|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Delaware
|
56-2358443
|
|
(State or other jurisdiction of
incorporation or organization)
|
(I.R.S. Employer
Identification No.)
|
|
15010 Avenue of Science, Suite 200
San Diego, CA
|
92128
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
Title of Each Class
|
Name of Each Exchange on Which Registered
|
|
Common Stock, par value $0.0001 per share
|
The NASDAQ Stock Market LLC
(The NASDAQ Global Market)
|
|
Large accelerated filer
|
o
|
Accelerated filer
|
x
|
|
Non-accelerated filer
|
o
(Do not check if a smaller reporting company)
|
Smaller reporting company
|
o
|
|
|
Page
|
|
|
Item 1.
|
||
|
Item 1A.
|
||
|
Item 1B.
|
||
|
Item 2.
|
||
|
Item 3.
|
||
|
Item 4.
|
||
|
Item 5.
|
||
|
Item 6.
|
||
|
Item 7.
|
||
|
Item 7A.
|
||
|
Item 8.
|
||
|
Item 9.
|
||
|
Item 9A.
|
||
|
Item 9B.
|
||
|
Item 10.
|
||
|
Item 11.
|
||
|
Item 12.
|
||
|
Item 13.
|
||
|
Item 14.
|
||
|
Item 15.
|
||
|
•
|
the initiation, cost, timing and results of our clinical programs for the ELAD
®
System, including statements related to our VTL-308 phase 3 clinical trial;
|
|
•
|
the timing of, and our ability to obtain and maintain regulatory approvals for the ELAD System;
|
|
•
|
regulatory developments in the United States and foreign countries;
|
|
•
|
the potential market for the ELAD System, including our anticipated gross margins if commercialized;
|
|
•
|
the rate and degree of market acceptance and clinical utility of the ELAD System;
|
|
•
|
our commercialization, marketing and manufacturing capabilities and strategy;
|
|
•
|
our plans to improve the ELAD System;
|
|
•
|
our plans to explore other uses for our VTL C3A cells;
|
|
•
|
our plans to obtain funding for our operations;
|
|
•
|
the performance of third parties in connection with the development of the ELAD System, including third parties involved in our clinical trials and third-party suppliers;
|
|
•
|
the development, regulatory approval, efficacy and commercialization of competing products;
|
|
•
|
our ability to retain key scientific or management personnel;
|
|
•
|
our intellectual property position;
|
|
•
|
our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; and
|
|
•
|
our ability to maintain effective internal control over financial reporting.
|
|
•
|
Successfully complete the ELAD System’s clinical development in severe acute alcoholic hepatitis (sAAH) subjects
. For VTL-308, we expect to enroll at least 150 subjects in an event-driven clinical design (a statistical plan that allows the study sample to be adjusted according to aggregate mortality) among roughly 40 sites in the United States and Europe, with first patient enrollment projected to occur in the first half of 2016, and we expect to report top-line data for VTL-308 around mid-2018.
|
|
•
|
Obtain regulatory approval for the ELAD System in the U.S. and Europe
. If our VTL-308 clinical trial is statistically and clinically successful, we plan to submit a Biologics License Application, or BLA, to the U.S. Food and Drug Administration, or FDA, and a Marketing Authorization Application, or MAA, to the European Medicines Agency, or EMA. An additional phase 3 trial may or may not be required for these approvals.
|
|
•
|
Maximize the commercial potential of the ELAD System in the U.S. and Europe
. If approved, we intend to directly commercialize the ELAD System in the U.S. and Europe with a targeted sales force focusing on liver transplant centers and other specialist intensive care centers. We believe that we can price the ELAD System in a range consistent with other currently marketed lifesaving therapies, such as left ventricular assist devices, orphan biologic medications for hereditary metabolic diseases and monoclonal antibody medications for cancer.
|
|
•
|
Opportunistically explore commercial opportunities for the ELAD System in other international markets
. We have completed a trial for the ELAD System that may be considered pivotal in China. The study predominantly enrolled subjects with viral hepatitis B, and our application for marketing approval, filed in 2007, is still under review. However, we do not currently anticipate receiving approval in China prior to our receipt of regulatory approval, if any, in the U.S. or Europe. We would expect to address the commercial opportunity in China following approval in the U.S. or Europe. We also plan to evaluate commercial opportunities in Australia, Japan, India, other Asian markets, the Middle East, Brazil and Africa.
|
|
•
|
Pursue development of the ELAD System in additional indications
. If the ELAD System is approved for use in patients with sAAH, we may pursue the ELAD System’s clinical development in viral hepatitis B, FHF, SILF and bridge-to-transplant.
|
|
•
|
Technical Improvements and New Applications
. We plan to continue our development of the ELAD System with the incorporation of technical improvements to our ancillary delivery device, customized disposable sets, and cells. In addition, we plan to explore the development of next generation systems and other uses for VTL C3A cells, including the potential commercialization of proteins and other C3A-produced compounds.
|

|
1.
|
Provide acute-phase response proteins to help dampen the pro-inflammatory environment and restore the patient’s immune responses.
The cells secrete several anti-inflammatory proteins, including alpha-1-antitrypsin (AAT) and interleukin-1 receptor antagonist (IL-1Ra), the latter of which is upregulated in response to pro-inflammatory cytokines typically found in alcoholic hepatitis patients. In addition, VTL C3A cells secrete gelsolin, which others have suggested may help dampen inflammation in response to damaged cells.
|
|
2.
|
Promote liver regeneration by providing factors known to be associated with liver repair.
The cells secrete a number of proteins involved in angiogenesis such as vascular endothelial growth factor, or VEGF, placental growth factor and angiopoietin 2. VEGF is reported to stimulate other cells to secrete hepatocyte growth factor, a known hepatocyte growth promoter, and we believe VEGF may be beneficial by providing improved vascularity to damaged liver sinusoids. Other factors secreted by VTL C3A cells that may be of importance for regeneration include transforming growth factor alpha, stem cell factor, erythropoietin, and platelet-derived growth factor.
|
|
3.
|
Produce blood coagulation factors to address blood clotting imbalances that are common in alcoholic hepatitis patients.
Factor V, Factor VII, Factor VIII, Factor IX, Factor X, Factor XII, fibrinogen, prothrombin, antithrombin III, Protein C and plasminogen activator inhibitor-1 have been shown to be produced by the VTL C3A cells.
|
|
4.
|
Assist in in the restoration of liver function by providing liver-specific metabolism and detoxification capabilities.
The VTL C3A cells express messenger RNA, or mRNA, for cytochrome P450, or CYP, isoenzymes CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4 and CYP3A5, which are collectively responsible for metabolizing nearly 90% of all drugs. Moreover, this CYP expression appears to respond dynamically as evidenced by different expression patterns in VTL C3A cells exposed to different clinical subjects.
|
|
•
|
Biologically active
. The ELAD System contains biologically active VTL C3A cells and is designed to replicate many liver functions. We believe that an acellular solution to liver failure is unlikely to effectively replace lost liver function. We believe that a cellular approach like ours, capable of replicating key biologic processes, is best able to provide the requisite flexibility and breadth of function to sufficiently supplement liver function and improve survival in patients with acute liver failure.
|
|
•
|
Human cellular therapy
. The ELAD System is based on human cells, which confer a considerable advantage over non-human, animal-based cell therapies. Given the widespread availability of animal tissues, much work has been done on the use of animal liver cells, often derived from pigs, to treat humans with liver failure. While immunological risk is always present in cellular therapy, the use of non-human animal tissues presents greater immunological risk compared to human cellular therapy. Humans possess naturally occurring antibodies that react with antigens on porcine cell surfaces. These antibodies can mount an immediate attack in the presence of porcine cells, causing these cells to rapidly lose function and die. Moreover, repeated treatments with a porcine cell may cause subsequent immune responses to become increasingly severe. The infusion of porcine enzymes into a patient’s blood stream also poses immunologic risk. We are not aware of any FDA-approved, non-human animal-based cellular therapy for use in patients.
|
|
•
|
Commercially scalable
. Our VTL C3A cells used in the ELAD System are immortal and can be expanded in quantities to scale production. Each set of four cartridges used to treat a single patient is grown in a production process that takes about six weeks. The process is carefully controlled and is performed under ultra-clean conditions to avoid contamination in our current Good Manufacturing Practices, or cGMP, compliant production plant. The process is scalable by modular units.
|
|
•
|
Minimal manipulation needed by site
. Prior to shipment, the ELAD cartridges are put into a dormant state and shipped under cool conditions. They have been validated to survive for up to 60 hours before being used for treatment. When the hospital receives the cartridges, they are unpacked by our ELAD System specialists on site, placed on the system, flushed with saline and are ready to be used for patient treatment. Our VTL C3A cells usually remain viable for the duration of the patient treatment.
|
|
1.
|
Primary Graft Non-Function, which occurs when a newly transplanted liver fails to function. This is a life threatening medical emergency, and can lead to death if a new organ does not become available quickly. We believe the ELAD System may provide patients with a bridge-to-transplant until a second liver becomes available.
|
|
2.
|
Small-For-Size or Split Liver Transplant occurs when the transplanted liver is functioning, but may be too small to sustain the patient, either because only a small donor liver was available, or because a live person donated a portion of their liver for transplantation. We believe the ELAD System may be able to support the patient’s liver function until the donated organ regenerates to a size large enough to become independent of external support. Moreover, the ELAD System may also enable transplantation of smaller liver fragments than typically used, potentially expanding the available pool of donor organs.
