large, multi-site clinical study evaluating the blood-based lung cancer diagnostic test. This collaboration involves the development of a prototype assay and a clinical study with over 2,000 blood samples obtained at six clinical sites from patients with a high-risk profile for development of lung cancer. Enrollment has continued since 2014 with all sites meeting or exceeding their collection goals. We started enrolling patients in clinical trials to supplement the Wistar sample collection and to provide the data needed for our analytical and clinical validations. As of March 2016, the clinical trial was being conducted at 20 sites and we anticipate adding additional sites in 2016. We anticipate that we will complete clinical trial enrollment by the third quarter of 2016.
Large clinical trials are needed to produce patient subsamples that ensure the development of a highly reliable, accurate diagnostic test. In the case of the lung cancer trials, samples are being collected from patients who are at risk for lung cancer, based on having positive or suspicious results from LDCT screening, and who have undergone biopsies to determine the pathology results or who have undergone a series of imaging procedures (LDCT or Petscans) to determine if the nodule is continuing to grow. Additionally, we will be collecting samples from patients who used alternative screening procedures such as chest x-rays and who were referred for biopsies.
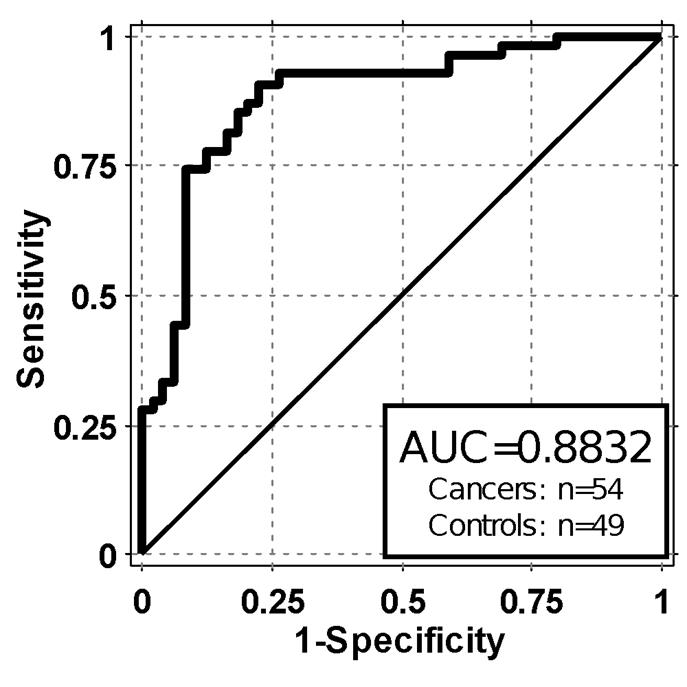
Wistar investigators and OncoCyte are currently assessing gene expression patterns in blood cells of patients with malignant lung disease and patients with non-malignant lung disease. Preliminary analysis of patient data from this study was completed during the first quarter of 2015 and preliminary findings from the research showed a sensitivity of 76% and a specificity of 88%. Sensitivity refers to the probability of detecting the presence of the disease accurately; while specificity refers to the probability of accurately predicting not having the disease. Data concerning the OncoCyte/Wistar preliminary lung assay performance with initial biomarkers and classifiers was presented at the American Thoracic Society (“ATS”) in May of 2015. The OncoCyte/Wistar preliminary lung assay had a false positive rate of only 12%. In comparison, National Lung Screening Test results reported in the New England Journal of Medicine (August 2011) showed that LDCTs have a very high false positive rate of approximately 96%. The study presented at ATS included both nodules and non-nodules and is the proof of concept for both our confirmatory and screening lung cancer diagnostic. We and Wistar are now assessing additional data, and we plan to analyze additional patient samples, for verification of the earlier preliminary analysis.
The lung confirmatory diagnostic is currently in the assay development stage. We anticipate that if our analysis of the most current data from the Wistar study and additional patient samples verify the Wistar preliminary data or support sufficient levels of sensitivity and specificity, the assay will move into the R&D validation study stage in mid-2016 and complete the CLIA validation stage in early 2017 but there can be no assurance that the development of that diagnostic test will advance in that time frame.
The ATS presentation data shows sensitivity of 76% and specificity of 88%. Sensitivity is the probability of detecting the presence of the disease accurately. A sensitivity of 76% means that three out of four cancers were detected. Specificity is the probability of accurately predicting not having the disease. In this case, approximately 9 out of 10 people without the disease were predicted. A false positive rate is a function of specificity and is equal to 1 minus specificity or 12% in this case.
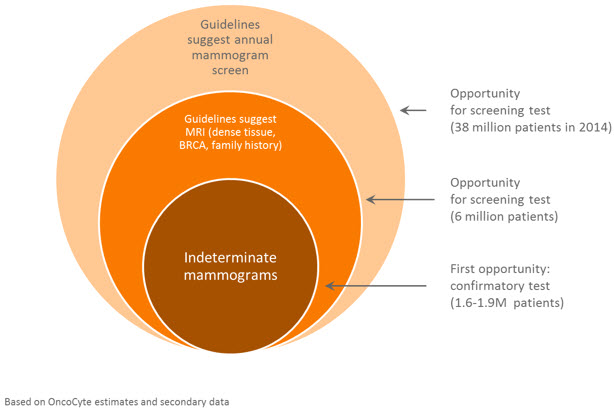
The early detection of cancer is associated with improved outcomes for patients. Mammography has been widely used since the 1970s for breast cancer screening in asymptomatic women; in 2013 over 38 million screening mammograms were performed in the US alone. Current US National Cancer Institute (“NCI”) guidelines recommend screening mammograms every one to two years in women 40 years and older, while the American Cancer Society and the National Comprehensive Cancer Network both recommend screening mammography every year starting at age 40. However in November of 2009, USPSTF revised their screening recommendations increasing the age to 50 and length of time between screenings from annual to biennial. This was partially driven by the concerns around false positives. Approximately 10% of women are recalled from screening mammography for further testing and approximately 95% of those women’s test results end up as false positives. Over the course of 10 years of screening, one out of every two women will experience a false positive with 7% to 17% of those women having unnecessary biopsies.
At the same time, mammogram screening in women aged 40 to 74 has been associated with relative reduction in breast cancer mortality of 15% to 20%. However, the NCI estimates that approximately 20% of all breast cancers are not detected by mammography during screening. These false negatives or missed diagnoses, together with the false positives or over diagnoses, indicate a strong unmet need for a breast cancer screening test with superior specificity and sensitivity when compared to standard screening mammography.
Additionally guidelines recommend MRI screening for approximately 6 million women who either have a family history, a BRCA gene mutation or dense breast tissue, since mammograms have been shown to miss cases of cancer in patients that meet these profiles.
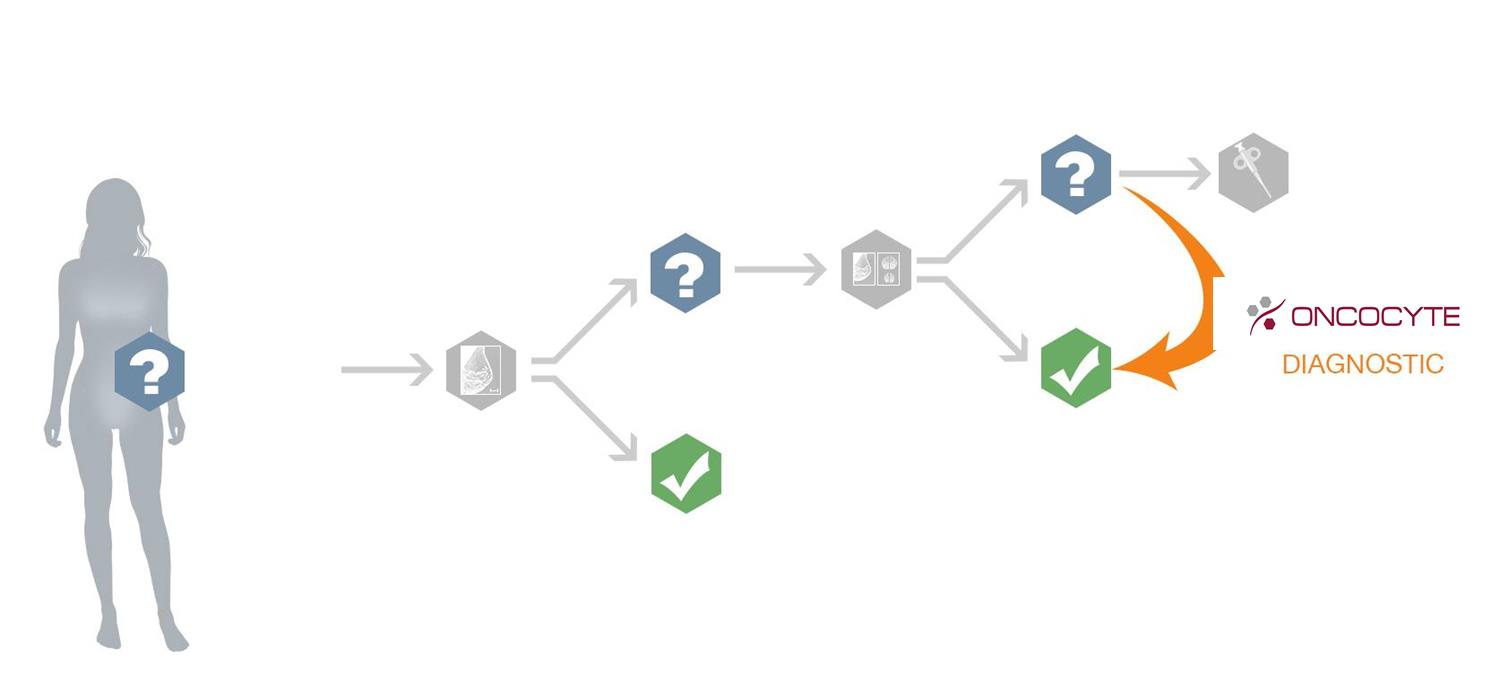
OncoCyte is developing a confirmatory diagnostic test that could be used with women who have an indeterminate mammogram result (BI-RADS 3 or 4). In the case of a mammogram BI-RADS 3 score, repeat imaging is recommended, which means that women may have to schedule another mammogram or they may be referred to a more costly MRI procedure. In the case of a mammogram BI-RADS 4 score, women are often referred for a biopsy. Our breast confirmatory diagnostic could be incorporated into breast screening protocols to confirm whether women with BI-RADS 3 or 4 scores need to undergo additional costly imaging or an invasive biopsy.
Each year approximately 5% of women have mammograms that are suspicious and many of these women are sent on to biopsies (Geller et al Radiology 222:2 2002). Currently it is estimated that about 16% or 250,000 of these biopsies will be cancerous. This is the focus of our initial research for our breast cancer confirmatory diagnostic as shown in the following graph. We are planning to expand our research efforts to include the second intended use – women who meet the guidelines for MRIs. There are over 6 million women in the U.S. for whom the guidelines recommend both a mammogram and a MRI yearly.
We plan to expand the use of our diagnostic in the future to meet the needs for a better breast cancer screening diagnostic, which could impact up to 38 million women each year. Research over the last 25 years has shown that large numbers of women are having unnecessary biopsies resulting in estimates of $4 billion a year being spent on false positives (Health Affairs, 34, no.4 (2015):576-583).
We tested the performance of our cancer markers in detecting breast cancer in a set of 134 samples from patients with confirmed cases of breast cancer. The outcome of this initial experiment led us to start prospective clinical trials during the first quarter of 2014 with our sponsorship of a 600 patient study at Scottsdale Medical Imaging Laboratories. As of March 2016, we had collected over 900 patient blood samples and acquired an additional 100 samples from biobanks. We will continue enrollment through 2016 until we meet the numbers needed to lock down an assay and validate the assay in a clinical environment. Data from this and ongoing studies will be used to support an initial use of the breast cancer diagnostic test by radiologists to aid in determining the malignancy potential of suspicious mammography findings. As part of the initial collaboration, we have retained all rights to develop and market our proprietary breast cancer diagnostic tests.
The current standard of care for bladder cancer diagnosis is cytology and cystoscopies. Urine cytology is a test to look for abnormal cells in a patient’s urine. Urine cytology is used along with other tests and procedures to diagnose urinary tract cancers. Cystoscopy is a procedure that allows a doctor to examine the lining of the bladder and the urethra, tube that carries urine out of the body. A hollow tube called a cystoscope, equipped with a lens, is inserted into the patient’s urethra and slowly advanced into the bladder. Increasingly over the years, cystoscopies have been used in conjunction with cytology which has resulted in increasing costs for the detection of bladder cancer.
Bladder cancer has the highest lifetime treatment costs per patient of all cancers. The high recurrence rate and ongoing invasive monitoring requirement are the key contributors to the economic and human toll of this disease.
Urothelial carcinoma constitutes more than 90% of bladder cancers in the Americas, Europe and Asia. Although most patients with bladder cancer can be treated with organ-sparing chemotherapy, UC has a relapse rate of nearly 70% and can progress to invasive, metastatic, and lethal disease. The regular surveillance and treatment of recurrent disease from the time of diagnosis for the remainder of a patient’s life makes urothelial Carcinoma the most costly malignancy on a per patient basis. The problem is amplified because the two standard methods for surveillance - microscopic assessment of urinary cytology specimens and bladder cystoscopy – which possess significant limitations with respect to both performance and cost. Although urine cytology does have a very high positive predictive value and low false positive rate, it has a low negative predictive value and a high indeterminate rate. Patients who have indeterminate urine cytology results commonly undergo cystoscopy, which is painful, time consuming, costly, and unnecessary in many cases since a neoplasm is often not present. In urothelial carcinoma, as in virtually all other cancers, earlier and more accurate diagnosis, including diagnosis of disease recurrence, is generally associated with better outcomes and lower cost.
Overall markets for bladder cancer diagnostics are large and growing. Based on National Cancer Institute statistics released in 2012, it was estimated that in 2013 over 72,000 new cases of bladder cancer would occur in the United States and a total of over 550,000 men and women alive would have a history of bladder cancer and be subject to recurrence surveillance testing using cystoscopy or urine cytology. Additionally, another 3 million patients present yearly with hematuria (blood in urine), an early symptom of bladder cancer and 500,000 patients have indeterminate cytology findings. These three patient profiles: indeterminate cytology, hematuria and surveillance, could result in a potential market opportunity of approximately 4.5 million tests yearly.
Sending urine specimens to us for analysis using our diagnostic tests instead of performing a cystoscopy procedure would be a significant departure from the current standard of care in the diagnosis of bladder cancer. Urologists may be reluctant or unwilling to change their practices and utilize our diagnostic test for bladder cancer even if our test is proven to have a high rate of accuracy in detecting the presence or absence of cancer.
The potential resistance of urologists to adopt the use of our bladder cancer diagnostic test means that marketing that test could require a substantial effort by a sales force. Due to this concern and our limited financial and marketing resources, we may seek to enter into an agreement with a larger company that has greater marketing resources for the marketing of our bladder cancer test. We may license out both completion of development and marketing to another company, retaining rights to receive a royalty on sales and possibly some sales related milestone payments, or we may complete the development of the test and seek to co-market the test with another company in an arrangement that might provide for a sharing of marketing costs and revenues. There is no assurance that we will be successful in entering into a licensing or co-marketing arrangement or that a licensee or co-marketing partner will succeed in marketing our bladder cancer diagnostic test. If we enter into a license or co-marketing agreement, our revenues from the sale of our bladder cancer diagnostic test may be substantially less than the amount of revenues and gross profits that we might receive if we were to market that diagnostic test ourselves.
As part of our clinical development of a urine-based bladder cancer diagnostic test, we initiated a clinical trial in January 2014 that has been expanded to a multi-site trial. The trial will involve up to 1,400 patient samples obtained from at least nine large urology clinics located throughout the United States. As of March 2016, we had approximately 1,275 samples in house. The clinical trial is designed to expand the potential use of our bladder cancer test beyond pathology laboratories and into urologic practices at the point of cystoscopy. The goal of the current clinical trial is to compare the performance of our bladder cancer markers to the performance of cystoscopy. Investigators in the trial are collecting urine samples from patients undergoing cystoscopy for the diagnosis of either primary or recurrent bladder cancer. Cystoscopy and biopsy results will be compared with the results of our proprietary diagnostic test panel in determining the overall performance of our classifier and markers.
In May of 2015, we presented preliminary findings of our bladder research at the American Association of Cancer Research. Preliminary findings showed a sensitivity of 90% and a specificity of 83%. Sensitivity is the probability of detecting the presence of the disease accurately. A sensitivity of 90% means that 9 out of 10 cancers were detected. Specificity is the probability of accurately predicting not having the disease. We expect that the inclusion of additional markers will improve the diagnostics sensitivity and specificity further.
Over the next two years, our goal is to achieve the following milestones relating to the development and commercialization of our cancer diagnostic tests:
Achieving the milestones will require expanding our research team to include diagnostic development personnel as well as sufficient clinical trial samples to conduct all the validation tests required.
In our liquid biopsy tests for lung, breast and bladder cancer, we are using the same general strategy for the identification of mRNA and miRNA biomarkers and are developing a gene expression classifier to interpret the differential gene expression or miRNA expression and yield a malignant versus benign determination. Ultimately our research may rely on only one type of biomarker in a specific indication. In the case of lung cancer, our test will be developed on mRNA biomarkers only. In the case of breast cancer, our study includes the use of both RNA markers and monoclonal antibodies directed to proteins.
Blood samples are collected by venipuncture into tubes and total RNA is isolated. mRNA biomarkers were identified using microarray equipment. The best performing mRNA biomarkers will be transferred to the commercial platform we will use in our CLIA laboratory. Differentially expressed miRNAs will be identified by screening the human V3 miRNA panel or alternative RNA detection methods. The optimal combination and final panel of mRNA and miRNA biomarkers together with potential protein-based assays will be determined using bioinformatics and machine learning strategies. The optimal classifier will be developed that yields the best discrimination between malignant and benign. The performance of the final biomarker panel and classifier will be tested on an independent set of samples to determine performance characteristics.
For bladder cancer, we are developing a urine test for use in recurrence screening and hematuria. The bladder cancer diagnostic is based on differential mRNA expression in urine samples. mRNA biomarkers were identified using microarray and top biomarkers were transferred to the commercial platform. A streamlined assay was developed that uses crude urine sediment lysates rather than purified RNA, eliminating the need for RNA isolation and amplification. The optimal classifier will be developed that yields the best discrimination between malignant and benign. The performance of the final biomarker panel and classifier will be tested on an independent set of samples to determine performance characteristics.
Biomarkers are important to the diagnosis of cancer in that their presence or absence in a specific patient sample drives the sensitivity and specificity scores of a molecular diagnostic. For example if a specific mRNA is only seen expressed in patients with cancer, it can be used to help make a malignant call on that sample. The use of biomarkers with a classifier can help ensure that the sensitivity score, which is a measure of correcting identifying the disease is sufficiently high to reduce false positives, ensuring that patients with the disease are correctly diagnosed. At the same time, biomarkers can be used to hone the specificity measure, which is a measure of correctly identifying patients without the disease, which reduces the number of patients who are unnecessarily referred to biopsy.
We have entered into a Sponsored Research Agreement (“SRA”), with Wistar pursuant to which Wistar investigators are conducting a multi-center patient study in which they are assessing gene expression patterns in blood cells of patients with malignant versus non-malignant lung disease. We have agreed to provide funding for the research that Wistar is conducting for us under the SRA.
We have agreed to indemnify Wistar, certain related persons, and the principal investigator against liabilities, damages, losses and expenses due to claims by any third party which result or arise out of the SRA, or any licenses of Wistar inventions.
The SRA will terminate on July 16, 2016 or upon the earlier completion of the sponsored research. The SRA may be terminated at an earlier date (a) by a party if the other party breaches the terms of the SRA and fails to timely cure the breach, (b) by Wistar in the event of certain insolvency or bankruptcy proceedings or similar events pertaining to us, or (c) by either party for any reason, subject to a specified period of prior written notice. Wistar will be entitled to retain payments made prior to the early termination of the SRA to the extent of its reasonable costs of work performed prior to the termination, plus the costs of non-cancellable commitments incurred by Wistar prior to the notice of termination.
We have entered into a License Agreement with Wistar that entitles us to use certain patents, know-how and data belonging to Wistar, including technology and data developed under the SRA.
Under the License Agreement, we have obtained an exclusive, worldwide license under certain patents, and under certain know-how and data (“Technical Information”) belonging to Wistar, for use in the field of molecular diagnostics for lung cancer, including, but not limited to confirmatory, companion and recurrence diagnostics for any type of lung cancer with detection through whole blood, fractionated blood, plasma, serum and/or other biological samples (the “Licensed Field”).
We have the right to grant sublicenses of the licensed patents and Technical Information. The sublicensee will be subject to Wistar’s approval, which will not be unreasonably withheld, if we are not selling a “Licensed Product.” As used in the License Agreement, a Licensed Product means any product that cannot be made, used, or sold, or any service, process or method that cannot be performed or provided, without infringing at least one pending or issued valid claim under the licensed patents in a particular country, or that incorporates or is made, identified, developed, optimized, characterized, selected, derived or determined to have utility, in whole or in part, by the use or modification of any licensed patent or any technology or invention covered thereby, any licensed Technical Information, or any other Licensed Product.
We have paid Wistar an initial license fee and will pay Wistar royalties on net sales, as defined in the License Agreement, of Licensed Products. The royalty rates will range from 3% to 5% depending upon the amount of our cumulative net sales of Licensed Products. If we are required to pay to royalties to a third party in order to manufacture or sell a Licensed Product in a particular country, the amount of royalties that we must pay Wistar on net sales of the Licensed Product will be reduced by the amount of royalties that we must pay to the third party, but subject to a maximum reduction of 50%. Our obligation to pay royalties to Wistar will terminate on a Licensed Product by-Licensed Product and country-by-country basis until the later of (i) the date a valid claim of a licensed patent covering the Licensed Product no longer exists, or (ii) the tenth (10th) anniversary of the first commercial sale of the Licensed Product in each country.
We will pay Wistar a minimum annual royalty during each subsequent year, which in each case will be credited against total royalties due on net sales of Licensed Products during the year in which the minimum royalty is paid.
We will also be obligated to pay Wistar an annual license maintenance fee each year unless we initiate sales of at least one Licensed Product by January 1, 2018.
In addition to royalties on net sales, if we grant any sublicense to the licensed patents or Technical Information, we will pay Wistar a portion of any non-royalty sublicensing income that we may receive from the sublicensee. Non-royalty sublicensing income will include any consideration we receive from a sublicensee for granting the sublicense, but excluding royalties on net sales of Licensed Products, the fair market value of any equity or debt securities we may sell to a sublicensee, and any payments we may receive from a sublicensee for research of a Licensed Product that we may conduct.
We also will pay Wistar (a) milestone payments upon the occurrence of certain milestone events in the development and commercialization of a Licensed Product, and (b) all past or ongoing costs incurred or to be incurred by Wistar, including government fees and attorneys’ fees, in the course of prosecuting the licensed patents.
Other Obligations
We have agreed to use commercially reasonable diligent efforts, directly or through sublicensees, to develop and commercialize Licensed Products. We will provide Wistar with written plans for the development and commercialization of Licensed Products and Wistar has the right to raise reasonable objections to our plans. We will also provide Wistar with annual reports on our progress in developing, evaluating, testing, and commercializing Licensed Products. We have agreed that we or a sublicensee will commence commercial sale of a Licensed Product by a specified date. If sales of a Licensed Product do not commence by the specified date, we may purchase up to three one-year extensions of the deadline by paying Wistar a designated fee for the applicable extension.