|
|
3.
|
Other forms of SILF. Primary liver cancer can sometimes be cured by resecting the cancerous part of the liver after which the remaining liver regenerates to full size. Currently, surgeons will typically only resect up to 50% of the liver in order to avoid death from liver failure. However, more extensive resections occasionally occur, and resection of smaller portions can also lead to liver failure. We believe the ELAD System may be able to support these patients while their liver regenerates and may also enable surgeons to perform larger tumor resections.
|
|
Trial
|
Date
|
Study Design
|
Indication(s)
|
Sites*
|
Location(s)
|
Total
Subjects
Enrolled
|
||||||
|
VTL-308
(phase 3)
|
Scheduled to commence first half of 2016
|
Randomized, controlled
|
sAAH
|
40+ planned
|
U.S., Europe
|
Minimum of 150
|
||||||
|
VTI-208
(phase 3)
|
2013-2015
|
Randomized, controlled
|
AILD/sAAH
|
59
|
U.S., Europe, Australia
|
203
|
||||||
|
VTI-210
(phase 3)
|
Commenced November 2014 and terminated August 2015
|
Randomized, controlled
|
sAAH
|
42
|
U.S., Europe
|
18
|
||||||
|
VTI-212
(phase 2/3)
|
Commenced June 2014 and terminated August 2015
|
Single-arm in phase 2 component
|
FHF and SILF
|
18
|
U.S.
|
8
|
||||||
|
VTI-206
(phase 2b)
|
2009 – 2011
|
Randomized, controlled
|
AILD and other
|
26
|
U.S., Europe
|
62
|
||||||
|
Compassionate-
use program
|
2008 – 2010
|
Single-arm
|
Various
|
8
|
U.S., U.K., Singapore, Saudi Arabia
|
18
|
||||||
|
VTI-201
(phase 2a)
|
2008 – 2009
|
Randomized, controlled
|
ACLF and other
|
6
|
U.S.
|
18
|
||||||
|
VTIC-301
(Pivotal)
|
2006 – 2007
|
Randomized, controlled
|
Various, primarily viral hepatitis
|
2
|
China
|
69
|
||||||
|
CR-202
(phase 2)
|
2001 – 2003
|
Randomized, controlled
|
FHF
|
8
|
U.S., U.K.
|
19
|
||||||
|
PS-0698
(phase 1)
|
1999 – 2000
|
Randomized, controlled
|
FHF
|
6
|
U.S., U.K.
|
25
|
||||||
|
*
|
For VTL-308, represents numbers of clinical sites which will fluctuate throughout the trials in part based on resources and competition between trials for subjects.
|


|
•
|
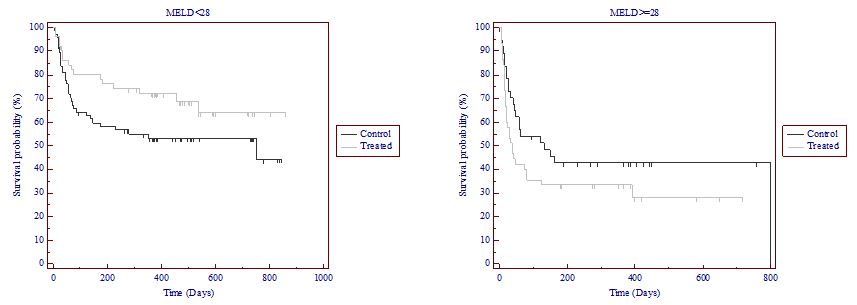
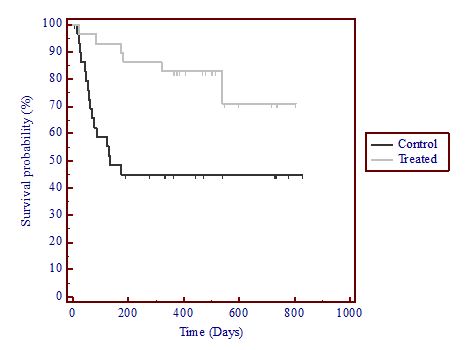
significant differences in 28 and 56-day survival using the log-rank test (p=0.015 and 0.026, respectively); (log-rank was not significant at 14 and 84 days, p=0.074 and 0.058, respectively);
|
|
•
|
significant differences in 84-day survival using the Wilcoxon test (p=0.049); and
|
|
•
|
no unexpected safety issues.
|

|
•
|
Significant differences in 28-day survival using the log-rank test (p=0.015);
|
|
•
|
No significant differences in 14, 56 and 84-day survival using the log-rank test; and
|
|
•
|
No unexpected safety issues.
|
|
•
|
our inability to recruit, train and retain adequate numbers of effective sales, marketing, training and support personnel;
|
|
•
|
the inability of sales personnel to obtain access to physicians, including key opinion leaders, or to persuade adequate numbers of physicians to use the ELAD System;
|
|
•
|
our inability to properly support the ELAD System therapy with our own qualified personnel at each customer site or our inability to properly train and support our customers to use the ELAD System effectively on their own;
|
|
•
|
the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive or integrated product offerings; and
|
|
•
|
unforeseen costs and expenses associated with creating an independent sales, marketing, training and support organization.
|
|
•
|
timeliness of contracting with clinical trial sites, and obtaining approval of the trial by the institutional review boards, or IRBs, at each site;
|
|
•
|
lack of a sufficient number of subjects who meet the enrollment criteria for our clinical trials;
|
|
•
|
perceived risks and benefits of the product candidate under study;
|
|
•
|
availability of competing therapies and clinical trials;
|
|
•
|
efforts to facilitate timely enrollment in clinical trials;
|
|
•
|
scheduling conflicts with participating clinicians; and
|
|
•
|
proximity and availability of clinical trial sites for prospective subjects.
|
|
•
|
delays or failures in designing an appropriate clinical trial protocol with sufficient statistical power and in reaching agreement on trial design with investigators and regulatory authorities;
|
|
•
|
delays or failure in reaching agreement on acceptable terms with prospective CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
|
|
•
|
delays or failure by CROs, investigators and clinical trial sites in ensuring the proper and timely conduct of our clinical trials;
|
|
•
|
delays or failure by us in manufacturing sufficient quantities of the ELAD cartridges pursuant to required quality standards for use in our clinical trials and by third-party manufacturers in supplying necessary and suitable components for the ELAD System;
|
|
•
|
delays or failure in transporting the ELAD System and cartridges to clinical trial sites with sufficient rapidity to enable treatment to begin early enough to have an opportunity for clinical benefit;
|
|
•
|
delays or failure in completing data analysis and achieving primary and secondary endpoints;
|
|
•
|
delays in subject enrollment or site initiation, including in light of, among other things, our negative results from VTI-208;
|
|
•
|
regulators or clinical site ethics committees or IRBs may not approve, delay, suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or concerns about patient safety;
|
|
•
|
we may suspend or terminate our clinical trials if we believe the ELAD System is exposing the participating subjects to unacceptable health risks or for other reasons;
|
|
•
|
subjects may not complete our clinical trials due to safety issues, adverse events, inconvenience or other reasons;
|
|
•
|
subjects in our clinical trials may die or suffer other adverse events for reasons that may be either related or unrelated to the ELAD System, particularly given the critically ill nature of these subjects;
|
|
•
|
we may have difficulty in maintaining contact with subjects after treatment, preventing us from collecting the data required by our study protocol; and
|
|
•
|
final analysis of the data of our clinical trials may conclude that the ELAD System lacks sufficient clinical efficacy or presents unacceptable safety risks.
|
|
•
|
the FDA may disagree with the design or implementation of our clinical trials or study endpoints. For example, it has expressed concern about the open-label design and multiplicity of confounding variables, including the need for delineating the standard of care that both treatment and controls will receive during our studies;
|
|
•
|
we may be unable to demonstrate to the satisfaction of the FDA that the ELAD System is safe and effective for its proposed indications or that the ELAD System provides significant clinically relevant benefits;
|
|
•
|
the results of our clinical trials may not meet the level of statistical significance required by the FDA for approval or may not support approval of a label that could command a price sufficient for us to be profitable;
|
|
•
|
the FDA may disagree with our interpretation of data from preclinical studies or clinical trials;
|
|
•
|
the opportunity for bias in the clinical trials as a result of the open-label design may not be adequately handled and may cause our trial to fail;
|
|
•
|
the ELAD System may be subject to an FDA advisory committee review, which is triggered by an FDA request and is solely within the FDA’s discretion, which may result in unexpected delays or hurdles to approval;
|
|
•
|
the FDA may determine that the manufacturing processes at our facilities or facilities of third party manufacturers with which we contract for clinical and commercial supplies are inadequate;
|
|
•
|
even if VTL-308 is successful in demonstrating a statistically significant improvement over standard of care, in light of the fact that certain confounding factors may be viewed by the FDA as limiting the persuasiveness of the study results, a single successful phase 3 clinical trial may not be sufficient to provide the substantial evidence of effectiveness necessary to support regulatory approval, and therefore we may need more than one phase 3 clinical trial to secure regulatory approval;
|
|
•
|
the FDA has commented that even if one of our phase 3 clinical trials is a statistical and clinical success, a second confirmatory trial that substantiates positive results may be necessary to support a BLA;
|
|
•
|
the approval policies or regulations of the FDA may significantly change in a manner rendering our clinical data insufficient for approval; and
|
|
•
|
the negative results from VTI-208 could result in more stringent requirements being imposed by the regulatory bodies and advisory groups.
|
|
•
|
delays in receipt of anticipated purchase orders;
|
|
•
|
our ability to recruit, train and retain sales, marketing, training and support personnel;
|
|
•
|
our inability to educate physicians about the ELAD System and drive the adoption of the ELAD System therapy for any approved indications;
|
|
•
|
performance of our targeted sales force in the U.S. and Europe and future partners in other markets;
|
|
•
|
results of clinical trials evaluating the ELAD System therapy;
|
|
•
|
positive or negative media coverage of the ELAD System or products of our competitors or our industry;
|
|
•
|
our ability to obtain further regulatory clearances or approvals, including for other indications;
|
|
•
|
delays in, or failure of, product and component deliveries by our subcontractors and suppliers;
|
|
•
|
changes in the length of the sales process;
|
|
•
|
changes in healthcare coverage and reimbursement policies;
|
|
•
|
customer response to the introduction of new product offerings; and
|
|
•
|
fluctuations in foreign currencies.
|
|
•
|
the federal healthcare program anti-kickback statute prohibits, among other things, persons or entities from knowingly and willfully soliciting, offering, receiving or paying any remuneration (including any kickback, bribe, or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce or in return for purchasing, leasing, ordering, or arranging for or recommending the purchase, lease, or order of any good, facility, service or item for which payment is made, in whole or in part, under a federal healthcare program;
|
|
•
|
the federal civil and criminal false claims laws and civil monetary penalties laws, including civil whistleblower or qui tam actions, prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, to the federal government, claims for payment or approval that are false or fraudulent or from knowingly making a false statement to improperly avoid, decrease or conceal an obligation to pay money to the federal government;
|
|
•
|
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal liability for, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or to obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program regardless of the payor (e.g., public or private) and knowingly or willfully falsifying, concealing, or covering up by any trick, scheme or device a material fact or making any materially false statement in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters;
|
|
•
|
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, and as amended again by the final HIPAA omnibus rule, Modifications to the HIPAA Privacy, Security, Enforcement, and Breach Notification Rules Under HITECH and the Genetic Information Nondiscrimination Act; Other Modifications to HIPAA, published in January 2013, imposes certain obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information without appropriate authorization by entities subject to the omnibus rule, such as health plans, clearinghouses and healthcare providers, and their associates;
|
|
•
|
the federal transparency law, enacted as part of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act (collectively, the ACA), and its implementing regulations, require manufacturers of drugs, devices, biologicals and medical supplies to report to the U.S. Department of Health and Human Services information related to payments and other transfers of value made to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members;
|
|
•
|
analogous state laws and regulations, including but not limited to: state anti-kickback and false claims laws, which may apply to our business practices, including but not limited to, research, distribution, sales and marketing arrangements and claims involving healthcare items or services reimbursed by state governmental and non-governmental third-party payors, including private insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government; and state laws and regulations that require manufacturers to file reports relating to pricing and marketing information, which requires tracking gifts and other remuneration and items of value provided to healthcare professionals and entities; and
|
|
•
|
European Union, or EU, data protection regulations, which may require member states of the EU to impose minimum restrictions on the collection and use of personal data that, in some respects, are more stringent, and impose more significant burdens on subject businesses, than current privacy standards in the U.S.
|
|
•
|
fluctuations in foreign currency exchange rates and controls;
|
|
•
|
competitive disadvantages to established foreign businesses with significant current market share and business and customer relationships;
|
|
•
|
nationalization;
|
|
•
|
tax and regulatory policies of local governments and the possibility of trade embargoes;
|
|
•
|
political instability, war or other hostilities; and
|
|
•
|
laws and policies of the U.S. and foreign governments affecting foreign trade and investment.
|
|
•
|
level of government involvement;
|
|
•
|
economic structure;
|
|
•
|
allocation of resources;
|
|
•
|
level of development;
|
|
•
|
inflation rates;
|
|
•
|
growth rate; and
|
|
•
|
control of foreign exchange.
|
|
•
|
complete clinical trials and related regulatory applications;
|
|
•
|
fund our operations;
|
|
•
|
commence and expand the commercialization of our products; and
|
|
•
|
further our research and development.
|
|
•
|
Successful and timely enrollment rates in our proposed clinical trial;
|
|
•
|
market acceptance of our products;
|
|
•
|
the cost of our research and development activities;
|
|
•
|
the cost and timing of our clinical development activities, in particular the rate of initiation of our clinical sites and the rate of enrollment of our clinical trials;
|
|
•
|
the cost of filing and prosecuting patent applications;
|
|
•
|
the cost of defending, litigation or any claims that we infringe third-party patents or violate other intellectual property rights;
|
|
•
|
the cost and timing of regulatory clearances or approvals, if any;
|
|
•
|
the cost and timing of establishing sales, marketing and distribution capabilities;
|
|
•
|
the cost and timing of establishing additional technical support capabilities;
|
|
•
|
the effect of competing technological and market developments; and
|
|
•
|
the extent to which we acquire or invest in businesses, products and technologies, although we currently have no commitments or agreements relating to any of these types of transactions.
|
|
•
|
clinical data and government approvals relating to the ELAD System;
|
|
•
|
changes in governmental regulations or in the status of our regulatory approvals or applications;
|
|
•
|
disputes or other developments with respect to our intellectual property rights or the intellectual property rights of others;
|
|
•
|
product liability claims or other litigation;
|
|
•
|
sales of large blocks of our common stock, including sales by our executive officers and directors;
|
|
•
|
changes in earnings estimates or recommendations by securities analysts;
|
|
•
|
our ability to meet investors expectations regarding our future operating performance;
|
|
•
|
media exposure of the ELAD System or products of our competitors;
|
|
•
|
volume and timing of sales of the ELAD System;
|
|
•
|
the introduction of new products or product enhancements by us or our competitors;
|
|
•
|
our ability to develop, obtain regulatory clearance or approval for and market new and enhanced products on a timely basis;
|
|
•
|
quarterly variations in our or our competitors’ results of operations;
|
|
•
|
developments in our industry; and
|
|
•
|
general market conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors.
|
|
•
|
authorize our board of directors to issue, without further action by our stockholders, up to 20,000,000 shares of undesignated preferred stock;
|
|
•
|
require that any action to be taken by our stockholders be effected at a duly called annual or special meeting and not by written consent;
|
|
•
|
specify that special meetings of our stockholders can be called only by a supermajority (75%) vote of our directors then in office;
|
|
•
|
specify that our board of directors may amend or repeal our bylaws only pursuant to a supermajority (75%) vote of our directors then in office;
|
|
•
|
specify that our stockholders may amend or repeal our bylaws only pursuant to a supermajority (75% and majority of the minority, if applicable) vote of the outstanding shares of our capital stock;
|
|
•
|
require in general the approval of a supermajority (75% and majority of the minority, if applicable) vote of our outstanding shares of capital stock to amend or repeal certain provisions of our certificate of incorporation;
|
|
•
|
require the approval of a supermajority (75% and majority of the minority, if applicable) vote of our outstanding shares of capital stock to approve the sale or liquidation of the company;
|
|
•
|
establish an advance notice procedure for stockholder approvals to be brought before an annual meeting of our stockholders, including proposed nominations of persons for election to our board of directors;
|
|
•
|
provide that directors may be removed only for cause by a supermajority (75%) vote of our outstanding shares of capital stock;
|
|
•
|
provide that vacancies on our board of directors may be filled only by a majority of directors then in office, even though less than a quorum;
|
|
•
|
provide that in general the number of directors on our board may only be fixed from time to time by a supermajority (75%) vote of our directors then in office;
|
|
•
|
establish that our board of directors is divided into three classes, Class I, Class II and Class III, with each class serving staggered terms; and
|
|
•
|
provide that certain stockholders affiliated with Muneer A. Satter, referred to as the Satter Investors, have rights to nominate up to a specific percentage of our directors (currently 30%) based on the Satter Investors’ ownership percentage in our Company.