We have agreed to indemnify Wistar and its trustees, managers, officers, agents, employees, faculty, affiliated investigators, personnel and staff (the “Indemnified Parties”), from and against any and all liability, loss, damage, action, claim or expense (including attorney’s fees) suffered or incurred by the Indemnified Parties due to claims which result from or arise out of (a) the License Agreement and the license granted to us, and any sublicense granted pursuant to the License Agreement, (b) the development, use, manufacture, promotion, sale or other disposition of the licensed patents, licensed Technical Information or any Licensed Products, (c) the breach of any our representations, warranties, or covenants in the License Agreement, or a breach of a sublicense by a sublicensee, or (d) the successful enforcement by an Indemnified Party of its indemnification rights under the License Agreement. This indemnification obligation shall apply to liabilities resulting from: (i) any product liability or other claim of any kind related to the use of a Licensed Product; (ii) any claim that the licensed patents or the design, composition, manufacture, use, sale or other disposition of any Licensed Product infringes or violates any patent, copyright, trademark or other intellectual property rights of any third party; or (iii) clinical trials or studies conducted by or on behalf of us or any sublicensee relating to the Licensed Products. Notwithstanding the foregoing, we will not be obligated to indemnify and hold harmless the Indemnified Parties from and against any liabilities that result from or arise out of an Indemnified Party’s gross negligence or willful misconduct.
Termination of the License Agreement
Wistar has the right to terminate the License Agreement, subject to certain notice and cure periods and
force majeure
delays in certain cases, if any of the following occur: (a) we fail to pay any amount payable to Wistar; (b) we materially breach any covenant or agreement or any continuing representation or warranty contained in the License Agreement; (c) we become subject to certain bankruptcy or insolvency events, (d) we dissolve or cease operations, (e) we or any of our affiliates or sublicensees or affiliates of any our sublicensees challenges the validity, patentability, scope, construction, enforceability, non-infringement, or Wistar’s ownership of any issued patent comprising the licensed patents, or assists any third party in any such challenge; or (f) we fail to fulfill our product development and commercialization diligence obligations and related performance milestones.
We have the right to terminate the License Agreement, subject to a notice and cure period, if Wistar materially breaches the License Agreement. At any time after the second anniversary date of the License Agreement we may terminate the License Agreement, with or without cause, upon the passage of a specified period of time after giving Wistar written notice of termination.
Wistar’s Retained Rights to Certain Proposed Products
Wistar has reserved the right to (i) make, use, practice and further develop the licensed patents and Technical Information for educational, research, and other internal purposes; (ii) grant to any academic, government, research or non-profit institution or organization the right to make, use and practice the licensed patents or Technical Information for non-commercial research and educational purposes; and (iii) grant licenses under the Licensed Patents or Technical Information to any party for any field, product, service or territory other than the Licensed Products in the Licensed Field.
In addition, if Wistar determines to develop or have developed an actual or potential Licensed Product that is for an application, product, sub-field or indication in the Licensed Field, but for which Wistar reasonably believes a Licensed Product is not being actively developed or commercialized by us or by our affiliates or sublicensees, Wistar may give us notice of the proposed product. If we timely inform Wistar of our election to develop the proposed product, and if we successfully negotiate a development plan and milestones for the proposed product, we will be entitled to develop the proposed product as a Licensed Product under the License Agreement. If we do not elect to develop the proposed product or do not reach agreement with Wistar for a development plan and milestones for the proposed product, Wistar may exclude the proposed product from our license under the License Agreement and may develop the proposed product itself or grant licenses to third parties under the licensed patents and Technical Information for the development and commercialization of the proposed product.
Manufacturing
Facilities Required
Under a Shared Facilities Agreement with BioTime, we have use of laboratory and office space at BioTime’s facility in Alameda, California. BioTime has leased approximately 30,795 square feet of office and laboratory space in two buildings located in Alameda, and will provide OncoCyte use of space sufficient for a CLIA compliant diagnostic laboratory.
Raw Materials
The processing of our diagnostics will use commercially available reagents that are sourced by a well-known manufacturer of molecular diagnostic analyzers, prep stations and reagents that has been in business for over 12 years. Although we do not believe that we will encounter sourcing issues for these supplies, if an interruption in supply were to occur, we might need to find a different source of supply of both the reagents and the analytic equipment that we will be using in our CLIA laboratory. An interruption in supply of reagents could cause us to suspend or limit laboratory operations, and a change in analytic equipment could require us to re-establish various testing procedures, which also could disrupt our operations.
Marketing
Following CLIA certification for our laboratory and diagnostic tests, we intend to market our diagnostic tests directly to health care providers working in the areas of lung cancer and in other areas of cancer where we will be developing molecular diagnostics. These health care providers will collect blood samples or send patients to laboratories to have blood or urine samples collected. These samples, also referred to as liquid biopsies, will be sent to our CLIA laboratory in California, either by the health care provider or the laboratory, where the sample will be run through an assay and a gene expression classifier to determine a binary result, either benign or suspicious. That result will be presented to the physician ordering the procedure in a standardized report.
We will ramp up sales and marketing teams over the next year. Over time, we will continue to grow our sales, market access and marketing organizations to increase the awareness and utilization of our diagnostic tests and to prepare for additional diagnostic test launches.
Patents and Trade Secrets
We rely primarily on patents and contractual obligations with employees and third parties to protect our proprietary rights. We have sought, and intend to continue to seek, appropriate patent protection for important and strategic components of our proprietary technologies by filing patent applications in the U.S. and certain foreign countries. There can be no assurance that any of our patents will guarantee protection or market exclusivity for our diagnostic tests and diagnostic test candidates. We may also use license agreements both to access technologies developed by other companies and universities and to convey certain intellectual property rights to others. Our financial success will be dependent in part on our ability to obtain commercially valuable patent claims and to protect our intellectual property rights and to operate without infringing upon the proprietary rights of others.
Our diagnostic patent portfolio includes 13 patent families owned by us with claims directed to compositions of matter and methods useful for detection of breast, bladder, colon, pancreatic, ovarian, and thyroid cancers using specific biomarkers or a panel of specific biomarkers. Patents are pending in various jurisdictions, including the United States, Europe, Australia, Canada, China, Hong Kong, Japan and Republic of Korea, with projected expiration dates ranging from 2032 to 2036. Additionally, we have one issued patent in Australia, with claims directed to a method of detecting bladder cancer; and one accepted patent application in Australia, with claims directed to a method of detecting breast cancer. The issued patent will expire in 2032.
We have also obtained an exclusive license from Wistar to certain pending patent applications in the field of molecular diagnostics for lung cancer. The pending claims are directed to compositions of matter and methods useful for detection of lung cancer using specific biomarkers or a panel of specific biomarkers, with projected expiration dates in 2036. Additionally, we have obtained from Wistar an exclusive option under which we may obtain licenses to additional issued and pending patents in the field of molecular diagnostics for lung cancer. Patents covered by the exclusive option have issued in the United States and Europe and are pending in the United States, Canada and India. Those patents are projected to expire in 2028 - 2030.
General Risks Related to Obtaining and Enforcing Patent Protection
Our patents and patent applications are directed to compositions of matter, formulations, methods of use and/or methods of manufacturing. The patent positions of pharmaceutical and biotechnology companies, including ours, are generally uncertain and involve complex legal and factual questions. Our business could be negatively impacted by any of the following:
|
|
·
|
The claims of any patents that are issued may not provide meaningful protection, may not provide a basis for commercially viable diagnostic tests or may not provide us with any competitive advantages;
|
|
|
·
|
Our patents may be challenged by third parties;
|
|
|
·
|
Others may have patents that relate to our technology or business that may prevent us from marketing our diagnostic test candidates unless we are able to obtain a license to those patents;
|
|
|
·
|
Patent applications to which we have rights may not result in issued patents; and
|
|
|
·
|
We may not be successful in developing additional proprietary technologies that are patentable.
|
In addition, others may independently develop similar or alternative technologies, duplicate any of our technologies and, if patents are licensed or issued to us, design around the patented technologies licensed to or developed by us. Moreover, we could incur substantial costs in litigation if we have to defend ourselves in patent lawsuits brought by third parties or if we initiate such lawsuits
The United States Supreme Court’s decisions in
Mayo Collaborative Services v. Prometheus Laboratories, Inc.
and
Association for Molecular Pathology v. Myriad Genetics
may limit our ability to obtain patent protection on diagnostic methods that merely recite a correlation between a naturally occurring event and a diagnostic outcome associated with that event. Our cancer diagnostic tests are based on the presence of certain genetic markers for a variety of cancers. In
Mayo Collaborative Services v. Prometheus Laboratories, Inc.
, the Supreme Court ruled that patent protection is not available for the use of a mathematical correlation of the presence of a well-known naturally occurring metabolite as a means of determining proper drug dosage. The claims in the contested patents that were the subject of that decision were directed to measuring the serum level of a drug metabolite and adjusting the dosing regimen of the drug based on the metabolite level. The Supreme Court said that a patent claim that merely claimed a correlation between the blood levels of a drug metabolite and the best dosage of the drug was not patentable subject matter because it did no more than recite a correlation that occurs in nature.
In
Association for Molecular Pathology v. Myriad Genetics,
the Supreme Court ruled that the discovery of the precise location and sequence of certain genes, mutations of which can dramatically increase the risk of breast and ovarian cancer, was not patentable. Knowledge of the gene location and sequences was used to determine the genes’ typical nucleotide sequence, which, in turn, enabled the development of medical tests useful for detecting mutations in these genes in a particular patient to assess the patient’s cancer risk. But the mere discovery of an important and useful gene did not render the genes patentable as a new composition of matter.
The United States Patent and Trademark Office (the “USPTO”) has issued interim guidelines in light of the Supreme Court decisions indicating that process claims having a natural principle as a limiting step will be evaluated to determine if the claim includes additional steps that practically apply the natural principle such that the claim amounts to significantly more than the natural principle itself. Because the diagnostic tests that we are developing combine an innovative methodology with newly discovered compositions of matter, we are hopeful that this Supreme Court decision will not preclude the availability of patent protection for our diagnostic tests.
There is a risk that any patent applications that we file and any patents that we hold or later obtain could be challenged by third parties and declared invalid or infringing of third party claims. A patent interference proceeding may be instituted with the USPTO when more than one person files a patent application covering the same technology, or if someone wishes to challenge the validity of an issued patent filed before March 16, 2013. At the completion of the interference proceeding, the USPTO will determine which competing applicant is entitled to the patent, or whether an issued patent is valid. Patent interference proceedings are complex, highly contested legal proceedings, and the USPTO’s decision is subject to appeal. This means that if an interference proceeding arises with respect to any of our patent applications, we may experience significant expenses and delay in obtaining a patent, and if the outcome of the proceeding is unfavorable to us, the patent could be issued to a competitor rather than to us. In addition to interference proceedings, the USPTO can reexamine issued patents at the request of a third party seeking to have the patent invalidated. After March 16, 2013 an inter partes review proceeding will allow third parties to challenge the validity of an issued patent where there is a reasonable likelihood of invalidity. This means that patents owned or licensed by us may be subject to re-examination and may be lost if the outcome of the re-examination is unfavorable to us.
Post Grant Review under the America Invents Act makes available opposition-like proceedings in the United States. As with the USPTO interference proceedings, Post Grant Review proceedings will be very expensive to contest and can result in significant delays in obtaining patent protection or can result in a denial of a patent application. Also, a derivation proceeding may be instituted by the USPTO or an inventor alleging that a patent or application was derived from the work of another inventor.
Oppositions to the issuance of patents may be filed under European patent law and the patent laws of certain other countries. As with the USPTO interference proceedings, these foreign proceedings can be very expensive to contest and can result in significant delays in obtaining a patent or can result in a denial of a patent application.
The enforcement of patent rights often requires litigation against third party infringers, and such litigation can be costly to pursue. Even if we succeed in having new patents issued or in defending any challenge to issued patents, there is no assurance that our patents will be comprehensive enough to provide us with meaningful patent protection against our competitors.
In addition to patents, we rely on trade secrets, know-how, and continuing technological advancement to maintain our competitive position. We have entered into intellectual property, invention, and non-disclosure agreements with our employees, and it is our practice to enter into confidentiality agreements with our consultants. There can be no assurance, however, that these measures will prevent the unauthorized disclosure or use of our trade secrets and know-how, or that others may not independently develop similar trade secrets and know-how or obtain access to our trade secrets, know-how, or proprietary technology.
Third-Party Payer Reimbursement
Billing, Coverage, and Reimbursement for Diagnostic tests
Revenues from our clinical laboratory testing will be derived from several different sources. Depending on the billing arrangement, the instruction of the ordering physician and applicable law, parties that may reimburse us for our services include:
|
|
·
|
Third-party payers that provide coverage to the patient, such as an insurance company, a managed care organization or a governmental payer program;
|
|
|
·
|
Physicians or other authorized parties, such as hospitals or independent laboratories, that order the testing service or otherwise refer the testing services to us; or
|
|
|
·
|
Patients in cases where the patient has no insurance, has insurance that partially covers the testing, or owes a co-payment, co-insurance or deductible amount.
|
Medicare
We expect that a substantial portion of the patients for whom we may perform diagnostic tests will have Medicare as their primary medical insurance. We cannot assure that, without Medicare reimbursement our planned tests will produce sufficient revenues to enable us to reach profitability and achieve our other commercial objectives.
Clinical diagnostic laboratory tests are generally reimbursed under the Medicare Clinical Laboratory Fee Schedule. Reimbursement under the Medicare program for the diagnostic tests that we will offer is based on the Medicare Clinical Laboratory Fee Schedule, which is subject to geographic adjustments and is updated annually.
Medicare payment amounts are established for each Current Procedural Terminology (“CPT”) code. CPT codes are the main data code set used by physicians, hospitals, laboratories and other health care professionals to report separately- payable clinical laboratory services for reimbursement purposes. The CPT coding system is maintained and updated on an annual basis by the American Medical Association (“AMA”). Each year, new laboratory test codes are added to the fee schedules and corresponding fees are developed in response to a public comment process. We will request a unique CPT code from the AMA for our diagnostic test. Any updates and changes in CPT coding and reimbursement methods could impact our revenues. The introduction of new codes by Centers for Medicare and Medicaid Services (“CMS”) in combination with other actions with regard to pricing could result in lower reimbursements to us than those we may anticipate, or could result in a reduction in the payments that we may receive, for our tests and could make it more difficult to obtain coverage from Medicare or other payers. There can be no guarantees that Medicare and other payers will establish positive or adequate coverage policies or reimbursement rates.
In addition, under the Clinical Laboratory Fee Schedule, Medicare also sets a cap on the amount that it will pay for any individual test. This cap, usually referred to as the National Limitation Amount, is set at a percentage of the median of all the contractor fee schedule amounts for each billing code.
Medicare has coverage policies that can be national or regional in scope. Coverage means that the test is approved as a benefit for Medicare beneficiaries. If there is no coverage, neither we nor any other party, such as a reference laboratory, may receive reimbursement from Medicare for the service.
Legislative and Regulatory Changes Impacting Medicare Reimbursements
From time to time, Congress has revised the Medicare statute and the formulas it establishes for both the Medicare Clinical Laboratory Fee Schedule. The payment amounts under the Medicare fee schedules are important because they not only will determine our reimbursement under Medicare, but those payment amounts are also often used as a basis for payment amounts set by other governmental and private third-party payers. For example, state Medicaid programs are prohibited from paying more than the Medicare fee schedule limit for clinical laboratory services furnished to Medicaid recipients.
Under the statutory formula for Medicare Clinical Laboratory Fee Schedule amounts, increases are made annually based on the Consumer Price Index for All Urban Consumers as of June 30 for the previous twelve-month period. The Affordable Care Act (“ACA”) has, among other things, imposed cuts to the Medicare reimbursement for clinical laboratories. The ACA replaced the 0.5% cut enacted by the Medicare Improvements for Patients and Providers Act with a “productivity adjustment” that will reduce the Consumer Price Index update in payments for clinical laboratory tests. The ACA includes a 1.75% reduction in the CPI update for clinical laboratories for the years 2011 through 2015. The Middle Class Tax Relief and Job Creation Act of 2012 (“MCTRJCA”), enacted in 2012, mandated an additional change in reimbursement for clinical laboratory service programs. This legislation required CMS to reduce the Medicare Clinical Laboratory Fee Schedule by 2% in 2013, which in turn has served as a base for subsequent years. As a consequence of the changes required by ACA and MCTRJCA, payment for clinical laboratory services has gone down in recent years.
Under the Protecting Access to Medicare Act of 2014 (“PAMA”), which was signed to law in April 2014, there will be major changes to the payment formula under the Medicare Clinical Laboratory Fee Schedule. Beginning January 1, 2016, each clinical laboratory must report laboratory test payment data for each Medicare-covered clinical diagnostic laboratory test that it furnishes during a time period to be defined by future regulations. The reported data must include the payment rate reflecting all discounts, rebates, coupons and other price concessions and the volume of each test that was paid by each private payer, such as health insurance issuers, group health plans, Medicare Advantage plans and Medicaid managed care organizations.
PAMA has the potential to significantly impact the way that laboratory tests are reimbursed by Medicare. Reimbursement rates for advanced diagnostic tests will initially be based on list prices or charges and then will be pegged to the average price paid by commercial third party payers such as health insurance companies. Diagnostics in this category must meet one of the following criteria:
|
|
·
|
Analysis of multiple biomarkers of DNA, RNA or proteins combined with a unique algorithm to yield a single patient-specific result;
|
|
|
·
|
Cleared or approved by the FDA; or
|
|
|
·
|
Meets other similar criteria established by the Secretary of Health and Human Services.
|
Beginning January 1, 2017 Medicare payment for any new advanced diagnostic test will be based on the list price/charge. After the test is commercially available for two quarters, the laboratory will be required to report payment and volume information and this data will be used to set payment for the test for the following year.
|
|
·
|
If data shows that the list price was greater than 130% of the payment using established methodology, generally a weighted median, CMS will recoup the difference from the laboratory through a payment claw back.
|
|
|
·
|
Payment will be updated annually based on the weighted median of commercial payer reimbursement.
|
Congress has proposed on several occasions to impose a 20% coinsurance charge on patients for clinical laboratory tests reimbursed under the Medicare Clinical Laboratory Fee Schedule, which would require us to bill patients for these amounts. Because of the relatively low reimbursement for many clinical laboratory tests, in the event that Congress were to ever enact such legislation, the cost of billing and collecting for these services would often exceed the amount actually received from the patient and effectively increase our costs of billing and collecting.
On September 25, 2015, CMS released preliminary determinations for the calendar year 2016 for the Medicare Clinical Laboratory Fee Schedule for some test codes, including some for oncology diagnostics, as had been anticipated. These preliminary determinations were based on a cross walk approach rather than a gap-fill approach. A cross walk approach matches a new code for a diagnostic against existing codes to determine the appropriate payment rate; while a gap-fill approach looks at local pricing patterns, including charges for the tests and any discounts on charges and payments determined by other payers. An example of this was Veracyte’s Afirma, which we believe can serve as a precedent for our lung cancer diagnostic. Afirma is classified as a Multivariate Assay with Algorithmic Analyses (“MAAA”). MAAAs are diagnostics comprised of multiple biomarkers with a gene expression classifier. The diagnostic that we are developing has the same characteristics as Afirma, which is why we believe that Afirma may serve as a precedent for the potential CMS reimbursement rate for our diagnostics test.
In October of 2015, Veracyte requested CMS to reconsider the decision to use a cross-walk method to set the reimbursement rate for Afirma based on the inconsistency of the decision with historical precedent and CMS’s own expert panel's previous recommendation to use the gap-fill pricing approach for new test codes. CMS published a final decision in its 2016 Clinical Laboratory Fee Schedule (CLFS) Final Determinations on November 17, 2015 reversing its previous decision to lower the 2016 Medicare reimbursement rate for Afirma based on the cross-walk approach.
Some Medicare claims may be subject to policies issued by the former and current Medicare Administrative Contractor (“MAC”) for California. CMS relies on a network of MACs to process Medicare claims, and MACs serve as the primary operational contact between the Medicare Fee-For-Service program, and approximately 1.5 million health care providers enrolled in the program. Palmetto GBA, acting on behalf of many MACs, issued a Local Coverage Determination that affects coverage, coding and billing of many molecular diagnostic tests. Under this Local Coverage Determination, Palmetto GBA stated that it would not cover any molecular diagnostic tests unless the test is expressly included in a National Coverage Determination issued by CMS or a Local Coverage Determination or coverage article issued by Palmetto GBA. Denial of coverage for our diagnostic tests by Palmetto GBA or its successor MAC, Noridian Healthcare Solutions, or reimbursement at inadequate levels, would have a material adverse impact on our business and on market acceptance our planned diagnostic tests.
Private and Governmental Third Party Payers
Where there is a private or governmental third-party payer coverage policy in place, we will bill the payer and the patient in accordance with the established policy. Where there is no coverage policy in place, we will pursue reimbursement on a case-by-case basis. Our efforts in obtaining reimbursement based on individual claims, including pursuing appeals or reconsiderations of claims denials, could take a substantial amount of time, and bills may not be paid for many months, if at all. Furthermore, if a third-party payer denies coverage after final appeal, payment may not be received at all.
Reimbursement rates paid by private third-party payers can vary based on whether the provider is considered to be an “in-network” provider, a participating provider, a covered provider, an “out-of-network” provider or a non-participating provider. These definitions can vary among payers. An in-network provider usually has a contract with the payor or benefits provider. This contract governs, among other things, service-level agreements and reimbursement rates. In certain instances an insurance company may negotiate an in-network rate for our testing. An in-network provider may have rates that are lower per test than those that are out-of-network, and that rate can vary widely. Rates vary based on the payor, the testing type and often the specifics of the patient’s insurance plan. If a laboratory agrees to contract as an in-network provider, it generally expects to receive quicker payment and access to additional covered patients. However, it is likely that we will initially be considered an “out-of-network” or non-participating provider by payers who cover the vast majority of lives until such time that we can negotiate contracts with these payers.
We cannot predict whether, or under what circumstances, payers will reimburse for all components of our tests. Full or partial denial of coverage by payers, or reimbursement at inadequate levels, would have a material adverse impact on our business and on market acceptance of our tests.
Direct Billing Laws and Other State Law Restrictions on Billing for Laboratory Services
Laws and regulations in certain states prohibit laboratories from billing physicians or other purchasers for testing that they order. Some states may allow laboratories to bill physicians directly but may prohibit the physician and, in some cases, other purchasers from charging more than the purchase price for the services, or may allow only for the recovery of acquisition costs, or may require disclosure of certain information on the invoice. In some cases, and if not prohibited by law or regulation, we may bill physicians, hospitals and other laboratories directly for the services that they order. An increase in the number of states that impose similar restrictions could adversely affect us by encouraging physicians to perform laboratory services in-house or by causing physicians to refer services to other laboratories that are not subject to the same restrictions.
Government Regulation
Clinical Laboratory Improvement Amendments of 1988 and State Regulation
As a provider of laboratory testing on human specimens for the purpose of disease diagnosis, prevention, or treatment, we will be required to hold certain federal, state and local licenses, certifications and permits to conduct our business. In 1988, Congress enacted CLIA, which established quality standards for all laboratories providing testing to ensure the accuracy, reliability and timeliness of patient test results regardless of where the test was performed. Our laboratory will obtain a CLIA certificate of accreditation. We also will be required to meet certain laboratory licensing and other requirements under state laws. Our laboratory will hold the required licenses from the applicable state agencies in the states in which we operate or from which we receive blood or urine samples for testing.
Under CLIA, a laboratory is defined as any facility which performs laboratory testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease, or the impairment of, or assessment of health of human beings. CLIA requires that we hold a certificate applicable to the complexity of the categories of testing we perform and that we comply with certain standards. CLIA further regulates virtually all clinical laboratories by requiring that they comply with various operational, personnel, facilities administration, quality and proficiency testing requirements intended to ensure that their clinical laboratory testing services are accurate, reliable and timely. CLIA certification is also a prerequisite to be eligible to be reimbursed for services provided to state and federal health care program beneficiaries. CLIA is user-fee funded. Therefore, all costs of administering the program must be covered by the regulated facilities, including certification and survey costs.
Under the CLIA, laboratory licensing requires a site inspection, review of standard operating procedures and verification that diagnostic results can be reproduced reliably across a number of different conditions. Before submitting for a license, extensive clinical testing, which is typically done in two phases, must be undertaken at a hospital or medical center to demonstrate optimal use, safety, and efficacy of the test in diagnosing a specific condition. Each clinical study is conducted under the auspices of an IRB that will consider, among other things, ethical factors, the safety of human subjects, and the possible liability of the institution.
Clinical studies are generally conducted in two phases. The first phase is analytical validation which is done in the research laboratory and involves the replication of consistent results for the same sample across a spectrum of different conditions. Once the analytical validation is completed, the assay moves into clinical validation. In clinical validation tests are run to confirm that consistent results for the same sample can be obtained in the actual laboratory that will conduct the commercial tests.
We will be subject to regular surveys and inspections to assess compliance with program standards. Laboratories performing high complexity testing are required to meet more stringent requirements than laboratories performing less complex tests.
CLIA and FDA Regulation of Diagnostic Tests
Our diagnostic tests will likely be classified as LDTs and consequently be governed under the CLIA regulations, as administered by CMS, as well as by applicable state laws. Historically, the FDA has exercised enforcement restraint with respect to most LDTs and has not required laboratories that offer LDTs to comply with FDA requirements for medical devices, such as registration, device listing, quality systems regulations, premarket clearance or premarket approval, and post-market controls. In recent years, however, the FDA has stated it intends to end its policy of enforcement restraint and begin regulating certain LDTs as medical devices. On October 3, 2014, the FDA issued two draft guidance documents, entitled “Framework for Regulatory Oversight of Laboratory Developed Tests (LDTs)” and “FDA Notification and Medical Device Reporting for Laboratory Developed Tests (LDTs)”, respectively, that set forth a proposed risk-based regulatory framework that would apply varying levels of FDA oversight to LDTs. The FDA has indicated that it does not intend to modify its policy of enforcement restraint until the draft guidance documents are finalized. It is unclear at this time when, or if, the draft guidance documents will be finalized, and even then, the new regulatory requirements are proposed to be phased-in consistent with the schedule set forth in the guidance, which may happen in as little as 12 months after the draft guidance is finalized for certain high-priority LDTs. Nevertheless, the FDA may decide to regulate certain LDTs on a case-by-case basis at any time.
Failure to comply with applicable FDA regulatory requirements may trigger a range of enforcement actions by the FDA, including warning letters, civil monetary penalties, injunctions, criminal prosecution, recall or seizure, operating restrictions, partial suspension or total shutdown of operations, and denial of or challenges to applications for clearance or approval, as well as significant adverse publicity. We cannot predict the ultimate form or impact of any such FDA guidance and the potential effect on our diagnostic test services.
We cannot provide any assurance that FDA regulation, including premarket review, will not be required in the future for our diagnostic tests, whether through additional guidance or regulations issued by the FDA, new enforcement policies adopted by the FDA or new legislation enacted by Congress. It is possible that proposed legislation discussed above or other new legislation could be enacted into law, or new regulations or guidance could be issued by the FDA. Such new legislation may result in new or increased regulatory requirements for us to continue to offer our diagnostic tests or to develop and introduce new tests or services.
If premarket review, including approval, is required, our business could be negatively affected until such review is completed and clearance to market or approval is obtained. If we are selling any of our diagnostic tests when new FDA approval requirements are implemented, we may be required to suspend sales until we obtain premarket clearance or approval. If our diagnostic tests are allowed to remain on the market but there is uncertainty about the legal status of those tests, if we are required by the FDA to label them investigational, or if labeling claims the FDA allows us to make are limited, order levels for the use of our tests may decline and reimbursement may be adversely affected.
New regulations could also require, among other things, additional clinical studies and submission of a premarket notification or filing a PMA application with the FDA. For example, LDTs with the same intended use as a cleared or approved companion diagnostic are defined in FDA’s draft guidance as “high-risk LDTs (Class III medical devices)” for which premarket review would be required. This may include the use of our LDTs for screening patients for cancer. See the discussion of FDA regulation of medical devices below under
In Vitro Diagnostics.
If premarket review is required by the FDA, there can be no assurance that our diagnostic tests will be cleared or approved on a timely basis, if at all, nor can there any be assurance that labeling claims allowed by the FDA will be consistent with our intended claims or will be adequate to support continued adoption of and reimbursement for our tests. Compliance with FDA regulations would increase the cost of conducting our business, and subject us to heightened requirements of the FDA and penalties for failure to comply with these requirements. We may also decide voluntarily to pursue FDA premarket review of our diagnostic tests if we determine that doing so would be appropriate.
California State Laboratory Licensing
In addition to federal certification requirements of laboratories under CLIA, licensure will be required and maintained under California law for the San Francisco Bay Area based laboratory that we plan to establish. Such laws include standards for the day-to-day operation of a clinical reference laboratory, including the training and skills required of personnel and quality control. In addition, California laws mandate proficiency testing, which involves testing of specimens that have been specifically prepared for the laboratory.
We will not be permitted to perform diagnostic tests at our California CLIA laboratory until it is certified by the state, and if after certification our laboratory falls out of compliance with California standards, the California Department of Health Services (“DHS”) may suspend, restrict or revoke our license to operate our laboratory, assess substantial civil money penalties, or impose specific corrective action plans. Any such actions could materially affect our business.
Other States’ Laboratory Licensing
Some states require licensure of out-of-state laboratories that accept specimens from those states. Our laboratories will need to pass various state inspections in order to get licensed to provide LDTs in each of state that requires licensure. In addition to the inspection requirements of the other states, Pennsylvania, Florida and Maryland have laws that require a certificate of compliance, and New York has its own special inspection requirements that must be met, in order to market our diagnostics in those states or to perform diagnostic tests on specimens received from patients residing in those states.
In Vitro Diagnostics
In the future, we may elect to develop IVDs, which are regulated by the FDA as medical devices. Medical devices marketed in the United States are subject to the regulatory controls under CLIA, the Federal Food, Drug, and Cosmetic Act, and regulations adopted by the FDA. Some requirements, known as premarket requirements, apply to medical devices before they are marketed, and other requirements, known as post-market requirements, apply to medical devices after they are marketed.
The particular premarket requirements that must be met to market a medical device in the United States will depend on the classification of the device under FDA regulations. Medical devices are categorized into one of three classes, based on the degree of risk they present. Devices that pose the lowest risk are designated as Class I devices, devices that pose moderate risk are designated as Class II devices and are subject to general controls and special controls, and the devices that pose the highest risk are designated as Class III devices and are subject to general controls and premarket approval.
A premarket submission to the FDA will be required for some Class I products, most Class II, and all Class III devices. Most Class I and some Class II devices are exempt from premarket submission requirements. Some Class I and most Class II devices may only be marketed after a 510(k) premarket notification, while a more extensive PMA or Premarket Approval is required to market Class III devices.
Unless and until the FDA’s draft guidance documents are finalized and the resulting new regulatory requirements are phased in our initial confirmatory diagnostics will not require FDA filing before launch. Since the tests are being developed as LDTs, the regulatory pathway that we will be following is the CLIA certification and inspection pathway. If the new requirements are phased in or if we elect to develop IVDs, our future screenings diagnostics may require a 510(k) submission or a PMA. In a 510(k) submission, the device sponsor must demonstrate that the new device is “substantially equivalent” to a predicate device in terms of intended use, technological characteristics, and performance testing. A 510(k) requires demonstration of substantial equivalence to another device that is legally marketed in the United States. Substantial equivalence means that the new device is at least as safe and effective as the predicate. A device is substantially equivalent if, in comparison to a predicate it (a) has the same intended use as the predicate; and has the same technological characteristics as the predicate; or (b) has the same intended use as the predicate; and has different technological characteristics and the information submitted to FDA; does not raise new questions of safety and effectiveness; and is demonstrated to be at least as safe and effective as the legally marketed predicate device.
A claim of substantial equivalence does not mean the new and predicate devices must be identical. Substantial equivalence is established with respect to intended use, design, energy used or delivered, materials, chemical composition, manufacturing process, performance, safety, effectiveness, labeling, biocompatibility, standards, and other characteristics. A device may not be marketed in the United States until the submitter receives a letter declaring the device substantially equivalent. If the FDA determines that a device is not substantially equivalent, the applicant may resubmit another 510(k) with new data, or request a Class I or II designation through the FDA’s
de novo
process which allows a new device without a valid predicate to be classified into Class I or II if it meets certain criteria, or file a reclassification petition, or submit a PMA.
A new 510(k) submission is required for changes or modifications to an existing approved device, where the modifications could significantly affect the safety or effectiveness of the device or the device is to be marketed for a new or different indication for use.
A PMA for Class III devices is the most stringent type of premarket submission. Before the FDA approves a PMA, the sponsor must provide valid scientific evidence demonstrating reasonable assurance of safety and effectiveness for the device’s intended use.
Submission of an application is no guarantee that the CMS or FDA will find it complete and accept it for filing. If an application is accepted for filing or licensing, following the CMS or FDA’s review, the CMS or FDA may grant marketing approval, request additional information or deny the application if it determines that the application does not provide an adequate basis for approval.
Health Insurance Portability and Accountability Act
Under the Health Insurance Portability and Accountability Act (“HIPAA”), the Department of Health and Human Services (“HHS”) has issued regulations to protect the privacy and security of protected health information used or disclosed by health care providers, such as us. HIPAA also regulates standardization of data content, codes and formats used in health care transactions and standardization of identifiers for health plans and providers. Penalties for violations of HIPAA regulations include civil and criminal penalties.
CMS and the Office of Civil Rights issued a final rule in February 2014 to amend both the HIPAA and CLIA regulations. The final rule amended the HIPAA privacy rule to remove the CLIA laboratory exceptions, and as a result, HIPAA-covered laboratories are now required to provide individuals, upon request, with access to their completed test reports. Under the new rules CLIA laboratories and CLIA-exempt laboratories may provide copies of a patient’s completed test reports that, using the laboratory’s authentication process, can be identified as belonging to that patient. These changes to the CLIA regulations and the HIPAA Privacy Rule provide individuals with a greater ability to access their health information, empowering them to take a more active role in managing their health and health care. CLIA laboratories must create and maintain policies, procedures, and other documentation necessary to inform patients of the right to access laboratory test reports and how to exercise that right.
We intend to fully comply with these regulations. The requirements under these regulations may periodically change and could have an effect on our business operations if compliance becomes substantially more costly than under current requirements.
New laws governing privacy may also be adopted in the future. We will take steps to comply with health information privacy requirements of which we are aware and to with which we must comply. However, we can provide no assurance that we will remain in compliance with diverse privacy requirements in all of the jurisdictions in which we do business. Failure to comply with privacy requirements could result in civil or criminal penalties, which could have a materially adverse effect on our business.
Physician Referral Prohibitions
Under a federal law directed at “self-referral,” commonly known as the Stark Law, there are prohibitions, with certain exceptions, on Medicare and Medicaid payments for laboratory tests referred by physicians who personally, or through a family member, have a “financial relationship”—including an investment or ownership interest or a compensation arrangement—with the clinical laboratory performing the tests. Several Stark Law exceptions are relevant to arrangements involving clinical laboratories, including: (1) fair market value compensation for the provision of items or services; (2) payments by physicians to a laboratory for clinical laboratory services; (3) certain space and equipment rental arrangements that satisfy certain requirements, and (4) personal services arrangements that satisfy certain requirements. The laboratory cannot submit claims to the Medicare Part B program for services furnished in violation of the Stark Law, and Medicaid reimbursements may be at risk as well. Penalties for violating the Stark Law include the return of funds received for all prohibited referrals, fines, civil monetary penalties and possible exclusion from the federal health care programs. Many states have comparable laws that are not limited to Medicare and Medicaid referrals.
Corporate Practice of Medicine
A number of states, including California, do not allow business corporations to employ physicians to provide professional services. This prohibition against the “corporate practice of medicine” is aimed at preventing corporations such as us from exercising control over the medical judgments or decisions of physicians. The state licensure statutes and regulations and agency and court decisions that enumerate the specific corporate practice rules vary considerably from state to state and are enforced by both the courts and regulatory authorities, each with broad discretion. If regulatory authorities or other parties in any jurisdiction successfully assert that we are engaged in the unauthorized corporate practice of medicine, we could be required to restructure our contractual and other arrangements. In addition, violation of these laws may result in sanctions imposed against us and/or the professional through licensure proceedings, and we could be subject to civil and criminal penalties that could result in exclusion from state and federal health care programs.
Federal and State Fraud and Abuse Laws
A variety of federal and state laws prohibit fraud and abuse. These laws are interpreted broadly and enforced aggressively by various state and federal agencies, including CMS, the Department of Justice, the Office of Inspector General for HHS, and various state agencies. In addition, the Medicare and Medicaid programs increasingly use a variety of contractors to review claims data and to identify improper payments as well as fraud and abuse. These contractors include Recovery Audit Contractors, Medicaid Integrity Contractors and Zone Program Integrity Contractors. In addition, CMS conducts Comprehensive Error Rate Testing audits, the purpose of which is to detect improper Medicare payments. Any overpayments identified must be repaid unless a favorable decision is obtained on appeal. In some cases, these overpayments can be used as the basis for an extrapolation, by which the error rate is applied to a larger universe of claims, and which can result in even higher repayments.
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting, receiving, or providing remuneration, directly or indirectly, to induce or in return for either the referral of an individual, or the furnishing, recommending, or arranging for the purchase, lease or order of any health care item or service reimbursable, in whole or in part, under a federal health care program. The definition of “remuneration” has been broadly interpreted to include anything of value, including gifts, discounts, credit arrangements, payments of cash, ownership interests and providing anything at less than its fair market value. Recognizing that the Anti- Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements within the health care industry, the Office of Inspector General for HHS has issued a series of regulatory “safe harbors.” These safe harbor regulations set forth certain requirements that, if met, will assure immunity from prosecution under the federal Anti-Kickback Statute. Although full compliance with these provisions ensures against prosecution under the federal Anti-Kickback Statute, the failure of a transaction or arrangement to fit within a specific safe harbor does not necessarily mean that the transaction or arrangement is illegal or that prosecution under the federal Anti-Kickback Statute will be pursued. For further discussion of the impact of federal and state health care fraud and abuse laws and regulations on our business, see the section entitled “Risk Factors.”
HIPAA also created new federal crimes, including health care fraud and false statements relating to health care matters. The health care fraud statute prohibits knowingly and willfully executing a scheme to defraud any health care benefit program, including private third-party payers. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from federal health care programs, such as the Medicare and Medicaid programs. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for health care benefits, items or services. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from federal health care programs.
Many states have laws similar to the federal laws described above and the state laws may be broader in scope and may apply regardless of payor.
Other Regulatory Requirements
Our laboratory will be subject to federal, state and local regulations relating to the handling and disposal of regulated medical waste, hazardous waste and biohazardous waste, including chemical, biological agents and compounds, blood samples and other human tissue. Typically, we will use outside vendors who are contractually obligated to comply with applicable laws and regulations to dispose of such waste. These vendors will be licensed or otherwise qualified to handle and dispose of such waste.
The Occupational Safety and Health Administration has established extensive requirements relating to workplace safety for health care employers, including requirements to develop and implement programs to protect workers from exposure to blood-borne pathogens by preventing or minimizing any exposure through needle stick or similar penetrating injuries.
Employees
As of March 16, 2016 we employed nine persons on a full-time basis and one person on a part-time basis. Four of our full-time employees hold Ph.D. degrees in one or more fields of science.
Our business is subject to various risks, including those described below. You should consider the following risk factors, together with all of the other information included in this Report, which could materially adversely affect our proposed operations, our business prospects, and financial condition, and the value of an investment in our business. There may be other factors that are not mentioned here or of which we are not presently aware that could also affect our business operations and prospects.
Risks Related to Our Business Operations
We are a development stage company and have incurred operating losses since inception and we do not know if we will attain profitability
Since our inception in September 2009, we have incurred operating losses and negative cash flow and we expect to continue to incur losses and negative cash flow in the future. Our net losses for the years ended December 31, 2015 and 2014 were approximately $8.7 million and $5.0 million and we had an accumulated deficit of approximately $24.1 million and $15.4 million as of December 31, 2015 and 2014, respectively. Since inception, we have financed our operations through the sale of our common stock to our current shareholders, loans from BioTime and BioTime affiliates, and sale of BioTime common shares that we hold as available-for-sale securities. Although BioTime may continue to provide administrative support to us on a reimbursable basis, there is no assurance that BioTime will provide future financing. There is no assurance that we will be able to obtain any additional financing that we may need, or that any such financing that may become available will be on terms that are favorable to us and our shareholders. Ultimately, our ability to generate sufficient operating revenue to earn a profit depends upon our success in developing and marketing or licensing our diagnostic tests and technology.
We will spend a substantial amount of our capital on research and development but we might not succeed in developing diagnostic tests and technologies that are useful in medicine
|
|
·
|
We are attempting to develop new medical diagnostic tests and technologies. The main focus of our business is on diagnostic tests for cancer. Our diagnostic tests are being developed through the use of blood and urine samples obtained in prospective and retrospective clinical trials involving humans, but none of our diagnostic tests have been used in medicine to diagnose cancer. Our technologies many not prove to be sufficiently efficacious to use in the diagnosis of cancer.
|
|
|
·
|
Some of our research could also have applications in new cancer therapeutics. None of our experimental therapeutic technologies have been applied in human medicine and have only been used in laboratory studies
in vitro
.
|
|
|
·
|
The experimentation we are doing is costly, time consuming, and uncertain as to its results. We incurred research and development expenses amounting to approximately, $4.5 million, and $4.0 million during years ended December 31, 2015 and 2014, respectively. Since 2011, most of our research has been devoted to the development of our lead diagnostic tests to detect lung cancer, breast cancer, and bladder cancer.
|
|
|
·
|
If we are successful in developing a new technology or diagnostic test, refinement of the new technology or diagnostic test and definition of the practical applications and limitations of the technology or diagnostic test may take years and require the expenditure of large sums of money.
|
We do not currently have any diagnostic tests on the market and have not yet generated any revenues from operations
|
|
·
|
We need to successfully develop and market or license the diagnostic tests that we are developing in order to earn revenues in sufficient amounts to meet our operating expenses.
|
|
|
·
|
Without diagnostic test sales or licensing fee revenues, we will not be able to operate at a profit, and we will not be able to cover our operating expenses without raising additional capital.
|
|
|
·
|
Should we be able to successfully develop and market our diagnostic tests we may not be able to receive reimbursement for them from payers, such as health insurance companies, health maintenance organizations and Medicare, or any reimbursement that we receive may be lower than we anticipate.
|
Sales of any diagnostic tests that we may develop could be adversely impacted by the reluctance of physicians to adopt the use of our tests and the availability of competing diagnostic tests
|
|
·
|
Physicians and hospitals may be reluctant to try a new diagnostic test due to the high degree of risk associated with the application of new technologies and diagnostic test in the field of human medicine, especially if the new test differs from the current standard of care for detecting cancer in patients.
|
|
|
·
|
Competing tests for the initial diagnosis, reoccurrence diagnosis and optimal treatment of cancer are being manufactured and marketed by established companies and by other smaller biotechnology companies.
|
|
|
·
|
Currently there are two diagnostic tests for lung cancer and multiple diagnostic tests for bladder cancer on the market. There is one diagnostic product for breast cancer that has been approved in Europe. In order to compete with other diagnostic tests, particularly any that sell at lower prices, our diagnostic tests will have to provide medically significant advantages or be more cost effective.
|
|
|
·
|
There also is a risk that our competitors may succeed in developing safer, more accurate or more cost effective diagnostic tests that could render our diagnostic tests and technologies obsolete or noncompetitive
|
We will need to issue additional equity or debt securities in order to raise additional capital needed to pay our operating expenses
|
|
·
|
We plan to continue to incur substantial research and development expenses and we anticipate that we will be incurring significant sales and marketing costs as we develop and commercialize our diagnostic test candidates. We will need to raise additional capital to pay operating expenses until we are able to generate sufficient revenues from diagnostic test sales, royalties, and license fees, and we will need to sell additional equity or debt securities to meet those capital needs.
|
|
|
·
|
Our ability to raise additional equity or debt capital will depend not only on progress made in developing our diagnostic tests, but also will depend on access to capital and conditions in the capital markets. There is no assurance that we will be able to raise capital at times and in amounts needed to finance the development and commercialization of our diagnostic tests, establishment of a CLIA certified diagnostic laboratory, and general operations. Even if capital is available, it may not be available on terms that we or our shareholders would consider favorable.
|
|
|
·
|
Sales of additional equity securities by us could result in the dilution of the interests of our shareholders.
|
If we fail to meet our obligations under license agreements, we may lose our rights to key technologies on which our business depends
Our business will depend on several critical technologies that have licensed from Wistar for our lung cancer diagnostic test. The license agreement imposes obligations on us, including payment obligations and obligations to pursue development and commercialization of diagnostic tests under the licensed patents and technology. If Wistar believes that we have failed to meet our obligations under a license agreement, Wistar could seek to limit or terminate our license rights, which could lead to costly and time-consuming litigation and, potentially, a loss of the licensed rights. During the period of any such litigation our ability to carry out the development and commercialization of potential diagnostic tests, and our ability to raise any capital that we might then need, could be significantly and negatively affected. If our license rights were restricted or ultimately lost, we would not be able to continue to use the licensed patents and technology in our business.
We do not yet have a certified diagnostic laboratory for use in conducting cancer diagnostic tests
We need to construct and equip or otherwise acquire a diagnostic laboratory, hire a staff to operate the laboratory, and obtain federal and state certification or licensing of the laboratory for use in conducting cancer diagnostic tests. We do not know how long it will take to build or acquire a diagnostic laboratory and obtain the required certifications and licenses for the laboratory. We will need to expend a substantial part of our cash on hand and management resources to complete construction, equipping, and staffing of the laboratory.
We have limited marketing and sales resources and no distribution resources for the commercialization of any diagnostic tests that we might successfully develop
If we are successful in developing marketable diagnostic tests, we will need to build our own marketing and sales capability, which would require the investment of significant financial and management resources to recruit, train, and manage a sales force.
Our business could be adversely affected if we lose the services of the key personnel upon whom we depend
Our diagnostics program is directed primarily by our Vice President of Research, Dr. Karen Chapman. Our commercial activities are directed primarily by our Chief Executive Officer William Annett and our Vice President of Marketing, Dr. Kristine C. Mechem. The loss of Dr. Chapman, Mr. Annett, or Dr. Mechem could have a material adverse effect on our business.
Our business and operations could suffer in the event of system failures
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Such events could cause interruption of our operations. For example, the loss of data for our diagnostic test candidates could result in delays in our regulatory filings and development efforts and significantly increase our costs. To the extent that any disruption or security breach was to result in a loss of or damage to our data, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the development of our diagnostic test candidates could be delayed.
Failure of our internal control over financial reporting could harm our business and financial results
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting for external purposes in accordance with accounting principles generally accepted in the U.S. Internal control over financial reporting includes maintaining records that in reasonable detail accurately and fairly reflect our transactions; providing reasonable assurance that transactions are recorded as necessary for preparation of our financial statements; providing reasonable assurance that receipts and expenditures of our assets are made in accordance with management authorization; and providing reasonable assurance that unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements would be prevented or detected on a timely basis. Because of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of our financial statements would be prevented or detected. Our growth and entry into new diagnostic tests, technologies and markets will place significant additional pressure on our system of internal control over financial reporting. Any failure to maintain an effective system of internal control over financial reporting could limit our ability to report our financial results accurately and timely or to detect and prevent fraud.
We will initially rely in part on financial systems maintained by BioTime and upon services provided by BioTime personnel. BioTime will allocate certain expenses among itself, us, and BioTime’s other subsidiaries, which creates a risk that the allocations may not accurately reflect the benefit of an expenditure or use of financial or other resources by us, BioTime as our parent company, and the BioTime subsidiaries among which the allocations are made.
Risks Related to Our Industry
We will face certain risks arising from regulatory, legal, and economic factors that affect our business and the business of other companies engaged in the development and marketing of diagnostic tests for human diseases. Because we are a small company without revenues and with limited capital resources, we may be less able to bear the financial impact of these risks than larger companies that have substantial income and available capital.