|
|
|
Price Range
|
||||||
|
|
High
|
Low
|
|||||
|
Year Ended December 31, 2014
|
|||||||
|
Second Quarter (commencing April 17, 2014)
|
$
|
35.20
|
|
$
|
10.66
|
|
|
|
Third Quarter
|
$
|
28.36
|
|
$
|
16.80
|
|
|
|
Fourth Quarter
|
$
|
26.90
|
|
$
|
13.40
|
|
|
|
|
Price Range
|
||||||
|
|
High
|
Low
|
|||||
|
Year Ended December 31, 2015
|
|||||||
|
First Quarter
|
$
|
28.93
|
|
$
|
18.81
|
|
|
|
Second Quarter
|
$
|
29.67
|
|
$
|
19.01
|
|
|
|
Third Quarter
|
$
|
28.00
|
|
$
|
2.81
|
|
|
|
Fourth Quarter
|
$
|
11.80
|
|
$
|
3.76
|
|
|

|
Year Ended December 31,
|
|||||||||||||||
|
2015
|
2014
|
2013
|
2012
|
||||||||||||
|
Consolidated Statement of Operations Data:
|
(in thousands, except share and per share amounts)
|
||||||||||||||
|
Operating expenses:
|
|||||||||||||||
|
Research and development
|
$
|
39,773
|
|
$
|
39,479
|
|
$
|
21,787
|
|
$
|
5,097
|
|
|||
|
General and administrative
|
12,347
|
|
10,863
|
|
9,615
|
|
4,483
|
|
|||||||
|
Total operating expenses
|
52,120
|
|
50,342
|
|
31,402
|
|
9,580
|
|
|||||||
|
Loss from operations
|
(52,120
|
)
|
(50,342
|
)
|
(31,402
|
)
|
(9,580
|
)
|
|||||||
|
Revaluation of future purchase rights liabilities
|
—
|
|
2,600
|
|
(1,306
|
)
|
3,101
|
|
|||||||
|
Other income (expense), net
|
97
|
|
75
|
|
(10
|
)
|
(222
|
)
|
|||||||
|
Net loss
|
(52,023
|
)
|
(47,667
|
)
|
(32,718
|
)
|
(6,701
|
)
|
|||||||
|
Amortization of deemed dividend
|
—
|
|
(4,744
|
)
|
(64
|
)
|
—
|
|
|||||||
|
Accretion to redemption value of redeemable convertible preferred stock
|
—
|
|
(4,410
|
)
|
(6,303
|
)
|
(942
|
)
|
|||||||
|
Net loss attributable to common stockholders
|
$
|
(52,023
|
)
|
$
|
(56,821
|
)
|
$
|
(39,085
|
)
|
$
|
(7,643
|
)
|
|||
|
Net loss per share attributable to common stockholders, basic and diluted
|
$
|
(2.07
|
)
|
$
|
(3.54
|
)
|
$
|
(74.86
|
)
|
$
|
(17.89
|
)
|
|||
|
Weighted –average common shares outstanding, basic and diluted
(1)
|
25,152,948
|
|
16,054,452
|
|
522,102
|
|
427,117
|
|
|||||||
|
(1)
|
Please refer to Note 2, "
Summary of Significant Accounting Policies
," in the notes to the consolidated financial statements, for an explanation of the method used to calculate basic and diluted net loss per share attributable to common stockholders and the number of shares used in the computation of the per share amounts.
|
|
As of December 31,
|
|||||||||||||||
|
2015
|
2014
|
2013
|
2012
|
||||||||||||
|
Consolidated Balance Sheet Data:
|
(in thousands)
|
||||||||||||||
|
Cash, cash equivalents and short-term investments
|
$
|
83,416
|
|
$
|
102,238
|
|
$
|
38,186
|
|
$
|
18,473
|
|
|||
|
Working capital
|
78,433
|
|
94,538
|
|
36,409
|
|
17,403
|
|
|||||||
|
Total assets
|
89,081
|
|
108,082
|
|
46,585
|
|
20,332
|
|
|||||||
|
Preferred stock
|
—
|
|
—
|
|
83,475
|
|
26,176
|
|
|||||||
|
Additional paid-in-capital
|
285,098
|
|
248,305
|
|
58,413
|
|
62,728
|
|
|||||||
|
Accumulated deficit
|
(202,856
|
)
|
(150,833
|
)
|
(103,166
|
)
|
(70,448
|
)
|
|||||||
|
Total stockholders’ equity (deficit)
|
82,325
|
|
97,563
|
|
(44,657
|
)
|
(7,632
|
)
|
|||||||
|
•
|
expenses incurred under agreements with clinical sites, clinical research organizations, or CROs, and statistical and regulatory consultants that assist us with our clinical trials;
|
|
•
|
employee-related expenses, which include salaries, benefits, travel and stock-based compensation;
|
|
•
|
the cost of acquiring and manufacturing clinical trial materials;
|
|
•
|
facilities, depreciation, and other allocated expenses, which include direct and allocated expenses for rent, information systems and maintenance of facilities and equipment, and depreciation of fixed assets; and
|
|
•
|
other costs associated with research and regulatory activities.
|
|
•
|
per subject trial costs;
|
|
•
|
the number of sites included in the trials;
|
|
•
|
the countries in which the trials are conducted;
|
|
•
|
the number of subjects that participate in the trials;
|
|
•
|
continuing quality assurance activities and standards consistent with the U.S. Food and Drug Administration, or FDA, and other regulatory requirements;
|
|
•
|
potential additional safety monitoring or other studies requested by regulatory agencies; and
|
|
•
|
the frequency and duration of subject follow-up visits.
|
|
|
Year Ended
December 31,
|
Change
|
||||||||||||
|
|
2015
|
2014
|
$
|
%
|
||||||||||
|
Operating expenses:
|
||||||||||||||
|
Research and development
|
$
|
39,773
|
|
$
|
39,479
|
|
$
|
294
|
|
1
|
%
|
|||
|
General and administrative
|
12,347
|
|
10,863
|
|
1,484
|
|
14
|
%
|
||||||
|
Total operating expenses
|
$
|
52,120
|
|
$
|
50,342
|
|
$
|
1,778
|
|
4
|
%
|
|||
|
|
Year Ended
December 31,
|
Change
|
||||||||||||
|
|
2014
|
2013
|
$
|
%
|
||||||||||
|
Operating expenses:
|
||||||||||||||
|
Research and development
|
$
|
39,479
|
|
$
|
21,787
|
|
$
|
17,692
|
|
81
|
%
|
|||
|
General and administrative
|
10,863
|
|
9,615
|
|
1,248
|
|
13
|
%
|
||||||
|
Total operating expenses
|
$
|
50,342
|
|
$
|
31,402
|
|
$
|
18,940
|
|
60
|
%
|
|||
|
2015
|
2014
|
2013
|
|||||||||
|
Cash (used in) provided by:
|
|||||||||||
|
Operating activities
|
$
|
(49,952
|
)
|
$
|
(40,825
|
)
|
$
|
(28,648
|
)
|
||
|
Investing activities
|
(1,281
|
)
|
(2,040
|
)
|
11,909
|
|
|||||
|
Financing activities
|
32,421
|
|
106,918
|
|
50,445
|
|
|||||
|
•
|
the scope, progress, results and costs of research and development and clinical trials related to the ELAD System or any future product candidates;
|
|
•
|
the cost and timing of scaling up and validating the manufacturing process for the ELAD System or any other product candidates for commercialization;
|
|
•
|
the cost and timing of commercialization activities, including reimbursement, marketing, sales and distribution costs, both before and after product approval (if any);
|
|
•
|
our ability to establish new collaborations, licensing or other arrangements and the financial terms of such agreements;
|
|
•
|
the number and characteristics of any future product candidates we pursue;
|
|
•
|
the costs involved with being a public company;
|
|
•
|
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patents, including litigation costs and the outcome of such litigation; and
|
|
•
|
the timing, receipt and amount of sales of, or royalties on the ELAD System and any future product candidates.
|
|
|
Payments Due by Period
|
||||||||||||||||||
|
|
Total
|
Less Than
1 Year |
2-3
Years |
3-5
Years |
More Than
5 Years |
||||||||||||||
|
|
(In thousands)
|
||||||||||||||||||
|
Operating lease obligations
|
$
|
1,380
|
|
$
|
889
|
|
$
|
491
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
Purchase obligations
|
621
|
|
621
|
|
—
|
|
—
|
|
—
|
|
|||||||||
|
Total contractual obligations
|
$
|
2,001
|
|
$
|
1,510
|
|
$
|
491
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
•
|
The Black-Scholes value of the Initial Award (as defined in our amended Outside Director Compensation Policy) has been increased from approximately $220,000 to approximately $250,000; and
|
|
•
|
The Black-Scholes value of the Annual Award (as defined in our amended Outside Director Compensation Policy) has been increased from approximately $110,000 to approximately $125,000, commencing with our 2016 annual meeting of stockholders.
|
|
Page
|
||
|
1. Financial Statements
. We have filed the following documents as part of this Annual Report:
|
||
|
|
||
|
Report of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm
|
||
|
Consolidated Balance Sheets
|
||
|
Consolidated Statements of Operations
|
||
|
Consolidated Statements of Comprehensive Loss
|
||
|
Consolidated Statements of Redeemable Convertible Preferred Stock and Stockholders’ Equity (Deficit)
|
||
|
Consolidated Statements of Cash Flows
|
||
|
Notes to Consolidated Financial Statements
|
||
|
2. Financial Statement Schedules.
None.
|
||
|
3. Exhibits.
The following exhibits are filed herewith or are incorporated by reference to exhibits previously
|
||
|
filed with the U.S. Securities and Exchange Commission.
|
||
|
|
|
Incorporated by Reference
|
|||||||||
|
Exhibit
Number
|
Exhibit Title
|
Form
|
File No.
|
Exhibit
|
Filing Date
|
||||||
|
3.1
|
Amended and Restated Certificate of Incorporation of the Registrant.
|
S-1/A
|
333-191711
|
3.2
|
|
November 6, 2013
|
|||||
|
3.2
|
Second Amended and Restated Bylaws of the Registrant.
|
S-1/A
|
333-191711
|
3.4
|
|
November 6, 2013
|
|||||
|
4.1
|
Specimen Common Stock Certificate of the Registrant.
|
S-1/A
|
333-191711
|
4.1
|
|
November 6, 2013
|
|||||
|
4.2
|
Fourth Amended and Restated Investors’ Rights Agreement, dated August 28, 2013.
|
S-1
|
333-191711
|
4.2
|
|
October 11, 2013
|
|||||
|
4.3
|
Investors’ Rights Agreement, dated February 23, 2012.
|
S-1
|
333-191711
|
4.3
|
|
October 11, 2013
|
|||||
|
4.4
|
Amended and Restated Investors’ Rights Agreement, dated June 7, 2011.
|
S-1
|
333-191711
|
4.4
|
|
October 11, 2013
|
|||||
|
10.1+
|
Form of Indemnification Agreement between the Registrant and its directors and officers.
|
S-1/A
|
333-191711
|
10.1
|
|
November 6, 2013
|
|||||
|
10.2+
|
Employment Letter Agreement between the Registrant and Duane Nash, dated October 30, 2013.
|
S-1/A
|
333-191711
|
10.2
|
|
November 6, 2013
|
|||||
|
10.3+
|
Employment Letter Agreement between the Registrant and Robert A. Ashley, dated October 30, 2013.
|
S-1/A
|
333-191711
|
10.3
|
|
November 6, 2013
|
|||||
|
10.4+
|
Employment Letter Agreement between the Registrant and Terence E. Winters, dated October 31, 2013.
|
S-1/A
|
333-191711
|
10.4
|
|
November 6, 2013
|
|||||
|
10.5+
|
Employment Letter Agreement between the Registrant and Michael V. Swanson, dated August 30, 2013.
|
S-1/A
|
333-191711
|
10.5
|
|
November 6, 2013
|
|||||
|
10.6+
|
Employment Letter Agreement between the Registrant and Andrew Henry, dated October 30, 2013.
|
S-1/A
|
333-191711
|
10.6
|
|
April 3, 2014
|
|||||
|
10.7+
|
Employment Letter Agreement between the Registrant and Aron P. Stern, dated October 30, 2013.
|
S-1/A
|
333-191711
|
10.7
|
|
March 11, 2014
|
|||||
|
10.8+
|
Employment Letter Agreement between the Registrant and Andrea Loewen, dated October 30, 2013.
|
S-1/A
|
333-191711
|
10.8
|
|
April 3, 2014
|
|||||
|
10.9+
|
Employment Letter Agreement between the Registrant and Richard Murawski, dated October 30, 2013.
|
S-1/A
|
333-191711
|
10.9
|
|
March 11, 2014
|
|||||
|
10.10+
|
Employment Letter Agreement between the Registrant and John Dunn, dated March 5, 2015.
|
10-K
|
001-36201
|
10.10
|
|
March 20, 2015
|
|||||
|
10.11+
|
2012 Stock Option Plan and form of agreements.
|
S-1
|
333-191711
|
10.6
|
|
October 11, 2013
|
|||||
|
10.12+
|
2014 Equity Incentive Plan and form of agreements.
|
S-1/A
|
333-191711
|
10.11
|
|
March 11, 2014
|
|||||
|
10.13+
|
Global Stock Option Agreement under 2014 Equity Incentive Plan.
|
10-K
|
001-36201
|
10.13
|
|
March 20, 2015
|
|||||
|
10.14+
|
Executive Incentive Compensation Plan.
|
S-1
|
333-191711
|
10.8
|
|
October 11, 2013
|
|||||
|
10.15+
|
Form Change of Control and Severance Agreement.
|
S-1
|
333-191711
|
10.9
|
|
October 11, 2013
|
|||||
|
10.16+
|
Non-Employee Director Compensation Policy.
|
S-1/A
|
333-191711
|
10.10
|
|
November 15, 2013
|
|||||
|
10.17
|
Standard Industrial/Commercial Multi-Tenant Lease and Addendum between DermTech International and R.E. Hazard Contracting Company, dated April 5, 2001, as amended.
|
S-1
|
333-191711
|
10.11
|
|
October 11, 2013
|
|||||
|
10.18
|
Standard Office Lease between Arden Realty Limited Partnership and the Registrant, dated May 7, 2013.
|
S-1
|
333-191711
|
10.12
|
|
October 11, 2013
|
|||||
|
10.19*+
|
Outside Director Compensation Policy
|
||||||||||
|
21.1*
|
List of subsidiaries of the Registrant.
|
||||||||||
|
23.1*
|
Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm.
|
||||||||||
|
24.1*
|
Power of Attorney (included on the signature page).
|
||||||||||
|
31.1*
|
Certification of Principal Executive Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
||||||||||
|
31.2*
|
Certification of Principal Financial Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
||||||||||
|
32.1**
|
Certification of Principal Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
||||||||||
|
32.2**
|
Certification of Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
||||||||||
|
101.INS*
|
XBRL Instance Document.
|
||||||||||
|
101.SCH*
|
XBRL Taxonomy Extension Schema Document.
|
||||||||||
|
101.CAL*
|
XBRL Taxonomy Extension Calculation Linkbase Document.
|
||||||||||
|
101.DEF*
|
XBRL Taxonomy Extension Definition Linkbase Database.
|
||||||||||
|
101.LAB*
|
XBRL Taxonomy Extension Label Linkbase Document.
|
||||||||||
|
101.PRE*
|
XBRL Taxonomy Extension Presentation Linkbase Document.
|
||||||||||
|
+
|
Indicates a management contract or compensatory plan or arrangement.
|
|
*
|
Filed herewith.
|
|
**
|
In accordance with Item 601(b)(32)(ii) of Regulation S-K and SEC Release No. 33-8238 and 34-47986, Final Rule: Management’s Reports on Internal Control Over Financial Reporting and Certification of Disclosure in Exchange Act Periodic Reports, the certifications furnished in Exhibits 32.1 and 32.2 hereto are deemed to accompany this Form 10-K and will not be deemed “filed” for purposes of Section 18 of the Exchange Act. Such certifications will not be deemed to be incorporated by reference into any filings under the Securities Act or the Exchange Act, except to the extent that the registrant specifically incorporates it by reference.
|
|
|
Page
|
|
F-2
|
|
|
F-3
|
|
|
F-4
|
|
|
F-5
|
|
|
F-6
|
|
|
F-7
|
|
|
F-8
|
|
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
83,416
|
|
$
|
102,238
|
|
|
|
Restricted cash
|
533
|
|
1,592
|
|
|||
|
Other current assets and prepaid expenses
|
1,139
|
|
986
|
|
|||
|
Total current assets
|
85,088
|
|
104,816
|
|
|||
|
Property and equipment, net
|
3,809
|
|
3,068
|
|
|||
|
Other assets
|
184
|
|
198
|
|
|||
|
Total assets
|
$
|
89,081
|
|
$
|
108,082
|
|
|
|
Liabilities and Stockholders’ Equity
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable
|
$
|
1,221
|
|
$
|
1,153
|
|
|
|
Accrued expenses
|
5,271
|
|
8,875
|
|
|||
|
Other current liabilities
|
163
|
|
250
|
|
|||
|
Total current liabilities
|
6,655
|
|
10,278
|
|
|||
|
Other long-term liabilities
|
101
|
|
241
|
|
|||
|
Commitments and contingencies
|
|
|
|||||
|
Stockholders’ equity:
|
|||||||
|
Preferred stock, $0.0001 par value; 20,000,000 authorized and no shares issued or outstanding at December 31, 2015 and 2014
|
—
|
|
—
|
|
|||
|
Common stock, $0.0001 par value; 130,000,000 shares authorized at December 31, 2015 and 2014; 30,473,083 and 23,982,786 shares issued and outstanding at December 31, 2015 and 2014, respectively
|
3
|
|
2
|
|
|||
|
Additional paid-in capital
|
285,098
|
|
248,305
|
|
|||
|
Accumulated other comprehensive income
|
80
|
|
89
|
|
|||
|
Accumulated deficit
|
(202,856
|
)
|
(150,833
|
)
|
|||
|
Total stockholders’ equity
|
82,325
|
|
97,563
|
|
|||
|
Total liabilities and stockholders’ equity
|
$
|
89,081
|
|
$
|
108,082
|
|
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Operating expenses:
|
|||||||||||
|
Research and development
|
$
|
39,773
|
|
$
|
39,479
|
|
$
|
21,787
|
|
||
|
General and administrative
|
12,347
|
|
10,863
|
|
9,615
|
|
|||||
|
Total operating expenses
|
52,120
|
|
50,342
|
|
31,402
|
|
|||||
|
Loss from operations
|
(52,120
|
)
|
(50,342
|
)
|
(31,402
|
)
|
|||||
|
Other income (expense):
|
|||||||||||
|
Interest income
|
58
|
|
19
|
|
5
|
|
|||||
|
Other income (expense), net
|
39
|
|
56
|
|
(15
|
)
|
|||||
|
Revaluation of future purchase rights liabilities
|
—
|
|
2,600
|
|
(1,306
|
)
|
|||||
|
Total other income (expense)
|
97
|
|
2,675
|
|
(1,316
|
)
|
|||||
|
Net loss
|
(52,023
|
)
|
(47,667
|
)
|
(32,718
|
)
|
|||||
|
Amortization of deemed dividend
|
—
|
|
(4,744
|
)
|
(64
|
)
|
|||||
|
Accretion to redemption value of redeemable convertible preferred stock
|
—
|
|
(4,410
|
)
|
(6,303
|
)
|
|||||
|
Net loss attributable to common stockholders