We will need to obtain regulatory approval of our diagnostic test candidates and laboratory facilities
We will need to receive certification for our diagnostic laboratory under the CLIA, and we will need to obtain FDA and other regulatory approvals for any
IVDs that we may develop, in order to market those diagnostic tests. The need to obtain regulatory approval to market a new diagnostic test means that:
|
|
·
|
The diagnostic tests that we may develop cannot be sold until the CMS or the FDA, and corresponding foreign regulatory authorities approve the laboratory tests or the IVDs for medical use.
|
|
|
·
|
We will have to obtain a CLIA certificate of registration license for our laboratory for the manufacture and use of diagnostic tests and as part of the submission, our laboratory will be inspected.
|
|
|
·
|
In addition to meeting federal regulatory requirements, each state has its own laboratory certification and inspection requirements for a CLIA laboratory that must be met in order to sell diagnostic tests in the state.
|
|
|
·
|
We will have to conduct expensive and time consuming clinical trials of new diagnostic tests. The full cost of conducting and completing clinical trials necessary to obtain FDA approval of IVD tests or CLIA certification of a new laboratory diagnostic test or for gaining reimbursement from health insurance companies, health maintenance organizations, Medicare, and other third party payers cannot be presently determined but could exceed our current financial resources.
|
|
|
·
|
Data obtained from preclinical and clinical studies is susceptible to varying interpretations that could delay, limit or prevent regulatory agency approvals. Delays or denials of the regulatory approvals may be encountered as a result of changes in regulatory agency policy, regulations, or laws.
|
|
|
·
|
A diagnostic test that is approved may be subject to restrictions on use.
|
|
|
·
|
The FDA can withdraw approval of an FDA regulated product if problems arise
|
|
|
·
|
CLIA licensed laboratories can lose their licenses if problems arise during a periodic inspection.
|
The FDA may impose additional regulations for laboratory developed tests such as the ones we are developing
|
|
·
|
The FDA issued two draft guidance documents that set forth a proposed risk-based regulatory framework that would apply varying levels of FDA oversight to LDTs such as those we are developing. If the FDA implements new regulatory measures:
|
|
|
·
|
We may be required to obtain pre-market clearance or approval before selling our diagnostic tests;
|
|
|
·
|
As a result of required FDA pre-market review, our tests may not be cleared or approved on a timely basis, if at all;
|
|
|
·
|
FDA labeling requirements may limit our claims about our diagnostic tests, which may have a negative effect on orders from physicians;
|
|
|
·
|
The regulatory approval process may involve, among other things, successfully completing additional clinical trials and making a 510(k) submission, or filing a pre-market approval application with the FDA; and,
|
|
|
·
|
If regulatory actions affect any of the reagents we obtain from suppliers and use in conducting our tests, our business could be adversely affected in the form of increased costs of testing or delays, limits or prohibitions on the purchase of reagents necessary to perform our testing.
|
If the FDA regulates LDTs and requires that we seek pre-market approval, there is no assurance that we will be able to comply with FDA requirements.
It may take two years or more to conduct the clinical studies and trials necessary to obtain pre-market approval from the FDA. Even if our clinical trials are completed as planned, we cannot be certain that the results will support our test claims or that the FDA will agree with our conclusions regarding our test results. Success in early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that the later trials will replicate the results of prior clinical trials and studies. If we are required to conduct pre-market clinical trials, delays in the commencement or completion of clinical testing could significantly increase our test development costs and delay commercialization. Many of the factors that may cause or lead to a delay in the commencement or completion of clinical trials may also ultimately lead to delay or denial of regulatory clearance or approval. The clinical trial process may fail to demonstrate that our tests are effective for the proposed indicated uses, which could cause us to abandon a test candidate and may delay development of other tests.
Clinical trial failures can occur at any stage of the testing and we may experience numerous unforeseen events during, or as a result of, the clinical trial process that could delay or prevent commercialization of our current or future diagnostic tests
Clinical trial failures or delays can occur at any stage of the trials, and may be directly or indirectly caused by a variety of factors, including but not limited to:
|
|
·
|
Delays in securing clinical investigators or trial sites for our clinical trials;
|
|
|
·
|
Delays in obtaining Institutional Review Board and other regulatory approvals to commence a clinical trial;
|
|
|
·
|
Slower than anticipated rates of patient recruitment and enrollment, or failing to reach the targeted number of patients due to competition for patients from other trials;
|
|
|
·
|
Limited or no availability of coverage, reimbursement and adequate payment from health maintenance organizations and other third party payers for the use of our diagnostic test candidates in our clinical trials;
|
|
|
·
|
Negative or inconclusive results from clinical trials;
|
|
|
·
|
Approval and introduction of new diagnostic or changes in standards of practice or regulatory guidance that render our clinical trial endpoints or the targeting of our proposed indications obsolete;
|
|
|
·
|
Inability to monitor patients adequately during or after treatment or problems with investigator or patient compliance with the trial protocols;
|
|
|
·
|
Inability to replicate in large controlled studies safety and efficacy data obtained from a limited number of patients in uncontrolled trials; and
|
|
|
·
|
Inability or unwillingness of medical investigators to follow our clinical protocols.
|
We will depend on Medicare and a limited number of private payers for a significant portion of our revenues, and our revenues could decline if these payers fail to provide timely and adequate payment for our diagnostic tests
We expect that a substantial portion of the patients for whom we will perform diagnostic tests will have Medicare as their primary medical insurance. Even if our planned tests are otherwise successful, reimbursement for the Medicare-covered portions of our planned tests might not, without Medicare reimbursement, produce sufficient revenues to enable us to reach profitability and achieve our other commercial objectives.
Medicare and other third-party payers may change their coverage policies or cancel future contracts with us at any time; review and adjust the rate of reimbursement; or stop paying for our tests altogether, which would reduce our total revenues. Payers have increased their efforts to control the cost, utilization, and delivery of health care services, and have undertaken measures to reduce payment rates for and decrease utilization of clinical laboratory testing. Because of the cost-trimming trends, any third-party payers that will cover and provide reimbursement for our diagnostic tests may suspend, revoke or discontinue coverage at any time, or may reduce the reimbursement rates payable to us. Any such action could have a negative impact on our revenues, which may have a material adverse effect on our financial condition, results of operations and cash flows.
Changes in healthcare laws and policies may have a material adverse effect on our financial condition, results of operations and cash flows
The ACA substantially changed the way health care is financed by both governmental and private insurers. Among the ACA’s key changes, the ACA reduced payment rates under the Medicare Clinical Laboratory Fee Schedule and established an Independent Payment Advisory Board to reduce the per capita rate of growth in Medicare spending if spending exceeds a target growth rate. Such provisions may negatively impact payment rates for our diagnostic tests.
PAMA significantly altered the payment methodology under the Clinical Laboratory Fee Schedule that determines Medicare coverage for laboratory tests. Under PAMA, clinical laboratories are required to report test payment data for each Medicare-covered clinical diagnostic laboratory test and beginning in 2017, the Medicare payment rate for each clinical diagnostic laboratory test will be equal to the weighted median amount for the test from the most recent data collection period.
Congress has proposed on several occasions to impose a 20% coinsurance payment requirement on patients for clinical laboratory tests reimbursed under the Medicare Clinical Laboratory Fee Schedule, which would require us to bill patients for these amounts. In the event that Congress were to ever enact such legislation, the cost of billing and collecting for our tests could often exceed the amount actually received from the patient.
On September 25, 2015, CMS released preliminary determinations for the calendar year 2016 for the Medicare Clinical Laboratory Fee Schedule for some test codes, including some for oncology diagnostics, as had been anticipated. These preliminary determinations were based on a cross walk approach rather than a gap-fill approach. A cross walk approach matches a new code for a diagnostic against existing codes to determine the appropriate payment rate; while a gap-fill approach looks at local pricing patterns, including charges for the tests and any discounts on charges and payments determined by other payers. At this point it is not clear what methodology CMS may use in their determinations for future diagnostics.
Beginning January 1, 2017, Medicare payment for any new advanced diagnostic test will be based on the list price or charge. After the test is commercially available for two quarters, the laboratory will be required to report payment and volume information and that data will be used to set payment for the test for the following year.
|
|
·
|
If data shows that the list price was greater than 130% of the payment using established methodology (a weighted median), CMS will recoup the difference from the laboratory through a payment claw back.
|
|
|
·
|
Payment will be updated annually based on the weighted median of commercial payer reimbursement.
|
We cannot predict whether future health care initiatives will be implemented at the federal or state level, or how any future legislation or regulation may affect us. The expansion of government’s role in the U.S. health care industry as a result of the ACA, and changes to the reimbursement amounts paid by Medicare and other payers for diagnostic tests may have a materially adverse effect on our business, financial condition, results of operations and cash flows.
Because of certain Medicare billing policies, we may not receive complete reimbursement for tests provided to Medicare patients
Medicare has coverage policies that can be national or regional in scope. Coverage means that the test or assay is approved as a benefit for Medicare beneficiaries. If there is no coverage, neither the supplier nor any other party, such as a diagnostic laboratory, may receive reimbursement from Medicare for the service. Regional policies are directed by Medicare’s regional Medicare Administrative Contractors (“MACs”). Reimbursement for our diagnostic testing may be negatively impacted by California MAC policies.
Long payment cycles of Medicare, Medicaid and/or other third-party payors, or other payment delays, could hurt our cash flows and increase our need for working capital
Medicare and Medicaid have complex billing and documentation requirements that we will have to satisfy in order to receive payment. Failure to comply with these requirements and other laws applicable to billing may result in, among other things, non-payment, refunds, exclusion from government healthcare programs, and civil or criminal liabilities, any of which may have a material adverse effect on our revenues and earnings. Similarly, the failure of private health insurers or other private third-party payers to properly process our payment claims in a timely manner could delay our receipt of payment for our diagnostic tests and services, which may have a material adverse effect on our cash flows.
Private health insurance company policies may deny coverage or limit the amount they will reimburse us for the performance of our diagnostic tests
Patients who are not covered by Medicare will generally rely on health insurance provided by private health insurance companies. If we are considered a “non-contracted provider” by a third-party payer, that payer may not reimburse patients for diagnostic tests performed by us or doctors within the payer’s network of covered physicians may not use our services to perform diagnostic tests for their patients. As a result we may need to enter into contracts with health insurance companies or other private payers to provide diagnostic tests to their insured patients at specified rates of reimbursement which may be lower than the rates we might otherwise collect.
We may be required to comply with federal and state laws governing the privacy of health information, and any failure to comply with these laws could result in material criminal and civil penalties
The HIPAA sets forth security regulations that establish administrative, physical and technical standards for maintaining the confidentiality, integrity and availability of Protected Health Information in electronic form. We also may be required to comply with state laws that are more stringent than HIPAA or that provide individuals with greater rights with respect to the privacy or security of, and access to, their health care records. The Health Information Technology for Economic and Clinical Health Act (“HITECH”) established certain health information security breach notification obligations that require covered entities to notify each individual whose “protected health information” is breached.
We may incur significant compliance costs related to HIPAA and HITECH privacy regulations and varying state privacy regulations and varying state privacy and security laws. Given the complexity of HIPAA and HITECH and their overlap with state privacy and security laws, and the fact that these laws are rapidly evolving and are subject to changing and potentially conflicting interpretation, our ability to comply with the HIPAA, HITECH and state privacy requirements is uncertain and the costs of compliance are significant. The costs of complying with any changes to the HIPAA, HITECH and state privacy restrictions may have a negative impact on our operations. Noncompliance could subject us to criminal penalties, civil sanctions and significant monetary penalties as well as reputational damage.
We are subject to federal and state healthcare fraud and abuse laws and regulations and could face substantial penalties if we are unable to fully comply with such laws
We are subject to health care fraud and abuse regulation and enforcement by both the federal government and the states in which we conduct our business. These health care laws and regulations include the following:
|
|
·
|
The federal Anti-Kickback Statute;
|
|
|
·
|
The federal physician self-referral prohibition, commonly known as the Stark Law;
|
|
|
·
|
The federal false claims and civil monetary penalties laws;
|
|
|
·
|
The federal Physician Payment Sunshine Act requirements under the ACA; and
|
|
|
·
|
State law equivalents of each of the federal laws enumerated above.
|
Any action brought against us for violation of these laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation of any of these laws and regulations, we may be subject to any applicable penalty associated with the violation, including, among others, administrative, civil and criminal penalties, damages and fines, and/or exclusion from participation in Medicare, Medicaid programs, including the California Medical Assistance Program (Medi-Cal—the California Medicaid program) or other state or federal health care programs. Additionally, we could be required to refund payments received by us, and we could be required to curtail or cease our operations.
Risks Related to Intellectual Property
If we are unable to obtain and enforce patents and to protect our trade secrets, others could use our technology to compete with us, which could limit opportunities for us to generate revenues by licensing our technology and selling diagnostic tests
|
|
·
|
Our success will depend in part on our ability to obtain and enforce patents and maintain trade secrets in the United States and in other countries. If we are unsuccessful in obtaining and enforcing patents, our competitors could use our technology and create diagnostic tests that compete with our diagnostic tests, without paying license fees or royalties to us.
|
|
|
·
|
The preparation, filing, and prosecution of patent applications can be costly and time consuming. Our limited financial resources may not permit us to pursue patent protection of all of our technology and diagnostic tests throughout the world.
|
|
|
·
|
Even if we are able to obtain issued patents covering our technology or diagnostic tests, we may have to incur substantial legal fees and other expenses to enforce our patent rights in order to protect our technology and diagnostic tests from infringing uses. We may not have the financial resources to finance the litigation required to preserve our patent and trade secret rights.
|
|
|
·
|
The Supreme Court decisions in
Mayo Collaborative Services v. Prometheus Laboratories, Inc.
and
Association for Molecular Pathology v. Myriad Genetics
may adversely impact our ability to obtain patent protection for some or all of our diagnostic tests, which use certain gene markers to indicate the presence of certain cancers. The claims in the contested patents that were the subject of the Supreme Court decision in
Mayo Collaborative Services v. Prometheus Laboratories, Inc.
were directed to measuring the serum level of a drug metabolite and adjusting the dosing regimen of the drug based on the metabolite level. The Supreme Court said that a patent claim that merely claimed a mathematical correlation between the blood levels of a drug metabolite and the best dosage of the drug was not patentable subject matter because it did no more than recite a correlation that occurs in nature. In
Association for Molecular Pathology v. Myriad Genetics,
the Supreme Court ruled that the discovery of the precise location and sequence of certain genes, mutations of which can dramatically increase the risk of breast and ovarian cancer, was not patentable. Knowledge of the gene location and sequences was used to determine the genes’ typical nucleotide sequence, which, in turn, enabled the development of medical tests useful for detecting mutations in these genes in a particular patient to assess the patient’s cancer risk. But the mere discovery of an important and useful gene did not render the genes patentable as a new composition of matter. The holdings in
Mayo Collaborative Services v. Prometheus Laboratories, Inc.
and
Association for Molecular Pathology v. Myriad Genetics
may limit our ability to obtain patent protection on diagnostic methods that merely recite a correlation between a naturally occurring event and a diagnostic outcome associated with that event.
|
There is no certainty that our pending or future patent applications will result in the issuance of patents
We have filed patent applications for technology that we have developed, and we may obtain licenses for patent applications covering genes that we or our partners have discovered, that we believe will be useful in producing new diagnostic tests. We may also file additional new patent applications in the future seeking patent protection for new technology or diagnostics tests or products that we develop ourselves or jointly with others. However, there is no assurance that any of our licensed patent applications, or any patent applications that we have filed or that we may file in the future in the United States or abroad, will result in the issuance of patents.
The process of applying for and obtaining patents can be expensive and slow
|
|
·
|
The preparation and filing of patent applications, and the maintenance of patents that are issued, may require substantial time and money.
|
|
|
·
|
A patent interference proceeding may be instituted with the USPTO when more than one person files a patent application covering the same technology, or if someone wishes to challenge the validity of an issued patent. At the completion of the interference proceeding, the USPTO will determine which competing applicant is entitled to the patent, or whether an issued patent is valid. Patent interference proceedings are complex, highly contested legal proceedings, and the USPTO’s decision is subject to appeal. This means that if an interference proceeding arises with respect to any of our patent applications, we may experience significant expenses and delay in obtaining a patent, and if the outcome of the proceeding is unfavorable to us, the patent could be issued to a competitor rather than to us.
|
|
|
·
|
A derivation proceeding may be instituted by the USPTO or an inventor alleging that a patent or application was derived from the work of another inventor.
|
|
|
·
|
Post Grant Review under the new America Invents Act will make available opposition-like proceedings in the United States. As with the USPTO interference proceedings, Post Grant Review proceedings will be very expensive to contest and can result in significant delays in obtaining patent protection or can result in a denial of a patent application.
|
|
|
·
|
Oppositions to the issuance of patents may be filed under European patent law and the patent laws of certain other countries. As with USPTO interference proceedings, these foreign proceedings can be very expensive to contest and can result in significant delays in obtaining a patent or can result in a denial of a patent application.
|
Our patents may not protect our diagnostic tests from competition
|
|
·
|
We might not be able to obtain any patents beyond the bladder cancer marker patent that has been issued by the USPTO, and any patents that we do obtain might not be comprehensive enough to provide us with meaningful patent protection.
|
|
|
·
|
There will always be a risk that our competitors might be able to successfully challenge the validity or enforceability of any patent issued to us.
|
|
|
·
|
In addition to interference proceedings, the USPTO can reexamine issued patents at the request of a third party. Our patents may be subject to inter partes review (replacing the reexamination proceeding), a proceeding in which a third party can challenge the validity of one of our patents to have the patent invalidated. This means that patents owned or licensed by us may be subject to reexamination and may be lost if the outcome of the reexamination is unfavorable to us.
|
We may be subject to patent infringement claims that could be costly to defend, which may limit our ability to use disputed technologies, and which could prevent us from pursuing research and development or commercialization of some of our diagnostic tests, require us to pay licensing fees to have freedom to operate and/or result in monetary damages or other liability for us
The success of our business depends significantly on our ability to operate without infringing patents and other proprietary rights of others. If the technology that we use infringes a patent held by others, we could be sued for monetary damages by the patent holder or its licensee, or we could be prevented from continuing research, development, and commercialization of diagnostic tests that rely on that technology, unless we are able to obtain a license to use the patent. The cost and availability of a license to a patent cannot be predicted, and the likelihood of obtaining a license at an acceptable cost would be lower if the patent holder or any of its licensees is using the patent to develop or market a diagnostic tests with which our diagnostic test would compete. If we could not obtain a necessary license, we would need to develop or obtain rights to alternative technologies, which could prove costly and could cause delays in diagnostic test development, or we could be forced to discontinue the development or marketing of any diagnostic tests that were developed using the technology covered by the patent.
Risks Related to Our Relationship with BioTime
We are a subsidiary of BioTime, and accordingly our business is substantially controlled by BioTime
BioTime owns approximately 57.7% of our issued and outstanding shares of common stock. This means that BioTime will have the voting power, through its ownership of shares of our common stock, to elect our entire Board of Directors and to control our management.
BioTime could cause corporate actions to be taken even if the interests of BioTime conflict with the interests of our other shareholders. This concentration of voting power could have the effect of deterring or preventing a change in control that might be beneficial to our other shareholders.
As the majority shareholder, BioTime will have the voting power to approve or disapprove any matter of corporate transaction presented to our shareholders for approval, including but not limited to:
|
|
·
|
Any amendment of our articles of incorporation or bylaws;
|
|
|
·
|
Any merger or consolidation of us with another company;
|
|
|
·
|
Any recapitalization or reorganization of our capital stock;
|
|
|
·
|
Any sale of assets or purchase of assets; or
|
|
|
·
|
A corporate dissolution or a plan of liquidation of our business.
|
We will initially rely upon BioTime for certain services and resources
Although we plan to have our own CLIA certified diagnostic laboratory, our own scientific personnel, and many critical management personnel, we will initially rely on BioTime to provide certain management and administrative services, including patent prosecution, certain legal services, accounting, financial management, and controls over financial accounting and reporting. We have entered into a Shared Facilities and Services Agreement (“Shared Facilities Agreement”) with BioTime under which we have agreed to bear costs allocated to us by BioTime for the use of BioTime office and research facilities, human resources, services, and materials provided for our benefit by BioTime. We will pay BioTime 105% of its costs of providing personnel and services to us, and for any use of its facilities by us, including an allocation of general overhead based on that use. We may also share the services of some research personnel with BioTime.
If BioTime’s human resources and facilities are not sufficient to serve both BioTime’s needs and ours, we will have to hire additional personnel of our own, either on a full-time or part-time basis, as employees or as consultants, and the cost of doing so could be greater than the costs that would be allocated to us by BioTime. Also, any new personnel that we may need to hire may not be as familiar with our business or operations as BioTime’s personnel, which means that we would incur the expense and inefficiencies related to training new employees or consultants.
Three of our directors are officers or directors of BioTime
Three of the seven members of our Board of Directors are also officers or directors of BioTime and one of our directors is our Chief Executive Officer. This commonality of directors means that we will not have a Board of Directors making business decisions on our behalf independent from BioTime and our management. Even those of our directors who do not serve on the BioTime Board of Directors will be elected to our Board of Directors by BioTime, and they may be removed from our Board of Directors by BioTime, as the majority shareholder.
Conflicts of interest may arise from our relationship with BioTime
Our relationship with BioTime could give rise to certain conflicts of interest that could have an impact on our research and development programs, business opportunities, and operations generally.
|
|
·
|
Even if we utilize different technologies than BioTime or its other subsidiaries, we could find ourselves in competition with them for research scientists, financing and other resources, licensing, manufacturing, and distribution arrangements, and for customers if we and BioTime or another BioTime subsidiary both bring diagnostic tests to market.
|
|
|
·
|
Because we are a subsidiary of BioTime, BioTime could prevent us from engaging in research and development programs, investments, business ventures, or agreements to develop, license, or acquire diagnostic tests or technologies that would or might compete with those owned, licensed, or under development by BioTime or any of its other subsidiaries.
|
|
|
·
|
BioTime and its other subsidiaries will engage for their own accounts in research and product development programs, investments, and business ventures, and we will not be entitled to participate or to receive an interest in those programs, investments, or business ventures. BioTime and its other subsidiaries will not be obligated to present any particular research and development, investment, or business opportunity to us, even if the opportunity would be within the scope of our research and development plans or programs, business objectives, or investment policies. These opportunities may include, for example, opportunities to acquire businesses or assets, including but not limited to patents and other intellectual property that could be used by us or by BioTime or by any of BioTime’s other subsidiaries. Our respective boards of directors will have to determine which company should pursue those opportunities, taking into account relevant facts and circumstances at the time, such as the financial and other resources of the companies available to acquire and utilize the opportunity, and the best “fit” between the opportunity and the business and research and development programs of the companies. However, since BioTime will have the ultimate power to elect the members of our Board of Directors, BioTime may have the ultimate say in decision making with respect to the allocation of opportunities.
|
|
|
·
|
If we enter into any patent or technology license or sublicense, or any other agreement with BioTime or with another BioTime subsidiary, the BioTime companies that are parties to the agreement may have a conflict of interest in determining how and when they should enforce their rights under the agreement if the other BioTime company that is a party were to default or otherwise fail to perform any of its obligations under the agreement.
|
|
|
·
|
One of our significant assets is 619,706 BioTime common shares that we acquired from BioTime in exchange for shares of our common stock. We may sell the BioTime shares from time to time, or pledge the shares as collateral for loans, to raise capital to finance our operations. Because a sale of those shares could have a depressing effect on the market value of BioTime common shares, BioTime will have a continuing interest in the number of shares we sell, the prices at which we sell the shares, and the time and manner in which the shares are sold. Further, we may need or find it desirable to sell BioTime common shares at the same time as BioTime, or other BioTime subsidiaries that hold BioTime common shares, also desire to sell some of their BioTime common shares. Concurrent sales of BioTime common shares by us, BioTime, or other BioTime subsidiaries cold have a depressing effect on the market price of the BioTime common shares, lower the price at which we and they are able to sell BioTime common shares, and result in lower net proceeds from the sales. We plan to coordinate any future sales of our BioTime common shares with BioTime and its other subsidiaries in order to provide an orderly and controlled process for raising capital through the sale of BioTime shares. This will include an agreement as to the number of shares to be sold, the time period or “market window” for selling shares, the use of a common securities broker-dealer, and a fair allocation of net sales based on average sales prices during any trading day on which we and they sell BioTime shares.
|
|
|
·
|
Each conflict of interest will be resolved by our respective boards of directors in keeping with their fiduciary duties and such policies as they may implement from time to time. However, the terms and conditions of patent and technology licenses and other agreements between us and BioTime or other BioTime subsidiaries will not be negotiated on an arm’s-length basis due to BioTime’s ownership of a controlling interest in us and due to the commonality of directors serving on our respective boards of directors.
|
Risks Related to Our Dependence on Third Parties
There is a limited number of manufacturers of molecular diagnostic equipment and related chemical reagents necessary for the provision of our diagnostic tests
In order to develop molecular diagnostics and to provision our diagnostic tests, we will need to acquire certain analytic equipment. There are only a few manufacturers of the equipment we will need and the chemical reagents that are required for use with a particular manufacturer’s equipment will be available only from that equipment manufacturer. If the manufacturer of the equipment we acquire discontinues operation or if we experience supply or quality issues with their equipment or reagents, it may become necessary for us to acquire different analytic equipment, which would require additional experiments to ensure reproducibility of our test results using the new equipment. As a result, we may be unable to provide our diagnostic tests for a period of time.
If we fail to enter into and maintain successful strategic alliances for diagnostic tests that we elect to co-develop, co-market, or out-license, we may have to reduce or delay our diagnostic test development or increase our expenditures
In order to facilitate the development, manufacture and commercialization of our diagnostic tests we may enter into strategic alliances with pharmaceutical companies or other industry participants to advance our programs and enable us to maintain our financial and operational capacity. We will face significant competition in seeking appropriate alliances. We may not be able to negotiate alliances on acceptable terms, if at all. If we fail to create and maintain suitable alliances, we may have to limit the size or scope of, or delay, one or more of our product development or research programs, or we will have to increase our expenditures and will need to obtain additional funding, which may be unavailable or available only on unfavorable terms.
If we are able to enter into development and marketing arrangements with pharmaceutical or medical device companies for our diagnostic tests, we may license product development, manufacturing, and marketing rights to the pharmaceutical or medical device company or to a joint venture company formed with the pharmaceutical or medical device company. Under such arrangements we might receive only a royalty on sales of the diagnostic tests developed or an equity interest in a joint venture company that develops the diagnostic test. As a result, our revenues from the sale of those diagnostic tests may be substantially less than the amount of revenues and gross profits that we might receive if we were to develop, manufacture, and market the diagnostic tests ourselves.
We may become dependent on possible future collaborations to develop and commercialize many of our diagnostic test candidates and to provide the manufacturing, regulatory compliance, sales, marketing and distribution capabilities required for the success of our business
We may enter into various kinds of collaborative research and development, manufacturing, and diagnostic test marketing agreements to develop and commercialize our diagnostic tests. Any future milestone payments and cost reimbursements from collaboration agreements could provide an important source of financing for our research and development programs, thereby facilitating the application of our technology to the development and commercialization of our diagnostic tests, but there are risks associated with entering into collaboration arrangements.
There is a risk that we could become dependent upon one or more collaborative arrangements for diagnostic test development or manufacturing or as a source of revenues from the sale of any diagnostic tests that may be developed by us alone or through one of the collaborative arrangements. A collaborative arrangement upon which we might depend might be terminated by our collaboration partner or they might determine not to actively pursue the development or commercialization of our diagnostic tests. A collaboration partner also may not be precluded from independently pursuing competing diagnostic tests or technologies.
There is a risk that a collaboration partner might fail to perform its obligations under the collaborative arrangements or may be slow in performing its obligations. In addition, a collaboration partner may experience financial difficulties at any time that could prevent it from having available funds to contribute to the collaboration. If a collaboration partner fails to conduct its diagnostic test development, manufacturing, commercialization, regulatory compliance, sales and marketing or distribution activities successfully and in a timely manner, or if it terminates or materially modifies its agreements with us, the development and commercialization of one or more diagnostic test candidates could be delayed, curtailed or terminated because we may not have sufficient financial resources or capabilities to continue diagnostic test development, manufacturing, and commercialization on our own.
Risks Pertaining to Our Common Stock
Ownership of our common stock will entail certain risks associated with the absence of a previous trading market for our common stock, volatility of prices for our shares, and the fact that we do not pay dividends.
Because we are engaged in the development of medical diagnostic tests, the price of our stock may rise and fall rapidly
The market price of our common stock, like that of the shares of many biotechnology companies, may be highly volatile. The price of our common stock may rise or fall rapidly as a result of a number of factors, including:
|
|
·
|
Sales or potential sales of substantial amounts of our common stock;
|
|
|
·
|
Results of preclinical testing or clinical trials of our diagnostic test candidates or those of our competitors;
|
|
|
·
|
Announcements about us or about our competitors, including clinical trial results, regulatory approvals, new diagnostic test introductions and commercial results;
|
|
|
·
|
The cost of our development programs;
|
|
|
·
|
The success of competitive diagnostic tests or technologies;
|
|
|
·
|
Litigation and other developments relating to our issued patents or patent applications or other proprietary rights or those of our competitors;
|
|
|
·
|
Conditions in the diagnostic, pharmaceutical or biotechnology industries;
|
|
|
·
|
Actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts;
|
|
|
·
|
Variations in our financial results or those of companies that are perceived to be similar to us, including the failure of our earnings to meet analysts’ expectations;
|
|
|
·
|
General economic, industry and market conditions; and
|
|
|
·
|
Changes in payer coverage and or reimbursement.
|
Many of these factors are beyond our control. The stock markets in general, and the market for pharmaceutical and biotechnological companies in particular, have been experiencing extreme price and volume fluctuations which have affected the market price of the equity securities without regard to the operating performance of the issuing companies. Broad market fluctuations, as well as industry factors and general economic and political conditions, may adversely affect the market price of our common stock.
Because we do not pay dividends, our stock may not be a suitable investment for anyone who needs to earn dividend income
We do not pay cash dividends on our common stock. For the foreseeable future we anticipate that any earnings generated in our business will be used to finance the growth of our business and will not be paid out as dividends to our shareholders. This means that our stock may not be a suitable investment for anyone who needs to earn income from their investments.
Securities analysts may not initiate coverage or continue to cover our common stock, and this may have a negative impact on the market price of our shares
The market for our common stock will depend, in part, on the research and reports that securities analysts publish about our business and our common stock. We do not have any control over these analysts. There is no guarantee that securities analysts will cover our common stock. If securities analysts do not cover our common stock, the lack of research coverage may adversely affect the market price of those shares. If securities analysts do cover our shares, they could issue reports or recommendations that are unfavorable to the price of our shares, and they could downgrade a previously favorable report or recommendation, and in either case our share price could decline as a result of the report. If one or more of these analysts ceases to cover our shares or fails to publish regular reports on our business, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
You may experience dilution of your ownership interests if we issue additional shares of common stock or preferred stock
In the future, we may issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our present shareholders. We are currently authorized to issue an aggregate of 55,000,000 shares of capital stock consisting of 50,000,000 shares of common stock and 5,000,000 “blank check” shares of preferred stock. At March 23, 2016 there were 25,411,800 shares of common stock outstanding and 2,854,000 shares of common stock reserved for issuance upon the exercise of options under our employee stock option plan. No shares of preferred stock are presently outstanding.
We may issue additional common stock or other securities that are convertible into or exercisable for common stock in order to raise additional capital, or in connection with hiring or retaining employees or consultants, or in connection with future acquisitions of licenses to technology or rights to acquire diagnostic tests in connection with future business acquisitions, or for other business purposes. The future issuance of any such additional common stock or other securities may create downward pressure on the trading price of our common stock.
We may also issue preferred stock having rights, preferences, and privileges senior to the rights of our common stock with respect to dividends, rights to share in distributions of our assets if we liquidate our company, or voting rights. Any preferred stock may also be convertible into common stock on terms that would be dilutive to holders of common stock.
Additional shares of our common stock will become eligible for public sale, and sales of those shares could create downward pressure on the trading price of our common stock
BioTime, directly and through a subsidiary, holds 14,866,888 shares of OncoCyte common stock and four other OncoCyte shareholders own a total of 6,003,000 shares of OncoCyte common stock that were issued without registration under the Securities Act, of which 4,500,000 shares are presently eligible to be publicly sold in compliance with the provisions of SEC Rule 144 and the balance of which will become eligible to be sold under Rule 144 after the shares have been beneficially owned for at least one year, or for at least six months if the shares are sold after we have been subject to the reporting requirements of Section 13 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), for a period of 90 days and have filed all reports required under the Exchange Act, other than form 8-K.
We have agreed to register for sale under the Securities Act 10,481,604 shares of common stock that we sold to certain investors, including BioTime, without registration under the Securities Act. We have agreed to file a registration statement covering those shares promptly after the date on which we first become eligible to register those securities on Form S-3. Under the rules for the use of Form S-3, on December 30, 2016 we will first become eligible to register securities on Form S-3.
Sales of OncoCyte common stock under Rule 144 or through an effective registration statement under the Securities Act could create downward pressure on the trading price of our common stock.
We are an “emerging growth company,” and may elect to comply with reduced public company reporting requirements applicable to emerging growth companies, which could make our common stock less attractive to investors
We are an “emerging growth company,” as defined in the JOBS Act, and we may to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an “emerging growth company.” We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.0 billion or more; (ii) the fifth anniversary of the completion of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act; (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the SEC.
We will incur costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives
As a public reporting company, we will incur significant legal, accounting and other expenses. The Sarbanes-Oxley Act of 2002 and rules subsequently implemented by the SEC, have imposed various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will entail significant legal and financial compliance costs and will make some activities more time consuming and costly. For example, we expect that these rules and regulations may make it difficult and expensive for us to obtain director and officer liability insurance, and we may be required to accept low policy limits and coverage.
|
Item 1B.
|
Unresolved Staff Comments
|
None
Under a Shared Facilities Agreement with BioTime, we have use of laboratory and office space at BioTime’s facility in Alameda, California. BioTime has leased approximately 30,795 square feet of office and laboratory space in two buildings located in Alameda, and will provide OncoCyte use of space sufficient for a CLIA compliant diagnostic laboratory.
|
Item 3.
|
Legal Proceedings
|
From time to time, we may be involved in routine litigation incidental to the conduct of our business. We are not presently involved in any material litigation or proceedings, and to our knowledge no such litigation or proceedings are contemplated.
|
Item 4.
|
Mine Safety Disclosures
|
Not applicable
PART II
|
|
Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities
|
Our common stock has been traded on the NYSE MKT under the symbol “OCX” since January 4, 2016.
As of March 23, 2016, we had 289 holders of record of our common stock. This number does not include shareholders whose shares of OncoCyte common stock are held in “street name” in accounts with securities broker-dealers or other financial institutions or fiduciaries.
The following table shows certain information concerning the options outstanding and available for issuance under all of our compensation plans and agreements as of December 31, 2015 (in thousands, except weighted average exercise price):
|
Plan Category
|
Number of Shares
to
be Issued upon
Exercise of
Outstanding
Options, Warrants,
and Rights
|
|
Weighted Average
Exercise Price of
the Outstanding
Options, Warrants,
and Rights
|
|
Number of Shares
Remaining Available
for Future Issuance
under Equity
Compensation Plans
|
|
|
OncoCyte Stock Option Plans Approved by Shareholders
|
|
|
2,240
|
|
|
$
|
2.03
|
|
|
|
1,757
|
|
Additional information concerning our Stock Option Plan and the stock options may be found in Note 7 to the Financial Statements.
Dividend Policy
We have never paid cash dividends on our capital stock and we do not anticipate paying cash dividends in the foreseeable future, but intend to retain our capital resources for reinvestment in our business. Any future determination to pay cash dividends will be at the discretion of our Board of Directors and will be dependent upon our financial condition, results of operations, capital requirements and other factors as our Board of Directors deems relevant.
|
Item 6.
|
Selected Financial Data (in thousands, except per share data)
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2015
|
|
|
2014
|
|
|
2013
|
|
|
OPERATING EXPENSES
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
$
|
4,527
|
|
|
$
|
3,962
|
|
|
$
|
2,943
|
|
|
General and administrative
|
|
|
4,191
|
|
|
|
1,011
|
|
|
|
552
|
|
|
Total operating expenses
|
|
|
8,718
|
|
|
|
4,973
|
|
|
|
3,495
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(8,718
|
)
|
|
|
(4,973
|
)
|
|
|
(3,495
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER EXPENSES, NET
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net
|
|
|
(19
|
)
|
|
|
(2
|
)
|
|
|
-
|
|
|
Other income (expense), net
|
|
|
2
|
|
|
|
(11
|
)
|
|
|
-
|
|
|
Total other expense, net
|
|
|
(17
|
)
|
|
|
(13
|
)
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET LOSS
|
|
$
|
(8,735
|
)
|
|
$
|
(4,986
|
)
|
|
$
|
(3,495
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per share
|
|
$
|
(0.42
|
)
|
|
$
|
(0.27
|
)
|
|
$
|
(0.19
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding: basic and diluted
|
|
|
21,009
|
|
|
|
18,200
|
|
|
|
18,200
|
|
|
|
|
December 31,
|
|
|
|
|
2015
|
|
2014
|
|
|
Balance Sheet Data (in thousands):
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
7,996
|
|
|
$
|
257
|
|
|
|
BioTime shares held as available-for-sale securities, at fair value
|
|
|
2,541
|
|
|
|
3,280
|
|
|
|
Intangible assets, net
|
|
|
1,230
|
|
|
|
1,472
|
|
|
|
Total assets
|
|
|
12,731
|
|
|
|
5,241
|
|
|
|
Total liabilities
|
|
|
2,314
|
|
|
|
6,315
|
|
|
|
Total stockholders’ equity (deficit)
|
|
$
|
10,417
|
|
|
$
|
(1,074
|
)
|
|
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations is intended to provide information necessary to understand our audited financial statements for the years ended December 31, 2015, 2014 and 2013, and highlight certain other information which, in the opinion of management, will enhance a reader's understanding of our financial condition, changes in financial condition and results of operations. These historical financial statements may not be indicative of our future performance. This Management's Discussion and Analysis of Financial Condition and Results of Operations contains a number of forward-looking statements, all of which are based on our current expectations and could be affected by the uncertainties and risks described throughout this filing, particularly in “Risk Factors.”
Emerging Growth Company Status
The Jumpstart our Business Startups Act of 2012 (“JOBS Act”) permits an "emerging growth company" such as OncoCyte to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. However, we will comply with newly adopted or revised accounting standards when they become applicable to public companies because our financial statements are consolidated with those of BioTime, which is not an emerging growth company under the JOBS Act and is therefore not permitted to delay the adoption of new or revised accounting standards that become applicable to public companies. This election under the JOBS Act to not delay the adoption of new or revised accounting standards is irrevocable.
Management’s Discussion and Analysis of Financial Condition and Results of Operations.
We were incorporated during September 2009. Our operations have included planning and launching research and diagnostic test development programs in house and with partners, pursuing patents, and conducting clinical trials.
The inherent uncertainties of developing new diagnostic tests for medical use make it impossible to predict the amount of time and expense that will be required to complete the development and commence commercialization of new diagnostic tests. There is no assurance that we will be successful in developing new technology or diagnostic tests, or that any technology or diagnostic tests that we may develop will be proven safe and effective in diagnosis of cancer in humans, or will be successfully commercialized.
We believe we have sufficient capital to carry out our current research and development plan at least through December 31, 2016. We will need to obtain additional financing in order to continue our operations after that date or if we proceed to construct a diagnostic laboratory and prepare to commercialize our first diagnostic test before the end of the year. Our determination as to when we will seek new financing and the amount of financing that we will need will be based on our evaluation of the progress we make in our research and development program, any changes to or the expansion of the scope and focus of our research, and our projection of future costs. See “Liquidity and Capital Resources” for a discussion of our available capital resources, our need for future financing, and possible sources of capital.
Critical Accounting Policies
The preparation of financial statements in conformity with accounting principles generally accepted in the United States (“GAAP”), requires management to make estimates and assumptions that affect the reported amounts in our financial statements and related notes. Our significant accounting policies are described in Note 2 to our financial statements included elsewhere in this Report. We have identified below our critical accounting policies and estimates that we believe require the greatest amount of judgment. On an ongoing basis, we evaluate our estimates that are subject to significant judgment including those related to the allocation of direct and indirect expenses, useful lives associated with long-lived assets, loss contingencies, valuation allowances related to deferred income taxes, and assumptions used to value stock-based awards, convertible debt, conversion features, warrants or other equity instruments. Actual results could differ materially from those estimates. On an ongoing basis, we evaluate our estimates compared to historical experience and trends, which form the basis for making judgments about the carrying value of assets and liabilities. To the extent that there are material differences between our estimates and our actual results, our future financial statement presentation, financial condition, results of operations and cash flows will be affected.
We believe the assumptions and estimates associated with the following have the greatest potential impact on our financial statements.
Related party transactions - Shared Facilities and Services Agreement
As more fully described in Note 4 to our financial statements, to the extent we do not employ our own human resources for operations, our parent company, BioTime, or BioTime commonly controlled and consolidated subsidiaries provide certain employees for administrative or operational services, as necessary, for our benefit, under the Shared Facilities Agreement. Accordingly, BioTime allocates expenses such as salaries and payroll related expenses incurred and paid on behalf of OncoCyte based on the amount of time that particular employees devote to our affairs. Other expenses such as legal, accounting, marketing, travel, and entertainment expenses are allocated to us to the extent that those expenses are incurred by or on behalf of OncoCyte. BioTime also allocates certain overhead expenses such as insurance, internet and telephone expenses based on a percentage determined by management. These allocations are made based upon activity-based allocation drivers such as time spent, percentage of square feet of office or laboratory space used, and percentage of personnel devoted to our operations or management. Management evaluates the appropriateness of the percentage allocations on a quarterly basis and believes that this basis for allocation is reasonable.
Stock-based compensation
We have adopted accounting standards governing share-based payments, which require the measurement and recognition of compensation expense for all share-based payment awards made to directors and employees, including employee stock options, based on estimated fair values. We utilize the Black-Scholes Merton option pricing model. Our determination of fair value of share-based payment awards on the date of grant using an option-pricing model is affected by our stock price as well as assumptions regarding a number of complex and subjective variables. These variables include, but are not limited to, expected stock price volatility over the term of the awards, and actual and projected employee stock option exercise behaviors. Because our common stock had no public trading history prior to December 31, 2015, for the years ended December 31, 2015 and 2014, we estimated the expected volatility of the awards from the historical volatility of selected public companies within the biotechnology industry with comparable characteristics to us, including similarity in size, lines of business, market capitalization, revenue and financial leverage. We determined the expected volatility assumption using the frequency of daily historical prices of comparable public company’s common stock for a period equal to the expected term of the options. The expected term of options granted is based upon the “simplified method” provided under
Staff Accounting Bulletin, Topic 14
, or SAB Topic 14. The risk-free rate is based on the U.S. Treasury rates in effect during the corresponding period of grant. Although the fair value of employee stock options is determined in accordance with FASB guidance, the key inputs and assumptions may change as we develop our own company estimates, experience and key inputs including our expected term, and stock price volatility based on the trading history of our stock on the NYSE:MKT. Changes in these subjective assumptions can materially affect the estimated value of equity grants and the stock-based compensation that we record in our financial statements.
Accounting for BioTime Shares
We account for the BioTime shares we hold as available-for-sale equity securities in accordance with ASC 320-10-25,
Investments – Debt and Equity Securities
, as the shares have a readily determinable fair value quoted on the NYSE MKT and are held principally for future working capital purposes, as necessary. These shares are measured at fair value and reported as current assets on the balance sheet based on the closing trading price of the security as of the date being presented. Unrealized holding gains and losses are excluded from the statements of operations and reported in equity as part of other comprehensive income or loss, net of income taxes, until realized. Realized gains and losses for shares sold are reclassified out of accumulated other comprehensive income or loss and included in equity, as an increase or decrease to common stock equity consistent with, and pursuant to, ASC 805-50
Business Combinations
(“ASC 805”), transactions between entities under common control.
Long-lived intangible assets
Long-lived intangible assets, primarily consisting of acquired patents, patent applications, and licenses to use certain patents are stated at acquired cost, less accumulated amortization. Amortization expense is computed using the straight-line method over the estimated useful lives of the assets over a period of 10 years.
Impairment of long-lived assets
We assess the impairment of long-lived assets, which consist primarily of long-lived intangible assets, furniture and equipment, whenever events or changes in circumstances indicate that such assets might be impaired and the carrying value may not be recoverable. If events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable and the expected undiscounted future cash flows attributable to the asset are less than the carrying amount of the asset, an impairment loss equal to the excess of the asset’s carrying value over its fair value is recorded. To date, there have been no such impairment losses.
Income taxes
We have filed a standalone U.S. federal income tax return since our inception. For California purposes, our activity for 2014 and 2015 has been included in BioTime’s California Combined tax return. The provision for income taxes has been determined as if we had filed separate tax returns for the periods presented. Accordingly, our effective tax rate in future years could vary from our historical effective tax rates depending on our future legal structure and related tax elections. The historical deferred tax assets, including the operating losses and credit carryforwards generated by us, will remain with us. We account for income taxes in accordance with GAAP, which prescribes the use of the asset and liability method, whereby deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect. Valuation allowances are established when necessary to reduce deferred tax assets when it is more likely than not that a portion or all of the deferred tax assets will not be realized. Our judgments regarding future taxable income may change over time due to changes in market conditions, changes in tax laws, tax planning strategies or other factors. If our assumptions and consequently our estimates change in the future, the valuation allowance may be increased or decreased, which may have a material impact on our statements of operations.
The guidance also prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not sustainable upon examination by taxing authorities. We will recognize accrued interest and penalties, if any, related to unrecognized tax benefits as income tax expense. No amounts were accrued for the payment of interest and penalties as of the financial statements periods presented herein. We are not aware of any uncertain tax positions that could result in significant additional payments, accruals, or other material deviation for the periods presented herein. We are currently unaware of any tax issues under review.
As further discussed in Notes 2 and 8 to our financial statements, OncoCyte early adopted the provisions of Accounting Standards Update, ASU 2015-17,
Income Taxes (Topic 740):
Balance Sheet Classification of Deferred Taxes
, on a retrospective basis and has adjusted the December 31, 2014 balances for the noncurrent deferred tax assets and current deferred tax liabilities as presented on the balance sheet to conform to the December 31, 2015 presentation. The early adoption of ASU 2015-17 had no impact to the statements of operations or cash flows for any period presented.
Research and development expenses
Research and development expenses consist of personnel costs and related benefits, including stock-based compensation, and expenses for outside consultants. These expenses include both direct and allocated or indirect overhead costs. Research and development costs are expensed as incurred.
General and administrative expenses
Our general and administrative expenses relate primarily to compensation and related benefits, including stock-based compensation, for executive and corporate personnel, including direct and allocated costs from BioTime; professional and consulting fees; direct and indirect allocated overhead.
Results of Operations
Comparison of the Years Ended December 31, 2015 and 2014
The following tables show our operating expenses for the years ended December 31, 2015 and 2014 (in thousands).
|
|
|
Years Ended
December 31,
|
|
$
|
|
%
|
|
|
|
|
2015
|
|
2014
|
|
Increase
|
|
Increase
|
|
|
Research and development expenses
|
|
$
|
4,527
|
|
|
$
|
3,962
|
|
|
$
|
+565
|
|
|
$
|
+14.3
|
%
|
|
|
General and administrative expenses
|
|
$
|
4,191
|
|
|
$
|
1,011
|
|
|
$
|
+3,180
|
|
|
$
|
+314.5
|
%
|
|
Research and development expenses
The following table shows the approximate amounts and percentages of our total research and development expenses of $4.5 million and $4.0 million allocated to our primary research and development projects during the years ended December 31, 2015 and 2014, respectively (in thousands).
|
|
|
Amount
(1)
|
|
Percent
|
|
|
Program
|
|
2015
|
|
2014
|
|
2015
|
|
2014
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General
|
|
$
|
1,719
|
|
|
$
|
1,621
|
|
|
|
38.0
|
%
|
|
|
40.9
|
%
|
|
|
Bladder Cancer Confirmatory Diagnostic
|
|
$
|
895
|
|
|
$
|
1,143
|
|
|
|
19.8
|
%
|
|
|
28.9
|
%
|
|
|
Breast Cancer Confirmatory Diagnostic
|
|
$
|
1,105
|
|
|
$
|
1,057
|
|
|
|
24.4
|
%
|
|
|
26.7
|
%
|
|
|
Lung Cancer Confirmatory Diagnostic
|
|
$
|
763
|
|
|
$
|
76
|
|
|
|
16.8
|
%
|
|
|
1.9
|
%
|
|
|
COLX
|
|
$
|
45
|
|
|
$
|
65
|
|
|
|
1.0
|
%
|
|
|
1.6
|
%
|
|
|
Total
|
|
$
|
4,527
|
|
|
$
|
3,962
|
|
|
|
100
|
%
|
|
|
100
|
%
|
|
|
|
(1)
|
Amount also includes certain general research and development expenses, such as laboratory supplies, laboratory expenses, rent allocated, and insurance allocated to research and development expenses, incurred directly by BioTime on behalf of OncoCyte and allocated to OncoCyte under the Shared Facilities Agreement.
|
Research and development expenses for the year ended December 31, 2015 increased to $4.5 million from $4.0 million for the same period in 2014. The increases in research and development expenses during 2015 are primarily attributable to the following increases: $309,000 of scientific consulting expenses, $277,000 of stock based compensation to employees and consultants, $235,000 of clinical trial related expenses; and a net increase of $101,000 in other miscellaneous expenses. These increases were in part offset by a $260,000 decrease in and patent, license, and trademark related fees and a $96,000 decrease in outside research services. Overall the increase in research and development expenses is due to increased staffing and costs of clinical trials as part of the development of our cancer diagnostic tests.