|
$
|
(52,023
|
)
|
$
|
(56,821
|
)
|
$
|
(39,085
|
)
|
||
|
Net loss per share attributable to common stockholders, basic and diluted
|
$
|
(2.07
|
)
|
$
|
(3.54
|
)
|
$
|
(74.86
|
)
|
||
|
Weighted-average common shares outstanding, basic and diluted
|
25,152,948
|
|
16,054,452
|
|
522,102
|
|
|||||
|
|
Years Ended
December 31, |
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Net loss
|
$
|
(52,023
|
)
|
$
|
(47,667
|
)
|
$
|
(32,718
|
)
|
||
|
Other comprehensive (loss) income:
|
|||||||||||
|
Foreign currency translation
|
(9
|
)
|
(7
|
)
|
8
|
|
|||||
|
Total comprehensive loss
|
$
|
(52,032
|
)
|
$
|
(47,674
|
)
|
$
|
(32,710
|
)
|
||
|
|
Junior
Convertible and Senior Redeemable Convertible Preferred Stock |
Common Stock
|
Additional
Paid-In Capital |
Accumulated
Other Comprehensive Income |
Accumulated
Deficit |
Total
Stockholders’ Equity (Deficit) |
|||||||||||||||||||||||
|
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||
|
Balance at January 1, 2013
|
7,019,599
|
|
$
|
26,176
|
|
467,167
|
|
$
|
—
|
|
$
|
62,728
|
|
$
|
88
|
|
$
|
(70,448
|
)
|
$
|
(7,632
|
)
|
|||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(32,718
|
)
|
(32,718
|
)
|
|||||||||||||
|
Other comprehensive income
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
8
|
|
—
|
|
8
|
|
|||||||||||||
|
Exercise of stock options, net of repurchase liability
|
—
|
|
—
|
|
139,071
|
|
—
|
|
135
|
|
—
|
|
—
|
|
135
|
|
|||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
948
|
|
—
|
|
—
|
|
948
|
|
|||||||||||||
|
Private placement senior redeemable convertible preferred stock — from February to December 2013
|
6,693,808
|
|
50,996
|
|
—
|
|
—
|
|
905
|
|
—
|
|
—
|
|
905
|
|
|||||||||||||
|
Accretion to redemption value of redeemable convertible preferred stock
|
—
|
|
6,303
|
|
—
|
|
—
|
|
(6,303
|
)
|
—
|
|
—
|
|
(6,303
|
)
|
|||||||||||||
|
Balance at December 31, 2013
|
13,713,407
|
|
83,475
|
|
606,238
|
|
—
|
|
58,413
|
|
96
|
|
(103,166
|
)
|
(44,657
|
)
|
|||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(47,667
|
)
|
(47,667
|
)
|
|||||||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(7
|
)
|
—
|
|
(7
|
)
|
|||||||||||||
|
Exercise of stock options and change in stock option early exercise repurchase liability
|
—
|
|
—
|
|
142,041
|
|
—
|
|
956
|
|
—
|
|
—
|
|
956
|
|
|||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
2,510
|
|
—
|
|
—
|
|
2,510
|
|
|||||||||||||
|
Private placement senior redeemable convertible preferred stock — from January to March 2014
|
2,296,016
|
|
18,167
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Amortization of deemed dividend
|
—
|
|
4,744
|
|
—
|
|
—
|
|
(4,744
|
)
|
—
|
|
—
|
|
(4,744
|
)
|
|||||||||||||
|
Accretion to redemption value of redeemable convertible preferred stock
|
—
|
|
4,410
|
|
—
|
|
—
|
|
(4,410
|
)
|
—
|
|
—
|
|
(4,410
|
)
|
|||||||||||||
|
Initial public offering, net of issuance costs
|
—
|
|
—
|
|
5,175,000
|
|
1
|
|
51,932
|
|
—
|
|
—
|
|
51,933
|
|
|||||||||||||
|
Conversion of redeemable convertible preferred stock to common stock
|
(16,009,423
|
)
|
(110,796
|
)
|
16,009,423
|
|
1
|
|
110,795
|
|
—
|
|
—
|
|
110,796
|
|
|||||||||||||
|
Adjustment for fractional shares
|
—
|
|
—
|
|
84
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
|||||||||||||
|
Issuance of common stock, net of issuance costs
|
—
|
|
—
|
|
2,050,000
|
|
—
|
|
32,853
|
|
—
|
|
—
|
|
32,853
|
|
|||||||||||||
|
Balance at December 31, 2014
|
—
|
|
—
|
|
23,982,786
|
|
2
|
|
248,305
|
|
89
|
|
(150,833
|
)
|
97,563
|
|
|||||||||||||
|
Net loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(52,023
|
)
|
(52,023
|
)
|
|||||||||||||
|
Other comprehensive loss
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
(9
|
)
|
—
|
|
(9
|
)
|
|||||||||||||
|
Exercise of stock options and change in stock option early exercise repurchase liability
|
—
|
|
—
|
|
217,570
|
|
—
|
|
615
|
|
—
|
|
—
|
|
615
|
|
|||||||||||||
|
Stock-based compensation
|
—
|
|
—
|
|
—
|
|
—
|
|
4,029
|
|
—
|
|
—
|
|
4,029
|
|
|||||||||||||
|
Issuance of common stock, net of issuance costs
|
—
|
|
—
|
|
6,272,727
|
|
1
|
|
32,149
|
|
—
|
|
—
|
|
32,150
|
|
|||||||||||||
|
Balance at December 31, 2015
|
—
|
|
$
|
—
|
|
30,473,083
|
|
$
|
3
|
|
$
|
285,098
|
|
$
|
80
|
|
$
|
(202,856
|
)
|
$
|
82,325
|
|
|||||||
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Cash flows from operating activities:
|
|||||||||||
|
Net loss
|
$
|
(52,023
|
)
|
$
|
(47,667
|
)
|
$
|
(32,718
|
)
|
||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|||||||||||
|
Depreciation and amortization
|
1,328
|
|
1,087
|
|
799
|
|
|||||
|
Stock-based compensation
|
4,029
|
|
2,510
|
|
948
|
|
|||||
|
Revaluation of future purchase rights liabilities
|
—
|
|
(2,600
|
)
|
1,306
|
|
|||||
|
Other
|
—
|
|
(7
|
)
|
(1
|
)
|
|||||
|
Changes in operating assets and liabilities:
|
|||||||||||
|
Other assets and prepaid expenses
|
143
|
|
279
|
|
(1,141
|
)
|
|||||
|
Accounts payable
|
281
|
|
46
|
|
(91
|
)
|
|||||
|
Accrued expenses
|
(3,584
|
)
|
5,396
|
|
2,266
|
|
|||||
|
Other liabilities
|
(126
|
)
|
131
|
|
(16
|
)
|
|||||
|
Net cash used in operating activities
|
(49,952
|
)
|
(40,825
|
)
|
(28,648
|
)
|
|||||
|
Cash flows from investing activities:
|
|||||||||||
|
Purchases of short-term investments
|
—
|
|
—
|
|
(2,999
|
)
|
|||||
|
Sales of short-term investments
|
—
|
|
—
|
|
17,000
|
|
|||||
|
Change in restricted cash
|
1,059
|
|
(631
|
)
|
(608
|
)
|
|||||
|
Purchases of property and equipment
|
(2,340
|
)
|
(1,409
|
)
|
(1,484
|
)
|
|||||
|
Net cash (used in) provided by investing activities
|
(1,281
|
)
|
(2,040
|
)
|
11,909
|
|
|||||
|
Cash flows from financing activities:
|
|||||||||||
|
Deferred financing costs
|
(283
|
)
|
—
|
|
(3,112
|
)
|
|||||
|
Proceeds from issuance of common stock, net of issuance costs
|
32,189
|
|
87,899
|
|
—
|
|
|||||
|
Proceeds from issuance of preferred stock, net of issuance costs
|
—
|
|
18,167
|
|
53,195
|
|
|||||
|
Proceeds from exercise of stock options
|
507
|
|
852
|
|
135
|
|
|||||
|
Proceeds from early exercise of stock options
|
8
|
|
—
|
|
227
|
|
|||||
|
Net cash provided by financing activities
|
32,421
|
|
106,918
|
|
50,445
|
|
|||||
|
Effect of exchange rate changes on cash and cash equivalents
|
(10
|
)
|
(1
|
)
|
3
|
|
|||||
|
Net change in cash and cash equivalents
|
(18,822
|
)
|
64,052
|
|
33,709
|
|
|||||
|
Cash and cash equivalents, beginning of period
|
102,238
|
|
38,186
|
|
4,477
|
|
|||||
|
Cash and cash equivalents, end of period
|
$
|
83,416
|
|
$
|
102,238
|
|
$
|
38,186
|
|
||
|
Supplemental disclosure of non-cash investing and financing activities:
|
|||||||||||
|
Offering costs included in liabilities
|
$
|
39
|
|
$
|
—
|
|
$
|
394
|
|
||
|
Purchase of property and equipment included in liabilities
|
$
|
9
|
|
$
|
277
|
|
$
|
170
|
|
||
|
Leasehold improvements paid for by landlord
|
$
|
—
|
|
$
|
—
|
|
$
|
478
|
|
||
|
Change in stock option early exercise repurchase liability
|
$
|
108
|
|
$
|
104
|
|
$
|
—
|
|
||
|
Conversion of redeemable convertible preferred stock to common stock
|
$
|
—
|
|
$
|
110,796
|
|
$
|
—
|
|
||
|