We expect to continue to incur a significant amount of research and development expenses.
General and administrative expenses
General and administrative expenses for the year ended December 31, 2015 increased to $4.2 million from $1.0 million for the same period in 2014. The increases in general and administrative expenses during 2015 are primarily attributable to the following increases: $1.2 million of stock based compensation expenses to employees and consultants allocated to general and administrative expense; $435,000 of salaries and payroll related expenses allocated to general and administrative expenses, $377,000 of accounting and audit related expenses; $332,000 of general consulting expenses; $258,000 of legal expenses; $189,000 of investor and public relations related expenses; $91,000 of recruiting expenses; $90,000 of cash and stock-based compensation to our independent directors, and a net increase of $210,000 in other miscellaneous expense. These increases are primarily as a result of increased staffing, including both management and consulting personnel, and costs related to our common stock becoming publicly traded in December 2015.
Income taxes
As of December 31, 2015, we have net operating loss carryforwards of approximately $20.1 million for U.S. federal income tax purposes and $14.8 million for state income tax purposes, which expire between 2030 and 2035. In addition, as of December 31, 2015, OncoCyte has research and development credit carryforwards for federal and state tax purposes of $664,000 and $698,000, respectively. The federal credits expire between 2029 and 2035, while the state credits have no expiration. Due to our losses incurred for all periods presented, we did not record any provision or benefit for income taxes.
During 2015 and 2014, we sold 259,712 and 406,756 BioTime common shares, respectively, in at-the-market transactions which resulted in taxable gains of approximately $815,000 and $1.3 million, respectively. These taxable gains were fully offset by current operating losses, thus resulting in no income taxes due from the sales. At December 31, 2015 and 2014, we recorded deferred tax liabilities of $864,000 and $1.1 million, respectively, resulting from the differences in the tax basis of BioTime shares held by OncoCyte as compared to the basis of such shares reported for financial reporting purposes.
A valuation allowance is provided when it is more likely than not that some portion of the deferred tax assets will not be realized. OncoCyte established a full valuation allowance for all periods presented due to the uncertainty of realizing future tax benefits from its net operating loss carryforwards and other deferred tax assets.
Comparison of the Years Ended December 31, 2014 and 2013
The following tables show our operating expenses for the year ended December 31, 2014 and 2013 (in thousands).
|
|
|
Year Ended
December 31,
|
|
|
|
|
|
|
|
|
|
|
2014
|
|
2013
|
|
$ Increase
|
|
%
Increase
|
|
|
Research and development expenses
|
|
$
|
3,962
|
|
|
$
|
2,943
|
|
|
$
|
+1,019
|
|
|
|
+35
|
%
|
|
|
General and administrative expenses
|
|
$
|
1,011
|
|
|
$
|
552
|
|
|
$
|
+459
|
|
|
|
+83
|
%
|
|
Research and development expenses
The following table shows the amounts and percentages of our total research and development expenses of $4.0 million and $2.9 million allocated to our primary research and development projects during the years ended December 31, 2014 and 2013, respectively (in thousands).
|
|
|
Amount
(1)
|
|
Percent
|
|
|
Program
|
|
2014
|
|
2013
|
|
2014
|
|
2013
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General
|
|
$
|
1,621
|
|
|
$
|
2,852
|
|
|
|
40.9
|
%
|
|
|
96.9
|
%
|
|
|
Bladder Cancer Confirmatory Diagnostic
|
|
$
|
1,143
|
|
|
$
|
27
|
|
|
|
28.9
|
%
|
|
|
0.9
|
%
|
|
|
Breast Cancer Confirmatory Diagnostic
|
|
$
|
1,057
|
|
|
$
|
49
|
|
|
|
26.7
|
%
|
|
|
1.7
|
%
|
|
|
Lung Cancer Confirmatory Diagnostic
|
|
$
|
76
|
|
|
$
|
-
|
|
|
|
1.9
|
%
|
|
|
-
|
%
|
|
|
COLX
|
|
$
|
65
|
|
|
$
|
15
|
|
|
|
1.6
|
%
|
|
|
0.5
|
%
|
|
|
Total
|
|
$
|
3,962
|
|
|
$
|
2,943
|
|
|
|
100
|
%
|
|
|
100
|
%
|
|
|
|
(1)
|
Amount also includes certain general research and development expenses, such as laboratory supplies, laboratory expenses, rent allocated, and insurance allocated to research and development expenses, incurred directly by BioTime on behalf of OncoCyte and allocated to OncoCyte under the Shared Facilities Agreement.
|
Research and development expenses for the year ended December 31, 2014 increased to $4.0 million from $2.9 million for the same period in 2013. The increases in research and development expenses during 2014 were due to increased staffing and costs of clinical trials as part of the development of our cancer diagnostic tests attributable to scientific consulting expenses, stock based compensation to employees and consultants, and clinical trial related expenses.
General and administrative expenses
General and administrative expenses for the year ended December 31, 2014 increased to $1.0 million from $552,000 for the same period in 2013. General and administrative expenses for the year ended December 31, 2014 reflect the following expense increases: $173,000 of salaries and payroll related expenses allocated to general and administrative expenses and $229,000 in salaries and related expenses for accounting services and building maintenance allocated from BioTime based on fixed rates evaluated on a quarterly basis by BioTime management and salaries for other services allocated based on monthly timesheets.
Liquidity and Capital Resources
At December 31, 2015, we had $8.0 million of cash and cash equivalents and held BioTime common shares as available-for-sale securities valued at $2.5 million. We will need to obtain additional debt or equity capital in order to finance our operations. We cannot assure that such financing will be available on favorable terms, if at all. Since inception, we have financed our operations through the sale of our common stock to our shareholders, loans from BioTime and BioTime affiliated entities, and the sale of BioTime common shares. The amount of revenue that may be earned through the licensing and sale of our diagnostic tests and technology, if any revenue is earned at all, the timing of the receipt of diagnostic test sales revenues, license fees, and royalty payments, if any at all, are uncertain.
Based on cash and other liquid assets currently on hand and projected rates of expenditure we believe that we will be able to fund our ongoing operations through December 31, 2016, including costs related to research and development and general and administrative expenses. If results of our research and development efforts are successful to the point where we believe that a commercial product can be launched successfully, then additional capital of up to $5.0 million may be required during 2016 for the building and certification of a diagnostic laboratory and for developing a sales and marketing team and launching our first diagnostic test. Dependent on results of any product commercialization and ongoing sales, additional capital might be required in 2017 or beyond to develop and launch other products, for working capital, and for other expenses.
The unavailability or inadequacy of financing or revenues to meet future capital needs could force us to modify, curtail, delay, or suspend some or all aspects of our planned operations. Sales of additional equity securities could result in the dilution of the interests of our shareholders.
Cash used in operations
During the years ended December 31, 2015 and 2014, our total research and development expenditures were $4.5 million and $4.0 million, respectively, and our general and administrative expenditures were $4.2 million and $1.0 million, respectively. Net loss for the years ended December 31, 2015 and 2014 amounted to $8.7 million and $5.0 million, respectively. Net cash used in operating activities during these periods amounted to $4.2 million and $1.2 million, respectively. The amount by which our net loss exceeded net cash used in our operations during 2015 is primarily due to the following: $1.8 million in stock-based compensation to employees, consultants and independent directors; $1.7 million in amounts owed to BioTime; $1.0 million in accounts payable and accrued expenses; and $242,000 in amortization of intangible assets. This overall difference was offset to some extent by $274,000 in prepaid expenses and other current assets, and $115,000 in amount due from affiliates.
Cash provided by investing activities
During the year ended December 31, 2015 and 2014, we received $815,000 and $1.3 million in proceeds from the sale of BioTime shares that we hold as available for sale securities, offset by $500,000 and $9,000 in cash payments made for purchases of machinery and equipment, respectively.
Cash provided by financing activities
During the year ended December 31, 2015 we received $8.3 million in cash from the sale of 2,710,857 shares of our common stock to BioTime, and $3.3 million in cash from the sale of 1,500,000 shares of our common stock to two other shareholders.
Contractual obligations
We had no contractual obligations as of December 31, 2015, with the exception of a Shared Facilities Agreement with BioTime under which we reimburse BioTime for a portion of the rent and other expenses of leasing our office and laboratory facility in Alameda, California, and for BioTime’s cost of providing us with the use of laboratory and office equipment and supplies, utilities, and personnel. We are presently being allocated from BioTime, 20% of certain general and administrative overhead expenses and 25% of certain general research and development expenses, which include BioTime’s insurance, general office and laboratory supplies, shipping and postage, internet and telephone expenses, certain office equipment repair and maintenance expenses, and base monthly rent and other costs arising under the lease of the laboratory and office facility that we share with BioTime. Our share of the base rent expense incurred by BioTime for the office and laboratory facility during the year ended December 31, 2015 was approximately $13,000 per month. Salaries and related expenses for accounting services and building maintenance are allocated based on a fixed percentage evaluated by BioTime management on a quarterly basis and adjusted based on the level of activity in each quarter. Salaries for any other services are allocated based on monthly timesheets.
Off-Balance Sheet Arrangements
As of December 31, 2015 and 2014, we did not have any off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of SEC Regulation S-K.
|
Item 7A.
|
Quantitative and Qualitative Disclosures about Market Risk
|
Foreign Currency Exchange Risk
We are not presently exposed in a significant degree to foreign exchange currency risks because we are not conducting international business at this time, and we do not engage in foreign currency hedging activities. If we engage in international transactions, we will need to translate foreign currencies into U.S. dollars for reporting purposes, and currency fluctuations could have an impact on our financial results.
Credit Risk
We place some of our cash in U.S. banks and invest most of our cash in money market funds. Deposits with banks may temporarily exceed the amount of insurance provided on such deposits. We will monitor the cash balances in the accounts and adjust the cash balances as appropriate, but if the amount of a deposit at any time exceeds the federally insured amount at a bank, the uninsured portion of the deposit could be lost, in whole or in part, if the bank were to fail. Our investments in money market funds are not insured or guaranteed by the United States government or any of its agencies.
Interest Rate Risk
We invest most of our cash in money market funds. The primary objective of our investments will be to preserve principal and liquidity while earning a return on our invested capital, without incurring significant risks. Our future investment income is not guaranteed and may fall short of expectations due to changes in prevailing interest rates, or we may suffer losses in principal if the net asset value of a money market fund falls below $1 per share.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
OncoCyte Corporation
We have audited the accompanying balance sheets of OncoCyte Corporation ("OncoCyte") as of December 31, 2015 and 2014, and the related statements of operations, comprehensive loss, stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2015. These financial statements are the responsibility of OncoCyte's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. OncoCyte is not required to have, nor were we engaged to perform, an audit
of its internal control over financial reporting.
Our audits included consideration
of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of OncoCyte's internal control over financial reporting. Accordingly we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
As discussed in Note 2 and Note 8 to the financial statements, OncoCyte changed the manner in which it classifies deferred taxes in 2015 due to the adoption of Accounting Standards Update 2015‑17,
Balance Sheet Classification of
Deferred Taxes
.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of OncoCyte Corporation at December 31, 2015 and 2014 and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2015, in conformity with accounting principles generally accepted in the United States of America.
/s/ OUM & CO. LLP
San Francisco, California
March 30, 2016
|
|
Financial Statements and Supplementary Data
|
ONCOCYTE CORPORATION
BALANCE SHEETS
(
In thousands
)
|
|
|
December 31,
|
|
|
|
|
2015
|
|
2014
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
CURRENT ASSETS
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
7,996
|
|
|
$
|
257
|
|
|
|
BioTime shares held as available-for-sale securities, at fair value
|
|
|
2,541
|
|
|
|
3,280
|
|
|
|
Prepaid expenses and other current assets
|
|
|
388
|
|
|
|
114
|
|
|
|
Total current assets
|
|
|
10,925
|
|
|
|
3,651
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NONCURRENT ASSETS
|
|
|
|
|
|
|
|
|
|
|
Intangible assets, net
|
|
|
1,230
|
|
|
|
1,472
|
|
|
|
Equipment and furniture, net
|
|
|
576
|
|
|
|
118
|
|
|
|
TOTAL ASSETS
|
|
$
|
12,731
|
|
|
$
|
5,241
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES
|
|
|
|
|
|
|
|
|
|
|
Amount due to parent, BioTime
|
|
$
|
807
|
|
|
$
|
5,735
|
|
|
|
Amount due to affiliates
|
|
|
40
|
|
|
|
154
|
|
|
|
Accounts payable
|
|
|
285
|
|
|
|
144
|
|
|
|
Accrued expenses and other current liabilities
|
|
|
1,182
|
|
|
|
282
|
|
|
|
Total current liabilities
|
|
|
2,314
|
|
|
|
6,315
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES
|
|
|
2,314
|
|
|
|
6,315
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies (see Note 9)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
|
|
|
Preferred stock, no par value, 5,000 shares authorized; none issued and outstanding
|
|
|
-
|
|
|
|
-
|
|
|
|
Common stock, no par value, 50,000 shares authorized; 25,391 and 18,200 shares issued and outstanding at December 31, 2015 and 2014, respectively
|
|
|
34,901
|
|
|
|
15,147
|
|
|
|
Accumulated other comprehensive loss on available-for-sale securities
|
|
|
(350
|
)
|
|
|
(822
|
)
|
|
|
Accumulated deficit
|
|
|
(24,134
|
)
|
|
|
(15,399
|
)
|
|
|
Total stockholders’ equity (deficit)
|
|
|
10,417
|
|
|
|
(1,074
|
)
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
$
|
12,731
|
|
|
$
|
5,241
|
|
|
The accompanying notes are an integral part of these financial statements.
ONCOCYTE CORPORATION
STATEMENTS OF OPERATIONS
(In thousands, except per share data)
|
|
|
Year Ended
December 31,
|
|
|
|
|
2015
|
|
2014
|
|
|
2013
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
$
|
4,527
|
|
|
$
|
3,962
|
|
|
$
|
2,943
|
|
|
|
General and administrative
|
|
|
4,191
|
|
|
|
1,011
|
|
|
|
552
|
|
|
|
Total operating expenses
|
|
|
8,718
|
|
|
|
4,973
|
|
|
|
3,495
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(8,718
|
)
|
|
|
(4,973
|
)
|
|
|
(3,495
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER EXPENSES, NET
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net
|
|
|
(19
|
)
|
|
|
(2
|
)
|
|
|
-
|
|
|
|
Other expenses, net
|
|
|
2
|
|
|
|
(11
|
)
|
|
|
-
|
|
|
|
Total other expenses, net
|
|
|
(17
|
)
|
|
|
(13
|
)
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET LOSS
|
|
$
|
(8,735
|
)
|
|
$
|
(4,986
|
)
|
|
$
|
(3,495
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per share
|
|
$
|
(0.42
|
)
|
|
$
|
(0.27
|
)
|
|
$
|
(0.19
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding: basic and diluted
|
|
|
21,009
|
|
|
|
18,200
|
|
|
|
18,200
|
|
|
The accompanying notes are an integral part of these financial statements.
ONCOCYTE CORPORATION
STATEMENTS OF COMPREHENSIVE LOSS
(In thousands)
|
|
|
Year Ended
December 31,
|
|
|
|
|
2015
|
|
2014
|
|
|
2013
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET LOSS
|
|
$
|
(8,735
|
)
|
|
$
|
(4,986
|
)
|
|
$
|
(3,495
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive loss, net of tax:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Realized loss on sale of BioTime shares
|
|
|
397
|
|
|
|
569
|
|
|
|
-
|
|
|
|
Unrealized gain (loss) on BioTime shares held as available-for-sale securities
|
|
|
75
|
|
|
|
(21
|
)
|
|
|
592
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COMPREHENSIVE LOSS
|
|
$
|
(8,263
|
)
|
|
$
|
(4,438
|
)
|
|
$
|
(2,903
|
)
|
|
The accompanying notes are an integral part of these financial statements.
ONCOCYTE CORPORATION
STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
(In thousands)
|
|
|
Common Stock
|
|
Accumulated Other
Comprehensive
Loss
|
|
Accumulated
Deficit
|
|
Total
Shareholders’
Equity (Deficit)
|
|
|
Shares
|
|
Amount
|
|
BALANCE AT JANUARY 1, 2013
|
|
|
18,200
|
|
|
$
|
15,072
|
|
|
$
|
(1,962
|
)
|
|
$
|
(6,918
|
)
|
|
$
|
6,192
|
|
|
|
Net Loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(3,495
|
)
|
|
|
(3,495
|
)
|
|
|
Unrealized gain on BioTime shares held as available-for-sale securities
|
|
|
-
|
|
|
|
-
|
|
|
|
592
|
|
|
|
-
|
|
|
|
592
|
|
|
|
Stock-based compensation
|
|
|
-
|
|
|
|
326
|
|
|
|
-
|
|
|
|
-
|
|
|
|
326
|
|
|
|
BALANCE AT DECEMBER 31, 2013
|
|
|
18,200
|
|
|
$
|
15,398
|
|
|
$
|
(1,370
|
)
|
|
$
|
(10,413
|
)
|
|
$
|
3,615
|
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(4,986
|
)
|
|
|
(4,986
|
)
|
|
|
Unrealized gain on BioTime shares held as available-for-sale securities
|
|
|
-
|
|
|
|
-
|
|
|
|
(21
|
)
|
|
|
-
|
|
|
|
(21
|
)
|
|
|
Stock-based compensation
|
|
|
-
|
|
|
|
318
|
|
|
|
-
|
|
|
|
-
|
|
|
|
318
|
|
|
|
Transfer of realized loss into equity from sale of BioTime shares
|
|
|
-
|
|
|
|
(569
|
)
|
|
|
569
|
|
|
|
-
|
|
|
|
-
|
|
|
|
BALANCE AT DECEMBER 31, 2014
|
|
|
18,200
|
|
|
|
15,147
|
|
|
|
(822
|
)
|
|
|
(15,399
|
)
|
|
|
(1,074
|
)
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(8,735
|
)
|
|
|
(8,735
|
)
|
|
|
Unrealized gain on BioTime shares held as available-for-sale securities
|
|
|
-
|
|
|
|
-
|
|
|
|
75
|
|
|
|
-
|
|
|
|
75
|
|
|
|
Stock-based compensation
|
|
|
-
|
|
|
|
1,815
|
|
|
|
-
|
|
|
|
-
|
|
|
|
1,815
|
|
|
|
Common stock issued to BioTime for extinguishment of debt
|
|
|
1,500
|
|
|
|
3,300
|
|
|
|
-
|
|
|
|
-
|
|
|
|
3,300
|
|
|
|
Common stock issued to investors for cash
|
|
|
1,500
|
|
|
|
3,300
|
|
|
|
-
|
|
|
|
-
|
|
|
|
3,300
|
|
|
|
Common stock issued to BioTime upon conversion of BioTime convertible note payable and accrued interest of $18
|
|
|
1,508
|
|
|
|
3,318
|
|
|
|
-
|
|
|
|
-
|
|
|
|
3,318
|
|
|
|
Common stock issued to BioTime for cash
|
|
|
2,711
|
|
|
|
8,349
|
|
|
|
-
|
|
|
|
-
|
|
|
|
8,349
|
|
|
|
Exercise of stock options
|
|
|
3
|
|
|
|
4
|
|
|
|
-
|
|
|
|
-
|
|
|
|
4
|
|
|
|
Fair value of contingently issuable warrant
|
|
|
-
|
|
|
|
65
|
|
|
|
-
|
|
|
|
-
|
|
|
|
65
|
|
|
|
OncoCyte common stock received as a dividend in kind from BioTime
|
|
|
(31
|
)
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
Transfer of realized loss into equity from sale of BioTime shares
|
|
|
-
|
|
|
|
(397
|
)
|
|
|
397
|
|
|
|
-
|
|
|
|
-
|
|
|
|
BALANCE AT DECEMBER 31, 2015
|
|
|
25,391
|
|
|
$
|
34,901
|
|
|
$
|
(350
|
)
|
|
$
|
(24,134
|
)
|
|
$
|
10,417
|
|
|
The accompanying notes are an integral part of these financial statements.
ONCOCYTE CORPORATION
STATEMENTS OF CASH FLOWS
(In thousands)
|
|
|
Year Ended
December 31
|
|
|
|
|
2015
|
|
2014
|
|
|
2013
|
|
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(8,735
|
)
|
|
$
|
(4,986
|
)
|
|
$
|
(3,495
|
)
|
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation expense
|
|
|
41
|
|
|
|
39
|
|
|
|
35
|
|
|
|
Amortization of intangible assets
|
|
|
242
|
|
|
|
242
|
|
|
|
242
|
|
|
|
Stock-based compensation
|
|
|
1,815
|
|
|
|
318
|
|
|
|
326
|
|
|
|
Contingently issuable warrant expense to investors
|
|
|
65
|
|
|
|
-
|
|
|
|
-
|
|
|
|
Interest expense
|
|
|
18
|
|
|
|
-
|
|
|
|
-
|
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amount due to parent, BioTime
|
|
|
1,672
|
|
|
|
2,823
|
|
|
|
2,136
|
|
|
|
Amount due to affiliates
|
|
|
(115
|
)
|
|
|
103
|
|
|
|
72
|
|
|
|
Prepaid expenses and other current assets
|
|
|
(274
|
)
|
|
|
6
|
|
|
|
(74
|
)
|
|
|
Accounts payable and accrued liabilities
|
|
|
1,042
|
|
|
|
291
|
|
|
|
48
|
|
|
|
Net cash used in operating activities
|
|
|
(4,229
|
)
|
|
|
(1,164
|
)
|
|
|
(710
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase of equipment
|
|
|
(500
|
)
|
|
|
(9
|
)
|
|
|
(25
|
)
|
|
|
Proceeds from sale of BioTime shares
|
|
|
815
|
|
|
|
1,329
|
|
|
|
-
|
|
|
|
Net cash provided by (used in) investing activities
|
|
|
315
|
|
|
|
1,320
|
|
|
|
(25
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from exercise of options
|
|
|
4
|
|
|
|
-
|
|
|
|
-
|
|
|
|
Proceeds from sale of common shares
|
|
|
11,649
|
|
|
|
-
|
|
|
|
-
|
|
|
|
Net cash provided by financing activities
|
|
|
11,653
|
|
|
|
-
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS
|
|
|
7,739
|
|
|
|
156
|
|
|
|
(735
|
)
|
|
|
CASH AND CASH EQUIVALENTS:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
At beginning of the period
|
|
|
257
|
|
|
|
101
|
|
|
|
836
|
|
|
|
At end of the period
|
|
$
|
7,996
|
|
|
$
|
257
|
|
|
$
|
101
|
|
|
|
SUPPLEMENTAL SCHEDULE OF NONCASH FINANCING AND INVESTING ACTIVITIES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued to BioTime for extinguishment of debt
|
|
$
|
3,300
|
|
|
$
|
-
|
|
|
$
|
-
|
|
|
|
Common stock issued to BioTime upon conversion of convertible note payable and accrued interest
|
|
|
3,318
|
|
|
|
-
|
|
|
|
-
|
|
|
|
Realized loss on sale of BioTime shares
|
|
|
397
|
|
|
|
569
|
|
|
|
-
|
|
|
The accompanying notes are an integral part of these financial statements.
ONCOCYTE CORPORATION
NOTES TO FINANCIAL STATEMENTS
1. Organization, Description of the Business and Liquidity
OncoCyte Corporation (“OncoCyte”) was incorporated in 2009 in the state of California and is a majority-owned subsidiary of BioTime, Inc. (“BioTime”), a publicly traded biotechnology company focused in the field of regenerative medicine. OncoCyte is developing molecular cancer diagnostics utilizing a discovery platform that focuses on identifying genetic markers broadly expressed in numerous types of cancer. OncoCyte is presently focusing its efforts on developing diagnostic tests for use in detecting a variety of cancers including lung, bladder, and breast cancers.
Liquidity
For all periods presented, OncoCyte had generated no revenues. Since inception, OncoCyte has financed its operations through the sale of its common stock to its shareholders, including BioTime, loans from BioTime and other BioTime affiliates, and sales of BioTime common shares that OncoCyte held as available-for-sale securities (see Note 2). OncoCyte has incurred operating losses and negative cash flows since inception, and had an accumulated deficit of $24.1 million and $15.4 million as December 31, 2015 and 2014, respectively.
OncoCyte plans to continue to invest significant resources in research and development in the field of molecular cancer diagnostics. OncoCyte expects to continue to incur operating losses and negative cash flows. If results of OncoCyte’s research and development efforts are successful to the point where it believes that a commercial product can be launched successfully, then additional capital of up to $5.0 million may be required during 2016 for the building and certification of a diagnostic laboratory and for developing a sales and marketing team and launching OncoCyte’s first diagnostic test. Dependent on results of any product commercialization and ongoing sales, additional capital might be required in 2017 or beyond to develop and launch other products, for working capital, and for other expenses. The unavailability or inadequacy of financing or revenues to meet future capital needs could force OncoCyte to modify, curtail, delay, or suspend some or all aspects of its planned operations. Sales of additional equity securities could result in the dilution of the interests of its shareholders. OncoCyte will need to obtain additional debt or equity capital in order to finance its operations. OncoCyte cannot assure that such financing will be available on favorable terms, if at all.
As of December 31, 2015, OncoCyte had $8.0 million in cash and cash equivalents and held BioTime shares available-for-sale, valued at $2.5 million, which OncoCyte may use for working capital purposes, as necessary. Based on cash and available for sale securities currently on hand and projected rates of expenditure, OncoCyte believes that it will be able to fund ongoing operations through at least December 31, 2016.
2. Summary of Significant Accounting Policies
Basis of presentation
The financial statements presented herein have been prepared on a separate, stand-alone basis. The financial statements are presented in accordance with U.S. generally accepted accounting principles (“GAAP”). BioTime has consolidated the results of OncoCyte into BioTime’s consolidated results based on BioTime’s ability to control OncoCyte’s operating and financial decisions and policies through its majority ownership of OncoCyte common stock throughout the periods presented. BioTime owned 57.7% and 75.3% of the outstanding common stock of OncoCyte at December 31, 2015 and 2014, respectively.
To the extent OncoCyte does not have its own employees or human resources for its operations, BioTime or BioTime subsidiaries provide certain employees for administrative or operational services, as necessary, for the benefit of OncoCyte (see Note 4). Accordingly, BioTime allocates expenses such as salaries and payroll related expenses incurred and paid on behalf of OncoCyte based on the amount of time that particular employees devote to OncoCyte affairs. Other expenses such as legal, accounting, marketing, travel, and entertainment expenses are allocated to OncoCyte to the extent that those expenses are incurred by or on behalf of OncoCyte. BioTime also allocates certain overhead expenses such as insurance, internet and telephone expenses based on a percentage determined by management. These allocations are made based upon activity-based allocation drivers such as time spent, percentage of square feet of office or laboratory space used, and percentage of personnel devoted to OncoCyte’s operations or management. Management evaluates the appropriateness of the percentage allocations on a quarterly basis and believes that this basis for allocation is reasonable.
As further discussed in Notes 4 and 7, OncoCyte grants stock options to employees of BioTime, or employees of other BioTime subsidiaries who perform services for OncoCyte, and OncoCyte recorded stock-based compensation expense in the accompanying statements of operations for these services performed in the periods presented.
Reverse stock split
On November 18, 2015 OncoCyte effected a one-for-two reverse stock split of its common stock.
All share, per-share and related information including the price at which shares of common stock have been sold or may be issued, including shares issuable upon the exercise of stock options or convertible debt, have been retroactively adjusted, in these financial statements and accompanying footnotes, where applicable, to reflect the impact of the reverse stock split.
Use of estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, management evaluates estimates which are subject to significant judgment, including those related to the allocation of direct and indirect expenses, useful lives associated with long-lived intangible assets, equipment and furniture, loss contingencies, valuation allowances related to deferred income taxes, and assumptions used to value stock-based awards, debt or other equity instruments. Actual results could differ materially from those estimates.
Fair value measurements
OncoCyte accounts for fair value measurements in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 820,
Fair Value Measurements
(“ASC 820”). ASC 820 establishes a single authoritative definition of fair value, sets out a framework for measuring fair value and expands on required disclosures about fair value measurement. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. ASC 820 describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value, which are the following:
|
|
·
|
Level 1
– Quoted prices in active markets for identical assets and liabilities.
|
|
|
·
|
Level 2
– Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted market prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
|
|
|
·
|
Level 3
– Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
|
In determining fair value, OncoCyte utilizes valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent possible, and also considers counterparty credit risk in its assessment of fair value. For the periods presented, OncoCyte has no financial assets or liabilities recorded at fair value on a recurring basis, except for cash and cash equivalents consisting of money market funds and the available-for-sale securities of BioTime common stock held by OncoCyte described below. These assets are measured at fair value using the period-end quoted market prices as a Level 1 input.
The carrying amounts of cash equivalents, prepaid expenses and other current assets, amounts due to BioTime and other affiliates, accounts payable, accrued expenses and other current liabilities approximate fair values because of the short-term nature of these items.
Cash and cash equivalents
Cash equivalents typically consist of highly liquid investments, with maturities of three months or less when purchased. At December 31, 2015 and 2014, OncoCyte's cash balances totaled $8.0 million and $257,000, respectively, and consist of bank account deposits, including $7.0 million held in money market funds at December 31, 2015.
Financial instruments that potentially subject OncoCyte to credit risk consist principally of cash and cash equivalents. OncoCyte, at times, maintains cash and cash equivalent balances at financial institutions in excess of amounts insured by United States government agencies. OncoCyte places its cash and cash equivalents with high credit quality financial institutions.
Accounting for BioTime shares
OncoCyte accounts for the BioTime shares it holds as available-for-sale equity securities in accordance with ASC 320-10-25,
Investments – Debt and Equity Securities
, as the shares have a readily determinable fair value quoted on the NYSE MKT and are held principally for sale to meet future working capital needs. These shares are measured at fair value and reported as current assets on the balance sheet based on the closing trading price of the security as of the date being presented. Unrealized holding gains and losses are excluded from the statements of operations and reported in equity as part of other comprehensive income or loss, net of income taxes, until realized. Realized gains and losses for shares sold are reclassified out of accumulated other comprehensive income or loss and included in equity, as an increase or decrease to equity in common stock consistent with, and pursuant to, ASC 805-50, transactions between entities under common control.
In 2015, OncoCyte sold 259,712 shares of BioTime common stock it held in at-the-market transactions for $815,000 in cash proceeds to be used for working capital purposes. The sale resulted in a $397,000 realized loss, which is recorded as a decrease to common stock equity on the dates of sale.
As of December 31, 2015, OncoCyte held 619,706 BioTime common shares as available-for-sale securities with a fair market value of $2.5 million.
Long-lived intangible assets
Long-lived intangible assets, primarily consisting of acquired patents, patent applications, and licenses to use certain patents are stated at acquired cost, less accumulated amortization (see Note 3). Amortization expense is computed using the straight-line method over the estimated useful lives of the assets over a period of 10 years.
Equipment and furniture
Equipment and furniture are stated at cost, less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, generally over a period of 36 to 120 months. Maintenance and repairs are expensed as incurred whereas significant renewals and betterments are capitalized. When assets are retired or otherwise disposed of, the cost and the related accumulated depreciation are removed from the respective accounts and any resulting gain or loss is reflected in OncoCyte’s results of operations.
Impairment of long-lived assets
OncoCyte assesses the impairment of long-lived assets, which consist primarily of long-lived intangible assets, furniture and equipment, whenever events or changes in circumstances indicate that such assets might be impaired and the carrying value may not be recoverable. If events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable and the expected undiscounted future cash flows attributable to the asset are less than the carrying amount of the asset, an impairment loss equal to the excess of the asset’s carrying value over its fair value is recorded. Through 2015, there have been no such impairment losses.
Income taxes
OncoCyte has filed a standalone U.S. federal income tax return since its inception. For California purposes, OncoCyte’s activity for 2014 and 2015 has been included in BioTime’s California Combined tax return. The provision for income taxes has been determined as if OncoCyte had filed separate tax returns for the periods presented. Accordingly, the effective tax rate of OncoCyte in future years could vary from its historical effective tax rates depending on the future legal structure of OncoCyte and related tax elections. The historical deferred tax assets, including the operating losses and credit carryforwards generated by OncoCyte, will remain with OncoCyte. OncoCyte accounts for income taxes in accordance with ASC 740,
Income Taxes
, which prescribes the use of the asset and liability method, whereby deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect. Valuation allowances are established when necessary to reduce deferred tax assets when it is more likely than not that a portion or all of the deferred tax assets will not be realized. OncoCyte’s judgments regarding future taxable income may change over time due to changes in market conditions, changes in tax laws, tax planning strategies or other factors. If OncoCyte’s assumptions and consequently its estimates change in the future, the valuation allowance may be increased or decreased, which may have a material impact on OncoCyte’s statements of operations.
The guidance also prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not sustainable upon examination by taxing authorities. OncoCyte will recognize accrued interest and penalties related to unrecognized tax benefits as income tax expense. No amounts were accrued for the payment of interest and penalties as of December 31, 2015 and 2014. OncoCyte is not aware of any uncertain tax positions that could result in significant additional payments, accruals, or other material deviation for the years ended December 31, 2015 and 2014. OncoCyte is currently unaware of any tax issues under review.
Research and development expenses
Research and development expenses consist primarily of personnel costs and related benefits, including stock-based compensation, and expenses for outside consultants. These expenses include both direct and allocated or indirect overhead costs allocated by BioTime (see Note 4). Research and development costs are expensed as incurred.
General and administrative expenses
OncoCyte’s general and administrative expenses relate primarily to compensation and related benefits, including stock-based compensation, for executive and corporate personnel, including direct costs and costs allocated by BioTime; professional and consulting fees; direct overhead and indirect overhead allocated by BioTime (see Note 4).
Stock-based compensation
OncoCyte recognizes compensation expense related to employee option grants and restricted stock grants, if any, in accordance with FASB ASC 718,
Compensation – Stock Compensation
(“ASC 718”).
OncoCyte estimates the fair value of employee stock-based payment awards on the grant-date and recognizes the resulting fair value, net of estimated forfeitures, over the requisite service period. OncoCyte uses the Black-Scholes option pricing model for estimating the fair value of options granted under OncoCyte’s Stock Option Plan. The fair value of each restricted stock grant, if any, is determined based on the value of the common stock granted or sold. OncoCyte has elected to treat stock-based payment awards with graded vesting schedules and time-based service conditions as a single award and recognizes stock-based compensation on a straight-line basis, net of estimated forfeitures, over the requisite service period.
Compensation expense for non-employee stock-based awards is recognized in accordance with ASC 718 and FASB ASC 505-50,
Equity-Based Payments to Non-Employees
. Stock option awards issued to non-employees, of principally of consultants and employees of BioTime or employees of BioTime subsidiaries who perform services for OncoCyte, are accounted for at fair value using the Black-Scholes option pricing model. Management believes that the fair value of the stock options is more reliably measured than the fair value of services received. OncoCyte records compensation expense based on the then-current fair values of the stock options at each financial reporting date. Compensation recorded during the service period is adjusted in subsequent periods for changes in the fair value of the stock options until the earlier of the date at which the non-employee’s performance is complete or a performance commitment is reached, which is generally when the stock option award vests. Compensation expense for non-employee grants is recorded on a straight-line basis in the statements of operations.
The Black-Scholes option pricing model requires OncoCyte to make certain assumptions including the fair value of the underlying common stock, the expected term, the expected volatility, the risk-free interest rate and the dividend yield (see Note 7).
The fair value of the shares of common stock underlying the stock options has historically been determined by the Board of Directors. Because there was no public market for OncoCyte’s common stock prior to December 31, 2015, the Board of Directors determined the fair value of the common stock at the time of the grant of options by considering a number of objective and subjective factors including contemporaneous sales of common stock to investors, valuation of comparable companies, operating and financial performance and general and industry-specific economic outlook, among other factors in accordance with applicable elements of the practice aid issued by the American Institute of Certified Public Accountants titled
Valuation of Privately Held Company Equity Securities Issued As Compensation
.
The expected term of employee stock options represents the weighted-average period that the stock options are expected to remain outstanding. OncoCyte estimates the expected term of options granted based upon the “simplified method” provided under
Staff Accounting Bulletin, Topic 14
, or SAB Topic 14.
Because OncoCyte’s common stock had no public trading history prior to December 31, 2015, for the years ended December 31, 2015 and 2014 OncoCyte estimated the expected volatility of the awards from the historical volatility of selected public companies within the biotechnology industry with comparable characteristics to OncoCyte, including similarity in size, lines of business, market capitalization, revenue and financial leverage. OncoCyte determined the expected volatility assumption using the frequency of daily historical prices of comparable public company’s common stock for a period equal to the expected term of the options.
The risk-free interest rate assumption is based upon observed interest rates on the United States government securities appropriate for the expected term of OncoCyte’s stock options.
The dividend yield assumption is based on OncoCyte’s history and expectation of dividend payouts. OncoCyte has never declared or paid any cash dividends on its common stock, and OncoCyte does not anticipate paying any cash dividends in the foreseeable future.
Net loss per common share
Basic net loss per common share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding for the period. Diluted net loss per share reflects the weighted-average number of shares of common stock outstanding plus the potential effect of dilutive securities or contracts which are convertible to common stock, such as stock options (using the treasury stock method) and shares issuable in future periods, except in cases where the effect would be anti-dilutive. Because OncoCyte reported net losses for all periods presented, all potentially dilutive common stock are antidilutive for those periods.
The computations of basic and diluted net loss per common share for the years ended December 31, 2015, 2014 and 2013 are as follows (in thousands, except per share amounts):
|
|
|
2015
|
|
2014
|
|
2013
|
|
|
Net loss
|
|
$
|
(8,735
|
)
|
|
$
|
(4,986
|
)
|
|
$
|
(3,495
|
)
|
|
|
Weighted average common shares outstanding – basic and diluted
|
|
|
21,009
|
|
|
|
18,200
|
|
|
|
18,200
|
)
|
|
|
Net loss per common share – basic and diluted
|
|
$
|
(0.42
|
)
|
|
$
|
(0.27
|
)
|
|
$
|
(0.19
|
)
|
|
The following common stock equivalents were excluded from the computation of diluted net loss per common share of common stock for the years ended December 31, 2015, 2014 and 2013 because including them would have been antidilutive (in thousands):
|
|
|
2015
|
|
2014
|
|
2013
|
|
|
Stock options under Stock Option Plan
|
|
|
2,240
|
|
|
|
1,361
|
|
|
|
1,375
|
|
|
Segments
OncoCyte’s executive management team, as a group, represents the entity’s chief operating decision makers. To date, OncoCyte’s executive management team has viewed OncoCyte’s operations as one segment that includes, the research and development of diagnostic tests for the detection of cancer. As a result, the financial information disclosed materially represents all of the financial information related to OncoCyte’s sole operating segment.
Recent accounting pronouncements
The following accounting standards, which are not yet effective, are presently being evaluated by OncoCyte to determine the impact that they might have on its financial statements.
In February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2016-02, “Leases (Topic 842)”, which requires lessees to recognize assets and liabilities for leases with lease terms greater than twelve months in the statement of financial position. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement. ASU 2016-02 also requires improved disclosures to help users of financial statements better understand the amount, timing and uncertainty of cash flows arising from leases. The update is effective for fiscal years beginning after December 15, 2018, including interim reporting periods. Early adoption is permitted. OncoCyte is currently evaluating the impact the adoption of ASU 2016-02 will have on its financial statements.
In November 2015, the FASB issued ASU 2015-17,
Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes
, which changes how deferred taxes are classified on a company’s balance sheet. The ASU eliminates the current requirement to present deferred tax liabilities and assets as current and noncurrent on the balance sheet. Instead, companies will be required to classify all deferred tax assets and liabilities as noncurrent. The amendments are effective for annual financial statements beginning after December 15, 2016, and interim periods within those annual periods. Early adoption is permitted for any interim and annual financial statements that have not yet been issued. As further discussed in Note 8, OncoCyte early adopted ASU 2015-17 as of December 31, 2015, on a retrospective basis.
In May 2014, the FASB issued ASU 2014-09,
Revenue from Contracts with Customers (Topic 606)
, which supersedes nearly all existing revenue recognition guidance under GAAP. The core principle of ASU 2014-09 is to recognize revenues when promised goods or services are transferred to customers in an amount that reflects the consideration to which an entity expects to be entitled for those goods or services. ASU 2014-09 defines a five-step process to achieve this core principle and, in doing so, more judgment and estimates may be required within the revenue recognition process than are required under existing GAAP.
The revised revenue standard is effective for public entities for annual periods beginning after December 15, 2017, and interim periods therein, using either of the following transition methods: (i) a full retrospective approach reflecting the application of the standard in each prior reporting period with the option to elect certain practical expedients, or (ii) a retrospective approach with the cumulative effect of initially adopting ASU 2014-09 recognized at the date of adoption (which includes additional footnote disclosures). OncoCyte is currently evaluating the impact of OncoCyte’s pending adoption of ASU 2014-09 on its financial statements and has not yet determined the method by which it will adopt the standard in fiscal 2018.
In August 2014, the FASB issued ASU No. 2014-15,
Presentation of Financial Statements – Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern
requiring management to evaluate on a regular basis whether any conditions or events have arisen that could raise substantial doubt about the entity’s ability to continue as a going concern. The guidance 1) provides a definition for the term “substantial doubt,” 2) requires an evaluation every reporting period, interim periods included, 3) provides principles for considering the mitigating effect of management’s plans to alleviate the substantial doubt, 4) requires certain disclosures if the substantial doubt is alleviated as a result of management’s plans, 5) requires an express statement, as well as other disclosures, if the substantial doubt is not alleviated, and 6) requires an assessment period of one year from the date the financial statements are issued. This standard is effective for the annual period ending after December 15, 2016, and for annual periods and interim periods thereafter. Early application is permitted. OncoCyte is currently evaluating the impact, if any, the adoption of ASU 2014-15 will have on its financial statements.
3. Selected Balance Sheet Components
Prepaid expenses and other current assets
At December 31, 2015 and 2014, prepaid expenses and other current assets were comprised of the following (in thousands):
|
|
|
2015
|
|
2014
|
|
|
Prepaid license fees
|
|
$
|
19
|
|
|
$
|
111
|
|
|
|
Outside research
|
|
|
366
|
|
|
|
-
|
|
|
|
Other prepaid expenses and current asset
|
|
|
3
|
|
|
|
3
|
|
|
|
Prepaid expenses and other current assets
|
|
$
|
388
|
|
|
$
|
114
|
|
|
Accrued expenses and other current liabilities
At December 31, 2015 and 2014, accrued expenses and other current liabilities were comprised of the following (in thousands):
|
|
|
2015
|
|
2014
|
|
|
Accrued bonuses and payroll related expenses
|
|
$
|
325
|
|
|
$
|
147
|
|
|
|
Other accrued expenses
|
|
|
857
|
|
|
|
135
|
|
|
|
Accrued expenses and other current liabilities
|
|
$
|
1,182
|
|
|
$
|
282
|
|
|
Intangible assets, net
In 2011, OncoCyte, through its parent, BioTime, acquired substantially all of the assets of Cell Targeting, Inc. (“CTI”), a company that was engaged in cancer therapy. The assets acquired consist primarily of patents, patent applications, and licenses to use certain patents. OncoCyte issued 261,959 shares of BioTime common stock to CTI with a market value of $2.3 million and paid CTI $250,000 in cash to acquire the assets. The asset purchase was accounted for as a business combination under the acquisition method of accounting. OncoCyte amortizes intangible assets over their useful lives estimated to be 10 year at the date of the acquisition.
At December 31, 2015 and 2014, intangible assets were comprised of the following (in thousands):
|
|
|
2015
|
|
2014
|
|
|
Intangible assets
|
|
$
|
$2,419
|
|
|
$
|
$2,419
|
|
|
|
Accumulated amortization
|
|
|
(1,189
|
)
|
|
|
(947
|
)
|
|
|
Intangible assets, net
|
|
$
|
$1,230
|
|
|
$
|
$1,472
|
|
|
Amortization expense amounted to approximately $242,000 annually.
Equipment and furniture, net
At December 31, 2015 and 2014, equipment and furniture were comprised of the following (in thousands):
|
|
|
2015
|
|
2014
|
|
|
Equipment and furniture
|
|
$
|
750
|
|
|
$
|
251
|
|
|
|
Accumulated depreciation
|
|
|
(174
|
)
|
|
|
(133
|
)
|
|
|
Equipment and furniture, net
|
|
$
|
576
|
|
|
$
|
118
|
|
|
Depreciation expense amounted to approximately $41,000, $39,000 and $35,000 for the years ended December 31, 2015, 2014 and 2013, respectively.
4. Related Party Transactions
Shared Facilities and Service Agreement
On October 8, 2009, OncoCyte and BioTime executed a Shared Facilities and Services Agreement (“Shared Facilities Agreement”). Under the terms of the Shared Facilities Agreement, BioTime will allow OncoCyte to use its premises and equipment located at Alameda, California for the sole purpose of conducting business. BioTime will also provide accounting, billing, bookkeeping, payroll, treasury, payment of accounts payable, and other similar administrative services to OncoCyte. BioTime may also provide the services of attorneys, accountants, and other professionals who may also provide professional services to BioTime and its other subsidiaries. BioTime will also provide OncoCyte with the services of its laboratory and research personnel, including BioTime employees and contractors, for the performance of research and development work for OncoCyte at the premises.
BioTime charges OncoCyte a Use Fee for services received and usage of facilities, equipment, and supplies. For each billing period, BioTime prorates and allocates costs incurred, as applicable, to OncoCyte, such costs include services of Bio Time employees, equipment, insurance, lease, professional, software, supplies and utilities. Allocation depends on key cost drivers including actual documented use, square footage of facilities used, time spent, costs incurred by or for OncoCyte, or upon proportionate usage by BioTime and OncoCyte, as reasonably estimated by BioTime (collectively “Use Fees”). BioTime, at its discretion, has the right to charge OncoCyte a 5% markup on such allocated costs although BioTime has not elected to charge this markup since the inception of the Shared Facilities Agreement. The allocated cost of BioTime employees and contractors who provide services is based upon records maintained of the number of hours of such personnel devoted to the performance of services.
The Use Fee is determined and invoiced to OncoCyte on a quarterly basis for each calendar quarter of each calendar year. If the Shared Facilities Agreement terminates prior to the last day of a billing period, the Use Fee will be determined for the number of days in the billing period elapsed prior to the termination of the Shared Facilities Agreement. Each invoice will be payable in full by OncoCyte within 30 days after receipt. Any invoice, or portion thereof, not paid in full when due will bear interest at the rate of 15% per annum until paid, unless the failure to make a payment is due to any inaction or delay in making a payment by BioTime employees from OncoCyte funds available for such purpose, rather than from the unavailability of sufficient funds legally available for payment or from an act, omission, or delay by any employee or agent of OncoCyte. Through December 31, 2015 BioTime has not charged OncoCyte any interest.