Valuation of future purchase rights upon issuance
|
$
|
—
|
|
$
|
—
|
|
$
|
1,294
|
|
||
|
Beneficial conversion underlying the senior preferred stock
|
$
|
—
|
|
$
|
—
|
|
$
|
969
|
|
||
|
Accretion to redemption value of redeemable convertible preferred stock
|
$
|
—
|
|
$
|
4,410
|
|
$
|
6,303
|
|
||
|
Amortization of deemed dividend
|
$
|
—
|
|
$
|
4,744
|
|
$
|
64
|
|
||
|
|
As of December 31,
|
|||||||
|
|
2015
|
2014
|
2013
|
|||||
|
Redeemable convertible preferred stock
|
—
|
|
—
|
|
13,713,407
|
|
||
|
Options to purchase common stock
|
3,716,520
|
|
3,210,693
|
|
3,098,573
|
|
||
|
Warrants to purchase common stock
|
250,646
|
|
250,646
|
|
250,646
|
|
||
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Manufacturing, clinical and laboratory equipment
|
$
|
7,040
|
|
$
|
5,292
|
|
|
|
Office furniture and equipment
|
135
|
|
113
|
|
|||
|
Computer equipment and software
|
129
|
|
152
|
|
|||
|
Leasehold improvements
|
4,441
|
|
3,367
|
|
|||
|
Construction in progress
|
124
|
|
922
|
|
|||
|
11,869
|
|
9,846
|
|
||||
|
Less: accumulated depreciation and amortization
|
(8,060
|
)
|
(6,778
|
)
|
|||
|
Total
|
$
|
3,809
|
|
$
|
3,068
|
|
|
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
Accrued clinical and related costs
|
$
|
2,675
|
|
$
|
6,072
|
|
|
|
Accrued compensation and related taxes
|
2,165
|
|
2,554
|
|
|||
|
Accrued other
|
431
|
|
249
|
|
|||
|
Total
|
$
|
5,271
|
|
$
|
8,875
|
|
|
|
|
Total
|
2016
|
2017
|
2018
|
2019
|
2020
|
|||||||||||||||||
|
Operating lease obligations
|
$
|
1,380
|
|
$
|
889
|
|
$
|
491
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
|||||
|
|
Payments Due by Period
|
||||||||||||||||||
|
|
Total
|
Less Than
1 Year |
2-3
Years |
3-5
Years |
More Than
5 Years |
||||||||||||||
|
Purchase obligations
|
$
|
621
|
|
$
|
621
|
|
$
|
—
|
|
$
|
—
|
|
$
|
—
|
|
||||
|
|
Fair Value Measurement at December 31, 2015
|
||||||||||||||
|
|
Fair Value
|
Level 1
|
Level 2
|
Level 3
|
|||||||||||
|
Assets
|
|||||||||||||||
|
Money market funds
|
$
|
80,078
|
|
$
|
80,078
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
|
Fair Value Measurement at December 31, 2014
|
||||||||||||||
|
|
Fair Value
|
Level 1
|
Level 2
|
Level 3
|
|||||||||||
|
Assets
|
|||||||||||||||
|
Money market funds
|
$
|
101,592
|
|
$
|
101,592
|
|
$
|
—
|
|
$
|
—
|
|
|||
|
|
Fair Value of
Future Purchase Rights Liabilities |
||
|
Balance at January 1, 2013
|
$
|
—
|
|
|
Initial valuation of additional future purchase rights
|
1,294
|
|
|
|
Re-measurement of future purchase rights
|
1,306
|
|
|
|
Balance at December 31, 2013
|
2,600
|
|
|
|
Re-measurement of future purchase rights
|
(2,600
|
)
|
|
|
Balance at December 31, 2014
|
$
|
—
|
|
|
|
December 31, 2013
|
||
|
Common stock fair value
|
$
|
5.93
|
|
|
Preferred stock price
|
$
|
8.00
|
|
|
Volatility
|
85.0
|
%
|
|
|
Risk-free interest rate
|
0.38
|
%
|
|
|
Contractual life (years)
|
2.08
|
|
|
|
Number of nodes
|
25
|
|
|
|
Dividend yield
|
—
|
%
|
|
|
Issuance Date
|
Number of
Shares
Subject to
Future Purchase
Rights Granted
|
Fair Value
per Share
of Rights
|
Grant Date Fair
Value Recorded
|
Common Stock
Value Used in
Determining
Fair Value
|
Beneficial
Conversion on
Underlying
Preferred Stock/
Options
|
|||||||||||||
|
September 25, 2012
|
3,750,000
|
|
$
|
2.80
|
|
$
|
3,101
|
|
$
|
2.30
|
|
$
|
—
|
|
||||
|
February 28, 2013
|
891,250
|
|
$
|
2.83
|
|
$
|
864
|
|
$
|
6.85
|
|
$
|
513
|
|
||||
|
April 30, 2013
|
461,271
|
|
$
|
2.40
|
|
$
|
379
|
|
$
|
6.85
|
|
$
|
192
|
|
||||
|
June 26, 2013
|
66,250
|
|
$
|
0.76
|
|
$
|
50
|
|
$
|
6.82
|
|
$
|
264
|
|
||||
|
|
As of December 31, 2015
|
|||||||
|
|
Warrants Outstanding
|
Exercise Price
|
Expiration Date
|
|||||
|
Series B
|
107
|
|
$
|
191.69
|
|
2/10/2016
|
||
|
Series C
|
9,919
|
|
$
|
147.91
|
|
11/20/2016
|
||
|
Series D
|
240,620
|
|
$
|
92.99
|
|
9/25/2019
|
||
|
Total warrants outstanding
|
250,646
|
|
||||||
|
|
Number of
Shares |
|
|
Common stock options outstanding
|
3,716,520
|
|
|
Common stock options available for future grant
|
370,775
|
|
|
Exercise of common stock warrants outstanding
|
250,646
|
|
|
Total common shares reserved for future issuance
|
4,337,941
|
|
|
•
|
1,200,000
shares of our common stock;
|
|
•
|
3%
of the outstanding shares of our common stock on the second-to-the-last day prior to each anniversary date of the effectiveness date of our IPO; or
|
|
•
|
an amount as our board of directors may determine.
|
|
Options
|
Weighted-
Average Exercise Price |
Weighted-
Average Remaining Contractual Term (Years) |
Aggregate
Intrinsic Value (In thousands) |
|||||||||
|
Outstanding as of January 1, 2015
|
3,210,693
|
|
$
|
7.54
|
|
|||||||
|
Granted
|
942,277
|
|
$
|
8.32
|
|
|||||||
|
Exercised
|
(217,570
|
)
|
$
|
2.36
|
|
|||||||
|
Forfeited and expired
|
(218,880
|
)
|
$
|
12.28
|
|
|||||||
|
Outstanding as of December 31, 2015
|
3,716,520
|
|
$
|
7.76
|
|
7.7
|
$
|
17,282
|
|
|||
|
Options vested and expected to vest as of December 31, 2015
|
3,621,869
|
|
$
|
7.72
|
|
7.6
|
$
|
16,852
|
|
|||
|
Options exercisable as of December 31, 2015
|
2,723,091
|
|
$
|
7.29
|
|
7.0
|
$
|
12,290
|
|
|||
|
|
Year Ended
December 31, |
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Aggregate intrinsic value of options exercised
|
$
|
1,235
|
|
$
|
1,972
|
|
$
|
633
|
|
||
|
|
Years Ended December 31,
|
|||||||
|
|
2015
|
2014
|
2013
|
|||||
|
Employees and Directors:
|
||||||||
|
Risk-free interest rate
|
1.71% - 1.85%
|
|
1.53% - 1.89%
|
|
0.76% - 1.10%
|
|
||
|
Expected dividend yield
|
—
|
%
|
—
|
%
|
—
|
%
|
||
|
Expected volatility
|
73% - 92%
|
|
80% - 85%
|
|
90% - 100%
|
|
||
|
Expected term of options (years)
|
5.8 - 6.0
|
|
6.0 - 6.2
|
|
5.0 - 5.5
|
|
||
|
Range of common stock value
|
$4.57 - $26.71
|
|
$7.55 – $24.04
|
|
$6.77 - $9.94
|
|
||
|
|
Years Ended December 31,
|
|||||||
|
|
2015
|
2014
|
2013
|
|||||
|
Non-Employees:
|
||||||||
|
Risk-free interest rate
|
0.10% - 1.93%
|
|
0.11% - 1.27%
|
|
0.13% - 0.86%
|
|
||
|
Expected dividend yield
|
—
|
%
|
—
|
%
|
—
|
%
|
||
|
Expected volatility
|
56% - 94%
|
|
73% - 85%
|
|
90% - 100%
|
|
||
|
Expected term of options (years)
|
0.3 - 6.0
|
|
1.0 - 4.0
|
|
1.0 - 4.0
|
|
||
|
Range of common stock value
|
$4.04 - $25.01
|
|
$11.31 - $27.24
|
|
$5.93 - $9.94
|
|
||
|
•
|
closing of an initial public offering;
|
|
•
|
sale to a strategic acquirer;
|
|
•
|
continuation as a private company with subsequent liquidation event; and
|
|
•
|
dissolution.
|
|
•
|
Scenarios: Expected future events were identified.
|
|
•
|
Scenario probabilities: Estimates of the probability of occurrence of each event were identified.
|
|
•
|
Valuation: Expected future values under each scenario were estimated.
|
|
•
|
Timing: Expected timing to the event under each scenario were estimated.
|
|
•
|
Risk adjusted discount rates: Risk-adjusted discount rates were selected for each equity class based on the rights and preferences of each equity class and market data.
|
|
•
|
Discounts: Appropriate minority or marketability discounts, if any, required to estimate the per share value of the various equity classes were determined.