In addition to the Use Fees, OncoCyte will reimburse BioTime for any out of pocket costs incurred by BioTime for the purchase of office supplies, laboratory supplies, and other goods and materials and services for the account or use of OncoCyte, provided that invoices documenting such costs are delivered to OncoCyte with each invoice for the Use Fee. Furthermore, BioTime will have no obligation to purchase or acquire any office supplies or other goods and materials or any services for OncoCyte, and if any such supplies, goods, materials or services are obtained for OncoCyte, BioTime may arrange for the suppliers thereof to invoice OncoCyte directly.
The Shared Facilities Agreement will remain in effect, unless either party gives the other party written notice stating that the Shared Facilities Agreement will terminate on December 31 of that year, or unless the agreement otherwise terminated under another provision of the agreement.
In aggregate, BioTime allocated and charged such Use Fees to OncoCyte approximating $595,000, $344,000 and $93,000 included in general and administrative expenses, and $565,000, $552,000 and $194,000 included in research and development expenses included in the statements of operations during the years ended December 31, 2015, 2014 and 2013, respectively.
As of December 31, 2015 and 2014, OncoCyte had $847,000 and $5.9 million outstanding and payable to BioTime and affiliates included in current liabilities in connection with the costs incurred under the Shared Facilities Agreement. Since these amounts are due and payable in 30 days of being invoiced, the payables are classified as current liabilities for all periods presented.
5. Related Party Convertible Promissory Note Payable
In May 2015, OncoCyte entered into Subscription Agreements with BioTime and two other shareholders (see Note 6). In connection with the Subscription Agreements, BioTime purchased 1,500,000 shares of OncoCyte common stock in exchange for the cancellation of $3.3 million of indebtedness owed to BioTime by OncoCyte, and OncoCyte delivered to BioTime a convertible promissory note (the “Note”) for an additional $3.3 million of OncoCyte’s indebtedness to BioTime. The cancellation of the aggregate $6.6 million of indebtedness owed to BioTime was an extinguishment of debt under the provisions of ASC 470-50,
Debt Modification and Extinguishment
. Based on a valuation performed by OncoCyte, the issuance date fair value of the Note was $3.3 million (see Note 2) and, the fair value of the OncoCyte common stock on the date of the exchange was $3.3 million. Accordingly, no gain or loss resulted from the debt extinguishment.
The Note had a stated interest rate of 1% per annum. Interest on the Note was due and payable on January 2 of each year until the maturity date and any accrued but unpaid interest, along with principal, is due on the maturity date. The Note was due and payable on November 30, 2016, if not converted sooner, and could be prepaid sooner only with the written consent of BioTime.
The Note was convertible into OncoCyte common stock at a conversion price of $2.20 per share. The conversion price was subject to pro rata adjustment in the event of a stock split, combination, reclassification, or similar event. BioTime, at its election, could convert the Note into common stock on or after the first to occur of (i) November 8, 2016, (ii) the date that is six months after the first closing date on which OncoCyte completes a firm commitment underwritten initial public offering of its common stock under the Securities Act of 1933, as amended, and (iii) the occurrence of an Event of Default, as defined in the Note. An Event of Default included OncoCyte’s failure to pay any amount due on the Note or the commencement of bankruptcy proceedings by or against OncoCyte or the occurrence of certain insolvency related events, OncoCyte’s corporate dissolution or liquidation, or any material breach or default by OncoCyte under any loan agreement, promissory note, or other instrument evidencing indebtedness payable to a third party.
On November 19, 2015, the Note was amended to permit BioTime to convert the Note and accrued interest into shares of OncoCyte common stock at any time prior to payment in full of the principal balance and accrued interest, and on November 20, 2015 BioTime converted the Note into 1,508,095 shares of OncoCyte common stock. The amendment to the Note was determined to be a debt conversion pursuant to ASC 470-20-40-5,
Debt with Conversion and Other Options
, and upon conversion the note balance and accrued interest was transferred to equity.
6. Shareholders’ Equity
Preferred Stock
OncoCyte is authorized to issue up to 5,000,000 shares of no par value preferred stock. As of December 31, 2015, no preferred shares were issued or outstanding.
Common Stock
OncoCyte has up to 50,000,000 shares of no par value common stock authorized. The holders of OncoCyte’s common stock are entitled to receive ratably dividends when, as, and if declared by the Board of Directors out of funds legally available. Upon liquidation, dissolution, or winding up, the holders of OncoCyte common stock are entitled to receive ratably the net assets available after the payment of all debts and other liabilities and subject to the prior rights of OncoCyte outstanding preferred shares, if any.
The holders of common stock are entitled to one vote for each share held on all matters submitted to a vote of OncoCyte stockholders. The holders of common stock have no preemptive, subscription, or redemption rights. The outstanding shares of common stock are fully paid and non-assessable.
In May 2015, OncoCyte entered into Subscription Agreements with Bio Time and two other shareholders (“Investors”). Under the Subscription Agreements, OncoCyte issued 1,500,000 shares of common stock to the Investors for $3.3 million cash, or $2.20 per share. Concurrently, BioTime purchased 1,500,000 shares of OncoCyte common stock in exchange for the cancellation of $3.3 million of indebtedness owed to BioTime by OncoCyte (see Note 5).
During September 2015, OncoCyte entered into a Subscription Agreement with BioTime pursuant to which BioTime purchased 2,710,857 shares of OncoCyte common stock for $8.3 million in cash, as part of a subscription offer made to all OncoCyte shareholders on a pro rata basis. The remaining shareholders had the right to purchase a total of 974,447 shares of OncoCyte common stock at the same price paid by BioTime, until November 16, 2015, when the right expired unexercised.
On December 31, 2015, in connection with BioTime’s distribution of OncoCyte common stock to BioTime shareholders, on a pro rata basis, OncoCyte received 30,985 shares of its own common stock from BioTime as a dividend in kind.
On that date, BioTime shareholders, including OncoCyte, received one share of OncoCyte common stock for every twenty shares of BioTime common stock held (see Note 2). The OncoCyte common stock distributed to OncoCyte was immediately retired on that date and reverted to the status of authorized but unissued common stock.
Contingently Issuable Common Stock Warrants
In June 2015, after the sale of stock under the Subscription Agreements described above was completed, OncoCyte and the Investors entered into a second agreement. Under the second agreement, the Investors agreed that if on or before June 30, 2016 OncoCyte conducts another rights offering to its shareholders at a pre-offer valuation of at least $40.0 million, the Investors will purchase shares in that offering with an aggregate purchase price equal to the lesser of (a) a percentage of total amount of capital which OncoCyte then seeks to raise in the rights offer and in any concurrent offering to third parties equal to the Investors’ aggregate pro rata share of the outstanding OncoCyte common stock on the record date for the rights offering, determined on a fully diluted basis, and (b) $3.0 million, or such lesser amount requested by OncoCyte.
The Investors also agreed that, for a period of one year from the date of the second agreement, neither of them shall invest or engage, directly or indirectly, whether as a partner, equity holder, lender, principal, agent, affiliate, consultant or otherwise, in any business anywhere in the world that develops products for the diagnosis and treatment of cancer or otherwise competes with OncoCyte in any way; provided, however, that the passive ownership of less than 5% of the outstanding stock of any publicly-traded corporation will not be deemed, solely by reason thereof, to be in violation of that agreement.
Under the second agreement, OncoCyte agreed that if shares of OncoCyte common stock were not publicly traded on any stock exchange or over the counter market by January 15, 2016, OncoCyte would issue to the Investors warrants to purchase, in the aggregate, 3,000,000 shares of OncoCyte common stock at an exercise price of $0.01 per share. For accounting purposes, the contingently issuable warrants, under the second agreement described above, were considered issued in June 2015 and classified as equity. OncoCyte estimated the issue date fair value of the warrants using a Black-Scholes option pricing model and management estimated that there was a low probability of not satisfying the contingency and having to issue the warrants. Accordingly, the probability-adjusted, fair value of the warrants was $65,400 on the issuance date and recognized as a general and administrative expense, with a corresponding increase to common stock equity. OncoCyte common stock began trading on the NYSE MKT on a “when issued” basis on December 30, 2015 in connection with BioTime’s distribution of a portion of its OncoCyte shares to BioTime shareholders, extinguishing the contingent obligation to issue the warrants.
7. Stock-based Compensation
Stock Option Plan
OncoCyte has adopted a 2010 Stock Option Plan (the “Plan”) under which OncoCyte initially authorized 2,000,000 shares of common stock for the grant of stock options or the sale of restricted stock. The Plan was amended to increase the authorized shares available for grant by 2,000,000 in 2015. The Plan also permits OncoCyte to issue such other securities as its Board of Directors or the Compensation Committee administering the Plan may determine.
No options may be granted under the Plan more than ten years after the date upon which the Plan was adopted by the Board of Directors, and no options granted under the Plan may be exercised after the expiration of ten years from the date of grant. Under the Plan, options to purchase common stock may be granted to employees, directors and certain consultants at exercise prices not less than the fair market value of common stock at date of grant, subject to certain limited exceptions for options granted in substitution of other options. Options may be fully exercisable immediately, or may be exercisable according to a schedule or conditions specified by the Board of Directors or the Compensation Committee. Generally, OncoCyte stock options have service related vesting conditions based on the continued performance of services for OncoCyte. The Plan also permits OncoCyte to award restricted stock for services rendered or to sell common stock to employees subject to vesting provisions under restricted stock agreements that provide for forfeiture of unvested shares upon the occurrence of specified events. OncoCyte may permit employees or consultants, but not officers or directors, who purchase stock under restricted stock purchase agreements, to pay for their shares by delivering a promissory note that is secured by a pledge of their shares. To date, only stock options have been issued under the Plan.
As discussed in Note 4, in connection with the services performed by employees of BioTime, or employees of other BioTime subsidiaries, OncoCyte grants stock options to those employees performing services for OncoCyte and records stock-based compensation expense in the accompanying statements of operations for these services performed in the periods presented.
Stock Options
Options granted under the Plan may be either “incentive stock options” within the meaning of Section 422(b) of the Internal Revenue Code of 1986, as amended (the “Code”), or non-qualified stock options. Incentive stock options may be granted only to OncoCyte employees and employees of its subsidiaries, if any. The exercise price of stock options granted under the Plan must be equal to the fair market value of OncoCyte common stock on the date the option is granted. In the case of an optionee who, at the time of grant, owns more than 10% of the combined voting power of all classes of OncoCyte stock, the exercise price of any incentive stock option must be at least 110% of the fair market value of the common stock on the grant date, and the term of the option may be no longer than five years. The aggregate fair market value of OncoCyte common stock (determined as of the grant date of the option) with respect to which incentive stock options become exercisable for the first time by an optionee in any calendar year may not exceed $100,000.
The options’ exercise price may be payable in cash or in common stock having a fair market value equal to the exercise price, or in a combination of cash and common stock, or other legal consideration for the issuance of stock as the Board of Directors or Compensation Committee may approve.
Incentive stock options granted under the Plan are nontransferable except by will or the laws of descent and distribution and may be exercised only during employment or within three months after termination of such employment, subject to certain exceptions in the event of the death or disability of the optionee.
Options other than incentive stock options under the Code are also nontransferable except by will or the laws of descent and distribution, except to the extent that the Board of Directors or Committee permits the optionee to transfer an option to a family member, a trust for family members, or other persons approved by the Board of Directors or Committee in its discretion.
Generally options will be exercisable only while the optionee remains an employee, director or consultant, or during a specific period thereafter as approved by the Board of Directors or Committee, but in the case of the termination of an employee, director, or consultant’s services due to death or disability, the period for exercising a vested option shall be extended to the earlier of 12 months after termination or the expiration date of the option.
The number of shares of common stock covered by the Plan, and the number of shares of common stock and the exercise price per share of each outstanding option, shall be proportionately adjusted for any increase or decrease in the number of issued and outstanding shares of common stock resulting from a subdivision or consolidation of shares or the payment of a stock dividend, or any other increase or decrease in the number of issued and outstanding shares of common stock effected without receipt of consideration by OncoCyte.
Options Granted
As of December 31, 2015, 1,757,417 shares were available for future grants under the Plan.
A summary of OncoCyte stock option activity under the Plan and related information follows (in thousands except weighted average exercise price):
|
Options
|
|
Available for
Grant
|
|
|
Number of
Shares
|
|
|
Weighted
Average
Exercise
Price
|
|
|
Outstanding at January 1, 2014
|
|
|
625
|
|
|
|
|
1,375
|
|
|
|
$
|
1.53
|
|
|
|
Granted
|
|
|
-
|
|
|
|
|
-
|
|
|
|
|
-
|
|
|
|
Options forfeited or cancelled
|
|
|
14
|
|
|
|
|
(14)
|
|
|
|
|
2.00
|
|
|
|
Outstanding at December 31, 2014
|
|
|
639
|
|
|
|
|
1,361
|
|
|
|
$
|
1.52
|
|
|
|
Increase in option pool
|
|
|
2,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options granted
|
|
|
(1,448)
|
|
|
|
|
1,448
|
|
|
|
|
2.21
|
|
|
|
Options exercised
|
|
|
-
|
|
|
|
|
(3)
|
|
|
|
|
1.34
|
|
|
|
Options forfeited or cancelled
|
|
|
566
|
|
|
|
|
(566)
|
|
|
|
|
1.59
|
|
|
|
Outstanding at December 31, 2015
|
|
|
1,757
|
|
|
|
|
2,240
|
|
|
|
$
|
2.03
|
|
|
|
Exercisable at December 31, 2015
|
|
|
|
|
|
|
972
|
|
|
|
$
|
1.61
|
|
|
There were 3,000 and no stock options exercised during the years ended December 31, 2015 and 2014, respectively.
At December 31, 2015 and 2014, OncoCyte had approximately $1.5 million and $87,000 of total unrecognized compensation expense, net of estimated forfeitures, related to the Plan that will be recognized over a weighted-average period of approximately 2.97 and 0.91 years, respectively.
OncoCyte recorded stock-based compensation expense in the following categories on the accompanying statements of operations for the years ended December 31, 2015, 2014 and 2013 (in thousands):
|
|
|
2015
|
|
2014
|
|
2013
|
|
|
Research and development
|
|
$
|
456
|
|
|
$
|
177
|
|
|
$
|
183
|
|
|
|
General and administrative
|
|
|
1,359
|
|
|
|
141
|
|
|
|
143
|
|
|
|
Total stock-based compensation expense
|
|
$
|
1,815
|
|
|
$
|
318
|
|
|
$
|
326
|
|
|
The assumptions that were used to calculate the grant date fair value of OncoCyte’s employee and non-employee stock option grants for the years ended December 31, 2015, 2014 and 2013 were as follows.
|
|
|
2015
|
|
2014
(1)
|
|
2013
|
|
|
Expected life (in years)
|
|
|
6.83
|
|
|
|
-
|
|
|
|
4.13
|
|
|
|
Risk-free interest rates
|
|
|
1.87
|
%
|
|
|
-
|
%
|
|
|
1.28
|
%
|
|
|
Volatility
|
|
|
74.15
|
%
|
|
|
-
|
%
|
|
|
70.68
|
%
|
|
|
Dividend yield
|
|
|
-
|
%
|
|
|
-
|
%
|
|
|
-
|
%
|
|
|
|
(1)
|
No stock options were granted in 2014.
|
Stock-based compensation expense is recognized based on awards that are ultimately expected to vest, and as a result, the amount has been reduced by estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures are estimated based on OncoCyte’s historical experience and future expectations.
The determination of stock-based compensation is inherently uncertain and subjective and involves the application of valuation models and assumptions requiring the use of judgment. If OncoCyte had made different assumptions, its stock-based compensation expense, and net loss for years ended December 31, 2015, 2014 and 2013, may have been significantly different.
There was no net income tax benefit recognized in the statements of operations for stock-based compensation expense for non-qualified stock options, as OncoCyte fully offsets net deferred tax assets with a valuation allowance (see Note 8). In addition, OncoCyte does not recognize deferred income taxes for incentive stock option compensation expense, and records a tax deduction only when a disqualified disposition has occurred.
8. Income Taxes
OncoCyte has filed standalone U.S. federal income tax returns since its inception. For California purposes, OncoCyte’s activity for 2014 and 2015 has been included in BioTime’s California Combined tax return. The provision for income taxes has been determined as if OncoCyte had filed separate tax returns for the periods presented. Accordingly, the effective tax rate of OncoCyte in future years could vary from its historical effective tax rates depending on the future legal structure of OncoCyte and related tax elections. The deferred tax assets, including the operating loss and credit carryforwards, generated by OncoCyte, will remain with OncoCyte.
OncoCyte early adopted ASU 2015-17 effective December 31, 2015, on a retrospective basis. Accordingly, OncoCyte adjusted the December 31, 2014, balance sheet for noncurrent deferred tax assets and current deferred tax liabilities to conform to the presentation for the current year due to the early adoption of ASU 2015-17 as follows (in thousands):
The adoption of this standard had no impact to the statements of operations or cash flows.
The primary components of the deferred tax assets and liabilities at December 31, 2015 and 2014 were as follows (in thousands):
Due to losses incurred for all periods presented, OncoCyte did not record any provision or benefit for income taxes.
Income taxes differed from the amounts computed by applying the U.S. federal income tax of 34% to pretax losses from operations as a result of the following:
As of December 31, 2015, OncoCyte has net operating loss carryforwards of approximately $20.1 million for U.S. federal income tax purposes and $14.8 million for state income tax purposes. Federal net operating loss carryforwards expire from 2030 and 2035, and state carryforwards expire from 2029 and 2035. In addition, as of December 31, 2015, OncoCyte has research and development credit carryforwards for federal and state purposes of $664,000 and $698,000, respectively. The federal credits will expire between 2030 and 2035, while the state credits have no expiration.
During 2015 and 2014, OncoCyte sold 259,712 and 406,756 BioTime common shares, respectively, in at-the-market transactions which resulted in taxable gains of approximately $815,000 and $1.3 million, respectively. These taxable gains were fully offset by current operating losses, thus resulting in no income taxes due from the sales. At December 31, 2015 and December 31, 2014, OncoCyte recorded deferred tax liabilities of $864,000 and $1.1 million, respectively, resulting from the difference in the tax basis of BioTime shares held as compared to the basis of those shares reported for financial reporting purposes (see Note 2).
A valuation allowance is provided when it is more likely than not that some portion of the deferred tax assets will not be realized. OncoCyte established a full valuation allowance for all periods presented due to the uncertainty of realizing future tax benefits from its net operating loss carryforwards and other deferred tax assets. The change in the valuation allowance was $3.9 million and $1.6 million for the years ended December 31, 2015 and 2014, respectively.
Internal Revenue Code Section 382 places a limitation (“Section 382 Limitation”) on the amount of taxable income that can be offset by net operating loss (“NOL”) carryforwards after a change in control (generally greater than 50% change in ownership within a three-year period) of a loss corporation. California has similar rules. Generally, after a control change, a loss corporation cannot deduct NOL carryforwards in excess of the Section 382 Limitation. Due to these “change in ownership” provisions, utilization of the NOL and tax credit carryforwards may be subject to an annual limitation regarding their utilization against taxable income in future periods. There has not been a change in ownership for any of the periods presented.
OncoCyte may be subject to potential income tax examination by U.S. federal or states authorities. These potential examinations may include inquiries regarding the timing and amount of deductions, and compliance with U.S. federal and state tax laws. In general, OncoCyte is no longer subject to tax examination by major taxing authorities for years before 2011. Although the statute is closed for purposes of assessing additional income and tax in those years, the taxing authorities may still make adjustments to the net operating loss and credit carryforwards used in open years. Any potential examinations may include inquiries regarding the timing and amount of deductions, and compliance with U.S. federal and state tax laws.
9. Commitments and Contingencies
OncoCyte had no commitments other than those under the Shared Facilities and Services Agreement described in Note 4. The minimum fixed payments due under the Shared Facilities Agreement are approximately $15,000 per month.
OncoCyte tax filings are subject to audit by taxing authorities in jurisdictions where it conducts business. These audits may result in assessments of additional taxes that are subsequently resolved with the authorities or potentially through the courts. Management believes OncoCyte has adequately provided for any ultimate amounts that are likely to result from these audits; however, final assessments, if any, could be significantly different than the amounts recorded in the financial statements.
OncoCyte will be subject to various claims and contingencies in the ordinary course of its business, including those related to litigation, business transactions, employee-related matters, and others. When OncoCyte is aware of a claim or potential claim, it assesses the likelihood of any loss or exposure. If it is probable that a loss will result and the amount of the loss can be reasonably estimated, OncoCyte will record a liability for the loss. If the loss is not probable or the amount of the loss cannot be reasonably estimated, OncoCyte discloses the claim if the likelihood of a potential loss is reasonably possible and the amount involved could be material. OncoCyte is not aware of any claims likely to have a material adverse effect on its financial condition or results of operations.
OncoCyte has entered into employment contracts with certain executive officers. Under the provisions of the contracts, OncoCyte may be required to incur severance obligations for matters relating to changes in control, as defined, and involuntary terminations. At December 31, 2015, total potential severance obligations in connection with the termination of employment contracts approximated $272,000 for termination without cause and $448,000 for termination due to a change in control.
In the normal course of business, OncoCyte may provide indemnification of varying scope under OncoCyte’s agreements with other companies or consultants, typically OncoCyte’s clinical research organizations, investigators, clinical sites, suppliers and others. Pursuant to these agreements, OncoCyte will generally agree to indemnify, hold harmless, and reimburse the indemnified parties for losses and expenses suffered or incurred by the indemnified parties arising from claims of third parties in connection with the use or testing of OncoCyte’s diagnostic tests. Indemnification provisions could also cover third party infringement claims with respect to patent rights, copyrights, or other intellectual property pertaining to OncoCyte’s diagnostic tests. The term of these indemnification agreements will generally continue in effect after the termination or expiration of the particular research, development, services, or license agreement to which they relate. The potential future payments OncoCyte could be required to make under these indemnification agreements will generally not be subject to any specified maximum amounts. Historically, OncoCyte has not been subject to any claims or demands for indemnification. OncoCyte also maintains various liability insurance policies that limit OncoCyte’s financial exposure. As a result, OncoCyte management believes that the fair value of these indemnification agreements is minimal. Accordingly, OncoCyte has not recorded any liabilities for these agreements as of December 31, 2015 and 2014.
10. Subsequent Events
On January 22, 2016, OncoCyte entered into a License Agreement with The Wistar Institute of Anatomy and Biology (“Wistar”). Under the License Agreement, OncoCyte has obtained an exclusive, worldwide license under certain patents, and under certain know-how and data belonging to Wistar, for use in the field of molecular diagnostics for lung cancer, including, but not limited to confirmatory, companion and recurrence diagnostics for any type of lung cancer with detection through whole blood, fractionated blood, plasma, serum and/or other biological samples.
On February 16, 2016, OncoCyte granted 637,000 stock options with an exercise price of $3.06 per share to certain executive officers under the Plan.
It is management’s responsibility to establish and maintain adequate internal control over all financial reporting pursuant to Rule 13a-15 under the Exchange Act. Our management, including our principal executive officer and our principal financial officer, have reviewed and evaluated the effectiveness of our disclosure controls and procedures as of the end of our fourth quarter. Following this review and evaluation
,
management collectively determined that our disclosure controls and procedures are effective to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act (i) is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms; and (ii) is accumulated and communicated to management, including our chief executive officer and our chief financial officer, as appropriate to allow timely decisions regarding required disclosure.
There were no changes in our internal control over financial reporting that occurred during the period covered by this Annual Report on Form 10-K that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting, as defined in Exchange Act Rule 13a-15(f), is a process designed by, or under the supervision of, our principal executive officer, our principal operations officer, and our principal financial officer, and effected by our Board of Directors, management, and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. The scope of management’s assessment of the effectiveness of internal control over financial reporting includes our consolidated subsidiaries.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2015, based on criteria established in the 2013 Internal Control - Integrated Framework issued by COSO. Based on this assessment, management believes that, as of that date, our internal control over financial reporting was effective.
The following financial statements of OncoCyte Corporation are filed in the Form 10-K:
All schedules are omitted because the required information is inapplicable or the information is presented in the financial statements or the notes thereto.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized on the 30
th
day of March, 2016.