|
|
Scenario
|
Weight
|
|
|
IPO by December 31, 2013
|
25
|
%
|
|
Sale by December 31, 2013
|
20
|
%
|
|
Private company
|
30
|
%
|
|
Dissolution
|
25
|
%
|
|
Scenario
|
Weight
|
|
|
IPO by November 15, 2013
|
35
|
%
|
|
Sale by November 15, 2013
|
10
|
%
|
|
Private company
|
30
|
%
|
|
Dissolution
|
25
|
%
|
|
Scenario
|
Weight
|
|
|
IPO by November 15, 2013
|
50
|
%
|
|
Sale by November 15, 2013
|
10
|
%
|
|
Private company
|
25
|
%
|
|
Dissolution
|
15
|
%
|
|
Scenario
|
Weight
|
|
|
IPO by November 15, 2013
|
50
|
%
|
|
Sale by November 15, 2013
|
10
|
%
|
|
Private company
|
25
|
%
|
|
Dissolution
|
15
|
%
|
|
Scenario
|
Weight
|
|
|
IPO by May 15, 2014
|
20
|
%
|
|
Sale by September 30, 2015
|
10
|
%
|
|
Private company
|
50
|
%
|
|
Dissolution
|
20
|
%
|
|
Scenario
|
Weight
|
|
|
IPO by May 15, 2014
|
20
|
%
|
|
Sale by September 30, 2015
|
10
|
%
|
|
Private company
|
50
|
%
|
|
Dissolution
|
20
|
%
|
|
Scenario
|
Weight
|
|
|
IPO by May 15, 2014
|
25
|
%
|
|
Sale by September 30, 2015
|
10
|
%
|
|
Private company
|
50
|
%
|
|
Dissolution
|
15
|
%
|
|
Scenario
|
Weight
|
|
|
IPO by April 15, 2014
|
65
|
%
|
|
Sale by September 30, 2015
|
10
|
%
|
|
Private company
|
15
|
%
|
|
Dissolution
|
10
|
%
|
|
|
Years Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
Employees and Directors:
|
|||||||||||
|
Research and development
|
$
|
1,412
|
|
$
|
763
|
|
$
|
262
|
|
||
|
General and administrative
|
2,012
|
|
937
|
|
503
|
|
|||||
|
Total
|
$
|
3,424
|
|
$
|
1,700
|
|
$
|
765
|
|
||
|
Non-Employees:
|
|||||||||||
|
Research and development
|
$
|
490
|
|
$
|
757
|
|
$
|
149
|
|
||
|
General and administrative
|
115
|
|
53
|
|
34
|
|
|||||
|
Total
|
$
|
605
|
|
$
|
810
|
|
$
|
183
|
|
||
|
|
Year Ended December 31,
|
||||||||||
|
|
2015
|
2014
|
2013
|
||||||||
|
United States
|
$
|
(51,779
|
)
|
$
|
(47,482
|
)
|
$
|
(32,524
|
)
|
||
|
Foreign
|
(244
|
)
|
(185
|
)
|
(194
|
)
|
|||||
|
$
|
(52,023
|
)
|
$
|
(47,667
|
)
|
$
|
(32,718
|
)
|
|||
|
|
Year Ended December 31,
|
|||||||
|
|
2015
|
2014
|
2013
|
|||||
|
Federal statutory rate
|
35.0
|
%
|
35.0
|
%
|
35.0
|
%
|
||
|
State tax (net of federal benefit)
|
5.8
|
%
|
5.4
|
%
|
5.3
|
%
|
||
|
Research and development credits
|
3.0
|
%
|
3.8
|
%
|
3.9
|
%
|
||
|
Warrants/purchase rights liabilities
|
0.0
|
%
|
2.2
|
%
|
(1.6
|
)%
|
||
|
Foreign net operating losses
|
(0.2
|
)%
|
(0.4
|
)%
|
(1.2
|
)%
|
||
|
382 limited net operating losses and credits
|
0.0
|
%
|
0.0
|
%
|
(0.6
|
)%
|
||
|
Change in valuation allowance
|
(43.3
|
)%
|
(45.1
|
)%
|
(40.0
|
)%
|
||
|
Other
|
(0.3
|
)%
|
(0.9
|
)%
|
(0.8
|
)%
|
||
|
Effective tax rate
|
0.0
|
%
|
0.0
|
%
|
0.0
|
%
|
||
|
|
December 31,
|
||||||
|
|
2015
|
2014
|
|||||
|
|
(in thousands)
|
||||||
|
Deferred tax assets:
|
|||||||
|
Net operating loss carryforwards
|
$
|
53,218
|
|
$
|
33,517
|
|
|
|
Research and development tax credits
|
4,903
|
|
3,269
|
|
|||
|
Stock compensation
|
2,000
|
|
879
|
|
|||
|
Foreign net operating loss carryforwards
|
333
|
|
377
|
|
|||
|
Other, net
|
1,823
|
|
1,715
|
|
|||
|
Total deferred tax assets
|
62,277
|
|
39,757
|
|
|||
|
Less valuation allowance
|
(62,277
|
)
|
(39,757
|
)
|
|||
|
$
|
—
|
|
$
|
—
|
|
||
|
|
Year Ended
December 31, |
||||||
|
|
2015
|
2014
|
|||||
|
Balance at beginning of year
|
$
|
938
|
|
$
|
407
|
|
|
|
Additions based on tax positions related to the current year
|
484
|
|
533
|
|
|||
|
Changes for prior period tax positions
|
—
|
|
(2
|
)
|
|||
|
Balance at end of year
|
$
|
1,422
|
|
$
|
938
|
|
|
|
|
For the Quarters Ended
|
|
|||||||||||||||||
|
|
March 31
|
June 30
|
September 30
|
December 31
|
Total Year
|
||||||||||||||
|
2015:
|
|||||||||||||||||||
|
Operating expenses
|
$
|
14,817
|
|
$
|
15,078
|
|
$
|
12,335
|
|
$
|
9,890
|
|
$
|
52,120
|
|
||||
|
Net loss
|
(14,758
|
)
|
(15,106
|
)
|
(12,300
|
)
|
(9,859
|
)
|
(52,023
|
)
|
|||||||||
|
Net loss attributable to common stockholders
|
(14,758
|
)
|
(15,106
|
)
|
(12,300
|
)
|
(9,859
|
)
|
(52,023
|
)
|
|||||||||
|
Basic and diluted net loss per share attributable to common stockholders (1)
|
$
|
(0.62
|
)
|
$
|
(0.63
|
)
|
$
|
(0.51
|
)
|
$
|
(0.34
|
)
|
|||||||
|
2014:
|
|||||||||||||||||||
|
Operating expenses
|
$
|
11,876
|
|
$
|
11,638
|
|
$
|
12,810
|
|
$
|
14,018
|
|
$
|
50,342
|
|
||||
|
Net loss
|
(10,748
|
)
|
(10,167
|
)
|
(12,798
|
)
|
(13,954
|
)
|
(47,667
|
)
|
|||||||||
|
Net loss attributable to common stockholders
|
(13,818
|
)
|
(16,251
|
)
|
(12,798
|
)
|
(13,954
|
)
|
(56,821
|
)
|
|||||||||
|
Basic and diluted net loss per share attributable to common stockholders (1)
|
$
|
(24.49
|
)
|
$
|
(0.91
|
)
|
$
|
(0.59
|
)
|
$
|
(0.59
|
)
|
|||||||
|
(1)
|
Net loss per share is computed independently for each of the quarters presented. Therefore, the sum of the quarterly per share calculations will not necessarily equal the annual per share calculation.
|
|
Vital Therapies, Inc.
|
||
|
Date: March 8, 2016
|
By:
|
/s/ Terence E. Winters, Ph.D.
|
|
Terence E. Winters, Ph.D.
|
||
|
Chief Executive Officer
|
||
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|||
|
/s/ TERENCE E. WINTERS, Ph.D.
|
|
Co-Chairman and Chief Executive Officer (Principal Executive Officer)
|
|
March 8, 2016
|
|
Terence E. Winters, Ph.D.
|
||||
|
/s/ MICHAEL V. SWANSON
|
|
Chief Financial Officer
(Principal Financial Officer and Principal Accounting Officer)
|
|
March 8, 2016
|
|
Michael V. Swanson
|
||||
|
/s/ MUNEER A. SATTER
|
|
Co-Chairman and Lead Director
|
|
March 8, 2016
|
|
Muneer A. Satter
|
||||
|
/s/ JEAN-JACQUES BIENAIMÉ
|
|
Director
|
|
March 8, 2016
|
|
Jean-Jacques Bienaimé
|
||||
|
/s/ CHERYL L. COHEN
|
|
Director
|
|
March 8, 2016
|
|
Cheryl L. Cohen
|
||||
|
/s/ PHILIP M. CROXFORD
|
|
Director
|
|
March 8, 2016
|
|
Philip M. Croxford
|
||||
|
/s/ DOUGLAS E. GODSHALL
|
|
Director
|
|
March 8, 2016
|
|
Douglas E. Godshall
|
||||
|
/s/ ERROL R. HALPERIN
|
|
Director
|
|
March 8, 2016
|
|
Errol R. Halperin
|
||||
|
/s/ J. MICHAEL MILLIS, M.D.
|
|
Director
|
|
March 8, 2016
|
|
J. Michael Millis, M.D.
|
||||
|
/s/ LOWELL E. SEARS
|
|
Director
|
|
March 8, 2016
|
|
Lowell E. Sears
|
||||
|
/s/ RANDOLPH C. STEER, M.D., PH.D.
|
|
Director
|
|
March 8, 2016
|
|
Randolph C. Steer, M.D., Ph.D.
|
||||